Medical Device Software: Establishing FDA Authority and Mobile Medical Apps
|
|
|
- Ernest Strickland
- 10 years ago
- Views:
Transcription
1 Medical Device Software: Establishing FDA Authority and Mobile Medical Apps Seth A. Mailhot, Partner Lead, FDA Regulatory Practice Overview Applying the Definition of a Device to Software Special Categories of Device Software Mobile Medical Applications Responsibilities of Mobile Medical App Manufacturers and Distributors 1
2 Definition of a Device Sec. 201(h) defines a device as an instrument, apparatus, implement, machine, contrivance,... or other similar or related article, including any component, part, or accessory, which is intended: for use in the diagnosis of disease or other conditions, in the cure, mitigation, treatment, or prevention of disease, to affect the structure or function of the body Software That is Not a Medical Device Electronic copies of medical textbooks, teaching aids or reference materials Software solely used to log, record, track, evaluate, or make decisions or suggestions related to developing or maintaining general health and wellness 2
3 Software That is Not a Medical Device (cont.) Software that only automates general office operations with functionalities that include billing, inventory, appointments, or insurance transactions, such as: Software that performs the functionality of an electronic health record system or personal health record system, including providing patients a means to access their health records Software That is Not a Medical Device (cont.) Software solely used to provide clinicians with training or reinforce training previously received Software that serves as a generic aid to assist users, but is not commercially marketed for a specific medical indication, such as serving as a magnifying glass, recording audio, note-taking, and replaying audio with amplification 3
4 Exceptions to Non Device Software Categories Software allowing the user to input patient-specific information along with reference material to automatically diagnose a disease or condition is a device Generic aids promoted for medical applications are devices: Software that controls a light emitting diode (LED) on a mobile platform is a general aid if the manufacturer intends the system to illuminate objects generally (without a specific device intended use), but If the LED-controlling software is promoted by the manufacturer through marketing and distribution for use as a light source to examine patients (such as an ophthalmoscope), then the software would meet the definition of a device Implications of FDA Regulation Registration and listing (including user fee) Premarket submission (unless exempt) Design controls (21 C.F.R ) Other good manufacturing practice requirements (Quality System Regulation) Reporting of adverse events (MDRs) Reporting of recalls (corrections or removals) Other requirements 4
5 Special Software Related Device Categories Medical Device Data System (MDDS) Mobile Medical Apps Laboratory Information System (LIS) Picture Archiving and Communications System (PACS) Remote Patient Monitoring System Medical Device Data System (MDDS) MDDS is intended to provide one or more of the following uses, without controlling or altering the functions or parameters of any connected medical devices: The electronic transfer, storage, conversion or display of medical device data of medical device data; Class I, 510(k) exempt (21 C.F.R ) 5
6 MDDS (cont.) MDDS may include software, electronic or electrical hardware such as a physical communications medium (including wireless hardware), modems, interfaces, and a communications protocol MDDS does not include devices intended to be used in connection with active patient monitoring Mobile Medical Application ( App ) Mobile application is either: a software application executed on a mobile platform, or a web-based software application tailored to a mobile platform, but executed on a server Mobile medical application is a mobile app that: Meets the definition of device in section 201(h), and Either: is used as an accessory to a regulated medical device; or transforms a mobile platform into a regulated medical device Mobile platform: Commercial off-the-shelf (COTS) computing platforms, with or without wireless connectivity, that are handheld in nature 6
7 Mobile Medical App (cont.) Mobile medical apps fit into the following categories: Displaying, storing, analyzing or transmitting patient-specific medical device data (if data is in original format, regulated as an MDDS) Controlling a connected medical device (accessory) Transforms the mobile platform into a regulated medical device (regulated as the device) Performs patient-specific analysis and diagnosis, or treatment recommendations (regulated same as non-mobile software type) Enforcement Discretion for Mobile Medical Apps FDA will exercise enforcement discretion for mobile apps that are not a mobile medical app, despite meeting the definition of a device FDA will also exercise enforcement discretion for mobile medical apps that: Help patients self-manage their disease or conditions without providing specific treatment or treatment suggestions Provide patients with simple tools to organize and track health information Provide easy access to information related to patients health conditions or treatments Help patients document, show, or communicate potential medical conditions to health care providers Automate simple tasks for health care providers; or Enable patients or providers to interact with Personal Health Record (PHR) or Electronic Health Record (EHR) systems 7
8 Examples of Simple Medical Calculations Enforcement discretion applies to simple medical calculations taught in medical schools and are routinely used in clinical practice Examples include medical calculators for: Body Mass Index (BMI) Total Body Water / Urea Volume of Distribution Mean arterial pressure Glascow Coma Scale score APGAR score NIH Stroke Scale Delivery date estimator Other Mobile Medical Apps Subject to Enforcement Discretion Help patients with diagnosed psychiatric conditions maintain their behavioral coping skills by providing behavioral techniques or audio messages Allow users to inquire about herb and drug interactions Use patient characteristics to provide screening, counseling and preventive recommendations from established authorities Track medications and provide user-configured reminders Allow users to collect blood pressure data and share, track, trend or upload it to a personal or electronic health record 8
