FIVE-YEAR BIOCHEMICAL OUTCOME FOLLOWING PERMANENT INTERSTITIAL BRACHYTHERAPY FOR CLINICAL T1 T3 PROSTATE CANCER
|
|
|
- Adrian Bond
- 8 years ago
- Views:
Transcription
1 PII S (01) Int. J. Radiation Oncology Biol. Phys., Vol. 51, No. 1, pp , 2001 Copyright 2001 Elsevier Science Inc. Printed in the USA. All rights reserved /01/$ see front matter CLINICAL INVESTIGATION Prostate FIVE-YEAR BIOCHEMICAL OUTCOME FOLLOWING PERMANENT INTERSTITIAL BRACHYTHERAPY FOR CLINICAL T1 T3 PROSTATE CANCER GREGORY S. MERRICK, M.D.,* WAYNE M. BUTLER, PH.D.,* ROBERT W. GALBREATH, PH.D.,* AND JONATHAN H. LIEF, PH.D.* *Schiffler Cancer Center, Wheeling Hospital, Wheeling, WV; George Washington University Medical Center, Division of Radiation Oncology and Biophysics, Washington, DC; Wheeling Jesuit University, Wheeling, WV Purpose: To evaluate 5-year biochemical disease-free outcome for men with clinical T1b T3a NxM American Joint Committee on Cancer (1997 AJCC) adenocarcinoma of the prostate gland who underwent transperineal ultrasound-guided permanent prostate brachytherapy. Methods and Materials: Four hundred twenty-five patients underwent transperineal ultrasound-guided prostate brachytherapy using either 103 Pd or 125 I, for clinical T1b T3a NxM0 (1997 AJCC) adenocarcinoma of the prostate gland, from April 1995 to October No patient underwent pathologic lymph-node staging. One hundred ninety patients were implanted with either 103 Pd or 125 I monotherapy; 235 patients received moderatedose external beam radiation therapy (EBRT), followed by a prostate brachytherapy boost; 163 patients received neoadjuvant hormonal manipulation, in conjunction with either 103 Pd or 125 I monotherapy (77 patients) or in conjunction with moderate-dose EBRT and a prostate brachytherapy boost (86 patients). The median patient age was 68.0 years (range, years). The median follow-up was 31 months (range, months). Follow-up was calculated from the day of implantation. No patient was lost to follow-up. Biochemical disease-free survival was defined by the American Society of Therapeutic Radiation and Oncology (ASTRO) consensus definition. Results: For the entire cohort, the 5-year actuarial biochemical no evidence of disease (bned) survival rate was 94%. For patients with low-, intermediate-, and high-risk disease, the 5-year biochemical disease-free rates were 97.1%, 97.5%, and 84.4%, respectively. For hormone-naive patients, 95.7%, 96.4%, and 79.9% of patients with low-, intermediate-, and high-risk disease were free of biochemical failure. Clinical and treatment parameters predictive of biochemical outcome included: clinical stage, pretreatment prostate-specific antigen (PSA), Gleason score, risk group, age > 65 years, and neoadjuvant hormonal therapy. Isotope choice was not a statistically significant predictor of disease-free survival for any risk group. The median postimplant PSA was < 0.2 for all risk groups, regardless of hormonal status. The mean posttreatment PSA, however, was significantly lower for men implanted with 103 Pd (0.14 ng/ml) than for those implanted with 125 I (0.25 ng/ml), p < Conclusion: With a median follow-up of 31 months, permanent prostate brachytherapy results in a high probability of actuarial 5-year biochemical disease-free survival (DFS) for patients with clinical T1b T3a (1997 AJCC) adenocarcinoma of the prostate gland, with an apparent plateau on the PSA survival curve Elsevier Science Inc. Prostate cancer, Brachytherapy, Biochemical outcome, 103 Pd, 125 I. INTRODUCTION Over the past decade, the field of prostate brachytherapy has experienced an unparalleled resurgence, secondary to several technologic advances, including the evolution of transrectal ultrasonography, the development of a closed transperineal approach, and the widespread availability of sophisticated treatment planning computers. These imaging and planning advances have significantly improved the accuracy of seed placement. In addition, CT-based postoperative dosimetry has provided a unique opportunity to evaluate quality and proactively predict outcome and complications. Reprint requests to: Dr. Gregory S. Merrick, Schiffler Cancer Center, Wheeling Hospital, 1 Medical Park, Wheeling, WV schifonc@wheelinghosp.com Prostate brachytherapy represents the ultimate three-dimensional (3D) conformal therapy, permitting dose escalation far exceeding other modalities. The majority of the prostate brachytherapy literature has reported results as favorable as those of the most positive radical prostatectomy and EBRT series (1 9). These brachytherapy results have been obtained with a variety of planning and intraoperative techniques, of which no method has proven superior. Our planning philosophy and intraoperative techniques have resulted in the delivery of therapeutic doses of radiation to a generous periprostatic margin by the placement of multiple periprostatic seeds (10). Received Jan 4, 2001, and in revised form Mar 21, Accepted for publication Mar 27,
2 42 I. J. Radiation Oncology Biology Physics Volume 51, Number 1, 2001 Table 1. Distribution of patients among various treatment groups Treatment group Brachytherapy as monotherapy External beam brachytherapy boost Overall 125 I mpd (Gy, TG-43)* Pd mpd (Gy, TG-43)* No. of 125 I patients No. of 103 Pd patients No. hormone naive No. neoadjuvant hormones Number overall patients Hormone naive Neoadjuvant hormones No. of 125 I patients No. of 103 Pd patients Number overall patients * mpd is the prescribed minimum peripheral dose in Gy based on AAPM TG-43 dosimetry. In this study, we report 5-year biochemical disease-free outcome using the American Society of Therapeutic Radiation and Oncology (ASTRO) consensus definition (11) for the first 425 men with clinical T1b T3a NxM0 (1997 American Joint Committee on Cancer [AJCC]) adenocarcinoma of the prostate gland undergoing transperineal ultrasoundguided permanent prostate brachytherapy at our institution. METHODS AND MATERIALS A total of 425 patients underwent transperineal ultrasound-guided permanent prostate brachytherapy, using either 103 Pd or 125 I, for clinical T1b T3a NxM0 adenocarcinoma of the prostate gland from April 1995 to October Patients were clinically staged by medical history, physical examination, including digital rectal examination, and serum prostate-specific antigen (PSA). Bone scans, computerized tomography (CT) of the pelvis, and prostatic acid phosphatase (PAP) were obtained as clinically indicated. No patient underwent pathologic lymph-node staging. Clinical stage was assigned according to the 1997 AJCC staging system (12). No patient was lost to follow-up. Because of well-documented inaccuracies in Gleason grading, all cases originating from outside institutions were reviewed before the formulation of a treatment plan (13). Calculation algorithms and seed parameters used in preplans and postoperative dosimetry were those recommended by the American Association of Physicists in Medicine (AAPM) Task Group 43 (TG-43) (14). Our patient selection, preplanning philosophies, intraoperative techniques, and dosimetric evaluation have been described previously (10, 15 22). Specifically, the brachytherapy procedure was performed with preloaded 18-gauge needles using transverse and sagittal ultrasonography along with fluoroscopy. On average, 40% of the seeds were placed in periprostatic locations (10). Table 1 summarizes the distribution of patients among various treatment groups: brachytherapy as monotherapy, brachytherapy boost following moderate-dose external beam radiation therapy (EBRT), and neoadjuvant hormonal manipulation status. EBRT consisted of 45 Gy to the prostate/periprostatic region/seminal vesicles/first echelon lymph nodes, in 1.8-Gy fractions, utilizing 15- to 18-MV photons delivered via a multifield technique, with shielding of the posterior one-half of the rectum via the lateral portals. The prescribed minimum peripheral doses (mpd), based on TG-43 dosimetry for 103 Pd monotherapy, 103 Pd boost, 125 I monotherapy, and 125 I boost, were 115 Gy, 90 Gy, 145 Gy, and 110 Gy, respectively. Those 125 I patients implanted before the implementation of the TG-43 formalism were recalculated. The number of patients implanted as monotherapy and boost were comparable, 190 patients and 235 patients, respectively, but 80% of the monotherapy patients were implanted with 125 I, while nearly 80% of the boost patients were implanted with 103 Pd. The distribution of patients receiving neoadjuvant hormonal manipulation was comparable between mono- and boost therapy and between isotopes. Hormonal manipulation consisted of an LHRH agonist and an antiandrogen. Hormones were prescribed for either urinary obstructive symptomatology, unfavorable geometry, or multiple poor prognosticators. Table 2 is a summary of patient clinical and implant characteristics stratified by risk group. Low-risk disease was defined as clinical T1c/T2a disease, Gleason score 6, and pretreatment PSA 10 ng/ml. Intermediate-risk patients presented with one unfavorable prognostic parameter, and high-risk patients presented with two or more unfavorable prognostic parameters (clinical stage T2b/T3a, PSA 10, Gleason score 7). The median patient age was 68.0 years (range, years). The mean follow-up was months, and the median follow-up was 31 months (range, months). Follow-up was calculated from the day of implantation. Clinical parameters evaluated for biochemical disease-free survival (DFS) included patient age, clinical T stage, Gleason score, and pretreatment PSA. Treatment parameters evaluated included the utilization of neoadjuvant hormonal manipulation, the utilization of moderate-dose EBRT before implantation, and the choice of isotope. Patients were monitored by physical examination, includ-
3 5-year biochemical prostate outcomes G. S. MERRICK et al. 43 Parameter Table 2. Patient clinical and implant characteristics stratified by risk group Overall n 425 Low risk n 160 Intermediate n 165 High risk n 100 Median Mean SD Mean SD Mean SD Mean SD Risk group p value Age at implant (years) Pretreatment prostate-specific antigen (ng/ml) Gleason score Clinical stage T2a T1c T2a T1c T2a T1c T2a T2a T2b Prostate US volume (cm 3 ) Planning target vol. (cm 3 ) PTV/US vol No. of needles used No. of seeds used I activity/seed (mci)* n 204 n 126 n 61 n Pd activity/seed (mci)* n 221 n 34 n 104 n 83 * 125 I activities are expressed in terms of the NIST 99 calibration standard, 1.27 U/mCi. 103 Pd activities are in terms of the manufacturer s calibration standard, U/mCi. The planning target volume (PTV) and the ratio of PTV to ultrasound (US) volume was determined for 304 patients planned on a computer system capable of performing three-dimensional volumetric analyses. ing digital rectal examination and PSA determinations at 3- to 6-month intervals. The endpoint of this analysis was disease-free survival with biochemical no evidence of disease (bned), as defined by the ASTRO consensus definition (11). An abnormal digital rectal examination and/or the development of distant metastases in the absence of PSA progression were also scored as failures. No patient underwent routine postimplant prostate biopsy. Biochemical DFS functions and comparisons of failure rates between patients across risk groups, hormonal therapy status, and the use of EBRT were calculated by Kaplan Meier analysis using SPSS 10.0 software (SPSS, Inc., Chicago, IL). Pearson s correlation coefficient, t tests, and 2 analysis techniques were used to determine the strengths of the relationships between biochemical outcome and clinical/ treatment parameters. Statistical significance was set at p 0.05 for all analyses. patients usually received a lower-dose, lower-seed-strength brachytherapy boost. Eighteen of 425 patients have failed treatment. Five of 18 have failed with distant metastases, including 2 men who died with prostate cancer, and 13 were classified as biochemical failure. To date, no patient has developed a clinically detectable local failure. There have been 12 deaths in the disease-free patient population, 3 of cancer other than prostate, and 9 of non cancer-related causes. Figure 1 displays a Kaplan Meier analysis of all 425 patients with an overall bned rate of 94%. Figure 2 illustrates biochemical outcome when stratified by risk group. RESULTS Table 2 outlines the clinical and treatment parameters and statistical analyses of the evaluated patient population in terms of risk group. Increasing risk was significantly correlated with increasing patient age. All other significant differences based on risk group are an expected consequence of either risk-group selection criteria (clinical stage, Gleason score, PSA) or planning target volume (PTV) definition (number of seeds and needles used). The PTV and the ratio of PTV to ultrasound volume was determined only for the 304 patients planned on a computer system capable of performing 3D volumetric analyses. The isotope activity was inversely related to risk group, because low-risk patients usually received monotherapy with an attendant higher mpd and higher seed strength, whereas high-risk Fig. 1. Kaplan Meier biochemical no evidence of disease (bned) survival curve for the 425 consecutive patients in the study population. Diamonds indicate the follow-up time for the patients in the series.
