DAIDS Appendix 2 No.: DWD-POL-DM-01.00A2. Data Management Requirements for Central Data Management Facilities
|
|
|
- Helen Bond
- 8 years ago
- Views:
Transcription
1 DAIDS Appendix 2 No.: DWD-POL-DM-01.00A2 Data Management Requirements for Central Data Management Facilities The following clinical trial data management requirements must be met in order to ensure the authenticity and integrity of data. The Division of Acquired Immunodeficiency Syndrome (DAIDS) may choose to conduct or to have a data management assessment visit to the facility conducted by an independent contractor. 1. Data Management Operations 1.1 Facility Staff Create and keep on file a current organizational chart for central data management personnel at the facility Appoint a Head of data management with the appropriate credentials and training in relation to the responsibilities assumed in the position Create and keep on file current job descriptions of key data management personnel Ensure that the assigned data management roles and responsibilities of the staff are reasonable compared with the experience/training of each individual. 1.2 Training Needs Develop an overall training plan that is relevant to the chosen data management system. Include what training is provided, to whom it is provided, how often it is provided, who provides the training, and how training effectiveness is monitored (e.g., written test after training, hands-on demonstration of new skills) Develop a training documentation system to track training given and received and to maintain training records. 1.3 Standard Operating Procedures (SOPs) Establish data management/information technology (IT) SOPs adequate to control the security, privacy, and accuracy of the data, including, but not limited to: System Development and Validation Installation and Validation of Software Facility, System, and Data Security System User Account Maintenance System Maintenance Change Control and Configuration Management Backup, Restore, and Disaster Recovery Data Collection Forms (review and approval process) Data Storage Database Design and Validation 20 DEC 06; Version of 9 No.: DWD-POL-DM-01.00A2
2 Coding Mechanism and Process Data Acquisition, Entry, and Processing Data Queries and Data Error Correction Adverse Event Reporting (AEs, SAEs, EAEs, Safety Reports) Database closure/archiving (not required prior to enrollment) Data audits (not required prior to enrollment) Data Management Training 1.4 Quality Assurance (QA)/Quality Control (QC) Develop and document (e.g., QA/QC SOPs, Data Quality Management Plan) the data management QA/QC process. Include how the accuracy, integrity, consistency, reliability, and completeness of data received from the data collection site(s) will be ensured; the frequency of quality review; the percentage of records reviewed; the error rate; and the measures taken to correct and resolve data errors. 2. Overall Data Management System 2.1 Hardware Choose a computer system platform, including workstations, servers (e.g., UNIX, Windows), and versions used for central storage, security, processing, and other data management, that is of sufficient size and speed to be able to efficiently support the chosen data management software and database needs Perform and document computer system validation. Include requirements/ specifications (e.g., validation protocol), test results, review of results, remediation taken to fix any problems, and test results after remediation Maintain system documentation and reference manuals used during the clinical trial. 2.2 Software Choose software to be used as the database system, including versions, capability, and associated tools, that has sufficient capacity to handle the anticipated data volume for the facility and has the capability of generating the data in the manner needed, keeping an audit trail, and performing process inspections and edit checks For each software package used in the data management process, perform and document software installation validation. A. For custom software validation, include requirements/specifications (e.g., validation protocol, test scripts), test results, review of results, remediation taken to fix any problems, and test results after remediation. B. For off-the-shelf software validation, follow the test scripts provided by the company and document the steps taken. 20 DEC 06; Version of 9 No.: DWD-POL-DM-01.00A2
3 2.2.3 Maintain software documentation and reference manuals used during the clinical trial. 2.3 Internet Capabilities Choose an Internet service that will provide the speed, reliability, security, and times of access necessary to facilitate the conduct of the clinical trial If possible, establish an Internet connection independent of the institution or organization (e.g., main university, main hospital), if applicable. 2.4 Physical Security Develop a security plan for the computer room and the main server room (if in a location separate from the computer room). Include who has access to the computer room/server room and how access is requested and approved; how access is monitored and documented; and how the computer room/server room is protected from fire, electrical power problems (e.g., power interruption, electrical surges), flooding, and theft. 2.5 Data Security Develop a security plan for data, both electronic and hard copy data For electronic data, include password protection, virus protection, firewalls, encryption, and authority checks (used to ensure only authorized individuals access the system) For hard copy data, include who has access to the storage area and how access is requested and approved; how access is monitored and documented; and how the storage area is protected from fire, flooding, and theft. 2.6 Rights Access, Roles, and Privileges Develop a process for how access levels for user accounts, roles, and groups are determined. Include who approves user accounts, roles, and groups; how user accounts, roles, and groups are created, modified, and privileges removed; how user accounts and groups are documented; and how visitor access is handled and documented. 2.7 Environment and Maintenance Develop environmental controls to support effective operation for computers, servers, and peripherals (i.e., temperature, humidity, power, etc.). Include how the environmental controls are monitored and how the environmental values are logged Develop a maintenance plan for the computer systems. Include who is responsible for maintenance, a description of the maintenance schedule, and how and where maintenance logs are maintained. 20 DEC 06; Version of 9 No.: DWD-POL-DM-01.00A2
4 2.8 Change Control Procedures Develop change control procedures to document changes made to the computer system, including software changes. Include how changes are requested, how the impact of the change is assessed, who is responsible for authorizing the change, how the change is tested and released, and how changes are documented. 2.9 Backup and Restoration Develop a database backup plan. Include who is responsible for the backup, the frequency of the backup, the log created during the backup, backup media rotation strategy, where the backup media are stored, and how backup activities are documented Develop a database restoration plan. Include how restorations are tested, how restoration requests are logged, and how database files are restored after loss Disaster Contingency Plan Develop a disaster contingency plan (e.g., in the case of a fire, theft). Include how the computer system will be restored/replaced and revalidated; how the software will be restored/replaced and revalidated; how the data will be restored/replaced and quality reviewed; and a description of the system or process that will be used while restoration/ replacement takes place (e.g., hand capturing data). 3. Protocol-specific Database(s) 3.1 Data Collection Tools Data Collection Tool Development Establish a development process for data collection tools (e.g., case report forms, questionnaires). Include design, development, coding, generation, review, revision, approval, testing, version control, and distribution Data Coding Develop a plan for data coding and text coding, including any standard dictionaries to be used Completion Instructions Develop completion instructions for data collection tools to accurately describe the way data should be collected, including a timeline for completion and submission Receipt Control and Tracking Procedures Develop a plan for receipt control and tracking of data collection tools (e.g., barcoding, numbering system). 20 DEC 06; Version of 9 No.: DWD-POL-DM-01.00A2
