Kirshner, et al DOI: /JCO
|
|
|
- Austin Horton
- 8 years ago
- Views:
Transcription
1 The following protocol information is provided solely to describe how the authors conducted the research underlying the published report associated with the following article: Prevention of Pegfilgrastim-Induced Bone Pain: A Phase III Double-Blind Placebo- Controlled Randomized Clinical Trial of the University of Rochester Cancer Center Clinical Community Oncology Program Research Base Kirshner, et al DOI: /JCO The information provided may not reflect the complete protocol or any previous amendments or modifications. As described in the Information for Contributors ( only specific elements of the most recent version of the protocol are requested by JCO. The protocol information is not intended to replace good clinical judgment in selecting appropriate therapy and in determining drug doses, schedules, and dose modifications. The treating physician or other health care provider is responsible for determining the best treatment for the patient. ASCO and JCO assume no responsibility for any injury or damage to persons or property arising out of the use of these protocol materials or due to any errors or omissions. Individuals seeking additional information about the protocol are encouraged to consult with the corresponding author directly.
2 UNIVERSITY OF ROCHESTER CANCER CENTER CCOP RESEARCH BASE Prevention of Pegfilgrastim-Induced Bone Pain (PIBP): A Phase III Double-Blind Placebo-Controlled Clinical Trial NCI Protocol # URCC URCC Protocol # URCC07079 Study Chair: Study Co-Chair: Biostatistics Chair: Jeffrey J. Kirshner, MD, HOACNY CCOP Gary R. Morrow, PhD, MS, URCC Jeffrey K. Giguere, MD, Greenville CCOP Pascal Jean-Pierre, PhD, URCC Charles Heckler, PhD, URCC James P. Wilmot Cancer Center URCC CCOP Research Base 601 Elmwood Avenue, Box 704 Rochester, NY (585) NCI Concept Submission 19-Dec-06 Revised Concept Submission 15-Feb-07 NCI Concept Approval 24-Apr-07 Protocol Submitted to NCI/PRC 2-Aug-07 Revised Protocol Submission 19-Dec-07 Amendment #1 29-Apr-08
3 Study Schema N=322 Phone call 3-5 days after pegfilgrastim administration Prior to first dose of pegfilgrastim -Consent -Questionnaires -Randomize to naproxen/placebo [Baseline] Day 2, 3 or 4 of chemotherapy cycle -Pegfilgrastim administered SC -Begin study medication -Return baseline questionnaires -Outcome questionnaires Study participation ends -Complete and mail outcome questionnaires [Outcome] 5 to 8 days of naproxen 500mg or matching placebo
4 3.0 Objectives 3.1 Primary Objectives: To determine if daily administration of naproxen, in an oral dose of 500 mg twice daily (BID) can prevent or reduce the severity and duration of PIBP compared to placebo. 3.2 Secondary Objectives: To identify potential risk factors for the development of PIBP To identify potential clinical predictors for the response or failure to respond to naproxen in an oral dose of 500 mg twice daily (BID) for prevention of PIBP To assess the toxicity of the administration of naproxen, in an oral dose of 500 mg twice daily (BID) in the preventive setting in this population of patients. 4.0 Participant Eligibility Study participants: 4.1 Must be diagnosed with a non-hematologic (non-myeloid) malignancy, be scheduled to receive chemotherapy and be scheduled to receive a first dose of pegfilgrastim (Neulasta ) (6 mg given sub-cutaneously [SC]) to ameliorate chemotherapy-induced neutropenia Chemotherapy may be for adjuvant, neoadjuvant, curative or palliative intent The pegfilgrastim must be administered on Day 2, 3 or 4 of the chemotherapy cycle (not more than 72 hours following chemotherapy). Date and time of pegfilgrastim administration must be recorded on the chemotherapy flow sheet. 4.2 Must not have clinical evidence of active gastrointestinal bleeding, a history of gastrointestinal bleeding, or gastric or duodenal ulcers. 4.3 Must not have undergone surgery on the heart within the past six months.
5 4.4 Must not have a known allergy to naproxen or have developed the triad of asthma, rhinitis, and nasal polyps after taking aspirin or other nonsteroidal antiinflammatory drugs. 4.5 Must not be currently taking any NSAID, such as aspirin and ibuprofen, any product containing naproxen (e.g. Naprosyn, EC-Naprosyn, Anaprox, Anaprox- DS, Naprosyn suspension, Aleve), or any steroid medication on a regular basis. 4.6 Must not be regularly taking any prescription or non-prescription medications for pre-existing chronic pain Patients who are on cardioprotective aspirin up to 325 mg per day are allowed. 4.7 Must not be currently taking therapeutic doses of Warfarin. 4.8 Must not have pre-chemotherapy serum creatinine level greater than 1.5 X the upper limit of normal (ULN). 4.9 Must not be pregnant or nursing. 4.9 Must be able to understand English (all assessment instruments are in English) Must be 18 years of age or older Must give written informed consent. 6.0 Treatment Protocol, Study Outline This is a randomized, double-blind, placebo-controlled Phase III clinical intervention trial to assess the efficacy of naproxen vs. placebo to prevent or ameliorate bone pain induced by pegfilgrastim (PIBP). Both groups of patients will keep a diary of the doses of study medication (naproxen or placebo) as well as any other pain medications and record their experience with PIBP. Details of the study design are as follows. 6.1 Patients must be approached for study participation before they receive their first dose of pegfilgrastim. Patients who are eligible and agree to participate will sign a consent form prior to completing baseline questionnaires or undergoing any other study procedures. 6.2 Participant instructions and tasks: Prior to (within two weeks of) the administration of their first dose of pegfilgrastim (baseline assessment), participants will complete baseline
6 questionnaires including questions concerning the presence, location and duration of any bone or joint pain as well as the manner in which the pain was treated When they receive their first dose of pegfilgrastim, (or at the chemotherapy visit if pegfilgrastim will be self-administered by the participant at home) each participant will be given a bottle containing all of their assigned study medication (16 tablets of naproxen or placebo) for up to 8 days of chemotherapy cycle one Participants will be instructed to take one tablet by mouth, twice daily, in the morning and in the evening, beginning on the day they receive their first dose of pegfilgrastim, and continuing for at least five days (up to a maximum of eight days) Participants will be given a total of 16 tablets of study medication so that they can take their medication for up to eight days if necessary for pain control Participants will be instructed to take their medication with food or milk The first dose of study medication should be taken prior to the administration of pegfilgrastim or as soon as possible after it is administered A missed dose of study drug may be taken if the time is within four hours of when the dose is customarily taken If the elapsed time since the missed dose is longer than four hours, that dose should not be taken and the next scheduled dose should be taken at the appropriate time Patients will be instructed to return their medication bottles at the following chemotherapy cycle At the study visit at which pegfilgrastim is administered, (or at the chemotherapy visit if pegfilgrastim will be self-administered by the participant at home) participants will be given a second set of questionnaires (outcome assessment) to complete at home and mail back to the treatment site. The set will include a questionnaire assessing any development of new bone or joint pain, its location, onset and duration, severity, and an assessment of the efficacy of the assigned medication Participants will mail the completed questionnaires back to each site in postage-paid envelopes provided.
