Pemetrexed for the treatment of malignant pleural mesothelioma
|
|
|
- Prosper Russell
- 8 years ago
- Views:
Transcription
1 Pemetrexed for the treatment of malignant pleural Issued: January 2008 guidance.nice.org.uk/ta135 NICE 2008
2 Contents 1 Guidance Clinical need and practice The technology Evidence and interpretation Clinical effectiveness Cost effectiveness Consideration of the evidence Implementation Recommendation for further research Related NICE guidance Review of guidance Appendix A: Appraisal Committee members and NICE project team A Appraisal Committee members B NICE project team Appendix B: Sources of evidence considered by the Committee Changes after publication About this guidance NICE All rights reserved. Last modified January 2008 Page 2 of 31
3 1 Guidance 1.1 Pemetrexed is recommended as a treatment option for malignant pleural only in people who have a World Health Organization (WHO) performance status of 0 or 1, who are considered to have advanced disease and for whom surgical resection is considered inappropriate. 1.2 Patients currently receiving pemetrexed who do not fall into the patient population defined in section 1.1 should have the option to continue therapy until they and their clinicians consider it appropriate to stop. NICE All rights reserved. Last modified January 2008 Page 3 of 31
4 2 Clinical need and practice 2.1 Malignant pleural (MPM) is a type of cancer that occurs in the pleura the mesothelium (membranous lining) surrounding the lungs. MPM is a rapidly progressive malignancy of insidious onset. 2.2 Approximately 90% of cases of MPM are linked to asbestos exposure. When asbestos fibres are inhaled or swallowed, they can cause scarring of the lung tissues, cancer of the bronchial tree (lung cancer) and sometimes cancers in the pleura and peritoneum. A wide range of occupations, notably shipbuilding, railway engineering and asbestos product manufacture, are associated with an increased risk of MPM. Family members of people whose work clothes were contaminated with asbestos fibres have also developed MPM. The condition is significantly more common in men, with a male to female ratio of 5:1. People with usually present with the disease between the ages of 60 and 79 years. 2.3 MPM usually develops years after exposure to asbestos. Data from 2004 suggest that about 1700 people in the UK are diagnosed with MPM each year. It is estimated that this figure will increase to a peak of more than 2000 cases each year between 2011 and 2015, reflecting a lag from the highest use of asbestos in the 1970s. An estimated 65,000 cases are expected to occur between 2002 and The use of asbestos was banned in the UK in Most people with MPM present with chest pain and dyspnoea and have pleural effusions evident on examination. Fatigue, profuse sweating, weight loss, anorexia and difficulty in swallowing become common as the disease progresses. Presentation and diagnosis often occur at an advanced stage and the prognosis for most patients is extremely poor. Median survival from diagnosis varies in studies, with a range of 9 13 months. Age, tumour histology, tumour stage at diagnosis and performance status have been shown to be independent prognostic factors. The most commonly used performance status scoring systems include the Karnofsky performance status (KPS) and the World Health Organization (WHO) scales. KPS is a 10-point scale ranging from 0 to 100, with higher scores representing normal day-to-day activity. The WHO system is a five-point scale with lower scores representing normal day- NICE All rights reserved. Last modified January 2008 Page 4 of 31
5 to-day activity. In general, WHO scores of 0 and 1 are considered equivalent to KPS scores of There is no standard treatment pathway for MPM in England and Wales. The clinical management is multimodal and a patient may receive a combination of treatments. Staging provides prognostic information and can help to determine an appropriate treatment strategy; however, it is complex, and surgical intervention is required to stage the disease fully. There is no universally accepted staging system, but the traditional Butchart system is gradually being replaced with a tumour nodes metastases (TNM) system developed by the International Mesothelioma Interest Group. In clinical practice, MPM is generally staged pragmatically based on whether or not surgical resection is considered an appropriate option. Extrapleural pneumonectomy is an option for the small proportion of patients (1 5%) whose tumours are at stage 1 or Surgery is not indicated for the majority of patients, so treatment aims to improve symptoms and maintain quality of life for as long as possible. Often, this does not involve treating the tumour with chemotherapy. Treatment that does not include a specific anti-cancer therapy is referred to as active symptom control (ASC) or best supportive care (BSC). For people with MPM, this may include interventions to manage pain and dyspnoea, and to address psychosocial problems. Treatments may include draining excess fluid from the pleural cavity and applying a talc pleurodesis (the insertion of talc to prevent further fluid accumulation), palliative radiotherapy, analgesics, steroids, appetite stimulants and bronchodilators. 2.7 There is no standard chemotherapy treatment for MPM. Pemetrexed in combination with cisplatin is the only chemotherapy regimen that is currently licensed for this indication. However, a variety of combination and single-agent regimens such as the mitomycin C, vinblastine and cisplatin combination (MVP) or vinorelbine are used. To date there have been no published randomised controlled trials (RCTs) comparing survival and symptom control in patients receiving chemotherapy with those receiving ASC. NICE All rights reserved. Last modified January 2008 Page 5 of 31
6 3 The technology 3.1 Pemetrexed (Alimta, Eli Lilly and Company) is licensed, in combination with cisplatin, for the treatment of chemotherapy-naive patients with unresectable MPM. Pemetrexed is a multi-targeted folate antagonist that inhibits DNA replication. Cisplatin is a platinum-based chemotherapeutic agent that has antitumour activity, either as a single agent or in combination, for a number of different cancers. The licensed dose of pemetrexed is 500 mg/m 2 body surface area, to be administered as a 10-minute intravenous infusion on the first day of a 21-day cycle. It is followed approximately 30 minutes later by cisplatin (recommended dose 75 mg/m 2 body surface area) infused over 2 hours. In order to reduce toxicity, patients treated with pemetrexed must receive folic acid and vitamin B 12 supplementation. To reduce the incidence and severity of skin reactions, patients are pre-medicated with a corticosteroid. 3.2 Adverse effects commonly associated with pemetrexed include nausea, vomiting, fatigue and neutropenia. Skin rash, mucositis and liver function abnormalities have also been reported. Cisplatin causes nausea and vomiting in the majority of patients. This is controllable in 50 80% of patients with antiemetic drugs. Serious toxic effects of cisplatin on the kidneys, bone marrow and ears are common, and serum electrolyte disturbances, hyperuricaemia, allergic reactions and cardiac abnormalities have also been reported. For full details of side effects and contraindications, see the summaries of product characteristics. 3.3 Pemetrexed costs 800 for a 500-mg vial (excluding VAT, 'British national formulary' [BNF]53rd edition). The cost per patient, assuming an average of five treatment cycles and a body surface area of 1.8 m 2, is approximately Costs may vary in different settings because of negotiated procurement discounts. NICE All rights reserved. Last modified January 2008 Page 6 of 31
7 4 Evidence and interpretation The Appraisal Committee (appendix A) considered evidence from a number of sources (appendix B). 4.1 Clinical effectiveness A single RCT of pemetrexed in MPM was identified. The EMPHACIS ('Evaluation of in a Phase III trial of pemetrexed with cisplatin') study compared pemetrexed plus cisplatin with cisplatin alone. This was a single-blind, international, multicentre trial in 448 patients. To be eligible, patients had to be 18 years or older, and were required to have a minimum life expectancy of 12 weeks, uni- or bi-dimensionally measurable disease, and a KPS of greater than or equal to 70. Patients who had had prior chemotherapy, those with a second primary malignancy or brain metastasis, and those unable to interrupt non-steroidal anti-inflammatory drugs were excluded Patients in the intervention arm (n = 226) received pemetrexed at a dose of 500 mg/m 2 followed 30 minutes later by cisplatin at a dose of 75 mg/m 2. Patients in the control arm (n = 222) received normal saline followed 30 minutes later by cisplatin at a dose of 75 mg/m 2. In both arms, treatment was administered on the first day of each 21-day cycle. The median number of cycles given was 6 (range 1 12) in the pemetrexed plus cisplatin arm and 4 (range 1 9) in the cisplatin arm. Median length of follow-up was 10 months During the early stages of the trial, incidences of severe toxicity (including drug-related death, neutropenia, febrile neutropenia and diarrhoea) were high in the combination arm. Folic acid and vitamin B 12 supplementation were therefore added to the trial protocol in both treatment arms to preserve blinding. With effect from the date of the protocol change, all patients received supplementation, resulting in three patient subgroups defined by supplementation status: never supplemented (n = 70), partially supplemented (those who started treatment before the protocol change; n = 47) and fully supplemented (those who started treatment after the protocol change; n = 331). The primary analysis was performed on all patients who were randomised and treated (intention-to-treat [ITT] population). A subgroup NICE All rights reserved. Last modified January 2008 Page 7 of 31
