Blood Collection and Handling Dried Blood Spot (DBS)
|
|
|
- Theodore Welch
- 7 years ago
- Views:
Transcription
1 Module 14 Blood Collection and Handling Dried Blood Spot (DBS) Purpose Pre-requisite Modules To provide you with the skills to collect and handle dried blood spots (DBS) for EQA purposes. Module 6: Safety at HIV Rapid Testing Site Module 8: Blood Collection Fingerprick Module 13: EQA Learning Objectives Content Outline Handout At the end of this module, you will be able to: Collect dried blood spots (DBS) Package and store DBS in a way to maintain specimen integrity Maintain DBS records Distinguish between valid and invalid DBS Required supplies How to collect and dry DBS How to package and store DBS Valid and invalid DBS Hands-on practice Example Transfer Log for Re-Testing Notes on Customization Module 14: EQA Dried Blood Spots 1 Participant Manual
2 What Is a Dried Blood Spot (DBS)? Dried Blood Spots (DBS) are whole blood collected on filter paper and dried. They are made directly from the client s whole blood. DBS are used for re-testing at a reference laboratory, which may be part of your country s External Quality Assessment plan. Testing site results are compared to reference laboratory results. DBS samples are useful for re-testing as they are easy to collect, store, and transport. EQA Re-testing Remember Two types of specimens may be collected at the site for referral to a reference lab for re-testing: serum/plasma or dried blood spots (DBS). What Are Your Responsibilities? Your EQA responsibilities include: Collecting valid specimens Labeling and store appropriately until transported for retesting Ensuring records are properly maintained Avoiding transcription errors A test result is only as good as the specimen collected. EQA Transfer Log An example EQA specimen transfer log is provided for your reference at the end of this module. Data recorded on transfer logs must be accurate. Care should be taken in transferring information from test records to EQA transfer log. Errors that are typically made include: ID on transfer log sheet doesn t match specimen ID filter paper Final result at testing site incorrectly transcribed from test records Required Supplies for DBS When collecting DBS, you need the following items: Blood collection card (filter paper) Glycine weighing paper Sealable plastic bags Humidity cards Desiccant packs Module 14: EQA Dried Blood Spots 2 Participant Manual
3 Other supplies include safety supplies such as discard bins and supplies for performing a fingerprick. While the collection card may include 5 circles, only one patient/client s blood may be collected on one card. How to Collect DBS Always use Universal Safety Precautions. This includes: Treat all blood samples as though they are infectious Wash hands Wear gloves and apron/lab coat Take precaution to avoid needle injury Dispose of contaminated sharps and waste appropriately It is critical that entire circle be uniformly saturated. Clearly label each card with appropriate identification number It is unacceptable to submit a blood card for testing that has not been properly labeled. Follow the fingerprick procedure and the additional steps below: Apply gentle pressure to the finger and allow a large drop of free flowing blood to collect at the puncture site. Working quickly, hold the filter paper by the edges and touch the filter paper gently against the large drop of blood and in one step allow a sufficient quantity of blood to soak through and completely fill or saturate a circle. A completed saturated spot will contain 100 µl of blood. Repeat, until you have collected enough blood to fill at least 3 circles on the blood collection card. Completing filling the circle is important because the laboratory will need to use a hole puncher to punch a section of the circle of blood for testing If collecting spots using a pipette, collect 100 µl of blood and gently apply to filter paper. Module 14: EQA Dried Blood Spots 3 Participant Manual
4 Additional tips: DO NOT press the filter paper against the puncture site. Apply blood to only one side of the filter paper. Do not layer successive drops of blood or apply blood more than once in the same collection circle. Do not milk the finger as excessive milking or squeezing the puncture site might cause hemolysis of the specimen or result in collection of tissue fluids with the specimen, which might adversely affect the test result. (NOTE: hemo- means red cells, -lysis means destruction, hemolysis means destruction of red cells.) NOTE: Two complete circles are better than five incomplete ones! While the collection card may include 5 circles, only one patient/client s blood may be collected on one card. How to Dry DBS Important tips for drying DBS: Avoid touching or smearing the blood spots Allow the specimen to fully air dry horizontally (at least 3 hours) at room temperature Keep away from direct sunlight - Care should be taken to avoid exposing DBS to environmental conditions that may compromise the integrity of the specimen. DBS should not be dried near an open window as sunlight, dust and in some cases flying insects may come in contact with the DBS during the drying procedure. Do not heat, stack or allow DBS to touch other surfaces during the drying process Dry Completely Before Packaging There are several ways in which you can effectively dry DBS The image on the left is one that was taken in India. DBS are dried horizontally between bamboo. The image on the right can be obtained from Schleicher & Schuell Bioscience, Inc. (Manufacturer of DBS collection cards) DBS change from bright red to dark red as they dry. Module 14: EQA Dried Blood Spots 4 Participant Manual
5 How to Package DBS for storage: An overview Follow these steps: 1. Appropriately stack DBS 2. Insert into sealable plastic bag 3. Add desiccant packets 4. Add humidity cards 5. Label contents of bag and seal. Step 1 - Stack DBS Place weighing or glycine paper between DBS cards before transport to prevent cross-contamination. Place filter paper between sheets of weighing paper Fold weigh ends of weighing paper Step 2 - Insert Into Sealable Plastic Bag You can typically place up to 15 DBS cards in each plastic bag. The bag should be just the right size to hold the cards. Avoid using bags that are too big as the cards will shuffle inside the bag. Sandwich bags will not work as many of these are of the type where you fold the top. The bag should be a sealable heavy duty plastic bag, one that will prevent moisture from entering. Module 14: EQA Dried Blood Spots 5 Participant Manual
6 Step 3 - Add Desiccant Packets Desiccant packets serve as a drying agent. At least 5 desiccant packs should be placed in each bag. Some sites may need more or fewer packets in each bag depending on environmental and storage conditions. Desiccant packets vary in size. The ones seen here are the smaller ones. Step 4 - Add Humidity Cards and Seal Bag Often humidity cards must be recharged before use. If the humidity card is pink at the 30% level, you must: Recharge card and desiccant pack by heating at C for 3-4 hours in a drying oven. Cool 10 minutes. IMMEDIATELY RETURN card and desiccant pack to sealable plastic bag. Note: If a drying oven is not available, place cards and excessive number of desiccant packets in a sealable bag or envelope Step 5 - Label Outside of Plastic Bag with Contents A permanent marker should be used to clearly label the contents on the bag. Module 14: EQA Dried Blood Spots 6 Participant Manual
7 How to Store DBS Keep packaged DBS (in sealable plastic bags) cool and dry until transported to reference laboratory. It is also acceptable to store DBS in a Styrofoam box. Avoid leaving it in a vehicle, as sun and heat will deteriorate DBS Avoid placing spots in an malfunctioning refrigerator where water may drip on or soak the spots How to Package DBS for Shipping Follow these steps: 1. Insert bundled DBS into rip-resistant envelope. The sealable plastic bag has a label on the outside indicating bio-hazardous contents. 2. Include appropriate documentation (such as EQA Transfer Log) with the shipment 3. Insert both into brown envelop and seal for shipment. Valid DBS This specimen is valid because: 100 µl of blood has been collected in each circle completely saturating or filling the circle The filter paper card has been labeled with appropriate identification. Blood is soaked through to the other side of the card. Module 14: EQA Dried Blood Spots 7 Participant Manual
