Acute relapses of multiple sclerosis
|
|
|
- Corey Carmella Wade
- 10 years ago
- Views:
Transcription
1 CHAPTER 27 Acute relapses of multiple sclerosis F. S e l l e b j e r g, 1 D. Barnes, 2 G. Filippini, 3 R. Midg ard, 4 X. Montalban, 5 P. Rieckmann, 6 K. Selmaj, 7 L. H. Vi s s e r, 8 P. So e lb e r g S ø re ns e n 1 1 University of Copenhagen and Rigshospitalet, Copenhagen, Denmark; 2 Atkinson Morley s Hospital, Wimbledon, UK; 3 Fondazione Istituto Nazionale Neurologico C. Besta, Milan, Italy; 4 Molde Hospital, Norway; 5 University Hospital Vall d Hebron, Barcelona, Spain; 6 University of British Columbia and Vancouver Coastal Health, Vancouver, British Columbia, Canada; 7 Medical University of Lodz, Poland; 8 St Elisabeth Hospital, Tilburg, The Netherlands Background Attacks or relapses are the dominating feature of relapsing remitting multiple sclerosis (MS), but are also observed in patients with secondary progressive MS with superimposed relapses. Even patients with primary progressive MS may experience relapses, becoming progressive - relapsing MS [1, 2]. In the McDonald criteria for the diagnosis of MS, a relapse is defined as an episode of neurological disturbance of the kind seen in MS, when the clinicopathological studies have established that the causative lesions are inflammatory and demyelinating in nature [3, 4]. An attack should last for at least 24 h. and, according to the McDonald criteria, there should be expert opinion that the event is not a pseudoattack as might be caused by an increase in body temperature or infection. Although the majority of relapses improve to some extent, incomplete recovery is an important determinant of irreversible, or at least long - lasting, neurological impairment in relapsing remitting MS [5, 6]. Glucocorticoid treatment is recommended as the first - line treatment of MS relapses in North American guidelines and in the recommendations of a European MS therapy consensus group [7, 8]. The aim of the European Federation of Neurological Societies (EFNS) task force was to review the current literature on relapse treatment. Key issues addressed are whether treatment of MS European Handbook of Neurological Management: Volume 1, 2nd Edition Edited by N. E. Gilhus, M. P. Barnes and M. Brainin 2011 Blackwell Publishing Ltd. ISBN: relapses: (1) can improve the speed of recovery; (2) can influence long - term recovery; (3) can influence subsequent disease activity; (4) has significant side effects. Furthermore, the task force sought to provide guidelines on whether all relapses should be treated and how relapses during pregnancy should be managed. Search s trategy We searched literature databases (EMBASE and PubMed), in English, for papers using the search terms multiple sclerosis, attack, relapse, exacerbation, and treatment in November 2004 and November The Cochrane Library and the reference lists of individual papers were searched for studies not identified in the EMBASE and PubMed searches. Studies of various treatments for patients suffering from relapses of MS were considered for the guidelines and were rated as Class I to Class IV studies according to the recommendations for EFNS scientific task forces [9]. Method for r eaching c onsensus The results of the literature searches were circulated by to the task force members for comments. The task force chairman prepared a first draft of the manuscript based on the results of the literature review and comments from the task force members. The draft and the recommendations were discussed during telephone conferences until consensus was reached within the task 411
2 412 SECTION 5 Neurological Problems force. Recommendations were rated from A to C according to the EFNS guidelines for scientific task forces [9]. Where there was insufficient evidence to support firm recommendations the term Good Practice Point (GPP) was used. Results Effect of g lucocorticoid and a drenocorticotrophic h ormone ( ACTH ) t reatment on MS r elapses Glucocorticoid or ACTH treatment of MS relapses was analysed in a Cochrane review that included results from six randomized, placebo - controlled clinical trials of either ACTH (two trials), intravenous (i.v.) methylprednisolone treatment (three trials) or oral methylprednisolone treatment (one trial) [10 15]. All trials reported a benefit in terms of rate of recovery compared to placebo [16]. A similar conclusion was reached in another meta - analysis, which used less stringent criteria for study inclusion than the Cochrane review [17]. Three trials have compared the efficacy of i.v. methylprednisolone and ACTH treatment in MS relapses [18 20]. One study including 14 patients treated with i.v. methylprednisolone (1 g daily for 7 days) and 11 patients treated with intramuscular (IM) ACTH (80 units, 60 units, 40 units, and 20 units daily, each for 1 week) reported more rapid improvement (after 3 and 28 days) after i.v. methylprednisolone than after ACTH treatment, but there was no significant difference after 3 months [19]. However, the patient blinding and the primary outcome were not clearly defined, for which reason this study should be considered a Class III study. One Class II study compared the administration of 1 g of methylprednisolone once daily for 3 days to ACTH treatment (80 units for 7 days, 40 units for 4 days and 20 units for 3 days) in 61 patients, and found no difference in terms of rate of recovery or final outcome after 12 weeks [20]. A Class III study including 60 patients, treated with either i.v. methylprednisolone (20 mg/kg day 1 3, 10 mg/kg day 4 7, 5 mg/kg day 8 10, and 1 mg/kg day 11 15) or ACTH (1 mg i.v. daily for 15 days), also found no difference in the efficacy of ACTH and methylprednisolone treatment [18]. The studies found no major differences in adverse events between methylprednisolone and ACTH treatment. Thus there is no evidence of any major difference in the efficacy of ACTH and methylprednisolone treatment from comparative studies, but the clinical trials were too small to rule out some difference in efficacy. Indeed, in the Cochrane review it was suggested that methylprednisolone treatment could still confer greater benefit than treatment with ACTH, and the administration of methylprednisolone is simpler than the more prolonged ACTH treatment regimen [16]. In a separate meta - analysis of three double - blind, randomized, placebo - controlled trials [21], it was concluded that treatment with i.v. methylprednisolone (15 mg/kg day 1 3, 10 mg/kg day 4 6, 5 mg/kg day 7 9, 2.5 mg/kg day 10 12, 1 mg/kg day followed by oral prednisone tapered slowly over 120 days [10] ), i.v. methylprednisolone without a tapering dose (500 mg once daily for 5 days [13] ), or oral methylprednisolone (500 mg once daily for 5 days followed by 400, 300, 200, 100, 64, 48, 32, 16, 8, and 8 mg once daily the subsequent 10 days [15] ) resulted in significantly faster recovery than did treatment with placebo (table 27.1 ). The first two trials provided follow - up data in a placebo - controlled design for 15 days [10] and 28 days [13]. The oral methylprednisolone study found significant differences between the methylprednisolone and the placebo group after 8 weeks and a trend to better improvement in the methylprednisolone group after 1 year [15]. In the latter trial there was no evidence that the 1 - year risk of subsequent relapses was influenced by oral high - dose methylprednisolone treatment. Specific g lucocorticoids, d ose, and r oute of a dministration The clinical trials of glucocorticoid treatment in relapses of MS have mainly assessed the effect of methylprednisolone treatment. Two trials have compared the effect of methylprednisolone treatment given i.v. or orally. One Class III study compared the effect of methylprednisolone (500 mg once daily for 5 days) given orally or i.v. in 35 patients with an MS relapse, and found no significant difference in recovery between the two treatment arms after 5 and 28 days [22]. Another study (Class I) compared the effect of oral methylprednisolone (48 mg daily for 7 days, 24 mg daily for 7 days, and 12 mg daily for 7 days) to treatment with i.v. methylprednisolone (1 g daily for 3 days) [23]. In this study, recovery from the relapse was similar in the 38 patients in the i.v. treatment group and the 42 patients in the oral treatment group at all time points for
