Public Health Management of Pertussis. HPA Guidelines for the Public Health Management of Pertussis Incidents in Healthcare Settings
|
|
|
- Adam Greer
- 8 years ago
- Views:
Transcription
1 Public Health Management of Pertussis HPA Guidelines for the Public Health Management of Pertussis Incidents in Healthcare Settings
2 Members of Pertussis Guideline Working Group Gayatri Amirthalingam (Chair) Girija Dabke (Author) Blandina Blackburn Fiona Bryce-Johnston Meera Chand rman Fry Tim Harrison Paul Heath David Jenkins Lesley McFarlane Giri Shankar Elizabeth Sheridan Kathy Wakefield William Welfare Consultant Epidemiologist, Immunisation, Hepatitis & Blood Safety department, HPA Colindale Consultant in Communicable Disease Control (CCDC), Hampshire & Isle of Wight Health Protection Unit (HPU) Consultant Occupational Physician, Royal Berkshire NHS Foundation Trust, Association of National Health Occupational Health Physicians Health Protection Nurse, Hampshire & Isle of Wight HPU Specialist Registrar in Microbiology, HPA Colindale Principal Clinical Scientist, Bordetella Reference Laboratory, Respiratory & Vaccine Preventable Bacteria Reference Unit (RVPBRU) Consultant Clinical Scientist, Bordetella Reference Laboratory, RVPBRU Reader in Paediatric Infectious Diseases, Honorary Consultant, St Georges Hospital, London Consultant Medical Microbiologist and Lead Infection Control Doctor, University Hospitals of Leicester NHS Trust, British Infection Association Health Protection Practitioner, East Midlands South HPU CCDC, rfolk, Suffolk and Cambridgeshire HPU Consultant Microbiologist and Head of HCAI and AMR, HPA Colindale Health Protection/Infection Prevention Manager (Immunisation Lead), Rotherham Public Health, Infection Prevention Society CCDC, Greater Manchester HPU This publication is also available in large print Health Protection Agency October 2012 PB64.01 Authorised by: Saurabh Gupta Effective Date: 25/10/12
3 Table of Contents 1. Summary of key recommendations Case management (for all clinically suspected, epidemiologically linked and lab confirmed cases) Definitions Priority groups for contact tracing and prophylaxis Identification of contacts for prophylaxis Action for priority contacts identified with a significant exposure Action for contacts with significant exposure NOT in priority groups Case finding and surveillance Two or more epidemiologically linked cases associated with a healthcare setting Background, risk of transmission and rationale for action Background Risk of transmission and rationale for public health action in a healthcare setting Definitions Case definitions Priority groups for public health action Partially immunised infants (< 12 months) Infectious period Case management Exclusion Laboratory investigation Treatment tification Management of household contacts Scenario 1: Single case in a Healthcare Worker Case finding and surveillance Risk assessment Identification of contacts in the healthcare setting for post-exposure prophylaxis Scenario 2: Single case in a hospitalised patient Case finding and surveillance Risk assessment Identification of contacts for post-exposure prophylaxis Special Settings: Neonatal Intensive Care Unit (NICU)/Special Care Baby Unit (SCBU) Paediatric Intensive Care Unit (ITU)/ High Dependency Unit (HDU) setting Chemoprophylaxis and Vaccination Chemoprophylaxis Vaccination Surveillance Two or more epidemiologically linked cases of pertussis in a healthcare setting References PB64.01 (Oct 2012) Page 2
4 11. Appendix Flow chart Flow chart Appendix Letter for parents/ guardians whose infant has been in contact with a case and does not require chemoprophylaxis Letter for parents/ guardians whose infant has been in contact with a case and requires immunisation / chemoprophylaxis Letter for HCWs that have been in contact with a case but do not require immunisation / chemoprophylaxis Letter for HCWs that have been in contact with a case and require immunisation / chemoprophylaxis PB64.01 (Oct 2012) Page 3
5 1. Summary of key recommendations This document provides specific guidance on the management of pertussis incidents in healthcare settings. The following summarises the key public health actions based on a review of the evidence by an expert working group Case management (for all clinically suspected, epidemiologically linked and lab confirmed cases) Case management including laboratory testing, antibiotic treatment and identification of household contacts should proceed according to the HPA guidelines for the public health management of pertussis (hereafter referred to as the HPA guidelines [1]). Healthcare workers (HCWs) who have been diagnosed with pertussis (either clinically suspected, epidemiologically linked or laboratory confirmed) should be excluded until 5 days of appropriate antibiotic treatment has been completed OR for 21 days from onset of symptoms, if appropriate antibiotic treatment has not been completed. Hospitalised patients diagnosed with pertussis (either clinically suspected, epidemiologically linked or laboratory confirmed) should be placed in respiratory isolation during the infectious period. As pertussis is a notifiable disease, all cases should be reported to the local Health Protection Unit (HPU) (and after April 2013 the Public Health England (PHE) Centre). Cases associated with a healthcare setting should also be reported to the relevant Infection Prevention and Control team (IPCT) and for HCWs, to Occupational Health (OH), even if it is too late for prophylaxis Definitions Public health action in this guidance applies to clinically suspected, epidemiologically linked or laboratory confirmed cases associated with health care settings. Action should not be delayed until the results of laboratory testing are available. Infectious period: A case is considered infectious from onset of symptoms until 5 days of appropriate antibiotic treatment OR for 21 days from onset of symptoms if appropriate antibiotic therapy has not been completed. Significant exposure in a Healthcare setting: Unprotected direct face-to-face contact (< 2 metre distance) for greater than a cumulative period of 1 hour with an infectious case OR Direct contact with respiratory secretions from an infectious case PB64.01 (Oct 2012) Page 4
6 e.g. performing an aerosol-generating procedure or examination of the nose and throat without appropriate personal protective equipment [2, 3] Priority groups for contact tracing and prophylaxis The priority groups for public health action in healthcare settings are defined as below: Group 1. Individuals at increased risk of severe complications ( Vulnerable ) Infants under one year of age who have received fewer than three doses of pertussis- containing vaccine. Group 2. Individuals at risk of transmitting to vulnerable individuals in group 1 who have not received a pertussis containing vaccine more than 1 week and less than 5 years ago: Pregnant women (> 32 weeks gestation). Health care staff working with infants or pregnant women Identification of contacts for prophylaxis Prophylaxis should be offered to those in priority groups WITH a significant exposure to an infectious case. However, the time/ duration criteria are only a guide and where unimmunised or partially immunised infants are exposed, a lower threshold should be applied e.g. prophylaxis should be considered for these infants in direct contact with an affected HCW regardless of duration of contact Action for priority contacts identified with a significant exposure Contacts in the priority groups defined above who have been identified within 21 days of a significant exposure (described in section 2.2) should be offered chemoprophylaxis/ vaccination as follows: Chemoprophylaxis Chemoprophylaxis should be offered (based on recommendations in the HPA guidelines) [1] to contacts within 21 days of a significant exposure in the following priority groups: Unimmunised and partially immunised infant contacts Pregnant women (>32 weeks gestation) who have not received a booster dose of pertussis vaccine more than 1 week and less than 5 years ago HCW contacts who have not received a booster dose of pertussis containing vaccine more than 1 week and less than 5 years ago PB64.01 (Oct 2012) Page 5
7 1.5.2 Vaccination Immunisation should be considered for those who have been offered chemoprophylaxis: Unimmunised and partially immunised infants should complete the primary course with the appropriate vaccine according to the recommended schedule. A booster dose of pertussis containing vaccine is recommended for pregnant women (> 32 weeks gestation) and HCW contacts who have not received a dose of pertussis containing vaccine in the preceding 5 years and no Td-IPV in the preceding month Communication Inform and advise letters should be provided along with the offer of prophylaxis (Appendix 2) Action for contacts with significant exposure NOT in priority groups Contacts with significant exposure who are not in risk groups should be informed and advised to seek medical attention if symptoms develop (Template letters included in Appendix 2) Case finding and surveillance The relevant Infection Prevention and Control team (IPCT) and Occupational Health (OH) should be informed of any cases of pertussis that occur in a healthcare setting, even if beyond 21 days of exposure and therefore too late for prophylaxis. The IPCT and OH will carry out case finding for existing cases among patient and HCW contacts and passive surveillance for further cases that may occur Two or more epidemiologically linked cases associated with a healthcare setting If two or more confirmed or epidemiologically linked cases of pertussis occur in a healthcare setting, an outbreak control team should be convened. If transmission is most likely to have occurred within the healthcare setting, then in addition to the actions taken as per algorithms for single cases, consideration should be given to offering vaccination more widely. The priority is active case finding and therefore in these circumstances a less specific case definition should be used to ensure no cases are missed. PB64.01 (Oct 2012) Page 6
8 Expert advice on outbreak investigation and response is available from the Immunisation, Hepatitis and Blood Safety Department, HPS-Colindale, HPA ( ) and on laboratory investigation from the Bordetella Reference Laboratory, MS-Colindale ( ) 1. 1 Laboratory Confirmation and Public Health action Appropriate Public Health action should not wait for laboratory results as negative results cannot be used to exclude pertussis infection. At the request of the HPU, the Bordetella Reference Laboratory will prioritise testing for evidence of B. pertussis infection in support of outbreaks or incident investigations as appropriate. However, please note these services are not available outside of regular working hours. Please contact Microbiology Services (MS)-Colindale on and discuss with senior staff prior to sending specimens. PB64.01 (Oct 2012) Page 7
9 2. Background, risk of transmission and rationale for action 2.1. Background Pertussis is an acute respiratory infection caused by the Gram-negative organism Bordetella pertussis. Pertussis can affect individuals of any age although the clinical presentation does vary. Young unvaccinated infants are at highest risk of severe complications (including pneumonia, apnoea and seizures) and death. Older vaccinated individuals often present with milder symptoms and atypical features which may go unrecognised leading to delays in treatment and timely public health action to prevent ongoing transmission. Protection conferred through natural infection or vaccination is not lifelong. The UK childhood immunisation schedule consists of three primary infant doses of pertussis containing vaccine and a preschool booster dose [4]. As protection following vaccination wanes over time, susceptible adults in the population can be an important source of infection for infants too young to be afforded direct protection from vaccination. Susceptible adults working in healthcare settings pose a potential risk of transmission to vulnerable infants and incidents in healthcare settings can be both challenging and resource intensive to manage. It is not currently national policy to routinely offer pertussis boosters to UK healthcare staff working in high risk settings. This document supplements the HPA Guidelines on the Public Health management of pertussis [1]. It seeks to provide additional specific guidance for managing incidents associated with healthcare settings based on a review of published literature and international guidance as well as the results of a survey of pertussis incidents in healthcare settings managed by local Health Protection Units 2 (HPUs) in Risk of transmission and rationale for public health action in a healthcare setting Outbreaks of pertussis in healthcare settings and exclusion of affected staff can be disruptive and costly to manage [5]. Several instances of pertussis transmission in health care settings have been reported in the literature and include transmission from healthcare worker (HCW) to HCW [5-8], HCW to patient [6, 9, 10] as well as patient to HCW [7, 11]. However the greatest risk of nosocomial transmission is likely to be from a HCW to a patient or to another member of staff. Patients with pertussis are most infectious in the initial catarrhal stage and during the first three weeks after the onset of cough [12]. Pertussis is transmitted by large 2 te: After April 2013, the functions of local HPUs will be transferred to Public Health England (PHE) Centres PB64.01 (Oct 2012) Page 8
