Molecular Diagnos/c Solu/ons for Urologic Cancer
|
|
|
- Rosanna Moody
- 10 years ago
- Views:
Transcription
1 Molecular Diagnos/c Solu/ons for Urologic Cancer 2016 Company Presentation Dr. Jan Groen, President & CEO
2 Forward Looking Statement 2 This presentation contains forward-looking statements & estimates made by the management of the Company with respect to the anticipated future performance of MDxHealth & the market in which it operates. Such statements & estimates are based on various assumptions & assessments of known & unknown risks, uncertainties & other factors, which were deemed reasonable when made but may or may not prove to be correct. Actual events are difficult to predict & may depend upon factors that are beyond the Company's control. Therefore, actual results, the financial condition, performance or achievements of MDxHealth, or industry results, may turn out to be materially different from any future results, performance or achievements expressed or implied by such statements & estimates. Given these uncertainties, no representations are made as to the accuracy or fairness of such forward-looking statements & estimates. MDxHealth disclaims any obligation to update any such forward-looking statement or estimates to reflect any change in the Company s expectations with regard thereto, or any change in events, conditions or circumstances on which any such statement or estimate is based, except to the extent required by Belgian law. Analyst Coverage Any opinions, estimates or forecasts made by analysts are theirs alone and do not represent opinions, forecasts or predictions of MDxHealth or its management. Requests for copies of analyst reports should be directed at the respective analyst & institution.
3 3 OUR MISSION To improve pa/ent outcomes by delivering molecular diagnos/c solu/ons for urologic cancers.
4 Company Highlights MDxHealth 4 Market leader Improving diagnosis Listed on Euronext Offices in molecular diagnostics with a focus on urology sales: $12M FY sales est.$18m of urological cancers with expanding suite of products - ConfirmMDx for Prostate - SelectMDx for Prostate publically traded multinational healthcare company - Euronext - (Ticker: MDXH.BR) Headquarters in Irvine, CA - Herstal, Belgium - Nijmegen, The Netherlands - 35,000 patients tested - AssureMDx for Bladder
5 5 Global Incidence of North America & Europe Annual Numbers Prostate and Bladder Cancer Annual Numbers 420, ,134 New cases of prostate cancer 288, , , , , ,211 New cases of bladder cancer 84,062 62,380 53,366 17,705 20,716 4,085 27,519 27, GLOBOCAN 2012 (IARC) Population forecasts were extracted from the United Nations, World Population prospects, the 2012 revision. Numbers are computed using age-specific rates and corresponding populations for 10 age-groups. India Australia New Zealand China Latin America Asia North America Europe
6 MDxHealth Business and Product Strategy US & EU 6 Instrumentation Technology & Products Commercial Biomarkers (CE & LDT) Channel Microtome Pathology Prostate Direct sales force PCR Epigenetic (DNA) Bladder Partnerships NGS Genetic (RNA) Kidney Distributors
7 7 Product Offering
8 Prostate Cancer Challenges Annual Numbers 8 Projected growth prostate drug market $8.7 billion $5.7 billion $4 billion $10 billion 27,540 $4 billion spent on the diagnosis and screening of prostate cancer spent on the treatment of prostate cancer men die annually from prostate cancer Source: Prostate Cancer Market Snapshot: More Then Provenge, The Pink Sheet, Nov 22, Elsevier Business Intelligence Publica/ons and Products
9 Early Detection is Important Significant Prostate Cancer 9 1 in 6 Men will develop prostate cancer in their lifetime 1 in 4 Men will be diagnosed with a false-negative biopsy 1. Taneja et al: AUA Optimal Techniques of Prostate Biopsy and Specimen Handling Shen et al: J. Ultrasound Med. 2008; Jun; 27(6):
10 Prostate Cancer in the United Sates Annual Numbers million Abnormal PSA tests 1 Who needs an initial biopsy? 1.3 million Biopsy procedures 2 Who needs a repeat biopsy? Biomarkers 241,000 Diagnosed with PCa 3 Who needs intervention? needed 27,540 Deaths Aubry W et al:. American Health Drug and Benefits (1): 15-24
11 11 Who Needs a Biopsy?
12 SelectMDx for Prostate Cancer Unmet Clinical Need million Improve patient selection for the men will undergo biopsy decision to biopsy
13 Need to Improve Patient Selection for Prostate Biopsy 13 Out of 1.3 Million Men Who Undergo Prostate Biopsy Each Year 25% will receive a false-negative result Image from AACR Founda/on 15% will suffer complications from biopsy (e.g. bleeding, infections, etc.) ~3% will be hospitalized in 30 days following biopsy Transrectal Ultrasound Guided Biopsy 1. Taneja et al: AUA Optimal Techniques of Prostate Biopsy and Specimen Handling 2013, 2. Shen et al: J. Ultrasound Med. 2008; Jun; 27(6): , 3. Loeb S, Carter HB, Berndt SI, et al:, J Urol. 2011;186:
14 14 Improve Patient Selection for Initial Prostate Biopsy Non-invasive Liquid Biopsy Assay RT qpcr method Urine-based test 3 mrna genes (HOXC3, DLX1, KLK3) LDT under CLIA CE marked IVD kit
15 SelectMDx for Prostate Cancer Provides actionable results for the decision to biopsy 15 Likelihood of high grade prostate cancer Biopsy? Elevated Serum PSA No Biopsy Likelihood of low grade prostate cancer
16 SelectMDx Performance SelectMDx for Prostate Cancer 16 ROC Curve for Clinically Significant Cancer Correlation with Gleason Score Sensi/vity SelectMDx Risk Profile p<0.001 p< Specificity No PCa GS 6 GS 7
