Carbohydrate Biochemistry Objectives -Recognize the features and functions of different classes of carbohydrates -Understand the concept of
|
|
|
- Marcus Park
- 7 years ago
- Views:
Transcription
1 Carbohydrate Biochemistry Objectives -Recognize the features and functions of different classes of carbohydrates -Understand the concept of asymmetric carbons and other diastereoisomers, epimers and enantiomers and anomers -Recognize the structure of glucose and its relationship with other monosaccharides -Understand the different reactions of monosaccharide -Understand the nature of glycosidic bond and recognize the structure of the common disaccharides -Recognize the different types and classes of polysaccharides -Understand the basic differences between amylose and cellulose -Understand the digestion of carbohydrates process, and recognize cases of abnormal degradation of disaccharides
2 *Functions of Carbohydrates * Classification of CHO - Monosaccharides - aldoses and ketoses * Structure of Monosaccharides -isomers, epimers and enantiomers -cyclic structure, hemiacetal and hemiketal *Reactions of monosaccharides * Derivatives of Hexoses -amino sugars -acidic sugars -Glycosides * Disaccharides -disaccharide formation and hydrolysis -nomenclature of disaccharides -glycosides * Polysaccharides (Glycans) -homo and hetero polysaccharides -Storage polysaccharides; starch & Glycogen -Structural polysaccharides, cellulose & chitin
3 Biochemistry of Carbohydrate Carbohydrates (CHO) are the most abundant biomolecules in nature CHO are the photosynthesis product nco2 +H2O (CH2O)n + no2 light Originally thought to have the formula (CH2O)n. Now known that only simple monosaccharides obey this rule. Carbohydrate- polyhydroxy aldehyde or ketone or a larger molecule which can be hydrolyzed to a polyhydroxy aldehyde or ketone. Functions of Carbohydrates 1. Energy source for plants and animals 2. Source of carbon in metabolic processes 3. Storage form of energy 4. Structural elements of cells and tissues 5. Some CHO participate in recognition and adhesion between cells and mediate some forms of inter cellular communications
4 Monosaccharides Classes of Saccharides = single polyhydroxy aldehyde or ketone unit, (eg. 6-carbon glucose, most abundant in nature) Oligosaccharides = short chain of 2- ~20 monosaccharides joined by glycosidic bonds (eg. disaccharide sucrose = glucose-fructose) - oligosaccharides > 3 residues are usually joined to protein or lipid in glycoconjugates Polysaccharides - chains > ~ 20 to 1000 s of monosaccharides in length - linear: eg. cellulose (glucose) n or chitin (N-acetylglucosamine) n - branched: eg. glycogen & starch (glucose) - depending on the sugar residues in a polysaccharide and the linkages between them, polysaccharides can have very different biological roles
5 Monosaccharides - Backbone = un-branched carbon chains in which all C atoms are linked by single bonds - Colorless, crystalline, solid freely soluble in water, insoluble in organic solvents - If it has keto group as the most oxidized functional group = Ketose - If it has aldehyde group as the most oxidized functional group = aldose According to the number of carbon atoms - 3 C = triose, 4 C = tetrose, 5 C = pentose, 6 C = hexose (eg. aldo- or ketohexoses) Simplest monosaccharides are 3 carbon... Common monosaccharides are 6 carbon... Aldotriose ketotriose...
