3-MoNTH FINANCIAL report 2011
|
|
|
- Kristin Baldwin
- 8 years ago
- Views:
Transcription
1 3-MoNTH FINANCIAL report 2011 Financials in line with expectations Funding through shareholder loan Interim analysis for efficacy of RENCAREX commenced Acquisition of Heidelberg Pharma AG successfully completed
2 WILEX GROUP 3-month financial report 2011 KEY group FIGURES Key Group figures (consolidated 2011) Q Q Change in % Earnings Sales revenue 71 0 n/a Other income (53.9) Operating expenses (6,217) (5,969) 4.2 of which research and development costs (4,649) (4,777) (2.7) Operating result (5,885) (5,405) 8.9 Earnings before tax (5,953) (5,394) 10.3 Net loss for the period (5,954) (5,400) 10.3 Earnings per share in (0.32) (0.34) (4.9) Balance sheet as of the end of the period Total assets 8,270 14,001 (40.9) Cash and cash equivalents 4,624 10,659 (56.6) Equity (7,212) 6,397 n/a Equity ratio 2 in % (87.2) 45.7 n/a Cash flow statement Cash flow from operating activities (7,291) (1,223) Cash flow from investing activities (10) (1) n/a Cash flow from financing activities 9,986 8, Employees (number) Employees as of the end of the period Employees average for the reporting period The reporting period begins on 1 December and ends on 28 February 2 Equity/total assets 3 Including WILEX Inc. (2011) and members of the Executive Management Board Rounding of exact figures may result in differences.
3 WILEX GROUP 3-month financial report 2011 LETTER TO THE SHAREHOLDERS 1 Letter to the shareholders An Extraordinary General Meeting was held on 15 December 2010, shortly after the start of the first quarter of the financial year. Our shareholders approved with a % majority the acquisition of Heidelberg Pharma AG by means of a capital increase in return for contributions in kind. A total of 3,200,000 new WILEX shares were issued subject to the exclusion of shareholders subscription rights. WILEX AG s new share capital of 21,613,035 was recorded in the Commercial Register on 17 March This completes the transaction. The acquisition of Heidelberg Pharma AG gives WILEX access to the company s novel conjugate platform technology for therapeutic antibodies (antibody drug conjugates, ADC) as well as its complementary preclinical services business. We have started to integrate Heidelberg Pharma AG, which will be managed as an independent subsidiary. In the Extraordinary General Meeting, the Executive Management Board was authorised to increase the Company s share capital, with the approval of the Supervisory Board, by up to 9,206, by issuing up to 9,206,517 new no par value bearer shares in return for cash contributions and/or contributions in kind on one or several occasions up to and including 14 December The resolution was adopted by % of the vote; it was also recorded in the Commercial Register. These forward-looking decisions were ratified with very large majorities by you, our shareholders, giving us the flexibility we need to implement our strategy. WILEX succeeded in securing additional financing shortly after the General Meeting through a loan agreement subject to subordination with its two main shareholders, dievini Hopp BioTech holding GmbH & Co. KG, Walldorf (dievini), and UCB Pharma S.A., Brussels, Belgium (UCB), signed on 17 December The loan is for 10 million. It is unsecured and not limited in time. In January 2011, the process related to the interim analysis for efficacy of RENCAREX was started and the milestone of the 343rd relapse was reached. We expect to receive the results of the interim analysis from the Independent Data Monitoring Committee during the second half of the year. These results could form the basis for the European application for marketing approval. We thank you for the trust you have placed in us and that you will continue to accompany us. Munich, 13 April 2011 Peter Llewellyn-Davies Chief Financial Officer
4 2 WILEX GROUP 3-month financial report 2011 INTERIM MANAGEMENT REPORT Interim management report Reporting period from 1 December 2010 to 28 February 2011 Introduction WILEX AG is a biopharmaceutical company focused on oncology with a broad portfolio of therapeutic and diagnostic products some of which are near to market for the targeted treatment and diagnosis of various types of cancer. The compounds are based on antibodies and small molecules. They are designed to inhibit tumour growth with a low side effect profile and prevent metastases. For a detailed description of the Company s business activities, please see the Annual Report page 16 WILEX AG will in future prepare consolidated financial statements that include WILEX AG and its US subsidiary WILEX Inc., Cambridge, MA, USA, starting with the financial statements for the 2010 financial year. In the current reporting period, the WILEX Group comprises two operating segments product development and diagnostics. The acquisition of Heidelberg Pharma AG was not completed until the end of the reporting period and will therefore be included in the consolidation starting with the half-yearly financial statements (see key events after the reporting period). Product development REDECTANE A phase III registration trial for the diagnostic candidate REDECTANE was completed and positive final data were announced in May The trial has shown that REDECTANE with positron emission tomography (PET) and computer tomography (CT) is clearly superior to the use of CT alone in diagnosing clear cell renal cell carcinomas. In the trial, 226 patients were examined with PET/CT and REDECTANE as well as with a diagnostic CT prior to kidney surgery. WILEX has completed preparations for the Pre-BLA Meeting, i. e. the official preliminary discussion of the approval application, with the US Food and Drug Administration (FDA). The documents for the meeting will be submitted as soon as WILEX has received the product documentation from its partner IBA. At the same time, WILEX is also preparing marketing activities in the United States in cooperation with its partner IBA. RENCAREX The therapeutic agent RENCAREX (INN: Girentuximab) is currently in a Phase III registration trial for the adjuvant therapy of non-metastatic clear cell renal cell carcinoma. The ARISER trial enrolled at more than 140 trial centres in 14 countries 864 patients who had either the whole kidney or the diseased part of the kidney removed and who had no detectable metastases after surgery. The process related to the interim analysis for efficacy of RENCAREX was started in January 2011, and the trial centres reported the 343rd relapse. Currently both the data and all radiological scans of all 864 patients are being collected and evaluated centrally. Subsequently, the interim analysis will be initiated and conducted by the Independent Data Monitoring Committee (IDMC). This analysis will provide critical information regarding the trial endpoint disease-free survival which could form the basis for the European application for marketing approval. MESUPRON WILEX made good progress in the Phase II trial of the serine protease inhibitor MESUPRON in patients with metastatic, HER2 receptor negative breast cancer, and patient recruitment is about to be completed. This randomised double-blind trial is designed to examine the efficacy of MESUPRON in combination with the chemotherapeutic agent Capecitabine (Xeloda, Hoffmann-La Roche AG, Basel, Switzerland) compared to Capecitabine alone. The patients receive the drugs in first-line treatment, which is the first treatment following the occurrence of metastases. MESUPRON was also tested in a Phase II trial in the locally advanced, inoperable and non-metastatic pancreatic cancer indication. The final data were published in the summer of 2010 and met with a very positive response.
5 WILEX GROUP 3-month financial report 2011 INTERIM MANAGEMENT REPORT 3 WX-554 MEK inhibitor WILEX took over UCB s MEK inhibitor as a preclinical project and brought it to the clinical development stage under the name WX-554. The mitogen-activated protein kinase (MEK) has been shown to play a central role in signal transduction. The MEK signalling pathway is over expressed in more than 30 % of cancers, resulting in uncontrolled tumour growth and proliferation. Clinical development of WX-554 will continue in 2011, which will see the start of trials in patients. Preclinical and research The preclinical development of the PI3K inhibitor WX-037, which was also taken over from UCB, were continued in the first quarter. The phosphatidylinositol-3-kinase/protein kinase (PI3K) signalling pathway sends a growth signal to the nucleus of a tumour cell. An inhibitor for the PI3K signalling pathway is of great therapeutic interest. Two antibody-based projects are currently in the research phase. The aim is to identify a specific antibody in each case that binds to a new target structure and thus might affect the spread of tumour cells of various types of cancer. Diagnostics WILEX Inc. s acquisition of Oncogene Science in November 2010 added diagnostics to WILEX s portfolio. WILEX Inc. focuses on the production and marketing of diagnostic tests in oncology. A distinction is made between ELISA assays, which measure proteins in the blood, and immunohistochemical (IHC) assays, which examine tissue. The Company s products RENCAREX and REDECTANE, which are based on the antibody Girentuximab, and the assays specialised in CA IX complement one another, as do the upa inhibitor MESUPRON and the ELISA assays for upa and PAI-1. With the ELISA assay, Oncogene to our knowledge has in its product range the only FDA-approved blood test for HER2/neu. WILEX Inc. offers approved tests for the clinical oncological immunodiagnostic market in order to improve treatment for cancer patients worldwide. For a detailed description of the Company s business activities, please see the Annual Report Market environment In the Company s view there have been no significant changes in the market environment for antibodies and small molecules. See pages 27, 28 and 29 of the 2010 annual report for further details.
6 4 WILEX GROUP 3-month financial report 2011 INTERIM MANAGEMENT REPORT Earnings, financial position and net assets The WILEX Group, comprising WILEX AG and the subsidiary WILEX Inc., reports consolidated figures for the first three months of the 2011 financial year (1 December 2010 to 28 February 2011). However, the comparative figures for the first quarter of 2010 refer to the separate financial statements of WILEX AG and thus are not directly comparable with the current consolidated figures for the first quarter of Heidelberg Pharma AG is not yet included in these figures as the acquisition date was after the end of the reporting period. Sales revenue and other income The business acquired from Oncogene Science in November 2010 is currently being newly built up by revitalising the existing customer base and gaining new customers worldwide. WILEX Inc. generated sales revenue of 71 k (previous year: 0 k) in the diagnostics segment in the first three months of the 2011 financial year. As in the previous year, the product development segment did not generate any sales revenue. Prepayments received for research projects are accrued and recognised as other income in line with project costs using the percentage-of-completion (PoC) method. At 260 k, other income fell 53.9 % compared to the previous year ( 564 k). Income realised from the US Department of Defense grants for the upa programme in the amount of 160 k (previous year: 57 k) increased year on year due to the degree of completion of the breast cancer trial of MESUPRON. At 64 k, income realisation from the licence agreements with Esteve and IBA is down from the previous year ( 500 k) and almost nearing its completion, as key milestones have been reached in both the ARISER trial and the REDECT trial. Income from the reversal of other provisions increased to 36 k year on year (previous year: 7 k). Other income in 000* Q Q Grant provided by the US Department of Defense Income realisation from licence agreements Reversal of other provisions * rounded Operating expenses Operating expenses including depreciation and amortisation amounted to 6.22 million, up 4.2 % from the previous year ( 5.97 million). Research and development costs were 4.65 million, corresponding to just under 75.0 % of operating expenses. They were 2.7 % lower year on year (previous year: 4.78 million). The ongoing clinical development of the monoclonal antibody Girentuximab (RENCAREX and REDECTANE ) accounted for 54.5 % of research and development costs. It was slightly higher year on year in relative terms (50.9%) despite the progress of the two Phase III trials because the costs for a production run of the antibody were incurred in the first quarter. The upa programme involving the small-molecule drug candidate MESUPRON specifically, the Phase II breast cancer trial accounted for about 30.8 %. Some costs for the completed Phase II trial in the pancreatic cancer indication were still being incurred in the prior-year period (29.4%). The other pro-
7 WILEX GROUP 3-month financial report 2011 INTERIM MANAGEMENT REPORT 5 jects, which mainly comprise the programmes acquired from UCB, account for 14.1 % of research and development costs. They accounted for 19.8 % in the prior-year period and were attributable to the Phase I trial of WX-554, preclinical work on WX-037 and research expenditures for three antibody-based projects, one of which has been discontinued in the meantime. Manufacturing and distribution costs of 0.32 million were recognised for the very first time in connection with WILEX Inc. s production and distribution of diagnostics. This is why there are no comparative figures for the prior-year period. Administrative costs were 1.25 million, up approx. 4.4 % from the previous year ( 1.19 million). The increase is due in particular to the operational launch of WILEX Inc. s business in the first quarter of 2011 as well as the costs of the Extraordinary General Meeting in December Operating expenses in million* Administrative costs Manufacturing and distribution costs Research and development costs * rounded Q Q Earnings In the first three months of the 2011 financial year, the WILEX Group posted a loss of 5.95 million for the period, up 10.3 % over the same period the previous year ( 5.40 million). This corresponds to earnings per share of 0.32 (previous year: 0.34). The WILEX Group s loss for the period comprises the following two segment losses: Product development ( 5.60 million) or 94.1 % of the loss for the period Diagnostics ( 0.35 million) or 5.9 % of the loss for the period Financing and liquidity WILEX signed a loan agreement for 10 million with its two main shareholders, dievini and UCB, on 17 December 2010 subject to subordination and payable in two instalments. The share of dievini in this loan is 7.5 million, and that of UCB 2.5 million. Both lenders will be paid interest of 6 % p. a. The unsecured loan is not limited in time. Finance income fell to just under 3 k in the reporting period (previous year: 12 k) due to the use of cash as planned and lower interest rates. The Company exclusively used short-term deposits for investing its liquid funds (e. g. overnight money). Finance costs comprising interest expense on the shareholder loan and the interest element of lease liabilities were approximately 70 k (previous year: 1 k). The financial result in the first quarter was 67 k (previous year: 11 k). At the end of the first quarter of 2011, the Company had cash and cash equivalents of 4.62 million (30 November 2010: 1.94 million; 28 February 2010: million).
