Probability for the distance between motif 1 and motif 0 in Immunoglobulin C2 domain
|
|
|
- Erik Ball
- 6 years ago
- Views:
Transcription
1 Submitted for publication 1 An investigation of distant homology detection methods for multidomain protein families Andrey Rzhetsky Columbia Genome Center and Department of Medical Informatics Columbia University ar345@columbia.edu Reina E. Riemann Columbia Genome Center Columbia University rer20@columbia.edu Abstract William Noble Grundy Department of Computer Science Columbia University bgrundy@cs.columbia.edu Andrea Califano IBM Computational Biology Center TJ Watson Research Center acal@us.ibm.com Homology detection methods first emerged as pairwise alignment algorithms, such as Needleman-Wunsch, Smith-Waterman, FASTA, and BLAST. Although these pairwise comparison methods continue to play the major role in heavy-duty genomics applications, there are a few recent more sensitive algorithms that rely on statistical models built from training sets of related sequences. Many interesting methods fit in this new category. The majority are based on hidden Markov models (HMMs), on position-specific scoring matrices (PSSMs), or on clustering approaches that extend the original pairwise analysis algorithms. To further complicate matters, there are a variety of methods that are capable of discovering and building protein domain models by analyzing families of related sequences. Given the rapid proliferation of these techniques, it is important to establish a sound methodology to uniformly compare the performance of such algorithms in a reasonable and objective fashion. The objective of this paper is twofold. It introduces a relatively simple framework for the comparative analysis of consensus-based homology detection algorithms and for the domain model discovery algorithms. It also introduces a novel PSSM-based technique called SPLASHSearch that compares favorably with other established techniques. To test these algorithms, training sets have been built from sequences that include related protein domains. Only subsequences that include the domain are used for domain model building and training, in order to avoid contamination of the tests from multiple, diverse domain signatures. The goal is to determine the ability of the various techniques to perform rapid, accurate sequence annotation given a correct domain definition by a set of training sequences. Three key observations arise from this exercise. First, algorithms based on consensus motifs are becoming as accurate and sensitive as the more computationally expensive methods based on HMMs. Second, the ability to identify motifs impacts the performance of the search methods. Finally, for the HMM method preprocessing the sequences with a multiple sequence alignment algorithm has a negative impact on the search performance for some difficult" domains. Keywords: Homology detection, proteins, motif analysis Introduction 1 Within the young field of computational biology, the protein homology detection task is a classic problem (Barsalou & Brutlag 1991). The task consists of finding, for a given protein or protein family, all distantly related protein sequences in a large database of unannotated sequences. Two sequences that share a common evolutionary ancestor are said to be homologous. Because we do not have access to ancestral protein sequences, the homology detection task is necessarily inferential. Initially, inferences of homology were made based upon pairwise comparisons of protein sequences, using dynamic programming algorithms such as the Needleman-Wunsch (Needleman & Wunsch 1970) and Smith-Waterman (Smith & Waterman 1981) algorithms. The popular database search tools BLAST (Altschul et al. 1990) and FASTA (Pearson 1985) are fast approximations of these dynamic programming algorithms. Unfortunately, many difficult" protein sequences exhibit no significant pairwise similarity to previously characterized sequences in public databases. However, evidence of significant homology of the difficult" sequences to known sequences often emerges from comparison of the difficult" sequences to statistical models describing families of known sequences. In this context, the ability to build complex consensus models from a training set of proteins that share a common function or structure is a powerful tool. There 1 Corresponding author: Andrey Rzhetsky, Department of Medical Informatics, Russ Berrie Pavilion, Unit 109, 1150 St. Nicholas Ave., Tel: (212) , Fax: (212)
2 Submitted for publication 2 are three key reasons for this power. First, in a consensus model, protein regions that are less functionally constrained contribute far less to the sequence-tomodel comparison than in a pairwise sequence analysis. This tends to reduce the impact of noisy" sequences that may contain multiple diverse functional signatures. Second, rather than using a generic model of amino acid substitution (Schwartz & Dayhoff 1978; Henikoff & Henikoff 1992), more accurate family- and site-specific models can be used. Finally, pairwise sequence analysis often assumes that sequences change mostly through amino acid substitution and insertion/deletion. However, in reality, proteins domains often can be swapped without a loss of protein function, and a domain exchange among non-homologous proteins is an important instrument ofmolecular evolution. The PROSITE database (Bairoch 1991) represents the first systematic attempt to identify biologically relevant consensus sequences, or motifs, in functionally related families. Once identified, motifs can be easily represented as PSSMs. Over the past five years, anumber of statistical and deterministic motif discovery algorithms have been introduced in the literature. These include MEME (Bailey & Elkan 1994), SPLASH (Califano 2000), the Gibbs sampler (Neuwald, Liu, & Lawrence 1995), Teiresias (Rigoutsos & Floratos 1998), PRATT (Jonassen, Collins, & Higgins 1995), and E- MOTIF (Nevill-Manning, Wu, & Brutlag 1998). The goal of this paper is to propose an objective framework for the comparison of consensus-based homology detection algorithms and of the underlying motif discovery algorithms. This framework allows for the analysis of the accuracy and computational efficiency of these approaches. The comparison methodology, training sequences, and results are available on the web, thereby simplifying the inclusion of new algorithms and new data sets over time. Clearly, given the large number of available algorithms, an exhaustive analysis of all available methods is impossible. Therefore, we have initially selected four independent algorithms. Three of them HMMER, MAST, and Meta-MEME are publicly available. The fourth, SPLASHSearch, is an additional contribution of this paper and compares favorably with the other methods. HMMER has been tested both with training sets formed by unaligned sequences and with ones that had been previously globally aligned using CLUSTALW (Thompson, Higgins, & Gibson 1994a). With the exception of HMMER, all methods rely on a set of PSSM models for the given functional sequence training set. In combination with these methods we have tested two independent motif discovery algorithms. The first is MEME, which is based on a probabilistic, maximum likelihood model. The second is SPLASH, which is based on a deterministic pattern discovery algorithm. SPLASH has been recently shown to offer an extremely efficient, yet exhaustive way to discover conserved motifs based on amino acid similarity metrics. It has also been shown to identify, with high probability, protein regions that are biologically relevant and highly conserved. In an exhaustive test against the PROSITE motif database (Hart et al. 2000) SPLASH produced motifs that were highly overlapping with PROSITE reported motifs in more than 75% of the cases. MEME, on the other hand, is the standard algorithm used to generate PSSM models for two ofthe four tested methods. Other choices are possible and will be added to this benchmark in successive iterations. Finally, Meta-MEME has been tested both with trained and untrained (uniform) transition probabilities between the individual PSSM models. This experimental setup yields a total of nine independent techniques: HMMER (aligned/unaligned), MAST with either MEME- or SPLASH-based PSSMs, Meta-MEME (trained/untrained) with either MEMEor SPLASH-based PSSMs, and SPLASHSearch with SPLASH-based PSSMs. The latter method extends the p-value integration method pioneered in MAST with a probabilistic model for the relative position of PSSM matches on the sequences. Since this additional probability model is not generated by MEME, it has not been possible to study the performance of SPLASH- Search with MEME-based PSSMs. Training sets in our analysis comprise individual protein domains rather than complete multi-domain proteins. This choice allows for a clear definition of a set of database sequences bearing genuine homology to the training sequences. The alternative approach, involving discovering and training domain models from complete multidomain protein sequences, would often result in statistical models reflecting more than one protein domain. Since pairs of multi-domain proteins frequently exhibit only partial homology, the training set is likely to contain domains that are not shared by all sequences in the set. Furthermore, the true positive" database matches to such training set would have to include all sequences having at least one of the domains represented in the training set. Note that the majority of currently existing methods are not designed to deal with domain models trained from multi-domain data sets. The domain sequences have been obtained by anau- tomated methods that analyzes the annotations of sequences in SWISSPROT 38. Five types of domains have been chosen for this exercise, including two simple domains with similarities identifiable with pairwise comparison approaches and three complex domains with lower global conservation that is often undetectable with pairwise comparison methods. The protein domain selection procedure is fairly laborious and requires some manual supervision to avoid potential contamination of the training sets and of the lists of true positive and true negative examples with spurious data. Therefore, the initial benchmark is limited to five domains. This should be considered an initial set that will grow over time to include more complex and discriminative examples. Clearly, this reduced set does not allow us to draw definitive conclusions about the
