Peptide bonds: resonance structure. Properties of proteins: Peptide bonds and side chains. Dihedral angles. Peptide bond. Protein physics, Lecture 5
|
|
|
- Nathan Hampton
- 8 years ago
- Views:
Transcription
1 Protein physics, Lecture 5 Peptide bonds: resonance structure Properties of proteins: Peptide bonds and side chains Proteins are linear polymers However, the peptide binds and side chains restrict conformational possibilities How do the peptide backbone and the amino acid side chains influence protein structure? Planarity of the peptide bonds is due to: Partial double bond character Large dipole moment that inhibits rotation Planar Resonance forms of a typical peptide group. The uncharged, single-bonded form (typically ~60%) is shown on the left, whereas the charged, double-bonded form (typically ~40%) is on the right. Peptide bond Dihedral angles Peptide bonds are stiff Rotation around a bond Planarity of bond means that cis and trans forms are possible Trans form is much more common due to less steric hindrance Cis form is most common in Proline residues Compare with bond angle
2 Dihedral angles In general there are 4 dihedral angles involved in describing protein structure Side chain C C N C Backbone and are flexible And account for freedom of protein structure One amino acid O Carbonyl group Peptide bond Planar = 0 or 180 GN Ramachandran ( ) G.N. Ramachandran (1963) used computer models of small polypeptides to systematically vary and with the objective of finding stable conformations For each conformation, the structure was examined for close contacts between atoms Atoms were treated as hard spheres with dimensions corresponding to their van der Waals radii Therefore, and angles which cause spheres to collide correspond to sterically disallowed conformations of the polypeptide backbone Ramachandran plot Plot of vs. The computed angles which are sterically allowed fall on certain regions of plot Dihedral angles C C N C O Protein conformation can be described in terms of the amino acid sequence and dihedral angles: i, i, i for each amino acid residue Flexibilty of the polypetide chain is restricted by Stiff peptide bond Steric hindrance (ie avoiding overlap of atoms) Ramachandran et al Ramachandran plot for glycine Allowed regions are in the 4 corners of the plot Prohibitive contacts are indicated Note that glycine has no side chain and is therefore the most flexible
3 Ramachandran plot for with C Just the crosshatched regions Allowed regions correspond to angles that yield sheet helix Left hand helix Repeating values of and along the chain result in regular structure Experimental Ramachandran plot, distribution from over 80,000 AA residues from high-resolution protein structures (x-ray crystallography) Prediction using potential energy function About 5% of observed conformations fall in forbidden regions Flexibility of peptide bond needs to be taken into account to improve this Deviations of 5 o bond angle; 0.05Å bond length or 12 o torsion angle () increases the potential energy by about 1/kcal/mol each Ramachandran plot for with C Can improve using potential energy function rather than hard sphere model. Protein structure example The structure of cytochrome C shows many segments of helix and the Ramachandran plot shows a tight grouping of, angles near -50,-50 alpha-helix cytochrome C Ramachandran plot
4 Protein structure example Side chain properties Similarly, repetitive values in the region of = -110 to 140 and = +110 to +135 give beta sheets. The structure of plastocyanin is composed mostly of beta sheets; the Ramachandran plot shows values in the 110, +130 region: How do the side chains influence protein structure? Glycine Side chain is just H Increases side chain flexibility (see Ramachandran plot) Can fit chain into small spaces Frequency restricted too much would render chains to flexible and lose 3D shape Alanine beta-sheet plastocyanin Ramachandran plot Has one methyl group as side chain Smallish nonpolar No real preference for inside or surface of protein Very abundant (due to simplicity and availability?) Side chain properties Side chain properties Branched side chains are stiffer Aromatic residues Val, Ile, Leu Reduce possibilities for chain folding Phe, Trp, Tyr, His Contain one methylene group spacer Without this severe steric hinderance Would make chain too stiff Intrinsically fluorescent particularly Trp Polar side chains Cysteine Cys, Ser, Thr, Asn, Gln, Tyr Can form hydrogen bonds Often on surface of protein Has unique property: only side chain with exposed S atom Can form disulfide bond with another Cys further along the chain Used to maintain a given structure of the protein or to respond to oxidation Large non-polar residues Leu, Ile, Phe, Pro, Trp Predominantly found in the interior of the molecule Disulfide bond
5 Side chain properties Proline Back bone is part of the cyclic group Backbone section has a bend Invokes bends in the protein chain or kinks in helices Histidine Side chain has pk value of 6.0 Can be charged or uncharged in the physiological ph range Two readily available states useful as a catalyst Involved in the active site of most enzymes Side chain properties Negative charged side chains Positive charged side chains Asp, Glu Negative charge at physiological ph (lose an H + ) Usually found on protein surface Lys, Arg Positive charge at physiological ph (gain an H + ) Usually found on protein surface Hydrophobicity Folding process of polypetide chain depends on hydrophobicity (non-polarity) of side chains Formation of hydrophobic core is an essential driving force in protein folding
6
Amino Acids. Amino acids are the building blocks of proteins. All AA s have the same basic structure: Side Chain. Alpha Carbon. Carboxyl. Group.