8 6 WILEX GROUP 3-month financial report 2011 INTERIM MANAGEMENT REPORT Cash flow statement The net cash flow from operating activities during the reporting period was 7.29 million (previous year: 1.22 million. At 10 k, the net cash used in investing activities was insignificant (previous year: 1 k). The net cash inflow from financing activities in the first three months was 9.99 million (previous year: 8.47 million) and was due to the shareholder loan. Total net inflow of cash and cash equivalents was 2.68 million (previous year: 7.25 million). This corresponds to an average cash inflow of 0.89 million per month during the first quarter of 2011 (previous year: 2.42 million). Adjusted for the effects of the shareholder loan granted in the first quarter of 2011, WILEX s average use of cash per month was 2.44 million (previous year: 2.07 million, adjusted for the non-cash capital increase in December 2009 and the second milestone payment from UCB). Cash flow Q in million* Cash as of 1 December 2010 Net cash flow from operating activities Cash flow from investing activities Cash flow from financing activities Cash as of 28 February 2011 ** rounded Assets Total assets as of 28 February 2011 were 8.27 million (30 November 2010: 5.59 million). Balance sheet structure assets in million* Non-current assets Other current assets Cash and cash equivalents * rounded Non-current assets at the end of the reporting period amounted to 2.14 million (30 November 2010: 2.19 million). Intangible assets essentially comprise licence fees and royalties from various cooperation agreements. At 1.14 million, these were slightly lower than on 30 November 2010 ( 1.17 million). Property, plant and equipment amounting to 0.83 million (30 November 2009: 0.86 million) primarily concerns laboratory and office equipment. The other non-current assets comprise the asset value of the reinsurance policy related to a pension obligation as well as an escrow account in favour of the landlord, which is blocked to the Company. At 0.16 million they differ only slightly from the previous year s figure ( 0.16 million). Current assets including cash and cash equivalents at the end of the reporting period were 6.13 million, up from the close of the 2010 financial year ( 3.40 million).
9 WILEX GROUP 3-month financial report 2011 INTERIM MANAGEMENT REPORT 7 Equity Equity at the end of the reporting period was 7.21 million (30 November 2010: 1.30 million). However, in accordance with the applicable provisions of the German Commercial Code, the Company s share capital has not been halved. The Company s equity situation improved after the reporting period due to the non-cash capital increase from the Heidelberg Pharma acquisition. The changes in equity are disclosed in the notes. page 14 Balance sheet structure equity and liabilities in million* Non-current liabilities Current liabilities Equity * rounded Liabilities Non-current liabilities as of 28 February 2011 were 0.22 million (30 November 2010: 0.38 million). Current liabilities rose to million at the close of the reporting period (30 November 2010: 6.50 million). This includes million in other current liabilities (30 November 2010: 4.41 million) resulting mainly from the 10 million shareholder loan from dievini and UCB. The obligation toward the US Department of Defense was recognised based on its contractually stipulated terms and accrued using a fixed trial endpoint; the corresponding income was reversed in accordance with the rate of progress. No obligation toward the cooperation partners Esteve and IBA was recognised anymore because both trials have reached the endpoints defined in connection with the accrual of income. Other current liabilities in million* Accruals US Department of Defense Accruals licence agreements Accrued liabilities Shareholder loan Other * rounded Trade payables were reduced to 1.40 million (30 November 2010: 2.04 million).
10 8 WILEX GROUP 3-month financial report 2011 INTERIM MANAGEMENT REPORT Employees and stock options At the end of the reporting period, 74 employees (30 November 2010: 80; 28 February 2010: 70), including Executive Management Board members, were employed by WILEX. A total of 45 employees worked in research and development (previous year: 50). Nineteen employees (previous year: 20) worked in administration and business development. Ten employees work in manufacturing and distribution, a unit which is being reported for the first time (previous year: 0). The Company has a performance-related compensation system for its employees comprising a fixed annual salary and a variable salary component. In addition, a stock option plan enables employees and Executive Management Board members to participate in the Company s success. WILEX has issued a total of 1,161,431 subscription rights to employees and members of the Executive Management Board, of which 984,944 options were outstanding at the end of the reporting period. No stock options were exercised to date. Currently no new stock options can be issued because the Annual General Meeting s authorisation to establish stock option plans or grant stock options has expired. No subscription rights were therefore issued to employees and members of the Executive Management Board during the reporting period. Report on risks and opportunities The risks and opportunities that arise in connection with WILEX s business are described in detail on pages 71 to 80 of the Annual Report We refer particularly to the financing risks and going concern risks described therein. WILEX uses an IT-based risk management system that complies with the requirements of the German Control and Transparency in Business Act (Gesetz zur Kontrolle und Transparenz im Unternehmensbereich) to monitor 16 different risk areas. WILEX is exposed to risks typical for the industry, namely those arising from the development and production of drug candidates used in cancer therapies. The time between the commencement of drug development and marketing approval usually spans many years. Even though our portfolio has matured further, there is a continued risk that none of the drug and diagnostic candidates in our current portfolio will receive marketing approval. Outlook REDECTANE filing is in preparation and expected in the coming months. The results of the interim analysis in the Phase III ARISER trial of RENCAREX are expected during the second half of We expect to complete patient recruitment in the Phase II trial of MESUPRON in the indication metastatic, HER2 receptor negative breast cancer shortly. Given the trial endpoint, progression-free survival, the final data from this study are expected in The Phase Ib trial of the MEK inhibitor WX-554 is likely to start in the second half of the year. The transition of Oncogene Science from Siemens Healthcare Diagnostics Inc. to WILEX Inc. and the integration in the WILEX Group has been completed. Full manufacturing capabilities are expected to be established within the next three to six months in order to increase sales.
11 WILEX GROUP 3-month financial report 2011 QUARTERLY FINANCIAL STATEMENTS 9 Consolidated statement of comprehensive income (IFRS) Reporting period from 1 December 2010 to 28 February 2011 Q Q Revenue 71,306 0 Other income 260, ,974 Income 331, ,974 Manufacturing and distribution costs (322,991) 0 Research and development costs (4,648,503) (4,776,506) Administrative costs (1,245,371) (1,192,483) Operating expenses (6,216,866) (5,968,989) Operating result (5,885,449) (5,405,015) Finance income 2,732 11,985 Finance costs (69,865) (1,373) Financial result (67,133) 10,613 Earnings before tax (5,952,582) (5,394,402) Income tax (1,613) (5,931) Net loss for the period (5,954,195) (5,400,333) Net currency gain from consolidation 7,385 n/a Comprehensive income (5,946,810) n/a Earnings per share Basic and diluted earnings per share (0.32) (0.34) Average number of shares issued 18,413,035 15,885,397 Rounding of exact figures may result in differences. Quarterly comparison Q Q Q Q Q Revenue Other income Operating expenses (6,217) (5,947) (6,001) (6,508) (5,969) Operating result (5,885) (5,877) (5,670) (6,160) (5,405) Earnings before tax (5,953) (5,875) (5,665) (6,157) (5,394) Net loss for the period (5,954) (5,876) (5,666) (6,156) (5,400) Basic and diluted earnings per share in (0.32) (0.32) (0.34) (0.39) (0.34) Average number of shares issued 18,413,035 18,413,035 16,678,475 15,957,965 15,885,397 1 Consolidated Rounding of exact figures may result in differences.
12 10 WILEX GROUP 3-month financial report 2011 QUARTERLY FINANCIAL STATEMENTS Consolidated balance sheet (IFRS) as of 28 February 2011 and as of 30 November 2010 Assets Property, plant and equipment 833, ,376 Intangible assets 1,140,719 1,165,644 Other non-current assets 162, ,942 Non-current assets 2,136,831 2,191,962 Inventories 143, ,599 Other assets and prepayments 1,107,669 1,123,569 Trade receivables 101,646 40,242 Other receivables 156, ,401 Cash and cash equivalents 4,624,260 1,943,151 Current assets 6,133,618 3,398,962 Total assets 8,270,448 5,590,924 Equity and liabilities Subscribed capital 18,413,035 18,413,035 Capital reserve 127,523, ,484,817 Accumulated losses (153,156,538) (147,202,343) Net currency gain/loss from consolidation 7,385 9,398 Equity (7,212,305) (1,295,093) Pension provisions 24,650 24,410 Lease liabilities 67,862 82,155 Other non-current liabilities 122, ,651 Non-current liabilities 215, ,216 Trade payables 1,408,428 2,039,573 Liabilities arising from leases 58,167 57,992 Other current liabilities 13,800,778 4,406,237 Current liabilities 15,267,373 6,503,801 Total equity and liabilities 8,270,448 5,590,924 Rounding of exact figures may result in differences.
