POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
|
|
|
- Loren Byrd
- 7 years ago
- Views:
Transcription
1 Original Issue Date (Created): 1/1/2012 Most Recent Review Date (Revised): 9/27/2016 Effective Date: 12/1/2016 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY I. POLICY TOP Semi-implantable and fully implantable middle ear hearing aids are considered investigational. There is insufficient evidence to support a conclusion concerning the health outcomes or benefits associated with this procedure. Cross-Reference MP Implantable Bone Conduction and Bone-Anchored Hearing Prosthetic Devices MP Cochlear Implants MP Auditory Brain Stem Implants II. PRODUCT VARIATIONS TOP This policy is applicable to all programs and products administered by Capital BlueCross unless otherwise indicated below. FEP PPO* * Refer to FEP Medical Policy Manual MP Semi-Implantable and Fully Implantable Middle Ear Hearing Aid. The FEP Medical Policy manual can be found at: Page 1
2 III. DESCRIPTION/BACKGROUND TOP Hearing loss is described as conductive, sensorineural, or mixed, and can be unilateral or bilateral. Normal hearing is the detection of sound at or below 20 decibels (db). The American Speech Language- Hearing Association has defined the degree of hearing loss based on pure tone average (PTA) detection thresholds as mild (20-40 db), moderate (40-60 db), severe (60-80 db), and profound ( 80 db). Sound amplification through the use of an air-conduction hearing aid can provide benefit to patients with sensorineural, conductive, or mixed hearing loss. Contralateral routing of signal is a system in which a microphone on the affected side transmits a signal to an air-conduction hearing aid on the normal or less affected side. Patients with moderate-to-severe sensorineural hearing loss are typically fitted with external acoustic hearing aids. Conductive hearing loss may be treated with acoustic or bone conduction hearing aids when surgical or medical interventions are unable to correct hearing loss. However, these hearing aids may not be acceptable to patients, either due to issues related to anatomic fit, sound quality, or personal preference. In some cases, external acoustic hearing aids cannot be used due to external ear pathologies (e.g., otitis externa). Semi-implantable and fully implantable middle ear hearing aids have been developed as an alternative to external acoustic hearing aids. Two semi-implantable devices have Food and Drug Administration (FDA) approval: the Vibrant Soundbridge (MED-EL Corp.) and the Maxum System (Ototronix). The devices consist of 3 components: a magnetic component that is implanted onto the ossicles of the middle ear, a receiver, and a sound processor. The Soundbridge device is implanted subcutaneously behind the ear while the processor is worn externally on the scalp over the receiver unit and held in place by a magnet. The Maxum System device is placed in the user s ear canal while the processor rests over the external ear. In general, the sound processor receives and amplifies the sound vibrations and transforms the sound pressure into electrical signals that are received by the receiver unit. The receiver unit then transduces these electrical signals into electromagnetic energy and creates an alternating electromagnetic field with the magnetic component (floating mass transducer) implanted on the ossicles of the middle ear. This electromagnetic field results in attractive and repulsive forces on the magnetic implant, causing vibration of the bones of the middle ear similar to normal hearing. One fully implantable middle ear hearing aid has FDA approval: the Esteem Implantable Hearing System (Envoy Medical). Similar to the semi-implantable devices, the fully implantable device consists of a sensor, a sound processor, and a driver connected to the ossicles. The sensor detects vibrations of the tympanic membrane and transforms the vibrations into electrical signals that are processed by the sound processor. The processor transduces these signals via piezoelectric transduction, as opposed to the electromagnetic transduction used in the semiimplantable devices. A piezoelectric transducer, the sensor, is placed at the head of the incus and converts mechanical vibrations detected from the tympanic membrane into electrical signals that are delivered to the stapes by another piezoelectric transducer, the driver. Page 2
3 REGULATORY STATUS Two semi-implantable devices were approved by the U.S. Food and Drug Administration (FDA) through the premarket approval process: the Vibrant Soundbridge in August 2000 and the Soundtec Direct System in September The Soundtec was discontinued by the manufacturer Ototronix in 2004 due to performance issues; it was re-released in 2009 under the name Maxum System. The FDA labeling approved for both devices states that the devices are intended for use in adults, 18 years of age or older, who have a moderate to severe sensorineural hearing loss and desire an alternative to an acoustic hearing aid." FDA product code: MPV. In March 2010, the Esteem Implantable Hearing System (Envoy Medical, St. Paul, MN), a fully implantable middle ear hearing aid, was approved by FDA through the premarket approval process. FDA-approved labeling for the Esteem hearing implant indicates it is intended to alleviate hearing loss... in adults 18 years of age or older with stable bilateral sensorineural hearing loss. FDA product code: OAF. Another fully implantable middle ear hearing aid, the Carina Fully Implantable Hearing Device, was in development (Otologics, Boulder, CO), but does not have FDA approval. IV. RATIONALE TOP The most recent literature review covers the period through December 17, Externally worn acoustic hearing aids are widely accepted devices for patients with hearing loss. Therefore this review of semi-implantable and fully implantable hearing aids focuses on various audiologic measures achieved with an externally worn hearing aid compared with a semi- or fully implantable hearing aid in the same patient. Studies of semi- and fully implantable middle ear hearing aids frequently report patient preference for an implantable device compared with an externally worn device. However, it must be determined to what extent patient preference is based on convenience compared with preference based on improved hearing. Only minimal safety concerns are related to external hearing aids. In contrast, an implantable hearing aid requires a surgical procedure for implantation. Potential risks cited for semi-implantable middle ear hearing aids include decrease in residual hearing in the implanted ear, infection in the ear and adjacent structures, and risks associated with general anesthesia. Major ear surgery may also result in numbness, swelling, or discomfort around the ear, the possibility of facial paresis, neck pain, and disturbance of balance and taste. Therefore, equivalency or improvement in audiologic outcomes associated with an implantable hearing aid must be balanced against the potential risks inherent in a surgical procedure. Page 3
4 Semi-Implantable Middle Ear Hearing Aids Trials Supporting Food and Drug Administration Approval of Semi-Implantable Hearing Aids The U.S. Food and Drug Administration (FDA) approval of the Soundbridge and Soundtec (now marketed as the Maxum System) devices was based in part on clinical trials of 53 and 108 patients, respectively, who had moderate-to-severe sensorineural hearing loss and who were dissatisfied with their existing external acoustic hearing aids. Results of these trials are available in the FDA Summaries of Safety and Effectiveness. 1,2 The results of the Soundbridge and Soundtec trials have also been reported in the peer-reviewed published literature. 3 The principal outcome measures were the audiologic outcomes before (with the hearing aid in use) and after the implant. The following audiologic outcomes were reported: functional gain, speech recognition, patient assessments, and safety. Each is discussed below. Functional Gain Functional gain is defined as the difference in sound field threshold (measured in decibels [db]) and is an indicator of functional benefit from an amplification device. For the Soundbridge device, the improvement in functional gain was 14.1 db, while for the Soundtec device, it was 7.9 db; both are considered a modest improvement. The clinical significance of this improvement is difficult to determine. For example, this level of improvement may be more clinically significant in patients with moderate hearing loss, for whom a 14-dB improvement in threshold might move them into the normal range for the spoken voice. Speech Recognition Speech recognition is assessed using the Speech Perception in Noise (SPIN) test and the Northwestern University 6 (NU-6) test, which consists of a 50-item word list. For the Soundbridge device, no significant difference in word recognition was found in quiet or noisy conditions between the implant and the acoustic hearing aid. For the Soundtec device, a statistically significant improvement was noted in NU-6 and SPIN test results at 52 weeks compared with an optimally fitted hearing aid. However, only 12 patients had completed the 52- week follow-up. Patient Assessments Patient self-evaluation was performed in a variety of ways. The Profile of Hearing Aid Performance (PHAP) consists of 7 subscales that measure several dimensions of hearing aid effectiveness, such as Ease of Communications, Reverberation, and Distortion of Sounds. The Hearing Device Satisfaction Scale (HDSS) was developed by Symphonix, the manufacturer. This scale evaluates hearing aid and Soundbridge use and general satisfaction level. The number of subjects who reported improvement was significant across all 7 PHAP subscales. The largest improvements in the Soundbridge compared with the acoustic hearing aid were reported for the Reverberation, Reduced Cues, and Background Noise subscales. Based on HDSS scores, 94% reported improved overall sound quality for the Soundbridge. For the Soundtec device, patient satisfaction was based on the Hough Ear Institute Profile. This profile assesses patient Page 4
