OPER H6001. Pharmaceutical Operations Mgt. APPROVED. Pharmaceutical Operations Management for Engineers. Credits: 5. NFQ Level: 9
|
|
|
- Bernard Richard
- 9 years ago
- Views:
Transcription
1 Short Title: Full Title: APPROVED Management for Engineers Module Code: OPER H6001 Credits: 5 NFQ Level: 9 Field of Study: Mechanics and metal work Module Delivered in 1 programme(s) Reviewed By: GERARD RYDER Module Author: HUGH CLAFFEY Module Description: To provide engineering students with an organisational structure for the management of pharmaceutical operations in the areas of validation, process control and regulation Learning Outcomes On successful completion of this module the learner will be able to: LO1 LO2 LO3 LO4 LO5 LO6 Design and interpret a pharmaceutical risk assessment Understand the requirements and implications of a pharmaceutical facility GMP inspection Apply the ICH guidelines to design a Pharmaceutical Management structure Validate GMP critical systems and processes Devise and interpret process validation documentation Apply regulatory guidelines to pharmaceutical manufacturing automation systems Pre-requisite learning Co-requisite Modules No Co-requisite modules listed
2 Module Content & Assessment Content (The percentage workload breakdown is inidcative and subject to change) % Pharmaceutical Management Structure Management systems compliant with best practice and current regulations. Use of ICH guidelines. Applications of Risk Management. CAPA and Six Sigma Regulatory Inspections IMB/FDA GMP facility inspections and GDP Rick Management Identify, assess and prioritize risks in a pharmaceutical manufacturing environment Process Validation QbD approach, Risk Management, ASTM 2500, ICH Q8,9 &10, CQAs, CPPs, biopharmaceutical, API and solid dosage processes, medical device manufacturing processes. Validation of GMP critical utilities Regulatory requirements and guidelines, sampling plan and frequency tests IQ, OQ and PQ requirements of HVAC, USP and WFI water, compressed gases and clean steam. Automated manufacturing process validation for pharmaceutical manufacturing Regulatory standards and guidelines; EU GMP Annex 11, FDA CFR 21 part 11, GAMP/lifecycle approach, documentation requirements % 20.00% Assessment Breakdown % Course Work 30.00% End of Module Formal Examination 70.00% Course Work Assessment Type Assessment Description Outcome addressed % of total Assessment Date Using FMEA case studies, identify, assess and prioritize risks in a pharmaceutical environment. Using WHO Case studies in GMP inspections, carry out an ad udit techniques, reference 21 CFR Part 11, GAMP Preparation of Marketing Authorization Applications and associated variations and renewals. Comparison of EU, PIC/S and FDA policy and procedures n/a n/a 5, n/a End of Module Formal Examination Assessment Type Assessment Description Outcome addressed % of total Assessment Date Formal Exam End-of-Semester Final Examination 1,2,3,4,5, End-of-Semester Reassessment Requirement Repeat examination Reassessment of this module will consist of a repeat examination. It is possible that there will also be a requirement to be reassessed in a coursework element. IT Tallaght reserves the right to alter the nature and timings of assessment
3 Module Workload Workload: Full Time Workload Type Workload Description Hours Frequency Average ly Learner Workload Lecture theory,, case studies, 3.00 Every Independent Learning supplemental reading, preparation of assignments 5.00 Every Total ly Learner Workload 8.00 Total ly Contact Hours 3.00 Workload: Part Time Workload Type Workload Description Hours Frequency Average ly Learner Workload Lecture theory, case studis 3.00 Every Independent Learning Time supplemental reading, preparation of assignments 5.00 Every Total ly Learner Workload 8.00 Total ly Contact Hours 3.00
4 Module Resources Recommended Book Resources Hamid Mollah, Harold Baseman, Mike Long, 2001, Application of Risk Management for Pharmaceutical and Biological Products Manufacturing, Wiley [ISBN: ] James L. Vesper, 2006, Risk Assessment and Risk Management in the Pharmaceutical Industry, GMP publishing [ISBN: ] Jackelyn Rodriguez, 2011, Capa in the Pharmaceutical and Biotech Industries, Biohealthcare Publishing [ISBN: ] edited by Joseph F. despautz 1998, Automation and validation of information in pharmaceutical processing, Marcel Dekker New York [ISBN: ] Stephen Robert Goldman, 2003, Handbook of Computer and Computerized System Validation for the Pharmaceutical Industry [ISBN: ] 2008, The GAMP Guide for Validation of Automated Systems [ISBN: ] Required Article/Paper Resources ICH 2011, ICH Q9 Quality Risk Management ICH 2008, ICH Q10 Pharmaceutical Quality System FDA 2009, White paper FDA Guidance for Industry update Process Validation, Pharmaceutical Engineering, 29 no 3 GHTF 2004, Quality Management Systems - Process Validation Guidance, Global Harmonisation Taskforce Guidelines This module does not have any other resources
5 Module Delivered in Programme Code Programme Semester Delivery TA_EAMEC_M Master of Engineering in Mechanical Engineering 9 Mandatory