5 3.1.5 Storage Establish storage space for both paper and electronic data collection tools that is adequate to store study documentation, complies with participant confidentiality, has adequate security in place, and has controlled access Source Documentation Develop source documentation requirements in relation to the data collection tools, including collection, verification, storage, and participant confidentiality Change Control Procedures Develop change control procedures to ensure quality control in changes made to the data collection tools. Include how changes are requested, how the impact of changes is assessed, who is responsible for authorizing the changes, how the changes are tested and released, and how the changes are documented. 3.2 Database Development Programming Standards A. Develop data programming plans to include programming standards for database development. Include application architecture, design, programs, database structure, and physical implementation details. B. Document adherence to the programming standards during database development Coding Criteria Establishment Develop the requirements for coding (coding plan) for the trial. Include actual coding, review, QA/QC, coding standards, and dictionary versioning Edit Check Development Process Establish an edit check development process that includes edit check requirements/specifications, design documentation, test results, review of results, remediation taken to fix any problems, and test results after remediation Protocol-Specific Database Validation A. Perform and document protocol-specific database validation. Include database requirements/specifications (e.g., validation protocol), design documentation, test results, review of results, remediation taken to fix any problems, and test results after remediation. B. Develop documentation for the approval of the database for production implementation. 20 DEC 06; Version of 9 No.: DWD-POL-DM-01.00A2
6 3.2.5 Technical Database System Documentation Develop and maintain system documentation that describes the database structure, variable names, data attributes, coding schemes, missing values, collected variables, calculated variables, transformed variables Change Control Procedures Develop change control procedures to document changes made to the database design. Include how changes are requested, how the impact of the change is assessed, who is responsible for authorizing the change, how the change is tested and released, and how the change is documented. 3.3 Enrollment/Randomization Procedures Develop and document enrollment procedures and, if applicable, randomization, and stratification procedures. Include how eligibility for enrollment will be checked; the methods used to minimize/avoid bias, management of randomization codes, and security and integrity of randomization; and how enrollment/randomization will be suspended or closed Perform and document validation of the randomization schema, if applicable. Include randomization requirements/specifications (e.g., validation protocol), design documentation, test results, review of results, remediation taken to fix any problems, and test results after remediation. 3.4 Blinding Procedures Develop and document blinding procedures, if applicable. Include how study agent is blinded, how the blinding codes are maintained, how the integrity of blinded data is maintained, and who is responsible for holding the blinding codes. 3.5 Data Processing Data Entry A. Develop a process for maintaining a log of data received from the data collection site(s). B. Determine a method of data entry (e.g., double data entry, split screen verification, electronic data capture) that will ensure the completeness, reliability, and accuracy of the data. C. Develop data entry instructions, including timelines for data entry. 20 DEC 06; Version of 9 No.: DWD-POL-DM-01.00A2
7 3.5.2 Electronic Data from Other Sources (i.e., Laboratory Data) A. Develop plan for receipt and processing of electronic data from other sources. Include how data will be transferred, received, quality controlled, loaded into the database, and synchronized with the clinical database. B. Document the process to be used to perform quality control checks on laboratory and other data received from other sources. C. Perform and document validation of systems/processes to be used to transfer, receive, load, and synchronize the data. Include requirements/specifications (e.g., validation protocol), design documentation, test results, review of results, remediation taken to fix any problems, and test results after remediation Data Error Correction A. Develop a process for how data errors are detected. Include query and edit check specifications (e.g., logic checks, out of range checks), how quality control reviews are performed, and how often the reviews are performed. B. Develop a process for how data errors are corrected. Include distribution and tracking of data queries (data clarifications) to the data collection site(s) and how data errors are documented, tracked, resolved, and corrected Quality Review of Data 3.6 Unblinding for Safety Develop a plan for retrospective quality review of data. Include the frequency of review, the percentage of records reviewed, the error rates, and the measures for error correction and resolution. Develop a plan for how to break the blinding code when the safety of a participant is at risk, if applicable, including who is responsible for making the decision to unblind and how to contact the responsible person outside of business hours. 3.7 Adverse Event Reporting Adverse Event Coding A. Choose an adverse event coding system (e.g., MedDRA). B. Perform and document installation validation of coding related software. Include coding system requirements/specifications (e.g., validation protocol), design documentation, test results, review of results, remediation taken to fix any problems, and test results after remediation. 20 DEC 06; Version of 9 No.: DWD-POL-DM-01.00A2
8 3.7.2 Adverse Event Reporting Procedures Develop a process for disseminating adverse events data, which includes adverse events (AEs), serious adverse events (SAEs), and expedited adverse events (EAEs). Include how adverse event data are received from the data collection site(s) (i.e., logged, tracked, entered, and processed), reported according to the specifications in the clinical protocol, documented, and quality controlled Data Reconciliation Develop a plan for reconciliation of data between the safety database (EAEs/SAEs) and the clinical database (AEs), if applicable. Include documentation of reconciliation efforts, test results, review of results, remediation taken to fix any problems, and test results after remediation Safety Reports 3.8 Study Reports Develop a process for writing and submitting safety reports. Include report specifications for reports to be sent to safety monitoring committees (e.g., Data and Safety Monitoring Board (DSMB), Institutional Review Board (IRB)/Ethics Committee (EC)) and regulatory agencies, how routine safety reports are generated and to whom they are provided, how report testing/validation is documented, the process for conducting quality control review of the data, and timeframes for submission. Establish a process for report development, including specifications, programming, testing/validation, and documentation. 4. Database Closure and Archiving 4.1 Final Database Audit Develop a plan for final database audit. Include the timing for the final database audit, the audit process, acceptable error rates, and how data errors are detected, documented, tracked, resolved, and corrected. 4.2 Database Closure Develop procedures for how the database is closed. Include who is responsible for making the decision to close the database, the steps that are followed, and how the activities are documented. 4.3 Database Review Describe the process for reviewing the database once the database is closed, if it is necessary to do so. Include who is responsible for making the decision; the steps for re-opening, reviewing, and re-closing the database; and how the activities are documented. 20 DEC 06; Version of 9 No.: DWD-POL-DM-01.00A2
9 4.4 Unblinding for Data Analysis Describe how the blinding code is broken for data analysis, if applicable. Include who is responsible for making the decision to break the code, the steps that are followed, and how the activities are documented. 4.5 Final Datasets Develop a plan for final datasets. Include location and security of final datasets, documentation for final datasets, and final disposition/archival of datasets. 4.6 Final Safety Data Develop a plan to provide final safety data to the DAIDS Regulatory Compliance Center (RCC) at the end of the clinical trial. 4.7 Record Retention Develop a plan for record retention, both electronic and hard copy. Include when record retention begins, the length of time the records are retained, where the records are retained, the security of the storage space, who has access to the storage space, who is responsible for approving access, how access is documented, how record retention is documented, and how records will be accessible for inspection by the sponsor, regulatory agencies, or other authorized individuals. 5. Data Audits Develop a process facility personnel will follow in the event of a data audit by a regulatory authority or a clinical trial sponsor. Include how to prepare for the data audit, how data audits are scheduled, who is notified upon the inspector s arrival at the facility, what documentation to expect from the inspector, how facility personnel should conduct themselves during the audit, how document and sample requests from the inspector should be handled, how a response to the regulatory authority/sponsor will be generated, and how remediation of contingencies will be performed and documented. 20 DEC 06; Version of 9 No.: DWD-POL-DM-01.00A2
IT General Controls Domain COBIT Domain Control Objective Control Activity Test Plan Test of Controls Results
 Acquire or develop application systems software Controls provide reasonable assurance that application and system software is acquired or developed that effectively supports financial reporting requirements.
Acquire or develop application systems software Controls provide reasonable assurance that application and system software is acquired or developed that effectively supports financial reporting requirements.