5 preference, acoustic feedback, perception of speech quality, occlusion, and tinnitus. At 20 weeks postimplant, improvements on all parameters were clinically significant. For example, 89% of patients preferred the implantable hearing aid to the acoustic hearing aid, although this result is not surprising because only patients who were dissatisfied with their previous acoustic hearing participated in the trial. A total of 67% of patients reported feedback with their previous acoustic hearing aid, while only 9% reported feedback with the implanted device. The clinical significance of the improvement in functional gain and speech perception is uncertain, although there appeared to be a clear patient preference for the implantable devices. 4 Safety Minimal safety issues were associated with either device. In the Soundbridge device, the most common complication was a fullness sensation in 18, which did not resolve in 13. Altered taste sensation was reported in 7 and transient pain in 13. Two patients reported a reduction in residual hearing. In the Soundtec device, the most common complication included device noise, ear pain, ear irritation, and processor failure. These complications resolved in almost all patients; no patient requested removal of the device. However, risks can only be adequately evaluated in broader populations over time. Additional Studies for Semi-Implantable Hearing Aids In 2013, Butler et al published results of a systematic review of comparative studies evaluating partially and fully implantable middle ear hearing devices for sensorineural hearing loss. 5 The review included 14 studies, none of which were randomized controlled trials, 13 of which evaluated a semi-implantable device, most often the Vibrant Soundbridge, with 1 study evaluating the Envoy fully implantable system. Outcomes reported across studies were heterogeneous. Among the 9 studies that reported on the primary outcome (functional hearing gain), 1 found that middle ear implants were statistically significantly better than hearing aids, 1 found that hearing aids were statistically significantly better than implants, and 6 studies found that middle ear implants were better than hearing aids, but without a clinically significant difference. The authors concluded that middle ear implants were at least as effective as hearing aids in improving hearing outcomes. A systematic review by Kahue et al (2014) evaluated studies of 3 FDA-approved middle ear hearing aids, the Vibrant Soundbridge, the Maxum System, and the Envoy Esteem (discussed in the following section). 6 Studies eligible for inclusion addressed purely sensorineural hearing loss, had at least 5 implanted ears, and reported comparative data between preoperative and postoperative audiometric performance. Seventeen studies (503 ears) were included, 3 of which evaluated the Soundtec System (now Maxum System, 190 ears), 5 of which evaluated the Envoy Esteem (102 ears), and 9 of which evaluated the Vibrant Soundbridge (211 ears). The 14 studies comparing preoperative unaided hearing and postoperative middle ear implant-assisted hearing demonstrated improvement in hearing thresholds (weight mean, 25.2 db improvement; range, db). However, for the 12 studies that compared the best aided preoperative condition with the postoperative assisted performance, the functional gain was smaller (weighted mean, 8.1 db improvement; range, -9.4 to 13 db), and only 1 reported statistically significant Page 5
6 improvements over optimally fitting hearing aids. Similarly, studies that compared the preoperative unaided condition and the postoperative middle ear implant-assisted hearing demonstrated improvements in speech recognition (weighted average, 44.8% improvement; range, 8.8%-64.0%), while speech recognition was similar for the middle ear implant-assisted condition and best aided preoperative condition. Ten studies reported on safety outcomes, including 5 studies that focused on partially implantable middle ear implants; in those studies 15 (11.4%) of 132 implants had a malfunction that led to explantation. One series with long-term follow-up (mean, 7.5 years) focused on middle ear implants in patients who failed external hearing aids. Zwartenkot et al (2013) described outcomes for 33 patients with moderate-to-severe sensorineural hearing loss who had severe chronic otitis externa and were implanted with the Vibrant Soundbridge system or the Otologics MET system, a middle ear implant system not available in the United States. 7 Compared with baseline, at long-term followup, subjects had statistically significant improvements in total scores on the Abbreviated Profile of Hearing Aid Benefit (63.3 at baseline vs 55.6 at follow-up, p<0.05). Eighty-five percent of subjects reported wearing the device more than 4 hours a day. Results of a 2002 phase 2 trial of the Soundtec system were published, 8 but this publication lags behind the data included in the FDA summary of safety and effectiveness. 2 An additional case series of 64 Soundtec implants was published in The average functional gain varied with frequency, with the lowest functional gain in the lower speech frequencies (7.9 db) and increasing functional gain at higher frequencies, ranging up to 27 db at the highest frequency of 6000 Hz. The functional gain of 7.9 db at the lower speech frequencies was similar to that reported in the FDA summary of safety and effectiveness, while the 27 db gain at the higher frequencies was higher than in the FDA summary. The cause of this marked discrepancy is not apparent. In this case series, the authors also reported that a high percentage of patients heard the magnet move inside the ear, resulting in a refinement of the surgical procedure to better stabilize the magnet. Semi-Implantable Hearing Aids for Conductive or Mixed Hearing Loss While the Vibrant Soundbridge received FDA approval for sensorineural hearing loss, several studies have evaluated it for off-label use in conductive or mixed hearing loss with coupling of the device s floating mass transducer to the middle ear s round or oval window, instead of the incus, bypassing the middle ear structures. In 2015, Ernst et al published results of a systematic review of studies reporting on the Vibrant Soundbridge for conductive or mixed hearing loss. 10 The authors included studies that compared the Vibrant Soundbridge with no intervention, bone conduction hearing implants (the Bonebridge implant, a fully implantable bone conduction hearing implant that uses a bone conduction floating mass transducer to transmit signals to the cochlea), and middle ear surgery plus hearing aids. Nineteen articles (total N=294 individuals) compared the Vibrant Soundbridge with no intervention were identified, including 16 cohort before-after studies, 2 concurrent cohort studies, and 1 nonrandomized clinical trial. No improvements in bone conduction Page 6
7 thresholds were reported. Studies reported a variety of methods for determining air conduction thresholds, precluding pooling of results, but hearing thresholds improved substantially in all studies. For speech recognition, a meta-analysis of results for change in score on the Italian disyllabic word lists and Freiburg Monosyllabic Word Test was conducted, with pooled mean improvements of 71.5% and 69%, respectively. No studies were identified that compared the Vibrant Soundbridge with the Bonebridge. Four studies (total N=43 individuals) compared the Vibrant Soundbridge and middle ear surgery plus hearing aids. Improvements in air conduction thresholds and functional gain were generally better with the Vibrant Soundbridge, but studies were mixed in terms of whether the Vibrant Soundbridge was associated with greater improvements in speech recognition. Since publication of the Ernst systematic review, Frenzel et al, using a single-subject repeatedmeasures design, reported outcomes on the Vibrant Soundbridge among 19 patients ages 5 to 17 with conductive or mixed hearing loss in a prospective study. 11 Younger children (age range, 5-9 years) improved monosyllable word recognition score from a mean of 28.9% preoperatively to 80% at the initial fitting (p=0.005) and to 95.5% at 6-month testing (p=0.001). Older children (age range, years) improved on word recognition score from a mean of 18.5% preoperatively to 80.5% at the initial fitting (p=0.001) and to 89% at 6 months postoperative (p=0.001). Improvements in speech recognition threshold and signal-to-noise ratio were also reported. Earlier series have reported within-subject comparisons of hearing outcomes before and after hearing aid amplification and patient-reported outcomes with implantable hearing devices. One study focused on implantable hearing aid outcomes in patients who failed external hearing aids. Marino et al, which was included in the Ernst systematic review, reported results of round window-coupled Vibrant Soundbridge implantation in 18 subjects with conductive or mixed hearing loss who could not derive benefit from conventional hearing aids due to chronic otitis externa, blind sac closure, pain with hearing aid mold use, and severe-to-profound mixed hearing loss. 12 Speech recognition in quiet settings with the Soundbridge device was similar to conventional hearing aids, while speech recognition in noisy settings was improved with the Soundbridge device. In the largest series identified, Colletti et al reported longer term outcomes for a case series of 50 patients ages 2 months to 74 years with severe conductive or mixed hearing loss due to ossicular chain defects who underwent coupling of the Vibrant Soundbridge system to the round window. 13 Although subjects demonstrated improvements in speech perception and pure-tone audiometry (in adults) and auditory brainstem response thresholds (in infants), the study s implications for practice are limited due to a large number of subjects with missing data (17/50). Other series, with sample sizes ranging from 9 to 25 subjects, have reported hearing outcomes with the Vibrant Soundbridge, using various coupling methods These studies generally report improvements in hearing measures and good patient satisfaction relative to external hearing aids. Page 7
8 One study, by Skarzynski et al, reported up to 3 years of follow-up in adults who received the Vibrant Soundbridge. 16 Over the 3 years, bone conduction hearing thresholds were stable. There were no cases of device extrusion or significant complications; 19% of patients had tinnitus, which resolved within 2 months postoperatively. Section Summary: Semi-Implantable Middle Ear Hearing Aids The evidence for use of semi-implantable middle ear hearing aids includes the clinical trials that supported FDA approval of the Vibrant Soundbridge and the Soundtec devices, along with a large number of observational series. Most available studies address the Vibrant Soundbridge device. For the use of semi-implantable middle ear hearing aids in patients with sensorineural hearing loss, the body of evidence suggests that these devices may be associated with a modest improvement in functional gain compared with external hearing aids, with similar improvements in speech recognition scores. Case series reporting on alternative coupling methods for the Vibrant Soundbridge for patients with conductive or mixed hearing loss also report improved hearing thresholds and word recognition. Although the devices appear to have a good safety profile in the short term, studies in larger series reporting on longer term durability, safety, and efficacy are needed to permit conclusions about the devices risks and benefits relative to external hearing aids. Fully Implantable Middle Ear Hearing Aid Trials Supporting FDA Approval of a Fully Implantable Hearing Aid FDA approval of the Esteem Hearing System was based on a prospective, nonrandomized, multicenter trial of 60 patients with moderate-to-severe sensorineural hearing loss designed to assess safety and efficacy. 27 Patients served as both control and test subjects as hearing was tested before (with and without hearing assistive devices) and after Esteem implantation. Results of this trial are available in the FDA summaries of safety and effectiveness. In this study, patients experienced an improvement of 11.4 db in mean speech reception threshold at 10 months postimplantation compared with preimplant-aided speech reception thresholds. Overall, word recognition scores were equal to or better than preimplant-aided scores in 93% of patients. The other 7% experienced lower word recognition scores than preimplant scores using hearing aids. Ninety-six adverse device events occurred and were not considered serious. Taste disturbance was the most common adverse effect reported at 42%, followed by tinnitus at 18% and facial paralysis/paresis at 7% of patients. Severe adverse device effects were experienced in 6 of the 57 patients implanted and included 3 revisions due to fibrous adhesions that limited implant benefit, 1 incision breakdown that required explantation, and 1 wound infection and 1 severe pain and facial weakness case, both of which resolved with medication. Overall, 70% of all adverse events resolved at 10-month follow-up. However, the serious adverse event of facial paralysis/palsy had not resolved in 2 patients. Kraus et al reported on 1-year follow-up of the Esteem study in Results were similar to those reported to FDA at 10-month follow-up. Mean speech reception thresholds improved Page 8
