Phy 231 Sp 03 Homework #11 Page 1 of 4
|
|
|
- Sharon Morrison
- 7 years ago
- Views:
Transcription
1 Phy 231 Sp 03 Homework #11 Page 1 of A) About how long would it take a 1 kw heating element to melt 1 kg of ice at 0 o C? (1 cal = 4.2 J; L m (ice) = 80 cal/g.) (a) 4.0 s (b) 36 s (c) 6 min (d) 1.0 hr (e) 10 hr 11-2A) How high must a 75.0 kg person climb to work off the equivalent of a large piece of chocolate cake rated at 500 (food) Calories? 1 (food) Calorie = 10 3 calories. (a) 2.8 m (b) 28 m (c) 280 m (d) 2,800 m (e) 28,000 m 11-3A) 1 kcal of heat is added to 10 g of ice initially at -5 o C. If none of the heat is lost to the surroundings, about what should be the final temperature of the system? (c(ice) = 0.5 cal/g o C; L m (ice) = 80 cal/g; c(h 2 0) = 1 cal/ g o C.) (a) 2 o C (b) 18 o C (c) 80 o C (d) 100 o C (e) None of these is close. 11-4A) A 100 gm block of copper, c(cu)= 0.1 cal/gm o C, initially at 100 o C, is quickly submerged in a tank containing 30 gm of water, c(h 2 0)= 1 cal/g o C initially at 0 o C. Neglect the heat capacity of the tank walls, assume that no heat leaks out the sides of the tank, and neglect any work due to thermal expansion. About what is the equilibrium temperature of the water plus block? (a) 50 o C (b) 25 o C (c) 2.5 o C (d) 75 o C (e) None of these. 11-5A) The specific heat of Aluminum (Al) is more than twice that of copper (Cu). Equal mass blocks of Al and Cu, both initially at 0 o C, are each dropped into identical calorimeters filled with water at 60 o C. When equilibrium is reached, (a) the temperature of the Cu is higher. (b) the temperature of the Al is higher. (c) the temperatures of the Cu and Al are the same. (d) which temperature is higher depends upon the masses of the blocks. (e) Yo u don t have enough information to tell. 11-6A) A 100 g cube of ice at 0 o C is dropped into 1.0 kg of water that is initially at 80 o C. If no heat flows into our out of the water-ice system, what is the final temperature of the system after the ice has melted?? Take L m (ice) = 80 cal/g and c(h 2 0) = 1 cal/g o C. (a) 6.5 o C (b) 80 o C (c) 85 o C (d) 0 o C (e) 65 o C 11-7A) An average person requires an energy per day of about 2500 Cal (1 (Food) Cal = 1000 usual cal). About how many Watts of continuous power generation does this energy/day correspond to? (a) 120 (b) 12 (c) 1.2 (d) 1200 (e) None of these is close. 11-8A) If the surface temperature of the Sun were reduced by half, about how much smaller would be the energy radiated by the sun? (a) ½ (b) ¼ (c) 1/8 (d) 1/16 (e) 1/ A) The thermal conductivities of glass (k = 0.84) and wood (0.10) and dry air (0.02) differ substantially from each other. The ratios of heat flow for wood (w) and glass (g) to that for dry air (da), through a wall of fixed length L, A, and T should be: H g /H da and H w /H da = (a) 42, 5 (b) 5, 42 (c) 0.024, 0.2 (d) 0.017,.002 (e) None of these is close A) About what is the rate of heat flow in J/s through a Cu block of length 8 cm and cross-sectional area 15 cm 2 with a temperature difference of 30 o C across the block? Take k(cu) = 400 J/s m o C. (a) 2300 (b) 230 (c) 23 (d) 2.3 (e) A) Which one of the following statements is WRONG? (a) Heat is energy that flows between a system and its environment by virtue of a temperature difference that exists between them. (b) Units that can be used for heat include cal, BTU, and J. (c) Most metals conduct heat better than wood does. (d) Adding a small amount of heat to any system always causes the temperature of that system to rise. (e) Dry air is a very good insulator, if it is constrained so that it can t move and thereby convect heat A) In a room at a uniform comfortable temperature, metallic objects normally feel colder to the touch than do wooden objects. The primary reason for this occurance is: (a) A given mass of wood contains more heat than the same mass of metal. (b) Heat tends to flow from metal to wood. (c) Metal conducts heat better than wood does. (d) The equilibrium temperature of metal is lower than that of wood. (e) The organic human body resembles wood more than it resembles metal.
2 Phy 231 Sp 03 Homework #11 Page 2 of A) Which one of the following statements about heat transport involving conduction, convection, or radiation, is WRONG? (a) The filament in an incandescent light bulb gives off most of its energy by radiation. (b) The heat transfer through a single pane of window glass occurs mostly by conduction. (c) Atmospheric convection has a strong effect on global climate patterns. (d) A furnace blower produces forced convection of the air in a room. (e) Energy from the Sun reaches us mostly by convection.
