Endovascular treatment of symptomatic atherosclerotic
|
|
|
- Osborn Morrison
- 9 years ago
- Views:
Transcription
1 Drug-Coated Balloon Versus Standard Percutaneous Transluminal Angioplasty for the Treatment of Superficial Femoral and Popliteal Peripheral Artery Disease 12-Month Results From the IN.PACT SFA Randomized Trial Gunnar Tepe, MD; John Laird, MD; Peter Schneider, MD; Marianne Brodmann, MD; Prakash Krishnan, MD; Antonio Micari, MD; Christopher Metzger, MD; Dierk Scheinert, MD; Thomas Zeller, MD; David J. Cohen, MD, MSc; David B. Snead, PhD; Beaux Alexander, MBA; Mario Landini, MS; Michael R. Jaff, DO; for the IN.PACT SFA Trial Investigators* Background Drug-coated balloons (DCBs) have shown promise in improving the outcomes for patients with peripheral artery disease. We compared a paclitaxel-coated balloon with percutaneous transluminal angioplasty (PTA) for the treatment of symptomatic superficial femoral and popliteal artery disease. Methods and Results The IN.PACT SFA Trial is a prospective, multicenter, single-blinded, randomized trial in which 331 patients with intermittent claudication or ischemic rest pain attributable to superficial femoral and popliteal peripheral artery disease were randomly assigned in a 2:1 ratio to treatment with DCB or PTA. The primary efficacy end point was primary patency, defined as freedom from restenosis or clinically driven target lesion revascularization at 12 months. Baseline characteristics were similar between the 2 groups. Mean lesion length and the percentage of total occlusions for the DCB and PTA arms were 8.94±4.89 and 8.81±5.12 cm (P=0.82) and 25.8% and 19.5% (P=0.22), respectively. DCB resulted in higher primary patency versus PTA (82.2% versus 52.4%; P<0.001). The rate of clinically driven target lesion revascularization was 2.4% in the DCB arm in comparison with 20.6% in the PTA arm (P<0.001). There was a low rate of vessel thrombosis in both arms (1.4% after DCB and 3.7% after PTA [P=0.10]). There were no device- or procedurerelated deaths and no major amputations. Conclusions In this prospective, multicenter, randomized trial, DCB was superior to PTA and had a favorable safety profile for the treatment of patients with symptomatic femoropopliteal peripheral artery disease. Clinical Trial Registration URL: Unique Identifiers: NCT and NCT (Circulation. 2015;131: DOI: /CIRCULATIONAHA ) Key Words: drug-eluting balloons peripheral arterial disease peripheral vascular diseases Endovascular treatment of symptomatic atherosclerotic peripheral artery disease (PAD) has gained widespread acceptance and is now recommended as the primary revascularization strategy in many clinical and anatomic scenarios. 1 3 Percutaneous transluminal angioplasty (PTA) of the superficial femoral and popliteal artery has a high initial success Clinical Perspective on p 502 rate, but restenosis occurs in up to 60% of cases. 4 Although randomized trials have demonstrated patency rates with bare metal stents and drug-eluting stents superior to those observed with PTA, 5 8 the optimal treatment for superficial femoral and popliteal artery disease remains controversial. Some practice guidelines advise against primary stenting in patients with intermittent claudication, 9 whereas others recommend primary stenting in short- or intermediate-length lesions 3 or Received May 5, 2014; accepted November 19, From the RodMed Klinikum, Rosenheim, Germany (G.T.); UC Davis, Sacramento, CA (J.L.); Kaiser Permanente Moanalua Medical Center and Clinic, Honolulu, HI (P.S.); Landeskrankenhaus Universitätsklinikum, Graz, Austria (M.B.); The Mount Sinai Medical Center, New York, NY (P.K.); GVM Care and Research, Lugo, Italy (A.M.); Maria Eleonora Hospital, Palermo, Italy (A.M.); Wellmont Holston Valley Medical Center, Kingsport, TN (C.M.); Park-Krankenhaus Leipzig, Germany (D.S.); Universitäts-Herzzentrum Freiburg - Bad Krozingen, Germany (T.Z.); Saint Luke s Mid America Heart Institute and University of Missouri-Kansas City School of Medicine, Kansas City, MO (D.J.C.); Medtronic, Inc., Santa Rosa, CA (D.B.S., B.A., M.L.); and Massachusetts General Hospital, Boston, MA (M.R.J.). *The principal investigators and clinical sites participating in the IN.PACT SFA Randomized Trial are listed in the online-only Data Supplement. The online-only Data Supplement is available with this article at /-/DC1. Correspondence to John R. Laird, MD, UC Davis Vascular Center, Lawrence J. Ellison Ambulatory Care Center, 4860 Y St, Suite 3400, Sacramento, CA [email protected] 2014 The Authors. Circulation is published on behalf of the American Heart Association, Inc., by Wolters Kluwer. This is an open access article under the terms of the Creative Commons Attribution Non-Commercial-NoDervis License, which permits use, distribution, and reproduction in any medium, provided that the original work is properly cited, the use is noncommercial, and no modifications or adaptations are made. Circulation is available at DOI: /CIRCULATIONAHA
2 496 Circulation February 3, 2015 in the event of acute PTA failure. 1 2 Despite the improved outcomes reported in some trials with stenting, the dynamic stresses applied by the superficial femoral and popliteal artery may result in stent fracture or in-stent restenosis. 12 Given the limitations of stenting, there has been considerable interest in identifying the approaches that could improve patency without the need for a permanent metallic implant. One approach to this challenge has been the development of the drug-coated balloon (DCB), which combines balloon dilatation with local delivery of an antiproliferative drug. Proofof-concept evidence has demonstrated the utility of different DCB technologies in reducing both restenosis and the need for reintervention in comparison with PTA Promising primary patency and target lesion revascularization rates up to 2 years postimplantation have been reported. 18 However, robust evidence from large randomized, controlled trials is lacking. The IN.PACT SFA Trial was designed to test the safety and efficacy of the IN.PACT Admiral DCB for the treatment of patients with symptomatic PAD in the superficial femoral and proximal popliteal artery. Methods Study Design The IN.PACT SFA Trial is a multicenter, international, singleblinded, randomized, controlled trial to assess the safety and efficacy of the IN.PACT Admiral DCB (Medtronic Inc, Santa Rosa, CA) versus standard PTA balloons in patients with symptomatic superficial femoral and proximal popliteal artery disease. The trial was prospectively designed to be conducted in 2 phases: IN.PACT SFA I (conducted in Europe) and IN.PACT SFA II (conducted in the United States), which are jointly referred to as IN.PACT SFA. The IN.PACT SFA Trial was prospectively analyzed according to a single statistical analysis plan. The 2 phases occurred sequentially in time with enrollment completed in the IN.PACT SFA I phase before the initiation of the IN.PACT SFA II phase. Minor differences between the IN.PACT SFA I phase and the IN.PACT SFA II phase eligibility criteria exist and include subtle variations in concomitant inflow and contralateral limb treatment, along with differences in predilatation requirements. A prespecified poolability test for treatment-by-trial phase interaction was established, with planned data pooling across the 2 phases in the event that there was no significant treatment-by-trial interaction. Both protocols were approved by the institutional review boards or ethics committees at each trial site. All patients provided written informed consent before enrollment. Both trial phases were conducted in accordance with the Declaration of Helsinki, good clinical practice guidelines, and applicable laws as specified by all relevant governmental bodies. An independent clinical events committee adjudicated all major adverse events. Independent core laboratories analyzed all images, including duplex ultrasonography (VasCore, Massachusetts General Hospital, Boston, MA) and angiography (SynvaCor, Springfield, IL). Patient Population Patients were eligible for enrollment if they had moderate to severe intermittent claudication or ischemic rest pain (Rutherford 2 4) and stenosis of 70% to 99% with lesion lengths between 4 and 18 cm or occlusion with lengths of 10 cm involving the superficial femoral and proximal popliteal arteries, and met all other eligibility criteria. Randomization and Blinding Randomization occurred after successful crossing of the lesion in the IN.PACT SFA I phase and after successful crossing and predilatation with a standard PTA balloon 1 mm smaller than the reference vessel diameter in the IN.PACT SFA II phase. A patient was considered enrolled at the time of randomization. Subjects were randomly assigned by an Interactive Voice Response System with the use of a method of permuted blocks to ensure that a 2:1 ratio was maintained across sites (Figure 1). The patients and the trial sponsor were blinded to the treatment assignments through the completion of all 12-month follow-up evaluations. The independent core laboratories and clinical events committee will remain blinded to the treatment assignments throughout the 60-month follow-up duration. Because of the visual difference between the IN.PACT DCB and standard PTA balloon, treating physicians, research coordinators, and catheterization laboratory staff were not blinded to the treatment assignment. Treating physicians, research coordinators, and catheterization laboratory staff received detailed and specific instructions and training on how to preserve the patients blinded status. Treatment and Medical Therapy Patients randomly assigned to the experimental arm were treated with the IN.PACT Admiral DCB. The IN.PACT DCB has a dual mode of action, comprising mechanical dilatation by the angioplasty balloon plus local drug delivery to the arterial wall intended to inhibit restenosis. The IN.PACT DCB coating includes paclitaxel as the antiproliferative agent at a dose of 3.5 μg/mm 2, with urea as the excipient. Available IN.PACT Admiral DCB sizes included 4-, 5-, 6-, and 7-mm diameters and 20-, 40-, 60-, 80 and 120-mm lengths (the 7-mm diameter device was not available in the 120-mm length). A minimum balloon inflation time of 180 seconds was required for both treatment groups. To avoid geographic miss, DCB length was chosen to exceed the target lesion length by 10 mm at the proximal and distal edges. The IN.PACT DCB is a single-inflation device, and, when treatment required multiple balloons, an overlap of 10 mm was applied for contiguous balloon inflations. Premedication included a loading dose of aspirin 300 to 325 mg and clopidogrel 300 mg within 24 hours of the index procedure or 2 hours postprocedure. Heparin was administered at the time of the procedure to maintain an activated clotting time 250 seconds. Figure 1. Trial flow diagram. The IN.PACT SFA Trial used a 2:1 randomized, control design, and intent-to-treat (ITT) analysis was conducted at 12 months. Three hundred thirty-one (331) patients with de novo or nonstented restenotic lesions in the superficial femoral and proximal popliteal artery were randomly assigned either to the IN.PACT Admiral drug-coated balloon or standard PTA treatment group. All subjects enrolled in the IN.PACT SFA Trial (n=331) will be followed for up to 5 years. Analysis at 1 year included subjects that provided end point data at the time of data snapshot. A subject was excluded under the following circumstances: (1) consent was withdrawn before the 1-year visit and no event had occurred before withdrawal or (2) there was no contact with the subject permitting a 1-year evaluation and no events had occurred before the 1-year evaluation. DCB indicates drug-coated balloon; PTA, percutaneous transluminal angioplasty; and SFA, superficial femoral artery.