9 11.8±1.8 db from a preimplant-aided score of 41.2 to 29.4 db (p 0.001). Mean word recognition scores improved by 19.8%±4.3% from preimplant-aided scores. The authors reported 133 adverse events, including 3 cases of facial paresis that resolved with medication. Additional Studies for a Fully Implantable Hearing Aid In 2014, Pulcherio et al reported results of a systematic review of studies of 2 fully implantable middle ear hearing devices: the FDA-approved Esteem device and the Carina device. 29 The review included 22 studies with a total of 244 patients, 134 implanted with the Esteem device and 110 with the Carina device. No randomized controlled trials were identified, and most studies were small, with the largest series including 57 subjects and 12 series including fewer than 10 subjects. All studies showed improvement of sound field threshold from unaided to aided conditions with the fully implantable device, but the magnitudes of the improvements varied. A 2012 systematic review of literature on the Esteem device included 7 articles that met inclusion criteria. 30 Complication rates with the Esteem device most commonly included taste disturbance. Clinically significant improvements in functional gain, speech reception, and speech recognition over the unaided condition were reported. In studies comparing the Esteem implant with conventional hearing aids, findings were mixed. Improvements in functional gain were similar to those for hearing aids; however, speech recognition and quality of life were greater with the implants. This limited evidence suggested these devices may offer a relatively safe and effective treatment option, particularly for patients medically unable to wear conventional hearing aids. However, the included studies were primarily quasi-experimental, pre/post comparisons of aided and unaided conditions. Furthermore, because of heterogeneity across studies, meta-analysis was not performed, and comparisons were made by structured review. Several representative case series are described next. Barbara et al reported on the use of the Esteem device in 21 patients with severe bilateral sensorineural hearing loss. 31 The authors reported mean hearing threshold levels improved overall from 70 to 48 db. In another article reporting on 6 patients implanted with the Esteem device, Barbara et al found the device improved hearing when assessed during postoperative fittings. 32 Chen et al reported on the phase 1 results of the Envoy Totally Implantable Hearing System in 7 patients followed at 2 and 4 months after activation of the device. 33 Improvements in word recognition and communication in background noise over best-fit hearing aid usage was perceived in 5 patients. Patient outcomes in functional gain and speech reception thresholds were comparable with best-fit hearing aid usage. Additional small case series do not provide significant additional evidence about outcome improvements associated with the Esteem device. A case series published since the Pulcherio and the Klein systematic reviews reported high rates of facial nerve palsies (10/34 subjects [29.4%]) after implantation of the Esteem device, which persisted to 3 months of follow-up in 6 (17.6%) of 34 subjects. 38 Page 9
10 Section Summary: Fully Implantable Middle Ear Hearing Aids The evidence for use of fully implantable middle ear hearing aids includes the clinical trial that supported FDA approval of the Esteem device, along with small observational series that report short-term results. These studies generally found improved hearing over unaided hearing, with modest improvements over hearing with best-fit aids. Ongoing and Unpublished Clinical Trials A search of ClinicalTrials.gov in January 2016 did not identify any ongoing or unpublished trials that would likely influence this review. Summary of Evidence The evidence for semi-implantable and fully implantable middle ear hearing aids in individuals who have hearing loss includes the single-arm interventional studies on which the devices Food and Drug Administration approvals were based, and a number of observational series. Relevant outcomes include symptoms, functional outcomes, quality of life, and treatment-related morbidity. The limited data suggest implantable middle ear hearing aids may provide marginal improvement in hearing compared with conventional external acoustic hearing aids in patients with sensorineural hearing loss. However, given the safety and effectiveness of external acoustic hearing aids and the increased risks inherent in a surgical procedure, the semi-implantable device must be associated with clinically significant improvement in various hearing parameters compared with external hearing aids. While safety concerns appear to be minimal, only a limited number of patients have been included in the clinical trials, and few have completed more than 1 year of follow-up. Given small sample sizes and limited safety data, risks cannot be adequately evaluated and compared with the marginal improvement in hearing. Studies of patients with conductive or mixed hearing loss and aural atresia, when external acoustic hearing aids are not an option, have also demonstrated hearing benefit with semi-implantable middle ear hearing aids. However, these studies are few and limited to small numbers of patients. Therefore, conclusions on the safety and effectiveness of semi-implantable hearing aids in these patients cannot be made, and further study with longer term follow-up is needed. Comparisons of semi-implantable devices with alternative hearing devices such as implantable bone-conduction and bone-anchored hearing aids would also be useful to determine device appropriateness for patients who are unable to use external air-conduction hearing aids. The evidence is insufficient to determine the effects of the technology on health outcomes. Practice Guidelines and Position Statements The American Academy of Otolaryngology Head and Neck Surgery issued a position statement on implantable hearing devices, most recently updated on January 8, 2013, which states 39 : The American Academy of Otolaryngology-Head and Neck Surgery, Inc. considers the implantation of a percutaneous of transcutaneous bone conduction hearing device, placement of a bone conduction oral appliance, and implantation of a semi-implantable or totally implantable hearing device to be acceptable procedures for the relief of hearing impairment Page 10
11 when performed by, or in collaboration with, a qualified otolaryngologist-head and neck surgeon. Use of any device must adhere to the restrictions and guidelines specified by the appropriate governing agency, such as the Food and Drug Administration in the United States and other similar agencies in countries other than the United States. Not applicable. U.S. Preventive Services Task Force Recommendations Medicare National Coverage No national coverage determination has been published. The Medicare Benefit Policy Manual references hearing aids and auditory implants, stating that hearing aids are excluded from coverage. 40 However, devices producing the perception of sound by replacing the function of the middle ear, cochlea, or auditory nerve are payable by Medicare as prosthetic devices. These devices are indicated only when hearing aids are medically inappropriate or cannot be used due to congenital malformations, chronic disease, severe sensorineural hearing loss, or surgery. The benefit manual does not specifically refer to semi- or fully implantable hearing aids as prosthetic devices. V. DEFINITIONS TOP HEARING AID is any device that does not produce as its output an electrical signal that directly stimulates the auditory nerve. Examples of hearing aids are devices that produce air-conducted sound into the external auditory canal, devices that produce sound by mechanically vibrating bone, or devices that produce sound by vibrating the cochlear fluid through stimulation of the round window. Devices such as cochlear implants, which produce as their output an electrical signal that directly stimulates the auditory nerve, are not considered to be hearing aids. OSSICLE refers to any small bone, especially one of the three bones of the ear. SENSORINEURAL HEARING LOSS refers to a form of hearing loss in which sound is conducted normally through the external and middle ear but a defect in the inner ear or auditory nerve results in hearing loss. The loss is measured in decibels and may be described as mild, moderate, severe, or profound. VI. BENEFIT VARIATIONS TOP The existence of this medical policy does not mean that this service is a covered benefit under the member's contract. Benefit determinations should be based in all cases on the applicable contract language. Medical policies do not constitute a description of benefits. A member s individual or group customer benefits govern which services are covered, which are excluded, Page 11
12 and which are subject to benefit limits and which require preauthorization. Members and providers should consult the member s benefit information or contact Capital for benefit information. VII. DISCLAIMER TOP Capital s medical policies are developed to assist in administering a member s benefits, do not constitute medical advice and are subject to change. Treating providers are solely responsible for medical advice and treatment of members. Members should discuss any medical policy related to their coverage or condition with their provider and consult their benefit information to determine if the service is covered. If there is a discrepancy between this medical policy and a member s benefit information, the benefit information will govern. Capital considers the information contained in this medical policy to be proprietary and it may only be disseminated as permitted by law. VIII. CODING INFORMATION TOP Note: This list of codes may not be all-inclusive, and codes are subject to change at any time. The identification of a code in this section does not denote coverage as coverage is determined by the terms of member benefit information. In addition, not all covered services are eligible for separate reimbursement. Investigational; therefore not covered: CPT Codes Current Procedural Terminology (CPT) copyrighted by American Medical Association. All Rights Reserved. HCPCS Codes S2230 V5095 Description Implantation of magnetic component of semi-implantable hearing device on ossicles in middle ear Semi-implantable middle ear hearing prosthesis IX. REFERENCES TOP 1. Administration FaD. Vibrant Soundbridge. Summary of Safety and Effectiveness. 2000; Accessed January 31, Page 12