8 analysis was performed on fully supplemented patients. Further post-hoc subgroup analyses were performed on fully supplemented patients with advanced disease (stage 3/4) because it was thought that most patients presenting to clinicians would fall into this category The primary endpoint of the EMPHACIS trial was survival. A statistically significant survival benefit was observed in patients randomised to pemetrexed plus cisplatin versus those receiving cisplatin alone. In the ITT population, median survival was 12.1 months (95% confidence interval [CI], 10.0 to 14.4) in the pemetrexed plus cisplatin arm versus 9.3 months (95% CI, 7.8 to 10.7) in the cisplatin arm (hazard ratio [HR] 0.77; 95% CI, 0.61 to 0.96; log rank test p value = 0.02). In fully supplemented patients, median survival was 13.3 months (95% CI, 11.4 to 14.9) in the combination arm versus 10 months (95% CI, 8.4 to 11.9) in the cisplatin arm (HR 0.75; 95% CI, 0.57 to 1.00; logrank test p value = 0.051). In fully supplemented patients with advanced disease, median survival was 13.2 months (95% CI, 9.3 to 14.9) in the combination arm versus 8.4 months (95% CI, 6.8 to 10.2) in the cisplatin arm (HR 0.63; 95% CI, 0.46 to 0.86; log rank test p value = 0.003) Secondary endpoints included 1-year survival, median time to progressive disease and tumour response rate. Pemetrexed plus cisplatin demonstrated statistically significant benefits versus cisplatin alone for all of these outcomes in the ITT population and in the subgroups. The results for these endpoints in the ITT population for the pemetrexed plus cisplatin group versus the cisplatin alone group, respectively, were as follows: 1-year survival: 50.3% versus 38.0% (p = 0.012) median time to progression: 5.7 months versus 3.9 months (p < 0.001) tumour response rate: 41.3% versus 16.7% (p < 0.001) Quality of life was evaluated using the Lung Cancer Symptom Scale Meso instrument. Several aspects of quality of life were evaluated, including pain, dyspnoea, fatigue, anorexia and cough. Over 18 weeks, patients treated with pemetrexed plus cisplatin demonstrated statistically significant symptomatic improvements when compared with those who received cisplatin alone. For NICE All rights reserved. Last modified January 2008 Page 8 of 31
9 global quality of life in the ITT population, a least squares mean score of 56 out of 100 was reported for patients randomised to pemetrexed plus cisplatin versus a score of 53 out of 100 for patients in the cisplatin arm (p value for the difference between arms = 0.012). A similar result was observed in the fully supplemented population Severe to life-threatening or disabling adverse events were statistically significantly more frequent in patients receiving pemetrexed plus cisplatin than in those receiving cisplatin alone. The most commonly reported of these in patients receiving pemetrexed plus cisplatin were: neutropenia (27.9%), leukopenia (17.7%), nausea (14.6%) and vomiting (13.3%). Supplementation with folic acid and vitamin B 12 resulted in a consistent reduction in the severity and incidence of adverse events (except for dehydration) in the pemetrexed plus cisplatin arm. The most common severe adverse events in fully supplemented patients randomised to pemetrexed plus cisplatin were: neutropenia (23.2%), leukopenia (14.9%), nausea (11.9%) and vomiting (10.7%) Supplementary documentation on pemetrexed provided by the manufacturer indicated that, in the ITT population, 42% (94 of 226) of patients randomised to pemetrexed plus cisplatin responded to treatment. Of those who experienced a response, 87% (82 of 94) did so within four cycles. Summary of the evidence on clinical effectiveness The results of the EMPHACIS trial suggest that pemetrexed plus cisplatin confers a survival benefit of approximately 3 months compared with cisplatin alone. The combination treatment also appears to demonstrate advantages in terms of 1-year survival, median time to progressive disease, tumour response rate and quality of life. Pemetrexed plus cisplatin appears to offer greater survival benefits than cisplatin alone in patients with advanced disease. 4.2 Cost effectiveness Estimates of cost effectiveness were provided by the manufacturer and by the Assessment Group. A review of the published literature identified a single cost- NICE All rights reserved. Last modified January 2008 Page 9 of 31
10 effectiveness study. This was a conference presentation/abstract that was a forerunner of the manufacturer's submission Two cost-effectiveness models were submitted by the manufacturer. Model 1 compared pemetrexed plus cisplatin with cisplatin alone. Model 2 compared pemetrexed plus cisplatin with standard care (as defined by the manufacturer on the basis of a market research survey). Both models had a 29-month time horizon (reflecting the trial follow-up period) and took a health service perspective. Both considered outcomes in terms of life years gained and quality-adjusted life years (QALYs). No discounting was applied to costs, because they were all incurred within 1 year. Outcomes were discounted at 3.5% Model 1 was based on individual patient data from the EMPHACIS trial. The model considered four subgroups: fully supplemented patients; fully supplemented patients with advanced disease; fully supplemented patients with good performance status (WHO performance status of 0 or 1); and fully supplemented patients with advanced disease and good performance status. Data for resource use were taken from the trial and unit costs were taken from Department of Health reference costs or official drug price lists (BNF,MIMS 2005). Mean survival was estimated from the trial data using Kaplan-Meier curves. Utility scores were taken from an ongoing observational study in patients with non-small-cell lung cancer (NSCLC) who completed the EQ-5D health-related quality of life questionnaire before chemotherapy. The basecase utility scores in both economic models were similar for both arms (0.68 for the pemetrexed plus cisplatin arm and 0.69 for the cisplatin alone arm) and did not take account of loss of quality of life in people with MPM as their disease progresses. A range of one-way and two-way sensitivity analyses was performed. No probabilistic sensitivity analysis was performed The incremental cost-effectiveness ratio (ICER) was 68,598 per QALY gained in the fully supplemented population. The ICER was more favourable in fully supplemented patients with advanced disease ( 53,314 per QALY gained), fully supplemented patients with good performance status ( 48,099 per QALY gained), and fully supplemented patients with advanced disease and good performance status ( 47,567 per QALY gained). NICE All rights reserved. Last modified January 2008 Page 10 of 31
11 4.2.5 Model 2 indirectly compared pemetrexed plus cisplatin with MVP, vinorelbine (with or without platinum) and ASC. Costs and outcomes for pemetrexed plus cisplatin were taken from the fully supplemented population in model 1. For the comparators, resource use data were gathered from market research surveys of oncologists, commissioned by the manufacturer. Zero cost was assumed for ASC, because it was reasoned that participants in chemotherapy trials would have received a similar level of ASC to patients receiving ASC alone. Median survival estimates were taken from a review of the published literature. Mean values for use in the cost-effectiveness analysis were derived by calculating a weighted average of reported medians and assuming the same mean to median ratio as that observed in the cisplatin only arm of the EMPHACIS trial. The same utility values were used as in model 1, with the utility for cisplatin (0.69) being applied to all comparators in model 2. A range of one-way and two-way sensitivity analyses was performed. The incremental cost per QALY gained for pemetrexed plus cisplatin was calculated to be 21,731 versus MVP, 28,391 versus vinorelbine with or without platinum and 32,066 versus ASC When the Assessment Group corrected the survival estimate for MVP for performance status, an ICER of 47,972 per QALY gained was obtained for pemetrexed plus cisplatin versus MVP. Using more favourable survival estimates and taking the number of cycles of chemotherapy from the literature rather than the manufacturer's market research survey, the ICERs versus MVP and vinorelbine were both above 60,000 per QALY gained. Using survival estimates for ASC taken from a meta-analysis designed to consider prognostic factors in MPM resulted in an ICER of 48,779 per QALY gained for pemetrexed plus cisplatin versus ASC The Assessment Group also carried out its own economic analysis of pemetrexed plus cisplatin compared with cisplatin alone. The four subgroups considered in the manufacturer's model 1 were analysed. Mean costs were derived from the individual patient data in model 1. Costs were not discounted. To derive mean effectiveness estimates, Weibull distributions were fitted to the Kaplan-Meier survival curves from the EMPHACIS trial, in order to model the survival distribution of patients at the end of the follow-up period. Mean survival was estimated using the weighted least squares method and a discount rate of NICE All rights reserved. Last modified January 2008 Page 11 of 31