10 droplets [13]. Traditionally, droplet transmission has been accepted as occurring within 3 feet of the infected patient. However, recent studies have suggested that droplets can be dispersed to a distance of 6 feet (1.9 metres) during coughing [14]. B. pertussis DNA has been detected as far away as 4 metres (13 feet) from a patient s bedside for up to 4 days following initiation of therapy although the clinical relevance of this remains uncertain [15]. The risk of transmission will therefore be dependent on the type of procedure undertaken and duration and proximity of exposure, with the risk being higher in settings involving close prolonged clinical contact e.g. intensive care and maternity settings. The recommendations for chemoprophylaxis and post exposure vaccination require consideration of the nature of the healthcare setting where the exposure has occurred including level/proximity of contact, the vulnerability of those exposed and the effectiveness of available interventions Young infants are at highest risk of severe complications, hospitalisation and death from pertussis. There is very little evidence supporting the increased risk of pertussis in other clinical risk groups such as individuals with underlying respiratory conditions, immunocompromised or pregnant women. The risk for pregnant women is in late pregnancy and relates to the potential transmission to the newborn. Pregnant women themselves do not appear to be at increased risk of severe pertussis compared with non-pregnant women. Although older children are at a lower risk of severe disease, they pose a risk of onward transmission. However, given the rapid turnover of patients in the paediatric setting, onward transmission within the healthcare setting is unlikely. Therefore chemoprophylaxis for exposed older children in the healthcare setting is not routinely recommended. The evidence of benefits of chemoprophylaxis is limited to close prolonged household type contact; evidence of effectiveness is limited outside of these settings [16]. The primary accelerated infant vaccination schedule in the UK is highly effective in preventing severe complications in infants and young children [17]. The current schedule (primary schedule plus preschool booster) is thought to provide protection in children until at least 10 years of age [17]. Although the duration of protection from boosters administered in adolescence and adulthood has not been clearly established, post exposure vaccination has the potential to provide longer term protection against current and future exposures. The use of pertussis booster vaccination in a hospital outbreak in the USA demonstrated rapid antibody responses and the potential to reduce susceptibility of the population within 1-2 weeks [18]. In light of the above evidence, these guidelines restrict the use of wider chemoprophylaxis and vaccination to healthcare settings where the risks from pertussis transmission are highest i.e. settings involving infants and pregnant women. In all other healthcare settings, case management and provision of information and advice to contacts with significant exposure to seek health advice if PB64.01 (Oct 2012) Page 9
11 they develop symptoms are recommended. Chemoprophylaxis and post exposure vaccination is NOT recommended in healthcare settings where pregnant women or infants are not involved. The two main scenarios considered here are: 1. The index case is a HCW 2. The index case is a hospitalised patient The following pages go through the risk assessment and management of cases and contacts for each of these scenarios. These are summarised in flow charts in Appendix 1. It is advised that the flow charts be read in conjunction with the full guidelines. Sample letters for HCW and patient contacts are included in Appendix 2. PB64.01 (Oct 2012) Page 10
12 3. Definitions 3.1. Case definitions Suspected case of pertussis: Any person in whom a clinician suspects pertussis infection OR Any person with an acute cough lasting for 14 days or more, without an apparent cause plus one or more of the following:- o Paroxysms of coughing o Post-tussive vomiting o Inspiratory whoop AND Absence of laboratory confirmation epidemiologic link to a laboratory confirmed case. Confirmed case of pertussis: Any person with signs and symptoms consistent with pertussis with: B. pertussis isolated from respiratory specimen (usually a nasopharyngeal swab/ per nasal swab or nasopharyngeal aspirate OR Anti- Pertussis toxin IgG titre >70 IU/ml in serum (in the absence of vaccination within the past year) 3 OR Confirmed B. pertussis PCR positive in a respiratory clinical specimen. Epidemiologically linked case of pertussis: A suspected case with signs and symptoms consistent with pertussis, but no laboratory confirmation, who was in contact with a laboratory confirmed case of pertussis in the 21 days before the onset of symptoms. 3 These data are currently under review and this advice will be updated accordingly. Interpretation of anti-pt IgG titres in recently vaccinated individuals is difficult. PB64.01 (Oct 2012) Page 11
13 3.2. Priority groups for public health action Priority groups for consideration of prophylaxis in healthcare settings are as below (based on the current HPA guidelines) [1]. Group 1. Individuals at increased risk of severe complications ( Vulnerable ) Infants under one year of age who have received fewer than three doses of pertussis-containing vaccine. Group 2. Individuals at risk of transmitting to vulnerable individuals in group 1 who have not received a pertussis containing vaccine more than 1 week and less than 5 years ago Pregnant women (> 32 weeks gestation) Health care staff working with infants or pregnant women 3.3. Partially immunised infants (< 12 months) Partially immunised infants are infants who have not completed the primary immunisation schedule i.e. not received 3 doses of pertussis-containing vaccine. This includes infants who are less than 4 months of age and are age-appropriately immunised (i.e. received one or two doses) but not fully protected Infectious period A case is considered infectious from onset of symptoms until 5 days of antibiotic treatment or for 21 days from onset of symptoms if they have not received appropriate antibiotic therapy. Chemoprophylaxis/vaccination should therefore be considered for contacts in priority groups with significant exposure if BOTH these conditions are met: Exposure occurred during infectious period Prophylaxis can be offered within 21 days of last exposure (incubation period) PB64.01 (Oct 2012) Page 12
14 3.5. Significant exposure in a healthcare setting Unprotected 4 direct face-to-face contact (< 2metre distance) for greater than a cumulative period of 1 hour with an infectious case OR Direct contact with respiratory secretions from an infectious case e.g. performing aerosol-generating procedures or examination of the nose and throat without appropriate personal protective equipment (PPE). The 2 metres (6.5 feet) distance is based on the evidence that coughing can result in dispersal of droplets up to a distance of 2 metres (Section 2.2). The 1 hour cut-off is arbitrary, based on the lower risk to transient contacts, and is consistent with other international guidelines [19]. However the time/distance criteria should be used as a guide and a lower threshold may be considered in some circumstances. For example where an infected HCW has provided direct clinical care to hospitalised infants (unimmunised/partially immunised), prophylaxis may be offered to these infants even when the duration of contact is less than 1 hour. Such circumstances should be discussed with the local HPU and after April 2013, the Public Health England (PHE) Centre. 4 Unprotected exposure refers to exposure in absence of appropriate PPE. Appropriate PPE for respiratory infections transmitted by droplet route includes surgical mask and use of gloves and apron if appropriate[2,3] PB64.01 (Oct 2012) Page 13
15 4. Case management Public health action should proceed on all clinically suspected, epidemiologically linked and laboratory confirmed cases of pertussis. Public health action should not be delayed until the results of laboratory testing are available. For details of case management, refer to current HPA guidelines [1] Exclusion If the case is a HCW, they should be excluded from work as soon as a diagnosis of pertussis is suspected, until 5 days of appropriate antibiotic treatment is completed or for 21 days from onset if not treated. If the case is a hospitalised patient, they should be placed in respiratory isolation until 5 days of treatment is completed or for 21 days from onset if not treated. HCWs looking after patients with pertussis should wear appropriate PPE [2, 3]. 4.2 Laboratory investigation The choice of testing method will depend on the age of the case and their duration of symptoms. For detailed information on the appropriate methods for confirming suspected cases, please refer to the current HPA guidelines [1]. For further advice, please contact the Bordetella Reference Laboratory, Colindale on Please note that the sensitivity and specificity of these tests are not 100%. Therefore negative results cannot be used to exclude pertussis infection. Public health action should proceed based on a risk assessment of the clinical and epidemiological factors (based on the case definitions stated above) Treatment Antibiotic therapy should be instituted according to current HPA guidelines [1] tification As a notifiable disease, all cases of pertussis should be reported to the local HPU and after April 2013, the PHE Centre. Cases associated with a healthcare setting should also be reported to the relevant infection prevention and control team (IPCT) and for HCWs, to occupational health (OH) even if it is too late for prophylaxis. This will heighten awareness and enable the IPCT and OH to detect and manage any further cases early. PB64.01 (Oct 2012) Page 14
16 4.5. Management of household contacts Identification and management of household contacts should proceed according to current HPA guidelines [1]. Household contacts who have symptoms suggestive of pertussis should be advised to seek medical attention and to avoid visiting the case in hospital until they have been assessed and managed appropriately. PB64.01 (Oct 2012) Page 15
17 5. Scenario 1: Single case in a Healthcare Worker (Clinically suspected /epidemiologically linked or laboratory confirmed) Case finding and surveillance Inform the relevant Infection Prevention and Control Team (IPCT) and Occupational Health (OH). The IPCT and/or OH will be responsible for case finding and passive surveillance for early detection of any further cases. The IPCT and OH should be informed even if it is beyond 21 days of exposure and too late for post exposure prophylaxis. The priority is case finding and therefore in these circumstances a less specific case definition should be used; a low threshold is recommended for referral of symptomatic contacts to a clinician for assessment, and for reporting to the IPCT Risk assessment i. Does the HCW provide care to high risk patients, i.e. pregnant women or infants? ii. Has the HCW worked during the infectious period? iii. Can action be taken for contacts (as defined below in section 5.3) within 21 days of exposure to the HCW case? If yes to all of the above, then the need for wider chemoprophylaxis and vaccination should be considered. If not then inform and advise contacts with significant exposure to seek medical attention if they develop symptoms suggestive of pertussis. HCW contacts who develop symptoms should also inform OH. Identification of contacts in priority groups with significant exposure in the healthcare setting and provision of information to contacts identified in order to facilitate early diagnosis should be carried out even if it is too late for chemoprophylaxis Identification of contacts in the healthcare setting for post-exposure prophylaxis Healthcare Worker (HCW) contacts HCWs who had greater than 1 hour of cumulative contact at a distance of less than 2 metres (6.5 feet) with the affected HCW whilst infectious AND who will be working with infants and/or pregnant women providing direct clinical care in the subsequent 3 weeks need to be identified. 5 See Appendix 1 for flow chart, to be read in conjunction with the text in this document. PB64.01 (Oct 2012) Page 16