4 44 I. J. Radiation Oncology Biology Physics Volume 51, Number 1, 2001 Fig. 2. Kaplan Meier biochemical no evidence of disease (bned) survival curves for the three risk groups. Dotted line and open circle markers low risk, n 160. Solid line and plus sign markers intermediate risk, n 165. Dashed line and diamond markers high risk, n 100. Markers indicate the follow-up time for the patients in the series. The difference between risk groups is significant, p Fig. 3. Kaplan Meier biochemical no evidence of disease (bned) survival curves for the three risk groups stratified by isotope implanted. a. Low risk. b. Intermediate risk. c. High risk. The dotted lines and diamond markers are for patients who were hormone naive. The solid lines and plus sign markers are for patients who were implanted with 125 I. The bned differences due to isotope in each risk group are not significant. Although the low- and intermediate-risk curves are virtually superimposed with plateaus at 97.1% and 97.5%, respectively, the disease-free survival (DFS) curve of the high-risk patients, 84.4% at 5 years, is statistically different, p The risk groups were further dissected into isotope status in Fig. 3. There was no statistically significant difference in DFS due to isotope choice in any risk group. When the risk groups were merged, there was also no overall differences in DFS due to isotope choice, p Disease-free survival as a function of risk group and hormonal status is shown in Figs. 4 and 5. For men receiving hormonal therapy, the DFS plateaus for low-, intermediate-, and high-risk groups are at 100%, 100%, and 97.1%, respectively. Of the 34 hormonally treated high-risk patients without evidence of biochemical failure, 29 (85.3%) had completed hormonal therapy, on average, 24.3 months (range, 5 61 months; median, 20 months) before data analysis. For hormone-naive patients, the DFS plateaus are at 95.7%, 96.4%, and 79.9% for low-, intermediate-, and highrisk groups, respectively. Hormonal status was not predictive of failure in any risk group, but in the overall population, patients receiving hormones were significantly less likely to fail, p Kaplan Meier analyses were also run for various Gleason score groups and PSA groups. For patients with Gleason score 2 4, 5 6, 7, and 8 10 histology, 100%, 97.9%, 93.6%, and 91.2% were free of biochemical failure at 5 years. For those patients with pretreatment PSAs of 0 4, 4 10, 10 20, and 20, 100%, 98.6%, 90.3%, and 83.3% were free of biochemical failure at 5 years. Table 3 documents treatment outcome by risk group in terms of follow-up, posttreatment PSA, the duration of hormonal manipulation (where appropriate), and the dosimetric parameters, V 100 and D 90.V 100 is the percentage of the evaluated target volume (prostate plus margin) receiving 100% of the prescribed mpd, using CT-based dosimetry obtained on the day of the implant (Day 0). D 90 is the
5 5-year biochemical prostate outcomes G. S. MERRICK et al. 45 Fig. 5. Kaplan Meier biochemical no evidence of disease (bned) survival curves as a function of neoadjuvant hormone use. The dotted line and open circle markers are for the 262 patients who were hormone naive. The solid line and plus sign markers are for the 163 patients who used neoadjuvant hormones for a median duration of 4 months and a mean of months. The bned difference due to hormone status is significant, p Fig. 4. Kaplan Meier biochemical no evidence of disease (bned) survival curves for the three risk groups stratified by neoadjuvant hormone status. a. Low risk. b. Intermediate risk. c. High risk. The dotted lines and open circles markers are for patients who were hormone naive. The solid lines and plus sign markers are for patients who used neoadjuvant hormones. The bned differences due to hormone status in each risk group are not significant. minimum dose received by 90% of the evaluated target volume. All implants were evaluated by CT-based dosimetry, but the V 100 and D 90 data reported in Table 3 are from 304 patients whose Day 0 dosimetry was performed on a computer system capable of calculating dose volume histograms. The mean V 100 was 93.8% 5.3%, and the mean D 90 was 111% 12% of mpd. Neither showed significant variation across risk groups; the variation between isotopes, although statistically significant for V 100, for 125 I vs for 103 Pd, is not considered to be clinically significant (17, 20). Total months of follow-up from the time of implant was significantly greater as a function of increasing risk group, and, within each risk group, patients implanted with 125 I were followed significantly longer than were those implanted with 103 Pd. For those men receiving neoadjuvant hormones, the total duration of hormone treatment also increased significantly with increasing risk group. The mean posttreatment PSAs decreased significantly with increasing risk group. This decrease across risk groups was significant not only overall, but also for patients stratified into hormone-naive and hormone-treated groups, but not when stratified by isotope used. Patients who received neoadjuvant hormones had significantly lower PSAs than their hormonenaive counterparts in each risk group cohort, and also when the risk groups were merged: ng/ml for hormone patients vs ng/ml for hormone-naive men, p Although insufficient time has elapsed for all patients to have reached their PSA nadir, the mean posttreatment PSA was significantly lower for men implanted with 103 Pd ( ng/ml) than for those with 125 I ( ng/ml), p The mean follow-up for the 103 Pd patients was about 6.8 months less than that of the 125 I patients. Although the mean PSAs in each risk group were consistently lower for 103 Pd patients, the isotope difference was only significant in the low-risk group. The influence of hormonal manipulation status is further delineated in Table 4 in terms of median PSAs. The median
6 46 I. J. Radiation Oncology Biology Physics Volume 51, Number 1, 2001 Table 3. Comparison of dosimetric and outcome parameters in terms of isotope and risk group Parameter Isotope or hormone status Low risk Intermediate risk High risk Overall n Mean SD n Mean SD n Mean SD n Mean SD Risk group p value V 100 (% vol) overall I Pd (Isotope p value) (0.661) (0.031) (0.400) (0.016) D 90 (% mpd) overall I Pd (Isotope p value) (0.723) (0.415) (0.615) (0.718) Last PSA (bned patients) overall I Pd (Isotope p value) (0.011) (0.084) (0.152) ( 0.001) Last PSA (bned patients) hormone naive neoadj. hormones (Hormone p value) (0.015) (0.024) (0.007) ( 0.001) Months follow-up overall I Pd (Isotope p value) ( 0.001) ( 0.001) (0.077) ( 0.001) Months hormones overall Abbreviations: SD standard deviation; PSA prostate-specific antigen; bned biochemical no evidence of disease. posttreatment PSAs for patients with low-, intermediate-, and high-risk disease were 0.2, 0.1, and 0.1, respectively. The median postimplant PSA was 0.2 for all risk groups, regardless of hormonal status. Overall, with a median follow-up of 31 months, 97.1% of patients had a posttreatment PSA 1.0 and 89.2% had a PSA 0.5. A slightly larger percentage of patients receiving neoadjuvant hormones achieved PSAs 1.0 ng/ml, when compared with those without hormonal therapy (98.8% vs. 95.9%) and 0.5 ng/ml (94.4% vs. 85.7%). The clinical and treatment parameters in Table 5 were evaluated in t tests to determine their impact on outcome. The same factors in Table 2 that classified patients into risk groups clinical stage, Gleason score, and pretreatment PSA were likewise predictors of failure. Using a cutoff point of 65 years produced a significant difference in DFS status, p 0.010, indicating that patients older than 65 years are at significantly greater risk of failure. Other factors that differed by risk group, such as planning volume and number of seeds and needles, were not predictive of failure. The dosimetric quality variables V 100 and D 90, which did not differ significantly by risk group, were not predictive of failure in this population. DISCUSSION Prostate brachytherapy represents the ultimate 3D conformal therapy, permitting dose escalation far exceeding other radiation modalities. In addition, the periprostatic region can be much more aggressively treated with brachytherapy than with radical prostatectomy because of the ability to place periprostatic seeds safely and accurately (10). The majority of the prostate brachytherapy literature has reported results as favorable as those of the most pos- Table 4. Postimplant prostate-specific antigen (PSA) in disease-free men stratified by hormonal manipulation status and risk group Parameter Hormonal status Low risk n 157 Intermediate risk n 162 High risk n 88 Overall n 407 Median last postimplant PSA overall no 0.2 (n 89) 0.1 (n 102) 0.1 (n 54) 0.1 (n 245) yes 0.1 (n 68) 0.1 (n 60) 0.1 (n 34) 0.1 (n 162) Percent of men with last PSA 1.0 overall no yes Percent of men with last PSA 0.5 overall no yes Median months duration of hormones yes
