FOR ACCESS CONTROL TECHNOLOGIES Docket No
|
|
|
- Alexander May
- 8 years ago
- Views:
Transcription
1 BEFORE THE UNITED STATES COPYRIGHT OFFICE LIBRARY OF CONGRESS PETITION OF A COALITION OF MEDICAL DEVICE RESEARCHERS FOR EXEMPTION TO PROHIBITION ON CIRCUMVENTION OF COPYRIGHT PROTECTION SYSTEMS Submitted by: Andrew F. Sellars Cyberlaw Clinic Berkman Center for Internet & Society Harvard Law School 23 Everett Street, Second Floor Cambridge, MA asellars@cyber.law.harvard.edu FOR ACCESS CONTROL TECHNOLOGIES Docket No A coalition of medical device patients and researchers (the Medical Device Research Coalition ) 1 submits this petition in response to the Notice of Inquiry on Exemption to Prohibition on Circumvention of Copyright Protection Systems for Access Control Technologies, 79 Fed. Reg. 55,687 (Sept. 17, 2014). Brief Overview of the Exemption The members of the Medical Device Research Coalition study the safety, security, and effectiveness of networked medical devices that are either implanted or attached to the body. This Coalition includes researchers who study device security at the design level, as well as those who study the safety and effectiveness of devices they personally use. Such research often requires the researcher to access the underlying source code and outputs from these devices, and device manufacturers are increasingly employing technologies that courts may classify as technological protection measures under 1201 of the Copyright Act. In order to make sure that this form of critical research continues, the Medical Device Research Coalition proposes the following exemption: Computer programs, in the form of firmware or software, including the outputs generated by those programs, that are contained within or generated by medical devices and their corresponding monitoring systems, when such devices are designed for attachment to or implantation in patients, and where such circumvention is at the 1 This coalition includes Hugo Campos, Stanford Medicine X; Jerome Radcliffe, Rapid7; Karen Sandler, Software Freedom Conservancy; and Benjamin West, an independent device researcher. The institutional affiliations provided here are for identification purposes only. 1 of 5
2 direction of a patient seeking access to information generated by his or her own device or at the direction of those conducting research into the safety, security, and effectiveness of such devices. Works Sought to Be Accessed in Medical Device Security Research This exemption seeks to allow researchers to access literary works, in particular, the software and firmware of medical devices and their corresponding monitoring systems, as well the outputs generated by such devices and monitoring systems, 2 when such devices are capable of transmitting or receiving information and are designed to be attached to or implanted in patients. Examples of such devices include pacemakers, implantable cardioverter defibrillators ( ICDs ), insulin pumps, and continuous glucose monitors ( CGMs ). Technological Protection Measures Governing Access to Medical Devices Many medical device manufacturers use measures to control access that a court may conclude are technological protection measures for purposes of The measures identified are wideranging, and include, inter alia, encryption on the outputs on medical devices or home monitoring systems, 3 password systems that control access to patient management software and devices, 4 and proprietary software and tools for extracting device information. 5 There is strong reason to believe that adoption of such technologies will increase over the coming three years. The Food and Drug Administration ( FDA ) published new guidelines last month for premarket submission of medical devices, which asks medical device manufacturers to implement and identify their technological security measures. 6 Such guidelines, while not 2 Many outputs on medical devices are not protectable as copyrighted works, and circumvention of protection measures over such data outputs would not implicate anti-circumvention law. See 17 U.S.C. 1201(a)(1)(A) (protecting only a work protected under this title ). This petition therefore seeks an exemption to circumvent the outputs of devices to the extent that a court ever would find the readouts of such devices to be embodied in a protectable expression. 3 See, e.g., VITALIO Pacemaker, BOSTON SCIENTIFIC, EU/products/pacemakers/vitalio.html (last visited Nov. 2, 2014); BIOTRONIK: Home Monitoring Service Center, BIOTRONIK, connect/en_us_web/biotronik/sub_top/patients/dianostics_and_therapies/home_montitoring (last visited Nov. 2, 2014). 4 See, e.g., Medtronic Paceart System: Technical Features, MEDTRONIC, (last visited Nov. 2, 2014). 5 See, e.g., BIOTRONIK, BIOTRONIK EHR DATASYNC FAQ 6 (2010), available at EHR_overview_EN_03Dec2010.pdf. 6 U.S. FOOD AND DRUG ADMINISTRATION, CONTENT OF PREMARKET SUBMISSIONS FOR MANAGEMENT OF CYBERSECURITY IN MEDICAL DEVICES: GUIDANCE FOR INDUSTRY AND FOOD AND DRUG ADMINISTRATION STAFF 3 (Oct. 2, 2014), available at 2 of 5
3 binding on medical device manufacturers, are highly likely to influence the development of these devices in the immediate future. Access of Medical Device Source Code and Outputs for General and Patient-Specific Research Does Not Infringe Copyright Members of the Medical Device Research Coalition and others in their fields use the computer programs and data outputs of medical devices to analyze the security and effectiveness of medical devices, both as patients analyzing devices they use for therapy and treatment and as general researchers. 7 The use of these works for purposes of researching and critiquing the safety and effectiveness of the devices that use them is clearly a lawful use, either as a fair use or as a use that only copies unprotectable elements from the underlying works. Accessing the source code and outputs of medical devices usually entails a form of reverse engineering, where a researcher intercepts the internally or externally transmitted binary object code of the program, and then uses a mix of techniques to reveal the underlying source code or data. 8 The act of reverse engineering might include the creation of incidental copies of the underlying work, but some techniques do not copy the underlying software or outputs at all. If a researcher only copies the data points from these devices, the user does not implicate the copyright owner s exclusive rights whatsoever. 9 Even if a researcher were to copy the full reports of data from a device, such reports may not have the necessary creativity in selection or arrangement of information to be protectable under the Copyright Act. 10 If a researcher does in fact copy protectable expression, it is usually only an interim copy made to access and examine the functional elements of the code for testing and analysis. Courts have held such interim copies to be a fair use. 11 A federal appellate court has also held the copying of a protectable database in order to extract the underlying unprotected data to be a fair use. 12 ments/ucm pdf ( Manufacturers should develop a set of cybersecurity controls to assure medical device cybersecurity and maintain medical device functionality and safety. ). 7 Examples of this sort of research and analysis include Wayne Burleson et al., Design Challenges for Secure Implantable Medical Devices, 49TH DESIGN AUTOMATION CONFERENCE 12, (June 2012); Hugo Campos, Hugo Campos Fights for the Right to Open His Heart's Data, TEDXCAMBRIDGE (Jan 20, 2012), Campos-fight. 8 ELDAD EILAM, REVERSING: SECRETS OF REVERSE ENGINEERING (2005). The term reverse engineering is meant in the more general way it is used in the computer science community, instead of the specific interoperability context used in the statute. See 1201(f). For a specific application of these techniques in an implantable medical device context, see Daniel Halperin et al., Pacemakers and Implantable Cardiac Defibrillators: Software Radio Attacks and Zero-Power Defenses, IEEE SYMPOSIUM ON SECURITY & PRIVACY 129, (2008). 9 See, e.g., N.Y. Mercantile Exch., Inc. v. IntercontinentalExchange, Inc., 497 F.3d 109, (2d Cir. 2007). 10 See id. 11 See, e.g., Sony Comp. Ent. v. Connectix Corp., 203 F.3d 596, 599 (9th Cir. 2000); Sega Enters. Ltd. v. Accolade, Inc., 977 F.2d 1510, 1526 (9th Cir. 1992). 12 Assessment Techs. of WI, LLC v. WIREdata, LLC, 350 F.3d 640, (7th Cir. 2003). 3 of 5
