MEDICATION SAFETY. December 2010 and the application has been requested to be given priority. [Australian Incident 117, December 2010]
|
|
|
- Kerry Kelley
- 8 years ago
- Views:
Transcription
1 MEDICATION SAFETY This series brings you up-to-date information about medication safety issues and strategies to prevent medication errors. It draws on Australian incidents and US experience, including (with permission) material from ISMP Medication Safety Alert! a bulletin published by the US Institute for Safe Medication Practices < This series is coordinated via the Committee of Specialty Practice in Medication Safety (Chair, Rosemary Burke, Director of Pharmacy, Concord Hospital, NSW). Australian incidents are collated and editorial recommendations made by Penny Thornton (Principal Advisor, Medication Safety, NSW Health; <medsafety@shpa.org.au>). AUSTRALIAN INCIDENTS Little green and white bottles! Two patients were admitted to hospital suffering from over-anticoagulation. One patient was found to have had dispensed Coumadin (warfarin) 5 mg instead of Coversyl (perindopril arginine) 5 mg at a community pharmacy. On reconciling the second s patient s own medicines, a hospital pharmacist found Coversyl and Coumadin tablets mixed in the same bottle when reconciling the second patient s medications. The problem relates to similarities in packaging used for Coumadin and Coversyl both come in white bottles with green lids and with Figure 1. Packaging similarity of Coumadin and Coversyl. green, black and white colouration on the manufacturers labels (Figure 1). Metoclopramide (Pramin) and amiloride (Kaluril) are also packed in a similar manner. Instances of this mix-up have occurred in several community pharmacies. Community pharmacies tend to store medications by alphabetical brand names and Coumadin and Coversyl are likely to be stored adjacent to one another in the dispensary. The chance of mix-up within hospital pharmacies is reduced as these products are stored by alphabetical generic names. Warfarin is a highrisk medication and the seriousness of these types of mix-ups cannot be underestimated. What can be done? Consider storing these products in a manner that reduces the risk of wrong product selection. Review the perindopril brand held in your pharmacy s inventory. To proactively avert this type of error, some pharmacies stock perindopril erbumine (APO- Perindopril) 4 mg instead of the Coversyl brand. Consider using alert stickers to ensure correct replacement of stock removed from pharmacy or imprest stock shelves. This includes other products that are similarly packaged. Continue to be vigilant regarding look-alike (and sound-alike) medicines and the potential for error. Risks with dispensing Coumadin/Coversyl have been communicated to community pharmacists via the Pharmaceutical Defense Limited. This issue will also be covered in the Pharmacy Guild s communication to members and in other pharmacy publications. Community pharmacists are being urged to store Coumadin under W and to use their scanners. The Australian Commission on Safety and Quality in Health Care has advised that the TGA has received an application for packaging changes from Servier (manufacturer of Coversyl) in mid December 2010 and the application has been requested to be given priority. [Australian Incident 117, December 2010] Care with chloral hydrate (again!) An incident has been reported involving a child who drank a full bottle of chloral hydrate syrup. This was a fatal overdose. Chloral hydrate bottles do not have childproof caps and until this situation is rectified, consider recapping all bottles with a child-proof cap at the point of dispensing, if possible. Always alert parents/carers to the need for safe storage to avoid children accessing what might be considered a nice flavoured drink. [Australian Incident 118, December 2010] US SAFETY BRIEFS Update on metal in drug patches An updated list of transdermal drug delivery systems with a metallic component was recently published (Hong I, et al. Hosp Pharm 2010; 45: 771-8l.). Serious burns may occur in patients undergoing magnetic resonance imaging (MRI) who have transdermal patches with metallic content applied to their skin. The metal acts as a conductor of radiofrequency pulses, inducing electric current that causes intense heat and burns. The US Food and Drug Administration (FDA) and ISMP issued an alert about burns from medication patches known to contain a metallic component in 2004 < acutecare/articles/ asp>. In 2009, the US FDA issued a public health advisory regarding removal of drug patches prior to MRI <www. fda.gov/newsevents/ Newsroom/PressAnnouncements/ucm htm>. The latest review, by pharmacists from the University of Illinois informs on the metallic content of available transdermal patches and recommendations regarding reapplication of patches after a scan. [Medication Safety Alert! 18 November 2010] Australian comment: Although the patches available in Australia may differ from those in the US, consider a blanket rule: Remove all patches before MRI even though some may cause no problems. Concerns are that the patch may not be replaced after the MRI or may be reapplied but has lost its stickiness. Allow for an extra unit usage if the patient has had an MRI as a new patch may need to be applied. Some dressings with a metallic thread may also cause the same burning effect. Top drugs that cause violence The link between violence and prescription drugs is the subject of a new study (Moore T, et al. PloS ONE. December 2010>. The study identified 31 drugs that are disproportionately associated with increased rates of reported violence. Case reports were obtained from the ISMP QuarterWatch database and composed of computer 52
2 extracts of all adverse drug event reports received by the US FDA. Inclusion criteria were reports of violence and aggressive behaviour from drugs that were submitted to the US FDA from 2004 through the third quarter of During the 5-year study period, 484 drugs accounted for serious adverse event reports; of which 1937 (0.25%) were cases of violence. Thirty-one drugs met the criteria for a disproportionate association with violence, i.e of the 1937 (79%) cases. Included were 387 reports of homicide, 404 reports of physical assaults, 223 cases of violence-related symptoms, and 896 reports of homicidal ideation. The drug that accounted for the most reports of violence was Champix (varenicline). Also associated with violence were two selective serotonin reuptake inhibitors used to treat depression, FLUoxetine (Prozac) and PARoxetine (Aropax, Extine, Paxtine, Paroxo), the attention deficit hyperactivity disorder drug atomoxetine (Strattera), amphetamines and the benzodiazepine triazolam. These data provide new evidence that violent acts are associated with a relatively small group of drugs, and that systematic studies are needed to establish the incidence, confirm differences among drugs, and identify additional common features. [Medication Safety Alert! 13 January 2011] documenting administration at the scheduled time, but actually giving the drug early or late; altering drug administration schedules to avoid documenting late administration; pre-pouring medications ahead of time to accelerate the drug administration process; skipping double-checks due to time constraints; failing to use barcode scanning to avoid documenting late administration; and leaving medications in the room for the patient to take at the right time. These and other at-risk behaviours, which are unintended consequences of attempts to comply with the CMS 30-minute rule have led to harmful errors. On the other hand, for some critical patient populations, certain first doses and time-critical scheduled medications (e.g. rapid-acting insulin), delays in administration can result in harm. Therein lies the dilemma faced by nurses and pharmacists responsible for dispensing and administering medications in a timely and safe manner. A one-size-fits-all, inflexible requirement to administer all scheduled medications within 30 minutes before or after their scheduled time rule is ill-suited for the majority of medications that are not time critical. A rigid, standard requirement also does not encourage or allow health professionals to use critical thinking to prioritise patient care or to manage the unexpected, which is commonplace in healthcare. In a setting of competing priorities, administration of non-time-critical medications may be lower on the list of priorities relative to other more crucial time-sensitive clinical tasks. Since publication of the survey results, ISMP has been working towards documenting what we believe would represent best practices associated with timely administration of scheduled medications. Scheduled medications include all maintenance doses administered according to a standard, repeated cycle of frequency (e.g. q4h, QID, TID, BID, daily, weekly, monthly, annually). For the purpose of ISMP s guidelines, scheduled medications do not include: stat and now dose; first doses and loading doses; one-time doses; specifically timed doses (e.g. antibiotic for surgical patient 10 minutes before incision); on-call doses (e.g. pre-procedure sedation); time-sequenced or concomitant medications (e.g. chemotherapy and rescue drugs, n-acetylcysteine and iodinated contrast media); drugs administered at specific times to ensure accurate peak/trough/serum drug levels; investigational drugs in clinical trials; and prn medications. The Guidelines for Timely Administration of Medications represent a synthesis of ISMP expertise, review of available literature on the risks associated with early and delayed administration of maintenance drug