3 Regime Prescription Practical considerations 4 Pre-infusion Assessment 4 Administration. 5 Checklist. 5 Administering the infusion
|
|
|
- George Shields
- 8 years ago
- Views:
Transcription
1 Guidelines for the use and administration of Rituximab in Rheumatoid Arthritis (RA) (To be used in conjunction with local policies for intravenous infusions) Contents Page Introduction 1 Licensed Indication 1 Pre-treatment screening 2 Contraindications 2 Evaluation of response 2 Definition of response 2 Repeated treatment 2 Treatment Dose and Co-medication 3 Regime Prescription Practical considerations 4 Pre-infusion Assessment 4 Administration 5 Checklist 5 Administering the infusion 5 Clinical observations 6 Infusion reactions 6 Post-infusion care 6 Adverse events 7 Patient information 7 References 8 Appendix 1 Documentation for Pre-infusion Assessment (example) 9 Appendix 2 Documentation for Administration of Infusion (example) 11 INTRODUCTION Rituximab is a genetically engineered chimeric mouse / human antibody. It acts by depleting antibody producing B cells. Rituximab has been licensed in the UK since 1998 for the treatment of non- Hodgkins lymphoma and in 2006 it was licensed for use in severe active RA following clinical trials 1, 2. A consensus statement on the use of Rituximab in rheumatoid arthritis (RA) was published in , the British Society for Rheumatology (BSR) have endorsed its use 4 and it is currently being evaluated by NICE.(NICE TA126 issued August 2007). LICENSED INDICATION Severe active RA* in combination with Methotrexate in adult patients who have had an inadequate response or intolerance to other disease-modifying anti-rheumatic drugs, including one or more tumour necrosis factor (TNF) inhibitor therapies
2 *Currently there is no disease activity score (DAS) requirement to qualify for treatment with Rituximab although Smolen et al 3 suggest >3.2. In the UK, if the patient has previously qualified for Anti-TNF a DAS of >5.2 has been required. PRE-TREATMENT SCREENING 3 Detailed history - including o chronic or recent co-morbidity such as cardiovascular and pulmonary disease o recurrent infections o allergies Physical examination to exclude contraindications CXR Routine blood tests Hepatitis B screen Immunoglobulin levels Baseline DAS28 (to monitor response) CD19 There may be some value in baseline CD19 levels, but measuring CD19 posttreatment is not necessary to determine when to repeat treatment, as this decision should be based on the individual patient s signs and symptoms (DAS28) NB: If patients need inactivated vaccinations e.g. Flu, the course should be completed 1 month prior to commencing Rituximab or given at least 7 months after treatment to ensure efficacy of immunisation. CONTRAINDICATIONS Hypersensitivity to Rituximab or other murine proteins Active acute or chronic infection Severe heart failure NYHA Class IV Pregnancy Use in children (safety not yet established) EVALUATION OF RESPONSE Rituximab may take up to 16 weeks from the first infusion to reach a response 3. Although the patient may have been seen prior to this time, any evaluation of response should wait until at least 16 weeks. It is worth noting that the IV glucocorticoid pre-medication may produce an early (usually temporary) effect for up to 8 weeks. DEFINITION OF RESPONSE Smolen et al 3 make some recommendations on defining a response including a minimum improvement of DAS28 of 1.2. REPEATED TREATMENT
3 Repeated infusion courses may be considered between 6-12 months 3, 4 after the initial course. The optimum repeated treatment schedule is being evaluated but currently there is only evidence for retreatment of responders 3. TREATMENT DOSE AND CO-MEDICATION Regime I.V. 1000mg Rituximab on Day 1 and Day 15 Methotrexate given weekly (dose as directed by Rheumatologist) Prescription The Physician should prescribe: - PRE-MEDICATION DRUGS Methylprednisolone 100mg IV (100mgs in 100mls normal saline infused over 30 minutes) to be commenced 60 minutes before Rituximab infusion Paracetamol 1gm orally - 60 minutes prior to infusion Chlorphenamine 10 mg IV - 60 minutes prior to infusion INFUSION THERAPY* The following prescription is based on 2mgs/ml (MabThera 10mg/ml dilution) First infusion - DAY 1 I.V. Rituximab 1000mg in 500mls of normal saline To be infused as follows: o 1 st 30 minutes 50mg/hour (25mls/hour) o 2 nd 30 minutes 100mg/hour (50mls/hour) o Thereafter the rate can be increased by 50mg/hour (25mls/hour) every 30 minutes to a maximum rate of 400mg/hour (200mls/hour) providing no adverse reactions occur Second infusion - DAY 15 (providing DAY 1 infusion was without adverse events) I.V. Rituximab 1000mg in 500mls of normal saline To be infused as follows: o 1 st 30 minutes 100mg/hour (50mls/hour) o 2 nd 30 minutes 200mg/hour (100mls/hour) o Thereafter the rate can be increased by 100mg/hour (50mls/hour) every 30 minutes to a maximum rate of 400mg/hr (200mls/hour) providing no adverse reactions occur AS REQUIRED Chlorphenamine 10 mg IV tds Hydrocortisone 100 mg IV tds Paracetamol 1G orally 4-5 hourly (max 4gm in 24 hours) Metoclopramide 10mg IV tds *NB: Rituximab can be diluted to a concentration of between 1-4mgs/ml normal saline
4 Concentration 1mg/ml 2mgs/ml (Preferred 4mgs/ml concentration above) Volume of fluid 1000mls 500mls 250mls
5 PRACTICAL CONSIDERATIONS Rituximab should only be administered in an area where full resuscitation facilities and close monitoring are available. This is usually done on an out-patient basis. Consideration should be given to the length of infusion time, ensuring that the patient arrives early enough in the day to complete the infusion. The first infusion may take between 6-7 hours to complete (i.e. IV Methylprednisolone 30 minutes; interval 30 minutes; 1 st infusion minimum 4 hours 15 minutes) or longer if the patient has any adverse reactions (see later section). The second infusion can be completed more quickly (Rituximab minimum of 3 hours 15 minutes) if the patient had no adverse effects during the first infusion. PRE-INFUSION ASSESSMENT This may be done 1-2 days prior to both of the infusions. The assessment will be undertaken by a member of the Rheumatology team to assess general health and to check for any sign of infection. Screening tests include: FBC, LFTs and U&Es Urinalysis (MSU, if any sign of infection) The results of blood and urine tests should be reviewed and documented in the patient s notes. Advise the patient to omit any oral anti-hypertensives for 12 hours prior to infusion (Rituximab may cause hypotension during infusion). Patients should bring these medications with them to take in the event of hypertension during the infusion. In hospitals where Pharmacy is preparing the infusion, the prescription should be sent to the Pharmacy Aseptics Facility at least 48 hours before the proposed infusion time. It is the responsibility of the Rheumatology team to then advise the Pharmacy to prepare the drug once all results are found to be satisfactory. If the Rituximab is being prepared by the ward / day unit staff*, the drugs should be ordered from pharmacy once the pre-assessment has been completed. * Rituximab can be classified as a.cytotoxic since it destroys B cells. However, it is different to the small molecules traditionally used as cytotoxic chemotherapy, which generally exert their effect by interfering with DNA replication. These effects are non-specific and can therefore result in adverse events when rapidly dividing healthy cells are also affected. By contrast Rituximab will only destroy CD20 positive B cells. Since the drug product does not contain any anti-microbial preservative or bacteriostatic agents, aseptic technique must be observed during preparation of the infusion solution. Rituximab does not require any special handling precautions beyond those described and is subject to the same considerations as any other preparation for intravenous use, including other monoclonal antibodies See an example of a pre-infusion assessment in Appendix 1
6 ADMINISTRATION On the day of the Rituximab infusion: The nurse should (see checklist Appendix 2): - Check pre-assessment has been performed Check that the patient has not received analgesics containing paracetamol within the last 4 hours and has omitted their morning dose of any anti-hypertensive medication. Take and record Temperature, Pulse, Blood Pressure and O 2 Saturation levels as baseline Insert venflon Ensure infusion pump is ready and working Administer pre-infusion medications as per drug chart, commencing 60 minutes before Rituximab is given In Units where the staff are preparing the infusion, make up as follows, using an aseptic technique: a. 1 single use vial of Rituximab contains 500mg in 50mls, therefore use 2 vials (1000mg) b. Remove an appropriate equivalent volume i.e. 100mls from the bag of I.V. normal saline c. Add Rituximab d. Gently invert the bag to mix and avoid foaming. Administering the infusion: Rituximab is infused through a peripheral cannula using an IV pump with a primed line. NB: The following regime is based on a concentration of 2mgs/ml i.e. 1000mgs in 500mls. The rate of the infusion will depend on the concentration of the Rituximab and whether it is the 1 st or 2 nd infusion. In the event of a reaction to the first infusion, the second infusion should be administered as per instructions for the first infusion. INFUSION RATE FOR DAY 1 INFUSION Time Mgs/hour Mls/hour 1 st 30 minutes 50mg/hour 25mls/hour 2 nd 30 minutes 100mg/hour 50mls/hour Thereafter the rate can be increased by 50mg/hour (25mls/hour) every 30 minutes to a maximum rate of 400mg/hour (200mls/hour) providing no adverse reactions occur (see page 6) The infusion should continue until completed (providing no adverse reactions occur). INFUSION RATE FOR DAY 15 INFUSION if the patient had no reaction to the first infusion Time Mgs/hour Mls/hour 1 st 30 minutes 100mg/hour 50mls/hour 2 nd 30 minutes 200mg/hour 100mls/hour Thereafter the rate can be increased by 100mg/hour (50mls/hour) every 30 minutes to a maximum rate of 400mg/hour (200mls/hour) providing no adverse reactions occur (see page 6) The infusion should continue until completed providing no adverse reactions occur.
