Picato gel. New Zealand Data Sheet. 1 Name of Medicine. 2 Qualitative and Quantitative Composition. 3 Pharmaceutical Form. 4 Clinical Particulars
|
|
|
- Jonah Dorsey
- 7 years ago
- Views:
Transcription
1 New Zealand Data Sheet Picato gel Ingenol mebutate 0.015% Ingenol mebutate 0.05% 1 Name of Medicine Picato gel, 0.015% Picato gel, 0.05% 2 Qualitative and Quantitative Composition Picato gel 0.015%: Each tube contains 70 mcg of ingenol mebutate in 0.47 g of gel. Picato gel 0.05%: Each tube contains 235 mcg of ingenol mebutate in 0.47 g of gel. Excipients: For the full list of excipients, see section Pharmaceutical Form Clear colourless gel. 4 Clinical Particulars 4.1 Therapeutic Indications Picato gel is indicated for the topical treatment of solar (actinic) keratoses in adults. 4.2 Posology and Method of Administration Posology Solar (actinic) keratoses on face and scalp in adults Picato gel 0.015% should be applied to the affected area once daily for 3 consecutive days. Solar (actinic) keratoses on the trunk and extremities (body) in adults Picato gel 0.05% should be applied to the affected area once daily for 2 consecutive days. Paediatric population There is no relevant use of Picato gel in the paediatric population. Elderly population No dose adjustment is required (see section 5.1). Method of administration Picato gel should be applied to the treatment area as defined by the treating physician. Each tube contains enough gel to cover an area of approximately 25 cm 2 (e.g. 5 cm x 5 cm). Picato Gel NZ Datasheet Page 1 of 10
2 The gel from the single dose tube should be squeezed onto a fingertip and spread evenly over the entire treatment area, allowing it to dry for 15 minutes. One single dose tube should be used for the treatment area. Patients should be instructed to wash their hands with soap and water immediately after applying Picato gel. If treating the hands, only the fingertip which is used for applying the gel should be washed. Washing and touching the treated area should be avoided for a period of 6 hours after application of Picato gel. After this period, the treatment area may be washed using mild soap and water. Picato gel should not be applied immediately after taking a shower or less than 2 hours before bedtime. The treated area should not be covered with occlusive bandages after Picato gel is applied. Optimal therapeutic effect can be assessed approximately 8 weeks after treatment. 4.3 Contraindications Hypersensitivity to the active substance or to any of the excipients, listed in section Special Warnings and Precautions for Use Avoid contact with, or inadvertent transfer to, the eyes during treatment and for 6 hours after treatment with Picato gel. If accidental exposure occurs, the eyes should be flushed immediately with large amounts of water, and the patient should seek medical care as soon as possible. Picato gel must not be ingested. If accidental ingestion occurs the patient should drink plenty of water. Local skin responses such as erythema, flaking/scaling, and crusting can occur after topical application of Picato gel (see section 4.8). These local skin responses have been shown to be associated with the clinical efficacy. Localised skin responses are transient and typically occur within 1 day of treatment initiation and peak in intensity up to 1 week following completion of treatment. Localised skin responses typically resolve within 2 weeks of treatment initiation when treating areas on the face and scalp and within 4 weeks of treatment initiation when treating areas on the trunk and extremities. Treatment effect may not be adequately assessed until resolution of local skin responses. Administration of Picato gel is not recommended until the skin is healed from treatment with any previous medicinal product or surgical treatment or from any open wounds. Clinical data on treatment for more that one treatment course of 2 to 3 consecutive days are not available. Studies have been conducted to assess the effects of UV irradiation of the skin following single and multiple applications of ingenol mebutate gel, 0.01%. Ingenol mebutate gel did not demonstrate any potential for photo irritation or photo allergic effects. However, due to the nature of the disease, excessive exposure to sunlight (including sunlamps and tanning beds) should be avoided or minimised. Picato Gel NZ Datasheet Page 2 of 10
3 4.5 Interaction with other Medicinal Products and Other Forms of Interaction No interaction studies have been performed. Interactions with systemically absorbed medicinal products are considered unlikely as Picato gel is not absorbed systemically. 4.6 Fertility, Pregnancy and Lactation Pregnancy There are no data from the use of ingenol mebutate in pregnant women. Animal studies do not indicate harmful effects with respect to reproductive toxicity (see section 5.3). Risks to humans receiving topical treatment with Picato gel are considered unlikely as ingenol mebutate gel is not absorbed systemically. As a precautionary measure, it is preferable to avoid the use of Picato gel during pregnancy. Breastfeeding No effects on the breastfed newborn/infant are anticipated as ingenol mebutate gel is not absorbed systemically. The nursing mother should be instructed that physical contact between her newborn/infant and the treated area should be avoided for a period of 6 hours after application of Picato gel. Fertility No fertility studies have been performed with ingenol mebutate. 4.7 Effects on ability to drive and use machines Picato gel has no influence on the ability to drive and use machines. 4.8 Undesirable Effects Summary of the safety profile The most frequently reported adverse reactions assessed by investigators are local skin responses including erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation and erosion/ulceration at the application site of ingenol mebutate gel, see Table 1 for MedDRA terms. Following the application of ingenol mebutate, most patients (>95%) experienced one or more local skin response(s). The local skin responses are transient and typically occur within 1 day of treatment initiation and peak in intensity up to 1 week following completion of treatment. These effects typically resolve within 2 weeks of treatment initiation for areas treated on the face and scalp and within 4 weeks of treatment initiation for areas treated on the trunk and extremities. Tabulated summary of adverse reactions Table 1 reflects exposure to Picato gel (0.015% or 0.05%) in 499 patients with solar (actinic) keratoses treated in four vehicle controlled phase 3 studies enrolling a total of 1002 patients. Patients received field treatment (area of 25 cm 2 ) with Picato gel at concentrations of 0.015% or 0.05%or vehicle once daily for 3 or 2 consecutive days respectively. The table below presents adverse reactions by MedDRA system organ class. Picato Gel NZ Datasheet Page 3 of 10
4 Frequencies have been defined according to the following convention: Very common ( 1/10); common ( 1/100 to <1/10); uncommon ( 1/1,000 to <1/100); rare ( 1/10,000 to <1/1,000); very rare (<1/10,000) Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness. Table 1 Adverse reactions by MedDRA System Organ Class Frequency System Organ Class Face and Scalp Trunk and extremities Infections and Infestations Application site pustules Very common Very common Application site infection Common Nervous system disorders Headache Common Eye Disorders* Eye lid oedema Common Eye pain Uncommon Periorbital oedema Common General disorders and administration site conditions Application site erosion Very common Very common Application site vesicles Very common Very common Application site swelling Very common Very common Application site exfoliation Very common Very common Application site scab Very common Very common Application site erythema Very common Very common Application site pain** Very common Common Application site pruritus Common Common Application site irritation Common Common Application site discharge Uncommon Application site paraesthesia Uncommon Uncommon Application site ulcer Uncommon Uncommon Application site warmth Uncommon * Application site swelling on the face or scalp may gravitate to the eye area ** Including application site burning Long-term follow up A total of 198 patients (184 treated with Picato gel and 14 treated with vehicle) were enrolled in long-term efficacy follow-up studies. Results from these studies did not change the safety profile of Picato gel (see section 5.1). Picato Gel NZ Datasheet Page 4 of 10
