The Drosophila Dpit47 protein is a nuclear Hsp90 cochaperone that interacts with DNA polymerase α
|
|
|
- Ashlie Roberts
- 8 years ago
- Views:
Transcription
1 RESEARCH ARTICLE 2015 The Drosophila Dpit47 protein is a nuclear Hsp90 cochaperone that interacts with DNA polymerase α Gilles Crevel, Helen Bates, Hella Huikeshoven and Sue Cotterill* Department of Biochemistry and Immunology, St Georges Hospital Medical School, Cranmer Terrace, London, SW17 0RE, UK These authors contributed equally to this paper *Author for correspondence ( s.cotterill@sghms.ac.uk) Accepted 10 March 2001 Journal of Cell Science 114, (2001) The Company of Biologists Ltd SUMMARY Hsp90 is gaining increasing importance as a protein involved in controlling the normal functioning of the cell. To do this it apparently interacts with a battery of cochaperone proteins that are involved in both substrate recognition and the progression of the Hsp90 catalytic pathway. In this report we have identified the Drosophila Dpit47 protein (DNA polymerase interacting tpr containing protein of 47 kda) through its interaction with the DNA polymerase α. This protein is a predominantly nuclear protein, which forms a tight and stoichiometric interaction with Hsp90 and shows interaction with Hsp70. It also has substantial homology to other known Hsp90 cochaperones, e.g. CNS1 and hop1, making it likely that this protein also functions as an Hsp90 co-chaperone. The interaction with the DNA polymerase α is not related to the special situation in early embryos where there are large amounts of maternal protein stockpiles of the polymerase, as it occurs to the same level in early and late embryos and also in proliferating cell culture. However, it does not occur in quiescent cells, making it likely that the protein is related to proliferation. This is also consistent with Dpit47 expression being higher in proliferating cells. The interaction between the Dpit47 and the polymerase takes place predominantly in the nucleoplasm, and seems to involve several subunits of the polymerase in comparable amounts, making it unlikely that it is solely required for the assembly of the polymerase complex. The polymerase can also be seen to interact with Hsp90, and the interaction between Dpit47 and the polymerase is increased by the specific Hsp90 inhibitor geldanamycin. This suggests that a complex of the Dpit47, Hsp90 and DNA polymerase exists in the cell. The interaction between DNA polymerase α and Dpit47 completely inhibits the activity of the polymerase. These results suggest that Hsp90 acts as a chaperone for DNA polymerase α and that this interaction is mediated through the novel co-chaperone Dpit47. This provides the first suggestion of a role for chaperones in DNA replication in higher eukaryotes. Key words: Replication, Heat shock proteins, DNA polymerase, Drosophila, Chaperone INTRODUCTION Heat shock proteins have traditionally been studied as factors that help proteins recover from high temperature or other stresses (Parsell and Lindquist, 1993). Hsp90 was first isolated by this route but in the last few years it has become apparent that this particular heat shock protein may have a more specialised role, and may be involved in controlling normal cellular functioning (in the absence of stress) of a variety of different proteins. The best studied of these is the glucocorticoid receptor, but Hsp90 activity has also been implicated in the control of a number of other proteins including a number of kinases (e.g. src; Buchner, 1999; Xu et al., 1999), cyclin D (Mahony et al., 1998) and telomerase (Holt et al., 1999). The effect of the Hsp90:substrate interaction varies; e.g. for src it is needed to direct phosphorylation of the kinase, whereas for the glucocorticoid receptor it is needed directly to allow the interaction with hormone. How Hsp90 achieves this in each case is not yet clear; however, its full activity seems to require collaboration with a number of cochaperone molecules. These are involved both in stimulating Hsp90 activity and providing specificity for the substrate (Buchner, 1999), and the Hsp90 pathway involves the formation of a number of dynamic complexes between the Hsp90, the co-chaperones and the client molecule. In this paper we provide evidence that the DNA polymerase α from Drosophila melanogaster appears to participate in such complexes and therefore falls into the ever-growing category of proteins controlled by Hsp90. DNA polymerases are essential enzymes involved in DNA replication and in repair. Although at least nine different polymerases have now been isolated from eukaryotes (Hubscher et al., 2000) only one of these the DNA polymerase α, contains a DNA primase activity. Therefore, DNA polymerase α is the only known DNA polymerase able to initiate de novo DNA synthesis (Foiani et al., 1997) and is required for both initiation at chromosomal origins and for the synthesis of Okasaki fragments on the lagging strand. In all species so far studied, the DNA polymerase α is composed of four subunits. The polymerase activity is associated with the largest (~180 kda) subunit, whereas the primase activity is associated with the smallest (~50 kda) (Bakkenist and Cotterill, 1994; Copeland and Wang, 1993; Nasheuer and Grosse, 1988; Santocanale et al., 1993). Little is known about
2 2016 JOURNAL OF CELL SCIENCE 114 (11) the activities associated with the other subunits (~60 and ~80 kda). For efficient initiation of replication, DNA polymerase α has to be loaded and active at specific points during the cell cycle; it also has to be prevented from inappropriate DNA synthesis. How this regulation is achieved is not yet clear, but extensive study in a number of organisms has indicated that it is liable to be at a number of different levels. Although the rate of transcription is cell-cycle controlled in budding yeast, protein levels remain constant throughout the cell cycle (Falconi et al., 1993). Differential association of the polymerase with the chromatin has been observed in budding yeast (Desdouets et al., 1998), human cell culture (Stokke et al., 1991) and Drosophila embryos (Melov et al., 1992). Phosphorylation may also be involved as phosphorylation of various subunits has been observed in vivo for budding yeast (Foiani et al., 1995), fission yeast (Bouvier et al., 1993), humans (Nasheuer and Grosse, 1988) and Drosophila (Kuroda and Ueda, 1999), and in vitro a number of kinases have been shown to phosphorylate the enzyme, in some cases having effects on the polymerase activity of the enzyme (Voitenleitner et al., 1999) (Weinreich and Stillman, 1999). In an effort to understand more about the mechanisms controlling the DNA polymerase α from Drosophila melanogaster, we have cloned and characterised all four subunits of the complex (Bakkenist and Cotterill, 1994; Cotterill et al., 1992; Huikeshoven and Cotterill, 1999; Melov et al., 1992) and carried out screens to identify interacting proteins. In this paper we present our data on one of the proteins that we isolated by two-hybrid interaction with the p180 subunit. This protein, which we have called Dpit47, appears to be an Hsp90 co-chaperone protein. We present our analysis of the characterisation of Dpit47 and its interaction with Hsp90. We also present data on the interaction of DNA polymerase α with Dpit47 and Hsp90. Complexes of DNA polymerase α with Dpit47 show severely suppressed polymerase activity in vitro. This interaction with Hsp90 could therefore provide an additional level of control for the polymerase. An effect of heat shock proteins is already well documented in a number of prokaryotic DNA replication systems (Konieczny and Zylicz, 1999), and is also thought to be involved in the control of replication of some eukaryotic viruses, e.g. SV40 (Campbell et al., 1997) and papilloma (Liu et al., 1998), but we believe this constitutes the first evidence that Hsp90 may have a function in DNA replication in higher eukaryotes. MATERIALS AND METHODS Cloning and sequencing Yeast two-hybrid screening was carried out as described (Gyuris et al., 1993; Zervos et al., 1993). Roger Brent kindly provided the yeast strain EGY48, the lacz reporter plasmid (psh18-34), the LexA fusion control plasmid (RFHM1) and the plasmid library (Drosophila ovarian cdna in pjg4-5 RFLY3). The plasmid used to make the LexA fusion bait was based on prs313 and pv44er-lex. The SacI- KpnI fragment of pv44er-lex containing the galactose inducible LexA protein and a multiple cloning site was ligated into SacI-KpnI cut prs313 in order to change the selectable marker from Trp to His. The Drosophila DNA polymerase α p180 subunit-coding region was then cloned in frame with the LexA protein. DNA sequencing was carried out using the Sequenase kit (US Biochemicals, Cleveland, OH, USA) or was sent to Advanced Biotechnology Centre, Charing Cross and Westminster Medical School, for ABI sequencing. Nucleotide sequence analysis and amino acid comparisons were performed using Geneworks (IntelliGenetics Inc.) or the Blast facility at NIH. Production of Dpit47 antibodies Dpit47 missing the first 20 amino acids was cloned into pqe31 (Qiagen) and the His-tagged protein expressed and purified according to the manufacturer s instructions. Purified protein was sent to Neosystem laboratoire (Strasbourg) for antibody production in two rabbits. The polyclonal antisera of these rabbits were affinity purified using purified Dpit47 cross-linked onto CNBr activated sepharose 4B (Amersham-Pharmacia) using standard procedures (Harlow and Lane, 1988). Immunoprecipitations Antibodies were cross-linked to protein A-sepharose beads using dimethylpimelimidate. 50 µl of packed beads were incubated for 1 hour at 4 C with 300 µl of extract. Beads were then washed ten times with 20 volumes of IP buffer: 10 mm Tris, ph 7.5, 100 mm NaCl, 0.05% triton X100 and 5% glycerol buffer (1 ml per wash). The beads were then incubated with 100 µl of IP buffer plus 250 mm NaCl. This eluate was kept and the beads washed ten times with IP buffer plus 250 mm NaCl. This treatment was repeated with 500 mm NaCl, the beads were incubated with 2% SDS and the pellet was resuspended in 100 µl SDS-PAGE loading buffer. For the experiment with the addition of geldanamycin or ATP, MgCl 2 was added to a final concentration of 1 mm and geldanamycin added at 3 µg/ml or ATP at 1 mm, final concentration. Calculation of Dpit47 concentration in extracts Dpit47 was immunoprecipitated from Drosophila embryo crude extract, as indicated above, so that it could be visualised on PAGE- SDS upon staining with sensitive Coomassie (Brilliant blue G colloidal) (e.g. Fig. 3; Fig. 4; Fig. 6b). The concentration of Dpit47 in the immunoprecipitate was estimated by comparison with known amounts of molecular weight markers. The immunoprecipitated Dpit47 was then used as a standard to estimate the amount of the protein in crude extracts by western blotting. In all cases, serial dilutions were loaded on the gels and the amounts estamated using the Alpha Innotech Corporation quantitation program. Imunocytochemistry and confocal microscopy Embryos were harvested, dechorionated in hypochlorite and rinsed in PBS, 0.1% triton X-100, and then fixed and stained essentially according to (Ashburner, 1989), except that fluorescent detection was used. Prior to hybridisation, embryos were blocked in PBS, 0.1% triton and 2% BSA for 1 hour. Primary antibodies (anti Dpit47 affinity purified) were added at a concentration of 1/500 in PBS, 0.1% triton and 2% BSA, and incubated at 4 C overnight. After washing in PBS plus 0.1% triton X-100, secondary antibody (FITC conjugated goat anti-rabbit, from Vectorlabs) was added at a concentration of 1/500 in PBS plus 0.1% tween-20 for 1 hour and the embryos washed in PBS, 0.1% tween-20. Embryos were counterstained with monoclonal anti-histones (1/1000, Chemicon) and horse anti-mouse Texas red monoclonal (1/500, Vectorlabs) following a similar procedure. Drosophila embryos, cell extracts and cellular fractionation For whole cell extract, Drosophila embryos of the ages indicated in the text were homogenised in 1 volume of IP buffer using a Dounce homogeniser with a tight pestle in order to break open the nuclei. These were filtered through Miracloth and then centrifuged for 5 minutes at 6.5 K rpm in a microfuge. For cellular fractionation the homogenisation was carried out using
