Protocol for an integrated data request of test results from the laboratories of pathological anatomy. SNOMED 3.5VF - users version January 2016
|
|
|
- Theodore May
- 8 years ago
- Views:
Transcription
1 Protocol for an integrated data request of test results from the laboratories of pathological anatomy SNOMED 3.5VF - users version January 2016 Cancer diagnoses & early detection of cancer (breast, colorectal and cervix) 1 SNOMED Request work year 2016 version January 2016
2 Table of contents Introduction... 3 Contact information Data transfer: general principles Which data should be transferred? Data format Means of delivery Data transfer: detailed overview Datasets A. Dataset for cancer diagnoses B. Dataset for breast and colorectal specimens C. Dataset for test results of cervical/vaginal smears and biopsies- including HPV-tests D. Summary of all datasets Inclusion criteria per project A. Inclusion criteria for the classic CANCER-file B. Inclusion criteria for the BREAST/COLON-file C. Inclusion criteria for the CERVIX-file Additional information regarding the variables A. Additional information regarding the COMMON variables B. Additional information regarding the specific CANCER variables C. Additional information regarding the specific BREAST/COLON variables C. Additional information regarding the specific CERVIX variables SNOMED Request work year 2016 version January 2016
3 Introduction This document will provide a set of instructions on how to transfer data on cancer diagnosis and test results of early cancer detection to the Belgian Cancer Registry. The aim is to provide a clear description of a standardized and integrated protocol describing how data should be transferred, allowing us to process this information and enabling the use of your data e.g. for national and international descriptive statistics on cancer incidence. Please transfer this document to your IT service responsible for assembling the dataset(s) and/or also to all the pathologists who deliver data. The legal basis for the work of the Belgian Cancer Registry can be found in: The law of 13 December 2006 called: Wet houdende diverse bepalingen betreffende gezondheid (1), hoofdstuk VI, Artikel 39 Loi portant dispositions diverses en matière de santé (1), chapitre VI, Article 39 Changes in this law relevant to this data request were published in the Belgisch Staatsblad / Moniteur Belge on June 2 nd, 2010 (art. 29). A Royal Decree, published in the Belgisch Staatsblad / Moniteur Belge on February 13 th, 2012, links the ratification of the laboratories for pathological anatomy to the participation in the cancer registration. Contact information For additional information or support, please contact the Belgian Cancer Registry at: 02/ or info@kankerregister.org / info@registreducancer.org Or by preference your contact person in the Belgian Cancer Registry. Additional information can also be found at : / SNOMED Request work year 2016 version January 2016
4 1. Data transfer: general principles 1.1 Which data should be transferred? The Belgian Cancer Registry kindly asks you to transfer all data concerning these 4 topics: 1. One structured file containing all encoded cancer diagnoses and a separate file with the written reports (i.e. protocols ); 2. One structured file containing the encoded test results of all breast specimens and a separate file with the written reports (i.e. protocols ); 3. One structured file containing the encoded test results of all colorectal specimens and a separate file with the written reports (i.e. protocols ); 4. One structured file containing the encoded test results of all cervical smears and biopsies and a separate file with the written reports (i.e. protocols ). A detailed description of each data set is stated below. Remark: following these procedures, it is normal that if a cancer is diagnosed it will result in double registration: once in the cancer file and once in the breast, colorectal or cervical file. For records with a date of specimen of 01/01/2014 onwards: Analyses done on request of other laboratories have to be delivered by either the laboratory that executed these tests OR by the laboratory that asked for the tests. This should be a mutual consent between laboratories but the applicant of the test has to guarantee the information reaches the BCR. 4 SNOMED Request work year 2016 version January 2016
5 1.2 Data format A. The format of the structured file is a tab separated text. B. The anonymous protocols should preferably be submitted in one file as a tab separated text format with a unique and clear delimiter between the different protocols. This delimiter should not appear in the protocols. The specimen number should be mentioned in the protocol. Only.doc and.txt files will be accepted. Separated.dot,.pdf,.html and other files are to be avoided. An example will be provided as attachment. 1.3 Means of delivery From March 2012 onwards, only the following means of delivery are accepted : 1) By sftp, a secure File Transfer Protocol: Please read the sftp-manual in which the simple procedure is explained. Once the data are ready to be sent, please call your contact in the Cancer Registry who will provide you with a username and password (temporary). The data you ll deliver by sftp have to be protected by a password (chosen by yourself) that you can communicate by telephone when you inform your contact that data have been placed on the sftp-server. 2) On CD-rom, Data can be delivered on CD-ROM secured by a password (to be communicated by telephone!) and sent to the Belgian Cancer Registry by a recorded delivery (aangetekende verzending / envoi recommandé) attended to the medical supervisor Dr. Liesbet Van Eycken at the following address. Stichting Kankerregister/Fondation Registre du Cancer For the attention of Dr. Liesbet Van Eycken, Medical Supervisor Koningsstraat 215 bus 7 / Rue Royale 215 boîte Brussel / 1210 Bruxelles Datatransfer by (a password secured zip file) has to be avoided. can only be used to transfer protocol-numbers or anonymized protocols. Once data allow identification of the patient, cannot be accepted! 5 SNOMED Request work year 2016 version January 2016
6 When a laboratory has several activity centres: - Separate delivery of the data of the different activity centres is possible when the name of the specific activity centre, responsible for the analysis and registration of the samples, is clearly mentioned. - One delivery, containing the activity of all centres, is also allowed provided that an extra variable is added, in particular the name of the activity centre that was responsible for the analysis and registration of the sample. - From working year 2015 on, only the main laboratory will receive a financial reimbursement for the collaboration with the Belgian Cancer Registry (not longer every single activity centre). 6 SNOMED Request work year 2016 version January 2016
7 2. Data transfer: detailed overview 2.1 Datasets 2.1.A. Dataset for cancer diagnoses Field Compulsory (C) Optional (O) Highly Recommended (HR) Format Short comment (for details see further) 1 INSZ/NISS C 11 characters, text format without space Leading zero's should be conserved! ( TEXT format therefore necessary) 2 Last name O/C Free text field Compulsory if INSZ/NISS unknown 3 First name O/C Free text field Compulsory if INSZ/NISS unknown 4 Sex C Male or Female 5 Date of birth C yyyymmdd 6 Date of death O yyyymmdd Only if applicable 7 Postal code C Free text field 8 Country code C 2 characters, text format 9 Specimen number C Free text field 10 Date specimen was taken 11 Requesting hospital/laboratory 12 Diagnostic procedure O/HR 13 Organ C 14 Laterality O/HR C O yyyymmdd Free text field Free text field ; P-code may be used Free text field ; T-code or ICDO-3 topo code 1 = left 2 = right G-code may be used 15 Morphology C Free text field; M-code ISO-code of the legal residence of the person Should match the specimen number in the protocol Name of the hospital/laboratory that requests the pathological examination. Please provide the significance of your codes in a separate file or mail All organ codes (Only use valid SNOMED organ codes) Only if applicable All histology codes (Only use valid SNOMED or ICD-O-3 morphology codes) 16 Differentiation grade O Free text field Histological grading and differentiation 17 pt O/C* Free text field TNM 7th edition from 2010 onwards 18 pn O/C* Free text field TNM 7th edition from 2010 onwards 19 pm O/C* Free text field TNM 7th edition from 2010 onwards 20 Degree of certainty (about morphology code) O 1 = uncertain 2 = differential diagnosis 3 = certain This field can be replaced by a comment field O=Optional C=Compulsory O/C=Compulsory if INSZ/NISS unknown O/C*=Compulsory if applicable HR=highly recommended 2.1.B. Dataset for breast and colorectal specimens 7 SNOMED Request work year 2016 version January 2016
8 Field Compulsory (C) Optional (O) Highly Recommended (HR) Format Short comment (for details see further) 1 INSZ/NISS C 11 characters, text format without space Leading zero's should be conserved! ( TEXT format therefore necessary) 2 Last name O/C Free text field Compulsory if INSZ/NISS unknown 3 First name O/C Free text field Compulsory if INSZ/NISS unknown 4 Sex C Male or Female 5 Date of birth C yyyymmdd 6 Date of death O yyyymmdd Only if applicable 7 Postal code C Free text field 8 Country code C 2 characters, text format 9 Specimen number C Free text field Code of the legal residence of the person Should match the specimen number in the protocol 10 Date specimen was taken C yyyymmdd 11 Requesting hospital/laboratory O Free text field 12 RIZIV/INAMI number of the applicant of the test 13 Diagnostic procedure O/HR 14 Organ C 15 Laterality O 16 Morphology C 17 Degree of certainty (about morphology code) C O 11 numbers text format without space Free text field ; P-code may be used Free text field ; T-code or ICDO-3 topo code 1 = left 2 = right or G-code Free text field; M-code 1 = uncertain 2 = differential diagnosis 3 = certain 18 Nomenclature number(s) O Text format without space Name of the hospital/laboratory that requests the pathological examination. Please provide the significance of your codes in a separate file or mail File breast: T-codes for breast File colorectal: T-codes for colon, rectum, anus (Only use valid SNOMED organ codes) Only if applicable All test results including negative results (Only use valid SNOMED or ICD-O-3 morphology codes) This field can be replaced by a comment field Different numbers to be separated by commas "," O=Optional C=Compulsory O/C=Compulsory if INSZ/NISS unknown HR=highly recommended 8 SNOMED Request work year 2016 version January 2016