3 CHAPTER 27 Acute relapses of multiple sclerosis 413 Table 27.1 Summary of three randomized, placebo - controlled trials comparing methylprednisolone ( MP ) treatment to placebo in patients with relapses of MS (Durelli et al. [10] ; Milligan et al. [12] ; Sellebjerg et al. [15] ). Data are changes in Kurtzke EDSS scores from baseline (mean and standard deviation in brackets) or differences (mean and 95% confidence intervals in brackets) between MP and placebo reported in a meta - analysis (Miller et al. [21] ). Study and treatment Change from baseline Difference (MP vs placebo) Day 5 7 Durelli, placebo ( n = 8) 0 (0) i.v. methylprednisolone ( n = 12) 1.00 (0.6) 1.00 ( 1.45 to 0.55) Milligan, placebo ( n = 9) 0.28 (0.51) i.v. methylprednisolone ( n = 13) 1.46 (1.38) 1.18 ( 2.19 to 0.17) Sellebjerg, placebo ( n = 25) 0.06 (0.44) Oral methylprednisolone ( n = 26) 0.58 (0.82) 0.52 ( 0.89 to 0.14) Pooled difference: 0.76 (standard error 0.14) Day Durelli, placebo ( n = 8) 0.38 (0.52) i.v. methylprednisolone ( n = 12) 2.04 (1.48) 1.67 ( 2.82 to 0.51) Milligan, placebo ( n = 8) 0.25 (1.22) i.v. methylprednisolone ( n = 13) 2.04 (1.51) 1.79 ( 3.11 to 0.46) Sellebjerg, placebo ( n = 25) 0.38 (0.81) Oral methylprednisolone ( n = 26) 0.94 (0.90) 0.56 ( 1.04 to 0.08) Pooled difference: 0.85 (standard error 0.21) up to 24 weeks of follow - up. The relapse rate the following 2 years was also similar in the oral and i.v. treatment group [24]. A Class III study comparing the effect of i.v. and oral methylprednisolone (1000 mg daily for 5 days) in 40 patients found comparable effect of the two treatment regimens on magnetic resonance imaging (MRI) outcomes [25]. A recent Cochrane review concluded that oral and i.v. treatment are both efficacious with respect to clinical outcomes (relapse recovery and subsequent relapse activity), radiological outcomes, and bioavailability measures, but that there is still insufficient evidence to prove equivalence of oral and i.v. treatment [26]. Oral tapering doses of glucocorticoids are commonly used, but no randomized controlled studies have yet compared the outcome of a relapse in patients treated with tapering doses of glucocorticoids or placebo following short-term, high-dose methylprednisolone treatment. However, a recent Class III study found no difference in recovery rate in 152 patients treated with high - dose methylprednisolone followed by an oral prednisone taper and 112 patients treated with high - dose methylprednisolone only [27]. Three studies have compared i.v. methylprednisolone treatment given in different doses. One Class III study found that recovery was faster after treatment with i.v. methylprednisolone (1 g once daily for 5 days) than after a single 1 g dose of i.v. methylprednisolone [28]. Two other studies (both Class III) have compared the effect of different doses of i.v. methylprednisolone in relapses of MS on a panel of different outcome measures. In the first study, treatment with i.v. methylprednisolone at a dose of 500 mg once daily for 5 days was compared to treatment with 2000 mg once daily for 5 days in 31 patients with a relapse of MS [29]. There was no difference in the efficacy of the low dose and the high dose of methylprednisolone in terms of clinical recovery or short - term suppression of MRI disease activity, but it was suggested that the high dose resulted in more pronounced suppression of MRI disease activity after 1 and 2 months. In the second study, i.v. methylprednisolone at a dose of 1 g or 2 g once daily for 5 days was compared in 24 patients who were followed up with clinical and neurophysiologic studies for 21 days after randomization to one of the two treatment arms [30]. This study showed no significant differences between the two methylprednisolone doses on the majority of diverse outcome measures, but a few favoured the higher dose over the lower dose. Two studies have compared the effect of treatment with different
4 414 SECTION 5 Neurological Problems doses of methylprednisolone and dexamethasone in relapses of MS [31, 32]. Due to the small sample sizes and differences in the baseline characteristics of the patients randomized to the different treatment arms, the results of these two studies are difficult to interpret. Glucocorticoid t reatment of a cute o ptic n euritis In the North American Optic Neuritis Treatment Trial (ONTT), 457 patients were randomized to receive treatment with i.v. methylprednisolone (250 mg four times daily for 3 days followed by oral prednisone, 1 mg/kg for 11 days, 20 mg on day 15, and 10 mg on days 16 and 18), oral prednisone (1 mg/kg for 14 days, 20 mg on day 15, and 10 mg on days 16 and 18), or oral placebo [33]. Treatment allocation was not blinded in patients randomized to treatment with i.v. methylprednisolone, while prednisone treatment and placebo was given in a double - blind design. Thus, the study was a Class II study investigating the efficacy of methylprednisolone treatment, but a Class I study in the comparison of oral prednisone and placebo. The study found no significant effect of i.v. methylprednisolone or oral prednisone treatment on the recovery of visual acuity, but the recovery of contrast sensitivity and visual fields was significantly faster in patients treated with i.v. methylprednisolone. After 6 months, patients treated with i.v. methylprednisolone had still recovered slightly better than patients treated with placebo, but no significant treatment effect was seen at follow - up after 1 year [34]. Oral prednisone treatment had no effect on the recovery from acute optic neuritis in either the ONTT or a Danish Class I study of oral prednisolone versus placebo in 128 patients with acute optic neuritis ( [33], J.L. Frederiksen, personal communication). Treatment with oral methylprednisolone (100 mg, 80 mg, 60 mg, 40 mg, 30 mg, 20 mg, 10 mg, and 5 mg daily for 3 days each) was not better than treatment with oral thiamine (100 mg daily for 24 days) on any of several outcome measures in a Class II study including 38 patients with acute optic neuritis [35]. Two additional studies (Class I) have compared the effect of treatment with high - dose methylprednisolone in acute optic neuritis. One study included 60 patients with acute optic neuritis who were treated with oral high - dose methylprednisolone (500 mg once daily for 5 days followed by 400, 300, 200, 100, 64, 48, 32, 16, 8, and 8 mg once daily the subsequent 10 days) or oral placebo [36]. Oral methylprednisolone treatment resulted in significantly better recovery of spatial visual function (visual acuity and contrast sensitivity), colour vision function, and visual symptoms after 1 week, but only borderline significant effects were observed after 3 weeks, and after 8 weeks there was no evidence of an effect of oral methylprednisolone treatment [36]. In a study of 66 patients with acute optic neuritis treatment with i.v. methylprednisolone (1 g once daily for 3 days) did not improve the outcome from acute optic neuritis after 26 weeks on either a panel of visual function and neurophysiologic variables or on MRI outcome measures [37]. A controversial finding in the ONTT was that patients treated with i.v. methylprednisolone appeared to have a lower risk of developing MS during 2 years of follow - up than patients treated with placebo. This was not statistically significant in the original trial report [33], but reached a significance level of p = 0.03 (not corrected for multiple comparisons) in a post hoc analysis where the baseline status of many patients had been reclassified [38, 39]. It was also suggested that oral prednisone treatment was associated with an increased risk of recurrent optic neuritis, but not an increased risk of subsequently developing MS [33, 38]. As there was no blinding to methylprednisolone treatment, and as the effect of treatment on MS risk was only observed after reanalysis and reclassification of the initial data, this part of the ONTT must be regarded as a Class III study. Another Class III study (a retrospective natural history study) has suggested that i.v. methylprednisolone treatment (1 g once daily for 3 days) could actually increase the risk of subsequently developing MS [40]. In the latter study, a surprisingly low risk of conversion to MS was, however, observed in the control group of untreated patients and patients treated with oral prednisone. Glucocorticoid t reatment in MS s ubgroups Whether subgroups of patients with MS relapses may benefit more from glucocorticoid treatment has been addressed in only a few studies. It has been suggested that patients with more severe relapses are more likely to respond to treatment with i.v. methylprednisolone (Class IV evidence) [41]. Another uncontrolled (Class IV) study suggested that patients with high cerebrospinal fluid (CSF) concentrations of myelin basic protein (MBP) are more likely to improve after i.v. methylprednisolone