3 Submitted for publication 3 relative performance of the various techniques. However, the results show a significant range of variability and several definite trends. With the exception of one or two of the simpler domain families that were easily identified by all methods, more complex domains yield a wide range of performance, with differences as large as 50%. In general, three observations emerge. First, the motif-based approaches offer accuracy and sensitivity that is comparable to that of the more computationally expensive HMM-based algorithms at a fraction of the computational cost. In particular, SPLASHSearch and HMMER unaligned offer the best results, with SPLASHSearch winning by about 15% in one of the 5 categories and tying in the remaining four. HMMER, however, is about an order of magnitude more computationally expensive. The MAST+SPLASH combination follows closely with a loss by 0.5% and one by 6.5% respectively. This approach is about 1.5 times faster than SPLASHSearch. MAST+MEME follows with four losses by 2%, 30%, 10%, and 11%. Finally, results for Meta-MEME in combination with MEME and SPLASH are mixed but, in general, it does not perform as well as the other techniques. It is likely that this algorithm would perform better with training sets composed of complete sequences and with additional fine-tuning of default parameters. Second, different choice of motif discovery methods can result in significant difference in terms of accuracy and sensitivity. In particular, MAST with SPLASHdiscovered motifs outperformed the same algorithm with MEME-discovered motifs in 4 out of 5 categories. Finally, training HMMER domain models with CLUSTALW-aligned sequences produces inferior results when the training set sequences belong to complex families that have low sequence conservation. For the most complex dataset, the immunoglobulinlike domains, performance drops by nearly 50% when CLUSTALW is used in conjunction with HMMER. This confirms that the substitution/deletion/insertion model of evolution, which is enforced in multiple sequence alignment, is inappropriate when complex functional signatures are involved. Methods Analyses were performed using five protein families extracted from the SWISSPROT database, version 38 (Bairoch 1994). Each family is defined by the presence of a particular type of domain or motif: CUB, c1q, galaptin, immunoglobulin and kringle. CUB domains are found in two types of proteins: proteins that regulate the developmental process and proteins in the complement system. The name CUB" indicates that this domain occurs in three complement components: C1r/C1s, Uegf, and bone morphogenic protein-1. The c1q μ domain μ participates μ in complement formation in vertebrates and occurs in the c1q subunit of the C1 enzyme complex. Galaptin domains are common to a class of soluble, developmentally regulated proteins called Family Seqs Sequence Domains Domain AAs AAs CUB c1q galaptin IG kringle Table 1: Protein families investigated. The columns contain the names of the families, number of sequences in each family, number of amino acids in each family, number of domains used in training, and the number of amino acids in those domains. galactoside-binding lectins. These proteins are synthesized by all vertebrates and seem to be involved in differentiation, cellular regulation and tissue construction. The immunoglobulin domain is common among a large superfamily of proteins, the most famous representatives of which are the antibodies in the human immune system. Immunoglobulins participate in specific recognition of other large molecules. Finally, kringles are triple-looped, disulfide, cross-linked domains found in a varying numbers of copies in some serine proteases and plasma proteins. Kringle domains are thought to play a role in binding mediators, such asmembranes, other proteins or phospholipids, and in the regulation of proteolytic activity. More information about these families is available at ttp:// Of the five families investigated, the first two are relatively closely clustered in sequence space, whereas the last three families contain highly dissimilar sequences whose global homology is nearly undetectable by pairwise sequence comparison methods (data not shown). Lists of sequences in each family were derived from SWISSPROT annotations by searching for corresponding keywords. Single domains were extracted from the sequences by exploiting the motif" and domain" features in SWISSPROT. For each family, the training set consists of approximately half the domains in the family. Some characteristics of these families are described in Table 1. The family members and training sets are available at families. For each family, MEME and SPLASH each discover one set of motifs. MEME, version 2.2.2, employs the default parameter settings from the web interface ( These settings include Dirichlet mixture priors (Brown et al. 1995) weighted by the megaprior heuristic (Bailey & Gribskov 1996), a minimum motif width of 12 and a maximum of 55. The motif model is a fully general, two-component mixture model that allows for a given motif to appear any number of times in the given sequences. MEME discovers a total of ten motifs for each family. SPLASH, version 1.0, also uses its default parameter settings to discover ten conserved motifs, as described in (Hart et al. 2000). Motifs are progressively discovered and masked
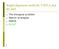
4 Submitted for publication 4 P15364: GQNLELTCHANGFPKPTISWAREHNAVMPAGGHLLAEPTLRIRSVHRMDRGGYYC IAQNGEGQPD Probability for the distance between motif 1 and motif 0 in Immunoglobulin C2 domain Distance in residues Figure 1: Relative motif distance probability for the first and second most likely motifs in the Immunoglobulin family. in the sequences, starting with the most statistically significant ones, so that the motifs are guaranteed to be non-overlapping. In particular, a BLOSUM50 matrix (Henikoff & Henikoff 1992) with a threshold of 0 is used as the similarity metric, one identity match is required, and reported motifs must have at least four tokens. The density constraint requires three matching tokens in any window of length 12. PSSMs are built using the amino acid counts for all the locations where the reported motifs occur in the training set. Pseudocounts are computed using the minimal risk approach (Wu, Nevill-Manning, & Brutlag 1999). The motifs are used in three different ways to search the SWISSPROT database, version 38. The first database search program, MAST (Bailey & Gribskov 1998), is part of the MEME software distribution package. MAST scans each database sequence with each motif separately and then combines the highest-scoring matches. MAST computes E-values by assuming that the motif matches within a given database sequence are independent of one another. MAST can search using motifs discovered either by MEME or SPLASH. The second program, SPLASHSearch, extends the MAST multiple p-value approach by taking into account the relative spacing of motifs, based on a probabiistic model. The model is computed empirically from the training set by determining the probability p(m j jm i ;d) of observing a motif m j at distance d residues from motif m i. This function is smothed using a gaussian filter with ff = 5 and renormalized. Figure 1, for instance, plots the final value of p(m0jm1;d) for the two PSSM models with the highest incidence in the Immunoglobulin C2 domain family. These correspond to the two SPLASH regular expressions [ILMFV]...G.Y.C and G..[ILMFV].[ILMFV].C, which have been underlined, as an example, in two sequence fragments: P04217:...SLLKPLANVTLTC QARLETPDFQLFKNGVAQEPVHLDSPAIKHQFLLTGDTQGRYRC RSGL... This information is then used in the context of an evidence integration framework such that motifs that occur in unlikely configurations are weighted independently rather than jointly, while motifs that appear in a configuration similar to one observed in the training set contribute jointly to the final score with the weighed product of their p-values. This weighting is accomplished as follows. Let us assume we have a set of motifs matches fm i g, where motif m i occurs at position l i on a given sequence and has a p-values p i. SPLASHSearch computes max i 2 4 X log(pi )+ j6=i [ log(p j ) Λ p(m j jm i ;l j l i )] 5 : Therefore, if p(m j jm i ;l j l i ) is close to zero for all js, the score is dominated by the term max[ log(p i )]. On the other hand, if there is an i such that p(m j jm i ;l j l i ) >> 0 for some js, then the contribution from the second term in the sum becomes not negligible. SPLASH- Search uses motifs discovered by SPLASH, but not by MEME because these additional probability density models are not produced by the latter algorithm. In the future, however, the output from MEME could be used to determine the probabilistic model in an intermediate step. The third search method uses Meta-MEME, version 2.1, to build motif-based HMMs. Each set of motifs is assembled into a motif-based HMM. The model topology is completely connected, meaning that transitions are included in the model from the end of every motif to the beginning of every other motif. This topology allows motifs to appear in any order and to appear multiple times within a single sequence. Sequence regions between motifs are modeled using a single free insertion module" (Hughey & Krogh 1996); i.e., a state with one self-transition and one outgoing transition, both with a transition probability" of 1.0. Initially, all motif-to-motif transition probabilities are initialized uniformly. Such a model is referred to below asan untrained" model. The motif-to-motif transition probabilities can be trained using expectationmaximization. Both the untrained and trained models are used to search the protein database. The Meta- MEME search tool, mhmms, computes the probability of the most likely path through the model. This score is reported as a log-odds scores in bits, using a simple, zero-order Markov chain as the background model (Grundy 1998). Meta-MEME can build models from motifs discovered by MEME or SPLASH. For comparison with the motif-based methods, homologs are also detected using profile HMMs. Two sets of HMMs are constructed by HMMER (Eddy 1995). The first set is derived by HMMER, version 2.0, from multiple alignments of each family, as produced by CLUSTALW (Thompson, Higgins, & Gibson 1994b) using neighbor-joining trees (Saitou & Nei 1987). The 3