13 2. Administration FaD. Soundtec Direct System. Summary of Safety and Effectiveness. 2001; Accessed January 31, Luetje CM, Brackman D, Balkany TJ, et al. Phase III clinical trial results with the Vibrant Soundbridge implantable middle ear hearing device: a prospective controlled multicenter study. Otolaryngol Head Neck Surg. Feb 2002;126(2): PMID Sterkers O, Boucarra D, Labassi S, et al. A middle ear implant, the Symphonix Vibrant Soundbridge: retrospective study of the first 125 patients implanted in France. Otol Neurotol. May 2003;24(3): PMID Butler CL, Thavaneswaran P, Lee IH. Efficacy of the active middle-ear implant in patients with sensorineural hearing loss. J Laryngol Otol. Jul 2013;127 Suppl 2:S8-16. PMID Kahue CN, Carlson ML, Daugherty JA, et al. Middle ear implants for rehabilitation of sensorineural hearing loss: a systematic review of FDA approved devices. Otol Neurotol. Aug 2014;35(7): PMID Zwartenkot JW, Hashemi J, Cremers CW, et al. Active middle ear implantation for patients with sensorineural hearing loss and external otitis: long-term outcome in patient satisfaction. Otol Neurotol. Jul 2013;34(5): PMID Hough JV, Matthews P, Wood MW, et al. Middle ear electromagnetic semi-implantable hearing device: results of the phase II SOUNDTEC direct system clinical trial. Otol Neurotol. Nov 2002;23(6): PMID Silverstein H, Atkins J, Thompson JH, Jr., et al. Experience with the SOUNDTEC implantable hearing aid. Otol Neurotol. Mar 2005;26(2): PMID Ernst A, Todt I, Wagner J. Safety and effectiveness of the Vibrant Soundbridge in treating conductive and mixed hearing loss: A systematic review. Laryngoscope. Oct PMID Frenzel H, Sprinzl G, Streitberger C, et al. The Vibrant Soundbridge in children and adolescents: preliminary european multicenter results. Otol Neurotol. Aug 2015;36(7): PMID Marino R, Linton N, Eikelboom RH, et al. A comparative study of hearing aids and round window application of the vibrant sound bridge (VSB) for patients with mixed or conductive hearing loss. Int J Audiol. Apr 2013;52(4): PMID Colletti L, Mandala M, Colletti V. Long-term outcome of round window Vibrant SoundBridge implantation in extensive ossicular chain defects. Otolaryngol Head Neck Surg. Jul 2013;149(1): PMID Vyskocil E, Riss D, Honeder C, et al. Vibroplasty in mixed and conductive hearing loss: Comparison of different coupling methods. Laryngoscope. Oct PMID Page 13
14 15. Atas A, Tutar H, Gunduz B, et al. Vibrant SoundBridge application to middle ear windows versus conventional hearing aids: a comparative study based on international outcome inventory for hearing aids. Eur Arch Otorhinolaryngol. Jan 2014;271(1): PMID Skarzynski H, Olszewski L, Skarzynski PH, et al. Direct round window stimulation with the Med-El Vibrant Soundbridge: 5 years of experience using a technique without interposed fascia. Eur Arch Otorhinolaryngol. Mar 2014;271(3): PMID de Abajo J, Sanhueza I, Giron L, et al. Experience with the active middle ear implant in patients with moderate-to-severe mixed hearing loss: indications and results. Otol Neurotol. Oct 2013;34(8): PMID Dillon MT, Tubbs RS, Adunka MC, et al. Round window stimulation for conductive and mixed hearing loss. Otol Neurotol. Oct 2014;35(9): PMID Beltrame AM, Martini A, Prosser S, et al. Coupling the Vibrant Soundbridge to cochlea round window: auditory results in patients with mixed hearing loss. Otol Neurotol. Feb 2009;30(2): PMID Bernardeschi D, Hoffman C, Benchaa T, et al. Functional results of Vibrant Soundbridge middle ear implants in conductive and mixed hearing losses. Audiol Neurootol. 2011;16(6): PMID Colletti L, Carner M, Mandala M, et al. The floating mass transducer for external auditory canal and middle ear malformations. Otol Neurotol. Jan 2011;32(1): PMID Gunduz B, Atas A, Bayazit YA, et al. Functional outcomes of Vibrant Soundbridge applied on the middle ear windows in comparison with conventional hearing aids. Acta Otolaryngol. Dec 2012;132(12): PMID Mandala M, Colletti L, Colletti V. Treatment of the atretic ear with round window vibrant soundbridge implantation in infants and children: electrocochleography and audiologic outcomes. Otol Neurotol. Oct 2011;32(8): PMID Roman S, Denoyelle F, Farinetti A, et al. Middle ear implant in conductive and mixed congenital hearing loss in children. Int J Pediatr Otorhinolaryngol. Dec 2012;76(12): PMID Sziklai I, Szilvassy J. Functional gain and speech understanding obtained by Vibrant Soundbridge or by open-fit hearing aid. Acta Otolaryngol. Apr 2011;131(4): PMID Zernotti ME, Arauz SL, Di Gregorio MF, et al. Vibrant Soundbridge in congenital osseous atresia: multicenter study of 12 patients with osseous atresia. Acta Otolaryngol. Jun 2013;133(6): PMID Page 14
15 27. Administration FaD. Esteem Implantable Hearing System. Summary of Safety and Effectiveness. 2010; Accessed January 31, Kraus EM, Shohet JA, Catalano PJ. Envoy Esteem Totally Implantable Hearing System: phase 2 trial, 1-year hearing results. Otolaryngol Head Neck Surg. Jul 2011;145(1): PMID Pulcherio JO, Bittencourt AG, Burke PR, et al. Carina(R) and Esteem(R): a systematic review of fully implantable hearing devices. PLoS One. 2014;9(10):e PMID Klein K, Nardelli A, Stafinski T. A systematic review of the safety and effectiveness of fully implantable middle ear hearing devices: the carina and esteem systems. Otol Neurotol. Aug 2012;33(6): PMID Barbara M, Biagini M, Monini S. The totally implantable middle ear device 'Esteem' for rehabilitation of severe sensorineural hearing loss. Acta Otolaryngol. Apr 2011;131(4): PMID Barbara M, Manni V, Monini S. Totally implantable middle ear device for rehabilitation of sensorineural hearing loss: preliminary experience with the Esteem, Envoy. Acta Otolaryngol. Apr 2009;129(4): PMID Chen DA, Backous DD, Arriaga MA, et al. Phase 1 clinical trial results of the Envoy System: a totally implantable middle ear device for sensorineural hearing loss. Otolaryngol Head Neck Surg. Dec 2004;131(6): PMID Gerard JM, Thill MP, Chantrain G, et al. Esteem 2 middle ear implant: our experience. Audiol Neurootol. 2012;17(4): PMID Kam AC, Sung JK, Yu JK, et al. Clinical evaluation of a fully implantable hearing device in six patients with mixed and sensorineural hearing loss: our experience. Clin Otolaryngol. Jun 2012;37(3): PMID Monini S, Biagini M, Atturo F, et al. Esteem(R) middle ear device versus conventional hearing aids for rehabilitation of bilateral sensorineural hearing loss. Eur Arch Otorhinolaryngol. Jul 2013;270(7): PMID Tsang WS, Yu JK, Wong TK, et al. Vibrant Soundbridge system: application of the stapes coupling technique. J Laryngol Otol. Jan 2013;127(1): PMID Barbara M, Volpini L, Monini S. Delayed facial nerve palsy after surgery for the Esteem((R)) fully implantable middle ear hearing device. Acta Otolaryngol. Apr 2014;134(4): PMID American Academy of Otolaryngology - Head and Neck Surgery. Implantable Hearing Devices 2013; Accessed February 19, Centers for Medicare and Medicaid Services. Medicare Policy Benefit Manual. Chapter 16 - General Exclusions from Coverage. 2014; Accessed January 31, Page 15
16 X. POLICY HISTORY TOP MP CAC 10/25/11 New policy. Semi-implantable middle ear hearing aid criteria previously were in MP Implantable Bone Conduction and Bone-Anchored Hearing Prosthetic Devices. For this review, the policies were separated. Fully implantable hearing aids were added to the policy and the title changed to reflect addition, Both fully implantable and semi-implantable hearing aids are considered investigational. CAC 10/30/12 Consensus. No change to policy statements which match BCBSA. References updated. With this review title changed to Semi-Implantable and Fully Implantable Middle Ear Hearing Aids (formerly Semi-Implantable and Fully Implantable Middle Ear Hearing Aid for Moderately to Severe Sensorineural Hearing Loss). codes reviewed 11/1/12 7/25/13 Admin coding review complete CAC 9/24/13 Consensus. No change to policy statements. Rationale section added. References updated. CAC 9/30/14. Consensus. No change to policy statements. References and rationale sections updated. CAC 9/29/15 Consensus review. No change to the policy statement. Background, references, and rationale updated. Coding Reviewed CAC 9/27/16 Consensus review. No change to the policy statement. Background, rationale, and references updated. Variations reformatted. Coding reviewed. Health care benefit programs issued or administered by Capital BlueCross and/or its subsidiaries, Capital Advantage Insurance Company, Capital Advantage Assurance Company and Keystone Health Plan Central. Independent licensees of the BlueCross BlueShield Association. Communications issued by Capital BlueCross in its capacity as administrator of programs and provider relations for all companies. Page 16
Semi-Implantable and Fully Implantable Middle Ear Hearing Aids
 MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES CODING DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES APPENDIX HISTORY Semi-Implantable and Fully Implantable Middle Ear Hearing Aids
MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES CODING DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES APPENDIX HISTORY Semi-Implantable and Fully Implantable Middle Ear Hearing Aids
Semi-Implantable and Fully Implantable Middle Ear Hearing Aids
 Semi-Implantable and Fully Implantable Middle Ear Hearing Aids Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively
Semi-Implantable and Fully Implantable Middle Ear Hearing Aids Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively
SEMI-IMPLANTABLE AND FULLY IMPLANTABLE MIDDLE EAR HEARING AIDS
 Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline must be read in its
Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline must be read in its
How To Know If A Cochlear Implant Is Right For You
 MEDICAL POLICY SUBJECT: COCHLEAR IMPLANTS AND PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
MEDICAL POLICY SUBJECT: COCHLEAR IMPLANTS AND PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
CONVENTIONAL AND DIGITAL HEARING AIDS
 CONVENTIONAL AND DIGITAL HEARING AIDS Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical
CONVENTIONAL AND DIGITAL HEARING AIDS Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical
Vibrant Soundbridge Implantable Hearing System
 Vibrant Soundbridge Implantable Hearing System Kristin M. Avitabile, MS, CCC-A Clinical Manager, Southeastern U.S. MED-EL Corporation Hearing Technology Hearing Aids Mild to severe HL Problems with feedback
Vibrant Soundbridge Implantable Hearing System Kristin M. Avitabile, MS, CCC-A Clinical Manager, Southeastern U.S. MED-EL Corporation Hearing Technology Hearing Aids Mild to severe HL Problems with feedback
Cochlear Implant, Bone Anchored Hearing Aids, and Auditory Brainstem Implant
 Origination: 06/23/08 Revised: 10/13/14 Annual Review: 11/12/15 Purpose: To provide cochlear implant, bone anchored hearing aids, and auditory brainstem implant guidelines for the Medical Department staff
Origination: 06/23/08 Revised: 10/13/14 Annual Review: 11/12/15 Purpose: To provide cochlear implant, bone anchored hearing aids, and auditory brainstem implant guidelines for the Medical Department staff
Medical Benefit Effective Date: 07/01/12 Next Review Date: 03/13 Preauthorization* Yes Review Dates: 11/07, 07/08, 05/09, 03/10, 03/11, 03/12
 Protocol Implantable Bone-Conduction and Bone-Anchored Hearing Aids (70103) Medical Benefit Effective Date: 07/01/12 Next Review Date: 03/13 Preauthorization* Yes Review Dates: 11/07, 07/08, 05/09, 03/10,
Protocol Implantable Bone-Conduction and Bone-Anchored Hearing Aids (70103) Medical Benefit Effective Date: 07/01/12 Next Review Date: 03/13 Preauthorization* Yes Review Dates: 11/07, 07/08, 05/09, 03/10,
Implantable Bone Conduction Clinical Coverage Policy No: 1A-36 Hearing Aids (BAHA) Amended Date: October 1, 2015.
 Implantable Bone Conduction Clinical Coverage Policy No: 1A-36 Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Conductive Hearing Loss... 1 1.2 Sensorineural Hearing Loss...
Implantable Bone Conduction Clinical Coverage Policy No: 1A-36 Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Conductive Hearing Loss... 1 1.2 Sensorineural Hearing Loss...
