mirror image non-superimposable on original molecule
|
|
|
- Britton Carter
- 7 years ago
- Views:
Transcription
1 tereochemistry eading: Wade chapter 5, sections tudy Problems: 5-8, 5-9, 5-0, 5-1, 5-5 Key oncepts and kills: Draw all stereoisomers of a given structure. dentify, diastereomers, and meso compounds; draw correct Fischer projections of asymmetric carbon atoms. Explain how the physical properties differ for different types of stereoisomers, and suggest how to separate different types of stereoisomers Lecture Topics:. onformational isomerism onsider cis-1,-dichlorocyclohexane; each chair conformation is chiral, but a ring flip will convert one stereoisomer to its mirror image. ince chair-chair interconversions are rapid at room temperature. A molecule cannot be optically active if its chiral conformations are in rapid equilibrium with their mirror images. different ring flip mirror image non-superimposable on original molecule consider only: σ identical to mirror image: A correct prediction about whether a molecule is chiral or achiral can be made by analyzing the most symmetrical conformation (in the case of a cyclic molecule, it is the flat conformation) Note that trans 1,-dibromo cyclohexane has chiral chair forms, the mirror images of which cannot be obtained by a ring flip: different consider only: ring flip mirror image non-superimposable on original molecule different from chiral ome molecules have no asymmetric carbon atoms (no centers of chirality) yet they are chiral. This is due to locked conformations of a molecule which are chiral and cannot interconvert because of steric hindrance: axial chirality An example is,,, -substituted biphenyls, in which the most symmetrical flat conformation cannot be obtained because of a very large degree of steric hindrance to rotation about the central - bond: a very high energy is required to rotate past the point
2 of planarity. The molecular conformations are thus locked with the two rings perpendicular to each other. The two conformations are enantiomeric; their mirror images cannot be superimposed. Very large degree of steric hindrance in most symmetrical planar conformation locked conformation ring planes are perpendicular non interconvertible conformations chiral conformations Allene is a molecule with one sp-hybridized carbon and two sp -hybridize carbon atoms. Because of the perpendicular p-orbitals on the central sp-hybridized carbon which overlap to form the pi orbitals, the pi orbitals are also perpendicular, and thus the end groups on the molecule are perpendicular. ubstituted allenes are thus chiral molecules with non-superimposable mirror images: mirror plane different compounds. Fischer Projections Fischer Projections are a shorthand method of drawing stereoisomers to facilitate their comparison when there are multiple asymmetric carbon atoms within a molecule. Fischer projections are drawn in cross form with the horizontal bonds coming toward the viewer and the vertical bonds going away from the viewer. For -lactic acid: Fischer projection Fischer projections that differ by are the same molecule. A 90 rotation, however, is not allowed. Fisher projections also my not be flipped over: they must be kept. same molecule 90 non-superimposable
3 A carbon chain must be drawn along the vertical line of the projection and given UPA numbering from top to bottom When looking at the mirror image of a Fischer projection, any groups that fail to superimpose after a turn indicates chirality and the two molecules are. 1 AL: non-superimposable: not the same molecule! AME MLEULE: AAL Assigning and configurations to Fischer Projections ame rules apply, but if the low priority group () is projecting forward (on a horizontal bond), a counterclockwise rotation indicates configuration and a clockwise rotation indicates configuration; if the low priority group is projecting backward (on a vertical bond) the regular ahn-ngold Prelog rule applies: 1 counterclockwise: projecting forward 1 clockwise: 1 1 clockwise: projecting backward clockwise
4 . are stereoisomers that are not mirror images; they are geometric isomers (like cis-trans isomers about a double bond) and usually compounds containing at least two centers of chirality. Examples: elationships: tereoisomers but not,,,, f the number of stereogenic carbon atoms in a molecule is n, there are a maximum of n possible stereoisomers. Thus, for two stereogenic carbon atoms, there are a maximum of or 4 possible stereoisomers. There are many cases where less than the maximum number of stereoisomers may be found, especially in symmetrical molecules. Example: ame molecule σ "meso-diastereomer",, (±)-diastereomers -stereogenic carbon atoms; a total of stereoisomers for,-dibromobutane! A meso compound is one that is achiral despite having centers of chirality: it usually has some element of symmetry, like an internal mirror plane
5 V. Absolute and elative onfiguration Absolute configuration is the detailed stereochemical picture of a molecule, specifying how the atoms are arranged in space through designating or configurations at each chirality center. elative configuration is the experimentally determined relationship between the configuration of two molecules or two stereogenic carbon atoms within a molecule, even though we may not know the absolute configuration of either. Example: trans,4-dimethylcyclohexanone relative configuration: trans configuration of two methyl groups determined by NM however, we don't know absolute stereochemistry: or V. Physical Properties of diastereomers, unlike, have different physical properties: melting points, boiling points, etc. can thus be separated by ordinary means: chromatography, distillation, crystallization, etc. Enantiomers, which are mirror-image molecules, have virtually identical physical properties and are thus very difficult to separate.. Pure of optically active compounds are often obtained by isolation from natural sources. Examples: carbohydrates (D-(+)-glucose) and amino acids (L-(+)- alanine) Many chemical reactions involve achiral reagents and thus can yield racemic mixtures as products. ow can one separate (resolve) of a given molecule? esolution of Enantiomers olumn hromatography can be employed using a chiral stationary phase as an adsobent: different form diastereomeric complexes with the chiral column packing and move through the column at different rates. n this way separation can be achieved without chemical modification of a racemic mixture. A racemic mixture can also be resolved by reacting the mixture of with a chiral resolving agent, which generates diasteromers that are easily separated. The pure can then be obtained by hydrolysis of the respective diastereomers. We illustrate this process for the formation of diastereomeric esters by reaction of a racemic diacid with a chiral alcohol:
6 inseparable acemic mixture esolving agent separable phenyl ethanol (,,,) + -phenyl ethanol + - hydrolysis: (,,,) + + (,,,) pure enantiomer
7 Additional Problems for practice: 1.) Draw a three-dimensional picture that corresponds to each of the following Fisher projections: a b c.) tate the relationship between each of the following pairs of structures (identical,, diasteromers, constitutional (structural) isomers, or different compounds that are not isomeric) a b c d d
Isomers Have same molecular formula, but different structures
 Isomers ave same molecular formula, but different structures Constitutional Isomers Differ in the order of attachment of atoms (different bond connectivity) Stereoisomers Atoms are connected in the same
Isomers ave same molecular formula, but different structures Constitutional Isomers Differ in the order of attachment of atoms (different bond connectivity) Stereoisomers Atoms are connected in the same
Molecule Projections
 Key Definitions ü Stereochemistry refers to the chemistry in 3 dimensions (greek stereos = solid). This science was created by Pasteur (1860), van Hoff et LeBel (1874). ü Stereisomers are isomeric molecules
Key Definitions ü Stereochemistry refers to the chemistry in 3 dimensions (greek stereos = solid). This science was created by Pasteur (1860), van Hoff et LeBel (1874). ü Stereisomers are isomeric molecules
cyclohexane cyclopentane Nomenclature Follows same rules as for stright-chain alkanes. Examples: name the following
 Structure and Stereochemistry of Alkanes Reading: Wade chapter 3, sections 3-10- 3-16 Study Problems: 3-43, 3-44, 3-45, 3-46 Key oncepts and Skills: ompare the energies of cycloalkanes, and explain ring
Structure and Stereochemistry of Alkanes Reading: Wade chapter 3, sections 3-10- 3-16 Study Problems: 3-43, 3-44, 3-45, 3-46 Key oncepts and Skills: ompare the energies of cycloalkanes, and explain ring
IR Applied to Isomer Analysis
 DiscovIR-LC TM Application Note 025 April 2008 Deposition and Detection System IR Applied to Isomer Analysis Infrared spectra provide valuable information about local configurations of atoms in molecules.
