National Institute for Clinical Excellence
|
|
|
- Karin Preston
- 8 years ago
- Views:
Transcription
1 National Institute for Clinical Excellence 11 Strand London WC2N 5HR Web: N k 1P July 01 (ABA)
2 Technology Appraisal Guidance - No.27 Guidance on the use of cyclo-oxygenase (Cox) II selective inhibitors, celecoxib, rofecoxib, meloxicam and etodolac for osteoarthritis and rheumatoid arthritis July 2001
3 Technology Appraisal No. 27 Guidance on the use of cyclo-oxygenase (Cox) II selective inhibitors, celecoxib, rofecoxib, meloxicam and etodolac for osteoarthritis and rheumatoid arthritis Issue date: July 2001 Review date: May 2004 Ordering Information Copies of this Guidance can be obtained from the NHS Response Line by telephoning and quoting ref. N0016. A patient version of this document can be obtained by quoting ref. N0018. A bi-lingual patient leaflet is also available ref. N0019. Distribution of Guidelines This document has been circulated to the following: Health Authority Chief Executives in England and Wales NHS Trust Chief Executives in England and Wales PCG Chief Executives Local Health Group General Managers Medical and Nursing Directors in England and Wales GPs in England and Wales Consultant Geriatricians in England and Wales Rheumatologists in England and Wales Chief Pharmacists, Heads of Drug Purchasing, Heads of Drug Information, Pharmaceutical Advisors, GP Prescribing Advisors and Purchase Advisors in England and Wales NHS Director Wales Chief Executive of the NHS in England NHS Executive Regional Directors Special Health Authority Chief Executives Community Health Councils in England and Wales Patient Advocacy Groups Commission for Health Improvement NHS Clinical Governance Support Team Chief Medical, Nursing Officers and Pharmaceutical Officers in England and Wales Medical Director & Head of NHS Quality National Assembly for Wales Representative bodies for health services, professional organisations and statutory bodies, Royal Colleges This Guidance is written in the following context: This guidance represents the view of the Institute which was arrived at after careful consideration of the available evidence. Health professionals are expected to take it fully into account when exercising their clinical judgement. This guidance does not, however, override the individual responsibility of health professionals to make appropriate decisions in the circumstances of the individual patient, in consultation with the patient and/or guardian or carer. National Institute for Clinical Excellence 11 Strand London WC2N 5HR Web: ISBN: Published by the National Institute for Clinical Excellence July 2001 Copyright National Institute for Clinical Excellence All rights reserved. This material may be freely reproduced for educational and not for profit purposes within the NHS. No reproduction by or for commercial organisations is permitted without the express written permission of the Institute.
4 Technology Appraisal Guidance No. 27 Issue Date July 2001 Review Date May 2004 Guidance on the use of cyclooxygenase (Cox) II selective inhibitors, celecoxib, rofecoxib, meloxicam and etodolac for osteoarthritis and rheumatoid arthritis This section (Section 1) constitutes the Institute's Guidance on the use of cyclooxygenase (Cox) II selective inhibitors, celecoxib, rofecoxib, meloxicam and etodolac for osteoarthritis and rheumatoid arthritis. The remainder of the document is structured in the following way: 2 Clinical Need 3 The Technology 4 Evidence 5 Implications for the NHS 6 Related Guidance 7 Further Research 8 Implementation 9 Review of Guidance Appendix A: Appraisal Committee Appendix B: Sources of Evidence Appendix C: Information for Patients The full document and a Summary of Evidence are available from our website at or by telephoning and quoting the reference number N0016. Mae r adran hon (adran 1) hefyd ar gael yn Gymraeg ar ein gwefan neu drwy gysylltu â , rhif cyfeirnod N Guidance 1.1 Cox II selective inhibitors and other non-steroidal anti-inflammatory drugs (NSAIDs) are indicated for pain and stiffness in inflammatory rheumatoid arthritis and for the short-term management of pain in osteoarthritis. All NSAIDs are associated with adverse events and should only be prescribed when there is a demonstrable clinical need and in accordance with their summary of product characteristics. Long-term use should be avoided without appropriate monitoring and re-evaluation of the clinical need. 1.2 Of particular concern is the propensity of NSAIDs, including the Cox II selective agents, to cause gastro-intestinal adverse events, which can include life threatening gastro-intestinal perforations, ulcers or bleeds. These agents should therefore only be prescribed after careful consideration of their risks and benefits, especially in patients who may be at increased risk of such adverse events. 1.3 Cox II selective inhibitors are not recommended for routine use in patients with rheumatoid arthritis (RA) or osteoarthritis (OA). They should be used, in preference to standard NSAIDs, when clearly indicated as part of the management of RA or OA only in patients who may be at high risk of developing serious gastrointestinal adverse effects. 1.4 Patients at high risk of developing serious gastrointestinal adverse events include those of 65 years of age and over, those using concomitant medications known to increase the likelihood of upper gastrointestinal adverse events, those with serious co-morbidity or those requiring the prolonged use of maximum recommended doses of standard NSAIDs (See Section 2.10). The risk of NSAID-induced complications is particularly increased in patients with a previous clinical history of gastroduodenal ulcer, gastrointestinal bleeding or gastroduodenal perforation. The use of even a Cox II selective agent should therefore be considered especially carefully in this situation. 1.5 In all patients with cardiovascular disease, there remains uncertainty over the use of Cox II selective inhibitors and they should not therefore be prescribed routinely in preference to standard NSAIDs where these are indicated in this group of patients. Furthermore, many patients with cardiovascular disease receive low dose aspirin and this carries an increased risk of gastro-intestinal events. In patients who are taking low dose aspirin, the benefit of using Cox II selective agents (to decrease gastrointestinal toxicity) is reduced. Prescribing Cox II selective agents preferentially over standard NSAIDs in this situation is therefore not justified on current evidence. 1.6 There is no evidence to justify the simultaneous prescription of gastroprotective agents with Cox II selective inhibitors as a means of further reducing potential gastrointestinal adverse events.
5 2 Clinical Need and Practice 2.1 Arthritis is a general term to describe the inflammatory or degenerative disease of one or more joints. Symptoms can include some or all of the following: pain, swelling, stiffness, restriction of movement and redness of the skin overlying the affected joint. RA and OA are the two most common forms. There are an estimated 1,325,000 1,750,000 OA patients and 250, ,000 RA patients in England and Wales. 2.2 RA is a progressive, destructive condition, for which therapy aims to control inflammation, pain and associated symptoms, thereby increasing quality of life. In the management of RA, NSAIDs are often used for extended periods of time and frequently combined with disease modifying agents. OA is a chronic condition in which the principal pathology is degenerative change. Although some modification of disease progression is possible the main therapeutic end point is pain control with simple analgesics. NSAIDs are often used in the short term to control acute inflammatory episodes. They are probably of little benefit in long-term continuous use for OA, which is likely to lead to increased risk of potentially harmful adverse effects. In both OA and RA pain control facilitates compliance with other treatment modalities such as weight loss and physiotherapy, which may influence disease progression. 2.3 Non-drug treatment is considered an important component of management in OA and RA and it includes patient education, physical and occupational therapy, dietary advice, selfmanagement programmes and weight loss. 2.4 Arthritis is a major cause of morbidity and disability in England and Wales and imposes a considerable financial burden on both patients and the NHS. The annual NHS expenditure on medical care for arthritis patients is estimated to be around 560m 920m, excluding indirect costs. 2.5 In 1999, over 18.5 million NSAID treatments were prescribed in England and Wales for various indications, at a cost of approximately 170m. This does not include the costs of coprescribing of gastroprotective agents. NSAIDs are also often used for conditions outside of their licensed indications and for pain not associated with inflammation. 2.6 All NSAIDs are believed to exert their anti-inflammatory and analgesic action by interfering with the formation of prostaglandins from their precursor (arachidonic acid) via inhibition of the catalysing enzyme cyclo-oxygenase (Cox). Prostaglandins are responsible for mediation of inflammation and pain, but also play a critical role in the protection of gastric mucosa, the support of renal function and in haemostasis through platelet activation. 2 NICE Technology Appraisal Guidance No.27
6 2.7 NSAIDs are associated with upper gastrointestinal side effects, ranging from mild dyspepsia to more severe complications such as gastric haemorrhage and perforation, which lead to hospitalisation, surgery or even death. Therefore, these drugs potentially have an important impact on both patient quality of life and health care expenditure. According to some estimates, around 2,000 deaths due to NSAID related side effects during treatment for arthritis occur each year in the UK. 2.8 Peptic ulcers can be detected endoscopically in 10-20% of people taking standard doses of traditional NSAIDs and simple erosions of the gastric mucosa in a further 20-40%. It is not clear how these endoscopic findings relate to the reported gastrointestinal adverse effects of NSAIDs or to the more serious complications such as perforation, ulcer or bleeding (PUB). 2.9 Gastro-protective agents (GPAs) are often co-prescribed with NSAIDs, with the aim of reducing the associated gastrointestinal adverse effects. It is estimated that coprescribing rates range from 17% to 34%. The most commonly used GPAs include proton pump inhibitors, H 2 receptor antagonists and misoprostol, a prostaglandin E1 analogue. However, these agents have been shown to be only partially effective in the prophylaxis and treatment of NSAID related gastrointestinal events. They are also not without additional side effects and add significantly to the total cost of drug therapy The following factors have been shown to be associated with a high risk of development of gastrointestinal complications following NSAID therapy: Age of 65 years and over; Previous clinical history of gastroduodenal ulcer, gastrointestinal bleeding or gastroduodenal perforation; Concomitant use of medications that are known to increase the likelihood of upper gastrointestinal adverse events e.g. steroids and anti-coagulants; Presence of serious co-morbidity, such as cardio-vascular disease, renal or hepatic impairment, diabetes and hypertension; Requirement for the prolonged use of maximum recommended doses of standard NSAIDs. NICE Technology Appraisal Guidance No.27 3
