Course syllabus for Chemistry W 109C Organic Chemistry
|
|
|
- Colin Lane
- 6 years ago
- Views:
Transcription
1 Course syllabus for Chemistry W 109C Organic Chemistry Class meets: On-line (with on-line discussions 3 days a week) Spring 2016 Instructor: Dr. Kalju Kahn, Office: PSB-N 2623, kalju@chem.ucsb.edu Phone: Office Hours: Friday 12:40 1:40 PM (on-line tele-conference) Course website: ilti.chem.ucsb.edu Textbooks and Materials: Required: Paula Y. Bruice, Organic Chemistry, 7 th Ed., Pearson 2014 Access to Mastering Chemistry based on Bruice 7 th edition Recommended: (a) Study Guide and Solutions Manual, (b) Molecular Model Kit Textbook website: Mastering Chemistry: masteringchemistry.com/ The Course: CHEM 109C is the last course of a three-course sequence (CHEM 109A-B-C). The CHEM 109 sequence provides the students fundamentals of organic chemistry and is mainly intended for students in the field of chemistry and biology. The current course, CHEM W 109C, focuses on: 1) Structure and reactions of organic compounds found commonly in living organisms: carbohydrates, amino acids, peptides, heterocyclic molecules, nucleotides, and coenzymes. These topics make up the bulk of the course 2) Principles of chemical and biochemical catalysis with focus on chemistry of coenzymes and enzymes. Organic chemistry of biochemical processes in the living cell will be discussed. Students who have successfully completed CHEM W 109C should be well prepared for subsequent college-level biochemistry courses. Because of a strong overlap between CHEM W 109C material and the new MCAT 2015 requirements, students completing this course are also in a strong position to tackle the Chemical and Physical Foundations of Biological Systems section of the new MCAT.
2 On-line format: This section of CHEM 109C is offered as an on-line course that follows a flipped-classroom instructional model. Students are expected to show a significant initiative by working independently with course materials (interactive course material slideshows, video lectures, online lessons) before meeting with the instructor for an on-line discussion. Each week, three 50- minute on-line discussions are offered during a pre-determined time. Students will earn points toward their grade during discussions. Technology Recommendations: The course utilizes standard technologies present in most modern operating systems and browsers. A meeting to discuss technical requirements will take place about a month before beginning of the course. For the best on-line experience, you want: A modern desktop or high-end laptop computer running a recent version of Windows, MacOS, or Linux. Computers in campus computer labs are well suited for this class. Many course components can be also accessed from tablet computers. A display of least (WXGA) to avoid horizontal scrolling. I strongly recommend a display at least 1920 pixels wide (HD) to allows simultaneous views of the discussion window and the course website. Dual-monitor setup is ideal. Please note that your experience on many smaller netbooks will be sub-optimal. Working speakers, a microphone, and a high-definition (at least ) webcam. An HD camcorder, connected via the FireWire port, may also work as a webcam. GPU-accelerated VP8 and Flash video decoding. For smooth video playback, I recommend that your video card is NVIDIA GeForce 600-series Kepler or newer, or AMD Radeon HD 7900-series Southern Islands or newer, or Intel HD 5300 Broadwell or higher. A modern up-to-date Firefox, Chrome, or Opera browser (for Safari and IE9, you need to install the WebM codec). Please make sure that a recent version of Adobe Flash is installed and enabled. Reliable high-bandwidth internet connection. Computers with wired connection to the campus network, a residential fiber optic network, or to a cable internet modem will be ideal. If you rely on Wi-Fi at home, please consider a dual-band router to relieve bandwidth congestion. While many course activities can be accessed via LTE mobile connections, mobile connections are not suitable for watching high-resolution course videos and participating in course discussions.
3 Expectations of Students and Study Advice 1) Individual and timely study based on digitally provided course materials is critical. In general, you should complete the required work each day before meeting with your instructor. Students who have taken comparable on-line courses in the past have indicated that the daily workload in the on-line class tends to be higher than in the regular lecture course. 2) Attendance in discussions and taking good notes is expected. You are able to earn correctness and participation points by answering questions during the discussion. You can preview the discussion material before the discussion, and you can review the discussion material after the discussion. Supplementing the discussion notes with study notes based on the textbook is a good way to improve your chances to be successful in this course. 3) For many people, writing down a clean set of notes after concluding each chapter is a powerful study strategy. When writing these clean notes, integrate your original notes, textbook material, practice problems, extra material found online, and your own thoughts into a cohesive and interesting story. You are encouraged to use the media-rich course website to write your notes and share these with fellow students, particularly when your independent research has uncovered aspects that are interesting to others. 4) The practice problems in the book and in the MasteringChemistry site are an excellent way to learn the material. Try to answer them as you read the textbook. I strongly recommend that you do at least the ones I have suggested. The answers to the problems at MasteringChemistry will contribute toward your grade. 5) Online lessons and quizzes in the course website have been designed by your instructor. These are graded assignments that you should complete by due date for credit. Finish each lesson preferably before the quiz and absolutely before the pertinent exam day. Work on the quizzes individually and recognize that questions like these are likely to show up on exams. 6) Vitamin problems, written by your instructor, are graded weekend homework assignments that you will work on either individually or together with a small group of students. If you work with a group, you will be posting your group answers on the course website; answer strategies will be discussed in the following week. Students in the class have an option to self-organize into small groups. 7) Online discussion forum is a place for students to collaborate and help each other while learning the material. Discussion openers such as the solvatochromism of carbonyl compounds in today s lecture was so over my head could anyone please explain it in plain language are most welcome. Because teaching others is a powerful way of solidifying your own understanding, some stronger students will play the role of instructor in these discussions. The course instructors will monitor discussions and assign credit based on meaningful participation. We may work together on sample practice problems during discussion and you can earn points by providing correct answers and helpful comments online. At the end of the course, students in the course will vote to nominate five peers who were most helpful with discussions for extra credit.