3 Tepe et al Drug-Coated Balloons for SFA Disease 497 Postdilatation with a standard PTA balloon was allowed at the discretion of the operator. In both treatment groups, provisional stenting was allowed only in the case of PTA failure after repeated and prolonged PTA inflations. PTA failure was defined as a residual stenosis 50% or major ( grade D) flow-limiting dissection confirmed by a peak translesional systolic pressure gradient of >10 mm Hg. In both arms, postprocedure medical therapy included aspirin 81 to 325 mg daily (for a minimum of 6 months) and clopidogrel 75 mg daily for a minimum duration of 1 month for nonstented patients and 3 months for patients who received stents. Usage of aspirin and antiplatelet drugs did not differ between treatment arms at discharge (97.6%), 30 days (87.6%), or 12 months (51.5%). Follow-Up For the primary end point analysis, patients were followed by the treating physician at 30 days, 6 months, and 12 months, including office visits with duplex ultrasonography functional testing and adverse event assessment. Reinterventions, if required within 12 months of the procedure, were performed according to standard practice by using PTA balloons and provisional stenting. Study End Points The primary efficacy end point was primary patency at 12 months following the index procedure, defined as freedom from clinically driven target lesion revascularization and restenosis as determined by a duplex ultrasonography derived peak systolic velocity ratio of Each component of the primary efficacy end point was independently adjudicated by the blinded Clinical Events Committee (for clinically driven target lesion revascularization) or by the core laboratories (for restenosis). Specifically, the independent Clinical Events Committee determined whether reinterventions at the target lesion were clinically driven on the basis of objective testing (ankle-brachial index decrease 20% or >0.15 in comparison with postprocedure ankle-brachial index) or symptoms of exertional limb discomfort. Safety end points included 30-day device- and procedure-related death, all-cause death, major target limb amputation, and target vessel thrombosis. These events were site reported and Clinical Events Committee adjudicated. Additional efficacy end points included acute procedural success, target vessel revascularization at 12 months, and primary sustained clinical improvement (defined as freedom from target limb amputation, target vessel revascularization, and increase in Rutherford class at 12 months). Functional assessments included general appraisal through administration of a 5-dimension (EQ-5D) health-related quality-of-life questionnaire and specific evaluation of walking capacity by using a Walking Impairment Questionnaire. A Six-Minute Walk Test was additionally conducted in the IN.PACT SFA II phase only. Statistical Analysis The planned enrollment of 330 subjects provided a power of 80% to detect a 50% improvement in the primary end point at 12 months (from 40% 4 in the PTA group to 60% in the DCB group) with a 1-sided type I error of 2.5%. From its inception, the trial was intended to have 2 phases under a single statistical analysis plan. Poolability of subjects across trial phases for the primary end point analysis was tested by using Cox proportional hazards regression. For this poolability analysis, model covariates included treatment group, phase, and a treatment-by-phase interaction effect. Because the treatmentby-trial phase interaction value for the primary end point was nonsignificant (P=0.428), the 2 trial phases were pooled for all analyses. All analyses were based on the intention-to-treat principle. Continuous variables are described as mean±standard deviation and were compared by t tests. Categorical variables are described as proportions and were compared by the Z test owing to the 1-sided testing. The Z test was used to test the hypothesis of equality of proportions in achieving the primary end point. Multiple imputation was performed by using the logistic regression approach for patients with missing primary end point data (29 DCB, 7 PTA). The following variables were included in the imputation model as covariates: age, sex, diabetes mellitus, lesion length, total occlusion, and Rutherford class at baseline. Five data sets were imputed from these covariates that mimic different realizations of the missing data. Within each imputed data set for the end point, the proportion experiencing the end point was statistically compared between treatment groups by using the 2-sample Z test. From these, an overall test statistic for the end point and its associated P value were calculated for the imputed data. The imputed difference (95% confidence interval) and P value are reported along with the as-observed numerator and denominator. A sensitivity analysis of the as-observed rates revealed a similar highly significant P value (P<0.001). As a secondary analysis, clinically driven target lesion revascularization was analyzed by the Kaplan-Meier method during the 12-month follow-up period. The difference in the survival curves between groups was assessed by using log-rank statistics. Statistical analyses were performed using SAS (SAS Institute, Cary, NC) version 9.2 or higher. Additional statistical information is found in Appendix I in the online-only Data Supplement. Role of the Funding Source The trial was designed by the principal investigators (G.T., J.L., P.S.) and the sponsor (Medtronic). The data were collected and analyzed by the sponsor. Harvard Clinical Research Institute independently validated the analyses, with funding from the sponsor. The first and last authors prepared the first draft, which was then reviewed and edited by the other coauthors. The sponsor had the right to review but not to approve the final manuscript. The authors had full access to all data and accept full responsibility for the accuracy and completeness of the reported analyses and interpretations of the data, and they vouch for the fidelity of the study to the protocol (available with full text of this article at Results Baseline and Procedural Characteristics Between September 2010 and April 2011 (IN.PACT SFA I phase) and between April 2012 and January 2013 (IN.PACT SFA II phase), 331 patients (220 in the DCB group and 111 in the PTA group) were enrolled at 13 sites in Europe and 44 sites in the United States, respectively. Patient flow through the 12-month follow-up is described in Figure 1. Demographics, comorbidities, and lesion characteristics were similar between the DCB and PTA groups (Table 1). The mean lesion length was 8.94±4.89 in the DCB arm and 8.81±5.12 cm in the PTA arm (P=0.82). Occlusions were treated in 25.8% and 19.5% (P=0.22) of patients in the DCB and PTA arms, respectively. No significant differences across the 2 arms were observed, with the exception of a lower number of patent infrapopliteal runoff vessels in the PTA group (P=0.04). Adjustment for differences in runoff between the treatment groups using propensity scores had no impact on the primary efficacy and safety outcomes. Efficacy Outcomes Procedural success, defined as a residual diameter stenosis of 50% for nonstented patients or 30% for stented patients, was achieved in 99.5% of subjects in the DCB arm and 98.2% of subjects in the PTA arm. In the intention-to-treat population, the 12-month primary patency rate was 82.2% in the DCB arm versus 52.4% in the PTA arm (P<0.001; Table 2). The DCB-treated patients demonstrated lower rates of clinically driven target lesion revascularization versus PTA-treated patients through 12 months (2.4% versus 20.6%; P<0.001; Figure 2). A significantly higher primary sustained clinical improvement (85.2%) was observed in the DCB arm in
4 498 Circulation February 3, 2015 Table 1. Baseline and Procedural Characteristics All Intention-to-Treat Subjects DCB (n=220 Subjects n=221 Lesions) PTA (n=111 Subjects n=113 Lesions) P Value* Clinical characteristics Age, y 67.5± ± Male sex,, % (m/n), 65.0 (143/220) 67.6 (75/111) 0.71 Diabetes mellitus, % (m/n) 40.5 (89/220) 48.6 (54/111) 0.16 Hypertension, % (m/n) 91.4 (201/220) 88.3 (98/111) 0.43 Hyperlipidemia, % (m/n) 84.5 (186/220) 82.0 (91/111) 0.64 Current smoker, % (m/n) 38.6 (85/220) 36.0 (40/111) 0.72 Coronary artery 57.0 (122/214) 55.0 (60/109) 0.81 disease, % (m/n) Carotid artery 34.9 (73/209) 31.7 (32/101) 0.61 disease, % (m/n) ABI / TBI 0.769± ± Rutherford class, % (m/n) (83/220) 37.8 (42/111) (126/220) 55.9 (62/111) (11/220) 5.4 (6/111) (0/220) 0.9 (1/111) Angiographic characteristics Lesion type de 95.0 (209/220) 94.6 (105/111) 0.88 novo, % (m/n) No. of patent runoff 0.04 vessels, % (m/n) (7/212) 4.5 (5/112) (29/212) 26.8 (30/112) (88/212) 33.0 (37/112) (88/212) 35.7 (40/112) Proximal popliteal 6.8 (15/221) 7.1 (8/113) 1.00 involvement, % (m/n) Lesion length, cm 8.94± ± Total occlusions, % (m/n) 25.8 (57/221) 19.5 (22/113) 0.22 Severe calcification, 8.1 (18/221) 6.2 (7/113) 0.66 % (m/n) Reference vessel 4.647± ± diameter, mm Minimum lumen diameter 0.900± ± (preprocedure), mm Diameter stenosis 81.1± ± (preprocedure), % Procedural characteristics Predilatation, % (m/n) 96.4 (212/220) 85.6 (95/111) <0.001 Postdilatation, % (m/n) 26.8 (59/220) 18.9 (21/111) 0.14 No. of treatment balloons 1.4± ±0.3 <0.001 per subject First treatment balloon 8.3± ± maximum pressure, atm Dissections, % (m/n) (80/221) 38.9 (44/113) A C 63.8 (141/221) 60.2 (68/113) D F 0.0 (0/221) 0.9 (1/113) (Continued) Table 1. Continued DCB (n=220 Subjects n=221 Lesions) PTA (n=111 Subjects n=113 Lesions) P Value* Provisional stenting, 7.3 (16/220) 12.6 (14/111) 0.11 % (m/n) Minimum lumen diameter 3.903± ± (postprocedure), mm Diameter stenosis 19.9± ± (postprocedure), % Device success,# % (m/n) 99.0 (308/311) 98.5 (128/130) 0.30 Procedural success,** 99.5 (219/220) 98.2 (109/111) 0.11 % (m/n) Clinical success, % (m/n) 99.1 (218/220) 97.3 (108/111) 0.10 ABI indicates ankle-brachial index; DCB, drug-coated balloon; m, numbers in category; n, number of available values; PTA, percutaneous transluminal angioplasty; and TBI, toe-brachial index. *All P values are 2-sided with the exception of success outcomes, which use 1-sided P values. Values represent mean±sd. Based on number of intention-to-treat subjects with available data. TBI allowed / used in cases of incompressible vessels in IN.PACT SFA II Trial phase. Site-reported. Normal-to-normal by Core Laboratory quantitative vascular angiography evaluation. #Successful delivery, inflation, deflation, and retrieval of the intact trial balloon without burst < rated burst pressure. **No procedural complications (death, major target limb amputation, thrombosis of target lesion, or target vessel revascularization) before discharge. Residual diameter stenosis 50% for nonstented subjects or 30% for stented subjects. comparison with the PTA arm (68.9%; P<0.001). The ankle-brachial index was significantly higher in the DCB arm at 12 months (Table 2). Implantation of provisional stents was similar in the DCB and PTA arms (7.3% versus 12.6%; P=0.11). When stented patients were excluded from the analyses, there were no changes in any of the conclusions. Efficacy outcomes by trial phase are included in Table I in the online-only Data Supplement. Safety Outcomes There were no procedure- or device-related deaths and no major amputations through 12 months in either arm. Sitereported and Clinical Events Committee adjudicated vessel thrombosis occurred in 1.4% of the subjects in the DCB arm and 3.7% of the subjects in the PTA arm (P=0.10). All-cause death through 12 months was 1.9% (n=4) versus 0.0% (n=0) in the DCB and PTA arms, respectively (P=0.93). Causes of death included cerebral infarction at 127 days, biliary sepsis at 168 days, sudden death at 287 days, and a perforated colon at 314 days following the index procedure. There were no untoward paclitaxel-related adverse effects as determined by the Clinical Events Committee. Safety outcomes by trial phase are included in Table I in the online-only Data Supplement. Functional Outcomes At 12 months, there was no significant difference between treatment groups in the change from baseline in quality-oflife by using the EQ-5D assessment, but the results trended in