7 3 The Technology 3.1 There are at least two isoforms of cyclo-oxygenase: Cox I (constitutive enzyme) and Cox II (inducible enzyme). High concentrations of Cox I are found in platelets, vascular endothelial cells, the stomach and in kidney collecting tubules. It is responsible for the production of prostaglandins, which are essential for maintenance of normal endocrine function, renal function, gastric mucosal integrity and haemostasis. In contrast, under physiological conditions, Cox II is virtually undetectable in most tissues, but its activity may be significantly increased by inflammatory and mitogenic stimuli. 3.2 Cox II selective inhibitors selectively block the Cox II iso-form of the cyclo-oxygenase enzyme, whilst largely sparing the Cox I isoform. Inhibition of Cox I is thought to be principally responsible for the gastrointestinal adverse effects of NSAIDs. 3.3 There is wide variation in the reported Cox II selectivity of the NSAIDs, as assessed by different assay techniques, and therefore classification of these agents according to their selectivity remains problematic. Celecoxib and rofecoxib, two recently introduced Cox II inhibitors, are often classified as "Cox II specific agents" due to claims of higher Cox II selectivity compared to the more established NSAIDs, meloxicam and etodolac, which are often referred to as "Cox II selective". This guidance refers to all four drugs as Cox II selective inhibitors. 3.4 Celecoxib and etodolac are licensed for symptomatic relief in the treatment of both OA and RA, whereas rofecoxib is licensed for OA only. Meloxicam is licensed for short-term symptomatic treatment of acute exacerbations of OA and long-term symptomatic treatment of RA. 3.5 The ranges of daily acquisition costs, based on the British National Formulary (September 2000) listed prices, are currently for meloxicam, 0.52 for etodolac, for celecoxib and 0.77 for rofecoxib. Consideration of differential daily acquisition costs should include the different dosage requirements for each drug within their licensed indications. 4 Evidence Clinical Effectiveness 4.1 A total of 53 randomised controlled trials (RCTs) involving 61,731 patients with RA and OA were reviewed (14 rofecoxib, 15 celecoxib, 3 rofecoxib versus celecoxib, 9 meloxicam and 12 etodolac). These trials included both placebo and NSAID comparators (naproxen, diclofenac, ibuprofen, piroxicam, tenoxicam, nabumetone, nimesulide and aspirin). 4 NICE Technology Appraisal Guidance No.27
8 4.2 There is evidence that the Cox II selective inhibitors referred to in this Guidance (rofecoxib, celecoxib, meloxicam and etodolac) are more effective than placebo and of equivalent efficacy to other NSAIDs in their ability to reduce pain (resting and active) and to improve physical and global function in both OA and RA patients. 4.3 There is evidence to suggest that, although the four Cox II selective inhibitors increase the incidence of gastrointestinal adverse events compared to placebo in both OA and RA patients, the magnitude of this effect is less than that of standard NSAID therapy. 4.4 Rofecoxib and celecoxib have been shown to be associated with a reduced incidence of gastric erosions on endoscopy compared to standard NSAIDs in patients with arthritis. However, the importance of this finding as a surrogate for the development of peptic ulceration or other major gastrointestinal adverse effects in patients treated with these drugs in routine clinical practice is uncertain. 4.5 Indirect comparison of the effects of meloxicam, rofecoxib and celecoxib relative to other NSAIDs based on available RCT evidence, does not demonstrate a difference in either efficacy or adverse events between them. 4.6 The RCT evidence for the incidence of gastrointestinal adverse events associated with etodolac compared to other NSAIDs cannot be considered conclusive as many of the studies were insufficiently powered to detect differences in serious gastrointestinal adverse events. There is however postmarketing data for etodolac, collected over 15 years, that provides pragmatic evidence of a reduced incidence of serious gastrointestinal events compared to standard NSAIDs. All of the Cox II selective agents exhibit some degree of dose dependency of the incidence of upper gastrointestinal adverse effects but published evidence indicates that the therapeutic window is much wider for rofecoxib and celecoxib than for meloxicam and etodolac. 4.7 Concerns have been raised over the cardiovascular and renal effects of Cox II selective inhibitors. In particular, this has been highlighted in the recent VIGOR trial (n=8,076), comparing rofecoxib and naproxen, which reported an increase in the number of myocardial infarctions in the rofecoxib group (0.4% compared to 0.1%; relative risk, 4.0: 95% CI, 1.1 to 14.2). However 38% of these infarctions occurred in only 4% of the trial patients who would have been candidates for cardioprophylaxis with low-dose aspirin, but which was not permitted by the strict inclusion criteria for the study. There were however other patients on rofecoxib who suffered increased numbers of myocardial infarctions in the trial who were not, in retrospect, candidates for low does aspirin. The issue remains unresolved NICE Technology Appraisal Guidance No.27 5
9 and further research is required. This potential increased risk should, however, be taken into consideration when prescribing selective Cox II inhibitors in patients with cardiovascular disease, as is the case with all NSAIDs. 4.8 Aspirin in low dose is frequently indicated for the secondary prevention of thrombotic cerebrovascular or cardiovascular disease in the same patients who may also be at increased risk of the gastrointestinal complications which are associated with all NSAIDS, including aspirin. Co-prescription of aspirin with standard NSAIDs is known to increase the risk of such complications and evidence from the CLASS trial (n=8,059) suggests that the risk reduction of upper gastrointestinal events associated with Cox II selective inhibitors may not be evident when they are combined with aspirin. In this study, the annualised incidence rates of upper gastrointestinal complications alone and combined with symptomatic ulcers for celecoxib versus ibuprofen/diclofenac in 1,645 patients who were also taking low dose aspirin were 2.0% vs. 2.1% (p=0.92) and 4.7% vs. 6.0% (p=0.49). In the 6,323 patients, who were not receiving aspirin the rates for upper gastrointestinal complications were significantly lower for celecoxib (0.4% vs. 1.3 p=0.04), although in both aspirin and non-aspirin groups combined there was no significant difference in the annualised incidence of upper gastrointestinal complications (0.8% vs. 1.45% p=0.09). In the VIGOR study (n=8,076), in which concomitant aspirin was not permitted, the rates of confirmed upper gastrointestinal events (ulcers, perforations or bleeds) were found to be 2.1 and 4.5 per 100 patient years (relative risk, 0.5: 95% confidence interval 0.3 to 0.6, p<0.001) in the patients receiving rofecoxib or naproxen respectively. 4.9 No evidence was found to justify the simultaneous prescription of gastro-protective agents with Cox II selective inhibitors as a means of further reducing potential gastrointestinal adverse events. Furthermore, the relative benefits of combinations of standard NSAIDs with GPAs compared to Cox II inhibitors alone has not been determined In summary, in the absence of evidence of significant differences in anti-inflammatory efficacy between the Cox IIs and standard NSAIDs, the avoidance of serious adverse effects, particularly on the gastro-intestinal tract, becomes the most relevant factor when considering their use. For the most part the evidence suggests that the Cox II selective agents are less likely to produce symptomatic gastrointestinal tract adverse events. However, there remains some concern regarding the potential cardiovascular risks associated with the Cox II selective agents and therefore caution is needed, as it is for standard NSAID therapy, when prescribing in patients with pre-existing cardiovascular disease. 6 NICE Technology Appraisal Guidance No.27
10 Cost effectiveness 4.11 There are three published cost-effectiveness studies on the selective Cox II inhibitors. Two of these compared meloxicam to modified release diclofenac, one in the UK and the other in a cross-national context (UK, Italy and France). The third compared the costs associated with the use of rofecoxib, celecoxib, ibuprofen, naproxen and diclofenac, in a simple decision analytic model based on local U.S. prices Economic evaluations submitted by the manufacturers generally report favourable incremental cost per gastrointestinal event averted and cost per life year gained (LYG) for the Cox II selective inhibitors versus standard NSAIDs in all (high and average risk) OA and RA patients. In the industry submissions, the upper limit of the cost per serious gastrointestinal event saved (i.e. perforation, ulceration and bleeding - PUB) ranges from 3,500 to 10,759 and LYG values for all patients range between 6,842 and 15,647.These models assume significant reductions in coprescribing rates of GPAs with Cox II selective inhibitors and all but one model ignored non-gastrointestinal side effects. Two submissions assessed the use of Cox II selective agents in 'high-risk' patients and reported improved cost-effectiveness ratios when compared with conventional NSAIDs for this patient group A recent, but as yet unpublished, study commissioned by the Canadian Coordinating Office for Health Technology Assessment (CCOHTA), estimated higher, hence less favourable, cost effectiveness ratios than those submitted by the manufacturers in average-risk patients. In this study, celecoxib was dominated by diclofenac (i.e. celecoxib was more costly and less effective). In addition the estimated cost per LYG ratios for rofecoxib were greater than 150,000 when compared with standard NSAIDs in average-risk patients. The CCOHTA report also includes incremental cost per quality adjusted life year (QALY) estimates for celecoxib and rofecoxib versus standard NSAIDs. These cost per QALY estimates were again not favourable for average-risk OA and RA patients, but became so for the 'high risk' group. These results support the potential for enhanced cost effectiveness of these agents in the 'high risk' group of patients. There are no other cost per QALY estimates available to date In summary, there is insufficient evidence in the literature to conclude specifically on the differential clinical effectiveness of the various Cox II selective inhibitors or of their differential risk of causing adverse upper gastrointestinal events in high risk patients. Evidence from economic models and the fact that the absolute number of gastrointestinal events averted is likely to be greater for high risk than for other patients indicates that the cost-effectiveness of the Cox II selective inhibitors would be more favourable in this high risk group. NICE Technology Appraisal Guidance No.27 7
