TOXICITY AND GENOTOXICITY STUDIES
|
|
|
- Sylvia Robertson
- 7 years ago
- Views:
Transcription
1 TOXICITY AND GENOTOXICITY STUDIES 1.0 Acute Toxicity Studies 1.1 Acute Toxicity and Toxicokinetic Study in Rat and Mice In order to determine LD 50 (cut-off) of test item, animals are dosed with one or more doses during a period not exceeding 24 hours at different dose levels. Different parameters like clinical signs, mortality and body weight is recorded during the study period. Detailed macroscopic examination is performed for found dead or terminally sacrificed animals. Satellite groups for toxicokinetic may be maintained to determine test item exposure. Study is conducted as per OECD guideline 423 / Repeated Dose Toxicity Studies or 7-Days Repeated Dose Toxicity and Toxicokinetic Study in Rat and Mice The information obtained from these studies is useful to provide preliminary identification of target organs for toxicity as well as for the dose selection for the long term studies. To evaluate the toxicity of test item, animals are dosed at three to four dose levels for 4 / 7 days. Different parameters like mortality, clinical signs, body weight, food consumption, hematology, clinical chemistry, coagulation parameters, urine analysis, organ weights, detailed macroscopic examination and histopathology of major organs is studied. Satellite groups are also maintained to determine toxicokinetic parameters on day 1 and day 4/ Days Repeated Dose Toxicity and Toxicokinetic Study in Rat and Mice To evaluate repeated dose toxicity of test item, animals are dosed at three or four dose levels for 14 days. Different parameters like mortality, clinical signs, body weight, food consumption, hematology, clinical chemistry, coagulation
2 parameters, urine analysis, organ weights, detailed macroscopic examination and histopathology of major organs are studied. Satellite groups are also maintained to determine toxicokinetic parameters on day 1 and day 14. MTD and NOAEL are determined based on the findings Days Repeated Dose Toxicity and Toxicokinetic Study in Rat and Mice with Recovery Period In order to determine sub-acute toxicity of test item, animals are dosed at three or four dose levels for 28 days. Different parameters like mortality, clinical signs, body weight, food consumption, hematology, clinical chemistry, coagulation parameters, urine analysis, organ weights, detailed macroscopic examination and histopathology of all organs are studied. Reversal of test item related effects are also evaluated. Satellite groups are maintained to determine toxicokinetic parameters on day 1 and day 28. MTD and NOAEL are determined based on the findings. 3.0 Genotoxicity Studies 3.1 In Vitro Bacterial Reverse Mutation Test - AMES (Standard & Mini) To evaluate the mutagenic potential of any compound, five strains of Salmonella typhimurium histidine auxotroph mutants (TA98, TA100, TA102, TA1535 and TA1537) that are deficient in the synthesis of histidine are used. A minimum of 5 concentrations of test item is evaluated using plate incorporation and pre incubation methods in the presence and absence of metabolic activation. By using bacterial strains that carry different mutations, it is possible to determine the type of genetic mutation (e.g. deletion, substitution, etc) induced of a mutagenic compound. Suven also offers a miniaturized version of the Ames test that requires a small amount of compound compared with the standard test. The Ames assay is performed in 6-well plates using two to five Salmonella strains to detect mutagenic potential of test items.
3
4 3.2 In Vitro Chromosome Aberration Test in CHO K1 cells or Human Peripheral Blood Lymphocytes In order to determine clastogenic potential of test item, CHO K1 cells are exposed to test item at various concentrations including negative and positive controls. Cell cycle is arrested in mitosis phase and, chromosomes are evaluated for presence any numeric as well as structural damage. 3.3 In Vitro Micronucleus Test in CHO K1 cells To evaluate genotoxic potential of test item, CHO K1 ells are exposed to test item at various concentrations including negative and positive controls. Cytochalasin treatment is given to arrest the cytokinesis. Binucleated cells are examined and counted for presence of micronuclei. 3.4 In Vivo Mammalian Erythrocyte Micronucleus Test in Swiss Albino Mice To evaluate genotoxic potential of test item, mice are dosed at six dose levels for 2 days including positive and negative controls. Bone marrow of femur bones is harvested and slides are prepared and scored for presence of micronucleus in polychromatic erythrocytes. Test item concentration at different time points are also determined from satellite groups. 3.5 In Vivo Chromosome Aberration Test in Rat and Mice In order to determine genotoxic potential of test item, animals are dosed at six dose levels for 2 days including positive and negative controls. Bone marrow from femur bones is harvested and slides are prepared and evaluated for presence any numeric as well as structural damage in chromosome. Test item concentration at different time points is also determined from satellite groups. 4.0 Method validation for Bio-analysis LC-MS/MS Method development for quantification of analyte of interest in plasma, urine or tissues and partial validation including linearity, accuracy and precision, recovery, and matrix stability.
5 Serene Chambers, Road No -5, Avenue-7 Banjara Hills, Hyderabad , INDIA
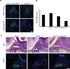
1) Aug 2005 In Vitro Chromosomal Aberration Study Cytotoxicity (File: TS39) Conclusion No cytotoxicity can be detected at concentrations up to 100%.
 Endotoxicity and Cytotoxicity Studies 1) Aug 2005 In Vitro Chromosomal Aberration Study Cytotoxicity (File: TS39) Chinese Hamster Ovary (CHO) cells were grown in monolayer with and without metabolic activation.
Endotoxicity and Cytotoxicity Studies 1) Aug 2005 In Vitro Chromosomal Aberration Study Cytotoxicity (File: TS39) Chinese Hamster Ovary (CHO) cells were grown in monolayer with and without metabolic activation.