7 6.2.6 A phone call will be made to participants on the third, fourth or fifth day following pegfilgrastim (Days 5, 6 or 7 of the chemotherapy cycle) to remind them to complete and mail back the questionnaires. 6.3 Treatment Duration Participants take study medication for one chemotherapy treatment only, i.e., the first chemotherapy treatment where pegfilgrastim is administered The trial ends when the completed measures have been returned following the participant s first dose of pegfilgrastim. 6.4 Ancillary Treatments Health care providers may prescribe acetaminophen, tramadol (Ultram ), narcotics or other analgesics except NSAIDs for participants as needed for control of pain If the use of any additional medication for bone pain becomes medically indicated, the participant may remain on the study medication and should record the name and dose of the additional pain medication as well as date(s) and time(s) they took the additional medication on the forms provided. 7.1 Primary Outcome Measure 7.0 Treatment Evaluation Our primary outcome measure will be the area under the curve for pain from baseline through day 5. (Day 1 = the day pegfilgrastim is administered.) Daily pain severity will be as recorded by the patient in the daily diary. Any rating of pain, from 1 to 10 on the scale will be considered a day with pain. This outcome measure will incorporate both the severity and the duration of the patient s bone pain. Pain measures after day 5 will be used for exploratory analysis only, to determine the proportion of patients whose pain lasts longer than 5 days. Pain duration will be measured as the number of days with pain. This information will be obtained from a daily diary given to each participant. Pain relief is also assessed in the BPI by asking patients what percent of their pain was relieved by medication, however the primary assessment about pain relief will be through the daily diary. As another way to assess the efficacy of the naproxen for pain relief, we will look at the amount of additional analgesia used by the patient, as recorded in the daily diary.
8 The Daily Diary is a three-page questionnaire. On each day for at least five days (and up to eight days) each participant will record the worst severity of any bone pain experienced that day ( using the worst pain question as written in the BPI), indicate the name and number of doses taken of any pain medication they took other than their assigned study medication, and will also indicate whether or not they took their study medication. 7.2 Secondary Outcome Measures Potential risk factors for the development of PIBP as well as for response or failure to respond to treatment, including participant age, sex, ethnicity, disease and stage, and chemotherapeutic regimen, will be obtained from the participant s medical record and from participant interview, and will be recorded on the On- Study form. We will also assess the presence or severity of symptoms prior to study enrollment and at study outcome using the Symptom Inventory. The Symptom Inventory was modified from measures created at MD Anderson and Memorial- Sloan Kettering Cancer Centers and is comprised of a list of 13 symptoms. The severity of each symptom is rated on an 11 point scale anchored by 0 = Not Present and 10 = As Bad As You Can Imagine. This questionnaire is used by our Clinical Trials Office in URCC investigator-initiated clinical trials. Oncologists at the URCC also use the measure in clinical practice in both Radiation and Medical Oncology clinics. Assessment of symptoms at baseline will be used to identify additional risk factors for the development of PIBP. Completion of the SI on Day 5 will be used to determine whether naproxen affects the presence or severity of symptoms other than pain (e.g. sleep, fatigue) and to identify potential adverse effects of the medication. The Brief Pain Inventory (Short Form) (BPI) will be used to measure general pain at baseline and on day 5 (or up to day 8 after pegfilgrastim administration, if the pain continues). The BPI is a self-report measure of pain intensity and the interference of pain with function. The original BPI was developed and validated by the Pain Research Group, WHO Collaborating Centre for Symptom Evaluation in Cancer Care. (6;7) Validity and reliability of the original BPI have been verified across several different cultures and languages. (7) We will use the short form of the BPI (BPI-SF). The reliability and validity of this modified, eight item, short form when used to assess pain on a daily basis has also been established. (8) It has been used extensively in clinical studies of patients with cancer. (9) In this questionnaire, pain intensity over the previous 24 hours is assessed at its worst, its least, on average and right now is assessed using an 11 point numerical rating scale anchored by 0 = No pain and 10 = Pain as bad as you can imagine. Interference of pain with general activity, mood, walking ability, normal work, interpersonal relations, sleep and enjoyment of life is assessed with an 11 point numerical rating scale anchored by 0 = Does not interfere and 10 = Completely interferes. A composite interference score will be calculated from the means of the individual responses.
9 We will track the occurrence and severity of toxicity from the medication using the AE reports submitted to the URCC Research Base. As stated in Section 6.0, toxicity will be assessed and reported using the NCI Common Toxicity Criteria. Patients will also record side effects from the study medication in the daily diary. 8.0 Drug Characteristics, Dosage and Administration 8.1 General Information Description: naproxen is a practically odorless, white to off-white crystalline substance. It is a lipid soluble, practically insoluble in water at low ph and freely soluble in water at high ph. The chemical formula is (+)-6-methoxy- -methyl-2-naphthleneacetic acid Clinical Pharmacology: naproxen is a non-steroidal anti-inflammatory drug with analgesic and antipyretic properties. Naproxen is rapidly and completely absorbed from the gastrointestinal tract with an in vivo bioavailability of 95%. The elimination half-life is hours and does not differ between products. Approximately 95% is excreted in the urine Mode of action: naproxen inhibits prostaglandin synthesis. Additional modes of action are possible, but have not been defined Pregnancy category C. There are no adequate and well-controlled studies in pregnant women. 8.2 Drug Formulation and Procurement All study medications (drug and placebo) will be supplied. The study drugs will be packaged in bottles of 16 tablets of naproxen 500 mg or placebo (identical in appearance, smell and taste to the study medication) each. The bottles will be distributed to the individual CCOPs from the Research Base. 8.3 Storage and Stability Naproxen is stable at normal room temperatures (between 68 F and 77 F). The study drug naproxen and the placebo must be stored at room temperature, in a dry place and in a securely locked, substantially constructed cabinet or enclosure. 8.4 Dosage and Administration
10 8.4.1 Prescription-strength naproxen and placebo will be administered in the dosage and manner specified for each arm in section Adverse Effects Adverse effects occurring in more than 3% - 9% of 960 patients treated for rheumatoid arthritis or osteoarthritis include constipation, heartburn, abdominal pain, nausea, headache, dizziness, drowsiness, itching, skin eruptions, ecchymoses, tinnitus, edema and dyspnea. Minor gastrointestinal problems such as dyspepsia are common and may develop early in the course of treatment. Serious gastrointestinal toxicity such as bleeding, ulceration and perforation have been reported in approximately 1% of individuals taking NSAIDs for 3-6 months, and in 2%-4% of patients taking these medications for one year, much longer periods than the time over which the drug will be administered in this study. In post-marketing studies of naproxen, photosensitivity reactions and changes in blood pressure (hypotension or hypotension) were observed in less than 1% of patients Patients will be instructed to watch for signs of gastrointestinal bleeding (blood in vomit or bloody, black, or tarry stools) Patients will be instructed to stop taking the study medication and call their treating oncologist immediately if any sign of gastrointestinal bleeding occurs Patients will be instructed to avoid drinking alcoholic beverages while taking the study medication Since drowsiness and dizziness may occur, patients will be urged to use care when driving or operating heavy equipment while taking the study drug Precautions Potential Drug Interactions Caution should be used in patients receiving methotrexate. These patients should be monitored for evidence of greater-thananticipated methotrexate-related hematologic and gastrointestinal toxicity Caution should be used in patients receiving ACE-inhibitors, as concomitant administration of naproxen may decrease the antihypertensive effect of these drugs.
