Import Health Standard
|
|
|
- Eleanore Watkins
- 7 years ago
- Views:
Transcription
1 Import Health Standard Poultry Hatching Eggs & Specific-Pathogen-Free Chicken Eggs COMEGIC.GEN Issued under the Biosecurity Act 1993
2 TITLE Import Health Standard: Poultry Hatching Eggs & Specific-Pathogen-Free Chicken Eggs COMMENCEMENT This Import Health Standard comes into force on ISSUING AUTHORITY This Import Health Standard is issued under section 24A of the Biosecurity Act Dated at Wellington this 24 th day of July Howard Pharo Manager, Import and Export Animals Ministry for Primary Industries (acting under delegated authority of the Director-General) Contact for further information Ministry for Primary Industries (MPI) Regulation & Assurance Branch Animal Imports PO Box 2526, Wellington Ministry for Primary Industries Page 1 of 20
3 Contents Page Introduction 3 Part 1: General Requirements Application Outcome Incorporation by reference Definitions Exporting country systems and certification Diagnostic testing and vaccination Inspection Labelling and packaging Pre-export isolation Treatment requirements Transport Permit to import The documentation that must accompany goods Biosecurity direction Post-arrival quarantine Biosecurity clearance 9 Part 2: Specified Requirements for Identified Risk Organisms Clinical inspection [all poultry] Avian influenza (AI) [all poultry] Avian paramyxoviruses type 1 (APMV-1), Newcastle disease (ND) [all poultry] Salmonella enterica sub species enterica serovar Gallinarum-Pullorum, Salmonella Enteritidis and Salmonella Typhimurium [all poultry] Salmonella arizonae [turkeys only] Mycoplasma [turkeys only] Duck virus enteritis (DVE) [ducks only] Goose parvovirus and Muscovy duck parvovirus [ducks only] Reovirus of Muscovy ducks [ducks only] Avian chlamydiosis [ducks only] 13 Schedule 1 Document History 14 Schedule 2 Definitions 15 Schedule 3 Compartment and Zone Requirements 18 Schedule 4 Post-Arrival Quarantine 19 Ministry for Primary Industries Page 2 of 20
4 Introduction This introduction is not part of the Import Health Standard, but is intended to indicate its general effect. Purpose This IHS specifies the minimum requirements that must be met when importing poultry hatching eggs and specific-pathogen-free (SPF) chicken eggs. Background The Biosecurity Act 1993 (the Act) provides the legal basis for excluding, eradicating and effectively managing pests and unwanted organisms. Import health standards issued under the Act set out requirements to be met to effectively manage biosecurity risks associated with importing goods. They include requirements that must be met in the exporting country, during transit, and during importation, before biosecurity clearance can be given. A guidance document accompanies this standard providing information on how the requirements may be met. Who should read this Import Health Standard? This IHS applies to importers of poultry hatching eggs and SPF chicken eggs. Why is this important? It is the importer s responsibility to ensure the requirements of this IHS are met. Consignments that do not comply with the requirements of this IHS may not be cleared for entry into New Zealand and/or further information may be sought from importers. Consignments that do not comply with the requirements of this IHS may be re-shipped or destroyed under the Act or treated in accordance with this IHS prior to release or equivalence determined. Importers are liable for all associated expenses. See guidance document for more information about importer responsibilities. Equivalence The Chief Technical Officer (CTO) may approve measures under section 27(1)(d) of the Act, different from those set out in this IHS, that may be applied to effectively manage risks associated with the importation of these goods. See guidance document for more information about equivalence and permits. Document History Refer to Schedule 1. Other information This is not an exhaustive list of compliance requirements and it is the importer s responsibility to be familiar with and comply with all New Zealand laws. See guidance document for more information about inspection and verification of consignments. Ministry for Primary Industries Page 3 of 20
5 Part 1: General Requirements 1.1 Application (1) This import health standard (IHS) applies to: a) hatching eggs of chickens (Gallus gallus), turkeys (Meleagris gallopavo) and ducks (Anas platyrhynchos domesticus and Cairina moschata), sourced from poultry breeding flocks compliant with the standards described in the World Organisation for Animal Health (OIE) Terrestrial Animal Health Code (the Code), Chapter on Biosecurity Procedures in Poultry Production; and b) specific-pathogen-free (SPF) eggs of chickens (Gallus gallus) produced by flocks free from specified pathogens. The flocks supplying SPF eggs must be kept under secure biosecurity controls at least equivalent to those required for breeders in the Code Chapter on Biosecurity in Poultry. (2) This IHS applies to imports of poultry hatching eggs and SPF chicken eggs from all countries. 1.2 Outcome (1) The outcome this IHS is seeking to achieve is the effective management of biosecurity risks associated with eligible consignments of poultry hatching eggs and SPF chicken eggs. (2) The biosecurity risk organisms associated with poultry hatching eggs and SPF chicken eggs that are managed by the requirements of this IHS are: a) For chicken hatching eggs: i) Notifiable avian influenza (NAI) viruses ii) Type 1 avian paramyxoviruses (Newcastle disease virus) iii) Salmonella Gallinarum-Pullorum iv) Salmonella Enteritidis and Salmonella Typhimurium b) For turkey hatching eggs: i) Avian influenza viruses ii) Type 1 avian paramyxoviruses (Newcastle disease virus) iii) Salmonella Gallinarum-Pullorum iv) Salmonella Enteritidis and Salmonella Typhimurium v) Salmonella arizonae vi) Mycoplasma iowae vii) Mycoplasma meleagridis c) For duck hatching eggs: i) Avian influenza viruses ii) Type 1 avian paramyxoviruses (Newcastle disease virus) iii) Salmonella Gallinarum-Pullorum iv) Salmonella Enteritidis and Salmonella Typhimurium v) Duck virus enteritis (DVE) virus vi) Goose and Muscovy duck parvoviruses (Muscovy ducks and their hybrid breeds only) vii) Reovirus of Muscovy ducks (Muscovy ducks and their hybrid breeds only) viii) Chlamydia psittaci Ministry for Primary Industries Page 4 of 20
6 1.3 Incorporation by reference (1) The following international standards are incorporated by reference in this IHS under section 142M of the Act: a) The World Organisation for Animal Health (OIE) Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (the Manual) (available at the OIE website: b) The OIE Terrestrial Animal Health Code (the Code) (available at the OIE Website: c) The International Air Transport Association (IATA) Live Animals Regulations (LAR): a copy is available for reading, free of charge, at MPI, Pastoral House, 25 The Terrace, Wellington. (2) The following MPI CTO material is incorporated by reference in this IHS under section 142M of the Act: a) MPI Approved Diagnostic Tests, Vaccines, Treatments and Post-arrival Testing Laboratories for Animal Import Health Standards (MPI-STD-TVTL). (3) Under section 142O(3) of the Act it is declared that section 142O(1) does not apply, that is, a notice under section 142O(2) of the Act is not required to be published before material that amends or replaces the above listed standards, guideline or lists has legal effect as part of these documents. See guidance document for more information about incorporation by reference and section 142O(1). 1.4 Definitions (1) For the purposes of this IHS and the attached guidance document, terms used that are defined in the Act have the meanings set out there. The Act is available at the following website: (2) See Schedule 2 for additional definitions that apply. 1.5 Exporting country systems and certification (1) Poultry hatching eggs and SPF chicken eggs to which this IHS applies may only be imported from a country where the Competent Authority has provided the following evidence to the satisfaction of the CTO: a) The verifiable animal health status of poultry populations in the exporting country, zone or compartment, with respect to biosecurity risk organisms of concern. b) The national systems/programmes and standards in the exporting country for regulatory oversight of the poultry industry. c) The capabilities and preferences of the exporting country s Competent Authority with respect to achieving equivalent outcomes to requirements stated in this IHS. See guidance document for more information about countries with CTO approved exporting systems that meet the requirements above. (2) Once satisfied, MPI and the Competent Authority may commence negotiation of a country-specific veterinary certificate. See guidance document for more information about country-specific veterinary certificates that have been agreed for trade. (1) Requirements in Part 2 may be met by a specific disease-free compartment or zone endorsed by the Competent Authority. Any specific disease free compartment or zone must meet the requirements in Schedule 3 of this IHS. Ministry for Primary Industries Page 5 of 20
7 MPI reserves the right to perform an in-country or desk-top audit at any time, including prior to the first shipment of ruminant meat and ruminant meat products. 1.6 Diagnostic testing and vaccination (1) Any laboratory conducting the pre-export and/or surveillance testing where required by this IHS must be approved by the Competent Authority of a country approved to export the product to New Zealand. (2) Laboratory samples must be collected, processed, and stored in accordance with the recommendations in the Code and/or the Manual or as described in MPI-STD-TVTL. (3) Diagnostic test(s) and vaccines used must be approved by MPI and listed in MPI-STD-TVTL. (4) Where flock testing options are used to satisfy specified requirements for identified risk organisms (Part 2), sampling of birds for diagnostic testing must be randomised, and representative of the flock from which the product is derived. The samples must be collected under the supervision of the Official Veterinarian. The sample size selected must be sufficiently large to give 95% confidence of detecting infection where there is at least 5% prevalence in the flock, unless otherwise stated. See guidance document for more information about diagnostic tests and vaccines for international trade. 1.7 Inspection Poultry hatching eggs (1) For pre-export inspection: a) The parent flock must be inspected by an Official Veterinarian within 28 days of commencement of collection of eggs for export. This inspection must be carried out once the birds are housed in the premise where egg collection will take place. b) An officer approved by the Competent Authority must inspect the consignment within 48 hours pre-shipment and verify that the consignment and accompanying documentation meets all the requirements of this import health standard. (2) For post-arrival inspection: a) Post-arrival inspection of packaging and document verification is required by an Inspector at the port of arrival. b) An Official Veterinarian must complete a full inspection of consignments of poultry hatching eggs and accompanying documentation once it is in the transitional facility SPF eggs (1) For pre-export inspection: a) The parent flock must have been held, under supervision of an Official Veterinarian, for at least 28 days immediately prior to the scheduled date of export in isolation facilities approved by the exporting country s Competent Authority. b) Any deaths or illnesses must have been investigated by an Official Veterinarian or by a registered veterinarian under the supervision of the Official Veterinarian. (2) For post-arrival inspection: a) Veterinary certificate inspection required by an Inspector at port of arrival in New Zealand. Ministry for Primary Industries Page 6 of 20
