7 Statistical Consultant, Montgomery Village, MD. 8 Ankle and Foot Medical Centers of the Delaware Valley, Philadelphia,
|
|
|
- Simon Smith
- 8 years ago
- Views:
Transcription
1 ORIGINAL RESEARCH Extracorporeal Shockwave Therapy Versus Placebo for the Treatment of Chronic Proximal Plantar Fasciitis: Results of a Randomized, Placebo-Controlled, Double- Blinded, Multicenter Intervention Trial D. Scot Malay, DPM, MSCE, 1 Martin M. Pressman, DPM, 2 Amir Assili, DPM, 3 Jason T. Kline, DPM, 4 Shane York, DPM, 5 Ben Buren, DPM, 6 Eugene R. Heyman, PhD, 7 Pam Borowsky, DPM, CPed, 8 and Carley LeMay, MA 9 Extracorporeal shockwave therapy (ESWT) has demonstrated efficacy in the treatment of recalcitrant proximal plantar fasciitis. The objective of this investigation was to compare the outcomes of participants treated with a new ESWT device with those treated with placebo. A total of 172 volunteer participants were randomized in a 2:1 active-to-placebo ratio in this prospective, double-blind, multicenter trial conducted between October 2003 and December ESWT (n 115) or placebo control (n 57) was administered on a single occasion without local or systemic anesthesia or sedation, after which follow-up was undertaken. The primary outcomes were the blind assessor s objective, and the participant s subjective assessments of heel pain during the first 3 months of follow-up. Participants were also followed up to 1 year to identify any adverse outcomes that may have been related to the shockwave device. On the visual analog scale, the blind assessor s objective assessment of heel pain displayed a mean reduction of 2.51 in the shockwave group and 1.57 in the placebo group; this difference was statistically significant (P.045). On the visual analog scale, the participant s self-assessment of heel pain displayed a mean reduction of 3.39 in the shockwave group and 1.78 in the placebo group; this difference was statistically significant (P.001). No serious adverse events were observed at any time. It was concluded that ESWT was both efficacious and safe for participants with chronic proximal plantar fasciitis that had been unresponsive to exhaustive conservative treatment. ( The Journal of Foot & Ankle Surgery 45(4): , 2006) Key words: extracorporeal shockwave therapy (ESWT), heel pain, Orthospec, proximal plantar fasciitis Address correspondence to: D. Scot Malay, DPM, MSCE, Ankle and Foot Medical Centers of the Delaware Valley, MAB #111, 38th at Market Streets, Philadelphia, PA malays@uphs.upenn.edu. 1 Ankle and Foot Medical Centers of the Delaware Valley, Penn-Presbyterian Medical Center, Postdoctoral Research Fellow, Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania School of Medicine, Philadelphia, PA. 2 Chief, Section of Podiatric Surgery, Yale New Haven Hospital, Clinical Assistant Professor, Orthopaedics and Rehabilitation, Yale School of Medicine, New Haven Foot Surgeons, New Haven, CT. 3 Podiatric Director, Shady Grove Wound Center, Shady Grove Podiatry, Gaithersburg, MD. 4 Chief Resident, Penn Presbyterian Medical Center, Philadelphia, PA. 5 Chief Resident, Podiatric Surgery, Yale New Haven Hospital, New Haven, CT. 6 Chief Resident, Podiatric Surgery, Yale New Haven Hospital, New Haven, CT. 7 Statistical Consultant, Montgomery Village, MD. 8 Ankle and Foot Medical Centers of the Delaware Valley, Philadelphia, PA. 9 Shady Grove Podiatry, Gaithersburg, MD. Disclosure: This multicenter, double-blinded clinical investigation, entitled protocol PF-01: A Comparative Randomized Placebo-Controlled Clinical Trial of Orthospec Versus Orthospec Placebo for the Relief of Pain in the Treatment of Proximal Plantar Fasciitis, was conducted under the United States Food and Drug Administration Investigational Device Exemption #G to determine the safety and efficacy of the Orthospec ESWT device. The study sponsor, Medispec LTD, Middlebrook Road, Suite 1, Germantown, MD 20874, provided the extracorporeal shockwave therapy and plantar pressure assessment devices, and funded the investigation. Copyright 2006 by the American College of Foot and Ankle Surgeons /06/ $32.00/0 doi: /j.jfas THE JOURNAL OF FOOT & ANKLE SURGERY
2 Proximal plantar fasciitis is a common complaint that confronts physicians treating the foot (1 6). Conservative therapies for this condition include various combinations of padding, strapping, nonsteroidal anti-inflammatory drugs (NSAIDS), physical therapy, night splints, and corticosteroid injections, and these therapies, for the majority of participants, prove to be beneficial (7 13). Nonetheless, approximately 10% of participants fail to respond satisfactorily to these conservative treatment strategies, and, for these participants, treatment options have traditionally evolved around surgical intervention for release of the plantar fascia at its attachment to the tuberosity of the calcaneus, with or without concomitant removal of a portion of the plantar calcaneus when there is radiographic evidence of a plantar calcaneal spur (14, 15). Moreover, postoperative complications such as recurrent pain, nerve injury, infection, and tarsal instability detract from the usefulness of surgical intervention. Since the mid-1990s, extracorporeal shockwave therapy (ESWT) has been successfully used in the treatment of chronic plantar fasciitis (16). Shockwaves are sound waves that are generated by a source that creates vibrations which are then transported through tissues via fluid and solid particle interaction. Proponents of shockwave therapy suggest that ESWT creates controlled local tissue injury that causes neovascularization, and is associated with increased amounts of tissue growth factors within the locally injured structures. It is therefore hypothesized that ESWT stimulates healing by creating a wound environment at the site of shockwave delivery (17 19). Other hypothesized mechanisms of action include the physical alteration of small axons, thereby inhibiting pain impulse conduction; chemical alteration of pain receptor neurotransmitter, thereby preventing pain perception; and hyperstimulation activation of the gate control mechanism, thereby affecting analgesia (20, 21). Although shockwaves used for lithotripsy are of higher energy than those used for the treatment of plantar fasciitis, animal studies have shown the development of an inflammatory response in tissues, ranging from tendon to physeal plate to trabecular bone, with energy levels ranging from 0.28 to 1.5 mj/mm 2 (22 28). It is generally understood that energy levels ranging from 0.22 to 0.36 mj/mm 2 are high enough to induce a therapeutic response in the plantar fascia by 3 to 6 weeks. However, randomized controlled trials of different shockwave delivery systems have yielded varying results (29 39). Currently, shockwave delivery systems delivering energy levels more than 0.34 to 0.36 mj/ mm 2 require the recipient to undergo regional nerve blocks combined with either intravenous sedation or general anesthesia, and the therapeutic guidelines for these devices call for one or more additional applications of ESWT should the participant not experience satisfactory resolution of his or her heel pain. Devices that do not deliver energy levels of at least 0.26 mj/mm 2 generally do not require local anesthesia at the site of delivery; however; they have been criticized as not being as efficacious as devices delivering shockwaves of higher energy (40). The Orthospec ESWT (Medispec LTD, Germantown, MD) device is an extracorporeal shockwave delivery system that is approved for distribution and use in the United States by the Food and Drug Administration (FDA). Although shockwave therapy has been available for the treatment of plantar fasciitis for about a decade in this country, the device under investigation in this clinical trial conveys unique features that distinguish it from other ESWT devices used for this condition. This device produces shockwaves electrohydraulically and delivers the energy to the treatment area through a rubber contact membrane (Fig 1). The energy is dispersed over a treatment area that is large enough that the intensity of the shockwaves reaches therapeutic levels while remaining generally well tolerated by recipient patients without the need for anesthesia or sedation. Moreover, the effective distribution of the shockwaves is over a broad enough anatomical area that there is no need for ultrasonic or radiographic targeting. The hypothesis of this industrysponsored FDA phase-3 investigation is that the Orthospec (active ESWT) device would provide greater relief of pain in comparison with a placebo control, after a one-time application for the treatment of recalcitrant proximal plantar fasciitis. Materials and Methods An FDA-approved, randomized, placebo-controlled, double-blinded, multicentered clinical trial to compare the efficacy and safety of the Orthospec device was designed and undertaken at clinical centers in Pennsylvania, Connecticut, and Maryland. Figure 2 schematically depicts the organization and flow of the investigation. Sample Size and Power Using 2.2 as the standard deviation for the change from baseline heel pain to postintervention heel pain, in accordance with previous experience, and allowing for a 5% loss to follow-up, a total of 183 participants, randomized 2:1 (122 active and 61 placebo), was required to provide 80% power to detect a difference of 1.0 at the 5% level of significance. Only one foot per participant was to be enrolled and treated in this study. Study Population To be included as a participant in the investigation, potential candidates had to be a man or woman older than 18 years of age; if female, not pregnant; diagnosed with prox- VOLUME 45, NUMBER 4, JULY/AUGUST
3 conditions such as osteoarthritis, diabetes mellitus, or peripheral vascular disease were considered eligible for participation only if the condition did not overtly or acutely affect their foot pain. Moreover, potential participants must have previously failed two pharmacologic (analgesic, antiinflammatory, or other) and two nonpharmacologic treatment modalities for relief of heel pain, and agree to avoid such treatments within the following time windows before the intervention visit: 6 weeks for local corticosteroid injections, 48 hours for NSAIDs or analgesic medications, and 2 weeks for physical therapy. Furthermore, potential participants had to agree to avoid the use of NSAIDs or analgesic medications for at least 48 hours before any follow-up visit. Potential participants were excluded if they had a recent history of significant cardiac, neurological, hepatic, renal, metabolic, or hematological disease or impairment as determined by pre-admission testing, medical history (recent and previous), and specialist evaluations. Potential participants with a history of previous surgery for plantar fasciitis or heel spur; those who chose to continue physical therapy or other conservative treatments for their heel pain during the time that they would be enrolled in the study; those having undergone a corticosteroid injection in the heel within 6 weeks of study treatment; those with neuropathic, malignant, or infectious causes of pain; those with a known coagulation disorder or those taking anticoagulant medications for either acute or chronic anticoagulant therapy; those with suspected tears of the plantar fascia, bilateral plantar fasciitis, an infection or malignancy at the area to be subjected to intervention; those who had any condition in which the exposure to radiation was not advisable (that is, pregnancy); those who were unable to provide informed consent themselves or who required a guardian to provide consent to be a volunteer participant in the study; and those simultaneously participating in another device or drug study or who had participated in any clinical trial involving an experimental device or drug within 30 days of entry into this study were also excluded. The investigators made the final determination of whether candidates were eligible to enroll in the study. FIGURE 1 (A) The ESWT device. (B) The Orthospec device positioned against the plantar heel. imal plantar fasciitis on the basis of history and physical examination with symptoms present for more than 6 months; have been treated by a licensed healthcare professional for at least 4 months; have a pain intensity score of 5 cm on the visual analog scale (VAS) in the investigator s heel pain assessment and the participant s self-assessment of pain on the first few minutes of walking in the morning; and have a single site of tenderness with local pressure over the plantar calcaneal tuberosity on passive dorsiflexion of the foot. Potential participants with chronic Data Collection Consent forms were reviewed and signed and demographic data were collected for all of the participants who met the inclusion criteria, in accordance with the institutional review board approved investigational protocol. Participants were randomized into two groups, either ESWT active or placebo, in a ratio of 2:1, as determined by computer-generated random numbers separately for each study center. A minimum of two investigators participated at each site. Because it was a double-blind study, both the participating patients and blinded investigator(s) were unaware of 198 THE JOURNAL OF FOOT & ANKLE SURGERY
4 Potential Participants from Clinical Practices in Connecticut, Pennsylvania, and Maryland Recruitment (0-3 mos.) Screening Visit (n = 196) Pre-treatment Evaluation Visit Informed Consent & Enrollment (1-4 mos.) Randomization (n = 172) Orthospec TM intervention (n = 115) Orthospec TM sham intervention (n = 57) 1 Month Efficacy Follow-up Visit (n = 111) 1 Month Efficacy Follow-up Visit (n = 54) 2-5 mos. (n = 165) 2 Month Efficacy Follow-up Visit (n = 97) 2 Month Efficacy Follow-up Visit (n = 48) 3-6 mos. (n = 145) 3 Month Efficacy Follow-up Visit (n = 101) 3 Month Efficacy Follow-up Visit (n = 51) 4-7 mos. (n = 152) 6 Month Safety Follow-up Visit (n = 87 ) 6 Month Safety Follow-up Visit (n = 45) 7-10 mos. (n = 132) 12 Month Safety Follow-up Visit (n = 78) 12 Month Safety Follow-up Visit (n = 40) mos. (n = 118) Data Analysis & Interpretation 16 mos. FIGURE 2 Protocol flow chart. treatment allocation. All evaluations, before and after the intervention, were carried out by blinded investigators who were responsible for performing the initial screening and history as well as all of the preintervention and postintervention assessments of heel pain. Investigators who were not blinded were responsible for administering the treatment and collecting data during the intervention. Data collected during the intervention included the amount of ESWT active or placebo intervention administered and whether any adverse events occurred during the intervention. Data collected preintervention and postintervention (blind assessors) included the participant s self-assessment and the investigator s assessment of heel pain, self-assessment of activity and function level, use of analgesics, radiographic and integument assessments, blinding assessment, and adverse event and complication data. VOLUME 45, NUMBER 4, JULY/AUGUST