9 Other Common Software Devices Laboratory Information System (LIS) Software that handles in vitro diagnostic data related to patient specimen identification, tests requested, results reported, quality control testing, and other aspects of sample analysis Class I, 510(k) exempt (21 C.F.R ) Picture Archiving and Communications System (PACS) Software capable of accepting, transferring, displaying, storing, or digitally processing medical images Class II (21 C.F.R ) Remote Patient Monitoring System Software that conditions physiological signals so that they can be transmitted via radiofrequency from one location to another for active patient monitoring Class II (21 C.F.R ) Who is a Software Manufacturer? Mobile Medical App Guidance states it is any person or entity that: Creates, designs, develops, labels, re-labels, remanufactures, modifies, or creates a mobile medical app software system from multiple components Creates a mobile medical app or a software system that provides users access to the medical device function through a website subscription, software as a service, or other similar means 9
10 Who is a Software Manufacturer? (cont.) Initiates specifications or requirements for mobile medical apps or procures product development/manufactu ring services from other individuals or entities (second party) for subsequent commercial distribution Who Is Not a Software Manufacturer? Manufacturers or distributors of mobile platforms who solely distribute or market their platform and do not intend (by marketing claims) the platform to be used for medical device functions Providers of tools, services or infrastructure used in the development, distribution, or use of a mobile medical app Licensed practitioners who manufacture or alter a mobile medical app solely for use in their professional practice or group and do not label or promote their mobile medical apps to be generally used by other licensed practitioners or other individuals outside of their group Persons who manufacture mobile medical apps solely for use in research, teaching, or analysis and do not introduce such devices into commercial distribution (although they may be subject to IDE requirements) 10
11 Mobile Medical App Distributors FDA revised the guidance redefining the operators of Google play, itunes store, and BlackBerry App World as online market places The draft guidance initially identified these entities as Mobile Medical App Distributors These entities are potentially exempt from the responsibilities of distributors: owners and operators that are only engaged in providing an online market place that allow mobile medical app manufacturers to commercially distribute their mobile medical apps FDA expects distributors of mobile medical apps who may or may not be a platform or service provider will cooperate with manufacturers in conducting corrections and removal actions QUESTIONS? 11
12 Seth A. Mailhot 601 Pennsylvania Avenue, N.W. Suite 700 South Washington, D.C Phone: (202) Cell: (617) Fax: (202) Seth Mailhot is a partner and lead of the FDA Regulatory Practice Group in Michael Best & Friedrich s Washington D.C. office. His 14 years working in the U.S. Food and Drug Administration (FDA) has provided him a unique perspective when counseling clients on a broad range of matters involving the FDA. Seth s practice includes representation of the medical device, pharmaceutical, dietary supplement, tobacco and food industries, and covers both premarket and post-market issues. His practice is focused on development of premarket submission strategies, and FDA enforcement of good manufacturing practices, both domestically and abroad. Admissions District of Columbia Massachusetts U.S. Patent and Trademark Office Education New England School of Law, J.D., Valedictorian, summa cum laude University of Massachusetts, B.S., Chemical Engineering 23 12
FDA Issues Final Guidance on Mobile Medical Apps
 ADVISORY September 2013 FDA Issues Final Guidance on Mobile Medical Apps On September 23, 2013, the U.S. Food & Drug Administration (FDA or the Agency) issued its final Guidance for Industry and Food and
ADVISORY September 2013 FDA Issues Final Guidance on Mobile Medical Apps On September 23, 2013, the U.S. Food & Drug Administration (FDA or the Agency) issued its final Guidance for Industry and Food and
Mobile Medical Applications: FDA s Final Guidance. M. Elizabeth Bierman Anthony T. Pavel Morgan, Lewis & Bockius, LLP
 Mobile Medical Applications: FDA s Final Guidance Michele L. Buenafe M. Elizabeth Bierman Anthony T. Pavel Morgan, Lewis & Bockius, LLP 1 Background FDA has a long-standing policy to regulate any computer
Mobile Medical Applications: FDA s Final Guidance Michele L. Buenafe M. Elizabeth Bierman Anthony T. Pavel Morgan, Lewis & Bockius, LLP 1 Background FDA has a long-standing policy to regulate any computer
Use of Mobile Medical Applications in Clinical Research
 Use of Mobile Medical Applications in Clinical Research Erin K. O Reilly, PhD RAC Associate Director, Regulatory Affairs Duke Translational Medicine Institute [email protected] September 10, 2014 (919)
Use of Mobile Medical Applications in Clinical Research Erin K. O Reilly, PhD RAC Associate Director, Regulatory Affairs Duke Translational Medicine Institute [email protected] September 10, 2014 (919)
Mobile Medical Applications
 Mobile Medical Applications What Is the Impact of FDA s New MMA Guidance for the Life Science Industry? June 6, 2014, 11:15 AM 12:15 PM Presented by: Mark Gardner, M.B.A., J.D. Agenda 1. How does FDA regulate
Mobile Medical Applications What Is the Impact of FDA s New MMA Guidance for the Life Science Industry? June 6, 2014, 11:15 AM 12:15 PM Presented by: Mark Gardner, M.B.A., J.D. Agenda 1. How does FDA regulate
CENTER FOR CONNECTED HEALTH POLICY
 CENTER FOR CONNECTED HEALTH POLICY The Center for Connected Health Policy (CCHP) is a public interest nonprofit organization that develops and advances telehealth policy solutions to promote improvements
CENTER FOR CONNECTED HEALTH POLICY The Center for Connected Health Policy (CCHP) is a public interest nonprofit organization that develops and advances telehealth policy solutions to promote improvements
Mobile Medical Applications. Guidance for Industry and Food and Drug Administration Staff
 Mobile Medical Applications Guidance for Industry and Food and Drug Administration Staff Document issued on: September 25, 2013 The draft of this guidance was issued on July 21, 2011. For questions regarding
