The Timing of Chemotherapy-Induced Neutropenia and Its Clinical and Economic Impact
|
|
|
- Edwin McCarthy
- 7 years ago
- Views:
Transcription
1 The Timing of Chemotherapy-Induced Neutropenia and Its Clinical and Economic Impact Review Article [1] April 30, 2006 By Howard Ozer, MD, PhD [2] Chemotherapy-induced neutropenia (CIN) and its complications exact a substantial toll on patients with cancer. Febrile neutropenia (FN), a sign of life-threatening infections, is associated with lengthy hospitalizations, early mortality, and high medical costs. In addition, neutropenia is the primary cause of dose reductions and dose delays, limiting the delivery of the chemotherapy at full dose and on schedule and thus compromising long-term survival in patients with potentially curable malignancies. Many recent studies in several major tumor types have documented that the greatest risk of neutropenia and its complications is in the first cycle of chemotherapy, with more than 50% of the first episodes of neutropenia and FN occurring in the first cycle. In addition to their other negative effects, these first-cycle events are also associated with early termination of the chemotherapy. The disproportionately high risk of neutropenia in the first cycle has important implications for managing CIN, as well as for the development and use of guidelines for supportive care. It highlights the importance of determining which patients are at high risk for neutropenia and its complications before the chemotherapy is initiated and implementing interventions, such as prophylactic growth factor support in the first and subsequent cycles, to reduce that risk. Chemotherapy-induced neutropenia (CIN) is the primary dose-limiting toxicity in patients with cancer treated with chemotherapy. It can lead to febrile neutropenia (FN), and it is associated with increased morbidity and early mortality, increased medical costs, and disruptions in potentially curative treatments.[1,2] In this article, I discuss the clinical and economic consequences of CIN and FN, as well as the risk and timing of CIN and FN in patients treated with myelosuppressive chemotherapy. Consequences of Chemotherapy-Induced Neutropenia Hospitalization, Mortality, and Costs The incidences of CIN and its complications, such as fever, infection, and chemotherapy dose alterations, vary by type of malignancy. One large prospective registry reported CIN rates of 15% to 65% in patients with five major tumor types: breast cancer, colon cancer, lymphoma, lung cancer, and ovarian cancer. The rates of FN ranged from 7% to 30%.[3] Hospitalization and treatment with empiric broad-spectrum antibiotics is typically required in patients with FN, and it has been estimated that in the United States more than 60,000 patients a year are hospitalized for FN.[4] These hospitalizations account for substantial mortality and medical costs. A review of discharge data from the University HealthSystem Consortium (UHC), which comprises 121 institutions, examined inpatient mortality in 41,779 nontransplant patients who had been hospitalized for FN in 1995 through 2000 and found that it was higher in patients with leukemia than in those with lymphoma and solid tumors (Figure 1).[1] The length of hospitalization followed a similar pattern, averaging 19.0 days in patients with leukemia, 10.8 days in those with lymphoma, and 8.5 days in those with solid tumors.[1] The average cost of hospitalization was $37,600 in patients with leukemia, $19,000 in patients with lymphoma, and $12,300 in patients with solid tumors.[1] Page 1 of 8
2 A minority of patients in the UHC database accounted for a disproportionate amount of medical costs. The mean cost of hospitalization in patients with complications that were related to FN was nearly double the mean cost in all patients ($18,400 vs $35,000). For some patients, the hospital stays were 30 to 40 days, costing as much as $60,000 to $100,000 for a single hospitalization for FN.[1] It should be noted that the medical costs in the UHC discharge records are only the direct costs of hospitalization and that other costs incurred by patients can substantially increase the total costs of an occurrence of FN. A survey of 26 patients with ovarian cancer in whom World Health Organization grade 3 or 4 CIN had occurred captured both its direct and its indirect costs in the 3 months after its occurrence.[5] The direct costs included out-of-pocket expenses such as physician visits, emergency room visits, drugs and medical devices, home healthcare, and laboratory tests. The indirect costs included patient work loss, family member work loss, and caregiver payments. The total direct costs were estimated at $1,340 and the total indirect costs at $3,840. Patient Quality of Life There is increasing evidence that neutropenia is also associated with important although more difficult to measure effects on patient quality of life (QOL). Hospitalization for FN and the fear of hospitalization have obvious negative effects on patient QOL,[6] but the effects of neutropenia are more subtle. Fortner and colleagues used several tools to evaluate the effects of CIN and FN on QOL in a heterogeneous group of patients. Lower absolute neutrophil counts were found to correlate with lower ability for physical work, worse physical symptoms, and greater psychological distress.[7,8] In another study, which analyzed 100 structured interviews with 34 patients in whom grade 4 neutropenia developed in the first cycle of chemotherapy, fatigue was the most common physical symptom reported, and interference with daily routine, negative self-evaluation, negative emotion, and social isolation were other common complaints that were associated with neutropenia.[9] A possible association between FN and other adverse events has also been shown, with the incidence and severity of common side effects such as nausea and vomiting, anorexia, and asthenia being increased in patients with FN.[10-12] When prophylactic growth factors were used to reduce the incidence of FN, the incidence of other adverse events was also reduced.