 Protein Structure Amino Acids Amino acids are the building blocks of proteins. All AA s have the same basic structure: Side Chain Alpha Carbon Amino Group Carboxyl Group Amino Acid Properties There are
Protein Structure Amino Acids Amino acids are the building blocks of proteins. All AA s have the same basic structure: Side Chain Alpha Carbon Amino Group Carboxyl Group Amino Acid Properties There are
Advanced Medicinal & Pharmaceutical Chemistry CHEM 5412 Dept. of Chemistry, TAMUK
 Advanced Medicinal & Pharmaceutical Chemistry CHEM 5412 Dept. of Chemistry, TAMUK Dai Lu, Ph.D. dlu@tamhsc.edu Tel: 361-221-0745 Office: RCOP, Room 307 Drug Discovery and Development Drug Molecules Medicinal
Advanced Medicinal & Pharmaceutical Chemistry CHEM 5412 Dept. of Chemistry, TAMUK Dai Lu, Ph.D. dlu@tamhsc.edu Tel: 361-221-0745 Office: RCOP, Room 307 Drug Discovery and Development Drug Molecules Medicinal
The peptide bond is rigid and planar
 Level Description Bonds Primary Sequence of amino acids in proteins Covalent (peptide bonds) Secondary Structural motifs in proteins: α- helix and β-sheet Hydrogen bonds (between NH and CO groups in backbone)
Level Description Bonds Primary Sequence of amino acids in proteins Covalent (peptide bonds) Secondary Structural motifs in proteins: α- helix and β-sheet Hydrogen bonds (between NH and CO groups in backbone)
IV. -Amino Acids: carboxyl and amino groups bonded to -Carbon. V. Polypeptides and Proteins
 IV. -Amino Acids: carboxyl and amino groups bonded to -Carbon A. Acid/Base properties 1. carboxyl group is proton donor! weak acid 2. amino group is proton acceptor! weak base 3. At physiological ph: H
IV. -Amino Acids: carboxyl and amino groups bonded to -Carbon A. Acid/Base properties 1. carboxyl group is proton donor! weak acid 2. amino group is proton acceptor! weak base 3. At physiological ph: H
Recap. Lecture 2. Protein conformation. Proteins. 8 types of protein function 10/21/10. Proteins.. > 50% dry weight of a cell
 Lecture 2 Protein conformation ecap Proteins.. > 50% dry weight of a cell ell s building blocks and molecular tools. More important than genes A large variety of functions http://www.tcd.ie/biochemistry/courses/jf_lectures.php
Lecture 2 Protein conformation ecap Proteins.. > 50% dry weight of a cell ell s building blocks and molecular tools. More important than genes A large variety of functions http://www.tcd.ie/biochemistry/courses/jf_lectures.php
Pipe Cleaner Proteins. Essential question: How does the structure of proteins relate to their function in the cell?
 Pipe Cleaner Proteins GPS: SB1 Students will analyze the nature of the relationships between structures and functions in living cells. Essential question: How does the structure of proteins relate to their
Pipe Cleaner Proteins GPS: SB1 Students will analyze the nature of the relationships between structures and functions in living cells. Essential question: How does the structure of proteins relate to their
Amino Acids, Peptides, Proteins
 Amino Acids, Peptides, Proteins Functions of proteins: Enzymes Transport and Storage Motion, muscle contraction Hormones Mechanical support Immune protection (Antibodies) Generate and transmit nerve impulses
Amino Acids, Peptides, Proteins Functions of proteins: Enzymes Transport and Storage Motion, muscle contraction Hormones Mechanical support Immune protection (Antibodies) Generate and transmit nerve impulses
Peptide Bonds: Structure
 Peptide Bonds: Structure Peptide primary structure The amino acid sequence, from - to C-terminus, determines the primary structure of a peptide or protein. The amino acids are linked through amide or peptide
Peptide Bonds: Structure Peptide primary structure The amino acid sequence, from - to C-terminus, determines the primary structure of a peptide or protein. The amino acids are linked through amide or peptide
Part A: Amino Acids and Peptides (Is the peptide IAG the same as the peptide GAI?)
 ChemActivity 46 Amino Acids, Polypeptides and Proteins 1 ChemActivity 46 Part A: Amino Acids and Peptides (Is the peptide IAG the same as the peptide GAI?) Model 1: The 20 Amino Acids at Biological p See
ChemActivity 46 Amino Acids, Polypeptides and Proteins 1 ChemActivity 46 Part A: Amino Acids and Peptides (Is the peptide IAG the same as the peptide GAI?) Model 1: The 20 Amino Acids at Biological p See
Protein Physics. A. V. Finkelstein & O. B. Ptitsyn LECTURE 1
 Protein Physics A. V. Finkelstein & O. B. Ptitsyn LECTURE 1 PROTEINS Functions in a Cell MOLECULAR MACHINES BUILDING BLOCKS of a CELL ARMS of a CELL ENZYMES - enzymatic catalysis of biochemical reactions
Protein Physics A. V. Finkelstein & O. B. Ptitsyn LECTURE 1 PROTEINS Functions in a Cell MOLECULAR MACHINES BUILDING BLOCKS of a CELL ARMS of a CELL ENZYMES - enzymatic catalysis of biochemical reactions
Paper: 6 Chemistry 2.130 University I Chemistry: Models Page: 2 of 7. 4. Which of the following weak acids would make the best buffer at ph = 5.0?