5 FIGURE 3 (A) The PressureSpec calibrated pressure applicator. (B) PressureSpec device used to apply and standardize plantar pressure. Investigator s pain assessments were accomplished with the PressureSpec (Medispec LTD) device (Fig 3), a calibrated handheld instrument that allowed standardized quantification of heel pain when it was applied to the symptomatic area. A 10-point VAS was used by the participants for the investigator s assessment and the participant s self-assessment of heel pain. The continuous nature of the data obtained with this scale was upheld by means of measuring the distance (centimeters) from the origin to the participant s mark on the scale. Participants were also provided with a diary so that they could record their heel pain on initial weight bearing in the morning. Activity and function were measured by the distance the patient was able to walk without heel pain. The diary was also used to record the participants use of anti-inflammatory and/or analgesic medications. Blind investigators assessed participant activity and function by means of a prescribed interview and examination, as well as review of the diary at 1, 2, and 3 months after the intervention visit. Intervention Protocol One Orthospec device and two different contact membranes were placed at each clinical site, and investigators who were not blinded administered either the active or sham shockwave intervention. During the intervention, all participants were positioned comfortably in front of the contact membrane of the device, and ultrasound gel was applied to both the membrane and the target heel. For participants randomized to the active intervention group, an unlined (noninsulated) contact membrane was used. For those participants randomized to the placebo group, a foam-insulated membrane was used to absorb the shockwaves and inhibit transmission of most of the energy. The visible appearance of both the sham and active contact membranes was identical, and the procedures for the intervention proceeded in an identical fashion for both treatment groups. Each patient, in both the ESWT active and placebo groups, underwent continuous shockwave transmission that started at the lowest energy level (level 1) and increased at 3-minute and 35- second intervals until the highest energy level (level 7) was achieved. The creation of shockwaves resulted in a sharp, audible sound, and participants and operators were provided with ear protection that they could use if desired. The intervention session lasted 25 minutes, during which 3800 shockwaves were administered at a rate of 150 shocks per minute. No form of systemic or local anesthesia, or sedation, was provided before, during, or after the intervention. If a participant was unable to tolerate a particular energy level, the unblinded investigator administering the intervention would decrease the energy level. Afterwards, the unblinded investigator recorded the highest energy level sustained and the number of shockwaves administered, and any adverse events or device malfunctions noted. At the end of the intervention session, in an effort to assess blinding, participants were asked whether they thought they had received the active or the placebo intervention. Outcomes The primary efficacy endpoint for this study was the change from baseline to 3 months postintervention in the investigator s assessment of heel pain. All randomized participants who had at least one follow-up evaluation for this outcome were included in the primary analysis of efficacy. If a participant did not have an assessment at 3 months postintervention, for whatever reason, then the last available assessment data were carried forward (last observation carried forward [LOCF] method). Secondary efficacy endpoints for this investigation included: 1. The investigator s heel pain assessment responder analysis, whereby participants who had a decrease from baseline to the third postintervention visit of 50% or more with a VAS score 4 cm were to be considered a success, whereas all others were to be considered a 200 THE JOURNAL OF FOOT & ANKLE SURGERY
6 failure. The last observation was carried forward for all participants who were missing a value at the third visit. 2. Change from baseline to 3 months posttreatment (third visit) in the participant s self-assessment of heel pain. If a participant did not have an assessment at the third visit, then the last available assessment was carried forward. 3. Participant s self-assessment of pain analyzed as a dichotomous variable, wherein participants who had a decrease from baseline to the third visit of 50% or more with a VAS score 4 cm were to be considered a success, whereas all others were to be considered a failure. The last observation was carried forward for all participants who were missing a value at the third visit. 4. Participant s self-assessment of activity level and function was treated as a dichotomous variable, where an improvement of 1 point or more over baseline, or maintaining a baseline score of 0 or 1 was considered a success. All other responses were to be considered a failure. 5. Change in use of pain medications from study enrollment was analyzed as a 3-point categorical variable representing increased, no change, or decreased use of analgesic medication. Reassessment of heel pain was performed with the PressureSpec device and the VAS at 1, 2, and 3 months postintervention. Prescribed questions regarding activity and function were also asked at these visits, in terms of the number of blocks walked before experiencing heel pain, and participant diaries were checked to assess and record the participant s use of pain medications. At the 1-, 2-, 3-, 6-, and 12-month follow-up visits, blinded investigators interviewed participants and examined the study heel for any signs of adverse events such as swelling, bruising, or paresthesia. Safety endpoints were recorded as the first occurrence of any adverse event or complication. Statistical Plan The protocol-defined primary endpoint was the change from baseline to 3 months posttreatment in the investigator s heel pain assessment. This endpoint was analyzed by an analysis of covariance that included the effects of treatment group, study center, and the baseline investigator s heel pain assessment. Continuous secondary endpoints were analyzed in the same way as the primary endpoint, and categorical secondary endpoints (responder analyses) were analyzed by a logistic regression that included the effects of treatment group, study center, and the corresponding baseline assessment. The change in the use of pain medication was analyzed by the Cochran-Mantel-Haenszel procedure, stratified by study center. Scores of 1, 0, and 1 were assigned to the response categories of increased, remained the same, and decreased, to create a test of the mean scores. Device safety was assessed by the first occurrence of all adverse events and all device-related adverse events. Each adverse event was presented as the rate per treatment group and tested using the Fisher s exact test. All analyses were based on randomized treatment and all statistical tests were 2-sided at the 5% level of significance. All of the analyses were performed with SAS Version 8.2 (SAS Institute, Cary, NC). Results A total of 196 participants were screened, 172 were randomized to treatment, and 152 completed the 12-week efficacy trial. A total of 168 participants had at least 1 follow-up visit and were included in analyses of efficacy. Inspection of the distributions of the data confirmed that our statistical assumptions were met. The 172 participants had a mean age of 51 years, 33% were men, the mean weight was 184 pounds, 87% were white, and the mean duration of foot pain was 30 months. Baseline demographic and clinical data are summarized in Table 1 by treatment group. Nineteen of the 172 participants (11.0%) had not used any oral medication to treat their foot pain. Primary Efficacy Results The mean change in the investigator s assessment of heel pain is summarized in Table 2, and these data indicate that the mean change from baseline in the ESWT group was significantly greater than that in the placebo group (difference 0.94; P.045; 95% confidence interval, 1.87 to 0.02). Moreover, the difference also achieved statistical significance at month 2. To assess the effect of the absence or presence of a radiographically evident plantar calcaneal spur, the mean changes from baseline by radiographic finding are summarized in Table 3. These data show that in the absence of a radiographically evident plantar calcaneal spur, the reduction in heel pain was statistically significantly greater for the ESWT group than for the placebo group, whereas in the presence of a plantar calcaneal spur, the reduction in heel pain was not statistically significantly different between the groups. To assess the dose-response relationship with respect to the primary endpoint, all placebo participants were pooled, and the mean changes from baseline in the investigator s assessment of heel pain, adjusted for study center and baseline assessment, are presented in Figure 4 by maximum shockwave energy applied. One participant was randomized to ESWT but received placebo by mistake and was included in the placebo group in this table. Of the 115 participants receiving active ESWT, 89 (77%) tolerated shockwave en- VOLUME 45, NUMBER 4, JULY/AUGUST
7 TABLE 1 Baseline and demographic summary ESWT Placebo (N 115) (N 57) TABLE 2 Mean change from baseline in investigator s assessment of heel pain (LOCF) ESWT Placebo P value Age (y) Mean SD Range Sex Men: n (%) 36 (31.3%) 21 (36.8%) Women: n (%) 79 (68.7%) 36 (63.2%) Race Black: n (%) 8 (7.1%) 5 (8.8%) White: n (%) 102 (90.3%) 48 (84.2%) Asian: n (%) 0 (0%) 3 (5.3%) Hispanic: n (%) 3 (2.7%) 1 (1.8%) Duration of foot pain (mo) Mean SD Range Weight (lb) Mean SD Range Calcaneal radiographs Plantar spur present 46 (40.0%) 21 (37.5%) Activity: blocks walked without pain No/Minor limitation: n (%) 12 (10.4%) 9 (15.8%) 6 10 Blocks: n (%) 14 (12.2%) 0 (0%) 4 6 Blocks: n (%) 6 (5.2%) 6 (10.5%) 1 3 Blocks: n (%) 18 (15.7%) 9 (15.8%) 1 Block: n (%) 65 (56.5%) 33 (57.9%) Preintervention analgesic use for heel pain No analgesic used 12 (10.4%) 7 (12.3%) Aspirin 4 (3.5%) 5 (8.8%) Celecoxib 9 (7.8%) 6 (10.5%) Flurbiprofen 1 (0.9%) 0 (0%) Ibuprofen 23 (20.0%) 17 (29.8%) Indomethacin 0 (0%) 1 (1.8%) Meloxicam 3 (2.6%) 0 (0%) Nambunetone 1 (0.9%) 0 (0%) Naproxen sodium 10 (8.7%) 5 (8.8%) Rofecoxib 9 (7.8%) 7 (12.3%) Other 59 (51.3%) 25 (43.9%) ergy levels 6 without any form of anesthesia, analgesia, or sedation (Table 4). A number of baseline factors were assessed to see if they had an effect on the investigator s assessment of heel pain. The only one found to be significant (P.05) was the baseline assessment, indicating that none of the baseline demographic factors influenced the investigator s assessment of heel pain. Table 5 summarizes the number and percent of responders (decrease from baseline of 50% or more with a VAS score 4 cm) at each visit with the last value carried forward for all missing values. These results show that the differences achieved statistical significance at months 2 and 3 of follow-up. Secondary efficacy endpoints included the participant s assessment of heel pain, the participant s assessment of Month 1 N Mean* Difference (95% CI) 0.34 ( 1.06, 0.37) Month 2 N Mean* Difference (95% CI) 0.99 ( 1.86, 0.12) Month 3 N Mean* Difference (95% CI) 0.94 ( 1.87, 0.02) shockwave therapy; CI, confidence interval. *Estimated from an analysis of variance and adjusted for baseline assessment and clinical site. TABLE 3 Mean change from baseline to month 3 in investigator s assessment of heel pain by the radiographic absence or presence of a plantar calcaneal spur (LOCF) ESWT Placebo P value Plantar spur absent N Mean* Difference (95% CI) 1.48 ( 2.63, 0.33) Plantar spur present N Mean* Difference (95% CI) 0.07 ( 1.66, 1.52) shockwave therapy; CI,confidence interval. *Estimated from an analysis of variance and adjusted for baseline assessment and clinical site. activity and function, and the change in use of pain medication. The participant s assessment of heel pain was handled both as a continuous variable and as a dichotomous variable, as was done for the primary endpoint. These data were analyzed by the same model that was used for the primary endpoint with the LOCF for all missing visits and are reported in Table 6. As with the primary endpoint, the difference at 3 months achieved statistical significance (P.001). Table 7 summarizes the results of the responder analyses for the participants assessment of heel pain. As with the continuous analysis, the results at 3 months achieved statistical significance (P.003). The protocol defined a responder, relative to the participant s self-assessment of activity and function, as a participant displaying an improvement of at least one category from baseline or a participant displaying maintenance of a baseline score of 0 or 1. These data were analyzed by a logistic regression that included the effects of treatment 202 THE JOURNAL OF FOOT & ANKLE SURGERY
