Contents. Introduction 2 Components What is ATP? Measuring ATP with Bioluminescence Technology Additional Uses of SystemSURE Plus 6
|
|
|
- Emery Austin
- 8 years ago
- Views:
Transcription
1
2 Contents Introduction 2 Components 3 Section 1: An Overview of ATP Cleaning Verification 1.1 What is ATP? Measuring ATP with Bioluminescence Technology Additional Uses of SystemSURE Plus 6 Section 2: Implementing an ATP Cleaning Verification System Appendices 2.1 Establishing Test Locations and Limits Broad Risk Categories and Limits Corrective Action Procedures Suggested Cleaning, Testing, and Corrective Action Procedure Flowchart Programming Locations Setting Up Test Plans Testing Frequency Daily Monitoring Using SureTrend Software to Maximize a Monitoring Program Additional Testing Calibration 22 Appendix A: Recommended Test Locations 23 Appendix B: Proper Sampling Procedure 24 11
3 Contents Introduction 2 Components 3 Section 1: An Overview of ATP Cleaning Verification 1.1 What is ATP? Measuring ATP with Bioluminescence Technology Additional Uses of SystemSURE Plus 6 Section 2: Implementing an ATP Cleaning Verification System Appendices 2.1 Establishing Test Locations and Limits Broad Risk Categories and Limits Corrective Action Procedures Suggested Cleaning, Testing, and Corrective Action Procedure Flowchart Programming Locations Setting Up Test Plans Testing Frequency Daily Monitoring Using SureTrend Software to Maximize a Monitoring Program Additional Testing Calibration 22 Appendix A: Recommended Test Locations 23 Appendix B: Proper Sampling Procedure
4 Introduction to the SystemSURE Plus ATP Cleaning Verification System Hygiena s SystemSURE Plus ATP Cleaning Verification System is a tool used to: Educate environmental services professionals and other personnel on proper cleaning technique Monitor and improve the cleanliness levels of surfaces in healthcare facilities Monitor the effects of changes within a cleaning program Document and track individual and overall cleaning performance Healthcare facilities that implement an objective monitoring system experience a 42% increase in cleaning thoroughness. i The Centers for Disease Control and Prevention (CDC) encourages all hospitals to develop preventative programs to optimize and monitor the thoroughness of high-touch surface cleaning. ii The SystemSURE Plus ATP Cleaning Verification System enables healthcare organizations to: Instantly assess the cleanliness of surfaces, allowing immediate corrective action to be taken Reduce or eliminate variation in surface cleaning performance by standardizing acceptable cleaning levels Improve and enhance the training of environmental services personnel Provide insight into whether current cleaning processes and tools are sufficient or below adequate Reduce the use of conventional microbiological testing methods that are slow, labor intensive, and costly Record and track test results to identify problem areas, make improvements, and show due diligence to auditors and compliance with regulations Enhance environmental cleaning programs which help to prevent the spread of harmful bacteria and viruses that are associated with healthcare associated infections Ensure patient safety and increase patient satisfaction Using Hygiena s SystemSURE Plus Cleaning Verification System, healthcare facilities are able to create a standard by which to measure cleaning effectiveness. i Carling, P.C., & Bartley, J.M. (2010). Evaluating hygienic cleaning in health care settings: What you do not know can harm your patients. American Journal of Infection Control; 38 : S41 ii 2
5 Components of the SystemSURE Plus ATP Cleaning Verification System The SystemSURE Plus ATP Cleaning Verification System consists of four parts: SystemSURE Plus Luminometer a user-friendly, handheld, light-reading unit that provides precise, on-site test results. Used with the UltraSnap testing device, extremely low levels of contamination can be detected in just 15 seconds. (Catalog # SS3H) 2. UltraSnap Testing Device a convenient, all-in-one ATP test device. Simply swab, snap, and squeeze, and the test is ready to be measured in the SystemSURE Plus. Packaged 100 tests per box. (Catalog # US2020) 3. SureTrend Data Analysis Software a powerful software program that allows users to upload test results to a database, analyze trends and generate reports for management and record-keeping. (Included with SystemSURE Plus Luminometer). 4. Calibration Devices - optional calibration rods confirm SystemSURE Plus is within specifications. (Catalog # PCD4000) See section 2.11 Calibration for more details. Hygiena s luminometer, testing devices, and software are designed to be easy to use, enabling operation by both technical and non-technical staff. 3 33
6 Section I: System Overview The SystemSURE Plus ATP Cleaning Verification System is a rapid cleaning monitoring system used to help hospitals and other healthcare organizations achieve optimal standardized cleaning levels. The system uses bioluminescence technology to identify and measure adenosine triphosphate, commonly known as ATP. 1.1 What is ATP? ATP is an energy molecule found in all living cells that allows cellular metabolism to take place. All organic matter contains ATP, including blood, saliva, and bacteria. In healthcare facilities, organic matter such as bodily fluids, blood, and bacteria left on surfaces can become a point of crosscontamination between patients and staff, leading to infections if not properly cleaned. Therefore the detection of ATP on a surface after cleaning is an indication of improper cleaning. 1.2 Measuring ATP with Bioluminescence Technology UltraSnap ATP surface tests contain an enzyme called luciferase which produces a bioluminescence (light-producing) reaction when it comes into contact with ATP. The light emitted from the reaction is measured and quantified in the SystemSURE Plus luminometer. The graphic below illustrates how ATP on a surface reacts with the enzyme in UltraSnap ATP test devices to emit light. 4
7 ATP Presence and RLU Measurement Fail Pass Clean Dirty Higher Contamination = Higher RLU The quantity of light generated by the bioluminescence reaction is directly proportional to the amount of ATP present in the sample. The reaction is immediate, allowing results to be processed in real-time. Results are then expressed numerically on the SystemSURE Plus screen as Relative Light Units (RLU). 5 55
8 1.3 Additional Uses of SystemSURE Plus In addition to routine ATP cleaning verification by Environmental Services (EVS), the SystemSURE Plus ATP Cleaning Verification System can be used for: Central/Sterile Services Used for verifying the cleanliness of flexible endoscopes and other reusable medical devices. Improper cleaning of equipment before sterilization can lead to non-sterile equipment. Hand Hygiene Compliance Used to measure levels of ATP present on personnel s hands before and after hand washing to demonstrate efficacy and thoroughness of hand washing efforts. Food Service/Cafeteria Food Safety Used to verify food preparation surfaces and dining areas have been cleaned properly. For more information on implementing the SystemSURE Plus in these and other areas of a hospital, visit 6
9 Section 2: Implementing an ATP Cleaning Verification System 2.1 Establishing Test Locations and Limits The SystemSURE Plus comes with a preset pass limit of 25 RLU and fail limit of 50 RLU. These limits are based on studies conducted in healthcare facilities, and a starting point from which custom limits can be refined. Pass Caution Fail 25 RLU RLU 50 RLU This section will guide users through identifying test locations and establishing appropriate pass/fail limits for those locations. To optimize an ATP cleaning verification program, many hospitals choose to set custom limits for test locations. Before testing begins, it is necessary to: 1. Identify areas within the facility that will be tested 2. Establish appropriate pass and fail limits for each location The CDC provides a list of recommended locations to test in hospitals (see Appendix A on page 23). Locations typically tested are high-touch surfaces where the chance of spreading infectious bacteria is high. Monitoring of low-risk surfaces on a less frequent basis is also essential to verifying a facility is being thoroughly cleaned. Prepare the list of locations on a piece of paper or Microsoft Excel worksheet. 7 77
10 Collecting Test Samples Once locations to be tested have been identified, pass and fail limits for each location can be established by collecting samples or assigning the locations to broad risk categories (see Section 2.2 on page 11). Before collecting initial samples, it is essential to master proper sampling procedure as detailed in Appendix B, page 24. Managers may choose to collect test samples in one of two ways: 1. After Routine Cleaning Time to implement: 1-4 weeks To use this method, sample each location a minimum of 10 times after routine cleaning. If locations are the same from room to room, it is acceptable to test the same location from different rooms. 2. Optimal cleaning Time to implement: 1-3 days To use this method, the manager overseeing the ATP cleaning verification program should clean each location that will be tested the way he or she wants the location to be cleaned each time. A minimum of 10 samples should be taken from the same locations in different rooms to ensure that no site is tested more than once. For example, the call buttons in ten different rooms could be sampled after the cleaning of these sites. Record RLU measurements on a spreadsheet (Example 1). (For an Excel spreadsheet that automatically calculates pass/fail limits, visit the resources section at Example 1: Collected Test Samples Tests Average Location RLU measurement Bedrail IV Pole Bedside Table Remote
11 Calculating Pass/Fail Limits with the Standard Deviation Method Result Calculation Instructions Pass Average To determine the lower (pass) limit of each location, calculate the average RLU score from the samples collected. Fail Average + (3 * Standard Deviation) To determine the upper (fail) limit, calculate the standard deviation of the samples collected and multiply by three (3) iii. Add that value to the average. Statistically, this calculation indicates that nearly all (99.7%) passing test results (clean surfaces) will be below the fail RLU limit. An RLU result higher than the fail limit would be a statistical outlier, indicating the location is not clean. Example 1 Continued: Calculated Limits Location Average = Pass Avg + (3 * Standard Deviation) = Fail Bedrail IV Pole Bedside Table Remote i. For calculations using Microsoft Excel: For lower limit (pass), use the function =AVERAGE For upper limit (fail) use the function =3*STDEV and add to =AVERAGE 9
