IR Conference Call on EYLEA (aflibercept) Injection in Diabetic Macular Edema. September 28, 2013
|
|
|
- Morgan Bridges
- 10 years ago
- Views:
Transcription
1 IR Conference Call on EYLEA (aflibercept) Injection in Diabetic Macular Edema September 28, 2013
2 Safe Harbor Statement This presentation includes forward-looking statements that involve risks and uncertainties relating to future events and the future performance of Regeneron, and actual events or results may differ materially from these forward-looking statements. Words such as "anticipate," "expect," "intend," "plan," "believe," "seek," "estimate," variations of such words and similar expressions are intended to identify such forward-looking statements, although not all forward-looking statements contain these identifying words. These statements concern, and these risks and uncertainties include, among others, the nature, timing, and possible success and therapeutic applications of Regeneron's products, product candidates, and research and clinical programs now underway or planned, including without limitation EYLEA (aflibercept) Injection; unforeseen safety issues resulting from the administration of products and product candidates in patients; the likelihood and timing of possible regulatory approval and commercial launch of Regeneron's latestage product candidates and new indications for marketed products, such as the application of EYLEA (aflibercept) Injection in the treatment of Diabetic Macular Edema; ongoing regulatory obligations and oversight and determinations by regulatory and administrative governmental authorities which may delay or restrict Regeneron's ability to continue to develop or commercialize Regeneron's products and product candidates; competing drugs and product candidates that may be superior to Regeneron's products and product candidates; uncertainty of market acceptance of Regeneron's products and product candidates; the ability of Regeneron to manufacture and manage supply chains for multiple products and product candidates; coverage and reimbursement determinations by third-party payers, including Medicare and Medicaid; unanticipated expenses; the costs of developing, producing, and selling products; the ability of Regeneron to meet any of its sales or other financial projections or guidance and changes to the assumptions underlying those projections or guidance; the potential for any license or collaboration agreement, including Regeneron's agreements with Sanofi and Bayer HealthCare, to be cancelled or terminated without any further product success; and risks associated with third party intellectual property and pending or future litigation relating thereto. A more complete description of these and. other material risks can be found in Regeneron's filings with the United States Securities and Exchange Commission, including its Form 10-K for the year ended December 31, 2012 and Form 10-Q for the quarter ended June 30, The reader is cautioned not to rely on any forward-looking statements made by Regeneron. Regeneron does not undertake any obligation to update publicly any forward-looking statement, including without limitation any financial projection or guidance, whether as a result of new information, future events, or otherwise.
3 Intravitreal Aflibercept Injection for Diabetic Macular Edema Primary Results David M. Brown, M.D. Houston Methodist Hospital Houston, TX For the -DME/-DME Study Investigators
4 Diabetes: An Epidemic Ø 25.8 million people in the United States (~8.3% of the population) Ø After 15 years of diabetes, prevalence is approximately Ø 20% in patients with Type 1 diabetes. Ø 14% to 25% in patients with Type 2 diabetes Ø Leading cause of blindness in workingage Americans Accessed Sept 2013 Data from the 2011 National Diabetes Fact Sheet (released Jan. 26, 2011) Klein R, Klein B, Moss S, Davis M, DeMets D. The Wisconsin Epidemiologic Study of Diabetic Retinopathy, IV: diabetic macular edema. Ophthalmology 1984;91:
5 TEXAS: Diabetes Statistics Over 2 million Texans affected.
6 Early Treatment Diabetic Retinopathy Study Vision Gain Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. - Arch Ophthalmol - 01-DEC-1985; 103(12):
7 ETDRS: Vision Loss Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. - Arch Ophthalmol - 01-DEC-1985; 103(12):
8 RIDE and RISE: Ranibizumab for Diabetic Macular Edema
9 Intravitreal Aflibercept Injection Fusion protein of key domains from human VEGF receptors 1 and 2 with human IgGFc Pre-clinical studies showed that aflibercept Traps multiple VEGF-A isoforms and placental growth factor (PlGF) Binds to VEGF-A and PlGF with higher affinity than native receptors Binds VEGF between its arms without risk of complex formation Purified and formulated preparation of aflibercept specifically for intravitreal injection Iso-osmotic solution compatible with intraocular environment Purified form to reduce unnecessary ingredients in the eye
10 Intravitreal Aflibercept Injection for Diabetic Macular Edema Primary Results
11 Study Sites Japan Europe Austria, Czech Republic, Denmark, France, Germany, Hungary, Italy, Poland, Spain USA 73 Centers 404 Patients Australia 54 Centers 461 Patients
12 Study Design Randomized, multicenter, double-masked trials in patients with clinically significant DME with central involvement and ETDRS BCVA 20/40 to 20/320 N=406 () N=466 () Patients randomized 1:1:1 IVT Aflibercept 2 mg q4 wks IVT Aflibercept 2 mg q8 wks* Laser Photocoagulation Primary endpoint: Mean change in BCVA Primary Endpoint: Week 52 Key Secondary endpoint: Change in DRSS *After 5 initial monthly doses Continued treatment through Year 3