5 Post-marketing Experience In addition to the adverse reactions reported during clinical studies and listed above, the following adverse reactions have been identified from post-marketing experience. It is not always possible to estimate their incidence. Application site pigmentation changes are uncommon but have been reported. Rare cases of application site scarring have been reported. Accidental eye exposure: Post-marketing reports of chemical conjunctivitis and corneal burn in connection with accidental eye exposure have been received (see section 4.4). 4.9 Overdose There has been no experience of overdose in clinical studies with Picato gel. In a clinical study 4 single dose tubes of Picato gel 0.05% was applied daily for 2 consecutive days to a 100 cm 2 area of skin for the treatment of solar (actinic) keratoses. The result demonstrated no change in the safety profile compared to the safety profile of Picato gel 0.05% when 1 tube is applied to a 25 cm 2 area for 2 consecutive days. 5 Pharmacological Properties 5.1 Pharmacodynamic properties Pharmacotherapeutic group: Antibiotics and chemotherapeutics for dermatological use, other chemotherapeutics, ATC code: D06BX02. Mechanism of action The mechanism of action of ingenol mebutate for use in solar (actinic) keratoses remains to be fully characterised. In vivo and in vitro models have shown a dual mechanism of action for the effects of ingenol mebutate: 1) induction of local lesion cell death and 2) promoting an inflammatory response characterised by local production of proinflammatory cytokines and chemokines and infiltration of immunocompetent cells. Pharmacodynamic effects Results from two clinical trials on biological effects of ingenol mebutate have shown that topical administration induced epidermal necrosis and a profound inflammatory response in both epidermis and the upper dermis of the treated skin, dominated by infiltrating T cells, neutrophils and macrophages. Necrosis in the dermis was rarely observed. Gene expression profiles of skin biopsies from the treated areas is suggestive of inflammatory responses and response to wounding, which is consistent with the histology assessments. Non-invasive examination of the treated skin by reflectance confocal microscopy have shown that the skin changes induced by ingenol mebutate were reversible, with almost complete normalisation of all measured parameters on day 57 after treatment, which is supported also by clinical findings and studies in animals. Picato Gel NZ Datasheet Page 5 of 10
6 Clinical efficacy and safety The efficacy and safety of Picato gel 0.015%, administered on the face or scalp for 3 consecutive days was studied in two double-blind, vehicle-controlled, clinical studies including 547 adult patients. Likewise the efficacy and safety of Picato gel 0.05%, administered on the trunk or extremities for 2 consecutive days was studied in two doubleblind, vehicle-controlled, clinical studies including 458 adult patients. Patients continued in the studies for an 8 week follow-up period during which they returned for clinical observations and safety monitoring. Efficacy, measured as complete and partial clearance rate, as well as median percent reduction, was assessed at day 57 (see Table 2). Patients had 4 to 8 clinically typical, visible, discrete, non-hyperkeratotic, non-hypertrophic, solar (actinic) keratoses lesions on the face or scalp within a contiguous 25 cm 2 treatment area on the face or scalp or on the trunk and extremities. On each scheduled dosing day, the study gel was applied to the entire treatment area. The compliance rate was high, with 98% of the patients completing these studies. Study patients ranged from 34 to 89 years of age (mean 64 and 66 years, respectively, for the two strengths) and 94% had Fitzpatrick skin type I, II, or III. At day 57, patients treated with Picato gel had higher complete and partial clearance rates than patients treated with vehicle gel (p<0.001). The median percent reduction in solar (actinic) keratoses lesions was higher in the group treated with ingenol mebutate compared to the vehicle group (see Table 2). Table 2 Rates of subjects with complete and partial clearance and median percent (%) lesion reduction in actinic keratoses Face and scalp Picato gel 0.015% (n=277) Vehicle (n=270) Trunk and Extremities Picato gel 0.05% (n=226) Vehicle (n=232) Complete Clearance Rate a 42.2% d 3.7% 34.1% d 4.7% Partial Clearance Rate b ( 75%) 63.9% d 7.4% 49.1% d 6.9% Median % Reduction c 83% 0% 75% 0% a Complete clearance rate was defined as the proportion of patients with no (zero) clinically visible solar (actinic) keratoses lesions in the treatment area. b Partial clearance rate was defined as the percentage of patients in whom 75% or more of the number of baseline solar (actinic) keratoses lesions were cleared. c Median percent (%) reduction in solar (actinic) keratoses lesions compared to baseline. d p<0.001; compared to vehicle by logistic regression with treatment, study and anatomical location. Safety of Picato gel 0.015% treatment for 3 days or Picato gel 0.05% treatment for 2 days was assessed up to day 57 and ingenol mebutate gel was found to be well tolerated. All adverse drug reactions and local skin responses resolved without sequelae. Picato Gel NZ Datasheet Page 6 of 10
7 Statistically significant differences in patient reported outcomes were observed in favour of patients receiving Picato gel compared to those receiving vehicle gel. Higher mean patient global satisfaction scores, indicating a higher level of overall satisfaction, were seen in the ingenol mebutate groups compared to the vehicle groups (p<0.001) as measured by the Treatment Satisfaction Questionnaire for Medication (TSQM). Long term efficacy Three prospective, observational long term 1 year follow-up studies were conducted to evaluate sustained efficacy by recurrence of solar (actinic) keratoses lesions in the treatment field, and safety in patients who had received treatment with Picato gel. One study included patients treated with Picato gel 0.015% on the face or scalp for 3 days and two studies included patients treated with Picato gel 0.05% on the truck or extremities (body) for 2 days. Only those patients who achieved complete clearance in the treated area at the end of the phase 3 studies (day 57) were eligible for long term follow-up. Patients were followed every 3 months for 12 months (see Table 3 and 4). Table 3 Rate of recurrence of solar (actinic) keratoses lesions on face and scalp Picato gel 0.015% (n=108) Recurrence Rate 12 months KM estimate (95% CI) a 53.9% ( ) Lesion Based Recurrence Rate b 12 months 12.8% (19.1) Mean (SD) a The recurrence rate is the Kaplan-Meier (KM) estimate at the target study date of the visit expressed as a percentage (95% CI). Recurrence was defined as any identified solar (actinic) keratoses lesion in the previously treated area for patients who achieved complete clearance at day 57 in the previous phase 3 studies. b The lesion-based recurrence rate for each patient defined as the ratio of the number of solar (actinic) keratoses lesions at 12 months to the number of lesions at baseline in the previous phase 3 studies. Picato Gel NZ Datasheet Page 7 of 10
8 Table 4 Rate of recurrence of solar (actinic) keratoses lesions on trunk and extremities (body) Picato gel 0.05% (n=76 c ) Recurrence Rate 12 months KM estimate (95% CI) a 56.0% ( ) Lesion Based Recurrence Rate b 12 months Mean (SD) 13.2% (23.0) a The recurrence rate is the Kaplan-Meier (KM) estimate at the target study date of the visit expressed as a percentage (95% CI). Recurrence was defined as any identified solar (actinic) keratoses lesion in the previously treated area for patients who achieved complete clearance at day 57 in a previous phase 3 studies. b The lesion-based recurrence rate for each patient defined as the ratio of the number of solar (actinic) keratoses lesions at 12 months to the number of lesions at baseline in the previous phase 3 studies. c Of these, 38 subjects were previously treated in a vehicle controlled phase 3 study and 38 subjects were previously treated in an uncontrolled phase 3 study. Risk of progression to squamous cell carcinoma At end of study (day 57), the rate of squamous cell carcinoma (SCC) reported in the treatment area was comparable in patients treated with ingenol mebutate gel (0.3%, 3 of 1165 patients) and in vehicle treated patients (0.3%, 2 of 632 patients) in the solar (actinic) keratoses clinical studies conducted with ingenol mebutate. SCC in the treatment area was reported in no patients (0 of 184 patients previously treated with ingenol mebutate gel) in the three prospective, observational long term 1 year follow-up studies. Experience with treatment of a larger area In a double-blind, vehicle-controlled study to evaluate systemic exposure, ingenol mebutate 0.05% gel, from 4 single dose tubes, was applied to a 100 cm 2 contiguous treatment area daily for 2 consecutive days. Results demonstrated no systemic absorption. Picato gel 0.05% was well tolerated when applied to a contiguous treatment area of 100 cm 2 on the trunk and extremities (body). Elderly population Of the 1165 patients treated with Picato gel in the solar (actinic) keratoses clinical studies conducted with ingenol mebutate gel, 656 patients (56%) were 65 years and older, while 241 patients (21%) were 75 years and older. No overall differences in safety or efficacy were observed between younger and older patients. 5.2 Pharmacokinetic properties The systemic pharmacokinetic profile of ingenol mebutate and its metabolites has not been characterised in humans due to the absence of quantifiable whole blood levels following topical administration. Picato Gel NZ Datasheet Page 8 of 10