11 Caution should be used in patients receiving lithium. These patients should be monitored for signs of lithium toxicity. (Diarrhea, vomiting, drowsiness, muscular weakness, and lack of coordination are early signs of lithium toxicity, whereas giddiness, ataxia, blurred vision, tinnitus or a large output of dilute urine may be seen at serum levels of lithium higher than 2 meq/l.) Caution should be used in patients receiving furosemide (Lasix) and thiazides as naproxen may decrease the natriuretic effect of these drugs in some patients. 9.0 Statistical Considerations 9.1 The assumptions underlying all statistical analyses will be thoroughly checked using appropriate graphical and numerical methods. (10;11) In the case of serious violations of the assumptions, appropriate nonparametric methods will be attempted. If outliers or influential data are detected, the accuracy of the data will be investigated. If no errors are found, analyses may be repeated after removing these cases to evaluate their impact on the results, however, the final analyses will include these data points. All statistical tests will be performed at the two-tailed 5% level of significance. Likewise, 95% confidence intervals will be constructed for estimation of effects (e.g., difference in mean change scores of pain severity between the treatment group and the placebo group). 9.2 Data will be analyzed on an "intent-to-treat" basis; participant data will be included in the treatment group to which the participant was randomized regardless of any subsequent changes to the treatment Every effort will be made to encourage and facilitate participants completion of questionnaires. In the event of missing data, the reasons for missing data will be recorded and tabulated according to treatment groups. 9.4 Statistical Analysis Baseline demographic and medical history variables will be summarized for each treatment group. Summaries for quantitative variables, such as age, will include the mean, median, standard deviation, minimum, and maximum. For qualitative variables, such as gender, summaries will include the number and percent of patients for each outcome. Treatment group comparisons will be based on either the two-sample t-tests for quantitative variables or chi-square tests for qualitative variables.
12 9.4.2 The primary outcome measure for this study is the area under the curve (AUC) for the patient s pain score from baseline through day five following pegfilgrastim administration. The AUC measure is the pain scores reported on the daily diary for the five days of observation. The X axis of the AUC represents duration of pain (5 days) and the Y axis represents severity (daily pain score.) This will be compared between the two treatment groups using the Analysis of Covariance (ANCOVA) method adjusting for baseline pain measures Additional analysis will address the secondary objectives of determining if there are any characteristics (age, sex, disease, type of chemotherapy regimen, et al.) that predict failure to prevent pain and if there are characteristics that correlate with the development and/or severity of pain. These will be analyzed using linear regression for the AUC of pain and the logisticregression for development of the pain. Baseline characteristics, treatment group and the interaction between baseline characteristics and treatment will be included as the covariates Differences in the proportion of patients developing serious toxicities in the two groups will be analyzed using chi-square tests There is no interim analysis planned. 9.5 Sample Size From preliminary data from the Syracuse CCOP (HOACNY), we collected data on the pain severity score at the start of the pain, the pain score on the last day of the pain and the duration of the pain. From these data we were able to calculate the area under the curve for the pain score assuming the pain score changed linearly over this period. The average AUC is 5.02 and the standard deviation is In order to have 80% power to detect a difference of 2.51 (a 50% reduction of the pain in the treatment group as compared to the control group) for the two treatment groups, using a twosided test with significance level of 0.05, we would need 145 evaluable people in each treatment group, thus 290 evaluable patients in total. Anticipating a 10% dropout rate, we will accrue approximately 322 patients, 161 in each group. 9.6 Representation of Women and Minorities None of the eligibility criteria for the study involve gender or ethnicity. However, the majority of participants may comprise women with breast cancer who are frequent recipients of chemotherapy. The Minority Based CCOP members affiliated with our group have expressed interest in participating in this protocol. Past enrollment in our CCOP studies has closely paralleled the gender and ethnic composition of the available population.
13 9.6.2 Careful review of the literature gives no reason to suspect differences in either outcome measures effects by gender or ethnicity. Subgroup analyses of study data will be conducted to check for such differences. The methods of analysis will follow those of the primary analysis, but power will be lower due to smaller sample sizes within subgroups.
PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain
 P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
Review of Pharmacological Pain Management
 Review of Pharmacological Pain Management CHAMP Activities are possible with generous support from The Atlantic Philanthropies and The John A. Hartford Foundation The WHO Pain Ladder The World Health Organization
Review of Pharmacological Pain Management CHAMP Activities are possible with generous support from The Atlantic Philanthropies and The John A. Hartford Foundation The WHO Pain Ladder The World Health Organization
Ask Your Doctor if There May Be a SMARTER CHOICE
 If you have osteoarthritis, rheumatoid arthritis or ankylosing spondylitis, Could Your NSAID Pain Medicine Be Hurting Your Stomach? Ask Your Doctor if There May Be a SMARTER CHOICE 1 of 8 Making Smart
If you have osteoarthritis, rheumatoid arthritis or ankylosing spondylitis, Could Your NSAID Pain Medicine Be Hurting Your Stomach? Ask Your Doctor if There May Be a SMARTER CHOICE 1 of 8 Making Smart
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
A. Ketorolac*** B. Naproxen C. Ibuprofen D. Celecoxib
 1. A man, 66 years of age, with a history of knee osteoarthritis (OA) is experiencing increasing pain at rest and with physical activity. He also has a history of depression and coronary artery disease.
1. A man, 66 years of age, with a history of knee osteoarthritis (OA) is experiencing increasing pain at rest and with physical activity. He also has a history of depression and coronary artery disease.
Nonsteroidal. Drugs (NSAIDs) Anti-Inflammatory. North American Spine Society Public Education Series
 Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) North American Spine Society Public Education Series Nonsteroidal Anti- Inflammatory Drugs Your healthcare provider has recommended that you take a nonsteroidal
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) North American Spine Society Public Education Series Nonsteroidal Anti- Inflammatory Drugs Your healthcare provider has recommended that you take a nonsteroidal
NIMULID MD. 1. Introduction. 2. Nimulid MD Drug delivery system
 NIMULID MD 1. Introduction Nimulid MD is a flavoured dispersible Nimesulide tablet with fast mouth dissolving characteristics thereby providing immediate relief. Nimesulide is a non-steroidal antiinflammatory
NIMULID MD 1. Introduction Nimulid MD is a flavoured dispersible Nimesulide tablet with fast mouth dissolving characteristics thereby providing immediate relief. Nimesulide is a non-steroidal antiinflammatory
Sponsor Novartis. Generic Drug Name Secukinumab. Therapeutic Area of Trial Psoriasis. Approved Indication investigational
 Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
2.0 Synopsis. Vicodin CR (ABT-712) M05-765 Clinical Study Report R&D/07/095. (For National Authority Use Only) to Part of Dossier: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Vicodin CR Name of Active Ingredient: Hydrocodone/Acetaminophen Extended Release (ABT-712) Individual Study Table Referring to Part of Dossier: Volume:
2.0 Synopsis Abbott Laboratories Name of Study Drug: Vicodin CR Name of Active Ingredient: Hydrocodone/Acetaminophen Extended Release (ABT-712) Individual Study Table Referring to Part of Dossier: Volume:
UW MEDICINE PATIENT EDUCATION. Xofigo Therapy. For metastatic prostate cancer. What is Xofigo? How does it work?
 UW MEDICINE PATIENT EDUCATION Xofigo Therapy For metastatic prostate cancer This handout explains how the drug Xofigo is used to treat metastatic prostate cancer. What is Xofigo? Xofigo is a radioactive
UW MEDICINE PATIENT EDUCATION Xofigo Therapy For metastatic prostate cancer This handout explains how the drug Xofigo is used to treat metastatic prostate cancer. What is Xofigo? Xofigo is a radioactive
Guidance on Investigational Medicinal Products (IMPs) and other medicinal products used in Clinical Trials
 EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Guidance on Investigational Medicinal Products (IMPs) and other medicinal products used in Clinical Trials
EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Guidance on Investigational Medicinal Products (IMPs) and other medicinal products used in Clinical Trials
Teriflunomide is the active metabolite of Leflunomide, a drug employed since 1994 for the treatment of rheumatoid arthritis (Baselt, 2011).
 Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Drugs for MS.Drug fact box cannabis extract (Sativex) Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug fact box cannabis extract (Sativex) Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further
Version History Policy Title Drugs for MS.Drug fact box cannabis extract (Sativex) Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further
Nursing 113. Pharmacology Principles
 Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Intravenous Methyl Prednisolone in Multiple Sclerosis
 Intravenous Methyl Prednisolone in Multiple Sclerosis Exceptional healthcare, personally delivered Relapse management in multiple sclerosis Relapses in multiple sclerosis (MS) are common and caused by
Intravenous Methyl Prednisolone in Multiple Sclerosis Exceptional healthcare, personally delivered Relapse management in multiple sclerosis Relapses in multiple sclerosis (MS) are common and caused by
Guidance for Industry
 Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
How To Take Methotrexate By Injection
 How To Take Methotrexate By Injection 1 how long does it take for methotrexate to work for abortion 2 methotrexate 15 mg hair loss 3 methotrexate injection dosage for rheumatoid arthritis 4 order methotrexate
How To Take Methotrexate By Injection 1 how long does it take for methotrexate to work for abortion 2 methotrexate 15 mg hair loss 3 methotrexate injection dosage for rheumatoid arthritis 4 order methotrexate
MANAGEMENT OF CHRONIC NON MALIGNANT PAIN
 MANAGEMENT OF CHRONIC NON MALIGNANT PAIN Introduction The Manitoba Prescribing Practices Program (MPPP) recognizes the important role served by physicians in relieving pain and suffering and acknowledges
MANAGEMENT OF CHRONIC NON MALIGNANT PAIN Introduction The Manitoba Prescribing Practices Program (MPPP) recognizes the important role served by physicians in relieving pain and suffering and acknowledges
Safety Information Card for Xarelto Patients
 Safety Information Card for Xarelto Patients 15mg Simply Protecting More Patients 20mg Simply Protecting More Patients Keep this card with you at all times Present this card to every physician or dentist
Safety Information Card for Xarelto Patients 15mg Simply Protecting More Patients 20mg Simply Protecting More Patients Keep this card with you at all times Present this card to every physician or dentist
understanding GI bleeding
 understanding GI bleeding a consumer education brochure American College of Gastroenterology 4900B South 31st Street, Arlington, VA 22206 703-820-7400 www.acg.gi.org American College of Gastroenterology
understanding GI bleeding a consumer education brochure American College of Gastroenterology 4900B South 31st Street, Arlington, VA 22206 703-820-7400 www.acg.gi.org American College of Gastroenterology
Breast Cancer. Breast Cancer Page 1
 Breast Cancer Summary Breast cancers which are detected early are curable by local treatments. The initial surgery will give the most information about the cancer; such as size or whether the glands (or
Breast Cancer Summary Breast cancers which are detected early are curable by local treatments. The initial surgery will give the most information about the cancer; such as size or whether the glands (or
Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada
 Background Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada The use of medications or drugs by non-physician health professionals is evolving and is linked to collaboration
Background Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada The use of medications or drugs by non-physician health professionals is evolving and is linked to collaboration
Medications for chronic pain
 Medications for chronic pain When it comes to treating chronic pain with medications, there are many to choose from. Different types of pain medications are used for different pain conditions. You may
Medications for chronic pain When it comes to treating chronic pain with medications, there are many to choose from. Different types of pain medications are used for different pain conditions. You may
DRUG INTERACTIONS: WHAT YOU SHOULD KNOW. Council on Family Health
 DRUG INTERACTIONS: WHAT YOU SHOULD KNOW Council on Family Health Drug Interactions There are more opportunities today than ever before to learn about your health and to take better care of yourself. It
DRUG INTERACTIONS: WHAT YOU SHOULD KNOW Council on Family Health Drug Interactions There are more opportunities today than ever before to learn about your health and to take better care of yourself. It
Adjunctive psychosocial intervention. Conditions requiring dose reduction. Immediate, peak plasma concentration is reached within 1 hour.
 Shared Care Guideline for Prescription and monitoring of Naltrexone Hydrochloride in alcohol dependence Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist,
Shared Care Guideline for Prescription and monitoring of Naltrexone Hydrochloride in alcohol dependence Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist,
Advances In Chemotherapy For Hormone Refractory Prostate Cancer. TAX 327 study results & SWOG 99-16 study results presented at ASCO 2004
 Ronald de Wit Rotterdam Cancer Institute The Netherlands Advances In Chemotherapy For Hormone Refractory Prostate Cancer TAX 327 study results & SWOG 99-16 study results presented at Slide 1 Prostate Cancer
Ronald de Wit Rotterdam Cancer Institute The Netherlands Advances In Chemotherapy For Hormone Refractory Prostate Cancer TAX 327 study results & SWOG 99-16 study results presented at Slide 1 Prostate Cancer
VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension
 VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension DESCRIPTION Hydroxyzine pamoate is designated chemically as 1-(p-chlorobenzhydryl) 4- [2-(2-hydroxyethoxy) ethyl] diethylenediamine salt of 1,1
VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension DESCRIPTION Hydroxyzine pamoate is designated chemically as 1-(p-chlorobenzhydryl) 4- [2-(2-hydroxyethoxy) ethyl] diethylenediamine salt of 1,1
1.0 Abstract. Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA. Keywords. Rationale and Background:
 1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
Clinical Algorithm & Preferred Medications to Treat Pain in Dialysis Patients
 Clinical Algorithm & Preferred Medications to Treat Pain in Dialysis Patients Developed by the Mid Atlantic Renal Coalition and the Kidney End of Life Coalition September 2009 This project was supported,
Clinical Algorithm & Preferred Medications to Treat Pain in Dialysis Patients Developed by the Mid Atlantic Renal Coalition and the Kidney End of Life Coalition September 2009 This project was supported,
Medicines To Treat Alcohol Use Disorder A Review of the Research for Adults
 Medicines To Treat Alcohol Use Disorder A Review of the Research for Adults Is This Information Right for Me? Yes, this information is right for you if: Your doctor* said you have alcohol use disorder
Medicines To Treat Alcohol Use Disorder A Review of the Research for Adults Is This Information Right for Me? Yes, this information is right for you if: Your doctor* said you have alcohol use disorder
Articles Presented. Journal Presentation. Dr Albert Lo. Dr Albert Lo
 * This presentation is prepared by the author in one s personal capacity for the purpose of academic exchange and does not represent the views of his/her organisations on the topic discussed. Journal Presentation
* This presentation is prepared by the author in one s personal capacity for the purpose of academic exchange and does not represent the views of his/her organisations on the topic discussed. Journal Presentation
CAMARILLO AQUATICS AND REHABILITATION SERVICES
 CAMARILLO AQUATICS AND REHABILITATION SERVICES Last Name First M.I. Address Apt.# City State Zip Code Phone # SS# Date of Birth Sex M F Driver s License # Marital Status: S M D W Spouse s Name How did
CAMARILLO AQUATICS AND REHABILITATION SERVICES Last Name First M.I. Address Apt.# City State Zip Code Phone # SS# Date of Birth Sex M F Driver s License # Marital Status: S M D W Spouse s Name How did
Emory Eye Center New Patient Questionnaire
 Patient Name: Date: Current Address: Current Phone: Date of Birth: Primary Care Physician: Referring Physician: (First & Last Name) (First & Last Name) Pharmacy Name: Phone #: ( ) Please answer all questions
Patient Name: Date: Current Address: Current Phone: Date of Birth: Primary Care Physician: Referring Physician: (First & Last Name) (First & Last Name) Pharmacy Name: Phone #: ( ) Please answer all questions