13 WILEX GROUP 3-month financial report 2011 QUARTERLY FINANCIAL STATEMENTS 11 Consolidated cash flow statement (IFRS) Reporting period from 1 December 2010 to 28 February 2011 Q Q Net loss for the period (5,954,195) (5,400,333) Adjustment for income statement items Measurement of stock options 38, ,763 Depreciation/amortisation 40,513 53,107 Increase in pension obligations Finance costs 78,629 1,373 Finance income (11,496) (11,985) Tax expense 1,613 5, , ,399 Changes in net working capital Inventories 16,796 0 Trade receivables (63,184) 4,975,864 Other receivables (41,299) 154,553 Prepayments 14,274 77,029 Other non-current assets (272,703) (309) Trade payables (629,140) 352,095 Other liabilities (510,479) (1,809,234) (1,485,732) 3,749,998 Cash flow from operating activities (7,291,432) (1,227,937) Finance costs paid (1,984) (23) Finance income received 2,732 4,652 Net cash flow from operating activities (7,290,685) (1,223,308) Cash flow from investing activities Purchase of property, plant and equipment (5,689) (822) Purchase of intangible assets (4,689) (182) Net cash flow from investing activities (10,378) (1,004) Cash flow from financing activities Proceeds from capital increases 0 8,925,823 Capital increase costs 0 (453,963) Receipt of shareholder loan 10,000,000 0 Repayment finance leases (14,118) 0 Net cash flow from financing activities 9,985,882 8,471,860 Influence of foreign exchange effects on cash and cash equivalents (3,711) 0 Net change in cash and cash equivalents 2,681,109 7,247,548 Cash and cash equivalents at beginning of period 1,943,151 3,411,063 at end of period 4,624,260 10,658,612 Rounding of exact figures may result in differences.
14 12 WILEX GROUP 3-month financial report 2011 QUARTERLY FINANCIAL STATEMENTS Consolidated statement of changes in equity (IFRS) Reporting period from 1 December 2010 to 28 February 2011 Capital reserve Shares Subscribed capital Capital measures/ premium Measurement of stock options Currency translation differences Accumulated losses Total As of 1 December ,172,673 2,194,945 13,780,935 13,780, ,367,618 0 (124,103,716) 3,044,837 Measurement of stock options 373, ,763 Net loss for the period (5,400,333) (5,400,333) Capital increase after accounting for capital procurement costs 2,177,030 2,177,030 6,201,630 8,378,660 Net change in equity 3,352,090 As of 28 February ,374,304 2,568,708 15,957,965 15,957, ,943,012 0 (129,504,049) 6,396,927 As of 1 December ,819,448 2,665,370 18,413,035 18,413, ,484,817 9,398 (147,202,343) (1,295,093) Measurement of stock options 38,996 38,996 Net currency gain/loss from consolidation (2,013) (2,013) Net loss for the period (5,954,195) (5,954,195) Net change in equity (5,917,212) As of 28 February ,819,448 2,704,366 18,413,035 18,413, ,523,814 7,385 (153,156,538) (7,212,305) Rounding of exact figures may result in differences.
15 WILEX GROUP 3-month financial report 2011 NOTES 13 Selected notes A. General disclosures Besides the initial reporting of operating segments, these interim financial statements as of 28 February 2011 were prepared using the same accounting policies that were applied to the consolidated financial statements as of 30 November The consolidated financial statements as of 28 February 2011 include WILEX AG, Munich, Germany, and WILEX Inc., Cambridge, MA, USA, jointly the Group. The Company s earnings, financial position and net assets as well as individual items of the financial statements for the first three months are explained in detail in the interim management report. The Company s business activities are not subject to seasonal influences. The interim financial statements reproduced in this report were prepared in accordance with the International Financial Reporting Standards (IFRS), IAS 34 Interim Financial Reporting issued by the International Accounting Standards Board (IASB) and in compliance with the Interpretations of the Standing Interpretations Committee (SIC) and the International Financial Reporting Interpretations Committee (IFRIC) as well as in accordance with the IFRS endorsed by the European Union. These interim financial statements must be read in the context of the IFRS consolidated financial statements as of 30 November 2010 published by WILEX AG for the 2010 financial year. The interim financial statements were not subjected to a review. Pursuant to our Declaration of Compliance from 14 February 2011 with Section of the German Corporate Governance Code, both the interim financial statements and the interim management report for the Group were discussed with the Supervisory Board s Audit Committee before being published. The 3-month financial report was approved for publication by the Executive Management Board on 13 April This is the first time that WILEX is reporting different operating segments in a financial report. An operating segment is a component of an entity (the Group) that engages in business activities, generates both sales revenue and income and incurs expenses. Its operating performance is regularly reviewed by the entity s managing directors or Executive Management Board. Discrete financial information is available for each operating segment by definition. The Group s management structure and structure of its intragroup reporting form the basis for segmentation. Segment result and segment assets contain components that may be directly attributable to a single segment or allocated to all segments on a reasonable basis. B. Segment reporting The WILEX Group consists of the following two operating segments: 1) Product development 2) Diagnostics Each individual operating segment, along with its core business and core projects, is set out below. 1) Product development WILEX AG is a biopharmaceutical company focused on oncology. It develops therapeutic and diagnostic products for the targeted treatment and detection of various types of cancer. The compounds are based on antibodies and small molecules aimed at inhibiting tumour growth and preventing metastases while displaying a low side-effect profile. This portfolio is described in more detail in the interim management report. pages 2 and 3
16 14 WILEX GROUP 3-month financial report 2011 NOTES page 3 2) Diagnostics WILEX Inc. s takeover of Oncogene Science in November 2010 added diagnostics to the Company s portfolio. WILEX Inc. focuses on the production and marketing of a multitude of diagnostics related to oncology. One differentiates between ELISA assays, which measure proteins in the blood, and immunohistochemical (IHC) assays, which examine tissue. It is the objective of WILEX to offer approved tests for the clinical oncological immunodiagnostic market in order to improve treatment for cancer patients worldwide (for more information see the interim management report). There was no intersegment sales revenue. The segment results were as follows: Product development Diagnostics Segment results Q * Q * Q * Q * Sales revenue (total) n/a External sales revenue n/a Intersegment sales revenue n/a Segment result before taxes (5,600) (5,394) (353) n/a * rounded C. Change in equity Equity at the end of the reporting period was 7.21 million (30 November 2010: 1.30 million). The subscribed capital amounted to million, as on 30 November The capital reserve was million (30 November 2010: million) and the losses accumulated since the Company s foundation totalled million (30 November 2010: million). The Company recognised a currency gain of 7 k in equity in connection with the consolidation of its U.S. subsidiary. The equity ratio at the end of the three-month period was 87.2 % (30 November 2010: 23.2 %). D. Acquisition of Heidelberg Pharma AG On 3 November 2010, WILEX signed an agreement, with the approval of the Supervisory Board, with all shareholders of Heidel berg Pharma AG regarding the acquisition of all shares in Heidelberg Pharma AG in return for WILEX shares. Prior to its integration into the WILEX Group, Heidelberg Pharma AG was a private company with 41 employees (34 FTEs) domiciled in Ladenburg near Heidelberg, Germany. Heidelberg Pharma has two business units. The first comprises an innovative conjugate platform technology for therapeutic antibodies (antibody drug conjugates, ADC). This ADC technology has the potential to enhance and improve the efficacy of many antibody-based therapies, including those currently on the market. This gives WILEX and Heidelberg Pharma the opportunity to utilise this technology platform for its own drug candidates and also generate revenue by out-licensing the technology to partners through Heidelberg Pharma. The second business unit comprises preclinical work on drug metabolism, pharmacology and pharmacokinetics in oncology and generates revenue by offering this infrastructure and expertise as a service to third parties.
17 WILEX GROUP 3-month financial report 2011 NOTES 15 Following the General Meeting s approval in December 2010 and the recording of the capital increase in the Commercial Register on 17 March 2011 (see note G), WILEX acquired all of the shares in Heidelberg Pharma AG by way of a non-cash capital increase in return for 3,200,000 new WILEX shares subject to the exclusion of shareholders subscription rights. The transaction price of 19.2 million for 100 % of the shares in Heidelberg Pharma AG is equivalent to a price of 6.00 per newly issued WILEX share, which is a premium of around 25 % on the share s closing price on 1 November This corresponds to a conversion ratio of 5.75 :1 in relation to the enterprise values of WILEX AG and Heidelberg Pharma AG. E. Consolidation and preliminary allocation of Heidelberg Pharma AG s goodwill The acquisition closing date was after the end of the reporting period. The profit/loss for the period of Heidelberg Pharma AG was therefore not included in the consolidated profit/loss as of 28 February Under IFRS 3 (Business Combinations), the purchase method shall be used to recognise and measure all identifiable assets acquired and liabilities assumed in connection with a business combination at their fair value. The Company consolidated its new business activities on a preliminary basis and will carry out the purchase price allocation in the course of the financial year. The outcome of that could result in an adjustment to the goodwill determined in measuring the transaction; pursuant to IFRS 3.45 any adjustments of the provisional amounts shall be made within 12 months from the acquisition date. The goodwill shall largely be allocated to the innovative conjugate platform technology for therapeutic antibodies as well as expected synergies from the integration of Heidelberg Pharma into the WILEX Group and the new staff s expertise. The following table shows the identifiable provisional assets and liabilities from the acquisition as of 17 March Given that the purchase price allocation has not yet been carried out, it is assumed that all carrying amounts shown in the preliminary opening balance sheet of Heidelberg Pharma AG prepared as of the acquisition date correspond to the fair value. Preliminary carrying amounts (fair value) as of the date of acquisition 000* Property, plant and equipment 876 Intangible assets 703 Inventories 100 Trade receivables 173 Other receivables 115 Cash and cash equivalents 886 Non-current liabilities (181) Trade payables (312) Other current liabilities (245) Total preliminary carrying amount (fair value) of the identified assets 2,115 * rounded
18 16 WILEX GROUP 3-month financial report 2011 NOTES page 15 The difference between the transaction price ( 19.2 million; see note D) and the total preliminary carrying amount shown in the opening balance sheet according to the table on page 15 ( 2.1 million) amounts to 17.1 million. This amount is attributable to the yet to be determined goodwill as of the acquisition date and intangible assets, such as patents, which have yet to be identified. As no purchase price allocation was carried out at the time this 3-month financial report was published, the difference cannot be specified or explained in more detail. The Company incurred 0.2 million in acquisition-related costs, mainly fees for the enterprise valuation and legal advice. All acquisition-related costs incurred by the reporting date are contained in the consolidated income statement by date of incurrence. Additional disclosures required under IFRS 3.B64 such as the total amount of goodwill that is expected to be deductible for tax purposes or an estimate of the range of outcomes (undiscounted), as well as pro forma disclosures on the acquiree, were not made because the period of time between the completion of the transaction and publication of this 3-month financial report was too short. F. Related party transactions The following reportable purchases were made by members of the Supervisory Board during the first quarter of Name Date Transaction Market place Price Number Volume Professor Christof Hettich* Purchase/ Subscription obligation OTC , , * NewMarket Venture Verwaltungs GmbH, to which Professor Christof Hettich is attributed, is the entity responsible for making the disclosure. The obligation to subscribe for the shares took effect by virtue of the resolution of the Extraordinary General Meeting of WILEX AG on 15 Decem ber 2010 to increase the Company s share capital by 3,200,000 in return for the contribution of all shares in Heidelberg Pharma AG, Ladenburg. No other relationships to related parties exist. G. Key events after the interim reporting period The non-cash capital increase related to Heidelberg Pharma AG was recorded in the Commercial Register on 17 March The acquisition thus has been completed. The issuance of 3,200,000 new WILEX shares raised the share capital of WILEX AG to 21,613,035 shares (see note D).