6 Monosaccharides have asymmetric centers - All monosaccharides (except dihydroxyacetone) have one or more asymmetric carbons - eg. glyceraldehyde: middle C is a chiral center, so molecule has 2 different optical isomers or enantiomers (= stereoisomers that are non-super imposable mirror images of one another) By convention, one enantiomer = the D isomer, the other is L, HORIZONTAL: PROJECTS OUT FROM PLANE VERTICAL: PROJECTS BEHIND PLANE Configurations of glyceraldehyde:
7 D and L configurations of monosaccharides - Stereoisomers of monosaccharides > 3 C divided into 2 groups: differ in the configuration about the chiral center most distant from the carbonyl C In biochemistry, we use the D-L system (similar principle to the R-S system usually used in organic, but everything is compared with glyceraldehyde - In general, a molecule with n chiral centers can have 2 n stereoisomers -OH GROUP ON RIGHT, CONFIGURATION = D -OH GROUP ON LEFT, CONFIGURATION = L IN ALDOHEXOSES, C-2, C-3, C-4, AND C-5 = CHIRAL CENTERS, SO 2 4 = 16 POSSIBLE ALDOHEXOSES: 8 D AND 8 L MIRROR Most of the hexoses in living organisms are D-isomers
8 Epimers - 2 sugars that differ only in the configuration around one carbon = epimers
9
10 Series of D-ketoses - Have 1 less chiral center than aldoses - C-4 and C-5 ketoses designated by adding ul into the name of their corresponding aldose, eg. D-ribulose = ketopentose corresponding to D-ribose
11 Stereochemistry The D and L designation of sugars with n > 3 are taken from the chiral carbon furthest from the carbonyl carbon. Other important terminology: Enantiomers- D- and L-sugars are enantiomers (mirror image molecules) Diastereomers- nonsuperimposable, non mirror image Epimers- differ in arrangement about one other chiral carbons. Conformational isomers:
12 Formation of the two cyclic forms of D-Glucose
13 Interconversion between anomers Anomers: Isomeric forms of monosaccharides that differ only in their configuration at the anomeric carbon. Mutarotation: The interconversion of α and β anomers of the monosaccharide - α and β anomers interconvert in solution via the linear form: = mutarotation - D-glucose solution forms an equilibrium mixture of ~ 64% β, 36% α
14 α-d- Mannose α -D Galactose
15 Reactions of Monosaccharides Reducing sugars. Reduction to polyols (Alditols). Reduction of Erythrose Erythritol Mannose Mannitol Glucose Sorbitol Oxidation into acidic sugars Oxidation the OH group at C6 -uronic acid derivative Glucose Glucuronic acid (COOH at C6) Oxidation the aldehyde group at C1 -onic acid Glucose Gluconic acid (COOH at C1) Reduction of hydroxyl group into deoxy sugars Ribose 2-deoxyribose Formation of acetals, also called glycosides Glycosidic bond- bond between a sugar and an alcohol (another sugar) or amine (a base) through an O- or N- linkage
16 Reducing sugars -The anomeric carbon of Glc and other sugars can be oxidized by mild oxidizing agents such as Cu 2+, providing the sugar is in its open chain form, with a free carbonyl carbon at C-1 -Sugars capable of reducing Cu 2+ are called reducing sugars -The end of a chain of sugars that has a free anomeric (C-1) carbon is called the reducing end This principle was used to detect qualitatively the presence of the reducing sugars these tests are not specific for Diabetes mellitus RED COLOR Blood glucose concentration is commonly determined by measuring the amount of H2O2
17 Complex carbohydrates Carbohydrate can be attached by glycosidic bond to non- carbohydrate structure through an O- or N- linkage to form complex carbohydrates -The non-carbohydrate portion is called Aglycone -The entire molecule is called Glycoside Glycosidic bond- bond between the anomeric carbon of a sugar and an alcohol (can be another sugar) or amine (a base) through an O- or N- linkage. Typical Aglycone - Purines, Pyrimrdines fo form Nucleotides - Aromatic rings (steroids) - Proteins to form glycoproteins, the polypeptide is joined to the sugar moiety via Asparagine to form N-glycoside and via serine to form O-glycosides - lipids to form and glycolipids
18 Complex carbohydrates N-glycoside O-glycosides
19 Joining of sugars via glycosidic bonds - O-glycosidic bonds are formed when the -OH group of one sugar reacts withthe anomeric carbon of the other - Reaction = formation of an acetal from a hemiacetal (eg. C- 1 of gluco pyranose) and an alcohol (-OH group on C-4 of second glucopyranose molecule)
20 Joining of sugars via glycosidic bonds - When an anomeric carbon participates in a glycosidic bond, it cannot exist in linear form, and can no longer act as a reducing sugar (ie. it can t reduce Cu 2+ ) - The end of a di- or polysaccharide chain with a free anomeric carbon (ie. not involved in a glycosidic bond) = the reducing end - In maltose, the configuration of the anomeric carbon in the glycosidic linkage is α - Glycosidic bonds are readily hydrolyzed by mild acid NON- REDUCING END REDUCING END a a OR b b/c MUTAROTATION
21 The name describes the sugar with its reducing end Glycosidic bonds are readily hydrolyzed by acids Non-reducing sugars named as Glycosides Trehalose: non-reducing sugar, a major constituents of the circulating fluid od insects
22 Polysaccharides: are polymers of monosaccharides units of medium to high molecular weight. Homopolysaccharides contain only a single type of monomers, as starch, glycogen, cellulose Heterpolysaccharides contain two or more different kinds monomers, as glycosaminoglycans Polysaccharides could be branched or un-branched Function: storage of energy, structural elements, animal exoskeleton and provide support +- - Important cell surface components, eg. in holding cells together in tissues - Important in molecular recognition events Polysacchs. usually don t have precisely defined molecular weights, unlike proteins
23 Storage polysaccharides: glycogen and starch Glycogen: polymer of α (1 4) linked glucoses with α (1 6) branches (one every 8-12 glucoses), average mol. wt. = several millions. Can be 7 % wet wt. of liver Starch: = mixture of amylose, a linear polymer of α (1 4) linked glucoses, and amylopectin, a linear polymer of α (1 4) linked glucoses with α (1 6) branches (one every 4-30 glucoses) Liver cells (hepatocytes) store glycogen equivalent to a [glucose] of 0.4 M Storage polysaccharides are essentially insoluble in the cell, so they don t raise the intracellular [glucose], which would set up a very high [glucose] gradient.
