BIOL 2457 A&P I CHAPTER 2 cont. (B) SI Monosaccharides = sugar; disaccharide = sugars; polysaccharide = sugars.
|
|
|
- Alberta Stanley
- 7 years ago
- Views:
Transcription
1 BIOL 2457 A&P I CHAPTER 2 cont. (B) SI 1 CARBOHYDRATES 1. Which elements are found in carbohydrates? What is the ratio of hydrogen to oxygen? 2. Monosaccharides = sugar; disaccharide = sugars; polysaccharide = sugars. 3. Dissaccharides and polysaccharides are formed by linking. What type of reaction links these monomers? What type of reaction splits the polymers into monomers? 4. A monosaccharide is a (complex or simple) carbohydrate. List 3 monosaccharides. 5. A disaccharide is a (complex or simple) carbohydrate. List 3 disaccharides 6. A polysaccharide is a (complex or simple) carbohydrate. List 3 polysaccharides 7. What are the functions of complex carbohydrates? and 8. is the sugar storage product of plants. This carbohydrate (can / cannot) be digested. 9. is the complex carbohydrate that gives structural support to plant cell walls. This carbohydrate is a good source of dietary fiber. It (can / cannot) be digested. 10. is the sugar storage product in animals. It is stored in the and. 11. Matching carbohydrates Monosaccharide found in RNA A Deoxyribose Storage form of energy in animals B Ribose Glucose + glucose C Maltose Preferred nutrient for making energy D Sucrose Fruit sugar E Lactose Monosaccharide found in DNA F Starch Polysaccharide that cannot be digested by humans G Glycogen Glucose + galactose H Cellulose Storage form of energy in plants I Glucose Part of milk sugar J Fructose Found in liver and muscle cells K Galactose Glucose + fructose
2 BIOL 2457 A&P I CHAPTER 2 cont. (B) SI 2 LIPIDS 12. Which elements are found in lipids? ; ratio of hydrogen to oxygen? Lipids are (hydrophilic or hydrophobic) and form in water. 13. Three categories of lipids are. 14. Triglycerides are made up of Triglycerides provide (more/less) energy than carbohydrates. 15. A triglyceride with all C-C single bonds is said to be (saturated/unsaturated). It is (liquid/solid) at room temperature. 16. A triglyceride with at least one C=C double bond is said to be (saturated/unsaturated). It is (liquid/solid) at room temperature. 17. (saturated/unsaturated) triglycerides build up an block arteries. 18. A lipid composed of glycerol, 2 fatty acids and phosphate is a. The phosphate groups make up the (hydrophilic/hydrophobic) heads which are (polar/nonpolar). The fatty acids make up the (hydrophilic/hydrophobic) tails which are (polar/non polar). 19. Cell membranes are made up of a with (hydrophilic/hydrophobic) heads on the outside and (hydrophilic/hydrophobic) tails on the inside. This structure gives the cell membranes its function of. 20. Lipids consisting of 4 interlocking rings with added functional groups that give certain properties to the lipid are called. They are the main components of testosterone and estrogen ( ) in both males and females. is a steroid found in between the phospholipids of the cell membrane and helps to maintain its fluidity. 21. What is the function of fatty acids? 22. What type of fatty acid acts as a local hormone for cell signaling? 23. What type of reaction combines glycerol with fatty acids? What type of reaction splits lipids into monomers? 24. Matching - lipids Glycerol + 3 fatty acids A Triglycerides One or more double bonds between carbons; liquid B Saturated triglycerides Glycerol + 2 fatty acids + phosphate C Unsaturated triglycerides Cell membrane D Phospholipids No double bonds between carbons; solid E Phospholipid bilayer Found between phospholipids in plasma membrane F Steroids Ring shaped lipids with added functional groups G Cholesterol
3 BIOL 2457 A&P I CHAPTER 2 cont. (B) SI 3 PROTEINS 25. Proteins are composed of ; a chain of these monomers is called a chain. 26. Dehydration synthesis reactions form bonds between the group of one amino acid and the group of another amino acid. 27. Give an example of these protein functions. support/structure storage transport regulation receptors carriers protection catalysts 28. The 20 different amino acids used to make proteins differ only in the groups attached to the central carbon. Different proteins are formed from different combinations and sequences of. 29. Protein structures Protein formed by bonding of 2 or more polypeptide A Primary chains Formed by sulfide bonds between distant amino acids B Secondary Sequence of amino acids that make up protein C Tertiary α helix; β pleated sheets formed by hydrogen bonds between amino acids D Quaternary 30. Hemoglobin, carrier proteins and enzymes are proteins. Skin, hair and nails are proteins. (globular/fibrous) 31. The loss of protein shape caused by changes in the physical or chemical environment is called. This causes the protein to be.