8 Mean Change group, clinical site, and baseline category; the results of which are presented in Table 8. The results indicated that participants in the ESWT group had a higher response rate than those in the placebo group at months 2 and 3. However, at neither month did the difference achieve statistical significance. At each posttreatment follow-up visit, investigators were asked to assess whether the participant had changed his or her use of pain medication in comparison with when he or Level *Placebo Level Level Level 7 Shock Wave Energy Applied FIGURE 4 Mean change from baseline to month 3 in investigator s assessment of heel pain by maximum shock wave energy applied (LOCF). *The placebo intervention did provide some degree of therapeutic effect. As demonstrated in this figure, the placebo mean decrease was equivalent to treatment levels 4.5. TABLE 4 Maximum shockwave energy level applied (N 115) Energy Level n (%) (12.2) (10.4) (47.0) 7 35 (30.9) TABLE 5 Investigator s assessment of heel pain responder s analysis* (LOCF) ESWT Placebo P value Month 1 Responders: n/n (%) 22/111 (19.8%) 9/54 (16.7%).58 Odds ratio (95% CI) 1.30 (0.52, 3.27) Month 2 Responders: n/n (%) 38/111 (34.2%) 9/54 (16.7%).019 Odds ratio (95% CI) 2.69 (1.17, 6.16) Month 3 Responders: n/n (%) 48/112 (42.9%) 11/56 (19.6%).003 Odds ratio (95% CI) 3.13 (1.46, 6.73) shockwave therapy; CI, confidence interval; VAS, visual analog score. *A responder is a participant with a 50% decrease from baseline and a VAS score 4 cm. Analysis was performed by a logistic regression including baseline assessment and clinical site as covariates. TABLE 6 Mean change from baseline in participant s assessment of heel pain (LOCF) ESWT Placebo P value Month 1 N Mean* Difference (95% CI) 0.11 ( 0.95, 0.72) Month 2 N Mean* Difference (95% CI) 0.73 ( 1.60, 0.15) Month 3 N Mean* Difference (95% CI) 1.61 ( 2.55, 0.67) shockwave therapy; CI, confidence interval. *Estimated from an analysis of variance and adjusted for baseline assessment and clinical site. TABLE 7 Participant s assessment of heel pain responder s analysis* (LOCF) ESWT Placebo P value Month 1 Responders: n/n (%) 39/110 (35.5%) 17/54 (31.5%).61 Odds ratio (95% CI) 1.20 (0.60, 2.41) Month 2 Responders: n/n (%) 48/111 (43.2%) 17/54 (31.5%).14 Odds ratio (95% CI) 1.67 (0.84, 3.32) Month 3 Responders: n/n (%) 59/112 (52.7%) 16/56 (28.6%).003 Odds ratio (95% CI) 2.85 (1.42, 5.69) shockwave therapy; CI, confidence interval. *A responder is a participant with a 50% decrease from baseline and a score 4. Analysis was performed by a logistic regression including baseline assessment and clinical site as covariates. she enrolled in the study. The numbers of participants who increased, had no change, or decreased their use of pain medication are summarized in Table 9. These data were analyzed by the Cochran-Mantel-Haenszel procedure, stratified by clinical site. Scores of 1, 0, and 1 were assigned to the 3 response categories to create tests of the mean scores. As seen, participants in the ESWT group had significantly greater decreases in the use of pain medication than participants in the placebo group at months 2 (P.028) and 3 (P.001). As seen in Figure 4, participants treated with an energy level of 4.5 appeared to have less pain relief than those treated with higher energy levels. To demonstrate the efficacy among participants receiving energy level 4.5, the primary analysis and each of the secondary analyses were repeated excluding ESWT participants who received an energy level of 4.5. Specifically, the mean change from VOLUME 45, NUMBER 4, JULY/AUGUST
9 TABLE 8 Participant s self-assessment of activity and function responder s analysis* (LOCF) ESWT Placebo P value Month 1 Responders: n/n (%) 60/111 (54.1%) 31/54 (57.4%).58 Odds ratio (95% CI) 0.82 (0.41, 1.66) Month 2 Responders: n/n (%) 64/111 (57.7%) 27/54 (50.0%).43 Odds ratio (95% CI) 1.32 (0.66, 2.65) Month 3 Responders: n/n (%) 72/112 (64.3%) 32/56 (57.1%).33 Odds ratio (95% CI) 1.42 (0.70, 2.85) shockwave therapy; CI, confidence interval. *Improvement of 1 point or maintenance of a baseline score of 0 or 1. Analysis was performed by a logistic regression including clinical site and baseline category as covariates. TABLE 9 Change in use of pain medication from study enrollment* ESWT Placebo P value Month 1 Increase: n/n (%) 7/111 (6.3%) 5/54 (9.3%).083 Decrease: n/n (%) 35/111 (31.5%) 10/54 (18.5%) Month 2 Increase: n/n (%) 1/95 (1.1%) 5/47 (10.6%).028 Decrease: n/n (%) 28/95 (29.5%) 9/47 (19.2%) Month 3 Increase: n/n (%) 1/100 (1.0%) 6/51 (11.8%).001 Decrease: n/n (%) 34/100 (34.0%) 7/51 (13.7%) Abbreviation: ESWT, extracorporeal shockwave therapy. *Analyzed with the Cochran-Mantel-Haenszel procedure stratified by clinical site and assigning scores of 1, 0, and 1 to the 3 response categories. baseline in the investigator s assessment of heel pain, overall, and responder s analysis; the mean change from baseline in the participant s assessment of heel pain, overall, and responder s analysis; and the change in the participant s activity and function and his or her change in analgesic use were reanalyzed (data not shown) for those participants receiving energy levels 4.5. As previously stated in regard to Figure 4, the participant who was randomized to ESWT but received placebo by mistake was included in the placebo group for these analyses. For each of these analyses, there was a higher level of improvement in pain relief, activity, and function when participants were administered a shockwave energy level 4.5. Figure 5 graphically depicts the mean change from baseline in the investigator s assessment of heel pain (LOCF, analysis of variance adjusted for baseline assessment and clinical site) for ESWT participants sustaining energy level 4.5. Similarly, Figure 6 graphically depicts the mean change from baseline in the participant s assessment of heel pain (LOCF, analysis of variance adjusted for baseline assessment and clinical site) for ESWT participants sustaining energy level 4.5. The results of each of the efficacy endpoints, and the method of analysis, are summarized in Table 10. We also considered it of interest to combine the 4 protocol-defined success criteria at 3 months (investigator s assessment of pain, participant s assessment of pain, participant s assessment of activity and function, and change in use of pain medication), and Figure 7 summarizes the numbers of participants by the number of efficacy endpoints for which they responded. As seen, the difference achieved statistical significance for each of the endpoints measured. Safety Results There were no serious adverse events encountered in this clinical trial. None of the participants in the placebo group (N 57) experienced an adverse event. Three participants in the active ESWT group (N 115) reported 1 adverse event each, all of which were either mild or moderate and were reported as being definitely, probably, or possibly related to the device. Two participants (1.7%) experienced bruising at the site of shockwave application on the heel, and these incidents were considered by the participant and the investigator to be device-related; one participant (0.9%) experienced local swelling that was determined to be unrelated to the device. The energy levels reached for these participants were reported to range from 4.5 to Month 1 (P=0.14) Month 2 (P=0.005) Month 3 (P=0.011) Orthospec Placebo FIGURE 5 Mean change from baseline in investigator s assessment of heel pain for ESWT Month 1 (P=0.42) Month 2 (P=0.0019) Month 3 (P=<0.001) Orthospec Placebo FIGURE 6 Mean change from baseline in participant s assessment of heel pain for ESWT level THE JOURNAL OF FOOT & ANKLE SURGERY
10 TABLE 10 Summary of efficacy results at 3 months for ESWT participants receiving energy level >4.5 versus placebo Measurement ESWT (N 88) Placebo (N 52) P value Investigator s assessment of pain (VAS)* Mean change from baseline Response rate 50.0% 21.2%.001 Participant s self-assessment of pain (VAS)* Mean change from baseline Response rate 62.1% 28.9%.001 Participant s self-assessment of activity and function (diary) Response rate 67.0% 56.1%.16 Change in use of analgesic medications (diary) Increased 1.0% 11.80%.001 No change 65.0% 74.50% Decreased 34.0% 13.70% Abbreviations: ESWT, extracorporeal shockwave therapy; VAS, visual analog scale. *Analysis of variance adjusted for baseline assessment and clinical site. Improvement of 1 point or maintenance of a baseline score of 0 or 1; analysis via a logistic regression including clinical site and baseline category as covariates. Cochran-Mantel-Haenszel procedure stratified by clinical site and assigning scores of 1, 0, and 1 to the 3 response categories. % Patients 90.00% 80.00% 70.00% 60.00% 50.00% 40.00% 30.00% 20.00% 10.00% 0.00% 77.30% 59.70% Subgroup Analyses 61.90% 29.80% 46.40% 19.30% 1 (P=0.015) 2 (P=<0.001) 3 (P=<0.001) Endpoints Achieved Orthospec Placebo FIGURE 7 Percentage of patients by the number of efficacy endpoints for which they responded for Orthospec levels 4.5. TABLE 11 Mean change from baseline to month 3 in investigator s assessment of heel pain by sex (LOCF) ESWT Placebo Difference Interaction P value Men N Mean* Women N Mean* shockwave therapy. *Adjusted for clinical site and baseline assessment. Subgroup analyses were done for sex, age group ( 45 years, years, 65 years), and body weight ( 160 lb, lb, 193 lb). Table 11 presents a subgroup analysis based on sex, while Table 12 is based on age and Table 13 is based on body weight tertiles (weight categories that encompass approximately 3 equal groups of TABLE 12 Mean change from baseline to month 3 in investigator s assessment of heel pain by age group (LOCF) participants). Included in these tables are the tests of treatment group-by-subgroup interaction, which assess the homogeneity of the differences between ESWT and placebo among subgroups. These data indicate that the differences between ESWT and placebo were consistent across the different subgroups. Discussion ESWT Placebo Difference Interaction P value 45 Years N Mean* Years N Mean* Years N 12 7 Mean* shockwave therapy. *Adjusted for clinical site and baseline assessment. The Orthospec ESWT device used in this study uses an electrohydraulic, or spark-gap, method of creating therapeutic shockwaves. In this system, an electrode ignites an electrical charge within a water-filled, stainless-steel, semiellipsoid chamber, evaporating a small portion of the water contained in the chamber and creating a shockwave that is reflected outward. The portable shockwave generator used in this study targets the shockwaves to a 35-mm diameter therapy zone that enables shockwaves of sufficient energy to be delivered to the tissues in a single therapeutic session, VOLUME 45, NUMBER 4, JULY/AUGUST
11 TABLE 13 Mean change from baseline to month 3 in investigator s assessment of heel pain by body weight (LOCF) ESWT Placebo Difference Interaction P value 160 lb N Mean* lb.35 N Mean* lb N Mean* shockwave therapy. *Adjusted for clinical site and baseline assessment. without the need for directional imaging, anesthesia, or sedation. The indications for ESWT include a wide range of conditions including plantar fasciitis, tendinosis calcarea (shoulder), lateral epicondylitis (tennis elbow), medial epicondylitis (golf elbow), chronic subcutaneous calcifications, patellar tendinitis, nonunion/pseudarthrosis, and nephrolithiasis, as well as uses related to the treatment of nervous system disorders. In this investigation, ESWT was used specifically for proximal plantar fasciitis, and was shown to be efficacious relative to the reduction of pain (Tables 2, 5, 6, and 10, and Figs 4, 5, and 6) and the reduction in use of analgesic medications for heel pain relief (Table 9). Moreover, the ESWT device was shown to be consistently effective at decreasing plantar heel pain regardless of the participant s sex, age, or body weight (Tables 11, 12, and 13). It is also interesting to note that the mean average weight of those participating in the active ESWT group was approximately 10 lb heavier in comparison with those in the placebo group (Table 1); however, this difference in weight was not statistically significant. Furthermore, although our data revealed greater qualitative improvements in activity and function in those participants in the ESWT group when compared with the placebo group, these differences were not statistically significant (Table 8). We also observed a difference in the effect of ESWT intervention on the change in heel pain for participants with and without a plantar spur (Table 3). For those participants with a plantar spur, the change from baseline to the month 3 follow-up for the investigator s assessment of heel pain was greater (a greater reduction in heel pain) in the ESWT group than in the placebo control group, although this difference did not meet statistical significance (P.96). For those participants without a plantar spur, the change from baseline to the month 3 follow-up for the investigator s assessment of heel pain was statistically significantly (P.012) greater (a greater reduction in heel pain) in the ESWT group than in the placebo control group. Despite noting a greater reduction in heel pain in the active ESWT group, for those with and without a plantar spur, the lack of a statistically significant reduction in pain when a spur was present prohibits us from saying that the efficacy was not diminished by the presence of the spur. Therefore, these results only suggest that the presence of a plantar spur did not alter the efficacy of ESWT and, perhaps, a greater sample size would enable a more definitive statement in this regard. Our data also indicated that those participants in the ESWT group responded to a greater number of efficacy endpoints (pain relief, improved activity and function, and reduced use of analgesics) than did those in the placebo group (Fig 7). The exclusion criteria for this investigation took into consideration the general contraindications to the use of ESWT, including pregnancy, children, nerve damage, tarsal tunnel syndrome, osteoporosis, rheumatoid arthritis, peripheral vascular disease, infection, tumor, bleeding diasthesis, cardiac pacemaker, and healing fracture. And, although the known adverse local effects of ESWT include subcutaneous hematoma, skin erosion, swelling, petechial hemorrhage, pain/paresthesia, and the systemic response of vasovagal syncope, we observed no serious adverse effects with the use of the ESWT device. During the course of this study, the ESWT device was still categorized as an investigational device under the scrutiny of the FDA and, therefore, safety was a key issue throughout our investigation. The shockwave energy used in this study was observed to be safe and not related to any significant adverse effects. This study also made clear the importance of administering shockwaves at sufficiently high energy levels, those being level 4 for the device tested in this trial. The relationship of the amount of energy administered to the amount of heel pain relief can be seen in Figure 4, which displays the mean change from baseline to the third month postintervention in the investigator s assessment of heel pain by the maximum amount of shockwave energy applied. These data depict the dose-response effect of the ESWT used in this investigation. Moreover, Tables 5, 7, and 10 and Figures 5, 6, and 7 show that the statistically significant difference changed from P.045 to P.003 (statistically significant to highly statistically significant) when the mean change from baseline in the investigator s assessment of heel pain was considered for all of the energy levels combined versus energy levels 4.5. In fact, the data in Table 10, as well as in Figures 5, 6, and 7, all support the finding that relief of heel pain was most efficacious among participants receiving shockwaves at levels 4.5. These data further reinforce the relationship of the amount of shockwave energy to pain reduction and suggest that a maximum energy level of 4.5 or less may be subtherapeutic. Inspection of Tables 2, 5, 6, and 7 also reveals a greater response to ESWT at 2 months versus 1 month, and again at 3 months versus 2 months. This finding is in keeping with the com- 206 THE JOURNAL OF FOOT & ANKLE SURGERY