4 The case for fair use is even stronger given that the uses here are so highly transformative. 13 The purpose of a medical device company s source code is to enable the function of a medical device; researchers like the members of this Coalition use the code in order to publish criticism on how safe, secure, and effective these products actually are. The use therefore resembles that of a book critic quoting excerpts of a book in her critique, a paradigm case of fair use. 14 The proposed uses also would never supplant the market for the original work. Only the original work (as embodied in a medical device) will administer therapy to a patient. To the extent research and criticism diminishes the market for the original work, it is only due to the effectiveness of such criticism, which is rightly excluded from a fair use analysis. 15 Allowing Anti-Circumvention Claims in this Area would Adversely Affect Critical Medical Research Developing safe, secure, and effective medical devices is a national priority. President Obama has declared it the policy of the United States to increase the volume, timeliness, and quality of cyber threat information, and has identified the Healthcare and Public Health sector as an industry of specific emphasis. 16 The FDA, in turn, has sought broad input from the Healthcare and Public Health... Sector on medical device and healthcare cybersecurity. 17 While the injuries resulting from device flaws to date have been due to design and programming errors rather than malicious attacks, medical device security is a pressing concern. According to recent reports, the Department of Homeland Security is currently conducting confidential investigations into some two dozen cases of cyber-security flaws in medical devices. 18 Hundreds of recalls of software-based medical devices have been issued previously, affecting over a million devices. More than 11% of all medical device recalls between 1999 and 2005 were attributed to software failures. 19 Medical device companies actively research these vulnerabilities, but it is often independent researchers, and not the manufacturers, that discover flaws, identify solutions, and inform the 13 See generally Neil Weinstock Netanel, Making Sense of Fair Use, 15 LEWIS & CLARK L. REV. 715, (2011) (noting that findings of transformativeness, while neither necessary nor dispositive, drive judicial analysis of fair use today). 14 Wainwright Securities, Inc. v. Wall St. Transcript Copr.,558 F.2d 91, 94 (2d Cir. 1977). 15 See, e.g., New Era Publ ns Int l v. Carol Publ g Grp., 904 F.2d 152, 160 (2d Cir. 1990). 16 See Exec. Order 13,636, 78 Fed. Reg. 11,739 (Feb. 12, 2013); WHITE HOUSE OFFICE OF THE PRESS SEC Y, PRESIDENTIAL POLICY DIRECTIVE 21 CRITICAL INFRASTRUCTURE SECURITY AND RESILIENCE (Feb. 12, 2013), available at 17 See Collaborative Approaches for Medical Device and Healthcare Cybersecurity; Public Workshop; Request for Comments, 79 Fed. Reg. 56,814, 58,814 (Sept. 23, 2014). 18 Jim Finkle, U.S. Government Probes Medical Devices for Possible Cyber Flaws, RECODE (Oct. 22, 2014), 19 Kevin Fu, Trustworthy Medical Device Software, in PUBLIC HEALTH EFFECTIVENESS OF THE FDA 510(K) CLEARANCE PROCESS (2011). 4 of 5
5 government and public about safety hazards in devices. For example, a group of university researchers in 2008 demonstrated for the first time that radio signals could be used to intercept communications from an ICD and reprogram the device, potentially with lethal effects. 20 Similar independent research showed potentially lethal vulnerabilities in insulin pumps. 21 These and other studies were cited in the Government Accountability Office s subsequent report to Congress on medical device security, which encouraged the FDA to expand its consideration of information security in its approval process. 22 On the individual monitoring level, a growing number of patients and medical professionals are demonstrating how patients can improve the effectiveness of their treatment outcomes by actively monitoring their own information. 23 Such research is rarely if ever done with manufacturer consent or authorization, no doubt in part due to the fact that discovery of flaws could lead to costly recalls, FDA investigations, and class action lawsuits. 24 Indeed, some companies have been shown to actively hide known vulnerabilities from patients and doctors. 25 Allowing independent research into this space is essential for public health and safety, and should be allowed to extend to those devices that employ technological measures to obfuscate their code or data outputs. Conclusion For the above reasons, the Medical Device Research Coalition requests that the Copyright Office include the exemption described above as part of its notice of proposed rulemaking. Respectfully submitted, Andrew F. Sellars Cyberlaw Clinic On behalf of Hugo Campos, Jerome Radcliffe, Karen Sandler, and Benjamin West See Halperin, supra note Jordan Robertson, The Trials of a Diabetic Hacker, BUSINESSWEEK.COM (Feb. 23, 2012), 22 U.S. GOV T ACCOUNTABILITY OFFICE, MEDICAL DEVICES: FDA SHOULD EXPAND ITS CONSIDERATION OF INFORMATION SECURITY FOR CERTAIN TYPES OF DEVICES (Aug. 2012), available at 23 See Campos, supra note For example, a 2005 investigation into device errors on infusion pumps made by Baxter Healthcare Corp. lead to massive recalls and fees for the company. See FDA Issues Statement on Baxter s Recall of Colleague Infusion Pumps, U.S. FOOD AND DRUG ADMINISTRATION (May 3, 2010), 25 For tragic examples of this, see Barry Meier, Maker of Heart Device Kept Flaw From Doctors, NEW YORK TIMES (May 24, 2005), 26 The Coalition with to thank Cyberlaw Clinic students Evita Grant and Megan Michaels for their invaluable contributions to this petition. 5 of 5
Multimedia evidence is not being provided in connection with this comment. These comments are submitted by the Coalition through their counsel:
 BEFORE THE UNITED STATES COPYRIGHT OFFICE LIBRARY OF CONGRESS LONG COMMENT REGARDING A PROPOSED EXEMPTION UNDER 17 U.S.C. 1201 Docket No. 2014-07 COMMENT OF A COALITION OF MEDICAL DEVICE RESEARCHERS IN
BEFORE THE UNITED STATES COPYRIGHT OFFICE LIBRARY OF CONGRESS LONG COMMENT REGARDING A PROPOSED EXEMPTION UNDER 17 U.S.C. 1201 Docket No. 2014-07 COMMENT OF A COALITION OF MEDICAL DEVICE RESEARCHERS IN
August 18, 2015. Re: Section 1201 Rulemaking Proposed Exemption for Medical Devices
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 August 18, 2015 Ms. Jacqueline C. Charlesworth General Counsel
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 August 18, 2015 Ms. Jacqueline C. Charlesworth General Counsel
White Paper on Medical Device Security. October 2013
 White Paper on Medical Device Security October 2013 TABLE OF CONTENTS Abstract... 3 Abbreviations... 4 Background... 5 Market... 7 Evidence of Lax Security Measures... 9 Challenges... 11 Solution... 13
White Paper on Medical Device Security October 2013 TABLE OF CONTENTS Abstract... 3 Abbreviations... 4 Background... 5 Market... 7 Evidence of Lax Security Measures... 9 Challenges... 11 Solution... 13
GAO MEDICAL DEVICES. FDA Should Expand Its Consideration of Information Security for Certain Types of Devices. Report to Congressional Requesters
 GAO United States Government Accountability Office Report to Congressional Requesters August 2012 MEDICAL DEVICES FDA Should Expand Its Consideration of Information Security for Certain Types of Devices
GAO United States Government Accountability Office Report to Congressional Requesters August 2012 MEDICAL DEVICES FDA Should Expand Its Consideration of Information Security for Certain Types of Devices
Before the U.S. COPYRIGHT OFFICE, LIBRARY OF CONGRESS
 Before the U.S. COPYRIGHT OFFICE, LIBRARY OF CONGRESS In the matter of Exemption to Prohibition on Circumvention of Copyright Protection Systems for Access Control Technologies Docket No. 2014-07 Petition
Before the U.S. COPYRIGHT OFFICE, LIBRARY OF CONGRESS In the matter of Exemption to Prohibition on Circumvention of Copyright Protection Systems for Access Control Technologies Docket No. 2014-07 Petition
Security and Privacy of Wireless Implantable Medical Devices
 Security and Privacy of Wireless Implantable Medical Devices Security Forum 2013 Hagenberg, 17.04.2013 Dipl.-Ing. Dr. Gregor Koenig Outlook Overview Device Hacking Safety & Utility Goals Security & Privacy
Security and Privacy of Wireless Implantable Medical Devices Security Forum 2013 Hagenberg, 17.04.2013 Dipl.-Ing. Dr. Gregor Koenig Outlook Overview Device Hacking Safety & Utility Goals Security & Privacy
New Devices Mean New Risks: The Potential for Liability When Software is a Component of Medical Devices. September 25, 2013
 New Devices Mean New Risks: The Potential for Liability When Software is a Component of Medical Devices September 25, 2013 The Hartford Insuring Innovation Joe Coray Dan Silverman Providing insurance solutions
New Devices Mean New Risks: The Potential for Liability When Software is a Component of Medical Devices September 25, 2013 The Hartford Insuring Innovation Joe Coray Dan Silverman Providing insurance solutions
Unpatchable: Living with a vulnerable implanted device
 Safer Sooner Together Unpatchable: Living with a vulnerable implanted device Marie Moe, PhD, Research ScienAst at SINTEF @MarieGMoe @iamthecavalry #safersoonertogether SINTEF ICT Safer Sooner Together
Safer Sooner Together Unpatchable: Living with a vulnerable implanted device Marie Moe, PhD, Research ScienAst at SINTEF @MarieGMoe @iamthecavalry #safersoonertogether SINTEF ICT Safer Sooner Together
DOES INTERMEDIATE COPYING OF COMPUTER SOFTWARE FOR THE PURPOSE OF REVERSE ENGINEERING A NON-INFRINGING PRODUCT INFRINGE THE COPYRIGHT IN THE SOFTWARE?