12 The range between the pass and fail values is the caution range. This could give different pass, caution, and fail levels for each location. This is typical when different surface types (plastic, stainless steel, etc.) are being tested and when the age of equipment varies. Thus, for Example 1 data, the limits for the locations using the standard deviation method are as follows: Example 1: Final Limits Location Pass Caution Fail Bedrail IV Pole Bedside Table Remote Eliminating the Caution Zone Many hospitals chose to eliminate the caution zone and consider anything greater than the pass score as a fail. Refer to the table below for an example. Example 1: Limits without Caution Range Location Pass Fail Bedrail 50 >50 IV Pole 30 >30 Bedside Table 15 >15 Remote 100 >
13 2.2 Broad Risk Categories and Limits While establishing custom limits for your facility would be the gold standard for setting up a monitoring program, many hospitals rely on limits for broad risk categories that have been validated by published, peer-reviewed, and third-party studies. For information on these studies, reference Technical Document: Establishing RLU Pass/Fail Limits available from the resource library on or contact your Hygiena technical representative. The general recommended limits below reflect an elimination of the caution zone. For hospitals that wish to use the caution zone, simply double the pass RLU (pass RLU x 2) to determine the fail RLU number. Public areas Application General Recommended Limits Pass (RLU) Fail (RLU) Examples: Elevator call buttons 50 >50 Hallway handrails Waiting room areas Patient rooms Examples: Call button Bed rails 25 >25 Patient restroom Monitor panels IV pole Sterile services 10 >10 Washer disinfector 5 >5 Food preparation and catering 10 >10 Hand washing 60 >60 11
14 2.3 Corrective Action Procedures Corrective action procedures provide clear instructions for what steps should be taken following pass, caution, or fail results. Recommended corrective action procedures are as follows: SYMBOL TEST RESULT CORRECTIVE ACTION Pass The surface has been adequately cleaned. No action required. Caution The surface may not have been adequately cleaned. The area may be recleaned and retested, or monitored for future problems. Cleaning personnel may require retraining on proper cleaning procedures. Fail The surface has not been cleaned to the cleaning standard and must be recleaned and retested. Cleaning personnel should also be retrained on proper cleaning procedures
15 2.4 Suggested Cleaning, Testing, and Corrective Action Procedure Flowchart 13
16 2.5 Programming Location Pass/Fail Limits into Software Once test locations have been identified and Pass/Fail limits determined, they must be entered into the SureTrend software and synced with the SystemSURE Plus luminometer. For instructions on how to install SureTrend software and add locations, see the installation guide and manual included with the SureTrend CD. See the SureTrend User Manual for steps on entering locations and limits. For assistance programming locations and limits in the SureTrend software, please contact your Hygiena representative. By default the SureTrend software assigns a pass limit of 10 and fail limit of 30 when you add a new location. Assign the correct limits to each location determined in Section 2.1 or 2.2. Group information can also be entered at this time. Group information is used for grouping locations together for reporting purposes. For example, groups can allow users to compare the performance of departments, wings, or facilities. Below is an example of the location setup in the SureTrend software. Prog # Location Group Pass Fail 1 Bedrail East Community Hospital East Remote Control - East Community Hospital East Sink East Community Hospital East Bedrail - West Community Hospital West Remote Control - West Community Hospital West Sink - West Community Hospital West
17 2.6 Setting up Test Plans Once locations and limits have been input into SureTrend software, test plans may then be set up. Test plans are helpful groups of locations that are tested one after another, grouped together, or tested on a specific day. Test plans help to keep testing and data analysis organized. See the SureTrend User Manual for directions on setting up test plans in SureTrend software. Here are some examples of test plans with locations: Nurses Station Keyboard Phone Countertop File cabinet handle Light switch ICU Patient Room Ventilator control panel IV Pole Monitor cables Call button Door handle Public Areas Handrails Door levers Waiting area chair Telephone Water cooler ER Mobile Workstations Crash cart ECG cart Laceration cart Bedside cart Trauma cart IV cart IV medication cart Cast cart West Wing Patient Room Bed tray table Patient phone Call button Bed rail Main light switch Sink handles Toilet flush handle Bathroom light switch Bathroom handrail Television remote Monday Bedrails IV Pole Bedside Table Remote Panels 15
18 2.7 Testing Frequency Once test plans are programmed into the SureTrend software, sync the SystemSURE Plus with the software and begin testing. The frequency of testing will be determined by: Budget Size of facility Importance of the cleaning standard Logistical and staff constraints Compliance with CDC environmental monitoring recommendations or other auditing bodies recommendations Desired statistical accuracy of reports (the greater the amount of data generated, the more representative the data will be) The CDC has a recommended formula for sample size determination. It is divided in two segments: Baseline Monitoring and Ongoing Monitoring. Section 2.8 outlines a Daily Monitoring routine that most hospitals will chose to fully leverage an ATP Cleaning Verification program. 1. Baseline Monitoring Getting Started Minimal Baseline Optimal This is the initial testing regiment that should be conducted to accurately assess the current level of cleanliness and compliance to cleaning processes. This initial testing will become the baseline to gauge improvements or deterioration of thoroughness of cleaning and will determine the number of points which must be monitored on a regular basis. While it would be ideal to identify small fluctuations in practice accurately (e.g., 10% relative change), such an approach takes slightly more time and testing. Instead, a meaningful change in cleaning practice (e.g., 20% relative change) can be detected without having to evaluate a substantial number of surfaces. The CDC recommends sampling all available surfaces identified in Section 2.1 or Appendix A (page 23) in a 10% - 15% sample of representative patient rooms in hospitals with over 150 beds. In hospitals with less than 150 beds, all available surfaces should be tested in a minimum of 15 rooms for baseline and ongoing monitoring. The CDC recommends baseline monitoring to be conducted at least three times per year. This is the minimum amount of testing that can be done to show a 20% relative change in cleaning levels. If pass levels decline, then more testing should be done in order to determine what has caused deterioration. (i.e., inefficient sanitizer, insufficient cleaning process, poor employee performance, etc.). Source: CDC Toolkit for Evaluating Environmental Cleaning, Appendix C, Evaluating-Environ-Cleaning.html 16 99
19 The following is an example of a baseline evaluation (to measure levels of cleanliness): Baseline Evaluation Calculation for a Typical 250-Bed Hospital Number of Patient Rooms Locations per Room Operating Room Locations Sterile Services Locations Total Locations Percent of Total Locations to Monitor For Example: 250 Patient Rooms 10 Test Locations in Each Room 100 Operating Room Locations 100 Sterile Services Locations 2,700 Total Locations Locations to Monitor Locations to Monitor 3 Evaluations per Year Totals Tests per Year For Example: Locations to Monitor 3 Evaluations per Year 810 1,215 Totals Tests per Year For a 250 bed hospital with 10 locations in each patient room, 100 operating room locations, and 100 sterile services locations, there are 2,700 total locations to be tested. To monitor 10-15% of locations, a total of locations will need to be tested three times per year (a total of 810-1,215 tests per year). 17
20 2. Ongoing Monitoring Minimalist Maintenance Minimal Baseline Optimal When hospitals have achieved more than 80% or higher pass results from baseline monitoring, the number of surfaces to be monitored can be decreased to those available in a 5% sample of rooms per evaluation cycle unless there is deterioration in practice. Though the CDC does outline minimal testing of 5% of potential test locations, Hygiena has found that hospitals that have a high cleaning thoroughness rate usually achieve such success through Daily Monitoring - see Section 2.8 on the following page. Below is an example of ongoing monitoring (once 80% or more of baseline test results are Passing ): Ongoing Monitoring for a Typical 250-Bed Hospital Number of Patient Rooms Locations per Room Operating Room Locations Sterile Services Locations Total Locations Percent of Total Locations to Monitor For Example: 250 Patient Rooms 10 Test Locations in Each Room 100 Operating Room Locations 100 Sterile Services Locations 2,700 Total Locations 135 Locations to Monitor Locations to Monitor 3 Evaluations per Year Totals Tests per Year For Example: 135 Locations to Monitor 3 Evaluations per Year 405 Totals Tests per Year For a 250 bed hospital with 10 locations in each room, 100 operating room locations, and 100 sterile services locations, there are 2700 total locations to be tested. To monitor 5% of locations, a total of 135 locations will need to be tested three times per year (a total of 405 tests per year). 1111
21 2.8 Daily Monitoring Optimal Program Minimal Baseline Optimal More and more hospitals are moving to daily or weekly ATP cleaning verification. Daily monitoring only requires a few tests per day, yet holds cleaning staff accountable for achieving an optimal level of cleanliness each and every day. Creating a reward system based on daily results can be a very powerful program that brings staff together and improves overall hospital cleanliness. Daily monitoring does take a greater time commitment and requires more consumables, so Hygiena will help every hospital to design a testing program within its resources and budget. Below are three scenarios breaking down the time and consumable use associated with a daily ATP monitoring program using 5, 10, or 15 tests per day. Scenario 1: Five Tests per Day Daily Monthly Yearly 5 tests 150 tests 1,800 tests # Tests Time (1 min/test) 5 minutes 2.5 hours 30 hours 19
