Newborn Screening. for Journalists, Policymakers, and Campaigns
|
|
|
- Lynette Caldwell
- 7 years ago
- Views:
Transcription
1 f r o m b i r t h to death a n d b e n c h to clinic THE HASTINGS CENTER Bioethics Briefing Book for Journalists, Policymakers, and Campaigns Chapter 27 Newborn Screening Mary Ann Baily, Newborn Screening, in From Birth to Death and Bench to Clinic: The Hastings Center Bioethics Briefing Book for Journalists, Policymakers, and Campaigns, ed. Mary Crowley (Garrison, NY: The Hastings Center, 2008), , The Hastings Center All rights reserved. No part of this book may be reproduced, stored in a retrieval system, or transmitted in any from or by any means (electronic, mechanical, photocopying, recording, or otherwise), without the prior written permission from the publisher, except for the inclusion of brief quotations in a review. We have attempted to provide complete and accurate information regarding the individuals, organizations, and agencies described in this book. However, we assume no liability for errors, omissions, or damages arising from the use of this Briefing Book.
2 newborn screening by Mary Ann Baily Framing the Issue State newborn screening programs test nearly all infants born in the United States for selected inherited and congenital conditions that may cause disability or death. Screening is mandatory in all but a few states. In addition to screening, the programs provide education, follow-up to definitive diagnosis, and long-term treatment and management, or link affected infants and parents to these services. The programs began in the 1960s after a simple blood test was developed for a genetic metabolic disorder called phenylketonuria (PKU). If infants with PKU are identified soon after birth and immediately put on a special diet, they can be protected from permanent mental retardation and other health problems. States began screening newborns for PKU and, over time, added other disorders in response to advocacy by parents of affected children, health professionals, and organizations concerned with child health. As individual state programs evolved, they came to vary considerably from each other in the conditions screened for, the services provided, and the modes of financing. Recently, a dramatic expansion of state newborn screening has been underway. The expansion is a response to new medical knowledge, new testing technologies, and political pressure by advocacy groups; however, its form and timing have been heavily influenced by the work of a federally funded expert group convened by the American College of Medical Genetics (ACMG). The group s charge was to develop a uniform test panel that could be adopted by all states. Before the work began, the majority of states screened for fewer than 10 conditions. The ACMG group s report recommended screening for 29 core conditions and another 25 conditions that could be detected incidentally in screening for the core group. By May 2008, most states had started screening for all or nearly all of the core conditions (see box, Core Conditions ). This expansion has been accompanied by ethical debates related to cost, evidence, and parental rights. While supporters believe that the expanded screening is important for children s health, critics charge that the ACMG group made its recommendations without a solid evidence base or adequate consideration of competing demands on public resources. Critics also object to the group s movement away from newborn screening s traditional reliance on significant benefit to the newborn from very early diagnosis and treatment as the criterion for adding a condition to a state screening panel. They argue that when this criterion is not met, there is no justification for omitting parental informed Mary Ann Baily, PhD, is a research scholar at The Hastings Center. H I G H L I G H T S n Newborn screening programs test nearly all infants born in this country for selected inherited and congenital conditions that can cause disability or death. n These state programs began in the 1960s with a blood test for phenylketonuria (PKU), a metabolic disorder that can cause permanent mental retardation and other problems unless it is diagnosed and treated early. n A dramatic expansion of newborn screening programs is under way, with most states testing for about 29 core conditions, up from fewer than 10 several years ago. n The expansion of newborn screening raises ethical controversies about its cost, evidence of its efficacy, and parental informed consent. n Policymakers have an ethical obligation to weigh benefits against costs when directing resources to newborn screening. n Although state governments create and manage screening programs, the federal government supports them with funding, help with improving program performance, and other kinds of assistance. n The Newborn Screening Saves Lives Act, signed into law in 2008, will help support state efforts to coordinate and improve their programs. ExpErts to ContaCt Mary ann Baily, phd, Research Scholar, The Hastings Center bailym@ thehastingscenter.org, , x 200 Jeffrey r. Botkin, MD, MpH, Professor of Pediatrics, Division of Medical Ethics, University of Utah School of Medicine jeffrey.botkin@hsc.utah.edu, Ellen Wright Clayton, JD, MD, Codirector, Center for Biomedical Ethics and Society, Vanderbilt University School of Law ellen.w.clayton@vanderbilt.edu, newborn screening 27 NewBorN ScreeNING 125
3 consent, and no obvious reason to give screening and treatment for a condition priority over other ethically important health care not readily available to all children, such as diagnosis and treatment of asthma or juvenile diabetes. The Ethical Controversies The debates about the expansion of newborn screening programs concentrate on three key issues: cost, evidence, and parental consent. Cost. Concern about cost is often seen as opposed to ethics in debates about newborn screening policy. Many advocates say that it is wrong to consider cost at all when infants lives are at stake. In contrast, ethicists say that it is wrong to ignore cost. Cost is an ethical issue because newborn screening uses collective resources (public and private) to pay for the screening, as well as the followup and treatment. Although screening newborns may be desirable, there are always other uses for resources that would also save lives and prevent disability there is an opportunity cost to their use. Policymakers thus have an ethical obligation of stewardship to weigh the benefits against the costs when directing resources to newborn screening. In stewardship, the total net benefit from screening isn t the only consideration; the fairness of the distribution of benefits and costs also matters. On the benefit side, fairness often comes up in discussions of state differences in test panels. To many people, it seems unfair that for an infant with a genetic disorder, being born in one state rather than another can be a matter of life or death. A major goal of the ACMG group was to make access to screening less variable from state to state. The variation in the quantity and quality of follow-up services received by affected children and their families is also a fairness issue. On the cost side, it is important to recognize that cost isn t just the screening test but also the entire cost of the program. There are also time and anxiety costs to families of newborns with false positive tests (infants who test positive but are shown by followup testing to be healthy). Currently, most people involved in newborn screening would agree that the distribution of these costs is arbitrary and does not conform to any reasonable standard of fairness. It imposes excessive burdens on some families and fails to distribute the total cost of the system equitably across the entire nation. Evidence. If cost is an ethical issue, then evidence is also an ethical issue. One cannot assess the opportunity cost of resources and the distributional fairness associated with a policy decision without detailed information about the decision s positive and negative effects. Some advocates are uncomfortable with calls for evidence-based newborn screening policy, however. They argue that it sets too high a bar, given that conditions considered for screening are relatively rare and, in the absence of screening, may be underdiagnosed. Since gathering evidence itself uses resources, everyone understands that policy must often be made without complete information. The debate is about how much information is enough. Strong advocates for screening are likely to say that screening should go ahead without any hard evidence if there is some hope of benefit. Others argue that ethics requires a cost-conscious, systematic effort before the introduction of a mandatory new test to gather and evaluate evidence on the consequences