MEDICAL POLICY I. POLICY POLICY GUIDELINES POLICY TITLE GENETIC TESTING FOR HEREDITARY HEMOCHROMATOSIS POLICY NUMBER MP 2.312
|
|
|
- Daniella Cobb
- 7 years ago
- Views:
Transcription
1 Original Issue Date (Created): 1/1/2013 Most Recent Review Date (Revised): 3/29/2016 Effective Date: 1/1/2017 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY I. POLICY Genetic testing for Hereditary Hemochromatosis HFE gene mutations may be considered medically necessary in a patient with abnormal serum iron indices indicating iron overload. (See policy guidelines Genetic testing for HFE gene mutations may be considered medically necessary in individuals with a family history of hemochromatosis in a first-degree relative. See policy guidelines). Genetic testing for hereditary hemochromatosis in screening of the general population is considered investigational. There is insufficient evidence to support a conclusion concerning the health outcomes or benefits associated with this procedure for this indication. POLICY GUIDELINES Serum iron indices in the diagnosis of HH (6) Elevated fasting transferrin saturation (the ratio of serum iron to total iron-binding capacity) is the most sensitive initial phenotypic screening test. A cut-off value of 45% will detect almost all affected C282Y homozygotes. Serum ferritin reflects body iron stores and generally rises later in the progression of iron overload. In the absence of confounding causes of hyperferritinemia (alcohol abuse, the metabolic syndrome, inflammatory states and acute and chronic hepatitis), serum ferritin is a good marker of the degree of iron overload. The negative predictive value of a normal transferrin saturation and serum ferritin is 97%. In this situation, no further testing is recommended. (6) 2011 Practice Guidelines by the American Association for the Study of Liver Diseases recommend HFE gene mutation testing in patients with abnormal serum iron indices, even Page 1
2 in the absence of symptoms (e.g., abnormal serum iron indices on routine screening chemistry panel). Genetic testing in an individual with a family history of HH The 2011 Practice Guidelines by the American Association for the Study of Liver Diseases recommend screening (iron studies and HFE mutation analysis) of first-degree relatives of patients with HFE-related HH to detect early disease and prevent complications. (5) For children of an identified proband, HFE testing of the other parent is generally recommended because if results are normal, the child is an obligate heterozygote and need not undergo further testing because there is no increased risk of iron loading. If C282Y homozygosity or compound heterozygosity is found in adult relatives of a proband, and if serum ferritin levels are increased, then therapeutic phlebotomy can be initiated. If ferritin level is normal in these patients, then yearly follow-up with iron studies is indicated. When identified, C282Y heterozygotes and H63D heterozygotes can be reassured that they are not at risk for developing progressive or symptomatic iron overload. Occasional H63D homozygotes can develop mild iron overload. Cross-references None II. PRODUCT VARIATIONS This policy is applicable to all programs and products administered by Capital BlueCross unless otherwise indicated below. BlueJourney HMO* BlueJourney PPO* FEP PPO** * Refer to Novitas Solutions Local Coverage Determination (LCD) L35062 Biomarkers Overview. **Refer to FEP Medical Policy Manual MP Genetic Testing for Hereditary Hemochromatosis. The FEP Medical Policy manual can be found at: III. DESCRIPTION/BACKGROUND Hereditary hemochromatosis, a common genetic disorder of iron metabolism, can lead to inappropriate iron absorption, toxic accumulation and organ damage. Genetic testing is available to assess mutations in the HFE gene, which are responsible for the majority of clinically significant cases of hereditary hemochromatosis. Serum iron indices in the diagnosis of hereditary hemochromatosis (HH) Page 2
3 Elevated fasting transferrin saturation (the ratio of serum iron to total iron-binding capacity) is the most sensitive initial phenotypic screening test. A cut-off value of 45% will detect almost all affected C282Y homozygotes. Serum ferritin reflects body iron stores and generally rises later in the progression of iron overload. In the absence of confounding causes of hyperferritinemia (alcohol abuse, the metabolic syndrome, inflammatory states and acute and chronic hepatitis), serum ferritin is a good marker of the degree of iron overload. The negative predictive value of a normal transferrin saturation and serum ferritin is 97%. In this situation, no further testing is recommended Practice Guidelines by the American Association for the Study of Liver Diseases recommend HFE gene mutation testing in patients with abnormal serum iron indices, even in the absence of symptoms (e.g., abnormal serum iron indices on routine screening chemistry panel). Genetic testing in an individual with a family history The 2011 Practice Guidelines by the American Association for the Study of Liver Diseases recommend screening (iron studies and HFE mutation analysis) of first-degree relatives of patients with HFE-related HH to detect early disease and prevent complications. For children of an identified proband, HFE testing of the other parent is generally recommended because if results are normal, the child is an obligate heterozygote and need not undergo further testing because there is no increased risk of iron loading. If C282Y homozygosity or compound heterozygosity is found in adult relatives of a proband, and if serum ferritin levels are increased, then therapeutic phlebotomy can be initiated. If ferritin level is normal in these patients, then yearly follow-up with iron studies is indicated. When identified, C282Y heterozygotes and H63D heterozygotes can be reassured that they are not at risk for developing progressive or symptomatic iron overload. Occasional H63D homozygotes can develop mild iron overload Iron overload Iron overload syndromes may be hereditary, secondary to some other disease (e.g. ironloading anemias, parenteral iron overload, chronic liver disease or dysmetabolic iron overload syndrome), or due to other miscellaneous conditions (e.g., neonatal iron overload, aceruloplasminemia, congenital atransferrinemia). Iron overload, if left untreated, can lead to secondary tissue damage in a wide range of organs resulting in chronic liver disease (hepatic fibrosis, cirrhosis, hepatocellular carcinoma), endocrine dysfunction (diabetes, hypogonadism), arthralgia or arthritis (typically involving the second and third metacarpo-phalangeal joints), and cardiomyopathy (either with symptomatic cardiac failure or arrhythmias). Hereditary hemochromatosis (HH), an autosomal recessive disorder, is the most common, identified, genetic disorder in Caucasians, and may be seen in approximately 1 in 250 Page 3
4 Caucasians. However, fully expressed disease with end-organ manifestations is seen in <10% of those individuals. The factors that influence phenotypic expression of HFErelated HH (that is the clinical appearance of iron overload) are not clearly defined. The low clinical penetrance appears to be due to a complex interplay of genetic status and other factors such as age, sex, environmental influences and the presence of other diseases. HH leads to inappropriate iron absorption from the intestine and progressive increase in intracellular iron concentrations. Untreated HH leads to premature death, usually by liver complications. Treatment by removing excess iron with serial phlebotomy is simple and effective, and if started before irreversible end organ damage, restores normal life expectancy. Diagnosis of hemochromatosis Patients with hemochromatosis may present with nonspecific systemic symptoms, specific organ-related symptoms, or they may be asymptomatic. The clinical diagnosis of hemochromatosis is based on documentation of increased iron stores as demonstrated by abnormal serum iron indices, specifically elevated transferrin saturation and elevated serum ferritin concentration. Liver biopsy has been used in the past to confirm diagnosis but is now generally limited to determining the degree of hepatic fibrosis and cirrhosis during management of the disease. Genetic testing can confirm a hereditary nature of the iron overload. Genetics of hereditary hemochromatosis The majority of patients with HH have mutations in the HFE gene, which is on the short arm of chromosome 6. The HFE gene was identified and cloned in The most common mutation in the HFE gene is C282Y, a missense mutation that substitutes a cysteine residue for tyrosine at amino acid position 282 on the HFE protein. Homozygosity for the C282Y mutation is associated with 60-90% of all cases of HH. Additionally, 3-8% of individuals affected with HH are heterozygous for this mutation. Penetrance for elevated serum iron indices among C282Y homozygotes is relatively high, but not 100%. However, the penetrance for the characteristic clinical endpoints (end organ damage) is quite low. There is no test that can predict whether a C282Y homozygote will develop clinical symptoms. The other significant mutation is referred to as H63D which results in the substitution of aspartic acid for histidine at position 63. Homozygosity for H63D is insufficient to cause clinically significant iron overload in the absence of modifying factors. However, heterozygosity for C282Y/H63D has been associated with increased hepatic iron concentrations; approximately 1-2% of patients with this genotype will develop clinical evidence of iron overload. The clinical significance of a third HFE mutation, S65C, appears to be minimal. This rare variant displays very low penetrance. Compound heterozygosity for C282Y and S65C Page 4