12 3.5% was applied. In both arms, the Assessment Group used mean utility values of for each subgroup. These values were calculated using an initial utility of 0.65, falling to 0.40 during a 100-day terminal period to account for lower quality of life in people with MPM towards the end of their life The Assessment Group's analysis resulted in an ICER of 60,600 per QALY gained in the fully supplemented population. The results were more favourable in fully supplemented patients with advanced disease ( 49,100 per QALY gained), fully supplemented patients with good performance status ( 50,400 per QALY gained) and fully supplemented patients with advanced disease and good performance status ( 37,700 per QALY gained). The Assessment Group also calculated the cost effectiveness of pemetrexed plus cisplatin versus cisplatin alone in fully supplemented patients with advanced disease and good performance status under the assumption that a smaller 100-mg vial of pemetrexed becomes available. In this case the ICER was 34,500 per QALY gained In a later document, the manufacturer suggested that the ICERs for pemetrexed plus cisplatin might be lower if treatment was stopped in patients who did not experience a tumour response after their fourth cycle. It was suggested that this would lower overall costs without reducing aggregate health benefit, because only those who respond to treatment would experience survival gains. No clinical evidence or economic analysis to support this proposal was submitted. Summary of the evidence on cost effectiveness The economic analyses carried out by the manufacturer and the Assessment Group, using model 1, both indicated an incremental cost per QALY gained of greater than 60,000 when pemetrexed plus cisplatin was compared with cisplatin alone in the fully supplemented population. Pemetrexed plus cisplatin, when compared with cisplatin alone, appears to have lower ICERs in patients with advanced disease and/or good performance status. The manufacturer's economic analyses (based on indirect comparisons) using model 2 indicated more favourable ICERs for pemetrexed plus cisplatin when compared with MVP, vinorelbine and ASC. However, the assumptions underpinning model 2 are subject to high levels of uncertainty. When the assumptions were modified NICE All rights reserved. Last modified January 2008 Page 12 of 31
13 to reflect performance-status-adjusted survival, and resource use based on published data, the ICERs from model 2 were in line with those of pemetrexed plus cisplatin versus cisplatin alone. 4.3 Consideration of the evidence The Committee reviewed the data available on the clinical and cost effectiveness of pemetrexed for the treatment of MPM, having considered evidence on the nature of the condition and the value placed on the benefits of pemetrexed by patient representatives and clinical specialists. It was also mindful of the need to take account of the effective use of NHS resources The Committee heard from clinical specialists and patient experts that pemetrexed plus cisplatin is valued as a potential treatment option in a disease area where it has demonstrated survival and quality of life advantages in an RCT and where there is incomplete evidence on the efficacy of alternative treatments The Committee discussed the relevant comparator for pemetrexed plus cisplatin in the context of the NHS. Clinical specialists advised that cisplatin monotherapy would not normally be used to treat MPM in clinical practice in England and Wales because of a lack of evidence of its effectiveness and its relatively unfavourable adverse-effect profile. The Committee heard that there is no standard care pathway for MPM; although some patients receive chemotherapy treatment, notably with MVP or vinorelbine, many patients receive ASC only. However, the Committee was also aware that there have been no published RCTs of MVP or vinorelbine in MPM, either versus ASC or against each other. The Committee noted that the results of a meta-analysis investigating prognostic factors for MPM suggest that survival with ASC without chemotherapy may be no worse than with chemotherapy. The Committee agreed that a direct RCT comparison of the efficacy of pemetrexed plus cisplatin versus other chemotherapy treatments and ASC would be an informative addition to the evidence base The Committee discussed whether pemetrexed should be recommended over treatments for MPM used most frequently in the UK, and therefore considered NICE All rights reserved. Last modified January 2008 Page 13 of 31
14 the indirect comparisons submitted by the manufacturer. It discussed the plausibility of the result that pemetrexed plus cisplatin had lower ICERs when compared with MVP, vinorelbine and ASC than when it was compared with cisplatin alone. The Committee noted the high degree of uncertainty surrounding the assumptions underpinning the model and observed that the survival estimates had been taken from relatively small, non-comparative and observational studies. It also noted that the study populations were unlikely to be comparable with the population of the EMPHACIS trial, particularly in terms of performance status, a key independent predictor of survival in MPM patients The Committee also noted that resource-use estimates for MVP and vinorelbine (based on the number of cycles of chemotherapy derived from the manufacturer's market research surveys) were higher than those reported in the studies from which the effectiveness estimates were taken, and considered the possibility that comparator costs may have been overestimated. The Committee saw that when the model assumptions were amended to incorporate more favourable survival estimates and resource use taken from the literature, ICERs were significantly higher. On balance, the Committee concluded that it could not base its decision on the indirect comparison model The Committee heard from clinical specialists that cisplatin could be considered a valid chemotherapeutic agent even though it is not favoured in the UK. The Committee discussed what could be inferred when the comparative evidence was limited to pemetrexed and cisplatin. It concluded that the survival benefit demonstrated by pemetrexed plus cisplatin in the EMPHACIS trial was likely to be robust because cisplatin was likely to be at least as effective as placebo or ASC, although, in terms of quality of life, cisplatin is likely to have adverse effects. The Committee also noted that cisplatin was likely to have higher costs than placebo or ASC and that this would affect the results of cost-effectiveness analysis. The Committee discussed the ICERs of pemetrexed plus cisplatin versus cisplatin alone produced by the manufacturer and the Assessment Group, and observed that the range of ICERs was higher than is normally considered acceptable. NICE All rights reserved. Last modified January 2008 Page 14 of 31
15 4.3.7 The Committee considered whether it was appropriate to accept economic results expressed in incremental costs per life year gained. The Committee noted that the 'Guide to the methods of technology appraisal' advises that the reference case measure of health benefits is the QALY, and that 'where health gain is expressed in terms of life years gained, the range of most plausible ICERs that are acceptable will be substantially lower...' ( ). The Committee did not consider it plausible that a patient with MPM on chemotherapy would have a full quality of life and this was confirmed by the experts present at the meeting. Furthermore, the Committee heard from clinical specialists that utility values derived from studies in NSCLC are a fair approximation of the utility values for people with MPM, and noted that sensitivity analyses indicated the ICERs from the manufacturer's economic model were not strongly influenced by the utility values. The Committee agreed that there are no reasons for it to change its preference for QALYs The Committee discussed the subgroup of patients with both advanced disease and good performance status, in view of the relatively favourable ICERs of pemetrexed plus cisplatin versus cisplatin alone ( 37,000 per QALY gained, or 34,500 per QALY gained assuming a 100-mg pemetrexed vial becomes available) that were calculated for this subgroup. The Committee was aware that most people with unresectable disease would be considered to have advanced disease and that this subgroup of patients comprised the majority of people with MPM seen in UK clinical practice. The Committee accepted that it was plausible that people with good performance status were likely to show a better response to treatment than those with poor performance status The Committee noted that not all patients respond to treatment with pemetrexed plus cisplatin and saw that, in the EMPHACIS trial, 87% of those who responded had done so within four cycles. Furthermore, the Committee noted from the consultation that it would be unusual for a UK oncologist to continue treatment beyond four cycles if there was disease progression or no response to treatment. The Committee therefore accepted that the mean number of cycles in clinical practice was likely to be less than the mean of six cycles reported in the EMPHACIS trial, and this would result in lower estimates of pemetrexed drug costs. NICE All rights reserved. Last modified January 2008 Page 15 of 31
16 The Committee discussed the possibility that differences in symptom relief (including pain and dyspnoea) and quality of life between pemetrexed plus cisplatin and cisplatin alone may not have been captured fully by the economic model because the utilities for both treatment and comparator had been estimated based on data from people with NSCLC. The Committee noted that there was some evidence from the EMPHACIS trial showing that pemetrexed plus cisplatin was associated with statistically significant symptomatic improvements (especially with pain relief) compared with cisplatin alone. The Committee agreed that the economic analyses may have underestimated the overall quality of life benefits of pemetrexed in people with MPM Having considered the likelihood of lower numbers of treatment cycles in clinical practice, the potential availability of a 100-mg pemetrexed vial and the likelihood of greater quality of life benefits than assumed by the costeffectiveness analyses, the Committee agreed that the ICER for pemetrexed plus cisplatin in the fully supplemented subgroup with advanced disease and good performance status was likely to fall within acceptable levels The Committee also noted that MPM is a rare and aggressive malignancy caused by occupational exposure to asbestos and was mindful that this disease has a very poor prognosis The Committee concluded that pemetrexed in combination with cisplatin should be recommended as an option for the treatment of MPM only in people who are considered to have advanced disease and who have a WHO performance status of 0 or 1, in whom surgical resection is not considered appropriate. NICE All rights reserved. Last modified January 2008 Page 16 of 31