of the decision. There is less controversy over what should happen after testing is introduced: most consider cost-conscious, systematic collection and evaluation of evidence on the effects of newborn screening to be an essential part of the ongoing management of these public programs. Parental consent. The United States has sturdy societal values respecting the rights of individuals to decide what treatments they will have, whether they will participate in research, and what can be done with their personal information and their bodily tissues, including blood samples. (Because only a fraction of each blood sample taken for newborn screening is used in the screening, the remainder is a valuable potential resource for research and program evaluation.) Since parents are normally considered the appropriate people to make decisions on behalf of their children, parental informed consent is ethically required for the medical treatment of children and for the involvement of children in research. Because parental consent is the ethical standard, the mandatory status of public newborn screening has always been controversial. Mandatory screening for PKU was originally sought on the grounds that the urgent need for early diagnosis and the great benefit of the treatment justified omitting parental informed consent. This criterion guided newborn screening programs for many years (although some ethicists maintained that states should obtain parental consent even in these cir- 126 THe HASTINGS center BIoeTHIcS BrIefING BooK
4 cumstances). The ACMG report argued that it was appropriate to depart from this criterion and consider benefits to the family or society, rather than to the infant. For example, early diagnosis of an untreatable genetic condition may allow parents to plan ahead for the time when the child s symptoms appear and perhaps to alter their reproductive decisions to avoid the birth of another affected child. Early identification of affected children can also benefit research on the condition by providing potential human research subjects and residual blood samples. When the traditional criterion is modified in these ways, however, parental informed consent to screening is the usual ethical standard. Looking to the Future: Federal and State Policy The ethical issues above are likely to persist in the debates over newborn screening policy. The policy action is at both the state and federal levels. Although state governments create and manage the programs, the federal government has played a supportive role in newborn screening from the beginning. This role includes funding research on newborn screening, promoting the development of policies and guidelines, and partnering with states to improve their programs performance. The federal government also supports newborn screening indirectly through Medicaid, a medical assistance program for certain categories of the poor that is financed with federal and state funds. Many state newborn screening programs charge fees to third party payers for testing and diagnostic services, and Medicaid is the third party payer for about onethird of babies born in the United States. The federal Health Resources and Services Administration (HRSA) funded the ACMG expert group s work. Through the Advisory Committee on Heritable Disorders in Newborns and Children, which advises the Secretary of Health and Human Services, HRSA and other federal agencies are now working with states as they implement the recommendations. Many states already had difficulty providing adequate follow-up services for the conditions in their existing test panels, and for all states, expanding programs to cover so many conditions is a major managerial and financing challenge. The Newborn Screening Saves Lives Act, signed into law in April 2008, will help support state efforts to coordinate and improve their programs. C o r E C o n D i t i o n s Blood disorders Sickle cell anemia S-beta thalassemia Sickle-c disease amino acid disorders Phenylketonuria Maple syrup urine disease Homocystinuria citrullinemia Argininosuccinic acidemia Tyrosinemia type I Fatty acid disorders Medium-chain acyl-coa dehydrogenase deficiency Very long-chain acyl-coa dehydrogenase deficiency Long-chain L-3-oH acyl-coa dehydrogenase deficiency Trifunctional protein deficiency carnitine uptake defect organic acid disorders Isovaleric acidemia Glutaric acidemia type I Hydroxymethylglutaric aciduria (also called HMG-coA lyase deficiency or 3-oH 3-cH3 glutaric aciduria) Multiple carboxylase deficiency Methylmalonic acidemia due to mutase deficiency 3-Methylcrotonyl-coA carboxylase deficiency Methylmalonic acidemia cbla and cblb forms Propionic acidemia Beta-Ketothiolase deficiency other conditions congenital hypothyroidism Biotinidase deficiency congenital adrenal hyperplasia Hearing loss cystic fibrosis classical galactosemia Going forward, the debate about the criteria to be used and the evidence required for adding new conditions to the nationally recommended newborn screening panel will continue. It is important to reach a societal consensus on an appropriate process. The Advisory Committee on Heritable Disorders has taken responsibility for overseeing the development and implementation of such a process. It would seem desirable to have the process include an evidence review that follows newborn screening 27 NewBorN ScreeNING 127
5 r E s o u r C E s Web sites The National Newborn Screening and Genetics research center. Includes fact sheets, a clickable map of state newborn screening programs, an up-to-date list of conditions screened for in the United States, and literature for parents and providers. Medline Plus, a service of the National Library of Medicine and the National Institutes of Health. Includes a gateway page under health topics for newborn screening that features an overview and tutorial, links to latest news and journal articles, research, and information on specific conditions. American Association of Pediatrics. Includes a gateway page under health topics for newborn screening that features overview articles, fact sheets and policy guidelines, information on specific conditions, and links. National conference of State Legislatures. Includes a page on newborn genetic and metabolic screening with links to newborn screening legislation in all fifty states. the Advisory committee on Heritable Disorders in Newborns and children. Includes reports and correspondence, governance, and educational materials. recent news Shirley S. wang, Addressing the fallout of Newborn Screening, Wall Street Journal, october 30, Jamie Talan, early Action Proving crucial to Hearing accepted scientific standards and is done (or is thoroughly reviewed by) people outside the field of newborn screening. Debate will also continue over the extent to which priority should be given to newborn screening given the constraints on the resources available for children s health. In today s health care system, there is no institutional structure that can force consideration of opportunity cost and no way to ensure responsible stewardship. Without stewardship, special interest groups such as health professional organizations, consumer groups, and makers of screening technologies can exercise inappropriate influence on policy. Many advocates provide important perspectives and are committed to the well-being of affected children; however, in the absence of a structure that forces comparison of newborn screening with other uses of public and private resources, it is difficult to make cost-conscious, evidence-based, fair decisions that balance the needs of all children. This situation is unlikely Success, New York Times, September 4, erin cline, Infant Screening rates rise in U.S., Los Angeles Times, June 11, Darshak Sanghavi, Newborn Screening Benefits Not So clear, Boston Globe, october 10, Further reading Mary Ann Baily and Thomas H. Murray, ethics, evidence, and cost in Newborn Screening, Hastings Center Report, Virginia A. Moyer et al., expanding Newborn Screening: Process, Policy, and Priorities, Hastings Center Report, Bruce K. Lin and Alan r. fleischman, Screening and caring for children with rare Disorders, Hastings Center Report, Newborn Screening: Toward a Uniform Screening Panel and System, eds. Michael S. watson et al., Genetics in Medicine supplement, May Scott D. Gross et al., from Public Health emergency to Public Health Service: The Implications of evolving criteria for Newborn Screening Panels, Pediatrics, March Jeffrey r. Botkin, research for Newborn Screening: Developing a National framework, Pediatrics, october American Academy of Pediatrics Newborn Screening Task force, Serving the family from Birth to the Medical Home, Pediatrics supplement, August to change without meaningful reform of American health care. The issue of parental consent will remain controversial. Many ethicists will continue to hold that programs must maintain clear benefit to the infant as the essential criterion for mandatory public newborn screening; if the benefit is to anyone else, or if the benefit to the infant is uncertain, parental informed consent is required. In the meantime, the expansion of the test panels has made the task of informing parents significantly more complex. Finally, there is an urgent need to clarify the ethical requirements with respect to parental consent for using leftover blood spots for newborn screening quality improvement, research related to newborn screening, and research on questions not directly related to newborn screening. The use of newborn screening blood spots is simply a specific instance of the larger issue of achieving a societal consensus on the ethical rules that should govern the use of bodily tissues for social purposes. 128 THe HASTINGS center BIoeTHIcS BrIefING BooK
