ACULAR Eye Drops. NAME OF THE DRUG Non-proprietary name: ketorolac trometamol. Chemical Structure:
|
|
|
- Earl Floyd
- 7 years ago
- Views:
Transcription
1 ACULAR Eye Drops NAME OF THE DRUG Non-proprietary name: ketorolac trometamol. Chemical Structure: Chemical Name: (±)-5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1) Empirical Formula: C 19 H 24 N 2 O 6 Molecular Weight: CAS No: pka: 3.54 DESCRIPTION Ketorolac trometamol is a white to off-white crystalline substance, which is a racemic mixture. It may exist in three crystalline forms, all of which are equally soluble in water. Ketorolac trometamol (ACULAR ) eye drops is a clear, colourless to pale yellow, sterile, isotonic aqueous solution. Active: ketorolac trometamol 5 mg/ml Excipients: benzalkonium chloride 0.01% (w/v), disodium edetate 0.1% (w/v), octoxinol 40, sodium chloride, sodium hydroxide/hydrochloric acid (to adjust ph) and purified water. PHARMACOLOGY Class: Ketorolac trometamol is a member of the pyrrolo-pyrolle group of non-steroidal antiinflammatory drugs for ophthalmic use. Pharmacological actions. Ketorolac trometamol is a nonsteroidal, anti-inflammatory agent demonstrating analgesic and anti-inflammatory activity. It is believed to inhibit the cyclo-oxygenase enzyme essential for prostaglandin biosynthesis. Ocular administration of ketorolac trometamol reduces prostaglandin E 2 levels in the aqueous humour. Ketorolac trometamol given systemically does not cause pupil constriction. Results from clinical studies indicate that ACULAR eye drops have no significant effect upon intraocular pressure. Pharmacokinetics. Two drops (0.1 ml) of 0.5% ACULAR eye drops instilled into the eyes of patients 12 hours and 1 hour prior to cataract extraction achieved measurable levels in 8 of 9 patients eyes (mean ketorolac concentrations 95 ng/ml aqueous humour, range ng/ml). One drop (0.05 ml) ACULAR (ketorolac trometamol 5 mg/ml) Product Information Version 2a Page 1 of 5
2 of 0.5% ketorolac trometamol solution was instilled into one eye and one drop of the vehicle into the other eye three times a day for 21 days in 26 normal subjects. Only 4 of 13 subjects had detectable amounts of ketorolac in their plasma (range 10.7 to 22.5 ng/ml) after 15 minutes at Day 10 during topical ocular treatment. Average peak plasma level following intramuscular administration of 30 mg ketorolac trometamol was 2.2 µg/ml 50 minutes after administration. Clinical Trials. Allergic conjunctivitis A total of 203 patients with seasonal allergic conjunctivitis were evaluable for efficacy in two randomised, controlled clinical trials of ACULAR (ketorolac) eye drops against placebo. The patients who received ketorolac demonstrated significant decreases in itching, and conjunctival inflammation over the 7 day treatment period. Post-operative inflammation A total of 206 patients who had undergone cataract surgery participated in two randomised, controlled clinical trials of ACULAR (ketorolac) eye drops against placebo. The patients who received ketorolac showed significantly greater decreases in anterior chamber cells and anterior chamber flare over the two weeks of treatment. 39% of ketorolac patients achieved a zero score for anterior cells and flare after 2 weeks of treatment compared to 12% of placebo patients. INDICATIONS ACULAR eye drops are indicated for the short term (2-4 weeks) relief of symptoms of seasonal allergic conjunctivitis. ACULAR eye drops are also indicated for the short term (2-4 weeks) prophylaxis and reduction in inflammation in patients undergoing cataract extraction. CONTRAINDICATIONS ACULAR eye drops are contraindicated in patients hypersensitive to any of the components of the medication. The potential exists for cross sensitivity to acetylsalicylic acid and other nonsteroidal antiinflammatory medicines. ACULAR eye drops are contraindicated in patients who have previously exhibited sensitivities to these drugs. PRECAUTIONS Patients with bleeding tendencies: With some nonsteroidal anti-inflammatory drugs a potential exists for increased bleeding time due to interference with thrombocyte aggregation. There have been reports that ocularly applied nonsteroidal anti-inflammatory drugs may cause increased bleeding of ocular tissues (including hyphemas) in conjunction with ocular surgery. ACULAR eye drops should be used with care in patients with known bleeding tendencies, or in patients who are receiving other medications which may prolong bleeding time, or patients with a known history of peptic ulceration. Bronchospasm: There have been post-marketing reports of bronchospasm or exacerbation of asthma in patients, who have either a known hypersensitivity to aspirin/non-steroidal antiinflammatory drugs or a past medical history of asthma associated with the use of ACULAR, which may be contributory. Caution is recommended in the use of ACULAR in these individuals. ACULAR (ketorolac trometamol 5 mg/ml) Product Information Version 2a. Page 2 of 5
3 Delayed Healing: All topical nonsteroidal anti-inflammatory drugs (NSAIDs) may slow or delay healing. Concomitant use of topical NSAIDs and topical steroids may increase the potential for healing problems. Corneal Effects: Use of topical NSAIDs may result in keratitis. In some susceptible patients, continued use of topical NSAIDs may result in epithelial breakdown, corneal thinning, corneal erosion, corneal ulceration or corneal perforation. These events may be sight threatening. Patients with evidence of corneal epithelial breakdown should immediately discontinue use of topical NSAIDs and should be closely monitored for corneal health. Topical NSAIDs should be used with caution in patients with complicated ocular surgeries, corneal denervation, corneal epithelial defects, diabetes mellitus, ocular surface diseases (e.g., dry eye syndrome), rheumatoid arthritis, or repeat ocular surgeries within a short period of time as they may be at increased risk for corneal adverse events which may become sight threatening. Post-marketing experience with topical NSAIDs also suggests that use more than 24 hours prior to surgery or use beyond 14 days post-surgery may increase patient risk for the occurrence and severity of corneal adverse events. Ability to drive: As ACULAR eye drops may cause transient blurring on instillation, caution is required with the use of hazardous machinery or driving, which are not recommended unless vision is clear. Masking of Infections: In common with other anti-inflammatory drugs, ACULAR eye drops may mask the usual signs of infections. Use in Pregnancy: Category C Ketorolac trometamol and its metabolites have been shown to pass into the foetus. Safety in human pregnancy has not been established. Not recommended in pregnancy. Reproduction studies have been performed in rabbits and rats at oral doses of 3.6 and 10 mg/kg/day respectively. The results from these studies did not reveal any significant evidence of harm to the foetus. However, studies in rabbits have shown a small increase in the incidence of major vessel anomalies. Use in Lactation: Ketorolac trometamol is excreted in the milk of lactating rats and rabbits, and has been detected in human breast milk. A peri/postnatal study in rats showed reductions in postnatal growth and survival of the offspring when ketorolac trometamol was administered to lactating rats at oral dose levels greater than 4.8 mg/kg/day. Thus, use of ketorolac trometamol in lactating mothers is not recommended. Use in children: Safety and efficacy have not been demonstrated in children aged less than 12 years. Carcinogenesis, Mutagenesis, Impairment of Fertility: An 18-month study in mice at oral doses of ketorolac trometamol of 2 mg/kg/day (equal to 1.2 times the human systemic exposure at the maximum recommended IM dose of 90 mg/day, based on AUC) and a 24 month study in rats at oral doses of 5 mg/kg (0.7 times the human parenteral AUC) showed no evidence of tumours. ACULAR (ketorolac trometamol 5 mg/ml) Product Information Version 2a. Page 3 of 5