24 Structure of Amylose Rotation is permitted about the two C-O bonds in a glycosidic linkage: most stable conformation for α (1 4) linked glucose = curved chain of rigid chairs - Conformation of a α (1 4) )-linkages in amylose and glycogen causes polymers to assume tightly coiled helical structures that form dense granules in cells. - Molecules heavily hydrated, b/c many OH groups available to H-bond to water.
25 Structural polysaccharides: cellulose In β(1 4)-linked polysaccharides, each residue is rotated 180 relative to its neighbors. The intra-chain H-bonds can form between ring O and the -OH of C-3 on adjacent glucoses: Cellobiose Glc β(1 4)-Glc H-bonds also form between -OH s on residues on neighboring chains....the stabilizing network of intra-chain and inter-chain H-bonds gives straight stable fibers with great tensile strength. Extensive H-bonding within molecule results in little H-bonding to water, hence fibers have low water content
26 Structural polysaccharides: chitin - Polymer of β(1 4)-)-linked N-acetylglucosamine - Each residue rotated 180 relative to its neighbors - Intra-chain H-bonds can form between ring O and C-3 s -OH s on adjacent sugars (as in cellulose) - Inter-chain -NH --- O=C- H-bonds between aminoacyl groups O CH 3 O=C H- N O O CH 2 OH
27 The End
3) How many monosaccharides are connected to each other in a disaccharide? A) 1 B) 2 C) 3 D) 4
 General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
CHEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) Page 1
 CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
I. Chapter 5 Summary. II. Nucleotides & Nucleic Acids. III. Lipids
 I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
Solid Phase Peptide Synthesis
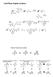 Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1
 CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
Chapter 3 Molecules of Cells
 Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Chapter 5. The Structure and Function of Macromolecule s
 Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
How To Understand The Chemistry Of Organic Molecules
 CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids
 The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
Organic Molecules of Life - Exercise 2
 Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Disaccharides consist of two monosaccharide monomers covalently linked by a glycosidic bond. They function in sugar transport.
 1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
The Chemistry of Carbohydrates
 The Chemistry of Carbohydrates Experiment #5 Objective: To determine the carbohydrate class of an unknown by carrying out a series of chemical reactions with the unknown and known compounds in each class
The Chemistry of Carbohydrates Experiment #5 Objective: To determine the carbohydrate class of an unknown by carrying out a series of chemical reactions with the unknown and known compounds in each class
A disaccharide is formed when a dehydration reaction joins two monosaccharides. This covalent bond is called a glycosidic linkage.
 CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
4. Which carbohydrate would you find as part of a molecule of RNA? a. Galactose b. Deoxyribose c. Ribose d. Glucose
 1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
Biochemistry of Cells
 Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Elements in Biological Molecules
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
BIOLOGICAL MOLECULES OF LIFE
 BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
Catalysis by Enzymes. Enzyme A protein that acts as a catalyst for a biochemical reaction.
 Catalysis by Enzymes Enzyme A protein that acts as a catalyst for a biochemical reaction. Enzymatic Reaction Specificity Enzyme Cofactors Many enzymes are conjugated proteins that require nonprotein portions
Catalysis by Enzymes Enzyme A protein that acts as a catalyst for a biochemical reaction. Enzymatic Reaction Specificity Enzyme Cofactors Many enzymes are conjugated proteins that require nonprotein portions
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Carbohydrates, proteins and lipids
 Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Name: Hour: Elements & Macromolecules in Organisms
 Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids
 VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
Chapter 3: Biological Molecules. 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Worksheet 13.1. Chapter 13: Human biochemistry glossary
 Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
The Molecules of Cells
 The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins
 Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
Biological molecules:
 Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Organic Compounds. Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for?
 Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
II. Chemistry of Sugars
 II. Chemistry of Sugars Sugars-or saccharides- are the most abundant bio-molecule on the planet. They are important in a number of biological roles. Most obviously you will know them as a major component
II. Chemistry of Sugars Sugars-or saccharides- are the most abundant bio-molecule on the planet. They are important in a number of biological roles. Most obviously you will know them as a major component
Qualitative Testing for Carbohydrates
 R E A 446 modular laboratory program in chemistry program editor:. A. Neidig Qualitative Testing for arbohydrates prepared by James. Schreck, University of Northern olorado, and William M. Loffredo, East
R E A 446 modular laboratory program in chemistry program editor:. A. Neidig Qualitative Testing for arbohydrates prepared by James. Schreck, University of Northern olorado, and William M. Loffredo, East
Lab 3 Organic Molecules of Biological Importance
 Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
10.1 The function of Digestion pg. 402
 10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Chapter 5: The Structure and Function of Large Biological Molecules Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four
Name Period Chapter 5: The Structure and Function of Large Biological Molecules Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four
Carbon-organic Compounds
 Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Water. Definition: A mole (or mol ) Water can IONIZE transiently. NONpolar covalent molecules do not dissolve in water + + + + + + + + + + + + + + + +
 Today s Topics Polar Covalent Bonds ydrogen bonding Properties of water p Water C bonds are Nonpolar Will these molecules dissolve in water? Start Macromolecules Carbohydrates & Lipids Sept 4, 05 Why are
Today s Topics Polar Covalent Bonds ydrogen bonding Properties of water p Water C bonds are Nonpolar Will these molecules dissolve in water? Start Macromolecules Carbohydrates & Lipids Sept 4, 05 Why are
Chapter 2 Chemical Principles
 Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
CHEM 2353 Fundamentals of Organic Chemistry. Carbohydrates. Organic and Biochemistry for Today (4 th ed.) Spencer L. Seager / Michael R.
 hapter 7 Notes EM 2353 Fundamentals of rganic hemistry hapter 7 arbohydrates rganic and Biochemistry for Today (4 th ed.) Spencer L. Seager / Michael R. Slabaugh Mr. Kevin A. Boudreaux Angelo State University
hapter 7 Notes EM 2353 Fundamentals of rganic hemistry hapter 7 arbohydrates rganic and Biochemistry for Today (4 th ed.) Spencer L. Seager / Michael R. Slabaugh Mr. Kevin A. Boudreaux Angelo State University
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Structure of proteins
 Structure of proteins Primary structure: is amino acids sequence or the covalent structure (50-2500) amino acids M.Wt. of amino acid=110 Dalton (56 110=5610 Dalton). Single chain or more than one polypeptide
Structure of proteins Primary structure: is amino acids sequence or the covalent structure (50-2500) amino acids M.Wt. of amino acid=110 Dalton (56 110=5610 Dalton). Single chain or more than one polypeptide
Elements & Macromolecules in Organisms
 Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
WATER CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" POLARITY HYDROGEN BONDING
 CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" WATER Compare the body of the jellyfish with our own bodies. The jellyfish will die if it is removed from its water environment, yet we can live in the
CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" WATER Compare the body of the jellyfish with our own bodies. The jellyfish will die if it is removed from its water environment, yet we can live in the
The Molecules of Life - Overview. The Molecules of Life. The Molecules of Life. The Molecules of Life
 The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
Proteins and Nucleic Acids
 Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
Isomers Have same molecular formula, but different structures
 Isomers ave same molecular formula, but different structures Constitutional Isomers Differ in the order of attachment of atoms (different bond connectivity) Stereoisomers Atoms are connected in the same
Isomers ave same molecular formula, but different structures Constitutional Isomers Differ in the order of attachment of atoms (different bond connectivity) Stereoisomers Atoms are connected in the same
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
lt\ Nutrition a-glncose www.cambridge.org in this web service Cambridge University Press Glucose+ fructose Glucose+ galactose
 P. T. Marshall and G. M. ughes 1 Nutrition 1.1 The basic biochemistry of mammalian metabolites The mammals are heterotrophic, that is to say they derive their essential nutrients, via food chains, from
P. T. Marshall and G. M. ughes 1 Nutrition 1.1 The basic biochemistry of mammalian metabolites The mammals are heterotrophic, that is to say they derive their essential nutrients, via food chains, from
Lab 2 Biochemistry. Learning Objectives. Introduction. Lipid Structure and Role in Food. The lab has the following learning objectives.