Newborn Screening in Manitoba. Information for Health Care Providers
 Newborn Screening in Manitoba Information for Health Care Providers Newborn screening: a healthy start leads to a healthier life Health care professionals have provided newborn screening for phenylketonuria
Newborn Screening in Manitoba Information for Health Care Providers Newborn screening: a healthy start leads to a healthier life Health care professionals have provided newborn screening for phenylketonuria
Newborn Screening in Saskatchewan Information for Health Care Providers
 Newborn Screening in Saskatchewan Information for Health Care Providers 2 Newborn screening: a healthy start leads to a healthier life Since the mid-1960s, health care providers have offered newborn screening
Newborn Screening in Saskatchewan Information for Health Care Providers 2 Newborn screening: a healthy start leads to a healthier life Since the mid-1960s, health care providers have offered newborn screening
Alabama Newborn Screening Program
 Alabama Newborn Screening Program Blood Spot Screening Hearing Screening Pulse Oximetry Screening Delivering You the Facts Alabama Department of Public Health Newborn Screening Program www.adph.org/newbornscreening
Alabama Newborn Screening Program Blood Spot Screening Hearing Screening Pulse Oximetry Screening Delivering You the Facts Alabama Department of Public Health Newborn Screening Program www.adph.org/newbornscreening
State of Board of Health Agenda March 23, 2012 9:00 a.m. Perimeter Center 9960 Mayland Drive Richmond, Virginia 23233
 State of Board of Health Agenda March 23, 2012 9:00 a.m. Perimeter Center 9960 Mayland Drive Richmond, Virginia 23233 Welcome and Introductions Review of Agenda Approval of December 2011 Minutes Commissioner
State of Board of Health Agenda March 23, 2012 9:00 a.m. Perimeter Center 9960 Mayland Drive Richmond, Virginia 23233 Welcome and Introductions Review of Agenda Approval of December 2011 Minutes Commissioner
Newborn screening policy and guidelines
 Newborn screening policy and guidelines 2011 Newborn screening policy and guidelines 2011 Newborn screening policy and guidelines 2011 Contacts Detailed newborn screening program information including
Newborn screening policy and guidelines 2011 Newborn screening policy and guidelines 2011 Newborn screening policy and guidelines 2011 Contacts Detailed newborn screening program information including
Your newborn baby s blood test
 Newborn Screening Free health checks for your baby Your newborn baby s blood test The Newborn Metabolic Screening Programme All babies are checked at birth to see that all is well. Some of your baby s
Newborn Screening Free health checks for your baby Your newborn baby s blood test The Newborn Metabolic Screening Programme All babies are checked at birth to see that all is well. Some of your baby s
Biobanks: DNA and Research
 f r o m b i r t h to death a n d b e n c h to clinic THE HASTINGS CENTER Bioethics Briefing Book for Journalists, Policymakers, and Campaigns Chapter 3 Biobanks: DNA and Research Karen J. Maschke, Biobanks:
f r o m b i r t h to death a n d b e n c h to clinic THE HASTINGS CENTER Bioethics Briefing Book for Journalists, Policymakers, and Campaigns Chapter 3 Biobanks: DNA and Research Karen J. Maschke, Biobanks:
Newborn Screening Test
 Important Information for Parents about the Newborn Screening Test Newborn Screening Branch Genetic Disease Screening Program http://cdph.ca.gov/nbs California Department of Public Health Publication Date:
Important Information for Parents about the Newborn Screening Test Newborn Screening Branch Genetic Disease Screening Program http://cdph.ca.gov/nbs California Department of Public Health Publication Date:
MICHIGAN NEWBORN SCREENING PROGRAM
 Michigan Department of Community Health MICHIGAN NEWBORN SCREENING PROGRAM Annual Report 2011 Acknowledgements State of Michigan Governor Rick Snyder Michigan Department of Community Health Director James
Michigan Department of Community Health MICHIGAN NEWBORN SCREENING PROGRAM Annual Report 2011 Acknowledgements State of Michigan Governor Rick Snyder Michigan Department of Community Health Director James
Inborn Errors of Metabolism
 351 Inborn Errors of Metabolism Definition/ cut-off value Inherited metabolic disorders caused by a defect in the enzymes or their cofactors that metabolize protein, carbohydrate, or fat. Inborn errors
351 Inborn Errors of Metabolism Definition/ cut-off value Inherited metabolic disorders caused by a defect in the enzymes or their cofactors that metabolize protein, carbohydrate, or fat. Inborn errors
Genetics in Family Medicine: The Australian Handbook for General Practitioners. Newborn screening
 Genetics in Family Medicine: The Australian Handbook for General Practitioners GP s role 3 The newborn screening process 3 Storage of newborn screening cards 3 results 5 Where no further testing is required
Genetics in Family Medicine: The Australian Handbook for General Practitioners GP s role 3 The newborn screening process 3 Storage of newborn screening cards 3 results 5 Where no further testing is required
Newborn Screening In America:
 Newborn Screening In America: Problems and Policies Vani Kilakkathi 2012 Council for Responsible Genetics 5 Upland Road, Suite 3 Cambridge, MA 02140 Email: crg@gene-watch.org www.councilforresponsiblegenetics.org
Newborn Screening In America: Problems and Policies Vani Kilakkathi 2012 Council for Responsible Genetics 5 Upland Road, Suite 3 Cambridge, MA 02140 Email: crg@gene-watch.org www.councilforresponsiblegenetics.org
MEDICAL NUTRITION FOR INHERITED METABOLIC DISEASE Corporate Medical Policy
 MEDICAL NUTRITION FOR INHERITED METABOLIC DISEASE Corporate Medical Policy File name: Medical Nutrition for Inherited Metabolic Disease File code: UM.NUT.02 Origination: 10/1998 Last Review: 11/2013 Next
MEDICAL NUTRITION FOR INHERITED METABOLIC DISEASE Corporate Medical Policy File name: Medical Nutrition for Inherited Metabolic Disease File code: UM.NUT.02 Origination: 10/1998 Last Review: 11/2013 Next
Senate Bill No. 1555 CHAPTER 484
 Senate Bill No. 1555 CHAPTER 484 An act to amend Sections 124977 and 125055 of, to add Sections 1604.6 and 125002 to, and to add Article 4 (commencing with Section 123370) to Chapter 1 of Part 2 of Division
Senate Bill No. 1555 CHAPTER 484 An act to amend Sections 124977 and 125055 of, to add Sections 1604.6 and 125002 to, and to add Article 4 (commencing with Section 123370) to Chapter 1 of Part 2 of Division
COLLECTION OF NEWBORN SCREENING SPECIMENS
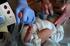 COLLECTION OF NEWBORN SCREENING SPECIMENS Newborn Screening is Mandatory Newborn screening has the potential to save or greatly improve the life of a newborn; therefore, Newborn Screening is mandatory
COLLECTION OF NEWBORN SCREENING SPECIMENS Newborn Screening is Mandatory Newborn screening has the potential to save or greatly improve the life of a newborn; therefore, Newborn Screening is mandatory
Genetic Testing in Research & Healthcare
 We Innovate Healthcare Genetic Testing in Research & Healthcare We Innovate Healthcare Genetic Testing in Research and Healthcare Human genetic testing is a growing science. It is used to study genes
We Innovate Healthcare Genetic Testing in Research & Healthcare We Innovate Healthcare Genetic Testing in Research and Healthcare Human genetic testing is a growing science. It is used to study genes
Fatty Acid Oxidation Disorders Galactosemia Biotinidase Deficiency
 Fatty Acid Oxidation Disorders Galactosemia Biotinidase Deficiency Dr. Kathy Grange, MD Division of Genetics and Genomic Medicine Department of Pediatrics Washington University School of Medicine What
Fatty Acid Oxidation Disorders Galactosemia Biotinidase Deficiency Dr. Kathy Grange, MD Division of Genetics and Genomic Medicine Department of Pediatrics Washington University School of Medicine What