8 Valid DBS On the card with MB/KP/120, the blood is spreading from one circle to another due to the anemia (anemic blood is more fluid). This is still considered a valid specimen. Blood has completely filled the circle. Notice that the third and fifth circles have been punched (hence the white area in the middle). Invalid DBS This specimen is invalid because quantity of blood is insufficient for testing. This may have been caused by:: Removing filter paper before blood has completely filled circle or before blood has soaked through to the other side Applying blood to filter paper with a capillary tube Filter paper coming in contact with gloved or ungloved hands or substances such as hand lotion or powder, either before or after blood specimen collection Invalid DBS This specimen is invalid because it appears scratched or abraded. This may have been caused by applying blood with a capillary tube or other device. Invalid DBS This specimen is invalid because the specimen was not dry before mailing. DBS must dry a minimum of 4 hours before packaging and shipping. Module 14: EQA Dried Blood Spots 8 Participant Manual
9 Invalid DBS This specimen is invalid because the specimen appears clotted or layered. This may have been caused by: Touching the same circle on the filter paper to blood drop several times Filling circle on both sides of filter paper The volume of specimen will not be uniform between spots resulting in errors during the testing process. Invalid DBS This specimen is invalid because the specimen appears hemolyzed, discolored, or contaminated. This may have been caused by: Squeezing or milking of area surrounding the puncture site Allowing filter paper to come in contact with glove or ungloved hands or substances either before or after blood collection Exposing blood spots to direct heat Invalid DBS This specimen is invalid because the specimen exhibits serum rings in other words, serum becomes separate from cells. This may have been caused by: Not allowing alcohol to dry at puncture site before making skin puncture Allowing filter paper to come in contact with alcohol, hand lotion, etc. Squeezing area surrounding puncture site excessively Drying specimen improperly Applying blood to filter paper with a capillary tube Module 14: EQA Dried Blood Spots 9 Participant Manual
10 Invalid DBS This specimen is invalid because no blood was applied to the filter paper. Key message DBS are used for re-testing at a reference laboratory, which may be part of your country s External Quality Assessment plan Care must be taken to collect, dry, store, and ship dried blood spots used for re-testing. Module 14: EQA Dried Blood Spots 10 Participant Manual
11 Module Review Find out how much you have learned by answering these questions. Describe the procedures for collecting DBS. Describe the procedures for drying DBS. Describe the procedures for packaging DBS for storage. Describe the procedures for storing DBS. Module 14: EQA Dried Blood Spots 11 Participant Manual
12 Module Review Find out how much you have learned by answering these questions. Describe the procedures for packaging DBS for shipping. Describe the criteria for valid DBS samples. What may have caused a DBS sample to be invalid? Module 14: EQA Dried Blood Spots 12 Participant Manual
13 Example Transfer Log for Re-testing [Insert Name of Referring Testing Site, Contact Name Address and Phone Number] Date: Referring Testing Site Tracking Number Test Subject ID* Final Result (Testing Site) Date Collected Type (DBS or Serum) Collected by Referral Lab Req Completed ( ) Date to referral lab Date Conf Result Received Result of Re-test *ID = Identification Lab Req = Laboratory Requisition Module 14: EQA Dried Blood Spots 13 Participant Manual
STANDARD OPERATING PROCEDURE
 TAKING BLOOD FROM INFANTS FOR THE HIV DNA PCR TEST STANDARD OPERATING PROCEDURE ACRONYMS ARV CCMT DBS Antiretrovirals Comprehensive care, management and treatment Dried blood spots This Standard Operating
TAKING BLOOD FROM INFANTS FOR THE HIV DNA PCR TEST STANDARD OPERATING PROCEDURE ACRONYMS ARV CCMT DBS Antiretrovirals Comprehensive care, management and treatment Dried blood spots This Standard Operating
Appendix H Managing Biohazardous Waste SOP
 Biohazardous waste is managed under the State of Rhode Island s Regulated Medical Waste Regulations (Regulation DEM-OWM-MW-1-2009, amended July, 2010). http://www.dem.ri.gov/pubs/regs/regs/waste/medwaste10.pdf
Biohazardous waste is managed under the State of Rhode Island s Regulated Medical Waste Regulations (Regulation DEM-OWM-MW-1-2009, amended July, 2010). http://www.dem.ri.gov/pubs/regs/regs/waste/medwaste10.pdf
How to safely collect blood samples from persons suspected to be infected with highly infectious blood-borne pathogens (e.g.
 How to safely collect blood samples from persons suspected to be infected with highly infectious blood-borne pathogens (e.g. Ebola) Step 1: Before entering patient room, assemble all equipment (1 st part)
How to safely collect blood samples from persons suspected to be infected with highly infectious blood-borne pathogens (e.g. Ebola) Step 1: Before entering patient room, assemble all equipment (1 st part)
To help you understand the role documents and records play in the quality system and the monitoring of programs.
 Module 15 Documents and Records Purpose Pre-requisite Modules Learning Objectives Content Outline Handout Notes on Customization To help you understand the role documents and records play in the quality
Module 15 Documents and Records Purpose Pre-requisite Modules Learning Objectives Content Outline Handout Notes on Customization To help you understand the role documents and records play in the quality
Appendix H IBC Managing Biohazardous Waste SOP
 Biohazardous waste is managed under the State of Rhode Island s Regulated Medical Waste Regulations (Regulation DEM-OWM-MW-1-2009, amended July, 2010). http://www.dem.ri.gov/pubs/regs/regs/waste/medwaste10.pdf
Biohazardous waste is managed under the State of Rhode Island s Regulated Medical Waste Regulations (Regulation DEM-OWM-MW-1-2009, amended July, 2010). http://www.dem.ri.gov/pubs/regs/regs/waste/medwaste10.pdf
Laboratory Biosafty In Molecular Biology and its levels
 Laboratory Biosafty In Molecular Biology and its levels Workshop 16-17 Oct..2012 Guidelines Does not mean optional Laboratory Biosafety The Laboratory Biosafety Manual is an important WHO publication
Laboratory Biosafty In Molecular Biology and its levels Workshop 16-17 Oct..2012 Guidelines Does not mean optional Laboratory Biosafety The Laboratory Biosafety Manual is an important WHO publication
Infectious Waste Management Plan
 Infectious Waste Management Plan Infectious Waste Management Plan USC Health & Safety Programs Unit 777-5269 POLICY: A. In keeping with the University of South Carolina's policy of providing protection
Infectious Waste Management Plan Infectious Waste Management Plan USC Health & Safety Programs Unit 777-5269 POLICY: A. In keeping with the University of South Carolina's policy of providing protection
Content Sheet 5-1: Overview of Sample Management
 Content Sheet 5-1: Overview of Management Role in quality management system management is a part of process control, one of the essentials of a quality management system. The quality of the work a laboratory
Content Sheet 5-1: Overview of Management Role in quality management system management is a part of process control, one of the essentials of a quality management system. The quality of the work a laboratory
INFECTION CONTROL POLICY
 INFECTION CONTROL POLICY Infection control is the name given to a wide range of policies, procedures and techniques intended to prevent the spread of infectious diseases amongst staff and service users.
INFECTION CONTROL POLICY Infection control is the name given to a wide range of policies, procedures and techniques intended to prevent the spread of infectious diseases amongst staff and service users.
