Ttreatment algorithms were discussed in a previous article. 1
|
|
|
- Bertram Lewis
- 8 years ago
- Views:
Transcription
1 The Texas Medication Algorithm Project: Report of the Texas Consensus Conference Panel on Medication Treatment of Major Depressive Disorder M. Lynn Crismon, Pharm.D.; Madhukar Trivedi, M.D.; Teresa A. Pigott, M.D.; A. John Rush, M.D.; Robert M. A. Hirschfeld, M.D.; David A. Kahn, M.D.; Charles DeBattista, D.M.H., M.D.; J. Craig Nelson, M.D.; Andrew A. Nierenberg, M.D.; Harold A. Sackeim, Ph.D.; Michael E. Thase, M.D.; and the Texas Consensus Conference Panel on Medication Treatment of Major Depressive Disorder Background: This article describes the development of consensus medication algorithms for the treatment of patients with major depressive disorder in the Texas public mental health system. To the best of our knowledge, the Texas Medication Algorithm Project (TMAP) is the first attempt to develop and prospectively evaluate consensus-based medication algorithms for the treatment of individuals with severe and persistent mental illnesses. The goals of the algorithm project are to increase the consistency of appropriate treatment of major depressive disorder and to improve clinical outcomes of patients with the disorder. Method: A consensus conference composed of academic clinicians and researchers, practicing clinicians, administrators, consumers, and families was convened to develop evidence-based consensus algorithms for the pharmacotherapy of major depressive disorder in the Texas mental health system. After a series of presentations and panel discussions, the consensus panel met and drafted the algorithms. Results: The panel consensually agreed on algorithms developed for both nonpsychotic and psychotic depression. The algorithms consist of systematic strategies to define appropriate treatment interventions and tactics to assure optimal implementation of the strategies. Subsequent to the consensus process, the algorithms were further modified and expanded iteratively to facilitate implementation on a local basis. Conclusion: These algorithms serve as the initial foundation for the development and implementation of medication treatment algorithms for patients treated in public mental health systems. Specific issues related to adaptation, implementation, feasibility testing, and evaluation of outcomes with the pharmacotherapeutic algorithms will be described in future articles. (J Clin Psychiatry 1999;60: ) Received June 26, 1997; accepted Dec. 9, From the College of Pharmacy, University of Texas at Austin, Austin (Dr. Crismon); the Department of Psychiatry, University of Texas Southwestern Medical Center, Dallas (Drs. Trivedi and Rush); the Department of Psychiatry, University of Texas Medical Branch, Galveston (Drs. Pigott and Hirshfeld); the Department of Psychiatry (Dr. Kahn) and the New York State Psychiatric Institute (Dr. Sackeim), Columbia University, New York; the Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Palo Alto, Calif. (Dr. DeBattista); the Department of Psychiatry, Yale University School of Medicine, New Haven, Conn. (Dr. Nelson); Massachusetts General Hospital, Boston (Dr. Nierenberg); the Western Psychiatric Institute and Clinic, Pittsburgh, Pa. (Dr. Thase); and the Texas Consensus Conference Panel on Medication Treatment of Major Depressive Disorder. A complete list of the members of the Texas Consensus Conference Panel on Medication Treatment of Major Depressive Disorder appears at the end of this article. Supported in part by grant 5 R24 MH from the National Institute of Mental Health, Rockville, Md., and grants from the Center for Mental Health Services, Rockville, Md., and the Texas Department of Mental Health and Mental Retardation, Austin. The authors thank Kenneth Z. Altshuler, M.D., for creativity and leadership in conceptualizing this project and Karla Starkweather for technical assistance. Reprint requests to: M. Lynn Crismon, Pharm.D., College of Pharmacy, MC A1910, The University of Texas at Austin, Austin, TX he rationale for the Texas Medication Algorithm Project and the various methods for developing Ttreatment algorithms were discussed in a previous article. 1 This article will address the development of pharmacotherapeutic algorithms for major depressive disorder. For major depressive disorder, it was decided to develop treatment algorithms through the consensus panel format. Numerous academic psychiatrists and clinical psychopharmacology specialists from around the country were invited to Galveston, Texas, to participate in the consensus conference. They were joined by physicians from the Texas public mental health system, administrators from the Texas Department of Mental Health and Mental Retardation (TDMHMR), invited mental health consumers, and family members to form the consensus panel. Before the conference, invited participants were sent a copy of the Agency for Health Care Policy and Research 142 COPYRIGHT 1999 PHYSICIANS POSTGRADUATE PRESS, INC. COPYRIGHT 1999 PHYSICIANS J Clin POSTGRADUATE Psychiatry 60:3, PRESS, March 1999 INC.
2 Texas Medication Algorithm Project (AHCPR) guidelines and selected articles or manuscripts that were solicited from the nationwide expert panel members Following a series of scheduled presentations and panel discussions, the consensus panel met until the medication algorithms were drafted. The algorithm draft was then presented for discussion with the remainder of the conference participants. One member of the consensus panel was then selected to develop the draft document into the consensus manuscript for further review by the consensus panel members. The consensus panel designated 3 levels of data for its deliberations. This categorization was a modification of the levels used in the American Psychiatric Association (APA) guideline, 14 AHCPR guidelines, 2 and the international psychopharmacology algorithm project. 15 Level A data consisted of randomized, controlled clinical trials; Level B data consisted of open trials and retrospective analyses; and Level C consisted of case reports and expert opinion or consensus of the panel based on widespread clinical practice. As a rule, in decision making, Level A evidence superseded Level B evidence, and Level B evidence superseded Level C evidence. However, depending on the context of the question being addressed, Level B data might address the question better than Level A, or Level C evidence better than Level B. Therefore, panel consensus was reached on any given point by utilizing the combination of levels of evidence that best addressed the question at hand. Additionally, consumer input was received and incorporated into the deliberations. Contributions from the consumers were clearly essential, since patient preference should always be considered when selecting and implementing treatment options. The consensus panel process was, above all, evidence based. In areas where the panel could not reach a consensus or where there was felt to be inadequate information for a consensus, no opinion was rendered, and the algorithm remained silent. This represents an essential point. Lack of inclusion of a treatment strategy or tactic in the algorithm does not necessarily imply that a particular treatment approach is irrational. It does indicate either that inadequate information was available for the panel to render a recommendation, or that the topic was controversial and insufficient agreement existed either in the literature or among experts for the panel to reach a consensus with respect to its inclusion in the algorithm. This approach is consistent with other evidence-based, consensus processes. 16 The panel recognized the difference between drug efficacy and effectiveness 16 : Efficacy is the ability of a drug to be proved effective in a population of patients during the course of a clinical study (preferably randomized, controlled trials). Efficacy, therefore, is determined by finding a statistically significant treatment effect in a group of patients being treated under optimal conditions. Effectiveness refers to the response to a medication in clinical practice, particularly with reference to an individual patient. Effectiveness may be influenced by many factors, such as adequate dosage, associated side effects, concomitant general medical problems or medications, and patient compliance. Thus, for a multitude of reasons, a drug with proven efficacy may or may not be effective in an individual patient. The concept of a restrictive formulary was rejected, and instead, all commercially available antidepressant medications were considered for possible inclusion in the algorithm. Although comparative efficacy and safety are paramount in traditional formulary decisions, many restrictive formulary recommendations appear to be largely influenced by drug acquisition costs and inventory control considerations. In other words, restrictive formularies are based on the perspective that within a therapeutic category, if medication choices with similar efficacy are limited, then acquisition costs can be decreased through large volume purchasing and by avoiding product duplication in the pharmacy s inventory. Considering that medications account for only 2% to 4% of mental health care expenditures, they represent a paltry expenditure in the total cost for mental heath care services. 10 With this in mind, the consensus panel decided that, when possible, drug use decisions should be based on pharmacoeconomic data. That is, a truly cost-efficient antidepressant is one that yields acceptable clinical outcomes for a patient and has the lowest overall health care costs. 10 Such analyses (cost-effectiveness, cost-benefit, or cost-utility) provide information about the relative value of care delivered per health care dollar expended. 8 Although opponents of such analyses argue that it is difficult in general terms to define what is acceptable or of value for an individual patient, these data are useful in developing health care policies that guide the expenditure of dollars toward interventions and practices that maximize clinical outcomes. Therefore, in many respects, the critical question is not whether an antidepressant is on the formulary, but where its use is most appropriately positioned in the treatment algorithm. Unfortunately, since there is a dearth of information regarding the pharmacoeconomics of treatment strategies and tactics in major depressive disorder, few data are available to facilitate this decisionmaking process. This is particularly true for the pharmacotherapy of patients with major depressive disorder treated in the public mental health sector, where there are no published cost-effectiveness analyses. One of the few reported prospective studies evaluating treatment guidelines in the management of depression compared a policy to use fluoxetine, a selective serotonin reuptake inhibitor (SSRI), or tricyclic antidepressants (TCAs), specifically desipramine or imipramine, for initial treatment of clinical depression by primary care physicians in a health maintenance organization. 17 After 6 months of treatment, the initial antidepressant choice showed no difference in patient outcomes. Both groups J Clin COPYRIGHT Psychiatry :3, PHYSICIANS March 1999 POSTGRADUATE PRESS, INC. COPYRIGHT 1999 PHYSICIANS POSTGRADUATE PRESS, INC. 143
3 were equivalent with respect to improvement in depressive symptoms as well as in the patients sense of well being. However, TCA-treated patients reported more side effects, and the TCAs were discontinued more often during the first month of treatment than was fluoxetine. When a TCA was discontinued, fluoxetine was most often the next drug prescribed. Interestingly, overall treatment costs were similar in the two groups, despite the higher acquisition cost for fluoxetine. This study lends support to claims that medications associated with reduced side effects promote patient adherence and that higher medication acquisition costs do not necessarily increase total health care costs. Given the paucity of information regarding the cost-effectiveness of antidepressants, the panel agreed that when equally efficacious medications are available, flexibility regarding specific drug choices at a given algorithm stage should be allowed. 18 After development and review, algorithms are usually disseminated for implementation, and in some cases they are published in the professional literature. Few attempts (none for the treatment of the seriously or persistently mentally ill) have been made to systematically evaluate the use and potential impact of algorithms in patient care. Since inadequate data currently exist to support the hypothesis that pharmacotherapeutic algorithms improve the outcomes of patients with severe mental disorders, TDMHMR, in collaboration with academic researchers and clinicians in the state of Texas, is currently evaluating the use of modifications (to be described in a subsequent article) of these algorithms in treating patients with major depressive disorder in the Texas public mental health system. Testing this hypothesis in a prospective empirical evaluation permits assessment of whether the implementation of algorithms, at least the one derived, improves outcomes for severe and persistently ill patients with major depressive disorder. 16 This type of evaluation is crucial to prevent a codification of ignorance and mandatory implementation of algorithms in patient care regardless of a lack of information concerning their potential effects on outcomes or costs. 3 Algorithm development does not ensure that physicians can or will use them. In fact, some studies have shown that merely providing or disseminating guidelines to physicians can result in minimal effect on practice behavior. 19 Instead, support systems must be enacted to facilitate the use of algorithms in patient care. Such support systems should ideally include the development of uniform outcome measures (e.g., rating scales) to evaluate patient response, physician education regarding the algorithm strategies and tactics, physician prompting regarding algorithm use (e.g., chart audit, physician feedback), improved patient follow-up systems to enhance patient adherence with treatment, and development of patient education materials and modalities to improve patient and family understanding of the disease state and treatment. Additional staff or a change in the staff mixture may be required to optimize patient care. Additional up-front costs may be necessary to successfully implement algorithms. However, if the algorithms successfully improve outcomes, decreased total costs (direct or indirect) should result over the long term. Algorithm development is a dynamic process; algorithms must be frequently reviewed and updated in a timely fashion. They should not be permanently codified. As studies and clinical experience dictate revisions in strategies and tactics to enhance patient care, and as new pharmacologic agents become available for use, algorithms must be modified. 20,21 At a minimum, practical considerations suggest that algorithms should be reviewed at least annually. For the pharmacotherapy of major depressive disorder, the Texas Consensus Conference Panel developed 2 algorithms: 1 for major depressive disorder without psychotic features and 1 for psychotic depression. General principles for algorithm development and implementation will be presented, and for each type of major depressive disorder, the treatment stages and tactics follow. TREATMENT ALGORITHMS FOR MAJOR DEPRESSIVE DISORDER The purpose of treatment algorithms is to integrate available research information and clinical experience into the development of user-friendly, step-by-step, medication decision trees. The assumption behind the development of treatment algorithms is that decreasing the variance and increasing the appropriateness of treatment approaches by the use of consensus treatment protocols results in enhanced patient outcomes. Algorithms do not decrease the need for clinicians to have adequate education and clinical training, nor are they necessarily intended to restrict treatment options. Rather, they are designed to facilitate a systematic approach to recommended treatment interventions. Algorithms are not one size fits all. Separate and distinct algorithms for management of the same illness or disorder may be necessary for different patient populations and different types of practitioners (e.g., primary care, private psychiatric practice, public mental health). Treatment algorithms do not substitute for clinical judgment. Instead, they represent a problem-solving aid for clinicians in making optimal clinical decisions. Each decision point in the algorithm requires professional decision making. It is assumed that the clinician must be skilled in diagnosis, recognition and interpretation of side effects, therapeutic response/nonresponse, drug interaction, and other important pharmacologic features. Furthermore, the clinician must know when patient-specific factors indicate a need to either deviate from the algorithm or disregard it entirely. In other words, the algo- 144 COPYRIGHT 1999 PHYSICIANS POSTGRADUATE PRESS, INC. COPYRIGHT 1999 PHYSICIANS J Clin POSTGRADUATE Psychiatry 60:3, PRESS, March 1999 INC.