3 Phy 231 Sp 03 Homework #11 Page 3 of B) About how long would it take a 1 kw heating element to boil 1 kg of water at 100 o C? (1 cal = 4.2 J; L v (H 2 0) = 540 cal/g.) (a) 40 min (b) 4 min (c) 7 hr (d) 70 hr (e) 3 s 11-2B) About how many 10 kg (22 lb) weights must a 75.0 kg person lift onto a 1 m high table to work off a large piece of chocolate cake rated at 500 (food) Calories? 1 (food) Calorie = 10 3 calories. (a) 210 (b) 21,000 (c) 2,100 (d) 21 (e) 210, B) About how many calories of heat are needed to change a 40 g ice cube from ice at 10 o C to steam at 110 o C? Take c(ice) = 0.5 cal/g o C, L m (ice) = 80 cal/g, c(h 2 0) = 1 cal/g o C, and L b (H 2 O) = 540 cal/g. (a) 2900 (b) 290 (c) 29,000 (d) 290,000 (e) B) About what mass of water at 25.0 o C must be allowed to come to thermal equilibrium with a 3.00 kg gold bar initia lly at 100 o C to reach a final equilibrium temperature of 50.0 o C? [Use c(au) = 0.03 cal/g o C, c(water) = 1 cal/g o C, and take no heat lost or gained.] (a) 0.18 g (b) 1800 g (c) 1.8 g (d) 18 g (e) 180 g 11-5B) The specific heat of Go ld (Au) is about one-third that of copper (Cu). Equal mass blocks of Au and Cu, both initially at 0 o C, are each dropped into identical calorimeters filled with water at 60 o C. When equilibrium is reached, (a) the temperature of the Cu is higher. (b) the temperature of the Au is higher. (c) the temperatures of the Cu and Au are the same. (d) which temperature is higher depends upon the masses of the blocks. (e) You don t have enough information to tell. 11-6B) If no heat flows into or out of the coffee-ice system, about how many 100 g ice cubes initially at 0 o C does it take to cool a 400 g glass of coffee (which is essentially just water) from an initial temperature of 100 o C to an equilibrium temperature of 0 o C and thereby make ice coffee? Take L m (ice) = 80 cal/g and c(h 2 0) = 1 cal/g o C. (a) 5 (b) 4 (c) 3 (d) 6 (e) You don t have enough information to tell. 11-7B) Each of 50 people sitting in a room puts out about 120 W of heat. About how many Megacal/hr does this correspond to? (a) 0.05 (b) 0.5 (c) 5 (d) 50 (e) B) Star A has twice the radius and twice the absolute temperature of start B. About what is the ratio of the power radiated by star A to that radiated by start B? (a) 4 (b) 8 (c) 16 (d) 32 (e) B) The thermal conductivities of glass (k = 0.84) and wood (0.10) and dry air (0.02) differ substantially from each other. The ratios of heat flow for dry air (da) and wood (w) to that for glass (g), through a wall of fixed length L, A, and T should be: H da /H g and H w /H g = (a) 42, 5 (b) 5, 42 (c) 0.024, 0.12 (d) 0.017,.002 (e) None of these is close B) What is the rate of heat flow in J/s through a wooden block of length 8 cm and cross-sectional area 15 cm 2 with a temperature difference of 30 o C across the block? Take k(wood) = 0.1 J/s m o C. (a) 5.6 (b) 5.6 x 10-3 (c) 56 (d) 5.6 x 10-2 (e) 5.6 x B) Which one of the following statements is WRONG? (a) Heat is energy that flows between a system and its environment by virtue of a temperature difference that exists between them. (b) Units that can be used for heat include cal, BTU, and J. (c) Most metals conduct heat less well than wood does. (d) Adding a small amount of heat to any system need not always cause the temperature of that system to rise. (e) Dry air is a very good insulator, if it is constrained so that it can t move and thereby convect heat B) Your Mom tells you not to stick your tongue on a freezing cold metal pole. What is the main reason why you shouldn t. (a) Metals generally have small heat capacities. (b) Heat tends to flow from the pole to your tongue. (c) The pole will taste bad. (d) Metals conduct heat away very well, so the water on your tongue could freeze and stick your tongue to the pole. (e) Mom is never wrong.
4 Phy 231 Sp 03 Homework #11 Page 4 of B) Which one of the following statements about heat transport involving conduction, convection, or radiation, is WRONG? (a) The filament in an incandescent light bulb gives off most of its energy by radiation. (b) The heat transfer through a single pane of window glass occurs mostly by convection. (c) Atmospheric convection has a strong effect on global climate patterns. (d) A furnace blower produces forced convection of the air in a room. (e) Energy from the Sun reaches us mostly by radiation.
5 11-1A) c 2A) d 3A) b 4A) b 5A) a 6A) e 7A) a 8A) d 9A) a 10A) b 11A) d 12A) c 13A) e 11-1B) a 2B) b 3B) c 4B) e 5B) b 6B) a 7B) c 8B) e 9B) c 10B) d 11B) c 12B) d 13B) b
Chapter 4: Transfer of Thermal Energy
 Chapter 4: Transfer of Thermal Energy Goals of Period 4 Section 4.1: To define temperature and thermal energy Section 4.2: To discuss three methods of thermal energy transfer. Section 4.3: To describe
Chapter 4: Transfer of Thermal Energy Goals of Period 4 Section 4.1: To define temperature and thermal energy Section 4.2: To discuss three methods of thermal energy transfer. Section 4.3: To describe
Phys222 W11 Quiz 1: Chapters 19-21 Keys. Name:
 Name:. In order for two objects to have the same temperature, they must a. be in thermal equilibrium.
Name:. In order for two objects to have the same temperature, they must a. be in thermal equilibrium.
Chapter 10: Temperature and Heat
 Chapter 10: Temperature and Heat 1. The temperature of a substance is A. proportional to the average kinetic energy of the molecules in a substance. B. equal to the kinetic energy of the fastest moving
Chapter 10: Temperature and Heat 1. The temperature of a substance is A. proportional to the average kinetic energy of the molecules in a substance. B. equal to the kinetic energy of the fastest moving
UNIT 6a TEST REVIEW. 1. A weather instrument is shown below.
 UNIT 6a TEST REVIEW 1. A weather instrument is shown below. Which weather variable is measured by this instrument? 1) wind speed 3) cloud cover 2) precipitation 4) air pressure 2. Which weather station
UNIT 6a TEST REVIEW 1. A weather instrument is shown below. Which weather variable is measured by this instrument? 1) wind speed 3) cloud cover 2) precipitation 4) air pressure 2. Which weather station
Chapter 18 Temperature, Heat, and the First Law of Thermodynamics. Problems: 8, 11, 13, 17, 21, 27, 29, 37, 39, 41, 47, 51, 57
 Chapter 18 Temperature, Heat, and the First Law of Thermodynamics Problems: 8, 11, 13, 17, 21, 27, 29, 37, 39, 41, 47, 51, 57 Thermodynamics study and application of thermal energy temperature quantity
Chapter 18 Temperature, Heat, and the First Law of Thermodynamics Problems: 8, 11, 13, 17, 21, 27, 29, 37, 39, 41, 47, 51, 57 Thermodynamics study and application of thermal energy temperature quantity
Answer, Key Homework 6 David McIntyre 1
 Answer, Key Homework 6 David McIntyre 1 This print-out should have 0 questions, check that it is complete. Multiple-choice questions may continue on the next column or page: find all choices before making
Answer, Key Homework 6 David McIntyre 1 This print-out should have 0 questions, check that it is complete. Multiple-choice questions may continue on the next column or page: find all choices before making
Melting ice Student sheet
 Melting ice Student sheet Predict Which ice cube will melt first? Observe Describe what you saw happen. Why? (Give a scientific explanation) Questions to think about: Why does ice melt? Why might one ice
Melting ice Student sheet Predict Which ice cube will melt first? Observe Describe what you saw happen. Why? (Give a scientific explanation) Questions to think about: Why does ice melt? Why might one ice
Learning outcomes. Students will be able to:
 Learning structure of the lesson The big picture This lesson is designed to exemplify an argumentation approach to practical work, using a predict-observe-explain framework. Students often think that some
Learning structure of the lesson The big picture This lesson is designed to exemplify an argumentation approach to practical work, using a predict-observe-explain framework. Students often think that some
Chapter 10 Temperature and Heat
 Chapter 10 Temperature and Heat GOALS When you have mastered the contents of this chapter, you will be able to achieve the following goals: Definitions Define each of the following terms, and use it an
Chapter 10 Temperature and Heat GOALS When you have mastered the contents of this chapter, you will be able to achieve the following goals: Definitions Define each of the following terms, and use it an
Chapter 10 Temperature and Heat
 Chapter 10 Temperature and Heat What are temperature and heat? Are they the same? What causes heat? What Is Temperature? How do we measure temperature? What are we actually measuring? Temperature and Its
Chapter 10 Temperature and Heat What are temperature and heat? Are they the same? What causes heat? What Is Temperature? How do we measure temperature? What are we actually measuring? Temperature and Its
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Sample Mid-Term 3 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) If you double the frequency of a vibrating object, its period A) is quartered.