DAIDS Bethesda, MD USA POLICY. Requirements for Clinical Quality Management Plans
 NOTE: This policy and associated appendices have been reviewed for accuracy and updated to meet 508 compliance guidelines. DAIDS has clearly stated the minimum requirements for the CQMP and provided examples
NOTE: This policy and associated appendices have been reviewed for accuracy and updated to meet 508 compliance guidelines. DAIDS has clearly stated the minimum requirements for the CQMP and provided examples
Clinical Data Management (Process and practical guide) Nguyen Thi My Huong, MD. PhD WHO/RHR/SIS
 Clinical Data Management (Process and practical guide) Nguyen Thi My Huong, MD. PhD WHO/RHR/SIS Training Course in Sexual and Reproductive Health Research Geneva 2013 OUTLINE Overview of Clinical Data
Clinical Data Management (Process and practical guide) Nguyen Thi My Huong, MD. PhD WHO/RHR/SIS Training Course in Sexual and Reproductive Health Research Geneva 2013 OUTLINE Overview of Clinical Data
EVALUATION REPORT. Weaknesses Identified During the FY 2014 Federal Information Security Management Act Review. March 13, 2015 REPORT NUMBER 15-07
 EVALUATION REPORT Weaknesses Identified During the FY 2014 Federal Information Security Management Act Review March 13, 2015 REPORT NUMBER 15-07 EXECUTIVE SUMMARY Weaknesses Identified During the FY 2014
EVALUATION REPORT Weaknesses Identified During the FY 2014 Federal Information Security Management Act Review March 13, 2015 REPORT NUMBER 15-07 EXECUTIVE SUMMARY Weaknesses Identified During the FY 2014
TEMPLATE DATA MANAGEMENT PLAN
 TEMPLATE DATA MANAGEMENT PLAN ICRIN (QM sub group) Version: XX Date: XXXXXXX Page 1 of 6 1.0 Document Ownership The Data Management Plan (DMP) will be initiated and subsequently owned by the Data Manager
TEMPLATE DATA MANAGEMENT PLAN ICRIN (QM sub group) Version: XX Date: XXXXXXX Page 1 of 6 1.0 Document Ownership The Data Management Plan (DMP) will be initiated and subsequently owned by the Data Manager
DAIDS Bethesda, MD USA. POLICY Requirements for Clinical Quality Management Plans
 CHANGE SUMMARY: This policy and associated appendices have been reviewed for accuracy and updated to meet 508 compliance guidelines. Key changes to the policy require all sites to submit a bi-annual Clinical
CHANGE SUMMARY: This policy and associated appendices have been reviewed for accuracy and updated to meet 508 compliance guidelines. Key changes to the policy require all sites to submit a bi-annual Clinical
Section 1 Project Management, Project Communication/Process Design, Mgmt, Documentation, Definition & Scope /CRO-Sponsor Partnership
 Section 1 Project Management, Project Communication/Process Design, Mgmt, Documentation, Definition & Scope /CRO-Sponsor Partnership PROJECT MANAGEMENT - SCOPE DEFINITION AND MANAGEMENT Understands the
Section 1 Project Management, Project Communication/Process Design, Mgmt, Documentation, Definition & Scope /CRO-Sponsor Partnership PROJECT MANAGEMENT - SCOPE DEFINITION AND MANAGEMENT Understands the
---Information Technology (IT) Specialist (GS-2210) IT Security Competency Model---
 ---Information Technology (IT) Specialist (GS-2210) IT Security Model--- TECHNICAL COMPETENCIES Computer Forensics Knowledge of tools and techniques pertaining to legal evidence used in the analysis of
---Information Technology (IT) Specialist (GS-2210) IT Security Model--- TECHNICAL COMPETENCIES Computer Forensics Knowledge of tools and techniques pertaining to legal evidence used in the analysis of
This is a controlled document. The master document is posted on the JRCO website and any print-off of this document will be classed as uncontrolled.
 This is a controlled document. The master document is posted on the JRCO website and any print-off of this document will be classed as uncontrolled. Researchers and their teams may print off this document
This is a controlled document. The master document is posted on the JRCO website and any print-off of this document will be classed as uncontrolled. Researchers and their teams may print off this document
Clinical Data Management (Process and practical guide) Dr Nguyen Thi My Huong WHO/RHR/RCP/SIS
 Clinical Data Management (Process and practical guide) Dr Nguyen Thi My Huong WHO/RHR/RCP/SIS Training Course in Sexual and Reproductive Health Research Geneva 2012 OUTLINE Clinical Data Management CDM
Clinical Data Management (Process and practical guide) Dr Nguyen Thi My Huong WHO/RHR/RCP/SIS Training Course in Sexual and Reproductive Health Research Geneva 2012 OUTLINE Clinical Data Management CDM
CHIS, Inc. Privacy General Guidelines
 CHIS, Inc. and HIPAA CHIS, Inc. provides services to healthcare facilities and uses certain protected health information (PHI) in connection with performing these services. Therefore, CHIS, Inc. is classified
CHIS, Inc. and HIPAA CHIS, Inc. provides services to healthcare facilities and uses certain protected health information (PHI) in connection with performing these services. Therefore, CHIS, Inc. is classified
The role, duties and responsibilities of clinical trials personnel Monitoring: rules and recommendations
 The role, duties and responsibilities of clinical trials personnel Monitoring: rules and recommendations Maria Luisa Paoloni OPBG Clinical & Research Services Monitoring and Responsible of monitoring:
The role, duties and responsibilities of clinical trials personnel Monitoring: rules and recommendations Maria Luisa Paoloni OPBG Clinical & Research Services Monitoring and Responsible of monitoring:
Managing & Validating Research Data
 Research Management Standard Operating Procedure ISOP-H02 VERSION / REVISION: 2.0 EFFECTIVE DATE: 01 03 12 REVIEW DATE: 01 03 14 AUTHOR(S): CONTROLLER(S): APPROVED BY: Information Officer; NBT Clinical
Research Management Standard Operating Procedure ISOP-H02 VERSION / REVISION: 2.0 EFFECTIVE DATE: 01 03 12 REVIEW DATE: 01 03 14 AUTHOR(S): CONTROLLER(S): APPROVED BY: Information Officer; NBT Clinical
STANDARD OPERATING PROCEDURE NO. CM.13-00 - 00
 STANDARD OPERATING PROCEDURE NO. CM.13-00 - 00 Version date: Effective Date: Replaces SOP No.: 1 5 January 20 13 15 February 20 13 Approved by: Date No: CM.13 00 00 Effective Date: 15 February 2013 Version
STANDARD OPERATING PROCEDURE NO. CM.13-00 - 00 Version date: Effective Date: Replaces SOP No.: 1 5 January 20 13 15 February 20 13 Approved by: Date No: CM.13 00 00 Effective Date: 15 February 2013 Version
SUBJECT: SECURITY OF ELECTRONIC MEDICAL RECORDS COMPLIANCE WITH THE HEALTH INSURANCE PORTABILITY AND ACCOUNTABILITY ACT OF 1996 (HIPAA)
 UNIVERSITY OF PITTSBURGH POLICY SUBJECT: SECURITY OF ELECTRONIC MEDICAL RECORDS COMPLIANCE WITH THE HEALTH INSURANCE PORTABILITY AND ACCOUNTABILITY ACT OF 1996 (HIPAA) DATE: March 18, 2005 I. SCOPE This
UNIVERSITY OF PITTSBURGH POLICY SUBJECT: SECURITY OF ELECTRONIC MEDICAL RECORDS COMPLIANCE WITH THE HEALTH INSURANCE PORTABILITY AND ACCOUNTABILITY ACT OF 1996 (HIPAA) DATE: March 18, 2005 I. SCOPE This
ROLES, RESPONSIBILITIES AND DELEGATION OF DUTIES IN CLINICAL TRIALS OF MEDICINAL PRODUCTS
 ROLES, RESPONSIBILITIES AND DELEGATION OF DUTIES IN CLINICAL TRIALS OF MEDICINAL PRODUCTS STANDARD OPERATING PROCEDURE NO SOP 09 DATE RATIFIED 4/7/13 NEXT REVIEW DATE 4/7/14 POLICY STATEMENT/KEY OBJECTIVES:
ROLES, RESPONSIBILITIES AND DELEGATION OF DUTIES IN CLINICAL TRIALS OF MEDICINAL PRODUCTS STANDARD OPERATING PROCEDURE NO SOP 09 DATE RATIFIED 4/7/13 NEXT REVIEW DATE 4/7/14 POLICY STATEMENT/KEY OBJECTIVES:
Version history Version number Version date Effective date 01 dd-mon-yyyy dd-mon-yyyy 02 dd-mon-yyyy dd-mon-yyyy 03 (current) dd-mon-yyyy dd-mon-yyyy