DiscovIR-LC TM Application Note 025 April 2008 Deposition and Detection System IR Applied to Isomer Analysis Infrared spectra provide valuable information about local configurations of atoms in molecules.
methyl RX example primary RX example secondary RX example secondary RX example tertiary RX example
 ucleophilic Substitution & Elimination hemistry 1 eginning patterns to knowfor S and E eactions - horizontal and vertical templates for practice Example 1 - two possible perspectives (deuterium and tritium
ucleophilic Substitution & Elimination hemistry 1 eginning patterns to knowfor S and E eactions - horizontal and vertical templates for practice Example 1 - two possible perspectives (deuterium and tritium
CHEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) Page 1
 CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
Final Examination, Organic Chemistry 1 (CHEM 2210) December 2000 Version *A* A. B. C. D.
 Final Examination, rganic hemistry 1 (EM 2210) December 2000 Version *A* 1. What are the hybridization of, and the geometrical shape around, the nitrogen atom in the following molecule? N 3 3 A. sp, linear
Final Examination, rganic hemistry 1 (EM 2210) December 2000 Version *A* 1. What are the hybridization of, and the geometrical shape around, the nitrogen atom in the following molecule? N 3 3 A. sp, linear
Stereochemistry Tutorial: Drawing Enantiomers and Diastereomers
 tereochemistry Tutorial: Drawing Enantiomers and Diastereomers Definitions for vocabulary words can be found in the Illustrated Glossary of rganic Chemistry, available on the course web site. A. Discussion
tereochemistry Tutorial: Drawing Enantiomers and Diastereomers Definitions for vocabulary words can be found in the Illustrated Glossary of rganic Chemistry, available on the course web site. A. Discussion
Chemistry 1110 Organic Chemistry IUPAC Nomenclature
 hemistry 1110 rganic hemistry IUPA Nomenclature 1 f the approximately 32 million unique chemical compounds presently known, over 95% of them can be classified as organic; i.e., containing carbon. The IUPA
hemistry 1110 rganic hemistry IUPA Nomenclature 1 f the approximately 32 million unique chemical compounds presently known, over 95% of them can be classified as organic; i.e., containing carbon. The IUPA
ORGANIC COMPOUNDS IN THREE DIMENSIONS
 (adapted from Blackburn et al., Laboratory Manual to Accompany World of hemistry, 2 nd ed., (1996) Saunders ollege Publishing: Fort Worth) Purpose: To become familiar with organic molecules in three dimensions
(adapted from Blackburn et al., Laboratory Manual to Accompany World of hemistry, 2 nd ed., (1996) Saunders ollege Publishing: Fort Worth) Purpose: To become familiar with organic molecules in three dimensions
Stereochemistry of Alkanes and Cycloalkanes
 Stereochemistry of Alkanes and ycloalkanes onformation of ethane: Rotation is possible around - Different arrangements of atoms that can be converted into one another by rotation about single bonds are
Stereochemistry of Alkanes and ycloalkanes onformation of ethane: Rotation is possible around - Different arrangements of atoms that can be converted into one another by rotation about single bonds are
1. What is the hybridization of the indicated atom in the following molecule?
 Practice Final Exam, Chemistry 2210, rganic Chem I 1. What is the hybridization of the indicated atom in the following molecule? A. sp 3 B. sp 2 C. sp D. not hybridized 2. Name the functional groups in
Practice Final Exam, Chemistry 2210, rganic Chem I 1. What is the hybridization of the indicated atom in the following molecule? A. sp 3 B. sp 2 C. sp D. not hybridized 2. Name the functional groups in
CHEM 208(Organic Chemistry I) Instructor: Dr. Niranjan Goswami. Tel: (618)545-3361. Email: Ngoswami@kaskaskia.edu. Web: www.kc.cc.il.
 CHEM 208(Organic Chemistry I) Instructor: Dr. Niranjan Goswami Tel: (618)545-3361 Email: Ngoswami@kaskaskia.edu Web: www.kc.cc.il.us/ngoswami CHEM 208 COURSE SYLLABUS KASKASKIA COLLEGE NAME TERM YEAR TEXT:
CHEM 208(Organic Chemistry I) Instructor: Dr. Niranjan Goswami Tel: (618)545-3361 Email: Ngoswami@kaskaskia.edu Web: www.kc.cc.il.us/ngoswami CHEM 208 COURSE SYLLABUS KASKASKIA COLLEGE NAME TERM YEAR TEXT:
Properties of a molecule. Absolute configuration, Cahn-Ingold Prelog. Planar, axial, topological chirality and chirality at atoms other than carbon
 Key stereochemical terminology Stereochemical terminology in organic chemistry can refer to structure of a molecule or to properties of a physical sample of the molecule. It is very important to recognize
Key stereochemical terminology Stereochemical terminology in organic chemistry can refer to structure of a molecule or to properties of a physical sample of the molecule. It is very important to recognize
Conjugation is broken completely by the introduction of saturated (sp3) carbon:
 Chapter 16 Conjugation, resonance, and dienes Conjugation relies on the partial overlap of p-orbitals on adjacent double or triple bonds. A common conjugated system involves 1,3-dienes, such as 1,3-butadiene.
Chapter 16 Conjugation, resonance, and dienes Conjugation relies on the partial overlap of p-orbitals on adjacent double or triple bonds. A common conjugated system involves 1,3-dienes, such as 1,3-butadiene.