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE S2(R1)
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDANCE ON GENOTOXICITY TESTING AND DATA INTERPRETATION
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDANCE ON GENOTOXICITY TESTING AND DATA INTERPRETATION
ICH guideline S2 (R1) on genotoxicity testing and data interpretation for pharmaceuticals intended for human use
 June 2012 EMA/CHMP/ICH/126642/2008 ICH guideline S2 (R1) on genotoxicity testing and data interpretation for pharmaceuticals intended for human use Step 5 Transmission to CHMP March 2008 Adoption by CHMP
June 2012 EMA/CHMP/ICH/126642/2008 ICH guideline S2 (R1) on genotoxicity testing and data interpretation for pharmaceuticals intended for human use Step 5 Transmission to CHMP March 2008 Adoption by CHMP
PART B: METHODS FOR THE DETERMINATION OF TOXICITY AND OTHER HEALTH EFFECTS GENERAL INTRODUCTION: PART B
 PART B: METHODS FOR THE DETERMINATION OF TOXICITY AND OTHER HEALTH EFFECTS GENERAL INTRODUCTION: PART B A. EXPLANATORY NOTE For the purpose of this General Introduction the following nymbering applies:
PART B: METHODS FOR THE DETERMINATION OF TOXICITY AND OTHER HEALTH EFFECTS GENERAL INTRODUCTION: PART B A. EXPLANATORY NOTE For the purpose of this General Introduction the following nymbering applies:
AMES TEST: Bacterial Reverse Mutation Assay
 AMES TEST: Bacterial Reverse Mutation Assay 1. Introduction The bacteria reversed mutation assay (Ames Test) is used to evaluate the mutagenic properties of test articles. The test uses amino acid-dependent
AMES TEST: Bacterial Reverse Mutation Assay 1. Introduction The bacteria reversed mutation assay (Ames Test) is used to evaluate the mutagenic properties of test articles. The test uses amino acid-dependent
DODECANEDIOIC ACID CAS N : 693-23-2
 OECD SIDS DODECANEDIOIC ACID FOREWORD INTRODUCTION DODECANEDIOIC ACID CAS N : 693-23-2 UNEP PUBLICATIONS Identifiers, Physical and Chemical properties 1 Substance End Point : IDENTIFIERS, PHYSICAL AND
OECD SIDS DODECANEDIOIC ACID FOREWORD INTRODUCTION DODECANEDIOIC ACID CAS N : 693-23-2 UNEP PUBLICATIONS Identifiers, Physical and Chemical properties 1 Substance End Point : IDENTIFIERS, PHYSICAL AND
Guidance Document on Revisions to OECD Genetic Toxicology Test Guidelines
 Guidance Document on Revisions to OECD Genetic Toxicology Test Guidelines TABLE OF CONTENTS GUIDANCE DOCUMENT ON REVISIONS TO OECD GENETIC TOXICOLOGY TEST GUIDELINES... 1 1 GENERAL INTRODUCTION (PREAMBLE)...
Guidance Document on Revisions to OECD Genetic Toxicology Test Guidelines TABLE OF CONTENTS GUIDANCE DOCUMENT ON REVISIONS TO OECD GENETIC TOXICOLOGY TEST GUIDELINES... 1 1 GENERAL INTRODUCTION (PREAMBLE)...
1,2-BUTANEDIOL CAS N : 584-03-2
 OECD SIDS 1,2-BUTANEDIOL FOREWORD INTRODUCTION 1,2-BUTANEDIOL CAS N : 584-03-2 UNEP PUBLICATIONS Identifiers, Physical and Chemical properties 243 Substance End Point : IDENTIFIERS, PHYSICAL AND CHEMICAL
OECD SIDS 1,2-BUTANEDIOL FOREWORD INTRODUCTION 1,2-BUTANEDIOL CAS N : 584-03-2 UNEP PUBLICATIONS Identifiers, Physical and Chemical properties 243 Substance End Point : IDENTIFIERS, PHYSICAL AND CHEMICAL
5.7 CHLOROTHALONIL (081)
 Chlorothalonil 89 5.7 CHLOROTHALONIL (081) TOXICOLOGY Chlorothalonil is the ISO approved common name for tetrachloroisophthalonitrile. Chlorothalonil (CAS No. 1897-45-6) is a non-systemic foliar fungicide
Chlorothalonil 89 5.7 CHLOROTHALONIL (081) TOXICOLOGY Chlorothalonil is the ISO approved common name for tetrachloroisophthalonitrile. Chlorothalonil (CAS No. 1897-45-6) is a non-systemic foliar fungicide
Overview on EFSA data requirements for the safety evaluation of food enzymes applications
 Overview on EFSA data requirements for the safety evaluation of food enzymes applications Fidel Toldrá and Klaus-Dieter Jany EFSA CEF Panel Info session on Food Enzymes applications Parma, 27 May 2014
Overview on EFSA data requirements for the safety evaluation of food enzymes applications Fidel Toldrá and Klaus-Dieter Jany EFSA CEF Panel Info session on Food Enzymes applications Parma, 27 May 2014
ISO 10993 Biological Evaluation of Medical Devices
 ISO 10993 Biological Evaluation of Medical Devices A Manager s Quiz Nancy J Stark, PhD Clinical Device Group Inc Evaluations for Medical Devices CDG Web Publications 28 May 02 1 Welcome to Clinical Device
ISO 10993 Biological Evaluation of Medical Devices A Manager s Quiz Nancy J Stark, PhD Clinical Device Group Inc Evaluations for Medical Devices CDG Web Publications 28 May 02 1 Welcome to Clinical Device
NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Veterinary Use EMEA/MRL/717/99-FINAL December 1999 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS TOLDIMFOS SUMMARY
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Veterinary Use EMEA/MRL/717/99-FINAL December 1999 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS TOLDIMFOS SUMMARY
Biodiesel by-products: A Case Study in BiodieselGlycerol and Cancer
 Journal of Proactive Medicine Vol 1. No 2. (2012) Journal of Proactive Medicinee The International Journal of cancer prevention, chemoprevec ention andd epidemiology Journal homepage: www.jproactive.hu
Journal of Proactive Medicine Vol 1. No 2. (2012) Journal of Proactive Medicinee The International Journal of cancer prevention, chemoprevec ention andd epidemiology Journal homepage: www.jproactive.hu
METHYL METHACRYLATE CAS N : 80-62-5
 FOREWORD INTRODUCTION CAS N : 80625 UNEP PUBLICATIONS SIDS INITIAL ASSESSMENT PROFILE CAS No. 80626 Chemical Name Methyl methacrylate Structural Formula CH 2 =CH(CH 3 )COOCH 3 RECOMMENDATIONS The chemical
FOREWORD INTRODUCTION CAS N : 80625 UNEP PUBLICATIONS SIDS INITIAL ASSESSMENT PROFILE CAS No. 80626 Chemical Name Methyl methacrylate Structural Formula CH 2 =CH(CH 3 )COOCH 3 RECOMMENDATIONS The chemical
Session 6 Clinical Trial Assessment Phase I Clinical Trial
 L1 Session 6 Clinical Trial Assessment Phase I Clinical Trial Presentation to APEC Preliminary Workshop on Review of Drug Development in Clinical Trials Celia Lourenco, PhD, Manager, Clinical Group I Office
L1 Session 6 Clinical Trial Assessment Phase I Clinical Trial Presentation to APEC Preliminary Workshop on Review of Drug Development in Clinical Trials Celia Lourenco, PhD, Manager, Clinical Group I Office
trans-1-chloro-3,3,3-trifluoropropene (1233zd(E)) (2013)
 WORKPLACE ENVIRONMENTAL EXPOSURE LEVEL trans-1-chloro-3,3,3-trifluoropropene (1233zd(E)) (2013) I. IDENTIFICATION Chemical Name: trans-1-chloro-3,3,3-trifluoropropylene Synonyms: HCFO-1233zd, 1233zd(E)
WORKPLACE ENVIRONMENTAL EXPOSURE LEVEL trans-1-chloro-3,3,3-trifluoropropene (1233zd(E)) (2013) I. IDENTIFICATION Chemical Name: trans-1-chloro-3,3,3-trifluoropropylene Synonyms: HCFO-1233zd, 1233zd(E)
