CALGB A Phase III Randomized Trial of Lobectomy versus Sublobar Resection for Small ( 2 cm) Peripheral Non-Small Cell Lung Cancer
|
|
|
- Vincent Holland
- 7 years ago
- Views:
Transcription
1 A Phase III Randomized Trial of Lobectomy versus Sublobar Resection for Small ( 2 cm) Peripheral Non-Small Cell Lung Cancer What is a research study or clinical trial? This is a clinical trial, a type of research study. Your study doctor will explain the clinical trial to you. Clinical trials include only people who choose to take part. Please take your time to make your decision about taking part. You may discuss your decision with your friends and family. You can also discuss it with your health care team. If you have any questions, you can ask your study doctor for more explanation. You are being asked to take part in this study because you have non-small cell lung cancer which needs to be surgically removed. Why is this study being done? The purpose of this study is to test a different way of doing surgery for lung cancer in patients who have small tumors with no evidence of spread. Recent studies have questioned whether removing a larger portion of the lung containing the tumor offers much better control of the cancer than removing a smaller section of the lung. The purpose of this clinical trial is to look at whether removal of a small section of lung (called a sublobar resection) is equal to a lobectomy (a larger surgery which takes out an entire section or lobe of the lung, or about one-quarter to one-half of one lung, depending on which lobe is taken out). This clinical trial will be studying the overall effects (good and/or bad) of a sublobar resection (smaller segment of your lung) compared to a lobectomy (entire lobe of your lung). How many people will take part in the study? About 1,400 people will take part in this study. What will happen if I take part in this research study? Routine Medical Tests The following tests and procedure must be done to make sure that you are eligible for this study. None of these tests are research. They are routine. Depending on when you last had them done you may need to repeat some of these tests. You will be asked to give your medical history and have a physical examination Pulmonary function tests (measures your lungs ability to take air in and out) Chest CT scan and x-ray, which can look inside of your chest to measure the tumor Your doctor may or may not decide to order a PET scan for you, which can show your doctor where a tumor is at in your body, and if it has spread beyond your lung Many of these tests will be repeated during the study. If you participate in this study, some of these tests may be done more frequently than if you were not taking part in this study. 6/15/07 Page 1 of 7 Participant Initials
2 Treatment Plan If you agree to participate you will be put into one of the two study groups described below. The group you are put into will be chosen by randomization. Randomization means that you are put into a group by chance. During the surgery, your surgeon will examine your tumor and determine if it is the correct size and be sure that the cancer has not spread. Once this is confirmed the research assistant will enter information into a computer program, and the computer program will then randomly place you into one of the study groups while you are still under anesthesia in the operating room. Neither you nor your doctor can choose the group you will be in. You will have an equal chance of being placed in either of the study groups. If you are in group 1 (often called Arm A ) you will have a standard operation for lung cancer called a lobectomy. If you are in group 2 (often called Arm B ) you will have a smaller portion of lung removed. Regardless of the type of surgery done, the entire tumor will be removed. The difference is the amount of surrounding normal lung tissue that is removed with the tumor. CALGB researchers are interested in reviewing your CT scans and PET scans (if obtained) to see how they relate to your prognosis. Therefore, copies of your scans and reports for the next 3 years will be sent to a CALGB central imaging center for review. How long will I be in the study? Before you have surgery on this study you will need to review and sign a separate permission form from your doctor/hospital for the surgery. If, during your operation, it is determined that you are not a candidate for randomization (if your tumor is too large, if it is not the right stage, etc.) then your doctor will decide which operation is right for you and you will no longer take part in this study. Before you have surgery your doctor will discuss what surgery will be done if the tumor does not meet the criteria for this study because of size or stage. Following your surgery the study doctor will ask you to visit the office for follow-up exams for at least every 3 months for 1 year, then every 6 months for 1 year, and then yearly for up to 5 years. You will need to have a pulmonary function test at 6 months following surgery. Additionally, you will need to have a chest CT at 6 and 12 months after surgery, and then yearly after that for up for 5 years. Can I stop being in the study? Yes. You can decide to stop at any time. Tell the study doctor if you are thinking about stopping or decide to stop. He or she will tell you how to stop safely. 6/15/07 Page 2 of 7 Participant Initials
3 It is important to tell the study doctor if you are thinking about stopping so any risks from the surgery can be evaluated by your doctor. Another reason to tell your doctor that you are thinking about stopping is to discuss what followup care and testing could be most helpful for you. The study doctor may stop you from taking part in this study at any time if he/she believes it is in your best interest; if you do not follow the study rules; or if the study is stopped. What side effects or risks can I expect from being in the study? You may have side effects while on the study. Everyone taking part in the study will be watched carefully for any side effects. However, doctors don t know all the side effects that may happen. Side effects may be mild or very serious. Some side effects may be unexpected or unforeseen. Your health care team may give you medicines to help lessen side effects. Both lobectomy and sublobar resection have risks associated with them. We believe that the risks may be less after sublobar resection, but it is possible they could be the same or even greater. There is a risk that sublobar resection will not be as effective at removing the cancer, which could possibly lead to a higher risk of the cancer returning. If the cancer returns, it may be less curable at that time. You should talk to your study doctor about any side effects that you have while taking part in the study. The risks of surgery include: Likely: Air leaking from the part of the lung that was operated on Less Likely: Pneumonia Rare: Infection in the area around the lung, wound infection or blood infection Bleeding Poor healing of the skin and/or muscles in the chest For more information about risks and side effects, ask your study doctor. Will I benefit from taking part in the study? Taking part in this study may or may not make your health better. While doctors hope a smaller resection will be as good as the usual treatment, this has not yet been proven. The reason for this study is to investigate this possibility. We do know that the information from this study will help doctors learn more about a sublobar resection when 6/15/07 Page 3 of 7 Participant Initials
4 compared to a lobectomy, as a treatment for cancer. This information could help future cancer patients. What other choices do I have if I do not take part in this study? Your other choices may include: Getting treatment or care for your cancer without being in this study (examples include having surgery without being on this study, or getting treatment other than surgery, like radiation or chemotherapy) Talk to your doctor about your choices before you decide if you will take part in this study. Will my medical information be kept private? We will do our best to make sure that the personal information in your medical record will be kept private. However, we cannot guarantee total privacy. Your personal information may be given out if required by law. If information from this study is published or presented at scientific meetings, your name and other personal information will not be used. A record of your progress will be kept in a confidential form at your hospital or doctor s office where you receive treatment. Organizations that may inspect and/or copy your research and medical records (blood samples, x-rays, scans, pathology slides, etc.) for quality assurance, research, and data analysis include groups such as: Southeast Cancer Control Consortium (SCCC) Operations Office Cancer and Leukemia Group B (CALGB) CALGB Imaging Core Laboratory National Cancer Institute (NCI) and its representatives Food and Drug Administration (FDA) Office for Human Research Protections (OHRP) Institutional Review Board (IRB) at Hospital Possible other federal or state government agencies If your record is used or given out for governmental purposes, it will be done under conditions that will protect your privacy to the fullest extent possible consistent with laws relating to public disclosure of information and law-enforcement responsibilities of the agency. These agencies may review the research to see that it is being done safely and correctly. You authorize the use of clinical information contained in your records, but any publication which includes such information or data shall not reveal your name, show your picture or contain any other personally identifying information, except as otherwise required by law. 6/15/07 Page 4 of 7 Participant Initials