22 Scenario 2: Ten Tests per Day Daily Monthly Yearly 10 tests 300 tests 3,600 tests # Tests Time (1 min/test) 10 minutes 5 hours 60 hours Scenario 3: Fifteen Tests per Day Daily Monthly Yearly 15 tests 450 tests 5,400 tests # Tests Time (1 min/test) 15 minutes 7.5 hours 90 hours
23 2.9 Use SureTrend Software to Maximize a Monitoring Program One of the greatest byproducts of a daily monitoring program is a robust database of test results to aid in decision making and management of hospital cleanliness programs. By frequently collecting test results, a hospital can build up a database of records to identify trends, asses training opportunities, and compare cleaning performance between departments, shifts, or facilities. Using these reports in regular performance feedback meetings with frontline personnel can be crucial in maintaining environmental cleaning effectiveness. SureTrend comes with dozens of preset reports so little setup is required by the user and reports can be automatically ed to stakeholders. Visit for a helpful guide for using SureTrend reports in hospitals: Quick Start Software Report Guide for the Healthcare Industry Additional Testing ATP cleaning verification can also be integrated into a hospital s emergency cleaning procedures as a final step to confirm thorough cleanliness. In the event of patient accidents, bio-waste spills, flood, new construction, outbreak, or other accidental contaminations involving blood, urine, or fecal waste, cleaning staff should perform an emergency cleaning of that area. To confirm thorough cleaning of the site, ATP tests can be taken. This will ensure that the spill site has been properly cleaned. 21
24 2.11 Calibration To verify instrument calibration, Hygiena offers two calibration kits that are recommended for periodic use with your system. Calibration Control Rod Kit (Catalog# PCD4000) Though Hygiena s ATP Cleaning Verification System automatically checks calibration at startup, it is recommended that calibration is verified with the Calibration Control Kit once a month for audit record-keeping purposes. Incorporating the Calibration Control Kit into a cleaning verification program will confirm that the instrument is within specifications and operating correctly. Each kit contains a positive rod and negative rod. The positive rod emits a very low level of constant light output that can be measured in RLUs to verify proper calibration of the unit. The negative rod produces zero (0) RLU and is used to check that background light is not entering the instrument, while ensuring that the light sensor is calibrated correctly. Positive Control Kit (Catalog # CK25) The Positive Control Kit is used for validating the efficacy and quality of the UltraSnap ATP Testing Device. Each kit comes with 25 sealed glass vials, containing a certain amount (approx. 5 x moles) of freeze-dried ATP and sugars to provide a predictable result if UltraSnap devices are used and stored correctly
25 Appendix A: Recommended Test Locations Patient Areas Airway cart Anesthesia cart Bathroom door knobs and levers* Bathroom handrails* Bathroom light switch* Bed control* Bed rails* Bedpan cleaner* Bedside chair* Bedside tables* BP machine cuff Cabinet door handles Call button* Door knobs* ECG cart Flush handle* IV cart IV pole* IV pump control* Laceration cart Light switch* Medication cart Monitor controls* Monitor touch screen* Monitor cables* Operating table base Operating table hand control Operating table mattress OR anesthesia machine OR boom OR keyboard OR door push plates OR spotlight Privacy curtain Phone* Sink top* Sink handles* Supply cart Toilet seat* Trauma cart Tray table* TV remote Ventilator control panel* Public Areas Drinking fountain buttons Elevator call buttons Hallway hand rails Nurses keyboard Nurses phone Vending machine buttons Visitor bathroom light switch Visitor bathroom door knobs Waiting room chairs Sterile Services Autoclave interior Biohazard receptacle Endoscope Sink Sterile tools Sterilizer handle Food Preparation: Cutlery Cutting board Dishwasher Freezer handles Fridge handles Knives Service trays Serving utensils Sink Sink handles Hand Hygiene: Preclean Post-clean Scrub sink Sink handles Soap dispenser * Source: CDC Environmental Checklist for Monitoring Terminal Cleaning available at 23
26 Appendix B: Proper Sampling Procedure Watch an instructional demo at The SystemSURE Plus luminometer is designed to detect contamination that cannot be seen by the naked eye. Excessive sample may interfere with the bioluminescence reaction and produce an inaccurate test result, which is why it is important to remove all visible soil from a surface before swabbing. Before collecting a sample for testing, the surface should be visibly clean. If any soiling or residue is present, reclean the area before testing. Turn on the SystemSURE Plus luminometer. The system will run through automatic calibration. Once calibration is complete, scroll through the program numbers (PROG) using the and arrows to find the programmed test location that correlates to the location being tested. This action should be taken prior to swabbing. Remove the individual UltraSnap device from the package. Next, remove the outer tube by holding onto the double ring base of the Snap-Valve while pulling down on the tube. The swab tip comes pre-moistened. Condensation may be visible on the inside of the swab tube. This is normal. Do not touch the swab tip or shaft with fingers or anything else, as this will contaminate the test. Discard any swabs that accidentally get tainted or activated. NOTE: For optimal performance, swabs that have been removed from cold storage should stand for 10 minutes at room temperature before use. Incorrect swabbing technique: Touching the swab shaft with your finger Lightly touching the swab to the sample area Collecting sample on only one side of the swab tip Correct swabbing technique: Sufficient pressure to create flex in the swab shaft (helps to break through any biofilm on the surface) Rotate the swab to collect sample on all sides of swab tip No contact with the swab shaft wand
27 Regular surfaces: Swab a 10 x 10 cm (4 x 4 in) square on the test surface, making a criss-cross pattern as shown. Irregular surfaces: Where 10 x 10 cm square sampling is not feasible, such as a bed rail, swab as much of the surface as possible to collect a representative sample. Note: Consistent sampling pattern on irregular surfaces is necessary to ensure reliable and repeatable results. All individuals responsible for performing swab tests should agree on similar sampling pattern. Re-insert the swab into the tube. Holding the device upright, activate UltraSnap by bending the bulb at the top until the plastic Snap-Valve breaks, then bend once more in the opposite direction. Squeeze the bulb twice to expel the liquid-stable reagent contained in the bulb and allow it to flow to the bottom of the tube. 25
28 Gently shake the device with a side-to-side motion for 5-10 seconds, bathing the swab bud in the liquid-stable reagent. The test is now activated and the bioluminescence reaction is taking place. For optimal results, the reading should be taken on the SystemSURE Plus luminometer within 30 seconds of activation. Open the lid on the SystemSURE Plus luminometer, and insert the activated UltraSnap device into the reading chamber. Close the lid. Taking care to hold the unit upright for an accurate reading, press OK on the SystemSURE Plus to initiate measurement. Results are displayed on the screen in 15 seconds
29 Gently shake the device with a side-to-side motion for 5-10 seconds, bathing the swab bud in the liquid-stable reagent. The test is now activated and the bioluminescence reaction is taking place. For optimal results, the reading should be taken on the SystemSURE Plus luminometer within 30 seconds of activation. Open the lid on the SystemSURE Plus luminometer, and insert the activated UltraSnap device into the reading chamber. Close the lid. Taking care to hold the unit upright for an accurate reading, press OK on the SystemSURE Plus to initiate measurement. Results are displayed on the screen in 15 seconds. 1919
30
31
32 INS0153 REVC
- WATER DAMAGE RESTORATION -
 - WATER DAMAGE RESTORATION - PROTOCOL FOR BIO-REVEAL SAMPLING OF CONDITION 1, 2 AND 3 MOLD & FUNGI CONTAMINATION Statement of Use The Bio-reveal Ultrasnap ATP swabs and the Bio-reveal Systemsure Plus luminometer
- WATER DAMAGE RESTORATION - PROTOCOL FOR BIO-REVEAL SAMPLING OF CONDITION 1, 2 AND 3 MOLD & FUNGI CONTAMINATION Statement of Use The Bio-reveal Ultrasnap ATP swabs and the Bio-reveal Systemsure Plus luminometer
- WATER DAMAGE RESTORATION -
 - WATER DAMAGE RESTORATION - PROTOCOL FOR BIO-REVEAL SAMPLING OF CATEGORY 1, 2 AND 3 WATER LOSS LIQUID/WATER & SURFACE TESTING Statement of Use The Bio-reveal Aquasnap ATP liquid sampling devices, Ultrasnap
- WATER DAMAGE RESTORATION - PROTOCOL FOR BIO-REVEAL SAMPLING OF CATEGORY 1, 2 AND 3 WATER LOSS LIQUID/WATER & SURFACE TESTING Statement of Use The Bio-reveal Aquasnap ATP liquid sampling devices, Ultrasnap
A Guide to ATP Hygiene Monitoring
 A Guide to ATP Hygiene Monitoring - i - TABLE OF CONTENTS QUICK START GUIDE...v Flow chart: Cleaning, Testing and Corrective Action Procedures...v INTRODUCTION...1 The EnSURE & SystemSURE Plus ATP Detection
A Guide to ATP Hygiene Monitoring - i - TABLE OF CONTENTS QUICK START GUIDE...v Flow chart: Cleaning, Testing and Corrective Action Procedures...v INTRODUCTION...1 The EnSURE & SystemSURE Plus ATP Detection
CARE HOMES AND NURSING HOMES
 Care Homes and Nursing Homes: This document provides guidelines and recommendations for cleaning care homes and nursing homes in the event of a disease outbreak in the vicinity. Cleaning and disinfection
Care Homes and Nursing Homes: This document provides guidelines and recommendations for cleaning care homes and nursing homes in the event of a disease outbreak in the vicinity. Cleaning and disinfection
CLEAN UP FOR VOMITING & DIARRHEAL EVENT IN RETAIL FOOD FACILITIES
 CLEAN UP FOR VOMITING & DIARRHEAL EVENT IN RETAIL FOOD FACILITIES GENERAL INFORMATION Noroviruses are a group of viruses that cause gastroenteritis [gas-trō-en-ter-ī-tis] in people. Gastroenteritis is
