SNEAK PEAK inside ACTIVITY OPTIONAL EXTRAS. EXTENSION Guess My Powder! (p. G - 49) Advance Preparation Set Up Activity Clean Up
|
|
|
- Hollie Hancock
- 7 years ago
- Views:
Transcription
1 Lost Labels Learning Objectives: Students collect and analyze data to identify substances. GRADE LEVEL 3 8 SCIENCE TOPICS Physical Properties Techniques Chemical Reactions PROCESS SKILLS Measuring Hypothesizing Inferring Evaluating GROUP SIZE 1 4 SNEAK PEAK inside ACTIVITY Students conduct chemical and physical tests on unknown white powders. STUDENT SUPPLIES see next page for more supplies various white powders plastic cups and spoons various liquids to test powders, etc. ADVANCE PREPARATION see next page for more details Cut up egg cartons. Fill ice cube trays with white powders. Prepare test solutions, etc. OPTIONAL EXTRAS DEMONSTRATION Modeling the Procedure (p. G - 42) Salt and Sugar Solubility (p. G - 42) Salt and Sugar Conductivity (p. G - 43) EXTENSION Guess My Powder! (p. G - 49) TIME REQUIRED Advance Preparation Set Up Activity Clean Up 20 minutes 10 minutes 50 minutes 10 minutes Lost Labels G 37 Chemistry in the K 8 Classroom
2 SUPPLIES Notes on activity setup: The instructions assume your students will use 6 different tests to identify 12 different powders. For a shorter activity, you may choose to use fewer tests to identify fewer powders. To make an abbreviated version of the activity, see the following pages: Use 3 tests to identify 8 powders (p. G - 53) Use 2 tests to identify 5 powders (p. G - 54) Use 2 tests to identify 6 powders (p. G - 54) In addition, there are different ways you can set up this activity. You can give each student group all of the powders they will test or you can set up stations with particular powders that students will rotate through. If each student group has all of the powders, the activity provides more substantial and meaningful experiences for each student but requires more preparation and a greater number of powders, plastic cups, spoons, etc. If students investigate all of the powders themselves in this way, they will take over twice as long to test them. But they probably get more out of the experience. On the other hand, student stations are easier to set up, require fewer materials, but require more management as students rotate. Item (this list assumes you will be testing all 12 powders; fewer supplies are needed when testing fewer powders) corn starch granulated sugar baking powder cream of tartar baking soda table salt laundry detergent (must contain sodium carbonate available in grocery stores) Epsom salts dry milk powder baby powder plaster of Paris Alka-Seltzer tablets ice cube trays (to distribute powders) small plastic cup (for mixing) Amount Needed 1 per group 1 per station or group Lost Labels G 38 Chemistry in the K 8 Classroom
3 plastic spoons (for powders) plastic spoon (for mixing) Styrofoam or plastic egg carton, preferably white in color pop-top squeeze bottles (e.g., water or sports drink) 6 oz. or larger vinegar tincture of iodine red cabbage water toothpicks magnifying glasses (optional) paper towels 1 12 per station or group 1 per station or group 1 per 3 4 stations or groups 1 4 per station ¼ cup per station or group 2 teaspoons per class ¼ cup per station or group 2 cups per station or group per station or group 1 per station or group 1 roll per station or group For Extension or Demonstration supplies, see the corresponding section. ADVANCE PREPARATION Please see the note on activity setup above. First CHOOSE how many tests your students will perform. All six tests will identify all 12 powders, but fewer tests still identify many powders. For instance, only two tests (iodine and cabbage) will identify up to six powders. See pages G - 48 to G - 50 for more information. Supplies Preparation Cabbage Juice Indicator: Finely chop cabbage with a knife. Place 2 cups or more in ½ gallon container. Add hot tap water until cabbage is just barely covered. Wait 2 5 minutes. Place strainer over large bowl. Pour cabbage and hot water through strainer, collecting water in bowl. The water should now be purple. Add ½ 1 cup salt to the mixture and stir until dissolved (this is to preserve the cabbage juice from mold). Store the purple water ( cabbage juice ) in a labeled container in the refrigerator until ready for use. It does not need to be refrigerated on day of use. Dispose of solid cabbage as you would other vegetable scraps. Lost Labels G 39 Chemistry in the K 8 Classroom
4 Iodine Solution: Add 1 teaspoon of tincture of iodine to 2 cups of water. Divide this solution into pop-top squeeze bottles. Use masking tape and permanent marker to label the bottles iodine solution. CAUTION: Iodine is poisonous and may stain skin and clothing. Alka-Seltzer: Put the tablets in a piece of paper folded in half. Crush with a jar or rolling pin. Egg Cartons: Use scissors to cut each egg carton into four pieces, so each piece has three egg wells. Students will use these wells for the iodine, cabbage juice, and vinegar tests. In place of egg cartons, white ice cube trays will also work. Don t attempt to cut the ice cube trays, however. Water: Fill pop-top squeeze bottles with room temperature water. Label these bottles water. Vinegar: Fill pop-top squeeze bottles with vinegar. Label these bottles vinegar. Ice Cube Trays of Powders: Use the masking tape and permanent marker to label up to 12 wells of ice cube trays with the numbers Use the masking tape and permanent marker to label up to 12 plastic spoons with the numbers Record which powder is which number. Add about one tablespoon of each powder into its corresponding well. Put the correct numbered spoon in each well. Properties of Powders Chart: Make copies to place at each station. You may choose to laminate these. Notes and Hints Laundry Detergent: The detergent must contain sodium carbonate (also called washing soda). Check the label. Try out these detergents first: Arm & Hammer Fabricare, BioKleen, Seventh Generation. Lost Labels G 40 Chemistry in the K 8 Classroom
5 SETUP For each group vinegar in pop-top squeeze bottle cabbage juice in pop-top squeeze bottle iodine solution in pop-top squeeze bottle water in pop-top squeeze bottle cut Styrofoam or plastic egg carton (ice cube trays as alternative) (to be shared by several stations) ice cube tray with powders 1 empty plastic cup 1 plastic spoon paper towels Properties of Powders Chart INTRODUCING THE ACTIVITY Let the students speculate before offering answers to any questions. The answers at right are provided for the teacher. Choose questions that are appropriate for your classroom. Tell the students the following story (or make up your own, better story!): A woman owns a cabin in the mountains. The only cupboards are at floor level, so she keeps her household supplies in labeled jars to protect them from moisture, mice, and insects. Unfortunately, some pipes burst and flooded the cupboards, washing the labels off all the jars. She has given us samples and would like us to figure out what is in each jar. We know what the twelve powders are, and we know some of their properties. By conducting chemical tests we should be able to determine which powder is which. What ideas do you have for testing these powders? Depending on the students experience, they may suggest many tests. CAUTION: Never put lab supplies in your mouth. Even if lab supplies are foods, they may be contaminated by other items in the lab. Lost Labels G 41 Chemistry in the K 8 Classroom
6 TEACHER DEMONSTRATION Modeling the Procedure You may wish to discuss with students the various procedures they will use to test and identify the different powders. For the Physical Properties, discuss the characteristics that can help distinguish one powder from another. For the Vinegar, Cabbage Juice, and Iodine, show students the amount of powder they need to use only a pea-sized amount. For the Temperature and Solubility, students need to add a bit more powder. If students do the Temperature test first, and the powder dissolves completely, then they don t need to do the Solubility since they know the powder will dissolve. The Powder Identification Chart lists the properties for all twelve different powders. Examine this chart with students to make sure they understand how to use it. Also, if you are not using all 12 powders, students can cross out the powders that are not included. Salt and Sugar Solubility This demonstration is best done after the activity, assuming the activity included both salt and sugar. Salt and sugar give the same results for all tests, so a further test is required to tell them apart. Students may suggest tasting powders, but this is not advisable since food items may be contaminated by other, poisonous substances in the lab. Compared to cold water, hot water will dissolve a much larger amount of sugar. This is not true for salt both cold and hot water will dissolve about the same amount. This difference can be used to distinguish salt from sugar. Supplies two microwaveable cups or jars water (at room temperature or colder) salt sugar two plastic spoons microwave or source of very hot water (microwave water to boiling beforehand and store in a thermos) Lost Labels G 42 Chemistry in the K 8 Classroom
7 Demonstration Add two spoonfuls of water to each cup. Add one heaping spoonful of salt to one cup and one heaping spoonful of sugar to the other cup. Stir to dissolve each powder as much as possible. Microwave both cups for seconds, taking them out to swirl the contents after the water starts to boil. (If a microwave is not available, add very hot water to both cups and swirl the contents.) The sugar should all dissolve in the water while some of the salt remains. Explanation Salt and sugar can both dissolve in water at room temperature, but sugar can dissolve at much higher concentrations in hot water than in cold. In contrast, salt dissolves to the same degree in both hot and cold water (this is actually a rather interesting and unusual property of common table salt). Students might suggest testing other powders with this simple temperature comparison. Salt and Sugar Conductivity You can also demonstrate how to distinguish salt and sugar by their conductivity. Salt will conduct electricity (allowing a light bulb to light) and sugar will not conduct electricity (and the light bulb will not light). Supplies two small glass jars (e.g., baby food jars) two batteries, AA size or larger three wires light bulb in socket with connectors alligator clips water plastic spoon sugar salt Figure 1 Sample conductivity set up. The wires in the solution and connected to the alligator clips are bare, exposed wires. Lost Labels G 43 Chemistry in the K 8 Classroom