4 Texas Medication Algorithm Project rithm represents only a framework for the clinician s decision making, not a substitute for decision making. While these algorithms are restricted to the use of medications in treating major depressive disorder, they are not intended to negate the role of the various forms of psychotherapy, either alone or in combination with medications, in the treatment of depression. Although multiple modalities are available for the treatment of major depressive disorder, it was decided, as a starting point, that the algorithm would initially be restricted to pharmacotherapy. Subsequent algorithms to include psychotherapeutic and rehabilitative services are planned. However, the consensus panel strongly endorsed the importance of patient and family psychoeducation as an indispensable part of any medication algorithm. This was endorsed as a means to enhance adherence, to reduce attrition, and to facilitate patients and families in learning how to manage their often recurrent or persistent disorder, as well as to enhance collaboration between patient and clinician to ensure optimal implementation of the algorithm. General Principles 1. A comprehensive psychiatric evaluation, general medical history, and relevant physical assessment and diagnostic tests should be completed, and a diagnosis of major depressive disorder made prior to entry of a patient into the treatment algorithm. Consultation should be considered when the diagnosis is in doubt. 2. In the presence of comorbid conditions, the clinician may determine that a patient may not be appropriate for entry into the algorithms. 3. The ultimate goals of treatment are to (a) achieve symptomatic remission and full return of psychosocial functioning, (b) prevent relapse, and (c) prevent recurrence. 4. The treatment algorithms are divided into different stages, which often include several strategic treatment options as well as specified tactics within each stage. The earlier stages in the algorithm involve less complex treatment regimens in terms of dosing regimens, side effect profiles, safety, etc., while the later stages generally involve more complicated regimens. 5. The various treatment options recommended at the various points in the algorithms are based on levels of evidence derived from (a) controlled clinical trials [Level A data], (b) open trials and retrospective data analyses [Level B data], and (c) case reports and clinical consensus [Level C data]. In addition, clinical consensus was reached by the panel in all algorithm treatment recommendations. 6. Patients may enter the algorithms at different stages. Entry is determined by specific clinical features and treatment history. 7. A patient s previous response to antidepressant treatments should always be considered when determining entry into an algorithm. If a patient responded well and tolerated a specific pharmacotherapy or other treatment intervention during a previous episode of depression, that same treatment is recommended again. Similarly, if a patient failed to respond to or tolerate an adequate trial of a specific medication during a previous episode of depression, that medication is not recommended for use during current or future depressive episodes. 8. Eligibility and point of entry into an algorithm for an individual patient should be determined by the clinician based on a review of relevant psychiatric factors (e.g., symptom severity, suicidality, comorbidity), general medical factors (e.g., concomitant medications or illnesses, age), and treatment history. 9. If efficacy is equivalent, medication selection is determined by medical factors such as side effect profile, potential toxicity and safety in overdose, drug interactions, and ease of use. When these considerations suggest that several medications are equivalent, patient preference (e.g., based upon side effect profile, dosing frequency) should dictate the medication choice. 10. An adequate discussion between the clinician and the patient of available treatment options and specific medications (including expected results, comparative side effect profiles, dosing strategies, etc.) must transpire before a specific treatment option is initiated. 11. At the beginning of entry into an algorithm, relatively frequent (e.g., weekly) outpatient follow-up appointments for further evaluation and assessment should be scheduled to facilitate optimal treatment outcomes by (a) monitoring and encouraging patient adherence with treatment and (b) rapidly identifying and correcting potential problems or adverse events associated with treatment. 12. to a medication is enhanced by assuring an adequate treatment trial (at least 4 6 weeks of administration at the recommended dosage range). However, if a patient fails to respond at all to an adequate treatment trial of a specific medication for 3 to 5 weeks or has partial but unsatisfactory response by weeks 6 to 8, an alternative plan should be initiated. 13. All patients with major depressive disorder who achieve a satisfactory clinical response (preferably symptom remission) should receive continuation phase treatment. 14. Maintenance phase medication treatment is indicated for patients with major depressive disorder J Clin COPYRIGHT Psychiatry :3, PHYSICIANS March 1999 POSTGRADUATE PRESS, INC. COPYRIGHT 1999 PHYSICIANS POSTGRADUATE PRESS, INC. 145
5 Figure 1. Strategies for the Treatment of Major Depressive Disorder Without Psychotic Features a Figure 2. Strategies for the Treatment of Major Depressive Disorder With Psychotic Features a Stage 1 Monotherapy SSRI, b bupropion, nefazodone, or venlafaxine Any stage(s) can be skipped depending on the clinical picture Partial response or nonresponse Alternate Monotherapy Stage 2 SSRI, bupropion, nefazodone, TCA, or venlafaxine Stage 1 Stage 2 TCA + antipsychotic SSRI + antipsychotic Amoxapine Venlafaxine + antipsychotic Nonresponse Efficacy Failure 1. If non-tca, go to TCA 2. If TCA, go to Stage 3 Side Effect Failure Different drug Stage 3 Partial response or nonresponse Alternate Monotherapy c SSRI, bupropion, nefazodone, TCA,venlafaxine, MAOI Drug from a class other than used in Stage 1 or 2 or Combination antidepressants: TCA + SSRI Stage 3 ECT Nonresponse Nonresponse Stage 4 Partial response or nonresponse ECT Stage 4 Previously Untried Primary agent + lithium Augmenting agent Stage 5 Partial response or nonresponse Other Maintenance Maintenance a The Texas Medication Algorithm Project (TMAP) algorithms are in the public domain, and these figures may be reproduced without permission, but with appropriate citation. a The Texas Medication Algorithm Project (TMAP) algorithms are in the public domain, and these figures may be reproduced without permission, but with appropriate citation. Abbreviations: ECT = electroconvulsive therapy, MAOI = monoamine oxidase inhibitor, SSRI = selective serotonin reuptake inhibitor, TCA = tricyclic antidepressant. b SSRIs preferred. c Consider TCA or venlafaxine if not tried. if they have had (a) 3 episodes of major depression or (b) 2 episodes of major depression and the presence of other factors suggesting that the risk of recurrence is substantial. 15. A clinical psychopharmacology consultation should be considered in treatment-resistant patients or at any time the clinician wants advice concerning treatment. 16. Adequate but minimal documentation should be completed for each algorithm stage and treatment choice (i.e., critical decision points). If algorithm stages are skipped or if treatment deviates from the algorithm, the rationale should be adequately documented. 17. The algorithms apply to both inpatients and outpatients since treatment setting is usually dictated by factors such as suicidality or psychosis rather than the type of medication to be administered. Therefore, the algorithms are independent of the treatment setting. 18. All patients should receive psychoeducation as a part of treatment. 19. At baseline and throughout treatment, patients should be evaluated for the need for further psychosocial interventions above and beyond psychoeducation. The algorithms are arranged into 3 major phases: acute treatment, continuation treatment, and maintenance treatment. For acute treatment, the strategies are organized into stages of treatment. The earlier stages typically utilize straightforward, easy to implement medications with favorable side effect profiles and limited toxicity. A patient progresses from one treatment stage to another either because of inadequate symptom improvement or intolerance to medication side effects. As one progresses down the algorithm, the strategies become more complicated, may carry a greater risk of side effects, and require closer monitoring and attention by the clinician. Treatment tactics that apply to each of the pharmacotherapeutic stages follow presentation of the strategies. All responding patients progress to the continuation phase, and the continuation and maintenance phase considerations are discussed in their respective sections. Figures 1 and 2 outline the treatment strategies. Recommended tactics are outlined in Table COPYRIGHT 1999 PHYSICIANS POSTGRADUATE PRESS, INC. COPYRIGHT 1999 PHYSICIANS J Clin POSTGRADUATE Psychiatry 60:3, PRESS, March 1999 INC.
6 Texas Medication Algorithm Project Table 1. Tactics for Acute Phase Treatment of Major Depressive Disorder: Within Each Pharmacotherapeutic Strategy Stage, Recommended Approaches to Conducting a Therapeutic Trial With an Antidepressant Assessment Point Clinical Status Plan a Weeks 1 3 Symptomatic Initiate medication: adjust dose to lower (Critical point 1) end of therapeutic dose range or serum level (Table 2) If patient shows rapid remission in first 2 3 weeks, this may be a placebo response; continue to offer treatment and encouragement Week 4 or remission Go to continuation phase (Critical point 2) Partial response b Satisfactory rate of improvement: observe Rate too slow, tolerating well: increase dose Minimal or no response; Discontinue, proceed to next stage patient intolerant of lowest therapeutic dose Minimal or no response; Increase dose c patient tolerating medication Week 6 or remission Go to continuation phase (Critical point 3) Partial response Satisfactory rate of improvement if previously increased dose: observe Rate too slow, tolerating well: increase dose; if dose already increased to maximum, augment with lithium Minimal response; patient Discontinue, proceed to next stage intolerant of higher dose Minimal response; patient Augment with lithium or alternative augmenting agent if tolerant previous nonresponse with lithium augmentation Week 8 or remission Go to continuation phase (Critical point 4) Partial response If tolerating regimen, augment with lithium (or alternative as above) if not previously done If not tolerating regimen, go to next stage Minimal response to lithium Discontinue, proceed to the next stage augmentation for 2 3 weeks Week 10 or remission Go to continuation phase (Critical point 5) Partial response Increase lithium dose if not previously done If on higher lithium dose, go to next stage No or minimal response Go to next stage Week 12 or remission Go to continuation phase (Critical point 6) Remains partial responder Go to next stage a For patients showing minimal or no response, total trial should not exceed 4 8 weeks. For patients with a partial response, the trial may last up to 12 weeks because decisions to increase the dose or augment with lithium may be reasonably postponed at each critical point if the patient appears to be improving. b With partial response, the clinician and patient assess both the absolute degree of improvement and the rate of improvement. No or minimal improvement is < 25% improvement in overall symptoms, partial response is between 25% and 49% improvement in symptoms, and response is 50% improvement. c In patients with psychotic depression, the clinician should assess whether to increase the dose of the antidepressant, the antipsychotic, or both. Definitions of While it is preferable to use systematic rating instruments such as the Hamilton Rating Scale for Depression (HAM-D) or the Inventory of Depressive Symptoms- Clinician Rated (IDS-CR) to evaluate patient response, it was recognized that the current managed care practice environment often does not allow adequate time for these assessments. Therefore, symptom response is based on a clinician s global impression. 9 Based on change from baseline severity, the following definitions are used to designate a patient s global level of symptom response: Remission: = a 75% global improvement in symptoms; : = a 50% 74% global improvement in symptoms; Partial response: = a 25% 49% global improvement in symptoms; Nonresponse: = a < 25% global improvement in symptoms. A thorough understanding of the basis for these descriptions and the evaluation of the goal of treatment for an individual patient requires consideration of the following caveats 4 : 1. Severely ill patients should be seen more often than patients who are less ill. Less ill but still symptomatic patients should be seen more often than patients whose depressive symptoms have remitted. 2. A single week of improvement may not represent a stable state. Since the next strategic step recommended (i.e., if the patient responds, go to continuation phase) assumes a stable response, the patient should be evaluated for several weeks after the first week of the response to ensure stability J Clin COPYRIGHT Psychiatry :3, PHYSICIANS March 1999 POSTGRADUATE PRESS, INC. COPYRIGHT 1999 PHYSICIANS POSTGRADUATE PRESS, INC. 147
7 of improvement before progressing to the continuation treatment phase. 3. The aim of treatment is symptom remission and normalization of function rather than just response or symptom improvement. 4 Although not all patients obtain a remission, every effort must be made to ensure maximal benefit for an individual patient. Even a patient achieving a 75% reduction in symptoms may not be asymptomatic. Therefore, once a response is seen, further tactical (e.g., dosage adjustment or augmentation) or strategic (e.g., addition of psychotherapy or rehabilitative services) options should be considered before accepting a response that is short of remission. ALGORITHM FOR MAJOR DEPRESSIVE DISORDER WITHOUT PSYCHOTIC FEATURES (see Figure 1) Acute Treatment Strategies Stage 1. Patients entered into the algorithm have a major depressive disorder of sufficient severity to indicate the need for medication. The algorithm begins with the assumption that the patient has received an adequate baseline evaluation, is appropriately diagnosed, and is appropriate for the major depressive disorder algorithm. Since all of the available antidepressants are similarly efficacious for major depressive disorder, treatment choice is based on other medication and patient factors. Patients are likely taking either no psychotropic medications currently or an antidepressant that is ineffective or which they poorly tolerate. Recommended antidepressants for Stage 1 include SSRIs (fluoxetine, paroxetine, sertraline) [Level A data], 2,11,14,22,23 or bupropion, nefazodone, or venlafaxine [Level A data]. 11 (Note: At the time of the consensus conference, mirtazapine and citalopram had not been approved by the Food and Drug Administration.) SSRIs are listed first because of data supporting their efficacy in both acute and maintenance phases, minimal need for dosage titration [Level A data], overall favorable side effect profile [Level A data], and length of available clinical experience [Level C data]. 4,23 However, when comparing the available Stage 1 antidepressants, all have relative advantages and disadvantages. Potential advantages for bupropion or nefazodone include a significantly lower incidence of sexual dysfunction as compared with SSRIs, TCAs, and monoamine oxidase inhibitors (MAOIs); a favorable effect on insomnia and anxiety (nefazodone) 24 ; a favorable effect on sleep architecture (nefazodone) 24 ; and minimal drug interactions (bupropion). 25 Potential advantages of venlafaxine include relatively few documented drug interactions and the suggestion of enhanced efficacy for severe and treatmentresistant depression. 25,26 Increased efficacy rates are found with increasing dosages of bupropion, nefazodone, and venlafaxine, whereas SSRIs have a relatively flat doseresponse curve. 26 SSRIs are usually given in once-daily doses, while twice-daily dosing is recommended for bupropion (< 150 mg per individual dose), nefazodone, and venlafaxine (Table 2). Depending on a patient s clinical presentation and personal preferences, a specific antidepressant that best suits the patient should be chosen. TCAs are not recommended for Stage 1 because of their relatively less favorable side effect profile, higher patient attrition rate in the acute phase compared with SSRIs and other newer agents, and high risk of toxicity, including lethality in overdose [Levels A and B data]. 4 Pharmacoeconomic data also indicate that SSRIs, at least in the primary care environment, may be associated with overall treatment costs at least as low as with TCAs [Levels A and B data]. 8,10,17 Augmentation versus alternative antidepressant monotherapy. The panel deliberated at length on 2 key issues: (1) when to add an augmenting agent to an antidepressant as opposed to switching from one antidepressant monotherapy to another and (2) which augmentation strategies to recommend. Arguments in favor of switching monotherapies as opposed to augmentation include lower medication-related costs, fewer potential side effects, and increased patient adherence with monotherapy. Additionally, evidence [Level B data] 9 indicates that switching from one monotherapy to another results in symptom response in approximately 50% of patients. Reasons in support of augmentation include avoidance of abandoning partial response with a monotherapy and the patient discouragement that might result over a failed treatment trial, fear of depressive symptoms worsening when a partially effective antidepressant is discontinued, and evidence that some augmentation strategies convert partial responders, and even nonresponders, to full remitters. 2,7,9,11,13,14,23,27 A consensus was reached that augmentation tactics should be available as an option even at Stage 1, because in some patients, especially those with a history of prior treatment failures, augmentation has potential advantages over switching antidepressant monotherapies. However, with other patients (e.g., patients without a history of treatment failures or those who quickly develop intolerance to the initial monotherapy), switching to an alternative monotherapy may be preferable. Additional details regarding augmentation tactics are discussed under the Treatment Tactics, Week 6. Stage 2. Stage 2 includes patients who did not improve clinically during Stage 1 owing to unsatisfactory symptom improvement or inability to tolerate side effects. Stage 2 also includes patients whose previous treatment history or specific medical or psychiatric features suggest that Stage 1 is not appropriate (see Figure 1). 1. If the patient s depressive symptoms did not respond with an SSRI during Stage 1, consider a 148 COPYRIGHT 1999 PHYSICIANS POSTGRADUATE PRESS, INC. COPYRIGHT 1999 PHYSICIANS J Clin POSTGRADUATE Psychiatry 60:3, PRESS, March 1999 INC.