5 may confer a low risk for mild HH. Individuals who are heterozygous for S65C and either the wild-type (normal) or H63D alleles do not seem to be at an increased risk for HH. With the advent of genetic testing in the late 1990s, HFE-related HH is now frequently identified in asymptomatic probands and in presymptomatic relatives of patients who are known to have the disease. (1) Therefore, a genetic diagnosis can be applied to individuals who have not yet developed phenotypic expression. These individuals have a genetic susceptibility to developing iron overload but may never do so. A consensus conference of the European Association for the Study of Liver Diseases in 2000 led to a recognition of the different stages and progression of hemochromatosis. These stages were defined as: 1. Stage 1: those patients with the genetic disorder with no increase in iron stores who have genetic susceptibility. 2. Stage 2: those patients with the genetic disorder who have phenotypic evidence of iron overload but who are without tissue or end organ damage. 3. Stage 3: those individuals who have the genetic disorder with iron overload and have iron deposition to the degree that tissue and end organ damage occurs. Regulatory Status No U.S. Food and Drug Administration (FDA)-cleared genotyping tests were found. Thus, genotyping is offered as a laboratory-developed test. Clinical laboratories may develop and validate tests in-house ( home-brew ) and market them as a laboratory service; such tests must meet the general regulatory standards of the Clinical Laboratory Improvement Act (CLIA). The laboratory offering the service must be licensed by CLIA for highcomplexity testing. IV. RATIONALE This policy was created in 2012 and is based on a search of the MEDLINE database through April 1, 2015 (see Appendix Table 1 for genetic testing categories). Recent reviews highlight the pathogenesis, diagnosis and management of hereditary hemochromatosis (HH). 1,9-11 Technology Assessments A 2001 TEC Assessment on genetic testing for HFE gene mutations related to HH concluded the following: Genetic testing and counseling for HFE mutations in the management of patients with symptoms of iron overload consistent with hereditary hemochromatosis, in the setting of 2 consecutive transferrin saturation values of 45% or more and a serum ferritin value of less than g/l, met the TEC criteria. Genetic testing and counseling for HFE mutations in asymptomatic relatives of subjects with hereditary hemochromatosis also met the TEC criteria. Page 5
6 The Assessment did not address the use of genetic testing for HFE gene mutations in screening of the general population. 12 Validation of the Clinical Use of a Genetic Test Validation of the clinical use of any genetic test focuses on 3 main principles: (1) analytic validity (technical accuracy of the test in detecting a mutation that is present or in excluding a mutation that is absent; (2) clinical validity (diagnostic performance of the test [sensitivity, specificity, positive and negative predictive values] in detecting clinical disease; and (3) clinical utility (ie, how results of the diagnostic test will be used to change management of the patient and whether these changes in management lead to clinically important improvements in health outcomes). Analytic Validity Stuhrmann et al (2005) initiated a pilot study on DNA-based screening of hereditary hemochromatosis in Germany. 13 A focus of the study was the analytic validity of different test methods. A total of 3961 subjects provided blood samples for testing of the C282Y HFE mutation; of these, 3930 samples were successfully tested by 2 independent test methods (either polymerase chain reaction and restriction digest, reverse allele-specific oligonucleotide hybridization, solid-phase oligonucleotide ligation assay, or microarray [DNA-chip]). In all, 67 of the tested subjects were homozygous for C282Y; 42.6% of the homozygotes already knew their clinical diagnosis of HH before sending the blood sample. Iron accumulation with further signs or symptoms of HH was present in 8 (24%) of 34 newly diagnosed C282Y homozygous subjects. Of 7860 tests performed, 7841 (99.6%) gave correct results. The overall error rate was 0.24% (95% confidence interval [CI], 0.15 to 0.38). Analytic specificity of the test methods for detecting homozygosity for C282Y was 100% (7726/7726 nonhomozygous test challenges; 95% CI, to 100), and analytic sensitivity was 97% (130/134 homozygous test challenges; 95% CI, 92.5 to 99.2). This evidence indicates that test methods for C282Y are robust and highly sensitive and specific. Clinical Validity Bryant et al (2008) evaluated the clinical validity and clinical utility of DNA testing in people suspected of having hereditary hemochromatosis and in family members of those diagnosed with the disorder by conducting a systematic review of 15 electronic databases (including MEDLINE and the Cochrane library) up to April Clinical validity, defined as the ability of the test to detect or predict the phenotype (disorder) of interest, involved establishing the probability that the test would be positive in people with clinical HH (sensitivity) and the probability that the test would be negative in people without the disease (specificity). Studies were included if they reported the use of DNA tests in whites of northern European origin with iron overload suggestive of HH compared with a control population, and reported or allowed the calculation of sensitivity and specificity. Clinical utility studies were included if they reported the use of DNA tests in whites with iron overload suggestive of HH (or relatives of suspected cases) compared with any case-identification strategy not involving DNA, and reported patient-based outcomes (eg, morbidity or mortality). Page 6
7 Eleven observational studies that evaluated the clinical validity of genotyping for the C282Y mutation in the diagnostic workup for HH were identified. Criteria used to define hemochromatosis varied among studies. Clinical sensitivity of C282Y homozygosity ranged from 28.4% to 100%; when considering studies that used strict criteria to classify HH, clinical sensitivity ranged from 91.3% to 92.4%. No clinical utility studies were found. The authors concluded that DNA testing for HH in at-risk populations has clinical validity and may have clinical utility. Clinical Utility The clinical utility of genetic testing for HH depends on how results can be used to improve patient management. Although there has never been a randomized controlled trial of phlebotomy versus no phlebotomy in the treatment of HH, there is evidence that initiation of phlebotomy before the development of cirrhosis and/or diabetes will significantly reduce HH-associated morbidity and mortality. 2,15,16 Data exists on the psychosocial aspect of genetic testing for HH. Picot et al (2009) conducted a systematic review of the psychosocial aspects of DNA testing for HH in at-risk subjects. 17 Databases were searched through 2007 for any quantitative or qualitative primary research that considered DNA testing of subjects considered at-risk for HH and that reported psychosocial outcomes. Three observational studies were included; each had methodologic limitations. After receiving test results, patient anxiety levels fell or were unchanged, and general health-related quality-of-life outcomes improved in some aspects from pretest assessments, or were unchanged. Outcomes were not reported separately for those referred for diagnosis and those with a family history of HH. The authors concluded that, although evidence is limited, results suggested that genetic testing for HH in at-risk subjects is accompanied by few negative psychosocial outcomes. Population Screening for HH General population screening for HH has been proposed because of the high prevalence of disease, absence of or nonspecific early clinical findings, specificity of findings once they appear, low cost of diagnosis and treatment, and high cost and low success rate of late diagnosis and treatment. However, because genotype penetrance is low, and the natural history of untreated individuals is unpredictable, support for population-based screening is lacking. The American Academy of Family Physicians, Centers for Disease Control and Prevention, and U.S. Preventive Services Task Force recommend against population-based general screening. 20 McLaren and Gordeuk (2009) conducted the Hemochromatosis and Iron Overload Screening (HEIRS) study to evaluate the prevalence, genetic and environmental determinants, and potential clinical, personal, and societal impact of hemochromatosis and iron overload in a multiethnic, primary care-based sample of 101,168 adults enrolled over a 2-year period at 4 centers in the United States and 1 in Canada. 21 Initial screening included genotyping for the HFE C282Y and H63D alleles, measurement of serum ferritin, and calculation of transferrin saturation. The yield of HFE genotyping for identifying persons with C282Y homozygosity was low in racial/ethnic groups other than non-hispanic whites. Overall frequency of homozygosity for the C282Y Page 7
8 mutation in non-hispanic whites was 4.4 per There was marked heterogeneity of disease expression in C282Y homozygotes. The authors concluded that (1) future studies to discover modifier genes that affect phenotypic expression in C282Y hemochromatosis should help identify patients who are at greatest risk of developing iron overload who may benefit from continued monitoring of iron status, and (2) although genetic testing is well-accepted and associated with minimal risk of discrimination, generalized population screening in a primary care population as performed in the HEIRS study is not recommended. In a substudy of white participants in the HEIRS study, Adams et al (2013) assessed the prevalence of HFE mutations in patients who had elevated serum ferritin levels less than 1000 g/l ( g/l for men, g/l for women). 22 Among 3359 men and 2416 women, prevalence of potential iron-loading HFE genotypes (defined as C282Y homozygote, C282Y/H63D compound heterozygote, or H63D homozygote) was 10% and 12% in men and women, respectively. Prevalence of C282Y homozygosity was 2% and 4% among men and women, respectively. Likelihood of C282Y homozygosity increased with increasing serum ferritin levels, from 0.3% to 16% in men, and from 0.3% to 30% in women. Posttest likelihood ratios (likelihood of C282Y homozygosity given a positive test result) exceeded 1 at serum ferritin levels of 500 g/l or more for men and at levels greater than 300 g/l for women. In white subjects with mild hyperferritinemia, causes of elevated serum ferritin level other than C282Y or H63D HFE mutations (eg, liver disease, diabetes) were more likely. Ongoing and Unpublished Clinical Trials A search of ClinicalTrials.gov did not identify any ongoing or unpublished trials that would likely influence this policy. Summary of Evidence Hereditary hemochromatosis (HH) is a common genetic disorder in the white population. Abnormal serum iron indices, clinical symptoms of iron overload or a family history of HH may provoke testing for diagnosis. Testing for mutations in the HFE gene, which contributes to most cases of HH, can confirm a genetic etiology; if clinically indicated, serial phlebotomy may be initiated, which can lead to a restored normal life expectancy. Therefore, genetic testing for HFE gene mutations may be considered medically necessary for patients with a clinical suspicion of hemochromatosis (signs and symptoms of iron overload) or in patients with fasting serum iron indices that are suggestive of iron overload, as well as in individuals with a family history of hemochromatosis. General population screening has been proposed because of the high prevalence of disease, absence of or nonspecific early clinical findings, simplicity and effectiveness of treatment, and low success rate of late diagnosis and treatment. However, because genotype penetrance is low, and the natural history of untreated individuals is unpredictable, support for population-based screening is lacking. Therefore, genetic testing for HH screening in the general population is considered investigational. Page 8