17 Patient Report Sample 17 Risk Profile: Low Risk Men who may avoid biopsy Decreased risk for aggressive cancer High Risk Men who may benefit from biopsy Increased risk for aggressive cancer Identification of a Candidate Gene Panel for the Early Diagnosis of Prostate Cancer. Leyten et al. Clin Cancer Res, 2015
18 Competition Who to Biopsy 18 Assay Characteris4cs Company Beckman Coulter Opko MDxHealth Specimen Blood Blood Urine Assay Characteris/cs Immuno assay Immuno assay qpcr Assay 3 Protein biomarkers 4 kallikriens biomarkers 3 mrna Biomarkers tpsa and fpsa, propsa PSA, fpsa, Intact PSA, HK-2 DLX1, HOXC6 Regulatory FDA/CE LDT/ CLIA/CE LDT/CLIA/CE List price ($) $499 $1,200 $500 Assay Performance (AUC) AUC 0.73 AUC 0.82 AUC 0.90 Comments Requires Phlebotomist Requires Phlebotomist No Phlebotomist Required Curr Opin Oncol May ; 26(3):
19 False Negative Biopsy Challenge
20 Need to Improve Patient Selection for Repeat Biopsy 20 Unmet Clinical Need ~1.3 million ~241k ~1 million Prostate biopsy procedures Diagnosed with PCa With negative biopsy ~25% with a false negative biopsy Aubry W et al:. American Health Drug and Benefits (1): 15-24
21 False Negative Biopsy Challenge ~25% of Men With Undetected Prostate Cancer million biopsy procedures per year Needle may miss cancer Pathologists can only interpret what is on the slide A biopsy procedure samples <1% of the gland Follow up is a repeat biopsy Image from AACR Founda/on Transrectal Ultrasound Guided 12 Core Biopsy
22 Fear of Undetected Cancer Leads to High Rate of Repeat Biopsy 22 Elevated Clinical Risk Factors Elevated PSA, Abnormal DRE, Family History TRUS Guided Prostate Biopsy Standard 12-core biopsy Unnecessary Repeat Biopsy Patient Compliance, Infections, Negative Prostate Biopsy Sampling error, Needle Missed Mark 43% of men undergo repeat biopsy Welch HG et al:. J Natl Cancer Inst 2007;99: Pinsky PF et al:. BJU International 99, no. 4 (April 2007):
23 Lead Product 23 ConfirmMDx for Prostate Cancer An LDT to Aid on the Decision for Repeat Biopsy RT MSP DNA Methylation assay Three epigenetic biomarkers (GSTPi, RASSF1 and APC) LDT under CLIA Uses residual tissue biopsy sample Tissue Biopsy
24 The Most Significant Independent Predictor for Prostate Cancer Detection on Repeat Biopsy 24 ConfirmMDx for Prostate Cancer Odds Ratio for GS 7 PCa 15.8 Area Under the Curve Age % + Biopsies PSA Atypia ConfirmMDx Risk Score Clinical Risk Factors PSA PTPTRC2 ConfirmMDx Risk Calculators 1) Van Criekinge et al; NOMCC: ; 2015 AUA Prostate Cancer: Detection and Screening V. 2) Partin AW et al; J Urol ) Stewart GD et al; J Urol 2013
25 Likelihood of Prostate Cancer on Repeat Biopsy 25 ConfirmMDx for Prostate Cancer 96% NPV for aggressive prostate cancer
26 Patient Report 26 Risk Profile: Sample Low Risk Men who may avoid biopsy Low likelihood of harboring aggressive cancer High Risk Men who benefit from biopsy High likelihood of harboring aggressive cancer
27 Competition Who to re-biopsy 27 Characteris4cs Company Hologic MDxHealth Specimen Urine Tissue Assay Characteris/cs' qpcr Methyla/on PCR mrna test, 1 biomarker DNA test, 3 biomarkers PCA3 GSTPi, RASFF1, APC Regulatory FDA/CE LDT/ CLIA List price ($) $500 $3,300 Reimbursement Medicare Medicare Assay Performance (NPV) NPV 90% NPV 96% for CS PCa Comments Indicates risk for cancer (any grade) Indicates loca/on & aggressiveness Curr Opin Oncol May ; 26(3):
28 28 Who requires cystoscopy?
29 Bladder Cancer in the US Unmet Clinical Need 29 1 million Hematuria pa/ents per year Methods for detection of bladder cancer are often inadequate, leaving many patients at risk for delayed diagnosis 72,000 Newly diagnosed bladder cancer cases
30 Need to Improve Bladder Cancer Diagnosis Diagnostic Modalities 30 Over 700,000 invasive cystoscopies procedures per year Cystoscopy Cytology Bladder wash or urine Histology of tissue biopsy
31 A Non-Invasive Approach to Stratify Patient Risk 31 Improving Patient Selection for Cystoscopy DNA Methylation Assay Provides physicians with actionable information to: Rule out: bladder cancer in patients with hematuria Reduce: invasive cystoscopy procedures Identify: patients at high-risk for bladder cancer Three Epigenetic Biomarkers TWIST ONECUT2 OTX1 LDT Under CLIA
32 Technical Validation AssureMDx for Bladder Cancer Performance 32 97% 76% 78% 82% 99% Negative Predictive Value 97% Sensitivity 44% 83% Specificity Cytology Fish NMP CxBladder ConfirmMDx AssureMDx BCa Comparison Sensitivity Van Kessel et al., JURO. Vol. 195, 1-7, 2016
33 33 99% NPV Confirming the absence of bladder cancer
34 Competitors Bladder Cancer Confirmation 34 Assay Characteris4cs Company Abbok Matritech Pacific Edge MDxHealth Specimen Urine Urine Urine Urine Assay Characteris/cs DNA hybridiza/on (FISH) Immuno assay qpcr Assay Methyla/on PCR 4 DNA Biomarkers 1 Protein biomarker 5 mrna Biomarkers 3 DNA Biomarkers aneuploidy of NMP22 IGFBP5, HOXA13, MDK TWIST, ONECUT2 and chromosomes 3, 7, 17, and CDK1 OTX1 & loss of both 9p21 loci Regulatory FDA/CE FDA/CE LDT/ CLIA/CE LDT/CLIA List price ($) ~$600 ~$25 ~$2,900 $500 Sensi/vity (%) Sens 76% Sens 78% Sens 82% Sens 97%
35 Prostate and Bladder Cancer Market U.S. 35 $2,750 $1,700 Revenue Millions $500 $250 $300 SelectMDx for Prostate Cancer ConfirmMDx for Prostate Cancer ConfirmMDx for Bladder Cancer RecurMDx for Bladder Cancer Total Available Market
36 Global Commercialization Experienced Commercial Team 36 ISO Lab CLIA MDxHealth Direct & Distributors Distributors Contract discussions with distributors
37 Commercialization US and EU 37 Laboratory developed test Large national sales force Established client base Reimbursement experience Strong KOL support CE marked in-vitro diagnostic kits Direct sales Distributors Reimbursement: gov, private ins, patient Launched in Benelux