3 Dpit47: a DNA polymerase chaperone 2017 a loose pestle in order to maintain the integrity of the nuclei. The filtered homogenate was centrifuged for 10 minutes at 13 K rpm. The supernatant is the cytoplasmic fraction. The nuclei pellet was washed several times using the same buffer and subjected to further fractionation, first using 1% Triton X100 and then increasing the NaCl concentration as indicated in the text. In each case the first supernatant was kept and the pellet washed several times with buffer supplemented with Triton and/or NaCl. All buffers were supplemented with protease inhibitors (Boehringer Mannheim, Complete TM, EDTA free). Cell culture S2 cells were propagated in 1X Schneider s Drosophila media (GIBCO) supplemented with 10% FBS, 50 units/ml penicillin and 50 µg/ml streptomycin. Cells either in exponentially growing phase or in stationary phase were harvested and treated as above for whole cell extracts or cellular fractionation. DNA polymerase assay A 35 bp oligonucleotide (TGAGTCGTATTACAATTCACTGGC- CGTCGTTTTAC) was annealed to a complementary 17 bp oligonucleotide (GTAAAACGACGGCCAGT). For each reaction 2.5 pmol of annealed primers were used. The reaction was carried out at 25 C in a final volume of 50 µl of 20 mm Tris-HCl, ph 8, 2 mm DTT, 200 µg/ml bovine serum albumin, 10 mm MgCl 2, 50 µm datp, 50 µm dgtp, 50 µm dttp and 32 PαdCTP. The reaction was stopped by the addition of 10 µl EDTA (0.5 M) and 5 µl SDS 20%. The free nucleotides were removed on a G50 spin column. The products of the reaction were ethanol precipitated and analysed on a 15% polyacrylamide gel. The bands were visualised either by autoradiography or using a phosphoimager screen. RESULTS Dpit47 is a novel polymerase α interacting protein Dpit47 was originally isolated using the two hybrid screen, with the 180 kda subunit of the Drosophila DNA polymerase α as a bait. The predicted amino acid sequence of the protein (396 aa; predicted molecular weight 46.5 kda) is shown in Fig. 1. The only distinguishable features in the Dpit47 sequence is a domain between positions 88 and 193 containing three TPR motifs (tetratrico peptide repeat). TPR motifs are highly degenerate 34 amino acid repeats thought to be involved in protein:protein interactions (Lamb et al., 1995), and are found in proteins with a wide range of functions such as cell cycle control, transcription and splicing, protein transport, regulatory phosphate turnover and protein folding. Searching of databases revealed that Dpit47 had homology both in the TPR motifs and in the surrounding sequence to the yeast CNS1 protein. CNS1 was isolated as a high-copynumber suppressor of the cpr7 slow-growth phenotype and it has been suggested to be an Hsp90 co-chaperone (Dolinski et al., 1998; Marsh et al., 1998). The closest homologue on the database, however, was a human protein called TTC4 (Su et al., 1999), which is in a region of the genome often deleted in breast cancer. The relative homology between Dpit47 and its yeast and human counterparts is shown in Fig. 1. Database searching also revealed several other Drosophila proteins related to the Dpit47 protein. It therefore seems likely that Dpit47 forms part of a protein family. Analysis of the region upstream of the Dpit47 gene did not reveal any sites for the E2F (Duronio et al., 1995) or DREF (Yamaguchi et al., 1995) transcription factors both of which have previously been implicated in the control of genes involved in cell cycle and replication in Drosophila. Dpit47 interacts with polymerase α in crude extracts To analyse Dpit47 further, an antibody was raised against an overexpressed hexa-his tagged version of the protein. In crude extract from Drosophila embryos, affinity purified antibody recognises a single band of approximately 50 kda (data not shown), which corresponds well to the size predicted from the sequence of 46.5 kda. Calculation of the amount of Dpit47 in the cell suggests that it is an abundant protein and is present at approximately molecules per cell in stage 14 embryos. (see Materials and methods) D.m. 1:MKQMAAQKTWTDEERLELAAQLDAELDAFIDGLEKKRYEEGWPEDRWQEEMDKHPFFMKR: 60 H.s. 1:...MEQPGQDP...TSDDVMDSFLEKFQSQPYRGGFHEDQWEKEFEKVPLFMSR: 48 S.c. 1:MSSVNANGGYTKPQ..K...YVPGPGDPELPP.QLSEFKD.KTSDEILKEMNRMPFFMTK: D.m. 60:.APQPGDDVH.PMFEGLQKLKYDPEENTRDELALNYKEDGNFYMKHKKFRMAIYSFTEGI:118 H.s. 48:.APSEIDPRENPDLACLQSIIFD.EERSPEEQAKTYKDEGNDYFKEKDYKKAVISYTEGL:106 S.c. 54:LDETDGAGGENVELEALKALAYE...GEPHEIAENFKKQGNELYKAKRFKDARELYSKGL: D.m. 119:KTKTDNPDVLAVLYNNRSAAHFFIKNYRSSLSDAQRALFYKPDYTKARWRSAQCAYELER:178 H.s. 107:KKKCADPDLNAVLYTNRAAAQYYLGNFRSALNDVTAARKLKPCHLKAIIRGALCHLELIH:166 S.c. 111:AVECEDKSINESLYANRAACELELKNYRRCIEDCSKALTINPKNVKCYYRTSKAFFQLNK: TPR motifs D.m. 179:FDLCTQMCEELLE.VDVDNEVAIALLHKNKMKKLEIERNQRKEAAEAKRRLTRFHRLRDA:237 H.s. 167:FAEAVNWCDEGLQ.IDAKEKKLLEMRAKADKLKRIEQRDVRKANLKEKKERNQNEALLQA:225 S.c. 171:LEEAKSAATFANQRIDPENKSILNMLSVIDRKEQELKAKEEKQQREAQERENKKIMLESA: D.m. 238:IEQRAIKFDDQKVGKKDVLSE...ELLYPKFLPLEDHPVHLDEDG.STLIWPAAFSYPEF:293 H.s. 226:IKARNIRLSEAACEDEDSASEGLGELFLDGLSTENPHGARLSLDGQGRLSWPVLFLYPEY:285 S.c. 231:MTLRNITN...IKTHSPVE...LLNEGKIRLEDP...MDFESQLIYPALIMYPTQ:276 Fig. 1. Predicted amino acid sequence of Dpit47, human TTC4 and S. cerevisiae CNS1. Shaded boxes indicate regions of identity. The TPR domain region is indicated below the sequence. D.m., Drosophila melanogaster; H.s., Homo sapiens; S.c., Saccharomyces cerevisiae; TPR, tetratrico peptide repeat D.m. 294:LYSDFYQQLPETTTMRDCLATLLT...EKLPYDKALNYRLGNVHVLENRKVG...CVHK:346 H.s. 286:AQSDFISAFHEDSRFIDHLMVMFG...ETPSWDLEQKYCLIIWRSTLRMRTG...QNYT:338 S.c. 277:DEFDFVGEVSELTTVQELVDLVLEGPQERFKKEGKENFTPKKVLVFMETKAGGLIKAGKK: D.m. 347:VDEEKQLAEIIAEKGFFVSGGALLFY.VVHKDSRVEQEFINEARRPMVYSC:396 H.s. 339:GCLPR...APCYRFYSTRGTL...:356 S.c. 337:LTFHDILKKESPDVPLFDNALKIYIVPKVESEGWISKWDKQKALERRSV..:385
4 2018 JOURNAL OF CELL SCIENCE 114 (11) Fig. 2. Co-immunoprecipitation of Dpit47 and DNA polymerase α. Drosophila embryo crude extract was subjected to immunoprecipitation using a polyclonal antibody against Dpit47 cross-linked to protein A sepharose. The immunocomplexes were washed extensively and then eluted with increasing salt concentrations and SDS as indicated in the figure. The top panel represents the elution profile of DNA polymerase α (polαp180), and the bottom represents that of the Dpit47 in the same experiment. c, control immunoprecipitation using an unrelated antibody. 1 and 2, immunoprecipitation using anti-dpit47 antibody (duplicate experiments are shown for each immunoprecipitate). To obtain confirmation of the interaction between DNA polymerase α and Dpit47, we carried out immunoprecipitations from crude 0-5 hour Drosophila embryo extract using the anti Dpit47 antibody. As shown in Fig. 2, Dpit47 is efficiently immunoprecipitated from embryo extract. The analysis of the eluted fractions by western blot using antibodies directed against DNA polymerase α reveals the presence of the 180 kda subunit. The interaction is of moderate strength as all of the 180 kda subunit has been eluted by 250 mm salt, and is further confirmed by the reverse immunoprecipitation of Dpit47 by anti-polymerase α antibodies (data not shown). This interaction between Dpit47 and the 180 kda subunit of the DNA polymerase α is specific, as it is not detected when pre-immune or unrelated sera are used for the immunoprecipitation. In addition, the precipitates do not contain PCNA, RPA, cdk2, topoisomerase 2 or lamin (data not shown). Calculation of the amount of the DNA polymerase α associated with Dpit47 suggests that it is about 10% of total cellular polymerase. Dpit47 immunoprecipitates after washing with 500 mm NaCl. Fig. 3 shows the results of such an analysis where the fractions were analysed on SDS-PAGE and stained with Coomassie Dpit47 is associated in a complex with Hsp90 and Hsp70 The CNS1 protein in yeast shows a physical and functional interaction with Hsp90. The homology of Dpit47 to the Hsp90 co-chaperone CNS1 prompted us to ask whether Dpit47 also interacted with the Drosophila Hsp90. The interaction between Hsp90 and its co-chaperones is quite strong; we therefore analysed those proteins that remained bound in the pellets of Fig. 3. Dpit47 forms a complex with Hsp90 and Hsp70. The pellets from two independent Dpit47 immunoprecipitations that had been washed in 500 mm NaCl were analysed by electrophoresis on SDS-PAGE and staining with sensitive Coomassie (Brilliant blue G colloidal). *, indicates the IgG heavy band. Sizes of molecular weight markers are shown to the left of the gel. Fig. 4. (A) The interaction between Dpit47 and DNA polymerase α is stabilised by geldanamycin (g) and destabilised by ATP. Dpit47 was immunoprecipitated from crude embryo extract with anti-dpit47 antibody cross-linked to protein A sepharose and eluted with 250 mm Nacl as in Fig. 2. The eluates were analysed on western blots using polyclonal anti-dna polymerase α antibody. (a) Dpit47 immunoprecipitates were carried out either in the presence (+g) or absence ( ) of 1 µg/ml geldanamycin as described in materials and methods. (b) Dpit47 immunoprecipitates were carried out as above either in the presence of 1 µg/ml geldanamycin (+g) or 1 mm ATP (+ATP). (B) The interaction between Dpit47 and Hsp70 is destabilised by ATP. The 500 mm washed pellets of the immunoprecipitation as described above were analysed on SDS- PAGE and stained with Coomassie blue. The bands corresponding to Hsp90 Hsp70 and Dpit47 are shown. a, no treatment of the immunoprecipitate; b, treatment with geldanamycin; c, treatment with ATP.
5 Dpit47: a DNA polymerase chaperone 2019 (Brilliant blue G). As shown, Dpit47 can be clearly identified and its identity confirmed by western blot using the antibody against Dpit47. Two other bands were seen in addition to Dpit47 and to the antibody bands. A band of around 85 kda was present in near stoichiometric amount to Dpit47, and another band of around 70 kda was visible at around 50% of the amount. These two bands were excised from the gel and analysed using MALDI-TOF. The 85 kda band was identified as the Drosophila Hsp90 homologue and the 70 kda as Drosophila Hsp70. From these results we conclude that Dpit47 forms a tight complex with Hsp90 and Hsp70, and by analogy to the function of the related budding yeast protein CNS1 is likely to function as an Hsp90 co-chaperone. Geldanamycin stabilises the interaction between Dpit47 and DNA polymerase α Because Dpit47 is likely to be an Hsp90 co-chaperone we were interested to know whether we could detect an interaction of Hsp90 with DNA polymerase α. Analysis of proteins immunoprecipitated with a monoclonal antibody against the DNA polymerase α did show a band that cross reacted with Hsp90 antibodies (data not shown). However, in our hands the Hsp90 antibodies produced a high background; we therefore sought another way to confirm that the DNA polymerase α interacted with Hsp90. Geldanamycin is a specific inhibitor of Hsp90 activity and acts by binding to its ATP binding site (Pearl and Prodromou, 2000). The presence of geldanamycin therefore interferes with the Hsp90 pathway and has been shown to alter the interactions between the component proteins of the pathway. For DNA polymerase α, direct measurement of the Fig. 5. Western blots show the abundance of Dpit47 throughout the developmental stages. Embryonic development is represented by the indicated age of embryos from 0 to 20 hours of development. L, larvae; P, pupae; M, males; F, females. Equal loading of samples was confirmed using a Drosophila anti-importin alpha-3 antibody (impα3) (Mathe et al., 2000). interaction with Hsp90 under various conditions was limited by the quality of the Hsp90 antibodies. We therefore decided to look for an effect of geldanamycin on the interaction between Dpit47 and DNA polymerase α. An observed effect should allow us to argue for an involvement of Hsp90 with the DNA polymerase α, mediated through Dpit47. DNA polymerase α complexed with Dpit47 was therefore isolated by immunoprecipitation in the presence or absence of geldanamycin. As can be seen in Fig. 4A,a, immunoprecipitations incubated with geldanamycin consistently contain more DNA polymerase α (about twofold) than is detected in the control. Although geldanamycin blocks the action of Hsp90, the addition of ATP to Hsp90 complexes has been shown to Fig. 6. DNA polymerase is associated with Dpit47 throughout development and in proliferating cell culture but not in quiescent cells. (a) A comparison of the amount of DNA polymerase α associated with Dpit47 complexes immunoprecipitated at different stages of the life cycle from 0 to 24 hours as indicated. The amounts of DNA polymerase α in each extract is indicated (loading controls). In each case equal amounts of the immunoprecipitated Dpit47 was loaded as determined by western blotting. (b) A comparison of the amount of DNA polymerase α associated with Dpit47 complexes immunoprecipitated from Drosophila embryo extract (E) and exponentially growing S2 cells (C). The two panels on the left are western blots showing the amounts of DNA polymerase α and Dpit47 in crude extracts from Drosophila embryos and S2 cells. The panel on the top right shows the amount of DNA polymerase α (as determined by western blot) immunoprecipitated with anti-dpit47 antibody from each extract. The bottom right panel has been included to show that equal amounts of Dpit47 (as analysed by coomassie staining) were analysed for each extract. (c) A comparison of the amount of DNA polymerase α associated with Dpit47 complexes immunoprecipitated from exponentially growing (D) or quiescent (Q) S2 cells. The two panels on the left are western blots showing the amounts of DNA polymerase α and Dpit47 in crude extracts from exponentially growing cells and quiescent cells. The panel on the top right shows the amount of DNA polymerase α (western blot) immunoprecipitated with anti Dpit47 antibody for each extract. As above the bottom right panel has been included to show that equal amounts of Dpit47 immunoprecipitate have been compared for each cell type (in this case by western blot).