4 BIOL 2457 A&P I CHAPTER 2 cont. (B) SI Matching proteins Specific to a particular protein A Amino acids Enzymatic action B Protein function Structural support C Peptide Building blocks of protein D Functional groups α helix; β pleated sheet E Primary structure Two or more polypeptide chains together F Secondary structure Hemoglobin transports oxygen G Tertiary structure Bonds formed by amino acids H Quaternary structure Loss of shape causes loss of protein function I Denaturation Sequence of amino acids J R groups Give proteins their properties Amino group + central carbon + carboxyl + R group Formed sulfide bonds ENZYMES 33. Substrates/reactants fit in on enzymes. 34. Enzymes (increase/decrease) the activation energy required for a chemical reaction. The enzyme is (changed/unchanged) at the end of a reaction. 35. stimulate enzymes to put substrates together to form products. Vitamins are an example. 36. List 2 things that will affect the activity of enzymes. 37. Define saturation kinetics. NUCLEIC ACIDS 38. The monomers making up nucleic acids are each of which is made up of a, and. 39. The two nucleic acids are and. What type of sugar is found in DNA? What type of sugar is found in RNA? 40. What is the function of DNA? 41. Name the five nitrogenous bases found in nucleic acids and give their symbols: 42. Which are found in DNA? Which are found in RNA?
5 BIOL 2457 A&P I CHAPTER 2 cont. (B) SI What is the difference between the structures of purines and pyrimidines: 44. Which bases are purines? Which bases are pyrimidines? 45. What type of bonds hold the bases together? 46. DNA has a strand; RNA has a strand. (single/double) 47. What is the Central Dogma? ATP - adenosine triphosphate 48. What is the structure of ATP? What type of bonds are between the phosphates? 49. What is the function of ATP? Breaking the bonds between the phosphates energy. (requires/releases) 50. Name another energy molecule used by the cell. 51. Give the structure of a coenzyme 52. What is the function of cyclic AMP? 53. Matching nucleic acids Sugar found in DNA; it lacks an oxygen atom A. Nucleotide 2 rings; adenine and guanine B. Purine Carries information from DNA to make proteins C. Pyrimidine Genetic information D. Deoxyribose supplies energy to cells E. Ribose 1 ring; thymine; cytosine, uracil F. DNA sugar, phosphate group, nitrogen base G. RNA sugar found in RNA H. ATP
6 BIOL 2457 A&P I CHAPTER 2 cont. (B) - Answers SI 6 1. CHO; 2 hydrogens : 1 oxygen 2. 1; 2; many 3. monosaccharides; dehydration synthesis; hydrolysis 4. simple; glucose, fructose, galactose 5. simple; maltose, sucrose, lactose 6. complex; starch, glycogen, cellulose 7. provides energy; structure 8. starch; can 9. cellulose; cannot 10. glycogen; liver; muscles 11. B, G, C, I, J, A, H, E, F, K, G, D 12. CHO; more than 2 hydrogens to each oxygen; hydrophobic; micelles 13. triglycerides, phospholipids, steroids 14. glycerol and 3 fatty acids; more 15. saturated; solid 16. unsaturated; liquid 17. saturated 18. phospholipid; hydrophilic; polar; hydrophobic; non polar 19. phospholipid bilayer; hydrophilic; hydrophobic; selective permeability 20. steroids; sex hormones; cholesterol 21. transport fats in body 22. prostaglandins 23. dehydration synthesis; hydrolysis 24. A, C, D, E, B, G, F 25. amino acids; polypeptide 26. peptide; amino; carboxyl 27. support/structure keratin in fingernails; collagen holds tissues together storage stores amino acids; e.g. albumin (white of egg); casein (in milk) transport hemoglobin transports O 2 and CO 2 between tissues and lungs; others transport substances across hydrophobic layer regulation synchronizes events in body; e.g. insulin (hormone) tells cells to take up sugar; glucagon (hormone) tells cells to release sugar receptors on cell surface; communicate with outside world carriers movement; e.g. muscles; actin and myosin interact with each other to contract protection antibodies (cells) recognize foreign invaders (bacteria, virus, pollen), bind to it and signal immune system response catalysts enzymes; speed up chemical reactions 28. R; amino acids 29. D, C, A, B 30. globular; fibrous 31. denaturation; non functional 32. A, B, B, A, F, H, B, C, I, E, J, A, H 33. active sites 34. decrease; unchanged 35. coenzymes 36. temperature; ph
7 BIOL 2457 A&P I CHAPTER 2 cont. (B) - Answers SI Enough substrate and enzymes are needed to make everything that is required by the body. 38. nucleotides; sugar; phosphate group; nitrogenous base 39. DNA; RNA; deoxyribose; ribose 40. carries the information to form all proteins required by the body 41. adenine (A), thymine (T), cytosine (C), guanine (G), uracil (U) 42. DNA A, T, C, G; RNA A, U, C, G 43. purines have two rings; pyrimidines have one ring 44. purines A, G; pyrimidines - C, U, T 45. hydrogen 46. double; single 47. DNA makes RNA makes Protein (DNA RNA protein) 48. adenine base; ribose; 3 phosphate groups; hydrogen 49. stores energy; releases 50. GTP (guanine triphosphate) 51. adenine + ribose + 2 phosphates + vitamin 52. cell communication 53. D, B, G, F, H, C, A, E
Biochemistry of Cells
 Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
How To Understand The Chemistry Of Organic Molecules
 CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
Biological molecules:
 Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Proteins and Nucleic Acids
 Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
Proteins and Nucleic Acids Chapter 5 Macromolecules: Proteins Proteins Most structurally & functionally diverse group of biomolecules. : o Involved in almost everything o Enzymes o Structure (keratin,
The Molecules of Cells
 The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
A disaccharide is formed when a dehydration reaction joins two monosaccharides. This covalent bond is called a glycosidic linkage.
 CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
Chapter 3 Molecules of Cells
 Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Disaccharides consist of two monosaccharide monomers covalently linked by a glycosidic bond. They function in sugar transport.
 1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
Elements in Biological Molecules
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 5. The Structure and Function of Macromolecule s
 Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Chapter 3: Biological Molecules. 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
BIOLOGICAL MOLECULES OF LIFE
 BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
4. Which carbohydrate would you find as part of a molecule of RNA? a. Galactose b. Deoxyribose c. Ribose d. Glucose
 1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
Name: Hour: Elements & Macromolecules in Organisms
 Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Organic Compounds. Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for?
 Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Chapter 2 Chemical Principles
 Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Carbohydrates, proteins and lipids
 Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Elements & Macromolecules in Organisms
 Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Carbon-organic Compounds
 Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
10.1 The function of Digestion pg. 402
 10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
I. Chapter 5 Summary. II. Nucleotides & Nucleic Acids. III. Lipids
 I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
Worksheet 13.1. Chapter 13: Human biochemistry glossary
 Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Proteins. Proteins. Amino Acids. Most diverse and most important molecule in. Functions: Functions (cont d)
 Proteins Proteins Most diverse and most important molecule in living i organisms Functions: 1. Structural (keratin in hair, collagen in ligaments) 2. Storage (casein in mother s milk) 3. Transport (HAEMOGLOBIN!)
Proteins Proteins Most diverse and most important molecule in living i organisms Functions: 1. Structural (keratin in hair, collagen in ligaments) 2. Storage (casein in mother s milk) 3. Transport (HAEMOGLOBIN!)
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids
 VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Chapter 5: The Structure and Function of Large Biological Molecules Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four
Name Period Chapter 5: The Structure and Function of Large Biological Molecules Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids
 The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
Carbohydrates Lipids Proteins Nucleic Acids
 Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
Chemical Basis of Life Module A Anchor 2
 Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
BIOMOLECULES. reflect
 reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
reflect A child s building blocks are relatively simple structures. When they come together, however, they can form magnifi cent structures. The elaborate city scene to the right is made of small, simple
Lab 3 Organic Molecules of Biological Importance
 Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
3120-1 - Page 1. Name:
 Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Name: 1) Which series is arranged in correct order according to decreasing size of structures? A) DNA, nucleus, chromosome, nucleotide, nitrogenous base B) chromosome, nucleus, nitrogenous base, nucleotide,
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins
 Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
Recognizing Organic Molecules: Carbohydrates, Lipids and Proteins Oct 15 8:05 PM What is an Organic Molecule? An Organic Molecule is a molecule that contains carbon and hydrogen and oxygen Carbon is found
WATER CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" POLARITY HYDROGEN BONDING
 CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" WATER Compare the body of the jellyfish with our own bodies. The jellyfish will die if it is removed from its water environment, yet we can live in the
CHAPTER 3 - BIOCHEMISTRY "THE CHEMISTRY OF LIFE" WATER Compare the body of the jellyfish with our own bodies. The jellyfish will die if it is removed from its water environment, yet we can live in the
Organic Molecules of Life - Exercise 2
 Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
The Molecules of Life - Overview. The Molecules of Life. The Molecules of Life. The Molecules of Life
 The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
1.1.2. thebiotutor. AS Biology OCR. Unit F211: Cells, Exchange & Transport. Module 1.2 Cell Membranes. Notes & Questions.
 thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
Genetic information (DNA) determines structure of proteins DNA RNA proteins cell structure 3.11 3.15 enzymes control cell chemistry ( metabolism )
 Biology 1406 Exam 3 Notes Structure of DNA Ch. 10 Genetic information (DNA) determines structure of proteins DNA RNA proteins cell structure 3.11 3.15 enzymes control cell chemistry ( metabolism ) Proteins
Biology 1406 Exam 3 Notes Structure of DNA Ch. 10 Genetic information (DNA) determines structure of proteins DNA RNA proteins cell structure 3.11 3.15 enzymes control cell chemistry ( metabolism ) Proteins
PRACTICE TEST QUESTIONS
 PART A: MULTIPLE CHOICE QUESTIONS PRACTICE TEST QUESTIONS DNA & PROTEIN SYNTHESIS B 1. One of the functions of DNA is to A. secrete vacuoles. B. make copies of itself. C. join amino acids to each other.
PART A: MULTIPLE CHOICE QUESTIONS PRACTICE TEST QUESTIONS DNA & PROTEIN SYNTHESIS B 1. One of the functions of DNA is to A. secrete vacuoles. B. make copies of itself. C. join amino acids to each other.
Chapter 2. The Chemistry of Life Worksheets
 Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
McMush. Testing for the Presence of Biomolecules
 Biology McMush Testing for the Presence of Biomolecules MATERIALS AND RESOURCES EACH GROUP aprons beaker, 250 ml 2 clamps, test tube goggles graduated cylinder, 50 ml paper towels test tube brush test
Biology McMush Testing for the Presence of Biomolecules MATERIALS AND RESOURCES EACH GROUP aprons beaker, 250 ml 2 clamps, test tube goggles graduated cylinder, 50 ml paper towels test tube brush test
Name Date Period. 2. When a molecule of double-stranded DNA undergoes replication, it results in
 DNA, RNA, Protein Synthesis Keystone 1. During the process shown above, the two strands of one DNA molecule are unwound. Then, DNA polymerases add complementary nucleotides to each strand which results
DNA, RNA, Protein Synthesis Keystone 1. During the process shown above, the two strands of one DNA molecule are unwound. Then, DNA polymerases add complementary nucleotides to each strand which results
DNA is found in all organisms from the smallest bacteria to humans. DNA has the same composition and structure in all organisms!
 Biological Sciences Initiative HHMI DNA omponents and Structure Introduction Nucleic acids are molecules that are essential to, and characteristic of, life on Earth. There are two basic types of nucleic
Biological Sciences Initiative HHMI DNA omponents and Structure Introduction Nucleic acids are molecules that are essential to, and characteristic of, life on Earth. There are two basic types of nucleic
The molecules of life. The molecules that make up living things are really big They are called macromolecules
 Food Labels All living things use materials and energy Our food comes from living things The food labels we see show us what our food is made of The stuff we are studying today can be found on food labels
Food Labels All living things use materials and energy Our food comes from living things The food labels we see show us what our food is made of The stuff we are studying today can be found on food labels
Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look
 OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
OpenStax-CNX module: m49457 1 Digestive System Module 7: Chemical Digestion and Absorption: A Closer Look Donna Browne Based on Chemical Digestion and Absorption: A Closer Look by OpenStax This work is
Keystone Review Practice Test Module A Cells and Cell Processes. 1. Which characteristic is shared by all prokaryotes and eukaryotes?
 Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY
 Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
Name PRESTWICK ACADEMY NATIONAL 5 BIOLOGY CELL BIOLOGY SUMMARY Cell Structure Identify animal, plant, fungal and bacterial cell ultrastructure and know the structures functions. Plant cell Animal cell
Nucleotides and Nucleic Acids
 Nucleotides and Nucleic Acids Brief History 1 1869 - Miescher Isolated nuclein from soiled bandages 1902 - Garrod Studied rare genetic disorder: Alkaptonuria; concluded that specific gene is associated
Nucleotides and Nucleic Acids Brief History 1 1869 - Miescher Isolated nuclein from soiled bandages 1902 - Garrod Studied rare genetic disorder: Alkaptonuria; concluded that specific gene is associated
Anatomy and Physiology Placement Exam 2 Practice with Answers at End!
 Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
2007 7.013 Problem Set 1 KEY
 2007 7.013 Problem Set 1 KEY Due before 5 PM on FRIDAY, February 16, 2007. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. Where in a eukaryotic cell do you
2007 7.013 Problem Set 1 KEY Due before 5 PM on FRIDAY, February 16, 2007. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. Where in a eukaryotic cell do you
NO CALCULATORS OR CELL PHONES ALLOWED
 Biol 205 Exam 1 TEST FORM A Spring 2008 NAME Fill out both sides of the Scantron Sheet. On Side 2 be sure to indicate that you have TEST FORM A The answers to Part I should be placed on the SCANTRON SHEET.
