An Overview for Closed System Outpatient Pharmacies
|
|
|
- Jasper Dorsey
- 7 years ago
- Views:
Transcription
1 The Transmucosal Immediate Release Fentanyl (TIRF) REMS Access Program An Overview for Closed System Outpatient Pharmacies To dispense TIRF medicines, your closed system outpatient pharmacy must enroll in the TIRF REMS Access program. What is the TIRF REMS Access program? The TIRF REMS (Risk Evaluation and Mitigation Strategy) Access program is designed to ensure informed risk-benefit decisions before initiating treatment, while patients are on treatment, and to ensure appropriate use of TIRF medicines. TIRF medicines are available only through a required Food and Drug Administration (FDA) restricted distribution program, because of the risk for misuse, abuse, addiction, overdose, and serious complications due to medication errors. A list of TIRF medicines available through the TIRF REMS Access program is located on the TIRF Products web page at How does the TIRF REMS Access program work? The TIRF REMS Access program requires pharmacies, prescribers, patients, and wholesalers to enroll in the program in order to utilize TIRF medications. The supply of TIRF medicines to pharmacies is controlled by enrolled distributors, who will verify the current enrollment status of the pharmacy prior to shipment of TIRF medicines. Pharmacies are required to verify the prescriber and the patient are enrolled in the TIRF REMS Access program before dispensing any TIRF medication. Does your institution qualify as a closed system outpatient pharmacy? For the purposes of this REMS, a closed system outpatient pharmacy is defined as an outpatient pharmacy that uses a pharmacy management system that does not support the process of electronically transmitting the validation and claim information currently required by the TIRF REMS Access program. For example, some pharmacies that are part of integrated healthcare delivery systems may qualify as closed system outpatient pharmacies. NOTE: There are different requirements for outpatient pharmacies that support the process of electronically transmitting claim information, and for inpatient pharmacies that only dispense for inpatient use. Please refer to An Overview for Chain Outpatient Pharmacies, An Overview for Independent Outpatient Pharmacies or An Overview for Inpatient Pharmacies for more information. If you do not qualify as a closed system outpatient pharmacy, please refer to the requirements for the other types of pharmacies. 1
2 The following two sections provide detailed information on the enrollment process (Section 1) and the dispensing processes (Section 2) for TIRF medicines in a closed system outpatient pharmacy. Section 1: Enrollment Process Summary of Enrollment Process 1. Confirm that your facility qualifies as a closed system outpatient pharmacy. 2. Select an individual to be your Authorized Closed System Outpatient Pharmacy Representative. 3. Complete the TIRF REMS Access Education Program and Knowledge Assessment. 4. Complete and submit a Closed System Outpatient Pharmacy Enrollment Form. 5. Train pharmacy staff. Detailed Enrollment Process Step 1: Confirm your facility qualifies as a closed system outpatient pharmacy Notify the TIRF REMS Access program by phone at or by to information@tirfremsaccess.com that you are a closed system outpatient pharmacy. When your pharmacy is validated as a closed system outpatient pharmacy, a Closed System Outpatient Pharmacy Enrollment Form will be provided. Step 2: Select an individual to be your authorized closed system outpatient pharmacy representative Select an authorized closed system outpatient pharmacy representative to establish and oversee the TIRF REMS Access program requirements. Step 3: Complete the TIRF REMS Access Education Program Review the TIRF REMS Access Education Program and successfully complete the Knowledge Assessment. The TIRF REMS Access Education Program is available online at the TIRF REMS Access program website or by contacting the TIRF REMS Access call center at If Knowledge Assessment was completed on paper, fax to , or the Knowledge Assessment to information@tirfremsaccess.com with enrollment form (see Step 4: Complete and submit enrollment form). 2
3 How do I complete the TIRF REMS Access Education Program online? Select the Create Account button on the home page. Complete the Create Account Information section. Already enrolled via fax and have an enrollment ID? select No. Create User ID and Password and select the Create My Account button. Select Pharmacy as the option to best describe you and select Continue. In response to question 2, select Pharmacy Staff. Review the content in the pop-up box and select Confirm to continue. Complete required fields in pharmacy staff details. Select Other from the dropdown list in the chain pharmacy name and populate the name of your closed system outpatient pharmacy organization in the Other field and submit form. Select the Start the TIRF REMS Access Education Program to proceed to the training. Once you have completed the Education Program, select the Go to Knowledge Assessment button and complete. A Knowledge Assessment confirmation code will be provided once the assessment is completed successfully. Step 4: Complete and submit enrollment form Complete and return the Closed System Outpatient Pharmacy Enrollment Form by fax to The authorized closed system outpatient pharmacy representative will receive an enrollment confirmation letter and instructions for enrolling dispensing locations within the closed system outpatient pharmacy by using a standard file template provided by the TIRF REMS Access program. If you did not complete the Education Program online, then you need to submit the Knowledge Assessment form with the enrollment form. Re-enroll every two (2) years. You will be notified by the TIRF REMS Access program in advance of the need to re-enroll. Step 5: Train pharmacy staff All closed system outpatient pharmacy staff involved in processing and dispensing of TIRF medications must be trained to dispense TIRF medicines in accordance with the TIRF REMS Access Education Program requirements available at Ensure that this training is documented and retained by the closed system outpatient pharmacy. This documentation should include the pharmacist/ pharmacy staff member s name, the date training was completed, and the method of training as a minimum. 3
4 Section 2: Dispensing Process Summary of Dispensing Process 1. Confirm pharmacy staff is trained. 2. Counsel patients and prescriber enrollment in TIRF REMS Access program. 3. Dispense TIRF medication. 4. Counsel patient and provide Medication Guide. Detailed Dispensing Process Step 1: Confirm that the pharmacy staff is trained Ensure all pharmacy staff involved in the processing and dispensing of TIRF medicines have been trained to specifically dispense TIRF medicines in accordance with the TIRF REMS Access program requirements available at (see Section 1, Step 5: Train pharmacy staff). Step 2: Confirm prescriber and patient enrollment Prior to dispensing each TIRF medicine prescription, confirm that the prescriber and patient are enrolled in the TIRF REMS Access program by contacting the TIRF REMS Access program by phone at or fax at This includes third party insurance claims, cash claims and any other claims (i.e.: workers compensation). To confirm enrollment confirmation by phone Contact the TIRF REMS Access program at and select option #2. Provide the following required data from the TIRF prescription to obtain an authorization to dispense: Dispensing Pharmacy DEA Dispensing Pharmacy NPI Dispensing Pharmacy Phone # Dispensing Pharmacy Fax # Prescriber DEA or NPI Patient Date of Birth Patient First Name Patient Last Name Patient Zip Code Prescriber Last Name Rx Date of Service Rx Number Rx NDC Days Supply Quantity for Dispense If validated, you will be supplied a prescription authorization number which indicates you can dispense TIRF medicine. If not validated, you will be provided a rejection reason and information regarding how to resolve the rejection. 4