9 No systemic absorption was detected at or above the lower limit of detection (0.1 ng/ml) when Picato gel 0.05% from 4 single dose tubes was applied to an area of 100 cm 2 on the dorsal forearm in solar (actinic) keratoses patients once daily for 2 consecutive days. In vitro study results demonstrate that ingenol mebutate does not inhibit or induce human cytochrome P450 isoforms. 5.3 Preclinical safety data Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity and genotoxicity. The non-clinical safety studies demonstrate that topical administration of ingenol mebutate gel is well tolerated with skin irritation potential and negligible risk of systemic toxicity under the recommended conditions of use. In rats, ingenol mebutate was not associated with foetal developmental effects at IV doses up to 5 mcg/kg/day (30 mcg/m 2 /day). In rabbits there were no major abnormalities. Minor foetal abnormalities or variants were observed in the foetuses of treated dams; however, the findings did not suggest a clear association with IV ingenol mebutate administration. The foetal NOAEL was 1 mcg/kg/day (12 mcg/m 2 /day). 6 Pharmaceutical Particulars 6.1 List of Excipients Isopropyl alcohol Hydroxyethylcellulose Citric acid monohydrate Sodium citrate Benzyl alcohol Purified water 6.2 Shelf life 2 years. Tubes should not be re-used once opened. 6.3 Special precautions for storage Store in refrigerator (2ºC 8ºC). 6.4 Nature and contents of container Single dose laminate tubes with inner layer of High Density Polyethylene (HDPE) and aluminium as the barrier layer. Caps of HDPE. Picato gel 0.015% for application to the face and scalp is available in a carton containing 3 single-dose tubes with 0.47 g of gel each. Picato gel 0.05% for application to the body is available in a carton containing 2 single-dose tubes with 0.47 g of gel each. Picato Gel NZ Datasheet Page 9 of 10
10 6.5 Special precautions for disposal and other handling Nil. 7 Medicine Classification Prescription medicine 8 Sponsor Details LEO Pharma Ltd Level 31, Vero Centre 48 Shortland Street Auckland 1010 New Zealand 9 Date of Preparation 12 September 2016 Picato Gel NZ Datasheet Page 10 of 10
ZOVIRAX Cold Sore Cream
 Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
Data Sheet ZOVIRAX Cold Sore Cream Aciclovir 5% w/w Presentation Topical cream Indications ZOVIRAX Cold Sore Cream is indicated for the treatment of Herpes simplex virus infections of the lips and face
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 10 g of solution contains 0.2 g of erythromycin. Structural formula of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 10 g of solution contains 0.2 g of erythromycin. Structural formula of
NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate.
 NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate. PRESENTATION Eye Drops: NAPHCON-A Eye Drops are a combination of an antihistamine (pheniramine maleate) and a decongestant
NEW ZEALAND DATA SHEET NAPHCON-A Naphazoline hydrochloride and pheniramine maleate. PRESENTATION Eye Drops: NAPHCON-A Eye Drops are a combination of an antihistamine (pheniramine maleate) and a decongestant
NEUROTONE THR 00904/0005 UKPAR
 NEUROTONE THR 00904/0005 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 10 Summary of Product Characteristics Page 11 Product Information Leaflet
NEUROTONE THR 00904/0005 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 10 Summary of Product Characteristics Page 11 Product Information Leaflet
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON HYPERICUM PERFORATUM L., HERBA (TRADITIONAL USE)
 European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMEA/HMPC/745582/2009 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH
European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMEA/HMPC/745582/2009 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013.
 The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013 Havrix 720 Junior 1 NAME OF THE MEDICINAL PRODUCT Havrix 720 Junior 2 QUALITATIVE
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in May 2013 Havrix 720 Junior 1 NAME OF THE MEDICINAL PRODUCT Havrix 720 Junior 2 QUALITATIVE
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prednicare Tablets 5mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active Substance(s) Prednisolone
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prednicare Tablets 5mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains: Active Substance(s) Prednisolone
One vial contains 500 U* of C1-inhibitor** After reconstitution the product contains 500 U/5 ml which correspond to a concentration of 100 U/ml.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cetor 100 U/ml powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 500 U* of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Cetor 100 U/ml powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 500 U* of
Hypromellose Eye Drops BP 0.3% w/v PL 23097/0006
 Hypromellose Eye Drops BP 0.3% w/v PL 23097/0006 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
Hypromellose Eye Drops BP 0.3% w/v PL 23097/0006 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
The clinical studies have been performed in children, adolescents and adults, from 4 years up to 55 years of age.
 NAME OF THE MEDICINE TdaP-Booster. Diphtheria, tetanus and pertussis (acellular mono-component) vaccine (adsorbed, reduced antigen content). DESCRIPTION TdaP-Booster is a suspension for injection in pre-filled
NAME OF THE MEDICINE TdaP-Booster. Diphtheria, tetanus and pertussis (acellular mono-component) vaccine (adsorbed, reduced antigen content). DESCRIPTION TdaP-Booster is a suspension for injection in pre-filled
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Isotrexin 2% + 0.05% w/w Gel 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Erythromycin Isotretinoin 2.00% w/w 0.05% w/w Excipients: Contains
Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Isotrexin 2% + 0.05% w/w Gel 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Erythromycin Isotretinoin 2.00% w/w 0.05% w/w Excipients: Contains
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON AVENA SATIVA L., FRUCTUS
 European Medicines Agency Evaluation of Medicines for Human Use London, 4 September 2008 Doc. Ref. EMEA/HMPC/368600/2007 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON
European Medicines Agency Evaluation of Medicines for Human Use London, 4 September 2008 Doc. Ref. EMEA/HMPC/368600/2007 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON
Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement
 Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement A Copy of this page signed by all three parties should be retained in the
Topical Tacrolimus or Pimecrolimus for the treatment of mild, moderate or severe atopic eczema. Effective Shared Care Agreement A Copy of this page signed by all three parties should be retained in the
Summary of the risk management plan (RMP) for Otezla (apremilast)
 EMA/741412/2014 Summary of the risk management plan (RMP) for Otezla (apremilast) This is a summary of the risk management plan (RMP) for Otezla, which details the measures to be taken in order to ensure
EMA/741412/2014 Summary of the risk management plan (RMP) for Otezla (apremilast) This is a summary of the risk management plan (RMP) for Otezla, which details the measures to be taken in order to ensure
RADIOPHARMACEUTICALS BASED ON MONOCLONAL ANTIBODIES
 RADIOPHARMACEUTICALS BASED ON MONOCLONAL ANTIBODIES Guideline Title Radiopharmaceuticals based on Monoclonal Antibodies Legislative basis Directives 65/65/EEC, 75/318/EEC as amended, Directive 89/343/EEC
RADIOPHARMACEUTICALS BASED ON MONOCLONAL ANTIBODIES Guideline Title Radiopharmaceuticals based on Monoclonal Antibodies Legislative basis Directives 65/65/EEC, 75/318/EEC as amended, Directive 89/343/EEC
RETIRIDES - Information for the user and usage instructions Translated from Spanish by Vaughter Wellness Ltd.
