(From the Biochemistry Department, Medical College of Alabama, Birmingham)
|
|
|
- Spencer Stafford
- 8 years ago
- Views:
Transcription
1 RELATIVE CONCENTRATIONS OF N is IN URINARY AMMONIA N AND UREA N AFTER FEEDING N15-LABELED COMPOUNDS BY I-ISIEN WU (From the Biochemistry Department, Medical College of Alabama, Birmingham) (Received for publication, July 27, 195) The concentrations of N ~5 in ammonia N and urea N in urine collected after feeding an amino acid or ammonia labeled with N 15 show an interesting relation which has not been interpreted or even clearly recognized. The purpose of the present paper is to point out this relation and to suggest a possible explanation. When/-leucine (1),/-lysine (2), or glycine (3, 4) labeled with N 15 in the amino group was incorporated in the food of rats, and the 24 hour urine was analyzed for N ~s and N, the N 15 concentration (expressed as ~- hereafter called the isotopic ratio) of ammonia (ra) was found to be higher than that of urea (r,). When aspartic acid (5) or ammonia (6) in the form of citrate was fed in similar experiments, the isotopic ratio of urea (r,) was found to be higher than that of ammonia. Thus, in these rat experiments the metabolic behavior of ammonia is the same as that of/-aspartic acid, but different from that of/-leucine, /-lysine, and glycine. When l-aspartic acid was fed to man in a single feeding (5), r, was much higher than r~ in urine collected shortly after feeding, but the relation was reversed later. It was pointed out that the relation between ra and r~ depended on the manner of feeding. Whereas in the human experiment the whole intake of aspartic acid was taken in a single feeding, in rat experiments the daily intake consisted of small portions spread over many hours, as the food was always accessible to the animals. With a single feeding, the change of isotopic ratio of a compound in urine with time can be described by a curve deducible from theory, while with continuous feeding, it is impossible to predict the isotopic ratio of the compound without knowing the feeding schedule or habit of the rats. In a recent paper Sprinson and Rittenberg (7) reported that after a single feeding of ammonium citrate in man "the N ~ concentration of the ammonia is always less than that of the urea," while after feeding isotopic glycine the concentration of N ~5 in ammonia is initially higher than that of urea. Thus it appears that in human experiments the metabolic behavior of glycine is the same as that of aspartic acid, but different from that of ammonia, while in rat 43 The Journal of General Physiology
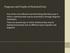
2 44 N z5 IN URINARY A~E~ONIA N AND UREA N experiments, aspartic acid and ammonia behave alike but differently from glycine. Obviously, a mere comparison of r, and ru without reference to quantitative time relations is not very revealing, and a detailed analysis of the ex- cd.7 (...) XO,6 I..c.I I-- 7 I.=J,5 n" LtJ n.4 O rc t~.3.. Oil o "2_ 1 J, J. o to,o TIME IN HOURS Fzo. 1. Isotopic ratios of ammonia and urea N after feeding isotopic/~aspartic acid (Experiment R reported in a previous paper (8).) The values of the isotopic ratios for an interval are plotted against the middle point of the interval. For the interval - to (to = 3), ~o and ~ atom percent excess and the ratioro:r, is.375 = 3.1. For the interval - t~, (t~ = 18), #, = #u =.124 atom per cent exces%.121 and the ratio r,:r~ = 1. These ia and #, values were calculated from the areas under the curves measured with a plaaimeter. perlmental data is called for. The purpose of this paper is to present such an analysis. Fig. 1 shows the curves for r, and r, after a single feeding of aspartic acid. (The data were obtained in an experiment R, reported in a previous paper (8).) It will be noted that the maximum r, occurs at about 1 hour while the maximum r, occurs at about 3 hours after feeding. It will be noted also that at the maximum, r, is several (six) times higher than r,. After reaching the maximum, I
3 HSIEN ~ 45 the ra curve falls off rapidly, while the r~ curve is still rising so that they cross at a point t~. In Fig. 1, tc is at about 3 hours. If urine is collected from t = to t = to, the average ra will be higher than the average r~, if the rates of ammonia and urea excretion remain unchanged. If urine is collected from tc to any time afterwards, the average r~ will be lower than the average r~. If urine is collected from t -- to a time t > to, the ratio r~:r~ will depend on t. This ratio is equal to the ratio of the areas below the r~ and r~ curves between t = and t. It can be seen that the greater the t, the smaller will be the ratio r~:r~. If urine is collected for the 24 hour interval after a single feeding this ratio should always be less than 1. For a given pair of ra and r~ curves, there is a time when the areas below the two curves from to t are equal. This time is about 18 hours in the experiment shown in Fig. 1. If before this time is reached, another dose of the isotopic compound is taken, then the ratio for the interval immediately afterward will be raised. By taking the isotopic compound at suitable intervals the ratio r~:r,, can be maintained at any value below the maximum. If the r~ and r~ curves are the same for the rat as for man, it is possible to calculate from the ratio r,:r,, the interval between feedings in the rat experiments. The shapes of the r, and r. curves after a single feeding are theoretically independent of the intake, but the absolute values of r, and r, must depend on the intake. When the amount fed at each feeding and the schedule of feeding are unknown, as they are in those rat experiments above referred to, no theoretical significance can be attached to the ratio ra:r, and interpretation is impossible. The above discussion on ra and r, curves for aspartic acid holds also for glycine. It is unfortunate that of the many experiments reported by Sprinson and Rittenberg only a few included both urea and ammonia analyses, and of these few the first collection of urine was too late to show the maximum points of r, and ra. However, in one experiment (DS-1) (7) the data did show a maximum point of r, at about 3 hours after feeding (Fig. 2, I). The maximum point of r, was missed, but from the slope of the r. curve, it can be seen that r. was falling when it crossed the r, curve. Since ra, like r,, must start at at t =, it must rise to a maximum before it began to fall, and it may be concluded that the same relation holds between r, and r, in Experiment DS-1 (glycine) as in the experiment with aspartic acid. Two other experiments with glycine, GL-1 and MZ-1 (Fig. 2, II, III), show the same general trend of the r. and r. curves. It may be concluded, therefore, that the same general relation holds between ro and r~ after glycine feeding as after aspartic acid feeding. Of the two experiments with ammonium citrate in man reported by Sprinson and Rittenberg (7), r~ showed a maximum in one experiment (subject with Addison's disease) but not in the other (normal subject). In both experiments
4 /, Ii.4 ii 46 N I5 IN URINARY AM~ONIA N AND UREA N U') O3 iii X i,i I-. Z i,i n- hj rl.3 II o.,i! / \ ~ 11/ I'/ "--- I j Y I I I r I I ~ O II II ', '\ I-- O.5 I n- O - I-- O3 "31- ; I I! /.1 V I I ~A f TIME IN HOURS FIG. 2. Isotopic ratio of ammonia and urea N after feeding isotopic glycine or ammonium citrate (reported by Sprinson and Rittenberg). I, Experiment DS-1, glycine; II, Experiment GL, glycine; III, Experiment MZ-1, glyeine; IV, Experiment on normal human subject, ammonium citrate.