 Trial name: HOVON xxx yyy Sponsor: HOVON Version history Version number Version date Effective date 01 dd-mon-yyyy dd-mon-yyyy 02 dd-mon-yyyy dd-mon-yyyy 03 (current) dd-mon-yyyy dd-mon-yyyy QRMP authors
Trial name: HOVON xxx yyy Sponsor: HOVON Version history Version number Version date Effective date 01 dd-mon-yyyy dd-mon-yyyy 02 dd-mon-yyyy dd-mon-yyyy 03 (current) dd-mon-yyyy dd-mon-yyyy QRMP authors
QUALITY CONTROL AND QUALITY ASSURANCE IN CLINICAL RESEARCH
 QUALITY CONTROL AND QUALITY ASSURANCE IN CLINICAL RESEARCH Martin Valania, Executive Director, Corporate QA and Compliance Introduction Pharmaceutical companies recognize the benefits of carefully managing
QUALITY CONTROL AND QUALITY ASSURANCE IN CLINICAL RESEARCH Martin Valania, Executive Director, Corporate QA and Compliance Introduction Pharmaceutical companies recognize the benefits of carefully managing
Information Technology General Controls Review (ITGC) Audit Program Prepared by:
 Information Technology General Controls Review (ITGC) Audit Program Date Prepared: 2012 Internal Audit Work Plan Objective: IT General Controls (ITGC) address the overall operation and activities of the
Information Technology General Controls Review (ITGC) Audit Program Date Prepared: 2012 Internal Audit Work Plan Objective: IT General Controls (ITGC) address the overall operation and activities of the
Reflection paper for laboratories that perform the analysis or evaluation of clinical trial samples
 28 February 2012 EMA/INS/GCP/532137/2010 GCP Inspectors Working Group Reflection paper for laboratories that perform the analysis or evaluation of clinical trial Draft agreed by GCP Inspectors Working
28 February 2012 EMA/INS/GCP/532137/2010 GCP Inspectors Working Group Reflection paper for laboratories that perform the analysis or evaluation of clinical trial Draft agreed by GCP Inspectors Working
Client Security Risk Assessment Questionnaire
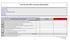 Select the appropriate answer from the drop down in the column, and provide a brief description in the section. 1 Do you have a member of your organization with dedicated information security duties? 2
Select the appropriate answer from the drop down in the column, and provide a brief description in the section. 1 Do you have a member of your organization with dedicated information security duties? 2
Guidance for Industry Computerized Systems Used in Clinical Investigations
 Guidance for Industry Computerized Systems Used in Clinical Investigations U.S. Department of Health and Human Services Food and Drug Administration (FDA) Office of the Commissioner (OC) May 2007 Guidance
Guidance for Industry Computerized Systems Used in Clinical Investigations U.S. Department of Health and Human Services Food and Drug Administration (FDA) Office of the Commissioner (OC) May 2007 Guidance
U. S. Department of Energy Consolidated Audit Program Checklist 5 Laboratory Information Management Systems Electronic Data Management
 U. S. Department of Energy Consolidated Audit Program Checklist 5 Laboratory Information Management Systems Electronic Data Management Revision 4.0 February 2014 Use of this DOECAP checklist is authorized
U. S. Department of Energy Consolidated Audit Program Checklist 5 Laboratory Information Management Systems Electronic Data Management Revision 4.0 February 2014 Use of this DOECAP checklist is authorized
OPERATIONAL CAPABILITY TECHNOLOGY QUESTIONNAIRE
 OPERATIONAL CAPABILITY TECHNOLOGY QUESTIONNAIRE Please provide all relevant documents responsive to the information requests listed within each area below. In addition to the specific documents requested,
OPERATIONAL CAPABILITY TECHNOLOGY QUESTIONNAIRE Please provide all relevant documents responsive to the information requests listed within each area below. In addition to the specific documents requested,
Evaluation Report. Weaknesses Identified During the FY 2013 Federal Information Security Management Act Review. April 30, 2014 Report Number 14-12
 Evaluation Report Weaknesses Identified During the FY 2013 Federal Information Security Management Act Review April 30, 2014 Report Number 14-12 U.S. Small Business Administration Office of Inspector General
Evaluation Report Weaknesses Identified During the FY 2013 Federal Information Security Management Act Review April 30, 2014 Report Number 14-12 U.S. Small Business Administration Office of Inspector General
Application Development within University. Security Checklist
 Application Development within University Security Checklist April 2011 The Application Development using data from the University Enterprise Systems or application Development for departmental use security
Application Development within University Security Checklist April 2011 The Application Development using data from the University Enterprise Systems or application Development for departmental use security
Guidance for Industry COMPUTERIZED SYSTEMS USED IN CLINICAL TRIALS
 Guidance for Industry COMPUTERIZED SYSTEMS USED IN CLINICAL TRIALS U.S. Department of Health and Human Services Food and Drug Administration Center for Biologic Evaluation and Research (CBER) Center for
Guidance for Industry COMPUTERIZED SYSTEMS USED IN CLINICAL TRIALS U.S. Department of Health and Human Services Food and Drug Administration Center for Biologic Evaluation and Research (CBER) Center for
Data Security Incident Response Plan. [Insert Organization Name]
![Data Security Incident Response Plan. [Insert Organization Name] Data Security Incident Response Plan. [Insert Organization Name]](/thumbs/34/17160420.jpg) Data Security Incident Response Plan Dated: [Month] & [Year] [Insert Organization Name] 1 Introduction Purpose This data security incident response plan provides the framework to respond to a security
Data Security Incident Response Plan Dated: [Month] & [Year] [Insert Organization Name] 1 Introduction Purpose This data security incident response plan provides the framework to respond to a security
This policy applies to all clinical research conducted at Beaumont Health System.
 CLINICAL RESEARCH QUALITY AND PROCESS IMPROVEMENT PROGRAM 113 1 of 6 PURPOSE Prior The purpose of this policy is to provide an overview of the Clinical Research Quality and Process Improvement Program
CLINICAL RESEARCH QUALITY AND PROCESS IMPROVEMENT PROGRAM 113 1 of 6 PURPOSE Prior The purpose of this policy is to provide an overview of the Clinical Research Quality and Process Improvement Program
CLINICAL DATA MONITORING PLAN (CDMoP) PROTOCOL # [0000] [TITLE]
![CLINICAL DATA MONITORING PLAN (CDMoP) PROTOCOL # [0000] [TITLE] CLINICAL DATA MONITORING PLAN (CDMoP) PROTOCOL # [0000] [TITLE]](/thumbs/39/18344673.jpg) CLINICAL DATA MONITORING PLAN (CDMoP) PROTOCOL # [0000] [TITLE] CONTRACT RESEARCH ORGANIZATION SPONSOR [NAME] [ADDRESS] 1 TABLE OF CONTENTS 1. Purpose 3 2. References 3 3. Study Roles and Responsibilities
CLINICAL DATA MONITORING PLAN (CDMoP) PROTOCOL # [0000] [TITLE] CONTRACT RESEARCH ORGANIZATION SPONSOR [NAME] [ADDRESS] 1 TABLE OF CONTENTS 1. Purpose 3 2. References 3 3. Study Roles and Responsibilities
SRA International Managed Information Systems Internal Audit Report
 SRA International Managed Information Systems Internal Audit Report Report #2014-03 June 18, 2014 Table of Contents Executive Summary... 3 Background Information... 4 Background... 4 Audit Objectives...
SRA International Managed Information Systems Internal Audit Report Report #2014-03 June 18, 2014 Table of Contents Executive Summary... 3 Background Information... 4 Background... 4 Audit Objectives...