9 2.1.C. Dataset for test results of cervical/vaginal smears and biopsies Field Compulsory (C) Optional (O) Highly Recommended (HR) Format Short comment (for details see further) 1 INSZ/NISS C 11 characters, text format without space Leading zero's should be conserved! ( TEXT format therefore necessary) 2 Last name O/C Free text field Compulsory if INSZ/NISS unknown 3 First name O/C Free text field Compulsory if INSZ/NISS unknown 4 Sex C Female 5 Date of birth C yyyymmdd 6 Date of death O yyyymmdd Only if applicable 7 Postal code C Free text field 8 Country code C 2 characters, text format 9 Specimen number C Free text field Code of the legal residence of the person Should match the specimen number in the protocol 10 Date specimen was taken C yyyymmdd 11 Requesting hospital/laboratory O Free text field 12 RIZIV/INAMI number of the applicant of the test C 11 numbers text format without space Name of the hospital/laboratory that requests the pathological examination. 13 Quality of the specimen C SUF, INSU Only for pap smears 14 Diagnostic procedure HR 15 Organ C Free text field: P-code may be used Free text field: T-code or ICDO-3 topo code 16 Morphology C Free text field: M-code 17 Degree of certainty (about morphology code) 18 HPV high risk test results O C if HPV test performed 19 HPV high risk types detected O 1 = uncertain 2 = differential diagnosis 3 = certain HPV-, HPV+, HPVi HP16, HP18,... Free text field 20 Nomenclature number(s) O** Text format without space O=Optional C=Compulsory O/C=Compulsory if INSZ/NISS unknown HR=highly recommended * the use of these codes is to be avoided, please use by preference the codes C52.X or C53.X ** From 01/01/2015 on, nomenclature numbers for cervical/vaginal samples are optional (Only applicable if certain conditions are fulfilled; see chapter 2.3.D) Please provide the significance of your codes in a separate file or mail T-code for cervix (only use valid SNOMED organ codes) or ICDO-3 topo code for cervix All test results including negative results, benign and premalignant lesions. (Only use valid SNOMED or ICD-O-3 morphology codes) This field can be replaced by a comment field Different HPV types to be separated by commas "," Different numbers to be separated by commas "," 9 SNOMED Request work year 2016 version January 2016
10 2.1.D. Summary of all datasets VARIABLES FOR SNOMED USERS DATASET FOR CANCER DIAGNOSES following international guidelines for cancer registries DATASET FOR BREAST AND COLON PREVENTION FILE DATASET FOR CERVIX PREVENTION FILE According to the need of the Centers for Cancer Detection in Belgium 1 (INSZ/NISS) C C C 2 Last name O/C O/C O/C 3 First name O/C O/C O/C 4 Sex C C C 5 Date of birth C C C 6 Date of death O O O 7 Zip code = postal code C C C 8 Country code C C C 9 Specimen number C C C 10 Date specimen was taken C C C 11 Requesting hospital/laboratory O O O 12 RIZIV/INAMI number of the applicant of the test 13 Quality of the specimen C 14 Diagnostic procedure O / HR O / HR HR 15 Organ C C C 16 Laterality O / HR O / HR for breast 17 Morphology C C C 18 Differentiation grade O 19 pt O/C* 20 pn O/C* 21 pm O/C* 22 Degree of certainty (about morphology code) 23 HPV high risk test results C O O O C C if HPV test performed 24 HPV high risk types detected O 25 Nomenclature number(s) O O=Optional C=Compulsory O/C=Compulsory if INSZ/NISS unknown O/C*=Compulsory if applicable HR=highly recommended O IF diagnostic procedure can be obtained by other variables 10 SNOMED Request work year 2016 version January 2016
11 2.2 Inclusion criteria per project 2.2.A. Inclusion criteria for the classic CANCER-file SELECTION BASED ON MORPHOLOGY-CODE Range to select: all morphology SNOMED codes M to M M M M (codes for severe dysplasia = carcinoma in situ) (equivalent for ICD-O-3: 8000/0 9999/9) All malignant tumours, invasive or in situ (inclusive severe dysplasia and high grade intra epithelial neoplasia) All hematological tumours including the myelodysplastic syndromes and myeloproliferative diseases. All tumours of the central nervous system whatever the behavior of the tumour (benign, low malignant potential, malignant). All urothelial cell tumours (low malignant potential, in situ, invasive). Ovary: malignant and borderline malignant tumours. If analyses of the skin are executed by a separate laboratory for dermatopathology, please transfer this mail with annexes to your colleagues so they can learn how to transfer their data (if you do not already deliver this data). These data are asked for once per year (demand in January data to be delivered by mid-february) 11 SNOMED Request work year 2016 version January 2016
12 2.2.B. Inclusion for the BREAST & COLON-file The datasets for the breast and colorectal specimens are identical; however the test results and protocols should be delivered in separate files. SELECTION BASED ON ORGAN-CODE Range to select: 1. For the structured file containing the encoded test results of all breast specimens: For users of the ICDO-3 topo codes: all C50.X For users of SNOMED 3.5 VF organ codes (T-codes) : All codes T-04xxx + T T T T-EA503 + T-EA509 In this selection, you should include all encoded test results of breast specimens, including negative, benign and (pre)malignant lesions. In July 2015 coding guidelines for breast samples were communicated through a newsletter sent to laboratories. This newsletter can be downloaded at the following link: 2. For the structured file containing the encoded test results of all colorectal specimens: For users of the ICDO-3 topo codes: all C18.X, C19.X, C20.X, C21.X For users of SNOMED 3.5 VF organ codes (T-codes): all codes T-59xxx + T In this selection, you should include all encoded test results of colorectal specimens, including negative, benign and (pre)malignant lesions. In April 2014 coding guidelines for colorectal samples were communicated through a newsletter sent to laboratories. This newsletter can be downloaded at the following link: These data are asked for once per work year. (demand in January data to be delivered by mid-february) 12 SNOMED Request work year 2016 version January 2016
13 2.2.C. Inclusion criteria for the CERVIX-file SELECTION BASED ON ORGAN-CODE Range to select: For users of the ICDO-3 topo codes: all C52.X, C53.X (and C57.8*-C57.9*) For users of SNOMED 3.5 VF organ codes (T-codes): All codes T-82xxx (exclusion of T-82050, T-82400, T-82410) + All codes T-832xx + T-EA557, T-EA559, T-EA561 (and T-EA563*) * the use of these codes is to be avoided during coding, please use by preference the codes C52.X or C53.X (ICD-O-3), or T-EA557, T-EA559, T-EA561 (SNOMED) In this selection, you should include: All cervical and vaginal cytology test results including negative tests (no abnormalities), light or moderate cell abnormalities and (pre)malignancies. All cervical and vaginal histology test results including negative tests (no abnormalities), light or moderate cell abnormalities and (pre)malignancies. Results of high risk HPV tests. For records with a date of specimen of 01/01/2014 onwards: HPV-analyses done on request of other laboratories have to be delivered by either the laboratory that executed these tests OR by the laboratory that asked for the tests. This should be a mutual consent between laboratories but the applicant of the test has to guarantee the information reaches the BCR. The cervix data can be asked several times per year in order to have recent data to be used in the screening programs. 13 SNOMED Request work year 2016 version January 2016
14 2.3 Additional information regarding the variables 2.3.A. Additional information regarding the COMMON variables National Social Security Number (INSZ/NISS) This variable is used for patient identification. TAKE CARE: this variable should always contain 11 characters and be a TEXT format, since otherwise leading zero s will be lost. In case the INSZ/NISS is lacking, one can deliver the last name, first name, sex, date of birth and postal code. Country code Please use the correct codes you can find on: The filled in country code refers to the country in which the patient is officially residing. The country code is a compulsory variable since malignancies are only taken into account for the incidence number of Belgium if the person has his/her legal residence in Belgium. Missing information about people residing outside Belgium will never be asked. Date specimen was taken Take care to fill in the date the specimen was taken. Only if this date is unknown, the date of reception of the specimen or the date of analysis can be used. When data is missing, data cannot be treated! Requesting hospital/laboratory Fill in the hospital/laboratory that sent the specimen, requested the pathological examination and to which the result is reported. If you use abbreviations, please provide us with a conversion table. If there is no requesting hospital/laboratory and the request originates from a private person, please mention PRIVATE for this variable. By providing us with this information, some missing or additional data can be asked (immediately) to the applicant of the analysis instead of the lab that performed the analysis. 14 SNOMED Request work year 2016 version January 2016
15 Diagnostic procedure The variable diagnostic procedure indicates if it concerns a cytology or a histology. This variable is not compulsory, but highly recommended when the diagnostic procedure cannot be deduced from other variables as the organ-code (eg. T-EA503 and T-AE509 = breast cytology) or nomenclature. You can use the P-axis of SNOMED 3.5VF to fill out this field. You can also use the following BD-codes (BD = Basis for Diagnosis) : Possible codes Meaning of the codes 1 Autopsy, found by hazard 2 Histology of the primary tumour 3 Histology of the metastasis 4 Cytology/hematology Important: if the diagnostic procedure is not clearly provided by means of organ, nomenclature or BD-code: all cervical samples will be considered by default as cytological examinations. For breast samples all organ codes will be considered as histological samples except for T-EA503 and T-EA509. Organ (Topography) Please use the code of the sample in which the diagnosis is made, regardless the organ in which the tumour takes it s origin. Please encode the organ as precisely as possible. For instance, sublocalisation for colon and skin and laterality for breast is important. For this variable we attend the T-axis (T-codes) of SNOMED 3.5VF (T=Topography). You can also use the topography code of the ICD-O-3. Without a topo-code, the record cannot be extracted for the prevention files (breast/colon and cervix), following the BCR-rules for extraction and cannot be treated by the Cancer Registry. 15 SNOMED Request work year 2016 version January 2016
16 If possible, you can include information about the diagnostic procedure within this variable, e.g. T-EA557: prélèvement cytologique du vagin, indicating a cytological examination. Morphology Please encode the morphology as precisely as possible. For this variable we attend the M-axis (M-codes) of SNOMED (M=Morphology) or ICD-O-3 morphology-codes. Without this M-code, a cancer cannot be extracted for the Cancer-file following the extraction rules of the BCR-protocol and cannot be treated by the Cancer Registry. For prevention files, please encode also negative, benign and (pre)malignant lesions. If no information on the lesion is available or only an additional test is performed without histological diagnosis (e.g. KRAS, HPV, EGFR, FISH,...), the value #NA (= not applicable) can be mentioned for the variable Morphology. To meet the quality criteria imposed by the BCR, other coding languages than CODAP/SNOMED 3.5VF will no longer be accepted. Only one coding language can be used in the delivered file (CODAP OR SNOMED). See also section 2.3.D. for additional information regarding the coding of cervixvariables. Laterality If possible, always mention the laterality. This can be done by means of the variable Laterality for which you can use the G-axis of SNOMED or the following codes: Possible codes Meaning of the codes 1 Left 2 Right #NK Not known #NA Not applicable Sometimes you can use a specific organ code from which laterality can be deduced, e.g. T for a left breast and T for a right breast. 16 SNOMED Request work year 2016 version January 2016