8 Demonstration Add about 1 inch of water to the jar. Add spoonfuls of sugar or salt to the water until no more dissolves. Set up the wires, clips, batteries, and light bulb according to the diagram. Place the wire leads in the solution. Don t let the wires touch each other. Ask the students to observe the jar. Do form at either of the wires? Are any changes visible in the wires? Does the light bulb glow? Does the solution change color? Explanation When salt dissolves in water, its molecules break apart into ions, small charged particles. These ions are capable of carrying a charge and can thus carry electricity. When sugar dissolves in water, its molecules separate from each other, but they do not break apart into ions. For this reason, sugar cannot conduct electricity. Lost Labels G 44 Chemistry in the K 8 Classroom
9 CLASSROOM ACTIVITY Have students follow the Scientific Procedure on page G - 55, working in groups of 1 4. Below are suggestions to help the teacher facilitate the activity. NOTES This handout is on p. G Running Suggestions Depending on age and skill level of students, each group can identify one powder, a few powders, or all twelve. Set out a number of powders appropriate for your students. CAUTION: Plaster of Paris clogs drains!!! If you use plaster of Paris in the activity, then: Caution students to treat all powders as if they cannot be poured down the drain! Instruct students to rinse their cups and egg cartons with a little water and then wipe them out with a paper towel. They should throw the paper towel away. Monitor students to see how much powder they are using. They only need a small amount (the size of a pea) for the Vinegar, Iodine, and Cabbage Juice. If you include both sugar and salt, students won t be able to identify them (see the Teacher Demonstration for info on identifying Lost Labels G 45 Chemistry in the K 8 Classroom
10 them). It s a great way to spark student inquiry or a discussion of how they might be identified. If your students have a lot of experience with related activities, they can devise their own tests to identify the powders. Advanced and experienced students can compare notes with each other and make educated guesses. Remind students to create an organized table to keep track of all their results. Ongoing Assessment Questions When students do the vinegar test, ask them what gas they think is in the and why they form. (The are carbon dioxide gas, which is created in the reaction.) When students do the iodine test, ask them why it would turn black. (The iodine reacts with starch, found in corn starch and also in baking powder.) (Remind students of the activity, Foam Peanuts, if they have done it.) When students do the temperature test, ask them why the solution changes temperature (the powder is arranged differently when mixed with water, and each different arrangement of molecules has a different energy; for some powders, this energy change is big enough to feel.) (Remind students of the activity, Matter of Degree, if they have done it.) When students do the cabbage juice test, ask them why the cabbage juice changes color (cabbage juice is an acid-base indicator, a chemical that interacts with acids and bases and changes color). (Remind students of the activity, Of Cabbages and Kings, if they have done it.) Listen to students reasoning when they identify the powders. They should refer to the Properties Chart and to their notes when identifying a powder. Safety and Disposal Information If you include plaster of Paris in the activity, do not allow students to put it down the sink. Plaster of Paris will permanently clog and destroy the drain. All other materials may be rinsed down the sink or thrown away. Students should wear safety goggles if possible. CAUTION: Plaster of Paris clogs drains PERMANENTLY. Caution students to throw all powders into the garbage unless they are absolutely certain the powder is NOT plaster of Paris. CAUTION: Iodine is poisonous and may stain skin or clothing. Lost Labels G 46 Chemistry in the K 8 Classroom
11 CLASSROOM DISCUSSION Ask for student observations and explanations. Let the students guide the discussion and present their hypotheses before discussing explanations. Choose questions that are appropriate for your classroom. List the numbers for each mystery powder on the board and have each group come up and write their identification for each powder. If there are conflicting results, you can discuss how each group came to the conclusion that they did. What reasoning did you use to identify the powders? Answers will vary. The results of each test narrow down the choices for the identity of the powders. In some cases, a powder can be identified with a single test (cream of tartar). In other cases, all six tests are required to identify a powder (baking soda vs. baking powder, for instance). In one particularly difficult case (sugar vs. salt) an extra test is required to identify the powders (see the Teacher Demonstration). Which tests used chemical changes to identify powders? How do you know? The Vinegar, Iodine, and Cabbage Juice all used chemical changes. Evidence of a chemical change can include a change in color or formation of. Which tests used physical changes to identify powders? How do you know? The Temperature and Solubility use physical properties. These don t create new substances. Looking at the physical properties of the powder (grain size, crystal formation, smell) is also not a chemical change. What powders were easy to determine? Alka-Seltzer with everything and is easy to spot. Cream of tartar is the only one that turns red with cabbage juice. What powders were difficult to determine? Baking soda, baking powder, and plaster of Paris don t show much of a temperature change, so they can be mistaken for other powders. Sugar and salt have the same results for all tests, so cannot be determined. After discussing the results for the powders, perform the demonstration to determine which of the powders is salt and which is sugar. Lost Labels G 47 Chemistry in the K 8 Classroom
12 EXPLANATION This background information is for teachers. Modify and communicate to students as necessary. This activity requires students to gather information from multiple chemical tests of various white powders. Each test shows a different physical or chemical property of the powders. Students then analyze their results and find the identity of each powder. Physical Properties Each powder is similar in color and composition, but students should still be able to find differences (especially if they have magnifying glasses). For instance, all of the powders are white, but the milk powder usually has a bit of a cream color. Also, the baby powder can have a faint smell. Upon inspection, students should notice that salt, sugar, and Epsom salts are crystals; they appear shiny on the flat surfaces of the crystal. Alka- Seltzer, detergent, and milk powder are mixtures of various powders, and they appear to be tiny, irregular chunks. The rest of the powders consist of very tiny particles and are classified as powders. Vinegar Several of the powders contain some form of the chemical carbonate, which will react with vinegar. There are several forms of carbonate in the powders, as shown in the following table: sodium bicarbonate is in sodium carbonate is in calcium carbonate is in baking soda Alka-Seltzer detergent plaster of Paris baking powder All these forms of carbonate react with vinegar to create carbon dioxide gas. Iodine Detergent is designed to remove stains, so the iodine fades when it is mixed with detergent. Milk removes the color of iodine because it contains a sugar called lactose. This sugar reacts with the iodine in solution, changing it from brown to colorless. Cornstarch and baking powder (which contains starch) will both turn blue or black. This is because starch and iodine react to form a blue or black compound. For a more in-depth explanation of the reaction of starch and iodine, see the Explanation section of Foam Peanuts. Lost Labels G 48 Chemistry in the K 8 Classroom
13 Cabbage Cabbage juice is an indicator and changes color depending on whether it is with an acid or a base. Baking powder, baking soda, and detergent are all basic and will turn the cabbage juice blue or green. Cream of tartar is an acid and turns the cabbage juice red or pink. For a more in-depth explanation of acid base indicators, see the Explanation section of Of Cabbages and Kings. Temperature Some powders require energy to dissolve (use an endothermic process) and feel cold when mixed with water. Some powders release energy (use an exothermic process) when dissolved and feel warm when mixed with water. Epsom salts is strongly endothermic; baking powder and baking soda are only slightly endothermic. Detergent is very exothermic; plaster of Paris is slightly exothermic. For a more in-depth explanation of chemical temperature change, see the Explanation section of Matter of Degree. Solubility Alka-Seltzer, Epsom salts, salt, and sugar all dissolve in water very easily; the powders can no longer be seen in the liquid. Baking powder, baking soda, cream of tartar, and detergent dissolve in water only a little, and some of the powder can still be seen in the liquid. Milk powder, plaster of Paris, and starch do not dissolve well in water, and all create uniform mixtures (the milk powder contains some solids that dissolve and some that don t). EXTENSIONS Extension A: Guess My Powder! Students use their knowledge of chemical properties to play a game. This can be done as a class or in partners. Extra Instructions One student, or the teacher, thinks of a chemical the students have identified and writes it on a hidden piece of paper. Other students ask yes or no questions. For instance, Does it dissolve completely in water? After answering questions, students guess the identity of the chemical. Explanation The game Guess Who? uses cards with people s faces printed on them. Players are challenged to identify a person based on their characteristics. Lost Labels G 49 Chemistry in the K 8 Classroom