Sample Mid-Term 3 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) If you double the frequency of a vibrating object, its period A) is quartered.
What Is Heat? What Is Heat?
 What Is Heat? Paul shivered inside the wood cabin. It was cold outside, and inside the cabin it wasn t much warmer. Paul could hear the rain beating down on the roof. Every few minutes there would be a
What Is Heat? Paul shivered inside the wood cabin. It was cold outside, and inside the cabin it wasn t much warmer. Paul could hear the rain beating down on the roof. Every few minutes there would be a
5 Answers and Solutions to Text Problems
 Energy and States of Matter 5 Answers and Solutions to Text Problems 5.1 At the top of the hill, all of the energy of the car is in the form of potential energy. As it descends down the hill, potential
Energy and States of Matter 5 Answers and Solutions to Text Problems 5.1 At the top of the hill, all of the energy of the car is in the form of potential energy. As it descends down the hill, potential
The First Law of Thermodynamics
 The First aw of Thermodynamics Q and W are process (path)-dependent. (Q W) = E int is independent of the process. E int = E int,f E int,i = Q W (first law) Q: + heat into the system; heat lost from the
The First aw of Thermodynamics Q and W are process (path)-dependent. (Q W) = E int is independent of the process. E int = E int,f E int,i = Q W (first law) Q: + heat into the system; heat lost from the
Name: Class: Date: 10. Some substances, when exposed to visible light, absorb more energy as heat than other substances absorb.
 Name: Class: Date: ID: A PS Chapter 13 Review Modified True/False Indicate whether the statement is true or false. If false, change the identified word or phrase to make the statement true. 1. In all cooling
Name: Class: Date: ID: A PS Chapter 13 Review Modified True/False Indicate whether the statement is true or false. If false, change the identified word or phrase to make the statement true. 1. In all cooling
OBJECTIVES THE STUDENTS WILL: Participate in cooperative problem solving in a group setting.
 ICE CAPADES THE POWER OF INSULATION GRADE LEVEL: Upper Elementary/Middle School (High School with extensions) SUBJECT AREA: Sciences, Mathematics DURATION: Preparation time 30 minutes Activity time: One
ICE CAPADES THE POWER OF INSULATION GRADE LEVEL: Upper Elementary/Middle School (High School with extensions) SUBJECT AREA: Sciences, Mathematics DURATION: Preparation time 30 minutes Activity time: One
Energy and Energy Transformations Test Review
 Energy and Energy Transformations Test Review Completion: 1. Mass 13. Kinetic 2. Four 14. thermal 3. Kinetic 15. Thermal energy (heat) 4. Electromagnetic/Radiant 16. Thermal energy (heat) 5. Thermal 17.
Energy and Energy Transformations Test Review Completion: 1. Mass 13. Kinetic 2. Four 14. thermal 3. Kinetic 15. Thermal energy (heat) 4. Electromagnetic/Radiant 16. Thermal energy (heat) 5. Thermal 17.
Rusty Walker, Corporate Trainer Hill PHOENIX
 Refrigeration 101 Rusty Walker, Corporate Trainer Hill PHOENIX Compressor Basic Refrigeration Cycle Evaporator Condenser / Receiver Expansion Device Vapor Compression Cycle Cooling by the removal of heat
Refrigeration 101 Rusty Walker, Corporate Trainer Hill PHOENIX Compressor Basic Refrigeration Cycle Evaporator Condenser / Receiver Expansion Device Vapor Compression Cycle Cooling by the removal of heat
UNIT (1) MEASUREMENTS IN CHEMISTRY
 UNIT (1) MEASUREMENTS IN CHEMISTRY Measurements are part of our daily lives. We measure our weights, driving distances, and gallons of gasoline. As a health professional you might measure blood pressure,
UNIT (1) MEASUREMENTS IN CHEMISTRY Measurements are part of our daily lives. We measure our weights, driving distances, and gallons of gasoline. As a health professional you might measure blood pressure,
1. At which temperature would a source radiate the least amount of electromagnetic energy? 1) 273 K 3) 32 K 2) 212 K 4) 5 K
 1. At which temperature would a source radiate the least amount of electromagnetic energy? 1) 273 K 3) 32 K 2) 212 K 4) 5 K 2. How does the amount of heat energy reflected by a smooth, dark-colored concrete
1. At which temperature would a source radiate the least amount of electromagnetic energy? 1) 273 K 3) 32 K 2) 212 K 4) 5 K 2. How does the amount of heat energy reflected by a smooth, dark-colored concrete
Note: You will receive no credit for late submissions. To learn more, read your instructor's Grading Policy
 1/7 2009/11/14 上 午 11:10 Manage this Assignment: Chapter 16 Due: 12:00am on Saturday, July 3, 2010 Note: You will receive no credit for late submissions. To learn more, read your instructor's Grading Policy
1/7 2009/11/14 上 午 11:10 Manage this Assignment: Chapter 16 Due: 12:00am on Saturday, July 3, 2010 Note: You will receive no credit for late submissions. To learn more, read your instructor's Grading Policy
(Walter Glogowski, Chaz Shapiro & Reid Sherman) INTRODUCTION
 Convection (Walter Glogowski, Chaz Shapiro & Reid Sherman) INTRODUCTION You know from common experience that when there's a difference in temperature between two places close to each other, the temperatures
Convection (Walter Glogowski, Chaz Shapiro & Reid Sherman) INTRODUCTION You know from common experience that when there's a difference in temperature between two places close to each other, the temperatures
Worksheet #17. 2. How much heat is released when 143 g of ice is cooled from 14 C to 75 C, if the specific heat capacity of ice is 2.087 J/(g C).