11 5 Implications for the NHS 6 Related Guidance 7 Further Research 5.1 The total budget impact of this guidance in the NHS in England and Wales will depend on a number of factors, including the extent of the reduction in GPA prescription, the relative risk reduction of gastrointestinal adverse events in high risk patients on changing to Cox II selective inhibitors, and the rate of uptake of the Cox II selective inhibitors. 5.2 Switching high-risk OA and RA patients to Cox II selective inhibitors would lead to an annual incremental cost of approximately 25 million to the NHS. This figure is based on the following assumptions: half of the high risk patients will be offered Cox II selective inhibitors; each product has an equal market share of 25%; the proportion of patients aged over 65 is 58% for OA and 45% for RA; the percentage of patients with previous gastrointestinal events is 8%; the average treatment duration is 180 days a year; and the relative risk reduction is constant across different risk groups. 6.1 The Institute issued guidance on the use of Proton Pump Inhibitors in the treatment of dyspepsia in June The Institute s clinical guideline for treatment of dyspepsia is in development. 7.1 There is a paucity of good quality comparative head to head randomised clinical trials of Cox II selective inhibitors in the treatment of arthritis, especially in patients at high risk of developing serious gastrointestinal adverse events. The subgroups of patients who are likely to benefit most from Cox II inhibitors need to be clearly defined. 7.2 There is a need for methodologically robust cost-effectiveness studies, possibly using data from head to head trials. These trials should specifically focus on adverse events and incorporate quality of life outcomes. 8 Implementation 7.3 The effectiveness of Cox II selective inhibitors in arthritis patients on low-dose aspirin treatment should also be further investigated. 8.1 Primary Care Groups, Local Health Groups and Trusts should take account of the acquisition costs of the individual products in determining the local prescribing practice of these selective Cox II inhibitors. 8.2 The use of Cox II selective inhibitors, and all other NSAIDs, outside of licensed indications should be discouraged. A significant proportion of the severe gastrointestinal events caused by NSAID treatment could be avoided by targeting appropriate patients. 8 NICE Technology Appraisal Guidance No.27
12 8.3 To enable clinicians to audit their implementation of this guidance, it is recommended that treatment outcomes be recorded for each patient receiving Cox II selective inhibitors who subsequently experience gastrointestinal adverse events and that these events are reported to the CSM through the yellow card scheme. 8.4 This information should be incorporated into local clinical audit data recording systems, and consideration given (if not already in place) to the establishment of appropriate categories in routine electronic record keeping systems used in primary care groups and hospitals. 9 Review of Guidance 8.5 Prospective clinical audit programmes should record the extent to which the recommendations of this guidance are implemented. Such programmes are likely to be more effective in improving patient care when they form part of the organisation s formal clinical governance arrangements and where they are linked to specific educational and training activities. 9.1 This guidance will be reviewed in May Andrew Dillon Chief Executive July 2001 NICE Technology Appraisal Guidance No.27 9
13 APPENDIX A Appraisal Committee Members The Appraisal Committee is a statutory committee whose members sit for 3 years. Two meetings are held per month and the majority of members attend one or the other. Declared interests may also exclude a member from individual technology appraisals. The committee are supplemented by technology specific experts as indicated in Appendix B. Professor R. L. Akehurst Dean, School of Health Related Research Sheffield University Professor David Barnett (Chairman) Professor of Clinical Pharmacology University of Leicester Professor Sir Colin Berry Professor of Morbid Anatomy St Bartholomew s and Royal London School of Medicine Dr Sheila Bird MRC Biostatistics Unit, Cambridge Professor Martin Buxton Director of Health Economics Research Group Brunel University Professor Yvonne Carter Professor of General Practice and Primary Care St Bartholomew s and Royal London School of Medicine Dr Karl Claxton Lecturer in Economics University of York Professor Duncan Colin-Jones Professor of Gastroenterology University of Southampton Professor Sarah Cowley Professor of Community Practice Development Kings College, London Dr Nicky Cullum Reader in Health Studies University of York Mr Chris Evennett Chief Executive Mid-Hampshire Primary Care Group Professor Terry Feest Clinical Director and Consultant Nephrologist Richard Bright Renal Unit and Chairman of the UK Renal Registry Ms Jean Gaffin Formerly Executive Director National Council for Hospice and Specialist Palliative Care Service Mrs Sue Gallagher Chief Executive Merton, Sutton and Wandsworth Health Authority Dr Trevor Gibbs International Medical Operations Director GlaxoWellcome R&D Ltd Mr John Goulston Director of Finance The Royal Free Hampstead NHS Trust Professor Philip Home Professor of Diabetes Medicine University of Newcastle Dr Terry John General Practitioner The Firs, London Dr Diane Ketley Clinical Governance Programme Leader Leicester Royal Infirmary Dr Mayur Lakhani General Practitioner, Highgate Surgery, Leicester and Lecturer, University of Leicester Mr M Mughal Consultant Surgeon Chorley and South Ribble NHS Trust Mr James Partridge Chief Executive Changing Faces Professor Philip Routledge Professor of Clinical Pharmacology University of Wales Professor Andrew Stevens Professor of Public Health University of Birmingham 10 NICE Technology Appraisal Guidance No.27
14 APPENDIX B Sources of Evidence The following documentation and opinion was made available to the Appraisals Committee: a. Assessment report prepared by the NICE Appraisals Team The Clinical Effectiveness and Cost-Effectiveness of Celecoxib, Rofecoxib, Meloxicam and Etodolac (Cox-II Inhibitors) for rheumatoid arthritis and osteoarthritis, October 2000 Assessment Report Addendum prepared by the NICE Appraisals Team, February 2001 d. External expert and patient advocate submissions from: Professor David L Scott, Professor of Clinical Rheumatology, King s College, London Dr John Dickson, Business Manager, Primary Care Rheumatology Society Neil Betteridge, Head of Policy and Campaigning,Arthritis Care b. Manufacturer/Sponsor submissions from: Boehringer Ingelheim Merck Sharp and Dohme Pharmacia and Pfizer Shire c. Professional/Specialist Group and Patient Group submissions from: British Geriatrics Society Chartered Society of Physiotherapy Royal College of General Practitioners BLAR (Arthritis Care,Arthritis Research Campaign, British League Against Rheumatism, British Society for Rheumatology and Primary Care Rheumatology Society) NICE Technology Appraisal Guidance No.27 11
15 APPENDIX C Guidance on the use of cyclo-oxygenase (Cox) II selective inhibitors, celecoxib, rofecoxib, meloxicam and etodolac for osteoarthritis and rheumatoid arthritis Patient information The patient information in this appendix has been designed to support the production of your own information leaflets. You can download it from our website at where it is available in English and Welsh. If you would like printed copies of the leaflets please ring the NHS Response Line on and quote reference number N0018 for the English patient leaflet and N0019 for the bi-lingual leaflet. What is NICE Guidance? The National Institute for Clinical Excellence (NICE) is a part of the NHS. It produces guidance for both the NHS and patients on the use of medicines, medical equipment, diagnostic tests and clinical and surgical procedures. When the Institute evaluates these things, it is called an appraisal. Each appraisal takes around 12 months to complete and involves the manufacturers of the drug or device, the professional organisations and the national groups who represent patients and their carers. NICE looked at the available evidence on the cyclo-oxygenase (Cox) II selective inhibitors, celecoxib, rofecoxib, meloxicam and etodolac and has provided guidance that will help the NHS in England and Wales decide when they should be used in the treatment of osteoarthritis and rheumatoid arthritis. What is arthritis? Arthritis is a general term used to describe a disease of the joints. Symptoms can include some or all of the following: pain, swelling, stiffness, difficulty in movement and redness of the skin over the affected joint. The two most common forms of arthritis are rheumatoid arthritis and osteoarthritis. There are 1,325,000 1,750,000 people with osteoarthritis and 250, ,000 people with rheumatoid arthritis in England and Wales. Rheumatoid arthritis is a condition in which the joints are inflamed and damaged over a long period of time. Treatment aims to improve quality of life by controlling the symptoms of the disease which can include inflammation (swelling of the joint) and pain. Nonsteroidal anti-inflammatory drugs (NSAIDs) are often used, and they can be combined with other medicines that can alter the way the disease progresses. Osteoarthritis is a form of arthritis that gradually damages the cartilage that lines the joints. Although some medicines are used with the aim of slowing down the disease, the main aim of treatment is to control pain with simple pain relieving drugs. 12 NICE Technology Appraisal Guidance No.27