5 Tepe et al Drug-Coated Balloons for SFA Disease 499 Table 2. Key Clinical and Safety Outcomes Outcome DCB (n=220) PTA (n=111) Difference [95% CI]* P Value Primary efficacy primary patency, % (m/n) 82.2 (157/191) 52.4 (54/103) 26.2% <0.001 [15.1% 37.3%] 12-month efficacy outcomes All TLR, % (m/n) 2.9 (6/207) 20.6 (22/107) <0.001 Clinically driven TLR, % (m/n) 2.4 (5/207) 20.6 (22/107) <0.001 Clinically driven TVR, % (m/n) 4.3 (9/207) 23.4 (25/107) <0.001 Primary sustained clinical improvement, % (m/n) 85.2 (167/196) 68.9 (73/106) <0.001 ABI/TBI 0.951±0.221# 0.886± month safety outcomes 30-day device- and procedure-related death, % (m/n) 0.0 (0/218) 0.0 (0/111) >0.999 Target limb major amputation, % (m/n) 0.0 (0/207) 0.0 (0/107) >0.999 All-cause death, % (m/n) 1.9 (4/207) 0.0 (0/107) 0.93 Thrombosis,** % (m/n) 1.4 (3/207) 3.7 (4/107) month functional outcomes Change from baseline by EQ-5D Index ±0.2089# ± Walking impairment, % 72.7±31.4# 73.6± Change in 6MWT from baseline to 12 mo, m 38.7±92.1# 59.1± ABI indicates ankle-brachial index; DCB, drug-coated balloon; EQ-5D, 5-dimension health-related quality-of-life questionnaire; m, numbers in category; 6MWT, 6-minute walk test; n, number of available values; PTA, percutaneous transluminal angioplasty; TBI, toe-brachial index; TLR, target lesion revascularization; and TVR, target vessel revascularization *Based on multiple imputation of missing data. One-sided P value. Based on number of subjects with available data (ie, as observed). Clinically driven TLR adjudicated by an independent Clinical Events Committee that was blinded to the assigned treatment, based on any reintervention at the target lesion attributable to symptoms or drop of ABI of 20% or >0.15 in comparison with postprocedure baseline ankle-brachial index. Freedom from target limb amputation, TVR, and increase in Rutherford class at 12 months postprocedure. TBI allowed/used in cases of incompressible vessels in IN.PACT SFA II. #Values represent mean±sd. **Defined as an occlusion attributable to thrombus formation that is rapidly evolving as confirmed by the sudden onset of symptoms within 14 days of imaging, and documented by duplex ultrasound and angiography of the index vessel. Data collected in IN.PACT SFA II phase only. favor of IN.PACT DCB (P=0.10, versus ). Both treatment groups also showed improvement from baseline in walking impairment at 12 months. Although the treatment groups showed similar functional outcomes at 12 months, 8.6 times more of the PTA subjects required a clinically driven target lesion revascularization to achieve the same levels of functional outcomes as the IN.PACT Admiral DCB subjects. In the IN.PACT SFA II phase, both treatment groups also showed improvement from baseline to 12 months in the walking distance assessed by the 6-Minute Walk Test, with no statistically significant difference between treatment groups. Functional outcomes by trial phase are included in Table I in the online-only Data Supplement. Discussion In this trial, the IN.PACT Admiral drug-coated balloon resulted in superior efficacy in comparison with a plain angioplasty balloon for the treatment of patients with symptomatic superficial femoral and proximal popliteal PAD. There was significantly better primary patency and a marked reduction in the need for target lesion revascularization at 12 months following treatment with the DCB. The efficacy of paclitaxel in reducing restenosis in the femoropopliteal artery has been previously reported by the use of different DCB technologies in various trials Although paclitaxel was the most commonly used antiproliferative drug in these preceding trials, each DCB was unique with respect to the paclitaxel dose (varying from 2 to 3.5 μg/mm 2 ), the carrier molecule (excipient), the balloon material, and the balloon and coating technology used. The findings from the present trial are consistent with these previous DCB proofof-concept trials that, although smaller because they were powered on a 6-month angiographic end point, enrolled a similar patient population with symptomatic femoropopliteal disease. All of these trials demonstrated significant reductions in late lumen loss at 6 months and lower rates of target lesion revascularization at 1 year for DCB versus PTA Moreover, the IN.PACT SFA Trial results are concordant with the 83.7% primary patency at 1 year reported by Micari et al 20 from a multicenter registry of IN.PACT DCB use in a similar patient population. Numerous modalities are available for the treatment of superficial femoral and popliteal artery disease, including implant-based technologies such as bare metal stents, 5 7,20,21
6 500 Circulation February 3, % 97.5% Freedom from Clinically-driven TLR 90% 80% 70% 60% 50% 40% 30% 20% IN.PACT PTA Log-rank P < % 10% Days After Index Procedure Number of subjects at risk IN.PACT PTA Figure 2. Twelve-month freedom from target lesion revascularization. Freedom from target lesion revascularization in 331 patients with superficial femoral and popliteal peripheral artery disease were randomly assigned to receive drug-coated balloons or standard angioplasty. The rate of freedom from clinically driven TLR was significantly higher in the group receiving drug-coated balloons than in the standard PTA group. PTA indicates percutaneous transluminal angioplasty; and TLR, target lesion revascularization. covered stents, 22,23 and drug-eluting stents, 8,24 and implant-free technology, as well, such as atherectomy devices. 25 DCB is an attractive alternative because it offers the promise of improved patency in comparison with PTA and a reduction in the need for stents. This is particularly important in the dynamic environment of the superficial femoral and popliteal arteries, where mechanical fatigue may lead to stent fracture and increased risk of in-stent restenosis Restenosis following superficial femoral and popliteal artery stenting has been reported to occur in recent trials with a frequency of 20% at 12 months with higher rates of 50% following stenting of long-segment disease.,12,20,21,26 Diffuse in-stent restenosis or instent occlusion is a very difficult problem to treat, 12,26,27 with recurrent restenosis rates of >70% at 1 to 2 years, although recent results have suggested improved outcomes with peripheral drug-eluting stents 28 and DCB. 29,30 Use of a DCB (and avoidance of stent implantation) does not limit future treatment options, an important consideration given the chronic and progressive nature of PAD. These findings compare favorably with other randomized clinical trials in this patient population. Despite the inclusion of longer lesion lengths that are at a higher risk of treatment failure, the 2.4% target lesion revascularization rate experienced in this trial is the lowest reported for an SFA device trial at 12 months. Clinically driven target lesion revascularization rates of 12.7% and 9.5% were reported in 2 recent randomized trials of bare metal and drug-eluting stents, despite their inclusion of shorter lesions (average lesion lengths of 7.0 and 5.4 cm, respectively). 7,8 IN.PACT DCB was associated with a low complication rate, including the absence of major amputations and a low rate of vessel thrombosis. Study Limitations The trial was deliberately and prospectively conducted in 2 sequential phases. The blinding of phase I was rigorously maintained until the completion of phase II. When the data were analyzed, there were no statistical differences between the 2 phases. Although improvements in the functional assessments of quality of life, walking impairment, and walking distance were observed in both treatment groups, the interpretation of these measures is complicated by the subjective nature of patient questionnaires and the influence of comorbidities, including progressive disease in nontreated vessels. The results of this trial cannot be generalized to patients not included in this trial. Future studies should encompass longer lesions and consider comparison with bare metal stents, drugeluting stents, and bypass, and optimal medical therapy and exercise, as well. Longer-term follow-up is needed to confirm the durability of the benefit. Conclusions In conclusion, in this large, prospective, multicenter, international, randomized trial, DCB was superior to PTA and had a favorable safety profile for the treatment of patients with symptomatic superficial femoral and proximal popliteal artery PAD. The IN.PACT DCB demonstrated impressive patency rates with low repeat revascularization rates in comparison with other modern endovascular therapies. DCB stands to become an important treatment option for patients with superficial femoral and popliteal artery disease. Acknowledgments We thank Judith Greengard, Victoria Rendon, and Melissa Hasenbank for editing assistance. Contributions: Drs Tepe, Jaff, and Laird prepared the first draft of this manuscript, which was then reviewed and edited by the other coauthors. Drs Snead and Cohen undertook the statistical analysis. All the authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; and also contributed to drafting the article or revising it critically for important intellectual content. Source of Funding The study was funded by Medtronic, Inc. Disclosures Dr Tepe holds research grants from Bard Peripheral Vascular, B. Braun, Biotronic, Covidien, Medrad, and Medtronic. He is a compensated advisory board member for Medtronic, and receives speaking honoraria from Bard Peripheral Vascular, Biotronic, Covidien,