Lead optimization services
 Lead optimization services The WIL Research Company (WRC) has extensive experience in fast track tailor-made screening strategies to help you with the challenging task of selecting your best candidate
Lead optimization services The WIL Research Company (WRC) has extensive experience in fast track tailor-made screening strategies to help you with the challenging task of selecting your best candidate
474 Adopted: 21st July 1997
 474 Adopted: 21st July 1997 OECD GUIDELINE FOR THE TESTING OF CHEMICALS Mammalian Erythrocyte Micronucleus Test INTRODUCTION 1. The mammalian in vivo micronucleus test is used for the detection of damage
474 Adopted: 21st July 1997 OECD GUIDELINE FOR THE TESTING OF CHEMICALS Mammalian Erythrocyte Micronucleus Test INTRODUCTION 1. The mammalian in vivo micronucleus test is used for the detection of damage
OPINION LAWSONE THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS SCCNFP/0798/04. COLIPA n C146
 THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS INTENDED FOR CONSUMERS OPINION CONCERNING LAWSONE COLIPA n C146 adopted by the SCCNFP on 16 February 2004 by means of the written procedure
THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS INTENDED FOR CONSUMERS OPINION CONCERNING LAWSONE COLIPA n C146 adopted by the SCCNFP on 16 February 2004 by means of the written procedure
MATERIAL SAFETY DATA SHEET
 Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Preventol - Preservatives
 Preventol - Preservatives Preventol CMK Summary of Toxicity and Ecotoxicity Preventol CMK Summary of Toxicity and Ecotoxicity Active ingredient: 4-chloro-3-methylphenol; p-chloro-m-cresol Synonyms: PCMC,
Preventol - Preservatives Preventol CMK Summary of Toxicity and Ecotoxicity Preventol CMK Summary of Toxicity and Ecotoxicity Active ingredient: 4-chloro-3-methylphenol; p-chloro-m-cresol Synonyms: PCMC,
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE PRE-CLINICAL EVALUATION OF ANTICANCER MEDICINAL PRODUCTS
 The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 23 July 1998 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE PRE-CLINICAL
The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 23 July 1998 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE PRE-CLINICAL
Guidance for Industry Safety Testing of Drug Metabolites
 Guidance for Industry Safety Testing of Drug Metabolites U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2008 Pharmacology
Guidance for Industry Safety Testing of Drug Metabolites U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2008 Pharmacology
Risk Assessment of Pesticides Residue by JMPR - To set ADI and ARfD-
 The FAO Regional Training Course: Strengthening Capacity of Data Collection and Generation for Food Safety Risk Analysis Support to Capacity Building and Implementation of International Safety Standards
The FAO Regional Training Course: Strengthening Capacity of Data Collection and Generation for Food Safety Risk Analysis Support to Capacity Building and Implementation of International Safety Standards
MATERIAL SAFETY DATA SHEET
 Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Risk Assessment in Chemical Food Safety. Dept. of Food Safety and Zoonoses (FOS) http://www.who.int/foodsafety/en/
 Risk Assessment in Chemical Food Safety Dept. of Food Safety and Zoonoses (FOS) http://www.who.int/foodsafety/en/ Risk Analysis Paradigm Internationally Scientific data analysis Risk Assessment WHO & FAO
Risk Assessment in Chemical Food Safety Dept. of Food Safety and Zoonoses (FOS) http://www.who.int/foodsafety/en/ Risk Analysis Paradigm Internationally Scientific data analysis Risk Assessment WHO & FAO
Guidance for Industry
 Guidance for Industry S9 Nonclinical Evaluation for Anticancer Pharmaceuticals U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center
Guidance for Industry S9 Nonclinical Evaluation for Anticancer Pharmaceuticals U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center
Cell Phone Radiation and Genomic Damage: In Vitro Exposure and Assessment
 Cell Phone Radiation and Genomic Damage: In Vitro Exposure and Assessment Chinar Shah 1, Anu Nair 1, Mehul Naik 2, Sonal Bakshi 3 Institute of Science, Nirma University, Ahmedabad, Gujarat, India 1 Electronics
Cell Phone Radiation and Genomic Damage: In Vitro Exposure and Assessment Chinar Shah 1, Anu Nair 1, Mehul Naik 2, Sonal Bakshi 3 Institute of Science, Nirma University, Ahmedabad, Gujarat, India 1 Electronics
User manual Database Import Wizard
 User manual For the latest news and the most up-todate information, please consult the b Document history Version Version 1.0 Comment for version 2.1 of the QSAR Toolbox Issue date: Language: English If
User manual For the latest news and the most up-todate information, please consult the b Document history Version Version 1.0 Comment for version 2.1 of the QSAR Toolbox Issue date: Language: English If
MATERIAL SAFETY DATA SHEET
 Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Basic Overview of Preclinical Toxicology Animal Models
 Basic Overview of Preclinical Toxicology Animal Models Charles D. Hebert, Ph.D., D.A.B.T. December 5, 2013 Outline Background In Vitro Toxicology In Vivo Toxicology Animal Models What is Toxicology? Background
Basic Overview of Preclinical Toxicology Animal Models Charles D. Hebert, Ph.D., D.A.B.T. December 5, 2013 Outline Background In Vitro Toxicology In Vivo Toxicology Animal Models What is Toxicology? Background
EFSA explains the carcinogenicity assessment of glyphosate
 PESTICIDES UNIT EFSA explains the carcinogenicity assessment of glyphosate Background 12 November 2015 During the EFSA peer-review process for the renewal of the approval of the pesticide active substance
PESTICIDES UNIT EFSA explains the carcinogenicity assessment of glyphosate Background 12 November 2015 During the EFSA peer-review process for the renewal of the approval of the pesticide active substance
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON ALOE BARBADENSIS MILLER
 European Medicines Agency Evaluation of Medicines for Human Use London, 26 October 2006 Doc. Ref. EMEA/HMPC/76310/2006 Corrigendum 1 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL
European Medicines Agency Evaluation of Medicines for Human Use London, 26 October 2006 Doc. Ref. EMEA/HMPC/76310/2006 Corrigendum 1 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL
JOINT COMMISSION INTERNATIONAL ACCREDITATION STANDARDS FOR. 2nd Edition
 JOINT COMMISSION INTERNATIONAL ACCREDITATION STANDARDS FOR CliniCAl laboratories 2nd Edition Effective 1 April 2010 International Patient Safety Goals (IPSG) Goals The following is a list of all goals.