CLEAN UP FOR VOMITING & DIARRHEAL EVENT IN RETAIL FOOD FACILITIES GENERAL INFORMATION Noroviruses are a group of viruses that cause gastroenteritis [gas-trō-en-ter-ī-tis] in people. Gastroenteritis is
Please, Wash Your Hands! We have a huge in issue in this world today and I don t think it gets addressed as
 Britney Boykin Persuasive Speech Please, Wash Your Hands! We have a huge in issue in this world today and I don t think it gets addressed as much as it should. Since we were little bitty we have been taught
Britney Boykin Persuasive Speech Please, Wash Your Hands! We have a huge in issue in this world today and I don t think it gets addressed as much as it should. Since we were little bitty we have been taught
Waters Corporation. Waters 2690/5 USER & TROUBLESHOOTING GUIDE
 Waters Corporation Waters 2690/5 USER & TROUBLESHOOTING GUIDE Contents 2690/5 Theory Setup procedures. Troubleshooting the 2690/5 User maintenance of the 2690/5 Spare Parts 2 2690/5 Theory 2690/5 Solvent
Waters Corporation Waters 2690/5 USER & TROUBLESHOOTING GUIDE Contents 2690/5 Theory Setup procedures. Troubleshooting the 2690/5 User maintenance of the 2690/5 Spare Parts 2 2690/5 Theory 2690/5 Solvent
MANITOBA PATIENT SERVICE CENTRE STANDARDS
 MANITOBA PATIENT SERVICE CENTRE STANDARDS February 2015 INTRODUCTION These Standards are derived from Z316.7-12 and are approved by the Council of the College of Physicians and Surgeons of Manitoba. These
MANITOBA PATIENT SERVICE CENTRE STANDARDS February 2015 INTRODUCTION These Standards are derived from Z316.7-12 and are approved by the Council of the College of Physicians and Surgeons of Manitoba. These
Living healthy with MRSA
 Stamford Health System Having MRSA means what? Living healthy with MRSA Discharge information for patients and families WASHING YOUR HANDS IS THE KEY!!! Staph aureus is a bacteria that lives on your skin
Stamford Health System Having MRSA means what? Living healthy with MRSA Discharge information for patients and families WASHING YOUR HANDS IS THE KEY!!! Staph aureus is a bacteria that lives on your skin
CHILDCARE SETTINGS. Childcare Settings
 Childcare Settings This document provides guidelines and recommendations for cleaning childcare settings such as nurseries, crèches and schools in the event of a disease outbreak in the vicinity. Cleaning
Childcare Settings This document provides guidelines and recommendations for cleaning childcare settings such as nurseries, crèches and schools in the event of a disease outbreak in the vicinity. Cleaning
Sanitary Food Preparation & Safe Food Handling
 70 Feeding Infants 10 Sanitary Food Preparation & Safe Food Handling Babies are more susceptible to bacteria than older children, and unsanitary food conditions can cause serious infections. General cleanliness,
70 Feeding Infants 10 Sanitary Food Preparation & Safe Food Handling Babies are more susceptible to bacteria than older children, and unsanitary food conditions can cause serious infections. General cleanliness,
VRE. Living with. Learning how to control the spread of Vancomycin-resistant enterococci (VRE)
 VRE Living with Learning how to control the spread of Vancomycin-resistant enterococci (VRE) CONTENTS IMPORTANT VRE is a serious infection that may become life-threatening if left untreated. If you or
VRE Living with Learning how to control the spread of Vancomycin-resistant enterococci (VRE) CONTENTS IMPORTANT VRE is a serious infection that may become life-threatening if left untreated. If you or
Ancillary Staff Training
 Ancillary Staff Training Goals of Infection Prevention Protect the patients Protect the staff Prevent spread of diseases How Does The Virus Spread Between People? Direct contact through broken skin, mouth,
Ancillary Staff Training Goals of Infection Prevention Protect the patients Protect the staff Prevent spread of diseases How Does The Virus Spread Between People? Direct contact through broken skin, mouth,
02.11 Food and Nutrition Services
 02.11 Purpose Audience To assure proper and safe food handling, storage, and preparation. All Department of employees. Personnel The following guidelines shall be used to monitor proper and safe food handling,
02.11 Purpose Audience To assure proper and safe food handling, storage, and preparation. All Department of employees. Personnel The following guidelines shall be used to monitor proper and safe food handling,
Environmental Monitoring
 Chapter 10 Environmental Monitoring By the end of this chapter, you will be able to: l List the types of environmental testing l Name three types of checks that are carried out to ensure the isolator is
Chapter 10 Environmental Monitoring By the end of this chapter, you will be able to: l List the types of environmental testing l Name three types of checks that are carried out to ensure the isolator is
Georgia Tech s Award-Winning Renewable Cleaning Program. Tommy Little Associate Director, Building Services
 Georgia Tech s Award-Winning Renewable Cleaning Program Tommy Little Associate Director, Building Services 2015 OUR AWARDS Winner of the 2006 National Association of Higher Education Facilities Officers
Georgia Tech s Award-Winning Renewable Cleaning Program Tommy Little Associate Director, Building Services 2015 OUR AWARDS Winner of the 2006 National Association of Higher Education Facilities Officers
Subpart 1. Installation. All plumbing systems must be. installed and tested according to this chapter and chapter 4715,
 4658.4500 PLUMBING SYSTEMS; NEW CONSTRUCTION. Subpart 1. Installation. All plumbing systems must be installed and tested according to this chapter and chapter 4715, the Minnesota Plumbing Code. Subp. 2.
4658.4500 PLUMBING SYSTEMS; NEW CONSTRUCTION. Subpart 1. Installation. All plumbing systems must be installed and tested according to this chapter and chapter 4715, the Minnesota Plumbing Code. Subp. 2.
Options for Evaluating Environmental Cleaning
 Options for Evaluating Environmental Cleaning Prepared by: Alice Guh, MD, MPH 1 Philip Carling, MD 2 Environmental Evaluation Workgroup 3 December 2010 1 Division of Healthcare Quality Promotion, National
Options for Evaluating Environmental Cleaning Prepared by: Alice Guh, MD, MPH 1 Philip Carling, MD 2 Environmental Evaluation Workgroup 3 December 2010 1 Division of Healthcare Quality Promotion, National
Standard Operating Procedures. Provincial Ebola Expert Working Group Feb 12 2015
 Recommendations for Environmental Services, Biohazardous Waste Management, and Food and Linen Management for Persons under Investigation and Confirmed Cases of Ebola Virus Disease: Standard Operating Procedures
Recommendations for Environmental Services, Biohazardous Waste Management, and Food and Linen Management for Persons under Investigation and Confirmed Cases of Ebola Virus Disease: Standard Operating Procedures
SEALED AIR LEARNING MANAGEMENT SYSTEM RE-IMAGINE TRAINING AND COMPLIANCE
 SEALED AIR LEARNING MANAGEMENT SYSTEM RE-IMAGINE TRAINING AND COMPLIANCE LEARNING MANAGEMENT SYSTEM RE-IMAGINE TRAINING AND COMPLIANCE A state of the art e-learning and data management solution that simplifies
SEALED AIR LEARNING MANAGEMENT SYSTEM RE-IMAGINE TRAINING AND COMPLIANCE LEARNING MANAGEMENT SYSTEM RE-IMAGINE TRAINING AND COMPLIANCE A state of the art e-learning and data management solution that simplifies
Hygiene and Infection. Control advice in the home
 Introduction The Infection Control Department Mid-Western Regional Hospital, Ennis, wrote this booklet with Clare Primary Community and Continuing Care and C-Diff Patients and Families Group. This booklet
Introduction The Infection Control Department Mid-Western Regional Hospital, Ennis, wrote this booklet with Clare Primary Community and Continuing Care and C-Diff Patients and Families Group. This booklet
How to Run the PacBio RS II Instrument. 1. Run Instrument. 1.1 How to Run the RS II Instrument
 How to Run the PacBio RS II Instrument 100 338 600 01 1. Run Instrument 1.1 How to Run the RS II Instrument Welcome to Pacific Biosciences' How to Run the PacBio RS II Instrument training module. This
How to Run the PacBio RS II Instrument 100 338 600 01 1. Run Instrument 1.1 How to Run the RS II Instrument Welcome to Pacific Biosciences' How to Run the PacBio RS II Instrument training module. This
#9. EFFECTIVE CLEANING, MAINTENANCE, AND PEST CONTROL PROGRAMS FOR YOUR HACCP PLAN
 #9. EFFECTIVE CLEANING, MAINTENANCE, AND PEST CONTROL PROGRAMS FOR YOUR HACCP PLAN by O. Peter Snyder, Jr., Ph.D. Hospitality Institute of Technology and Management In past articles, I have discussed supplier
#9. EFFECTIVE CLEANING, MAINTENANCE, AND PEST CONTROL PROGRAMS FOR YOUR HACCP PLAN by O. Peter Snyder, Jr., Ph.D. Hospitality Institute of Technology and Management In past articles, I have discussed supplier
Care and use guide. Contents. a handy solution made easy. www.getbluelab.com. page
 Care and use guide Contents page Features Quick guide Before use To operate Important - ph pen probe care 4 Cleaning 5 Battery replacement 5 Hydration 6 Calibration 6 Error messages 7 Troubleshooting guide
Care and use guide Contents page Features Quick guide Before use To operate Important - ph pen probe care 4 Cleaning 5 Battery replacement 5 Hydration 6 Calibration 6 Error messages 7 Troubleshooting guide
DIALYSIS SPECIFIC EOC OBSERVATION Data Definition Tool
 DIALYSIS SPECIFIC EOC OBSERVATION Data Definition Tool This audit is to be completed by the manager or designee on a monthly basis. "Dialysis Specific EOC Observation" audits are due by the last day of
DIALYSIS SPECIFIC EOC OBSERVATION Data Definition Tool This audit is to be completed by the manager or designee on a monthly basis. "Dialysis Specific EOC Observation" audits are due by the last day of
Norwalk-Like Viruses Decontamination Guidelines for Environmental Services
 Norwalk-Like Viruses Decontamination Guidelines for Environmental Services This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended
Norwalk-Like Viruses Decontamination Guidelines for Environmental Services This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended
CYTOTOXIC PRECAUTIONS A GUIDE FOR PATIENTS & FAMILIES
 Perth and Smiths Falls District Hospital Attention: Manager, Quality 60 Cornelia Street, West Smiths Falls, Ontario K7A 2H9 CYTOTOXIC PRECAUTIONS A GUIDE FOR PATIENTS & FAMILIES This guide has been prepared
Perth and Smiths Falls District Hospital Attention: Manager, Quality 60 Cornelia Street, West Smiths Falls, Ontario K7A 2H9 CYTOTOXIC PRECAUTIONS A GUIDE FOR PATIENTS & FAMILIES This guide has been prepared
How to safely collect blood samples from persons suspected to be infected with highly infectious blood-borne pathogens (e.g.