Mobile Medical Applications Guidance for Industry and Food and Drug Administration Staff Document issued on: September 25, 2013 The draft of this guidance was issued on July 21, 2011. For questions regarding
The Shifting Sands of Medical Software Regulation
 The Shifting Sands of Medical Software Regulation Suzanne O Shea Ralph Hall September 10, 2014 What Software is Regulated by FDA? FDA regulates medical devices. FDA regulates software that meets the definition
The Shifting Sands of Medical Software Regulation Suzanne O Shea Ralph Hall September 10, 2014 What Software is Regulated by FDA? FDA regulates medical devices. FDA regulates software that meets the definition
Regulation of Mobile Medical Apps
 Regulation of Mobile Medical Apps May 30, 2014 Copyright 2014 Software Quality Consulting Inc. Slide 1 Speaker Bio Steven R. Rakitin has over 35 years experience as a software engineer and 25 years in
Regulation of Mobile Medical Apps May 30, 2014 Copyright 2014 Software Quality Consulting Inc. Slide 1 Speaker Bio Steven R. Rakitin has over 35 years experience as a software engineer and 25 years in
Mobile Medical Application Development: FDA Regulation
 Mobile Medical Application Development: FDA Regulation Mobile Medical Applications: Current Environment Currently over 100,000 mobile health related applications are available for download; there is an
Mobile Medical Application Development: FDA Regulation Mobile Medical Applications: Current Environment Currently over 100,000 mobile health related applications are available for download; there is an
Mobile Medical Applications: An Overview of FDA Regulation
 Mobile Medical Applications: An Overview of FDA Regulation RAPS Annual Convention 2014 Austin, Texas Michael A. Swit, Esq. Special Counsel, FDA Law Practice Duane Morris LLP Standard Disclaimers The views
Mobile Medical Applications: An Overview of FDA Regulation RAPS Annual Convention 2014 Austin, Texas Michael A. Swit, Esq. Special Counsel, FDA Law Practice Duane Morris LLP Standard Disclaimers The views
US & CANADA: REGULATION AND GUIDELINES ON MEDICAL SOFTWARE AND APPS OR
 US & CANADA: REGULATION AND GUIDELINES ON MEDICAL SOFTWARE AND APPS OR A MEDICAL DEVICE IS A MEDICAL DEVICE IS A MEDICAL DEVICE AHWP Medical SW Workshop Taipei, Taiwan November 3, 2012 John G. Abbott,
US & CANADA: REGULATION AND GUIDELINES ON MEDICAL SOFTWARE AND APPS OR A MEDICAL DEVICE IS A MEDICAL DEVICE IS A MEDICAL DEVICE AHWP Medical SW Workshop Taipei, Taiwan November 3, 2012 John G. Abbott,
Medical Device Data Systems, Medical Image Storage Devices, and Medical Image Communications Devices
 Medical Device Data Systems, Medical Image Storage Devices, and Medical Image Communications Devices Draft Guidance for Industry and Food and Drug Administration Staff DRAFT GUIDANCE This guidance document
Medical Device Data Systems, Medical Image Storage Devices, and Medical Image Communications Devices Draft Guidance for Industry and Food and Drug Administration Staff DRAFT GUIDANCE This guidance document
Regulatory Considerations for Medical Device Software. Medical Device Software
 Medtec Ireland 2015 Wireless Medical Devices Regulatory Considerations for Medical Device Software Kenneth L. Block, RAC October 7, 2015 Galway, Ireland Offices: Dallas, Texas (12 employees) Tokyo, Japan
Medtec Ireland 2015 Wireless Medical Devices Regulatory Considerations for Medical Device Software Kenneth L. Block, RAC October 7, 2015 Galway, Ireland Offices: Dallas, Texas (12 employees) Tokyo, Japan
Regulation of Medical Apps FDA and European Union
 JHW Comment - The content of below article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances. Erik Vollebregt Erik is a
JHW Comment - The content of below article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances. Erik Vollebregt Erik is a
Medical Device Software
 Medical Device Software Bakul Patel Senior Policy Advisor 1 Overview Medical devices and software Oversight principles and Current approach Trends, Challenges and opportunities Addressing challenges 2
Medical Device Software Bakul Patel Senior Policy Advisor 1 Overview Medical devices and software Oversight principles and Current approach Trends, Challenges and opportunities Addressing challenges 2
REFERENCE DOCUMENT. (DRAFT for Public Comments) Title: White Paper on Medical Device Software Regulation Software Qualification and Classification
 AHWP/WG1/R001:2014 REFERENCE DOCUMENT (DRAFT for Public Comments) Title: White Paper on Medical Device Software Regulation Software Qualification and Classification Author: Work Group 1, Pre-Market Submission
AHWP/WG1/R001:2014 REFERENCE DOCUMENT (DRAFT for Public Comments) Title: White Paper on Medical Device Software Regulation Software Qualification and Classification Author: Work Group 1, Pre-Market Submission
The U.S. FDA s Regulation and Oversight of Mobile Medical Applications
 The U.S. FDA s Regulation and Oversight of Mobile Medical Applications The U.S. FDA s Regulation and Oversight of Mobile Medical Applications As smart phones and portable tablet computers become the preferred
The U.S. FDA s Regulation and Oversight of Mobile Medical Applications The U.S. FDA s Regulation and Oversight of Mobile Medical Applications As smart phones and portable tablet computers become the preferred
Breakout Sessions: FDA s Regulation of Mobile Health and Medical Applications
 Breakout Sessions: FDA s Regulation of Mobile Health and Medical Applications 2015 Annual Conference Washington, DC Bakul Patel, Associate Director for Digital Health, Office of Center Director, Center
Breakout Sessions: FDA s Regulation of Mobile Health and Medical Applications 2015 Annual Conference Washington, DC Bakul Patel, Associate Director for Digital Health, Office of Center Director, Center
Thomas Conroy, RPh., J.D. Director, Promotion Compliance Global Regulatory Affairs MARCH 11, 2015
 Thomas Conroy, RPh., J.D. Director, Promotion Compliance Global Regulatory Affairs MARCH 11, 2015 All slides and accompanying comments, ideas, arguments and other statements of any kind are personal to
Thomas Conroy, RPh., J.D. Director, Promotion Compliance Global Regulatory Affairs MARCH 11, 2015 All slides and accompanying comments, ideas, arguments and other statements of any kind are personal to
Risk based 12/1/2015. Digital Health Bakul Patel Associate Director for Digital Health Office of Center Director.