therapy, and advice from survey participants and an expert clinical advisory group of more than 20 clinicians many of whom are practising in healthcare settings. The guidelines may seem detailed but timely medication administration is a multifaceted issue that cannot be managed appropriately with a single standard. We also plan to interact with CMS to discuss possible alterations to their guidelines to best facilitate both timely and safe drug administration. The Guidelines for Timely Administration of Medications (summary in Table 1) recommend: Guidelines for timely medication administration In our 9 September 2010 newsletter < letters/acutecare/articles/ pdf> we discussed the results of our survey regarding the requirement in the Centers for Medicare & Medicaid Services (CMS) Conditions of Participation Interpretive Guidelines to administer medications within 30 minutes before or after the scheduled time < som107ap_a_hospitals.pdf>, which directs surveyors to determine whether drugs [are] administered within 30 minutes of the scheduled time for administration. The survey elicited responses from almost nurses. Respondents made it clear that changes to drug delivery methods and gradual increases in the complexity of care, number of prescribed medications per patient, and number of patients assigned to each nurse have made the long-standing CMS 30-minute rule troublesome. Many nurses felt the rule was unsafe, impossible to follow, and often unnecessary from a clinical perspective. Aware that nurses were struggling to comply with the rule and that surveyors were sometimes citing hospitals for noncompliance, we conducted the survey to prepare for interaction with CMS leaders to discuss our concerns regarding the rule s unintended effects on patient safety. Preliminary interaction has taken place, and CMS has indicated openness to suggestions for improvement. Respondent nurses reported feeling pressured to take these and other short cuts to comply with the CMS 30- minute rule, such as: preparing IV solutions on the unit, and bypassing pharmacy verification and specialised aseptic techniques; administering medications before conducting a physical assessment of the patient and/or checking vital signs, lab values, weight, and allergy status; removing medications from automated dispensing cabinets via override without a pharmacist s review; borrowing medications from one patient to administer to another patient; not thinking critically about drug administration while rushing through verification of new orders and actual drug administration; 53
3 1. Identify a hospital-specific list of time-critical scheduled medications (those where early or delayed administration of maintenance doses of greater than 30 minutes may cause harm or result in substantial suboptimal therapy or pharmacological effect). While the list of time-critical scheduled medications will include a limited number of drugs, a universal list is not desirable because hospitals that treat different patient populations (e.g. mental health, oncology, pediatrics, premature infants) may need to include different medications to address respective risks. Similarly, some hospitals that serve very diverse patient populations may decide to identify both hospital-wide and unit-specific time-critical medications. Examples of time-critical scheduled medications include: immunosuppressives used to prevention solidorgan transplant rejection or to treat myasthenia gravis; and drugs that require administration within a specified short period of time before, after, or with meals, e.g. rapid-, short-, or ultra-short-acting insulins, certain oral antidiabetics (e.g. acarbose, repaglinide, glimepiride), alendronate and pancrelipase. Additionally, because some scheduled medications can be time-critical for certain patients given their diagnoses (e.g. scheduled parenteral anti-infectives for worsening sepsis), the list may include drugs that are time-critical only when used for a specific diagnosis or indication. Further, policies should allow prescribers, pharmacists, or nurses to declare any medication to be time-critical (i.e. must be given within 30 minutes before or after the scheduled time) by including this designation with the medication order and/or medication administration record (MAR) entry. 2. Establish guidelines that facilitate pharmacy order review, dispensing, and nurse administration of the hospital-identified, time-critical scheduled medications within 30 minutes before or 30 minutes after the scheduled time (or more exact timing when indicated, as with rapid-, short-, and ultrashort-acting insulin). MAR entries for hospital-identified timecritical scheduled medications should be clearly designated to remind staff that these drugs require meticulous attention to timely administration (e.g. administered at the designated time, or plus or minus 30 minutes from the scheduled time, if appropriate). 3. Establish guidelines for timely drug administration of scheduled non-time critical medications as follows: administer daily, weekly, or monthly medications plus or minus 2 hours from the scheduled time. (It is generally safe to administer daily/weekly/monthly medications within a timeframe that exceeds 2 hours, ISMP recommends keeping the timeframe to 2 hours before or after the scheduled time to prevent accidental omission of doses that might be more easily forgotten if delayed more than 2 hours.) administer medications given more frequently than daily but not more frequently than every 4 hours (e.g. BID, TID, q4h, q6h) plus or minus 1 hour from the scheduled time. administer medications given more frequently than every 4 hours (e.g. q1h, q2h, q3h) within 25% of the dosing interval (e.g. ± 15 minutes for hourly doses, ± 30 minutes for every 2 hours dosing, ± 45 minutes for every 3 hours dosing). As an alternative to the guidelines in item 3 (above), hospitals with emars may choose to establish a standard allowable percent of difference between the scheduled and administration times not to exceed 25% for all non-time-critical medications if the emars can alert nurses in advance to an impending late dose and the allowable timeframe for administration. 4. Although not associated with the timing of scheduled medications, hospitals should also define targeted timeframes for administering first doses and loading doses of key medications, such as IV antiinfectives, IV anticoagulants, and IV antiepileptics where timeliness is critical (e.g. ED patient with suspected sepsis should not wait several hours for administration of a prescribed anti-infective). Where electronic prescribing systems are available, the prescriber can also be queried regarding when the first dose should be administered given the standard administration times. While timely administration of first or loading doses of these drugs may be critical, many are not necessarily time-critical when it comes to subsequent maintenance doses. The targeted timeframes for first or loading doses of medications should be accompanied by procedures that facilitate achievement of the administration time goals. [Medication Safety Alert! 13 January 2011] Adjust Pradaxa dose for renal impairment We hope health professionals are paying as much attention to safe prescribing of the oral direct thrombin inhibitor Pradaxa (dabigatran etexilate mesylate) as they would warfarin. In a recent report, Pradaxa was prescribed by a cardiologist for a patient with Stage IV chronic kidney disease. The dose was not adjusted for the patient s renal impairment. Instead of the recommended dose of 75 mg BID, the patient received 150 mg BID. On a follow-up visit with the cardiologist, the patient complained of weakness, black tarry stools, and was found to have a low haemoglobin and haematocrit. The cardiologist immediately sent the patient to a local ED. The patient was admitted to the intensive care unit with a massive gastrointestinal bleed and given multiple units of fresh frozen plasma and packed red blood cells. His haematocrit and haemoglobin returned to safe levels, allowing transfer to a progressive care unit. Among the precautions for Pradaxa are several drug interactions and a recommended dose of 75 mg BID for patients with a creatinine clearance of 15 to 30 ml/min. Dosing recommendations for patients with a creatinine clearance below 15 ml/min or on dialysis are not provided on the label. 54