4 Ketorolac trometamol was not genotoxic in a series of assays for gene mutations and DNA damage. Ketorolac trometamol did not cause chromosome breakage in the mouse micronucleus test in vivo at 1590 µg/ml, approximately 1000 times the average human plasma levels, but increased the incidence of chromosomal aberrations in Chinese hamster ovarian cells in vitro at higher concentrations. Impairment of fertility did not occur in male or female rats at oral doses of 9 mg/kg (1.2 times human parenteral AUC) and 16 mg/kg (2.1 times the human parenteral AUC) respectively. Interactions with other drugs: No specific clinical studies on interactions with other drugs have been conducted with ACULAR eye drops. There were no reports in the controlled studies of interactions of ACULAR eye drops with systemic and ophthalmic medications such as antibiotics, sedatives, beta blockers, carbonic anhydrase inhibitors, miotics, mydriatics, cycloplegics, local anaesthetics and corticosteroids. Concomitant use of topical NSAIDs and topical steroids may increase the potential for healing problems. Information for Patients: ACULAR should not be administered while wearing contact lenses. ADVERSE REACTIONS In controlled clinical studies, the most frequently reported adverse events with the use of ACULAR eye drops have been transient stinging and burning on instillation (eye pain). These events were reported by up to 40% of subjects treated with ACULAR. Other adverse events reported in controlled clinical studies (at an incidence of >1%) included conjunctivitis (scratching, foreign body sensation, itching, erythema), local allergic reactions, superficial keratitis, corneal oedema, eye infection, eye inflammation, eye irritation and hypersensitivity. Ptosis, blepharitis, photophobia, blurred vision, eye dryness, corneal lesion, iritis, and glaucoma were also reported in >1% of patients in some studies. Uncommon (>0.1% and <1%) adverse events reported were eye dryness, corneal infiltrates, ulcerative keratitis, visual disturbance and headaches. In post-marketing experience ocular burning and stinging, ocular irritation, local allergic reactions, superficial ocular infections and superficial keratitis were the most frequently reported adverse reactions. Systemic allergic reactions have been reported very rarely.there have been post-marketing reports of bronchospasm or exacerbation of asthma in patients who have either a known hypersensitivity to aspirin/non-steroidal anti-inflammatory drugs or a past medical history of asthma, associated with the use of ACULAR which may be contributory (see PRECAUTIONS). DOSAGE AND ADMINISTRATION Seasonal allergic conjunctivitis: The recommended dose of ACULAR eye drops for the relief of symptoms of seasonal allergic conjunctivitis is one drop (0.25 mg) instilled in the eye four times daily. Treatment may be continued for up to four weeks. Post-operative inflammation: The recommended dose of ACULAR eye drops for the prophylaxis and treatment of inflammation in patients who have undergone cataract extraction is one to two drops ( mg) four times daily, starting 24 hours before surgery and continuing for 2-4 weeks. ACULAR (ketorolac trometamol 5 mg/ml) Product Information Version 2a. Page 4 of 5
5 Discard any unused contents 28 days after opening the bottle. OVERDOSAGE There is no experience of overdosage by the ophthalmic route. If accidentally ingested, fluids should be taken to dilute the effects, if any. PRESENTATION ACULAR (ketorolac trometamol) eye drops is supplied in white opaque plastic dropper bottles with dropper applicators. Eye drops: 5 and 10 ml Storage: Store below 25 C. Protect from light. Shelf life: 2 years POISONS SCHEDULE: S4 AUST R NAME AND ADDRESS Allergan Australia Pty. Ltd. 810 Pacific Highway Gordon NSW 2072 ABN TGA Approval Date: 6 March, 1998 Date of Most recent Amendment: 26 September 2011 Registered Trade Mark of Allergan Inc. ACULAR (ketorolac trometamol 5 mg/ml) Product Information Version 2a. Page 5 of 5
DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief
 DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief Common causes of itching due to eye allergies include: Pollen from trees, grasses, and ragweed Dust mites Cat dander Approximately 10 million
DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief Common causes of itching due to eye allergies include: Pollen from trees, grasses, and ragweed Dust mites Cat dander Approximately 10 million
VOLTAREN OPHTHA EYE DROPS
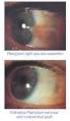 פורמט עלון זה נקבע ע"ימשרד הבריאות ותוכנו נבדק ואושר על ידו באפריל 2008 PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
פורמט עלון זה נקבע ע"ימשרד הבריאות ותוכנו נבדק ואושר על ידו באפריל 2008 PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate.
 NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate. PRESENTATION Eye Drops: NAPHCON-A Eye Drops are a combination of an antihistamine (pheniramine maleate) and a decongestant
NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate. PRESENTATION Eye Drops: NAPHCON-A Eye Drops are a combination of an antihistamine (pheniramine maleate) and a decongestant
VOLTAREN OPHTHA EYE DROPS
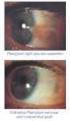 2013 במאי משרד הבריאות ותוכנו נבדק ואושר על ידו ע"י עלון זה נקבע ע פורמט PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
2013 במאי משרד הבריאות ותוכנו נבדק ואושר על ידו ע"י עלון זה נקבע ע פורמט PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
Revised: 9/2014. LUMIGAN (bimatoprost ophthalmic solution) 0.01% for topical ophthalmic use. Initial U.S. Approval: 2001
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LUMIGAN (bimatoprost ophthalmic solution) 0.01% safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LUMIGAN (bimatoprost ophthalmic solution) 0.01% safely and effectively. See full prescribing information
ZOVIRAX Cold Sore Cream
 Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
FULL PRESCRIBING INFORMATION: CONTENTS*
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PHOTREXA VISCOUS and PHOTREXA safely and effectively. See full prescribing information for PHOTREXA
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PHOTREXA VISCOUS and PHOTREXA safely and effectively. See full prescribing information for PHOTREXA
Revised: 9/2014 HIGHLIGHTS OF PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LATISSE safely and effectively. See full prescribing information for LATISSE. LATISSE (bimatoprost
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LATISSE safely and effectively. See full prescribing information for LATISSE. LATISSE (bimatoprost
Hypromellose Eye Drops BP 0.3% w/v PL 23097/0006
 Hypromellose Eye Drops BP 0.3% w/v PL 23097/0006 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
Hypromellose Eye Drops BP 0.3% w/v PL 23097/0006 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Losartan Potassium and Hydrochlorothiazide Tablets 100 mg/12.5 mg Lupin Limited Goa 403 722
MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Losartan Potassium and Hydrochlorothiazide Tablets 100 mg/12.5 mg Lupin Limited Goa 403 722
used to treat inflammation, corneal injury and bacterial infections in the external part of the eye.