 Digital Health Bakul Patel Associate Director for Digital Health Office of Center Director Center for Devices and Radiological Health 1 Oversight Approach Platform independent Promote innovation Promote
Digital Health Bakul Patel Associate Director for Digital Health Office of Center Director Center for Devices and Radiological Health 1 Oversight Approach Platform independent Promote innovation Promote
MOBILE MEDICAL APPLICATIONS
 October 7, 2013 EVOKE HEALTH POINT OF VIEW MOBILE MEDICAL APPLICATIONS FDA GUIDANCE FOR INDUSTRY FOR MORE INFORMATION: Mark McConaghy, VP, Strategy Evoke Health 267.765.4998 [email protected]
October 7, 2013 EVOKE HEALTH POINT OF VIEW MOBILE MEDICAL APPLICATIONS FDA GUIDANCE FOR INDUSTRY FOR MORE INFORMATION: Mark McConaghy, VP, Strategy Evoke Health 267.765.4998 [email protected]
CDRH Regulated Software
 CDRH Regulated Software An Introduction John F. Murray Jr. CDRH Software Compliance Expert CDRH Regulates Software in the following areas Medical Devices Automation of Production Systems Automation of
CDRH Regulated Software An Introduction John F. Murray Jr. CDRH Software Compliance Expert CDRH Regulates Software in the following areas Medical Devices Automation of Production Systems Automation of
Developing a Mobile Medical App? How to determine if it is a medical device and get it cleared by the US FDA
 Developing a Mobile Medical App? How to determine if it is a medical device and get it cleared by the US FDA In this presentation: App stats: Explosive growth Examples already cleared by the US FDA Is
Developing a Mobile Medical App? How to determine if it is a medical device and get it cleared by the US FDA In this presentation: App stats: Explosive growth Examples already cleared by the US FDA Is
CDRH Regulated Software Looking back, looking forward
 CDRH Regulated Software Looking back, looking forward Medical Device Software Compliance Expert US Food & Drug Administration at the Regulatory Affairs Professional Society Indianapolis, Indiana Goal of
CDRH Regulated Software Looking back, looking forward Medical Device Software Compliance Expert US Food & Drug Administration at the Regulatory Affairs Professional Society Indianapolis, Indiana Goal of
Rethinking the FDA s Regulation of. By Scott D. Danzis and Christopher Pruitt
 Rethinking the FDA s Regulation of Mobile Medical Apps By Scott D. Danzis and Christopher Pruitt Smartphones and mobile devices have rapidly become part of everyday life in the United States. It is no
Rethinking the FDA s Regulation of Mobile Medical Apps By Scott D. Danzis and Christopher Pruitt Smartphones and mobile devices have rapidly become part of everyday life in the United States. It is no
Templates. FDA Mobile Medical App Regulations. Your own sub headline This is an example text. Your Logo
 Templates FDA Mobile Medical App Regulations Your own sub headline This is an example text Your Logo FDA Oversight of Medical Devices The latest Guidance from the FDA Tom Richards MD/MS [email protected]
Templates FDA Mobile Medical App Regulations Your own sub headline This is an example text Your Logo FDA Oversight of Medical Devices The latest Guidance from the FDA Tom Richards MD/MS [email protected]
Mobile Medical Apps. Purpose. Diane Romza Kutz Fredric E. Roth V. Regulation and Risks. Purpose of today s presentation
 Mobile Medical Apps Regulation and Risks Diane Romza Kutz Fredric E. Roth V Purpose Purpose of today s presentation Identify the newly-regulated industry Identify the newly regulated products and the basis
Mobile Medical Apps Regulation and Risks Diane Romza Kutz Fredric E. Roth V Purpose Purpose of today s presentation Identify the newly-regulated industry Identify the newly regulated products and the basis
International Medical Device Regulators Forum (IMDRF) US FDA Center for Devices and Radiological Health - Update
 International Medical Device Regulators Forum (IMDRF) US FDA Center for Devices and Radiological Health - Update Kimberly A. Trautman Associate Director, International Affairs Office of the Center Director
International Medical Device Regulators Forum (IMDRF) US FDA Center for Devices and Radiological Health - Update Kimberly A. Trautman Associate Director, International Affairs Office of the Center Director
How To Regulate A Medical Device From A Cell Phone
 On Behalf of: InTouch Health White Paper FDA Regulation of Mobile Health Technologies The Current Regulatory Framework as Applied to InTouch Health s Telemedicine Solution June 15, 2012 TABLE OF CONTENTS
On Behalf of: InTouch Health White Paper FDA Regulation of Mobile Health Technologies The Current Regulatory Framework as Applied to InTouch Health s Telemedicine Solution June 15, 2012 TABLE OF CONTENTS
Final Document. Software as a Medical Device (SaMD): Key Definitions. Date: 9 December 2013. Despina Spanou, IMDRF Chair. IMDRF/SaMD WG/N10FINAL:2013