38 Proven Sales Team Molecular Diagnostic Specialists MDxHealth Managed Care - 3 MDxHealth Sales Force - 37 MDxHealth Client Services - 6 Sales reps with a proven track record Diverse clientele of small and large group practices Sales Team: 83 Focused guideline strategy Strategically utilizing co-marketing partnership labs Partnerships Sales Force - 37
39 Annual Sales Volume MDxHealth Lead Product 39 >15,000 12,300 7,000 Product launched in June 2012 >35,000 patients tested 1,100 Guidance P
40 40 US Male Population Age % of at-risk population covered 43,605,000 Medicare Coverage: Nov. 3rd, 2014 Medicare Male Popula/on: 24,717,805 Expanding Commercial Insurance Coverage Extensive PPO/TPA Network Contracts United States Census Bureau 2014 National Projections Kaiser Family Foundation, 2012 Palmetto GBA LCD #L35368 MDxHealth Internal Data
41 ConfirmMDx Payor Mix (US) Selected Payers Reimbursing for Testing 41 1% 1% 32% 33% Commercial Medicare Medicaid Medicare Advantage 33% Tricare
42 Continued Revenue Growth MDxHealth 42 $16 $ Millions $11.7 $7.6 $5.2 $3.6 Guidance 2011Y 2012Y 2013Y 2014Y 2015P P = Results pending
43 2015 Performance Financials 43 Revenue for the first 9 months up 44% ($12M) ConfirmMDx Q4 sales volume rose 50% over Q Net loss decreased by 16% in the first nine months of 2015 $35M in cash and cash equivalents Subsequently, acquired NovioGendix for $8.8M, inclusive of MDxHealth shares and milestones
44 MDxHealth News Flow Publication of SelectMDx validation study Launch SelectMDx on US market in H1 Expansion contracts US payors Publication of bladder cancer validation study Launch AssureMDx in Q4
45 45 Appendix
46 The Foundation of Exact Science s Cologuard 46 Non-invasive Colon Cancer Screening Test Technology licensed to Exact Sciences Exact Sciences test uses methods, based on MDxHealth s technology Exclusive use of MDxHealth biomarker for stool-based detection Non-exclusive access to MDxHealth s MSP platform Licensing Terms Up to $3M in milestones and maintenance fees Royalties in the mid-single digit range The license agreement runs through 2028 FDA Approved / Medicare Covered
47 PredictMDx for Glioblastoma 47 MGMT Biomarker Test Technology licensed to LabCorp LabCorp test uses methods based on MDxHealth s technology and biomarker Exclusive North America rights to use MDxHealth biomarker in brain tissue Royalties in the double digit range Clinical Use Included in the NCCN guidelines Tier 1 reimbursement code LDT, tissue based test for Test required in newly diagnosed stage IV Glioma Brain Cancer
Investor Day September 2015
 1 Molecular Diagnostic Solutions for Urologic Cancer Investor Day September 2015 Agenda 2 Introduc)on and Company update Jan Groen Development and Valida)on of SelectMDx by Prof. Dr. Jack Schalken Commercial
1 Molecular Diagnostic Solutions for Urologic Cancer Investor Day September 2015 Agenda 2 Introduc)on and Company update Jan Groen Development and Valida)on of SelectMDx by Prof. Dr. Jack Schalken Commercial
The 4Kscore blood test for risk of aggressive prostate cancer
 The 4Kscore blood test for risk of aggressive prostate cancer Prostate cancer tests When to use the 4Kscore Test? Screening Prior to 1 st biopsy Prior to negative previous biopsy Prognosis in Gleason 6
The 4Kscore blood test for risk of aggressive prostate cancer Prostate cancer tests When to use the 4Kscore Test? Screening Prior to 1 st biopsy Prior to negative previous biopsy Prognosis in Gleason 6
The 4Kscore blood test for risk of aggressive prostate cancer
 The 4Kscore blood test for risk of aggressive prostate cancer Early detection of aggressive prostate cancer Challenges Serum PSA has a high false positive rate Over 1 million prostate biopsies performed
The 4Kscore blood test for risk of aggressive prostate cancer Early detection of aggressive prostate cancer Challenges Serum PSA has a high false positive rate Over 1 million prostate biopsies performed
Biomarkers for Prostate Cancer. Eric Wallen, MD Department of Urology
 Biomarkers for Prostate Cancer Eric Wallen, MD Department of Urology Disclosure MDxHealth Scientific Advisor 55-year-old man: Poor Guy Risk of prostate cancer? 1 in 6 Risk of prostate cancer death? 1 in
Biomarkers for Prostate Cancer Eric Wallen, MD Department of Urology Disclosure MDxHealth Scientific Advisor 55-year-old man: Poor Guy Risk of prostate cancer? 1 in 6 Risk of prostate cancer death? 1 in
PSA Screening for Prostate Cancer Information for Care Providers
 All men should know they are having a PSA test and be informed of the implications prior to testing. This booklet was created to help primary care providers offer men information about the risks and benefits
All men should know they are having a PSA test and be informed of the implications prior to testing. This booklet was created to help primary care providers offer men information about the risks and benefits
Advances in Diagnostic and Molecular Testing in Prostate Cancer
 Advances in Diagnostic and Molecular Testing in Prostate Cancer Ashley E. Ross MD PhD Assistant Professor Urology, Oncology, Pathology Johns Hopkins School of Medicine September 24, 2015 1 Disclosures
Advances in Diagnostic and Molecular Testing in Prostate Cancer Ashley E. Ross MD PhD Assistant Professor Urology, Oncology, Pathology Johns Hopkins School of Medicine September 24, 2015 1 Disclosures
PSA Testing 101. Stanley H. Weiss, MD. Professor, UMDNJ-New Jersey Medical School. Director & PI, Essex County Cancer Coalition. weiss@umdnj.