9 Practice Guidelines and Position Statements American Academy of Family Physicians The American Academy of Family Physicians recommends against routine genetic screening for in the asymptomatic general population (grade D recommendation: at least fair evidence that [the service] is ineffective or that harms outweigh benefits). 18 American Association for the Study of Liver Diseases A 2011 practice guideline from the American Association for the Study of Liver Diseases recommends 2 : Patients with abnormal iron studies should be evaluated as patients with hemochromatosis, even in the absence of symptoms (based on high quality evidence [A]). In a patient with suggestive symptoms, physical findings, or family history of HH, a combination of transferrin saturation and ferritin should be obtained rather than relying on a single test, and if either is abnormal (transferrin saturation 45% or ferritin above the upper limit of normal), then HFE mutation analysis should be performed (strength of recommendation 1 [strong] by the classification of the Grading of Recommendation Assessment, Development, and Evaluation [GRADE] workgroup; based on moderate quality evidence [B]). Screening (iron studies and HFE mutation analysis) of first-degree relatives of patients with HFE-related HH is recommended to detect early disease and prevent complications. (1A) Screening for non-hfe-related HH is not recommended. Average risk population screening for HH is not recommended. (1B) Centers for Disease Control and Prevention The Centers for Disease Control and Prevention does not currently recommend population screening for HFE mutations. 19 U.S. Preventive Services Task Force Recommendations The U.S. Preventive Services Task Force has decided not to review the evidence and update its recommendations for hemochromatosis screening. 23 Medicare National Coverage There is no national coverage determination (NCD). V. DEFINITIONS FIRST-DEGREE RELATIVE refers to a parent, sibling, or child. Page 9
10 VI. BENEFIT VARIATIONS The existence of this medical policy does not mean that this service is a covered benefit under the member's contract. Benefit determinations should be based in all cases on the applicable contract language. Medical policies do not constitute a description of benefits. A member s individual or group customer benefits govern which services are covered, which are excluded, and which are subject to benefit limits and which require preauthorization. Members and providers should consult the member s benefit information or contact Capital for benefit information. VII. DISCLAIMER Capital s medical policies are developed to assist in administering a member s benefits, do not constitute medical advice and are subject to change. Treating providers are solely responsible for medical advice and treatment of members. Members should discuss any medical policy related to their coverage or condition with their provider and consult their benefit information to determine if the service is covered. If there is a discrepancy between this medical policy and a member s benefit information, the benefit information will govern. Capital considers the information contained in this medical policy to be proprietary and it may only be disseminated as permitted by law. VIII. CODING INFORMATION Note: This list of codes may not be all-inclusive, and codes are subject to change at any time. The identification of a code in this section does not denote coverage as coverage is determined by the terms of member benefit information. In addition, not all covered services are eligible for separate reimbursement. Covered when medically necessary: CPT Codes Current Procedural Terminology (CPT) copyrighted by American Medical Association. All Rights Reserved. ICD-10- CM Diagnosis Description Code* E Hereditary hemochromatosis E Hemochromatosis due to repeated red blood cell transfusions E Other hemochromatosis E Hemochromatosis, unspecified Page 10
11 E83.10 Disorder of iron metabolism, unspecified E83.19 Other disorders of iron metabolism R79.0 Abnormal level of blood mineral Z83.49 Family history of other endocrine, nutritional and metabolic diseases *If applicable, please see Medicare LCD or NCD for additional covered diagnoses. IX. REFERENCES 1. Clark P, Britton LJ, Powell LW. The diagnosis and management of hereditary haemochromatosis. Clin Biochem Rev. Feb 2010;31(1):3-8. PMID Bacon BR, Adams PC, Kowdley KV, et al. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. Jul 2011;54(1): PMID Stickel F, Buch S, Zoller H, et al. Evaluation of genome-wide loci of iron metabolism in hereditary hemochromatosis identifies PCSK7 as a host risk factor of liver cirrhosis. Hum Mol Genet. Jul ;23(14): PMID Kanwar P, Kowdley KV. Metal storage disorders: Wilson disease and hemochromatosis. Med Clin North Am. Jan 2014;98(1): PMID Sood R, Bakashi R, Hegade VS, et al. Diagnosis and management of hereditary haemochromatosis. British Journal of General Practice. June 1, ;63(611): Vujic M. Molecular basis of HFE-hemochromatosis. Front Pharmacol. 2014;5:42. PMID Radio FC, Majore S, Binni F, et al. TFR2-related hereditary hemochromatosis as a frequent cause of primary iron overload in patients from Central-Southern Italy. Blood Cells Mol Dis. Feb-Mar 2014;52(2-3): PMID Ekanayake D, Roddick C, Powell LW. Recent advances in hemochromatosis: a 2015 update : A summary of proceedings of the 2014 conference held under the auspices of Hemochromatosis Australia. Hepatol Int. Mar PMID Alexander J, Kowdley KV. HFE-associated hereditary hemochromatosis. Genet Med. May 2009;11(5): PMID Gan EK, Powell LW, Olynyk JK. Natural history and management of HFE-hemochromatosis. Semin Liver Dis. Aug 2011;31(3): PMID Crownover BK, Covey CJ. Hereditary hemochromatosis. Am Fam Physician. Feb ;87(3): PMID Blue Cross and Blue Shield Association Technology Evaluation Center (TEC). Genetic testing for HFE gene mutations related to hereditary hemochromatosis. TEC Assessments 2001; Volume 16, Tab Stuhrmann M, Strassburg C, Schmidtke J. Genotype-based screening for hereditary haemochromatosis. I: Technical performance, costs and clinical relevance of a German pilot study. Eur J Hum Genet. Jan 2005;13(1): PMID Page 11
12 14. Bryant J, Cooper K, Picot J, et al. A systematic review of the clinical validity and clinical utility of DNA testing for hereditary haemochromatosis type 1 in at-risk populations. J Med Genet. Aug 2008;45(8): PMID Adams PC, Speechley M, Kertesz AE. Long-term survival analysis in hereditary hemochromatosis. Gastroenterology. Aug 1991;101(2): PMID Niederau C, Fischer R, Purschel A, et al. Long-term survival in patients with hereditary hemochromatosis. Gastroenterology. Apr 1996;110(4): PMID Picot J, Bryant J, Cooper K, et al. Psychosocial aspects of DNA testing for hereditary hemochromatosis in at-risk individuals: a systematic review. Genet Test Mol Biomarkers. Feb 2009;13(1):7-14. PMID American Academy of Family Physicians. Hemochromatosis, Accessed April 2, Centers for Disease Control and Prevention. Hemochromatosis (iron storage disease). Training & education - epidemiology prevalence. Page last updated: September 3, Accessed April 2, Whitlock EP, Garlitz BA, Harris EL, et al. Screening for hereditary hemochromatosis: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. Aug ;145(3): PMID McLaren GD, Gordeuk VR. Hereditary hemochromatosis: insights from the Hemochromatosis and Iron Overload Screening (HEIRS) Study. Hematology Am Soc Hematol Educ Program. 2009: PMID Adams PC, McLaren CE, Speechley M, et al. HFE mutations in Caucasian participants of the Hemochromatosis and Iron Overload Screening study with serum ferritin level <1000 microg/l. Can J Gastroenterol. Jul 2013;27(7): PMID U.S. Preventive Services Task Force (USPSTF). Screening for hemochromatosis, August Accessed March Other sources: Novitas Solutions. Local Coverage Determination (LCD) L35062 Biomarkers Overview. Effective 10/01/15. Accessed October 22, January 12, X. POLICY HISTORY MP CAC 8/28/12 New Policy. Adopting BCBSA. Previously silent on this testing. New medically necessary statement for a patient with abnormal serum iron indices indicating iron overload or with a family history of hemochromatosis in a first-degree relative. Investigational for screening in the general population. Page 12
13 Policy guidelines added. CAC 7/30/13- Consensus review. References updated but no change to policy statements. FEP variation revised to refer to the FEP manual. Admin code review completed. CAC 3/25/14 Consensus review. References updated but no change to the policy statements. Rationale added. Codes reviewed. CAC 3/24/15 Consensus review. Added Medicare variation referencing LCD L3360 Biomarkers Overview. Updated rationale and reference list. No change to policy statements. Policy coded. 11/2/15 Administrative change. LCD number changed from L33640 to L35062 due to Novitas update to ICD-10. CAC 3/29/16 Consensus review. No changes to the policy statements. References and rationale updated. Coding reviewed. 1/1/17 Administrative-variations reformatted. Health care benefit programs issued or administered by Capital BlueCross and/or its subsidiaries, Capital Advantage Insurance Company, Capital Advantage Assurance Company and Keystone Health Plan Central. Independent licensees of the BlueCross BlueShield Association. Communications issued by Capital BlueCross in its capacity as administrator of programs and provider relations for all companies. Page 13
Protocol. Genetic Testing for Hereditary Hemochromatosis
 Protocol Genetic Testing for Hereditary Hemochromatosis (20480) Medical Benefit Effective Date: 01/01/15 Next Review Date: 09/16 Preauthorization Yes Review Dates: 09/12, 09/13, 09/14, 09/15 Preauthorization
Protocol Genetic Testing for Hereditary Hemochromatosis (20480) Medical Benefit Effective Date: 01/01/15 Next Review Date: 09/16 Preauthorization Yes Review Dates: 09/12, 09/13, 09/14, 09/15 Preauthorization
MEDICAL POLICY POLICY TITLE DIABETIC SELF-MANAGEMENT TRAINING PROGRAM POLICY NUMBER MP- 2.076
 Original Issue Date (Created): July 1, 2005 Most Recent Review Date (Revised): Effective Date: May 24, 2011 August 31, 2011- RETIRED I. POLICY Initial diabetic self-management training (DSMT) may be considered
Original Issue Date (Created): July 1, 2005 Most Recent Review Date (Revised): Effective Date: May 24, 2011 August 31, 2011- RETIRED I. POLICY Initial diabetic self-management training (DSMT) may be considered
Hemochromatosis. National Digestive Diseases Information Clearinghouse
 Hemochromatosis National Digestive Diseases Information Clearinghouse What is hemochromatosis? Hemochromatosis is the most common form of iron overload disease. Too much iron in the body causes hemochromatosis.