InForm On Demand Single Trial Services Description
 InForm On Demand Single Trial Services Description Version 7.0 Effective Date: 0 25-Sep-2014 This is the Services Description for Oracle InForm On Demand Single Trial ( Schedule ) to Your Study Order for
InForm On Demand Single Trial Services Description Version 7.0 Effective Date: 0 25-Sep-2014 This is the Services Description for Oracle InForm On Demand Single Trial ( Schedule ) to Your Study Order for
A Best Practices Point of View from. Data Backup and Disaster Recovery Planning
 A Best Practices Point of View from Data Backup and Disaster Recovery Planning Security Protect Your Data Expertise Support Patient Privacy Business Continuity Plan and Restore Peace of Mind Backup and
A Best Practices Point of View from Data Backup and Disaster Recovery Planning Security Protect Your Data Expertise Support Patient Privacy Business Continuity Plan and Restore Peace of Mind Backup and
Computer System Validation for Clinical Trials:
 Computer System Validation for Clinical Trials: Framework Standard Operating Procedure (F-SOP) Author: Tim Cross Version History: 0.1di DRAFT 24-April-2013 0.2 DRAFT 12-June-2013 Current Version: 1.0 17-June-2013
Computer System Validation for Clinical Trials: Framework Standard Operating Procedure (F-SOP) Author: Tim Cross Version History: 0.1di DRAFT 24-April-2013 0.2 DRAFT 12-June-2013 Current Version: 1.0 17-June-2013
Data Management Unit Research Institute for Health Sciences, Chiang Mai University
 Data Management Unit Research Institute for Health Sciences, Chiang Mai University Clinical Data Management is the process of handling data from clinical trials. The inherent goal of any clinical data
Data Management Unit Research Institute for Health Sciences, Chiang Mai University Clinical Data Management is the process of handling data from clinical trials. The inherent goal of any clinical data
Supplier IT Security Guide
 Revision Date: 28 November 2012 TABLE OF CONTENT 1. INTRODUCTION... 3 2. PURPOSE... 3 3. GENERAL ACCESS REQUIREMENTS... 3 4. SECURITY RULES FOR SUPPLIER WORKPLACES AT AN INFINEON LOCATION... 3 5. DATA
Revision Date: 28 November 2012 TABLE OF CONTENT 1. INTRODUCTION... 3 2. PURPOSE... 3 3. GENERAL ACCESS REQUIREMENTS... 3 4. SECURITY RULES FOR SUPPLIER WORKPLACES AT AN INFINEON LOCATION... 3 5. DATA
Information Systems and Technology
 As public servants, it is our responsibility to use taxpayers dollars in the most effective and efficient way possible while adhering to laws and regulations governing those processes. There are many reasons
As public servants, it is our responsibility to use taxpayers dollars in the most effective and efficient way possible while adhering to laws and regulations governing those processes. There are many reasons
Service Children s Education
 Service Children s Education Data Handling and Security Information Security Audit Issued January 2009 2009 - An Agency of the Ministry of Defence Information Security Audit 2 Information handling and
Service Children s Education Data Handling and Security Information Security Audit Issued January 2009 2009 - An Agency of the Ministry of Defence Information Security Audit 2 Information handling and
Data-management and Biostatistics
 Data-management and Biostatistics OnQ Data prides itself in being able to offer a cost-effective flexible service that result in the hassle-free generation of clean, high quality scientific data in the
Data-management and Biostatistics OnQ Data prides itself in being able to offer a cost-effective flexible service that result in the hassle-free generation of clean, high quality scientific data in the
Office of Inspector General
 DEPARTMENT OF HOMELAND SECURITY Office of Inspector General Security Weaknesses Increase Risks to Critical United States Secret Service Database (Redacted) Notice: The Department of Homeland Security,
DEPARTMENT OF HOMELAND SECURITY Office of Inspector General Security Weaknesses Increase Risks to Critical United States Secret Service Database (Redacted) Notice: The Department of Homeland Security,
Information Security Risk Assessment Checklist. A High-Level Tool to Assist USG Institutions with Risk Analysis
 Information Security Risk Assessment Checklist A High-Level Tool to Assist USG Institutions with Risk Analysis Updated Oct 2008 Introduction Information security is an important issue for the University
Information Security Risk Assessment Checklist A High-Level Tool to Assist USG Institutions with Risk Analysis Updated Oct 2008 Introduction Information security is an important issue for the University
Clinical database/ecrf validation: effective processes and procedures
 TITOLO SLIDE Testo Slide Testo Slide Testo Slide Clinical database/ecrf validation: effective processes and procedures IV BIAS ANNUAL CONGRESS Padova September, 26 th 2012 PQE WORKSHOP: What's new in Computerized
TITOLO SLIDE Testo Slide Testo Slide Testo Slide Clinical database/ecrf validation: effective processes and procedures IV BIAS ANNUAL CONGRESS Padova September, 26 th 2012 PQE WORKSHOP: What's new in Computerized
SWAP EXECUTION FACILITY OPERATIONAL CAPABILITY TECHNOLOGY QUESTIONNAIRE
 SWAP EXECUTION FACILITY OPERATIONAL CAPABILITY TECHNOLOGY QUESTIONNAIRE Please provide all relevant documents responsive to the information requests listed within each area below. In addition to the specific
SWAP EXECUTION FACILITY OPERATIONAL CAPABILITY TECHNOLOGY QUESTIONNAIRE Please provide all relevant documents responsive to the information requests listed within each area below. In addition to the specific
Keyfort Cloud Services (KCS)
 Keyfort Cloud Services (KCS) Data Location, Security & Privacy 1. Executive Summary The purposes of this document is to provide a common understanding of the data location, security, privacy, resiliency
Keyfort Cloud Services (KCS) Data Location, Security & Privacy 1. Executive Summary The purposes of this document is to provide a common understanding of the data location, security, privacy, resiliency
DATA MANAGEMENT IN CLINICAL TRIALS: GUIDELINES FOR RESEARCHERS
 Reference Number: UHB 139 Version Number: 2 Date of Next Review: 14 Apr 2018 Previous Trust/LHB Reference Number: N/A DATA MANAGEMENT IN CLINICAL TRIALS: GUIDELINES FOR RESEARCHERS Introduction and Aim
Reference Number: UHB 139 Version Number: 2 Date of Next Review: 14 Apr 2018 Previous Trust/LHB Reference Number: N/A DATA MANAGEMENT IN CLINICAL TRIALS: GUIDELINES FOR RESEARCHERS Introduction and Aim
How To Use A Court Record Electronically In Idaho
 Idaho Judicial Branch Scanning and Imaging Guidelines DRAFT - October 25, 2013 A. Introduction Many of Idaho s courts have considered or implemented the use of digital imaging systems to scan court documents
Idaho Judicial Branch Scanning and Imaging Guidelines DRAFT - October 25, 2013 A. Introduction Many of Idaho s courts have considered or implemented the use of digital imaging systems to scan court documents
SOUTHWEST VIRGINIA COMMUNITY COLLEGE RECORDS MANAGEMENT POLICY
 SOUTHWEST VIRGINIA COMMUNITY COLLEGE RECORDS MANAGEMENT POLICY Statement of Intent This policy establishes the general responsibilities for management, retention, and disposition of SOUTHWEST VIRGINIA
SOUTHWEST VIRGINIA COMMUNITY COLLEGE RECORDS MANAGEMENT POLICY Statement of Intent This policy establishes the general responsibilities for management, retention, and disposition of SOUTHWEST VIRGINIA
EMA Clinical Laboratory Guidance - Key points and Clarifications PAUL STEWART
 EMA Clinical Laboratory Guidance - Key points and Clarifications PAUL STEWART Framework Labs generate data that are used to make decisions on the safety and efficacy of medicinal products; consequently,
EMA Clinical Laboratory Guidance - Key points and Clarifications PAUL STEWART Framework Labs generate data that are used to make decisions on the safety and efficacy of medicinal products; consequently,
Human Research Protection Program Good Clinical Practice Guidance for Investigators Investigator & Research Staff Responsibilities
 This Guidance Document is to ensure that investigators and research personnel recognize their responsibilities associated with the conduct of human subject research by outlining their responsibilities,
This Guidance Document is to ensure that investigators and research personnel recognize their responsibilities associated with the conduct of human subject research by outlining their responsibilities,
REGULATIONS COMPLIANCE ASSESSMENT
 ALIX is free software: you can redistribute it and/or modify it under the terms of the GNU General Public License as published by the Free Software Foundation. REGULATIONS COMPLIANCE ASSESSMENT BUSINESS
ALIX is free software: you can redistribute it and/or modify it under the terms of the GNU General Public License as published by the Free Software Foundation. REGULATIONS COMPLIANCE ASSESSMENT BUSINESS
HIPAA: In Plain English
 HIPAA: In Plain English Material derived from a presentation by Kris K. Hughes, Esq. Posted with permission from the author. The Health Insurance Portability and Accountability Act of 1996 (HIPAA), Pub.
HIPAA: In Plain English Material derived from a presentation by Kris K. Hughes, Esq. Posted with permission from the author. The Health Insurance Portability and Accountability Act of 1996 (HIPAA), Pub.
HIPAA 203: Security. An Introduction to the Draft HIPAA Security Regulations
 HIPAA 203: Security An Introduction to the Draft HIPAA Security Regulations Presentation Agenda Security Introduction Security Component Requirements and Impacts Administrative Procedures Physical Safeguards
HIPAA 203: Security An Introduction to the Draft HIPAA Security Regulations Presentation Agenda Security Introduction Security Component Requirements and Impacts Administrative Procedures Physical Safeguards
TECHNICAL AND ORGANIZATIONAL DATA SECURITY MEASURES
 TECHNICAL AND ORGANIZATIONAL DATA SECURITY MEASURES Contents Introduction... 3 The Technical and Organizational Data Security Measures... 3 Access Control of Processing Areas (Physical)... 3 Access Control
TECHNICAL AND ORGANIZATIONAL DATA SECURITY MEASURES Contents Introduction... 3 The Technical and Organizational Data Security Measures... 3 Access Control of Processing Areas (Physical)... 3 Access Control
INTERIM SITE MONITORING PROCEDURE
 INTERIM SITE MONITORING PROCEDURE 1. PURPOSE The purpose of this SOP is to describe the interim monitoring procedures conducted at Institution, according to GCP and other applicable local regulations.