17 5 Implementation 5.1 The Healthcare Commission assesses the performance of NHS organisations in meeting core and developmental standards set by the Department of Health in 'Standards for better health'issued in July The Secretary of State has directed that the NHS provides funding and resources for medicines and treatments that have been recommended by s normally within 3 months from the date that NICE publishes the guidance. Core standard C5 states that healthcare organisations should ensure they conform to s. 5.2 'Healthcare standards for Wales' was issued by the Welsh Assembly Government in May 2005 and provides a framework both for self-assessment by healthcare organisations and for external review and investigation by Healthcare Inspectorate Wales. Standard 12a requires healthcare organisations to ensure that patients and service users are provided with effective treatment and care that conforms to guidance. The Assembly Minister for Health and Social Services issued a Direction in October 2003 that requires local health boards and NHS trusts to make funding available to enable the implementation of NICE technology appraisal guidance, normally within 3 months. 5.3 When NICE recommends a treatment 'as an option', the NHS must make sure it is available within the period set out in the paragraph above. This means that, if a patient has malignant pleural and the doctor responsible for their care thinks that pemetrexed is the right treatment, it should be available for use, in line with NICE's recommendations. 5.4 NICE has developed tools to help organisations implement this guidance (listed below). A costing statement explaining the resource impact of this guidance. Audit criteria to monitor local practice. NICE All rights reserved. Last modified January 2008 Page 17 of 31
18 6 Recommendation for further research 6.1 The Committee identified a need for RCTs comparing alternative chemotherapy regimens in MPM. Specifically, the Committee recommended that trials be conducted in which pemetrexed plus cisplatin is compared with treatments that are currently commonly used in clinical practice in England and Wales in order to determine its relative effectiveness. The Committee also recommended that comparative trials of pemetrexed plus cisplatin versus other promising treatments be conducted. NICE All rights reserved. Last modified January 2008 Page 18 of 31
19 7 Related NICE guidance 7.1 There is no related guidance for this technology. NICE All rights reserved. Last modified January 2008 Page 19 of 31
20 8 Review of guidance 8.1 The review date for a technology appraisal refers to the month and year in which the Guidance Executive will consider whether the technology should be reviewed. This decision will be taken in the light of information gathered by the Institute, and in consultation with consultees and commentators. 8.2 The guidance on this technology was reviewed in December Andrew Dillon Chief Executive January 2008 NICE All rights reserved. Last modified January 2008 Page 20 of 31
Natalizumab for the treatment of adults with highly active relapsing remitting multiple sclerosis
 Natalizumab for the treatment of adults with highly active Issued: August 2007 guidance.nice.org.uk/ta127 NICE 2007 Contents 1 Guidance... 3 2 The technology... 4 3 The manufacturer's submission... 5 4
Natalizumab for the treatment of adults with highly active Issued: August 2007 guidance.nice.org.uk/ta127 NICE 2007 Contents 1 Guidance... 3 2 The technology... 4 3 The manufacturer's submission... 5 4
Scottish Medicines Consortium
 Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Scottish Medicines Consortium pemetrexed 500mg infusion (Alimta ) No. (192/05) Eli Lilly 8 July 2005 The Scottish Medicines Consortium has completed its assessment of the above product and advises NHS
Natalizumab for the treatment of adults with highly active relapsing remitting multiple sclerosis
 Issue date: August 2007 Review date: June 2010 Natalizumab for the treatment of adults with highly active relapsing remitting multiple sclerosis This guidance was developed using the single technology
Issue date: August 2007 Review date: June 2010 Natalizumab for the treatment of adults with highly active relapsing remitting multiple sclerosis This guidance was developed using the single technology
Pemetrexed for the maintenance treatment of non-small-cell lung cancer
 Pemetrexed for the maintenance treatment of non-small-cell lung cancer Issued: June 2010 guidance.nice.org.uk/ta190 NICE has accredited the process used by the Centre for Health Technology Evaluation at
Pemetrexed for the maintenance treatment of non-small-cell lung cancer Issued: June 2010 guidance.nice.org.uk/ta190 NICE has accredited the process used by the Centre for Health Technology Evaluation at
Malignant Mesothelioma
 Malignant Malignant mesothelioma is a tumour originating from mesothelial cells. 85 95% of mesotheliomas are caused by asbestos exposure. It occurs much more commonly in the chest (malignant pleural mesothelioma)
Malignant Malignant mesothelioma is a tumour originating from mesothelial cells. 85 95% of mesotheliomas are caused by asbestos exposure. It occurs much more commonly in the chest (malignant pleural mesothelioma)
Malignant Mesothelioma
 Malignant mesothelioma is a tumour originating from mesothelial cells. 85 95% of mesotheliomas are caused by asbestos exposure. It occurs much more commonly in the chest (malignant pleural mesothelioma)
Malignant mesothelioma is a tumour originating from mesothelial cells. 85 95% of mesotheliomas are caused by asbestos exposure. It occurs much more commonly in the chest (malignant pleural mesothelioma)
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Erlotinib for the treatment of nonsmall-cell
 Erlotinib for the treatment of nonsmall-cell lung Issued: November 2008 last modified: December 2012 guidance.nice.org.uk/ta NICE has accredited the process used by the Centre for Health Technology Evaluation
Erlotinib for the treatment of nonsmall-cell lung Issued: November 2008 last modified: December 2012 guidance.nice.org.uk/ta NICE has accredited the process used by the Centre for Health Technology Evaluation
How To Treat Mesothelioma
 Mesothelioma in the UK Liz Darlison Mesothelioma UK The National Macmillan Mesothelioma Resource Centre June 2010 Mesothelioma Applied Research Foundation - 2010 www.curemeso.org Contents Size of Mesothelioma
Mesothelioma in the UK Liz Darlison Mesothelioma UK The National Macmillan Mesothelioma Resource Centre June 2010 Mesothelioma Applied Research Foundation - 2010 www.curemeso.org Contents Size of Mesothelioma
Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-hodgkin's lymphoma: Review of technology appraisal guidance 37
 Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-hodgkin's lymphoma: Review of technology appraisal Issued: February 2008 guidance.nice.org.uk/ta NICE 2008 Contents
Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-hodgkin's lymphoma: Review of technology appraisal Issued: February 2008 guidance.nice.org.uk/ta NICE 2008 Contents
Summary of treatment benefits
 Risk Management Plan PEMETREXED Powder for concentrate for Solution for infusion Pemetrexed is also indicated as monotherapy for the maintenance treatment of locally advanced or metastatic non small cell
Risk Management Plan PEMETREXED Powder for concentrate for Solution for infusion Pemetrexed is also indicated as monotherapy for the maintenance treatment of locally advanced or metastatic non small cell
Activity of pemetrexed in thoracic malignancies
 Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Mesothelioma Priority Setting Partnership. PROTOCOL November 2013
 Mesothelioma Priority Setting Partnership PROTOCOL November 2013 Purpose The purpose of this protocol is to set out the aims, objectives and commitments of the Mesothelioma Priority Setting Partnership
Mesothelioma Priority Setting Partnership PROTOCOL November 2013 Purpose The purpose of this protocol is to set out the aims, objectives and commitments of the Mesothelioma Priority Setting Partnership
2. Background This was the fourth submission for everolimus requesting listing for clear cell renal carcinoma.
 PUBLIC SUMMARY DOCUMENT Product: Everolimus, tablets, 5 mg and 10 mg, Afinitor Sponsor: Novartis Pharmaceuticals Australia Pty Ltd Date of PBAC Consideration: November 2011 1. Purpose of Application To
PUBLIC SUMMARY DOCUMENT Product: Everolimus, tablets, 5 mg and 10 mg, Afinitor Sponsor: Novartis Pharmaceuticals Australia Pty Ltd Date of PBAC Consideration: November 2011 1. Purpose of Application To
Rituximab for the first-line maintenance treatment of follicular non-hodgkin s lymphoma
 Issue date: June 2011 Rituximab for the first-line maintenance treatment of follicular non-hodgkin s lymphoma This guidance was developed using the the single technology appraisal process NICE technology
Issue date: June 2011 Rituximab for the first-line maintenance treatment of follicular non-hodgkin s lymphoma This guidance was developed using the the single technology appraisal process NICE technology
Technology appraisal guidance Published: 27 June 2007 nice.org.uk/guidance/ta121
 Carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma Technology appraisal guidance Published: 27 June 2007 nice.org.uk/guidance/ta121 NICE 2007. All rights reserved.
Carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma Technology appraisal guidance Published: 27 June 2007 nice.org.uk/guidance/ta121 NICE 2007. All rights reserved.
The NCPE has issued a recommendation regarding the use of pertuzumab for this indication. The NCPE does not recommend reimbursement of pertuzumab.