6 2020 JOURNAL OF CELL SCIENCE 114 (11) catalyse release of the substrate. Fig. 4A,b shows that the replacement of geldanamycin with ATP reduced the DNA polymerase α level below detection levels. This is also consistent with what we would expect for the interaction of an Hsp90 complex with DNA polymerase α. We therefore conclude that the appearance of Hsp90 in DNA polymerase α immunoprecipitates, plus the observed effects of geldanamycin and ATP on the interaction of DNA polymerase α with Dpit47, are consistent with what would be expected if Hsp90 acted as a chaperone for DNA polymerase α and if Dpit47 was a co-chaperone that mediated this interaction. Geldanamycin affects the interaction of Dpit47 with Hsp70 The specific interaction with DNA polymerase α only represents a small percentage of the Dpit47 in the cell. We were therefore interested to determine the effect of geldanamycin in general on the interaction of Dpit47 with other components of the Hsp90 pathway. We looked at the relative amounts of Hsp90 and Hsp70 immunoprecipitated in the pellets under the various conditions (Fig. 4B). In the control immunoprecipitation Hsp90 and Hsp70 can clearly be identified. When the immunoprecipitation is performed in the presence of geldanamycin, Hsp90 and Hsp 70 are pulled down in similar amounts as in the control. In the presence of ATP, similar amounts of Hsp90 are detected but the level of Hsp70 is much reduced. These results further support the involvement of Dpit47 in the Hsp90 pathway. DNA polymerase α interacts with Dpit47 at all stages of the life cycle in Drosophila and in cell cultures The interaction between DNA polymerase α and Dpit47 that we have detected so far was in total cell extracts obtained from Drosophila embryos aged between 0 and 5 hours. In such extracts the amount of proteins provided by maternal input is still very high, especially for those proteins involved in proliferation. To determine if the interaction between DNA polymerase α and Dpit47 was limited to embryos and was provided by maternal input we analysed the Dpit47:DNA polymerase α interaction in conditions where the maternal input is considerably reduced (older embryos) or where there is no maternal input (cell culture). Fig. 6 shows the results of such an analysis. For Fig. 6a we made whole cell extracts from embryos up to 24 hours old (larval stage), and carried out immunoprecipitations using the anti-dpit47 antibody, in the same conditions as above. In all cases the interaction between DNA polymerase α and Dpit47 is detected at a comparable level. Similarly, we made whole cell extracts from a Drosophila cell line S2. Cells were collected in exponentially growing phase and homogenised as indicated in materials and methods. In this case Dpit47 developmental profile To determine the cellular function of the interaction between Dpit47 and DNA polymerase α we needed to get more information about the behaviour of the Dpit47 protein in Drosophila. Total crude extracts from all stages of Drosophila life cycle were analysed on western blot with antibody directed against Dpit47. Fig. 5 shows the profile consistently obtained with Dpit47 antibodies. The protein is more abundant in young embryos, in pupae and in females and lower in late embryos, larvae and males. This pattern is characteristic of a protein having a role in proliferation. Fig. 7. Subcellular and cell cycle distribution of Dpit47. (a) Confocal micrographs of Dpit47 stained embryos that have been counterstained with anti-α tubulin monoclonal antibodies. (b) Confocals micrographs of Dpit47 stained embryos which have been counterstained with anti-histone monoclonal antibodies. In the merged images in each case the Dpit47 is green whereas the counterstain is red. Stages of the cell cycle are indicated to the left of the panels.
7 Dpit47: a DNA polymerase chaperone 2021 the interaction between Dpit47 and DNA polymerase α was also detected to a similar level (Fig. 6b). In all cases the interactions showed the same elution characteristics (data not shown). From these results we can conclude that the interaction between DNA polymerase α and Dpit47 is not limited to young embryos but also takes place in older embryos and in cell culture. The interaction between DNA polymerase α and Dpit47 is not detected in non-proliferating cells We next investigated whether the Dpit47:DNA polymerase α interaction also occurred in non-proliferating cells. We made whole cell extracts from quiescent S2 cells and carried out immunoprecipitation using the anti-dpit47 antibody. As a positive control we used proliferating S2 cells as above. Fig. 6c compares the level of DNA polymerase α in equivalent amounts of Dpit47 immunoprecipitates from exponentially growing and quiescent cells and shows that in quiescent cells the amount of DNA polymerase α is very much reduced. This is not due to the absence of DNA polymerase α as it is also detected in quiescent cells (Fig. 6c, loading control). From this result we can conclude that the interaction between Dpit47 and DNA polymerase α is confined to proliferating cells and therefore, as was originally suggested by the expression profile of the Dpit47, the interaction is likely to have some role in proliferation. Dpit47 is found in the nucleus but is only loosely associated with the chromatin Much of the activity of Hsp90 co-chaperones takes place in the cytoplasm. We therefore determined the cellular location of Dpit47 as a guide to where it was likely to carry out its function. In embryos prior to cellularisation where the protein was most abundant, Dpit47 protein was visible throughout the embryo in the nucleus and cytoplasm (data not shown). After cellularisation, however, the protein was seen predominantly in the nucleus. Fig. 7a shows the staining pattern using affinity purified Dpit47 antibodies and counterstained with anti-tubulin antibodies to clearly visualise the stages of the cell cycle. Throughout the cell cycle even during mitosis when the nuclear envelope has partially broken down Dpit47 staining appears to be confined within the nuclear region. In Fig. 7b where the embryos are counterstained with histone it is apparent that the Dpit47 staining only partially overlaps that of the DNA in interphase and telophase and in the mitotic stages forms a cloud in the vicinity of, but not directly associated with, the DNA. In both Fig. 7a and Fig. 7b it is possible to see that two different patterns are obtained for Dpit47 staining in mitosis. In the upper of the two metaphase panels in each case the protein appears more proximal to the DNA than the other. Although both these patterns are visible regularly we have not been able to determine definitively which of them preceeds the other in the cell cycle. To confirm the cellular location of Dpit47 using another method we performed cellular fractionation of embryos and Drosophila S2 cells. The initial fractionation was between cytoplasm and nucleus. The nuclear pellet was further fractionated into the nucleoplasm and chromatin associated protein by nonionic detergent treatment. Increasingly stringent salt washes were then used to release proteins binding to the nuclear pellet with different affinities. The biochemical analysis of the S2 cells is shown in Fig. 8. About 50% of the Dpit47 is seen in the cytoplasmic fractions. This most likely represents leakage from the nuclear area, particularly during mitosis when the nuclear envelope is not intact. On further fractionation it can be seen that Dpit47 is released totally from the nucleus by triton treatment and is not detected in the chromatin-associated fractions. By comparison, the DNA polymerase α is found to be much more tightly associated with the nucleus. The fractionation pattern from
8 2022 JOURNAL OF CELL SCIENCE 114 (11) Fig. 8. Cellular fractionation of Dpit47 and DNA polymerase α. Drosophila embryo extracts were subjected to salt and detergent fractionation and analysed by western blot using anti Dpit47 and anti-dna polymerase (pol) antibodies as described in Materials and Methods. Ce, total cellular extract as a control; 50 mm NaCl, cytoplasmic fraction; Triton, protein released on treatment of nucleus with triton, representing the nucleoplasmic fraction; Triton final, last triton wash before salt extractions to show that there is no carry over between fractions; 250 mm NaCl, Triton pellet extracted with 250 mm NaCl and 1% Triton; pellet, remainder after all extractions. embryos is largely similar (data not shown), except that a slightly higher percentage of the Dpit47 was found in the cytoplasm, probably due to maternal input in early embryos. The cellular fractionation results are therefore consistent with the data obtained from immunofluoresence and suggest that the bulk of the Dpit47 is located in the nucleoplasm but is not tightly associated with the chromatin. The interaction between Dpit47 and DNA polymerase α is detected both in cytoplasmic and nucleoplasmic fractions Because Dpit47 is detected in the cytoplasm and the nucleoplasm we were interested to determine whether the interaction that we had observed with DNA polymerase α was also detected in both compartments. Immunoprecipitations were therefore performed on cytoplasmic and their corresponding nucleoplasmic fractions from embryos 0-18 Fig. 9. Analysis of Dpit47 DNA polymerase complexes in the cytoplasm and in the nucleoplasm. Cytoplasmic and nucleoplasmic fraction were subjected to immunoprecipitations using anti-dpit47 antibody. The DNA polymerase eluted in 250 mm NaCl from two separate experiments fractions was determined by western blots using anti-dna polymerase antibody. For the cytoplasmic fraction (top panel) the lane ce on the left corresponds to shorter exposure time in order to visualise the band. The same exposure time as the eluted fractions is shown on the right. i and ii, two separate elutions; ce, crude extract control. Fig. 10. The primase subunit p50 is detected in the complex immunoprecipitated by anti-dpit47 antibody. Dpit47 immunoprecipitates as described in Materials and Methods were analysed using anti-p180 (polαp180) and anti-p50 (polαp50) antibodies. In each case two seperate experiments are shown. The lane after the ce contains the last wash of the immunoprecipitate prior to elution to show that there is no carryover between fractions. 250 mm and 500 mm, the 250 mm and 500 mm NaCl elutions from the immunoprecipitate, respectively; ce, crude extract as a control. hours old and analysed as described above. As shown in Fig. 9 the interaction between DNA polymerase α and Dpit47 is detected in both fractions. By comparison of the amount of DNA polymerase α present in the precipitate and in the crude cytoplasmic and nucleoplasmic fractions, it is possible to see that significantly more p180 is found associated with Dpit47 in the nucleoplasmic than in the cytoplasmic fraction. Other subunits of DNA polymerase α are detected in the eluates DNA polymeraseα is a multisubunit enzyme and therefore one possible function of the interaction with Dpit47 is related to the assembly of the complex. If this was the case we would expect that immunoprecipitates of Dpit47 would contain substoichiometric amounts of the other polymerase subunits. Fractions in which the 180 kda subunit of the DNA polymerase α was detected in Dpit47 immunoprecipitates were therefore analysed by western blotting for the presence of the other subunits. Fig. 10 shows that the primase subunit p50 was also present in the precipitates and eluted with the same profile as the polymerase subunit. Calculation of the amount of the primase present suggested that the levels of the two proteins were also present in similar amounts. Similar results were obtained for the 73kDa subunit (data not shown). In view of this result and because of the very strong interaction between the p50 and p60 subunits and the p180 and p73 subunits, it seems likely that the interaction detected is between Dpit47 and the whole DNA polymerase α enzyme, and makes it less likely that the interaction is involved in the assembly of the polymerase complex. The DNA polymerase α associated with Dpit47 is inactive We also wished to determine whether the association between Dpit47 and DNA polymerase α had any effect on its enzymatic activity. We therefore looked at the ability of the DNA polymerase α co-immunoprecipitated with Dpit47 to perform an end filling reaction on a 35 mer substrate. As a control we immunoprecipitated DNA polymerase α with a monoclonal antibody known not to affect its activity. The levels of
9 Dpit47: a DNA polymerase chaperone 2023 Fig. 11. The DNA polymerase α complexed with Dpit47 is inactive. DNA polymerase α was immunoprecipitated using anti-dpit47 antibody or monoclonal anti-dna polymerase α antibody. Top panel, DNA polymerase assay: immunoprecipitated DNA polymerase α was used to carry out an end filling polymerase assay on a 35 mer as described in Materials and Methods. The reaction was stopped after 5, 15 or 30 minutes. The product of the reaction was analysed on a 15% polyacrylamide gel. Lower panel shows by western blotting the amount of DNA polymerase α contained in the Dpit47 (Dpit47 IP) and DNA polymerase α (polα IP) immunoprecipitates used in the assay. polymerase were adjusted to be equivalent in both cases (Fig. 11, loading control). Fig. 11 clearly demonstrates that the DNA polymerase associated with Dpit47 has no detectable polymerase activity. This is not due to an inhibitory protein present in the Dpit47 immunoprecipitates as addition of Dpit47 precipitates has no effect on the level of polymerase activity detected in the DNA polymerase immunoprecipitates (data not shown). DISCUSSION Dpit47 is an Hsp90 interacting protein We have identified the Drosophila Dpit47 protein as a novel Hsp90 co-chaperone protein that interacts with the DNA polymerase α. Like other Hsp90 co-chaperone proteins, Dpit47 possesses a TPR domain. TPR domains are found in a wide variety of proteins and are thought to be involved in protein:protein interactions. In the Hsp90 co-activators they are thought to be involved in the interaction with Hsp90 (Scheufler et al., 2000), and the co-chaperone TPR domains form a distinct subset of TPR motif containing proteins that are more closely related to each other than to other TPR proteins, e.g. the APC proteins. Quite a large number of proteins have now been identified as Hsp90 co-chaperones; however, this blanket term covers a number of different subgroups. A more detailed analysis of the sequence of Dpit47 suggests that it falls into that class of co-chaperones that contains proteins such as Hop. These are thought to be involved in the early part of the reaction, and in recognition of the client proteins, although how closely these proteins are related to each other functionally and whether they also possess other biochemical activities remains to be determined. Our suggestion of a co-chaperone role for Dpit47 is strengthened by our isolation of tight and stoichiometric complex of Dpit47 with Hsp90 in extracts, and further supported by the observed interaction between Dpit47 and Hsp70, another protein which has been shown to act in the Hsp90 pathway. The isolation of a complex containing only these two proteins, the relative levels of the Hsp90 and 70 in the complex and the effects of the addition of ATP or the specific Hsp90 inhibitor geldanamycin on these interactions is also consistent with