7 Clinical observations on DAY 1 and DAY 15 1 st hour Blood pressure, Pulse, Temperature and O 2 sats every 15 minutes Thereafter, every 30 minutes prior to increasing the rate of infusion and throughout the course of the infusion once maximum rate is reached. Most reactions have been noted during the first few minutes of the infusion, so the patient should be observed carefully during this time and following increases in infusion rates. INFUSION REACTIONS Acute infusion reactions may occur within 1-2 hrs of the first Rituximab infusion. These consist of fever, headache, rigors, flushing, nausea, rash, and URTI symptoms. Transient hypotension and bronchospasm are usually related to the infusion rate If the patient experiences an infusion reaction Mild to moderate reactions e.g. low grade fever; hypotension <30mmHg from baseline o Halve the infusion rate and o Consider giving prn medication Moderate to severe reactions e.g. fever >38.5ºC; chills; mucosal swelling; shortness of breath; hypotension by >30mmHg from baseline o STOP the infusion and treat the symptoms. o Contact the doctor. o The infusion should be restarted at half the previous rate only when the symptoms have resolved. Note: in the case of extravasation, Rituximab is not an irritant and no special action is needed POST INFUSION 1. Remove venflon 2. Advise patient to seek medical help if they have any symptoms that could be due to an infection e.g. fever in the hours or days after the infusion ensure they have appropriate contact numbers for the Rheumatology Department or otherwise to contact GP and / or attend A & E 3. Advise patient to restart any anti-hypertensive drugs the day after infusion 4. Organise infusion 2 or follow up appointment as required 5. Ensure patient is having regular methotrexate monitoring according to BSR and local guidelines 6. Ensure the patient has a follow up assessment at 16 weeks to assess response
8 ADVERSE EVENTS 3, 5 Infusion reactions o Mild to moderate infusion reactions 30-35% at 1 st infusion; less with the 2 nd (see above) o Severe infusion reactions are uncommon frequency is reduced by the concomitant use of IV steroids Infections o Small increase in serious infections (not opportunistic infections e.g. TB) See Summary of Product Characteristics (SPC) for full updated list of adverse events ( PATIENT INFORMATION The Arthritis Research Campaign (arc) have produced a patient information leaflet on the use of Rituximab in RA The National Rheumatoid Arthritis Society (NRAS) has patient information about Rituximab on the website
9 REFERENCES 1. Edwards CWE, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-Cell-targeted therapy with Rituximab in patients with rheumatoid arthritis N.Engl J Med 2004;350: Edwards JC, Leandro MJ, Cambridge G. Repeated B Lymphocte depletion therapy in rheumatoid arthritis:5 year follow-up 2005 Arthritis and Rheumatism Volume 52:9(Supplement) 3. Smolen JS, Keystone EC, Emery P et al. Consensus statement on the use of Rituximab in patients with rheumatoid arthritis ARD Online First as /ARD British Society of Rheumatology (BSR) Statement on Rituximab for Refractory Rheumatoid Arthritis Summary of Product Characteristics Rituximab (Mabthera) July These guidelines have been developed by Maggie Carr, Consultant Nurse in Rheumatology, Ashford and St Peter s NHS Hospitals Trust and Diane Home, Consultant Nurse in Rheumatology, West Middlesex University Hospital in consultation with medical and pharmacy colleagues. Acknowledgements Rheumatology Departments at University College London Hospitals, St Georges Hospital NHS Trust, London and Wirral Hospital NHS Trust Drug Information at Roche Products ltd Ashford and St Peter s Hospitals (ASPH) NHS Trust 2007 West Middlesex University Hospital NHS Trust 2007 Copyright and other intellectual rights in this document belong to the ASPH NHS Trust and West Middlesex University Hospitals University NHS Trust. The Trusts authorise healthcare organisations to reproduce this material for educational and non-commercial use. Minor update modification made: 22nd July, 2009 (by Susan Oliver)
10 Appendix 1 RITUXIMAB PRE-TREATMENT ASSESSMENT (1-2 days prior to infusion) Patient details label DATE: ASSESSMENT PERFORMED BY: Temp ºC Pulse bpm Resps rpm BP mm/hg Urinalysis IS THE PATIENT PREGNANT /BREASTFEEDING ANY CURRENT INFECTION E.G. COLD, COUGH, DENTAL ABSCESS, UTI ANY KNOWN RECENT EXPOSURE TO CHICKEN POX /SHINGLES ANY VACCINATIONS IN THE LAST 4 WEEKS YES / NO / NA YES / NO YES / NO YES / NO BLOOD TESTS RESULTS: List FBC, LFTs, U&Es ESR CRP Hep B Screen Igs CD19 Page 10 of 13 Version 13 March 2007
11 CHECKLIST: Does the patient have an information leaflet about Rituximab? Does the patient know details of first infusion Date Time Venue Has the patient arranged transport home? Does the patient know that any anti-hypertensive medication needs to be omitted on the morning of infusion? Does the patient know dates for second infusion second pre-treatment visit Are the necessary medications prescribed ready for the infusion? Has the chart been taken to pharmacy (preferably 48 hours before infusion due) Has pharmacy been told to proceed with making up of Rituximab once preassessment satisfactory? Have notes gone to ward? To be filed in patient notes following infusion Y/N - details Page 1 Version 13 March 2007
12 Appendix 2 WARD: ADMINISTRATION OF RITUXIMAB DATE: Rituximab infusion 1 2 (circle) Affix patient details label Checklist prior to commencing infusion 1. Normal FBC Y / N 2. Pre- assessment review documented in notes Y / N 3. Drug chart completed for Rituximab, pre-infusion and prn Y / N medications 4. Baseline observations on arrival within normal parameters Y / N 5. Patient is wearing a hospital name band Y/N If NO to questions 1-4 please contact the Rheumatology Department Insert relevant contact details If YES proceed with Rituximab infusion as per guidelines Document all observations and infusion rates on the record sheet and any other information on the nursing record sheet. (S) Page 12 of 13 Version 13 March 2007
13 DATE NAME HOPSITAL NUMBER Nursing Observations Record Sheet - Rituximab Infusions Time Duration of infusion hr/mins Rate of infusion mls/hr BP mm/hg Pulse bpm Temp ºC O 2 sats % Any adverse reaction? Initials Page 13 of 13 Version 13 March 2007
Gloucestershire Hospitals
 Gloucestershire Hospitals NHS Foundation Trust TRUST GUIDELINE INFLIXIMAB PRESCRIBING AND ADMINISTRATION 1. INTRODUCTION Infliximab is an anti-tumour necrosis factor-α (Anti-TNF) antibody. It is from a
Gloucestershire Hospitals NHS Foundation Trust TRUST GUIDELINE INFLIXIMAB PRESCRIBING AND ADMINISTRATION 1. INTRODUCTION Infliximab is an anti-tumour necrosis factor-α (Anti-TNF) antibody. It is from a
Biologic Treatments for Rheumatoid Arthritis
 Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