 Paper: 6 Chemistry 2.130 University I Chemistry: Models Page: 2 of 7 4. Which of the following weak acids would make the best buffer at ph = 5.0? A) Acetic acid (Ka = 1.74 x 10-5 ) B) H 2 PO - 4 (Ka =
Paper: 6 Chemistry 2.130 University I Chemistry: Models Page: 2 of 7 4. Which of the following weak acids would make the best buffer at ph = 5.0? A) Acetic acid (Ka = 1.74 x 10-5 ) B) H 2 PO - 4 (Ka =
Ionization of amino acids
 Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
A. A peptide with 12 amino acids has the following amino acid composition: 2 Met, 1 Tyr, 1 Trp, 2 Glu, 1 Lys, 1 Arg, 1 Thr, 1 Asn, 1 Ile, 1 Cys
 Questions- Proteins & Enzymes A. A peptide with 12 amino acids has the following amino acid composition: 2 Met, 1 Tyr, 1 Trp, 2 Glu, 1 Lys, 1 Arg, 1 Thr, 1 Asn, 1 Ile, 1 Cys Reaction of the intact peptide
Questions- Proteins & Enzymes A. A peptide with 12 amino acids has the following amino acid composition: 2 Met, 1 Tyr, 1 Trp, 2 Glu, 1 Lys, 1 Arg, 1 Thr, 1 Asn, 1 Ile, 1 Cys Reaction of the intact peptide
Shu-Ping Lin, Ph.D. E-mail: splin@dragon.nchu.edu.tw
 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute te of Biomedical Engineering ing E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ edu tw/pweb/users/splin/ Date: 10.13.2010
Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute te of Biomedical Engineering ing E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ edu tw/pweb/users/splin/ Date: 10.13.2010
Combinatorial Biochemistry and Phage Display
 Combinatorial Biochemistry and Phage Display Prof. Valery A. Petrenko Director - Valery Petrenko Instructors Galina Kouzmitcheva and I-Hsuan Chen Auburn 2006, Spring semester COMBINATORIAL BIOCHEMISTRY
Combinatorial Biochemistry and Phage Display Prof. Valery A. Petrenko Director - Valery Petrenko Instructors Galina Kouzmitcheva and I-Hsuan Chen Auburn 2006, Spring semester COMBINATORIAL BIOCHEMISTRY
The peptide bond Peptides and proteins are linear polymers of amino acids. The amino acids are
 Introduction to Protein Structure Proteins are large heteropolymers usually comprised of 50 2500 monomer units, although larger proteins are observed 7. The monomer units of proteins are amino acids. The
Introduction to Protein Structure Proteins are large heteropolymers usually comprised of 50 2500 monomer units, although larger proteins are observed 7. The monomer units of proteins are amino acids. The
BOC334 (Proteomics) Practical 1. Calculating the charge of proteins
 BC334 (Proteomics) Practical 1 Calculating the charge of proteins Aliphatic amino acids (VAGLIP) N H 2 H Glycine, Gly, G no charge Hydrophobicity = 0.67 MW 57Da pk a CH = 2.35 pk a NH 2 = 9.6 pi=5.97 CH
BC334 (Proteomics) Practical 1 Calculating the charge of proteins Aliphatic amino acids (VAGLIP) N H 2 H Glycine, Gly, G no charge Hydrophobicity = 0.67 MW 57Da pk a CH = 2.35 pk a NH 2 = 9.6 pi=5.97 CH
Role of Hydrogen Bonding on Protein Secondary Structure Introduction
 Role of Hydrogen Bonding on Protein Secondary Structure Introduction The function and chemical properties of proteins are determined by its three-dimensional structure. The final architecture of the protein
Role of Hydrogen Bonding on Protein Secondary Structure Introduction The function and chemical properties of proteins are determined by its three-dimensional structure. The final architecture of the protein
(c) How would your answers to problem (a) change if the molecular weight of the protein was 100,000 Dalton?
 Problem 1. (12 points total, 4 points each) The molecular weight of an unspecified protein, at physiological conditions, is 70,000 Dalton, as determined by sedimentation equilibrium measurements and by
Problem 1. (12 points total, 4 points each) The molecular weight of an unspecified protein, at physiological conditions, is 70,000 Dalton, as determined by sedimentation equilibrium measurements and by
AMINO ACIDS & PEPTIDE BONDS STRUCTURE, CLASSIFICATION & METABOLISM
 AMINO ACIDS & PEPTIDE BONDS STRUCTURE, CLASSIFICATION & METABOLISM OBJECTIVES At the end of this session the student should be able to, recognize the structures of the protein amino acid and state their
AMINO ACIDS & PEPTIDE BONDS STRUCTURE, CLASSIFICATION & METABOLISM OBJECTIVES At the end of this session the student should be able to, recognize the structures of the protein amino acid and state their
Amino Acids and Proteins
 Amino Acids and Proteins Proteins are composed of amino acids. There are 20 amino acids commonly found in proteins. All have: N2 C α R COO Amino acids at neutral p are dipolar ions (zwitterions) because
Amino Acids and Proteins Proteins are composed of amino acids. There are 20 amino acids commonly found in proteins. All have: N2 C α R COO Amino acids at neutral p are dipolar ions (zwitterions) because
Disulfide Bonds at the Hair Salon
 Disulfide Bonds at the Hair Salon Three Alpha Helices Stabilized By Disulfide Bonds! In order for hair to grow 6 inches in one year, 9 1/2 turns of α helix must be produced every second!!! In some proteins,
Disulfide Bonds at the Hair Salon Three Alpha Helices Stabilized By Disulfide Bonds! In order for hair to grow 6 inches in one year, 9 1/2 turns of α helix must be produced every second!!! In some proteins,
The Organic Chemistry of Amino Acids, Peptides, and Proteins
 Essential rganic Chemistry Chapter 16 The rganic Chemistry of Amino Acids, Peptides, and Proteins Amino Acids a-amino carboxylic acids. The building blocks from which proteins are made. H 2 N C 2 H Note:
Essential rganic Chemistry Chapter 16 The rganic Chemistry of Amino Acids, Peptides, and Proteins Amino Acids a-amino carboxylic acids. The building blocks from which proteins are made. H 2 N C 2 H Note:
Peptide Bond Amino acids are linked together by peptide bonds to form polypepetide chain.
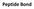 Peptide Bond Peptide Bond Amino acids are linked together by peptide bonds to form polypepetide chain. + H 2 O 2 Peptide bonds are strong and not broken by conditions that denature proteins, such as heating.
Peptide Bond Peptide Bond Amino acids are linked together by peptide bonds to form polypepetide chain. + H 2 O 2 Peptide bonds are strong and not broken by conditions that denature proteins, such as heating.