 Final Document Title: Authoring Group: Software as a Medical Device (SaMD): Key Definitions IMDRF SaMD Working Group Date: 9 December 2013 Despina Spanou, IMDRF Chair This document was produced by the
Final Document Title: Authoring Group: Software as a Medical Device (SaMD): Key Definitions IMDRF SaMD Working Group Date: 9 December 2013 Despina Spanou, IMDRF Chair This document was produced by the
2014 Annual Report on Inspections of Establishments
 2014 Annual Report on Inspections of Establishments Table of Contents Introduction... 2 Data Collection and Definitions... 3 Section 510(h)(6)(A)(i) Number of Domestic and Foreign Establishments Registered
2014 Annual Report on Inspections of Establishments Table of Contents Introduction... 2 Data Collection and Definitions... 3 Section 510(h)(6)(A)(i) Number of Domestic and Foreign Establishments Registered
General Wellness: Policy for Low Risk Devices. Draft Guidance for Industry and Food and Drug Administration Staff
 General Wellness: Policy for Low Risk Devices Draft Guidance for Industry and Food and Drug Administration Staff DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Document
General Wellness: Policy for Low Risk Devices Draft Guidance for Industry and Food and Drug Administration Staff DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Document
How To Know If A Mobile App Is A Medical Device
 The Regulation of Medical Device Apps Prepared for West of England Academic Health Science Network and University of Bristol June 2014 June 2014 1 Table of Contents 1 Purpose...3 2 Scope...3 3 The Regulation
The Regulation of Medical Device Apps Prepared for West of England Academic Health Science Network and University of Bristol June 2014 June 2014 1 Table of Contents 1 Purpose...3 2 Scope...3 3 The Regulation
Cutting Edge Issues in Health Care Technology & mhealth. Agenda
 Cutting Edge Issues in Health Care Technology & mhealth Vernessa Pollard July 2014 Agenda The Regulatory Landscape Jurisdiction and Authority Over Mobile Health Products Requirements, Compliance and Enforcement
Cutting Edge Issues in Health Care Technology & mhealth Vernessa Pollard July 2014 Agenda The Regulatory Landscape Jurisdiction and Authority Over Mobile Health Products Requirements, Compliance and Enforcement
Medical Product Software Development and FDA Regulations Software Development Practices and FDA Compliance
 Medical Product Development and FDA Regulations IEEE Orange County Computer Society March 27, 2006 Carl R. Wyrwa Medical Product Development and FDA Regulations Introduction Regulated FDA Overview Medical
Medical Product Development and FDA Regulations IEEE Orange County Computer Society March 27, 2006 Carl R. Wyrwa Medical Product Development and FDA Regulations Introduction Regulated FDA Overview Medical
Sarah Chandler A/Head, Regulatory and Scientific Section Medical Devices Bureau [email protected]
 Software Regulated as a Medical Device Sarah Chandler A/Head, Regulatory and Scientific Section Medical Devices Bureau [email protected] Therapeutic Products Directorate Director General S. Sharma
Software Regulated as a Medical Device Sarah Chandler A/Head, Regulatory and Scientific Section Medical Devices Bureau [email protected] Therapeutic Products Directorate Director General S. Sharma
Understanding Medical Device Regulation for mhealth A Guide for Mobile Operators
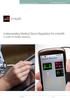 for mhealth A Guide for Mobile Operators 1 Foreword Mobile technologies will make a significant contribution to addressing the enormous challenges of healthcare provision worldwide. Early efforts in mobile
for mhealth A Guide for Mobile Operators 1 Foreword Mobile technologies will make a significant contribution to addressing the enormous challenges of healthcare provision worldwide. Early efforts in mobile
FDA Regulation of Whole Slide Imaging (WSI) Devices: Current Thoughts
 FDA Regulation of Whole Slide Imaging (WSI) Devices: Current Thoughts Clinical Laboratory Improvement Advisory Committee Meeting Centers for Disease Control and Prevention February 15, 2012 Tremel A. Faison,
FDA Regulation of Whole Slide Imaging (WSI) Devices: Current Thoughts Clinical Laboratory Improvement Advisory Committee Meeting Centers for Disease Control and Prevention February 15, 2012 Tremel A. Faison,
Considerations for using the Web for Medical Device Applications
 Considerations for using the Web for Medical Device Applications MEDS, San Diego August 23 rd, 2012 Daniel Sterling, President Who is Sterling? Your Partner in Medical Device Development What we do: o
Considerations for using the Web for Medical Device Applications MEDS, San Diego August 23 rd, 2012 Daniel Sterling, President Who is Sterling? Your Partner in Medical Device Development What we do: o
What is a medical device? Medical Devices: Roadmap to Market. Kathryn Klaus, Esq.