In non-hodgkin s lymphoma, MabThera is used to treat two types of the disease, both of which affect B-lymphocytes:
 EMA/614203/2010 EMEA/H/C/000165 EPAR summary for the public rituximab This is a summary of the European public assessment report (EPAR) for. It explains how the Committee for Medicinal Products for Human
EMA/614203/2010 EMEA/H/C/000165 EPAR summary for the public rituximab This is a summary of the European public assessment report (EPAR) for. It explains how the Committee for Medicinal Products for Human
New Evidence reports on presentations given at EULAR 2012. Rituximab for the Treatment of Rheumatoid Arthritis
 New Evidence reports on presentations given at EULAR 2012 Rituximab for the Treatment of Rheumatoid Arthritis Report on EULAR 2012 presentations Long-term safety of rituximab: 10-year follow-up in the
New Evidence reports on presentations given at EULAR 2012 Rituximab for the Treatment of Rheumatoid Arthritis Report on EULAR 2012 presentations Long-term safety of rituximab: 10-year follow-up in the
Care Pathway for the Administration of Intravenous Iron Sucrose (Venofer )
 Departments of Haematology, Nephrology and Pharmacy Care Pathway for the Administration of Intravenous Iron Sucrose (Venofer ) [Care Pathway Review Date] Guidance for use This Care Pathway is intended
Departments of Haematology, Nephrology and Pharmacy Care Pathway for the Administration of Intravenous Iron Sucrose (Venofer ) [Care Pathway Review Date] Guidance for use This Care Pathway is intended
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC)
 BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) September 2014 Review date: September 2017 Bulletin 203: Tocilizumab (subcutaneous) in combination with methotrexate or as monotherapy for the treatment
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) September 2014 Review date: September 2017 Bulletin 203: Tocilizumab (subcutaneous) in combination with methotrexate or as monotherapy for the treatment
Evidence-based Management of Rheumatoid Arthritis (2009)
 CPLD reviews its distance learning programmes every twelve months to ensure currency. This update has been produced by an expert and should be read in conjunction with the Evidencebased Management of distance
CPLD reviews its distance learning programmes every twelve months to ensure currency. This update has been produced by an expert and should be read in conjunction with the Evidencebased Management of distance
Eastern Health MS Service. Tysabri Therapy. Information for People with MS and their Families
 Eastern Health MS Service Tysabri Therapy Information for People with MS and their Families The Eastern Health MS Service has developed this information for you as a guide through what will happen to you
Eastern Health MS Service Tysabri Therapy Information for People with MS and their Families The Eastern Health MS Service has developed this information for you as a guide through what will happen to you
Severe rheumatoid arthritis (a disease that causes inflammation of the joints),where MabThera is given intravenously together with methotrexate.
 EMA/614203/2010 EMEA/H/C/000165 EPAR summary for the public rituximab This is a summary of the European public assessment report (EPAR) for. It explains how the Committee for Medicinal Products for Human
EMA/614203/2010 EMEA/H/C/000165 EPAR summary for the public rituximab This is a summary of the European public assessment report (EPAR) for. It explains how the Committee for Medicinal Products for Human
Beaumont Hospital Department of Nephrology and Renal Nursing. Guideline for administering Ferinject
 Beaumont Hospital Department of Nephrology and Renal Nursing Guideline Name: Guideline for administering Ferinject Guideline Number: 18 Guideline Version: a Developed By: Louise Kelly CNM 1 Renal Day Care
Beaumont Hospital Department of Nephrology and Renal Nursing Guideline Name: Guideline for administering Ferinject Guideline Number: 18 Guideline Version: a Developed By: Louise Kelly CNM 1 Renal Day Care
Shared care protocol for the management of patients with Rheumatoid Arthritis treated with disease modifying antirheumatic drugs (DMARDs)
 Tameside Hospital NHS Foundation Trust and NHS Tameside and Glossop Shared care protocol for the management of patients with Rheumatoid Arthritis treated with disease modifying antirheumatic drugs (DMARDs)
Tameside Hospital NHS Foundation Trust and NHS Tameside and Glossop Shared care protocol for the management of patients with Rheumatoid Arthritis treated with disease modifying antirheumatic drugs (DMARDs)
Rituximab. Drug information Rituximab. This leaflet provides information on rituximab and will answer any questions you have about the treatment.
 Drug information Rituximab Rituximab This leaflet provides information on rituximab and will answer any questions you have about the treatment. Arthritis Research UK produce and print our booklets entirely
Drug information Rituximab Rituximab This leaflet provides information on rituximab and will answer any questions you have about the treatment. Arthritis Research UK produce and print our booklets entirely
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Health Technology Appraisal Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure
Multiple sclerosis disease-modifying drugs second line treatments
 Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Multiple sclerosis disease-modifying drugs second line treatments The following information should be read in conjunction
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Multiple sclerosis disease-modifying drugs second line treatments The following information should be read in conjunction
Cycle frequency: Every four weeks Total number of cycles: 6-8
 Fludarabine Low Grade non-hodgkin s Lymphoma and CLL Fludarabine 25mg/m 2 oral Days 1-5 Cycle frequency: Every four weeks Total number of cycles: 6-8 Anti-emetic group Low Prophylactic co-trimoxazole and
Fludarabine Low Grade non-hodgkin s Lymphoma and CLL Fludarabine 25mg/m 2 oral Days 1-5 Cycle frequency: Every four weeks Total number of cycles: 6-8 Anti-emetic group Low Prophylactic co-trimoxazole and
Current Rheumatoid Arthritis Treatment Options: Update for Managed Care and Specialty Pharmacists
 Current Rheumatoid Arthritis Treatment Options: Update for Managed Care and Specialty Pharmacists 1. Which of the following matches of biologic targets that contribute to rheumatoid arthritis (RA) and
Current Rheumatoid Arthritis Treatment Options: Update for Managed Care and Specialty Pharmacists 1. Which of the following matches of biologic targets that contribute to rheumatoid arthritis (RA) and
How long will it take to work? You may begin to feel better within a few days or it may take up to six weeks after your first treatment session.