19 WILEX GROUP 3-month financial report 2011 NOTES 17 Responsibility statement of the Executive Management Board To the best of our knowledge, and in accordance with the applicable reporting principles, the financial statements for the first three months give a true and fair view of the assets, liabilities, financial position and profit or loss of the WILEX Group, and the interim management report includes a fair review of the development and performance of the business and the position of the WILEX Group, together with a description of the material opportunities and risks associated with the expected development of the WILEX Group. Munich, 13 April 2011 Executive Management Board Professor Olaf G. Wilhelm Peter Llewellyn-Davies Dr Paul Bevan Dr Thomas Borcholte
20 18 WILEX GROUP 3-month financial report 2011 SHARE WILEX s share WILEX s share price performance, indexed as of 1 January 2011 % WILEX AG DAX Subsector Biotechnology NASDAQ Biotechnology January February March WILEX s share started the year 2011 at a price of 4.60 and closed on 31 March 2011 at This means that it lost 20 % of its value in the last four months. Whilst the NASDAQ Biotechnology Index rose 6 % in the first quarter, continuing the previous year s good performance, the DAX Subsector Biotechnology Index developed rather unevenly and closed just under 3 %. The Company can only speculate on the reasons for the decline in WILEX s share, for there were no events at WILEX that would justify its share s decline. WILEX s market capitalisation was about 79.3 million at the end of March At 24,486 shares, the average daily trading volume of WILEX s shares in the first three months of the current financial year decreased year on year (26,204 shares). Key share figures as of the end of the reporting period Q Q Shares issued Number 18,413,035 15,957,965 Market capitalisation million Closing price (XETRA) High (all stock exchanges) ( ) Low (all stock exchanges) ( ) ( ) ( ) Volatility (260 days, XETRA) % Average daily trading volume 1 Shares 24,486 26,204 Average daily trading volume 1 104, ,883 Earnings per share (0.32) (0.34) 2 1 all stock exchanges 2 based on an average of 15,885,397 shares outstanding Source: Bloomberg
21 WILEX GROUP 3-month financial report 2011 SHARE 19 Shareholder structure The Heidelberg Pharma non-cash capital increase was recorded in the Commercial Register following the end of the reporting period. The Company s share capital has been 21,613, since 17 March Our shareholder structure changed as a result of the Heidelberg Pharma transaction. The equity interest of dievini Hopp BioTech and related parties rose from % to %. The shareholder structure is as follows after completing the Heidelberg Pharma transaction: Shareholder structure of WILEX AG dievini Hopp BioTech % % UCB 4.28 % TVM Capital Funds % Free float (less than 3 %) Corporate bodies (held directly) 1.58 % 3.95 % Merlin Funds 1 dievini Hopp BioTech holding GmbH & Co. KG + related parties Annual General Meeting 2011 We invite all shareholders to the Annual General Meeting of WILEX AG on Wednesday, 18 May 2011, at 11:00 a. m. at Haus der Bayerischen Wirtschaft (HBW), Europasaal, Max-Joseph-Strasse 5, Munich. All information on the Annual General Meeting can be found on the Company s website under Investor Relations > Annual General Meeting. Financial calendar Date 18 May 2011 Annual General Meeting 2011, 11:00 a.m. 14 July 2011 Half-yearly Financial Report October month Financial Report 2011
22 20 WILEX GROUP 3-month financial report 2011 For your NOTES
23 Contact WILEX AG Grillparzerstr Munich, Germany Tel (0) Fax + 49 (0) investors@wilex.com Peter Llewellyn-Davies Katja Arnold (CIRO) Chief Financial Officer Senior Manager Corporate Communications Tel (0) Tel (0) Fax + 49 (0) Fax + 49 (0) pld@wilex.com katja.arnold@wilex.com Publishing information Published by: Responsible for the project: Design by: WILEX AG, Grillparzerstr. 10, Munich, Germany Katja Arnold, WILEX AG annika Häussler, Artdirektion und Grafikdesign, Hamburg The 3-month Financial Report is also published in German and is available for download from our website at The English translation of the 3-month Financial Report is provided for convenience only. The German original is definitive. As of: 13 April 2011
24 WILEX AG Grillparzerstr Munich Germany
9-MONTH FINANCIAL REPORT 2010
 9-MONTH FINANCIAL REPORT 2010 Highlights Q3: Mandatory offer from dievini Hopp BioTech closed Rights issue successfully executed GMP certification renewed Financials in line with expectations WILEX AG
9-MONTH FINANCIAL REPORT 2010 Highlights Q3: Mandatory offer from dievini Hopp BioTech closed Rights issue successfully executed GMP certification renewed Financials in line with expectations WILEX AG
Employees Employees as at 31 August 53 44 20.5 Employees average during the reporting period 52 44 18.2
 9M 2007 9M 2006 Change in % Earnings key figures in EUR 000 Other operating income 2,158 966 123.4 Operating expenses (19,486) (13,149) 48.2 of which research and development (16,606) (10,527) 57.8 Operating
9M 2007 9M 2006 Change in % Earnings key figures in EUR 000 Other operating income 2,158 966 123.4 Operating expenses (19,486) (13,149) 48.2 of which research and development (16,606) (10,527) 57.8 Operating
WILEX AG: Interim management statement on the first quarter of 2016
 WILEX AG: Interim management statement on the first quarter of 2016 Munich, Germany, 14 April 2016. WILEX AG (ISIN DE000A11QVV0 / WL6 / FSE) today reported on the first three months of the 2016 financial
WILEX AG: Interim management statement on the first quarter of 2016 Munich, Germany, 14 April 2016. WILEX AG (ISIN DE000A11QVV0 / WL6 / FSE) today reported on the first three months of the 2016 financial
9-MoNth financial report 2013
 9-MoNth financial report 2013 UCB acquires rights to an antibody programme for non-oncology indications Cooperation and licence agreement for ADC signed with Roche Nuclea acquires WILEX Inc. and will develop
9-MoNth financial report 2013 UCB acquires rights to an antibody programme for non-oncology indications Cooperation and licence agreement for ADC signed with Roche Nuclea acquires WILEX Inc. and will develop
9-Month financial report 2014
 9-Month financial report 2014 Licence agreement signed with RedHill Biopharma for MESUPRON Reverse share split completed UCB waives repayment of shareholder loan Significant extension of the ADC licence
9-Month financial report 2014 Licence agreement signed with RedHill Biopharma for MESUPRON Reverse share split completed UCB waives repayment of shareholder loan Significant extension of the ADC licence
3-MONTH FINANCIAL REPORT 2015
 3-MONTH FINANCIAL REPORT 2015 Sales revenue and earnings up; costs reduced substantially Subsidiary Heidelberg Pharma awarded grants to support ADC technology Rights issue successfully completed WILEX
3-MONTH FINANCIAL REPORT 2015 Sales revenue and earnings up; costs reduced substantially Subsidiary Heidelberg Pharma awarded grants to support ADC technology Rights issue successfully completed WILEX
Company update. German Equity Forum, Frankfurt November 2011
 Company update German Equity Forum, Frankfurt November 2011 At a glance Oncology-focused biopharmaceutical company Transition from R&D to commercially driven business Product candidates with strong competitive
Company update German Equity Forum, Frankfurt November 2011 At a glance Oncology-focused biopharmaceutical company Transition from R&D to commercially driven business Product candidates with strong competitive
2013 1 million. Sales revenue 13.3 16.1 9.9. Other income 5.8 1.7 1.8. Operating expenses (24.1) (26.8) (25.1)
 Annual Report 2013 New start 2014 Key figures 2013 1 million 2012 1 million 2011 million Earnings Sales revenue 13.3 16.1 9.9 Other income 5.8 1.7 1.8 Operating expenses (24.1) (26.8) (25.1) of which research
Annual Report 2013 New start 2014 Key figures 2013 1 million 2012 1 million 2011 million Earnings Sales revenue 13.3 16.1 9.9 Other income 5.8 1.7 1.8 Operating expenses (24.1) (26.8) (25.1) of which research
Interim consolidated financial statements as of September 30, 2007
 1 Interim consolidated financial statements as of September 30, 2007 January 1 through September 30, 2007 MeVis Medical Solutions AG laying the foundation for further dynamic growth: Sales plus other operating
1 Interim consolidated financial statements as of September 30, 2007 January 1 through September 30, 2007 MeVis Medical Solutions AG laying the foundation for further dynamic growth: Sales plus other operating
TO OUR SHAREHOLDERS DYNAMIC FIRST HALF YEAR
 HALF YEAR REPORT AS OF JUNE 30, 2015 TO OUR SHAREHOLDERS Patrik Heider, Spokesman of the Executive Board and CFOO The Nemetschek Group maintained its dynamic development from the first quarter of 2015
HALF YEAR REPORT AS OF JUNE 30, 2015 TO OUR SHAREHOLDERS Patrik Heider, Spokesman of the Executive Board and CFOO The Nemetschek Group maintained its dynamic development from the first quarter of 2015
Overview of the key figures for the first half of the year
 Half-Year Report 2015 Q2 Revenues increase in the first half of the year by 23% EBIT increased by 1.5 million euros compared to the previous year Order book is growing Overall annual forecast remains unchanged
Half-Year Report 2015 Q2 Revenues increase in the first half of the year by 23% EBIT increased by 1.5 million euros compared to the previous year Order book is growing Overall annual forecast remains unchanged
TO OUR SHAREHOLDERS PROFITABLE GROWTH COURSE INTERNATIONALIZATION FURTHER EXTENDED US MARKET IN FOCUS
 QUARTERLY STATEMENT AS OF MARCH 31, 2015 TO OUR SHAREHOLDERS Patrik Heider, Spokesman of the Executive Board and CFOO The Nemetschek Group has made a dynamic start in the 2015 financial year and continues
QUARTERLY STATEMENT AS OF MARCH 31, 2015 TO OUR SHAREHOLDERS Patrik Heider, Spokesman of the Executive Board and CFOO The Nemetschek Group has made a dynamic start in the 2015 financial year and continues
HALF YEAR REPORT AS OF JUNE 30
 2 0 1 4 HALF YEAR REPORT AS OF JUNE 30 T O O U R S H A R E H O L D E R S Dear shareholders, ladies and gentlemen, The Nemetschek Group continued its successful development in the second quarter of 2014
2 0 1 4 HALF YEAR REPORT AS OF JUNE 30 T O O U R S H A R E H O L D E R S Dear shareholders, ladies and gentlemen, The Nemetschek Group continued its successful development in the second quarter of 2014
Annual Report 2007. Milestones achieved Focus on commercialisation
 Annual Report 2007 Milestones achieved Focus on commercialisation CONTENTS ABOUT US Foreword by the Executive Management Board 2 Strategy 4 Expertise on board 6 VALUES WILEX s share 8 Corporate Governance
Annual Report 2007 Milestones achieved Focus on commercialisation CONTENTS ABOUT US Foreword by the Executive Management Board 2 Strategy 4 Expertise on board 6 VALUES WILEX s share 8 Corporate Governance
Note 2 SIGNIFICANT ACCOUNTING
 Note 2 SIGNIFICANT ACCOUNTING POLICIES BASIS FOR THE PREPARATION OF THE FINANCIAL STATEMENTS The consolidated financial statements have been prepared in accordance with International Financial Reporting