13 Treatment Schedule IAI 2q4 Week IAI 2q8 Laser Laser (no more often than every 12 weeks) Sham laser (if re-treatment criteria met) Laser (as needed based on pre-specified criteria) Primary Endpoint IAI 2mg Year 2 Year 3 Sham injection IAI 2 mg (as needed based on pre-specified criteria) or sham Starting at Week 24 : Additional (rescue) treatment available to all patients Patients with BCVA below BSL and loss from previous best BCVA score of 15 letters at 1 visit or 10 letters on 2 consecutive visits
14 Key Eligibility Criteria Inclusion Criteria: Adults 18 years with Type 1 or 2 diabetes with DME involving central subfield ETDRS BCVA 20/40 to 20/320 Exclusion Criteria: No previous treatment for DME in last 3 months Active PDR IOP 25 mm Hg No previous vitreoretinal surgery Uncontrolled diabetes or blood pressure History of CVA or MI within 6 months
15 Patient Disposition Laser IAI 2q4 IAI 2q8 ALL IAI Randomized, n Received Study Medication, n Completed Week 52, n (%) 115 (85.2%) 125 (91.9%) 120 (88.9%) 360 (88.7%) Randomized, n Received Study Medication, n Completed Week 52, n (%) 145 (92.9%) 146 (93.6%) 144 (93.5%) 435 (93.3%)
16 Baseline Demographics Laser IAI 2q4 IAI 2q8 Laser IAI 2q4 IAI 2q8 N Age, years (SD) 63.9 (8.6) 62.6 (8.6) 64.2 (7.8) 61.7 (8.7) 62.0 (11.2) 63.1 (9.4) Gender (Women), n (%) 54 (40.9%) 53 (39.0%) 47 (34.8%) 69 (44.8%) 67 (43.5%) 73 (48.3%) Race, n (%) White 106 (80.3%) 109 (80.1%) 106 (78.5%) 131 (85.1%) 128 (83.1%) 125 (82.8%) Black 1 (0.8%) 0 1 (0.7%) 16 (10.4%) 16 (10.4%) 19 (12.6%) Asian 25 (18.9%) 27 (19.9%) 27 (20.0%) 3 (1.9%) 5 (3.2%) 2 (1.3%) Other HbA1c, mean % (SD) 7.7 (1.26) 7.8 (1.46) 7.7 (1.43) 7.6 (1.68) 7.9 (1.65) 7.9 (1.56) Proportion w/ >8%, n (%) 42 (31.8%) 55 (40.4%) 44 (32.6%) 45 (29.2%) 57 (37.0%) 57 (37.7%) Duration of Diabetes, years (SD) Full analysis set 14.5 (9.8) 14.3 (9.2) 14.1 (8.9) 17.2 (9.55) 16.5 (9.94) 17.6 (11.46)
17 Baseline Disease Characteristics Laser IAI 2q4 IAI 2q8 Laser IAI 2q4 IAI 2q8 N BCVA, ETDRS Letter score (SD) 60.8 (10.6) 60.8 (10.7) 58.8 (11.2) 59.7 (10.9) 58.9 (10.8) 59.4 (10.9) CRT, µm (SD) 540 (152) 502 (144) 518 (147) 483 (153) 485 (157) 479 (154) Prior Anti-VEGF Treatment, n (%) 11 (8.3%) 7 (5.1%) 14 (10.4%) 63 (40.9%) 66 (42.9%) 68 (45.0%) BCVA: Best-Corrected Visual Acuity CRT: Central Retinal Thickness (Central Subfield) Full analysis set
18 Baseline Disease Characteristics N DRSS, n (%) (27.3%) 31 (22.8%) 28 (20.7%) 60 (39.0%) 49 (31.8%) 52 (34.4%) (18.2%) 18 (13.2%) 27 (20.0%) 26 (16.9%) 26 (16.9%) 32 (21.2%) (26.5%) 44 (32.4%) 42 (31.1%) 42 (27.3%) 52 (33.8%) 40 (26.5%) Cannot grade 33 (25.0%) 39 (28.7%) 34 (25.2%) 4 (2.6%) 2 (1.3%) 3 (2.0%) 40 Laser IAI 2q4 IAI 2q8 Laser IAI 2q4 IAI 2q8 40 Proportion of Patients Level About 74% of patients in, and 98% of patients in could be graded DRSS: Diabetic Retinopathy Severity Score; Full analysis set Level
19 Exposure and Rescue Treatment
20 Treatment Experience Mean # of Active Injections* Mean # of Active Injections* Number of Treatments Mean # of Active Lasers* Mean # of Active Lasers* 2.7 Laser 2q4 2q8 0 SAF: Laser: n=133; 2q4: n=136; 2q8: n=135; SAF: Laser: n=154; 2q4: n=155; 2q8: n=152 *Not considering Rescue Treatment; 13 Injections possible; Minimum # of lasers = 1, maximum = 4/5
21 Additional (Rescue) Treatment Experience SAF Laser: n=133; 2q4: n=136; 2q8: n=135 SAF Laser: n=154; 2q4: n=155; 2q8: n=151 *Patients in IAI arms received laser and patients in the laser arm received IAI if Rescue Treatment Criteria were met. Proportion of Patients Receiving Rescue Treatment Proportion of Patients 100% 80% 60% 40% 20% 0% 24.1% 31.2% 4.4% 8.1% 2.6% 0.7% Laser 2q4 2q8
22 Fellow Eye Treatment Proportion of Patients Receiving anti-vegf Treatment Proportion of Patients 100% 80% 60% 40% 20% 24.1% 26.5% 25.2% 59.1% 59.1% 70.9% Laser 2q4 2q8 0% Laser: n=132; 2q4: n=136; 2q8: n=135 SAF Laser: n=154; 2q4: n=154; 2q8: n=151
23 Efficacy
24 Mean Change in Best-Corrected Visual Acuity ETDRS letters 14 Laser 2q4 2q Laser Laser Week ETDRS; Compared to baseline; FAS; LOCF Laser: n=154; 2q4: n=154; 2q8: n=151 - Laser: n=132; 2q4: n=136; 2q8; n=135
25 Mean Change in Best-Corrected Visual Acuity ETDRS letters Week ETDRS; Compared to baseline; FAS; LOCF Laser: n=154; 2q4: n=154; 2q8: n=151 - Laser: n=132; 2q4: n=136; 2q8; n=135 *P < vs. laser *P < vs. laser Laser 2q4 2q q q4 1.2 Laser q q Laser
26 Proportion of Patients Gaining 10 Letters 100% Proportion of Patients 80% 60% 40% 20% 25.8% 54.4%* 53.3%* 19.5% 64.9%* 58.3%* Laser 2q4 2q8 0% FAS- Laser: n=132; 2q4: n=136; 2q8: n=135 FAS- Laser: n=154; 2q4: n=154; 2q8: n=151 Compared to baseline; LOCF *P < vs. laser *P < vs. laser
27 Proportion of Patients Gaining 15 Letters 100% Proportion of Patients 80% 60% 40% 20% 32.4%* 41.6%* 33.3%* 31.1%* 9.1% 7.8% Laser 2q4 2q8 0% FAS- Laser: n=132; 2q4: n=136; 2q8: n=135 FAS- Laser: n=154; 2q4: n=154; 2q8: n=151 Compared to baseline; LOCF *P < vs. laser *P < vs. laser
28 Proportion of Patients Losing 15 Letters 50% Proportion of Patients 40% 30% 20% 10% 0% 10.6% 9.1% 0.7% 0.0% 0.6% 0.7% Laser 2q4 2q8 FAS- Laser: n=132; 2q4: n=136; 2q8: n=135 FAS- Laser: n=154; 2q4: n=154; 2q8: n=151 Compared to baseline; LOCF
29 0 Mean Change in Central Retinal Thickness Week Laser 2q4 2q *P < vs. laser Laser µm q q Laser q q8 - Laser: n=132; 2q4: n=136; 2q8: n=135 Laser: n=154; 2q4: n=154; 2q8: n=151 Central Sub-field; Compared to baseline; FAS; LOCF; SD-OCT *P < vs. laser
30 DRSS: Diabetic Retinopathy Severity Score Compared to baseline; LOCF Proportion of Patients with 2 Step Improvement in DRSS 100% Proportion of Patients 80% 60% 40% 20% 7.5% 33.3% 33.8% 27.7% 29.1% 14.3% Laser 2q4 2q8 0% n=80 n=81 n=83 P < q4 vs. laser P = q8 vs. laser n=154 n=151 n=154 P < q4 vs. laser P = q8 vs. laser
31 Safety