Combinatorial Biochemistry and Phage Display
 Combinatorial Biochemistry and Phage Display Prof. Valery A. Petrenko Director - Valery Petrenko Instructors Galina Kouzmitcheva and I-Hsuan Chen Auburn 2006, Spring semester COMBINATORIAL BIOCHEMISTRY
Combinatorial Biochemistry and Phage Display Prof. Valery A. Petrenko Director - Valery Petrenko Instructors Galina Kouzmitcheva and I-Hsuan Chen Auburn 2006, Spring semester COMBINATORIAL BIOCHEMISTRY
Assessment Schedule 2013 Chemistry: Demonstrate understanding of the properties of organic compounds (91391)
 NCEA Level 3 Chemistry (91391) 2013 page 1 of 8 Assessment Schedule 2013 Chemistry: Demonstrate understanding of the properties of organic compounds (91391) Evidence Statement Q Evidence Achievement Achievement
NCEA Level 3 Chemistry (91391) 2013 page 1 of 8 Assessment Schedule 2013 Chemistry: Demonstrate understanding of the properties of organic compounds (91391) Evidence Statement Q Evidence Achievement Achievement
2/10/2011. Stability of Cycloalkanes: Ring Strain. Stability of Cycloalkanes: Ring Strain. 4.3 Stability of Cycloalkanes: Ring Strain
 4.3 Stability of Cycloalkanes: Ring Strain Angle strain The strain induced in a molecule when bond angles are forced to deviate from the ideal 109º tetrahedral value (Adolf von Baeyer 1885) Stability of
4.3 Stability of Cycloalkanes: Ring Strain Angle strain The strain induced in a molecule when bond angles are forced to deviate from the ideal 109º tetrahedral value (Adolf von Baeyer 1885) Stability of
Solid Phase Peptide Synthesis
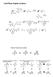 Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
On Factoring Chirality and Stereoisomerism
 Topics in Stereochemisty, Volume 14 Edited by Ernest L. Eliel, Norman L. Allinger, Samuel H. Wilen Copyright 1983 by John Wiley & Sons, Inc. On Factoring Chirality and Stereoisomerism HANS HIRSCHMANN Department
Topics in Stereochemisty, Volume 14 Edited by Ernest L. Eliel, Norman L. Allinger, Samuel H. Wilen Copyright 1983 by John Wiley & Sons, Inc. On Factoring Chirality and Stereoisomerism HANS HIRSCHMANN Department
17.2 REACTIONS INVOLVING ALLYLIC AND BENZYLIC RADICALS
 17. REACTINS INVLVING ALLYLIC AND BENZYLIC RADICALS 793 As Eq. 17. shows, the products derived from the reaction of water at the ring carbons are not formed. The reason is that these products are not aromatic
17. REACTINS INVLVING ALLYLIC AND BENZYLIC RADICALS 793 As Eq. 17. shows, the products derived from the reaction of water at the ring carbons are not formed. The reason is that these products are not aromatic
EXPERIMENT 5: DIPEPTIDE RESEARCH PROJECT
 EXPERIMENT 5: DIPEPTIDE RESEARCH PROJECT Pre-Lab Questions: None. 64 I. Background Information DIPEPTIDE RESEARCH PROJECT Methods developed by organic chemists for the synthesis of biopolymers have had
EXPERIMENT 5: DIPEPTIDE RESEARCH PROJECT Pre-Lab Questions: None. 64 I. Background Information DIPEPTIDE RESEARCH PROJECT Methods developed by organic chemists for the synthesis of biopolymers have had
STANDARD ANSWERS AND DEFINITIONS
 Evidence for Kekule s model to be wrong: STANDARD ANSWERS AND DEFINITIONS All C-C bond lengths are the same length, between C-C and C=C. Only reacts with Br2 with a halogen carrier Benzene is lower in
Evidence for Kekule s model to be wrong: STANDARD ANSWERS AND DEFINITIONS All C-C bond lengths are the same length, between C-C and C=C. Only reacts with Br2 with a halogen carrier Benzene is lower in
PRACTICE PROBLEMS, CHAPTERS 1-3
 PRATIE PRBLEMS, APTERS 1-3 (overed from h. 3: Alkane and Alkyl alide nomenclature only) 1. The atomic number of boron is 5. The correct electronic configuration of boron is: A. 1s 2 2s 3 B. 1s 2 2p 3.
PRATIE PRBLEMS, APTERS 1-3 (overed from h. 3: Alkane and Alkyl alide nomenclature only) 1. The atomic number of boron is 5. The correct electronic configuration of boron is: A. 1s 2 2s 3 B. 1s 2 2p 3.
Sugar Identification using Polarimetry
 Sugar Identification using Polarimetry Safety Concerns: No special safety precautions need to be implemented. Standard organic laboratory safety measures need to be in place. Purpose The purpose of this
Sugar Identification using Polarimetry Safety Concerns: No special safety precautions need to be implemented. Standard organic laboratory safety measures need to be in place. Purpose The purpose of this
Dr.B.R.AMBEDKAR OPEN UNVERSITY FACULTY OF SCIENCE M.Sc. I year -CHEMISTRY (2013-14) Course I: Inorganic Chemistry
 M.Sc. I year -CHEMISTRY (2013-14) Course I: Inorganic Chemistry Maximum Marks 15 Minimum Marks - 06 Section A 1X10=10 Answer any One question from the following Two questions a. What is symmetry operation?
M.Sc. I year -CHEMISTRY (2013-14) Course I: Inorganic Chemistry Maximum Marks 15 Minimum Marks - 06 Section A 1X10=10 Answer any One question from the following Two questions a. What is symmetry operation?