GAMP5 - a lifecycle management framework for customized bioprocess solutions
 GE Healthcare Life Sciences GAMP5 - a lifecycle management framework for customized bioprocess solutions imagination at work GE Healthcare s engineering department, Customized Bioprocess Solutions (CBS),
GE Healthcare Life Sciences GAMP5 - a lifecycle management framework for customized bioprocess solutions imagination at work GE Healthcare s engineering department, Customized Bioprocess Solutions (CBS),
Advanced Web Application Development
 Long Title: Language of Instruction: Advanced Web English Module Code: H8AWA Credits: 5 NFQ Level: LEVEL 8 Field of Study: Computer use Taxonomy: Blooms Module Delivered in 1 programme(s) Module Coordinator:
Long Title: Language of Instruction: Advanced Web English Module Code: H8AWA Credits: 5 NFQ Level: LEVEL 8 Field of Study: Computer use Taxonomy: Blooms Module Delivered in 1 programme(s) Module Coordinator:
Industry Implications of Pharmaceutical Quality ICH Guidelines
 EIPG General Assembly Industry Implications of Pharmaceutical Quality ICH Guidelines 20 th April 2008 Pharmaceutical Quality Develop a harmonised pharmaceutical quality system applicable across the lifecycle
EIPG General Assembly Industry Implications of Pharmaceutical Quality ICH Guidelines 20 th April 2008 Pharmaceutical Quality Develop a harmonised pharmaceutical quality system applicable across the lifecycle
Library Guide: Pharmaceutical GMPs
 Library Guide: Pharmaceutical GMPs Table of Contents Overview...3 Courses Listed by Functional Area... 4 Course Descriptions: A Step-by-Step Approach to Process Validation (PHDV79)... 7 A Tour of the FDA
Library Guide: Pharmaceutical GMPs Table of Contents Overview...3 Courses Listed by Functional Area... 4 Course Descriptions: A Step-by-Step Approach to Process Validation (PHDV79)... 7 A Tour of the FDA
Principles. of Pharmaceutical Facility Design. Full Time Part Time Online. www.dpseng.com.sg
 Principles of Pharmaceutical Facility Design Full Time Part Time Online www.dpseng.com.sg Contents 1. Welcome 2. Programme Overview 3. Programme Content www.dpseng.com.sg 2 Welcome Transition into a new
Principles of Pharmaceutical Facility Design Full Time Part Time Online www.dpseng.com.sg Contents 1. Welcome 2. Programme Overview 3. Programme Content www.dpseng.com.sg 2 Welcome Transition into a new
Programme Code Programme Title Delivery Certificate in Tourism and Event Mandatory. Event Management. Event Management
 6.5 Event Management Programme Code Programme Title Delivery TEM601 Certificate in Tourism and Event Mandatory Management Module Title (short) Module Title (full) Event Management Event Management Module
6.5 Event Management Programme Code Programme Title Delivery TEM601 Certificate in Tourism and Event Mandatory Management Module Title (short) Module Title (full) Event Management Event Management Module
Electronic GMP Systems
 Electronic GMP Systems Specification - Implementation Validation 18 20 March 2015, Prague, Czech Republic SPEAKERS: Kai Kiefer fme AG, Germany Dr Bob McDowall McDowall Consulting, UK LEARNING OBJECTIVES:
Electronic GMP Systems Specification - Implementation Validation 18 20 March 2015, Prague, Czech Republic SPEAKERS: Kai Kiefer fme AG, Germany Dr Bob McDowall McDowall Consulting, UK LEARNING OBJECTIVES:
State of Control Over the Lifecycle and Process Validation (New and Legacy Products)
 State of Control Over the Lifecycle and Process Validation (New and Legacy Products) Grace McNally Branch Chief (acting), Regulatory Policy and Collaboration Branch FDA/CDER/Office of Compliance ICH Q10,
State of Control Over the Lifecycle and Process Validation (New and Legacy Products) Grace McNally Branch Chief (acting), Regulatory Policy and Collaboration Branch FDA/CDER/Office of Compliance ICH Q10,
Impact Assessment in a Science & Risk Based Environment. R. Legland 11/04/11
 Impact Assessment in a Science & Risk Based Environment R. Legland 11/04/11 Background US GMP s EU GMP s Japan GMP s ICH Q8, Q9, Q10 Guidance ASTM Standard E2500-07 Science and Risk Based Approach to Determine
Impact Assessment in a Science & Risk Based Environment R. Legland 11/04/11 Background US GMP s EU GMP s Japan GMP s ICH Q8, Q9, Q10 Guidance ASTM Standard E2500-07 Science and Risk Based Approach to Determine
ASSESSMENT OF QUALITY RISK MANAGEMENT IMPLEMENTATION
 PHARMACEUTICAL INSPECTION CONVENTION PHARMACEUTICAL INSPECTION CO-OPERATION SCHEME PI 038-1 26 March 2012 AIDE-MEMOIRE ASSESSMENT OF QUALITY RISK MANAGEMENT IMPLEMENTATION PIC/S March 2012 Reproduction
PHARMACEUTICAL INSPECTION CONVENTION PHARMACEUTICAL INSPECTION CO-OPERATION SCHEME PI 038-1 26 March 2012 AIDE-MEMOIRE ASSESSMENT OF QUALITY RISK MANAGEMENT IMPLEMENTATION PIC/S March 2012 Reproduction
 1 The quality management system (QMS) is the corner stone of compliance to GMP. The QMS is made up of several documents, that when followed ensures the GMP compliance of the process, facility and company.
1 The quality management system (QMS) is the corner stone of compliance to GMP. The QMS is made up of several documents, that when followed ensures the GMP compliance of the process, facility and company.
