Brain and CNS Cancer. Measurability of Quality Performance Indicators Version 2.3
|
|
|
- Eunice Norton
- 7 years ago
- Views:
Transcription
1 Brain and CNS Cancer Measurability of Quality Permance Indicators Version 2.3 To be read in conjunction with: Brain and Central Nervous System Cancer Clinical Quality Permance Indicators Brain and Central Nervous System Cancer Data Definitions (latest published version) Brain/CNS Cancer QPI Measurability v2.3 1
2 Measurability of Quality Permance Indicators Brain & CNS Cancer Please refer to the Brain and CNS Cancer Quality Permance Indicators published by Healthcare Improvement Scotland a full description of individual QPIs Please refer to the Brain and CNS Cancer QPI Dataset published by ISD Scotland a full description of individual data items Document control: This version Title Brain & CNS Cancer Measurability of QPIs Version/Issue Number 2.3 Effective From December 2014 Author Charlotte Anthony, ISD Document Type Guidance Document status Final Document Purpose Publication Summary of changes Final Revision History Version Date Status Summary of Changes QPI (s) 0.1 June 2013 Draft First version All 0.2 Dec 2013 Draft Changes following public engagement 1.2 June 2014 Final Amendments made out-with review, incorporating additional data item into the dataset updating the MDT 2 QPI. 2.0 Nov 2014 Final Change to version number due to changes in dataset n/a 2.1 May 2015 Final Amendments made out-with review 1, 2, August 2015 Final Amendments made out-with review 6,9 2.3 March 2016 Final Amendments made following Baseline Review 3,5,6,7,8,9,10 Updates from Previous Version QPI Summary of changes (excluding matting changes) (March 2016) 3 Description, Numerator & Denominator changed reference numbers to letters and inserted reference text to align with the QPI document - a This includes: - oligodendroglioma (WHO Grade II) - anaplastic oligodendroglioma (WHO Grade III) - oligoastrocytomas (WHO Grade II) - anaplastic oligoastrocytoma (WHO Grade III) - glioblastoma with an oligodendroglial component (WHO Grade IV) b including subtypes (WHO Grade IV) c The O(6)-methylguanine-DNA methyltransferase (MGMT) gene 5 Denominator removed and Patients undergoing biopsy only ; Main type of Definitive Operation changed to Main Type of Definitive Surgery ; deleted SCAN from MRI SCAN changed equation from [SCAN <> (95 OR...)] to [MRI <> (95 OR...)] 6 Deleted high grade (world health organisation (WHO) Grades III and IV) from QPI title and inserted d after malignant gliomas. Description deleted high grade and after malignant glioma inserted d (with enhancing component on pre-operative imaging) also after 90% inserted resection of the measurable enhancing component Numerator and Denominator have now been split into 2 specifications. Numerator (i) is Number of patients with resectable malignant gliomad (with enhancing component on pre-operative imaging) undergoing surgical resection where >90%* reduction in tumour volume is achieved. Denominator (i) is All patients with malignant gliomad (with enhancing component on pre-operative imaging) undergoing surgical resection. (Excluding patients undergoing biopsy only 1 ). Date of Diagnosis [DIAGDATE] in range specified comparative analysis; AND Morphology of Tumour coded as Glioblastoma, NOS; Giant cell glioblastoma, Oligodendroglioma, NOS; Oligodendroglioma, anaplastic; AND Main Type of Definitive Surgery {Brain/CNS Cancer} coded as Craniotomy lesion of frontal lobe, Craniotomy lesion of temporal lobe, Craniotomy lesion of parietal lobe, Craniotomy lesion of occipital lobe, Craniotomy lesion of cerebellum, Craniotomy lesion of brain tissue - other site AND Enhancing component present on pre-operative imaging coded as yes AND Post Surgical MRI coded as Yes - [MORPHOL = 9440/3 OR 9441/3 OR 9442/3 OR 9382/3 OR 9450/3 OR 9451/3] AND [OPCODE1 OR OPCODE2 OR OPCODE3 OR OPCODE4 = A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8] Brain/CNS Cancer QPI Measurability v2.3 2
3 AND [ENHANC = 1] AND [POSTMRI = 1]. Numerator (ii) is Number of patients with resectable malignant glioma d (with enhancing component on pre-operative imaging) undergoing surgery (biopsy and surgical resection) where >90%* reduction in tumour volume is achieved. - Reduction in Tumour Volume {Brain/CNS Cancer} coded as 90-99% or 100% - [REDUCT = 3 OR 4]. Denominator (ii) is All patients with malignant glioma d (with enhancing component on pre-operative imaging) undergoing surgery (biopsy and surgical resection) (No exclusions).- Date of Diagnosis [DIAGDATE] in range specified comparative analysis; AND Morphology of Tumour coded as Glioblastoma, NOS; Giant cell glioblastoma, Oligodendroglioma, NOS; Oligodendroglioma, anaplastic; AND Main Type of Definitive Surgery {Brain/CNS Cancer} coded as Craniotomy lesion of frontal lobe, Craniotomy lesion of temporal lobe, Craniotomy lesion of parietal lobe, Craniotomy lesion of occipital lobe, Craniotomy lesion of cerebellum, Craniotomy lesion of brain tissue - other site, Biopsy only AND Enhancing component present on pre-operative imaging coded as yes AND Post Surgical MRI coded as Yes - [MORPHOL = 9440/3 OR 9441/3 OR 9442/3 OR 9382/3 OR 9450/3 OR 9451/3] AND [OPCODE1 OR OPCODE2 OR OPCODE3 OR OPCODE4 = A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8 OR 98] AND [ENHANC = 1] AND [POSTMRI = 1]. NR Exclusion replaced [OPCODE1 OR OPCODE2 OR OPCODE3 OR OPCODE4 =99] with N/A; NR Denominator changed to 2 specs (i) [MORPHOL = 9999/9] OR [OPCODE1 = 99 AND OPCODE2-4 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8) OR OPCODE2 = 99 AND OPCODE1, 3, 4 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8) OR OPCODE3 = 99 AND OPCODE 1, 2, 4 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8) OR OPCODE4 = 99 AND OPCODE1-3 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8)] OR [ENHANC = 99] OR [POSTMRI = 99] and (ii) [MORPHOL = 9999/9] OR [OPCODE1 = 99 AND OPCODE2-4 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8 OR 98) OR OPCODE2 = 99 AND OPCODE1, 3, 4 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8 OR 98) OR OPCODE3 = 99 AND OPCODE 1, 2, 4 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8 OR 98) OR OPCODE4 = 99 AND OPCODE1-3 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8 OR 98)] OR [ENHANC = 99] OR [POSTMRI = 99] References have now changed from Malignant gliomas include: Grade IV (Glioblastoma multime- GBM and its variants e.g. gliosarcoma), Grade III (Anaplastic Astrocytoma- AA), Grade III anaplastic oligodendrogliomas, Grade III or IV mixed tumours e.g. oligoastrocytoma, glioblastoma with oligodenroglial component, Highgrade ependymoma To d Malignant gliomas include: Glioblastoma multime- GBM and its variants e.g. gliosarcoma, Anaplastic Astrocytoma- AA, Anaplastic oligodendrogliomas, Mixed tumours e.g.oligoastrocytoma, glioblastoma with oligodenroglial component, High-grade ependymoma e Patients undergoing biopsy only are excluded by default due to the operation codes used within this measurability * Percentage tumour reduction should be assessed by comparing pre surgical imaging to post surgical 72hr Magnetic Resonance Imaging (MRI) 7 QPI title, Description and Numerator inserted d after malignant glioma, removed (WHO Grades II, III and IV) and inserted (with enhancing component on pre-operative imaging); Denominator inserted d after malignant glioma, removed (WHO Grades II, III and IV) and inserted (with enhancing component on pre-operative imaging), deleted AND WHO Grade coded as Grade II, Grade III or Grade IV, Main type of Definitive Operation changed to Main Type of Definitive Surgery, removed or Craniotomy lesion of cerebellum, inserted Enhancing component present on pre-operative imaging coded as yes; AND, removed Not applicable patients undergoing biopsy only, removed AND [GRADE = 2 OR 3 OR 4], OPCODE OR A02.5, POSTMRI OR 96 inserted AND [ENHANC =1]; NR Denominator removed OR [GRADE = 99] and inserted OR [ENHANC = 99] Reference d Malignant gliomas include: Glioblastoma multime- GBM and its variants e.g. gliosarcoma, Anaplastic Astrocytoma- AA, Anaplastic oligodendrogliomas, Mixed tumours e.g.oligoastrocytoma, glioblastoma with oligodenroglial component, High-grade ependymoma is now inserted. 8 Denominator removed radiotherapy course type coded as Adjuvant, Neo adjuvant 1 OR 2 OR 9 Changed from Access to Adjuvant Treatment to Access to Oncological Treatment ; QPI Title removed surgical resection and inserted surgery ; Description changed surgical resection to surgery throughout and receive to commence ; Numerator changed from Number of patients with high grade glioma (WHO grades III and IV) who undergo surgical resection who commence oncological treatment (chemotherapy, radiotherapy or chemoradiotherapy) within 6 weeks of surgical resection. - Radiotherapy Course Type {Brain/CNS Cancer} 1; or Type of Systemic Anti-Cancer Therapy (SACT) {Brain/CNS Cancer} 1 coded as adjuvant or chemoradiotherapy; AND Date Treatment Started {Cancer} (Radiotherapy) 1; or Date Treatment Started Systemic Anti-Cancer Therapy (SACT) 1 minus Date of Definitive Surgery is less than or equal to 42 - ( [RADIO1 = 1 OR 7] AND [RADDATE1 DSURG 42] ) OR ( [CHEMTYPE1 = 1 OR 5] AND [CHEMDATE1 DSURG 42] ) To Number of patients with high grade glioma (WHO grades III and IV) who undergo oncological treatment (chemotherapy, radiotherapy or chemoradiotherapy) who commence treatment within 6 weeks of surgery. - Date Treatment Started {Cancer} (Radiotherapy) 1; or Date Treatment Started Systemic Anti-Cancer Therapy (SACT) 1 minus Date of Definitive Surgery is less than or equal to 42 - [RADDATE1 DSURG 42] OR [CHEMDATE1 DSURG 42] ) ; Denominator changed from All patients with high grade glioma (WHO grades 3 and 4) who undergo surgical resection. (No exclusions) - Date of Diagnosis [DIAGDATE] in range specified comparative analysis; AND Morphology of Tumour coded as Glioblastoma, NOS; Giant cell glioblastoma, Oligodendroglioma, NOS; Oligodendroglioma, anaplastic; AND WHO Grade coded as Grade III or Grade IV; AND Main Type of Definitive Surgery {Brain/CNS Cancer} 1-4 coded as Craniotomy lesion of; frontal lobe, temporal lobe, parietal lobe, occipital lobe, cerebellum or brain tissue other site. - [MORPHOL = 9382/3 OR 9440/3 OR 9441/3 OR 9442/3 OR 9450/3 OR 9451/3] AND [GRADE = 3 OR 4] AND ([OPCODE1 OR OPCODE2 OR OPCODE3 OR OPCODE4 = A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8]) ; NR Numerator equation changed from [RADDATE1 = 09/09/0909] OR [CHEMDATE1 = 09/09/0909] OR [DSURG = 09/09/0909] OR [RADIO1 = 99] OR [CHEMTYPE1 = 99] To ([RADDATE1 = 09/09/0909] AND [CHEMDATE1 = 09/09/0909]) OR [DSURG = 09/09/0909] ; NR Brain/CNS Cancer QPI Measurability v2.3 3