Conformational Properties of Polypeptide Chains
 Conformational Properties of Polypeptide Chains Levels of Organization Primary structure Amino acid sequence of the protein Secondary structure H bonds in the peptide chain backbone α helix and β sheets
Conformational Properties of Polypeptide Chains Levels of Organization Primary structure Amino acid sequence of the protein Secondary structure H bonds in the peptide chain backbone α helix and β sheets
Structure of proteins
 Structure of proteins Primary structure: is amino acids sequence or the covalent structure (50-2500) amino acids M.Wt. of amino acid=110 Dalton (56 110=5610 Dalton). Single chain or more than one polypeptide
Structure of proteins Primary structure: is amino acids sequence or the covalent structure (50-2500) amino acids M.Wt. of amino acid=110 Dalton (56 110=5610 Dalton). Single chain or more than one polypeptide
H H N - C - C 2 R. Three possible forms (not counting R group) depending on ph
 Amino acids - 0 common amino acids there are others found naturally but much less frequently - Common structure for amino acid - C, -N, and functional groups all attached to the alpha carbon N - C - C
Amino acids - 0 common amino acids there are others found naturally but much less frequently - Common structure for amino acid - C, -N, and functional groups all attached to the alpha carbon N - C - C
In addition to being shorter than a single bond, the double bonds in ethylene don t twist the way single bonds do. In other words, the other atoms
 In addition to being shorter than a single bond, the double bonds in ethylene don t twist the way single bonds do. In other words, the other atoms attached to the carbons (hydrogens in this case) can no
In addition to being shorter than a single bond, the double bonds in ethylene don t twist the way single bonds do. In other words, the other atoms attached to the carbons (hydrogens in this case) can no
This class deals with the fundamental structural features of proteins, which one can understand from the structure of amino acids, and how they are
 This class deals with the fundamental structural features of proteins, which one can understand from the structure of amino acids, and how they are put together. 1 A more detailed view of a single protein
This class deals with the fundamental structural features of proteins, which one can understand from the structure of amino acids, and how they are put together. 1 A more detailed view of a single protein
Introduction to Chemical Biology
 Professor Stuart Conway Introduction to Chemical Biology University of xford Introduction to Chemical Biology ecommended books: Professor Stuart Conway Department of Chemistry, Chemistry esearch Laboratory,
Professor Stuart Conway Introduction to Chemical Biology University of xford Introduction to Chemical Biology ecommended books: Professor Stuart Conway Department of Chemistry, Chemistry esearch Laboratory,
Covalent bonds are the strongest chemical bonds contributing to the protein structure A peptide bond is formed between with of the following?
 MCAT Question Covalent bonds are the strongest chemical bonds contributing to the protein structure A peptide bond is formed between with of the following? A. Carboxylic group and amino group B. Two carboxylic
MCAT Question Covalent bonds are the strongest chemical bonds contributing to the protein structure A peptide bond is formed between with of the following? A. Carboxylic group and amino group B. Two carboxylic
PROTEINS STRUCTURE AND FUNCTION (DR. TRAISH)
 Introduction to Proteins - Proteins are abundant and functionally diverse molecules - They participate in cell regulation at all levels - They share a common structural feature: all are linear polymers
Introduction to Proteins - Proteins are abundant and functionally diverse molecules - They participate in cell regulation at all levels - They share a common structural feature: all are linear polymers
18.2 Protein Structure and Function: An Overview
 18.2 Protein Structure and Function: An Overview Protein: A large biological molecule made of many amino acids linked together through peptide bonds. Alpha-amino acid: Compound with an amino group bonded
18.2 Protein Structure and Function: An Overview Protein: A large biological molecule made of many amino acids linked together through peptide bonds. Alpha-amino acid: Compound with an amino group bonded
Chapter 12 - Proteins
 Roles of Biomolecules Carbohydrates Lipids Proteins 1) Catalytic 2) Transport 3) Regulatory 4) Structural 5) Contractile 6) Protective 7) Storage Nucleic Acids 12.1 -Amino Acids Chapter 12 - Proteins Amino
Roles of Biomolecules Carbohydrates Lipids Proteins 1) Catalytic 2) Transport 3) Regulatory 4) Structural 5) Contractile 6) Protective 7) Storage Nucleic Acids 12.1 -Amino Acids Chapter 12 - Proteins Amino
Built from 20 kinds of amino acids
 Built from 20 kinds of amino acids Each Protein has a three dimensional structure. Majority of proteins are compact. Highly convoluted molecules. Proteins are folded polypeptides. There are four levels
Built from 20 kinds of amino acids Each Protein has a three dimensional structure. Majority of proteins are compact. Highly convoluted molecules. Proteins are folded polypeptides. There are four levels
Acidic amino acids: Those whose side chains can carry a negative charge at certain ph values. Typically aspartic acid, glutamic acid.
 A Acidic amino acids: Those whose side chains can carry a negative charge at certain ph values. Typically aspartic acid, glutamic acid. Active site: Usually applied to catalytic site of an enzyme or where
A Acidic amino acids: Those whose side chains can carry a negative charge at certain ph values. Typically aspartic acid, glutamic acid. Active site: Usually applied to catalytic site of an enzyme or where
PROTEINS THE PEPTIDE BOND. The peptide bond, shown above enclosed in the blue curves, generates the basic structural unit for proteins.
 Ca 2+ The contents of this module were developed under grant award # P116B-001338 from the Fund for the Improvement of Postsecondary Education (FIPSE), United States Department of Education. However, those
Ca 2+ The contents of this module were developed under grant award # P116B-001338 from the Fund for the Improvement of Postsecondary Education (FIPSE), United States Department of Education. However, those
Lecture 4: Peptides and Protein Primary Structure [PDF] Key Concepts. Objectives See also posted Peptide/pH/Ionization practice problems.
![Lecture 4: Peptides and Protein Primary Structure [PDF] Key Concepts. Objectives See also posted Peptide/pH/Ionization practice problems. Lecture 4: Peptides and Protein Primary Structure [PDF] Key Concepts. Objectives See also posted Peptide/pH/Ionization practice problems.](/thumbs/25/5655226.jpg) Lecture 4: Peptides and Protein Primary Structure [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 2, pp. 34-37 Practice problems (peptide ionization) [PDF]; problems in textbook: chapter 2, pp. 63-64,
Lecture 4: Peptides and Protein Primary Structure [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 2, pp. 34-37 Practice problems (peptide ionization) [PDF]; problems in textbook: chapter 2, pp. 63-64,
Proteins the primary biological macromolecules of living organisms
 Proteins the primary biological macromolecules of living organisms Protein structure and folding Primary Secondary Tertiary Quaternary structure of proteins Structure of Proteins Protein molecules adopt
Proteins the primary biological macromolecules of living organisms Protein structure and folding Primary Secondary Tertiary Quaternary structure of proteins Structure of Proteins Protein molecules adopt
Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush
 Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush α-keratins, bundles of α- helices Contain polypeptide chains organized approximately parallel along a single axis: Consist
Nafith Abu Tarboush DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush α-keratins, bundles of α- helices Contain polypeptide chains organized approximately parallel along a single axis: Consist
Amino Acids as Acids, Bases and Buffers:
 Amino Acids as Acids, Bases and Buffers: - Amino acids are weak acids - All have at least 2 titratable protons (shown below as fully protonated species) and therefore have 2 pka s o α-carboxyl (-COOH)
Amino Acids as Acids, Bases and Buffers: - Amino acids are weak acids - All have at least 2 titratable protons (shown below as fully protonated species) and therefore have 2 pka s o α-carboxyl (-COOH)
Structure and properties of proteins. Vladimíra Kvasnicová
 Structure and properties of proteins Vladimíra Kvasnicová Chemical nature of proteins biopolymers of amino acids macromolecules (M r > 10 000) Classification of proteins 1) by localization in an organism
Structure and properties of proteins Vladimíra Kvasnicová Chemical nature of proteins biopolymers of amino acids macromolecules (M r > 10 000) Classification of proteins 1) by localization in an organism
Proteins. Proteins. Amino Acids. Most diverse and most important molecule in. Functions: Functions (cont d)
 Proteins Proteins Most diverse and most important molecule in living i organisms Functions: 1. Structural (keratin in hair, collagen in ligaments) 2. Storage (casein in mother s milk) 3. Transport (HAEMOGLOBIN!)