Biol 205 Exam 1 TEST FORM A Spring 2008 NAME Fill out both sides of the Scantron Sheet. On Side 2 be sure to indicate that you have TEST FORM A The answers to Part I should be placed on the SCANTRON SHEET.
Exam 4 Outline CH 105 Spring 2012
 Exam 4 Outline CH 105 Spring 2012 You need to bring a pencil and your ACT card. Chapter 24: Lipids 1. Describe the properties and types of lipids a. All are hydrophobic b. Fatty acid-based typically contain
Exam 4 Outline CH 105 Spring 2012 You need to bring a pencil and your ACT card. Chapter 24: Lipids 1. Describe the properties and types of lipids a. All are hydrophobic b. Fatty acid-based typically contain
Nutrients: Carbohydrates, Proteins, and Fats. Chapter 5 Lesson 2
 Nutrients: Carbohydrates, Proteins, and Fats Chapter 5 Lesson 2 Carbohydrates Definition- the starches and sugars found in foods. Carbohydrates are the body s preferred source of energy providing four
Nutrients: Carbohydrates, Proteins, and Fats Chapter 5 Lesson 2 Carbohydrates Definition- the starches and sugars found in foods. Carbohydrates are the body s preferred source of energy providing four
http://faculty.sau.edu.sa/h.alshehri
 http://faculty.sau.edu.sa/h.alshehri Definition: Proteins are macromolecules with a backbone formed by polymerization of amino acids. Proteins carry out a number of functions in living organisms: - They
http://faculty.sau.edu.sa/h.alshehri Definition: Proteins are macromolecules with a backbone formed by polymerization of amino acids. Proteins carry out a number of functions in living organisms: - They
8/20/2012 H C OH H R. Proteins
 Proteins Rubisco monomer = amino acids 20 different amino acids polymer = polypeptide protein can be one or more polypeptide chains folded & bonded together large & complex 3-D shape hemoglobin Amino acids
Proteins Rubisco monomer = amino acids 20 different amino acids polymer = polypeptide protein can be one or more polypeptide chains folded & bonded together large & complex 3-D shape hemoglobin Amino acids
Preliminary MFM Quiz
 Preliminary MFM Quiz 1. The major carrier of chemical energy in all cells is: A) adenosine monophosphate B) adenosine diphosphate C) adenosine trisphosphate D) guanosine trisphosphate E) carbamoyl phosphate
Preliminary MFM Quiz 1. The major carrier of chemical energy in all cells is: A) adenosine monophosphate B) adenosine diphosphate C) adenosine trisphosphate D) guanosine trisphosphate E) carbamoyl phosphate
Lipids. Classes of Lipids. Types of Lipids. Saturated and Unsaturated Fatty Acids. Fatty Acids. 15.1 Lipids 15.2 Fatty Acids
 hapter 15 15.1 15.2 Fatty Acids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but not in water. named for the Greek word lipos, which means fat. extracted
hapter 15 15.1 15.2 Fatty Acids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but not in water. named for the Greek word lipos, which means fat. extracted
Energy Production In A Cell (Chapter 25 Metabolism)
 Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
Energy Production In A Cell (Chapter 25 Metabolism) Large food molecules contain a lot of potential energy in the form of chemical bonds but it requires a lot of work to liberate the energy. Cells need
THE HISTORY OF CELL BIOLOGY
 SECTION 4-1 REVIEW THE HISTORY OF CELL BIOLOGY Define the following terms. 1. cell 2. cell theory Write the correct letter in the blank. 1. One early piece of evidence supporting the cell theory was the
SECTION 4-1 REVIEW THE HISTORY OF CELL BIOLOGY Define the following terms. 1. cell 2. cell theory Write the correct letter in the blank. 1. One early piece of evidence supporting the cell theory was the
Macromolecules in my food!!
 Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
Cellular Respiration Worksheet 1. 1. What are the 3 phases of the cellular respiration process? Glycolysis, Krebs Cycle, Electron Transport Chain.
 Cellular Respiration Worksheet 1 1. What are the 3 phases of the cellular respiration process? Glycolysis, Krebs Cycle, Electron Transport Chain. 2. Where in the cell does the glycolysis part of cellular
Cellular Respiration Worksheet 1 1. What are the 3 phases of the cellular respiration process? Glycolysis, Krebs Cycle, Electron Transport Chain. 2. Where in the cell does the glycolysis part of cellular
AP BIOLOGY 2008 SCORING GUIDELINES
 AP BIOLOGY 2008 SCORING GUIDELINES Question 1 1. The physical structure of a protein often reflects and affects its function. (a) Describe THREE types of chemical bonds/interactions found in proteins.