4 8) Two 50-minute mid-terms will be given. Both mid-term exams are open-book test that you take at a common time online. The exams will be proctored (either physically by course TAs or remotely via your webcam) to ensure that you answer your questions without help from other people. The two mid-terms mainly test your knowledge of topics covered prior to exam. 9) There is a large 3-hour written final exam at the end of the course. The closed-book final exam will cover all the topics that were taught in this course and also test your ability to understand the material. Exam questions will be largely based on the material covered in the recorded lectures, on-line lessons, quizzes, and discussions. You can take the final on campus or at a remote proctoring center. 10) Study your graded mid-term exams and corresponding answer keys carefully as soon as these are available. Go back to your clean notes and analyze if the concept or problem that you did not answer perfectly was well covered in your notes. This way, you may be able to identify areas of weaknesses and find solutions that allow being more successful in the next test. Please join me during my on-line office hours to bounce off your thoughts on best study strategies. 11) There are no make-up exams. If you know in advance that you will have a legitimate reason (UCSB sports team, field trip, surgery, etc.) to miss an exam, please inform the instructor at least one week in advance and provide the appropriate written documentation. In case of medical emergency, provide a verifiable doctors excuse stating that you could not take the exam due to an illness. 12) Honesty and academic integrity must be always preserved. While working with others is encouraged outside the classroom, you must answer the graded quiz questions and exam questions individually. No calculators or other materials are permitted on closed-book final. 13) All course materials and the intellectual content of the course are protected by United States Federal Copyright Law. No student (and all other persons) shall give, sell, or otherwise distribute to others, or publish any electronically available course materials or recordings made during any course presentation without the written consent of the instructor. For example, you are not allowed to copy quiz/exam questions and post them in public or private websites. 14) No students shall share access privileges (such as UCSBNetID and password that authenticates them at the course website) with other individuals, including fellow students. Students should take reasonable precautions against unauthorized access to their electronic devices that are used to access or store course materials. 15) No students shall give, sell, or otherwise distribute names, pictures, addresses, or any other personal data about fellow students taking the course without an explicit permission. While student s access to course resources is closely monitored by the instructor for educational purposes, students in the course should generally expect privacy rights comparable to these outlined for K-12 students in the CA Senate Bill No
5 16) Respectful behavior in online discussion sections is expected of all students. As in a physical classroom, offensive language, actions, or harassment will not be tolerated and may result in a referral to the Office of Judicial Affairs for disciplinary action. 17) The grade is based on the number of points out of 900 points total. Grading will be based on the curve but you have to meet a certain level to get grade higher than F. Students who have earned over 350 points through the online work and midterms but get between 125 and 150 points on the final will be given an opportunity to re-take the final two days later and may receive grade no higher than C+ in the course. 18) Your points will come from the following sources: Online lessons and quizzes at course website Online discussions along w/ discussion questions 100 pts 100 pts Online lessons and quizzes at Mastering Chemistry 20 pts Weekend homework problems Midterm 1: Midterm 2: Final exam: 80 pts 100 pts 100 pts 400 pts 19) I will post course histograms periodically to help you assess where you are standing relative to your peers.
6 Course topics: The selection of topics in CHEM W 109C depends on the background of students in the course. Majority of the students should have learned the benzene chemistry already, and we will start with heterocyclic compounds. Students who have not covered the chemistry of electrophilic aromatic substitutions shall independently cover this topics with the help of resources provided. If the first quiz shows that many students lack a deeper understanding of reactivity principles, a brief review of key organic reactivity principles will be done during the first week. The list of topics (along w/ 7 th ed chapters) planned for CHEM W 109C is below: Section 0 (mainly for independent review): Review of principles of organic reactivity. Discussion of a variety of topics that are critical in understanding the principles of organic reactivity. Hopefully, most of this is a review for majority of students while other topics introduce familiar ideas at deeper level. Electronic structure of organic compounds (e.g. 5.3, 6.2, 6.8, 8.2, 8.5, 8.10) Acids and bases; electrophiles and nucleophiles (e.g 2.1, 2.12, 5.6, 6.8, 16.5 ) Potential energy surfaces for chemical reactions (e.g. 5.7, 5.10) Reaction intermediates and transition states (6.3) Stability and properties of carbocation intermediates (6.2, 6.4, 6.7, 8.13) Kinetically and thermodynamically controlled reactions (8.18) Valence bond and molecular orbital language in describing chemistry ( ) Molecular orbitals and chemical reactivity (not directly in the book) Electrostatic potential maps on electron density (many colorful pictures in the book) Section 1: Heterocyclic Compounds; More about Amines, Ethers, and Thiols The goal is to understand correlations between electronic structure, acid-base properties, and chemical reactivity of nitrogen-, oxygen, and sulfur containing heterocyclic compounds. This chapter gives you plenty of chances to review ideas such as hybridization, resonance, molecular geometry, pronation equilibrium, and tautomerism. In addition, you can exercise your brain cells by learning names and structures of several similar-sounding molecules. Review of acid-base properties of alcohols, amines, thiols, esters, and amides (20.2, 20.3 at al) Nomenclature of heterocyclic compounds ( ) Properties and reactivity of aliphatic N-, O-, and S-containing heterocycles (not in the book) Lactones and lactams (review, see also 16.15) Properties and reactivity of aromatic N-, O-, and S-containing heterocycles (20.5, 20.6) Electrophilic aromatic substitution in pyrrole, furan, and thiophene (20.5) Electrophilic and nucleophilic aromatic substitutions in pyrimidine (20.6) Electronic structure and properties of imidazoles (20.7) Electronic structure and properties of pyrimidines: uracil, thymine, cytosine ( extra) Electronic structure and properties of indole and purines ( extra)
7 Section 2: Carbohydrates. Focus in on structure, stereochemistry, and reactions. Essentially all of Chapter 21 is relevant. If you have modeling kit, practice building open and closed-chain forms of D-glucose, D-mannose, and D-galactose. Chemical nature of carbohydrates (21.0) Classification of carbohydrates: aldoses vs ketoses; trioses, tetroses (21.1) Conformations and configurations, R/S and D/L nomenclature (21.2) Simple trioses: glyceraldehyde and dihydroxyacetone (21.2, 21.4) Simple tetroses: erythrose, threose and erythrulose (21.3, 21.4) Simple pentoses: ribose, arabinose and ribulose (21.3, 21.4) Simple hexoses: glucose, galactose, mannose and fructose (21.3, 21.4) Recognize enantiomeric and diastereomeric pairs (e.g. erythrose and threose) Spectropolarimetry as a method to study carbohydrates in solution (lesson) Epimerization and enediol rearrangements (21.5) Reduction of aldoses and ketoses to alditols, optical activity of alditols (21.6) Oxidation of aldoses to aldonic acids by Br 2 (21.6) Oxidation of aldoses and ketoses to aldonic acids by Tollens reagent (21.6) Carbonyl carbon as electrophilic reaction center (background) Kiliani-Fisher synthesis to extend chain (21.7) Wohl degradation to shorten chain (21.8) Reactions of the hydroxyl group (background) Mechanism of formation of hemiacetals (21.10) Formation of furanoses and pyranoses; ring strain considerations (21.10) Haworth projection of cyclic forms (21.10) Anomeric carbon and anomers: structures of α-d-glucose and β-d-glucose (21.11) Acetals; mechanism of formation of glycosides via an oxocarbenium ion (21.12) Stability of axial and equatorial positions; anomeric effect and solvent effects (21.13) Formation of the glycosidic bond in disaccharides Reducing disaccharides: maltose and cellobiose (21.14, 21.15) Non-reducing disaccharide: Trehalose Lactose; how to determine connectivity of a disaccharide (21.15) Fructofuranose as the cyclic form of fructose; structure of sucrose (21.15) General structure of polysaccharides (21.16) Structure and function of glycogen (21.16) Composition and function of scratch (21.16) Amylose and amylopectin as polymers of α-d-glucose (21.16) Structure of cellulose and the composition of the plant cell wall Structure of chitin and the composition of fungal cell wall Structure of peptidoglycan and composition of the bacterial cell wall Glycoproteins and the determinants of human A/B/O blood types (21.18) Structure and properties of some synthetic sweeteners (21.19)