8 1.8 Labelling and packaging (1) The consignment must be clearly identified and identifiable to the veterinary certificate; and Poultry hatching eggs (1) Must be placed in a spill proof container and sealed into clean and disinfected crates, using an official seal attached by an Official Veterinarian of the country of origin before departure SPF eggs (1) The Official Veterinarian must confirm after due enquiry that the eggs were placed into clean spill proof containers and sealed into clean and disinfected crates inside the facilities before dispatch. 1.9 Pre-export isolation (1) The parent flock must be kept in a country, zone or compartment free of the risk organisms, as described in Part 2 of this IHS, for at least 21 days prior to collection of eggs for export. (2) Parent birds must be kept isolated from all other birds, as described in the Code Chapter on biosecurity procedures for poultry Treatment requirements (1) The eggs must be clean when collected, unwashed and have intact (uncracked) shells. (2) They must be collected separately from dirty and broken or cracked eggs. (3) Hatching eggs must be cleaned and sanitised as soon as possible after collection using an approved sanitising agent, in accordance with the manufacturer s instructions, or equivalent. Details of the treatment must be attached to the veterinary certificate Transport (1) Details of transport and arrival times of the eggs must be supplied to an Inspector and/or Official Veterinarian at the port of entry not less than 7 days in advance of importation. (2) Transport containers must meet the design and species specification published in the International Air Transport Association (IATA) Live Animals Regulations (LAR), unless otherwise agreed by MPI. (3) The vehicle which the eggs are to be transported to the port of departure in must be cleaned and disinfected. The date of treatment, the chemical(s) used, and the active ingredients must be appended to the veterinary certificate. (4) During transport to the port of departure, and during transit or stopovers en route to New Zealand, hatching eggs must only be transported or stored with animals of equivalent health status. (5) Eggs transiting a third country en route to New Zealand must receive MPI approval prior to export. MPI approval and any special conditions must be noted on the import permit. (6) Containers made of timber must be inspected by an authorised Inspector at the arrival port and must comply with the IHS: Woodware from All Countries. Ministry for Primary Industries Page 7 of 20
9 1.13 Permit to import (1) A permit to import is required. Applications must be submitted to MPI prior to importation. (2) The importer must supply the following information to obtain a permit: a) The name and address of exporter. b) The number, sex, age, species and microchip transponder identification of the animal. c) The date of proposed importation. d) The name and address of the transitional facility in New Zealand to which the consignment is to proceed following importation. e) The port of arrival, and route and means of transport to the transitional facility. f) A letter from the authorised Supervisor of the post-arrival quarantine, stating that the facility meets the facility approval requirements of MPI Standard Standard for Avian Transitional Facilities and is able to accept the proposed number of hatching eggs to be imported on the proposed import date. (3) Permit to import application forms can be found on the MPI website: Application for Permit to Import. (4) Completed applications can be submitted to Animal Imports animalimports@mpi.govt.nz The documentation that must accompany goods (1) Poultry hatching eggs and SPF chicken eggs must arrive in New Zealand with: a) A permit to import (copy acceptable). b) A veterinary certificate, that must include all of the following: i) a unique consignment identifier ii) the description, species, and number of eggs iii) the name and address of the importer (consignee) and exporter (consignor) iv) the name, signature and contact details of the Official Veterinarian v) certification and endorsements that the requirements outlined in Part 1 and Part 2 of this IHS have been met vi) the name of diagnostic test(s), treatments and vaccines used to meet this IHS, and date of application to the parent flock. c) Original laboratory reports; copies of laboratory reports endorsed by the Official Veterinarian; or a tabulated summary of laboratory results endorsed by the Official Veterinarian must include: i) the relevant risk organism tested for ii) sample size iii) dates of sample collection iv) test type v) test result. d) Vaccination records, that are attached to the veterinary certificate and include: i) details of any vaccination of the parent flock as required in part 2 of the IHS ii) date of administration iii) name and nature of vaccine. (2) A country-specific veterinary certificate must accompany the consignment where equivalent measures have been negotiated and approved by a CTO under section 27(1)(d) of the Act. See guidance document for more information about equivalence and country-specific veterinary certificates. (3) A separate veterinary certificate must be supplied for each parent flock from which the eggs for export are sourced. Ministry for Primary Industries Page 8 of 20
10 (4) All documents must: a) be original, unless otherwise stated b) accompany the imported goods c) be in English or have an English translation that is clear and legible d) be endorsed on every page by the Official Veterinarian with their original stamp, signature and date or be endorsed in the space allocated and all pages have paper based alternative security features (except for the permit to import). (5) Documentation copies must be sent to the Biosecurity Inspector at the airport/port of arrival at least one working day in advance of importation Biosecurity direction (1) Upon arrival in New Zealand, an Inspector must inspect the consignment to ensure the packaging is intact and the accompanying documentation is as described in clause An Official Veterinarian will complete a full inspection of poultry hatching egg consignments and accompanying documentation once they are in the transitional facility. (2) Providing the documentation meets all requirements listed in clause 1.12, an MPI Inspector under section 25 of the Act, may give a biosecurity direction authorising the consignment to be moved to the approved avian or biological transitional facility named in the import permit Post-arrival quarantine (1) Poultry hatching eggs must be incubated, hatched and the hatchlings be raised in a transitional facility approved and supervised by the authorised Supervisor to MPI Standard Standard for Avian Transitional Facilities. Consignments must comply with post-arrival quarantine (PAQ) requirements as described in Schedule 4. (2) SPF eggs may be used within a transitional facility approved to MPI Standard Transitional Facilities for Biological Products; and operate at a minimum physical containment level 2 (PC2). The eggs and any resultant chickens shall remain in the transitional facility and be destroyed at the conclusion of the work, triple bagged and incinerated. Alternatively SPF eggs may be hatched in an Avian Transitional Facility ( ) and released as per the requirements for poultry hatching eggs in (1) above. See guidance document for more information about post-arrival quarantine sampling and testing protocols Biosecurity clearance (1) A biosecurity clearance, under section 26 of the Act, may be issued when the poultry hatching eggs and SPF chicken eggs meet all the requirements of this IHS, provided the applicable requirements of the section 27 of the Act are met. (2) No clearance shall be issued for SPF eggs, products derived from those eggs or any resultant chickens held in a Transitional Facilities for Biological Products. Ministry for Primary Industries Page 9 of 20
11 Part 2: Specified Requirements for Identified Risk Organisms (1) The Competent Authority of the exporting country is required to issue a signed, stamped and dated veterinary certificate containing declarations regarding the following diseases: 2.1 Clinical inspection [all poultry] (1) For poultry hatching eggs: a) Within the 28 days prior to the commencement of collection of the eggs, the birds in the parent flock must have been inspected and found to be free of clinical evidence of infectious disease. b) The parent flock must be compliant with the standards described in the Code Chapter on biosecurity procedures in poultry production. c) The inspection must be undertaken in the premise the birds will be housed in during the egg collection period. (2) For SPF eggs: a) The parent flock must have been held, under supervision of an Official Veterinarian, for at least 28 days immediately prior to the scheduled date of export in isolation facilities approved by the exporting countries Competent Authority. b) Any deaths or illnesses must have been investigated by an Official Veterinarian. 2.2 Avian influenza (AI) [all poultry] (1) The eggs for export must be derived from parent flocks: a) With a vaccination status of either: i) Not vaccinated for avian influenza; or ii) Vaccinated for avian influenza in accordance with the provisions of the Manual and the nature of the vaccine used and the date of vaccination have been attached to the veterinary certificate; and either b) or c) must apply. b) That have been resident for at least the 21 days before, and during, egg collection in a country, zone or compartment that was free from AI, with current Code surveillance requirements being met for avian influenza; or c) Demonstrated to be free from infection with AI by testing a statistically valid sample, selected in accordance with the Code s recommended Surveillance Strategies, with a test for AI listed in MPI-STD-TVTL, within the 21 days prior to commencement of egg collection and at a maximum of 21 day intervals during the egg collection period. Important information for importers 20 December 2016 Avian Influenza Outbreaks There have been recent outbreaks of avian influenza in the Netherlands and the United Kingdom. Until these outbreaks are resolved these countries are unable to declare country freedom from avian influenza. Ministry for Primary Industries Page 10 of 20
12 2.3 Avian paramyxoviruses type 1 (APMV-1), Newcastle disease (ND) [all poultry] (1) The eggs for export must be derived from parent flocks: a) With a vaccination status of either: i) Not vaccinated for APMV-1; or ii) Vaccinated for APMV-1 using an inactivated vaccine; and/or iii) Vaccinated with a live, lentogenic, APMV-1 vaccine strain, in accordance with the provisions of the Manual, and the nature of the vaccine used and the date of vaccination have been attached to the veterinary certificate. The master seed virus for the vaccine used must have an intracerebral pathogenicity index (ICPI) <0.4; and either b) or c) must apply. b) That have been resident for at least the 21 days before, and during, egg collection in a country, zone or compartment that was free from Newcastle disease (as defined in the Code) with current ND Code surveillance requirements being met; or c) Demonstrated to be free from infection with APMV-1 by carrying out testing on a statistically valid sample, selected in accordance with the Code s Surveillance Strategies, with a test listed in MPI- STD-TVTL, within the 21 days prior to commencement of egg collection and at a maximum of 21 day intervals during the egg collection period. 2.4 Salmonella enterica sub species enterica serovar Gallinarum- Pullorum, Salmonella Enteritidis and Salmonella Typhimurium [all poultry] (1) The eggs for export must be derived from parent flocks in a country, zone or compartment free from Salmonella Gallinarum-Pullorum, Salmonella Enteritidis and Salmonella Typhimurium as demonstrated by surveillance, conducted in accordance with the Code requirements for monitoring poultry breeding flocks for Salmonella, and approved by an MPI CTO; or (2) The eggs for export must be derived from a parent flock certified as free from Salmonella Gallinarum- Pullorum, Salmonella Enteritidis and Salmonella Typhimurium. Flock monitoring must have been carried out in accordance with the Code requirements for monitoring poultry breeding flocks for Salmonella. 2.5 Salmonella arizonae [turkeys only] (1) The turkey hatching eggs must be: a) Derived from parent flocks in a country, zone or compartment free from Salmonella arizonae as demonstrated by surveillance, conducted in accordance with the Code requirements for monitoring poultry breeding flocks for Salmonella, and approved by an MPI CTO; or b) Derived from a parent flock certified free from Salmonella arizonae. Flock monitoring must have been carried out in accordance with the Code requirements for monitoring poultry breeding flocks for Salmonella; or c) Derived from parent flocks demonstrated to be free from Salmonella arizonae by testing a statistically valid, randomly selected sample of turkeys within the 7 day period before collection of the hatching eggs commenced with an approved diagnostic test listed in the MPI document MPI-STD-TVTL. Ministry for Primary Industries Page 11 of 20