17 Degree of certainty This applies to the certainty about the morphology, not about the HPV test. Missing information: If certain information is not present in your database you can fill out the column with: #NS not stated or not present #NK unknown #NA not applicable 17 SNOMED Request work year 2016 version January 2016
18 2.3.B. Additional information regarding the specific CANCER variables Differentiation grade When not specified in the morphology code (e.g. M-83313: adénocarcinome vésiculaire bien différencié) this optional variable can be delivered in a separate field: Histological grading and differentiation Possible codes Meaning of the codes 1 well differentiated 2 moderately differentiated 3 poorly differentiated 4 undifferentiated / anaplastic #NA Not applicable #NK Not known #NS Not stated ptnm-variables Please use different, separate fields to deliver these variables. Use the TNM 7th edition from 2010 onwards and take care of the errata you can easily find on The TNM is not applicable for every tumour or specimen. That is the reason why we cannot make these variables compulsory for every registration. However, when applicable (and present in the report), the TNM-variables should also be present in the structured file and not only in the protocol. Extraction of these variables out of the protocols by means of text-recognition is possible but this procedure is time-consuming and is always accompanied by a loss of quality, what has to be avoided as much as possible. 18 SNOMED Request work year 2016 version January 2016
19 2.3.C. Additional information regarding the specific BREAST/COLON variables RIZIV/INAMI number of the applicant of the test A fail-safe system is a back-up mechanism which ensures that patients with an abnormal screening result will have the appropriate follow up. Within this 'fail safe' mechanism, the variables requesting hospital/laboratory and RIZIV/INAMI number of the applicant of the test are necessary to be able to contact the persons responsible for the follow-up of the patient. TAKE CARE: this variable should be a TEXT format and should by preference contain 11 characters. Nomenclature number(s) Nomenclature can be useful to provide us with the information about the diagnostic procedure (difference between cytology/histology), especially for breast specimens. In case of more than one nomenclature number, they should be entered separated by a comma (, ). TAKE CARE: this variable should be a TEXT format. From 01/01/2014 on, delivery of nomenclature numbers for breast and colorectal samples is not longer compulsory but optional. 19 SNOMED Request work year 2016 version January 2016
20 2.3.D. Additional information regarding the specific CERVIX variables RIZIV/INAMI number of the applicant of the test See also section 2.3.C. Quality of the specimen This variable is obligatory for cervical smears. For registrations of smear taken from 01/01/2013 on, the codes SUF+ and SUF- are no longer used. Both codes are pooled into only one code SUF which indicates the sample is of sufficient quality to result in a reliable diagnosis. Possible codes SUF INSU Meaning of the codes Sufficient: the sample is of sufficient quality to conclude a reliable diagnosis. Insufficient: no reliable test result could be determined or the specimen could not be evaluated at all because it was broken or incorrectly labelled Organ (Topography) Please encode the organ (topography) as precisely as possible. For ICD-O: Avoid the use of the unspecific codes C57.8 and C57.9. Use by preference the code C52.X and C53.X. For SNOMED: for the coding of cytology samples, use by preference the following codes: T-EA557 Frottis vaginal T-EA559 Frottis cervical T-EA561 Frottis endocervical Avoid the use of the unspecific code T-EA SNOMED Request work year 2016 version January 2016
21 Morphology Also see section 2.3.A. For correct coding of the cervical smears (cytology) and biopsies (histology), please follow the systematic overview of diagnostic CERVIBASE 2011 codes as mentioned below. By coding the test results, always take care to make a clear distinction between cytology and histology. If for a patient both a cytological and a histological analysis has been performed and described in the same protocol, both the cytological and the histological diagnosis should be delivered in different records with the same sample/reference number. If the cytological and histological results are mentioned in different protocols, both the cytological and histological diagnosis should be delivered in different records with the appropriate reference number and the correct sample date. For screening programs, it is important to know which cytological exams are followed by a histological confirmation. 21 SNOMED Request work year 2016 version January 2016
22 SNOMED/CERVIBASE shortlist : POSSIBLE CODES FOR CYTOLOGICAL AND HISTOLOGICAL LESIONS CYTOLOGY Morphology code Meaning of the code #NA Not applicable (e.g. if HPV test performed on the request of another laboratory) No cytological Insufficient (no reliable test result could be determined or the specimen could diagnosis INSU not be evaluated at all) No cell NILM Negative for epithelial cell abnormalities or any other malignancy abnormalities M No evidence of neoplasm ASCU Atypical squamous cells of undetermined significance (ASCUS) ASCH Atypical squamous cells, cannot exclude HSIL LSIL Low-grade squamous intraepithelial lesion HSIL High-grade squamous intraepithelial lesion (incl. in situ) Squamous cell M Atypical squamous cells of undetermined significance (ASCUS) abnormalities M Low-grade squamous intraepithelial lesion M High-grade squamous intraepithelial lesion (incl. in situ) M Atypical squamous cells of uncertain significance, probably malignant (ASCUS, probably malignant) M Squamous cell carcinoma AGLC Atypical glandular cells (no distinction between any favor or origin) Glandular cell abnormalities M Atypical glandular cells of undetermined significance M Adenocarcinoma in situ, high grade glandular dysplasia M Adenocarcinoma Other malignancies existing codes Any other malignancy To avoid M Cytological atypia, please consider if it is not feasible to specify more (ASCU, ASCH, AGLC,...) HISTOLOGY Code Meaning of the code No cell ABST No dysplasia nor tumour (absent) abnormalities M No evidence of neoplasm CIN1 Mild dysplasia (CIN1) CIN2 Moderate dysplasia (CIN2) M Mild dysplasia (CIN1) M Moderate dysplasia (CIN2) M Squamous intraepithelial neoplasia, high grade / Squamous intraepithelial neoplasia Grade III / Severe dysplasia (CIN3) / Carcinoma in situ Squamous cervical lesions Glandular cervical lesions Other malignancies M Squamous cell carcinoma in situ / high grade squamous dysplasia M Squamous cell carcinoma M Squamous cell carcinoma, keratinizing M Squamous cell carcinoma, large cell, nonkeratinizing M Squamous cell carcinoma, verrucous M Squamous cell carcinoma, basaloid M Squamous cell carcinoma, papillary M Squamous cell carcinoma, early invasive (micro-invasive) existing codes Squamous carcinoma, other types CGIN Endocervical glandular dysplasia M Atypical glandular cells of undetermined significance M Adenocarcinoma in situ, high grade glandular dysplasia M Adenocarcinoma M Adenocarcinoma, mucinous M Adenocarcinoma, mucinous, endocervical type M Adenocarcinoma, endometrioid M Adenocarcinoma, clear cell M Adenosquamous carcinoma existing codes Adenocarcinoma, other types M Metastasis of a non-cervical cancer existing codes Any other malignancy 22 SNOMED Request work year 2016 version January 2016
23 HPV high risk test results HPV high risk test results: select only one code Possible codes HPV- HPV- HPV+ HPVi Meaning of the codes Test performed, no HPV detected Test performed, HPV detected but only HPV low risk Test performed, HPV high risk detected Test performed, no analysis or no reliable test result possible HPV high risk types detected Specific HPV high risk types detected Possible codes Meaning of the codes HP16 HPV Type 16 HP18 HPV Type 18 HP16,HP18* HPV Type 16 AND 18 HPxx HPV Type xx * Multiple HPV types to be entered in a text field separated by commas, Following HPV-types are considered by the BCR as high risk HPV-types, based on the recommendations by the IARC (2014): HP16,HP18,HP31,HP33,HP35,HP39,HP45,HP51,HP52,HP56,HP58,HP59,HP68 This variable is not required to be filled out when no search for specific HPV high risk subtypes is performed by you or another laboratory or when only a range of possible HPV-types can be given. As cervical cancer is HPV-related and prevention campaigns include vaccination of young girls, data on HPV test results are very useful to evaluate the effect of the vaccination program. Nomenclature number(s) In case of more than one nomenclature number, they should be entered separated by a comma (, ). A list with useful nomenclature numbers will you be provided (as 23 SNOMED Request work year 2016 version January 2016
24 attachment). Take care: this variable should be a TEXT format to allow correct treatment of the data. From 01/01/2015 on, delivery of nomenclature numbers for cervical/vaginal samples is not longer compulsory BUT if your laboratory decides not to deliver this variable in the future, it is obligatory to indicate very clearly the difference between cytology and histology by means of the organ code or the diagnostic procedure. Important : if the diagnostic procedure is not clearly provided by means of organ code, nomenclature or diagnostic procedure-code: the cervical samples will be considered by default as cytological examinations. If your laboratory will continue to deliver this variable, the field of the nomenclature codes has to be filled in as completely as possible according to the following recommendations. a. Official nomenclature code: if reimbursed by RIZIV/INAMI b. Non-official (dummy) code: if not reimbursed by RIZIV/INAMI (please provide us with the meaning of each used dummy code) c. #NK: if the reimbursement by the RIZIV/INAMI is uncertain It is recommended to send all the samples of the requested period even when no certainty exists about the nomenclature. Delivery of an incomplete file in which a lot of samples are missing has to be avoided. Additional information about cervical smears For the cervical smears we want to know the following information: o Which method do you use to analyze the samples (conventional or liquid based)? o Which laboratory performs the HPV tests? o Which test is used for HPV detection? These questions are repeated every year in order to have the most up to date information. If changes in the treatment of HPV testing took place during the year, please inform us. 24 SNOMED Request work year 2016 version January 2016
Pap smears, cytology and CCHC lab work and follow up
 Pap smears, cytology and CCHC lab work and follow up What is a Pap Smear? A Pap smear (also known as the Pap test) is a medical procedure in which a sample of cells from a woman's cervix (the end of the
Pap smears, cytology and CCHC lab work and follow up What is a Pap Smear? A Pap smear (also known as the Pap test) is a medical procedure in which a sample of cells from a woman's cervix (the end of the
Cervical Cancer Screening and Management Guidelines: Changing Again, Huh?