8 Texas Medication Algorithm Project Table 2. Doses of Medications Used for Acute Phase Treatment of Major Depressive Disorder Usual Higher Dose (Serum Level) Usual Target Dose (Serum Level) for Treatment-Resistant Patients Type/Class Medication to Achieve in Wk 1 3 (mg/d) in Wk 4 8 (total mg/d) Usual Dose Schedule Antidepressants SSRIs Fluoxetine qam Paroxetine qam Sertraline qam TCAs Amitriptyline qhs Clomipramine qhs Desipramine 150 (> 125 ng/ml) 300 ( ng/ml) qhs Imipramine 150 (imipramine ( ng/ml) qhs desipramine > 200 ng/ml) Nortriptyline ( ng/ml) 150 ( ng/ml) qhs Others Amoxapine qhs Bupropion bid or tid, 150 mg/dose Nefazodone bid Venlafaxine bid or tid MAOIs Phenelzine qd, bid, or tid Tranylcypromine qd, bid, or tid Antipsychotics for psychotic depression a High potency Haloperidol qhs Medium potency Perphenazine qhs Augmentation for Lithium ( meq/l) ( meq/l) qhs inadequate response Dose range, Options Medication mg/d Schedule Adjuncts for associated symptoms of depression Insomnia Medium-acting benzodiazepine Lorazepam b hs; taper after several weeks or as soon as possible Anxiety or panic attacks Medium-acting or long-acting Lorazepam b q 4 6 h as needed throughout day benzodiazepine Anxiety, if benzodiazepine Serotonin 1A partial agonist Buspirone bid or tid contraindicated Severe agitation Benzodiazepines or very low Lorazepam b q 4 6 h as needed throughout the day doses of antipsychotics Haloperidol b 1 2 q 4 6 h as needed throughout the day Treatment-emergent side effects Insomnia due to antidepressant Medium-acting benzodiazepine Lorazepam b qhs (especially SSRI, bupropion, Small dose of sedating antide- Trazodone qhs venlafaxine) pressant added to primary treatment Extrapyramidal symptoms from Anticholinergic Benztropine 2 4 qhs or bid antipsychotics Representative examples only. Example only. trial with a different SSRI, bupropion, nefazodone, or venlafaxine. 2. If the patient has prominent symptoms of atypical depression a. Consider an SSRI if not used in Stage 1 [Level B data]. 4,14,23 b. If an SSRI was used in Stage 1, consider an MAOI [Level A data]. 4,9,11,14,23 If this is not feasible because of the patient s inability or unwillingness to follow dietary restrictions, consider a trial with an alternate SSRI. 3. If the patient had worsening of depressive symptoms during Stage 1, consider either a TCA or venlafaxine. 4,9,23 Some studies suggest that TCAs [Level B data] and perhaps venlafaxine [Levels B and C data] may be more effective than other antidepressants in severely depressed patients. If a TCA is chosen, the secondary amine TCAs (desipramine and nortriptyline) are preferred over the tertiary amine TCAs because of their advantageous side effect profile [Level A data]. 4,23 The TCA should be initially titrated as tolerated to a dose that attains a steady-state serum concentration within the ranges specified in Table If the patient s response during Stage 1 was unsatisfactory due to an inability to tolerate side effects, consider choosing an antidepressant from a different class or with a substantially different side effect profile. 4,23 For example, if the patient experienced sexual dysfunction on an SSRI, consider changing to bupropion or nefazodone. Many SSRI and venlafaxine side effects are similar, J Clin COPYRIGHT Psychiatry :3, PHYSICIANS March 1999 POSTGRADUATE PRESS, INC. COPYRIGHT 1999 PHYSICIANS POSTGRADUATE PRESS, INC. 149
9 except that venlafaxine has also been reported to elevate blood pressure in some patients (especially with doses > 225 mg/day). 28 Nefazodone may be a preferable choice as an alternate antidepressant for a patient who does not tolerate the side effects (e.g., nausea, sexual dysfunction, insomnia) commonly associated with the SSRIs or venlafaxine. 24 Because of the potential for drug interactions, changing from an SSRI to nefazodone should be performed cautiously. However, if the SSRI-related side effects during Stage 1 were primarily nausea, excessive restlessness, or agitation, an alternate SSRI initiated at a lower dose might be considered. Stage 3. Stage 3 includes patients who did not improve clinically during Stage 2 owing to unsatisfactory symptom improvement or inability to tolerate side effects. Stage 3 also includes patients whose previous treatment history or current clinical features suggest that Stages 1 or 2 are not appropriate (see Figure 1). 1. If a TCA or venlafaxine has not already been used, consider monotherapy with one of these agents If a TCA or venlafaxine has been previously used, consider a TCA/SSRI combination [Level B data]. 9,11,13,14,23 Since the SSRIs may inhibit the metabolism of TCAs, the combination should be used cautiously and with monitoring of serum TCA concentrations [Level A data]. Because of norfluoxetine s long elimination half-life, the maximum effects of fluoxetine on serum TCA concentrations may not be observed for 4 to 6 weeks after addition of fluoxetine to a TCA. Stage 4. Stage 4 includes patients who did not improve clinically during Stage 3 owing to unsatisfactory symptom improvement or inability to tolerate side effects. Stage 4 also includes patients whose previous treatment history or current clinical features suggest that Stages 1, 2, or 3 are not appropriate. A severely depressed patient with significant suicidal features should be considered for initial treatment with electroconvulsive therapy (ECT) after discussion with the patient and, when possible, the family (Table 3). Stage 4 treatment is ECT. 6,9,14,23 Since cognitive side effects are generally lessened when right unilateral ECT is used compared with bilateral ECT, treatment may begin with right unilateral ECT. However, before declaring a patient resistant to ECT, a course of bilateral treatment should be attempted. The electrical dose with right unilateral ECT should be at least 2.5 times the threshold dose, while bilateral ECT should be no more than 2.5 times the initial threshold. A patient whose symptoms improve with ECT should generally receive 1 to 2 treatments after the symptoms have gone into remission or have not shown Table 3. Tactics for Electroconvulsive Therapy (ECT) Modality and Dosage: May start with either: right unilateral ECT (dose 2.5 seizure threshold) bilateral (dose 2.5 seizure threshold). Duration: Continue until the patient is asymptomatic or shows no further improvement over 2 treatments. Treatment Resistance: Do not declare that a patient is resistant to ECT without administering a course of bilateral ECT. If the patient receiving right unilateral ECT is without benefit after 6 to 8 treatments, it is recommended to switch to bilateral ECT. Attempt at least 10 treatments with bilateral ECT before concluding that ECT is unsuccessful. further improvement. With either modality, the patient should receive 6 to 10 treatments before being declared resistant to treatment. Stage 5. Stage 5 includes patients who either did not improve clinically during Stage 4 or were not administered ECT because of either nonavailability or patient/ family preference. Treatment options at Stage 5 include other augmentation strategies (e.g., dextroamphetamine, methylphenidate) or other combinations of antidepressants (e.g., SSRI + bupropion, TCA + MAOI). 9,14,27 As stated in Stage 3, because of the possibility of drug interactions and relatively increased risk of adverse effects, antidepressant combinations should be used cautiously and with close monitoring. Treatment Tactics Assuring an appropriate approach to implementing a treatment stage in an individual patient is extremely important. Individual patient factors must be addressed while utilizing an adequate medication dose and duration of treatment to fully evaluate response. The treatment tactics are intended to assist the clinician in assuring that the patient receives an adequate therapeutic trial at the treatment stage. With the exception of ECT, the tactics for each treatment stage are similar (see Table 1). Weeks 1 3 (critical decision point 1). Dosing. The dose response curve for SSRIs is reasonably flat, but side effects increase with increasing dose. Therefore, SSRI treatment should start at the lower end of the dosage range with the dose increased as tolerated in patients whose symptoms do not improve with lower doses 11,23 (see Tables 1 and 2). With venlafaxine, although some patients will respond at 75 mg daily (in divided doses), response rates increase with increasing doses within the therapeutic range. If tolerated, venlafaxine may be initially titrated to 150 mg daily. Doses as high as 375 mg daily have been used in severely depressed patients, but the risk of side effects is substantially greater, especially with doses higher than 225 mg daily. 11,26 Nefazodone is initially started at 50 mg once or twice daily with titration to 300 to 600 mg daily (in divided doses) at a rate appropriate to minimize side 150 COPYRIGHT 1999 PHYSICIANS POSTGRADUATE PRESS, INC. COPYRIGHT 1999 PHYSICIANS J Clin POSTGRADUATE Psychiatry 60:3, PRESS, March 1999 INC.
10 Texas Medication Algorithm Project effects and increase the likelihood of antidepressant response. 11 The initial bupropion dose is usually 150 to 200 mg daily (in divided doses), with a maximum daily dose of 450 mg for patients not responding to lower doses. No single dose should exceed 150 mg 11,14,23 (see Table 2). Monitoring. The panel believes that patients seen more often early in treatment (e.g., every 1 2 weeks) may have more favorable outcomes owing to increased adherence as compared with patients seen less often. 2,4 Antidepressant side effects often occur early in treatment, and patients should be monitored closely for emergence of side effects, especially during the initial treatment period. Since early intervention can increase the likelihood of patient adherence with the treatment plan, both patient and family should be encouraged to contact the clinic if side effects occur. Additionally, more frequent visits during initial treatment allow the clinician to assess for potential worsening of symptoms or the emergence of suicidality and other complicating factors. The increased frequency of visits also provides the opportunity to offer encouragement and education to the depressed patient, and, when appropriate, to family members and significant others. If a patient shows rapid remission of depressive symptoms within the first 3 weeks, appropriate education and positive reinforcement should be given to the patient to encourage continuation treatment. Week 4 (critical decision point 2). 1. If the patient has had a response (> than 50% improvement in symptoms) of depressive symptoms after 4 weeks of treatment, proceed to continuation phase treatment. 2. If the patient has a partial response (e.g., 25% 49% improvement in symptoms) in depressive symptoms within the first 4 weeks, the medication should be continued for an additional 2 to 4 more weeks. 2,4 If the rate of symptom response is deemed too slow and the patient does not have substantial side effects, a dose increase should be considered. 3. If the patient has shown no or minimal antidepressant response (i.e., < 25% improvement in symptoms) after 4 weeks at therapeutic doses, remission or even clinically significant improvement is unlikely if the same dose is continued for an additional 4 weeks [Level B data]. 4,7 Some patients will benefit from an antidepressant dosage increase. 11 In the poorly responding patient, a dose increase at week 4 enhances the possibility of response. 7,23 a. If no or minimal response has occurred after 4 weeks, and the patient is tolerating (i.e., minimal side effects) the medication, the antidepressant dose should be increased and the patient treated for 2 to 4 more weeks. 7 This principle generally applies with all antidepressants, except that the dose-response range is wider with some antidepressants (e.g., nefazodone, TCAs, venlafaxine) than others (e.g., SSRIs). b. If the patient has shown minimal or no response and is not tolerating the antidepressant at week 4, treatment should progress to the next acute treatment stage. Week 6 (critical decision point 3). 1. If the patient has had a response, proceed to the continuation phase of treatment. 2. If the patient is a partial responder a. And has a satisfactory rate of improvement with the antidepressant dose increased at week 4, continue the medication and observe for 2 more weeks. b. And has had a slow rate of symptom improvement but is tolerating the drug, consider a dosage increase and continue for 2 more weeks. c. And has had a slow rate of symptom improvement, even after the antidepressant dose was increased to the usual maximum at week 4 with good tolerance, consider augmentation with lithium [Levels A and B data]. 2,7,9,11,13,14,23 In young to middle-aged adults with normal renal function, the initial lithium augmentation dose is generally 300 mg (8 meq) 2 or 3 times daily. This usually achieves a steady-state serum lithium concentration of 0.4 to 0.8 meq/l. However, it is questionable with lithium augmentation whether there is a relationship between serum lithium concentration and improvement in depressive symptoms. Lithium, if tolerated, should be continued for 2 weeks. If there is no or minimal response after 2 weeks, the lithium dose should be increased as needed to achieve a steady-state serum concentration of 0.8 to 1.0 meq/l (see Table 2). Lithium is recommended as the initial choice for augmentation treatment because of the body of literature supporting its use. 7,9,11,13,23 If a patient s symptoms did not improve with lithium augmentation during previous treatment stages, then a trial of thyroid hormone supplementation [Level B data] or buspirone augmentation may be used instead [Level B data]. 7,9,11,13,23,27 However, depending on the patient s general medical status, ability to tolerate potential lithium side effects, the availability of resources to monitor lithium therapy, and patient preference, thyroid hormone supplementation or buspirone may be chosen as the initial augmenting agent. In general, however, patients should receive a trial of lithium aug- J Clin COPYRIGHT Psychiatry :3, PHYSICIANS March 1999 POSTGRADUATE PRESS, INC. COPYRIGHT 1999 PHYSICIANS POSTGRADUATE PRESS, INC. 151
11 mentation before proceeding to ECT (i.e., Stage 4). 3. If the patient has shown no or minimal antidepressant response a. And the antidepressant dose was increased to the usual maximum at week 4 with good tolerance, consider augmentation as above. b. If the patient is not tolerating the higher antidepressant dose, proceed to the next acute treatment stage. 7,9 Week 8 (critical decision point 4). 1. If the patient has shown response or remission, proceed to the continuation phase of treatment. 2. If the patient is a partial responder a. And is tolerating the antidepressant at a high dose and augmentation was not used at week 6, consider an augmenting medication now. b. And is not tolerating the antidepressant, proceed to the next acute treatment stage. 3. If the patient has shown no or minimal response a. And is tolerating the antidepressant/lithium combination, increase lithium dose as above. b. And is not tolerating the antidepressant/ lithium combination, proceed to the next acute treatment stage. Week 10 (critical decision point 5). All of these patients were either partial or nonresponders at the week 8 visit. At the week 10 visit, all of these patients should be receiving higher doses of the antidepressant and also receiving lithium (or an alternative augmenting agent). 1. If the patient is a partial responder a. And the patient is receiving the lower lithium dose, increase dose to achieve steady-state serum lithium concentration of 0.8 to 1.0 meq/l. b. And is receiving the higher lithium dose, proceed to the next acute treatment stage. 2. If the patient has shown no or minimal response, proceed to the next acute treatment stage. Week 12 (critical decision point 6). If the patient is still a partial responder, proceed to the next acute treatment stage. Phase Treatment Patient received pharmacotherapy during acute phase. During the acute treatment phase, if a patient achieves a clinical response, but not remission, further strategies or tactics should be considered to achieve the best possible response for an individual patient (see definitions of response). Because of a lack of research evidence or clinical consensus regarding methods to fine tune clinical response, the panel did not make specific recommendations. At baseline and throughout treatment, patients psychosocial needs, including the utility of psychotherapy or other rehabilitative interventions, should be assessed. Medication(s) should be continued for 6 to 9 months after symptom remission (i.e., after end of the acute treatment phase) at the acute phase full therapeutic doses. 2,4,11,14 Patients should be seen by the clinician at least once every 3 months (preferably every 1 2 months) during continuation treatment. If this was the first major depressive episode, the patient can be evaluated for tapering and medication discontinuation at the end of the continuation phase treatment. If previous depressive episodes have occurred, the patient should be evaluated for maintenance treatment. When discontinuing the antidepressant, the dosage should be tapered no more rapidly than 25% per week (or as slow as practical with available dosage forms), starting no earlier than 6 to 8 months after the end of acute phase treatment. Tapering and discontinuation usually occur over a 2- to 3-month period. 2,4 Patients should be taught to monitor for recurrence of depressive symptoms. Since a new depressive episode is most likely to occur within the first 8 months of medication discontinuation, patients should be seen every 2 to 4 months during this period. If depression recurs, prompt treatment with the medication previously effective should be reinitiated (i.e., initiate algorithm stage and tactic that previously resulted in remission of depressive symptoms). Patient received ECT during acute phase. treatment with an antidepressant is recommended. 6 It is preferable to select an antidepressant that the patient has not received or one that the patient has responded to during a previous episode of depression. However, if necessary, a previously ineffective antidepressant may be used in combination with lithium. Dosing, duration of treatment, monitoring, and medication tapering are as described above. If a patient relapses during antidepressant continuation treatment after successful ECT during the acute phase, continuation ECT should be considered. Maintenance Phase Treatment Patients experiencing an initial episode of major depressive disorder have at least a 50% chance of having a second episode, and by the third episode of major depression, there is a 90% chance of recurrence. 2,14 Therefore, all patients experiencing their third depressive episode and some patients having a second episode should be considered for maintenance treatment (Table 4). 2,4,11,14 Maintenance medication should continue at full therapeutic doses and, as in the continuation phase, the regimen associated with symptom remission is recommended. The optimal duration of maintenance medication has not been established, but depending on risk factors, is generally between 1 year past continuation phase and lifetime administration. 152 COPYRIGHT 1999 PHYSICIANS POSTGRADUATE PRESS, INC. COPYRIGHT 1999 PHYSICIANS J Clin POSTGRADUATE Psychiatry 60:3, PRESS, March 1999 INC.