7 Tepe et al Drug-Coated Balloons for SFA Disease 501 Medrad, and Medtronic. Dr Laird holds research grants from W.L. Gore and Medtronic. He is a compensated advisory board member and consultant for Abbott Vascular, Bard Peripheral Vascular, Boston Scientific, Covidien, and Medtronic. Dr Micari holds research grants from Medtronic, and is a compensated consultant for Medtronic. Dr Metzger receives speaking honoraria from Bard Peripheral Vascular and Medtronic and is compensated for participation in training courses sponsored by Abbott Vascular and Medtronic. He is a compensated consultant for Abbott Vascular. Dr Scheinert holds research grants from Abbott Vascular, Angioslide, Atheromed, Biotronik, Boston Scientific, Cook Medical, Cordis, Covidien, CR Bard, Gardia Medical, Heoteq, Intact Vascular, Medtronic, TriReme Medical, and Upstream Peripheral Technologies. He is a compensated consultant/ advisory board member for Abbott Vascular, Angioslide, Atheromed, Biotronik, Boston Scientific, Cook Medical, Cordis, Covidien, CR Bard, Gardia Medical, Heoteq, Intact Vascular, Medtronic, TriReme Medical, and Upstream Peripheral Technologies. Dr Zeller holds research grants from Bard-Lutonix, Biotronik, Cook Medical, and Medtronic. He receives speaking honoraria from Bard-Lutonix, Biotronik, Cook Medical, and Medtronic. Dr Cohen holds research grants from Abbott Vascular, Boston Scientific, Covidien, and Medtronic and is a compensated consultant for Abbott Vascular and Medtronic. Dr Snead, B. Alexander, and M. Landini are full-time employees of Medtronic. Dr Jaff is a noncompensated Advisor for Medtronic and is the Medical Director of VasCore, the Vascular Ultrasound Core Laboratory. He is a compensated member of VIVA Physicians, a not-for-profit 501c3 education/research organization. The other authors report no conflicts. References 1. Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM Jr, White CJ, White J, White RA, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B; American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; Vascular Disease Foundation. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463 e654. doi: /CIRCULATIONAHA Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(suppl S):S5 67. doi: /j.jvs European Society of Cardiology (ESC), Tendera M, Aboyans V, Bartelink ML, Baumgartner I, Clément D, Collet JP, Cremonesi A, De Carlo M, Erbel R, Fowkes FG, Heras M, Kownator S, Minar E, Ostergren J, Poldermans D, Riambau V, Roffi M, Röther J, Sievert H, van Sambeek M, Zeller T; ESC Committee for Practice Guidelines. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2011;32: Rocha-Singh KJ, Jaff MR, Crabtree TR, Bloch DA, Ansel G; VIVA Physicians, Inc. Performance goals and endpoint assessments for clinical trials of femoropopliteal bare nitinol stents in patients with symptomatic peripheral arterial disease. Catheter Cardiovasc Interv. 2007;69: doi: /ccd Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, Schlager O, Cejna M, Lammer J, Minar E. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354: doi: /NEJMoa Dick P, Wallner H, Sabeti S, Loewe C, Mlekusch W, Lammer J, Koppensteiner R, Minar E, Schillinger M. Balloon angioplasty versus stenting with nitinol stents in intermediate length superficial femoral artery lesions. Catheter Cardiovasc Interv. 2009;74: doi: /ccd Laird JR, Katzen BT, Scheinert D, Lammer J, Carpenter J, Buchbinder M, Dave R, Ansel G, Lansky A, Cristea E, Collins TJ, Goldstein J, Jaff MR; RESILIENT Investigators. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve-month results from the RESILIENT randomized trial. Circ Cardiovasc Interv. 2010;3: doi: / CIRCINTERVENTIONS Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, Zeller T, Roubin GS, Burket MW, Khatib Y, Snyder SA, Ragheb AO, White JK, Machan LS; Zilver PTX Investigators. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circ Cardiovasc Interv. 2011;4: doi: / CIRCINTERVENTIONS Layden J, Michaels J, Bermingham S, Higgins B; Guideline Development Group. Diagnosis and management of lower limb peripheral arterial disease: summary of NICE guidance. BMJ. 2012;345:e Klein AJ, Chen SJ, Messenger JC, Hansgen AR, Plomondon ME, Carroll JD, Casserly IP. Quantitative assessment of the conformational change in the femoropopliteal artery with leg movement. Catheter Cardiovasc Interv. 2009;74: doi: /ccd Scheinert D, Scheinert S, Sax J, Piorkowski C, Bräunlich S, Ulrich M, Biamino G, Schmidt A. Prevalence and clinical impact of stent fractures after femoropopliteal stenting. J Am Coll Cardiol. 2005;45: doi: /j.jacc Laird JR, Yeo KK. The treatment of femoropopliteal in-stent restenosis: back to the future. J Am Coll Cardiol. 2012;59: doi: /j. jacc Tepe G, Zeller T, Albrecht T, Heller S, Schwarzwälder U, Beregi JP, Claussen CD, Oldenburg A, Scheller B, Speck U. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med. 2008;358: doi: /NEJMoa Werk M, Langner S, Reinkensmeier B, Boettcher HF, Tepe G, Dietz U, Hosten N, Hamm B, Speck U, Ricke J. Inhibition of restenosis in femoropopliteal arteries: paclitaxel-coated versus uncoated balloon: femoral paclitaxel randomized pilot trial. Circulation. 2008;118: doi: /CIRCULATIONAHA Werk M, Albrecht T, Meyer DR, Ahmed MN, Behne A, Dietz U, Eschenbach G, Hartmann H, Lange C, Schnorr B, Stiepani H, Zoccai GB, Hänninen EL. Paclitaxel-coated balloons reduce restenosis after femoro-popliteal angioplasty: evidence from the randomized PACIFIER trial. Circ Cardiovasc Interv. 2012;5: doi: /CIRCINTERVENTIONS Scheinert D, Duda S, Zeller T, Krankenberg H, Ricke J, Bosiers M, Tepe G, Naisbitt S, Rosenfield K. The LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first-in-human randomized trial of lowdose drug-coated balloon versus uncoated balloon angioplasty. JACC Cardiovasc Interv. 2014;7: doi: /j.jcin Fanelli F, Cannavale A, Boatta E, Corona M, Lucatelli P, Wlderk A, Cirelli C, Salvatori FM. Lower limb multilevel treatment with drug-eluting balloons: 6-month results from the DEBELLUM randomized trial. J Endovasc Ther. 2012;19: doi: /JEVT MR Micari A, Cioppa A, Vadalà G, Castriota F, Liso A, Marchese A, Grattoni C, Pantaleo P, Cremonesi A, Rubino P, Biamino G. 2-year results of paclitaxel-eluting balloons for femoropopliteal artery disease: evidence from a multicenter registry. JACC Cardiovasc Interv. 2013;6: doi: /j.jcin Schlager O, Francesconi M, Haumer M, Dick P, Sabeti S, Amighi J, Mlekusch W, Koppensteiner R, Minar E, Schillinger M. Duplex sonography versus angiography for assessment of femoropopliteal arterial disease in a real-world setting. J Endovasc Ther. 2007;14: doi: / (2007)14[452:DSVAFA]2.0.CO; Bosiers M, Deloose K, Callaert J, Moreels N, Keirse K, Verbist J, Peeters P. Results of the Protégé EverFlex 200-mm-long nitinol stent (ev3) in
8 502 Circulation February 3, 2015 TASC C and D femoropopliteal lesions. J Vasc Surg. 2011;54: doi: /j.jvs Armstrong EJ, Saeed H, Alvandi B, Singh S, Singh GD, Yeo KK, Anderson D, Westin GG, Dawson DL, Pevec WC, Laird JR. Nitinol selfexpanding stents vs. balloon angioplasty for very long femoropopliteal lesions. J Endovasc Ther. 2014;21: doi: / MR Saxon R, Chervu A, Jones P, Bajwa TK, Gable DR, Soukas PA, Begg RJ, Adams JG, Ansel GM, Schneider DB, Eichler CM, Rush MJ. Heparinbonded, expanded polytetrafluoroethylene-lined stent graft in the treatment of femoropopliteal artery disease: 1-year results of the VIPER (Viabahn Endoprosthesis with Heparin Bioactive Surface in the Treatment of Superficial Femoral Artery Obstructive Disease) trial. J Vasc Interv Radiol. 2013; 24: Lammer J, Zeller T, Hausegger K, Schaefer PJ, Gschwendtner M, Mueller- Huelsbeck S, Rand T, Funovics M, Wolf F, Rastan A, Gschwandtner M, Puchner S, Ristl R, Schoder M. Heparin-bonded covered stents versus bare metal stents for complex femoro-popliteal artery lesions: the randomized VIASTAR trial. J Am Coll Cardiol. 2013;62: doi: /j. jacc Dake MD, Scheinert D, Tepe G, Tessarek J, Fanelli F, Bosiers M, Ruhlmann C, Kavteladze Z, Lottes AE, Ragheb AO, Zeller T; Zilver PTX Single-Arm Study Investigators. Nitinol stents with polymer-free paclitaxel coating for lesions in the superficial femoral and popliteal arteries above the knee: twelve-month safety and effectiveness results from the Zilver PTX single-arm clinical study. J Endovasc Ther. 2011;18: doi: / Garcia LA, Lyden SP. Atherectomy for infrainguinal peripheral artery disease. J Endovasc Ther. 2009; 16(suppl 2): II Tosaka A, Soga Y, Iida O, Ishihara T, Hirano K, Suzuki K, Yokoi H, Nanto S, Nobuyoshi M. Classification and clinical impact of restenosis after femoropopliteal stenting. J Am Coll Cardiol. 2012;59: doi: /j. jacc Laird JR Jr, Yeo KK, Rocha-Singh K, Das T, Joye J, Dippel E, Reddy B, Botti C, Jaff MR. Excimer laser with adjunctive balloon angioplasty and heparincoated self-expanding stent grafts for the treatment of femoropopliteal artery in-stent restenosis: twelve-month results from the SALVAGE study. Catheter Cardiovasc Interv. 2012;80: doi: /ccd Zeller T, Dake MD, Tepe G, Brechtel K, Noory E, Beschorner U, Kultgen PL, Rastan A. Treatment of femoropopliteal in-stent restenosis with paclitaxel-eluting stents. JACC Cardiovasc Interv. 2013;6: doi: /j.jcin Virga V, Stabile E, Biamino G, Salemme L, Cioppa A, Giugliano G, Tesorio T, Cota L, Popusoi G, Pucciarelli A, Esposito G, Trimarco B, Rubino P. Drug-eluting balloons for the treatment of the superficial femoral artery in-stent restenosis: 2-year follow-up. JACC Cardiovasc Interv. 2014;7: doi: /j.jcin Liistro F, Angioli P, Porto I, Ricci L, Ducci K, Grotti S, Falsini G, Ventoruzzo G, Turini F, Bellandi G, Bolognese L. Paclitaxel-eluting balloon vs. standard angioplasty to reduce recurrent restenosis in diabetic patients with in-stent restenosis of the superficial femoral and proximal popliteal arteries: the DEBATE-ISR study. J Endovasc Ther. 2014;21:1 8. doi: / R.1. Clinical Perspective The clinical data from the IN.PACT SFA Trial, which was designed as a 2-phase, global, multicenter, single-blind, randomized trial, provide valid scientific evidence to conclusively demonstrate the effectiveness and safety of the IN.PACT Admiral Drug-Coated Balloon (DCB) in the treatment of patients with peripheral artery disease in the superficial femoral and proximal popliteal artery. The IN.PACT Admiral DCB achieved the primary effectiveness and safety end points of the trial, demonstrating superiority of IN.PACT Admiral DCB to percutaneous transluminal angioplasty. Despite the inclusion of longer lesion lengths that are at a higher risk of treatment failure, the 2.4% target lesion revascularization rate is the lowest reported for a superficial femoral artery device trial at 12 months. The significant sustained clinical improvement, the low rate of clinically driven target lesion revascularization, the absence of amputation, and the minimal incidence of thrombosis observed in the IN.PACT Admiral DCB group further underscore the clinical benefits of IN.PACT Admiral DCB and validate the favorable results generated by previous clinical trials. The IN.PACT SFA Trial data demonstrate an important therapeutic advantage over the existing alternative treatments for peripheral artery disease. DCB is an attractive alternative, because it offers the promise of improved patency in comparison with percutaneous transluminal angioplasty and a reduction in the need for stents. This is particularly important in the dynamic environment of the superficial femoral and popliteal arteries, where mechanical fatigue may lead to stent fracture and the increased risk of in-stent restenosis. The results of this trial support DCB as an important treatment option for patients with superficial femoral and popliteal artery disease.
Majestic Trial 12 Month Results
 Majestic Trial 12 Month Results S.Müller-Hülsbeck, MD, EBIR, FCIRSE, FICA ACADEMIC HOSPITALS Flensburg of Kiel University Ev.-Luth. Diakonissenanstalt zu Flensburg Knuthstraße 1, 24939 FLENSBURG Dept.
Majestic Trial 12 Month Results S.Müller-Hülsbeck, MD, EBIR, FCIRSE, FICA ACADEMIC HOSPITALS Flensburg of Kiel University Ev.-Luth. Diakonissenanstalt zu Flensburg Knuthstraße 1, 24939 FLENSBURG Dept.
Popliteal artery: to stent or not to stent?