 PSA Testing 101 Stanley H. Weiss, MD Professor, UMDNJ-New Jersey Medical School Director & PI, Essex County Cancer Coalition [email protected] September 23, 2010 Screening: 3 tests for PCa A good screening
PSA Testing 101 Stanley H. Weiss, MD Professor, UMDNJ-New Jersey Medical School Director & PI, Essex County Cancer Coalition [email protected] September 23, 2010 Screening: 3 tests for PCa A good screening
REGISTRATION DOCUMENT 2014. Epigenetic Diagnostics for Oncology
 REGISTRATION DOCUMENT 2014 Epigenetic Diagnostics for Oncology L E T T E R T O S H A R E H O L D E R S DEAR SHAREHOLDERS, 2014 was a year of substantial progress and achievements for your Company. During
REGISTRATION DOCUMENT 2014 Epigenetic Diagnostics for Oncology L E T T E R T O S H A R E H O L D E R S DEAR SHAREHOLDERS, 2014 was a year of substantial progress and achievements for your Company. During
Annual Report 2015. Molecular Diagnostics for Urological Cancer
 Annual 2015 Molecular Diagnostics for Urological Cancer LETTER TO SHAREHOLDERS Dear Shareholders, 2015 was another exciting year with substantial progress and achievements for your Company. During the
Annual 2015 Molecular Diagnostics for Urological Cancer LETTER TO SHAREHOLDERS Dear Shareholders, 2015 was another exciting year with substantial progress and achievements for your Company. During the
Update on Prostate Cancer: Screening, Diagnosis, and Treatment Making Sense of the Noise and Directions Forward
 Update on Prostate Cancer: Screening, Diagnosis, and Treatment Making Sense of the Noise and Directions Forward 33 rd Annual Internal Medicine Update December 5, 2015 Ryan C. Hedgepeth, MD, MS Chief of
Update on Prostate Cancer: Screening, Diagnosis, and Treatment Making Sense of the Noise and Directions Forward 33 rd Annual Internal Medicine Update December 5, 2015 Ryan C. Hedgepeth, MD, MS Chief of
Prostate Cancer Screening. Dr. J. McCracken, Urologist
 Prostate Cancer Screening Dr. J. McCracken, Urologist USPSTF Lifetime risk for diagnosis currently estimated at 15.9% Llifetime risk of dying of prostate cancer is 2.8% Seventy percent of deaths due to
Prostate Cancer Screening Dr. J. McCracken, Urologist USPSTF Lifetime risk for diagnosis currently estimated at 15.9% Llifetime risk of dying of prostate cancer is 2.8% Seventy percent of deaths due to
Guidelines for Cancer Prevention, Early detection & Screening. Prostate Cancer
 Guidelines for Cancer Prevention, Early detection & Screening Prostate Cancer Intervention Comments & Recommendations For primary prevention, it has been suggested that diets low in meat & other fatty
Guidelines for Cancer Prevention, Early detection & Screening Prostate Cancer Intervention Comments & Recommendations For primary prevention, it has been suggested that diets low in meat & other fatty
Epigenomics AG. March 2015. www.epigenomics.com
 Epigenomics AG March 2015 www.epigenomics.com Safe Harbor Forward Looking Statements This communication contains certain forward-looking statements, including, without limitation, statements containing
Epigenomics AG March 2015 www.epigenomics.com Safe Harbor Forward Looking Statements This communication contains certain forward-looking statements, including, without limitation, statements containing
Craig Hallum Conference Investor Presentation
 Craig Hallum Conference Investor Presentation Improving cancer outcomes with groundbreaking precision in molecular testing Paul Kinnon President and CEO Sept 2015 1 Forward-Looking Statements Certain statements
Craig Hallum Conference Investor Presentation Improving cancer outcomes with groundbreaking precision in molecular testing Paul Kinnon President and CEO Sept 2015 1 Forward-Looking Statements Certain statements
MEDICAL POLICY SUBJECT: PROSTATE CANCER SCREENING, DETECTION AND MONITORING
 MEDICAL POLICY PAGE: 1 OF: 8 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
MEDICAL POLICY PAGE: 1 OF: 8 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
Corporate Medical Policy Urinary Tumor Markers for Bladder Cancer
 Corporate Medical Policy Urinary Tumor Markers for Bladder Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: urinary_tumor_markers_for_bladder_cancer 5/2011 11/2015 11/2016
Corporate Medical Policy Urinary Tumor Markers for Bladder Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: urinary_tumor_markers_for_bladder_cancer 5/2011 11/2015 11/2016
Name of Policy: Genetic and Protein Biomarkers for the Diagnosis and Cancer Risk Assessment of Prostate Cancer
 Name of Policy: Genetic and Protein Biomarkers for the Diagnosis and Cancer Risk Assessment of Prostate Cancer Policy #: 534 Latest Review Date: June 2015 Category: Laboratory Policy Grade: B Background/Definitions:
Name of Policy: Genetic and Protein Biomarkers for the Diagnosis and Cancer Risk Assessment of Prostate Cancer Policy #: 534 Latest Review Date: June 2015 Category: Laboratory Policy Grade: B Background/Definitions:
The PSA Controversy: Defining It, Discussing It, and Coping With It
 The PSA Controversy: Defining It, Discussing It, and Coping With It 11 TH ANNUAL SYMPOSIUM ON MEN S HEALTH June 12, 2013 The PSA Controversy Defining It, Discussing It and Coping With It As of May 2012,
The PSA Controversy: Defining It, Discussing It, and Coping With It 11 TH ANNUAL SYMPOSIUM ON MEN S HEALTH June 12, 2013 The PSA Controversy Defining It, Discussing It and Coping With It As of May 2012,
1. What is the prostate-specific antigen (PSA) test?
 1. What is the prostate-specific antigen (PSA) test? Prostate-specific antigen (PSA) is a protein produced by the cells of the prostate gland. The PSA test measures the level of PSA in the blood. The doctor
1. What is the prostate-specific antigen (PSA) test? Prostate-specific antigen (PSA) is a protein produced by the cells of the prostate gland. The PSA test measures the level of PSA in the blood. The doctor
FAQ About Prostate Cancer Treatment and SpaceOAR System
 FAQ About Prostate Cancer Treatment and SpaceOAR System P. 4 Prostate Cancer Background SpaceOAR Frequently Asked Questions (FAQ) 1. What is prostate cancer? The vast majority of prostate cancers develop
FAQ About Prostate Cancer Treatment and SpaceOAR System P. 4 Prostate Cancer Background SpaceOAR Frequently Asked Questions (FAQ) 1. What is prostate cancer? The vast majority of prostate cancers develop
Science Highlights. To PSA or not to PSA: That is the Question.
 Science Highlights June 2012 by Ann A. Kiessling, PhD at the To PSA or not to PSA: That is the Question. The current raucous debate over the commonly used PSA blood test to screen for prostate cancer,
Science Highlights June 2012 by Ann A. Kiessling, PhD at the To PSA or not to PSA: That is the Question. The current raucous debate over the commonly used PSA blood test to screen for prostate cancer,
IV. UNAUDITED CONSOLIDATED STATEMENT OF CASH FLOWS. 6 VII. STATUTORY AUDITOR'S LIMITED REVIEW REPORT... 10
 2015 INTERIM REPORT TABLE OF CONTENTS I. INTERIM MANAGEMENT REPORT.. 2 II. III. CONDENSED UNAUDITED CONSOLIDATED STATEMENT OF FINANCIAL POSITION.. 4 CONDENSED UNAUDITED CONSOLIDATED STATEMENT OF COMPREHENSIVE
2015 INTERIM REPORT TABLE OF CONTENTS I. INTERIM MANAGEMENT REPORT.. 2 II. III. CONDENSED UNAUDITED CONSOLIDATED STATEMENT OF FINANCIAL POSITION.. 4 CONDENSED UNAUDITED CONSOLIDATED STATEMENT OF COMPREHENSIVE
Epigenomics AG. May 2015. www.epigenomics.com
 Epigenomics AG May 2015 www.epigenomics.com Safe Harbor Forward Looking Statements This communication contains certain forward-looking statements, including, without limitation, statements containing the
Epigenomics AG May 2015 www.epigenomics.com Safe Harbor Forward Looking Statements This communication contains certain forward-looking statements, including, without limitation, statements containing the
Role of MRI in the Diagnosis of Prostate Cancer, A Proposal
 Clinical and Experimental Medical Sciences, Vol. 1, 2013, no. 3, 111 116 HIKARI Ltd, www.m-hikari.com Role of MRI in the Diagnosis of Prostate Cancer, A Proposal W. Akhter Research Fellow Urology, Bartshealth
Clinical and Experimental Medical Sciences, Vol. 1, 2013, no. 3, 111 116 HIKARI Ltd, www.m-hikari.com Role of MRI in the Diagnosis of Prostate Cancer, A Proposal W. Akhter Research Fellow Urology, Bartshealth
Corporate Medical Policy Saturation Biopsy for Diagnosis and Staging of Prostate Cancer
 Corporate Medical Policy Saturation Biopsy for Diagnosis and Staging of Prostate Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: saturation_biopsy_for_diagnosis_and_staging_of_prostate_cancer
Corporate Medical Policy Saturation Biopsy for Diagnosis and Staging of Prostate Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: saturation_biopsy_for_diagnosis_and_staging_of_prostate_cancer
PCA3 DETECTION TEST FOR PROSTATE CANCER DO YOU KNOW YOUR RISK OF HAVING CANCER?