JOINT COMMISSION INTERNATIONAL ACCREDITATION STANDARDS FOR CliniCAl laboratories 2nd Edition Effective 1 April 2010 International Patient Safety Goals (IPSG) Goals The following is a list of all goals.
In vivo dose response assays
 In vivo dose response assays Tumor assays 1. Tumor growth measurements; tumor growth delay. After irradiation, the tumor is measured daily to determine the mean diameter, or volume. Plot tumor size versus
In vivo dose response assays Tumor assays 1. Tumor growth measurements; tumor growth delay. After irradiation, the tumor is measured daily to determine the mean diameter, or volume. Plot tumor size versus
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
FAO SPECIFICATIONS AND EVALUATIONS FOR AGRICULTURAL PESTICIDES
 FOR AGRICULTURAL PESTICIDES ALPHA-CYPERMETHRIN A racemic mixture of: (S)-α-cyano-3-phenoxybenzyl-(1R,3R)-3-(2,2-dichlorovinyl)- 2,2-dimethylcyclopropane-carboxylate and (R)-α-cyano-3-phenoxybenzyl-(1S,3S)-3-(2,2-dichlorovinyl)-
FOR AGRICULTURAL PESTICIDES ALPHA-CYPERMETHRIN A racemic mixture of: (S)-α-cyano-3-phenoxybenzyl-(1R,3R)-3-(2,2-dichlorovinyl)- 2,2-dimethylcyclopropane-carboxylate and (R)-α-cyano-3-phenoxybenzyl-(1S,3S)-3-(2,2-dichlorovinyl)-
GUIDANCE ON A STRATEGY FOR GENOTOXICITY TESTING OF CHEMICAL SUBSTANCES
 GUIDANCE ON A STRATEGY FOR GENOTOXICITY TESTING OF CHEMICAL SUBSTANCES CONTENTS PARAGRAPH I. Preface 1-5 II. Introduction 6-10 III. Significance of Chemical Induced Mutation for Human Health 11-12 IV.
GUIDANCE ON A STRATEGY FOR GENOTOXICITY TESTING OF CHEMICAL SUBSTANCES CONTENTS PARAGRAPH I. Preface 1-5 II. Introduction 6-10 III. Significance of Chemical Induced Mutation for Human Health 11-12 IV.
Risk Assessment Report on (3-CHLORO-2-HYDROXYPROPYL)TRIMETHYLAMMONIUM CHLORIDE (CHPTAC)
 Scientific Committee on Health and Environmental Risks SCHER Risk Assessment Report on (3-CHLORO-2-HYDROXYPROPYL)TRIMETHYLAMMONIUM CHLORIDE (CHPTAC) Human Health Part CAS No.: 3327-22-8 EINECS No.: 222-048-3
Scientific Committee on Health and Environmental Risks SCHER Risk Assessment Report on (3-CHLORO-2-HYDROXYPROPYL)TRIMETHYLAMMONIUM CHLORIDE (CHPTAC) Human Health Part CAS No.: 3327-22-8 EINECS No.: 222-048-3
FRENCH AGENCY FOR VETERINARY MEDICINAL PRODUCTS DECENTRALISED PROCEDURE PUBLICLY AVAILABLE ASSESSMENT REPORT FOR A VETERINARY MEDICINAL PRODUCT
 FRENCH AGENCY FOR VETERINARY MEDICINAL PRODUCTS DECENTRALISED PROCEDURE FOR A VETERINARY MEDICINAL PRODUCT EMEDOG, 1 mg/ml, solution for injection for dogs Date: 20/08/2015 French agency for food, environnemental
FRENCH AGENCY FOR VETERINARY MEDICINAL PRODUCTS DECENTRALISED PROCEDURE FOR A VETERINARY MEDICINAL PRODUCT EMEDOG, 1 mg/ml, solution for injection for dogs Date: 20/08/2015 French agency for food, environnemental
GENOTOXICITY TESTING, A REGULATORY REQUIREMENT FOR DRUG DISCOVERY AND DEVELOPMENT: IMPACT OF ICH GUIDELINES
 Indian Journal of Pharmacology 2002; 34: 86-99 EDUCATIONAL FORUM GENOTOXICITY TESTING IN DRUG SAFETY EVALUATION GENOTOXICITY TESTING, A REGULATORY REQUIREMENT FOR DRUG DISCOVERY AND DEVELOPMENT: IMPACT
Indian Journal of Pharmacology 2002; 34: 86-99 EDUCATIONAL FORUM GENOTOXICITY TESTING IN DRUG SAFETY EVALUATION GENOTOXICITY TESTING, A REGULATORY REQUIREMENT FOR DRUG DISCOVERY AND DEVELOPMENT: IMPACT
dixa a data infrastructure for chemical safety Jos Kleinjans Dept of Toxicogenomics Maastricht University
 dixa a data infrastructure for chemical safety Jos Kleinjans Dept of Toxicogenomics Maastricht University Current protocol for chemical safety testing Short Term Tests for Genetic Toxicity Bacterial Reverse
dixa a data infrastructure for chemical safety Jos Kleinjans Dept of Toxicogenomics Maastricht University Current protocol for chemical safety testing Short Term Tests for Genetic Toxicity Bacterial Reverse
Permethrin in Drinking-water: Use for Vector Control in Drinking-water Sources and Containers
 WHO/SDE/WSH/05.08/111/Rev/1 Permethrin in Drinking-water: Use for Vector Control in Drinking-water Sources and Containers Background document for development of WHO Guidelines for Drinking-water Quality
WHO/SDE/WSH/05.08/111/Rev/1 Permethrin in Drinking-water: Use for Vector Control in Drinking-water Sources and Containers Background document for development of WHO Guidelines for Drinking-water Quality
MATERIAL SAFETY DATA SHEET
 Page 1 of 8 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 8 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
The Science of Chemical Safety Essential Toxicology - 4. Hazard and Risk. John Duffus & Howard Worth. IUPAC Educators Resource Material IUPAC
 The Science of Chemical Safety Essential Toxicology - 4 Hazard and Risk John Duffus & Howard Worth IUPAC Educators Resource Material IUPAC Hazard and Risk - 1 Hazard is the potential of a substance to
The Science of Chemical Safety Essential Toxicology - 4 Hazard and Risk John Duffus & Howard Worth IUPAC Educators Resource Material IUPAC Hazard and Risk - 1 Hazard is the potential of a substance to
ICH M3 (R2) Guideline on Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals
 ICH M3 (R2) Guideline on Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals International Conference on Harmonisation of Technical Requirements
ICH M3 (R2) Guideline on Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals International Conference on Harmonisation of Technical Requirements
MATERIAL SAFETY DATA SHEET SECTION 1. IDENTIFICATION OF SUBSTANCE AND CONTACT INFORMATION
 Merck Animal Health One Merck Dr. Whitehouse Station, NJ 08889 MATERIAL SAFETY DATA SHEET Merck Animal Health urges each user or recipient of this MSDS to read the entire data sheet to become aware of