13 2.6 Mycoplasma [turkeys only] (1) For Mycoplasma iowae: a) The eggs for export must be derived from parent flocks in a country where Mycoplasma iowae is not recognised to be present; or b) Ten percent (10%) of the birds in the parent flock must be subjected, with negative results 1 in each case, to cloacal swab culture for Mycoplasma iowae within the 28 days prior to commencement of collection of eggs for export and the flock must have a negative test history for Mycoplasma iowae; or c) The turkey hatching eggs must be derived from a parent flock demonstrated to be free from Mycoplasma iowae by carrying out testing on a statistically valid sample with a test listed in MPI-STD-TVTL within the 28 days prior to commencement of collection of eggs for export. (2) For Mycoplasma meleagridis: a) The eggs for export must be derived from parent flocks in a country where Mycoplasma meleagridis is not recognised to be present; or b) The turkey hatching eggs must be derived from a parent flock demonstrated to be free from Mycoplasma meleagridis by testing a statistically valid sample of the flock with a test listed in MPI-STD-TVTL within the 28 days prior to commencement of collection of eggs for export; or c) The turkey hatching eggs must be derived from a parent flock demonstrated to be free from Mycoplasma meleagridis with testing undertaken in accordance with a Competent Authority supervised poultry health scheme with consistently negative results for the past 12 months. Testing must be carried out on a statistically valid sample, with a test listed in MPI-STD-TVTL. 2.7 Duck virus enteritis (DVE) [ducks only] (1) The duck hatching eggs for export must be derived from a parent flock not vaccinated for DVE; and a) The duck hatching eggs must be derived from a parent flock in a country where DVE is not recognised; or b) The duck hatching eggs must be derived from parent flocks demonstrated free from DVE by testing a statistically valid sample of the flock with a test for DVE listed in MPI-STD-TVTL within the 21 days prior to the commencement of collection of eggs for export. 2.8 Goose parvovirus and Muscovy duck parvovirus [ducks only] (1) The duck hatching eggs must be from breeds other than Muscovy duck (Cairina moshata) and their hybrids; or (2) The Muscovy duck hatching eggs (or hybrid Muscovy duck hatching eggs) must be derived from a parent flock in a country where Muscovy duck parvovirus and goose parvovirus is not recognised; or (3) The Muscovy duck hatching eggs (or hybrid Muscovy duck hatching eggs) must be derived from an establishment that has maintained a closed flock with a negative surveillance history for goose parvovirus and Muscovy duck parvovirus for the past 3 years. The parent flock must be demonstrated free from goose parvovirus and Muscovy duck parvovirus by testing a statistically valid sample of the flock with a test for goose parvovirus and Muscovy duck parvovirus listed in MPI-STD-TVTL within the 21 days prior to the commencement of collection of eggs for export. 1 In the case of positive or inconclusive results, a further sample must be taken and retested by a test listed in MPI-STD- TVTL at a Competent Authority approved laboratory. Any bird positive to this test must be subject to post mortem and bacteriological examination and show no evidence of Mycoplasma infection. Ministry for Primary Industries Page 12 of 20
14 2.9 Reovirus of Muscovy ducks [ducks only] (1) The duck hatching eggs must be from breeds other than Muscovy duck (Cairina moshata) and their hybrids; or (2) The Muscovy duck hatching eggs (or hybrid Muscovy duck hatching eggs) must be derived from a parent flock in a country where duck reovirus (DRV) has not been recognised; or (3) The Muscovy duck hatching eggs (or hybrid Muscovy duck hatching eggs) must be imported from an establishment that has maintained a closed flock with a negative surveillance history for reovirus of Muscovy ducks for the past 3 years. The parent flock must be demonstrated free from reovirus of Muscovy ducks by testing a statistically valid sample of the flock with a test for DRV listed in MPI-STD-TVTL within the 21 days prior to the commencement of collection of eggs for export Avian chlamydiosis [ducks only] (1) The duck hatching eggs must be derived from parent flocks in a country where Chlamydia psittaci (serovar C and E) in ducks is not recognised; or (2) The duck hatching eggs must be derived from parent flocks which are kept as closed flocks with a negative surveillance history for Chlamydia psittaci. a) The parent flock must be demonstrated free from Chlamydia psittaci by testing a statistically valid sample of the flock with a test for Chlamydia psittaci listed in MPI-STD-TVTL within the 21 days prior to the commencement of collection of eggs for export. b) The sample size must be sufficient to detect 10% prevalence with 99% confidence. Post arrival quarantine testing is required as per Schedule 4. Ministry for Primary Industries Page 13 of 20
15 Schedule 1 Document History Date First Issued Title Shortcode 5 February 2013 Poultry Hatching Eggs and Specific-Pathogen- Free Chicken Eggs COMEGIC.GEN Date of Issued Amendments Title Shortcode 12 July 2013 Poultry Hatching Eggs and Specific-Pathogen- Free Chicken Eggs 29 July 2013 Poultry Hatching Eggs and Specific-Pathogen- Free Chicken Eggs August 2014 October 2014 December 2014 July 2015 Poultry Hatching Eggs and Specific-Pathogen- Free Chicken Eggs Poultry Hatching Eggs and Specific-Pathogen- Free Chicken Eggs Poultry Hatching Eggs and Specific-Pathogen- Free Chicken Eggs Poultry Hatching Eggs and Specific-Pathogen- Free Chicken Eggs COMEGIC.GEN COMEGIC.GEN COMEGIC.GEN COMEGIC.GEN COMEGIC.GEN COMEGIC.GEN Ministry for Primary Industries Page 14 of 20
16 Schedule 2 Definitions Approved Countries Countries from which New Zealand enables imports of a particular commodity. The countries must be included in the scope of the most recent import risk analysis/assessment for the commodity and be specifically approved by MPI. Biosecurity Authority Written authority from an Inspector, given under section 25 of the Act, to move uncleared goods from a transitional facility, containment facility or biosecurity control area to another transitional facility, containment facility or biosecurity control area, or to export those goods from New Zealand. (Note: Goods given a Biosecurity Clearance by an Inspector are released to the importer without restriction). Biosecurity Plan A plan that identifies potential pathways for the introduction and spread of disease in a zone or compartment, and describes the measures which are being or will be applied to mitigate the disease risks, if applicable, in accordance with the recommendations in the Code. A Biosecurity Manual must comply with the Code Chapters on zoning and compartmentalisation, and application of compartmentalisation. Closed Flock A flock that is managed on an all-in, all-out basis, in a single epidemiological unit with no introductions of birds from different epidemiological units. A biosecurity plan as described in the Code must be adhered to during the management of the flock. The Code The World Organisation for Animal Health Terrestrial Animal Health Code. Any reference in this standard to the Code is to the most current as found on the OIE website. Compartment An animal subpopulation contained in one or more establishments under a common biosecurity management system with a distinct health status with respect to a specific disease or specific diseases for which required surveillance, control and biosecurity measures have been applied for the purpose of international trade. Competent Authority The Veterinary or other Governmental Authority of an OIE Member, that has the responsibility and competence for ensuring or supervising the implementation of animal health and welfare measures, international veterinary certification and other standards and recommendations in the Code in the whole territory. Official Veterinarian A veterinarian authorised by the Competent Authority of the country to perform certain designated official tasks associated with animal health and/or public health and inspections of commodities and, when appropriate, to certify in conformity with the provisions of the OIE Code Chapter for certification procedures. OIE Ministry for Primary Industries Page 15 of 20
17 The World Organisation for Animal Health. Parent Flock A group of birds of one species kept for producing fertile eggs, as a single epidemiological unit, in accordance with the Code Chapter on biosecurity in poultry, recommendations applicable to the operation of poultry establishments and additional measures for breeders. Permit to Import A permit issued by the Director General of MPI pursuant to section 24(D)(2) of the Act. Poultry Hatching Eggs Hatching eggs of chickens (Gallus gallus), turkeys (Meleagris gallopavo) or ducks (Anas platyrhynchos domesticus and Cairina moshata), sourced from poultry breeding flocks compliant with the standards described in the Code chapter on biosecurity procedures in poultry production. Prescribed Diagnostic Test The diagnostic test(s) which can be used for international trade when the OIE Code recommends a testing procedure for international trade in a commodity. Production Cycle The production cycle refers to all operations between and including the hatching, breeder farms and the egg handling facilities associated with a consignment for export to New Zealand. Specific-Pathogen-Free (SPF) Eggs Eggs produced by chicken flocks free from specified pathogens. The flocks supplying SPF eggs must be kept under secure biosecurity controls at least equivalent to those required for breeders in the Code Chapter on Biosecurity in Poultry. Supervisor As defined in the MPI Standard for Avian Transitional Facilities , a registered veterinarian and inspector employed, or contracted by the supplier, who inspects the transitional facility and audits the operation of quarantine. Surveillance Competent Authority supervised systematic ongoing collection, collation, and analysis of information related to animal health and the timely dissemination of information so that action can be taken. For the purposes of this IHS, for risk organisms, where disease specific surveillance recommendations are made in the Code, the Code recommendations must be met. For other risk organisms, surveillance must meet the recommendations in the Code Chapter for animal health surveillance. Transitional Facility Any place approved as a transitional facility in accordance with section 39 of the Act for the purpose of inspection, testing, storage, treatment, holding or destruction of uncleared goods; or A part of a port declared to be a transitional facility in accordance with section 39 of the Act. The Manual Ministry for Primary Industries Page 16 of 20
18 The World Organisation for Animal Health (OIE) Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Veterinary Certificate A certificate, issued in conformity with the provisions of the OIE Code Chapter for certification procedures, describing the animal health and/or public health requirements which are fulfilled by the exported commodities. Zone A clearly defined part of a territory containing an animal subpopulation with a distinct health status with respect to a specific disease for which required surveillance, control and biosecurity measures have been applied for the purpose of international trade. Ministry for Primary Industries Page 17 of 20
19 Schedule 3 Compartment and Zone Requirements (1) If the option of importing from a specified disease free compartment or zone is selected for any of the identified risk organisms a biosecurity plan must be provided, to the satisfaction of a CTO prior to commencing the production cycle for product for export to New Zealand. (2) A biosecurity plan must: a) Be prepared in accordance with the Code. b) Be officially endorsed by the exporting country s Competent Authority. c) Meet the requirements of a specific disease free compartment or zone. d) Include an original letter dated, officially stamped and signed by the Competent Authority of the exporting country: i) Stating that the compartment s or zone s biosecurity plan under which trade is eligible to occur has been officially endorsed. ii) Stating that the surveillance and monitoring programme in place has been audited against the biosecurity plan and that the Competent Authority is satisfied that it can verify that the compartment is free of the disease for which the compartment is formed. e) Include an original letter dated, officially stamped and signed by the Competent Authority of the exporting country: i) Certifying that the compartment or zone has been maintained free of the disease for which the compartment is formed for at least the 12 months prior to commencing the production cycle for product for export to New Zealand. ii) Stating that records of procedures and systems (including test results) of the establishment(s) forming the compartment (or zone where applicable), for at least the 12 months prior to commencing the production cycle for product for export to New Zealand, are available to MPI upon request. iii) Stating that all procedures, systems and characteristics of the establishment(s) forming the compartment (or zone where applicable) have been maintained and are identical to those described in the approved biosecurity plan. (3) Any change to the biosecurity plan or production system outline must be notified to the Competent Authority and re-approved. Re-approval must be to the satisfaction of a CTO. (4) MPI reserves the right to audit facilities prior to the export of product to New Zealand. (5) MPI reserves the right to audit compartments or zones prior to the export of product to New Zealand. Ministry for Primary Industries Page 18 of 20