5 CHAPTER 27 Acute relapses of multiple sclerosis 415 treatment [42]. This finding was confirmed using 1 - week follow - up data in a post hoc analysis of patients included in two randomized, placebo - controlled trials of oral high-dose methylprednisolone treatment [43]. However, the additional benefit of methylprednisolone treatment in patients with high CSF concentrations of MBP was not sustained at follow - up after 8 weeks, while patients who had an active gadolinium - enhanced MRI at baseline appeared to benefit from treatment even at follow - up after 8 weeks (Class III evidence) [43]. Side e ffects of g lucocorticoid t reatment In the placebo - controlled trials serious adverse events were not observed after high - dose methylprednisolone treatment [10, 13, 15]. Milligan et al. did not report the precise frequency of adverse events, but noted that treatment was surprisingly free from serious adverse events [13]. Those most frequently reported were a slight reddening of the face, transient ankle swelling, and a metallic taste in the mouth during infusion. In the Cochrane review it was concluded that the oral administration of methylprednisolone is associated with a higher frequency of side effects (mainly gastrointestinal and psychic disorders), and that oral administration should be avoided for this reason [16]. In the study of Durelli et al. the incidence of elevated mood and insomnia increased during the study from two out of 11 patients (18%) treated with intravenous high - dose methylprednisolone at day 5 to five out of 11 patients (45%) at day 15 [10]. In the study of oral high - dose treatment with an oral tapering dose and a total treatment duration of 15 days, disturbed sleep was observed in 65% and slight mood changes in 23% [15], which is not significantly different from the frequency observed by Durelli and coworkers. Durelli and coworkers (1986) did not report gastrointestinal side effects, but all patients received prophylactic antacid treatment. In the study of oral high - dose methylprednisolone treatment, gastrointestinal side effects (mainly heartburn not requiring symptomatic treatment) were observed in 38% of patients treated with oral methylprednisolone and 8% in the placebo group [15]. The randomized comparisons of i.v. and oral treatment with methylprednisolone at equivalent doses found that the side effects of oral and i.v. methylprednisolone treatment were similar [22, 25]. This is supported by the results of a smaller, non - randomized Class III study comparing treatment with i.v. methylprednisolone and oral prednisone at equivalent doses, which also failed to detect any difference in the side effects of oral and i.v. treatment [44]. In a review of 240 patients, who had been treated with one or more courses of i.v. methylprednisolone (1 g daily for 5 days followed by 10 days of oral prednisone treatment), minor infections were observed in four patients; one patient had a single seizure within 12 h of treatment, 11 patients were noted to have glucosuria during treatment, five had gastrointestinal symptoms that required antacid or H2 antagonist treatment, three patients had an exacerbation of acne, ankle oedema was recorded in two patients, and one patient was hypertensive during treatment. A feeling of wellbeing was common (frequency not given), and four patients had episodes of euphoria, whereas two patients were depressive. Transient facial flushing, a transient disturbance of taste, distal paraesthesia, insomnia, and mild weight gain occurred in a significant proportion of patients, but the exact frequency was not stated [45]. Severe side effects of methylprednisolone treatment are rare, but psychosis, acute pancreatitis, and anaphylactoid reactions to i.v. treatment have been reported [46 49]. Short-term methylprednisolone treatment in patients with MS appears to be safe in terms of long - term effects on bone mineralization, but pulsed methylprednisolone treatment has short - term effects on bone metabolism [50], and a recent study indicates that the risk of avascular bone necrosis after methylprednisolone treatment may not be negligible [51]. Other t reatments A single Class I crossover study of 22 patients with severe relapses of inflammatory demyelination (including 12 with MS) who were refractory to treatment with high - dose methylprednisolone suggested a beneficial effect of treatment with plasma exchange [52]. In this study there was moderate or marked improvement during plasma exchange treatment in eight out of 19 patients (42%), whereas such improvement was observed only after one out of 17 courses (6%) of sham treatment ( p = 0.01). Open (Class IV) studies have also reported an effect of plasma exchange in patients with acute optic neuritis who had not improved after high - dose i.v. methylprednisolone treatment [53, 54]. One study found that an effect of plasma exchange that was sustained after 6
6 416 SECTION 5 Neurological Problems months was more likely when treatment was initiated early and in patients who had shown improvement already at hospital discharge [55]. Intravenous immunoglobulin (IVIg) treatment is widely used in a variety of neurological diseases. A single Class IV study of intravenous IVIg treatment in relapses of MS suggested that as many as 68% of patients improved within 24 h of treatment [56]. Two studies have investigated if IVIg treatment as add - on to therapy with high - dose i.v. methylprednisolone is superior to add - on placebo treatment [57, 58]. Both studies were negative on primary and secondary end - points. A phase III study of IVIg treatment reported marked improvement in 23 patients with severe visual impairment after methylprednisolone treatment of optic neuritis, whereas there was no improvement in a control group comprising 24 patients treated with methylprednisolone only [59]. However, in this study spontaneous recovery in the control group was surprisingly poor, and a randomized Class I trial of treatment with IVIg or placebo in 68 patients with acute optic neuritis failed to detect any treatment effect [60]. Whereas treatment with natalizumab lowers the frequency of relapses in MS, natalizumab was not efficacious in the treatment of relapses in a randomized, placebo - controlled Class I study of 180 patients with an MS relapse [61]. A single Class II study compared the effect of multidisciplinary rehabilitation with the effect of standard therapy in a randomized clinical trial design, where both treatment arms received i.v. methylprednisolone treatment. The study suggested that a multidisciplinary team rehabilitation programme results in better functional recovery after 3 months than does treatment with i.v. methylprednisolone in a standard setting [62]. Treatment of r elapses d uring p regnancy There are no specific studies on relapse treatment in pregnant patients with MS, but short - term treatment with glucocorticoids is generally considered safe in pregnant women, and treatment may be considered in patients with a relapse of sufficient severity to warrant treatment, although treatment during the first trimester should probably be avoided (Class IV evidence [63] ). Recommendations There is consistent evidence from several Class I studies and meta - analyses for a beneficial effect of glucocorticoid treatment in relapses of MS. Hence, treatment with intravenous or oral methylprednisolone in a dose of at least 500 mg daily for 5 days should be considered for treatment of relapses (Level A). Treatment with i.v. methylprednisolone (1 g once daily for 3 days with an oral tapering dose) may be considered for treatment of acute optic neuritis (Level B). Treatment with i.v. methylprednisolone (1 g once daily for 3 days) should be considered as an alternative treatment (GPP; [8]). There is no evidence of major differences in the efficacy of methylprednisolone treatment given i.v. or orally in terms of clinical efficacy or side effects, but prolonged oral treatment may possibly be associated with a higher prevalence of side effects. Furthermore, due to the low number of patients included in the available clinical trials, some efficacy differences between the i.v. and oral route of administration cannot be excluded. The optimal dosage, the specific glucocorticoid to use, and whether to use a taper after initial pulse therapy, has not been adequately addressed in randomized controlled trials. This implies a need for new randomized studies assessing risk/ benefit ratios and adverse effects of specific glucocorticoids, dose, and route of administration for treatment of MS relapses. There is insufficient data to clearly define patient subgroups who are more likely to respond to methylprednisolone treatment, but treatment may be more efficacious in patients with clinical, MRI, or CSF evidence indicating higher disease activity (Level C recommendation). Home versus outpatient administration of i.v. steroids was evaluated in one clinical trial [64], and in one large French survey of home - based treatment of MS relapses with i.v. methylprednisolone [65]. The results of both studies indicate that treatment with i.v. steroids can be effectively and safely administered at home. Consideration may, however, sometimes be given to administering the first course of methylprednisolone as an inpatient (GPP). In patients who fail to respond to therapy with methylprednisolone in the dose range used in the randomized, placebo - controlled trials [10, 11, 13, 15], treatment with higher doses (up to 2 g daily for 5 days) should be considered (Level C recommendation [8] ).