8 Section 3: Nucleobases, nucleotides, and nucleic acids Biological function of nucleotides and nucleic acids (26.1, 26.2) General structure of nucleosides and nucleotides (26.1) Purine and pyrimidine nucleobases (20.7, 26.1) Tautomerism in nucleobases (not in the book, see 77 for keto-enol tautomers) Purine degradation pathway (not in the book) N-glycosidic bond and its acid-catalyzed hydrolysis (26.1) Biosynthesis of pyrimidine and purine nucleotides (not in the book) Nucleotides in DNA and RNA (26.3) ATP and camp as two important nucleotides (26.2) Polynucleotides: DNA and RNA (26.3) Structure and function of DNA (26.3, 26.4) Structure and function of mrna (26.8) Structure and function of trna (26.8) Structure and function of rrna (26.9) Hydrolysis of RNA backbone (26.4) Chemistry of spontaneous mutagenesis (not in the book) Chemistry of UV-induced mutagenesis: cyclobutadiene thymine dimers (28.6) Biosynthesis of DNA (26.4) Chemical synthesis of DNA via phosphoramidate chemistry (not in the book) Section 4: Amino Acids Biological function of amino acids (Ch. 22) General structure and stereochemistry of amino acids (22.1, 22.2) Classification of 20 common amino acids based on side chain structure (Ch. 22) Structures of 20 common amino acids and of selenocysteine (22.1) Common post-translational modifications of L-amino acids (not in the book) Occurrence of D-amino acids in natural products (22.2) Ionization of the carboxylic acid moiety in carboxylic and amino acids (22.3) Ionization of the amino group in aliphatic amines and amino acids (22.3) Ionization of ionizable amino acid side chain (22.3) The concept of pi; calculation of pi of amino acids (22.4) Migration of amino acids in electric field; electrophoresis (22.5) Isoelectric focusing Separation of amino acids by ion exchange chromatography (22.5) Separation of amino acids by paper chromatography (22.5) Visualization of amino acids with ninhydrin and phenylisothiocyanate (22.5) Synthesis of amino acids using the Hell Volhard Zelinsky reaction (22.6) Synthesis of amino acids using reductive amination (22.6) Synthesis of amino acids using α-bromomalonic esters and phtalimide (22.6) Synthesis of amino acids using the Strecker method (22.6) Kinetic resolution of enantiomers using aminoacylase (22.7)
9 Peptides and proteins Formation and structure of the peptide bond (22.8) Reaction of the thiol group; formation and reduction of the disulfide bond (22.8) Solution-phase synthesis of peptides (22.10) Solid-phase synthesis of peptides by the Merrifield method (22.11) Identification of the N-terminal AA in peptides and proteins with Edman s reagent (22.13) Cleavage of the N-terminal AA with aminopeptidase (22.13) Cleavage of the C-terminal AA with carboxypeptidase A or carboxypeptidase B (22.13) Cleavage of peptides and proteins by trypsin, chymotrypsin and cyanogen bromide (22.13) Strategies to sequence peptides and proteins (22.13) Structure and function of peptides and proteins (22.9, 22.12) Separation of peptides and proteins by electrophoresis, chromatography, and MS Common secondary structure elements in proteins (22.14) Tertiary structure; folding of proteins; ligand binding sites (22.15) Quaternary structure of proteins (22.16) Section 5: Catalysis and Cofactors Principles of chemical and enzymatic catalysis (Ch. 23) Benefits of catalysis in biological systems (23.8) Acid-base catalysis with examples (23.2, 23.4, 23.9) Covalent catalysis with examples (23.4, 23.10) Metal ion catalysis with examples; EDTA as metal chelator (23.5) Electrostatic stabilization of transition states with examples Importance of reactant positioning / proximity with examples (23.6, 23.7) Mechanism of haloalkane dehalogenase catalysis (not in the book) Mechanism and substrate specificity of chymotrypsin catalysis (23.9) Mechanism and biological importance of lysozyme catalysis (23.10) Mechanism and stereochemistry of alcohol dehydrogenase catalysis (24.1) Regulation of activity of biocatalysts (not in the book) Vitamins and coenzymes (Ch 24) Redox chemistry of NAD + /NADH and NADP + /NADPH (24.1) FMN and FAD are oxidizing cofactors in dehydrogenases (24.2) Reaction of reduced flavin with molecular oxygen (not in the book) Role of thiamine pyrophosphate in pyruvate decarboxylase reaction (24.3) Pyridoxal phosphate in transamination and decarboxylation reactions (24.6) Tetrahydrofolate as cofactor in one-carbon transfer reactions (24.7) Section 6: Lipids Nomenclature of lipids: fatty acids, waxes, fats and oils Structure of phosphoglycerides; lipid membranes Prostaglandins: structure, function and biosynthesis Terpenes and terpenoids; terpene biosynthesis (25.16, 25.17) Cholesterol biosynthesis (25.18)
10 Section 7: The Organic Chemistry of Metabolic Pathways Distinction between catabolism and anabolisms (Ch. 25) The importance of ATP in driving biological reactions ( ) The four stages of catabolism (25.5) The catabolism of fats (25.6) The catabolism of carbohydrates: glycolysis (25.7) The fate of pyruvate (25.8) Select reactions of the citric acid cycle (25.10) Section 8: Synthetic Polymers Two major classes of synthetic polymers (27.1) Chain-growth polymers (27.2) Step-growth polymers (27.3) Polymerization of dienes; rubber (27.4) Radical polymerization, cationic polymerization, and anionic polymerization with examples (Ch. 27) Properties and applications of common polymers like polyethylene, polystyrene, and polyvinyl chloride (Ch. 27) Biodegradable polymers (27.10) Section X (independent study w/ supporting discussion for students who need to cover these topics): Reactions of Substituted Benzenes. The goal is to understand some principles that govern the reactivity of aromatic rings toward electrophiles. Focus is on reaction mechanism-based prediction of outcomes of electrophilic substitution reactions Electrophilic aromatic substitutions: what electrophiles react with benzene and how fast (19.X) The mechanism of electrophilic aromatic substitution involving the arenium ion. (19.3) The halogenation of benzene (19.4) The nitration, sulfonation, and deuterium exchange in benzene (19.5; 19.6) The Friedel Crafts acylation of benzene (19.7) The Gatterman Koch synthesis of benzaldehyde (19.7) The Friedel Crafts alkylation of benzene (19.8) The Friedel Crafts acylation followed by reduction (19.9) Nomenclature of substituted aromatic compounds (19.10) Electrophilic substitutions in naphthalene (not in the book) Reactions of Substituted Benzenes and Naphthalene. Reactivity of substituted aromatic compounds: chemical properties of substituents (19.14) How substituents in the aromatic ring affect the electron density in the ring (19.14)
11 How different substituents affect the pk a of benzoic acids (19.16) How different substituents affect the reactivity of the ring toward electrophiles (19.15) Steric effect of substituents and the ortho para ratio (19.17) Reactions directly at the substituent; not in the ring (19.12) The arenediazonium ion ( ) Synthetic utility of electrophilic aromatic substitutions and synthetic strategies ( ) Nucleophilic aromatic substitution (19.24) Biological hydroxylation/epoxidation of aromatic rings with P450 enzymes (review of 11.8) Good luck! Kalju.