 Cost Effectiveness of Pertuzumab (Perjeta ) in Combination with Trastuzumab and Docetaxel in Adults with HER2-Positive Metastatic or Locally Recurrent Unresectable Breast Cancer Who Have Not Received Previous
Cost Effectiveness of Pertuzumab (Perjeta ) in Combination with Trastuzumab and Docetaxel in Adults with HER2-Positive Metastatic or Locally Recurrent Unresectable Breast Cancer Who Have Not Received Previous
Trastuzumab for the treatment of HER2-positive metastatic gastric cancer
 Trastuzumab for the treatment of HER2-positive metastatic gastric cancer Issued: November 2010 guidance.nice.org.uk/ta208 NICE has accredited the process used by the Centre for Health Technology Evaluation
Trastuzumab for the treatment of HER2-positive metastatic gastric cancer Issued: November 2010 guidance.nice.org.uk/ta208 NICE has accredited the process used by the Centre for Health Technology Evaluation
National Horizon Scanning Centre. Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer. December 2007
 Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer
 LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
British Thoracic Oncology Group (BTOG) Annual Report - April 2014 to March 2015
 0116 250 2811 dawn.mckinley@uhl-tr.nhs.uk www.btog.org @BTOGORG British Thoracic Oncology Group (BTOG) Annual Report - April 2014 to March 2015 BTOG s Aim BTOG is the multi-disciplinary group for professionals
0116 250 2811 dawn.mckinley@uhl-tr.nhs.uk www.btog.org @BTOGORG British Thoracic Oncology Group (BTOG) Annual Report - April 2014 to March 2015 BTOG s Aim BTOG is the multi-disciplinary group for professionals
How To Understand The Cost Effectiveness Of Bortezomib
 DOI: 1.331/hta13suppl1/5 Health Technology Assessment 29; Vol. 13: Suppl. 1 Bortezomib for the treatment of multiple myeloma patients C Green, J Bryant,* A Takeda, K Cooper, A Clegg, A Smith and M Stephens
DOI: 1.331/hta13suppl1/5 Health Technology Assessment 29; Vol. 13: Suppl. 1 Bortezomib for the treatment of multiple myeloma patients C Green, J Bryant,* A Takeda, K Cooper, A Clegg, A Smith and M Stephens
بسم هللا الرحمن الرحيم
 بسم هللا الرحمن الرحيم Updates in Mesothelioma By Samieh Amer, MD Professor of Cardiothoracic Surgery Faculty of Medicine, Cairo University History Wagner and his colleagues (1960) 33 cases of mesothelioma
بسم هللا الرحمن الرحيم Updates in Mesothelioma By Samieh Amer, MD Professor of Cardiothoracic Surgery Faculty of Medicine, Cairo University History Wagner and his colleagues (1960) 33 cases of mesothelioma
Nalmefene for reducing alcohol consumption in people with alcohol dependence
 Nalmefene for reducing alcohol consumption in people with alcohol dependence Issued: November 2014 guidance.nice.org.uk/ta325 NICE has accredited the process used by the Centre for Health Technology Evaluation
Nalmefene for reducing alcohol consumption in people with alcohol dependence Issued: November 2014 guidance.nice.org.uk/ta325 NICE has accredited the process used by the Centre for Health Technology Evaluation
Technology appraisal guidance Published: 27 January 2016 nice.org.uk/guidance/ta376
 Radium-223 dichloride for treating hormone-relapsed prostate cancer with bone metastases Technology appraisal guidance Published: 27 January 2016 nice.org.uk/guidance/ta376 NICE 2016. All rights reserved.
Radium-223 dichloride for treating hormone-relapsed prostate cancer with bone metastases Technology appraisal guidance Published: 27 January 2016 nice.org.uk/guidance/ta376 NICE 2016. All rights reserved.
Best supportive care: Do we know what it is?
 Best supportive care: Do we know what it is? Angela Boland Rumona Dickson Barbara Jack James Stevenson Edge Hill University Faculty of Health www.liv.ac.uk/lrig Collaborative partners Liverpool Reviews
Best supportive care: Do we know what it is? Angela Boland Rumona Dickson Barbara Jack James Stevenson Edge Hill University Faculty of Health www.liv.ac.uk/lrig Collaborative partners Liverpool Reviews
January 2013 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW. Summary. Contents
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
Teriflunomide for treating relapsing remitting multiple sclerosis
 Teriflunomide for treating relapsing remitting multiple Issued: January 2014 last modified: June 2014 guidance.nice.org.uk/ta NICE has accredited the process used by the Centre for Health Technology Evaluation
Teriflunomide for treating relapsing remitting multiple Issued: January 2014 last modified: June 2014 guidance.nice.org.uk/ta NICE has accredited the process used by the Centre for Health Technology Evaluation
How To Write A Review Of Pemetrex
 Health Technology Assessment 2007; Vol. 11: No. 1 Pemetrexed disodium for the treatment of malignant pleural mesothelioma: a systematic review and economic evaluation Y Dundar, A Bagust, R Dickson, S Dodd,
Health Technology Assessment 2007; Vol. 11: No. 1 Pemetrexed disodium for the treatment of malignant pleural mesothelioma: a systematic review and economic evaluation Y Dundar, A Bagust, R Dickson, S Dodd,
1. Comparative effectiveness of alemtuzumab
 Cost-effectiveness of alemtuzumab (Lemtrada ) for the treatment of adult patients with relapsing remitting multiple sclerosis with active disease defined by clinical or imaging features The NCPE has issued
Cost-effectiveness of alemtuzumab (Lemtrada ) for the treatment of adult patients with relapsing remitting multiple sclerosis with active disease defined by clinical or imaging features The NCPE has issued
the standard of care 2009 5/1/2009 Mesothelioma: The standard of care take home messages PILC 2006 Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009
 Mesothelioma: The standard of care Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009 take home messages PILC 2006 All patients should receive adequate palliation of dyspnea and pain before starting chemotherapy
Mesothelioma: The standard of care Jan.vanmeerbeeck@ugent.be Brussels, March 7, 2009 take home messages PILC 2006 All patients should receive adequate palliation of dyspnea and pain before starting chemotherapy
DECISION AND SUMMARY OF RATIONALE
 DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Clofarabine in the treatment of relapsed acute myeloid leukaemia (AML) The application was for clofarabine to remain in
DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Clofarabine in the treatment of relapsed acute myeloid leukaemia (AML) The application was for clofarabine to remain in
Dimethyl fumarate for treating relapsing-remitting multiple sclerosis
 Dimethyl fumarate for treating relapsing-remitting multiple Issued: August 2014 guidance.nice.org.uk/ta320 NICE has accredited the process used by the Centre for Health Technology Evaluation at NICE to
Dimethyl fumarate for treating relapsing-remitting multiple Issued: August 2014 guidance.nice.org.uk/ta320 NICE has accredited the process used by the Centre for Health Technology Evaluation at NICE to
Dimethyl fumarate for treating relapsing remitting multiple sclerosis
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Final appraisal determination Dimethyl fumarate for treating relapsing remitting multiple sclerosis This guidance was developed using the single technology
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Final appraisal determination Dimethyl fumarate for treating relapsing remitting multiple sclerosis This guidance was developed using the single technology
Adalimumab for the treatment of psoriasis
 DOI: 10.3310/hta13suppl2/07 Health Technology Assessment 2009; Vol. 13: Suppl. 2 Adalimumab for the treatment of psoriasis D Turner, J Picot,* K Cooper and E Loveman Southampton Health Technology Assessments
DOI: 10.3310/hta13suppl2/07 Health Technology Assessment 2009; Vol. 13: Suppl. 2 Adalimumab for the treatment of psoriasis D Turner, J Picot,* K Cooper and E Loveman Southampton Health Technology Assessments
Cost-effectiveness of dimethyl fumarate (Tecfidera ) for the treatment of adult patients with relapsing remitting multiple sclerosis
 Cost-effectiveness of dimethyl fumarate (Tecfidera ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness
Cost-effectiveness of dimethyl fumarate (Tecfidera ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness
Prucalopride for the treatment of chronic constipation in women
 Prucalopride for the treatment of chronic constipation in Issued: December 2010 guidance.nice.org.uk/ta NICE has accredited the process used by the Centre for Health Technology Evaluation at NICE to produce
Prucalopride for the treatment of chronic constipation in Issued: December 2010 guidance.nice.org.uk/ta NICE has accredited the process used by the Centre for Health Technology Evaluation at NICE to produce
Cost-effectiveness of teriflunomide (Aubagio ) for the treatment of adult patients with relapsing remitting multiple sclerosis
 Cost-effectiveness of teriflunomide (Aubagio ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness of teriflunomide
Cost-effectiveness of teriflunomide (Aubagio ) for the treatment of adult patients with relapsing remitting multiple sclerosis The NCPE has issued a recommendation regarding the cost-effectiveness of teriflunomide
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE. Final Appraisal Determination. Anakinra for rheumatoid arthritis
 NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE Final Appraisal Determination Anakinra for rheumatoid arthritis 1 Guidance 1.1 On the balance of its clinical benefits and cost effectiveness, anakinra is not
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE Final Appraisal Determination Anakinra for rheumatoid arthritis 1 Guidance 1.1 On the balance of its clinical benefits and cost effectiveness, anakinra is not
How To Treat Lung Cancer At Cleveland Clinic