Proteins Proteins Most diverse and most important molecule in living i organisms Functions: 1. Structural (keratin in hair, collagen in ligaments) 2. Storage (casein in mother s milk) 3. Transport (HAEMOGLOBIN!)
Sidechain Torsional Potentials and Motion of Amino Acids in Proteins:
 Proc. Nat. Acad. Sci. USA Vol. 72, No. 6, pp. 2002-2006, June 1975 Sidechain Torsional Potentials and Motion of Amino Acids in Proteins: Bovine Pancreatic Trypsin Inhibitor (nuclear magnetic resonance
Proc. Nat. Acad. Sci. USA Vol. 72, No. 6, pp. 2002-2006, June 1975 Sidechain Torsional Potentials and Motion of Amino Acids in Proteins: Bovine Pancreatic Trypsin Inhibitor (nuclear magnetic resonance
INTRODUCTION TO PROTEIN STRUCTURE
 Name Class: Partner, if any: INTRODUCTION TO PROTEIN STRUCTURE PRIMARY STRUCTURE: 1. Write the complete structural formula of the tripeptide shown (frame 10). Circle and label the three sidechains which
Name Class: Partner, if any: INTRODUCTION TO PROTEIN STRUCTURE PRIMARY STRUCTURE: 1. Write the complete structural formula of the tripeptide shown (frame 10). Circle and label the three sidechains which
Invariant residue-a residue that is always conserved. It is assumed that these residues are essential to the structure or function of the protein.
 Chapter 6 The amino acid side chains have polar and nonpolar properties, and the relative hydrophobicity of the amino acid side chains is critical for the folding and stability of a protein. The more hydrophobic
Chapter 6 The amino acid side chains have polar and nonpolar properties, and the relative hydrophobicity of the amino acid side chains is critical for the folding and stability of a protein. The more hydrophobic
Peptides & Proteins. (thanks to Hans Börner)
 Peptides & Proteins (thanks to Hans Börner) 1 Proteins & Peptides Proteuos: Proteus (Gr. mythological figure who could change form) proteuo: "first, ref. the basic constituents of all living cells peptos:
Peptides & Proteins (thanks to Hans Börner) 1 Proteins & Peptides Proteuos: Proteus (Gr. mythological figure who could change form) proteuo: "first, ref. the basic constituents of all living cells peptos:
CSC 2427: Algorithms for Molecular Biology Spring 2006. Lecture 16 March 10
 CSC 2427: Algorithms for Molecular Biology Spring 2006 Lecture 16 March 10 Lecturer: Michael Brudno Scribe: Jim Huang 16.1 Overview of proteins Proteins are long chains of amino acids (AA) which are produced
CSC 2427: Algorithms for Molecular Biology Spring 2006 Lecture 16 March 10 Lecturer: Michael Brudno Scribe: Jim Huang 16.1 Overview of proteins Proteins are long chains of amino acids (AA) which are produced
Chapter 26 Biomolecules: Amino Acids, Peptides, and Proteins
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 26 Biomolecules: Amino Acids, Peptides, and Proteins Proteins Amides from Amino Acids Amino acids contain a basic amino group and an acidic carboxyl
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 26 Biomolecules: Amino Acids, Peptides, and Proteins Proteins Amides from Amino Acids Amino acids contain a basic amino group and an acidic carboxyl
--not necessarily a protein! (all proteins are polypeptides, but the converse is not true)
 00Note Set 5b 1 PEPTIDE BONDS AND POLYPEPTIDES OLIGOPEPTIDE: --chain containing only a few amino acids (see tetrapaptide, Fig 5.9) POLYPEPTIDE CHAINS: --many amino acids joined together --not necessarily
00Note Set 5b 1 PEPTIDE BONDS AND POLYPEPTIDES OLIGOPEPTIDE: --chain containing only a few amino acids (see tetrapaptide, Fig 5.9) POLYPEPTIDE CHAINS: --many amino acids joined together --not necessarily
Hydrogen Bonds The electrostatic nature of hydrogen bonds
 Hydrogen Bonds Hydrogen bonds have played an incredibly important role in the history of structural biology. Both the structure of DNA and of protein a-helices and b-sheets were predicted based largely
Hydrogen Bonds Hydrogen bonds have played an incredibly important role in the history of structural biology. Both the structure of DNA and of protein a-helices and b-sheets were predicted based largely
Patrick, An Introduction to Medicinal Chemistry 4e Chapter 13 Drug design: optimizing target interactions. Pyrrole ring N H
 Patrick, An Introduction to dicinal hemistry 4e hapter 13 Drug design: optimizing target interactions Answers to end-of-chapter questions 1) The pyrrole ring of DU 122290 serves to increase the rigidity
Patrick, An Introduction to dicinal hemistry 4e hapter 13 Drug design: optimizing target interactions Answers to end-of-chapter questions 1) The pyrrole ring of DU 122290 serves to increase the rigidity
Carbohydrates, proteins and lipids
 Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
From Sequence to Structure
 1 From Sequence to Structure The genomics revolution is providing gene sequences in exponentially increasing numbers. onverting this sequence information into functional information for the gene products
1 From Sequence to Structure The genomics revolution is providing gene sequences in exponentially increasing numbers. onverting this sequence information into functional information for the gene products
Previously published in Biophysical Society On-line Textbook PROTEINS CHAPTER 1. PROTEIN STRUCTURE. Section 1. Primary structure, secondary motifs,
 Previously published in Biophysical Society On-line Textbook PROTEINS CHAPTER 1. PROTEIN STRUCTURE Section 1. Primary structure, secondary motifs, tertiary architecture, and quaternary organization Jannette