20 Schedule 4 Post-Arrival Quarantine Requirements for poultry for hatching eggs (1) The post arrival quarantine (PAQ) facility must be approved by MPI as a transitional facility approved to MPI Standard Standard for Avian Transitional Facilities and be under the supervision of an authorised Supervisor. (2) Written approval/advice from the authorised Supervisor must be provided with the application for an import permit. (3) PAQ stay, testing, treatments and procedures must be undertaken under supervision of the Authorised Supervisor. (4) Regular inspections will be made by the authorised Supervisor, as set out in the MPI Standard Standard for Avian Transitional Facilities. (5) Any unusual deaths, increase in mortality rate or illness must be immediately reported to the Authorised Supervisor or the MPI hotline and carcasses retained for possible postmortem examination. (6) Any positive results must be reported to MPIs Investigation and Diagnostic Centre (IDC). Any APMV isolate must be identified to serotype level, particularly for APMV-1, otherwise any APMV isolated will be assumed to be one of 9 APMV types (APMV1- APMV 9) including NDV. Any influenza A isolate must be identified to haemagglutinin (HA) subtype, particularly for H5 or H7, otherwise any influenza A isolated will be assumed to be one of 16 subtypes including AI. (7) Testing must be conducted by laboratories approved by MPI to conduct the specific tests required. See additional details in the guidance document for laboratory approval requirements. (8) No compensation will be paid for any birds slaughtered as a result of testing for disease or for diagnosis. (9) MPI reserves the right to remove birds and or specimens for any tests that may be desired at any time. (10) A person authorised under the Act shall issue biosecurity clearance once the consignment has met all of the conditions for import, including negative test results received for AI, ND and Chlamydia psittaci (where required). Testing requirements Newcastle disease (APMV-1) (1) The consignment must be tested negative for ND by one of the following options: a) MPI-approved PCR testing of a representative sample of hatch debris, dead-in-shell chicks, embryos and dead chicks; or b) Testing a representative sample of chicks at or after 21 days of hatching for ND by a MPIapproved PCR test. The sample size must be sufficient to confirm that the flock is free from infection with at least 95% confidence of detecting 5% prevalence; or c) Serological testing of chicks for APMV at or after 28 days of hatching. The sample size must be sufficient to confirm that the flock is free from infection with at least 95% confidence of detecting 5% prevalence. (Testing must be conducted after 28 days to avoid interference of maternal antibodies. If maternal antibodies are suspected in samples collected at or after 28 days, the Authorised Supervisor must be notified immediately and repeat testing must be carried out on or before 42 days of age or as agreed with MPI. Rising serum titres, or any clinical evidence of disease must be reported immediately in accordance with the requirements in the avian transitional facility standard) Note: Option c) cannot be used for ducks as serology is not reliable in this species. Ministry for Primary Industries Page 19 of 20
21 Avian influenza (1) The consignment must be tested negative for AI by one of the following options: a) MPI-approved PCR testing of a representative sample of hatch debris, dead-in-shell chicks, embryos and dead chicks; or b) Testing a representative sample of chicks at or after 21 days of hatching for AI by a MPIapproved PCR test. The sample size must be sufficient to confirm that the flock is free from infection with at least 95% confidence of detecting 5% prevalence; or c) Serological testing of chicks for AI at or after 28 days of hatching. The sample size must be sufficient to confirm that the flock is free of infection with at least 95% confidence of detecting 5% prevalence. (Testing must be conducted after 28 days to avoid interference of maternal antibodies.) Chlamydia psittaci [ducks only] (1) The consignment must be tested negative for C. psittaci by one of the following options: a) A representative sample of dead hatchlings and dead-in-shell chicks must be examined to detect the presence of C. psittaci using histochemical staining of liver and spleen impression smears; or b) At hatching a representative sample of the consignment, including dead-in-shell chicks, embryos, dead and live chicks must be demonstrated free from C. psittaci by testing a statistically valid sample with a test for Chlamydia psittaci listed in MPI-STD-TVTL. Note: The sample size must be sufficient to detect 10% prevalence with 99% confidence. Ministry for Primary Industries Page 20 of 20
DEPARTMENT OF VETERINARY SERVICES MALAYSIA
 DEPARTMENT OF VETERINARY SERVICES MALAYSIA Ministry of Agricutlure and Agro-Based Industry Malaysia Wisma Tani, Podium Block, Lot 4G1, Precinct 4 Federal Government Administration Centre 62630 PUTRAJAYA,
DEPARTMENT OF VETERINARY SERVICES MALAYSIA Ministry of Agricutlure and Agro-Based Industry Malaysia Wisma Tani, Podium Block, Lot 4G1, Precinct 4 Federal Government Administration Centre 62630 PUTRAJAYA,
Intra-Union Trade in Poultry for Slaughter. Notes for Guidance of the Official Veterinarian (OV) and Exporters
 Department for Environment, Food and Rural Affairs March 2015 Intra-Union Trade in Poultry for Slaughter Notes for Guidance of the Official Veterinarian (OV) and Exporters Page 1 of 8 Contents 1. Key Documents...
Department for Environment, Food and Rural Affairs March 2015 Intra-Union Trade in Poultry for Slaughter Notes for Guidance of the Official Veterinarian (OV) and Exporters Page 1 of 8 Contents 1. Key Documents...
BIOSECURITY PROCEDURES IN POULTRY PRODUCTION
 1 Annex VII CHAPTER 6.4. BIOSECURITY PROCEDURES IN POULTRY PRODUCTION Article 6.4.1. Introduction This chapter provides recommended biosecurity procedures in poultry production and is not specifically
1 Annex VII CHAPTER 6.4. BIOSECURITY PROCEDURES IN POULTRY PRODUCTION Article 6.4.1. Introduction This chapter provides recommended biosecurity procedures in poultry production and is not specifically
IMPORT HEALTH STANDARD FOR PIG MEAT AND PIG MEAT PRODUCTS FOR HUMAN CONSUMPTION FROM CANADA AND/OR THE UNITED STATES OF AMERICA
 IMPT HEALTH STANDARD F PIG MEAT AND PIG MEAT PRODUCTS F HUMAN CONSUMPTION FROM CANADA AND/ THE UNITED STATES OF AMERICA Issued pursuant to Section 22 of the Biosecurity Act 1993 Dated: 18 March 2011 Certification
IMPT HEALTH STANDARD F PIG MEAT AND PIG MEAT PRODUCTS F HUMAN CONSUMPTION FROM CANADA AND/ THE UNITED STATES OF AMERICA Issued pursuant to Section 22 of the Biosecurity Act 1993 Dated: 18 March 2011 Certification
Guidelines for Animal Disease Control
 Guidelines for Animal Disease Control 1. Introduction and objectives The guidelines are intended to help countries identify priorities, objectives and the desired goal of disease control programmes. Disease
Guidelines for Animal Disease Control 1. Introduction and objectives The guidelines are intended to help countries identify priorities, objectives and the desired goal of disease control programmes. Disease
Quarantine Requirements for the Importation of Laboratory Animals
 Appendix 1-8 Quarantine Requirements for the Importation of Laboratory Animals (In case of any discrepancy between the English version and the Chinese text of these Requirements, the Chinese text shall
Appendix 1-8 Quarantine Requirements for the Importation of Laboratory Animals (In case of any discrepancy between the English version and the Chinese text of these Requirements, the Chinese text shall
The Danish veterinary preparedness for avian influenza and Newcastle disease
 The Danish veterinary preparedness for avian influenza and Newcastle disease Sten Mortensen, Veterinary R&D manager, Animal Health Division, Deputy head 19-04-2016 Livestock statistics, Denmark 2015 Species
The Danish veterinary preparedness for avian influenza and Newcastle disease Sten Mortensen, Veterinary R&D manager, Animal Health Division, Deputy head 19-04-2016 Livestock statistics, Denmark 2015 Species
DEVISING IMPORT HEALTH MEASURES FOR ANIMAL COMMODITIES
 DEVISING IMPORT HEALTH MEASURES FOR ANIMAL COMMODITIES This paper provides guidance to OIE Members on the use of the animal health information in the OIE World Animal Health Information Database (WAHID)
DEVISING IMPORT HEALTH MEASURES FOR ANIMAL COMMODITIES This paper provides guidance to OIE Members on the use of the animal health information in the OIE World Animal Health Information Database (WAHID)
Compensation for Salmonella Enteritidis & Typhimurium to owners in England
 Department for Environment, Food and Rural Affairs Compensation for Salmonella Enteritidis & Typhimurium to owners in England January 2015 Contents 1. Introduction... 1 2. Aims and objectives... 1 3. Legal
Department for Environment, Food and Rural Affairs Compensation for Salmonella Enteritidis & Typhimurium to owners in England January 2015 Contents 1. Introduction... 1 2. Aims and objectives... 1 3. Legal
SANITARY AND PHYTOSANITARY MEASURES (SPS)
 TEXTUAL PROPOSAL SANITARY AND PHYTOSANITARY MEASURES (SPS) Article 1 Scope and coverage This Chapter applies to all SPS measures that may, directly or indirectly, affect trade between the Parties. This
TEXTUAL PROPOSAL SANITARY AND PHYTOSANITARY MEASURES (SPS) Article 1 Scope and coverage This Chapter applies to all SPS measures that may, directly or indirectly, affect trade between the Parties. This
http://www.who.int/csr/disease/avian_influenza/phase/en 4 http://new.paho.org/hq/index.php?option=com_content&task=view&id=1283&itemid=569
 Food and Agriculture Organization of the United Nations International Food Safety Authorities Network (INFOSAN) (Update) 30 April 2009 INFOSAN Information Note No. 2/2009 Human-animal interface aspects
Food and Agriculture Organization of the United Nations International Food Safety Authorities Network (INFOSAN) (Update) 30 April 2009 INFOSAN Information Note No. 2/2009 Human-animal interface aspects
http:www.aphis.gov/animal_health/vet_accreditation/training_modules.shtml
 APHIS Approved Supplemental Training for Accredited Veterinarians has now been approved for Continuing Education Credit by the Texas Board of Veterinary Medical Examiners In the new accreditation process,
APHIS Approved Supplemental Training for Accredited Veterinarians has now been approved for Continuing Education Credit by the Texas Board of Veterinary Medical Examiners In the new accreditation process,
General Information and Suggested Procedural Checklist for Florida Importers of SVC-Susceptible Fish Species. (As of October 13, 2006)
 Spring Viremia of Carp (SVC) Interim Rule and the Importation of Susceptible Fish Species General Information and Suggested Procedural Checklist for Florida Importers of SVC-Susceptible Fish Species (As
Spring Viremia of Carp (SVC) Interim Rule and the Importation of Susceptible Fish Species General Information and Suggested Procedural Checklist for Florida Importers of SVC-Susceptible Fish Species (As
Newcastle Disease Outbreaks in Denmark 2002 Final Report
 Newcastle Disease Outbreaks in Denmark 2002 Final Report Danish Veterinary and Food Administration 2003 For further information on the Newcastle Disease Outbreaks in Denmark 2002, please contact: Preben
Newcastle Disease Outbreaks in Denmark 2002 Final Report Danish Veterinary and Food Administration 2003 For further information on the Newcastle Disease Outbreaks in Denmark 2002, please contact: Preben
Import Health Standard
 Import Health Standard Ballast Water from All Countries 17 May 2016 Issued under the Biosecurity Act 1993 TITLE Import Health Standard: Ballast Water from All Countries COMMENCEMENT This Import Health
Import Health Standard Ballast Water from All Countries 17 May 2016 Issued under the Biosecurity Act 1993 TITLE Import Health Standard: Ballast Water from All Countries COMMENCEMENT This Import Health
How To Control A Dog
 TITLE 8: AGRICULTURE AND ANIMALS CHAPTER I: DEPARTMENT OF AGRICULTURE : ANIMALS AND ANIMAL PRODUCTS (EXCEPT MEAT AND POULTRY INSPECTION ACT REGULATIONS) PART 30 ANIMAL CONTROL ACT Section 30.10 Definitions
TITLE 8: AGRICULTURE AND ANIMALS CHAPTER I: DEPARTMENT OF AGRICULTURE : ANIMALS AND ANIMAL PRODUCTS (EXCEPT MEAT AND POULTRY INSPECTION ACT REGULATIONS) PART 30 ANIMAL CONTROL ACT Section 30.10 Definitions
PROCEDURE MANUAL: DAFF APPROVAL OF LABORATORIES
 DEPARTMENT OF AGRICULTURE, FISHERIES AND FORESTRY DIRECTORATE ANIMAL HEALTH EPIDEMIOLOGY COMPILED BY: Helen Booker, Riette Theron FIRST DOCUMENT August 2015 APPROVED BY: Director Animal Health APPROVAL
DEPARTMENT OF AGRICULTURE, FISHERIES AND FORESTRY DIRECTORATE ANIMAL HEALTH EPIDEMIOLOGY COMPILED BY: Helen Booker, Riette Theron FIRST DOCUMENT August 2015 APPROVED BY: Director Animal Health APPROVAL
SES Introduction. SES: Who? SES: Introduction. SES: Development. Preparedness/Response Goals. Control Area: What is it? 11/7/14.