INTERIM SITE MONITORING PROCEDURE 1. PURPOSE The purpose of this SOP is to describe the interim monitoring procedures conducted at Institution, according to GCP and other applicable local regulations.
INFORMATION TECHNOLOGY SECURITY STANDARDS
 INFORMATION TECHNOLOGY SECURITY STANDARDS Version 2.0 December 2013 Table of Contents 1 OVERVIEW 3 2 SCOPE 4 3 STRUCTURE 5 4 ASSET MANAGEMENT 6 5 HUMAN RESOURCES SECURITY 7 6 PHYSICAL AND ENVIRONMENTAL
INFORMATION TECHNOLOGY SECURITY STANDARDS Version 2.0 December 2013 Table of Contents 1 OVERVIEW 3 2 SCOPE 4 3 STRUCTURE 5 4 ASSET MANAGEMENT 6 5 HUMAN RESOURCES SECURITY 7 6 PHYSICAL AND ENVIRONMENTAL
An Overview of Information Security Frameworks. Presented to TIF September 25, 2013
 An Overview of Information Security Frameworks Presented to TIF September 25, 2013 What is a framework? A framework helps define an approach to implementing, maintaining, monitoring, and improving information
An Overview of Information Security Frameworks Presented to TIF September 25, 2013 What is a framework? A framework helps define an approach to implementing, maintaining, monitoring, and improving information
AUSTIN INDEPENDENT SCHOOL DISTRICT INTERNAL AUDIT DEPARTMENT TRANSPORTATION AUDIT PROGRAM
 GENERAL: The Technology department is responsible for the managing of electronic devices and software for the District, as well as the Help Desk for resolution of employee-created help tickets. The subgroups
GENERAL: The Technology department is responsible for the managing of electronic devices and software for the District, as well as the Help Desk for resolution of employee-created help tickets. The subgroups
IT Best Practices Audit TCS offers a wide range of IT Best Practices Audit content covering 15 subjects and over 2200 topics, including:
 IT Best Practices Audit TCS offers a wide range of IT Best Practices Audit content covering 15 subjects and over 2200 topics, including: 1. IT Cost Containment 84 topics 2. Cloud Computing Readiness 225
IT Best Practices Audit TCS offers a wide range of IT Best Practices Audit content covering 15 subjects and over 2200 topics, including: 1. IT Cost Containment 84 topics 2. Cloud Computing Readiness 225
SAS 70 Exams Of EBT Controls And Processors
 Appendix VIII SAS 70 Examinations of EBT Service Organizations Background States must obtain an examination by an independent auditor of the State electronic benefits transfer (EBT) service providers (service
Appendix VIII SAS 70 Examinations of EBT Service Organizations Background States must obtain an examination by an independent auditor of the State electronic benefits transfer (EBT) service providers (service
DHHS/NIH/OD/OIR/OHSRP 1/2/2015
 DHHS/NIH/OD/OIR/OHSRP 1/2/2015 The audience for this course is Principal Investigators (PIs), investigators and Research Coordinators (RCs) serving on the study team of human clinical studies and trials.
DHHS/NIH/OD/OIR/OHSRP 1/2/2015 The audience for this course is Principal Investigators (PIs), investigators and Research Coordinators (RCs) serving on the study team of human clinical studies and trials.
micros MICROS Systems, Inc. Enterprise Information Security Policy (MEIP) August, 2013 Revision 8.0 MICROS Systems, Inc. Version 8.
 micros MICROS Systems, Inc. Enterprise Information Security Policy (MEIP) Revision 8.0 August, 2013 1 Table of Contents Overview /Standards: I. Information Security Policy/Standards Preface...5 I.1 Purpose....5
micros MICROS Systems, Inc. Enterprise Information Security Policy (MEIP) Revision 8.0 August, 2013 1 Table of Contents Overview /Standards: I. Information Security Policy/Standards Preface...5 I.1 Purpose....5
Supplier Security Assessment Questionnaire
 HALKYN CONSULTING LTD Supplier Security Assessment Questionnaire Security Self-Assessment and Reporting This questionnaire is provided to assist organisations in conducting supplier security assessments.
HALKYN CONSULTING LTD Supplier Security Assessment Questionnaire Security Self-Assessment and Reporting This questionnaire is provided to assist organisations in conducting supplier security assessments.
Private Runtime Environment
 Private Runtime Environment 1. Principles A Private Runtime Environment (PRE) is an environment which enables Contractors to locate their resources in a segregated environment within premises provided
Private Runtime Environment 1. Principles A Private Runtime Environment (PRE) is an environment which enables Contractors to locate their resources in a segregated environment within premises provided
DESIGNATED CONTRACT MARKET OPERATIONAL CAPABILITY TECHNOLOGY QUESTIONNAIRE
 DESIGNATED CONTRACT MARKET OPERATIONAL CAPABILITY TECHNOLOGY QUESTIONNAIRE Please provide all relevant documents responsive to the information requests listed within each area below. In addition to the
DESIGNATED CONTRACT MARKET OPERATIONAL CAPABILITY TECHNOLOGY QUESTIONNAIRE Please provide all relevant documents responsive to the information requests listed within each area below. In addition to the
PBGC Information Security Policy
 PBGC Information Security Policy 1. Purpose. The Pension Benefit Guaranty Corporation (PBGC) Information Security Policy (ISP) defines the security and protection of PBGC information resources. 2. Reference.
PBGC Information Security Policy 1. Purpose. The Pension Benefit Guaranty Corporation (PBGC) Information Security Policy (ISP) defines the security and protection of PBGC information resources. 2. Reference.