Dpit47 being involved in the early stages of the Hsp90 pathway. The recent release of the Drosophila genome sequence (Adams et al., 2000) has further allowed us to determine that Dpit47 is a member of a family of proteins in Drosophila. Seven other homologues can be identified from their genome sequence, although in most cases the homology is largely confined to the TPR region. One of these proteins has already been defined as the Drosophila Hop homologue; the others are as yet uncharacterised. Database searching has also allowed us to identify Dpit47 homologues in other species from budding yeast to mammals. The closest of these is a human protein TTC4 (accession number NM_ ), which was first identified as a putative tumour suppressor gene frequently deleted in breast cancer (Su et al., 1999). Since this is closely related to Dpit47 it would be interesting to determine if TTC4 has retained functional characteristics of the Dpit47 protein. Dpit47 is likely to function as a DNA polymerase α co-chaperone Most of the work presented here centres around the study of the interaction of the Dpit47 protein with the DNA polymerase α. Although the interaction was first seen in the two hybrid system, we have also confirmed that the interaction can be detected in extracts from various types of cells. We have detected the interaction in both cytoplasmic and nucleoplasmic fractions, suggesting the interaction may occur in both compartments. However, we cannot completely rule out the possibility that Dpit47 associates with DNA polymerase α predominantly in the nucleoplasmic fraction, and that the interactions observed in cytoplasmic fractions are caused by leakage from the nucleus during the nuclear preparation, particularly from cells in mitosis. Although both proteins are quite abundant, the interaction only involves about 10-20% of the polymerase and 1-2% of the Dpit47. This is consistent with the Dpit47 having other substrates (see later). For the DNA polymerase α this could represent an instability of the complex to the isolation conditions, alternatively only a subpopulation of the DNA polymerase α molecules may bind to Dpit47. In addition to showing the interaction between Dpit47 and DNA polymerase α, we have also been able to immunoprecipitate Hsp90 directly with DNA polymerase α antibodies. Although we have not formally isolated the trimeric complex, this observation, taken together with the observed effect of geldanamycin on the Dpit47:polymerase interaction, strongly suggests that the DNA polymerase α:dpit47 complex is likely to also include Hsp90. Cellular function of Dpit47: DNA polymerase α interaction Our data is suggestive of a role for the Hsp90 pathway in
10 2024 JOURNAL OF CELL SCIENCE 114 (11) DNA polymerase α function. Interestingly, it is also consistent with a much earlier report in which geldanamycin was shown to inhibit DNA polymerase α activity (Yamaki et al., 1982). However, an important question is the function of this interaction in a cellular context. Among Hsp90 client proteins the purpose of the interaction with Hsp90 varies depending on the protein concerned. For a large group of proteins, including the widely studied glucocorticoid receptor (Buchner, 1999) and telomerase, it seems that the interaction is involved in the conversion of the protein from the inactive to the active state. For other proteins it is involved in holding the protein in a particular configuration so that it can interact with protein partners or be modified in some way (e.g. phosphorylated). The interaction with the DNA polymerase that we have studied here causes a severe inhibition of the polymerase activity in the complex (the specific activity is at least 50x less than that normally seen). This suggests that the most likely role of the interaction in this case is to sequester polymerase in an inactive form. What still remains to be determined is the role this plays in the normal functioning of the cell. It is likely to be involved in proliferation, as the interaction does not occur in quiescent cells. The presence of the interaction in late embryos/larvae and cell culture also suggests that it is not just related to the unusual situation in early embryos in Drosophila where excessive amounts of maternal proteins are present. In the normal progression of the cell cycle there are a number of places where such an activity could be useful. Inactive polymerase must be maintained prior to initiation of DNA replication, or after the completion of one complete round, therefore Dpit47 could function at either of these stages. Because the observed location of Dpit47 is predominantly nucleoplasmic but not chromatin bound, any observed effect must take place prior to or subsequent to the association of the polymerase with the replication complex, rather than actually in the complex itself. Therefore, Dpit47 could function by facilitating rapid binding of the polymerase to the chromatin during initiation of DNA replication by concentrating the polymerase in an inactive form close to its potential substrate. Alternatively, it could function by allowing rapid sequestration of the polymerase after it has finished synthesis. A sequestration role for the Dpit47 DNA polymerase α interaction does not preclude the possibility that the interaction may have other additional effects on the polymerase that are more in line with the effects that the Hsp90 pathway has been seen to have on other client proteins. We cannot rule out completely that passage through the Hsp90 complex is required for activation of all polymerase molecules (as for the glucocorticoid receptor). However, the high level of activity of the polymerase isolated from early embryos when only small amounts need to have been activated owing to the small number of genomes to replicate, and the observation that polymerases from other organisms can be overproduced and are active, makes it less likely. Equally, the observation that the Dpit47 immunoprecipitates contain all four subunits in equal amounts makes it unlikely that interaction is required for the assembly of the complex. However, it is still possible that the Dpit47 interaction is required for modification of the polymerase, or for allowing its interaction with other components of the replication complex. Cellular function of Dpit47 Hsp90 is an essential abundant molecular chaperone involved in the folding, assembly and activation of number of proteins involved in signal transduction, cell cycle control, telomerase activity Hsp90 (Holt et al., 1999) or transcriptional regulation (Pratt, 1998). Here we have presented evidence that links the Hsp90 pathway with a mainstream replication protein, thereby providing the first report of a possible role for chaperones in the process of DNA replication in higher eukaryotes. However, the amount of Dpit47 is far greater than that of DNA polymerase α, making it very likely that Dpit47 has other client partners. Sensitive Coomassie analysis of the fractions where DNA polymerase α is detected eluting from the anti-dpit47 antibody reveal the presence of about a dozen bands of comparable intensity to DNA polymerase α. It would therefore be interesting to identify these proteins to determine if any others are involved in DNA replication and to see what other cellular processes might involve Dpit47. The authors would like to thank S. Kearsey and C. Donaghue for critical reading of the manuscript, and M. Arbeitman for communicating results prior to publication. Thanks also to C. Prodomou, I. R. Lehman and K. Kuroda for antibodies and R. Brent for two hybrid libraries. This work was supported by a grant from the AICR and Marie Curie Cancer Care. REFERENCES Adams, M. D., Celniker, S. E., Holt, R. A., Evans, C. A., Gocayne, J. D., Amanatides, P. G., Scherer, S. E., Li, P. W., Hoskins, R. A., Galle, R. F. et al. (2000). The genome sequence of Drosophila melanogaster. Science 287, Ashburner, M. (1989). In Drosophila: a laboratory manual (Vol. 2), Cold Spring Harbor Laboratory Press. Bakkenist, C. J. and Cotterill, S. (1994). The 50-kDa primase subunit of Drosophila melanogaster DNA polymerase alpha. Molecular characterization of the gene and functional analysis of the overexpressed protein. J. Biol. Chem. 269, Bouvier, D., De Recondo, A. M. and Baldacci, G. (1993). In vivo phosphorylation, mitotic behavior, and nuclear binding of the catalytic subunit of DNA polymerase alpha in fission yeast. Exp. Cell Res. 207, Buchner, J. (1999). Hsp90 & Co. a holding for folding. Trends Biochem. Sci. 24, Campbell, K. S., Mullane, K. P., Aksoy, I. A., Stubdal, H., Zalvide, J., Pipas, J. M., Silver, P. A., Roberts, T. M., Schaffhausen, B. S. and DeCaprio, J. A. (1997). DnaJ/hsp40 chaperone domain of SV40 large T antigen promotes efficient viral DNA replication. Genes Dev. 11, Copeland, W. C. and Wang, T. S. (1993). Enzymatic characterization of the individual mammalian primase subunits reveals a biphasic mechanism for initiation of DNA replication. J. Biol. Chem. 268, Cotterill, S., Lehman, I. R. and McLachlan, P. (1992). Cloning of the gene for the 73 kd subunit of the DNA polymerase alpha primase of Drosophila melanogaster. Nucleic Acids Res. 20, Desdouets, C., Santocanale, C., Drury, L. S., Perkins, G., Foiani, M., Plevani, P. and Diffley, J. F. (1998). Evidence for a Cdc6p-independent mitotic resetting event involving DNA polymerase alpha. EMBO J. 17, Dolinski, K. J., Cardenas, M. E. and Heitman, J. (1998). CNS1 encodes an essential p60/sti1 homolog in Saccharomyces cerevisiae that suppresses cyclophilin 40 mutations and interacts with Hsp90. Mol. Cell Biol. 18, Duronio, R. J., O Farrell, P. H., Xie, J. E., Brook, A. and Dyson, N. (1995). The transcription factor E2F is required for S phase during Drosophila embryogenesis. Genes Dev. 9, Falconi, M. M., Piseri, A., Ferrari, M., Lucchini, G., Plevani, P. and Foiani, M. (1993). De novo synthesis of budding yeast DNA polymerase alpha and
11 Dpit47: a DNA polymerase chaperone 2025 POL1 transcription at the G1/S boundary are not required for entrance into S phase. Proc. Natl. Acad. Sci. USA 90, [published erratum appears in Proc. Natl. Acad. Sci. USA (1994) 91,1598.] Foiani, M., Liberi, G., Lucchini, G. and Plevani, P. (1995). Cell cycledependent phosphorylation and dephosphorylation of the yeast DNA polymerase alpha-primase B subunit. Mol. Cell Biol. 15, Foiani, M., Lucchini, G. and Plevani, P. (1997). The DNA polymerase alphaprimase complex couples DNA replication, cell- cycle progression and DNA-damage response. Trends Biochem. Sci. 22, Gyuris, J., Golemis, E., Chertkov, H. and Brent, R. (1993). Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell 75, Harlow, E. and Lane, D. (1988). In Antibodies: A laboratory manual, Cold Spring Harbor Laboratory Press. Holt, S. E., Aisner, D. L., Baur, J., Tesmer, V. M., Dy, M., Ouellette, M., Trager, J. B., Morin, G. B., Toft, D. O., Shay, J. W. et al. (1999). Functional requirement of p23 and Hsp90 in telomerase complexes. Genes Dev. 13, Hubscher, U., Nasheuer, H. P. and Syvaoja, J. E. (2000). Eukaryotic DNA polymerases, a growing family. Trends Biochem. Sci. 25, Huikeshoven, H. and Cotterill, S. (1999). Cloning and characterisation of the gene for the large subunit of the DNA primase from Drosophila melanogaster. Biochim. Biophys. Acta 1445, Konieczny, I. and Zylicz, M. (1999). Role of bacterial chaperones in DNA replication. Genet. Eng. 21, Kuroda, K. and Ueda, R. (1999). Phosphorylation and dephosphorylation of the B subunit of DNA polymerase alpha-primase complex in the early embryogenesis of Drosophila. Biochem. Biophys. Res. Commun. 254, Lamb, J. R., Tugendreich, S. and Hieter, P. (1995). Tetratrico peptide repeat interactions: to TPR or not to TPR? Trends Biochem. Sci. 20, Liu, J. S., Kuo, S. R., Makhov, A. M., Cyr, D. M., Griffith, J. D., Broker, T. R. and Chow, L. T. (1998). Human Hsp70 and Hsp40 chaperone proteins facilitate human papillomavirus-11 E1 protein binding to the origin and stimulate cell-free DNA replication. J. Biol. Chem. 273, Mahony, D., Parry, D. A. and Lees, E. (1998). Active cdk6 complexes are predominantly nuclear and represent only a minority of the cdk6 in T cells. Oncogene 16, Marsh, J. A., Kalton, H. M. and Gaber, R. F. (1998). Cns1 is an essential protein associated with the hsp90 chaperone complex in Saccharomyces cerevisiae that can restore cyclophilin 40-dependent functions in cpr7delta cells. Mol. Cell Biol. 18, Mathe, E., Bates, H., Huikeshoven, H., Deak, P., Glover, D. M. and Cotterill, S. (2000). Importin-alpha3 is required at multiple stages of Drosophila development and has a role in the completion of oogenesis. Dev. Biol. 223, Melov, S., Vaughan, H. and Cotterill, S. (1992). Molecular characterisation of the gene for the 180 kda subunit of the DNA polymerase-primase of Drosophila melanogaster. J. Cell Sci. 102, Nasheuer, H. P. and Grosse, F. (1988). DNA polymerase alpha-primase from calf thymus. Determination of the polypeptide responsible for primase activity. J. Biol. Chem. 263, Parsell, D. A. and Lindquist, S. (1993). The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu. Rev. Genet. 27, Pearl, L. H. and Prodromou, C. (2000). Structure and in vivo function of Hsp90. Curr. Opin. Struct. Biol. 10, Pratt, W. B. (1998). The hsp90-based chaperone system: involvement in signal transduction from a variety of hormone and growth factor receptors. Proc. Soc. Exp. Biol. Med. 217, Santocanale, C., Foiani, M., Lucchini, G. and Plevani, P. (1993). The isolated 48,000-dalton subunit of yeast DNA primase is sufficient for RNA primer synthesis. J. Biol. Chem. 268, Scheufler, C., Brinker, A., Bourenkov, G., Pegoraro, S., Moroder, L., Bartunik, H., Hartl, F. U. and Moarefi, I. (2000). Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70- Hsp90 multichaperone machine. Cell 101, Stokke, T., Erikstein, B., Holte, H., Funderud, S. and Steen, H. B. (1991). Cell cycle-specific expression and nuclear binding of DNA polymerase alpha. Mol. Cell Biol. 11, Su, G., Roberts, T. and Cowell, J. K. (1999). TTC4, a novel human gene containing the tetratricopeptide repeat and mapping to the region of chromosome 1p31 that is frequently deleted in sporadic breast cancer. Genomics 55, Voitenleitner, C., Rehfuess, C., Hilmes, M., O Rear, L., Liao, P. C., Gage, D. A., Ott, R., Nasheuer, H. P. and Fanning, E. (1999). Cell cycledependent regulation of human DNA polymerase alpha-primase activity by phosphorylation. Mol. Cell Biol. 19, Weinreich, M. and Stillman, B. (1999). Cdc7p-Dbf4p kinase binds to chromatin during S phase and is regulated by both the APC and the RAD53 checkpoint pathway. EMBO J. 18, Xu, Y., Singer, M. A. and Lindquist, S. (1999). Maturation of the tyrosine kinase c-src as a kinase and as a substrate depends on the molecular chaperone Hsp90. Proc. Natl. Acad. Sci. USA 96, Yamaguchi, M., Hayashi, Y., Nishimoto, Y., Hirose, F. and Matsukage, A. (1995). A nucleotide sequence essential for the function of DRE, a common promoter element for Drosophila DNa replication-related genes. J. Biol. Chem. 270, Yamaki, H., Suzuki, H., Choi, E. C. and Tanaka, N. (1982). Inhibition of DNA synthesis in murine tumor cells by geldanamycin, an antibiotic of the benzoquinoid ansamycin group. J. Antibiot. 35, Zervos, A. S., Gyuris, J. and Brent, R. (1993). Mxi1, a protein that specifically interacts with Max to bind Myc-Max recognition sites. Cell 72, [published erratum appears in Cell (1994) 79, following 388].