 DOES INTERMEDIATE COPYING OF COMPUTER SOFTWARE FOR THE PURPOSE OF REVERSE ENGINEERING A NON-INFRINGING PRODUCT INFRINGE THE COPYRIGHT IN THE SOFTWARE? Robert V. Donahoe INTRODUCTION Researchers and engineers
DOES INTERMEDIATE COPYING OF COMPUTER SOFTWARE FOR THE PURPOSE OF REVERSE ENGINEERING A NON-INFRINGING PRODUCT INFRINGE THE COPYRIGHT IN THE SOFTWARE? Robert V. Donahoe INTRODUCTION Researchers and engineers
Cyber Insurance and Your Data Ted Claypoole, Partner, Womble Carlyle and Jack Freund, PhD, InfoSec Mgr, TIAA-CREF
 Cyber Insurance and Your Data Ted Claypoole, Partner, Womble Carlyle and Jack Freund, PhD, InfoSec Mgr, TIAA-CREF October 9, 2013 1 Cyber Insurance Why? United States Department of Commerce: Cyber Insurance
Cyber Insurance and Your Data Ted Claypoole, Partner, Womble Carlyle and Jack Freund, PhD, InfoSec Mgr, TIAA-CREF October 9, 2013 1 Cyber Insurance Why? United States Department of Commerce: Cyber Insurance
John O. Brennan Central Intelligence Agency Office of Public Affairs Washington, D.C. 20505. November 4, 2015. Mr. Brennan:
 John O. Brennan Central Intelligence Agency Office of Public Affairs Washington, D.C. 20505 November 4, 2015 Mr. Brennan: On March 31, 2015 several organizations called on the Central Intelligence Agency
John O. Brennan Central Intelligence Agency Office of Public Affairs Washington, D.C. 20505 November 4, 2015 Mr. Brennan: On March 31, 2015 several organizations called on the Central Intelligence Agency
UNITED STATES TAX COURT. LATTICE SEMICONDUCTOR CORPORATION AND SUBSIDIARIES, Petitioner v. COMMISSIONER OF INTERNAL REVENUE, Respondent
 T.C. Memo. 2011-100 UNITED STATES TAX COURT LATTICE SEMICONDUCTOR CORPORATION AND SUBSIDIARIES, Petitioner v. COMMISSIONER OF INTERNAL REVENUE, Respondent Docket No. 13109-08. Filed May 9, 2011. Steven
T.C. Memo. 2011-100 UNITED STATES TAX COURT LATTICE SEMICONDUCTOR CORPORATION AND SUBSIDIARIES, Petitioner v. COMMISSIONER OF INTERNAL REVENUE, Respondent Docket No. 13109-08. Filed May 9, 2011. Steven
AUTHORED BY: George W. Gray CTO, VP Software & Information Systems Ivenix, Inc. ADDRESSING CYBERSECURITY IN INFUSION DEVICES
 AUTHORED BY: George W. Gray CTO, VP Software & Information Systems Ivenix, Inc. ADDRESSING CYBERSECURITY IN INFUSION DEVICES INTRODUCTION Cybersecurity has become an increasing concern in the medical device
AUTHORED BY: George W. Gray CTO, VP Software & Information Systems Ivenix, Inc. ADDRESSING CYBERSECURITY IN INFUSION DEVICES INTRODUCTION Cybersecurity has become an increasing concern in the medical device
MEDICAL DEVICE Cybersecurity.
 MEDICAL DEVICE Cybersecurity. 2 MEDICAL DEVICE CYBERSECURITY Introduction Wireless technology and the software in medical devices have greatly increased healthcare providers abilities to efficiently and
MEDICAL DEVICE Cybersecurity. 2 MEDICAL DEVICE CYBERSECURITY Introduction Wireless technology and the software in medical devices have greatly increased healthcare providers abilities to efficiently and
COMMENTS OF THE ELECTRONIC PRIVACY INFORMATION CENTER
 COMMENTS OF THE ELECTRONIC PRIVACY INFORMATION CENTER to THE CYBER SECURITY AND INFORMATION ASSURANCE RESEARCH AND DEVELOPMENT SENIOR STEERING GROUP OF THE FEDERAL NETWORKING AND INFROMATION TECHNOLOGY
COMMENTS OF THE ELECTRONIC PRIVACY INFORMATION CENTER to THE CYBER SECURITY AND INFORMATION ASSURANCE RESEARCH AND DEVELOPMENT SENIOR STEERING GROUP OF THE FEDERAL NETWORKING AND INFROMATION TECHNOLOGY
Auto Care and the Copyright Conundrum
 Short Comment of the Auto Care Association and the Automotive Parts Remanufacturers Association in Neither Support Nor Opposition to Proposed Exemption Under 17 U.S.C. 1201 (Proposed Exemption 21 Vehicle
Short Comment of the Auto Care Association and the Automotive Parts Remanufacturers Association in Neither Support Nor Opposition to Proposed Exemption Under 17 U.S.C. 1201 (Proposed Exemption 21 Vehicle
THE FREEDOM OF INFORMATION ACT (FOIA) AND DISCOVERY TWO DIFFERENT AVENUES FOR ACCESSING AGENCY RECORDS AND THE BENEFITS OF LEVERAGING E-
 THE FREEDOM OF INFORMATION ACT (FOIA) AND DISCOVERY TWO DIFFERENT AVENUES FOR ACCESSING AGENCY RECORDS AND THE BENEFITS OF LEVERAGING E- DISCOVERY TOOLS FOR FOIA The Freedom of Information Act (FOIA) and
THE FREEDOM OF INFORMATION ACT (FOIA) AND DISCOVERY TWO DIFFERENT AVENUES FOR ACCESSING AGENCY RECORDS AND THE BENEFITS OF LEVERAGING E- DISCOVERY TOOLS FOR FOIA The Freedom of Information Act (FOIA) and
Before the Federal Communications Commission Washington, D.C. 20554
 Before the Federal Communications Commission Washington, D.C. 20554 In the Matter of ) ) Inquiry Concerning High Speed Access to ) GN Docket No. 00-185 the Internet Over Cable and Other Facilities ) Proprietary
Before the Federal Communications Commission Washington, D.C. 20554 In the Matter of ) ) Inquiry Concerning High Speed Access to ) GN Docket No. 00-185 the Internet Over Cable and Other Facilities ) Proprietary
MEDICAL DEVICE SECURITY
 Medical devices should be secure. Doctors are gentlemen and therefore their computers are always secure. Dr. Ignaz Semmelweis 1818-1865 Dr. Charles Meigs 1792-1869 St. Jude Medical, the third major defibrillator
Medical devices should be secure. Doctors are gentlemen and therefore their computers are always secure. Dr. Ignaz Semmelweis 1818-1865 Dr. Charles Meigs 1792-1869 St. Jude Medical, the third major defibrillator
The U.S. FDA s Regulation and Oversight of Mobile Medical Applications
 The U.S. FDA s Regulation and Oversight of Mobile Medical Applications The U.S. FDA s Regulation and Oversight of Mobile Medical Applications As smart phones and portable tablet computers become the preferred
The U.S. FDA s Regulation and Oversight of Mobile Medical Applications The U.S. FDA s Regulation and Oversight of Mobile Medical Applications As smart phones and portable tablet computers become the preferred
Software-Enabled Consumer Products Study: Notice and Request for Public Comment
 This document is scheduled to be published in the Federal Register on 12/15/2015 and available online at http://federalregister.gov/a/2015-31411, and on FDsys.gov LIBRARY OF CONGRESS U.S. Copyright Office
This document is scheduled to be published in the Federal Register on 12/15/2015 and available online at http://federalregister.gov/a/2015-31411, and on FDsys.gov LIBRARY OF CONGRESS U.S. Copyright Office
l'tlbl1c COP"t u. S. Citizenship OCT 2 6 2010 and Immigration Services
 identifying data deleted to prevent clearly unwarranted invasion of personal privacy l'tlbl1c COP"t U.S. Department of Homeland Security lj.s. Citizenship and Immigration Services OjJice of Administrative
identifying data deleted to prevent clearly unwarranted invasion of personal privacy l'tlbl1c COP"t U.S. Department of Homeland Security lj.s. Citizenship and Immigration Services OjJice of Administrative
Risk Management and Cybersecurity for Devices that Contain Software. Seth D. Carmody, Ph.D. 12 th Medical Device Quality Congress March 18, 2015
 Risk Management and Cybersecurity for Devices that Contain Software Seth D. Carmody, Ph.D. 12 th Medical Device Quality Congress March 18, 2015 Main Points Establish a Cybersecurity Risk Management Program
Risk Management and Cybersecurity for Devices that Contain Software Seth D. Carmody, Ph.D. 12 th Medical Device Quality Congress March 18, 2015 Main Points Establish a Cybersecurity Risk Management Program
GE Healthcare. Medical Device Product Security. Patient Safety Patient Privacy Patient Care
 GE Healthcare Medical Device Product Security Patient Safety Patient Privacy Patient Care Presentation to the: Information Security and Privacy Advisory Board 03-March-2011 Steven Abrahamson Program Manager,
GE Healthcare Medical Device Product Security Patient Safety Patient Privacy Patient Care Presentation to the: Information Security and Privacy Advisory Board 03-March-2011 Steven Abrahamson Program Manager,
Second Annual Conference September 16, 2015 to September 18, 2015 Chicago, IL
 Second Annual Conference September 16, 2015 to September 18, 2015 Chicago, IL Using Insurance Coverage to Mitigate Cybersecurity Risks To Warranty and Service Contract Businesses Barry Buchman, Partner
Second Annual Conference September 16, 2015 to September 18, 2015 Chicago, IL Using Insurance Coverage to Mitigate Cybersecurity Risks To Warranty and Service Contract Businesses Barry Buchman, Partner
UNITED STATES DISTRICT COURT EASTERN DISTRICT OF WISCONSIN
 UNITED STATES DISTRICT COURT EASTERN DISTRICT OF WISCONSIN LENNETT GENSEL, Plaintiff, -vs- Case No. 13-C-1196 PERFORMANT TECHNOLOGIES, Inc., Defendant. DECISION AND ORDER On January 28, 2015, the Court
UNITED STATES DISTRICT COURT EASTERN DISTRICT OF WISCONSIN LENNETT GENSEL, Plaintiff, -vs- Case No. 13-C-1196 PERFORMANT TECHNOLOGIES, Inc., Defendant. DECISION AND ORDER On January 28, 2015, the Court
S. ll IN THE SENATE OF THE UNITED STATES A BILL
 TH CONGRESS ST SESSION S. ll To codify mechanisms for enabling cybersecurity threat indicator sharing between private and government entities, as well as among private entities, to better protect information
TH CONGRESS ST SESSION S. ll To codify mechanisms for enabling cybersecurity threat indicator sharing between private and government entities, as well as among private entities, to better protect information
Healthcare Information Technology - A Risky Business?