Merck Animal Health One Merck Dr. Whitehouse Station, NJ 08889 MATERIAL SAFETY DATA SHEET Merck Animal Health urges each user or recipient of this MSDS to read the entire data sheet to become aware of
FAO SPECIFICATIONS AND EVALUATIONS FOR AGRICULTURAL PESTICIDES. 1-Methylcyclopropene
 FAO SPECIFICATIONS AND EVALUATIONS FOR AGRICULTURAL PESTICIDES 1-Methylcyclopropene TABLE OF CONTENTS 1-METHYLCYCLOPROPENE Page DISCLAIMER INTRODUCTION 1 PART ONE SPECIFICATIONS FOR 1-METHYLCYCLOPROPENE
FAO SPECIFICATIONS AND EVALUATIONS FOR AGRICULTURAL PESTICIDES 1-Methylcyclopropene TABLE OF CONTENTS 1-METHYLCYCLOPROPENE Page DISCLAIMER INTRODUCTION 1 PART ONE SPECIFICATIONS FOR 1-METHYLCYCLOPROPENE
Oxymetholone: I. Evaluation in a Comprehensive Battery of Genetic Toxicology and In Vitro Transformation Assays*
 Oxymetholone: I. Evaluation in a Comprehensive Battery of Genetic Toxicology and In Vitro Transformation Assays HENRY E. HOLDEN, DEBBIE STUDWELL, AND JENNESS B. MAJESKA Department of Toxicology and Safety
Oxymetholone: I. Evaluation in a Comprehensive Battery of Genetic Toxicology and In Vitro Transformation Assays HENRY E. HOLDEN, DEBBIE STUDWELL, AND JENNESS B. MAJESKA Department of Toxicology and Safety
Bifenthrin 41 5.2 BIFENTHRIN (178)
 Bifenthrin 41 5.2 BIFENTHRIN (178) TOXICOLOGY Bifenthrin is the International Organization for Standardization (ISO) approved name for 2-methyl-3- phenylphenyl) methyl (1RS, 3RS)-3-[(Z)-2-chloro-3, 3,
Bifenthrin 41 5.2 BIFENTHRIN (178) TOXICOLOGY Bifenthrin is the International Organization for Standardization (ISO) approved name for 2-methyl-3- phenylphenyl) methyl (1RS, 3RS)-3-[(Z)-2-chloro-3, 3,
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE S1A. Current Step 4 version
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDELINE ON THE NEED FOR CARCINOGENICITY STUDIES
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDELINE ON THE NEED FOR CARCINOGENICITY STUDIES
Bio 102 Practice Problems Genetic Code and Mutation
 Bio 102 Practice Problems Genetic Code and Mutation Multiple choice: Unless otherwise directed, circle the one best answer: 1. Beadle and Tatum mutagenized Neurospora to find strains that required arginine
Bio 102 Practice Problems Genetic Code and Mutation Multiple choice: Unless otherwise directed, circle the one best answer: 1. Beadle and Tatum mutagenized Neurospora to find strains that required arginine
Risk assessment and regulation of tattoo inks in the EU
 Risk assessment and regulation of tattoo inks in the EU Paul Janssen Current situation: Tattoo application in a regulatory vacuum : Is not a medical treatment Is not a cosmetic treatment No EU-regulation
Risk assessment and regulation of tattoo inks in the EU Paul Janssen Current situation: Tattoo application in a regulatory vacuum : Is not a medical treatment Is not a cosmetic treatment No EU-regulation
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Transgenic Rodent Gene Mutation Assays: Current State of Validation. George R. Douglas
 Transgenic Rodent Gene Mutation Assays: Current State of Validation Prepared for Organization for Economic Cooperation and Development (OECD) by George R. Douglas Mechanistic Studies Division Environmental
Transgenic Rodent Gene Mutation Assays: Current State of Validation Prepared for Organization for Economic Cooperation and Development (OECD) by George R. Douglas Mechanistic Studies Division Environmental
September 19, 1984 FOOD PRODUCTION AND DIRECTION GÉNÉRALE, SECTION INSPECTION BRANCH PRODUCTION ET INSPECTION PESTICIDES DES ALIMENTS TRADE MEMORANDUM
 Agriculture Canada September 19, 1984 T-1-245 FOOD PRODUCTION AND DIRECTION GÉNÉRALE, SECTION INSPECTION BRANCH PRODUCTION ET INSPECTION PESTICIDES DES ALIMENTS TRADE MEMORANDUM RE: Guidelines for Developing
Agriculture Canada September 19, 1984 T-1-245 FOOD PRODUCTION AND DIRECTION GÉNÉRALE, SECTION INSPECTION BRANCH PRODUCTION ET INSPECTION PESTICIDES DES ALIMENTS TRADE MEMORANDUM RE: Guidelines for Developing
OECD GUIDELINE FOR THE TESTING OF CHEMICALS DRAFT PROPOSAL FOR A NEW GUIDELINE XXX: Transgenic Rodent Gene Mutation Assay
 OECD GUIDELINE FOR THE TESTING OF CHEMICALS DRAFT PROPOSAL FOR A NEW GUIDELINE XXX: Transgenic Rodent Gene Mutation Assay INTRODUCTION 1. OECD guidelines are available for a wide range of in vitro mutation
OECD GUIDELINE FOR THE TESTING OF CHEMICALS DRAFT PROPOSAL FOR A NEW GUIDELINE XXX: Transgenic Rodent Gene Mutation Assay INTRODUCTION 1. OECD guidelines are available for a wide range of in vitro mutation
SISTER CHROMATID EXCHANGES: A STUDY IN FLUOROTIC INDIVIDUALS OF NORTH GUJARAT
 Fluoride Vol.27 No.4 215-219 1994 Research Report 215 SISTER CHROMATID EXCHANGES: A STUDY IN FLUOROTIC INDIVIDUALS OF NORTH GUJARAT F J Sheth, A S Multani and N J Chinoy Ahmedabad, India SUMMARY: The purpose
Fluoride Vol.27 No.4 215-219 1994 Research Report 215 SISTER CHROMATID EXCHANGES: A STUDY IN FLUOROTIC INDIVIDUALS OF NORTH GUJARAT F J Sheth, A S Multani and N J Chinoy Ahmedabad, India SUMMARY: The purpose
SCANTIBODIES Laboratory, Inc. Contract Monoclonal Antibody Production
 A Technical Publication of SCANTIBODIES Laboratory, Inc. Volume 1 Number 4 9336 Abraham Way Santee, CA 92071 USA (619) 258-9300 fax (619) 258-9366 www.scantibodies.com SCANTIBODIES Laboratory, Inc. Contract
A Technical Publication of SCANTIBODIES Laboratory, Inc. Volume 1 Number 4 9336 Abraham Way Santee, CA 92071 USA (619) 258-9300 fax (619) 258-9366 www.scantibodies.com SCANTIBODIES Laboratory, Inc. Contract
LECTURE 6 Gene Mutation (Chapter 16.1-16.2)