I. Chapter 5 Summary. II. Nucleotides & Nucleic Acids. III. Lipids
 I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
Biological molecules:
 Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
How To Understand The Chemistry Of Organic Molecules
 CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
Chapter 3 Molecules of Cells
 Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
BIOLOGICAL MOLECULES OF LIFE
 BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
Disaccharides consist of two monosaccharide monomers covalently linked by a glycosidic bond. They function in sugar transport.
 1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
Biochemistry of Cells
 Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
A disaccharide is formed when a dehydration reaction joins two monosaccharides. This covalent bond is called a glycosidic linkage.
 CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
3) How many monosaccharides are connected to each other in a disaccharide? A) 1 B) 2 C) 3 D) 4
 General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
Lab 3 Organic Molecules of Biological Importance
 Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Chapter 5. The Structure and Function of Macromolecule s
 Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
The Molecules of Cells
 The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Catalysis by Enzymes. Enzyme A protein that acts as a catalyst for a biochemical reaction.
 Catalysis by Enzymes Enzyme A protein that acts as a catalyst for a biochemical reaction. Enzymatic Reaction Specificity Enzyme Cofactors Many enzymes are conjugated proteins that require nonprotein portions
Catalysis by Enzymes Enzyme A protein that acts as a catalyst for a biochemical reaction. Enzymatic Reaction Specificity Enzyme Cofactors Many enzymes are conjugated proteins that require nonprotein portions
Worksheet 13.1. Chapter 13: Human biochemistry glossary
 Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Nucleotides and Nucleic Acids
 Nucleotides and Nucleic Acids Brief History 1 1869 - Miescher Isolated nuclein from soiled bandages 1902 - Garrod Studied rare genetic disorder: Alkaptonuria; concluded that specific gene is associated
Nucleotides and Nucleic Acids Brief History 1 1869 - Miescher Isolated nuclein from soiled bandages 1902 - Garrod Studied rare genetic disorder: Alkaptonuria; concluded that specific gene is associated
Bioenergetics. Free Energy Change
 Bioenergetics Energy is the capacity or ability to do work All organisms need a constant supply of energy for functions such as motion, transport across membrane barriers, synthesis of biomolecules, information
Bioenergetics Energy is the capacity or ability to do work All organisms need a constant supply of energy for functions such as motion, transport across membrane barriers, synthesis of biomolecules, information
Elements in Biological Molecules
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 8: Energy and Metabolism
 Chapter 8: Energy and Metabolism 1. Discuss energy conversions and the 1 st and 2 nd law of thermodynamics. Be sure to use the terms work, potential energy, kinetic energy, and entropy. 2. What are Joules
Chapter 8: Energy and Metabolism 1. Discuss energy conversions and the 1 st and 2 nd law of thermodynamics. Be sure to use the terms work, potential energy, kinetic energy, and entropy. 2. What are Joules
Electrophilic Aromatic Substitution Reactions
 Electrophilic Aromatic Substitution Reactions, Course Notes Archive, 1 Electrophilic Aromatic Substitution Reactions An organic reaction in which an electrophile substitutes a hydrogen atom in an aromatic
Electrophilic Aromatic Substitution Reactions, Course Notes Archive, 1 Electrophilic Aromatic Substitution Reactions An organic reaction in which an electrophile substitutes a hydrogen atom in an aromatic
CHEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) Page 1
 CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
Carbon-organic Compounds
 Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Energy Production In A Cell (Chapter 25 Metabolism)
 Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
Chapter 16 The Citric Acid Cycle
 Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Which of the following is not true of the reaction catalyzed by the pyruvate dehydrogenase complex? A) Biotin participates in the decarboxylation.
Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Which of the following is not true of the reaction catalyzed by the pyruvate dehydrogenase complex? A) Biotin participates in the decarboxylation.
Chapter 2 Chemical Principles
 Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Plant Biochemistry, Spring 2013 BOT 6935, section 4264, 4 credits
 Plant Biochemistry, Spring 2013 BOT 6935, section 4264, 4 credits Meeting time and place MTWTh, 4 th Period, 133 Cancer/Genetics Research Complex Instructors Dr. Alice Harmon, 621 Carr Hall, harmon@ufl.edu,
Plant Biochemistry, Spring 2013 BOT 6935, section 4264, 4 credits Meeting time and place MTWTh, 4 th Period, 133 Cancer/Genetics Research Complex Instructors Dr. Alice Harmon, 621 Carr Hall, harmon@ufl.edu,
Name: Hour: Elements & Macromolecules in Organisms
 Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Methods of Grading S/N Style of grading Percentage Score 1 Attendance, class work and assignment 10 2 Test 20 3 Examination 70 Total 100
 COURSE: MIB 303 Microbial Physiology and Metabolism (3 Units- Compulsory) Course Duration: Three hours per week for 15 weeks (45 hours). Lecturer: Jimoh, S.O. B.Sc., M.Sc, Ph.D Microbiology (ABU, Zaria)
COURSE: MIB 303 Microbial Physiology and Metabolism (3 Units- Compulsory) Course Duration: Three hours per week for 15 weeks (45 hours). Lecturer: Jimoh, S.O. B.Sc., M.Sc, Ph.D Microbiology (ABU, Zaria)
Syllabus for MCB 3010/5001: Biochemistry Fall Semester 2011
 Syllabus for MCB 3010/5001: Biochemistry Fall Semester 2011 Instructor: Dr. Wolf-Dieter Reiter Office: TLS 406 Phone: 486-5733 E-mail: wdreiter@uconn.edu Office hours: Wednesday, 11:00 12:00 a.m., Thursday,
Syllabus for MCB 3010/5001: Biochemistry Fall Semester 2011 Instructor: Dr. Wolf-Dieter Reiter Office: TLS 406 Phone: 486-5733 E-mail: wdreiter@uconn.edu Office hours: Wednesday, 11:00 12:00 a.m., Thursday,
Carbohydrates, proteins and lipids
 Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Energy & Enzymes. Life requires energy for maintenance of order, growth, and reproduction. The energy living things use is chemical energy.