 Healthcare Information Technology A Risky Business? Healthcare Security Summit 12 March 2014 Disclaimers» This presentation is for educational purposes only.» This presentation does not constitute legal
Healthcare Information Technology A Risky Business? Healthcare Security Summit 12 March 2014 Disclaimers» This presentation is for educational purposes only.» This presentation does not constitute legal
DOL Whistleblower Rule Will Have Far-Reaching Effects
 Portfolio Media. Inc. 860 Broadway, 6th Floor New York, NY 10003 www.law360.com Phone: +1 646 783 7100 Fax: +1 646 783 7161 customerservice@law360.com DOL Whistleblower Rule Will Have Far-Reaching Effects
Portfolio Media. Inc. 860 Broadway, 6th Floor New York, NY 10003 www.law360.com Phone: +1 646 783 7100 Fax: +1 646 783 7161 customerservice@law360.com DOL Whistleblower Rule Will Have Far-Reaching Effects
Medical Devices. Safe, but are they secure? Dan Stoker, Consultant Professional Services, Coalfire
 Medical Devices Safe, but are they secure? Dan Stoker, Consultant Professional Services, Coalfire Introduction This perspective paper aims to help organizations understand the emerging issue of security
Medical Devices Safe, but are they secure? Dan Stoker, Consultant Professional Services, Coalfire Introduction This perspective paper aims to help organizations understand the emerging issue of security
The Ethical Implications of NSA Surveillance for Lawyers. David G. Ries Clark Hill Thorp Reed
 The Ethical Implications of NSA Surveillance for Lawyers David G. Ries Clark Hill Thorp Reed 2 3 The June 2013 Headlines: NSA collecting phone records of millions of Verizon customers daily The Guardian,
The Ethical Implications of NSA Surveillance for Lawyers David G. Ries Clark Hill Thorp Reed 2 3 The June 2013 Headlines: NSA collecting phone records of millions of Verizon customers daily The Guardian,
No. 03 Civ. 2183(NRB). Feb. 23, 2004. * * * MEMORANDUM AND ORDER
 307 F.Supp.2d 521 United States District Court, S.D. New York. I.M.S. INQUIRY MANAGEMENT SYSTEMS, LTD., Plaintiff, v. BERKSHIRE INFORMATION SYSTEMS, INC., Defendant. BUCHWALD, District Judge. No. 03 Civ.
307 F.Supp.2d 521 United States District Court, S.D. New York. I.M.S. INQUIRY MANAGEMENT SYSTEMS, LTD., Plaintiff, v. BERKSHIRE INFORMATION SYSTEMS, INC., Defendant. BUCHWALD, District Judge. No. 03 Civ.
Increase In Vulnerabilities Of Mobile Broadband Network Infrastructure
 THE AVALANCHE OF VULNERABILITIES A PERSPECTIVE Mike Ahmadi Global Director of Critical Systems Security, Codenomicon Ltd @codenomicon UNKNOWN VULNERABILITIES ARE BAD KNOWN VULNERABILITIES ARE A HUGE PROBLEM
THE AVALANCHE OF VULNERABILITIES A PERSPECTIVE Mike Ahmadi Global Director of Critical Systems Security, Codenomicon Ltd @codenomicon UNKNOWN VULNERABILITIES ARE BAD KNOWN VULNERABILITIES ARE A HUGE PROBLEM
In This Issue: Finance & Legal Edition. Voice. Cybersecurity Developments Raise Growing Regulatory Concerns For Undersea Cable Industry
 Voice of the Industry 69 m a r 2013 ISSN 1948-3031 Finance & Legal Edition In This Issue: Cybersecurity Developments Raise Growing Regulatory Concerns For Undersea Cable Industry Current Legal Trends And
Voice of the Industry 69 m a r 2013 ISSN 1948-3031 Finance & Legal Edition In This Issue: Cybersecurity Developments Raise Growing Regulatory Concerns For Undersea Cable Industry Current Legal Trends And
Special Topics in Security and Privacy of Medical Information. Reminders. Medical device security. Sujata Garera
 Special Topics in Security and Privacy of Medical Information Sujata Garera Reminders Assignment due today Project part 1 due on next Tuesday Assignment 2 will be online today evening 2nd Discussion session
Special Topics in Security and Privacy of Medical Information Sujata Garera Reminders Assignment due today Project part 1 due on next Tuesday Assignment 2 will be online today evening 2nd Discussion session
COMMENTS OF THE ELECTRONIC PRIVACY INFORMATION CENTER THE DEPARTMENT OF DEFENSE [DOD-2009-OS-0183/RIN 0790-AI60]
![COMMENTS OF THE ELECTRONIC PRIVACY INFORMATION CENTER THE DEPARTMENT OF DEFENSE [DOD-2009-OS-0183/RIN 0790-AI60] COMMENTS OF THE ELECTRONIC PRIVACY INFORMATION CENTER THE DEPARTMENT OF DEFENSE [DOD-2009-OS-0183/RIN 0790-AI60]](/thumbs/25/5930167.jpg) COMMENTS OF THE ELECTRONIC PRIVACY INFORMATION CENTER to THE DEPARTMENT OF DEFENSE Defense Industrial Base (DIB) Voluntary Cyber Security and Information Assurance (CS/IA) Activities By notice published
COMMENTS OF THE ELECTRONIC PRIVACY INFORMATION CENTER to THE DEPARTMENT OF DEFENSE Defense Industrial Base (DIB) Voluntary Cyber Security and Information Assurance (CS/IA) Activities By notice published
Outline to HIPAA presentation
 Outline to HIPAA presentation I) Overview of the HIPAA Privacy Rule regulations that relate to obtaining a person s medical records for court proceedings and law enforcement purposes. A) Entities covered
Outline to HIPAA presentation I) Overview of the HIPAA Privacy Rule regulations that relate to obtaining a person s medical records for court proceedings and law enforcement purposes. A) Entities covered
Technical Help Desk Terms of Service
 Technical Help Desk Terms of Service This esecuritel Technical Help Desk Terms of Service (the Agreement ) is provided in connection with the eligible tablet enrolled in either the Advanced Protection
Technical Help Desk Terms of Service This esecuritel Technical Help Desk Terms of Service (the Agreement ) is provided in connection with the eligible tablet enrolled in either the Advanced Protection
Special Topics in Security and Privacy of Medical Information. Reminders. Last lecture: Recap. Sujata Garera. Project part 1 submission
 Special Topics in Security and Privacy of Medical Information Sujata Garera Reminders Project part 1 submission Assignment 2 is online Last lecture: Recap Medical Telemetry Infrastructure Devices capturing
Special Topics in Security and Privacy of Medical Information Sujata Garera Reminders Project part 1 submission Assignment 2 is online Last lecture: Recap Medical Telemetry Infrastructure Devices capturing
NATIONAL CYBERSECURITY PROTECTION ACT OF 2014
 PUBLIC LAW 113 282 DEC. 18, 2014 NATIONAL CYBERSECURITY PROTECTION ACT OF 2014 VerDate Mar 15 2010 21:01 Feb 12, 2015 Jkt 049139 PO 00282 Frm 00001 Fmt 6579 Sfmt 6579 E:\PUBLAW\PUBL282.113 PUBL282 128
PUBLIC LAW 113 282 DEC. 18, 2014 NATIONAL CYBERSECURITY PROTECTION ACT OF 2014 VerDate Mar 15 2010 21:01 Feb 12, 2015 Jkt 049139 PO 00282 Frm 00001 Fmt 6579 Sfmt 6579 E:\PUBLAW\PUBL282.113 PUBL282 128
THE SCHOOL DISTRICT OF PHILADELPHIA By: Miles H. Shore, Esquire Identification No. 03274 440 N. Broad Street, Suite 313 Attorney for Appellant
 Filed and Attested by PROTHONOTARY 07 OCT 2013 04:10 pm K. PERMSAP THE SCHOOL DISTRICT OF PHILADELPHIA By: Miles H. Shore, Esquire Identification No. 03274 440 N. Broad Street, Suite 313 Attorney for Appellant
Filed and Attested by PROTHONOTARY 07 OCT 2013 04:10 pm K. PERMSAP THE SCHOOL DISTRICT OF PHILADELPHIA By: Miles H. Shore, Esquire Identification No. 03274 440 N. Broad Street, Suite 313 Attorney for Appellant
Medical Devices: Security & Privacy Concerns. NIST Information Security and Privacy Advisory Board (ISPAB) Meeting July 14, 2011
 Medical Devices: Security & Privacy Concerns Kevin Fu Associate Professor Security & Privacy Research Lab UMass Amherst Computer Science http://spqr.cs.umass.edu/ http://secure-medicine.org/ NIST Information
Medical Devices: Security & Privacy Concerns Kevin Fu Associate Professor Security & Privacy Research Lab UMass Amherst Computer Science http://spqr.cs.umass.edu/ http://secure-medicine.org/ NIST Information
DIVISION N CYBERSECURITY ACT OF 2015
 H. R. 2029 694 DIVISION N CYBERSECURITY ACT OF 2015 SEC. 1. SHORT TITLE; TABLE OF CONTENTS. (a) SHORT TITLE. This division may be cited as the Cybersecurity Act of 2015. (b) TABLE OF CONTENTS. The table