 Crohn's and Colitis UK Drug Treatment Information Improving life for people affected by inflammatory bowel diseases Infliximab This information leaflet aims to answer common questions you may have if you
Crohn's and Colitis UK Drug Treatment Information Improving life for people affected by inflammatory bowel diseases Infliximab This information leaflet aims to answer common questions you may have if you
Shared Care Guideline-Use of Donepezil, Galantamine, Rivastigmine and Memantine in Dementia
 Shared Care Guideline-Use of Donepezil, Galantamine, Rivastigmine and Memantine in Dementia Version: 3.0 Ratified by: Medicines Committee Date ratified: 16 th November 2011 Name of originator/author: James
Shared Care Guideline-Use of Donepezil, Galantamine, Rivastigmine and Memantine in Dementia Version: 3.0 Ratified by: Medicines Committee Date ratified: 16 th November 2011 Name of originator/author: James
Original Policy Date
 MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
Gilenya. Exceptional healthcare, personally delivered
 Gilenya Exceptional healthcare, personally delivered Your Consultant Neurologist has suggested that you may benefit from treatment with Gilenya. The decision to start this form of treatment can be difficult.
Gilenya Exceptional healthcare, personally delivered Your Consultant Neurologist has suggested that you may benefit from treatment with Gilenya. The decision to start this form of treatment can be difficult.
Guideline for the use of Biological Therapies in the Treatment of Psoriasis
 1 Date of Production: March1 st 2011 Date of 1 st review: July 10 th 2015 Date for next review: March1 st 2018 Local Contact Dermatology Consultant Shanti Ayob Patient group to which this applies: Patients
1 Date of Production: March1 st 2011 Date of 1 st review: July 10 th 2015 Date for next review: March1 st 2018 Local Contact Dermatology Consultant Shanti Ayob Patient group to which this applies: Patients
I B2.4. Design of the patient information leaflet for VariQuin
 (English translation of official Dutch version) I B2.4. Design of the patient information leaflet for VariQuin Information for the Patient: Read this package leaflet carefully when you have some time to
(English translation of official Dutch version) I B2.4. Design of the patient information leaflet for VariQuin Information for the Patient: Read this package leaflet carefully when you have some time to
Intravenous Methyl Prednisolone in Multiple Sclerosis
 Intravenous Methyl Prednisolone in Multiple Sclerosis Exceptional healthcare, personally delivered Relapse management in multiple sclerosis Relapses in multiple sclerosis (MS) are common and caused by
Intravenous Methyl Prednisolone in Multiple Sclerosis Exceptional healthcare, personally delivered Relapse management in multiple sclerosis Relapses in multiple sclerosis (MS) are common and caused by
Issue date: August 2010
 Issue date: August 2010 Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a TNF inhibitor Part review of NICE technology appraisal
Issue date: August 2010 Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a TNF inhibitor Part review of NICE technology appraisal
Guy s, King s and St Thomas Cancer Centre The Cancer Outpatient Clinic Maintenance BCG for nonmuscle invasive bladder cancer
 Guy s, King s and St Thomas Cancer Centre The Cancer Outpatient Clinic Maintenance BCG for nonmuscle invasive bladder cancer This information sheet has been given to you to explain the use of maintenance
Guy s, King s and St Thomas Cancer Centre The Cancer Outpatient Clinic Maintenance BCG for nonmuscle invasive bladder cancer This information sheet has been given to you to explain the use of maintenance
VAD Chemotherapy Regimen for Multiple Myeloma Information for Patients
 VAD Chemotherapy Regimen for Multiple Myeloma Information for Patients The Regimen contains: V = vincristine (Oncovin ) A = Adriamycin (doxorubicin) D = Decadron (dexamethasone) How Is This Regimen Given?
VAD Chemotherapy Regimen for Multiple Myeloma Information for Patients The Regimen contains: V = vincristine (Oncovin ) A = Adriamycin (doxorubicin) D = Decadron (dexamethasone) How Is This Regimen Given?
Clinical Performance Director of Nursing Allison Bussey
 PGD 0314 Patient Group Direction Administration of Adrenaline (Epinephrine) 1:1000 (1mg/ml) Injection By Registered Nurses employed by South Staffordshire & Shropshire Healthcare Foundation NHS Trust This
PGD 0314 Patient Group Direction Administration of Adrenaline (Epinephrine) 1:1000 (1mg/ml) Injection By Registered Nurses employed by South Staffordshire & Shropshire Healthcare Foundation NHS Trust This
Methotrexate treatment
 Methotrexate treatment Oral methotrexate pre-treatment patient information leaflet This leaflet has been prepared to support information given to you as part of your discussions with the doctor, nurse
Methotrexate treatment Oral methotrexate pre-treatment patient information leaflet This leaflet has been prepared to support information given to you as part of your discussions with the doctor, nurse
Lung Pathway Group Pemetrexed and Cisplatin in Non-Small Cell Lung Cancer (NSCLC)
 Indication: NICE TA181 First line treatment option in advanced or metastatic non-squamous NSCLC (histology confirmed as adenocarcinoma or large cell carcinoma) Performance status 0-1 Regimen details: Pemetrexed
Indication: NICE TA181 First line treatment option in advanced or metastatic non-squamous NSCLC (histology confirmed as adenocarcinoma or large cell carcinoma) Performance status 0-1 Regimen details: Pemetrexed
Shared Care Guideline for the use of Leflunomide for Rheumatoid Arthritis
 Shared Care Guideline for the use of Leflunomide for Rheumatoid Section 1: Agreement for transfer of prescribing to GP Please sign this form and return it to the named consultant if you are willing to
Shared Care Guideline for the use of Leflunomide for Rheumatoid Section 1: Agreement for transfer of prescribing to GP Please sign this form and return it to the named consultant if you are willing to
POAC CLINICAL GUIDELINE
 POAC CLINICAL GUIDELINE Acute Pylonephritis DIAGNOSIS COMPLICATED PYELONEPHRITIS EXCLUSION CRITERIA: Male Known or suspected renal impairment (egfr < 60) Abnormality of renal tract Known or suspected renal
POAC CLINICAL GUIDELINE Acute Pylonephritis DIAGNOSIS COMPLICATED PYELONEPHRITIS EXCLUSION CRITERIA: Male Known or suspected renal impairment (egfr < 60) Abnormality of renal tract Known or suspected renal
Patient Sticker Multiple Sclerosis Ambulatory Emergency Care Pathway
 Multiple Sclerosis Ambulatory Emergency Care Pathway 1 Consultant: Dr M Oldfield Consultant: Dr D Harris Lead Nurse: Catie Paterson Ambulatory Emergency Care (AEC) Unit Patient From ED (Emergency Department)
Multiple Sclerosis Ambulatory Emergency Care Pathway 1 Consultant: Dr M Oldfield Consultant: Dr D Harris Lead Nurse: Catie Paterson Ambulatory Emergency Care (AEC) Unit Patient From ED (Emergency Department)
EMEA PUBLIC STATEMENT ON LEFLUNOMIDE (ARAVA) - SEVERE AND SERIOUS HEPATIC REACTIONS -
 The European Agency for the Evaluation of Medicinal Products Post-authorisation evaluation of medicines for human use London, 12 March 2001 Doc. Ref: EMEA/H/5611/01/en EMEA PUBLIC STATEMENT ON LEFLUNOMIDE
The European Agency for the Evaluation of Medicinal Products Post-authorisation evaluation of medicines for human use London, 12 March 2001 Doc. Ref: EMEA/H/5611/01/en EMEA PUBLIC STATEMENT ON LEFLUNOMIDE
Vincristine by short infusion Doxorubicin by injection Cyclophosphamide by injection Rituximab by an infusion over between 60 minutes to a few hours