18 Patient contacts Patients exposed to the affected HCW during the infectious period should be identified from the following groups: Unimmunised and partially immunised infants who received direct clinical care from the infected HCW (for these groups the <2 metre/>1 hour definition may not be appropriate and prophylaxis should be considered regardless of duration of contact) Pregnant women (>32 weeks gestation) with significant exposure (<2 metre/>1 hour). Post exposure prophylaxis should be offered to those identified as recommended in section 7. Any other contacts identified that had significant exposure (<2 metre/>1 hour) but are not in priority groups (as defined in section 3), do not require prophylaxis. They should be informed and advised to seek early medical attention if they develop symptoms suggestive of pertussis. PB64.01 (Oct 2012) Page 17
19 6. Scenario 2: Single case in a hospitalised patient (Clinically suspected/ epidemiologically linked or laboratory confirmed) Case finding and surveillance Inform the relevant Infection Prevention and Control team (IPCT) and Occupational Health (OH). The IPCT and/or OH will be responsible for case finding and surveillance for early detection of any further cases. The priority is case finding and therefore in these circumstances a less specific case definition should be used; a low threshold is recommended for referral of symptomatic contacts to a clinician for assessment, and for reporting to the IPCT Risk assessment i. Can action be taken within 21 days of exposure? ii. Is there a possibility that infants, pregnant women (>32 weeks gestation) or HCWs working with either of these two groups have had significant exposure during the infectious period? (i.e. a period when respiratory isolation has not been implemented). If yes to both of the above, then the need for wider chemoprophylaxis and vaccination should be considered. If not then inform and advise contacts with significant exposure to seek early medical attention if they develop symptoms suggestive of pertussis. HCW contacts who develop symptoms should also inform OH. Identification of contacts in priority groups with significant exposure in the healthcare setting and provision of information to contacts identified in order to enable early diagnosis should be carried out even if it is too late for post-exposure prophylaxis Identification of contacts for post-exposure prophylaxis Significant exposure to other patients or staff will not occur where a case has been in respiratory isolation from the time of admission and in these circumstances no postexposure prophylaxis is required. Healthcare Worker (HCW) contacts HCWs with significant exposure (<2 metre/>1 hour) to the affected patient whilst infectious without wearing appropriate PPE AND who will be working with infants 6 See Appendix 1 for flowchart, to be read in conjunction with the text in this document. PB64.01 (Oct 2012) Page 18
20 and/or pregnant women providing direct clinical care in the subsequent 3 weeks need to be identified. Patient contacts Patients who had exposure to the index case during the infectious period should be identified from the following groups: All unimmunised and partially immunised infants admitted to the same bay should be offered prophylaxis Pregnant women (>32 weeks gestation) who have not received a booster dose of pertussis-containing vaccine more than 1 week and less than 5 years ago, with significant exposure to the index case (<2 metre/>1 hour) 6.4. Special Settings: Neonatal Intensive Care Unit (NICU)/Special Care Baby Unit (SCBU) Paediatric Intensive Care Unit (ITU)/ High Dependency Unit (HDU) setting For a child admitted to NICU/ SCBU/Paediatric HDU or ITU with pertussis, a risk assessment needs to be undertaken to determine the need for wider prophylaxis. This will include identifying whether the case was ventilated, if this was a closed or open circuit and whether there is a possibility of breaks in the ventilatory circuit leading to exposure risk. If in doubt, contact the local HPU 7 for assistance in risk assessment. If a risk is deemed to exist, given the vulnerability of the patient population, prophylaxis is recommended for all unimmunised and partially immunised infants in the same bay as the case (regardless of the time/duration of exposure). Chemoprophylaxis and/or vaccination should be offered to those identified as recommended in section 7. Any other contacts identified that had significant exposure (<2 metre/>1 hour) but are not in priority groups (as defined in section 3), do not need prophylaxis. They should be informed and advised to seek early medical advice if symptoms appear. Household contacts of cases who have symptoms suggestive of pertussis should be advised to seek medical attention and to avoid visiting the case in hospital until they have been assessed and managed appropriately. 7 (and after April 2013 the PHE Centre) PB64.01 (Oct 2012) Page 19
21 7. Chemoprophylaxis and Vaccination 7.1. Chemoprophylaxis Chemoprophylaxis should offered (based on recommendations in current HPA guidelines [1] to contacts identified with significant exposure in the following groups: Unimmunised and partially immunised infant contacts Pregnant women (>32 weeks gestation) who have not received a booster dose of pertussis-containing vaccine more than 1 week and less than 5 years ago. HCW contacts who have not received a booster dose of pertussis-containing vaccine more than 1 week and less than 5 years ago Vaccination Immunisation should be considered for those who have been offered chemoprophylaxis: Unimmunised and partially immunised infants should complete the schedule with the appropriate vaccine. A booster dose of pertussis containing vaccine is recommended for pregnant women (> 32 weeks gestation) and HCW contacts who have not received a dose of pertussis containing vaccine in the preceding 5 years and no Td-IPV in the preceding month. Chemoprophylaxis is not recommended for fully immunised infants (who have received 3 doses of pertussis-containing vaccine). PB64.01 (Oct 2012) Page 20
22 8. Surveillance OH and the relevant IPCT should be informed of any cases of pertussis occurring in healthcare settings. All contacts identified with significant exposure should be informed and advised of symptoms to enable early detection of illness. The IPCT/OH will need to undertake passive surveillance amongst staff and patients to ensure early detection and management of any further cases. This would normally be for a period of 42 days (two incubation periods) from onset of symptoms in the index case. te: Although chemoprophylaxis is likely to be more effective when implemented early, it is not 100% effective. Vaccination does not provide 100% protection against infection; immunity takes 1-2 weeks to develop and is known to wane over time. Therefore a diagnosis of pertussis should still be considered in an exposed individual who develops signs and symptoms compatible with pertussis, despite receiving chemoprophylaxis and despite previous vaccination. If further clinically suspected cases arise during the surveillance period (42 days from onset of index case), testing should be discussed with the Bordetella Reference Laboratory 8. However, public health action including exclusion and treatment of cases should proceed based on clinical diagnosis. 8 Laboratory confirmation and public health action Appropriate public health action should not wait for laboratory results as negative results cannot be used to exclude pertussis infection. At the request of the HPU, the Bordetella Reference Laboratory will prioritise testing for evidence of B. pertussis infection in support of outbreaks or incident investigations as appropriate. However, please note these services are not available outside of regular working hours. Please contact Microbiology Services (MS)-Colindale on and discuss with senior staff prior to sending specimens. PB64.01 (Oct 2012) Page 21
23 9. Two or more epidemiologically linked cases of pertussis in a healthcare setting If two or more confirmed or epidemiologically linked cases of pertussis occur in a healthcare setting, an outbreak control team should be convened. An appropriate outbreak control team is likely to include: Director of Infection Prevention and Control Hospital Microbiologist (if different) Infection control nurse Consultant/s from relevant clinical specialties Occupational health physician / nurse HPU representative Communications Outbreak Control Team (OCT) risk assessment should consider the following: i. Are any of the cases confirmed? ii. Is transmission likely to have occurred in the healthcare setting or in the community? iii. Is the transmission from HCW to HCW/HCW to patient/or patient to HCW? iv. What was the nature of contact between the cases? v. Is there a risk of ongoing transmission in the setting? Attempts should be made to confirm diagnosis following discussion with the Bordetella Reference Laboratory at Colindale 9. If transmission is most likely to have occurred within the healthcare setting, then in addition to the actions taken as per algorithms for single cases, consideration should be given to offering vaccination more widely. Active surveillance will need to be in place to detect new cases early to enable quick diagnosis, exclusion or isolation and treatment. The priority is active case finding and therefore in these circumstances a less specific case definition should be used to ensure no cases are missed. 9 Laboratory confirmation and public health action Appropriate public health action should not wait for laboratory results as negative results cannot be used to exclude pertussis infection. At the request of the HPU, the Bordetella Reference Laboratory will prioritise testing for evidence of B. pertussis infection in support of outbreaks or incident investigations as appropriate. However, please note these services are not available outside of regular working hours. Please contact Microbiology Services (MS)-Colindale on and discuss with senior staff prior to sending specimens. PB64.01 (Oct 2012) Page 22
24 Expert advice on outbreak investigation and response is available from the Immunisation, Hepatitis and Blood Safety Department, HPS-Colindale, HPA ( ) and on laboratory investigation from the Bordetella Reference Laboratory, MS-Colindale ( ). PB64.01 (Oct 2012) Page 23
25 10. References 1. HPA guidelines for the public health management of pertussis. ugh/guidelines/. 2. Infection control precautions to minimise transmission of seasonal influenza in the healthcare setting, Health Protection Agency Information on Droplet/Contact/Airborne Precautions. Health Protection Scotland. April 2009 Date of re-issue: April Immunisation against infectious disease "The Green Book" D.o. Health, Editor Leekha, S., R.L. Thompson, and P. Sampathkumar, Epidemiology and control of pertussis outbreaks in a tertiary care center and the resource consumption associated with these outbreaks. Infect Control Hosp Epidemiol, (5): p Bassinet, L., et al., socomial pertussis outbreak among adult patients and healthcare workers. Infect Control Hosp Epidemiol, (11): p Baugh, V. and N. McCarthy, Outbreak of Bordetella pertussis among oncology nurse specialists. Occup Med (Lond), (5): p Pascual, F.B., et al., Outbreak of pertussis among healthcare workers in a hospital surgical unit. Infect Control Hosp Epidemiol, (6): p Alexander, E.M., et al., Pertussis outbreak on a neonatal unit: identification of a healthcare worker as the likely source. J Hosp Infect, (2): p Paterson, J.M. and V. Sheppeard, socomial pertussis infection of infants: still a risk in Commun Dis Intell, (4): p Outbreaks of Pertussis Associated with Hospitals --- Kentucky, Pennsylvania, and Oregon, 2003 MMWR Weekly January 28, 2005 / 54(03); Tiwari, T., T.V. Murphy, and J. Moran, Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC Guidelines. MMWR Recomm Rep, (RR-14): p Sandora, T.J., C.A. Gidengil, and G.M. Lee, Pertussis vaccination for health care workers. Clin Microbiol Rev, (3): p Xie, X., et al., How far droplets can move in indoor environments--revisiting the Wells evaporation-falling curve. Indoor Air, (3): p Aintablian, N., P. Walpita, and M.H. Sawyer, Detection of Bordetella pertussis and respiratory synctial virus in air samples from hospital rooms. Infect Control Hosp Epidemiol, (12): p Dodhia, H. and E. Miller, Review of the evidence for the use of erythromycin in the management of persons exposed to pertussis. Epidemiol Infect, (2): p Campbell, H., et al., Accelerating control of pertussis in England and Wales. Emerg Infect Dis, (1): p Kirkland, K.B., et al., Kinetics of pertussis immune responses to tetanus-diphtheriaacellular pertussis vaccine in health care personnel: implications for outbreak control. Clin Infect Dis, (4): p Pertussis. National guidelines for public health units, Australia PB64.01 (Oct 2012) Page 24