H. R. 2029 694 DIVISION N CYBERSECURITY ACT OF 2015 SEC. 1. SHORT TITLE; TABLE OF CONTENTS. (a) SHORT TITLE. This division may be cited as the Cybersecurity Act of 2015. (b) TABLE OF CONTENTS. The table
White Paper on Financial Industry Regulatory Climate
 White Paper on Financial Industry Regulatory Climate According to a 2014 report on threats to the financial services sector, 45% of financial services organizations polled had suffered economic crime during
White Paper on Financial Industry Regulatory Climate According to a 2014 report on threats to the financial services sector, 45% of financial services organizations polled had suffered economic crime during
Suzanne B. Schwartz, MD, MBA Director Emergency Preparedness/Operations & Medical Countermeasures (EMCM Program) CDRH/FDA
 8 th Annual Safeguarding Health Information: Building Assurance through HIPAA Security HHS Office of Civil Rights and National Institute of Standards & Technology Wednesday September 2, 2015 Suzanne B.
8 th Annual Safeguarding Health Information: Building Assurance through HIPAA Security HHS Office of Civil Rights and National Institute of Standards & Technology Wednesday September 2, 2015 Suzanne B.
Kevin Fu Associate Professor Security & Privacy Research Lab UMass Amherst Computer Science http://spqr.cs.umass.edu/
 Security and Privacy for Implantable Medical Devices Kevin Fu Associate Professor Security & Privacy Research Lab UMass Amherst Computer Science http://spqr.cs.umass.edu/ SRC/NSF/SFI Forum on Integrated
Security and Privacy for Implantable Medical Devices Kevin Fu Associate Professor Security & Privacy Research Lab UMass Amherst Computer Science http://spqr.cs.umass.edu/ SRC/NSF/SFI Forum on Integrated
IN THE COURT OF CHANCERY OF THE STATE OF DELAWARE
 IN THE COURT OF CHANCERY OF THE STATE OF DELAWARE : AL JAZEERA AMERICA, LLC, : : Plaintiff, : : v. : C.A. No. 8823-VCG : AT&T SERVICES, INC., : : Defendant. : : MOTION TO STAY OCTOBER 14, 2013 LETTER OPINION
IN THE COURT OF CHANCERY OF THE STATE OF DELAWARE : AL JAZEERA AMERICA, LLC, : : Plaintiff, : : v. : C.A. No. 8823-VCG : AT&T SERVICES, INC., : : Defendant. : : MOTION TO STAY OCTOBER 14, 2013 LETTER OPINION
Privacy Year in Review: Privacy and VoIP Technology
 Privacy Year in Review: Privacy and VoIP Technology JOHN B. MORRIS, JR. ABSTRACT Voice over internet protocol ( VoIP ) technology is increasingly being used throughout the nation. VoIP technology provides
Privacy Year in Review: Privacy and VoIP Technology JOHN B. MORRIS, JR. ABSTRACT Voice over internet protocol ( VoIP ) technology is increasingly being used throughout the nation. VoIP technology provides
HCCA Research Compliance Conference May 31-June 3, 2015
 Cybersecurity of Medical Devices and the Impact on Research Ken Briggs, Esq. Polsinelli PC, Phoenix kbriggs@polsinelli.com 602.650.2042 One East Washington St., Suite 1200 Phoenix, AZ 85004-2568 June 2015
Cybersecurity of Medical Devices and the Impact on Research Ken Briggs, Esq. Polsinelli PC, Phoenix kbriggs@polsinelli.com 602.650.2042 One East Washington St., Suite 1200 Phoenix, AZ 85004-2568 June 2015
Westlaw Journal. What is the Cybersecurity Framework? Risk Management Process And Pathway to Corporate Liability? Expert Analysis
 Westlaw Journal Computer & Internet Litigation News and Analysis Legislation Regulation Expert Commentary VOLUME 31, ISSUE 14 / DECEMBER 12, 2013 Expert Analysis The Cybersecurity Framework: Risk Management
Westlaw Journal Computer & Internet Litigation News and Analysis Legislation Regulation Expert Commentary VOLUME 31, ISSUE 14 / DECEMBER 12, 2013 Expert Analysis The Cybersecurity Framework: Risk Management
HEALTH INFORMATION TECHNOLOGY AND HIPAA: CAN WE SATISFY SECURITY AND PRIVACY STANDARDS IN THE DIGITAL AGE? 2007 Robert Malone I.
 Abstract: Robert Bond Malone is currently pursuing a J.D. at The University of Oklahoma College of Law as part of the Class of 2007. Below, Mr. Malone expands upon his previous publication, Health Information
Abstract: Robert Bond Malone is currently pursuing a J.D. at The University of Oklahoma College of Law as part of the Class of 2007. Below, Mr. Malone expands upon his previous publication, Health Information
Viva Energy may from time to time amend, delete or supplement these Terms and Conditions. Any change takes effect from the earlier of:
 SHELL CARD ONLINE TERMS AND CONDITIONS VERSION: AUGUST 2014 1. SCOPE 1.1 These Terms and Conditions apply to use of the Shell Card Online (SCOL) web programme accessible via www.vivaenergy.com.au, by a
SHELL CARD ONLINE TERMS AND CONDITIONS VERSION: AUGUST 2014 1. SCOPE 1.1 These Terms and Conditions apply to use of the Shell Card Online (SCOL) web programme accessible via www.vivaenergy.com.au, by a
UNITED STATES OF AMERICA Before the SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549
 INITIAL DECISION RELEASE NO. 536 ADMINISTRATIVE PROCEEDING File No. 3-15416 UNITED STATES OF AMERICA Before the SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 In the Matter of : INITIAL DECISION
INITIAL DECISION RELEASE NO. 536 ADMINISTRATIVE PROCEEDING File No. 3-15416 UNITED STATES OF AMERICA Before the SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 In the Matter of : INITIAL DECISION
Rethinking the FDA s Regulation of. By Scott D. Danzis and Christopher Pruitt
 Rethinking the FDA s Regulation of Mobile Medical Apps By Scott D. Danzis and Christopher Pruitt Smartphones and mobile devices have rapidly become part of everyday life in the United States. It is no
Rethinking the FDA s Regulation of Mobile Medical Apps By Scott D. Danzis and Christopher Pruitt Smartphones and mobile devices have rapidly become part of everyday life in the United States. It is no
ILLINOIS OFFICIAL REPORTS
 ILLINOIS OFFICIAL REPORTS Appellate Court Hart v. Kieu Le, 2013 IL App (2d) 121380 Appellate Court Caption LYNETTE Y. HART, Plaintiff-Appellant, v. LOAN KIEU LE, Defendant-Appellee. District & No. Second
ILLINOIS OFFICIAL REPORTS Appellate Court Hart v. Kieu Le, 2013 IL App (2d) 121380 Appellate Court Caption LYNETTE Y. HART, Plaintiff-Appellant, v. LOAN KIEU LE, Defendant-Appellee. District & No. Second
CONCERNS WITH THE LEAKED INTERNET CHAPTER OF ACTA
 CONCERNS WITH THE LEAKED INTERNET CHAPTER OF ACTA The U.S. proposal for an Internet chapter in the Anti-Counterfeiting Trade Agreement (ACTA) has been leaked to the press and widely disseminated on the
CONCERNS WITH THE LEAKED INTERNET CHAPTER OF ACTA The U.S. proposal for an Internet chapter in the Anti-Counterfeiting Trade Agreement (ACTA) has been leaked to the press and widely disseminated on the
Billing Code: 3510-EA
 Billing Code: 3510-EA DEPARTMENT OF COMMERCE Office of the Secretary National Institute of Standards and Technology National Telecommunications and Information Administration [Docket Number: 130206115-3115-01]
Billing Code: 3510-EA DEPARTMENT OF COMMERCE Office of the Secretary National Institute of Standards and Technology National Telecommunications and Information Administration [Docket Number: 130206115-3115-01]
Case 4:11-cv-10504-TSH Document 13 Filed 08/21/12 Page 1 of 7 UNITED STATES DISTRICT COURT DISTRICT OF MASSACHUSETTS
 Case 4:11-cv-10504-TSH Document 13 Filed 08/21/12 Page 1 of 7 UNITED STATES DISTRICT COURT DISTRICT OF MASSACHUSETTS JOE HAND PROMOTIONS, INC., Plaintiff, v. CIVIL ACTION NO. 11-10504-TSH KEVIN J. LENIHAN,
Case 4:11-cv-10504-TSH Document 13 Filed 08/21/12 Page 1 of 7 UNITED STATES DISTRICT COURT DISTRICT OF MASSACHUSETTS JOE HAND PROMOTIONS, INC., Plaintiff, v. CIVIL ACTION NO. 11-10504-TSH KEVIN J. LENIHAN,
Trials@uspto.gov Paper No. 9 571-272-7822 Date Entered: August 26, 2013 UNITED STATES PATENT AND TRADEMARK OFFICE
 Trials@uspto.gov Paper No. 9 571-272-7822 Date Entered: August 26, 2013 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD UNIVERSAL REMOTE CONTROL, INC. Petitioner v. UNIVERSAL
Trials@uspto.gov Paper No. 9 571-272-7822 Date Entered: August 26, 2013 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD UNIVERSAL REMOTE CONTROL, INC. Petitioner v. UNIVERSAL
What is Quantified Self (QS)?