 Worksheet #17 Calculating Heat 1. How much heat is needed to bring 12.0 g of water from 28.3 C to 43.87 C, if the specific heat capacity of water is 4.184 /(g? 2. How much heat is released when 143 g of
Worksheet #17 Calculating Heat 1. How much heat is needed to bring 12.0 g of water from 28.3 C to 43.87 C, if the specific heat capacity of water is 4.184 /(g? 2. How much heat is released when 143 g of
TEACHER BACKGROUND INFORMATION THERMAL ENERGY
 TEACHER BACKGROUND INFORMATION THERMAL ENERGY In general, when an object performs work on another object, it does not transfer all of its energy to that object. Some of the energy is lost as heat due to
TEACHER BACKGROUND INFORMATION THERMAL ENERGY In general, when an object performs work on another object, it does not transfer all of its energy to that object. Some of the energy is lost as heat due to
FXA 2008. Candidates should be able to : Define and apply the concept of specific heat capacity. Select and apply the equation : E = mcδθ
 UNIT G484 Module 3 4.3.3 Thermal Properties of Materials 1 Candidates should be able to : Define and apply the concept of specific heat capacity. Select and apply the equation : E = mcδθ The MASS (m) of
UNIT G484 Module 3 4.3.3 Thermal Properties of Materials 1 Candidates should be able to : Define and apply the concept of specific heat capacity. Select and apply the equation : E = mcδθ The MASS (m) of
EXPERIMENT 4 THE DETERMINATION OF THE CALORIC CONTENT OF A CASHEW NUT
 EXPERIMENT 4 THE DETERMINATION OF THE CALORIC CONTENT OF A CASHEW NUT Textbook reference: pp103-105 Purpose: In this Activity, students determine how many calories are released per gram when cashews burn
EXPERIMENT 4 THE DETERMINATION OF THE CALORIC CONTENT OF A CASHEW NUT Textbook reference: pp103-105 Purpose: In this Activity, students determine how many calories are released per gram when cashews burn
2. Room temperature: C. Kelvin. 2. Room temperature:
 Temperature I. Temperature is the quantity that tells how hot or cold something is compared with a standard A. Temperature is directly proportional to the average kinetic energy of molecular translational
Temperature I. Temperature is the quantity that tells how hot or cold something is compared with a standard A. Temperature is directly proportional to the average kinetic energy of molecular translational
Type: Single Date: Homework: READ 12.8, Do CONCEPT Q. # (14) Do PROBLEMS (40, 52, 81) Ch. 12
 Type: Single Date: Objective: Latent Heat Homework: READ 12.8, Do CONCEPT Q. # (14) Do PROBLEMS (40, 52, 81) Ch. 12 AP Physics B Date: Mr. Mirro Heat and Phase Change When bodies are heated or cooled their
Type: Single Date: Objective: Latent Heat Homework: READ 12.8, Do CONCEPT Q. # (14) Do PROBLEMS (40, 52, 81) Ch. 12 AP Physics B Date: Mr. Mirro Heat and Phase Change When bodies are heated or cooled their
The Water Cycle Now You See It, Now You Don t
 The Water Cycle Now You See It, Now You Don t Unit: Salinity Patterns & the Water Cycle l Grade Level: Elementary l Time Required: Introduction - 30 min. - Activity as groups 45min Wrap Up 20 min l Content
The Water Cycle Now You See It, Now You Don t Unit: Salinity Patterns & the Water Cycle l Grade Level: Elementary l Time Required: Introduction - 30 min. - Activity as groups 45min Wrap Up 20 min l Content
1. The Kinetic Theory of Matter states that all matter is composed of atoms and molecules that are in a constant state of constant random motion
 Physical Science Period: Name: ANSWER KEY Date: Practice Test for Unit 3: Ch. 3, and some of 15 and 16: Kinetic Theory of Matter, States of matter, and and thermodynamics, and gas laws. 1. The Kinetic
Physical Science Period: Name: ANSWER KEY Date: Practice Test for Unit 3: Ch. 3, and some of 15 and 16: Kinetic Theory of Matter, States of matter, and and thermodynamics, and gas laws. 1. The Kinetic
Thermodynamics AP Physics B. Multiple Choice Questions
 Thermodynamics AP Physics B Name Multiple Choice Questions 1. What is the name of the following statement: When two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium
Thermodynamics AP Physics B Name Multiple Choice Questions 1. What is the name of the following statement: When two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium
Convection, Conduction & Radiation
 Convection, Conduction & Radiation There are three basic ways in which heat is transferred: convection, conduction and radiation. In gases and liquids, heat is usually transferred by convection, in which
Convection, Conduction & Radiation There are three basic ways in which heat is transferred: convection, conduction and radiation. In gases and liquids, heat is usually transferred by convection, in which
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Tidal forces in general are the result of A) unequal forces acting on different parts
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Tidal forces in general are the result of A) unequal forces acting on different parts
Test Bank - Chapter 3 Multiple Choice
 Test Bank - Chapter 3 The questions in the test bank cover the concepts from the lessons in Chapter 3. Select questions from any of the categories that match the content you covered with students. The
Test Bank - Chapter 3 The questions in the test bank cover the concepts from the lessons in Chapter 3. Select questions from any of the categories that match the content you covered with students. The
How does a microwave oven work?
 last lecture Electromagnetic waves oscillating electric and magnetic fields c = c = 3x10 8 m/s or 186,282 miles/sec Radios using the tank circuit to emit and receive electromagnetic waves of a specific
last lecture Electromagnetic waves oscillating electric and magnetic fields c = c = 3x10 8 m/s or 186,282 miles/sec Radios using the tank circuit to emit and receive electromagnetic waves of a specific
Chapter 4 Practice Quiz
 Chapter 4 Practice Quiz 1. Label each box with the appropriate state of matter. A) I: Gas II: Liquid III: Solid B) I: Liquid II: Solid III: Gas C) I: Solid II: Liquid III: Gas D) I: Gas II: Solid III:
Chapter 4 Practice Quiz 1. Label each box with the appropriate state of matter. A) I: Gas II: Liquid III: Solid B) I: Liquid II: Solid III: Gas C) I: Solid II: Liquid III: Gas D) I: Gas II: Solid III:
ES 106 Laboratory # 2 HEAT AND TEMPERATURE
 ES 106 Laboratory # 2 HEAT AND TEMPERATURE Introduction Heat transfer is the movement of heat energy from one place to another. Heat energy can be transferred by three different mechanisms: convection,
ES 106 Laboratory # 2 HEAT AND TEMPERATURE Introduction Heat transfer is the movement of heat energy from one place to another. Heat energy can be transferred by three different mechanisms: convection,
UNIT 2 GCSE PHYSICS 2.2.1 Forces and Energy 2011 FXA WORK DONE (J) = ENERGY TRANSFERRED (J) WORK