 PCA3 DETECTION TEST FOR PROSTATE CANCER DO YOU KNOW YOUR RISK OF HAVING CANCER? PCA3 DETECTION TEST FOR PROSTATE CANCER There is a range of methods available to your healthcare professional to verify the
PCA3 DETECTION TEST FOR PROSTATE CANCER DO YOU KNOW YOUR RISK OF HAVING CANCER? PCA3 DETECTION TEST FOR PROSTATE CANCER There is a range of methods available to your healthcare professional to verify the
Detecting Cancer in Blood. Company presentation
 Detecting Cancer in Blood Company presentation Safe harbor statement Forward Looking Statements This communication contains certain forward-looking statements, including, without limitation, statements
Detecting Cancer in Blood Company presentation Safe harbor statement Forward Looking Statements This communication contains certain forward-looking statements, including, without limitation, statements
Saturation Biopsy for Diagnosis and Staging of Prostate Cancer. Original Policy Date
 MP 7.01.101 Saturation Biopsy for Diagnosis and Staging of Prostate Cancer Medical Policy Section Surgery Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date /12/2013 Return to Medical Policy
MP 7.01.101 Saturation Biopsy for Diagnosis and Staging of Prostate Cancer Medical Policy Section Surgery Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date /12/2013 Return to Medical Policy
Historical Basis for Concern
 Androgens After : Are We Ready? Mohit Khera, MD, MBA Assistant Professor of Urology Division of Male Reproductive Medicine and Surgery Scott Department of Urology Baylor College of Medicine Historical
Androgens After : Are We Ready? Mohit Khera, MD, MBA Assistant Professor of Urology Division of Male Reproductive Medicine and Surgery Scott Department of Urology Baylor College of Medicine Historical
Screening for Prostate Cancer
 Screening for Prostate Cancer It is now clear that screening for Prostate Cancer discovers the disease at an earlier and more curable stage. It is not yet clear whether this translates into reduced mortality
Screening for Prostate Cancer It is now clear that screening for Prostate Cancer discovers the disease at an earlier and more curable stage. It is not yet clear whether this translates into reduced mortality
PSA Testing for Prostate Cancer An information sheet for men considering a PSA Test
 PSA Testing for Prostate Cancer An information sheet for men considering a PSA Test What is the aim of this leaflet? Prostate cancer is a serious condition. The PSA test, which can give an early indication
PSA Testing for Prostate Cancer An information sheet for men considering a PSA Test What is the aim of this leaflet? Prostate cancer is a serious condition. The PSA test, which can give an early indication
Prostate Cancer 2014
 Prostate Cancer 2014 Eric A. Klein, M.D. Chairman Glickman Urological and Kidney Institute Professor of Surgery Cleveland Clinic Lerner College of Medicine Incidence rates, US Men Mortality Rates, US Men
Prostate Cancer 2014 Eric A. Klein, M.D. Chairman Glickman Urological and Kidney Institute Professor of Surgery Cleveland Clinic Lerner College of Medicine Incidence rates, US Men Mortality Rates, US Men
Early Detection Research Network: Validation Infrastructure. Jo Ann Rinaudo, Ph.D. Division of Cancer Prevention
 Early Detection Research Network: Validation Infrastructure Jo Ann Rinaudo, Ph.D. Division of Cancer Prevention 15 October 2015 Mission The NCI Early Detection Research Network s (EDRN) mission is to implement
Early Detection Research Network: Validation Infrastructure Jo Ann Rinaudo, Ph.D. Division of Cancer Prevention 15 October 2015 Mission The NCI Early Detection Research Network s (EDRN) mission is to implement
Screening for Prostate Cancer
 Cancer Expert Working Group on Cancer Prevention and Screening Screening for Prostate Cancer Information for men and their families 1 What is the prostate? 2 What is prostate cancer? prostate The prostate
Cancer Expert Working Group on Cancer Prevention and Screening Screening for Prostate Cancer Information for men and their families 1 What is the prostate? 2 What is prostate cancer? prostate The prostate
The Centers for Medicare & Medicaid Services (CMS) seeks stakeholder comments on the following clinical quality measure under development:
 The Centers for Medicare & Medicaid Services (CMS) seeks stakeholder comments on the following clinical quality measure under development: Title: Non-Recommended PSA-Based Screening Description: The percentage
The Centers for Medicare & Medicaid Services (CMS) seeks stakeholder comments on the following clinical quality measure under development: Title: Non-Recommended PSA-Based Screening Description: The percentage
A New Biomarker in Prostate Cancer Care: Oncotype Dx. David M Albala, MD Chief of Urology Crouse Hospital Syracuse, NY
 A New Biomarker in Prostate Cancer Care: Oncotype Dx David M Albala, MD Chief of Urology Crouse Hospital Syracuse, NY Learning Objectives Review the current challenges in the prediction and prognosis of
A New Biomarker in Prostate Cancer Care: Oncotype Dx David M Albala, MD Chief of Urology Crouse Hospital Syracuse, NY Learning Objectives Review the current challenges in the prediction and prognosis of
Prostate Health Index Literature
 Prostate Health Index Literature Update June 2013 Preoperative Prostate-Specific Antigen Isoform p2psa and Its Derivatives, %p2psa and Prostate Health Index, Predict Pathologic Outcomes in Patients Undergoing
Prostate Health Index Literature Update June 2013 Preoperative Prostate-Specific Antigen Isoform p2psa and Its Derivatives, %p2psa and Prostate Health Index, Predict Pathologic Outcomes in Patients Undergoing
Local Coverage Determination (LCD): MolDX: Genomic Health Oncotype DX Prostate Cancer Assay (L36153)
 Local Coverage Determination (LCD): MolDX: Genomic Health Oncotype DX Prostate Cancer Assay (L36153) Contractor Information Contractor Name Palmetto GBA LCD Information Document Information LCD ID L36153
Local Coverage Determination (LCD): MolDX: Genomic Health Oncotype DX Prostate Cancer Assay (L36153) Contractor Information Contractor Name Palmetto GBA LCD Information Document Information LCD ID L36153
Beyond the PSA: Genomic Testing in Localized Prostate Cancer
 Beyond the PSA: Genomic Testing in Localized Prostate Cancer Kelvin A. Moses, MD, PhD Vanderbilt University Medical Center Wednesday, December 2, 2015 5:00 p.m. ET/2:00 p.m. PT About ZERO ZERO s mission
Beyond the PSA: Genomic Testing in Localized Prostate Cancer Kelvin A. Moses, MD, PhD Vanderbilt University Medical Center Wednesday, December 2, 2015 5:00 p.m. ET/2:00 p.m. PT About ZERO ZERO s mission
Molecular Diagnostics in Cancer Testing
 Product Sheet More information at http://www.biomarketgroup.com/market-research-report/molecular-diagnostics-in-cancertesting.html Molecular Diagnostics in Cancer Testing Published: 2015-AUG-01 Pages:
Product Sheet More information at http://www.biomarketgroup.com/market-research-report/molecular-diagnostics-in-cancertesting.html Molecular Diagnostics in Cancer Testing Published: 2015-AUG-01 Pages:
HEALTH NEWS PROSTATE CANCER THE PROSTATE
 HEALTH NEWS PROSTATE CANCER THE PROSTATE Prostate comes from the Greek meaning to stand in front of ; this is very different than prostrate which means to lie down flat. The prostate is a walnut-sized
HEALTH NEWS PROSTATE CANCER THE PROSTATE Prostate comes from the Greek meaning to stand in front of ; this is very different than prostrate which means to lie down flat. The prostate is a walnut-sized
HAVE YOU BEEN NEWLY DIAGNOSED with DCIS?