 Energy & Enzymes Life requires energy for maintenance of order, growth, and reproduction. The energy living things use is chemical energy. 1 Energy exists in two forms - potential and kinetic. Potential
Energy & Enzymes Life requires energy for maintenance of order, growth, and reproduction. The energy living things use is chemical energy. 1 Energy exists in two forms - potential and kinetic. Potential
Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids
 VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Proteins and Nucleic Acids
 Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
Chapter 3: Biological Molecules. 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Organic Compounds. Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for?
 Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
4. Which carbohydrate would you find as part of a molecule of RNA? a. Galactose b. Deoxyribose c. Ribose d. Glucose
 1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
Chemical Basis of Life Module A Anchor 2
 Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
Summary of Metabolism. Mechanism of Enzyme Action
 Summary of Metabolism Mechanism of Enzyme Action 1. The substrate contacts the active site 2. The enzyme-substrate complex is formed. 3. The substrate molecule is altered (atoms are rearranged, or the
Summary of Metabolism Mechanism of Enzyme Action 1. The substrate contacts the active site 2. The enzyme-substrate complex is formed. 3. The substrate molecule is altered (atoms are rearranged, or the
10.1 The function of Digestion pg. 402
 10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
Lecture 15: Enzymes & Kinetics Mechanisms
 ROLE OF THE TRANSITION STATE Lecture 15: Enzymes & Kinetics Mechanisms Consider the reaction: H-O-H + Cl - H-O δ- H Cl δ- HO - + H-Cl Reactants Transition state Products Margaret A. Daugherty Fall 2004
ROLE OF THE TRANSITION STATE Lecture 15: Enzymes & Kinetics Mechanisms Consider the reaction: H-O-H + Cl - H-O δ- H Cl δ- HO - + H-Cl Reactants Transition state Products Margaret A. Daugherty Fall 2004
MULTIPLE CHOICE QUESTIONS
 MULTIPLE CHOICE QUESTIONS 1. Most components of energy conversion systems evolved very early; thus, the most fundamental aspects of energy metabolism tend to be: A. quite different among a diverse group
MULTIPLE CHOICE QUESTIONS 1. Most components of energy conversion systems evolved very early; thus, the most fundamental aspects of energy metabolism tend to be: A. quite different among a diverse group
4/18/2011. 9.8 Substituent Effects in Electrophilic Substitutions. Substituent Effects in Electrophilic Substitutions
 9.8 Substituent effects in the electrophilic substitution of an aromatic ring Substituents affect the reactivity of the aromatic ring Some substituents activate the ring, making it more reactive than benzene
9.8 Substituent effects in the electrophilic substitution of an aromatic ring Substituents affect the reactivity of the aromatic ring Some substituents activate the ring, making it more reactive than benzene
Chapter 16 The Citric Acid Cycle
 Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Production of acetyl-coa (activated acetate) Page: 603 Difficulty: 2 Ans: A Which of the following is not true of the reaction catalyzed by
Chapter 16 The Citric Acid Cycle Multiple Choice Questions 1. Production of acetyl-coa (activated acetate) Page: 603 Difficulty: 2 Ans: A Which of the following is not true of the reaction catalyzed by
Benzene Benzene is best represented as a resonance hybrid:
 Electrophilic Aromatic Substitution (EAS) is a substitution reaction usually involving the benzene ring; more specifically it is a reaction in which the hydrogen atom of an aromatic ring is replaced as
Electrophilic Aromatic Substitution (EAS) is a substitution reaction usually involving the benzene ring; more specifically it is a reaction in which the hydrogen atom of an aromatic ring is replaced as
Elements & Macromolecules in Organisms
 Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
BIO 361 Biochemistry. Oficina: CABD Building 20 Room 133 First Floor Fall 2015 Email: csanoca@upo.es Thursday 16.00-17-00 Office Hours:
 Centro Universitario Internacional BIO 361 Biochemistry Carlos Santos Ocaña Course Information: Oficina: CABD Building 20 Room 133 First Floor Fall 2015 Email: csanoca@upo.es Thursday 16.00-17-00 Office
Centro Universitario Internacional BIO 361 Biochemistry Carlos Santos Ocaña Course Information: Oficina: CABD Building 20 Room 133 First Floor Fall 2015 Email: csanoca@upo.es Thursday 16.00-17-00 Office
WATER CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" POLARITY HYDROGEN BONDING
 CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" WATER Compare the body of the jellyfish with our own bodies. The jellyfish will die if it is removed from its water environment, yet we can live in the
CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" WATER Compare the body of the jellyfish with our own bodies. The jellyfish will die if it is removed from its water environment, yet we can live in the
Electrophilic Aromatic Substitution
 Electrophilic Aromatic Substitution Electrophilic Aromatic Substitution: a reaction in which the hydrogen atom of an aromatic ring is replaced as a result of an electrophilic attack on the aromatic ring
Electrophilic Aromatic Substitution Electrophilic Aromatic Substitution: a reaction in which the hydrogen atom of an aromatic ring is replaced as a result of an electrophilic attack on the aromatic ring
CHEM 208(Organic Chemistry I) Instructor: Dr. Niranjan Goswami. Tel: (618)545-3361. Email: Ngoswami@kaskaskia.edu. Web: www.kc.cc.il.