4 Nicotine nitroglycerin patch mix-up A pharmacist told us about two incidents in which nicotine transdermal patches were dispensed to patients instead of nitroglycerin patches. The patches were stored alphabetically by generic name in the pharmacy where technicians manually pulled the medications; they were not dispensed by an automated robot. The nicotine and nitroglycerin patches wound up side-by-side and were in similar-looking packages. Since the incident, the pharmacy has changed and re-labelled the shelving locations of these products and made staff aware of the newly located stock. Nicotine patches kept in some clinical areas have also been removed since providing nicotine in place of nitroglycerin, or nitroglycerin in place of nicotine, is likely to cause serious adverse effects. the expected dose in 16% of syringes, suggesting that unrecognised medication errors may be a major source of morbidity and mortality in resuscitated patients, including children. The types of errors that occur during codes are varied. However, numerous studies have suggested that the most common types of errors that occur during codes are dosing errors, drug selection errors, drug preparation errors, administration technique errors and omissions. Around a quarter of these errors originate during dispensing/preparation of the drug; about half originate during administration; and about 10% occur during prescribing. In addition, a study of mock pediatric resuscitations showed that approximately 1 in 5 verbal orders did not specify an exact dose and around half did not include the route of administration. The wrong dose prescribing errors that occurred during this study included 10-fold overdoses, total daily doses Preventing medication errors during codes Problem: Cardiac and/or respiratory resuscitation is an extremely stressful situation during which health professionals have little time for discussion and verification of the patient s treatment plan, including medications. During these medical emergencies, often referred to as a code blue every second counts and every ordered as a single dose, and ordering the wrong concentration of dextrose for an infant. Furthermore, around 12% of the ordered drugs in this study were never administered. Examples of actual errors that have been reported to the ISMP Medication Errors Reporting Program or errors published in the referenced journal articles are provided in Table 1. Most often, these errors errant action or inaction can result in patient harm or involve reliance on human calculations, death. While studies on medication errors during codes have documented variable incidence rates from less than 1% when studying reported events to 15% from observations during simulations two facts are clear: patient harm from medication errors during code conditions is high. Serious errors are often missed, even if the patient died or is harmed, because there was no suspicion that the drugs caused or contributed to the morbidity or mortality. One study documented that medication errors during codes are 39 times more likely to result in harm and 51 times more likely to result in death than non-code related medication errors. This finding is not surprising because many of the drugs administered during medical emergencies are high-alert and patients are at their most vulnerable. Another study analysed the actual drug content in syringes used during code simulations and found substantial deviations from miscommunication, protocol deviation, knowledge deficit, or a dispensing device problem. Commonly reported contributing factors include: look-alike packaging or drug names; disorganised and non-standard code carts; excessive stock in code carts; distractions caused by an hectic environment; poorly communicated verbal orders; inexperienced staff; alternative drugs in code carts during a drug shortage; confusing or missing information about drugs; and multiple concentrations of a drug in code cart drawers. A 2008 study also highlighted that patients involved in codes are not the only ones who may be affected by medication errors. Other patients assigned to staff who attend the code may also be victims of errors, i.e. collateral damage. These medication errors, which were rarely harmful, were mostly missed respiratory treatments that were supposed to be administered by respiratory therapists who were busy attending a code. Safe Practice Recommendations: Ideally, reducing the incidence of emergency resuscitations would simultaneously reduce the incidence of errors during codes. Interventions such as ensuring adequate patientto-staff ratios for appropriate monitoring and the ability to activate rapid response teams to address ongoing concerns with the patient s condition before it deteriorates into a code situation can help. However, there will always be unstable patients who are vulnerable to codes, and patients who arrive at the hospital in full cardiac and/or respiratory arrest. Thus, organisations need to address medication error risks that may be present during these unique and often chaotic situations. One study that examined medication errors associated with codes showed that action to reduce the risk of similar errors was documented for only 29% of the errors. When action was taken, the most common strategies were to inform staff who made the error and provide additional education both low leverage strategies. Less than 5% of the errors resulted in system- or policy-level action. Table 2 provides a list of strategies to decrease the risk of medication errors during codes. The list can be used to assess current practices and establish an action plan with high-leverage strategies that are more likely to reduce the risk of errors during codes. 55
5 56
Office of Clinical Standards and Quality / Survey & Certification Group
 DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services 7500 Security Boulevard, Mail Stop C2-21-16 Baltimore, Maryland 21244-1850 Office of Clinical Standards and Quality / Survey
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services 7500 Security Boulevard, Mail Stop C2-21-16 Baltimore, Maryland 21244-1850 Office of Clinical Standards and Quality / Survey
Administrative Policies and Procedures for MOH hospitals /PHC Centers. TITLE: Organization & Management Of Medication Use APPLIES TO: Hospital-wide
 Administrative Policies and Procedures for MOH hospitals /PHC Centers TITLE: Organization & Management Of Medication Use APPLIES TO: Hospital-wide NO. OF PAGES: ORIGINAL DATE: REVISION DATE : السیاسات
Administrative Policies and Procedures for MOH hospitals /PHC Centers TITLE: Organization & Management Of Medication Use APPLIES TO: Hospital-wide NO. OF PAGES: ORIGINAL DATE: REVISION DATE : السیاسات
Evolution of a Closed Loop Medication Use Process
 Evolution of a Closed Loop Medication Use Process Paul J. Vitale, Pharm.D. pvitale@mdmercy.com Vice President and Chief Pharmacy Officer The Mercy Medical Center Baltimore, Maryland Agenda Hospital Background
Evolution of a Closed Loop Medication Use Process Paul J. Vitale, Pharm.D. pvitale@mdmercy.com Vice President and Chief Pharmacy Officer The Mercy Medical Center Baltimore, Maryland Agenda Hospital Background
DISPENSING HIGH RISK/ALERT MEDICATIONS. Lana Gordineer, MSN, RN Diabetes Educator
 DISPENSING HIGH RISK/ALERT MEDICATIONS Lana Gordineer, MSN, RN Diabetes Educator HIGH RISK/ALERT MEDICATIONS (or DRUGS) Medications that have a high risk of causing serious injury or death to a patient
DISPENSING HIGH RISK/ALERT MEDICATIONS Lana Gordineer, MSN, RN Diabetes Educator HIGH RISK/ALERT MEDICATIONS (or DRUGS) Medications that have a high risk of causing serious injury or death to a patient
Guidelines on Counseling. Approved by PEIPB
 Guidelines on Counseling Approved by PEIPB November 2005 1 Patient Counseling Patient counseling is a key competency element of the Pharmaceutical Care process. Given the advertising for medication in
Guidelines on Counseling Approved by PEIPB November 2005 1 Patient Counseling Patient counseling is a key competency element of the Pharmaceutical Care process. Given the advertising for medication in
Introduction. Background to this event. Raising awareness 09/11/2015
 Introduction Primary Care Medicines Governance HSCB Background to this event New class of medicines Availability of training Increasing volume of prescriptions Reports of medication incidents Raising awareness
Introduction Primary Care Medicines Governance HSCB Background to this event New class of medicines Availability of training Increasing volume of prescriptions Reports of medication incidents Raising awareness
Overview of emar Electronic Medication Administration Record
 Overview of emar Electronic Medication Administration Record March 2006 WHAT IS emar? emar Electronic Medication Administration Record - Replaces the paper MAR MAK Medication Administration Check (Siemens)
Overview of emar Electronic Medication Administration Record March 2006 WHAT IS emar? emar Electronic Medication Administration Record - Replaces the paper MAR MAK Medication Administration Check (Siemens)
SECTION N: MEDICATIONS. N0300: Injections. Item Rationale Health-related Quality of Life. Planning for Care. Steps for Assessment. Coding Instructions
 SECTION N: MEDICATIONS Intent: The intent of the items in this section is to record the number of days, during the last 7 days (or since admission/reentry if less than 7 days) that any type of injection,
SECTION N: MEDICATIONS Intent: The intent of the items in this section is to record the number of days, during the last 7 days (or since admission/reentry if less than 7 days) that any type of injection,
Medicines reconciliation on admission and discharge from hospital policy April 2013. WHSCT medicines reconciliation policy 1
 Medicines reconciliation on admission and discharge from hospital policy April 2013 WHSCT medicines reconciliation policy 1 Policy Title Policy Reference Number Medicines reconciliation on admission and
Medicines reconciliation on admission and discharge from hospital policy April 2013 WHSCT medicines reconciliation policy 1 Policy Title Policy Reference Number Medicines reconciliation on admission and
Center for Clinical Standards and Quality/Survey & Certification Group
 DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services 7500 Security Boulevard, Mail Stop C2-21-16 Baltimore, Maryland 21244-1850 Center for Clinical Standards and Quality/Survey
DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services 7500 Security Boulevard, Mail Stop C2-21-16 Baltimore, Maryland 21244-1850 Center for Clinical Standards and Quality/Survey
A Pharmacy Pharmacist, Ms B. A Report by the Health and Disability Commissioner. (Case 04HDC13191)
 A Pharmacy Pharmacist, Ms B A Report by the Health and Disability Commissioner (Case 04HDC13191) Opinion/04HDC13191 Parties involved Mrs A Miss A Ms B Pharmacy Complainant Consumer Provider Pharmacy Complaint