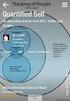 Subtitle Title Content Quantified Self (QS) (Sensitive) Personal data Security risks QS Privacy risks QS Art. 29 Working Party (WP29) on QS WP29 on ehealth WP29 on Internet of Things (IoT) QS data at risk
Subtitle Title Content Quantified Self (QS) (Sensitive) Personal data Security risks QS Privacy risks QS Art. 29 Working Party (WP29) on QS WP29 on ehealth WP29 on Internet of Things (IoT) QS data at risk
COMMENTS OF THE ELECTRONIC PRIVACY INFORMATION CENTER THE FEDERAL TRADE COMMISSION. In the Matter of Myspace, LLC. FTC File No. 102 3058.
 COMMENTS OF THE ELECTRONIC PRIVACY INFORMATION CENTER to THE FEDERAL TRADE COMMISSION In the Matter of Myspace, LLC FTC File No. 102 3058 June 8, 2012 By notice published on May 14, 2012, the Federal Trade
COMMENTS OF THE ELECTRONIC PRIVACY INFORMATION CENTER to THE FEDERAL TRADE COMMISSION In the Matter of Myspace, LLC FTC File No. 102 3058 June 8, 2012 By notice published on May 14, 2012, the Federal Trade
To improve cybersecurity in the United States through enhanced sharing of information about cybersecurity threats, and for other purposes.
 BAG15121 Discussion Draft S.L.C. 114TH CONGRESS 1ST SESSION S. XXXX To improve cybersecurity in the United States through enhanced sharing of information about cybersecurity threats, and for other purposes.
BAG15121 Discussion Draft S.L.C. 114TH CONGRESS 1ST SESSION S. XXXX To improve cybersecurity in the United States through enhanced sharing of information about cybersecurity threats, and for other purposes.
The Department of Homeland Security The Department of Justice
 The Department of Homeland Security The Department of Justice to Assist Non-Federal Entities to Share Cyber Threat Indicators and Defensive Measures with Federal Entities under the Cybersecurity Information
The Department of Homeland Security The Department of Justice to Assist Non-Federal Entities to Share Cyber Threat Indicators and Defensive Measures with Federal Entities under the Cybersecurity Information
How To Write A National Cybersecurity Act
 ROCKEFELLER SNOWE CYBERSECURITY ACT SUBSTITUTE AMENDMENT FOR S.773 March 17, 2010 BACKGROUND & WHY THIS LEGISLATION IS IMPORTANT: Our nation is at risk. The networks that American families and businesses
ROCKEFELLER SNOWE CYBERSECURITY ACT SUBSTITUTE AMENDMENT FOR S.773 March 17, 2010 BACKGROUND & WHY THIS LEGISLATION IS IMPORTANT: Our nation is at risk. The networks that American families and businesses
The Problems With SEC s Cybersecurity Approach
 Portfolio Media. Inc. 860 Broadway, 6th Floor New York, NY 10003 www.law360.com Phone: +1 646 783 7100 Fax: +1 646 783 7161 customerservice@law360.com The Problems With SEC s Cybersecurity Approach Law360,
Portfolio Media. Inc. 860 Broadway, 6th Floor New York, NY 10003 www.law360.com Phone: +1 646 783 7100 Fax: +1 646 783 7161 customerservice@law360.com The Problems With SEC s Cybersecurity Approach Law360,
In an age where so many businesses and systems are reliant on computer systems,
 Cyber Security Laws and Policy Implications of these Laws In an age where so many businesses and systems are reliant on computer systems, there is a large incentive for maintaining the security of their
Cyber Security Laws and Policy Implications of these Laws In an age where so many businesses and systems are reliant on computer systems, there is a large incentive for maintaining the security of their
MORGENSTERN & BLUE, LLC
 Case 3:09-cv-00298-N Document 836 Filed 10/15/2009 Page 1 of 11 IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF TEXAS DALLAS DIVISION SECURITIES AND EXCHANGE COMMISSION, Plaintiff, v.
Case 3:09-cv-00298-N Document 836 Filed 10/15/2009 Page 1 of 11 IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF TEXAS DALLAS DIVISION SECURITIES AND EXCHANGE COMMISSION, Plaintiff, v.
Healthcare Cybersecurity Perspectives from the Michigan Healthcare Cybersecurity Council
 Healthcare Cybersecurity Perspectives from the Michigan Healthcare Cybersecurity Council Presented by Doug Copley, Chairman Michigan Healthcare Cybersecurity Council Mr. Chairman and Committee Members,
Healthcare Cybersecurity Perspectives from the Michigan Healthcare Cybersecurity Council Presented by Doug Copley, Chairman Michigan Healthcare Cybersecurity Council Mr. Chairman and Committee Members,
PUBLIC LAW 105 304 OCT. 28, 1998 DIGITAL MILLENNIUM COPYRIGHT ACT
 PUBLIC LAW 105 304 OCT. 28, 1998 DIGITAL MILLENNIUM COPYRIGHT ACT 112 STAT. 2860 PUBLIC LAW 105 304 OCT. 28, 1998 Oct. 28, 1998 [H.R. 2281] Digital Millennium Copyright Act. 17 USC 101 note. Public Law
PUBLIC LAW 105 304 OCT. 28, 1998 DIGITAL MILLENNIUM COPYRIGHT ACT 112 STAT. 2860 PUBLIC LAW 105 304 OCT. 28, 1998 Oct. 28, 1998 [H.R. 2281] Digital Millennium Copyright Act. 17 USC 101 note. Public Law
Case 6:05-bk-03360-KSJ Doc 24 Filed 09/26/05 Page 1 of 6 UNITED STATES BANKRUPTCY COURT MIDDLE DISTRICT OF FLORIDA ORLANDO DIVISION ) ) ) ) ) ) )
 Case 6:05-bk-03360-KSJ Doc 24 Filed 09/26/05 Page 1 of 6 UNITED STATES BANKRUPTCY COURT MIDDLE DISTRICT OF FLORIDA ORLANDO DIVISION In re JOHN DORGAN, MARYANN DORGAN, Debtors. Case No. 6:05-bk-03360-KSJ
Case 6:05-bk-03360-KSJ Doc 24 Filed 09/26/05 Page 1 of 6 UNITED STATES BANKRUPTCY COURT MIDDLE DISTRICT OF FLORIDA ORLANDO DIVISION In re JOHN DORGAN, MARYANN DORGAN, Debtors. Case No. 6:05-bk-03360-KSJ
Much Ado About Injury: Making Sense Of FTAIA Circuit Split
 Portfolio Media. Inc. 860 Broadway, 6th Floor New York, NY 10003 www.law360.com Phone: +1 646 783 7100 Fax: +1 646 783 7161 customerservice@law360.com Much Ado About Injury: Making Sense Of FTAIA Circuit
Portfolio Media. Inc. 860 Broadway, 6th Floor New York, NY 10003 www.law360.com Phone: +1 646 783 7100 Fax: +1 646 783 7161 customerservice@law360.com Much Ado About Injury: Making Sense Of FTAIA Circuit
UNITED STATES BANKRUPTCY APPELLATE PANEL OF THE TENTH CIRCUIT
 BAP Appeal No. 05-36 Docket No. 29 Filed: 01/20/2006 Page: 1 of 7 UNITED STATES BANKRUPTCY APPELLATE PANEL OF THE TENTH CIRCUIT IN RE RICHARD A. FORD and TONDA L. FORD, also known as Tonda Yung, Debtors.