 TOBRADEX * Eye Drops Tobramycin and Dexamethasone CONSUMER MEDICINE INFORMATION What is in this Leaflet This leaflet answers some common questions about TOBRADEX* Eye Drops. It does not contain all the
TOBRADEX * Eye Drops Tobramycin and Dexamethasone CONSUMER MEDICINE INFORMATION What is in this Leaflet This leaflet answers some common questions about TOBRADEX* Eye Drops. It does not contain all the
160S01105, Page 1 of 7. Human Hepatitis B Immunoglobulin, solution for intramuscular injection.
 160S01105, Page 1 of 7 New Zealand Data Sheet Hepatitis B Immunoglobulin-VF NAME OF THE MEDICINE Human Hepatitis B Immunoglobulin, solution for intramuscular injection. DESCRIPTION Hepatitis B Immunoglobulin-VF
160S01105, Page 1 of 7 New Zealand Data Sheet Hepatitis B Immunoglobulin-VF NAME OF THE MEDICINE Human Hepatitis B Immunoglobulin, solution for intramuscular injection. DESCRIPTION Hepatitis B Immunoglobulin-VF
PRODUCT INFORMATION (This PI contains all registered presentations of Avamys.) AVAMYS Nasal Spray
 PRODUCT INFORMATION (This PI contains all registered presentations of Avamys.) AVAMYS Nasal Spray NAME OF THE MEDICINE: Fluticasone furoate Structure: 21 F O 2 3 28 HO 19 1 10 5 4 O 20 S 18 12 O 11 13
PRODUCT INFORMATION (This PI contains all registered presentations of Avamys.) AVAMYS Nasal Spray NAME OF THE MEDICINE: Fluticasone furoate Structure: 21 F O 2 3 28 HO 19 1 10 5 4 O 20 S 18 12 O 11 13
PHENYLEPHRINE HYDROCHLORIDE INJECTION USP
 PRESCRIBING INFORMATION PHENYLEPHRINE HYDROCHLORIDE INJECTION USP 10 mg/ml Sandoz Canada Inc. Date of Preparation: September 1992 145 Jules-Léger Date of Revision : January 13, 2011 Boucherville, QC, Canada
PRESCRIBING INFORMATION PHENYLEPHRINE HYDROCHLORIDE INJECTION USP 10 mg/ml Sandoz Canada Inc. Date of Preparation: September 1992 145 Jules-Léger Date of Revision : January 13, 2011 Boucherville, QC, Canada
UNIVERSIDAD DE GUAYAQUIL DEPARTMENT OF CHEMICAL SCIENCES
 CODE: 38/05 TITLE: Establishment of the potential anti-inflammatory effect of the product known as CUMANDA, originating from NutraMedix Laboratories, LLC, Florida OBJECTIVES: To study the possible anti-inflammatory
CODE: 38/05 TITLE: Establishment of the potential anti-inflammatory effect of the product known as CUMANDA, originating from NutraMedix Laboratories, LLC, Florida OBJECTIVES: To study the possible anti-inflammatory
PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement)
 1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
UNIVERSIDAD DE GUAYAQUIL DEPARTMENT OF CHEMICAL SCIENCES
 CODE: 38/05 TITLE: Establishment of the potential anti-inflammatory effect of the product known as SAMENTO, originating from NutraMedix Laboratories, LLC, Florida OBJECTIVES: To study the possible anti-inflammatory
CODE: 38/05 TITLE: Establishment of the potential anti-inflammatory effect of the product known as SAMENTO, originating from NutraMedix Laboratories, LLC, Florida OBJECTIVES: To study the possible anti-inflammatory
MATERIAL SAFETY DATA SHEET
 Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
PACKAGE LEAFLET: INFORMATION FOR THE USER. ADRENALINE (HCl) STEROP 0,8mg/1ml. Solution for injection. Adrenaline (Levorenine, Epinephrine)
 PACKAGE LEAFLET: INFORMATION FOR THE USER ADRENALINE (HCl) STEROP 0,4mg/1ml ADRENALINE (HCl) STEROP 0,8mg/1ml Solution for injection Adrenaline (Levorenine, Epinephrine) Read all of this leaflet carefully
PACKAGE LEAFLET: INFORMATION FOR THE USER ADRENALINE (HCl) STEROP 0,4mg/1ml ADRENALINE (HCl) STEROP 0,8mg/1ml Solution for injection Adrenaline (Levorenine, Epinephrine) Read all of this leaflet carefully
White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other.