A Pharmacy Pharmacist, Ms B A Report by the Health and Disability Commissioner (Case 04HDC13191) Opinion/04HDC13191 Parties involved Mrs A Miss A Ms B Pharmacy Complainant Consumer Provider Pharmacy Complaint
Community Pharmacies
 High-Alert Medication Modeling and Error-Reduction Scorecards (HAMMERS ) Workbook For Community Pharmacies Data Entry Errors (Patient) Data Entry Errors (Drug) Prescribing Errors Point of Sale Errors Drug
High-Alert Medication Modeling and Error-Reduction Scorecards (HAMMERS ) Workbook For Community Pharmacies Data Entry Errors (Patient) Data Entry Errors (Drug) Prescribing Errors Point of Sale Errors Drug
SafetyFirst Alert. Errors in Transcribing and Administering Medications
 SafetyFirst Alert Massachusetts Coalition for the Prevention of Medical Errors January 2001 This issue of Safety First Alert is a publication of the Massachusetts Coalition for the Prevention of Medical
SafetyFirst Alert Massachusetts Coalition for the Prevention of Medical Errors January 2001 This issue of Safety First Alert is a publication of the Massachusetts Coalition for the Prevention of Medical
Key Element VIII: Staff Competency and Education
 Key Element VIII: Staff Competency and Education Practitioners and support staff receive sufficient training and orientation to the dispensing process and medication error prevention, and undergo baseline
Key Element VIII: Staff Competency and Education Practitioners and support staff receive sufficient training and orientation to the dispensing process and medication error prevention, and undergo baseline
Reducing the risk of patient harm: A focus on insulin
 Reducing the risk of patient harm: A focus on insulin New York State Partnership for Patients (NYSPFP) Initiative Regional Educational Session November 2013 1 1 Disclosure Matt Fricker, Matt Grissinger,
Reducing the risk of patient harm: A focus on insulin New York State Partnership for Patients (NYSPFP) Initiative Regional Educational Session November 2013 1 1 Disclosure Matt Fricker, Matt Grissinger,
Medication Safety and Error Prevention
 Medication Safety and Error Prevention 16 LEARNING OBJECTIVES By the end of this chapter, students will be able to competently: 1. Explain the process for reporting errors. 2. Explain the difference between
Medication Safety and Error Prevention 16 LEARNING OBJECTIVES By the end of this chapter, students will be able to competently: 1. Explain the process for reporting errors. 2. Explain the difference between
Dabigatran (Pradaxa) Guidelines
 Dabigatran (Pradaxa) Guidelines Dabigatran is a new anticoagulant for reducing the risk of stroke in patients with atrial fibrillation. Dabigatran is a direct thrombin inhibitor, similar to warfarin, without
Dabigatran (Pradaxa) Guidelines Dabigatran is a new anticoagulant for reducing the risk of stroke in patients with atrial fibrillation. Dabigatran is a direct thrombin inhibitor, similar to warfarin, without
Licensed Pharmacy Technician Scope of Practice
 Licensed Scope of Practice Adapted from: Request for Regulation of s Approved by Council April 24, 2015 Definitions In this policy: Act means The Pharmacy and Pharmacy Disciplines Act means an unregulated
Licensed Scope of Practice Adapted from: Request for Regulation of s Approved by Council April 24, 2015 Definitions In this policy: Act means The Pharmacy and Pharmacy Disciplines Act means an unregulated
Safe IV Compounding Procedures: The Release of ISMP Guidelines
 Safe IV Compounding Procedures: The Release of ISMP Guidelines Matthew P. Fricker, Jr., MS, RPh, FASHP, Program Director Institute for Safe Medication Practices 1 Objectives List system based causes of
Safe IV Compounding Procedures: The Release of ISMP Guidelines Matthew P. Fricker, Jr., MS, RPh, FASHP, Program Director Institute for Safe Medication Practices 1 Objectives List system based causes of
CNA and NSO Risk Control Self-assessment Checklist for Nurse Practitioners 1. Self-assessment topic Yes No Actions needed to reduce risks
 Risk Control Self-assessment Checklist for Nurse Practitioners This checklist is designed to help nurse practitioners evaluate risk exposures associated with their current practice. For additional nurse
Risk Control Self-assessment Checklist for Nurse Practitioners This checklist is designed to help nurse practitioners evaluate risk exposures associated with their current practice. For additional nurse
Guidance on adverse drug reactions
 Guidance on adverse drug reactions Classification of adverse drug reactions Adverse drug reactions are frequently classified as type A and type B reactions. An extended version of this classification system
Guidance on adverse drug reactions Classification of adverse drug reactions Adverse drug reactions are frequently classified as type A and type B reactions. An extended version of this classification system
CORONER S REPORT REPORT
 CORONER S REPORT 26 THE CORONER HAS RECOMMENDED THAT THE ONTARIO COLLEGE OF PHARMACISTS EDUCATE CLINICIANS ON THE DEFINITION OF OPIOID TOLERANCE, AND REVIEW THE PATIENT CONDITIONS AND COMORBIDITIES THAT
CORONER S REPORT 26 THE CORONER HAS RECOMMENDED THAT THE ONTARIO COLLEGE OF PHARMACISTS EDUCATE CLINICIANS ON THE DEFINITION OF OPIOID TOLERANCE, AND REVIEW THE PATIENT CONDITIONS AND COMORBIDITIES THAT
What Is Patient Safety?
 Patient Safety Research Introductory Course Session 1 What Is Patient Safety? David W. Bates, MD, MSc External Program Lead for Research, WHO Professor of Medicine, Harvard Medical School Professor of
Patient Safety Research Introductory Course Session 1 What Is Patient Safety? David W. Bates, MD, MSc External Program Lead for Research, WHO Professor of Medicine, Harvard Medical School Professor of
An introduction to High Risk Medications
 An introduction to High Risk Medications Une politique sure des médicaments : déjà deux approches 25 octobre 2013 Danguy Christine Hôpital A. Vésale An introduction to High Risk Medications 1. High Risk
An introduction to High Risk Medications Une politique sure des médicaments : déjà deux approches 25 octobre 2013 Danguy Christine Hôpital A. Vésale An introduction to High Risk Medications 1. High Risk
NCC MERP Taxonomy of Medication Errors 1
 Preamble NCC MERP Taxonomy of Medication Errors 1 This document provides a standard taxonomy of medication errors to be used in combination with systems analysis in recording and tracking of medication
Preamble NCC MERP Taxonomy of Medication Errors 1 This document provides a standard taxonomy of medication errors to be used in combination with systems analysis in recording and tracking of medication
Evidence Based Practice Information Sheets for Health Professionals. Strategies to reduce medication errors with reference to older adults
 Volume 9, issue 4, 2005 ISSN 1329-1874 BestPractice Evidence Based Practice Information Sheets for Health Professionals Information source Strategies to reduce medication errors with reference to older
Volume 9, issue 4, 2005 ISSN 1329-1874 BestPractice Evidence Based Practice Information Sheets for Health Professionals Information source Strategies to reduce medication errors with reference to older
Health Professions Act BYLAWS SCHEDULE F. PART 3 Residential Care Facilities and Homes Standards of Practice. Table of Contents
 Health Professions Act BYLAWS SCHEDULE F PART 3 Residential Care Facilities and Homes Standards of Practice Table of Contents 1. Application 2. Definitions 3. Supervision of Pharmacy Services in a Facility
Health Professions Act BYLAWS SCHEDULE F PART 3 Residential Care Facilities and Homes Standards of Practice Table of Contents 1. Application 2. Definitions 3. Supervision of Pharmacy Services in a Facility
Key Element V: Drug Standardization, Storage, and Distribution
 Key Element V: Drug Standardization, Storage, and Distribution Prescribed medications are accessible to patients and dispensed in a safe and secure manner. Medications and other necessary drug supplies
Key Element V: Drug Standardization, Storage, and Distribution Prescribed medications are accessible to patients and dispensed in a safe and secure manner. Medications and other necessary drug supplies
MEDICATION GUIDE WELLBUTRIN (WELL byu-trin) (bupropion hydrochloride) Tablets
 MEDICATION GUIDE WELLBUTRIN (WELL byu-trin) (bupropion hydrochloride) Tablets Read this Medication Guide carefully before you start using WELLBUTRIN and each time you get a refill. There may be new information.
MEDICATION GUIDE WELLBUTRIN (WELL byu-trin) (bupropion hydrochloride) Tablets Read this Medication Guide carefully before you start using WELLBUTRIN and each time you get a refill. There may be new information.
Safety indicators for inpatient and outpatient oral anticoagulant care
 Safety indicators for inpatient and outpatient oral anticoagulant care 1 Recommendations from the British Committee for Standards in Haematology (BCSH) & National Patient Safety Agency (NPSA) Address for
Safety indicators for inpatient and outpatient oral anticoagulant care 1 Recommendations from the British Committee for Standards in Haematology (BCSH) & National Patient Safety Agency (NPSA) Address for
ExCPT Certified Pharmacy Technician (CPhT) Detailed Test Plan* 100 scored items, 20 pretest items Exam time: 2 hours 10 minutes
 ExCPT Certified Pharmacy Technician (CPhT) Detailed Test Plan* 100 scored items, 20 pretest items Exam time: 2 hours 10 minutes # scored items 1. Regulations and Pharmacy Duties 35 A. Overview of technician
ExCPT Certified Pharmacy Technician (CPhT) Detailed Test Plan* 100 scored items, 20 pretest items Exam time: 2 hours 10 minutes # scored items 1. Regulations and Pharmacy Duties 35 A. Overview of technician
Revised: X Date: 04/07/14. 1. All medication orders shall be reviewed, signed, or co-signed by a Registered Nurse (RN).