Anti-ATF6 α antibody, mouse monoclonal (1-7)
 Anti-ATF6 α antibody, mouse monoclonal (1-7) 73-500 50 ug ATF6 (activating transcription factor 6) is an endoplasmic reticulum (ER) membrane-bound transcription factor activated in response to ER stress.
Anti-ATF6 α antibody, mouse monoclonal (1-7) 73-500 50 ug ATF6 (activating transcription factor 6) is an endoplasmic reticulum (ER) membrane-bound transcription factor activated in response to ER stress.
Classic Immunoprecipitation
 292PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Classic Immunoprecipitation Utilizes Protein A/G Agarose for Antibody Binding (Cat.
292PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Classic Immunoprecipitation Utilizes Protein A/G Agarose for Antibody Binding (Cat.
Chromatin Immunoprecipitation
 Chromatin Immunoprecipitation A) Prepare a yeast culture (see the Galactose Induction Protocol for details). 1) Start a small culture (e.g. 2 ml) in YEPD or selective media from a single colony. 2) Spin
Chromatin Immunoprecipitation A) Prepare a yeast culture (see the Galactose Induction Protocol for details). 1) Start a small culture (e.g. 2 ml) in YEPD or selective media from a single colony. 2) Spin
Chromatin Immunoprecipitation (ChIP)
 Chromatin Immunoprecipitation (ChIP) Day 1 A) DNA shearing 1. Samples Dissect tissue (One Mouse OBs) of interest and transfer to an eppendorf containing 0.5 ml of dissecting media (on ice) or PBS but without
Chromatin Immunoprecipitation (ChIP) Day 1 A) DNA shearing 1. Samples Dissect tissue (One Mouse OBs) of interest and transfer to an eppendorf containing 0.5 ml of dissecting media (on ice) or PBS but without
KMS-Specialist & Customized Biosimilar Service
 KMS-Specialist & Customized Biosimilar Service 1. Polyclonal Antibody Development Service KMS offering a variety of Polyclonal Antibody Services to fit your research and production needs. we develop polyclonal
KMS-Specialist & Customized Biosimilar Service 1. Polyclonal Antibody Development Service KMS offering a variety of Polyclonal Antibody Services to fit your research and production needs. we develop polyclonal
CUSTOM ANTIBODIES. Fully customised services: rat and murine monoclonals, rat and rabbit polyclonals, antibody characterisation, antigen preparation
 CUSTOM ANTIBODIES Highly competitive pricing without compromising quality. Rat monoclonal antibodies for the study of gene expression and proteomics in mice and in mouse models of human diseases available.
CUSTOM ANTIBODIES Highly competitive pricing without compromising quality. Rat monoclonal antibodies for the study of gene expression and proteomics in mice and in mouse models of human diseases available.
THE His Tag Antibody, mab, Mouse
 THE His Tag Antibody, mab, Mouse Cat. No. A00186 Technical Manual No. TM0243 Update date 01052011 I Description.... 1 II Key Features. 2 III Storage 2 IV Applications.... 2 V Examples - ELISA..... 2 VI
THE His Tag Antibody, mab, Mouse Cat. No. A00186 Technical Manual No. TM0243 Update date 01052011 I Description.... 1 II Key Features. 2 III Storage 2 IV Applications.... 2 V Examples - ELISA..... 2 VI
Pure-IP Western Blot Detection Kit
 Product Manual Pure-IP Western Blot Detection Kit Catalog Number PRB-5002 20 blots FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction The technique of immunoprecipitation (IP) is used
Product Manual Pure-IP Western Blot Detection Kit Catalog Number PRB-5002 20 blots FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction The technique of immunoprecipitation (IP) is used
WESTERN BLOTTING TIPS AND TROUBLESHOOTING GUIDE TROUBLESHOOTING GUIDE
 WESTERN BLOTTING TIPS AND TROUBLESHOOTING GUIDE TIPS FOR SUCCESSFUL WESTERB BLOTS TROUBLESHOOTING GUIDE 1. Suboptimal protein transfer. This is the most common complaint with western blotting and could
WESTERN BLOTTING TIPS AND TROUBLESHOOTING GUIDE TIPS FOR SUCCESSFUL WESTERB BLOTS TROUBLESHOOTING GUIDE 1. Suboptimal protein transfer. This is the most common complaint with western blotting and could
TECHNICAL BULLETIN. HIS-Select Nickel Affinity Gel. Catalog Number P6611 Storage Temperature 2 8 C
 HIS-Select Nickel Affinity Gel Catalog Number P6611 Storage Temperature 2 8 C TECHNICAL BULLETIN Product Description HIS-Select Nickel Affinity Gel is an immobilized metalion affinity chromatography (IMAC)
HIS-Select Nickel Affinity Gel Catalog Number P6611 Storage Temperature 2 8 C TECHNICAL BULLETIN Product Description HIS-Select Nickel Affinity Gel is an immobilized metalion affinity chromatography (IMAC)
Angelo Peschiaroli, N. Valerio Dorrello, Daniele Guardavaccaro, Monica Venere, Thanos Halazonetis, Nicholas E. Sherman, and Michele Pagano
 Molecular Cell, Volume 23 Supplemental Data SCF βtrcp -Mediated Degradation of Claspin Regulates Recovery from the DNA Replication Checkpoint Response Angelo Peschiaroli, N. Valerio Dorrello, Daniele Guardavaccaro,
Molecular Cell, Volume 23 Supplemental Data SCF βtrcp -Mediated Degradation of Claspin Regulates Recovery from the DNA Replication Checkpoint Response Angelo Peschiaroli, N. Valerio Dorrello, Daniele Guardavaccaro,
How many of you have checked out the web site on protein-dna interactions?
 How many of you have checked out the web site on protein-dna interactions? Example of an approximately 40,000 probe spotted oligo microarray with enlarged inset to show detail. Find and be ready to discuss
How many of you have checked out the web site on protein-dna interactions? Example of an approximately 40,000 probe spotted oligo microarray with enlarged inset to show detail. Find and be ready to discuss
Chapter 2 Antibodies. Contents. Introduction
 Chapter 2 Antibodies Keywords Immunohistochemistry Antibody labeling Fluorescence microscopy Fluorescent immunocytochemistry Fluorescent immunohistochemistry Indirect immunocytochemistry Immunostaining
Chapter 2 Antibodies Keywords Immunohistochemistry Antibody labeling Fluorescence microscopy Fluorescent immunocytochemistry Fluorescent immunohistochemistry Indirect immunocytochemistry Immunostaining
Expression and Purification of Recombinant Protein in bacteria and Yeast. Presented By: Puspa pandey, Mohit sachdeva & Ming yu
 Expression and Purification of Recombinant Protein in bacteria and Yeast Presented By: Puspa pandey, Mohit sachdeva & Ming yu DNA Vectors Molecular carriers which carry fragments of DNA into host cell.
Expression and Purification of Recombinant Protein in bacteria and Yeast Presented By: Puspa pandey, Mohit sachdeva & Ming yu DNA Vectors Molecular carriers which carry fragments of DNA into host cell.
Protein extraction from Tissues and Cultured Cells using Bioruptor Standard & Plus
 Protein extraction from Tissues and Cultured Cells using Bioruptor Standard & Plus Introduction Protein extraction from tissues and cultured cells is the first step for many biochemical and analytical
Protein extraction from Tissues and Cultured Cells using Bioruptor Standard & Plus Introduction Protein extraction from tissues and cultured cells is the first step for many biochemical and analytical
Biotechnology and Recombinant DNA (Chapter 9) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College
 Biotechnology and Recombinant DNA (Chapter 9) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Primary Source for figures and content: Eastern Campus Tortora, G.J. Microbiology
Biotechnology and Recombinant DNA (Chapter 9) Lecture Materials for Amy Warenda Czura, Ph.D. Suffolk County Community College Primary Source for figures and content: Eastern Campus Tortora, G.J. Microbiology
Sample Questions for Exam 3
 Sample Questions for Exam 3 1. All of the following occur during prometaphase of mitosis in animal cells except a. the centrioles move toward opposite poles. b. the nucleolus can no longer be seen. c.
Sample Questions for Exam 3 1. All of the following occur during prometaphase of mitosis in animal cells except a. the centrioles move toward opposite poles. b. the nucleolus can no longer be seen. c.
QUANTITATIVE RT-PCR. A = B (1+e) n. A=amplified products, B=input templates, n=cycle number, and e=amplification efficiency.
 QUANTITATIVE RT-PCR Application: Quantitative RT-PCR is used to quantify mrna in both relative and absolute terms. It can be applied for the quantification of mrna expressed from endogenous genes, and
QUANTITATIVE RT-PCR Application: Quantitative RT-PCR is used to quantify mrna in both relative and absolute terms. It can be applied for the quantification of mrna expressed from endogenous genes, and
Fluorescein Isothiocyanate (FITC)- conjugated Antibodies
 USER GUIDE Fluorescein Isothiocyanate (FITC)- conjugated Antibodies Catalog Numbers R933-25, R953-25, R963-25 Document Part Number 25-0376 Publication Number MAN0000194 Revision 2.0 For Research Use Only.
USER GUIDE Fluorescein Isothiocyanate (FITC)- conjugated Antibodies Catalog Numbers R933-25, R953-25, R963-25 Document Part Number 25-0376 Publication Number MAN0000194 Revision 2.0 For Research Use Only.