Hemochromatosis National Digestive Diseases Information Clearinghouse What is hemochromatosis? Hemochromatosis is the most common form of iron overload disease. Too much iron in the body causes hemochromatosis.
MEDICAL POLICY POLICY TITLE DENTAL AND ORAL SURGERY SERVICES AFTER AN ACCIDENT POLICY NUMBER MP- 1.108
 Original Issue Date (Created): July 1, 2005 Most Recent Review Date (Revised): Effective Date: June 29, 2010 May 25, 2011- RETIRED I. POLICY II. Dental and/or oral surgery services (on a limited basis)
Original Issue Date (Created): July 1, 2005 Most Recent Review Date (Revised): Effective Date: June 29, 2010 May 25, 2011- RETIRED I. POLICY II. Dental and/or oral surgery services (on a limited basis)
Corporate Medical Policy Genetic Testing for Alpha-1 Antitrypsin Deficiency
 Corporate Medical Policy Genetic Testing for Alpha-1 Antitrypsin Deficiency File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_alpha_1_antitrypsin_deficiency 5/2012
Corporate Medical Policy Genetic Testing for Alpha-1 Antitrypsin Deficiency File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_alpha_1_antitrypsin_deficiency 5/2012
Corporate Medical Policy Genetic Testing for Fanconi Anemia
 Corporate Medical Policy Genetic Testing for Fanconi Anemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_fanconi_anemia 03/2015 3/2016 3/2017 3/2016 Description
Corporate Medical Policy Genetic Testing for Fanconi Anemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_fanconi_anemia 03/2015 3/2016 3/2017 3/2016 Description
MEDICAL POLICY POLICY TITLE POLICY NUMBER ACUTE INPATIENT REHABILITATION MP-8.003
 Original Issue Date (Created): July 1, 2002 Most Recent Review Date (Revised): Effective Date: May 27, 2008 May 1, 2008- RETIRED I. DESCRIPTION/BACKGROUND Inpatient rehabilitation hospitals provide an
Original Issue Date (Created): July 1, 2002 Most Recent Review Date (Revised): Effective Date: May 27, 2008 May 1, 2008- RETIRED I. DESCRIPTION/BACKGROUND Inpatient rehabilitation hospitals provide an
NP/PA Clinical Hepatology Fellowship Summary of Year-Long Curriculum
 OVERVIEW OF THE FELLOWSHIP The goal of the AASLD NP/PA Fellowship is to provide a 1-year postgraduate hepatology training program for nurse practitioners and physician assistants in a clinical outpatient
OVERVIEW OF THE FELLOWSHIP The goal of the AASLD NP/PA Fellowship is to provide a 1-year postgraduate hepatology training program for nurse practitioners and physician assistants in a clinical outpatient
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): October 25, 2011 Most Recent Review Date (Revised): January 27, 2015 Effective Date: April 1, 2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS
Original Issue Date (Created): October 25, 2011 Most Recent Review Date (Revised): January 27, 2015 Effective Date: April 1, 2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS
Epi procolon The Blood Test for Colorectal Cancer Screening
 Epi procolon The Blood Test for Colorectal Cancer Screening Epi procolon is an approved blood test for colorectal cancer screening. The US Preventive Services Task Force, the American Cancer Society and
Epi procolon The Blood Test for Colorectal Cancer Screening Epi procolon is an approved blood test for colorectal cancer screening. The US Preventive Services Task Force, the American Cancer Society and
AASLD PRACTICE GUIDELINE
 AASLD PRACTICE GUIDELINE Diagnosis and Management of Hemochromatosis: 2011 Practice Guideline by the American Association for the Study of Liver Diseases Bruce R. Bacon, 1 Paul C. Adams, 2 Kris V. Kowdley,
AASLD PRACTICE GUIDELINE Diagnosis and Management of Hemochromatosis: 2011 Practice Guideline by the American Association for the Study of Liver Diseases Bruce R. Bacon, 1 Paul C. Adams, 2 Kris V. Kowdley,
Genetic testing for Gilbert s syndrome: how useful is it in determining the cause of jaundice?
 Clinical Chemistry 44:8 1604 1609 (1998) Test Utilization and Outcomes Genetic testing for Gilbert s syndrome: how useful is it in determining the cause of jaundice? Aram S. Rudenski * and David J. Halsall
Clinical Chemistry 44:8 1604 1609 (1998) Test Utilization and Outcomes Genetic testing for Gilbert s syndrome: how useful is it in determining the cause of jaundice? Aram S. Rudenski * and David J. Halsall
MEDICAL POLICY I. POLICY OCCUPATIONAL THERAPY (OUTPATIENT) MP-8.004 POLICY TITLE POLICY NUMBER
 Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 3/24/2015 Effective Date: 11/2/2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS
Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 3/24/2015 Effective Date: 11/2/2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS
BACKGROUND MEDIA INFORMATION Fast facts about liver disease
 BACKGROUND MEDIA INFORMATION Fast facts about liver disease Liver, or hepatic, disease comprises a wide range of complex conditions that affect the liver. Liver diseases are extremely costly in terms of
BACKGROUND MEDIA INFORMATION Fast facts about liver disease Liver, or hepatic, disease comprises a wide range of complex conditions that affect the liver. Liver diseases are extremely costly in terms of
Figure 1. Location of the liver in the body. Figure 2. Deposition of iron in the liver; A, Gross liver; B, Histological view.
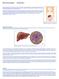 Hemochromatosis: Introduction Hemochromatosis was first identified in the 1800s, and by 1935 it was understood to be an inherited disease resulting in iron overload and deposition. Today, hemochromatosis
Hemochromatosis: Introduction Hemochromatosis was first identified in the 1800s, and by 1935 it was understood to be an inherited disease resulting in iron overload and deposition. Today, hemochromatosis
GENETIC TESTING FOR INHERITED MUTATIONS OR SUSCEPTIBILITY TO CANCER OR OTHER CONDITIONS MED207.110
 GENETIC TESTING FOR INHERITED MUTATIONS OR SUSCEPTIBILITY TO CANCER OR OTHER CONDITIONS MED207.110 COVERAGE: Pre- and post-genetic test counseling may be eligible for coverage in addition to the genetic
GENETIC TESTING FOR INHERITED MUTATIONS OR SUSCEPTIBILITY TO CANCER OR OTHER CONDITIONS MED207.110 COVERAGE: Pre- and post-genetic test counseling may be eligible for coverage in addition to the genetic
1.5 Function of analyte For albumin, see separate entry. The immunoglobulins are components of the humoral arm of the immune system.
 Total protein (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Total protein 1.2 Alternative names None 1.3 NMLC code 1.4 Description of analyte This is a quantitative measurement
Total protein (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Total protein 1.2 Alternative names None 1.3 NMLC code 1.4 Description of analyte This is a quantitative measurement
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: testing_serum_vitamin_d_levels 9/2015 2/2016 2/2017 2/2016 Description of Procedure or Service Vitamin D,
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: testing_serum_vitamin_d_levels 9/2015 2/2016 2/2017 2/2016 Description of Procedure or Service Vitamin D,
MEDICAL POLICY HIGH-DOSE CHEMOTHERAPY WITH OR WITHOUT AUTOLOGOUS STEM CELL RESCUE FOR AUTOIMMUNE DISEASES, INCLUDING MULTIPLE SCLEROSIS MP-9.
 Original Issue Date (Created): August 23, 2002 Most Recent Review Date (Revised): Effective Date: June 17, 2008 July 1, 2009- RETIRED I. DESCRIPTION/BACKGROUND High dose chemotherapy (HDC) involves the
Original Issue Date (Created): August 23, 2002 Most Recent Review Date (Revised): Effective Date: June 17, 2008 July 1, 2009- RETIRED I. DESCRIPTION/BACKGROUND High dose chemotherapy (HDC) involves the
Diagnostic Scoring System for LQTS
 Medical Coverage Policy Genetic Testing: Congenital Long QT Syndrome Device/Equipment Drug Medical Surgery Test Other Effective Date: 2/15/2011 Policy Last Updated: 2/21/2012 Prospective review is recommended/required.
Medical Coverage Policy Genetic Testing: Congenital Long QT Syndrome Device/Equipment Drug Medical Surgery Test Other Effective Date: 2/15/2011 Policy Last Updated: 2/21/2012 Prospective review is recommended/required.