 29 When a force causes an object to move through a distance, work is done. Work done, force and distance are related by the equation : W = F x d WORK When a force is applied to an object and cause it to
29 When a force causes an object to move through a distance, work is done. Work done, force and distance are related by the equation : W = F x d WORK When a force is applied to an object and cause it to
The Ideal Gas Law. Gas Constant. Applications of the Gas law. P = ρ R T. Lecture 2: Atmospheric Thermodynamics
 Lecture 2: Atmospheric Thermodynamics Ideal Gas Law (Equation of State) Hydrostatic Balance Heat and Temperature Conduction, Convection, Radiation Latent Heating Adiabatic Process Lapse Rate and Stability
Lecture 2: Atmospheric Thermodynamics Ideal Gas Law (Equation of State) Hydrostatic Balance Heat and Temperature Conduction, Convection, Radiation Latent Heating Adiabatic Process Lapse Rate and Stability
Heat and Temperature: Front End Evaluation Report. Joshua Gutwill. October 1999
 Heat and Temperature: Front End Evaluation Report Joshua Gutwill October 1999 Keywords: 1 Heat and Temperature Front End Evaluation Report October 28, 1999 Goal:
Heat and Temperature: Front End Evaluation Report Joshua Gutwill October 1999 Keywords: 1 Heat and Temperature Front End Evaluation Report October 28, 1999 Goal:
Practical Applications of Freezing by Boiling Process
 Practical Applications of Freezing by Boiling Process Kenny Gotlieb, Sasha Mitchell and Daniel Walsh Physics Department, Harvard-Westlake School 37 Coldwater Canyon, N. Hollywood, CA 9164 Introduction
Practical Applications of Freezing by Boiling Process Kenny Gotlieb, Sasha Mitchell and Daniel Walsh Physics Department, Harvard-Westlake School 37 Coldwater Canyon, N. Hollywood, CA 9164 Introduction
Heat Transfer: Conduction, Convection, and Radiation
 Heat Transfer: Conduction, Convection, and Radiation Introduction We have learned that heat is the energy that makes molecules move. Molecules with more heat energy move faster, and molecules with less
Heat Transfer: Conduction, Convection, and Radiation Introduction We have learned that heat is the energy that makes molecules move. Molecules with more heat energy move faster, and molecules with less
14 HEAT AND HEAT TRANSFER METHODS
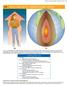 CHAPTER 14 HEAT AND HEAT TRANSFER METHODS 469 14 HEAT AND HEAT TRANSFER METHODS Figure 14.1 (a) The chilling effect of a clear breezy night is produced by the wind and by radiative heat transfer to cold
CHAPTER 14 HEAT AND HEAT TRANSFER METHODS 469 14 HEAT AND HEAT TRANSFER METHODS Figure 14.1 (a) The chilling effect of a clear breezy night is produced by the wind and by radiative heat transfer to cold
Energy Matters Heat. Changes of State
 Energy Matters Heat Changes of State Fusion If we supply heat to a lid, such as a piece of copper, the energy supplied is given to the molecules. These start to vibrate more rapidly and with larger vibrations
Energy Matters Heat Changes of State Fusion If we supply heat to a lid, such as a piece of copper, the energy supplied is given to the molecules. These start to vibrate more rapidly and with larger vibrations
Home Energy Evaluation Report
 Home Energy Evaluation Report Nate Begeman, 1550 Saint Francis Dr San Jose, CA 95125 Air Infiltration Current Air Leakage 2,852 Current Air Changes Per Hour 0.91 Recommended Air Changes Per Hour 0.35 Percent
Home Energy Evaluation Report Nate Begeman, 1550 Saint Francis Dr San Jose, CA 95125 Air Infiltration Current Air Leakage 2,852 Current Air Changes Per Hour 0.91 Recommended Air Changes Per Hour 0.35 Percent
Test 5 Review questions. 1. As ice cools from 273 K to 263 K, the average kinetic energy of its molecules will
 Name: Thursday, December 13, 2007 Test 5 Review questions 1. As ice cools from 273 K to 263 K, the average kinetic energy of its molecules will 1. decrease 2. increase 3. remain the same 2. The graph below
Name: Thursday, December 13, 2007 Test 5 Review questions 1. As ice cools from 273 K to 263 K, the average kinetic energy of its molecules will 1. decrease 2. increase 3. remain the same 2. The graph below
Chapter 2 Measurements in Chemistry. Standard measuring device. Standard scale gram (g)
 1 Chapter 2 Measurements in Chemistry Standard measuring device Standard scale gram (g) 2 Reliability of Measurements Accuracy closeness to true value Precision reproducibility Example: 98.6 o F 98.5 o
1 Chapter 2 Measurements in Chemistry Standard measuring device Standard scale gram (g) 2 Reliability of Measurements Accuracy closeness to true value Precision reproducibility Example: 98.6 o F 98.5 o
Heat Energy FORMS OF ENERGY LESSON PLAN 2.7. Public School System Teaching Standards Covered
 FORMS OF ENERGY LESSON PLAN 2.7 Heat Energy This lesson is designed for 3rd 5th grade students in a variety of school settings (public, private, STEM schools, and home schools) in the seven states served
FORMS OF ENERGY LESSON PLAN 2.7 Heat Energy This lesson is designed for 3rd 5th grade students in a variety of school settings (public, private, STEM schools, and home schools) in the seven states served
Ice Cream Lab & Application Questions
 Deep Freeze 1 Ice Cream Lab & Application Questions Name: Period: Date: Overview Have you ever wondered what it is about throwing salt on ice that makes it melt? And just why does it melt? Where does the
Deep Freeze 1 Ice Cream Lab & Application Questions Name: Period: Date: Overview Have you ever wondered what it is about throwing salt on ice that makes it melt? And just why does it melt? Where does the
Humidity, Condensation, Clouds, and Fog. Water in the Atmosphere
 Humidity, Condensation, Clouds, and Fog or Water in the Atmosphere The Hydrologic Cycle Where the Water Exists on Earth Evaporation From the Oceans and Land The Source of Water Vapor for the Atmosphere
Humidity, Condensation, Clouds, and Fog or Water in the Atmosphere The Hydrologic Cycle Where the Water Exists on Earth Evaporation From the Oceans and Land The Source of Water Vapor for the Atmosphere
Physics PH1FP. (Jun15PH1FP01) General Certificate of Secondary Education Foundation Tier June 2015. Unit Physics P1. Unit Physics P1 TOTAL
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Question Mark Science A Unit Physics P1 Physics Unit Physics P1 Friday 12 June 2015 General
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Question Mark Science A Unit Physics P1 Physics Unit Physics P1 Friday 12 June 2015 General
Specific Heat Capacity and Latent Heat Questions A2 Physics
 1. An electrical heater is used to heat a 1.0 kg block of metal, which is well lagged. The table shows how the temperature of the block increased with time. temp/ C 20.1 23.0 26.9 30.0 33.1 36.9 time/s