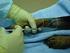 R-CHOP R-CHOP This leaflet is offered as a guide to you and your family. The possible benefits of treatment vary; for some people chemotherapy may reduce the risk of the cancer coming back, for others
R-CHOP R-CHOP This leaflet is offered as a guide to you and your family. The possible benefits of treatment vary; for some people chemotherapy may reduce the risk of the cancer coming back, for others
Rheumatoid Arthritis
 Rheumatoid Arthritis Carole Callaghan Principal Pharmacist NHS Lothian Aim To update pharmacists on the current management of rheumatoid arthritis and explore ways to implement pharmaceutical care for
Rheumatoid Arthritis Carole Callaghan Principal Pharmacist NHS Lothian Aim To update pharmacists on the current management of rheumatoid arthritis and explore ways to implement pharmaceutical care for
Speaking Plainly. Biologic treatment options for rheumatoid arthritis
 in association with Plain English Campaign Speaking Plainly Biologic treatment options for rheumatoid arthritis A guide to help healthcare professionals talking to patients with rheumatoid arthritis Foreword
in association with Plain English Campaign Speaking Plainly Biologic treatment options for rheumatoid arthritis A guide to help healthcare professionals talking to patients with rheumatoid arthritis Foreword
Immune modulation in rheumatology. Geoff McColl University of Melbourne/Australian Rheumatology Association
 Immune modulation in rheumatology Geoff McColl University of Melbourne/Australian Rheumatology Association A traditional start to a presentation on biological agents in rheumatic disease is Plasma cell
Immune modulation in rheumatology Geoff McColl University of Melbourne/Australian Rheumatology Association A traditional start to a presentation on biological agents in rheumatic disease is Plasma cell
Donepezil (Aricept ), Galantamine (Reminyl XL ), Rivastigmine (Exelon ) and Memantine (Ebixa )
 Donepezil (Aricept ), Galantamine (Reminyl XL ), Rivastigmine (Exelon ) and Memantine (Ebixa ) ESCA: For the treatment of Alzheimer s disease. SECONDARY CARE SECTION TO BE COMPLETED BY INITIATING DOCTOR
Donepezil (Aricept ), Galantamine (Reminyl XL ), Rivastigmine (Exelon ) and Memantine (Ebixa ) ESCA: For the treatment of Alzheimer s disease. SECONDARY CARE SECTION TO BE COMPLETED BY INITIATING DOCTOR
Oxford University Hospitals. NHS Trust. Department of Neurology Natalizumab (Tysabri) for Multiple Sclerosis. Information for patients
 Oxford University Hospitals NHS Trust Department of Neurology Natalizumab (Tysabri) for Multiple Sclerosis Information for patients page 2 What is Natalizumab and what is it used for? Natalizumab is an
Oxford University Hospitals NHS Trust Department of Neurology Natalizumab (Tysabri) for Multiple Sclerosis Information for patients page 2 What is Natalizumab and what is it used for? Natalizumab is an
PATIENT MEDICATION INFORMATION
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr CYRAMZA ramucirumab Read this carefully before you receive CYRAMZA (pronounced "si ram - ze"). This leaflet is a
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr CYRAMZA ramucirumab Read this carefully before you receive CYRAMZA (pronounced "si ram - ze"). This leaflet is a
Biologics... The Story So Far. Biologics. The Story So Far. A Patient Guide to Biologic Therapies in the Treatment of Rheumatoid Arthritis
 Biologics... The Story So Far Biologics The Story So Far A Patient Guide to Biologic Therapies in the Treatment of Rheumatoid Arthritis September 2013 Help & information from NRAS NRAS is the only patient-led
Biologics... The Story So Far Biologics The Story So Far A Patient Guide to Biologic Therapies in the Treatment of Rheumatoid Arthritis September 2013 Help & information from NRAS NRAS is the only patient-led
Adjunctive psychosocial intervention. Conditions requiring dose reduction. Immediate, peak plasma concentration is reached within 1 hour.
 Shared Care Guideline for Prescription and monitoring of Naltrexone Hydrochloride in alcohol dependence Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist,
Shared Care Guideline for Prescription and monitoring of Naltrexone Hydrochloride in alcohol dependence Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist,
Regimen : Fludarabine Cyclophosphamide Rituximab (FCR-oral)
 Regimen : Fludarabine Cyclophosphamide Rituximab (FCR-oral) Indication CLL 1st line as per NICE TAG 174 CLL (relapsed) as per NICE TAG 193 Regimen details Day Drug Dose Route Cycle 1 1 Rituximab 375mg/m
Regimen : Fludarabine Cyclophosphamide Rituximab (FCR-oral) Indication CLL 1st line as per NICE TAG 174 CLL (relapsed) as per NICE TAG 193 Regimen details Day Drug Dose Route Cycle 1 1 Rituximab 375mg/m
Teriflunomide (Aubagio) 14mg once daily tablet
 Teriflunomide (Aubagio) 14mg once daily tablet Exceptional healthcare, personally delivered Your Consultant Neurologist has suggested that you may benefit from treatment with Teriflunomide. The decision
Teriflunomide (Aubagio) 14mg once daily tablet Exceptional healthcare, personally delivered Your Consultant Neurologist has suggested that you may benefit from treatment with Teriflunomide. The decision
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
Patient Information Leaflet
 Patient Information Leaflet METHOTREXATE We hope this fact sheet will provide you with some information about Methotrexate and answer some of the questions you may have. Methotrexate is available in tablet
Patient Information Leaflet METHOTREXATE We hope this fact sheet will provide you with some information about Methotrexate and answer some of the questions you may have. Methotrexate is available in tablet
A Survey of Barriers to Treatment Access in Rheumatoid Arthritis. Country Annex Report: UK
 A Survey of Barriers to Treatment Access in Rheumatoid Arthritis Country Annex Report: UK October 2009 1 Interviews In the UK, five rheumatologists and one patient representative were interviewed. The
A Survey of Barriers to Treatment Access in Rheumatoid Arthritis Country Annex Report: UK October 2009 1 Interviews In the UK, five rheumatologists and one patient representative were interviewed. The
Leflunomide Leflunomide
 Drug information Leflunomide Leflunomide This leaflet provides information on leflunomide and will answer any questions you have about the treatment. Arthritis Research UK produce and print our booklets
Drug information Leflunomide Leflunomide This leaflet provides information on leflunomide and will answer any questions you have about the treatment. Arthritis Research UK produce and print our booklets
Carla Duff, CPNP MSN CCRP Clinical Advanced Registered Nurse Practitioner University of South Florida Division of Allergy, Immunology, and
 Carla Duff, CPNP MSN CCRP Clinical Advanced Registered Nurse Practitioner University of South Florida Division of Allergy, Immunology, and Rheumatology Intravenous Subcutaneous IVIg SCIg What should you
Carla Duff, CPNP MSN CCRP Clinical Advanced Registered Nurse Practitioner University of South Florida Division of Allergy, Immunology, and Rheumatology Intravenous Subcutaneous IVIg SCIg What should you
METHOTREXATE TREATMENT
 METHOTREXATE TREATMENT This leaflet has been prepared to support information given to you as part of your discussions with the doctor, nurse or pharmacist before you start treatment with oral methotrexate.
METHOTREXATE TREATMENT This leaflet has been prepared to support information given to you as part of your discussions with the doctor, nurse or pharmacist before you start treatment with oral methotrexate.
Human Normal Immunoglobulin Solution for Intravenous Infusion.