5 HS~N wu 47 the maximum point of ra was not observed. But it can be seen that at 1 to 2 hours after feeding in both experiments ra was falling (and not rising) and it was falling much more rapidly than r, (Fig. 2, IV). x Extrapolation of the curves (shown in dotted line) would give pictures quite comparable to those in experiments with glycine and aspartic acid. The only difference between these amino acids, on the one hand, and ammonia on the other would be a quantitative one--in the times at which maximum r, and maximum r, occur and the values of these maxima. From the results in the rat experiments it would appear that the maximum r, and tq occur earlier with ammonium citrate and aspartic acid than with glycine. Equality of the r, and r~ at the Maximum Point of the Latter While the initially high r~:r~ ratio has been reported for glycine as for aspartic acid, a significant relation has not been noticed before. As shown in Fig. 2, I, the r, curve and the r, curve intersect (that is, ra is equal to r,) at the maximum point of r, in the glycine experiment (DS-1) as in the aspartie acid experiment (Fig. 1). In other experiments with glycine (MF-1) and (GL-1) for which urea and ammonia analyses were reported, the data are not sufficient to show this relation clearly. But it can be seen (Fig. 2) in both these experiments that ro and r~ when extrapolated (shown in dotted lines) would intersect at somewhere near the ma~mum point of r~ which was missed in these experiments. In one ammonium citrate experiment, with normal human subject, similar relation can be seen between the r~ and r, curves. In the other ammonium citrate experiment, this relation is not shown. However, not much weight should be given to this experiment for several reasons. (1) The first sample of urine was collected from 1 hour before, to 2 hours after, feeding. This makes uncertain the r~ and r~ values for the first urine sample. (2) The total N intake of this subject was unusually, low (2.5 gin. per day). (3) The r~ and r. reached much higher values in this subject than in the normal subject with the same intake of isotopic ammonium citrate, and it seems possible that there was some abnormality in ammonia metabolism in the subject with Addison's disease. Zilversmit et al. (9), in their studies with phospholipids labeled with radioactive P, have pointed out that when a compound A is the immediate precursor of another compound B, the specific activity of B is equal to that of A at the maximum point of B. In the present instance, the isotopic ratio of urea, at the maximum point, is equal to that of ammonia. This suggests that urea is formed from ammonia which is nothing new, but the observed relation can be true only when urea is formed exclusively from ammonia. x Fig. 2, IV shows the curves for the normal subject. The curve for the patient with Addison's disease is not shown here. There is some uncertainty in the first point of the curve for this subject because the first sample of urine covered the period from 1 hour before, to 2 hours after, feeding.
6 48 N 15 IN URINARY AMMONIA N AND UREA N Rather and Poppas (1) have shown that the amino N of aspartic acid is transferred to citrulline in the arginine cycle and that aspartic acid is the main pathway of urea formation. Wu and Rittenberg (5) have shown that the aspartic acid N behaves metabolically like ammonia N in some respects. In view of these findings, it may be surmised that malic acid readily accepts ammonia and transfers it to citrulline. However, this explanation is probably not correct as the experiment with glycine (DS-1) shows the same relation between ro and r,. A more plausible explanation is that the isotopic ratio of ammonia in urine is not the same as that in the metabolic pool (which is negligible in view of the low ammonia content of blood) but is the same as the isotopic ratio of the amino acid from which ammonia is formed and is directly excreted by the kidneys. If it is assumed that the aspartic acid or glycine fed comes rapidly into equilibrium with other amino acids in the pool, and it is from this mixture of amino acids rather than the particular amino acid fed that the ammonia is derived, the relation between r= and r, after feeding either glycine or aspartic acid is explained. When two different compounds B1 and B~ are both immediately derived from A, the relative rate of fall of the concentration of B1 should be the same as that of B2. In the present instance, if both urea and ammonia are immediately derived from the amino acid fed, r= and r, should fall at the same relative rate. This is definitely not the case in the first few hours after feeding. As shown in Figs. 1 and 2, r, falls much more rapidly than r~. Instead of both being immediately derived from the isotopic compound fed, urea appears to be derived from ammonia. The curves shown in Figs. 1 and 2 are plotted with the average r of an interval against the time at the middle point of the interval. In other words, the average r is taken as equal to the instantaneous r at the middle point of the interval. This assumption is open to some error unless urine has been collected at short intervals. While there is no doubt that the ra is initially always higher than r~, the exact equality of r, and r= at the point of maximum r~ requires further investigation with other N15-1abeled compounds. SUMMARY {N15~ Available data on the isotopic ratio~-~-. ) of ammonia (r,) and that of urea. (r,) after a single feeding of glycine, aspartic acid, and ammonium citrate are analyzed. From this analysis the following conclusions are drawn. [NlS\ 1. The isotopic ratio~-~-) of ammonia (r~) is always higher than that of urea (r,) in the initial period after a single feeding of isotopic glycine or aspartic acid, but the relation is reversed later. A similar relation probably holds after feeding isotopic ammonia.
7 ~,SmN WV It is pointed out that the ratio of average r, to average r, depends on the time interval for which urine is collected, on the schedule of feeding, and probably also on the amount taken at each feeding. When the amount fed and the feeding schedule are unknown, theoretical interpretation of the ratio of average r, to average r, is impossible. 3. At the point of maximum isotopic ratio of urea, it is very probably equal to the isotopic ratio of ammonia. A possible explanation is suggested. BIBLIOGRAPHY 1. Schoenheimer, R., Ratner, S., and Rittenberg, D., J. Biol. Chem., 1939, 13, Weissman, N., and Schoenheimer, R., J, Biol. Chem., 1941, 14, Ratner, S., Rittenberg, D., Keston, A. S., and Schoenheimer, R., J. Biol. Chem., 194, 134, Shemin, D., and Rittenberg, D., J. Biol. Chem., 1944, 1~, Wu, H., and Rittenberg, D., J. Biol. Chem., 1949, 179, Schoenheimer, R., The Dynamic State of Body Constituents, Cambridge, Harvard University Press, 1946, Sprinson, D., and Rittenberg, D., J. Biol. Chem., 1949, 18, Wu, H., and Snyderman, S. E., J. Gen. Physiol., 1951, 34, Zilversmit, D. B., Entenman, C., and Fisher, M. C., J. Gen. Physiol., 1943, 26, Ratner, S., and Poppas, A., J. Biol. Chem., 1949, 179, 1183, 1199.
RATE OF EXCRETION OF N 15 AFTER FEEDING N16-LABELED /-ASPARTIC ACID IN MAN BY HSIEN WU AND SELMA E. SNYDERMAN
 RATE OF EXCRETION OF N 15 AFTER FEEDING N16-LABELED /-ASPARTIC ACID IN MAN BY HSIEN WU AND SELMA E. SNYDERMAN (From the Biochemistry Department, Medical College of Alabama, Birmingham, and the Department
RATE OF EXCRETION OF N 15 AFTER FEEDING N16-LABELED /-ASPARTIC ACID IN MAN BY HSIEN WU AND SELMA E. SNYDERMAN (From the Biochemistry Department, Medical College of Alabama, Birmingham, and the Department
THE EFFECT OF SODIUM CHLORIDE ON THE GLUCOSE TOLERANCE OF THE DIABETIC RAT*
 THE EFFECT OF SODIUM CHLORIDE ON THE GLUCOSE TOLERANCE OF THE DIABETIC RAT* BY JAMES M. ORTEN AND HENRY B. DEVLINt (From the Deparkment of Physiological Chemistry, Wayne University College of Medicine,
THE EFFECT OF SODIUM CHLORIDE ON THE GLUCOSE TOLERANCE OF THE DIABETIC RAT* BY JAMES M. ORTEN AND HENRY B. DEVLINt (From the Deparkment of Physiological Chemistry, Wayne University College of Medicine,
AP Physics 1 and 2 Lab Investigations
 AP Physics 1 and 2 Lab Investigations Student Guide to Data Analysis New York, NY. College Board, Advanced Placement, Advanced Placement Program, AP, AP Central, and the acorn logo are registered trademarks
AP Physics 1 and 2 Lab Investigations Student Guide to Data Analysis New York, NY. College Board, Advanced Placement, Advanced Placement Program, AP, AP Central, and the acorn logo are registered trademarks
Chemistry 201. Practical aspects of buffers. NC State University. Lecture 15
 Chemistry 201 Lecture 15 Practical aspects of buffers NC State University The everyday ph scale To review what ph means in practice, we consider the ph of everyday substances that we know from experience.
Chemistry 201 Lecture 15 Practical aspects of buffers NC State University The everyday ph scale To review what ph means in practice, we consider the ph of everyday substances that we know from experience.