Note 2 SIGNIFICANT ACCOUNTING POLICIES BASIS FOR THE PREPARATION OF THE FINANCIAL STATEMENTS The consolidated financial statements have been prepared in accordance with International Financial Reporting
Consolidated Statement of Financial Position
 INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS 30 JUNE 2014 Consolidated Statement of Financial Position in CHF 1,000 Note 30 June 2014 31 December 2013 (unaudited) (audited) Assets Non-current assets
INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS 30 JUNE 2014 Consolidated Statement of Financial Position in CHF 1,000 Note 30 June 2014 31 December 2013 (unaudited) (audited) Assets Non-current assets
2015 Quarterly Report II
 2015 Quarterly Report II 2 Key data Eckert & Ziegler 01 06/2015 01 06/2014 Change Sales million 69.0 61.9 + 11 % Return on revenue before tax % 16 % 9 % + 87 % EBITDA million 15.6 9.7 + 61 % EBIT million
2015 Quarterly Report II 2 Key data Eckert & Ziegler 01 06/2015 01 06/2014 Change Sales million 69.0 61.9 + 11 % Return on revenue before tax % 16 % 9 % + 87 % EBITDA million 15.6 9.7 + 61 % EBIT million
ANNUAL REPORT 2015. Amanitin. Amanitin. Antibody Targeted Amanitin Conjugates. Innovative cancer therapies
 ANNUAL REPORT 2015 Amanitin Amanitin Antibody Targeted Amanitin Conjugates Innovative cancer therapies Key figures 2015 1 000 2014 1 000 Change in % Earnings Sales revenue 2,284 3,597 (36 %) Other income
ANNUAL REPORT 2015 Amanitin Amanitin Antibody Targeted Amanitin Conjugates Innovative cancer therapies Key figures 2015 1 000 2014 1 000 Change in % Earnings Sales revenue 2,284 3,597 (36 %) Other income
ANNUAL REPORT 2014. Amanitin. Amanitin. Next Generation ADCs
 ANNUAL REPORT 2014 Amanitin Amanitin Next Generation ADCs Key figures 2014 1 million 2013 1 million 2012 1 million Earnings Sales revenue 3.6 13.3 16.1 Other income 1.4 5.8 1.7 Operating expenses (10.6)
ANNUAL REPORT 2014 Amanitin Amanitin Next Generation ADCs Key figures 2014 1 million 2013 1 million 2012 1 million Earnings Sales revenue 3.6 13.3 16.1 Other income 1.4 5.8 1.7 Operating expenses (10.6)
3 M O N T H S R E P O R T 2 O O 3 / 2 O O 4
 3 M O N T H S R E P O R T 2 O O 3 / 2 O O 4 Content 03 Hönle at a glance 04 Letter to the Shareholders 06 Management Report 09 Consolidated financial statement 17 Shareholdings of the corporate bodies
3 M O N T H S R E P O R T 2 O O 3 / 2 O O 4 Content 03 Hönle at a glance 04 Letter to the Shareholders 06 Management Report 09 Consolidated financial statement 17 Shareholdings of the corporate bodies
Group 9-month report Bastei Lübbe AG 1 Apr - 31 Dec 2015
 Group 9-month report Bastei Lübbe AG 1 Apr - 31 Dec 2015 At a Glance Key figures (IFRS) 01/04/2015-2015 01/04/- Change in % Business development in million Group turnover 79.4 86.8-8.5 % EBITDA 14.0 11.5
Group 9-month report Bastei Lübbe AG 1 Apr - 31 Dec 2015 At a Glance Key figures (IFRS) 01/04/2015-2015 01/04/- Change in % Business development in million Group turnover 79.4 86.8-8.5 % EBITDA 14.0 11.5
PROMETIC LIFE SCIENCES INC.
 PROMETIC LIFE SCIENCES INC. Investor Update July to September 2002 This present release must be considered as the quarterly report to shareholders. 1. Letter to Shareholders Dated November 21, 2002 2.
PROMETIC LIFE SCIENCES INC. Investor Update July to September 2002 This present release must be considered as the quarterly report to shareholders. 1. Letter to Shareholders Dated November 21, 2002 2.
9-MONTHS REPORT. Stable development of business in Q3 Lila Logistik confirms full-year forecast
 /08 9-MONTHS REPORT Stable development of business in Q3 Lila Logistik confirms full-year forecast Key figures for the first three quarters of 2008 in accordance with IFRS 01.01. 01.01. Change in Change
/08 9-MONTHS REPORT Stable development of business in Q3 Lila Logistik confirms full-year forecast Key figures for the first three quarters of 2008 in accordance with IFRS 01.01. 01.01. Change in Change
2014/2015 The IndusTrIal Group
 Q2 2014/2015 Half-Year Interim Report 2014/2015 1 April to 30 September 2014 The Industrial Group The first six months of financial year 2014/2015 at a glance Incoming orders increased in the first half
Q2 2014/2015 Half-Year Interim Report 2014/2015 1 April to 30 September 2014 The Industrial Group The first six months of financial year 2014/2015 at a glance Incoming orders increased in the first half
Letter from the Management Board 3. Key Financial Figures 4. Management Report 5. Consolidated Income Statement (IFRS) 9
 3-Months Report 2015 Content Letter from the Management Board 3 Key Financial Figures 4 Management Report 5 Consolidated Income Statement (IFRS) 9 Consolidated Statement of Comprehensive Income (IFRS)
3-Months Report 2015 Content Letter from the Management Board 3 Key Financial Figures 4 Management Report 5 Consolidated Income Statement (IFRS) 9 Consolidated Statement of Comprehensive Income (IFRS)
Transition to International Financial Reporting Standards
 Transition to International Financial Reporting Standards Topps Tiles Plc In accordance with IFRS 1, First-time adoption of International Financial Reporting Standards ( IFRS ), Topps Tiles Plc, ( Topps
Transition to International Financial Reporting Standards Topps Tiles Plc In accordance with IFRS 1, First-time adoption of International Financial Reporting Standards ( IFRS ), Topps Tiles Plc, ( Topps
2014 Quarterly Report II
 2014 Quarterly Report II 2 Key data Eckert & Ziegler 01 06/2014 01 06/2013 Change Sales million 61.9 55.3 12% Return on revenue before tax % 9 % 12 % 26 % EBITDA million 9.7 10.2 5 % EBIT million 6.2 6.9
2014 Quarterly Report II 2 Key data Eckert & Ziegler 01 06/2014 01 06/2013 Change Sales million 61.9 55.3 12% Return on revenue before tax % 9 % 12 % 26 % EBITDA million 9.7 10.2 5 % EBIT million 6.2 6.9
ico Therapeutics Inc. Condensed Interim Financial Statements (Unaudited) September 30, 2012 and 2011 (in Canadian dollars)
 Condensed Interim Financial Statements (Unaudited) September 30, 2012 and 2011 Balance Sheet (Unaudited) Assets Note September 30, 2012 December 31, 2011 Current assets Cash and cash equivalents 1,478,167
Condensed Interim Financial Statements (Unaudited) September 30, 2012 and 2011 Balance Sheet (Unaudited) Assets Note September 30, 2012 December 31, 2011 Current assets Cash and cash equivalents 1,478,167
Overview of the key figures for the first nine months
 Continued revenue growth: up 12% on previous year Results impacted by revenue structure and one-off effects High volume of orders: outlook remains optimistic Q3 Overview of the key figures for the first
Continued revenue growth: up 12% on previous year Results impacted by revenue structure and one-off effects High volume of orders: outlook remains optimistic Q3 Overview of the key figures for the first
Interim Report. January - September
 Interim Report January - September LETTER TO THE SHAREHOLDERS RIB SOFTWARE AG LETTER TO THE SHAREHOLDERS Dear Shareholders, With two strategic acquisitions in the third quarter of, we have taken a further
Interim Report January - September LETTER TO THE SHAREHOLDERS RIB SOFTWARE AG LETTER TO THE SHAREHOLDERS Dear Shareholders, With two strategic acquisitions in the third quarter of, we have taken a further
3-month report January - March 2007 Published on August 10, 2007
 3-month report January - March 2007 Published on August 10, 2007 3-month report January March 2007 1. Group management report for the first quarter of 2007 Overview of the first quarter in 2007 Continued
3-month report January - March 2007 Published on August 10, 2007 3-month report January March 2007 1. Group management report for the first quarter of 2007 Overview of the first quarter in 2007 Continued
In addition, Outokumpu has adopted the following amended standards as of January 1, 2009:
 1. Corporate information Outokumpu Oyj is a Finnish public limited liability company organised under the laws of Finland and domiciled in Espoo. The parent company, Outokumpu Oyj, has been listed on the
1. Corporate information Outokumpu Oyj is a Finnish public limited liability company organised under the laws of Finland and domiciled in Espoo. The parent company, Outokumpu Oyj, has been listed on the
3. CONSOLIDATED QUARTERLY FINANCIAL STATEMENTS
 3. CONSOLIDATED QUARTERLY FINANCIAL STATEMENTS (1) Consolidated Quarterly Balance Sheets September 30, 2014 and March 31, 2014 Supplementary Information 2Q FY March 2015 March 31, 2014 September 30, 2014
3. CONSOLIDATED QUARTERLY FINANCIAL STATEMENTS (1) Consolidated Quarterly Balance Sheets September 30, 2014 and March 31, 2014 Supplementary Information 2Q FY March 2015 March 31, 2014 September 30, 2014
Ahlers AG, Herford. ISIN DE0005009708 and DE0005009732 INTERIM REPORT
 Ahlers AG, Herford ISIN DE0005009708 and DE0005009732 on the first nine months of fiscal 2005/06 (December 1, 2005, to August 31, 2006) BUSINESS DEVELOPMENT IN THE FIRST NINE MONTHS OF FISCAL 2005/06 According
Ahlers AG, Herford ISIN DE0005009708 and DE0005009732 on the first nine months of fiscal 2005/06 (December 1, 2005, to August 31, 2006) BUSINESS DEVELOPMENT IN THE FIRST NINE MONTHS OF FISCAL 2005/06 According
2014/2015 The IndusTrIal Group
 Q1 2014/2015 Interim Report 1 April to 30 june 2014 The Industrial Group The essentials at a glance in the first quarter Big increase in incoming orders, sales on par with previous year, earnings considerably
Q1 2014/2015 Interim Report 1 April to 30 june 2014 The Industrial Group The essentials at a glance in the first quarter Big increase in incoming orders, sales on par with previous year, earnings considerably
CONSOLIDATED PROFIT AND LOSS ACCOUNT For the six months ended June 30, 2002
 CONSOLIDATED PROFIT AND LOSS ACCOUNT For the six months ended June 30, 2002 Unaudited Unaudited Note Turnover 2 5,576 5,803 Other net losses (1) (39) 5,575 5,764 Direct costs and operating expenses (1,910)
CONSOLIDATED PROFIT AND LOSS ACCOUNT For the six months ended June 30, 2002 Unaudited Unaudited Note Turnover 2 5,576 5,803 Other net losses (1) (39) 5,575 5,764 Direct costs and operating expenses (1,910)
ANNUAL FINANCIAL RESULTS
 ANNUAL FINANCIAL RESULTS For the year ended 31 July 2013 ANNUAL FINANCIAL RESULTS 2013 FONTERRA CO-OPERATIVE GROUP LIMITED Contents: DIRECTORS STATEMENT... 1 INCOME STATEMENT... 2 STATEMENT OF COMPREHENSIVE
ANNUAL FINANCIAL RESULTS For the year ended 31 July 2013 ANNUAL FINANCIAL RESULTS 2013 FONTERRA CO-OPERATIVE GROUP LIMITED Contents: DIRECTORS STATEMENT... 1 INCOME STATEMENT... 2 STATEMENT OF COMPREHENSIVE
FINANCIAL REPORT H1 2014
 FINANCIAL REPORT H1 2014 HIGH SPEED BY PASSION 02_Key Figures 03_Group Status Report 05_Consolidated Financial Statements 10_Notes 11_Declaration of the Legal Representatives 02 PANKL KEY FIGURES EARNING
FINANCIAL REPORT H1 2014 HIGH SPEED BY PASSION 02_Key Figures 03_Group Status Report 05_Consolidated Financial Statements 10_Notes 11_Declaration of the Legal Representatives 02 PANKL KEY FIGURES EARNING
THERATECHNOLOGIES INC.