7 5-year biochemical prostate outcomes G. S. MERRICK et al. 47 Table 5. Clinical and treatment parameters tested for effect on outcome in univariate analysis Significant parameters p value Parameters not significant p value Clinical stage Ultrasound volume Pretreatment PSA Planning volume Gleason score No. of needles used Risk group No. of seeds implanted Age at implant 65 years V External beam therapy D Neoadjuvant hormonal therapy PSA prostate-specific antigen. itive radical prostatectomy and EBRT series (1 9). Our results compare favorably with those reports. For patients undergoing palladium monotherapy, Blasko and colleagues (2) reported 5-year biochemical control rates of 94%, 82%, and 65% for patients with low-, intermediate-, and high-risk disease. In our series, patients undergoing prostate brachytherapy with or without EBRT, who did not receive hormonal manipulation and were classified as low-, intermediate-, and high-risk disease, had actuarial biochemical disease-free rates at 5 years of 95.7%, 96.4%, and 79.9%, respectively. For patients managed with dose-escalated 3D conformal EBRT, Zelefsky et al. (23) reported 4-year biochemical disease-free survival rates of approximately 95%, 80%, and 55% for low-, intermediate-, and high-risk patients who received Gy. Critz and colleagues (4) managed all patients with 125 I brachytherapy followed by supplemental radiation therapy, with a 5-year disease-free survival rate of 88%. For patients with Gleason score 2 4, 5 6, 7, and 8 10 histology, 93%, 89%, 82%, and 67% were free of biochemical failure at 5 years. Our results for these Gleason score groups were 100%, 97.9%, 93.6%, and 91.2% at 5 years. For those patients with pretreatment PSAs of 0 4, 4 10, 10 20, and 20, Critz et al. reported that 94%, 93%, 75%, and 69% were free of biochemical failure at 5 years. Our results for these PSA groups were 100%, 98.6%, 90.3%, and 83.3% freedom from biochemical failure at 5 years. Our 5-year biochemical disease-free survival compares favorably with previous reports (1 8). In addition, our absolute PSA values also compare favorably with a previous report (2). Blasko and colleagues (2) reported a median PSA of 0.3 for patients with a median follow-up of 41.5 months, and 82% of those patients had a PSA 1.0 and 68% had a PSA 0.5. In the Seattle experience, posttreatment PSAs continued to decline for 48 months following brachytherapy. In our study population, 95.9% of men not receiving hormonal manipulation had a posttreatment PSA 1.0, and 85.7% had a posttreatment PSA 0.5, with a median follow-up of 31 months. It is probable that our mean and median PSA values will continue to decline for at least another year. In multivariate analysis, age greater than 65 years predicted for biochemical failure. Although not all studies have reported age to be a predictor of recurrence (24), age has been reported to be of prognostic significance in both the radical prostatectomy (25, 26) and EBRT (27) literature. Smith and colleagues (26) recently reported a statistically significant reduction in biochemical failures for men 59 years of age or younger undergoing prostatectomy. The mean follow-up of those young patients (32 months) is comparable to our mean follow-up of months and our median follow-up of 31 months. We are especially encouraged by the 79.9% 5-year freedom from biochemical failure rate (median posttreatment PSA, 0.1) for the 65 hormone-naive high-risk patients. Although some patients with high Gleason scores may have subclinical distant metastatic disease at diagnosis, a recent analysis of Radiation Therapy Oncology Group (RTOG) trials reported improved survival for patients with high Gleason scores who received higher doses of EBRT (28). This finding does not support the hypothesis that the majority of high Gleason score patients have subclinical distant metastases at diagnosis, and it supports an aggressive locoregional approach. We believe that our favorable results for all risk groups may be a result of delivering therapeutic doses of radiation to a generous periprostatic margin by the placement of multiple periprostatic seeds (10). Stock and colleagues (29) have shown a dose response for biochemical failures based on Day 30 D 90 dosimetry of 125 I implants exceeding 97% mpd. In our study population, the mean dosimetric quality variables V 100 and D 90 were 93.8% 5.3% volume and 111% 12% mpd, respectively, on Day 0, and were not predictive of failure. The values of these implant parameters are expected to increase with time as operative edema resolves (30). Because the mean D 90 of the failures was 115% 28% of mpd, we believe that the number of inadequate implants is too small in the relatively small failure group for statistical analysis to detect a dose response. Our 5-year actuarial biochemical results add additional support to the literature (1 9) regarding the effectiveness of permanent prostate brachytherapy for patients with carcinoma of the prostate gland. Additional follow-up of our patient cohort will be mandatory to confirm the durability of these results. CONCLUSION With a median follow-up of 31 months, permanent prostate brachytherapy results in a high probability of actuarial 5-year biochemical disease-free survival, with an apparent plateau on the freedom-from-psa failure curve.
8 48 I. J. Radiation Oncology Biology Physics Volume 51, Number 1, 2001 REFERENCES 1. Merrick GS, Butler WM, Lief JH, et al. Is brachytherapy comparable with radical prostatectomy and external beam radiation for clinically localized prostate cancer? Tech Urol 2001;7: Blasko JC, Grimm PD, Sylvester JE, et al. Palladium-103 brachytherapy for prostate carcinoma. Int J Radiat Oncol Biol Phys 2000;46: Ragde H, Korb LJ, Elgamal AA, et al. Modern prostate brachytherapy: Prostate specific antigen results in 219 patients with up to 12 years of observed follow-up. Cancer 2000;89: Critz FA, Williams WH, Levinson AK, et al. Simultaneous irradiation for prostate cancer: Intermediate results with modern techniques. J Urol 2000;164: Blasko JC, Wallner K, Grimm PD, et al. Prostate specific antigen based disease control following ultrasound guided 125iodine implantation for stage T1/T2 prostatic carcinoma. J Urol 1995;154: Dattoli M, Wallner K, True L, et al. Prognostic role of serum prostatic acid phosphatase for 103Pd-based radiation for prostatic carcinoma. Int J Radiat Oncol Biol Phys 1999;45: Zelefsky MJ, Hollister T, Raben A, et al. Five-year biochemical outcome, and toxicity with transperineal CT-planned permanent I-125 prostate implantation for patients with localized prostate cancer. Int J Radiat Oncol Biol Phys 2000;47: Potters L, Cha C, Oshinsky G, et al. Risk profiles to predict PSA relapse-free survival for patients undergoing permanent prostate brachytherapy. Cancer J Sci Am 1999;5: Merrick GS, Butler WM, Lief JH, et al. Five-year biochemical outcome after prostate brachytherapy for hormone-naive men 62 years of age. Int J Radiat Oncol Biol Phys In press. 10. Merrick GS, Butler WM, Dorsey AT, et al. Seed fixity in the prostate/periprostatic region following brachytherapy. Int J Radiat Oncol Biol Phys 2000;46: American Society for Therapeutic Radiology, and Oncology Consensus Panel. Consensus Statement: Guidelines for PSA following radiation therapy. Int J Radiat Oncol Biol Phys 1997;37: Fleming ID, Cooper JS, Henson DE, et al. AJCC cancer staging handbook. 5th ed. Philadelphia: Lippincott-Raven Press; Merrick GS, Butler WM, Farthing WH, et al. The impact of Gleason score accuracy as a criterion for prostate brachytherapy patient selection. J Brachytherapy Int 1998;14: Nath R, Anderson LL, Luxton G, et al. Dosimetry of interstitial brachytherapy sources: Recommendations of the AAPM Radiation Therapy CommitteeTask Group No. 43. Med Phys 1995;22: Butler WM, Merrick GS, Lief JH, et al. Comparison of seed loading approaches in prostate brachytherapy. Med Phys 2000;27: Merrick GS, Butler WM. Modified uniform seed loading for prostate brachytherapy: Rationale, design, and evaluation. 2000;6: Merrick GS, Butler WM, Dorsey AT, et al. Potential role of various dosimetric quality indicators in prostate brachytherapy. Int J Radiat Oncol Biol Phys 1999;44: Merrick GS, Butler WM, Dorsey AT, et al. Rectal dosimetric analysis following prostatic conformal brachytherapy. Int J Radiat Oncol Biol Phys 1999;43: Merrick GS, Butler WM, Dorsey AT, et al. The influence of timing on the dosimetric analysis of transperineal ultrasound guided prostatic conformal brachytherapy. Rad Oncol Invest 1998;6: Merrick GS, Butler WM, Dorsey AT, et al. The effect of prostate size and isotope selection on dosimetric quality following permanent seed implantation. Tech Urol In press. 21. Merrick GS, Butler WM, Dorsey AT, et al. The influence of prophylactic dexamethasone on edema following prostate brachytherapy. Tech Urol 2000;6: Butler WM, Dorsey AT, Nelson KR, et al. Quality assurance calibration of I-125 rapid strand in a sterile environment. Int J Radiat Oncol Biol Phys 1998;41: Zelefsky MJ, Leibel SA, Gaudin PB, et al. Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys 1998;41: Catalona WJ, Smith D. Five-year tumor recurrence rates after anatomical radical retropubic prostatectomy for prostate cancer. J Urol 1998;160: Carter HB, Epstein JI, and Partin AW. Influence of age and prostate-specific antigen on the chance of curable prostate cancer among men with nonpalpable disease. Urol 1999;53: Smith CV, Bauer JJ, Connelly RR, et al. Prostate cancer in men age 50 years or younger: A review of the department of defense center for prostate disease research multi-center prostate cancer database. J Urol 2000;164: Herold DM, Hanlon AL, Movsas B, et al. Age related prostate cancer metastases. Urology 1998;51: Valicenti R, Lu J, Pilepich M, et al. Survival advantage from higher-dose radiation therapy for clinically localized prostate cancer treated in the Radiation Therapy Oncology Group trials. J Clin Oncol 2000;18: Stock RG, Stone NN, Tabert A, et al. A dose response study for I-125 prostate implants. Int J Radiat Oncol Biol Phys 1998;41: Waterman FM, Yue N, Corn BW, et al. Edema associated with I-125 or Pd-103 prostate brachytherapy and its impact on post-implant dosimetry: An analysis based on serial CT acquisition. Int J Radiat Oncol Biol Phys 1999;41:
NATURAL HISTORY OF CLINICALLY STAGED LOW- AND INTERMEDIATE-RISK PROSTATE CANCER TREATED WITH MONOTHERAPEUTIC PERMANENT INTERSTITIAL BRACHYTHERAPY
 doi:1.116/j.ijrobp.9..1 Int. J. Radiation Oncology Biol. Phys., Vol. 76, No., pp. 349 354, 1 Copyright Ó 1 Elsevier Inc. Printed in the USA. All rights reserved 36-316/1/$ see front matter CLINICAL INVESTIGATION
doi:1.116/j.ijrobp.9..1 Int. J. Radiation Oncology Biol. Phys., Vol. 76, No., pp. 349 354, 1 Copyright Ó 1 Elsevier Inc. Printed in the USA. All rights reserved 36-316/1/$ see front matter CLINICAL INVESTIGATION
doi:10.1016/j.ijrobp.2006.07.1382 CLINICAL INVESTIGATION
 doi:10.1016/j.ijrobp.2006.07.1382 Int. J. Radiation Oncology Biol. Phys., Vol. 67, No. 1, pp. 57 64, 2007 Copyright 2007 Elsevier Inc. Printed in the USA. All rights reserved 0360-3016/07/$ see front matter
doi:10.1016/j.ijrobp.2006.07.1382 Int. J. Radiation Oncology Biol. Phys., Vol. 67, No. 1, pp. 57 64, 2007 Copyright 2007 Elsevier Inc. Printed in the USA. All rights reserved 0360-3016/07/$ see front matter
The optimal treatment for clinically localized
 REVIEW COMBINING EXTERNAL BEAM RADIOTHERAPY WITH PROSTATE BRACHYTHERAPY: ISSUES AND RATIONALE CLARISSA FEBLES AND RICHARD K. VALICENTI The optimal treatment for clinically localized prostate cancer remains
REVIEW COMBINING EXTERNAL BEAM RADIOTHERAPY WITH PROSTATE BRACHYTHERAPY: ISSUES AND RATIONALE CLARISSA FEBLES AND RICHARD K. VALICENTI The optimal treatment for clinically localized prostate cancer remains
How To Know If You Should Get A Brachytherapy Or Radioactive Seed Implantation
 MEDICAL POLICY SUBJECT: BRACHYTHERAPY OR PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
MEDICAL POLICY SUBJECT: BRACHYTHERAPY OR PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
Implementation Date: April 2015 Clinical Operations
 National Imaging Associates, Inc. Clinical guideline PROSTATE CANCER Original Date: March 2011 Page 1 of 5 Radiation Oncology Last Review Date: March 2015 Guideline Number: NIA_CG_124 Last Revised Date:
National Imaging Associates, Inc. Clinical guideline PROSTATE CANCER Original Date: March 2011 Page 1 of 5 Radiation Oncology Last Review Date: March 2015 Guideline Number: NIA_CG_124 Last Revised Date:
Patient Selection for Prostate Brachytherapy
 Patient Selection for Prostate Brachytherapy a report by Gregory S Merrick, MD 1, Wayne M Butler, PhD 1 and K ent E Wallner, MD 2 1. Schiffler Cancer Center and Wheeling Jesuit University, 2. Puget Sound
Patient Selection for Prostate Brachytherapy a report by Gregory S Merrick, MD 1, Wayne M Butler, PhD 1 and K ent E Wallner, MD 2 1. Schiffler Cancer Center and Wheeling Jesuit University, 2. Puget Sound
PROTON THERAPY FOR PROSTATE CANCER: THE INITIAL LOMA LINDA UNIVERSITY EXPERIENCE
 doi:10.1016/j.ijrobp.2003.10.011 Int. J. Radiation Oncology Biol. Phys., Vol. 59, No. 2, pp. 348 352, 2004 Copyright 2004 Elsevier Inc. Printed in the USA. All rights reserved 0360-3016/04/$ see front
doi:10.1016/j.ijrobp.2003.10.011 Int. J. Radiation Oncology Biol. Phys., Vol. 59, No. 2, pp. 348 352, 2004 Copyright 2004 Elsevier Inc. Printed in the USA. All rights reserved 0360-3016/04/$ see front
LATE MORBIDITY PROFILES IN PROSTATE CANCER PATIENTS TREATED TO 79 84 GY BY A SIMPLE FOUR-FIELD COPLANAR BEAM ARRANGEMENT
 PII S0360-3016(02)03822-1 Int. J. Radiation Oncology Biol. Phys., Vol. 55, No. 1, pp. 71 77, 2003 Copyright 2003 Elsevier Science Inc. Printed in the USA. All rights reserved 0360-3016/03/$ see front matter
PII S0360-3016(02)03822-1 Int. J. Radiation Oncology Biol. Phys., Vol. 55, No. 1, pp. 71 77, 2003 Copyright 2003 Elsevier Science Inc. Printed in the USA. All rights reserved 0360-3016/03/$ see front matter
PROSTATE CANCER WITH LARGE GLANDS TREATED WITH 3- DIMENSIONAL COMPUTERIZED TOMOGRAPHY GUIDED PARARECTAL BRACHYTHERAPY: UP TO 8 YEARS OF FOLLOWUP
 0022-5347/03/1694-1331/0 Vol. 169, 1331 1336, April 2003 THE JOURNAL OF UROLOGY Printed in U.S.A. Copyright 2003 by AMERICAN UROLOGICAL ASSOCIATION DOI: 10.1097/01.ju.0000055773.91290.e8 PROSTATE CANCER
0022-5347/03/1694-1331/0 Vol. 169, 1331 1336, April 2003 THE JOURNAL OF UROLOGY Printed in U.S.A. Copyright 2003 by AMERICAN UROLOGICAL ASSOCIATION DOI: 10.1097/01.ju.0000055773.91290.e8 PROSTATE CANCER
JAMA. 1998;280:969-974
 Original Contributions Biochemical Outcome After Radical Prostatectomy, Radiation Therapy, or Interstitial for Clinically Localized Prostate Cancer Anthony V. D Amico, MD, PhD; Richard Whittington, MD;
Original Contributions Biochemical Outcome After Radical Prostatectomy, Radiation Therapy, or Interstitial for Clinically Localized Prostate Cancer Anthony V. D Amico, MD, PhD; Richard Whittington, MD;
The Evolving Role of Prostate Brachytherapy
 Brachytherapy is rapidly becoming a preferred choice by patients for the management of their localized prostate cancer. Faith Krucina. Power of Sound. 36 24. Acrylic on canvas, Courtesy of Artistic Images
Brachytherapy is rapidly becoming a preferred choice by patients for the management of their localized prostate cancer. Faith Krucina. Power of Sound. 36 24. Acrylic on canvas, Courtesy of Artistic Images
7. Prostate cancer in PSA relapse
 7. Prostate cancer in PSA relapse A patient with prostate cancer in PSA relapse is one who, having received a primary treatment with intent to cure, has a raised PSA (prostate-specific antigen) level defined
7. Prostate cancer in PSA relapse A patient with prostate cancer in PSA relapse is one who, having received a primary treatment with intent to cure, has a raised PSA (prostate-specific antigen) level defined
Medical Policy Brachytherapy for Clinically Localized Prostate Cancer Using Permanently Implanted Seeds
 Medical Policy Brachytherapy for Clinically Localized Prostate Cancer Using Permanently Implanted Seeds Table of Contents Policy: Commercial Coding Information Information Pertaining to All Policies Policy:
Medical Policy Brachytherapy for Clinically Localized Prostate Cancer Using Permanently Implanted Seeds Table of Contents Policy: Commercial Coding Information Information Pertaining to All Policies Policy:
These rare variants often act aggressively and may respond differently to therapy than the more common prostate adenocarcinoma.
 Prostate Cancer OVERVIEW Prostate cancer is the second most common cancer diagnosed among American men, accounting for nearly 200,000 new cancer cases in the United States each year. Greater than 65% of
Prostate Cancer OVERVIEW Prostate cancer is the second most common cancer diagnosed among American men, accounting for nearly 200,000 new cancer cases in the United States each year. Greater than 65% of
Long-term outcomes and prognostic factors in patients treated with intraoperatively planned prostate brachytherapy
 Brachytherapy - (2012) - Long-term outcomes and prognostic factors in patients treated with intraoperatively planned prostate brachytherapy Carlos Vargas 1, *, Douglas Swartz 2, Apoorva Vashi 2, Mark Blasser
Brachytherapy - (2012) - Long-term outcomes and prognostic factors in patients treated with intraoperatively planned prostate brachytherapy Carlos Vargas 1, *, Douglas Swartz 2, Apoorva Vashi 2, Mark Blasser
American College Of Radiology ACR Appropriateness Criteria PERMANENT SOURCE BRACHYTHERAPY FOR PROSTATE CANCER
 American College Of Radiology ACR Appropriateness Criteria PERMANENT SOURCE BRACHYTHERAPY FOR PROSTATE CANCER Expert Panel on Radiation Oncology Prostate Work Group: Louis Potters, MD 1 ; Carlos A. Perez,
American College Of Radiology ACR Appropriateness Criteria PERMANENT SOURCE BRACHYTHERAPY FOR PROSTATE CANCER Expert Panel on Radiation Oncology Prostate Work Group: Louis Potters, MD 1 ; Carlos A. Perez,
Protons vs. CyberKnife. Protons vs. CyberKnife. Page 1 UC SF. What are. Alexander R. Gottschalk, M.D., Ph.D.
 Protons vs. CyberKnife UC SF Protons vs. CyberKnife UC SF Alexander R. Gottschalk, M.D., Ph.D. Associate Professor and Director of the CyberKnife Radiosurgery Program Department of Radiation Oncology University
Protons vs. CyberKnife UC SF Protons vs. CyberKnife UC SF Alexander R. Gottschalk, M.D., Ph.D. Associate Professor and Director of the CyberKnife Radiosurgery Program Department of Radiation Oncology University
Prostatectomy, pelvic lymphadenect. Med age 63 years Mean followup 53 months No other cancer related therapy before recurrence. Negative.