5 What are the costs of taking part in this study? You and/or your health plan/insurance provider (Medicare should be considered a health insurance provider) will need to pay for some or all of the costs of treating your cancer in this study. Some health plans will not pay these costs for people taking part in studies. Check with your health plan or insurance company to find out what they will pay for. Taking part in this study may or may not cost your insurance company more than the cost of getting regular cancer treatment. You or your insurance carrier will be responsible for the costs of clinic visits, any hospital admissions, laboratory tests, x- rays, scans, chemotherapy treatments, radiation treatments, and any other tests. You will not be charged for the shipment of the x-rays and scans to the CALGB central imaging center. Please ask your doctor about any added costs or insurance problems. For more information on clinical trials and insurance coverage, you can visit the National Cancer Institute s Web site at You can print a copy of the Clinical Trials and Insurance Coverage information from this Web site. You will not be paid to participate in this study. What happens if I am injured because I took part in this study? It is important that you tell your study doctor, if you feel that you have been injured because of taking part in this study. You can tell the doctor in person or call him/her at #. You will get medical treatment if you are injured as a result of taking part in this study. You and/or your health plan will be charged for this treatment. The study will not pay for medical treatment. Although no funds or monies have been set aside to compensate you in the event of injury or illness related to the study treatment or procedures, you do not waive any of your legal rights for compensation by signing this form. You or your insurance company will be charged for continuing medical care and/or hospitalization. What are my rights if I take part in this study? Taking part in this study is voluntary. You may choose to take part, not to take part, or may leave the study at any time. No matter what decision you make, there will be no penalty to you and you will not lose any of your regular benefits. Leaving the study will not affect your medical care or result in any penalty or loss of benefits to which you are entitled. Even after you agree to take part in this study, you may withdraw at any time. Before you withdraw, you should talk to one of the researchers or nurses involved. This will allow them to inform you of any medical problems that could result from stopping your treatment. You can choose to withdraw one of two ways. In the first, you can stop your study treatment, but still allow the study doctor to follow your care. In the second, you 6/15/07 Page 5 of 7 Participant Initials
6 can stop your study treatment and not have any further contact with the study staff. Either way, there will be no penalty to you. Your decision will not affect your medical treatment or your relationship with those treating you or with this institution. If you withdraw from the study, you will still be offered all available care that suits your needs and medical condition. You are free to seek care from a doctor of your choice at any time. A Data Safety and Monitoring Committee (DSMC), an independent group of experts, will be reviewing the data from this research throughout the study. The DSMC is a committee assigned to a randomized clinical trial charged or obligated with the responsibility of monitoring performance of the trial, safety of the participants, and effectiveness of the treatments being tested. We will tell you about new information that may affect your health, welfare or willingness to stay in this study. You may be asked to sign another consent form in response to new information. Who can answer my questions about the study? For questions about the study or a research-related injury, contact your doctor,, at #. You may ask your doctor for further information on the risks, benefits or alternative treatments. For questions about your rights as a research participant, contact the Institutional Review Board (which is a group of people at the hospital in the community where you receive treatment who review the research to protect your rights) at # (the office of ). Where can I get more information? You may call the National Cancer Institute s (NCI s) Cancer Information Service at: CANCER ( ) or TTY: You may also visit the NCI Web site at For the NCI s clinical trials information, go to: For the NCI s general information about cancer, go to: 6/15/07 Page 6 of 7 Participant Initials
7 Participant Agreement I have been offered the opportunity to ask questions about this study and all questions have been answered to my satisfaction. The contents of this form have been explained to me and I understand them. I agree to allow the research personnel specified above the access to my medical records. It may be necessary for my doctor to contact me at a future date regarding new information about the treatment I received; therefore I agree to notify my doctor of any change of address and/or telephone number. My signature below means that I have voluntarily agreed to participate in this research study. I will be given a copy of all 7 pages of this consent. I have read it or it has been read to me. I may also request a copy of the study (complete study plan). (Date) (Participant Signature) I certify that I have explained to the above individual the nature and purpose, the potential benefits, and possible risks associated with participation in the research study and have answered any questions that have been raised. (Date) (Signature of Person Obtaining Consent) 6/15/07 Page 7 of 7
CORD BLOOD TRANSPLANTATION STUDY EXPANDED ACCESS PROTOCOL APPENDIX A SAMPLE CONSENT FORM
 APPENDIX A SAMPLE CONSENT FORM CORD BLOOD TRANSPLANTATION (COBLT) STUDY SAMPLE CONSENT FORM FOR THE EXPANDED ACCESS PROTOCOL You (your child) are being asked to take part in a clinical research study.
APPENDIX A SAMPLE CONSENT FORM CORD BLOOD TRANSPLANTATION (COBLT) STUDY SAMPLE CONSENT FORM FOR THE EXPANDED ACCESS PROTOCOL You (your child) are being asked to take part in a clinical research study.
If you are signing for a minor child, you refers to your child throughout the consent document.
 CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY Adult Patient or Parent, for Minor Patient INSTITUTE: National Cancer Institute PRINCIPAL INVESTIGATOR: Raffit Hassan, M.D. STUDY TITLE: Tissue Procurement
CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY Adult Patient or Parent, for Minor Patient INSTITUTE: National Cancer Institute PRINCIPAL INVESTIGATOR: Raffit Hassan, M.D. STUDY TITLE: Tissue Procurement
National Emphysema Treatment Trial (NETT) Consent for Screening and Patient Registry
 National Emphysema Treatment Trial (NETT) Consent for Screening and Patient Registry Instructions: This consent statement is to be signed and dated by the patient in the presence of a certified study staff
National Emphysema Treatment Trial (NETT) Consent for Screening and Patient Registry Instructions: This consent statement is to be signed and dated by the patient in the presence of a certified study staff
SAMPLE CONSENT A. Informed Consent Template for Cancer Treatment Trials
 SAMPLE CONSENT A Informed Consent Template for Cancer Treatment Trials PHASE III RANDOMIZED STUDY OF CONCURRENT CHEMOTHERAPY AND PELVIC RADIATION THERAPY WITH OR WITHOUT ADJUVANT CHEMOTHERAPY IN HIGH-RISK
SAMPLE CONSENT A Informed Consent Template for Cancer Treatment Trials PHASE III RANDOMIZED STUDY OF CONCURRENT CHEMOTHERAPY AND PELVIC RADIATION THERAPY WITH OR WITHOUT ADJUVANT CHEMOTHERAPY IN HIGH-RISK
Consent Form: Example 2 (DNA Sequencing)
 Consent Form: Example 2 (DNA Sequencing) Important note: This model language was developed for the NHGRI Medical Sequencing Project (MSP). It is included here only as an example of how to describe a sequencing
Consent Form: Example 2 (DNA Sequencing) Important note: This model language was developed for the NHGRI Medical Sequencing Project (MSP). It is included here only as an example of how to describe a sequencing