 How to safely collect blood samples from persons suspected to be infected with highly infectious blood-borne pathogens (e.g. Ebola) Step 1: Before entering patient room, assemble all equipment (1 st part)
How to safely collect blood samples from persons suspected to be infected with highly infectious blood-borne pathogens (e.g. Ebola) Step 1: Before entering patient room, assemble all equipment (1 st part)
Page 1 of 6 COMPLIANCE CHECKLIST. > Long Term Care Facility - Nursing Unit
 COMPLIANCE CHECKLIST Page 1 of 6 > Long Term Care Facility - Nursing Unit The following checklist is for plan review of new long-term care facilities and new additions to existing long-term care facilities.
COMPLIANCE CHECKLIST Page 1 of 6 > Long Term Care Facility - Nursing Unit The following checklist is for plan review of new long-term care facilities and new additions to existing long-term care facilities.
RECOMMENDED CLEANING PROCEDURES FOR NOROVIRUS OUTBREAKS. General Recommendations:
 RECOMMENDED CLEANING PROCEDURES FOR NOROVIRUS OUTBREAKS Please use the following as a guide to increase standard infection control and housekeeping activities during gastrointestinal illness outbreaks,
RECOMMENDED CLEANING PROCEDURES FOR NOROVIRUS OUTBREAKS Please use the following as a guide to increase standard infection control and housekeeping activities during gastrointestinal illness outbreaks,
Your Guide to Peritoneal Dialysis Module 3: Doing Peritoneal Dialysis at Home
 Your Guide to Peritoneal Dialysis Module 3: 6.0959 in Preparing to do PD One of the most important things about PD is to keep the dialysis area and anything that comes in contact with the PD equipment
Your Guide to Peritoneal Dialysis Module 3: 6.0959 in Preparing to do PD One of the most important things about PD is to keep the dialysis area and anything that comes in contact with the PD equipment
STEP-BY-STEP INSTRUCTIONS FOR INVESTIGATIONAL USE. Rapid HCV Antibody Test FOR ORAQUICK RAPID HCV ANTIBODY TEST
 Before performing testing, all operators MUST read and become familiar with Universal Precautions for Prevention of Transmission of Human Immunodeficiency Virus, Hepatitis B Virus, and other Blood-borne
Before performing testing, all operators MUST read and become familiar with Universal Precautions for Prevention of Transmission of Human Immunodeficiency Virus, Hepatitis B Virus, and other Blood-borne
ENVIRONMENTAL MONITORING
 ENVIRONMENTAL MONITORING Assessment and verification of the adequacy of the aseptic compounding environment is essential. Environmental monitoring programs are designed to promptly identify potential sources
ENVIRONMENTAL MONITORING Assessment and verification of the adequacy of the aseptic compounding environment is essential. Environmental monitoring programs are designed to promptly identify potential sources
Environmental Cleaning Top 10 Best Practices
 Environmental Cleaning Top 10 Best Practices Overview Environmental Cleaning Top 10 Practices PIDAC document Auditing environmental cleaning practices Environmental Cleaning toolkit 2 WHAT DO WE KNOW?
Environmental Cleaning Top 10 Best Practices Overview Environmental Cleaning Top 10 Practices PIDAC document Auditing environmental cleaning practices Environmental Cleaning toolkit 2 WHAT DO WE KNOW?
What Is. Norovirus? Learning how to control the spread of norovirus. Web Sites
 Web Sites Centers for Disease Control and Prevention (CDC) http://www.cdc.gov/norovirus/index.html Your Local Health Department http://www.azdhs.gov/diro/lhliaison/countymap.htm What Is Ocument dn Norovirus?
Web Sites Centers for Disease Control and Prevention (CDC) http://www.cdc.gov/norovirus/index.html Your Local Health Department http://www.azdhs.gov/diro/lhliaison/countymap.htm What Is Ocument dn Norovirus?
Methods of Verification of Cleaning in CSSD. Dr. Winfried Michels Mechelen Oct.2012
 Methods of Verification of Cleaning in CSSD Dr. Winfried Michels Mechelen Oct.2012 Excerpts from ISO EN 15883-1 GTZ/PI/Dr. Michels 2 Before we consider which tracer to be monitored as a criterion for cleanliness
Methods of Verification of Cleaning in CSSD Dr. Winfried Michels Mechelen Oct.2012 Excerpts from ISO EN 15883-1 GTZ/PI/Dr. Michels 2 Before we consider which tracer to be monitored as a criterion for cleanliness
ArtisanLink Staining System is an automated special stains slide
 Technical Tips Tips on using the ArtisanLink Special Staining System Jamie Nowacek, BS, HT(ASCP) CM, QIHC, PMP Dako North America, Inc. Carpinteria, CA, USA ArtisanLink Staining System is an automated
Technical Tips Tips on using the ArtisanLink Special Staining System Jamie Nowacek, BS, HT(ASCP) CM, QIHC, PMP Dako North America, Inc. Carpinteria, CA, USA ArtisanLink Staining System is an automated
Infection Control in the Dental Practice through Proper Sterilization
 Midmark Corporation 60 Vista Drive P.O. Box 286 Versailles, Ohio 45380-0286 937-526-3662 midmark.com Infection Control in the Dental Practice through Proper Sterilization In today's world, the need for
Midmark Corporation 60 Vista Drive P.O. Box 286 Versailles, Ohio 45380-0286 937-526-3662 midmark.com Infection Control in the Dental Practice through Proper Sterilization In today's world, the need for
Achieving Independence. A Guide to Self-Catheterization with the Bard Touchless Plus Intermittent Catheter System
 Bard: Intermittent Self-Catheterization A Guide to Self-Catheterization with the Bard Touchless Plus Intermittent Catheter System Achieving Independence Introducing the Bard Touchless Plus Catheter One
Bard: Intermittent Self-Catheterization A Guide to Self-Catheterization with the Bard Touchless Plus Intermittent Catheter System Achieving Independence Introducing the Bard Touchless Plus Catheter One
Achieving Independence
 Bard: Intermittent Self-Catheterization A Guide to Self-Catheterization Achieving Independence Introduction This brochure is provided by Bard, a leading provider of urology products since 1907. The best
Bard: Intermittent Self-Catheterization A Guide to Self-Catheterization Achieving Independence Introduction This brochure is provided by Bard, a leading provider of urology products since 1907. The best
IRISH ACCOMMODATION SERVICE AWARDS
 IRISH ACCOMMODATION SERVICE AWARDS CHECKLIST Property Name: Category: 3* hotel 4* hotel 5* hotel Boutique hotel B&B Hospital Nursing Home Colleges / Schools Irish Accommodation Service Awards IASI Accommodation
IRISH ACCOMMODATION SERVICE AWARDS CHECKLIST Property Name: Category: 3* hotel 4* hotel 5* hotel Boutique hotel B&B Hospital Nursing Home Colleges / Schools Irish Accommodation Service Awards IASI Accommodation
A Sanitarian s Role During Disaster Relief Efforts
 A Sanitarian s Role During Disaster Relief Efforts Experience Following Hurricane Katrina at the George R. Brown Convention Center in Houston Texas Houston Department of Health and Human Services Disaster
A Sanitarian s Role During Disaster Relief Efforts Experience Following Hurricane Katrina at the George R. Brown Convention Center in Houston Texas Houston Department of Health and Human Services Disaster
High Dose Radioactive Iodine (I-131) Therapy for Treatment of Thyroid Cancer
 12 High Dose Radioactive Iodine (I-131) Therapy for Treatment of Thyroid Cancer Please arrive 15 minutes early to allow for parking and registration. If you have questions or need to cancel your appointment
12 High Dose Radioactive Iodine (I-131) Therapy for Treatment of Thyroid Cancer Please arrive 15 minutes early to allow for parking and registration. If you have questions or need to cancel your appointment
TRAINING MANUAL for Clean Business Environments
 TRAINING MANUAL for Clean Business Environments Phone: (630) 771-1123 Cell: (708) 903-2799 Email: rtmcb86@sbcglobal.net Website: mcbroomscleaning.com Proud Member of the Bolingbrook Area Chamber of Commerce
TRAINING MANUAL for Clean Business Environments Phone: (630) 771-1123 Cell: (708) 903-2799 Email: rtmcb86@sbcglobal.net Website: mcbroomscleaning.com Proud Member of the Bolingbrook Area Chamber of Commerce
Laboratory Line. Vertical and Bench-Top Life Science Autoclaves
 Laboratory Line Vertical and Bench-Top Life Science Autoclaves The Advanced Laboratory Line The Full Spectrum of Laboratory Sterilizers Sterilization in a laboratory environment has its unique requirements.