7 CHAPTER 27 Acute relapses of multiple sclerosis 417 There is insufficient data to support the use of IVIg therapy as monotherapy for relapses of MS. Treatment with IVIg as an add - on to treatment of MS relapses with methylprednisolone or as monotherapy for acute optic neuritis is not efficacious (Level A recommendation). Neither is natalizumab as monotherapy efficacious in MS relapses. Patients with inflammatory demyelination, including patients with MS, who have not responded to treatment with methylprednisolone may benefit from plasma exchange treatment, but only about one - third of treated patients are likely to respond. This treatment regimen should probably be restricted to a subgroup of patients with severe relapses (Level B recommendation). A randomized controlled study specifically addressing the effect of plasma exchange and IVIg treatment in patients with severe relapses of MS not responding to methylprednisolone treatment would be desirable. A more intense, interdisciplinary rehabilitation programme should be considered after treatment with i.v. methylprednisolone as evidence from a single trial suggests that this probably further improves recovery (Level B recommendation). Conflicts of i nterest Authors of this chapter have received the following: honoraria for lecturing, serving on advisory councils or trial steering committees; covering of travel expenses for attending meetings; or research grants from Almirall, Bayer Schering Pharma, Biogen Idec, EMD, Genentech, GenMab, Genzyme, Lundbeck, Merck Serono, Novartis, Novo Nordisk, Sanofi-aventis or Teva. References 1. Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Engl J Med 2000 ;343 (20 ): Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996 ;46 (4 ): McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001 ;50 (1 ): Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the McDonald Criteria. Ann Neurol 2005 ;58 (6 ): Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain 2003 ;126 ( Pt 4 ): Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology 2003 ;61 (11 ): Goodin DS, Frohman EM, Garmany GP Jr, et al. Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology 2002 ;58 (2 ): Rieckmann P, Toyka KV, Bassetti C, et al. Escalating immunotherapy of multiple sclerosis new aspects and practical application. J Neurol 2004 ;251 (11 ): Brainin M, Barnes M, Baron JC, et al. Guidance for the preparation of neurological management guidelines by EFNS scientific task forces revised recommendations Eur J Neurol 2004 ;11 (9 ): Durelli L, Cocito D, Riccio A, et al. High-dose intravenous methylprednisolone in the treatment of multiple sclerosis: clinical-immunologic correlations. Neurology 1986 ;36 (2 ): Filipovic SR, Drulovic J, Stojsavljevic N, Levic Z. The effects of high - dose intravenous methylprednisolone on event - related potentials in patients with multiple sclerosis. J Neurol Sci 1997 ;152 (2 ): Miller H, Newell DJ, Ridley A. Multiple sclerosis. Treatment of acute exacerbations with corticotrophin (A.C.T.H.). Lancet 1961 ;2 (7212 ): Milligan NM, Newcombe R, Compston DA. A double-blind controlled trial of high dose methylprednisolone in patients with multiple sclerosis: 1. Clinical effects. J Neurol Neurosurg Psychiatry 1987 ;50 (5 ): Rose AS, Kuzma JW, Kurtzke JF, Namerow NS, Sibley WA, Tourtellotte WW. Cooperative study in the evaluation of therapy in multiple sclerosis. ACTH vs. placebo final report. Neurology 1970 ;20 (5 ): Sellebjerg F, Frederiksen JL, Nielsen PM, Olesen J. Doubleblind, randomized, placebo - controlled study of oral, high - dose methylprednisolone in attacks of MS. Neurology 1998 ;51 (2 ): Filippini G, Brusaferri F, Sibley WA et al. Corticosteroids or ACTH for acute exacerbations in multiple sclerosis. Cochrane Database Syst Rev 2000 ;(4 ):CD Brusaferri F, Candelise L. Steroids for multiple sclerosis and optic neuritis: a meta - analysis of randomized controlled clinical trials. J Neurol 2000 ;247 (6 ):
8 418 SECTION 5 Neurological Problems 18. Abbruzzese G, Gandolfo C, Loeb C. Bolus methylprednisolone versus ACTH in the treatment of multiple sclerosis. Ital J Neurol Sci 1983 ;4 (2 ): Barnes MP, Bateman DE, Cleland PG, et al. Intravenous methylprednisolone for multiple sclerosis in relapse. J Neurol Neurosurg Psychiatry 1985 ;48 (2 ): Thompson AJ, Kennard C, Swash M, et al. Relative efficacy of intravenous methylprednisolone and ACTH in the treatment of acute relapse in MS. Neurology 1989 ;39 (7 ): Miller DM, Weinstock - Guttman B, Bethoux F, et al. A meta - analysis of methylprednisolone in recovery from multiple sclerosis exacerbations. Mult Scler 2000 ;6 (4 ): Alam SM, Kyriakides T, Lawden M, Newman PK. Methylprednisolone in multiple sclerosis: a comparison of oral with intravenous therapy at equivalent high dose. J Neurol Neurosurg Psychiatry 1993 ;56 (11 ): Barnes D, Hughes RA, Morris RW, et al. Randomised trial of oral and intravenous methylprednisolone in acute relapses of multiple sclerosis. Lancet 1997 ;349 (9056 ): Sharrack B, Hughes RA, Morris RW, et al. The effect of oral and intravenous methylprednisolone treatment on subsequent relapse rate in multiple sclerosis. J Neurol Sci 2000 ;173 (1 ): Martinelli V, Rocca MA, Annovazzi P, et al. A short-term randomized MRI study of high - dose oral vs intravenous methylprednisolone in MS. Neurology 2009 ;73 (22 ): Burton JM, O Connor PW, Hohol M, Beyene J. Oral versus intravenous steroids for treatment of relapses in multiple sclerosis. Cochrane Database Syst Rev 2009 ;(3 ):CD Perumal JS, Caon C, Hreha S, et al. Oral prednisone taper following intravenous steroids fails to improve disability or recovery from relapses in multiple sclerosis. Eur J Neurol 2008 ;15 (7 ): Bindoff L, Lyons PR, Newman PK, Saunders M. Methylprednisolone in multiple sclerosis: a comparative dose study. J Neurol Neurosurg Psychiatry 1988 ;51 (8 ): Oliveri RL, Valentino P, Russo C, et al. Randomized trial comparing two different high doses of methylprednisolone in MS: a clinical and MRI study. Neurology 1998 ;50 (6 ): Fierro B, Salemi G, Brighina F, et al. A transcranial magnetic stimulation study evaluating methylprednisolone treatment in multiple sclerosis. Acta Neurol Scand 2002 ;105 (3 ): La Mantia L, Eoli M, Milanese C, Salmaggi A, Dufour A, Torri V. Double-blind trial of dexamethasone versus methylprednisolone in multiple sclerosis acute relapses. Eur Neurol 1994 ;34 (4 ): Milanese C, La ML, Salmaggi A, et al. Double-blind randomized trial of ACTH versus dexamethasone versus methylprednisolone in multiple sclerosis bouts. Clinical, cerebrospinal fluid and neurophysiological results. Eur Neurol 1989 ;29 (1 ): Beck RW, Cleary PA, Anderson MM Jr, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The Optic Neuritis Study Group. N Engl J Med 1992 ;326 (9 ): Beck RW, Cleary PA. Optic neuritis treatment trial. One-year follow-up results. Arch Ophthalmol 1993 ;111 (6 ): Trauzettel-Klosinski S, Axman D, Diener HC. The Tübingen study on optic neuritis a prospective, randomized and controlled trial. Clin. Vis. Sci : Sellebjerg F, Nielsen HS, Frederiksen JL, Olesen J. A randomized, controlled trial of oral high - dose methylprednisolone in acute optic neuritis. Neurology 1999 ;52 (7 ): Kapoor R, Miller DH, Jones SJ, et al. Effects of intravenous methylprednisolone on outcome in MRI - based prognostic subgroups in acute optic neuritis. Neurology 1998 ;50 (1 ): Beck RW, Cleary PA, Trobe JD, et al. The effect of corticosteroids for acute optic neuritis on the subsequent development of multiple sclerosis. The Optic Neuritis Study Group. N Engl J Med 1993 ;329 (24 ): Goodin DS. Perils and pitfalls in the interpretation of clinical trials: a reflection on the recent experience in multiple sclerosis. Neuroepidemiology 1999 ;18 (2 ): Herishanu YO, Badarna S, Sarov B, Abarbanel JM, Segal S, Bearman JE. A possible harmful late effect of methylprednisolone therapy on a time cluster of optic neuritis. Acta Neurol Scand 1989 ;80 (6 ): Nos C, Sastre-Garriga J, Borras C, Rio J, Tintore M, Montalban X. Clinical impact of intravenous methylprednisolone in attacks of multiple sclerosis. Mult Scler 2004 ; 10 (4 ): Whitaker JN, Layton BA, Herman PK, Kachelhofer RD, Burgard S, Bartolucci AA. Correlation of myelin basic protein - like material in cerebrospinal fluid of multiple sclerosis patients with their response to glucocorticoid treatment. Ann Neurol 1993 ;33 (1 ): Sellebjerg F, Jensen CV, Larsson HB, Frederiksen JL. Gadolinium - enhanced magnetic resonance imaging predicts response to methylprednisolone in multiple sclerosis. Mult Scler 2003 ;9 (1 ): Metz LM, Sabuda D, Hilsden RJ, Enns R, Meddings JB. Gastric tolerance of high - dose pulse oral prednisone in multiple sclerosis. Neurology 1999 ;53 (9 ): Lyons PR, Newman PK, Saunders M. Methylprednisolone therapy in multiple sclerosis: a profile of adverse effects. J Neurol Neurosurg Psychiatry 1988 ;51 (2 ): Chrousos GA, Kattah JC, Beck RW, Cleary PA. Side effects of glucocorticoid treatment. Experience of the