Genetic testing. The difference diagnostics can make. The British In Vitro Diagnostics Association
 6 Genetic testing The difference diagnostics can make The British In Vitro Diagnostics Association Genetic INTRODUCTION testing The Department of Health published Our Inheritance, Our Future - Realising
6 Genetic testing The difference diagnostics can make The British In Vitro Diagnostics Association Genetic INTRODUCTION testing The Department of Health published Our Inheritance, Our Future - Realising
Insurance coverage of medical foods for treatment of inherited metabolic disorders
 May 18, 2012 Insurance coverage of medical foods for treatment of inherited metabolic disorders Secretary s Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC) Susan A. Berry,
May 18, 2012 Insurance coverage of medical foods for treatment of inherited metabolic disorders Secretary s Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC) Susan A. Berry,
Texas Newborn Screening Performance Measures Project
 Texas Newborn Screening Performance Measures Project Susan Tanksley, PhD MSGRCC Annual Meeting July 14, 2011 The Texas Newborn Screening Performance Measure Project (TNSPMP) is funded through a cooperative
Texas Newborn Screening Performance Measures Project Susan Tanksley, PhD MSGRCC Annual Meeting July 14, 2011 The Texas Newborn Screening Performance Measure Project (TNSPMP) is funded through a cooperative
State Policy and Finance Framework for Newborn Screening Programs:
 State Policy and Finance Framework for Newborn Screening Programs: Case Studies of Select States Presentation by: Kay Johnson, MPH, EdM President, Johnson Group Consulting Research Assistant Professor,
State Policy and Finance Framework for Newborn Screening Programs: Case Studies of Select States Presentation by: Kay Johnson, MPH, EdM President, Johnson Group Consulting Research Assistant Professor,
PUBLIC HEALTH IMPROVEMENT PARTNERSHIP
 PUBLIC HEALTH IMPROVEMENT PARTNERSHIP PUBLIC HEALTH ACTIVITIES & SERVICES INVENTORY TECHNICAL NOTES HEALTHY FAMILY DEVELOPMENT Nurse-Family Partnership Nurse-Family Partnership is a voluntary program of
PUBLIC HEALTH IMPROVEMENT PARTNERSHIP PUBLIC HEALTH ACTIVITIES & SERVICES INVENTORY TECHNICAL NOTES HEALTHY FAMILY DEVELOPMENT Nurse-Family Partnership Nurse-Family Partnership is a voluntary program of
Newborn Screening Public Health Service, Research and Public Trust
 Newborn Screening Public Health Service, Research and Public Trust Anne Marie Comeau, Ph.D Deputy Director, New England Newborn Screening Program Professor of Pediatrics, UMMS A National Conversation on
Newborn Screening Public Health Service, Research and Public Trust Anne Marie Comeau, Ph.D Deputy Director, New England Newborn Screening Program Professor of Pediatrics, UMMS A National Conversation on
Newborn Screening and Health Information Technology
 Newborn Screening and Health Information Technology Alan E Zuckerman MD FAAP Georgetown University Medical Center SACHDNC HIT Workgroup Co-Chair AAP Council on Clinical Information Technology (COCIT) Executive
Newborn Screening and Health Information Technology Alan E Zuckerman MD FAAP Georgetown University Medical Center SACHDNC HIT Workgroup Co-Chair AAP Council on Clinical Information Technology (COCIT) Executive
NEWBORN GENETIC SCREENING: EVOLVING TECHNOLOGIES AND PUBLIC HEALTH POLICY
 NEWBORN GENETIC SCREENING: EVOLVING TECHNOLOGIES AND PUBLIC HEALTH POLICY Patricia C. Kuszler, MD, JD * University of Washington School of Law William H. Gates Hall, Box 353020 Seattle, Washington U.S.A
NEWBORN GENETIC SCREENING: EVOLVING TECHNOLOGIES AND PUBLIC HEALTH POLICY Patricia C. Kuszler, MD, JD * University of Washington School of Law William H. Gates Hall, Box 353020 Seattle, Washington U.S.A
Newborn Screening Issues
 Newborn Screening Issues - The Public Health Aspect - Dr. Helmut Brand Msc, MFPHM Institute of Public Health NRW, Bielefeld Public Health Genetics is like Mr Tur Tur, the phantom giant: the further you
Newborn Screening Issues - The Public Health Aspect - Dr. Helmut Brand Msc, MFPHM Institute of Public Health NRW, Bielefeld Public Health Genetics is like Mr Tur Tur, the phantom giant: the further you
New Estimates of the Economic Benefits of Newborn Screening for Congenital Hypothyroidism in the US
 The findings and conclusions in this presentation have not been formally disseminated by the Centers for Disease Control and Prevention and should not be construed to represent any agency determination
The findings and conclusions in this presentation have not been formally disseminated by the Centers for Disease Control and Prevention and should not be construed to represent any agency determination
FORMULA & SPECIALIZED FOOD
 FORMULA & SPECIALIZED FOOD ADMINISTRATIVE POLICY Policy Number: HOME 005.16 T2 Effective Date: December 1, 2014 Table of Contents CONDITIONS OF COVERAGE... COVERAGE RATIONALE BENEFIT CONSIDERATIONS...
FORMULA & SPECIALIZED FOOD ADMINISTRATIVE POLICY Policy Number: HOME 005.16 T2 Effective Date: December 1, 2014 Table of Contents CONDITIONS OF COVERAGE... COVERAGE RATIONALE BENEFIT CONSIDERATIONS...
STEP 2 IMPLEMENTATION: HIE TOOLS USER GUIDE HIE TOOLS USER GUIDE
 STEP 2 IMPLEMENTATION: HIE TOOLS USER GUIDE HIE TOOLS USER GUIDE LIST OF REVISIONS REVISION NO. DATE REVISED BY: PAGE/S DESCRIPTION 1 TBD GDSP 13 Initial Version II STEP 2 IMPLEMENTATION: HIE TOOLS USER
STEP 2 IMPLEMENTATION: HIE TOOLS USER GUIDE HIE TOOLS USER GUIDE LIST OF REVISIONS REVISION NO. DATE REVISED BY: PAGE/S DESCRIPTION 1 TBD GDSP 13 Initial Version II STEP 2 IMPLEMENTATION: HIE TOOLS USER
Newborn Screening Update for Health Care Practitioners
 Newborn Screening Update for Health Care Practitioners Changes in regulations affecting your practice for newborns transferred to neonatal intensive care units. Changes for newborn screening of premature,
Newborn Screening Update for Health Care Practitioners Changes in regulations affecting your practice for newborns transferred to neonatal intensive care units. Changes for newborn screening of premature,
One Hundred Tenth Congress of the United States of America
 S. 1858 One Hundred Tenth Congress of the United States of America AT THE SECOND SESSION Begun and held at the City of Washington on Thursday, the third day of January, two thousand and eight An Act To
S. 1858 One Hundred Tenth Congress of the United States of America AT THE SECOND SESSION Begun and held at the City of Washington on Thursday, the third day of January, two thousand and eight An Act To
Colorado Legislative Council Staff
 Colorado Legislative Council Staff Room 029 State Capitol, Denver, CO 80203-1784 (303) 866-3521 FAX: 866-3855 TDD: 866-3472 MEMORANDUM July 1, 2011 TO: Interested Persons FROM: Kelly Stapleton, Senior
Colorado Legislative Council Staff Room 029 State Capitol, Denver, CO 80203-1784 (303) 866-3521 FAX: 866-3855 TDD: 866-3472 MEMORANDUM July 1, 2011 TO: Interested Persons FROM: Kelly Stapleton, Senior
Newborn Screening Program Study On Comprehensive and Sustainable Long-Term Storage and Use
 Public Health Laboratory, Newborn Screening Program 601 Robert Street North St. Paul, MN 55155-2531 651-201-5466 www.health.state.mn.us/newbornscreening Newborn Screening Program Study On Comprehensive
Public Health Laboratory, Newborn Screening Program 601 Robert Street North St. Paul, MN 55155-2531 651-201-5466 www.health.state.mn.us/newbornscreening Newborn Screening Program Study On Comprehensive
STATE STATUTES AND REGULATIONS ON DIETARY TREATMENT OF DISORDERS IDENTIFIED THROUGH NEWBORN SCREENING JURISDICTION AND CITATION
 Alabama AC 22 20 3 AAC 40 20 10.01 et seq. PKU, CH, GALT, CAH, hearing loss, hemoglobinopathy, BD, CF, AAD, FAOD, OAD, and other heritable diseases Statutes authorize board of health to create regulations
Alabama AC 22 20 3 AAC 40 20 10.01 et seq. PKU, CH, GALT, CAH, hearing loss, hemoglobinopathy, BD, CF, AAD, FAOD, OAD, and other heritable diseases Statutes authorize board of health to create regulations
A Parent s Guide to Understanding Congenital Hypothyroidism. Children s of Alabama Department of Pediatric Endocrinology
 A Parent s Guide to Understanding Congenital Hypothyroidism Children s of Alabama Department of Pediatric Endocrinology How did you get here? Every baby born in the state of Alabama is required by law
A Parent s Guide to Understanding Congenital Hypothyroidism Children s of Alabama Department of Pediatric Endocrinology How did you get here? Every baby born in the state of Alabama is required by law
Organic Acid Disorders
 Genetic Fact Sheets for Parents Organic Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
Genetic Fact Sheets for Parents Organic Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
CCS - Does CCS provide Counseling or Support Services? CCS - Are there any costs to me? CCS - What papers should I bring?