5 Submitted for publication 5 Method Motif discovery by SPLASH Motif discovery by MEME Model building and search by HMMER Database search by MAST Database search by Meta-MEME Database search by SPLASHSearch Time < 1 minute 30 minutes 20 minutes < 1 minute 30 minutes < 1 minute Table 4: Comparison of computational requirements of motif discovery and homology detection methods. Times are averages for the 5 families. Notable exceptions are reported in the text. second set of profile HMMs is built directly from the unaligned domain sequences using HMMER, version 1.8. Model calibration and database searching are carried out for both types of models using HMMER 2.0. For each program, the default parameter settings are used. Each homology detection method produces as output a ranked list of putative homologs. These lists are summarized using receiver operating characteristic (ROC) analysis (Gribskov & Robinson 1996). The ROC score is the area under a curve that plots true positives versus false positives for varying score thresholds. ROC analysis combines measures of a search's sensitivity and selectivity. The ROC100 score is the area under the ROC curve, up to the first 100 false positives. ROC100 scores are normalized to range from 0 to 1, with 1 corresponding to the most sensitive and selective search. In total, nine homology detection methods were investigated, as summarized in Table 2. None of the parameter settings was varied prior to reporting these results. Hence, it is likely that alternate values would provide better homology detection performance. In practice, however, the correct set of homologs is not known a priori, and parameter optimization is therefore not possible. Results and discussion The results of our experiments are summarized in Table 3. Between the two motif discovery algorithms, MEME and SPLASH, neither consistently provides superior homology detection performance, but SPLASH appears to be on average significantly faster than MEME (the exception is analysis for the set of fulllength CUB-containing protein sequences). Also, in combination with MAST, it outperforms MEME in 4 out of the 5 tests. Among the five database search techniques, MAST, SPLASHSearch and HMMER all provide excellent performance, although the MAST and SPLASHSearch search programs are significantly faster than their HMMER and Meta-MEME counterparts. Motif discovery and search techniques In combination with MAST, SPLASH is a winner over MEME in 4 out of 5 categories, in two of these by more than 5%. When these algorithms are used in combina- Method CUB c1q gal IG krin MAST s S s S Meta-MEME (un) M S M s M Meta-MEME (tr) S M S S Table 5: No motif discovery algorithm provides significantly superior homology detection performance. The table lists, for each of three database search methods, the motif detection program that provides better relative performance (S=SPLASH, M=MEME). A lowercase letter is used when ROC100 values are better by more than 0.01 but less than When two searches produced ROC100 values within 0.01 of one another, no winner is declared. tion with Meta-MEME, results are more mixed and no clear trend emerges. In general, however, the combination of MAST and SPLASH seems to offer the best overall accuracy coupled with the best overall computational speed. Table 5 compares the homology detection performance provided by motifs discovered by these two programs. Analysis of the domain models produced by SPLASH and MEME indicate that SPLASH tends to generate motif models that more completely cover the training sequences than do the models produced by MEME. Surprisingly, estimation of non-uniform transition probabilities between PSSMs by either SPLASH or MEME does not provide any apparent advantage in selectivity of search (Tables 3, 5), even though this technique is more expensive computationally (data not shown). A plausible explanation for this result is that the training set has to be significantly larger to provide for reliable estimates of these parameters, and the currently obtained estimates include large sampling errors. It appears that with the current size of training datasets uniform transition probabilities appear to be the better choice in PSSM-based searches. Furthermore, comparison of the overall performance of search methods (Tables 3) with their computation cost (Tables 4) indicates that MAST and SPLASHSearch provide a near-optimum tradeoff between database search quality (sensitivity and selectivity) and search computational complexity. In Table 6 we report the results of motif discovery, using SPLASH and MEME, for the C2 domain of the Immunoglobulin family. The most notable difference between the two motif discovery methods is that motifs reported by MEME seem to be shorter and have higher rates of occurence than those reported by SPLASH. The occurence rates reported by SPLASH are somewhat misleading because SPLASH reports regular expression matches rather than the expected number of occurrences of the corresponding PSSM model. Once generated from the corresponding regular expression, SPLASH motifs usually have a significantly higher incidence in the dataset. For instance, the first regular
6 Submitted for publication 6 Model building Search tool Scores 1 MEME MAST E-value 2 MEME + Meta-MEME (untrained) mhmms Viterbi log-odds 3 MEME + Meta-MEME (trained) mhmms Viterbi log-odds 4 SPLASH MAST E-value 5 SPLASH + Meta-MEME (untrained) mhmms Viterbi log-odds 6 SPLASH + Meta-MEME (trained) mhmms Viterbi log-odds 7 SPLASH SPLASHSearch see text 8 HMMER from unaligned sequences hmmsearch Viterbi log-odds 9 HMMER from CLUSTALW alignment hmmsearch Viterbi log-odds Table 2: Summary of homology detection methods. See the text for a detailed description of each method. Method CUB c1q galaptin IG kringle MEME + MAST MEME + Meta-MEME (untrained) MEME + Meta-MEME (trained) SPLASH + MAST SPLASH + Meta-MEME (untrained) SPLASH + Meta-MEME (trained) SPLASH + SPLASHSearch HMMER from unaligned sequences HMMER from CLUSTALW alignment Table 3: Comparison of homology detection methods. Values reported are ROC100 scores. SPLASH Id Seq. Support ZScore Width Motif e [ILMFV]...G.Y.C e+30 8 G..[ILMFV].[ILMFV].C e+25 9 G.P.P.[ILMFV].[FWY] e [DQEK]...L..Q...[ANST]...S.[ILV][NST]...[DEK][NDS][RQK][REK]...[DQK][RQK] e [RQEHK][QEK]..[ILV].[LWY]T..Q[AS]...[LY]...[NT][LF]T e [ILMV].[HFWY][ILMFV]..NG.[RQK]...[ND]...[IV][LW]...[NS] e [DQEK]...[RQEHK][QEK][QEK]V.L[ILMV][MV][LMF] e L[RQEHK]...F...[REHK][IL].G.[RHK]...[AS].[IFV] e [LFWY]..DG..[ILMV]..[NDHS][NDHS][REK]..[ILMF] e [AST].P.[ANST]..[ILMFV][QK][LFWY]S..[ANS] MEME Id Seq. Support ZScore Width Motif D[AS]GTYTC GETVTL.C G.P.P.[IV]TW [IV].WYK[ND]GK.L LTCEV[LWM]GPTSP PPAQYSWLING PPA.Y.WYING PPANFT[WI]QKNG LSYRWLLNE KQAKAVWVLNP Table 6: Motifs Identified by SPLASH and MEME in Immunoglobulin C2 domain.
7 Submitted for publication 7 Method ROC100 MEME + MAST MEME + Meta-MEME (ut) MEME + Meta-MEME (tr) SPLASH + Meta-MEME (ut) SPLASH + Meta-MEME (tr) Table 7: Comparison of domain discovery and database search methods trained from complete sequences of proteins containing at least one CUB domain. Values reported are ROC100 scores. The table does not contain results for HMMER package because it was not intended for discovering motifs in full-length sequences. expression reported by SPLASH ocurs 228 times in the training set. However, it largely overlaps with the motif found by MEME and, once converted into a PSSM, has a comparable incidence. The same can be said for motifs 2 and 3. These motifs also correspond to equivalent regions on the sequence to the corresponding motif identified by MEME. However, from 4 to 10, the motifs found by SPLASH are significantly longer and have better support than comparable MEME motifs. For instance MEME motifs 6, 7, and 8 are virtually identical, and the last two motifs have an incidence lower than 4. This may in part explain the better performance on the C2 domain of the MAST/SPLASH combination versus the MAST/MEME combination. In other words, while the programs are somewhat equivalent at finding highly conserved regions in the family, SPLASH has an advantage when it comes to discover motifs conserved in a small subset of the sequences. Also, overall SPLASH motifs are longer on average and may therefore have a slightly better discriminative power. Modeling whole sequences Discovering protein domains in full-length sequences is a difficult problem that apparently still remains open. To illustrate the difficulties associated with training domain models from full-length proteins, we present ROC100 scores obtained from sequences containing CUB domain (Tables 7). To explain the comparison results, let us examine possible formulations of the problem of motif discovery from full-length sequences. One can formulate the goal of such analysis as identification of all protein domains encountered at least once in the training set. In this case, the set of true positive database matches would include all sequences having at least one of the domains encountered in the training set. This formulation would inevitably lead to attempts to build a model of a protein domain that is represented in the training set by asingle sequence and therefore appears flawed. Alternatively, one can formulate the problem as identifying domains that are common for all sequences in the training set, which is the case with the CUB domains in our dataset, and the set of true positive database matches would include only all CUB-containing proteins. We have chosen the latter formulation of the problem. However, both motif discovery methods, MEME and SPLASH, apparently identify a number of motifs situated outside of the CUB domain. It is not surprising then that database searches with the resulting models identify a large number of false positive" matches corresponding to training set domains other than CUB. These are indeed false positive with respect to the CUB family but they are correct matches to other domain signatures also present in members of the CUB family. This tendency to pick up large numbers of false positive database matches appears to be stronger for the SPLASH algorithm than MEME because SPLASH on average tends to give fuller coverage of the training sequences with the discovered motifs. In conclusion we note that, currently, the largest obstacles to a rigorous comparison of domain discovery methods are the incompleteness of sequence annotations in the databases and the presence of circular logic in defining a set of true-positive database matches based on sequence annotations derived from earlier database searches. References Altschul, S. F.; Gish, W.; Miller, W.; Myers, E. W.; and Lipman, D. J A basic local alignment search tool. Journal of Molecular Biology 215: Bailey, T. L., and Elkan, C. P Fitting a mixture model by expectation-maximization to discover motifs in biopolymers. In Altman, R.; Brutlag, D.; Karp, P.; Lathrop, R.; and Searls, D., eds., Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology, AAAI Press. Bailey, T. L., and Gribskov, M The megaprior heuristic for discovering protein sequence patterns. In States, D. J.; Agarwal, P.; Gaasterland, T.; Hunter, L.; and Smith, R., eds., Proceedings of the Fourth International Conference on Intelligent Systems for Molecular Biology, AAAI Press. Bailey, T. L., and Gribskov, M Combining evidence using p-values: Application to sequence homology searches. Bioinformatics 14(1): Bairoch, A PROSITE: A dictionary of sites and patterns in proteins. Nucleic Acids Research 19: Bairoch, A The SWISS-PROT protein sequence data bank: Current status. Nucleic Acids Research 22(17): Barsalou, T., and Brutlag, D Searching gene and protein sequence databases. M. D. Computing 8(3): Brown, M.; Hughey, R.; Krogh, A.; Mian, I.; Sjolander, K.; and Haussler, D Using Dirichlet mixture priors to derive hidden Markov models for protein families. In Rawlings, C., ed., Proceedings of the