 Popliteal artery: to stent or not to stent? Karl-Ludwig Schulte Vascular Center Berlin Ev. Hospital Königin Elisabeth St. Gertrauden Hospital University Hospital Charité, CC11 Humboldt-University Berlin
Popliteal artery: to stent or not to stent? Karl-Ludwig Schulte Vascular Center Berlin Ev. Hospital Königin Elisabeth St. Gertrauden Hospital University Hospital Charité, CC11 Humboldt-University Berlin
IN.PACT SFA: A Prospective Randomized Trial of a Drug- Coated Balloon for Femoropopliteal Lesions Two-Year Outcomes
 IN.PACT SFA: A Prospective Randomized Trial of a Drug- Coated Balloon for Femoropopliteal Lesions Two-Year Outcomes John Laird, MD UC Davis, Sacramento, California USA On behalf of the IN.PACT SFA Trial
IN.PACT SFA: A Prospective Randomized Trial of a Drug- Coated Balloon for Femoropopliteal Lesions Two-Year Outcomes John Laird, MD UC Davis, Sacramento, California USA On behalf of the IN.PACT SFA Trial
ESC Guidelines on the diagnosis and treatment of peripheral artery diseases Lower extremity artery disease. Erich Minar Medical University Vienna
 ESC Guidelines on the diagnosis and treatment of peripheral artery diseases Lower extremity artery disease Erich Minar Medical University Vienna for the Task Force on the Diagnosis and Treatment of Peripheral
ESC Guidelines on the diagnosis and treatment of peripheral artery diseases Lower extremity artery disease Erich Minar Medical University Vienna for the Task Force on the Diagnosis and Treatment of Peripheral
Antiplatelet and anticoagulation treatment of patients undergoing carotid and peripheral artery angioplasty
 Round Table: Antithrombotic therapy beyond ACS Antiplatelet and anticoagulation treatment of patients undergoing carotid and peripheral artery angioplasty M. Matsagkas, MD, PhD, EBSQ-Vasc Associate Professor
Round Table: Antithrombotic therapy beyond ACS Antiplatelet and anticoagulation treatment of patients undergoing carotid and peripheral artery angioplasty M. Matsagkas, MD, PhD, EBSQ-Vasc Associate Professor
Health Policy Advisory Committee on Technology Technology Brief
 Health Policy Advisory Committee on Technology Technology Brief LifeStent vascular stent for symptomatic lesions of the superficial femoral or proximal popliteal artery August 2012 State of Queensland
Health Policy Advisory Committee on Technology Technology Brief LifeStent vascular stent for symptomatic lesions of the superficial femoral or proximal popliteal artery August 2012 State of Queensland
Mechanical Properties of Nitinol Stents and Stent-grafts. Comparison of 6 mm Diameter Devices
 Mechanical Properties of Nitinol Stents and Stent-grafts Comparison of 6 mm Diameter Devices P E R F O R M A N C E b y d e s i g n Purpose The association of target vessel restenosis with stent fractures
Mechanical Properties of Nitinol Stents and Stent-grafts Comparison of 6 mm Diameter Devices P E R F O R M A N C E b y d e s i g n Purpose The association of target vessel restenosis with stent fractures
Drug-Eluting Balloons. Klaus Bonaventura Department of Cardiology and Angiology Heart Thorax Vascular Center, Klinikum Ernst von Bergmann, Potsdam
 Drug-Eluting Balloons Klaus Bonaventura Department of Cardiology and Angiology Heart Thorax Vascular Center, Klinikum Ernst von Bergmann, Potsdam Potential conflicts of interest Speaker s name: Klaus Bonaventura
Drug-Eluting Balloons Klaus Bonaventura Department of Cardiology and Angiology Heart Thorax Vascular Center, Klinikum Ernst von Bergmann, Potsdam Potential conflicts of interest Speaker s name: Klaus Bonaventura
PRECOMBAT Trial. Seung-Whan Lee, MD, PhD On behalf of the PRECOMBAT Investigators
 Premier of Randomized Comparison of Bypass Surgery versus Angioplasty Using Sirolimus-Eluting Stent in Patients with Left Main Coronary Artery Disease PRECOMBAT Trial Seung-Whan Lee, MD, PhD On behalf
Premier of Randomized Comparison of Bypass Surgery versus Angioplasty Using Sirolimus-Eluting Stent in Patients with Left Main Coronary Artery Disease PRECOMBAT Trial Seung-Whan Lee, MD, PhD On behalf
Cardiac Rehabilitation An Underutilized Class I Treatment for Cardiovascular Disease
 Cardiac Rehabilitation An Underutilized Class I Treatment for Cardiovascular Disease What is Cardiac Rehabilitation? Cardiac rehabilitation is a comprehensive exercise, education, and behavior modification
Cardiac Rehabilitation An Underutilized Class I Treatment for Cardiovascular Disease What is Cardiac Rehabilitation? Cardiac rehabilitation is a comprehensive exercise, education, and behavior modification
Measure #257 (NQF 1519): Statin Therapy at Discharge after Lower Extremity Bypass (LEB) National Quality Strategy Domain: Effective Clinical Care
 Measure #257 (NQF 1519): Statin Therapy at Discharge after Lower Extremity Bypass (LEB) National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Measure #257 (NQF 1519): Statin Therapy at Discharge after Lower Extremity Bypass (LEB) National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY
Ostial LAD: Single stent approach is the best. Antonio A. Pocoví, MD, FSCAI, MTSAC, Advisory Council Member, CACI
 Ostial LAD: Single stent approach is the best Antonio A. Pocoví, MD, FSCAI, MTSAC, Advisory Council Member, CACI Chair, Interventional Cardiology Sanatorio San Lucas Instituto Alexander Fleming Buenos
Ostial LAD: Single stent approach is the best Antonio A. Pocoví, MD, FSCAI, MTSAC, Advisory Council Member, CACI Chair, Interventional Cardiology Sanatorio San Lucas Instituto Alexander Fleming Buenos
Supera Peripheral Stent System Instructions for Use
 Supera Peripheral Stent System Instructions for Use Table of Contents 1.0 DEVICE DESCRIPTION 2.0 HOW SUPPLIED 3.0 INDICATIONS 4.0 CONTRAINDICATIONS 5.0 WARNINGS 6.0 PRECAUTIONS 6.1 Stent Delivery System
Supera Peripheral Stent System Instructions for Use Table of Contents 1.0 DEVICE DESCRIPTION 2.0 HOW SUPPLIED 3.0 INDICATIONS 4.0 CONTRAINDICATIONS 5.0 WARNINGS 6.0 PRECAUTIONS 6.1 Stent Delivery System
University of Ulsan College of Medicine, Asan Medical Center on behalf of the REAL-LATE and the ZEST-LATE trial
 Duration of Dual Antiplatelet Therapy After Drug-Eluting Stent Implantation A Pooled Analysis of the REAL-LATE and the ZEST-LATE Trial Seung-Jung Park MD PhD Seung-Jung Park, MD, PhD, University of Ulsan
Duration of Dual Antiplatelet Therapy After Drug-Eluting Stent Implantation A Pooled Analysis of the REAL-LATE and the ZEST-LATE Trial Seung-Jung Park MD PhD Seung-Jung Park, MD, PhD, University of Ulsan
Cilostazol versus Clopidogrel after Coronary Stenting
 Cilostazol versus Clopidogrel after Coronary Stenting Seong-Wook Park, MD, PhD, FACC Division of Cardiology, Asan Medical Center University of Ulsan College of Medicine Seoul, Korea AMC, 2004 Background
Cilostazol versus Clopidogrel after Coronary Stenting Seong-Wook Park, MD, PhD, FACC Division of Cardiology, Asan Medical Center University of Ulsan College of Medicine Seoul, Korea AMC, 2004 Background
Intracoronary Stenting and. Robert A. Byrne, Julinda Mehilli, Salvatore Cassese, Franz-Josef Neumann, Susanne Pinieck, Tomohisa Tada,
 Prospective, Randomized Trial of Paclitaxel-Eluting Balloon versus Paclitaxel-Eluting Stent versus Balloon Angioplasty for Treatment of Coronary Restenosis in Limus- Eluting Stents Intracoronary Stenting
Prospective, Randomized Trial of Paclitaxel-Eluting Balloon versus Paclitaxel-Eluting Stent versus Balloon Angioplasty for Treatment of Coronary Restenosis in Limus- Eluting Stents Intracoronary Stenting
Clinical Research Intracoronary Stenting with Crushing in Coronary Artery Bifurcation Lesions: Initial Results and Medium-Term Follow Up
 Hellenic J Cardiol 45: 379-383, 2004 Clinical Research Intracoronary Stenting with Crushing in Coronary Artery Bifurcation Lesions: Initial Results and Medium-Term Follow Up PETROS S. DARDAS, DIMITRIS
Hellenic J Cardiol 45: 379-383, 2004 Clinical Research Intracoronary Stenting with Crushing in Coronary Artery Bifurcation Lesions: Initial Results and Medium-Term Follow Up PETROS S. DARDAS, DIMITRIS
EXCITE ISR: Initial Results. Eric J. Dippel, MD FACC On behalf of the EXCITE ISR Investigators
 EXCITE ISR: Initial Results Eric J. Dippel, MD FACC On behalf of the EXCITE ISR Investigators Disclosure Statement of Financial Interest Within the past 12 months, I or my spouse/partner have had a financial
EXCITE ISR: Initial Results Eric J. Dippel, MD FACC On behalf of the EXCITE ISR Investigators Disclosure Statement of Financial Interest Within the past 12 months, I or my spouse/partner have had a financial
Duration of Dual Antiplatelet Therapy After Coronary Stenting
 Duration of Dual Antiplatelet Therapy After Coronary Stenting C. DEAN KATSAMAKIS, DO, FACC, FSCAI INTERVENTIONAL CARDIOLOGIST ADVOCATE LUTHERAN GENERAL HOSPITAL INTRODUCTION Coronary artery stents are
Duration of Dual Antiplatelet Therapy After Coronary Stenting C. DEAN KATSAMAKIS, DO, FACC, FSCAI INTERVENTIONAL CARDIOLOGIST ADVOCATE LUTHERAN GENERAL HOSPITAL INTRODUCTION Coronary artery stents are
on behalf of the AUGMENT-HF Investigators
 One Year Follow-Up Results from AUGMENT-HF: A Multicenter Randomized Controlled Clinical Trial of the Efficacy of Left Ventricular Augmentation with Algisyl-LVR in the Treatment of Heart Failure* Douglas
One Year Follow-Up Results from AUGMENT-HF: A Multicenter Randomized Controlled Clinical Trial of the Efficacy of Left Ventricular Augmentation with Algisyl-LVR in the Treatment of Heart Failure* Douglas
MEDICAL POLICY No. 91580-R1 DRUG-ELUTING STENTS FOR ISCHEMIC HEART DISEASE
 DRUG-ELUTING STENTS FOR ISCHEMIC HEART DISEASE Effective Date: October 1, 2015 Review Dates: 10/11, 10/12, 10/13, 8/14, 8/15 Date Of Origin: October 12, 2011 Status: Current Summary of Changes Clarifications:
DRUG-ELUTING STENTS FOR ISCHEMIC HEART DISEASE Effective Date: October 1, 2015 Review Dates: 10/11, 10/12, 10/13, 8/14, 8/15 Date Of Origin: October 12, 2011 Status: Current Summary of Changes Clarifications:
Rethink Atherectomy: Expert Insights Into Clinical Application and Use of the JETSTREAM System
 , LLC an HMP Communications Holdings Company September 2013 Volume 25/ Supplement B www.invasivecardiology.com The Official Journal of the International Andreas Gruentzig Society Rethink Atherectomy: Expert