 HAVE YOU BEEN NEWLY DIAGNOSED with DCIS? Jen D. Mother and volunteer. Diagnosed with DCIS breast cancer in 2012. An educational guide prepared by Genomic Health This guide is designed to educate women
HAVE YOU BEEN NEWLY DIAGNOSED with DCIS? Jen D. Mother and volunteer. Diagnosed with DCIS breast cancer in 2012. An educational guide prepared by Genomic Health This guide is designed to educate women
Early Detection of Colorectal Cancer Made Easy with a Blood Test
 INFORMATION FOR PHYSICIANS Early Detection of Colorectal Cancer Made Easy with a Blood Test Epi pro Colon 2.0 : 2 nd Generation Septin 9 Test CRC SCREENING SAVES LIVES Colorectal cancer is a major health
INFORMATION FOR PHYSICIANS Early Detection of Colorectal Cancer Made Easy with a Blood Test Epi pro Colon 2.0 : 2 nd Generation Septin 9 Test CRC SCREENING SAVES LIVES Colorectal cancer is a major health
Epigenomics AG Corporate Presentation
 Epigenomics AG Corporate Presentation September 2013 www.epigenomics.com Safe Harbor Forward Looking Statements This communication contains certain forward-looking statements, including, without limitation,
Epigenomics AG Corporate Presentation September 2013 www.epigenomics.com Safe Harbor Forward Looking Statements This communication contains certain forward-looking statements, including, without limitation,
PSA screening in asymptomatic men the debate continues www.bpac.org.nz keyword: psa
 PSA screening in asymptomatic men the debate continues www.bpac.org.nz keyword: psa Key messages: PSA is present in the benign and malignant prostate There is currently no national screening programme
PSA screening in asymptomatic men the debate continues www.bpac.org.nz keyword: psa Key messages: PSA is present in the benign and malignant prostate There is currently no national screening programme
Detection and staging of recurrent prostate cancer is still one of the important clinical problems in prostate cancer. A rise in PSA or biochemical
 Summary. 111 Detection and staging of recurrent prostate cancer is still one of the important clinical problems in prostate cancer. A rise in PSA or biochemical recurrence (BCR) is the first sign of recurrent
Summary. 111 Detection and staging of recurrent prostate cancer is still one of the important clinical problems in prostate cancer. A rise in PSA or biochemical recurrence (BCR) is the first sign of recurrent
Us TOO University Presents: Understanding Diagnostic Testing
 Us TOO University Presents: Understanding Diagnostic Testing for Prostate Cancer Patients Today s speaker is Manish Bhandari, MD Program moderator is Pam Barrett, Us TOO International Made possible by
Us TOO University Presents: Understanding Diagnostic Testing for Prostate Cancer Patients Today s speaker is Manish Bhandari, MD Program moderator is Pam Barrett, Us TOO International Made possible by
Progress and Prospects in Ovarian Cancer Screening and Prevention
 Progress and Prospects in Ovarian Cancer Screening and Prevention Rebecca Stone, MD MS Assistant Professor Kelly Gynecologic Oncology Service The Johns Hopkins Hospital 1 No Disclosures 4/12/2016 2 Ovarian
Progress and Prospects in Ovarian Cancer Screening and Prevention Rebecca Stone, MD MS Assistant Professor Kelly Gynecologic Oncology Service The Johns Hopkins Hospital 1 No Disclosures 4/12/2016 2 Ovarian
Introduction to Pathology and Diagnostic Medicine
 Harvard-MIT Division of Health Sciences and Technology HST.035: Principle and Practice of Human Pathology Dr. Badizadegan Introduction to Pathology and Diagnostic Medicine Spring 2003 What is pathology?
Harvard-MIT Division of Health Sciences and Technology HST.035: Principle and Practice of Human Pathology Dr. Badizadegan Introduction to Pathology and Diagnostic Medicine Spring 2003 What is pathology?
Screening for Cancer in Light of New Guidelines and Controversies. Christopher Celio, MD St. Jude Heritage Medical Group
 Screening for Cancer in Light of New Guidelines and Controversies Christopher Celio, MD St. Jude Heritage Medical Group Screening Tests The 2 major objectives of a good screening program are: (1) detection
Screening for Cancer in Light of New Guidelines and Controversies Christopher Celio, MD St. Jude Heritage Medical Group Screening Tests The 2 major objectives of a good screening program are: (1) detection
Early Prostate Cancer: Questions and Answers. Key Points
 CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Early Prostate Cancer:
CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Early Prostate Cancer:
PROSTATE CANCER. Normal-risk men: No family history of prostate cancer No history of prior screening Not African-American
 PROSTATE CANCER 1. Guidelines for Screening Risk Factors Normal-risk men: No family history of prostate cancer No history of prior screening Not African-American High-risk men: Family history of prostate
PROSTATE CANCER 1. Guidelines for Screening Risk Factors Normal-risk men: No family history of prostate cancer No history of prior screening Not African-American High-risk men: Family history of prostate
How prostate cancer is diagnosed
 How prostate cancer is diagnosed This information is an extract from the booklet Having tests for prostate cancer. You may find the full booklet helpful. We can send you a free copy see page 7. Contents
How prostate cancer is diagnosed This information is an extract from the booklet Having tests for prostate cancer. You may find the full booklet helpful. We can send you a free copy see page 7. Contents
MEDICAL POLICY SUBJECT: MOLECULAR MARKERS IN FINE NEEDLE ASPIRATES OF THE THYROID EFFECTIVE DATE: 11/19/15
 MEDICAL POLICY SUBJECT: MOLECULAR MARKERS IN FINE NEEDLE PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases,
MEDICAL POLICY SUBJECT: MOLECULAR MARKERS IN FINE NEEDLE PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases,
Product: Point-of-care immunoassay system yielding rapid labquality quantitative blood test results with unprecedented ease-ofuse.