BAP Appeal No. 05-36 Docket No. 29 Filed: 01/20/2006 Page: 1 of 7 UNITED STATES BANKRUPTCY APPELLATE PANEL OF THE TENTH CIRCUIT IN RE RICHARD A. FORD and TONDA L. FORD, also known as Tonda Yung, Debtors.
DIVISION N CYBERSECURITY ACT OF 2015
 U:\0REPT\OMNI\FinalOmni\CPRT--HPRT-RU00-SAHR0-AMNT.xml DIVISION N CYBERSECURITY ACT OF 0 SEC.. SHORT TITLE; TABLE OF CONTENTS. (a) SHORT TITLE. This division may be cited as the Cybersecurity Act of 0.
U:\0REPT\OMNI\FinalOmni\CPRT--HPRT-RU00-SAHR0-AMNT.xml DIVISION N CYBERSECURITY ACT OF 0 SEC.. SHORT TITLE; TABLE OF CONTENTS. (a) SHORT TITLE. This division may be cited as the Cybersecurity Act of 0.
Cybersecurity for Medical Devices
 Cybersecurity for Medical Devices Suzanne O Shea Kathleen Rice January 29, 2015 Why Is This Important? Security Risks in the Sensors of Implantable Medical Devices Over the last year, we ve seen an uptick
Cybersecurity for Medical Devices Suzanne O Shea Kathleen Rice January 29, 2015 Why Is This Important? Security Risks in the Sensors of Implantable Medical Devices Over the last year, we ve seen an uptick
LEGAL UPDATE THIRD PARTY POP-UP ADVERTISEMENTS: U-HAUL INT L, INC. V. WHENU.COM. Andrew J. Sinclair
 LEGAL UPDATE THIRD PARTY POP-UP ADVERTISEMENTS: U-HAUL INT L, INC. V. WHENU.COM Andrew J. Sinclair I. INTRODUCTION Pop-up advertising has been an enormous success for internet advertisers 1 and a huge
LEGAL UPDATE THIRD PARTY POP-UP ADVERTISEMENTS: U-HAUL INT L, INC. V. WHENU.COM Andrew J. Sinclair I. INTRODUCTION Pop-up advertising has been an enormous success for internet advertisers 1 and a huge
UNITED STATES OF AMERICA Before the SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549
 INITIAL DECISION RELEASE NO. 808 ADMINISTRATIVE PROCEEDING File No. 3-16511 UNITED STATES OF AMERICA Before the SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 In the Matter of The Registration
INITIAL DECISION RELEASE NO. 808 ADMINISTRATIVE PROCEEDING File No. 3-16511 UNITED STATES OF AMERICA Before the SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 In the Matter of The Registration
PRESENTATION TO THE UNIVERSITY SYSTEM OF MARYLAND S BOARD OF REGENTS
 CYBERSECURITY PRESENTATION TO THE UNIVERSITY SYSTEM OF MARYLAND S BOARD OF REGENTS by Dr. Lawrence A. Gordon (Lgordon@rhsmith.umd.edu) EY Professor of Managerial Accounting and Information Assurance Affiliate
CYBERSECURITY PRESENTATION TO THE UNIVERSITY SYSTEM OF MARYLAND S BOARD OF REGENTS by Dr. Lawrence A. Gordon (Lgordon@rhsmith.umd.edu) EY Professor of Managerial Accounting and Information Assurance Affiliate
Comment submission: Date for submission of comments: Comments will be received until 5:00 P.M., Eastern Time, on August 18, 2008.
 THE PORT AUTHORITY OF NY & NJ John F. Kennedy International Airport, LaGuardia Airport and Newark Liberty International Airport NOTICE OF PROPOSED ACTION Issued: August 4, 2008 TAKE NOTICE THAT, The Port
THE PORT AUTHORITY OF NY & NJ John F. Kennedy International Airport, LaGuardia Airport and Newark Liberty International Airport NOTICE OF PROPOSED ACTION Issued: August 4, 2008 TAKE NOTICE THAT, The Port
Legislative Language
 Legislative Language SEC. 1. COORDINATION OF FEDERAL INFORMATION SECURITY POLICY. (a) IN GENERAL. Chapter 35 of title 44, United States Code, is amended by striking subchapters II and III and inserting
Legislative Language SEC. 1. COORDINATION OF FEDERAL INFORMATION SECURITY POLICY. (a) IN GENERAL. Chapter 35 of title 44, United States Code, is amended by striking subchapters II and III and inserting
causes of actions based on Travelers own tortious conduct and not directly related to the Manville insurance policies.[12]
![causes of actions based on Travelers own tortious conduct and not directly related to the Manville insurance policies.[12] causes of actions based on Travelers own tortious conduct and not directly related to the Manville insurance policies.[12]](/thumbs/24/3265665.jpg) Portfolio Media, Inc. 648 Broadway, Suite 200 New York, NY 10012 www.law360.com Phone: +1 212 537 6331 Fax: +1 212 537 6371 customerservice@portfoliomedia.com The Significance Of Travelers V. Bailey Law360,
Portfolio Media, Inc. 648 Broadway, Suite 200 New York, NY 10012 www.law360.com Phone: +1 212 537 6331 Fax: +1 212 537 6371 customerservice@portfoliomedia.com The Significance Of Travelers V. Bailey Law360,
NOT FOR PUBLICATION UNITED STATES COURT OF APPEALS FOR THE NINTH CIRCUIT. Plaintiff - Appellee, MEMORANDUM *
 NOT FOR PUBLICATION UNITED STATES COURT OF APPEALS FOR THE NINTH CIRCUIT FILED FEB 04 2015 LIFE ALERT EMERGENCY RESPONSE, INC., a California corporation, No. 14-55930 D.C. No. 2:13-cv-03455-JAK-SS MOLLY
NOT FOR PUBLICATION UNITED STATES COURT OF APPEALS FOR THE NINTH CIRCUIT FILED FEB 04 2015 LIFE ALERT EMERGENCY RESPONSE, INC., a California corporation, No. 14-55930 D.C. No. 2:13-cv-03455-JAK-SS MOLLY
Cyber and CGL Insurance Coverage for Data Breach Claims
 Cyber and CGL Insurance Coverage for Data Breach Claims Paula Weseman Theisen, Partner Data breach overview Definition of data breach/types Data breach costs Data breach legal claims and damages Cyber-insurance
Cyber and CGL Insurance Coverage for Data Breach Claims Paula Weseman Theisen, Partner Data breach overview Definition of data breach/types Data breach costs Data breach legal claims and damages Cyber-insurance
ehealth Privacy & Security Interest Group Monthly Call Friday November 14, 2014
 ehealth Privacy & Security Interest Group Monthly Call Friday November 14, 2014 Medical Device Security in a Connected World Kevin McDonald 1 www.americanbar.org ehealth Privacy & Security Interest Group
ehealth Privacy & Security Interest Group Monthly Call Friday November 14, 2014 Medical Device Security in a Connected World Kevin McDonald 1 www.americanbar.org ehealth Privacy & Security Interest Group
CELL PHONE UNLOCKING: A LEGAL PRIMER. of the Librarian of Congress not to allow consumers to unlock their cell phones to access
 Jonathan Band jband@policybandwidth.com On March 4, 2012, the White House announced that it disagreed with the decision of the Librarian of Congress not to allow consumers to unlock their cell phones to
Jonathan Band jband@policybandwidth.com On March 4, 2012, the White House announced that it disagreed with the decision of the Librarian of Congress not to allow consumers to unlock their cell phones to
Public Law 108 330 108th Congress An Act
 PUBLIC LAW 108 330 OCT. 16, 2004 118 STAT. 1275 Public Law 108 330 108th Congress An Act To amend title 31, United States Code, to improve the financial accountability requirements applicable to the Department
PUBLIC LAW 108 330 OCT. 16, 2004 118 STAT. 1275 Public Law 108 330 108th Congress An Act To amend title 31, United States Code, to improve the financial accountability requirements applicable to the Department
Medical Device Data Systems, Medical Image Storage Devices, and Medical Image Communications Devices
 Medical Device Data Systems, Medical Image Storage Devices, and Medical Image Communications Devices Draft Guidance for Industry and Food and Drug Administration Staff DRAFT GUIDANCE This guidance document
Medical Device Data Systems, Medical Image Storage Devices, and Medical Image Communications Devices Draft Guidance for Industry and Food and Drug Administration Staff DRAFT GUIDANCE This guidance document
Cybersecurity and Data Breach: Mitigating Risk and How Government Policymakers Approach These Critical Issues
 Cybersecurity and Data Breach: Mitigating Risk and How Government Policymakers Approach These Critical Issues Todd Bertoson Daniel Gibb Erin Sheppard Principal Senior Managing Associate Counsel todd.bertoson@dentons.com
Cybersecurity and Data Breach: Mitigating Risk and How Government Policymakers Approach These Critical Issues Todd Bertoson Daniel Gibb Erin Sheppard Principal Senior Managing Associate Counsel todd.bertoson@dentons.com
United States Court of Appeals for the Federal Circuit
 NOTE: This disposition is nonprecedential. United States Court of Appeals for the Federal Circuit ORACLE AMERICA, INC., Appellant v. GOOGLE, INC., Appellee 2014-1351 Appeal from the United States Patent
NOTE: This disposition is nonprecedential. United States Court of Appeals for the Federal Circuit ORACLE AMERICA, INC., Appellant v. GOOGLE, INC., Appellee 2014-1351 Appeal from the United States Patent
FACTUAL BACKGROUND. former co-workers of the decedents with whom they worked at common job sites, in common
 SUPREME COURT OF THE STATE OF NEW YORK COUNTY OF NEW YORK IN RE: NEW YORK CITY ASBESTOS LITIGATION This Document Refers To: WALTER SKY x Index No.: 105281/2000 RECOMMENDATION OF THE SPECIAL MASTER FACTUAL
SUPREME COURT OF THE STATE OF NEW YORK COUNTY OF NEW YORK IN RE: NEW YORK CITY ASBESTOS LITIGATION This Document Refers To: WALTER SKY x Index No.: 105281/2000 RECOMMENDATION OF THE SPECIAL MASTER FACTUAL
140-2-.04 Criminal Justice Information Exchange and Dissemination.