9 CHAPTER 27 Acute relapses of multiple sclerosis 419 Optic Neuritis Treatment Trial. JAMA 1993 ;269 (16 ): Deruaz CA, Spertini F, Souza LF, Du Pasquier RA, Schluep M. Anaphylactic reaction to methylprednisolone in multiple sclerosis: a practical approach to alternative corticosteroids. Mult Scler 2007 ;13 (4 ): Pryse - Phillips WE, Chandra RK, Rose B. Anaphylactoid reaction to methylprednisolone pulsed therapy for multiple sclerosis. Neurology 1984 ;34 (8 ): van den Berg JS, van Eikema Hommes OR, Wuis EW, Stapel S, van der Valk PG. Anaphylactoid reaction to intravenous methylprednisolone in a patient with multiple sclerosis. J Neurol Neurosurg Psychiatry 1997 ; 63 (6 ): Dovio A, Perazzolo L, Osella G, et al. Immediate fall of bone formation and transient increase of bone resorption in the course of high - dose, short - term glucocorticoid therapy in young patients with multiple sclerosis. J Clin Endocrinol Metab 2004 ;89 (10 ): Ce P, Gedizlioglu M, Gelal F, Coban P, Ozbek G. Avascular necrosis of the bones: an overlooked complication of pulse steroid treatment of multiple sclerosis. Eur J Neurol 2006 ;13 (8 ): Weinshenker BG, O Brien PC, Petterson TM, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol 1999 ;46 (6 ): Ruprecht K, Klinker E, Dintelmann T, Rieckmann P, Gold R. Plasma exchange for severe optic neuritis: treatment of 10 patients. Neurology 2004 ;63 (6 ): Trebst C, Reising A, Kielstein JT, Hafer C, Stangel M. Plasma exchange therapy in steroid - unresponsive relapses in patients with multiple sclerosis. Blood Purif 2009 ;28 (2 ): Llufriu S, Castillo J, Blanco Y, et al. Plasma exchange for acute attacks of CNS demyelination: predictors of improvement at 6 months. Neurology 2009 ;73 (12 ): Soukop W, Tschabitscher H. Gamma globulin therapy in multiple sclerosis. Theoretical considerations and initial clinical experiences with 7S immunoglobulins in MS therapy. Wien Med Wochenschr 1986 ;136 (18 ): S ø rensen PS, Haas J, Sellebjerg F, Olsson T, Ravnborg M. IV immunoglobulins as add - on treatment to methylprednisolone for acute relapses in MS. Neurology 2004 ;63 (11 ): Visser LH, Beekman R, Tijssen CC, et al. A randomized, double - blind, placebo - controlled pilot study of i.v. immune globulins in combination with i. v. methylprednisolone in the treatment of relapses in patients with MS. Mult Scler 2004 ;10 (1 ): Tselis A, Perumal J, Caon C, et al. Treatment of corticosteroid refractory optic neuritis in multiple sclerosis patients with intravenous immunoglobulin. Eur J Neurol 2008 ; 15 (11 ): Roed HG, Langkilde A, Sellebjerg F, et al. A double-blind, randomized trial of IV immunoglobulin treatment in acute optic neuritis. Neurology 2005 ;64 (5 ): O Connor PW, Goodman A, Willmer - Hulme AJ, et al. Randomized multicenter trial of natalizumab in acute MS relapses: clinical and MRI effects. Neurology 2004 ;62 (11 ): Craig J, Young CA, Ennis M, Baker G, Boggild M. A randomised controlled trial comparing rehabilitation against standard therapy in multiple sclerosis patients receiving intravenous steroid treatment. J Neurol Neurosurg Psychiatry 2003 ;74 (9 ): Ferrero S, Pretta S, Ragni N. Multiple sclerosis: management issues during pregnancy. Eur J Obstet Gynecol Reprod Biol 2004 ;115 (1 ): Chataway J, Porter B, Riazi A, et al. Home versus outpatient administration of intravenous steroids for multiple - sclerosis relapses: a randomised controlled trial. Lancet Neurol 2006 ;5 (7 ): Creange A, Debouverie M, Jaillon-Riviere V, et al. Home administration of intravenous methylprednisolone for multiple sclerosis relapses: the experience of French multiple sclerosis networks. Mult Scler 2009 ;15 (9 ):
RELAPSE MANAGEMENT. Pauline Shaw MS Nurse Specialist 25 th June 2010
 RELAPSE MANAGEMENT Pauline Shaw MS Nurse Specialist 25 th June 2010 AIMS OF SESSION Relapsing/Remitting MS Definition of relapse/relapse rate Relapse Management NICE Guidelines Regional Clinical Guidelines
RELAPSE MANAGEMENT Pauline Shaw MS Nurse Specialist 25 th June 2010 AIMS OF SESSION Relapsing/Remitting MS Definition of relapse/relapse rate Relapse Management NICE Guidelines Regional Clinical Guidelines
PROPOSED REVISIONS TO THE CRITERIA. multiple sclerosis (RRMS)] Chapter 6 6 It is recognised that use is in only exceptional circumstances in RRMS.
![PROPOSED REVISIONS TO THE CRITERIA. multiple sclerosis (RRMS)] Chapter 6 6 It is recognised that use is in only exceptional circumstances in RRMS. PROPOSED REVISIONS TO THE CRITERIA. multiple sclerosis (RRMS)] Chapter 6 6 It is recognised that use is in only exceptional circumstances in RRMS.](/thumbs/33/16408576.jpg) Specialist Working Group for Neurology Proposed changes to the Criteria for the clinical use of intravenous immunoglobulin in Australia, Second Edition ITEM Condition Name Multiple Sclerosis (MS) Multiple
Specialist Working Group for Neurology Proposed changes to the Criteria for the clinical use of intravenous immunoglobulin in Australia, Second Edition ITEM Condition Name Multiple Sclerosis (MS) Multiple
Treatment Optimization in MS: When to Start, When to Shift, when to Stop
 Treatment Optimization in MS: When to Start, When to Shift, when to Stop Mark S. Freedman MSc MD FAAN FANA FRCPC Director, Multiple Sclerosis Research Unit University of Ottawa Sr. Scientist, Ottawa Hospital
Treatment Optimization in MS: When to Start, When to Shift, when to Stop Mark S. Freedman MSc MD FAAN FANA FRCPC Director, Multiple Sclerosis Research Unit University of Ottawa Sr. Scientist, Ottawa Hospital
Reversibility of Acute Demyelinating Lesions in relapsingremitting
 Reversibility of Acute Demyelinating Lesions in relapsingremitting Multiple Sclerosis Omar A. Khan ( Division of Neuroimmunology, Department of Neurology, Neurology and Research Services. Veterans Affairs
Reversibility of Acute Demyelinating Lesions in relapsingremitting Multiple Sclerosis Omar A. Khan ( Division of Neuroimmunology, Department of Neurology, Neurology and Research Services. Veterans Affairs
Disclosures. Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
 Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Gold R, Giovannoni G, Selmaj K, et al, for
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Gold R, Giovannoni G, Selmaj K, et al, for
Medication Policy Manual. Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012
 Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru283 Topic: Aubagio, teriflunomide Date of Origin: November 9, 2012 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON
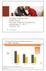 Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Multiple Sclerosis (MS) Aprile Royal, Novartis Pharma Canada Inc. September 21, 2011 Toronto, ON First-line DMTs Reduce Relapse Frequency by ~30% vs. Placebo Frequency of relapse with various DMTs, based
Committee Approval Date: December 12, 2014 Next Review Date: December 2015
 Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
Medication Policy Manual Policy No: dru299 Topic: Tecfidera, dimethyl fumarate Date of Origin: May 16, 2013 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective Date: January
TITLE: Treatment of Patients with Multiple Sclerosis: A Review of Guidelines
 TITLE: Treatment of Patients with Multiple Sclerosis: A Review of Guidelines DATE: 13 March 2013 CONTEXT AND POLICY ISSUES Multiple sclerosis (MS) is an unpredictable, often disabling disease of the central
TITLE: Treatment of Patients with Multiple Sclerosis: A Review of Guidelines DATE: 13 March 2013 CONTEXT AND POLICY ISSUES Multiple sclerosis (MS) is an unpredictable, often disabling disease of the central
Medication Policy Manual. Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004
 Medication Policy Manual Policy No: dru108 Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective
Medication Policy Manual Policy No: dru108 Topic: Betaseron, Extavia, interferon beta-1b Date of Origin: June 18, 2004 Committee Approval Date: December 12, 2014 Next Review Date: December 2015 Effective
Medication Policy Manual. Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014
 Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
Medication Policy Manual Policy No: dru376 Topic: Plegridy, peginterferon beta-1a Date of Origin: December 12, 2014 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective
ß-interferon and. ABN Guidelines for 2007 Treatment of Multiple Sclerosis with. Glatiramer Acetate
 ABN Guidelines for 2007 Treatment of Multiple Sclerosis with ß-interferon and Glatiramer Acetate Published by the Association of British Neurologists Ormond House, 27 Boswell Street, London WC1N 3JZ Contents
ABN Guidelines for 2007 Treatment of Multiple Sclerosis with ß-interferon and Glatiramer Acetate Published by the Association of British Neurologists Ormond House, 27 Boswell Street, London WC1N 3JZ Contents
Intravenous Methyl Prednisolone in Multiple Sclerosis
 Intravenous Methyl Prednisolone in Multiple Sclerosis Exceptional healthcare, personally delivered Relapse management in multiple sclerosis Relapses in multiple sclerosis (MS) are common and caused by
Intravenous Methyl Prednisolone in Multiple Sclerosis Exceptional healthcare, personally delivered Relapse management in multiple sclerosis Relapses in multiple sclerosis (MS) are common and caused by
Steroids. What are steroids?
 Steroids What are steroids? Also known as corticosteroids and glucocorticoids, steroids are hormones that are normally produced by the adrenal glands. They are mainly used in multiple sclerosis (MS) because
Steroids What are steroids? Also known as corticosteroids and glucocorticoids, steroids are hormones that are normally produced by the adrenal glands. They are mainly used in multiple sclerosis (MS) because
Dr. Morrow has no disclosures relevant to this presentation.