Cystic Fibrosis Webquest Sarah Follenweider, The English High School 2009 Summer Research Internship Program
 Cystic Fibrosis Webquest Sarah Follenweider, The English High School 2009 Summer Research Internship Program Introduction: Cystic fibrosis (CF) is an inherited chronic disease that affects the lungs and
Cystic Fibrosis Webquest Sarah Follenweider, The English High School 2009 Summer Research Internship Program Introduction: Cystic fibrosis (CF) is an inherited chronic disease that affects the lungs and
190.25 - Alpha-fetoprotein
 Other Names/Abbreviations AFP 190.25 - Alpha-fetoprotein Alpha-fetoprotein (AFP) is a polysaccharide found in some carcinomas. It is effective as a biochemical marker for monitoring the response of certain
Other Names/Abbreviations AFP 190.25 - Alpha-fetoprotein Alpha-fetoprotein (AFP) is a polysaccharide found in some carcinomas. It is effective as a biochemical marker for monitoring the response of certain
Genetic Testing in Research & Healthcare
 We Innovate Healthcare Genetic Testing in Research & Healthcare We Innovate Healthcare Genetic Testing in Research and Healthcare Human genetic testing is a growing science. It is used to study genes
We Innovate Healthcare Genetic Testing in Research & Healthcare We Innovate Healthcare Genetic Testing in Research and Healthcare Human genetic testing is a growing science. It is used to study genes
Molecular Genetic Testing in Public Health and Clinical Settings
 Molecular Genetic Testing in Public Health and Clinical Settings Ira M. Lubin, PhD, FACMG Division of Laboratory Systems NCPDCID, CCID Centers for Disease Control and Prevention Atlanta, Georgia Disclaimers
Molecular Genetic Testing in Public Health and Clinical Settings Ira M. Lubin, PhD, FACMG Division of Laboratory Systems NCPDCID, CCID Centers for Disease Control and Prevention Atlanta, Georgia Disclaimers
Genetic testing. The difference diagnostics can make. The British In Vitro Diagnostics Association
 6 Genetic testing The difference diagnostics can make The British In Vitro Diagnostics Association Genetic INTRODUCTION testing The Department of Health published Our Inheritance, Our Future - Realising
6 Genetic testing The difference diagnostics can make The British In Vitro Diagnostics Association Genetic INTRODUCTION testing The Department of Health published Our Inheritance, Our Future - Realising
Amylase and Lipase Tests
 Amylase and Lipase Tests Also known as: Amy Formal name: Amylase Related tests: Lipase The Test The blood amylase test is ordered, often along with a lipase test, to help diagnose and monitor acute or
Amylase and Lipase Tests Also known as: Amy Formal name: Amylase Related tests: Lipase The Test The blood amylase test is ordered, often along with a lipase test, to help diagnose and monitor acute or
BURDEN OF LIVER DISEASE IN BRAZIL
 BURDEN OF LIVER DISEASE IN BRAZIL Burden of Liver Disease in Europe Blachier et al. J Hepatol 58:593, 2013 Review of 260 epidemiologic studies of the 5 previous years Cirrhosis is responsible for 170.000
BURDEN OF LIVER DISEASE IN BRAZIL Burden of Liver Disease in Europe Blachier et al. J Hepatol 58:593, 2013 Review of 260 epidemiologic studies of the 5 previous years Cirrhosis is responsible for 170.000
Hereditary Hemochromatosis
 CLINICAL REVIEW Hereditary Hemochromatosis Robert B. Hash, MD Background: The understanding of hereditary hemochromatosis, along with the availability of genetic testing, is changing the approach to diagnosis
CLINICAL REVIEW Hereditary Hemochromatosis Robert B. Hash, MD Background: The understanding of hereditary hemochromatosis, along with the availability of genetic testing, is changing the approach to diagnosis
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
Corporate Medical Policy
 Corporate Medical Policy Quantitative Electroencephalography as a Diagnostic Aid for Attention File Name: Origination: Last CAP Review: Next CAP Review: Last Review: quantitative_electroencephalography_as_a_diagnostic_aid_for_adhd
Corporate Medical Policy Quantitative Electroencephalography as a Diagnostic Aid for Attention File Name: Origination: Last CAP Review: Next CAP Review: Last Review: quantitative_electroencephalography_as_a_diagnostic_aid_for_adhd
Corporate Medical Policy Cord Blood as a Source of Stem Cells
 Corporate Medical Policy Cord Blood as a Source of Stem Cells File Name: Origination: Last CAP Review: Next CAP Review: Last Review cord_blood_as_a_source_of_stem_cells 2/2001 3/2015 3/2016 3/2015 Description
Corporate Medical Policy Cord Blood as a Source of Stem Cells File Name: Origination: Last CAP Review: Next CAP Review: Last Review cord_blood_as_a_source_of_stem_cells 2/2001 3/2015 3/2016 3/2015 Description
Recommendations for the Identification of Chronic Hepatitis C virus infection Among Persons Born During 1945-1965
 Recommendations for the Identification of Chronic Hepatitis C virus infection Among Persons Born During 1945-1965 MMWR August 17, 2012 Prepared by : The National Viral Hepatitis Technical Assistance Center
Recommendations for the Identification of Chronic Hepatitis C virus infection Among Persons Born During 1945-1965 MMWR August 17, 2012 Prepared by : The National Viral Hepatitis Technical Assistance Center
INFORMATION FOR PATIENTS AND THEIR FAMILIES. You can live a healthy life, if you get treatment early
 INFORMATION FOR PATIENTS AND THEIR FAMILIES Hemochromatosis AN IRON OVERLOAD DISEASE You can live a healthy life, if you get treatment early Department of Health and Human Services Centers for Disease
INFORMATION FOR PATIENTS AND THEIR FAMILIES Hemochromatosis AN IRON OVERLOAD DISEASE You can live a healthy life, if you get treatment early Department of Health and Human Services Centers for Disease
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 1/27/2015 Effective Date: 6/1/2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER
Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 1/27/2015 Effective Date: 6/1/2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER
Corporate Medical Policy
 Corporate Medical Policy Proteomics-based Testing Related to Ovarian Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: proteomics_based_testing_related_to_ovarian_cancer 7/2010
Corporate Medical Policy Proteomics-based Testing Related to Ovarian Cancer File Name: Origination: Last CAP Review: Next CAP Review: Last Review: proteomics_based_testing_related_to_ovarian_cancer 7/2010
Title: Genetics and Hearing Loss: Clinical and Molecular Characteristics
 Session # : 46 Day/Time: Friday, May 1, 2015, 1:00 4:00 pm Title: Genetics and Hearing Loss: Clinical and Molecular Characteristics Presenter: Kathleen S. Arnos, PhD, Gallaudet University This presentation
Session # : 46 Day/Time: Friday, May 1, 2015, 1:00 4:00 pm Title: Genetics and Hearing Loss: Clinical and Molecular Characteristics Presenter: Kathleen S. Arnos, PhD, Gallaudet University This presentation
IRON METABOLISM DISORDERS
 IRON METABOLISM DISORDERS ANEMIA Definition Decrease in the number of circulating red blood cells Most common hematologic disorder by Most common hematologic disorder by far 1 Blood loss ANEMIA Causes
IRON METABOLISM DISORDERS ANEMIA Definition Decrease in the number of circulating red blood cells Most common hematologic disorder by Most common hematologic disorder by far 1 Blood loss ANEMIA Causes
MEDICAL POLICY POLICY TITLE
 Original Issue Date (Created): July 1, 2002 Most Recent Review Date (Revised): Effective Date: April 15, 2008 July 1, 2009- RETIRED I. DESCRIPTION/BACKGROUND High dose chemotherapy (HDC) involves the administration
Original Issue Date (Created): July 1, 2002 Most Recent Review Date (Revised): Effective Date: April 15, 2008 July 1, 2009- RETIRED I. DESCRIPTION/BACKGROUND High dose chemotherapy (HDC) involves the administration
The sensitive marker for glomerular filtration rate (GFR) Estimation of GFR from Serum Cystatin C:
 The sensitive marker for glomerular filtration rate (GFR) Estimation of GFR from Serum Cystatin C: The good correlation allows close estimation of GFR Cystatin C GFR GFR in serum estimated* measured* n
The sensitive marker for glomerular filtration rate (GFR) Estimation of GFR from Serum Cystatin C: The good correlation allows close estimation of GFR Cystatin C GFR GFR in serum estimated* measured* n
Mendelian inheritance and the
 Mendelian inheritance and the most common genetic diseases Cornelia Schubert, MD, University of Goettingen, Dept. Human Genetics EUPRIM-Net course Genetics, Immunology and Breeding Mangement German Primate
Mendelian inheritance and the most common genetic diseases Cornelia Schubert, MD, University of Goettingen, Dept. Human Genetics EUPRIM-Net course Genetics, Immunology and Breeding Mangement German Primate
Lecture 6: Single nucleotide polymorphisms (SNPs) and Restriction Fragment Length Polymorphisms (RFLPs)
 Lecture 6: Single nucleotide polymorphisms (SNPs) and Restriction Fragment Length Polymorphisms (RFLPs) Single nucleotide polymorphisms or SNPs (pronounced "snips") are DNA sequence variations that occur
Lecture 6: Single nucleotide polymorphisms (SNPs) and Restriction Fragment Length Polymorphisms (RFLPs) Single nucleotide polymorphisms or SNPs (pronounced "snips") are DNA sequence variations that occur
Patterns of abnormal LFTs and their differential diagnosis
 Patterns of abnormal LFTs and their differential diagnosis Professor Matthew Cramp South West Liver Unit and Peninsula Schools of Medicine and Dentistry, Plymouth Summary liver function / liver function
Patterns of abnormal LFTs and their differential diagnosis Professor Matthew Cramp South West Liver Unit and Peninsula Schools of Medicine and Dentistry, Plymouth Summary liver function / liver function
Medicare Advantage Risk Adjustment Data Validation CMS-HCC Pilot Study. Report to Medicare Advantage Organizations
 Medicare Advantage Risk Adjustment Data Validation CMS-HCC Pilot Study Report to Medicare Advantage Organizations JULY 27, 2004 JULY 27, 2004 PAGE 1 Medicare Advantage Risk Adjustment Data Validation CMS-HCC
Medicare Advantage Risk Adjustment Data Validation CMS-HCC Pilot Study Report to Medicare Advantage Organizations JULY 27, 2004 JULY 27, 2004 PAGE 1 Medicare Advantage Risk Adjustment Data Validation CMS-HCC
Number 12.04.516 Effective Date August 11, 2015 Revision Date(s) Replaces 2.04.133 (not adopted)
 MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDI HISTORY Genetic Testing for CHEK2 Mutations for Breast Cancer Number 12.04.516
MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES CODING APPENDI HISTORY Genetic Testing for CHEK2 Mutations for Breast Cancer Number 12.04.516
Alanine aminotransferase (serum, plasma)
 Alanine aminotransferase (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Alanine aminotransferase (ALT) 1.2 Alternative names Systematic name L alanine:2 oxoglutarate aminotransferase
Alanine aminotransferase (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Alanine aminotransferase (ALT) 1.2 Alternative names Systematic name L alanine:2 oxoglutarate aminotransferase
Medicare Coverage of Genomic Testing
 Medicare Coverage of Genomic Testing Louis B. Jacques, MD Director, DID/CAG/OCSQ With acknowledgements to Jeff Roche, MD Social Security Act 1862(a)(1)(A) Notwithstanding any other provision of this title,
Medicare Coverage of Genomic Testing Louis B. Jacques, MD Director, DID/CAG/OCSQ With acknowledgements to Jeff Roche, MD Social Security Act 1862(a)(1)(A) Notwithstanding any other provision of this title,
Coding and Billing. Commonly Asked Questions. Physician Office Reimbursement Guideline Q1. A1. Q2. A2.