 Preparedness/Response Goals Section 2: SES Introduction Note from Dr. Buswell: This is only one of three sec;ons of the Security Egg Supply presenta;on. The en;re presenta;on is available online at hcp://secureeggsupply.com/wp-
Preparedness/Response Goals Section 2: SES Introduction Note from Dr. Buswell: This is only one of three sec;ons of the Security Egg Supply presenta;on. The en;re presenta;on is available online at hcp://secureeggsupply.com/wp-
HPAI Response HPAI Response Goals November 18, 2015
 HPAI Response HPAI Response Goals November 18, 2015 Please note: These may be revised as the situation continues to change. USDA APHIS will work to achieve these goals for critical activities in a highly
HPAI Response HPAI Response Goals November 18, 2015 Please note: These may be revised as the situation continues to change. USDA APHIS will work to achieve these goals for critical activities in a highly
B I O S E C U R I T Y F O R B R E E D E R F A R M S
 Issue No.1 / February 2010 B I O S E C U R I T Y F O R B R E E D E R F A R M S ~ An Integral Part of the Modern Production System ~ Marcelo PANIAGO, DVM, MSc, MBA. Regional Market Manager - Poultry Ceva
Issue No.1 / February 2010 B I O S E C U R I T Y F O R B R E E D E R F A R M S ~ An Integral Part of the Modern Production System ~ Marcelo PANIAGO, DVM, MSc, MBA. Regional Market Manager - Poultry Ceva
EXPORT CERTIFICATION SYSTEM FOR ANIMAL & ANIMAL PRODUCTS THE MALAYSIAN PERSPECTIVE
 EXPORT CERTIFICATION SYSTEM FOR ANIMAL & ANIMAL PRODUCTS THE MALAYSIAN PERSPECTIVE Dr. Norlizan Mohd Noor Biosecurity Management and SPS Division Department of Veterinary Services Malaysia Putrajaya 1
EXPORT CERTIFICATION SYSTEM FOR ANIMAL & ANIMAL PRODUCTS THE MALAYSIAN PERSPECTIVE Dr. Norlizan Mohd Noor Biosecurity Management and SPS Division Department of Veterinary Services Malaysia Putrajaya 1
12.7.2007 Official Journal of the European Union L 182/19 DIRECTIVES
 12.7.2007 Official Journal of the European Union L 182/19 DIRECTIVES COUNCIL DIRECTIVE 2007/43/EC of 28 June 2007 laying down minimum rules for the protection of chickens kept for meat production (Text
12.7.2007 Official Journal of the European Union L 182/19 DIRECTIVES COUNCIL DIRECTIVE 2007/43/EC of 28 June 2007 laying down minimum rules for the protection of chickens kept for meat production (Text
Animal health requirements for the exported pig meat, etc. to Japan from Spain are as follows.
 Animal health requirements for meat and viscera derived from pigs and sausages, ham and bacon made from the said meat and viscera as raw materials to be exported to Japan from Spain Animal health requirements
Animal health requirements for meat and viscera derived from pigs and sausages, ham and bacon made from the said meat and viscera as raw materials to be exported to Japan from Spain Animal health requirements
Executive Summary Conclusions and Recommendations. June 22-24, 2015 Holiday Inn Inner Harbor Baltimore, Maryland, USA
 Executive Summary Conclusions and Recommendations June 22-24, 2015 Holiday Inn Inner Harbor Baltimore, Maryland, USA Suggested bibliographic citation for this report: USDA (2015). International conference
Executive Summary Conclusions and Recommendations June 22-24, 2015 Holiday Inn Inner Harbor Baltimore, Maryland, USA Suggested bibliographic citation for this report: USDA (2015). International conference
FEDERATIVE REPUBLIC OF BRAZIL Ministry of Agriculture, Livestock and Food Supply Secretariat of Animal and Plant Health and Inspection
 FEDERATIVE REPUBLIC OF BRAZIL Ministry of Agriculture, Livestock and Food Supply Secretariat of Animal and Plant Health and Inspection Eradication of a FMD outbreak in Mato Grosso do Sul, Brazil Animal
FEDERATIVE REPUBLIC OF BRAZIL Ministry of Agriculture, Livestock and Food Supply Secretariat of Animal and Plant Health and Inspection Eradication of a FMD outbreak in Mato Grosso do Sul, Brazil Animal
Control of Newcastle and Infectious Bursal diseases in Poultry: Vaccines, Vaccination and Bio security
 Control of Newcastle and Infectious Bursal diseases in Poultry: Vaccines, Vaccination and Bio security Nick Nwankpa, Karim Tounkara and Charles Bodjo OUTLINE OF PRESENTATION 1. INTRODUCTION 2. DEFINITIONS
Control of Newcastle and Infectious Bursal diseases in Poultry: Vaccines, Vaccination and Bio security Nick Nwankpa, Karim Tounkara and Charles Bodjo OUTLINE OF PRESENTATION 1. INTRODUCTION 2. DEFINITIONS
Appendix II (Concerning the Article 21): Biosecurity Standards 1. Pigs and wild boar
 Appendix II (Concerning the Article 21): Biosecurity Standards 1. Pigs and wild boar I. Gaining the latest information regarding livestock epidemic control, etc. 1. Always confirm the information provided
Appendix II (Concerning the Article 21): Biosecurity Standards 1. Pigs and wild boar I. Gaining the latest information regarding livestock epidemic control, etc. 1. Always confirm the information provided
QUALITY MANAGEMENT IN VETERINARY TESTING LABORATORIES
 NB: Version adopted by the World Assembly of Delegates of the OIE in May 2012 CHAPTER 1.1.4. QUALITY MANAGEMENT IN VETERINARY TESTING LABORATORIES SUMMARY Valid laboratory results are essential for diagnosis,
NB: Version adopted by the World Assembly of Delegates of the OIE in May 2012 CHAPTER 1.1.4. QUALITY MANAGEMENT IN VETERINARY TESTING LABORATORIES SUMMARY Valid laboratory results are essential for diagnosis,
SEROLOGICAL SERVICE IN POULTRY INDUSTRY
 Issue No.30 / May 2010 SEROLOGICAL SERVICE IN POULTRY INDUSTRY By Dr Vincent TURBLIN, Deputy Regional Market Manager Poultry - CEVA Animal Health Asia Pacific In avian diagnostic medicine, the usual immediate
Issue No.30 / May 2010 SEROLOGICAL SERVICE IN POULTRY INDUSTRY By Dr Vincent TURBLIN, Deputy Regional Market Manager Poultry - CEVA Animal Health Asia Pacific In avian diagnostic medicine, the usual immediate
MINISTRY OF AGRICULTURE, LIVESTOCK AND FOOD SUPPLY. NORMATIVE INSTRUCTION NUMBER 44 OF OCTOBER 2 nd, 2007
 MINISTRY OF AGRICULTURE, LIVESTOCK AND FOOD SUPPLY NORMATIVE INSTRUCTION NUMBER 44 OF OCTOBER 2 nd, 2007 (Published in the Official Gazette Number 191 - Section 1, page 2 to 10, Wednesday, October 3 rd,
MINISTRY OF AGRICULTURE, LIVESTOCK AND FOOD SUPPLY NORMATIVE INSTRUCTION NUMBER 44 OF OCTOBER 2 nd, 2007 (Published in the Official Gazette Number 191 - Section 1, page 2 to 10, Wednesday, October 3 rd,
INFORMATION FROM SPAIN ON LOW PATHOGENIC AVIAN INFLUENZA SITUATION
 MINISTERIO DE MEDIO AMBIENTE Y MEDIO RURAL Y MARINO INFORMATION FROM SPAIN ON LOW PATHOGENIC AVIAN INFLUENZA SITUATION Luis José Romero González S. G. Sanidad de la Producción Primaria SCoFCAH,, July 1st,
MINISTERIO DE MEDIO AMBIENTE Y MEDIO RURAL Y MARINO INFORMATION FROM SPAIN ON LOW PATHOGENIC AVIAN INFLUENZA SITUATION Luis José Romero González S. G. Sanidad de la Producción Primaria SCoFCAH,, July 1st,
The U.S. Trichinella Certification Program
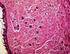 The U.S. Trichinella Certification Program H. Ray Gamble U.S. National Academies 500 Fifth Street NW Washington, DC 20001 rgamble@nas.edu 1-202-334-2787 Pre-Slaughter Control (Prevention) of Trichinella
The U.S. Trichinella Certification Program H. Ray Gamble U.S. National Academies 500 Fifth Street NW Washington, DC 20001 rgamble@nas.edu 1-202-334-2787 Pre-Slaughter Control (Prevention) of Trichinella
National Pig Health Council. Code of Practice for Importation of Live Pigs into Ireland
 National Pig Health Council Code of Practice for Importation of Live Pigs into Ireland : Irish Farm Centre, Bluebell, Dublin 12 Telephone: 01-4500266 Fax: 01-4551043 April 2014 1 1. Introduction Through
National Pig Health Council Code of Practice for Importation of Live Pigs into Ireland : Irish Farm Centre, Bluebell, Dublin 12 Telephone: 01-4500266 Fax: 01-4551043 April 2014 1 1. Introduction Through
STATUTORY INSTRUMENTS SUPPLEMENT No. 1 11th May, 2012.