Syllabus for General Organic Chemistry M07A- Fall 2013 Prof. Robert Keil
 Syllabus for General Organic Chemistry M07A- Fall 2013 Prof. Robert Keil Textbook and Materials What you must buy: Organic Chemistry 4 th Ed. Janice G. Smith, McGraw Hill. (Older edition is fine) Chem
Syllabus for General Organic Chemistry M07A- Fall 2013 Prof. Robert Keil Textbook and Materials What you must buy: Organic Chemistry 4 th Ed. Janice G. Smith, McGraw Hill. (Older edition is fine) Chem
An Introduction to Organic Chemistry
 An Introduction to Organic Chemistry 81 Organic Chemistry Organic chemistry is the study of compounds containing carbon with the exception of simple compounds e.g. carbonates (CO 3 2- ), carbon dioxide
An Introduction to Organic Chemistry 81 Organic Chemistry Organic chemistry is the study of compounds containing carbon with the exception of simple compounds e.g. carbonates (CO 3 2- ), carbon dioxide
F322: Chains, Energy and Resources 2.2.4 Alcohols
 F322: hains, Energy and Resources 2.2.4 Alcohols 167 marks 1. This question is about the six alcohols below. butan-2-ol 2-methylpentan-3-ol propan-1-ol ethane-1,2-diol 2-methylpropan-2-ol propan-2-ol Which
F322: hains, Energy and Resources 2.2.4 Alcohols 167 marks 1. This question is about the six alcohols below. butan-2-ol 2-methylpentan-3-ol propan-1-ol ethane-1,2-diol 2-methylpropan-2-ol propan-2-ol Which
Chapter 13 Organic Chemistry
 Chapter 13 Organic Chemistry Introduction Organic chemistry is the study of carbon based compounds. The structural and genetic materials of living organisms are organic compounds. Many of the substances
Chapter 13 Organic Chemistry Introduction Organic chemistry is the study of carbon based compounds. The structural and genetic materials of living organisms are organic compounds. Many of the substances
Chapter 15 Radical Reactions. Radicals are reactive species with a single unpaired electron, formed by
 Chapter 15 Radical Reactions Radicals are reactive species with a single unpaired electron, formed by homolysis of a covalent bond; a radical contains an atom that does not have an octet of electrons,
Chapter 15 Radical Reactions Radicals are reactive species with a single unpaired electron, formed by homolysis of a covalent bond; a radical contains an atom that does not have an octet of electrons,
Chem 11 Oa-Section 2. Exam #2. 1 October 2004
 " Chem 11 Oa-Section 2 Exam #2 1 October 2004 Name: K~ Instructions Please read each question carefully and answer it completely and clearly. Do the problems in the order that is easiest for you. Point
" Chem 11 Oa-Section 2 Exam #2 1 October 2004 Name: K~ Instructions Please read each question carefully and answer it completely and clearly. Do the problems in the order that is easiest for you. Point
the double or triple bond. If the multiple bond is CH 3 C CCHCCH 3
 Alkenes, Alkynes, and Aromatic ompounds Alkenes and Alkynes Unsaturated contain carbon-carbon double and triple bond to which more hydrogen atoms can be added. Alkenes: carbon-carbon double bonds Alkynes:
Alkenes, Alkynes, and Aromatic ompounds Alkenes and Alkynes Unsaturated contain carbon-carbon double and triple bond to which more hydrogen atoms can be added. Alkenes: carbon-carbon double bonds Alkynes:
Principles of Drug Action 1, Spring 2005, Aromatics HYDROCARBON STRUCTURE AND CHEMISTRY: AROMATICS. Jack DeRuiter
 I. Introduction YDOABON STUTUE AND EMISTY: AOMATIS Jack Deuiter ydrocarbons are organic compounds consisting of - and - bonds. arbon has a valence of four and thus requires four electrons or bonds to complete
I. Introduction YDOABON STUTUE AND EMISTY: AOMATIS Jack Deuiter ydrocarbons are organic compounds consisting of - and - bonds. arbon has a valence of four and thus requires four electrons or bonds to complete
2.4 Enantiomeric Isomers
 2.4 Enantiomeric Isomers There is an additional type of isomer that can exist when we have an atom with 4 different groups attached to that atom in tetrahedral geometry. Consider a methane molecule with
2.4 Enantiomeric Isomers There is an additional type of isomer that can exist when we have an atom with 4 different groups attached to that atom in tetrahedral geometry. Consider a methane molecule with
Alkanes. Chapter 1.1
 Alkanes Chapter 1.1 Organic Chemistry The study of carbon-containing compounds and their properties What s so special about carbon? Carbon has 4 bonding electrons. Thus, it can form 4 strong covalent bonds
Alkanes Chapter 1.1 Organic Chemistry The study of carbon-containing compounds and their properties What s so special about carbon? Carbon has 4 bonding electrons. Thus, it can form 4 strong covalent bonds
Chapter 10 Conjugation in Alkadienes and Allylic Systems
 . 0 onjugated Systems hapter 0 onjugation in Alkadienes and Allylic Systems onjugated systems are those in which a π-bond is connected or conjugated (from the Latin conjugare which means to link r yoke
. 0 onjugated Systems hapter 0 onjugation in Alkadienes and Allylic Systems onjugated systems are those in which a π-bond is connected or conjugated (from the Latin conjugare which means to link r yoke
3) How many monosaccharides are connected to each other in a disaccharide? A) 1 B) 2 C) 3 D) 4
 General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
General, Organic, and Biochemistry, 2e (Frost) HOMEWORK Chapter 6 Carbohydrates Life s Sweet Molecules 6.1 Multiple-Choice 1) Which of the following is a polysaccharide? Glucose Sucrose C) Starch D) Maltose
Absolute Structure Absolute Configuration
 Absolute Structure Absolute Configuration Some definitions Absolute Configuration -> spatial arrangement of the atoms for a chiral molecule (R/S, P/M or D/L assignment). Absolute Structure -> spatial arrangement
Absolute Structure Absolute Configuration Some definitions Absolute Configuration -> spatial arrangement of the atoms for a chiral molecule (R/S, P/M or D/L assignment). Absolute Structure -> spatial arrangement
 2814 hains, Rings and Spectroscopy June 2003 Mark Scheme 2814 Mark Scheme June 2003 The following annotations may be used when marking: X = incorrect response (errors may also be underlined) ^ = omission
2814 hains, Rings and Spectroscopy June 2003 Mark Scheme 2814 Mark Scheme June 2003 The following annotations may be used when marking: X = incorrect response (errors may also be underlined) ^ = omission
Chapter 22 Carbonyl Alpha-Substitution Reactions
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 22 Carbonyl Alpha-Substitution Reactions The α Position The carbon next to the carbonyl group is designated as being in the α position Electrophilic
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 22 Carbonyl Alpha-Substitution Reactions The α Position The carbon next to the carbonyl group is designated as being in the α position Electrophilic
11.4 NUCLEOPHILIC SUBSTITUTION REACTIONS OF EPOXIDES
 .4 NUEPII SUBSTITUTIN REATINS F EPXIDES 495 (d When tert-butyl methyl ether is heated with sulfuric acid, methanol and -methylpropene distill from the solution. (e Tert-butyl methyl ether cleaves much
.4 NUEPII SUBSTITUTIN REATINS F EPXIDES 495 (d When tert-butyl methyl ether is heated with sulfuric acid, methanol and -methylpropene distill from the solution. (e Tert-butyl methyl ether cleaves much
CHEM 101 Exam 4. Page 1
 CEM 101 Exam 4 Form 1 (White) November 30, 2001 Page 1 Section This exam consists of 8 pages. When the exam begins make sure you have one of each. Print your name at the top of each page now. Show your
CEM 101 Exam 4 Form 1 (White) November 30, 2001 Page 1 Section This exam consists of 8 pages. When the exam begins make sure you have one of each. Print your name at the top of each page now. Show your
The Synthesis of trans-dichlorobis(ethylenediamine)cobalt(iii) Chloride
 CHEM 122L General Chemistry Laboratory Revision 2.0 The Synthesis of trans-dichlorobis(ethylenediamine)cobalt(iii) Chloride To learn about Coordination Compounds and Complex Ions. To learn about Isomerism.