Guidance on Withdrawal of Subjects from Research: Data Retention and Other Related Issues
 Office for Human Research Protections (OHRP) Department of Health and Human Services (HHS) Guidance on Withdrawal of Subjects from Research: Data Retention and Other Related Issues This guidance represents
Office for Human Research Protections (OHRP) Department of Health and Human Services (HHS) Guidance on Withdrawal of Subjects from Research: Data Retention and Other Related Issues This guidance represents
[Informed Consent Form for ] Name the group of individuals for whom this consent is written. Explanation Example
![[Informed Consent Form for ] Name the group of individuals for whom this consent is written. Explanation Example [Informed Consent Form for ] Name the group of individuals for whom this consent is written. Explanation Example](/thumbs/40/20833128.jpg) [YOUR INSTITUTIONAL LETTERHEAD] Please do not submit consent forms on the WHO letter head [Name of Principle Investigator] [Informed Consent Form for ] Name the group of individuals for whom this consent
[YOUR INSTITUTIONAL LETTERHEAD] Please do not submit consent forms on the WHO letter head [Name of Principle Investigator] [Informed Consent Form for ] Name the group of individuals for whom this consent
RESEARCH PARTICIPANT INFORMED CONSENT AND PRIVACY AUTHORIZATION FORM
 If you are using Epic for this study, fax a copy of the signed consent form to 410-367-7382. Patient I.D. Plate RESEARCH PARTICIPANT INFORMED CONSENT AND PRIVACY AUTHORIZATION FORM Protocol Title: Application
If you are using Epic for this study, fax a copy of the signed consent form to 410-367-7382. Patient I.D. Plate RESEARCH PARTICIPANT INFORMED CONSENT AND PRIVACY AUTHORIZATION FORM Protocol Title: Application
Sample Consent. Transfusion of Prematures (TOP)
 Final: October 8. 2012 Revised: February 8. 2013 Appendix A: Sample Consent ~ Penn Medicine,~. Informed Consent Form and HIPAA Authorization Protocol Title: Short Title: Principal Investigator: Emergency
Final: October 8. 2012 Revised: February 8. 2013 Appendix A: Sample Consent ~ Penn Medicine,~. Informed Consent Form and HIPAA Authorization Protocol Title: Short Title: Principal Investigator: Emergency
RESEARCH SUBJECT INFORMATION AND CONSENT FORM
 1 1 1 1 1 1 1 0 1 0 1 0 RESEARCH SUBJECT INFORMATION AND CONSENT FORM TITLE: PROTOCOL NR: SPONSOR: INVESTIGATOR: WIRB VCU tracking number This template is based on a drug or device research study. The
1 1 1 1 1 1 1 0 1 0 1 0 RESEARCH SUBJECT INFORMATION AND CONSENT FORM TITLE: PROTOCOL NR: SPONSOR: INVESTIGATOR: WIRB VCU tracking number This template is based on a drug or device research study. The
Ask Us About Clinical Trials
 Ask Us About Clinical Trials Clinical Trials and You. Our specialists and researchers are at the forefront of their fields and are leading the way in developing new therapies and procedures for diagnosing
Ask Us About Clinical Trials Clinical Trials and You. Our specialists and researchers are at the forefront of their fields and are leading the way in developing new therapies and procedures for diagnosing
CONSENT FORM. Cord Blood Banking for Transplantation
 Cord Blood Program Northwest Tissue Center/Puget Sound Blood Center 921 Terry Avenue Seattle, WA 98104 (206) 292-1896 Swedish Medical Center 747 Broadway Seattle, WA 98122 (206) 386-6000 CONSENT FORM Cord
Cord Blood Program Northwest Tissue Center/Puget Sound Blood Center 921 Terry Avenue Seattle, WA 98104 (206) 292-1896 Swedish Medical Center 747 Broadway Seattle, WA 98122 (206) 386-6000 CONSENT FORM Cord
CONSENT FORM 12/19/08
 12/19/08 1001 University Place Evanston, Illinois 60201 www.northshore.org CONSENT FORM Phone (224) 364-7100 Fax (847) 570-8011 Intravesical Alkalized Lidocaine for the Treatment of Overactive Bladder
12/19/08 1001 University Place Evanston, Illinois 60201 www.northshore.org CONSENT FORM Phone (224) 364-7100 Fax (847) 570-8011 Intravesical Alkalized Lidocaine for the Treatment of Overactive Bladder
Patient Handbook on Stem Cell Therapies
 Patient Handbook on Stem Cell Therapies Appendix I of the Guidelines for the Clinical Translation of Stem Cells www.isscr.org 2008, International Society for Stem Cell Research 2 Introduction We have all
Patient Handbook on Stem Cell Therapies Appendix I of the Guidelines for the Clinical Translation of Stem Cells www.isscr.org 2008, International Society for Stem Cell Research 2 Introduction We have all
1. General Information About The Mitochondrial Disease Biobank
 Name and Clinic Number IRB # 09-002265 00 Consent form approved November 6, 2013; This consent valid through August 6, 2014; 1. General Information About The Mitochondrial Disease Biobank Study Title:
Name and Clinic Number IRB # 09-002265 00 Consent form approved November 6, 2013; This consent valid through August 6, 2014; 1. General Information About The Mitochondrial Disease Biobank Study Title:
Tuberculosis: FAQs. What is the difference between latent TB infection and TB disease?
 Tuberculosis: FAQs What is TB disease? Tuberculosis (TB) is a disease caused by bacteria (germs) that are spread from person to person through the air. TB usually affects the lungs, but it can also affect
Tuberculosis: FAQs What is TB disease? Tuberculosis (TB) is a disease caused by bacteria (germs) that are spread from person to person through the air. TB usually affects the lungs, but it can also affect
Research Ethics Review Committee (WHO ERC)
 Research Ethics Review Committee (WHO ERC) 20, AVENUE APPIA CH-1211 GENEVA 27 SWITZERLAND HTTP://INTRANET.WHO.INT/HOMES/RPC/ERC HTTP://WWW.WHO.INT/RPC/RESEARCH_ETHICS Informed Consent Template for Clinical
Research Ethics Review Committee (WHO ERC) 20, AVENUE APPIA CH-1211 GENEVA 27 SWITZERLAND HTTP://INTRANET.WHO.INT/HOMES/RPC/ERC HTTP://WWW.WHO.INT/RPC/RESEARCH_ETHICS Informed Consent Template for Clinical
Probe: Could you tell me about when?
 PERIODIC ASSESSMENT OF TREATMENT AND VITAL/DISEASE STATUS Periodic Assessment of Cancer Treatment and Disease Status (To be administered to patient at 3 months and reviewed at 6, 9 and 12 months) Instructions:
PERIODIC ASSESSMENT OF TREATMENT AND VITAL/DISEASE STATUS Periodic Assessment of Cancer Treatment and Disease Status (To be administered to patient at 3 months and reviewed at 6, 9 and 12 months) Instructions:
A guide for the patient
 Understanding series LUNG CANCER CLINICAL TRIALS 1-800-298-2436 LungCancerAlliance.org A guide for the patient TABLE OF CONTENTS The Basics What is a Clinical Trial?...3 Types of Clinical Trials... 3 Phases
Understanding series LUNG CANCER CLINICAL TRIALS 1-800-298-2436 LungCancerAlliance.org A guide for the patient TABLE OF CONTENTS The Basics What is a Clinical Trial?...3 Types of Clinical Trials... 3 Phases
Clinical Trials: What You Need to Know
 Clinical Trials: What You Need to Know Clinical trials are studies in which people volunteer to test new drugs or devices. Doctors use clinical trials to learn whether a new treatment works and is safe
Clinical Trials: What You Need to Know Clinical trials are studies in which people volunteer to test new drugs or devices. Doctors use clinical trials to learn whether a new treatment works and is safe
Patient Information Leaflet: Part 1 select-d
 Patient Information Leaflet: Part 1 select-d Anticoagulation Therapy in SELECTeD Cancer Patients at Risk of Recurrence of Venous Thromboembolism Introduction This
Patient Information Leaflet: Part 1 select-d Anticoagulation Therapy in SELECTeD Cancer Patients at Risk of Recurrence of Venous Thromboembolism Introduction This
APPENDIX B SAMPLE INFORMED CONSENT FORM
 APPENDIX B SAMPLE INFORMED CONSENT FORM B.1 INFORMED CONSENT TO PARTICIPATE IN A RESEARCH STUDY Umbilical Cord Blood Banking for Transplantation You are being asked to take part in a research study that
APPENDIX B SAMPLE INFORMED CONSENT FORM B.1 INFORMED CONSENT TO PARTICIPATE IN A RESEARCH STUDY Umbilical Cord Blood Banking for Transplantation You are being asked to take part in a research study that
Questions to Ask My Doctor About My Cancer
 Questions to Ask My Doctor Being told you have cancer can be scary and stressful. You probably have a lot of questions and concerns. Learning about the disease, how it s treated, and how this information
Questions to Ask My Doctor Being told you have cancer can be scary and stressful. You probably have a lot of questions and concerns. Learning about the disease, how it s treated, and how this information
RESEARCH PARTICIPANT INFORMED CONSENT AND PRIVACY AUTHORIZATION FORM
 Patient I.D. Plate RESEARCH PARTICIPANT INFORMED CONSENT AND PRIVACY AUTHORIZATION FORM Protocol Title: Application No.: Sponsor: The National Simulation Study: Evaluating Simulated Clinical Experiences
Patient I.D. Plate RESEARCH PARTICIPANT INFORMED CONSENT AND PRIVACY AUTHORIZATION FORM Protocol Title: Application No.: Sponsor: The National Simulation Study: Evaluating Simulated Clinical Experiences
Recruiting now. Could you help by joining this study?