 Sarah A. Morrow M.D., M.S., FRCPC Assistant Professor of Neurology Western University London, ON CANADA Dr. Morrow has no disclosures relevant to this presentation. 1 Treatment of relapses Cognitive relapses
Sarah A. Morrow M.D., M.S., FRCPC Assistant Professor of Neurology Western University London, ON CANADA Dr. Morrow has no disclosures relevant to this presentation. 1 Treatment of relapses Cognitive relapses
Version History. Previous Versions. Policy Title. Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields
Version History Policy Title Drugs for MS.Drug facts box Glatiramer Acetate Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields
Oral versus intravenous steroids for treatment of relapses in multiple sclerosis (Review)
 Oral versus intravenous steroids for treatment of relapses in multiple sclerosis (Review) Burton JM, O Connor PW, Hohol M, Beyene J This is a reprint of a Cochrane review, prepared and maintained by The
Oral versus intravenous steroids for treatment of relapses in multiple sclerosis (Review) Burton JM, O Connor PW, Hohol M, Beyene J This is a reprint of a Cochrane review, prepared and maintained by The
Natural history of multiple sclerosis: risk factors and prognostic indicators Sandra Vukusic a,b,c,d and Christian Confavreux a,b,c,d
 Natural history of multiple sclerosis: risk factors and prognostic indicators Sandra Vukusic a,b,c,d and Christian Confavreux a,b,c,d Purpose of review To highlight progress in the description of the natural
Natural history of multiple sclerosis: risk factors and prognostic indicators Sandra Vukusic a,b,c,d and Christian Confavreux a,b,c,d Purpose of review To highlight progress in the description of the natural
Version History. Previous Versions. for secondary progressive MS (SPMS) Policy Title. Drugs for MS.Drug facts box Interferon beta 1b
 Version History Policy Title Drugs for MS.Drug facts box Interferon beta 1b for secondary progressive MS (SPMS) Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review
Version History Policy Title Drugs for MS.Drug facts box Interferon beta 1b for secondary progressive MS (SPMS) Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review
How To Use A Drug In Multiple Sclerosis
 Revised (2009) guidelines for prescribing in multiple sclerosis INTRODUCTION In January 2001, the (ABN) first published guidelines for the use of licensed disease modifying treatments (ß-interferon and
Revised (2009) guidelines for prescribing in multiple sclerosis INTRODUCTION In January 2001, the (ABN) first published guidelines for the use of licensed disease modifying treatments (ß-interferon and
Mariusz Stasiołek Neurology Department Polish Mother s Memorial Hospital Research Institute, Lodz
 Therapeutic Plasma Exchange in Multiple Sclerosis Relapses Mariusz Stasiołek Neurology Department Polish Mother s Memorial Hospital Research Institute, Lodz MS heterogeneity Multiple Sclerosis differences
Therapeutic Plasma Exchange in Multiple Sclerosis Relapses Mariusz Stasiołek Neurology Department Polish Mother s Memorial Hospital Research Institute, Lodz MS heterogeneity Multiple Sclerosis differences
Accuracy in Space and Time: Diagnosing Multiple Sclerosis. 2012 Genzyme Corporation, a Sanofi company.
 Accuracy in Space and Time: Diagnosing Multiple Sclerosis 2012 Genzyme Corporation, a Sanofi company. Brought All rights to reserved. you by www.msatrium.com, MS.US.PO876.1012 your gateway to MS knowledge.
Accuracy in Space and Time: Diagnosing Multiple Sclerosis 2012 Genzyme Corporation, a Sanofi company. Brought All rights to reserved. you by www.msatrium.com, MS.US.PO876.1012 your gateway to MS knowledge.
acquired chronic immune-mediated inflammatory condition of CNS. MS in children: 10% +secondary progressive MS: rare +primary progressive MS: rare
 Immunomodulatory Therapies in Pediatric MS Vuong Chinh Quyen Neurology Department Medscape Mar 8, 2013 Multiple Sclerosis in Children. Iran J Child Neurol. 2013 Spring Introduction acquired chronic immune-mediated
Immunomodulatory Therapies in Pediatric MS Vuong Chinh Quyen Neurology Department Medscape Mar 8, 2013 Multiple Sclerosis in Children. Iran J Child Neurol. 2013 Spring Introduction acquired chronic immune-mediated
Multiple sclerosis (MS) is a leading cause of nontraumatic
 REVIEW ARTICLE Ben W. Thrower, MD Abstract: Relapses, exacerbations, and attacks are synonymous for new or worsened neurologic symptoms that are the hallmark of relapsing-remitting multiple sclerosis.
REVIEW ARTICLE Ben W. Thrower, MD Abstract: Relapses, exacerbations, and attacks are synonymous for new or worsened neurologic symptoms that are the hallmark of relapsing-remitting multiple sclerosis.
A blood sample will be collected annually for up to 2 years for JCV antibody testing.
 Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
Mellen Center Currently Enrolling Non-Treatment Trials STRATIFY-2 JCV Antibody Program in Patients with Relapsing Multiple Sclerosis Receiving or Considering Treatment with Tysabri Primary Investigator:
Version History. Previous Versions. Drugs for MS.Drug facts box fampridine Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box fampridine Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Version History Policy Title Drugs for MS.Drug facts box fampridine Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Treatment in Relapsing MS: Choosing Among the Options. Donald Negroski, MD
 Treatment in Relapsing MS: Choosing Among the Options Donald Negroski, MD Disclosures Research Grants Educational activities and lectures Consulting or other services including Continuing Medical Education
Treatment in Relapsing MS: Choosing Among the Options Donald Negroski, MD Disclosures Research Grants Educational activities and lectures Consulting or other services including Continuing Medical Education
Acute demyelinating optic neuritis Rod Foroozan, MD, Lawrence M. Buono, MD, Peter J. Savino, MD, and Robert C. Sergott, MD
 Acute demyelinating optic neuritis Rod Foroozan, MD, Lawrence M. Buono, MD, Peter J. Savino, MD, and Robert C. Sergott, MD Acute demyelinating optic neuritis associated with multiple sclerosis (MS) is
Acute demyelinating optic neuritis Rod Foroozan, MD, Lawrence M. Buono, MD, Peter J. Savino, MD, and Robert C. Sergott, MD Acute demyelinating optic neuritis associated with multiple sclerosis (MS) is
Issues Regarding Use of Placebo in MS Drug Trials. Peter Scott Chin, MD Novartis Pharmaceuticals Corporation
 Issues Regarding Use of Placebo in MS Drug Trials Peter Scott Chin, MD Novartis Pharmaceuticals Corporation Context of the Guidance The draft EMA Guidance mentions placebo as a comparator for superiority
Issues Regarding Use of Placebo in MS Drug Trials Peter Scott Chin, MD Novartis Pharmaceuticals Corporation Context of the Guidance The draft EMA Guidance mentions placebo as a comparator for superiority
Version History. Previous Versions. Drugs for MS.Drug facts box fingolimod Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Version History Policy Title Drugs for MS.Drug facts box fingolimod Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further fields as required
Managing Relapsing Remitting MS Risks & benefits of emerging therapies. Dr Mike Boggild The Walton Centre
 Managing Relapsing Remitting MS Risks & benefits of emerging therapies Dr Mike Boggild The Walton Centre MS: Facts and figures Affects 1 in 800 in the UK Commonest cause of acquired neurological disability
Managing Relapsing Remitting MS Risks & benefits of emerging therapies Dr Mike Boggild The Walton Centre MS: Facts and figures Affects 1 in 800 in the UK Commonest cause of acquired neurological disability
Natalizumab (Tysabri)
 Natalizumab (Tysabri) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 Natalizumab (Tysabri) Date of issue: July 2010 Review date: July 2011 Contents Section
Natalizumab (Tysabri) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 Natalizumab (Tysabri) Date of issue: July 2010 Review date: July 2011 Contents Section
Using the MS Clinical Course Descriptions in Clinical Practice
 Using the MS Clinical Course Descriptions in Clinical Practice Mark J. Tullman, MD Director of Clinical Research The MS Center for Innovations in Care Missouri Baptist Medical Center Disclosures Consultant/speaking
Using the MS Clinical Course Descriptions in Clinical Practice Mark J. Tullman, MD Director of Clinical Research The MS Center for Innovations in Care Missouri Baptist Medical Center Disclosures Consultant/speaking
Laquinimod Polman, C. et al. Neurology 2005;64:987-991