 Coding and Billing oorasure Technologies is pleased to provide you information on billing and reimbursement for HCV testing with the OraQuick HCV Rapid Antibody Test. Correctly identifying services delivered
Coding and Billing oorasure Technologies is pleased to provide you information on billing and reimbursement for HCV testing with the OraQuick HCV Rapid Antibody Test. Correctly identifying services delivered
Genetic Testing for CHEK2 Mutations for Breast Cancer
 Genetic Testing for CHEK2 Mutations for Breast Cancer Policy Number: 2.04.133 Last Review: 8/2015 Origination: 8/2015 Next Review: 8/2016 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Genetic Testing for CHEK2 Mutations for Breast Cancer Policy Number: 2.04.133 Last Review: 8/2015 Origination: 8/2015 Next Review: 8/2016 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
2.1 AST can be measured in heparin plasma or serum. 3 Summary of clinical applications and limitations of measurements
 Aspartate aminotransferase (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Aspartate aminotransferase (AST) 1.2 Alternative names Systematic name L aspartate:2 oxoglutarate aminotransferase
Aspartate aminotransferase (serum, plasma) 1 Name and description of analyte 1.1 Name of analyte Aspartate aminotransferase (AST) 1.2 Alternative names Systematic name L aspartate:2 oxoglutarate aminotransferase
Treatment of Low Risk MDS. Overview. Myelodysplastic Syndromes (MDS)
 Overview Amy Davidoff, Ph.D., M.S. Associate Professor Pharmaceutical Health Services Research Department, Peter Lamy Center on Drug Therapy and Aging University of Maryland School of Pharmacy Clinical
Overview Amy Davidoff, Ph.D., M.S. Associate Professor Pharmaceutical Health Services Research Department, Peter Lamy Center on Drug Therapy and Aging University of Maryland School of Pharmacy Clinical
Corporate Medical Policy Genetic Testing for Hereditary Hearing Loss
 Corporate Medical Policy Genetic Testing for Hereditary Hearing Loss File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_hereditary_hearing_loss 10/2013 8/2015 8/2016
Corporate Medical Policy Genetic Testing for Hereditary Hearing Loss File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_hereditary_hearing_loss 10/2013 8/2015 8/2016
Coding with. Snayhil Rana
 Coding with ICD-9-CM CM Snayhil Rana ICD-9-CM CM Index Pre-Test Introduction to ICD-9-CM Coding The Three Volumes of the ICD-9-CM ICD-9-CM Coding Conventions Other ICD-9-CM Sections ICD-9-CM for Claim
Coding with ICD-9-CM CM Snayhil Rana ICD-9-CM CM Index Pre-Test Introduction to ICD-9-CM Coding The Three Volumes of the ICD-9-CM ICD-9-CM Coding Conventions Other ICD-9-CM Sections ICD-9-CM for Claim
MEDICAL POLICY No. 91104-R7 DETOXIFICATION I. POLICY/CRITERIA
 DETOXIFICATION MEDICAL POLICY Effective Date: January 7, 2013 Review Dates: 1/93, 2/97, 4/99, 2/01, 12/01, 2/02, 2/03, 1/04, 1/05, 12/05, 12/06, 12/07, 12/08, 12/09, 12/10, 12/11, 12/12, 12/13, 11/14 Date
DETOXIFICATION MEDICAL POLICY Effective Date: January 7, 2013 Review Dates: 1/93, 2/97, 4/99, 2/01, 12/01, 2/02, 2/03, 1/04, 1/05, 12/05, 12/06, 12/07, 12/08, 12/09, 12/10, 12/11, 12/12, 12/13, 11/14 Date
MEDICAL POLICY I. POLICY POLICY TITLE HOME HEALTH POLICY NUMBER MP-3.002
 Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 1/27/2015 Effective Date: 6/1/2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER
Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 1/27/2015 Effective Date: 6/1/2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER
DIAGNOSING CHILDHOOD MUSCULAR DYSTROPHIES
 DIAGNOSING CHILDHOOD MUSCULAR DYSTROPHIES Extracts from a review article by KN North and KJ Jones: Recent advances in diagnosis of the childhood muscular dystrophies Journal of Paediatrics and Child Health
DIAGNOSING CHILDHOOD MUSCULAR DYSTROPHIES Extracts from a review article by KN North and KJ Jones: Recent advances in diagnosis of the childhood muscular dystrophies Journal of Paediatrics and Child Health
NORD Guides for Physicians #1. Physician s Guide to. Tyrosinemia. Type 1
 NORD Guides for Physicians #1 The National Organization for Rare Disorders Physician s Guide to Tyrosinemia Type 1 The original version of this booklet was made possible by donations in honor of Danielle
NORD Guides for Physicians #1 The National Organization for Rare Disorders Physician s Guide to Tyrosinemia Type 1 The original version of this booklet was made possible by donations in honor of Danielle
Oral Zinc Supplementation as an Adjunct Therapy in the Management of Hepatic Encephalopathy: A Randomized Controlled Trial
 Oral Zinc Supplementation as an Adjunct Therapy in the Management of Hepatic Encephalopathy: A Randomized Controlled Trial Marcus R. Pereira A. Study Purpose Hepatic encephalopathy is a common complication
Oral Zinc Supplementation as an Adjunct Therapy in the Management of Hepatic Encephalopathy: A Randomized Controlled Trial Marcus R. Pereira A. Study Purpose Hepatic encephalopathy is a common complication
ICD-9-CM/ICD-10-CM Codes for MNT
 / Codes for MNT ICD (International Classification of Diseases) codes are used by physicians and medical coders to assign medical diagnoses to individual patients. It is not within the scope of practice
/ Codes for MNT ICD (International Classification of Diseases) codes are used by physicians and medical coders to assign medical diagnoses to individual patients. It is not within the scope of practice
Delivering the power of the world s most successful genomics platform
 Delivering the power of the world s most successful genomics platform NextCODE Health is bringing the full power of the world s largest and most successful genomics platform to everyday clinical care NextCODE
Delivering the power of the world s most successful genomics platform NextCODE Health is bringing the full power of the world s largest and most successful genomics platform to everyday clinical care NextCODE
Hepatology Guidelines for Primary Care November 2011
 Common requests for hepatology opinion/advice 1. Abnormal LFTs (is this fatty liver?) 2. Fatty Liver Disease (is this serious liver disease?) 3. Abnormal liver ultrasound 4. Alcohol related liver disease
Common requests for hepatology opinion/advice 1. Abnormal LFTs (is this fatty liver?) 2. Fatty Liver Disease (is this serious liver disease?) 3. Abnormal liver ultrasound 4. Alcohol related liver disease
Detecting HFE Mutations in Human Genomic DNA: Instructor s Manual
 Detecting HFE Mutations in Human Genomic DNA: Instructor s Manual I. Purpose and Concepts Covered...1 II. Background on Hereditary Hemochromatosis...1 III. PCR Amplification of Exons 2 and 4 from the HFE
Detecting HFE Mutations in Human Genomic DNA: Instructor s Manual I. Purpose and Concepts Covered...1 II. Background on Hereditary Hemochromatosis...1 III. PCR Amplification of Exons 2 and 4 from the HFE
Genetics Lecture Notes 7.03 2005. Lectures 1 2
 Genetics Lecture Notes 7.03 2005 Lectures 1 2 Lecture 1 We will begin this course with the question: What is a gene? This question will take us four lectures to answer because there are actually several
Genetics Lecture Notes 7.03 2005 Lectures 1 2 Lecture 1 We will begin this course with the question: What is a gene? This question will take us four lectures to answer because there are actually several
HEALTH DEPARTMENT BILLING GUIDELINES
 HEALTH DEPARTMENT BILLING GUIDELINES Acknowledgement: Current Procedural Terminology (CPT ) is copyright 2015 American Medical Association. All Rights Reserved. No fee schedules, basic units, relative
HEALTH DEPARTMENT BILLING GUIDELINES Acknowledgement: Current Procedural Terminology (CPT ) is copyright 2015 American Medical Association. All Rights Reserved. No fee schedules, basic units, relative
Genetic Testing: Scientific Background for Policymakers
 Genetic Testing: Scientific Background for Policymakers Amanda K. Sarata Specialist in Health Policy December 19, 2011 CRS Report for Congress Prepared for Members and Committees of Congress Congressional
Genetic Testing: Scientific Background for Policymakers Amanda K. Sarata Specialist in Health Policy December 19, 2011 CRS Report for Congress Prepared for Members and Committees of Congress Congressional
Cirrhosis and HCV. Jonathan Israel M.D.