INSTRUCTIONS FOR USE HUMIRA 40 MG/0.8 ML, 20 MG/0.4 ML AND 10 MG/0.2 ML SINGLE-USE PREFILLED SYRINGE
 INSTRUCTIONS FOR USE HUMIRA (Hu-MARE-ah) (adalimumab) 40 MG/0.8 ML, 20 MG/0.4 ML AND 10 MG/0.2 ML SINGLE-USE PREFILLED SYRINGE Do not try to inject HUMIRA yourself until you have been shown the right way
INSTRUCTIONS FOR USE HUMIRA (Hu-MARE-ah) (adalimumab) 40 MG/0.8 ML, 20 MG/0.4 ML AND 10 MG/0.2 ML SINGLE-USE PREFILLED SYRINGE Do not try to inject HUMIRA yourself until you have been shown the right way
Waste Management. Course Description
 Waste Management Course Description After completing this training course you will be familiar with the correct handling and disposal of waste materials generated through the provision of PoCT (Point of
Waste Management Course Description After completing this training course you will be familiar with the correct handling and disposal of waste materials generated through the provision of PoCT (Point of
STEP-BY-STEP INSTRUCTIONS FOR INVESTIGATIONAL USE. Rapid HCV Antibody Test FOR ORAQUICK RAPID HCV ANTIBODY TEST
 Before performing testing, all operators MUST read and become familiar with Universal Precautions for Prevention of Transmission of Human Immunodeficiency Virus, Hepatitis B Virus, and other Blood-borne
Before performing testing, all operators MUST read and become familiar with Universal Precautions for Prevention of Transmission of Human Immunodeficiency Virus, Hepatitis B Virus, and other Blood-borne
MANITOBA PATIENT SERVICE CENTRE STANDARDS
 MANITOBA PATIENT SERVICE CENTRE STANDARDS February 2015 INTRODUCTION These Standards are derived from Z316.7-12 and are approved by the Council of the College of Physicians and Surgeons of Manitoba. These
MANITOBA PATIENT SERVICE CENTRE STANDARDS February 2015 INTRODUCTION These Standards are derived from Z316.7-12 and are approved by the Council of the College of Physicians and Surgeons of Manitoba. These
6 Body Fluid Stains and Standards
 6 Body Fluid Stains and Standards Laboratory examination of body fluids (i.e., blood, semen, saliva, etc.) may produce significant information in certain investigations. This chapter considers the recognition,
6 Body Fluid Stains and Standards Laboratory examination of body fluids (i.e., blood, semen, saliva, etc.) may produce significant information in certain investigations. This chapter considers the recognition,
Biohazardous, Medical & Biological Waste Guidance Chart
 CSULA Environmental Health and Safety Biohazardous, Medical & Biological Waste Guidance Chart The chart below provides information on how to handle most, if not all, of the items that frequently are collectively
CSULA Environmental Health and Safety Biohazardous, Medical & Biological Waste Guidance Chart The chart below provides information on how to handle most, if not all, of the items that frequently are collectively
Use and Disposal of Sharps
 From Infection Prevention: A Reference Booklet for Health Care Providers 2001 EngenderHealth Use and Disposal of Sharps In health care settings, injuries from needles and other sharp items are the number-one
From Infection Prevention: A Reference Booklet for Health Care Providers 2001 EngenderHealth Use and Disposal of Sharps In health care settings, injuries from needles and other sharp items are the number-one
Sheep@Purdue. Blood Sampling in Sheep AS-557-W
 Sheep@Purdue AS-557-W Blood Sampling in Sheep Becky Mitchell, Animal Sciences Student Mike Neary, Extension Sheep Specialist Gerald Kelly, Manager of Sheep Teaching and Research Flock Purdue University
Sheep@Purdue AS-557-W Blood Sampling in Sheep Becky Mitchell, Animal Sciences Student Mike Neary, Extension Sheep Specialist Gerald Kelly, Manager of Sheep Teaching and Research Flock Purdue University
Hand Hygiene and Infection Control
 C Hand Hygiene and Infection Control Sirius Business Services Ltd www.siriusbusinessservices.co.uk Tel 01305 769969 info@siriusbusinessservices.co.uk Whatever your First Aid, Fire Safety or Health & Safety
C Hand Hygiene and Infection Control Sirius Business Services Ltd www.siriusbusinessservices.co.uk Tel 01305 769969 info@siriusbusinessservices.co.uk Whatever your First Aid, Fire Safety or Health & Safety
Annual Biomedical Waste Code Training
 Annual Biomedical Waste Code Training Provided by: Barbara D. Will, MPH Biomedical Waste Program Supervisor To protect, promote and improve the health of all people in Florida through integrated state,
Annual Biomedical Waste Code Training Provided by: Barbara D. Will, MPH Biomedical Waste Program Supervisor To protect, promote and improve the health of all people in Florida through integrated state,
THE UNIVERSITY OF MAINE BIOMEDICAL WASTE MANAGEMENT PLAN
 THE UNIVERSITY OF MAINE BIOMEDICAL WASTE MANAGEMENT PLAN Department: The University of Maine Safety and Environmental Management Department Page i TABLE OF CONTENTS Section Title Page 1. Purpose... 1 2.
THE UNIVERSITY OF MAINE BIOMEDICAL WASTE MANAGEMENT PLAN Department: The University of Maine Safety and Environmental Management Department Page i TABLE OF CONTENTS Section Title Page 1. Purpose... 1 2.