8 Submitted for publication 8 Third International Conference on Intelligent Systems for Molecular Biology, AAAI Press. Califano, A SPLASH: Structural pattern localization analysis by sequential histograms. Bioinformatics. Eddy, S. R Multiple alignment using hidden Markov models. In Rawlings, C., ed., Proceedings of the Third International Conference on Intelligent Systems for Molecular Biology, AAAI Press. Gribskov, M., and Robinson, N. L Use of receiver operating characteristic (ROC) analysis to evaluate sequence matching. Computers and Chemistry 20(1): Grundy, W. N A Bayesian Approach to Motifbased Protein Modeling. Ph.D. Dissertation, University of California, San Diego, La Jolla, CA. Hart, R.; Royyuru, A.; Stolovitsky, G.; and Califano, A Systematic and automated discovery of patterns in PROSITE families. In Proceedings of the Fourth Annual International Conference on Computational Molecular Biology. Henikoff, S., and Henikoff, J. G Amino acid substitution matrices from protein blocks. Proceedings of the National Academy of Sciences of the United States of America 89: Hughey, R., and Krogh, A Hidden Markov models for sequence analysis: Extension and analysis of the basic method. Computer Applications in the Biosciences 12(2): Jonassen, I.; Collins, J. F.; and Higgins, D. G Finding flexible patterns in unaligned protein sequences. Protein Science 4: Needleman, S., and Wunsch, C A general method applicable to the search for similarities in the amino acid sequences of two proteins. Journal of Molecular Biology 48: Neuwald, A. F.; Liu, J. S.; and Lawrence, C. E Gibbs motif sampling: Detection of bacterial outer membrane protein repeats. Protein Science 4: Nevill-Manning, C. G.; Wu, T. D.; and Brutlag, D. L Highly specific protein sequence motifs for genome analysis. Proceedings of the National Academy of Sciences of the United States of America 95(11): Pearson, W. R Rapid and sensitive sequence comparisions with FASTP and FASTA. Methods in Enzymology 183: Rigoutsos, I., and Floratos, A Combinatorial pattern discovery in biological sequences: the TEIRE- SIAS algorithm. Bioinformatics 14(1): Saitou, N., and Nei, M The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 4: Schwartz, R. M., and Dayhoff, M. O Atlas of Protein Sequence and Structure. Silver Spring, MD: National Biomedical Research Foundation. chapter Matrices for detecing distant relationships, Smith, T., and Waterman, M Identification of common molecular subsequences. Journal of Molecular Biology 147: Thompson, J. D.; Higgins, D. G.; and Gibson, T. J. 1994a. CLUSTAL W: Improving the sensitivity of progressive multiple alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22(22): Thompson, J. D.; Higgins, D. G.; and Gibson, T. J. 1994b. CLUSTAL W: Improving the sensitivity of progressivemultiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Research 22: Wu, T. D.; Nevill-Manning, C. G.; and Brutlag, D. L Minimum-risk profiles of protein families based on statistical decision theory. Journal of Computational Biology 6(2):
Sequence homology search tools on the world wide web
 44 Sequence Homology Search Tools Sequence homology search tools on the world wide web Ian Holmes Berkeley Drosophila Genome Project, Berkeley, CA email: ihh@fruitfly.org Introduction Sequence homology
44 Sequence Homology Search Tools Sequence homology search tools on the world wide web Ian Holmes Berkeley Drosophila Genome Project, Berkeley, CA email: ihh@fruitfly.org Introduction Sequence homology
Pairwise Sequence Alignment
 Pairwise Sequence Alignment carolin.kosiol@vetmeduni.ac.at SS 2013 Outline Pairwise sequence alignment global - Needleman Wunsch Gotoh algorithm local - Smith Waterman algorithm BLAST - heuristics What
Pairwise Sequence Alignment carolin.kosiol@vetmeduni.ac.at SS 2013 Outline Pairwise sequence alignment global - Needleman Wunsch Gotoh algorithm local - Smith Waterman algorithm BLAST - heuristics What
Network Protocol Analysis using Bioinformatics Algorithms
 Network Protocol Analysis using Bioinformatics Algorithms Marshall A. Beddoe Marshall_Beddoe@McAfee.com ABSTRACT Network protocol analysis is currently performed by hand using only intuition and a protocol
Network Protocol Analysis using Bioinformatics Algorithms Marshall A. Beddoe Marshall_Beddoe@McAfee.com ABSTRACT Network protocol analysis is currently performed by hand using only intuition and a protocol
PROC. CAIRO INTERNATIONAL BIOMEDICAL ENGINEERING CONFERENCE 2006 1. E-mail: msm_eng@k-space.org
 BIOINFTool: Bioinformatics and sequence data analysis in molecular biology using Matlab Mai S. Mabrouk 1, Marwa Hamdy 2, Marwa Mamdouh 2, Marwa Aboelfotoh 2,Yasser M. Kadah 2 1 Biomedical Engineering Department,
BIOINFTool: Bioinformatics and sequence data analysis in molecular biology using Matlab Mai S. Mabrouk 1, Marwa Hamdy 2, Marwa Mamdouh 2, Marwa Aboelfotoh 2,Yasser M. Kadah 2 1 Biomedical Engineering Department,
SGI. High Throughput Computing (HTC) Wrapper Program for Bioinformatics on SGI ICE and SGI UV Systems. January, 2012. Abstract. Haruna Cofer*, PhD
 White Paper SGI High Throughput Computing (HTC) Wrapper Program for Bioinformatics on SGI ICE and SGI UV Systems Haruna Cofer*, PhD January, 2012 Abstract The SGI High Throughput Computing (HTC) Wrapper
White Paper SGI High Throughput Computing (HTC) Wrapper Program for Bioinformatics on SGI ICE and SGI UV Systems Haruna Cofer*, PhD January, 2012 Abstract The SGI High Throughput Computing (HTC) Wrapper
BLAST. Anders Gorm Pedersen & Rasmus Wernersson
 BLAST Anders Gorm Pedersen & Rasmus Wernersson Database searching Using pairwise alignments to search databases for similar sequences Query sequence Database Database searching Most common use of pairwise
BLAST Anders Gorm Pedersen & Rasmus Wernersson Database searching Using pairwise alignments to search databases for similar sequences Query sequence Database Database searching Most common use of pairwise
Similarity Searches on Sequence Databases: BLAST, FASTA. Lorenza Bordoli Swiss Institute of Bioinformatics EMBnet Course, Basel, October 2003
 Similarity Searches on Sequence Databases: BLAST, FASTA Lorenza Bordoli Swiss Institute of Bioinformatics EMBnet Course, Basel, October 2003 Outline Importance of Similarity Heuristic Sequence Alignment:
Similarity Searches on Sequence Databases: BLAST, FASTA Lorenza Bordoli Swiss Institute of Bioinformatics EMBnet Course, Basel, October 2003 Outline Importance of Similarity Heuristic Sequence Alignment:
Bioinformatics Grid - Enabled Tools For Biologists.
 Bioinformatics Grid - Enabled Tools For Biologists. What is Grid-Enabled Tools (GET)? As number of data from the genomics and proteomics experiment increases. Problems arise for the current sequence analysis
Bioinformatics Grid - Enabled Tools For Biologists. What is Grid-Enabled Tools (GET)? As number of data from the genomics and proteomics experiment increases. Problems arise for the current sequence analysis
T cell Epitope Prediction
 Institute for Immunology and Informatics T cell Epitope Prediction EpiMatrix Eric Gustafson January 6, 2011 Overview Gathering raw data Popular sources Data Management Conservation Analysis Multiple Alignments
Institute for Immunology and Informatics T cell Epitope Prediction EpiMatrix Eric Gustafson January 6, 2011 Overview Gathering raw data Popular sources Data Management Conservation Analysis Multiple Alignments
UCHIME in practice Single-region sequencing Reference database mode
 UCHIME in practice Single-region sequencing UCHIME is designed for experiments that perform community sequencing of a single region such as the 16S rrna gene or fungal ITS region. While UCHIME may prove
UCHIME in practice Single-region sequencing UCHIME is designed for experiments that perform community sequencing of a single region such as the 16S rrna gene or fungal ITS region. While UCHIME may prove
Rapid alignment methods: FASTA and BLAST. p The biological problem p Search strategies p FASTA p BLAST
 Rapid alignment methods: FASTA and BLAST p The biological problem p Search strategies p FASTA p BLAST 257 BLAST: Basic Local Alignment Search Tool p BLAST (Altschul et al., 1990) and its variants are some
Rapid alignment methods: FASTA and BLAST p The biological problem p Search strategies p FASTA p BLAST 257 BLAST: Basic Local Alignment Search Tool p BLAST (Altschul et al., 1990) and its variants are some
Bio-Informatics Lectures. A Short Introduction
 Bio-Informatics Lectures A Short Introduction The History of Bioinformatics Sanger Sequencing PCR in presence of fluorescent, chain-terminating dideoxynucleotides Massively Parallel Sequencing Massively
Bio-Informatics Lectures A Short Introduction The History of Bioinformatics Sanger Sequencing PCR in presence of fluorescent, chain-terminating dideoxynucleotides Massively Parallel Sequencing Massively
Protein Protein Interaction Networks
 Functional Pattern Mining from Genome Scale Protein Protein Interaction Networks Young-Rae Cho, Ph.D. Assistant Professor Department of Computer Science Baylor University it My Definition of Bioinformatics
Functional Pattern Mining from Genome Scale Protein Protein Interaction Networks Young-Rae Cho, Ph.D. Assistant Professor Department of Computer Science Baylor University it My Definition of Bioinformatics
BIO 3350: ELEMENTS OF BIOINFORMATICS PARTIALLY ONLINE SYLLABUS
 BIO 3350: ELEMENTS OF BIOINFORMATICS PARTIALLY ONLINE SYLLABUS NEW YORK CITY COLLEGE OF TECHNOLOGY The City University Of New York School of Arts and Sciences Biological Sciences Department Course title:
BIO 3350: ELEMENTS OF BIOINFORMATICS PARTIALLY ONLINE SYLLABUS NEW YORK CITY COLLEGE OF TECHNOLOGY The City University Of New York School of Arts and Sciences Biological Sciences Department Course title:
Frequently Asked Questions Next Generation Sequencing
 Frequently Asked Questions Next Generation Sequencing Import These Frequently Asked Questions for Next Generation Sequencing are some of the more common questions our customers ask. Questions are divided
Frequently Asked Questions Next Generation Sequencing Import These Frequently Asked Questions for Next Generation Sequencing are some of the more common questions our customers ask. Questions are divided
Sequence Analysis 15: lecture 5. Substitution matrices Multiple sequence alignment
 Sequence Analysis 15: lecture 5 Substitution matrices Multiple sequence alignment A teacher's dilemma To understand... Multiple sequence alignment Substitution matrices Phylogenetic trees You first need
Sequence Analysis 15: lecture 5 Substitution matrices Multiple sequence alignment A teacher's dilemma To understand... Multiple sequence alignment Substitution matrices Phylogenetic trees You first need
Phylogenetic Trees Made Easy
 Phylogenetic Trees Made Easy A How-To Manual Fourth Edition Barry G. Hall University of Rochester, Emeritus and Bellingham Research Institute Sinauer Associates, Inc. Publishers Sunderland, Massachusetts
Phylogenetic Trees Made Easy A How-To Manual Fourth Edition Barry G. Hall University of Rochester, Emeritus and Bellingham Research Institute Sinauer Associates, Inc. Publishers Sunderland, Massachusetts
Guide for Bioinformatics Project Module 3
 Structure- Based Evidence and Multiple Sequence Alignment In this module we will revisit some topics we started to look at while performing our BLAST search and looking at the CDD database in the first
Structure- Based Evidence and Multiple Sequence Alignment In this module we will revisit some topics we started to look at while performing our BLAST search and looking at the CDD database in the first
Clone Manager. Getting Started
 Clone Manager for Windows Professional Edition Volume 2 Alignment, Primer Operations Version 9.5 Getting Started Copyright 1994-2015 Scientific & Educational Software. All rights reserved. The software
Clone Manager for Windows Professional Edition Volume 2 Alignment, Primer Operations Version 9.5 Getting Started Copyright 1994-2015 Scientific & Educational Software. All rights reserved. The software
Current Motif Discovery Tools and their Limitations
 Current Motif Discovery Tools and their Limitations Philipp Bucher SIB / CIG Workshop 3 October 2006 Trendy Concepts and Hypotheses Transcription regulatory elements act in a context-dependent manner.
Current Motif Discovery Tools and their Limitations Philipp Bucher SIB / CIG Workshop 3 October 2006 Trendy Concepts and Hypotheses Transcription regulatory elements act in a context-dependent manner.