CHEM 122L General Chemistry Laboratory Revision 2.0 The Synthesis of trans-dichlorobis(ethylenediamine)cobalt(iii) Chloride To learn about Coordination Compounds and Complex Ions. To learn about Isomerism.
Quality. Now Certified to ISO 9001:2008
 Quality Now Certified to ISO 90012008 Quality Policy It is Peptides International's goal is to achieve complete customer satisfaction by addressing customer needs and delivering what we promise. The company
Quality Now Certified to ISO 90012008 Quality Policy It is Peptides International's goal is to achieve complete customer satisfaction by addressing customer needs and delivering what we promise. The company
Chapter 4 Lecture Notes
 Chapter 4 Lecture Notes Chapter 4 Educational Goals 1. Given the formula of a molecule, the student will be able to draw the line-bond (Lewis) structure. 2. Understand and construct condensed structural
Chapter 4 Lecture Notes Chapter 4 Educational Goals 1. Given the formula of a molecule, the student will be able to draw the line-bond (Lewis) structure. 2. Understand and construct condensed structural
Molecular Structures. Chapter 9 Molecular Structures. Using Molecular Models. Using Molecular Models. C 2 H 6 O structural isomers: .. H C C O..
 John W. Moore onrad L. Stanitski Peter. Jurs http://academic.cengage.com/chemistry/moore hapter 9 Molecular Structures Stephen. oster Mississippi State University Molecular Structures 2 6 structural isomers:
John W. Moore onrad L. Stanitski Peter. Jurs http://academic.cengage.com/chemistry/moore hapter 9 Molecular Structures Stephen. oster Mississippi State University Molecular Structures 2 6 structural isomers:
1.15 Bonding in Methane and Orbital Hybridization
 1.15 Bonding in Methane and Orbital Hybridization Structure of Methane tetrahedral bond angles = 109.5 bond distances = 110 pm but structure seems inconsistent with electron configuration of carbon Electron
1.15 Bonding in Methane and Orbital Hybridization Structure of Methane tetrahedral bond angles = 109.5 bond distances = 110 pm but structure seems inconsistent with electron configuration of carbon Electron
Chapter 5 Classification of Organic Compounds by Solubility
 Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Molecular Orbital Theory
 Molecular Orbital Theory To date, we have looked at three different theories of molecular boning. They are the VSEPR Theory (with Lewis Dot Structures), the Valence Bond Theory (with hybridization) and
Molecular Orbital Theory To date, we have looked at three different theories of molecular boning. They are the VSEPR Theory (with Lewis Dot Structures), the Valence Bond Theory (with hybridization) and
Chapter 10. Conjugation in Alkadienes and Allylic Systems. Class Notes. B. The allyl group is both a common name and an accepted IUPAC name
 Chapter 10 Conjugation in Alkadienes and Allylic Systems Chapter 10 suggested problems: I. The allyl group Class Notes A. B. The allyl group is both a common name and an accepted IUPAC name 1. Allyl alcohol
Chapter 10 Conjugation in Alkadienes and Allylic Systems Chapter 10 suggested problems: I. The allyl group Class Notes A. B. The allyl group is both a common name and an accepted IUPAC name 1. Allyl alcohol
Molecular Geometry and VSEPR We gratefully acknowledge Portland Community College for the use of this experiment.
 Molecular and VSEPR We gratefully acknowledge Portland ommunity ollege for the use of this experiment. Objectives To construct molecular models for covalently bonded atoms in molecules and polyatomic ions
Molecular and VSEPR We gratefully acknowledge Portland ommunity ollege for the use of this experiment. Objectives To construct molecular models for covalently bonded atoms in molecules and polyatomic ions
Chapter 12 Organic Compounds with Oxygen and Sulfur
 Chapter 12 Organic Compounds with Oxygen and Sulfur 1 Alcohols An alcohol contains a hydroxyl group ( OH) that replaces a hydrogen atom in a hydrocarbon. A phenol contains a hydroxyl group ( OH) attached
Chapter 12 Organic Compounds with Oxygen and Sulfur 1 Alcohols An alcohol contains a hydroxyl group ( OH) that replaces a hydrogen atom in a hydrocarbon. A phenol contains a hydroxyl group ( OH) attached
Examination of Proton NMR Spectra
 Examination of Proton NMR Spectra What to Look For 1) Number of Signals --- indicates how many "different kinds" of protons are present. 2) Positions of the Signals --- indicates something about magnetic
Examination of Proton NMR Spectra What to Look For 1) Number of Signals --- indicates how many "different kinds" of protons are present. 2) Positions of the Signals --- indicates something about magnetic
Chapter 10 Molecular Geometry and Chemical Bonding Theory
 Chem 1: Chapter 10 Page 1 Chapter 10 Molecular Geometry and Chemical Bonding Theory I) VSEPR Model Valence-Shell Electron-Pair Repulsion Model A) Model predicts Predicts electron arrangement and molecular
Chem 1: Chapter 10 Page 1 Chapter 10 Molecular Geometry and Chemical Bonding Theory I) VSEPR Model Valence-Shell Electron-Pair Repulsion Model A) Model predicts Predicts electron arrangement and molecular
Chapter 9. Chemical reactivity of molecules depends on the nature of the bonds between the atoms as well on its 3D structure
 Chapter 9 Molecular Geometry & Bonding Theories I) Molecular Geometry (Shapes) Chemical reactivity of molecules depends on the nature of the bonds between the atoms as well on its 3D structure Molecular
Chapter 9 Molecular Geometry & Bonding Theories I) Molecular Geometry (Shapes) Chemical reactivity of molecules depends on the nature of the bonds between the atoms as well on its 3D structure Molecular
CHEM 322 Organic Chemistry II - Professor Kathleen V. Kilway
 CHEM 322 Organic Chemistry II - Professor Kathleen V. Kilway "Organic Chemistry" by Maitland Jones, 4th edition Chapter 12 Homework: 1, 2, 4, 5, 6, 7, 15, 16, 17, 19, 21, 24, 26, 28, 29, 30, 38, 39, 44,
CHEM 322 Organic Chemistry II - Professor Kathleen V. Kilway "Organic Chemistry" by Maitland Jones, 4th edition Chapter 12 Homework: 1, 2, 4, 5, 6, 7, 15, 16, 17, 19, 21, 24, 26, 28, 29, 30, 38, 39, 44,
A Grignard reagent formed would deprotonate H of the ethyl alcohol OH.