5 To confirm enrollment confirmation by fax: Populate all of the required fields on the TIRF REMS Access Prescription Authorization Form and fax to To obtain a TIRF REMS Access Prescription Authorization Form which may be reproduced to use continually, please information@tirfremsaccess.com. If validated, you will be supplied a prescription authorization number via fax within one (1) business day which indicates you can dispense the TIRF medicine. If not validated, you will be provided a rejection reason and information regarding how to resolve the rejection using the phone number provided on the request. Step 3: Dispensing Receive the prescription authorization number from the TIRF REMS Access program and then prepare, label, and dispense the medication. Step 4: Counsel patient and provide Medication Guide Counsel the patient on the appropriate use, safe storage, and the proper disposal procedures of TIRF medicines. Provide a copy of the product specific Medication Guide to the patient with each prescription. Reporting Adverse Events and Monitoring To report any adverse events including the misuse, abuse, addiction, or overdose of TIRF medication, contact: TIRF REMS Access program at and/or FDA MedWatch program by phone at FDA-1088 or online at If you have any questions, need additional information, or need additional copies of any TIRF REMS Access documents, please visit or call the TIRF REMS Access program at
TRANSMUCOSAL IMMEDIATE RELEASE FENTANYL (TIRF) RISK EVALUATION AND MITIGATION STRATEGY (REMS)
 Initial REMS approval: 12/2011 Most recent modification: /2014 TRANSMUCOSAL IMMEDIATE RELEASE FENTANYL (TIRF) RISK EVALUATION AND MITIGATION STRATEGY (REMS) Page 1 of 16 I. GOALS The goals of the TIRF
Initial REMS approval: 12/2011 Most recent modification: /2014 TRANSMUCOSAL IMMEDIATE RELEASE FENTANYL (TIRF) RISK EVALUATION AND MITIGATION STRATEGY (REMS) Page 1 of 16 I. GOALS The goals of the TIRF
The TIRF REMS Access program is a Food and Drug Administration (FDA) required risk management program
 Subject: Important Drug Warning Announcement of a single shared REMS (Risk Evaluation and Mitigation Strategy) program for all Transmucosal Immediate Release Fentanyl (TIRF) products due to the potential
Subject: Important Drug Warning Announcement of a single shared REMS (Risk Evaluation and Mitigation Strategy) program for all Transmucosal Immediate Release Fentanyl (TIRF) products due to the potential
Buprenorphine-containing Transmucosal products for Opioid Dependence (BTOD) Risk Evaluation and Mitigation Strategy (REMS)
 Initial REMS approval: 02/2013 Most recent modification: 06/2015 Buprenorphine-containing Transmucosal products for Opioid Dependence (BTOD) Risk Evaluation and Mitigation Strategy (REMS) This REMS applies
Initial REMS approval: 02/2013 Most recent modification: 06/2015 Buprenorphine-containing Transmucosal products for Opioid Dependence (BTOD) Risk Evaluation and Mitigation Strategy (REMS) This REMS applies
RISK EVALUATION AND MITIGATION STRATEGY (REMS)
 Initial REMS approval: 08/2015 NDA 22526 Addyi TM (flibanserin) Serotonin 1A receptor agonist and a Serotonin 2A receptor antagonist Sprout Pharmaceuticals, Inc. 4208 Six Forks Road, Suite 1010 Raleigh,
Initial REMS approval: 08/2015 NDA 22526 Addyi TM (flibanserin) Serotonin 1A receptor agonist and a Serotonin 2A receptor antagonist Sprout Pharmaceuticals, Inc. 4208 Six Forks Road, Suite 1010 Raleigh,
Manual of Instructions
 NEW YORK STATE DEPARTMENT OF HEALTH OFFICIAL NEW YORK STATE PRESCRIPTION PROGRAM ELECTRONIC DATA TRANSMISSION Manual of Instructions New York State Department of Health Bureau of Narcotic Enforcement 433
NEW YORK STATE DEPARTMENT OF HEALTH OFFICIAL NEW YORK STATE PRESCRIPTION PROGRAM ELECTRONIC DATA TRANSMISSION Manual of Instructions New York State Department of Health Bureau of Narcotic Enforcement 433
RHODE ISLAND PRESCRIPTION MONITORING PROGRAM DATA COLLECTION MANUAL
 RHODE ISLAND PRESCRIPTION MONITORING PROGRAM DATA COLLECTION MANUAL Effective Date: October 13, 2014 Optimum Technology, Inc. Contact Information Phone: 866-683-2476 Fax: 866-282-7076 RIRxReport@otech.com
RHODE ISLAND PRESCRIPTION MONITORING PROGRAM DATA COLLECTION MANUAL Effective Date: October 13, 2014 Optimum Technology, Inc. Contact Information Phone: 866-683-2476 Fax: 866-282-7076 RIRxReport@otech.com
How To Get A Tirf
 Transmucosal Immediate Release Fentanyl (TIRF) Products Risk Evaluation and Mitigation Strategy (REMS) Education Program for Prescribers and Pharmacists Products Covered Under This Program Abstral (fentanyl)
Transmucosal Immediate Release Fentanyl (TIRF) Products Risk Evaluation and Mitigation Strategy (REMS) Education Program for Prescribers and Pharmacists Products Covered Under This Program Abstral (fentanyl)
PROPOSED REGULATION OF THE STATE BOARD OF PHARMACY. LCB File No. R047-15. September 15, 2015
 PROPOSED REGULATION OF THE STATE BOARD OF PHARMACY LCB File No. R047-15 September 15, 2015 EXPLANATION Matter in italics is new; matter in brackets [omitted material] is material to be omitted. AUTHORITY:
PROPOSED REGULATION OF THE STATE BOARD OF PHARMACY LCB File No. R047-15 September 15, 2015 EXPLANATION Matter in italics is new; matter in brackets [omitted material] is material to be omitted. AUTHORITY:
Real-time Pre and Post Claim Edits: Improve Reimbursement, Compliance and Safety
 Real-time Pre and Post Claim Edits: Improve Reimbursement, Compliance and Safety An ESI Healthcare Business Solutions White Paper by Thomas Renshaw R.Ph. Introduction Outpatient pharmacies submitting claims
Real-time Pre and Post Claim Edits: Improve Reimbursement, Compliance and Safety An ESI Healthcare Business Solutions White Paper by Thomas Renshaw R.Ph. Introduction Outpatient pharmacies submitting claims
Table of Contents. 2 P a g e
 Table of Contents Introduction... 3 Important Contact Information... 3 Pharmacy Rights... 3 Claims Adjudication... 3 Reversals... 4 Required Data Fields... 4 Identification cards... 4 Required Identification
Table of Contents Introduction... 3 Important Contact Information... 3 Pharmacy Rights... 3 Claims Adjudication... 3 Reversals... 4 Required Data Fields... 4 Identification cards... 4 Required Identification
Pharmacy Program Pre-Test
 Last Name: Pharmacy Program Pre-Test * For each question, put a check mark for the one option that you think is correct. 1. A pharmacist receives a security prescription from a known local medical group
Last Name: Pharmacy Program Pre-Test * For each question, put a check mark for the one option that you think is correct. 1. A pharmacist receives a security prescription from a known local medical group
PRESCRIPTION MONITORING PROGRAM (PMP)
 PRESCRIPTION MONITORING PROGRAM (PMP) DATA REPORTING MANUAL Effective Date: April 1, 2012 Contact Information: (505) 222-9830 larry.loring@state.nm.us 1 P a g e Table of Contents Reporting requirements
PRESCRIPTION MONITORING PROGRAM (PMP) DATA REPORTING MANUAL Effective Date: April 1, 2012 Contact Information: (505) 222-9830 larry.loring@state.nm.us 1 P a g e Table of Contents Reporting requirements
LOUISIANA PRESCRIPTION MONITORING PROGRAM
 LOUISIANA PRESCRIPTION MONITORING PROGRAM DATA COLLECTION MANUAL - VERSION 1.0 Revision History Date Version Description Author 12/13/2013 1.0 Initial version Lena Roe (Otech) 1/28/2014 1.1 Updates Lena
LOUISIANA PRESCRIPTION MONITORING PROGRAM DATA COLLECTION MANUAL - VERSION 1.0 Revision History Date Version Description Author 12/13/2013 1.0 Initial version Lena Roe (Otech) 1/28/2014 1.1 Updates Lena
Controlled Substances Prescription Monitoring Program. Dean Wright, RPh Arizona State Board of Pharmacy
 Controlled Substances Prescription Monitoring Program Dean Wright, RPh Arizona State Board of Pharmacy Controlled Substances Prescription Monitoring Program Arizona s Forty-eighth Legislature passed H.B.