32 Safety Overview Laser IAI 2q4 IAI 2q8 ALL IAI Laser IAI 2q4 IAI 2q8 ALL IAI N Ocular AEs (study eye), n (%) Systemic AEs, n (%) Ocular SAEs (study eye), n (%) Systemic SAEs, n (%) 82 (61.7%) 76 (55.9%) 80 (59.3%) 156 (57.6%) 103 (66.9%) 96 (61.9%) 87 (57.2%) 183 (59.6%) 81 (60.9%) 92 (67.6%) 98 (72.6%) 190 (70.1%) 132 (85.7%) 125 (80.6%) 119 (78.3%) 244 (79.5%) 6 (4.5%) 2 (1.5%) 3 (2.2%) 5 (1.8%) 6 (3.9%) 3 (1.9%) 2 (1.3%) 5 (1.6%) 18 (13.5%) 19 (14.0%) 25 (18.5%) 44 (16.2%) 47 (30.5%) 48 (31.0%) 39 (25.7%) 87 (28.3%) (S)AE = (Serious) Adverse Event Safety analyses set
33 Intraocular Inflammation Study Eye Laser IAI 2q4 IAI 2q8 Laser IAI 2q4 IAI 2q8 Number of Injections Number of events, n (%) 1 (0.4%)* 4 (0.2%) 5 (0.4%) 1 (0.2%)* 4 (0.2%) 1 (0.1%) Endophthalmitis, n (%) *Patient did not receive an IVT injections in study eye Safety analyses set
34 APTC Events* N Any APTC event, n (%) 2 (1.5%) 2 (1.5%) 4 (3.0%) 6 (2.2%) Non-fatal MI 1 (0.8%) 1 (0.7%) 0 1 (0.4%) Non-fatal stroke 0 1 (0.7%) 2 (1.5%) 3 (1.1%) Vascular deaths 1 (0.8%) 0 2 (1.5%) 2 (0.7%) Laser IAI 2q4 IAI 2q8 ALL IAI N Any APTC event, n (%) 6 (3.9%) 7 (4.5%) 6 (3.9%) 13 (4.2%) Non-fatal MI 4 (2.6%) 3 (1.9%) 3 (2.0%) 6 (2.0%) Non-fatal stroke 2 (1.3%) 2 (1.3%) 3 (2.0%) 5 (1.6%) Vascular deaths 1 (0.6%) 2 (1.3%) 0 2 (0.7%) *As adjudicated by a masked committee; Only treatment emergent events included; APTC=Anti-platelet thromboembolic Collaboration Safety analyses set
35 Deaths Through Week 52 N Death, n (%) 1 (0.8%) 0 4 (3.0%)* 4 (1.5%) Acute MI 1 (0.8%) Cardiac failure (0.7%) 1 (0.4%) Hypertensive heart disease (0.7%) 1 (0.4%) Lung neoplasm (0.7%) 1 (0.4%) B-cell lymphoma (0.7%) 1 (0.4%) Laser N Death, n (%) 1 (0.6%) 2 (1.3%) 0 2 (0.7%) Death 0 1 (0.6%) 0 1 (0.3%) Myocardial infarction 0 1 (0.6%) 0 1 (0.3%) Sudden cardiac death 1 (0.6%) *Note: One additional death (MI) occurred in the 2q8 group in in year 2 (406 and 77 days after the first and last active injections, respectively), but due to data conventions it was included in the Year 1 clinical database Safety analyses set IAI 2q4 IAI 2q8 ALL IAI
36 Deaths Through Week 52 Kaplan Meier Plot of Time to Death Note: One additional death (MI) occurred in the 2q8 group in -DME in year 2 (406 and 77 days after the first and last active injections, respectively), but due to data conventions it was included in the Year 1 clinical database. Safety analyses set
37 Conclusions ETDRS letters q q q q Laser Laser Superiority of IAI over laser treatment seen through Week 52 On average, IAI 2q8 group performed similarly to the IAI 2q4 group No systemic safety signal in both IAI treatment groups evident through Week 52
38 Q&A 38
FDA approves Lucentis (ranibizumab injection) for treatment of diabetic macular edema
 Media Release Basel, 13 August 2012 FDA approves Lucentis (ranibizumab injection) for treatment of diabetic macular edema First major treatment advance in more than 25 years for sight-threatening condition
Media Release Basel, 13 August 2012 FDA approves Lucentis (ranibizumab injection) for treatment of diabetic macular edema First major treatment advance in more than 25 years for sight-threatening condition
Ohr Pharmaceutical Reports Fiscal Year 2015 Financial and Business Results
 December 10, 2015 Ohr Pharmaceutical Reports Fiscal Year 2015 Financial and Business Results OHR-102 Phase 3 Program in Wet-AMD to be Initiated Upon Completion of SPA; Enroll Patients in Q1 2016 Conference
December 10, 2015 Ohr Pharmaceutical Reports Fiscal Year 2015 Financial and Business Results OHR-102 Phase 3 Program in Wet-AMD to be Initiated Upon Completion of SPA; Enroll Patients in Q1 2016 Conference
Dexamethasone intravitreal implant for the treatment of macular oedema secondary to retinal vein occlusion
 Dexamethasone intravitreal implant for the treatment of macular oedema secondary to retinal vein occlusion Issued: July 2011 guidance.nice.org.uk/ta229 NICE has accredited the process used by the Centre
Dexamethasone intravitreal implant for the treatment of macular oedema secondary to retinal vein occlusion Issued: July 2011 guidance.nice.org.uk/ta229 NICE has accredited the process used by the Centre
Charles C Wykoff, David M Brown, Maria E Maldonado, Daniel E Croft. Clinical science
 Aflibercept treatment for patients with exudative age-related macular degeneration who were incomplete responders to multiple ranibizumab injections (TURF trial) Charles C Wykoff, David M Brown, Maria
Aflibercept treatment for patients with exudative age-related macular degeneration who were incomplete responders to multiple ranibizumab injections (TURF trial) Charles C Wykoff, David M Brown, Maria
Peptide and Sustained Release Technology to Treat Ocular Diseases and Cancer
 Peptide and Sustained Release Technology to Treat Ocular Diseases and Cancer Jordan Green, PhD, Co-Founder and CEO Wendy Perrow, MBA, Senior Business Advisor February 11, 2015 www.asclepix.com Advantages
Peptide and Sustained Release Technology to Treat Ocular Diseases and Cancer Jordan Green, PhD, Co-Founder and CEO Wendy Perrow, MBA, Senior Business Advisor February 11, 2015 www.asclepix.com Advantages
Diabetes & blindness. due to DME BLINDNESS IN EUROPE
 Diabetes & blindness due to DME BLINDNESS IN EUROPE Blindness is a life-changing disability which puts a heavy strain on the daily lives of sufferers, their families, and society at large. Today, 284 million
Diabetes & blindness due to DME BLINDNESS IN EUROPE Blindness is a life-changing disability which puts a heavy strain on the daily lives of sufferers, their families, and society at large. Today, 284 million
An Informational Guide to CENTRAL RETINAL VEIN OCCLUSION
 Science of CRVO www.scienceofcrvo.org An Informational Guide to CENTRAL RETINAL VEIN OCCLUSION This brochure will guide you in understanding CRVO and the treatment options available to prevent vision loss.
Science of CRVO www.scienceofcrvo.org An Informational Guide to CENTRAL RETINAL VEIN OCCLUSION This brochure will guide you in understanding CRVO and the treatment options available to prevent vision loss.