Laboratory Line Vertical and Bench-Top Life Science Autoclaves The Advanced Laboratory Line The Full Spectrum of Laboratory Sterilizers Sterilization in a laboratory environment has its unique requirements.
http://www.coastalamusements.com
 Nexus is a Registered Trademark of Coastal Amusements. OWNERS MANUAL 1935 Swarthmore Avenue, Lakewood, NJ 08701 (732)-905-6662 FAX (732)-905-6815 http://www.coastalamusements.com GAME DESCRIPTION Nexus
Nexus is a Registered Trademark of Coastal Amusements. OWNERS MANUAL 1935 Swarthmore Avenue, Lakewood, NJ 08701 (732)-905-6662 FAX (732)-905-6815 http://www.coastalamusements.com GAME DESCRIPTION Nexus
All Apartments River Village Community Bedroom Bathroom Kitchen Living Room Commons/Hallways/Stairwells Doors Windows
 All Apartments Paint cost per linear foot $3.79 Drywall repair per linear foot $10.25 Drywall repair with paint cost per linear foot $14.04 Flooring cost per square foot $8.95 Item Removal Fee (Per Item)
All Apartments Paint cost per linear foot $3.79 Drywall repair per linear foot $10.25 Drywall repair with paint cost per linear foot $14.04 Flooring cost per square foot $8.95 Item Removal Fee (Per Item)
 Overhead #7 Overhead #8 Key Points of This Training You will learn more about: Identifying and reducing hazards on the job Identifying and reducing hazards in healthcare jobs Laws that protect teens from
Overhead #7 Overhead #8 Key Points of This Training You will learn more about: Identifying and reducing hazards on the job Identifying and reducing hazards in healthcare jobs Laws that protect teens from
Moving to a hospital or skilled nursing facility
 H Moving to a hospital or skilled nursing facility What to expect when you have MRSA (Methicillin-resistant Staphylococcus aureus) A booklet for patients, residents, family members, and caregivers About
H Moving to a hospital or skilled nursing facility What to expect when you have MRSA (Methicillin-resistant Staphylococcus aureus) A booklet for patients, residents, family members, and caregivers About
To provide direction for the safe handling, administration and disposal of hazardous drugs.
 Subsection: MEDICATION Related terms: Cytotoxic Drugs, Antineoplastic Drugs Authorized by: Clinical Directors CS-04-02-01 Page 1 of 9 Date Established: October 2006 Date For Review: September 2014 Dates
Subsection: MEDICATION Related terms: Cytotoxic Drugs, Antineoplastic Drugs Authorized by: Clinical Directors CS-04-02-01 Page 1 of 9 Date Established: October 2006 Date For Review: September 2014 Dates
Healthcare workers report that various factors contribute to poor compliance with hand hygiene. These include:
 RISKTOPICS Hand hygiene in the healthcare setting January 2013 Proper hand hygiene is the best way to keep from getting sick and prevent germs from being spread to others. For hospitals, nursing homes
RISKTOPICS Hand hygiene in the healthcare setting January 2013 Proper hand hygiene is the best way to keep from getting sick and prevent germs from being spread to others. For hospitals, nursing homes
Bacterial Transformation with Green Fluorescent Protein. Table of Contents Fall 2012
 Bacterial Transformation with Green Fluorescent Protein pglo Version Table of Contents Bacterial Transformation Introduction..1 Laboratory Exercise...3 Important Laboratory Practices 3 Protocol...... 4
Bacterial Transformation with Green Fluorescent Protein pglo Version Table of Contents Bacterial Transformation Introduction..1 Laboratory Exercise...3 Important Laboratory Practices 3 Protocol...... 4
Deluxe Bottle Warmer
 Deluxe Bottle Warmer IMPORTANT SAFEGUARDS This product is for household use only. When using electrical appliances, basic safety precautions should always be followed including the following: Read all
Deluxe Bottle Warmer IMPORTANT SAFEGUARDS This product is for household use only. When using electrical appliances, basic safety precautions should always be followed including the following: Read all
Cleaning Can Be Programmatically Improved
 Cleaning Can Be Programmatically Improved Improving cleaning of the environment surrounding patients in 36 acute care hospitals. 80 % of Objects Cleaned 70 60 50 40 PRE INTERVENTION POST INTERVENTION P
Cleaning Can Be Programmatically Improved Improving cleaning of the environment surrounding patients in 36 acute care hospitals. 80 % of Objects Cleaned 70 60 50 40 PRE INTERVENTION POST INTERVENTION P
BODY SUBSTANCE ISOLATION (BSI): THE STANDARD OF CARE
 CRAIG HOSPITAL POLICY/PROCEDURE Approved: NPC, IC, MEC, P&P 05/06 Effective Date: 02/88 P&P 06/09 Attachments: Revised Date: 05/03, 04/06 Decision Tree for Isolation Precautions Comments on Specific Diseases
CRAIG HOSPITAL POLICY/PROCEDURE Approved: NPC, IC, MEC, P&P 05/06 Effective Date: 02/88 P&P 06/09 Attachments: Revised Date: 05/03, 04/06 Decision Tree for Isolation Precautions Comments on Specific Diseases
ClikSTAR - Important facts about your new insulin delivery device.
 ClikSTAR - Important facts about your new insulin delivery device. Instruction for Use ClikSTAR Insulin delivery device Before you start: Read these instructions and follow them completely each time you
ClikSTAR - Important facts about your new insulin delivery device. Instruction for Use ClikSTAR Insulin delivery device Before you start: Read these instructions and follow them completely each time you
Section 6: Your Hemodialysis Catheter
 Section 6: Your Hemodialysis Catheter What you should know about your dialysis catheter How to change your catheter TEGO connectors Starting dialysis using a catheter End of dialysis using a catheter Changing
Section 6: Your Hemodialysis Catheter What you should know about your dialysis catheter How to change your catheter TEGO connectors Starting dialysis using a catheter End of dialysis using a catheter Changing
Nursing Home Care and Cleaning
 Article for: New England HealthCare Article Due Date: June 15 or sooner Article Type: Small Feature Magazine Editor: Shawn Client: Enviro Subject: Cleaning Nursing Homes/bring in Green cleaning Word Count:
Article for: New England HealthCare Article Due Date: June 15 or sooner Article Type: Small Feature Magazine Editor: Shawn Client: Enviro Subject: Cleaning Nursing Homes/bring in Green cleaning Word Count:
R432. Health, Health Systems Improvement, Licensing. R432-5. Nursing Facility Construction. R432-5-1. Legal Authority. R432-5-2. Purpose.
 R432. Health, Health Systems Improvement, Licensing. R432-5. Nursing Facility Construction. R432-5-1. Legal Authority. This rule is promulgated pursuant to Title 26, Chapter 21. R432-5-2. Purpose. The
R432. Health, Health Systems Improvement, Licensing. R432-5. Nursing Facility Construction. R432-5-1. Legal Authority. This rule is promulgated pursuant to Title 26, Chapter 21. R432-5-2. Purpose. The
Flexible Endoscopes: The A,B,C s of Monitoring Manual Cleaning Efficacy!
 Flexible Endoscopes: The A,B,C s of Monitoring Manual Cleaning Efficacy! Dr. Michelle J. Alfa, Ph.D., FCCM Medical Director, Clinical Microbiology, Diagnostic Services of Manitoba, Winnipeg, Canada Disclosure:
Flexible Endoscopes: The A,B,C s of Monitoring Manual Cleaning Efficacy! Dr. Michelle J. Alfa, Ph.D., FCCM Medical Director, Clinical Microbiology, Diagnostic Services of Manitoba, Winnipeg, Canada Disclosure:
The Drink-Aide The NEW look of independence
 The Drink-Aide The NEW look of independence Introducing Drink-Aide What is Drink-Aide? The Drink-Aide is a water bottle that attaches easily to a wheelchair for use by persons with little or no upper
The Drink-Aide The NEW look of independence Introducing Drink-Aide What is Drink-Aide? The Drink-Aide is a water bottle that attaches easily to a wheelchair for use by persons with little or no upper
Installation Instructions 6028.801
 DAZZLE Installation Instructions 08.80 Spread Lavatory Faucet with Speed Connect Drain* Congratulations on purchasing your American Standard faucet with Speed Connect drain, a feature found only on American
DAZZLE Installation Instructions 08.80 Spread Lavatory Faucet with Speed Connect Drain* Congratulations on purchasing your American Standard faucet with Speed Connect drain, a feature found only on American
SANITATION AND PEST CONTROL INSPECTION REPORT INSPECTOR: BUSINESS NAME:
 SANITATION AND PEST CONTROL INSPECTION REPORT INSPECTOR: BUSINESS NAME: A. EXTERIOR AREAS 1. Evidence of pest activity 2. Pest harborage 3. Adequate garbage handling 4. Adequate garbage container design
SANITATION AND PEST CONTROL INSPECTION REPORT INSPECTOR: BUSINESS NAME: A. EXTERIOR AREAS 1. Evidence of pest activity 2. Pest harborage 3. Adequate garbage handling 4. Adequate garbage container design
Name of Equipment Silver King Model SKMCD1P/C1. This equipment chapter is to be inserted in the appropriate section of the Equipment Manual.