 Medical Devices: Roadmap to Market Kathryn Klaus, Esq. The last installment of Regulatory 360 discussed the FDA organization in general where it came from and a broad overview of how it operates, as well
Medical Devices: Roadmap to Market Kathryn Klaus, Esq. The last installment of Regulatory 360 discussed the FDA organization in general where it came from and a broad overview of how it operates, as well
Guidance for Sponsors, Institutional Review Boards, Clinical Investigators and FDA Staff
 Guidance for Sponsors, Institutional Review Boards, Clinical Investigators and FDA Staff Guidance on Informed Consent for In Vitro Diagnostic Device Studies Using Leftover Human Specimens that are Not
Guidance for Sponsors, Institutional Review Boards, Clinical Investigators and FDA Staff Guidance on Informed Consent for In Vitro Diagnostic Device Studies Using Leftover Human Specimens that are Not
Role of the Investigational Drug Services (IDS) in the Management of Investigational Drugs
 Role of the Investigational Drug Services (IDS) in the Management of Investigational Drugs Charlesworth Rae, BS, PharmD, JD Investigational Drug Pharmacist July 2012 1 Outline of Presentation I. Introduction
Role of the Investigational Drug Services (IDS) in the Management of Investigational Drugs Charlesworth Rae, BS, PharmD, JD Investigational Drug Pharmacist July 2012 1 Outline of Presentation I. Introduction
On Behalf of: InTouch Health
 On Behalf of: InTouch Health White Paper FDA Regulation of Mobile Health Technologies The Current Regulatory Framework as Applied to InTouch Health s Telehealth Solutions June 15, 2012; Updated June 15,
On Behalf of: InTouch Health White Paper FDA Regulation of Mobile Health Technologies The Current Regulatory Framework as Applied to InTouch Health s Telehealth Solutions June 15, 2012; Updated June 15,
Fra udvikling til kommercialisering af kliniske apps Apps som medicinsk udstyr? 29/1-2015. Brian Hedegaard, DELTA
 Fra udvikling til kommercialisering af kliniske apps Apps som medicinsk udstyr? 29/1-2015 Brian Hedegaard, DELTA Awareness please.. Brian Hedegaard Senior Consultant - Medical devices Approval Management
Fra udvikling til kommercialisering af kliniske apps Apps som medicinsk udstyr? 29/1-2015 Brian Hedegaard, DELTA Awareness please.. Brian Hedegaard Senior Consultant - Medical devices Approval Management
Combination Products Regulation in the United States
 Combination Products Regulation in the United States Presenter: Scott Sardeson RAC US/EU 3M Health Care St. Paul, MN USA 1 Presentation Outline Combination products Definitions and Regulations Jurisdiction
Combination Products Regulation in the United States Presenter: Scott Sardeson RAC US/EU 3M Health Care St. Paul, MN USA 1 Presentation Outline Combination products Definitions and Regulations Jurisdiction
Development and Validation of In Vitro Diagnostic Tests. YC Lee, Ph.D. CEO
 Development and Validation of In Vitro Diagnostic Tests YC Lee, Ph.D. CEO 1 Validation of In Vitro Diagnostic Tests Validated d Diagnostic Test should: Provides test results that identify if positive i
Development and Validation of In Vitro Diagnostic Tests YC Lee, Ph.D. CEO 1 Validation of In Vitro Diagnostic Tests Validated d Diagnostic Test should: Provides test results that identify if positive i
Guidance for Industry Computerized Systems Used in Clinical Investigations
 Guidance for Industry Computerized Systems Used in Clinical Investigations U.S. Department of Health and Human Services Food and Drug Administration (FDA) Office of the Commissioner (OC) May 2007 Guidance
Guidance for Industry Computerized Systems Used in Clinical Investigations U.S. Department of Health and Human Services Food and Drug Administration (FDA) Office of the Commissioner (OC) May 2007 Guidance
Draft Guidance for Industry, Food and Drug Administration Staff, and Clinical Laboratories
 14 1 2 3 4 5 6 7 8 9 10 11 12 13 15 16 17 18 19 20 21 22 23 24 25 26 27 Draft Guidance for Industry, Food and Drug Administration Staff, and Clinical Laboratories Framework for Regulatory Oversight of
14 1 2 3 4 5 6 7 8 9 10 11 12 13 15 16 17 18 19 20 21 22 23 24 25 26 27 Draft Guidance for Industry, Food and Drug Administration Staff, and Clinical Laboratories Framework for Regulatory Oversight of
Regulation and Risk Management of Combination Products
 Regulation and Risk Management of Combination Products Katherine Ulman Associate Scientist Global Regulatory Compliance Manager Dow Corning Healthcare Jim Curtis Senior Specialist, Healthcare Applications
Regulation and Risk Management of Combination Products Katherine Ulman Associate Scientist Global Regulatory Compliance Manager Dow Corning Healthcare Jim Curtis Senior Specialist, Healthcare Applications
User Agreement. Quality. Value. Efficiency.
 User Agreement Quality. Value. Efficiency. Welcome to QVuE, the Leaders Network on Quality, Value and Efficiency website sponsored by The Medicines Company. The information provided in this Webinar Series
User Agreement Quality. Value. Efficiency. Welcome to QVuE, the Leaders Network on Quality, Value and Efficiency website sponsored by The Medicines Company. The information provided in this Webinar Series
RESEARCH INVOLVING DATA AND/OR BIOLOGICAL SPECIMENS
 RESEARCH INVOLVING DATA AND/OR BIOLOGICAL SPECIMENS 1. Overview IRB approval and participant informed consent are required to collect biological specimens for research purposes. Similarly, IRB approval
RESEARCH INVOLVING DATA AND/OR BIOLOGICAL SPECIMENS 1. Overview IRB approval and participant informed consent are required to collect biological specimens for research purposes. Similarly, IRB approval
Total Product Lifecycle Solutions from NSF Health Sciences Medical Devices
 Total Product Lifecycle Solutions from NSF Health Sciences Medical Devices Experts in medical device quality systems, compliance, regulatory affairs, auditing and training www.nsf.org The Right People.
Total Product Lifecycle Solutions from NSF Health Sciences Medical Devices Experts in medical device quality systems, compliance, regulatory affairs, auditing and training www.nsf.org The Right People.