14 CROSS-CURRICULAR CONNECTIONS LANGUAGE ARTS BIOLOGY Write a Mystery Compound Story Students can write a story that involves the identification of a mystery compound. Taxonomy: Classify Your World Give students multiple objects or pictures to group according to their characteristics. Younger students can identify a trait and group the blocks, tools, foods, plants, pictures of animals, rocks, etc., by the traits they share. Middle students can classify items according to common traits. Older students can use the traits of the items to build a dichotomous key a series of yes or no questions that allows the identification of the item. Use one of the supplied resources, or find your own, as a starting place. RESOURCES Web This Web site has many ideas for classification exercises using plants and animals. It suggests properties to use to group species. Lessons can be used for all ages. Web The Linnean Society of London is concerned with taxonomy, which involves the examination and collection of a wide range of scientific evidence for accurate identification that is essential to any research. The Society finds much importance in identification of species for biodiversity conservation. Information about Carl Linnaeus, the founder of modern taxonomy, can be found on the site by choosing History from the main page. Watts, Tom, Pacific Coast Tree Finder Reading level: 3 rd grade to adult This is a classic key to identifying the native trees of the Pacific Coast, from British Columbia to Baja California. It uses characteristics such as leaf shape, tree height, and geographical location to determine the species of tree. It also includes pictures for easier identification. This book uses a dichotomous key format for accurate identification. The Finders series of pocket guides also includes guides to identify flowers, berries, mammals, and ferns. Dussling, Jennifer, Looking at Rocks Reading level: 1 st grade to 4 th grade This is a great introduction to rocks and rock hounding. The pictures are clear and enhance the text, which is written at a good level for beginning readers. Lost Labels G 50 Chemistry in the K 8 Classroom
15 VOCABULARY acid: base: a compound with an excess of available hydrogen ions; often sour in taste a chemical or compound that takes up hydrogen ions; often bitter in taste carbonate: a chemical made of one carbon atom and three oxygen atoms; CO3 2- crystal: dissolve: endothermic: exothermic: indicator: ion: lactose powder: a chemical in solid form; usually refers to pure solids with shiny facets and faces, such as diamonds when the molecules of a solid separate and become completely surrounded by the molecules of a liquid a chemical change that absorbs energy, causing the reaction container to feel cool a chemical change that releases energy, causing the reaction container to feel warm a substance that changes color to indicate the presence or concentration of a certain chemical an electrically charged atom or group of atoms sugar found in milk products composed of particles that are too small to be seen easily Lost Labels G 51 Chemistry in the K 8 Classroom
16 Lost Labels POWDER IDENTIFICATION CHART Powder Physical Properties Vinegar Iodine Cabbage Temp. Solubility Alka- Seltzer coarse, irregular chunks orangebrown, stays purple, stays same temp yes, baby powder powder, may have a smell no orangebrown stays purple stays same temp no baking powder fine powder black, blue, colder a little baking soda fine powder orangebrown blue colder a little cream of tartar fine powder no orangebrown red stays same temp a little detergent Epsom salts coarse, irregular chunks small, sharp edged crystals no milk powder fine powder no plaster of Paris fine powder orangebrown color fades orangebrown orangebrown color fades orangebrown green warmer a little stays purple colder yes stays purple stays same temp milky stays purple warmer paste salt small, sharp edged crystals no orangebrown stays purple stays same temp yes starch fine powder no black stays purple stays same temp paste sugar small, sharp edged crystals no orangebrown stays purple stays same temp yes
17 Lost Labels THESE 3 TESTS IDENTIFY 8 POWDERS Powder Vinegar Iodine Cabbage Alka-Seltzer brown, one of: Epsom salts, baby powder, salt, sugar, milk powder no orangebrown baking powder black, cream of tartar no baking soda orangebrown orangebrown detergent orangebrown color fades plaster of Paris orangebrown purple, stays purple blue, blue red green stays purple starch no black stays purple
18 Lost Labels THESE 2 TESTS IDENTIFY 5 POWDERS Powder Vinegar Cabbage Alka Seltzer purple, cream of tartar no red detergent green baking powder or baking soda one of: Epsom salts, baby powder, plaster of Paris, milk powder, salt, sugar, starch no blue stays purple THESE 2 TESTS IDENTIFY 6 POWDERS Powder Iodine Cabbage Alka-Seltzer brown, no color change, baking powder black, blue, baking soda orange-brown blue cream of tartar orange-brown red detergent orange-brown color fades green starch black stays purple baby powder, Epsom salts,, sugar, plaster of Paris, salt, milk powder orange-brown stays purple
19 Lost Labels SCIENTIFIC PROCEDURE Six tests are listed below. You can conduct any test, on any powder, in any order. Your goal is to collect enough information on each powder to identify it. Be careful not to mix powders with each other in the ice cube tray, the spoons, or in the egg carton. Record your observations on the data sheet. Be sure to clean out the plastic cup and egg carton between tests. In each case, pour any chemicals from your test into the trash first. Then add a little water and wipe out the container with a paper towel. Throw away the paper towel. Physical Properties What does the powder look like? Does it have a smell? Are there crystals or small, irregular chunks? Is it a fine or coarse powder? Vinegar Put a pea-sized amount of the powder in a well of the egg carton. Add ¼ spoonful of vinegar. Stir with a toothpick. What happens? Cabbage Juice Put a pea-sized amount of the powder in a well of the egg carton. Add ¼ spoonful of cabbage juice. Stir with a toothpick. What happens? Iodine Put a pea-sized amount of the powder in a well of the egg carton. Add ¼ spoonful of iodine solution. Stir with a toothpick. What happens? Temperature Add one spoonful of water to the plastic cup. Feel the plastic cup. Add one spoonful of powder to the cup. Mix with a toothpick. Feel the plastic cup now. Did the temperature change? Solubility Add one spoonful of water to the plastic cup. Add ½ spoonful of powder to the water. Mix with a toothpick. Does the powder dissolve in the water?
20 Lost Labels DATA COLLECTION SHEET Powder (write your prediction under the number) 1 Physical Properties Vinegar Iodine Cabbage Temp. Solubility
21 SUPPLY WORKSHEET This worksheet is also available online at Lost Labels Recommended group size: 1 3 Number of Students: Number of Groups: Supplies Amount Needed Supplies on Hand note: This list assumes you will be testing all 12 powders; modify supplies as needed when testing fewer powders cornstarch granulated sugar baking powder cream of tartar baking soda table salt laundry detergent (must contain sodium carbonate available in grocery stores) Epsom salts dry milk powder baby powder plaster of Paris Alka-Seltzer tablets ice cube trays (to distribute powders) small plastic cup (for mixing) plastic spoons (for powders) plastic spoon (for mixing) Styrofoam or plastic egg carton, preferably white in color pop-top squeeze bottles (e.g., water or sports drink) 6 oz. or larger vinegar tincture of iodine red cabbage water toothpicks magnifying glasses (optional) paper towels 1 per group 1 per station or group 1 12 per station or group 1 per station or group 1 per 3 4 stations or groups 1 4 per station ¼ cup per station or group 2 tsp per class ¼ cup per station or group 2 cups per station or group per station or group 1 per station or group 1 roll per station or group Supplies Needed Supply Worksheet continues on next page. Lost Labels G 57 Chemistry in the K 8 Classroom
22 Supplies Amount Needed Supplies on Hand Extension A no extra supplies needed Teacher Demonstration Modeling the Procedure no extra supplies needed Salt and Sugar Solubility microwaveable cups or jars water (at room temperature or colder) salt sugar plastic spoons microwave or source of very hot water (microwave water to boiling beforehand and store in a thermos) Salt and Sugar Conductivity small glass jars (e.g., baby food jars) batteries, AA size or larger wires light bulb in socket with connectors alligator clips water plastic spoon sugar salt 2 cups or jars ¼ cup heaping spoonful heaping spoonful 2 spoons 2 for class 2 for class 3 or more depending on set up 1 for class 3 or more depending on set up ¼ cup 1 for class ¼ cup ¼ cup Supplies Needed Lost Labels G 58 Chemistry in the K 8 Classroom
Acids and Bases. AND a widemouth container of the following solids:
 Acids and Bases GOAL To introduce students to acids and bases. MATERIALS: 3 10oz clear plastic cups 1 4 oz. bottle white vinegar - labeled Acid 1 4 oz. bottle of water - labeled Water 1 4 oz. bottle of
Acids and Bases GOAL To introduce students to acids and bases. MATERIALS: 3 10oz clear plastic cups 1 4 oz. bottle white vinegar - labeled Acid 1 4 oz. bottle of water - labeled Water 1 4 oz. bottle of
Of Cabbages and Kings
 Of Cabbages and Kings Learning Objectives: Students will learn about indicators, acids, bases, and the ph scale. GRADE LEVEL K 8 SCIENCE TOPICS Physical Properties Techniques Chemical Reactions PROCESS
Of Cabbages and Kings Learning Objectives: Students will learn about indicators, acids, bases, and the ph scale. GRADE LEVEL K 8 SCIENCE TOPICS Physical Properties Techniques Chemical Reactions PROCESS
Review and apply Investigation 5. Let s review Pages 311-312
 Review and apply Investigation 5 Let s review Pages 311-312 1. After you tested all the known powders with all the test liquids, describe what you did to identify the unknown powder. Students should have
Review and apply Investigation 5 Let s review Pages 311-312 1. After you tested all the known powders with all the test liquids, describe what you did to identify the unknown powder. Students should have
SNEAK PEAK inside ACTIVITY. ADVANCE PREPARATION see next page for more details Dilute alcohol with water Set out plastic cups, etc.