 Cervical Cancer Screening and Management Guidelines: Changing Again, Huh? Summary of 2013 recommendations from ASC (American Cancer Society), ASCCP (American Society for Colposcopy and Cervical Pathology),
Cervical Cancer Screening and Management Guidelines: Changing Again, Huh? Summary of 2013 recommendations from ASC (American Cancer Society), ASCCP (American Society for Colposcopy and Cervical Pathology),
Explanation of your PAP smear
 Explanation of your PAP smear Approximately 5-10% of PAP smears in the United States are judged to be abnormal. Too often, the woman who receives this news worries that she already has, or will develop,
Explanation of your PAP smear Approximately 5-10% of PAP smears in the United States are judged to be abnormal. Too often, the woman who receives this news worries that she already has, or will develop,
The Diagnosis of Cancer in the Pathology Laboratory
 The Diagnosis of Cancer in the Pathology Laboratory Dr Edward Sheffield Christmas Select 74 Meeting, Queen s Hotel Cheltenham, 3 rd December 2014 Agenda Overview of the pathology of cancer How specimens
The Diagnosis of Cancer in the Pathology Laboratory Dr Edward Sheffield Christmas Select 74 Meeting, Queen s Hotel Cheltenham, 3 rd December 2014 Agenda Overview of the pathology of cancer How specimens
Cervical Cancer The Importance of Cervical Screening and Vaccination
 Cervical Cancer The Importance of Cervical Screening and Vaccination Cancer Cells Cancer begins in cells, the building blocks that make up tissues. Tissues make up the organs of the body. Sometimes, this
Cervical Cancer The Importance of Cervical Screening and Vaccination Cancer Cells Cancer begins in cells, the building blocks that make up tissues. Tissues make up the organs of the body. Sometimes, this
Cervical Cancer Screening Guideline
 Cervical Cancer Screening Guideline Prevention 2 Abbreviations Used 2 Specimen Collection Techniques 3 Screening 4 Management Women 21 Years and Older Pap results 5 findings: ASC-US and LSIL 6 findings:
Cervical Cancer Screening Guideline Prevention 2 Abbreviations Used 2 Specimen Collection Techniques 3 Screening 4 Management Women 21 Years and Older Pap results 5 findings: ASC-US and LSIL 6 findings:
PROPERTY OF ELSEVIER SAMPLE CONTENT - NOT FINAL ABNORMAL PAP SMEAR (ABNORMAL CERVICAL CYTOLOGIC FINDINGS) Kathleen Dor
 1 ABNORMAL PAP SMEAR (ABNORMAL CERVICAL CYTOLOGIC FINDINGS) Kathleen Dor Cervical cytology screening has significantly decreased rates of mortality from cervical cancer; however, 400 women die each year
1 ABNORMAL PAP SMEAR (ABNORMAL CERVICAL CYTOLOGIC FINDINGS) Kathleen Dor Cervical cytology screening has significantly decreased rates of mortality from cervical cancer; however, 400 women die each year
The society for lower genital tract disorders since 1964.
 The society for lower genital tract disorders since 1964. Updated Consensus Guidelines for Managing Abnormal Cervical Cancer Screening Tests and Cancer Precursors American Society for and Cervical Pathology
The society for lower genital tract disorders since 1964. Updated Consensus Guidelines for Managing Abnormal Cervical Cancer Screening Tests and Cancer Precursors American Society for and Cervical Pathology
Understanding. Cervical Changes A Health Guide for Women. National Cancer Institute U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES
 National Cancer Institute Understanding Cervical Changes A Health Guide for Women U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health This guide helped me talk with my doctor after
National Cancer Institute Understanding Cervical Changes A Health Guide for Women U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health This guide helped me talk with my doctor after
ALABAMA BREAST AND CERVICAL CANCER EARLY DETECTION PROGRAM PROVIDER MANUAL
 ALABAMA BREAST AND CERVICAL CANCER EARLY DETECTION PROGRAM PROVIDER MANUAL Revised January 2012 1 TABLE OF CONTENTS PROGRAM OVERVIEW.. 3 INTRODUCTION 5 SCREENING ELIGIBILITY GUIDELINES 6 PATIENT RIGHTS..
ALABAMA BREAST AND CERVICAL CANCER EARLY DETECTION PROGRAM PROVIDER MANUAL Revised January 2012 1 TABLE OF CONTENTS PROGRAM OVERVIEW.. 3 INTRODUCTION 5 SCREENING ELIGIBILITY GUIDELINES 6 PATIENT RIGHTS..
Management of Abnormal PAP Smears. K Chacko, MD, FACP 2010 GIM Conference
 Management of Abnormal PAP Smears K Chacko, MD, FACP 2010 GIM Conference Scope of the Problem About 7-10% 7 of PAPs will come back abnormal 3.5 to 4 million in the US each year Approximate 4000 deaths
Management of Abnormal PAP Smears K Chacko, MD, FACP 2010 GIM Conference Scope of the Problem About 7-10% 7 of PAPs will come back abnormal 3.5 to 4 million in the US each year Approximate 4000 deaths
PROTOCOL OF THE RITA DATA QUALITY STUDY
 PROTOCOL OF THE RITA DATA QUALITY STUDY INTRODUCTION The RITA project is aimed at estimating the burden of rare malignant tumours in Italy using the population based cancer registries (CRs) data. One of
PROTOCOL OF THE RITA DATA QUALITY STUDY INTRODUCTION The RITA project is aimed at estimating the burden of rare malignant tumours in Italy using the population based cancer registries (CRs) data. One of
Cervical Screening and HPV Vaccine Guidelines In Saudi Arabia. Prof. Mohammed Addar Chairmen Gyneoncology section KKUH, King Saud University
 Cervical Screening and HPV Vaccine Guidelines In Saudi Arabia Prof. Mohammed Addar Chairmen Gyneoncology section KKUH, King Saud University Burden of HPV related cancers l l Cervical Cancer of the cervix
Cervical Screening and HPV Vaccine Guidelines In Saudi Arabia Prof. Mohammed Addar Chairmen Gyneoncology section KKUH, King Saud University Burden of HPV related cancers l l Cervical Cancer of the cervix
Cervical Cancer Prevention and Early Detection What is cervical cancer?
 Cervical Cancer Prevention and Early Detection What is cervical cancer? Cervical cancer starts in cells lining the cervix. The cervix is the lower part of the uterus (womb). It is sometimes called the
Cervical Cancer Prevention and Early Detection What is cervical cancer? Cervical cancer starts in cells lining the cervix. The cervix is the lower part of the uterus (womb). It is sometimes called the
American Academy of Family Physicians
 American Academy of Family Physicians Barbara E. Stanford MD Grand Rapids Family Medicine Residency Wege Family Medicine HPV is transient in most women HPV-75% Normal ASCUS LSIL HSIL Cancer 80-90% 75%???
American Academy of Family Physicians Barbara E. Stanford MD Grand Rapids Family Medicine Residency Wege Family Medicine HPV is transient in most women HPV-75% Normal ASCUS LSIL HSIL Cancer 80-90% 75%???