Current Management of Retinal Vein Occlusion Robert A. Mittra, MD VRS Course 2015
 Central Retinal Vein Occlusion Hemi-Central Vein Occlusion Current Management of Retinal Vein Occlusion Robert A. Mittra, MD VRS Course 2015 Branch Retinal Vein Occlusion Ischemic vs. non-ischemic BRVO
Central Retinal Vein Occlusion Hemi-Central Vein Occlusion Current Management of Retinal Vein Occlusion Robert A. Mittra, MD VRS Course 2015 Branch Retinal Vein Occlusion Ischemic vs. non-ischemic BRVO
Ranibizumab for Macular Edema following Central Retinal Vein Occlusion
 for Macular Edema following Central Retinal Vein Occlusion Six-Month Primary End Point Results of a Phase III Study David M. Brown, MD, FACS, 1 Peter A. Campochiaro, MD, 2 Rishi P. Singh, MD, 3 Zhengrong
for Macular Edema following Central Retinal Vein Occlusion Six-Month Primary End Point Results of a Phase III Study David M. Brown, MD, FACS, 1 Peter A. Campochiaro, MD, 2 Rishi P. Singh, MD, 3 Zhengrong
ACTVE INTERVENTIONAL CLINICAL TRIALS - 03/01/2015. Principal Investigator Study Title and Sponsor Study Description
 Cornea and External Disease Service Li, Jennifer A Prospective, Multicenter Post-Approval Study (PAS) of VisionCare s Implantable Miniature Telescope (by Dr. Isaac Lipshitz) in Patients with Bilateral
Cornea and External Disease Service Li, Jennifer A Prospective, Multicenter Post-Approval Study (PAS) of VisionCare s Implantable Miniature Telescope (by Dr. Isaac Lipshitz) in Patients with Bilateral
Riociguat Clinical Trial Program
 Riociguat Clinical Trial Program Riociguat (BAY 63-2521) is an oral agent being investigated as a new approach to treat chronic thromboembolic pulmonary hypertension (CTEPH) and pulmonary arterial hypertension
Riociguat Clinical Trial Program Riociguat (BAY 63-2521) is an oral agent being investigated as a new approach to treat chronic thromboembolic pulmonary hypertension (CTEPH) and pulmonary arterial hypertension
Sponsor Novartis. Generic Drug Name Secukinumab. Therapeutic Area of Trial Psoriasis. Approved Indication investigational
 Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K CURRENT REPORT
 UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 OR 15(d) of The Securities Exchange Act of 1934 Date of Report (Date of earliest event
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 OR 15(d) of The Securities Exchange Act of 1934 Date of Report (Date of earliest event
Sanofi Reports Positive Phase 3 Results for Toujeo (insulin glargine [rdna origin] injection, 300 U/mL)
![Sanofi Reports Positive Phase 3 Results for Toujeo (insulin glargine [rdna origin] injection, 300 U/mL) Sanofi Reports Positive Phase 3 Results for Toujeo (insulin glargine [rdna origin] injection, 300 U/mL)](/thumbs/26/8616095.jpg) PRESS RELEASE Sanofi Reports Positive Phase 3 Results for Toujeo (insulin glargine [rdna origin] injection, 300 U/mL) Meta-analysis of three late-stage trials in people with type 2 diabetes shows decreases
PRESS RELEASE Sanofi Reports Positive Phase 3 Results for Toujeo (insulin glargine [rdna origin] injection, 300 U/mL) Meta-analysis of three late-stage trials in people with type 2 diabetes shows decreases
Avastin (Bevacizumab) Intravitreal Injection
 Avastin (Bevacizumab) Intravitreal Injection This handout describes how Avastin may be used to treat wet age related macular degeneration (AMD) or macular edema due to retinal vascular disease such as
Avastin (Bevacizumab) Intravitreal Injection This handout describes how Avastin may be used to treat wet age related macular degeneration (AMD) or macular edema due to retinal vascular disease such as
U.S. Food and Drug Administration
 U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
U.S. Food and Drug Administration Notice: Archived Document The content in this document is provided on the FDA s website for reference purposes only. It was current when produced, but is no longer maintained
Completed Clinical Research Trials Prema Abraham, M.D.
 Completed Clinical Research Trials Prema Abraham, M.D. AMD Studies 2010 Present Principal Investigator: A Phase 2, randomized, double-masked, controlled trial to establish the safety and efficacy of intravitreous
Completed Clinical Research Trials Prema Abraham, M.D. AMD Studies 2010 Present Principal Investigator: A Phase 2, randomized, double-masked, controlled trial to establish the safety and efficacy of intravitreous
The Long-term Effects of Laser Photocoagulation Treatment in Patients with Diabetic Retinopathy
 The Long-term Effects of Laser Photocoagulation Treatment in Patients with Diabetic Retinopathy The Early Treatment Diabetic Retinopathy Follow-up Study Emily Y. Chew, MD, 1 Frederick L. Ferris III, MD,
The Long-term Effects of Laser Photocoagulation Treatment in Patients with Diabetic Retinopathy The Early Treatment Diabetic Retinopathy Follow-up Study Emily Y. Chew, MD, 1 Frederick L. Ferris III, MD,
LUCENTIS ranibizumab (rbe)
 1 LUCENTIS ranibizumab (rbe) NAME OF THE MEDICINE Active ingredient: Ranibizumab Chemical name: Immunoglobulin G1, anti-(human vascular endothelial growth factor) Fab fragment (human-mouse monoclonal rhufab
1 LUCENTIS ranibizumab (rbe) NAME OF THE MEDICINE Active ingredient: Ranibizumab Chemical name: Immunoglobulin G1, anti-(human vascular endothelial growth factor) Fab fragment (human-mouse monoclonal rhufab
Guidance for Industry Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes
 Guidance for Industry Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes U.S. Department of Health and Human Services Food and Drug Administration Center
Guidance for Industry Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes U.S. Department of Health and Human Services Food and Drug Administration Center
1981-1982 Ophthalmic Consultants Northwest, Seattle, Washington Retinal and vitreous consultation and surgery
 EDUCATION Richard S. Munsen Jr., MD University of Washington Eye Institute HMC Box 359608, 325 Ninth Ave, Seattle WA 98104-2499 (206) 744-2020 for patient appointments, Fax (206) 897-4320 Academic phone
EDUCATION Richard S. Munsen Jr., MD University of Washington Eye Institute HMC Box 359608, 325 Ninth Ave, Seattle WA 98104-2499 (206) 744-2020 for patient appointments, Fax (206) 897-4320 Academic phone
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Ophthalmic Innovation 2015 A View from the AAO
 Ophthalmic Innovation 215 A View from the AAO Ophthalmic Innovation 215: a view from the AAO IRIS David W. Parke II, MD CEO American Academy of Ophthalmology Introduction to AAO s IRIS Registry IRIS Registry
Ophthalmic Innovation 215 A View from the AAO Ophthalmic Innovation 215: a view from the AAO IRIS David W. Parke II, MD CEO American Academy of Ophthalmology Introduction to AAO s IRIS Registry IRIS Registry