16 What are Cox II inhibitors? Cox II inhibitors are a type of non-steroidal anti-inflammatory drug (NSAID) used for the short-term treatment of acute inflammation in the joints caused by arthritis. They include celecoxib (Celebrex) and etodolac (Lodine SR) which can be used for rheumatoid arthritis and osteoarthritis, rofecoxib (Vioxx) which is only used for osteoarthritis and meloxicam (Mobic) which is used for acute osteoarthritis and the long-term treatment of rheumatoid arthritis. Cox II selective inhibitors and other non-steroidal antiinflammatory drugs (NSAIDs) are used to treat pain and stiffness in inflammatory rheumatoid arthritis and for the short-term management of pain in osteoarthritis. What has NICE recommended? Cox II selective inhibitors are not recommended for routine (regular) use in patients with rheumatoid arthritis or osteoarthritis. They should only be used, instead of standard NSAIDs, in people with rheumatoid arthritis or osteoarthritis who may be at high risk of developing serious gastrointestinal problems. All NSAIDs can cause side effects (other problems) and they should only be prescribed when there is a demonstrable clinical need and they should only be used for the type of disease that they are licensed for. Long-term use of these products should be avoided unless the person taking the medicine is monitored and their condition is checked to see if these medicines are still required. All NSAIDs, including the Cox II selective agents, can cause side effects such as gastro-intestinal problems (problems in the stomach or intestine). These problems can include stomach ulcers or bleeding, and possibly life threatening perforations (rips or holes) in the wall of the stomach or intestine. These drugs should therefore only be prescribed after a careful consideration of the risks and benefits for the person who would be taking them. Special care should be taken in people who are considered at a high-risk of such gastro-intestinal problems. People at high risk of developing serious gastro-intestinal problems include: those aged 65 years and over; those using other medicines that are known to increase the likelihood of gastro-intestinal problems; those with some other diseases related to their osteoarthritis or rheumatoid arthritis (these are called serious co-morbidities); and those requiring the long term use of standard NSAIDs at the maximum dose. NICE Technology Appraisal Guidance No.27 13
17 What should I do? Will NICE review its guidance? Further Information If you, or someone you care for, has rheumatoid or osteoarthritis then you should discuss this advice at your next appointment. Yes. The guidance will be reviewed in May Further information on NICE, and the full guidance on Cox II selective inhibitors, issued to the NHS is available on the NICE web site ( The guidance can also be requested from , quoting reference N0016. If you would like the full guidance on PPIs please quote reference 21492, and reference for the patient leaflet. If you have access to the Internet and would like to find out more about osteoarthritis or rheumatoid arthritis please visit the NHS Direct website: If you would like to speak to NHS direct please call them on NICE Technology Appraisal Guidance No.27
18
Technology appraisal guidance Published: 10 August 2000 nice.org.uk/guidance/ta1
 Guidance on the Extraction of Wisdom Teeth Technology appraisal guidance Published: 10 August 2000 nice.org.uk/guidance/ta1 NICE 2000. All rights reserved. Contents 1 Guidance... 3 2 Clinical Need and
Guidance on the Extraction of Wisdom Teeth Technology appraisal guidance Published: 10 August 2000 nice.org.uk/guidance/ta1 NICE 2000. All rights reserved. Contents 1 Guidance... 3 2 Clinical Need and
National Institute for Clinical Excellence
 National Institute for Clinical Excellence 90 Long Acre Covent Garden London WC2E 9RZ Web: www.nice.org.uk 22197 1P 45k Sept 00 (ABA) Guidance on the use of inhaler systems (devices) in children under
National Institute for Clinical Excellence 90 Long Acre Covent Garden London WC2E 9RZ Web: www.nice.org.uk 22197 1P 45k Sept 00 (ABA) Guidance on the use of inhaler systems (devices) in children under
Articles Presented. Journal Presentation. Dr Albert Lo. Dr Albert Lo
 * This presentation is prepared by the author in one s personal capacity for the purpose of academic exchange and does not represent the views of his/her organisations on the topic discussed. Journal Presentation
* This presentation is prepared by the author in one s personal capacity for the purpose of academic exchange and does not represent the views of his/her organisations on the topic discussed. Journal Presentation
A. Ketorolac*** B. Naproxen C. Ibuprofen D. Celecoxib
 1. A man, 66 years of age, with a history of knee osteoarthritis (OA) is experiencing increasing pain at rest and with physical activity. He also has a history of depression and coronary artery disease.
1. A man, 66 years of age, with a history of knee osteoarthritis (OA) is experiencing increasing pain at rest and with physical activity. He also has a history of depression and coronary artery disease.
Psoriasis and Psoriatic Arthritis Alliance
 Psoriasis and Psoriatic Arthritis Alliance A principal source of information on psoriasis and psoriatic arthritis ) Treatments for Psoriatic Arthritis overview Although psoriatic arthritis is a chronic
Psoriasis and Psoriatic Arthritis Alliance A principal source of information on psoriasis and psoriatic arthritis ) Treatments for Psoriatic Arthritis overview Although psoriatic arthritis is a chronic
CHAPTER 1 INTRODUCTION
 CHAPTER 1 INTRODUCTION Rheumatoid Arthritis (RA) is a chronic syndrome characterised by non-specific, usually symmetric inflammation of the peripheral joints, potentially resulting in progressive destruction
CHAPTER 1 INTRODUCTION Rheumatoid Arthritis (RA) is a chronic syndrome characterised by non-specific, usually symmetric inflammation of the peripheral joints, potentially resulting in progressive destruction
Comparative Effectiveness Review Number 4. Comparative Effectiveness and Safety of Analgesics for Osteoarthritis
 This report is based on research conducted by the Oregon Evidence-based Practice Center (EPC) under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. 290-02-0024).
This report is based on research conducted by the Oregon Evidence-based Practice Center (EPC) under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. 290-02-0024).
Low back pain. Quick reference guide. Issue date: May 2009. Early management of persistent non-specific low back pain
 Issue date: May 2009 Low back pain Early management of persistent non-specific low back pain Developed by the National Collaborating Centre for Primary Care About this booklet This is a quick reference
Issue date: May 2009 Low back pain Early management of persistent non-specific low back pain Developed by the National Collaborating Centre for Primary Care About this booklet This is a quick reference
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and
 Health Technology Assessment 2008; Vol. 12: No. 11 Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for
Health Technology Assessment 2008; Vol. 12: No. 11 Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for
Using big data to identify opportunity and drive informed change in secondary care
 Using big data to identify opportunity and drive informed change in secondary care Exploring the changing patterns of Non-Steroidal Anti-Inflammatory Drug dispensing in UK hospitals ABOUT IMS HEALTH IMS
Using big data to identify opportunity and drive informed change in secondary care Exploring the changing patterns of Non-Steroidal Anti-Inflammatory Drug dispensing in UK hospitals ABOUT IMS HEALTH IMS
What is Rheumatoid Arthritis?
 Name: A Case Study in Pharmaceutical Research Date: Class Work: Part I Aim: How do Biologists Select their Projects? What is Rheumatoid Arthritis? Rheumatoid arthritis (rue-ma-toyd arth-write-tis) is a
Name: A Case Study in Pharmaceutical Research Date: Class Work: Part I Aim: How do Biologists Select their Projects? What is Rheumatoid Arthritis? Rheumatoid arthritis (rue-ma-toyd arth-write-tis) is a
What s the Deal with NSAIDs?
 Focus on CME at the Memorial xxx University of Newfoundland What s the Deal with NSAIDs? Majed M. Khraishi, MB, BCh, FRCPC Presented at Wednesday at Noon Ask the Consultant Arthritis is one of the most
Focus on CME at the Memorial xxx University of Newfoundland What s the Deal with NSAIDs? Majed M. Khraishi, MB, BCh, FRCPC Presented at Wednesday at Noon Ask the Consultant Arthritis is one of the most
Rheumatoid arthritis inadults
 Understanding NICE guidance Information for people who use NHS services Rheumatoid arthritis inadults NICE clinical guidelines advise the NHS on caring for people with specific conditions or diseases and
Understanding NICE guidance Information for people who use NHS services Rheumatoid arthritis inadults NICE clinical guidelines advise the NHS on caring for people with specific conditions or diseases and
Apixaban for the prevention of stroke and systemic embolism in people with non-valvular atrial fibrillation
 Apixaban for the prevention of stroke and systemic embolism in people with non-valvular atrial fibrillation ERRATUM This report was commissioned by the NIHR HTA Programme as project number 11/49 This document
Apixaban for the prevention of stroke and systemic embolism in people with non-valvular atrial fibrillation ERRATUM This report was commissioned by the NIHR HTA Programme as project number 11/49 This document
Analgesics for Osteoarthritis: An Update of the
 Comparative Effectiveness Review Number 38 Analgesics for Osteoarthritis: An Update of the 2006 Comparative Effectiveness Review Comparative Effectiveness Review Number 38 Analgesics for Osteoarthritis:
Comparative Effectiveness Review Number 38 Analgesics for Osteoarthritis: An Update of the 2006 Comparative Effectiveness Review Comparative Effectiveness Review Number 38 Analgesics for Osteoarthritis:
What Effects do Provincial Drug Plan Coverage Policies for New Drugs have on Patterns of Use and Cost?