MEDICAL POLICY POLICY TITLE DENTAL AND ORAL SURGERY SERVICES AFTER AN ACCIDENT POLICY NUMBER MP- 1.108
 Original Issue Date (Created): July 1, 2005 Most Recent Review Date (Revised): Effective Date: June 29, 2010 May 25, 2011- RETIRED I. POLICY II. Dental and/or oral surgery services (on a limited basis)
Original Issue Date (Created): July 1, 2005 Most Recent Review Date (Revised): Effective Date: June 29, 2010 May 25, 2011- RETIRED I. POLICY II. Dental and/or oral surgery services (on a limited basis)
HEARING AIDS AND DEVICES INCLUDING WEARABLE, BONE-ANCHORED AND SEMI- IMPLANTABLE
 MEDICAL POLICY HEARING AIDS AND DEVICES INCLUDING WEARABLE, BONE-ANCHORED AND SEMI- IMPLANTABLE Policy Number: 2015T0396O Effective Date: December 1, 2015 Table of Contents BENEFIT CONSIDERATIONS COVERAGE
MEDICAL POLICY HEARING AIDS AND DEVICES INCLUDING WEARABLE, BONE-ANCHORED AND SEMI- IMPLANTABLE Policy Number: 2015T0396O Effective Date: December 1, 2015 Table of Contents BENEFIT CONSIDERATIONS COVERAGE
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 11/24/2015 Effective Date: 2/1/2016 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS
Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 11/24/2015 Effective Date: 2/1/2016 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS
Hearing Aids - Adult HEARING AIDS - ADULT HS-159. Policy Number: HS-159. Original Effective Date: 3/18/2010. Revised Date(s): 3/18/2011; 3/1/2012
 Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
Harmony Behavioral Health, Inc. Harmony Behavioral Health of Florida, Inc. Harmony Health Plan of Illinois, Inc. HealthEase of Florida, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance
Clinical Commissioning Policy: Bone Anchored Hearing Aids. April 2013. Reference: NHSCB/ D09/P/a
 Clinical Commissioning Policy: Bone Anchored Hearing Aids April 2013 Reference: NHSCB/ D09/P/a NHS Commissioning Board Clinical Commissioning Policy: Bone Anchored Hearing Aids First published: April 2013
Clinical Commissioning Policy: Bone Anchored Hearing Aids April 2013 Reference: NHSCB/ D09/P/a NHS Commissioning Board Clinical Commissioning Policy: Bone Anchored Hearing Aids First published: April 2013
Comparison of Traditional Bone-Conduction Hearing Aids with the Baha â System DOI: 10.3766/jaaa.21.4.5
 J Am Acad Audiol 21:267 273 (2010) Comparison of Traditional Bone-Conduction Hearing Aids with the Baha â System DOI: 10.3766/jaaa.21.4.5 Lisa Christensen* Laura Smith-Olinde Jillian Kimberlain* Gresham
J Am Acad Audiol 21:267 273 (2010) Comparison of Traditional Bone-Conduction Hearing Aids with the Baha â System DOI: 10.3766/jaaa.21.4.5 Lisa Christensen* Laura Smith-Olinde Jillian Kimberlain* Gresham
GONCA SENNAROĞLU PhD LEVENT SENNAROĞLU MD. Department of Otolaryngology Hacettepe University ANKARA, TURKEY
 GONCA SENNAROĞLU PhD LEVENT SENNAROĞLU MD Department of Otolaryngology Hacettepe University ANKARA, TURKEY To present the audiological findings and rehabilitative outcomes of CI in children with cochlear
GONCA SENNAROĞLU PhD LEVENT SENNAROĞLU MD Department of Otolaryngology Hacettepe University ANKARA, TURKEY To present the audiological findings and rehabilitative outcomes of CI in children with cochlear
HEARING & HEARING LOSS. Dr I Butler 2015
 HEARING & HEARING LOSS Dr I Butler 2015 DISCLOSURE Sponsorship to attend local and international workshops Cochlear (Southern ENT) Med el TOPICS Anatomy Classification of hearing loss Congenital hearing
HEARING & HEARING LOSS Dr I Butler 2015 DISCLOSURE Sponsorship to attend local and international workshops Cochlear (Southern ENT) Med el TOPICS Anatomy Classification of hearing loss Congenital hearing
KANSAS MEDICAL ASSISTANCE PROGRAM PROVIDER MANUAL. Audiology
 KANSAS MEDICAL ASSISTANCE PROGRAM PROVIDER MANUAL Audiology PART II Introduction Section BILLING INSTRUCTIONS Page 7000 Audiology Billing Instructions............... 7-1 Submission of Claim................
KANSAS MEDICAL ASSISTANCE PROGRAM PROVIDER MANUAL Audiology PART II Introduction Section BILLING INSTRUCTIONS Page 7000 Audiology Billing Instructions............... 7-1 Submission of Claim................
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): October 25, 2011 Most Recent Review Date (Revised): January 27, 2015 Effective Date: April 1, 2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS
Original Issue Date (Created): October 25, 2011 Most Recent Review Date (Revised): January 27, 2015 Effective Date: April 1, 2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS
Anatomy and Physiology of Hearing (added 09/06)
 Anatomy and Physiology of Hearing (added 09/06) 1. Briefly review the anatomy of the cochlea. What is the cochlear blood supply? SW 2. Discuss the effects of the pinna, head and ear canal on the transmission
Anatomy and Physiology of Hearing (added 09/06) 1. Briefly review the anatomy of the cochlea. What is the cochlear blood supply? SW 2. Discuss the effects of the pinna, head and ear canal on the transmission
BONE-CONDUCTION HEARING AIDS
 BONE-CONDUCTION HEARING AIDS Introduction Conventional hearing aids fit in the ear canal and amplify sounds, which the hearing aid user then hears in the normal way. However, these hearing aids are not
BONE-CONDUCTION HEARING AIDS Introduction Conventional hearing aids fit in the ear canal and amplify sounds, which the hearing aid user then hears in the normal way. However, these hearing aids are not
Implantable Bone-Conduction and Bone-Anchored Hearing Aids. Original Policy Date
 MP 7.01.02 Implantable Bone-Conduction and Bone-Anchored Hearing Aids Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013
MP 7.01.02 Implantable Bone-Conduction and Bone-Anchored Hearing Aids Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013
GUIDELINES DEFINITIONS:
 U n i t e d H e a l t h C a r e G u i d e l i n e Division Departments Community Plan Products State :Arizona UnitedHealthcare Children s Rehabilitative Services Title: CRS IMPLANTABLE BONE CONDUCTION
U n i t e d H e a l t h C a r e G u i d e l i n e Division Departments Community Plan Products State :Arizona UnitedHealthcare Children s Rehabilitative Services Title: CRS IMPLANTABLE BONE CONDUCTION
Table of Contents. 1.0 Description of the Procedure, Product, or Service... 1 1.1 Definitions... 1
 Soft Band and Implantable Bone Clinical Coverage Policy No: 13 B Conduction Hearing Aid External Amended Date: October 1, 2015 Parts Replacement and Repair Table of Contents 1.0 Description of the Procedure,
Soft Band and Implantable Bone Clinical Coverage Policy No: 13 B Conduction Hearing Aid External Amended Date: October 1, 2015 Parts Replacement and Repair Table of Contents 1.0 Description of the Procedure,
Middle Ear Implants for the Treatment of Hearing Loss
 Middle Ear Implants for the Treatment of Hearing Loss FINAL STE REPORT December 2011 Submitted to: The Alberta Health Technologies Decision Process Health Technologies and Services Policy Unit Clinical
Middle Ear Implants for the Treatment of Hearing Loss FINAL STE REPORT December 2011 Submitted to: The Alberta Health Technologies Decision Process Health Technologies and Services Policy Unit Clinical
Unilateral (Hearing Loss in One Ear) Hearing Loss Guidance
 Unilateral (Hearing Loss in One Ear) Hearing Loss Guidance Indiana s Early Hearing Detection and Intervention Program Before universal newborn hearing screening, most children with unilateral hearing loss
Unilateral (Hearing Loss in One Ear) Hearing Loss Guidance Indiana s Early Hearing Detection and Intervention Program Before universal newborn hearing screening, most children with unilateral hearing loss
Implantable Bone Conduction and Bone-Anchored Hearing Aids
 MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES CODING DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES APPENDIX HISTORY Implantable Bone Conduction and Bone-Anchored Hearing Aids Number
MEDICAL POLICY POLICY RELATED POLICIES POLICY GUIDELINES CODING DESCRIPTION SCOPE BENEFIT APPLICATION RATIONALE REFERENCES APPENDIX HISTORY Implantable Bone Conduction and Bone-Anchored Hearing Aids Number
Understanding Hearing Loss 404.591.1884. www.childrensent.com
 Understanding Hearing Loss 404.591.1884 www.childrensent.com You just found out your child has a hearing loss. You know what the Audiologist explained to you, but it is hard to keep track of all the new
Understanding Hearing Loss 404.591.1884 www.childrensent.com You just found out your child has a hearing loss. You know what the Audiologist explained to you, but it is hard to keep track of all the new
Light wear for a powerful hearing. Bone Conduction Headset
 Light wear for a powerful hearing Bone Conduction Headset 2 Light wear for a powerful hearing Melody Flex, the new bone conduction headset is AUTEL s solution to improve hearing quality of those affected
Light wear for a powerful hearing Bone Conduction Headset 2 Light wear for a powerful hearing Melody Flex, the new bone conduction headset is AUTEL s solution to improve hearing quality of those affected
Hearing Screening Coding Fact Sheet for Primary Care Pediatricians
 Hearing Screening Coding Fact Sheet for Primary Care Pediatricians While coding for hearing screening is relatively straightforward, ensuring that appropriate payment is received for such services is a
Hearing Screening Coding Fact Sheet for Primary Care Pediatricians While coding for hearing screening is relatively straightforward, ensuring that appropriate payment is received for such services is a
Hearing Tests for Children with Multiple or Developmental Disabilities by Susan Agrawal
 www.complexchild.com Hearing Tests for Children with Multiple or Developmental Disabilities by Susan Agrawal Hearing impairment is a common problem in children with developmental disabilities or who have
www.complexchild.com Hearing Tests for Children with Multiple or Developmental Disabilities by Susan Agrawal Hearing impairment is a common problem in children with developmental disabilities or who have
Mississippi Medicaid. Provider Reference Guide. For Part 203. Physician Services
 Mississippi Medicaid Provider Reference Guide For Part 203 Physician Services This is a companion document to the Mississippi Administrative Code Title 23 and must be utilized as a reference only. January
Mississippi Medicaid Provider Reference Guide For Part 203 Physician Services This is a companion document to the Mississippi Administrative Code Title 23 and must be utilized as a reference only. January
ORIGINAL ARTICLE. Long-term Results of Bone-Anchored Hearing Aid Recipients Who Had Previously Used Air-Conduction Hearing Aids
 ORIGINAL ARTICLE Long-term Results of Bone-Anchored Hearing Aid Recipients Who Had Previously Used Air-Conduction Hearing Aids Myrthe K. S. Hol, MD; Ad F. M. Snik, MSc, PhD; Emmanuel A. M. Mylanus, MD,
ORIGINAL ARTICLE Long-term Results of Bone-Anchored Hearing Aid Recipients Who Had Previously Used Air-Conduction Hearing Aids Myrthe K. S. Hol, MD; Ad F. M. Snik, MSc, PhD; Emmanuel A. M. Mylanus, MD,
Introduction Bone Anchored Implants (BAI), Candidacy and Pre-Operative Testing for Adult Patients
 Introduction Bone Anchored Implants (BAI), Candidacy and Pre-Operative Testing for Adult Patients Developed by Hakanssonand his colleagues in Sweden in the late 1970s 3 Components Sound Processor (#1)
Introduction Bone Anchored Implants (BAI), Candidacy and Pre-Operative Testing for Adult Patients Developed by Hakanssonand his colleagues in Sweden in the late 1970s 3 Components Sound Processor (#1)
Michigan Ear Institute. Cochlear Implant.