 Adjuvante und Salvage Radiotherapie Ludwig Plasswilm Klinik für Radio-Onkologie, KSSG CANCER CONTROL WITH RADICAL PROSTATECTOMY ALONE IN 1,000 CONSECUTIVE PATIENTS 1983 1998 Clinical stage T1 and T2 Mean
Adjuvante und Salvage Radiotherapie Ludwig Plasswilm Klinik für Radio-Onkologie, KSSG CANCER CONTROL WITH RADICAL PROSTATECTOMY ALONE IN 1,000 CONSECUTIVE PATIENTS 1983 1998 Clinical stage T1 and T2 Mean
Detection and staging of recurrent prostate cancer is still one of the important clinical problems in prostate cancer. A rise in PSA or biochemical
 Summary. 111 Detection and staging of recurrent prostate cancer is still one of the important clinical problems in prostate cancer. A rise in PSA or biochemical recurrence (BCR) is the first sign of recurrent
Summary. 111 Detection and staging of recurrent prostate cancer is still one of the important clinical problems in prostate cancer. A rise in PSA or biochemical recurrence (BCR) is the first sign of recurrent
CYBERKNIFE RADIOSURGERY FOR EARLY PROSTATE CANCER Rationale and Results. Alan Katz MD JD Flushing, NY USA
 CYBERKNIFE RADIOSURGERY FOR EARLY PROSTATE CANCER Rationale and Results Alan Katz MD JD Flushing, NY USA Prostate Ablative Therapy Over the last 10 years our therapy has improved bned rates for LDR/HDR
CYBERKNIFE RADIOSURGERY FOR EARLY PROSTATE CANCER Rationale and Results Alan Katz MD JD Flushing, NY USA Prostate Ablative Therapy Over the last 10 years our therapy has improved bned rates for LDR/HDR
LONG-TERM URINARY TOXICITY AFTER 3-DIMENSIONAL CONFORMAL RADIOTHERAPY FOR PROSTATE CANCER IN PATIENTS WITH PRIOR HISTORY OF TRANSURETHRAL RESECTION
 PII S0360-3016(00)00714-8 Int. J. Radiation Oncology Biol. Phys., Vol. 48, No. 3, pp. 643 647, 2000 Copyright 2000 Elsevier Science Inc. Printed in the USA. All rights reserved 0360-3016/00/$ see front
PII S0360-3016(00)00714-8 Int. J. Radiation Oncology Biol. Phys., Vol. 48, No. 3, pp. 643 647, 2000 Copyright 2000 Elsevier Science Inc. Printed in the USA. All rights reserved 0360-3016/00/$ see front
PSA After Radiation for Prostate Cancer
 Review Article [1] May 01, 2004 By Deborah A. Kuban, MD [2], Howard D. Thames, PhD [3], and Larry B. Levy, MS [4] The introduction of prostate-specific antigen (PSA) as a reliable tumor marker for prostate
Review Article [1] May 01, 2004 By Deborah A. Kuban, MD [2], Howard D. Thames, PhD [3], and Larry B. Levy, MS [4] The introduction of prostate-specific antigen (PSA) as a reliable tumor marker for prostate
馬 偕 紀 念 醫 院 新 竹 分 院 前 列 腺 癌 放 射 治 療 指 引
 馬 偕 紀 念 醫 院 新 竹 分 院 前 列 腺 癌 放 射 治 療 指 引 2009.12.02 修 訂 2013.05.13 四 版 前 言 新 竹 馬 偕 醫 院 放 射 腫 瘤 科 藉 由 跨 院 聯 合 會 議 機 制 進 行 討 論, 以 制 定 符 合 現 狀 之 前 列 腺 癌 放 射 治 療 指 引 本 院 前 列 腺 癌 放 射 治 療 指 引 的 建 立, 係 參 考 國 內
馬 偕 紀 念 醫 院 新 竹 分 院 前 列 腺 癌 放 射 治 療 指 引 2009.12.02 修 訂 2013.05.13 四 版 前 言 新 竹 馬 偕 醫 院 放 射 腫 瘤 科 藉 由 跨 院 聯 合 會 議 機 制 進 行 討 論, 以 制 定 符 合 現 狀 之 前 列 腺 癌 放 射 治 療 指 引 本 院 前 列 腺 癌 放 射 治 療 指 引 的 建 立, 係 參 考 國 內
Historical Basis for Concern
 Androgens After : Are We Ready? Mohit Khera, MD, MBA Assistant Professor of Urology Division of Male Reproductive Medicine and Surgery Scott Department of Urology Baylor College of Medicine Historical
Androgens After : Are We Ready? Mohit Khera, MD, MBA Assistant Professor of Urology Division of Male Reproductive Medicine and Surgery Scott Department of Urology Baylor College of Medicine Historical
External-Beam Radiotherapy in the Management of Carcinoma of the Prostate
 Technological advances in external-beam radiation therapy and the developments in equipment and computers allow for safer delivery of higher radiation doses to the prostate. Tina Sotis. The Tropic of Capricorn
Technological advances in external-beam radiation therapy and the developments in equipment and computers allow for safer delivery of higher radiation doses to the prostate. Tina Sotis. The Tropic of Capricorn
Oncology Annual Report: Prostate Cancer 2005 Update By: John Konefal, MD, Radiation Oncology
 Oncology Annual Report: Prostate Cancer 25 Update By: John Konefal, MD, Radiation Oncology Prostate cancer is the most common cancer in men, with 232,9 new cases projected to be diagnosed in the U.S. in
Oncology Annual Report: Prostate Cancer 25 Update By: John Konefal, MD, Radiation Oncology Prostate cancer is the most common cancer in men, with 232,9 new cases projected to be diagnosed in the U.S. in
Brachytherapy for Prostate Cancer
 Urol Int 1999;63:87 91 Manfred P. Wirth Oliver W. Hakenberg Klinik und Poliklinik für Urologie, Universitätsklinikum Carl-Gustav-Carus der Technischen Universität Dresden, Deutschland Brachytherapy for
Urol Int 1999;63:87 91 Manfred P. Wirth Oliver W. Hakenberg Klinik und Poliklinik für Urologie, Universitätsklinikum Carl-Gustav-Carus der Technischen Universität Dresden, Deutschland Brachytherapy for
Saturation Biopsy for Diagnosis and Staging of Prostate Cancer. Original Policy Date
 MP 7.01.101 Saturation Biopsy for Diagnosis and Staging of Prostate Cancer Medical Policy Section Surgery Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date /12/2013 Return to Medical Policy
MP 7.01.101 Saturation Biopsy for Diagnosis and Staging of Prostate Cancer Medical Policy Section Surgery Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date /12/2013 Return to Medical Policy
PROSTATE CANCER. Get the facts, know your options. Samay Jain, MD, Assistant Professor,The University of Toledo Chief, Division of Urologic Oncology
 PROSTATE CANCER Get the facts, know your options Samay Jain, MD, Assistant Professor,The University of Toledo Chief, Division of Urologic Oncology i What is the Prostate? Unfortunately, you have prostate
PROSTATE CANCER Get the facts, know your options Samay Jain, MD, Assistant Professor,The University of Toledo Chief, Division of Urologic Oncology i What is the Prostate? Unfortunately, you have prostate
Brachytherapy for prostate cancer
 Brachytherapy for prostate cancer Findings by SBU Alert Published Jun 7, 2000 Version 1 Brachytherapy is not widely used in Sweden to treat localized prostate cancer. This treatment method has been available
Brachytherapy for prostate cancer Findings by SBU Alert Published Jun 7, 2000 Version 1 Brachytherapy is not widely used in Sweden to treat localized prostate cancer. This treatment method has been available
Prostate Cancer Screening in Taiwan: a must
 Prostate Cancer Screening in Taiwan: a must 吳 俊 德 基 隆 長 庚 醫 院 台 灣 醫 學 會 105 th What is the PSA test? The blood level of PSA is often elevated in men with prostate cancer, and the PSA test was originally
Prostate Cancer Screening in Taiwan: a must 吳 俊 德 基 隆 長 庚 醫 院 台 灣 醫 學 會 105 th What is the PSA test? The blood level of PSA is often elevated in men with prostate cancer, and the PSA test was originally
Prostate Cancer What Are the Outcomes of Radical Prostatectomy for High-risk Prostate Cancer?
 Prostate Cancer What Are the Outcomes of Radical Prostatectomy for High-risk Prostate Cancer? Stacy Loeb, Edward M. Schaeffer, Bruce J. Trock, Jonathan I. Epstein, Elizabeth B. Humphreys, and Patrick C.
Prostate Cancer What Are the Outcomes of Radical Prostatectomy for High-risk Prostate Cancer? Stacy Loeb, Edward M. Schaeffer, Bruce J. Trock, Jonathan I. Epstein, Elizabeth B. Humphreys, and Patrick C.
A new score predicting the survival of patients with spinal cord compression from myeloma
 A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; sarah_douglas@gmx.de (2) Steven
A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; sarah_douglas@gmx.de (2) Steven
Proton Therapy for Prostate Cancer
 Proton Therapy for Prostate Cancer Andrew K. Lee, MD, MPH Director, Proton Therapy Center Associate Professor Department of Radiation Oncology M.D. Anderson Cancer Center Randomized studies showing benefit
Proton Therapy for Prostate Cancer Andrew K. Lee, MD, MPH Director, Proton Therapy Center Associate Professor Department of Radiation Oncology M.D. Anderson Cancer Center Randomized studies showing benefit
Corporate Medical Policy Intensity-Modulated Radiation Therapy (IMRT) of the Prostate
 Corporate Medical Policy Intensity-Modulated Radiation Therapy (IMRT) of the Prostate File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intensity_modulated_radiation_therapy_imrt_of_the_prostate
Corporate Medical Policy Intensity-Modulated Radiation Therapy (IMRT) of the Prostate File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intensity_modulated_radiation_therapy_imrt_of_the_prostate
PCa Commentary. Volume 73 January-February 2012 PSA AND TREATMENT DECISIONS:
 1101 Madison Street Suite 1101 Seattle, WA 98104 P 206-215-2480 www.seattleprostate.com PCa Commentary Volume 73 January-February 2012 CONTENTS PSA SCREENING & BASIC SCIENCE PSA AND TREATMENT 1 DECISIONS
1101 Madison Street Suite 1101 Seattle, WA 98104 P 206-215-2480 www.seattleprostate.com PCa Commentary Volume 73 January-February 2012 CONTENTS PSA SCREENING & BASIC SCIENCE PSA AND TREATMENT 1 DECISIONS
Clinical Trials and Radiation Treatment. Gerard Morton Odette Cancer Centre Sunnybrook Research Institute University of Toronto
 Clinical Trials and Radiation Treatment Gerard Morton Odette Cancer Centre Sunnybrook Research Institute University of Toronto What I will cover.. A little about radiation treatment The clinical trials
Clinical Trials and Radiation Treatment Gerard Morton Odette Cancer Centre Sunnybrook Research Institute University of Toronto What I will cover.. A little about radiation treatment The clinical trials
A918: Prostate: adenocarcinoma
 A918: Prostate: adenocarcinoma General facts of prostate cancer The prostate is about the size of a walnut. It is just below the bladder and in front of the rectum. The tube that carries urine (the urethra)
A918: Prostate: adenocarcinoma General facts of prostate cancer The prostate is about the size of a walnut. It is just below the bladder and in front of the rectum. The tube that carries urine (the urethra)
Prostate Cancer. Patient Information
 Prostate Cancer Patient Information 1 The Prostate & Prostate Cancer The prostate is a small gland in the male reproductive system, approximately the size and shape of a walnut. It is located directly
Prostate Cancer Patient Information 1 The Prostate & Prostate Cancer The prostate is a small gland in the male reproductive system, approximately the size and shape of a walnut. It is located directly
Prostate cancer is the most common cause of death from cancer in men over age 75. Prostate cancer is rarely found in men younger than 40.