 Reaction: Yes or No? Learning Objectives: Students will list and identify three characteristics of chemical reactions. GRADE LEVEL 3 8 SCIENCE TOPICS Solutions and Mixtures Chemical Reactions PROCESS SKILLS
Reaction: Yes or No? Learning Objectives: Students will list and identify three characteristics of chemical reactions. GRADE LEVEL 3 8 SCIENCE TOPICS Solutions and Mixtures Chemical Reactions PROCESS SKILLS
SNEAK PEAK inside ACTIVITY. ADVANCE PREPARATION see next page for more details Cut up steel wool Mix Kool-Aid solutions OPTIONAL EXTRAS
 Kool Colors Learning Objectives: Students learn how different factors affect the rate of chemical reactions. GRADE LEVEL 2 8 SCIENCE TOPICS Solutions and Mixtures Techniques Chemical Reactions PROCESS
Kool Colors Learning Objectives: Students learn how different factors affect the rate of chemical reactions. GRADE LEVEL 2 8 SCIENCE TOPICS Solutions and Mixtures Techniques Chemical Reactions PROCESS
Physical and Chemical Changes
 Physical and Chemical Changes Jana Barrow West Point Jr. High 2775 W 550 N 801-402-8100 West Point, UT 84015 jbarrow@dsdmail.net Eighth Grade Integrated Science Standard I: Students will understand the
Physical and Chemical Changes Jana Barrow West Point Jr. High 2775 W 550 N 801-402-8100 West Point, UT 84015 jbarrow@dsdmail.net Eighth Grade Integrated Science Standard I: Students will understand the
Chemistry 101. Chemistry Experiments for the Home Acidity Determination Using Indicators
 Chemistry 101 Chemistry Experiments for the Home Acidity Determination Using Indicators I. Objective: To determine the acidity of a variety of common substances by the use of indicators. To prepare your
Chemistry 101 Chemistry Experiments for the Home Acidity Determination Using Indicators I. Objective: To determine the acidity of a variety of common substances by the use of indicators. To prepare your
Chemquest: Physical Changes or Chemical Reactions
 Chemquest: Physical Changes or Chemical Reactions Erik Misner May 9, 2005 Background: This lesson is designed to be an interactive and fun way to learn the difference between physical changes and chemical
Chemquest: Physical Changes or Chemical Reactions Erik Misner May 9, 2005 Background: This lesson is designed to be an interactive and fun way to learn the difference between physical changes and chemical
Lesson 4. Temperature change
 54 Lesson 4 Temperature change T E A C H E R G U I D E Lesson summary Students meet scientist Jason Williams, an industrial chemist who designs the materials and processes for making solar cells. He explains
54 Lesson 4 Temperature change T E A C H E R G U I D E Lesson summary Students meet scientist Jason Williams, an industrial chemist who designs the materials and processes for making solar cells. He explains
Chapter 5, Lesson 3 Why Does Water Dissolve Salt?
 Chapter 5, Lesson 3 Why Does Water Dissolve Salt? Key Concepts The polarity of water molecules enables water to dissolve many ionically bonded substances. Salt (sodium chloride) is made from positive sodium
Chapter 5, Lesson 3 Why Does Water Dissolve Salt? Key Concepts The polarity of water molecules enables water to dissolve many ionically bonded substances. Salt (sodium chloride) is made from positive sodium
Ontario Science and Technology Curriculum 1999 Strand: Matter and Materials Topic: Properties of Liquids and Solids Grade: 2
 Name: Ontario Science and Technology Curriculum 1999 Strand: Matter and Materials Topic: Properties of Liquids and Solids Grade: 2 All rights reserved Developed by T Tasker May be photocopied for classroom
Name: Ontario Science and Technology Curriculum 1999 Strand: Matter and Materials Topic: Properties of Liquids and Solids Grade: 2 All rights reserved Developed by T Tasker May be photocopied for classroom
Acids & Bases Around the House Use a ph indicator to find acids and bases
 Use a ph indicator to find acids and bases Description: Visitors predict whether various household solutions are acids or bases, and test their hypotheses using a universal ph indicator. Then, visitors
Use a ph indicator to find acids and bases Description: Visitors predict whether various household solutions are acids or bases, and test their hypotheses using a universal ph indicator. Then, visitors
Chapter 6, Lesson 4: Temperature and the Rate of a Chemical Reaction
 Chapter 6, Lesson 4: Temperature and the Rate of a Chemical Reaction Key Concepts Reactants must be moving fast enough and hit each other hard enough for a chemical reaction to take place. Increasing the
Chapter 6, Lesson 4: Temperature and the Rate of a Chemical Reaction Key Concepts Reactants must be moving fast enough and hit each other hard enough for a chemical reaction to take place. Increasing the
First Grade Unit A: PHYSICAL SCIENCE Chapter 1: Observing Solids, Liquids and Gases Lessons 1 to 5
 First Grade Unit A: PHYSICAL SCIENCE Chapter 1: Observing Solids, Liquids and Gases Lessons 1 to 5 Physical Science Overview Materials (matter) come in different forms. Water can be rain falling (liquid)
First Grade Unit A: PHYSICAL SCIENCE Chapter 1: Observing Solids, Liquids and Gases Lessons 1 to 5 Physical Science Overview Materials (matter) come in different forms. Water can be rain falling (liquid)
Name. Lab 3: ENZYMES. In this lab, you ll investigate some of the properties of enzymes.
 Name Lab 3: ENZYMES In this lab, you ll investigate some of the properties of enzymes. So what are enzymes? Enzymes are large protein molecules (macromolecules) They catalyze or speed up chemical reactions
Name Lab 3: ENZYMES In this lab, you ll investigate some of the properties of enzymes. So what are enzymes? Enzymes are large protein molecules (macromolecules) They catalyze or speed up chemical reactions
OBJECTIVES: Visitors learn what an antioxidant is and how it behaves. They also learn how to test for the presence of vitamin C..
 Vitamin C Visitors use iodine to compare the reactivity of two starch solutions one with vitamin C added, one without vitamin C. OBJECTIVES: Visitors learn what an antioxidant is and how it behaves. They
Vitamin C Visitors use iodine to compare the reactivity of two starch solutions one with vitamin C added, one without vitamin C. OBJECTIVES: Visitors learn what an antioxidant is and how it behaves. They
Properties of Acids and Bases
 Lab 22 Properties of Acids and Bases TN Standard 4.2: The student will investigate the characteristics of acids and bases. Have you ever brushed your teeth and then drank a glass of orange juice? What
Lab 22 Properties of Acids and Bases TN Standard 4.2: The student will investigate the characteristics of acids and bases. Have you ever brushed your teeth and then drank a glass of orange juice? What
Chapter 5 Student Reading
 Chapter 5 Student Reading THE POLARITY OF THE WATER MOLECULE Wonderful water Water is an amazing substance. We drink it, cook and wash with it, swim and play in it, and use it for lots of other purposes.
Chapter 5 Student Reading THE POLARITY OF THE WATER MOLECULE Wonderful water Water is an amazing substance. We drink it, cook and wash with it, swim and play in it, and use it for lots of other purposes.
Introduction. ph = log [H + ]
![Introduction. ph = log [H + ] Introduction. ph = log [H + ]](/thumbs/40/21282159.jpg) Visualizing ph 2010, 1992 by David A. Katz. All rights reserved. Permission granted for classroom use. All reproductions must include original copyright. David A. Katz Chemist, Educator, Science Communicator,
Visualizing ph 2010, 1992 by David A. Katz. All rights reserved. Permission granted for classroom use. All reproductions must include original copyright. David A. Katz Chemist, Educator, Science Communicator,
Reaction in a Bag. Scientific Method Demonstrations
 elearning 2009 Introduction Reaction in a Bag Scientific Method Demonstrations Publication No. 91419 Careful observation is the foundation of science, leading to questions about what we have observed how,
elearning 2009 Introduction Reaction in a Bag Scientific Method Demonstrations Publication No. 91419 Careful observation is the foundation of science, leading to questions about what we have observed how,
Chemical versus Physical Changes
 Chemical versus Physical Changes Permission to Copy - This document may be reproduced for non-commercial educational purposes Copyright 2009 General Electric Company What are physical and chemical changes?
Chemical versus Physical Changes Permission to Copy - This document may be reproduced for non-commercial educational purposes Copyright 2009 General Electric Company What are physical and chemical changes?