Process Validation: Practical Aspects of the New FDA Guidance
 Process Validation: Practical Aspects of the New FDA Guidance ISPE Boston Chapter Meeting April 18, 2013 Rusty Morrison Commissioning Agents, Inc. Objectives / Summary What is Process Validation? Regulatory
Process Validation: Practical Aspects of the New FDA Guidance ISPE Boston Chapter Meeting April 18, 2013 Rusty Morrison Commissioning Agents, Inc. Objectives / Summary What is Process Validation? Regulatory
This interpretation of the revised Annex
 Reprinted from PHARMACEUTICAL ENGINEERING The Official Magazine of ISPE July/August 2011, Vol. 31 No. 4 www.ispe.org Copyright ISPE 2011 The ISPE GAMP Community of Practice (COP) provides its interpretation
Reprinted from PHARMACEUTICAL ENGINEERING The Official Magazine of ISPE July/August 2011, Vol. 31 No. 4 www.ispe.org Copyright ISPE 2011 The ISPE GAMP Community of Practice (COP) provides its interpretation
[ABOUT THE AUTHOR. FDA Lifecycle Approach to Process Validation What, Why, and How? PQ Forum. Paul L. Pluta]
![[ABOUT THE AUTHOR. FDA Lifecycle Approach to Process Validation What, Why, and How? PQ Forum. Paul L. Pluta] [ABOUT THE AUTHOR. FDA Lifecycle Approach to Process Validation What, Why, and How? PQ Forum. Paul L. Pluta]](/thumbs/32/15497330.jpg) Paul L. Pluta] FDA Lifecycle Approach to Process Validation What, Why, and How? Paul L. Pluta PQ Forum provides a mechanism for validation practitioners to share information about Stage 2 process qualification
Paul L. Pluta] FDA Lifecycle Approach to Process Validation What, Why, and How? Paul L. Pluta PQ Forum provides a mechanism for validation practitioners to share information about Stage 2 process qualification
ICH Q10 Pharmaceutical Quality System (PQS)
 ICH Q10 Pharmaceutical Quality System (PQS) International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use ICH Q10 PQS Guideline Background Objectives
ICH Q10 Pharmaceutical Quality System (PQS) International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use ICH Q10 PQS Guideline Background Objectives
Workshop B Control Strategy
 ICH-GCG ASEAN Workshop B Control Strategy Jean-Louis ROBERT, Ph.D. National Health Laboratory, Luxembourg Chair-person ICH IWG Q8, Q9, Q10 Kuala Lumpur, 26-28 July 2010 International Conference on Harmonisation
ICH-GCG ASEAN Workshop B Control Strategy Jean-Louis ROBERT, Ph.D. National Health Laboratory, Luxembourg Chair-person ICH IWG Q8, Q9, Q10 Kuala Lumpur, 26-28 July 2010 International Conference on Harmonisation
GAMP 4 to GAMP 5 Summary
 GAMP 4 to GAMP 5 Summary Introduction This document provides summary information on the GAMP 5 Guide and provides a mapping to the previous version, GAMP 4. It specifically provides: 1. Summary of Need
GAMP 4 to GAMP 5 Summary Introduction This document provides summary information on the GAMP 5 Guide and provides a mapping to the previous version, GAMP 4. It specifically provides: 1. Summary of Need
White paper: FDA Guidance for Industry Update Process Validation
 White paper: FDA Guidance for Industry Update Process Validation In January 2011, the FDA released the final version of its long-awaited update to its Process Validation Guidance for Industry. Since then,
White paper: FDA Guidance for Industry Update Process Validation In January 2011, the FDA released the final version of its long-awaited update to its Process Validation Guidance for Industry. Since then,
Title:: Effective GMP AUDITS for APIs and Formulation Pharma Companies By G.Sundar-Director/Consultant PharmQA Compliance solutions
 WELCOME Title:: Effective GMP AUDITS for APIs and Formulation Pharma Companies By G.Sundar-Director/Consultant PharmQA Compliance solutions Contents: Introduction GMP Audit GMP Audit plan GMP Auditing
WELCOME Title:: Effective GMP AUDITS for APIs and Formulation Pharma Companies By G.Sundar-Director/Consultant PharmQA Compliance solutions Contents: Introduction GMP Audit GMP Audit plan GMP Auditing
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE Q4B ANNEX 12
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE EVALUATION AND RECOMMENDATION OF PHARMACOPOEIAL
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE EVALUATION AND RECOMMENDATION OF PHARMACOPOEIAL
Computerised Systems in Analytical Laboratories
 ECA Certified Computer Validation Manager Course* New EU Annex 11 and Chapter 4 Requirements will be covered Computerised Systems in Analytical Laboratories Foto: DRK The Electronic Analytical GMP : Integrating
ECA Certified Computer Validation Manager Course* New EU Annex 11 and Chapter 4 Requirements will be covered Computerised Systems in Analytical Laboratories Foto: DRK The Electronic Analytical GMP : Integrating
GUIDE TO GOOD MANUFACTURING PRACTICE FOR MEDICINAL PRODUCTS ANNEX 15 *
 PHARMACEUTICAL INSPECTION CONVENTION PHARMACEUTICAL INSPECTION CO-OPERATION SCHEME PS/INF 11/2015 1 April 2015 GUIDE TO GOOD MANUFACTURING PRACTICE FOR MEDICINAL PRODUCTS ANNEX 15 * * Entry into force:
PHARMACEUTICAL INSPECTION CONVENTION PHARMACEUTICAL INSPECTION CO-OPERATION SCHEME PS/INF 11/2015 1 April 2015 GUIDE TO GOOD MANUFACTURING PRACTICE FOR MEDICINAL PRODUCTS ANNEX 15 * * Entry into force:
Work plan for GMP/GDP Inspectors Working Group for 2016
 21 December 2015 EMA/INS/GMP/738756/2015 Compliance and Inspection Work plan for GMP/GDP Inspectors Working Group for 2016 Chairperson: Status David Cockburn January 2016 1. Meetings scheduled for 2016
21 December 2015 EMA/INS/GMP/738756/2015 Compliance and Inspection Work plan for GMP/GDP Inspectors Working Group for 2016 Chairperson: Status David Cockburn January 2016 1. Meetings scheduled for 2016
In 2001, ISPE issued Baseline Guide Volume
 In today s biopharma and pharmaceutical industries, three related, but distinct terms are in common use: commissioning, qualification, and verification. Inconsistent interpretation and application of these
In today s biopharma and pharmaceutical industries, three related, but distinct terms are in common use: commissioning, qualification, and verification. Inconsistent interpretation and application of these
ICH Q10 - Pharmaceutical Quality System
 WCC PDA Dinner Meeting Jan 2012 ICH Q10 - Pharmaceutical Quality System Neil Wilkinson NSF-DBA www.nsf-dba.com ICHQ10.1 WCC PDA Dinner Meeting Jan 2012 Your Presenter Partner at NSF-DBA USA Training, Consultancy,
WCC PDA Dinner Meeting Jan 2012 ICH Q10 - Pharmaceutical Quality System Neil Wilkinson NSF-DBA www.nsf-dba.com ICHQ10.1 WCC PDA Dinner Meeting Jan 2012 Your Presenter Partner at NSF-DBA USA Training, Consultancy,
Commercial Manufacturing - Qualification & Validation-related GMP Deficiencies and Other Lifecycle Considerations
 Commercial Manufacturing - Qualification & Validation-related GMP Deficiencies and Other Lifecycle Considerations Kevin O Donnell PhD Market Compliance Manager, IMB PDA / FDA Conference Pharmaceutical
Commercial Manufacturing - Qualification & Validation-related GMP Deficiencies and Other Lifecycle Considerations Kevin O Donnell PhD Market Compliance Manager, IMB PDA / FDA Conference Pharmaceutical
Building an Effective Supplier Control Program: A review of key program elements & their implementation.