 A.D.A.M. Medical Encyclopedia. Prostate cancer Cancer - prostate; Biopsy - prostate; Prostate biopsy; Gleason score Last reviewed: October 2, 2013. Prostate cancer is cancer that starts in the prostate
A.D.A.M. Medical Encyclopedia. Prostate cancer Cancer - prostate; Biopsy - prostate; Prostate biopsy; Gleason score Last reviewed: October 2, 2013. Prostate cancer is cancer that starts in the prostate
The 4Kscore blood test for risk of aggressive prostate cancer
 The 4Kscore blood test for risk of aggressive prostate cancer Prostate cancer tests When to use the 4Kscore Test? Screening Prior to 1 st biopsy Prior to negative previous biopsy Prognosis in Gleason 6
The 4Kscore blood test for risk of aggressive prostate cancer Prostate cancer tests When to use the 4Kscore Test? Screening Prior to 1 st biopsy Prior to negative previous biopsy Prognosis in Gleason 6
Prostate IMRT: Promises and Problems Chandra Burman, Ph.D. Memorial Sloan-Kettering Cancer Center, New York, NY 10021
 Prostate IMRT: Promises and Problems Chandra Burman, Ph.D. Memorial Sloan-Kettering Cancer Center, New York, NY 10021 Introduction Prostate is one of the treatment sites that is well suited for IMRT. For
Prostate IMRT: Promises and Problems Chandra Burman, Ph.D. Memorial Sloan-Kettering Cancer Center, New York, NY 10021 Introduction Prostate is one of the treatment sites that is well suited for IMRT. For
DIAGNOSIS OF PROSTATE CANCER
 DIAGNOSIS OF PROSTATE CANCER Determining the presence of prostate cancer generally involves a series of tests and exams. Before starting the testing process, the physician will ask questions about the
DIAGNOSIS OF PROSTATE CANCER Determining the presence of prostate cancer generally involves a series of tests and exams. Before starting the testing process, the physician will ask questions about the
Robert Bristow MD PhD FRCPC
 Robert Bristow MD PhD FRCPC Clinician-Scientist and Professor, Radiation Oncology and Medical Biophysics, University of Toronto and Ontario Cancer Institute/ (UHN) Head, PMH-CFCRI Prostate Cancer Research
Robert Bristow MD PhD FRCPC Clinician-Scientist and Professor, Radiation Oncology and Medical Biophysics, University of Toronto and Ontario Cancer Institute/ (UHN) Head, PMH-CFCRI Prostate Cancer Research
Analysis of Prostate Cancer at Easter Connecticut Health Network Using Cancer Registry Data
 The 2014 Cancer Program Annual Public Reporting of Outcomes/Annual Site Analysis Statistical Data from 2013 More than 70 percent of all newly diagnosed cancer patients are treated in the more than 1,500
The 2014 Cancer Program Annual Public Reporting of Outcomes/Annual Site Analysis Statistical Data from 2013 More than 70 percent of all newly diagnosed cancer patients are treated in the more than 1,500
radiotherapy in locally advanced prostatic cancer: A dosimetric point of view
 Radiotherapy and Oncology 78 (2006) 47 52 www.thegreenjournal.com Prostate brachytherapy 192 Ir or 125 I prostate brachytherapy as a boost to external beam radiotherapy in locally advanced prostatic cancer:
Radiotherapy and Oncology 78 (2006) 47 52 www.thegreenjournal.com Prostate brachytherapy 192 Ir or 125 I prostate brachytherapy as a boost to external beam radiotherapy in locally advanced prostatic cancer:
Forum. Advances in radiation therapy for prostate. cancer. Abstract. Radiation therapy for localised prostate. cancer
 Advances in radiation therapy for prostate cancer Nitya Patanjali 1 and Scott Williams 2 1. Radiation Oncology, Sydney Cancer Centre, Royal Prince Alfred Hospital, Sydney. 2. Radiation Oncology, Peter
Advances in radiation therapy for prostate cancer Nitya Patanjali 1 and Scott Williams 2 1. Radiation Oncology, Sydney Cancer Centre, Royal Prince Alfred Hospital, Sydney. 2. Radiation Oncology, Peter
Effective for dates of service on or after May 1, 2015 refer to: Blue Cross and Blue Shield of Alabama Radiation Therapy Management RTM Policies
 Effective for dates of service on or after May 1, 2015 refer to: Blue Cross and Blue Shield of Alabama Radiation Therapy Management RTM Policies Name of Policy: Brachytherapy for Clinically Localized Prostate
Effective for dates of service on or after May 1, 2015 refer to: Blue Cross and Blue Shield of Alabama Radiation Therapy Management RTM Policies Name of Policy: Brachytherapy for Clinically Localized Prostate
SECOND MALIGNANCIES AFTER PROSTATE BRACHYTHERAPY: INCIDENCE OF BLADDER AND COLORECTAL CANCERS IN PATIENTS WITH 15 YEARS OF POTENTIAL FOLLOW-UP
 doi:10.1016/j.ijrobp.2006.05.016 Int. J. Radiation Oncology Biol. Phys., Vol. 66, No. 3, pp. 669 673, 2006 Copyright 2006 Elsevier Inc. Printed in the USA. All rights reserved 0360-3016/06/$ see front
doi:10.1016/j.ijrobp.2006.05.016 Int. J. Radiation Oncology Biol. Phys., Vol. 66, No. 3, pp. 669 673, 2006 Copyright 2006 Elsevier Inc. Printed in the USA. All rights reserved 0360-3016/06/$ see front
Newly Diagnosed Prostate Cancer: Understanding Your Risk
 Newly Diagnosed Prostate Cancer: Understanding Your Risk When the urologist calls with the life-changing news that your prostate biopsy is positive for prostate cancer, an office appointment is made to
Newly Diagnosed Prostate Cancer: Understanding Your Risk When the urologist calls with the life-changing news that your prostate biopsy is positive for prostate cancer, an office appointment is made to
Prostate Cancer. Treatments as unique as you are
 Prostate Cancer Treatments as unique as you are UCLA Prostate Cancer Program Prostate cancer is the second most common cancer among men. The UCLA Prostate Cancer Program brings together the elements essential
Prostate Cancer Treatments as unique as you are UCLA Prostate Cancer Program Prostate cancer is the second most common cancer among men. The UCLA Prostate Cancer Program brings together the elements essential
Michael J. Zelefsky,*, Heather Chan, Margie Hunt, Yoshiya Yamada, Alison M. Shippy and Howard Amols
 Long-Term Outcome of High Dose Intensity Modulated Radiation Therapy for Patients With Clinically Localized Prostate Cancer Michael J. Zelefsky,*, Heather Chan, Margie Hunt, Yoshiya Yamada, Alison M. Shippy
Long-Term Outcome of High Dose Intensity Modulated Radiation Therapy for Patients With Clinically Localized Prostate Cancer Michael J. Zelefsky,*, Heather Chan, Margie Hunt, Yoshiya Yamada, Alison M. Shippy
Treatment Options After Failure of Radiation Therapy A Review Daniel B. Rukstalis, MD
 MANAGEMENT OF RADIATION FAILURE IN PROSTATE CANCER Treatment Options After Failure of Radiation Therapy A Review Daniel B. Rukstalis, MD Division of Urology, MCP Hahnemann University, Philadelphia, PA
MANAGEMENT OF RADIATION FAILURE IN PROSTATE CANCER Treatment Options After Failure of Radiation Therapy A Review Daniel B. Rukstalis, MD Division of Urology, MCP Hahnemann University, Philadelphia, PA
Prostate Cancer. What is prostate cancer?
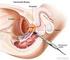 Scan for mobile link. Prostate Cancer Prostate cancer is a tumor of the prostate gland, which is located in front of the rectum and below the bladder. Your doctor may perform a physical exam, prostate-specific
Scan for mobile link. Prostate Cancer Prostate cancer is a tumor of the prostate gland, which is located in front of the rectum and below the bladder. Your doctor may perform a physical exam, prostate-specific
PSA Screening for Prostate Cancer Information for Care Providers
 All men should know they are having a PSA test and be informed of the implications prior to testing. This booklet was created to help primary care providers offer men information about the risks and benefits
All men should know they are having a PSA test and be informed of the implications prior to testing. This booklet was created to help primary care providers offer men information about the risks and benefits
Adjuvant radiation therapy for recurrent PSA after radical prostatectomy in T1±T2 prostate cancer
 Adjuvant radiation therapy for recurrent after radical prostatectomy in T1±T2 prostate cancer Prostate Cancer and Prostatic Diseases (1998) 1, 321±325 ß 1998 Stockton Press All rights reserved 1365±7852/98
Adjuvant radiation therapy for recurrent after radical prostatectomy in T1±T2 prostate cancer Prostate Cancer and Prostatic Diseases (1998) 1, 321±325 ß 1998 Stockton Press All rights reserved 1365±7852/98
Current and Future Trends in Proton Treatment of Prostate Cancer
 Current and Future Trends in Proton Treatment of Prostate Cancer Reinhard W. Schulte Assistant Professor Department of Radiation Medicine Loma Linda University Medical Center Loma Linda, CA, USA Outline
Current and Future Trends in Proton Treatment of Prostate Cancer Reinhard W. Schulte Assistant Professor Department of Radiation Medicine Loma Linda University Medical Center Loma Linda, CA, USA Outline
A STATISTICAL EVALUATION OF RULES FOR BIOCHEMICAL FAILURE AFTER RADIOTHERAPY IN MEN TREATED FOR PROSTATE CANCER
 doi:10.1016/j.ijrobp.2009.01.013 Int. J. Radiation Oncology Biol. Phys., Vol. 75, No. 5, pp. 1357 1363, 2009 Copyright Ó 2009 Elsevier Inc. Printed in the USA. All rights reserved 0360-3016/09/$ see front
doi:10.1016/j.ijrobp.2009.01.013 Int. J. Radiation Oncology Biol. Phys., Vol. 75, No. 5, pp. 1357 1363, 2009 Copyright Ó 2009 Elsevier Inc. Printed in the USA. All rights reserved 0360-3016/09/$ see front
Does my patient need more therapy after prostate cancer surgery?