Previously published in Biophysical Society On-line Textbook PROTEINS CHAPTER 1. PROTEIN STRUCTURE Section 1. Primary structure, secondary motifs, tertiary architecture, and quaternary organization Jannette
Myoglobin and Hemoglobin
 Myoglobin and Hemoglobin Myoglobin and hemoglobin are hemeproteins whose physiological importance is principally related to their ability to bind molecular oxygen. Myoglobin (Mb) The oxygen storage protein
Myoglobin and Hemoglobin Myoglobin and hemoglobin are hemeproteins whose physiological importance is principally related to their ability to bind molecular oxygen. Myoglobin (Mb) The oxygen storage protein
Exam 4 Outline CH 105 Spring 2012
 Exam 4 Outline CH 105 Spring 2012 You need to bring a pencil and your ACT card. Chapter 24: Lipids 1. Describe the properties and types of lipids a. All are hydrophobic b. Fatty acid-based typically contain
Exam 4 Outline CH 105 Spring 2012 You need to bring a pencil and your ACT card. Chapter 24: Lipids 1. Describe the properties and types of lipids a. All are hydrophobic b. Fatty acid-based typically contain
Structures of Proteins. Primary structure - amino acid sequence
 Structures of Proteins Primary structure - amino acid sequence Secondary structure chain of covalently linked amino acids folds into regularly repeating structures. Secondary structure is the result of
Structures of Proteins Primary structure - amino acid sequence Secondary structure chain of covalently linked amino acids folds into regularly repeating structures. Secondary structure is the result of
Preliminary MFM Quiz
 Preliminary MFM Quiz 1. The major carrier of chemical energy in all cells is: A) adenosine monophosphate B) adenosine diphosphate C) adenosine trisphosphate D) guanosine trisphosphate E) carbamoyl phosphate
Preliminary MFM Quiz 1. The major carrier of chemical energy in all cells is: A) adenosine monophosphate B) adenosine diphosphate C) adenosine trisphosphate D) guanosine trisphosphate E) carbamoyl phosphate
http://faculty.sau.edu.sa/h.alshehri
 http://faculty.sau.edu.sa/h.alshehri Definition: Proteins are macromolecules with a backbone formed by polymerization of amino acids. Proteins carry out a number of functions in living organisms: - They
http://faculty.sau.edu.sa/h.alshehri Definition: Proteins are macromolecules with a backbone formed by polymerization of amino acids. Proteins carry out a number of functions in living organisms: - They
AP BIOLOGY 2008 SCORING GUIDELINES
 AP BIOLOGY 2008 SCORING GUIDELINES Question 1 1. The physical structure of a protein often reflects and affects its function. (a) Describe THREE types of chemical bonds/interactions found in proteins.
AP BIOLOGY 2008 SCORING GUIDELINES Question 1 1. The physical structure of a protein often reflects and affects its function. (a) Describe THREE types of chemical bonds/interactions found in proteins.
THE CHEMICAL SYNTHESIS OF PEPTIDES
 TE EMIAL SYTESIS F PEPTIDES Peptides are the long molecular chains that make up proteins. Synthetic peptides are used either as drugs (as they are biologically active) or in the diagnosis of disease. Peptides
TE EMIAL SYTESIS F PEPTIDES Peptides are the long molecular chains that make up proteins. Synthetic peptides are used either as drugs (as they are biologically active) or in the diagnosis of disease. Peptides
Helices From Readily in Biological Structures
 The α Helix and the β Sheet Are Common Folding Patterns Although the overall conformation each protein is unique, there are only two different folding patterns are present in all proteins, which are α
The α Helix and the β Sheet Are Common Folding Patterns Although the overall conformation each protein is unique, there are only two different folding patterns are present in all proteins, which are α
Refinement of a pdb-structure and Convert
 Refinement of a pdb-structure and Convert A. Search for a pdb with the closest sequence to your protein of interest. B. Choose the most suitable entry (or several entries). C. Convert and resolve errors
Refinement of a pdb-structure and Convert A. Search for a pdb with the closest sequence to your protein of interest. B. Choose the most suitable entry (or several entries). C. Convert and resolve errors
Amino Acids, Proteins, and Enzymes. Primary and Secondary Structure Tertiary and Quaternary Structure Protein Hydrolysis and Denaturation
 Amino Acids, Proteins, and Enzymes Primary and Secondary Structure Tertiary and Quaternary Structure Protein Hydrolysis and Denaturation 1 Primary Structure of Proteins H 3 N The particular sequence of
Amino Acids, Proteins, and Enzymes Primary and Secondary Structure Tertiary and Quaternary Structure Protein Hydrolysis and Denaturation 1 Primary Structure of Proteins H 3 N The particular sequence of
Chemistry 110. Bettelheim, Brown, Campbell & Farrell. Introduction to General, Organic and Biochemistry Chapter 22 Proteins
 hemistry 110 Bettelheim, Brown, ampbell & Farrell Ninth Edition Introduction to General, rganic and Biochemistry hapter 22 Proteins Step-growth polyamide (polypeptide) polymers or oligomers of L-α-aminoacids.
hemistry 110 Bettelheim, Brown, ampbell & Farrell Ninth Edition Introduction to General, rganic and Biochemistry hapter 22 Proteins Step-growth polyamide (polypeptide) polymers or oligomers of L-α-aminoacids.
NO CALCULATORS OR CELL PHONES ALLOWED
 Biol 205 Exam 1 TEST FORM A Spring 2008 NAME Fill out both sides of the Scantron Sheet. On Side 2 be sure to indicate that you have TEST FORM A The answers to Part I should be placed on the SCANTRON SHEET.