 Michigan Ear Institute Cochlear Implant www.michiganear.com DOCTORS Jack M. Kartush, MD Dennis I. Bojrab, MD Michael J. LaRouere, MD John J. Zappia, MD, FACS Eric W. Sargent, MD, FACS Seilesh C. Babu,
Michigan Ear Institute Cochlear Implant www.michiganear.com DOCTORS Jack M. Kartush, MD Dennis I. Bojrab, MD Michael J. LaRouere, MD John J. Zappia, MD, FACS Eric W. Sargent, MD, FACS Seilesh C. Babu,
Prevalence of otological disorders in diabetic patients with hearing loss
 Prevalence of otological disorders in diabetic patients with hearing loss Manche Santoshi Kumari *, Jangala Madhavi *, Koralla Raja Meganadh *, Akka Jyothy Institute of Genetics and Hospital for Genetic
Prevalence of otological disorders in diabetic patients with hearing loss Manche Santoshi Kumari *, Jangala Madhavi *, Koralla Raja Meganadh *, Akka Jyothy Institute of Genetics and Hospital for Genetic
So, how do we hear? outer middle ear inner ear
 The ability to hear is critical to understanding the world around us. The human ear is a fully developed part of our bodies at birth and responds to sounds that are very faint as well as sounds that are
The ability to hear is critical to understanding the world around us. The human ear is a fully developed part of our bodies at birth and responds to sounds that are very faint as well as sounds that are
8.Audiological Evaluation
 8. A U D I O L O G I C A L E V A L U A T I O N 8.Audiological Evaluation The external ear of the child with Progeria Behavioral testing for assessing hearing thresholds Objective electrophysiologic tests
8. A U D I O L O G I C A L E V A L U A T I O N 8.Audiological Evaluation The external ear of the child with Progeria Behavioral testing for assessing hearing thresholds Objective electrophysiologic tests
Bone Anchored Hearing Aids (BAHA)
 B-ENT, 2007, 3, Suppl. 6, 45-50 Bone Anchored Hearing Aids (BAHA) G. E. J. Forton* and P. H. Van de Heyning** *E.N.T. department, Heilig Hart General Hospital, Roeselare; **University E.N.T. department,
B-ENT, 2007, 3, Suppl. 6, 45-50 Bone Anchored Hearing Aids (BAHA) G. E. J. Forton* and P. H. Van de Heyning** *E.N.T. department, Heilig Hart General Hospital, Roeselare; **University E.N.T. department,
PURE TONE AUDIOMETRY Andrew P. McGrath, AuD
 PURE TONE AUDIOMETRY Andrew P. McGrath, AuD Pure tone audiometry is the standard behavioral assessment of an individual s hearing. The results of pure tone audiometry are recorded on a chart or form called
PURE TONE AUDIOMETRY Andrew P. McGrath, AuD Pure tone audiometry is the standard behavioral assessment of an individual s hearing. The results of pure tone audiometry are recorded on a chart or form called
Protocol. Implantable Bone-Conduction and Bone-Anchored Hearing Aids
 Protocol Implantable Bone-Conduction and Bone-Anchored Hearing Aids (70103) Medical Benefit Effective Date: 04/01/13 Next Review Date: 01/17 Preauthorization Yes Review Dates: 11/07, 07/08, 05/09, 03/10,
Protocol Implantable Bone-Conduction and Bone-Anchored Hearing Aids (70103) Medical Benefit Effective Date: 04/01/13 Next Review Date: 01/17 Preauthorization Yes Review Dates: 11/07, 07/08, 05/09, 03/10,
REGULATIONS FOR THE DEGREE OF MASTER OF SCIENCE IN AUDIOLOGY (MSc[Audiology])
![REGULATIONS FOR THE DEGREE OF MASTER OF SCIENCE IN AUDIOLOGY (MSc[Audiology]) REGULATIONS FOR THE DEGREE OF MASTER OF SCIENCE IN AUDIOLOGY (MSc[Audiology])](/thumbs/27/10327872.jpg) 224 REGULATIONS FOR THE DEGREE OF MASTER OF SCIENCE IN AUDIOLOGY (MSc[Audiology]) (See also General Regulations) Any publication based on work approved for a higher degree should contain a reference to
224 REGULATIONS FOR THE DEGREE OF MASTER OF SCIENCE IN AUDIOLOGY (MSc[Audiology]) (See also General Regulations) Any publication based on work approved for a higher degree should contain a reference to
Your Hearing ILLUMINATED
 Your Hearing ILLUMINATED INFORMATION FROM YOUR HEARING CARE PROFESSIONAL REDISCOVER your hearing and reconnect 1 with the important things you might have been missing. Your sense of hearing is a vital
Your Hearing ILLUMINATED INFORMATION FROM YOUR HEARING CARE PROFESSIONAL REDISCOVER your hearing and reconnect 1 with the important things you might have been missing. Your sense of hearing is a vital
Diseases of the middle ear
 Diseases of the middle ear Acute Suppurative Otitis Media: Acute suppurative otitis media may be viral or bacterial and is accompanied by signs of pain, pressure sensation, diminished hearing and occasional
Diseases of the middle ear Acute Suppurative Otitis Media: Acute suppurative otitis media may be viral or bacterial and is accompanied by signs of pain, pressure sensation, diminished hearing and occasional
Surgery for Conductive Hearing Loss
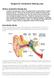 Surgery for Conductive Hearing Loss What is conductive hearing loss Conductive hearing loss is a form of hearing loss due to abnormalities in mobile portions of the ear. Theses are the movable parts (including
Surgery for Conductive Hearing Loss What is conductive hearing loss Conductive hearing loss is a form of hearing loss due to abnormalities in mobile portions of the ear. Theses are the movable parts (including
Hearing Tests And Your Child
 HOW EARLY CAN A CHILD S HEARING BE TESTED? Most parents can remember the moment they first realized that their child could not hear. Louise Tracy has often told other parents of the time she went onto
HOW EARLY CAN A CHILD S HEARING BE TESTED? Most parents can remember the moment they first realized that their child could not hear. Louise Tracy has often told other parents of the time she went onto
Learners Who are Deaf or Hard of Hearing Kalie Carlisle, Lauren Nash, and Allison Gallahan
 Learners Who are Deaf or Hard of Hearing Kalie Carlisle, Lauren Nash, and Allison Gallahan Definition Deaf A deaf person is one whose hearing disability precludes successful processing of linguistic information
Learners Who are Deaf or Hard of Hearing Kalie Carlisle, Lauren Nash, and Allison Gallahan Definition Deaf A deaf person is one whose hearing disability precludes successful processing of linguistic information
Questions and Answers for Parents
 Questions and Answers for Parents There are simple, inexpensive tests available to detect hearing impairment in infants during the first days of life. In the past, most hearing deficits in children were
Questions and Answers for Parents There are simple, inexpensive tests available to detect hearing impairment in infants during the first days of life. In the past, most hearing deficits in children were
Bone Conduction Implants Recent Changes in Surgical Techniques, Hearing Technology & Implant Options
 2 0 1 5 A G B e l l L S L S y m p o s i u m Bone Conduction Implants Recent Changes in Surgical Techniques, Hearing Technology & Implant Options J u l y 1 0, 2 0 1 5 The Oticon Medical company Part of
2 0 1 5 A G B e l l L S L S y m p o s i u m Bone Conduction Implants Recent Changes in Surgical Techniques, Hearing Technology & Implant Options J u l y 1 0, 2 0 1 5 The Oticon Medical company Part of
Cochlear Implants. Policy Number: 7.01.05 Last Review: 10/2015 Origination: 10/1988 Next Review: 10/2016
 Cochlear Implants Policy Number: 7.01.05 Last Review: 10/2015 Origination: 10/1988 Next Review: 10/2016 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for cochlear implants
Cochlear Implants Policy Number: 7.01.05 Last Review: 10/2015 Origination: 10/1988 Next Review: 10/2016 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for cochlear implants
Patient: A 65-year-old male who is a Medicare Part B beneficiary, whose testing was ordered by his internist
 The following examples are to assist you with PQRS reporting. These examples were created in collaboration with the Academy of Doctors of Audiology and the American Speech-Language-Hearing Association
The following examples are to assist you with PQRS reporting. These examples were created in collaboration with the Academy of Doctors of Audiology and the American Speech-Language-Hearing Association
MEDICAL POLICY POLICY TITLE POLICY NUMBER ACUTE INPATIENT REHABILITATION MP-8.003
 Original Issue Date (Created): July 1, 2002 Most Recent Review Date (Revised): Effective Date: May 27, 2008 May 1, 2008- RETIRED I. DESCRIPTION/BACKGROUND Inpatient rehabilitation hospitals provide an
Original Issue Date (Created): July 1, 2002 Most Recent Review Date (Revised): Effective Date: May 27, 2008 May 1, 2008- RETIRED I. DESCRIPTION/BACKGROUND Inpatient rehabilitation hospitals provide an
Bone Anchored Hearing Aids B.A.H.A
 Bone Anchored Hearing Aids B.A.H.A Dr. Abdulrahman Hagr MBBS FRCS(c) Assistant Professor King Saud University Otolaryngology Consultant Otologist, Neurotologist & Skull Base Surgeon King Abdulaziz Hospital
Bone Anchored Hearing Aids B.A.H.A Dr. Abdulrahman Hagr MBBS FRCS(c) Assistant Professor King Saud University Otolaryngology Consultant Otologist, Neurotologist & Skull Base Surgeon King Abdulaziz Hospital
Cochlear Implants: A Communication Choice. Cochlear Implants: A Communication Tool. www.cochlear.com
 Cochlear Ltd ABN 96 002 618 073 14 Mars Road, PO Box 629 Lane Cove NSW 2066 Australia Tel: 61 2 9428 6555 Fax: 61 2 9428 6353 Cochlear Americas 400 Inverness Parkway Suite 400 Englewood CO 80112 USA Tel:
Cochlear Ltd ABN 96 002 618 073 14 Mars Road, PO Box 629 Lane Cove NSW 2066 Australia Tel: 61 2 9428 6555 Fax: 61 2 9428 6353 Cochlear Americas 400 Inverness Parkway Suite 400 Englewood CO 80112 USA Tel:
62 Hearing Impaired MI-SG-FLD062-02
 62 Hearing Impaired MI-SG-FLD062-02 TABLE OF CONTENTS PART 1: General Information About the MTTC Program and Test Preparation OVERVIEW OF THE TESTING PROGRAM... 1-1 Contact Information Test Development
62 Hearing Impaired MI-SG-FLD062-02 TABLE OF CONTENTS PART 1: General Information About the MTTC Program and Test Preparation OVERVIEW OF THE TESTING PROGRAM... 1-1 Contact Information Test Development
Hearing Aids. What Is a Hearing Aid? How Common Is Hearing Loss and What Causes It? How Do We Hear?