AP BIOLOGY 2008 SCORING GUIDELINES Question 1 1. The physical structure of a protein often reflects and affects its function. (a) Describe THREE types of chemical bonds/interactions found in proteins.
Get It Right. Answers. Chapter 1: The Science of Life. A biologist studies all living things.
 Discover Biology 'N' Level Science Chapter 1 Chapter 1: The Science of Life A biologist studies all living things. In order to carry out the scientific method, we need to ask questions. Discover Biology
Discover Biology 'N' Level Science Chapter 1 Chapter 1: The Science of Life A biologist studies all living things. In order to carry out the scientific method, we need to ask questions. Discover Biology
Digestive System Functions
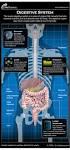 Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
Digestive System Functions A. Gastrointestinal Processes 1. Ingestion: placing food in mouth (voluntary) 2. Propulsion: moving food through GI tract a. Peristalsis: alternating waves of contraction and
Digestive System Lecture 5 Winter 2014
 Digestive System Lecture 5 Winter 2014 This lecture tells the story of the Flow of Matter from Food to Cells. The pictures are only there to help you visualize structures don t worry about names of structures
Digestive System Lecture 5 Winter 2014 This lecture tells the story of the Flow of Matter from Food to Cells. The pictures are only there to help you visualize structures don t worry about names of structures
Biological Molecules
 Biological Molecules I won t lie. This is probably the most boring topic you have ever done in any science. It s pretty much as simple as this: learn the material deal with it. Enjoy don t say I didn t
Biological Molecules I won t lie. This is probably the most boring topic you have ever done in any science. It s pretty much as simple as this: learn the material deal with it. Enjoy don t say I didn t
Cells & Cell Organelles
 Cells & Cell Organelles The Building Blocks of Life H Biology Types of cells bacteria cells Prokaryote - no organelles Eukaryotes - organelles animal cells plant cells Cell size comparison Animal cell
Cells & Cell Organelles The Building Blocks of Life H Biology Types of cells bacteria cells Prokaryote - no organelles Eukaryotes - organelles animal cells plant cells Cell size comparison Animal cell
Amino Acids, Proteins, and Enzymes. Primary and Secondary Structure Tertiary and Quaternary Structure Protein Hydrolysis and Denaturation
 Amino Acids, Proteins, and Enzymes Primary and Secondary Structure Tertiary and Quaternary Structure Protein Hydrolysis and Denaturation 1 Primary Structure of Proteins H 3 N The particular sequence of
Amino Acids, Proteins, and Enzymes Primary and Secondary Structure Tertiary and Quaternary Structure Protein Hydrolysis and Denaturation 1 Primary Structure of Proteins H 3 N The particular sequence of
The Chemical Basis of Life. Chemical Bonds
 The Chemical Basis of Life white=hydrogen red=oxygen gray=carbon yellow=phosphorus blue=nitrogen green=sulfur All organisms are made up of water, inorganic ions, small molecules (77% by weight) and macromolecules
The Chemical Basis of Life white=hydrogen red=oxygen gray=carbon yellow=phosphorus blue=nitrogen green=sulfur All organisms are made up of water, inorganic ions, small molecules (77% by weight) and macromolecules
Chapter 8: An Introduction to Metabolism
 Chapter 8: An Introduction to Metabolism Name Period Concept 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 1. Define metabolism. The totality of an organism
Chapter 8: An Introduction to Metabolism Name Period Concept 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 1. Define metabolism. The totality of an organism
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES
 LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Ch23_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following statements concerning digestion are correct except A) The major physical
Unit 2: Cells, Membranes and Signaling CELL MEMBRANE. Chapter 5 Hillis Textbook
 Unit 2: Cells, Membranes and Signaling CELL MEMBRANE Chapter 5 Hillis Textbook HOW DOES THE LAB RELATE TO THE NEXT CHAPTER? SURFACE AREA: the entire outer covering of a cell that enables materials pass.
Unit 2: Cells, Membranes and Signaling CELL MEMBRANE Chapter 5 Hillis Textbook HOW DOES THE LAB RELATE TO THE NEXT CHAPTER? SURFACE AREA: the entire outer covering of a cell that enables materials pass.
BSC 2010 - Exam I Lectures and Text Pages. The Plasma Membrane Structure and Function. Phospholipids. I. Intro to Biology (2-29) II.