Biol 205 Exam 1 TEST FORM A Spring 2008 NAME Fill out both sides of the Scantron Sheet. On Side 2 be sure to indicate that you have TEST FORM A The answers to Part I should be placed on the SCANTRON SHEET.
A reduced model of short range interactions in polypeptide chains
 A reduced model of short range interactions in polypeptide chains Andrzej Kolinski a) Department of Chemistry, University of Warsaw, Pasteura 1, 0-093 Warsaw, Poland (and Department of Molecular Biology,
A reduced model of short range interactions in polypeptide chains Andrzej Kolinski a) Department of Chemistry, University of Warsaw, Pasteura 1, 0-093 Warsaw, Poland (and Department of Molecular Biology,
The Molecules of Cells
 The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
Communicated March 31, 1951 CHR CHR CHR. *H* zz '" *H _ 0.-...H / k C,.. CHR CNR CHR CHR CHR *HN/' N 'H_N/' H_./ - H-(H.
 VOL. 37, 1951 CHEMISTR Y: PA ULING AND COREY 251 THE PLEATED SHEET, A NEW LAYER CONFIGURATION OF POL YPEPTIDE CHAINS BY LINUS PAULING AND ROBERT B. COREY GATES AND CRELLIN LABORATORIES OF CHEMISTRY,* CALIFORNIA
VOL. 37, 1951 CHEMISTR Y: PA ULING AND COREY 251 THE PLEATED SHEET, A NEW LAYER CONFIGURATION OF POL YPEPTIDE CHAINS BY LINUS PAULING AND ROBERT B. COREY GATES AND CRELLIN LABORATORIES OF CHEMISTRY,* CALIFORNIA
A disaccharide is formed when a dehydration reaction joins two monosaccharides. This covalent bond is called a glycosidic linkage.
 CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
Chapter 2: Biochemistry Problems
 hapter 2: Biochemistry Problems Biochemistry Problems If you were a biochemist, you would study chemical substances and vital processes that occur in living organisms. You might study macromolecules such
hapter 2: Biochemistry Problems Biochemistry Problems If you were a biochemist, you would study chemical substances and vital processes that occur in living organisms. You might study macromolecules such
Chapter 3 Molecules of Cells
 Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Outline. Market & Technology Trends. LifeTein Technology Portfolio. LifeTein Services
 1 Outline Market & Technology Trends LifeTein Technology Portfolio LifeTein Services 2 Synthetic Therapeutic Peptides More than 60 synthetic therapeutic peptides under 50 amino acids in size have reached
1 Outline Market & Technology Trends LifeTein Technology Portfolio LifeTein Services 2 Synthetic Therapeutic Peptides More than 60 synthetic therapeutic peptides under 50 amino acids in size have reached
2007 7.013 Problem Set 1 KEY
 2007 7.013 Problem Set 1 KEY Due before 5 PM on FRIDAY, February 16, 2007. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. Where in a eukaryotic cell do you
2007 7.013 Problem Set 1 KEY Due before 5 PM on FRIDAY, February 16, 2007. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. Where in a eukaryotic cell do you
Chemical Bonds and Groups - Part 1
 hemical Bonds and Groups - Part 1 ARB SKELETS arbon has a unique role in the cell because of its ability to form strong covalent bonds with other carbon atoms. Thus carbon atoms can join to form chains.
hemical Bonds and Groups - Part 1 ARB SKELETS arbon has a unique role in the cell because of its ability to form strong covalent bonds with other carbon atoms. Thus carbon atoms can join to form chains.
Chapter 3: Biological Molecules. 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
How To Understand The Chemistry Of Organic Molecules
 CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
Non-Covalent Bonds (Weak Bond)
 Non-Covalent Bonds (Weak Bond) Weak bonds are those forces of attraction that, in biological situations, do not take a large amount of energy to break. For example, hydrogen bonds are broken by energies
Non-Covalent Bonds (Weak Bond) Weak bonds are those forces of attraction that, in biological situations, do not take a large amount of energy to break. For example, hydrogen bonds are broken by energies
PROTEIN SEQUENCING. First Sequence
 PROTEIN SEQUENCING First Sequence The first protein sequencing was achieved by Frederic Sanger in 1953. He determined the amino acid sequence of bovine insulin Sanger was awarded the Nobel Prize in 1958
PROTEIN SEQUENCING First Sequence The first protein sequencing was achieved by Frederic Sanger in 1953. He determined the amino acid sequence of bovine insulin Sanger was awarded the Nobel Prize in 1958
8/20/2012 H C OH H R. Proteins
 Proteins Rubisco monomer = amino acids 20 different amino acids polymer = polypeptide protein can be one or more polypeptide chains folded & bonded together large & complex 3-D shape hemoglobin Amino acids
Proteins Rubisco monomer = amino acids 20 different amino acids polymer = polypeptide protein can be one or more polypeptide chains folded & bonded together large & complex 3-D shape hemoglobin Amino acids
Biochemistry 462a Hemoglobin Structure and Function Reading - Chapter 7 Practice problems - Chapter 7: 1-6; Proteins extra problems
 Biochemistry 462a Hemoglobin Structure and Function Reading - Chapter 7 Practice problems - Chapter 7: 1-6; Proteins extra problems Myoglobin and Hemoglobin Oxygen is required for oxidative metabolism
Biochemistry 462a Hemoglobin Structure and Function Reading - Chapter 7 Practice problems - Chapter 7: 1-6; Proteins extra problems Myoglobin and Hemoglobin Oxygen is required for oxidative metabolism
Introduction to Protein Folding
 Introduction to Protein Folding Chapter 4 Proteins: Three Dimensional Structure and Function Conformation - three dimensional shape Native conformation - each protein folds into a single stable shape (physiological
Introduction to Protein Folding Chapter 4 Proteins: Three Dimensional Structure and Function Conformation - three dimensional shape Native conformation - each protein folds into a single stable shape (physiological
BASIC CONCEPTS IN BIOCHEMISTRY A STUDENT'S SURVIVAL GUIDE. Second Edition. HIRAM F. GILBERT, Ph.D.