 1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
Chapter 13 Organic Chemistry
 Chapter 13 Organic Chemistry 13-1. Carbon Bonds 13-2. Alkanes 13-3. Petroleum Products 13-4. Structural Formulas 13-5. Isomers 13-6. Unsaturated Hydrocarbons 13-7. Benzene 13-8. Hydrocarbon Groups 13-9.
Chapter 13 Organic Chemistry 13-1. Carbon Bonds 13-2. Alkanes 13-3. Petroleum Products 13-4. Structural Formulas 13-5. Isomers 13-6. Unsaturated Hydrocarbons 13-7. Benzene 13-8. Hydrocarbon Groups 13-9.
Photosynthesis and Sucrose Production
 Photosynthesis and Sucrose Production 2 Starch and sucrose, key substrates for the development of dental caries, are exclusively synthesized by plants. They are made in plant leaves by a process called
Photosynthesis and Sucrose Production 2 Starch and sucrose, key substrates for the development of dental caries, are exclusively synthesized by plants. They are made in plant leaves by a process called
Molecule Projections
 Key Definitions ü Stereochemistry refers to the chemistry in 3 dimensions (greek stereos = solid). This science was created by Pasteur (1860), van Hoff et LeBel (1874). ü Stereisomers are isomeric molecules
Key Definitions ü Stereochemistry refers to the chemistry in 3 dimensions (greek stereos = solid). This science was created by Pasteur (1860), van Hoff et LeBel (1874). ü Stereisomers are isomeric molecules
BIOMOLECULES. reflect
 reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
Chapter 5 Classification of Organic Compounds by Solubility
 Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein
 Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein Biology Leaving Cert Experiments Materials/Equipment Starch solution (1%) Iodine Solution Glucose Solution (1%) 100 C) Benedict s
Conduct A Qualitative Test For Starch, Fat, A Reducing Sugar, A Protein Biology Leaving Cert Experiments Materials/Equipment Starch solution (1%) Iodine Solution Glucose Solution (1%) 100 C) Benedict s
Macromolecules in my food!!
 Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
Carbohydrates Lipids Proteins Nucleic Acids
 Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
Ch24_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch24_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Substances originating in plant or animal material and soluble in non-polar organic solvents
Ch24_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Substances originating in plant or animal material and soluble in non-polar organic solvents
Sugars, Starches, and Fibers Are All Carbohydrates
 Sugars, Starches, and Fibers Are All Carbohydrates What are carbohydrates? Today's food advertisements call them carbs, but they are not all the same. They are a group of compounds that have some similarities
Sugars, Starches, and Fibers Are All Carbohydrates What are carbohydrates? Today's food advertisements call them carbs, but they are not all the same. They are a group of compounds that have some similarities
Unit Vocabulary: o Organic Acid o Alcohol. o Ester o Ether. o Amine o Aldehyde
 Unit Vocabulary: Addition rxn Esterification Polymer Alcohol Ether Polymerization Aldehyde Fermentation Primary Alkane Functional group Saponification Alkene Halide (halocarbon) Saturated hydrocarbon Alkyne
Unit Vocabulary: Addition rxn Esterification Polymer Alcohol Ether Polymerization Aldehyde Fermentation Primary Alkane Functional group Saponification Alkene Halide (halocarbon) Saturated hydrocarbon Alkyne
IR Applied to Isomer Analysis
 DiscovIR-LC TM Application Note 025 April 2008 Deposition and Detection System IR Applied to Isomer Analysis Infrared spectra provide valuable information about local configurations of atoms in molecules.
DiscovIR-LC TM Application Note 025 April 2008 Deposition and Detection System IR Applied to Isomer Analysis Infrared spectra provide valuable information about local configurations of atoms in molecules.
The molecules of life. The molecules that make up living things are really big They are called macromolecules
 Food Labels All living things use materials and energy Our food comes from living things The food labels we see show us what our food is made of The stuff we are studying today can be found on food labels
Food Labels All living things use materials and energy Our food comes from living things The food labels we see show us what our food is made of The stuff we are studying today can be found on food labels
Chemical Basis of Life Module A Anchor 2
 Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Chapter 2. The Chemistry of Life Worksheets
 Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Biofuel Feedstocks and Production
 Lecture Five Cellulose, Hemicellulose and Lignin Biological and Ecological Engineering Department Summary of Lecture Four Synthesis of starch in plants. Amylose and amylopectin. Crystalline and amorphous
Lecture Five Cellulose, Hemicellulose and Lignin Biological and Ecological Engineering Department Summary of Lecture Four Synthesis of starch in plants. Amylose and amylopectin. Crystalline and amorphous
1.1.2. thebiotutor. AS Biology OCR. Unit F211: Cells, Exchange & Transport. Module 1.2 Cell Membranes. Notes & Questions.
 thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
Combinatorial Biochemistry and Phage Display
 Combinatorial Biochemistry and Phage Display Prof. Valery A. Petrenko Director - Valery Petrenko Instructors Galina Kouzmitcheva and I-Hsuan Chen Auburn 2006, Spring semester COMBINATORIAL BIOCHEMISTRY
Combinatorial Biochemistry and Phage Display Prof. Valery A. Petrenko Director - Valery Petrenko Instructors Galina Kouzmitcheva and I-Hsuan Chen Auburn 2006, Spring semester COMBINATORIAL BIOCHEMISTRY
Molecular Cell Biology
 Harvey Lodish Arnold Berk Paul Matsudaira Chris A. Kaiser Monty Krieger Matthew P. Scott Lawrence Zipursky James Darnell Molecular Cell Biology Fifth Edition Chapter 2: Chemical Foundations Copyright 2004
Harvey Lodish Arnold Berk Paul Matsudaira Chris A. Kaiser Monty Krieger Matthew P. Scott Lawrence Zipursky James Darnell Molecular Cell Biology Fifth Edition Chapter 2: Chemical Foundations Copyright 2004
Anatomy and Physiology Placement Exam 2 Practice with Answers at End!
 Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Biological Molecules
 Biological Molecules I won t lie. This is probably the most boring topic you have ever done in any science. It s pretty much as simple as this: learn the material deal with it. Enjoy don t say I didn t
Biological Molecules I won t lie. This is probably the most boring topic you have ever done in any science. It s pretty much as simple as this: learn the material deal with it. Enjoy don t say I didn t
18.2 Protein Structure and Function: An Overview
 18.2 Protein Structure and Function: An Overview Protein: A large biological molecule made of many amino acids linked together through peptide bonds. Alpha-amino acid: Compound with an amino group bonded
18.2 Protein Structure and Function: An Overview Protein: A large biological molecule made of many amino acids linked together through peptide bonds. Alpha-amino acid: Compound with an amino group bonded
3120-1 - Page 1. Name:
 Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Ch18_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch18_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following can be classified as biomolecules except A) lipids. B) proteins. C)
Ch18_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following can be classified as biomolecules except A) lipids. B) proteins. C)
Chapter 12 Organic Compounds with Oxygen and Sulfur
 Chapter 12 Organic Compounds with Oxygen and Sulfur 1 Alcohols An alcohol contains a hydroxyl group ( OH) that replaces a hydrogen atom in a hydrocarbon. A phenol contains a hydroxyl group ( OH) attached
Chapter 12 Organic Compounds with Oxygen and Sulfur 1 Alcohols An alcohol contains a hydroxyl group ( OH) that replaces a hydrogen atom in a hydrocarbon. A phenol contains a hydroxyl group ( OH) attached
Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch
 Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch Introduction Enzymes are proteins composed of amino acid building blocks. Enzymes catalyze or increase the rate of metabolic
Human Physiology Lab (Biol 236L) Digestive Physiology: Amylase hydrolysis of starch Introduction Enzymes are proteins composed of amino acid building blocks. Enzymes catalyze or increase the rate of metabolic
Keystone Review Practice Test Module A Cells and Cell Processes. 1. Which characteristic is shared by all prokaryotes and eukaryotes?
 Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
CHEM 121. Chapter 19, Name: Date:
 CHEM 121. Chapter 19, Name: Date: 1. A lipid is any substance of biochemical origin that is A) soluble in water but insoluble in nonpolar solvents B) insoluble in both water and nonpolar solvents C) insoluble
CHEM 121. Chapter 19, Name: Date: 1. A lipid is any substance of biochemical origin that is A) soluble in water but insoluble in nonpolar solvents B) insoluble in both water and nonpolar solvents C) insoluble
MCAT Organic Chemistry - Problem Drill 23: Amino Acids, Peptides and Proteins
 MCAT rganic Chemistry - Problem Drill 23: Amino Acids, Peptides and Proteins Question No. 1 of 10 Question 1. Which amino acid does not contain a chiral center? Question #01 (A) Serine (B) Proline (C)
MCAT rganic Chemistry - Problem Drill 23: Amino Acids, Peptides and Proteins Question No. 1 of 10 Question 1. Which amino acid does not contain a chiral center? Question #01 (A) Serine (B) Proline (C)
Cellular Respiration: Practice Questions #1
 Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
Cellular Respiration: Practice Questions #1 1. Which statement best describes one of the events taking place in the chemical reaction? A. Energy is being stored as a result of aerobic respiration. B. Fermentation
Nucleotides and Nucleic Acids
 Nucleotides and Nucleic Acids Brief History 1 1869 - Miescher Isolated nuclein from soiled bandages 1902 - Garrod Studied rare genetic disorder: Alkaptonuria; concluded that specific gene is associated
Nucleotides and Nucleic Acids Brief History 1 1869 - Miescher Isolated nuclein from soiled bandages 1902 - Garrod Studied rare genetic disorder: Alkaptonuria; concluded that specific gene is associated
3Reactions of Sugars. Introduction. Mutarotation
 3eactions of Sugars Introduction 35 Mutarotation 35 xidation of Sugars 39 Glycoside Formation 40 Acid atalyzed Sugar eactions 42 Alkaline-atalyzed Sugar eactions 43 Summary 45 Vocabulary 47 eferences 47
3eactions of Sugars Introduction 35 Mutarotation 35 xidation of Sugars 39 Glycoside Formation 40 Acid atalyzed Sugar eactions 42 Alkaline-atalyzed Sugar eactions 43 Summary 45 Vocabulary 47 eferences 47
IV. -Amino Acids: carboxyl and amino groups bonded to -Carbon. V. Polypeptides and Proteins
 IV. -Amino Acids: carboxyl and amino groups bonded to -Carbon A. Acid/Base properties 1. carboxyl group is proton donor! weak acid 2. amino group is proton acceptor! weak base 3. At physiological ph: H
IV. -Amino Acids: carboxyl and amino groups bonded to -Carbon A. Acid/Base properties 1. carboxyl group is proton donor! weak acid 2. amino group is proton acceptor! weak base 3. At physiological ph: H
Chapter 14 Glycolysis. Glucose. 2 Pyruvate 2 Lactate (sent to liver to be converted back to glucose) TCA Cycle
 Chapter 14 Glycolysis Requires mitochondria and O 2 Glucose glycolysis anaerobic respiration 2 Pyruvate 2 Lactate (sent to liver to be converted back to glucose) pyruvate dehydrogenase acetyl-coa TCA Cycle
Chapter 14 Glycolysis Requires mitochondria and O 2 Glucose glycolysis anaerobic respiration 2 Pyruvate 2 Lactate (sent to liver to be converted back to glucose) pyruvate dehydrogenase acetyl-coa TCA Cycle
Unit 2: Cells, Membranes and Signaling CELL MEMBRANE. Chapter 5 Hillis Textbook
 Unit 2: Cells, Membranes and Signaling CELL MEMBRANE Chapter 5 Hillis Textbook HOW DOES THE LAB RELATE TO THE NEXT CHAPTER? SURFACE AREA: the entire outer covering of a cell that enables materials pass.
Unit 2: Cells, Membranes and Signaling CELL MEMBRANE Chapter 5 Hillis Textbook HOW DOES THE LAB RELATE TO THE NEXT CHAPTER? SURFACE AREA: the entire outer covering of a cell that enables materials pass.
UNIT (11) MOLECULES OF LIFE: LIPIDS AND PROTEINS
 UNIT (11) MOLECULES OF LIFE: LIPIDS AND PROTEINS 11.1 Types of Lipids Lipids are also biochemical compounds that contain carbon, hydrogen, and oxygen. But lipids, unlike carbohydrates, share no common
UNIT (11) MOLECULES OF LIFE: LIPIDS AND PROTEINS 11.1 Types of Lipids Lipids are also biochemical compounds that contain carbon, hydrogen, and oxygen. But lipids, unlike carbohydrates, share no common
1. Essay: The Digestive and Absorption Processes of Macronutrients
 Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
NO CALCULATORS OR CELL PHONES ALLOWED
 Biol 205 Exam 1 TEST FORM A Spring 2008 NAME Fill out both sides of the Scantron Sheet. On Side 2 be sure to indicate that you have TEST FORM A The answers to Part I should be placed on the SCANTRON SHEET.
Biol 205 Exam 1 TEST FORM A Spring 2008 NAME Fill out both sides of the Scantron Sheet. On Side 2 be sure to indicate that you have TEST FORM A The answers to Part I should be placed on the SCANTRON SHEET.
DNA is found in all organisms from the smallest bacteria to humans. DNA has the same composition and structure in all organisms!