12 mon understanding that the maximum benefit of ESWT is usually not observed until at least 6 to 8 weeks after administration of the acoustic energy. This investigation also showed that 89 of 115 participants (77%) in the active ESWT group were able to safely tolerate shockwave energy levels 4.5 without the use of anesthesia, analgesia, or sedation (Table 4). To our knowledge, this is the first report of shockwave energy levels of this magnitude being safely tolerated without concomitant administration of anesthesia, analgesia, sedation, or combinations thereof by a large proportion of patients with proximal plantar fasciitis. Although the randomized controlled trial is generally considered the highest form of clinical investigation of a therapeutic intervention or a diagnostic test, and is therefore accepted as the most likely study design to yield valid results, we understand that every trial conveys certain limitations that are specific to the individual investigation. We would like to mention limitations that we have considered in relationship to this study. First, we did not use a healthrelated quality-of-life measurement, such as the short-form health survey (36 items) (41), the Bristol Foot Score (42), or the Foot Function Index (43), all of which have become generally accepted as reliable instruments that can produce valid information. Our emphasis was on measuring pain before and after shockwave therapy and observing whether alteration of pain affected each participant s function. Therefore, we combined pain measurement on the VAS with a number of functional measurements, namely ambulatory ability and the use of analgesic medication. Despite their wide acceptance and use, VASs have been criticized for a number of biases, including end-digit aversion and spacing-out partiality (44, 45). Key to our decision to use the VAS, however, was its ease of application and traditionally high levels of participant acceptance, understanding, and high rates of completion, and wide acceptance as a useful tool for measuring a variety of health states, including pain. Furthermore, the FDA commonly accepts the use of the VAS as a means of measuring pain in clinical trials related to musculoskeletal disorders (46). Our use of the participant diary to record walking activity and analgesic use could have been biased by inaccuracies, either purposeful or inadvertent. However, we took particular care at the time of enrollment to explain the use of the diary, and, at each follow-up visit, the diary was reviewed with the participant and its purpose and usage, again, reiterated and clarified. Second, we only measured efficacy outcomes up to 3 months after the intervention. This is in keeping with many phase-3 device trials conducted under the auspices of the FDA. The goal of this trial was to establish whether the shockwave device was efficacious and safe within the confines of the investigation. As with any one-time intervention, the longer the period between administration of the intervention and the follow-up evaluation, the more likely it is that other interventions, including time itself, could contaminate the results. Moreover, in patients who have already failed typical conservative strategies, diminished or alleviated pain within 3 months after shockwave administration seems to be a clinically reasonable amount of time by which one could expect to identify an alteration of plantar heel pain. Further, previous experience has indicated that the usual time required before shockwave therapy affects an alteration in pain is about 6 weeks. Therefore, measuring efficacy outcomes at 12 weeks would reasonably be expected to be a sufficient period of time in which to identify pain relief, without unduly increasing the risk of contaminating the intervention. Our efforts to combine efficacy endpoints and to report the number of endpoints achieved depending on the intervention received (Table 10) was undertaken in an effort to qualitatively get a sense of the number of symptoms affected by ESWT. We consider this information with the understanding that we are, in this investigation, weighing all of the endpoints evenly and that it is likely that these endpoints should probably be weighted differently. Several methodological techniques used in this investigation biased toward the null and imparted a conservative tone to our results. These methods pertained to the way in which the placebo was applied and to the data analysis. By design, it was understood that some degree of shockwave energy, possibly even a therapeutic dose, could be experienced in the placebo group despite the use of the energyabsorbing foam insert in the contact membrane. In essence, the sham intervention was not a pure placebo. This biased the results toward the null when comparing the active intervention with the sham therapy. Similarly, use of the LOCF method of analysis also biased toward the null in that participants who did not have an assessment at 3 months postintervention, for whatever reason, had their last available data carried forward for use in the computation of efficacy. Understanding that ESWT typically does not result in notable clinical improvement until at least 3 to 6 weeks postintervention, missing the third month follow-up visit would likely result in carrying forward a pain measurement that would be higher than a measurement actually taken at the 3-month follow-up visit, and would therefore minimize the apparent effect of the intervention relative to the reduction of heel pain. Furthermore, we performed a responder analysis wherein a responder was defined as a participant who had a decrease from baseline to follow-up at 3 months of 50% or more with a VAS score 4.0 cm. Participants meeting this definition were considered a therapeutic success, whereas all others were considered a failure. Here, again, handling the outcomes in this fashion imparted a conservative influence on the results in that the definition of a responder was linked to pain relief, and the responder analysis only considered the dichotomous variable, success or failure. Understanding that success required a minimum VOLUME 45, NUMBER 4, JULY/AUGUST
13 of a 50% reduction in pain, with a score of 4.0 cm or less on the VAS, participants who noted reductions in pain that did not reach a magnitude consistent with being a responder were counted as therapeutic failures regardless of whether they had clinically improved. Moreover, the LOCF method of analysis was also used in the responder analysis, and, as previously noted, this further biased toward the null. In regard to external validity, our protocol excluded patients with bilateral proximal plantar fasciitis despite the fact that patients commonly have bilateral plantar heel pain when they present to the clinic, and this may have diminished the generalizability of our findings. We focused on patients with just one painful heel, because this was an investigational new device study and we did not want to put any volunteer participants at undue risk. We also wanted to avoid analytical issues that may have obscured interpretation of the results or diminished the statistical power to determine a significant difference. Moreover, we believe that collecting data from 3 different clinical centers, having minimal loss to follow-up, and controlling for center effects in the analysis increased the generalizability of our findings. For all of these reasons, we believe that the methods used to measure the primary and secondary outcomes in this clinical trial were acceptable, and, along with the bias-reducing methods of randomization and blinding of participants and outcomes assessors, as well as the multicenter nature of this study, contributed to the generation of valid results that convey external validity. Summary and Conclusions The results of this clinical investigation demonstrate the safety and efficacy of the Orthospec Extracorporeal Shockwave Therapy device for treatment of recalcitrant proximal plantar fasciitis. The study achieved statistical significance in its protocol-defined primary endpoint (P.045), change from baseline in the investigator s assessment of heel pain at 3 months. When participants were assessed by whether they achieved a clinical response in the primary endpoint, the number of responders was statistically significantly greater (P.003) in the ESWT group (42.9%) than in the placebo group (19.6%). These results were further confirmed in each of the secondary measures of heel pain, the participant s assessment of pain, and the change in use of pain medication. The mean decrease from baseline in the participant s assessment of pain was statistically significantly greater (P.001) in the ESWT group ( 3.39) than in the placebo group ( 1.78), and the proportion of participants who achieved a clinical response in this endpoint was also statistically significantly greater (P.003) in the ESWT group (52.7%) than in the placebo group (28.6%). When we assessed the change from study enrollment in the use of pain medication to treat heel pain, participants in the ESWT group had a smaller proportion with an increase (1.0% vs 11.8%) and a larger proportion with a decrease (34.0% vs 13.7%), and these differences were highly statistically significant (P.001). Participants in the ESWT group also had a higher response rate at 3 months than those in the placebo group in the participant s assessment of activity and function; however, this endpoint did not achieve statistical significance. Although the placebo intervention did provide some degree of therapeutic effect, the ESWT group demonstrated a stronger effect in comparison with the placebo. These results indicate that ESWT remains an encouraging alternative to surgical intervention for treating pain caused by proximal plantar fasciitis. This randomized, doubleblinded, placebo-controlled, multicenter trial demonstrated clinical success in an unbiased fashion. All assessments of the reduction of heel pain were found to be statistically significant when compared with placebo in participants who had already failed standard conservative treatments. Moreover, significant pain relief was achieved with a single treatment session without the use of local anesthetics or systemic analgesics or sedatives, or combinations of these agents. Furthermore, in this study, there were no participant deaths or other serious adverse events observed in the follow-up period (an important aspect of the investigation of any new therapeutic device), and only 3 transient adverse events were reported: 2 cases of bruising (relatedness code: definitely and probably related) and 1 case of mild local swelling (relatedness code: not related). All 3 cases were considered mild and displayed complete resolution of symptomatology after several days. The FDA-approved Orthospec ESWT device used in this clinical trial is considered an alternative treatment intervention in the management of recalcitrant proximal plantar fasciitis with or without a plantar calcaneal spur. Acknowledgments We would like to thank Donald R. Green, DPM, Luke D. Cicchinelli, DPM, Alan J. Mlodzienski, DPM, and Michael S. Downey, DPM, for their thoughtful critiques of our manuscript. We would also like to thank Jacqueline Rosenzweig for her assistance in scheduling participants and organizing and maintaining case report forms, and Sheryl Skinner of Medispec LTD for her site management and monitoring efforts. References 1. McCarthy DJ, Gorecki GE. The anatomical basis of inferior calcaneal lesions. J Am Podiatr Med Assoc 69: , Sherer PR. Heel pain syndrome: pathomechanics and nonsurgical treatment. J Am Podiatr Med Assoc 81:68 72, Malay DS. Plantar fasciitis and heel spur syndrome: a retrospective 208 THE JOURNAL OF FOOT & ANKLE SURGERY