Certification Practice Statement
 FernUniversität in Hagen: Certification Authority (CA) Certification Practice Statement VERSION 1.1 Ralph Knoche 18.12.2009 Contents 1. Introduction... 4 1.1. Overview... 4 1.2. Scope of the Certification
FernUniversität in Hagen: Certification Authority (CA) Certification Practice Statement VERSION 1.1 Ralph Knoche 18.12.2009 Contents 1. Introduction... 4 1.1. Overview... 4 1.2. Scope of the Certification
ACDM GUIDELINES TO FACILITATE PRODUCTION OF A DATA HANDLING PROTOCOL
 ACDM GUIDELINES TO FACILITATE PRODUCTION OF A DATA HANDLING PROTOCOL BACKGROUND The need was identified by the Electronic Data Transfer Special Interest Group (SIG) for each company or organisation to
ACDM GUIDELINES TO FACILITATE PRODUCTION OF A DATA HANDLING PROTOCOL BACKGROUND The need was identified by the Electronic Data Transfer Special Interest Group (SIG) for each company or organisation to
Delphi Information 3 rd Party Security Requirements Summary. Classified: Public 5/17/2012. Page 1 of 11
 Delphi Information 3 rd Party Security Requirements Summary Classified: Public 5/17/2012 Page 1 of 11 Contents Introduction... 3 Summary for All Users... 4 Vendor Assessment Considerations... 7 Page 2
Delphi Information 3 rd Party Security Requirements Summary Classified: Public 5/17/2012 Page 1 of 11 Contents Introduction... 3 Summary for All Users... 4 Vendor Assessment Considerations... 7 Page 2
Document Number: SOP/RAD/SEHSCT/007 Page 1 of 17 Version 2.0
 Standard Operating Procedures (SOPs) Research and Development Office Title of SOP: Computerised Systems for Clinical Trials SOP Number: 7 Version Number: 2.0 Supercedes: 1.0 Effective date: August 2013
Standard Operating Procedures (SOPs) Research and Development Office Title of SOP: Computerised Systems for Clinical Trials SOP Number: 7 Version Number: 2.0 Supercedes: 1.0 Effective date: August 2013
How To Protect Decd Information From Harm
 Policy ICT Security Please note this policy is mandatory and staff are required to adhere to the content Summary DECD is committed to ensuring its information is appropriately managed according to the
Policy ICT Security Please note this policy is mandatory and staff are required to adhere to the content Summary DECD is committed to ensuring its information is appropriately managed according to the
April 2010. promoting efficient & effective local government
 Department of Public Works and Environmental Services Department of Information Technology Fairfax Inspections Database Online (FIDO) Application Audit Final Report April 2010 promoting efficient & effective
Department of Public Works and Environmental Services Department of Information Technology Fairfax Inspections Database Online (FIDO) Application Audit Final Report April 2010 promoting efficient & effective
No. 706. Page 1 of 5. Issue Date 4/21/2014
 Subject: ROUTINE MONITORING VISITS Standard Operating Procedure Prepared By: Beaumont Research Coordinating Center, Research Institute PURPOSE Prior Issue Date 8/15/2011 No. 706 Issue Date 4/21/2014 Page
Subject: ROUTINE MONITORING VISITS Standard Operating Procedure Prepared By: Beaumont Research Coordinating Center, Research Institute PURPOSE Prior Issue Date 8/15/2011 No. 706 Issue Date 4/21/2014 Page
BOARD ACTION MEMORANDUM. TO: NCUA Board DATE: April 12, 2011 FROM: Director, Office of Corporate Credit Unions
 BOARD ACTION MEMORANDUM TO: NCUA Board DATE: April 12, 2011 FROM: Director, Office of Corporate Credit Unions SUBJ: Approval of Credit Union Service Organization (CUSO) Activities ACTION ITEM: NCUA Board
BOARD ACTION MEMORANDUM TO: NCUA Board DATE: April 12, 2011 FROM: Director, Office of Corporate Credit Unions SUBJ: Approval of Credit Union Service Organization (CUSO) Activities ACTION ITEM: NCUA Board
CTR System Report - 2008 FISMA
 CTR System Report - 2008 FISMA February 27, 2009 TABLE of CONTENTS BACKGROUND AND OBJECTIVES... 5 BACKGROUND... 5 OBJECTIVES... 6 Classes and Families of Security Controls... 6 Control Classes... 7 Control
CTR System Report - 2008 FISMA February 27, 2009 TABLE of CONTENTS BACKGROUND AND OBJECTIVES... 5 BACKGROUND... 5 OBJECTIVES... 6 Classes and Families of Security Controls... 6 Control Classes... 7 Control
APPENDIX P: MODEL QUALITY ASSURANCE PLAN
 APPENDIX P: MODEL QUALITY ASSURANCE PLAN Centers for Medicare & Medicaid Services Appendix P: Model Quality Assurance Plan January 2016 This page intentionally left blank. Centers for Medicare & Medicaid
APPENDIX P: MODEL QUALITY ASSURANCE PLAN Centers for Medicare & Medicaid Services Appendix P: Model Quality Assurance Plan January 2016 This page intentionally left blank. Centers for Medicare & Medicaid
REGULATIONS FOR THE SECURITY OF INTERNET BANKING
 REGULATIONS FOR THE SECURITY OF INTERNET BANKING PAYMENT SYSTEMS DEPARTMENT STATE BANK OF PAKISTAN Table of Contents PREFACE... 3 DEFINITIONS... 4 1. SCOPE OF THE REGULATIONS... 6 2. INTERNET BANKING SECURITY
REGULATIONS FOR THE SECURITY OF INTERNET BANKING PAYMENT SYSTEMS DEPARTMENT STATE BANK OF PAKISTAN Table of Contents PREFACE... 3 DEFINITIONS... 4 1. SCOPE OF THE REGULATIONS... 6 2. INTERNET BANKING SECURITY
Electronic Data Capture - MACRO. Anja Fischer, Thomas Müller
 Electronic Data Capture - MACRO Anja Fischer, Thomas Müller Agenda Purpose of Electronic Data Capture (EDC) Regulatory Requirements EDC-System MACRO Clinical Trial Support for ELN by WP 3 (CICS) Costs
Electronic Data Capture - MACRO Anja Fischer, Thomas Müller Agenda Purpose of Electronic Data Capture (EDC) Regulatory Requirements EDC-System MACRO Clinical Trial Support for ELN by WP 3 (CICS) Costs
Decision on adequate information system management. (Official Gazette 37/2010)
 Decision on adequate information system management (Official Gazette 37/2010) Pursuant to Article 161, paragraph (1), item (3) of the Credit Institutions Act (Official Gazette 117/2008, 74/2009 and 153/2009)
Decision on adequate information system management (Official Gazette 37/2010) Pursuant to Article 161, paragraph (1), item (3) of the Credit Institutions Act (Official Gazette 117/2008, 74/2009 and 153/2009)
FINAL May 2005. Guideline on Security Systems for Safeguarding Customer Information
 FINAL May 2005 Guideline on Security Systems for Safeguarding Customer Information Table of Contents 1 Introduction 1 1.1 Purpose of Guideline 1 2 Definitions 2 3 Internal Controls and Procedures 2 3.1
FINAL May 2005 Guideline on Security Systems for Safeguarding Customer Information Table of Contents 1 Introduction 1 1.1 Purpose of Guideline 1 2 Definitions 2 3 Internal Controls and Procedures 2 3.1
Procedure Title: TennDent HIPAA Security Awareness and Training
 Procedure Title: TennDent HIPAA Security Awareness and Training Number: TD-QMP-P-7011 Subject: Security Awareness and Training Primary Department: TennDent Effective Date of Procedure: 9/23/2011 Secondary
Procedure Title: TennDent HIPAA Security Awareness and Training Number: TD-QMP-P-7011 Subject: Security Awareness and Training Primary Department: TennDent Effective Date of Procedure: 9/23/2011 Secondary
SERVICE SCHEDULE INFRASTRUCTURE AND PLATFORM SERVICES
 SERVICE SCHEDULE INFRASTRUCTURE AND PLATFORM SERVICES This Product Schedule Terms & Conditions is incorporated into a Services Agreement also comprising the General Terms and Conditions which the Customer
SERVICE SCHEDULE INFRASTRUCTURE AND PLATFORM SERVICES This Product Schedule Terms & Conditions is incorporated into a Services Agreement also comprising the General Terms and Conditions which the Customer
University of California, Riverside Computing and Communications. IS3 Local Campus Overview Departmental Planning Template
 University of California, Riverside Computing and Communications IS3 Local Campus Overview Departmental Planning Template Last Updated April 21 st, 2011 Table of Contents: Introduction Security Plan Administrative
University of California, Riverside Computing and Communications IS3 Local Campus Overview Departmental Planning Template Last Updated April 21 st, 2011 Table of Contents: Introduction Security Plan Administrative
Supplier Information Security Addendum for GE Restricted Data
 Supplier Information Security Addendum for GE Restricted Data This Supplier Information Security Addendum lists the security controls that GE Suppliers are required to adopt when accessing, processing,