 Nausicalm Cyclizine hydrochloride Ph. Eur. 50 mg Presentation White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other. Uses Actions The active ingredient-cyclizine
Nausicalm Cyclizine hydrochloride Ph. Eur. 50 mg Presentation White, circular, biconvex, uncoated tablets with a score line on one side, plain on the other. Uses Actions The active ingredient-cyclizine
CHLORHEXIDINE ACETATE WITH CETRIMIDE (%) ANTISEPTIC SOLUTION
 CHLORHEXIDINE ACETATE WITH CETRIMIDE (%) ANTISEPTIC SOLUTION NAME OF THE MEDICINE Proprietary name: Non-proprietary name: Chemical Name: Molecular formula: Baxter Chlorhexidine Cetrimide Antiseptic Solution
CHLORHEXIDINE ACETATE WITH CETRIMIDE (%) ANTISEPTIC SOLUTION NAME OF THE MEDICINE Proprietary name: Non-proprietary name: Chemical Name: Molecular formula: Baxter Chlorhexidine Cetrimide Antiseptic Solution
UBISTESIN 1:200,000 and UBISTESIN FORTE 1:100,000
 UBISTESIN 1:200,000 and UBISTESIN FORTE 1:100,000 Articaine hydrochloride and adrenaline hydrochloride Consumer Medicine Information WHAT IS IN THIS LEAFLET Please read this leaflet carefully before you
UBISTESIN 1:200,000 and UBISTESIN FORTE 1:100,000 Articaine hydrochloride and adrenaline hydrochloride Consumer Medicine Information WHAT IS IN THIS LEAFLET Please read this leaflet carefully before you
VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension
 VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension DESCRIPTION Hydroxyzine pamoate is designated chemically as 1-(p-chlorobenzhydryl) 4- [2-(2-hydroxyethoxy) ethyl] diethylenediamine salt of 1,1
VISTARIL (hydroxyzine pamoate) Capsules and Oral Suspension DESCRIPTION Hydroxyzine pamoate is designated chemically as 1-(p-chlorobenzhydryl) 4- [2-(2-hydroxyethoxy) ethyl] diethylenediamine salt of 1,1
NIZORAL (KETOCONAZOLE) 2% SHAMPOO DESCRIPTION
 NIZORAL (KETOCONAZOLE) 2% SHAMPOO DESCRIPTION NIZORAL (ketoconazole) 2% Shampoo is a red-orange liquid for topical application, containing the broad spectrum synthetic antifungal agent ketoconazole in
NIZORAL (KETOCONAZOLE) 2% SHAMPOO DESCRIPTION NIZORAL (ketoconazole) 2% Shampoo is a red-orange liquid for topical application, containing the broad spectrum synthetic antifungal agent ketoconazole in
See 17 for PATIENT COUNSELING INFORMATION and FDAapproved. Revised: 4/2015
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DUAC Gel safely and effectively. See full prescribing information for DUAC Gel. DUAC (clindamycin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DUAC Gel safely and effectively. See full prescribing information for DUAC Gel. DUAC (clindamycin
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prednicare Tablets 5mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active Substance(s) Prednisolone
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prednicare Tablets 5mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active Substance(s) Prednisolone
Package leaflet: Information for the patient. Dorzolamide Mylan 20 mg/ml Eye drops, Solution Dorzolamide
 Package leaflet: Information for the patient Dorzolamide Mylan 20 mg/ml Eye drops, Solution Dorzolamide Read all of this leaflet carefully before you start using this medicine because it contains important
Package leaflet: Information for the patient Dorzolamide Mylan 20 mg/ml Eye drops, Solution Dorzolamide Read all of this leaflet carefully before you start using this medicine because it contains important
8.5 Geriatric Use * Sections or subsections omitted from the full prescribing information are not listed.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Injectafer safely and effectively. See full prescribing information for Injectafer. INJECTAFER (ferric
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Injectafer safely and effectively. See full prescribing information for Injectafer. INJECTAFER (ferric
Use of Nepafenac in Lasek Johnny L. Gayton, MD
 Use of Nepafenac in Lasek Johnny L. Gayton, MD Kathrine Jackson, COA Eyesight Associates, Warner Robins, GA Analgesic Effect NSAIDs provide analgesic effect 1-3 Minimize pain and discomfort following cataract
Use of Nepafenac in Lasek Johnny L. Gayton, MD Kathrine Jackson, COA Eyesight Associates, Warner Robins, GA Analgesic Effect NSAIDs provide analgesic effect 1-3 Minimize pain and discomfort following cataract
How To Know If You Can See Without Glasses Or Contact Lense After Lasik
 The LASIK experience I WHO CAN HAVE LASIK? To be eligible for LASIK you should be at least 21 years of age, have healthy eyes and be in good general health. Your vision should not have deteriorated significantly
The LASIK experience I WHO CAN HAVE LASIK? To be eligible for LASIK you should be at least 21 years of age, have healthy eyes and be in good general health. Your vision should not have deteriorated significantly
The clinical studies have been performed in children, adolescents and adults, from 4 years up to 55 years of age.
 NAME OF THE MEDICINE TdaP-Booster. Diphtheria, tetanus and pertussis (acellular mono-component) vaccine (adsorbed, reduced antigen content). DESCRIPTION TdaP-Booster is a suspension for injection in pre-filled
NAME OF THE MEDICINE TdaP-Booster. Diphtheria, tetanus and pertussis (acellular mono-component) vaccine (adsorbed, reduced antigen content). DESCRIPTION TdaP-Booster is a suspension for injection in pre-filled
It can therefore be bought without a prescription but should be used correctly to ensure its efficacy and to reduce side effects.
 140 mg Medicated plaster Diclofenac Sodium BEFORE USE, CAREFULLY READ ALL THE INFORMATION GIVEN ON THE INFORMATIVE LEAFLET This medicinal product is intended for SELF-MEDICATION. It can be used to treat
140 mg Medicated plaster Diclofenac Sodium BEFORE USE, CAREFULLY READ ALL THE INFORMATION GIVEN ON THE INFORMATIVE LEAFLET This medicinal product is intended for SELF-MEDICATION. It can be used to treat
There is a risk of renal impairment in dehydrated children and adolescents.
 PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
REFRACTIVE SURGERY NIGHTMARES Dr.ATHIYA AGARWAL
 REFRACTIVE SURGERY NIGHTMARES Dr.ATHIYA AGARWAL POST LASIK INFECTION Infection occurring after photorefractive keratectomy (PRK) may be 1. Secondary to the defect in the epithelium as well as the use of
REFRACTIVE SURGERY NIGHTMARES Dr.ATHIYA AGARWAL POST LASIK INFECTION Infection occurring after photorefractive keratectomy (PRK) may be 1. Secondary to the defect in the epithelium as well as the use of
Public Assessment Report. Decentralised Procedure
 Public Assessment Report Decentralised Procedure Opticrom Allergy Single Dose 2% w/v Eye Drops, Solution (sodium cromoglicate) Procedure No: UK Licence No: PL 04425/0688 Aventis Pharma Limited (T/A Sanofi-aventis
Public Assessment Report Decentralised Procedure Opticrom Allergy Single Dose 2% w/v Eye Drops, Solution (sodium cromoglicate) Procedure No: UK Licence No: PL 04425/0688 Aventis Pharma Limited (T/A Sanofi-aventis
FRENCH AGENCY FOR VETERINARY MEDICINAL PRODUCTS DECENTRALISED PROCEDURE PUBLICLY AVAILABLE ASSESSMENT REPORT FOR A VETERINARY MEDICINAL PRODUCT
 FRENCH AGENCY FOR VETERINARY MEDICINAL PRODUCTS DECENTRALISED PROCEDURE FOR A VETERINARY MEDICINAL PRODUCT EMEDOG, 1 mg/ml, solution for injection for dogs Date: 20/08/2015 French agency for food, environnemental
FRENCH AGENCY FOR VETERINARY MEDICINAL PRODUCTS DECENTRALISED PROCEDURE FOR A VETERINARY MEDICINAL PRODUCT EMEDOG, 1 mg/ml, solution for injection for dogs Date: 20/08/2015 French agency for food, environnemental
PACKAGE LEAFLET: INFORMATION FOR THE USER. PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion. Paracetamol
 PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
PACKAGE LEAFLET: INFORMATION FOR THE USER PARACETAMOL MACOPHARMA 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Isotrexin 2% + 0.05% w/w Gel 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Erythromycin Isotretinoin 2.00% w/w 0.05% w/w Excipients: Contains
Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Isotrexin 2% + 0.05% w/w Gel 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Erythromycin Isotretinoin 2.00% w/w 0.05% w/w Excipients: Contains
MATERIAL SAFETY DATA SHEET
 Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013.