Paracetamol apollo +9191 46 950 950. Paracetamol apollo +9191 46 950 950. Paracetamol
 Paracetamol apollo +9191 46 950 950 Paracetamol apollo +9191 46 950 950 Paracetamol CAS Number : 103-90-2 Molecular Weight : 151.17 g/mol Molecular Formula : C8H9NO2 Systematic (IUPAC) : N-(4- hydroxyphenyl)ethanamide
Paracetamol apollo +9191 46 950 950 Paracetamol apollo +9191 46 950 950 Paracetamol CAS Number : 103-90-2 Molecular Weight : 151.17 g/mol Molecular Formula : C8H9NO2 Systematic (IUPAC) : N-(4- hydroxyphenyl)ethanamide
PATIENT HEALTH QUESTIONNAIRE Radiation Oncology (Patient Label)
 REVIEWED DATE / INITIALS SAFETY: Are you at risk for falls? Do you have a Pacemaker? Females; Is there a possibility you may be pregnant? ALLERGIES: Do you have any allergies to medications? If, please
REVIEWED DATE / INITIALS SAFETY: Are you at risk for falls? Do you have a Pacemaker? Females; Is there a possibility you may be pregnant? ALLERGIES: Do you have any allergies to medications? If, please
POST-TEST Pain Resource Professional Training Program University of Wisconsin Hospital & Clinics
 POST-TEST University of Wisconsin Hospital & Clinics True/False/Don't Know - Circle the correct answer T F D 1. Changes in vital signs are reliable indicators of pain severity. T F D 2. Because of an underdeveloped
POST-TEST University of Wisconsin Hospital & Clinics True/False/Don't Know - Circle the correct answer T F D 1. Changes in vital signs are reliable indicators of pain severity. T F D 2. Because of an underdeveloped
An Introduction to the Improved FDA Prescription Drug Labeling
 An Introduction to the Improved FDA Prescription Drug Labeling 1 Introduction Mary E. Kremzner, Pharm.D. CDR, U.S. Public Health Service Deputy Director, Division of Drug Information Center for Drug Evaluation
An Introduction to the Improved FDA Prescription Drug Labeling 1 Introduction Mary E. Kremzner, Pharm.D. CDR, U.S. Public Health Service Deputy Director, Division of Drug Information Center for Drug Evaluation
A Guide to pain relief medicines For patients receiving Palliative Care
 A Guide to pain relief medicines For patients receiving Palliative Care 1 Which pain medicines are you taking? Contents Page No. Amitriptyline 8 Codeine 9 Co-codamol 10 Co-dydramol 11 Diclofenac (Voltarol
A Guide to pain relief medicines For patients receiving Palliative Care 1 Which pain medicines are you taking? Contents Page No. Amitriptyline 8 Codeine 9 Co-codamol 10 Co-dydramol 11 Diclofenac (Voltarol
Clinical Trials Network
 National Drug Abuse Treatment Clinical Trials Network STOP SMOKING STUDY Should I Join? NATIONAL INSTITUTES OF HEALTH U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Introduction Many people who abuse drugs
National Drug Abuse Treatment Clinical Trials Network STOP SMOKING STUDY Should I Join? NATIONAL INSTITUTES OF HEALTH U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Introduction Many people who abuse drugs
Dallas Neurosurgical and Spine Associates, P.A Patient Health History
 Dallas Neurosurgical and Spine Associates, P.A Patient Health History DOB: Date: Reason for your visit (Chief complaint): Past Medical History Please check corresponding box if you have ever had any of
Dallas Neurosurgical and Spine Associates, P.A Patient Health History DOB: Date: Reason for your visit (Chief complaint): Past Medical History Please check corresponding box if you have ever had any of
Stowe School Medications Policy
 INTRODUCTION Most pupils will need medication at some stage of their school life. Although this will mainly be for short periods there are a few pupils with chronic conditions who may require regular medication
INTRODUCTION Most pupils will need medication at some stage of their school life. Although this will mainly be for short periods there are a few pupils with chronic conditions who may require regular medication
Series 1 Case Studies Adverse Events that Represent Unanticipated Problems: Reporting Required
 Welcome! This document contains three (3) series of Case Study examples that will demonstrate all four OHSU reporting categories (#1 4) as well as examples of events that are considered not reportable.
Welcome! This document contains three (3) series of Case Study examples that will demonstrate all four OHSU reporting categories (#1 4) as well as examples of events that are considered not reportable.
patient group direction
 DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
There is a risk of renal impairment in dehydrated children and adolescents.
 PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
Peptic Ulcer. Anatomy The stomach is a hollow organ. It is located in the upper abdomen, under the ribs.
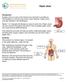 Peptic Ulcer Introduction A peptic ulcer is a sore in the lining of your stomach or duodenum. The duodenum is the first part of your small intestine. Peptic ulcers may also develop in the esophagus. Nearly
Peptic Ulcer Introduction A peptic ulcer is a sore in the lining of your stomach or duodenum. The duodenum is the first part of your small intestine. Peptic ulcers may also develop in the esophagus. Nearly
UNIT VIII NARCOTIC ANALGESIA
 UNIT VIII NARCOTIC ANALGESIA Objective Review the definitions of Analgesic, Narcotic and Antagonistic. List characteristics of Opioid analgesics in terms of mechanism of action, indications for use and
UNIT VIII NARCOTIC ANALGESIA Objective Review the definitions of Analgesic, Narcotic and Antagonistic. List characteristics of Opioid analgesics in terms of mechanism of action, indications for use and
IMPORTANT DRUG WARNING Regarding Mycophenolate-Containing Products
 Dear Healthcare Provider: Mycophenolate REMS (Risk Evaluation and Mitigation Strategy) has been mandated by the FDA (Food and Drug Administration) due to postmarketing reports showing that exposure to
Dear Healthcare Provider: Mycophenolate REMS (Risk Evaluation and Mitigation Strategy) has been mandated by the FDA (Food and Drug Administration) due to postmarketing reports showing that exposure to
Controlling Pain Part 2: Types of Pain Medicines for Your Prostate Cancer
 Controlling Pain Part 2: Types of Pain Medicines for Your Prostate Cancer The following information is based on the general experiences of many prostate cancer patients. Your experience may be different.
Controlling Pain Part 2: Types of Pain Medicines for Your Prostate Cancer The following information is based on the general experiences of many prostate cancer patients. Your experience may be different.
Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid
 Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
St. Luke s MS Center New Patient Questionnaire. Name: Date: Birth date: Right or Left handed? Who is your Primary Doctor?
 St. Luke s MS Center New Patient Questionnaire Name: Date: Birth date: Right or Left handed? Who is your Primary Doctor? Who referred you to the MS Center? List any other doctors you see: Reason you have
St. Luke s MS Center New Patient Questionnaire Name: Date: Birth date: Right or Left handed? Who is your Primary Doctor? Who referred you to the MS Center? List any other doctors you see: Reason you have
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Presence and extent of fatty liver or other metabolic liver diseases
 UC San Diego Health System Patient Information Sheet: Liver Biopsy What is a Liver Biopsy? A liver biopsy is a procedure where a qualified doctor (typically a hepatologist, radiologist or gastroenterologist)
UC San Diego Health System Patient Information Sheet: Liver Biopsy What is a Liver Biopsy? A liver biopsy is a procedure where a qualified doctor (typically a hepatologist, radiologist or gastroenterologist)
Medication Guide EQUETRO (ē-kwĕ-trō) (carbamazepine) Extended-Release Capsules
 Medication Guide EQUETRO (ē-kwĕ-trō) (carbamazepine) Extended-Release Capsules Read this Medication Guide before you start taking EQUETRO and each time you get a refill. There may be new information. This
Medication Guide EQUETRO (ē-kwĕ-trō) (carbamazepine) Extended-Release Capsules Read this Medication Guide before you start taking EQUETRO and each time you get a refill. There may be new information. This
VAD Chemotherapy Regimen for Multiple Myeloma Information for Patients
 VAD Chemotherapy Regimen for Multiple Myeloma Information for Patients The Regimen contains: V = vincristine (Oncovin ) A = Adriamycin (doxorubicin) D = Decadron (dexamethasone) How Is This Regimen Given?