EUROPEAN COMMISSION HEALTH & CONSUMER PROTECTION DIRECTORATE-GENERAL
 EUROPEAN COMMISSION HEALTH & CONSUMER PROTECTION DIRECTORATE-GENERAL Directorate C - Scientific Opinions C2 - Management of scientific committees II; scientific co-operation and networks Revision of the
EUROPEAN COMMISSION HEALTH & CONSUMER PROTECTION DIRECTORATE-GENERAL Directorate C - Scientific Opinions C2 - Management of scientific committees II; scientific co-operation and networks Revision of the
(From the Department of Biochemistry, University of Nebraska College of Medicine, Omaha)
 THE INHIBITION OF THE ADENOSINE TRIPHOSPHATASE ACTIVITY OF ACTOMYOSIN BY MAGNESIUM IONS BY IRVIN BRAVERMAN A.~ SERGIUS MORGULIS (From the Department of Biochemistry, University of Nebraska College of Medicine,
THE INHIBITION OF THE ADENOSINE TRIPHOSPHATASE ACTIVITY OF ACTOMYOSIN BY MAGNESIUM IONS BY IRVIN BRAVERMAN A.~ SERGIUS MORGULIS (From the Department of Biochemistry, University of Nebraska College of Medicine,
were shown (1).- Figure 3 shows four typical levels, of intravenous administration of glucose (5). Figure 4 shows a typical result obtained after
 IV. RESPONSE OF CITRIC ACID LEVELS OF NORMAL ADULTS AND CHILDREN TO INTRAMUSCULAR INJECTION OF EPINEPHRINE' By JOSEPH B. PINCUS, SAMUEL NATELSON, AND JULIUS K. LUGOVOY (Frost the Pediatric Research Laboratory
IV. RESPONSE OF CITRIC ACID LEVELS OF NORMAL ADULTS AND CHILDREN TO INTRAMUSCULAR INJECTION OF EPINEPHRINE' By JOSEPH B. PINCUS, SAMUEL NATELSON, AND JULIUS K. LUGOVOY (Frost the Pediatric Research Laboratory
Anatomy/Physiology Course Summary Department: Science. Semester 1
 Anatomy/Physiology Course Summary Department: Science Semester 1 Learning Objective #1 Students will be able to understand and use the language of the discipline in addition to understanding the concept
Anatomy/Physiology Course Summary Department: Science Semester 1 Learning Objective #1 Students will be able to understand and use the language of the discipline in addition to understanding the concept
The Urea Cycle. April 11, 2003 Bryant Miles
 The Urea ycle April 11, 2003 Bryant Miles I. Ammonia Toxicity Every amino acid contains at least one amino group. Therefore every amino acid degradation pathway has a key step where the amino group is
The Urea ycle April 11, 2003 Bryant Miles I. Ammonia Toxicity Every amino acid contains at least one amino group. Therefore every amino acid degradation pathway has a key step where the amino group is
Published on: 07/04/2015 Page 1 of 5
 Bladder Stones A DNA test for Hyperunicosuria (HUU) to find the gene which is implicated in the development of URATE stones has been developed which work with Bulldogs and many other breeds including Black
Bladder Stones A DNA test for Hyperunicosuria (HUU) to find the gene which is implicated in the development of URATE stones has been developed which work with Bulldogs and many other breeds including Black
III. THE MICROBIAL BIOMASS
 III. THE MICROBIAL BIOMASS Required Readings: Ley, R.E., D.A. Lipson and S.K. Schmidt. 2001. Microbial biomass levels in barren and vegetated high altitude talus soils. Soil Sci. Soc. Am. J. 65:111 117.
III. THE MICROBIAL BIOMASS Required Readings: Ley, R.E., D.A. Lipson and S.K. Schmidt. 2001. Microbial biomass levels in barren and vegetated high altitude talus soils. Soil Sci. Soc. Am. J. 65:111 117.
Mass Spectrometry Signal Calibration for Protein Quantitation
 Cambridge Isotope Laboratories, Inc. www.isotope.com Proteomics Mass Spectrometry Signal Calibration for Protein Quantitation Michael J. MacCoss, PhD Associate Professor of Genome Sciences University of
Cambridge Isotope Laboratories, Inc. www.isotope.com Proteomics Mass Spectrometry Signal Calibration for Protein Quantitation Michael J. MacCoss, PhD Associate Professor of Genome Sciences University of
Compound extracted from plant Aristolochia. Nephrotoxin and carcinogen. Page 2
 Mariana Babayeva MD, PhD Touro College of Pharmacy, New York, NY, USA Page 1 Compound extracted from plant Aristolochia Nephrotoxin and carcinogen Page 2 AA-I is an organic anion eliminated by the kidney
Mariana Babayeva MD, PhD Touro College of Pharmacy, New York, NY, USA Page 1 Compound extracted from plant Aristolochia Nephrotoxin and carcinogen Page 2 AA-I is an organic anion eliminated by the kidney
ph. Weak acids. A. Introduction
 ph. Weak acids. A. Introduction... 1 B. Weak acids: overview... 1 C. Weak acids: an example; finding K a... 2 D. Given K a, calculate ph... 3 E. A variety of weak acids... 5 F. So where do strong acids
ph. Weak acids. A. Introduction... 1 B. Weak acids: overview... 1 C. Weak acids: an example; finding K a... 2 D. Given K a, calculate ph... 3 E. A variety of weak acids... 5 F. So where do strong acids
Separation of Amino Acids by Paper Chromatography
 Separation of Amino Acids by Paper Chromatography Chromatography is a common technique for separating chemical substances. The prefix chroma, which suggests color, comes from the fact that some of the
Separation of Amino Acids by Paper Chromatography Chromatography is a common technique for separating chemical substances. The prefix chroma, which suggests color, comes from the fact that some of the
Quan%ta%ve proteomics. Maarten Altelaar, 2014
 Quan%ta%ve proteomics Maarten Altelaar, 2014 Proteomics Altelaar et al. Nat Rev Gen 14, 2013, 35-48 Quan%ta%ve proteomics Quan%ta%ve proteomics Control Diseased, s%mulated, Knock down, etc. How quan%ta%ve
Quan%ta%ve proteomics Maarten Altelaar, 2014 Proteomics Altelaar et al. Nat Rev Gen 14, 2013, 35-48 Quan%ta%ve proteomics Quan%ta%ve proteomics Control Diseased, s%mulated, Knock down, etc. How quan%ta%ve
ACID-BASE BALANCE AND ACID-BASE DISORDERS. I. Concept of Balance A. Determination of Acid-Base status 1. Specimens used - what they represent
 ACID-BASE BALANCE AND ACID-BASE DISORDERS I. Concept of Balance A. Determination of Acid-Base status 1. Specimens used - what they represent II. Electrolyte Composition of Body Fluids A. Extracellular
ACID-BASE BALANCE AND ACID-BASE DISORDERS I. Concept of Balance A. Determination of Acid-Base status 1. Specimens used - what they represent II. Electrolyte Composition of Body Fluids A. Extracellular