 CHEM 208(Organic Chemistry I) Instructor: Dr. Niranjan Goswami Tel: (618)545-3361 Email: Ngoswami@kaskaskia.edu Web: www.kc.cc.il.us/ngoswami CHEM 208 COURSE SYLLABUS KASKASKIA COLLEGE NAME TERM YEAR TEXT:
CHEM 208(Organic Chemistry I) Instructor: Dr. Niranjan Goswami Tel: (618)545-3361 Email: Ngoswami@kaskaskia.edu Web: www.kc.cc.il.us/ngoswami CHEM 208 COURSE SYLLABUS KASKASKIA COLLEGE NAME TERM YEAR TEXT:
Chapter 2. The Chemistry of Life Worksheets
 Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Chemistry and Biochemistry
 SUBJECT OUTLINE Subject Name: Chemistry and Biochemistry SECTION 1 GENERAL INFORMATION Subject Code: BIOB111 Award/s: Total course credit points: Level: Bachelor of Health Science (Naturopathy) 128 Core
SUBJECT OUTLINE Subject Name: Chemistry and Biochemistry SECTION 1 GENERAL INFORMATION Subject Code: BIOB111 Award/s: Total course credit points: Level: Bachelor of Health Science (Naturopathy) 128 Core
How To Learn Chemistry And Biochemistry
 SUBJECT OUTLINE Subject Name: Chemistry and Biochemistry SECTION 1 GENERAL INFORMATION Subject Code: BIOB111 Award/s: Total course credit points: Level: Bachelor of Health Science (Naturopathy) 128 Core
SUBJECT OUTLINE Subject Name: Chemistry and Biochemistry SECTION 1 GENERAL INFORMATION Subject Code: BIOB111 Award/s: Total course credit points: Level: Bachelor of Health Science (Naturopathy) 128 Core
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Chapter 5: The Structure and Function of Large Biological Molecules Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four
Name Period Chapter 5: The Structure and Function of Large Biological Molecules Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four
The molecules of life. The molecules that make up living things are really big They are called macromolecules
 Food Labels All living things use materials and energy Our food comes from living things The food labels we see show us what our food is made of The stuff we are studying today can be found on food labels
Food Labels All living things use materials and energy Our food comes from living things The food labels we see show us what our food is made of The stuff we are studying today can be found on food labels
Preliminary MFM Quiz
 Preliminary MFM Quiz 1. The major carrier of chemical energy in all cells is: A) adenosine monophosphate B) adenosine diphosphate C) adenosine trisphosphate D) guanosine trisphosphate E) carbamoyl phosphate
Preliminary MFM Quiz 1. The major carrier of chemical energy in all cells is: A) adenosine monophosphate B) adenosine diphosphate C) adenosine trisphosphate D) guanosine trisphosphate E) carbamoyl phosphate
Solid Phase Peptide Synthesis
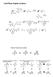 Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
1. Enzymes. Biochemical Reactions. Chapter 5: Microbial Metabolism. 1. Enzymes. 2. ATP Production. 3. Autotrophic Processes
 Chapter 5: Microbial Metabolism 1. Enzymes 2. ATP Production 3. Autotrophic Processes 1. Enzymes Biochemical Reactions All living cells depend on biochemical reactions to maintain homeostasis. All of the
Chapter 5: Microbial Metabolism 1. Enzymes 2. ATP Production 3. Autotrophic Processes 1. Enzymes Biochemical Reactions All living cells depend on biochemical reactions to maintain homeostasis. All of the
How To Understand The Chemistry Of An Enzyme
 Chapt. 8 Enzymes as catalysts Ch. 8 Enzymes as catalysts Student Learning Outcomes: Explain general features of enzymes as catalysts: Substrate -> Product Describe nature of catalytic sites general mechanisms
Chapt. 8 Enzymes as catalysts Ch. 8 Enzymes as catalysts Student Learning Outcomes: Explain general features of enzymes as catalysts: Substrate -> Product Describe nature of catalytic sites general mechanisms
H H N - C - C 2 R. Three possible forms (not counting R group) depending on ph
 Amino acids - 0 common amino acids there are others found naturally but much less frequently - Common structure for amino acid - C, -N, and functional groups all attached to the alpha carbon N - C - C
Amino acids - 0 common amino acids there are others found naturally but much less frequently - Common structure for amino acid - C, -N, and functional groups all attached to the alpha carbon N - C - C
BERGEN COMMUNITY COLLEGE DIVISION OF MATHEMATICS, SCIENCE AND TECHNOLOGY DEPARTMENT OF PHYSICAL SCIENCES STUDENT COURSE OUTLINE
 BERGEN COMMUNITY COLLEGE DIVISION OF MATHEMATICS, SCIENCE AND TECHNOLOGY DEPARTMENT OF PHYSICAL SCIENCES STUDENT COURSE OUTLINE Course Title: Prerequisites: Course Description: Textbook: CHM 212 Organic
BERGEN COMMUNITY COLLEGE DIVISION OF MATHEMATICS, SCIENCE AND TECHNOLOGY DEPARTMENT OF PHYSICAL SCIENCES STUDENT COURSE OUTLINE Course Title: Prerequisites: Course Description: Textbook: CHM 212 Organic
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids
 The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1
 CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
Organic Molecules of Life - Exercise 2
 Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Chemistry 20 Chapters 15 Enzymes
 Chemistry 20 Chapters 15 Enzymes Enzymes: as a catalyst, an enzyme increases the rate of a reaction by changing the way a reaction takes place, but is itself not changed at the end of the reaction. An
Chemistry 20 Chapters 15 Enzymes Enzymes: as a catalyst, an enzyme increases the rate of a reaction by changing the way a reaction takes place, but is itself not changed at the end of the reaction. An
Figure 5. Energy of activation with and without an enzyme.
 Biology 20 Laboratory ENZYMES & CELLULAR RESPIRATION OBJECTIVE To be able to list the general characteristics of enzymes. To study the effects of enzymes on the rate of chemical reactions. To demonstrate
Biology 20 Laboratory ENZYMES & CELLULAR RESPIRATION OBJECTIVE To be able to list the general characteristics of enzymes. To study the effects of enzymes on the rate of chemical reactions. To demonstrate
Biochemistry - I. Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture-11 Enzyme Mechanisms II
 Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture-11 Enzyme Mechanisms II In the last class we studied the enzyme mechanisms of ribonuclease A
Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture-11 Enzyme Mechanisms II In the last class we studied the enzyme mechanisms of ribonuclease A
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins
 Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
Reactions of Fats and Fatty Acids
 Reactions of Fats and Fatty Acids Outline Fats and Oils Fatty Acid Biosynthesis Biodiesel Homework We hear quite a lot about the place of fats and oils in human nutrition. Foods high in fat are at the
Reactions of Fats and Fatty Acids Outline Fats and Oils Fatty Acid Biosynthesis Biodiesel Homework We hear quite a lot about the place of fats and oils in human nutrition. Foods high in fat are at the
Chem 306 Chapter 21 Bioenergetics Lecture Outline III
 Chem 306 Chapter 21 Bioenergetics Lecture Outline III I. HOW IS ATP GENERATED IN THE FINAL STAGE CATABOLISM? A. OVERVIEW 1. At the end of the citric acid cycle, all six carbons of glucose have been oxidized
Chem 306 Chapter 21 Bioenergetics Lecture Outline III I. HOW IS ATP GENERATED IN THE FINAL STAGE CATABOLISM? A. OVERVIEW 1. At the end of the citric acid cycle, all six carbons of glucose have been oxidized
NO CALCULATORS OR CELL PHONES ALLOWED
 Biol 205 Exam 1 TEST FORM A Spring 2008 NAME Fill out both sides of the Scantron Sheet. On Side 2 be sure to indicate that you have TEST FORM A The answers to Part I should be placed on the SCANTRON SHEET.