 Does my patient need more therapy after prostate cancer surgery? Contact the GenomeDx Patient Care Team at: 1.888.792.1601 (toll-free) or e-mail: client.service@genomedx.com Prostate Cancer Classifier
Does my patient need more therapy after prostate cancer surgery? Contact the GenomeDx Patient Care Team at: 1.888.792.1601 (toll-free) or e-mail: client.service@genomedx.com Prostate Cancer Classifier
Dosimetry on the Intraoperative Procedure
 LDR-Brachytherapy of Prostate Cancer: Impact of Post-Implant Dosimetry on the Intraoperative Procedure Dr. med. Armin Thöni Dr. phil. nat. Hans Neuenschwander PD Dr. med. Jörn Wulf Radio-Onkologie, Lindenhofspital
LDR-Brachytherapy of Prostate Cancer: Impact of Post-Implant Dosimetry on the Intraoperative Procedure Dr. med. Armin Thöni Dr. phil. nat. Hans Neuenschwander PD Dr. med. Jörn Wulf Radio-Onkologie, Lindenhofspital
1. What is the prostate-specific antigen (PSA) test?
 1. What is the prostate-specific antigen (PSA) test? Prostate-specific antigen (PSA) is a protein produced by the cells of the prostate gland. The PSA test measures the level of PSA in the blood. The doctor
1. What is the prostate-specific antigen (PSA) test? Prostate-specific antigen (PSA) is a protein produced by the cells of the prostate gland. The PSA test measures the level of PSA in the blood. The doctor
CURRICULUM VITAE. PETER D. GRIMM, D.O. Prostate Cancer Treatment Center 1-877-330-7722 Email: peter@grimm.com
 CURRICULUM VITAE PETER D. GRIMM, D.O. Prostate Cancer Treatment Center 1-877-330-7722 Email: peter@grimm.com CURRENT STATUS Executive Director (July 2009-present) Chief Executive Officer: Prostate Cancer
CURRICULUM VITAE PETER D. GRIMM, D.O. Prostate Cancer Treatment Center 1-877-330-7722 Email: peter@grimm.com CURRENT STATUS Executive Director (July 2009-present) Chief Executive Officer: Prostate Cancer
American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy
 Brachytherapy 11 (2012) 6e19 American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy Brian J. Davis 1, *, Eric M. Horwitz 2, W. Robert Lee
Brachytherapy 11 (2012) 6e19 American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy Brian J. Davis 1, *, Eric M. Horwitz 2, W. Robert Lee
PSA Testing 101. Stanley H. Weiss, MD. Professor, UMDNJ-New Jersey Medical School. Director & PI, Essex County Cancer Coalition. weiss@umdnj.
 PSA Testing 101 Stanley H. Weiss, MD Professor, UMDNJ-New Jersey Medical School Director & PI, Essex County Cancer Coalition weiss@umdnj.edu September 23, 2010 Screening: 3 tests for PCa A good screening
PSA Testing 101 Stanley H. Weiss, MD Professor, UMDNJ-New Jersey Medical School Director & PI, Essex County Cancer Coalition weiss@umdnj.edu September 23, 2010 Screening: 3 tests for PCa A good screening
Prostate Cancer Treatment
 Scan for mobile link. Prostate Cancer Treatment Prostate cancer is a tumor of the prostate gland, which is located in front of the rectum and below the bladder. Your doctor may perform a physical exam,
Scan for mobile link. Prostate Cancer Treatment Prostate cancer is a tumor of the prostate gland, which is located in front of the rectum and below the bladder. Your doctor may perform a physical exam,
The 4Kscore blood test for risk of aggressive prostate cancer
 The 4Kscore blood test for risk of aggressive prostate cancer Early detection of aggressive prostate cancer Challenges Serum PSA has a high false positive rate Over 1 million prostate biopsies performed
The 4Kscore blood test for risk of aggressive prostate cancer Early detection of aggressive prostate cancer Challenges Serum PSA has a high false positive rate Over 1 million prostate biopsies performed
CMScript. Member of a medical scheme? Know your guaranteed benefits! Issue 7 of 2014
 Background CMScript Member of a medical scheme? Know your guaranteed benefits! Issue 7 of 2014 Prostate cancer is second only to lung cancer as the leading cause of cancer-related deaths in men. It is
Background CMScript Member of a medical scheme? Know your guaranteed benefits! Issue 7 of 2014 Prostate cancer is second only to lung cancer as the leading cause of cancer-related deaths in men. It is
Radiation Therapy for Prostate Cancer: Treatment options and future directions
 Radiation Therapy for Prostate Cancer: Treatment options and future directions David Weksberg, M.D., Ph.D. PinnacleHealth Cancer Institute September 12, 2015 Radiation Therapy for Prostate Cancer: Treatment
Radiation Therapy for Prostate Cancer: Treatment options and future directions David Weksberg, M.D., Ph.D. PinnacleHealth Cancer Institute September 12, 2015 Radiation Therapy for Prostate Cancer: Treatment
BRACHYTHERAPY OR CONFORMAL EXTERNAL RADIOTHERAPY FOR PROSTATE CANCER: A SINGLE-INSTITUTION MATCHED-PAIR ANALYSIS
 doi:10.1016/j.ijrobp.2009.01.081 Int. J. Radiation Oncology Biol. Phys., Vol. 76, No. 1, pp. 43 49, 2010 Copyright Ó 2010 Elsevier Inc. Printed in the USA. All rights reserved 0360-3016/10/$ see front
doi:10.1016/j.ijrobp.2009.01.081 Int. J. Radiation Oncology Biol. Phys., Vol. 76, No. 1, pp. 43 49, 2010 Copyright Ó 2010 Elsevier Inc. Printed in the USA. All rights reserved 0360-3016/10/$ see front
Cyberknife Information Guide. Prostate Cancer Treatment
 Cyberknife Information Guide Prostate Cancer Treatment CYBERKNIFE INFORMATION GUIDE PROSTATE CANCER TREATMENT As a patient recently diagnosed with localized prostate cancer, it is important that you familiarize
Cyberknife Information Guide Prostate Cancer Treatment CYBERKNIFE INFORMATION GUIDE PROSTATE CANCER TREATMENT As a patient recently diagnosed with localized prostate cancer, it is important that you familiarize
Issues Concerning Development of Products for Treatment of Non-Metastatic Castration- Resistant Prostate Cancer (NM-CRPC)
 Issues Concerning Development of Products for Treatment of Non-Metastatic Castration- Resistant Prostate Cancer (NM-CRPC) FDA Presentation ODAC Meeting September 14, 2011 1 Review Team Paul G. Kluetz,
Issues Concerning Development of Products for Treatment of Non-Metastatic Castration- Resistant Prostate Cancer (NM-CRPC) FDA Presentation ODAC Meeting September 14, 2011 1 Review Team Paul G. Kluetz,
Role of Radiation after Radical Prostatectomy Review of Literature
 Vol. 9, No: 1 Jan - Jun 2013. Page 1-44 Role of Radiation after Radical Prostatectomy Review of Literature S.K. Raghunath, N. Srivatsa Abstract Biochemical relapse after radical prostatectomy occurs in
Vol. 9, No: 1 Jan - Jun 2013. Page 1-44 Role of Radiation after Radical Prostatectomy Review of Literature S.K. Raghunath, N. Srivatsa Abstract Biochemical relapse after radical prostatectomy occurs in
Radiation Therapy in Prostate Cancer Current Status and New Advances
 Radiation Therapy in Prostate Cancer Current Status and New Advances Arno J. Mundt MD Professor and Chairman Dept Radiation Oncology Moores Cancer Center UCSD Presentation Welcome Overview of UCSD, Moores
Radiation Therapy in Prostate Cancer Current Status and New Advances Arno J. Mundt MD Professor and Chairman Dept Radiation Oncology Moores Cancer Center UCSD Presentation Welcome Overview of UCSD, Moores
doi:10.1016/j.ijrobp.2003.10.050 CLINICAL INVESTIGATION
 doi:10.1016/j.ijrobp.2003.10.050 Int. J. Radiation Oncology Biol. Phys., Vol. 59, No. 2, pp. 419 425, 2004 Copyright 2004 Elsevier Inc. Printed in the USA. All rights reserved 0360-3016/04/$ see front
doi:10.1016/j.ijrobp.2003.10.050 Int. J. Radiation Oncology Biol. Phys., Vol. 59, No. 2, pp. 419 425, 2004 Copyright 2004 Elsevier Inc. Printed in the USA. All rights reserved 0360-3016/04/$ see front
Radiation therapy is a well-established treatment option for patients with
 Hematol Oncol Clin N Am 20 (2006) 857 878 HEMATOLOGY/ONCOLOGY CLINICS OF NORTH AMERICA Update on Radiation Therapy in Prostate Cancer Andrew K. Lee, MD, MPH*, Steven J. Frank, MD Division of Radiation
Hematol Oncol Clin N Am 20 (2006) 857 878 HEMATOLOGY/ONCOLOGY CLINICS OF NORTH AMERICA Update on Radiation Therapy in Prostate Cancer Andrew K. Lee, MD, MPH*, Steven J. Frank, MD Division of Radiation
Prostate Cancer Treatment: What s Best for You?
 Prostate Cancer Treatment: What s Best for You? Prostate Cancer: Radiation Therapy Approaches I. Choices There is really a variety of options in prostate cancer management overall and in radiation therapy.
Prostate Cancer Treatment: What s Best for You? Prostate Cancer: Radiation Therapy Approaches I. Choices There is really a variety of options in prostate cancer management overall and in radiation therapy.
Prostate Cancer Results Study Group 2014 Results Comparing Treatment of. About This Review Study
 Prostate Cancer Results Study Group Results Comparing Treatment of Prostate Cancer Peter Grimm, DO Seattle, WA About This Review Study,000+ prostate studies were published between 00 and June 3, of those
Prostate Cancer Results Study Group Results Comparing Treatment of Prostate Cancer Peter Grimm, DO Seattle, WA About This Review Study,000+ prostate studies were published between 00 and June 3, of those
A new score predicting the survival of patients with spinal cord compression from myeloma
 A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; sarah_douglas@gmx.de (2) Steven
A new score predicting the survival of patients with spinal cord compression from myeloma (1) Sarah Douglas, Department of Radiation Oncology, University of Lubeck, Germany; sarah_douglas@gmx.de (2) Steven
Transperineal Permanent Brachytherapy of Localised Prostate Cancer
 european urology supplements 7 (2008) 732 741 available at www.sciencedirect.com journal homepage: www.europeanurology.com Transperineal Permanent Brachytherapy of Localised Prostate Cancer Nader Naderi
european urology supplements 7 (2008) 732 741 available at www.sciencedirect.com journal homepage: www.europeanurology.com Transperineal Permanent Brachytherapy of Localised Prostate Cancer Nader Naderi
Us TOO University Presents: Understanding Diagnostic Testing
 Us TOO University Presents: Understanding Diagnostic Testing for Prostate Cancer Patients Today s speaker is Manish Bhandari, MD Program moderator is Pam Barrett, Us TOO International Made possible by
Us TOO University Presents: Understanding Diagnostic Testing for Prostate Cancer Patients Today s speaker is Manish Bhandari, MD Program moderator is Pam Barrett, Us TOO International Made possible by
Curriculum Vitae John Edward Sylvester, M.D.