 CCS - Does CCS provide Counseling or Support Services? CCS - Are there any costs to me? CCS - What papers should I bring? CCS - What are the steps to CCS services? CCS - How is my privacy protected? CCS
CCS - Does CCS provide Counseling or Support Services? CCS - Are there any costs to me? CCS - What papers should I bring? CCS - What are the steps to CCS services? CCS - How is my privacy protected? CCS
Child Abuse and Neglect AAP Policy Recommendations
 Child Abuse and Neglect AAP Policy Recommendations When Inflicted Skin Injuries Constitute Child Abuse Committee on Child Abuse and Neglect PEDIATRICS Vol. 110 No. 3 September 2002, pp. 644-645 Recommendations
Child Abuse and Neglect AAP Policy Recommendations When Inflicted Skin Injuries Constitute Child Abuse Committee on Child Abuse and Neglect PEDIATRICS Vol. 110 No. 3 September 2002, pp. 644-645 Recommendations
Suggested answers to questions
 Teacher Notes Introduction This activity encourages students to read an article which provides a good overview of the issues involved in genetic testing. It will also teach them useful reading skills,
Teacher Notes Introduction This activity encourages students to read an article which provides a good overview of the issues involved in genetic testing. It will also teach them useful reading skills,
Parents Guide To Primary Congenital Hypothyroidism
 Parents Guide To Primary Congenital Hypothyroidism California Department of Health Services Genetic Disease Branch www.dhs.ca.gov/gdb To Parents: California State Law requires that all babies have the
Parents Guide To Primary Congenital Hypothyroidism California Department of Health Services Genetic Disease Branch www.dhs.ca.gov/gdb To Parents: California State Law requires that all babies have the
Promoting Family Planning
 Promoting Family Planning INTRODUCTION Voluntary family planning has been widely adopted throughout the world. More than half of all couples in the developing world now use a modern method of contraception
Promoting Family Planning INTRODUCTION Voluntary family planning has been widely adopted throughout the world. More than half of all couples in the developing world now use a modern method of contraception
House Resolution No. 37
 california legislature regular session House Resolution No. Introduced by Assembly Member Hill August, House Resolution No. Relative to umbilical cord blood banking. WHEREAS, Since the first umbilical
california legislature regular session House Resolution No. Introduced by Assembly Member Hill August, House Resolution No. Relative to umbilical cord blood banking. WHEREAS, Since the first umbilical
DRAFT. CHILDREN S ENVIRONMENTAL HEALTH EHSC-GA 2051 Thursdays, 4:55-7:35 PM Bobst Library, Lower Level Room 151. SYLLABUS, Spring 2014
 DRAFT CHILDREN S ENVIRONMENTAL HEALTH EHSC-GA 2051 Thursdays, 4:55-7:35 PM Bobst Library, Lower Level Room 151 SYLLABUS, Spring 2014 Michael Weitzman MD, Professor of Environmental Medicine and Pediatrics
DRAFT CHILDREN S ENVIRONMENTAL HEALTH EHSC-GA 2051 Thursdays, 4:55-7:35 PM Bobst Library, Lower Level Room 151 SYLLABUS, Spring 2014 Michael Weitzman MD, Professor of Environmental Medicine and Pediatrics
INTERNATIONAL FRAMEWORK FOR ASSURANCE ENGAGEMENTS CONTENTS
 INTERNATIONAL FOR ASSURANCE ENGAGEMENTS (Effective for assurance reports issued on or after January 1, 2005) CONTENTS Paragraph Introduction... 1 6 Definition and Objective of an Assurance Engagement...
INTERNATIONAL FOR ASSURANCE ENGAGEMENTS (Effective for assurance reports issued on or after January 1, 2005) CONTENTS Paragraph Introduction... 1 6 Definition and Objective of an Assurance Engagement...
Health Reform Minnesota. Health Care Home Care Coordination Tiering-Billing Workshop October 1, 2014
 Health Reform Minnesota Health Care Home Care Coordination Tiering-Billing Workshop October 1, 2014 Objectives Gain a better understanding of how to use the HCH Care Coordination Tier Assignment tool for
Health Reform Minnesota Health Care Home Care Coordination Tiering-Billing Workshop October 1, 2014 Objectives Gain a better understanding of how to use the HCH Care Coordination Tier Assignment tool for
Methods for Promoting Public Dialogue on the Use of Residual Newborn Screening Samples for Research
 Methods for Promoting Public Dialogue on the Use of Residual Newborn Screening Samples for Research Rebecca A. Anderson, BS, GeneSIS Center, Department of Pediatrics, University of Utah, rebecca.anderson@hsc.utah.edu
Methods for Promoting Public Dialogue on the Use of Residual Newborn Screening Samples for Research Rebecca A. Anderson, BS, GeneSIS Center, Department of Pediatrics, University of Utah, rebecca.anderson@hsc.utah.edu
Clinical Trials: Improving the Care of People Living With Cancer
 CLINICAL TRIALS Clinical Trials: Improving the Care of People Living With Cancer Presented by Mary McCabe, RN, MA Memorial Sloan-Kettering Cancer Center Carolyn Messner, DSW CancerCare Learn about: Stages
CLINICAL TRIALS Clinical Trials: Improving the Care of People Living With Cancer Presented by Mary McCabe, RN, MA Memorial Sloan-Kettering Cancer Center Carolyn Messner, DSW CancerCare Learn about: Stages
November 2008 / Common Sense Media. Media + Child and Adolescent Health: A Systematic Review
 November 2008 / Common Sense Media Media + Child and Adolescent Health: A Systematic Review Media + Child and Adolescent Health: A Systematic Review Executive Summary Media are increasingly pervasive in
November 2008 / Common Sense Media Media + Child and Adolescent Health: A Systematic Review Media + Child and Adolescent Health: A Systematic Review Executive Summary Media are increasingly pervasive in
Consultation Response Medical profiling and online medicine: the ethics of 'personalised' healthcare in a consumer age Nuffield Council on Bioethics
 Consultation Response Medical profiling and online medicine: the ethics of 'personalised' healthcare in a consumer age Nuffield Council on Bioethics Response by the Genetic Interest Group Question 1: Health
Consultation Response Medical profiling and online medicine: the ethics of 'personalised' healthcare in a consumer age Nuffield Council on Bioethics Response by the Genetic Interest Group Question 1: Health
Organ Transplantation
 f r o m b i r t h to death a n d b e n c h to clinic THE HASTINGS CENTER Bioethics Briefing Book for Journalists, Policymakers, and Campaigns Chapter 28 Organ Transplantation Arthur Caplan, Organ Transplantation,
f r o m b i r t h to death a n d b e n c h to clinic THE HASTINGS CENTER Bioethics Briefing Book for Journalists, Policymakers, and Campaigns Chapter 28 Organ Transplantation Arthur Caplan, Organ Transplantation,
Genetic Aspects of Mental Retardation and Developmental Disabilities
 Prepared by: Chahira Kozma, MD Associate Professor of Pediatrics Medical Director/DCHRP Kozmac@georgetown.edu cck2@gunet.georgetown.edu Genetic Aspects of Mental Retardation and Developmental Disabilities
Prepared by: Chahira Kozma, MD Associate Professor of Pediatrics Medical Director/DCHRP Kozmac@georgetown.edu cck2@gunet.georgetown.edu Genetic Aspects of Mental Retardation and Developmental Disabilities
Molecular Genetic Testing in Public Health and Clinical Settings
 Molecular Genetic Testing in Public Health and Clinical Settings Ira M. Lubin, PhD, FACMG Division of Laboratory Systems NCPDCID, CCID Centers for Disease Control and Prevention Atlanta, Georgia Disclaimers
Molecular Genetic Testing in Public Health and Clinical Settings Ira M. Lubin, PhD, FACMG Division of Laboratory Systems NCPDCID, CCID Centers for Disease Control and Prevention Atlanta, Georgia Disclaimers
206-478-8227 www.healthdataconsulting.com. Getting Specific. New ICD-10 codes. Will they make a difference?