 Vermont Psychiatric Care Hospital Procedure Revised: X Date: 04/07/14 I. Medication Orders 1. All medication orders shall be reviewed, signed, or co-signed by a Registered Nurse (RN). 2. Medication orders
Vermont Psychiatric Care Hospital Procedure Revised: X Date: 04/07/14 I. Medication Orders 1. All medication orders shall be reviewed, signed, or co-signed by a Registered Nurse (RN). 2. Medication orders
ORAL ANTICOAGULANTS RIVAROXABAN (XARELTO) FOR PULMONARY EMBOLISM (PE)
 ORAL ANTICOAGULANTS RIVAROXABAN (XARELTO) FOR PULMONARY EMBOLISM (PE) Information Leaflet Your Health. Our Priority. Page 2 of 6 What Are Anticoagulants And What Do They Do? This information leaflet has
ORAL ANTICOAGULANTS RIVAROXABAN (XARELTO) FOR PULMONARY EMBOLISM (PE) Information Leaflet Your Health. Our Priority. Page 2 of 6 What Are Anticoagulants And What Do They Do? This information leaflet has
ORAL ANTICOAGULANTS - RIVAROXABAN (XARELTO) FOR ATRIAL FIBRILLATION
 ORAL ANTICOAGULANTS - RIVAROXABAN (XARELTO) FOR ATRIAL FIBRILLATION Information Leaflet Your Health. Our Priority. Page 2 of 6 What Are Anticoagulants And What Do They Do? This information leaflet has
ORAL ANTICOAGULANTS - RIVAROXABAN (XARELTO) FOR ATRIAL FIBRILLATION Information Leaflet Your Health. Our Priority. Page 2 of 6 What Are Anticoagulants And What Do They Do? This information leaflet has
How To Improve Safety
 Medication safety in Australia an overview Margaret Duguid Pharmaceutical Advisor Australian Commission on Safety and Quality in Health Care 30 October 2009 Medication safety and quality Medications are
Medication safety in Australia an overview Margaret Duguid Pharmaceutical Advisor Australian Commission on Safety and Quality in Health Care 30 October 2009 Medication safety and quality Medications are
Paxil/Paxil-CR (paroxetine)
 Generic name: Paroxetine Available strengths: 10 mg, 20 mg, 30 mg, 40 mg tablets; 10 mg/5 ml oral suspension; 12.5 mg, 25 mg, 37.5 mg controlled-release tablets (Paxil-CR) Available in generic: Yes, except
Generic name: Paroxetine Available strengths: 10 mg, 20 mg, 30 mg, 40 mg tablets; 10 mg/5 ml oral suspension; 12.5 mg, 25 mg, 37.5 mg controlled-release tablets (Paxil-CR) Available in generic: Yes, except
Keeping patients safe when they transfer between care providers getting the medicines right
 PART 1 Keeping patients safe when they transfer between care providers getting the medicines right Good practice guidance for healthcare professions July 2011 Endorsed by: Foreword Taking a medicine is
PART 1 Keeping patients safe when they transfer between care providers getting the medicines right Good practice guidance for healthcare professions July 2011 Endorsed by: Foreword Taking a medicine is
Reducing harm from high-alert medications
 Institute for Healthcare Improvement s 5 Million Lives Campaign Best-practice protocols: Reducing harm from high-alert medications The Institute for Healthcare Improvement challenges clinicians and administrators
Institute for Healthcare Improvement s 5 Million Lives Campaign Best-practice protocols: Reducing harm from high-alert medications The Institute for Healthcare Improvement challenges clinicians and administrators
ADMINISTRATION OF MEDICATIONS POLICY
 Policy 6.007. ADMINISTRATION OF MEDICATIONS POLICY It is the policy of Cooperative Educational Services (C.E.S.) that students who require any medications to be administered during school hours, including
Policy 6.007. ADMINISTRATION OF MEDICATIONS POLICY It is the policy of Cooperative Educational Services (C.E.S.) that students who require any medications to be administered during school hours, including
Adverse Drug Events and Medication Safety: Diabetes Agents and Hypoglycemia
 Adverse Drug Events and Medication Safety: Diabetes Agents and Hypoglycemia Date: October 20, 2015 Presented by Mike Crooks, PharmD., PCMH-CCE Pharmacy Interventions, Technical Lead 11/9/2015 1 Objectives:
Adverse Drug Events and Medication Safety: Diabetes Agents and Hypoglycemia Date: October 20, 2015 Presented by Mike Crooks, PharmD., PCMH-CCE Pharmacy Interventions, Technical Lead 11/9/2015 1 Objectives:
Service Specification Template Department of Health, updated June 2015
 Service Specification Template Department of Health, updated June 2015 Service Specification No. : 2 Service: Commissioner Lead: Provider Lead: Period: Anti-coagulation monitoring Date of Review: 31 st
Service Specification Template Department of Health, updated June 2015 Service Specification No. : 2 Service: Commissioner Lead: Provider Lead: Period: Anti-coagulation monitoring Date of Review: 31 st
Medications: A Double-Edged Sword Family Caregiver Alliance
 Any symptom in an elderly patient should be considered a drug side effect until proved otherwise. Brown University Long-term Care Quality Letter, 1995. Modern medicines have contributed to longer life
Any symptom in an elderly patient should be considered a drug side effect until proved otherwise. Brown University Long-term Care Quality Letter, 1995. Modern medicines have contributed to longer life
NHS Lanarkshire Care Homes Protocol Group. Care Home Prescriptions - Good Practice Guide
 NHS Lanarkshire Care Homes Protocol Group Care Home Prescriptions - Good Practice Guide Date of Publication Review Date August 2015 Responsible Author Francesca Aaen Care Homes Pharmacist on behalf of
NHS Lanarkshire Care Homes Protocol Group Care Home Prescriptions - Good Practice Guide Date of Publication Review Date August 2015 Responsible Author Francesca Aaen Care Homes Pharmacist on behalf of
Reducing Medical Errors with an Electronic Medical Records System
 Reducing Medical Errors with an Electronic Medical Records System A recent report by the Institute of Medicine estimated that as many as 98,000 people die in any given year from medical errors in hospitals
Reducing Medical Errors with an Electronic Medical Records System A recent report by the Institute of Medicine estimated that as many as 98,000 people die in any given year from medical errors in hospitals
Standards of Practice for Pharmacists and Pharmacy Technicians
 Standards of Practice for Pharmacists and Pharmacy Technicians Introduction These standards are made under the authority of Section 133 of the Health Professions Act. They are one component of the law
Standards of Practice for Pharmacists and Pharmacy Technicians Introduction These standards are made under the authority of Section 133 of the Health Professions Act. They are one component of the law
Understanding Alberta s Drug Schedules
 Understanding Alberta s Drug Schedules Preface In May 2002, the provincial drug schedules to the Pharmaceutical Profession Act were amended. In April 2007, the Alberta Regulation 66/2007 to the Pharmacy
Understanding Alberta s Drug Schedules Preface In May 2002, the provincial drug schedules to the Pharmaceutical Profession Act were amended. In April 2007, the Alberta Regulation 66/2007 to the Pharmacy
Recommendations for the Prevention and Control of Influenza in Nursing Homes Virginia Department of Health
 Recommendations for the Prevention and Control of Influenza in Nursing Homes Virginia Department of Health Settings such as nursing homes that house persons at high risk for influenza-related complications
Recommendations for the Prevention and Control of Influenza in Nursing Homes Virginia Department of Health Settings such as nursing homes that house persons at high risk for influenza-related complications
UNIVERSITY OF WISCONSIN HOSPITAL AND CLINICS DEPARTMENT OF PHARMACY SCOPE OF PATIENT CARE SERVICES FY 2014 October 1 st, 2014
 UNIVERSITY OF WISCONSIN HOSPITAL AND CLINICS DEPARTMENT OF PHARMACY SCOPE OF PATIENT CARE SERVICES FY 2014 October 1 st, 2014 Department Name: Department of Pharmacy Department Director: Steve Rough, MS,
UNIVERSITY OF WISCONSIN HOSPITAL AND CLINICS DEPARTMENT OF PHARMACY SCOPE OF PATIENT CARE SERVICES FY 2014 October 1 st, 2014 Department Name: Department of Pharmacy Department Director: Steve Rough, MS,
IT requirement specification Safety alert. Oral methotrexate 2.5mg and 10mg tablets
 IT requirement specification Safety alert Oral methotrexate 2.5mg and 10mg tablets Synopsis This document sets out the IT requirements for addressing methotrexate-related patient safety incidents that
IT requirement specification Safety alert Oral methotrexate 2.5mg and 10mg tablets Synopsis This document sets out the IT requirements for addressing methotrexate-related patient safety incidents that
Arkansas Emergency Department Opioid Prescribing Guidelines
 Arkansas Emergency Department Opioid Prescribing Guidelines 1. One medical provider should provide all opioids to treat a patient s chronic pain. 2. The administration of intravenous and intramuscular
Arkansas Emergency Department Opioid Prescribing Guidelines 1. One medical provider should provide all opioids to treat a patient s chronic pain. 2. The administration of intravenous and intramuscular
Inpatient Anticoagulation Safety. To provide safe and effective anticoagulation therapy through a collaborative approach.