 Hearing Aids What Is a Hearing Aid? A hearing aid is an electronic, battery-operated device that amplifies and changes sound to allow for improved communication. Hearing aids receive sound through a microphone,
Hearing Aids What Is a Hearing Aid? A hearing aid is an electronic, battery-operated device that amplifies and changes sound to allow for improved communication. Hearing aids receive sound through a microphone,
FOR PUBLIC CONSULTATION ONLY BONE-CONDUCTION HEARING DEVICES IN PEOPLE WITH HEARING IMPAIRMENT
 1 EVIDENCE SUMMARY REPORT FOR PUBLIC CONSULTATION ONLY BONE-CONDUCTION HEARING DEVICES IN PEOPLE WITH HEARING IMPAIRMENT QUESTIONS TO BE ADDRESSED: 1. Are the following bone-conduction hearing devices
1 EVIDENCE SUMMARY REPORT FOR PUBLIC CONSULTATION ONLY BONE-CONDUCTION HEARING DEVICES IN PEOPLE WITH HEARING IMPAIRMENT QUESTIONS TO BE ADDRESSED: 1. Are the following bone-conduction hearing devices
A new method of partial deafness treatment
 Signature: Med Sci Monit, 3; 9(4): CS-24 PMID: 129676 WWW.MEDSCIMONIT.COM Case Study Received: 2.12.5 Accepted: 3.2. Published: 3.4.23 A new method of partial deafness treatment Henryk Skarżyński, Artur
Signature: Med Sci Monit, 3; 9(4): CS-24 PMID: 129676 WWW.MEDSCIMONIT.COM Case Study Received: 2.12.5 Accepted: 3.2. Published: 3.4.23 A new method of partial deafness treatment Henryk Skarżyński, Artur
MEDICAL POLICY SUBJECT: COGNITIVE REHABILITATION. POLICY NUMBER: 8.01.19 CATEGORY: Therapy/Rehabilitation
 MEDICAL POLICY SUBJECT: COGNITIVE REHABILITATION PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical
MEDICAL POLICY SUBJECT: COGNITIVE REHABILITATION PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical
- Review ear anatomy. Evaluation of Hearing. - Specific causes of hearing loss
 Hearing Loss in Primary Care Aaron C. Moberly, MD Otolaryngologist Department of Otorhinolaryngology The Ohio State University Wexner Medical Center Overview - Review ear anatomy - Evaluation of hearing
Hearing Loss in Primary Care Aaron C. Moberly, MD Otolaryngologist Department of Otorhinolaryngology The Ohio State University Wexner Medical Center Overview - Review ear anatomy - Evaluation of hearing
Surgical Treatment of Chronic Rhinosinusitis in. Children
 Surgical Treatment of Chronic Rhinosinusitis in Children Fuad M. Baroody, M.D., F.A.C.S. Professor of Otolaryngology-Head and Neck Surgery and Pediatrics The University of Chicago Medicine and Biological
Surgical Treatment of Chronic Rhinosinusitis in Children Fuad M. Baroody, M.D., F.A.C.S. Professor of Otolaryngology-Head and Neck Surgery and Pediatrics The University of Chicago Medicine and Biological
SUBJECT: MANAGEMENT OF BREAST EFFECTIVE DATE: 12/16/99 IMPLANTS REVISED DATE:
 MEDICAL POLICY SUBJECT: MANAGEMENT OF BREAST PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
MEDICAL POLICY SUBJECT: MANAGEMENT OF BREAST PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy
Middle ear conditions
 Middle ear conditions Middle ear conditions This factsheet is part of our Ears and ear problems range. It is written for people who have been diagnosed with a condition that affects the middle ear. Read
Middle ear conditions Middle ear conditions This factsheet is part of our Ears and ear problems range. It is written for people who have been diagnosed with a condition that affects the middle ear. Read
Cochlear Implant and Aural Rehabilitation Corporate Medical Policy
 Cochlear Implant and Aural Rehabilitation Corporate Medical Policy File name: Cochlear Implant & Aural Rehabilitation File code: UM.REHAB.06 Origination: Adoption of BCBSA policy 2015 Last Review: 03/2015
Cochlear Implant and Aural Rehabilitation Corporate Medical Policy File name: Cochlear Implant & Aural Rehabilitation File code: UM.REHAB.06 Origination: Adoption of BCBSA policy 2015 Last Review: 03/2015
Pure-Tone Assessment and Screening of Children with Middle-Ear Effusion
 J Am Acad Audiol 5 : 173-182 (1994) Pure-Tone Assessment and Screening of Children with Middle-Ear Effusion Shlomo Silman* t Carol A. Silvermantt Daniel S. Arick Abstract The purpose of this prospective
J Am Acad Audiol 5 : 173-182 (1994) Pure-Tone Assessment and Screening of Children with Middle-Ear Effusion Shlomo Silman* t Carol A. Silvermantt Daniel S. Arick Abstract The purpose of this prospective
Audiology (0340) Test at a Glance. About this test. Test Guide Available. See Inside Back Cover. Test Code 0340
 Audiology (0340) Test Guide Available See Inside Back Cover Test at a Glance Test Name Audiology Test Code 0340 Time 2 hours Number of Questions 150 Format Multiple-choice questions Approximate Approximate
Audiology (0340) Test Guide Available See Inside Back Cover Test at a Glance Test Name Audiology Test Code 0340 Time 2 hours Number of Questions 150 Format Multiple-choice questions Approximate Approximate
Protocol. Cochlear Implant
 Protocol Cochlear Implant (70105) Medical Benefit Effective Date: 10/01/14 Next Review Date: 07/16 Preauthorization Yes Review Dates: 04/07, 05/08, 05/09, 03/10, 03/11, 03/12, 03/13, 07/13, 03/14, 07/14,
Protocol Cochlear Implant (70105) Medical Benefit Effective Date: 10/01/14 Next Review Date: 07/16 Preauthorization Yes Review Dates: 04/07, 05/08, 05/09, 03/10, 03/11, 03/12, 03/13, 07/13, 03/14, 07/14,
Guidance on professional practice for Hearing Aid Audiologists
 Guidance on professional practice for Hearing Aid Audiologists Assuring High Quality Professional Hearing Care Introduction This booklet is intended to be guidance on good professional practices for Registered
Guidance on professional practice for Hearing Aid Audiologists Assuring High Quality Professional Hearing Care Introduction This booklet is intended to be guidance on good professional practices for Registered
Cochlear implants for children and adults with severe to profound deafness
 Issue date: January 2009 Review date: February 2011 Cochlear implants for children and adults with severe to profound deafness National Institute for Health and Clinical Excellence Page 1 of 41 Final appraisal
Issue date: January 2009 Review date: February 2011 Cochlear implants for children and adults with severe to profound deafness National Institute for Health and Clinical Excellence Page 1 of 41 Final appraisal
Vibrant Soundbridge and Bone Conduction Hearing Aid in Patients with Bilateral Malformation of External Ear
 34 Original Research THIEME Vibrant Soundbridge and Bone Conduction Hearing Aid in Patients with Bilateral Malformation of External Ear Maria Fernanda Capoani Garcia Mondelli 1 Thais Cristina Barbosa Mariano
34 Original Research THIEME Vibrant Soundbridge and Bone Conduction Hearing Aid in Patients with Bilateral Malformation of External Ear Maria Fernanda Capoani Garcia Mondelli 1 Thais Cristina Barbosa Mariano
Dr. Abdel Aziz Hussein Lecturer of Physiology Mansoura Faculty of Medicine
 Physiological Basis of Hearing Tests By Dr. Abdel Aziz Hussein Lecturer of Physiology Mansoura Faculty of Medicine Introduction Def: Hearing is the ability to perceive certain pressure vibrations in the
Physiological Basis of Hearing Tests By Dr. Abdel Aziz Hussein Lecturer of Physiology Mansoura Faculty of Medicine Introduction Def: Hearing is the ability to perceive certain pressure vibrations in the
A PROFESSIONAL PRACTICE PROFILE
 A PROFESSIONAL PRACTICE PROFILE FOR HEARING HEALTH PROFESSIONALS The International Hearing Society has adopted the following practice profile as a comprehensive declaration of dispensing characteristics
A PROFESSIONAL PRACTICE PROFILE FOR HEARING HEALTH PROFESSIONALS The International Hearing Society has adopted the following practice profile as a comprehensive declaration of dispensing characteristics
The Disability Tax Credit Certificate Tip sheet for Audiologists
 The Disability Tax Credit Certificate Tip sheet for Audiologists Developed by: The Canadian Academy of Audiology (CAA) & Speech- Language and Audiology Canada (SAC) Purpose of This Document The Canada
The Disability Tax Credit Certificate Tip sheet for Audiologists Developed by: The Canadian Academy of Audiology (CAA) & Speech- Language and Audiology Canada (SAC) Purpose of This Document The Canada
Ear Disorders and Problems
 Ear Disorders and Problems Introduction Your ear has three main parts: outer, middle and inner. You use all of them to hear. There are many disorders and problems that can affect the ear. The symptoms
Ear Disorders and Problems Introduction Your ear has three main parts: outer, middle and inner. You use all of them to hear. There are many disorders and problems that can affect the ear. The symptoms
Position Paper on Cochlear Implants in Children
 Position Paper on Cochlear Implants in Children Position: The Canadian Association of Speech-Language Pathologists and Audiologists (CASLPA) supports cochlear implantation in children where appropriate
Position Paper on Cochlear Implants in Children Position: The Canadian Association of Speech-Language Pathologists and Audiologists (CASLPA) supports cochlear implantation in children where appropriate
The NAL Percentage Loss of Hearing Scale
 The NAL Percentage Loss of Hearing Scale Anne Greville Audiology Adviser, ACC February, 2010 The NAL Percentage Loss of Hearing (PLH) Scale was developed by John Macrae of the Australian National Acoustic
The NAL Percentage Loss of Hearing Scale Anne Greville Audiology Adviser, ACC February, 2010 The NAL Percentage Loss of Hearing (PLH) Scale was developed by John Macrae of the Australian National Acoustic
My child has a hearing loss
 My child has a hearing loss A guide for parents Content You are not alone 3 Hearing impairment 5 Methods of testing hearing 6 Audiogram 7 Types and causes of hearing loss 8 Degree of hearing loss 10 Where
My child has a hearing loss A guide for parents Content You are not alone 3 Hearing impairment 5 Methods of testing hearing 6 Audiogram 7 Types and causes of hearing loss 8 Degree of hearing loss 10 Where
Chapter 8. Hearing and Ear Abnormalities in Fanconi Anemia. Introduction. Anatomy and Function of the Ear
 Chapter 8 Hearing and Ear Abnormalities in Fanconi Anemia H. Jeffrey Kim, MD, FACS, Christopher Zalewski, MA, and Carmen C. Brewer, PhD Introduction In 1927, Guido Fanconi, MD, noticed an association between
Chapter 8 Hearing and Ear Abnormalities in Fanconi Anemia H. Jeffrey Kim, MD, FACS, Christopher Zalewski, MA, and Carmen C. Brewer, PhD Introduction In 1927, Guido Fanconi, MD, noticed an association between
Hearing Tests And Your Child