26 11 Appendix Flowchart 1 Index Case is Healthcare Worker Clinically suspected/ epi linked or lab confirmed case of pertussis in a HCW Arrange lab testing to confirm (If required, public health action should not be delayed while waiting for results) Inform Occupational Health and Infection Control. Passive surveillance for further cases Worked while infectious Direct clinical contact with infants or pregnant women or HCWs working with these groups Exclude case until non infectious Manage as case as per standard national guidance Passive surveillance for new cases amongst staff Inform and advise Is action possible within 21 days of last exposure Significant contact (>1 hour at <2m) Exclude case until non infectious Manage case as per standard national guidance Exclude case until non infectious Manage case as per standard national guidance Passive case surveillance Inform and advise Exclude case until non infectious Manage case as per standard national guidance Exclude case until non infectious Manage case as per standard national guidance Patient Contacts Management of Contacts HCW Contacts Vulnerable contacts? not fully immunised infant or pregnant woman >32 weeks gestation Due to work with either infants or pregnant woman >32 weeks gestation Inform and advise contacts Passive case surveillance Significant Contact (>1 hour at <2m) [Less if young infants] Significant Contact (>1 hour at <2m) Significant Contact (>1 hour at <2m) Inform and advise contacts Passive case surveillance Offer chemoprophylaxis and vaccination action for contacts Inform and advise action for contacts Offer chemoprophylaxis and vaccination 25
27 11.2 Flowchart 2 Index Case is a hospitalised patient Clinically suspected/ epi linked or lab confirmed case of pertussis in an admitted patient Arrange lab testing to confirm (If required, public health action should not be delayed while waiting for results) Manage case as per national guidance Inform Occupational Health and Infection Control. Passive surveillance for further cases Was the patient in respiratory isolation during their admission while infectious? action for contacts Is action possible within 21 days of last exposure Inform and advise vulnerable contacts. Passive case surveillance Patient Contacts Management of Contacts HCW Contacts Vulnerable contacts? not fully immunised infant or pregnant woman >32 weeks gestation Due to work with either infants or pregnant woman >32 weeks gestation Inform and advise contacts Passive case surveillance Significant Contact (>1 hour at <2m) [Less if young infants](see main text for ICU/HDU) Significant Contact (>1 hour at <2m) Significant Contact (>1 hour at <2m) Inform and advise contacts Passive case surveillance Offer chemoprophylaxis and vaccination action for contacts Inform and advise Inform and advise Offer chemoprophylaxis and vaccination 26
28 12. Appendix Letter for parents/ guardians whose infant has been in contact with a case and does not require chemoprophylaxis Dear Parent/Guardian Your child has been identified as a contact of a case of whooping cough (pertussis) at XXX hospital/centre/practice. Whooping cough is a lung infection caused by Bordetella pertussis bacteria. Whooping cough usually begins like a common cold with a runny nose, low fever, sneezing and mild occasional coughing. Over the next one to two weeks, this progresses to fits of coughing which may be followed by choking and/or vomiting. The cough often comes in short bursts followed by a gasp for air (when the characteristic whooping noise may be made) and the feeling of not being able to catch your breath. Whooping cough doesn t always cause the typical whoop or vomiting after coughing. As your child has been in contact with a case, there is a small possibility that your child may develop whooping cough. If your child is well and has been fully immunised/ is over 1 year old (DELETE AS APPROPRIATE), the risk from the recent contact is low and, we would not recommend antibiotics at this stage based on the national guidance. Please check that their immunisations are up to date (if unsure, check in their red book or with your GP or health visitor) If your child is unwell or develops a cough illness in the next 3 weeks, please contact your general practitioner. They will arrange appropriate testing for whooping cough and management. Further information on pertussis is available from or Yours faithfully PB64.01 (Oct 2012) Page 27
29 12.2. Letter for parents/ guardians whose infant has been in contact with a case and requires immunisation / chemoprophylaxis Dear Parent/Guardian Your child has been identified as a contact of a case of whooping cough (pertussis) at XXX hospital/centre/practice. Whooping cough is a lung infection caused by Bordetella pertussis bacteria. Whooping cough usually begins like a common cold with a runny nose, low fever, sneezing and mild occasional coughing. Over the next one to two weeks, this progresses to fits of coughing which may be followed by choking and/or vomiting. The cough often comes in short bursts followed by a gasp for air (when the characteristic whooping noise may be made) and the feeling of not being able to catch your breath. Whooping cough doesn t always cause the typical whoop or vomiting after coughing. As your child has been in contact with a case, there is a possibility that your child may develop whooping cough. To reduce the risk, we recommend that your child has a short course of antibiotics. Your child should attend for their routine immunisations as usual as the vaccines offer long term protection for your child against whooping cough. If your child is over 4 months and has not received 3 doses of a pertussis containing vaccine (the normal schedule includes pertussis vaccines at 2, 3 and 4 months), we will arrange for your child to complete this schedule/ please contact your GP or health visitor. ADD DETAILS OF ARRANGEMENTS If your child is unwell or develops a cough illness in the next 3 weeks, please contact your general practitioner. They will arrange appropriate testing for pertussis and management. Further information on pertussis is available from or Yours faithfully PB64.01 (Oct 2012) Page 28
30 12.3. Letter for HCWs that have been in contact with a case but do not require immunisation / chemoprophylaxis Dear You have been identified as a contact of a case of whooping cough (pertussis). Although the risk that you will develop whooping cough is low, we are writing to you to provide information about whooping cough and what you should do in the unlikely event that you develop symptoms Whooping cough is a respiratory infection caused by Bordetella pertussis bacteria. Whooping cough usually begins like a common cold with a runny nose, low fever, sneezing and mild occasional coughing. Over the next one to two weeks, this progresses to fits of coughing which may be followed by choking and/or vomiting. The cough often comes in short bursts (paroxysms) followed by a gasp for air (when the characteristic whooping noise may be made) and the feeling of not being able to catch your breath. Whooping cough doesn t always cause the typical whoop or vomiting after coughing. Whooping cough most commonly affects infants and is most serious in this group. Whooping cough does, however, also occur in older children, adolescents and adults. Across the UK, the number of cases of whooping cough in these older age groups is currently high. If a healthcare worker develops whooping cough, they may transmit whooping cough to vulnerable patients, particularly young children and pregnant women. In line with national guidance, antibiotics and immunisation is being offered to those with close contact with the case and who are due to work with children or pregnant women in the next 3 weeks. Based on the information available to us, you are not part of this group and thus do not require antibiotics or immunisation as a result of the recent contact If you develop a cough illness in the next 3 weeks, please contact occupational health on XXXXXXXXXXX to ensure appropriate testing for whooping cough and management. Further information on whooping cough is available from or Yours faithfully Occupational Health PB64.01 (Oct 2012) Page 29
31 12.4 Letter for HCWs that have been in contact with a case and require immunisation / chemoprophylaxis Dear You have been identified as a contact of a case of whooping cough (pertussis). Although the risk that you will develop whooping cough is low, we are writing to provide you with information about the infection and how you can protect yourself and your patients. Whooping cough is a respiratory infection caused by Bordetella pertussis bacteria. Whooping cough usually begins like a common cold with a runny nose, low fever, sneezing and mild occasional coughing. Over the next one to two weeks, this progresses to fits of coughing which may be followed by choking and/or vomiting. The cough often comes in short bursts (paroxysms) followed by a gasp for air (when the characteristic whooping noise may be made) and the feeling of not being able to catch your breath. Whooping cough doesn t always cause the typical whoop or vomiting after coughing. Whooping cough most commonly affects infants and is most serious in this group. Whooping cough does, however, also occur in older children, adolescents and adults. Across the UK, the number of cases of whooping cough in these older age groups is currently high. If a healthcare worker develops whooping cough, they may transmit whooping cough to patients, particularly young children and pregnant women. As you have been in close contact with a case and you are due to work with infants or pregnant women in the next 3 weeks, we recommend that you have a short course of antibiotics and a booster dose of vaccine (if you have not had a pertussis vaccine in the last 5 years). ADD DETAILS OF ARRANGEMENTS If you develop a cough illness in the next 3 weeks, please contact occupational health on XXXXXXXXXXX to ensure appropriate testing for whooping cough and management. Further information on whooping cough is available from or Yours faithfully Occupational Health PB64.01 (Oct 2012) Page 30
PROTOCOL FOR THE MANAGEMENT OF CLOSE CONTACTS OF PERTUSSIS INFECTION
 PROTOCOL FOR THE MANAGEMENT OF CLOSE CONTACTS OF PERTUSSIS INFECTION Printed copies must not be considered the definitive version DOCUMENT CONTROL PROTOCOL NO. 1.03 Policy Group Infection Control Committee
PROTOCOL FOR THE MANAGEMENT OF CLOSE CONTACTS OF PERTUSSIS INFECTION Printed copies must not be considered the definitive version DOCUMENT CONTROL PROTOCOL NO. 1.03 Policy Group Infection Control Committee
The Newcastle upon Tyne Hospitals NHS Foundation Trust. Management and post-exposure prophylaxis for suspected cases of pertussis (Whooping Cough)
 The Newcastle upon Tyne Hospitals NHS Foundation Trust Management and post-exposure prophylaxis for suspected cases of pertussis (Whooping Cough) Version No.: 5.0 Effective From: 2 May 2013 Expiry Date:
The Newcastle upon Tyne Hospitals NHS Foundation Trust Management and post-exposure prophylaxis for suspected cases of pertussis (Whooping Cough) Version No.: 5.0 Effective From: 2 May 2013 Expiry Date:
Laboratory confirmation requires isolation of Bordetella pertussis or detection of B. pertussis nucleic acid, preferably from a nasopharyngeal swab.
 Pertussis Epidemiology in New Zealand New Zealand has continued to experience outbreaks of pertussis in recent decades. This is in part due to historically low immunisation rates and in part because immunity
Pertussis Epidemiology in New Zealand New Zealand has continued to experience outbreaks of pertussis in recent decades. This is in part due to historically low immunisation rates and in part because immunity
Pertussis Information for GPs and other Health Care Providers on Clinical and Public Health Management. March 2010
 Pertussis Information for GPs and other Health Care Providers on Clinical and Public Health Management March 2010 Infectious Agent Bordetella pertussis (a bacterium) Clinical Features Infants and Young
Pertussis Information for GPs and other Health Care Providers on Clinical and Public Health Management March 2010 Infectious Agent Bordetella pertussis (a bacterium) Clinical Features Infants and Young
PENNSYLVANIA DEPARTMENT OF HEALTH 2015 PAHAN 307 04-02-ADV Pertussis in Centre County
 PENNSYLVANIA DEPARTMENT OF HEALTH 2015 PAHAN 307 04-02-ADV Pertussis in Centre County DATE: 04/02/2015 TO: Health Alert Network FROM: Karen M. Murphy, PhD, RN, Acting Secretary of Health SUBJECT: DISTRIBUTION:
PENNSYLVANIA DEPARTMENT OF HEALTH 2015 PAHAN 307 04-02-ADV Pertussis in Centre County DATE: 04/02/2015 TO: Health Alert Network FROM: Karen M. Murphy, PhD, RN, Acting Secretary of Health SUBJECT: DISTRIBUTION:
Health Professionals Advice: Azithromycin now fully funded 3 December 2012
 Health Professionals Advice: Azithromycin now fully funded 3 December 2012 SITUATION UPDATE The Pertussis epidemic has continued all year, and is expected to continue throughout 2013. Between 1 January
Health Professionals Advice: Azithromycin now fully funded 3 December 2012 SITUATION UPDATE The Pertussis epidemic has continued all year, and is expected to continue throughout 2013. Between 1 January
Remove this cover sheet before redistributing and replace it with your own. Please ensure that DPHHS is included on your HAN distribution list.