 BASIC CONCEPTS IN BIOCHEMISTRY A STUDENT'S SURVIVAL GUIDE Second Edition HIRAM F. GILBERT, Ph.D. Professor of Biochemistry Baylor College of Medicine Houston, Texas McGraw-Hill Health Professions Division
BASIC CONCEPTS IN BIOCHEMISTRY A STUDENT'S SURVIVAL GUIDE Second Edition HIRAM F. GILBERT, Ph.D. Professor of Biochemistry Baylor College of Medicine Houston, Texas McGraw-Hill Health Professions Division
Papers listed: Cell2. This weeks papers. Chapt 4. Protein structure and function
 Papers listed: Cell2 During the semester I will speak of information from several papers. For many of them you will not be required to read these papers, however, you can do so for the fun of it (and it
Papers listed: Cell2 During the semester I will speak of information from several papers. For many of them you will not be required to read these papers, however, you can do so for the fun of it (and it
Biochemistry - I. Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture-11 Enzyme Mechanisms II
 Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture-11 Enzyme Mechanisms II In the last class we studied the enzyme mechanisms of ribonuclease A
Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture-11 Enzyme Mechanisms II In the last class we studied the enzyme mechanisms of ribonuclease A
I N V E S T I C E D O R O Z V O J E V Z D Ě L Á V Á N Í
 I V E S T I E D Z V J E V Z D Ě L Á V Á Í AMIAIDS PEPTIDES AMIAIDS = substitutional/functional derivatives of carboxylic acids = basic units of proteins (2-aminoacids) General formula of 2-aminoacids (α-aminoacids):
I V E S T I E D Z V J E V Z D Ě L Á V Á Í AMIAIDS PEPTIDES AMIAIDS = substitutional/functional derivatives of carboxylic acids = basic units of proteins (2-aminoacids) General formula of 2-aminoacids (α-aminoacids):
Rapid and Reproducible Amino Acid Analysis of Physiological Fluids for Clinical Research Using LC/MS/MS with the atraq Kit
 Rapid and Reproducible Amino Acid Analysis of Physiological Fluids for Clinical Research Using LC/MS/MS with the atraq Kit Fast, simple and cost effective analysis Many areas of biochemical research and
Rapid and Reproducible Amino Acid Analysis of Physiological Fluids for Clinical Research Using LC/MS/MS with the atraq Kit Fast, simple and cost effective analysis Many areas of biochemical research and
CHAPTER 29 AMINO ACIDS, POLYPEPTIDES, AND PROTEINS SOLUTIONS TO REVIEW QUESTIONS
 APTER 29 AMI AIDS, PLYPEPTIDES, AD PRTEIS SLUTIS T REVIEW QUESTIS 1. The designation, α, means that the amine group in common amino acids is connected to the carbon immediately adjacent to the carboxylic
APTER 29 AMI AIDS, PLYPEPTIDES, AD PRTEIS SLUTIS T REVIEW QUESTIS 1. The designation, α, means that the amine group in common amino acids is connected to the carbon immediately adjacent to the carboxylic
Lecture 19: Proteins, Primary Struture
 CPS260/BGT204.1 Algorithms in Computational Biology November 04, 2003 Lecture 19: Proteins, Primary Struture Lecturer: Pankaj K. Agarwal Scribe: Qiuhua Liu 19.1 The Building Blocks of Protein [1] Proteins
CPS260/BGT204.1 Algorithms in Computational Biology November 04, 2003 Lecture 19: Proteins, Primary Struture Lecturer: Pankaj K. Agarwal Scribe: Qiuhua Liu 19.1 The Building Blocks of Protein [1] Proteins
Lecture 13-14 Conformation of proteins Conformation of a protein three-dimensional structure native state. native condition
 Lecture 13-14 Conformation of proteins Conformation of a protein refers to the three-dimensional structure in its native state. There are many different possible conformations for a molecule as large as
Lecture 13-14 Conformation of proteins Conformation of a protein refers to the three-dimensional structure in its native state. There are many different possible conformations for a molecule as large as
Chapter 05 The Three-Dimensional Structure of Proteins
 Chapter 05 The Three-Dimensional Structure of Proteins The covalent backbone of a typical protein contains hundreds of individual bonds. Because free rotation is possible around many of these bonds, the
Chapter 05 The Three-Dimensional Structure of Proteins The covalent backbone of a typical protein contains hundreds of individual bonds. Because free rotation is possible around many of these bonds, the
Elements in Biological Molecules
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
The chemistry of insulin
 FREDERICK S ANGER The chemistry of insulin Nobel Lecture, December 11, 1958 It is great pleasure and privilege for me to give an account of my work on protein structure and I am deeply sensitive of the
FREDERICK S ANGER The chemistry of insulin Nobel Lecture, December 11, 1958 It is great pleasure and privilege for me to give an account of my work on protein structure and I am deeply sensitive of the
Chapter 5. The Structure and Function of Macromolecule s
 Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Biological Molecules
 Biological Molecules I won t lie. This is probably the most boring topic you have ever done in any science. It s pretty much as simple as this: learn the material deal with it. Enjoy don t say I didn t
Biological Molecules I won t lie. This is probably the most boring topic you have ever done in any science. It s pretty much as simple as this: learn the material deal with it. Enjoy don t say I didn t
Section I Using Jmol as a Computer Visualization Tool
 Section I Using Jmol as a Computer Visualization Tool Jmol is a free open source molecular visualization program used by students, teachers, professors, and scientists to explore protein structures. Section
Section I Using Jmol as a Computer Visualization Tool Jmol is a free open source molecular visualization program used by students, teachers, professors, and scientists to explore protein structures. Section
Secondary Structure Prediction. Michael Tress CNIO
 Secondary Structure Prediction Michael Tress CNIO Why do we Need to Know About Secondary Structure? Secondary structure prediction is a step towards deducing the fold. In order to arrive at the correct
Secondary Structure Prediction Michael Tress CNIO Why do we Need to Know About Secondary Structure? Secondary structure prediction is a step towards deducing the fold. In order to arrive at the correct
4. Which carbohydrate would you find as part of a molecule of RNA? a. Galactose b. Deoxyribose c. Ribose d. Glucose
 1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