 Building an Effective Supplier Control Program: A review of key program elements & their implementation. Jonathan Lee VP RQCS Medtronic Surgical Technologies Building an Effective Supplier Control Program
Building an Effective Supplier Control Program: A review of key program elements & their implementation. Jonathan Lee VP RQCS Medtronic Surgical Technologies Building an Effective Supplier Control Program
What is the correct title of this publication? What is the current status of understanding and implementation?
 GMP Rules and Guidelines in 2013 for Computer System Validation / Computerises Systems / Electronic Records and Signatures/ IT Infrastructure and Application Compliance: What is the correct title of this
GMP Rules and Guidelines in 2013 for Computer System Validation / Computerises Systems / Electronic Records and Signatures/ IT Infrastructure and Application Compliance: What is the correct title of this
UNICEF s Quality Assurance System for procurement of medicines
 UNICEF s Quality Assurance System for procurement of medicines QA Specialist Peter Svarrer Jakobsen Joint UNICEF, UNFPA and WHO Meeting with Manufacturers and Suppliers UN City, Copenhagen 23 September
UNICEF s Quality Assurance System for procurement of medicines QA Specialist Peter Svarrer Jakobsen Joint UNICEF, UNFPA and WHO Meeting with Manufacturers and Suppliers UN City, Copenhagen 23 September
Implementing Lifecycle Validation Practices at Contract Manufacturing Organizations
 Implementing Lifecycle Validation Practices at Contract Manufacturing Organizations This paper discusses the nuances of lifecycle validation implementation at contract manufacturing organizations (CMOs).
Implementing Lifecycle Validation Practices at Contract Manufacturing Organizations This paper discusses the nuances of lifecycle validation implementation at contract manufacturing organizations (CMOs).
GE Healthcare Life Sciences. Validation Services. Compliance support through life cycle management
 GE Healthcare Life Sciences Validation Services Compliance support through life cycle management Validation Services Validation Services is an independent product and service provider within GE Healthcare
GE Healthcare Life Sciences Validation Services Compliance support through life cycle management Validation Services Validation Services is an independent product and service provider within GE Healthcare
Pharmaceutical Auditor Training
 www.nsf.org Formerly NSF-DBA The right people. The right solution. The first time. Pharmaceutical or Training Pharmaceutical GMP s and Self-Inspections PQMS or/lead or Training Course A17638 Why Choose
www.nsf.org Formerly NSF-DBA The right people. The right solution. The first time. Pharmaceutical or Training Pharmaceutical GMP s and Self-Inspections PQMS or/lead or Training Course A17638 Why Choose
FDA Guidance for Industry Update - Process Validation
 FDA Guidance Update: Process Validation: General Principles and Practices White Paper FDA Guidance for Industry Update - Process Validation The changing face of Validation; are IQ, OQ and PQ really dead
FDA Guidance Update: Process Validation: General Principles and Practices White Paper FDA Guidance for Industry Update - Process Validation The changing face of Validation; are IQ, OQ and PQ really dead
A NUSAGE-PharmEng Pharmaceutical and Biotechnology Training Program
 A NUSAGE-PharmEng Pharmaceutical and Biotechnology Training Program Shaping Human Capital for Challenges in the Pharmaceutical Industry CLEANING VALIDATION Instructors Loh Kean Chong Rick Ng Date and Time
A NUSAGE-PharmEng Pharmaceutical and Biotechnology Training Program Shaping Human Capital for Challenges in the Pharmaceutical Industry CLEANING VALIDATION Instructors Loh Kean Chong Rick Ng Date and Time
Guidance for Industry
 Guidance for Industry Q8, Q9, and Q10 Questions and Answers(R4) U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics
Guidance for Industry Q8, Q9, and Q10 Questions and Answers(R4) U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics
EVALUATION AND RECOMMENDATION OF PHARMACOPOEIAL TEXTS FOR USE IN THE ICH REGIONS ON TABLET FRIABILITY GENERAL CHAPTER Q4B ANNEX 9(R1)
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE EVALUATION AND RECOMMENDATION OF PHARMACOPOEIAL
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE EVALUATION AND RECOMMENDATION OF PHARMACOPOEIAL
Pharmaceutical Engineering: The Lisbon Masters Program
 EAFP Catania June 24-26, 26, 2010 Pharmaceutical Engineering: The Lisbon Masters Program José Menezes, Rogério Gaspar, João Bordado,, José Morais Technical University of Lisbon (Portugal) Faculty of Pharmacy,
EAFP Catania June 24-26, 26, 2010 Pharmaceutical Engineering: The Lisbon Masters Program José Menezes, Rogério Gaspar, João Bordado,, José Morais Technical University of Lisbon (Portugal) Faculty of Pharmacy,
3rd ANNUAL SEMINAR PHARMASSIST Ltd in collaboration with PHARMA D&S Srl
 Venue: To be announced 3rd ANNUAL SEMINAR Quality Risk Management & Quality Risk Management (ICH Q9) & Applications in the Pharmaceutical Quality System (ICH Q10): Applications in the Pharmaceutical Sector
Venue: To be announced 3rd ANNUAL SEMINAR Quality Risk Management & Quality Risk Management (ICH Q9) & Applications in the Pharmaceutical Quality System (ICH Q10): Applications in the Pharmaceutical Sector
LIBRARY GUIDE. Online Courses. March 2012
 LIBRARY GUIDE Online Courses March 2012 i Table of Contents OVERVIEW..................................................................................... 1 COURSE DESCRIPTIONS (LISTED ALPHABETICALLY)...............................................
LIBRARY GUIDE Online Courses March 2012 i Table of Contents OVERVIEW..................................................................................... 1 COURSE DESCRIPTIONS (LISTED ALPHABETICALLY)...............................................