 The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013 Havrix 720 Junior 1 NAME OF THE MEDICINAL PRODUCT Havrix 720 Junior 2 QUALITATIVE
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013 Havrix 720 Junior 1 NAME OF THE MEDICINAL PRODUCT Havrix 720 Junior 2 QUALITATIVE
Learn More About Product Labeling
 Learn More About Product Labeling Product label The product label is developed during the formal process of review and approval by regulatory agencies of any medicine or medical product. There are specific
Learn More About Product Labeling Product label The product label is developed during the formal process of review and approval by regulatory agencies of any medicine or medical product. There are specific
Perfalgan 10 mg/ml, solution for infusion
 PACKAGE LEAFLET: INFORMATION FOR THE USER Perfalgan 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need
PACKAGE LEAFLET: INFORMATION FOR THE USER Perfalgan 10 mg/ml, solution for infusion Paracetamol Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need
Disclosures. Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
 Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Mitzi Joi Williams, MD Neurologist MS Center of Atlanta, Atlanta, GA Disclosures Consultant and Speaker for Biogen Idec, TEVA Neuroscience, EMD Serrono, Mallinckrodt, Novartis, Genzyme, Accorda Therapeutics
Perrigo Pharmaceuticals Co.
 Ibuprofen Page 1 of 7 Perrigo Pharmaceuticals Co. U.S. Pharmaceuticals Division MATERIAL SAFETY DATA SHEET NDC Product(s) Code(s): 0121-4774-05 0121-4774-10 Product Name: Ibuprofen Oral Suspension 100mg/5mL
Ibuprofen Page 1 of 7 Perrigo Pharmaceuticals Co. U.S. Pharmaceuticals Division MATERIAL SAFETY DATA SHEET NDC Product(s) Code(s): 0121-4774-05 0121-4774-10 Product Name: Ibuprofen Oral Suspension 100mg/5mL
Calcium Folinate Ebewe Data Sheet
 NAME OF THE MEDICINE Calcium folinate injection Composition Active: Calcium folinate (equivalent to 10 mg folinic acid per ml) Inactive: Sodium chloride (7.7mg/mL), qs Water for Injections. Preservative
NAME OF THE MEDICINE Calcium folinate injection Composition Active: Calcium folinate (equivalent to 10 mg folinic acid per ml) Inactive: Sodium chloride (7.7mg/mL), qs Water for Injections. Preservative
PATIENT CONSENT FOR LASER IN-SITU KERATOMILEUSIS (LASIK)
 INTRODUCTION: You have been diagnosed with myopia (nearsightedness) or hyperopia (farsightedness) with or without astigmatism, or astigmatism alone. Myopia is a result of light entering the eye and focusing
INTRODUCTION: You have been diagnosed with myopia (nearsightedness) or hyperopia (farsightedness) with or without astigmatism, or astigmatism alone. Myopia is a result of light entering the eye and focusing
NSAID PREPARATIONS. COMPOSITION : Each enteric coated tablet contains Diclofenac Sodium BP 25 mg. Clofenac 50. Clofenac SR. Sodium BP 50 mg.
 Diclofenac Analgesic & Anti-inflammatory COMPOSITION Clofenac 25 : Each enteric coated tablet contains Diclofenac Sodium BP 25 mg. Clofenac 50 : Each enteric coated tablet contains Diclofenac Sodium BP
Diclofenac Analgesic & Anti-inflammatory COMPOSITION Clofenac 25 : Each enteric coated tablet contains Diclofenac Sodium BP 25 mg. Clofenac 50 : Each enteric coated tablet contains Diclofenac Sodium BP
1g cream or ointment contains 1 mg methylprednisolone aceponate.
 CONSUMER MEDICINE INFORMATION ADVANTAN 1g cream or ointment contains 1 mg methylprednisolone aceponate. What is in this leaflet Please read this leaflet carefully before you start using ADVANTAN. It will
CONSUMER MEDICINE INFORMATION ADVANTAN 1g cream or ointment contains 1 mg methylprednisolone aceponate. What is in this leaflet Please read this leaflet carefully before you start using ADVANTAN. It will
Public Assessment Report. Decentralised Procedure
 Public Assessment Report Decentralised Procedure Olopatadine Zentiva 1 mg/ml eye drops, solution (olopatadine hydrochloride) Procedure No: UK Licence No: PL 17780/0568 Winthrop Pharmaceuticals UK Limited
Public Assessment Report Decentralised Procedure Olopatadine Zentiva 1 mg/ml eye drops, solution (olopatadine hydrochloride) Procedure No: UK Licence No: PL 17780/0568 Winthrop Pharmaceuticals UK Limited
Glycopyrronium Bromide 0.5mg/mL and Neostigmine Metilsulfate 2.5mg/mL Solution for Injection
 NEW ZEALAND DATA SHEET Glycopyrronium Bromide 0.5mg/mL and Neostigmine Metilsulfate 2.5mg/mL Solution for Injection Each 1mL contains Glycopyrrolate USP 0.5mg (Glycopyrronium Bromide) and Neostigmine Metilsulfate
NEW ZEALAND DATA SHEET Glycopyrronium Bromide 0.5mg/mL and Neostigmine Metilsulfate 2.5mg/mL Solution for Injection Each 1mL contains Glycopyrrolate USP 0.5mg (Glycopyrronium Bromide) and Neostigmine Metilsulfate
Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel
 Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel PL 10972/0089 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 11 Summary of Product Characteristics
Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel PL 10972/0089 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 11 Summary of Product Characteristics
FULL PRESCRIBING INFORMATION
 HIGHLIGHTS F PRESCRIBING INFRMATIN These highlights do not include all the information needed to use AzaSite safely and effectively. See full prescribing information for AzaSite. AzaSite (azithromycin
HIGHLIGHTS F PRESCRIBING INFRMATIN These highlights do not include all the information needed to use AzaSite safely and effectively. See full prescribing information for AzaSite. AzaSite (azithromycin
Psoriasis and Psoriatic Arthritis Alliance
 Psoriasis and Psoriatic Arthritis Alliance A principal source of information on psoriasis and psoriatic arthritis ) Treatments for Psoriatic Arthritis overview Although psoriatic arthritis is a chronic
Psoriasis and Psoriatic Arthritis Alliance A principal source of information on psoriasis and psoriatic arthritis ) Treatments for Psoriatic Arthritis overview Although psoriatic arthritis is a chronic
Material Safety data sheet
 EMERGENCY OVERVIEW METFORMIN HYDROCHLORIDE TABLET contain Metformin and excipients generally considered to be non- toxic and non-hazardous in small quantities and under conditions of normal occupational
EMERGENCY OVERVIEW METFORMIN HYDROCHLORIDE TABLET contain Metformin and excipients generally considered to be non- toxic and non-hazardous in small quantities and under conditions of normal occupational
Naloxone Hydrochloride Injection PRODUCT INFORMATION
 Naloxone Hydrochloride Injection PRODUCT INFORMATION DESCRIPTION Naloxone hydrochloride is 17-allyl-4,5α-epoxy-3,14-dihydroxymorphinan-6-one hydrochloride; C 19 H 21 NO 4.HCl. It is an off-white powder
Naloxone Hydrochloride Injection PRODUCT INFORMATION DESCRIPTION Naloxone hydrochloride is 17-allyl-4,5α-epoxy-3,14-dihydroxymorphinan-6-one hydrochloride; C 19 H 21 NO 4.HCl. It is an off-white powder
1. What Xylocaine with adrenaline is and what it is used for
 Package leaflet: Information for the user Xylocaine 1% and 2% with adrenaline (epinephrine) 1:200,000 Solution for Injection lidocaine, adrenaline (epinephrine) Read all of this leaflet carefully before
Package leaflet: Information for the user Xylocaine 1% and 2% with adrenaline (epinephrine) 1:200,000 Solution for Injection lidocaine, adrenaline (epinephrine) Read all of this leaflet carefully before
A normal eye is protected by a layer of natural tears.