 THE EAST AFRICAN COMMUNITY STATUTORY INSTRUMENTS SUPPLEMENT No. 1 11th May, 2012. to the East African Community Gazette No. 7 of 11th May, 2012. Printed by the Uganda Printing and Publishing Corporation,
THE EAST AFRICAN COMMUNITY STATUTORY INSTRUMENTS SUPPLEMENT No. 1 11th May, 2012. to the East African Community Gazette No. 7 of 11th May, 2012. Printed by the Uganda Printing and Publishing Corporation,
Connecticut Veterinary Medical Diagnostic Laboratory
 Connecticut Veterinary Medical Diagnostic Laboratory Department of Pathobiology and Veterinary Science College of Agriculture and Natural Resources University of Connecticut SL Bushmich, MS, DVM CVMDL:
Connecticut Veterinary Medical Diagnostic Laboratory Department of Pathobiology and Veterinary Science College of Agriculture and Natural Resources University of Connecticut SL Bushmich, MS, DVM CVMDL:
Conditions for Payment of Highly Pathogenic Avian Influenza Indemnity Claims
 This document is scheduled to be published in the Federal Register on 02/09/2016 and available online at http://federalregister.gov/a/2016-02530, and on FDsys.gov BILLING CODE: 3410-34-P DEPARTMENT OF
This document is scheduled to be published in the Federal Register on 02/09/2016 and available online at http://federalregister.gov/a/2016-02530, and on FDsys.gov BILLING CODE: 3410-34-P DEPARTMENT OF
WHO Regional Office for Europe update on avian influenza A (H7N9) virus
 WHO Regional Office for Europe update on avian influenza A (H7N9) virus Situation update 2: 30 April 2013 Address requests about publications of the WHO Regional Office for Europe to: Publications WHO
WHO Regional Office for Europe update on avian influenza A (H7N9) virus Situation update 2: 30 April 2013 Address requests about publications of the WHO Regional Office for Europe to: Publications WHO
Pandemic Influenza Cleaning and Disinfection Protocol
 This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
This document has been developed in accordance with current applicable infection control and regulatory guidelines. It is intended for use as a guideline only. At no time should this document replace existing
HPAI H5N8 outbreak in layers in the Netherlands. 20 November 2014, Ruth Bouwstra
 HPAI H5N8 outbreak in layers in the Netherlands 20 November 2014, Ruth Bouwstra Avian Influenza Surveillance in the Netherlands Passive surveillance (notification of suspect situation) Acute infections
HPAI H5N8 outbreak in layers in the Netherlands 20 November 2014, Ruth Bouwstra Avian Influenza Surveillance in the Netherlands Passive surveillance (notification of suspect situation) Acute infections
AU/IBAR-CHINA ASSISTANCE PROTOCOL ON THE PREVENTION AND CONTROL OF HPAI IN AFRICA
 African Union Interafrican Bureau of Animal Resources Regional SPINAP-AHI Coordination for Eastern Africa AU/IBAR-CHINA ASSISTANCE PROTOCOL ON THE PREVENTION AND CONTROL OF HPAI IN AFRICA CHINA-AFRICA
African Union Interafrican Bureau of Animal Resources Regional SPINAP-AHI Coordination for Eastern Africa AU/IBAR-CHINA ASSISTANCE PROTOCOL ON THE PREVENTION AND CONTROL OF HPAI IN AFRICA CHINA-AFRICA
European Regulations for Animal Tracking
 European Regulations for Animal Tracking Tony Sephton 04/02/2015 Issue/Revision: 1.0 Reference: ESA-TIAA-HO-2015-451 Status: Issued Agenda EU Legislation. National Legislation based on UK example. International
European Regulations for Animal Tracking Tony Sephton 04/02/2015 Issue/Revision: 1.0 Reference: ESA-TIAA-HO-2015-451 Status: Issued Agenda EU Legislation. National Legislation based on UK example. International
Anhang 1.3 Animal health requirements for pig meat etc. to be exported to Japan from Austria
 Anhang 1.3 Animal health requirements for pig meat etc. to be exported to Japan from Austria 1 This document defines animal health requirements for pig meat etc. and its products to be exported to Japan
Anhang 1.3 Animal health requirements for pig meat etc. to be exported to Japan from Austria 1 This document defines animal health requirements for pig meat etc. and its products to be exported to Japan
Facts about the production of Poultry Meat in Denmark 4. July 2014
 Facts about the production of Poultry Meat in Denmark 4. July 2014 Birthe Steenberg Manager Danish Poultry Meat Association Tlf. 24631673; E-mail: bsb@lf.dk Poultry Meat from stable to table Breeding animals
Facts about the production of Poultry Meat in Denmark 4. July 2014 Birthe Steenberg Manager Danish Poultry Meat Association Tlf. 24631673; E-mail: bsb@lf.dk Poultry Meat from stable to table Breeding animals
GUIDELINES FOR FOOD IMPORT CONTROL SYSTEMS
 GUIDELINES FOR FOOD IMPORT CONTROL SYSTEMS SECTION 1 SCOPE CAC/GL 47-2003 1. This document provides a framework for the development and operation of an import control system to protect consumers and facilitate
GUIDELINES FOR FOOD IMPORT CONTROL SYSTEMS SECTION 1 SCOPE CAC/GL 47-2003 1. This document provides a framework for the development and operation of an import control system to protect consumers and facilitate
POULTRY MANAGEMENT Manage poultry rearing operations
 1 of 6 level: 4 credit: 32 planned review date: December 2008 sub-field: purpose: entry information: accreditation option: moderation option: Poultry Production People credited with this unit standard
1 of 6 level: 4 credit: 32 planned review date: December 2008 sub-field: purpose: entry information: accreditation option: moderation option: Poultry Production People credited with this unit standard
POULTRY MANAGEMENT Manage poultry layer unit operations
 1 of 6 level: 4 credit: 32 planned review date: December 2008 sub-field: purpose: entry information: accreditation option: moderation option: Poultry Production People credited with this unit standard
1 of 6 level: 4 credit: 32 planned review date: December 2008 sub-field: purpose: entry information: accreditation option: moderation option: Poultry Production People credited with this unit standard
REFERENCE LABORATORY TESTING
 Diagnostic Services Price List Tel: +44 (0) 1483 232441 Fax: +44 (0) 1483 232621 Reference Laboratories Incoming.Samples@pirbright.ac.uk October 2014 1 REFERENCE LABORATORY TESTING The Pirbright Institute
Diagnostic Services Price List Tel: +44 (0) 1483 232441 Fax: +44 (0) 1483 232621 Reference Laboratories Incoming.Samples@pirbright.ac.uk October 2014 1 REFERENCE LABORATORY TESTING The Pirbright Institute
PLEASE NOTE. For more information concerning the history of this Act, please see the Table of Public Acts.
 PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this Act, current to December 2, 2015. It is intended for information and reference purposes only. This
PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this Act, current to December 2, 2015. It is intended for information and reference purposes only. This
SADC GUIDELINES ON IMPORT AND EXPORT PROCEDURES FOR PHARMACEUTICAL PRODUCTS
 SADC GUIDELINES ON IMPORT AND EXPORT PROCEDURES FOR PHARMACEUTICAL PRODUCTS June 2006 1 INTRODUCTION Public health concerns demand that the manufacture of pharmaceutical products and their subsequent handling
SADC GUIDELINES ON IMPORT AND EXPORT PROCEDURES FOR PHARMACEUTICAL PRODUCTS June 2006 1 INTRODUCTION Public health concerns demand that the manufacture of pharmaceutical products and their subsequent handling
National FMD Response Planning
 National FMD Response Planning Proactive Risk Assessment to Support and Managed Preparedness Movement of Livestock and Poultry Timothy J. Goldsmith DVM, MPH, DACVPM Center for Animal Health and Food Safety
National FMD Response Planning Proactive Risk Assessment to Support and Managed Preparedness Movement of Livestock and Poultry Timothy J. Goldsmith DVM, MPH, DACVPM Center for Animal Health and Food Safety
RECOMMENDATIONS. - Livestock is important in food security, income generation, small holder s livelihoods and poverty alleviation.
 RECOMMENDATIONS CONSIDERING THAT: - Livestock is important in food security, income generation, small holder s livelihoods and poverty alleviation. - Major livestock diseases are of social and economic
RECOMMENDATIONS CONSIDERING THAT: - Livestock is important in food security, income generation, small holder s livelihoods and poverty alleviation. - Major livestock diseases are of social and economic
Laws and Regulations Relating to RABIES. Excerpts from the. California Health and Safety Code. and the. California Code of Regulations
 Laws and Regulations Relating to RABIES Excerpts from the California Health and Safety Code and the California Code of Regulations CALIFORNIA DEPARTMENT OF PUBLIC HEALTH DIVISION OF COMMUNICABLE DISEASE
Laws and Regulations Relating to RABIES Excerpts from the California Health and Safety Code and the California Code of Regulations CALIFORNIA DEPARTMENT OF PUBLIC HEALTH DIVISION OF COMMUNICABLE DISEASE
Appendix 1 ATTACHMENT A REVISED OPERATIONAL CERTIFICATION PROCEDURES (OCP) FOR THE RULES OF ORIGIN OF THE ASEAN-CHINA FREE TRADE AREA
 Appendix 1 ATTACHMENT A REVISED OPERATIONAL CERTIFICATION PROCEDURES (OCP) FOR THE RULES OF ORIGIN OF THE ASEAN-CHINA FREE TRADE AREA For the purpose of implementing the Rules of Origin for the ASEAN-China
Appendix 1 ATTACHMENT A REVISED OPERATIONAL CERTIFICATION PROCEDURES (OCP) FOR THE RULES OF ORIGIN OF THE ASEAN-CHINA FREE TRADE AREA For the purpose of implementing the Rules of Origin for the ASEAN-China
Guidelines for the Safe Transport of Infectious Substances and Diagnostic Specimens
 Distr.: General Orig.: English Guidelines for the Safe Transport of Infectious Substances and Diagnostic Specimens WORLD HEALTH ORGANIZATION Division of Emerging and Other Communicable Diseases Surveillance
Distr.: General Orig.: English Guidelines for the Safe Transport of Infectious Substances and Diagnostic Specimens WORLD HEALTH ORGANIZATION Division of Emerging and Other Communicable Diseases Surveillance
animals importation into bermuda www.bermudatourism.com - 1.800.bermuda
 animals importation into bermuda www.bermudatourism.com - 1.800.bermuda The information pertaining to the importation of animals contained herein is correct as of the time of preparing this document (May
animals importation into bermuda www.bermudatourism.com - 1.800.bermuda The information pertaining to the importation of animals contained herein is correct as of the time of preparing this document (May
EVALUATION OF VETERINARY SERVICES
 1 TERRESTRIAL ANIMAL HEALTH STANDARDS COMMISSION SEPTEMBER 2010 REPORT CHAPTER 3.2. EVALUATION OF VETERINARY SERVICES General considerations Article 3.2.1. 1. Evaluation of Veterinary Services is an important
1 TERRESTRIAL ANIMAL HEALTH STANDARDS COMMISSION SEPTEMBER 2010 REPORT CHAPTER 3.2. EVALUATION OF VETERINARY SERVICES General considerations Article 3.2.1. 1. Evaluation of Veterinary Services is an important
Animal Health Programs: Combining Surveillance, Detection, and Response
 United States Department of Agriculture Animal and Plant Health Inspection Service Animal Health Programs: Combining Surveillance, Detection, and Response The Animal and Plant Health Inspection Service
United States Department of Agriculture Animal and Plant Health Inspection Service Animal Health Programs: Combining Surveillance, Detection, and Response The Animal and Plant Health Inspection Service
Crisis Management Centre - Animal Health (CMC-AH)
 Crisis Management Centre - Animal Health (CMC-AH) Rapid Missions Update October 2014 October 2015 The Crisis Management Centre Animal Health (CMC-AH) is a joint platform of the Food and Agriculture Organization
Crisis Management Centre - Animal Health (CMC-AH) Rapid Missions Update October 2014 October 2015 The Crisis Management Centre Animal Health (CMC-AH) is a joint platform of the Food and Agriculture Organization
TERRESTRIAL ANIMAL HEALTH CODE
 WORLD ORGANISATION FOR ANIMAL HEALTH Protecting animals, preserving our future TERRESTRIAL ANIMAL HEALTH CODE VOLUME I General provisions Twenty-third edition, 2014 First edition, 1968 Second edition,
WORLD ORGANISATION FOR ANIMAL HEALTH Protecting animals, preserving our future TERRESTRIAL ANIMAL HEALTH CODE VOLUME I General provisions Twenty-third edition, 2014 First edition, 1968 Second edition,
ISO/IEC 17025 QUALITY MANUAL
 1800 NW 169 th Pl, Beaverton, OR 97006 Revision F Date: 9/18/06 PAGE 1 OF 18 TABLE OF CONTENTS Quality Manual Section Applicable ISO/IEC 17025:2005 clause(s) Page Quality Policy 4.2.2 3 Introduction 4
1800 NW 169 th Pl, Beaverton, OR 97006 Revision F Date: 9/18/06 PAGE 1 OF 18 TABLE OF CONTENTS Quality Manual Section Applicable ISO/IEC 17025:2005 clause(s) Page Quality Policy 4.2.2 3 Introduction 4
SLOVAKIA Population 1999: 5 395 324 Population 2000: 5 402 547 Area: 48 845 km 2
 WHO Surveillance Programme for Control of Foodborne Infections and Intoxications in Europe Country Reports: Slovakia BfR SLOVAKIA Population 1999: 5 395 324 Population 2: 5 42 547 Area: 48 845 km 2 1.