Biol 205 Exam 1 TEST FORM A Spring 2008 NAME Fill out both sides of the Scantron Sheet. On Side 2 be sure to indicate that you have TEST FORM A The answers to Part I should be placed on the SCANTRON SHEET.
Anabolic and Catabolic Reactions are Linked by ATP in Living Organisms
 Chapter 5: Microbial Metabolism Microbial Metabolism Metabolism refers to all chemical reactions that occur within a living a living organism. These chemical reactions are generally of two types: Catabolic:
Chapter 5: Microbial Metabolism Microbial Metabolism Metabolism refers to all chemical reactions that occur within a living a living organism. These chemical reactions are generally of two types: Catabolic:
AP BIOLOGY CHAPTER 7 Cellular Respiration Outline
 AP BIOLOGY CHAPTER 7 Cellular Respiration Outline I. How cells get energy. A. Cellular Respiration 1. Cellular respiration includes the various metabolic pathways that break down carbohydrates and other
AP BIOLOGY CHAPTER 7 Cellular Respiration Outline I. How cells get energy. A. Cellular Respiration 1. Cellular respiration includes the various metabolic pathways that break down carbohydrates and other
Foothill College Winter 2015 Survey of Organic and Biochemistry 30B Sections 01 and 02
 Foothill College Winter 2015 Survey of Organic and Biochemistry 30B Sections 01 and 02 Welcome to Chemistry 30B! Office hours: Tuesday and Thursday 10:30-11:45 AM Room 4431 or by appointment Instructor:
Foothill College Winter 2015 Survey of Organic and Biochemistry 30B Sections 01 and 02 Welcome to Chemistry 30B! Office hours: Tuesday and Thursday 10:30-11:45 AM Room 4431 or by appointment Instructor:
Georgia Perimeter College - Dunwoody Campus Chemistry 1152-200 Fall 2011 Course Syllabus(revised)
 Georgia Perimeter College - Dunwoody Campus Chemistry 1152-200 Fall 2011 Course Syllabus(revised) Course Title: Survey of Chemistry II, TR 1000 1115 Room NE-1260 Instructor: Dr. Jerry L. Poteat, Associate
Georgia Perimeter College - Dunwoody Campus Chemistry 1152-200 Fall 2011 Course Syllabus(revised) Course Title: Survey of Chemistry II, TR 1000 1115 Room NE-1260 Instructor: Dr. Jerry L. Poteat, Associate
Microbial Metabolism. Biochemical diversity
 Microbial Metabolism Biochemical diversity Metabolism Define Requirements Energy Enzymes Rate Limiting step Reaction time Types Anabolic Endergonic Dehydration Catabolic Exergonic Hydrolytic Metabolism
Microbial Metabolism Biochemical diversity Metabolism Define Requirements Energy Enzymes Rate Limiting step Reaction time Types Anabolic Endergonic Dehydration Catabolic Exergonic Hydrolytic Metabolism
Student name ID # 2. (4 pts) What is the terminal electron acceptor in respiration? In photosynthesis? O2, NADP+
 1. Membrane transport. A. (4 pts) What ion couples primary and secondary active transport in animal cells? What ion serves the same function in plant cells? Na+, H+ 2. (4 pts) What is the terminal electron
1. Membrane transport. A. (4 pts) What ion couples primary and secondary active transport in animal cells? What ion serves the same function in plant cells? Na+, H+ 2. (4 pts) What is the terminal electron
Copyright 2010 Pearson Education, Inc. Chapter Twenty Three 1
 23.2 Glucose Metabolism: An Overview When glucose enters a cell from the bloodstream, it is immediately converted to glucose 6- phosphate. Once this phosphate is formed, glucose is trapped within the cell
23.2 Glucose Metabolism: An Overview When glucose enters a cell from the bloodstream, it is immediately converted to glucose 6- phosphate. Once this phosphate is formed, glucose is trapped within the cell
CHEM 451 BIOCHEMISTRY I. SUNY Cortland Fall 2010
 CHEM 451 BIOCHEMISTRY I SUNY Cortland Fall 2010 Instructor: Dr. Frank Rossi Office: Bowers 135 Office Hours: Mon. 2:30-4:00, Wed. 4:00-5:30, Friday 2:30-3:00, or by appointment. Extra evening office hours
CHEM 451 BIOCHEMISTRY I SUNY Cortland Fall 2010 Instructor: Dr. Frank Rossi Office: Bowers 135 Office Hours: Mon. 2:30-4:00, Wed. 4:00-5:30, Friday 2:30-3:00, or by appointment. Extra evening office hours
What affects an enzyme s activity? General environmental factors, such as temperature and ph. Chemicals that specifically influence the enzyme.
 CH s 8-9 Respiration & Metabolism Metabolism A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme is a catalytic protein. Hydrolysis of sucrose by
CH s 8-9 Respiration & Metabolism Metabolism A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction. An enzyme is a catalytic protein. Hydrolysis of sucrose by
Copyright 2000-2003 Mark Brandt, Ph.D. 54
 Pyruvate Oxidation Overview of pyruvate metabolism Pyruvate can be produced in a variety of ways. It is an end product of glycolysis, and can be derived from lactate taken up from the environment (or,
Pyruvate Oxidation Overview of pyruvate metabolism Pyruvate can be produced in a variety of ways. It is an end product of glycolysis, and can be derived from lactate taken up from the environment (or,
BIOMOLECULES. reflect
 reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
Cellular Respiration Worksheet 1. 1. What are the 3 phases of the cellular respiration process? Glycolysis, Krebs Cycle, Electron Transport Chain.