 A normal eye is protected by a layer of natural tears. If you need to use eye drops several times a day to make your eyes comfortably moist, you should consult your eye doctor. Helps increase tear production
A normal eye is protected by a layer of natural tears. If you need to use eye drops several times a day to make your eyes comfortably moist, you should consult your eye doctor. Helps increase tear production
See 17 for PATIENT COUNSELING INFORMATION. Revised: 3/2016 FULL PRESCRIBING INFORMATION: CONTENTS* WARNING: ADRENAL CRISIS IN THE SETTING OF SHOCK OR
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LYSODREN safely and effectively. See full prescribing information for LYSODREN. LYSODREN (mitotane)
MATERIAL SAFETY DATA SHEET
 Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Managing Challenging Cases in Refractive Surgery
 Managing Challenging Cases in Refractive Surgery Missouri Optometric Association Stephen A. Wexler, MD Eric E. Polk, OD, FAAO Outline The presenters will review challenging cases they have managed in refractive
Managing Challenging Cases in Refractive Surgery Missouri Optometric Association Stephen A. Wexler, MD Eric E. Polk, OD, FAAO Outline The presenters will review challenging cases they have managed in refractive
3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP
 PRESCRIBING INFORMATION 3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of Revision:
PRESCRIBING INFORMATION 3% Sodium Chloride Injection, USP 5% Sodium Chloride Injection, USP IV Fluid and Electrolyte Replenisher Baxter Corporation Mississauga, Ontario L5N 0C2 Canada Date of Revision:
A Genetic Analysis of Rheumatoid Arthritis
 A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
A Genetic Analysis of Rheumatoid Arthritis Introduction to Rheumatoid Arthritis: Classification and Diagnosis Rheumatoid arthritis is a chronic inflammatory disorder that affects mainly synovial joints.
patient group direction
 DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
DICLOFENAC v01 1/8 DICLOFENAC PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product)
 PART VI SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product) Format and content of the summary of the RMP The summary of the RMP part VI contains information based on RMP modules SI, SVIII and RMP
PART VI SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product) Format and content of the summary of the RMP The summary of the RMP part VI contains information based on RMP modules SI, SVIII and RMP
Chemical Name: (±)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6- carboxylic acid.
![Chemical Name: (±)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6- carboxylic acid. Chemical Name: (±)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6- carboxylic acid.](/thumbs/40/20977501.jpg) OCUFLOX (ofloxacin ophthalmic solution) 0.3% sterile DESCRIPTION OCUFLOX (ofloxacin ophthalmic solution) 0.3% is a sterile ophthalmic solution. It is a fluorinated carboxyquinolone anti-infective for topical
OCUFLOX (ofloxacin ophthalmic solution) 0.3% sterile DESCRIPTION OCUFLOX (ofloxacin ophthalmic solution) 0.3% is a sterile ophthalmic solution. It is a fluorinated carboxyquinolone anti-infective for topical
Public Assessment Report. Pharmacy to General Sales List Reclassification. Pirinase Hayfever Relief for Adults 0.05% Nasal Spray.
 Public Assessment Report Pharmacy to General Sales List Reclassification Pirinase Hayfever Relief for Adults 0.05% Nasal Spray (Fluticasone) PL 00079/0688 Glaxo Wellcome UK Limited TABLE OF CONTENTS Introduction
Public Assessment Report Pharmacy to General Sales List Reclassification Pirinase Hayfever Relief for Adults 0.05% Nasal Spray (Fluticasone) PL 00079/0688 Glaxo Wellcome UK Limited TABLE OF CONTENTS Introduction
New Zealand Datasheet
 New Zealand Datasheet Name of Medicine PRAXBIND Idarucizumab 50 mg/ml solution for injection/infusion Presentation Idarucizumab is a humanized monoclonal antibody fragment (Fab) molecule derived from an
New Zealand Datasheet Name of Medicine PRAXBIND Idarucizumab 50 mg/ml solution for injection/infusion Presentation Idarucizumab is a humanized monoclonal antibody fragment (Fab) molecule derived from an
ALLERGENIC EXTRACT. Prescription Set of Serial Dilutions (or Maintenance Vial (s)) INSTRUCTIONS FOR USE. U.S. Government License No.