 PROFESSIONAL EXPERIENCE Clinical Assistant Professor Florida State University (May 2011-Present) Medical Director Lakewood Ranch Oncology Center (March 2010-Present) Medical Director Prostate Cancer Treatment
PROFESSIONAL EXPERIENCE Clinical Assistant Professor Florida State University (May 2011-Present) Medical Director Lakewood Ranch Oncology Center (March 2010-Present) Medical Director Prostate Cancer Treatment
9. Discuss guidelines for follow-up post-thyroidectomy for cancer (labs/tests) HH
 9. Discuss guidelines for follow-up post-thyroidectomy for cancer (labs/tests) HH Differentiated thyroid cancer expresses the TSH receptor on the cell membrane and responds to TSH stimulation by increasing
9. Discuss guidelines for follow-up post-thyroidectomy for cancer (labs/tests) HH Differentiated thyroid cancer expresses the TSH receptor on the cell membrane and responds to TSH stimulation by increasing
Bard: Prostate Cancer Treatment. Bard: Pelvic Organ Prolapse. Prostate Cancer. An overview of. Treatment. Prolapse. Information and Answers
 Bard: Prostate Cancer Treatment Bard: Pelvic Organ Prolapse Prostate Cancer An overview of Pelvic Treatment Organ Prolapse Information and Answers A Brief Overview Prostate Anatomy The prostate gland,
Bard: Prostate Cancer Treatment Bard: Pelvic Organ Prolapse Prostate Cancer An overview of Pelvic Treatment Organ Prolapse Information and Answers A Brief Overview Prostate Anatomy The prostate gland,
Prostate Cancer. Guideline for the Management of Clinically Localized Prostate Cancer: 2007 Update
 Prostate Cancer Members, (specialty): Ian Thompson, M.D., Chair, (Urology) James Brantley Thrasher, M.D., Co-Chair, (Urology) Gunnar Aus, M.D., (Urology) Arthur L. Burnett, M.D., (Sexual Medicine) Edith
Prostate Cancer Members, (specialty): Ian Thompson, M.D., Chair, (Urology) James Brantley Thrasher, M.D., Co-Chair, (Urology) Gunnar Aus, M.D., (Urology) Arthur L. Burnett, M.D., (Sexual Medicine) Edith
Prostate Cancer In-Depth
 Prostate Cancer In-Depth Introduction Prostate cancer is the most common visceral malignancy among American men. In the year 2003, there are expected to be 220,000 new cases and nearly 29,000 deaths in
Prostate Cancer In-Depth Introduction Prostate cancer is the most common visceral malignancy among American men. In the year 2003, there are expected to be 220,000 new cases and nearly 29,000 deaths in
Local Coverage Determination (LCD): MolDX: Genomic Health Oncotype DX Prostate Cancer Assay (L36153)
 Local Coverage Determination (LCD): MolDX: Genomic Health Oncotype DX Prostate Cancer Assay (L36153) Contractor Information Contractor Name Palmetto GBA LCD Information Document Information LCD ID L36153
Local Coverage Determination (LCD): MolDX: Genomic Health Oncotype DX Prostate Cancer Assay (L36153) Contractor Information Contractor Name Palmetto GBA LCD Information Document Information LCD ID L36153
Gleason Score. Oncotype DX GPS. identified for. about surveillance. time to get sophisticated
 patient: MARK SMITH PSA 6.2 Gleason Score 6 Oncotype DX GPS 8 identified for active surveillance time to get sophisticated about surveillance Accurate prediction of prostate cancer risk is needed at the
patient: MARK SMITH PSA 6.2 Gleason Score 6 Oncotype DX GPS 8 identified for active surveillance time to get sophisticated about surveillance Accurate prediction of prostate cancer risk is needed at the
Neoadjuvant hormonal therapy in prostate cancer impact of PSA level before radiotherapy
 JBUON 2013; 18(4): 949-953 ISSN: 1107-0625, online ISSN: 2241-6293 www.jbuon.com E-mail: editorial_office@jbuon.com ORIGINAL ARTICLE Neoadjuvant hormonal therapy in prostate cancer impact of PSA level
JBUON 2013; 18(4): 949-953 ISSN: 1107-0625, online ISSN: 2241-6293 www.jbuon.com E-mail: editorial_office@jbuon.com ORIGINAL ARTICLE Neoadjuvant hormonal therapy in prostate cancer impact of PSA level
An Introduction to PROSTATE CANCER
 An Introduction to PROSTATE CANCER Being diagnosed with prostate cancer can be a life-altering experience. It requires making some very difficult decisions about treatments that can affect not only the
An Introduction to PROSTATE CANCER Being diagnosed with prostate cancer can be a life-altering experience. It requires making some very difficult decisions about treatments that can affect not only the
Salvage Conformal Radiotherapy for Biochemical Recurrent Prostate Cancer after Radical Prostatectomy
 Clinical Urology Salvage Conformal Radiotherapy for Prostate Cancer International Braz J Urol Vol. 32 (4): 46-427, July - August, 2006 Salvage Conformal Radiotherapy for Biochemical Recurrent Prostate
Clinical Urology Salvage Conformal Radiotherapy for Prostate Cancer International Braz J Urol Vol. 32 (4): 46-427, July - August, 2006 Salvage Conformal Radiotherapy for Biochemical Recurrent Prostate
Radiation Therapy in Prostate Cancer Current Status and New Advances
 Radiation Therapy in Prostate Cancer Current Status and New Advances Arno J. Mundt MD Professor and Chairman Dept Radiation Oncology Moores Cancer Center UCSD Radiation Therapy Wilhelm Roentgen (1845-1923)
Radiation Therapy in Prostate Cancer Current Status and New Advances Arno J. Mundt MD Professor and Chairman Dept Radiation Oncology Moores Cancer Center UCSD Radiation Therapy Wilhelm Roentgen (1845-1923)
IAEA HUMAN HEALTH REPORTS No. 11. Strategies for the Management of Localized Prostate Cancer: A Guide for Radiation Oncologists
 IAEA HUMAN HEALTH REPORTS No. 11 Strategies for the Management of Localized Prostate Cancer: A Guide for Radiation Oncologists @ IAEA HUMAN HEALTH SERIES PUBLICATIONS The mandate of the IAEA human health
IAEA HUMAN HEALTH REPORTS No. 11 Strategies for the Management of Localized Prostate Cancer: A Guide for Radiation Oncologists @ IAEA HUMAN HEALTH SERIES PUBLICATIONS The mandate of the IAEA human health
TITLE: Comparison of the dosimetric planning of partial breast irradiation with and without the aid of 3D virtual reality simulation (VRS) software.
 SAMPLE CLINICAL RESEARCH APPLICATION ABSTRACT: TITLE: Comparison of the dosimetric planning of partial breast irradiation with and without the aid of 3D virtual reality simulation (VRS) software. Hypothesis:
SAMPLE CLINICAL RESEARCH APPLICATION ABSTRACT: TITLE: Comparison of the dosimetric planning of partial breast irradiation with and without the aid of 3D virtual reality simulation (VRS) software. Hypothesis:
BRACHYTHERAPY OR RADIOACTIVE SEED IMPLANTATION CANCER OF THE PROSTATE
 BRACHYTHERAPY OR RADIOACTIVE SEED IMPLANTATION CANCER OF THE PROSTATE Prostate cancer, like other cancers, is a disease of the body's cells. Normal cells reproduce themselves by dividing--facilitating
BRACHYTHERAPY OR RADIOACTIVE SEED IMPLANTATION CANCER OF THE PROSTATE Prostate cancer, like other cancers, is a disease of the body's cells. Normal cells reproduce themselves by dividing--facilitating
J Clin Oncol 23:6992-6998. 2005 by American Society of Clinical Oncology INTRODUCTION
 VOLUME 23 NUMBER 28 OCTOBER 1 2005 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T Predictors of Prostate Cancer Specific Mortality After Radical Prostatectomy or Radiation Therapy Ping Zhou,
VOLUME 23 NUMBER 28 OCTOBER 1 2005 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T Predictors of Prostate Cancer Specific Mortality After Radical Prostatectomy or Radiation Therapy Ping Zhou,
Report with statistical data from 2007
 2008 Cancer Program Annual Report with statistical data from 2007 Lake Cumberland Regional Hospital 305 Langdon Streett Somerset, KY 42503 Telephone: 606-679-7441 Fax: 606-678-9919 Cancer Committee Mullai,
2008 Cancer Program Annual Report with statistical data from 2007 Lake Cumberland Regional Hospital 305 Langdon Streett Somerset, KY 42503 Telephone: 606-679-7441 Fax: 606-678-9919 Cancer Committee Mullai,
Understanding the. Controversies of. testosterone replacement. therapy in hypogonadal men with prostate cancer. controversies surrounding
 Controversies of testosterone replacement therapy in hypogonadal men with prostate cancer Samuel Deem, DO CULTURA CREATIVE (RF) / ALAMY Understanding the controversies surrounding testosterone replacement
Controversies of testosterone replacement therapy in hypogonadal men with prostate cancer Samuel Deem, DO CULTURA CREATIVE (RF) / ALAMY Understanding the controversies surrounding testosterone replacement
Cancer in Primary Care: Prostate Cancer Screening. How and How often? Should we and in which patients?
 Cancer in Primary Care: Prostate Cancer Screening How and How often? Should we and in which patients? PLCO trial (Prostate, Lung, Colorectal and Ovarian) Results In the screening group, rates of compliance
Cancer in Primary Care: Prostate Cancer Screening How and How often? Should we and in which patients? PLCO trial (Prostate, Lung, Colorectal and Ovarian) Results In the screening group, rates of compliance
Subject: Proton Beam Therapy for Prostate Cancer
 Subject: Proton Beam Therapy for Prostate Cancer Guidance Number: MCG-153 Revision Date(s): Original Effective Date: 10/30/13 Medical Coverage Guidance Approval Date: 10/30/13 PREFACE This Medical Guidance
Subject: Proton Beam Therapy for Prostate Cancer Guidance Number: MCG-153 Revision Date(s): Original Effective Date: 10/30/13 Medical Coverage Guidance Approval Date: 10/30/13 PREFACE This Medical Guidance
A New Biomarker in Prostate Cancer Care: Oncotype Dx. David M Albala, MD Chief of Urology Crouse Hospital Syracuse, NY
 A New Biomarker in Prostate Cancer Care: Oncotype Dx David M Albala, MD Chief of Urology Crouse Hospital Syracuse, NY Learning Objectives Review the current challenges in the prediction and prognosis of
A New Biomarker in Prostate Cancer Care: Oncotype Dx David M Albala, MD Chief of Urology Crouse Hospital Syracuse, NY Learning Objectives Review the current challenges in the prediction and prognosis of