BIO MEDICAL WASTE MANAGEMENT
 Bio Medical Waste Management MODULE 5 BIO MEDICAL WASTE MANAGEMENT 5.1 INTRODUCTION Bio medical waste (BMW) may be defined as any solid, fluid or liquid waste material including its container and any other
Bio Medical Waste Management MODULE 5 BIO MEDICAL WASTE MANAGEMENT 5.1 INTRODUCTION Bio medical waste (BMW) may be defined as any solid, fluid or liquid waste material including its container and any other
OCCUPATIONAL SAFETY AND ENVIRONMENTAL HEALTH GUIDELINE
 OSEH Occupational Safety & Environmental Health OCCUPATIONAL SAFETY AND ENVIRONMENTAL HEALTH GUIDELINE Subject: Biohazardous (Medical) Waste Disposal Date: 08/19/09 Revision: 03 Page: 1 of 7 TABLE OF Section
OSEH Occupational Safety & Environmental Health OCCUPATIONAL SAFETY AND ENVIRONMENTAL HEALTH GUIDELINE Subject: Biohazardous (Medical) Waste Disposal Date: 08/19/09 Revision: 03 Page: 1 of 7 TABLE OF Section
TEMPLE UNIVERSITY ENVIRONMENTAL HEALTH AND RADIATION SAFETY
 Page 1 of 7 ISSUED: 5/00 REVISED: 08/06 1. Potential Releases of Radioactive Materials to Unrestricted Areas The Environmental Health and Safety Department (EHRS) must be notified immediately if an emergency
Page 1 of 7 ISSUED: 5/00 REVISED: 08/06 1. Potential Releases of Radioactive Materials to Unrestricted Areas The Environmental Health and Safety Department (EHRS) must be notified immediately if an emergency
Pesticide Spills. Chapter 26. In This Chapter. Keywords. Accidents. Pesticides Act and Environmental Protection Act
 Chapter 26 Pesticide Spills In This Chapter Keywords Accidents After learning the information in this chapter, you will be able to: 1. Define a spill. 2. Describe when a spill must be reported to the Spills
Chapter 26 Pesticide Spills In This Chapter Keywords Accidents After learning the information in this chapter, you will be able to: 1. Define a spill. 2. Describe when a spill must be reported to the Spills
Newborn screening sample collection guidelines
 Newborn screening sample collection guidelines Detailed information about the newborn screening program, including correct sample collection techniques, can be found in the e-learning tool available at:
Newborn screening sample collection guidelines Detailed information about the newborn screening program, including correct sample collection techniques, can be found in the e-learning tool available at:
Standard Operating Procedure (SOP) Work Package 8. Sample Collection and Storage
 Standard Operating Procedure (SOP) Work Package 8 Sample Collection and Storage May 2008 SOP WP 8 - Sample collection Table of Contents 1 SAMPLE COLLECTION... 3 1.1 Sample collection -summary... 3 1.2
Standard Operating Procedure (SOP) Work Package 8 Sample Collection and Storage May 2008 SOP WP 8 - Sample collection Table of Contents 1 SAMPLE COLLECTION... 3 1.1 Sample collection -summary... 3 1.2
How To Understand And Understand The Rules Of Hazardous Waste
 Understanding Regulated Medical Waste & Best Management Practices Regulations & References The information provided in this presentation is based on the referenced Code of Federal Regulations and State
Understanding Regulated Medical Waste & Best Management Practices Regulations & References The information provided in this presentation is based on the referenced Code of Federal Regulations and State
Prevention and control of infection in care homes. Summary for staff
 Prevention and control of infection in care homes Summary for staff 1 DH INFORMATION READER BOX Policy Clinical Estates HR / Workforce Commissioner Development IM & T Management Provider Development Finance
Prevention and control of infection in care homes Summary for staff 1 DH INFORMATION READER BOX Policy Clinical Estates HR / Workforce Commissioner Development IM & T Management Provider Development Finance
INSTRUCTIONS FOR USE HUMIRA 40 MG/0.8 ML SINGLE-USE PEN
 INSTRUCTIONS FOR USE HUMIRA (Hu-MARE-ah) (adalimumab) 40 MG/0.8 ML SINGLE-USE PEN Do not try to inject HUMIRA yourself until you have been shown the right way to give the injections and have read and understand
INSTRUCTIONS FOR USE HUMIRA (Hu-MARE-ah) (adalimumab) 40 MG/0.8 ML SINGLE-USE PEN Do not try to inject HUMIRA yourself until you have been shown the right way to give the injections and have read and understand
Guidelines for Ethidium Bromide Waste Management & Disposal. University of Tennessee-Knoxville
 1. Background Guidelines for Ethidium Bromide Waste Management & Disposal University of Tennessee-Knoxville Ethidium bromide (3,8 diamino-5-ethyl-6-phenyl phenanthridinium bromide, dromilac, CAS #1239-45-8),
1. Background Guidelines for Ethidium Bromide Waste Management & Disposal University of Tennessee-Knoxville Ethidium bromide (3,8 diamino-5-ethyl-6-phenyl phenanthridinium bromide, dromilac, CAS #1239-45-8),
FACT SHEET : Using Autoclaves Safely
 CSULA Environmental Health and Safety Biosafety Office FACT SHEET : Using Autoclaves Safely Most science research laboratories on campus require the use of autoclaves. The primary purpose of the autoclave
CSULA Environmental Health and Safety Biosafety Office FACT SHEET : Using Autoclaves Safely Most science research laboratories on campus require the use of autoclaves. The primary purpose of the autoclave
How Does a Doctor Test for AIDS?
 Edvo-Kit #S-70 How Does a Doctor Test for AIDS? S-70 Experiment Objective: The Human Immunodefi ciency Virus (HIV) is an infectious agent that causes Acquired Immunodefi ciency Syndrome (AIDS) in humans.
Edvo-Kit #S-70 How Does a Doctor Test for AIDS? S-70 Experiment Objective: The Human Immunodefi ciency Virus (HIV) is an infectious agent that causes Acquired Immunodefi ciency Syndrome (AIDS) in humans.
A Guide to Managing Your Biological Waste at the University at Albany
 A Guide to Managing Your Biological Waste at the University at Albany Section 1 - What you need to know: Definition: "Regulated Medical Waste (RMW) shall mean any of the following waste which is generated
A Guide to Managing Your Biological Waste at the University at Albany Section 1 - What you need to know: Definition: "Regulated Medical Waste (RMW) shall mean any of the following waste which is generated
Conservation of Momentum Greg Kifer
 SCIENCE EXPERIMENTS ON FILE Revised Edition 6.7-1 Conservation of Momentum Greg Kifer Topic Conservation of momentum Time 1 hour! Safety Please click on the safety icon to view the safety precautions.
SCIENCE EXPERIMENTS ON FILE Revised Edition 6.7-1 Conservation of Momentum Greg Kifer Topic Conservation of momentum Time 1 hour! Safety Please click on the safety icon to view the safety precautions.
Bloodborne Pathogens Program Revised July, 5 2012
 Bloodborne Pathogens Program Revised July, 5 2012 Page 1 of 16 Table of Contents 1.0 INTRODUCTION...3 1.1 Purpose...3 1.2 Policy.3 2.0 EXPOSURE CONTROL METHODS 4 2.1 Universal Precautions.4 2.2 Engineering
Bloodborne Pathogens Program Revised July, 5 2012 Page 1 of 16 Table of Contents 1.0 INTRODUCTION...3 1.1 Purpose...3 1.2 Policy.3 2.0 EXPOSURE CONTROL METHODS 4 2.1 Universal Precautions.4 2.2 Engineering
Training on Standard Operating Procedures for Health Care Waste Management Swaziland 12 May, 2011
 Training on Standard Operating Procedures for Health Care Waste Management Swaziland 12 May, 2011 Safe Infectious Waste Handling and Transport Objective Waste Overview Roles and Responsibilities of Waste
Training on Standard Operating Procedures for Health Care Waste Management Swaziland 12 May, 2011 Safe Infectious Waste Handling and Transport Objective Waste Overview Roles and Responsibilities of Waste
BSL 1 Laboratory Biosafety Manual
 BSL 1 Laboratory Biosafety Manual Version 1.0 Idaho State University, Office for Research Institutional Biosafety Committee (IBC) 1651 Alvin Ricken Drive, Pocatello, ID 83201-8046 Phone: 208-282-2179 Fax:
BSL 1 Laboratory Biosafety Manual Version 1.0 Idaho State University, Office for Research Institutional Biosafety Committee (IBC) 1651 Alvin Ricken Drive, Pocatello, ID 83201-8046 Phone: 208-282-2179 Fax:
Blood Collection and Processing SOP
 Brisbane Breast Bank Blood Collection and Processing SOP Breast Pathology Laboratory University of Queensland Centre for Clinical Research Blood Collection We collect 30ml of blood from patients who have
Brisbane Breast Bank Blood Collection and Processing SOP Breast Pathology Laboratory University of Queensland Centre for Clinical Research Blood Collection We collect 30ml of blood from patients who have
Medical or Biological Waste: Storage, Treatment, Disposal and Transportation Plan
 Medical or Biological Waste: Storage, Treatment, Disposal and Transportation Plan 1. Scope This program covers all departments at Wellesley College who generate medical or biological waste to include Health
Medical or Biological Waste: Storage, Treatment, Disposal and Transportation Plan 1. Scope This program covers all departments at Wellesley College who generate medical or biological waste to include Health