 Non-Small Cell Lung Cancer Recruiting now AstraZeneca is looking for men and women with locally advanced or metastatic non-small cell lung cancer (NSCLC) to join ATLANTIC, a clinical study to help investigate
Non-Small Cell Lung Cancer Recruiting now AstraZeneca is looking for men and women with locally advanced or metastatic non-small cell lung cancer (NSCLC) to join ATLANTIC, a clinical study to help investigate
Aspirin Dosing: A Patient Centric Trial Assessing Benefits and Long term Effectiveness (ADAPTABLE)
 Aspirin Dosing: A Patient Centric Trial Assessing Benefits and Long term Effectiveness (ADAPTABLE) We are asking you to join a research study called ADAPTABLE. The information below explains the study
Aspirin Dosing: A Patient Centric Trial Assessing Benefits and Long term Effectiveness (ADAPTABLE) We are asking you to join a research study called ADAPTABLE. The information below explains the study
Pl"OtocolDirector: Iris Schrijver _ IRB Approval Date: _June 20 2006 IRE Expiration Date: June 19, 2007 _ STANFORD SAMPLE CONSENT FORM
 Protocol Title: Molecular genetic basis of sensorineural hearing Pl"OtocolDirector: Iris Schrijver IRB Approval Date: June 20 2006 IRE Expiration Date: June 19, 2007 STANFORD SAMPLE CONSENT FORM Please
Protocol Title: Molecular genetic basis of sensorineural hearing Pl"OtocolDirector: Iris Schrijver IRB Approval Date: June 20 2006 IRE Expiration Date: June 19, 2007 STANFORD SAMPLE CONSENT FORM Please
TAKING PART IN CANCER TREATMENT RESEARCH STUDIES
 For more infomation about Cancer Clinical Trials at Upstate Cancer Center please call Upstate Connect 1.800.464.8668 TAKING PART IN CANCER TREATMENT RESEARCH STUDIES Information provided by: National Cancer
For more infomation about Cancer Clinical Trials at Upstate Cancer Center please call Upstate Connect 1.800.464.8668 TAKING PART IN CANCER TREATMENT RESEARCH STUDIES Information provided by: National Cancer
National Cancer Institute
 National Cancer Institute Taking Part in Cancer Treatment Research Studies U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health Taking Part in Cancer Treatment Research Studies If
National Cancer Institute Taking Part in Cancer Treatment Research Studies U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health Taking Part in Cancer Treatment Research Studies If
INFORMED CONSENT FOR SLEEVE GASTRECTOMY
 INFORMED CONSENT FOR SLEEVE GASTRECTOMY This informed-consent document has been prepared to help inform you about your Sleeve Gastrectomy including the risks and benefits, as well as alternative treatments.
INFORMED CONSENT FOR SLEEVE GASTRECTOMY This informed-consent document has been prepared to help inform you about your Sleeve Gastrectomy including the risks and benefits, as well as alternative treatments.
Saint Francis Kidney Transplant Program Issue Date: 6/9/15
 Kidney Transplant Candidate Informed Consent Education Here are educational materials about Kidney Transplant. Please review and read these before your evaluation visit. The RN Transplant Coordinator will
Kidney Transplant Candidate Informed Consent Education Here are educational materials about Kidney Transplant. Please review and read these before your evaluation visit. The RN Transplant Coordinator will
Thymus Cancer. This reference summary will help you better understand what thymus cancer is and what treatment options are available.
 Thymus Cancer Introduction Thymus cancer is a rare cancer. It starts in the small organ that lies in the upper chest under the breastbone. The thymus makes white blood cells that protect the body against
Thymus Cancer Introduction Thymus cancer is a rare cancer. It starts in the small organ that lies in the upper chest under the breastbone. The thymus makes white blood cells that protect the body against
Questions to Ask My Doctor About Breast Cancer
 Questions to Ask My Doctor Being told you have breast cancer can be scary and stressful. You probably have a lot of questions and concerns. Learning about the disease, how it s treated, and how this information
Questions to Ask My Doctor Being told you have breast cancer can be scary and stressful. You probably have a lot of questions and concerns. Learning about the disease, how it s treated, and how this information
Surgery. Wedge resection only part of the lung, not. not a lobe, is removed. Cancer Council NSW
 The treatment you receive will depend on your lung cancer type, for example, whether you have a non-small cell lung cancer Adenocarcinoma or Squamous cell carcinoma, and if this is a sub-type with a mutation.
The treatment you receive will depend on your lung cancer type, for example, whether you have a non-small cell lung cancer Adenocarcinoma or Squamous cell carcinoma, and if this is a sub-type with a mutation.
METASTASES TO THE BONE
 RADIATION THERAPY FOR METASTASES TO THE BONE Facts to Help Patients Make an Informed Decision TARGETING CANCER CARE AMERICAN SOCIETY FOR RADIATION ONCOLOGY WHAT ARE BONE METASTASES? Cancer that starts
RADIATION THERAPY FOR METASTASES TO THE BONE Facts to Help Patients Make an Informed Decision TARGETING CANCER CARE AMERICAN SOCIETY FOR RADIATION ONCOLOGY WHAT ARE BONE METASTASES? Cancer that starts
What If I Have a Spot on My Lung? Do I Have Cancer? Patient Education Guide
 What If I Have a Spot on My Lung? Do I Have Cancer? Patient Education Guide A M E R I C A N C O L L E G E O F C H E S T P H Y S I C I A N S Lung cancer is one of the most common cancers. About 170,000
What If I Have a Spot on My Lung? Do I Have Cancer? Patient Education Guide A M E R I C A N C O L L E G E O F C H E S T P H Y S I C I A N S Lung cancer is one of the most common cancers. About 170,000
IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN
 + IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
+ IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
Nova Southeastern University IRB - Consent Form Checklist Version Date: 02/17/2010
 Nova Southeastern University IRB - Consent Form Checklist Version Date: 02/17/2010 This form is intended to assist researchers in creating consent and assent forms. Informed consent is one of the primary
Nova Southeastern University IRB - Consent Form Checklist Version Date: 02/17/2010 This form is intended to assist researchers in creating consent and assent forms. Informed consent is one of the primary
What Cancer Patients Need To Know
 Taking Part in Clinical Trials What Cancer Patients Need To Know NATIONAL INSTITUTES OF HEALTH National Cancer Institute Generous support for this publication was provided by Novartis Oncology. Taking
Taking Part in Clinical Trials What Cancer Patients Need To Know NATIONAL INSTITUTES OF HEALTH National Cancer Institute Generous support for this publication was provided by Novartis Oncology. Taking
Disease/Illness GUIDE TO ASBESTOS LUNG CANCER. What Is Asbestos Lung Cancer? www.simpsonmillar.co.uk Telephone 0844 858 3200
 GUIDE TO ASBESTOS LUNG CANCER What Is Asbestos Lung Cancer? Like tobacco smoking, exposure to asbestos can result in the development of lung cancer. Similarly, the risk of developing asbestos induced lung
GUIDE TO ASBESTOS LUNG CANCER What Is Asbestos Lung Cancer? Like tobacco smoking, exposure to asbestos can result in the development of lung cancer. Similarly, the risk of developing asbestos induced lung
Document Template #1 Adult/General Informed Consent (Rev. 9/20/2011)
 Document Template #1 Adult/General Informed Consent (Rev. 9/20/2011) Consent Form for Participation in the Research Study Entitled XYZ (or can be written in the XYZ study ) Funding Source: List complete
Document Template #1 Adult/General Informed Consent (Rev. 9/20/2011) Consent Form for Participation in the Research Study Entitled XYZ (or can be written in the XYZ study ) Funding Source: List complete
Colon Cancer. What Is Colon Cancer? What Are the Screening Methods?