Controlled Substances Prescription Monitoring Program Dean Wright, RPh Arizona State Board of Pharmacy Controlled Substances Prescription Monitoring Program Arizona s Forty-eighth Legislature passed H.B.
Statement BAR CODE LABEL REQUIREMENTS FOR HUMAN DRUG AND BIOLOGIC PRODUCTS
 Statement on BAR CODE LABEL REQUIREMENTS FOR HUMAN DRUG AND BIOLOGIC PRODUCTS Edith Rosato, R.Ph. Vice President Pharmacy Affairs National Association of Chain Drug Stores Alexandria, VA July 26,2002 Submitted
Statement on BAR CODE LABEL REQUIREMENTS FOR HUMAN DRUG AND BIOLOGIC PRODUCTS Edith Rosato, R.Ph. Vice President Pharmacy Affairs National Association of Chain Drug Stores Alexandria, VA July 26,2002 Submitted
Dispenser s Implementation Guide
 Alabama Department of Public Health Prescription Drug Monitoring Program October 2014 This document is formatted for duplex printing. This page intentionally left blank. Copyright 2009-2014 Health Information
Alabama Department of Public Health Prescription Drug Monitoring Program October 2014 This document is formatted for duplex printing. This page intentionally left blank. Copyright 2009-2014 Health Information
PMP AWAR X E. User Support Manual V 1.2
 PMP AWAR X E User Support Manual V 1.2 04/09/2014 1 Contents 1 What Is a Requestor?... 3 2 Registration... 3 2.1 Registration Process... 3 2.2 Registering as a Delegate... 7 2.3 Email Verification... 11
PMP AWAR X E User Support Manual V 1.2 04/09/2014 1 Contents 1 What Is a Requestor?... 3 2 Registration... 3 2.1 Registration Process... 3 2.2 Registering as a Delegate... 7 2.3 Email Verification... 11
Addyi REMS Program Prescriber and Pharmacy Training
 Addyi REMS Program Prescriber and Pharmacy Training The Food and Drug Administration (FDA) has required a Risk Evaluation and Mitigation Strategy (REMS) to ensure the benefits of Addyi outweigh the increased
Addyi REMS Program Prescriber and Pharmacy Training The Food and Drug Administration (FDA) has required a Risk Evaluation and Mitigation Strategy (REMS) to ensure the benefits of Addyi outweigh the increased
Evaluating the impact of REMS on burden and patient access
 Evaluating the impact of REMS on burden and patient access Doris Auth, Pharm.D. Team Leader, Division of Risk Management Office of Medication Error Prevention and Risk Management Center for Drug Evaluation
Evaluating the impact of REMS on burden and patient access Doris Auth, Pharm.D. Team Leader, Division of Risk Management Office of Medication Error Prevention and Risk Management Center for Drug Evaluation
FAQs on the Required National Provider Identifier (NPI)
 FAQs on the Required National Provider Identifier (NPI) Provided by the National Community Pharmacists Association (NCPA) and the National Council for Prescription Drug Programs (NCPDP) At-A-Glance: Important!
FAQs on the Required National Provider Identifier (NPI) Provided by the National Community Pharmacists Association (NCPA) and the National Council for Prescription Drug Programs (NCPDP) At-A-Glance: Important!
DIVISION OF CORPORATIONS, BUSINESS AND PROFESSIONAL P.O. BOX 110806 JUNEAU, ALASKA 99811-0806 ALASKA STATE BOARD OF PHARMACY
 DIVISION OF CORPORATIONS, BUSINESS AND PROFESSIONAL P.O. BOX 110806 JUNEAU, ALASKA 99811-0806 ALASKA STATE BOARD OF PHARMACY Remote Pharmacy Owner Name: DBA Name: Address: Telephone Number: Fax Number:
DIVISION OF CORPORATIONS, BUSINESS AND PROFESSIONAL P.O. BOX 110806 JUNEAU, ALASKA 99811-0806 ALASKA STATE BOARD OF PHARMACY Remote Pharmacy Owner Name: DBA Name: Address: Telephone Number: Fax Number:
Medical College of Georgia SOP NUMBER: 03 INVESTIGATIONAL DRUG HANDLING Version Number: 1.0, 1.1 Effective Date: 09/12/06, 08/02/10, 3/2/11
 Effective Date: 09/12/06, 08/02/10, 3/2/11 Title: 1.0 OBJECTIVE: 1.1 This SOP describes the methods and policies for: Handling investigational drug Dispensing investigational drug 1.2. This procedure applies
Effective Date: 09/12/06, 08/02/10, 3/2/11 Title: 1.0 OBJECTIVE: 1.1 This SOP describes the methods and policies for: Handling investigational drug Dispensing investigational drug 1.2. This procedure applies
Guidance Medication Guides Distribution Requirements and Inclusion in Risk Evaluation and Mitigation Strategies (REMS)
 Guidance s Distribution Requirements and Inclusion in Risk Evaluation and Mitigation Strategies (REMS) U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Guidance s Distribution Requirements and Inclusion in Risk Evaluation and Mitigation Strategies (REMS) U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation
Review of Maine Pharmacy Rules. An update of new rules adopted 12/11/2013
 Review of Maine Pharmacy Rules An update of new rules adopted 12/11/2013 This review is meant to highlight important changes and additions to the rules as adopted on 12/11/2013. We will cover the rules
Review of Maine Pharmacy Rules An update of new rules adopted 12/11/2013 This review is meant to highlight important changes and additions to the rules as adopted on 12/11/2013. We will cover the rules
Table of Contents. User Request for Access... 3. User Login... 3. Upload a Data File... 4
 Table of Contents User Request for Access... 3 User Login... 3 Upload a Data File... 4 Add Patient Records Direct Dispenser Users... 5 Pharmacy... 8 Search for Patient Records... 10 Search for Error Records...
Table of Contents User Request for Access... 3 User Login... 3 Upload a Data File... 4 Add Patient Records Direct Dispenser Users... 5 Pharmacy... 8 Search for Patient Records... 10 Search for Error Records...