The Cervical Screening Manual
 The Cervical Screening Manual A Guide for Health Departments and Providers Collaboration Partners: Chronic Disease and Injury Section Breast and Cervical Cancer Control Program Women s and Children s Health
The Cervical Screening Manual A Guide for Health Departments and Providers Collaboration Partners: Chronic Disease and Injury Section Breast and Cervical Cancer Control Program Women s and Children s Health
Cancer of the Cervix
 Cancer of the Cervix WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 A woman's cervix (the opening of the uterus) is lined with cells. Cancer of the cervix occurs when those cells change,
Cancer of the Cervix WOMENCARE A Healthy Woman is a Powerful Woman (407) 898-1500 A woman's cervix (the opening of the uterus) is lined with cells. Cancer of the cervix occurs when those cells change,
Abnormal Pap Smear Tracking in General Internal Medicine Clinic
 Abnormal Pap Smear Tracking in General Internal Medicine Clinic J A C O B K U R L A N D E R & T A R A O B R I E N C A R Q I P R O J E C T J A N U A R Y 2 0, 2 0 1 0 PDSA cycle Plan Act Do Study Our Charge
Abnormal Pap Smear Tracking in General Internal Medicine Clinic J A C O B K U R L A N D E R & T A R A O B R I E N C A R Q I P R O J E C T J A N U A R Y 2 0, 2 0 1 0 PDSA cycle Plan Act Do Study Our Charge
NATIONAL GUIDELINE FOR CERVICAL CANCER SCREENING PROGRAMME
 NATIONAL GUIDELINE FOR CERVICAL CANCER SCREENING PROGRAMME CERVICAL CANCER Introduction Cancer of the cervix is the second most common form of cancer amongst South African women. Approximately one in every
NATIONAL GUIDELINE FOR CERVICAL CANCER SCREENING PROGRAMME CERVICAL CANCER Introduction Cancer of the cervix is the second most common form of cancer amongst South African women. Approximately one in every
Management of Abnormal Pap Smear Clinical Practice Guideline
 Management of Abnormal Pap Smear Clinical Guideline General Principles: The Papanicolaou (Pap) smear is widely credited with reducing mortality from cervical cancer, and remains the single best method
Management of Abnormal Pap Smear Clinical Guideline General Principles: The Papanicolaou (Pap) smear is widely credited with reducing mortality from cervical cancer, and remains the single best method
Sage Screening Program. Provider Manual
 Sage Screening Program Provider Manual Sage Screening Program Minnesota Department of Health 85 E. 7th Place, Suite 400 P.O. Box 64882 St. Paul, Minnesota 55164-0882 (651) 201-5600 (phone) (651) 201-5601-
Sage Screening Program Provider Manual Sage Screening Program Minnesota Department of Health 85 E. 7th Place, Suite 400 P.O. Box 64882 St. Paul, Minnesota 55164-0882 (651) 201-5600 (phone) (651) 201-5601-
Cervical Cancer Screening. Clinical Practice Guidelines for Average Risk Women
 QEYGYN051 Cervical Cancer Screening Clinical Practice Guidelines for Average Risk Women For Approval of the Provincial Medical Affairs Committee October 2013 Table of Contents Page Background Information
QEYGYN051 Cervical Cancer Screening Clinical Practice Guidelines for Average Risk Women For Approval of the Provincial Medical Affairs Committee October 2013 Table of Contents Page Background Information
OBJECTIVES By the end of this segment, the community participant will be able to:
 Cancer 101: Cancer Diagnosis and Staging Linda U. Krebs, RN, PhD, AOCN, FAAN OCEAN Native Navigators and the Cancer Continuum (NNACC) (NCMHD R24MD002811) Cancer 101: Diagnosis & Staging (Watanabe-Galloway
Cancer 101: Cancer Diagnosis and Staging Linda U. Krebs, RN, PhD, AOCN, FAAN OCEAN Native Navigators and the Cancer Continuum (NNACC) (NCMHD R24MD002811) Cancer 101: Diagnosis & Staging (Watanabe-Galloway
Screening for Cancer of the Cervix
 Screening for Cancer of the Cervix An Office Manual for Health Professionals Cervical Cancer Screening Program tenth Edition 2013 Contact Information Cervical Cancer Screening Program (CCSP) Administration
Screening for Cancer of the Cervix An Office Manual for Health Professionals Cervical Cancer Screening Program tenth Edition 2013 Contact Information Cervical Cancer Screening Program (CCSP) Administration
Guidelines for reporting histopathology of cervical carcinoma
 Guidelines for reporting histopathology of cervical carcinoma Naveena Singh, Consultant Pathologist Introduction Cancer management is multidisciplinary Histopathology report has a MAJOR impact on management
Guidelines for reporting histopathology of cervical carcinoma Naveena Singh, Consultant Pathologist Introduction Cancer management is multidisciplinary Histopathology report has a MAJOR impact on management
2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors
 2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors L. Stewart Massad, MD, Mark H. Einstein, MD, Warner K. Huh, MD, Hormuzd A. Katki,
2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors L. Stewart Massad, MD, Mark H. Einstein, MD, Warner K. Huh, MD, Hormuzd A. Katki,
Cervical Cancer Screening
 Clinical in Oncology Cervical Cancer Screening V.1.2009 Continue www.nccn.org Panel Members * Edward E. Partridge, MD/Chair University of Alabama at Birmingham Comprehensive Cancer Center Nadeem Abu-Rustum,
Clinical in Oncology Cervical Cancer Screening V.1.2009 Continue www.nccn.org Panel Members * Edward E. Partridge, MD/Chair University of Alabama at Birmingham Comprehensive Cancer Center Nadeem Abu-Rustum,
Cervical Cancer. Cervical smear test. The cervix. Dysplasia. Cervical cancer. The female reproductive system
 INFORMATION SHEET Cervical Cancer This information sheet has been written to provide you with information about cervical cancer (cancer of the cervix). The sheet has information about the different types
INFORMATION SHEET Cervical Cancer This information sheet has been written to provide you with information about cervical cancer (cancer of the cervix). The sheet has information about the different types
Multiple Primary and Histology Site Specific Coding Rules KIDNEY. FLORIDA CANCER DATA SYSTEM MPH Kidney Site Specific Coding Rules
 Multiple Primary and Histology Site Specific Coding Rules KIDNEY 1 Prerequisites 2 Completion of Multiple Primary and Histology General Coding Rules 3 There are many ways to view the Multiple l Primary/Histology
Multiple Primary and Histology Site Specific Coding Rules KIDNEY 1 Prerequisites 2 Completion of Multiple Primary and Histology General Coding Rules 3 There are many ways to view the Multiple l Primary/Histology
Introduction: Tumor Swelling / new growth / mass. Two types of growth disorders: Non-Neoplastic. Secondary / adaptation due to other cause.
 Disorders of Growth Introduction: Tumor Swelling / new growth / mass Two types of growth disorders: Non-Neoplastic Secondary / adaptation due to other cause. Neoplastic. Primary growth abnormality. Non-Neoplastic
Disorders of Growth Introduction: Tumor Swelling / new growth / mass Two types of growth disorders: Non-Neoplastic Secondary / adaptation due to other cause. Neoplastic. Primary growth abnormality. Non-Neoplastic
CARDCARE PROTECTOR CERTIFICATE OF INSURANCE
 CARDCARE PROTECTOR CERTIFICATE OF INSURANCE DBS Bank Ltd ( DBS ) is the master policyholder under Group Policy No. MD00000002 (the Policy ), underwritten by Manulife (Singapore) Pte. Ltd. (the Insurer
CARDCARE PROTECTOR CERTIFICATE OF INSURANCE DBS Bank Ltd ( DBS ) is the master policyholder under Group Policy No. MD00000002 (the Policy ), underwritten by Manulife (Singapore) Pte. Ltd. (the Insurer
chapter 5. Quality control at the population-based cancer registry
 chapter 5. Quality control at the population-based cancer registry All cancer registries should be able to give some objective indication of the quality of the data that they have collected. The methods
chapter 5. Quality control at the population-based cancer registry All cancer registries should be able to give some objective indication of the quality of the data that they have collected. The methods
Multiple Primary and Histology Coding Rules
 Multiple Primary and Histology Coding Rules January 10, 2008 National Cancer Institute Surveillance Epidemiology and End Results Program Bethesda, MD PLEASE NOTE This PDF of the 2007 Multiple Primaries
Multiple Primary and Histology Coding Rules January 10, 2008 National Cancer Institute Surveillance Epidemiology and End Results Program Bethesda, MD PLEASE NOTE This PDF of the 2007 Multiple Primaries
HUMAN PAPILLOMAVIRUS (HPV) FACT SHEET
 HUMAN PAPILLOMAVIRUS (HPV) FACT SHEET Background Information - Human Papillomavirus HPV is the name of a group of viruses that include more than 80 different types associated with a variety of epidermal
HUMAN PAPILLOMAVIRUS (HPV) FACT SHEET Background Information - Human Papillomavirus HPV is the name of a group of viruses that include more than 80 different types associated with a variety of epidermal
Cervical cancer screening with the HPV test and the Pap test in women ages 30 and older
 Cervical cancer screening with the HPV test and the Pap test in women ages 30 and older When to get tested and how to make sense of your test results If you are 30 years or older and your Pap test is normal
Cervical cancer screening with the HPV test and the Pap test in women ages 30 and older When to get tested and how to make sense of your test results If you are 30 years or older and your Pap test is normal
Biomedical Engineering for Global Health. Lecture Thirteen
 Biomedical Engineering for Global Health Lecture Thirteen Outline The burden of cancer How does cancer develop? Why is early detection so important? Strategies for early detection Example cancers/technologies
Biomedical Engineering for Global Health Lecture Thirteen Outline The burden of cancer How does cancer develop? Why is early detection so important? Strategies for early detection Example cancers/technologies
XI International Workshop of Lower Genital Tract Pathology HPV Disease: The global battle Rome 19-21 April 2012
 XI International Workshop of Lower Genital Tract Pathology HPV Disease: The global battle Rome 19-21 April 2012 Thursday 19 nd April 2012 Morning Sessions 07.50-08.20 Registration 08.20-08.40 Welcome:
XI International Workshop of Lower Genital Tract Pathology HPV Disease: The global battle Rome 19-21 April 2012 Thursday 19 nd April 2012 Morning Sessions 07.50-08.20 Registration 08.20-08.40 Welcome:
Rare Thoracic Tumours
 Rare Thoracic Tumours 1. Epithelial Tumour of Trachea 1 1.1 General Results Table 1. Epithelial Tumours of Trachea: Incidence, Trends, Survival Flemish Region 2001-2010 Both Sexes Incidence Trend EAPC
Rare Thoracic Tumours 1. Epithelial Tumour of Trachea 1 1.1 General Results Table 1. Epithelial Tumours of Trachea: Incidence, Trends, Survival Flemish Region 2001-2010 Both Sexes Incidence Trend EAPC
Vaccination and Screening for the Prevention of Cervical Cancer: Health Effects and Cost-effectiveness
 Vaccination and Screening for the Prevention of Cervical Cancer: Health Effects and Cost-effectiveness Vaccinatie en screening voor de preventie van baarmoederhalskanker: Gezondheidseffecten en kosteneffectiviteit
Vaccination and Screening for the Prevention of Cervical Cancer: Health Effects and Cost-effectiveness Vaccinatie en screening voor de preventie van baarmoederhalskanker: Gezondheidseffecten en kosteneffectiviteit
Changes in Breast Cancer Reports After Second Opinion. Dr. Vicente Marco Department of Pathology Hospital Quiron Barcelona. Spain
 Changes in Breast Cancer Reports After Second Opinion Dr. Vicente Marco Department of Pathology Hospital Quiron Barcelona. Spain Second Opinion in Breast Pathology Usually requested when a patient is referred
Changes in Breast Cancer Reports After Second Opinion Dr. Vicente Marco Department of Pathology Hospital Quiron Barcelona. Spain Second Opinion in Breast Pathology Usually requested when a patient is referred
GLOBAL CONCERNS ABOUT HPV VACCINES FACT SHEET
 GLOBAL CONCERNS ABOUT HPV VACCINES FACT SHEET When detected, HPV infection is easily managed and rarely proceeds to cancer Very few women with HPV develop cervical cancer HPV infections are only one of
GLOBAL CONCERNS ABOUT HPV VACCINES FACT SHEET When detected, HPV infection is easily managed and rarely proceeds to cancer Very few women with HPV develop cervical cancer HPV infections are only one of
Cytopathology of the Glandular Lesions of the Female Genital Tract
 Cytopathology of the Glandular Lesions of the Female Genital Tract Matías Jiménez-Ayala Madrid Beatriz Jiménez-Ayala Portillo Madrid Including contributions by Francesc Alameda Barcelona Carmen Alvarez-Santín
Cytopathology of the Glandular Lesions of the Female Genital Tract Matías Jiménez-Ayala Madrid Beatriz Jiménez-Ayala Portillo Madrid Including contributions by Francesc Alameda Barcelona Carmen Alvarez-Santín
Policy Wording. Together, all the way.