The Effective Management of Change Across the ICHQ10 Lifecycle
 The Effective Management of Change Across the ICHQ10 Lifecycle Rob Hughes AstraZeneca 1 Change Management the guide This presentation will: describe a structured approach to change across the ICH Q10 lifecycle
The Effective Management of Change Across the ICHQ10 Lifecycle Rob Hughes AstraZeneca 1 Change Management the guide This presentation will: describe a structured approach to change across the ICH Q10 lifecycle
Calibration & Preventative Maintenance. Sally Wolfgang Manager, Quality Operations Merck & Co., Inc.
 Calibration & Preventative Maintenance Sally Wolfgang Manager, Quality Operations Merck & Co., Inc. Calibration: A comparison of two instruments or measuring devices one of which is a standard of known
Calibration & Preventative Maintenance Sally Wolfgang Manager, Quality Operations Merck & Co., Inc. Calibration: A comparison of two instruments or measuring devices one of which is a standard of known
On-Site GMP Training GMP COMPLIANCE TECHNICAL
 PharmaNet On-Site GMP Training GMP COMPLIANCE TECHNICAL 284 E Lake Mead Pkwy Suite C-278 Henderson, NV 89015 Phone: 702-558-0094 Fax: 702-558-0079 www.gmpseminars.com Key to Level of Program Level A: Program
PharmaNet On-Site GMP Training GMP COMPLIANCE TECHNICAL 284 E Lake Mead Pkwy Suite C-278 Henderson, NV 89015 Phone: 702-558-0094 Fax: 702-558-0079 www.gmpseminars.com Key to Level of Program Level A: Program
The Technical Compliance Manager
 The Technical Compliance Manager Avoiding Non-Compliance in the technical area 3-5 June 2014, Berlin, Germany SPEAKERS: Dr Gerhard Hauser European Hygienic Engineering and Design Group (EHEDG) Dr Jean
The Technical Compliance Manager Avoiding Non-Compliance in the technical area 3-5 June 2014, Berlin, Germany SPEAKERS: Dr Gerhard Hauser European Hygienic Engineering and Design Group (EHEDG) Dr Jean
Module Documentation
 Module Documentation MARK07010 Contents of this document are copyright of Galway Mayo Institute of Technology Page 1 of 5 MARK07010 Short Title Full Title Attendance N/A Discipline 342 MARKETING & ADVERTISING
Module Documentation MARK07010 Contents of this document are copyright of Galway Mayo Institute of Technology Page 1 of 5 MARK07010 Short Title Full Title Attendance N/A Discipline 342 MARKETING & ADVERTISING
Comparative analysis between the possible regulatory approaches to GMP compliance TITOLO PRESENTAZIONE
 Comparative analysis between the possible regulatory approaches to GMP compliance TITOLO PRESENTAZIONE Dr. Fulvio CARLOTTI, GNOSIS SpA, Corporate QA Director September 26, 2014 Scope of GMP GMP compliance
Comparative analysis between the possible regulatory approaches to GMP compliance TITOLO PRESENTAZIONE Dr. Fulvio CARLOTTI, GNOSIS SpA, Corporate QA Director September 26, 2014 Scope of GMP GMP compliance
Harmonizing Change Control Processes Globally
 Quality & Compliance Associates, LLC Harmonizing Change Control Processes Globally President Quality & Compliance Associates, LLC When We Deal In A Global Environment, How Do We Design A System That Addresses
Quality & Compliance Associates, LLC Harmonizing Change Control Processes Globally President Quality & Compliance Associates, LLC When We Deal In A Global Environment, How Do We Design A System That Addresses
Job description. Job title. Process Engineer. Responsible to. Process Engineering Manager. Hours/sessions per week 37.
 Job description Job title Directorate Pay band Responsible to Base/location Process Engineer Production SEO Process Engineering Manager Porton Hours/sessions per week 37.5 Job type Permanent INTRODUCTION
Job description Job title Directorate Pay band Responsible to Base/location Process Engineer Production SEO Process Engineering Manager Porton Hours/sessions per week 37.5 Job type Permanent INTRODUCTION
Testing Automated Manufacturing Processes
 Testing Automated Manufacturing Processes (PLC based architecture) 1 ❶ Introduction. ❷ Regulations. ❸ CSV Automated Manufacturing Systems. ❹ PLCs Validation Methodology / Approach. ❺ Testing. ❻ Controls
Testing Automated Manufacturing Processes (PLC based architecture) 1 ❶ Introduction. ❷ Regulations. ❸ CSV Automated Manufacturing Systems. ❹ PLCs Validation Methodology / Approach. ❺ Testing. ❻ Controls
Training Course Computerized System Validation in the Pharmaceutical Industry Istanbul, 16-17 January 2003. Change Control
 Training Course Computerized System Validation in the Pharmaceutical Industry Istanbul, 16-17 January 2003 Change Control Wolfgang Schumacher Roche Pharmaceuticals, Basel Agenda Change Control Definitions
Training Course Computerized System Validation in the Pharmaceutical Industry Istanbul, 16-17 January 2003 Change Control Wolfgang Schumacher Roche Pharmaceuticals, Basel Agenda Change Control Definitions
Computer-Aided Facilities Management - A Logical Trend
 Computer-Aided Facilities Management - A Logical Trend Two major organisational changes are part of the modern pharmaceutical industry scene. Firstly, mergers as companies seek to consolidate resources