 RETIRIDES - Information for the user and usage instructions Translated from Spanish by Vaughter Wellness Ltd. Table of Contents 1. Name of the product...2 2. Qualitative and quantitative composition...2
RETIRIDES - Information for the user and usage instructions Translated from Spanish by Vaughter Wellness Ltd. Table of Contents 1. Name of the product...2 2. Qualitative and quantitative composition...2
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT ACEGON, 50 microgram/ml, solution for injection for cattle. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains: Active
Summary of Product Characteristics 1 NAME OF THE VETERINARY MEDICINAL PRODUCT ACEGON, 50 microgram/ml, solution for injection for cattle. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains: Active
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Plus 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 100 g of solution contains: Erythromycin 4.0 g, Tretinoin 0.025 g. For a full
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Aknemycin Plus 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 100 g of solution contains: Erythromycin 4.0 g, Tretinoin 0.025 g. For a full
PACKAGE LEAFLET: INFORMATION FOR THE USER. Elidel 10 mg/g Cream. pimecrolimus
 PACKAGE LEAFLET: INFORMATION FOR THE USER Elidel 10 mg/g Cream pimecrolimus Read all of this leaflet carefully before you start using Elidel cream Keep this leaflet. You may need to read it again. If you
PACKAGE LEAFLET: INFORMATION FOR THE USER Elidel 10 mg/g Cream pimecrolimus Read all of this leaflet carefully before you start using Elidel cream Keep this leaflet. You may need to read it again. If you
SUMMARY OF PRODUCT CHARACTERISTICS. Albuman 200 g/l is a solution containing 200 g/l (20%) of total protein of which at least 95% is human albumin.
 Albuman 200 g/l SPC 01 December 2015 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Albuman 200 g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Albuman 200 g/l
Albuman 200 g/l SPC 01 December 2015 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Albuman 200 g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Albuman 200 g/l
Community herbal monograph on Commiphora molmol Engler, gummi-resina
 12 July 2011 EMA/HMPC/96911/2010 Committee on Herbal Medicinal Products (HMPC) Community herbal monograph on Commiphora molmol Engler, gummi-resina Final Discussion in Working Party on Community monographs
12 July 2011 EMA/HMPC/96911/2010 Committee on Herbal Medicinal Products (HMPC) Community herbal monograph on Commiphora molmol Engler, gummi-resina Final Discussion in Working Party on Community monographs
Annex II. Scientific conclusions and grounds for revocation of the marketing authorisations
 Annex II Scientific conclusions and grounds for revocation of the marketing authorisations 4 Scientific conclusions and grounds for revocation of the marketing authorisations Overall summary of the scientific
Annex II Scientific conclusions and grounds for revocation of the marketing authorisations 4 Scientific conclusions and grounds for revocation of the marketing authorisations Overall summary of the scientific
Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid
 Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
Package Leaflet: Information for the User Thioctacid 600 T Solution for Injection contains 600 mg alpha-lipoic acid For use in adults Active substance: Alpha-lipoic acid, Trometamol salt (1:1) Read all
Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel
 Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel PL 10972/0089 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 11 Summary of Product Characteristics
Ibuprofen 10% w/w gel Nurofen Maximum Strength 10 % Gel PL 10972/0089 UKPAR TABLE OF CONTENTS Lay Summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 11 Summary of Product Characteristics
PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement)
 1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
1 PHOSPHATE-SANDOZ Tablets (High dose phosphate supplement) PHOSPHATE-SANDOZ PHOSPHATE-SANDOZ Tablets are a high dose phosphate supplement containing sodium phosphate monobasic. The CAS registry number
Having regard to the Treaty establishing the European Economic Community, and in particular Article 100 thereof;
 DIRECTIVE 65/65/EEC Council Directive 65/65/EEC of 26 January 1965 on the approximation of provisions laid down by law, regulation or administrative action relating to medicinal products (OJ L No 22 of
DIRECTIVE 65/65/EEC Council Directive 65/65/EEC of 26 January 1965 on the approximation of provisions laid down by law, regulation or administrative action relating to medicinal products (OJ L No 22 of
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON HYPERICUM PERFORATUM L., HERBA (WELL-ESTABLISHED MEDICINAL USE)
 European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMA/HMPC/101304/2008 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON
European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMA/HMPC/101304/2008 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON
VOLTAREN OPHTHA EYE DROPS
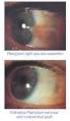 פורמט עלון זה נקבע ע"ימשרד הבריאות ותוכנו נבדק ואושר על ידו באפריל 2008 PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
פורמט עלון זה נקבע ע"ימשרד הבריאות ותוכנו נבדק ואושר על ידו באפריל 2008 PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
Always take this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse have told you.
 leaflet: Information for the user Macrogol 4000 10 g powder for oral solution in sachet Macrogol 4000
leaflet: Information for the user Macrogol 4000 10 g powder for oral solution in sachet Macrogol 4000
SUMMARYOF PRODUCT CHARACTERISTICS
 SUMMARYOF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Excis 10 mg/ml concentrate for solution for fish treatment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance: Cypermethrin
SUMMARYOF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Excis 10 mg/ml concentrate for solution for fish treatment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance: Cypermethrin
MATERIAL SAFETY DATA SHEET
 Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Summary of the risk management plan (RMP) for Orkambi (lumacaftor and ivacaftor)
 EMA/662624/2015 Summary of the risk management plan (RMP) for Orkambi (lumacaftor and ivacaftor) This is a summary of the risk management plan (RMP) for Orkambi, which details the measures to be taken
EMA/662624/2015 Summary of the risk management plan (RMP) for Orkambi (lumacaftor and ivacaftor) This is a summary of the risk management plan (RMP) for Orkambi, which details the measures to be taken
Public Assessment Report Scientific discussion. Tenofovir disoproxil Teva (tenofovir disoproxil) SE/H/1432/01/DC
 Public Assessment Report Scientific discussion Tenofovir disoproxil Teva (tenofovir disoproxil) SE/H/1432/01/DC This module reflects the scientific discussion for the approval of Tenofovir disoproxil Teva.
Public Assessment Report Scientific discussion Tenofovir disoproxil Teva (tenofovir disoproxil) SE/H/1432/01/DC This module reflects the scientific discussion for the approval of Tenofovir disoproxil Teva.