 206-478-8227 www.healthdataconsulting.com Getting Specific New ICD-10 codes. Will they make a difference? [First in a series on getting to specific documentation and coding] Joseph C Nichols MD Principal
206-478-8227 www.healthdataconsulting.com Getting Specific New ICD-10 codes. Will they make a difference? [First in a series on getting to specific documentation and coding] Joseph C Nichols MD Principal
Children s Health and Nursing:
 Children s Health and Nursing: A Summary of the Issues What s the issue? The foundation for healthy growth and development in later years is established to a large degree in the first six years of life.
Children s Health and Nursing: A Summary of the Issues What s the issue? The foundation for healthy growth and development in later years is established to a large degree in the first six years of life.
June 20, 2012. Testimony of. Vera F. Tait MD, FAAP. On behalf of the. American Academy of Pediatrics. Before the
 Testimony of Vera F. Tait MD, FAAP On behalf of the Before the Subcommittee on Personnel, Senate Armed Services Committee Department of Federal Affairs 601 13th Street NW, Suite 400 North Washington, DC
Testimony of Vera F. Tait MD, FAAP On behalf of the Before the Subcommittee on Personnel, Senate Armed Services Committee Department of Federal Affairs 601 13th Street NW, Suite 400 North Washington, DC
Colorado s Integrated Data System: one state s approach to a comprehensive health information exchange for short-term and long-term follow-up
 Colorado s Integrated Data System: one state s approach to a comprehensive health information exchange for short-term and long-term follow-up Erica L. Wright, MS, CGC Certified Genetic Counselor, Instructor
Colorado s Integrated Data System: one state s approach to a comprehensive health information exchange for short-term and long-term follow-up Erica L. Wright, MS, CGC Certified Genetic Counselor, Instructor
On the Ethics of Clinical Whole Genome Sequencing of Children
 PEDIATRICS PERSPECTIVES On the Ethics of Clinical Whole Genome Sequencing of Children AUTHORS: Thomas May, PhD, Kaija L. Zusevics, PhD, and Kimberly A. Strong, PhD Program in Genomics and Ethics, Center
PEDIATRICS PERSPECTIVES On the Ethics of Clinical Whole Genome Sequencing of Children AUTHORS: Thomas May, PhD, Kaija L. Zusevics, PhD, and Kimberly A. Strong, PhD Program in Genomics and Ethics, Center
Rio Political Declaration on Social Determinants of Health
 Rio Political Declaration on Social Determinants of Health Rio de Janeiro, Brazil, 21 October 2011 1. Invited by the World Health Organization, we, Heads of Government, Ministers and government representatives
Rio Political Declaration on Social Determinants of Health Rio de Janeiro, Brazil, 21 October 2011 1. Invited by the World Health Organization, we, Heads of Government, Ministers and government representatives
Research Involving Human Biological Materials: Ethical Issues and Policy Guidance Executive Summary
 Research Involving Human Biological Materials: Ethical Issues and Policy Guidance Executive Summary Introduction Biomedical researchers have long studied human biological materials such as cells collected
Research Involving Human Biological Materials: Ethical Issues and Policy Guidance Executive Summary Introduction Biomedical researchers have long studied human biological materials such as cells collected
By Avery Comarow FOR PARENTS AND OTHER CAREGIVERS. Why does U.S. News rank children's hospitals?
 How and Why: A 2013-14 Best Children's Hospitals FAQ The facts and methodology behind our latest pediatric rankings (cancer survival description corrected). By Avery Comarow Why should anyone care about
How and Why: A 2013-14 Best Children's Hospitals FAQ The facts and methodology behind our latest pediatric rankings (cancer survival description corrected). By Avery Comarow Why should anyone care about
The Search for Causes and Cures
 ISSUE REPORT BIRTH DEFECTS AND DEVELOPMENTAL DISABILITIES: The Search for Causes and Cures JULY 2005 PREVENTING EPIDEMICS. PROTECTING PEOPLE. REPORT AUTHORS Kim Elliott, MA Deputy Director Trust for America
ISSUE REPORT BIRTH DEFECTS AND DEVELOPMENTAL DISABILITIES: The Search for Causes and Cures JULY 2005 PREVENTING EPIDEMICS. PROTECTING PEOPLE. REPORT AUTHORS Kim Elliott, MA Deputy Director Trust for America
Preconception Clinical Care for Women Medical Conditions
 Preconception Clinical Care for Women All women of reproductive age are candidates for preconception care; however, preconception care must be tailored to meet the needs of the individual. Given that preconception
Preconception Clinical Care for Women All women of reproductive age are candidates for preconception care; however, preconception care must be tailored to meet the needs of the individual. Given that preconception
Exhibit A Scope of Work. Vendor agrees to provide to the California Department of Public Health (CDPH) the services described herein.
 1. Service Overview Vendor agrees to provide to the (CDPH) the services described herein. The Vendor shall provide diagnosis, treatment, and outcome data for metabolic patients identified through the Newborn
1. Service Overview Vendor agrees to provide to the (CDPH) the services described herein. The Vendor shall provide diagnosis, treatment, and outcome data for metabolic patients identified through the Newborn
Oregon s Strategic Plan for Genetics and Public Health
 Oregon s Strategic Plan for Genetics and Public Health November 2002 Supported by a grant from the Maternal and Child Health Bureau Grant # 4 H46 MC 00172-02-1 Introduction to Oregon s Strategic Plan for
Oregon s Strategic Plan for Genetics and Public Health November 2002 Supported by a grant from the Maternal and Child Health Bureau Grant # 4 H46 MC 00172-02-1 Introduction to Oregon s Strategic Plan for
WRITTEN STATEMENT ON BEHALF OF THE AMERICAN ACADEMY OF PEDIATRICS PRESENTED TO THE INSTITUTE OF MEDICINE COMMITTEE ON DISABILITY IN AMERICA
 WRITTEN STATEMENT ON BEHALF OF THE AMERICAN ACADEMY OF PEDIATRICS PRESENTED TO THE INSTITUTE OF MEDICINE COMMITTEE ON DISABILITY IN AMERICA JANUARY 9, 2006 PAUL LIPKIN, MD, FAAP CHAIR, AAP COUNCIL ON CHILDREN
WRITTEN STATEMENT ON BEHALF OF THE AMERICAN ACADEMY OF PEDIATRICS PRESENTED TO THE INSTITUTE OF MEDICINE COMMITTEE ON DISABILITY IN AMERICA JANUARY 9, 2006 PAUL LIPKIN, MD, FAAP CHAIR, AAP COUNCIL ON CHILDREN
Carrier Screening For Genetic Diseases Preconception Consent (Female and/or Male Partner)
 Carrier Screening For Genetic Diseases Preconception Consent (Female and/or Male Partner) The goal of our practice at ARMS is to make sure that you receive optimal care to improve your chances of having
Carrier Screening For Genetic Diseases Preconception Consent (Female and/or Male Partner) The goal of our practice at ARMS is to make sure that you receive optimal care to improve your chances of having
Ethical Guidelines for the Development of Emergency Plans
 Ethical Guidelines for the Development of Emergency Plans FOR MORE INFORMATION CONTACT: Peggy Connorton Director, Quality and Long Term Care Trend Tracker 1201 L St. NW Washington, D.C. 20005 Tel: 202-898-4000
Ethical Guidelines for the Development of Emergency Plans FOR MORE INFORMATION CONTACT: Peggy Connorton Director, Quality and Long Term Care Trend Tracker 1201 L St. NW Washington, D.C. 20005 Tel: 202-898-4000
The Sickle Cell Treatment Act of 2003: The Law s Provisions and Opportunities for Advocacy Policy Brief
 The Sickle Cell Treatment Act of 2003: The Law s Provisions and Opportunities for Advocacy Policy Brief Introduction The Sickle Cell Treatment Act (SCTA) provides an important opportunity to work with
The Sickle Cell Treatment Act of 2003: The Law s Provisions and Opportunities for Advocacy Policy Brief Introduction The Sickle Cell Treatment Act (SCTA) provides an important opportunity to work with
Testimony of. Before The Subcommittee on Labor, Health and Human Services and Education of the Committee on Appropriations United States Senate
 Testimony of James F. Childress, Ph.D. Commissioner, National Bioethics Advisory Commission and Kyle Professor of Religious Studies Professor of Medical Education University of Virginia Charlottesville,
Testimony of James F. Childress, Ph.D. Commissioner, National Bioethics Advisory Commission and Kyle Professor of Religious Studies Professor of Medical Education University of Virginia Charlottesville,
HEALTH RESEARCH, INC. New York State Department of Health Wadsworth Center/Division of Genetics
 HEALTH RESEARCH, INC. New York State Department of Health Wadsworth Center/Division of Genetics New York-Mid-Atlantic Consortium for Genetic and Newborn Screening Services Program Evaluation Consultant
HEALTH RESEARCH, INC. New York State Department of Health Wadsworth Center/Division of Genetics New York-Mid-Atlantic Consortium for Genetic and Newborn Screening Services Program Evaluation Consultant
Easy Read. How can we make sure everyone gets the right health care? How can we make NHS care better?