Utah Division of Solid and Hazardous Waste Solid Waste Management Program
 Utah Division of Solid and Hazardous Waste Solid Waste Management Program Mailing Address Office Location Phone (801) 536-0200 P.O. Box 144880 195 North 1950 West Fax (801) 536-0222 Salt Lake City, Utah
Utah Division of Solid and Hazardous Waste Solid Waste Management Program Mailing Address Office Location Phone (801) 536-0200 P.O. Box 144880 195 North 1950 West Fax (801) 536-0222 Salt Lake City, Utah
Policy for the Disposal of Biological Waste
 Policy for the Disposal of Biological Waste I. Biological Waste II. Regulated Medical Waste Prepared by: Rutgers Environmental Health and Safety 24 Street 1603 Building 4127, Livingston Campus Piscataway,
Policy for the Disposal of Biological Waste I. Biological Waste II. Regulated Medical Waste Prepared by: Rutgers Environmental Health and Safety 24 Street 1603 Building 4127, Livingston Campus Piscataway,
Biosafety Level 2 (BSL-2) Safety Guidelines
 BLS-4 Biosafety Level 2 (BSL-2) Safety Guidelines BSL-3 BSL-2 BSL-1 BSL-2 builds upon BSL-1. If you work in a lab that is designated a BSL-2, the microbes used pose moderate hazards to laboratory staff
BLS-4 Biosafety Level 2 (BSL-2) Safety Guidelines BSL-3 BSL-2 BSL-1 BSL-2 builds upon BSL-1. If you work in a lab that is designated a BSL-2, the microbes used pose moderate hazards to laboratory staff
Waste Management Policy
 Waste Management Policy Revised April 2013 1 Contents Page Content Page No. Clinical Waste 3 - The handling and disposal of Clinical and Soiled 3 - Policy 3 - Warning - The collection of Clinical Waste
Waste Management Policy Revised April 2013 1 Contents Page Content Page No. Clinical Waste 3 - The handling and disposal of Clinical and Soiled 3 - Policy 3 - Warning - The collection of Clinical Waste
Caring for Your PleurX Pleural Catheter
 Caring for Your PleurX Pleural Catheter A PleurX Pleural Catheter has been placed in your chest through a small incision in your skin into the pleural space (see picture below). This allows you to drain
Caring for Your PleurX Pleural Catheter A PleurX Pleural Catheter has been placed in your chest through a small incision in your skin into the pleural space (see picture below). This allows you to drain
Cold Agglutination Titer detecting Cold Reacting Antibodies
 Objectives: Cold Agglutination Titer detecting Cold Reacting Antibodies 1. Perform a serial dilution to determine the amount of cold reacting antibody present in a patient specimen with the results obtained
Objectives: Cold Agglutination Titer detecting Cold Reacting Antibodies 1. Perform a serial dilution to determine the amount of cold reacting antibody present in a patient specimen with the results obtained
REGULATED MEDICAL WASTE MANAGEMENT. Title 15, Article 27, Environmental Conservation Law (DEC)
 REGULATED MEDICAL WASTE MANAGEMENT Medical Waste Laws/Regulations: The definitions of medical waste, requirements for handling, disposal and tracking are primarily determined by: Title XIII, Article 13,
REGULATED MEDICAL WASTE MANAGEMENT Medical Waste Laws/Regulations: The definitions of medical waste, requirements for handling, disposal and tracking are primarily determined by: Title XIII, Article 13,
UV100A Ultraviolet Air Treatment System
 UV100A Ultraviolet Air Treatment System INSTALLATION INSTRUCTIONS APPLICATION When installed in forced air heating and cooling systems, the UV100A Ultraviolet Air Treatment System kills airborne microorganism
UV100A Ultraviolet Air Treatment System INSTALLATION INSTRUCTIONS APPLICATION When installed in forced air heating and cooling systems, the UV100A Ultraviolet Air Treatment System kills airborne microorganism
BLOOD BORNE PATHOGENS
 BLOOD BORNE PATHOGENS AIDS and other blood-borne pathogens such as Hepatitis B and C are deadly diseases that are present in today s society. All blood-borne pathogens are transmitted in blood and other
BLOOD BORNE PATHOGENS AIDS and other blood-borne pathogens such as Hepatitis B and C are deadly diseases that are present in today s society. All blood-borne pathogens are transmitted in blood and other
CHAPTER 5 SHIPPING PROCEDURES
 CHAPTER 5 SHIPPING PROCEDURES Chapter 5 SHIPPING PROCEDURES 5.1 SHIPPING CBUS FROM COLLECTION CENTERS TO CORD BLOOD BANKS Principle Collected umbilical cord blood has been found to maintain cell viability
CHAPTER 5 SHIPPING PROCEDURES Chapter 5 SHIPPING PROCEDURES 5.1 SHIPPING CBUS FROM COLLECTION CENTERS TO CORD BLOOD BANKS Principle Collected umbilical cord blood has been found to maintain cell viability
REGULATED MEDICAL WASTE
 REGULATED MEDICAL WASTE GENERATOR FACT SHEET (Revised November 2013) THE NEW JERSEY REGULATED MEDICAL WASTE PROGRAM IS A COMPREHENSIVE MANAGEMENT SYSTEM THAT PROVIDES FOR THE PROPER AND SAFE TRACKING,
REGULATED MEDICAL WASTE GENERATOR FACT SHEET (Revised November 2013) THE NEW JERSEY REGULATED MEDICAL WASTE PROGRAM IS A COMPREHENSIVE MANAGEMENT SYSTEM THAT PROVIDES FOR THE PROPER AND SAFE TRACKING,
MEDICAL WASTE MANAGEMENT
 MEDICAL WASTE MANAGEMENT I. INTRODUCTION Medical waste disposal has become a growing concern for most medical facilities because of increasing regulations and growing public perception. To address these
MEDICAL WASTE MANAGEMENT I. INTRODUCTION Medical waste disposal has become a growing concern for most medical facilities because of increasing regulations and growing public perception. To address these
Managing Regulated Medical Waste in New Mexico
 Managing Regulated Medical Waste in New Mexico Prepared by: Dr. Joe King Camino Real Environmental Research Center Sunland Park, New Mexico History of Medical Waste Regulations 1988 Legislation in response
Managing Regulated Medical Waste in New Mexico Prepared by: Dr. Joe King Camino Real Environmental Research Center Sunland Park, New Mexico History of Medical Waste Regulations 1988 Legislation in response
Instructions for Drawing Blood Samples
 Instructions for Drawing Blood Samples For Blood Collection Supplies please contact us. *Whatever means of restraint is used it should cause minimal distress and should be quick and safe for both the animal
Instructions for Drawing Blood Samples For Blood Collection Supplies please contact us. *Whatever means of restraint is used it should cause minimal distress and should be quick and safe for both the animal
Protocols for Collecting and Storing DNA Samples. Created: March 28, 1998 Revised December 12, 2006
 Protocols for Collecting and Storing DNA Samples Michael K. Schwartz Research Ecologist Rocky Mountain Research Station Missoula Montana Kristy Pilgrim Laboratory Supervisor Rocky Mountain Research Station
Protocols for Collecting and Storing DNA Samples Michael K. Schwartz Research Ecologist Rocky Mountain Research Station Missoula Montana Kristy Pilgrim Laboratory Supervisor Rocky Mountain Research Station
ABORhCard. ABORhCard Package Insert ABO and Rh Blood Grouping Device
 ABORhCard Package Insert ABO and Rh Blood Grouping Device ABORhCard Intended Use The ABORhCard is a qualitative in vitro test that provides a simultaneous ABO and Rh determination of an individual s ABO
ABORhCard Package Insert ABO and Rh Blood Grouping Device ABORhCard Intended Use The ABORhCard is a qualitative in vitro test that provides a simultaneous ABO and Rh determination of an individual s ABO
Leader s Guide E4017. Bloodborne Pathogens: Always Protect Yourself
 E4017 Bloodborne Pathogens: Always Protect Yourself 1 Table of Contents Introduction 3 Video Overview.3 Video Outline.4 Preparing for and Conducting a Presentation. 7 Discussion Ideas..8 Quiz..9 Quiz Answers...11
E4017 Bloodborne Pathogens: Always Protect Yourself 1 Table of Contents Introduction 3 Video Overview.3 Video Outline.4 Preparing for and Conducting a Presentation. 7 Discussion Ideas..8 Quiz..9 Quiz Answers...11
9.Pediatric Procedures
 9.Pediatric Procedures A. Introduction 1. Pediatric blood collection may be by skin puncture or venipuncture. 2. Skill in pediatric phlebotomy is gained by knowledge of special collection equipment, observation
9.Pediatric Procedures A. Introduction 1. Pediatric blood collection may be by skin puncture or venipuncture. 2. Skill in pediatric phlebotomy is gained by knowledge of special collection equipment, observation
Hazardous Materials Disposal Handbook Biohazardous Waste
 Created by: Stuart Elle Date: September 10, 2009 Revised by: Hazardous Materials Disposal Handbook Biohazardous Waste n/a Date: n/a 1. Introduction... 2 1.1. Purpose... 2 1.2. The Role of Hazardous Materials
Created by: Stuart Elle Date: September 10, 2009 Revised by: Hazardous Materials Disposal Handbook Biohazardous Waste n/a Date: n/a 1. Introduction... 2 1.1. Purpose... 2 1.2. The Role of Hazardous Materials
MEDICAL WASTE MANAGEMENT
 MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
MEDICAL WASTE MANAGEMENT Biological Safety INTRODUCTION PURPOSE Regulated medical waste is a designation for wastes that may contain pathogenic microorganisms which was previously termed infectious waste.