 Name of Equipment Silver King Model SKMCD1P/C1 This equipment chapter is to be inserted in the appropriate section of the Equipment Manual. Manufactured exclusively for McDonald s By Silver King Refrigeration,
Name of Equipment Silver King Model SKMCD1P/C1 This equipment chapter is to be inserted in the appropriate section of the Equipment Manual. Manufactured exclusively for McDonald s By Silver King Refrigeration,
NEW YORK. Downloaded 01.15.11
 Nurses Station & Resident Call System PRE 1975: NEW YORK Downloaded 01.15.11 713 1.3 Nursing units. Each nursing unit shall include the following service areas and shall meet the following minimum requirements:
Nurses Station & Resident Call System PRE 1975: NEW YORK Downloaded 01.15.11 713 1.3 Nursing units. Each nursing unit shall include the following service areas and shall meet the following minimum requirements:
Lead Testing and On Site Calibration for Water Testing Detection Range: 2 100ppb
 Document: AND Lead 100 7 2013 Lead Testing and On Site Calibration for Water Testing Detection Range: 2 100ppb July, 2013 Edition 1 ANDalyze, Inc., 2012. All rights reserved. Printed in USA. Table of Contents
Document: AND Lead 100 7 2013 Lead Testing and On Site Calibration for Water Testing Detection Range: 2 100ppb July, 2013 Edition 1 ANDalyze, Inc., 2012. All rights reserved. Printed in USA. Table of Contents
Biohazardous, Medical & Biological Waste Guidance Chart
 CSULA Environmental Health and Safety Biohazardous, Medical & Biological Waste Guidance Chart The chart below provides information on how to handle most, if not all, of the items that frequently are collectively
CSULA Environmental Health and Safety Biohazardous, Medical & Biological Waste Guidance Chart The chart below provides information on how to handle most, if not all, of the items that frequently are collectively
NIH Clinical Center Patient Education Materials Giving a subcutaneous injection
 NIH Clinical Center Patient Education Materials What is a subcutaenous injection? A subcutaneous injection is given in the fatty layer of tissue just under the skin. A subcutaneous injection into the fatty
NIH Clinical Center Patient Education Materials What is a subcutaenous injection? A subcutaneous injection is given in the fatty layer of tissue just under the skin. A subcutaneous injection into the fatty
Content Sheet 5-1: Overview of Sample Management
 Content Sheet 5-1: Overview of Management Role in quality management system management is a part of process control, one of the essentials of a quality management system. The quality of the work a laboratory
Content Sheet 5-1: Overview of Management Role in quality management system management is a part of process control, one of the essentials of a quality management system. The quality of the work a laboratory
Conservation of Momentum Greg Kifer
 SCIENCE EXPERIMENTS ON FILE Revised Edition 6.7-1 Conservation of Momentum Greg Kifer Topic Conservation of momentum Time 1 hour! Safety Please click on the safety icon to view the safety precautions.
SCIENCE EXPERIMENTS ON FILE Revised Edition 6.7-1 Conservation of Momentum Greg Kifer Topic Conservation of momentum Time 1 hour! Safety Please click on the safety icon to view the safety precautions.
PATIENT GUIDE. Care and Maintenance Drainage Frequency: Max. Drainage Volume: Dressing Option: Clinician s Signature: Every drainage Weekly
 PATIENT GUIDE Care and Maintenance Drainage Frequency: Max. Drainage Volume: Dressing Option: Every drainage Weekly Clinician s Signature: ACCESS SYSTEMS Pleural Space Insertion Site Cuff Exit Site Catheter
PATIENT GUIDE Care and Maintenance Drainage Frequency: Max. Drainage Volume: Dressing Option: Every drainage Weekly Clinician s Signature: ACCESS SYSTEMS Pleural Space Insertion Site Cuff Exit Site Catheter
Instructions for Use. Components of the GENOTROPIN PEN 12
 Instructions for Use Important Note Please read these instructions completely before using the GENOTROPIN PEN 12. If there is anything you do not understand or cannot do, call the toll-free number listed
Instructions for Use Important Note Please read these instructions completely before using the GENOTROPIN PEN 12. If there is anything you do not understand or cannot do, call the toll-free number listed
Biological Safety Program
 Risk Management & Safety Main Office, Wyoming Hall Phone: (307) 766-3277 Fax: (307)766-6116 Regulated Materials Management Center Phone: (307)766-3696 Fax: (307)766-3699 Web: www.uwyo.edu/ehs Email: UWEHS@uwyo.edu
Risk Management & Safety Main Office, Wyoming Hall Phone: (307) 766-3277 Fax: (307)766-6116 Regulated Materials Management Center Phone: (307)766-3696 Fax: (307)766-3699 Web: www.uwyo.edu/ehs Email: UWEHS@uwyo.edu
Advice for Colleges, Universities, and Students about Ebola in West Africa For Colleges and Universities
 Advice for Colleges, Universities, and Students about Ebola in West Africa For Colleges and Universities Advice for Study Abroad, Foreign Exchange, or Other Education-related Travel Is it safe to travel
Advice for Colleges, Universities, and Students about Ebola in West Africa For Colleges and Universities Advice for Study Abroad, Foreign Exchange, or Other Education-related Travel Is it safe to travel
INTRODUCTION TO CLINICAL PRACTICE AND CLINICAL SKILLS 2nd Year MEDICAL YEAR 2009/2010
 INTRODUCTION TO CLINICAL PRACTICE AND CLINICAL SKILLS 2nd Year MEDICAL YEAR 2009/2010 POINT OF CARE TESTING This session is designed to teach you the principals of point of care testing. This is common
INTRODUCTION TO CLINICAL PRACTICE AND CLINICAL SKILLS 2nd Year MEDICAL YEAR 2009/2010 POINT OF CARE TESTING This session is designed to teach you the principals of point of care testing. This is common
FMI Listeria Action Plan for Retail Delis
 FMI Listeria Action Plan for Retail Delis November 2012 Listeria control at retail is an ongoing challenge. FMI and our members are committed to finding ways to control the growth and if possible, eliminate
FMI Listeria Action Plan for Retail Delis November 2012 Listeria control at retail is an ongoing challenge. FMI and our members are committed to finding ways to control the growth and if possible, eliminate
Response to Biological Spills in the Laboratory (Intentional or Accidental)
 Response to Biological Spills in the Laboratory (Intentional or Accidental) Exposure Management For splash to eyes, mucous membranes, or broken area of the skin Irrigate eyes with clean water, saline or
Response to Biological Spills in the Laboratory (Intentional or Accidental) Exposure Management For splash to eyes, mucous membranes, or broken area of the skin Irrigate eyes with clean water, saline or
ATI Skills Modules Checklist for Urinary Catheter Care
 For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Urinary Catheter Care Student s name Date Verify order Patient record Assess for procedure need Identify, gather, and prepare
For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Urinary Catheter Care Student s name Date Verify order Patient record Assess for procedure need Identify, gather, and prepare