 Treatment Guide Lung Cancer Management The Chest Cancer Center at Cleveland Clinic, which includes specialists from the Respiratory Institute, Taussig Cancer Institute and Miller Family Heart & Vascular
Treatment Guide Lung Cancer Management The Chest Cancer Center at Cleveland Clinic, which includes specialists from the Respiratory Institute, Taussig Cancer Institute and Miller Family Heart & Vascular
Issue date: October 2009. Guide to the single technology appraisal process
 Issue date: October 2009 Guide to the single technology appraisal process Guide to the single technology appraisal process Issued: October 2009 This document is one of a series describing the processes
Issue date: October 2009 Guide to the single technology appraisal process Guide to the single technology appraisal process Issued: October 2009 This document is one of a series describing the processes
Treating Mesothelioma - A Quick Guide
 Treating Mesothelioma - A Quick Guide Contents This is a brief summary of the information on Treating mesothelioma from CancerHelp UK. You will find more detailed information on the website. In this information
Treating Mesothelioma - A Quick Guide Contents This is a brief summary of the information on Treating mesothelioma from CancerHelp UK. You will find more detailed information on the website. In this information
Maintenance therapy in in Metastatic NSCLC. Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai
 Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Asbestos Related Diseases. Asbestosis Mesothelioma Lung Cancer Pleural Disease. connecting raising awareness supporting advocating
 Asbestos Related Diseases Asbestosis Mesothelioma Lung Cancer Pleural Disease connecting raising awareness supporting advocating 1800 017 758 www.asbestosassociation.com.au Asbestos lagging was widely
Asbestos Related Diseases Asbestosis Mesothelioma Lung Cancer Pleural Disease connecting raising awareness supporting advocating 1800 017 758 www.asbestosassociation.com.au Asbestos lagging was widely
EVIDENCE IN BRIEF OVERALL CLINICAL BENEFIT
 perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
boceprevir 200mg capsule (Victrelis ) Treatment experienced patients SMC No. (722/11) Merck, Sharpe and Dohme Ltd
 boceprevir 200mg capsule (Victrelis ) Treatment experienced patients SMC No. (722/11) Merck, Sharpe and Dohme Ltd 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment
boceprevir 200mg capsule (Victrelis ) Treatment experienced patients SMC No. (722/11) Merck, Sharpe and Dohme Ltd 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment
Summary 1. Comparative-effectiveness
 Cost-effectiveness of Delta-9-tetrahydrocannabinol/cannabidiol (Sativex ) as add-on treatment, for symptom improvement in patients with moderate to severe spasticity due to MS who have not responded adequately
Cost-effectiveness of Delta-9-tetrahydrocannabinol/cannabidiol (Sativex ) as add-on treatment, for symptom improvement in patients with moderate to severe spasticity due to MS who have not responded adequately
Trastuzumab (Herceptin ) for patients with metastatic breast cancer
 JULY 2007 Incorporates published evidence to November 2006 INFORMATION ABOUT Trastuzumab (Herceptin ) for patients with metastatic breast cancer This information has been developed to help you understand
JULY 2007 Incorporates published evidence to November 2006 INFORMATION ABOUT Trastuzumab (Herceptin ) for patients with metastatic breast cancer This information has been developed to help you understand
Mesothelioma: Questions and Answers
 CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Mesothelioma: Questions
CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Mesothelioma: Questions
Natalizumab for the treatment of adults with highly active relapsing remitting multiple sclerosis
 Natalizumab for the treatment of adults with highly active relapsing remitting multiple sclerosis Premeeting briefing This briefing presents major issues arising from the manufacturer s submission, Evidence
Natalizumab for the treatment of adults with highly active relapsing remitting multiple sclerosis Premeeting briefing This briefing presents major issues arising from the manufacturer s submission, Evidence
Etanercept and infliximab for the treatment of adults with psoriatic arthritis. NICE technology appraisal guidance 104. Issue date: July 2006
 Issue date: July 2006 Review date: July 2007 Etanercept and infliximab for the treatment of adults with psoriatic arthritis NICE technology appraisal guidance 104 NICE technology appraisal guidance 104
Issue date: July 2006 Review date: July 2007 Etanercept and infliximab for the treatment of adults with psoriatic arthritis NICE technology appraisal guidance 104 NICE technology appraisal guidance 104
West of Scotland Cancer Network Chemotherapy Protocol. Cisplatin and Pemetrexed for Malignant Mesothelioma (LUWOS 0021)
 West of Scotland Cancer Network Chemotherapy Protocol Cisplatin and Pemetrexed for Malignant Mesothelioma (LUWOS 0021) Indication Palliative chemotherapy for malignant mesothelioma of the pleura Eligibility
West of Scotland Cancer Network Chemotherapy Protocol Cisplatin and Pemetrexed for Malignant Mesothelioma (LUWOS 0021) Indication Palliative chemotherapy for malignant mesothelioma of the pleura Eligibility
Use of medicines outside of their UK marketing authorisation in pain management and palliative medicine
 The British Pain Society Use of medicines outside of their UK marketing authorisation in pain management and palliative medicine This is a consensus document prepared on behalf of the British Pain Society
The British Pain Society Use of medicines outside of their UK marketing authorisation in pain management and palliative medicine This is a consensus document prepared on behalf of the British Pain Society
How To Contact The Lung Cancer And Mesothelioma Multi-Disciplinary Team
 Oxford University Hospitals NHS Trust Oxford Lung Cancer and Mesothelioma Multi- Disciplinary Team (MDT) This information leaflet explains the role of the Multi-Disciplinary Team and Specialist Nursing
Oxford University Hospitals NHS Trust Oxford Lung Cancer and Mesothelioma Multi- Disciplinary Team (MDT) This information leaflet explains the role of the Multi-Disciplinary Team and Specialist Nursing
L Lang-Lazdunski, A Bille, S Marshall, R Lal, D Landau, J Spicer
 Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine and systemic chemotherapy in malignant pleural mesothelioma. A 10-year experience. L Lang-Lazdunski, A Bille, S Marshall, R Lal,
Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine and systemic chemotherapy in malignant pleural mesothelioma. A 10-year experience. L Lang-Lazdunski, A Bille, S Marshall, R Lal,
Please see the LUCADA data manual v3.1.3, available in the downloads section
 National Lung Cancer Audit Frequently Asked Questions What dataset should be used? Please see the LUCADA data manual v3.1.3, available in the downloads section What does LUCADA stand for? LUCADA stands
National Lung Cancer Audit Frequently Asked Questions What dataset should be used? Please see the LUCADA data manual v3.1.3, available in the downloads section What does LUCADA stand for? LUCADA stands
B10/S/a Cancer: Malignant Mesothelioma (Adult)
 B10/S/a 2013/14 NHS STANDARD CONTRACT FOR CANCER: MALIGNANT MESOTHELIOMA (ADULT) SECTION B PART 1 - SERVICE SPECIFICATIONS Service Specification No. Service Commissioner Lead Provider Lead Period Date
B10/S/a 2013/14 NHS STANDARD CONTRACT FOR CANCER: MALIGNANT MESOTHELIOMA (ADULT) SECTION B PART 1 - SERVICE SPECIFICATIONS Service Specification No. Service Commissioner Lead Provider Lead Period Date
Introduction Objective Methods Results Conclusion
 Introduction Objective Methods Results Conclusion 2 Malignant pleural mesothelioma (MPM) is the most common form of mesothelioma a rare cancer associated with long latency period (i.e. 20 to 40 years),
Introduction Objective Methods Results Conclusion 2 Malignant pleural mesothelioma (MPM) is the most common form of mesothelioma a rare cancer associated with long latency period (i.e. 20 to 40 years),
Exploring the Role of Vitamins in Achieving a Healthy Heart
 Exploring the Role of Vitamins in Achieving a Healthy Heart There are many avenues you can take to keep your heart healthy. The first step you should take is to have a medical professional evaluate the
Exploring the Role of Vitamins in Achieving a Healthy Heart There are many avenues you can take to keep your heart healthy. The first step you should take is to have a medical professional evaluate the
To: Interested Parties. Our reference: MLX 310 Date: 2 August 2004. Dear Sir/Madam
 To: Interested Parties 020 7084 2642 020 7084 2121 anne.thyer@mhra.gsi.gov.uk Our reference: MLX 310 Date: 2 August 2004 Dear Sir/Madam PROPOSALS TO ENABLE THE USE OF ELECTRONIC SIGNATURES ON PRESCRIPTIONS
To: Interested Parties 020 7084 2642 020 7084 2121 anne.thyer@mhra.gsi.gov.uk Our reference: MLX 310 Date: 2 August 2004 Dear Sir/Madam PROPOSALS TO ENABLE THE USE OF ELECTRONIC SIGNATURES ON PRESCRIPTIONS
Asbestos and your lungs
 This information describes what asbestos is and the lung conditions that are caused by exposure to it. It also includes information about what to do if you have been exposed to asbestos, and the benefits
This information describes what asbestos is and the lung conditions that are caused by exposure to it. It also includes information about what to do if you have been exposed to asbestos, and the benefits
Cancer patients waiting for potentially live-saving treatments in UK
 Cancer patients waiting for potentially live-saving treatments in UK 29 May 2005 UK patients are waiting too long for new treatments, according to a 'Dossier of Delay' compiled by information charity CancerBACUP.