Lesson 10: Mixtures of Matter - Part 2
 Science Unit: Matter Lesson 10: Mixtures of Matter - Part 2 School year: 2004/2005 Developed for: Developed by: Grade level: Duration of lesson: Notes: Queen Alexandra Elementary School, Vancouver School
Science Unit: Matter Lesson 10: Mixtures of Matter - Part 2 School year: 2004/2005 Developed for: Developed by: Grade level: Duration of lesson: Notes: Queen Alexandra Elementary School, Vancouver School
WHAT S NEW, CO? Thanks for the opportunity to work with your students. Our goal is to teach developmentally TEACHER S GUIDE
 TEACHER S GUIDE WHAT S NEW, CO? GET TO KNOW A CHEMICAL REACTION 2 Thanks for the opportunity to work with your students. Our goal is to teach developmentally appropriate chemistry concepts that support
TEACHER S GUIDE WHAT S NEW, CO? GET TO KNOW A CHEMICAL REACTION 2 Thanks for the opportunity to work with your students. Our goal is to teach developmentally appropriate chemistry concepts that support
Energetic Reactions: Ice Cream Experiment Teacher Guide
 Module Overview Heat transfer is an important part of many chemical reactions, but it is often not directly observed. In this module students conduct an experiment making homemade ice cream that requires
Module Overview Heat transfer is an important part of many chemical reactions, but it is often not directly observed. In this module students conduct an experiment making homemade ice cream that requires
Leavener Lineup. Getting started. How do we use chemical reactions in the kitchen? Hands-on experiment. Year levels 4 5. Curriculum Links.
 rise and Shine: what Makes Bread Rise? Lesson 2 Leavener Lineup Year levels 4 5 Curriculum Links Science Science knowledge helps people to understand the effect of their actions (Yr 4, ACSHE062). Solids,
rise and Shine: what Makes Bread Rise? Lesson 2 Leavener Lineup Year levels 4 5 Curriculum Links Science Science knowledge helps people to understand the effect of their actions (Yr 4, ACSHE062). Solids,
Return to Lab Menu. Acids and Bases in Your House
 Return to Lab Menu Acids and Bases in Your House OBJECTIVES Isolate a natural acid-base indicator. Determine the acid-base properties of common household solutions. INTRODUCTION Acids and bases are among
Return to Lab Menu Acids and Bases in Your House OBJECTIVES Isolate a natural acid-base indicator. Determine the acid-base properties of common household solutions. INTRODUCTION Acids and bases are among
Dry Ice Color Show Dry Ice Demonstrations
 elearning 2009 Introduction Dry Ice Color Show Dry Ice Demonstrations Publication No. 95016 Add a small piece of solid carbon dioxide to a colored indicator solution and watch as the solution immediately
elearning 2009 Introduction Dry Ice Color Show Dry Ice Demonstrations Publication No. 95016 Add a small piece of solid carbon dioxide to a colored indicator solution and watch as the solution immediately
Acids, Bases, and ph
 CHAPTER 9 1 SECTION Acids, Bases, and Salts Acids, Bases, and ph KEY IDEAS As you read this section, keep these questions in mind: What properties do acids have? What properties do bases have? How can
CHAPTER 9 1 SECTION Acids, Bases, and Salts Acids, Bases, and ph KEY IDEAS As you read this section, keep these questions in mind: What properties do acids have? What properties do bases have? How can
Lesson Plan: How Do We Know What is Healthy Water?
 Lesson Plan: How Do We Know What is Healthy Water? Estimated Time: 1-3 days ph /Chlorine / Hardness State Standards taught and addressed Grade 8: Standards Taught (and evaluated at end of lesson) Science
Lesson Plan: How Do We Know What is Healthy Water? Estimated Time: 1-3 days ph /Chlorine / Hardness State Standards taught and addressed Grade 8: Standards Taught (and evaluated at end of lesson) Science
CHM 130LL: ph, Buffers, and Indicators
 CHM 130LL: ph, Buffers, and Indicators Many substances can be classified as acidic or basic. Acidic substances contain hydrogen ions, H +, while basic substances contain hydroxide ions, OH. The relative
CHM 130LL: ph, Buffers, and Indicators Many substances can be classified as acidic or basic. Acidic substances contain hydrogen ions, H +, while basic substances contain hydroxide ions, OH. The relative
Properties of Acids and Bases
 Properties of Acids and Bases (Adapted from Flinn Scientific Acid Base Test Kit I #AP4567) Introduction Battery acid, stomach acid, acid rain just a few acids in our everyday life! What does it mean when
Properties of Acids and Bases (Adapted from Flinn Scientific Acid Base Test Kit I #AP4567) Introduction Battery acid, stomach acid, acid rain just a few acids in our everyday life! What does it mean when
ANSWER KEY. Acids, Bases, and Solutions. Chapter Project Worksheet 1 1. Answers will vary. Sample: cherries, blueberries,
 Chapter Project Worksheet 1 1. Answers will vary. Sample: cherries, blueberries, and grass 2. Answers will vary. Sample: Cut 5 g of cherries into small pieces and place in blender. Blend for two minutes,
Chapter Project Worksheet 1 1. Answers will vary. Sample: cherries, blueberries, and grass 2. Answers will vary. Sample: Cut 5 g of cherries into small pieces and place in blender. Blend for two minutes,
Unit 1 - Pure Substances and Mixtures Chapter 2: Solutions
 2.1 Solutes & Solvents Vocabulary: Unit 1 - Pure Substances and Mixtures Chapter 2: Solutions solvent the larger part of a solution - the part of a solution into which the solutes dissolve solute the smaller
2.1 Solutes & Solvents Vocabulary: Unit 1 - Pure Substances and Mixtures Chapter 2: Solutions solvent the larger part of a solution - the part of a solution into which the solutes dissolve solute the smaller
Solids, Liquids, and Gases
 Solids, Liquids, and Gases nd Intended for Grade: 2 Grade Subject: Science Description: Activities to help students understand solids, liquids, gases, and the changes between these states. Objective: The
Solids, Liquids, and Gases nd Intended for Grade: 2 Grade Subject: Science Description: Activities to help students understand solids, liquids, gases, and the changes between these states. Objective: The
VANDERBILT STUDENT VOLUNTEERS FOR SCIENCE. Acids and Bases. Fall 2012
 VANDERBILT STUDENT VOLUNTEERS FOR SCIENCE Acids and Bases Fall 2012 GOAL: To introduce students to acids and bases. MATERIALS 3 10oz clear plastic cups 1 4 oz. bottle white vinegar - labeled Acid 1 4 oz.
VANDERBILT STUDENT VOLUNTEERS FOR SCIENCE Acids and Bases Fall 2012 GOAL: To introduce students to acids and bases. MATERIALS 3 10oz clear plastic cups 1 4 oz. bottle white vinegar - labeled Acid 1 4 oz.
PHYSICAL AND CHEMICAL PROPERTIES AND CHANGES
 PHYSICAL AND CHEMICAL PROPERTIES AND CHANGES Name Key PHYSICAL PROPERTY CHEMICAL PROPERTY 1. observed with senses 1. indicates how a substance 2. determined without destroying matter reacts with something
PHYSICAL AND CHEMICAL PROPERTIES AND CHANGES Name Key PHYSICAL PROPERTY CHEMICAL PROPERTY 1. observed with senses 1. indicates how a substance 2. determined without destroying matter reacts with something
Household Acids and Bases
 Household Acids and Bases GRADE LEVEL INDICATORS Experiment Demonstrate that the ph scale (0-14) is used to measure acidity and classify substances or solutions as acidic, basic, or neutral. 21 Develop
Household Acids and Bases GRADE LEVEL INDICATORS Experiment Demonstrate that the ph scale (0-14) is used to measure acidity and classify substances or solutions as acidic, basic, or neutral. 21 Develop
Luminol Test PROCESS SKILLS SCIENCE TOPICS VOCABULARY
 EXPERIMENT: LUMINOL TEST Luminol Test Visitors mix a solution of luminol with fake blood (hydrogen peroxide) to produce a reaction that gives off blue light. OBJECTIVES: Visitors learn that some chemical
EXPERIMENT: LUMINOL TEST Luminol Test Visitors mix a solution of luminol with fake blood (hydrogen peroxide) to produce a reaction that gives off blue light. OBJECTIVES: Visitors learn that some chemical
Chem 100 Lab Experiment #9 - ACID/BASE INDICATORS
 Lab #9 Chem 100 Lab Experiment #9 - ACID/BASE INDICATORS Name: Purpose: In this laboratory we will investigate how indicators can be used to test for the presence of acids or bases in a number of common
Lab #9 Chem 100 Lab Experiment #9 - ACID/BASE INDICATORS Name: Purpose: In this laboratory we will investigate how indicators can be used to test for the presence of acids or bases in a number of common
CHEMICAL DETERMINATION OF EVERYDAY HOUSEHOLD CHEMICALS
 CHEMICAL DETERMINATION OF EVERYDAY HOUSEHOLD CHEMICALS Purpose: It is important for chemists to be able to determine the composition of unknown chemicals. This can often be done by way of chemical tests.
CHEMICAL DETERMINATION OF EVERYDAY HOUSEHOLD CHEMICALS Purpose: It is important for chemists to be able to determine the composition of unknown chemicals. This can often be done by way of chemical tests.
Hands-On Labs SM-1 Lab Manual
 EXPERIMENT 4: Separation of a Mixture of Solids Read the entire experiment and organize time, materials, and work space before beginning. Remember to review the safety sections and wear goggles when appropriate.