 Inpatient Anticoagulation Safety Purpose: Policy: To provide safe and effective anticoagulation therapy through a collaborative approach. Upon the written order of a physician, Heparin, Low Molecular Weight
Inpatient Anticoagulation Safety Purpose: Policy: To provide safe and effective anticoagulation therapy through a collaborative approach. Upon the written order of a physician, Heparin, Low Molecular Weight
PHARMACY TECHNICIAN CCAPP Accredited Program Provisional Status
 PHARMACY TECHNICIAN CCAPP Accredited Program Provisional Status Program Overview As a result of pharmacists taking a more active role in clinical drug therapy and the counselling of their patients, the
PHARMACY TECHNICIAN CCAPP Accredited Program Provisional Status Program Overview As a result of pharmacists taking a more active role in clinical drug therapy and the counselling of their patients, the
East & South East England Specialist Pharmacy Services Medicines Use and Safety Division Community Health Services Transcribing
 East & outh East England pecialist Pharmacy ervices East of England, London, outh Central & outh East Coast Medicines Use and afety Division Community Health ervices Transcribing Guidance to support the
East & outh East England pecialist Pharmacy ervices East of England, London, outh Central & outh East Coast Medicines Use and afety Division Community Health ervices Transcribing Guidance to support the
ICD-10-CM Official Guidelines for Coding and Reporting
 2013 Narrative changes appear in bold text Items underlined have been moved within the guidelines since the 2012 version Italics are used to indicate revisions to heading changes The Centers for Medicare
2013 Narrative changes appear in bold text Items underlined have been moved within the guidelines since the 2012 version Italics are used to indicate revisions to heading changes The Centers for Medicare
Stowe School Medications Policy
 INTRODUCTION Most pupils will need medication at some stage of their school life. Although this will mainly be for short periods there are a few pupils with chronic conditions who may require regular medication
INTRODUCTION Most pupils will need medication at some stage of their school life. Although this will mainly be for short periods there are a few pupils with chronic conditions who may require regular medication
MEDICATION GUIDE. Bupropion Hydrochloride (bue-proe-pee-on HYE-droe-KLOR-ide) Extended-Release Tablets, USP (SR)
 MEDICATION GUIDE Bupropion Hydrochloride (bue-proe-pee-on HYE-droe-KLOR-ide) Extended-Release Tablets, USP (SR) Read this Medication Guide carefully before you start taking bupropion hydrochloride extendedrelease
MEDICATION GUIDE Bupropion Hydrochloride (bue-proe-pee-on HYE-droe-KLOR-ide) Extended-Release Tablets, USP (SR) Read this Medication Guide carefully before you start taking bupropion hydrochloride extendedrelease
Professional Standards and Guidance for the Sale and Supply of Medicines
 Professional Standards and Guidance for the Sale and Supply of Medicines About this document The Code of Ethics sets out seven principles of ethical practice that you must follow as a pharmacist or pharmacy
Professional Standards and Guidance for the Sale and Supply of Medicines About this document The Code of Ethics sets out seven principles of ethical practice that you must follow as a pharmacist or pharmacy
Optimizing Medication Administration in a Pediatric ER
 Optimizing Medication Administration in a Pediatric ER ER Pharmacist Review of First Dose Non-Emergent Medications Penny Williams, RN, MS Clinical Program Manager, Emergency Center Children s Medical Center
Optimizing Medication Administration in a Pediatric ER ER Pharmacist Review of First Dose Non-Emergent Medications Penny Williams, RN, MS Clinical Program Manager, Emergency Center Children s Medical Center
Nursing 113. Pharmacology Principles
 Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
Nursing 113 Pharmacology Principles 1. The study of how drugs enter the body, reach the site of action, and are removed from the body is called a. pharmacotherapeutics b. pharmacology c. pharmacodynamics
medication errors CNE 1.1 contact hours
 Preventing high-alert medication errors in hospital patients We ve made strides in preventing these errors but haven t reached our goal. By Pamela Anderson, MSN, RN, APRN-BC, CCRN, and Terri Townsend,
Preventing high-alert medication errors in hospital patients We ve made strides in preventing these errors but haven t reached our goal. By Pamela Anderson, MSN, RN, APRN-BC, CCRN, and Terri Townsend,
SELECTED OPIATES TOXICITY A MODERN DAY EPIDEMIC
 SELECTED OPIATES TOXICITY A MODERN DAY EPIDEMIC Learning Objectives: 1. Identify the names and reasons/circumstances for additional toxicity of SELECTED OPIATES hydromorphone DILAUDID Methadone Fentanyl/DURAGESIC
SELECTED OPIATES TOXICITY A MODERN DAY EPIDEMIC Learning Objectives: 1. Identify the names and reasons/circumstances for additional toxicity of SELECTED OPIATES hydromorphone DILAUDID Methadone Fentanyl/DURAGESIC
BOARD OF PHARMACY DIVISION 41 OPERATION OF PHARMACIES (RETAIL AND INSTITUTIONAL DRUG OUTLETS) CONSULTING PHARMACISTS AND OPERATION OF DRUG ROOMS
 BOARD OF PHARMACY DIVISION 41 OPERATION OF PHARMACIES (RETAIL AND INSTITUTIONAL DRUG OUTLETS) CONSULTING PHARMACISTS AND OPERATION OF DRUG ROOMS 855-041-6050 Definitions Hospitals with Pharmacies (1) In
BOARD OF PHARMACY DIVISION 41 OPERATION OF PHARMACIES (RETAIL AND INSTITUTIONAL DRUG OUTLETS) CONSULTING PHARMACISTS AND OPERATION OF DRUG ROOMS 855-041-6050 Definitions Hospitals with Pharmacies (1) In
Prescribing Standards for Nurse Practitioners (NPs)
 Prescribing Standards for Nurse Practitioners (NPs) July 2014 Approved by the College and Association of Registered Nurses of Alberta Provincial Council, (July 2014) Permission to reproduce this document
Prescribing Standards for Nurse Practitioners (NPs) July 2014 Approved by the College and Association of Registered Nurses of Alberta Provincial Council, (July 2014) Permission to reproduce this document
Disease Management Identifications and Stratification Health Risk Assessment Level 1: Level 2: Level 3: Stratification
 Disease Management UnitedHealthcare Disease Management (DM) programs are part of our innovative Care Management Program. Our Disease Management (DM) program is guided by the principles of the UnitedHealthcare
Disease Management UnitedHealthcare Disease Management (DM) programs are part of our innovative Care Management Program. Our Disease Management (DM) program is guided by the principles of the UnitedHealthcare
Reducing Medication Risks of Electronic Medication Systems
 Program Date: August 10, 2012 Geriatric Grand Rounds Topic: Reducing Medication Risks of Electronic Medication Systems Presenter: Laura Finn, CGP, FASCP, Consultant Pharmacist Adjunct Associate Professor
Program Date: August 10, 2012 Geriatric Grand Rounds Topic: Reducing Medication Risks of Electronic Medication Systems Presenter: Laura Finn, CGP, FASCP, Consultant Pharmacist Adjunct Associate Professor
RN CONSULTATION. KEY TERMS: Assessment Basic tasks Consultation Home health services PRN Written parameters OBJECTIVES:
 RN CONSULTATION The purpose of this section is to assist the learner in understanding the role of a registered nurse (RN) consultant and when to seek RN consultation. KEY TERMS: Assessment Basic tasks