 Easy Read How can we make NHS care better? How can we make sure everyone gets the right health care? What can we do to make the NHS good now and in the future? How can we afford to keep the NHS going?
Easy Read How can we make NHS care better? How can we make sure everyone gets the right health care? What can we do to make the NHS good now and in the future? How can we afford to keep the NHS going?
National Survey of Pre- and Post- Analytical Performance Measures Used in Newborn Screening Programs
 National Survey of Pre- and Post- Analytical Performance Measures Used in Newborn Screening Programs Simran Tiwana, MBA* Susan Tanksley, PhD* Brad Therrell, PhD Donna Williams, BS* Mirsa Douglass, MBA*
National Survey of Pre- and Post- Analytical Performance Measures Used in Newborn Screening Programs Simran Tiwana, MBA* Susan Tanksley, PhD* Brad Therrell, PhD Donna Williams, BS* Mirsa Douglass, MBA*
4. All cord blood banks should be subject to the same standards, regulations and accreditation requirements.
 WMDA Policy Statement on the Utility of Autologous or Family Cord Blood Unit Storage The WMDA Board adopted this policy on 25 th of May 2006. Policy updated _April 2011 The Cord Blood Working Group and
WMDA Policy Statement on the Utility of Autologous or Family Cord Blood Unit Storage The WMDA Board adopted this policy on 25 th of May 2006. Policy updated _April 2011 The Cord Blood Working Group and
American College of Medical Genetics and Genomics Strategic Plan
 American College of Medical Genetics and Genomics Strategic Plan The American College of Medical Genetics and Genomics (ACMG) is the specialty society for diplomates certified by the American Board of
American College of Medical Genetics and Genomics Strategic Plan The American College of Medical Genetics and Genomics (ACMG) is the specialty society for diplomates certified by the American Board of
HEALTH TRANSITION AND ECONOMIC GROWTH IN SRI LANKA LESSONS OF THE PAST AND EMERGING ISSUES
 HEALTH TRANSITION AND ECONOMIC GROWTH IN SRI LANKA LESSONS OF THE PAST AND EMERGING ISSUES Dr. Godfrey Gunatilleke, Sri Lanka How the Presentation is Organized An Overview of the Health Transition in Sri
HEALTH TRANSITION AND ECONOMIC GROWTH IN SRI LANKA LESSONS OF THE PAST AND EMERGING ISSUES Dr. Godfrey Gunatilleke, Sri Lanka How the Presentation is Organized An Overview of the Health Transition in Sri
Refer to https://www.bcbsal.org/providers/hcreform/hcrpreventivecod ing.pdf for the Quick Reference Guide for HCR Preventive Care Services
 Refer to https://www.bcbsal.org/providers/hcreform/hcrpreventivecod ing.pdf for the Quick Reference Guide for HCR Preventive Care Services Name of Policy: Preventive Care Services under Health Care Reform
Refer to https://www.bcbsal.org/providers/hcreform/hcrpreventivecod ing.pdf for the Quick Reference Guide for HCR Preventive Care Services Name of Policy: Preventive Care Services under Health Care Reform
Practitioner s Manual
 Oregon Practitioner s Manual The Oregon Practitioner s Manual Oregon Health & Science University Cary Harding, MD Stephen L. LaFranchi, MD Gregory Thomas, MD Michael Wall, MD Oregon Health Authority Public
Oregon Practitioner s Manual The Oregon Practitioner s Manual Oregon Health & Science University Cary Harding, MD Stephen L. LaFranchi, MD Gregory Thomas, MD Michael Wall, MD Oregon Health Authority Public
Newborn Blood Spot Standards for newborn blood spot screening
 Newborn Blood Spot Standards for newborn blood spot screening Version 1.0 / August 2013 About the NHS Newborn Blood Spot Screening Programme The NHS Newborn Blood Spot Screening Programme has responsibility
Newborn Blood Spot Standards for newborn blood spot screening Version 1.0 / August 2013 About the NHS Newborn Blood Spot Screening Programme The NHS Newborn Blood Spot Screening Programme has responsibility
U.K. Familial Ovarian Cancer Screening Study (UK FOCSS) Phase 2 Patient Information Sheet
 U.K. Familial Ovarian Cancer Screening Study (UK FOCSS) Phase 2 Patient Information Sheet 1. Invitation You are being invited to take part in a research study. Before you decide it is important for you
U.K. Familial Ovarian Cancer Screening Study (UK FOCSS) Phase 2 Patient Information Sheet 1. Invitation You are being invited to take part in a research study. Before you decide it is important for you
ROUTINE HEART EXAM AND
 INFORMATION FOR PARENTS ROUTINE HEART EXAM AND BIOBANK IN ALL NEWBORNS In the Copenhagen area 2016-2018 You have the option to let your child join a research study conducted by doctors with expertise in
INFORMATION FOR PARENTS ROUTINE HEART EXAM AND BIOBANK IN ALL NEWBORNS In the Copenhagen area 2016-2018 You have the option to let your child join a research study conducted by doctors with expertise in
Cord blood banking: information for parents
 Cord blood banking: information for parents Published August 2006 by the RCOG Contents Page number Key points 1 About this information 2 What is cord blood? 2 Why is cord blood useful? 3 How is cord blood
Cord blood banking: information for parents Published August 2006 by the RCOG Contents Page number Key points 1 About this information 2 What is cord blood? 2 Why is cord blood useful? 3 How is cord blood
ARMENIA. Albert Matevosyan MD,PhD
 Albert Matevosyan MD,PhD Neurohereditary Diseases Charity Association Head of Republic Center of Medical Genetic and Department of Medical Genetic Yerevan State Medical University Rome EUROPLAN (Italy)
Albert Matevosyan MD,PhD Neurohereditary Diseases Charity Association Head of Republic Center of Medical Genetic and Department of Medical Genetic Yerevan State Medical University Rome EUROPLAN (Italy)
Overview of Genetic Testing and Screening
 Integrating Genetics into Your Practice Webinar Series Overview of Genetic Testing and Screening Genetic testing is an important tool in the screening and diagnosis of many conditions. New technology is
Integrating Genetics into Your Practice Webinar Series Overview of Genetic Testing and Screening Genetic testing is an important tool in the screening and diagnosis of many conditions. New technology is
Preventive Care Coverage Wondering what preventive care your plan covers?