 Cancer cover Policy Wording Together, all the way. Cancer Cover Insurance Policy Wording 1. Introducing your Policy 2. What is Cancer Cover Insurance? 3. About your Policy 4. Some terms defined 5. What
Cancer cover Policy Wording Together, all the way. Cancer Cover Insurance Policy Wording 1. Introducing your Policy 2. What is Cancer Cover Insurance? 3. About your Policy 4. Some terms defined 5. What
Dictionary of SEER*Stat Variables November 2011 Submission (released April 2012) http://seer.cancer.gov/data/seerstat/nov2011/ 1 of 18
 November 2011 Data Submission Item # refers to the item - see http://www.naaccr.org/standardsandregistryoperations/volumeii.aspx CS= Collaborative Staging SSF = Site-specific Factor Field Item # Description
November 2011 Data Submission Item # refers to the item - see http://www.naaccr.org/standardsandregistryoperations/volumeii.aspx CS= Collaborative Staging SSF = Site-specific Factor Field Item # Description
Introduction to Gynecologic Oncology
 Objectives Cervical Dysplasia and Cancer Introduction to Gynecologic Oncology Department of Obstetrics and Gynecology University of Western Ontario Discuss the incidence and risk factors for cervical dysplasia
Objectives Cervical Dysplasia and Cancer Introduction to Gynecologic Oncology Department of Obstetrics and Gynecology University of Western Ontario Discuss the incidence and risk factors for cervical dysplasia
Breast and Cervical Cancer Treatment Program (BCCTP) Application Guide
 Breast and Cervical Cancer Treatment Program (BCCTP) Application Guide 2012 Created January 1, 2012 BCCTP APPLICATION GUIDE Table of Contents Page Application Process 3 Application Checklist 4 Federal
Breast and Cervical Cancer Treatment Program (BCCTP) Application Guide 2012 Created January 1, 2012 BCCTP APPLICATION GUIDE Table of Contents Page Application Process 3 Application Checklist 4 Federal
Examples of good screening tests include: mammography for breast cancer screening and Pap smears for cervical cancer screening.
 CANCER SCREENING Dr. Tracy Sexton (updated July 2010) What is screening? Screening is the identification of asymptomatic disease or risk factors by history taking, physical examination, laboratory tests
CANCER SCREENING Dr. Tracy Sexton (updated July 2010) What is screening? Screening is the identification of asymptomatic disease or risk factors by history taking, physical examination, laboratory tests
The Ontario Cancer Registry moves to the 21 st Century
 The Ontario Cancer Registry moves to the 21 st Century Rebuilding the OCR Public Health Ontario Grand Rounds Oct. 14, 2014 Diane Nishri, MSc Mary Jane King, MPH, CTR Outline 1. What is the Ontario Cancer
The Ontario Cancer Registry moves to the 21 st Century Rebuilding the OCR Public Health Ontario Grand Rounds Oct. 14, 2014 Diane Nishri, MSc Mary Jane King, MPH, CTR Outline 1. What is the Ontario Cancer
Singapore Cancer Registry Annual Registry Report Trends in Cancer Incidence in Singapore 2009 2013. National Registry of Diseases Office (NRDO)
 Singapore Cancer Registry Annual Registry Report Trends in Cancer Incidence in Singapore 2009 2013 National Registry of Diseases Office (NRDO) Released November 3, 2014 Acknowledgement This report was
Singapore Cancer Registry Annual Registry Report Trends in Cancer Incidence in Singapore 2009 2013 National Registry of Diseases Office (NRDO) Released November 3, 2014 Acknowledgement This report was
Colposcopic Management of Abnormal Cervical Cytology and Histology
 No. 284, December 2012 Colposcopic Management of Abnormal Cervical Cytology and Histology This clinical practice guideline has been prepared by the Executive Council of the Society of Canadian Colposcopists
No. 284, December 2012 Colposcopic Management of Abnormal Cervical Cytology and Histology This clinical practice guideline has been prepared by the Executive Council of the Society of Canadian Colposcopists
Information Model Requirements of Post-Coordinated SNOMED CT Expressions for Structured Pathology Reports
 Information Model Requirements of Post-Coordinated SNOMED CT Expressions for Structured Pathology Reports W. Scott Campbell, Ph.D., MBA James R. Campbell, MD Acknowledgements Steven H. Hinrichs, MD Chairman
Information Model Requirements of Post-Coordinated SNOMED CT Expressions for Structured Pathology Reports W. Scott Campbell, Ph.D., MBA James R. Campbell, MD Acknowledgements Steven H. Hinrichs, MD Chairman
Pleural and Pericardial fluids a one year analysis
 Pleural and Pericardial fluids a one year analysis Dr Olafsdottir Dr J Williams Department of Cytology, Llandough Hospital Contents Background Standards Aims/Questions Investigation group Results Conclusions
Pleural and Pericardial fluids a one year analysis Dr Olafsdottir Dr J Williams Department of Cytology, Llandough Hospital Contents Background Standards Aims/Questions Investigation group Results Conclusions
Guidelines for Preventative Health Care in LGBT Populations
 + Guidelines for Preventative Health Care in LGBT Populations Katie Imborek, MD Department of Family Medicine Co-Director UI LGBTQ Clinic April 2 nd, 2014 + Objectives n Understand CDC screening guidelines
+ Guidelines for Preventative Health Care in LGBT Populations Katie Imborek, MD Department of Family Medicine Co-Director UI LGBTQ Clinic April 2 nd, 2014 + Objectives n Understand CDC screening guidelines
Making Sense of Your Pap and HPV Test Results
 Making Sense of Your Pap and HPV Test Results Keep this booklet until you get your test results back from your doctor. U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Disease Control and Prevention
Making Sense of Your Pap and HPV Test Results Keep this booklet until you get your test results back from your doctor. U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Disease Control and Prevention
Outline. Workup for metastatic breast cancer. Metastatic breast cancer
 Metastatic breast cancer Immunostain Update: Diagnosis of metastatic breast carcinoma, emphasizing distinction from GYN primary 1/3 of breast cancer patients will show metastasis 1 st presentation or 20-30
Metastatic breast cancer Immunostain Update: Diagnosis of metastatic breast carcinoma, emphasizing distinction from GYN primary 1/3 of breast cancer patients will show metastasis 1 st presentation or 20-30
In The Abstract A quarterly newsletter from the
 In The Abstract A quarterly newsletter from the Kentucky Cancer Registry Large Hospital Edition July, 2000 KCR FALL WORKSHOP Sept. 14-16, 2000 Enclosed with this newsletter is a packet of information containing
In The Abstract A quarterly newsletter from the Kentucky Cancer Registry Large Hospital Edition July, 2000 KCR FALL WORKSHOP Sept. 14-16, 2000 Enclosed with this newsletter is a packet of information containing
Update on the Belgian Cancer Registry,
 Update on the Belgian Cancer Registry, breast cancer data... Belgian Breast Meeting 3-4/10/2008 Liesbet Van Eycken Overview Introduction, history of the BCR Breast Cancer Incidence - Mortality Europe Belgium
Update on the Belgian Cancer Registry, breast cancer data... Belgian Breast Meeting 3-4/10/2008 Liesbet Van Eycken Overview Introduction, history of the BCR Breast Cancer Incidence - Mortality Europe Belgium
Polyps. Hyperplasias. CAP 2011: Course AP104. The High Risk Benign Endometrium. Mutter and Nucci 1
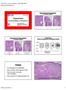 Course AP104 Endometrial Hyperplasia A morphologic Definition Hyperplasias Hormonal Effect or Precancer? George L. Mutter, MD Harvard Medical School and Brigham and Women s Hospital Boston, MA Endometrial
Course AP104 Endometrial Hyperplasia A morphologic Definition Hyperplasias Hormonal Effect or Precancer? George L. Mutter, MD Harvard Medical School and Brigham and Women s Hospital Boston, MA Endometrial
HPV and the Future of Cervical Screening
 HPV and the Future of Cervical Screening John Tidy, Professor of Gynaecological Oncology Chair, National Colposcopy QA Committee, Sheffield What is HPV? Small ds DNA virus Over 140 genotypes described
HPV and the Future of Cervical Screening John Tidy, Professor of Gynaecological Oncology Chair, National Colposcopy QA Committee, Sheffield What is HPV? Small ds DNA virus Over 140 genotypes described
Chapter 14 Cancer of the Cervix Uteri
 Carol L. Kosary Introduction Despite the existence of effective screening through the use of Pap smears since the 195 s, there were 9,71 estimated cases of invasive cervical cancer and 3,7 deaths in 26
Carol L. Kosary Introduction Despite the existence of effective screening through the use of Pap smears since the 195 s, there were 9,71 estimated cases of invasive cervical cancer and 3,7 deaths in 26
Cervical Cancer Screening
 TOC NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Cervical Cancer Screening Version 2.2012 NCCN.g Continue Version 2.2012, 05/02/12 National Comprehensive Cancer Netwk, Inc. 2012, All
TOC NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Cervical Cancer Screening Version 2.2012 NCCN.g Continue Version 2.2012, 05/02/12 National Comprehensive Cancer Netwk, Inc. 2012, All
Chapter 13. The hospital-based cancer registry
 Chapter 13. The hospital-based cancer registry J.L. Young California Tumor Registry, 1812 14th Street, Suite 200, Sacramento, CA 95814, USA Introduction The purposes of a hospital-based cancer registry