 Laquinimod Polman, C. et al. Neurology 2005;64:987-991 Multicenter, double-blind, randomized trial, patients with RR MS received 0.1 mg or 0.3 mg laquinimod or placebo as three daily tablets for 24 weeks
Laquinimod Polman, C. et al. Neurology 2005;64:987-991 Multicenter, double-blind, randomized trial, patients with RR MS received 0.1 mg or 0.3 mg laquinimod or placebo as three daily tablets for 24 weeks
Summary HTA. Interferons and Natalizumab for Multiple Sclerosis Clar C, Velasco-Garrido M, Gericke C. HTA-Report Summary
 Summary HTA HTA-Report Summary Interferons and Natalizumab for Multiple Sclerosis Clar C, Velasco-Garrido M, Gericke C Health policy background Multiple sclerosis (MS) is a chronic inflammatory disease
Summary HTA HTA-Report Summary Interferons and Natalizumab for Multiple Sclerosis Clar C, Velasco-Garrido M, Gericke C Health policy background Multiple sclerosis (MS) is a chronic inflammatory disease
Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple Sclerosis
 Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
Your contact News Release Barbara Fry Phone +1 905 919 0163 April 29/30, 2009 Two-Year Phase III Data Presented at AAN 61st Annual Meeting Show Positive Outcome of Cladribine Tablets in Patients with Multiple
Drugs for MS.Drug fact box cannabis extract (Sativex) Version 1.0 Author
 Version History Policy Title Drugs for MS.Drug fact box cannabis extract (Sativex) Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further
Version History Policy Title Drugs for MS.Drug fact box cannabis extract (Sativex) Version 1.0 Author West Midlands Commissioning Support Unit Publication Date Jan 2013 Review Date Supersedes/New (Further
The Nuts and Bolts of Multiple Sclerosis. Rebecca Milholland, M.D., Ph.D. Center for Neurosciences
 The Nuts and Bolts of Multiple Sclerosis Rebecca Milholland, M.D., Ph.D. Center for Neurosciences Objectives Discuss which patients are at risk for Multiple Sclerosis Discuss the diagnostic criteria for
The Nuts and Bolts of Multiple Sclerosis Rebecca Milholland, M.D., Ph.D. Center for Neurosciences Objectives Discuss which patients are at risk for Multiple Sclerosis Discuss the diagnostic criteria for
Ulf Kallweit, MD; Ilijas Jelcic, MD; Nathalie Braun, MD, PhD; Heike Fischer, MD; Björn Zörner,
 Sustained efficacy of Natalizumab in the treatment of relapsing-remitting multiple sclerosis independent of disease activity and disability at baseline: real-life data from a Swiss cohort Ulf Kallweit,
Sustained efficacy of Natalizumab in the treatment of relapsing-remitting multiple sclerosis independent of disease activity and disability at baseline: real-life data from a Swiss cohort Ulf Kallweit,
Original Policy Date
 MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
MP 5.01.20 Tysabri (natalizumab) Medical Policy Section Prescription Drug Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Local Policy/12:2013 Return to Medical Policy Index Disclaimer
Clinically isolated syndrome (CIS)
 Clinically isolated syndrome (CIS) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 We hope you find the information in this factsheet helpful. If you would
Clinically isolated syndrome (CIS) Spirella Building, Letchworth, SG6 4ET 01462 476700 www.mstrust.org.uk reg charity no. 1088353 We hope you find the information in this factsheet helpful. If you would
Clinical features. Chapter 2. Clinical manifestations. Course
 Chapter 2 Clinical features Clinical manifestations The wide range of symptoms and signs of multiple sclerosis (MS) reflect multifocal lesions in the central nervous system (CNS), including in the afferent
Chapter 2 Clinical features Clinical manifestations The wide range of symptoms and signs of multiple sclerosis (MS) reflect multifocal lesions in the central nervous system (CNS), including in the afferent
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
Treatment guidelines for relapsing MS and the two step approach for disease modifying therapy
 Treatment guidelines for relapsing MS and the two step approach for disease modifying therapy Klaus Schmierer, PhD FRCP Blizard Institute, Barts and The London School of Medicine & Dentistry Barts Health
Treatment guidelines for relapsing MS and the two step approach for disease modifying therapy Klaus Schmierer, PhD FRCP Blizard Institute, Barts and The London School of Medicine & Dentistry Barts Health
MS ECHO: Update on MS treatment. Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology 10 14 2015
 MS ECHO: Update on MS treatment Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology 10 14 2015 Conflict of Interest Dr. Stobbe has no conflicts of interest to disclose
MS ECHO: Update on MS treatment Gary Stobbe, MD Medical Director, MS Project ECHO Clinical Assistant Professor, UW Neurology 10 14 2015 Conflict of Interest Dr. Stobbe has no conflicts of interest to disclose
Patient Sticker Multiple Sclerosis Ambulatory Emergency Care Pathway
 Multiple Sclerosis Ambulatory Emergency Care Pathway 1 Consultant: Dr M Oldfield Consultant: Dr D Harris Lead Nurse: Catie Paterson Ambulatory Emergency Care (AEC) Unit Patient From ED (Emergency Department)
Multiple Sclerosis Ambulatory Emergency Care Pathway 1 Consultant: Dr M Oldfield Consultant: Dr D Harris Lead Nurse: Catie Paterson Ambulatory Emergency Care (AEC) Unit Patient From ED (Emergency Department)
IV immunoglobulins as add-on treatment to methylprednisolone for acute relapses in MS
 IV immunoglobulins as add-on treatment to methylprednisolone for acute relapses in MS P. Soelberg Sorensen, MD, DMSc; J. Haas, MD; F. Sellebjerg, MD, DMSc; T. Olsson, MD; M. Ravnborg, MD, DMSc; and the
IV immunoglobulins as add-on treatment to methylprednisolone for acute relapses in MS P. Soelberg Sorensen, MD, DMSc; J. Haas, MD; F. Sellebjerg, MD, DMSc; T. Olsson, MD; M. Ravnborg, MD, DMSc; and the
Medication Policy Manual. Topic: Gilenya, fingolimod Date of Origin: November 22, 2010
 Medication Policy Manual Policy No: dru229 Topic: Gilenya, fingolimod Date of Origin: November 22, 2010 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Medication Policy Manual Policy No: dru229 Topic: Gilenya, fingolimod Date of Origin: November 22, 2010 Committee Approval Date: December 11, 2015 Next Review Date: December 2016 Effective Date: January
Understanding Relapse in Multiple Sclerosis. A guide for people with MS and their families
 Understanding Relapse in Multiple Sclerosis A guide for people with MS and their families Introduction You have been given this booklet because you have been diagnosed with Multiple Sclerosis (MS) and
Understanding Relapse in Multiple Sclerosis A guide for people with MS and their families Introduction You have been given this booklet because you have been diagnosed with Multiple Sclerosis (MS) and
The submission positioned dimethyl fumarate as a first-line treatment option.
 Product: Dimethyl Fumarate, capsules, 120 mg and 240 mg, Tecfidera Sponsor: Biogen Idec Australia Pty Ltd Date of PBAC Consideration: July 2013 1. Purpose of Application The major submission sought an
Product: Dimethyl Fumarate, capsules, 120 mg and 240 mg, Tecfidera Sponsor: Biogen Idec Australia Pty Ltd Date of PBAC Consideration: July 2013 1. Purpose of Application The major submission sought an
Multiple Sclerosis (MS) Class Update
 Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Multiple Sclerosis (MS) Class Update Month/Year of
Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119 Multiple Sclerosis (MS) Class Update Month/Year of
New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents
 New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents William Tyor, M.D. Chief, Neurology Atlanta VA Medical Center Professor, Department of Neurology Emory University School of Medicine
New and Emerging Immunotherapies for Multiple Sclerosis: Oral Agents William Tyor, M.D. Chief, Neurology Atlanta VA Medical Center Professor, Department of Neurology Emory University School of Medicine
AUBAGIO (teriflunomide) oral tablet
 AUBAGIO (teriflunomide) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
AUBAGIO (teriflunomide) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
CLINICALLY ISOLATED SYNDROME
 CLINICALLY ISOLATED SYNDROME: WHY TO TREAT? * Jacquelyn Bainbridge, PharmD ABSTRACT Clinically isolated syndrome (CIS) is the first symptomatic neurologic episode consistent with multiple sclerosis (MS).
CLINICALLY ISOLATED SYNDROME: WHY TO TREAT? * Jacquelyn Bainbridge, PharmD ABSTRACT Clinically isolated syndrome (CIS) is the first symptomatic neurologic episode consistent with multiple sclerosis (MS).