WHO Surveillance Programme for Control of Foodborne Infections and Intoxications in Europe Country Reports: Slovakia BfR SLOVAKIA Population 1999: 5 395 324 Population 2: 5 42 547 Area: 48 845 km 2 1.
Drinking Water Quality Management Plan Review and Audit Guideline
 Drinking Water Quality Management Plan Review and Audit Guideline This publication has been compiled by Queensland Water Supply Regulator, Department of Energy and Water Supply. State of Queensland, 2013.
Drinking Water Quality Management Plan Review and Audit Guideline This publication has been compiled by Queensland Water Supply Regulator, Department of Energy and Water Supply. State of Queensland, 2013.
The Disease is a commonly diagnosed infectious disease that affects all equidae worldwide. Caused by the bacterium Streptococcus equi (S.equi), this d
 ADVICE ON The Disease is a commonly diagnosed infectious disease that affects all equidae worldwide. Caused by the bacterium Streptococcus equi (S.equi), this disease is highly contagious and is spread
ADVICE ON The Disease is a commonly diagnosed infectious disease that affects all equidae worldwide. Caused by the bacterium Streptococcus equi (S.equi), this disease is highly contagious and is spread
VETERINARY TECHNICIAN
 MICHIGAN CIVIL SERVICE COMMISSION JOB SPECIFICATION VETERINARY TECHNICIAN JOB DESCRIPTION Employees in this job perform various tasks to facilitate and support the work and research of professional veterinarians,
MICHIGAN CIVIL SERVICE COMMISSION JOB SPECIFICATION VETERINARY TECHNICIAN JOB DESCRIPTION Employees in this job perform various tasks to facilitate and support the work and research of professional veterinarians,
SURVEILLANCE AND CONTROL METHODS FOR INFECTIOUS SALMON ANEMIA (ISA)
 EURL FOR FISH DISEASES SURVEILLANCE AND CONTROL METHODS FOR INFECTIOUS SALMON ANEMIA (ISA) SURVEILLANCE AND CONTROL METHODS FOR INFECTIOUS SALMON ANEMIA (ISA) I. Requirements for surveillance and eradication
EURL FOR FISH DISEASES SURVEILLANCE AND CONTROL METHODS FOR INFECTIOUS SALMON ANEMIA (ISA) SURVEILLANCE AND CONTROL METHODS FOR INFECTIOUS SALMON ANEMIA (ISA) I. Requirements for surveillance and eradication
AHCPRK201A Care for health and welfare of pigs
 AHCPRK201A Care for health and welfare of pigs Release: 1 AHCPRK201A Care for health and welfare of pigs Modification History Not Applicable Unit Descriptor Unit descriptor This unit covers the process
AHCPRK201A Care for health and welfare of pigs Release: 1 AHCPRK201A Care for health and welfare of pigs Modification History Not Applicable Unit Descriptor Unit descriptor This unit covers the process
COMMISSION IMPLEMENTING DECISION. of XXX. (Text with EEA relevance)
 EUROPEAN COMMISSION Brussels, XXX SANCO/12230/2014 CIS (POOL/G2/2014/12230/12230-EN CIS.doc) [ ](2014) XXX draft COMMISSION IMPLEMENTING DECISION of XXX establishing specific transitional measures for
EUROPEAN COMMISSION Brussels, XXX SANCO/12230/2014 CIS (POOL/G2/2014/12230/12230-EN CIS.doc) [ ](2014) XXX draft COMMISSION IMPLEMENTING DECISION of XXX establishing specific transitional measures for
Example of a food company quality
 Appendix A manual Example of a food company quality Contents Date: 13/03/95 RME-QLMN-OO Page 1 of 3 Section Title ISO 9001 reference 01 In trod uction 02 Purpose 03 Scope 04 Definitions 05 Management responsibility
Appendix A manual Example of a food company quality Contents Date: 13/03/95 RME-QLMN-OO Page 1 of 3 Section Title ISO 9001 reference 01 In trod uction 02 Purpose 03 Scope 04 Definitions 05 Management responsibility
Notifiable Diseases. A Guide for Official Veterinarians
 Notifiable Diseases A Guide for Official Veterinarians Learning Objectives To understand the mechanisms in place for dealing with notifiable disease To improve awareness of which diseases are notifiable
Notifiable Diseases A Guide for Official Veterinarians Learning Objectives To understand the mechanisms in place for dealing with notifiable disease To improve awareness of which diseases are notifiable
Comparison Chart of FDA and EPA Good Laboratory Practice (GLP) Regulations and the OECD Principles of GLP
 Comparison Chart of FDA and EPA Good Laboratory Practice (GLP) Regulations and the OECD Principles of GLP Document issued on: June 2004 U.S. Department of Health and Human Services Food and Drug Administration
Comparison Chart of FDA and EPA Good Laboratory Practice (GLP) Regulations and the OECD Principles of GLP Document issued on: June 2004 U.S. Department of Health and Human Services Food and Drug Administration
TRANSPORT FOR LONDON (TfL) LOW EMISSIONS CERTIFICATE (LEC) GUIDANCE NOTES FOR THE COMPANY AUDIT PROCESS. LEC (Company Audit) Guidance Notes
 TRANSPORT FOR LONDON (TfL) LOW EMISSIONS CERTIFICATE (LEC) GUIDANCE NOTES FOR THE COMPANY AUDIT PROCESS LEC (Company Audit) Guidance Notes Glossary of Terms Transport for London (TfL) London Low Emission
TRANSPORT FOR LONDON (TfL) LOW EMISSIONS CERTIFICATE (LEC) GUIDANCE NOTES FOR THE COMPANY AUDIT PROCESS LEC (Company Audit) Guidance Notes Glossary of Terms Transport for London (TfL) London Low Emission
OH&S Management Systems Audit Checklist (NAT, E3)
 3.1.2 3.1.1 Introduction OH&S Management Systems Audit Checklist (NAT, E3) This audit checklist is based on Element 3 (Implementation) of the National Self-Insurers OHS Audit Tool. For a full copy of the
3.1.2 3.1.1 Introduction OH&S Management Systems Audit Checklist (NAT, E3) This audit checklist is based on Element 3 (Implementation) of the National Self-Insurers OHS Audit Tool. For a full copy of the
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL
 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Directorate F - Food and Veterinary Office Ares(2014)567860 DG(SANCO) 2013-6703 - MR FINAL FINAL REPORT OF AN AUDIT CARRIED OUT IN BRAZIL FROM
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Directorate F - Food and Veterinary Office Ares(2014)567860 DG(SANCO) 2013-6703 - MR FINAL FINAL REPORT OF AN AUDIT CARRIED OUT IN BRAZIL FROM
CONDITIONS FOR THE OVERSEAS TRANSFER OF WOMBATS
 CONDITIONS FOR THE OVERSEAS TRANSFER OF WOMBATS March 2008 1 Contents Page Contents 2 Preamble 3 Selection of Export Stock 4 Pre Export Requirements 4 Transportation Requirements 5 Crates/Boxes 5 Acclimatisation
CONDITIONS FOR THE OVERSEAS TRANSFER OF WOMBATS March 2008 1 Contents Page Contents 2 Preamble 3 Selection of Export Stock 4 Pre Export Requirements 4 Transportation Requirements 5 Crates/Boxes 5 Acclimatisation
Adrian Cooper. Defra Update
 Adrian Cooper Defra Update Defra update Defra and the FSA have recently (14 Feb) issued a general authorisation on imports from third countries of glucosamine, chondroitin and chitosan. A CIN, OVS note
Adrian Cooper Defra Update Defra update Defra and the FSA have recently (14 Feb) issued a general authorisation on imports from third countries of glucosamine, chondroitin and chitosan. A CIN, OVS note
I. Flock Health Management A. A. Bickford California Veterinary Diagnostic Laboratory System Turlock, CA
 California Egg Quality Assurance Program Educational Training Material Training Session 3 Flock Health Management I. Flock Health Management A. A. Bickford California Veterinary Diagnostic Laboratory System
California Egg Quality Assurance Program Educational Training Material Training Session 3 Flock Health Management I. Flock Health Management A. A. Bickford California Veterinary Diagnostic Laboratory System
OIE standards : procedures, model certificates OIE Regional Seminar on Food Safety Sofia, Bulgaria, 22-24 April 2009
 OIE standards : procedures, model certificates OIE Regional Seminar on Food Safety Sofia, Bulgaria, 22-24 April 2009 Dr. Caroline Planté Sub-Regional Representation in Brussels REF IN THE TERR. CODE Specific
OIE standards : procedures, model certificates OIE Regional Seminar on Food Safety Sofia, Bulgaria, 22-24 April 2009 Dr. Caroline Planté Sub-Regional Representation in Brussels REF IN THE TERR. CODE Specific
GUIDE TO IMPLEMENTING A REGULATORY FOOD SAFETY AUDITOR SYSTEM
 GUIDE TO IMPLEMENTING A REGULATORY FOOD SAFETY AUDITOR SYSTEM FEBRUARY 2016 2 Contents Introduction... 4 Scope and objectives... 5 Scope... 5 Objectives... 5 Responsibilities... 5 The role of the licensee
GUIDE TO IMPLEMENTING A REGULATORY FOOD SAFETY AUDITOR SYSTEM FEBRUARY 2016 2 Contents Introduction... 4 Scope and objectives... 5 Scope... 5 Objectives... 5 Responsibilities... 5 The role of the licensee
CHAPTER 7. 1. Section 2 of P.L.1999, c.336 (C.56:8-93) is amended to read as follows:
 CHAPTER 7 AN ACT concerning the sale of cats and dogs, and amending and supplementing P.L.1999, c.336. BE IT ENACTED by the Senate and General Assembly of the State of New Jersey: 1. Section 2 of P.L.1999,
CHAPTER 7 AN ACT concerning the sale of cats and dogs, and amending and supplementing P.L.1999, c.336. BE IT ENACTED by the Senate and General Assembly of the State of New Jersey: 1. Section 2 of P.L.1999,
Decree N 152 (24 January 2013) Administrative Measure on Inspection, Quarantine and Supervision of Imports and Exports of Dairy products
 Decree N 152 (24 January 2013) Administrative Measure on Inspection, Quarantine and Supervision of Imports and Exports of Dairy products Chapter 1 General Principle Article 1 In order to enhance inspection,
Decree N 152 (24 January 2013) Administrative Measure on Inspection, Quarantine and Supervision of Imports and Exports of Dairy products Chapter 1 General Principle Article 1 In order to enhance inspection,
Have you ever wanted to help animals and people stay healthy? Have you ever thought about working in veterinary medicine? Well, I m here to explain
 Have you ever wanted to help animals and people stay healthy? Have you ever thought about working in veterinary medicine? Well, I m here to explain what veterinarians do and answer some of your questions.