Chapter 13. The hospital-based cancer registry J.L. Young California Tumor Registry, 1812 14th Street, Suite 200, Sacramento, CA 95814, USA Introduction The purposes of a hospital-based cancer registry
PRIMARY SEROUS CARCINOMA OF PERITONEUM: A CASE REPORT
 PRIMARY SEROUS CARCINOMA OF PERITONEUM: A CASE REPORT Dott. Francesco Pontieri (*) U.O. di Anatomia Patologica P.O. di Rossano (CS) Dott. Gian Franco Zannoni Anatomia Patologica Facoltà di Medicina e Chirurgia
PRIMARY SEROUS CARCINOMA OF PERITONEUM: A CASE REPORT Dott. Francesco Pontieri (*) U.O. di Anatomia Patologica P.O. di Rossano (CS) Dott. Gian Franco Zannoni Anatomia Patologica Facoltà di Medicina e Chirurgia
GUIDELINE DOCUMENT CERVICAL CANCER SCREENING IN SOUTH AFRICA 2015
 GUIDELINE DOCUMENT CERVICAL CANCER SCREENING IN SOUTH AFRICA 2015 Cervical cancer remains an important cause of morbidity and mortality in South Africa. At present the national cervical cancer prevention
GUIDELINE DOCUMENT CERVICAL CANCER SCREENING IN SOUTH AFRICA 2015 Cervical cancer remains an important cause of morbidity and mortality in South Africa. At present the national cervical cancer prevention
Lung Carcinomas New 2015 WHO Classification. Spasenija Savic Pathology
 Lung Carcinomas New 2015 WHO Classification Spasenija Savic Pathology ***EXPECTED SPRING 2015*** This authoritative, concise reference book provides an international standard for oncologists and pathologists
Lung Carcinomas New 2015 WHO Classification Spasenija Savic Pathology ***EXPECTED SPRING 2015*** This authoritative, concise reference book provides an international standard for oncologists and pathologists
The Pap Smear: Guidelines for Screening and Follow-up
 The Pap Smear: Guidelines for Screening and Follow-up The incidence of and mortality from cervical cancers have decreased with the Pap smear's opportunistic use in Canada. The rate of decline has eased,
The Pap Smear: Guidelines for Screening and Follow-up The incidence of and mortality from cervical cancers have decreased with the Pap smear's opportunistic use in Canada. The rate of decline has eased,
Routine Vaginal Cuff Smear Testing in Post Hysterectomy Patients With Benign Uterine Conditions: When Is It Indicated?
 ORIGINAL ARTICLES Routine Vaginal Cuff Smear Testing in Post Hysterectomy Patients With Benign Uterine Conditions: When Is It Indicated? Andrea Videleftky, MD, Nonnan GrossI, MD, Maxine Denniston, MSPH,
ORIGINAL ARTICLES Routine Vaginal Cuff Smear Testing in Post Hysterectomy Patients With Benign Uterine Conditions: When Is It Indicated? Andrea Videleftky, MD, Nonnan GrossI, MD, Maxine Denniston, MSPH,
Breast Imaging Made Brief and Simple. Jane Clayton MD Associate Professor Department of Radiology LSUHSC New Orleans, LA
 Breast Imaging Made Brief and Simple Jane Clayton MD Associate Professor Department of Radiology LSUHSC New Orleans, LA What women are referred for breast imaging? Two groups of women are referred for
Breast Imaging Made Brief and Simple Jane Clayton MD Associate Professor Department of Radiology LSUHSC New Orleans, LA What women are referred for breast imaging? Two groups of women are referred for
Cancer Screening. Robert L. Robinson, MD, MS. Ambulatory Conference SIU School of Medicine Department of Internal Medicine.
 Cancer Screening Robert L. Robinson, MD, MS Ambulatory Conference SIU School of Medicine Department of Internal Medicine March 13, 2003 Why screen for cancer? Early diagnosis often has a favorable prognosis
Cancer Screening Robert L. Robinson, MD, MS Ambulatory Conference SIU School of Medicine Department of Internal Medicine March 13, 2003 Why screen for cancer? Early diagnosis often has a favorable prognosis
The Ontario Cancer Registry and its Data Quality. Diane Nishri Senior Research Associate, Surveillance February, 2011
 The Ontario Cancer Registry and its Data Quality Diane Nishri Senior Research Associate, Surveillance February, 2011 Objectives Become familiar with cancer registration in Ontario, including issues related
The Ontario Cancer Registry and its Data Quality Diane Nishri Senior Research Associate, Surveillance February, 2011 Objectives Become familiar with cancer registration in Ontario, including issues related
2015 RN.ORG, S.A., RN.ORG, LLC
 Cervical Cancer WWW.RN.ORG Reviewed September, 2015, Expires September, 2017 Provider Information and Specifics available on our Website Unauthorized Distribution Prohibited 2015 RN.ORG, S.A., RN.ORG,
Cervical Cancer WWW.RN.ORG Reviewed September, 2015, Expires September, 2017 Provider Information and Specifics available on our Website Unauthorized Distribution Prohibited 2015 RN.ORG, S.A., RN.ORG,
An abnormal Pap smear - what does it mean?
 An abnormal Pap smear - what does it mean? It is natural to feel worried if you have just found out that your Pap smear result is not normal (abnormal). Around 1 in 10 Pap smears will show changes in the
An abnormal Pap smear - what does it mean? It is natural to feel worried if you have just found out that your Pap smear result is not normal (abnormal). Around 1 in 10 Pap smears will show changes in the
Provider Reimbursement for Women's Cancer Screening Program
 Reimbursement Schedule July 1, 2015 June 30, 2016 Office Visits - Established Patients Office Visit / Minimal / no physician 99211 $ 16.70 Office Visit / Problem focused History / exam 99212 $ 36.46 Preventive
Reimbursement Schedule July 1, 2015 June 30, 2016 Office Visits - Established Patients Office Visit / Minimal / no physician 99211 $ 16.70 Office Visit / Problem focused History / exam 99212 $ 36.46 Preventive
General Rules SEER Summary Stage 2000. Objectives. What is Staging? 5/8/2014
 General Rules SEER Summary Stage 2000 Linda Mulvihill Public Health Advisor NCRA Annual Meeting May 2014 National Center for Chronic Disease Prevention and Health Promotion Division of Cancer Prevention
General Rules SEER Summary Stage 2000 Linda Mulvihill Public Health Advisor NCRA Annual Meeting May 2014 National Center for Chronic Disease Prevention and Health Promotion Division of Cancer Prevention
Cytology : first alert of mesothelioma? Professor B. Weynand, UCL Yvoir, Belgium
 Cytology : first alert of mesothelioma? Professor B. Weynand, UCL Yvoir, Belgium Introduction 3 cavities with the same embryologic origin the mesoderme Pleura Exudates Pleura Peritoneum Pericardium 22%
Cytology : first alert of mesothelioma? Professor B. Weynand, UCL Yvoir, Belgium Introduction 3 cavities with the same embryologic origin the mesoderme Pleura Exudates Pleura Peritoneum Pericardium 22%
Cancer in Ireland 2013: Annual report of the National Cancer Registry
 Cancer in 2013: Annual report of the National Cancer Registry ABBREVIATIONS Acronyms 95% CI 95% confidence interval APC Annual percentage change ASR Age standardised rate (European standard population)
Cancer in 2013: Annual report of the National Cancer Registry ABBREVIATIONS Acronyms 95% CI 95% confidence interval APC Annual percentage change ASR Age standardised rate (European standard population)
Cancer Survival in Belgium
 B E L G I A N C A N C E R R E G I S T R Y Cancer Survival in Belgium Belgium 24-28 Brussels-Capital Region 24-28 Walloon Region 24-28 Flemish Region 1999-28 Cancer Survival in Belgium Belgium 24-28 Brussels-Capital
B E L G I A N C A N C E R R E G I S T R Y Cancer Survival in Belgium Belgium 24-28 Brussels-Capital Region 24-28 Walloon Region 24-28 Flemish Region 1999-28 Cancer Survival in Belgium Belgium 24-28 Brussels-Capital
worry When to Cervical Abnormalities CME Workshop What s the situation? What are the trends? By Dianne Miller, MD, FRCSC In this article:
 CME Workshop When to worry Cervical Abnormalities By Dianne Miller, MD, FRCSC What s the situation? Over 600,000 Papanicolaou s (Pap) smears were performed in British Columbia in 2000. Approximately 13,400
CME Workshop When to worry Cervical Abnormalities By Dianne Miller, MD, FRCSC What s the situation? Over 600,000 Papanicolaou s (Pap) smears were performed in British Columbia in 2000. Approximately 13,400
Screening for Cancer in Light of New Guidelines and Controversies. Christopher Celio, MD St. Jude Heritage Medical Group
 Screening for Cancer in Light of New Guidelines and Controversies Christopher Celio, MD St. Jude Heritage Medical Group Screening Tests The 2 major objectives of a good screening program are: (1) detection
Screening for Cancer in Light of New Guidelines and Controversies Christopher Celio, MD St. Jude Heritage Medical Group Screening Tests The 2 major objectives of a good screening program are: (1) detection
Cancer Screening and Early Detection Guidelines
 Cancer Screening and Early Detection Guidelines Guillermo Tortolero Luna, MD, PhD Director Cancer Control and Population Sciences Program University of Puerto Rico Comprehensive Cancer Center ASPPR Clinical
Cancer Screening and Early Detection Guidelines Guillermo Tortolero Luna, MD, PhD Director Cancer Control and Population Sciences Program University of Puerto Rico Comprehensive Cancer Center ASPPR Clinical
METROPOLITAN LIFE INSURANCE COMPANY NEW YORK, NEW YORK