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE Q5B
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE QUALITY OF BIOTECHNOLOGICAL PRODUCTS: ANALYSIS
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE QUALITY OF BIOTECHNOLOGICAL PRODUCTS: ANALYSIS
ChIP TROUBLESHOOTING TIPS
 ChIP TROUBLESHOOTING TIPS Creative Diagnostics Abstract ChIP dissects the spatial and temporal dynamics of the interactions between chromatin and its associated factors CD Creative Diagnostics info@creative-
ChIP TROUBLESHOOTING TIPS Creative Diagnostics Abstract ChIP dissects the spatial and temporal dynamics of the interactions between chromatin and its associated factors CD Creative Diagnostics info@creative-
The immune response Antibodies Antigens Epitopes (antigenic determinants) the part of a protein antigen recognized by an antibody Haptens small
 The immune response Antibodies Antigens Epitopes (antigenic determinants) the part of a protein antigen recognized by an antibody Haptens small molecules that can elicit an immune response when linked
The immune response Antibodies Antigens Epitopes (antigenic determinants) the part of a protein antigen recognized by an antibody Haptens small molecules that can elicit an immune response when linked
Chapter 18: Applications of Immunology
 Chapter 18: Applications of Immunology 1. Vaccinations 2. Monoclonal vs Polyclonal Ab 3. Diagnostic Immunology 1. Vaccinations What is Vaccination? A method of inducing artificial immunity by exposing
Chapter 18: Applications of Immunology 1. Vaccinations 2. Monoclonal vs Polyclonal Ab 3. Diagnostic Immunology 1. Vaccinations What is Vaccination? A method of inducing artificial immunity by exposing
Genomic DNA Clean & Concentrator Catalog Nos. D4010 & D4011
 Page 0 INSTRUCTION MANUAL Catalog Nos. D4010 & D4011 Highlights Quick (5 minute) spin column recovery of large-sized DNA (e.g., genomic, mitochondrial, plasmid (BAC/PAC), viral, phage, (wga)dna, etc.)
Page 0 INSTRUCTION MANUAL Catalog Nos. D4010 & D4011 Highlights Quick (5 minute) spin column recovery of large-sized DNA (e.g., genomic, mitochondrial, plasmid (BAC/PAC), viral, phage, (wga)dna, etc.)
Approaches that can be used to study expression of specific proteins
 Approaches that can be used to study expression of specific proteins Receptors and transporters Homogenate binding studies Receptor autoradiography Radiochemical Western blotting Immunohistochemistry/cytochemistry
Approaches that can be used to study expression of specific proteins Receptors and transporters Homogenate binding studies Receptor autoradiography Radiochemical Western blotting Immunohistochemistry/cytochemistry
竞 争 性 分 析 Epitope Mapping 实 验 方 法
 竞 争 性 分 析 Epitope Mapping 实 验 方 法 ABSTRACT The simplest way to determine whether two monoclonal antibodies bind to distinct sites on a protein antigen is to carry out a competition assay. The assay can
竞 争 性 分 析 Epitope Mapping 实 验 方 法 ABSTRACT The simplest way to determine whether two monoclonal antibodies bind to distinct sites on a protein antigen is to carry out a competition assay. The assay can
Investigating the role of a Cryptosporidium parum apyrase in infection
 Investigating the role of a Cryptosporidium parum apyrase in infection David Riccardi and Patricio Manque Abstract This project attempted to characterize the function of a Cryptosporidium parvum apyrase
Investigating the role of a Cryptosporidium parum apyrase in infection David Riccardi and Patricio Manque Abstract This project attempted to characterize the function of a Cryptosporidium parvum apyrase
In vitro analysis of pri-mirna processing. by Drosha-DGCR8 complex. (Narry Kim s lab)
 In vitro analysis of pri-mirna processing by Drosha-DGCR8 complex (Narry Kim s lab) 1-1. Preparation of radiolabeled pri-mirna transcript The RNA substrate for a cropping reaction can be prepared by in
In vitro analysis of pri-mirna processing by Drosha-DGCR8 complex (Narry Kim s lab) 1-1. Preparation of radiolabeled pri-mirna transcript The RNA substrate for a cropping reaction can be prepared by in
Ubiquitin Interact Kit
 Ubiquitin Interact Kit Item No. 15978 Customer Service 800.364.9897 * Technical Support 888.526.5351 www.caymanchem.com TABLE OF CONTENTS GENERAL INFORMATION 3 Materials Supplied 3 Precautions 4 If You
Ubiquitin Interact Kit Item No. 15978 Customer Service 800.364.9897 * Technical Support 888.526.5351 www.caymanchem.com TABLE OF CONTENTS GENERAL INFORMATION 3 Materials Supplied 3 Precautions 4 If You
Recombinant Enterokinase Kits
 Table of Contents About the Kits...2 Description 2 Components 2 rek Cleavage...3 Small scale optimization 3 Scale-up 4 Monitoring cleavage 4 rek Capture...5 Capture buffer considerations 5 Monitoring rek
Table of Contents About the Kits...2 Description 2 Components 2 rek Cleavage...3 Small scale optimization 3 Scale-up 4 Monitoring cleavage 4 rek Capture...5 Capture buffer considerations 5 Monitoring rek
Interaktionen von RNAs und Proteinen
 Sonja Prohaska Computational EvoDevo Universitaet Leipzig June 9, 2015 Studying RNA-protein interactions Given: target protein known to bind to RNA problem: find binding partners and binding sites experimental
Sonja Prohaska Computational EvoDevo Universitaet Leipzig June 9, 2015 Studying RNA-protein interactions Given: target protein known to bind to RNA problem: find binding partners and binding sites experimental
Protocol for Western Blotting
 Protocol for Western Blotting Materials Materials used on Day 3 Protease inhibitor stock: 1 μg/μl pepstatin A in DMSO 200 μm leupeptin in OG Buffer 200 mm PMSF: Freshly made. Ex) 34.8 mg PMSF in 1 ml isopropanol
Protocol for Western Blotting Materials Materials used on Day 3 Protease inhibitor stock: 1 μg/μl pepstatin A in DMSO 200 μm leupeptin in OG Buffer 200 mm PMSF: Freshly made. Ex) 34.8 mg PMSF in 1 ml isopropanol
Molecular Biology Techniques: A Classroom Laboratory Manual THIRD EDITION
 Molecular Biology Techniques: A Classroom Laboratory Manual THIRD EDITION Susan Carson Heather B. Miller D.Scott Witherow ELSEVIER AMSTERDAM BOSTON HEIDELBERG LONDON NEW YORK OXFORD PARIS SAN DIEGO SAN
Molecular Biology Techniques: A Classroom Laboratory Manual THIRD EDITION Susan Carson Heather B. Miller D.Scott Witherow ELSEVIER AMSTERDAM BOSTON HEIDELBERG LONDON NEW YORK OXFORD PARIS SAN DIEGO SAN
RevertAid Premium First Strand cdna Synthesis Kit
 RevertAid Premium First Strand cdna Synthesis Kit #K1651, #K1652 CERTIFICATE OF ANALYSIS #K1651 Lot QUALITY CONTROL RT-PCR using 100 fg of control GAPDH RNA and GAPDH control primers generated a prominent
RevertAid Premium First Strand cdna Synthesis Kit #K1651, #K1652 CERTIFICATE OF ANALYSIS #K1651 Lot QUALITY CONTROL RT-PCR using 100 fg of control GAPDH RNA and GAPDH control primers generated a prominent
Appendix C DNA Replication & Mitosis
 K.Muma Bio 6 Appendix C DNA Replication & Mitosis Study Objectives: Appendix C: DNA replication and Mitosis 1. Describe the structure of DNA and where it is found. 2. Explain complimentary base pairing:
K.Muma Bio 6 Appendix C DNA Replication & Mitosis Study Objectives: Appendix C: DNA replication and Mitosis 1. Describe the structure of DNA and where it is found. 2. Explain complimentary base pairing:
Antibody Production Price List
 Antibody Production Price List Presenting Insight Biotechnology s price list for custom polyclonal and monoclonal antibody production services. We are happy to tailor individual packages towards the specific
Antibody Production Price List Presenting Insight Biotechnology s price list for custom polyclonal and monoclonal antibody production services. We are happy to tailor individual packages towards the specific
Rubisco; easy Purification and Immunochemical Determination
 Rubisco; easy Purification and Immunochemical Determination Ulrich Groß Justus-Liebig-Universität Gießen, Institute of Plant Nutrition, Department of Tissue Culture, Südanlage 6, D-35390 Giessen e-mail:
Rubisco; easy Purification and Immunochemical Determination Ulrich Groß Justus-Liebig-Universität Gießen, Institute of Plant Nutrition, Department of Tissue Culture, Südanlage 6, D-35390 Giessen e-mail:
First Strand cdna Synthesis
 380PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name First Strand cdna Synthesis (Cat. # 786 812) think proteins! think G-Biosciences
380PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name First Strand cdna Synthesis (Cat. # 786 812) think proteins! think G-Biosciences
APPLICATION FOCUS. Application Solutions for Western Blotting
 APPLICATION FOCUS Application Solutions for Western Blotting WESTERN BLOTTING Companion Products Solutions for consistently better blotting. Companion products from Cell Signaling Technology (CST) are
APPLICATION FOCUS Application Solutions for Western Blotting WESTERN BLOTTING Companion Products Solutions for consistently better blotting. Companion products from Cell Signaling Technology (CST) are
Protein/Protein and Protein/RNA Co-Immunoprecipitations (Kenny Watkins and Alice Barkan, updated 12/09)
 Protein/Protein and Protein/RNA Co-Immunoprecipitations (Kenny Watkins and Alice Barkan, updated 12/09) Part 1 is a simple coip method for directed coip assays, to determine whether two known proteins
Protein/Protein and Protein/RNA Co-Immunoprecipitations (Kenny Watkins and Alice Barkan, updated 12/09) Part 1 is a simple coip method for directed coip assays, to determine whether two known proteins
The Need for a PARP in vivo Pharmacodynamic Assay
 The Need for a PARP in vivo Pharmacodynamic Assay Jay George, Ph.D., Chief Scientific Officer, Trevigen, Inc., Gaithersburg, MD For further infomation, please contact: William Booth, Ph.D. Tel: +44 (0)1235
The Need for a PARP in vivo Pharmacodynamic Assay Jay George, Ph.D., Chief Scientific Officer, Trevigen, Inc., Gaithersburg, MD For further infomation, please contact: William Booth, Ph.D. Tel: +44 (0)1235
MEF Nucleofector Kit 1 and 2
 page 1 of 7 MEF Nucleofector Kit 1 and 2 for Mouse Embryonic Fibroblasts (MEF) MEF display significant phenotypic variations which depend on the strain, the genetic background of the they are isolated
page 1 of 7 MEF Nucleofector Kit 1 and 2 for Mouse Embryonic Fibroblasts (MEF) MEF display significant phenotypic variations which depend on the strain, the genetic background of the they are isolated
MEF Starter Nucleofector Kit
 page 1 of 7 MEF Starter Nucleofector Kit for Mouse Embryonic Fibroblasts (MEF) MEF display significant phenotypic variations which depend on the strain, the genetic background of the mice they are isolated
page 1 of 7 MEF Starter Nucleofector Kit for Mouse Embryonic Fibroblasts (MEF) MEF display significant phenotypic variations which depend on the strain, the genetic background of the mice they are isolated
Western Blotting. USA: proteintech@ptglab.com UK & Europe: europe@ptglab.com China: service@ptglab.com. www.ptglab.com
 Western Blotting All steps are carried out at room temperature unless otherwise indicated. Recipes for all solutions highlighted bold are included at the end of the protocol. SDS-PAGE 1. Construct an SDS-PAGE
Western Blotting All steps are carried out at room temperature unless otherwise indicated. Recipes for all solutions highlighted bold are included at the end of the protocol. SDS-PAGE 1. Construct an SDS-PAGE
Optimal Conditions for F(ab ) 2 Antibody Fragment Production from Mouse IgG2a
 Optimal Conditions for F(ab ) 2 Antibody Fragment Production from Mouse IgG2a Ryan S. Stowers, 1 Jacqueline A. Callihan, 2 James D. Bryers 2 1 Department of Bioengineering, Clemson University, Clemson,
Optimal Conditions for F(ab ) 2 Antibody Fragment Production from Mouse IgG2a Ryan S. Stowers, 1 Jacqueline A. Callihan, 2 James D. Bryers 2 1 Department of Bioengineering, Clemson University, Clemson,
Genolution Pharmaceuticals, Inc. Life Science and Molecular Diagnostic Products
 Genolution Pharmaceuticals, Inc. Revolution through genes, And Solution through genes. Life Science and Molecular Diagnostic Products www.genolution1.com TEL; 02-3010-8670, 8672 Geno-Serum Hepatitis B
Genolution Pharmaceuticals, Inc. Revolution through genes, And Solution through genes. Life Science and Molecular Diagnostic Products www.genolution1.com TEL; 02-3010-8670, 8672 Geno-Serum Hepatitis B
Molecular Cloning, Product Brochure
 , Product Brochure Interest in any of the products, request or order them at Bio-Connect. Bio-Connect B.V. T NL +31 (0)26 326 44 50 T BE +32 (0)2 503 03 48 Begonialaan 3a F NL +31 (0)26 326 44 51 F BE
, Product Brochure Interest in any of the products, request or order them at Bio-Connect. Bio-Connect B.V. T NL +31 (0)26 326 44 50 T BE +32 (0)2 503 03 48 Begonialaan 3a F NL +31 (0)26 326 44 51 F BE
European Medicines Agency
 European Medicines Agency July 1996 CPMP/ICH/139/95 ICH Topic Q 5 B Quality of Biotechnological Products: Analysis of the Expression Construct in Cell Lines Used for Production of r-dna Derived Protein
European Medicines Agency July 1996 CPMP/ICH/139/95 ICH Topic Q 5 B Quality of Biotechnological Products: Analysis of the Expression Construct in Cell Lines Used for Production of r-dna Derived Protein
HCS604.03 Exercise 1 Dr. Jones Spring 2005. Recombinant DNA (Molecular Cloning) exercise:
 HCS604.03 Exercise 1 Dr. Jones Spring 2005 Recombinant DNA (Molecular Cloning) exercise: The purpose of this exercise is to learn techniques used to create recombinant DNA or clone genes. You will clone
HCS604.03 Exercise 1 Dr. Jones Spring 2005 Recombinant DNA (Molecular Cloning) exercise: The purpose of this exercise is to learn techniques used to create recombinant DNA or clone genes. You will clone
Rapid GST Inclusion Body Solubilization and Renaturation Kit
 Product Manual Rapid GST Inclusion Body Solubilization and Renaturation Kit Catalog Number AKR-110 FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Bacteria are widely used for His
Product Manual Rapid GST Inclusion Body Solubilization and Renaturation Kit Catalog Number AKR-110 FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Bacteria are widely used for His
4. DNA replication Pages: 979-984 Difficulty: 2 Ans: C Which one of the following statements about enzymes that interact with DNA is true?