Mucodyne-Clear 250 mg/5 ml Syrup PL 04425/0665
 Mucodyne-Clear 250 mg/5 ml Syrup PL 04425/0665 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
Mucodyne-Clear 250 mg/5 ml Syrup PL 04425/0665 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 9 Summary of product characteristics Page 10 Patient
NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE NONCLINICAL EVALUATION FOR ANTICANCER PHARMACEUTICALS
SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product)
 PART VI SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product) Format and content of the summary of the RMP The summary of the RMP part VI contains information based on RMP modules SI, SVIII and RMP
PART VI SUMMARY OF THE RISK MANAGEMENT PLAN (by medicinal product) Format and content of the summary of the RMP The summary of the RMP part VI contains information based on RMP modules SI, SVIII and RMP
Facts About Chickenpox and Shingles for Adults
 Facts About Chickenpox and Shingles for Adults What is chickenpox? Chickenpox, also known as varicella, is a very contagious disease caused by the varicella-zoster virus. It is spread easily through the
Facts About Chickenpox and Shingles for Adults What is chickenpox? Chickenpox, also known as varicella, is a very contagious disease caused by the varicella-zoster virus. It is spread easily through the
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 Temporary Core Product Information Inde- (13May2013) Page 1 / 7 1 NAME OF THE MEDICINAL PRODUCT XYLONOR SPRAY, oromucosal spray, solution. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each gram contains:
Temporary Core Product Information Inde- (13May2013) Page 1 / 7 1 NAME OF THE MEDICINAL PRODUCT XYLONOR SPRAY, oromucosal spray, solution. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each gram contains:
DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief
 DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief Common causes of itching due to eye allergies include: Pollen from trees, grasses, and ragweed Dust mites Cat dander Approximately 10 million
DO YOU HAVE ITCHY ALLERGY EYES? Find out about lasting relief Common causes of itching due to eye allergies include: Pollen from trees, grasses, and ragweed Dust mites Cat dander Approximately 10 million
Biologic Treatments for Rheumatoid Arthritis
 Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
Biologic Treatments Rheumatoid Arthritis (also known as cytokine inhibitors, TNF inhibitors, IL 1 inhibitor, or Biologic Response Modifiers) Description Biologics are new class of drugs that have been
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE PRE-CLINICAL EVALUATION OF ANTICANCER MEDICINAL PRODUCTS
 The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 23 July 1998 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE PRE-CLINICAL
The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit London, 23 July 1998 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON THE PRE-CLINICAL
MATERIAL SAFETY DATA SHEET TOILET BOWL SANITIZER
 MATERIAL SAFETY DATA SHEET TOILET BOWL SANITIZER No. 1 Date Issued: 07/03/2011 COMPANY DETAILS NAME: Hygiene Systems (Pty) Ltd EMERGENCY TEL: 0860 494 4363 ADDRESS: 7 Hargan Road, TEL: 011 552 8403 SECTION
MATERIAL SAFETY DATA SHEET TOILET BOWL SANITIZER No. 1 Date Issued: 07/03/2011 COMPANY DETAILS NAME: Hygiene Systems (Pty) Ltd EMERGENCY TEL: 0860 494 4363 ADDRESS: 7 Hargan Road, TEL: 011 552 8403 SECTION
160S01105, Page 1 of 7. Human Hepatitis B Immunoglobulin, solution for intramuscular injection.
 160S01105, Page 1 of 7 New Zealand Data Sheet Hepatitis B Immunoglobulin-VF NAME OF THE MEDICINE Human Hepatitis B Immunoglobulin, solution for intramuscular injection. DESCRIPTION Hepatitis B Immunoglobulin-VF
160S01105, Page 1 of 7 New Zealand Data Sheet Hepatitis B Immunoglobulin-VF NAME OF THE MEDICINE Human Hepatitis B Immunoglobulin, solution for intramuscular injection. DESCRIPTION Hepatitis B Immunoglobulin-VF
MATERIAL SAFETY DATA SHEET
 Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 5 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
VOLTAREN OPHTHA EYE DROPS
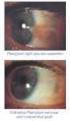 2013 במאי משרד הבריאות ותוכנו נבדק ואושר על ידו ע"י עלון זה נקבע ע פורמט PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
2013 במאי משרד הבריאות ותוכנו נבדק ואושר על ידו ע"י עלון זה נקבע ע פורמט PRESCRIBING INFORMATION VOLTAREN OPHTHA EYE DROPS 1 Name of the medicinal product Voltaren Ophtha Eye Drops. 2 Qualitative and quantitative
PACKAGE LEAFLET: INFORMATION FOR THE USER. VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution. Cyanocobalamin
 PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
PACKAGE LEAFLET: INFORMATION FOR THE USER VITAMINE B12 STEROP 1mg/1ml Solution for injection / oral solution Cyanocobalamin Read all of this leaflet carefully before you start using this medicine because
Public Assessment Report Scientific discussion. Tostrex (Testosterone) SE/H/571/01
 Public Assessment Report Scientific discussion Tostrex (Testosterone) SE/H/571/01 This module reflects the scientific discussion for the approval of Tostrex. The procedure was finalised at 2006-04-07.
Public Assessment Report Scientific discussion Tostrex (Testosterone) SE/H/571/01 This module reflects the scientific discussion for the approval of Tostrex. The procedure was finalised at 2006-04-07.
1g cream or ointment contains 1 mg methylprednisolone aceponate.
 CONSUMER MEDICINE INFORMATION ADVANTAN 1g cream or ointment contains 1 mg methylprednisolone aceponate. What is in this leaflet Please read this leaflet carefully before you start using ADVANTAN. It will
CONSUMER MEDICINE INFORMATION ADVANTAN 1g cream or ointment contains 1 mg methylprednisolone aceponate. What is in this leaflet Please read this leaflet carefully before you start using ADVANTAN. It will
GENERAL CONSIDERATIONS FOR CLINICAL TRIALS E8
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GENERAL CONSIDERATIONS FOR CLINICAL TRIALS E8 Current
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE GENERAL CONSIDERATIONS FOR CLINICAL TRIALS E8 Current
Medication Guide Testim (TĔS tim) CIII (testosterone gel)
 Medication Guide Testim (TĔS tim) CIII (testosterone gel) Read this Medication Guide that comes with Testim before you start using it and each time you get a refill. There may be new information. This
Medication Guide Testim (TĔS tim) CIII (testosterone gel) Read this Medication Guide that comes with Testim before you start using it and each time you get a refill. There may be new information. This
Kalms Tablets THR 01074/0235 UKPAR
 Kalms Tablets THR 01074/0235 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 10 Summary of Product Characteristics Page 11 Patient Information Leaflet
Kalms Tablets THR 01074/0235 UKPAR TABLE OF CONTENTS Lay summary Page 2 Scientific discussion Page 3 Steps taken for assessment Page 10 Summary of Product Characteristics Page 11 Patient Information Leaflet
Formulations and Strengths Topical gel containing isotretinoin 0.05 % w/w (0.05 g per 100 g gel)
 ISOTREX Gel Formulations and Strengths Topical gel containing isotretinoin 0.05 % w/w (0.05 g per 100 g gel) Excipients Butylated hydroxytoluene Hydroxypropylcellulose Ethanol CLINICAL INFORMATION Indications
ISOTREX Gel Formulations and Strengths Topical gel containing isotretinoin 0.05 % w/w (0.05 g per 100 g gel) Excipients Butylated hydroxytoluene Hydroxypropylcellulose Ethanol CLINICAL INFORMATION Indications
OCCUPATIONAL SKIN DISEASES IN NURSES
 International Journal of Occupational Medicine and Environmental Health, 2003; 16(3): 241 247 OCCUPATIONAL SKIN DISEASES IN NURSES RUTA TELKSNIENE 1 and VIDMANTAS JANUSKEVICIUS 2 1 Department of Environmental
International Journal of Occupational Medicine and Environmental Health, 2003; 16(3): 241 247 OCCUPATIONAL SKIN DISEASES IN NURSES RUTA TELKSNIENE 1 and VIDMANTAS JANUSKEVICIUS 2 1 Department of Environmental
SUMMARY OF PRODUCT CHARACTERISTICS
 Registration No. : 2C 22/47 (N) Importer / Manufacturer: Sanofi Pasteur Ltd., Thailand/Sanofi Pasteur S.A., France SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICAL PRODUCT : PENTAXIM (Diphtheria,
Registration No. : 2C 22/47 (N) Importer / Manufacturer: Sanofi Pasteur Ltd., Thailand/Sanofi Pasteur S.A., France SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICAL PRODUCT : PENTAXIM (Diphtheria,
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE. Current Step 4 version
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE THE EXTENT OF POPULATION EXPOSURE TO ASSESS CLINICAL
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE THE EXTENT OF POPULATION EXPOSURE TO ASSESS CLINICAL
It can therefore be bought without a prescription but should be used correctly to ensure its efficacy and to reduce side effects.