 Interim Consolidated Financial Statements of (In thousands of Canadian dollars) THERATECHNOLOGIES INC. Table of Contents Page Interim Consolidated Financial Positions 1 Interim Consolidated Statements
Interim Consolidated Financial Statements of (In thousands of Canadian dollars) THERATECHNOLOGIES INC. Table of Contents Page Interim Consolidated Financial Positions 1 Interim Consolidated Statements
CEMATRIX CORPORATION Consolidated Financial Statements (in Canadian dollars) September 30, 2015
 Consolidated Financial Statements September 30, 2015 Management s Responsibility for Financial Reporting and Notice of No Auditor Review of the Interim Consolidated Financial Statements for the Three and
Consolidated Financial Statements September 30, 2015 Management s Responsibility for Financial Reporting and Notice of No Auditor Review of the Interim Consolidated Financial Statements for the Three and
Unaudited Financial Report
 RECRUITING SERVICES Amadeus FiRe AG Unaudited Financial Report Quarter I - 2015 Temporary Staffing. Permanent Placement Interim Management. Training www.amadeus-fire.de Unaudited Amadeus FiRe Group Financial
RECRUITING SERVICES Amadeus FiRe AG Unaudited Financial Report Quarter I - 2015 Temporary Staffing. Permanent Placement Interim Management. Training www.amadeus-fire.de Unaudited Amadeus FiRe Group Financial
IV. UNAUDITED CONSOLIDATED STATEMENT OF CASH FLOWS. 6 VII. STATUTORY AUDITOR'S LIMITED REVIEW REPORT... 10
 2015 INTERIM REPORT TABLE OF CONTENTS I. INTERIM MANAGEMENT REPORT.. 2 II. III. CONDENSED UNAUDITED CONSOLIDATED STATEMENT OF FINANCIAL POSITION.. 4 CONDENSED UNAUDITED CONSOLIDATED STATEMENT OF COMPREHENSIVE
2015 INTERIM REPORT TABLE OF CONTENTS I. INTERIM MANAGEMENT REPORT.. 2 II. III. CONDENSED UNAUDITED CONSOLIDATED STATEMENT OF FINANCIAL POSITION.. 4 CONDENSED UNAUDITED CONSOLIDATED STATEMENT OF COMPREHENSIVE
RNS Number : 4336R 3Legs Resources plc 29 June 2015
 RNS Number : 4336R 3Legs Resources plc 29 June 2015 3Legs Resources plc ("3Legs" or "the Company") Final Results The Board of 3Legs is pleased to announce the Company's final audited results for the year
RNS Number : 4336R 3Legs Resources plc 29 June 2015 3Legs Resources plc ("3Legs" or "the Company") Final Results The Board of 3Legs is pleased to announce the Company's final audited results for the year
Logwin AG. Interim Financial Report as of 31 March 2015
 Logwin AG Interim Financial Report as of 31 March 2015 Key Figures 1 January 31 March 2015 Earnings position In thousand EUR 2015 2014 Revenues Group 274,433 278,533 Change on 2014-1.5% Solutions 101,821
Logwin AG Interim Financial Report as of 31 March 2015 Key Figures 1 January 31 March 2015 Earnings position In thousand EUR 2015 2014 Revenues Group 274,433 278,533 Change on 2014-1.5% Solutions 101,821
MASUPARIA GOLD CORPORATION
 CONDENSED CONSOLIDATED INTERIM FINANCIAL STATEMENTS THREE MONTHS ENDED DECEMBER 31, 2011 and 2010 (expressed in Canadian Dollars) NOTICE TO READERS Under National Instrument 51-102, Part 4.3 (3)(a), if
CONDENSED CONSOLIDATED INTERIM FINANCIAL STATEMENTS THREE MONTHS ENDED DECEMBER 31, 2011 and 2010 (expressed in Canadian Dollars) NOTICE TO READERS Under National Instrument 51-102, Part 4.3 (3)(a), if
BIOMARK DIAGNOSTICS INC. CONDENSED CONSOLIDATED INTERIM FINANCIAL STATEMENTS. June 30, 2015. (Stated in Canadian Dollars)
 CONDENSED CONSOLIDATED INTERIM FINANCIAL STATEMENTS (Unaudited Prepared by Management) NOTICE OF NO AUDITOR REVIEW OF CONDENSED CONSOLIDATED INTERIM FINANCIAL STATEMENTS Under National Instrument 51-102,
CONDENSED CONSOLIDATED INTERIM FINANCIAL STATEMENTS (Unaudited Prepared by Management) NOTICE OF NO AUDITOR REVIEW OF CONDENSED CONSOLIDATED INTERIM FINANCIAL STATEMENTS Under National Instrument 51-102,
ARM Holdings plc Consolidated balance sheet - IFRS
 ARM Holdings plc Consolidated balance sheet - IFRS 30 June 31 December 2010 2009 Unaudited Audited 000 000 Assets Current assets: Financial assets: Cash and cash equivalents 53,746 34,489 Short-term investments
ARM Holdings plc Consolidated balance sheet - IFRS 30 June 31 December 2010 2009 Unaudited Audited 000 000 Assets Current assets: Financial assets: Cash and cash equivalents 53,746 34,489 Short-term investments
ANNOUNCEMENT OF THE RESULTS FOR THE THREE AND NINE MONTHS ENDED 30 SEPTEMBER 2011
 Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness
Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this announcement, make no representation as to its accuracy or completeness
IMMEDIA GROUP PLC. ( Immedia or the Company ) INTERIM RESULTS
 IMMEDIA GROUP PLC ( Immedia or the Company ) INTERIM RESULTS Immedia Group Plc (AIM: IME), which provides bespoke digital networks, music strategies and brand conversation, today announces its interim
IMMEDIA GROUP PLC ( Immedia or the Company ) INTERIM RESULTS Immedia Group Plc (AIM: IME), which provides bespoke digital networks, music strategies and brand conversation, today announces its interim
nine months statement
 nine months statement 22 Brüder Mannesmann Aktiengesellschaft Remscheid Brüder Mannesmann AG is a trading group with two orientations the worldwide trading in tools and the trading in pipe fittings for
nine months statement 22 Brüder Mannesmann Aktiengesellschaft Remscheid Brüder Mannesmann AG is a trading group with two orientations the worldwide trading in tools and the trading in pipe fittings for
Interim Financial Statements
 [Type text] Interim Financial Statements KCA Deutag Alpha Limited For the twelve months ended 31 December 2015 Page 1 of 11 Table of Contents Consolidated income statement... 3 Consolidated statement of
[Type text] Interim Financial Statements KCA Deutag Alpha Limited For the twelve months ended 31 December 2015 Page 1 of 11 Table of Contents Consolidated income statement... 3 Consolidated statement of
Interim Report as of 30 June 2006 Q2/2006
 Interim Report as of 30 June 2006 Q2/2006 GFT Group Summary Financial figures according to IFRS in e(k) 01/01 30/06/2006 01/01 30/06/2005 Revenues 80,123 58,290 Earnings before interest, taxes, depreciation
Interim Report as of 30 June 2006 Q2/2006 GFT Group Summary Financial figures according to IFRS in e(k) 01/01 30/06/2006 01/01 30/06/2005 Revenues 80,123 58,290 Earnings before interest, taxes, depreciation
For personal use only
 APPENDIX 4D INTERIM FINANCIAL REPORT RESULTS FOR ANNOUNCEMENT TO THE MARKET Appendix 4D item 2.1 Revenue from ordinary activities. Appendix 4D item 2.2 Profit (loss) from ordinary activities after tax
APPENDIX 4D INTERIM FINANCIAL REPORT RESULTS FOR ANNOUNCEMENT TO THE MARKET Appendix 4D item 2.1 Revenue from ordinary activities. Appendix 4D item 2.2 Profit (loss) from ordinary activities after tax
Global Value Fund Limited A.B.N. 90 168 653 521. Appendix 4E - Preliminary Financial Report for the year ended 30 June 2015
 A.B.N. 90 168 653 521 Appendix 4E - Preliminary Financial Report for the year ended 30 June 2015 Appendix 4E - Preliminary Financial Report For the year ended 30 June 2015 Preliminary Report This preliminary
A.B.N. 90 168 653 521 Appendix 4E - Preliminary Financial Report for the year ended 30 June 2015 Appendix 4E - Preliminary Financial Report For the year ended 30 June 2015 Preliminary Report This preliminary
Travel24.com AG. Quarterly Report Q1 2015
 Travel24.com AG Quarterly Report Q1 2015 2 Selected Key Group Data January 1 - March 31 Change In thousands of euro 2015 2014 % Revenue 4,494 7,810-42 % EBIT 806 1,231-35 % Net profit 66 518-87 % Earnings
Travel24.com AG Quarterly Report Q1 2015 2 Selected Key Group Data January 1 - March 31 Change In thousands of euro 2015 2014 % Revenue 4,494 7,810-42 % EBIT 806 1,231-35 % Net profit 66 518-87 % Earnings
Consolidated financial statements
 Summary of significant accounting policies Basis of preparation DSM s consolidated financial statements have been prepared in accordance with International Financial Reporting Standards (IFRS) as adopted
Summary of significant accounting policies Basis of preparation DSM s consolidated financial statements have been prepared in accordance with International Financial Reporting Standards (IFRS) as adopted
PRELIMINARY RESULTS FOR HALF YEAR ENDED 30 SEPTEMBER 2015
 Page 1 PRELIMINARY RESULTS FOR HALF YEAR ENDED 30 SEPTEMBER 2015 Reporting Period 6 months to 30 September 2015 Reporting Period 6 months to 30 September 2014 Amount NZ$ 000 Percentage Change % Revenue
Page 1 PRELIMINARY RESULTS FOR HALF YEAR ENDED 30 SEPTEMBER 2015 Reporting Period 6 months to 30 September 2015 Reporting Period 6 months to 30 September 2014 Amount NZ$ 000 Percentage Change % Revenue
21 August 2007. Company Announcements Office Australian Stock Exchange Limited, Melbourne. By E-lodgement. Preliminary Final Report
 21 August 2007 Company Announcements Office Australian Stock Exchange Limited, Melbourne By E-lodgement Preliminary Final Report This release contains an announcement to the Australian Stock Exchange Limited
21 August 2007 Company Announcements Office Australian Stock Exchange Limited, Melbourne By E-lodgement Preliminary Final Report This release contains an announcement to the Australian Stock Exchange Limited
QUARTERLY REPORT 1/2001
 Industrial Automation 4CONTROL Automotive Electronics We set Standards in Control and Communication Technology Softing Everything under 4CONTROL For the first three months of 2001, turnover and EBIT of
Industrial Automation 4CONTROL Automotive Electronics We set Standards in Control and Communication Technology Softing Everything under 4CONTROL For the first three months of 2001, turnover and EBIT of
International Accounting Standard 7 Statement of cash flows *
 International Accounting Standard 7 Statement of cash flows * Objective Information about the cash flows of an entity is useful in providing users of financial statements with a basis to assess the ability
International Accounting Standard 7 Statement of cash flows * Objective Information about the cash flows of an entity is useful in providing users of financial statements with a basis to assess the ability
ATS AUTOMATION TOOLING SYSTEMS INC.