 State of Montana Health Alert Network DPHHS HAN ADVISORY Cover Sheet DATE: May 15, 2012 SUBJECT: Pertussis INSTRUCTIONS: DISTRIBUTE to your local HAN contacts. This HAN is intended for general sharing
State of Montana Health Alert Network DPHHS HAN ADVISORY Cover Sheet DATE: May 15, 2012 SUBJECT: Pertussis INSTRUCTIONS: DISTRIBUTE to your local HAN contacts. This HAN is intended for general sharing
PERTUSSIS SURVEILLANCE AND RESPONSE PROTOCOL
 PERTUSSIS SURVEILLANCE AND RESPONSE PROTOCOL Public Health Action 1. Educate the public, particularly parents of infants, about the dangers of whooping cough and the advantages of initiating immunization
PERTUSSIS SURVEILLANCE AND RESPONSE PROTOCOL Public Health Action 1. Educate the public, particularly parents of infants, about the dangers of whooping cough and the advantages of initiating immunization
HPA Guidelines for the Public Health Management of Pertussis
 HPA Guidelines for the Public Health Management of Pertussis Author Gayatri Amirthalingam and the Pertussis Guidelines Group Pertussis Guidelines Group Gayatri Amirthalingam Consultant Epidemiologist,
HPA Guidelines for the Public Health Management of Pertussis Author Gayatri Amirthalingam and the Pertussis Guidelines Group Pertussis Guidelines Group Gayatri Amirthalingam Consultant Epidemiologist,
FREQUENTLY ASKED QUESTIONS ABOUT PERTUSSIS (WHOOPING COUGH)
 FREQUENTLY ASKED QUESTIONS ABOUT PERTUSSIS (WHOOPING COUGH) What is pertussis? General Questions About Pertussis Pertussis, or whooping cough, is a contagious illness that is spread when an infected person
FREQUENTLY ASKED QUESTIONS ABOUT PERTUSSIS (WHOOPING COUGH) What is pertussis? General Questions About Pertussis Pertussis, or whooping cough, is a contagious illness that is spread when an infected person
TEMPORARY PROGRAMME PERTUSSIS VACCINATION FOR PREGNANT WOMEN
 TEMPORARY PROGRAMME PERTUSSIS VACCINATION FOR PREGNANT WOMEN Richard Smithson Neil Irvine Maureen McCartney Consultant Health Protection October 2012 Pertussis/whooping cough The disease Whooping Cough
TEMPORARY PROGRAMME PERTUSSIS VACCINATION FOR PREGNANT WOMEN Richard Smithson Neil Irvine Maureen McCartney Consultant Health Protection October 2012 Pertussis/whooping cough The disease Whooping Cough
Pertussis (whooping cough) immunisation for pregnant women
 Factsheet September 2012 Pertussis (whooping cough) immunisation for pregnant women Immunisation DEPARTMENT OF HEALTH Rheynn Slaynt The routine childhood immunisation programme has been very effective
Factsheet September 2012 Pertussis (whooping cough) immunisation for pregnant women Immunisation DEPARTMENT OF HEALTH Rheynn Slaynt The routine childhood immunisation programme has been very effective
Alberta Health and Wellness Public Health Notifiable Disease Management Guidelines August 2011
 August 2011 Pertussis Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) August 2011 August 2011 December 2005 Case Definition
August 2011 Pertussis Revision Dates Case Definition Reporting Requirements Remainder of the Guideline (i.e., Etiology to References sections inclusive) August 2011 August 2011 December 2005 Case Definition
Pertussis (whooping cough) immunisation for pregnant women the safest way to protect yourself and your baby
 Factsheet September 2012 Pertussis (whooping cough) immunisation for pregnant women the safest way to protect yourself and your baby The routine childhood immunisation programme has been very effective
Factsheet September 2012 Pertussis (whooping cough) immunisation for pregnant women the safest way to protect yourself and your baby The routine childhood immunisation programme has been very effective
What is whooping cough. (pertussis)? Information and Prevention. Ocument dn
 What is whooping cough Ocument dn (pertussis)? Information and Prevention IMPORTANT Pertussis (or whooping cough) is a highly contagious infection that can cause uncontrollable, violent coughing. If you
What is whooping cough Ocument dn (pertussis)? Information and Prevention IMPORTANT Pertussis (or whooping cough) is a highly contagious infection that can cause uncontrollable, violent coughing. If you
Whooping cough and pregnancy
 Whooping cough and pregnancy Your questions answered on how to help protect your baby 2014 edition the safest way to protect yourself and your baby 1 There is a lot of whooping cough around at the moment
Whooping cough and pregnancy Your questions answered on how to help protect your baby 2014 edition the safest way to protect yourself and your baby 1 There is a lot of whooping cough around at the moment
APIC Practice Guidance Committee: Implementation Insights Prevention & Control of Pertussis
 1275 K Street, NW, Suite 1000 Washington, DC 20005-4006 Phone: 202/789-1890 Fax: 202/789-1899 apicinfo@apic.org www.apic.org APIC Practice Guidance Committee: Implementation Insights Prevention & Control
1275 K Street, NW, Suite 1000 Washington, DC 20005-4006 Phone: 202/789-1890 Fax: 202/789-1899 apicinfo@apic.org www.apic.org APIC Practice Guidance Committee: Implementation Insights Prevention & Control
Prevention of Pertussis Among Pregnant & Post Partum Women and Their Infants. Marilyn Michels RN MSN CIC Kathleen Curtis MS RN
 Prevention of Pertussis Among Pregnant & Post Partum Women and Their Infants Marilyn Michels RN MSN CIC Kathleen Curtis MS RN Pertussis and Adults Pertussis (whooping cough) a poorly controlled vaccine-preventable
Prevention of Pertussis Among Pregnant & Post Partum Women and Their Infants Marilyn Michels RN MSN CIC Kathleen Curtis MS RN Pertussis and Adults Pertussis (whooping cough) a poorly controlled vaccine-preventable
Whooping Cough Vaccine for Pregnant Women
 Whooping Cough Vaccine for Pregnant Women What is whooping cough (pertussis)? Whooping cough (also known as pertussis) is a highly contagious illness that can be life threatening. The disease is most serious
Whooping Cough Vaccine for Pregnant Women What is whooping cough (pertussis)? Whooping cough (also known as pertussis) is a highly contagious illness that can be life threatening. The disease is most serious
Factsheet September 2012. Pertussis immunisation for pregnant women. Introduction
 Factsheet September 2012 Pertussis immunisation for pregnant women Introduction The routine childhood immunisation programme has been very effective in reducing the overall numbers of cases of pertussis.
Factsheet September 2012 Pertussis immunisation for pregnant women Introduction The routine childhood immunisation programme has been very effective in reducing the overall numbers of cases of pertussis.
Vaccination against pertussis (whooping cough) - the replacement of Repevax with Boostrix -IPV an update for registered healthcare practitioners
 Vaccination against pertussis (whooping cough) - the replacement of Repevax with Boostrix -IPV an update for registered healthcare practitioners Questions and Answers May 2014 Health Protection Scotland
Vaccination against pertussis (whooping cough) - the replacement of Repevax with Boostrix -IPV an update for registered healthcare practitioners Questions and Answers May 2014 Health Protection Scotland
Whooping cough (Pertussis) Information for you
 Whooping cough (Pertussis) Information for you Visit our website: www.nhsaaa.net All our publications are available in other formats ...Information for you...information for you...information for you.
Whooping cough (Pertussis) Information for you Visit our website: www.nhsaaa.net All our publications are available in other formats ...Information for you...information for you...information for you.
New Jersey Department of Health Vaccine Preventable Disease Program Pertussis FAQs. Date: January 10, 2013
 New Jersey Department of Health Vaccine Preventable Disease Program Pertussis FAQs Date: January 10, 2013 KEY MESSAGES: 1. Pertussis is a very contagious vaccine-preventable disease that can cause serious
New Jersey Department of Health Vaccine Preventable Disease Program Pertussis FAQs Date: January 10, 2013 KEY MESSAGES: 1. Pertussis is a very contagious vaccine-preventable disease that can cause serious
Frequently asked questions about whooping cough (pertussis)
 Frequently asked questions about whooping cough (pertussis) About whooping cough What is whooping cough? Whooping cough is a highly contagious illness caused by bacteria. It mainly affects the respiratory
Frequently asked questions about whooping cough (pertussis) About whooping cough What is whooping cough? Whooping cough is a highly contagious illness caused by bacteria. It mainly affects the respiratory
Vaccination against pertussis (Whooping cough) for pregnant women- 2014. Information for healthcare professionals
 Vaccination against pertussis (Whooping cough) for pregnant women- 2014 Information for healthcare professionals About Public Health England Public Health England s mission is to protect and improve the
Vaccination against pertussis (Whooping cough) for pregnant women- 2014 Information for healthcare professionals About Public Health England Public Health England s mission is to protect and improve the
I am reaching out to you with some preventative information that you might be interested in sharing with your school community.
 Reported cases of pertussis (whooping cough) are on the rise in Colorado and El Paso County. As of October 23, more than 1,000 cases of pertussis have been reported across the state. El Paso County cases
Reported cases of pertussis (whooping cough) are on the rise in Colorado and El Paso County. As of October 23, more than 1,000 cases of pertussis have been reported across the state. El Paso County cases
Pertussis: halting the epidemic by protecting infants. 34 BPJ Issue 51
 Pertussis: halting the epidemic by protecting infants 34 BPJ Issue 51 Infants who have not yet completed the National Immunisation Schedule and children who are not immunised, or only partially immunised
Pertussis: halting the epidemic by protecting infants 34 BPJ Issue 51 Infants who have not yet completed the National Immunisation Schedule and children who are not immunised, or only partially immunised
A review of the public health response to two pertussis outbreaks in Maryland: Lessons Learned
 A review of the public health response to two pertussis outbreaks in Maryland: Lessons Learned Presenter: Kristen George MPH Candidate Johns Hopkins University Bloomberg School of Public Health Preceptor:
A review of the public health response to two pertussis outbreaks in Maryland: Lessons Learned Presenter: Kristen George MPH Candidate Johns Hopkins University Bloomberg School of Public Health Preceptor:
Pertussis Whooping Cough Update
 May 3, 1 Dear Kitsap County Providers, This is an update about our current status of pertussis cases here in Kitsap County. We currently have 35 cases as outlined below. See the epidemiology curve (Figure
May 3, 1 Dear Kitsap County Providers, This is an update about our current status of pertussis cases here in Kitsap County. We currently have 35 cases as outlined below. See the epidemiology curve (Figure
Information on Measles and Whooping Cough: Vaccination and Disease
 Information on Measles and Whooping Cough: Vaccination and Disease Vaccine s Mechanism of Action Vaccines expose the recipient to a small amount of an weakened organism. Through this exposure, the body
Information on Measles and Whooping Cough: Vaccination and Disease Vaccine s Mechanism of Action Vaccines expose the recipient to a small amount of an weakened organism. Through this exposure, the body
Whooping cough and pregnancy
 Whooping cough and pregnancy Your questions answered on how to help protect your baby the safest way to protect yourself and your baby There is a lot of whooping cough around at the moment and babies who
Whooping cough and pregnancy Your questions answered on how to help protect your baby the safest way to protect yourself and your baby There is a lot of whooping cough around at the moment and babies who
NSW Pertussis Control Program 2015
 NSW Pertussis Control Program 2015 Pertussis Disease Pertussis ( whooping cough ) is a bacterial infection affecting the respiratory system, caused by the organism Bordetella pertussis It affects individuals
NSW Pertussis Control Program 2015 Pertussis Disease Pertussis ( whooping cough ) is a bacterial infection affecting the respiratory system, caused by the organism Bordetella pertussis It affects individuals
The Reality Pertussis can be a serious illness, part icularly for babies and young children.