 Chapter 25 DNA Metabolism Multiple Choice Questions 1. DNA replication Page: 977 Difficulty: 2 Ans: C The Meselson-Stahl experiment established that: A) DNA polymerase has a crucial role in DNA synthesis.
Chapter 25 DNA Metabolism Multiple Choice Questions 1. DNA replication Page: 977 Difficulty: 2 Ans: C The Meselson-Stahl experiment established that: A) DNA polymerase has a crucial role in DNA synthesis.
CD3/TCR stimulation and surface detection Determination of specificity of intracellular detection of IL-7Rα by flow cytometry
 CD3/TCR stimulation and surface detection Stimulation of HPB-ALL cells with the anti-cd3 monoclonal antibody OKT3 was performed as described 3. In brief, antibody-coated plates were prepared by incubating
CD3/TCR stimulation and surface detection Stimulation of HPB-ALL cells with the anti-cd3 monoclonal antibody OKT3 was performed as described 3. In brief, antibody-coated plates were prepared by incubating
Western Blotting: Mini-gels
 Western Blotting: Mini-gels Materials a Protein Extraction Buffer (for callus or kernel), Solution Stock Final Volume Tris-HCl ph 80 1 M 200 mm 20 ml NaCl 4 M 100 mm 25 ml Sucrose 2 M 400 mm 20 ml EDTA
Western Blotting: Mini-gels Materials a Protein Extraction Buffer (for callus or kernel), Solution Stock Final Volume Tris-HCl ph 80 1 M 200 mm 20 ml NaCl 4 M 100 mm 25 ml Sucrose 2 M 400 mm 20 ml EDTA
INSTRUCTION Probemaker
 INSTRUCTION Probemaker Instructions for Duolink In Situ Probemaker PLUS (Art. no. 92009-0020) and Duolink In Situ Probemaker MINUS (Art. no. 92010-0020) Table of content 1. Introduction 4 2. Applications
INSTRUCTION Probemaker Instructions for Duolink In Situ Probemaker PLUS (Art. no. 92009-0020) and Duolink In Situ Probemaker MINUS (Art. no. 92010-0020) Table of content 1. Introduction 4 2. Applications
Peptide Antibody Production
 Peptide Antibody Production A) Peptide BioSynthesis (http://www.biosyn.com, 800-227-0627) B) Conjugation of peptide to KLH (Imject Maleimide Activated KLH, PIERCE=Thermo #77605, 10 mg) C) Peptide affinity
Peptide Antibody Production A) Peptide BioSynthesis (http://www.biosyn.com, 800-227-0627) B) Conjugation of peptide to KLH (Imject Maleimide Activated KLH, PIERCE=Thermo #77605, 10 mg) C) Peptide affinity
STUDIES ON SEED STORAGE PROTEINS OF SOME ECONOMICALLY MINOR PLANTS
 STUDIES ON SEED STORAGE PROTEINS OF SOME ECONOMICALLY MINOR PLANTS THESIS SUBMITTED FOR THE DEGREB OF DOCTOR OF PHILOSOPHY (SCIENCE) OF THE UNIVERSITY OF CALCUTTA 1996 NRISINHA DE, M.Sc DEPARTMENT OF BIOCHEMISTRY
STUDIES ON SEED STORAGE PROTEINS OF SOME ECONOMICALLY MINOR PLANTS THESIS SUBMITTED FOR THE DEGREB OF DOCTOR OF PHILOSOPHY (SCIENCE) OF THE UNIVERSITY OF CALCUTTA 1996 NRISINHA DE, M.Sc DEPARTMENT OF BIOCHEMISTRY
How To Understand How Gene Expression Is Regulated
 What makes cells different from each other? How do cells respond to information from environment? Regulation of: - Transcription - prokaryotes - eukaryotes - mrna splicing - mrna localisation and translation
What makes cells different from each other? How do cells respond to information from environment? Regulation of: - Transcription - prokaryotes - eukaryotes - mrna splicing - mrna localisation and translation
Chapter 5. Characterization of. Topoisomerase II alpha and beta. Kinase activities
 Chapter 5 Characterization of Topoisomerase II alpha and beta Kinase activities The results incorporated in the previous chapter demonstrated the presence of two molecular species of kinases in the purified
Chapter 5 Characterization of Topoisomerase II alpha and beta Kinase activities The results incorporated in the previous chapter demonstrated the presence of two molecular species of kinases in the purified
DNA Fingerprinting. Unless they are identical twins, individuals have unique DNA
 DNA Fingerprinting Unless they are identical twins, individuals have unique DNA DNA fingerprinting The name used for the unambiguous identifying technique that takes advantage of differences in DNA sequence
DNA Fingerprinting Unless they are identical twins, individuals have unique DNA DNA fingerprinting The name used for the unambiguous identifying technique that takes advantage of differences in DNA sequence
Supplemental Information. McBrayer et al. Supplemental Data
 1 Supplemental Information McBrayer et al. Supplemental Data 2 Figure S1. Glucose consumption rates of MM cell lines exceed that of normal PBMC. (A) Normal PBMC isolated from three healthy donors were
1 Supplemental Information McBrayer et al. Supplemental Data 2 Figure S1. Glucose consumption rates of MM cell lines exceed that of normal PBMC. (A) Normal PBMC isolated from three healthy donors were
Chapter 18 Regulation of Gene Expression
 Chapter 18 Regulation of Gene Expression 18.1. Gene Regulation Is Necessary By switching genes off when they are not needed, cells can prevent resources from being wasted. There should be natural selection
Chapter 18 Regulation of Gene Expression 18.1. Gene Regulation Is Necessary By switching genes off when they are not needed, cells can prevent resources from being wasted. There should be natural selection
NimbleGen DNA Methylation Microarrays and Services
 NimbleGen DNA Methylation Microarrays and Services Sample Preparation Instructions Outline This protocol describes the process for preparing samples for NimbleGen DNA Methylation microarrays using the
NimbleGen DNA Methylation Microarrays and Services Sample Preparation Instructions Outline This protocol describes the process for preparing samples for NimbleGen DNA Methylation microarrays using the
6 Characterization of Casein and Bovine Serum Albumin
 6 Characterization of Casein and Bovine Serum Albumin (BSA) Objectives: A) To separate a mixture of casein and bovine serum albumin B) to characterize these proteins based on their solubilities as a function
6 Characterization of Casein and Bovine Serum Albumin (BSA) Objectives: A) To separate a mixture of casein and bovine serum albumin B) to characterize these proteins based on their solubilities as a function
Interim Progress Report R&D Project 348. Development of a Field Test Kit for Detection of Blue-Green Algal Toxins
 Interim Progress Report R&D Project 348 Development of a Field Test Kit for Detection of Blue-Green Algal Toxins Biocode Limited November 1992 R&D 348/04/A ENVIRONMENT AGENCY 135357 CONTENTS SUMMARY KEYWORDS
Interim Progress Report R&D Project 348 Development of a Field Test Kit for Detection of Blue-Green Algal Toxins Biocode Limited November 1992 R&D 348/04/A ENVIRONMENT AGENCY 135357 CONTENTS SUMMARY KEYWORDS
Publikationsliste Claudia Götz
 Publikationsliste Claudia Götz 1. Reinhard,B., Götz, C., and Faillard, H.: Synthesis of N-Acetyl-9-Oacetylneuraminic acid α-p-aminophenylthioketoside and its application as ligand in the affinity chromatography
Publikationsliste Claudia Götz 1. Reinhard,B., Götz, C., and Faillard, H.: Synthesis of N-Acetyl-9-Oacetylneuraminic acid α-p-aminophenylthioketoside and its application as ligand in the affinity chromatography
VLLM0421c Medical Microbiology I, practical sessions. Protocol to topic J10
 Topic J10+11: Molecular-biological methods + Clinical virology I (hepatitis A, B & C, HIV) To study: PCR, ELISA, your own notes from serology reactions Task J10/1: DNA isolation of the etiological agent
Topic J10+11: Molecular-biological methods + Clinical virology I (hepatitis A, B & C, HIV) To study: PCR, ELISA, your own notes from serology reactions Task J10/1: DNA isolation of the etiological agent
Genomic DNA Extraction Kit INSTRUCTION MANUAL
 Genomic DNA Extraction Kit INSTRUCTION MANUAL Table of Contents Introduction 3 Kit Components 3 Storage Conditions 4 Recommended Equipment and Reagents 4 Introduction to the Protocol 4 General Overview
Genomic DNA Extraction Kit INSTRUCTION MANUAL Table of Contents Introduction 3 Kit Components 3 Storage Conditions 4 Recommended Equipment and Reagents 4 Introduction to the Protocol 4 General Overview
Methionine Sulfoxide Immunoblotting Kit
 Methionine Sulfoxide Immunoblotting Kit Item No. 600160 Customer Service 800.364.9897 * Technical Support 888.526.5351 www.caymanchem.com TABLE OF CONTENTS GENERAL INFORMATION 3 Materials Supplied 4 Precautions
Methionine Sulfoxide Immunoblotting Kit Item No. 600160 Customer Service 800.364.9897 * Technical Support 888.526.5351 www.caymanchem.com TABLE OF CONTENTS GENERAL INFORMATION 3 Materials Supplied 4 Precautions
pcas-guide System Validation in Genome Editing
 pcas-guide System Validation in Genome Editing Tagging HSP60 with HA tag genome editing The latest tool in genome editing CRISPR/Cas9 allows for specific genome disruption and replacement in a flexible
pcas-guide System Validation in Genome Editing Tagging HSP60 with HA tag genome editing The latest tool in genome editing CRISPR/Cas9 allows for specific genome disruption and replacement in a flexible
DP419 RNAsimple Total RNA Kit. RNAprep pure Series. DP501 mircute mirna Isolation Kit. DP438 MagGene Viral DNA / RNA Kit. DP405 TRNzol Reagent
 Overview of TIANGEN Products DP419 RNAsimple Total RNA Kit DP430 RNAprep pure Kit(For Cell/Bacteria) DP315/DP315-R TIANamp Virus DNA/RNA Kit DP431 RNAprep pure Kit (For Tissue) Silica-membrane Technology
Overview of TIANGEN Products DP419 RNAsimple Total RNA Kit DP430 RNAprep pure Kit(For Cell/Bacteria) DP315/DP315-R TIANamp Virus DNA/RNA Kit DP431 RNAprep pure Kit (For Tissue) Silica-membrane Technology
Taq98 Hot Start 2X Master Mix
 Taq98 Hot Start 2X Master Mix Optimized for 98C Denaturation Lucigen Corporation 2905 Parmenter St, Middleton, WI 53562 USA Toll Free: (888) 575-9695 (608) 831-9011 FAX: (608) 831-9012 lucigen@lucigen.com
Taq98 Hot Start 2X Master Mix Optimized for 98C Denaturation Lucigen Corporation 2905 Parmenter St, Middleton, WI 53562 USA Toll Free: (888) 575-9695 (608) 831-9011 FAX: (608) 831-9012 lucigen@lucigen.com
Notch 1 -dependent regulation of cell fate in colorectal cancer
 Notch 1 -dependent regulation of cell fate in colorectal cancer Referees: PD Dr. Tobias Dick Prof. Dr. Wilfried Roth http://d-nb.info/1057851272 CONTENTS Summary 1 Zusammenfassung 2 1 INTRODUCTION 3 1.1
Notch 1 -dependent regulation of cell fate in colorectal cancer Referees: PD Dr. Tobias Dick Prof. Dr. Wilfried Roth http://d-nb.info/1057851272 CONTENTS Summary 1 Zusammenfassung 2 1 INTRODUCTION 3 1.1
Western Blot Analysis with Cell Samples Grown in Channel-µ-Slides
 Western Blot Analysis with Cell Samples Grown in Channel-µ-Slides Polyacrylamide gel electrophoresis (PAGE) and subsequent analyses are common tools in biochemistry and molecular biology. This Application
Western Blot Analysis with Cell Samples Grown in Channel-µ-Slides Polyacrylamide gel electrophoresis (PAGE) and subsequent analyses are common tools in biochemistry and molecular biology. This Application
ab185915 Protein Sumoylation Assay Ultra Kit
 ab185915 Protein Sumoylation Assay Ultra Kit Instructions for Use For the measuring in vivo protein sumoylation in various samples This product is for research use only and is not intended for diagnostic
ab185915 Protein Sumoylation Assay Ultra Kit Instructions for Use For the measuring in vivo protein sumoylation in various samples This product is for research use only and is not intended for diagnostic
Control of Gene Expression
 Home Gene Regulation Is Necessary? Control of Gene Expression By switching genes off when they are not needed, cells can prevent resources from being wasted. There should be natural selection favoring
Home Gene Regulation Is Necessary? Control of Gene Expression By switching genes off when they are not needed, cells can prevent resources from being wasted. There should be natural selection favoring
Genetic information (DNA) determines structure of proteins DNA RNA proteins cell structure 3.11 3.15 enzymes control cell chemistry ( metabolism )
 Biology 1406 Exam 3 Notes Structure of DNA Ch. 10 Genetic information (DNA) determines structure of proteins DNA RNA proteins cell structure 3.11 3.15 enzymes control cell chemistry ( metabolism ) Proteins
Biology 1406 Exam 3 Notes Structure of DNA Ch. 10 Genetic information (DNA) determines structure of proteins DNA RNA proteins cell structure 3.11 3.15 enzymes control cell chemistry ( metabolism ) Proteins
PROTOCOL. Immunostaining for Flow Cytometry. Background. Materials and equipment required.