[DOCKET NO.96N-0002] DRAFT
![[DOCKET NO.96N-0002] DRAFT [DOCKET NO.96N-0002] DRAFT](/thumbs/26/7603707.jpg) [DOCKET NO.96N-0002] DRAFT DRAFT DOCUMENT CONCERNING THE REGULATION OF PLACENTAL/UMBILICAL CORD BLOOD STEM CELL PRODUCTS INTENDED FOR TRANSPLANTATION OR FURTHER MANUFACTURE INTO INJECTABLE PRODUCTS DECEMBER,
[DOCKET NO.96N-0002] DRAFT DRAFT DOCUMENT CONCERNING THE REGULATION OF PLACENTAL/UMBILICAL CORD BLOOD STEM CELL PRODUCTS INTENDED FOR TRANSPLANTATION OR FURTHER MANUFACTURE INTO INJECTABLE PRODUCTS DECEMBER,
Health Information Technology & Management Chapter 2 HEALTH INFORMATION SYSTEMS
 Health Information Technology & Management Chapter 2 HEALTH INFORMATION SYSTEMS INFORMATION SYSTEM *Use of computer hardware and software to process data into information. *Healthcare information system
Health Information Technology & Management Chapter 2 HEALTH INFORMATION SYSTEMS INFORMATION SYSTEM *Use of computer hardware and software to process data into information. *Healthcare information system
Regulatory Issues in Genetic Testing and Targeted Drug Development
 Regulatory Issues in Genetic Testing and Targeted Drug Development Janet Woodcock, M.D. Deputy Commissioner for Operations Food and Drug Administration October 12, 2006 Genetic and Genomic Tests are Types
Regulatory Issues in Genetic Testing and Targeted Drug Development Janet Woodcock, M.D. Deputy Commissioner for Operations Food and Drug Administration October 12, 2006 Genetic and Genomic Tests are Types
Activating Standardization Bodies Around Medical Apps
 Activating Standardization Bodies Around Medical Apps Michael J. Ackerman, Ph.D. Assistant Director High Performance Computing and Communications U.S. National Library of Medicine The views and opinions
Activating Standardization Bodies Around Medical Apps Michael J. Ackerman, Ph.D. Assistant Director High Performance Computing and Communications U.S. National Library of Medicine The views and opinions
FDA Regulation of Hearing Aids. Eric A. Mann, MD, PhD Clinical Deputy Director Division of Ophthalmic and ENT Devices ODE/CDRH/FDA
 FDA Regulation of Hearing Aids Eric A. Mann, MD, PhD Clinical Deputy Director Division of Ophthalmic ENT ODE/CDRH/FDA U.S. Food Drug Administration Presentation Outline Overview of device regulations riskbased
FDA Regulation of Hearing Aids Eric A. Mann, MD, PhD Clinical Deputy Director Division of Ophthalmic ENT ODE/CDRH/FDA U.S. Food Drug Administration Presentation Outline Overview of device regulations riskbased
FDA s Final Compliance Policy Guide for Marketed Unapproved Drugs Is Agency Enforcement at a Crossroads, or Stuck in a Traffic Circle
 FDA s Final Compliance Policy Guide for Marketed Unapproved Drugs Is Agency Enforcement at a Crossroads, or Stuck in a Traffic Circle Kurt R. Karst Associate Hyman, Phelps & McNamara, P.C. 700 Thirteenth
FDA s Final Compliance Policy Guide for Marketed Unapproved Drugs Is Agency Enforcement at a Crossroads, or Stuck in a Traffic Circle Kurt R. Karst Associate Hyman, Phelps & McNamara, P.C. 700 Thirteenth
Guidance for Industry
 Guidance for Industry Electronic Source Data in Clinical Investigations U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for
Guidance for Industry Electronic Source Data in Clinical Investigations U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for
KIZ 2,33-3 p8 ~ 510(k) Summary. As required by 21 CFR 807.92(c) 510(k) Premarket Notification Submission AFrame Digital MobileCare Monitor.
 KIZ 2,33-3 p8 ~ 510(k) Premarket Notification Submission AFrame Digital MobileCare Monitor 510(k) Summary f 1 As required by 21 CFR 807.92(c) Submitter: 510(k) Owner: AFrame Digital, Inc. Address:.1889
KIZ 2,33-3 p8 ~ 510(k) Premarket Notification Submission AFrame Digital MobileCare Monitor 510(k) Summary f 1 As required by 21 CFR 807.92(c) Submitter: 510(k) Owner: AFrame Digital, Inc. Address:.1889
Information Sheet Guidance For IRBs, Clinical Investigators, and Sponsors
 Information Sheet Guidance For IRBs, Clinical Investigators, and Sponsors Frequently Asked Questions About Medical Devices Additional copies are available from: Office of Good Clinical Practice Office
Information Sheet Guidance For IRBs, Clinical Investigators, and Sponsors Frequently Asked Questions About Medical Devices Additional copies are available from: Office of Good Clinical Practice Office
Privacy Policy Version 1.0, 1 st of May 2016
 Privacy Policy Version 1.0, 1 st of May 2016 THIS PRIVACY POLICY APPLIES TO PERSONAL INFORMATION COLLECTED BY GOCIETY SOLUTIONS FROM USERS OF THE GOCIETY SOLUTIONS APPLICATIONS (GoLivePhone and GoLiveAssist)
Privacy Policy Version 1.0, 1 st of May 2016 THIS PRIVACY POLICY APPLIES TO PERSONAL INFORMATION COLLECTED BY GOCIETY SOLUTIONS FROM USERS OF THE GOCIETY SOLUTIONS APPLICATIONS (GoLivePhone and GoLiveAssist)
Security of Medical Device Applications
 Security of Medical Device Applications Dennis M. Seymour, CISSP, PMP Senior Security Architect Ellumen, Inc. Prepared for 14th Semi-Annual Software Assurance Forum Objectives Recent Article (ISC)2 FDA
Security of Medical Device Applications Dennis M. Seymour, CISSP, PMP Senior Security Architect Ellumen, Inc. Prepared for 14th Semi-Annual Software Assurance Forum Objectives Recent Article (ISC)2 FDA
Brainreader ApS February 4, 2015 C/O Mette Munch QA Consultant Skagenvej 21 Egaa, 8250 DENMARK
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center - WO66-G609 Silver Spring, MD 20993-0002 Brainreader ApS February
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center - WO66-G609 Silver Spring, MD 20993-0002 Brainreader ApS February
510(k) Summary GlobalMedia Group, LIC. MAY 0 7 2013 CONiM