 LECTURE 6 Gene Mutation (Chapter 16.1-16.2) 1 Mutation: A permanent change in the genetic material that can be passed from parent to offspring. Mutant (genotype): An organism whose DNA differs from the
LECTURE 6 Gene Mutation (Chapter 16.1-16.2) 1 Mutation: A permanent change in the genetic material that can be passed from parent to offspring. Mutant (genotype): An organism whose DNA differs from the
SALONPAS PAIN RELEF PATCH, MEDICATED PLASTER PL 23168/0001 UKPAR TABLE OF CONTENTS
 SALONPAS PAIN RELEF PATCH, MEDICATED PLASTER PL 23168/0001 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 14 Steps taken after authorisation summary
SALONPAS PAIN RELEF PATCH, MEDICATED PLASTER PL 23168/0001 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 14 Steps taken after authorisation summary
FAO SPECIFICATIONS AND EVALUATIONS FOR AGRICULTURAL PESTICIDES CARBARYL. 1-naphthyl methylcarbamate
 FOR AGRICULTURAL PESTICIDES CARBARYL 1-naphthyl methylcarbamate TABLE OF CONTENTS CARBARYL DISCLAIMER Page INTRODUCTION 1 PART ONE SPECIFICATIONS 2 CARBARYL INFORMATION 3 CARBARYL TECHNICAL MATERIAL (MARCH
FOR AGRICULTURAL PESTICIDES CARBARYL 1-naphthyl methylcarbamate TABLE OF CONTENTS CARBARYL DISCLAIMER Page INTRODUCTION 1 PART ONE SPECIFICATIONS 2 CARBARYL INFORMATION 3 CARBARYL TECHNICAL MATERIAL (MARCH
Safety Report of GelRed and GelGreen
 Safety Report of GelRed and GelGreen A Summary of Mutagenicity and Environmental Safety Test Results from Three Independent Laboratories Last updated: October 16, 2013 Overview Ethidium bromide (EB) has
Safety Report of GelRed and GelGreen A Summary of Mutagenicity and Environmental Safety Test Results from Three Independent Laboratories Last updated: October 16, 2013 Overview Ethidium bromide (EB) has
Biological importance of metabolites. Safety and efficacy aspects
 Biological importance of metabolites Safety and efficacy aspects Bernard Walther Technologie Servier Biological importance of metabolites Safety testing of drug metabolites Bioanalytical strategy Structural
Biological importance of metabolites Safety and efficacy aspects Bernard Walther Technologie Servier Biological importance of metabolites Safety testing of drug metabolites Bioanalytical strategy Structural
MATERIAL SAFETY DATA SHEET
 Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Non-clinical development of biologics
 Aurigon Life Science GmbH Non-clinical development of biologics Requirements, challenges and case studies Committed to Life. Sigrid Messemer vet. med. M4 Seminar March 10 th 2014 Aurigon - your full service
Aurigon Life Science GmbH Non-clinical development of biologics Requirements, challenges and case studies Committed to Life. Sigrid Messemer vet. med. M4 Seminar March 10 th 2014 Aurigon - your full service
Re: GRAS Notice No. AGRN 000-009
 May 4, 2012 Dr. Hardy Edwards III Vitamin Derivatives, Inc. 625 Lem Edwards Road Winterville, Georgia 30683 Re: GRAS Notice No. AGRN 000-009 Dear Dr. Edwards: The Food and Drug Administration (FDA) is
May 4, 2012 Dr. Hardy Edwards III Vitamin Derivatives, Inc. 625 Lem Edwards Road Winterville, Georgia 30683 Re: GRAS Notice No. AGRN 000-009 Dear Dr. Edwards: The Food and Drug Administration (FDA) is
ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals. Step 5
 European Medicines Agency July 1996 CPMP/ICH/140/95 ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON THE NEED FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS
European Medicines Agency July 1996 CPMP/ICH/140/95 ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON THE NEED FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS
WHO SPECIFICATIONS AND EVALUATIONS FOR PUBLIC HEALTH PESTICIDES PYRIPROXYFEN. 4-phenoxyphenyl (RS)-2-(2-pyridyloxy)propyl ether
 WHO SPECIFICATIONS AND EVALUATIONS FOR PUBLIC HEALTH PESTICIDES PYRIPROXYFEN 4-phenoxyphenyl (RS)-2-(2-pyridyloxy)propyl ether Page 1 of 19 TABLE OF CONTENTS PYRIPROXYFEN Page DISCLAIMER 3 INTRODUCTION
WHO SPECIFICATIONS AND EVALUATIONS FOR PUBLIC HEALTH PESTICIDES PYRIPROXYFEN 4-phenoxyphenyl (RS)-2-(2-pyridyloxy)propyl ether Page 1 of 19 TABLE OF CONTENTS PYRIPROXYFEN Page DISCLAIMER 3 INTRODUCTION
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE M3(R2)
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDANCE ON NONCLINICAL SAFETY STUDIES FOR THE
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GUIDANCE ON NONCLINICAL SAFETY STUDIES FOR THE
MDM. Metabolic Drift Mutations - Attenuation Technology
 MDM Metabolic Drift Mutations - Attenuation Technology Seite 2 Origin of MDM attenuation technology Prof. Dr. Klaus Linde Pioneer in R&D of human and animal vaccines University of Leipzig Germany Origin
MDM Metabolic Drift Mutations - Attenuation Technology Seite 2 Origin of MDM attenuation technology Prof. Dr. Klaus Linde Pioneer in R&D of human and animal vaccines University of Leipzig Germany Origin
BASF Corporation. Material Safety Data Sheet
 BASF Corporation Material Safety Data Sheet Page : 1 Original Date: 10/30/1992 Revision Date: 07/23/2001 BASF CORPORATION 3000 CONTINENTAL DRIVE NORTH MOUNT OLIVE, NJ 07828 (973) 426-4671 EMERGENCY TELEPHONE:
BASF Corporation Material Safety Data Sheet Page : 1 Original Date: 10/30/1992 Revision Date: 07/23/2001 BASF CORPORATION 3000 CONTINENTAL DRIVE NORTH MOUNT OLIVE, NJ 07828 (973) 426-4671 EMERGENCY TELEPHONE:
In Silico Models: Risk Assessment With Non-Testing Methods in OSIRIS Opportunities and Limitations
 In Silico Models: Risk Assessment With Non-Testing Methods in OSIRIS Opportunities and Limitations Mark Cronin, Mark Hewitt, Katarzyna Przybylak School of Pharmacy and Chemistry Liverpool John Moores University