4 Denominator equation changed from [MORPHOL = 9999/9] OR [GRADE = 99] OR [OPCODE1 OR OPCODE2 OR OPCODE3 OR OPCODE4 = 99] To [MORPHOL = 9999/9] OR [GRADE = 99] OR [OPCODE1 = 99 AND OPCODE2-4 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8 OR 98) OR OPCODE2 = 99 AND OPCODE1, 3, 4 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8 OR 98) OR OPCODE3 = 99 AND OPCODE 1, 2, 4 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8 OR 98) OR OPCODE4 = 99 AND OPCODE1-3 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8 OR 98)] OR [(RADIO1 = 99 AND CHEMTYPE1 <>(1 OR 5)] OR [(CHEMTYPE1 = 99 AND RADIO1 <> (3 OR 7)] 10 Denominator added Chemoradiotherapy Radiotherapy Course Type [RADIO1 OR RADIO2 = 3 OR 7] QPI Summary of changes (excluding matting changes) (August 2015) 6 Remove duplicate 'or Craniotomy lesion of cerebellum' and duplicate opcode 'OR A NR Denominator MORPHOL = 9999/9 instead of 99 and OPCODE = 99 instead of 9999/9. QPI Summary of changes (excluding matting changes) (May 2015) 1 NR Num changed to [PSTATUS <>9] AND ([WHODATE = 09/09/0909] OR [MDTDATE = 09/09/0909]), NR Den changed to [ICDSITE = C99.X] OR [MDTDATE = 09/09/0909] 2 Numerator changed to [MDTDATE <> 10/10/1010] AND ([MDTDATE DEFTREATDATE] OR [DEFTREATDATE = 10/10/1010]) 9 RADIO2 and CHEMTYPE2 removed, chemoradiotherapy added QPI Summary of changes (excluding matting changes) (June 2014) 2 Inserted new MDT QPI. 8 Numerator amended Specialist Neuro-oncologist {Brain/CNS Cancer} coded as Yes [SPECNEURO = 01], NR Numerator amended [SPECNEURO = 99] Brain/CNS Cancer QPI Measurability v2.3 4
5 QPI 1 - Documentation of Permance Status QPI Title: Patients with newly-diagnosed brain/central nervous system (CNS) cancer should have a world health organisation (WHO) permance status documented at time of diagnosis. Numerator: Proportion of newly-diagnosed patients with brain/cns cancer who have a documented WHO permance status at the time of multidisciplinary team (MDT) discussion. Number of newly-diagnosed patients with brain/cns cancer discussed at MDT meeting with a documented WHO permance status at the time of MDT discussion. WHO/ECOG Permance Status not coded as Not Applicable; AND Date of Assessment of WHO/ECOG Permance Status (KPS) is less than or equal to Date Discussed by Care Team (MDT) [PSTATUS <> 9] AND [WHODATE MDTDATE] Denominator: All newly-diagnosed patients with brain/cns cancer discussed at MDT meeting (No exclusions). Date of Diagnosis [DIAGDATE] in range specified comparative analysis; AND Site of Origin of Primary Tumour {Brain/CNS Cancer} 1-4 coded as Brain/CNS Cancer; AND Date Discussed by Care Team (MDT) not coded as Inapplicable [ICDSITE = C700 OR C701 OR C709 OR C71 OR C72 OR C75.2 OR C75.3] AND [MDTDATE <> 10/10/1010] numerator exclusion Include in the measurement against the target. Present as not recorded only if the patient cannot otherwise be identified as [PSTATUS <>9] AND ([ WHODATE = 09/09/0909] OR [MDTDATE = 09/09/0909]) Include in the measurement against the target unless there is other definitive evidence that the record should be excluded. Present as not recorded only where the record cannot otherwise be definitively identified as an inclusion/exclusion this standard. N/A Exclude from the measurement against the target. Present as not recorded only where the patient cannot otherwise be definitively identified as an inclusion/exclusion this standard [ICDSITE = C99.X] Brain/CNS Cancer QPI Measurability v2.3 5
6 QPI 2 Multi-Disciplinary Team Meeting QPI Title: Patients with brain/cns cancer should be discussed by a multidisciplinary (MDT) team prior to definitive management. Numerator: Denominator: Proportion of patients with Brain/CNS cancer who are discussed at MDT meeting bee definitive management Number of patients with Brain/CNS cancer discussed at the MDT bee definitive treatment. Date Discussed by Care Team (MDT) not coded as not applicable; AND Date Discussed by Care Team (MDT) is bee or equal to Date of Definitive Treatment OR Date of Definitive Treatment is equal to not applicable. [MDTDATE <> 10/10/1010] AND ([MDTDATE DEFTREATDATE] OR [DEFTREATDATE = 10/10/1010]) All patients with Brain/CNS cancer (Excluding patients who died bee first treatment). Date of Diagnosis [DIAGDATE] in range specified comparative analysis; AND Site of Origin of Primary Tumour {Brain/CNS Cancer} 1-4 coded as Brain/CNS Cancer; AND Type of First Treatment not coded as Patient died bee treatment. numerator exclusion ( [ICDSITE = C700 OR C701 OR C709 OR C71 OR C72 OR C75.2 OR C75.3] ) AND [FIRSTTREATTYPE <>94] Include in the measurement against the target. Present as not recorded only if the patient cannot otherwise be identified as [MDTDATE = 09/09/0909] OR [DEFTREATDATE = 09/09/0909] Include in the measurement against the target unless there is other definitive evidence that the record should be excluded. Present as not recorded only where the record cannot otherwise be definitively identified as an inclusion/exclusion this standard. [FIRSTTREATTYPE = 99] Exclude from the measurement against the target. Present as not recorded only where the patient cannot otherwise be definitively identified as an inclusion/exclusion this standard [ICDSITE = C99.X] Brain/CNS Cancer QPI Measurability v2.3 6
7 b c QPI 3 Molecular Analysis QPI Title: Patients with biopsied or resected gliomas should have molecular analysis permed on the tumour tissue within 21 days of surgery to inm treatment decision making. Proportion of patients with biopsied or resected gliomas who undergo relevant molecular analysis of tumour tissue within 21 days of surgery. Please note: This QPI measures 2 distinct elements: (i): Patients with gliomas with an oligodendroglial a component who have the tumour tested combined loss of 1p/19q; and (ii): Patients with glioblastomas b who have the tumour tested MGMT c promoter methylation status. Specification (i) Numerator (i) Number of patients with glioma with an oligodendroglial a component where tissue sample is tested 1p/19q within 21 days of surgery. 1P/19Q Tissue Analysis {Brain/CNS Cancer} not coded as Not Done or Insufficient Sample; and Date of 1P/19Q Tissue Analysis {Brain/CNS Cancer} minus Date of Definitive Surgery less than or equal to 21 days Specifications (ii): Denominator (i) Numerator (ii) [SAMP1P <> 03 OR 04] AND [SAMP1PDATE DSURG 21] All patients with glioma with an oligodendroglial a component undergoing surgery (No exclusions). Date of Diagnosis [DIAGDATE] in range specified comparative analysis; AND Morphology of Tumour coded as Mixed glioma; Oligodendroglioma, NOS; Oligodendroglioma, anaplastic; AND WHO Grade coded as Grade II, Grade III or Grade IV; and Date of Definitive Surgery not coded as Inapplicable. [MORPHOL = 9382/3 OR 9450/3 OR 9451/3] AND [GRADE = 2 OR 3 OR 4] AND [DSURG <> 10/10/1010] Number of patients with glioblastomas where tissue sample is assessed MGMT promoter hypermethylation status within 21 days of surgery. MGMT Tissue Analysis not coded as Not Done or Insufficient Sample; and Date of MGMT Tissue Analysis minus Date of Definitive Surgery less than or equal to 21 days [MGMTSAMP <> 03 OR 04] AND [MGMTSAMPDATE DSURG 21] a This includes: - oligodendroglioma (WHO Grade II) - anaplastic oligodendroglioma (WHO Grade III) - oligoastrocytomas (WHO Grade II) - anaplastic oligoastrocytoma (WHO Grade III) - glioblastoma with an oligodendroglial component (WHO Grade IV) b including subtypes (WHO Grade IV) c The O(6)-methylguanine-DNA methyltransferase (MGMT) gene a Brain/CNS Cancer QPI Measurability v2.3 7
8 Denominator (ii) All patients with glioblastoma undergoing surgery (No exclusions). Date of Diagnosis [DIAGDATE] in range specified comparative analysis; AND Morphology of Tumour coded as Glioblastoma, NOS; Giant cell glioblastoma; or Gliosarcoma (Glioblastoma with Sarcomatous Component); AND WHO Grade coded as Grade IV, and Date of Definitive Surgery not coded as Inapplicable. [MORPHOL = 9440/3 OR 9441/3 OR 9442/3] AND [GRADE = 4] AND [DSURG <> 10/10/1010] numerator Include in the measurement against the target. Present as not recorded only if the patient cannot otherwise be identified as (i) [SAMP1P = 99] OR [SAMP1PDATE = 09/09/0909] OR [DSURG = 09/09/0909] (ii) [MGMTSAMP = 99] OR [MGMTSAMPDATE = 09/09/0909] OR [DSURG = 09/09/0909] exclusion Include in the measurement against the target unless there is other definitive evidence that the record should be excluded. Present as not recorded only where the record cannot otherwise be definitively identified as an inclusion/exclusion this standard. (i) and (ii) N/A Exclude from the measurement against the target. Present as not recorded only where the patient cannot otherwise be definitively identified as an inclusion/exclusion this standard (i) and (ii) [MORPHOL = 9999/9] OR [GRADE = 99] OR [DSURG = 09/09/0909] Brain/CNS Cancer QPI Measurability v2.3 8
9 QPI 4 Neuropathological Diagnosis QPI Title: Numerator: All pathology reports brain/central nervous system (CNS) cancer should contain full pathology inmation (including WHO grade) to inm patient management. Proportion of patients diagnosed with brain/cns cancer where the pathology report contains a full set of data items as defined by the Royal College of Pathologists Number of patients with a histological diagnosis of brain/cns cancer where histological pathology report contains all data items, as defined by relevant Royal College of Pathologists publication. Histopathology Report Complete {Brain/CNS Cancer} coded as Complete [PATHCOMPL = 1] Denominator: All patients with a histological diagnosis of brain/cns cancer (No exclusions). Date of Diagnosis [DIAGDATE] in range specified comparative analysis; AND Site of Origin of Primary Tumour {Cancer} coded as Brain/CNS Cancer; AND Most Valid Basis of Diagnosis {Cancer} coded as Histology of metastasis, or Histology of primary [ICDSITE = C700 OR C701 OR C709 OR C71 OR C72 OR C75.2 OR C75.3] AND [VALID = 6 OR 7] numerator exclusion Include in the measurement against the target. Present as not recorded only if the patient cannot otherwise be identified as [PATHCOMPL = 99] Include in the measurement against the target unless there is other definitive evidence that the record should be excluded. Present as not recorded only where the record cannot otherwise be definitively identified as an inclusion/exclusion this standard. N/A Exclude from the measurement against the target. Present as not recorded only where the patient cannot otherwise be definitively identified as an inclusion/exclusion this standard [ICDSITE = C99.X] OR [VALID = 99] Brain/CNS Cancer QPI Measurability v2.3 9