State of South Dakota Board of Pharmacy Prescription Drug Monitoring Program. User Support Manual
 State of South Dakota Board of Pharmacy Prescription Drug Monitoring Program User Support Manual Contents 1 What Is a Requestor?... 3 2 Registration... 4 2.1 Registration Process... 4 2.2 Registering as
State of South Dakota Board of Pharmacy Prescription Drug Monitoring Program User Support Manual Contents 1 What Is a Requestor?... 3 2 Registration... 4 2.1 Registration Process... 4 2.2 Registering as
FDA Advisory Committee Hearing on Potential Rescheduling of Hydrocodone: Pharmacist and Pharmacy Issues to Consider
 FDA Advisory Committee Hearing on Potential Rescheduling of Hydrocodone: Pharmacist and Pharmacy Issues to Consider January 24, 2013 FDA White Oak Campus Steve Simenson, BPharm, FAPhA, DPNAP APhA President
FDA Advisory Committee Hearing on Potential Rescheduling of Hydrocodone: Pharmacist and Pharmacy Issues to Consider January 24, 2013 FDA White Oak Campus Steve Simenson, BPharm, FAPhA, DPNAP APhA President
Implementation of Florida s PDMP. Rebecca Poston Program Manager
 Implementation of Florida s PDMP Rebecca Poston Program Manager Disclaimer I have no relevant financial relationships or commercial interest in the content presented in this program. Learning Objectives:
Implementation of Florida s PDMP Rebecca Poston Program Manager Disclaimer I have no relevant financial relationships or commercial interest in the content presented in this program. Learning Objectives:
TABLE OF CONTENTS CHAPTER 8 PRESCRIPTION DRUG MONITORING PROGRAM
 TABLE OF CONTENTS CHAPTER 8 PRESCRIPTION DRUG MONITORING PROGRAM Section 1. Authority 8-1 Section 2. Transmission of Information Regarding Dispensing of Controlled Substances to Certain Persons 8-1 Section
TABLE OF CONTENTS CHAPTER 8 PRESCRIPTION DRUG MONITORING PROGRAM Section 1. Authority 8-1 Section 2. Transmission of Information Regarding Dispensing of Controlled Substances to Certain Persons 8-1 Section
Southlake Psychiatry. Suboxone Contract
 Suboxone Contract Thank you for considering Southlake Psychiatry for your Suboxone treatment. Opiate Addiction is a serious condition for which you may find relief with Suboxone treatment. In order to
Suboxone Contract Thank you for considering Southlake Psychiatry for your Suboxone treatment. Opiate Addiction is a serious condition for which you may find relief with Suboxone treatment. In order to
Substance Use: Addressing Addiction and Emerging Issues
 MODULE 6: SUBSTANCE USE: ADDRESSING ADDICTION AND EMERGING ISSUES Substance Use: Addressing Addiction and Emerging Issues Martha C. Romney, RN, MS, JD, MPH Assistant Professor Jefferson School of Population
MODULE 6: SUBSTANCE USE: ADDRESSING ADDICTION AND EMERGING ISSUES Substance Use: Addressing Addiction and Emerging Issues Martha C. Romney, RN, MS, JD, MPH Assistant Professor Jefferson School of Population
Covered Entities Guide for Public Users. Registering a Contract Pharmacy
 Registering a Contract Pharmacy Major Sections in This Guide: To jump to a specific section in this guide, click one of these links: Searching for a Covered Entity (page 4) Terminating an Existing Contract
Registering a Contract Pharmacy Major Sections in This Guide: To jump to a specific section in this guide, click one of these links: Searching for a Covered Entity (page 4) Terminating an Existing Contract
PHARMACY MANUAL. WHP Health Initiatives, Inc. 2275 Half Day Road, Suite 250 Bannockburn, IL 60015
 PHARMACY MANUAL WHP Health Initiatives, Inc. 2275 Half Day Road, Suite 250 Bannockburn, IL 60015 Welcome WHP Health Initiatives, Inc. ( WHI ) is pleased to welcome you to our network of participating pharmacies.
PHARMACY MANUAL WHP Health Initiatives, Inc. 2275 Half Day Road, Suite 250 Bannockburn, IL 60015 Welcome WHP Health Initiatives, Inc. ( WHI ) is pleased to welcome you to our network of participating pharmacies.
Frequently Asked Questions
 This document provides answers to some common questions regarding the reporting of controlled substances to the New Hampshire (PDMP) database. A list of the questions answered in this document is provided
This document provides answers to some common questions regarding the reporting of controlled substances to the New Hampshire (PDMP) database. A list of the questions answered in this document is provided
POS Helpdesk Operational Procedure
 POS Helpdesk Operational Procedure Purpose: To describe the tools and scenarios associated with IME Pharmacy Point of Sale (POS) Help Desk operations. Identification of Roles: Pharmacy Point of Sale (POS)
POS Helpdesk Operational Procedure Purpose: To describe the tools and scenarios associated with IME Pharmacy Point of Sale (POS) Help Desk operations. Identification of Roles: Pharmacy Point of Sale (POS)
Welcome to. Prompt Fulfillment and Delivery 1-844-CUBIST-CARES (1-844-282-4782)
 Welcome to When you prescribe SIVEXTRO (tedizolid phosphate) to your patients, our goal is to ensure they have access. That is why AccessSIVEXTRO is committed to helping eligible patients so they can receive
Welcome to When you prescribe SIVEXTRO (tedizolid phosphate) to your patients, our goal is to ensure they have access. That is why AccessSIVEXTRO is committed to helping eligible patients so they can receive
Medicare Part D Plan Finder instructions
 Medicare Part D Plan Finder instructions You can use these instructions to help you find the lowest cost Part D plans (both stand alone and Advantage plans) for the prescription drugs that you take. You
Medicare Part D Plan Finder instructions You can use these instructions to help you find the lowest cost Part D plans (both stand alone and Advantage plans) for the prescription drugs that you take. You
Bureau of Justice Assistance Harold Rogers Prescription Drug Monitoring Program National Meeting 9/23/2014
 Bureau of Justice Assistance Harold Rogers Prescription Drug Monitoring Program National Meeting 9/23/2014 Jeanne Tuttle, R.Ph. National Pharmacist Program Managers Pharmacy Benefits Management Services
Bureau of Justice Assistance Harold Rogers Prescription Drug Monitoring Program National Meeting 9/23/2014 Jeanne Tuttle, R.Ph. National Pharmacist Program Managers Pharmacy Benefits Management Services
Inhaler Technique Check
 Protocol October 2015 Version 1.3 Table of Contents Service Information... 2 Service objective... 2 Clinical service overview... 2 Documentation... 3 Staff Roles... 3 Facilities to support the program...
Protocol October 2015 Version 1.3 Table of Contents Service Information... 2 Service objective... 2 Clinical service overview... 2 Documentation... 3 Staff Roles... 3 Facilities to support the program...