 ALLERGENIC EXTRACT Prescription Set of Serial Dilutions (or Maintenance Vial (s)) INSTRUCTIONS FOR USE U.S. Government License No. 308 Revised 07/04 PO Box 800 Lenoir, NC 28645 USA DESCRIPTION This set
ALLERGENIC EXTRACT Prescription Set of Serial Dilutions (or Maintenance Vial (s)) INSTRUCTIONS FOR USE U.S. Government License No. 308 Revised 07/04 PO Box 800 Lenoir, NC 28645 USA DESCRIPTION This set
Treating the Eye with Autologous and Allogeneic Serum Administration. Clarke D. Newman, OD, FAAO
 Treating the Eye with Autologous and Allogeneic Serum Administration cdnewman@ earthlink.net Abstract: This one-hour course will detail the use of blood serum in the treatment of anterior ocular surface
Treating the Eye with Autologous and Allogeneic Serum Administration cdnewman@ earthlink.net Abstract: This one-hour course will detail the use of blood serum in the treatment of anterior ocular surface
PACKAGE LEAFLET: INFORMATION FOR THE USER. VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution. Cyanocobalamin
 PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid
 Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
PACKAGE LEAFLET: INFORMATION FOR THE USER. ADRENALINE (TARTRATE) STEROP 1 mg/1 ml Solution for injection. Adrenaline (Levorenine, Epinephrine)
 PACKAGE LEAFLET: INFORMATION FOR THE USER ADRENALINE (TARTRATE) STEROP 1 mg/1 ml Solution for injection Adrenaline (Levorenine, Epinephrine) Read all of this leaflet carefully before you start using this
PACKAGE LEAFLET: INFORMATION FOR THE USER ADRENALINE (TARTRATE) STEROP 1 mg/1 ml Solution for injection Adrenaline (Levorenine, Epinephrine) Read all of this leaflet carefully before you start using this
Section 1: Identification Product information Product Name Active substance Intended Uses
 Section 1: Identification Product information Product Name Active substance Intended Uses Company Details Manufacturer SAFETY DATA SHEET Levetiracetam Tablets, 250 mg, 500 mg, 750 mg & 1000 mg Levetiracetam
Section 1: Identification Product information Product Name Active substance Intended Uses Company Details Manufacturer SAFETY DATA SHEET Levetiracetam Tablets, 250 mg, 500 mg, 750 mg & 1000 mg Levetiracetam
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 10 g of solution contains 0.2 g of erythromycin. Structural formula of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 10 g of solution contains 0.2 g of erythromycin. Structural formula of
Teriflunomide is the active metabolite of Leflunomide, a drug employed since 1994 for the treatment of rheumatoid arthritis (Baselt, 2011).
 Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Yes This controlled document shall not be copied in part or whole without the express permission of the author or the author s representative.
 Title: Patient Group Direction for the administration of lidocaine hydrochloride 1% injection as infiltration anaesthesia for insertion/removal of central venous catheters by nurses/radiographers working
Title: Patient Group Direction for the administration of lidocaine hydrochloride 1% injection as infiltration anaesthesia for insertion/removal of central venous catheters by nurses/radiographers working
One vial contains 500 U* of C1-inhibitor** After reconstitution the product contains 500 U/5 ml which correspond to a concentration of 100 U/ml.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cetor 100 U/ml powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 500 U* of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cetor 100 U/ml powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 500 U* of
PRODUCT INFORMATION. Silver sulfadiazine is a sulfonamide and has broad antimicrobial activity against both Grampositive and Gram-negative organisms.
 PRODUCT INFORMATION Name of the Medicine: FLAMAZINE CREAM 1.0% w/w Silver sulfadiazine 1% w/w Composition: Active ingredient. Silver sulfadiazine. Excipients. Polysorbate 60 Ph. Eur, Polysorbate 80 Ph.
PRODUCT INFORMATION Name of the Medicine: FLAMAZINE CREAM 1.0% w/w Silver sulfadiazine 1% w/w Composition: Active ingredient. Silver sulfadiazine. Excipients. Polysorbate 60 Ph. Eur, Polysorbate 80 Ph.
Consent for Bilateral Simultaneous Refractive Surgery PRK
 Consent for Bilateral Simultaneous Refractive Surgery PRK Please sign and return Patient Copy While many patients choose to have both eyes treated at the same surgical setting, there may be risks associated
Consent for Bilateral Simultaneous Refractive Surgery PRK Please sign and return Patient Copy While many patients choose to have both eyes treated at the same surgical setting, there may be risks associated
EFFIMET 1000 XR Metformin Hydrochloride extended release tablet
 BRAND NAME: Effimet XR. THERAPEUTIC CATEGORY: Anti-Diabetic PHARMACOLOGIC CLASS: Biguanides EFFIMET 1000 XR Metformin Hydrochloride extended release tablet COMPOSITION AND PRESENTATION Composition Each
BRAND NAME: Effimet XR. THERAPEUTIC CATEGORY: Anti-Diabetic PHARMACOLOGIC CLASS: Biguanides EFFIMET 1000 XR Metformin Hydrochloride extended release tablet COMPOSITION AND PRESENTATION Composition Each
1 Always test and record vision wearing distance spectacles test each eye separately A 1mm pinhole will improve acuity in refractive errors
 Golden eye rules Examination techniques 1 Always test and record vision wearing distance spectacles test each eye separately A 1mm pinhole will improve acuity in refractive errors Snellen chart (6 metre)
Golden eye rules Examination techniques 1 Always test and record vision wearing distance spectacles test each eye separately A 1mm pinhole will improve acuity in refractive errors Snellen chart (6 metre)
Biologic Treatments for Rheumatoid Arthritis
 Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
WHAT SHOULD TREATMENT ACHIEVE?
 Uveitis Information Group Factsheet The Treatment of Uveitis (2) Drug Treatments Please use these factsheets as background information to help discussion with your doctors. Individual cases may vary enormously
Uveitis Information Group Factsheet The Treatment of Uveitis (2) Drug Treatments Please use these factsheets as background information to help discussion with your doctors. Individual cases may vary enormously
Salbutamol 1mg/ml Nebuliser Solution. Salbutamol 2mg/ml Nebuliser Solution PL 36390/0035 PL 36390/0036
 Salbutamol 1mg/ml Nebuliser Solution Salbutamol 2mg/ml Nebuliser Solution PL 36390/0035 PL 36390/0036 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment
Salbutamol 1mg/ml Nebuliser Solution Salbutamol 2mg/ml Nebuliser Solution PL 36390/0035 PL 36390/0036 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment
Cyclooxygenase and NSAIDs
 Cyclooxygenase and NSAIDs Cyclooxygenase An enzyme responsible for the production of prostaglandins Two forms, COX1 and COX2 Contains two separate active sites for prostaglandin synthase One side contains
Cyclooxygenase and NSAIDs Cyclooxygenase An enzyme responsible for the production of prostaglandins Two forms, COX1 and COX2 Contains two separate active sites for prostaglandin synthase One side contains
SUMMARY OF PRODUCT CHARACTERISTICS. Buprenovet 0.3 mg/ml Solution for Injection for Dogs and Cats (AT, DE)
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Buprecare 0.3 mg/ml Solution for Injection for Dogs and Cats (UK, BE, FR, IE, LU, NL, ES) Buprenovet 0.3 mg/ml Solution for
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Buprecare 0.3 mg/ml Solution for Injection for Dogs and Cats (UK, BE, FR, IE, LU, NL, ES) Buprenovet 0.3 mg/ml Solution for
NEUROTONE THR 00904/0005 UKPAR
 NEUROTONE THR 00904/0005 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 10 Summary of Product Characteristics Page 11 Product Information Leaflet
NEUROTONE THR 00904/0005 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 10 Summary of Product Characteristics Page 11 Product Information Leaflet
Keep Up With Your Treatment and Keep Track of Your Eye Pressure
 Keep Up With Your Treatment and Keep Track of Your Eye Pressure Get into the habit of applying your XAATAN (latanoprost ophthalmic solution) eye drops at the same time every day DAY 1 DAY 2 DAY 3 DAY 4
Keep Up With Your Treatment and Keep Track of Your Eye Pressure Get into the habit of applying your XAATAN (latanoprost ophthalmic solution) eye drops at the same time every day DAY 1 DAY 2 DAY 3 DAY 4
PERIOCULAR (SUBTENON) STEROID INJECTION ERIC S. MANN M.D.,Ph.D.