 How Early Can A Child s Hearing Be Tested? Most parents can remember the moment they first realized that their child could not hear. Louise Tracy has often told other parents of the time she went onto
How Early Can A Child s Hearing Be Tested? Most parents can remember the moment they first realized that their child could not hear. Louise Tracy has often told other parents of the time she went onto
Treatment Guide Understanding Hearing Loss. Cleveland Clinic Hearing Specialists. Choosing Care for Hearing Loss
 Treatment Guide Understanding Hearing Loss Good hearing is part of a full and active life. Let us help you achieve a world of better hearing and improve your quality of life. Choosing Care for Hearing
Treatment Guide Understanding Hearing Loss Good hearing is part of a full and active life. Let us help you achieve a world of better hearing and improve your quality of life. Choosing Care for Hearing
Bone-Anchored (BAHA) and Semi Implantable Hearing Aids
 Bone-Anchored (BAHA) and Semi Implantable Hearing Aids Policy Number: 2016M0023B Effective Date: February 1, 2016 Table of Contents: Page: Cross Reference Policy: POLICY DESCRIPTION 2 Cochlear Implants,
Bone-Anchored (BAHA) and Semi Implantable Hearing Aids Policy Number: 2016M0023B Effective Date: February 1, 2016 Table of Contents: Page: Cross Reference Policy: POLICY DESCRIPTION 2 Cochlear Implants,
Hearing Loss in Geriatric Primary Care Mary Ann Forciea MD Josh Uy MD
 Hearing Loss in Geriatric Primary Care Mary Ann Forciea MD Josh Uy MD Q: In my office practice, I screen for hearing loss with A Level of difficulty in office conversation Questionnaire Hand held hldaudiometer
Hearing Loss in Geriatric Primary Care Mary Ann Forciea MD Josh Uy MD Q: In my office practice, I screen for hearing loss with A Level of difficulty in office conversation Questionnaire Hand held hldaudiometer
MEDICAL POLICY POLICY TITLE DIABETIC SELF-MANAGEMENT TRAINING PROGRAM POLICY NUMBER MP- 2.076
 Original Issue Date (Created): July 1, 2005 Most Recent Review Date (Revised): Effective Date: May 24, 2011 August 31, 2011- RETIRED I. POLICY Initial diabetic self-management training (DSMT) may be considered
Original Issue Date (Created): July 1, 2005 Most Recent Review Date (Revised): Effective Date: May 24, 2011 August 31, 2011- RETIRED I. POLICY Initial diabetic self-management training (DSMT) may be considered
National Medical Policy
 National Medical Policy Subject: Policy Number: Cochlear Implantation NMP298 Effective Date*: November 2006 Updated: May 2016 This National Medical Policy is subject to the terms in the IMPORTANT NOTICE
National Medical Policy Subject: Policy Number: Cochlear Implantation NMP298 Effective Date*: November 2006 Updated: May 2016 This National Medical Policy is subject to the terms in the IMPORTANT NOTICE
COCHLEAR NERVE APLASIA : THE AUDIOLOGIC PERSPECTIVE A CASE REPORT. Eva Orzan, MD Pediatric Audiology University Hospital of Padova, Italy
 COCHLEAR NERVE APLASIA : THE AUDIOLOGIC PERSPECTIVE A CASE REPORT Eva Orzan, MD Pediatric Audiology University Hospital of Padova, Italy Congenital absence or underdevelopment of the cochlear nerve has
COCHLEAR NERVE APLASIA : THE AUDIOLOGIC PERSPECTIVE A CASE REPORT Eva Orzan, MD Pediatric Audiology University Hospital of Padova, Italy Congenital absence or underdevelopment of the cochlear nerve has
Pure Tone Hearing Screening in Schools: Revised Notes on Main Video. IMPORTANT: A hearing screening does not diagnose a hearing loss.
 Pure Tone Hearing Screening in Schools: Revised Notes on Main Video (Notes are also available for Video segments: Common Mistakes and FAQs) IMPORTANT: A hearing screening does not diagnose a hearing loss.
Pure Tone Hearing Screening in Schools: Revised Notes on Main Video (Notes are also available for Video segments: Common Mistakes and FAQs) IMPORTANT: A hearing screening does not diagnose a hearing loss.
Audio Examination. Place of Exam:
 Audio Examination Name: Date of Exam: SSN: C-number: Place of Exam: The Handbook of Standard Procedures and Best Practices for Audiology Compensation and Pension Exams is available online. ( This is a
Audio Examination Name: Date of Exam: SSN: C-number: Place of Exam: The Handbook of Standard Procedures and Best Practices for Audiology Compensation and Pension Exams is available online. ( This is a
MEDICAL POLICY I. POLICY OCCUPATIONAL THERAPY (OUTPATIENT) MP-8.004 POLICY TITLE POLICY NUMBER
 Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 3/24/2015 Effective Date: 11/2/2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS
Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 3/24/2015 Effective Date: 11/2/2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS
X-Plain Perforated Ear Drum Reference Summary
 X-Plain Perforated Ear Drum Reference Summary Introduction Perforated eardrum is a common condition. The eardrum is a thin layer of tissue at the end of the ear canal inside the ear. Eardrum perforations
X-Plain Perforated Ear Drum Reference Summary Introduction Perforated eardrum is a common condition. The eardrum is a thin layer of tissue at the end of the ear canal inside the ear. Eardrum perforations
Early vs. Late Onset Hearing Loss: How Children Differ from Adults. Andrea Pittman, PhD Arizona State University
 Early vs. Late Onset Hearing Loss: How Children Differ from Adults Andrea Pittman, PhD Arizona State University Heterogeneity of Children with Hearing Loss Chronological age Age at onset Age at identification
Early vs. Late Onset Hearing Loss: How Children Differ from Adults Andrea Pittman, PhD Arizona State University Heterogeneity of Children with Hearing Loss Chronological age Age at onset Age at identification
MEDICAL POLICY I. POLICY POLICY TITLE HOME HEALTH POLICY NUMBER MP-3.002
 Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 1/27/2015 Effective Date: 6/1/2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER
Original Issue Date (Created): 7/1/2002 Most Recent Review Date (Revised): 1/27/2015 Effective Date: 6/1/2015 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER
Coding Fact Sheet for Primary Care Pediatricians
 1/1/2015 Hearing Testing Coding Fact Sheet Coding Fact Sheet for Primary Care Pediatricians While coding for hearing screening is relatively straightforward, ensuring that appropriate payment is received
1/1/2015 Hearing Testing Coding Fact Sheet Coding Fact Sheet for Primary Care Pediatricians While coding for hearing screening is relatively straightforward, ensuring that appropriate payment is received
NEW YORK STATE MEDICAID PROGRAM HEARING AID/ AUDIOLOGY SERVICES PROCEDURE CODES
 NEW YORK STATE MEDICAID PROGRAM HEARING AID/ AUDIOLOGY SERVICES PROCEDURE CODES Table of Contents WHAT S NEW FOR THE 2016 MANUAL? --------------------------------------------------------------------------------
NEW YORK STATE MEDICAID PROGRAM HEARING AID/ AUDIOLOGY SERVICES PROCEDURE CODES Table of Contents WHAT S NEW FOR THE 2016 MANUAL? --------------------------------------------------------------------------------
Pediatric Hearing Assessment
 Pediatric Hearing Assessment Stanton Jones Key Points This chapter outlines the methods of hearing assessment that are appropriate for children from birth to adolescence. The importance of timely referral
Pediatric Hearing Assessment Stanton Jones Key Points This chapter outlines the methods of hearing assessment that are appropriate for children from birth to adolescence. The importance of timely referral
NEW YORK STATE MEDICAID PROGRAM HEARING AID/ AUDIOLOGY SERVICES PROCEDURE CODES
 NEW YORK STATE MEDICAID PROGRAM HEARING AID/ AUDIOLOGY SERVICES PROCEDURE CODES Table of Contents GENERAL INFORMATION AND INSTRUCTIONS----------------------------------------------- 2 A. DIAGNOSTIC SERVICES
NEW YORK STATE MEDICAID PROGRAM HEARING AID/ AUDIOLOGY SERVICES PROCEDURE CODES Table of Contents GENERAL INFORMATION AND INSTRUCTIONS----------------------------------------------- 2 A. DIAGNOSTIC SERVICES
Florida Medicaid. Hearing Services Coverage Policy
 Florida Medicaid Agency for Health Care Administration June 2016 Table of Contents 1.0 Introduction... 1 1.1 Description... 1 1.2 Legal Authority... 1 1.3 Definitions... 1 2.0 Eligible Recipient... 2 2.1
Florida Medicaid Agency for Health Care Administration June 2016 Table of Contents 1.0 Introduction... 1 1.1 Description... 1 1.2 Legal Authority... 1 1.3 Definitions... 1 2.0 Eligible Recipient... 2 2.1
Cochlear Implants: Bilateral versus Unilateral
 Health Technology Assessment Cochlear Implants: Bilateral versus Unilateral Final Evidence Report April 17, 2013 Health Technology Assessment Program (HTA) Washington State Health Care Authority PO Box
Health Technology Assessment Cochlear Implants: Bilateral versus Unilateral Final Evidence Report April 17, 2013 Health Technology Assessment Program (HTA) Washington State Health Care Authority PO Box
A Guide to Otoacoustic Emissions (OAEs) for Physicians
 A Guide to Otoacoustic Emissions (OAEs) for Physicians Introduction Hearing loss is not uncommon in children and adults. According to recent estimates, 31.5 million people in the United States report difficulty
A Guide to Otoacoustic Emissions (OAEs) for Physicians Introduction Hearing loss is not uncommon in children and adults. According to recent estimates, 31.5 million people in the United States report difficulty
AP Psychology ~ Ms. Justice
 AP Psychology ~ Ms. Justice 8: What are the characteristics of air pressure waves that we hear as sound? Audition Audition, or hearing, is highly adaptive. We hear a wide range of sounds, but we hear best
AP Psychology ~ Ms. Justice 8: What are the characteristics of air pressure waves that we hear as sound? Audition Audition, or hearing, is highly adaptive. We hear a wide range of sounds, but we hear best
NEW YORK STATE MEDICAID PROGRAM HEARING AID/ AUDIOLOGY SERVICES FEE SCHEDULE
 NEW YORK STATE MEDICAID PROGRAM HEARING AID/ AUDIOLOGY SERVICES FEE SCHEDULE Table of Contents GENERAL INFORMATION AND INSTRUCTIONS----------------------------------------------- 2 CODES -------------------------------------------------------------------------------------------------------
NEW YORK STATE MEDICAID PROGRAM HEARING AID/ AUDIOLOGY SERVICES FEE SCHEDULE Table of Contents GENERAL INFORMATION AND INSTRUCTIONS----------------------------------------------- 2 CODES -------------------------------------------------------------------------------------------------------