 Cancer of the colon or rectum (colorectal cancer) is the second most common cancer in the U.S. In fact, of all people born, 1 in 40 will die of the disease. What Is Colon Cancer? Colon cancer begins with
Cancer of the colon or rectum (colorectal cancer) is the second most common cancer in the U.S. In fact, of all people born, 1 in 40 will die of the disease. What Is Colon Cancer? Colon cancer begins with
RADIATION THERAPY FOR BRAIN METASTASES. Facts to Help Patients Make an Informed Decision TARGETING CANCER CARE AMERICAN SOCIETY FOR RADIATION ONCOLOGY
 RADIATION THERAPY FOR BRAIN METASTASES Facts to Help Patients Make an Informed Decision TARGETING CANCER CARE AMERICAN SOCIETY FOR RADIATION ONCOLOGY FACTS ABOUT BRAIN METASTASIS Brain metastases are clusters
RADIATION THERAPY FOR BRAIN METASTASES Facts to Help Patients Make an Informed Decision TARGETING CANCER CARE AMERICAN SOCIETY FOR RADIATION ONCOLOGY FACTS ABOUT BRAIN METASTASIS Brain metastases are clusters
The lungs What is lung cancer? How common is it? Risks & symptoms Diagnosis & treatment options
 Why We re Here The lungs What is lung cancer? How common is it? Risks & symptoms Diagnosis & treatment options What Are Lungs? What Do They Do? 1 Located in the chest Allow you to breathe Provide oxygen
Why We re Here The lungs What is lung cancer? How common is it? Risks & symptoms Diagnosis & treatment options What Are Lungs? What Do They Do? 1 Located in the chest Allow you to breathe Provide oxygen
Moving forward, where are we with Clinical Trials?
 Moving forward, where are we with Clinical Trials? Dennis A. Wigle Division of Thoracic Surgery Mayo Clinic AATS/STS General Thoracic Surgery Symposium Sunday, April 27 th 2014 2012 MFMER slide-1 Where
Moving forward, where are we with Clinical Trials? Dennis A. Wigle Division of Thoracic Surgery Mayo Clinic AATS/STS General Thoracic Surgery Symposium Sunday, April 27 th 2014 2012 MFMER slide-1 Where
CONSENT TO PARTICIPATE IN A CLINICAL RESEARCH STUDY
 The Feinstein Institute for Medical Research North Shore-Long Island Jewish Health System (NS-LIJHS) TITLE OF PROTOCOL: Genetics and Epidemiology of Absolute Pitch and Related Cognitive Traits Principal
The Feinstein Institute for Medical Research North Shore-Long Island Jewish Health System (NS-LIJHS) TITLE OF PROTOCOL: Genetics and Epidemiology of Absolute Pitch and Related Cognitive Traits Principal
A Practical Guide to Advances in Staging and Treatment of NSCLC
 A Practical Guide to Advances in Staging and Treatment of NSCLC Robert J. Korst, M.D. Director, Thoracic Surgery Medical Director, The Blumenthal Cancer Center The Valley Hospital Objectives Revised staging
A Practical Guide to Advances in Staging and Treatment of NSCLC Robert J. Korst, M.D. Director, Thoracic Surgery Medical Director, The Blumenthal Cancer Center The Valley Hospital Objectives Revised staging
Surgery Choices. National Cancer Institute. For Women with DCIS or Breast Cancer. National Institutes of Health
 National Cancer Institute Surgery Choices For Women with DCIS or Breast Cancer U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health The National Cancer Institute is grateful for our
National Cancer Institute Surgery Choices For Women with DCIS or Breast Cancer U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health The National Cancer Institute is grateful for our
THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS INFORMATION.
 Notice of Privacy Practices KAISER PERMANENTE NORTHERN CALIFORNIA REGION THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS INFORMATION.
Notice of Privacy Practices KAISER PERMANENTE NORTHERN CALIFORNIA REGION THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS INFORMATION.
MULTICARE ASSOCIATES OF THE TWIN CITIES, P.A. NOTICE OF PRIVACY PRACTICES
 MULTICARE ASSOCIATES OF THE TWIN CITIES, P.A. NOTICE OF PRIVACY PRACTICES THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS INFORMATION.
MULTICARE ASSOCIATES OF THE TWIN CITIES, P.A. NOTICE OF PRIVACY PRACTICES THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS INFORMATION.
Cancer Clinical Trials: In-Depth Information
 Cancer Clinical Trials: In-Depth Information The Drug Development and Approval Process 1. Early research and preclinical testing 2. IND application filed with FDA 3. Clinical trials (phases 1, 2, and 3)
Cancer Clinical Trials: In-Depth Information The Drug Development and Approval Process 1. Early research and preclinical testing 2. IND application filed with FDA 3. Clinical trials (phases 1, 2, and 3)
The RADICALS trial Radiotherapy Timing Randomisation (RADICALS-RT) Clinical trial of treatment after surgery for prostate cancer
 Stoke Mandeville Hospital Mandeville Road Aylesbury Buckinghamshire HP21 8AL Tel: 01296 315 908 www.buckshealthcare.nhs.uk The RADICALS trial Radiotherapy Timing Randomisation (RADICALS-RT) Clinical trial
Stoke Mandeville Hospital Mandeville Road Aylesbury Buckinghamshire HP21 8AL Tel: 01296 315 908 www.buckshealthcare.nhs.uk The RADICALS trial Radiotherapy Timing Randomisation (RADICALS-RT) Clinical trial
PRIVACY NOTICE. In certain situations, we may also disclose patient information to another provider or health plan for their health care operations.
 1 PRIVACY NOTICE THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS INFORMATION. PLEASE REVIEW IT CAREFULLY. This Privacy Notice is being
1 PRIVACY NOTICE THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS INFORMATION. PLEASE REVIEW IT CAREFULLY. This Privacy Notice is being
RESEARCH EDUCATE ADVOCATE. Just Diagnosed with Melanoma Now What?