, LLC an HMP Communications Holdings Company September 2013 Volume 25/ Supplement B www.invasivecardiology.com The Official Journal of the International Andreas Gruentzig Society Rethink Atherectomy: Expert
IN.PACT ADMIRAL DRUG-COATED BALLOON NEW TECHNOLOGY ADD-ON PAYMENT (NTAP)
 IN.PACT ADMIRAL DRUG-COATED BALLOON NEW TECHNOLOGY ADD-ON PAYMENT (NTAP) New Technology Add-on (NTAP) for DCB OVERVIEW Effective October 1, 2015, hospital inpatient cases using a drug-coated balloon (DCB)
IN.PACT ADMIRAL DRUG-COATED BALLOON NEW TECHNOLOGY ADD-ON PAYMENT (NTAP) New Technology Add-on (NTAP) for DCB OVERVIEW Effective October 1, 2015, hospital inpatient cases using a drug-coated balloon (DCB)
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
A Post-market Study to Assess the STENTYS Self-exPanding COronary Stent In AcuTe myocardial InfarctiON in Real Life APPOSITION III
 A Post-market Study to Assess the STENTYS Self-exPanding COronary Stent In AcuTe myocardial InfarctiON in Real Life APPOSITION III Gilles Montalescot, MD, PhD Pitié-Salpêtrière Hospital, Paris, France
A Post-market Study to Assess the STENTYS Self-exPanding COronary Stent In AcuTe myocardial InfarctiON in Real Life APPOSITION III Gilles Montalescot, MD, PhD Pitié-Salpêtrière Hospital, Paris, France
Credentials for Peripheral Angioplasty: Comments on Society of Cardiac Angiography and Intervention Revisions
 Credentials for Peripheral Angioplasty: Comments on Society of Cardiac Angiography and Intervention Revisions David Sacks, MD, Gary J. Becker, MD, and Terence A.S. Matalon, MD J Vasc Interv Radiol 2003;
Credentials for Peripheral Angioplasty: Comments on Society of Cardiac Angiography and Intervention Revisions David Sacks, MD, Gary J. Becker, MD, and Terence A.S. Matalon, MD J Vasc Interv Radiol 2003;
REPORTING STENT PLACEMENT FOR NONOCCLUSIVE VASCULAR DISEASE IN LOWER EXTREMITIES
 REPORTING STENT PLACEMENT FOR NONOCCLUSIVE VASCULAR DISEASE IN LOWER EXTREMITIES Effective January 1, 2015, there was a change in CPT that affects reporting specific endovascular services provided in the
REPORTING STENT PLACEMENT FOR NONOCCLUSIVE VASCULAR DISEASE IN LOWER EXTREMITIES Effective January 1, 2015, there was a change in CPT that affects reporting specific endovascular services provided in the
STONY BROOK UNIVERSITY HOSPITAL VASCULAR CENTER CREDENTIALING POLICY
 STONY BROOK UNIVERSITY HOSPITAL VASCULAR CENTER CREDENTIALING POLICY Per Medical Board decision March 18, 2008: These credentialing standards do NOT apply to peripheral angiography performed in the context
STONY BROOK UNIVERSITY HOSPITAL VASCULAR CENTER CREDENTIALING POLICY Per Medical Board decision March 18, 2008: These credentialing standards do NOT apply to peripheral angiography performed in the context
Antonio Colombo MD on behalf of the SECURITY Investigators
 Second Generation Drug-Eluting Stents Implantation Followed by Six Versus Twelve-Month - Dual Antiplatelet Therapy - The SECURITY Randomized Clinical Trial Antonio Colombo MD on behalf of the SECURITY
Second Generation Drug-Eluting Stents Implantation Followed by Six Versus Twelve-Month - Dual Antiplatelet Therapy - The SECURITY Randomized Clinical Trial Antonio Colombo MD on behalf of the SECURITY
Steven J. Yakubov, MD FACC For the CoreValve US Clinical Investigators
 Long-Term Outcomes Using a Self- Expanding Bioprosthesis in Patients With Severe Aortic Stenosis Deemed Extreme Risk for Surgery: Two-Year Results From the CoreValve US Pivotal Trial Steven J. Yakubov,
Long-Term Outcomes Using a Self- Expanding Bioprosthesis in Patients With Severe Aortic Stenosis Deemed Extreme Risk for Surgery: Two-Year Results From the CoreValve US Pivotal Trial Steven J. Yakubov,
Copenhagen University Hospital Rigshospitalet Aarhus University Hospital Skejby Denmark
 Long-term outcome after drug-eluting versus bare-metal stent implantation in patients with ST-elevation myocardial infarction 3 year follow-up of the randomised trial Peter Clemmensen, Henning Kelbæk,
Long-term outcome after drug-eluting versus bare-metal stent implantation in patients with ST-elevation myocardial infarction 3 year follow-up of the randomised trial Peter Clemmensen, Henning Kelbæk,
The Pantera Lux Paclitaxel DEB Device Description and Clinical Studies. Christoph Hehrlein, University Clinic Freiburg i.br.
 The Pantera Lux Paclitaxel DEB Device Description and Clinical Studies Christoph Hehrlein, University Clinic Freiburg i.br. Germany Disclosure Statement of Financial Interest Within the past 12 months,
The Pantera Lux Paclitaxel DEB Device Description and Clinical Studies Christoph Hehrlein, University Clinic Freiburg i.br. Germany Disclosure Statement of Financial Interest Within the past 12 months,
Patients suffering from critical limb ischemia (CLI)
 Building a Successful Amputation Prevention Program Our single-center experience implementing an amputation prevention algorithm and how it has led to a trend in reduced amputation rates. By Jihad A. Mustapha,
Building a Successful Amputation Prevention Program Our single-center experience implementing an amputation prevention algorithm and how it has led to a trend in reduced amputation rates. By Jihad A. Mustapha,
Main Effect of Screening for Coronary Artery Disease Using CT
 Main Effect of Screening for Coronary Artery Disease Using CT Angiography on Mortality and Cardiac Events in High risk Patients with Diabetes: The FACTOR-64 Randomized Clinical Trial Joseph B. Muhlestein,
Main Effect of Screening for Coronary Artery Disease Using CT Angiography on Mortality and Cardiac Events in High risk Patients with Diabetes: The FACTOR-64 Randomized Clinical Trial Joseph B. Muhlestein,
How To Determine Pad
 Process Representation #1 : The PAD algorithm as a sequential flow thru all sections An exploded version of the above scoped section flow is shown below. Notes: The flow presupposes existing services (
Process Representation #1 : The PAD algorithm as a sequential flow thru all sections An exploded version of the above scoped section flow is shown below. Notes: The flow presupposes existing services (
Atrial Fibrillation 2014 How to Treat How to Anticoagulate. Allan Anderson, MD, FACC, FAHA Division of Cardiology
 Atrial Fibrillation 2014 How to Treat How to Anticoagulate Allan Anderson, MD, FACC, FAHA Division of Cardiology Projection for Prevalence of Atrial Fibrillation: 5.6 Million by 2050 Projected number of
Atrial Fibrillation 2014 How to Treat How to Anticoagulate Allan Anderson, MD, FACC, FAHA Division of Cardiology Projection for Prevalence of Atrial Fibrillation: 5.6 Million by 2050 Projected number of
JUL 2 2008. Ms. Kendra Basler Regulatory Affairs Associate Abbott Vascular Cardiac Therapies 3200 Lakeside Drive Santa Clara, CA 95054-2807
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service JUL 2 2008 Food and Drug Administration 9200 Corporate Boulevard Rockville MD 20850 Ms. Kendra Basler Regulatory Affairs Associate Abbott Vascular
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service JUL 2 2008 Food and Drug Administration 9200 Corporate Boulevard Rockville MD 20850 Ms. Kendra Basler Regulatory Affairs Associate Abbott Vascular
SAMPLE. Asia-Pacific Interventional Cardiology Procedures Outlook to 2020. Reference Code: GDMECR0061PDB. Publication Date: May 2014
 Asia-Pacific Interventional Cardiology Procedures Outlook to 2020 Reference Code: GDMECR0061PDB Publication Date: May 2014 Page 1 1 Table of Contents 1 Table of Contents... 2 1.1 List of Tables... 4 1.2
Asia-Pacific Interventional Cardiology Procedures Outlook to 2020 Reference Code: GDMECR0061PDB Publication Date: May 2014 Page 1 1 Table of Contents 1 Table of Contents... 2 1.1 List of Tables... 4 1.2
Endovascular Repair of the Superficial Femoral Artery
 Supplement to Produced under an educational grant from W. L. Gore & Associates September 2008 Endovascular Repair of the Superficial Femoral Artery A roundtable discussion of the challenges in treating
Supplement to Produced under an educational grant from W. L. Gore & Associates September 2008 Endovascular Repair of the Superficial Femoral Artery A roundtable discussion of the challenges in treating
Non-surgical treatment of severe varicose veins
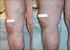 Non-surgical treatment of severe varicose veins Yasu Harasaki UCHSC Department of Surgery General Surgery Grand Rounds March 19, 2007 Definition Dilated, palpable, subcutaneous veins generally >3mm in
Non-surgical treatment of severe varicose veins Yasu Harasaki UCHSC Department of Surgery General Surgery Grand Rounds March 19, 2007 Definition Dilated, palpable, subcutaneous veins generally >3mm in
Rotational Atherectomy for the Treatment of In-Stent Restenosis
 Home SVCC Area: English - Español - Português Rotational Atherectomy for the Treatment of In-Stent Restenosis Steven L. Goldberg, MD Cardiac Catheterization Laboratory, University of Washington Medical
Home SVCC Area: English - Español - Português Rotational Atherectomy for the Treatment of In-Stent Restenosis Steven L. Goldberg, MD Cardiac Catheterization Laboratory, University of Washington Medical
California Health and Safety Code, Section 1256.01
 California Health and Safety Code, Section 1256.01 1256.01. (a) The Elective Percutaneous Coronary Intervention (PCI) Pilot Program is hereby established in the department. The purpose of the pilot program
California Health and Safety Code, Section 1256.01 1256.01. (a) The Elective Percutaneous Coronary Intervention (PCI) Pilot Program is hereby established in the department. The purpose of the pilot program
Interventional Cardiology Peripheral Interventions Rhythm Management
 FY2016 Hospital Inpatient Rule (IPPS) Interventional Cardiology Peripheral Interventions Rhythm Management On April 17, 2015 the Centers for Medicare and Medicaid Services (CMS) released the Hospital Inpatient
FY2016 Hospital Inpatient Rule (IPPS) Interventional Cardiology Peripheral Interventions Rhythm Management On April 17, 2015 the Centers for Medicare and Medicaid Services (CMS) released the Hospital Inpatient
Carotid Artery Stenting? Comparison of the outcome of octogenarian patients according to the stent type. Data from a multicentre registry