THREE DIMENSIONAL REPRESENTATION OF AMINO ACID CHARAC- TERISTICS
 THREE DIMENSIONAL REPRESENTATION OF AMINO ACID CHARAC- TERISTICS O.U. Sezerman 1, R. Islamaj 2, E. Alpaydin 2 1 Laborotory of Computational Biology, Sabancı University, Istanbul, Turkey. 2 Computer Engineering
THREE DIMENSIONAL REPRESENTATION OF AMINO ACID CHARAC- TERISTICS O.U. Sezerman 1, R. Islamaj 2, E. Alpaydin 2 1 Laborotory of Computational Biology, Sabancı University, Istanbul, Turkey. 2 Computer Engineering
RETRIEVING SEQUENCE INFORMATION. Nucleotide sequence databases. Database search. Sequence alignment and comparison
 RETRIEVING SEQUENCE INFORMATION Nucleotide sequence databases Database search Sequence alignment and comparison Biological sequence databases Originally just a storage place for sequences. Currently the
RETRIEVING SEQUENCE INFORMATION Nucleotide sequence databases Database search Sequence alignment and comparison Biological sequence databases Originally just a storage place for sequences. Currently the
Cross-references to the corresponding SWISS-PROT entries as well as to matched sequences from the PDB 3D-structure database 2 are also provided.
 Amos Bairoch and Philipp Bucher are group leaders at the Swiss Institute of Bioinformatics (SIB), whose mission is to promote research, development of software tools and databases as well as to provide
Amos Bairoch and Philipp Bucher are group leaders at the Swiss Institute of Bioinformatics (SIB), whose mission is to promote research, development of software tools and databases as well as to provide
Consensus alignment server for reliable comparative modeling with distant templates
 W50 W54 Nucleic Acids Research, 2004, Vol. 32, Web Server issue DOI: 10.1093/nar/gkh456 Consensus alignment server for reliable comparative modeling with distant templates Jahnavi C. Prasad 1, Sandor Vajda
W50 W54 Nucleic Acids Research, 2004, Vol. 32, Web Server issue DOI: 10.1093/nar/gkh456 Consensus alignment server for reliable comparative modeling with distant templates Jahnavi C. Prasad 1, Sandor Vajda
ParaMEME: A Parallel Implementation and a Web Interface for a DNA and Protein Motif Discovery Tool
 From: CABIOS 12:303-310 ParaMEME: A Parallel Implementation and a Web Interface for a DNA and Protein Motif Discovery Tool William N. Grundy email: bgrundy@cs.ucsd.edu Department of Computer Science and
From: CABIOS 12:303-310 ParaMEME: A Parallel Implementation and a Web Interface for a DNA and Protein Motif Discovery Tool William N. Grundy email: bgrundy@cs.ucsd.edu Department of Computer Science and
Final Project Report
 CPSC545 by Introduction to Data Mining Prof. Martin Schultz & Prof. Mark Gerstein Student Name: Yu Kor Hugo Lam Student ID : 904907866 Due Date : May 7, 2007 Introduction Final Project Report Pseudogenes
CPSC545 by Introduction to Data Mining Prof. Martin Schultz & Prof. Mark Gerstein Student Name: Yu Kor Hugo Lam Student ID : 904907866 Due Date : May 7, 2007 Introduction Final Project Report Pseudogenes
Protein & DNA Sequence Analysis. Bobbie-Jo Webb-Robertson May 3, 2004
 Protein & DNA Sequence Analysis Bobbie-Jo Webb-Robertson May 3, 2004 Sequence Analysis Anything connected to identifying higher biological meaning out of raw sequence data. 2 Genomic & Proteomic Data Sequence
Protein & DNA Sequence Analysis Bobbie-Jo Webb-Robertson May 3, 2004 Sequence Analysis Anything connected to identifying higher biological meaning out of raw sequence data. 2 Genomic & Proteomic Data Sequence
Inferring Probabilistic Models of cis-regulatory Modules. BMI/CS 776 www.biostat.wisc.edu/bmi776/ Spring 2015 Colin Dewey cdewey@biostat.wisc.
 Inferring Probabilistic Models of cis-regulatory Modules MI/S 776 www.biostat.wisc.edu/bmi776/ Spring 2015 olin Dewey cdewey@biostat.wisc.edu Goals for Lecture the key concepts to understand are the following
Inferring Probabilistic Models of cis-regulatory Modules MI/S 776 www.biostat.wisc.edu/bmi776/ Spring 2015 olin Dewey cdewey@biostat.wisc.edu Goals for Lecture the key concepts to understand are the following
ID of alternative translational initiation events. Description of gene function Reference of NCBI database access and relative literatures
 Data resource: In this database, 650 alternatively translated variants assigned to a total of 300 genes are contained. These database records of alternative translational initiation have been collected
Data resource: In this database, 650 alternatively translated variants assigned to a total of 300 genes are contained. These database records of alternative translational initiation have been collected
Genome Explorer For Comparative Genome Analysis
 Genome Explorer For Comparative Genome Analysis Jenn Conn 1, Jo L. Dicks 1 and Ian N. Roberts 2 Abstract Genome Explorer brings together the tools required to build and compare phylogenies from both sequence
Genome Explorer For Comparative Genome Analysis Jenn Conn 1, Jo L. Dicks 1 and Ian N. Roberts 2 Abstract Genome Explorer brings together the tools required to build and compare phylogenies from both sequence
MASCOT Search Results Interpretation
 The Mascot protein identification program (Matrix Science, Ltd.) uses statistical methods to assess the validity of a match. MS/MS data is not ideal. That is, there are unassignable peaks (noise) and usually
The Mascot protein identification program (Matrix Science, Ltd.) uses statistical methods to assess the validity of a match. MS/MS data is not ideal. That is, there are unassignable peaks (noise) and usually
FART Neural Network based Probabilistic Motif Discovery in Unaligned Biological Sequences
 FART Neural Network based Probabilistic Motif Discovery in Unaligned Biological Sequences M. Hemalatha, P. Ranjit Jeba Thangaiah and K. Vivekanandan, Member IEEE Abstract Finding Motif in bio-sequences
FART Neural Network based Probabilistic Motif Discovery in Unaligned Biological Sequences M. Hemalatha, P. Ranjit Jeba Thangaiah and K. Vivekanandan, Member IEEE Abstract Finding Motif in bio-sequences
Algorithms in Computational Biology (236522) spring 2007 Lecture #1
 Algorithms in Computational Biology (236522) spring 2007 Lecture #1 Lecturer: Shlomo Moran, Taub 639, tel 4363 Office hours: Tuesday 11:00-12:00/by appointment TA: Ilan Gronau, Taub 700, tel 4894 Office
Algorithms in Computational Biology (236522) spring 2007 Lecture #1 Lecturer: Shlomo Moran, Taub 639, tel 4363 Office hours: Tuesday 11:00-12:00/by appointment TA: Ilan Gronau, Taub 700, tel 4894 Office
Introduction to Bioinformatics 3. DNA editing and contig assembly
 Introduction to Bioinformatics 3. DNA editing and contig assembly Benjamin F. Matthews United States Department of Agriculture Soybean Genomics and Improvement Laboratory Beltsville, MD 20708 matthewb@ba.ars.usda.gov
Introduction to Bioinformatics 3. DNA editing and contig assembly Benjamin F. Matthews United States Department of Agriculture Soybean Genomics and Improvement Laboratory Beltsville, MD 20708 matthewb@ba.ars.usda.gov
BIOINF 525 Winter 2016 Foundations of Bioinformatics and Systems Biology http://tinyurl.com/bioinf525-w16
 Course Director: Dr. Barry Grant (DCM&B, bjgrant@med.umich.edu) Description: This is a three module course covering (1) Foundations of Bioinformatics, (2) Statistics in Bioinformatics, and (3) Systems
Course Director: Dr. Barry Grant (DCM&B, bjgrant@med.umich.edu) Description: This is a three module course covering (1) Foundations of Bioinformatics, (2) Statistics in Bioinformatics, and (3) Systems
Choices, choices, choices... Which sequence database? Which modifications? What mass tolerance?