 140-2-.04 Criminal Justice Information Exchange and Dissemination. (1) Exchange and dissemination of criminal justice information by criminal justice agencies: (a) Criminal justice agencies shall exchange
140-2-.04 Criminal Justice Information Exchange and Dissemination. (1) Exchange and dissemination of criminal justice information by criminal justice agencies: (a) Criminal justice agencies shall exchange
Case 8:09-bk-11551-MGW Doc 53 Filed 07/30/13 Page 1 of 7 UNITED STATES BANKRUPTCY COURT MIDDLE DISTRICT OF FLORIDA TAMPA DIVISION
 Case 8:09-bk-11551-MGW Doc 53 Filed 07/30/13 Page 1 of 7 UNITED STATES BANKRUPTCY COURT MIDDLE DISTRICT OF FLORIDA TAMPA DIVISION IN RE: Randy L. Jones, Case No. 8:09-bk-11551-MGW Chapter 13 Debtor. /
Case 8:09-bk-11551-MGW Doc 53 Filed 07/30/13 Page 1 of 7 UNITED STATES BANKRUPTCY COURT MIDDLE DISTRICT OF FLORIDA TAMPA DIVISION IN RE: Randy L. Jones, Case No. 8:09-bk-11551-MGW Chapter 13 Debtor. /
DEPARTMENT OF THE TREASURY. March 2, 2001 CC:PA:DPL DL-103615-99. Chief, Branch 1 Assistant Chief Counsel (Disclosure & Privacy Law)
 (I DEPARTMENT OF THE TREASURY OFFICE OF INTERNAL REVENUE SERVICE CHIEF COUNSEL WASHINGTON, D.C. 20224 March 2, 2001 CC:PA:DPL DL-103615-99 MEMORANDUM'FOR CAROL GOLD DIRECTOR, EMPLOYEE PLANS DIVISION Attention:
(I DEPARTMENT OF THE TREASURY OFFICE OF INTERNAL REVENUE SERVICE CHIEF COUNSEL WASHINGTON, D.C. 20224 March 2, 2001 CC:PA:DPL DL-103615-99 MEMORANDUM'FOR CAROL GOLD DIRECTOR, EMPLOYEE PLANS DIVISION Attention:
UNITED STATES OF AMERICA Before the SECURITIES AND EXCHANGE COMMISSION
 SECURITIES ACT OF 1933 Release No. 9812 / June 18, 2015 UNITED STATES OF AMERICA Before the SECURITIES AND EXCHANGE COMMISSION SECURITIES EXCHANGE ACT OF 1934 Release No. 75206 / June 18, 2015 ADMINISTRATIVE
SECURITIES ACT OF 1933 Release No. 9812 / June 18, 2015 UNITED STATES OF AMERICA Before the SECURITIES AND EXCHANGE COMMISSION SECURITIES EXCHANGE ACT OF 1934 Release No. 75206 / June 18, 2015 ADMINISTRATIVE
National Institute of Standards and Technology-- Use of Electronic Data Interchange Technology to Create Valid Obligations
 OF COMPTROLLER T H E UN IT ED GENERAL S TAT ES Comptroller General of the United States Washington, D.C. 20548 Decision Matter of: National Institute of Standards and Technology-- Use of Electronic Data
OF COMPTROLLER T H E UN IT ED GENERAL S TAT ES Comptroller General of the United States Washington, D.C. 20548 Decision Matter of: National Institute of Standards and Technology-- Use of Electronic Data
UNITED STATES DISTRICT COURT DISTRICT OF NEW JERSEY
 UNITED STATES DISTRICT COURT DISTRICT OF NEW JERSEY DEAN SMITH, on behalf of himself and Others similarly situated, v. Michael Harrison, Esquire, Plaintiff, Defendant. OPINION Civ. No. 07-4255 (WHW) Walls,
UNITED STATES DISTRICT COURT DISTRICT OF NEW JERSEY DEAN SMITH, on behalf of himself and Others similarly situated, v. Michael Harrison, Esquire, Plaintiff, Defendant. OPINION Civ. No. 07-4255 (WHW) Walls,
CYBER-SURVEILLANCE BILL SET TO MOVE TO SENATE FLOOR
 CYBER-SURVEILLANCE BILL SET TO MOVE TO SENATE FLOOR July 28, 2015 The Senate is expected to consider the Cybersecurity Information Sharing Act (CISA, S. 754 1 ) on the Senate floor soon. The bill was marked
CYBER-SURVEILLANCE BILL SET TO MOVE TO SENATE FLOOR July 28, 2015 The Senate is expected to consider the Cybersecurity Information Sharing Act (CISA, S. 754 1 ) on the Senate floor soon. The bill was marked
S. 2519 AN ACT. To codify an existing operations center for cybersecurity.
 TH CONGRESS D SESSION S. 1 AN ACT To codify an existing operations center for cybersecurity. 1 Be it enacted by the Senate and House of Representa- tives of the United States of America in Congress assembled,
TH CONGRESS D SESSION S. 1 AN ACT To codify an existing operations center for cybersecurity. 1 Be it enacted by the Senate and House of Representa- tives of the United States of America in Congress assembled,
Myths and Facts about the Cyber Intelligence Sharing and Protection Act (CISPA)
 Myths and Facts about the Cyber Intelligence Sharing and Protection Act (CISPA) MYTH: The cyber threat is being exaggerated. FACT: Cyber attacks are a huge threat to American lives, national security,
Myths and Facts about the Cyber Intelligence Sharing and Protection Act (CISPA) MYTH: The cyber threat is being exaggerated. FACT: Cyber attacks are a huge threat to American lives, national security,
Ed McMurray, CISA, CISSP, CTGA CoNetrix
 Ed McMurray, CISA, CISSP, CTGA CoNetrix AGENDA Introduction Cybersecurity Recent News Regulatory Statements NIST Cybersecurity Framework FFIEC Cybersecurity Assessment Questions Information Security Stats
Ed McMurray, CISA, CISSP, CTGA CoNetrix AGENDA Introduction Cybersecurity Recent News Regulatory Statements NIST Cybersecurity Framework FFIEC Cybersecurity Assessment Questions Information Security Stats
Before the FEDERAL COMMUNICATIONS COMMISSION Washington, D.C. 20554. Comments of CTIA The Wireless Association
 Before the FEDERAL COMMUNICATIONS COMMISSION Washington, D.C. 20554 In the Matter of CSRIC IV Cybersecurity Risk Management and Assurance Recommendations ) ) ) PS Docket No. 15-68 ) ) Comments of CTIA
Before the FEDERAL COMMUNICATIONS COMMISSION Washington, D.C. 20554 In the Matter of CSRIC IV Cybersecurity Risk Management and Assurance Recommendations ) ) ) PS Docket No. 15-68 ) ) Comments of CTIA