 RESEARCH EDUCATE ADVOCATE Just Diagnosed with Melanoma Now What? INTRODUCTION If you are reading this, you have undergone a biopsy (either of a skin lesion or a lymph node) or have had other tests in which
RESEARCH EDUCATE ADVOCATE Just Diagnosed with Melanoma Now What? INTRODUCTION If you are reading this, you have undergone a biopsy (either of a skin lesion or a lymph node) or have had other tests in which
PATIENT INFORMATION SHEET
 PATIENT INFORMATION SHEET Surgical and large bore pleural procedures in Malignant pleural Mesothelioma And Radiotherapy Trial (SMART trial) Stoke Mandeville Hospital Mandeville Road Aylesbury Buckinghamshire
PATIENT INFORMATION SHEET Surgical and large bore pleural procedures in Malignant pleural Mesothelioma And Radiotherapy Trial (SMART trial) Stoke Mandeville Hospital Mandeville Road Aylesbury Buckinghamshire
Blue Cross of NEPA: Custom PPO Option 10014 Coverage Period: 03/01/2015-02/29/2016
 This is only a summary. If you want more detail about your coverage and costs, you can get the complete terms in the policy or plan document at www.bcnepa.com or by calling 1-888-345-2346. Important Questions
This is only a summary. If you want more detail about your coverage and costs, you can get the complete terms in the policy or plan document at www.bcnepa.com or by calling 1-888-345-2346. Important Questions
Small cell lung cancer
 Small cell lung cancer Small cell lung cancer is a disease in which malignant (cancer) cells form in the tissues of the lung. The lungs are a pair of cone-shaped breathing organs that are found within
Small cell lung cancer Small cell lung cancer is a disease in which malignant (cancer) cells form in the tissues of the lung. The lungs are a pair of cone-shaped breathing organs that are found within
Clinical Trials at PMH
 Clinical Trials at PMH What You Need To Know UHN Patient Education Improving Health Through Education A Guide for Patients, Their Families and Friends in the PMH Cancer Program This information is to be
Clinical Trials at PMH What You Need To Know UHN Patient Education Improving Health Through Education A Guide for Patients, Their Families and Friends in the PMH Cancer Program This information is to be
INTRAPERITONEAL HYPERTHERMIC CHEMOTHERAPY (IPHC) FOR PERITONEAL CARCINOMATOSIS AND MALIGNANT ASCITES. INFORMATION FOR PATIENTS AND FAMILY MEMBERS
 INTRAPERITONEAL HYPERTHERMIC CHEMOTHERAPY (IPHC) FOR PERITONEAL CARCINOMATOSIS AND MALIGNANT ASCITES. INFORMATION FOR PATIENTS AND FAMILY MEMBERS Description of Treatment A major difficulty in treating
INTRAPERITONEAL HYPERTHERMIC CHEMOTHERAPY (IPHC) FOR PERITONEAL CARCINOMATOSIS AND MALIGNANT ASCITES. INFORMATION FOR PATIENTS AND FAMILY MEMBERS Description of Treatment A major difficulty in treating
HOSPITAL OF THE UNIVERSITY OF PENNSYLVANIA CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION
 CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: A5322, Version 2.0, 01/28/15 Long-term Follow-up of Older HIV-infected Adults in the ACTG: Addressing
CONSENT TO PARTICIPATE IN A RESEARCH STUDY AND RESEARCH SUBJECT HIPAA AUTHORIZATION Protocol Title: A5322, Version 2.0, 01/28/15 Long-term Follow-up of Older HIV-infected Adults in the ACTG: Addressing
CLINICAL TRIALS SHOULD YOU PARTICIPATE? by Gwen L. Nichols, MD
 CLINICAL TRIALS SHOULD YOU PARTICIPATE? by Gwen L. Nichols, MD Gwen L. Nichols, M.D., is currently the Oncology Site Head of the Roche Translational Clinical Research Center at Hoffman- LaRoche. In this
CLINICAL TRIALS SHOULD YOU PARTICIPATE? by Gwen L. Nichols, MD Gwen L. Nichols, M.D., is currently the Oncology Site Head of the Roche Translational Clinical Research Center at Hoffman- LaRoche. In this
HIPAA Notice of Privacy Practices
 HIPAA Notice of Privacy Practices THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS INFORMATION. PLEASE REVIEW IT CAREFULLY. This Notice
HIPAA Notice of Privacy Practices THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS INFORMATION. PLEASE REVIEW IT CAREFULLY. This Notice
Mesothelioma. 1995-2013, The Patient Education Institute, Inc. www.x-plain.com ocft0101 Last reviewed: 03/21/2013 1
 Mesothelioma Introduction Mesothelioma is a type of cancer. It starts in the tissue that lines your lungs, stomach, heart, and other organs. This tissue is called mesothelium. Most people who get this
Mesothelioma Introduction Mesothelioma is a type of cancer. It starts in the tissue that lines your lungs, stomach, heart, and other organs. This tissue is called mesothelium. Most people who get this
NOTICE OF PATIENT RIGHTS AND PRIVACY PRACTICES
 1303 NE Cushing Dr. Suite 200 Bend, Oregon 97701 Phone (541) 318-0858 Fax (541) 318-6740 NOTICE OF PATIENT RIGHTS AND PRIVACY PRACTICES THIS INFORMATION IS PROVIDED TO YOU BY BEND SURGERY CENTER THIS NOTICE
1303 NE Cushing Dr. Suite 200 Bend, Oregon 97701 Phone (541) 318-0858 Fax (541) 318-6740 NOTICE OF PATIENT RIGHTS AND PRIVACY PRACTICES THIS INFORMATION IS PROVIDED TO YOU BY BEND SURGERY CENTER THIS NOTICE
U.K. Familial Ovarian Cancer Screening Study (UK FOCSS) Phase 2 Patient Information Sheet
 U.K. Familial Ovarian Cancer Screening Study (UK FOCSS) Phase 2 Patient Information Sheet 1. Invitation You are being invited to take part in a research study. Before you decide it is important for you
U.K. Familial Ovarian Cancer Screening Study (UK FOCSS) Phase 2 Patient Information Sheet 1. Invitation You are being invited to take part in a research study. Before you decide it is important for you
Clinical Trials: Questions and Answers
 Clinical Trials: Questions and Answers Key Points Clinical trials are research studies that test how well new medical approaches work in people (see Question 1). Every clinical trial has a protocol, which
Clinical Trials: Questions and Answers Key Points Clinical trials are research studies that test how well new medical approaches work in people (see Question 1). Every clinical trial has a protocol, which
Medicare Patient Information. Patient Name: SS#: - - Date of Birth: / / Sex: Female Male. City: State: Zip Code:
 Medicare Patient Information Patient Name: SS#: - - Date of Birth: / / Sex: Female Male Address: Street: City: State: Zip Code: Home Phone: ( ) - Work/Mobile Phone: ( ) - Please print your name as it Appears
Medicare Patient Information Patient Name: SS#: - - Date of Birth: / / Sex: Female Male Address: Street: City: State: Zip Code: Home Phone: ( ) - Work/Mobile Phone: ( ) - Please print your name as it Appears
Colon and Rectal Cancer
 Colon and Rectal Cancer What is colon or rectal cancer? Colon or rectal cancer is the growth of abnormal cells in your large intestine, which is also called the large bowel. The colon is the last 5 feet
Colon and Rectal Cancer What is colon or rectal cancer? Colon or rectal cancer is the growth of abnormal cells in your large intestine, which is also called the large bowel. The colon is the last 5 feet
Questions to Ask My Doctor About Prostate Cancer
 Questions to Ask My Doctor Being told you have prostate cancer can be scary and stressful. You probably have a lot of questions and concerns. Learning about the disease, how it s treated, and how this
Questions to Ask My Doctor Being told you have prostate cancer can be scary and stressful. You probably have a lot of questions and concerns. Learning about the disease, how it s treated, and how this
UnitedHealthcare Insurance Company of the River Valley Attachment D - Schedule of Benefits
 UnitedHealthcare Insurance Company of the River Valley Attachment D - Schedule of Benefits Please refer to your Provider Directory for listings of Participating Physicians, Hospitals, and other Providers.