 Interim Consolidated Financial Statements For the period ended June 29, 2014 (Unaudited) (Condensed) Interim Consolidated Statements of Financial Position (in thousands of Canadian dollars unaudited) June
Interim Consolidated Financial Statements For the period ended June 29, 2014 (Unaudited) (Condensed) Interim Consolidated Statements of Financial Position (in thousands of Canadian dollars unaudited) June
Volex Group plc. Transition to International Financial Reporting Standards Supporting document for 2 October 2005 Interim Statement. 1.
 Volex Group plc Transition to International Financial Reporting Standards Supporting document for 2 October 2005 Interim Statement 1. Introduction The consolidated financial statements of Volex Group plc
Volex Group plc Transition to International Financial Reporting Standards Supporting document for 2 October 2005 Interim Statement 1. Introduction The consolidated financial statements of Volex Group plc
NOTES TO THE COMPANY FINANCIAL STATEMENTS
 FINANCIAL S 78 79 80 81 82 CONSOLIDATED INCOME CONSOLIDATED OF COMPREHENSIVE INCOME CONSOLIDATED OF FINANCIAL POSITION CONSOLIDATED OF CONSOLIDATED OF CHANGES IN EQUITY 83 NOTES TO THE CONSOLIDATED FINANCIAL
FINANCIAL S 78 79 80 81 82 CONSOLIDATED INCOME CONSOLIDATED OF COMPREHENSIVE INCOME CONSOLIDATED OF FINANCIAL POSITION CONSOLIDATED OF CONSOLIDATED OF CHANGES IN EQUITY 83 NOTES TO THE CONSOLIDATED FINANCIAL
Suruhanjaya Syarikat Malaysia Taxonomy Tagging List Templates ssmt_20131231
 Suruhanjaya Syarikat Malaysia Taxonomy Tagging List Templates ssmt_20131231 A view of financial and non financial elements as may be presented in set of financial statements. Content Page [010000] Filing
Suruhanjaya Syarikat Malaysia Taxonomy Tagging List Templates ssmt_20131231 A view of financial and non financial elements as may be presented in set of financial statements. Content Page [010000] Filing
CONSOLIDATED INTERIM FINANCIAL STATEMENTS
 CONSOLIDATED INTERIM FINANCIAL STATEMENTS AND GROUP INTERIM MANAGEMENT REPORT SECOND QUARTER OF 2008 JUNE 30, 2008 FRANCONOFURT AG FRANKFURT AM MAIN FRANCONOFURT AG, FRANKFURT AM MAIN CONSOLIDATED INTERIM
CONSOLIDATED INTERIM FINANCIAL STATEMENTS AND GROUP INTERIM MANAGEMENT REPORT SECOND QUARTER OF 2008 JUNE 30, 2008 FRANCONOFURT AG FRANKFURT AM MAIN FRANCONOFURT AG, FRANKFURT AM MAIN CONSOLIDATED INTERIM
Interim Report 2014 January - June
 Interim Report 2014 January - June Letter to the shareholders Interim Report Jan Jun 2014 RIB Software AG Dear Shareholders, The Chinese Year of the Horse has met the high expectations placed on it within
Interim Report 2014 January - June Letter to the shareholders Interim Report Jan Jun 2014 RIB Software AG Dear Shareholders, The Chinese Year of the Horse has met the high expectations placed on it within
IFrS. Disclosure checklist. July 2011. kpmg.com/ifrs
 IFrS Disclosure checklist July 2011 kpmg.com/ifrs Contents What s new? 1 1. General presentation 2 1.1 Presentation of financial statements 2 1.2 Changes in equity 12 1.3 Statement of cash flows 13 1.4
IFrS Disclosure checklist July 2011 kpmg.com/ifrs Contents What s new? 1 1. General presentation 2 1.1 Presentation of financial statements 2 1.2 Changes in equity 12 1.3 Statement of cash flows 13 1.4
MOLOGEN AG interim report as of March 31, 2015 Key data 2
 Interim report as of March 31, 2015 MOLOGEN AG interim report as of March 31, 2015 Key data 2 Highlights Slight decline in research and development expenses EBIT improved accordingly Ongoing recruitment
Interim report as of March 31, 2015 MOLOGEN AG interim report as of March 31, 2015 Key data 2 Highlights Slight decline in research and development expenses EBIT improved accordingly Ongoing recruitment
ANNUAL FINANCIAL RESULTS FOR THE YEAR ENDED 31 JULY 2014 FONTERRA ANNUAL FINANCIAL RESULTS 2014 A
 ANNUAL FINANCIAL RESULTS FOR THE YEAR ENDED 31 JULY 2014 FONTERRA ANNUAL FINANCIAL RESULTS 2014 A CONTENTS DIRECTORS STATEMENT 1 INCOME STATEMENT 2 STATEMENT OF COMPREHENSIVE INCOME 3 STATEMENT OF FINANCIAL
ANNUAL FINANCIAL RESULTS FOR THE YEAR ENDED 31 JULY 2014 FONTERRA ANNUAL FINANCIAL RESULTS 2014 A CONTENTS DIRECTORS STATEMENT 1 INCOME STATEMENT 2 STATEMENT OF COMPREHENSIVE INCOME 3 STATEMENT OF FINANCIAL
D. Consolidated Financial Statements
 Consolidated 254 D.1 Consolidated Statements of Income 255 D.2 Consolidated Statements of Comprehensive Income 256 D.3 Consolidated Statements of Financial Position 257 D.4 Consolidated Statements of Cash
Consolidated 254 D.1 Consolidated Statements of Income 255 D.2 Consolidated Statements of Comprehensive Income 256 D.3 Consolidated Statements of Financial Position 257 D.4 Consolidated Statements of Cash
Contact 6-Month Report 2005
 Contact 6-Month Report 2005 Security Networks AG Kronprinzenstrasse 30 45128 ssen Germany Phone: +49 (0) 201 54 54-0 Fax: +49 (0) 201 54 54-456 Internet: www..com -mail: investor.relations@.com Key figures
Contact 6-Month Report 2005 Security Networks AG Kronprinzenstrasse 30 45128 ssen Germany Phone: +49 (0) 201 54 54-0 Fax: +49 (0) 201 54 54-456 Internet: www..com -mail: investor.relations@.com Key figures
Restated Consolidated Financial Statements as at December 31, 2011
 Restated Consolidated Financial Statements as at December 31, 2011 (including the adjusted adidas AG Opening Consolidated Statement of Financial Position (IFRS) as at January 1, 2011) This excerpt from
Restated Consolidated Financial Statements as at December 31, 2011 (including the adjusted adidas AG Opening Consolidated Statement of Financial Position (IFRS) as at January 1, 2011) This excerpt from
Interim Report 201. Celesio AG. report as of 30 September 2015
 Interim Report 201 Celesio AG H1 Half-year financial report as of 30 September 2015 The Celesio Group Celesio is a leading international wholesale and retail company and provider of logistics and services
Interim Report 201 Celesio AG H1 Half-year financial report as of 30 September 2015 The Celesio Group Celesio is a leading international wholesale and retail company and provider of logistics and services
An exploration stage company. Condensed Interim Consolidated Financial Statements
 An exploration stage company Condensed Interim Consolidated Financial Statements (Expressed in US Dollars - unaudited) Condensed interim consolidated statements of financial position (Expressed in United
An exploration stage company Condensed Interim Consolidated Financial Statements (Expressed in US Dollars - unaudited) Condensed interim consolidated statements of financial position (Expressed in United
NEPAL ACCOUNTING STANDARDS ON CASH FLOW STATEMENTS
 NAS 03 NEPAL ACCOUNTING STANDARDS ON CASH FLOW STATEMENTS CONTENTS Paragraphs OBJECTIVE SCOPE 1-3 BENEFITS OF CASH FLOWS INFORMATION 4-5 DEFINITIONS 6-9 Cash and cash equivalents 7-9 PRESENTATION OF A
NAS 03 NEPAL ACCOUNTING STANDARDS ON CASH FLOW STATEMENTS CONTENTS Paragraphs OBJECTIVE SCOPE 1-3 BENEFITS OF CASH FLOWS INFORMATION 4-5 DEFINITIONS 6-9 Cash and cash equivalents 7-9 PRESENTATION OF A
Quarterly Financial Report. as of March 31, 2010. Qarterly Financial Report. as of March 31, 2010
 Quarterly Financial Report as of March 31, 2010 Qarterly Financial Report as of March 31, 2010 PULSION Quarterly Financial Report as at March 31, 2010 1 PULSION at a glance PULSION (Group) according to
Quarterly Financial Report as of March 31, 2010 Qarterly Financial Report as of March 31, 2010 PULSION Quarterly Financial Report as at March 31, 2010 1 PULSION at a glance PULSION (Group) according to
Notes to Consolidated Financial Statements Notes to Non-Consolidated Financial Statements
 [Translation: Please note that the following purports to be a translation from the Japanese original Notice of Convocation of the Annual General Meeting of Shareholders 2013 of Chugai Pharmaceutical Co.,
[Translation: Please note that the following purports to be a translation from the Japanese original Notice of Convocation of the Annual General Meeting of Shareholders 2013 of Chugai Pharmaceutical Co.,
Symbility Solutions Inc. Interim Condensed Consolidated Financial Statements (Unaudited) Quarter ended March 31, 2016
 Interim Condensed Consolidated Financial Statements (Unaudited) Quarter ended Interim Consolidated Statements of Financial Position (Unaudited - In thousands of Canadian dollars) As at Note March 31, 2016
Interim Condensed Consolidated Financial Statements (Unaudited) Quarter ended Interim Consolidated Statements of Financial Position (Unaudited - In thousands of Canadian dollars) As at Note March 31, 2016
Oasmia Pharmaceutical AB (publ)
 Oasmia Pharmaceutical AB (publ) Interim report for the period May 2014 January 2015 Increased focus on Docecal. THIRD QUARTER November 1, 2014 January 31, 2015 Consolidated Net sales amounted to TSEK 482
Oasmia Pharmaceutical AB (publ) Interim report for the period May 2014 January 2015 Increased focus on Docecal. THIRD QUARTER November 1, 2014 January 31, 2015 Consolidated Net sales amounted to TSEK 482
Interim report as at 31 March 2014. Unit sales, revenue and profit increase Dividend increases to 2.90 per share Stock split ratio of two-for-one
 Interim report as at 31 March 2014 Unit sales, revenue and profit increase Dividend increases to 2.90 per share Stock split ratio of two-for-one Fielmann Aktiengesellschaft Group interim report as at 31
Interim report as at 31 March 2014 Unit sales, revenue and profit increase Dividend increases to 2.90 per share Stock split ratio of two-for-one Fielmann Aktiengesellschaft Group interim report as at 31
G8 Education Limited ABN: 95 123 828 553. Accounting Policies
 G8 Education Limited ABN: 95 123 828 553 Accounting Policies Table of Contents Note 1: Summary of significant accounting policies... 3 (a) Basis of preparation... 3 (b) Principles of consolidation... 3
G8 Education Limited ABN: 95 123 828 553 Accounting Policies Table of Contents Note 1: Summary of significant accounting policies... 3 (a) Basis of preparation... 3 (b) Principles of consolidation... 3
KYODO PRINTING CO., LTD. and Consolidated Subsidiaries
 KYODO PRINTING CO., LTD. and Consolidated Subsidiaries Interim Consolidated Financial Statements (Unaudited) for the, Interim Consolidated Balance Sheets, as compared with March 31, (Unaudited) ASSETS,
KYODO PRINTING CO., LTD. and Consolidated Subsidiaries Interim Consolidated Financial Statements (Unaudited) for the, Interim Consolidated Balance Sheets, as compared with March 31, (Unaudited) ASSETS,
Quarterly Financial Report March 31, 2009. MBB Industries AG. Berlin
 Quarterly Financial Report March 31, 2009 MBB Industries AG. Berlin Quarterly Financial Report March 31, 2009 MBB Industries AG MBB Industries in Numbers 03 MBB Industries in Numbers Three Month (Jan.