 CONSUMER MEDICINE INFORMATION (CMI) OCTAGAM Human Normal Immunoglobulin Solution for Intravenous Infusion. OCTAGAM is available in single use bottles of 20 ml, 50 ml, 100 ml and 200 ml. OCTAGAM contains
CONSUMER MEDICINE INFORMATION (CMI) OCTAGAM Human Normal Immunoglobulin Solution for Intravenous Infusion. OCTAGAM is available in single use bottles of 20 ml, 50 ml, 100 ml and 200 ml. OCTAGAM contains
NHS FORTH VALLEY Multiple Sclerosis Service Management of MS Relapses
 NHS FORTH VALLEY Multiple Sclerosis Service Management of MS Relapses Approved 22/06/2010 Version Version 2 Date of First Issue 2002 Review Date 10/05/2016 Date of Issue 01/02/2010 EQIA Yes 22.06.10 Author
NHS FORTH VALLEY Multiple Sclerosis Service Management of MS Relapses Approved 22/06/2010 Version Version 2 Date of First Issue 2002 Review Date 10/05/2016 Date of Issue 01/02/2010 EQIA Yes 22.06.10 Author
Rivaroxaban: Amber Drug Guidance for the prevention of stroke and systemic embolism in patients with non-valvular AF
 Leeds Rivaroxaban: Amber Drug Guidance for the prevention of stroke and systemic embolism in patients with non-valvular AF Amber Drug Level 3 (amber drug with monitoring requirements) We have started your
Leeds Rivaroxaban: Amber Drug Guidance for the prevention of stroke and systemic embolism in patients with non-valvular AF Amber Drug Level 3 (amber drug with monitoring requirements) We have started your
Other treatments for chronic myeloid leukaemia
 Other treatments for chronic myeloid leukaemia This information is an extract from the booklet Understanding chronic myeloid leukaemia. You may find the full booklet helpful. We can send you a copy free
Other treatments for chronic myeloid leukaemia This information is an extract from the booklet Understanding chronic myeloid leukaemia. You may find the full booklet helpful. We can send you a copy free
RHEUMATOID ARTHRITIS. Dr Bruce Kirkham Rheumatology Clinical Lead
 RHEUMATOID ARTHRITIS Dr Bruce Kirkham Rheumatology Clinical Lead RHEUMATOID ARTHRITIS (RA) RA is a common disease: 0.8 per cent of the population RA more common in females: female to male ratio 3:1 RA
RHEUMATOID ARTHRITIS Dr Bruce Kirkham Rheumatology Clinical Lead RHEUMATOID ARTHRITIS (RA) RA is a common disease: 0.8 per cent of the population RA more common in females: female to male ratio 3:1 RA
Intravenous Immunoglobulin in Neurological disorders
 Intravenous Immunoglobulin in Neurological disorders Exceptional healthcare, personally delivered What is Intravenous Immunoglobulin (IVIg)? Intravenous immunoglobulin (IVIg) is a blood product that combines
Intravenous Immunoglobulin in Neurological disorders Exceptional healthcare, personally delivered What is Intravenous Immunoglobulin (IVIg)? Intravenous immunoglobulin (IVIg) is a blood product that combines
Rheumatoid Arthritis. GP workshop 15 January 2011
 Rheumatoid Arthritis GP workshop 15 January 2011 Case 1 A 72 year old Malay woman with RA comes for routine follow up. She feels generally unwell in the last 5 days. Appetite is fair. Her joints are fine.
Rheumatoid Arthritis GP workshop 15 January 2011 Case 1 A 72 year old Malay woman with RA comes for routine follow up. She feels generally unwell in the last 5 days. Appetite is fair. Her joints are fine.
MEDICATION GUIDE. ACTEMRA (AC-TEM-RA) (tocilizumab) Solution for Intravenous Infusion
 MEDICATION GUIDE ACTEMRA (AC-TEM-RA) (tocilizumab) Solution for Intravenous Infusion ACTEMRA (AC-TEM-RA) (tocilizumab) Injection, Solution for Subcutaneous Administration Read this Medication Guide before
MEDICATION GUIDE ACTEMRA (AC-TEM-RA) (tocilizumab) Solution for Intravenous Infusion ACTEMRA (AC-TEM-RA) (tocilizumab) Injection, Solution for Subcutaneous Administration Read this Medication Guide before
ACCIDENT AND EMERGENCY DEPARTMENT/CARDIOLOGY
 Care Pathway Triage category ATRIAL FIBRILLATION PATHWAY ACCIDENT AND EMERGENCY DEPARTMENT/CARDIOLOGY AF/ FLUTTER IS PRIMARY REASON FOR PRESENTATION YES NO ONSET SYMPTOMS OF AF./../ TIME DURATION OF AF
Care Pathway Triage category ATRIAL FIBRILLATION PATHWAY ACCIDENT AND EMERGENCY DEPARTMENT/CARDIOLOGY AF/ FLUTTER IS PRIMARY REASON FOR PRESENTATION YES NO ONSET SYMPTOMS OF AF./../ TIME DURATION OF AF
Package leaflet: Information for the patient. Entyvio 300 mg powder for concentrate for solution for infusion vedolizumab
 Package leaflet: Information for the patient Entyvio 300 mg powder for concentrate for solution for infusion vedolizumab This medicine is subject to additional monitoring. This will allow quick identification
Package leaflet: Information for the patient Entyvio 300 mg powder for concentrate for solution for infusion vedolizumab This medicine is subject to additional monitoring. This will allow quick identification
Radioactive Ra 223 therapy. Information for patients Weston Park Hospital
 Radioactive Ra 223 therapy Information for patients Weston Park Hospital page 2 of 8 This leaflet contains information about radioactive Ra 223 therapy. If you have any questions about your treatment,
Radioactive Ra 223 therapy Information for patients Weston Park Hospital page 2 of 8 This leaflet contains information about radioactive Ra 223 therapy. If you have any questions about your treatment,
Humulin R (U500) insulin: Prescribing Guidance
 Leeds Humulin R (U500) insulin: Prescribing Guidance Amber Drug Level 2 We have started your patient on Humulin R (U500) insulin for the treatment of diabetic patients with marked insulin resistance requiring
Leeds Humulin R (U500) insulin: Prescribing Guidance Amber Drug Level 2 We have started your patient on Humulin R (U500) insulin for the treatment of diabetic patients with marked insulin resistance requiring
Drug Treatment Information for patients with Inflammatory Bowel Disease. Infliximab
 Drug Treatment Information for patients with Inflammatory Bowel Disease Infliximab Improving life for people affected by Colitis and Crohn s Disease National Association For Colitis and Crohn s Disease
Drug Treatment Information for patients with Inflammatory Bowel Disease Infliximab Improving life for people affected by Colitis and Crohn s Disease National Association For Colitis and Crohn s Disease
Guidelines for Nurse Led HIV Clinic
 Guidelines for Nurse Led HIV Clinic Produced and approved by (Committee and Date) Kieran Sharkey Ratified by (Committee and Date) Reviewed Review date: Guidelines for Nurse Led HIV Clinic Introduction
Guidelines for Nurse Led HIV Clinic Produced and approved by (Committee and Date) Kieran Sharkey Ratified by (Committee and Date) Reviewed Review date: Guidelines for Nurse Led HIV Clinic Introduction
patient group direction
 DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
Summary of the risk management plan (RMP) for Accofil (filgrastim)
 EMA/475472/2014 Summary of the risk management plan (RMP) for Accofil (filgrastim) This is a summary of the risk management plan (RMP) for Accofil, which details the measures to be taken in order to ensure
EMA/475472/2014 Summary of the risk management plan (RMP) for Accofil (filgrastim) This is a summary of the risk management plan (RMP) for Accofil, which details the measures to be taken in order to ensure
påçííáëü=jéçáåáåéë=`çåëçêíáìã==
 påçííáëü=jéçáåáåéë=`çåëçêíáìã== adalimumab 40mg pre-filled syringe for subcutaneous injection (Humira ) No. (218/05) Abbott New indication: treatment of active and progressive psoriatic arthritis in adults