14 analysis. In Reconstructive Surgery of the Foot and Leg: Update 1996, pp , edited by NS Vickers, Podiatry Institute, Tucker, GA, Pfeffer G, Bacchetti P, Deland J, Lewis A, Anderson R, Davis W, Alvarez R, Brodsky J, Cooper P, Frey C, Herrick R, Myerson M, Sammarco J, Janecki C, Ross S, Bowman M, Smith R. Comparison of custom and prefabricated orthoses in the initial treatment of proximal plantar fasciitis. Foot Ankle Int 20: , Riddle DL, Pulisic M, Pidcoe P, Johnson RE. Risk factors for plantar fasciitis: a matched case-control study. J Bone Joint Surg Am 85-A: , Riddle DL, Schappert SM. Volume of ambulatory care visits and patterns of care for patients diagnosed with plantar fasciitis: a national study of medical doctors. Foot Ankle Int 25: , Govindarajan R, Bakalova T, Doss NW, Splain SH, Michael R, Abadir AR. Posterior tibial nerve block in the therapeutic management of painful calcaneal spur (plantar fasciitis): a preliminary experience. Can J Anaesth 50: , DiGiovanni BF, Nawoczenski DA, Lintal ME, Moore EA, Murray JC, Wilding GE, Baumhauer JF. Tissue-specific plantar fascia-stretching exercise enhances outcomes in patients with chronic heel pain. A prospective, randomized study. J Bone Joint Surg Am 85-A: , Martin JE, Hosch JC, Goforth WP, Murff RT, Lynch DM, Odom RD. Mechanical treatment of plantar fasciitis. A prospective study. J Am Podiatr Med Assoc 91:55 62, Probe RA, Baca M, Adams R, Preece C. Night splint treatment for plantar fasciitis. A prospective randomized study. Clin Orthop 368: , Crawford F, Atkins D, Young P, Edwards J. Steroid injection for heel pain: evidence of short-term effectiveness. A randomized controlled trial. Rheumatology (Oxford) 38: , Lynch DM, Goforth WP, Martin JE, Odom RD, Preece CK, Kotter MW. Conservative treatment of plantar fasciitis. A prospective study. J Am Podiatr Med Assoc 88: , Gudeman SD, Eisele SA, Heidt RS Jr, Colosimo AJ, Stroupe AL. Treatment of plantar fasciitis by iontophoresis of 0.4% dexamethasone. A randomized, double-blind, placebo-controlled study. Am J Sports Med 25: , Tomczak RL, Haverstock BD. A retrospective comparison of endoscopic plantar fasciotomy to open plantar fasciotomy with heel spur resection for chronic plantar fasciitis/heel spur syndrome. J Foot Ankle Surg 34: , Kinley S, Frascone S, Calderone D, Wertheimer SJ, Squire MA, Wiseman FA. Endoscopic plantar fasciotomy versus traditional open heel spur surgery: a prospective study. J Foot Ankle Surg 32: , Rompe JD, Hopf C, Nafe B, Burger R. Low-energy extracorporeal shockwave therapy for painful heel: a prospective controlled singleblind study. Arch Orthop Trauma Surg 115:75 79, Perez M, Weiner R, Gilley JC. Extracorporeal shockwave therapy for plantar fasciitis. Clin Podiatr Med Surg 20: , Daecke W, Kusnierczak D, Loew M. Long-term effects of extracorporeal shockwave therapy in chronic calcific tendinitis of the shoulder. J Shoulder Elbow Surg 11: , Haake M, Wessel C, Wilke A. [Effects of extracorporeal shockwaves (ESW) on human bone marrow cell cultures]. Biomed Tech (Berl) 44: , Plaisier PW, van der Hul RL, Terpstra OT, Bruining HA. Current role of extracorporeal shockwave therapy in surgery. Br J Surg 81: , Wilner JM, Strash WW. Extracorporeal shockwave therapy for plantar fasciitis and other musculoskeletal conditions utilizing the Ossatron an update. Clin Podiatr Med Surg 21: , Delius M, Draenert K, Al Diek Y, Draenert Y. Biological effects of shockwaves: in vivo effect of high energy pulses on rabbit bone. Ultrasound Med Biol 21: , McCormack D, Lane H, McElwain J. The osteogenic potential of extracorporeal shockwave therapy: an in-vivo study. Ir J Med Sci 165:20 22, Rompe JD, Eysel P, Hopf C, Vogel J, Kullmer K. [Extracorporeal shockwave treatment of delayed bone healing. A critical assessment]. Unfallchirurg 100: , Orhan Z, Alper M, Akman Y, Yavuz O, Yalciner A. An experimental study on the application of extracorporeal shockwaves in the treatment of tendon injuries: preliminary report. J Orthop Sci 6: , Perlick L, Schiffmann R, Kraft CN, Wallny T, Diedrich O. [Extracorporeal shockwave treatment of the achilles tendinitis: experimental and preliminary clinical results]. [Article in German]. Z Orthop Ihre Grenzgeb 140: , Martini L, Fini M, Giavaresi G, Torricelli P, de Pretto M, Rimondini L, Giardino R. Primary osteoblasts response to shockwave therapy using different parameters. Artif Cells Blood Substit Immobil Biotechnol 31: , Orhan Z, Ozturan K, Guven A, Cam K. The effect of extracorporeal shockwaves on a rat model of injury to tendoachillis. A histological and biomechanical study. J Bone Joint Surg Br 86: , Hyer CF, VanCourt R, Block A. Evaluation of ultrasound-guided extracorporeal shockwave therapy (ESWT) in the treatment of chronic plantar fasciitis. J Foot Ankle Surg 44: , Alvarez RG, Ogden JA, Jaakkola J, Cross GL. Symptom duration of plantar fasciitis and the effectiveness of Orthotripsy. Foot Ankle Int 24: , Haake M, Buch M, Schoellner C, Goebel F, Vogel M, Mueller I, Hausdorf J, Zamzow K, Schade-Brittinger C, Mueller HH. Extracorporeal shockwave therapy for plantar fasciitis: randomized controlled multicentre trial. BMJ 327: , Speed CA, Nichols D, Wies J, Humphreys H, Richards C, Burnet S, Hazleman BL. Extracorporeal shockwave therapy for plantar fasciitis. A double blind randomized controlled trial. J Orthop Res 21: , Hammer DS, Adam F, Kreutz A, Kohn D, Seil R. Extracorporeal shockwave therapy (ESWT) in patients with chronic proximal plantar fasciitis: a 2-year follow-up. Foot Ankle Int 24: , Speed CA, Nichols D, Wies J, Humphreys H, Richards C, Burnet S, Hazleman BL. Extracorporeal shockwave therapy for plantar fasciitis. A double blind randomized controlled trial. J Orthop Res 21: , Rompe JD, Decking J, Schoellner C, Nafe B. Shockwave application for chronic plantar fasciitis in running athletes. A prospective, randomized, placebo-controlled trial. Am J Sports Med 31: , Buchbinder R, Ptasznik R, Gordon J, Buchanan J, Prabaharan V, Forbes A. Ultrasound-guided extracorporeal shockwave therapy for plantar fasciitis: a randomized controlled trial. JAMA 288: , Hammer DS, Rupp S, Kreutz A, Pape D, Kohn D, Seil R. Extracorporeal shockwave therapy (ESWT) in patients with chronic proximal plantar fasciitis. Foot Ankle Int 23: , Rompe JD, Schoellner C, Nafe B. Evaluation of low-energy extracorporeal shock-wave application for treatment of chronic plantar fasciitis. J Bone Joint Surg Am 84-A: , Ogden JA, Alvarez R, Levitt R, Cross GL, Marlow M. Shockwave therapy for chronic proximal plantar fasciitis. Clin Orthop 387:47 59, Haake M, Buch M, Schoellner C, Goebel F, Vogel M, Mueller I, VOLUME 45, NUMBER 4, JULY/AUGUST
15 Hausdorf J, Zamzow K, Schade-Brittinger C, Mueller HH. Extracorporeal shockwave therapy for plantar fasciitis: randomised controlled multicentre trial. BMJ 327:75 79, Jenkinson C, Coulter A, Wright L. Short form 36 (SF36) health survey questionnaire: normative data for adults of working age. BMJ 306: , Barnett S, Campbell R, Harvey I. The Bristol Foot Score: developing a patient-based foot-health measure. J Am Podiatr Med Assoc 95: , Bennett PJ, Patterson C, Wearing S, Baglioni T. Development and validation of a questionnaire designed to measure foot-health status. J Am Podiatr Med Assoc 88: , Shmueli A. The visual analog rating scale of health-related quality of life: an examination of end-digit preferences. Health Qual Life Outcomes 3:71, Paul-Dauphin A, Guillemin F, Vuruib JM, Briancon S. Bias and precision in visual analogue scales: a randomized controlled trial. Am J Epidemiol 150: , Guidance for industry: clinical development programs for drugs, devices, and biological products intended for the treatment of osteoarthritis (OA). U.S. Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health (CDRH), July 1999, p. 3. (Available at dockets/98fr/980077g2.pdf/ last accessed May 23, 2006). 210 THE JOURNAL OF FOOT & ANKLE SURGERY
Physician Labeling. ( Clinical Application of the Duolith SD1 will be added to Operating Manuals upon PMA Approval.)
 Physician Labeling ( Clinical Application of the Duolith SD1 will be added to Operating Manuals upon PMA Approval.) CAUTION: Federal law restricts this device to sale by or on the order of a physician.
Physician Labeling ( Clinical Application of the Duolith SD1 will be added to Operating Manuals upon PMA Approval.) CAUTION: Federal law restricts this device to sale by or on the order of a physician.
Treatment of Recalcitrant Intermetatarsal Neuroma With 4% Sclerosing Alcohol Injection: A Pilot Study
 Treatment of Recalcitrant Intermetatarsal Neuroma With 4% Sclerosing Alcohol Injection: A Pilot Study Christopher F. Hyer, DPM,' Lynette R. Mehl, DPM,2 Alan J. Block, DPM, MS, FACFAS,3 and Robert B. Vancourt,
Treatment of Recalcitrant Intermetatarsal Neuroma With 4% Sclerosing Alcohol Injection: A Pilot Study Christopher F. Hyer, DPM,' Lynette R. Mehl, DPM,2 Alan J. Block, DPM, MS, FACFAS,3 and Robert B. Vancourt,
.org. Plantar Fasciitis and Bone Spurs. Anatomy. Cause
 Plantar Fasciitis and Bone Spurs Page ( 1 ) Plantar fasciitis (fashee-eye-tiss) is the most common cause of pain on the bottom of the heel. Approximately 2 million patients are treated for this condition
Plantar Fasciitis and Bone Spurs Page ( 1 ) Plantar fasciitis (fashee-eye-tiss) is the most common cause of pain on the bottom of the heel. Approximately 2 million patients are treated for this condition
Sponsor Novartis. Generic Drug Name Secukinumab. Therapeutic Area of Trial Psoriasis. Approved Indication investigational
 Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
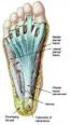
Plantar Fasciitis. Plantar Fascia
 Plantar Fasciitis Introduction Plantar fasciitis is an inflammation of the thick band of tissue that connects your heel bone to your toes. This thick band of tissue is called the plantar fascia. Plantar
Plantar Fasciitis Introduction Plantar fasciitis is an inflammation of the thick band of tissue that connects your heel bone to your toes. This thick band of tissue is called the plantar fascia. Plantar
PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain
 P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
P a g e 1 PROTOCOL SYNOPSIS Evaluation of long-term opioid efficacy for chronic pain Clinical Phase 4 Study Centers Study Period 25 U.S. sites identified and reviewed by the Steering Committee and Contract
Guidance for Industry Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes
 Guidance for Industry Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes U.S. Department of Health and Human Services Food and Drug Administration Center
Guidance for Industry Diabetes Mellitus Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes U.S. Department of Health and Human Services Food and Drug Administration Center
Plantar fasciitis: p. 1/6. E.M.S. Electro Medical Systems S.A. Ch. de la Vuarpillière 31 CH-1260 Nyon Switzerland
 Clinical trials published in the international peer-reviewed literature 1 demonstrating efficacy and safety of treatment with the EMS Swiss Dolorclast according to Evidence Based Medicine criteria 2 :
Clinical trials published in the international peer-reviewed literature 1 demonstrating efficacy and safety of treatment with the EMS Swiss Dolorclast according to Evidence Based Medicine criteria 2 :
.org. Achilles Tendinitis. Description. Cause. Achilles tendinitis is a common condition that causes pain along the back of the leg near the heel.
 Achilles Tendinitis Page ( 1 ) Achilles tendinitis is a common condition that causes pain along the back of the leg near the heel. The Achilles tendon is the largest tendon in the body. It connects your
Achilles Tendinitis Page ( 1 ) Achilles tendinitis is a common condition that causes pain along the back of the leg near the heel. The Achilles tendon is the largest tendon in the body. It connects your
Plantar Fascia Release
 Plantar Fascia Release Introduction Plantar fasciitis is a common condition that causes pain around the heel. It may be severe enough to affect regular activities. If other treatments are unsuccessful,
Plantar Fascia Release Introduction Plantar fasciitis is a common condition that causes pain around the heel. It may be severe enough to affect regular activities. If other treatments are unsuccessful,
2.0 Synopsis. Vicodin CR (ABT-712) M05-765 Clinical Study Report R&D/07/095. (For National Authority Use Only) to Part of Dossier: Volume:
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Vicodin CR Name of Active Ingredient: Hydrocodone/Acetaminophen Extended Release (ABT-712) Individual Study Table Referring to Part of Dossier: Volume:
2.0 Synopsis Abbott Laboratories Name of Study Drug: Vicodin CR Name of Active Ingredient: Hydrocodone/Acetaminophen Extended Release (ABT-712) Individual Study Table Referring to Part of Dossier: Volume:
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
A Guide to Heel Pain
 The Society of Chiropodists and Podiatrists A Guide to Heel Pain The Society of Chiropodists and Podiatrists Heel pain may be caused by a number of different problems; for effective treatment you need
The Society of Chiropodists and Podiatrists A Guide to Heel Pain The Society of Chiropodists and Podiatrists Heel pain may be caused by a number of different problems; for effective treatment you need
Plantar fascia. Plantar Fasciitis (pain in the heel of the foot)
 ! Plantar fascia Plantar Fasciitis (pain in the heel of the foot) Plantar Fasciitis is the most common foot problem seen in runners and is often associated with an increase in running mileage. Typically
! Plantar fascia Plantar Fasciitis (pain in the heel of the foot) Plantar Fasciitis is the most common foot problem seen in runners and is often associated with an increase in running mileage. Typically
The Diagnosis and Management of Heel Pain in Active People
 The Diagnosis and Management of Heel Pain in Active People Perry Julien, D.P.M. Past President, American Academy of Podiatric Sports Medicine Podiatry Coordinator, 1996 Summer Olympic Games Atlanta Georgia
The Diagnosis and Management of Heel Pain in Active People Perry Julien, D.P.M. Past President, American Academy of Podiatric Sports Medicine Podiatry Coordinator, 1996 Summer Olympic Games Atlanta Georgia
Heel Pain: Heal! Amie C. Scantlin, DPM, MS, FACFAS Glencoe Regional Health Services (320) 864-3121 ext. 1933
 Heel Pain: Heal! Amie C. Scantlin, DPM, MS, FACFAS Glencoe Regional Health Services (320) 864-3121 ext. 1933 www.grhsonline.org Important Notice The information contained in this document is for informational
Heel Pain: Heal! Amie C. Scantlin, DPM, MS, FACFAS Glencoe Regional Health Services (320) 864-3121 ext. 1933 www.grhsonline.org Important Notice The information contained in this document is for informational
DIVISION OF RHEUMATOLOGY DEPARTMENT OF MEDICINE UNIVERSITY OF WESTERN ONTARIO POSTGRADUATE EDUCTION ORTHOPAEDIC OFF-SERVICE GOALS & OBJECTIVES
 DIVISION OF RHEUMATOLOGY DEPARTMENT OF MEDICINE UNIVERSITY OF WESTERN ONTARIO POSTGRADUATE EDUCTION ORTHOPAEDIC OFF-SERVICE GOALS & OBJECTIVES GOAL #1 develop the ability to order and understand interpretation
DIVISION OF RHEUMATOLOGY DEPARTMENT OF MEDICINE UNIVERSITY OF WESTERN ONTARIO POSTGRADUATE EDUCTION ORTHOPAEDIC OFF-SERVICE GOALS & OBJECTIVES GOAL #1 develop the ability to order and understand interpretation
How To Treat Heel Pain
 Plantar Fasciitis, Heel Spurs, Heel Pain The Plantar Fasciitis Organization is dedicated to the understanding of Plantar Fasciitis, Heel Spurs, and all other forms of Heel Pain. Welcome to the Plantar
Plantar Fasciitis, Heel Spurs, Heel Pain The Plantar Fasciitis Organization is dedicated to the understanding of Plantar Fasciitis, Heel Spurs, and all other forms of Heel Pain. Welcome to the Plantar
Endoscopic Plantar Fasciotomy
 Endoscopic Plantar Fasciotomy Introduction Plantar fasciitis is a common condition that causes pain centralized around the heel. It may be severe enough to affect regular activities. Health care providers
Endoscopic Plantar Fasciotomy Introduction Plantar fasciitis is a common condition that causes pain centralized around the heel. It may be severe enough to affect regular activities. Health care providers
THE INTERNET STROKE CENTER PRESENTATIONS AND DISCUSSIONS ON STROKE MANAGEMENT
 THE INTERNET STROKE CENTER PRESENTATIONS AND DISCUSSIONS ON STROKE MANAGEMENT Stroke Prevention in Atrial Fibrillation Gregory Albers, M.D. Director Stanford Stroke Center Professor of Neurology and Neurological
THE INTERNET STROKE CENTER PRESENTATIONS AND DISCUSSIONS ON STROKE MANAGEMENT Stroke Prevention in Atrial Fibrillation Gregory Albers, M.D. Director Stanford Stroke Center Professor of Neurology and Neurological
Outline. The Agony of the Foot: Disclosure. Plantar Fasciitis. Top 5 Foot and Ankle Problems in Primary Care. Daniel Thuillier, M.D.