American Journal of Business Education January 2010 Volume 3, Number 1
 Improving Pedagogy Through The Use Of Dynamic Excel Presentations In Financial Management Courses George A. Mangiero, PhD, Iona College, USA John Manley, PhD, Iona College, USA J. T. Mollica, Boston University,
Improving Pedagogy Through The Use Of Dynamic Excel Presentations In Financial Management Courses George A. Mangiero, PhD, Iona College, USA John Manley, PhD, Iona College, USA J. T. Mollica, Boston University,
Reporting Low-level Analytical Data
 W. Horwitz, S. Afr. J. Chem., 2000, 53 (3), 206-212, , . [formerly: W. Horwitz, S. Afr. J. Chem.,
W. Horwitz, S. Afr. J. Chem., 2000, 53 (3), 206-212, , . [formerly: W. Horwitz, S. Afr. J. Chem.,
High Blood Pressure. Dr. Rath s Cellular Health Recommendations for Prevention and Adjunct Therapy
 4 High Blood Pressure Dr. Rath s Cellular Health Recommendations for Prevention and Adjunct Therapy The Facts About High Blood Pressure Dr. Rath s Cellular Health Recommendations: - Documented Health Benefits
4 High Blood Pressure Dr. Rath s Cellular Health Recommendations for Prevention and Adjunct Therapy The Facts About High Blood Pressure Dr. Rath s Cellular Health Recommendations: - Documented Health Benefits
3.4 BRØNSTED LOWRY ACIDS AND BASES
 96 CAPTER 3 ACIDS AND BASES. TE CURVED-ARROW NOTATION and that the unshared electron pair (and negative charge) is shared equally by the two terminal carbons. C L C A C 1 allyl anion (c) Using the curved-arrow
96 CAPTER 3 ACIDS AND BASES. TE CURVED-ARROW NOTATION and that the unshared electron pair (and negative charge) is shared equally by the two terminal carbons. C L C A C 1 allyl anion (c) Using the curved-arrow
Patients with COPD were recruited according to the inclusion criteria; aged 40-75, current or
 Online Data Supplement Materials and Methods The ECLIPSE Cohort Patients with COPD were recruited according to the inclusion criteria; aged 40-75, current or ex-smokers with >10 pack-year history; a post
Online Data Supplement Materials and Methods The ECLIPSE Cohort Patients with COPD were recruited according to the inclusion criteria; aged 40-75, current or ex-smokers with >10 pack-year history; a post
Chemical Name: Acetone CAS: 67-64-1 Synonyms: propanone, β-ketopropane, dimethyl ketone, dimethylformaldehyde, DMK, 2-propanone, propan-2-one
 2011 Health Risk Limits for Groundwater Health Risk Assessment Unit, Environmental Health Division 651-201-4899 651-201-5797 TDD Web Publication Date: March 21, 2011 Chemical Name: Acetone CAS: 67-64-1
2011 Health Risk Limits for Groundwater Health Risk Assessment Unit, Environmental Health Division 651-201-4899 651-201-5797 TDD Web Publication Date: March 21, 2011 Chemical Name: Acetone CAS: 67-64-1
LAB 7 MOSFET CHARACTERISTICS AND APPLICATIONS
 LAB 7 MOSFET CHARACTERISTICS AND APPLICATIONS Objective In this experiment you will study the i-v characteristics of an MOS transistor. You will use the MOSFET as a variable resistor and as a switch. BACKGROUND
LAB 7 MOSFET CHARACTERISTICS AND APPLICATIONS Objective In this experiment you will study the i-v characteristics of an MOS transistor. You will use the MOSFET as a variable resistor and as a switch. BACKGROUND
Chapter 6 Notes. Chemical Composition
 Chapter 6 Notes Chemical Composition Section 6.1: Counting By Weighing We can weigh a large number of the objects and find the average mass. Once we know the average mass we can equate that to any number
Chapter 6 Notes Chemical Composition Section 6.1: Counting By Weighing We can weigh a large number of the objects and find the average mass. Once we know the average mass we can equate that to any number
Reflection and Refraction
 Equipment Reflection and Refraction Acrylic block set, plane-concave-convex universal mirror, cork board, cork board stand, pins, flashlight, protractor, ruler, mirror worksheet, rectangular block worksheet,
Equipment Reflection and Refraction Acrylic block set, plane-concave-convex universal mirror, cork board, cork board stand, pins, flashlight, protractor, ruler, mirror worksheet, rectangular block worksheet,
1333 Plaza Blvd, Suite E, Central Point, OR 97502 * www.mountainviewvet.net
 1333 Plaza Blvd, Suite E, Central Point, OR 97502 * www.mountainviewvet.net Diabetes Mellitus (in cats) Diabetes, sugar Affected Animals: Most diabetic cats are older than 10 years of age when they are
1333 Plaza Blvd, Suite E, Central Point, OR 97502 * www.mountainviewvet.net Diabetes Mellitus (in cats) Diabetes, sugar Affected Animals: Most diabetic cats are older than 10 years of age when they are
II. DISTRIBUTIONS distribution normal distribution. standard scores
 Appendix D Basic Measurement And Statistics The following information was developed by Steven Rothke, PhD, Department of Psychology, Rehabilitation Institute of Chicago (RIC) and expanded by Mary F. Schmidt,
Appendix D Basic Measurement And Statistics The following information was developed by Steven Rothke, PhD, Department of Psychology, Rehabilitation Institute of Chicago (RIC) and expanded by Mary F. Schmidt,
Introduction, Noncovalent Bonds, and Properties of Water
 Lecture 1 Introduction, Noncovalent Bonds, and Properties of Water Reading: Berg, Tymoczko & Stryer: Chapter 1 problems in textbook: chapter 1, pp. 23-24, #1,2,3,6,7,8,9, 10,11; practice problems at end
Lecture 1 Introduction, Noncovalent Bonds, and Properties of Water Reading: Berg, Tymoczko & Stryer: Chapter 1 problems in textbook: chapter 1, pp. 23-24, #1,2,3,6,7,8,9, 10,11; practice problems at end
VITAMIN C AND INFECTIOUS DISEASE: A REVIEW OF THE LITERATURE AND THE RESULTS OF A RANDOMIZED, DOUBLE-BLIND, PROSPECTIVE STUDY OVER 8 YEARS
 39 Chapter 3 VITAMIN C AND INFECTIOUS DISEASE: A REVIEW OF THE LITERATURE AND THE RESULTS OF A RANDOMIZED, DOUBLE-BLIND, PROSPECTIVE STUDY OVER 8 YEARS Maxine Briggs TABLE OF CONTENTS I. Review of the
39 Chapter 3 VITAMIN C AND INFECTIOUS DISEASE: A REVIEW OF THE LITERATURE AND THE RESULTS OF A RANDOMIZED, DOUBLE-BLIND, PROSPECTIVE STUDY OVER 8 YEARS Maxine Briggs TABLE OF CONTENTS I. Review of the
Teriflunomide is the active metabolite of Leflunomide, a drug employed since 1994 for the treatment of rheumatoid arthritis (Baselt, 2011).
 Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Review of Fundamental Mathematics
 Review of Fundamental Mathematics As explained in the Preface and in Chapter 1 of your textbook, managerial economics applies microeconomic theory to business decision making. The decision-making tools
Review of Fundamental Mathematics As explained in the Preface and in Chapter 1 of your textbook, managerial economics applies microeconomic theory to business decision making. The decision-making tools
Answer Key to Problem Set #2: Expected Value and Insurance
 Answer Key to Problem Set #2: Expected Value and Insurance 1. (a) We have u (w) = 1 2 w 1 2, so u (w) = 1 4 w 3 2. As we will see below, u (w) < 0 indicates that the individual is risk-averse. (b) The
Answer Key to Problem Set #2: Expected Value and Insurance 1. (a) We have u (w) = 1 2 w 1 2, so u (w) = 1 4 w 3 2. As we will see below, u (w) < 0 indicates that the individual is risk-averse. (b) The
Diagrams and Graphs of Statistical Data
 Diagrams and Graphs of Statistical Data One of the most effective and interesting alternative way in which a statistical data may be presented is through diagrams and graphs. There are several ways in
Diagrams and Graphs of Statistical Data One of the most effective and interesting alternative way in which a statistical data may be presented is through diagrams and graphs. There are several ways in
AP CHEMISTRY 2007 SCORING GUIDELINES. Question 6
 AP CHEMISTRY 2007 SCORING GUIDELINES Question 6 Answer the following questions, which pertain to binary compounds. (a) In the box provided below, draw a complete Lewis electron-dot diagram for the IF 3
AP CHEMISTRY 2007 SCORING GUIDELINES Question 6 Answer the following questions, which pertain to binary compounds. (a) In the box provided below, draw a complete Lewis electron-dot diagram for the IF 3
WM2012 Conference, February 26 March 1, 2012, Phoenix, Arizona, USA
 ABSTRACT Comparison of Activity Determination of Radium 226 in FUSRAP Soil using Various Energy Lines - 12299 Brian Tucker*, Jough Donakowski**, David Hays*** *Shaw Environmental & Infrastructure, Stoughton,
ABSTRACT Comparison of Activity Determination of Radium 226 in FUSRAP Soil using Various Energy Lines - 12299 Brian Tucker*, Jough Donakowski**, David Hays*** *Shaw Environmental & Infrastructure, Stoughton,
CH 10 - REVIEW QUESTIONS
 CH 10 - REVIEW QUESTIONS 1. The short-run aggregate supply curve is horizontal at: A) a level of output determined by aggregate demand. B) the natural level of output. C) the level of output at which the
CH 10 - REVIEW QUESTIONS 1. The short-run aggregate supply curve is horizontal at: A) a level of output determined by aggregate demand. B) the natural level of output. C) the level of output at which the
Ethyl Glucuronide. Where is the Drug? Detection Windows. Urine. Blood. Absorption and Distribution. Metabolism. Excretion
 Where is the Drug? Ethyl Absorption and Distribution A Biomarker for Ethanol Use Metabolism Excretion Anthony G. Costantino, Ph.D. D-ABFT NMS Labs,Willow Grove, PA John J. Treuting, Ph.D. Treuting & Assoc.
Where is the Drug? Ethyl Absorption and Distribution A Biomarker for Ethanol Use Metabolism Excretion Anthony G. Costantino, Ph.D. D-ABFT NMS Labs,Willow Grove, PA John J. Treuting, Ph.D. Treuting & Assoc.
ph: Measurement and Uses
 ph: Measurement and Uses One of the most important properties of aqueous solutions is the concentration of hydrogen ion. The concentration of H + (or H 3 O + ) affects the solubility of inorganic and organic
ph: Measurement and Uses One of the most important properties of aqueous solutions is the concentration of hydrogen ion. The concentration of H + (or H 3 O + ) affects the solubility of inorganic and organic
Measuring Unilateral Market Power in Wholesale Electricity Markets: The California Market, 1998 2000
 Measuring Unilateral Market Power in Wholesale Electricity Markets: The California Market, 1998 2000 By FRANK A. WOLAK* * Department of Economics, Stanford University, Stanford, CA 94305-6072, and NBER
Measuring Unilateral Market Power in Wholesale Electricity Markets: The California Market, 1998 2000 By FRANK A. WOLAK* * Department of Economics, Stanford University, Stanford, CA 94305-6072, and NBER
Amino Acid Metabolism (Chapter 20) Lecture 8:
 Amino Acid Metabolism (Chapter 20) Lecture 8: Nitrogen Fixation (20.7); Nitrite Assimilation (not in text?); Protein Digestion in the Gut (5.3b, 11.5, 20.2); Amino Acid Degradation in Cells (20.2); Next:
Amino Acid Metabolism (Chapter 20) Lecture 8: Nitrogen Fixation (20.7); Nitrite Assimilation (not in text?); Protein Digestion in the Gut (5.3b, 11.5, 20.2); Amino Acid Degradation in Cells (20.2); Next:
DIGITAL FOREX OPTIONS
 DIGITAL FOREX OPTIONS OPENGAMMA QUANTITATIVE RESEARCH Abstract. Some pricing methods for forex digital options are described. The price in the Garhman-Kohlhagen model is first described, more for completeness
DIGITAL FOREX OPTIONS OPENGAMMA QUANTITATIVE RESEARCH Abstract. Some pricing methods for forex digital options are described. The price in the Garhman-Kohlhagen model is first described, more for completeness
TOTAL PROTEIN FIBRINOGEN
 UNIT: Proteins 16tproteins.wpd Task Determination of Total Protein, Albumin and Globulins Objectives Upon completion of this exercise, the student will be able to: 1. Explain the ratio of albumin and globulin
UNIT: Proteins 16tproteins.wpd Task Determination of Total Protein, Albumin and Globulins Objectives Upon completion of this exercise, the student will be able to: 1. Explain the ratio of albumin and globulin
CHI-SQUARE: TESTING FOR GOODNESS OF FIT
 CHI-SQUARE: TESTING FOR GOODNESS OF FIT In the previous chapter we discussed procedures for fitting a hypothesized function to a set of experimental data points. Such procedures involve minimizing a quantity
CHI-SQUARE: TESTING FOR GOODNESS OF FIT In the previous chapter we discussed procedures for fitting a hypothesized function to a set of experimental data points. Such procedures involve minimizing a quantity
Course Specification (Master in Urology/Physiology)
 Course Specification (Master in Urology/Physiology) ١ University/ Academy: Al-Azhar University Faculty/ Institute: Faculty of Medicine (Assuit) Department: physiology Course Specification (Master in Urology/Physiology)
Course Specification (Master in Urology/Physiology) ١ University/ Academy: Al-Azhar University Faculty/ Institute: Faculty of Medicine (Assuit) Department: physiology Course Specification (Master in Urology/Physiology)
DESCRIPTIVE STATISTICS. The purpose of statistics is to condense raw data to make it easier to answer specific questions; test hypotheses.
 DESCRIPTIVE STATISTICS The purpose of statistics is to condense raw data to make it easier to answer specific questions; test hypotheses. DESCRIPTIVE VS. INFERENTIAL STATISTICS Descriptive To organize,
DESCRIPTIVE STATISTICS The purpose of statistics is to condense raw data to make it easier to answer specific questions; test hypotheses. DESCRIPTIVE VS. INFERENTIAL STATISTICS Descriptive To organize,
Experiment 10. Radioactive Decay of 220 Rn and 232 Th Physics 2150 Experiment No. 10 University of Colorado
 1 Radioactive Decay of 220 Rn and 232 Th Physics 2150 Experiment No. 10 University of Colorado Introduction Some radioactive isotopes formed billions of years ago have half- lives so long that they are
1 Radioactive Decay of 220 Rn and 232 Th Physics 2150 Experiment No. 10 University of Colorado Introduction Some radioactive isotopes formed billions of years ago have half- lives so long that they are
SpikeTides TM Peptides for relative and absolute quantification in SRM and MRM Assays
 Protocol SpikeTides TM Peptides for relative and absolute quantification in SRM and MRM Assays Contact us: InfoLine: +49-30-6392-7878 Order per fax: +49-30-6392-7888 or e-mail: www: peptide@jpt.com www.jpt.com
Protocol SpikeTides TM Peptides for relative and absolute quantification in SRM and MRM Assays Contact us: InfoLine: +49-30-6392-7878 Order per fax: +49-30-6392-7888 or e-mail: www: peptide@jpt.com www.jpt.com
Chapter 1: Chemistry: Measurements and Methods
 Chapter 1: Chemistry: Measurements and Methods 1.1 The Discovery Process o Chemistry - The study of matter o Matter - Anything that has mass and occupies space, the stuff that things are made of. This
Chapter 1: Chemistry: Measurements and Methods 1.1 The Discovery Process o Chemistry - The study of matter o Matter - Anything that has mass and occupies space, the stuff that things are made of. This