 140 mg Medicated plaster Diclofenac Sodium BEFORE USE, CAREFULLY READ ALL THE INFORMATION GIVEN ON THE INFORMATIVE LEAFLET This medicinal product is intended for SELF-MEDICATION. It can be used to treat
140 mg Medicated plaster Diclofenac Sodium BEFORE USE, CAREFULLY READ ALL THE INFORMATION GIVEN ON THE INFORMATIVE LEAFLET This medicinal product is intended for SELF-MEDICATION. It can be used to treat
CORE SmPC FOR RADIOPHARMACEUTICALS
 CORE SmPC FOR RADIOPHARMACEUTICALS The QRD Product Information template with explanatory notes and the convention to be followed for QRD templates provide general guidance on format and text and should
CORE SmPC FOR RADIOPHARMACEUTICALS The QRD Product Information template with explanatory notes and the convention to be followed for QRD templates provide general guidance on format and text and should
Medication Guide Rebif (Re-bif) Interferon beta-1a (in-ter-feer-on beta-one-â)
 Medication Guide Rebif (Re-bif) Interferon beta-1a (in-ter-feer-on beta-one-â) Please read this leaflet carefully before you start to use Rebif and each time your prescription is refilled since there may
Medication Guide Rebif (Re-bif) Interferon beta-1a (in-ter-feer-on beta-one-â) Please read this leaflet carefully before you start to use Rebif and each time your prescription is refilled since there may
MEDICATION GUIDE. PROTOPIC [pro-top-ik] (tacrolimus) Ointment 0.03% Ointment 0.1%
![MEDICATION GUIDE. PROTOPIC [pro-top-ik] (tacrolimus) Ointment 0.03% Ointment 0.1% MEDICATION GUIDE. PROTOPIC [pro-top-ik] (tacrolimus) Ointment 0.03% Ointment 0.1%](/thumbs/27/10084771.jpg) MEDICATION GUIDE PROTOPIC [pro-top-ik] (tacrolimus) Ointment 0.03% Ointment 0.1% Read the Medication Guide every time you or a family member gets PROTOPIC Ointment. There may be new information. This Medication
MEDICATION GUIDE PROTOPIC [pro-top-ik] (tacrolimus) Ointment 0.03% Ointment 0.1% Read the Medication Guide every time you or a family member gets PROTOPIC Ointment. There may be new information. This Medication
1. NAME OF THE MEDICINAL PRODUCT. Impavido 10 mg capsules Impavido 50 mg capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION. 1 capsule contains:
 1. NAME OF THE MEDICINAL PRODUCT Impavido 10 mg capsules Impavido 50 mg capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 capsule contains: Impavido 10 mg capsules 10 mg Miltefosine. Impavido 50 mg
1. NAME OF THE MEDICINAL PRODUCT Impavido 10 mg capsules Impavido 50 mg capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 capsule contains: Impavido 10 mg capsules 10 mg Miltefosine. Impavido 50 mg
PRODUCT INFORMATION Stieva-A Creams 0.025% w/w, 0.05% w/w and 0.1% w/w
 NAME OF THE MEDICINE Stieva-A Cream PRODUCT INFORMATION Stieva-A Creams 0.025% w/w, 0.05% w/w and 0.1% w/w DESCRIPTION Chemical names: 3, 7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexene-1-yl)-2,4,6,8-non-tetraenoic
NAME OF THE MEDICINE Stieva-A Cream PRODUCT INFORMATION Stieva-A Creams 0.025% w/w, 0.05% w/w and 0.1% w/w DESCRIPTION Chemical names: 3, 7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexene-1-yl)-2,4,6,8-non-tetraenoic
Lung Pathway Group Nintedanib (Vargatef) in advanced Non-Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Nintedanib (Vargatef) in advanced Non-Small Cell Lung Cancer (NSCLC) Indication: In combination with docetaxel in locally advanced, metastatic or locally recurrent NSCLC of adenocarcinoma
Lung Pathway Group Nintedanib (Vargatef) in advanced Non-Small Cell Lung Cancer (NSCLC) Indication: In combination with docetaxel in locally advanced, metastatic or locally recurrent NSCLC of adenocarcinoma
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in September 2008 HAVRIX 1440
 The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in September 2008 HAVRIX 1440 HAVRIX 720 Junior Monodose TITLE Hepatitis A (inactivated) vaccine
The format of this leaflet was determined by the Ministry of Health and its content was checked and approved in September 2008 HAVRIX 1440 HAVRIX 720 Junior Monodose TITLE Hepatitis A (inactivated) vaccine
Sponsor Novartis. Generic Drug Name Secukinumab. Therapeutic Area of Trial Psoriasis. Approved Indication investigational
 Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
Clinical Trial Results Database Page 2 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Psoriasis Approved Indication investigational Clinical Trial Results Database Page 3 Study
FRENCH AGENCY FOR VETERINARY MEDICINAL PRODUCTS DECENTRALISED PROCEDURE PUBLICLY AVAILABLE ASSESSMENT REPORT FOR A VETERINARY MEDICINAL PRODUCT
 FRENCH AGENCY FOR VETERINARY MEDICINAL PRODUCTS DECENTRALISED PROCEDURE FOR A VETERINARY MEDICINAL PRODUCT EMEDOG, 1 mg/ml, solution for injection for dogs Date: 20/08/2015 French agency for food, environnemental
FRENCH AGENCY FOR VETERINARY MEDICINAL PRODUCTS DECENTRALISED PROCEDURE FOR A VETERINARY MEDICINAL PRODUCT EMEDOG, 1 mg/ml, solution for injection for dogs Date: 20/08/2015 French agency for food, environnemental
Summary of Product Characteristics Medical Helium
 Summary of Product Characteristics Medical Helium www.uk.airliquide.com 1. NAME OF THE MEDICINAL PRODUCT Medical Helium 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Helium BP 1988 100%. 3. PHARMACEUTICAL
Summary of Product Characteristics Medical Helium www.uk.airliquide.com 1. NAME OF THE MEDICINAL PRODUCT Medical Helium 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Helium BP 1988 100%. 3. PHARMACEUTICAL
INITIATING ORAL AUBAGIO (teriflunomide) THERAPY
 FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
FOR YOUR PATIENTS WITH RELAPSING FORMS OF MS INITIATING ORAL AUBAGIO (teriflunomide) THERAPY WARNING: HEPATOTOXICITY AND RISK OF TERATOGENICITY Severe liver injury including fatal liver failure has been
Felimazole 5 mg Coated Tablet
 .. CARTON TEXT - FRONT PANEL PRESCRIPTION ANIMAL REMEDY READ SAFETY DIRECTIONS BEFORE OPENING OR USING KEEP OUT OF REACH OF CHILDREN FOR ANIMAL TREATMENT ONLY RLP Approved Felimazole 5 mg Coated Tablet
.. CARTON TEXT - FRONT PANEL PRESCRIPTION ANIMAL REMEDY READ SAFETY DIRECTIONS BEFORE OPENING OR USING KEEP OUT OF REACH OF CHILDREN FOR ANIMAL TREATMENT ONLY RLP Approved Felimazole 5 mg Coated Tablet
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY : Product name: EZ Clean Wipes for lens cleaning Supplier: Ophir Optronics Ltd. 6 Hartom St. Har-Hotzvim Jerusalem 91450, Israel
MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY : Product name: EZ Clean Wipes for lens cleaning Supplier: Ophir Optronics Ltd. 6 Hartom St. Har-Hotzvim Jerusalem 91450, Israel
Teriflunomide is the active metabolite of Leflunomide, a drug employed since 1994 for the treatment of rheumatoid arthritis (Baselt, 2011).
 Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
Page 1 of 10 ANALYTE NAME AND STRUCTURE TERIFLUNOMIDE Teriflunomide TRADE NAME Aubagio CATEGORY Antimetabolite TEST CODE PURPOSE Therapeutic Drug Monitoring GENERAL RELEVANCY BACKGROUND sclerosis. The
VITAMIN C AND INFECTIOUS DISEASE: A REVIEW OF THE LITERATURE AND THE RESULTS OF A RANDOMIZED, DOUBLE-BLIND, PROSPECTIVE STUDY OVER 8 YEARS
 39 Chapter 3 VITAMIN C AND INFECTIOUS DISEASE: A REVIEW OF THE LITERATURE AND THE RESULTS OF A RANDOMIZED, DOUBLE-BLIND, PROSPECTIVE STUDY OVER 8 YEARS Maxine Briggs TABLE OF CONTENTS I. Review of the
39 Chapter 3 VITAMIN C AND INFECTIOUS DISEASE: A REVIEW OF THE LITERATURE AND THE RESULTS OF A RANDOMIZED, DOUBLE-BLIND, PROSPECTIVE STUDY OVER 8 YEARS Maxine Briggs TABLE OF CONTENTS I. Review of the
ICH Topic E 8 General Considerations for Clinical Trials. Step 5 NOTE FOR GUIDANCE ON GENERAL CONSIDERATIONS FOR CLINICAL TRIALS (CPMP/ICH/291/95)
 European Medicines Agency March 1998 CPMP/ICH/291/95 ICH Topic E 8 General Considerations for Clinical Trials Step 5 NOTE FOR GUIDANCE ON GENERAL CONSIDERATIONS FOR CLINICAL TRIALS (CPMP/ICH/291/95) TRANSMISSION
European Medicines Agency March 1998 CPMP/ICH/291/95 ICH Topic E 8 General Considerations for Clinical Trials Step 5 NOTE FOR GUIDANCE ON GENERAL CONSIDERATIONS FOR CLINICAL TRIALS (CPMP/ICH/291/95) TRANSMISSION
Disease Modifying Therapies for MS
 Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Disease Modifying Therapies for MS The term disease-modifying therapy means a drug that can modify or change the course of a disease. In other words a DMT should be able to reduce the number of attacks
Abstral Prescriber and Pharmacist Guide
 Abstral Prescriber and Pharmacist Guide fentanyl citrate sublingual tablets Introduction The Abstral Prescriber and Pharmacist Guide is designed to support healthcare professionals in the diagnosis of
Abstral Prescriber and Pharmacist Guide fentanyl citrate sublingual tablets Introduction The Abstral Prescriber and Pharmacist Guide is designed to support healthcare professionals in the diagnosis of
Pharmacology skills for drug discovery. Why is pharmacology important?
 skills for drug discovery Why is pharmacology important?, the science underlying the interaction between chemicals and living systems, emerged as a distinct discipline allied to medicine in the mid-19th
skills for drug discovery Why is pharmacology important?, the science underlying the interaction between chemicals and living systems, emerged as a distinct discipline allied to medicine in the mid-19th
Decentralised Procedure. Public Assessment Report. Gadojaco Handvist Gadopentetat Dimeglumin Jacobsen 500 Mikromol/ml Injektionslösung
 Bundesinstitut für Arzneimittel und Medizinprodukte Decentralised Procedure Public Assessment Report Gadojaco Handvist Gadopentetat Dimeglumin Jacobsen 500 Mikromol/ml Injektionslösung Gadopentetate dimeglumine
Bundesinstitut für Arzneimittel und Medizinprodukte Decentralised Procedure Public Assessment Report Gadojaco Handvist Gadopentetat Dimeglumin Jacobsen 500 Mikromol/ml Injektionslösung Gadopentetate dimeglumine
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Sativex Cannabis sativa L. extracts (delta-9-tetrahydrocannabinol and cannabidiol) Oromucosal Spray 5.5 / 10 ml
 New Zealand Consumer Medicine Information Sativex Cannabis sativa L. extracts (delta-9-tetrahydrocannabinol and cannabidiol) Oromucosal Spray 5.5 / 10 ml What is in this leaflet Please read all of this
New Zealand Consumer Medicine Information Sativex Cannabis sativa L. extracts (delta-9-tetrahydrocannabinol and cannabidiol) Oromucosal Spray 5.5 / 10 ml What is in this leaflet Please read all of this
Manual: Policies Document ID: PI013 Date Created: Jun 08 Section: Investigator No. Pages: 7 Review Date: Aug 15 Future Review Date: Aug 17
 P0LICYI013 PREGNANCY AND SEXUAL HEALTH POLICY Manual: Policies Document ID: PI013 Date Created: Jun 08 Section: Investigator No. Pages: 7 Review Date: Aug 15 Future Review Date: Aug 17 PURPOSE To describe
P0LICYI013 PREGNANCY AND SEXUAL HEALTH POLICY Manual: Policies Document ID: PI013 Date Created: Jun 08 Section: Investigator No. Pages: 7 Review Date: Aug 15 Future Review Date: Aug 17 PURPOSE To describe
New Zealand Datasheet
 New Zealand Datasheet Name of Medicine PRAXBIND Idarucizumab 50 mg/ml solution for injection/infusion Presentation Idarucizumab is a humanized monoclonal antibody fragment (Fab) molecule derived from an
New Zealand Datasheet Name of Medicine PRAXBIND Idarucizumab 50 mg/ml solution for injection/infusion Presentation Idarucizumab is a humanized monoclonal antibody fragment (Fab) molecule derived from an
SAFETY DATA SHEET CLEENOL LIFT SPRAY CLEANER WITH BACTERICIDE
 Revision Date 18/06/2010 Revision 1 Supersedes date 04/06/2010 SAFETY DATA SHEET CLEENOL LIFT SPRAY CLEANER WITH BACTERICIDE SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING
Revision Date 18/06/2010 Revision 1 Supersedes date 04/06/2010 SAFETY DATA SHEET CLEENOL LIFT SPRAY CLEANER WITH BACTERICIDE SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING
Neutral Superoxidized solution vs. benzoyl peroxide gel 5% in the treatment of. Superoxidized solution (SOS) is an electrochemically processed aqueous
 Neutral Superoxidized solution vs. benzoyl peroxide gel 5% in the treatment of acne vulgaris: a randomized open-label clinical trial. Summary. Superoxidized solution (SOS) is an electrochemically processed
Neutral Superoxidized solution vs. benzoyl peroxide gel 5% in the treatment of acne vulgaris: a randomized open-label clinical trial. Summary. Superoxidized solution (SOS) is an electrochemically processed
PATIENT INFORMATION LEAFLET. CEFALEXIN 250 mg AND 500 mg CAPSULES CEFALEXIN
 PATIENT INFORMATION LEAFLET CEFALEXIN 250 mg AND 500 mg CAPSULES CEFALEXIN Read all of this leaflet carefully before you start taking this medicine. - Keep this leaflet. You may need to read it again.