 216 S11-E2 Page 2 Name Key I. (9 points) Answer in the boxes below the following questions for the Grignard reagent C 3 -Mg. (1) (2 points) Is the carbon atom associated with magnesium electrophilic or
216 S11-E2 Page 2 Name Key I. (9 points) Answer in the boxes below the following questions for the Grignard reagent C 3 -Mg. (1) (2 points) Is the carbon atom associated with magnesium electrophilic or
PROTEINS THE PEPTIDE BOND. The peptide bond, shown above enclosed in the blue curves, generates the basic structural unit for proteins.
 Ca 2+ The contents of this module were developed under grant award # P116B-001338 from the Fund for the Improvement of Postsecondary Education (FIPSE), United States Department of Education. However, those
Ca 2+ The contents of this module were developed under grant award # P116B-001338 from the Fund for the Improvement of Postsecondary Education (FIPSE), United States Department of Education. However, those
Boston University Dresden Science Program ORGANIC CHEMISTRY CAS CH 203 Lecture
 Boston University Dresden Science Program ORGANIC CHEMISTRY CAS CH 203 Lecture Instructor: Professor Wolf D. Habicher, Professor Claus Rüger Meeting Times Lectures: twice a week at 90 minutes each Discussions:
Boston University Dresden Science Program ORGANIC CHEMISTRY CAS CH 203 Lecture Instructor: Professor Wolf D. Habicher, Professor Claus Rüger Meeting Times Lectures: twice a week at 90 minutes each Discussions:
Ionization of amino acids
 Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
Unit Vocabulary: o Organic Acid o Alcohol. o Ester o Ether. o Amine o Aldehyde
 Unit Vocabulary: Addition rxn Esterification Polymer Alcohol Ether Polymerization Aldehyde Fermentation Primary Alkane Functional group Saponification Alkene Halide (halocarbon) Saturated hydrocarbon Alkyne
Unit Vocabulary: Addition rxn Esterification Polymer Alcohol Ether Polymerization Aldehyde Fermentation Primary Alkane Functional group Saponification Alkene Halide (halocarbon) Saturated hydrocarbon Alkyne
Ultraviolet Spectroscopy
 Ultraviolet Spectroscopy The wavelength of UV and visible light are substantially shorter than the wavelength of infrared radiation. The UV spectrum ranges from 100 to 400 nm. A UV-Vis spectrophotometer
Ultraviolet Spectroscopy The wavelength of UV and visible light are substantially shorter than the wavelength of infrared radiation. The UV spectrum ranges from 100 to 400 nm. A UV-Vis spectrophotometer
Chapter 2 Polar Covalent Bond Covalent bond in which the electron pairs are not shared equally.
 hapter 2 Polar ovalent Bond ovalent bond in which the electron pairs are not shared equally. Pure ovalent Bond (non-polar) increasing bond polarity Ionic Bond X X X Y X + Y - Electronegativity, c ability
hapter 2 Polar ovalent Bond ovalent bond in which the electron pairs are not shared equally. Pure ovalent Bond (non-polar) increasing bond polarity Ionic Bond X X X Y X + Y - Electronegativity, c ability
Determination of Molecular Structure by MOLECULAR SPECTROSCOPY
 Determination of Molecular Structure by MOLEULAR SPETROSOPY hemistry 3 B.Z. Shakhashiri Fall 29 Much of what we know about molecular structure has been learned by observing and analyzing how electromagnetic
Determination of Molecular Structure by MOLEULAR SPETROSOPY hemistry 3 B.Z. Shakhashiri Fall 29 Much of what we know about molecular structure has been learned by observing and analyzing how electromagnetic
CORK INSTITUTE OF TECHNOLOGY INSTITIÚID TEICNEOLAÍOCHTA CHORCAÍ
 CORK INSTITUTE OF TECHNOLOGY INSTITIÚID TEICNEOLAÍOCHTA CHORCAÍ Module Title: Topics in Organic Chemistry Module Code: CHEO 7003 School : Science Programme Title: Bachelor of Science in Analytical & Pharmaceutical
CORK INSTITUTE OF TECHNOLOGY INSTITIÚID TEICNEOLAÍOCHTA CHORCAÍ Module Title: Topics in Organic Chemistry Module Code: CHEO 7003 School : Science Programme Title: Bachelor of Science in Analytical & Pharmaceutical
Figure 3-1-1: Alkaline hydrolysis (saponification) of oil to make soaps.
 Chapter 3 CHEMICAL MODIFICATION OF OILS AND FATS From the fats and oils obtained from natural resources, the majority of them are used directly or just after refinement. While the others are used after
Chapter 3 CHEMICAL MODIFICATION OF OILS AND FATS From the fats and oils obtained from natural resources, the majority of them are used directly or just after refinement. While the others are used after
18.2 Protein Structure and Function: An Overview
 18.2 Protein Structure and Function: An Overview Protein: A large biological molecule made of many amino acids linked together through peptide bonds. Alpha-amino acid: Compound with an amino group bonded
18.2 Protein Structure and Function: An Overview Protein: A large biological molecule made of many amino acids linked together through peptide bonds. Alpha-amino acid: Compound with an amino group bonded
Molecular Models Experiment #1
 Molecular Models Experiment #1 Objective: To become familiar with the 3-dimensional structure of organic molecules, especially the tetrahedral structure of alkyl carbon atoms and the planar structure of
Molecular Models Experiment #1 Objective: To become familiar with the 3-dimensional structure of organic molecules, especially the tetrahedral structure of alkyl carbon atoms and the planar structure of
Survival Organic Chemistry Part I: Molecular Models
 Survival Organic Chemistry Part I: Molecular Models The goal in this laboratory experience is to get you so you can easily and quickly move between empirical formulas, molecular formulas, condensed formulas,
Survival Organic Chemistry Part I: Molecular Models The goal in this laboratory experience is to get you so you can easily and quickly move between empirical formulas, molecular formulas, condensed formulas,
SHAPES OF MOLECULES (VSEPR MODEL)
 1 SAPES MLEULES (VSEPR MDEL) Valence Shell Electron-Pair Repulsion model - Electron pairs surrounding atom spread out as to minimize repulsion. - Electron pairs can be bonding pairs (including multiple