A Phase 2 Study of HTX-011 in the Management of Post-Operative Pain Positive Top-Line Results
 A Phase 2 Study of HTX-011 in the Management of Post-Operative Pain Positive Top-Line Results September 22, 2015 Forward-Looking Statements This presentation contains "forward-looking statements" as defined
A Phase 2 Study of HTX-011 in the Management of Post-Operative Pain Positive Top-Line Results September 22, 2015 Forward-Looking Statements This presentation contains "forward-looking statements" as defined
Sponsor Novartis Pharmaceuticals
 Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Generic Drug Name Indacaterol Therapeutic Area of Trial Chronic Obstructive Pulmonary Disease (COPD) Indication studied: COPD Study
Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Generic Drug Name Indacaterol Therapeutic Area of Trial Chronic Obstructive Pulmonary Disease (COPD) Indication studied: COPD Study
Effect of liraglutide on body weight in overweight or obese subjects with type 2 diabetes: SCALE - Diabetes
 Effect of liraglutide on body weight in overweight or obese subjects with type 2 diabetes: SCALE - Diabetes This trial is conducted in Africa, Asia, Europe and the United States of America (USA). The aim
Effect of liraglutide on body weight in overweight or obese subjects with type 2 diabetes: SCALE - Diabetes This trial is conducted in Africa, Asia, Europe and the United States of America (USA). The aim
SYNOPSIS. 2-Year (0.5 DB + 1.5 OL) Addendum to Clinical Study Report
 Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier (For National Authority Use
Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier (For National Authority Use
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
- Patients treated with alemtuzumab in CARE-MS II were more than twice as likely to experience disability improvement compared to Rebif -
 PRESS RELEASE Significant Improvement in Disability Scores Observed in Multiple Sclerosis Patients Who Received Lemtrada TM* (Alemtuzumab) Compared With Rebif in Phase lll Trial - Patients treated with
PRESS RELEASE Significant Improvement in Disability Scores Observed in Multiple Sclerosis Patients Who Received Lemtrada TM* (Alemtuzumab) Compared With Rebif in Phase lll Trial - Patients treated with
Affiliation : ASSOCIATION FOR RESEARCH IN VISION AND OPHTHALMOLOGY (ARVO). AMERICAN ACADEMY OF OPHTHALMOLOGY (AAO)
 Curriculum Vitae Federico Ricci, MD Name: Federico Ricci, MD. Titolo Accademico /Title: Professore Aggregato di Oftalmologia / Aggregate Professor of Ophthalmology Direttore UOSD Patologie Retiniche [Centro
Curriculum Vitae Federico Ricci, MD Name: Federico Ricci, MD. Titolo Accademico /Title: Professore Aggregato di Oftalmologia / Aggregate Professor of Ophthalmology Direttore UOSD Patologie Retiniche [Centro
NC DIVISION OF SERVICES FOR THE BLIND POLICIES AND PROCEDURES VOCATIONAL REHABILITATION
 NC DIVISION OF SERVICES FOR THE BLIND POLICIES AND PROCEDURES VOCATIONAL REHABILITATION Section: E Revision History Revised 01/97; 05/03; 02/08; 04/08; 03/09; 05/09; 12/09; 01/11; 12/14 An individual is
NC DIVISION OF SERVICES FOR THE BLIND POLICIES AND PROCEDURES VOCATIONAL REHABILITATION Section: E Revision History Revised 01/97; 05/03; 02/08; 04/08; 03/09; 05/09; 12/09; 01/11; 12/14 An individual is
Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators
 Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators Shaikha Al Naimi Doctor of Pharmacy Student College of Pharmacy Qatar University
Journal Club: Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy by the AIM-HIGH Investigators Shaikha Al Naimi Doctor of Pharmacy Student College of Pharmacy Qatar University
2013 Diabetes and Eye Disease Fact Sheet
 The Georgia Department of Public Health 2013 Diabetes and Eye Disease Fact Sheet Diabetes is the leading cause of new cases of blindness among United States adults. 1-3 As Georgia s population ages and
The Georgia Department of Public Health 2013 Diabetes and Eye Disease Fact Sheet Diabetes is the leading cause of new cases of blindness among United States adults. 1-3 As Georgia s population ages and
Week 12 study results
 Week 12 study results 15 April 2015 Copyright 2015 Galapagos NV Disclaimer This document may contain certain statements, including forward-looking statements, such as statements concerning the safety and
Week 12 study results 15 April 2015 Copyright 2015 Galapagos NV Disclaimer This document may contain certain statements, including forward-looking statements, such as statements concerning the safety and
on behalf of the AUGMENT-HF Investigators
 One Year Follow-Up Results from AUGMENT-HF: A Multicenter Randomized Controlled Clinical Trial of the Efficacy of Left Ventricular Augmentation with Algisyl-LVR in the Treatment of Heart Failure* Douglas
One Year Follow-Up Results from AUGMENT-HF: A Multicenter Randomized Controlled Clinical Trial of the Efficacy of Left Ventricular Augmentation with Algisyl-LVR in the Treatment of Heart Failure* Douglas
Addendum to Clinical Review for NDA 22-512
 Addendum to Clinical Review for DA 22-512 Drug: Sponsor: Indication: Division: Reviewers: dabigatran (Pradaxa) Boehringer Ingelheim Prevention of stroke and systemic embolism in atrial fibrillation Division
Addendum to Clinical Review for DA 22-512 Drug: Sponsor: Indication: Division: Reviewers: dabigatran (Pradaxa) Boehringer Ingelheim Prevention of stroke and systemic embolism in atrial fibrillation Division
Medical Devices. Driving Growth With Transformational Technology
 Medical Devices Driving Growth With Transformational Technology Forward-Looking Statement Some statements in this presentation may be forward-looking statements for purposes of the Private Securities Litigation
Medical Devices Driving Growth With Transformational Technology Forward-Looking Statement Some statements in this presentation may be forward-looking statements for purposes of the Private Securities Litigation
Preliminary Results from a Phase 2 Study of ARQ 197 in Patients with Microphthalmia Transcription Factor Family (MiT) Associated Tumors
 Preliminary Results from a Phase 2 Study of ARQ 197 in Patients with Microphthalmia Transcription Factor Family (MiT) Associated Tumors John Goldberg 1 *, George Demetri 2, Edwin Choy 3, Lee Rosen 4, Alberto
Preliminary Results from a Phase 2 Study of ARQ 197 in Patients with Microphthalmia Transcription Factor Family (MiT) Associated Tumors John Goldberg 1 *, George Demetri 2, Edwin Choy 3, Lee Rosen 4, Alberto
U.S. Scientific Update Aricept 23 mg Tablets. Dr. Lynn Kramer President NeuroScience Product Creation Unit Eisai Inc.