1. An electrical heater is used to heat a 1.0 kg block of metal, which is well lagged. The table shows how the temperature of the block increased with time. temp/ C 20.1 23.0 26.9 30.0 33.1 36.9 time/s
Mechanical Energy and Heat
 Mechanical Energy and Heat Purpose: Students will observe the conversion of mechanical energy to thermal energy. Introduction: The principle of conservation of energy is surprisingly new. No one person
Mechanical Energy and Heat Purpose: Students will observe the conversion of mechanical energy to thermal energy. Introduction: The principle of conservation of energy is surprisingly new. No one person
Forms of Energy. Freshman Seminar
 Forms of Energy Freshman Seminar Energy Energy The ability & capacity to do work Energy can take many different forms Energy can be quantified Law of Conservation of energy In any change from one form
Forms of Energy Freshman Seminar Energy Energy The ability & capacity to do work Energy can take many different forms Energy can be quantified Law of Conservation of energy In any change from one form
REASONING AND SOLUTION
 39. REASONING AND SOLUTION The heat released by the blood is given by Q cm T, in which the specific heat capacity c of the blood (water) is given in Table 12.2. Then Therefore, T Q cm 2000 J 0.8 C [4186
39. REASONING AND SOLUTION The heat released by the blood is given by Q cm T, in which the specific heat capacity c of the blood (water) is given in Table 12.2. Then Therefore, T Q cm 2000 J 0.8 C [4186
The Three Heat Transfer Modes in Reflow Soldering
 Section 5: Reflow Oven Heat Transfer The Three Heat Transfer Modes in Reflow Soldering There are three different heating modes involved with most SMT reflow processes: conduction, convection, and infrared
Section 5: Reflow Oven Heat Transfer The Three Heat Transfer Modes in Reflow Soldering There are three different heating modes involved with most SMT reflow processes: conduction, convection, and infrared
Science test KEY STAGE 2 LEVELS 3 5. Test B. First name. Last name. School. For marker s use only 5 7 9 11 13 15 17 19 21 TOTAL
 Sc KEY STAGE 2 Science test LEVELS 3 5 Test B First name Last name School 2009 For marker s use only Page 5 7 9 11 13 15 17 19 21 TOTAL Marks INSTRUCTIONS Read this carefully. You have 45 minutes for this
Sc KEY STAGE 2 Science test LEVELS 3 5 Test B First name Last name School 2009 For marker s use only Page 5 7 9 11 13 15 17 19 21 TOTAL Marks INSTRUCTIONS Read this carefully. You have 45 minutes for this
Energy Conversions I. Unit of measure (most common one) Form Definition Example
 Energy Conversions I Energy can take many forms, but any one form can usually be converted into another. And no matter what form we talk about, we can use conversion factors to calculate equivalent amounts
Energy Conversions I Energy can take many forms, but any one form can usually be converted into another. And no matter what form we talk about, we can use conversion factors to calculate equivalent amounts
GATEWAY SCIENCE B651/01 PHYSICS B Unit 1 Modules P1 P2 P3 (Foundation Tier)
 F GENERAL CERTIFICATE OF SECONDARY EDUCATION GATEWAY SCIENCE B651/01 PHYSICS B Unit 1 Modules P1 P2 P3 (Foundation Tier) *CUP/T63931* Candidates answer on the question paper A calculator may be used for
F GENERAL CERTIFICATE OF SECONDARY EDUCATION GATEWAY SCIENCE B651/01 PHYSICS B Unit 1 Modules P1 P2 P3 (Foundation Tier) *CUP/T63931* Candidates answer on the question paper A calculator may be used for
Chapter Test A. States of Matter MULTIPLE CHOICE. a fixed amount of STAs2 a. a solid. b. a liquid. c. a gas. d. any type of matter.
 Assessment Chapter Test A States of Matter MULTIPLE CHOICE Write the letter of the correct answer in the space provided. 1. Boyle s law explains the relationship between volume and pressure for a fixed
Assessment Chapter Test A States of Matter MULTIPLE CHOICE Write the letter of the correct answer in the space provided. 1. Boyle s law explains the relationship between volume and pressure for a fixed
Specific Volume of Liquid (Column 7). The volume per unit of mass in cubic feet per pound.
 Steam Tables What They Are How to Use Them The heat quantities and temperature/ pressure relationships referred to in this Handbook are taken from the Properties of Saturated Steam table. Definitions of
Steam Tables What They Are How to Use Them The heat quantities and temperature/ pressure relationships referred to in this Handbook are taken from the Properties of Saturated Steam table. Definitions of
CHAPTER 7 THE SECOND LAW OF THERMODYNAMICS. Blank
 CHAPTER 7 THE SECOND LAW OF THERMODYNAMICS Blank SONNTAG/BORGNAKKE STUDY PROBLEM 7-1 7.1 A car engine and its fuel consumption A car engine produces 136 hp on the output shaft with a thermal efficiency
CHAPTER 7 THE SECOND LAW OF THERMODYNAMICS Blank SONNTAG/BORGNAKKE STUDY PROBLEM 7-1 7.1 A car engine and its fuel consumption A car engine produces 136 hp on the output shaft with a thermal efficiency
Specific Heat (slope and steepness)
 1 Specific Heat (slope and steepness) 10 pages. According to the Physical Science text book, the Specific Heat of a material is DEFINED as the following: Specific heat is the amount of heat energy required
1 Specific Heat (slope and steepness) 10 pages. According to the Physical Science text book, the Specific Heat of a material is DEFINED as the following: Specific heat is the amount of heat energy required
What is Energy? What is the relationship between energy and work?
 What is Energy? What is the relationship between energy and work? Compare kinetic and potential energy What are the different types of energy? What is energy? Energy is the ability to do work. Great, but
What is Energy? What is the relationship between energy and work? Compare kinetic and potential energy What are the different types of energy? What is energy? Energy is the ability to do work. Great, but
1/2/3. Finding out about the Water Cycle
 The Water Cycle 1/2/3. Finding out about the Water Cycle Aims: To enable pupils to learn and understand what happens at each stage of the Water Cycle To introduce specific vocabulary related to the Water
The Water Cycle 1/2/3. Finding out about the Water Cycle Aims: To enable pupils to learn and understand what happens at each stage of the Water Cycle To introduce specific vocabulary related to the Water
Energy is the basic necessity for the economic
 CHAPTER 1 Introduction 1.1 Importance of Electrical Energy 1.2 Generation of Electrical Energy 1.3 Sources of Energy 1.4 Comparison of Energy Sources 1.5 Units of Energy 1.6 Relationship Among Energy Units
CHAPTER 1 Introduction 1.1 Importance of Electrical Energy 1.2 Generation of Electrical Energy 1.3 Sources of Energy 1.4 Comparison of Energy Sources 1.5 Units of Energy 1.6 Relationship Among Energy Units
Buoyancy and Archimedes Principle. Buoyancy and Archimedes Principle Assume block is in equilibrium.