10 QPI 5 Pre-Treatment Magnetic Resonance Imaging (MRI) QPI Title: Numerator: Patients with brain/central nervous system (CNS) cancer should have Magnetic Resonance Imaging (MRI) prior to treatment. Proportion of patients with brain/cns cancer undergoing resection and/or radical radiotherapy or chemotherapy, who have an MRI prior to treatment. Number of patients with brain/cns cancer undergoing resection of tumour, radical radiotherapy or chemotherapy who receive an MRI prior to treatment. MRI SCAN (Pre-treatment) coded as Yes [MRI = 1] Denominator: All patients with brain/cns cancer undergoing resection of tumour, radical radiotherapy or chemotherapy (Excluding Patients unable to undergo an MRI scan (e.g.pacemaker or other MRI incompatible implanted device; Cerebral aneurysm clip; Metal in eye; Claustrophobia; Unable to fit bore of scanner; Too heavy MRI table); Patients who refuse MRI; and Patients with Contraindication to intravenous contrast medium;. Date of Diagnosis [DIAGDATE] in range specified comparative analysis; AND Site of Origin of Primary Tumour {Cancer} coded as Brain/CNS Cancer; AND (Main Type of Definitive Surgery{Brain/CNS Cancer} 1-4 coded as Craniotomy lesion of frontal lobe, Craniotomy lesion of temporal lobe, Craniotomy lesion of parietal lobe, Craniotomy lesion of occipital lobe, Craniotomy lesion of cerebellum, Craniotomy lesion of brain tissue - other site, or Craniotomy lesion of cerebellum; OR Radiotherapy Course Type {Brain/CNS Cancer} 1-2 coded as Radical, or Chemoradiotherapy; OR Type of Systemic Anti-Cancer Therapy (SACT) (Brain/CNS Cancer) 1-2 coded as Neoadjuvant, or Chemoradiotherapy; AND MRI (Pre-treatment) not coded as Patient refused investigation, Contraindication to intravenous contrast medium, or Clinically inappropriate [ICDSITE = C700 OR C701 OR C709 OR C71 OR C72 OR C75.2 OR C75.3] AND ( [OPCODE1 OR OPCODE2 OR OPCODE3 OR OPCODE4 = A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.8 OR A02.5] OR [RADIO1 OR RADIO2 = 3 OR 7] OR [CHEMTYPE1 OR CHEMTYPE2 = 02 OR 05] ) AND [MRI<> (95 OR 97 OR 98)] numerator exclusion Include in the measurement against the target. Present as not recorded only if the patient cannot otherwise be identified as [MRI = 99] Include in the measurement against the target unless there is other definitive evidence that the record should be excluded. Present as not recorded only where the record cannot otherwise be definitively identified as an inclusion/exclusion this standard. [MRI = 99] Exclude from the measurement against the target. Present as not recorded only where the patient cannot otherwise be definitively identified as an inclusion/exclusion this standard [ICDSITE = C99.X] OR [OPCODE1 OR OPCODE2 OR OPCODE3 OR OPCODE4 = 99] OR [RADIO1 OR RADIO2 = 99] OR [CHEMTYPE1 OR CHEMTYPE2 = 99] Brain/CNS Cancer QPI Measurability v2.3 10
11 QPI 6 Maximal Surgical Resection QPI Title: Wherever possible patients should undergo maximal surgical resection of malignant gliomas d. Proportion of patients with malignant glioma d (with enhancing component on pre-operative imaging) who undergo maximal surgical resection (>90% resection of the measurable enhancing component), provided it is considered consistent with safe outcome. Numerator (i): Number of patients with resectable malignant glioma d (with enhancing component on pre-operative imaging) undergoing surgical resection where >90%* reduction in tumour volume is achieved. Reduction in Tumour Volume {Brain/CNS Cancer} coded as 90-99% or 100% Specification (i): [REDUCT = 3 OR 4] Denominator (i): All patients with malignant glioma d (with enhancing component on pre-operative imaging) undergoing surgical resection. (Excluding patients undergoing biopsy only d ). Date of Diagnosis [DIAGDATE] in range specified comparative analysis; AND Morphology of Tumour coded as Glioblastoma, NOS; Giant cell glioblastoma, Oligodendroglioma, NOS; Oligodendroglioma, anaplastic; ; AND Main Type of Definitive Surgery {Brain/CNS Cancer} coded as Craniotomy lesion of frontal lobe, Craniotomy lesion of temporal lobe, Craniotomy lesion of parietal lobe, Craniotomy lesion of occipital lobe, Craniotomy lesion of cerebellum, Craniotomy lesion of brain tissue - other site AND Enhancing component present on pre-operative imaging coded as yes AND Post Surgical MRI coded as Yes [MORPHOL = 9440/3 OR 9441/3 OR 9442/3 OR 9382/3 OR 9450/3 OR 9451/3] AND [OPCODE1 OR OPCODE2 OR OPCODE3 OR OPCODE4 = A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8] AND [ENHANC = 1] AND [POSTMRI = 1] Numerator (ii): Number of patients with resectable malignant glioma d (with enhancing component on pre-operative imaging) undergoing surgery (biopsy and surgical resection) where >90%* reduction in tumour volume is achieved. Reduction in Tumour Volume {Brain/CNS Cancer} coded as 90-99% or 100% Specification [REDUCT = 3 OR 4] d Malignant gliomas include: Glioblastoma multime- GBM and its variants e.g. gliosarcoma) Anaplastic Astrocytoma- AA) Aanaplastic oligodendrogliomas Mixed tumours e.g. oligoastrocytoma, glioblastoma with oligodenroglial component High-grade ependymoma e Patients undergoing biopsy only are excluded by default due to the operation codes used within this measurability Brain/CNS Cancer QPI Measurability v2.3 11
12 (ii): Denominator (ii): All patients with malignant glioma d (with enhancing component on pre-operative imaging) undergoing surgery (biopsy and surgical resection) (No exclusions). Date of Diagnosis [DIAGDATE] in range specified comparative analysis; AND Morphology of Tumour coded as Glioblastoma, NOS; Giant cell glioblastoma, Oligodendroglioma, NOS; Oligodendroglioma, anaplastic; AND Main Type of Definitive Surgery {Brain/CNS Cancer} coded as Craniotomy lesion of frontal lobe, Craniotomy lesion of temporal lobe, Craniotomy lesion of parietal lobe, Craniotomy lesion of occipital lobe, Craniotomy lesion of cerebellum, Craniotomy lesion of brain tissue - other site, Biopsy only AND Enhancing component present on pre-operative imaging coded as yes AND Post Surgical MRI coded as Yes [MORPHOL = 9440/3 OR 9441/3 OR 9442/3 OR 9382/3 OR 9450/3 OR 9451/3] AND [OPCODE1 OR OPCODE2 OR OPCODE3 OR OPCODE4 = A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8 OR 98] AND [ENHANC = 1] AND [POSTMRI = 1] numerator exclusion Include in the measurement against the target. Present as not recorded only if the patient cannot otherwise be identified as [REDUCT = 99] Include in the measurement against the target unless there is other definitive evidence that the record should be excluded. Present as not recorded only where the record cannot otherwise be definitively identified as an inclusion/exclusion this standard. N/A Exclude from the measurement against the target. Present as not recorded only where the patient cannot otherwise be definitively identified as an inclusion/exclusion this standard (i) [MORPHOL = 9999/9] OR [OPCODE1 = 99 AND OPCODE2-4 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8) OR OPCODE2 = 99 AND OPCODE1, 3, 4 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8) OR OPCODE3 = 99 AND OPCODE 1, 2, 4 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8) OR OPCODE4 = 99 AND OPCODE1-3 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8)] OR [ENHANC = 99] OR [POSTMRI = 99] (ii) [MORPHOL = 9999/9] OR [OPCODE1 = 99 AND OPCODE2-4 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8 OR 98) OR OPCODE2 = 99 AND OPCODE1, 3, 4 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8 OR 98) OR OPCODE3 = 99 AND OPCODE 1, 2, 4 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8 OR 98) OR OPCODE4 = 99 AND OPCODE1-3 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8 OR 98)] OR [ENHANC = 99] OR [POSTMRI = 99] *Percentage tumour reduction should be assessed by comparing pre surgical imaging to post surgical 72hr Magnetic Resonance Imaging (MRI) Brain/CNS Cancer QPI Measurability v2.3 12
13 QPI 7 Early Post-Operative Imaging QPI Title: Numerator: Patients with malignant glioma d (with enhancing component on pre-operative imaging) undergoing surgical resection should be subject to early post-operative imaging. Proportion of patients with malignant glioma d, (with enhancing component on pre-operative imaging) who receive early post operative imaging with Magnetic Resonance Imaging (MRI) within 3 days (72hrs) of surgical resection. Number of patients with malignant glioma d, (with enhancing component on pre-operative imaging) undergoing surgical resection receiving MRI within 3 days (72hrs) of surgical resection. Date of Post Surgical MRI SCAN minus Date of Definitive Surgery less than or equal to 3 [POSTMRIDATE DSURG 3] Denominator: NB Dates do not allow specification in hours All patients with malignant glioma d, (with enhancing component on pre-operative imaging), undergoing surgical resection. (Excluding Patients unable to undergo an MRI scan (e.g.pacemaker or other MRI incompatible implanted device; Cerebral aneurysm clip; Metal in eye; Claustrophobia; Unable to fit bore of scanner; Too heavy MRI table); Patients who refuse MRI; Contraindication to intravenous contrast medium; and Patients undergoing biopsy only e ). Date of Diagnosis [DIAGDATE] in range specified comparative analysis; AND Morphology of Tumour coded as Glioblastoma, NOS; Giant cell glioblastoma, Oligodendroglioma, NOS; Oligodendroglioma, anaplastic; ; AND Main Type of Definitive Surgery {Brain/CNS Cancer} coded as Craniotomy lesion of frontal lobe, Craniotomy lesion of temporal lobe, Craniotomy lesion of parietal lobe, Craniotomy lesion of occipital lobe, Craniotomy lesion of cerebellum, Craniotomy lesion of brain tissue - other site ; AND Enhancing component present on pre-operative imaging coded as yes; AND Post Surgical MRI SCAN not coded as Patient refused investigation,, Contraindication to intravenous contrast medium, or Clinically inappropriate. [MORPHOL = 9440/3 OR 9441/3 OR 9442/3 OR 9382/3 OR 9450/3 OR 9451/3] AND [OPCODE1 OR OPCODE2 OR OPCODE3 OR OPCODE4 = A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8 ] AND [ENHANC = 1] AND [POSTMRI <> (95 OR 97 OR 98)] numerator Include in the measurement against the target. Present as not recorded only if the patient cannot otherwise be identified as [POSTMRIDATE = 09/09/0909] OR [DSURG = 09/09/0909] d Malignant gliomas include: Glioblastoma multime- GBM and its variants e.g. gliosarcoma) Anaplastic Astrocytoma- AA) Aanaplastic oligodendrogliomas Mixed tumours e.g. oligoastrocytoma, glioblastoma with oligodenroglial component High-grade ependymoma e Patients undergoing biopsy only are excluded by default due to the operation codes used within this measurability Brain/CNS Cancer QPI Measurability v2.3 13
14 exclusion Include in the measurement against the target unless there is other definitive evidence that the record should be excluded. Present as not recorded only where the record cannot otherwise be definitively identified as an inclusion/exclusion this standard. [POSTMRI = 99] Exclude from the measurement against the target. Present as not recorded only where the patient cannot otherwise be definitively identified as an inclusion/exclusion this standard [MORPHOL = 9999/9] OR [OPCODE1 OR OPCODE2 OR OPCODE3 OR OPCODE4 = 99] OR [ENHANC = 99] Brain/CNS Cancer QPI Measurability v2.3 14