12 Texas Medication Algorithm Project Table 4. Considerations for Maintenance Medication a Feature 3 or more episodes of major depressive disorder 2 episodes of major depressive disorder and 1 or more of the following: Family history of bipolar disorder History of recurrence within 1 year after previously effective medication was discontinued A family history of recurrent major depressive disorder Early onset (before age 20) of the first depressive episode Severe, sudden, or life-threatening depressive episodes within the past 3 years a Adapted from reference 2, page 111. Strength of Indication Very strongly recommended Strongly recommended Strongly recommended Strongly recommended Strongly recommended Strongly recommended Active discussions regarding the initiation and duration of maintenance treatment are an important element in the clinician-patient collaboration for this as well as other phases of pharmacologic treatment of major depressive disorder. The patient s personal preference as well as the risk factors for recurrence must be considered in the decision process. ALGORITHM FOR MAJOR DEPRESSIVE DISORDER WITH PSYCHOTIC FEATURES (see Figure 2) Acute Treatment Phase Stage 1. The algorithm begins with the assumption that the patient has received an adequate baseline evaluation and is appropriately diagnosed. The patient entered into the algorithm at Stage 1 is most likely experiencing the first episode of major depression with psychotic features or has adequately responded to a Stage 1 regimen during a previous episode (see Figure 2). The options for Stage 1 are TCA (amitriptyline, clomipramine, desipramine, imipramine, or nortriptyline) plus an antipsychotic [Level A data], 4,11,12,14,23 an SSRI or venlafaxine plus an antipsychotic [Levels B and C data], 4,6,12,23 or amoxapine [Levels A and B data]. 4,12,23 In psychotic depression, antidepressant plus antipsychotic combinations are more efficacious than antidepressants or antipsychotics alone. In patients with concomitant, serious, general medical conditions, one may wish to start an antipsychotic alone and then add an antidepressant in a few days as tolerated. The treatment regimen should be based on tolerability, safety, need for dosage adjustment, patient compliance, potential drug interactions, and patient s age, general medical status, and preference. TCAs are the only antidepressants that have been systematically evaluated in more than one randomized controlled clinical trial in patients with psychotic depression [Level A data]. 12 The tertiary amine TCAs, amitriptyline and imipramine, are included as first-line agents since studies have demonstrated a positive relationship between their serum concentrations and response in psychotic depression (when coadministered with an antipsychotic). Although scant data exist for their efficacy in psychotic depression [Level C data], 12 SSRIs and venlafaxine are included owing to their preferential side effect profiles and the fact that psychotic depression is often complicated by general medical problems and a high risk of suicide. Preliminary data suggest that venlafaxine may be more effective in severely depressed or treatment-resistant patients [Levels B and C data], and it has few documented clinically significant drug interactions. Amoxapine monotherapy has been shown to be effective in one randomized clinical trial of patients with psychotic depression [Level B data]. 11,12 Medium- to high-potency typical antipsychotics (e.g., haloperidol or perphenazine) are suggested because of their lower incidence of orthostatic hypotension, electrocardiographic changes, and anticholinergic effects as compared with low-potency antipsychotics. 12 Such factors may be particularly important in patients taking concomitant medications or with general medical conditions. At the time of the consensus conference, risperidone and clozapine were the only atypical antipsychotics available, and experience with their use in psychotic depression was limited. For this reason, the panel decided to be silent with respect to their use, but acknowledged that risperidone might be useful in patients unable to tolerate traditional antipsychotics because of extrapyramidal side effects. If the patient s clinical presentation dictates a need for more immediate clinical response (e.g., emergent suicidality) or if the patient has a history of previous response to ECT, going directly to Stage 3 should be considered. 5,6,12 Stage 2. Stage 2 includes patients who did not improve clinically during Stage 1 due to (1) lack of depressive symptom improvement or (2) inability to tolerate side effects. Patients may enter the algorithm at Stage 2 if their history of response during previous depressive episodes suggests that Stage 1 is not appropriate. Stage 2 options are 1. If the patient s depressive symptoms did not improve during Stage 1 a. And the patient received a TCA during Stage 1, consider treatment with venlafaxine plus an antipsychotic or proceed to Stage 3. b. And an SSRI was the antidepressant used in Stage 1, consider treatment with a TCA plus an antipsychotic. 12,23 c. And amoxapine was the antidepressant used in Stage 1, consider treatment with a TCA plus an antipsychotic. 12,23 2. If the patient did not improve during Stage 1 owing to intolerable side effects, select an antidepres- J Clin COPYRIGHT Psychiatry :3, PHYSICIANS March 1999 POSTGRADUATE PRESS, INC. COPYRIGHT 1999 PHYSICIANS POSTGRADUATE PRESS, INC. 153
13 sant from a different class with a different side effect profile (e.g., from a TCA to an SSRI). 23 If the patient is unable to tolerate 2 different antidepressants from different chemical classes, consider proceeding to Stage 3. If the patient s clinical presentation dictates a need for more immediate clinical response (e.g., emergent suicidality) or if the patient has a history of previous response to ECT, skipping Stage 2 and going directly to Stage 3 should be considered. 5,6,12 Stage 3. Stage 3 includes patients who did not improve clinically at Stage 2 owing to (1) lack of depressive symptom improvement or (2) inability to tolerate side effects. Patients may enter the algorithm at Stage 3 if their history of response during previous depressive episodes suggests that Stage 1 or 2 is not appropriate. If the patient s clinical presentation dictates a need for more immediate clinical response (e.g., emergent suicidality) or if the patient has a history of previous response to ECT, entry at Stage 3 should be considered (see Figure 2). Stage 3 treatment is ECT. 6,12,14,23 ECT dosing is the same as for nonpsychotic depression. In general, any antidepressant or antipsychotic medication should be discontinued before initiating ECT. If a patient does not give informed consent for ECT or fails to respond to ECT, proceed to Stage 4. Stage 4. If a patient did not demonstrate response during Stage 3 (or did not give consent for ECT), lithium augmentation of an antidepressant plus antipsychotic combination should be considered 12,13 Therapeutic doses of the antidepressant and the antipsychotic should be attained before lithium augmentation is initiated. If the patient was able to tolerate a TCA in a previous stage, a TCA should be considered as the antidepressant. Lithium augmentation dosing is the same as for nonpsychotic depression (see Tables 1 and 2). Treatment Tactics The tactics for treatment of psychotic depression are in many respects similar to those for nonpsychotic depression. To reduce redundancy, the reader is referred to the tactics previously described for a more detailed discussion. With the exception of ECT, the tactics for each treatment stage are similar (see Tables 1 and 3). Weeks 1 3 (critical decision point 1). Dosing. The TCA dosage should be titrated as rapidly as tolerated to provide a serum concentration within the usual therapeutic range. With outpatients, this will usually take a week or more. It takes approximately 5 days after a dose change to achieve a steady-state or plateau serum TCA concentration 12 (see Table 2). Amoxapine has a definite doseresponse curve, and dosage titration is necessary to minimize side effects and increase the likelihood of achieving both antidepressant and antipsychotic effects. The amoxapine dose should be titrated to at least 200 mg daily 11,12 (see Table 2). Suggested initial antipsychotic doses for haloperidol are 5 to 10 mg daily and for perphenazine, 24 to 36 mg daily. 12 Week 4 (critical decision point 2). 1. If the patient has a response ( 50% improvement in symptoms) of depressive symptoms within 4 weeks of treatment, proceed to continuation phase treatment. 2. If the patient has a partial response (e.g., 25% 49% improvement) in depressive symptoms within the first 4 weeks, the medication should be continued for 2 to 4 more weeks. If the rate of symptom response is deemed too slow and the patient does not have substantial side effects, the antidepressant dose should be increased as tolerated. 3. If the patient has had no or minimal antidepressant response (i.e., < 25% improvement in symptoms) after 4 weeks of treatment, remission or even clinically significant improvement (e.g., 50% improvement in depressive symptoms) is unlikely to occur if the same dose is continued. In these patients, a dose increase at week 4 enhances the possibility of response. Serum drug concentrations should be monitored during TCA treatment. Nortriptyline has a therapeutic window, and serum concentrations either below or above the therapeutic range are associated with poorer clinical response. If psychotic symptoms persist, a modest antipsychotic dose increase may be considered at this point as well (see Tables 1 and 2). a. If no or minimal response occurs after 4 weeks, and the patient is tolerating (i.e., minimal side effects) the medication, the antidepressant dose should be increased and the patient treated for 2 to 4 more weeks. b. If the patient has shown minimal or no response and is not tolerating the antidepressant at week 4, proceed to the next acute treatment stage. Week 6 (critical decision point 3). 1. If the patient has responded (i.e., > 50% improvement in symptoms), proceed to the continuation phase of treatment. 2. If the patient is a partial responder (i.e., 25% 49% improvement in symptoms) a. And has a satisfactory rate of improvement with the antidepressant dose increased at week 4, continue medication and observe for 2 more weeks. b. And has a slow rate of symptom improvement, but the patient is tolerating the drug, consider a dosage increase and continue for 2 more weeks. 154 COPYRIGHT 1999 PHYSICIANS POSTGRADUATE PRESS, INC. COPYRIGHT 1999 PHYSICIANS J Clin POSTGRADUATE Psychiatry 60:3, PRESS, March 1999 INC.