 Sounds of Pertussis Pertussis, also known as whooping cough, is a poten tially deadly infection that can strike at any age, but is particularly dangerous for babies. The sounds of pertussis are like no
Sounds of Pertussis Pertussis, also known as whooping cough, is a poten tially deadly infection that can strike at any age, but is particularly dangerous for babies. The sounds of pertussis are like no
Community Health Administration
 Community Health Administration 300 North San Antonio Road Santa Barbara, CA 93110-1332 805/681-5439 FAX 805/681-5200 Takashi M. Wada, MD, MPH Director/Health Officer Anne M. Fearon Deputy Director Suzanne
Community Health Administration 300 North San Antonio Road Santa Barbara, CA 93110-1332 805/681-5439 FAX 805/681-5200 Takashi M. Wada, MD, MPH Director/Health Officer Anne M. Fearon Deputy Director Suzanne
Parents and Grandparents
 Parents and Grandparents Vaccination for parents and grandparents is vital to help protect newborn babies against whooping cough. Protect your baby by being immunised too. To find out more about Boostrix
Parents and Grandparents Vaccination for parents and grandparents is vital to help protect newborn babies against whooping cough. Protect your baby by being immunised too. To find out more about Boostrix
BE SURE. BE SAFE. VACCINATE.
 DON T GET OR GIVE THE FLU THIS YEAR THANK YOU Vaccination is the only protection. www.immunisation.ie BE SURE. BE SAFE. VACCINATE. FLU VACCINE 2013-2014 Healthcare workers prevent the spread of flu and
DON T GET OR GIVE THE FLU THIS YEAR THANK YOU Vaccination is the only protection. www.immunisation.ie BE SURE. BE SAFE. VACCINATE. FLU VACCINE 2013-2014 Healthcare workers prevent the spread of flu and
VARICELLA ZOSTER (VZ) VIRUS, CHICKENPOX & SHINGLES GUIDANCE
 VARICELLA ZOSTER (VZ) VIRUS, CHICKENPOX & SHINGLES GUIDANCE Summary This guidance provides background information on varicella zoster (VZ), chickenpox and shingles and sets out the infection control measures
VARICELLA ZOSTER (VZ) VIRUS, CHICKENPOX & SHINGLES GUIDANCE Summary This guidance provides background information on varicella zoster (VZ), chickenpox and shingles and sets out the infection control measures
Recommendations for the Prevention and Control of Influenza in Nursing Homes Virginia Department of Health
 Recommendations for the Prevention and Control of Influenza in Nursing Homes Virginia Department of Health Settings such as nursing homes that house persons at high risk for influenza-related complications
Recommendations for the Prevention and Control of Influenza in Nursing Homes Virginia Department of Health Settings such as nursing homes that house persons at high risk for influenza-related complications
MANAGEMENT OF TUBERCULOSIS IN PRISONS: Guidance for prison healthcare teams
 MANAGEMENT OF TUBERCULOSIS IN PRISONS: Guidance for prison healthcare teams Document control Title Type Author/s Management of tuberculosis in prisons: Guidance for prison healthcare teams Operational
MANAGEMENT OF TUBERCULOSIS IN PRISONS: Guidance for prison healthcare teams Document control Title Type Author/s Management of tuberculosis in prisons: Guidance for prison healthcare teams Operational
Pertussis. Chapter 15 Pertussis. August 2015. Pertussis. Vaccine introduced in 1952/53 (DTP) and 1996 (DTaP) NOTIFIABLE
 Chapter 15 15 Vaccine introduced in 1952/53 (DTP) and 1996 (DTaP) NOTIFIABLE In some circumstances, advice in these guidelines may differ from that in the Summary of Product Characteristics of the vaccines.
Chapter 15 15 Vaccine introduced in 1952/53 (DTP) and 1996 (DTaP) NOTIFIABLE In some circumstances, advice in these guidelines may differ from that in the Summary of Product Characteristics of the vaccines.
HPA National Measles Guidelines Local & Regional Services
 HPA National Measles Guidelines Local & Regional Services Version 1.2: 28 th October 2010 1 Table of Contents Title: Page No: 1. INTRODUCTION 3 2. RATIONALE FOR PUBLIC HEALTH ACTION...3 3. SURVEILLANCE
HPA National Measles Guidelines Local & Regional Services Version 1.2: 28 th October 2010 1 Table of Contents Title: Page No: 1. INTRODUCTION 3 2. RATIONALE FOR PUBLIC HEALTH ACTION...3 3. SURVEILLANCE
ECDC INTERIM GUIDANCE
 ECDC INTERIM GUIDANCE Interim ECDC public health guidance on case and contact management for the new influenza A(H1N1) virus infection Version 3, 19 May 2009 ECDC intends to produce a series of interim
ECDC INTERIM GUIDANCE Interim ECDC public health guidance on case and contact management for the new influenza A(H1N1) virus infection Version 3, 19 May 2009 ECDC intends to produce a series of interim
Tdap booster vaccine for Health Care Workers Frequently Asked Questions for Health Professionals
 Tdap booster vaccine for Health Care Workers Frequently Asked Questions for Health Professionals NEW items in 2013 Immunisation Guidelines for Ireland are in RED What is Tdap booster vaccine? Tdap is a
Tdap booster vaccine for Health Care Workers Frequently Asked Questions for Health Professionals NEW items in 2013 Immunisation Guidelines for Ireland are in RED What is Tdap booster vaccine? Tdap is a
Pertussis Toolkit for Schools
 Pertussis Toolkit for Schools Cerro Gordo County experienced a significant pertussis outbreak in 2012. To lessen the effects of future outbreaks, several community partners worked together to improve our
Pertussis Toolkit for Schools Cerro Gordo County experienced a significant pertussis outbreak in 2012. To lessen the effects of future outbreaks, several community partners worked together to improve our
Updated Recommendations for Use of Tdap in Pregnant Women
 From the Texas Department of State Health Services Immunization Branch The goal of the Vaccine Advisory is to disseminate, in a timely manner, practical information related to vaccines, vaccine-preventable
From the Texas Department of State Health Services Immunization Branch The goal of the Vaccine Advisory is to disseminate, in a timely manner, practical information related to vaccines, vaccine-preventable
Pertussis and Pregnancy
 Pertussis and Pregnancy Protect Your Patients From Pertussis (Whooping Cough) 1 Pertussis Video https://www.youtube.com/watch?v=f1g5woy5qce#a ction=share 2 1 Class Objectives By the end of this class the
Pertussis and Pregnancy Protect Your Patients From Pertussis (Whooping Cough) 1 Pertussis Video https://www.youtube.com/watch?v=f1g5woy5qce#a ction=share 2 1 Class Objectives By the end of this class the
Summary of the risk management plan (RMP) for Tritanrix HB [Diphtheria, tetanus, pertussis (whole cell) and hepatitis B (rdna) vaccine (adsorbed)]
![Summary of the risk management plan (RMP) for Tritanrix HB [Diphtheria, tetanus, pertussis (whole cell) and hepatitis B (rdna) vaccine (adsorbed)] Summary of the risk management plan (RMP) for Tritanrix HB [Diphtheria, tetanus, pertussis (whole cell) and hepatitis B (rdna) vaccine (adsorbed)]](/thumbs/27/12172665.jpg) EMA/14365/2014 Summary of the risk management plan (RMP) for Tritanrix HB [Diphtheria, tetanus, pertussis (whole cell) and hepatitis B (rdna) vaccine (adsorbed)] Overview of disease epidemiology Diphtheria
EMA/14365/2014 Summary of the risk management plan (RMP) for Tritanrix HB [Diphtheria, tetanus, pertussis (whole cell) and hepatitis B (rdna) vaccine (adsorbed)] Overview of disease epidemiology Diphtheria
Illinois Long Term Care Facilities and Assisted Living Facilities
 TO: FROM: RE: Illinois Long Term Care Facilities and Assisted Living Facilities Richard Dees, Chief, Bureau of Long Term Care Karen McMahon, Immunization Section Chief Craig Conover, MD, Medical Director,
TO: FROM: RE: Illinois Long Term Care Facilities and Assisted Living Facilities Richard Dees, Chief, Bureau of Long Term Care Karen McMahon, Immunization Section Chief Craig Conover, MD, Medical Director,
Pertussis or Whooping Cough
 The Emily Center Diagnosis/Disease/Illness Este folleto se encuentra traducido al español. Solicitelo al enfermero o médico. #1336 Name of Child: Date: What is pertussis? Pertussis, also called whooping
The Emily Center Diagnosis/Disease/Illness Este folleto se encuentra traducido al español. Solicitelo al enfermero o médico. #1336 Name of Child: Date: What is pertussis? Pertussis, also called whooping
INCREASED INCIDENCE OF PERTUSSIS (WHOOPING COUGH) IN MARATHON COUNTY
 November 25, 2013 INCREASED INCIDENCE OF PERTUSSIS (WHOOPING COUGH) IN MARATHON COUNTY Since November 1, 2013, Marathon County Health Department has investigated 20 cases of laboratory confirmed pertussis
November 25, 2013 INCREASED INCIDENCE OF PERTUSSIS (WHOOPING COUGH) IN MARATHON COUNTY Since November 1, 2013, Marathon County Health Department has investigated 20 cases of laboratory confirmed pertussis
Is your family at risk for pertussis?
 Is your family at risk for pertussis? Help protect your family against a serious disease and talk to your health-care professional about adult and adolescent immunization with Adacel vaccine. Please click
Is your family at risk for pertussis? Help protect your family against a serious disease and talk to your health-care professional about adult and adolescent immunization with Adacel vaccine. Please click
Pertussis in Babies - 2011
 Guidelines for the Public Health Management of Pertussis Public Health Medicine Communicable Disease Group HSE October 2013 Version Date Originator Reviewer Comment 1.0 August 2012 Pertussis Subgroup of
Guidelines for the Public Health Management of Pertussis Public Health Medicine Communicable Disease Group HSE October 2013 Version Date Originator Reviewer Comment 1.0 August 2012 Pertussis Subgroup of
Whooping cough. If you are pregnant you should get vaccinated to protect your baby
 Whooping cough If you are pregnant you should get vaccinated to protect your baby Cases of whooping cough are on the increase by getting the vaccine while pregnant you can protect your baby In the last
Whooping cough If you are pregnant you should get vaccinated to protect your baby Cases of whooping cough are on the increase by getting the vaccine while pregnant you can protect your baby In the last
New Jersey Department of Health and Senior Services Communicable Disease Service Vaccine Preventable Disease Program (VPDP)
 Pertussis (Whooping Cough) Control Guidelines Infectious Agent: Bordetella pertussis Clinical Manifestations: Fever is usually mild. Children and infants are most severely affected. Older adolescents and
Pertussis (Whooping Cough) Control Guidelines Infectious Agent: Bordetella pertussis Clinical Manifestations: Fever is usually mild. Children and infants are most severely affected. Older adolescents and
safest place for your baby is in your arms...