 METROPOLITAN LIFE INSURANCE COMPANY NEW YORK, NEW YORK POLICYHOLDER: Your Employer Group Policy Form No: GPNP14-CI (Referred to as the Group Policy ) Certificate Form No: GCERT14-CI (Referred to as the
METROPOLITAN LIFE INSURANCE COMPANY NEW YORK, NEW YORK POLICYHOLDER: Your Employer Group Policy Form No: GPNP14-CI (Referred to as the Group Policy ) Certificate Form No: GCERT14-CI (Referred to as the
YOUR LUNG CANCER PATHOLOGY REPORT
 UNDERSTANDING YOUR LUNG CANCER PATHOLOGY REPORT 1-800-298-2436 LungCancerAlliance.org A GUIDE FOR THE PATIENT 1 CONTENTS What is a Pathology Report?...3 The Basics...4 Sections of a Pathology Report...7
UNDERSTANDING YOUR LUNG CANCER PATHOLOGY REPORT 1-800-298-2436 LungCancerAlliance.org A GUIDE FOR THE PATIENT 1 CONTENTS What is a Pathology Report?...3 The Basics...4 Sections of a Pathology Report...7
The 2015 Cancer System Performance Report
 The 2015 Cancer System Performance Report June 2015 Technical Appendix Page 1 of 20 1. Prevention Smoking prevalence Definition: Percentage of population aged 12 years and older in each specified group
The 2015 Cancer System Performance Report June 2015 Technical Appendix Page 1 of 20 1. Prevention Smoking prevalence Definition: Percentage of population aged 12 years and older in each specified group
Diagnostic Challenge. Department of Pathology,
 Cytology of Pleural Fluid as a Diagnostic Challenge Paavo Pääkkö,, MD, PhD Chief Physician and Head of the Department Department of Pathology, Oulu University Hospital,, Finland Oulu University Hospital
Cytology of Pleural Fluid as a Diagnostic Challenge Paavo Pääkkö,, MD, PhD Chief Physician and Head of the Department Department of Pathology, Oulu University Hospital,, Finland Oulu University Hospital
HPV, Cervical Dysplasia and Cancer
 FACTSHEET HPV, Cervical Dysplasia and Cancer Summary Cervical dysplasia is an abnormal change in the cells of the cervix in the uterus. Early changes, called low-grade lesions by doctors, may persist and
FACTSHEET HPV, Cervical Dysplasia and Cancer Summary Cervical dysplasia is an abnormal change in the cells of the cervix in the uterus. Early changes, called low-grade lesions by doctors, may persist and
Protocol for the Examination of Specimens From Patients With Carcinoma of the Uterine Cervix
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Uterine Cervix Protocol applies to all invasive carcinomas of the cervix. Based on AJCC/UICC TNM, 7th edition Protocol web
Protocol for the Examination of Specimens From Patients With Carcinoma of the Uterine Cervix Protocol applies to all invasive carcinomas of the cervix. Based on AJCC/UICC TNM, 7th edition Protocol web
Guidelines for. Quality Assurance in Cervical Screening. Second Edition
 Guidelines for Assurance in Cervical Screening Second Edition Chapter 2 assurance in programme operation 2.1 Introduction 2.2 assurance s and standards 2.2.1 Screening population and screening intervals
Guidelines for Assurance in Cervical Screening Second Edition Chapter 2 assurance in programme operation 2.1 Introduction 2.2 assurance s and standards 2.2.1 Screening population and screening intervals
Cancer screening: indications, benefits and myths
 Cancer screening: indications, benefits and myths Silvia Deandrea Institute for Health and Consumer Protection Public Health Policy Support Unit Healthcare Quality Team Joint Research Centre The European
Cancer screening: indications, benefits and myths Silvia Deandrea Institute for Health and Consumer Protection Public Health Policy Support Unit Healthcare Quality Team Joint Research Centre The European
Wisconsin Cancer Data Bulletin Wisconsin Department of Health Services Division of Public Health Office of Health Informatics
 Wisconsin Cancer Data Bulletin Wisconsin Department of Health Services Division of Public Health Office of Health Informatics In Situ Breast Cancer in Wisconsin INTRODUCTION This bulletin provides information
Wisconsin Cancer Data Bulletin Wisconsin Department of Health Services Division of Public Health Office of Health Informatics In Situ Breast Cancer in Wisconsin INTRODUCTION This bulletin provides information
SCREENING FOR CANCER OF THE CERVIX
 SCREENING FOR CANCER OF THE CERVIX An Office Manual for Health Professionals This manual has been prepared by the Cervical Cancer Screening Program of the BC Cancer Agency to support effective use of the
SCREENING FOR CANCER OF THE CERVIX An Office Manual for Health Professionals This manual has been prepared by the Cervical Cancer Screening Program of the BC Cancer Agency to support effective use of the
Innersense Voice 139 Jan 15, 2013 Screen to Prevent: Cervical & Prostate Cancer
 Screening test to detect cancer at its earliest stage has been one of the most studied areas in our fight against cancer. However, the benefits of screening in terms of cancer prevention, early detection
Screening test to detect cancer at its earliest stage has been one of the most studied areas in our fight against cancer. However, the benefits of screening in terms of cancer prevention, early detection
Cervical Screening Programme
 Cervical Screening Programme England 2010-11 1 The NHS Information Centre is England s central, authoritative source of health and social care information. Acting as a hub for high quality, national, comparative
Cervical Screening Programme England 2010-11 1 The NHS Information Centre is England s central, authoritative source of health and social care information. Acting as a hub for high quality, national, comparative
The Role of Genetic Testing in the Evaluation of Thyroid Nodules. Thyroid Cancer and FNA. Thyroid Cancer. Pure Follicular Cancers.
 Where does Molecular Analysis of FNA Specimens fit into the evaluation of thyroid nodules? The Role of Genetic Testing in the Evaluation of Thyroid Nodules Ultrasound TSH Risk factors Jill E. Langer, MD
Where does Molecular Analysis of FNA Specimens fit into the evaluation of thyroid nodules? The Role of Genetic Testing in the Evaluation of Thyroid Nodules Ultrasound TSH Risk factors Jill E. Langer, MD
CHAPTER 2. Neoplasms (C00-D49) March 2014. 2014 MVP Health Care, Inc.
 Neoplasms (C00-D49) March 2014 2014 MVP Health Care, Inc. CHAPTER SPECIFIC CATEGORY CODE BLOCKS C00-C14 Malignant neoplasms of lip, oral cavity and pharynx C15-C26 Malignant neoplasms of digestive organs
Neoplasms (C00-D49) March 2014 2014 MVP Health Care, Inc. CHAPTER SPECIFIC CATEGORY CODE BLOCKS C00-C14 Malignant neoplasms of lip, oral cavity and pharynx C15-C26 Malignant neoplasms of digestive organs
Hospital-Based Tumor Registry. Srinagarind Hospital, Khon Kaen University
 Hospital-Based Tumor Registry Srinagarind Hospital, Khon Kaen University Statistical Report 2012 Cancer Unit, Faculty of Medicine Khon Kaen University Khon Kaen, Thailand Tel & Fax:+66(43)-202485 E-mail:
Hospital-Based Tumor Registry Srinagarind Hospital, Khon Kaen University Statistical Report 2012 Cancer Unit, Faculty of Medicine Khon Kaen University Khon Kaen, Thailand Tel & Fax:+66(43)-202485 E-mail:
Liquid-based cytology
 Liquid-based cytology Claire Bourgain UZBrussel Introduction LBC for gynaecological cytology has been introduced in Belgium a decade ago There is no consensus On performance compared to conventional cytology
Liquid-based cytology Claire Bourgain UZBrussel Introduction LBC for gynaecological cytology has been introduced in Belgium a decade ago There is no consensus On performance compared to conventional cytology
Frozen Section Diagnosis
 Frozen Section Diagnosis Dr Catherine M Corbishley Honorary Consultant Histopathologist St George s Healthcare NHS Trust and lead examiner final FRCPath Practical 2008-2011 Frozen Section Diagnosis The
Frozen Section Diagnosis Dr Catherine M Corbishley Honorary Consultant Histopathologist St George s Healthcare NHS Trust and lead examiner final FRCPath Practical 2008-2011 Frozen Section Diagnosis The
SUPPLEMENTARY NOTES. Personal General Insurance (4 th Edition) Date Of Issue: 1 October 2014
 SUPPLEMENTARY NOTES Personal General Insurance (4 th Edition) Date Of Issue: 1 October 2014 The following amendments have NOT been incorporated in the Study Guide. They should be marked up in the Study
SUPPLEMENTARY NOTES Personal General Insurance (4 th Edition) Date Of Issue: 1 October 2014 The following amendments have NOT been incorporated in the Study Guide. They should be marked up in the Study
LDMAS (LOH Data Management and Analysis Software) User Guide
 LDMAS (LOH Data Management and Analysis Software) User Guide Written by : Rifat Hamoudi, Division of Molecular Histopathology, Department of Pathology, University of Cambridge, Cambridge, CB2 2QQ. Email
LDMAS (LOH Data Management and Analysis Software) User Guide Written by : Rifat Hamoudi, Division of Molecular Histopathology, Department of Pathology, University of Cambridge, Cambridge, CB2 2QQ. Email
Report series: General cancer information
 Fighting cancer with information Report series: General cancer information Eastern Cancer Registration and Information Centre ECRIC report series: General cancer information Cancer is a general term for
Fighting cancer with information Report series: General cancer information Eastern Cancer Registration and Information Centre ECRIC report series: General cancer information Cancer is a general term for