14 Texas Medication Algorithm Project 3. If the patient has shown no or minimal antidepressant response (i.e., < 25% improvement in symptoms) a. And the antidepressant dose was increased to the usual maximum at week 4, proceed to the next acute treatment stage. b. And the dose was not increased to the usual maximum at week 4, but the patient is currently tolerating the antidepressant, increase to the usual maximum dose (based on serum concentration if a TCA). c. And the patient is not tolerating the antidepressant, proceed to the next acute treatment stage. Week 8 (critical decision point 4). 1. If the patient has responded (i.e., > 50% improvement in symptoms), proceed to continuation phase treatment. 2. If the patient is a partial responder (i.e., 25% 49% improvement in symptoms) a. And is tolerating the antidepressant at high doses, continue the medication 2 more weeks. b. And is not tolerating the antidepressant, proceed to the next acute treatment phase. 3. If the patient has shown no or minimal response (i.e., < 25% improvement in symptoms), proceed to the next acute treatment phase. Week 10 (critical decision point 5). Only patients who were partial responders at the week 8 visit should remain at this point. At the week 10 visit, patients are receiving an antipsychotic and high doses of an antidepressant. 1. If the patient has responded (i.e., > 50% improvement in symptoms) after 10 weeks of treatment, proceed to the continuation phase of treatment. 2. If the patient continues to demonstrate only a partial response, proceed to the next acute treatment phase. Phase Treatment Patient received pharmacotherapy during acute phase. Antidepressants should be maintained during the continuation phase as was discussed under nonpsychotic depression. No systematic studies have been reported regarding antipsychotic treatment during the continuation phase. However, it is recommended that the acute phase antipsychotic dose be continued for 1 to 2 months and then slowly tapered over the continuation phase. 12 The duration of antipsychotic treatment should be limited as the patient s clinical situation permits in order to reduce the risk of tardive dyskinesia. If a patient is receiving a TCA, the serum concentration should be monitored, and the dose adjusted as necessary, after discontinuing the antipsychotic. Patient received ECT during acute phase. Recommendations for patients having received ECT during the acute phase are the same as discussed under nonpsychotic depression. Maintenance Phase Recommendations for maintenance phase treatment are the same as those suggested for patients with major depressive disorder without psychotic features. 2,4,11,14 CONCLUSION We found it entirely feasible to develop specific recommendations with regard to the type of treatments (strategies) and the preferred methods by which to deliver them (tactics) in both psychotic and nonpsychotic forms of major depressive disorder for patients in the public sector. However, beyond the second stage, the level of evidence for the recommendations is modest at best. Thus, the proposed algorithms represent a tentative foundation for a sequenced medication plan. Whether these algorithms actually yield a more uniform approach to patient care or improve clinical outcomes for major depressive disorder is currently being prospectively evaluated in a systematic manner within the Texas public mental health system. It is anticipated that revisions in these algorithms will evolve as they are piloted in the clinical setting and as new drugs and advances in treatment occur.* Ongoing revisions in the algorithms can be accessed on the TMAP Web site at It is hoped that the consensus panel recommendations will serve as an initial foundation for the development and implementation of pharmacotherapeutic treatment algorithms for patients treated in public mental health environments. Drug names: amitriptyline (Elavil and others), amoxapine (Asendin), benztropine (Cogentin and others), bupropion (Wellbutrin), buspirone (BuSpar), citalopram (Celexa), clomipramine (Anafranil), clozapine (Clozaril), desipramine (Norpramin and others), dextroamphetamine (Dexedrine and others), fluoxetine (Prozac), haloperidol (Haldol and others), imipramine (Tofranil and others), lorazepam (Ativan and others), methylphenidate (Ritalin), mirtazapine (Remeron), nefazodone (Serzone), nortriptyline (Pamelor and others), paroxetine (Paxil), perphenazine (Trilafon), phenelzine (Nardil), risperidone (Risperdal), sertraline (Zoloft), tranylcypromine (Parnate), trazodone (Desyrel and others), venlafaxine (Effexor). REFERENCES 1. Gilbert DA, Altshuler KZ, Rago WV, et al. Texas Medication Algorithm Project: definitions, rationale, and methods to develop medication algorithms. J Clin Psychiatry 1998;59: Clinical Practice Guideline Number 5: Depression in Primary Care, vol 2. Treatment of Major Depression. Rockville, Md: US Dept Health Human Services, Agency for Health Care Policy and Research; April AHCPR publication *The authors welcome feedback from clinicians regarding their experiences with the algorithms, as well as modifications made to improve their utility in particular practice settings. J Clin COPYRIGHT Psychiatry :3, PHYSICIANS March 1999 POSTGRADUATE PRESS, INC. COPYRIGHT 1999 PHYSICIANS POSTGRADUATE PRESS, INC. 155
15 3. Rush AJ, Prien RF. From scientific knowledge to the clinical practice of psychopharmacology: can the gap be bridged? Psychopharmacol Bull 1995;31: Rush AJ, Kupfer DJ. Strategies and tactics in the treatment of depression. In: Gabbard GO, ed. Treatments of Psychiatric Disorders, vol 1. 2nd ed. Washington, DC: American Psychiatric Press; 1995: Sobin C, Prudic J, Devanand DP, et al. Who responds to electroconvulsive therapy? a comparison of effective and ineffective forms of treatment. Br J Psychiatry 1996;169: Prudic J, Sackeim HA, Rifas S. Medication resistance, response to ECT, and prevention of relapse. Psychiatr Ann 1994;24: Nierenberg AA, McLean NE, Alpert JA, et al. Early nonresponse to fluoxetine as a predictor of poor 8-week outcome. Am J Psychiatry 1995;152: Conner TM, Crismon ML, Still DJ. A critical review of selected pharmacoeconomic analyses of antidepressant therapy. Ann Pharmacother. In press 9. Thase ME, Rush AJ. Treatment-resistant depression. In: Bloom FE, Kupfer DJ, eds. Psychopharmacology: The Fourth Generation of Progress. New York, NY: Raven Press; 1995: Still DJ, Crismon ML. Pharmacoeconomics of depression. Clin Pharm Newswatch 1995;2(11): Fava M, Rosenbaum JF. Pharmacotherapy and somatic therapies. In: Beckham EE, Leber WR, eds. Handbook of Depression. New York, NY: Guilford Publications; 1995: DeBattista C, Belanoff J, Schatzberg AF. Treatment of psychotic depression. In: Montgomery S, Halbreich U, eds. Hormones, Brain, and Neuropsychopharmacology, Book 4: Pharmacotherapy of Mood and Cognition. Washington, DC: American Psychiatric Press. In press 13. Nelson JC. Augmentation strategies for treatment of unipolar major depression. Mod Probl Pharmacopsychiatry 1997;25: American Psychiatric Association. Practice Guideline for Major Depressive Disorder in Adults. Am J Psychiatry 1993;150(4, suppl):s1 S Jobson KO, Potter WZ. International psychopharmacology algorithm project report. Psychopharmacol Bull 1995;31: Rush AJ. Clinical practice guidelines: good news, bad news, or no news? Arch Gen Psychiatry 1993;50: Simon GE, VonKorff M, Heiligenstein JH, et al. Initial antidepressant choice in primary care: effectiveness and cost of fluoxetine vs tricyclic antidepressants. JAMA 1996;275: Horn SD, Sharkey PD, Tracy DM, et al. Intended and unintended consequences of HMO cost-containment strategies: results from the managed care outcomes project. Am J Managed Care 1996;2: Lomas J. Words without action? the production, dissemination, and impact of consensus recommendations. Annu Rev Public Health 1991;12: Dotson LR, Witmer DR. Development of ASHP therapeutic guidelines. Am J Health Syst Pharm 1995;52: Field JF, Lohr KN, eds. Clinical Practice Guidelines: Directions for a New Program. Washington, DC: National Academic Press; Rey JA, Crismon ML, Dopheide JA, et al. Management of major depression in adults. In: Manolakis P, ed. APhA Guide to Drug Treatment Protocols: A Resource for Creating and Using Disease Specific Pathways. Washington, DC: American Pharmaceutical Association; 1997: MD-i MD Nelson JC, Docherty JP, Henschen GM, et al. C. Algorithms for the treatment of subtypes of unipolar major depression. Psychopharmacol Bull 1995;31: Ellingrod VL, Perry PJ. Nefazodone: a new antidepressant. Am J Health Syst Pharm 1995;52: Ereshefsky L, Riesenman C, Lam YW. Antidepressant drug interactions and the cytochrome P450 system: the role of cytochrome P450 2D6. Clin Pharmacokinet 1995;29(suppl 1): Nemeroff CB. Evolutionary trends in the pharmacotherapeutic management of depression. J Clin Psychiatry 1994;55(12, suppl): Harvey KV, Balon R. Augmentation with buspirone: a review. Ann Clin Psychiatry 1995;7: Cohen LJ. Rational drug use in the treatment of depression. Pharmacotherapy 1997;17:45 61 Acknowledgment: The Texas Consensus Conference Panel on Medication Treatment of Major Depressive Disorder A. John Rush, M.D. (chair), Department of Psychiatry, University of Texas Southwestern Medical Center, Dallas; Robert M. A. Hirschfeld, M.D. (co-chair), Department of Psychiatry, University of Texas Medical Branch, Galveston; Diane Ammons, Houston, Tex.; Salvador A. Contreras, M.D., Lubbock Regional MHMR Center and Department of Psychiatry, Texas Tech Health Science Center, Lubbock; M. Lynn Crismon, Pharm.D., College of Pharmacy, The University of Texas at Austin, Austin; Charles DeBattista, D.M.H., M.D., Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Palo Alto, Calif.; Clifford Gay, Depressive Manic-Depressive Association of Texas, Dallas; Margaret Meyer, M.D., psychiatrist, Austin, Tex.; J. Craig Nelson, M.D., Department of Psychiatry, Yale University School of Medicine, New Haven, Conn.; Andrew A. Nierenberg, M.D., Massachusetts General Hospital, Boston; Teresa A. Pigott, M.D., Department of Psychiatry, University of Texas Medical Branch, Galveston; Harold A. Sackeim, Ph.D., New York State Psychiatric Institute, Columbia University, New York; Steven P. Shon, M.D., Texas Department of Mental Health and Mental Retardation (MHMR), Austin; Kathleen Stanley, M.D., Tarrant County MHMR Services, Ft. Worth, Tex.; Michael E. Thase, M.D., Western Psychiatric Institute and Clinic, Pittsburgh, Pa.; Madhukar Trivedi, M.D., Department of Psychiatry, University of Texas Southwestern Medical Center, Dallas; and James Van Norman, M.D., Austin-Travis County MHMR Center, Austin, Tex. Members ex officio include David A. Kahn, M.D., Department of Psychiatry, Columbia University, New York, N.Y.; William V. Rago, Jr., Ph.D., M.B.A., Susan Stone, J.D., M.D., and Marcia G. Toprac, Ph.D., Texas Department of Mental Health and Mental Retardation, Austin. 156 COPYRIGHT 1999 PHYSICIANS POSTGRADUATE PRESS, INC. COPYRIGHT 1999 PHYSICIANS J Clin POSTGRADUATE Psychiatry 60:3, PRESS, March 1999 INC.
TREATING MAJOR DEPRESSIVE DISORDER
 TREATING MAJOR DEPRESSIVE DISORDER A Quick Reference Guide Based on Practice Guideline for the Treatment of Patients With Major Depressive Disorder, Second Edition, originally published in April 2000.
TREATING MAJOR DEPRESSIVE DISORDER A Quick Reference Guide Based on Practice Guideline for the Treatment of Patients With Major Depressive Disorder, Second Edition, originally published in April 2000.
TEXAS IMPLEMENTATION OF MEDICATION ALGORITHMS (TIMA) Guidelines for Treating Major Depressive Disorder
 TEXAS IMPLEMENTATION OF MEDICATION ALGORITHMS (TIMA) Guidelines for Treating Major Depressive Disorder TIMA PHYSICIAN PROCEDURAL MANUAL Madhukar H. Trivedi, M.D. Steven Shon, M.D. M. Lynn Crismon, Pharm.
TEXAS IMPLEMENTATION OF MEDICATION ALGORITHMS (TIMA) Guidelines for Treating Major Depressive Disorder TIMA PHYSICIAN PROCEDURAL MANUAL Madhukar H. Trivedi, M.D. Steven Shon, M.D. M. Lynn Crismon, Pharm.
TREATMENT-RESISTANT DEPRESSION AND ANXIETY
 University of Washington 2012 TREATMENT-RESISTANT DEPRESSION AND ANXIETY Catherine Howe, MD, PhD University of Washington School of Medicine Definition of treatment resistance Failure to remit after 2
University of Washington 2012 TREATMENT-RESISTANT DEPRESSION AND ANXIETY Catherine Howe, MD, PhD University of Washington School of Medicine Definition of treatment resistance Failure to remit after 2
Update on guidelines on biological treatment of depressive disorder. Dr. Henry CHEUNG Psychiatrist in private practice
 Update on guidelines on biological treatment of depressive disorder Dr. Henry CHEUNG Psychiatrist in private practice 2013 update International Task Force of World Federation of Societies of Biological
Update on guidelines on biological treatment of depressive disorder Dr. Henry CHEUNG Psychiatrist in private practice 2013 update International Task Force of World Federation of Societies of Biological
(4) To characterize the course of illness after adequate response to and continuation on the treatments found effective for individual participants.
 I. Specific Aims/Objectives STAR*D has several main objectives and is powered to assess the effectiveness of a sequence of treatments at various levels of treatment. Most of these objectives entail evaluating
I. Specific Aims/Objectives STAR*D has several main objectives and is powered to assess the effectiveness of a sequence of treatments at various levels of treatment. Most of these objectives entail evaluating
MOH CLINICAL PRACTICE GUIDELINES 6/2011 DEPRESSION
 MOH CLINICAL PRACTICE GUIDELINES 6/2011 DEPRESSION Executive summary of recommendations Details of recommendations can be found in the main text at the pages indicated. Clinical evaluation D The basic
MOH CLINICAL PRACTICE GUIDELINES 6/2011 DEPRESSION Executive summary of recommendations Details of recommendations can be found in the main text at the pages indicated. Clinical evaluation D The basic
NICE Clinical guideline 23
 NICE Clinical guideline 23 Depression Management of depression in primary and secondary care Consultation on amendments to recommendations concerning venlafaxine On 31 May 2006 the MHRA issued revised
NICE Clinical guideline 23 Depression Management of depression in primary and secondary care Consultation on amendments to recommendations concerning venlafaxine On 31 May 2006 the MHRA issued revised
Best Practices Treatment Guideline for Major Depression
 Best Practices Treatment Guideline for Major Depression Special Report on New Depression Treatment Technology Based on 2010 APA Practice Guidelines Best Practices Guideline for the Treatment of Patients
Best Practices Treatment Guideline for Major Depression Special Report on New Depression Treatment Technology Based on 2010 APA Practice Guidelines Best Practices Guideline for the Treatment of Patients
What are the best treatments?