 PROTOCOL Immunostaining for Flow Cytometry 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 Rev.0 Background The combination of single cell analysis using flow cytometry and the specificity of antibody-based
PROTOCOL Immunostaining for Flow Cytometry 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 Rev.0 Background The combination of single cell analysis using flow cytometry and the specificity of antibody-based
Chapter 3. Protein Structure and Function
 Chapter 3 Protein Structure and Function Broad functional classes So Proteins have structure and function... Fine! -Why do we care to know more???? Understanding functional architechture gives us POWER
Chapter 3 Protein Structure and Function Broad functional classes So Proteins have structure and function... Fine! -Why do we care to know more???? Understanding functional architechture gives us POWER
Lecture 8. Protein Trafficking/Targeting. Protein targeting is necessary for proteins that are destined to work outside the cytoplasm.
 Protein Trafficking/Targeting (8.1) Lecture 8 Protein Trafficking/Targeting Protein targeting is necessary for proteins that are destined to work outside the cytoplasm. Protein targeting is more complex
Protein Trafficking/Targeting (8.1) Lecture 8 Protein Trafficking/Targeting Protein targeting is necessary for proteins that are destined to work outside the cytoplasm. Protein targeting is more complex
Custom Polyclonal Anti-Peptide Antibody, Brochure
 Custom Polyclonal Anti-Peptide Antibody, Brochure Interest in any of the products, request or order them at Bio-Connect. Bio-Connect B.V. T NL +31 (0)26 326 44 50 T BE +32 (0)2 503 03 48 Begonialaan 3a
Custom Polyclonal Anti-Peptide Antibody, Brochure Interest in any of the products, request or order them at Bio-Connect. Bio-Connect B.V. T NL +31 (0)26 326 44 50 T BE +32 (0)2 503 03 48 Begonialaan 3a
PCR was carried out in a reaction volume of 20 µl using the ABI AmpliTaq GOLD kit (ABI,
 Supplemental Text/Tables PCR Amplification and Sequencing PCR was carried out in a reaction volume of 20 µl using the ABI AmpliTaq GOLD kit (ABI, Foster City, CA). Each PCR reaction contained 20 ng genomic
Supplemental Text/Tables PCR Amplification and Sequencing PCR was carried out in a reaction volume of 20 µl using the ABI AmpliTaq GOLD kit (ABI, Foster City, CA). Each PCR reaction contained 20 ng genomic
Protein transfer from SDS-PAGE to nitrocellulose membrane using the Trans-Blot SD cell (Western).
 Western Blot SOP Protein transfer from SDS-PAGE to nitrocellulose membrane using the Trans-Blot SD cell (Western). Date: 8/16/05, 10/31/05, 2/6/06 Author: N.Oganesyan, R. Kim Edited by: R. Kim Summary:
Western Blot SOP Protein transfer from SDS-PAGE to nitrocellulose membrane using the Trans-Blot SD cell (Western). Date: 8/16/05, 10/31/05, 2/6/06 Author: N.Oganesyan, R. Kim Edited by: R. Kim Summary:
ELISA BIO 110 Lab 1. Immunity and Disease
 ELISA BIO 110 Lab 1 Immunity and Disease Introduction The principal role of the mammalian immune response is to contain infectious disease agents. This response is mediated by several cellular and molecular
ELISA BIO 110 Lab 1 Immunity and Disease Introduction The principal role of the mammalian immune response is to contain infectious disease agents. This response is mediated by several cellular and molecular
Gene Expression Assays
 APPLICATION NOTE TaqMan Gene Expression Assays A mpl i fic ationef ficienc yof TaqMan Gene Expression Assays Assays tested extensively for qpcr efficiency Key factors that affect efficiency Efficiency
APPLICATION NOTE TaqMan Gene Expression Assays A mpl i fic ationef ficienc yof TaqMan Gene Expression Assays Assays tested extensively for qpcr efficiency Key factors that affect efficiency Efficiency
STANDARD OPERATING PROCEDURE
 STANDARD OPERATING PROCEDURE Title: Evaluation using Western Blot SOP#: M-103 Version #: 1 Author: R. Saul Date Approved: Feb. 5, 2009 Date Modified: 1. PURPOSE The purpose of this document is to describe
STANDARD OPERATING PROCEDURE Title: Evaluation using Western Blot SOP#: M-103 Version #: 1 Author: R. Saul Date Approved: Feb. 5, 2009 Date Modified: 1. PURPOSE The purpose of this document is to describe
SUPPLEMENTARY DATA 1
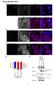 SUPPLEMENTARY DATA 1 Supplementary Figure S1. Overexpression of untagged Ago2 inhibits the nuclear transport of the dotted foci of GFP-signal of myc-gfp-tnrc6a-nes-mut. (A C) HeLa cells expressing myc-gfp-tnrc6a-nes-mut
SUPPLEMENTARY DATA 1 Supplementary Figure S1. Overexpression of untagged Ago2 inhibits the nuclear transport of the dotted foci of GFP-signal of myc-gfp-tnrc6a-nes-mut. (A C) HeLa cells expressing myc-gfp-tnrc6a-nes-mut
ELUTION OF DNA FROM AGAROSE GELS
 ELUTION OF DNA FROM AGAROSE GELS OBTECTIVE: To isolate specific bands or regions of agarose-separated DNA for use in subsequent experiments and/or procedures. INTRODUCTION: It is sometimes necessary to
ELUTION OF DNA FROM AGAROSE GELS OBTECTIVE: To isolate specific bands or regions of agarose-separated DNA for use in subsequent experiments and/or procedures. INTRODUCTION: It is sometimes necessary to
HighPure Maxi Plasmid Kit
 HighPure Maxi Plasmid Kit For purification of high pure plasmid DNA with high yields www.tiangen.com PP120109 HighPure Maxi Plasmid Kit Kit Contents Storage Cat.no. DP116 Contents RNaseA (100 mg/ml) Buffer
HighPure Maxi Plasmid Kit For purification of high pure plasmid DNA with high yields www.tiangen.com PP120109 HighPure Maxi Plasmid Kit Kit Contents Storage Cat.no. DP116 Contents RNaseA (100 mg/ml) Buffer
The Biotechnology Education Company
 EDVTEK P.. Box 1232 West Bethesda, MD 20827-1232 The Biotechnology 106 EDV-Kit # Principles of DNA Sequencing Experiment bjective: The objective of this experiment is to develop an understanding of DNA
EDVTEK P.. Box 1232 West Bethesda, MD 20827-1232 The Biotechnology 106 EDV-Kit # Principles of DNA Sequencing Experiment bjective: The objective of this experiment is to develop an understanding of DNA
Proteomics Research with BIOCHAIN
 Proteomics Research with IOCHIN Protein Extraction CNMCS Compartmental Protein Extraction Kit Cytoplasmic, Nuclear, Membrane, and Cytoskeleton Protein One kit isolates four different proteins sequentially
Proteomics Research with IOCHIN Protein Extraction CNMCS Compartmental Protein Extraction Kit Cytoplasmic, Nuclear, Membrane, and Cytoskeleton Protein One kit isolates four different proteins sequentially
Protein Analysis. -Detection and quantification. Toby M Holmes
 Protein Analysis -Detection and quantification Toby M Holmes Clinical Research Unit UCD school of Medicine and Medical Sciences Mater Misericordiae University Hospital Dublin Protein structure General
Protein Analysis -Detection and quantification Toby M Holmes Clinical Research Unit UCD school of Medicine and Medical Sciences Mater Misericordiae University Hospital Dublin Protein structure General
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/7/339/ra80/dc1 Supplementary Materials for Manipulation of receptor oligomerization as a strategy to inhibit signaling by TNF superfamily members Julia T. Warren,
www.sciencesignaling.org/cgi/content/full/7/339/ra80/dc1 Supplementary Materials for Manipulation of receptor oligomerization as a strategy to inhibit signaling by TNF superfamily members Julia T. Warren,
Aviva Systems Biology
 Aviva Custom Antibody Service and Price Mouse Monoclonal Antibody Service Package Number Description Package Contents Time Price Customer provides antigen protein $6,174 Monoclonal package1 (From protein
Aviva Custom Antibody Service and Price Mouse Monoclonal Antibody Service Package Number Description Package Contents Time Price Customer provides antigen protein $6,174 Monoclonal package1 (From protein
Antibody Function & Structure
 Antibody Function & Structure Specifically bind to antigens in both the recognition phase (cellular receptors) and during the effector phase (synthesis and secretion) of humoral immunity Serology: the
Antibody Function & Structure Specifically bind to antigens in both the recognition phase (cellular receptors) and during the effector phase (synthesis and secretion) of humoral immunity Serology: the
Cell Division Mitosis and the Cell Cycle
 Cell Division Mitosis and the Cell Cycle A Chromosome and Sister Chromatids Key Points About Chromosome Structure A chromosome consists of DNA that is wrapped around proteins (histones) and condensed Each
Cell Division Mitosis and the Cell Cycle A Chromosome and Sister Chromatids Key Points About Chromosome Structure A chromosome consists of DNA that is wrapped around proteins (histones) and condensed Each
Recombinant DNA Technology
 Recombinant DNA Technology Dates in the Development of Gene Cloning: 1965 - plasmids 1967 - ligase 1970 - restriction endonucleases 1972 - first experiments in gene splicing 1974 - worldwide moratorium
Recombinant DNA Technology Dates in the Development of Gene Cloning: 1965 - plasmids 1967 - ligase 1970 - restriction endonucleases 1972 - first experiments in gene splicing 1974 - worldwide moratorium
Plant Genomic DNA Extraction using CTAB
 Plant Genomic DNA Extraction using CTAB Introduction The search for a more efficient means of extracting DNA of both higher quality and yield has lead to the development of a variety of protocols, however
Plant Genomic DNA Extraction using CTAB Introduction The search for a more efficient means of extracting DNA of both higher quality and yield has lead to the development of a variety of protocols, however
Profiling of non-coding RNA classes Gunter Meister
 Profiling of non-coding RNA classes Gunter Meister RNA Biology Regensburg University Universitätsstrasse 31 93053 Regensburg Overview Classes of non-coding RNAs Profiling strategies Validation Protein-RNA
Profiling of non-coding RNA classes Gunter Meister RNA Biology Regensburg University Universitätsstrasse 31 93053 Regensburg Overview Classes of non-coding RNAs Profiling strategies Validation Protein-RNA
Integrated Protein Services
 Integrated Protein Services Custom protein expression & purification Version DC04-0012 Expression strategy The first step in the recombinant protein generation process is to design an appropriate expression
Integrated Protein Services Custom protein expression & purification Version DC04-0012 Expression strategy The first step in the recombinant protein generation process is to design an appropriate expression
FAK phospho S732 Antibody, Rabbit Polyclonal Antibody
 GenWay Biotech, Inc. 6777 Nancy Ridge Drive San Diego, CA 92121 Tel: (858)458-0866 Fax: (858)458-0833 sales@genwaybio.com FAK phospho S732 Antibody, Rabbit Polyclonal Antibody Catalog Number: 18-272-197354
GenWay Biotech, Inc. 6777 Nancy Ridge Drive San Diego, CA 92121 Tel: (858)458-0866 Fax: (858)458-0833 sales@genwaybio.com FAK phospho S732 Antibody, Rabbit Polyclonal Antibody Catalog Number: 18-272-197354