 The Agony of the Foot: Top 5 Foot and Ankle Problems in Primary Care Daniel Thuillier, M.D. Assistant Professor of Clinical Orthopaedics University of California San Francisco Plantar Fasciitis Achilles
The Agony of the Foot: Top 5 Foot and Ankle Problems in Primary Care Daniel Thuillier, M.D. Assistant Professor of Clinical Orthopaedics University of California San Francisco Plantar Fasciitis Achilles
.org. Posterior Tibial Tendon Dysfunction. Anatomy. Cause. Symptoms
 Posterior Tibial Tendon Dysfunction Page ( 1 ) Posterior tibial tendon dysfunction is one of the most common problems of the foot and ankle. It occurs when the posterior tibial tendon becomes inflamed
Posterior Tibial Tendon Dysfunction Page ( 1 ) Posterior tibial tendon dysfunction is one of the most common problems of the foot and ankle. It occurs when the posterior tibial tendon becomes inflamed
Subject: No. Page PROTOCOL AND CASE REPORT FORM DEVELOPMENT AND REVIEW Standard Operating Procedure
 703 1 of 11 POLICY The Beaumont Research Coordinating Center (BRCC) will provide advice to clinical trial investigators on protocol development, content and format. Upon request, the BRCC will review a
703 1 of 11 POLICY The Beaumont Research Coordinating Center (BRCC) will provide advice to clinical trial investigators on protocol development, content and format. Upon request, the BRCC will review a
CLINICAL PRACTICE GUIDELINES FOR MANAGEMENT OF LOW BACK PAIN
 CLINICAL PRACTICE GUIDELINES FOR MANAGEMENT OF LOW BACK PAIN Low back pain is very common, up to 90+% of people are affected by back pain at some time in their lives. Most often back pain is benign and
CLINICAL PRACTICE GUIDELINES FOR MANAGEMENT OF LOW BACK PAIN Low back pain is very common, up to 90+% of people are affected by back pain at some time in their lives. Most often back pain is benign and
Study Design. Date: March 11, 2003 Reviewer: Jawahar Tiwari, Ph.D. Ellis Unger, M.D. Ghanshyam Gupta, Ph.D. Chief, Therapeutics Evaluation Branch
 BLA: STN 103471 Betaseron (Interferon β-1b) for the treatment of secondary progressive multiple sclerosis. Submission dated June 29, 1998. Chiron Corp. Date: March 11, 2003 Reviewer: Jawahar Tiwari, Ph.D.
BLA: STN 103471 Betaseron (Interferon β-1b) for the treatment of secondary progressive multiple sclerosis. Submission dated June 29, 1998. Chiron Corp. Date: March 11, 2003 Reviewer: Jawahar Tiwari, Ph.D.
Guidance for Industry Migraine: Developing Drugs for Acute Treatment
 Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
Heel pain and Plantar fasciitis
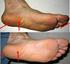 A patient s guide Heel pain and Plantar fasciitis Fred Robinson BSc FRCS FRCS(orth) Consultant Trauma & Orthopaedic Surgeon Alex Wee BSc FRCS(orth) Consultant Trauma & Orthopaedic Surgeon. What causes
A patient s guide Heel pain and Plantar fasciitis Fred Robinson BSc FRCS FRCS(orth) Consultant Trauma & Orthopaedic Surgeon Alex Wee BSc FRCS(orth) Consultant Trauma & Orthopaedic Surgeon. What causes
PROVEN NON-INVASIVE TREATMENT FOR RHEUMATOID ARTHRITIS
 PROVEN NON-INVASIVE TREATMENT FOR RHEUMATOID ARTHRITIS BIONICARE HAND SYSTEM Non-drug, non-invasive Adjunctive therapy For patients symptomatic despite current therapy For patients intolerant to drug
PROVEN NON-INVASIVE TREATMENT FOR RHEUMATOID ARTHRITIS BIONICARE HAND SYSTEM Non-drug, non-invasive Adjunctive therapy For patients symptomatic despite current therapy For patients intolerant to drug
Heel Pain Syndromes DELLON INSTITUTES FOR PERIPHERAL NERVE SURGERY
 Heel Pain s 5 3333 N CALVERT ST, SUITE 370, BALTIMORE, MD 21218 T410 467 5400 F410 366 9826 delloninstitutes.com your complaints are Pain, numbness or burning in your heel. The timing of this pain and
Heel Pain s 5 3333 N CALVERT ST, SUITE 370, BALTIMORE, MD 21218 T410 467 5400 F410 366 9826 delloninstitutes.com your complaints are Pain, numbness or burning in your heel. The timing of this pain and
PODIATRIC SURGERY INFORMATION GUIDE: MANAGEMENT OF PLANTAR FASCIITIS/HEEL PAIN
 PODIATRIC SURGERY INFORMATION GUIDE: MANAGEMENT OF PLANTAR FASCIITIS/HEEL PAIN What is Plantar Fasciitis? Plantar fasciitis is pain in the heel and arch area of the foot. The plantar fascia is a strong
PODIATRIC SURGERY INFORMATION GUIDE: MANAGEMENT OF PLANTAR FASCIITIS/HEEL PAIN What is Plantar Fasciitis? Plantar fasciitis is pain in the heel and arch area of the foot. The plantar fascia is a strong
1.0 Abstract. Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA. Keywords. Rationale and Background:
 1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
1.0 Abstract Title: Real Life Evaluation of Rheumatoid Arthritis in Canadians taking HUMIRA Keywords Rationale and Background: This abbreviated clinical study report is based on a clinical surveillance
Study Design and Statistical Analysis
 Study Design and Statistical Analysis Anny H Xiang, PhD Department of Preventive Medicine University of Southern California Outline Designing Clinical Research Studies Statistical Data Analysis Designing
Study Design and Statistical Analysis Anny H Xiang, PhD Department of Preventive Medicine University of Southern California Outline Designing Clinical Research Studies Statistical Data Analysis Designing
Antipsychotic drugs are the cornerstone of treatment
 Article Effectiveness of Olanzapine, Quetiapine, Risperidone, and Ziprasidone in Patients With Chronic Schizophrenia Following Discontinuation of a Previous Atypical Antipsychotic T. Scott Stroup, M.D.,
Article Effectiveness of Olanzapine, Quetiapine, Risperidone, and Ziprasidone in Patients With Chronic Schizophrenia Following Discontinuation of a Previous Atypical Antipsychotic T. Scott Stroup, M.D.,
Elbow Injuries and Disorders
 Elbow Injuries and Disorders Introduction Your elbow joint is made up of bone, cartilage, ligaments and fluid. Muscles and tendons help the elbow joint move. There are many injuries and disorders that
Elbow Injuries and Disorders Introduction Your elbow joint is made up of bone, cartilage, ligaments and fluid. Muscles and tendons help the elbow joint move. There are many injuries and disorders that
Rheumatoid Arthritis: Symptoms, Causes, and Treatments of Rheumatoid Foot and Ankle
 Rheumatoid arthritis is the most common form of inflammatory arthritis, affecting about two to three million Americans. Rheumatoid arthritis is a symmetric disease, meaning that it will usually involve
Rheumatoid arthritis is the most common form of inflammatory arthritis, affecting about two to three million Americans. Rheumatoid arthritis is a symmetric disease, meaning that it will usually involve
Spinal Cord Stimulation (SCS) Therapy: Fact Sheet
 Spinal Cord Stimulation (SCS) Therapy: Fact Sheet What is SCS Therapy? Spinal cord stimulation (SCS) may be a life-changing 1 surgical option for patients to control their chronic neuropathic pain and
Spinal Cord Stimulation (SCS) Therapy: Fact Sheet What is SCS Therapy? Spinal cord stimulation (SCS) may be a life-changing 1 surgical option for patients to control their chronic neuropathic pain and
Objectives Learn the anatomy of the foot. Identify key terms associated with plantar fasciitis. Determine the causes of plantar fasciitis and understa
 Plantar Fasciitis Objectives Learn the anatomy of the foot. Identify key terms associated with plantar fasciitis. Determine the causes of plantar fasciitis and understand why it occurs. Recognize the injury
Plantar Fasciitis Objectives Learn the anatomy of the foot. Identify key terms associated with plantar fasciitis. Determine the causes of plantar fasciitis and understand why it occurs. Recognize the injury
Accelerated recovery of post-operative dental implant patients by means of pulsed shortwave (SWT) therapy: An Observational Study
 Bartolomeo Operti, MD and Dr Tiziano Tealdo Accelerated recovery of post-operative dental implant patients by means of pulsed shortwave (SWT) therapy: Bartolomeo Operti, MD Chief of Anesthesia Department
Bartolomeo Operti, MD and Dr Tiziano Tealdo Accelerated recovery of post-operative dental implant patients by means of pulsed shortwave (SWT) therapy: Bartolomeo Operti, MD Chief of Anesthesia Department
W40 Total prosthetic replacement of knee joint using cement
 Bedfordshire and Hertfordshire Priorities Forum statement Number: 33 Subject: Referral criteria for patients from primary care presenting with knee pain due to ostoarthritis, and clinical threshold for
Bedfordshire and Hertfordshire Priorities Forum statement Number: 33 Subject: Referral criteria for patients from primary care presenting with knee pain due to ostoarthritis, and clinical threshold for
The Role of Acupuncture with Electrostimulation in the Prozen Shoulder
 The Role of Acupuncture with Electrostimulation in the Prozen Shoulder Yu-Te Lee A. Aim To evaluate the efficacy of acupuncture with electrostimulation in conjunction with physical therapy in improving
The Role of Acupuncture with Electrostimulation in the Prozen Shoulder Yu-Te Lee A. Aim To evaluate the efficacy of acupuncture with electrostimulation in conjunction with physical therapy in improving
Informed Patient Tutorial Copyright 2012 by the American Academy of Orthopaedic Surgeons
 Informed Patient Tutorial Copyright 2012 by the American Academy of Orthopaedic Surgeons Informed Patient - Carpal Tunnel Release Surgery Introduction Welcome to the American Academy of Orthopaedic Surgeons'
Informed Patient Tutorial Copyright 2012 by the American Academy of Orthopaedic Surgeons Informed Patient - Carpal Tunnel Release Surgery Introduction Welcome to the American Academy of Orthopaedic Surgeons'
6/3/2011. High Prevalence and Incidence. Low back pain is 5 th most common reason for all physician office visits in the U.S.