15 QPI 8 Specialist Neuro-Oncology Access QPI Title: Numerator: Patients with brain/central nervous system (CNS) cancer undergoing oncological treatment should be managed by a site specialist neurooncologist. Proportion of patients with brain/cns cancer undergoing oncological treatment (chemotherapy or radiotherapy) who are managed by a specialist neuro-oncologist. Number of patients with brain/cns cancer undergoing oncological treatment (chemotherapy or radiotherapy) who are managed by specialist neuro-oncologist. Specialist Neuro-oncologist {Brain/CNS Cancer} coded as Yes [SPECNEURO = 01] Denominator: All patients with brain/cns cancer undergoing oncological treatment (chemotherapy or radiotherapy) (No exclusions). Date of Diagnosis [DIAGDATE] in range specified comparative analysis; AND Site of Origin of Primary Tumour {Cancer} coded as Brain/CNS Cancer; AND (Radiotherapy Course Type {Brain/CNS Cancer} 1-2 coded as Radical, Palliative, or Chemoradiotherapy; OR Type of Systemic Anti-Cancer Therapy (SACT) (Brain/CNS Cancer) 1-2 coded as Adjuvant, Neoadjuvant, Palliative, Chemoradiotherapy, or Biological Therapy) [ICDSITE = C700 OR C701 OR C709 OR C71 OR C72 OR C75.2 OR C75.3] AND ( [RADIO1 OR RADIO2 = 3 OR 4 OR 7] OR [CHEMTYPE1 OR CHEMTYPE2 = 1 OR 2 OR 4 OR 5 OR 7] ) numerator exclusion Include in the measurement against the target. Present as not recorded only if the patient cannot otherwise be identified as [SPECNEURO = 99] Include in the measurement against the target unless there is other definitive evidence that the record should be excluded. Present as not recorded only where the record cannot otherwise be definitively identified as an inclusion/exclusion this standard. N/A Exclude from the measurement against the target. Present as not recorded only where the patient cannot otherwise be definitively identified as an inclusion/exclusion this standard [ICDSITE = 99] OR [RADIO1 OR RADIO2 = 99] OR [CHEMTYPE1 OR CHEMTYPE2= 99] Brain/CNS Cancer QPI Measurability v2.3 15
16 QPI 9 Access to Oncological Treatment QPI Title: The maximum time between surgery and oncological treatment patients with high grade glioma (WHO grades III and IV) should be 6 weeks. Numerator: Denominator: numerator exclusion Proportion of patients with high grade glioma (WHO grades III and IV) undergoing surgery who commence their oncological treatment (chemotherapy, radiotherapy or chemoradiotherapy) within 6 weeks of surgery.. Number of patients with high grade glioma (WHO grades III and IV) who undergo oncological treatment (chemotherapy, radiotherapy or chemoradiotherapy) who commence treatment within 6 weeks of surgery. Date Treatment Started {Cancer} (Radiotherapy) 1; or Date Treatment Started Systemic Anti-Cancer Therapy (SACT) 1 minus Date of Definitive Surgery is less than or equal to 42 [RADDATE1 DSURG 42] OR [CHEMDATE1 DSURG 42] ) All patients with high grade glioma (WHO grades 3 and 4) who undergooncological treatment (chemotherapy, radiotherapy or chemoradiotherapy) following surgery.. (No exclusions) Date of Diagnosis [DIAGDATE] in range specified comparative analysis; AND Morphology of Tumour coded as Glioblastoma, NOS; Giant cell glioblastoma, Oligodendroglioma, NOS; Oligodendroglioma, anaplastic; AND WHO Grade coded as Grade III or Grade IV; AND Main Type of Definitive Surgery {Brain/CNS Cancer} 1-4 coded as Craniotomy lesion of; frontal lobe, temporal lobe, parietal lobe, occipital lobe, cerebellum or brain tissue other site, biopsy only AND Radiotherapy Course Type {Brain/CNS Cancer} 1 coded as radical or chemoradiotherapy; or Type of Systemic Anti-Cancer Therapy (SACT) {Brain/CNS Cancer} 1 coded as adjuvant or chemoradiotherapy. [MORPHOL = 9382/3 OR 9440/3 OR 9441/3 OR 9442/3 OR 9450/3 OR 9451/3] AND [GRADE = 3 OR 4] AND ([OPCODE1 OR OPCODE2 OR OPCODE3 OR OPCODE4 = A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8 OR 98]) AND ([RADIO1 =3 or 7] OR [CHEMTYPE1 = 1 or 5]) Include in the measurement against the target. Present as not recorded only if the patient cannot otherwise be identified as ([RADDATE1 = 09/09/0909] AND [CHEMDATE1 = 09/09/0909]) OR [DSURG = 09/09/0909] Include in the measurement against the target unless there is other definitive evidence that the record should be excluded. Present as not recorded only where the record cannot otherwise be definitively identified as an inclusion/exclusion this standard. N/A Exclude from the measurement against the target. Present as not recorded only where the patient cannot otherwise be definitively identified as an inclusion/exclusion this standard [MORPHOL = 9999/9] OR [GRADE = 99] OR [OPCODE1 = 99 AND OPCODE2-4 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8 OR 98) OR OPCODE2 = 99 AND OPCODE1, 3, 4 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8 OR 98) OR OPCODE3 = 99 AND OPCODE 1, 2, 4 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8 OR 98) OR OPCODE4 = 99 AND OPCODE1-3 <> (A02.1 OR A02.2 OR A02.3 OR A02.4 OR A02.5 OR A02.8 OR 98)] OR [(RADIO1 = 99 AND CHEMTYPE1 <>(1 OR 5)] OR [(CHEMTYPE1 = 99 AND RADIO1 <> (3 OR 7)] Brain/CNS Cancer QPI Measurability v2.3 16
17 QPI 10 Radical Radiotherapy Planning Process QPI Title: Numerator: The radical radiotherapy planning process patients with primary brain/cns cancer should include Magnetic Resonance Imaging (MRI) fusion. Proportion of patients with primary brain/central nervous system (CNS) cancer undergoing radical radiotherapy whom the radiotherapy planning process includes MRI fusion. Number of patients with primary brain/cns cancer undergoing radical radiotherapy whom radiotherapy planning includes MRI fusion. MRI Fusion in Radiotherapy Planning {Brain/CNS Cancer} coded as Yes MRI fusion included [MRIFUS = 1] Denominator: All patients with primary brain/cns cancer undergoing radical radiotherapy (Excluding Patients unable to undergo an MRI scan e.g. Pacemaker or other MRI incompatible implanted device; Cerebral aneurysm clip; Metal in eye; Claustrophobia; Unable to fit bore of scanner; Too heavy MRI table; Patients who refuse MRI, Contraindication to intravenous contrast medium) Date of Diagnosis [DIAGDATE] in range specified comparative analysis; AND Site of Origin of Primary Tumour {Cancer} coded as Brain/CNS Cancer;; AND (Radiotherapy Course Type {Brain/CNS Cancer} 1-2 coded as Radical or Chemoradiotherapy; AND MRI Fusion in Radiotherapy Planning {Brain/CNS Cancer} not coded as Clinically inappropriate or Not applicable or Patient Refused or Contraindication to Intravenous Contrast Medium [ICDSITE = C700 OR C701 OR C709 OR C71 OR C72 OR C75.2 OR C75.3] AND [RADIO1 OR RADIO2 = 3 or 7] AND [MRIFUS <> 95 OR 96 OR 97 OR 98] numerator exclusion Include in the measurement against the target. Present as not recorded only if the patient cannot otherwise be identified as [MRIFUS = 99] Include in the measurement against the target unless there is other definitive evidence that the record should be excluded. Present as not recorded only where the record cannot otherwise be definitively identified as an inclusion/exclusion this standard. [MRIFUS = 99] Exclude from the measurement against the target. Present as not recorded only where the patient cannot otherwise be definitively identified as an inclusion/exclusion this standard [ICDSITE = 99] OR [RADIO1 OR RADIO2 = 99] Brain/CNS Cancer QPI Measurability v2.3 17
18 QPI 11 Seizure Management QPI Title: Patients with brain/central nervous system (CNS) cancer presenting with seizures at diagnosis should be seen by a neurologist and/or a nurse with expertise in epilepsy management. Proportion of patients with brain/cns cancer presenting with seizures at diagnosis who are seen by a neurologist or a nurse with expertise in epilepsy management. Numerator Number of patients presenting with seizures at diagnosis seen by a neurologist or a nurse with expertise in epilepsy management. Seen by Neurologist and/or Nurse with Expertise in Epilepsy Management {Brain/CNS Cancer} coded as seen by Nurse with expertise in epilepsy management or Neurologist or Seen by both (nurse with expertise in epilepsy management and Neurologist). [EPILNESN = 1 OR 2 OR 3] Denominator All brain/cns cancer patients presenting with seizures at diagnosis (No exclusions) Date of Diagnosis [DIAGDATE] in range specified comparative analysis; AND Site of Origin of Primary Tumour {Cancer} coded as Brain/CNS Cancer; AND Seizure Presentation coded as Yes [ICDSITE = C700 OR C701 OR C709 OR C71 OR C72 OR C75.2 OR C75.3] AND [EPIL = 01] numerator exclusion Include in the measurement against the target. Present as not recorded only if the patient cannot otherwise be identified as [EPILNESN = 99] Include in the measurement against the target unless there is other definitive evidence that the record should be excluded. Present as not recorded only where the record cannot otherwise be definitively identified as an inclusion/exclusion this standard. N/A Exclude from the measurement against the target. Present as not recorded only where the patient cannot otherwise be definitively identified as an inclusion/exclusion this standard [ICDSITE = C99.X] OR [EPIL = 99] Brain/CNS Cancer QPI Measurability v2.3 18
Endometrial Cancer. Measurability of Quality Performance Indicators Version 2.0
 Endometrial Cancer Measurability of Quality Performance Indicators Version 2.0 To be read in conjunction with: Endometrial Cancer QPIs Final Publication v2 Endometrial QPI Dataset (latest published version)
Endometrial Cancer Measurability of Quality Performance Indicators Version 2.0 To be read in conjunction with: Endometrial Cancer QPIs Final Publication v2 Endometrial QPI Dataset (latest published version)
Lymphoma. Measurability of Quality Performance Indicators Version 2.0
 Lymphoma Measurability of Quality Performance Indicators Version 2.0 To be read in conjunction with: Lymphoma Clinical Quality Performance Indicators (September 2013) Lymphoma Data Definitions V2.0 (September
Lymphoma Measurability of Quality Performance Indicators Version 2.0 To be read in conjunction with: Lymphoma Clinical Quality Performance Indicators (September 2013) Lymphoma Data Definitions V2.0 (September
PRIMARY GLIOMA (oligodendroglioma, astrocytoma, oligodendroglioma, oligoastrocytoma, including anaplastic, gliosarcoma and glioblastoma multiforme)
 Protocol for Planning and Treatment The process to be followed when a course of chemotherapy is required to treat: PRIMARY GLIOMA (oligodendroglioma, astrocytoma, oligodendroglioma, oligoastrocytoma, including
Protocol for Planning and Treatment The process to be followed when a course of chemotherapy is required to treat: PRIMARY GLIOMA (oligodendroglioma, astrocytoma, oligodendroglioma, oligoastrocytoma, including
Technology appraisal guidance Published: 27 June 2007 nice.org.uk/guidance/ta121
 Carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma Technology appraisal guidance Published: 27 June 2007 nice.org.uk/guidance/ta121 NICE 2007. All rights reserved.
Carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma Technology appraisal guidance Published: 27 June 2007 nice.org.uk/guidance/ta121 NICE 2007. All rights reserved.
Supportive Care For Patients With High-Grade Glioma (primary brain tumours) Dr Susan Catt & Professor Lesley Fallowfield
 Supportive Care For Patients With High-Grade Glioma (primary brain tumours) Dr Susan Catt & Professor Lesley Fallowfield Partners Mr Giles Critchley Consultant Neurosurgeon Hurstwood Park Neurological
Supportive Care For Patients With High-Grade Glioma (primary brain tumours) Dr Susan Catt & Professor Lesley Fallowfield Partners Mr Giles Critchley Consultant Neurosurgeon Hurstwood Park Neurological
Brain Tumours. High Grade Gliomas. Information for patients. Exceptional healthcare, personally delivered
 Brain Tumours High Grade Gliomas Information for patients Exceptional healthcare, personally delivered Contents Page Types of Brain Tumours... 4 What is a Glioma... 4 How common are these tumours and who
Brain Tumours High Grade Gliomas Information for patients Exceptional healthcare, personally delivered Contents Page Types of Brain Tumours... 4 What is a Glioma... 4 How common are these tumours and who
Anatomic locations in high grade glioma
 256 Oslobanu, Florian Anatomic locations in high grade glioma Anatomic locations in high grade glioma A. Oslobanu 1, St.I. Florian 2 University of Medicine and Pharmacy, Iuliu Hatieganu Cluj-Napoca 1 Assistant
256 Oslobanu, Florian Anatomic locations in high grade glioma Anatomic locations in high grade glioma A. Oslobanu 1, St.I. Florian 2 University of Medicine and Pharmacy, Iuliu Hatieganu Cluj-Napoca 1 Assistant
Living with a Brain Tumor: A Guide for Patients and Families. Dana-Farber/Brigham and Women s Cancer Center Center for Neuro-Oncology
 Living with a Brain Tumor: A Guide for Patients and Families Dana-Farber/Brigham and Women s Cancer Center Center for Neuro-Oncology www.dana-farber.org/braintumor Introduction Dear patient and family
Living with a Brain Tumor: A Guide for Patients and Families Dana-Farber/Brigham and Women s Cancer Center Center for Neuro-Oncology www.dana-farber.org/braintumor Introduction Dear patient and family
Management of low grade glioma s: update on recent trials
 Management of low grade glioma s: update on recent trials M.J. van den Bent The Brain Tumor Center at Erasmus MC Cancer Center Rotterdam, the Netherlands Low grades Female, born 1976 1 st seizure 2005,
Management of low grade glioma s: update on recent trials M.J. van den Bent The Brain Tumor Center at Erasmus MC Cancer Center Rotterdam, the Netherlands Low grades Female, born 1976 1 st seizure 2005,
Lung Cancer Treatment Guidelines
 Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
Updated June 2014 Derived and updated by consensus of members of the Providence Thoracic Oncology Program with the aid of evidence-based National Comprehensive Cancer Network (NCCN) national guidelines,
Mesothelioma. 1. Introduction. 1.1 General Information and Aetiology
 Mesothelioma 1. Introduction 1.1 General Information and Aetiology Mesotheliomas are tumours that arise from the mesothelial cells of the pleura, peritoneum, pericardium or tunica vaginalis [1]. Most are
Mesothelioma 1. Introduction 1.1 General Information and Aetiology Mesotheliomas are tumours that arise from the mesothelial cells of the pleura, peritoneum, pericardium or tunica vaginalis [1]. Most are
Please see the LUCADA data manual v3.1.3, available in the downloads section
 National Lung Cancer Audit Frequently Asked Questions What dataset should be used? Please see the LUCADA data manual v3.1.3, available in the downloads section What does LUCADA stand for? LUCADA stands
National Lung Cancer Audit Frequently Asked Questions What dataset should be used? Please see the LUCADA data manual v3.1.3, available in the downloads section What does LUCADA stand for? LUCADA stands
Non-Small Cell Lung Cancer Treatment Comparison to NCCN Guidelines
 Non-Small Cell Lung Cancer Treatment Comparison to NCCN Guidelines April 2008 (presented at 6/12/08 cancer committee meeting) By Shelly Smits, RHIT, CCS, CTR Conclusions by Dr. Ian Thompson, MD Dr. James
Non-Small Cell Lung Cancer Treatment Comparison to NCCN Guidelines April 2008 (presented at 6/12/08 cancer committee meeting) By Shelly Smits, RHIT, CCS, CTR Conclusions by Dr. Ian Thompson, MD Dr. James
A practical guide to understanding cancer
 A practical guide to understanding cancer Contents 1 Contents About this booklet 2 2 The brain and brain tumours 5 Diagnosing brain tumours 21 Treating brain tumours 37 After your treatment 77 Your feelings
A practical guide to understanding cancer Contents 1 Contents About this booklet 2 2 The brain and brain tumours 5 Diagnosing brain tumours 21 Treating brain tumours 37 After your treatment 77 Your feelings
National Bowel Cancer Audit Report 2008 Public and Executive Summary
 National Bowel Cancer Audit Report 2008 Public and Executive Summary Prepared in association with: Healthcare Quality Improvement Partnership HQIP Association of Coloproctology of Great Britain and Ireland
National Bowel Cancer Audit Report 2008 Public and Executive Summary Prepared in association with: Healthcare Quality Improvement Partnership HQIP Association of Coloproctology of Great Britain and Ireland
Brain Tumor 101. Shanna Armstrong, RN Neuro Oncology Nurse Clinician UC Brain Tumor Center
 Brain Tumor 101 Shanna Armstrong, RN Neuro Oncology Nurse Clinician UC Brain Tumor Center Objectives Identify the different parts of the brain Describe how each part of the brain works Connect each part
Brain Tumor 101 Shanna Armstrong, RN Neuro Oncology Nurse Clinician UC Brain Tumor Center Objectives Identify the different parts of the brain Describe how each part of the brain works Connect each part
The Brain and Spine CenTer
 The Br ain and Spine Center Choosing the right treatment partner is important for patients facing tumors involving the brain, spine or skull base. The Brain and Spine Center at The University of Texas
The Br ain and Spine Center Choosing the right treatment partner is important for patients facing tumors involving the brain, spine or skull base. The Brain and Spine Center at The University of Texas
How To Prepare A Meeting For A Health Care Conference
 THORACIC ONCOLOGY MULTIDISCIPLINARY TEAM MEETINGS: OPERATIONAL POLICY EXECUTIVE SUMMARY 1. All patients in Lothian with thoracic malignancies should be discussed at designated times in pathway (see App
THORACIC ONCOLOGY MULTIDISCIPLINARY TEAM MEETINGS: OPERATIONAL POLICY EXECUTIVE SUMMARY 1. All patients in Lothian with thoracic malignancies should be discussed at designated times in pathway (see App
High-grade glioma in a patient with breast cancer
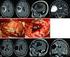 Asian Journal of Surgery (2014) 37, 162e166 Available online at www.sciencedirect.com journal homepage: www.e-asianjournalsurgery.com CASE REPORT High-grade glioma in a patient with breast cancer Che-Chao
Asian Journal of Surgery (2014) 37, 162e166 Available online at www.sciencedirect.com journal homepage: www.e-asianjournalsurgery.com CASE REPORT High-grade glioma in a patient with breast cancer Che-Chao
Clinical Management Guideline Management of locally advanced or recurrent Renal cell carcinoma. Protocol for Planning and Treatment
 Protocol for Planning and Treatment The process to be followed in the management of: LOCALLY ADVANCED OR METASTATIC RENAL CELL CARCINOMA Patient information given at each stage following agreed information
Protocol for Planning and Treatment The process to be followed in the management of: LOCALLY ADVANCED OR METASTATIC RENAL CELL CARCINOMA Patient information given at each stage following agreed information
AMERICAN BRAIN TUMOR ASSOCIATION. Glioblastoma and Malignant Astrocytoma
 AMERICAN BRAIN TUMOR ASSOCIATION Glioblastoma and Malignant Astrocytoma ACKNOWLEDGEMENTS ABOUT THE AMERICAN BRAIN TUMOR ASSOCIATION Founded in 1973, the American Brain Tumor Association (ABTA) was the
AMERICAN BRAIN TUMOR ASSOCIATION Glioblastoma and Malignant Astrocytoma ACKNOWLEDGEMENTS ABOUT THE AMERICAN BRAIN TUMOR ASSOCIATION Founded in 1973, the American Brain Tumor Association (ABTA) was the
AMERICAN BRAIN TUMOR ASSOCIATION. Ependymoma
 AMERICAN BRAIN TUMOR ASSOCIATION Ependymoma ACKNOWLEDGEMENTS ABOUT THE AMERICAN BRAIN TUMOR ASSOCIATION Founded in 1973, the American Brain Tumor Association (ABTA) was the first national nonprofit organization
AMERICAN BRAIN TUMOR ASSOCIATION Ependymoma ACKNOWLEDGEMENTS ABOUT THE AMERICAN BRAIN TUMOR ASSOCIATION Founded in 1973, the American Brain Tumor Association (ABTA) was the first national nonprofit organization
Foreword. Professor Michael Barton OAM Chair, Brain Tumour Guidelines Working Party Professor of Radiation Oncology, UNSW
 Foreword Brain tumours are uncommon, but they can have a devastating effect on patients lives and the lives of their families and carers. This guideline has been developed to provide information about
Foreword Brain tumours are uncommon, but they can have a devastating effect on patients lives and the lives of their families and carers. This guideline has been developed to provide information about
New Options to Treat Brain Tumors
 New Options to Treat Brain Tumors Guest Expert: Joachim, MD Associate Professor of Neuro-Oncology Director, Yale Cancer Center Brain Tumor Program www.wnpr.org www.yalecancercenter.org Welcome to Yale
New Options to Treat Brain Tumors Guest Expert: Joachim, MD Associate Professor of Neuro-Oncology Director, Yale Cancer Center Brain Tumor Program www.wnpr.org www.yalecancercenter.org Welcome to Yale
What is Glioblastoma? How is GBM classified according to the WHO Grading System? What risk factors pertain to GBM?