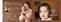 Help protect your baby by helping to protect yourself. Photo: John Bentham Imagine a cough barreling through your infant s body at up to 100 mph. Help protect yourself and your family by getting an adult
Help protect your baby by helping to protect yourself. Photo: John Bentham Imagine a cough barreling through your infant s body at up to 100 mph. Help protect yourself and your family by getting an adult
Why Pertussis matters..
 Why Pertussis matters.. Pertussis (Whooping cough) outbreaks continue to impact children & adults, both locally and across the US. Cases have been on the rise since the 1980 s. However, recent data on
Why Pertussis matters.. Pertussis (Whooping cough) outbreaks continue to impact children & adults, both locally and across the US. Cases have been on the rise since the 1980 s. However, recent data on
Adult Vaccination Frequently Asked Questions: The Basics
 The Basics Why should I get vaccinated? Vaccination is the best way to protect against infections that can make you sick and be passed on to those around you. 1 What kinds of side effects will I get from
The Basics Why should I get vaccinated? Vaccination is the best way to protect against infections that can make you sick and be passed on to those around you. 1 What kinds of side effects will I get from
Whooping Cough. The Lungs Whooping cough is an infection of the lungs and breathing tubes, both of which are parts of the respiratory system.
 Whooping Cough Introduction Whooping cough is a serious bacterial infection of the lungs and breathing tubes. It is also called pertussis. About 16 million cases of whooping cough happen worldwide each
Whooping Cough Introduction Whooping cough is a serious bacterial infection of the lungs and breathing tubes. It is also called pertussis. About 16 million cases of whooping cough happen worldwide each
SWINE FLU: FROM CONTAINMENT TO TREATMENT
 SWINE FLU: FROM CONTAINMENT TO TREATMENT SWINE FLU: FROM CONTAINMENT TO TREATMENT INTRODUCTION As Swine Flu spreads and more people start to catch it, it makes sense to move from intensive efforts to contain
SWINE FLU: FROM CONTAINMENT TO TREATMENT SWINE FLU: FROM CONTAINMENT TO TREATMENT INTRODUCTION As Swine Flu spreads and more people start to catch it, it makes sense to move from intensive efforts to contain
2 P age. Babies from Birth to Age 2
 Contents Babies from Birth to Age 2... 2 Vaccines give parents the power... 2 Vaccines are recommended throughout our lives... 3 Talk to your doctor... 3 Vaccines are very safe... 3 Whooping Cough (Pertussis)...
Contents Babies from Birth to Age 2... 2 Vaccines give parents the power... 2 Vaccines are recommended throughout our lives... 3 Talk to your doctor... 3 Vaccines are very safe... 3 Whooping Cough (Pertussis)...
The flu vaccination WINTER 2016/17. Who should have it and why. Flu mmunisation 2016/17
 The flu vaccination WINTER 2016/17 Who should have it and why Flu mmunisation 2016/17 The flu vaccination 1 Winter 2016/17 Helping to protect everyone, every winter This leaflet explains how you can help
The flu vaccination WINTER 2016/17 Who should have it and why Flu mmunisation 2016/17 The flu vaccination 1 Winter 2016/17 Helping to protect everyone, every winter This leaflet explains how you can help
INFECTION CONTROL MANUAL
 Page 1 of 19 Key Words: staff, communicable diseases, diseases, infectious diseases Policy Applies to: All staff employed by Mercy Hospital, Credentialed Specialists and Allied Health Professionals involved
Page 1 of 19 Key Words: staff, communicable diseases, diseases, infectious diseases Policy Applies to: All staff employed by Mercy Hospital, Credentialed Specialists and Allied Health Professionals involved
2 months Diptheria; Tetanus; Whooping Cough; Hib & Polio 1st dose Pneumococcal Conjugate Vaccination
 IMMUNISATIONS You may want to know if your child should have routine immunisations and whether there could be an increased risk of complications because of the heart condition. We have sought the opinions
IMMUNISATIONS You may want to know if your child should have routine immunisations and whether there could be an increased risk of complications because of the heart condition. We have sought the opinions
Pertussis 1. DI PROTOCOL CHECKLIST
 1. DI PROTOCOL CHECKLIST Enter available information into Merlin upon receipt of initial report Review background on disease, case definition and laboratory testing Contact provider to acquire medical
1. DI PROTOCOL CHECKLIST Enter available information into Merlin upon receipt of initial report Review background on disease, case definition and laboratory testing Contact provider to acquire medical
Protecting your baby against meningitis and septicaemia
 Protecting your baby against meningitis and septicaemia caused by meningococcal B bacteria MenB vaccine now available! Information about the MenB vaccine and recommended paracetamol use mmunisation The
Protecting your baby against meningitis and septicaemia caused by meningococcal B bacteria MenB vaccine now available! Information about the MenB vaccine and recommended paracetamol use mmunisation The
Vaccines in Pregnancy MARK H. SAWYER, MD UCSD SCHOOL OF MEDICINE RADY CHILDREN S HOSPITAL SAN DIEGO
 Vaccines in Pregnancy MARK H. SAWYER, MD UCSD SCHOOL OF MEDICINE RADY CHILDREN S HOSPITAL SAN DIEGO 1 Objectives List vaccines that should be given either during pregnancy or immediately post-partum in
Vaccines in Pregnancy MARK H. SAWYER, MD UCSD SCHOOL OF MEDICINE RADY CHILDREN S HOSPITAL SAN DIEGO 1 Objectives List vaccines that should be given either during pregnancy or immediately post-partum in
Pertussis (Whooping Cough) http://www.cdc.gov/pertussis/index.html. Causes and Transmission. Causes. Transmission
 Pertussis (Whooping Cough) http://www.cdc.gov/pertussis/index.html Pertussis, also known as whooping cough, is a highly contagious respiratory disease. It is caused by the bacterium Bordetella pertussis.
Pertussis (Whooping Cough) http://www.cdc.gov/pertussis/index.html Pertussis, also known as whooping cough, is a highly contagious respiratory disease. It is caused by the bacterium Bordetella pertussis.
Pertussis: Resurgence, Prevention and Role of the Ob/Gyn
 Pertussis: Resurgence, Prevention and Role of the Ob/Gyn Kimberly B. Fortner, MD Vanderbilt University Department OB/Gyn Division Maternal-Fetal Medicine Vanderbilt Vaccine Research Program Learning Objectives
Pertussis: Resurgence, Prevention and Role of the Ob/Gyn Kimberly B. Fortner, MD Vanderbilt University Department OB/Gyn Division Maternal-Fetal Medicine Vanderbilt Vaccine Research Program Learning Objectives
Pertussis Yukon Communicable Disease Control 4 Hospital Rd., Whitehorse, YT Y1A 3H8 Phone: (867) 667-8323 Fax: (867) 667-8349 May 2015
 TABLE OF CONTENTS 1.0 AUTHORITY... 2 2.0 GOAL... 2 3.0 PERTUSSIS FLOW CHART... 3 4.0 CASE MANAGEMENT... 4 4.1 Confirm the Diagnosis... 4 4.2 Laboratory Testing... 5 4.3 Case History... 5 4.4 Case Treatment...
TABLE OF CONTENTS 1.0 AUTHORITY... 2 2.0 GOAL... 2 3.0 PERTUSSIS FLOW CHART... 3 4.0 CASE MANAGEMENT... 4 4.1 Confirm the Diagnosis... 4 4.2 Laboratory Testing... 5 4.3 Case History... 5 4.4 Case Treatment...
Childhood Diseases and potential risks during pregnancy: (All information available on the March of Dimes Web Site.) http://www.modimes.
 Childhood Diseases and potential risks during pregnancy: (All information available on the March of Dimes Web Site.) http://www.modimes.org/ Fifth disease (erythema infectiosum) is a common, mild, childhood
Childhood Diseases and potential risks during pregnancy: (All information available on the March of Dimes Web Site.) http://www.modimes.org/ Fifth disease (erythema infectiosum) is a common, mild, childhood
2. THE DISEASE AND ITS EPIDEMIOLOGY
 1. DISEASE REPORTING 1.1 Purpose of Reporting and Surveillance 1. To prevent illness and death among exposed, high risk persons. 2. To vaccinate exposed, under immunized children. Pertussis 3. To educate
1. DISEASE REPORTING 1.1 Purpose of Reporting and Surveillance 1. To prevent illness and death among exposed, high risk persons. 2. To vaccinate exposed, under immunized children. Pertussis 3. To educate
Communicable Disease Control Chapter I Management of Specific Diseases Pertussis June 2010
 TABLE OF CONTENTS 1.0 AUTHORITY...1 2.0 GOAL...1 3.0 PERTUSSIS FLOW CHART...2 4.0 CASE IDENTIFICATION...3 4.1 CONFIRM THE DIAGNOSIS...3 5.0 CASE MANAGEMENT...4 5.1 LABORATORY TESTING...4 5.2 CASE HISTORY...4
TABLE OF CONTENTS 1.0 AUTHORITY...1 2.0 GOAL...1 3.0 PERTUSSIS FLOW CHART...2 4.0 CASE IDENTIFICATION...3 4.1 CONFIRM THE DIAGNOSIS...3 5.0 CASE MANAGEMENT...4 5.1 LABORATORY TESTING...4 5.2 CASE HISTORY...4
Tuberculosis Exposure Control Plan for Low Risk Dental Offices
 Tuberculosis Exposure Control Plan for Low Risk Dental Offices A. BACKGROUND According to the CDC, approximately one-third of the world s population, almost two billion people, are infected with tuberculosis.
Tuberculosis Exposure Control Plan for Low Risk Dental Offices A. BACKGROUND According to the CDC, approximately one-third of the world s population, almost two billion people, are infected with tuberculosis.
Know the Symptoms. Call Your Doctor, Treat Whooping Gough Early. Whooping Cough Signs and Symptoms. Whooping Cough Shots Prevent the Disease
 What ls Whooping Cough? Whooping cough disease (also called pertussis) causes coughing fits that make it hard to breathe. It spreads easily when someone with the disease coughs or sneezes. It can kill
What ls Whooping Cough? Whooping cough disease (also called pertussis) causes coughing fits that make it hard to breathe. It spreads easily when someone with the disease coughs or sneezes. It can kill
CATHOLIC CHARITIES MAINE FAMILY CHILD CARE
 CATHOLIC CHARITIES MAINE FAMILY CHILD CARE October 2012 In accordance with Federal law and U.S. Department of Agriculture policy, this institution is prohibited from discriminating on the basis of race,
CATHOLIC CHARITIES MAINE FAMILY CHILD CARE October 2012 In accordance with Federal law and U.S. Department of Agriculture policy, this institution is prohibited from discriminating on the basis of race,
Pertussis. 1. DISEASE REPORTING A. Purpose of Reporting and Surveillance
 1. DISEASE REPORTING A. Purpose of Reporting and Surveillance Pertussis 1. To prevent illness and death, particularly among infants younger than 1 year, and among persons who may transmit pertussis to
1. DISEASE REPORTING A. Purpose of Reporting and Surveillance Pertussis 1. To prevent illness and death, particularly among infants younger than 1 year, and among persons who may transmit pertussis to
swine flu vaccination:
 swine flu vaccination: what you need to know Flu. Protect yourself and others. Contents What is swine flu?............... 3 About the swine flu vaccine....... 4 What else do I need to know?...... 8 What
swine flu vaccination: what you need to know Flu. Protect yourself and others. Contents What is swine flu?............... 3 About the swine flu vaccine....... 4 What else do I need to know?...... 8 What
Vaccination: It s what your child would choose. Your guide to childhood vaccinations.