Massachusetts Substance Abuse Policy and Practices. Senator Jennifer L. Flanagan Massachusetts Worcester and Middlesex District
 Massachusetts Substance Abuse Policy and Practices Senator Jennifer L. Flanagan Massachusetts Worcester and Middlesex District November 2014 Substance Abuse and Addiction National and State opioid abuse
Massachusetts Substance Abuse Policy and Practices Senator Jennifer L. Flanagan Massachusetts Worcester and Middlesex District November 2014 Substance Abuse and Addiction National and State opioid abuse
NEW JERSEY PRESCRIPTION MONITORING PROGRAM (NJPMP)
 NEW JERSEY PRESCRIPTION MONITORING PROGRAM (NJPMP) DATA COLLECTION MANUAL Effective Date: January 1, 2015 Optimum Technology, Inc. Contact Information Phone: 866-683-2476 NJRxReport@otech.com Fax: 866-282-7076
NEW JERSEY PRESCRIPTION MONITORING PROGRAM (NJPMP) DATA COLLECTION MANUAL Effective Date: January 1, 2015 Optimum Technology, Inc. Contact Information Phone: 866-683-2476 NJRxReport@otech.com Fax: 866-282-7076
Prescription Drug Monitoring Program Center of Excellence at Brandeis
 Prescription Drug Monitoring Program Center of Excellence at Brandeis Notes from the Field NF 4.1 Using PDMPs to Improve Medical Care: Washington State s Data Sharing Initiative with Medicaid and Workers
Prescription Drug Monitoring Program Center of Excellence at Brandeis Notes from the Field NF 4.1 Using PDMPs to Improve Medical Care: Washington State s Data Sharing Initiative with Medicaid and Workers
2015 REPORT Steven W. Schierholt, Esq. Executive Director www.pharmacy.ohio.gov
 OHIO AUTOMATED RX REPORTING SYSTEM 2015 REPORT Steven W. Schierholt, Esq. Executive Director www.pharmacy.ohio.gov OHIO AUTOMATED RX REPORTING SYSTEM What is OARRS? To address the growing misuse and diversion
OHIO AUTOMATED RX REPORTING SYSTEM 2015 REPORT Steven W. Schierholt, Esq. Executive Director www.pharmacy.ohio.gov OHIO AUTOMATED RX REPORTING SYSTEM What is OARRS? To address the growing misuse and diversion
Investigational Drugs: Investigational Drugs and Biologics
 : I. PURPOSE The purpose of this policy is to establish procedures for the proper control, storage, use and handling of investigational drugs and biologics to ensure that adequate safeguards are in place
: I. PURPOSE The purpose of this policy is to establish procedures for the proper control, storage, use and handling of investigational drugs and biologics to ensure that adequate safeguards are in place
LOUISIANA PRESCRIPTION MONITORING PROGRAM DATA COLLECTION MANUAL
 LOUISIANA PRESCRIPTION MONITORING PROGRAM DATA COLLECTION MANUAL Effective Date: January 1, 2015 Optimum Technology, Inc. Contact Information Phone: 866-683-2476 LABPPMP@otech.com Fax: 866-282-7076 Table
LOUISIANA PRESCRIPTION MONITORING PROGRAM DATA COLLECTION MANUAL Effective Date: January 1, 2015 Optimum Technology, Inc. Contact Information Phone: 866-683-2476 LABPPMP@otech.com Fax: 866-282-7076 Table
247 CMR: BOARD OF REGISTRATION IN PHARMACY
 247 CMR 9.00: CODE OF PROFESSIONAL CONDUCT; PROFESSIONAL STANDARDS FOR REGISTERED PHARMACISTS, PHARMACIES AND PHARMACY DEPART- MENTS Section 9.01: Code of Professional Conduct for Registered Pharmacists,
247 CMR 9.00: CODE OF PROFESSIONAL CONDUCT; PROFESSIONAL STANDARDS FOR REGISTERED PHARMACISTS, PHARMACIES AND PHARMACY DEPART- MENTS Section 9.01: Code of Professional Conduct for Registered Pharmacists,
An Overview of Risk Evaluation and Mitigation Strategy (REMS): Elements Related to Medication Use and Dispensing
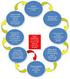 INDIANA PHARMACISTS ALLIANCE (IPA) CONTINUING PHARMACY EDUCATION (CPE) 2012 Article #9 An Overview of Risk Evaluation and Mitigation Strategy (REMS): Elements Related to Medication Use and Dispensing Authors:
INDIANA PHARMACISTS ALLIANCE (IPA) CONTINUING PHARMACY EDUCATION (CPE) 2012 Article #9 An Overview of Risk Evaluation and Mitigation Strategy (REMS): Elements Related to Medication Use and Dispensing Authors:
WellDyneRx Mail Service General Questions and Answers
 WellDyneRx Mail Service General Questions and Answers I. Location/ Hours of Operation 1. Where is WellDyneRx Mail Pharmacy located? WellDyneRx mail pharmacy has two locations: 1) Centennial, CO, a suburb
WellDyneRx Mail Service General Questions and Answers I. Location/ Hours of Operation 1. Where is WellDyneRx Mail Pharmacy located? WellDyneRx mail pharmacy has two locations: 1) Centennial, CO, a suburb
Printed copies are for reference only. Please refer to the electronic copy of this policy for the latest version.
 340B Pharmacy Audit Policy Version: 1.4 Date Created: 01/05/2015 Date Approved: 02/18/2015 Printed copies are for reference only. Please refer to the electronic copy of this policy for the latest version.
340B Pharmacy Audit Policy Version: 1.4 Date Created: 01/05/2015 Date Approved: 02/18/2015 Printed copies are for reference only. Please refer to the electronic copy of this policy for the latest version.
11/26/2012. Implementation of Florida s PDMP. Disclosure
 Implementation of Florida s PDMP Rebecca R. Poston Program Manager December 1, 2012 Disclosure I have no vested interest in or affiliation with any corporate organizations offering financial support or
Implementation of Florida s PDMP Rebecca R. Poston Program Manager December 1, 2012 Disclosure I have no vested interest in or affiliation with any corporate organizations offering financial support or
Frequently Asked Questions
 This document provides answers to some common questions regarding the reporting of controlled substances to the Hawaii (HI PDMP) database. A list of the questions answered in this document is provided
This document provides answers to some common questions regarding the reporting of controlled substances to the Hawaii (HI PDMP) database. A list of the questions answered in this document is provided
Medicare Part D Plan Finder instructions
 Medicare Part D Plan Finder instructions These instructions may help you find the lowest cost Part D coverage (in both stand alone and Advantage plans). You may need these detailed instructions to get
Medicare Part D Plan Finder instructions These instructions may help you find the lowest cost Part D coverage (in both stand alone and Advantage plans). You may need these detailed instructions to get
PHARMACY. billing module
 PHARMACY billing module Pharmacy Billing Module Coding...2 Basic Rules...2 Before You Begin...2 Reimbursement and Copayment...3 Point of Sale Billing...4 Billing for Split Prescriptions...5 Billing of
PHARMACY billing module Pharmacy Billing Module Coding...2 Basic Rules...2 Before You Begin...2 Reimbursement and Copayment...3 Point of Sale Billing...4 Billing for Split Prescriptions...5 Billing of
U.S. Bureau of Labor Statistics. Pharmacy Tech
 From the: U.S. Bureau of Labor Statistics Pharmacy Tech Pharmacy technicians fill prescriptions and check inventory. Pharmacy technicians help licensed pharmacists dispense prescription medication. They
From the: U.S. Bureau of Labor Statistics Pharmacy Tech Pharmacy technicians fill prescriptions and check inventory. Pharmacy technicians help licensed pharmacists dispense prescription medication. They
E-Control Medicine Prescription Manual
 E-Control Medicine Prescription Manual 1 P a g e Contents 1. Introduction... 3 1.1 Purpose & Objective... 3 1.2 Assumptions... 3 2. Login to the System... 3 3. Account Registration... 5 3.1 Register a
E-Control Medicine Prescription Manual 1 P a g e Contents 1. Introduction... 3 1.1 Purpose & Objective... 3 1.2 Assumptions... 3 2. Login to the System... 3 3. Account Registration... 5 3.1 Register a
Health Literacy & Medication Safety
 Health Literacy & Medication Safety Can We Confuse Patients Less? Michael S. Wolf, MA MPH PhD Assistant Professor and Director Health Literacy and Learning Program (HeLP) Division of General Internal Medicine