GEORGIA INSTITUTE OF TECHNOLOGY ENVIRONMENTAL HEALTH AND SAFETY PERSONAL PROTECTIVE EQUIPMENT
 PURPOSE To protect the health and welfare of GEORGIA TECH employees in areas where there may be a risk of injury or exposure to hazardous substances or conditions employees who work in areas where physical
PURPOSE To protect the health and welfare of GEORGIA TECH employees in areas where there may be a risk of injury or exposure to hazardous substances or conditions employees who work in areas where physical
1. Preparing thin and thick blood films with capillary or venous blood
 Methods in Parasitology 1. Preparing thin and thick blood films with capillary or venous blood Swiss Tropical Institute, Basel April 2005 1.0 Preparing thin and thick blood films Contents 1.1 Preparatory
Methods in Parasitology 1. Preparing thin and thick blood films with capillary or venous blood Swiss Tropical Institute, Basel April 2005 1.0 Preparing thin and thick blood films Contents 1.1 Preparatory
BIOMEDICAL WASTE MANAGEMENT
 BIOMEDICAL WASTE MANAGEMENT Facilitator: Dr. NAVPREET Assistant Professor, Department of Community Medicine Govt. Medical College & Hospital, Chandigarh. Specific Learning Objectives At the end of session,
BIOMEDICAL WASTE MANAGEMENT Facilitator: Dr. NAVPREET Assistant Professor, Department of Community Medicine Govt. Medical College & Hospital, Chandigarh. Specific Learning Objectives At the end of session,
Title: Fingerstick Glucose by Accu-Chek Inform
 Title: Fingerstick Glucose by Accu-Chek Inform Target Audience: This module is available to aid in assessing competency for all clinical staff who perform fingerstick glucose testing. Contents Instructions...
Title: Fingerstick Glucose by Accu-Chek Inform Target Audience: This module is available to aid in assessing competency for all clinical staff who perform fingerstick glucose testing. Contents Instructions...
Using a Pendulum to Measure Gravity s Acceleration Elizabeth B. Chesick
 SCIENCE EXPERIMENTS ON FILE Revised Edition 6.33-1 Using a Pendulum to Measure Gravity s Acceleration Elizabeth B. Chesick Topic Motion of a pendulum; gravity Time 1 2 hour! Safety Please click on the
SCIENCE EXPERIMENTS ON FILE Revised Edition 6.33-1 Using a Pendulum to Measure Gravity s Acceleration Elizabeth B. Chesick Topic Motion of a pendulum; gravity Time 1 2 hour! Safety Please click on the
Mouse Creatine Kinase MB isoenzyme (CKMB) ELISA
 KAMIYA BIOMEDICAL COMPANY Mouse Creatine Kinase MB isoenzyme (CKMB) ELISA For the quantitative determination of mouse CKMB in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-57681
KAMIYA BIOMEDICAL COMPANY Mouse Creatine Kinase MB isoenzyme (CKMB) ELISA For the quantitative determination of mouse CKMB in serum, plasma, cell culture fluid and other biological fluids Cat. No. KT-57681
VC 104+ Rigid Grade / Rigid Grade Imagine VC 104 Rigid Grade Commercial Customised
 Page 1 of 6 instructions VC 104+ Rigid Grade / VC 104+ Rigid Grade Imagine / (for the rest of this document referred to as VC 104) can be applied to new and used trucks and trailers with painted rigid
Page 1 of 6 instructions VC 104+ Rigid Grade / VC 104+ Rigid Grade Imagine / (for the rest of this document referred to as VC 104) can be applied to new and used trucks and trailers with painted rigid
Fiber Analysis 2005, 2004, 2003, 2001, 1999 by David A. Katz. All rights reserved.
 Fiber Analysis 2005, 2004, 2003, 2001, 1999 by David A. Katz. All rights reserved. Fiber evidence can be found at crime scenes in a number of different ways. In personal contact between the clothing of
Fiber Analysis 2005, 2004, 2003, 2001, 1999 by David A. Katz. All rights reserved. Fiber evidence can be found at crime scenes in a number of different ways. In personal contact between the clothing of
Biohazardous Waste and Sharps Disposal
 Biohazardous Waste and Sharps Disposal Federal OSHA Occupational Exposure to Bloodborne Pathogens Standard 29 CFR 1910.1030 State California Code of Regulations (CCR), Medical Waste Management Act, Chapter
Biohazardous Waste and Sharps Disposal Federal OSHA Occupational Exposure to Bloodborne Pathogens Standard 29 CFR 1910.1030 State California Code of Regulations (CCR), Medical Waste Management Act, Chapter
Contents. Introduction... A Note About Heatsinks... Precautions...
 Contents Introduction... A Note About Heatsinks... Precautions... Application Instructions... Heatsink Preparation... CPU Preparation... Tinting the Heatsink and Metal cap... Applying Thermal Compound...
Contents Introduction... A Note About Heatsinks... Precautions... Application Instructions... Heatsink Preparation... CPU Preparation... Tinting the Heatsink and Metal cap... Applying Thermal Compound...