 What are the best treatments? Description of Condition Depression is a common medical condition with a lifetime prevalence in the United States of 15% among adults. Symptoms include feelings of sadness,
What are the best treatments? Description of Condition Depression is a common medical condition with a lifetime prevalence in the United States of 15% among adults. Symptoms include feelings of sadness,
Treatments for Major Depression. Drug Treatments The two (2) classes of drugs that are typical antidepressants are:
 Treatments for Major Depression Drug Treatments The two (2) classes of drugs that are typical antidepressants are: 1. 2. These 2 classes of drugs increase the amount of monoamine neurotransmitters through
Treatments for Major Depression Drug Treatments The two (2) classes of drugs that are typical antidepressants are: 1. 2. These 2 classes of drugs increase the amount of monoamine neurotransmitters through
Algorithm for Initiating Antidepressant Therapy in Depression
 Algorithm for Initiating Antidepressant Therapy in Depression Refer for psychotherapy if patient preference or add cognitive behavioural office skills to antidepressant medication Moderate to Severe depression
Algorithm for Initiating Antidepressant Therapy in Depression Refer for psychotherapy if patient preference or add cognitive behavioural office skills to antidepressant medication Moderate to Severe depression
Clinical Guideline / Formulary Document Pharmacy Department Medicines Management Services
 Clinical Guideline / Formulary Document Pharmacy Department Medicines Management Services DEPRESSION Pharmacological Treatment of Depression NICE guidelines suggest the following stepped care model also
Clinical Guideline / Formulary Document Pharmacy Department Medicines Management Services DEPRESSION Pharmacological Treatment of Depression NICE guidelines suggest the following stepped care model also
TEXAS MEDICATION ALGORITHM PROJECT PROCEDURAL MANUAL
 TEXAS MEDICATION ALGORITHM PROJECT PROCEDURAL MANUAL MAJOR DEPRESSIVE DISORDER ALGORITHMS Brandon Suehs, PharmD Tami R. Argo, PharmD, MS, BCPP Sherrie D. Bendele, BS M. Lynn Crismon, PharmD, BCPP Madhukar
TEXAS MEDICATION ALGORITHM PROJECT PROCEDURAL MANUAL MAJOR DEPRESSIVE DISORDER ALGORITHMS Brandon Suehs, PharmD Tami R. Argo, PharmD, MS, BCPP Sherrie D. Bendele, BS M. Lynn Crismon, PharmD, BCPP Madhukar
Antidepressant treatment in adults
 Antidepressant treatment in adults A NICE pathway brings together all NICE guidance, quality standards and materials to support implementation on a specific topic area. The pathways are interactive and
Antidepressant treatment in adults A NICE pathway brings together all NICE guidance, quality standards and materials to support implementation on a specific topic area. The pathways are interactive and
Depression: management of depression in primary and secondary care
 Issue date: December 2004 Quick reference guide Depression: management of depression in primary and secondary care Clinical Guideline 23 Developed by the National Collaborating Centre for Mental Health
Issue date: December 2004 Quick reference guide Depression: management of depression in primary and secondary care Clinical Guideline 23 Developed by the National Collaborating Centre for Mental Health
Amendments to recommendations concerning venlafaxine
 Amendments to recommendations concerning venlafaxine On 31 May 2006 the MHRA issued revised prescribing advice for venlafaxine*. This amendment brings the guideline into line with the new advice but does
Amendments to recommendations concerning venlafaxine On 31 May 2006 the MHRA issued revised prescribing advice for venlafaxine*. This amendment brings the guideline into line with the new advice but does
Psychopharmacology. Psychopharmacology. Hamish McAllister-Williams Reader in Clinical. Department of Psychiatry, RVI
 Regional Affective Disorders Service Psychopharmacology Northumberland, Tyne and Wear NHS Trust Hamish McAllister-Williams Reader in Clinical Psychopharmacology Department of Psychiatry, RVI Intro NOT
Regional Affective Disorders Service Psychopharmacology Northumberland, Tyne and Wear NHS Trust Hamish McAllister-Williams Reader in Clinical Psychopharmacology Department of Psychiatry, RVI Intro NOT
Step 4: Complex and severe depression in adults
 Step 4: Complex and severe depression in adults A NICE pathway brings together all NICE guidance, quality standards and materials to support implementation on a specific topic area. The pathways are interactive
Step 4: Complex and severe depression in adults A NICE pathway brings together all NICE guidance, quality standards and materials to support implementation on a specific topic area. The pathways are interactive
Depression in adults with a chronic physical health problem
 Depression in adults with a chronic physical health problem Treatment and management Issued: October 2009 NICE clinical guideline 91 guidance.nice.org.uk/cg91 NICE has accredited the process used by the
Depression in adults with a chronic physical health problem Treatment and management Issued: October 2009 NICE clinical guideline 91 guidance.nice.org.uk/cg91 NICE has accredited the process used by the
Depre r s e sio i n o i n i a dults Yousuf Al Farsi
 Depression in adults Yousuf Al Farsi Objectives 1. Aetiology 2. Classification 3. Major depression 4. Screening 5. Differential diagnosis 6. Treatment approach 7. When to refer 8. Complication 9. Prognosis
Depression in adults Yousuf Al Farsi Objectives 1. Aetiology 2. Classification 3. Major depression 4. Screening 5. Differential diagnosis 6. Treatment approach 7. When to refer 8. Complication 9. Prognosis
Medication Management of Depressive Disorders in Children and Adolescents. Satya Tata, M.D. Kansas University Medical Center
 Medication Management of Depressive Disorders in Children and Adolescents Satya Tata, M.D. Kansas University Medical Center First Line Medications SSRIs Prozac (Fluoxetine): 5-605 mg Zoloft (Sertraline):
Medication Management of Depressive Disorders in Children and Adolescents Satya Tata, M.D. Kansas University Medical Center First Line Medications SSRIs Prozac (Fluoxetine): 5-605 mg Zoloft (Sertraline):
Depression is a common biological brain disorder and occurs in 7-12% of all individuals over
 Depression is a common biological brain disorder and occurs in 7-12% of all individuals over the age of 65. Specific groups have a much higher rate of depression including the seriously medically ill (20-40%),
Depression is a common biological brain disorder and occurs in 7-12% of all individuals over the age of 65. Specific groups have a much higher rate of depression including the seriously medically ill (20-40%),
Treatment and management of depression in adults, including adults with a chronic physical health problem
 Issue date: October 2009 Depression Treatment and management of depression in adults, including adults with a chronic physical health problem This is an update of NICE clinical guideline 23 Developed by
Issue date: October 2009 Depression Treatment and management of depression in adults, including adults with a chronic physical health problem This is an update of NICE clinical guideline 23 Developed by
IMR ISSUES, DECISIONS AND RATIONALES The Final Determination was based on decisions for the disputed items/services set forth below:
 Case Number: CM13-0018009 Date Assigned: 10/11/2013 Date of Injury: 06/11/2004 Decision Date: 01/13/2014 UR Denial Date: 08/16/2013 Priority: Standard Application Received: 08/29/2013 HOW THE IMR FINAL
Case Number: CM13-0018009 Date Assigned: 10/11/2013 Date of Injury: 06/11/2004 Decision Date: 01/13/2014 UR Denial Date: 08/16/2013 Priority: Standard Application Received: 08/29/2013 HOW THE IMR FINAL
DEPRESSION CARE PROCESS STEP EXPECTATIONS RATIONALE
 1 DEPRESSION CARE PROCESS STEP EXPECTATIONS RATIONALE ASSESSMENT/PROBLEM RECOGNITION 1. Did the staff and physician seek and document risk factors for depression and any history of depression? 2. Did staff
1 DEPRESSION CARE PROCESS STEP EXPECTATIONS RATIONALE ASSESSMENT/PROBLEM RECOGNITION 1. Did the staff and physician seek and document risk factors for depression and any history of depression? 2. Did staff
The first treatment regimen for depression is frequently ineffective or
 12 5 Mood Disorders Treatment of Major Depression and Dysthymia: What to Do When the Initial Intervention Fails The first treatment regimen for depression is frequently ineffective or inadequate. In fact,
12 5 Mood Disorders Treatment of Major Depression and Dysthymia: What to Do When the Initial Intervention Fails The first treatment regimen for depression is frequently ineffective or inadequate. In fact,
Preferred Practice Guidelines Bipolar Disorder in Children and Adolescents
 These Guidelines are based in part on the following: American Academy of Child and Adolescent Psychiatry s Practice Parameter for the Assessment and Treatment of Children and Adolescents With Bipolar Disorder,
These Guidelines are based in part on the following: American Academy of Child and Adolescent Psychiatry s Practice Parameter for the Assessment and Treatment of Children and Adolescents With Bipolar Disorder,
NICE clinical guideline 90
 Depression in adults The treatment and management of depression in adults Issued: October 2009 NICE clinical guideline 90 guidance.nice.org.uk/cg90 NHS Evidence has accredited the process used by the Centre
Depression in adults The treatment and management of depression in adults Issued: October 2009 NICE clinical guideline 90 guidance.nice.org.uk/cg90 NHS Evidence has accredited the process used by the Centre
Psychopharmacotherapy for Children and Adolescents
 TREATMENT GUIDELINES Psychopharmacotherapy for Children and Adolescents Guideline 7 Psychopharmacotherapy for Children and Adolescents Description There are few controlled trials to guide practitioners
TREATMENT GUIDELINES Psychopharmacotherapy for Children and Adolescents Guideline 7 Psychopharmacotherapy for Children and Adolescents Description There are few controlled trials to guide practitioners
Major Depression. What is major depression?
 Major Depression What is major depression? Major depression is a serious medical illness affecting 9.9 million American adults, or approximately 5 percent of the adult population in a given year. Unlike
Major Depression What is major depression? Major depression is a serious medical illness affecting 9.9 million American adults, or approximately 5 percent of the adult population in a given year. Unlike
Treatment of Patients With Major Depressive Disorder Second Edition
 PRACTICE GUIDELINE FOR THE Treatment of Patients With Major Depressive Disorder Second Edition WORK GROUP ON MAJOR DEPRESSIVE DISORDER T. Byram Karasu, M.D., Chair Alan Gelenberg, M.D. Arnold Merriam,
PRACTICE GUIDELINE FOR THE Treatment of Patients With Major Depressive Disorder Second Edition WORK GROUP ON MAJOR DEPRESSIVE DISORDER T. Byram Karasu, M.D., Chair Alan Gelenberg, M.D. Arnold Merriam,
CLINICIAN INTERVIEW COMPLEXITIES OF BIPOLAR DISORDER. Interview with Charles B. Nemeroff, MD, PhD
 COMPLEXITIES OF BIPOLAR DISORDER Interview with Charles B. Nemeroff, MD, PhD Dr Nemeroff is the Reunette W. Harris Professor and Chairman of the Department of Psychiatry and Behavioral Sciences at Emory
COMPLEXITIES OF BIPOLAR DISORDER Interview with Charles B. Nemeroff, MD, PhD Dr Nemeroff is the Reunette W. Harris Professor and Chairman of the Department of Psychiatry and Behavioral Sciences at Emory
Elizabeth A. Crocco, MD Assistant Clinical Professor Chief, Division of Geriatric Psychiatry Department of Psychiatry and Behavioral Sciences Miller
 Elizabeth A. Crocco, MD Assistant Clinical Professor Chief, Division of Geriatric Psychiatry Department of Psychiatry and Behavioral Sciences Miller School of Medicine/University of Miami Question 1 You
Elizabeth A. Crocco, MD Assistant Clinical Professor Chief, Division of Geriatric Psychiatry Department of Psychiatry and Behavioral Sciences Miller School of Medicine/University of Miami Question 1 You
Performance Improvement Strategies in Clinical Depression
 This activity was sponsored by Med-IQ and developed in collaboration with the National Committee for Quality Assurance (NCQA). Performance Improvement Strategies in Clinical Depression Community of Practice
This activity was sponsored by Med-IQ and developed in collaboration with the National Committee for Quality Assurance (NCQA). Performance Improvement Strategies in Clinical Depression Community of Practice
Treating Major Depressive Disorder
 Treating Major Depressive Disorder A Quick Reference Guide Based on Practice Guideline for the Treatment of Patients With Major Depressive Disorder, Third Edition, originally published in October 2010.
Treating Major Depressive Disorder A Quick Reference Guide Based on Practice Guideline for the Treatment of Patients With Major Depressive Disorder, Third Edition, originally published in October 2010.