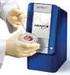 Mission Provide Clinicians, Patients and Payors with a Point-of-Care Diagnostic Solution Coupling Clinically Relevant Performance with Unparalleled Ease-of-Use and Cost Effectiveness Product: Point-of-care
Mission Provide Clinicians, Patients and Payors with a Point-of-Care Diagnostic Solution Coupling Clinically Relevant Performance with Unparalleled Ease-of-Use and Cost Effectiveness Product: Point-of-care
Official reprint from UpToDate www.uptodate.com 2013 UpToDate
 Official reprint from UpToDate www.uptodate.com 2013 UpToDate The content on the UpToDate website is not intended nor recommended as a substitute for medical advice, diagnosis, or treatment. Always seek
Official reprint from UpToDate www.uptodate.com 2013 UpToDate The content on the UpToDate website is not intended nor recommended as a substitute for medical advice, diagnosis, or treatment. Always seek
STATE OF MICHIGAN DEPARTMENT OF INSURANCE AND FINANCIAL SERVICES Before the Director of Insurance and Financial Services
 STATE OF MICHIGAN DEPARTMENT OF INSURANCE AND FINANCIAL SERVICES Before the Director of Insurance and Financial Services In the matter of: Petitioner, v Blue Care Network of Michigan, Respondent. File
STATE OF MICHIGAN DEPARTMENT OF INSURANCE AND FINANCIAL SERVICES Before the Director of Insurance and Financial Services In the matter of: Petitioner, v Blue Care Network of Michigan, Respondent. File
Does my patient need more therapy after prostate cancer surgery?
 Does my patient need more therapy after prostate cancer surgery? Contact the GenomeDx Patient Care Team at: 1.888.792.1601 (toll-free) or e-mail: [email protected] Prostate Cancer Classifier
Does my patient need more therapy after prostate cancer surgery? Contact the GenomeDx Patient Care Team at: 1.888.792.1601 (toll-free) or e-mail: [email protected] Prostate Cancer Classifier
Tumour Markers. What are Tumour Markers? How Are Tumour Markers Used?
 Dr. Anthony C.H. YING What are? Tumour markers are substances that can be found in the body when cancer is present. They are usually found in the blood or urine. They can be products of cancer cells or
Dr. Anthony C.H. YING What are? Tumour markers are substances that can be found in the body when cancer is present. They are usually found in the blood or urine. They can be products of cancer cells or
BRAF in the diagnostic evaluation of thyroid nodules
 Symposium 13 Molecular markers in thyroid cancer: current role in clinical practice BRAF in the diagnostic evaluation of thyroid nodules Laura Fugazzola University of Milan, Italy Papillary carcinoma BRAF
Symposium 13 Molecular markers in thyroid cancer: current role in clinical practice BRAF in the diagnostic evaluation of thyroid nodules Laura Fugazzola University of Milan, Italy Papillary carcinoma BRAF
CONTENTS: WHAT S IN THIS BOOKLET
 Q Questions & A & Answers About Your Prostate Having a biopsy test to find out if you may have prostate cancer can bring up a lot of questions. This booklet will help answer those questions. CONTENTS:
Q Questions & A & Answers About Your Prostate Having a biopsy test to find out if you may have prostate cancer can bring up a lot of questions. This booklet will help answer those questions. CONTENTS:
Corporate Overview. April 2014 OTCQB: LCDX
 Corporate Overview April 2014 OTCQB: LCDX Company Snapshot biopsy Provides non-invasive, point-of-care cellular imaging technologies VivaScope systems can improve patient outcomes while reducing costs
Corporate Overview April 2014 OTCQB: LCDX Company Snapshot biopsy Provides non-invasive, point-of-care cellular imaging technologies VivaScope systems can improve patient outcomes while reducing costs
U.S. Clinical Laboratory and Pathology Testing 2013-2015:
 U.S. Clinical Laboratory and Pathology Testing 2013-2015: Market Analysis, Trends, and Forecasts Now more than ever, it s critical to understand the industry at a macro level. Prepare your organization
U.S. Clinical Laboratory and Pathology Testing 2013-2015: Market Analysis, Trends, and Forecasts Now more than ever, it s critical to understand the industry at a macro level. Prepare your organization
Cancer Screening and Early Detection Guidelines
 Cancer Screening and Early Detection Guidelines Guillermo Tortolero Luna, MD, PhD Director Cancer Control and Population Sciences Program University of Puerto Rico Comprehensive Cancer Center ASPPR Clinical
Cancer Screening and Early Detection Guidelines Guillermo Tortolero Luna, MD, PhD Director Cancer Control and Population Sciences Program University of Puerto Rico Comprehensive Cancer Center ASPPR Clinical
Robert Bristow MD PhD FRCPC
 Robert Bristow MD PhD FRCPC Clinician-Scientist and Professor, Radiation Oncology and Medical Biophysics, University of Toronto and Ontario Cancer Institute/ (UHN) Head, PMH-CFCRI Prostate Cancer Research
Robert Bristow MD PhD FRCPC Clinician-Scientist and Professor, Radiation Oncology and Medical Biophysics, University of Toronto and Ontario Cancer Institute/ (UHN) Head, PMH-CFCRI Prostate Cancer Research
Prostate cancer is the most common cause of death from cancer in men over age 75. Prostate cancer is rarely found in men younger than 40.
 A.D.A.M. Medical Encyclopedia. Prostate cancer Cancer - prostate; Biopsy - prostate; Prostate biopsy; Gleason score Last reviewed: October 2, 2013. Prostate cancer is cancer that starts in the prostate
A.D.A.M. Medical Encyclopedia. Prostate cancer Cancer - prostate; Biopsy - prostate; Prostate biopsy; Gleason score Last reviewed: October 2, 2013. Prostate cancer is cancer that starts in the prostate
Newly Diagnosed Prostate Cancer: Understanding Your Risk
 Newly Diagnosed Prostate Cancer: Understanding Your Risk When the urologist calls with the life-changing news that your prostate biopsy is positive for prostate cancer, an office appointment is made to
Newly Diagnosed Prostate Cancer: Understanding Your Risk When the urologist calls with the life-changing news that your prostate biopsy is positive for prostate cancer, an office appointment is made to
CXCA-MSP. The next step in cervical cancer prevention! GynTect : Epigenetic biomarkers for reliable cancer diagnostics. www.gbo.