EXPERIMENT 4: Separation of a Mixture of Solids Read the entire experiment and organize time, materials, and work space before beginning. Remember to review the safety sections and wear goggles when appropriate.
Chemical Changes. Measuring a Chemical Reaction. Name(s)
 Chemical Changes Name(s) In the particle model of matter, individual atoms can be bound tightly to other atoms to form molecules. For example, water molecules are made up of two hydrogen atoms bound to
Chemical Changes Name(s) In the particle model of matter, individual atoms can be bound tightly to other atoms to form molecules. For example, water molecules are made up of two hydrogen atoms bound to
O o. Thomas Jefferson National Accelerator Facility - Office of Science Education http://education.jlab.org/
 O o b l ekk c What is Oobleck? Can you use THE SCIENTIFIC METHOD AND your senses to solve the mystery of Oobleck? Problem Three liquids are mixed together in a plastic bag. Using your senses (except for
O o b l ekk c What is Oobleck? Can you use THE SCIENTIFIC METHOD AND your senses to solve the mystery of Oobleck? Problem Three liquids are mixed together in a plastic bag. Using your senses (except for
Who took Jerell s ipod? -- An organic compound mystery 1
 Who took Jerell s ipod? -- An organic compound mystery 1 Jerell is a 10 th grade student who works at McDonald s on the weekends. While on break, Jerell was studying for his biology test and listening
Who took Jerell s ipod? -- An organic compound mystery 1 Jerell is a 10 th grade student who works at McDonald s on the weekends. While on break, Jerell was studying for his biology test and listening
The Amazing Elephant Toothpaste! Lesson Overview
 The Amazing Elephant Toothpaste! Lesson Overview Students will investigate chemical change. Suggested Grade Levels: 3-8 Standards for Lesson Content Standard A: Science as Inquiry Content Standard B: Physical
The Amazing Elephant Toothpaste! Lesson Overview Students will investigate chemical change. Suggested Grade Levels: 3-8 Standards for Lesson Content Standard A: Science as Inquiry Content Standard B: Physical
Lesson Plan 16 Cool Chemistry - DIY ph Indicator
 Lesson Plan 16 Cool Chemistry - DIY ph Indicator Brief description Red cabbage juice is a natural ph indicator making this classic activity one of the most popular chemistry experiments for children. The
Lesson Plan 16 Cool Chemistry - DIY ph Indicator Brief description Red cabbage juice is a natural ph indicator making this classic activity one of the most popular chemistry experiments for children. The
FIRST GRADE CHEMISTRY
 FIRST GRADE CHEMISTRY 1 WEEK LESSON PLANS AND ACTIVITIES ROCK CYCLE OVERVIEW OF FIRST GRADE CHEMISTRY WEEK 1. PRE: Comparing solids, gases, liquids, and plasma. LAB: Exploring how states of matter can
FIRST GRADE CHEMISTRY 1 WEEK LESSON PLANS AND ACTIVITIES ROCK CYCLE OVERVIEW OF FIRST GRADE CHEMISTRY WEEK 1. PRE: Comparing solids, gases, liquids, and plasma. LAB: Exploring how states of matter can
Teachers Notes BATH BOMB FACTORY
 Teachers Notes BATH BOMB FACTORY BATH BOMB FACTORY 2 BATH BOMB FACTORY INTRODUCTION BATH BOMB FACTORY is a fun activity that promotes scientific thinking. It allows children to explore materials and find
Teachers Notes BATH BOMB FACTORY BATH BOMB FACTORY 2 BATH BOMB FACTORY INTRODUCTION BATH BOMB FACTORY is a fun activity that promotes scientific thinking. It allows children to explore materials and find
Acids & Bases: Using Purple Cabbage as a ph indicator. Grade 9 Activity Plan
 Acids & Bases: Using Purple Cabbage as a ph indicator Grade 9 Activity Plan 1 Acids, Bases & Purple Cabbage Objectives: 1. To demonstrate the basic physical and chemical properties of acids and bases.
Acids & Bases: Using Purple Cabbage as a ph indicator Grade 9 Activity Plan 1 Acids, Bases & Purple Cabbage Objectives: 1. To demonstrate the basic physical and chemical properties of acids and bases.
Ice Cream Lab- A Tasty Phase Change!
 Ice Cream Lab- A Tasty! Name Date EN Class Purpose: To investigate the effects of heat transfer on phase changes. To investigate the effects of temperature changes on physical changes. Materials: ½ cup
Ice Cream Lab- A Tasty! Name Date EN Class Purpose: To investigate the effects of heat transfer on phase changes. To investigate the effects of temperature changes on physical changes. Materials: ½ cup
5s Solubility & Conductivity
 5s Solubility & Conductivity OBJECTIVES To explore the relationship between the structures of common household substances and the kinds of solvents in which they dissolve. To demonstrate the ionic nature
5s Solubility & Conductivity OBJECTIVES To explore the relationship between the structures of common household substances and the kinds of solvents in which they dissolve. To demonstrate the ionic nature
sciencemuseumoutreach Kitchen Science 1 Demonstrations to do at home
 sciencemuseumoutreach Kitchen Science 1 Demonstrations to do at home The Creative Canal Project (CCP) is part of the Science Museum s Outreach Department, which works with teachers, students, families
sciencemuseumoutreach Kitchen Science 1 Demonstrations to do at home The Creative Canal Project (CCP) is part of the Science Museum s Outreach Department, which works with teachers, students, families
Ice Cream Lab & Application Questions
 Deep Freeze 1 Ice Cream Lab & Application Questions Name: Period: Date: Overview Have you ever wondered what it is about throwing salt on ice that makes it melt? And just why does it melt? Where does the
Deep Freeze 1 Ice Cream Lab & Application Questions Name: Period: Date: Overview Have you ever wondered what it is about throwing salt on ice that makes it melt? And just why does it melt? Where does the
KITCHEN CHEMISTRY Chemical reaction with vinegar and baking soda
 KITCHEN CHEMISTRY Chemical reaction with vinegar and baking soda By Darby Sloss and Marianne Smith Edited by Anne Starace Abstract Chemistry is an important part of our lives. Kitchen Chemistry uses some
KITCHEN CHEMISTRY Chemical reaction with vinegar and baking soda By Darby Sloss and Marianne Smith Edited by Anne Starace Abstract Chemistry is an important part of our lives. Kitchen Chemistry uses some
Chemical Formulas, Equations, and Reactions Test Pre-AP Write all answers on your answer document.
 Name: Period: Chemical Formulas, Equations, and Reactions Test Pre-AP Write all answers on your answer document. 1. Which of the following is a NOT a physical property of hydrogen? A. It is gas C. It is
Name: Period: Chemical Formulas, Equations, and Reactions Test Pre-AP Write all answers on your answer document. 1. Which of the following is a NOT a physical property of hydrogen? A. It is gas C. It is
SECOND GRADE 1 WEEK LESSON PLANS AND ACTIVITIES
 SECOND GRADE 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF SECOND GRADE WATER WEEK 1. PRE: Exploring the properties of water. LAB: Experimenting with different soap mixtures. POST: Analyzing
SECOND GRADE 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF SECOND GRADE WATER WEEK 1. PRE: Exploring the properties of water. LAB: Experimenting with different soap mixtures. POST: Analyzing
Enzyme Pre-Lab. Using the Enzyme worksheet and Enzyme lab handout answer the Pre-Lab questions the pre-lab must be complete before beginning the lab.
 Enzyme Pre-Lab Using the Enzyme worksheet and Enzyme lab handout answer the Pre-Lab questions the pre-lab must be complete before beginning the lab. Background: In this investigation, you will study several
Enzyme Pre-Lab Using the Enzyme worksheet and Enzyme lab handout answer the Pre-Lab questions the pre-lab must be complete before beginning the lab. Background: In this investigation, you will study several
Solutions and Suspensions
 Science Unit: Lesson 11: Matter Solutions and Suspensions School year: 2005/2006 Developed for: Developed by: Grade level: Duration of Lesson McBride Elementary School, Vancouver School District Catriona
Science Unit: Lesson 11: Matter Solutions and Suspensions School year: 2005/2006 Developed for: Developed by: Grade level: Duration of Lesson McBride Elementary School, Vancouver School District Catriona
Water Cycle. DELTA SCIENCE READER Overview... 123 Before Reading... 124 Guide the Reading... 125 After Reading... 130
 Water Cycle T ABLE OF CONTENTS ABOUT DELTA SCIENCE MODULES Program Introduction................... iii Teacher s Guide..................... iv Delta Science Readers............... vi Equipment and Materials
Water Cycle T ABLE OF CONTENTS ABOUT DELTA SCIENCE MODULES Program Introduction................... iii Teacher s Guide..................... iv Delta Science Readers............... vi Equipment and Materials
Chapter 2, Lesson 5: Changing State Melting
 Chapter 2, Lesson 5: Changing State Melting Key Concepts Melting is a process that causes a substance to change from a solid to a liquid. Melting occurs when the molecules of a solid speed up enough that
Chapter 2, Lesson 5: Changing State Melting Key Concepts Melting is a process that causes a substance to change from a solid to a liquid. Melting occurs when the molecules of a solid speed up enough that
Objectives: Vocabulary: Materials: Students will: Safety: Element Mineral Streak. Absolute Hardness
 : Finding Clues Like a Scientist Author: Kris Poduska Date Created: 2000 Subject: Earth Science, Classification Level: Elementary and Middle School Standards: New York State- Intermediate Science (www.emsc.nysed.gov/ciai/)
: Finding Clues Like a Scientist Author: Kris Poduska Date Created: 2000 Subject: Earth Science, Classification Level: Elementary and Middle School Standards: New York State- Intermediate Science (www.emsc.nysed.gov/ciai/)
Chapter 4, Lesson 5: Energy Levels, Electrons, and Ionic Bonding
 Chapter 4, Lesson 5: Energy Levels, Electrons, and Ionic Bonding Key Concepts The attractions between the protons and electrons of atoms can cause an electron to move completely from one atom to the other.