 U.S. Scientific Update Aricept 23 mg Tablets Dr. Lynn Kramer President NeuroScience Product Creation Unit Eisai Inc. Unmet Need in Moderate to Severe Alzheimer s Disease (AD) Ongoing clinical deterioration
U.S. Scientific Update Aricept 23 mg Tablets Dr. Lynn Kramer President NeuroScience Product Creation Unit Eisai Inc. Unmet Need in Moderate to Severe Alzheimer s Disease (AD) Ongoing clinical deterioration
DEMONSTRATING MEANINGFUL USE STAGE 1 REQUIREMENTS FOR ELIGIBLE PROVIDERS USING CERTIFIED EHR TECHNOLOGY IN 2014
 DEMONSTRATING MEANINGFUL USE STAGE 1 REQUIREMENTS FOR ELIGIBLE PROVIDERS USING CERTIFIED EHR TECHNOLOGY IN 2014 The chart below lists the measures (and specialty exclusions) that eligible providers must
DEMONSTRATING MEANINGFUL USE STAGE 1 REQUIREMENTS FOR ELIGIBLE PROVIDERS USING CERTIFIED EHR TECHNOLOGY IN 2014 The chart below lists the measures (and specialty exclusions) that eligible providers must
Laser Treatment for Proliferative Diabetic Retinopathy (PDR) Pan Retinal Photocoagulation (PRP)
 Laser Treatment for Proliferative Diabetic Retinopathy (PDR) Pan Retinal Photocoagulation (PRP) Ophthalmology Department Page 12 Patient Information Further Information We endeavour to provide an excellent
Laser Treatment for Proliferative Diabetic Retinopathy (PDR) Pan Retinal Photocoagulation (PRP) Ophthalmology Department Page 12 Patient Information Further Information We endeavour to provide an excellent
THE INTERNET STROKE CENTER PRESENTATIONS AND DISCUSSIONS ON STROKE MANAGEMENT
 THE INTERNET STROKE CENTER PRESENTATIONS AND DISCUSSIONS ON STROKE MANAGEMENT Stroke Prevention in Atrial Fibrillation Gregory Albers, M.D. Director Stanford Stroke Center Professor of Neurology and Neurological
THE INTERNET STROKE CENTER PRESENTATIONS AND DISCUSSIONS ON STROKE MANAGEMENT Stroke Prevention in Atrial Fibrillation Gregory Albers, M.D. Director Stanford Stroke Center Professor of Neurology and Neurological
THE EYE INSTITUTE. Dear Patient:
 THE EYE INSTITUTE Eye Associates of Wayne P.A. 968 Hamburg Turnpike Wayne, NJ 07470 p. 973-696-0300 f. 973-696-0464 Eye Institute North, LLC 5677 Berkshire Valley Rd. Oak Ridge, NJ 07438 p. 973-208-0600
THE EYE INSTITUTE Eye Associates of Wayne P.A. 968 Hamburg Turnpike Wayne, NJ 07470 p. 973-696-0300 f. 973-696-0464 Eye Institute North, LLC 5677 Berkshire Valley Rd. Oak Ridge, NJ 07438 p. 973-208-0600
INTERNATIONAL CLINICAL DIABETIC RETINOPATHY DISEASE SEVERITY SCALE DETAILED TABLE
 Proposed Disease Severity Level No apparent Mild Non- Proliferative Moderate Nonproliferative Severe Non- Proliferative Proliferative INTERNATIONAL CLINICAL DIABETIC RETINOPATHY DISEASE SEVERITY SCALE
Proposed Disease Severity Level No apparent Mild Non- Proliferative Moderate Nonproliferative Severe Non- Proliferative Proliferative INTERNATIONAL CLINICAL DIABETIC RETINOPATHY DISEASE SEVERITY SCALE
An introduction to Optos
 An introduction to Optos Building The Retina Company Roy Davis, CEO Christine Soden, CFO 1 Forward-Looking Statements Certain statements made in this presentation are forward-looking statements. These
An introduction to Optos Building The Retina Company Roy Davis, CEO Christine Soden, CFO 1 Forward-Looking Statements Certain statements made in this presentation are forward-looking statements. These
Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM
 Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM This report was commissioned by the NIHR HTA Programme as project number 12/78
Dabigatran etexilate for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism ERRATUM This report was commissioned by the NIHR HTA Programme as project number 12/78
Patient-Reported Outcomes with LASIK (PROWL-1) Results
 Patient-Reported Outcomes with LASIK (PROWL-1) Results Elizabeth M. Hofmeister, MD CAPT, MC, USN Naval Medical Center San Diego Refractive Surgery Advisor for Navy Ophthalmology Assistant Professor of
Patient-Reported Outcomes with LASIK (PROWL-1) Results Elizabeth M. Hofmeister, MD CAPT, MC, USN Naval Medical Center San Diego Refractive Surgery Advisor for Navy Ophthalmology Assistant Professor of
Bios 6648: Design & conduct of clinical research
 Bios 6648: Design & conduct of clinical research Section 1 - Specifying the study setting and objectives 1. Specifying the study setting and objectives 1.0 Background Where will we end up?: (a) The treatment
Bios 6648: Design & conduct of clinical research Section 1 - Specifying the study setting and objectives 1. Specifying the study setting and objectives 1.0 Background Where will we end up?: (a) The treatment
Investor News. Not intended for U.S. and UK media
 Investor News Not intended for U.S. and UK media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer s Xarelto (Rivaroxaban) Approved for the Treatment of Pulmonary Embolism
Investor News Not intended for U.S. and UK media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer s Xarelto (Rivaroxaban) Approved for the Treatment of Pulmonary Embolism
Sponsor. Novartis Generic Drug Name. Vildagliptin. Therapeutic Area of Trial. Type 2 diabetes. Approved Indication. Investigational.