UnitedHealthcare Insurance Company of the River Valley Attachment D - Schedule of Benefits Please refer to your Provider Directory for listings of Participating Physicians, Hospitals, and other Providers.
You Can Quit Smoking. U.S. Department of Health and Human Services Public Health Service
 You Can Quit Smoking C O N S U M E R G U I D E U.S. Department of Health and Human Services Public Health Service NICOTINE: A POWERFUL ADDICTION If you have tried to quit smoking, you know how hard it
You Can Quit Smoking C O N S U M E R G U I D E U.S. Department of Health and Human Services Public Health Service NICOTINE: A POWERFUL ADDICTION If you have tried to quit smoking, you know how hard it
Electronic Medical Records & Litigation
 Electronic Medical Records & Litigation Victor R. Cotton MD, JD President Law & Medicine Hershey, PA www.lawandmed.com Procedures Delays in Diagnosis Procedures Violation of Informed Consent Improper Performance
Electronic Medical Records & Litigation Victor R. Cotton MD, JD President Law & Medicine Hershey, PA www.lawandmed.com Procedures Delays in Diagnosis Procedures Violation of Informed Consent Improper Performance
Breast Cancer. Sometimes cells keep dividing and growing without normal controls, causing an abnormal growth called a tumor.
 Breast Cancer Introduction Cancer of the breast is the most common form of cancer that affects women but is no longer the leading cause of cancer deaths. About 1 out of 8 women are diagnosed with breast
Breast Cancer Introduction Cancer of the breast is the most common form of cancer that affects women but is no longer the leading cause of cancer deaths. About 1 out of 8 women are diagnosed with breast
This factsheet aims to outline the characteristics of some rare lung cancers, and highlight where each type of lung cancer may be different.
 There are several different kinds of lung cancer, often referred to as lung cancer subtypes. Some of these occur more often than others. In this factsheet we will specifically look at the subtypes of cancers
There are several different kinds of lung cancer, often referred to as lung cancer subtypes. Some of these occur more often than others. In this factsheet we will specifically look at the subtypes of cancers
A Checklist for Patients with Breast Cancer
 A Checklist for Patients with Breast Cancer Questions to Ask the Doctor 1 and Quick Help Resources 1 Adapted from: American Cancer Society. Detailed Guide: Breast Cancer - What Should You Ask Your Doctor
A Checklist for Patients with Breast Cancer Questions to Ask the Doctor 1 and Quick Help Resources 1 Adapted from: American Cancer Society. Detailed Guide: Breast Cancer - What Should You Ask Your Doctor
PATIENT INFORMATION INSURANCE INFORMATION
 (mm/dd/yyyy): Have you been to Physicians Urgent Care before? Yes No Arrival Time: If yes, when? Is this a follow-up to a previous visit: Yes No PATIENT INFORMATION Patient s First Name: Middle Name: Last
(mm/dd/yyyy): Have you been to Physicians Urgent Care before? Yes No Arrival Time: If yes, when? Is this a follow-up to a previous visit: Yes No PATIENT INFORMATION Patient s First Name: Middle Name: Last
Endoscopic Plantar Fasciotomy
 Endoscopic Plantar Fasciotomy Introduction Plantar fasciitis is a common condition that causes pain centralized around the heel. It may be severe enough to affect regular activities. Health care providers
Endoscopic Plantar Fasciotomy Introduction Plantar fasciitis is a common condition that causes pain centralized around the heel. It may be severe enough to affect regular activities. Health care providers
CONSENT FORM TEMPLATE. Derivation and Distribution of Induced Pluripotent Stem (ips) Cell Lines Created from Donor Specimens
 CONSENT FORM TEMPLATE Derivation and Distribution of Induced Pluripotent Stem (ips) Cell Lines Created from Donor Specimens INTRODUCTION We invite you to take part in a research study at [name of research
CONSENT FORM TEMPLATE Derivation and Distribution of Induced Pluripotent Stem (ips) Cell Lines Created from Donor Specimens INTRODUCTION We invite you to take part in a research study at [name of research
PERIPHERAL STEM CELL TRANSPLANT INTRODUCTION
 PERIPHERAL STEM CELL TRANSPLANT INTRODUCTION This booklet was designed to help you and the important people in your life understand the treatment of high dose chemotherapy with stem cell support: a procedure
PERIPHERAL STEM CELL TRANSPLANT INTRODUCTION This booklet was designed to help you and the important people in your life understand the treatment of high dose chemotherapy with stem cell support: a procedure
Medicare. Medicare Overview. Medicare Part D Prescription Plans. Medicare
 58 requires enrollment as soon as a retiree, spouse or dependent of a retiree is eligible for. Parts A & B MUST be elected. Overview There are three parts to : Hospital Insurance (also called Part A. Your
58 requires enrollment as soon as a retiree, spouse or dependent of a retiree is eligible for. Parts A & B MUST be elected. Overview There are three parts to : Hospital Insurance (also called Part A. Your
KAISER PERMANENTE SOUTHERN CALIFORNIA REGION
 Notice of Privacy Practices KAISER PERMANENTE SOUTHERN CALIFORNIA REGION THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS INFORMATION.
Notice of Privacy Practices KAISER PERMANENTE SOUTHERN CALIFORNIA REGION THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS INFORMATION.
PATIENT INFORMATION SHEET KEY FACTS
 PATIENT INFORMATION SHEET KEY FACTS Please read this carefully and refer to the full information sheet You are invited to take part in a research study, comparing subcutaneously (injection under skin)
PATIENT INFORMATION SHEET KEY FACTS Please read this carefully and refer to the full information sheet You are invited to take part in a research study, comparing subcutaneously (injection under skin)
ADVOCATE HEALTH CARE NOTICE OF PRIVACY PRACTICES
 THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS INFORMATION. PLEASE REVIEW IT CAREFULLY. I have received the attached Advocate Health
THIS NOTICE DESCRIBES HOW MEDICAL INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED AND HOW YOU CAN GET ACCESS TO THIS INFORMATION. PLEASE REVIEW IT CAREFULLY. I have received the attached Advocate Health
Medicals c i e n t i f i c study
 Medicals c i e n t i f i c study design risks medical-ethics review board 2 Table of contents M e d i c a l - s c i e n t i f i c study Preface 2 Introduction 4 Medical-scientific study 5 Why participate?
Medicals c i e n t i f i c study design risks medical-ethics review board 2 Table of contents M e d i c a l - s c i e n t i f i c study Preface 2 Introduction 4 Medical-scientific study 5 Why participate?
TREATING LUNG CANCER AT VA PITTSBURGH HEALTHCARE SYSTEM A HERO S GUIDE
 Providing the best in cancer care for Veterans TREATING LUNG CANCER AT VA PITTSBURGH HEALTHCARE SYSTEM A HERO S GUIDE VA Pittsburgh s state-of-the-art TomoTherapy machine, which delivers radiation to patients.