PATIENT INFORMATION LEAFLET CEFALEXIN 250 mg AND 500 mg CAPSULES CEFALEXIN Read all of this leaflet carefully before you start taking this medicine. - Keep this leaflet. You may need to read it again.
ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals. Step 5
 European Medicines Agency July 1996 CPMP/ICH/140/95 ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON THE NEED FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS
European Medicines Agency July 1996 CPMP/ICH/140/95 ICH Topic S 1 A The Need for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON THE NEED FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS
BREAST CANCER AWARENESS FOR WOMEN AND MEN by Samar Ali A. Kader. Two years ago, I was working as a bedside nurse. One of my colleagues felt
 Ali A. Kader, S. (2010). Breast cancer awareness for women and men. UCQ Nursing Journal of Academic Writing, Winter 2010, 70 76. BREAST CANCER AWARENESS FOR WOMEN AND MEN by Samar Ali A. Kader Two years
Ali A. Kader, S. (2010). Breast cancer awareness for women and men. UCQ Nursing Journal of Academic Writing, Winter 2010, 70 76. BREAST CANCER AWARENESS FOR WOMEN AND MEN by Samar Ali A. Kader Two years
skin and soft tissue infections (skinfold pyoderma, impetigo, folliculitis, furunculosis, cellulitis) caused by susceptible strains of organisms.
 Part II SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MARBOCYL P 5 mg tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active Ingredient Marbofloxacin 5mg per tablet
Part II SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MARBOCYL P 5 mg tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active Ingredient Marbofloxacin 5mg per tablet
MEDICATION GUIDE Testosterone (tes-tos-te-rōn) Gel, CIII
 MEDICATION GUIDE Testosterone (tes-tos-te-rōn) Gel, CIII Read this Medication Guide that comes with testosterone gel before you start using it and each time you get a refill. There may be new information.
MEDICATION GUIDE Testosterone (tes-tos-te-rōn) Gel, CIII Read this Medication Guide that comes with testosterone gel before you start using it and each time you get a refill. There may be new information.
Potulaca (Portulaca oleracea) detergent and other formulations that disrupt the active anthraquinones by regulating the Nitric
 Natural effective support for acne, oily and inflamed skin care At the heart of Frutarom's TopicRange for oily and acne skin problems, we focus Z-Care - a botanical blend based on traditions of Chinese
Natural effective support for acne, oily and inflamed skin care At the heart of Frutarom's TopicRange for oily and acne skin problems, we focus Z-Care - a botanical blend based on traditions of Chinese
Learn More About Product Labeling
 Learn More About Product Labeling Product label The product label is developed during the formal process of review and approval by regulatory agencies of any medicine or medical product. There are specific
Learn More About Product Labeling Product label The product label is developed during the formal process of review and approval by regulatory agencies of any medicine or medical product. There are specific
FAQs on Influenza A (H1N1-2009) Vaccine
 FAQs on Influenza A (H1N1-2009) Vaccine 1) What is Influenza A (H1N1-2009) (swine flu) 1? Influenza A (H1N1-2009), previously known as "swine flu", is a new strain of influenza virus that spreads from
FAQs on Influenza A (H1N1-2009) Vaccine 1) What is Influenza A (H1N1-2009) (swine flu) 1? Influenza A (H1N1-2009), previously known as "swine flu", is a new strain of influenza virus that spreads from
Community herbal monograph on Oenothera biennis L.; Oenothera lamarckiana L., oleum
 16 December 2011 EMA/HMPC/277792/2009 Committee on Herbal Medicinal Products (HMPC) Community herbal monograph on Oenothera biennis L.; Oenothera lamarckiana L., Final Discussion in Working Party on Community
16 December 2011 EMA/HMPC/277792/2009 Committee on Herbal Medicinal Products (HMPC) Community herbal monograph on Oenothera biennis L.; Oenothera lamarckiana L., Final Discussion in Working Party on Community
INFUSE Bone Graft (rhbmp-2/acs)
 1 INFUSE Bone Graft (rhbmp-2/acs) For patients who need more bone to place dental implants Enjoy living with INFUSE Bone Graft. www.medtronic.com Medtronic Spinal and Biologics Business Worldwide Headquarters
1 INFUSE Bone Graft (rhbmp-2/acs) For patients who need more bone to place dental implants Enjoy living with INFUSE Bone Graft. www.medtronic.com Medtronic Spinal and Biologics Business Worldwide Headquarters
There is a risk of renal impairment in dehydrated children and adolescents.
 PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
PACKAGE LEAFLET: INFORMATION FOR THE USER MELFEN 200mg FILM-COATED TABLETS MELFEN 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking this medicine because it
[For POM]: Read all of this leaflet carefully before you start using this medicine.
![[For POM]: Read all of this leaflet carefully before you start using this medicine. [For POM]: Read all of this leaflet carefully before you start using this medicine.](/thumbs/40/20645119.jpg) Package Leaflet: Information for the User Duraphat 500mg/100g Toothpaste Active Substance: Sodium Fluoride [For POM]: Read all of this leaflet carefully before you start using this medicine. - Keep this
Package Leaflet: Information for the User Duraphat 500mg/100g Toothpaste Active Substance: Sodium Fluoride [For POM]: Read all of this leaflet carefully before you start using this medicine. - Keep this
STD Treatment Chart Nancy Harris, N.P. Women s Health Coordinator August 2003 Disease Treatment Alternative treatment
 STD Treatment Chart Nancy Harris, N.P. Women s Health Coordinator August 2003 Disease Treatment Alternative Pregnancy Chlamydia Chancroid Epdidymitis Gonorrhea: Uncomplicated (cx, urethra, and rectum):
STD Treatment Chart Nancy Harris, N.P. Women s Health Coordinator August 2003 Disease Treatment Alternative Pregnancy Chlamydia Chancroid Epdidymitis Gonorrhea: Uncomplicated (cx, urethra, and rectum):
FastTest. You ve read the book... ... now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. Please refer back to relevant sections
BOWEN S DISEASE (SQUAMOUS CELL CARCINOMA IN SITU)
 BOWEN S DISEASE (SQUAMOUS CELL CARCINOMA IN SITU) What are the aims of this leaflet? This leaflet has been written to help you understand more about squamous cell carcinoma in situ (Bowen s disease). It
BOWEN S DISEASE (SQUAMOUS CELL CARCINOMA IN SITU) What are the aims of this leaflet? This leaflet has been written to help you understand more about squamous cell carcinoma in situ (Bowen s disease). It
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Biological importance of metabolites. Safety and efficacy aspects
 Biological importance of metabolites Safety and efficacy aspects Bernard Walther Technologie Servier Biological importance of metabolites Safety testing of drug metabolites Bioanalytical strategy Structural
Biological importance of metabolites Safety and efficacy aspects Bernard Walther Technologie Servier Biological importance of metabolites Safety testing of drug metabolites Bioanalytical strategy Structural
I B2.4. Design of the patient information leaflet for VariQuin
 (English translation of official Dutch version) I B2.4. Design of the patient information leaflet for VariQuin Information for the Patient: Read this package leaflet carefully when you have some time to
(English translation of official Dutch version) I B2.4. Design of the patient information leaflet for VariQuin Information for the Patient: Read this package leaflet carefully when you have some time to