VAD Chemotherapy Regimen for Multiple Myeloma Information for Patients The Regimen contains: V = vincristine (Oncovin ) A = Adriamycin (doxorubicin) D = Decadron (dexamethasone) How Is This Regimen Given?
FREEDOM C: A 16-Week, International, Multicenter, Double-Blind, Randomized, Placebo-Controlled Comparison of the Efficacy and Safety of Oral UT-15C
 FREEDOM C: A 16-Week, International, Multicenter, Double-Blind, Randomized, Placebo-Controlled Comparison of the Efficacy and Safety of Oral UT-15C SR in Combination with an ERA and/or a PDE-5 Inhibitor
FREEDOM C: A 16-Week, International, Multicenter, Double-Blind, Randomized, Placebo-Controlled Comparison of the Efficacy and Safety of Oral UT-15C SR in Combination with an ERA and/or a PDE-5 Inhibitor
PREMIER PAIN CARE PA Carlos J Garcia MD 2435 W. Oak Street # 103 Denton, TX 76201 Phone 940-323-9404 Fax 940-323-9422 PATIENT REGISTRATION
 PREMIER PAIN CARE PA Carlos J Garcia MD 2435 W. Oak Street # 103 Denton, TX 76201 Phone 940-323-9404 Fax 940-323-9422 PATIENT REGISTRATION Last Name First Name MI Mailing Address City Zip code Home Phone
PREMIER PAIN CARE PA Carlos J Garcia MD 2435 W. Oak Street # 103 Denton, TX 76201 Phone 940-323-9404 Fax 940-323-9422 PATIENT REGISTRATION Last Name First Name MI Mailing Address City Zip code Home Phone
NEW PATIENT CLINICAL INFORMATION FORM. Booth Gardner Parkinson s Care & Movement Disorders Center Evergreen Neuroscience Institute
 NEW PATIENT CLINICAL INFORMATION FORM Booth Gardner Parkinson s Care & Movement Disorders Center Evergreen Neuroscience Institute Date: Name: Referring Doctor: How did you hear about us? NWPF Your Physician:
NEW PATIENT CLINICAL INFORMATION FORM Booth Gardner Parkinson s Care & Movement Disorders Center Evergreen Neuroscience Institute Date: Name: Referring Doctor: How did you hear about us? NWPF Your Physician:
Getting the best result from Opioid medicine. in the management of chronic pain
 Getting the best result from Opioid medicine in the management of chronic pain Your doctor has prescribed you opioid medicine to help you manage your chronic pain. This patient information leaflet gives
Getting the best result from Opioid medicine in the management of chronic pain Your doctor has prescribed you opioid medicine to help you manage your chronic pain. This patient information leaflet gives
Chemotherapy Side Effects Worksheet
 Page 1 of 6 Chemotherapy Side Effects Worksheet Medicines or drugs that destroy cancer cells are called cancer chemotherapy. It is sometimes the first choice for treating many cancers. Chemotherapy differs
Page 1 of 6 Chemotherapy Side Effects Worksheet Medicines or drugs that destroy cancer cells are called cancer chemotherapy. It is sometimes the first choice for treating many cancers. Chemotherapy differs
Herniated Cervical Disc
 Herniated Cervical Disc North American Spine Society Public Education Series What Is a Herniated Disc? The backbone, or spine, is composed of a series of connected bones called vertebrae. The vertebrae
Herniated Cervical Disc North American Spine Society Public Education Series What Is a Herniated Disc? The backbone, or spine, is composed of a series of connected bones called vertebrae. The vertebrae
A Phase 2 Study of Interferon Beta-1a (Avonex ) in Ulcerative Colitis
 A Phase 2 Study of (Avonex ) in Ulcerative Colitis - Study Results - ClinicalTrials.gov A Phase 2 Study of (Avonex ) in Ulcerative Colitis This study has been completed. Sponsor: Biogen Idec Information
A Phase 2 Study of (Avonex ) in Ulcerative Colitis - Study Results - ClinicalTrials.gov A Phase 2 Study of (Avonex ) in Ulcerative Colitis This study has been completed. Sponsor: Biogen Idec Information
CVP Chemotherapy Regimen for Lymphoma Information for Patients
 CVP Chemotherapy Regimen for Lymphoma Information for Patients The Regimen Contains: C: Cyclophosphamide (Cytoxan ) V: Vincristine (Oncovin ) P: Prednisone How Is This Regimen Given? CVP is given every
CVP Chemotherapy Regimen for Lymphoma Information for Patients The Regimen Contains: C: Cyclophosphamide (Cytoxan ) V: Vincristine (Oncovin ) P: Prednisone How Is This Regimen Given? CVP is given every
Subject: No. Page PROTOCOL AND CASE REPORT FORM DEVELOPMENT AND REVIEW Standard Operating Procedure
 703 1 of 11 POLICY The Beaumont Research Coordinating Center (BRCC) will provide advice to clinical trial investigators on protocol development, content and format. Upon request, the BRCC will review a
703 1 of 11 POLICY The Beaumont Research Coordinating Center (BRCC) will provide advice to clinical trial investigators on protocol development, content and format. Upon request, the BRCC will review a
Dr. David Y. Liao, D.O. Orthopedic Center, LLC. Release of Information
 Release of Information The purpose of this form is to alert our office as to those family members and/or friends who may be scheduling or canceling appointments on your behalf and/or will need to have
Release of Information The purpose of this form is to alert our office as to those family members and/or friends who may be scheduling or canceling appointments on your behalf and/or will need to have
Maintenance of abstinence in alcohol dependence
 Shared Care Guideline for Prescription and monitoring of Acamprosate Calcium Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist, Alcohol Services Dr Donnelly
Shared Care Guideline for Prescription and monitoring of Acamprosate Calcium Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist, Alcohol Services Dr Donnelly
Clinical Spotlight in Breast Cancer
 2015 European Oncology Congress in Vienna Clinical Spotlight in Breast Cancer Reference Slide Deck Abstract #1815 Impact of Palbociclib Plus Fulvestrant on Global QOL, Functioning, and Symptoms Compared
2015 European Oncology Congress in Vienna Clinical Spotlight in Breast Cancer Reference Slide Deck Abstract #1815 Impact of Palbociclib Plus Fulvestrant on Global QOL, Functioning, and Symptoms Compared
Arthritis www.patientedu.org
 written by Harvard Medical School Arthritis www.patientedu.org Arthritis is the most common chronic disease in the world, and it s the leading cause of disability in the United States. There are more than
written by Harvard Medical School Arthritis www.patientedu.org Arthritis is the most common chronic disease in the world, and it s the leading cause of disability in the United States. There are more than
PATIENT HISTORY FORM
 PATIENT HISTORY FORM If you are new to the office, have not been seen in over one (1) year, or are returning for a new problem, please complete this form in full. If there have been any changes since your
PATIENT HISTORY FORM If you are new to the office, have not been seen in over one (1) year, or are returning for a new problem, please complete this form in full. If there have been any changes since your
Gruppo di lavoro: Malattie Tromboemboliche
 Gruppo di lavoro: Malattie Tromboemboliche 2381 Soluble Recombinant Thrombomodulin Ameliorates Hematological Malignancy-Induced Disseminated Intravascular Coagulation More Promptly Than Conventional Anticoagulant
Gruppo di lavoro: Malattie Tromboemboliche 2381 Soluble Recombinant Thrombomodulin Ameliorates Hematological Malignancy-Induced Disseminated Intravascular Coagulation More Promptly Than Conventional Anticoagulant
Subcutaneous Testosterone-Anastrozole Therapy in Breast Cancer Survivors. 2010 ASCO Breast Cancer Symposium Abstract 221 Rebecca L. Glaser M.D.