Depression Flow Chart
 Depression Flow Chart SCREEN FOR DEPRESSION ANNUALLY Assess for depression annually with the PHQ-9. Maintain a high index of suspicion in high risk older adults. Consider suicide risk and contributing
Depression Flow Chart SCREEN FOR DEPRESSION ANNUALLY Assess for depression annually with the PHQ-9. Maintain a high index of suspicion in high risk older adults. Consider suicide risk and contributing
Recognition and Treatment of Depression in Parkinson s Disease
 Recognition and Treatment of Depression in Parkinson s Disease Web Ross VA Pacific Islands Health Care System What is depression? Depression is a serious medical condition that affects a person s feelings,
Recognition and Treatment of Depression in Parkinson s Disease Web Ross VA Pacific Islands Health Care System What is depression? Depression is a serious medical condition that affects a person s feelings,
SECTION N: MEDICATIONS. N0300: Injections. Item Rationale Health-related Quality of Life. Planning for Care. Steps for Assessment. Coding Instructions
 SECTION N: MEDICATIONS Intent: The intent of the items in this section is to record the number of days, during the last 7 days (or since admission/reentry if less than 7 days) that any type of injection,
SECTION N: MEDICATIONS Intent: The intent of the items in this section is to record the number of days, during the last 7 days (or since admission/reentry if less than 7 days) that any type of injection,
PSYCHOPHARMACOLOGY AND WORKING WITH PSYCHIATRY PROVIDERS. Juanaelena Garcia, MD Psychiatry Director Institute for Family Health
 PSYCHOPHARMACOLOGY AND WORKING WITH PSYCHIATRY PROVIDERS Juanaelena Garcia, MD Psychiatry Director Institute for Family Health Learning Objectives Learn basics about the various types of medications that
PSYCHOPHARMACOLOGY AND WORKING WITH PSYCHIATRY PROVIDERS Juanaelena Garcia, MD Psychiatry Director Institute for Family Health Learning Objectives Learn basics about the various types of medications that
Depression in Long-Term Care
 Depression in Long-Term Care Annette Carron, DO, CMD, FACOI, FAAHPM Director Geriatrics and Palliative Care Botsford Hospital Slide 1 OBJECTIVES Know and understand: Incidence and morbidity of depressive
Depression in Long-Term Care Annette Carron, DO, CMD, FACOI, FAAHPM Director Geriatrics and Palliative Care Botsford Hospital Slide 1 OBJECTIVES Know and understand: Incidence and morbidity of depressive
TITLE: Cannabinoids for the Treatment of Post-Traumatic Stress Disorder: A Review of the Clinical Effectiveness and Guidelines
 TITLE: Cannabinoids for the Treatment of Post-Traumatic Stress Disorder: A Review of the Clinical Effectiveness and Guidelines DATE: 01 December 2009 CONTEXT AND POLICY ISSUES: Post-traumatic stress disorder
TITLE: Cannabinoids for the Treatment of Post-Traumatic Stress Disorder: A Review of the Clinical Effectiveness and Guidelines DATE: 01 December 2009 CONTEXT AND POLICY ISSUES: Post-traumatic stress disorder
Clinical Practice Guideline: Depression in Primary Care, Adult 4 Taft Court Rockville, MD 20850 www.mamsi.com
 Clinical Practice Guideline: Depression in 4 Taft Court Rockville, MD 20850 www.mamsi.com 40 05 17 035 3/03 Once a primary care patient presents with depressive symptoms, the primary care physician makes
Clinical Practice Guideline: Depression in 4 Taft Court Rockville, MD 20850 www.mamsi.com 40 05 17 035 3/03 Once a primary care patient presents with depressive symptoms, the primary care physician makes
CLINICAL PRACTICE GUIDELINES. Depression
 CLINICAL PRACTICE GUIDELINES Depression MOH Clinical Practice Guidelines 6/2011 3 1 Published by Ministry of Health, Singapore 16 College Road, College of Medicine Building Singapore 169854 Printed by
CLINICAL PRACTICE GUIDELINES Depression MOH Clinical Practice Guidelines 6/2011 3 1 Published by Ministry of Health, Singapore 16 College Road, College of Medicine Building Singapore 169854 Printed by
PTSD Evidence Based Practice Recommendations
 PTSD Evidence ased Practice Recommendations Jason's ox est Practice Group Dr. Sam Moreno, Michelle Schnack Compilation & consolidation of the research and recommendations located in: The VA/DoD Clinical
PTSD Evidence ased Practice Recommendations Jason's ox est Practice Group Dr. Sam Moreno, Michelle Schnack Compilation & consolidation of the research and recommendations located in: The VA/DoD Clinical
MAJOR DEPRESSION DURING CONCEPTION AND PREGNANCY: A Guide for Patients and Families
 MAJOR DEPRESSION DURING CONCEPTION AND PREGNANCY: A Guide for Patients and Families David A. Kahn, MD, Margaret L. Moline, PhD, Ruth W. Ross, MA, Lee S. Cohen, MD, and Lori L. Altshuler, MD www.womensmentalhealth.org
MAJOR DEPRESSION DURING CONCEPTION AND PREGNANCY: A Guide for Patients and Families David A. Kahn, MD, Margaret L. Moline, PhD, Ruth W. Ross, MA, Lee S. Cohen, MD, and Lori L. Altshuler, MD www.womensmentalhealth.org
Community Center Readiness Guide Additional Resource #17 Protocol for Physician Assistants and Advanced Practice Nurses
 Community Center Readiness Guide Additional Resource #17 Protocol for Physician Assistants and Advanced Practice Nurses PROTOCOL FOR PHYSICIAN ASSISTANTS AND ADVANCED PRACTICE NURSES 1. POLICY Advanced
Community Center Readiness Guide Additional Resource #17 Protocol for Physician Assistants and Advanced Practice Nurses PROTOCOL FOR PHYSICIAN ASSISTANTS AND ADVANCED PRACTICE NURSES 1. POLICY Advanced
DEMENTIA EDUCATION & TRAINING PROGRAM
 The pharmacological management of aggression in the nursing home requires careful assessment and methodical treatment to assure maximum safety for patients, nursing home residents and staff. Aggressive
The pharmacological management of aggression in the nursing home requires careful assessment and methodical treatment to assure maximum safety for patients, nursing home residents and staff. Aggressive
MOH CLINICAL PRACTICE GUIDELINES 2/2008 Prescribing of Benzodiazepines
 MOH CLINICL PRCTICE GUIELINES 2/2008 Prescribing of Benzodiazepines College of Family Physicians, Singapore cademy of Medicine, Singapore Executive summary of recommendations etails of recommendations
MOH CLINICL PRCTICE GUIELINES 2/2008 Prescribing of Benzodiazepines College of Family Physicians, Singapore cademy of Medicine, Singapore Executive summary of recommendations etails of recommendations
Member Rights & Responsibilities
 Member Rights & Responsibilities Member Rights and Responsibilities Blue KC has updated our Member Rights & Responsibilities to be consistent across all of our HMO and PPO products. Blue KC members have:
Member Rights & Responsibilities Member Rights and Responsibilities Blue KC has updated our Member Rights & Responsibilities to be consistent across all of our HMO and PPO products. Blue KC members have:
TREATING PANIC DISORDER
 TREATING PANIC DISORDER A Quick Reference Guide Based on Practice Guideline for the Treatment of Patients With Panic Disorder, originally published in May 1998. A guideline watch, summarizing significant
TREATING PANIC DISORDER A Quick Reference Guide Based on Practice Guideline for the Treatment of Patients With Panic Disorder, originally published in May 1998. A guideline watch, summarizing significant
1. Which of the following SSRIs requires up to a 5-week washout period because of the
 1 Chapter 38. Major Depressive Disorders, Self-Assessment Questions 1. Which of the following SSRIs requires up to a 5-week washout period because of the long half-life of its potent active metabolite?
1 Chapter 38. Major Depressive Disorders, Self-Assessment Questions 1. Which of the following SSRIs requires up to a 5-week washout period because of the long half-life of its potent active metabolite?
Major Depressive Disorders Questions submitted for consideration by workshop participants
 Major Depressive Disorders Questions submitted for consideration by workshop participants Prioritizing Comparative Effectiveness Research Questions: PCORI Stakeholder Workshops June 9, 2015 Patient-Centered
Major Depressive Disorders Questions submitted for consideration by workshop participants Prioritizing Comparative Effectiveness Research Questions: PCORI Stakeholder Workshops June 9, 2015 Patient-Centered
in young people Management of depression in primary care Key recommendations: 1 Management
 Management of depression in young people in primary care Key recommendations: 1 Management A young person with mild or moderate depression should typically be managed within primary care services A strength-based
Management of depression in young people in primary care Key recommendations: 1 Management A young person with mild or moderate depression should typically be managed within primary care services A strength-based
TREATING BIPOLAR DISORDER
 TREATING BIPOLAR DISORDER A Quick Reference Guide Based on Practice Guideline for the Treatment of Patients With Bipolar Disorder, Second Edition, originally published in April 2002. 1 For Continuing Medical
TREATING BIPOLAR DISORDER A Quick Reference Guide Based on Practice Guideline for the Treatment of Patients With Bipolar Disorder, Second Edition, originally published in April 2002. 1 For Continuing Medical
TEXAS MEDICATION ALGORITHM PROJECT PROCEDURAL MANUAL
 TEXAS MEDICATION ALGORITHM PROJECT PROCEDURAL MANUAL BIPOLAR DISORDER ALGORITHMS M. Lynn Crismon, PharmD, BCPP Tami R. Argo, PharmD, MS, BCPP Sherrie D. Bendele, BS Trisha Suppes, MD, PhD 2007, Texas Department
TEXAS MEDICATION ALGORITHM PROJECT PROCEDURAL MANUAL BIPOLAR DISORDER ALGORITHMS M. Lynn Crismon, PharmD, BCPP Tami R. Argo, PharmD, MS, BCPP Sherrie D. Bendele, BS Trisha Suppes, MD, PhD 2007, Texas Department
GUIDELINES FOR USE OF PSYCHOTHERAPEUTIC MEDICATIONS IN OLDER ADULTS
 GUIDELINES GUIDELINES FOR USE OF PSYCHOTHERAPEUTIC MEDICATIONS IN OLDER ADULTS Preamble The American Society of Consultant Pharmacists has developed these guidelines for use of psychotherapeutic medications
GUIDELINES GUIDELINES FOR USE OF PSYCHOTHERAPEUTIC MEDICATIONS IN OLDER ADULTS Preamble The American Society of Consultant Pharmacists has developed these guidelines for use of psychotherapeutic medications
POPULAR DEPRESSION MEDICATIONS
 Popular Depression Medications A Helpful Guide to Antidepressant Drugs POPULAR DEPRESSION MEDICATIONS A Helpful Guide to Antidepressant Drugs Popular Depression Medications A Helpful Guide to Antidepressant
Popular Depression Medications A Helpful Guide to Antidepressant Drugs POPULAR DEPRESSION MEDICATIONS A Helpful Guide to Antidepressant Drugs Popular Depression Medications A Helpful Guide to Antidepressant
THE DEPRESSION RESEARCH CLINIC Department of Psychiatry and Behavioral Sciences Stanford University, School of Medicine
 THE DEPRESSION RESEARCH CLINIC Department of Psychiatry and Behavioral Sciences Stanford University, School of Medicine Volume 1, Issue 1 August 2007 The Depression Research Clinic at Stanford University
THE DEPRESSION RESEARCH CLINIC Department of Psychiatry and Behavioral Sciences Stanford University, School of Medicine Volume 1, Issue 1 August 2007 The Depression Research Clinic at Stanford University
Recognizing and Treating Depression in Children and Adolescents.
 Recognizing and Treating Depression in Children and Adolescents. KAREN KANDO, MD Division of Child and Adolescent Psychiatry Center for Neuroscience and Behavioral Medicine Phoenix Children s Hospital
Recognizing and Treating Depression in Children and Adolescents. KAREN KANDO, MD Division of Child and Adolescent Psychiatry Center for Neuroscience and Behavioral Medicine Phoenix Children s Hospital
These guidelines are intended to support General Practitioners in the care of their patients with dementia both in the community and in care homes.
 This is a new guideline. These guidelines are intended to support General Practitioners in the care of their patients with dementia both in the community and in care homes. It incorporates NICE clinical
This is a new guideline. These guidelines are intended to support General Practitioners in the care of their patients with dementia both in the community and in care homes. It incorporates NICE clinical
Depression in the Elderly: Recognition, Diagnosis, and Treatment
 Depression in the Elderly: Recognition, Diagnosis, and Treatment LOUIS A. CANCELLARO, PhD, MD, EFAC Psych Professor Emeritus and Interim Chair ETSU Department of Psychiatry & Behavioral Sciences Diagnosis
Depression in the Elderly: Recognition, Diagnosis, and Treatment LOUIS A. CANCELLARO, PhD, MD, EFAC Psych Professor Emeritus and Interim Chair ETSU Department of Psychiatry & Behavioral Sciences Diagnosis
DEPRESSION Depression Assessment PHQ-9 Screening tool Depression treatment Treatment flow chart Medications Patient Resource
 E-Resource March, 2015 DEPRESSION Depression Assessment PHQ-9 Screening tool Depression treatment Treatment flow chart Medications Patient Resource Depression affects approximately 20% of the general population
E-Resource March, 2015 DEPRESSION Depression Assessment PHQ-9 Screening tool Depression treatment Treatment flow chart Medications Patient Resource Depression affects approximately 20% of the general population
This continuing education activity is co-sponsored by Indiana University School of Medicine and by CME Outfitters, LLC.