Providing the best in cancer care for Veterans TREATING LUNG CANCER AT VA PITTSBURGH HEALTHCARE SYSTEM A HERO S GUIDE VA Pittsburgh s state-of-the-art TomoTherapy machine, which delivers radiation to patients.
UNIVERSITY OF MICHIGAN CONSENT TO BE PART OF A RESEARCH STUDY
 UNIVERSITY OF MICHIGAN CONSENT TO BE PART OF A RESEARCH STUDY INFORMATION ABOUT THIS FORM You may be eligible to take part in a research study. This form gives you important information about the study.
UNIVERSITY OF MICHIGAN CONSENT TO BE PART OF A RESEARCH STUDY INFORMATION ABOUT THIS FORM You may be eligible to take part in a research study. This form gives you important information about the study.
The Michigan State University Institute for Health Policy (IHP) is recruiting a sample of office based primary care providers to be interviewed
 The Michigan State University Institute for Health Policy (IHP) is recruiting a sample of office based primary care providers to be interviewed concerning their perceptions of the Meaningful Use of electronic
The Michigan State University Institute for Health Policy (IHP) is recruiting a sample of office based primary care providers to be interviewed concerning their perceptions of the Meaningful Use of electronic
MANCHESTER Lung Cancer Screening Program Dartmouth-Hitchcock Manchester 100 Hitchcock Way Manchester, NH 03104 (603) 695-2850
 LEBANON Lung Cancer Screening Program One Medical Center Drive Lebanon, NH 03756 (603) 650-4400 (866) 966-1601 Toll-free cancer.dartmouth.edu/lungscreening MANCHESTER Lung Cancer Screening Program Dartmouth-Hitchcock
LEBANON Lung Cancer Screening Program One Medical Center Drive Lebanon, NH 03756 (603) 650-4400 (866) 966-1601 Toll-free cancer.dartmouth.edu/lungscreening MANCHESTER Lung Cancer Screening Program Dartmouth-Hitchcock
Plantar Fascia Release
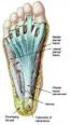 Plantar Fascia Release Introduction Plantar fasciitis is a common condition that causes pain around the heel. It may be severe enough to affect regular activities. If other treatments are unsuccessful,
Plantar Fascia Release Introduction Plantar fasciitis is a common condition that causes pain around the heel. It may be severe enough to affect regular activities. If other treatments are unsuccessful,
NOTICE OF PRIVACY PRACTICE
 Effective Date: September 23, 2013 NOTICE OF PRIVACY PRACTICE UNIVERSITY OF CALIFORNIA SAN FRANCISCO UCSF HEALTH SYSTEM THIS NOTICE DESCRIBES HOW HEALTH INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED
Effective Date: September 23, 2013 NOTICE OF PRIVACY PRACTICE UNIVERSITY OF CALIFORNIA SAN FRANCISCO UCSF HEALTH SYSTEM THIS NOTICE DESCRIBES HOW HEALTH INFORMATION ABOUT YOU MAY BE USED AND DISCLOSED
Treating Mesothelioma - A Quick Guide
 Treating Mesothelioma - A Quick Guide Contents This is a brief summary of the information on Treating mesothelioma from CancerHelp UK. You will find more detailed information on the website. In this information
Treating Mesothelioma - A Quick Guide Contents This is a brief summary of the information on Treating mesothelioma from CancerHelp UK. You will find more detailed information on the website. In this information
Pancreatic Cancer Information for patients and their families
 Pancreatic Cancer Information for patients and their families This handout answers common questions that are often asked by our patients and families. The information in this booklet is what we talked
Pancreatic Cancer Information for patients and their families This handout answers common questions that are often asked by our patients and families. The information in this booklet is what we talked
STANDARD OPERATING POLICY AND PROCEDURE
 STANDARD OPERATING POLICY AND PROCEDURE SUBJECT: Biospecimen Request and Release Policy Number: 500.0 Policy Date: 1/16/2009 Amendment Date: N/A Revision Date: 5-3-2010 I. INTRODUCTION AND PURPOSE The
STANDARD OPERATING POLICY AND PROCEDURE SUBJECT: Biospecimen Request and Release Policy Number: 500.0 Policy Date: 1/16/2009 Amendment Date: N/A Revision Date: 5-3-2010 I. INTRODUCTION AND PURPOSE The
Questions to ask my doctor: About prostate cancer
 Questions to ask my doctor: About prostate cancer Being diagnosed with prostate cancer can be scary and stressful. You probably have a lot of questions and concerns. Learning about the disease, how it
Questions to ask my doctor: About prostate cancer Being diagnosed with prostate cancer can be scary and stressful. You probably have a lot of questions and concerns. Learning about the disease, how it
PATIENT REGISTRATION FORM PATIENT INFORMATION
 Siepser Laser Eye Care PATIENT REGISTRATION FORM : PATIENT INFORMATION First Name Middle Initial: Last Name: Birth : Gender: Male Female Marital Status: SSN: Driver s License #: Address: City: State: Zip:
Siepser Laser Eye Care PATIENT REGISTRATION FORM : PATIENT INFORMATION First Name Middle Initial: Last Name: Birth : Gender: Male Female Marital Status: SSN: Driver s License #: Address: City: State: Zip:
A CONSUMER'S GUIDE TO CANCER INSURANCE. from YOUR North Carolina Department of Insurance CONSUMER'SGUIDE
 A CONSUMER'S GUIDE TO from YOUR North Carolina Department of Insurance CONSUMER'SGUIDE IMPORTANT INFORMATION WHAT IS? Cancer insurance provides benefits only if you are diagnosed with cancer, as defined
A CONSUMER'S GUIDE TO from YOUR North Carolina Department of Insurance CONSUMER'SGUIDE IMPORTANT INFORMATION WHAT IS? Cancer insurance provides benefits only if you are diagnosed with cancer, as defined
YOU RE A POTENTIAL MATCH
 YOU RE A POTENTIAL MATCH Explore the Journey to Donation Edward, PBSC donor, with his wife, Andrea TABLE OF CONTENTS A patient needs you You re a potential match and next steps 3 Steps of the donation
YOU RE A POTENTIAL MATCH Explore the Journey to Donation Edward, PBSC donor, with his wife, Andrea TABLE OF CONTENTS A patient needs you You re a potential match and next steps 3 Steps of the donation
Department of State Academic Exchanges Participant Medical History and Examination Form
 Department of State Academic Exchanges Participant Medical History and Examination Form Having been selected to participate in a U.S. Department of State educational exchange program, you are required
Department of State Academic Exchanges Participant Medical History and Examination Form Having been selected to participate in a U.S. Department of State educational exchange program, you are required
General Information About Non-Small Cell Lung Cancer
 General Information About Non-Small Cell Lung Cancer Non-small cell lung cancer is a disease in which malignant (cancer) cells form in the tissues of the lung. The lungs are a pair of cone-shaped breathing
General Information About Non-Small Cell Lung Cancer Non-small cell lung cancer is a disease in which malignant (cancer) cells form in the tissues of the lung. The lungs are a pair of cone-shaped breathing
Blue MedicareRx SM (PDP) Medicare Prescription Drug Plan 2012 Enrollment Form
 Official Use Only: Date Stamp Blue MedicareRx SM (PDP) Medicare Prescription Drug Plan 2012 Enrollment Form Return completed applications to your Employer Please refer to the Blue MedicareRx (PDP) Evidence
Official Use Only: Date Stamp Blue MedicareRx SM (PDP) Medicare Prescription Drug Plan 2012 Enrollment Form Return completed applications to your Employer Please refer to the Blue MedicareRx (PDP) Evidence