Computer-Aided Facilities Management - A Logical Trend Two major organisational changes are part of the modern pharmaceutical industry scene. Firstly, mergers as companies seek to consolidate resources
Process Validation Guidance Requirements (FDA and EU Annex 15: Qualification and Validation)
 Global CompliancePanel Knowledge, a Way Forward 2-day In-person Seminar: Process Validation Guidance Requirements (FDA and EU Annex 15: Qualification and Validation) Los Angeles, CA July 28th & 29th, 2016
Global CompliancePanel Knowledge, a Way Forward 2-day In-person Seminar: Process Validation Guidance Requirements (FDA and EU Annex 15: Qualification and Validation) Los Angeles, CA July 28th & 29th, 2016
OMCL Network of the Council of Europe QUALITY ASSURANCE DOCUMENT
 OMCL Network of the Council of Europe QUALITY ASSURANCE DOCUMENT PA/PH/OMCL (08) 69 3R Full document title and reference Document type VALIDATION OF COMPUTERISED SYSTEMS Legislative basis - CORE DOCUMENT
OMCL Network of the Council of Europe QUALITY ASSURANCE DOCUMENT PA/PH/OMCL (08) 69 3R Full document title and reference Document type VALIDATION OF COMPUTERISED SYSTEMS Legislative basis - CORE DOCUMENT
Working Party on Control of Medicines and Inspections. Final Version of Annex 15 to the EU Guide to Good Manufacturing Practice
 EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Single market, regulatory environment, industries under vertical legislation Pharmaceuticals and cosmetics Brussels, July 2001 Working Party on Control
EUROPEAN COMMISSION ENTERPRISE DIRECTORATE-GENERAL Single market, regulatory environment, industries under vertical legislation Pharmaceuticals and cosmetics Brussels, July 2001 Working Party on Control
UNICEF s Quality Assurance System for Procurement of Micronutrient Powders (MNP)
 UNICEF s Quality Assurance System for Procurement of Micronutrient Powders (MNP) Nutrition Supplier Meeting, June 30, 2015 Dimitris Catsoulacos Quality Assurance Specialist PRESENTATION OVERVIEW Quality
UNICEF s Quality Assurance System for Procurement of Micronutrient Powders (MNP) Nutrition Supplier Meeting, June 30, 2015 Dimitris Catsoulacos Quality Assurance Specialist PRESENTATION OVERVIEW Quality
Quality Risk Management Tools Quality Risk Management Tool Selection When to Select FMEA: QRM Tool Selection Matrix
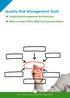 Quality Risk Management Tools Quality Risk Management Tool Selection When to Select FMEA: QRM Tool Selection Matrix 26 Quality Risk Management Tools The ICH Q9 guideline, Quality Risk Management, provides
Quality Risk Management Tools Quality Risk Management Tool Selection When to Select FMEA: QRM Tool Selection Matrix 26 Quality Risk Management Tools The ICH Q9 guideline, Quality Risk Management, provides
GCP - Records Managers Association
 GCP - Records Managers Association Guidance on the Scanning and Destruction of Paper Records 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 The introduction
GCP - Records Managers Association Guidance on the Scanning and Destruction of Paper Records 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 The introduction
INTRODUCTION. This book offers a systematic, ten-step approach, from the decision to validate to
 INTRODUCTION This book offers a systematic, ten-step approach, from the decision to validate to the assessment of the validation outcome, for validating configurable off-the-shelf (COTS) computer software
INTRODUCTION This book offers a systematic, ten-step approach, from the decision to validate to the assessment of the validation outcome, for validating configurable off-the-shelf (COTS) computer software
Staying Current in Cold Chain Management
 Staying Current in Cold Chain Management Changes to USP, EU GDPs and Storage and Shipping Practices for Drugs Table of Contents AUTHORS...3 Paul Daniel...3 Piritta Maunu...3 EDITOR...3 John Ferreira...3
Staying Current in Cold Chain Management Changes to USP, EU GDPs and Storage and Shipping Practices for Drugs Table of Contents AUTHORS...3 Paul Daniel...3 Piritta Maunu...3 EDITOR...3 John Ferreira...3
Rx-360 Supplier Assessment Questionnaire (SAQ)
 Rx-360 Supplier Assessment Questionnaire (SAQ) The Rx-360 Supplier Assessment Questionnaire (SAQ) was created by the Rx-360 Supplier-Led Working Group. The Supplier-Led Working Group is an Rx-360 working
Rx-360 Supplier Assessment Questionnaire (SAQ) The Rx-360 Supplier Assessment Questionnaire (SAQ) was created by the Rx-360 Supplier-Led Working Group. The Supplier-Led Working Group is an Rx-360 working
LabChip GX/GXII with LabChip GxP Software
 Regulatory Compliance LabChip GX/GXII with LabChip GxP Software Supporting Regulatory Compliance Caliper LabChip GX/GXII suite of instruments provides automated electrophoresis to analyze quality, size,
Regulatory Compliance LabChip GX/GXII with LabChip GxP Software Supporting Regulatory Compliance Caliper LabChip GX/GXII suite of instruments provides automated electrophoresis to analyze quality, size,
CONCEPT HEIDELBERG. Pharmaceutical Quality Training. Conferences. Services.