In Silico Models: Risk Assessment With Non-Testing Methods in OSIRIS Opportunities and Limitations Mark Cronin, Mark Hewitt, Katarzyna Przybylak School of Pharmacy and Chemistry Liverpool John Moores University
1. When new cells are formed through the process of mitosis, the number of chromosomes in the new cells
 Cell Growth and Reproduction 1. When new cells are formed through the process of mitosis, the number of chromosomes in the new cells A. is half of that of the parent cell. B. remains the same as in the
Cell Growth and Reproduction 1. When new cells are formed through the process of mitosis, the number of chromosomes in the new cells A. is half of that of the parent cell. B. remains the same as in the
MATERIAL SAFETY DATA SHEET
 Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
ELISA BIO 110 Lab 1. Immunity and Disease
 ELISA BIO 110 Lab 1 Immunity and Disease Introduction The principal role of the mammalian immune response is to contain infectious disease agents. This response is mediated by several cellular and molecular
ELISA BIO 110 Lab 1 Immunity and Disease Introduction The principal role of the mammalian immune response is to contain infectious disease agents. This response is mediated by several cellular and molecular
US Environmental Protection Agency Office of Pesticide Programs
 US Environmental Protection Agency Office of Pesticide Programs BIOPESTICIDE REGISTRATION ACTION DOCUMENT 1- Methylcyclopropene (PC Code 224459) BIOPESTICIDE REGISTRATION ACTION DOCUMENT 1- Methylcyclopropene
US Environmental Protection Agency Office of Pesticide Programs BIOPESTICIDE REGISTRATION ACTION DOCUMENT 1- Methylcyclopropene (PC Code 224459) BIOPESTICIDE REGISTRATION ACTION DOCUMENT 1- Methylcyclopropene
Ethyl Glucuronide. Where is the Drug? Detection Windows. Urine. Blood. Absorption and Distribution. Metabolism. Excretion
 Where is the Drug? Ethyl Absorption and Distribution A Biomarker for Ethanol Use Metabolism Excretion Anthony G. Costantino, Ph.D. D-ABFT NMS Labs,Willow Grove, PA John J. Treuting, Ph.D. Treuting & Assoc.
Where is the Drug? Ethyl Absorption and Distribution A Biomarker for Ethanol Use Metabolism Excretion Anthony G. Costantino, Ph.D. D-ABFT NMS Labs,Willow Grove, PA John J. Treuting, Ph.D. Treuting & Assoc.
FAO SPECIFICATIONS AND EVALUATIONS FOR AGRICULTURAL PESTICIDES FLUSILAZOLE. bis(4-fluorophenyl)(methyl)(1h-1,2,4-triazol-1- ylmethyl)silane
 FOR AGRICULTURAL PESTICIDES FLUSILAZOLE bis(4-fluorophenyl)(methyl)(1h-1,2,4-triazol-1- ylmethyl)silane TABLE OF CONTENTS DISCLAIMER Page INTRODUCTION 1 PART ONE SPECIFICATIONS 2 FLUSILAZOLE INFORMATION
FOR AGRICULTURAL PESTICIDES FLUSILAZOLE bis(4-fluorophenyl)(methyl)(1h-1,2,4-triazol-1- ylmethyl)silane TABLE OF CONTENTS DISCLAIMER Page INTRODUCTION 1 PART ONE SPECIFICATIONS 2 FLUSILAZOLE INFORMATION
Uses of Flow Cytometry
 Uses of Flow Cytometry 1. Multicolour analysis... 2 2. Cell Cycle and Proliferation... 3 a. Analysis of Cellular DNA Content... 4 b. Cell Proliferation Assays... 5 3. Immunology... 6 4. Apoptosis... 7
Uses of Flow Cytometry 1. Multicolour analysis... 2 2. Cell Cycle and Proliferation... 3 a. Analysis of Cellular DNA Content... 4 b. Cell Proliferation Assays... 5 3. Immunology... 6 4. Apoptosis... 7
My Sister s s Keeper. Science Background Talk
 My Sister s s Keeper Science Background Talk Outline Acute promyelocytic leukemia (APL) APL Treatment Savior Siblings In vitro fertilization (IVF) Pre-implantation Genetic Diagnosis (PGD) Risks of donating
My Sister s s Keeper Science Background Talk Outline Acute promyelocytic leukemia (APL) APL Treatment Savior Siblings In vitro fertilization (IVF) Pre-implantation Genetic Diagnosis (PGD) Risks of donating
Name of Chemical: Tebufenpyrad Reason for Issuance: Unconditional Registration Date Issued: August 26, 2002
 United States Environmental Protection Agency Office of Prevention, Pesticides and Toxic Substances (7501C) Pesticide Fact Sheet Name of Chemical: Tebufenpyrad Reason for Issuance: Unconditional Registration
United States Environmental Protection Agency Office of Prevention, Pesticides and Toxic Substances (7501C) Pesticide Fact Sheet Name of Chemical: Tebufenpyrad Reason for Issuance: Unconditional Registration
Guidance for Industry
 Guidance for Industry M3(R2) Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals U.S. Department of Health and Human Services Food and Drug
Guidance for Industry M3(R2) Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals U.S. Department of Health and Human Services Food and Drug
IRON OXIDE NANOPARTICLES AND THEIR TOXICOLOGICAL EFFECTS: IN VIVO AND IN VITRO STUDIES
 IRON OXIDE NANOPARTICLES AND THEIR TOXICOLOGICAL EFFECTS: IN VIVO AND IN VITRO STUDIES PhD Thesis Brigitta Szalay Department of Public Health Faculty of Medicine University of Szeged 2012 IRON OXIDE NANOPARTICLES
IRON OXIDE NANOPARTICLES AND THEIR TOXICOLOGICAL EFFECTS: IN VIVO AND IN VITRO STUDIES PhD Thesis Brigitta Szalay Department of Public Health Faculty of Medicine University of Szeged 2012 IRON OXIDE NANOPARTICLES
Chemical Name: Acetone CAS: 67-64-1 Synonyms: propanone, β-ketopropane, dimethyl ketone, dimethylformaldehyde, DMK, 2-propanone, propan-2-one
 2011 Health Risk Limits for Groundwater Health Risk Assessment Unit, Environmental Health Division 651-201-4899 651-201-5797 TDD Web Publication Date: March 21, 2011 Chemical Name: Acetone CAS: 67-64-1
2011 Health Risk Limits for Groundwater Health Risk Assessment Unit, Environmental Health Division 651-201-4899 651-201-5797 TDD Web Publication Date: March 21, 2011 Chemical Name: Acetone CAS: 67-64-1
Drug Development Services