Health Literacy & Medication Safety Can We Confuse Patients Less? Michael S. Wolf, MA MPH PhD Assistant Professor and Director Health Literacy and Learning Program (HeLP) Division of General Internal Medicine
What Every Practitioner Needs to Know About Controlled Substance Prescribing
 What Every Practitioner Needs to Know About Controlled Substance Prescribing New York State Department of Health Use of Controlled Substances Controlled substances can be effective in the treatment of
What Every Practitioner Needs to Know About Controlled Substance Prescribing New York State Department of Health Use of Controlled Substances Controlled substances can be effective in the treatment of
Alaska Prescription Drug Monitoring Program
 Alaska 1. What is a PDMP? PDMP stands for. This is a commonly used term for the programs implemented by various states to monitor the dispensing of controlled substances within their borders. For this
Alaska 1. What is a PDMP? PDMP stands for. This is a commonly used term for the programs implemented by various states to monitor the dispensing of controlled substances within their borders. For this
ExCPT Certified Pharmacy Technician (CPhT) Detailed Test Plan* 100 scored items, 20 pretest items Exam time: 2 hours 10 minutes
 ExCPT Certified Pharmacy Technician (CPhT) Detailed Test Plan* 100 scored items, 20 pretest items Exam time: 2 hours 10 minutes # scored items 1. Regulations and Pharmacy Duties 35 A. Overview of technician
ExCPT Certified Pharmacy Technician (CPhT) Detailed Test Plan* 100 scored items, 20 pretest items Exam time: 2 hours 10 minutes # scored items 1. Regulations and Pharmacy Duties 35 A. Overview of technician
Controlled II Substance Rules & Regulations. Controlled II Substance Rules & Regulations
 North Carolina Board of Pharmacy Controlled Substance Pocketcard A brief summary of pertinent rules and regulations impacting the practice of pharmacy ~ Controlled II Substance Controlled II Substance
North Carolina Board of Pharmacy Controlled Substance Pocketcard A brief summary of pertinent rules and regulations impacting the practice of pharmacy ~ Controlled II Substance Controlled II Substance
Medicare Part D Hospice Care Hospice Information for Medicare Part D Plans
 P. O. Box 31397 Tampa, FL 33631 Medicare Part D Hospice Care Hospice Information for Medicare Part D Plans Table of Contents Introduction...2 Background...2 Purpose...2 1) To document that a drug is unrelated
P. O. Box 31397 Tampa, FL 33631 Medicare Part D Hospice Care Hospice Information for Medicare Part D Plans Table of Contents Introduction...2 Background...2 Purpose...2 1) To document that a drug is unrelated
Prescription Drug Information for Patients: History
 Prescription Drug Information for Patients: History 1500s: Ethical Statutes of Royal College of Physicians Let no physician teach the people about medicines or even tell them the names of the medicines,
Prescription Drug Information for Patients: History 1500s: Ethical Statutes of Royal College of Physicians Let no physician teach the people about medicines or even tell them the names of the medicines,
340B Drug Discount Program Improving Compliance to Protect Savings and Be Audit Ready. Suzanne Herzog Founding Director Rx X Consulting
 340B Drug Discount Program Improving Compliance to Protect Savings and Be Audit Ready Suzanne Herzog Founding Director Rx X Consulting What is 340B? 340B Overview A drug discount program that allows covered
340B Drug Discount Program Improving Compliance to Protect Savings and Be Audit Ready Suzanne Herzog Founding Director Rx X Consulting What is 340B? 340B Overview A drug discount program that allows covered
Why should I report issues directly through my pharmacy management system vendor and not Surescripts?
 E-Prescribing FAQ Table of Contents p.4 How do I work with my pharmacy management system vendor to enable my pharmacy for e-prescribing via the Surescripts network? Why should I report issues directly
E-Prescribing FAQ Table of Contents p.4 How do I work with my pharmacy management system vendor to enable my pharmacy for e-prescribing via the Surescripts network? Why should I report issues directly
September 13, 2013. Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm.1061 Rockville, MD 20852
 September 13, 2013 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm.1061 Rockville, MD 20852 [Submitted electronically to www.regulations.gov] Re: Standardizing
September 13, 2013 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm.1061 Rockville, MD 20852 [Submitted electronically to www.regulations.gov] Re: Standardizing
Medicare Plan Finder - General Tips
 Medicare Plan Finder - General Tips 1. For security purposes, your Medicare Plan Finder session will time out after 30 minutes of inactivity. However, you have the option to extend your session if you
Medicare Plan Finder - General Tips 1. For security purposes, your Medicare Plan Finder session will time out after 30 minutes of inactivity. However, you have the option to extend your session if you
Instructions for Completing the Initial System Assessment for Upcoming HIPAA Changes Due Date: (specify date)
 for Completing the Initial System Assessment for Upcoming HIPAA Changes Due Date: (specify date) Some major changes to the HIPAA federally mandated regulations are forthcoming. Therefore, it is essential
for Completing the Initial System Assessment for Upcoming HIPAA Changes Due Date: (specify date) Some major changes to the HIPAA federally mandated regulations are forthcoming. Therefore, it is essential
INSTRUCTIONS FOR USE: OA-RX
 P a g e 1 INSTRUCTIONS FOR USE: OA-RX Welcome to Office Ally s Electronic Prescription module, called OA-Rx. This web-based program has been designed to integrate into Office Ally s Practice Mate and EHR
P a g e 1 INSTRUCTIONS FOR USE: OA-RX Welcome to Office Ally s Electronic Prescription module, called OA-Rx. This web-based program has been designed to integrate into Office Ally s Practice Mate and EHR
DEPARTMENT OF LICENSING AND REGULATORY AFFAIRS DIRECTOR S OFFICE PHARMACY PROGRAM FOR UTILIZATION OF UNUSED PRESCRIPTION DRUGS
 DEPARTMENT OF LICENSING AND REGULATORY AFFAIRS DIRECTOR S OFFICE PHARMACY PROGRAM FOR UTILIZATION OF UNUSED PRESCRIPTION DRUGS (By authority conferred on the director of the department of licensing and
DEPARTMENT OF LICENSING AND REGULATORY AFFAIRS DIRECTOR S OFFICE PHARMACY PROGRAM FOR UTILIZATION OF UNUSED PRESCRIPTION DRUGS (By authority conferred on the director of the department of licensing and
DC DEPARTMENT OF HEALTH Pharmaceutical Procurement and Distribution Pharmaceutical Warehouse. DC Health Care Safety Net ALLIANCE PROGRAM
 DC DEPARTMENT OF HEALTH Pharmaceutical Warehouse DC Health Care Safety Net ALLIANCE PROGRAM OPERATIONAL PROTOCOLS Operational protocols for the DC Health Care Alliance program through the DOH Pharmaceutical
DC DEPARTMENT OF HEALTH Pharmaceutical Warehouse DC Health Care Safety Net ALLIANCE PROGRAM OPERATIONAL PROTOCOLS Operational protocols for the DC Health Care Alliance program through the DOH Pharmaceutical
Pharmacy Handbook. Understanding Your Prescription Benefit
 Pharmacy Handbook Understanding Your Prescription Benefit 1 Welcome to Your Prescription Drug Plan! Health Republic Insurance of New York has partnered with US Script to manage your prescription drug benefits.
Pharmacy Handbook Understanding Your Prescription Benefit 1 Welcome to Your Prescription Drug Plan! Health Republic Insurance of New York has partnered with US Script to manage your prescription drug benefits.
NOTE: In the event that the seal is accidentally broken, the narcotic may be wasted via narcotic wastage procedures.