Chapter 4, Lesson 5: Energy Levels, Electrons, and Ionic Bonding Key Concepts The attractions between the protons and electrons of atoms can cause an electron to move completely from one atom to the other.
Freezing Point Depression: Why Don t Oceans Freeze? Teacher Advanced Version
 Freezing Point Depression: Why Don t Oceans Freeze? Teacher Advanced Version Freezing point depression describes the process where the temperature at which a liquid freezes is lowered by adding another
Freezing Point Depression: Why Don t Oceans Freeze? Teacher Advanced Version Freezing point depression describes the process where the temperature at which a liquid freezes is lowered by adding another
Household Acids and Bases
 Household Acids and Bases Computer 28 Many common household solutions contain acids and bases. Acid-base indicators, such as litmus and red cabbage juice, turn different colors in acidic and basic solutions.
Household Acids and Bases Computer 28 Many common household solutions contain acids and bases. Acid-base indicators, such as litmus and red cabbage juice, turn different colors in acidic and basic solutions.
Neutralizing an Acid and a Base
 Balancing Act Teacher Information Objectives In this activity, students neutralize a base with an acid. Students determine the point of neutralization of an acid mixed with a base while they: Recognize
Balancing Act Teacher Information Objectives In this activity, students neutralize a base with an acid. Students determine the point of neutralization of an acid mixed with a base while they: Recognize
TEACHER ACTIVITY GUIDE
 Page 1/5 EXPECTED OUTCOMES TEACHER ACTIVITY GUIDE ROOT BEER PRODUCTION Taken from IFT Experiments in Food Science Series This activity will allow student an opportunity to explore yeast fermentation by
Page 1/5 EXPECTED OUTCOMES TEACHER ACTIVITY GUIDE ROOT BEER PRODUCTION Taken from IFT Experiments in Food Science Series This activity will allow student an opportunity to explore yeast fermentation by
Letter to the Student... 5 Test-Taking Checklist... 6 Next Generation Sunshine State Standards Correlation Chart... 7
 Table of Contents Letter to the Student..................................... 5 Test-Taking Checklist.................................... 6 Next Generation Sunshine State Standards Correlation Chart...
Table of Contents Letter to the Student..................................... 5 Test-Taking Checklist.................................... 6 Next Generation Sunshine State Standards Correlation Chart...
Questions for Discussion. Introduction. What is ph? Neutralization
 Questions for Discussion Why do leaves change color in autumn? What are acids and bases? How does red cabbage fit in? How are all these things related? Introduction The questions above may not seem related,
Questions for Discussion Why do leaves change color in autumn? What are acids and bases? How does red cabbage fit in? How are all these things related? Introduction The questions above may not seem related,
A PRIMER ON ph. A Presentation for ASTA Conference September 30-October 1, 2008
 A PRIMER ON ph A Presentation for ASTA Conference September 30-October 1, 2008 Rebecca Richardson Martha Gothard Director Science Specialist Regional In-Service Education Center AMSTI University of Montevallo
A PRIMER ON ph A Presentation for ASTA Conference September 30-October 1, 2008 Rebecca Richardson Martha Gothard Director Science Specialist Regional In-Service Education Center AMSTI University of Montevallo
Kool Demo for Acid-Base Reactions
 Kool Demo for Acid-Base Reactions Kool Demo for Acid-Base Reactions Adapted from : http://www.stevespanglerscience.com/lab/experiments/color-changing-milk-of-magnesia Materials: Red cabbage juice indicator
Kool Demo for Acid-Base Reactions Kool Demo for Acid-Base Reactions Adapted from : http://www.stevespanglerscience.com/lab/experiments/color-changing-milk-of-magnesia Materials: Red cabbage juice indicator
Bay Area Scientists in Schools Presentation Plan
 Bay Area Scientists in Schools Presentation Plan Lesson Name Presenter(s) Grade Level 5th The Chemical Workout/Blow it Up Chemistry Graduate Students from the Maimone Group at UC Berkeley Standards Connection(s):
Bay Area Scientists in Schools Presentation Plan Lesson Name Presenter(s) Grade Level 5th The Chemical Workout/Blow it Up Chemistry Graduate Students from the Maimone Group at UC Berkeley Standards Connection(s):
The Acid Test Grade Nine
 Ohio Standards Connection: Physical Sciences Benchmark B Explain how atoms react with each other to form other substances and how molecules react with each other or other atoms to form even different substances.
Ohio Standards Connection: Physical Sciences Benchmark B Explain how atoms react with each other to form other substances and how molecules react with each other or other atoms to form even different substances.
Can Gases Act Like a Greenhouse?
 Can Gases Act Like a Greenhouse? Activity 1 Following a discussion that enables student to express what they already know about the greenhouse effect, students conduct a controlled experiment to confirm
Can Gases Act Like a Greenhouse? Activity 1 Following a discussion that enables student to express what they already know about the greenhouse effect, students conduct a controlled experiment to confirm
Sugar or Salt? Ionic and Covalent Bonds
 Lab 11 Sugar or Salt? Ionic and Covalent Bonds TN Standard 2.1: The student will investigate chemical bonding. Have you ever accidentally used salt instead of sugar? D rinking tea that has been sweetened
Lab 11 Sugar or Salt? Ionic and Covalent Bonds TN Standard 2.1: The student will investigate chemical bonding. Have you ever accidentally used salt instead of sugar? D rinking tea that has been sweetened
WHAT IS THE SCIENTIFIC METHOD?
 WHAT IS THE SCIENTIFIC METHOD? A lesson to introduce the application of the Scientific Method to High School Chemistry Students Karen Balbierer CCMR RET I August 15, 2003 Lesson Plan Summary Lesson Subject:
WHAT IS THE SCIENTIFIC METHOD? A lesson to introduce the application of the Scientific Method to High School Chemistry Students Karen Balbierer CCMR RET I August 15, 2003 Lesson Plan Summary Lesson Subject:
How do you digest milk? In this experiment you will test the ability of two substances, an acid and enzyme, to break down protein.
 3.3 (page 1) Science Projects For ALL Students Digestion How do you digest milk? In this experiment you will test the ability of two substances, an acid and enzyme, to break down protein. Digestion is
3.3 (page 1) Science Projects For ALL Students Digestion How do you digest milk? In this experiment you will test the ability of two substances, an acid and enzyme, to break down protein. Digestion is
Physical and Chemical Changes Pre Test Questions
 Pre Test Questions Name: Period: Date: 1. Which of the following is an example of physical change? a. Mixing baking soda and vinegar together, and this causes bubbles and foam. b. A glass cup falls from
Pre Test Questions Name: Period: Date: 1. Which of the following is an example of physical change? a. Mixing baking soda and vinegar together, and this causes bubbles and foam. b. A glass cup falls from
ph Measurements of Common Substances
 Chem 100 Section Experiment 10 Name Partner s Name Introduction ph Measurements of Common Substances The concentration of an acid or base is frequently expressed as ph. Historically, ph stands for the
Chem 100 Section Experiment 10 Name Partner s Name Introduction ph Measurements of Common Substances The concentration of an acid or base is frequently expressed as ph. Historically, ph stands for the
The Digestive System Grade 5
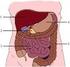 TEACHING LEARNING COLLABORATIVE (TLC) LIFE SCIENCE The Digestive System Grade 5 Created by: Shelly Bell (Kelseyville Elementary School), Bart Pontoni (Riviera Elementary School), Shane Lee (Pomo Elementary
TEACHING LEARNING COLLABORATIVE (TLC) LIFE SCIENCE The Digestive System Grade 5 Created by: Shelly Bell (Kelseyville Elementary School), Bart Pontoni (Riviera Elementary School), Shane Lee (Pomo Elementary
Activity: How Do We Clean Up an Oil Spill?