 CXCA-MSP The next step in cervical cancer prevention! GynTect : Epigenetic biomarkers for reliable cancer diagnostics www.gbo.com/diagnostics H 3 C NH 2 NH H 3 C 2 N mc N mc N H N O H O The challenge of
CXCA-MSP The next step in cervical cancer prevention! GynTect : Epigenetic biomarkers for reliable cancer diagnostics www.gbo.com/diagnostics H 3 C NH 2 NH H 3 C 2 N mc N mc N H N O H O The challenge of
Prostate Cancer Gene 3 (PCA3) the first highly specific genetic test* improves the diagnosis of prostate cancer. * CE marked
 Prostate Cancer Gene 3 (PCA3) the first highly specific genetic test* improves the diagnosis of prostate cancer * CE marked Introduction Prostate biopsy dilemma Prostate cancer (PCa) is the most commonly
Prostate Cancer Gene 3 (PCA3) the first highly specific genetic test* improves the diagnosis of prostate cancer * CE marked Introduction Prostate biopsy dilemma Prostate cancer (PCa) is the most commonly
Prostate Cancer Screening in Taiwan: a must
 Prostate Cancer Screening in Taiwan: a must 吳 俊 德 基 隆 長 庚 醫 院 台 灣 醫 學 會 105 th What is the PSA test? The blood level of PSA is often elevated in men with prostate cancer, and the PSA test was originally
Prostate Cancer Screening in Taiwan: a must 吳 俊 德 基 隆 長 庚 醫 院 台 灣 醫 學 會 105 th What is the PSA test? The blood level of PSA is often elevated in men with prostate cancer, and the PSA test was originally
TO SCREEN OR NOT TO SCREEN: THE PROSTATE CANCER
 TO SCREEN OR NOT TO SCREEN: THE PROSTATE CANCER DILEMMA Thomas J Stormont MD January 2012 http://www.youtube.com/watch?v=8jd 7bAHVp0A&feature=related related INTRODUCTION A government health panel (the
TO SCREEN OR NOT TO SCREEN: THE PROSTATE CANCER DILEMMA Thomas J Stormont MD January 2012 http://www.youtube.com/watch?v=8jd 7bAHVp0A&feature=related related INTRODUCTION A government health panel (the
Corporate Medical Policy Molecular Markers in Fine Needle Aspirates of the Thyroid
 Corporate Medical Policy Molecular Markers in Fine Needle Aspirates of the Thyroid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_markers_in_fine_needle_aspirates_of_the_thyroid
Corporate Medical Policy Molecular Markers in Fine Needle Aspirates of the Thyroid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_markers_in_fine_needle_aspirates_of_the_thyroid
Prostate Cancer Screening. A Decision Guide for African Americans
 Prostate Cancer Screening A Decision Guide for African Americans This booklet was developed by the U.S. Department of Health and Human Services, Centers for Disease Control and Prevention (CDC). Published
Prostate Cancer Screening A Decision Guide for African Americans This booklet was developed by the U.S. Department of Health and Human Services, Centers for Disease Control and Prevention (CDC). Published
MediPoint: In-Vitro Colorectal Cancer Screening Tests - Current and Future Players
 Brochure More information from http://www.researchandmarkets.com/reports/2669701/ MediPoint: In-Vitro Colorectal Cancer Screening Tests - Current and Future Players Description: MediPoint: In-Vitro Colorectal
Brochure More information from http://www.researchandmarkets.com/reports/2669701/ MediPoint: In-Vitro Colorectal Cancer Screening Tests - Current and Future Players Description: MediPoint: In-Vitro Colorectal
The PSA Test for Prostate Cancer Screening:
 For more information, please contact your local VA Medical Center or Health Clinic. U.S. Department of Veterans Affairs Veterans Health Administration Patient Care Services Health Promotion and Disease
For more information, please contact your local VA Medical Center or Health Clinic. U.S. Department of Veterans Affairs Veterans Health Administration Patient Care Services Health Promotion and Disease
An Introduction to PROSTATE CANCER
 An Introduction to PROSTATE CANCER Being diagnosed with prostate cancer can be a life-altering experience. It requires making some very difficult decisions about treatments that can affect not only the
An Introduction to PROSTATE CANCER Being diagnosed with prostate cancer can be a life-altering experience. It requires making some very difficult decisions about treatments that can affect not only the
Prostate Cancer. Treatments as unique as you are
 Prostate Cancer Treatments as unique as you are UCLA Prostate Cancer Program Prostate cancer is the second most common cancer among men. The UCLA Prostate Cancer Program brings together the elements essential
Prostate Cancer Treatments as unique as you are UCLA Prostate Cancer Program Prostate cancer is the second most common cancer among men. The UCLA Prostate Cancer Program brings together the elements essential
How To Combine The Two Companies Into A Single Company
 Announcement no. 22/2007 To OMX The Nordic Exchange, Copenhagen, and the press Vedbæk, November 27, 2007 EXIQON TO ACQUIRE ONCOTECH INC AND ENTER THE MARKET FOR CANCER MOLECULAR DIAGNOSTICS IN 2008 Summary:
Announcement no. 22/2007 To OMX The Nordic Exchange, Copenhagen, and the press Vedbæk, November 27, 2007 EXIQON TO ACQUIRE ONCOTECH INC AND ENTER THE MARKET FOR CANCER MOLECULAR DIAGNOSTICS IN 2008 Summary:
Prostate Cancer Guide. A resource to help answer your questions about prostate cancer
 Prostate Cancer Guide A resource to help answer your questions about prostate cancer Thank you for downloading this guide to prostate cancer treatment. We know that all the information provided online
Prostate Cancer Guide A resource to help answer your questions about prostate cancer Thank you for downloading this guide to prostate cancer treatment. We know that all the information provided online
NCCN Prostate Cancer Early Detection Guideline
 NCCN Prostate Cancer Early Detection Guideline Joan McClure Senior Vice President National Comprehensive Cancer Network African American Prostate Cancer Disparity Summit September 22, 2006 Washington,
NCCN Prostate Cancer Early Detection Guideline Joan McClure Senior Vice President National Comprehensive Cancer Network African American Prostate Cancer Disparity Summit September 22, 2006 Washington,
Prostate cancer. Christopher Eden. The Royal Surrey County Hospital, Guildford & The Hampshire Clinic, Old Basing.
 Prostate cancer Christopher Eden The Royal Surrey County Hospital, Guildford & The Hampshire Clinic, Old Basing. Screening Screening men for PCa (prostate cancer) using PSA (Prostate Specific Antigen blood
Prostate cancer Christopher Eden The Royal Surrey County Hospital, Guildford & The Hampshire Clinic, Old Basing. Screening Screening men for PCa (prostate cancer) using PSA (Prostate Specific Antigen blood