 CONCEPT HEIDELBERG Pharmaceutical Quality Training. Conferences. Services. About Us Helping you to Comply with GMP Since our foundation on 1 April 1978, CONCEPT HEIDELBERG has been concentrating on providing
CONCEPT HEIDELBERG Pharmaceutical Quality Training. Conferences. Services. About Us Helping you to Comply with GMP Since our foundation on 1 April 1978, CONCEPT HEIDELBERG has been concentrating on providing
Introduction to Q10 Pharmaceutical Quality System
 ICH-GCG Asean Training Workshop on ICH Guidelines Q8,Q9 and Q10 (New Paradigm) Introduction to Q10 Pharmaceutical Quality System Georges FRANCE, Q- IWG Kuala Lumpur, Malaysia 26-28 July 2010 International
ICH-GCG Asean Training Workshop on ICH Guidelines Q8,Q9 and Q10 (New Paradigm) Introduction to Q10 Pharmaceutical Quality System Georges FRANCE, Q- IWG Kuala Lumpur, Malaysia 26-28 July 2010 International
Using CITI Program Content: Good Clinical Practice (GCP)
 CONTENT AUTHOR Jaime A. Arango, EdD, CIP CITI Program at the University of Miami INTRODUCTION Like all CITI Program educational materials, the components of the Good Clinical Practice (GCP) series can
CONTENT AUTHOR Jaime A. Arango, EdD, CIP CITI Program at the University of Miami INTRODUCTION Like all CITI Program educational materials, the components of the Good Clinical Practice (GCP) series can
ICH guideline Q10 on pharmaceutical quality system
 September 2015 EMA/CHMP/ICH/214732/2007 Committee for Human Medicinal Products Step 5 Transmission to CHMP May 2007 Transmission to interested parties May 2007 Deadline for comments November 2007 Final
September 2015 EMA/CHMP/ICH/214732/2007 Committee for Human Medicinal Products Step 5 Transmission to CHMP May 2007 Transmission to interested parties May 2007 Deadline for comments November 2007 Final
GOOD PRACTICES FOR COMPUTERISED SYSTEMS IN REGULATED GXP ENVIRONMENTS
 PHARMACEUTICAL INSPECTION CONVENTION PHARMACEUTICAL INSPECTION CO-OPERATION SCHEME PI 011-3 25 September 2007 PIC/S GUIDANCE GOOD PRACTICES FOR COMPUTERISED SYSTEMS IN REGULATED GXP ENVIRONMENTS PIC/S
PHARMACEUTICAL INSPECTION CONVENTION PHARMACEUTICAL INSPECTION CO-OPERATION SCHEME PI 011-3 25 September 2007 PIC/S GUIDANCE GOOD PRACTICES FOR COMPUTERISED SYSTEMS IN REGULATED GXP ENVIRONMENTS PIC/S
PharmAssess Audit Reports Operational Consultancy GMP Auditing Services Regulatory Affairs. www.rephine.com
 PharmAssess Audit Reports Operational Consultancy GMP Auditing Services Regulatory Affairs www.rephine.com The Market Leader in GMP Audit Reporting PharmAssess audit reports are accepted by all Regulatory
PharmAssess Audit Reports Operational Consultancy GMP Auditing Services Regulatory Affairs www.rephine.com The Market Leader in GMP Audit Reporting PharmAssess audit reports are accepted by all Regulatory
TRAINING TITLE: Internal Auditing Workshop (WORK-008)
 TRAINING TITLE: Internal Auditing Workshop (WORK-008) OVERVIEW: GMP regulations worldwide as well as FDA and ICH guidances require that companies have in place an internal quality audit program. Auditing
TRAINING TITLE: Internal Auditing Workshop (WORK-008) OVERVIEW: GMP regulations worldwide as well as FDA and ICH guidances require that companies have in place an internal quality audit program. Auditing
A Quality System Approach to Retrospective Validation of Manufacturing Support Systems William Lodato, P.E.
 A Quality System Approach to Retrospective Validation of Manufacturing Support Systems William Lodato, P.E. Abstract As manufacturing support systems (HVAC, Electrical, Compressed Air, Nitrogen, WFI, Control/Monitoring
A Quality System Approach to Retrospective Validation of Manufacturing Support Systems William Lodato, P.E. Abstract As manufacturing support systems (HVAC, Electrical, Compressed Air, Nitrogen, WFI, Control/Monitoring
ICH Public Meeting. Joseph C. Famulare. October 2, 2006. Acting Deputy Director Office of Compliance CDER / FDA Office of Compliance
 ICH Public Meeting October 2, 2006 Joseph C. Famulare Acting Deputy Director Office of Compliance CDER / FDA Office of Compliance The Current State of Pharmaceutical Manufacturing Inability to predict
ICH Public Meeting October 2, 2006 Joseph C. Famulare Acting Deputy Director Office of Compliance CDER / FDA Office of Compliance The Current State of Pharmaceutical Manufacturing Inability to predict
Quality Risk Management
 PS/INF 1/2010 * * Quality Risk Management Quality Risk Management Implementation of ICH Q9 in the pharmaceutical field an example of methodology from PIC/S Document > Authors: L. Viornery (AFSSAPS) Ph.
PS/INF 1/2010 * * Quality Risk Management Quality Risk Management Implementation of ICH Q9 in the pharmaceutical field an example of methodology from PIC/S Document > Authors: L. Viornery (AFSSAPS) Ph.
PES INSTITUTE OF TECHNOLOGY B.E 5TH SEMESTER (AUTONOMOUS) - PROVISIONAL RESULTS JANUARY 2015 COMPUTER SCIENCE AND ENGINEERING BRANCH
 1 1PI12CS002 A A A A A B A A NA S NA NA NA NA NA NA NA 25.0 25.0 9.04 2 1PI12CS004 B I B C B A A A NA NA NA S NA NA NA NA NA 21.0 21.0 8.14 3 1PI12CS005 B B C B B B A B A NA NA NA NA NA NA NA NA 25.0 25.0
1 1PI12CS002 A A A A A B A A NA S NA NA NA NA NA NA NA 25.0 25.0 9.04 2 1PI12CS004 B I B C B A A A NA NA NA S NA NA NA NA NA 21.0 21.0 8.14 3 1PI12CS005 B B C B B B A B A NA NA NA NA NA NA NA NA 25.0 25.0
Post-Approval Change Management: Challenges and Opportunities An FDA Perspective
 CMC Workshop From Drug Development to Global Supply to Patients April 15-17, 2013, Washington, DC Post-Approval Change Management: Challenges and Opportunities An FDA Perspective Christine M. V. Moore,
CMC Workshop From Drug Development to Global Supply to Patients April 15-17, 2013, Washington, DC Post-Approval Change Management: Challenges and Opportunities An FDA Perspective Christine M. V. Moore,
Guidance for Industry. Q10 Pharmaceutical Quality System
 Guidance for Industry Q10 Pharmaceutical Quality System U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation
Guidance for Industry Q10 Pharmaceutical Quality System U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation
EQUIPMENT QUALIFICATION AND VALIDATION OF TABLET COMPRESSION MACHINE
 WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES P.Ramasubramaniyan et al. Volume 2, Issue 6, 5412-5418. Research Article ISSN 2278 4357 EQUIPMENT QUALIFICATION AND VALIDATION OF TABLET COMPRESSION
WORLD JOURNAL OF PHARMACY AND PHARMACEUTICAL SCIENCES P.Ramasubramaniyan et al. Volume 2, Issue 6, 5412-5418. Research Article ISSN 2278 4357 EQUIPMENT QUALIFICATION AND VALIDATION OF TABLET COMPRESSION
Pharmaceutical Quality Management System: Current Concept
 Pharmaceutical Quality Management System: Current Concept Neetu Dubey 1, *, Himanshu Gupta 3, R.K. Sharma 2, Nitin Dubey 1, Nidhi Dubey 4 1. IPS Academy, Indore, Madhya Pradesh, India. 2. Prestige Institute
Pharmaceutical Quality Management System: Current Concept Neetu Dubey 1, *, Himanshu Gupta 3, R.K. Sharma 2, Nitin Dubey 1, Nidhi Dubey 4 1. IPS Academy, Indore, Madhya Pradesh, India. 2. Prestige Institute
Lifecycle CMC Management: ICH Q12 Progress to date
 Lifecycle CMC Management: ICH Q12 Progress to date BWP/QWP/GMDP IWG Industry European workshop 28-29 October 2015 Jean-Louis ROBERT (EU) Graham Cook (EFPIA) Please take note The following slides represent
Lifecycle CMC Management: ICH Q12 Progress to date BWP/QWP/GMDP IWG Industry European workshop 28-29 October 2015 Jean-Louis ROBERT (EU) Graham Cook (EFPIA) Please take note The following slides represent
GOOD DOCUMENTATION AND QUALITY MANAGEMENT PRINCIPLES. Vimal Sachdeva Technical Officer (Inspector), WHO Prequalification of Medicines Programme
 GOOD DOCUMENTATION AND QUALITY MANAGEMENT PRINCIPLES Vimal Sachdeva Technical Officer (Inspector), WHO Prequalification of Medicines Programme Contents 1. Why good documentation is essential? 2. What constitutes
GOOD DOCUMENTATION AND QUALITY MANAGEMENT PRINCIPLES Vimal Sachdeva Technical Officer (Inspector), WHO Prequalification of Medicines Programme Contents 1. Why good documentation is essential? 2. What constitutes
Computer System Validation - It s More Than Just Testing
 Computer System Validation - It s More Than Just Testing Introduction Computer System Validation is the technical discipline that Life Science companies use to ensure that each Information Technology application
Computer System Validation - It s More Than Just Testing Introduction Computer System Validation is the technical discipline that Life Science companies use to ensure that each Information Technology application
GMP Pharma BV. Netherlands
 GMP Pharma BV Netherlands Connecting the European & Indian Pharmaceutical, Biotechnology and Biopharmaceutical Industry for parallel growth and solicitation. About Us: GMP Pharma BV is a multisource organization
GMP Pharma BV Netherlands Connecting the European & Indian Pharmaceutical, Biotechnology and Biopharmaceutical Industry for parallel growth and solicitation. About Us: GMP Pharma BV is a multisource organization
Analytical Instrument Qualification
 Analytical Instrument Qualification Participate in 4 Workshops! Foto: BÜCHI Practical Approaches for USP General Chapter Compliance in the QC Laboratory 19-21 May 2014, Berlin, Germany SPEAKERS:
Analytical Instrument Qualification Participate in 4 Workshops! Foto: BÜCHI Practical Approaches for USP General Chapter Compliance in the QC Laboratory 19-21 May 2014, Berlin, Germany SPEAKERS:
ICH Q 10 Pharmaceutical Quality System PQS DIA China Annual Meeting, May 2010
 ICH Q 10 Pharmaceutical Quality System PQS DIA China Annual Meeting, May 2010 Joseph C. Famulare Head of External Relations and Collaboration Pharma Global Technical Operations Global Quality, F. Hoffmann-La
ICH Q 10 Pharmaceutical Quality System PQS DIA China Annual Meeting, May 2010 Joseph C. Famulare Head of External Relations and Collaboration Pharma Global Technical Operations Global Quality, F. Hoffmann-La
Validation Approach and Scope for Business Process System Validation. Epitome Technologies Private Limited
 Validation Approach and Scope for Business Validation Epitome Technologies Private Limited PROPOSAL Page 2 of 8 Table Of Contents: 1.0 GENERAL... 3 2.0 SCOPE MODULES COVERED... 3 3.0 QUALIFICATION APPROACH...
Validation Approach and Scope for Business Validation Epitome Technologies Private Limited PROPOSAL Page 2 of 8 Table Of Contents: 1.0 GENERAL... 3 2.0 SCOPE MODULES COVERED... 3 3.0 QUALIFICATION APPROACH...
GAMP 5 and the Supplier Leveraging supplier advantage out of compliance
 GAMP 5 and the Supplier Leveraging supplier advantage out of compliance Paul Osborne Performance PharmaTech Ltd. Overview This document is designed to assist suppliers who wish to sell computer based equipment
GAMP 5 and the Supplier Leveraging supplier advantage out of compliance Paul Osborne Performance PharmaTech Ltd. Overview This document is designed to assist suppliers who wish to sell computer based equipment
PROPOSAL FOR REVISION OF THE SUPPLEMENTARY GUIDELINES ON GOOD MANUFACTURING PRACTICES: VALIDATION, APPENDIX 7: NON-STERILE PROCESS VALIDATION
 April 2013 RESTRICTED 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 PROPOSAL FOR REVISION OF THE SUPPLEMENTARY GUIDELINES ON GOOD MANUFACTURING PRACTICES:
April 2013 RESTRICTED 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 PROPOSAL FOR REVISION OF THE SUPPLEMENTARY GUIDELINES ON GOOD MANUFACTURING PRACTICES:
Using the ISPE s GAMP Methodology to Validate Environmental Monitoring System Software
 Using the ISPE s GAMP Methodology to Validate Environmental Monitoring System Software TEST DOCUMENTS DETAILED DOCUMENTS FUNCTIONAL SPECS USER REQUIREMENTS Introduction Continuous Monitoring Systems (CMS)
Using the ISPE s GAMP Methodology to Validate Environmental Monitoring System Software TEST DOCUMENTS DETAILED DOCUMENTS FUNCTIONAL SPECS USER REQUIREMENTS Introduction Continuous Monitoring Systems (CMS)