 High Prevalence and Incidence Prevalence 85% of Americans will experience low back pain at some time in their life. Incidence 5% annual Timothy C. Shen, M.D. Physical Medicine and Rehabilitation Sub-specialty
High Prevalence and Incidence Prevalence 85% of Americans will experience low back pain at some time in their life. Incidence 5% annual Timothy C. Shen, M.D. Physical Medicine and Rehabilitation Sub-specialty
A. Ketorolac*** B. Naproxen C. Ibuprofen D. Celecoxib
 1. A man, 66 years of age, with a history of knee osteoarthritis (OA) is experiencing increasing pain at rest and with physical activity. He also has a history of depression and coronary artery disease.
1. A man, 66 years of age, with a history of knee osteoarthritis (OA) is experiencing increasing pain at rest and with physical activity. He also has a history of depression and coronary artery disease.
.org. Ankle Fractures (Broken Ankle) Anatomy
 Ankle Fractures (Broken Ankle) Page ( 1 ) A broken ankle is also known as an ankle fracture. This means that one or more of the bones that make up the ankle joint are broken. A fractured ankle can range
Ankle Fractures (Broken Ankle) Page ( 1 ) A broken ankle is also known as an ankle fracture. This means that one or more of the bones that make up the ankle joint are broken. A fractured ankle can range
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
PLANTAR FASCITIS (Heel Spur Syndrome)
 PLANTAR FASCITIS (Heel Spur Syndrome) R. Amadeus Mason MD Description Plantar fascitis is characterized by stiffness and inflammation of the main fascia (fibrous connective [ligament-like] tissue) on the
PLANTAR FASCITIS (Heel Spur Syndrome) R. Amadeus Mason MD Description Plantar fascitis is characterized by stiffness and inflammation of the main fascia (fibrous connective [ligament-like] tissue) on the
Herniated Cervical Disc
 Herniated Cervical Disc North American Spine Society Public Education Series What Is a Herniated Disc? The backbone, or spine, is composed of a series of connected bones called vertebrae. The vertebrae
Herniated Cervical Disc North American Spine Society Public Education Series What Is a Herniated Disc? The backbone, or spine, is composed of a series of connected bones called vertebrae. The vertebrae
Get Rid of Elbow Pain
 Get Rid of Elbow Pain Self Regional Healthcare Optimum Life Center 115 Academy Avenue Greenwood, SC 29646 Office: (864) 725-7088 Self Regional Healthcare Physical Therapy Savannah Lakes 207 Holiday Road
Get Rid of Elbow Pain Self Regional Healthcare Optimum Life Center 115 Academy Avenue Greenwood, SC 29646 Office: (864) 725-7088 Self Regional Healthcare Physical Therapy Savannah Lakes 207 Holiday Road
CHPN Review Course Pain Management Part 1 Hospice and Palliative Nurses Association
 CHPN Review Course Pain Management Part 1 Disclosures Bonnie Morgan has no real or perceived conflicts of interest that relate to this presentation. Copyright 2015 by the. HPNA has the exclusive rights
CHPN Review Course Pain Management Part 1 Disclosures Bonnie Morgan has no real or perceived conflicts of interest that relate to this presentation. Copyright 2015 by the. HPNA has the exclusive rights
Predislocation syndrome
 Predislocation syndrome Sky Ridge Medical Center, Aspen Building Pre-dislocation syndrome, capsulitis, and metatarsalgia are all similar problems usually at the ball of the foot near the second and third
Predislocation syndrome Sky Ridge Medical Center, Aspen Building Pre-dislocation syndrome, capsulitis, and metatarsalgia are all similar problems usually at the ball of the foot near the second and third
Guidance for Industry
 Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
Guidance for Industry Cancer Drug and Biological Products Clinical Data in Marketing Applications U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and
NIMULID MD. 1. Introduction. 2. Nimulid MD Drug delivery system
 NIMULID MD 1. Introduction Nimulid MD is a flavoured dispersible Nimesulide tablet with fast mouth dissolving characteristics thereby providing immediate relief. Nimesulide is a non-steroidal antiinflammatory
NIMULID MD 1. Introduction Nimulid MD is a flavoured dispersible Nimesulide tablet with fast mouth dissolving characteristics thereby providing immediate relief. Nimesulide is a non-steroidal antiinflammatory
A Patient s Guide to Diffuse Idiopathic Skeletal Hyperostosis (DISH)
 A Patient s Guide to Diffuse Idiopathic Skeletal Hyperostosis (DISH) Introduction Diffuse Idiopathic Skeletal Hyperostosis (DISH) is a phenomenon that more commonly affects older males. It is associated
A Patient s Guide to Diffuse Idiopathic Skeletal Hyperostosis (DISH) Introduction Diffuse Idiopathic Skeletal Hyperostosis (DISH) is a phenomenon that more commonly affects older males. It is associated
FAQ About Prostate Cancer Treatment and SpaceOAR System
 FAQ About Prostate Cancer Treatment and SpaceOAR System P. 4 Prostate Cancer Background SpaceOAR Frequently Asked Questions (FAQ) 1. What is prostate cancer? The vast majority of prostate cancers develop
FAQ About Prostate Cancer Treatment and SpaceOAR System P. 4 Prostate Cancer Background SpaceOAR Frequently Asked Questions (FAQ) 1. What is prostate cancer? The vast majority of prostate cancers develop
Guidance on Investigational Medicinal Products (IMPs) and other medicinal products used in Clinical Trials
 EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Guidance on Investigational Medicinal Products (IMPs) and other medicinal products used in Clinical Trials
EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Guidance on Investigational Medicinal Products (IMPs) and other medicinal products used in Clinical Trials
Temple Physical Therapy
 Temple Physical Therapy A General Overview of Common Neck Injuries For current information on Temple Physical Therapy related news and for a healthy and safe return to work, sport and recreation Like Us
Temple Physical Therapy A General Overview of Common Neck Injuries For current information on Temple Physical Therapy related news and for a healthy and safe return to work, sport and recreation Like Us
Version History. Previous Versions. Policy Title. Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields
Version History Policy Title Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields
Participating in Alzheimer s Disease Clinical Trials and Studies
 Participating in Alzheimer s Disease Clinical Trials and Studies FACT SHEET When Margaret was diagnosed with earlystage Alzheimer s disease at age 68, she wanted to do everything possible to combat the
Participating in Alzheimer s Disease Clinical Trials and Studies FACT SHEET When Margaret was diagnosed with earlystage Alzheimer s disease at age 68, she wanted to do everything possible to combat the
SYNOPSIS. 2-Year (0.5 DB + 1.5 OL) Addendum to Clinical Study Report
 Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier (For National Authority Use
Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier (For National Authority Use
Acute Low Back Pain. North American Spine Society Public Education Series
 Acute Low Back Pain North American Spine Society Public Education Series What Is Acute Low Back Pain? Acute low back pain (LBP) is defined as low back pain present for up to six weeks. It may be experienced
Acute Low Back Pain North American Spine Society Public Education Series What Is Acute Low Back Pain? Acute low back pain (LBP) is defined as low back pain present for up to six weeks. It may be experienced
University of Hawai i Human Studies Program. Guidelines for Developing a Clinical Research Protocol
 University of Hawai i Human Studies Program Guidelines for Developing a Clinical Research Protocol Following are guidelines for writing a clinical research protocol for submission to the University of
University of Hawai i Human Studies Program Guidelines for Developing a Clinical Research Protocol Following are guidelines for writing a clinical research protocol for submission to the University of
ELECTROMYOGRAPHY (EMG), NEEDLE, NERVE CONDUCTION STUDIES (NCS) AND QUANTITATIVE SENSORY TESTING (QST)
 AND QUANTITATIVE SENSORY TESTING (QST) Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical
AND QUANTITATIVE SENSORY TESTING (QST) Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical
Sample Treatment Protocol
 Sample Treatment Protocol 1 Adults with acute episode of LBP Definition: Acute episode Back pain lasting
Sample Treatment Protocol 1 Adults with acute episode of LBP Definition: Acute episode Back pain lasting
Summary ID# 13614. Clinical Study Summary: Study F3Z-JE-PV06
 CT Registry ID# Page 1 Summary ID# 13614 Clinical Study Summary: Study F3Z-JE-PV06 INSIGHTS; INSulin-changing study Intending to Gain patients insights into insulin treatment with patient-reported Health
CT Registry ID# Page 1 Summary ID# 13614 Clinical Study Summary: Study F3Z-JE-PV06 INSIGHTS; INSulin-changing study Intending to Gain patients insights into insulin treatment with patient-reported Health
Ankle Injury/Sprains in Youth Soccer Players Elite Soccer Community Organization (ESCO) November 14, 2013
 Ankle Injury/Sprains in Youth Soccer Players Elite Soccer Community Organization (ESCO) November 14, 2013 Jeffrey R. Baker, DPM, FACFAS Weil Foot and Ankle Institute Des Plaines, IL Ankle Injury/Sprains
Ankle Injury/Sprains in Youth Soccer Players Elite Soccer Community Organization (ESCO) November 14, 2013 Jeffrey R. Baker, DPM, FACFAS Weil Foot and Ankle Institute Des Plaines, IL Ankle Injury/Sprains
Heel Steroid Injection
 Heel Steroid Injection Evidence of effectiveness 2 systematic reviews (2 included) ~/ ~ 2 experimental studies (2 included) ~/ -/~ 1 observational (1 included) 2.5/3 Evidence of safety and harm Generic
Heel Steroid Injection Evidence of effectiveness 2 systematic reviews (2 included) ~/ ~ 2 experimental studies (2 included) ~/ -/~ 1 observational (1 included) 2.5/3 Evidence of safety and harm Generic
Gruppo di lavoro: Malattie Tromboemboliche
 Gruppo di lavoro: Malattie Tromboemboliche 2381 Soluble Recombinant Thrombomodulin Ameliorates Hematological Malignancy-Induced Disseminated Intravascular Coagulation More Promptly Than Conventional Anticoagulant
Gruppo di lavoro: Malattie Tromboemboliche 2381 Soluble Recombinant Thrombomodulin Ameliorates Hematological Malignancy-Induced Disseminated Intravascular Coagulation More Promptly Than Conventional Anticoagulant
Aesthetic Facial Plastic Surgery, PLLC 1810 116th Ave NE #102 Bellevue, WA 98004
 Aesthetic Facial Plastic Surgery, PLLC 1810 116th Ave NE #102 Bellevue, WA 98004 GENERAL, HIPAA, PHOTO VIDEO INFORMED CONSENT FORM, AND RELEASE AGREEMENT Aesthetic Facial Plastic Surgery, PLLC s ("AFPS"),
Aesthetic Facial Plastic Surgery, PLLC 1810 116th Ave NE #102 Bellevue, WA 98004 GENERAL, HIPAA, PHOTO VIDEO INFORMED CONSENT FORM, AND RELEASE AGREEMENT Aesthetic Facial Plastic Surgery, PLLC s ("AFPS"),
TASK FORCE SUPPLEMENT FOR FUNCTIONAL CAPACITY EVALUATION
 TASK FORCE SUPPLEMENT FOR FUNCTIONAL CAPACITY EVALUATION A. GENERAL PRINCIPLES Use of a Functional Capacity Evaluation (FCE) is to determine the ability of a patient to safely function within a work environment.
TASK FORCE SUPPLEMENT FOR FUNCTIONAL CAPACITY EVALUATION A. GENERAL PRINCIPLES Use of a Functional Capacity Evaluation (FCE) is to determine the ability of a patient to safely function within a work environment.