 HOSPITAL NAME INSTITUTIONAL POLICY AND PROCEDURE (IPP) Department: Manual: Section: TITLE/DESCRIPTION POLICY NUMBER NARCOTICS AND CONTROLLED DRUGS EFFECTIVE DATE REVIEW DUE REPLACES NUMBER NO. OF PAGES
HOSPITAL NAME INSTITUTIONAL POLICY AND PROCEDURE (IPP) Department: Manual: Section: TITLE/DESCRIPTION POLICY NUMBER NARCOTICS AND CONTROLLED DRUGS EFFECTIVE DATE REVIEW DUE REPLACES NUMBER NO. OF PAGES
Opioid Prescribing Practices and Pain Management: Role of FDA Douglas C. Throckmorton, MD Deputy Director for Regulator Programs, CDER, FDA
 Opioid Prescribing Practices and Pain Management: Role of FDA Douglas C. Throckmorton, MD Deputy Director for Regulator Programs, CDER, FDA American Academy of Hospice and Palliative Care Medicine October,
Opioid Prescribing Practices and Pain Management: Role of FDA Douglas C. Throckmorton, MD Deputy Director for Regulator Programs, CDER, FDA American Academy of Hospice and Palliative Care Medicine October,
Medicare Advantage Plan Finder:
 Medicare Advantage Plan Finder: 1. Visit www.medicare.gov; scroll half-way down the page and on the left hand side under the large font Find doctors, providers, hospitals, plans & suppliers click on Find
Medicare Advantage Plan Finder: 1. Visit www.medicare.gov; scroll half-way down the page and on the left hand side under the large font Find doctors, providers, hospitals, plans & suppliers click on Find
MEETING OF THE DRUG SAFETY AND RISK MANAGEMENT ADVISORY COMMITTEE MEETING: RISKS AND BENEFITS OF HYDROCODONE COMBINATION ANALGESIC PRODUCTS
 MEETING OF THE DRUG SAFETY AND RISK MANAGEMENT ADVISORY COMMITTEE MEETING: RISKS AND BENEFITS OF HYDROCODONE COMBINATION ANALGESIC PRODUCTS David Gaugh, R.Ph. Senior Vice President for Sciences and Regulatory
MEETING OF THE DRUG SAFETY AND RISK MANAGEMENT ADVISORY COMMITTEE MEETING: RISKS AND BENEFITS OF HYDROCODONE COMBINATION ANALGESIC PRODUCTS David Gaugh, R.Ph. Senior Vice President for Sciences and Regulatory
A New Generation of Prescription Monitoring Programs: Best & Promising Practices
 A New Generation of Prescription Monitoring Programs: Best & Promising Practices National PDMP Meeting Washington, DC September 25, 2013 Presented by John L Eadie Why Is a New Generation of PDMPs Being
A New Generation of Prescription Monitoring Programs: Best & Promising Practices National PDMP Meeting Washington, DC September 25, 2013 Presented by John L Eadie Why Is a New Generation of PDMPs Being
Frequently Asked Questions (FAQs) Treatment Authorization Request (TAR) Restriction on Antipsychotic Medications for the 0-17 Population
 Frequently Asked Questions (FAQs) Treatment Authorization Request (TAR) Restriction on Antipsychotic Medications for the 0-17 Population Prescriber FAQs Update January 22, 2015 1. What information is needed
Frequently Asked Questions (FAQs) Treatment Authorization Request (TAR) Restriction on Antipsychotic Medications for the 0-17 Population Prescriber FAQs Update January 22, 2015 1. What information is needed
Blue Medicare Advantage
 Blue Medicare Advantage Part D Drugs in Part B Setting TransAct RX Questions and Answers www.transactrx.com Enrollment Questions 1. Is there a cost to enroll or to process claims through the portal? No:
Blue Medicare Advantage Part D Drugs in Part B Setting TransAct RX Questions and Answers www.transactrx.com Enrollment Questions 1. Is there a cost to enroll or to process claims through the portal? No:
Technical Assistance Guide. PDMP Suggested Practices to Ensure Pharmacy Compliance and Improve Data Integrity
 Technical Assistance Guide PDMP Suggested Practices to Ensure Pharmacy Compliance and Improve Data Integrity This project was supported by Grant No. 2011-PM-BX-K001 awarded by the Bureau of Justice Assistance.
Technical Assistance Guide PDMP Suggested Practices to Ensure Pharmacy Compliance and Improve Data Integrity This project was supported by Grant No. 2011-PM-BX-K001 awarded by the Bureau of Justice Assistance.
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE April 8, 2011 EFFECTIVE DATE April 8, 2011 MEDICAL ASSISTANCE BULLETIN NUMBER 03-11-01, 09-11-02, 14-11-01, 18-11-01 24-11-03, 27-11-02, 31-11-02, 33-11-02 SUBJECT Electronic Prescribing Internet-based
ISSUE DATE April 8, 2011 EFFECTIVE DATE April 8, 2011 MEDICAL ASSISTANCE BULLETIN NUMBER 03-11-01, 09-11-02, 14-11-01, 18-11-01 24-11-03, 27-11-02, 31-11-02, 33-11-02 SUBJECT Electronic Prescribing Internet-based
Co-Pay Assistance Program for CUBICIN (daptomycin for injection) for Intravenous Use Enrollment Form
 1. PATIENT INFORMATION Name Gender: o Male o Female Date of Birth: / / Address City State ZIP Email Home Phone Cell Phone Work Phone Alternate Contact Person (Optional) Alternate Phone Number (Optional)
1. PATIENT INFORMATION Name Gender: o Male o Female Date of Birth: / / Address City State ZIP Email Home Phone Cell Phone Work Phone Alternate Contact Person (Optional) Alternate Phone Number (Optional)
GUIDE FOR SORTING RX HISTORY REPORTS IN MICROSOFT EXCEL
 GUIDE FOR SORTING RX HISTORY REPORTS IN MICROSOFT EXCEL 1. Log in to your INSPECT WebCenter Account. 2. Go to the Requests tab on the left, and select New Request. 3. Select Practitioner from the drop-down
GUIDE FOR SORTING RX HISTORY REPORTS IN MICROSOFT EXCEL 1. Log in to your INSPECT WebCenter Account. 2. Go to the Requests tab on the left, and select New Request. 3. Select Practitioner from the drop-down
Pharmacy Operating Guidelines & Information
 Pharmacy Operating Guidelines & Information RxAMERICA PHARMACY BENEFIT MANAGEMENT Pharmacy Operating Guidelines & Information Table of Contents I. Quick Reference List...3 C. D. E. Important Phone Numbers...
Pharmacy Operating Guidelines & Information RxAMERICA PHARMACY BENEFIT MANAGEMENT Pharmacy Operating Guidelines & Information Table of Contents I. Quick Reference List...3 C. D. E. Important Phone Numbers...