Have you ever wanted to help animals and people stay healthy? Have you ever thought about working in veterinary medicine? Well, I m here to explain what veterinarians do and answer some of your questions.
MINISTRY OF AGRICULTURE AND RURAL DEVELOPMENT
 MINISTRY OF AGRICULTURE AND RURAL DEVELOPMENT VETERINARY SERVICES THE PINE, ST MICHAEL BB11091 BARBADOS ANIMAL HEALTH ADMINISTRATION TEL. NOS: (246) 427-5073 VETERINARY SERVICES LABORATORY (246) 427-5492
MINISTRY OF AGRICULTURE AND RURAL DEVELOPMENT VETERINARY SERVICES THE PINE, ST MICHAEL BB11091 BARBADOS ANIMAL HEALTH ADMINISTRATION TEL. NOS: (246) 427-5073 VETERINARY SERVICES LABORATORY (246) 427-5492
Final report. EFTA Surveillance Authority mission to. Norway. from 11 to 18 June 2013. regarding application of EEA legislation
 Brussels, 7 October 2013 Case No: 73395 Event No: 685540 Final report EFTA Surveillance Authority mission to Norway from 11 to 18 June 2013 regarding application of EEA legislation related to Pure bred
Brussels, 7 October 2013 Case No: 73395 Event No: 685540 Final report EFTA Surveillance Authority mission to Norway from 11 to 18 June 2013 regarding application of EEA legislation related to Pure bred
The economic and social impact of the Institute for Animal Health s work on Avian Infectious Diseases
 The economic and social impact of the Institute for Animal Health s work on Avian Infectious Diseases Donald Webb DTZ One Marsden Street Manchester M2 1HW 0161 235 7639 February 2010 Contents 1. Introduction
The economic and social impact of the Institute for Animal Health s work on Avian Infectious Diseases Donald Webb DTZ One Marsden Street Manchester M2 1HW 0161 235 7639 February 2010 Contents 1. Introduction
University of Ottawa Pandemic Plan
 University of Ottawa Pandemic Plan August 2009 Introduction A disease epidemic occurs when there are more cases of a disease than normal. A pandemic is a worldwide disease epidemic. A pandemic may occur
University of Ottawa Pandemic Plan August 2009 Introduction A disease epidemic occurs when there are more cases of a disease than normal. A pandemic is a worldwide disease epidemic. A pandemic may occur
Management is designed to produce veterinarians and veterinary officers who are
 Graduate Diploma Program in Veterinary in Livestock Diseases and Health Management International Program (New curriculum 2009) 1. Title of curriculum Graduate Diploma Program in Veterinary in Livestock
Graduate Diploma Program in Veterinary in Livestock Diseases and Health Management International Program (New curriculum 2009) 1. Title of curriculum Graduate Diploma Program in Veterinary in Livestock
INCIDENT REPORT Smithfield Beef Group Souderton, INC., Establishment 1311 Export of Ineligible Beef to Japan Report
 INCIDENT REPORT Smithfield Beef Group Souderton, INC., Establishment 1311 Export of Ineligible Beef to Japan Report EXECUTIVE SUMMARY August 13, 2008 Shipments from Smithfield Beef Group (Souderton), Establishment
INCIDENT REPORT Smithfield Beef Group Souderton, INC., Establishment 1311 Export of Ineligible Beef to Japan Report EXECUTIVE SUMMARY August 13, 2008 Shipments from Smithfield Beef Group (Souderton), Establishment
Guest Editorial Historical Note on the Origin of the LaSota Strain of Newcastle Disease Virus
 AVIAN DISEASES vol. 24 no. 2 Guest Editorial Historical Note on the Origin of the LaSota Strain of Newcastle Disease Virus Tevis M. Goldhaft 2245 East Landis Avenue Vineland, New Jersey 08360 Received
AVIAN DISEASES vol. 24 no. 2 Guest Editorial Historical Note on the Origin of the LaSota Strain of Newcastle Disease Virus Tevis M. Goldhaft 2245 East Landis Avenue Vineland, New Jersey 08360 Received
Guidance Document Infectious Substances
 Guidance Document Infectious Substances Note: 1. The following Guidance Document was developed by the ICAO DGP. The original ICAO document reflects references to the ICAO Technical Instructions these have
Guidance Document Infectious Substances Note: 1. The following Guidance Document was developed by the ICAO DGP. The original ICAO document reflects references to the ICAO Technical Instructions these have
ANIMAL HEALTH ACT 1981 THE DISEASE CONTROL (ENGLAND) ORDER 2003 (AS AMENDED) GENERAL LICENCE FOR THE MOVEMENT OF PIGS PART I
 ANIMAL HEALTH ACT 1981 THE DISEASE CONTROL (ENGLAND) ORDER 2003 (AS AMENDED) GENERAL LICENCE FOR THE MOVEMENT OF PIGS PART I In accordance with Article 12 of the Disease Control (England) Order 2003, (the
ANIMAL HEALTH ACT 1981 THE DISEASE CONTROL (ENGLAND) ORDER 2003 (AS AMENDED) GENERAL LICENCE FOR THE MOVEMENT OF PIGS PART I In accordance with Article 12 of the Disease Control (England) Order 2003, (the
Guideline on good pharmacovigilance practices (GVP)
 1 2 20 February 2012 EMA/541760/2011 3 4 Guideline on good pharmacovigilance practices (GVP) Module I Pharmacovigilance systems and their quality systems Draft finalised by the Agency in collaboration
1 2 20 February 2012 EMA/541760/2011 3 4 Guideline on good pharmacovigilance practices (GVP) Module I Pharmacovigilance systems and their quality systems Draft finalised by the Agency in collaboration
What Roles & Benefits for UOFA?
 Increasing Contribution of Private Poultry Sector to Disease Prevention & Control: What Roles & Benefits for UOFA? Dr. Baba Soumare; Regional Influenza Advisor, USAID West Africa Office Outline 1. USAID
Increasing Contribution of Private Poultry Sector to Disease Prevention & Control: What Roles & Benefits for UOFA? Dr. Baba Soumare; Regional Influenza Advisor, USAID West Africa Office Outline 1. USAID
OIE Reference Laboratory Reports Activities
 OIE Reference Laboratory Reports Activities Activities in 2013 This report has been submitted : 2014-01-23 10:22:43 Name of disease (or topic) for which you are a designated OIE Reference Laboratory: Enzootic
OIE Reference Laboratory Reports Activities Activities in 2013 This report has been submitted : 2014-01-23 10:22:43 Name of disease (or topic) for which you are a designated OIE Reference Laboratory: Enzootic
MINISTRY OF FISHERIES CRITERIA FOR REGISTRATION OF IMPORTERS
 MINISTRY OF FISHERIES CRITERIA FOR REGISTRATION OF IMPORTERS The Import of fish and fish products is controlled by the Ministry of Fisheries in accordance with the Fisheries and Marine Resources (Import
MINISTRY OF FISHERIES CRITERIA FOR REGISTRATION OF IMPORTERS The Import of fish and fish products is controlled by the Ministry of Fisheries in accordance with the Fisheries and Marine Resources (Import
1. INTRODUCTION. Momona Ethiopian Journal of Science (MEJS), V6(2)33-38,2014 CNCS, Mekelle University, ISSN:2220-184X
 Seroprevalence of Salmonella Gallinarum Infection in Chicken Population of parts of Tigray and Addis Ababa by Plate Agglutination and Microagglutination Tests Ashwani Kumar*, Etsay Kebede, Yohaness Tekle,
Seroprevalence of Salmonella Gallinarum Infection in Chicken Population of parts of Tigray and Addis Ababa by Plate Agglutination and Microagglutination Tests Ashwani Kumar*, Etsay Kebede, Yohaness Tekle,
Health and welfare of Finnish pigs. Mari Heinonen Professor in swine medicine Department of Production Animal Medicine University of Helsinki
 Health and welfare of Finnish pigs Mari Heinonen Professor in swine medicine Department of Production Animal Medicine University of Helsinki FINLAND 60-70 latitude 338 000 sq.km 69 % forest 10 % water
Health and welfare of Finnish pigs Mari Heinonen Professor in swine medicine Department of Production Animal Medicine University of Helsinki FINLAND 60-70 latitude 338 000 sq.km 69 % forest 10 % water
Impact of Avian Influenza UK and Global
 Impact of Avian Influenza UK and Global Recent history of AI outbreaks The scale of the problem Impact on birds, PEOPLE, commercial Two examples Nafferton, Preston Thanks Nigel Gibbens Dan Pearson Brian
Impact of Avian Influenza UK and Global Recent history of AI outbreaks The scale of the problem Impact on birds, PEOPLE, commercial Two examples Nafferton, Preston Thanks Nigel Gibbens Dan Pearson Brian
STUDY GUIDE: POULTRY MEAT EXAMINATION
 STUDY GUIDE: POULTRY MEAT EXAMINATION In Collaboration with the Directorate Veterinary Services National Department of Agriculture January 2007 Study guide Poultry meat examination January 2007 Studiegids
STUDY GUIDE: POULTRY MEAT EXAMINATION In Collaboration with the Directorate Veterinary Services National Department of Agriculture January 2007 Study guide Poultry meat examination January 2007 Studiegids
Copa-Cogeca s views on Critically Important Antibiotics Miguel Angel Higuera (ASAJA, ES)
 AHW(14)1987:1 Copa-Cogeca s views on Critically Important Antibiotics Miguel Angel Higuera (ASAJA, ES) Vice-Chair of the Copa-Cogeca Working Party on Animal Health and Welfare London 28 th February 2014
AHW(14)1987:1 Copa-Cogeca s views on Critically Important Antibiotics Miguel Angel Higuera (ASAJA, ES) Vice-Chair of the Copa-Cogeca Working Party on Animal Health and Welfare London 28 th February 2014