 Cellular Respiration Worksheet 1 1. What are the 3 phases of the cellular respiration process? Glycolysis, Krebs Cycle, Electron Transport Chain. 2. Where in the cell does the glycolysis part of cellular
Cellular Respiration Worksheet 1 1. What are the 3 phases of the cellular respiration process? Glycolysis, Krebs Cycle, Electron Transport Chain. 2. Where in the cell does the glycolysis part of cellular
A. A peptide with 12 amino acids has the following amino acid composition: 2 Met, 1 Tyr, 1 Trp, 2 Glu, 1 Lys, 1 Arg, 1 Thr, 1 Asn, 1 Ile, 1 Cys
 Questions- Proteins & Enzymes A. A peptide with 12 amino acids has the following amino acid composition: 2 Met, 1 Tyr, 1 Trp, 2 Glu, 1 Lys, 1 Arg, 1 Thr, 1 Asn, 1 Ile, 1 Cys Reaction of the intact peptide
Questions- Proteins & Enzymes A. A peptide with 12 amino acids has the following amino acid composition: 2 Met, 1 Tyr, 1 Trp, 2 Glu, 1 Lys, 1 Arg, 1 Thr, 1 Asn, 1 Ile, 1 Cys Reaction of the intact peptide
COURSE OUTLINE. The course description is online @ http://camosun.ca/learn/calendar/current/web/chem.html
 School of Arts & Science CHEMISTRY AND GEOSCIENCE DEPARTMENT CHEM 255 Biochemistry Winter Semester 2014 COURSE OUTLINE This course concerns fundamental aspects of biomolecules and biochemical processes
School of Arts & Science CHEMISTRY AND GEOSCIENCE DEPARTMENT CHEM 255 Biochemistry Winter Semester 2014 COURSE OUTLINE This course concerns fundamental aspects of biomolecules and biochemical processes
CHAPTER 6 AN INTRODUCTION TO METABOLISM. Section B: Enzymes
 CHAPTER 6 AN INTRODUCTION TO METABOLISM Section B: Enzymes 1. Enzymes speed up metabolic reactions by lowering energy barriers 2. Enzymes are substrate specific 3. The active site in an enzyme s catalytic
CHAPTER 6 AN INTRODUCTION TO METABOLISM Section B: Enzymes 1. Enzymes speed up metabolic reactions by lowering energy barriers 2. Enzymes are substrate specific 3. The active site in an enzyme s catalytic
Microbial Metabolism. Chapter 5. Enzymes. Enzyme Components. Mechanism of Enzymatic Action
 Chapter 5 Microbial Metabolism Metabolism is the sum of all chemical reactions within a living organism, including anabolic (biosynthetic) reactions and catabolic (degradative) reactions. Anabolism is
Chapter 5 Microbial Metabolism Metabolism is the sum of all chemical reactions within a living organism, including anabolic (biosynthetic) reactions and catabolic (degradative) reactions. Anabolism is
I N V E S T I C E D O R O Z V O J E V Z D Ě L Á V Á N Í ENZYMES
 = substances that... biological reactions 1. Provide an alternative reaction route which has a lower... energy 2. Reactions catalysed by enzymes occur under mild conditions + good yield + fast 3. Enzymes
= substances that... biological reactions 1. Provide an alternative reaction route which has a lower... energy 2. Reactions catalysed by enzymes occur under mild conditions + good yield + fast 3. Enzymes
Previous lecture: Today:
 Previous lecture: The energy requiring step from substrate to transition state is an energy barrier called the free energy of activation G Transition state is the unstable (10-13 seconds) highest energy
Previous lecture: The energy requiring step from substrate to transition state is an energy barrier called the free energy of activation G Transition state is the unstable (10-13 seconds) highest energy
Lecture 26: Overview of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) structure
 Lecture 26: Overview of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) structure Nucleic acids play an important role in the storage and expression of genetic information. They are divided into
Lecture 26: Overview of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) structure Nucleic acids play an important role in the storage and expression of genetic information. They are divided into
The Molecules of Life - Overview. The Molecules of Life. The Molecules of Life. The Molecules of Life
 The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Two Forms of Energy
 Module 2D - Energy and Metabolism Objective # 19 All living organisms require energy for survival. In this module we will examine some general principles about chemical reactions and energy usage within
Module 2D - Energy and Metabolism Objective # 19 All living organisms require energy for survival. In this module we will examine some general principles about chemical reactions and energy usage within
CHAPTER 15: ANSWERS TO SELECTED PROBLEMS
 CHAPTER 15: ANSWERS T SELECTED PRBLEMS SAMPLE PRBLEMS ( Try it yourself ) 15.1 ur bodies can carry out the second reaction, because it requires less energy than we get from breaking down a molecule of
CHAPTER 15: ANSWERS T SELECTED PRBLEMS SAMPLE PRBLEMS ( Try it yourself ) 15.1 ur bodies can carry out the second reaction, because it requires less energy than we get from breaking down a molecule of
Chapter 2 Polar Covalent Bonds; Acids and Bases
 John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 2 Polar Covalent Bonds; Acids and Bases Javier E. Horta, M.D., Ph.D. University of Massachusetts Lowell Polar Covalent Bonds: Electronegativity
John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 2 Polar Covalent Bonds; Acids and Bases Javier E. Horta, M.D., Ph.D. University of Massachusetts Lowell Polar Covalent Bonds: Electronegativity
Course Outline. 1. COURSE INFORMATION Session Offered Winter 2012 Course Name Biochemistry
 Course Outline 1. COURSE INFORMATION Session Offered Winter 2012 Course Name Biochemistry Course Code BIOTECH 2BC3 Program Name Biotechnology Calendar Description Biochemistry and biotechnology; amino
Course Outline 1. COURSE INFORMATION Session Offered Winter 2012 Course Name Biochemistry Course Code BIOTECH 2BC3 Program Name Biotechnology Calendar Description Biochemistry and biotechnology; amino
Enzymes: Introduction
 Enzymes: Introduction Firefly bioluminescence is produced by an oxidation reaction catalyzed by the enzyme firefly luciferase. The oxidized substrate (product of the reaction) is in an electronically excited
Enzymes: Introduction Firefly bioluminescence is produced by an oxidation reaction catalyzed by the enzyme firefly luciferase. The oxidized substrate (product of the reaction) is in an electronically excited
School of Kinesiology Faculty of Health Sciences Western University. EXERCISE BIOCHEMISTRY Kin 3360B Winter, 2015
 2014-15 School of Kinesiology Faculty of Health Sciences Western University EXERCISE BIOCHEMISTRY Kin 3360B Winter, 2015 Instructor: J.M. Kowalchuk Office: HSB 411C Location: SH 3317 Office Hours: by appointment
2014-15 School of Kinesiology Faculty of Health Sciences Western University EXERCISE BIOCHEMISTRY Kin 3360B Winter, 2015 Instructor: J.M. Kowalchuk Office: HSB 411C Location: SH 3317 Office Hours: by appointment
Chapter 8: An Introduction to Metabolism
 Chapter 8: An Introduction to Metabolism Name Period Concept 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 1. Define metabolism. The totality of an organism
Chapter 8: An Introduction to Metabolism Name Period Concept 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 1. Define metabolism. The totality of an organism