To Cope with Stress? DEMONS of Traders DEMONS. The Mysteries of the Trend Using the Opening Price. The Thought
 ASIA PACIFIC S PREEMINENT TRADING MAGAZINE ASIA PACIFIC S PREEMINENT TRADING MAGAZINE November / December 2005 www.traders-journal.com MICA (P) 352/05/2005 The Mysteries of the Trend Using the Opening
ASIA PACIFIC S PREEMINENT TRADING MAGAZINE ASIA PACIFIC S PREEMINENT TRADING MAGAZINE November / December 2005 www.traders-journal.com MICA (P) 352/05/2005 The Mysteries of the Trend Using the Opening
MultiQuant Software 2.0 for Targeted Protein / Peptide Quantification
 MultiQuant Software 2.0 for Targeted Protein / Peptide Quantification Gold Standard for Quantitative Data Processing Because of the sensitivity, selectivity, speed and throughput at which MRM assays can
MultiQuant Software 2.0 for Targeted Protein / Peptide Quantification Gold Standard for Quantitative Data Processing Because of the sensitivity, selectivity, speed and throughput at which MRM assays can
Calcium (serum, plasma, blood)
 Calcium (serum, plasma, blood) 1 Name and description of analyte 1.1 Name of analyte Calcium (total in serum, plasma, ionised in blood (see 2.1 (2)). 1.2 Alternative names None 1.3 NMLC code 1.4 Description
Calcium (serum, plasma, blood) 1 Name and description of analyte 1.1 Name of analyte Calcium (total in serum, plasma, ionised in blood (see 2.1 (2)). 1.2 Alternative names None 1.3 NMLC code 1.4 Description
2. Simple Linear Regression
 Research methods - II 3 2. Simple Linear Regression Simple linear regression is a technique in parametric statistics that is commonly used for analyzing mean response of a variable Y which changes according
Research methods - II 3 2. Simple Linear Regression Simple linear regression is a technique in parametric statistics that is commonly used for analyzing mean response of a variable Y which changes according
It has been contended that it would be possible for a socialist economy to solve
 THE EQUATIONS OF MATHEMATICAL ECONOMICS AND THE PROBLEM OF ECONOMIC CALCULATION IN A SOCIALIST STATE LUDWIG VON MISES I It has been contended that it would be possible for a socialist economy to solve
THE EQUATIONS OF MATHEMATICAL ECONOMICS AND THE PROBLEM OF ECONOMIC CALCULATION IN A SOCIALIST STATE LUDWIG VON MISES I It has been contended that it would be possible for a socialist economy to solve
Preventing Catheter Blockages: A Guide for Health Professionals
 Introduction Preventing Catheter Blockages: A Guide for Health Professionals and Long term catheterisation is common for someone with a spinal cord injury (SCI). However, it poses a concern as this method
Introduction Preventing Catheter Blockages: A Guide for Health Professionals and Long term catheterisation is common for someone with a spinal cord injury (SCI). However, it poses a concern as this method
Risk Assessment in Chemical Food Safety. Dept. of Food Safety and Zoonoses (FOS) http://www.who.int/foodsafety/en/
 Risk Assessment in Chemical Food Safety Dept. of Food Safety and Zoonoses (FOS) http://www.who.int/foodsafety/en/ Risk Analysis Paradigm Internationally Scientific data analysis Risk Assessment WHO & FAO
Risk Assessment in Chemical Food Safety Dept. of Food Safety and Zoonoses (FOS) http://www.who.int/foodsafety/en/ Risk Analysis Paradigm Internationally Scientific data analysis Risk Assessment WHO & FAO
Statistics I for QBIC. Contents and Objectives. Chapters 1 7. Revised: August 2013
 Statistics I for QBIC Text Book: Biostatistics, 10 th edition, by Daniel & Cross Contents and Objectives Chapters 1 7 Revised: August 2013 Chapter 1: Nature of Statistics (sections 1.1-1.6) Objectives
Statistics I for QBIC Text Book: Biostatistics, 10 th edition, by Daniel & Cross Contents and Objectives Chapters 1 7 Revised: August 2013 Chapter 1: Nature of Statistics (sections 1.1-1.6) Objectives
A Greener Synthesis of Creatine
 A Greener Synthesis of Creatine Carl S Lecher 1 and Ryan J Bernhardt, 2 Marian College, Indianapolis, I Chemical Concepts Addition to nitriles, vacuum filtration, melting point determination Green Lessons
A Greener Synthesis of Creatine Carl S Lecher 1 and Ryan J Bernhardt, 2 Marian College, Indianapolis, I Chemical Concepts Addition to nitriles, vacuum filtration, melting point determination Green Lessons
EXPERIMENT 5. Molecular Absorption Spectroscopy: Determination of Iron With 1,10-Phenanthroline
 EXPERIMENT 5 Molecular Absorption Spectroscopy: Determination of Iron With 1,10-Phenanthroline UNKNOWN Submit a clean, labeled 100-mL volumetric flask to the instructor so that your unknown iron solution
EXPERIMENT 5 Molecular Absorption Spectroscopy: Determination of Iron With 1,10-Phenanthroline UNKNOWN Submit a clean, labeled 100-mL volumetric flask to the instructor so that your unknown iron solution
Lactic Acid Dehydrogenase
 Lactic Acid Dehydrogenase Pyruvic Acid Dehydrogenase Complex Pyruvate to ACETYL coa CC CoA + CO 2 Mitochondria 3 carbon Pyruvate to 2 carbon ACETYL Coenzyme A Pyruvate Acetyl CoA + CO 2 + NADH + H + CO2
Lactic Acid Dehydrogenase Pyruvic Acid Dehydrogenase Complex Pyruvate to ACETYL coa CC CoA + CO 2 Mitochondria 3 carbon Pyruvate to 2 carbon ACETYL Coenzyme A Pyruvate Acetyl CoA + CO 2 + NADH + H + CO2
ACID-BASE TITRATIONS: DETERMINATION OF CARBONATE BY TITRATION WITH HYDROCHLORIC ACID BACKGROUND
 #3. Acid - Base Titrations 27 EXPERIMENT 3. ACID-BASE TITRATIONS: DETERMINATION OF CARBONATE BY TITRATION WITH HYDROCHLORIC ACID BACKGROUND Carbonate Equilibria In this experiment a solution of hydrochloric
#3. Acid - Base Titrations 27 EXPERIMENT 3. ACID-BASE TITRATIONS: DETERMINATION OF CARBONATE BY TITRATION WITH HYDROCHLORIC ACID BACKGROUND Carbonate Equilibria In this experiment a solution of hydrochloric
ANNEX 2: Assessment of the 7 points agreed by WATCH as meriting attention (cover paper, paragraph 9, bullet points) by Andy Darnton, HSE
 ANNEX 2: Assessment of the 7 points agreed by WATCH as meriting attention (cover paper, paragraph 9, bullet points) by Andy Darnton, HSE The 7 issues to be addressed outlined in paragraph 9 of the cover
ANNEX 2: Assessment of the 7 points agreed by WATCH as meriting attention (cover paper, paragraph 9, bullet points) by Andy Darnton, HSE The 7 issues to be addressed outlined in paragraph 9 of the cover
Impact of Genetic Testing on Life Insurance
 Agenda, Volume 10, Number 1, 2003, pages 61-72 Impact of Genetic Testing on Life Insurance he Human Genome project generates immense interest in the scientific community though there are also important
Agenda, Volume 10, Number 1, 2003, pages 61-72 Impact of Genetic Testing on Life Insurance he Human Genome project generates immense interest in the scientific community though there are also important
Radiation Sciences Nuclear Medicine Technology Program Hot Lab Log Book (Material)
 Radiation Sciences Nuclear Medicine Technology Program Hot Lab Log Book (Material) Table of Content Receiving a Radioactive Package Constancy Test Daily Survey Weekly Wipes Accuracy Test Linearity Geometric
Radiation Sciences Nuclear Medicine Technology Program Hot Lab Log Book (Material) Table of Content Receiving a Radioactive Package Constancy Test Daily Survey Weekly Wipes Accuracy Test Linearity Geometric
BEC Feed Solutions. Steve Blake BEC Feed Solutions
 BEC Feed Solutions Presenter: Steve Blake BEC Feed Solutions Nutritional Role of Phosphorus Phosphorus (P) is present in all cells in the body Essential for many digestive and metabolic processes, including
BEC Feed Solutions Presenter: Steve Blake BEC Feed Solutions Nutritional Role of Phosphorus Phosphorus (P) is present in all cells in the body Essential for many digestive and metabolic processes, including
Comment of Consumer Reports Regarding Draft Guidance for Industry: Arsenic In Apple Juice Action Level Docket No. FDA-2012-D-0322
 November 12, 2013 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane Room 1061 Rockville, MD 20852. Comment of Consumer Reports Regarding Draft Guidance for Industry:
November 12, 2013 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane Room 1061 Rockville, MD 20852. Comment of Consumer Reports Regarding Draft Guidance for Industry:
Chemical Bonds and Groups - Part 1
 hemical Bonds and Groups - Part 1 ARB SKELETS arbon has a unique role in the cell because of its ability to form strong covalent bonds with other carbon atoms. Thus carbon atoms can join to form chains.