 This continuing education activity is co-sponsored by Indiana University School of Medicine and by CME Outfitters, LLC. Indiana University School of Medicine and CME Outfitters, LLC, gratefully acknowledge
This continuing education activity is co-sponsored by Indiana University School of Medicine and by CME Outfitters, LLC. Indiana University School of Medicine and CME Outfitters, LLC, gratefully acknowledge
Care Manager Resources: Common Questions & Answers about Treatments for Depression
 Care Manager Resources: Common Questions & Answers about Treatments for Depression Questions about Medications 1. How do antidepressants work? Antidepressants help restore the correct balance of certain
Care Manager Resources: Common Questions & Answers about Treatments for Depression Questions about Medications 1. How do antidepressants work? Antidepressants help restore the correct balance of certain
Clinical guideline Published: 28 October 2009 nice.org.uk/guidance/cg90
 Depression in adults: recognition and management Clinical guideline Published: 28 October 2009 nice.org.uk/guidance/cg90 NICE 2009. All rights reserved. Last updated April 2016 Your responsibility The
Depression in adults: recognition and management Clinical guideline Published: 28 October 2009 nice.org.uk/guidance/cg90 NICE 2009. All rights reserved. Last updated April 2016 Your responsibility The
CURRENT AND EMERGING THERAPEUTICS FOR DEPRESSION
 75 CURRENT AND EMERGING THERAPEUTICS FOR DEPRESSION A. JOHN RUSH NEAL D. RYAN This chapter discusses critical conceptual and practical issues confronting clinicians who must distill the massive neuroscientific,
75 CURRENT AND EMERGING THERAPEUTICS FOR DEPRESSION A. JOHN RUSH NEAL D. RYAN This chapter discusses critical conceptual and practical issues confronting clinicians who must distill the massive neuroscientific,
Depression Pathway. Patient Education Box 4. Guided self help Box 18. pg 11
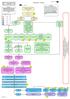 LCFT localised Map pathway June 2009 Depression Pathway Instructions: Throughout this pathway if you click on the Bookmarks tab to the left of the screen and then click on the various documents you will
LCFT localised Map pathway June 2009 Depression Pathway Instructions: Throughout this pathway if you click on the Bookmarks tab to the left of the screen and then click on the various documents you will
Evidence-based best practice interventions for the treatment of mood disorders: Annotated Information Package
 Evidence-based best practice interventions for the treatment of mood disorders: Annotated Information Package September 2007 Evidence-based best practice interventions for the treatment of mood disorders:
Evidence-based best practice interventions for the treatment of mood disorders: Annotated Information Package September 2007 Evidence-based best practice interventions for the treatment of mood disorders:
MOLINA HEALTHCARE OF CALIFORNIA
 MOLINA HEALTHCARE OF CALIFORNIA MAJOR DEPRESSION IN ADULTS IN PRIMARY CARE HEALTH CARE GUIDELINE (ICSI) Health Care Guideline Twelfth Edition May 2009. The guideline was reviewed and adopted by the Molina
MOLINA HEALTHCARE OF CALIFORNIA MAJOR DEPRESSION IN ADULTS IN PRIMARY CARE HEALTH CARE GUIDELINE (ICSI) Health Care Guideline Twelfth Edition May 2009. The guideline was reviewed and adopted by the Molina
Unipolar Depression Management Protocol
 Unipolar Depression Management Protocol All Team Members: Patient Self-Management Education & Support Unipolar depression or Major Depressive Disorder means that the patient feels persistent low mood or
Unipolar Depression Management Protocol All Team Members: Patient Self-Management Education & Support Unipolar depression or Major Depressive Disorder means that the patient feels persistent low mood or
TREATING BORDERLINE PERSONALITY DISORDER
 TREATING BORDERLINE PERSONALITY DISORDER A Quick Reference Guide Based on Practice Guideline for the Treatment of Patients With Borderline Personality Disorder, originally published in October 2001. 1
TREATING BORDERLINE PERSONALITY DISORDER A Quick Reference Guide Based on Practice Guideline for the Treatment of Patients With Borderline Personality Disorder, originally published in October 2001. 1
Fixing Mental Health Care in America
 Fixing Mental Health Care in America A National Call for Measurement Based Care in Behavioral Health and Primary Care An Issue Brief Released by The Kennedy Forum Prepared by: John Fortney PhD, Rebecca
Fixing Mental Health Care in America A National Call for Measurement Based Care in Behavioral Health and Primary Care An Issue Brief Released by The Kennedy Forum Prepared by: John Fortney PhD, Rebecca
Workshop: Management of Depression in the Primary Care Setting, Kaiser Permanente of Ohio s Multidisciplinary Model
 Workshop: Management of Depression in the Primary Care Setting, Kaiser Permanente of Ohio s Multidisciplinary Model Larissa Elgudin, MD, Chief of Behavioral Health Services Colleen O Malley RN, BSN, Regional
Workshop: Management of Depression in the Primary Care Setting, Kaiser Permanente of Ohio s Multidisciplinary Model Larissa Elgudin, MD, Chief of Behavioral Health Services Colleen O Malley RN, BSN, Regional
BEST in MH clinical question-answering service
 Best Evidence Summaries of Topics in Mental Healthcare BEST in MH clinical question-answering service Question In adults with a diagnosis of psychotic / delusional depression how effective is electroconvulsive
Best Evidence Summaries of Topics in Mental Healthcare BEST in MH clinical question-answering service Question In adults with a diagnosis of psychotic / delusional depression how effective is electroconvulsive
Department of Psychiatry, Harvard Medical School,
 This article was downloaded by: [VA Medical Centre] On: 30 November 2009 Access details: Access Details: [subscription number 790388541] Publisher Informa Healthcare Informa Ltd Registered in England and
This article was downloaded by: [VA Medical Centre] On: 30 November 2009 Access details: Access Details: [subscription number 790388541] Publisher Informa Healthcare Informa Ltd Registered in England and
Depression. The treatment and management of depression in adults. This is a partial update of NICE clinical guideline 23
 Issue date: October 2009 Depression The treatment and management of depression in adults This is a partial update of NICE clinical guideline 23 NICE clinical guideline 90 Developed by the National Collaborating
Issue date: October 2009 Depression The treatment and management of depression in adults This is a partial update of NICE clinical guideline 23 NICE clinical guideline 90 Developed by the National Collaborating
Step 2: Recognised depression in adults persistent subthreshold depressive symptoms or mild to moderate depression
 Step 2: Recognised depression in adults persistent subthreshold depressive symptoms or mild to moderate depression A NICE pathway brings together all NICE guidance, quality standards and materials to support
Step 2: Recognised depression in adults persistent subthreshold depressive symptoms or mild to moderate depression A NICE pathway brings together all NICE guidance, quality standards and materials to support
Practice guideline for the treatment of patients with bipolar disorder (revision).
 Brief Summary GUIDELINE TITLE Practice guideline for the treatment of patients with bipolar disorder (revision). BIBLIOGRAPHIC SOURCE(S) Practice guideline for the treatment of patients with bipolar disorder
Brief Summary GUIDELINE TITLE Practice guideline for the treatment of patients with bipolar disorder (revision). BIBLIOGRAPHIC SOURCE(S) Practice guideline for the treatment of patients with bipolar disorder
ADHD PRACTISE PARAMETER. IRSHAAD SHAFFEEULLAH, M.D. A diplomate American Board of CHILD AND ADOLESCENT PSYCHIATRY
 ADHD PRACTISE PARAMETER IRSHAAD SHAFFEEULLAH, M.D. A diplomate American Board of CHILD AND ADOLESCENT PSYCHIATRY Similar type of idea Similar document Similar document AACAP document Neurobiological condition
ADHD PRACTISE PARAMETER IRSHAAD SHAFFEEULLAH, M.D. A diplomate American Board of CHILD AND ADOLESCENT PSYCHIATRY Similar type of idea Similar document Similar document AACAP document Neurobiological condition
Treatment: Healing Actions, Healing Words
 Treatment: Healing Actions, Healing Words 15 : Insight-Oriented Therapy Psychoanalysis (p.687) First use of a talking cure Developed by Sigmund Freud Identify unconscious motivations Free association Dream
Treatment: Healing Actions, Healing Words 15 : Insight-Oriented Therapy Psychoanalysis (p.687) First use of a talking cure Developed by Sigmund Freud Identify unconscious motivations Free association Dream
Major Depressive Disorder (MDD) Guideline Diagnostic Nomenclature for Clinical Depressive Conditions
 Major Depressive Disorder Major Depressive Disorder (MDD) Guideline Diagnostic omenclature for Clinical Depressive Conditions Conditions Diagnostic Criteria Duration Major Depression 5 of the following
Major Depressive Disorder Major Depressive Disorder (MDD) Guideline Diagnostic omenclature for Clinical Depressive Conditions Conditions Diagnostic Criteria Duration Major Depression 5 of the following
BOARD OF PHARMACY SPECIALITIES 2215 Constitution Avenue, NW Washington, DC 20037-2985 202-429-7591 FAX 202-429-6304 bps@aphanet.org www.bpsweb.
 BOARD OF PHARMACY SPECIALITIES 2215 Constitution Avenue, NW Washington, DC 20037-2985 202-429-7591 FAX 202-429-6304 bps@aphanet.org www.bpsweb.org Content Outline for the PSYCHIATRIC PHARMACY SPECIALTY
BOARD OF PHARMACY SPECIALITIES 2215 Constitution Avenue, NW Washington, DC 20037-2985 202-429-7591 FAX 202-429-6304 bps@aphanet.org www.bpsweb.org Content Outline for the PSYCHIATRIC PHARMACY SPECIALTY
Pharmacologic Treatment of Acute Major Depression and Dysthymia
 POSITION PAPERS CLINICAL GUIDELINE, PART 1 Pharmacologic Treatment of Acute Major Depression and Dysthymia Vincenza Snow, MD; Steven Lascher, DVM, MPH; and Christel Mottur-Pilson, PhD, for the American
POSITION PAPERS CLINICAL GUIDELINE, PART 1 Pharmacologic Treatment of Acute Major Depression and Dysthymia Vincenza Snow, MD; Steven Lascher, DVM, MPH; and Christel Mottur-Pilson, PhD, for the American
Medical Malpractice Treatment Alprazolam benzodiazepine - A Case Study
 Improving Outcomes in Patients Who are Prescribed Alprazolam with Concurrent Use of Opioids Pik-Sai Yung, M.D. Staff Psychiatrist Center for Counseling at Walton Background and Rationale Alprazolam is
Improving Outcomes in Patients Who are Prescribed Alprazolam with Concurrent Use of Opioids Pik-Sai Yung, M.D. Staff Psychiatrist Center for Counseling at Walton Background and Rationale Alprazolam is
Paxil/Paxil-CR (paroxetine)
 Generic name: Paroxetine Available strengths: 10 mg, 20 mg, 30 mg, 40 mg tablets; 10 mg/5 ml oral suspension; 12.5 mg, 25 mg, 37.5 mg controlled-release tablets (Paxil-CR) Available in generic: Yes, except
Generic name: Paroxetine Available strengths: 10 mg, 20 mg, 30 mg, 40 mg tablets; 10 mg/5 ml oral suspension; 12.5 mg, 25 mg, 37.5 mg controlled-release tablets (Paxil-CR) Available in generic: Yes, except
Appendix to Tennessee Department of Health: Tennessee Clinical Practice Guidelines for Outpatient Management of Chronic Non- Malignant Pain
 Appendix to Tennessee Department of Health: Tennessee Clinical Practice Guidelines for Outpatient Management of Chronic Non- Malignant Pain Division of Workers Compensation 04.01.2015 Background Opioids
Appendix to Tennessee Department of Health: Tennessee Clinical Practice Guidelines for Outpatient Management of Chronic Non- Malignant Pain Division of Workers Compensation 04.01.2015 Background Opioids
Pharmacological management of unipolar depression
 Acta Psychiatr Scand 2013: 127 (Suppl. 443): 6 23 All rights reserved DOI: 10.1111/acps.12122 2013 John Wiley & Sons A/S. Published by Blackwell Publishing Ltd ACTA PSYCHIATRICA SCANDINAVICA Clinical overview
Acta Psychiatr Scand 2013: 127 (Suppl. 443): 6 23 All rights reserved DOI: 10.1111/acps.12122 2013 John Wiley & Sons A/S. Published by Blackwell Publishing Ltd ACTA PSYCHIATRICA SCANDINAVICA Clinical overview
POST-TRAUMATIC STRESS DISORDER PTSD Diagnostic Criteria PTSD Detection and Diagnosis PC-PTSD Screen PCL-C Screen PTSD Treatment Treatment Algorithm
 E-Resource March, 2014 POST-TRAUMATIC STRESS DISORDER PTSD Diagnostic Criteria PTSD Detection and Diagnosis PC-PTSD Screen PCL-C Screen PTSD Treatment Treatment Algorithm Post-traumatic Stress Disorder
E-Resource March, 2014 POST-TRAUMATIC STRESS DISORDER PTSD Diagnostic Criteria PTSD Detection and Diagnosis PC-PTSD Screen PCL-C Screen PTSD Treatment Treatment Algorithm Post-traumatic Stress Disorder
The Pharmacological Management of Depression in the Adult Person with Mental Retardation and Developmental Disabilities (MR/DD)
 The Pharmacological Management of Depression in the Adult Person with Mental Retardation and (MR/DD) 1. Overview Multiple classes of medications are available for treatment of depression in the patient
The Pharmacological Management of Depression in the Adult Person with Mental Retardation and (MR/DD) 1. Overview Multiple classes of medications are available for treatment of depression in the patient
Treatment Modalities As in younger patients, the goals of treating depression in elderly patients include alleviating
 Iranian Rehabilitation Journal, Vol. 10, No. 16, Oct. 2012 Reviews/Short communication Treatment of Depression in the Elderly: A systematic review Arash Mirabzadeh, MD. University of Social Welfare and
Iranian Rehabilitation Journal, Vol. 10, No. 16, Oct. 2012 Reviews/Short communication Treatment of Depression in the Elderly: A systematic review Arash Mirabzadeh, MD. University of Social Welfare and
Hypothyroidism and Depression: Use of TSH as a Diagnostic Tool and the Role of Thyroid Supplement Therapy in Psychiatric Practice
 Hypothyroidism and Depression: Use of TSH as a Diagnostic Tool and the Role of Thyroid Supplement Therapy in Psychiatric Practice By Scott McDonald, DO PGY1 Hypothyroidism General medicine texts always
Hypothyroidism and Depression: Use of TSH as a Diagnostic Tool and the Role of Thyroid Supplement Therapy in Psychiatric Practice By Scott McDonald, DO PGY1 Hypothyroidism General medicine texts always
Escitalopram (Lexapro) for major depressive disorder
 Escitalopram (Lexapro) for major depressive disorder Summary PBS listing Reason for listing Place in therapy Restricted benefit: Major depressive disorders. Escitalopram was listed on the basis of cost-minimisation
Escitalopram (Lexapro) for major depressive disorder Summary PBS listing Reason for listing Place in therapy Restricted benefit: Major depressive disorders. Escitalopram was listed on the basis of cost-minimisation
ADVANCED BEHAVIORAL HEALTH, INC. Clinical Level of Care Guidelines - 2015
 The Clinical Level of Care Guidelines contained on the following pages have been developed as a guide to assist care managers, physicians and providers in making medical necessity decisions about the least
The Clinical Level of Care Guidelines contained on the following pages have been developed as a guide to assist care managers, physicians and providers in making medical necessity decisions about the least
Comorbid Conditions in Autism Spectrum Illness. David Ermer MD June 13, 2014
 Comorbid Conditions in Autism Spectrum Illness David Ermer MD June 13, 2014 Overview Diagnosing comorbidities in autism spectrum illnesses Treatment issues specific to autism spectrum illnesses Treatment
Comorbid Conditions in Autism Spectrum Illness David Ermer MD June 13, 2014 Overview Diagnosing comorbidities in autism spectrum illnesses Treatment issues specific to autism spectrum illnesses Treatment
Depression in Older Persons
 Depression in Older Persons How common is depression in later life? Depression affects more than 6.5 million of the 35 million Americans aged 65 or older. Most people in this stage of life with depression
Depression in Older Persons How common is depression in later life? Depression affects more than 6.5 million of the 35 million Americans aged 65 or older. Most people in this stage of life with depression
ATYPICALS ANTIPSYCHOTIC MEDICATIONS
 The atypical antipsychotics are a class of drugs that are used to treat a number of behavioral health disorders, including schizophrenia, other psychotic disorders, mood disorders, and behavioral agitation
The atypical antipsychotics are a class of drugs that are used to treat a number of behavioral health disorders, including schizophrenia, other psychotic disorders, mood disorders, and behavioral agitation
Primary Care Guide For Prescription Of Anxiolytic Medications For Persons With Mental Retardation and Developmental Disabilities (MR/DD)
 Primary Care Guide For Prescription Of Anxiolytic Medications For Persons With Mental Retardation and Developmental Disabilities (MR/DD) Overview of Prescription The DD/MR patient may develop symptoms
Primary Care Guide For Prescription Of Anxiolytic Medications For Persons With Mental Retardation and Developmental Disabilities (MR/DD) Overview of Prescription The DD/MR patient may develop symptoms
Preferred Practice Guidelines for the Identification and Treatment of Depressive Disorder
 Preferred Practice Guidelines for the Identification and Treatment of Depressive Disorder These Guidelines were based in part from on following: Treatment of Patients With Major Depressive Disorder from
Preferred Practice Guidelines for the Identification and Treatment of Depressive Disorder These Guidelines were based in part from on following: Treatment of Patients With Major Depressive Disorder from