påçííáëü=jéçáåáåéë=`çåëçêíáìã== adalimumab 40mg pre-filled syringe for subcutaneous injection (Humira ) No. (218/05) Abbott New indication: treatment of active and progressive psoriatic arthritis in adults
Dabigatran: Amber Drug Guidance for the prevention of stroke and systemic embolism in patients with non-valvular AF
 Leeds Dabigatran: Amber Drug Guidance for the prevention of stroke and systemic embolism in patients with non-valvular AF Amber Drug Level 3 (amber drug with monitoring requirements) We have started your
Leeds Dabigatran: Amber Drug Guidance for the prevention of stroke and systemic embolism in patients with non-valvular AF Amber Drug Level 3 (amber drug with monitoring requirements) We have started your
Maintenance of abstinence in alcohol dependence
 Shared Care Guideline for Prescription and monitoring of Acamprosate Calcium Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist, Alcohol Services Dr Donnelly
Shared Care Guideline for Prescription and monitoring of Acamprosate Calcium Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist, Alcohol Services Dr Donnelly
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la]
![READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la] READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la]](/thumbs/35/17235501.jpg) READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] mepolizumab lyophilized powder for subcutaneous injection Read this carefully before you start
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] mepolizumab lyophilized powder for subcutaneous injection Read this carefully before you start
PACKAGE LEAFLET: INFORMATION FOR THE USER. PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion. Paracetamol
 PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
Cytotoxic and Biotherapies Credentialing Programme Module 6
 Cytotoxic and Biotherapies Credentialing Programme Module 6 1. Safe Handling and Administration of Cytotoxic and Biotherapies 2. Waste and Spill Management At the completion of this module the RN will
Cytotoxic and Biotherapies Credentialing Programme Module 6 1. Safe Handling and Administration of Cytotoxic and Biotherapies 2. Waste and Spill Management At the completion of this module the RN will
Media Release. Basel, 11 June 2009. RA patients with enhanced response identified
 Media Release Basel, 11 June 2009 New data demonstrate the ability of MabThera to reduce the progression of joint damage when used as a first-line biologic treatment in rheumatoid arthritis RA patients
Media Release Basel, 11 June 2009 New data demonstrate the ability of MabThera to reduce the progression of joint damage when used as a first-line biologic treatment in rheumatoid arthritis RA patients
Your Treatment with Bacillus Calmette- Guérin (BCG)
 Your Treatment with Bacillus Calmette- Guérin (BCG) Patient Information Introduction Your consultant has prescribed BCG for the treatment of your bladder tumour. This booklet does not replace the discussion
Your Treatment with Bacillus Calmette- Guérin (BCG) Patient Information Introduction Your consultant has prescribed BCG for the treatment of your bladder tumour. This booklet does not replace the discussion
Paracetamol apollo +9191 46 950 950. Paracetamol apollo +9191 46 950 950. Paracetamol
 Paracetamol apollo +9191 46 950 950 Paracetamol apollo +9191 46 950 950 Paracetamol CAS Number : 103-90-2 Molecular Weight : 151.17 g/mol Molecular Formula : C8H9NO2 Systematic (IUPAC) : N-(4- hydroxyphenyl)ethanamide
Paracetamol apollo +9191 46 950 950 Paracetamol apollo +9191 46 950 950 Paracetamol CAS Number : 103-90-2 Molecular Weight : 151.17 g/mol Molecular Formula : C8H9NO2 Systematic (IUPAC) : N-(4- hydroxyphenyl)ethanamide
BE SURE. BE SAFE. VACCINATE.
 DON T GET OR GIVE THE FLU THIS YEAR THANK YOU Vaccination is the only protection. www.immunisation.ie BE SURE. BE SAFE. VACCINATE. FLU VACCINE 2013-2014 Healthcare workers prevent the spread of flu and
DON T GET OR GIVE THE FLU THIS YEAR THANK YOU Vaccination is the only protection. www.immunisation.ie BE SURE. BE SAFE. VACCINATE. FLU VACCINE 2013-2014 Healthcare workers prevent the spread of flu and
LEFLUNOMIDE (Adults)
 Shared Care Guideline DRUG: Introduction: LEFLUNOMIDE (Adults) Indication: Disease modifying drug for rheumatoid arthritis and psoriatic arthritis Licensing Information: Disease modifying drug for active
Shared Care Guideline DRUG: Introduction: LEFLUNOMIDE (Adults) Indication: Disease modifying drug for rheumatoid arthritis and psoriatic arthritis Licensing Information: Disease modifying drug for active
Infl ectra for rheumatoid arthritis
 Infl ectra for rheumatoid arthritis Some important information to get you started with your treatment This booklet is intended only for use by patients who have been prescribed Inflectra. Introduction
Infl ectra for rheumatoid arthritis Some important information to get you started with your treatment This booklet is intended only for use by patients who have been prescribed Inflectra. Introduction
ANNE ARUNDEL MEDICAL CENTER CRITICAL CARE MEDICATION MANUAL DEPARTMENT OF NURSING AND PHARMACY. Guidelines for Use of Intravenous Isoproterenol
 ANNE ARUNDEL MEDICAL CENTER CRITICAL CARE MEDICATION MANUAL DEPARTMENT OF NURSING AND PHARMACY Guidelines for Use of Intravenous Isoproterenol Major Indications Status Asthmaticus As a last resort for
ANNE ARUNDEL MEDICAL CENTER CRITICAL CARE MEDICATION MANUAL DEPARTMENT OF NURSING AND PHARMACY Guidelines for Use of Intravenous Isoproterenol Major Indications Status Asthmaticus As a last resort for
Naltrexone Shared Care Guideline for the treatment of alcohol dependence and opioid dependance
 Naltrexone Shared Care Guideline for the treatment of alcohol dependence and opioid dependance Introduction Indication/Licensing information: Naltrexone is licensed for use as an additional therapy, within
Naltrexone Shared Care Guideline for the treatment of alcohol dependence and opioid dependance Introduction Indication/Licensing information: Naltrexone is licensed for use as an additional therapy, within
Mifamurtide (Mepact ) for Osteosarcoma
 Mifamurtide (Mepact ) for Osteosarcoma Mifamurtide (Mepact ) for Osteosarcoma This leaflet is offered as a guide to you and your family. Your treatment will be fully explained to you by your doctor or
Mifamurtide (Mepact ) for Osteosarcoma Mifamurtide (Mepact ) for Osteosarcoma This leaflet is offered as a guide to you and your family. Your treatment will be fully explained to you by your doctor or
Summary of the risk management plan (RMP) for Otezla (apremilast)
 EMA/741412/2014 Summary of the risk management plan (RMP) for Otezla (apremilast) This is a summary of the risk management plan (RMP) for Otezla, which details the measures to be taken in order to ensure
EMA/741412/2014 Summary of the risk management plan (RMP) for Otezla (apremilast) This is a summary of the risk management plan (RMP) for Otezla, which details the measures to be taken in order to ensure
ENDORSED BY THE GOVERNANCE COMMITTEE
 Guideline for the Preparation or Manipulation of Monoclonal Antibodies (MABs) and related compounds such as Fusion Proteins, used in the Treatment of Cancer Date Approved by Network Governance July 2012