Quarterly Financial Report March 31, 2009 MBB Industries AG. Berlin Quarterly Financial Report March 31, 2009 MBB Industries AG MBB Industries in Numbers 03 MBB Industries in Numbers Three Month (Jan.
NedSense enterprises n.v. Condensed consolidated Interim financial statements
 NED NEDSENSE enterprises n.v. NedSense enterprises n.v. Condensed consolidated Interim financial statements 30 June 2012 NedSense enterprises n.v. Half-year Report 2012 (unaudited) Report of the Board
NED NEDSENSE enterprises n.v. NedSense enterprises n.v. Condensed consolidated Interim financial statements 30 June 2012 NedSense enterprises n.v. Half-year Report 2012 (unaudited) Report of the Board
Statutory Financial Statements
 Statutory Financial Statements for the year ended December 31, 2007 by Kardan NV, Amsterdam, the Netherlands Consolidated IFRS Financial Statements Consolidated IFRS Balance Sheet 54 Consolidated IFRS
Statutory Financial Statements for the year ended December 31, 2007 by Kardan NV, Amsterdam, the Netherlands Consolidated IFRS Financial Statements Consolidated IFRS Balance Sheet 54 Consolidated IFRS
A&W Food Services of Canada Inc. Consolidated Financial Statements December 30, 2012 and January 1, 2012 (in thousands of dollars)
 A&W Food Services of Canada Inc. Consolidated Financial Statements December 30, and January 1, (in thousands of dollars) February 12, 2013 Independent Auditor s Report To the Shareholders of A&W Food Services
A&W Food Services of Canada Inc. Consolidated Financial Statements December 30, and January 1, (in thousands of dollars) February 12, 2013 Independent Auditor s Report To the Shareholders of A&W Food Services
Interim Report HORNBACH HOLDING AG GROUP. 1st QUARTER 2004/2005 (March 1 to May 31, 2004)
 Interim Report HORNBACH HOLDING AG GROUP 1st QUARTER 2004/2005 (March 1 to May 31, 2004) page 2 HORNBACH HOLDING AG Group Interim Report (IFRS) for the First Quarter of 2004/2005 (March 1 to May 31, 2004)
Interim Report HORNBACH HOLDING AG GROUP 1st QUARTER 2004/2005 (March 1 to May 31, 2004) page 2 HORNBACH HOLDING AG Group Interim Report (IFRS) for the First Quarter of 2004/2005 (March 1 to May 31, 2004)
SAGICOR FINANCIAL CORPORATION
 Interim Financial Statements Nine-months ended September 30, 2015 FINANCIAL RESULTS FOR THE CHAIRMAN S REVIEW The Sagicor Group recorded net income from continuing operations of US $60.4 million for the
Interim Financial Statements Nine-months ended September 30, 2015 FINANCIAL RESULTS FOR THE CHAIRMAN S REVIEW The Sagicor Group recorded net income from continuing operations of US $60.4 million for the
condensed consolidated interim financial statements 2015
 January march 2015 condensed consolidated interim financial statements 2015 (unaudited) contents 1. Income Statement 1 2. Statement of Comprehensive Income 2 3. Balance Sheet 3 4. Statement of Changes
January march 2015 condensed consolidated interim financial statements 2015 (unaudited) contents 1. Income Statement 1 2. Statement of Comprehensive Income 2 3. Balance Sheet 3 4. Statement of Changes
September 30, 2014 FIRST HALF-YEAR REPORT 2014
 September 30, 2014 FIRST HALF-YEAR REPORT 2014 as of June 30, 2014 Financial summary YTD* 2014 YTD* 2013 Revenue CHF million 0.5 0.5 Net operating costs CHF million (16.5) (10.5) Net loss CHF million (23.4)
September 30, 2014 FIRST HALF-YEAR REPORT 2014 as of June 30, 2014 Financial summary YTD* 2014 YTD* 2013 Revenue CHF million 0.5 0.5 Net operating costs CHF million (16.5) (10.5) Net loss CHF million (23.4)
Condensed Interim Financial Statements Fiscal 2013 First Quarter (Unaudited) For the three months ended July 31, 2012 and 2011
 Condensed Interim Financial Statements Fiscal 2013 First Quarter (Unaudited) CRITICAL OUTCOME TECHNOLOGIES INC Page 2 Condensed Interim Financial Statements Table of Contents Notice of No Audit or Review
Condensed Interim Financial Statements Fiscal 2013 First Quarter (Unaudited) CRITICAL OUTCOME TECHNOLOGIES INC Page 2 Condensed Interim Financial Statements Table of Contents Notice of No Audit or Review
IFRS Illustrative Consolidated Financial Statements 2014
 IFRS Illustrative Consolidated Financial Statements 2014 1 PKF International Limited administers a network of legally independent member firms which carry on separate businesses under the PKF Name. PKF
IFRS Illustrative Consolidated Financial Statements 2014 1 PKF International Limited administers a network of legally independent member firms which carry on separate businesses under the PKF Name. PKF
MOLOGEN. Our research - for you. Interim Report as of March 31, 2013
 MOLOGEN. Our research - for you Interim Report as of March 31, 2013 MOLOGEN AG interim report as of March 31, 2013 Key data 2 KEY DATA of MOLOGEN AG according to IFRS as of March 31, 2013 Statement of
MOLOGEN. Our research - for you Interim Report as of March 31, 2013 MOLOGEN AG interim report as of March 31, 2013 Key data 2 KEY DATA of MOLOGEN AG according to IFRS as of March 31, 2013 Statement of
Interim report as at 30 September 2015
 Interim report as at 30 September 2015 Fielmann improves unit sales, revenue and profit Specialists of tomorrow: 3,000 apprentices Fielmann creates 500 new jobs Fielmann Aktiengesellschaft Group interim
Interim report as at 30 September 2015 Fielmann improves unit sales, revenue and profit Specialists of tomorrow: 3,000 apprentices Fielmann creates 500 new jobs Fielmann Aktiengesellschaft Group interim
Symbility Solutions Inc. Interim Condensed Consolidated Financial Statements (Unaudited) Quarter ended June 30, 2015
 Interim Condensed Consolidated Financial Statements (Unaudited) Quarter ended Interim Consolidated Statements of Financial Position (Unaudited - In thousands of Canadian dollars) 2015 As at December 31,
Interim Condensed Consolidated Financial Statements (Unaudited) Quarter ended Interim Consolidated Statements of Financial Position (Unaudited - In thousands of Canadian dollars) 2015 As at December 31,
eqube Gaming Limited Condensed Interim Consolidated Financial Statements For the Three and Nine Months Ended November 30, 2015 (Unaudited)
 Condensed Interim Consolidated Financial Statements For the Three and Nine Months Ended November 30, 2015 Notice to Reader The following interim consolidated financial statements and notes have not been
Condensed Interim Consolidated Financial Statements For the Three and Nine Months Ended November 30, 2015 Notice to Reader The following interim consolidated financial statements and notes have not been
Opening doors to new ideas. Interim Report 2007/08
 Opening doors to new ideas Interim Report 2007/08 SPG Media Group Plc Interim Report 2007/08 Contents 2 Chairman s Statement 4 Consolidated Interim Income Statement 5 Consolidated Interim Balance Sheet
Opening doors to new ideas Interim Report 2007/08 SPG Media Group Plc Interim Report 2007/08 Contents 2 Chairman s Statement 4 Consolidated Interim Income Statement 5 Consolidated Interim Balance Sheet
Consolidated Balance Sheets
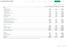 Consolidated Balance Sheets March 31 2015 2014 2015 Assets: Current assets Cash and cash equivalents 726,888 604,571 $ 6,057,400 Marketable securities 19,033 16,635 158,608 Notes and accounts receivable:
Consolidated Balance Sheets March 31 2015 2014 2015 Assets: Current assets Cash and cash equivalents 726,888 604,571 $ 6,057,400 Marketable securities 19,033 16,635 158,608 Notes and accounts receivable:
AcuityAds Inc. Condensed Consolidated Interim Financial Statements. Three months ended March 31, 2014 and 2013 (Unaudited)
 AcuityAds Inc. Condensed Consolidated Interim Financial Statements Condensed Consolidated Interim Statements of Financial Position March 31, December 31, 2014 2013 Assets Current assets: Cash $ 446,034
AcuityAds Inc. Condensed Consolidated Interim Financial Statements Condensed Consolidated Interim Statements of Financial Position March 31, December 31, 2014 2013 Assets Current assets: Cash $ 446,034