RN CONSULTATION The purpose of this section is to assist the learner in understanding the role of a registered nurse (RN) consultant and when to seek RN consultation. KEY TERMS: Assessment Basic tasks
West Midlands Centre for ADRs. Jeffrey Aronson. Robin Ferner. Side Effects of Drugs Annuals. Editor Meyler s Side Effects of Drugs
 Do we have a common understanding of medication errors? Editor Meyler s Side Effects of Drugs Jeffrey Aronson Co-editor: Stephens Detection and Evaluation of Adverse Drug Reactions Side Effects of Drugs
Do we have a common understanding of medication errors? Editor Meyler s Side Effects of Drugs Jeffrey Aronson Co-editor: Stephens Detection and Evaluation of Adverse Drug Reactions Side Effects of Drugs
QUM and Continuity of Care A Prescribed Medicines Services and Programs
 Community Pharmacy Roadmap Program Development Template Program/Service: Quadrant: 1. Program/Service Description QUM and Continuity of Care A Prescribed Medicines Services and Programs a) Background The
Community Pharmacy Roadmap Program Development Template Program/Service: Quadrant: 1. Program/Service Description QUM and Continuity of Care A Prescribed Medicines Services and Programs a) Background The
Maintenance of abstinence in alcohol dependence
 Shared Care Guideline for Prescription and monitoring of Acamprosate Calcium Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist, Alcohol Services Dr Donnelly
Shared Care Guideline for Prescription and monitoring of Acamprosate Calcium Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist, Alcohol Services Dr Donnelly
PRESCRIBING FOR SMOKING CESSATION. (Adapted from the Self-Limiting Conditions Independent Study Program for Manitoba Pharmacists)
 PRESCRIBING FOR SMOKING CESSATION (Adapted from the Self-Limiting Conditions Independent Study Program for Manitoba Pharmacists) Acknowledgements The Self-Limiting Conditions Independent Study Program
PRESCRIBING FOR SMOKING CESSATION (Adapted from the Self-Limiting Conditions Independent Study Program for Manitoba Pharmacists) Acknowledgements The Self-Limiting Conditions Independent Study Program
Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada
 Background Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada The use of medications or drugs by non-physician health professionals is evolving and is linked to collaboration
Background Over the Counter Drugs (OTCs): Considerations for Physical Therapy Practice in Canada The use of medications or drugs by non-physician health professionals is evolving and is linked to collaboration
Cancer services children s CSCF v3.2
 Cancer services children s CSCF v3.2 Module overview Please note: This module should be read in conjunction with the Fundamentals of the Framework (including glossary and acronym list), Children s Services
Cancer services children s CSCF v3.2 Module overview Please note: This module should be read in conjunction with the Fundamentals of the Framework (including glossary and acronym list), Children s Services
www.uhs.nhs.uk/commercial
 Sue Ladds & Mark Pepperrell Chief Pharmacist & Deputy Chief Pharmacist University Hospital Southampton NHS Foundation Trust www.nhs.uhs.uk/commercial Medicines and Technology Medicines are the most common
Sue Ladds & Mark Pepperrell Chief Pharmacist & Deputy Chief Pharmacist University Hospital Southampton NHS Foundation Trust www.nhs.uhs.uk/commercial Medicines and Technology Medicines are the most common
Medication Management Guidelines for Nurses and Midwives
 Medication Management Guidelines for Nurses and Midwives 1. Introduction As the statutory body responsible for the regulation of nursing and midwifery practice in Western Australia (WA), the Nurses & Midwives
Medication Management Guidelines for Nurses and Midwives 1. Introduction As the statutory body responsible for the regulation of nursing and midwifery practice in Western Australia (WA), the Nurses & Midwives
MASSACHUSETTS. Downloaded January 2011
 MASSACHUSETTS Downloaded January 2011 150.007 NURSING SERVICES (G) Nursing and Supportive Routines and Practices. (2) No medication, treatment or therapeutic diet shall be administered to a patient or
MASSACHUSETTS Downloaded January 2011 150.007 NURSING SERVICES (G) Nursing and Supportive Routines and Practices. (2) No medication, treatment or therapeutic diet shall be administered to a patient or
COMPUTERIZED PROVIDER ORDER ENTRY: AD-HOC ORDERS
 COMPUTERIZED PROVIDER ORDER ENTRY: AD-HOC ORDERS Find Field Enter the desired order in the Find field. You may press Enter or select the Binoculars Icon to view all existing orderables. Orderable This
COMPUTERIZED PROVIDER ORDER ENTRY: AD-HOC ORDERS Find Field Enter the desired order in the Find field. You may press Enter or select the Binoculars Icon to view all existing orderables. Orderable This
Electronic Medication Administration Record (emar) (For Cerner Sites Only)
 POLICY NO. 1009 Approved: 12/05 Effective: 12/05 Reviewed: 9/10; 5/12 1. Purpose: Electronic Medication Administration Record (emar) (For Cerner Sites Only) To provide direction for the transcription and
POLICY NO. 1009 Approved: 12/05 Effective: 12/05 Reviewed: 9/10; 5/12 1. Purpose: Electronic Medication Administration Record (emar) (For Cerner Sites Only) To provide direction for the transcription and
LOUISIANA STATE UNIVERSITY HEALTH SCIENCES CENTER - SHREVEPORT MEDICAL RECORDS CONTENT/DOCUMENTATION
 LOUISIANA STATE UNIVERSITY HEALTH SCIENCES CENTER - SHREVEPORT MEDICAL RECORDS CONTENT/DOCUMENTATION Hospital Policy Manual Purpose: To define the components of the paper and electronic medical record
LOUISIANA STATE UNIVERSITY HEALTH SCIENCES CENTER - SHREVEPORT MEDICAL RECORDS CONTENT/DOCUMENTATION Hospital Policy Manual Purpose: To define the components of the paper and electronic medical record
GUIDELINES ON PREVENTING MEDICATION ERRORS IN PHARMACIES AND LONG-TERM CARE FACILITIES THROUGH REPORTING AND EVALUATION
 GUIDELINES GUIDELINES ON PREVENTING MEDICATION ERRORS IN PHARMACIES AND LONG-TERM CARE FACILITIES THROUGH REPORTING AND EVALUATION Preamble The purpose of this document is to provide guidance for the pharmacist
GUIDELINES GUIDELINES ON PREVENTING MEDICATION ERRORS IN PHARMACIES AND LONG-TERM CARE FACILITIES THROUGH REPORTING AND EVALUATION Preamble The purpose of this document is to provide guidance for the pharmacist
Program Approved by AoA, NCOA. Website: www.homemeds.org
 MEDICATION MANAGEMENT IMPROVEMENT SYSTEM: HomeMeds SM The HomeMeds SM system is a collaborative approach to identifying, assessing, and resolving medication problems in community-dwelling older adults.
MEDICATION MANAGEMENT IMPROVEMENT SYSTEM: HomeMeds SM The HomeMeds SM system is a collaborative approach to identifying, assessing, and resolving medication problems in community-dwelling older adults.
Automating the Pharmacy Medication Cycle in Acute Care Settings
 Automating the Pharmacy Medication Cycle in Acute Care Settings Costs, Benefits and Potential Unintended Consequences Enterprise Information Systems Steering Committee Nursing Informatics Committee and
Automating the Pharmacy Medication Cycle in Acute Care Settings Costs, Benefits and Potential Unintended Consequences Enterprise Information Systems Steering Committee Nursing Informatics Committee and
Adjunctive psychosocial intervention. Conditions requiring dose reduction. Immediate, peak plasma concentration is reached within 1 hour.