 Assume block is in equilibrium. Then upward forces must equal downward forces. Upward force: pressure from fluid Downward force: atmospheric pressure plus weight Therefore In this case, the object is less
Assume block is in equilibrium. Then upward forces must equal downward forces. Upward force: pressure from fluid Downward force: atmospheric pressure plus weight Therefore In this case, the object is less
Density Lab. If you get stuck or are uncertain, please ask questions and/or refer to the hints at the end of the lab. Name: Section: Due Date:
 Name: Section: Due Date: Lab 01B-1 If you get stuck or are uncertain, please ask questions and/or refer to the hints at the end of the lab. Density Lab Density is an important concept in oceanography,
Name: Section: Due Date: Lab 01B-1 If you get stuck or are uncertain, please ask questions and/or refer to the hints at the end of the lab. Density Lab Density is an important concept in oceanography,
Preview of Period 5: Thermal Energy, the Microscopic Picture
 Preview of Period 5: Thermal Energy, the Microscopic Picture 5.1 Temperature and Molecular Motion What is evaporative cooling? 5.2 Temperature and Phase Changes How much energy is required for a phase
Preview of Period 5: Thermal Energy, the Microscopic Picture 5.1 Temperature and Molecular Motion What is evaporative cooling? 5.2 Temperature and Phase Changes How much energy is required for a phase
Tsawwassen BC Arena Before & After installation of the Low-E ceiling
 LOW EMISSIVITY CEILINGS Emissivity- Describes the ability of a certain material to radiate heat rather then absorb it. Materials that radiate 100% have an (EM) of 1.0, while materials that radiate no heat
LOW EMISSIVITY CEILINGS Emissivity- Describes the ability of a certain material to radiate heat rather then absorb it. Materials that radiate 100% have an (EM) of 1.0, while materials that radiate no heat
PREPARATION FOR CHEMISTRY LAB: COMBUSTION
 1 Name: Lab Instructor: PREPARATION FOR CHEMISTRY LAB: COMBUSTION 1. What is a hydrocarbon? 2. What products form in the complete combustion of a hydrocarbon? 3. Combustion is an exothermic reaction. What
1 Name: Lab Instructor: PREPARATION FOR CHEMISTRY LAB: COMBUSTION 1. What is a hydrocarbon? 2. What products form in the complete combustion of a hydrocarbon? 3. Combustion is an exothermic reaction. What
Chapter 3 Student Reading
 Chapter 3 Student Reading If you hold a solid piece of lead or iron in your hand, it feels heavy for its size. If you hold the same size piece of balsa wood or plastic, it feels light for its size. The
Chapter 3 Student Reading If you hold a solid piece of lead or iron in your hand, it feels heavy for its size. If you hold the same size piece of balsa wood or plastic, it feels light for its size. The
What Causes Climate? Use Target Reading Skills
 Climate and Climate Change Name Date Class Climate and Climate Change Guided Reading and Study What Causes Climate? This section describes factors that determine climate, or the average weather conditions
Climate and Climate Change Name Date Class Climate and Climate Change Guided Reading and Study What Causes Climate? This section describes factors that determine climate, or the average weather conditions
Chemistry Unit 3 Reading Assignment Energy and Kinetic Molecular Theory
 Chemistry Unit 3 Reading Assignment Energy and Kinetic Molecular Theory The story behind the difficulty we have with energy is fascinating to those of us who struggle with trying to teach energy in a coherent
Chemistry Unit 3 Reading Assignment Energy and Kinetic Molecular Theory The story behind the difficulty we have with energy is fascinating to those of us who struggle with trying to teach energy in a coherent
Advice to consumer Reference 50.1 May 2013. Condensation. Some causes, some advice.
 Advice to consumer Reference 50.1 May 2013 Condensation Some causes, some advice. 03 Contents The issue 04 What is condensation 06 The factors governing condensation 09 How double or triple glazing helps
Advice to consumer Reference 50.1 May 2013 Condensation Some causes, some advice. 03 Contents The issue 04 What is condensation 06 The factors governing condensation 09 How double or triple glazing helps
Peltier Application Note
 Peltier Application Note Early 19th century scientists, Thomas Seebeck and Jean Peltier, first discovered the phenomena that are the basis for today s thermoelectric industry. Seebeck found that if you
Peltier Application Note Early 19th century scientists, Thomas Seebeck and Jean Peltier, first discovered the phenomena that are the basis for today s thermoelectric industry. Seebeck found that if you
Calculating Heat Loss by Mark Crombie, Chromalox
 Calculating Heat Loss by Mark Crombie, Chromalox Posted: January 30, 2006 This article deals with the basic principles of heat transfer and the calculations used for pipes and vessels. By understanding
Calculating Heat Loss by Mark Crombie, Chromalox Posted: January 30, 2006 This article deals with the basic principles of heat transfer and the calculations used for pipes and vessels. By understanding
Video Killed the Radio Star! Watch a video of me explaining the difference between static and kinetic friction by clicking here.
 Lesson 26: Friction Friction is a force that always exists between any two surfaces in contact with each other. There is no such thing as a perfectly frictionless environment. Even in deep space, bits
Lesson 26: Friction Friction is a force that always exists between any two surfaces in contact with each other. There is no such thing as a perfectly frictionless environment. Even in deep space, bits
UNIT 1 GCSE PHYSICS 1.1.1 Infrared Radiation 2011 FXA
 1 All objects emit and absorb thermal radiation. The hotter an object is the infrared radiation it radiates in a given time. It is continually being transferred to and from all objects. The hotter the
1 All objects emit and absorb thermal radiation. The hotter an object is the infrared radiation it radiates in a given time. It is continually being transferred to and from all objects. The hotter the
Solar Thermal Systems
 Solar Thermal Systems Design and Applications in the UAE Murat Aydemir Viessmann Middle East FZE General Manager (M.Sc. Mech.Eng., ASHRAE) Dubai Knowledge Village Congress Centre, Dubai 20.4.2009 Viessmann
Solar Thermal Systems Design and Applications in the UAE Murat Aydemir Viessmann Middle East FZE General Manager (M.Sc. Mech.Eng., ASHRAE) Dubai Knowledge Village Congress Centre, Dubai 20.4.2009 Viessmann
Sample Questions Chapter 2. Stoker
 Sample Questions Chapter 2. Stoker 1. The mathematical meaning associated with the metric system prefixes centi, milli, and micro is, respectively, A) 2, 4, and 6. B) 2, 3, and 6. C) 3, 6, and 9. D) 3,
Sample Questions Chapter 2. Stoker 1. The mathematical meaning associated with the metric system prefixes centi, milli, and micro is, respectively, A) 2, 4, and 6. B) 2, 3, and 6. C) 3, 6, and 9. D) 3,
Mission 7: Saving Energy
 Mission 7: Saving Energy How can we save energy? Converting one type of energy to another often damages the environment. For example, burning coal to make electricity causes air pollution. That s why we
Mission 7: Saving Energy How can we save energy? Converting one type of energy to another often damages the environment. For example, burning coal to make electricity causes air pollution. That s why we
Practice final for Basic Physics spring 2005 answers on the last page Name: Date:
 Practice final for Basic Physics spring 2005 answers on the last page Name: Date: 1. A 12 ohm resistor and a 24 ohm resistor are connected in series in a circuit with a 6.0 volt battery. Assuming negligible
Practice final for Basic Physics spring 2005 answers on the last page Name: Date: 1. A 12 ohm resistor and a 24 ohm resistor are connected in series in a circuit with a 6.0 volt battery. Assuming negligible