 Cirrhosis and HCV Jonathan Israel M.D. Outline Relationship of fibrosis and cirrhosisprevalence and epidemiology. Sequelae of cirrhosis Diagnosis of cirrhosis Effect of cirrhosis on efficacy of treatment
Cirrhosis and HCV Jonathan Israel M.D. Outline Relationship of fibrosis and cirrhosisprevalence and epidemiology. Sequelae of cirrhosis Diagnosis of cirrhosis Effect of cirrhosis on efficacy of treatment
RDW-- Interpreting the Full Blood Count
 RDW-- Interpreting the Full Blood Count The most important components of a Full Blood Count report are, of course, the Haemoglobin, the White Cell Count and Differential and the Platelet Count. However,
RDW-- Interpreting the Full Blood Count The most important components of a Full Blood Count report are, of course, the Haemoglobin, the White Cell Count and Differential and the Platelet Count. However,
Tuesday 14 May 2013 Morning
 THIS IS A NEW SPECIFICATION H Tuesday 14 May 2013 Morning GCSE TWENTY FIRST CENTURY SCIENCE BIOLOGY A A161/02 Modules B1 B2 B3 (Higher Tier) *A137150613* Candidates answer on the Question Paper. A calculator
THIS IS A NEW SPECIFICATION H Tuesday 14 May 2013 Morning GCSE TWENTY FIRST CENTURY SCIENCE BIOLOGY A A161/02 Modules B1 B2 B3 (Higher Tier) *A137150613* Candidates answer on the Question Paper. A calculator
Behavioral Health Policy Phototherapy Light for the Treatment of Seasonal Affective (SAD) and Other Depressive Disorders
 Behavioral Health Policy Phototherapy Light for the Treatment of Seasonal Affective (SAD) and Other Depressive Disorders Table of Contents Policy: Commercial Coding Information Information Pertaining to
Behavioral Health Policy Phototherapy Light for the Treatment of Seasonal Affective (SAD) and Other Depressive Disorders Table of Contents Policy: Commercial Coding Information Information Pertaining to
Genetic Mutations Cause Many Birth Defects:
 Genetic Mutations Cause Many Birth Defects: What We Learned from the FORGE Canada Project Jan M. Friedman, MD, PhD University it of British Columbia Vancouver, Canada I have no conflicts of interest related
Genetic Mutations Cause Many Birth Defects: What We Learned from the FORGE Canada Project Jan M. Friedman, MD, PhD University it of British Columbia Vancouver, Canada I have no conflicts of interest related
SHAHID AZIZ DO, FACOI.
 SHAHID AZIZ DO, FACOI. ASSOCIATE CLINICAL PROFESSOR OF GI AND LIVER DISEASE. UT SOUTHWESTERN HEALTH SCIENCE CENTER, DALLAS ADJUNCT CLINICAL ASSISTANT PROFESSOR DEPT OF MEDICINE UNT HEALTH SCIENCE CENTER
SHAHID AZIZ DO, FACOI. ASSOCIATE CLINICAL PROFESSOR OF GI AND LIVER DISEASE. UT SOUTHWESTERN HEALTH SCIENCE CENTER, DALLAS ADJUNCT CLINICAL ASSISTANT PROFESSOR DEPT OF MEDICINE UNT HEALTH SCIENCE CENTER
Hepatitis C Glossary of Terms
 Acute Hepatitis C A short-term illness that usually occurs within the first six months after someone is exposed to the hepatitis C virus (HCV). 1 Antibodies Proteins produced as part of the body s immune
Acute Hepatitis C A short-term illness that usually occurs within the first six months after someone is exposed to the hepatitis C virus (HCV). 1 Antibodies Proteins produced as part of the body s immune
Overview of Genetic Testing and Screening
 Integrating Genetics into Your Practice Webinar Series Overview of Genetic Testing and Screening Genetic testing is an important tool in the screening and diagnosis of many conditions. New technology is
Integrating Genetics into Your Practice Webinar Series Overview of Genetic Testing and Screening Genetic testing is an important tool in the screening and diagnosis of many conditions. New technology is
Male New Patient Package
 Male New Patient Package The contents of this package are your first step to restore your vitality. Please take time to read this carefully and answer all the questions as completely as possible. Thank
Male New Patient Package The contents of this package are your first step to restore your vitality. Please take time to read this carefully and answer all the questions as completely as possible. Thank
HEALTH EVIDENCE REVIEW COMMISSION (HERC) COVERAGE GUIDANCE: DIAGNOSIS OF SLEEP APNEA IN ADULTS DATE: 5/9/2013 HERC COVERAGE GUIDANCE
 HEALTH EVIDENCE REVIEW COMMISSION (HERC) COVERAGE GUIDANCE: DIAGNOSIS OF SLEEP APNEA IN ADULTS DATE: 5/9/2013 HERC COVERAGE GUIDANCE The following diagnostic tests for Obstructive Sleep Apnea (OSA) should
HEALTH EVIDENCE REVIEW COMMISSION (HERC) COVERAGE GUIDANCE: DIAGNOSIS OF SLEEP APNEA IN ADULTS DATE: 5/9/2013 HERC COVERAGE GUIDANCE The following diagnostic tests for Obstructive Sleep Apnea (OSA) should
Genetic Testing for Duchenne and Becker Muscular Dystrophy
 Corporate Medical Policy Genetic Testing for Duchenne and Becker Muscular Dystrophy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_duchenne_and_becker_muscular_dystrophy
Corporate Medical Policy Genetic Testing for Duchenne and Becker Muscular Dystrophy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_duchenne_and_becker_muscular_dystrophy
Oral Health Coding Fact Sheet for Primary Care Physicians
 2015 Oral Health Coding Fact Sheet for Primary Care Physicians CPT Codes: Current Procedural Terminology (CPT) codes are developed and maintained by the American Medical Association. The codes consist
2015 Oral Health Coding Fact Sheet for Primary Care Physicians CPT Codes: Current Procedural Terminology (CPT) codes are developed and maintained by the American Medical Association. The codes consist
Patient Information. for Childhood
 Patient Information Genetic Testing for Childhood Hearing Loss Introduction This document describes the most common genetic cause of childhood hearing loss and explains the role of genetic testing. Childhood
Patient Information Genetic Testing for Childhood Hearing Loss Introduction This document describes the most common genetic cause of childhood hearing loss and explains the role of genetic testing. Childhood
Innovation Platform: Sudden Cardiac Death
 Innovation Platform: Sudden Cardiac Death Prof. dr. Bart Loeys CRC Antwerp General Assembly VzW Board of Directors Strategic Advisory Board Staff: 1 executive director 1 ICT manager 1 financial administration
Innovation Platform: Sudden Cardiac Death Prof. dr. Bart Loeys CRC Antwerp General Assembly VzW Board of Directors Strategic Advisory Board Staff: 1 executive director 1 ICT manager 1 financial administration
Policy Product Variations Description/Background Rationale Definitions Benefit Variations Disclaimer Coding Information References Policy History
 Original Issue Date (Created): 4/1/2014 Most Recent Review Date (Revised): 11/24/2015 Effective Date: 2/1/2016 Policy Product Variations Description/Background Rationale Definitions Benefit Variations
Original Issue Date (Created): 4/1/2014 Most Recent Review Date (Revised): 11/24/2015 Effective Date: 2/1/2016 Policy Product Variations Description/Background Rationale Definitions Benefit Variations
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_cll_and_sll
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_cll_and_sll
ELEMENTS FOR A PUBLIC SUMMARY. Overview of disease epidemiology. Summary of treatment benefits
 VI: 2 ELEMENTS FOR A PUBLIC SUMMARY Bicalutamide (CASODEX 1 ) is a hormonal therapy anticancer agent, used for the treatment of prostate cancer. Hormones are chemical messengers that help to control the
VI: 2 ELEMENTS FOR A PUBLIC SUMMARY Bicalutamide (CASODEX 1 ) is a hormonal therapy anticancer agent, used for the treatment of prostate cancer. Hormones are chemical messengers that help to control the
School-age child 5-1 THE BLOOD
 C A S E S T U D Y 5 : School-age child Adapted from Thomson Delmar Learning s Case Study Series: Pediatrics, by Bonita E. Broyles, RN, BSN, MA, PhD. Copyright 2006 Thomson Delmar Learning, Clifton Park,
C A S E S T U D Y 5 : School-age child Adapted from Thomson Delmar Learning s Case Study Series: Pediatrics, by Bonita E. Broyles, RN, BSN, MA, PhD. Copyright 2006 Thomson Delmar Learning, Clifton Park,
Essential Nursing Competencies and Curricula Guidelines for Genetics and Genomics: Outcome Indicators