hemical Bonds and Groups - Part 1 ARB SKELETS arbon has a unique role in the cell because of its ability to form strong covalent bonds with other carbon atoms. Thus carbon atoms can join to form chains.
Recall this chart that showed how most of our course would be organized:
 Chapter 4 One-Way ANOVA Recall this chart that showed how most of our course would be organized: Explanatory Variable(s) Response Variable Methods Categorical Categorical Contingency Tables Categorical
Chapter 4 One-Way ANOVA Recall this chart that showed how most of our course would be organized: Explanatory Variable(s) Response Variable Methods Categorical Categorical Contingency Tables Categorical
Liver Function Tests. Dr Stephen Butler Paediatric Advance Trainee TDHB
 Liver Function Tests Dr Stephen Butler Paediatric Advance Trainee TDHB Introduction Case presentation What is the liver? Overview of tests used to measure liver function RJ 10 month old European girl
Liver Function Tests Dr Stephen Butler Paediatric Advance Trainee TDHB Introduction Case presentation What is the liver? Overview of tests used to measure liver function RJ 10 month old European girl
What Do We Learn about Hepatotoxicity Using Coumarin-Treated Rat Model?
 What Do We Learn about Hepatotoxicity Using Coumarin-Treated Rat Model? authors M. David Ho 1, Bob Xiong 1, S. Stellar 2, J. Proctor 2, J. Silva 2, H.K. Lim 2, Patrick Bennett 1, and Lily Li 1 Tandem Labs,
What Do We Learn about Hepatotoxicity Using Coumarin-Treated Rat Model? authors M. David Ho 1, Bob Xiong 1, S. Stellar 2, J. Proctor 2, J. Silva 2, H.K. Lim 2, Patrick Bennett 1, and Lily Li 1 Tandem Labs,
PHAR 7633 Chapter 19 Multi-Compartment Pharmacokinetic Models
 Student Objectives for this Chapter PHAR 7633 Chapter 19 Multi-Compartment Pharmacokinetic Models To draw the scheme and write the differential equations appropriate to a multi-compartment pharmacokinetic
Student Objectives for this Chapter PHAR 7633 Chapter 19 Multi-Compartment Pharmacokinetic Models To draw the scheme and write the differential equations appropriate to a multi-compartment pharmacokinetic
Supplementation of Wheat Gluten Protein1
 Supplementation of Wheat Gluten Protein1 W. L. BANKS, JR.," J. B. ALLISON AND R. W. WANNEMACHER, JR. Bureau of Biological Research, Rutgers â The State University, New Brunswick, New Jersey ABSTRACT Male
Supplementation of Wheat Gluten Protein1 W. L. BANKS, JR.," J. B. ALLISON AND R. W. WANNEMACHER, JR. Bureau of Biological Research, Rutgers â The State University, New Brunswick, New Jersey ABSTRACT Male
This unit will lay the groundwork for later units where the students will extend this knowledge to quadratic and exponential functions.
 Algebra I Overview View unit yearlong overview here Many of the concepts presented in Algebra I are progressions of concepts that were introduced in grades 6 through 8. The content presented in this course
Algebra I Overview View unit yearlong overview here Many of the concepts presented in Algebra I are progressions of concepts that were introduced in grades 6 through 8. The content presented in this course
Chapter 1 The Atomic Nature of Matter
 Chapter 1 The Atomic Nature of Matter 6. Substances that cannot be decomposed into two or more simpler substances by chemical means are called a. pure substances. b. compounds. c. molecules. d. elements.
Chapter 1 The Atomic Nature of Matter 6. Substances that cannot be decomposed into two or more simpler substances by chemical means are called a. pure substances. b. compounds. c. molecules. d. elements.
Chapter 2: Linear Equations and Inequalities Lecture notes Math 1010
 Section 2.1: Linear Equations Definition of equation An equation is a statement that equates two algebraic expressions. Solving an equation involving a variable means finding all values of the variable
Section 2.1: Linear Equations Definition of equation An equation is a statement that equates two algebraic expressions. Solving an equation involving a variable means finding all values of the variable
Oral Zinc Supplementation as an Adjunct Therapy in the Management of Hepatic Encephalopathy: A Randomized Controlled Trial
 Oral Zinc Supplementation as an Adjunct Therapy in the Management of Hepatic Encephalopathy: A Randomized Controlled Trial Marcus R. Pereira A. Study Purpose Hepatic encephalopathy is a common complication
Oral Zinc Supplementation as an Adjunct Therapy in the Management of Hepatic Encephalopathy: A Randomized Controlled Trial Marcus R. Pereira A. Study Purpose Hepatic encephalopathy is a common complication
UNIVERSITY OF BIRMINGHAM BIRMINGHAM BUSINESS SCHOOL. GUIDELINES FOR PREPARING A PhD RESEARCH PROPOSAL
 UNIVERSITY OF BIRMINGHAM BIRMINGHAM BUSINESS SCHOOL GUIDELINES FOR PREPARING A PhD RESEARCH PROPOSAL PhD degrees are by research only, with candidates completing their work under the personal supervision
UNIVERSITY OF BIRMINGHAM BIRMINGHAM BUSINESS SCHOOL GUIDELINES FOR PREPARING A PhD RESEARCH PROPOSAL PhD degrees are by research only, with candidates completing their work under the personal supervision
Mass Spectrometry. Overview
 Mass Spectrometry Overview Mass Spectrometry is an analytic technique that utilizes the degree of deflection of charged particles by a magnetic field to find the relative masses of molecular ions and fragments.2
Mass Spectrometry Overview Mass Spectrometry is an analytic technique that utilizes the degree of deflection of charged particles by a magnetic field to find the relative masses of molecular ions and fragments.2
Supply and Demand Fundamental tool of economic analysis Used to discuss unemployment, value of $, protection of the environment, etc.