Cancer patients waiting for potentially live-saving treatments in UK 29 May 2005 UK patients are waiting too long for new treatments, according to a 'Dossier of Delay' compiled by information charity CancerBACUP.
Medical Technologies Evaluation Programme Methods guide
 Issue date: April 2011 Medical Technologies Evaluation Programme Methods guide National Institute for Health and Clinical Excellence MidCity Place 71 High Holborn London WC1V 6NA www.nice.org.uk National
Issue date: April 2011 Medical Technologies Evaluation Programme Methods guide National Institute for Health and Clinical Excellence MidCity Place 71 High Holborn London WC1V 6NA www.nice.org.uk National
Asbestos Related Diseases
 Asbestos Related Diseases Asbestosis Mesothelioma Lung Cancer Pleural Disease Asbestosis and Mesothelioma (LUNG CANCER) Support Group 1800 017 758 www.amsg.com.au ii Helping you and your family through
Asbestos Related Diseases Asbestosis Mesothelioma Lung Cancer Pleural Disease Asbestosis and Mesothelioma (LUNG CANCER) Support Group 1800 017 758 www.amsg.com.au ii Helping you and your family through
Salisbury Lung Cancer Service (1 of 5)
 Salisbury Lung Cancer Service (1 of 5) i If you need this information in another language or medium (audio, large print, etc) please contact Customer Care on 0800 374 208 email: customercare@ salisbury.nhs.uk.
Salisbury Lung Cancer Service (1 of 5) i If you need this information in another language or medium (audio, large print, etc) please contact Customer Care on 0800 374 208 email: customercare@ salisbury.nhs.uk.
PATIENT INFORMATION SHEET
 PATIENT INFORMATION SHEET Surgical and large bore pleural procedures in Malignant pleural Mesothelioma And Radiotherapy Trial (SMART trial) Stoke Mandeville Hospital Mandeville Road Aylesbury Buckinghamshire
PATIENT INFORMATION SHEET Surgical and large bore pleural procedures in Malignant pleural Mesothelioma And Radiotherapy Trial (SMART trial) Stoke Mandeville Hospital Mandeville Road Aylesbury Buckinghamshire
Evaluation of a Primary Care Dermatology Service: final report
 Evaluation of a Primary Care Dermatology Service: final report Report for the National Co-ordinating Centre for NHS Service Delivery and Organisation R&D (NCCSDO) December 2005 prepared by Chris Salisbury*
Evaluation of a Primary Care Dermatology Service: final report Report for the National Co-ordinating Centre for NHS Service Delivery and Organisation R&D (NCCSDO) December 2005 prepared by Chris Salisbury*
Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation
 Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation Issued: May 2012 guidance.nice.org.uk/ta256 NICE has accredited the process used by the Centre for Health
Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation Issued: May 2012 guidance.nice.org.uk/ta256 NICE has accredited the process used by the Centre for Health
DECISION AND SUMMARY OF RATIONALE
 DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Crizotinib as 2nd line treatment for patients with anaplastic lymphoma kinase (ALK) positive lung cancer Score The application
DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Crizotinib as 2nd line treatment for patients with anaplastic lymphoma kinase (ALK) positive lung cancer Score The application
boceprevir 200mg capsule (Victrelis ) Treatment naïve patients SMC No. (723/11) Merck Sharpe and Dohme Ltd
 boceprevir 200mg capsule (Victrelis ) Treatment naïve patients SMC No. (723/11) Merck Sharpe and Dohme Ltd 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the
boceprevir 200mg capsule (Victrelis ) Treatment naïve patients SMC No. (723/11) Merck Sharpe and Dohme Ltd 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the
Donepezil, galantamine, rivastigmine (review) and memantine for the treatment of Alzheimer s disease (amended)
 Issue date: November 2006 (amended September 2007, August 2009) Donepezil, galantamine, rivastigmine (review) and memantine for the treatment of Alzheimer s disease (amended) Includes a review of NICE
Issue date: November 2006 (amended September 2007, August 2009) Donepezil, galantamine, rivastigmine (review) and memantine for the treatment of Alzheimer s disease (amended) Includes a review of NICE
GIVING INFORMATION TO LUNG CANCER PATIENTS
 GIVING INFORMATION TO LUNG CANCER PATIENTS Guidance For Healthcare Professionals Discussing Options For Patients On The Lung Cancer Pathway British Thoracic Society Lung Cancer and Mesothelioma Specialist
GIVING INFORMATION TO LUNG CANCER PATIENTS Guidance For Healthcare Professionals Discussing Options For Patients On The Lung Cancer Pathway British Thoracic Society Lung Cancer and Mesothelioma Specialist
Supportive Care For Patients With High-Grade Glioma (primary brain tumours) Dr Susan Catt & Professor Lesley Fallowfield
 Supportive Care For Patients With High-Grade Glioma (primary brain tumours) Dr Susan Catt & Professor Lesley Fallowfield Partners Mr Giles Critchley Consultant Neurosurgeon Hurstwood Park Neurological
Supportive Care For Patients With High-Grade Glioma (primary brain tumours) Dr Susan Catt & Professor Lesley Fallowfield Partners Mr Giles Critchley Consultant Neurosurgeon Hurstwood Park Neurological
Randomized trials versus observational studies
 Randomized trials versus observational studies The case of postmenopausal hormone therapy and heart disease Miguel Hernán Harvard School of Public Health www.hsph.harvard.edu/causal Joint work with James
Randomized trials versus observational studies The case of postmenopausal hormone therapy and heart disease Miguel Hernán Harvard School of Public Health www.hsph.harvard.edu/causal Joint work with James
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE. Final Appraisal Determination. Patient-education models for diabetes
 NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE 1 Guidance 1.1 It is recommended that structured patient education is made available to all people with diabetes at the time of initial diagnosis and then as
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE 1 Guidance 1.1 It is recommended that structured patient education is made available to all people with diabetes at the time of initial diagnosis and then as
NHS. Surgical repair of vaginal wall prolapse using mesh. National Institute for Health and Clinical Excellence. 1 Guidance.
 Issue date: June 2008 NHS National Institute for Health and Clinical Excellence Surgical repair of vaginal wall prolapse using mesh 1 Guidance 1.1 The evidence suggests that surgical repair of vaginal
Issue date: June 2008 NHS National Institute for Health and Clinical Excellence Surgical repair of vaginal wall prolapse using mesh 1 Guidance 1.1 The evidence suggests that surgical repair of vaginal
Management of Non-Small Cell Lung Cancer Guide for General Practitioners
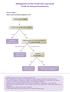 Management of n-small Cell Lung Cancer Guide for General Practitioners Clinical Stage I Cancer only in one lobe of lung and
Management of n-small Cell Lung Cancer Guide for General Practitioners Clinical Stage I Cancer only in one lobe of lung and
Daclatasvir for treating chronic hepatitis C
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Appraisal consultation document Daclatasvir for treating chronic hepatitis C The Department of Health has asked the National Institute for Health and Care
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Appraisal consultation document Daclatasvir for treating chronic hepatitis C The Department of Health has asked the National Institute for Health and Care
Malignant Mesothelioma: an Update
 Malignant Mesothelioma: an Update Nico van Zandwijk Asbestos Diseases Research Institute Bernie Banton Centre University of Sydney Australia Physicians Week RACP 19-5-2009 Health Risks of Asbestos Fibers
Malignant Mesothelioma: an Update Nico van Zandwijk Asbestos Diseases Research Institute Bernie Banton Centre University of Sydney Australia Physicians Week RACP 19-5-2009 Health Risks of Asbestos Fibers
COST OF SKIN CANCER IN ENGLAND MORRIS, S., COX, B., AND BOSANQUET, N.