 Closed- or Open-cell stents in Carotid Artery Stenting? Comparison of the outcome of octogenarian patients according to the stent type. Data from a multicentre registry Nikas D 1, Reimers B 2, Iakovou
Closed- or Open-cell stents in Carotid Artery Stenting? Comparison of the outcome of octogenarian patients according to the stent type. Data from a multicentre registry Nikas D 1, Reimers B 2, Iakovou
STROKE PREVENTION IN ATRIAL FIBRILLATION. TARGET AUDIENCE: All Canadian health care professionals. OBJECTIVE: ABBREVIATIONS: BACKGROUND:
 STROKE PREVENTION IN ATRIAL FIBRILLATION TARGET AUDIENCE: All Canadian health care professionals. OBJECTIVE: To guide clinicians in the selection of antithrombotic therapy for the secondary prevention
STROKE PREVENTION IN ATRIAL FIBRILLATION TARGET AUDIENCE: All Canadian health care professionals. OBJECTIVE: To guide clinicians in the selection of antithrombotic therapy for the secondary prevention
Is There A LIfe for DES after discontinuation of Clopidogrel
 Chicago 2014 Is There A LIfe for DES after discontinuation of Clopidogrel Six-month versus 24-month dual antiplatelet therapy after implantation of drug eluting stents in patients non-resistant to aspirin:
Chicago 2014 Is There A LIfe for DES after discontinuation of Clopidogrel Six-month versus 24-month dual antiplatelet therapy after implantation of drug eluting stents in patients non-resistant to aspirin:
Dual Antiplatelet Therapy. Stephen Monroe, MD FACC Chattanooga Heart Institute
 Dual Antiplatelet Therapy Stephen Monroe, MD FACC Chattanooga Heart Institute Scope of Talk Identify the antiplatelet drugs and their mechanisms of action Review dual antiplatelet therapy in: The medical
Dual Antiplatelet Therapy Stephen Monroe, MD FACC Chattanooga Heart Institute Scope of Talk Identify the antiplatelet drugs and their mechanisms of action Review dual antiplatelet therapy in: The medical
FFR CT : Clinical studies
 FFR CT : Clinical studies Bjarne Nørgaard Department Cardiology B Aarhus University Hospital Skejby, Denmark Disclosures: Research grants: Edwards and Siemens Coronary CTA: High diagnostic sensitivity
FFR CT : Clinical studies Bjarne Nørgaard Department Cardiology B Aarhus University Hospital Skejby, Denmark Disclosures: Research grants: Edwards and Siemens Coronary CTA: High diagnostic sensitivity
Coronary Bifurcation Treatment: Update from the European Bifurcation Club. Remo Albiero, MD Ist. Clinico S. Rocco Brescia (Italy)
 Coronary Bifurcation Treatment: Update from the European Bifurcation Club Remo Albiero, MD Ist. Clinico S. Rocco Brescia (Italy) Disclosure Statement of Financial Interest Within the past 12 months, I
Coronary Bifurcation Treatment: Update from the European Bifurcation Club Remo Albiero, MD Ist. Clinico S. Rocco Brescia (Italy) Disclosure Statement of Financial Interest Within the past 12 months, I
A Patient s Guide to Primary and Secondary Prevention of Cardiovascular Disease Using Blood-Thinning (Anticoagulant) Drugs
 A Patient s Guide to Primary and Secondary Prevention of PATIENT EDUCATION GUIDE What Is Cardiovascular Disease? Cardiovascular disease (CVD) is a broad term that covers any disease of the heart and circulatory
A Patient s Guide to Primary and Secondary Prevention of PATIENT EDUCATION GUIDE What Is Cardiovascular Disease? Cardiovascular disease (CVD) is a broad term that covers any disease of the heart and circulatory
Evolu'on of Balloon Angioplasty From POBA to newer technologies NCVH May 26, 2015
 Evolu'on of Balloon Angioplasty From POBA to newer technologies NCVH May 26, 2015 Cezar Staniloae, MD Heart and Vascular Ins7tute New York University Medical Center Overview 1. Physics of balloon angioplasty
Evolu'on of Balloon Angioplasty From POBA to newer technologies NCVH May 26, 2015 Cezar Staniloae, MD Heart and Vascular Ins7tute New York University Medical Center Overview 1. Physics of balloon angioplasty
Apixaban Plus Mono vs. Dual Antiplatelet Therapy in Acute Coronary Syndromes: Insights from the APPRAISE-2 Trial
 Apixaban Plus Mono vs. Dual Antiplatelet Therapy in Acute Coronary Syndromes: Insights from the APPRAISE-2 Trial Connie N. Hess, MD, MHS, Stefan James, MD, PhD, Renato D. Lopes, MD, PhD, Daniel M. Wojdyla,
Apixaban Plus Mono vs. Dual Antiplatelet Therapy in Acute Coronary Syndromes: Insights from the APPRAISE-2 Trial Connie N. Hess, MD, MHS, Stefan James, MD, PhD, Renato D. Lopes, MD, PhD, Daniel M. Wojdyla,
OHTAC Recommendation
 OHTAC Recommendation Multiple Sclerosis and Chronic Cerebrospinal Venous Insufficiency Presented to the Ontario Health Technology Advisory Committee in May 2010 May 2010 Issue Background A review on the
OHTAC Recommendation Multiple Sclerosis and Chronic Cerebrospinal Venous Insufficiency Presented to the Ontario Health Technology Advisory Committee in May 2010 May 2010 Issue Background A review on the
Atherosclerosis of the aorta. Artur Evangelista
 Atherosclerosis of the aorta Artur Evangelista Atherosclerosis of the aorta Diagnosis Classification Prevalence Risk factors Marker of generalized atherosclerosis Risk of embolism Therapy Diagnosis Atherosclerosis
Atherosclerosis of the aorta Artur Evangelista Atherosclerosis of the aorta Diagnosis Classification Prevalence Risk factors Marker of generalized atherosclerosis Risk of embolism Therapy Diagnosis Atherosclerosis
Prognostic impact of uric acid in patients with stable coronary artery disease
 Prognostic impact of uric acid in patients with stable coronary artery disease Gjin Ndrepepa, Siegmund Braun, Martin Hadamitzky, Massimiliano Fusaro, Hans-Ullrich Haase, Kathrin A. Birkmeier, Albert Schomig,
Prognostic impact of uric acid in patients with stable coronary artery disease Gjin Ndrepepa, Siegmund Braun, Martin Hadamitzky, Massimiliano Fusaro, Hans-Ullrich Haase, Kathrin A. Birkmeier, Albert Schomig,
Presenter: Marco Valgimigli, MD PhD, FESC Erasmus MC, Thoraxcenter Rotterdam The Netherlands
 Comparing zotarolimus-eluting and bare-metal stent efficacy in selected high bleeding risk patients treated with a short dual antiplatelet therapy duration. A pre-specified analysis from the The Zotarolimuseluting
Comparing zotarolimus-eluting and bare-metal stent efficacy in selected high bleeding risk patients treated with a short dual antiplatelet therapy duration. A pre-specified analysis from the The Zotarolimuseluting
Comparison of Second-Generation Stents for Application in the Superficial Femoral Artery: An In Vitro Evaluation Focusing on Stent Design
 Comparison of Second-Generation Stents for Application in the Superficial Femoral Artery: An In Vitro Evaluation Focusing on Stent Design Müller-Hülsbeck S, Schäfer PJ, Charalambous N, Yagi H, Heller M,
Comparison of Second-Generation Stents for Application in the Superficial Femoral Artery: An In Vitro Evaluation Focusing on Stent Design Müller-Hülsbeck S, Schäfer PJ, Charalambous N, Yagi H, Heller M,
LEADERS: 5-Year Follow-up
 LEADERS: -Year Follow-up from a Prospective, Randomized Trial of Biolimus A9-eluting Stents with a Biodegradable Polymer vs. Sirolimus-eluting Stents with a Durable Polymer : Final Report of the LEADERS
LEADERS: -Year Follow-up from a Prospective, Randomized Trial of Biolimus A9-eluting Stents with a Biodegradable Polymer vs. Sirolimus-eluting Stents with a Durable Polymer : Final Report of the LEADERS
Therapeutic Approach in Patients with Diabetes and Coronary Artery Disease
 Home SVCC Area: English - Español - Português Therapeutic Approach in Patients with Diabetes and Coronary Artery Disease Martial G. Bourassa, MD Research Center, Montreal Heart Institute, Montreal, Quebec,
Home SVCC Area: English - Español - Português Therapeutic Approach in Patients with Diabetes and Coronary Artery Disease Martial G. Bourassa, MD Research Center, Montreal Heart Institute, Montreal, Quebec,
Renovascular Hypertension
 Renovascular Hypertension Philip Stockwell, MD Assistant Professor of Medicine (Clinical) Warren Alpert School of Medicine Cardiology for the Primary Care Provider September 28, 201 Renovascular Hypertension
Renovascular Hypertension Philip Stockwell, MD Assistant Professor of Medicine (Clinical) Warren Alpert School of Medicine Cardiology for the Primary Care Provider September 28, 201 Renovascular Hypertension
Vascular Stent System (6F, 20 100mm stents)
 100000000922.2 Instructions for Use Cordis S.M.A.R.T. CONTROL Vascular Stent System (6F, 20 100mm stents) Cordis S.M.A.R.T. Vascular Stent System (6F, 120 150mm stents) Symbols Catalog Number LOT NonPyrogenic
100000000922.2 Instructions for Use Cordis S.M.A.R.T. CONTROL Vascular Stent System (6F, 20 100mm stents) Cordis S.M.A.R.T. Vascular Stent System (6F, 120 150mm stents) Symbols Catalog Number LOT NonPyrogenic
Understanding the Pain Trajectory During Treadmill Testing in Peripheral Artery Disease
 Understanding the Pain Trajectory During Treadmill Testing in Peripheral Artery Disease Diane Treat-Jacobson, PhD, RN, FAHA, FAAN Susan J. Henly, PhD, RN Ulf G. Bronas, PhD, ATC, ATR Arthur S. Leon, MD,
Understanding the Pain Trajectory During Treadmill Testing in Peripheral Artery Disease Diane Treat-Jacobson, PhD, RN, FAHA, FAAN Susan J. Henly, PhD, RN Ulf G. Bronas, PhD, ATC, ATR Arthur S. Leon, MD,
The Minvasys Amazonia Pax & Nile Pax Polymer Free Paclitaxel Eluting Stent Program
 The Minvasys Amazonia Pax & Nile Pax Polymer Free Paclitaxel Eluting Stent Program Jean Fajadet, MD, FESC Clinique Pasteur - Toulouse - France Disclosure statement Nothing to disclose Dedicated Delivery
The Minvasys Amazonia Pax & Nile Pax Polymer Free Paclitaxel Eluting Stent Program Jean Fajadet, MD, FESC Clinique Pasteur - Toulouse - France Disclosure statement Nothing to disclose Dedicated Delivery
Adult Cardiology. Diagnosis of Arterial Disease of the Lower Extremities With Duplex Scanning: A Validation Study
 Adult Cardiology Diagnosis of Arterial Disease of the Lower Extremities With Duplex Scanning: A Validation Study Rosella S. Arellano, MD; Ma. Teresa B. Abola, MD. Background --- While standard x-ray arteriography
Adult Cardiology Diagnosis of Arterial Disease of the Lower Extremities With Duplex Scanning: A Validation Study Rosella S. Arellano, MD; Ma. Teresa B. Abola, MD. Background --- While standard x-ray arteriography