 Vaccination: It s what your child would choose No-one likes an injection, but your child would never choose to suffer from serious conditions like measles, mumps or whooping cough. Your guide to childhood
Vaccination: It s what your child would choose No-one likes an injection, but your child would never choose to suffer from serious conditions like measles, mumps or whooping cough. Your guide to childhood
PERTUSSIS SURVEILLANCE PROTOCOL FOR ONTARIO HOSPITALS
 PERTUSSIS SURVEILLANCE PROTOCOL FOR ONTARIO HOSPITALS Developed by the Ontario Hospital Association and the Ontario Medical Association Joint Communicable Diseases Surveillance Protocols Committee Approved
PERTUSSIS SURVEILLANCE PROTOCOL FOR ONTARIO HOSPITALS Developed by the Ontario Hospital Association and the Ontario Medical Association Joint Communicable Diseases Surveillance Protocols Committee Approved
Influenza Vaccine Frequently Asked Questions. Influenza Control Program
 Influenza Vaccine Frequently Asked Questions Influenza Control Program Influenza or the flu can be a serious contagious disease, which is spread by droplet transmission through close contact with an infected
Influenza Vaccine Frequently Asked Questions Influenza Control Program Influenza or the flu can be a serious contagious disease, which is spread by droplet transmission through close contact with an infected
Haemophilus Influenzae (meningitis and invasive
 Haemophilus Influenzae (meningitis and invasive disease)! Report immediately 24/7 by phone upon initial suspicion or laboratory test order PROTOCOL CHECKLIST Enter available information into Merlin upon
Haemophilus Influenzae (meningitis and invasive disease)! Report immediately 24/7 by phone upon initial suspicion or laboratory test order PROTOCOL CHECKLIST Enter available information into Merlin upon
AV1300 STAFF INFLUENZA IMMUNIZATION AND EXCLUSION POLICY
 AV1300 STAFF INFLUENZA IMMUNIZATION AND EXCLUSION POLICY 1.0 PURPOSE To help ensure that those at greatest risk of complications and death from influenza are optimally protected through the appropriate
AV1300 STAFF INFLUENZA IMMUNIZATION AND EXCLUSION POLICY 1.0 PURPOSE To help ensure that those at greatest risk of complications and death from influenza are optimally protected through the appropriate
Research articles. to the ongoing increased pertussis activity, primarily to minimise further infant hospitalisations and deaths.
 Research articles The number of deaths among infants under one year of age in England with pertussis: results of a capture/ recapture analysis for the period 2001 to 2011 A J van Hoek (albertjan.vanhoek@hpa.org.uk)
Research articles The number of deaths among infants under one year of age in England with pertussis: results of a capture/ recapture analysis for the period 2001 to 2011 A J van Hoek (albertjan.vanhoek@hpa.org.uk)
Connection with other policy areas and (How does it fit/support wider early years work and partnerships)
 Illness such as gastroenteritis and upper respiratory tract infections, along with injuries caused by accidents in the home, are the leading causes of attendances at Accident & Emergency and hospitalisation
Illness such as gastroenteritis and upper respiratory tract infections, along with injuries caused by accidents in the home, are the leading causes of attendances at Accident & Emergency and hospitalisation
MUMPS PUBLIC FREQUENTLY ASKED QUESTIONS
 New Jersey Department of Health Vaccine Preventable Disease Program MUMPS PUBLIC FREQUENTLY ASKED QUESTIONS Date: April 17, 2014 DESCRIPTION OF MUMPS What is mumps? Mumps is a disease that is caused by
New Jersey Department of Health Vaccine Preventable Disease Program MUMPS PUBLIC FREQUENTLY ASKED QUESTIONS Date: April 17, 2014 DESCRIPTION OF MUMPS What is mumps? Mumps is a disease that is caused by
Ambulance Service. Patient Care. and. Transportation Standards
 Ambulance Service Patient Care and Transportation Standards Ministry of Health and Long-Term Care Emergency Health Services Branch Patient Care A. General Each operator and each emergency medical attendant
Ambulance Service Patient Care and Transportation Standards Ministry of Health and Long-Term Care Emergency Health Services Branch Patient Care A. General Each operator and each emergency medical attendant
Clinical description 2 Laboratory test for diagnosis 3. Incubation period 4 Mode of transmission 4 Period of communicability 4
 Tuberculosis Contents Epidemiology in New Zealand 2 Case definition 2 Clinical description 2 Laboratory test for diagnosis 3 Case classification 3 Spread of infection 4 Incubation period 4 Mode of transmission
Tuberculosis Contents Epidemiology in New Zealand 2 Case definition 2 Clinical description 2 Laboratory test for diagnosis 3 Case classification 3 Spread of infection 4 Incubation period 4 Mode of transmission
School of Health & Rehabilitation Sciences INFORMATION FOR HEALTH CARE STUDENTS RE: VACCINATIONS AND INFECTIOUS DISEASES
 School of Health & Rehabilitation Sciences INFORMATION FOR HEALTH CARE STUDENTS RE: VACCINATIONS AND INFECTIOUS DISEASES Adults as well as children need protection against infectious diseases. As Health
School of Health & Rehabilitation Sciences INFORMATION FOR HEALTH CARE STUDENTS RE: VACCINATIONS AND INFECTIOUS DISEASES Adults as well as children need protection against infectious diseases. As Health
Guidance for School Responses to Influenza - 2009 2010
 Guidance for School Responses to Influenza - 2009 2010 West Virginia Department of Education and West Virginia Department of Health and Human Resources August 19, 2009 8/19/2009 1 Purpose To provide local
Guidance for School Responses to Influenza - 2009 2010 West Virginia Department of Education and West Virginia Department of Health and Human Resources August 19, 2009 8/19/2009 1 Purpose To provide local
How Many Tdap Vaccines Are Given to San Diego County People?
 Immunizations and Pregnancy Mark H. Sawyer, MD San Diego Immunization Partnership San Diego County Immunization Branch Objectives List vaccines that should be given either during pregnancy or immediately
Immunizations and Pregnancy Mark H. Sawyer, MD San Diego Immunization Partnership San Diego County Immunization Branch Objectives List vaccines that should be given either during pregnancy or immediately
Developed in response to: Health and Social Act 2008 Contributes to CQC Core Standard Outcome 8
 Management of H1N1 (Swine Flu) Patients Policy Register No: 09061 Status: Public Developed in response to: Health and Social Act 2008 Contributes to CQC Core Standard Outcome 8 number: Consulted With Post/Committee/Group
Management of H1N1 (Swine Flu) Patients Policy Register No: 09061 Status: Public Developed in response to: Health and Social Act 2008 Contributes to CQC Core Standard Outcome 8 number: Consulted With Post/Committee/Group
Solid Organ Transplantation
 Solid Organ Transplantation Infection Prevention And Control Transplant Atlantic 2011 October 13/2011 Kathy Hart Introduction In the past several years, the drugs that we use, the surgeries themselves,
Solid Organ Transplantation Infection Prevention And Control Transplant Atlantic 2011 October 13/2011 Kathy Hart Introduction In the past several years, the drugs that we use, the surgeries themselves,
Interim Guidance for Medical Schools on health checks for active and latent Tuberculosis in medical students returning from elective periods overseas
 Interim Guidance for Medical Schools on health checks for active and latent Tuberculosis in medical students returning from elective periods overseas This document has been developed by: Dr Mathina Darmalingam
Interim Guidance for Medical Schools on health checks for active and latent Tuberculosis in medical students returning from elective periods overseas This document has been developed by: Dr Mathina Darmalingam
Public Health Monitoring of Returning Travellers
 Introduction Ebola virus disease (EVD) is associated with a high fatality rate, and is currently affecting several countries in West Africa. Although the risk in Canada is very low, Ontario s health care
Introduction Ebola virus disease (EVD) is associated with a high fatality rate, and is currently affecting several countries in West Africa. Although the risk in Canada is very low, Ontario s health care
STAFF SCREENING AND IMMUNISATION POLICY
 2GETHER NHS FOUNDATION TRUST GLOUCESTERSHIRE HOSPITALS NHS FOUNDATION TRUST NHS GLOUCESTERSHIRE GLOUCESTERSHIRE CARE SERVICES STAFF SCREENING AND IMMUNISATION POLICY Version: 1 Consultation: Ratified by:
2GETHER NHS FOUNDATION TRUST GLOUCESTERSHIRE HOSPITALS NHS FOUNDATION TRUST NHS GLOUCESTERSHIRE GLOUCESTERSHIRE CARE SERVICES STAFF SCREENING AND IMMUNISATION POLICY Version: 1 Consultation: Ratified by:
Explanation of Immunization Requirements
 Explanation of Immunization Requirements CONTENTS Hepatitis A... 2 Hepatitis B... 3 Influenza... 4 Measles (Rubella), Mumps, and Rubella (MMR)... 5 Pertussis (Tdap)... 6 Tuberculosis (TB) Test... 7 Varicella/Chicken
Explanation of Immunization Requirements CONTENTS Hepatitis A... 2 Hepatitis B... 3 Influenza... 4 Measles (Rubella), Mumps, and Rubella (MMR)... 5 Pertussis (Tdap)... 6 Tuberculosis (TB) Test... 7 Varicella/Chicken
Employee/Occupational Health Management of Healthcare Workers who are Close Contacts or Contacts of Ebola Virus Disease (EVD)
 Employee/Occupational Health Management of Healthcare Workers who are Close Contacts or Contacts of Ebola Virus Disease (EVD) Version 2.0: 17-December-2014 Approval: Provincial EVD Preparedness Task Group
Employee/Occupational Health Management of Healthcare Workers who are Close Contacts or Contacts of Ebola Virus Disease (EVD) Version 2.0: 17-December-2014 Approval: Provincial EVD Preparedness Task Group
Guidance: Infection Prevention and Control Measures for Healthcare Workers in Acute Care and Long-term Care Settings. Seasonal Influenza
 Guidance: Infection Prevention and Control Measures for Healthcare Workers in Acute Care and Long-term Care Settings Seasonal Influenza To promote and protect the health of Canadians through leadership,
Guidance: Infection Prevention and Control Measures for Healthcare Workers in Acute Care and Long-term Care Settings Seasonal Influenza To promote and protect the health of Canadians through leadership,
Ebola: Teaching Points for Nurse Educators
 Ebola: Teaching Points for Nurse Educators Heightened media attention on emerging disease outbreaks such as Ebola may raise concerns among students. During outbreaks such as Ebola, nursing faculty are
Ebola: Teaching Points for Nurse Educators Heightened media attention on emerging disease outbreaks such as Ebola may raise concerns among students. During outbreaks such as Ebola, nursing faculty are