 BSC 2010 - Exam I Lectures and Text Pages I. Intro to Biology (2-29) II. Chemistry of Life Chemistry review (30-46) Water (47-57) Carbon (58-67) Macromolecules (68-91) III. Cells and Membranes Cell structure
BSC 2010 - Exam I Lectures and Text Pages I. Intro to Biology (2-29) II. Chemistry of Life Chemistry review (30-46) Water (47-57) Carbon (58-67) Macromolecules (68-91) III. Cells and Membranes Cell structure
A. A peptide with 12 amino acids has the following amino acid composition: 2 Met, 1 Tyr, 1 Trp, 2 Glu, 1 Lys, 1 Arg, 1 Thr, 1 Asn, 1 Ile, 1 Cys
 Questions- Proteins & Enzymes A. A peptide with 12 amino acids has the following amino acid composition: 2 Met, 1 Tyr, 1 Trp, 2 Glu, 1 Lys, 1 Arg, 1 Thr, 1 Asn, 1 Ile, 1 Cys Reaction of the intact peptide
Questions- Proteins & Enzymes A. A peptide with 12 amino acids has the following amino acid composition: 2 Met, 1 Tyr, 1 Trp, 2 Glu, 1 Lys, 1 Arg, 1 Thr, 1 Asn, 1 Ile, 1 Cys Reaction of the intact peptide
Molecular Genetics. RNA, Transcription, & Protein Synthesis
 Molecular Genetics RNA, Transcription, & Protein Synthesis Section 1 RNA AND TRANSCRIPTION Objectives Describe the primary functions of RNA Identify how RNA differs from DNA Describe the structure and
Molecular Genetics RNA, Transcription, & Protein Synthesis Section 1 RNA AND TRANSCRIPTION Objectives Describe the primary functions of RNA Identify how RNA differs from DNA Describe the structure and
How To Understand The Human Body
 Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
1. When applying the process of science, which of these is tested? a. an observation b. a result c. a hypothesis d. a question e.
 BCOR 11 Exam 1, 2004 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1. When applying the process of science, which of these is tested? a. an observation
BCOR 11 Exam 1, 2004 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1. When applying the process of science, which of these is tested? a. an observation
Genetics Test Biology I
 Genetics Test Biology I Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Avery s experiments showed that bacteria are transformed by a. RNA. c. proteins.
Genetics Test Biology I Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Avery s experiments showed that bacteria are transformed by a. RNA. c. proteins.
CELL MEMBRANES, TRANSPORT, and COMMUNICATION. Teacher Packet
 AP * BIOLOGY CELL MEMBRANES, TRANSPORT, and COMMUNICATION Teacher Packet AP* is a trademark of the College Entrance Examination Board. The College Entrance Examination Board was not involved in the production
AP * BIOLOGY CELL MEMBRANES, TRANSPORT, and COMMUNICATION Teacher Packet AP* is a trademark of the College Entrance Examination Board. The College Entrance Examination Board was not involved in the production
Ch24_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch24_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Substances originating in plant or animal material and soluble in non-polar organic solvents
Ch24_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Substances originating in plant or animal material and soluble in non-polar organic solvents
Lab 2 Biochemistry. Learning Objectives. Introduction. Lipid Structure and Role in Food. The lab has the following learning objectives.
 1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
Helices From Readily in Biological Structures
 The α Helix and the β Sheet Are Common Folding Patterns Although the overall conformation each protein is unique, there are only two different folding patterns are present in all proteins, which are α
The α Helix and the β Sheet Are Common Folding Patterns Although the overall conformation each protein is unique, there are only two different folding patterns are present in all proteins, which are α
Transcription and Translation of DNA
 Transcription and Translation of DNA Genotype our genetic constitution ( makeup) is determined (controlled) by the sequence of bases in its genes Phenotype determined by the proteins synthesised when genes
Transcription and Translation of DNA Genotype our genetic constitution ( makeup) is determined (controlled) by the sequence of bases in its genes Phenotype determined by the proteins synthesised when genes
THE LIVING CELL. Cells also have variety of shapes. Plant cells are often rectangular or polygonal, while egg cells are usually spherical.