 Subcutaneous Testosterone-Anastrozole Therapy in Breast Cancer Survivors 2010 ASCO Breast Cancer Symposium Abstract 221 Rebecca L. Glaser M.D., FACS Learning Objectives After reading and reviewing this
Subcutaneous Testosterone-Anastrozole Therapy in Breast Cancer Survivors 2010 ASCO Breast Cancer Symposium Abstract 221 Rebecca L. Glaser M.D., FACS Learning Objectives After reading and reviewing this
to Part of Dossier: Name of Active Ingredient: Title of Study: Quality of life study with adalimumab in rheumatoid arthritis. ESCALAR.
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Individual Study Table Referring to Part of Dossier: Adalimumab (HUMIRA) (For National Authority Use Only) Name of Active Ingredient: Adalimumab Title
2.0 Synopsis Abbott Laboratories Name of Study Drug: Individual Study Table Referring to Part of Dossier: Adalimumab (HUMIRA) (For National Authority Use Only) Name of Active Ingredient: Adalimumab Title
Study Design. Date: March 11, 2003 Reviewer: Jawahar Tiwari, Ph.D. Ellis Unger, M.D. Ghanshyam Gupta, Ph.D. Chief, Therapeutics Evaluation Branch
 BLA: STN 103471 Betaseron (Interferon β-1b) for the treatment of secondary progressive multiple sclerosis. Submission dated June 29, 1998. Chiron Corp. Date: March 11, 2003 Reviewer: Jawahar Tiwari, Ph.D.
BLA: STN 103471 Betaseron (Interferon β-1b) for the treatment of secondary progressive multiple sclerosis. Submission dated June 29, 1998. Chiron Corp. Date: March 11, 2003 Reviewer: Jawahar Tiwari, Ph.D.
Test Content Outline Effective Date: June 9, 2014. Pain Management Nursing Board Certification Examination
 Pain Management Nursing Board Certification Examination There are 175 questions on this examination. Of these, 150 are scored questions and 25 are pretest questions that are not scored. Pretest questions
Pain Management Nursing Board Certification Examination There are 175 questions on this examination. Of these, 150 are scored questions and 25 are pretest questions that are not scored. Pretest questions
A Prospective Pilot Study Describing the Use of Performance-Enhancing Drugs in Adolescent and Young Adult (AYA) Male Oncology Patients
 Clinical Research Rotation The IRB Protocol A Prospective Pilot Study Describing the Use of Performance-Enhancing Drugs in Adolescent and Young Adult (AYA) Male Oncology Patients Version 1.0 (rev 9/20/2012)
Clinical Research Rotation The IRB Protocol A Prospective Pilot Study Describing the Use of Performance-Enhancing Drugs in Adolescent and Young Adult (AYA) Male Oncology Patients Version 1.0 (rev 9/20/2012)
Tracy Walker, RN, BSN, CCRP Research Nurse OHSU Knight Cancer Institute. 503-346-1183 walkertr@ohsu.edu
 Tracy Walker, RN, BSN, CCRP Research Nurse OHSU Knight Cancer Institute 503-346-1183 walkertr@ohsu.edu Exercise Questions to Keep in Mind Is there an adverse event? What is the severity? What is the relationship
Tracy Walker, RN, BSN, CCRP Research Nurse OHSU Knight Cancer Institute 503-346-1183 walkertr@ohsu.edu Exercise Questions to Keep in Mind Is there an adverse event? What is the severity? What is the relationship
Lumbar Spinal Stenosis
 Lumbar Spinal Stenosis North American Spine Society Public Education Series What Is Lumbar Spinal Stenosis? The vertebrae are the bones that make up the lumbar spine (low back). The spinal canal runs through
Lumbar Spinal Stenosis North American Spine Society Public Education Series What Is Lumbar Spinal Stenosis? The vertebrae are the bones that make up the lumbar spine (low back). The spinal canal runs through
Treatment with Apixaban
 UW MEDICINE PATIENT EDUCATION Treatment with Apixaban Eliquis This handout explains the medicine apixaban, a drug that helps prevent blood clots. What is apixaban? Apixaban (brand name Eliquis) is an anticoagulant
UW MEDICINE PATIENT EDUCATION Treatment with Apixaban Eliquis This handout explains the medicine apixaban, a drug that helps prevent blood clots. What is apixaban? Apixaban (brand name Eliquis) is an anticoagulant
Daily aspirin therapy: Understand the benefits and risks
 Daily aspirin therapy: Understand the benefits and risks Is an aspirin a day the right thing for you? It's not as easy a decision as it sounds. Know the benefits and risks before considering daily aspirin
Daily aspirin therapy: Understand the benefits and risks Is an aspirin a day the right thing for you? It's not as easy a decision as it sounds. Know the benefits and risks before considering daily aspirin
Treatment with Rivaroxaban
 UW MEDICINE PATIENT EDUCATION Treatment with Rivaroxaban Xarelto This handout explains the medicine rivaroxaban, a drug that helps prevent blood clots. What is rivaroxaban? Rivaroxaban (brand name Xarelto)
UW MEDICINE PATIENT EDUCATION Treatment with Rivaroxaban Xarelto This handout explains the medicine rivaroxaban, a drug that helps prevent blood clots. What is rivaroxaban? Rivaroxaban (brand name Xarelto)
University of Hawai i Human Studies Program. Guidelines for Developing a Clinical Research Protocol
 University of Hawai i Human Studies Program Guidelines for Developing a Clinical Research Protocol Following are guidelines for writing a clinical research protocol for submission to the University of
University of Hawai i Human Studies Program Guidelines for Developing a Clinical Research Protocol Following are guidelines for writing a clinical research protocol for submission to the University of
A ragweed pollen as a treatment for a ragweed allergy? It s called immunotherapy.
 A ragweed pollen as a treatment for a ragweed allergy? It s called immunotherapy. RAGWITEK is a prescription medicine used for sublingual (under the tongue) immunotherapy to treat ragweed pollen allergies
A ragweed pollen as a treatment for a ragweed allergy? It s called immunotherapy. RAGWITEK is a prescription medicine used for sublingual (under the tongue) immunotherapy to treat ragweed pollen allergies
Antipsychotic drugs are the cornerstone of treatment
 Article Effectiveness of Olanzapine, Quetiapine, Risperidone, and Ziprasidone in Patients With Chronic Schizophrenia Following Discontinuation of a Previous Atypical Antipsychotic T. Scott Stroup, M.D.,
Article Effectiveness of Olanzapine, Quetiapine, Risperidone, and Ziprasidone in Patients With Chronic Schizophrenia Following Discontinuation of a Previous Atypical Antipsychotic T. Scott Stroup, M.D.,
October 2012. We hope that our tool will be a useful aid in your efforts to improve pain management in your setting. Sincerely,
 October 2012 he Knowledge and Attitudes Survey Regarding Pain tool can be used to assess nurses and other professionals in your setting and as a pre and post test evaluation measure for educational programs.
October 2012 he Knowledge and Attitudes Survey Regarding Pain tool can be used to assess nurses and other professionals in your setting and as a pre and post test evaluation measure for educational programs.