 Optimization 1 Choices, choices, choices... Which sequence database? Which modifications? What mass tolerance? Where to begin? 2 Sequence Databases Swiss-prot MSDB, NCBI nr dbest Species specific ORFS
Optimization 1 Choices, choices, choices... Which sequence database? Which modifications? What mass tolerance? Where to begin? 2 Sequence Databases Swiss-prot MSDB, NCBI nr dbest Species specific ORFS
Facebook Friend Suggestion Eytan Daniyalzade and Tim Lipus
 Facebook Friend Suggestion Eytan Daniyalzade and Tim Lipus 1. Introduction Facebook is a social networking website with an open platform that enables developers to extract and utilize user information
Facebook Friend Suggestion Eytan Daniyalzade and Tim Lipus 1. Introduction Facebook is a social networking website with an open platform that enables developers to extract and utilize user information
Tutorial for proteome data analysis using the Perseus software platform
 Tutorial for proteome data analysis using the Perseus software platform Laboratory of Mass Spectrometry, LNBio, CNPEM Tutorial version 1.0, January 2014. Note: This tutorial was written based on the information
Tutorial for proteome data analysis using the Perseus software platform Laboratory of Mass Spectrometry, LNBio, CNPEM Tutorial version 1.0, January 2014. Note: This tutorial was written based on the information
Amino Acids and Their Properties
 Amino Acids and Their Properties Recap: ss-rrna and mutations Ribosomal RNA (rrna) evolves very slowly Much slower than proteins ss-rrna is typically used So by aligning ss-rrna of one organism with that
Amino Acids and Their Properties Recap: ss-rrna and mutations Ribosomal RNA (rrna) evolves very slowly Much slower than proteins ss-rrna is typically used So by aligning ss-rrna of one organism with that
Database searching with DNA and protein sequences: An introduction Clare Sansom Date received (in revised form): 12th November 1999
 Dr Clare Sansom works part time at Birkbeck College, London, and part time as a freelance computer consultant and science writer At Birkbeck she coordinates an innovative graduate-level Advanced Certificate
Dr Clare Sansom works part time at Birkbeck College, London, and part time as a freelance computer consultant and science writer At Birkbeck she coordinates an innovative graduate-level Advanced Certificate
Next Generation Sequencing: Technology, Mapping, and Analysis
 Next Generation Sequencing: Technology, Mapping, and Analysis Gary Benson Computer Science, Biology, Bioinformatics Boston University gbenson@bu.edu http://tandem.bu.edu/ The Human Genome Project took
Next Generation Sequencing: Technology, Mapping, and Analysis Gary Benson Computer Science, Biology, Bioinformatics Boston University gbenson@bu.edu http://tandem.bu.edu/ The Human Genome Project took
Core Bioinformatics. Degree Type Year Semester. 4313473 Bioinformàtica/Bioinformatics OB 0 1
 Core Bioinformatics 2014/2015 Code: 42397 ECTS Credits: 12 Degree Type Year Semester 4313473 Bioinformàtica/Bioinformatics OB 0 1 Contact Name: Sònia Casillas Viladerrams Email: Sonia.Casillas@uab.cat
Core Bioinformatics 2014/2015 Code: 42397 ECTS Credits: 12 Degree Type Year Semester 4313473 Bioinformàtica/Bioinformatics OB 0 1 Contact Name: Sònia Casillas Viladerrams Email: Sonia.Casillas@uab.cat
Statistical Machine Translation: IBM Models 1 and 2
 Statistical Machine Translation: IBM Models 1 and 2 Michael Collins 1 Introduction The next few lectures of the course will be focused on machine translation, and in particular on statistical machine translation
Statistical Machine Translation: IBM Models 1 and 2 Michael Collins 1 Introduction The next few lectures of the course will be focused on machine translation, and in particular on statistical machine translation
ProteinPilot Report for ProteinPilot Software
 ProteinPilot Report for ProteinPilot Software Detailed Analysis of Protein Identification / Quantitation Results Automatically Sean L Seymour, Christie Hunter SCIEX, USA Pow erful mass spectrometers like
ProteinPilot Report for ProteinPilot Software Detailed Analysis of Protein Identification / Quantitation Results Automatically Sean L Seymour, Christie Hunter SCIEX, USA Pow erful mass spectrometers like
BBSRC TECHNOLOGY STRATEGY: TECHNOLOGIES NEEDED BY RESEARCH KNOWLEDGE PROVIDERS
 BBSRC TECHNOLOGY STRATEGY: TECHNOLOGIES NEEDED BY RESEARCH KNOWLEDGE PROVIDERS 1. The Technology Strategy sets out six areas where technological developments are required to push the frontiers of knowledge
BBSRC TECHNOLOGY STRATEGY: TECHNOLOGIES NEEDED BY RESEARCH KNOWLEDGE PROVIDERS 1. The Technology Strategy sets out six areas where technological developments are required to push the frontiers of knowledge
CD-HIT User s Guide. Last updated: April 5, 2010. http://cd-hit.org http://bioinformatics.org/cd-hit/
 CD-HIT User s Guide Last updated: April 5, 2010 http://cd-hit.org http://bioinformatics.org/cd-hit/ Program developed by Weizhong Li s lab at UCSD http://weizhong-lab.ucsd.edu liwz@sdsc.edu 1. Introduction
CD-HIT User s Guide Last updated: April 5, 2010 http://cd-hit.org http://bioinformatics.org/cd-hit/ Program developed by Weizhong Li s lab at UCSD http://weizhong-lab.ucsd.edu liwz@sdsc.edu 1. Introduction
Introduction to Bioinformatics AS 250.265 Laboratory Assignment 6
 Introduction to Bioinformatics AS 250.265 Laboratory Assignment 6 In the last lab, you learned how to perform basic multiple sequence alignments. While useful in themselves for determining conserved residues
Introduction to Bioinformatics AS 250.265 Laboratory Assignment 6 In the last lab, you learned how to perform basic multiple sequence alignments. While useful in themselves for determining conserved residues
SPECIAL PERTURBATIONS UNCORRELATED TRACK PROCESSING
 AAS 07-228 SPECIAL PERTURBATIONS UNCORRELATED TRACK PROCESSING INTRODUCTION James G. Miller * Two historical uncorrelated track (UCT) processing approaches have been employed using general perturbations
AAS 07-228 SPECIAL PERTURBATIONS UNCORRELATED TRACK PROCESSING INTRODUCTION James G. Miller * Two historical uncorrelated track (UCT) processing approaches have been employed using general perturbations
Integrating DNA Motif Discovery and Genome-Wide Expression Analysis. Erin M. Conlon
 Integrating DNA Motif Discovery and Genome-Wide Expression Analysis Department of Mathematics and Statistics University of Massachusetts Amherst Statistics in Functional Genomics Workshop Ascona, Switzerland
Integrating DNA Motif Discovery and Genome-Wide Expression Analysis Department of Mathematics and Statistics University of Massachusetts Amherst Statistics in Functional Genomics Workshop Ascona, Switzerland
BIOINFORMATICS TUTORIAL
 Bio 242 BIOINFORMATICS TUTORIAL Bio 242 α Amylase Lab Sequence Sequence Searches: BLAST Sequence Alignment: Clustal Omega 3d Structure & 3d Alignments DO NOT REMOVE FROM LAB. DO NOT WRITE IN THIS DOCUMENT.
Bio 242 BIOINFORMATICS TUTORIAL Bio 242 α Amylase Lab Sequence Sequence Searches: BLAST Sequence Alignment: Clustal Omega 3d Structure & 3d Alignments DO NOT REMOVE FROM LAB. DO NOT WRITE IN THIS DOCUMENT.
When you install Mascot, it includes a copy of the Swiss-Prot protein database. However, it is almost certain that you and your colleagues will want
 1 When you install Mascot, it includes a copy of the Swiss-Prot protein database. However, it is almost certain that you and your colleagues will want to search other databases as well. There are very
1 When you install Mascot, it includes a copy of the Swiss-Prot protein database. However, it is almost certain that you and your colleagues will want to search other databases as well. There are very
DATA MINING TECHNOLOGY. Keywords: data mining, data warehouse, knowledge discovery, OLAP, OLAM.
 DATA MINING TECHNOLOGY Georgiana Marin 1 Abstract In terms of data processing, classical statistical models are restrictive; it requires hypotheses, the knowledge and experience of specialists, equations,
DATA MINING TECHNOLOGY Georgiana Marin 1 Abstract In terms of data processing, classical statistical models are restrictive; it requires hypotheses, the knowledge and experience of specialists, equations,
Lecture/Recitation Topic SMA 5303 L1 Sampling and statistical distributions
 SMA 50: Statistical Learning and Data Mining in Bioinformatics (also listed as 5.077: Statistical Learning and Data Mining ()) Spring Term (Feb May 200) Faculty: Professor Roy Welsch Wed 0 Feb 7:00-8:0
SMA 50: Statistical Learning and Data Mining in Bioinformatics (also listed as 5.077: Statistical Learning and Data Mining ()) Spring Term (Feb May 200) Faculty: Professor Roy Welsch Wed 0 Feb 7:00-8:0
Hidden Markov Models in Bioinformatics. By Máthé Zoltán Kőrösi Zoltán 2006
 Hidden Markov Models in Bioinformatics By Máthé Zoltán Kőrösi Zoltán 2006 Outline Markov Chain HMM (Hidden Markov Model) Hidden Markov Models in Bioinformatics Gene Finding Gene Finding Model Viterbi algorithm
Hidden Markov Models in Bioinformatics By Máthé Zoltán Kőrösi Zoltán 2006 Outline Markov Chain HMM (Hidden Markov Model) Hidden Markov Models in Bioinformatics Gene Finding Gene Finding Model Viterbi algorithm
BASIC STATISTICAL METHODS FOR GENOMIC DATA ANALYSIS
 BASIC STATISTICAL METHODS FOR GENOMIC DATA ANALYSIS SEEMA JAGGI Indian Agricultural Statistics Research Institute Library Avenue, New Delhi-110 012 seema@iasri.res.in Genomics A genome is an organism s
BASIC STATISTICAL METHODS FOR GENOMIC DATA ANALYSIS SEEMA JAGGI Indian Agricultural Statistics Research Institute Library Avenue, New Delhi-110 012 seema@iasri.res.in Genomics A genome is an organism s
FlipFlop: Fast Lasso-based Isoform Prediction as a Flow Problem
 FlipFlop: Fast Lasso-based Isoform Prediction as a Flow Problem Elsa Bernard Laurent Jacob Julien Mairal Jean-Philippe Vert September 24, 2013 Abstract FlipFlop implements a fast method for de novo transcript
FlipFlop: Fast Lasso-based Isoform Prediction as a Flow Problem Elsa Bernard Laurent Jacob Julien Mairal Jean-Philippe Vert September 24, 2013 Abstract FlipFlop implements a fast method for de novo transcript
Extend Table Lens for High-Dimensional Data Visualization and Classification Mining
 Extend Table Lens for High-Dimensional Data Visualization and Classification Mining CPSC 533c, Information Visualization Course Project, Term 2 2003 Fengdong Du fdu@cs.ubc.ca University of British Columbia