Media Release. Basel, 8 October 2015
 Media Release Basel, 8 October 2015 Roche s ocrelizumab first investigational medicine to show positive pivotal study results in both relapsing and primary progressive forms of multiple sclerosis Ocrelizumab
Media Release Basel, 8 October 2015 Roche s ocrelizumab first investigational medicine to show positive pivotal study results in both relapsing and primary progressive forms of multiple sclerosis Ocrelizumab
March 30, 2007. I. IVIg IS AN ACCEPTED THERAPY FOR RRMS, AND IS PRESCRIBED ALONG WITH A DISEASE-MODIFYING AGENT SUCH AS COPAXONE
 18 Timberline Drive Farmington, CT 06032 (860) 674-1370 (phone) (860) 674-1378 (fax) (860) 305-9835 (cell) www.advocacyforpatients.org [email protected] AZ Benefit Options ATTN: Appeals PO
18 Timberline Drive Farmington, CT 06032 (860) 674-1370 (phone) (860) 674-1378 (fax) (860) 305-9835 (cell) www.advocacyforpatients.org [email protected] AZ Benefit Options ATTN: Appeals PO
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
The role of focal white matter lesions on magnetic resonance
 ORIGINAL ARTICLE Treatment Effect on Brain Atrophy Correlates with Treatment Effect on Disability in Multiple Sclerosis Maria Pia Sormani, PhD, 1 Douglas L. Arnold, MD, 2 and Nicola De Stefano, MD 3 Objective:
ORIGINAL ARTICLE Treatment Effect on Brain Atrophy Correlates with Treatment Effect on Disability in Multiple Sclerosis Maria Pia Sormani, PhD, 1 Douglas L. Arnold, MD, 2 and Nicola De Stefano, MD 3 Objective:
How To Get Ivig To Treat Neuromyelitis Optica
 Aetna Customer Resolution Team PO Box 14002 Lexington, KY 40512 RE: Patient Type of service: IVIg Dates of service: To be determined (prior authorization) Dear Sir or Madam: I am writing on behalf of your
Aetna Customer Resolution Team PO Box 14002 Lexington, KY 40512 RE: Patient Type of service: IVIg Dates of service: To be determined (prior authorization) Dear Sir or Madam: I am writing on behalf of your
How To Treat Multiple Sclerosis
 presents The Treatment and Management of MS Exacerbations Live Webinar February 2, 2012 7 pm Eastern Guest Presenter Ben Thrower M.D. MD Andrew C. Carlos MS Institute Shepherd Center This program is made
presents The Treatment and Management of MS Exacerbations Live Webinar February 2, 2012 7 pm Eastern Guest Presenter Ben Thrower M.D. MD Andrew C. Carlos MS Institute Shepherd Center This program is made
fingolimod, 0.5mg, hard capsules (Gilenya ) SMC No. (992/14) Novartis Pharmaceuticals UK
 fingolimod, 0.5mg, hard capsules (Gilenya ) SMC No. (992/14) Novartis Pharmaceuticals UK 08 August 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
fingolimod, 0.5mg, hard capsules (Gilenya ) SMC No. (992/14) Novartis Pharmaceuticals UK 08 August 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
The Clinical Characteristics of Optic Neuritis in Korean Children
 pissn: 1011-8942 eissn: 2092-9382 Korean J Ophthalmol 2011;25(2):116-120 DOI: 10.3341/kjo.2011.25.2.116 The Clinical Characteristics of Optic Neuritis in Korean Children Original Article Dong Hyun Jo 1,2,
pissn: 1011-8942 eissn: 2092-9382 Korean J Ophthalmol 2011;25(2):116-120 DOI: 10.3341/kjo.2011.25.2.116 The Clinical Characteristics of Optic Neuritis in Korean Children Original Article Dong Hyun Jo 1,2,
Immunex Corporation Novantrone (Mitoxantrone HCL) P&CNS Advisory Committee Briefing Document. Page 020
 Page 020 4.0 Efficacy of Mitoxantrone in Multiple Sclerosis The efficacy of mitoxantrone in MS was demonstrated in two well-designed, randomized trials: Studies 901 and 902. The study design and efficacy
Page 020 4.0 Efficacy of Mitoxantrone in Multiple Sclerosis The efficacy of mitoxantrone in MS was demonstrated in two well-designed, randomized trials: Studies 901 and 902. The study design and efficacy
GENENTECH S OCRELIZUMAB FIRST INVESTIGATIONAL MEDICINE TO SHOW EFFICACY IN PEOPLE WITH PRIMARY PROGRESSIVE MULTIPLE SCLEROSIS IN LARGE PHASE III STUDY
 NEWS RELEASE Media Contact: Tara Iannuccillo (650) 467-6800 Investor Contacts: Stefan Foser Karl Mahler (650) 467-2016 011 41 61 687 8503 GENENTECH S OCRELIZUMAB FIRST INVESTIGATIONAL MEDICINE TO SHOW
NEWS RELEASE Media Contact: Tara Iannuccillo (650) 467-6800 Investor Contacts: Stefan Foser Karl Mahler (650) 467-2016 011 41 61 687 8503 GENENTECH S OCRELIZUMAB FIRST INVESTIGATIONAL MEDICINE TO SHOW
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Daclizumab for treating relapsing-remitting multiple Draft scope (pre-referral) Draft remit/appraisal objective To
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Daclizumab for treating relapsing-remitting multiple Draft scope (pre-referral) Draft remit/appraisal objective To
Which injectable medication should I take for relapsing-remitting multiple sclerosis?
 Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
Which injectable medication should I take for relapsing-remitting multiple sclerosis? A decision aid to discuss options with your doctor This decision aid is for you if you: Have multiple sclerosis Have
OHTAC Recommendation
 OHTAC Recommendation Multiple Sclerosis and Chronic Cerebrospinal Venous Insufficiency Presented to the Ontario Health Technology Advisory Committee in May 2010 May 2010 Issue Background A review on the
OHTAC Recommendation Multiple Sclerosis and Chronic Cerebrospinal Venous Insufficiency Presented to the Ontario Health Technology Advisory Committee in May 2010 May 2010 Issue Background A review on the
Relapsing-remitting multiple sclerosis Ambulatory with or without aid
 AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
AVONEX/BETASERON/COPAXONE/EXTAVIA/GILENYA/REBIF/TYSABRI Applicant must be covered on an Alberta Government sponsored drug program. Page 1 of 5 PATIENT INFMATION Surname First Name Middle Initial Sex Date
How To Treat Ms With Ifnb-1B
 Disease Modifying Therapies in Multiple Sclerosis Report of The Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and The MS Council for Clinical Practice Guidelines
Disease Modifying Therapies in Multiple Sclerosis Report of The Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and The MS Council for Clinical Practice Guidelines
J.P. Morgan Cazenove Therapeutic Seminar
 Jannan, MS J.P. Morgan Cazenove Therapeutic Seminar David Meeker - CEO, Genzyme June 25, 2012 Forward Looking Statements This presentation contains forward-looking statements as defined in the Private
Jannan, MS J.P. Morgan Cazenove Therapeutic Seminar David Meeker - CEO, Genzyme June 25, 2012 Forward Looking Statements This presentation contains forward-looking statements as defined in the Private
Study Design. Date: March 11, 2003 Reviewer: Jawahar Tiwari, Ph.D. Ellis Unger, M.D. Ghanshyam Gupta, Ph.D. Chief, Therapeutics Evaluation Branch
 BLA: STN 103471 Betaseron (Interferon β-1b) for the treatment of secondary progressive multiple sclerosis. Submission dated June 29, 1998. Chiron Corp. Date: March 11, 2003 Reviewer: Jawahar Tiwari, Ph.D.
BLA: STN 103471 Betaseron (Interferon β-1b) for the treatment of secondary progressive multiple sclerosis. Submission dated June 29, 1998. Chiron Corp. Date: March 11, 2003 Reviewer: Jawahar Tiwari, Ph.D.
Optic Neuritis. The optic nerve fibers are coated with myelin to help them conduct the electrical signals back to your brain.
 Optic Neuritis Your doctor thinks that you have had an episode of optic neuritis. This is the most common cause of sudden visual loss in a young patient. It is often associated with discomfort in or around
Optic Neuritis Your doctor thinks that you have had an episode of optic neuritis. This is the most common cause of sudden visual loss in a young patient. It is often associated with discomfort in or around
What is Multiple Sclerosis? Gener al information
 What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
What is Multiple Sclerosis? Gener al information Kim, diagnosed in 1986 What is MS? Multiple sclerosis (or MS) is a chronic, often disabling disease that attacks the central nervous system (brain and spinal
Progress in MS: Current and Emerging Therapies
 Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
Progress in MS: Current and Emerging Therapies Presented by: Dr. Kathryn Giles, MD MSc FRCPC The MS Society gratefully acknowledges the grant received from Biogen Idec Canada, which makes possible the
Guidance for evaluation of new neurological symptoms in patients receiving TYSABRI
 Guidance for evaluation of new neurological symptoms in patients receiving TYSABRI Background information Progressive multifocal leukoencephalopathy (PML) PML is a demyelinating disease that attacks the
Guidance for evaluation of new neurological symptoms in patients receiving TYSABRI Background information Progressive multifocal leukoencephalopathy (PML) PML is a demyelinating disease that attacks the
ESCMID Online Lecture Library. by author
 Do statins improve outcomes of patients with sepsis and pneumonia? Jordi Carratalà Department of Infectious Diseases Statins for sepsis & community-acquired pneumonia Sepsis and CAP are major healthcare
Do statins improve outcomes of patients with sepsis and pneumonia? Jordi Carratalà Department of Infectious Diseases Statins for sepsis & community-acquired pneumonia Sepsis and CAP are major healthcare
Chapter 10. Summary & Future perspectives
 Summary & Future perspectives 123 Multiple sclerosis is a chronic disorder of the central nervous system, characterized by inflammation and axonal degeneration. All current therapies modulate the peripheral
Summary & Future perspectives 123 Multiple sclerosis is a chronic disorder of the central nervous system, characterized by inflammation and axonal degeneration. All current therapies modulate the peripheral