Women s Continence and Pelvic Health Center
 Women s Continence and Pelvic Health Center Committed to Caring 580-590 Court Street Keene, New Hampshire 03431 (603) 354-5454 Ext. 6643 URINARY INCONTINENCE QUESTIONNAIRE The purpose of this questionnaire
Women s Continence and Pelvic Health Center Committed to Caring 580-590 Court Street Keene, New Hampshire 03431 (603) 354-5454 Ext. 6643 URINARY INCONTINENCE QUESTIONNAIRE The purpose of this questionnaire
Abstral Prescriber and Pharmacist Guide
 Abstral Prescriber and Pharmacist Guide fentanyl citrate sublingual tablets Introduction The Abstral Prescriber and Pharmacist Guide is designed to support healthcare professionals in the diagnosis of
Abstral Prescriber and Pharmacist Guide fentanyl citrate sublingual tablets Introduction The Abstral Prescriber and Pharmacist Guide is designed to support healthcare professionals in the diagnosis of
General Internal Medicine Clinic New Patient Questionnaire
 General Internal Medicine Clinic New Patient Questionnaire Date: Name: What would you like to be called by the doctor? Marital Status: Please list how you would like to be contacted, for test results:
General Internal Medicine Clinic New Patient Questionnaire Date: Name: What would you like to be called by the doctor? Marital Status: Please list how you would like to be contacted, for test results:
Sponsor. Novartis Generic Drug Name. Vildagliptin. Therapeutic Area of Trial. Type 2 diabetes. Approved Indication. Investigational.
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Investigational Study Number CLAF237A2386 Title A single-center,
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Investigational Study Number CLAF237A2386 Title A single-center,
Definition of Investigational Medicinal Products (IMPs) Definition of Non Investigational Medicinal Products (NIMPs)
 EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Definition of Investigational Medicinal Products (IMPs) Definition of Non Investigational Medicinal Products
EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Definition of Investigational Medicinal Products (IMPs) Definition of Non Investigational Medicinal Products
Painkillers (analgesics)
 Drug information (analgesics) This leaflet provides information on painkillers and will answer any questions you have about the treatment. Arthritis Research UK produce and print our booklets entirely
Drug information (analgesics) This leaflet provides information on painkillers and will answer any questions you have about the treatment. Arthritis Research UK produce and print our booklets entirely
CHOP Chemotherapy Regimen for Lymphoma Information for Patients
 CHOP Chemotherapy Regimen for Lymphoma Information for Patients The Regimen Contains: C: Cytoxan (cyclophosphamide) H: Adriamycin (hydroxy doxorubicin) O: vincristine (Oncovin ) P: Prednisone How Is This
CHOP Chemotherapy Regimen for Lymphoma Information for Patients The Regimen Contains: C: Cytoxan (cyclophosphamide) H: Adriamycin (hydroxy doxorubicin) O: vincristine (Oncovin ) P: Prednisone How Is This
Joanna L. Starrels. 2 ND YEAR RESEARCH ELECTIVE RESIDENT S JOURNAL Volume VIII, 2003-2004. A. Study Purpose and Rationale
 Outpatient Treatment of Opiate Dependence with Sublingual Buprenorphine/Naloxone versus Methadone Maintenance: a Randomized Trial of Alternative Treatments in Real Life Settings Joanna L. Starrels A. Study
Outpatient Treatment of Opiate Dependence with Sublingual Buprenorphine/Naloxone versus Methadone Maintenance: a Randomized Trial of Alternative Treatments in Real Life Settings Joanna L. Starrels A. Study
Clinical Study Report. Clinical Efficacy of the e-bright Tooth Whitening Accelerator Home Edition: a randomized placebo controlled clinical trial
 Clinical Study Report Clinical Efficacy of the e-bright Tooth Whitening Accelerator Home Edition: a randomized placebo controlled clinical trial (Clinical Study Protocol Number: EBRIGHT-2007-01) Study
Clinical Study Report Clinical Efficacy of the e-bright Tooth Whitening Accelerator Home Edition: a randomized placebo controlled clinical trial (Clinical Study Protocol Number: EBRIGHT-2007-01) Study
Children s Cancer Pain Can Be Relieved A Guide for Parents and Families
 Children s Cancer Pain Can Be Relieved A Guide for Parents and Families This booklet is dedicated to Shaney Banks and all other children with cancer. Wisconsin Cancer Pain Initiative 1989 This booklet
Children s Cancer Pain Can Be Relieved A Guide for Parents and Families This booklet is dedicated to Shaney Banks and all other children with cancer. Wisconsin Cancer Pain Initiative 1989 This booklet
TAKING CARE OF YOUR RHEUMATOID ARTHRITIS
 TAKING CARE OF YOUR RHEUMATOID ARTHRITIS RHEUMATOID ARTHRITIS (RA) FAST FACTS What is Rheumatoid Arthritis? Rheumatoid arthritis (RA) is a chronic disease that can affect your ability to function and be
TAKING CARE OF YOUR RHEUMATOID ARTHRITIS RHEUMATOID ARTHRITIS (RA) FAST FACTS What is Rheumatoid Arthritis? Rheumatoid arthritis (RA) is a chronic disease that can affect your ability to function and be
Methotrexate Dose For Juvenile Rheumatoid Arthritis
 Methotrexate Dose For Juvenile Rheumatoid Arthritis should i take methotrexate for my ra methotrexate 50 mg/ml methotrexate sodium 2.5mg tablets what is the usual dosage of methotrexate for ra methotrexate
Methotrexate Dose For Juvenile Rheumatoid Arthritis should i take methotrexate for my ra methotrexate 50 mg/ml methotrexate sodium 2.5mg tablets what is the usual dosage of methotrexate for ra methotrexate
ORAL MEDICATIONS FOR MS! Gilenya and Aubagio
 ORAL MEDICATIONS FOR MS! Gilenya and Aubagio Champions against MS 4/20/13 Alexandra Goodyear, MD Stanford University Oral Medications Since 2010, 3 new oral medications for MS: Gilenya 2010 Aubagio 2012
ORAL MEDICATIONS FOR MS! Gilenya and Aubagio Champions against MS 4/20/13 Alexandra Goodyear, MD Stanford University Oral Medications Since 2010, 3 new oral medications for MS: Gilenya 2010 Aubagio 2012
Medication Guide. Serious loss of body fluid (dehydration) and changes in blood salts (electrolytes) in your blood.
 Medication Guide MoviPrep (moo-vee-prěp) (PEG 3350, sodium sulfate, sodium chloride, potassium chloride, sodium ascorbate, and ascorbic acid for oral solution) Read this Medication Guide before you start
Medication Guide MoviPrep (moo-vee-prěp) (PEG 3350, sodium sulfate, sodium chloride, potassium chloride, sodium ascorbate, and ascorbic acid for oral solution) Read this Medication Guide before you start
Enhancing the Child s Voice in Clinical Care and Research
 Enhancing the Child s Voice in Clinical Care and Research Bryce B. Reeve, Ph.D. Associate Professor, Health Policy and Management Member, Lineberger Comprehensive Cancer Center bbreeve@email.unc.edu Standardized,
Enhancing the Child s Voice in Clinical Care and Research Bryce B. Reeve, Ph.D. Associate Professor, Health Policy and Management Member, Lineberger Comprehensive Cancer Center bbreeve@email.unc.edu Standardized,
A. Approval for the indications of osteoarthritis and rheumatoid arthritis at a dose of 10mg/day and dysmenorrhea at a dose of 20-mg bid as needed.
 Agent: Valdecoxib Indication: Analgesia, Dysmenorrhea Osteoarthritis, and Rheumatoid Arthritis Reviewer: Kent Johnson, MD Date: November 7, 2001 NDA: 21,341 EXECUTIVE SUMMARY 1-RECOMMENDATIONS A. Approval
Agent: Valdecoxib Indication: Analgesia, Dysmenorrhea Osteoarthritis, and Rheumatoid Arthritis Reviewer: Kent Johnson, MD Date: November 7, 2001 NDA: 21,341 EXECUTIVE SUMMARY 1-RECOMMENDATIONS A. Approval