Giving safe injections
 Giving safe injections A guide for nurses and others who give injections World Health Organization International Council of Nurses The World Health Organization (WHO) defines a safe injection to be one
Giving safe injections A guide for nurses and others who give injections World Health Organization International Council of Nurses The World Health Organization (WHO) defines a safe injection to be one
Decontamination and Waste Management www.biosecurity.sandia.gov
 Decontamination and Waste Management www.biosecurity.sandia.gov SAND No. 2006-3684C Sandia is a multiprogram laboratory operated by Sandia Corporation, a Lockheed Martin Company, for the United States
Decontamination and Waste Management www.biosecurity.sandia.gov SAND No. 2006-3684C Sandia is a multiprogram laboratory operated by Sandia Corporation, a Lockheed Martin Company, for the United States
School of Chemistry and Chemical Biology Hazardous Waste Management Plan
 School of Chemistry and Chemical Biology Hazardous Waste Management Plan SOPs for the disposal of hazardous waste generated in the School of Chemistry and Chemical Biology Updated 29/11/12 Page 1 Contents:
School of Chemistry and Chemical Biology Hazardous Waste Management Plan SOPs for the disposal of hazardous waste generated in the School of Chemistry and Chemical Biology Updated 29/11/12 Page 1 Contents:
Beware that ordinary prescription glasses do not provide adequate protection. This is also true with poorly fitting safety glasses.
 Ethidium Bromide Introduction Ethidium bromide (EtBr) is widely used for visualization of nucleic acids in electrophoretic gels. EtBr forms fluorescent complexes, by intercalation of DNA, which are readily
Ethidium Bromide Introduction Ethidium bromide (EtBr) is widely used for visualization of nucleic acids in electrophoretic gels. EtBr forms fluorescent complexes, by intercalation of DNA, which are readily
4. Cryogenic gloves are generally designed to protect the hands from intense cold or heat.
 PROTECTIVE GLOVES In many University laboratories, exposure to chemicals, infectious agents, sharp objects, extreme temperatures and other hazards can create a potential for injury to the hand. Wherever
PROTECTIVE GLOVES In many University laboratories, exposure to chemicals, infectious agents, sharp objects, extreme temperatures and other hazards can create a potential for injury to the hand. Wherever
Policies. Prep Room Policies
 Introduction INTRODUCTION The Microbiology Prep Room is located in 531A Life Sciences Building. The telephone number is 372-8609. It is open from 7:30 a.m. to 4:30 p.m. during the fall and spring semesters.
Introduction INTRODUCTION The Microbiology Prep Room is located in 531A Life Sciences Building. The telephone number is 372-8609. It is open from 7:30 a.m. to 4:30 p.m. during the fall and spring semesters.
Federal Wage System Job Grading Standard For Laboratory Working, 3511. Table of Contents
 Federal Wage System Job Grading Standard For Laboratory Working, 3511 Table of Contents WORK COVERED... 2 WORK NOT COVERED...2 TITLES... 2 GRADE LEVELS... 2 NOTE TO USERS... 3 LABORATORY WORKER, GRADE
Federal Wage System Job Grading Standard For Laboratory Working, 3511 Table of Contents WORK COVERED... 2 WORK NOT COVERED...2 TITLES... 2 GRADE LEVELS... 2 NOTE TO USERS... 3 LABORATORY WORKER, GRADE
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT
 UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT June 2008 Table of Contents Section Page Background 1 Definitions 1-2
UNIVERSITY OF NORTH FLORIDA BIOMEDICAL WASTE MANAGEMENT PLAN DEVELOPED BY: ENVIRONMENTAL HEALTH, SAFETY, INSURANCE & RISK MANAGEMENT June 2008 Table of Contents Section Page Background 1 Definitions 1-2
Parallel Circuits Charles Lang
 SCIENCE EXPERIMENTS ON FILE Revised Edition 6.20-1 Parallel Circuits Charles Lang Topic Parallel circuits Time 1 1 2 hours! Safety Adult supervision is required. Please click on the safety icon to view
SCIENCE EXPERIMENTS ON FILE Revised Edition 6.20-1 Parallel Circuits Charles Lang Topic Parallel circuits Time 1 1 2 hours! Safety Adult supervision is required. Please click on the safety icon to view
VENOUS BLOOD COLLECTION
 Venous Blood Collection p. 1 of 5 Johns Hopkins Medical Laboratories Department of Pathology Core Lab Clinical Services Zd7: Word: JHH Venous Blood Collection Procedure Signature Date Signature Date Written
Venous Blood Collection p. 1 of 5 Johns Hopkins Medical Laboratories Department of Pathology Core Lab Clinical Services Zd7: Word: JHH Venous Blood Collection Procedure Signature Date Signature Date Written
Hemoglobin Testing using the Data Management HemoCue Analyzer
 The Johns Hopkins Medical Institutions Hemoglobin Testing using the Data Management HemoCue Analyzer A Self-Study Packet for The Johns Hopkins Hospital Point-of-Care Testing Program Revision: 12/02 copyright
The Johns Hopkins Medical Institutions Hemoglobin Testing using the Data Management HemoCue Analyzer A Self-Study Packet for The Johns Hopkins Hospital Point-of-Care Testing Program Revision: 12/02 copyright
Minimizing Regulated Medical Waste
 Minimizing Regulated Medical Waste Marcy Yeshnowski Tetra Tech EM Inc. Take Home Message 1. While we re talking about RMW minimization, NEVER compromise safety and compliance. 2. Focus on what you can
Minimizing Regulated Medical Waste Marcy Yeshnowski Tetra Tech EM Inc. Take Home Message 1. While we re talking about RMW minimization, NEVER compromise safety and compliance. 2. Focus on what you can
Parent & Healthcare Professional Instructions for the collection of Maternal & Umbilical Cord Blood
 Parent & Healthcare Professional Instructions for the collection of Maternal & Umbilical Cord Blood 1 2 3 Contents List of Umbilical Cord Blood Collection Kit Thermally insulated transportation box - do
Parent & Healthcare Professional Instructions for the collection of Maternal & Umbilical Cord Blood 1 2 3 Contents List of Umbilical Cord Blood Collection Kit Thermally insulated transportation box - do
ClikSTAR - Important facts about your new insulin delivery device.
 ClikSTAR - Important facts about your new insulin delivery device. Instruction for Use ClikSTAR Insulin delivery device Before you start: Read these instructions and follow them completely each time you
ClikSTAR - Important facts about your new insulin delivery device. Instruction for Use ClikSTAR Insulin delivery device Before you start: Read these instructions and follow them completely each time you
Hemodialysis Access: What You Need to Know
 Hemodialysis Access: What You Need to Know Hemodialysis Access: What You Need To Know Whether you already get hemodialysis treatment, or you will need to start dialysis soon, this booklet will help you
Hemodialysis Access: What You Need to Know Hemodialysis Access: What You Need To Know Whether you already get hemodialysis treatment, or you will need to start dialysis soon, this booklet will help you
The University of Texas at San Antonio Office of Environmental Health, Safety and Risk Management. Part A. Biological Waste Management Safety Plan
 The University of Texas at San Antonio Office of Environmental Health, Safety and Risk Management Part A Biological Waste Management Safety Plan i. SIGNATURE PAGE This Biological Waste Management Safety
The University of Texas at San Antonio Office of Environmental Health, Safety and Risk Management Part A Biological Waste Management Safety Plan i. SIGNATURE PAGE This Biological Waste Management Safety
call 811 to get advice from a nurse, or have someone drive the patient to a hospital Emergency Department. Patients should NOT drive themselves.