Guideline for the Preparation or Manipulation of Monoclonal Antibodies (MABs) and related compounds such as Fusion Proteins, used in the Treatment of Cancer Date Approved by Network Governance July 2012
POST EXPOSURE PROPHYLAXI S
 Departments of Infectious Diseases & Emergency Medicine POST EXPOSURE PROPHYLAXI S QUI CK GUI DE FOR EMERGENCY DEPT Adapted from Irish National PEP Guidelines and St James s Hospital GUIDE Clinic/Emergency
Departments of Infectious Diseases & Emergency Medicine POST EXPOSURE PROPHYLAXI S QUI CK GUI DE FOR EMERGENCY DEPT Adapted from Irish National PEP Guidelines and St James s Hospital GUIDE Clinic/Emergency
Procedure for Inotrope Administration in the home
 Procedure for Inotrope Administration in the home Purpose This purpose of this procedure is to define the care used when administering inotropic agents intravenously in the home This includes: A. Practice
Procedure for Inotrope Administration in the home Purpose This purpose of this procedure is to define the care used when administering inotropic agents intravenously in the home This includes: A. Practice
Stowe School Medications Policy
 INTRODUCTION Most pupils will need medication at some stage of their school life. Although this will mainly be for short periods there are a few pupils with chronic conditions who may require regular medication
INTRODUCTION Most pupils will need medication at some stage of their school life. Although this will mainly be for short periods there are a few pupils with chronic conditions who may require regular medication
FAQs on Influenza A (H1N1-2009) Vaccine
 FAQs on Influenza A (H1N1-2009) Vaccine 1) What is Influenza A (H1N1-2009) (swine flu) 1? Influenza A (H1N1-2009), previously known as "swine flu", is a new strain of influenza virus that spreads from
FAQs on Influenza A (H1N1-2009) Vaccine 1) What is Influenza A (H1N1-2009) (swine flu) 1? Influenza A (H1N1-2009), previously known as "swine flu", is a new strain of influenza virus that spreads from
Medicines Management
 Medicines Management Patient Group Direction for the Supply/administration of Adrenaline (Epinephrine) for Treatment of Anaphylaxis by accredited community Pharmacists. Rationale To enable a pharmacist,
Medicines Management Patient Group Direction for the Supply/administration of Adrenaline (Epinephrine) for Treatment of Anaphylaxis by accredited community Pharmacists. Rationale To enable a pharmacist,
Prescribing Framework for Donepezil in the Treatment and Management of Dementia
 Hull & East Riding Prescribing Committee Prescribing Framework for Donepezil in the Treatment and Management of Dementia Patients Name:.. NHS Number: Patients Address:... (Use addressograph sticker) GP
Hull & East Riding Prescribing Committee Prescribing Framework for Donepezil in the Treatment and Management of Dementia Patients Name:.. NHS Number: Patients Address:... (Use addressograph sticker) GP
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy (DMT) means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Upstate University Health System Medication Exam - Version A
 Upstate University Health System Medication Exam - Version A Name: ID Number: Date: Unit: Directions: Please read each question below. Choose the best response for each of the Multiple Choice and Medication
Upstate University Health System Medication Exam - Version A Name: ID Number: Date: Unit: Directions: Please read each question below. Choose the best response for each of the Multiple Choice and Medication
BEAUMONT HOSPITAL DEPARTMENT OF NEPHROLOGY RENAL BIOPSY
 1 BEAUMONT HOSPITAL DEPARTMENT OF NEPHROLOGY GUIDELINES ON ADMINISTRATION OF INTRAVENOUS IRON SUCROSE (VENOFER) AS A BOLUS DOSE IN THE RENAL OUTPATIENT SETTING Date Developed: August- October 2007 RENAL
1 BEAUMONT HOSPITAL DEPARTMENT OF NEPHROLOGY GUIDELINES ON ADMINISTRATION OF INTRAVENOUS IRON SUCROSE (VENOFER) AS A BOLUS DOSE IN THE RENAL OUTPATIENT SETTING Date Developed: August- October 2007 RENAL
Page 1 of 15 Origination Date: 09/14 Revision Date(s): 10/2015, 02/2016 Developed By: Medical Criteria Committee 10/28/2015
 Moda Health Plan, Inc. Medical Necessity Criteria Subject: Actemra (tocilizumab) Page 1 of 15 Origination Date: 09/14 Revision Date(s): 10/2015, 02/2016 Developed By: Medical Criteria Committee 10/28/2015
Moda Health Plan, Inc. Medical Necessity Criteria Subject: Actemra (tocilizumab) Page 1 of 15 Origination Date: 09/14 Revision Date(s): 10/2015, 02/2016 Developed By: Medical Criteria Committee 10/28/2015
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
UW MEDICINE PATIENT EDUCATION. Xofigo Therapy. For metastatic prostate cancer. What is Xofigo? How does it work?
 UW MEDICINE PATIENT EDUCATION Xofigo Therapy For metastatic prostate cancer This handout explains how the drug Xofigo is used to treat metastatic prostate cancer. What is Xofigo? Xofigo is a radioactive
UW MEDICINE PATIENT EDUCATION Xofigo Therapy For metastatic prostate cancer This handout explains how the drug Xofigo is used to treat metastatic prostate cancer. What is Xofigo? Xofigo is a radioactive
Rituximab (MabThera ) in rheumatoid arthritis: non-nice approved indications
 Rituximab (MabThera ) in rheumatoid arthritis: non-nice approved indications Lead author: Stephen Erhorn NHS Regional Drug & Therapeutics Centre (Newcastle) July 2011 2011 Summary NICE guidance is extensive
Rituximab (MabThera ) in rheumatoid arthritis: non-nice approved indications Lead author: Stephen Erhorn NHS Regional Drug & Therapeutics Centre (Newcastle) July 2011 2011 Summary NICE guidance is extensive
Methotrexate Dose For Juvenile Rheumatoid Arthritis
 Methotrexate Dose For Juvenile Rheumatoid Arthritis should i take methotrexate for my ra methotrexate 50 mg/ml methotrexate sodium 2.5mg tablets what is the usual dosage of methotrexate for ra methotrexate
Methotrexate Dose For Juvenile Rheumatoid Arthritis should i take methotrexate for my ra methotrexate 50 mg/ml methotrexate sodium 2.5mg tablets what is the usual dosage of methotrexate for ra methotrexate
New England Pain Management Consultants At New England Baptist Hospital
 New England Pain Management Consultants At New England Baptist Hospital Pain Management Center Health Assessment Dear New Pain Management Patient, Welcome to the New England Pain Management Consultants
New England Pain Management Consultants At New England Baptist Hospital Pain Management Center Health Assessment Dear New Pain Management Patient, Welcome to the New England Pain Management Consultants
Improvement in Quality of Life of Rheumatoid Arthritis Patients on Biologic Therapy
 Improvement in Quality of Life of Rheumatoid Arthritis Patients on Biologic Therapy R Adams 1, Ct Ng 2, A Gibbs 2, L Tilson 1, D Veale 2, B Bresnihan 2, O FitzGerald 2, M Barry 1 1. National Centre for
Improvement in Quality of Life of Rheumatoid Arthritis Patients on Biologic Therapy R Adams 1, Ct Ng 2, A Gibbs 2, L Tilson 1, D Veale 2, B Bresnihan 2, O FitzGerald 2, M Barry 1 1. National Centre for
DISEASE MODIFYING THERAPY CARE PATHWAY. Multiple Sclerosis Service
 DISEASE MODIFYING THERAPY CARE PATHWAY Multiple Sclerosis Service CONTENTS Disease modifying therapy care pathway ABN guidelines Appendix 1 MS Service DMT Sheet Appendix 2 Who to contact info Appendix
DISEASE MODIFYING THERAPY CARE PATHWAY Multiple Sclerosis Service CONTENTS Disease modifying therapy care pathway ABN guidelines Appendix 1 MS Service DMT Sheet Appendix 2 Who to contact info Appendix