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Investigational Study Number CLAF237A2386 Title A single-center,
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Investigational Study Number CLAF237A2386 Title A single-center,
Stent for Life Initiative How can we improve system delay and patients delay in STEMI
 Stent for Life Initiative How can we improve system delay and patients delay in STEMI Z. Kaifoszova SFL Initiative Europe 2011 Stent for Life Initiative 10 countries participate in the program Declaration
Stent for Life Initiative How can we improve system delay and patients delay in STEMI Z. Kaifoszova SFL Initiative Europe 2011 Stent for Life Initiative 10 countries participate in the program Declaration
Main Effect of Screening for Coronary Artery Disease Using CT
 Main Effect of Screening for Coronary Artery Disease Using CT Angiography on Mortality and Cardiac Events in High risk Patients with Diabetes: The FACTOR-64 Randomized Clinical Trial Joseph B. Muhlestein,
Main Effect of Screening for Coronary Artery Disease Using CT Angiography on Mortality and Cardiac Events in High risk Patients with Diabetes: The FACTOR-64 Randomized Clinical Trial Joseph B. Muhlestein,
FORM 6-K. SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549. Report of Foreign Private Issuer
 FORM 6-K SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 Report of Foreign Private Issuer Pursuant to Rule 13a-16 or 15d-16 under the Securities Exchange Act of 1934 For the month of August 2011
FORM 6-K SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 Report of Foreign Private Issuer Pursuant to Rule 13a-16 or 15d-16 under the Securities Exchange Act of 1934 For the month of August 2011
Background. t 1/2 of 3.7 4.7 days allows once-daily dosing (1.5 mg) with consistent serum concentration 2,3 No interaction with CYP3A4 inhibitors 4
 Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Medical management of CHF: A New Class of Medication. Al Timothy, M.D. Cardiovascular Institute of the South
 Medical management of CHF: A New Class of Medication Al Timothy, M.D. Cardiovascular Institute of the South Disclosures Speakers Bureau for Amgen Background Chronic systolic congestive heart failure remains
Medical management of CHF: A New Class of Medication Al Timothy, M.D. Cardiovascular Institute of the South Disclosures Speakers Bureau for Amgen Background Chronic systolic congestive heart failure remains
NAVILAS Laser System Focal Laser Treatment for Diabetic Macular Edema - One Year Results of a Case Series
 Send Orders for Reprints to [email protected] 48 The Open Ophthalmology Journal, 2013, 7, 48-53 Open Access NAVILAS Laser System Focal Laser Treatment for Diabetic Macular Edema - One Year Results
Send Orders for Reprints to [email protected] 48 The Open Ophthalmology Journal, 2013, 7, 48-53 Open Access NAVILAS Laser System Focal Laser Treatment for Diabetic Macular Edema - One Year Results
Bayer Pharma AG 13342 Berlin Germany Tel. +49 30 468-1111 www.bayerpharma.com. News Release. Not intended for U.S. and UK Media
 News Release Not intended for U.S. and UK Media Bayer Pharma AG 13342 Berlin Germany Tel. +49 30 468-1111 www.bayerpharma.com Bayer Forms Collaboration with Academic and Governmental Institutions for Rivaroxaban
News Release Not intended for U.S. and UK Media Bayer Pharma AG 13342 Berlin Germany Tel. +49 30 468-1111 www.bayerpharma.com Bayer Forms Collaboration with Academic and Governmental Institutions for Rivaroxaban
Trial Description. Organizational Data. Secondary IDs
 Trial Description Title A randomized, double-blind, multicenter study to demonstrate equivalent efficacy and to compare safety and immunogenicity of a biosimilar etanercept (GP2015) and Enbrel in patients
Trial Description Title A randomized, double-blind, multicenter study to demonstrate equivalent efficacy and to compare safety and immunogenicity of a biosimilar etanercept (GP2015) and Enbrel in patients
2.0 Synopsis. Vicodin CR (ABT-712) M05-765 Clinical Study Report R&D/07/095. (For National Authority Use Only) to Part of Dossier: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Vicodin CR Name of Active Ingredient: Hydrocodone/Acetaminophen Extended Release (ABT-712) Individual Study Table Referring to Part of Dossier: Volume:
2.0 Synopsis Abbott Laboratories Name of Study Drug: Vicodin CR Name of Active Ingredient: Hydrocodone/Acetaminophen Extended Release (ABT-712) Individual Study Table Referring to Part of Dossier: Volume:
EXPANDING THE EVIDENCE BASE IN OUTCOMES RESEARCH: USING LINKED ELECTRONIC MEDICAL RECORDS (EMR) AND CLAIMS DATA
 EXPANDING THE EVIDENCE BASE IN OUTCOMES RESEARCH: USING LINKED ELECTRONIC MEDICAL RECORDS (EMR) AND CLAIMS DATA A CASE STUDY EXAMINING RISK FACTORS AND COSTS OF UNCONTROLLED HYPERTENSION ISPOR 2013 WORKSHOP
EXPANDING THE EVIDENCE BASE IN OUTCOMES RESEARCH: USING LINKED ELECTRONIC MEDICAL RECORDS (EMR) AND CLAIMS DATA A CASE STUDY EXAMINING RISK FACTORS AND COSTS OF UNCONTROLLED HYPERTENSION ISPOR 2013 WORKSHOP
The largest clinical study of Bayer's Xarelto (rivaroxaban) Wednesday, 14 November 2012 07:38
 Bayer HealthCare has announced the initiation of the COMPASS study, the largest clinical study of its oral anticoagulant Xarelto (rivaroxaban) to date, investigating the prevention of major adverse cardiac
Bayer HealthCare has announced the initiation of the COMPASS study, the largest clinical study of its oral anticoagulant Xarelto (rivaroxaban) to date, investigating the prevention of major adverse cardiac
Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, MD 21244
 March 7, 2014 Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, MD 21244 Re: Dear Sir or Madam: On behalf of the American Heart Association (AHA), including the American Stroke
March 7, 2014 Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, MD 21244 Re: Dear Sir or Madam: On behalf of the American Heart Association (AHA), including the American Stroke
Efficacy, safety and preference study of a insulin pen PDS290 vs. a Novo Nordisk marketed insulin pen in diabetics
 Efficacy, safety and preference study of a insulin pen PDS290 vs. a Novo Nordisk marketed insulin pen in diabetics This trial is conducted in the United States of America (USA). The aim of this clinical
Efficacy, safety and preference study of a insulin pen PDS290 vs. a Novo Nordisk marketed insulin pen in diabetics This trial is conducted in the United States of America (USA). The aim of this clinical
FREEDOM C: A 16-Week, International, Multicenter, Double-Blind, Randomized, Placebo-Controlled Comparison of the Efficacy and Safety of Oral UT-15C
 FREEDOM C: A 16-Week, International, Multicenter, Double-Blind, Randomized, Placebo-Controlled Comparison of the Efficacy and Safety of Oral UT-15C SR in Combination with an ERA and/or a PDE-5 Inhibitor
FREEDOM C: A 16-Week, International, Multicenter, Double-Blind, Randomized, Placebo-Controlled Comparison of the Efficacy and Safety of Oral UT-15C SR in Combination with an ERA and/or a PDE-5 Inhibitor
Investor News. Phase III J-ROCKET AF Study of Bayer s Xarelto (rivaroxaban) Meets Primary Endpoint. Not intended for U.S.
 Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Phase III J-ROCKET AF Study of Bayer s Xarelto (rivaroxaban) Meets Primary Endpoint
Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Phase III J-ROCKET AF Study of Bayer s Xarelto (rivaroxaban) Meets Primary Endpoint
Systolic Blood Pressure Intervention Trial (SPRINT) Principal Results
 Systolic Blood Pressure Intervention Trial (SPRINT) Principal Results Paul K. Whelton, MB, MD, MSc Chair, SPRINT Steering Committee Tulane University School of Public Health and Tropical Medicine, and
Systolic Blood Pressure Intervention Trial (SPRINT) Principal Results Paul K. Whelton, MB, MD, MSc Chair, SPRINT Steering Committee Tulane University School of Public Health and Tropical Medicine, and
Demonstrating Meaningful Use Stage 1 Requirements for Eligible Providers Using Certified EMR Technology
 Demonstrating Meaningful Use Stage 1 Requirements for Eligible Providers Using Certified EMR Technology The chart below lists the measures (and specialty exclusions) that eligible providers must demonstrate
Demonstrating Meaningful Use Stage 1 Requirements for Eligible Providers Using Certified EMR Technology The chart below lists the measures (and specialty exclusions) that eligible providers must demonstrate
Xarelto (Rivaroxaban): Effective in a broad spectrum. Joep Hufman, MD Medical Scientific Liason
 Xarelto (Rivaroxaban): Effective in a broad spectrum Joep Hufman, MD Medical Scientific Liason Xarelto : Effective in a broad spectrum Introduction Therapeutic areas SPAF VTE Prevention VTE treatment Practical
Xarelto (Rivaroxaban): Effective in a broad spectrum Joep Hufman, MD Medical Scientific Liason Xarelto : Effective in a broad spectrum Introduction Therapeutic areas SPAF VTE Prevention VTE treatment Practical
Involution of Threshold Retinopathy of Prematurity after Diode Laser Photocoagulation
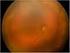 Involution of Threshold Retinopathy of Prematurity after Diode Laser Photocoagulation David K. Coats, MD, 1,2 Aaron M. Miller, MD, 1 Kathryn M. Brady McCreery, MD, 1 Eric R. Holz, MD, 1 Evelyn A. Paysse,
Involution of Threshold Retinopathy of Prematurity after Diode Laser Photocoagulation David K. Coats, MD, 1,2 Aaron M. Miller, MD, 1 Kathryn M. Brady McCreery, MD, 1 Eric R. Holz, MD, 1 Evelyn A. Paysse,
Clinical Study Synopsis
 Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website. It is provided for patients and healthcare professionals to increase the transparency of
Clinical Study Synopsis This file is posted on the Bayer HealthCare Clinical Trials Registry and Results website. It is provided for patients and healthcare professionals to increase the transparency of
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
The Consequences of Missing Data in the ATLAS ACS 2-TIMI 51 Trial
 The Consequences of Missing Data in the ATLAS ACS 2-TIMI 51 Trial In this white paper, we will explore the consequences of missing data in the ATLAS ACS 2-TIMI 51 Trial and consider if an alternative approach
The Consequences of Missing Data in the ATLAS ACS 2-TIMI 51 Trial In this white paper, we will explore the consequences of missing data in the ATLAS ACS 2-TIMI 51 Trial and consider if an alternative approach
Visual Disorders in Middle-Age and Elderly Patients with Diabetic Retinopathy
 Medical Care for the Elderly Visual Disorders in Middle-Age and Elderly Patients with Diabetic Retinopathy JMAJ 46(1): 27 32, 2003 Shigehiko KITANO Professor, Department of Ophthalmology, Diabetes Center,
Medical Care for the Elderly Visual Disorders in Middle-Age and Elderly Patients with Diabetic Retinopathy JMAJ 46(1): 27 32, 2003 Shigehiko KITANO Professor, Department of Ophthalmology, Diabetes Center,
Majestic Trial 12 Month Results
 Majestic Trial 12 Month Results S.Müller-Hülsbeck, MD, EBIR, FCIRSE, FICA ACADEMIC HOSPITALS Flensburg of Kiel University Ev.-Luth. Diakonissenanstalt zu Flensburg Knuthstraße 1, 24939 FLENSBURG Dept.
Majestic Trial 12 Month Results S.Müller-Hülsbeck, MD, EBIR, FCIRSE, FICA ACADEMIC HOSPITALS Flensburg of Kiel University Ev.-Luth. Diakonissenanstalt zu Flensburg Knuthstraße 1, 24939 FLENSBURG Dept.
Please allow us to welcome you to our practice. Our first priority is to provide you with the best care possible.
 PAUL L. TREGER, M.D. RANDALL CONRAD, O.D. GLENN B. COOK, M.D., PhD TARA BROWN, M.D. 7877 PARKWAY DRIVE SUITE 100 - LA MESA, CA 91942 619.286.3711 FAX 619.286.2184 Dear Please allow us to welcome you to
PAUL L. TREGER, M.D. RANDALL CONRAD, O.D. GLENN B. COOK, M.D., PhD TARA BROWN, M.D. 7877 PARKWAY DRIVE SUITE 100 - LA MESA, CA 91942 619.286.3711 FAX 619.286.2184 Dear Please allow us to welcome you to
J.P. Morgan Cazenove Therapeutic Seminar
 Jannan, MS J.P. Morgan Cazenove Therapeutic Seminar David Meeker - CEO, Genzyme June 25, 2012 Forward Looking Statements This presentation contains forward-looking statements as defined in the Private
Jannan, MS J.P. Morgan Cazenove Therapeutic Seminar David Meeker - CEO, Genzyme June 25, 2012 Forward Looking Statements This presentation contains forward-looking statements as defined in the Private
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
The Role of Insurance in Providing Access to Cardiac Care in Maryland. Samuel L. Brown, Ph.D. University of Baltimore College of Public Affairs
 The Role of Insurance in Providing Access to Cardiac Care in Maryland Samuel L. Brown, Ph.D. University of Baltimore College of Public Affairs Heart Disease Heart Disease is the leading cause of death
The Role of Insurance in Providing Access to Cardiac Care in Maryland Samuel L. Brown, Ph.D. University of Baltimore College of Public Affairs Heart Disease Heart Disease is the leading cause of death
CORONADO EYE ASSOCIATES GLENN B. COOK, M.D., PhD 801 ORANGE AVENUE, STE. 204 - CORONADO, CA 92118 619.437-4406 FAX 619.522-7983
 Dear Please allow us to welcome you to our practice. Our first priority is to provide you with the best care possible. Enclosed is your patient information sheet and medical history questionnaire. Please
Dear Please allow us to welcome you to our practice. Our first priority is to provide you with the best care possible. Enclosed is your patient information sheet and medical history questionnaire. Please
Understanding Diseases and Treatments with Canadian Real-world Evidence
 Understanding Diseases and Treatments with Canadian Real-world Evidence Real-World Evidence for Successful Market Access WHITEPAPER REAL-WORLD EVIDENCE Generating real-world evidence requires the right
Understanding Diseases and Treatments with Canadian Real-world Evidence Real-World Evidence for Successful Market Access WHITEPAPER REAL-WORLD EVIDENCE Generating real-world evidence requires the right
ICD-10 Transition & Implementation Information
 ICD-10 Transition & Implementation Information I ICD-10 Implementation Compliance Date: October 1, 2015 ICD-10-CM diagnoses codes will be used by all providers in every health care setting. ICD-10-PCS
ICD-10 Transition & Implementation Information I ICD-10 Implementation Compliance Date: October 1, 2015 ICD-10-CM diagnoses codes will be used by all providers in every health care setting. ICD-10-PCS
Bayer Initiates Rivaroxaban Phase III Study to Support Dose Selection According to Individual Benefit-Risk Profile in Long- Term VTE Prevention
 Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Long-term prevention of venous blood clots (VTE): Bayer Initiates Rivaroxaban
Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Long-term prevention of venous blood clots (VTE): Bayer Initiates Rivaroxaban