 510(k) Summary GlobalMedia Group, LIC. MAY 0 7 2013 CONiM Date Prepared: January 8, 2013 Submitter's Information: GlobalMedia Group, LLC 15020 N. 74th St. Scottsdale, AZ 85260 Contact: Nicholas Campbell
510(k) Summary GlobalMedia Group, LIC. MAY 0 7 2013 CONiM Date Prepared: January 8, 2013 Submitter's Information: GlobalMedia Group, LLC 15020 N. 74th St. Scottsdale, AZ 85260 Contact: Nicholas Campbell
Leveraging Partners and Open Source Technology in your Mobility Strategy. emids webinar Thursday, August 11, 2011 1:00 pm 2:00 pm EDT
 Leveraging Partners and Open Source Technology in your Mobility Strategy emids webinar Thursday, August 11, 2011 1:00 pm 2:00 pm EDT Presenters Jerry Buchanan Account Director emids Technologies Ambarish
Leveraging Partners and Open Source Technology in your Mobility Strategy emids webinar Thursday, August 11, 2011 1:00 pm 2:00 pm EDT Presenters Jerry Buchanan Account Director emids Technologies Ambarish
Mobile Medical Apps. 34% of Adults 34% App = Application; = a self contained program or piece of software designed to fulfill a particular purpose;
 Mobile Medical Apps CONSTANTINE GEAN, MD, MS, MBA, FACOEM AOHC 2014 April 30, 2014 DISCLAIMERS ( Aka, My lawyer told me to say nothing I say matters and it s not my fault :) THESE APPS ARE PRESENTED FOR
Mobile Medical Apps CONSTANTINE GEAN, MD, MS, MBA, FACOEM AOHC 2014 April 30, 2014 DISCLAIMERS ( Aka, My lawyer told me to say nothing I say matters and it s not my fault :) THESE APPS ARE PRESENTED FOR
EU and US regulation of health information technology, software and mobile apps
 EU and US regulation of health information technology, software and mobile apps www.practicallaw.com/3-518-3154 Lincoln Tsang, Vernessa Pollard and Daniel Kracov Arnold & Porter LLP The emergence of integrated
EU and US regulation of health information technology, software and mobile apps www.practicallaw.com/3-518-3154 Lincoln Tsang, Vernessa Pollard and Daniel Kracov Arnold & Porter LLP The emergence of integrated
RAPS ONLINE UNIVERSITY
 RAPS ONLINE UNIVERSITY Practical education and training for business success. For regulatory professionals, there is only one name to know and trust for online education and training RAPS Online University.
RAPS ONLINE UNIVERSITY Practical education and training for business success. For regulatory professionals, there is only one name to know and trust for online education and training RAPS Online University.
Marketed Unapproved Drugs: FDA to Take Immediate Enforcement Action at Any Time, Without Prior Notice
 Marketed Unapproved Drugs: FDA to Take Immediate Enforcement Action at Any Time, Without Prior Notice Kurt R. Karst Hyman, Phelps & McNamara, P.C. 700 Thirteenth Street, N.W., Suite 1200 Washington, D.C.
Marketed Unapproved Drugs: FDA to Take Immediate Enforcement Action at Any Time, Without Prior Notice Kurt R. Karst Hyman, Phelps & McNamara, P.C. 700 Thirteenth Street, N.W., Suite 1200 Washington, D.C.
30 In Compliance October 2014 www.incompliancemag.com
 30 In Compliance October 2014 www.incompliancemag.com Medical Devices in a Wireless World BY IVAYLO TANKOV While wireless technology is now an integral component of a wide variety of manufactured products,
30 In Compliance October 2014 www.incompliancemag.com Medical Devices in a Wireless World BY IVAYLO TANKOV While wireless technology is now an integral component of a wide variety of manufactured products,
Correspondence between ISO 13485:2003 and the US Quality System Regulation
 Correspondence between ISO 13485:2003 and the US Quality System Regulation Correspondence between ISO 13485:2003 and the US Quality System Regulation 1 Scope 1.1 General This International Standard specifies
Correspondence between ISO 13485:2003 and the US Quality System Regulation Correspondence between ISO 13485:2003 and the US Quality System Regulation 1 Scope 1.1 General This International Standard specifies
Access to Electronic Health Records Policy Franciscan Health System
 Access to Electronic Health Records Policy Franciscan Health System PURPOSE: The purpose of the Access to Electronic Health Records Policy ( EHR Policy ) is to establish processes and procedures for permitting
Access to Electronic Health Records Policy Franciscan Health System PURPOSE: The purpose of the Access to Electronic Health Records Policy ( EHR Policy ) is to establish processes and procedures for permitting
March 31, 2016. Etiometry, Inc. Richard Galgon Independent Consulting Associate Quintiles 5846 Cobblestone Lane Waunakee, Wisconsin 53597
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service March 31, 2016 Food and Drug Administration 10903 New Hampshire Avenue Document Control Center - WO66-G609 Silver Spring, MD 20993-0002 Etiometry,
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service March 31, 2016 Food and Drug Administration 10903 New Hampshire Avenue Document Control Center - WO66-G609 Silver Spring, MD 20993-0002 Etiometry,
College of Pharmacy. Pharmacy Practice and Science
 520 SPECIAL TOPICS IN PHARMACY LAW. (2) Discussion of the legal framework and special legal issues in pharmacy practice. Topics will include application of antitrust laws to pharmacy, patent and trademark
520 SPECIAL TOPICS IN PHARMACY LAW. (2) Discussion of the legal framework and special legal issues in pharmacy practice. Topics will include application of antitrust laws to pharmacy, patent and trademark
Electronic Health Records
 What Do Electronic Health Records Mean for Our Practice? What are Electronic Health Records? Electronic Health Records (EHRs) are computer systems that medical practices use instead of paper charts. All
What Do Electronic Health Records Mean for Our Practice? What are Electronic Health Records? Electronic Health Records (EHRs) are computer systems that medical practices use instead of paper charts. All