 Shared Care Guideline for Prescription and monitoring of Naltrexone Hydrochloride in alcohol dependence Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist,
Shared Care Guideline for Prescription and monitoring of Naltrexone Hydrochloride in alcohol dependence Author(s)/Originator(s): (please state author name and department) Dr Daly - Consultant Psychiatrist,
Guidelines for dispensing of medicines 6585 08/10
 for dispensing of medicines 6585 08/10 for dispensing of medicines Contents Introduction 1 Who needs to use these guidelines? 1 Summary of guidelines 1 1 1 Dispensing precaution safety of prescriptions
for dispensing of medicines 6585 08/10 for dispensing of medicines Contents Introduction 1 Who needs to use these guidelines? 1 Summary of guidelines 1 1 1 Dispensing precaution safety of prescriptions
Rivaroxaban (Xarelto) for preventing blood clots after hip or knee replacement surgery
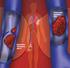 medicineupdate Asking the right questions about new medicines Rivaroxaban (Xarelto) for preventing blood clots after hip or knee replacement surgery This Medicine Update is for people who have been prescribed
medicineupdate Asking the right questions about new medicines Rivaroxaban (Xarelto) for preventing blood clots after hip or knee replacement surgery This Medicine Update is for people who have been prescribed
Stroke/VTE Quality Measure Build for Meaningful Use Stage 1
 Stroke/VTE Quality Measure Build for Meaningful Use Stage 1 Presented by Susan Haviland, BSN RN Senior Consult, Santa Rosa Consulting Meaningful Use Quality Measures Centers for Medicare and Medicaid Services
Stroke/VTE Quality Measure Build for Meaningful Use Stage 1 Presented by Susan Haviland, BSN RN Senior Consult, Santa Rosa Consulting Meaningful Use Quality Measures Centers for Medicare and Medicaid Services
FDA Approved Oral Anticoagulants
 FDA Approved Oral Anticoagulants Generic (Trade Name) Warfarin (Coumadin, Jantoven ) 1 FDA approved indication Prophylaxis and treatment of venous thromboembolism (VTE) Prophylaxis and treatment of thromboembolic
FDA Approved Oral Anticoagulants Generic (Trade Name) Warfarin (Coumadin, Jantoven ) 1 FDA approved indication Prophylaxis and treatment of venous thromboembolism (VTE) Prophylaxis and treatment of thromboembolic
ORAL ANTICOAGULANTS - RIVAROXABAN (XARELTO) FOR DEEP VEIN THROMBOSIS (DVT)
 ORAL ANTICOAGULANTS - RIVAROXABAN (XARELTO) FOR DEEP VEIN THROMBOSIS (DVT) Information Leaflet Your Health. Our Priority. Page 2 of 6 What Are Anticoagulants And What Do They Do? This information leaflet
ORAL ANTICOAGULANTS - RIVAROXABAN (XARELTO) FOR DEEP VEIN THROMBOSIS (DVT) Information Leaflet Your Health. Our Priority. Page 2 of 6 What Are Anticoagulants And What Do They Do? This information leaflet
ARTICLE 10. OUTPATIENT TREATMENT CENTERS
 Section R9-10-1001. R9-10-1002. R9-10-1003. R9-10-1004. R9-10-1005. R9-10-1006. R9-10-1007. R9-10-1008. R9-10-1009. R9-10-1010. R9-10-1011. R9-10-1012. R9-10-1013. R9-10-1014. R9-10-1015. R9-10-1016. R9-10-1017.
Section R9-10-1001. R9-10-1002. R9-10-1003. R9-10-1004. R9-10-1005. R9-10-1006. R9-10-1007. R9-10-1008. R9-10-1009. R9-10-1010. R9-10-1011. R9-10-1012. R9-10-1013. R9-10-1014. R9-10-1015. R9-10-1016. R9-10-1017.
The Massachusetts Coalition for the Prevention of Medical Errors. MHA Best Practice Recommendations to Reduce Medication Errors
 The Massachusetts Coalition for the Prevention of Medical Errors MHA Best Practice Recommendations to Reduce Medication Errors Executive Summary In 1997, the Massachusetts Coalition for the Prevention
The Massachusetts Coalition for the Prevention of Medical Errors MHA Best Practice Recommendations to Reduce Medication Errors Executive Summary In 1997, the Massachusetts Coalition for the Prevention
Seven steps to patient safety The full reference guide. Second print August 2004
 Seven steps to patient safety The full reference guide Second print August 2004 National Patient Safety Agency Seven steps to patient safety 113 Appendix Four F Examples of events according to severity
Seven steps to patient safety The full reference guide Second print August 2004 National Patient Safety Agency Seven steps to patient safety 113 Appendix Four F Examples of events according to severity
Breakfast symposium: From hospital to home - the focus on the patient
 Breakfast symposium: From hospital to home - the focus on the patient Nadya Hamedi DARZI Fellow UCLPartners and Barts Health NHS Trust in collaboration with North Central London Local Pharmaceutical Committee
Breakfast symposium: From hospital to home - the focus on the patient Nadya Hamedi DARZI Fellow UCLPartners and Barts Health NHS Trust in collaboration with North Central London Local Pharmaceutical Committee
What You Need to KnowWhen Taking Anticoagulation Medicine
 What You Need to KnowWhen Taking Anticoagulation Medicine What are anticoagulant medicines? Anticoagulant medicines are a group of medicines that inhibit blood clotting, helping to prevent blood clots.
What You Need to KnowWhen Taking Anticoagulation Medicine What are anticoagulant medicines? Anticoagulant medicines are a group of medicines that inhibit blood clotting, helping to prevent blood clots.
Memantine (Ebixa) Drug treatment for Alzheimer s disease
 IS 20 October 2011 Information sheet Memantine (Ebixa) Drug treatment for Alzheimer s disease Introduction... 1 How does Ebixa work?... 1 Who might benefit?... 2 What effect might Ebixa have?... 2 How
IS 20 October 2011 Information sheet Memantine (Ebixa) Drug treatment for Alzheimer s disease Introduction... 1 How does Ebixa work?... 1 Who might benefit?... 2 What effect might Ebixa have?... 2 How
Medication Guidelines
 Medication Guidelines January 2014 Approved by the College and Association of Registered Nurses of Alberta Provincial Council, January 2014. Second printing with editorial change (p16), November 2014.
Medication Guidelines January 2014 Approved by the College and Association of Registered Nurses of Alberta Provincial Council, January 2014. Second printing with editorial change (p16), November 2014.
Dorset Cardiac Centre
 P a g e 1 Dorset Cardiac Centre Patients with Atrial Fibrillation/Flutter undergoing DC Cardioversion or Ablation procedures- Guidelines for Novel Oral Anti-coagulants (NOACS) licensed for this use February
P a g e 1 Dorset Cardiac Centre Patients with Atrial Fibrillation/Flutter undergoing DC Cardioversion or Ablation procedures- Guidelines for Novel Oral Anti-coagulants (NOACS) licensed for this use February
Question & Answer Guide
 Joint Commission Primary Care Medical Home (PCMH) Certification for Accredited Ambulatory Health Care Organizations Question & Answer Guide A. SCORING/DECISION-RELATED Question: We are already Joint Commission
Joint Commission Primary Care Medical Home (PCMH) Certification for Accredited Ambulatory Health Care Organizations Question & Answer Guide A. SCORING/DECISION-RELATED Question: We are already Joint Commission
CONNECTICUT. Downloaded January 2011 19 13 D8T. CHRONIC AND CONVALESCENT NURSING HOMES AND REST HOMES WITH NURSING SUPERVISION
 CONNECTICUT Downloaded January 2011 19 13 D8T. CHRONIC AND CONVALESCENT NURSING HOMES AND REST HOMES WITH NURSING SUPERVISION (d) General Conditions. (6) All medications shall be administered only by licensed
CONNECTICUT Downloaded January 2011 19 13 D8T. CHRONIC AND CONVALESCENT NURSING HOMES AND REST HOMES WITH NURSING SUPERVISION (d) General Conditions. (6) All medications shall be administered only by licensed
Agency # 070.00 REGULATION 9 PHARMACEUTICAL CARE/PATIENT COUNSELING
 Agency # 070.00 REGULATION 9 PHARMACEUTICAL CARE/PATIENT COUNSELING 09-00: PATIENT COUNSELING 09-00-0001--PATIENT INFORMATION, DRUG USE EVALUATION, AND PATIENT COUNSELING The intent of this regulation
Agency # 070.00 REGULATION 9 PHARMACEUTICAL CARE/PATIENT COUNSELING 09-00: PATIENT COUNSELING 09-00-0001--PATIENT INFORMATION, DRUG USE EVALUATION, AND PATIENT COUNSELING The intent of this regulation
Overview of Mental Health Medication Trends
 America s State of Mind Report is a Medco Health Solutions, Inc. analysis examining trends in the utilization of mental health related medications among the insured population. The research reviewed prescription
America s State of Mind Report is a Medco Health Solutions, Inc. analysis examining trends in the utilization of mental health related medications among the insured population. The research reviewed prescription
Remeron (mirtazapine)
 Remeron (mirtazapine) FDA ALERT [07/2005] Suicidal Thoughts or Actions in Children and Adults Patients with depression or other mental illnesses often think about or attempt suicide. Closely watch anyone
Remeron (mirtazapine) FDA ALERT [07/2005] Suicidal Thoughts or Actions in Children and Adults Patients with depression or other mental illnesses often think about or attempt suicide. Closely watch anyone