 Biological Sciences Initiative HHMI DNA omponents and Structure Introduction Nucleic acids are molecules that are essential to, and characteristic of, life on Earth. There are two basic types of nucleic
Biological Sciences Initiative HHMI DNA omponents and Structure Introduction Nucleic acids are molecules that are essential to, and characteristic of, life on Earth. There are two basic types of nucleic
Preliminary MFM Quiz
 Preliminary MFM Quiz 1. The major carrier of chemical energy in all cells is: A) adenosine monophosphate B) adenosine diphosphate C) adenosine trisphosphate D) guanosine trisphosphate E) carbamoyl phosphate
Preliminary MFM Quiz 1. The major carrier of chemical energy in all cells is: A) adenosine monophosphate B) adenosine diphosphate C) adenosine trisphosphate D) guanosine trisphosphate E) carbamoyl phosphate
1. When applying the process of science, which of these is tested? a. an observation b. a result c. a hypothesis d. a question e.
 BCOR 11 Exam 1, 2004 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1. When applying the process of science, which of these is tested? a. an observation
BCOR 11 Exam 1, 2004 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1. When applying the process of science, which of these is tested? a. an observation
Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look
 OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
Carbohydrates. at CordenPharma
 Carbohydrates at CordenPharma Carbohydrates in ature Carbohydrates constitute the most abundant and ubiquitous group of natural products. The nutritive value of mono-and polysaccharides such as glucose
Carbohydrates at CordenPharma Carbohydrates in ature Carbohydrates constitute the most abundant and ubiquitous group of natural products. The nutritive value of mono-and polysaccharides such as glucose
The Lipid Bilayer Is a Two-Dimensional Fluid
 The Lipid Bilayer Is a Two-Dimensional Fluid The aqueous environment inside and outside a cell prevents membrane lipids from escaping from bilayer, but nothing stops these molecules from moving about and
The Lipid Bilayer Is a Two-Dimensional Fluid The aqueous environment inside and outside a cell prevents membrane lipids from escaping from bilayer, but nothing stops these molecules from moving about and
How To Understand The Human Body
 Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Overview of Glycolysis Under anaerobic conditions, the glycolytic pathway present in most species results in a balanced reaction:
 Glycolysis Glucose is a valuable molecule. It can be used to generate energy (in red blood cells and in brain under normal conditions, glucose is the sole energy source), and it can be used to generate
Glycolysis Glucose is a valuable molecule. It can be used to generate energy (in red blood cells and in brain under normal conditions, glucose is the sole energy source), and it can be used to generate
BIOLOGICAL MEMBRANES: FUNCTIONS, STRUCTURES & TRANSPORT
 BIOLOGICAL MEMBRANES: FUNCTIONS, STRUCTURES & TRANSPORT UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY BMLS II / B Pharm II / BDS II VJ Temple
BIOLOGICAL MEMBRANES: FUNCTIONS, STRUCTURES & TRANSPORT UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY BMLS II / B Pharm II / BDS II VJ Temple
http://faculty.sau.edu.sa/h.alshehri
 http://faculty.sau.edu.sa/h.alshehri Definition: Proteins are macromolecules with a backbone formed by polymerization of amino acids. Proteins carry out a number of functions in living organisms: - They
http://faculty.sau.edu.sa/h.alshehri Definition: Proteins are macromolecules with a backbone formed by polymerization of amino acids. Proteins carry out a number of functions in living organisms: - They
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES
 LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
Copyright 2010 Pearson Education, Inc. Chapter Fourteen 1
 An alcohol has an OH bonded to an alkyl group; a phenol has an OH bonded directly to an aromatic ring; and an ether has an O bonded to two organic groups. Chapter Fourteen 1 Ethyl alcohol, dimethyl ether,
An alcohol has an OH bonded to an alkyl group; a phenol has an OH bonded directly to an aromatic ring; and an ether has an O bonded to two organic groups. Chapter Fourteen 1 Ethyl alcohol, dimethyl ether,
Reactions of Fats and Fatty Acids
 Reactions of Fats and Fatty Acids Outline Fats and Oils Fatty Acid Biosynthesis Biodiesel Homework We hear quite a lot about the place of fats and oils in human nutrition. Foods high in fat are at the
Reactions of Fats and Fatty Acids Outline Fats and Oils Fatty Acid Biosynthesis Biodiesel Homework We hear quite a lot about the place of fats and oils in human nutrition. Foods high in fat are at the
Bioenergetics. Free Energy Change
 Bioenergetics Energy is the capacity or ability to do work All organisms need a constant supply of energy for functions such as motion, transport across membrane barriers, synthesis of biomolecules, information
Bioenergetics Energy is the capacity or ability to do work All organisms need a constant supply of energy for functions such as motion, transport across membrane barriers, synthesis of biomolecules, information