Cold as an adjunctive therapy for headache
 Cold as an adjunctive therapy for headache Seymour Diamond, MD Frederick G. Freltag, DO Preview Patients with acute headache are often so vexed by pain that they seek out numerous physicians and headache
Cold as an adjunctive therapy for headache Seymour Diamond, MD Frederick G. Freltag, DO Preview Patients with acute headache are often so vexed by pain that they seek out numerous physicians and headache
HIGH INTENSITY LASER A REVOLUTION IN THERAPEUTIC LASER TECHNOLOGY
 HIGH INTENSITY LASER A REVOLUTION IN THERAPEUTIC LASER TECHNOLOGY sales@btlnet.com www.btlnet.com All rights reserved. Although every care has been taken to provide accurate and up-to-date information,
HIGH INTENSITY LASER A REVOLUTION IN THERAPEUTIC LASER TECHNOLOGY sales@btlnet.com www.btlnet.com All rights reserved. Although every care has been taken to provide accurate and up-to-date information,
ABOUT XARELTO CLINICAL STUDIES
 ABOUT XARELTO CLINICAL STUDIES FAST FACTS Xarelto (rivaroxaban) is a novel, oral direct Factor Xa inhibitor. On September 30, 2008, the European Commission granted marketing approval for Xarelto for the
ABOUT XARELTO CLINICAL STUDIES FAST FACTS Xarelto (rivaroxaban) is a novel, oral direct Factor Xa inhibitor. On September 30, 2008, the European Commission granted marketing approval for Xarelto for the
Cilostazol versus Clopidogrel after Coronary Stenting
 Cilostazol versus Clopidogrel after Coronary Stenting Seong-Wook Park, MD, PhD, FACC Division of Cardiology, Asan Medical Center University of Ulsan College of Medicine Seoul, Korea AMC, 2004 Background
Cilostazol versus Clopidogrel after Coronary Stenting Seong-Wook Park, MD, PhD, FACC Division of Cardiology, Asan Medical Center University of Ulsan College of Medicine Seoul, Korea AMC, 2004 Background
Test Content Outline Effective Date: June 9, 2014. Pain Management Nursing Board Certification Examination
 Pain Management Nursing Board Certification Examination There are 175 questions on this examination. Of these, 150 are scored questions and 25 are pretest questions that are not scored. Pretest questions
Pain Management Nursing Board Certification Examination There are 175 questions on this examination. Of these, 150 are scored questions and 25 are pretest questions that are not scored. Pretest questions
Information on Rheumatoid Arthritis
 Information on Rheumatoid Arthritis Table of Contents About Rheumatoid Arthritis 1 Definition 1 Signs and symptoms 1 Causes 1 Risk factors 1 Test and diagnosis 2 Treatment options 2 Lifestyle 3 References
Information on Rheumatoid Arthritis Table of Contents About Rheumatoid Arthritis 1 Definition 1 Signs and symptoms 1 Causes 1 Risk factors 1 Test and diagnosis 2 Treatment options 2 Lifestyle 3 References
Articles Presented. Journal Presentation. Dr Albert Lo. Dr Albert Lo
 * This presentation is prepared by the author in one s personal capacity for the purpose of academic exchange and does not represent the views of his/her organisations on the topic discussed. Journal Presentation
* This presentation is prepared by the author in one s personal capacity for the purpose of academic exchange and does not represent the views of his/her organisations on the topic discussed. Journal Presentation
YALE UNIVERSITY SCHOOL OF MEDICINE: SECTION OF OTOLARYNGOLOGY PATIENT INFORMATION FUNCTIONAL ENDOSCOPIC SINUS SURGERY
 YALE UNIVERSITY SCHOOL OF MEDICINE: SECTION OF OTOLARYNGOLOGY PATIENT INFORMATION FUNCTIONAL ENDOSCOPIC SINUS SURGERY What is functional endoscopic sinus surgery (FESS)? Functional endoscopic sinus surgery
YALE UNIVERSITY SCHOOL OF MEDICINE: SECTION OF OTOLARYNGOLOGY PATIENT INFORMATION FUNCTIONAL ENDOSCOPIC SINUS SURGERY What is functional endoscopic sinus surgery (FESS)? Functional endoscopic sinus surgery
A Genetic Analysis of Rheumatoid Arthritis
 A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
Spinal Cord Stimulators (SCS) for Injured Workers with Chronic Back and Leg
 Spinal Cord Stimulators (SCS) for Injured Workers with Chronic Back and Leg Pain after Lumbar Surgery A Prospective Study to Describe Costs, Complications, and Patient Outcomes: Final Report September
Spinal Cord Stimulators (SCS) for Injured Workers with Chronic Back and Leg Pain after Lumbar Surgery A Prospective Study to Describe Costs, Complications, and Patient Outcomes: Final Report September
Medical Treatment Guidelines Washington State Department of Labor and Industries
 Complex regional pain syndrome (CRPS) Formerly known as reflex sympathetic dystrophy 1. Introduction This bulletin outlines the Department of Labor and Industries guidelines for diagnosing and treating
Complex regional pain syndrome (CRPS) Formerly known as reflex sympathetic dystrophy 1. Introduction This bulletin outlines the Department of Labor and Industries guidelines for diagnosing and treating
Herniated Lumbar Disc
 Herniated Lumbar Disc North American Spine Society Public Education Series What Is a Herniated Disc? The spine is made up of a series of connected bones called vertebrae. The disc is a combination of strong
Herniated Lumbar Disc North American Spine Society Public Education Series What Is a Herniated Disc? The spine is made up of a series of connected bones called vertebrae. The disc is a combination of strong
Humulin (LY041001) Page 1 of 1
 (LY041001) These clinical study results are supplied for informational purposes only in the interests of scientific disclosure. They are not intended to substitute for the FDA-approved package insert or
(LY041001) These clinical study results are supplied for informational purposes only in the interests of scientific disclosure. They are not intended to substitute for the FDA-approved package insert or
Medical management of CHF: A New Class of Medication. Al Timothy, M.D. Cardiovascular Institute of the South
 Medical management of CHF: A New Class of Medication Al Timothy, M.D. Cardiovascular Institute of the South Disclosures Speakers Bureau for Amgen Background Chronic systolic congestive heart failure remains
Medical management of CHF: A New Class of Medication Al Timothy, M.D. Cardiovascular Institute of the South Disclosures Speakers Bureau for Amgen Background Chronic systolic congestive heart failure remains
Clinical bottom line. For more detailed evidence on the effectiveness of injections for tennis elbow, please see the CAT on:
 Short Question: Specific Question: In patients presenting with acute or chronic tendinopathies, what is the incidence of harm for those receiving steroid injections compared to those receiving usual care?
Short Question: Specific Question: In patients presenting with acute or chronic tendinopathies, what is the incidence of harm for those receiving steroid injections compared to those receiving usual care?
Plantar Fasciitis Information Leaflet. Maneesh Bhatia. Consultant Orthopaedic Surgeon
 Plantar Fasciitis Information Leaflet Maneesh Bhatia Consultant Orthopaedic Surgeon What is plantar fasciitis? The plantar fascia is a strong band of tissue that stretches from the heel to the toes. It
Plantar Fasciitis Information Leaflet Maneesh Bhatia Consultant Orthopaedic Surgeon What is plantar fasciitis? The plantar fascia is a strong band of tissue that stretches from the heel to the toes. It
Behavioral and Physical Treatments for Tension-type and Cervicogenic Headache
 Evidence Report: Behavioral and Physical Treatments for Tension-type and Cervicogenic Headache Douglas C. McCrory, MD, MHSc Donald B. Penzien, PhD Vic Hasselblad, PhD Rebecca N. Gray, DPhil Duke University
Evidence Report: Behavioral and Physical Treatments for Tension-type and Cervicogenic Headache Douglas C. McCrory, MD, MHSc Donald B. Penzien, PhD Vic Hasselblad, PhD Rebecca N. Gray, DPhil Duke University
DIFFERENTIAL DIAGNOSIS OF LOW BACK PAIN. Arnold J. Weil, M.D., M.B.A. Non-Surgical Orthopaedics, P.C. Atlanta, GA
 DIFFERENTIAL DIAGNOSIS OF LOW BACK PAIN Arnold J. Weil, M.D., M.B.A. Non-Surgical Orthopaedics, P.C. Atlanta, GA MEDICAL ALGORITHM OF REALITY LOWER BACK PAIN Yes Patient will never get better until case
DIFFERENTIAL DIAGNOSIS OF LOW BACK PAIN Arnold J. Weil, M.D., M.B.A. Non-Surgical Orthopaedics, P.C. Atlanta, GA MEDICAL ALGORITHM OF REALITY LOWER BACK PAIN Yes Patient will never get better until case
Preventing Potential Claims of Negligence What Would You Do Settle or Go to Trial?
 Preventing Potential Claims of Negligence What Would You Do Settle or Go to Trial? Ross E. Taubman, DPM PICA President and Chief Medical Officer 2013 APMA YOUNG PHYSICIANS INSTITUTE April 12 14 PICA Headquarters
Preventing Potential Claims of Negligence What Would You Do Settle or Go to Trial? Ross E. Taubman, DPM PICA President and Chief Medical Officer 2013 APMA YOUNG PHYSICIANS INSTITUTE April 12 14 PICA Headquarters
X Stop Spinal Stenosis Decompression
 X Stop Spinal Stenosis Decompression Am I a candidate for X Stop spinal surgery? You may be a candidate for the X Stop spinal surgery if you have primarily leg pain rather than mostly back pain and your
X Stop Spinal Stenosis Decompression Am I a candidate for X Stop spinal surgery? You may be a candidate for the X Stop spinal surgery if you have primarily leg pain rather than mostly back pain and your
Other Noninfectious Diseases. Chapter 31 Lesson 3
 Other Noninfectious Diseases Chapter 31 Lesson 3 Diabetes Diabetes- a chronic disease that affects the way body cells convert food into energy. Diabetes is the seventh leading cause of death by disease
Other Noninfectious Diseases Chapter 31 Lesson 3 Diabetes Diabetes- a chronic disease that affects the way body cells convert food into energy. Diabetes is the seventh leading cause of death by disease
5.07.09. Aubagio. Aubagio (teriflunomide) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.09 Subject: Aubagio Page: 1 of 6 Last Review Date: December 5, 2014 Aubagio Description Aubagio (teriflunomide)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.07.09 Subject: Aubagio Page: 1 of 6 Last Review Date: December 5, 2014 Aubagio Description Aubagio (teriflunomide)
CARPAL TUNNEL SYNDROME A PATIENT GUIDE TO THE NURSE-LED CARPAL TUNNEL SERVICE
 CARPAL TUNNEL SYNDROME A PATIENT GUIDE TO THE NURSE-LED CARPAL TUNNEL SERVICE Information Leaflet Your Health. Our Priority. Page 2 of 6 What is carpal tunnel syndrome? It is entrapment of a nerve at the
CARPAL TUNNEL SYNDROME A PATIENT GUIDE TO THE NURSE-LED CARPAL TUNNEL SERVICE Information Leaflet Your Health. Our Priority. Page 2 of 6 What is carpal tunnel syndrome? It is entrapment of a nerve at the
Bios 6648: Design & conduct of clinical research
 Bios 6648: Design & conduct of clinical research Section 1 - Specifying the study setting and objectives 1. Specifying the study setting and objectives 1.0 Background Where will we end up?: (a) The treatment
Bios 6648: Design & conduct of clinical research Section 1 - Specifying the study setting and objectives 1. Specifying the study setting and objectives 1.0 Background Where will we end up?: (a) The treatment
Initial results of treatment with Linear Shockwave Therapy (LSWT) by Renova in patients with Erectile Dysfunction A pilot clinical study
 Initial results of treatment with Linear Shockwave Therapy (LSWT) by Renova in patients with Erectile Dysfunction A pilot clinical study Investigators Dr. Ahmed Hind, MD Consultant to Pr.Catanzaro Milano
Initial results of treatment with Linear Shockwave Therapy (LSWT) by Renova in patients with Erectile Dysfunction A pilot clinical study Investigators Dr. Ahmed Hind, MD Consultant to Pr.Catanzaro Milano
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 18 December 2002 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 18 December 2002 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE
Case Series on Chronic Whiplash Related Neck Pain Treated with Intraarticular Zygapophysial Joint Regeneration Injection Therapy
 Pain Physician 2007; 10:313-318 ISSN 1533-3159 Case Series Case Series on Chronic Whiplash Related Neck Pain Treated with Intraarticular Zygapophysial Joint Regeneration Injection Therapy R. Allen Hooper
Pain Physician 2007; 10:313-318 ISSN 1533-3159 Case Series Case Series on Chronic Whiplash Related Neck Pain Treated with Intraarticular Zygapophysial Joint Regeneration Injection Therapy R. Allen Hooper
.org. Herniated Disk in the Lower Back. Anatomy. Description
 Herniated Disk in the Lower Back Page ( 1 ) Sometimes called a slipped or ruptured disk, a herniated disk most often occurs in your lower back. It is one of the most common causes of low back pain, as
Herniated Disk in the Lower Back Page ( 1 ) Sometimes called a slipped or ruptured disk, a herniated disk most often occurs in your lower back. It is one of the most common causes of low back pain, as
If several different trials are mentioned in one publication, the data of each should be extracted in a separate data extraction form.
 General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
TENS, Electroacupuncture and Ice Massage: Comparison of Treatment for Osteoarthritis of the Knee
 TENS, Electroacupuncture and Ice Massage: Comparison of Treatment for Osteoarthritis of the Knee Merih Yurtkuran, Tuncer Kocagil Uludag University Medical Faculty Department of Physical Therapy and Rehabilitation,
TENS, Electroacupuncture and Ice Massage: Comparison of Treatment for Osteoarthritis of the Knee Merih Yurtkuran, Tuncer Kocagil Uludag University Medical Faculty Department of Physical Therapy and Rehabilitation,
Spine Vol. 30 No. 16; August 15, 2005, pp 1799-1807
 A Randomized Controlled Trial of an Educational Intervention to Prevent the Chronic Pain of Whiplash Associated Disorders Following Rear-End Motor Vehicle Collisions 1 Spine Vol. 30 No. 16; August 15,
A Randomized Controlled Trial of an Educational Intervention to Prevent the Chronic Pain of Whiplash Associated Disorders Following Rear-End Motor Vehicle Collisions 1 Spine Vol. 30 No. 16; August 15,