 Enhancing the effectiveness of health care for Ontarians through research What Effects do Provincial Drug Plan Coverage Policies for New Drugs have on Patterns of Use and Cost? November 2003 What effects
Enhancing the effectiveness of health care for Ontarians through research What Effects do Provincial Drug Plan Coverage Policies for New Drugs have on Patterns of Use and Cost? November 2003 What effects
Choosing Pain Medicine for Osteoarthritis. A Guide for Consumers
 Choosing Pain Medicine for Osteoarthritis A Guide for Consumers Fast Facts on Pain Relievers Acetaminophen (Tylenol ) works on mild pain and has fewer risks than other pain pills. Prescription (Rx) pain
Choosing Pain Medicine for Osteoarthritis A Guide for Consumers Fast Facts on Pain Relievers Acetaminophen (Tylenol ) works on mild pain and has fewer risks than other pain pills. Prescription (Rx) pain
A. Approval for the indications of osteoarthritis and rheumatoid arthritis at a dose of 10mg/day and dysmenorrhea at a dose of 20-mg bid as needed.
 Agent: Valdecoxib Indication: Analgesia, Dysmenorrhea Osteoarthritis, and Rheumatoid Arthritis Reviewer: Kent Johnson, MD Date: November 7, 2001 NDA: 21,341 EXECUTIVE SUMMARY 1-RECOMMENDATIONS A. Approval
Agent: Valdecoxib Indication: Analgesia, Dysmenorrhea Osteoarthritis, and Rheumatoid Arthritis Reviewer: Kent Johnson, MD Date: November 7, 2001 NDA: 21,341 EXECUTIVE SUMMARY 1-RECOMMENDATIONS A. Approval
VIOXX Gastrointestinal Outcomes Research Trial (VIGOR) Bonnie Goldmann, M.D. Regulatory Affairs Merck Research Laboratories
 VIOXX Gastrointestinal Outcomes Research Trial (VIGOR) Bonnie Goldmann, M.D. Regulatory Affairs Merck Research Laboratories 1 Arachidonic Acid CO 2 H COX-1 NSAIDs COX-2 Prostanoids Prostanoids Protection
VIOXX Gastrointestinal Outcomes Research Trial (VIGOR) Bonnie Goldmann, M.D. Regulatory Affairs Merck Research Laboratories 1 Arachidonic Acid CO 2 H COX-1 NSAIDs COX-2 Prostanoids Prostanoids Protection
patient group direction
 DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
Shared care protocol for the management of patients with Rheumatoid Arthritis treated with disease modifying antirheumatic drugs (DMARDs)
 Tameside Hospital NHS Foundation Trust and NHS Tameside and Glossop Shared care protocol for the management of patients with Rheumatoid Arthritis treated with disease modifying antirheumatic drugs (DMARDs)
Tameside Hospital NHS Foundation Trust and NHS Tameside and Glossop Shared care protocol for the management of patients with Rheumatoid Arthritis treated with disease modifying antirheumatic drugs (DMARDs)
Rheumatology through the ages. Dr Laurel Young Consultant Rheumatologist Royal Berkshire Hospital
 Rheumatology through the ages Dr Laurel Young Consultant Rheumatologist Royal Berkshire Hospital Outline of talk History of rheumatology Old therapies History of rheumatology in Reading Water therapy History
Rheumatology through the ages Dr Laurel Young Consultant Rheumatologist Royal Berkshire Hospital Outline of talk History of rheumatology Old therapies History of rheumatology in Reading Water therapy History
Cost-effectiveness of dimethyl fumarate (Tecfidera ) for the treatment of adult patients with relapsing remitting multiple sclerosis
 Cost-effectiveness of dimethyl fumarate (Tecfidera ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness
Cost-effectiveness of dimethyl fumarate (Tecfidera ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness
Health Care Needs Assessment of Services for Adults with Rheumatoid Arthritis
 Health Care Needs Assessment of Services for Adults with Rheumatoid Arthritis PART D: Cost Implications for NHS Rheumatoid Arthritis Services in Scotland Scottish Public Health Network August 2013 1 Table
Health Care Needs Assessment of Services for Adults with Rheumatoid Arthritis PART D: Cost Implications for NHS Rheumatoid Arthritis Services in Scotland Scottish Public Health Network August 2013 1 Table
Identifying and treating long-term kidney problems (chronic kidney disease)
 Understanding NICE guidance Information for people who use NHS services Identifying and treating long-term kidney problems (chronic kidney disease) NICE clinical guidelines advise the NHS on caring for
Understanding NICE guidance Information for people who use NHS services Identifying and treating long-term kidney problems (chronic kidney disease) NICE clinical guidelines advise the NHS on caring for
W40 Total prosthetic replacement of knee joint using cement
 Bedfordshire and Hertfordshire Priorities Forum statement Number: 33 Subject: Referral criteria for patients from primary care presenting with knee pain due to ostoarthritis, and clinical threshold for
Bedfordshire and Hertfordshire Priorities Forum statement Number: 33 Subject: Referral criteria for patients from primary care presenting with knee pain due to ostoarthritis, and clinical threshold for
TA 256: Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation
 Service Notification in response to DHSSPS endorsed NICE Technology Appraisals TA 256: Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation 1 Name of Commissioning
Service Notification in response to DHSSPS endorsed NICE Technology Appraisals TA 256: Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation 1 Name of Commissioning
Radiofrequency ablation of varicose veins
 NHS National Institute for Clinical Excellence Radiofrequency ablation of varicose veins Understanding NICE guidance information for people considering the procedure, and for the public September 2003
NHS National Institute for Clinical Excellence Radiofrequency ablation of varicose veins Understanding NICE guidance information for people considering the procedure, and for the public September 2003
Ask Your Doctor if There May Be a SMARTER CHOICE
 If you have osteoarthritis, rheumatoid arthritis or ankylosing spondylitis, Could Your NSAID Pain Medicine Be Hurting Your Stomach? Ask Your Doctor if There May Be a SMARTER CHOICE 1 of 8 Making Smart
If you have osteoarthritis, rheumatoid arthritis or ankylosing spondylitis, Could Your NSAID Pain Medicine Be Hurting Your Stomach? Ask Your Doctor if There May Be a SMARTER CHOICE 1 of 8 Making Smart
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE. Final Appraisal Determination. Anakinra for rheumatoid arthritis
 NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE Final Appraisal Determination Anakinra for rheumatoid arthritis 1 Guidance 1.1 On the balance of its clinical benefits and cost effectiveness, anakinra is not
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE Final Appraisal Determination Anakinra for rheumatoid arthritis 1 Guidance 1.1 On the balance of its clinical benefits and cost effectiveness, anakinra is not
understanding GI bleeding
 understanding GI bleeding a consumer education brochure American College of Gastroenterology 4900B South 31st Street, Arlington, VA 22206 703-820-7400 www.acg.gi.org American College of Gastroenterology
understanding GI bleeding a consumer education brochure American College of Gastroenterology 4900B South 31st Street, Arlington, VA 22206 703-820-7400 www.acg.gi.org American College of Gastroenterology
Leukapheresis for inflammatory bowel disease
 Issue date: June 2005 Leukapheresis for inflammatory bowel disease Understanding NICE guidance information for people considering the procedure, and for the public Information about NICE Interventional
Issue date: June 2005 Leukapheresis for inflammatory bowel disease Understanding NICE guidance information for people considering the procedure, and for the public Information about NICE Interventional
Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada
 Background Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada The use of medications or drugs by non-physician health professionals is evolving and is linked to collaboration
Background Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada The use of medications or drugs by non-physician health professionals is evolving and is linked to collaboration
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Multiple Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Multiple Technology Appraisal Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Multiple Technology Appraisal Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional
NIMULID MD. 1. Introduction. 2. Nimulid MD Drug delivery system
 NIMULID MD 1. Introduction Nimulid MD is a flavoured dispersible Nimesulide tablet with fast mouth dissolving characteristics thereby providing immediate relief. Nimesulide is a non-steroidal antiinflammatory
NIMULID MD 1. Introduction Nimulid MD is a flavoured dispersible Nimesulide tablet with fast mouth dissolving characteristics thereby providing immediate relief. Nimesulide is a non-steroidal antiinflammatory
boceprevir 200mg capsule (Victrelis ) Treatment experienced patients SMC No. (722/11) Merck, Sharpe and Dohme Ltd
 boceprevir 200mg capsule (Victrelis ) Treatment experienced patients SMC No. (722/11) Merck, Sharpe and Dohme Ltd 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment
boceprevir 200mg capsule (Victrelis ) Treatment experienced patients SMC No. (722/11) Merck, Sharpe and Dohme Ltd 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment
Low Molecular Weight Heparin. All Wales Medicines Strategy Group (AWMSG) Recommendations and advice
 Low Molecular Weight Heparin All Wales Medicines Strategy Group (AWMSG) Recommendations and advice Starting Point Low Molecular Weight Heparin (LMWH): Inhibits factor Xa and factor IIa (thrombin) Small
Low Molecular Weight Heparin All Wales Medicines Strategy Group (AWMSG) Recommendations and advice Starting Point Low Molecular Weight Heparin (LMWH): Inhibits factor Xa and factor IIa (thrombin) Small
Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation
 Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation Issued: May 2012 guidance.nice.org.uk/ta256 NICE has accredited the process used by the Centre for Health
Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation Issued: May 2012 guidance.nice.org.uk/ta256 NICE has accredited the process used by the Centre for Health
NHS. Surgical repair of vaginal wall prolapse using mesh. National Institute for Health and Clinical Excellence. 1 Guidance.