Extend Table Lens for High-Dimensional Data Visualization and Classification Mining CPSC 533c, Information Visualization Course Project, Term 2 2003 Fengdong Du fdu@cs.ubc.ca University of British Columbia
Analysis of kiva.com Microlending Service! Hoda Eydgahi Julia Ma Andy Bardagjy December 9, 2010 MAS.622j
 Analysis of kiva.com Microlending Service! Hoda Eydgahi Julia Ma Andy Bardagjy December 9, 2010 MAS.622j What is Kiva? An organization that allows people to lend small amounts of money via the Internet
Analysis of kiva.com Microlending Service! Hoda Eydgahi Julia Ma Andy Bardagjy December 9, 2010 MAS.622j What is Kiva? An organization that allows people to lend small amounts of money via the Internet
Data Integration via Constrained Clustering: An Application to Enzyme Clustering
 Data Integration via Constrained Clustering: An Application to Enzyme Clustering Elisa Boari de Lima Raquel Cardoso de Melo Minardi Wagner Meira Jr. Mohammed Javeed Zaki Abstract When multiple data sources
Data Integration via Constrained Clustering: An Application to Enzyme Clustering Elisa Boari de Lima Raquel Cardoso de Melo Minardi Wagner Meira Jr. Mohammed Javeed Zaki Abstract When multiple data sources
Using Illumina BaseSpace Apps to Analyze RNA Sequencing Data
 Using Illumina BaseSpace Apps to Analyze RNA Sequencing Data The Illumina TopHat Alignment and Cufflinks Assembly and Differential Expression apps make RNA data analysis accessible to any user, regardless
Using Illumina BaseSpace Apps to Analyze RNA Sequencing Data The Illumina TopHat Alignment and Cufflinks Assembly and Differential Expression apps make RNA data analysis accessible to any user, regardless
A Learning Based Method for Super-Resolution of Low Resolution Images
 A Learning Based Method for Super-Resolution of Low Resolution Images Emre Ugur June 1, 2004 emre.ugur@ceng.metu.edu.tr Abstract The main objective of this project is the study of a learning based method
A Learning Based Method for Super-Resolution of Low Resolution Images Emre Ugur June 1, 2004 emre.ugur@ceng.metu.edu.tr Abstract The main objective of this project is the study of a learning based method
Just the Facts: A Basic Introduction to the Science Underlying NCBI Resources
 1 of 8 11/7/2004 11:00 AM National Center for Biotechnology Information About NCBI NCBI at a Glance A Science Primer Human Genome Resources Model Organisms Guide Outreach and Education Databases and Tools
1 of 8 11/7/2004 11:00 AM National Center for Biotechnology Information About NCBI NCBI at a Glance A Science Primer Human Genome Resources Model Organisms Guide Outreach and Education Databases and Tools
Protein Prospector and Ways of Calculating Expectation Values
 Protein Prospector and Ways of Calculating Expectation Values 1/16 Aenoch J. Lynn; Robert J. Chalkley; Peter R. Baker; Mark R. Segal; and Alma L. Burlingame University of California, San Francisco, San
Protein Prospector and Ways of Calculating Expectation Values 1/16 Aenoch J. Lynn; Robert J. Chalkley; Peter R. Baker; Mark R. Segal; and Alma L. Burlingame University of California, San Francisco, San
1.1 Difficulty in Fault Localization in Large-Scale Computing Systems
 Chapter 1 Introduction System failures have been one of the biggest obstacles in operating today s largescale computing systems. Fault localization, i.e., identifying direct or indirect causes of failures,
Chapter 1 Introduction System failures have been one of the biggest obstacles in operating today s largescale computing systems. Fault localization, i.e., identifying direct or indirect causes of failures,
2.3 Identify rrna sequences in DNA
 2.3 Identify rrna sequences in DNA For identifying rrna sequences in DNA we will use rnammer, a program that implements an algorithm designed to find rrna sequences in DNA [5]. The program was made by
2.3 Identify rrna sequences in DNA For identifying rrna sequences in DNA we will use rnammer, a program that implements an algorithm designed to find rrna sequences in DNA [5]. The program was made by
Random Forest Based Imbalanced Data Cleaning and Classification
 Random Forest Based Imbalanced Data Cleaning and Classification Jie Gu Software School of Tsinghua University, China Abstract. The given task of PAKDD 2007 data mining competition is a typical problem
Random Forest Based Imbalanced Data Cleaning and Classification Jie Gu Software School of Tsinghua University, China Abstract. The given task of PAKDD 2007 data mining competition is a typical problem
Globally Optimal Crowdsourcing Quality Management
 Globally Optimal Crowdsourcing Quality Management Akash Das Sarma Stanford University akashds@stanford.edu Aditya G. Parameswaran University of Illinois (UIUC) adityagp@illinois.edu Jennifer Widom Stanford
Globally Optimal Crowdsourcing Quality Management Akash Das Sarma Stanford University akashds@stanford.edu Aditya G. Parameswaran University of Illinois (UIUC) adityagp@illinois.edu Jennifer Widom Stanford
Support Vector Machines with Profile-Based Kernels for Remote Protein Homology Detection
 TECHNICAL REPORT Report No. CSAI2004-01 Date: August 2004 Support Vector Machines with Profile-Based Kernels for Remote Protein Homology Detection Steven Busuttil John Abela Gordon J. Pace University of
TECHNICAL REPORT Report No. CSAI2004-01 Date: August 2004 Support Vector Machines with Profile-Based Kernels for Remote Protein Homology Detection Steven Busuttil John Abela Gordon J. Pace University of
Using Data Mining for Mobile Communication Clustering and Characterization
 Using Data Mining for Mobile Communication Clustering and Characterization A. Bascacov *, C. Cernazanu ** and M. Marcu ** * Lasting Software, Timisoara, Romania ** Politehnica University of Timisoara/Computer
Using Data Mining for Mobile Communication Clustering and Characterization A. Bascacov *, C. Cernazanu ** and M. Marcu ** * Lasting Software, Timisoara, Romania ** Politehnica University of Timisoara/Computer
Guidance for Industry
 Guidance for Industry Interpreting Sameness of Monoclonal Antibody Products Under the Orphan Drug Regulations U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Guidance for Industry Interpreting Sameness of Monoclonal Antibody Products Under the Orphan Drug Regulations U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Trading Strategies and the Cat Tournament Protocol
 M A C H I N E L E A R N I N G P R O J E C T F I N A L R E P O R T F A L L 2 7 C S 6 8 9 CLASSIFICATION OF TRADING STRATEGIES IN ADAPTIVE MARKETS MARK GRUMAN MANJUNATH NARAYANA Abstract In the CAT Tournament,
M A C H I N E L E A R N I N G P R O J E C T F I N A L R E P O R T F A L L 2 7 C S 6 8 9 CLASSIFICATION OF TRADING STRATEGIES IN ADAPTIVE MARKETS MARK GRUMAN MANJUNATH NARAYANA Abstract In the CAT Tournament,
Graph Mining and Social Network Analysis
 Graph Mining and Social Network Analysis Data Mining and Text Mining (UIC 583 @ Politecnico di Milano) References Jiawei Han and Micheline Kamber, "Data Mining: Concepts and Techniques", The Morgan Kaufmann
Graph Mining and Social Network Analysis Data Mining and Text Mining (UIC 583 @ Politecnico di Milano) References Jiawei Han and Micheline Kamber, "Data Mining: Concepts and Techniques", The Morgan Kaufmann
Module 1. Sequence Formats and Retrieval. Charles Steward
 The Open Door Workshop Module 1 Sequence Formats and Retrieval Charles Steward 1 Aims Acquaint you with different file formats and associated annotations. Introduce different nucleotide and protein databases.
The Open Door Workshop Module 1 Sequence Formats and Retrieval Charles Steward 1 Aims Acquaint you with different file formats and associated annotations. Introduce different nucleotide and protein databases.
SPATIAL DATA CLASSIFICATION AND DATA MINING
 , pp.-40-44. Available online at http://www. bioinfo. in/contents. php?id=42 SPATIAL DATA CLASSIFICATION AND DATA MINING RATHI J.B. * AND PATIL A.D. Department of Computer Science & Engineering, Jawaharlal
, pp.-40-44. Available online at http://www. bioinfo. in/contents. php?id=42 SPATIAL DATA CLASSIFICATION AND DATA MINING RATHI J.B. * AND PATIL A.D. Department of Computer Science & Engineering, Jawaharlal
Predict the Popularity of YouTube Videos Using Early View Data
 000 001 002 003 004 005 006 007 008 009 010 011 012 013 014 015 016 017 018 019 020 021 022 023 024 025 026 027 028 029 030 031 032 033 034 035 036 037 038 039 040 041 042 043 044 045 046 047 048 049 050
000 001 002 003 004 005 006 007 008 009 010 011 012 013 014 015 016 017 018 019 020 021 022 023 024 025 026 027 028 029 030 031 032 033 034 035 036 037 038 039 040 041 042 043 044 045 046 047 048 049 050
Healthcare Measurement Analysis Using Data mining Techniques
 www.ijecs.in International Journal Of Engineering And Computer Science ISSN:2319-7242 Volume 03 Issue 07 July, 2014 Page No. 7058-7064 Healthcare Measurement Analysis Using Data mining Techniques 1 Dr.A.Shaik
www.ijecs.in International Journal Of Engineering And Computer Science ISSN:2319-7242 Volume 03 Issue 07 July, 2014 Page No. 7058-7064 Healthcare Measurement Analysis Using Data mining Techniques 1 Dr.A.Shaik
Focusing on results not data comprehensive data analysis for targeted next generation sequencing
 Focusing on results not data comprehensive data analysis for targeted next generation sequencing Daniel Swan, Jolyon Holdstock, Angela Matchan, Richard Stark, John Shovelton, Duarte Mohla and Simon Hughes
Focusing on results not data comprehensive data analysis for targeted next generation sequencing Daniel Swan, Jolyon Holdstock, Angela Matchan, Richard Stark, John Shovelton, Duarte Mohla and Simon Hughes
INDIRECT INFERENCE (prepared for: The New Palgrave Dictionary of Economics, Second Edition)
 INDIRECT INFERENCE (prepared for: The New Palgrave Dictionary of Economics, Second Edition) Abstract Indirect inference is a simulation-based method for estimating the parameters of economic models. Its
INDIRECT INFERENCE (prepared for: The New Palgrave Dictionary of Economics, Second Edition) Abstract Indirect inference is a simulation-based method for estimating the parameters of economic models. Its
Data Mining Analysis of HIV-1 Protease Crystal Structures
 Data Mining Analysis of HIV-1 Protease Crystal Structures Gene M. Ko, A. Srinivas Reddy, Sunil Kumar, and Rajni Garg AP0907 09 Data Mining Analysis of HIV-1 Protease Crystal Structures Gene M. Ko 1, A.
Data Mining Analysis of HIV-1 Protease Crystal Structures Gene M. Ko, A. Srinivas Reddy, Sunil Kumar, and Rajni Garg AP0907 09 Data Mining Analysis of HIV-1 Protease Crystal Structures Gene M. Ko 1, A.