 Drug Development Services USING BLOOD AND BONE MARROW PRIMARY CELL SYSTEMS Clinically Relevant In Vitro Assays Broad Spectrum of Drug Classes Multi-Species Platforms Enhancing Drug Development through
Drug Development Services USING BLOOD AND BONE MARROW PRIMARY CELL SYSTEMS Clinically Relevant In Vitro Assays Broad Spectrum of Drug Classes Multi-Species Platforms Enhancing Drug Development through
Protective effect of vitamin C and or vitamin E on micronuclei induction by rifampicin in mice
 Protective effect of vitamin C and or vitamin E on micronuclei induction by rifampicin in mice O. AWODELE 1*, S.O. OLAYEMI 1, C.G. ALIMBA 2, C. EGBEJIOGU 1 and A. AKINTONWA 1 1 Department of Pharmacology,
Protective effect of vitamin C and or vitamin E on micronuclei induction by rifampicin in mice O. AWODELE 1*, S.O. OLAYEMI 1, C.G. ALIMBA 2, C. EGBEJIOGU 1 and A. AKINTONWA 1 1 Department of Pharmacology,
GUIDELINES FOR THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
 GUIDELINES FOR THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS -ii- GUIDELINES ON THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND
GUIDELINES FOR THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS -ii- GUIDELINES ON THE REGISTRATION OF BIOLOGICAL PEST CONTROL AGENTS FOOD AND
MATERIAL SAFETY DATA SHEET
 Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
FAO SPECIFICATIONS AND EVALUATIONS FOR AGRICULTURAL PESTICIDES PROCHLORAZ. N-propyl-N-[2-(2,4,6-trichlorophenoxy)ethyl]imidazole-1- carboxamide
![FAO SPECIFICATIONS AND EVALUATIONS FOR AGRICULTURAL PESTICIDES PROCHLORAZ. N-propyl-N-[2-(2,4,6-trichlorophenoxy)ethyl]imidazole-1- carboxamide FAO SPECIFICATIONS AND EVALUATIONS FOR AGRICULTURAL PESTICIDES PROCHLORAZ. N-propyl-N-[2-(2,4,6-trichlorophenoxy)ethyl]imidazole-1- carboxamide](/thumbs/32/15635834.jpg) FOR AGRICULTURAL PESTICIDES PROCHLORAZ N-propyl-N-[2-(2,4,6-trichlorophenoxy)ethyl]imidazole-1- carboxamide TABLE OF CONTENTS DISCLAIMER Page INTRODUCTION 1 PART ONE SPECIFICATIONS 2 PROCHLORAZ INFORMATION
FOR AGRICULTURAL PESTICIDES PROCHLORAZ N-propyl-N-[2-(2,4,6-trichlorophenoxy)ethyl]imidazole-1- carboxamide TABLE OF CONTENTS DISCLAIMER Page INTRODUCTION 1 PART ONE SPECIFICATIONS 2 PROCHLORAZ INFORMATION
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Losartan Potassium and Hydrochlorothiazide Tablets 100 mg/12.5 mg Lupin Limited Goa 403 722
MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Losartan Potassium and Hydrochlorothiazide Tablets 100 mg/12.5 mg Lupin Limited Goa 403 722
Uncertainty factors:
 The Interdepartmental Group on Health Risks from Chemicals Uncertainty factors: Their use in human health risk assessment by UK Government cr9 The Interdepartmental Group on Health Risks from Chemicals
The Interdepartmental Group on Health Risks from Chemicals Uncertainty factors: Their use in human health risk assessment by UK Government cr9 The Interdepartmental Group on Health Risks from Chemicals
Product Development Services for Global Registration of Drugs & Biologics. 16-17 th April 2014. Contact: kevin.breesch@toxikon.be
 Product Development Services for Global Registration of Drugs & Biologics 16-17 th April 2014 Contact: kevin.breesch@toxikon.be CONTENT OF PRESENTATION» History» Company Profile» Current Services» Proof-of-Concept:
Product Development Services for Global Registration of Drugs & Biologics 16-17 th April 2014 Contact: kevin.breesch@toxikon.be CONTENT OF PRESENTATION» History» Company Profile» Current Services» Proof-of-Concept:
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON PHOTOSAFETY TESTING
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 27 June 2002 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON PHOTOSAFETY
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 27 June 2002 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON PHOTOSAFETY
1. Identification of the substance/mixture and of the company/undertaking
 1. Identification of the substance/mixture and of the company/undertaking Designation : Sifrol, solid (Bulk),
1. Identification of the substance/mixture and of the company/undertaking Designation : Sifrol, solid (Bulk),
IAEA-TECDOC-934. Effects of ionizing radiation on blood and blood components: A survey. í ) INTERNATIONAL ATOMIC ENERGY AGENCY
 IAEA-TECDOC-934 Effects of ionizing radiation on blood and blood components: A survey í ) INTERNATIONAL ATOMIC ENERGY AGENCY The IAEA does The originating Section of this publication in the IAEA was: Industrial
IAEA-TECDOC-934 Effects of ionizing radiation on blood and blood components: A survey í ) INTERNATIONAL ATOMIC ENERGY AGENCY The IAEA does The originating Section of this publication in the IAEA was: Industrial
MATERIAL SAFETY DATA SHEET
 Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Rodent Toxicity/Carcinogenicity Studies on Cell Phone Radio Frequency Radiation in Reverberation Chambers
 Rodent Toxicity/Carcinogenicity Studies on Cell Phone Radio Frequency Radiation in Reverberation Chambers Ronald L. Melnick, Christopher Portier National Institute of Environmental Health Sciences National
Rodent Toxicity/Carcinogenicity Studies on Cell Phone Radio Frequency Radiation in Reverberation Chambers Ronald L. Melnick, Christopher Portier National Institute of Environmental Health Sciences National
COPPER MONOCHLORIDE CAS N : 7758-89-6
 FOREWORD ITRODUCTIO CAS : 7758-89-6 UEP PUBLICATIOS SIDS Initial Assessment Report For SIAM 2 Washington D.C., USA, 8-2 October 25. Chemical ame: Copper monochloride 2. CAS umber: 7758-89-6 3. Sponsor
FOREWORD ITRODUCTIO CAS : 7758-89-6 UEP PUBLICATIOS SIDS Initial Assessment Report For SIAM 2 Washington D.C., USA, 8-2 October 25. Chemical ame: Copper monochloride 2. CAS umber: 7758-89-6 3. Sponsor