 GBM (English) What is Glioblastoma? Glioblastoma or glioblastoma multiforme is one of the most common brain tumors accounting for approximately 12 to 15 percent of all brain tumors. The name of the tumor
GBM (English) What is Glioblastoma? Glioblastoma or glioblastoma multiforme is one of the most common brain tumors accounting for approximately 12 to 15 percent of all brain tumors. The name of the tumor
Rotation Specific Goals & Objectives: University Health Network-Princess Margaret Hospital/ Sunnybrook Breast/Melanoma
 Rotation Specific Goals & Objectives: University Health Network-Princess Margaret Hospital/ Sunnybrook Breast/Melanoma Medical Expert: Breast Rotation Specific Competencies/Objectives 1.0 Medical History
Rotation Specific Goals & Objectives: University Health Network-Princess Margaret Hospital/ Sunnybrook Breast/Melanoma Medical Expert: Breast Rotation Specific Competencies/Objectives 1.0 Medical History
How To Predict Prognosis From An Eortc Gb
 1 di 5 15/10/2011 9.22 Radiation Oncology/CNS/High grade glioma/overview Front Page: Radiation Oncology RTOG Trials Randomized Trials Glioblastoma and High Grade Gliomas Overview Pathology University of
1 di 5 15/10/2011 9.22 Radiation Oncology/CNS/High grade glioma/overview Front Page: Radiation Oncology RTOG Trials Randomized Trials Glioblastoma and High Grade Gliomas Overview Pathology University of
National Cancer Institute. What You Need TM. To Know About. Brain Tumors. U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health
 National Cancer Institute What You Need TM To Know About Brain Tumors U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health For more publications This is only one of many free booklets
National Cancer Institute What You Need TM To Know About Brain Tumors U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health For more publications This is only one of many free booklets
LCA Brain/CNS Cancer Clinical Guidelines
 LCA Brain/CNS Cancer Clinical Guidelines June 2014 LCA BRAIN/CNS CANCER CLINICAL GUIDELINES Contents Introduction... 4 Executive Summary... 7 1 Early Diagnosis... 8 1.1 Criteria for referral... 8 1.2 Onward
LCA Brain/CNS Cancer Clinical Guidelines June 2014 LCA BRAIN/CNS CANCER CLINICAL GUIDELINES Contents Introduction... 4 Executive Summary... 7 1 Early Diagnosis... 8 1.1 Criteria for referral... 8 1.2 Onward
Surgery. Wedge resection only part of the lung, not. not a lobe, is removed. Cancer Council NSW
 The treatment you receive will depend on your lung cancer type, for example, whether you have a non-small cell lung cancer Adenocarcinoma or Squamous cell carcinoma, and if this is a sub-type with a mutation.
The treatment you receive will depend on your lung cancer type, for example, whether you have a non-small cell lung cancer Adenocarcinoma or Squamous cell carcinoma, and if this is a sub-type with a mutation.
CMScript. Member of a medical scheme? Know your guaranteed benefits! Issue 7 of 2014
 Background CMScript Member of a medical scheme? Know your guaranteed benefits! Issue 7 of 2014 Prostate cancer is second only to lung cancer as the leading cause of cancer-related deaths in men. It is
Background CMScript Member of a medical scheme? Know your guaranteed benefits! Issue 7 of 2014 Prostate cancer is second only to lung cancer as the leading cause of cancer-related deaths in men. It is
Glioblastoma: Is Survival Possible? By Ben A. Williams Copyright, 2014
 Glioblastoma: Is Survival Possible? By Ben A. Williams Copyright, 2014 Glioblastoma multiforme are among the most deadly neoplasms and continue to be regarded as incurable and universally fatal. This reputation
Glioblastoma: Is Survival Possible? By Ben A. Williams Copyright, 2014 Glioblastoma multiforme are among the most deadly neoplasms and continue to be regarded as incurable and universally fatal. This reputation
High Grade Gliomas: Update in Treatment and Care Ryan T. Merrell, M.D. Clinical Assistant Professor of Neurology NorthShore University HealthSystem
 High Grade Gliomas: Update in Treatment and Care Ryan T. Merrell, M.D. Clinical Assistant Professor of Neurology NorthShore University HealthSystem rmerrell@northshore.org Objectives Provide updates on
High Grade Gliomas: Update in Treatment and Care Ryan T. Merrell, M.D. Clinical Assistant Professor of Neurology NorthShore University HealthSystem rmerrell@northshore.org Objectives Provide updates on
Phyllodes tumours: borderline malignant and malignant
 Phyllodes tumours: borderline malignant and malignant This booklet is for people who would like more information on borderline malignant or malignant phyllodes tumours. It describes what they are, the
Phyllodes tumours: borderline malignant and malignant This booklet is for people who would like more information on borderline malignant or malignant phyllodes tumours. It describes what they are, the
Invasive lobular breast cancer
 Invasive lobular breast cancer This booklet is about invasive lobular breast cancer. It describes what invasive lobular breast cancer is, the symptoms, how it s diagnosed and possible treatments. Diagnosed
Invasive lobular breast cancer This booklet is about invasive lobular breast cancer. It describes what invasive lobular breast cancer is, the symptoms, how it s diagnosed and possible treatments. Diagnosed
Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy) for Paediatric Cancer Treatment
 Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy) for Paediatric Cancer Treatment Reference: NHS England xxx/x/x 1 Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy)
Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy) for Paediatric Cancer Treatment Reference: NHS England xxx/x/x 1 Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy)
International Journal of Pharma and Bio Sciences REVIEW OF BRAIN AND BRAIN CANCER TREATMENT
 International Journal of Pharma and Bio Sciences REVIEW ARTICLE ARTICALTICLE PHARMACOLOGY REVIEW OF BRAIN AND BRAIN CANCER TREATMENT Corresponding Author R.Srinivasan Siddhartha institute of pharmaceutical
International Journal of Pharma and Bio Sciences REVIEW ARTICLE ARTICALTICLE PHARMACOLOGY REVIEW OF BRAIN AND BRAIN CANCER TREATMENT Corresponding Author R.Srinivasan Siddhartha institute of pharmaceutical
Case Report. Central Neurocytoma. Fotis Souslian, MD; Dino Terzic, MD; Ramachandra Tummala, MD. Department of Neurosurgery, University of Minnesota
 1 Case Report Central Neurocytoma Fotis, MD; Dino Terzic, MD; Ramachandra Tummala, MD Department of Neurosurgery, University of Minnesota Case This is a previously healthy 20 year old female, with 3 months
1 Case Report Central Neurocytoma Fotis, MD; Dino Terzic, MD; Ramachandra Tummala, MD Department of Neurosurgery, University of Minnesota Case This is a previously healthy 20 year old female, with 3 months
What Is an Arteriovenous Malformation (AVM)?
 What Is an Arteriovenous Malformation (AVM)? From the Cerebrovascular Imaging and Intervention Committee of the American Heart Association Cardiovascular Council Randall T. Higashida, M.D., Chair 1 What
What Is an Arteriovenous Malformation (AVM)? From the Cerebrovascular Imaging and Intervention Committee of the American Heart Association Cardiovascular Council Randall T. Higashida, M.D., Chair 1 What
Glioblastoma (cancer affecting the brain) A guide for journalists on glioblastoma and its treatment
 Glioblastoma (cancer affecting the brain) A guide for journalists on glioblastoma and its treatment Section 1 Glioblastoma Section 2 Epidemiology and prognosis Section 3 Treatment Contents Contents 2 3
Glioblastoma (cancer affecting the brain) A guide for journalists on glioblastoma and its treatment Section 1 Glioblastoma Section 2 Epidemiology and prognosis Section 3 Treatment Contents Contents 2 3
Brain Cancer. This reference summary will help you understand how brain tumors are diagnosed and what options are available to treat them.
 Brain Cancer Introduction Brain tumors are not rare. Thousands of people are diagnosed every year with tumors of the brain and the rest of the nervous system. The diagnosis and treatment of brain tumors
Brain Cancer Introduction Brain tumors are not rare. Thousands of people are diagnosed every year with tumors of the brain and the rest of the nervous system. The diagnosis and treatment of brain tumors
Targeted Therapy What the Surgeon Needs to Know
 Targeted Therapy What the Surgeon Needs to Know AATS Focus in Thoracic Surgery 2014 David R. Jones, M.D. Professor & Chief, Thoracic Surgery Memorial Sloan Kettering Cancer Center I have no disclosures
Targeted Therapy What the Surgeon Needs to Know AATS Focus in Thoracic Surgery 2014 David R. Jones, M.D. Professor & Chief, Thoracic Surgery Memorial Sloan Kettering Cancer Center I have no disclosures
AMERICAN BRAIN TUMOR ASSOCIATION. Metastatic Brain Tumors
 AMERICAN BRAIN TUMOR ASSOCIATION Metastatic Brain Tumors AcKnoWLeDGemenTs about the american BRaIn tumor association Founded in 1973, the American Brain Tumor Association (ABTA) was the first national
AMERICAN BRAIN TUMOR ASSOCIATION Metastatic Brain Tumors AcKnoWLeDGemenTs about the american BRaIn tumor association Founded in 1973, the American Brain Tumor Association (ABTA) was the first national
NEW HYBRID IMAGING TECHNOLOGY MAY HAVE BIG POTENTIAL FOR IMPROVING DIAGNOSIS OF PROSTATE CANCER
 Media Release April 7, 2009 For Immediate Release NEW HYBRID IMAGING TECHNOLOGY MAY HAVE BIG POTENTIAL FOR IMPROVING DIAGNOSIS OF PROSTATE CANCER London, Ontario Improved hybrid imaging techniques developed
Media Release April 7, 2009 For Immediate Release NEW HYBRID IMAGING TECHNOLOGY MAY HAVE BIG POTENTIAL FOR IMPROVING DIAGNOSIS OF PROSTATE CANCER London, Ontario Improved hybrid imaging techniques developed
The following information is only meant for people who have been diagnosed with advanced non-small cell
 Important information for people with advanced non-small cell lung cancer The following information is only meant for people who have been diagnosed with advanced non-small cell lung cancer (NSCLC). NSCLC
Important information for people with advanced non-small cell lung cancer The following information is only meant for people who have been diagnosed with advanced non-small cell lung cancer (NSCLC). NSCLC
Clinical Practice Guidelines. for the management of adult gliomas: astrocytomas and oligodendrogliomas
 Clinical Practice Guidelines for the management of adult gliomas: astrocytomas and oligodendrogliomas August 2009 Cancer Council Australia/Australian Cancer Network/Clinical Oncological Society of Australia
Clinical Practice Guidelines for the management of adult gliomas: astrocytomas and oligodendrogliomas August 2009 Cancer Council Australia/Australian Cancer Network/Clinical Oncological Society of Australia
Brain Tumors. Patient Education Radiation Oncology
 Patient Education This booklet provides information and resources that University of Washington Medical Center (UWMC) and Harborview Medical Center patients and family members have told us have been helpful.
Patient Education This booklet provides information and resources that University of Washington Medical Center (UWMC) and Harborview Medical Center patients and family members have told us have been helpful.
MesoPDT. Photodynamic Therapy for malignant pleural mesothelioma ONCO-THAI. Image Assisted Laser Therapy for Oncology
 MesoPDT Photodynamic Therapy for malignant pleural mesothelioma ONCO-THAI Image Assisted Laser Therapy for Oncology Unité Inserm ONCO-THAI «Image Assisted Laser Therapy for Oncology» Inserm ONCO-THAI "Image
MesoPDT Photodynamic Therapy for malignant pleural mesothelioma ONCO-THAI Image Assisted Laser Therapy for Oncology Unité Inserm ONCO-THAI «Image Assisted Laser Therapy for Oncology» Inserm ONCO-THAI "Image
Chapter 5 Treatment of epilepsy in brain tumor patients
 Chapter 5 Treatment of epilepsy in brain tumor patients Het drinken van wijn met uitwerpselen van een ezel erin vermengd (Egypte) Wrijf een rokende ui of een kous van iemand met zweetvoeten onder de neus
Chapter 5 Treatment of epilepsy in brain tumor patients Het drinken van wijn met uitwerpselen van een ezel erin vermengd (Egypte) Wrijf een rokende ui of een kous van iemand met zweetvoeten onder de neus
4.2 Spinal Cord Compression
 4.2 Spinal Cord Compression AO Protocol Name: Metastatic Spinal Cord Compression (MSCC) AO Type: Type I (New Cancer), Type III (Cancer Complication) Author: ST Introduction: MSCC can cause irreversible
4.2 Spinal Cord Compression AO Protocol Name: Metastatic Spinal Cord Compression (MSCC) AO Type: Type I (New Cancer), Type III (Cancer Complication) Author: ST Introduction: MSCC can cause irreversible
Brain Tumors A HANDBOOK FOR THE NEWLY DIAGNOSED
 Brain Tumors A HANDBOOK FOR THE NEWLY DIAGNOSED INSURANCE CARD Insurance PART ONE: THE DIAGNOSIS Introduction...3 Where do I start?...5 Types of brain tumor treatments...5 Your healthcare team.................