 Taking Care at Home After Surgery This checklist is to help you and your support person know what to do after you go home following your surgery. If you are given instructions verbally or in writing by
Taking Care at Home After Surgery This checklist is to help you and your support person know what to do after you go home following your surgery. If you are given instructions verbally or in writing by
Connecticut Biomedical Waste (BMW) Requirements (22a-209-15) and Common Industry Practices
 Connecticut Biomedical Waste (BMW) Requirements (22a-209-15) and Common Industry Practices Mark Latham CT DEP, Waste Engineering and Enforcement Division Common Synonyms for BMW Regulated Medical Waste
Connecticut Biomedical Waste (BMW) Requirements (22a-209-15) and Common Industry Practices Mark Latham CT DEP, Waste Engineering and Enforcement Division Common Synonyms for BMW Regulated Medical Waste
IgM ELISA. For the quantitative determination of IgM in human serum and plasma. For Research Use Only. Not For Use In Diagnostic Procedures.
 IgM ELISA For the quantitative determination of IgM in human serum and plasma For Research Use Only. Not For Use In Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Calibrator
IgM ELISA For the quantitative determination of IgM in human serum and plasma For Research Use Only. Not For Use In Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Calibrator
Ancillary Staff Training
 Ancillary Staff Training Goals of Infection Prevention Protect the patients Protect the staff Prevent spread of diseases How Does The Virus Spread Between People? Direct contact through broken skin, mouth,
Ancillary Staff Training Goals of Infection Prevention Protect the patients Protect the staff Prevent spread of diseases How Does The Virus Spread Between People? Direct contact through broken skin, mouth,
HEEL PRICK PROCEDURE FOR NEWBORN BLOOD SPOT SCREENING
 HEEL PRICK PROCEDURE FOR NEWBORN BLOOD SPOT SCREENING First Issued April 2007 Issue Version Two Purpose of Issue/Description of Change To promote a safe and effective blood spot screening procedure Planned
HEEL PRICK PROCEDURE FOR NEWBORN BLOOD SPOT SCREENING First Issued April 2007 Issue Version Two Purpose of Issue/Description of Change To promote a safe and effective blood spot screening procedure Planned
Percentage of the Medical Waste Stream That Is Regulated Medical Waste Microbiological Waste Pathological Waste Blood and Body Fluids
 Percentage of the Medical Waste Stream That Is Regulated Medical Waste Most medical waste may be handled as general solid waste and does not require treatment. Regulated medical waste makes up only a very
Percentage of the Medical Waste Stream That Is Regulated Medical Waste Most medical waste may be handled as general solid waste and does not require treatment. Regulated medical waste makes up only a very
UNIVERSITY OF RICHMOND REGULATED MEDICAL WASTE MANAGEMENT GUIDELINES
 UNIVERSITY OF RICHMOND REGULATED MEDICAL WASTE MANAGEMENT GUIDELINES November 2003 Table of Contents Section Page I. Introduction.... 1 II. Characteristics of Regulated Medical Waste 1-2 III. Exclusions...2-3
UNIVERSITY OF RICHMOND REGULATED MEDICAL WASTE MANAGEMENT GUIDELINES November 2003 Table of Contents Section Page I. Introduction.... 1 II. Characteristics of Regulated Medical Waste 1-2 III. Exclusions...2-3
Ward 29 guide to the safe preparation and administration of intravenous (IV) antibiotics at home
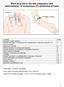 Ward 29 guide to the safe preparation and administration of intravenous (IV) antibiotics at home Contents Page Important contact numbers 1 General information on preparing and administering IV antibiotics
Ward 29 guide to the safe preparation and administration of intravenous (IV) antibiotics at home Contents Page Important contact numbers 1 General information on preparing and administering IV antibiotics
Body Art Facility Infection Prevention And Control Plan Guideline
 Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
Body Art Facility Infection Prevention And Control Plan Guideline In accordance with the California Health and Safety Code, Section 119313, a body art facility shall maintain and follow a written Infection
URINE COLLECTION, PREPARATION AND HANDLING
 URINE III-38 URINE COLLECTION, PREPARATION AND HANDLING I. SPECIMEN COLLECTION A. Introduction Laboratory tests requiring urine specimens involve a wide variety of procedures. A basic urinalysis is almost
URINE III-38 URINE COLLECTION, PREPARATION AND HANDLING I. SPECIMEN COLLECTION A. Introduction Laboratory tests requiring urine specimens involve a wide variety of procedures. A basic urinalysis is almost
"ADOPTED STANDARDS FOR THE REGULATION OF MEDICAL WASTE" IN HEALTH CARE FACILITIES LICENSED BY THE MISSISSIPPI STATE DEPARTMENT OF HEALTH
 "ADOPTED STANDARDS FOR THE REGULATION OF MEDICAL WASTE" IN HEALTH CARE FACILITIES LICENSED BY THE MISSISSIPPI STATE DEPARTMENT OF HEALTH REGULATED MEDICAL WASTE "Infectious medical wastes" includes solid
"ADOPTED STANDARDS FOR THE REGULATION OF MEDICAL WASTE" IN HEALTH CARE FACILITIES LICENSED BY THE MISSISSIPPI STATE DEPARTMENT OF HEALTH REGULATED MEDICAL WASTE "Infectious medical wastes" includes solid
FLORENCE TOWNSHIP BOARD OF EDUCATION FILE CODE: 4112.4/4212.4 Florence, New Jersey
 FLORENCE TOWNSHIP BOARD OF EDUCATION FILE CODE: 4112.4/4212.4 Florence, New Jersey Regulation Exposure Control Administration BLOODBORNE PATHOGENS A. The district safety and health program officer, district
FLORENCE TOWNSHIP BOARD OF EDUCATION FILE CODE: 4112.4/4212.4 Florence, New Jersey Regulation Exposure Control Administration BLOODBORNE PATHOGENS A. The district safety and health program officer, district
Introduction 1 The system 1 The meter 2 The display 3 The mode 3 The measurement 4 Coding the meter 4 How to obtain a drop of blood 6 Application of
 Introduction 1 The system 1 The meter 2 The display 3 The mode 3 The measurement 4 Coding the meter 4 How to obtain a drop of blood 6 Application of the blood 7 Procedure to test glucose 7 Procedure to
Introduction 1 The system 1 The meter 2 The display 3 The mode 3 The measurement 4 Coding the meter 4 How to obtain a drop of blood 6 Application of the blood 7 Procedure to test glucose 7 Procedure to
Bacterial Transformation with Green Fluorescent Protein. Table of Contents Fall 2012
 Bacterial Transformation with Green Fluorescent Protein pglo Version Table of Contents Bacterial Transformation Introduction..1 Laboratory Exercise...3 Important Laboratory Practices 3 Protocol...... 4
Bacterial Transformation with Green Fluorescent Protein pglo Version Table of Contents Bacterial Transformation Introduction..1 Laboratory Exercise...3 Important Laboratory Practices 3 Protocol...... 4
Safe Work Procedure Sharps and Bio-hazardous waste handling and disposal
 Safe Work Procedure Sharps and Bio-hazardous waste handling and disposal Task/Activity: Acupuncture and Musculoskeletal therapy (MST) clinics and practical classrooms (Acupuncture & MST Skin Infection
Safe Work Procedure Sharps and Bio-hazardous waste handling and disposal Task/Activity: Acupuncture and Musculoskeletal therapy (MST) clinics and practical classrooms (Acupuncture & MST Skin Infection