 Issue date: June 2008 NHS National Institute for Health and Clinical Excellence Surgical repair of vaginal wall prolapse using mesh 1 Guidance 1.1 The evidence suggests that surgical repair of vaginal
Issue date: June 2008 NHS National Institute for Health and Clinical Excellence Surgical repair of vaginal wall prolapse using mesh 1 Guidance 1.1 The evidence suggests that surgical repair of vaginal
Medical Insurance Long Term (chronic) Conditions Explained
 Medical Insurance Long Term (chronic) Conditions Explained October 2013 FS 28452 wpa.org.uk EMS 505226 IS 553152 Introduction This leaflet explains how we manage claims for our policyholders whose medical
Medical Insurance Long Term (chronic) Conditions Explained October 2013 FS 28452 wpa.org.uk EMS 505226 IS 553152 Introduction This leaflet explains how we manage claims for our policyholders whose medical
Survey conducted into patient experience of life with osteoarthritis. A collaboration between Pro Bono Bio, Arthritis Research UK, and LloydsPharmacy
 Survey conducted into patient experience of life with osteoarthritis. A collaboration between Pro Bono Bio, Arthritis Research UK, and LloydsPharmacy Executive summary Over 400 people with joint conditions
Survey conducted into patient experience of life with osteoarthritis. A collaboration between Pro Bono Bio, Arthritis Research UK, and LloydsPharmacy Executive summary Over 400 people with joint conditions
Summary of the risk management plan (RMP) for Ofev (nintedanib)
 EMA/738120/2014 Summary of the risk management plan (RMP) for Ofev (nintedanib) This is a summary of the risk management plan (RMP) for Ofev, which details the measures to be taken in order to ensure that
EMA/738120/2014 Summary of the risk management plan (RMP) for Ofev (nintedanib) This is a summary of the risk management plan (RMP) for Ofev, which details the measures to be taken in order to ensure that
Improving healthcare for people with long-term conditions
 Improving healthcare for people with long-term conditions UWe-book: Number Two 1 Health and Well-being At UWE our research and development is real and dynamic, identifying problems and providing solutions
Improving healthcare for people with long-term conditions UWe-book: Number Two 1 Health and Well-being At UWE our research and development is real and dynamic, identifying problems and providing solutions
THE COMPARATIVE OUTCOME OF TOPICAL FORMULATIONS IN RHEUMATOID ARTHRITIS: A PROSPECTIVE STUDY IN 125 PATIENTS OF AGE GROUPS 40-60 YEARS:
 Page972 Indo American Journal of Pharmaceutical Research, 2015 ISSN NO: 2231-6876 THE COMPARATIVE OUTCOME OF TOPICAL FORMULATIONS IN RHEUMATOID ARTHRITIS: A PROSPECTIVE STUDY IN 125 PATIENTS OF AGE GROUPS
Page972 Indo American Journal of Pharmaceutical Research, 2015 ISSN NO: 2231-6876 THE COMPARATIVE OUTCOME OF TOPICAL FORMULATIONS IN RHEUMATOID ARTHRITIS: A PROSPECTIVE STUDY IN 125 PATIENTS OF AGE GROUPS
Donepezil, galantamine, rivastigmine (review) and memantine for the treatment of Alzheimer s disease (amended)
 Issue date: November 2006 (amended September 2007, August 2009) Donepezil, galantamine, rivastigmine (review) and memantine for the treatment of Alzheimer s disease (amended) Includes a review of NICE
Issue date: November 2006 (amended September 2007, August 2009) Donepezil, galantamine, rivastigmine (review) and memantine for the treatment of Alzheimer s disease (amended) Includes a review of NICE
Introduction. Background to this event. Raising awareness 09/11/2015
 Introduction Primary Care Medicines Governance HSCB Background to this event New class of medicines Availability of training Increasing volume of prescriptions Reports of medication incidents Raising awareness
Introduction Primary Care Medicines Governance HSCB Background to this event New class of medicines Availability of training Increasing volume of prescriptions Reports of medication incidents Raising awareness
Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM
 Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM This report was commissioned by the NIHR HTA Programme as project number 12/78
Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM This report was commissioned by the NIHR HTA Programme as project number 12/78
Single Blind, Comparative Study of Ketoprofen Cream Vs Diclofenac and Piroxicam Cream in Management of Rheumatoid Arthritis Patients
 International Journal of Pharma Sciences Vol. 4, No. 5 (2014): 697-701 Research Article Open Access ISSN: 2320-6810 Single Blind, Comparative Study of Ketoprofen Vs Diclofenac and Piroxicam in Management
International Journal of Pharma Sciences Vol. 4, No. 5 (2014): 697-701 Research Article Open Access ISSN: 2320-6810 Single Blind, Comparative Study of Ketoprofen Vs Diclofenac and Piroxicam in Management
New Oral Anticoagulants. How safe are they outside the trials?
 New Oral Anticoagulants How safe are they outside the trials? Objectives The need for anticoagulant therapy Indications for anticoagulation Traditional anticoagulant therapies Properties of new oral anticoagulants
New Oral Anticoagulants How safe are they outside the trials? Objectives The need for anticoagulant therapy Indications for anticoagulation Traditional anticoagulant therapies Properties of new oral anticoagulants
Wales Patient Access Scheme: Process Guidance
 Wales Patient Access Scheme: Process Guidance July 2012 (Updated September 2014) This guidance document has been prepared by the Patient Access Scheme Wales Group, with support from the All Wales Therapeutics
Wales Patient Access Scheme: Process Guidance July 2012 (Updated September 2014) This guidance document has been prepared by the Patient Access Scheme Wales Group, with support from the All Wales Therapeutics
Scottish Medicines Consortium
 Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Overview of Rheumatology
 Overview of Rheumatology Griffin Hospital Mini Med School Stephen Moses, MD Valley Medical Associates 135 Division St. Ansonia, CT 06401 203.735.9354 Topics I. Anatomy of a Joint II. Osteoarthritis III.
Overview of Rheumatology Griffin Hospital Mini Med School Stephen Moses, MD Valley Medical Associates 135 Division St. Ansonia, CT 06401 203.735.9354 Topics I. Anatomy of a Joint II. Osteoarthritis III.
Essential Shared Care Agreement Drugs for Dementia
 Ref No. E040 Essential Shared Care Agreement Drugs for Dementia Please complete the following details: Patient s name, address, date of birth Consultant s contact details (p.3) And send One copy to: 1.
Ref No. E040 Essential Shared Care Agreement Drugs for Dementia Please complete the following details: Patient s name, address, date of birth Consultant s contact details (p.3) And send One copy to: 1.
TAKING CARE OF YOUR RHEUMATOID ARTHRITIS
 TAKING CARE OF YOUR RHEUMATOID ARTHRITIS RHEUMATOID ARTHRITIS (RA) FAST FACTS What is Rheumatoid Arthritis? Rheumatoid arthritis (RA) is a chronic disease that can affect your ability to function and be
TAKING CARE OF YOUR RHEUMATOID ARTHRITIS RHEUMATOID ARTHRITIS (RA) FAST FACTS What is Rheumatoid Arthritis? Rheumatoid arthritis (RA) is a chronic disease that can affect your ability to function and be
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Health Technology Appraisal. Drugs for the treatment of pulmonary arterial hypertension
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Drugs for the treatment of Remit / Appraisal objective: Final scope To appraise the clinical and cost effectiveness of
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Drugs for the treatment of Remit / Appraisal objective: Final scope To appraise the clinical and cost effectiveness of
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure
HEALTH CARE COSTS 11
 2 Health Care Costs Chronic health problems account for a substantial part of health care costs. Annually, three diseases, cardiovascular disease (including stroke), cancer, and diabetes, make up about
2 Health Care Costs Chronic health problems account for a substantial part of health care costs. Annually, three diseases, cardiovascular disease (including stroke), cancer, and diabetes, make up about
The NSAID Drugs Prescriptions and Prices 2004 through 1 st Quarter 2005
 EMBARGOED FOR RELEASE 6 p.m. EST, June 2 Summary The NSAID Drugs Prescriptions and Prices 2004 through 1 st Quarter 2005 An Analysis by Consumers Union and Consumer Reports Best Buy Drugs June 2005 Doctors
EMBARGOED FOR RELEASE 6 p.m. EST, June 2 Summary The NSAID Drugs Prescriptions and Prices 2004 through 1 st Quarter 2005 An Analysis by Consumers Union and Consumer Reports Best Buy Drugs June 2005 Doctors
UCB. Certolizumab pegol (CIMZIA ) for the treatment of Rheumatoid Arthritis PATIENT ACCESS SCHEME (PAS) SUBMISSION TO NICE
 UCB Certolizumab pegol (CIMZIA ) for the treatment of Rheumatoid Arthritis PATIENT ACCESS SCHEME (PAS) SUBMISSION TO NICE July 23 d 2009 1 Executive summary UCB have proposed a patient access scheme (PAS)
UCB Certolizumab pegol (CIMZIA ) for the treatment of Rheumatoid Arthritis PATIENT ACCESS SCHEME (PAS) SUBMISSION TO NICE July 23 d 2009 1 Executive summary UCB have proposed a patient access scheme (PAS)
boceprevir 200mg capsule (Victrelis ) Treatment naïve patients SMC No. (723/11) Merck Sharpe and Dohme Ltd
 boceprevir 200mg capsule (Victrelis ) Treatment naïve patients SMC No. (723/11) Merck Sharpe and Dohme Ltd 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the
boceprevir 200mg capsule (Victrelis ) Treatment naïve patients SMC No. (723/11) Merck Sharpe and Dohme Ltd 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the
Dimethyl fumarate for treating relapsing remitting multiple sclerosis
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Final appraisal determination Dimethyl fumarate for treating relapsing remitting multiple sclerosis This guidance was developed using the single technology
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Final appraisal determination Dimethyl fumarate for treating relapsing remitting multiple sclerosis This guidance was developed using the single technology
Appendix C Factors to consider when choosing between anticoagulant options and FAQs