Brain Tumors A HANDBOOK FOR THE NEWLY DIAGNOSED INSURANCE CARD Insurance PART ONE: THE DIAGNOSIS Introduction...3 Where do I start?...5 Types of brain tumor treatments...5 Your healthcare team.................
Case Number: RT2009-124(M) Potential Audiences: Intent Doctor, Oncology Special Nurse, Resident Doctor
 Renal Cell Carcinoma of the Left Kidney Post Radical Surgery with pt4 Classification with Multiple Lung and Single Brain Metastases: the Role and Treatment Consideration of Radiotherapy Case Number: RT2009-124(M)
Renal Cell Carcinoma of the Left Kidney Post Radical Surgery with pt4 Classification with Multiple Lung and Single Brain Metastases: the Role and Treatment Consideration of Radiotherapy Case Number: RT2009-124(M)
Baylor Radiosurgery Center
 Radiosurgery Center Baylor Radiosurgery Center Sophisticated Radiosurgery for both Brain and Body University Medical Center at Dallas Radiosurgery Center 3500 Gaston Avenue Hoblitzelle Hospital, First
Radiosurgery Center Baylor Radiosurgery Center Sophisticated Radiosurgery for both Brain and Body University Medical Center at Dallas Radiosurgery Center 3500 Gaston Avenue Hoblitzelle Hospital, First
2015/16 NHS STANDARD CONTRACT FOR ACUTE, AMBULANCE, COMMUNITY AND MENTAL HEALTH AND LEARNING DISABILITY SERVICES (MULTILATERAL)-
 2015/16 NHS STANDARD CONTRACT FOR ACUTE, AMBULANCE, COMMUNITY AND MENTAL HEALTH AND LEARNING DISABILITY SERVICES (MULTILATERAL)- SECTION B PART 1 - SERVICE SPECIFICATION FOR LUNG CANCER Service specification
2015/16 NHS STANDARD CONTRACT FOR ACUTE, AMBULANCE, COMMUNITY AND MENTAL HEALTH AND LEARNING DISABILITY SERVICES (MULTILATERAL)- SECTION B PART 1 - SERVICE SPECIFICATION FOR LUNG CANCER Service specification
Management of Non-Small Cell Lung Cancer Guide for General Practitioners
 Management of n-small Cell Lung Cancer Guide for General Practitioners Clinical Stage I Cancer only in one lobe of lung and
Management of n-small Cell Lung Cancer Guide for General Practitioners Clinical Stage I Cancer only in one lobe of lung and
Surgical guidelines for the management of breast cancer
 Available online at www.sciencedirect.com EJSO xx (2009) S1eS22 www.ejso.com Guidelines Surgical guidelines for the management of breast cancer Contents Association of Breast Surgery at BASO 2009 Introduction...
Available online at www.sciencedirect.com EJSO xx (2009) S1eS22 www.ejso.com Guidelines Surgical guidelines for the management of breast cancer Contents Association of Breast Surgery at BASO 2009 Introduction...
AA Life Insurance providing access to Best Doctors. AA Life Insurance is provided by Friends Life and Pensions Limited
 AA Life Insurance is provided by Friends Life and Pensions Limited What would be the first thing you might think about if you were diagnosed with a serious illness? 2 It is very likely that the first thing
AA Life Insurance is provided by Friends Life and Pensions Limited What would be the first thing you might think about if you were diagnosed with a serious illness? 2 It is very likely that the first thing
Corporate Medical Policy
 Corporate Medical Policy Intensity Modulated Radiation Therapy for Tumors of the Central File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intensity_modulated_radiation_therapy_for_tumors
Corporate Medical Policy Intensity Modulated Radiation Therapy for Tumors of the Central File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intensity_modulated_radiation_therapy_for_tumors
AA Life Insurance providing access to Best Doctors. AA Life Insurance is provided by Friends Life Limited
 AA Life Insurance is provided by Friends Life Limited What would be the first thing you might think about if you were diagnosed with a serious illness? It is very likely that the first thing you might
AA Life Insurance is provided by Friends Life Limited What would be the first thing you might think about if you were diagnosed with a serious illness? It is very likely that the first thing you might
National Lung Cancer Audit Report
 National Lung Cancer Audit Report 2013 Report for the audit period 2012 Copyright 2013, Health and Social Care Information Centre, National Lung Cancer Audit. All rights reserved. 1 Prepared in partnership
National Lung Cancer Audit Report 2013 Report for the audit period 2012 Copyright 2013, Health and Social Care Information Centre, National Lung Cancer Audit. All rights reserved. 1 Prepared in partnership
Technological Innovation: High Field Open Magnetic Resonance Spectroscopy
 Technological Innovation: High Field Open Magnetic Resonance Spectroscopy Student Investigator: Thomas R. Pace UVM COM Class of 2012 Faculty Mentor: Christopher Filippi Department of Radiology Fletcher
Technological Innovation: High Field Open Magnetic Resonance Spectroscopy Student Investigator: Thomas R. Pace UVM COM Class of 2012 Faculty Mentor: Christopher Filippi Department of Radiology Fletcher
Scottish Cancer Taskforce National Cancer Quality Steering Group. Prostate Cancer Clinical Quality Performance Indicators Engagement Document
 Scottish Cancer Taskforce National Cancer Quality Steering Group Prostate Cancer Clinical Quality Performance Indicators Engagement Document May 2016 Contents Page 1. National Cancer Quality Programme...
Scottish Cancer Taskforce National Cancer Quality Steering Group Prostate Cancer Clinical Quality Performance Indicators Engagement Document May 2016 Contents Page 1. National Cancer Quality Programme...
The brain structure and function
 The brain structure and function This information is an extract from the booklet Understanding brain tumours. You may find the full booklet helpful. We can send you a copy free see page 5. Contents Introduction
The brain structure and function This information is an extract from the booklet Understanding brain tumours. You may find the full booklet helpful. We can send you a copy free see page 5. Contents Introduction
Brain Tumor Guide for the Newly Diagnosed
 Brain Tumor Guide for the Newly Diagnosed Version 6.0 Updated June 9, 2009 Copyright 2009 Grey Ribbon Crusade Virtualtrials.com Tablle of Contents Chapter 1: Introduction: Where, When, How and Why Me?
Brain Tumor Guide for the Newly Diagnosed Version 6.0 Updated June 9, 2009 Copyright 2009 Grey Ribbon Crusade Virtualtrials.com Tablle of Contents Chapter 1: Introduction: Where, When, How and Why Me?
RESEARCH EDUCATE ADVOCATE. Just Diagnosed with Melanoma Now What?
 RESEARCH EDUCATE ADVOCATE Just Diagnosed with Melanoma Now What? INTRODUCTION If you are reading this, you have undergone a biopsy (either of a skin lesion or a lymph node) or have had other tests in which
RESEARCH EDUCATE ADVOCATE Just Diagnosed with Melanoma Now What? INTRODUCTION If you are reading this, you have undergone a biopsy (either of a skin lesion or a lymph node) or have had other tests in which
Information booklet Symptomatic brain cavernomas
 Cavernoma Alliance UK Helping the Cavernoma Community Information booklet Symptomatic brain cavernomas For people with symptoms due to their brain cavernoma www.cavernoma.org.uk info@cavernoma.org.uk Registered
Cavernoma Alliance UK Helping the Cavernoma Community Information booklet Symptomatic brain cavernomas For people with symptoms due to their brain cavernoma www.cavernoma.org.uk info@cavernoma.org.uk Registered
Prostate Cancer. Treatments as unique as you are
 Prostate Cancer Treatments as unique as you are UCLA Prostate Cancer Program Prostate cancer is the second most common cancer among men. The UCLA Prostate Cancer Program brings together the elements essential
Prostate Cancer Treatments as unique as you are UCLA Prostate Cancer Program Prostate cancer is the second most common cancer among men. The UCLA Prostate Cancer Program brings together the elements essential
GUIDELINES ON ONCOLOGY TREATMENT RATES. 1. Refer to Central Organisation ECHS following letter Nos :-
 GUIDELINES ON ONCOLOGY TREATMENT RATES 1. Refer to Central Organisation ECHS following letter Nos :- (a) B/49774/AG/ECHS/Referral dated 01 Dec 2009. (b) B/49773/AG/ECHS/Rates/Policy dated 10 Jan 2011.
GUIDELINES ON ONCOLOGY TREATMENT RATES 1. Refer to Central Organisation ECHS following letter Nos :- (a) B/49774/AG/ECHS/Referral dated 01 Dec 2009. (b) B/49773/AG/ECHS/Rates/Policy dated 10 Jan 2011.
National Oesophago-Gastric Cancer Audit Frequently Asked Questions Data Collection System
 Please also see the General Validation Rules tab on the Validation Workbook Question National Oesophago-Gastric Cancer Audit Answer In order to access the data collection system, all users are required
Please also see the General Validation Rules tab on the Validation Workbook Question National Oesophago-Gastric Cancer Audit Answer In order to access the data collection system, all users are required
Brain and Spinal Cord Tumours in Adults
 Brain and Spinal Cord Tumours in Adults WHAT IS BRAIN/CNS TUMORS IN ADULTS? The body is made up of trillions of living cells. Normal body cells grow, divide to make new cells, and die in an orderly way.
Brain and Spinal Cord Tumours in Adults WHAT IS BRAIN/CNS TUMORS IN ADULTS? The body is made up of trillions of living cells. Normal body cells grow, divide to make new cells, and die in an orderly way.
Family Handout for Children with a Brain Tumor
 Family Handout for Children with a Brain Tumor Table of Contents What do we know about childhood brain tumors? Symptoms of Brain Tumors Anatomy and Function of the Brain How Brain Tumors are Diagnosed
Family Handout for Children with a Brain Tumor Table of Contents What do we know about childhood brain tumors? Symptoms of Brain Tumors Anatomy and Function of the Brain How Brain Tumors are Diagnosed
Novel Surgery for Inoperable Brain Tumors. A Patient Guide to NeuroBlate
 Novel Surgery for Inoperable Brain Tumors A Patient Guide to NeuroBlate rose ella burkhardt brain tumor and neuro-oncology center Pioneering a Promising Surgical Treatment A brain tumor diagnosis is overwhelming
Novel Surgery for Inoperable Brain Tumors A Patient Guide to NeuroBlate rose ella burkhardt brain tumor and neuro-oncology center Pioneering a Promising Surgical Treatment A brain tumor diagnosis is overwhelming
The National Clinical Lung Cancer Audit (LUCADA)
 The National Clinical Lung Cancer Audit (LUCADA) DATA MANUAL Title: Version: 3.1.5 Date: September 2013 LUCADA Lung Cancer Audit VERSION HISTORY Version Date Issued Brief Summary of Change Owner s Name
The National Clinical Lung Cancer Audit (LUCADA) DATA MANUAL Title: Version: 3.1.5 Date: September 2013 LUCADA Lung Cancer Audit VERSION HISTORY Version Date Issued Brief Summary of Change Owner s Name
The Center for Prostate Cancer. Personalized Treatment. Clinical Excellence.