Data Layouts and Formats
 Data Layouts and Formats Pharmacy, Dental, and Provider Files March 13, 2008 INSTITUTE FOR CHILD HEALTH POLICY 1 ENCOUNTERS SUBMISSION GUIDELINES 03/13//2008 Table of Contents 1. INTRODUCTION 3 2. GENERAL
Data Layouts and Formats Pharmacy, Dental, and Provider Files March 13, 2008 INSTITUTE FOR CHILD HEALTH POLICY 1 ENCOUNTERS SUBMISSION GUIDELINES 03/13//2008 Table of Contents 1. INTRODUCTION 3 2. GENERAL
eprescribe FAQs General Application FAQs
 Allscripts eprescribe eprescribe FAQs General Application FAQs What does eprescribe allow me to do? Based on Allscripts' award winning EMR technology, eprescribe enables physicians to improve patient care
Allscripts eprescribe eprescribe FAQs General Application FAQs What does eprescribe allow me to do? Based on Allscripts' award winning EMR technology, eprescribe enables physicians to improve patient care
Drug Supply Chain Security Act (Title II of the Drug Quality and Security Act) Overview and Implementation
 Drug Supply Chain Security Act (Title II of the Drug Quality and Security Act) Overview and Implementation Connie Jung, RPh, PhD U.S. Food and Drug Administration NACDS Total Store Expo August 24, 2014
Drug Supply Chain Security Act (Title II of the Drug Quality and Security Act) Overview and Implementation Connie Jung, RPh, PhD U.S. Food and Drug Administration NACDS Total Store Expo August 24, 2014
Enhance Controlled Substance Inventory Control While Improving Workflow Efficiency
 WHITE PAPER Enhance Controlled Substance Inventory Control While Improving Workflow Efficiency Most ambulatory pharmacies still use a manual process to record the receipt and disposition of controlled
WHITE PAPER Enhance Controlled Substance Inventory Control While Improving Workflow Efficiency Most ambulatory pharmacies still use a manual process to record the receipt and disposition of controlled
Massachusetts Department of Public Health (MDPH) Prescription Monitoring Program (MA PMP) and Drug Control Program (DCP) April 8, 2014
 MA PMP Pharmacy Data Entry and Data Submitter s FAQ Utilizing ASAP 4.2 Supplement to the MA PMP Pharmacy Data Entry and Data Submitter s Guide Utilizing ASAP 4.2 Massachusetts Department of Public Health
MA PMP Pharmacy Data Entry and Data Submitter s FAQ Utilizing ASAP 4.2 Supplement to the MA PMP Pharmacy Data Entry and Data Submitter s Guide Utilizing ASAP 4.2 Massachusetts Department of Public Health
Inventory Management
 Inventory Management Chapter Outline Inventory Management Inventory Systems Computer & Inventory Ordering Forms Stocking & Storing Inventory Management Inventory A listing of medication of the goods or
Inventory Management Chapter Outline Inventory Management Inventory Systems Computer & Inventory Ordering Forms Stocking & Storing Inventory Management Inventory A listing of medication of the goods or
These are just some of the eligibility requirements meeting these criteria does not guarantee acceptance.
 BARACLUDE PATIENT ASSISTANCE PROGRAM The Baraclude Patient Assistance Program is designed to provide free medication to qualifying patients who do not have prescription drug coverage and are having a hard
BARACLUDE PATIENT ASSISTANCE PROGRAM The Baraclude Patient Assistance Program is designed to provide free medication to qualifying patients who do not have prescription drug coverage and are having a hard
2014-15 EMPLOYEE BENEFITS GUIDE
 2014-15 EMPLOYEE BENEFITS GUIDE ELIGIBILITY All People 2.0 employees are eligible to enroll in The American Worker program within 30 days of your first pay check. People 2.0 offers a variety of affordable
2014-15 EMPLOYEE BENEFITS GUIDE ELIGIBILITY All People 2.0 employees are eligible to enroll in The American Worker program within 30 days of your first pay check. People 2.0 offers a variety of affordable
Electronic Signature Guidance
 National Council for Prescription Drug Programs White Paper Electronic Signature Guidance Version 1.0 February 2014 This document provides clarification and guidance to the industry for the use of electronic
National Council for Prescription Drug Programs White Paper Electronic Signature Guidance Version 1.0 February 2014 This document provides clarification and guidance to the industry for the use of electronic
WHITE PAPER The Impact of Rising Generic Drug Prices on the U.S. Drug Supply Chain
 WHITE PAPER The Impact of Rising Generic Drug Prices on the U.S. Drug Supply Chain Over the past two years, the pharmacy industry has seen unprecedented increases in the prices of generic drugs, causing
WHITE PAPER The Impact of Rising Generic Drug Prices on the U.S. Drug Supply Chain Over the past two years, the pharmacy industry has seen unprecedented increases in the prices of generic drugs, causing
Enroll in Interconnect
 Enroll in Interconnect Enrollment Form Checklist In this packet, you will find all of the necessary forms to enroll your patients in Interconnect and give them access to a full suite of support services
Enroll in Interconnect Enrollment Form Checklist In this packet, you will find all of the necessary forms to enroll your patients in Interconnect and give them access to a full suite of support services
RULE. The Administration of Medication in Louisiana Public Schools
 RULE The Administration of Medication in Louisiana Public Schools Developed in 1994 by The Louisiana State Board of Elementary and Secondary Education and The Louisiana State Board of Nursing Amendments
RULE The Administration of Medication in Louisiana Public Schools Developed in 1994 by The Louisiana State Board of Elementary and Secondary Education and The Louisiana State Board of Nursing Amendments
PHARMACEUTICAL MANAGEMENT PROCEDURES
 PHARMACEUTICAL MANAGEMENT PROCEDURES THE FORMULARY The purpose of Coventry Health Care s formulary is to encourage use of the most cost-effective drugs. The formulary is necessary because the cost of prescription
PHARMACEUTICAL MANAGEMENT PROCEDURES THE FORMULARY The purpose of Coventry Health Care s formulary is to encourage use of the most cost-effective drugs. The formulary is necessary because the cost of prescription
DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only.
 Guidance for Industry Format and Content of Proposed Risk Evaluation and Mitigation Strategies (REMS), REMS Assessments, and Proposed REMS Modifications DRAFT GUIDANCE This guidance document is being distributed
Guidance for Industry Format and Content of Proposed Risk Evaluation and Mitigation Strategies (REMS), REMS Assessments, and Proposed REMS Modifications DRAFT GUIDANCE This guidance document is being distributed
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE September 4, 2015 SUBJECT EFFECTIVE DATE September 9, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Opiate Dependence Treatments, Oral Buprenorphine Agents - Pharmacy
ISSUE DATE September 4, 2015 SUBJECT EFFECTIVE DATE September 9, 2015 MEDICAL ASSISTANCE BULLETIN NUMBER *See below BY Prior Authorization of Opiate Dependence Treatments, Oral Buprenorphine Agents - Pharmacy
Cultural Vistas Inbound Frequently Asked Claim Questions (FAQ) (For New J-1 Visa Holders in the U.S.A. as of 5-1-2012)
 Specializing in international health insurance for groups. TOLL FREE: 866-433-7462 (within USA) Phone: 607-272-2707 (collect from overseas) FAX: 607-272-2703 EMAIL: claims@iees.com WEB: www.iees.com PO
Specializing in international health insurance for groups. TOLL FREE: 866-433-7462 (within USA) Phone: 607-272-2707 (collect from overseas) FAX: 607-272-2703 EMAIL: claims@iees.com WEB: www.iees.com PO
Health Care Job Information Sheet #10. Pharmacy
 Health Care Job Information Sheet #10 Pharmacy A. Occupations A. Occupations 1) Pharmacist 2) Pharmacy Technician/ Assistant B. Labour Market Prospects C. ITPs in the Field D. Links 1) Pharmacist Regulated
Health Care Job Information Sheet #10 Pharmacy A. Occupations A. Occupations 1) Pharmacist 2) Pharmacy Technician/ Assistant B. Labour Market Prospects C. ITPs in the Field D. Links 1) Pharmacist Regulated