 PERIOCULAR (SUBTENON) STEROID INJECTION ERIC S. MANN M.D.,Ph.D. A. INDICATIONS: Periocular steroid injection involves placement of steroid around the eye to treat intraocular inflammation or swelling of
PERIOCULAR (SUBTENON) STEROID INJECTION ERIC S. MANN M.D.,Ph.D. A. INDICATIONS: Periocular steroid injection involves placement of steroid around the eye to treat intraocular inflammation or swelling of
TABLE OF CONTENTS: LASER EYE SURGERY CONSENT FORM
 1 BoydVision TABLE OF CONTENTS: LASER EYE SURGERY CONSENT FORM Risks and Side Effects... 2 Risks Specific to PRK... 3 Risks Specific to LASIK... 4 Patient Statement of Consent... 5 Consent for Laser Eye
1 BoydVision TABLE OF CONTENTS: LASER EYE SURGERY CONSENT FORM Risks and Side Effects... 2 Risks Specific to PRK... 3 Risks Specific to LASIK... 4 Patient Statement of Consent... 5 Consent for Laser Eye
FLIXONASE ALLERGY Non Drowsy Nasal Spray 24 hour Effective Relief and Prevention Available in 60 & 150 sprays
 FLIXONASE ALLERGY Non Drowsy Nasal Spray 24 hour Effective Relief and Prevention Available in 60 & 150 sprays CONSUMER MEDICINE INFORMATION WHAT IS IN THIS LEAFLET? Please read this leaflet carefully before
FLIXONASE ALLERGY Non Drowsy Nasal Spray 24 hour Effective Relief and Prevention Available in 60 & 150 sprays CONSUMER MEDICINE INFORMATION WHAT IS IN THIS LEAFLET? Please read this leaflet carefully before
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE S1A. Current Step 4 version
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDELINE ON THE NEED FOR CARCINOGENICITY STUDIES
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDELINE ON THE NEED FOR CARCINOGENICITY STUDIES
PATIENT INFORMATION LEAFLET. CEFALEXIN 250 mg AND 500 mg CAPSULES CEFALEXIN
 PATIENT INFORMATION LEAFLET CEFALEXIN 250 mg AND 500 mg CAPSULES CEFALEXIN Read all of this leaflet carefully before you start taking this medicine. - Keep this leaflet. You may need to read it again.
PATIENT INFORMATION LEAFLET CEFALEXIN 250 mg AND 500 mg CAPSULES CEFALEXIN Read all of this leaflet carefully before you start taking this medicine. - Keep this leaflet. You may need to read it again.
Stepping toward a different treatment option LEARN WHAT ACTHAR CAN DO FOR YOU
 FOR MS RELAPSES Stepping toward a different treatment option LEARN WHAT ACTHAR CAN DO FOR YOU As a person with multiple sclerosis (MS), you know firsthand the profound impact MS relapses can have on your
FOR MS RELAPSES Stepping toward a different treatment option LEARN WHAT ACTHAR CAN DO FOR YOU As a person with multiple sclerosis (MS), you know firsthand the profound impact MS relapses can have on your
How To Treat Eye Problems With A Laser
 1550 Oak St., Suite 5 1515 Oak St., St Eugene, OR 97401 Eugene, OR 97401 (541) 687-2110 (541) 344-2010 INFORMED CONSENT FOR PHOTOREFRACTIVE KERATECTOMY (PRK) This information is to help you make an informed
1550 Oak St., Suite 5 1515 Oak St., St Eugene, OR 97401 Eugene, OR 97401 (541) 687-2110 (541) 344-2010 INFORMED CONSENT FOR PHOTOREFRACTIVE KERATECTOMY (PRK) This information is to help you make an informed
BECONASE AQ (beclomethasone dipropionate, monohydrate) Nasal Spray, 42 mcg
 BECONASE AQ (beclomethasone dipropionate, monohydrate) Nasal Spray, 42 mcg For Intranasal Use Only. PRESCRIBING INFORMATION SHAKE WELL BEFORE USE. DESCRIPTION Beclomethasone dipropionate, monohydrate,
BECONASE AQ (beclomethasone dipropionate, monohydrate) Nasal Spray, 42 mcg For Intranasal Use Only. PRESCRIBING INFORMATION SHAKE WELL BEFORE USE. DESCRIPTION Beclomethasone dipropionate, monohydrate,
2 DOSAGE AND ADMINISTRATION 2.1 Dosing and Dose Adjustment
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Auryxia safely and effectively. See full prescribing information for Auryxia. Auryxia (ferric citrate)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Auryxia safely and effectively. See full prescribing information for Auryxia. Auryxia (ferric citrate)
Summary of the risk management plan (RMP) for Orkambi (lumacaftor and ivacaftor)
 EMA/662624/2015 Summary of the risk management plan (RMP) for Orkambi (lumacaftor and ivacaftor) This is a summary of the risk management plan (RMP) for Orkambi, which details the measures to be taken
EMA/662624/2015 Summary of the risk management plan (RMP) for Orkambi (lumacaftor and ivacaftor) This is a summary of the risk management plan (RMP) for Orkambi, which details the measures to be taken
FLAMAZINE CREAM 1.0% w/w
 PRODUCT INFORMATION NAME OF THE MEDICINE: FLAMAZINE CREAM 1.0% w/w Silver sulfadiazine 1% w/w Composition: Active ingredient. Silver sulfadiazine. Excipients. Polysorbate 60 Ph. Eur, Polysorbate 80 Ph.
PRODUCT INFORMATION NAME OF THE MEDICINE: FLAMAZINE CREAM 1.0% w/w Silver sulfadiazine 1% w/w Composition: Active ingredient. Silver sulfadiazine. Excipients. Polysorbate 60 Ph. Eur, Polysorbate 80 Ph.