 THE LIVING CELL A Tour of the cell The cell is the smallest and the basic unit of structure of all organisms. There are two main types or categories of cells: prokaryotic cells and eukaryotic cells. Prokaryotic
THE LIVING CELL A Tour of the cell The cell is the smallest and the basic unit of structure of all organisms. There are two main types or categories of cells: prokaryotic cells and eukaryotic cells. Prokaryotic
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1
 CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
CHEMISTRY AND BIOLOGICAL ROLE OF CARBOHYDRATES IN THE BODY-1 Chiral centers: Asymmetric carbons, i.e carbon atom with four different substituents Enantiomers : Mirror images Stereoisomers MONOSACCHARIDE
BIOLOGICAL MEMBRANES: FUNCTIONS, STRUCTURES & TRANSPORT
 BIOLOGICAL MEMBRANES: FUNCTIONS, STRUCTURES & TRANSPORT UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY BMLS II / B Pharm II / BDS II VJ Temple
BIOLOGICAL MEMBRANES: FUNCTIONS, STRUCTURES & TRANSPORT UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY BMLS II / B Pharm II / BDS II VJ Temple
DNA, RNA, Protein synthesis, and Mutations. Chapters 12-13.3
 DNA, RNA, Protein synthesis, and Mutations Chapters 12-13.3 1A)Identify the components of DNA and explain its role in heredity. DNA s Role in heredity: Contains the genetic information of a cell that can
DNA, RNA, Protein synthesis, and Mutations Chapters 12-13.3 1A)Identify the components of DNA and explain its role in heredity. DNA s Role in heredity: Contains the genetic information of a cell that can
Academic Nucleic Acids and Protein Synthesis Test
 Academic Nucleic Acids and Protein Synthesis Test Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Each organism has a unique combination
Academic Nucleic Acids and Protein Synthesis Test Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Each organism has a unique combination
STRUCTURES OF NUCLEIC ACIDS
 CHAPTER 2 STRUCTURES OF NUCLEIC ACIDS What is the chemical structure of a deoxyribonucleic acid (DNA) molecule? DNA is a polymer of deoxyribonucleotides. All nucleic acids consist of nucleotides as building
CHAPTER 2 STRUCTURES OF NUCLEIC ACIDS What is the chemical structure of a deoxyribonucleic acid (DNA) molecule? DNA is a polymer of deoxyribonucleotides. All nucleic acids consist of nucleotides as building
Transmembrane proteins span the bilayer. α-helix transmembrane domain. Multiple transmembrane helices in one polypeptide
 Transmembrane proteins span the bilayer α-helix transmembrane domain Hydrophobic R groups of a.a. interact with fatty acid chains Multiple transmembrane helices in one polypeptide Polar a.a. Hydrophilic
Transmembrane proteins span the bilayer α-helix transmembrane domain Hydrophobic R groups of a.a. interact with fatty acid chains Multiple transmembrane helices in one polypeptide Polar a.a. Hydrophilic
Structure of proteins
 Structure of proteins Primary structure: is amino acids sequence or the covalent structure (50-2500) amino acids M.Wt. of amino acid=110 Dalton (56 110=5610 Dalton). Single chain or more than one polypeptide
Structure of proteins Primary structure: is amino acids sequence or the covalent structure (50-2500) amino acids M.Wt. of amino acid=110 Dalton (56 110=5610 Dalton). Single chain or more than one polypeptide
1. Essay: The Digestive and Absorption Processes of Macronutrients
 Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Lecture 26: Overview of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) structure
 Lecture 26: Overview of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) structure Nucleic acids play an important role in the storage and expression of genetic information. They are divided into
Lecture 26: Overview of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) structure Nucleic acids play an important role in the storage and expression of genetic information. They are divided into
Biological cell membranes
 Unit 14: Cell biology. 14 2 Biological cell membranes The cell surface membrane surrounds the cell and acts as a barrier between the cell s contents and the environment. The cell membrane has multiple
Unit 14: Cell biology. 14 2 Biological cell membranes The cell surface membrane surrounds the cell and acts as a barrier between the cell s contents and the environment. The cell membrane has multiple
2. The number of different kinds of nucleotides present in any DNA molecule is A) four B) six C) two D) three
 Chem 121 Chapter 22. Nucleic Acids 1. Any given nucleotide in a nucleic acid contains A) two bases and a sugar. B) one sugar, two bases and one phosphate. C) two sugars and one phosphate. D) one sugar,
Chem 121 Chapter 22. Nucleic Acids 1. Any given nucleotide in a nucleic acid contains A) two bases and a sugar. B) one sugar, two bases and one phosphate. C) two sugars and one phosphate. D) one sugar,
Biology 12 June 2003 Provincial Examination
 Biology 12 June 2003 rovincial Examination ANWER KEY / CORING GUIDE CURRICULUM: Organizers 1. Cell Biology 2. Cell rocesses and Applications 3. Human Biology ub-organizers A, B, C, D E, F, G, H I, J, K,
Biology 12 June 2003 rovincial Examination ANWER KEY / CORING GUIDE CURRICULUM: Organizers 1. Cell Biology 2. Cell rocesses and Applications 3. Human Biology ub-organizers A, B, C, D E, F, G, H I, J, K,
FIGURE 2.18. A. The phosphate end of the molecule is polar (charged) and hydrophilic (attracted to water).
 PLASMA MEMBRANE 1. The plasma membrane is the outermost part of a cell. 2. The main component of the plasma membrane is phospholipids. FIGURE 2.18 A. The phosphate end of the molecule is polar (charged)
PLASMA MEMBRANE 1. The plasma membrane is the outermost part of a cell. 2. The main component of the plasma membrane is phospholipids. FIGURE 2.18 A. The phosphate end of the molecule is polar (charged)