Odyssey of the Mind Technology Fair. Simple Electronics
 Simple Electronics 1. Terms volts, amps, ohms, watts, positive, negative, AC, DC 2. Matching voltages a. Series vs. parallel 3. Battery capacity 4. Simple electronic circuit light bulb 5. Chose the right
Simple Electronics 1. Terms volts, amps, ohms, watts, positive, negative, AC, DC 2. Matching voltages a. Series vs. parallel 3. Battery capacity 4. Simple electronic circuit light bulb 5. Chose the right
Physical and Chemical Properties of Matter
 Physical and Chemical Properties of Matter What is matter? Anything that has mass and takes up space Chemical or Physical Property? Physical properties of matter: characteristics that can be observed or
Physical and Chemical Properties of Matter What is matter? Anything that has mass and takes up space Chemical or Physical Property? Physical properties of matter: characteristics that can be observed or
1. Fahrenheit and Celsius. 2. Converting Fahrenheit values into Celsius. Name:
 Name: Skill Sheet 25.2 Temperature Scales Temperature, a measure of the average kinetic energy of the molecules of a substance, has an important role in our daily lives. Whether we are cooking dinner,
Name: Skill Sheet 25.2 Temperature Scales Temperature, a measure of the average kinetic energy of the molecules of a substance, has an important role in our daily lives. Whether we are cooking dinner,
Temperature. Temperature
 Chapter 8 Temperature Temperature a number that corresponds to the warmth or coldness of an object measured by a thermometer is a per-particle property no upper limit definite limit on lower end Temperature
Chapter 8 Temperature Temperature a number that corresponds to the warmth or coldness of an object measured by a thermometer is a per-particle property no upper limit definite limit on lower end Temperature
Topic Page Contents Page
 Heat energy (11-16) Contents Topic Page Contents Page Heat energy and temperature 3 Latent heat energy 15 Interesting temperatures 4 Conduction of heat energy 16 A cooling curve 5 Convection 17 Expansion
Heat energy (11-16) Contents Topic Page Contents Page Heat energy and temperature 3 Latent heat energy 15 Interesting temperatures 4 Conduction of heat energy 16 A cooling curve 5 Convection 17 Expansion
BB-18 Black Body High Vacuum System Technical Description
 BB-18 Black Body High Vacuum System Technical Description The BB-18 Black Body is versatile and is programmed for use as a fixed cold target at 80 K or variable target, at 80 K- 350 K no extra cost. The
BB-18 Black Body High Vacuum System Technical Description The BB-18 Black Body is versatile and is programmed for use as a fixed cold target at 80 K or variable target, at 80 K- 350 K no extra cost. The
Exam on Heat and Energy
 Exam on Heat and Energy True/False Indicate whether the statement is true or false. 1. Energy is the ability to cause change. 2. Energy is measured in joules. 3. When you ride a playground swing, your
Exam on Heat and Energy True/False Indicate whether the statement is true or false. 1. Energy is the ability to cause change. 2. Energy is measured in joules. 3. When you ride a playground swing, your
Characteristics of Evaporators
 Characteristics of Evaporators Roger D. Holder, CM, MSME 10-28-2003 Heat or Energy In this paper, we will discuss the characteristics of an evaporator coil. The variance of the operational condenses of
Characteristics of Evaporators Roger D. Holder, CM, MSME 10-28-2003 Heat or Energy In this paper, we will discuss the characteristics of an evaporator coil. The variance of the operational condenses of
Provided by TryEngineering - www.tryengineering.org
 Provided by TryEngineering - Lesson Focus Lesson focuses on the engineering behind keeping food and other items cool. Students work in teams to develop a system to make an insulated liquid container that
Provided by TryEngineering - Lesson Focus Lesson focuses on the engineering behind keeping food and other items cool. Students work in teams to develop a system to make an insulated liquid container that
SOLAR ENERGY FUNDAMENTALS
 Radiantec SOLAR ENERGY FUNDAMENTALS G E N E R A L S U P P L E M E N T 420 by Radiantec Company What is Solar Energy? What is the Sun? The sun is a star, not much different from the billions of others in
Radiantec SOLAR ENERGY FUNDAMENTALS G E N E R A L S U P P L E M E N T 420 by Radiantec Company What is Solar Energy? What is the Sun? The sun is a star, not much different from the billions of others in
Solar Cooking. A Design and Technology project for Key Stage 2
 Solar Cooking A Design and Technology project for Key Stage 2 Project Sheet pg. 1 Objectives To learn about the problems which come with cooking in the developing world and how solar power can help. To
Solar Cooking A Design and Technology project for Key Stage 2 Project Sheet pg. 1 Objectives To learn about the problems which come with cooking in the developing world and how solar power can help. To
Practice Test. 4) The planet Earth loses heat mainly by A) conduction. B) convection. C) radiation. D) all of these Answer: C
 Practice Test 1) Increase the pressure in a container of oxygen gas while keeping the temperature constant and you increase the A) molecular speed. B) molecular kinetic energy. C) Choice A and choice B
Practice Test 1) Increase the pressure in a container of oxygen gas while keeping the temperature constant and you increase the A) molecular speed. B) molecular kinetic energy. C) Choice A and choice B
THE STUDY OF THE EFFECT OF DRY ICE ON THE TEMPERATURE OF WATER
 THE STUDY OF THE EFFECT OF DRY ICE ON THE TEMPERATURE OF WATER Justin Tunley Cary Academy ABSTRACT: The purpose of this study was to find out how much the temperature of water would change over time after
THE STUDY OF THE EFFECT OF DRY ICE ON THE TEMPERATURE OF WATER Justin Tunley Cary Academy ABSTRACT: The purpose of this study was to find out how much the temperature of water would change over time after
1. The Determination of Boiling Point
 1. The Determination of Boiling Point Objective In this experiment, you will first check your thermometer for errors by determining the temperature of two stable equilibrium systems. You will then use
1. The Determination of Boiling Point Objective In this experiment, you will first check your thermometer for errors by determining the temperature of two stable equilibrium systems. You will then use
1/9/2013. Terminology Calculating Heat Transfer Code Requirements Design Examples and Sustainability
 1/9/13 By Brett T. Fagan, P.E. Presented by Marie Horan, P.E. Building Diagnostics, Inc. Building Diagnostics Terminology Calculating Heat Transfer Code Requirements Design Examples and Sustainability
1/9/13 By Brett T. Fagan, P.E. Presented by Marie Horan, P.E. Building Diagnostics, Inc. Building Diagnostics Terminology Calculating Heat Transfer Code Requirements Design Examples and Sustainability
Thermochemistry. r2 d:\files\courses\1110-20\99heat&thermorans.doc. Ron Robertson
 Thermochemistry r2 d:\files\courses\1110-20\99heat&thermorans.doc Ron Robertson I. What is Energy? A. Energy is a property of matter that allows work to be done B. Potential and Kinetic Potential energy
Thermochemistry r2 d:\files\courses\1110-20\99heat&thermorans.doc Ron Robertson I. What is Energy? A. Energy is a property of matter that allows work to be done B. Potential and Kinetic Potential energy
Energy Test Study Guide
 Name: Energy Test Study Guide (Test Dates: A Day May 5 th B Day May 6 th ) USE YOUR INTERACTIVE NOTEBOOK TO STUDY CLASSROOM ASSIGNMENTS, LABS, FORMATIVE ASSESSMENTS, AND HOMEWORK. ENERGY AND THE TWO MAIN
Name: Energy Test Study Guide (Test Dates: A Day May 5 th B Day May 6 th ) USE YOUR INTERACTIVE NOTEBOOK TO STUDY CLASSROOM ASSIGNMENTS, LABS, FORMATIVE ASSESSMENTS, AND HOMEWORK. ENERGY AND THE TWO MAIN