Supplier Information Security Addendum for GE Restricted Data This Supplier Information Security Addendum lists the security controls that GE Suppliers are required to adopt when accessing, processing,
Corporate ICT Availability
 Policy Corporate ICT Availability Please note this policy is mandatory and staff are required to adhere to the content Summary DECD ICT facilities and information must be available during agreed operational
Policy Corporate ICT Availability Please note this policy is mandatory and staff are required to adhere to the content Summary DECD ICT facilities and information must be available during agreed operational
Domain 1 The Process of Auditing Information Systems
 Certified Information Systems Auditor (CISA ) Certification Course Description Our 5-day ISACA Certified Information Systems Auditor (CISA) training course equips information professionals with the knowledge
Certified Information Systems Auditor (CISA ) Certification Course Description Our 5-day ISACA Certified Information Systems Auditor (CISA) training course equips information professionals with the knowledge
ensure prompt restart of critical applications and business activities in a timely manner following an emergency or disaster
 Security Standards Symantec shall maintain administrative, technical, and physical safeguards for the Symantec Network designed to (i) protect the security and integrity of the Symantec Network, and (ii)
Security Standards Symantec shall maintain administrative, technical, and physical safeguards for the Symantec Network designed to (i) protect the security and integrity of the Symantec Network, and (ii)
Archiving of Research Documentation
 Suspension, Termination & Completion Standard Operating Procedure VERSION / REVISION: 2.0 EFFECTIVE DATE: 28 05 12 REVIEW DATE: 28 05 14 AUTHOR(S): CONTROLLER: APPROVED BY: Clinical Trials Manager; Recruitment
Suspension, Termination & Completion Standard Operating Procedure VERSION / REVISION: 2.0 EFFECTIVE DATE: 28 05 12 REVIEW DATE: 28 05 14 AUTHOR(S): CONTROLLER: APPROVED BY: Clinical Trials Manager; Recruitment
GCP INSPECTORS WORKING GROUP <DRAFT> REFLECTION PAPER ON EXPECTATIONS FOR ELECTRONIC SOURCE DOCUMENTS USED IN CLINICAL TRIALS
 European Medicines Agency London, 17 October 2007 Doc. Ref. EMEA/505620/2007 GCP INSPECTORS WORKING GROUP REFLECTION PAPER ON EXPECTATIONS FOR ELECTRONIC SOURCE DOCUMENTS USED IN CLINICAL TRIALS
European Medicines Agency London, 17 October 2007 Doc. Ref. EMEA/505620/2007 GCP INSPECTORS WORKING GROUP REFLECTION PAPER ON EXPECTATIONS FOR ELECTRONIC SOURCE DOCUMENTS USED IN CLINICAL TRIALS
HIPAA Compliance Guide
 HIPAA Compliance Guide Important Terms Covered Entities (CAs) The HIPAA Privacy Rule refers to three specific groups as covered entities, including health plans, healthcare clearinghouses, and health care
HIPAA Compliance Guide Important Terms Covered Entities (CAs) The HIPAA Privacy Rule refers to three specific groups as covered entities, including health plans, healthcare clearinghouses, and health care
Master Document Audit Program
 Activity Code 11510 B-1 Planning Considerations Information Technology General System Controls Audit Specific Independence Determination Members of the audit team and internal specialists consulting on
Activity Code 11510 B-1 Planning Considerations Information Technology General System Controls Audit Specific Independence Determination Members of the audit team and internal specialists consulting on
Validating Enterprise Systems: A Practical Guide
 Table of Contents Validating Enterprise Systems: A Practical Guide Foreword 1 Introduction The Need for Guidance on Compliant Enterprise Systems What is an Enterprise System The Need to Validate Enterprise
Table of Contents Validating Enterprise Systems: A Practical Guide Foreword 1 Introduction The Need for Guidance on Compliant Enterprise Systems What is an Enterprise System The Need to Validate Enterprise
Tk20 Backup Procedure
 Tk20 Backup Procedure 1 TK20 BACKUP PROCEDURE OVERVIEW 3 FEATURES AND ADVANTAGES: 3 TK20 BACKUP PROCEDURE 4 DAILY BACKUP CREATION 4 TRANSFER OF BACKUPS 5 AUDITING PROCESS 5 BACKUP REPOSITORY 5 WRITE TO
Tk20 Backup Procedure 1 TK20 BACKUP PROCEDURE OVERVIEW 3 FEATURES AND ADVANTAGES: 3 TK20 BACKUP PROCEDURE 4 DAILY BACKUP CREATION 4 TRANSFER OF BACKUPS 5 AUDITING PROCESS 5 BACKUP REPOSITORY 5 WRITE TO
MICHIGAN AUDIT REPORT OFFICE OF THE AUDITOR GENERAL THOMAS H. MCTAVISH, C.P.A. AUDITOR GENERAL
 MICHIGAN OFFICE OF THE AUDITOR GENERAL AUDIT REPORT THOMAS H. MCTAVISH, C.P.A. AUDITOR GENERAL ...The auditor general shall conduct post audits of financial transactions and accounts of the state and of
MICHIGAN OFFICE OF THE AUDITOR GENERAL AUDIT REPORT THOMAS H. MCTAVISH, C.P.A. AUDITOR GENERAL ...The auditor general shall conduct post audits of financial transactions and accounts of the state and of
SOP Number: SOP-QA-20 Version No: 1. Author: Date: 1-9-15 (Patricia Burns, Research Governance Manager, University of Aberdeen)
 Standard Operating Procedure: SOP Number: SOP-QA-20 Version No: 1 Author: Date: 1-9-15 (Patricia Burns, Research Governance Manager, University of Aberdeen) Approved by: Date: 1-9-15 (Professor Julie Brittenden,
Standard Operating Procedure: SOP Number: SOP-QA-20 Version No: 1 Author: Date: 1-9-15 (Patricia Burns, Research Governance Manager, University of Aberdeen) Approved by: Date: 1-9-15 (Professor Julie Brittenden,
Protecting Official Records as Evidence in the Cloud Environment. Anne Thurston
 Protecting Official Records as Evidence in the Cloud Environment Anne Thurston Introduction In a cloud computing environment, government records are held in virtual storage. A service provider looks after
Protecting Official Records as Evidence in the Cloud Environment Anne Thurston Introduction In a cloud computing environment, government records are held in virtual storage. A service provider looks after
SUPPLIER SECURITY STANDARD
 SUPPLIER SECURITY STANDARD OWNER: LEVEL 3 COMMUNICATIONS AUTHOR: LEVEL 3 GLOBAL SECURITY AUTHORIZER: DALE DREW, CSO CURRENT RELEASE: 12/09/2014 Purpose: The purpose of this Level 3 Supplier Security Standard
SUPPLIER SECURITY STANDARD OWNER: LEVEL 3 COMMUNICATIONS AUTHOR: LEVEL 3 GLOBAL SECURITY AUTHORIZER: DALE DREW, CSO CURRENT RELEASE: 12/09/2014 Purpose: The purpose of this Level 3 Supplier Security Standard
IT - General Controls Questionnaire
 IT - General Controls Questionnaire Internal Control Questionnaire Question Yes No N/A Remarks G1. ACCESS CONTROLS Access controls are comprised of those policies and procedures that are designed to allow
IT - General Controls Questionnaire Internal Control Questionnaire Question Yes No N/A Remarks G1. ACCESS CONTROLS Access controls are comprised of those policies and procedures that are designed to allow
UNIVERSITY OF LEICESTER, UNIVERSITY OF LOUGHBOROUGH & UNIVERSITY HOSPITALS OF LEICESTER NHS TRUST JOINT RESEARCH & DEVELOPMENT SUPPORT OFFICE
 UNIVERSITY OF LEICESTER, UNIVERSITY OF LOUGHBOROUGH & UNIVERSITY HOSPITALS OF LEICESTER NHS TRUST JOINT RESEARCH & DEVELOPMENT SUPPORT OFFICE STANDARD OPERATING PROCEDURES University of Leicester (UoL)
UNIVERSITY OF LEICESTER, UNIVERSITY OF LOUGHBOROUGH & UNIVERSITY HOSPITALS OF LEICESTER NHS TRUST JOINT RESEARCH & DEVELOPMENT SUPPORT OFFICE STANDARD OPERATING PROCEDURES University of Leicester (UoL)
CLINICAL DATA MANAGEMENT
 J * Edition Practical Guide to CLINICAL DATA MANAGEMENT Susanne Prokscha (g) CRC Press Taylor Francis Croup London York CRC Press is an imprint of the Taylor Francis Croup, an buslness Preface Introduction
J * Edition Practical Guide to CLINICAL DATA MANAGEMENT Susanne Prokscha (g) CRC Press Taylor Francis Croup London York CRC Press is an imprint of the Taylor Francis Croup, an buslness Preface Introduction
U.S. Department of the Interior's Federal Information Systems Security Awareness Online Course
 U.S. Department of the Interior's Federal Information Systems Security Awareness Online Course Rules of Behavior Before you print your certificate of completion, please read the following Rules of Behavior
U.S. Department of the Interior's Federal Information Systems Security Awareness Online Course Rules of Behavior Before you print your certificate of completion, please read the following Rules of Behavior