 Appendix C Factors to consider when choosing between anticoagulant options and FAQs Choice of anticoagulant for non-valvular* atrial fibrillation: Clinical decision aid Patients should already be screened
Appendix C Factors to consider when choosing between anticoagulant options and FAQs Choice of anticoagulant for non-valvular* atrial fibrillation: Clinical decision aid Patients should already be screened
Research Article Practice of Pain Management by Indian Healthcare Practitioners: Results of a Paper Based Questionnaire Survey
 Pain Research and Treatment Volume 2015, Article ID 891092, 8 pages http://dx.doi.org/10.1155/2015/891092 Research Article Practice of Pain Management by Indian Healthcare Practitioners: Results of a Paper
Pain Research and Treatment Volume 2015, Article ID 891092, 8 pages http://dx.doi.org/10.1155/2015/891092 Research Article Practice of Pain Management by Indian Healthcare Practitioners: Results of a Paper
Shared Care Guideline-Use of Donepezil, Galantamine, Rivastigmine and Memantine in Dementia
 Shared Care Guideline-Use of Donepezil, Galantamine, Rivastigmine and Memantine in Dementia Version: 3.0 Ratified by: Medicines Committee Date ratified: 16 th November 2011 Name of originator/author: James
Shared Care Guideline-Use of Donepezil, Galantamine, Rivastigmine and Memantine in Dementia Version: 3.0 Ratified by: Medicines Committee Date ratified: 16 th November 2011 Name of originator/author: James
Costing statement: Depression: the treatment and management of depression in adults. (update) and
 Costing statement: Depression: the treatment and management of depression in adults (update) and Depression in adults with a chronic physical health problem: treatment and management Summary It has not
Costing statement: Depression: the treatment and management of depression in adults (update) and Depression in adults with a chronic physical health problem: treatment and management Summary It has not
Rivaroxaban for the treatment of deep vein thrombosis and prevention of recurrent deep vein thrombosis and pulmonary embolism
 Rivaroxaban for the treatment of deep vein thrombosis and prevention of recurrent deep vein thrombosis and pulmonary Issued: July 2012 guidance.nice.org.uk/ta NHS Evidence has accredited the process used
Rivaroxaban for the treatment of deep vein thrombosis and prevention of recurrent deep vein thrombosis and pulmonary Issued: July 2012 guidance.nice.org.uk/ta NHS Evidence has accredited the process used
National Digestive Diseases Information Clearinghouse
 Gastritis National Digestive Diseases Information Clearinghouse U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH What is gastritis? Gastritis is a condition in which the stomach
Gastritis National Digestive Diseases Information Clearinghouse U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH What is gastritis? Gastritis is a condition in which the stomach
Peninsula Commissioning Priorities Group. Commissioning Policy Varicose Vein Referral
 NHS Devon NHS Plymouth Torbay Care Trust Peninsula Commissioning Priorities Group Commissioning Policy Varicose Vein Referral Varicose Vein Referral Guidelines 1. Description of service/treatment Most
NHS Devon NHS Plymouth Torbay Care Trust Peninsula Commissioning Priorities Group Commissioning Policy Varicose Vein Referral Varicose Vein Referral Guidelines 1. Description of service/treatment Most
Rivaroxaban for the treatment of deep vein thrombosis and prevention of recurrent deep vein thrombosis and pulmonary embolism
 Rivaroxaban for the treatment of deep vein thrombosis and prevention of recurrent deep vein thrombosis and pulmonary Issued: July 2012 guidance.nice.org.uk/ta NICE has accredited the process used by the
Rivaroxaban for the treatment of deep vein thrombosis and prevention of recurrent deep vein thrombosis and pulmonary Issued: July 2012 guidance.nice.org.uk/ta NICE has accredited the process used by the
Guidelines for the Use of Controlled Substances in the Treatment of Pain Adopted by the New Hampshire Medical Society, July 1998
 Guidelines for the Use of Controlled Substances in the Treatment of Pain Adopted by the New Hampshire Medical Society, July 1998 Section I: Preamble The New Hampshire Medical Society believes that principles
Guidelines for the Use of Controlled Substances in the Treatment of Pain Adopted by the New Hampshire Medical Society, July 1998 Section I: Preamble The New Hampshire Medical Society believes that principles
Step 4: Complex and severe depression in adults
 Step 4: Complex and severe depression in adults A NICE pathway brings together all NICE guidance, quality standards and materials to support implementation on a specific topic area. The pathways are interactive
Step 4: Complex and severe depression in adults A NICE pathway brings together all NICE guidance, quality standards and materials to support implementation on a specific topic area. The pathways are interactive
Time to Act Urgent Care and A&E: the patient perspective
 Time to Act Urgent Care and A&E: the patient perspective May 2015 Executive Summary The NHS aims to put patients at the centre of everything that it does. Indeed, the NHS Constitution provides rights to
Time to Act Urgent Care and A&E: the patient perspective May 2015 Executive Summary The NHS aims to put patients at the centre of everything that it does. Indeed, the NHS Constitution provides rights to
COST OF SKIN CANCER IN ENGLAND MORRIS, S., COX, B., AND BOSANQUET, N.
 ISSN 1744-6783 COST OF SKIN CANCER IN ENGLAND MORRIS, S., COX, B., AND BOSANQUET, N. Tanaka Business School Discussion Papers: TBS/DP05/39 London: Tanaka Business School, 2005 1 Cost of skin cancer in
ISSN 1744-6783 COST OF SKIN CANCER IN ENGLAND MORRIS, S., COX, B., AND BOSANQUET, N. Tanaka Business School Discussion Papers: TBS/DP05/39 London: Tanaka Business School, 2005 1 Cost of skin cancer in
PG Certificate / PG Diploma / MSc in Clinical Pharmacy
 PG Certificate / PG Diploma / MSc in Clinical Pharmacy Programme Information September 2014 Entry School of Pharmacy Queen s University Belfast Queen s University Belfast - Clinical Pharmacy programme
PG Certificate / PG Diploma / MSc in Clinical Pharmacy Programme Information September 2014 Entry School of Pharmacy Queen s University Belfast Queen s University Belfast - Clinical Pharmacy programme
(Intro to Arthritis with a. Arthritis) Manager of Education & Services for the Vancouver Island Region of The Arthritis Society
 Arthritis 101 (Intro to Arthritis with a Focus on Rheumatoid Arthritis) by Cari Taylor by Cari Taylor Manager of Education & Services for the Vancouver Island Region of The Arthritis Society What You Will
Arthritis 101 (Intro to Arthritis with a Focus on Rheumatoid Arthritis) by Cari Taylor by Cari Taylor Manager of Education & Services for the Vancouver Island Region of The Arthritis Society What You Will
Alcohol-use disorders overview
 Alcohol-use disorders overview A NICE pathway brings together all NICE guidance, quality standards and materials to support implementation on a specific topic area. The pathways are interactive and designed
Alcohol-use disorders overview A NICE pathway brings together all NICE guidance, quality standards and materials to support implementation on a specific topic area. The pathways are interactive and designed
Post-traumatic stress disorder (PTSD): the treatment of PTSD in adults and children
 Post-traumatic stress disorder (PTSD): the treatment of PTSD in adults and children Understanding NICE guidance information for people with PTSD, their advocates and carers, and the public March 2005 Information
Post-traumatic stress disorder (PTSD): the treatment of PTSD in adults and children Understanding NICE guidance information for people with PTSD, their advocates and carers, and the public March 2005 Information
A Genetic Analysis of Rheumatoid Arthritis
 A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
DECISION AND SUMMARY OF RATIONALE
 DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Clofarabine in the treatment of relapsed acute myeloid leukaemia (AML) The application was for clofarabine to remain in
DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Clofarabine in the treatment of relapsed acute myeloid leukaemia (AML) The application was for clofarabine to remain in
Drug treatments for neuropathic pain
 Understanding NICE guidance Information for people who use NHS services Drug treatments for neuropathic pain NICE clinical guidelines advise the NHS on caring for people with specific conditions or diseases
Understanding NICE guidance Information for people who use NHS services Drug treatments for neuropathic pain NICE clinical guidelines advise the NHS on caring for people with specific conditions or diseases
Dimethyl fumarate for treating relapsing-remitting multiple sclerosis
 Dimethyl fumarate for treating relapsing-remitting multiple Issued: August 2014 guidance.nice.org.uk/ta320 NICE has accredited the process used by the Centre for Health Technology Evaluation at NICE to
Dimethyl fumarate for treating relapsing-remitting multiple Issued: August 2014 guidance.nice.org.uk/ta320 NICE has accredited the process used by the Centre for Health Technology Evaluation at NICE to
Service Specification Template Department of Health, updated June 2015
 Service Specification Template Department of Health, updated June 2015 Service Specification No. : 2 Service: Commissioner Lead: Provider Lead: Period: Anti-coagulation monitoring Date of Review: 31 st
Service Specification Template Department of Health, updated June 2015 Service Specification No. : 2 Service: Commissioner Lead: Provider Lead: Period: Anti-coagulation monitoring Date of Review: 31 st
Cyclooxygenase and NSAIDs
 Cyclooxygenase and NSAIDs Cyclooxygenase An enzyme responsible for the production of prostaglandins Two forms, COX1 and COX2 Contains two separate active sites for prostaglandin synthase One side contains
Cyclooxygenase and NSAIDs Cyclooxygenase An enzyme responsible for the production of prostaglandins Two forms, COX1 and COX2 Contains two separate active sites for prostaglandin synthase One side contains
Peptic Ulcer. Anatomy The stomach is a hollow organ. It is located in the upper abdomen, under the ribs.