1 SAPES MLEULES (VSEPR MDEL) Valence Shell Electron-Pair Repulsion model - Electron pairs surrounding atom spread out as to minimize repulsion. - Electron pairs can be bonding pairs (including multiple
Hybrid Molecular Orbitals
 Hybrid Molecular Orbitals Last time you learned how to construct molecule orbital diagrams for simple molecules based on the symmetry of the atomic orbitals. Molecular orbitals extend over the entire molecule
Hybrid Molecular Orbitals Last time you learned how to construct molecule orbital diagrams for simple molecules based on the symmetry of the atomic orbitals. Molecular orbitals extend over the entire molecule
Draft agreed by the QWP February 2015. Draft adopted by the CHMP for release for consultation March 2015. Draft endorsed by the CMD(h) March 2015
 1 2 3 26 March 2015 EMA/CHMP/QWP/104223/2015 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Reflection paper on the chemical structure and properties criteria to be considered for the evaluation
1 2 3 26 March 2015 EMA/CHMP/QWP/104223/2015 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Reflection paper on the chemical structure and properties criteria to be considered for the evaluation
Synthesis of Isopentyl Acetate
 Experiment 8 Synthesis of Isopentyl Acetate Objectives To prepare isopentyl acetate from isopentyl alcohol and acetic acid by the Fischer esterification reaction. Introduction Esters are derivatives of
Experiment 8 Synthesis of Isopentyl Acetate Objectives To prepare isopentyl acetate from isopentyl alcohol and acetic acid by the Fischer esterification reaction. Introduction Esters are derivatives of
Bonding Models. Bonding Models (Lewis) Bonding Models (Lewis) Resonance Structures. Section 2 (Chapter 3, M&T) Chemical Bonding
 Bonding Models Section (Chapter, M&T) Chemical Bonding We will look at three models of bonding: Lewis model Valence Bond model M theory Bonding Models (Lewis) Bonding Models (Lewis) Lewis model of bonding
Bonding Models Section (Chapter, M&T) Chemical Bonding We will look at three models of bonding: Lewis model Valence Bond model M theory Bonding Models (Lewis) Bonding Models (Lewis) Lewis model of bonding
Page 1. 6. Which hydrocarbon is a member of the alkane series? (1) 1. Which is the structural formula of methane? (1) (2) (2) (3) (3) (4) (4)
 1. Which is the structural formula of methane? 6. Which hydrocarbon is a member of the alkane series? 7. How many carbon atoms are contained in an ethyl group? 1 3 2 4 2. In the alkane series, each molecule
1. Which is the structural formula of methane? 6. Which hydrocarbon is a member of the alkane series? 7. How many carbon atoms are contained in an ethyl group? 1 3 2 4 2. In the alkane series, each molecule
An Adventure in Stereochemistry: Alice in Mirror Image Land*
 An Adventure in Stereochemistry: Alice in Mirror Image Land* by Frank J. Dinan Department of Chemistry and Biochemistry Canisius College, Buffalo, NY Gordon T. Yee Department of Chemistry Virginia Polytechnic
An Adventure in Stereochemistry: Alice in Mirror Image Land* by Frank J. Dinan Department of Chemistry and Biochemistry Canisius College, Buffalo, NY Gordon T. Yee Department of Chemistry Virginia Polytechnic
Answer Key, Problem Set 11
 Chemistry 122 Mines, Spring 2010 Answer Key, Problem Set 11 1. Write the electron configuration for each of the following atoms and ions (use the noble gas abbreviation): (a) Mn: [Ar] 4s 2 3d 5 (Note:
Chemistry 122 Mines, Spring 2010 Answer Key, Problem Set 11 1. Write the electron configuration for each of the following atoms and ions (use the noble gas abbreviation): (a) Mn: [Ar] 4s 2 3d 5 (Note:
Where Is My Lone Pair?
 Where Is My Lone Pair? Goal: In this tutorial we'll learn how to determine which orbital contains a lone pair. This is important for resonance, conjugation, and aromaticity. To master this subject you'll
Where Is My Lone Pair? Goal: In this tutorial we'll learn how to determine which orbital contains a lone pair. This is important for resonance, conjugation, and aromaticity. To master this subject you'll
Q.1 Carbonyl compounds are formed by oxidation of alcohols;
 arbonyl compounds 814 1 ARBYL MPUDS - Aldehydes and Ketones Q.1 arbonyl compounds are formed by oxidation of alcohols; a) Which type of alcohol is oxidised to an aldehyde? b) Which type of alcohol is oxidised
arbonyl compounds 814 1 ARBYL MPUDS - Aldehydes and Ketones Q.1 arbonyl compounds are formed by oxidation of alcohols; a) Which type of alcohol is oxidised to an aldehyde? b) Which type of alcohol is oxidised
Name Lab #3: Solubility of Organic Compounds Objectives: Introduction: soluble insoluble partially soluble miscible immiscible
 Lab #3: Solubility of rganic Compounds bjectives: - Understanding the relative solubility of organic compounds in various solvents. - Exploration of the effect of polar groups on a nonpolar hydrocarbon
Lab #3: Solubility of rganic Compounds bjectives: - Understanding the relative solubility of organic compounds in various solvents. - Exploration of the effect of polar groups on a nonpolar hydrocarbon
Suggested solutions for Chapter 7
 s for Chapter 7 7 PRBLEM 1 Are these molecules conjugated? Explain your answer in any reasonable way. C Et C Et C Et Revision of the basic kinds of conjugation and how to show conjugation with curly arrows.
s for Chapter 7 7 PRBLEM 1 Are these molecules conjugated? Explain your answer in any reasonable way. C Et C Et C Et Revision of the basic kinds of conjugation and how to show conjugation with curly arrows.
Ch18_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch18_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following can be classified as biomolecules except A) lipids. B) proteins. C)
Ch18_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) All of the following can be classified as biomolecules except A) lipids. B) proteins. C)
But in organic terms: Oxidation: loss of H 2 ; addition of O or O 2 ; addition of X 2 (halogens).
 Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
TRANSITION METALS AND COORDINATION CHEMISTRY
 CHAPTER TWENTY-ONE TRANSITION METALS AND COORDINATION CHEMISTRY For Review 1. Chromium ([Ar]:4s 0 3d 5 ) and copper [Ar]:4s 1 3d 10 ) have electron configurations which are different from that predicted
CHAPTER TWENTY-ONE TRANSITION METALS AND COORDINATION CHEMISTRY For Review 1. Chromium ([Ar]:4s 0 3d 5 ) and copper [Ar]:4s 1 3d 10 ) have electron configurations which are different from that predicted
Bonding & Molecular Shape Ron Robertson
 Bonding & Molecular Shape Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\00bondingtrans.doc The Nature of Bonding Types 1. Ionic 2. Covalent 3. Metallic 4. Coordinate covalent Driving
Bonding & Molecular Shape Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\00bondingtrans.doc The Nature of Bonding Types 1. Ionic 2. Covalent 3. Metallic 4. Coordinate covalent Driving
Solving Spectroscopy Problems
 Solving Spectroscopy Problems The following is a detailed summary on how to solve spectroscopy problems, key terms are highlighted in bold and the definitions are from the illustrated glossary on Dr. Hardinger
Solving Spectroscopy Problems The following is a detailed summary on how to solve spectroscopy problems, key terms are highlighted in bold and the definitions are from the illustrated glossary on Dr. Hardinger
Lecture 8 : Coordinate Geometry. The coordinate plane The points on a line can be referenced if we choose an origin and a unit of 20
 Lecture 8 : Coordinate Geometry The coordinate plane The points on a line can be referenced if we choose an origin and a unit of 0 distance on the axis and give each point an identity on the corresponding
Lecture 8 : Coordinate Geometry The coordinate plane The points on a line can be referenced if we choose an origin and a unit of 0 distance on the axis and give each point an identity on the corresponding
Nuclear Magnetic Resonance Spectroscopy
 Nuclear Magnetic Resonance Spectroscopy Introduction NMR is the most powerful tool available for organic structure determination. It is used to study a wide variety of nuclei: 1 H 13 C 15 N 19 F 31 P 2
Nuclear Magnetic Resonance Spectroscopy Introduction NMR is the most powerful tool available for organic structure determination. It is used to study a wide variety of nuclei: 1 H 13 C 15 N 19 F 31 P 2
Q.1 Draw out some suitable structures which fit the molecular formula C 6 H 6
 Aromatic compounds GE 1 BENZENE Structure Primary analysis revealed benzene had an... empirical formula of and a molecular formula of 6 6 Q.1 Draw out some suitable structures which fit the molecular formula
Aromatic compounds GE 1 BENZENE Structure Primary analysis revealed benzene had an... empirical formula of and a molecular formula of 6 6 Q.1 Draw out some suitable structures which fit the molecular formula
Chapter 1 Structure and Bonding. Modified by Dr. Daniela Radu
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 1 Structure and Bonding Modified by Dr. Daniela Radu What is Organic Chemistry? Living things are made of organic chemicals Proteins that make
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 1 Structure and Bonding Modified by Dr. Daniela Radu What is Organic Chemistry? Living things are made of organic chemicals Proteins that make
Molecular Geometry and Chemical Bonding Theory
 Chapter 10 Molecular Geometry and Chemical Bonding Theory Concept Check 10.1 An atom in a molecule is surrounded by four pairs of electrons, one lone pair and three bonding pairs. Describe how the four
Chapter 10 Molecular Geometry and Chemical Bonding Theory Concept Check 10.1 An atom in a molecule is surrounded by four pairs of electrons, one lone pair and three bonding pairs. Describe how the four
Carbon-Carbon bonds: Hybridization
 Carbon-Carbon bonds: Hybridization Abstract: Gina 05/05/11 Molecular binding behavior has a large inuence on the structure of a material and their properties. As a exclusion, carbon bind themself not in
Carbon-Carbon bonds: Hybridization Abstract: Gina 05/05/11 Molecular binding behavior has a large inuence on the structure of a material and their properties. As a exclusion, carbon bind themself not in
Course Prerequisite: Chemistry 141 or 143.
 Instructor: Matthias Brewer; Office: Cook A316; email: Matthias.Brewer@uvm.edu BlackBoard Site: bb.uvm.edu Lecture: 10:40am 11:30am MWF, Angell B106 Review Sessions: 5:30pm Thur., Angell B106 Laboratory
Instructor: Matthias Brewer; Office: Cook A316; email: Matthias.Brewer@uvm.edu BlackBoard Site: bb.uvm.edu Lecture: 10:40am 11:30am MWF, Angell B106 Review Sessions: 5:30pm Thur., Angell B106 Laboratory
Covalent Bonding and Molecular Geometry
 Name Section # Date of Experiment Covalent Bonding and Molecular Geometry When atoms combine to form molecules (this also includes complex ions) by forming covalent bonds, the relative positions of the
Name Section # Date of Experiment Covalent Bonding and Molecular Geometry When atoms combine to form molecules (this also includes complex ions) by forming covalent bonds, the relative positions of the
Peptide Bond Amino acids are linked together by peptide bonds to form polypepetide chain.
 Peptide Bond Peptide Bond Amino acids are linked together by peptide bonds to form polypepetide chain. + H 2 O 2 Peptide bonds are strong and not broken by conditions that denature proteins, such as heating.
Peptide Bond Peptide Bond Amino acids are linked together by peptide bonds to form polypepetide chain. + H 2 O 2 Peptide bonds are strong and not broken by conditions that denature proteins, such as heating.
Enantiomers: Synthesis, characterization, and resolution of tris(ethylenediamine)cobalt(iii) chloride Introduction:
 Enantiomers: Synthesis, characterization, and resolution of tris(ethylenediamine)cobalt(iii) chloride Introduction: The development of coordination chemistry prior to 1950 involved the synthesis and characterization
Enantiomers: Synthesis, characterization, and resolution of tris(ethylenediamine)cobalt(iii) chloride Introduction: The development of coordination chemistry prior to 1950 involved the synthesis and characterization
Draft agreed by the QWP February 2015. Draft adopted by the CHMP for release for consultation March 2015. Draft endorsed by the CMD(h) March 2015
 17 December 2015 EMA/CHMP/QWP/104223/2015 Committee for Medicinal Products for Human Use (CHMP) Reflection paper on the chemical structure and properties criteria to be considered for the evaluation of
17 December 2015 EMA/CHMP/QWP/104223/2015 Committee for Medicinal Products for Human Use (CHMP) Reflection paper on the chemical structure and properties criteria to be considered for the evaluation of
EXPERIMENT 3 (Organic Chemistry II) Nitration of Aromatic Compounds: Preparation of methyl-m-nitrobenzoate
 EXPERIMENT 3 (Organic Chemistry II) Nitration of Aromatic Compounds: Preparation of methyl-m-nitrobenzoate Pahlavan/Cherif Purpose a) Study electrophilic aromatic substitution reaction (EAS) b) Study regioselectivity
EXPERIMENT 3 (Organic Chemistry II) Nitration of Aromatic Compounds: Preparation of methyl-m-nitrobenzoate Pahlavan/Cherif Purpose a) Study electrophilic aromatic substitution reaction (EAS) b) Study regioselectivity
Prentice Hall. Chemistry (Wilbraham) 2008, National Student Edition - South Carolina Teacher s Edition. High School. High School
 Prentice Hall Chemistry (Wilbraham) 2008, National Student Edition - South Carolina Teacher s Edition High School C O R R E L A T E D T O High School C-1.1 Apply established rules for significant digits,
Prentice Hall Chemistry (Wilbraham) 2008, National Student Edition - South Carolina Teacher s Edition High School C O R R E L A T E D T O High School C-1.1 Apply established rules for significant digits,