 STAYING WELL Regence BlueCross BlueShield of Oregon is an Independent Licensee of the Blue Cross and Blue Shield Association Preventive Care Coverage Wondering what preventive care your plan covers? Our
STAYING WELL Regence BlueCross BlueShield of Oregon is an Independent Licensee of the Blue Cross and Blue Shield Association Preventive Care Coverage Wondering what preventive care your plan covers? Our
1 ALPHA-1. The Liver and Alpha-1 Antitrypsin Deficiency (Alpha-1) FOUNDATION FOUNDATION. A patient s guide to Alpha-1 liver disease
 The Liver and Alpha-1 Antitrypsin Deficiency (Alpha-1) 1 ALPHA-1 FOUNDATION The Alpha-1 Foundation is committed to finding a cure for Alpha-1 Antitrypsin Deficiency and to improving the lives of people
The Liver and Alpha-1 Antitrypsin Deficiency (Alpha-1) 1 ALPHA-1 FOUNDATION The Alpha-1 Foundation is committed to finding a cure for Alpha-1 Antitrypsin Deficiency and to improving the lives of people
Comments from EASAC and FEAM on the In vitro diagnostic medical devices Regulation
 Comments from EASAC and FEAM on the In vitro diagnostic medical devices Regulation Introduction The proposed Regulations on medical devices represent an important initiative to strengthen the characterisation
Comments from EASAC and FEAM on the In vitro diagnostic medical devices Regulation Introduction The proposed Regulations on medical devices represent an important initiative to strengthen the characterisation
Editorial Commentary ~ The Case for Universal Newborn Screening Charles P. Hehmeyer
 EP (Exceptional Parent) rarely endorses the positions expressed in a guest editorial but in this case, we not only concur with Mr. Hehmeyer's position on Universal Newborn Screening but we wholeheartedly
EP (Exceptional Parent) rarely endorses the positions expressed in a guest editorial but in this case, we not only concur with Mr. Hehmeyer's position on Universal Newborn Screening but we wholeheartedly
On Time / Every Time. A Partnership of Safety and Reliability for Newborn Screening
 On Time / Every Time A Partnership of Safety and Reliability for Newborn Screening Welcome As a result of this program, the participant will be able to: Discuss the importance of obtaining a quality specimen
On Time / Every Time A Partnership of Safety and Reliability for Newborn Screening Welcome As a result of this program, the participant will be able to: Discuss the importance of obtaining a quality specimen
1. General Information About The Mitochondrial Disease Biobank
 Name and Clinic Number IRB # 09-002265 00 Consent form approved November 6, 2013; This consent valid through August 6, 2014; 1. General Information About The Mitochondrial Disease Biobank Study Title:
Name and Clinic Number IRB # 09-002265 00 Consent form approved November 6, 2013; This consent valid through August 6, 2014; 1. General Information About The Mitochondrial Disease Biobank Study Title:
ENTERAL FORMULAE AND PARENTERAL NUTRITIONAL SOLUTIONS, DME
 ENTERAL FORMULAE AND PARENTERAL NUTRITIONAL SOLUTIONS, DME Policy NHP only reimburses participating DME vendors for the provision of medically necessary enteral and parenteral formulae and nutritional
ENTERAL FORMULAE AND PARENTERAL NUTRITIONAL SOLUTIONS, DME Policy NHP only reimburses participating DME vendors for the provision of medically necessary enteral and parenteral formulae and nutritional
HUMAN FERTILISATION AND EMBRYOLOGY AUTHORITY REPORT:
 HUMAN FERTILISATION AND EMBRYOLOGY AUTHORITY REPORT: Introduction 1. Preimplantation tissue typing is a new technique which allows the selection of embryos in order to bring about the birth of a child
HUMAN FERTILISATION AND EMBRYOLOGY AUTHORITY REPORT: Introduction 1. Preimplantation tissue typing is a new technique which allows the selection of embryos in order to bring about the birth of a child
Bioethics Education in Professional Science Master s Programs at California State University Channel Islands
 ETHICS AND THE PSM Bioethics Education in Professional Science Master s Programs at California State University Channel Islands Ching-Hua Wang, M.D., Ph.D., Director of MS Biotechnology and Bioinformatics
ETHICS AND THE PSM Bioethics Education in Professional Science Master s Programs at California State University Channel Islands Ching-Hua Wang, M.D., Ph.D., Director of MS Biotechnology and Bioinformatics
First Trimester Screening for Down Syndrome
 First Trimester Screening for Down Syndrome What is first trimester risk assessment for Down syndrome? First trimester screening for Down syndrome, also known as nuchal translucency screening, is a test
First Trimester Screening for Down Syndrome What is first trimester risk assessment for Down syndrome? First trimester screening for Down syndrome, also known as nuchal translucency screening, is a test
1st Session 114 242 STEM CELL THERAPEUTIC AND RESEARCH REAUTHORIZATION ACT OF 2015
 114TH CONGRESS REPORT " HOUSE OF REPRESENTATIVES! 1st Session 114 242 STEM CELL THERAPEUTIC AND RESEARCH REAUTHORIZATION ACT OF 2015 SEPTEMBER 8, 2015. Committed to the Committee of the Whole House on
114TH CONGRESS REPORT " HOUSE OF REPRESENTATIVES! 1st Session 114 242 STEM CELL THERAPEUTIC AND RESEARCH REAUTHORIZATION ACT OF 2015 SEPTEMBER 8, 2015. Committed to the Committee of the Whole House on
Executive summary. Climate change assessments Review of the processes and procedures of the IPCC
 Executive summary Climate change is a long-term challenge that will require every nation to make decisions about how to respond. The Intergovernmental Panel on Climate Change (IPCC) was established by
Executive summary Climate change is a long-term challenge that will require every nation to make decisions about how to respond. The Intergovernmental Panel on Climate Change (IPCC) was established by
Screening for Critical Congenital Heart Disease in the Apparently Healthy Newborn
 Screening for Critical Congenital Heart Disease in the Apparently Healthy Newborn A presentation of Texas Pulse Oximetry Project: A Joint Educational Initiative of The University of Texas Health Science
Screening for Critical Congenital Heart Disease in the Apparently Healthy Newborn A presentation of Texas Pulse Oximetry Project: A Joint Educational Initiative of The University of Texas Health Science
Master of Bioethics Degree Program
 Master of Bioethics Degree Program Offered as a full-time (one-year) or part-time (two-year) course of study This degree program, offered by the Center for Bioethics at Harvard Medical School, offers education,
Master of Bioethics Degree Program Offered as a full-time (one-year) or part-time (two-year) course of study This degree program, offered by the Center for Bioethics at Harvard Medical School, offers education,
Seriously, talk. tell. test. Celiac Disease.
 Seriously, Celiac Disease. talk. If you have celiac disease, your family members might have it too. Talk to them about your experience and how celiac disease runs in families. Tell them the facts. Urge
Seriously, Celiac Disease. talk. If you have celiac disease, your family members might have it too. Talk to them about your experience and how celiac disease runs in families. Tell them the facts. Urge
PREVENTIVE HEALTHCARE GUIDELINES INTRODUCTION
 PREVENTIVE HEALTHCARE GUIDELINES INTRODUCTION Health Plan of Nevada and Sierra Health and Life suggest that health plan members get certain screening tests, exams and shots to stay healthy. This document
PREVENTIVE HEALTHCARE GUIDELINES INTRODUCTION Health Plan of Nevada and Sierra Health and Life suggest that health plan members get certain screening tests, exams and shots to stay healthy. This document
Breathless The Whys and Wherefores Living with Alpha-1-Antitrypsin Deficiency
 Breathless The Whys and Wherefores Living with Deficiency Patient Information Program Breathlessness Short of breath after running the marathon? Short of breath after climbing a flight of stairs? Short
Breathless The Whys and Wherefores Living with Deficiency Patient Information Program Breathlessness Short of breath after running the marathon? Short of breath after climbing a flight of stairs? Short