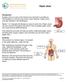 Peptic Ulcer Introduction A peptic ulcer is a sore in the lining of your stomach or duodenum. The duodenum is the first part of your small intestine. Peptic ulcers may also develop in the esophagus. Nearly
Peptic Ulcer Introduction A peptic ulcer is a sore in the lining of your stomach or duodenum. The duodenum is the first part of your small intestine. Peptic ulcers may also develop in the esophagus. Nearly
Polymyalgia Rheumatica www.arthritis.org.nz
 Polymyalgia Rheumatica www.arthritis.org.nz Did you know? Arthritis affects one in six New Zealanders over the age of 15 years. Polymyalgia rheumatica is a common rheumatic condition. It affects more women
Polymyalgia Rheumatica www.arthritis.org.nz Did you know? Arthritis affects one in six New Zealanders over the age of 15 years. Polymyalgia rheumatica is a common rheumatic condition. It affects more women
Article details. Abstract. Version 1
 Article details Title Authors Abstract Version 1 Osteoarthritis in Family Physician Practices in Canada: A Report of the Canadian Primary Care Sentinel Surveillance Network (CPCSSN) Morkem, Rachael; Birtwhistle,
Article details Title Authors Abstract Version 1 Osteoarthritis in Family Physician Practices in Canada: A Report of the Canadian Primary Care Sentinel Surveillance Network (CPCSSN) Morkem, Rachael; Birtwhistle,
How To Understand The Cost Effectiveness Of Bortezomib
 DOI: 1.331/hta13suppl1/5 Health Technology Assessment 29; Vol. 13: Suppl. 1 Bortezomib for the treatment of multiple myeloma patients C Green, J Bryant,* A Takeda, K Cooper, A Clegg, A Smith and M Stephens
DOI: 1.331/hta13suppl1/5 Health Technology Assessment 29; Vol. 13: Suppl. 1 Bortezomib for the treatment of multiple myeloma patients C Green, J Bryant,* A Takeda, K Cooper, A Clegg, A Smith and M Stephens
MANAGEMENT OF CHRONIC NON MALIGNANT PAIN
 MANAGEMENT OF CHRONIC NON MALIGNANT PAIN Introduction The Manitoba Prescribing Practices Program (MPPP) recognizes the important role served by physicians in relieving pain and suffering and acknowledges
MANAGEMENT OF CHRONIC NON MALIGNANT PAIN Introduction The Manitoba Prescribing Practices Program (MPPP) recognizes the important role served by physicians in relieving pain and suffering and acknowledges
THE CLINICAL USE OF NATURAL ANTI-INFLAMMATORY HERBS AND SUPPLEMENTS JAMES MESCHINO DC, MS, ND
 THE CLINICAL USE OF NATURAL ANTI-INFLAMMATORY HERBS AND SUPPLEMENTS JAMES MESCHINO DC, MS, ND As reported by Gottlieb in 1997, the management of osteoarthritis should include specific dietary and supplementation
THE CLINICAL USE OF NATURAL ANTI-INFLAMMATORY HERBS AND SUPPLEMENTS JAMES MESCHINO DC, MS, ND As reported by Gottlieb in 1997, the management of osteoarthritis should include specific dietary and supplementation
Adalimumab for the treatment of psoriasis
 DOI: 10.3310/hta13suppl2/07 Health Technology Assessment 2009; Vol. 13: Suppl. 2 Adalimumab for the treatment of psoriasis D Turner, J Picot,* K Cooper and E Loveman Southampton Health Technology Assessments
DOI: 10.3310/hta13suppl2/07 Health Technology Assessment 2009; Vol. 13: Suppl. 2 Adalimumab for the treatment of psoriasis D Turner, J Picot,* K Cooper and E Loveman Southampton Health Technology Assessments
National Rheumatoid Arthritis Society. THE ECONOMIC BURDEN OF RHEUMATOID ARTHRITIS March 2010
 National Rheumatoid Arthritis Society THE ECONOMIC BURDEN OF RHEUMATOID ARTHRITIS March 2010 ABOUT NRAS NRAS provides support, information, education and advocacy for people with rheumatoid arthritis (RA)
National Rheumatoid Arthritis Society THE ECONOMIC BURDEN OF RHEUMATOID ARTHRITIS March 2010 ABOUT NRAS NRAS provides support, information, education and advocacy for people with rheumatoid arthritis (RA)
2. Characteristics of staff Qualifications required. Additional requirements. Continued education & training requirements
 Patient Group Direction The supply of Azithromycin 1g as a single dose by accredited Community Pharmacists to patients in receipt of a positive test result to Chlamydia trachomatis, and treatment of their
Patient Group Direction The supply of Azithromycin 1g as a single dose by accredited Community Pharmacists to patients in receipt of a positive test result to Chlamydia trachomatis, and treatment of their
Canadian consensus guidelines on long-term nonsteroidal anti-inflammatory drug therapy and the need for gastroprotection: benefits versus risks
 Alimentary Pharmacology & Therapeutics Canadian consensus guidelines on long-term nonsteroidal anti-inflammatory drug therapy and the need for gastroprotection: benefits versus risks A. ROSTOM*, P. MOAYYEDI
Alimentary Pharmacology & Therapeutics Canadian consensus guidelines on long-term nonsteroidal anti-inflammatory drug therapy and the need for gastroprotection: benefits versus risks A. ROSTOM*, P. MOAYYEDI
Nursing 113. Pharmacology Principles
 Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Duration of Dual Antiplatelet Therapy After Coronary Stenting
 Duration of Dual Antiplatelet Therapy After Coronary Stenting C. DEAN KATSAMAKIS, DO, FACC, FSCAI INTERVENTIONAL CARDIOLOGIST ADVOCATE LUTHERAN GENERAL HOSPITAL INTRODUCTION Coronary artery stents are
Duration of Dual Antiplatelet Therapy After Coronary Stenting C. DEAN KATSAMAKIS, DO, FACC, FSCAI INTERVENTIONAL CARDIOLOGIST ADVOCATE LUTHERAN GENERAL HOSPITAL INTRODUCTION Coronary artery stents are
Donepezil (Aricept ), Galantamine (Reminyl XL ), Rivastigmine (Exelon ) and Memantine (Ebixa )
 Donepezil (Aricept ), Galantamine (Reminyl XL ), Rivastigmine (Exelon ) and Memantine (Ebixa ) ESCA: For the treatment of Alzheimer s disease. SECONDARY CARE SECTION TO BE COMPLETED BY INITIATING DOCTOR
Donepezil (Aricept ), Galantamine (Reminyl XL ), Rivastigmine (Exelon ) and Memantine (Ebixa ) ESCA: For the treatment of Alzheimer s disease. SECONDARY CARE SECTION TO BE COMPLETED BY INITIATING DOCTOR
Gastrointestinal bleeding: the management of acute upper gastrointestinal bleeding
 Gastrointestinal bleeding: the management of acute upper gastrointestinal bleeding NICE guideline Draft for consultation, December 2011 If you wish to comment on this version of the guideline, please be
Gastrointestinal bleeding: the management of acute upper gastrointestinal bleeding NICE guideline Draft for consultation, December 2011 If you wish to comment on this version of the guideline, please be
Safety indicators for inpatient and outpatient oral anticoagulant care
 Safety indicators for inpatient and outpatient oral anticoagulant care 1 Recommendations from the British Committee for Standards in Haematology (BCSH) & National Patient Safety Agency (NPSA) Address for
Safety indicators for inpatient and outpatient oral anticoagulant care 1 Recommendations from the British Committee for Standards in Haematology (BCSH) & National Patient Safety Agency (NPSA) Address for
Michigan Guidelines for the Use of Controlled Substances for the Treatment of Pain
 Michigan Guidelines for the Use of Controlled Substances for the Treatment of Pain Section I: Preamble The Michigan Boards of Medicine and Osteopathic Medicine & Surgery recognize that principles of quality
Michigan Guidelines for the Use of Controlled Substances for the Treatment of Pain Section I: Preamble The Michigan Boards of Medicine and Osteopathic Medicine & Surgery recognize that principles of quality
2. Background This was the fourth submission for everolimus requesting listing for clear cell renal carcinoma.
 PUBLIC SUMMARY DOCUMENT Product: Everolimus, tablets, 5 mg and 10 mg, Afinitor Sponsor: Novartis Pharmaceuticals Australia Pty Ltd Date of PBAC Consideration: November 2011 1. Purpose of Application To
PUBLIC SUMMARY DOCUMENT Product: Everolimus, tablets, 5 mg and 10 mg, Afinitor Sponsor: Novartis Pharmaceuticals Australia Pty Ltd Date of PBAC Consideration: November 2011 1. Purpose of Application To