EM Clustering Approach for Multi-Dimensional Analysis of Big Data Set
 EM Clustering Approach for Multi-Dimensional Analysis of Big Data Set Amhmed A. Bhih School of Electrical and Electronic Engineering Princy Johnson School of Electrical and Electronic Engineering Martin
EM Clustering Approach for Multi-Dimensional Analysis of Big Data Set Amhmed A. Bhih School of Electrical and Electronic Engineering Princy Johnson School of Electrical and Electronic Engineering Martin
Hidden Markov models in biological sequence analysis
 Hidden Markov models in biological sequence analysis by E. Birney The vast increase of data in biology has meant that many aspects of computational science have been drawn into the field. Two areas of
Hidden Markov models in biological sequence analysis by E. Birney The vast increase of data in biology has meant that many aspects of computational science have been drawn into the field. Two areas of
Multiple Sequence Alignment and Analysis: Part I An Introduction to the Theory and Application of Multiple Sequence Analysis.
 Steven M. Thompson Manuscript for Multiple Sequence Alignment and Analysis Page 1 3/31/04 Multiple Sequence Alignment and Analysis: Part I An Introduction to the Theory and Application of Multiple Sequence
Steven M. Thompson Manuscript for Multiple Sequence Alignment and Analysis Page 1 3/31/04 Multiple Sequence Alignment and Analysis: Part I An Introduction to the Theory and Application of Multiple Sequence
Comparative genomic hybridization Because arrays are more than just a tool for expression analysis
 Microarray Data Analysis Workshop MedVetNet Workshop, DTU 2008 Comparative genomic hybridization Because arrays are more than just a tool for expression analysis Carsten Friis ( with several slides from
Microarray Data Analysis Workshop MedVetNet Workshop, DTU 2008 Comparative genomic hybridization Because arrays are more than just a tool for expression analysis Carsten Friis ( with several slides from
Supplementary material: A benchmark of multiple sequence alignment programs upon structural RNAs Paul P. Gardner a Andreas Wilm b Stefan Washietl c
 Supplementary material: A benchmark of multiple sequence alignment programs upon structural RNAs Paul P. Gardner a Andreas Wilm b Stefan Washietl c a Department of Evolutionary Biology, University of Copenhagen,
Supplementary material: A benchmark of multiple sequence alignment programs upon structural RNAs Paul P. Gardner a Andreas Wilm b Stefan Washietl c a Department of Evolutionary Biology, University of Copenhagen,
Effect of Using Neural Networks in GA-Based School Timetabling
 Effect of Using Neural Networks in GA-Based School Timetabling JANIS ZUTERS Department of Computer Science University of Latvia Raina bulv. 19, Riga, LV-1050 LATVIA janis.zuters@lu.lv Abstract: - The school
Effect of Using Neural Networks in GA-Based School Timetabling JANIS ZUTERS Department of Computer Science University of Latvia Raina bulv. 19, Riga, LV-1050 LATVIA janis.zuters@lu.lv Abstract: - The school
MORPHEUS. http://biodev.cea.fr/morpheus/ Prediction of Transcription Factors Binding Sites based on Position Weight Matrix.
 MORPHEUS http://biodev.cea.fr/morpheus/ Prediction of Transcription Factors Binding Sites based on Position Weight Matrix. Reference: MORPHEUS, a Webtool for Transcripton Factor Binding Analysis Using
MORPHEUS http://biodev.cea.fr/morpheus/ Prediction of Transcription Factors Binding Sites based on Position Weight Matrix. Reference: MORPHEUS, a Webtool for Transcripton Factor Binding Analysis Using
Software that writes Software Stochastic, Evolutionary, MultiRun Strategy Auto-Generation. TRADING SYSTEM LAB Product Description Version 1.
 Software that writes Software Stochastic, Evolutionary, MultiRun Strategy Auto-Generation TRADING SYSTEM LAB Product Description Version 1.1 08/08/10 Trading System Lab (TSL) will automatically generate
Software that writes Software Stochastic, Evolutionary, MultiRun Strategy Auto-Generation TRADING SYSTEM LAB Product Description Version 1.1 08/08/10 Trading System Lab (TSL) will automatically generate
Web Data Extraction: 1 o Semestre 2007/2008
 Web Data : Given Slides baseados nos slides oficiais do livro Web Data Mining c Bing Liu, Springer, December, 2006. Departamento de Engenharia Informática Instituto Superior Técnico 1 o Semestre 2007/2008
Web Data : Given Slides baseados nos slides oficiais do livro Web Data Mining c Bing Liu, Springer, December, 2006. Departamento de Engenharia Informática Instituto Superior Técnico 1 o Semestre 2007/2008
CENG 734 Advanced Topics in Bioinformatics
 CENG 734 Advanced Topics in Bioinformatics Week 9 Text Mining for Bioinformatics: BioCreative II.5 Fall 2010-2011 Quiz #7 1. Draw the decompressed graph for the following graph summary 2. Describe the
CENG 734 Advanced Topics in Bioinformatics Week 9 Text Mining for Bioinformatics: BioCreative II.5 Fall 2010-2011 Quiz #7 1. Draw the decompressed graph for the following graph summary 2. Describe the
How To Find Local Affinity Patterns In Big Data
 Detection of local affinity patterns in big data Andrea Marinoni, Paolo Gamba Department of Electronics, University of Pavia, Italy Abstract Mining information in Big Data requires to design a new class
Detection of local affinity patterns in big data Andrea Marinoni, Paolo Gamba Department of Electronics, University of Pavia, Italy Abstract Mining information in Big Data requires to design a new class
Activity IT S ALL RELATIVES The Role of DNA Evidence in Forensic Investigations
 Activity IT S ALL RELATIVES The Role of DNA Evidence in Forensic Investigations SCENARIO You have responded, as a result of a call from the police to the Coroner s Office, to the scene of the death of
Activity IT S ALL RELATIVES The Role of DNA Evidence in Forensic Investigations SCENARIO You have responded, as a result of a call from the police to the Coroner s Office, to the scene of the death of
Pipeline Pilot Enterprise Server. Flexible Integration of Disparate Data and Applications. Capture and Deployment of Best Practices
 overview Pipeline Pilot Enterprise Server Pipeline Pilot Enterprise Server (PPES) is a powerful client-server platform that streamlines the integration and analysis of the vast quantities of data flooding
overview Pipeline Pilot Enterprise Server Pipeline Pilot Enterprise Server (PPES) is a powerful client-server platform that streamlines the integration and analysis of the vast quantities of data flooding
Numerical Field Extraction in Handwritten Incoming Mail Documents
 Numerical Field Extraction in Handwritten Incoming Mail Documents Guillaume Koch, Laurent Heutte and Thierry Paquet PSI, FRE CNRS 2645, Université de Rouen, 76821 Mont-Saint-Aignan, France Laurent.Heutte@univ-rouen.fr
Numerical Field Extraction in Handwritten Incoming Mail Documents Guillaume Koch, Laurent Heutte and Thierry Paquet PSI, FRE CNRS 2645, Université de Rouen, 76821 Mont-Saint-Aignan, France Laurent.Heutte@univ-rouen.fr
Extraction and Visualization of Protein-Protein Interactions from PubMed
 Extraction and Visualization of Protein-Protein Interactions from PubMed Ulf Leser Knowledge Management in Bioinformatics Humboldt-Universität Berlin Finding Relevant Knowledge Find information about Much
Extraction and Visualization of Protein-Protein Interactions from PubMed Ulf Leser Knowledge Management in Bioinformatics Humboldt-Universität Berlin Finding Relevant Knowledge Find information about Much
Using MATLAB: Bioinformatics Toolbox for Life Sciences
 Using MATLAB: Bioinformatics Toolbox for Life Sciences MR. SARAWUT WONGPHAYAK BIOINFORMATICS PROGRAM, SCHOOL OF BIORESOURCES AND TECHNOLOGY, AND SCHOOL OF INFORMATION TECHNOLOGY, KING MONGKUT S UNIVERSITY
Using MATLAB: Bioinformatics Toolbox for Life Sciences MR. SARAWUT WONGPHAYAK BIOINFORMATICS PROGRAM, SCHOOL OF BIORESOURCES AND TECHNOLOGY, AND SCHOOL OF INFORMATION TECHNOLOGY, KING MONGKUT S UNIVERSITY
Early Cloud Experiences with the Kepler Scientific Workflow System
 Available online at www.sciencedirect.com Procedia Computer Science 9 (2012 ) 1630 1634 International Conference on Computational Science, ICCS 2012 Early Cloud Experiences with the Kepler Scientific Workflow
Available online at www.sciencedirect.com Procedia Computer Science 9 (2012 ) 1630 1634 International Conference on Computational Science, ICCS 2012 Early Cloud Experiences with the Kepler Scientific Workflow
Protein Analysis and WEKA Data Mining
 GenMiner: a Data Mining Tool for Protein Analysis Gerasimos Hatzidamianos 1, Sotiris Diplaris 1,2, Ioannis Athanasiadis 1,2, Pericles A. Mitkas 1,2 1 Department of Electrical and Computer Engineering Aristotle
GenMiner: a Data Mining Tool for Protein Analysis Gerasimos Hatzidamianos 1, Sotiris Diplaris 1,2, Ioannis Athanasiadis 1,2, Pericles A. Mitkas 1,2 1 Department of Electrical and Computer Engineering Aristotle
INTRUSION PREVENTION AND EXPERT SYSTEMS
 INTRUSION PREVENTION AND EXPERT SYSTEMS By Avi Chesla avic@v-secure.com Introduction Over the past few years, the market has developed new expectations from the security industry, especially from the intrusion
INTRUSION PREVENTION AND EXPERT SYSTEMS By Avi Chesla avic@v-secure.com Introduction Over the past few years, the market has developed new expectations from the security industry, especially from the intrusion
HENIPAVIRUS ANTIBODY ESCAPE SEQUENCING REPORT
 HENIPAVIRUS ANTIBODY ESCAPE SEQUENCING REPORT Kimberly Bishop Lilly 1,2, Truong Luu 1,2, Regina Cer 1,2, and LT Vishwesh Mokashi 1 1 Naval Medical Research Center, NMRC Frederick, 8400 Research Plaza,
HENIPAVIRUS ANTIBODY ESCAPE SEQUENCING REPORT Kimberly Bishop Lilly 1,2, Truong Luu 1,2, Regina Cer 1,2, and LT Vishwesh Mokashi 1 1 Naval Medical Research Center, NMRC Frederick, 8400 Research Plaza,