 Activity: How Do We Clean Up an Oil Spill? Summary In this activity, students simulate an oil spill and test different materials abilities to clean the oil spill. Resource Type Activity Grade Level High
Activity: How Do We Clean Up an Oil Spill? Summary In this activity, students simulate an oil spill and test different materials abilities to clean the oil spill. Resource Type Activity Grade Level High
ACIDS AND BASES SAFETY PRECAUTIONS
 ACIDS AND BASES Mild acids and bases are used in cooking (their reaction makes biscuits and bread rise). Acids such as those in our stomachs eat away at food or digest it. Strong acids and bases are used
ACIDS AND BASES Mild acids and bases are used in cooking (their reaction makes biscuits and bread rise). Acids such as those in our stomachs eat away at food or digest it. Strong acids and bases are used
The Properties of Water (Instruction Sheet)
 The Properties of Water (Instruction Sheet) Property : High Polarity Activity #1 Surface Tension: PILE IT ON. Materials: 1 DRY penny, 1 eye dropper, water. 1. Make sure the penny is dry. 2. Begin by estimating
The Properties of Water (Instruction Sheet) Property : High Polarity Activity #1 Surface Tension: PILE IT ON. Materials: 1 DRY penny, 1 eye dropper, water. 1. Make sure the penny is dry. 2. Begin by estimating
THE ACTIVITY OF LACTASE
 THE ACTIVITY OF LACTASE Lab VIS-8 From Juniata College Science in Motion Enzymes are protein molecules which act to catalyze the chemical reactions in living things. These chemical reactions make up the
THE ACTIVITY OF LACTASE Lab VIS-8 From Juniata College Science in Motion Enzymes are protein molecules which act to catalyze the chemical reactions in living things. These chemical reactions make up the
EXAMPLE EXERCISE 4.1 Change of Physical State
 EXAMPLE EXERCISE 4.1 Change of Physical State State the term that applies to each of the following changes of physical state: (a) Snow changes from a solid to a liquid. (b) Gasoline changes from a liquid
EXAMPLE EXERCISE 4.1 Change of Physical State State the term that applies to each of the following changes of physical state: (a) Snow changes from a solid to a liquid. (b) Gasoline changes from a liquid
Teacher Demo: Turning Water into Wine into Milk into Beer
 SNC2D/2P Chemical Reactions/Chemical Reactions and their Practical Applications Teacher Demo: Turning Water into Wine into Milk into Beer Topics evidence of chemical change types of chemical reactions
SNC2D/2P Chemical Reactions/Chemical Reactions and their Practical Applications Teacher Demo: Turning Water into Wine into Milk into Beer Topics evidence of chemical change types of chemical reactions
Experiment 16-Acids, Bases and ph
 Definitions acid-an ionic compound that releases or reacts with water to form hydrogen ion (H + ) in aqueous solution. They taste sour and turn litmus red. Acids react with certain metals such as zinc,
Definitions acid-an ionic compound that releases or reacts with water to form hydrogen ion (H + ) in aqueous solution. They taste sour and turn litmus red. Acids react with certain metals such as zinc,
ANSWER KEY. Energy Levels, Electrons and IONIC Bonding It s all about the Give and Take!
 ANSWER KEY Energy Levels, Electrons and IONIC Bonding It s all about the Give and Take! From American Chemical Society Middle School Chemistry Unit: Chapter 4 Content Statements: Distinguish the difference
ANSWER KEY Energy Levels, Electrons and IONIC Bonding It s all about the Give and Take! From American Chemical Society Middle School Chemistry Unit: Chapter 4 Content Statements: Distinguish the difference
Chapter 3: Separating Mixtures (pg. 54 81)
 Chapter 3: Separating Mixtures (pg. 54 81) 3.2: Separating Mechanical Mixtures (PB Pg. 40 5 & TB Pg. 58 61): Name: Date: Check Your Understanding & Learning (PB pg. 40 & TB pg. 61): 1. What are four methods
Chapter 3: Separating Mixtures (pg. 54 81) 3.2: Separating Mechanical Mixtures (PB Pg. 40 5 & TB Pg. 58 61): Name: Date: Check Your Understanding & Learning (PB pg. 40 & TB pg. 61): 1. What are four methods
EXPERIMENT 20: Determination of ph of Common Substances
 Materials: ph paper and color chart (ph range 3 to 12) or ph meter distilled water white vinegar household ammonia (or baking soda) spot plate test or 3 small test tubes stirring rod solutions / fruits
Materials: ph paper and color chart (ph range 3 to 12) or ph meter distilled water white vinegar household ammonia (or baking soda) spot plate test or 3 small test tubes stirring rod solutions / fruits
The Digestive System: Where does food go? Teacher Version
 The Digestive System: Where does food go? Teacher Version In this lab you will learn about your digestive system. We will use everyday objects like yarn and a ziplock bag to understand how long our digestive
The Digestive System: Where does food go? Teacher Version In this lab you will learn about your digestive system. We will use everyday objects like yarn and a ziplock bag to understand how long our digestive
Return to Lab Menu. Stoichiometry Exploring the Reaction between Baking Soda and Vinegar
 Return to Lab Menu Stoichiometry Exploring the Reaction between Baking Soda and Vinegar Objectives -to observe and measure mass loss in a gas forming reaction -to calculate CO 2 loss and correlate to a
Return to Lab Menu Stoichiometry Exploring the Reaction between Baking Soda and Vinegar Objectives -to observe and measure mass loss in a gas forming reaction -to calculate CO 2 loss and correlate to a
ROCKS, FOSSILS AND SOILS SECTION 8: FOSSILS From Hands on Science by Linda Poore, 2003
 ROCKS, FOSSILS AND SOILS SECTION 8: FOSSILS From Hands on Science by Linda Poore, 2003 STANDARDS: Westminster College Students will write or draw descriptions of a sequence of steps, events and observations,
ROCKS, FOSSILS AND SOILS SECTION 8: FOSSILS From Hands on Science by Linda Poore, 2003 STANDARDS: Westminster College Students will write or draw descriptions of a sequence of steps, events and observations,
ANALYSIS OF VITAMIN C
 Purpose To learn how to analyze food for vitamin C content and to examine various sources for vitamin C content. Caution Handle the glassware with caution to prevent breakage. When using a burner in the
Purpose To learn how to analyze food for vitamin C content and to examine various sources for vitamin C content. Caution Handle the glassware with caution to prevent breakage. When using a burner in the
Lesson Plan for Lava Lamps
 Lesson Plan for Lava Lamps Written by Liz Roth-Johnson and Perry Roth-Johnson Introduction & Background Information Although we cannot see them with our eyes, all things are made up of molecules. Different
Lesson Plan for Lava Lamps Written by Liz Roth-Johnson and Perry Roth-Johnson Introduction & Background Information Although we cannot see them with our eyes, all things are made up of molecules. Different
Physical and Chemical Properties and Changes
 Physical and Chemical Properties and Changes An understanding of material things requires an understanding of the physical and chemical characteristics of matter. A few planned experiments can help you
Physical and Chemical Properties and Changes An understanding of material things requires an understanding of the physical and chemical characteristics of matter. A few planned experiments can help you
The Structure of Water Introductory Lesson
 Dana V. Middlemiss Fall 2002 The Structure of Water Introductory Lesson Abstract: This is an introduction to the chemical nature of water and its interactions. In particular, this lesson will explore evaporation,
Dana V. Middlemiss Fall 2002 The Structure of Water Introductory Lesson Abstract: This is an introduction to the chemical nature of water and its interactions. In particular, this lesson will explore evaporation,
KINDERGARTEN WATER 1 WEEK LESSON PLANS AND ACTIVITIES
 KINDERGARTEN WATER 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF KINDERGARTEN WEEK 1. PRE: Defining the states of matter. LAB: Discovering the properties of water. POST: Analyzing the water
KINDERGARTEN WATER 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF KINDERGARTEN WEEK 1. PRE: Defining the states of matter. LAB: Discovering the properties of water. POST: Analyzing the water
Mixtures. reflect. How is seawater different from pure water? How is it different from rocky soil?
 reflect Everything around us is made out of tiny bits of matter. These particles may combine in different ways to produce new materials. Sometimes we need to separate the parts of a material. If we know
reflect Everything around us is made out of tiny bits of matter. These particles may combine in different ways to produce new materials. Sometimes we need to separate the parts of a material. If we know
CHAPTER 3: MATTER. Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64
 CHAPTER 3: MATTER Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64 3.1 MATTER Matter: Anything that has mass and occupies volume We study
CHAPTER 3: MATTER Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64 3.1 MATTER Matter: Anything that has mass and occupies volume We study
OPEN WIDE! Fun Science Activities Inside!
 OPEN WIDE! Fun Science Activities Inside! THINK BEFORE YOU DRINK Overview: In this experiment, kids may be surprised to learn how much sugar is in popular drinks and how this hidden sugar can damage teeth!
OPEN WIDE! Fun Science Activities Inside! THINK BEFORE YOU DRINK Overview: In this experiment, kids may be surprised to learn how much sugar is in popular drinks and how this hidden sugar can damage teeth!