 ISSN 1744-6783 COST OF SKIN CANCER IN ENGLAND MORRIS, S., COX, B., AND BOSANQUET, N. Tanaka Business School Discussion Papers: TBS/DP05/39 London: Tanaka Business School, 2005 1 Cost of skin cancer in
ISSN 1744-6783 COST OF SKIN CANCER IN ENGLAND MORRIS, S., COX, B., AND BOSANQUET, N. Tanaka Business School Discussion Papers: TBS/DP05/39 London: Tanaka Business School, 2005 1 Cost of skin cancer in
Pain and symptom management in pleural mesothelioma
 Pain and symptom management in pleural mesothelioma MARF October 2006 Helen Clayson Hospice of St Mary of Furness University of Sheffield Outline Background to the study Symptoms in mesothelioma What is
Pain and symptom management in pleural mesothelioma MARF October 2006 Helen Clayson Hospice of St Mary of Furness University of Sheffield Outline Background to the study Symptoms in mesothelioma What is
Surgery. Wedge resection only part of the lung, not. not a lobe, is removed. Cancer Council NSW
 The treatment you receive will depend on your lung cancer type, for example, whether you have a non-small cell lung cancer Adenocarcinoma or Squamous cell carcinoma, and if this is a sub-type with a mutation.
The treatment you receive will depend on your lung cancer type, for example, whether you have a non-small cell lung cancer Adenocarcinoma or Squamous cell carcinoma, and if this is a sub-type with a mutation.
PRIMARY LUNG CANCER TREATMENT
 PRIMARY LUNG CANCER TREATMENT Cancer Care Pathways Directorate Tailored Information in Cancer Care (TICC) Sir Anthony Mamo Oncology Centre December 2014 Contents About this booklet 1 Types of Lung Cancer
PRIMARY LUNG CANCER TREATMENT Cancer Care Pathways Directorate Tailored Information in Cancer Care (TICC) Sir Anthony Mamo Oncology Centre December 2014 Contents About this booklet 1 Types of Lung Cancer
How To Prepare A Meeting For A Health Care Conference
 THORACIC ONCOLOGY MULTIDISCIPLINARY TEAM MEETINGS: OPERATIONAL POLICY EXECUTIVE SUMMARY 1. All patients in Lothian with thoracic malignancies should be discussed at designated times in pathway (see App
THORACIC ONCOLOGY MULTIDISCIPLINARY TEAM MEETINGS: OPERATIONAL POLICY EXECUTIVE SUMMARY 1. All patients in Lothian with thoracic malignancies should be discussed at designated times in pathway (see App
Clinical cases in Malignant Pleural Mesothelioma: Adherence to the ESMO Clinical Practice Guidelines
 Clinical cases in Malignant Pleural Mesothelioma: Adherence to the ESMO Clinical Practice Guidelines Wieneke Buikhuisen The Netherlands Cancer Institute Amsterdam The Netherlands Case (1) Male, 56 year
Clinical cases in Malignant Pleural Mesothelioma: Adherence to the ESMO Clinical Practice Guidelines Wieneke Buikhuisen The Netherlands Cancer Institute Amsterdam The Netherlands Case (1) Male, 56 year
Alemtuzumab for treating relapsing-remitting multiple sclerosis
 Alemtuzumab for treating relapsing-remitting multiple Issued: May 2014 guidance.nice.org.uk/ta NICE has accredited the process used by the Centre for Health Technology Evaluation at NICE to produce technology
Alemtuzumab for treating relapsing-remitting multiple Issued: May 2014 guidance.nice.org.uk/ta NICE has accredited the process used by the Centre for Health Technology Evaluation at NICE to produce technology
Treatment for pleural mesothelioma
 Treatment for pleural mesothelioma This information is an extract from the booklet Understanding mesothelioma. You may find the full booklet helpful. We can send you a free copy see page 9. Contents Treatment
Treatment for pleural mesothelioma This information is an extract from the booklet Understanding mesothelioma. You may find the full booklet helpful. We can send you a free copy see page 9. Contents Treatment
Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost Effectiveness
 Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost Effectiveness Investigators: Paul G. Shekelle, MD, PhD, Director Alicia R. Maher, MD Clinical
Treatment of Metastatic Non-Small Cell Lung Cancer: A Systematic Review of Comparative Effectiveness and Cost Effectiveness Investigators: Paul G. Shekelle, MD, PhD, Director Alicia R. Maher, MD Clinical
Advances In Chemotherapy For Hormone Refractory Prostate Cancer. TAX 327 study results & SWOG 99-16 study results presented at ASCO 2004
 Ronald de Wit Rotterdam Cancer Institute The Netherlands Advances In Chemotherapy For Hormone Refractory Prostate Cancer TAX 327 study results & SWOG 99-16 study results presented at Slide 1 Prostate Cancer
Ronald de Wit Rotterdam Cancer Institute The Netherlands Advances In Chemotherapy For Hormone Refractory Prostate Cancer TAX 327 study results & SWOG 99-16 study results presented at Slide 1 Prostate Cancer
SMALL CELL LUNG CANCER
 Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Pre-workshop exercise
 Setting research priorities for mesothelioma workshop 10 th November 2014 Pre-workshop exercise Your individual ranking of unanswered questions about the diagnosis, treatment and care of mesothelioma Please
Setting research priorities for mesothelioma workshop 10 th November 2014 Pre-workshop exercise Your individual ranking of unanswered questions about the diagnosis, treatment and care of mesothelioma Please
How valuable is a cancer therapy? It depends on who you ask.
 How valuable is a cancer therapy? It depends on who you ask. Comparing and contrasting the ESMO Magnitude of Clinical Benefit Scale with the ASCO Value Framework in Cancer Ram Subramanian Kevin Schorr
How valuable is a cancer therapy? It depends on who you ask. Comparing and contrasting the ESMO Magnitude of Clinical Benefit Scale with the ASCO Value Framework in Cancer Ram Subramanian Kevin Schorr
London Cancer. Mesothelioma Lung Protocols
 London Cancer Mesothelioma Lung Protocols Version 0.9 Contents 1. Staging... 3 2. Mesothelioma Summary of Chemotherapy Protocols... 4 3. Mesothelioma Chemotherapy Protocols... 7 3.1. Pemetrexed (Alimta
London Cancer Mesothelioma Lung Protocols Version 0.9 Contents 1. Staging... 3 2. Mesothelioma Summary of Chemotherapy Protocols... 4 3. Mesothelioma Chemotherapy Protocols... 7 3.1. Pemetrexed (Alimta
Cancer Treatments Subcommittee of PTAC Meeting held 18 September 2015. (minutes for web publishing)
 Cancer Treatments Subcommittee of PTAC Meeting held 18 September 2015 (minutes for web publishing) Cancer Treatments Subcommittee minutes are published in accordance with the Terms of Reference for the
Cancer Treatments Subcommittee of PTAC Meeting held 18 September 2015 (minutes for web publishing) Cancer Treatments Subcommittee minutes are published in accordance with the Terms of Reference for the
REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group
 REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group In the 2002 edition of the ASCO meeting, a total of 315 abstracts in the field of respiratory
REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group In the 2002 edition of the ASCO meeting, a total of 315 abstracts in the field of respiratory
DECISION AND SUMMARY OF RATIONALE
 DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Everolimus in combination with exemestane hormone therapy for oestrogen receptor positive locally advanced or metastatic
DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Everolimus in combination with exemestane hormone therapy for oestrogen receptor positive locally advanced or metastatic
How To Understand How Cancer Works
 Mesothelioma Understanding your diagnosis Mesothelioma Understanding your diagnosis When you first hear that you have cancer, you may feel alone and afraid. You may be overwhelmed by the large amount of
Mesothelioma Understanding your diagnosis Mesothelioma Understanding your diagnosis When you first hear that you have cancer, you may feel alone and afraid. You may be overwhelmed by the large amount of
Alcohol-use disorders overview
 Alcohol-use disorders overview A NICE pathway brings together all NICE guidance, quality standards and materials to support implementation on a specific topic area. The pathways are interactive and designed
Alcohol-use disorders overview A NICE pathway brings together all NICE guidance, quality standards and materials to support implementation on a specific topic area. The pathways are interactive and designed
POLICY A. INDICATIONS
 Alimta (pemetrexed) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 09/01/2007 Current Effective Date: 10/01/2015 POLICY A. INDICATIONS The indications below
Alimta (pemetrexed) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 09/01/2007 Current Effective Date: 10/01/2015 POLICY A. INDICATIONS The indications below