 Essential Nursing Competencies and Curricula Guidelines for Genetics and Genomics: Outcome Indicators Introduction The Outcome Indicators are an adjunct to the Essential Nursing Competencies and Curricula
Essential Nursing Competencies and Curricula Guidelines for Genetics and Genomics: Outcome Indicators Introduction The Outcome Indicators are an adjunct to the Essential Nursing Competencies and Curricula
SMF Awareness Seminar 2014
 SMF Awareness Seminar 2014 Clinical Evaluation for In Vitro Diagnostic Medical Devices Dr Jiang Naxin Health Sciences Authority Medical Device Branch 1 In vitro diagnostic product means Definition of IVD
SMF Awareness Seminar 2014 Clinical Evaluation for In Vitro Diagnostic Medical Devices Dr Jiang Naxin Health Sciences Authority Medical Device Branch 1 In vitro diagnostic product means Definition of IVD
The National Institute of Genomic Medicine (INMEGEN) was
 Genome is...... the complete set of genetic information contained within all of the chromosomes of an organism. It defines the particular phenotype of an individual. What is Genomics? The study of the
Genome is...... the complete set of genetic information contained within all of the chromosomes of an organism. It defines the particular phenotype of an individual. What is Genomics? The study of the
Chromosomes, Mapping, and the Meiosis Inheritance Connection
 Chromosomes, Mapping, and the Meiosis Inheritance Connection Carl Correns 1900 Chapter 13 First suggests central role for chromosomes Rediscovery of Mendel s work Walter Sutton 1902 Chromosomal theory
Chromosomes, Mapping, and the Meiosis Inheritance Connection Carl Correns 1900 Chapter 13 First suggests central role for chromosomes Rediscovery of Mendel s work Walter Sutton 1902 Chromosomal theory
CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA
 CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA Cytogenetics is the study of chromosomes and their structure, inheritance, and abnormalities. Chromosome abnormalities occur in approximately:
CHROMOSOMES Dr. Fern Tsien, Dept. of Genetics, LSUHSC, NO, LA Cytogenetics is the study of chromosomes and their structure, inheritance, and abnormalities. Chromosome abnormalities occur in approximately:
Unexplained Hepatic, Neurologic or Psychiatric Symptoms?
 A Diagnostic Tool for Physicians Unexplained Hepatic, Neurologic or Psychiatric Symptoms? Think WILSON DISEASE ALGORITHMS FOR ASSESSMENT OF WILSON DISEASE Table 1. Clinical Features in Patients with Wilson
A Diagnostic Tool for Physicians Unexplained Hepatic, Neurologic or Psychiatric Symptoms? Think WILSON DISEASE ALGORITHMS FOR ASSESSMENT OF WILSON DISEASE Table 1. Clinical Features in Patients with Wilson
Guidelines for Cancer Prevention, Early detection & Screening. Prostate Cancer
 Guidelines for Cancer Prevention, Early detection & Screening Prostate Cancer Intervention Comments & Recommendations For primary prevention, it has been suggested that diets low in meat & other fatty
Guidelines for Cancer Prevention, Early detection & Screening Prostate Cancer Intervention Comments & Recommendations For primary prevention, it has been suggested that diets low in meat & other fatty
Celiac Disease. Donald Schoch, M.D. Ohio ACP Meeting October 17, 2014
 Celiac Disease Donald Schoch, M.D. Ohio ACP Meeting October 17, 2014 None to disclose Conflicts of Interest Format Present a case Do a pretest about the evaluation Review case Discuss the questions & answers
Celiac Disease Donald Schoch, M.D. Ohio ACP Meeting October 17, 2014 None to disclose Conflicts of Interest Format Present a case Do a pretest about the evaluation Review case Discuss the questions & answers
Differential Diagnosis of NAFLD- A Short Summary:
 Differential Diagnosis of NAFLD- A Short Summary: Almost a fifth of our general pediatric population is now classified as overweight in the United States. When such children present with elevated liver
Differential Diagnosis of NAFLD- A Short Summary: Almost a fifth of our general pediatric population is now classified as overweight in the United States. When such children present with elevated liver
ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials)
 ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials) 3 Integrated Trials Testing Targeted Therapy in Early Stage Lung Cancer Part of NCI s Precision Medicine Effort in
ALCHEMIST (Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials) 3 Integrated Trials Testing Targeted Therapy in Early Stage Lung Cancer Part of NCI s Precision Medicine Effort in
Big Data for Population Health and Personalised Medicine through EMR Linkages
 Big Data for Population Health and Personalised Medicine through EMR Linkages Zheng-Ming CHEN Professor of Epidemiology Nuffield Dept. of Population Health, University of Oxford Big Data for Health Policy
Big Data for Population Health and Personalised Medicine through EMR Linkages Zheng-Ming CHEN Professor of Epidemiology Nuffield Dept. of Population Health, University of Oxford Big Data for Health Policy
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_epithelial_ovarian_cancer 2/2001 11/2015 11/2016 11/2015 Description
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_epithelial_ovarian_cancer 2/2001 11/2015 11/2016 11/2015 Description
Wilson Disease. National Digestive Diseases Information Clearinghouse
 Wilson Disease National Digestive Diseases Information Clearinghouse U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH What is Wilson disease? Wilson disease is a genetic disorder
Wilson Disease National Digestive Diseases Information Clearinghouse U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH What is Wilson disease? Wilson disease is a genetic disorder
MEDICAL POLICY No. 91503-R4 BLOOD PRESSURE MONITORS & AMBULATORY BLOOD PRESSURE MONITORING
 BLOOD PRESSURE MONITORS & Effective Date: December 21, 2015 Review Dates: 01/05, 12/05, 12/06, 12/07, 12/08, 12/09, 12/10, 12/11, 12/12, 12/13, 11/14, 11/15 Date Of Origin: January 19, 2005 Status: Current
BLOOD PRESSURE MONITORS & Effective Date: December 21, 2015 Review Dates: 01/05, 12/05, 12/06, 12/07, 12/08, 12/09, 12/10, 12/11, 12/12, 12/13, 11/14, 11/15 Date Of Origin: January 19, 2005 Status: Current
Rheumatology Labs for Primary Care Providers. Robert Monger, M.D., F.A.C.P. 2015 Frontiers in Medicine
 Rheumatology Labs for Primary Care Providers Robert Monger, M.D., F.A.C.P. 2015 Frontiers in Medicine Objectives Review the Indications for and Interpretation of lab testing for the following diseases:
Rheumatology Labs for Primary Care Providers Robert Monger, M.D., F.A.C.P. 2015 Frontiers in Medicine Objectives Review the Indications for and Interpretation of lab testing for the following diseases:
Appendix B: Provincial Case Definitions for Reportable Diseases
 Infectious Diseases Protocol Appendix B: Provincial Case Definitions for Reportable Diseases Disease: Hepatitis B Revised Hepatitis B 1.0 Provincial Reporting Confirmed, chronic and probable cases of disease
Infectious Diseases Protocol Appendix B: Provincial Case Definitions for Reportable Diseases Disease: Hepatitis B Revised Hepatitis B 1.0 Provincial Reporting Confirmed, chronic and probable cases of disease
American Society of Addiction Medicine
 American Society of Addiction Medicine Public Policy Statement On Drug Testing as a Component of Addiction Treatment and Monitoring Programs and in other Clinical Settings [Note: ASAM also has a Public
American Society of Addiction Medicine Public Policy Statement On Drug Testing as a Component of Addiction Treatment and Monitoring Programs and in other Clinical Settings [Note: ASAM also has a Public
Approach to Abnormal Liver Tests
 Approach to Abnormal Liver Tests Naga P. Chalasani, MD, FACG Professor of Medicine and Cellular & Integrative Physiology Director, Division of Gastroenterology and Hepatology Indiana University School
Approach to Abnormal Liver Tests Naga P. Chalasani, MD, FACG Professor of Medicine and Cellular & Integrative Physiology Director, Division of Gastroenterology and Hepatology Indiana University School