FREEDOM C: A 16-Week, International, Multicenter, Double-Blind, Randomized, Placebo-Controlled Comparison of the Efficacy and Safety of Oral UT-15C
 FREEDOM C: A 16-Week, International, Multicenter, Double-Blind, Randomized, Placebo-Controlled Comparison of the Efficacy and Safety of Oral UT-15C SR in Combination with an ERA and/or a PDE-5 Inhibitor
FREEDOM C: A 16-Week, International, Multicenter, Double-Blind, Randomized, Placebo-Controlled Comparison of the Efficacy and Safety of Oral UT-15C SR in Combination with an ERA and/or a PDE-5 Inhibitor
SeQuent Please balloon catheter for in-stent coronary restenosis. NICE medical technology guidance 1. Issue date: December 2010
 Issue date: December 2010 SeQuent Please balloon catheter for in-stent coronary restenosis NICE medical technology guidance 1 1 NICE medical technology guidance 1 NICE medical technology guidance 1 SeQuent
Issue date: December 2010 SeQuent Please balloon catheter for in-stent coronary restenosis NICE medical technology guidance 1 1 NICE medical technology guidance 1 NICE medical technology guidance 1 SeQuent
ANESTHESIA FOR PATIENTS WITH CORONARY STENTS FOR NON CARDIAC SURGERY. Dr. Mahesh Vakamudi. Professor and Head
 ANESTHESIA FOR PATIENTS WITH CORONARY STENTS FOR NON CARDIAC SURGERY Dr. Mahesh Vakamudi Professor and Head Department of Anesthesiology, Critical Care and Pain Medicine Sri Ramachandra University INTRODUCTION
ANESTHESIA FOR PATIENTS WITH CORONARY STENTS FOR NON CARDIAC SURGERY Dr. Mahesh Vakamudi Professor and Head Department of Anesthesiology, Critical Care and Pain Medicine Sri Ramachandra University INTRODUCTION
Talent Thoracic Stent Graft with THE Xcelerant Delivery System. Expanding the Indications for TEVAR
 Talent Thoracic with THE Xcelerant Delivery System Expanding the Indications for TEVAR Talent Thoracic Precise placement 1 Broad patient applicability 1 Excellent clinical outcomes 1, a + Xcelerant Delivery
Talent Thoracic with THE Xcelerant Delivery System Expanding the Indications for TEVAR Talent Thoracic Precise placement 1 Broad patient applicability 1 Excellent clinical outcomes 1, a + Xcelerant Delivery
The Bioresorbable Vascular Stent Dr Albert Ko
 The Bioresorbable Vascular Stent Dr Albert Ko Dr Albert Ko MB BS, FRACP, FCSANZ Interventional/General Cardiologist Ascot Cardiology Symposium 2013 Treatment Goals for Coronary Artery Disease Relieve of
The Bioresorbable Vascular Stent Dr Albert Ko Dr Albert Ko MB BS, FRACP, FCSANZ Interventional/General Cardiologist Ascot Cardiology Symposium 2013 Treatment Goals for Coronary Artery Disease Relieve of
Is Stenting or Coronary Artery By-pass Grafting the Better Treatment for This Patient?
 Is Stenting or Coronary Artery By-pass Grafting the Better Treatment for This Patient? --- NIRS-IVUS TVC Imaging Adds Additional Information for the Heart Team Dr. Luis Tami Memorial Regional Hospital
Is Stenting or Coronary Artery By-pass Grafting the Better Treatment for This Patient? --- NIRS-IVUS TVC Imaging Adds Additional Information for the Heart Team Dr. Luis Tami Memorial Regional Hospital
INSTEAD at 5-year follow-up shifts the expectations for endovascular treatment
 INSTEAD at 5-year follow-up shifts the expectations for endovascular treatment Christoph A. Nienaber, MD, FACC University Heart Center Rostock Department of Medicine I - Cardiology [email protected]
INSTEAD at 5-year follow-up shifts the expectations for endovascular treatment Christoph A. Nienaber, MD, FACC University Heart Center Rostock Department of Medicine I - Cardiology [email protected]
Overview of Newer Stent Devices for Aneurysm Treatment
 Overview of Newer Stent Devices for Aneurysm Treatment Randall C. Edgell, M.D. Associate Professor Vascular and Interventional Neurology Saint Louis University Disclosure Outcome adjudication for THERAPY,
Overview of Newer Stent Devices for Aneurysm Treatment Randall C. Edgell, M.D. Associate Professor Vascular and Interventional Neurology Saint Louis University Disclosure Outcome adjudication for THERAPY,
Repeat Coronary Revascularization Procedures after Successful Bare-Metal or Drug-Eluting Stent Implantation
 Original Contribution Repeat Coronary Revascularization Procedures after Successful Bare-Metal or Drug-Eluting Stent Implantation Cynthia A. Yock, MS, J. Michael Isbill, MS, Spencer B. King III, MD, Mark
Original Contribution Repeat Coronary Revascularization Procedures after Successful Bare-Metal or Drug-Eluting Stent Implantation Cynthia A. Yock, MS, J. Michael Isbill, MS, Spencer B. King III, MD, Mark
Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators
 Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators Shaikha Al Naimi Doctor of Pharmacy Student College of Pharmacy Qatar University
Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators Shaikha Al Naimi Doctor of Pharmacy Student College of Pharmacy Qatar University
Review of Intracoronary Radiation for In-Stent Restenosis
 Review of Intracoronary Radiation for In-Stent Restenosis Robert Lew, MBBS, FRACP, Andrew Ajani, MD, Ron Waksman, MD, FACC Vascular Brachytherapy using beta and gamma radiation is the standard care for
Review of Intracoronary Radiation for In-Stent Restenosis Robert Lew, MBBS, FRACP, Andrew Ajani, MD, Ron Waksman, MD, FACC Vascular Brachytherapy using beta and gamma radiation is the standard care for
INSTRUCTIONS FOR USE
 LUTONIX 035 Drug Coated Balloon PTA Catheter INSTRUCTIONS FOR USE Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. Table of Contents 1 DEVICE DESCRIPTION... 2
LUTONIX 035 Drug Coated Balloon PTA Catheter INSTRUCTIONS FOR USE Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician. Table of Contents 1 DEVICE DESCRIPTION... 2
Objectives. Preoperative Cardiac Risk Stratification for Noncardiac Surgery. History
 Preoperative Cardiac Risk Stratification for Noncardiac Surgery Kimberly Boddicker, MD FACC Essentia Health Heart and Vascular Center 27 th Heart and Vascular Conference May 13, 2011 Objectives Summarize
Preoperative Cardiac Risk Stratification for Noncardiac Surgery Kimberly Boddicker, MD FACC Essentia Health Heart and Vascular Center 27 th Heart and Vascular Conference May 13, 2011 Objectives Summarize
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
Listen to your heart: Good Cardiovascular Health for Life
 Listen to your heart: Good Cardiovascular Health for Life Luis R. Castellanos MD, MPH Assistant Clinical Professor of Medicine University of California San Diego School of Medicine Sulpizio Family Cardiovascular
Listen to your heart: Good Cardiovascular Health for Life Luis R. Castellanos MD, MPH Assistant Clinical Professor of Medicine University of California San Diego School of Medicine Sulpizio Family Cardiovascular
Antiplatelet and Antithrombotics From clinical trials to guidelines
 Antiplatelet and Antithrombotics From clinical trials to guidelines Ashraf Reda, MD, FESC Prof and head of Cardiology Dep. Menofiya University Preisedent of EGYBAC Chairman of WGLVR One of the big stories
Antiplatelet and Antithrombotics From clinical trials to guidelines Ashraf Reda, MD, FESC Prof and head of Cardiology Dep. Menofiya University Preisedent of EGYBAC Chairman of WGLVR One of the big stories
Cardiac Assessment for Renal Transplantation: Pre-Operative Clearance is Only the Tip of the Iceberg
 Cardiac Assessment for Renal Transplantation: Pre-Operative Clearance is Only the Tip of the Iceberg 2 nd Annual Duke Renal Transplant Symposium March 1, 2014 Durham, NC Joseph G. Rogers, M.D. Associate
Cardiac Assessment for Renal Transplantation: Pre-Operative Clearance is Only the Tip of the Iceberg 2 nd Annual Duke Renal Transplant Symposium March 1, 2014 Durham, NC Joseph G. Rogers, M.D. Associate
If several different trials are mentioned in one publication, the data of each should be extracted in a separate data extraction form.
 General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
Guidance for Industry Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes
 Guidance for Industry Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes U.S. Department of Health and Human Services Food and Drug Administration Center
Guidance for Industry Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes U.S. Department of Health and Human Services Food and Drug Administration Center
Cardiac Rehabilitation The Best Medicine for Your CAD Patients. James A. Stone
 James A. Stone BPHE, BA, MSc, MD, PhD, FRCPC, FAACVPR, FACC Clinical Professor of Medicine, University of Calgary Total Cardiology, Calgary Acknowledgements and Disclosures Acknowledgements Jacques Genest
James A. Stone BPHE, BA, MSc, MD, PhD, FRCPC, FAACVPR, FACC Clinical Professor of Medicine, University of Calgary Total Cardiology, Calgary Acknowledgements and Disclosures Acknowledgements Jacques Genest
Systolic Blood Pressure Intervention Trial (SPRINT) Principal Results
 Systolic Blood Pressure Intervention Trial (SPRINT) Principal Results Paul K. Whelton, MB, MD, MSc Chair, SPRINT Steering Committee Tulane University School of Public Health and Tropical Medicine, and
Systolic Blood Pressure Intervention Trial (SPRINT) Principal Results Paul K. Whelton, MB, MD, MSc Chair, SPRINT Steering Committee Tulane University School of Public Health and Tropical Medicine, and
Abdominal Aortic Aneurysm (AAA) Screening Guideline
 Abdominal Aortic Aneurysm (AAA) Screening Guideline Prevention 2 Screening Recommendations and Tests 2 Follow-up/Monitoring 2 Evidence Summary and References 3 Guideline Development Process and Team 6
Abdominal Aortic Aneurysm (AAA) Screening Guideline Prevention 2 Screening Recommendations and Tests 2 Follow-up/Monitoring 2 Evidence Summary and References 3 Guideline Development Process and Team 6
Riociguat Clinical Trial Program
 Riociguat Clinical Trial Program Riociguat (BAY 63-2521) is an oral agent being investigated as a new approach to treat chronic thromboembolic pulmonary hypertension (CTEPH) and pulmonary arterial hypertension
Riociguat Clinical Trial Program Riociguat (BAY 63-2521) is an oral agent being investigated as a new approach to treat chronic thromboembolic pulmonary hypertension (CTEPH) and pulmonary arterial hypertension