 Supply and emand Fundamental tool of economic analysis Used to discuss unemployment, value of $, protection of the environment, etc. Chapter Outline: (a) emand is the consumer side of the market. (b) Supply
Supply and emand Fundamental tool of economic analysis Used to discuss unemployment, value of $, protection of the environment, etc. Chapter Outline: (a) emand is the consumer side of the market. (b) Supply
Anti-Gaming in the OnePipe Optimal Liquidity Network
 1 Anti-Gaming in the OnePipe Optimal Liquidity Network Pragma Financial Systems I. INTRODUCTION With careful estimates 1 suggesting that trading in so-called dark pools now constitutes more than 7% of
1 Anti-Gaming in the OnePipe Optimal Liquidity Network Pragma Financial Systems I. INTRODUCTION With careful estimates 1 suggesting that trading in so-called dark pools now constitutes more than 7% of
The commercial culture of -citrus in most soils of California requires
 NITROGEN IN RELATION TO THE GROWTH OF CITRUS CUTTINGS IN SOLUTION CULTURES A. R. C. HA AS (WITH FIVE FIGURES) The commercial culture of -citrus in most soils of California requires some form of nitrogen
NITROGEN IN RELATION TO THE GROWTH OF CITRUS CUTTINGS IN SOLUTION CULTURES A. R. C. HA AS (WITH FIVE FIGURES) The commercial culture of -citrus in most soils of California requires some form of nitrogen
How To Understand The Human Body
 Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Continuous Cooling Transformation (CCT) Diagrams
 Continuous Cooling Transformation (CCT) Diagrams R. Manna Assistant Professor Centre of Advanced Study Department of Metallurgical Engineering Institute of Technology, Banaras Hindu University Varanasi-221
Continuous Cooling Transformation (CCT) Diagrams R. Manna Assistant Professor Centre of Advanced Study Department of Metallurgical Engineering Institute of Technology, Banaras Hindu University Varanasi-221
THE BODY TEMPERATURE OF THE FROG
 55 THE BODY TEMPERATURE OF THE FROG BY KENNETH MELLANBY 1 The University of Sheffield (Received 25 October 1940) (With Four Text-figures) INTRODUCTION IT is well known that poikilothermous animals may
55 THE BODY TEMPERATURE OF THE FROG BY KENNETH MELLANBY 1 The University of Sheffield (Received 25 October 1940) (With Four Text-figures) INTRODUCTION IT is well known that poikilothermous animals may
Chapter 16 The Citric Acid Cycle
 Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Production of acetyl-coa (activated acetate) Page: 603 Difficulty: 2 Ans: A Which of the following is not true of the reaction catalyzed by
Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Production of acetyl-coa (activated acetate) Page: 603 Difficulty: 2 Ans: A Which of the following is not true of the reaction catalyzed by
Copyright 2000-2003 Mark Brandt, Ph.D. 35
 Amino acid breakdown Amino acids comprise one of the three major energy sources for animals. They are an especially important energy source for carnivorous animals, and for all animals during early starvation
Amino acid breakdown Amino acids comprise one of the three major energy sources for animals. They are an especially important energy source for carnivorous animals, and for all animals during early starvation
A Comparison of an HPGe-based and NaI-based Radionuclide Identifier (RID) for Radioactive Materials
 Illicit Trafficking, Sub/cross-national threats, Poster presentation A Comparison of an HPGe-based and NaI-based Radionuclide Identifier (RID) for Radioactive Materials Ronald M. Keyser, Timothy R. Twomey,
Illicit Trafficking, Sub/cross-national threats, Poster presentation A Comparison of an HPGe-based and NaI-based Radionuclide Identifier (RID) for Radioactive Materials Ronald M. Keyser, Timothy R. Twomey,
Acids and Bases. but we will use the term Lewis acid to denote only those acids to which a bond can be made without breaking another bond
 Acids and Bases. Brønsted acids are proton donors, and Brønsted bases are proton acceptors. Examples of Brønsted acids: HCl, HBr, H 2 SO 4, HOH, H 3 O +, + NH 4, NH 3, CH 3 CO 2 H, H CH 2 COCH 3, H C CH,
Acids and Bases. Brønsted acids are proton donors, and Brønsted bases are proton acceptors. Examples of Brønsted acids: HCl, HBr, H 2 SO 4, HOH, H 3 O +, + NH 4, NH 3, CH 3 CO 2 H, H CH 2 COCH 3, H C CH,
Course Curriculum for Master Degree in Food Science and Technology/ Department of Nutrition and Food Technology
 Course Curriculum for Master Degree in Food Science and Technology/ Department of Nutrition and Food Technology The Master Degree in Food Science and Technology / Department of Nutrition and Food Technology,
Course Curriculum for Master Degree in Food Science and Technology/ Department of Nutrition and Food Technology The Master Degree in Food Science and Technology / Department of Nutrition and Food Technology,
How to Win the Stock Market Game
 How to Win the Stock Market Game 1 Developing Short-Term Stock Trading Strategies by Vladimir Daragan PART 1 Table of Contents 1. Introduction 2. Comparison of trading strategies 3. Return per trade 4.
How to Win the Stock Market Game 1 Developing Short-Term Stock Trading Strategies by Vladimir Daragan PART 1 Table of Contents 1. Introduction 2. Comparison of trading strategies 3. Return per trade 4.
What is the purpose of this document? What is in the document? How do I send Feedback?
 This document is designed to help North Carolina educators teach the Common Core (Standard Course of Study). NCDPI staff are continually updating and improving these tools to better serve teachers. Statistics
This document is designed to help North Carolina educators teach the Common Core (Standard Course of Study). NCDPI staff are continually updating and improving these tools to better serve teachers. Statistics
Lab 2 Biochemistry. Learning Objectives. Introduction. Lipid Structure and Role in Food. The lab has the following learning objectives.
 1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
Econ 102 Aggregate Supply and Demand
 Econ 102 ggregate Supply and Demand 1. s on previous homework assignments, turn in a news article together with your summary and explanation of why it is relevant to this week s topic, ggregate Supply
Econ 102 ggregate Supply and Demand 1. s on previous homework assignments, turn in a news article together with your summary and explanation of why it is relevant to this week s topic, ggregate Supply
3. Data Analysis, Statistics, and Probability
 3. Data Analysis, Statistics, and Probability Data and probability sense provides students with tools to understand information and uncertainty. Students ask questions and gather and use data to answer
3. Data Analysis, Statistics, and Probability Data and probability sense provides students with tools to understand information and uncertainty. Students ask questions and gather and use data to answer
Ammonia (plasma, blood)
 Ammonia (plasma, blood) 1 Name and description of analyte 1.1 Name of analyte Ammonia 1.2 Alternative names None 1.3 NLMC code 1.4 Description of analyte Ammonia has the formula NH 3. At physiological
Ammonia (plasma, blood) 1 Name and description of analyte 1.1 Name of analyte Ammonia 1.2 Alternative names None 1.3 NLMC code 1.4 Description of analyte Ammonia has the formula NH 3. At physiological
method has been used for all uric acid determinations in the Medical
 THE BLOOD URIC ACID IN DISEASE By EDWIN P. JORDAN AND DOROTHY GASTON (From the Department of Medicine of the University of Chicago, Chicago) (Received for publication March 28, 1932) Because of the large
THE BLOOD URIC ACID IN DISEASE By EDWIN P. JORDAN AND DOROTHY GASTON (From the Department of Medicine of the University of Chicago, Chicago) (Received for publication March 28, 1932) Because of the large
Guidance for Industry
 Guidance for Industry Q2B Validation of Analytical Procedures: Methodology November 1996 ICH Guidance for Industry Q2B Validation of Analytical Procedures: Methodology Additional copies are available from:
Guidance for Industry Q2B Validation of Analytical Procedures: Methodology November 1996 ICH Guidance for Industry Q2B Validation of Analytical Procedures: Methodology Additional copies are available from:
URINARY (RENAL) STONE (NEPHROLITHOISIS) An Overview
 URINARY (RENAL) STONE (NEPHROLITHOISIS) An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY PLB MBBS
URINARY (RENAL) STONE (NEPHROLITHOISIS) An Overview UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY PLB MBBS
Reflections A PAPER IN A SERIES COMMISSIONED TO CELEBRATE THE CENTENARY OF THE JBC IN 2005
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 277, No. 37, Issue of September 13, pp. 33531 33536, 2002 2002 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Reflections
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 277, No. 37, Issue of September 13, pp. 33531 33536, 2002 2002 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Reflections
A Short review of steel demand forecasting methods
 A Short review of steel demand forecasting methods Fujio John M. Tanaka This paper undertakes the present and past review of steel demand forecasting to study what methods should be used in any future
A Short review of steel demand forecasting methods Fujio John M. Tanaka This paper undertakes the present and past review of steel demand forecasting to study what methods should be used in any future