 The Center for Prostate Cancer Personalized Treatment. Clinical Excellence. The Center for Prostate Cancer Leaders in Prostate Cancer Treatment and Research The Center for Prostate Cancer at the North
The Center for Prostate Cancer Personalized Treatment. Clinical Excellence. The Center for Prostate Cancer Leaders in Prostate Cancer Treatment and Research The Center for Prostate Cancer at the North
Helen Joseph Breast Care Clinic - Johannesburg, South Africa
 - Johannesburg, South Africa General Information New breast cancer cases treated per year 360 Breast multidisciplinarity team members 12 Radiologists, surgeons, pathologists, medical oncologists, radiotherapists
- Johannesburg, South Africa General Information New breast cancer cases treated per year 360 Breast multidisciplinarity team members 12 Radiologists, surgeons, pathologists, medical oncologists, radiotherapists
AMERICAN BRAIN TUMOR ASSOCIATION. Proton Therapy
 AMERICAN BRAIN TUMOR ASSOCIATION Proton Therapy Acknowledgements About the American Brain Tumor Association Founded in 1973, the American Brain Tumor Association (ABTA) was the first national nonprofit
AMERICAN BRAIN TUMOR ASSOCIATION Proton Therapy Acknowledgements About the American Brain Tumor Association Founded in 1973, the American Brain Tumor Association (ABTA) was the first national nonprofit
Glioblastoma, brain metastases, spine metastases
 VOL. III Issue 1 2012 This issue focuses on: Neurologic-Oncology Welcome to the Spring 2012 edition of Oncology News from Memorial Regional Cancer Center. This issue focuses on Neurologic-Oncology. At
VOL. III Issue 1 2012 This issue focuses on: Neurologic-Oncology Welcome to the Spring 2012 edition of Oncology News from Memorial Regional Cancer Center. This issue focuses on Neurologic-Oncology. At
PRIMARY LUNG CANCER TREATMENT
 PRIMARY LUNG CANCER TREATMENT Cancer Care Pathways Directorate Tailored Information in Cancer Care (TICC) Sir Anthony Mamo Oncology Centre December 2014 Contents About this booklet 1 Types of Lung Cancer
PRIMARY LUNG CANCER TREATMENT Cancer Care Pathways Directorate Tailored Information in Cancer Care (TICC) Sir Anthony Mamo Oncology Centre December 2014 Contents About this booklet 1 Types of Lung Cancer
Guidelines for Management of Renal Cancer
 Guidelines for Management of Renal Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Versions 2 and 3 Section 5 updated bullets 5.3 and 5.4 Section 6 updated
Guidelines for Management of Renal Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Versions 2 and 3 Section 5 updated bullets 5.3 and 5.4 Section 6 updated
Prostate Cancer Guide. A resource to help answer your questions about prostate cancer
 Prostate Cancer Guide A resource to help answer your questions about prostate cancer Thank you for downloading this guide to prostate cancer treatment. We know that all the information provided online
Prostate Cancer Guide A resource to help answer your questions about prostate cancer Thank you for downloading this guide to prostate cancer treatment. We know that all the information provided online
Quality in Nursing Clinical Nurse Specialists in Cancer Care; Provision, Proportion and Performance
 Ensuring Better Treatment National Cancer Action Team Part of the National Cancer Programme Quality in Nursing Clinical Nurse Specialists in Cancer Care; Provision, Proportion and Performance A census
Ensuring Better Treatment National Cancer Action Team Part of the National Cancer Programme Quality in Nursing Clinical Nurse Specialists in Cancer Care; Provision, Proportion and Performance A census
GUIDELINES FOR ASSESSMENT OF SPINAL STABILITY THE CHRISTIE, GREATER MANCHESTER & CHESHIRE. CP57 Version: V3
 GUIDELINES FOR ASSESSMENT OF SPINAL STABILITY THE CHRISTIE, GREATER MANCHESTER & CHESHIRE Procedure Reference: Document Owner: CP57 Version: V3 Dr V. Misra Accountable Committee: Acute Oncology Group Network
GUIDELINES FOR ASSESSMENT OF SPINAL STABILITY THE CHRISTIE, GREATER MANCHESTER & CHESHIRE Procedure Reference: Document Owner: CP57 Version: V3 Dr V. Misra Accountable Committee: Acute Oncology Group Network
Dr. Henry Friedman and Dr. Linda Liau reviewed and approved the contents of this guide.
 Version 7 If you will be having a brain tumor surgery soon, there are a few things to consider which can keep your options open. See Chapter 4 for new treatment options! Updated November 1, 2012 Copyright
Version 7 If you will be having a brain tumor surgery soon, there are a few things to consider which can keep your options open. See Chapter 4 for new treatment options! Updated November 1, 2012 Copyright
How do I find the best place to get treatment for my lymphoma?
 Produced November 2010 Next revision due November 2012 How do I find the best place to get treatment for my lymphoma? Introduction Fortunately this is not a question that patients with cancers of the blood
Produced November 2010 Next revision due November 2012 How do I find the best place to get treatment for my lymphoma? Introduction Fortunately this is not a question that patients with cancers of the blood
Welcome to the Children s Epilepsy Surgery Service
 Welcome to the Children s Epilepsy Surgery Service Information for families Great Ormond Street Hospital for Children NHS Foundation Trust 2 Contents Introduction 3 My Team 4 The Epilepsy Team 5 Data collection
Welcome to the Children s Epilepsy Surgery Service Information for families Great Ormond Street Hospital for Children NHS Foundation Trust 2 Contents Introduction 3 My Team 4 The Epilepsy Team 5 Data collection
Cancer Treatment Benefit
 Cancer Treatment Benefit In Hong Kong, more than 27,000 men and women are newly-diagnosed with cancer each year 1, which means on average one new case is recorded every 20 minutes. Currently 1 in 4 men
Cancer Treatment Benefit In Hong Kong, more than 27,000 men and women are newly-diagnosed with cancer each year 1, which means on average one new case is recorded every 20 minutes. Currently 1 in 4 men
AMERICAN BRAIN TUMOR ASSOCIATION. Meningioma
 AMERICAN BRAIN TUMOR ASSOCIATION Meningioma AcKnoWLeDGemenTs about the american BraIn tumor association Founded in 1973, the American Brain Tumor Association (ABTA) was the first national nonprofit organization
AMERICAN BRAIN TUMOR ASSOCIATION Meningioma AcKnoWLeDGemenTs about the american BraIn tumor association Founded in 1973, the American Brain Tumor Association (ABTA) was the first national nonprofit organization
Fact sheet 10. Borderline ovarian tumours. The difficult cases. What is borderline ovarian cancer (BOC)?
 For this reason, some doctors prefer the term borderline ovarian tumour rather than borderline ovarian cancer. Fact sheet 10 Borderline ovarian tumours We, Ovacome, are a support network for people affected
For this reason, some doctors prefer the term borderline ovarian tumour rather than borderline ovarian cancer. Fact sheet 10 Borderline ovarian tumours We, Ovacome, are a support network for people affected
YOUR MRI EXAM: QUESTIONS AND ANSWERS
 YOUR MRI EXAM: QUESTIONS AND ANSWERS What is MRI? MRI is short for Magnetic Resonance Imaging. MRI is an advanced technology that lets your doctor see internal organs, blood vessels, muscles, joints, tumors,
YOUR MRI EXAM: QUESTIONS AND ANSWERS What is MRI? MRI is short for Magnetic Resonance Imaging. MRI is an advanced technology that lets your doctor see internal organs, blood vessels, muscles, joints, tumors,
Inova. Breast Care Institute
 Inova Breast Care Institute At the Inova Breast Care Institute, our commitment is to provide expert care for you, every step of the way. Our multidisciplinary team of more than 80 experts provides a full
Inova Breast Care Institute At the Inova Breast Care Institute, our commitment is to provide expert care for you, every step of the way. Our multidisciplinary team of more than 80 experts provides a full
A918: Prostate: adenocarcinoma
 A918: Prostate: adenocarcinoma General facts of prostate cancer The prostate is about the size of a walnut. It is just below the bladder and in front of the rectum. The tube that carries urine (the urethra)
A918: Prostate: adenocarcinoma General facts of prostate cancer The prostate is about the size of a walnut. It is just below the bladder and in front of the rectum. The tube that carries urine (the urethra)
Brain Tumor Center. A Team Approach to Treating Brain Tumors
 Brain Tumor Center A Team Approach to Treating Brain Tumors Introducing Our Brain Tumor Center Making an appointment with the Brain Tumor Center at the Center for Advanced Medicine is the important first
Brain Tumor Center A Team Approach to Treating Brain Tumors Introducing Our Brain Tumor Center Making an appointment with the Brain Tumor Center at the Center for Advanced Medicine is the important first
Magnetic Resonance Imaging
 Magnetic Resonance Imaging North American Spine Society Public Education Series What Is Magnetic Resonance Imaging (MRI)? Magnetic resonance imaging (MRI) is a valuable diagnostic study that has been used
Magnetic Resonance Imaging North American Spine Society Public Education Series What Is Magnetic Resonance Imaging (MRI)? Magnetic resonance imaging (MRI) is a valuable diagnostic study that has been used
ARRO Case: Low Grade Glioma (LGG)
 ARRO Case: Low Grade Glioma (LGG) Stephanie Rice, BS (MSIV) Abigail T. Berman, MD Michelle Alonso-Basanta, MD, PhD University of Pennsylvania October 25, 2013 44 F, h/o hypothyroidism Presentation: Case
ARRO Case: Low Grade Glioma (LGG) Stephanie Rice, BS (MSIV) Abigail T. Berman, MD Michelle Alonso-Basanta, MD, PhD University of Pennsylvania October 25, 2013 44 F, h/o hypothyroidism Presentation: Case
Lung Cancer & Mesothelioma 2011-2015
 Lung Cancer & Mesothelioma 2011-2015 Annex G Mesothelioma 1. The vision for mesothelioma services is set out in the Mesothelioma Framework issued by DH on 26 February 2007 (supported by the British Thoracic
Lung Cancer & Mesothelioma 2011-2015 Annex G Mesothelioma 1. The vision for mesothelioma services is set out in the Mesothelioma Framework issued by DH on 26 February 2007 (supported by the British Thoracic
Skin cancer Patient information
 Skin cancer Patient information What is cancer? The human body is made up of billions of cells. In healthy people, cells grow, divide and die. New cells constantly replace old ones in an orderly way. This
Skin cancer Patient information What is cancer? The human body is made up of billions of cells. In healthy people, cells grow, divide and die. New cells constantly replace old ones in an orderly way. This
Tubular breast cancer
 Tubular breast cancer This booklet is for people who would like more information about tubular breast cancer. It describes what tubular breast cancer is, its symptoms, how a diagnosis is made and the possible
Tubular breast cancer This booklet is for people who would like more information about tubular breast cancer. It describes what tubular breast cancer is, its symptoms, how a diagnosis is made and the possible
Breast cancer research and a changing treatment pathway
 Breast cancer research and a changing treatment pathway Stuart McIntosh Clinical Senior Lecturer in Surgical Oncology, QUB Consultant Breast Surgeon, BCH What is the breast surgeon s role in 2016? Surgery
Breast cancer research and a changing treatment pathway Stuart McIntosh Clinical Senior Lecturer in Surgical Oncology, QUB Consultant Breast Surgeon, BCH What is the breast surgeon s role in 2016? Surgery