2. Does the patient have one of the following appropriate indications for placing indwelling urinary catheters?
 A. Decision to Insert a Urinary Catheter: 1. Before placing an indwelling catheter, please consider if these alternatives would be more appropriate: Bladder scanner: to assess and confirm urinary retention,
A. Decision to Insert a Urinary Catheter: 1. Before placing an indwelling catheter, please consider if these alternatives would be more appropriate: Bladder scanner: to assess and confirm urinary retention,
Quick Start Guide See Inside for Use and Safety Information
 3 rd Generation Personal 3D Printer Quick Start Guide See Inside for Use and Safety Information The USB Mass Storage Device Contains the User Guide and Quick Start Guide in other Languages Congratulations
3 rd Generation Personal 3D Printer Quick Start Guide See Inside for Use and Safety Information The USB Mass Storage Device Contains the User Guide and Quick Start Guide in other Languages Congratulations
Department: Manager: Safety Coordinator: Date:
 SAFETY and staff, regardless of hiring status or job position 1 1. Can staff name their department safety coordinator? 2. Is the Department Safety Binder current? 3. Do slip/trip hazards exist? 4. How
SAFETY and staff, regardless of hiring status or job position 1 1. Can staff name their department safety coordinator? 2. Is the Department Safety Binder current? 3. Do slip/trip hazards exist? 4. How
JAC-CEN-DEL COMMUNITY SCHOOLS BLOODBORNE PATHOGENS UNIVERSAL PRECAUTIONS A BACK TO SCHOOL TRADITION
 JAC-CEN-DEL COMMUNITY SCHOOLS BLOODBORNE PATHOGENS UNIVERSAL PRECAUTIONS A BACK TO SCHOOL TRADITION UNIVERSAL PRECAUTIONS AGAINST BLOODBORNE PATHOGENS Employees working in a school system are potentially
JAC-CEN-DEL COMMUNITY SCHOOLS BLOODBORNE PATHOGENS UNIVERSAL PRECAUTIONS A BACK TO SCHOOL TRADITION UNIVERSAL PRECAUTIONS AGAINST BLOODBORNE PATHOGENS Employees working in a school system are potentially
Best Practices for Environmental Cleaning for Prevention and Control of Infections In All Health Care Settings: Time for Review
 Best Practices for Environmental Cleaning for Prevention and Control of Infections In All Health Care Settings: Time for Review Provincial Infectious Diseases Advisory Committee on Infection Prevention
Best Practices for Environmental Cleaning for Prevention and Control of Infections In All Health Care Settings: Time for Review Provincial Infectious Diseases Advisory Committee on Infection Prevention
The FreeStyle Lite Meter and How It Works
 ENGLISH The FreeStyle Lite Meter and How It Works System Check Screen This screen always appears when the meter is turned on so that you can make sure the display is working properly. Do not use the meter
ENGLISH The FreeStyle Lite Meter and How It Works System Check Screen This screen always appears when the meter is turned on so that you can make sure the display is working properly. Do not use the meter
Start Here USB. (802.11) (Ethernet)
 Start Here 1 USB 802.11 Ethernet USB cable users: Do not connect the USB cable until Section A. USB A USB Wireless (802.11) or wired (Ethernet) network users : you must follow the instructions in this
Start Here 1 USB 802.11 Ethernet USB cable users: Do not connect the USB cable until Section A. USB A USB Wireless (802.11) or wired (Ethernet) network users : you must follow the instructions in this
Care and use guide. Contents. page
 Care and use guide Contents page Features Bluelab Combo Meter Introduction Preparing for use Calibration Changing nutrient and temperature display units 6 Measuring hydroponic elements 7 Battery replacement
Care and use guide Contents page Features Bluelab Combo Meter Introduction Preparing for use Calibration Changing nutrient and temperature display units 6 Measuring hydroponic elements 7 Battery replacement
Recommendations for the Safe Use of Handling of Cytotoxic Drugs
 Recommendations for the Safe Use of Handling of Cytotoxic Drugs Introduction Cytotoxic drugs are toxic compounds and are known to have carcinogenic, mutagenic and/or teratogenic potential. With direct
Recommendations for the Safe Use of Handling of Cytotoxic Drugs Introduction Cytotoxic drugs are toxic compounds and are known to have carcinogenic, mutagenic and/or teratogenic potential. With direct
INSULIN INJECTION KNOW-HOW
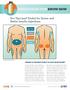 0-0- 0 INSULIN INJECTION KNOW-HOW pro tips (and tricks) for easier and better Insulin Injections ABDOMEN THIGHS BUTTOCKS ARMS recommended injection sites WHERE IS THE BEST PLACE TO GIVE INJECTIONS? 0-
0-0- 0 INSULIN INJECTION KNOW-HOW pro tips (and tricks) for easier and better Insulin Injections ABDOMEN THIGHS BUTTOCKS ARMS recommended injection sites WHERE IS THE BEST PLACE TO GIVE INJECTIONS? 0-
Revision 5. Calvin College Medical Waste Management Plan. Date: Health and Safety
 Calvin College Medical Waste Management Plan Prepared by: Date: Environmental 6/10/1 Health and Safety Approved By: Date: Revision 1.0 Policy The following medical waste management plan has been established
Calvin College Medical Waste Management Plan Prepared by: Date: Environmental 6/10/1 Health and Safety Approved By: Date: Revision 1.0 Policy The following medical waste management plan has been established
DEPARTMENT OF AIRPORT OPERATIONS CLEANING SCHEDULE FOR CONTRACT 2014
 FOR CONTRACT 2014 AIRSIDE LANDSIDE Arrival/Departure Gate/ Windows cleaned in & out 104 Walkway Concourse swept 365 Trash collected 730 Floors washed twice a week 104 Baggage Make up area Sweep 730 Empty
FOR CONTRACT 2014 AIRSIDE LANDSIDE Arrival/Departure Gate/ Windows cleaned in & out 104 Walkway Concourse swept 365 Trash collected 730 Floors washed twice a week 104 Baggage Make up area Sweep 730 Empty
Installing the Video Input and TV Tuner Cards in a Compact Computer or a Dual PCI-Slot Tower Computer
 Installing the Video Input and TV Tuner Cards in a Compact Computer or a Dual PCI-Slot Tower Computer This booklet describes how to install the video input and TV tuner cards in a compact Macintosh computer
Installing the Video Input and TV Tuner Cards in a Compact Computer or a Dual PCI-Slot Tower Computer This booklet describes how to install the video input and TV tuner cards in a compact Macintosh computer
Ultrasonic Jewelry/DVD Cleaner. make jewelry, dvds and more sparkle like new
 Ultrasonic Jewelry/DVD Cleaner make jewelry, dvds and more sparkle like new Table of contents Cautions & Warnings...................................................... 2-4 Location of Controls........................................................
Ultrasonic Jewelry/DVD Cleaner make jewelry, dvds and more sparkle like new Table of contents Cautions & Warnings...................................................... 2-4 Location of Controls........................................................
Parent & Healthcare Professional Instructions for the collection of Maternal & Umbilical Cord Blood
 Parent & Healthcare Professional Instructions for the collection of Maternal & Umbilical Cord Blood 1 2 3 Contents List of Umbilical Cord Blood Collection Kit Thermally insulated transportation box - do
Parent & Healthcare Professional Instructions for the collection of Maternal & Umbilical Cord Blood 1 2 3 Contents List of Umbilical Cord Blood Collection Kit Thermally insulated transportation box - do
C. difficile. Answers to frequently asked questions about. at the Jewish General Hospital. www.jgh.ca SIR MORTIMER B. DAVIS JEWISH GENERAL HOSPITAL
 Answers to frequently asked questions about C. difficile at the Jewish General Hospital SIR MORTIMER B. DAVIS JEWISH GENERAL HOSPITAL A McGill University Teaching Hospital www.jgh.ca 1. CLOSTRIDIUM DIFFICILE
Answers to frequently asked questions about C. difficile at the Jewish General Hospital SIR MORTIMER B. DAVIS JEWISH GENERAL HOSPITAL A McGill University Teaching Hospital www.jgh.ca 1. CLOSTRIDIUM DIFFICILE
Report Index PROPERTY DESCRIPTION 1 ELECTRICAL SYSTEM 2 COMMON INTERIOR AREAS 3 INTERIOR ROOMS 6 KITCHEN 8 BATHROOMS 10
 Report Index PROPERTY DESCRIPTION 1 ELECTRICAL SYSTEM 2 COMMON INTERIOR AREAS 3 INTERIOR ROOMS 6 KITCHEN 8 BATHROOMS 10 PROPERTY DESCRIPTION PROPERTY INSPECTED: INSPECTION ADDRESS: 4722 Baltimore Avenue
Report Index PROPERTY DESCRIPTION 1 ELECTRICAL SYSTEM 2 COMMON INTERIOR AREAS 3 INTERIOR ROOMS 6 KITCHEN 8 BATHROOMS 10 PROPERTY DESCRIPTION PROPERTY INSPECTED: INSPECTION ADDRESS: 4722 Baltimore Avenue
Sterile Dressing Change with Tegaderm CHG for Central Venous Catheter (CVC)
 Sterile Dressing Change with Tegaderm CHG for Central Venous Catheter (CVC) The dressing protects your catheter site. It also helps prevent infection at the site. Keep your dressing clean and dry at all
Sterile Dressing Change with Tegaderm CHG for Central Venous Catheter (CVC) The dressing protects your catheter site. It also helps prevent infection at the site. Keep your dressing clean and dry at all
Cleaning Guidelines for Care Homes. Includes cleaning standards for the general environment and equipment
 Cleaning Guidelines for Care Homes Includes cleaning standards for the general environment and equipment Contents Page 1. Background 2. Setting the Standard 3. Introduction to Cleaning 4. Cleaning 5. Disinfection
Cleaning Guidelines for Care Homes Includes cleaning standards for the general environment and equipment Contents Page 1. Background 2. Setting the Standard 3. Introduction to Cleaning 4. Cleaning 5. Disinfection
Manual for GlobePharma Mini-Press II Rotary Tablet Press
 1 of 13 Preparing the Rotary Press 1. Make sure the rotary press is unplugged. 2. Open the bottom cabinet of the rotary press and take out the grey tool kit, and the beige box of punches and dies. 3. Take
1 of 13 Preparing the Rotary Press 1. Make sure the rotary press is unplugged. 2. Open the bottom cabinet of the rotary press and take out the grey tool kit, and the beige box of punches and dies. 3. Take
BOSTON COLLEGE ENVIRONMENTAL HEALTH AND SAFETY LABORATORY GUIDE FOR RENOVATIONS, REMODELS, MOVES AND TERMINATIONS
 BOSTON COLLEGE ENVIRONMENTAL HEALTH AND SAFETY LABORATORY GUIDE FOR RENOVATIONS, REMODELS, MOVES AND TERMINATIONS I. Policy for Termination of Laboratory Use of Hazardous Materials The Principal Investigator
BOSTON COLLEGE ENVIRONMENTAL HEALTH AND SAFETY LABORATORY GUIDE FOR RENOVATIONS, REMODELS, MOVES AND TERMINATIONS I. Policy for Termination of Laboratory Use of Hazardous Materials The Principal Investigator
Biosafety Level 2 (BSL-2) Safety Guidelines
 BLS-4 Biosafety Level 2 (BSL-2) Safety Guidelines BSL-3 BSL-2 BSL-1 BSL-2 builds upon BSL-1. If you work in a lab that is designated a BSL-2, the microbes used pose moderate hazards to laboratory staff
BLS-4 Biosafety Level 2 (BSL-2) Safety Guidelines BSL-3 BSL-2 BSL-1 BSL-2 builds upon BSL-1. If you work in a lab that is designated a BSL-2, the microbes used pose moderate hazards to laboratory staff