[12] Reduced Dose Intensity The most insidious effect of CIN may, however, be the disruption of the cancer treatment with potentially serious consequences for long-term survival. Dose reductions and delays are common in patients treated with chemotherapy. A retrospective analysis of data on 20,799 patients who were treated with chemotherapy after surgery for early-stage breast cancer between 1997 and 2000 found that dose delays of 7 days or longer were required in 25% of patients, dose reductions of 15% or more were required in 37%, and relative dose intensity (RDI) was less than 85% in 56% (Figure 2).[13] A similar analysis of data on 4,522 patients who were treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), CHOP plus rituximab (Rituxan) (CHOP-R), or CNOP (mitoxantrone [Novantrone] substituted for doxorubicin) for aggressive non-hodgkin's lymphoma (NHL) found dose delays of 7 days or longer in 24% of patients, dose reductions of 15% or more in 40%, and RDI less than 85% in 53%. [14] Multivariate analyses in both studies found that advanced age ( 60 or 65 years) was a significant predictor of RDI less than 85%, independent of other risk factors such as poor Eastern Cooperative Oncology Group performance status, advanced tumor stage, increased body surface area, and absence of treatment with CSFs.[13,14] Page 2 of 8
3 Most clinicians would name CIN and its complications as the most frequent causes of chemotherapy dose delays and dose reductions, and recent data support this. A retrospective analysis of practice patterns examined regimen alterations in 1,111 patients who were treated with adjuvant therapy with cyclophosphamide, methotrexate, and fluorouracil (CMF), doxorubicin and cyclophosphamide (AC), or other regimens for early-stage breast cancer. Across all regimens, the RDI was less than 85% of that planned in 22% of patients. Chemotherapy-induced neutropenia was reported as the most frequent cause of the dose reductions and delays, accounting for 61% of these regimen alterations.[15] An analysis of similar data from 492 patients treated with CHOP or CNOP for intermediate-grade NHL had similar findings. With both regimens combined, there were dose reductions or delays in 48% of patients; CIN was once again the most frequent cause, accounting for 56% of the delays and 59% of the reductions.[16] There were similar findings in a recent trial of vinorelbine plus cisplatin in 242 patients with resected non-small-cell lung cancer. World Health Organization grade 3 or 4 neutropenia occurred in 73% of patients and FN occurred in 7%. There was at least one dose delay in 55% of patients, generally due to persistent neutropenia at the time of the planned dose of vinorelbine on day 15 of the cycle.[17] Such alterations in chemotherapy regimens may help ameliorate the immediate problem of neutropenia, but their long-term effects are clearly negative. The most recent update of the classic study by Bonadonna and colleagues well illustrates this point. This study, a retrospective analysis of data on 207 patients treated with 12 cycles of CMF for node-positive breast cancer, found significantly lower long-term survival in patients who were treated with less than 85% of the planned dose. At a median follow-up of 28.5 years, relapse-free survival was 26% in patients treated with less than 85% of the planned dose and 42% in those treated with a higher RDI, and overall survival was 21% and 40%.[18] Analysis of data on another frequently curable malignancy, previously untreated NHL, had similar results. In 115 patients treated with CHOP, methotrexate, bleomycin (Blenoxane), doxorubicin, cyclophosphamide, vincristine, and dexamethasone ([M]BACOD), or methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone, and bleomycin (MACOP-B) chemotherapy, a doxorubicin RDI of 75% or less in the first three cycles was found to be the strongest predictor of mortality at a median follow-up of 3 years (P =.001). Even when the analysis was restricted to patients in whom a complete response had been achieved, a low RDI of doxorubicin remained a predictor of poor survival.[19] In addition to these frequently curable tumors, alterations in chemotherapy regimens appear to also compromise the treatment outcomes in settings in which treatment is less commonly curative. In a randomized trial of full-dose and attenuated-dose etoposide and cisplatin in 95 patients with small-cell lung cancer, the response rate was lower in patients treated with the attenuated dose (39% vs 68%) as was 1-year survival (18% vs 39%).[20] Page 3 of 8
4 Perhaps the best evidence for the importance of delivered chemotherapy dose intensity comes from Cancer and Leukemia Group B (CALGB) 9741, a large clinical trial in women with node-positive early-stage breast cancer that tested the regimen of concurrent doxorubicin and cyclophosphamide followed by paclitaxel (AC-T) given in dose-dense 14-day cycles and in standard 21-day cycles.[21] To reduce the risk of neutropenia, proactive CSFs were given to all patients treated with the 14-day regimen. The protocol-specified 36-month analysis showed significantly higher disease-free survival (risk ratio 0.74, P =.010) and overall survival (risk ratio 0.69, P =.013) in the patients treated with the 14-day regimen. Five-year results were recently reported, with these differences in survival remaining significant and of similar magnitude.[22] On the basis of these results, many specialists in breast cancer have adopted dose-dense AC-T with routine growth factor support as a standard regimen in women with node-positive early-stage breast cancer. Many are also using once-per-cycle pegfilgrastim rather than daily filgrastim, a strategy that has been shown to be safe and effective.[23] Risk in the First Cycle A growing body of evidence supports the conclusion that, contrary to common belief, the risk of CIN and FN is highest in the first cycle of chemotherapy rather than cumulative across cycles. An analysis of data on 577 patients with intermediate-grade NHL treated with up to six cycles of CHOP found that more than half of the first occurrences of FN-58% were in the first cycle (Figure 3).[24] Similar results were seen in a randomized trial of CHOP and CHOP-R in 528 patients with NHL, in which the largest proportion of all occurrences of FN, 39%, were in the first cycle of the chemotherapy.[25] In both studies, most of the occurrences of FN in the first cycle were by day 14. A similar pattern has been seen in a large prospective registry, the Awareness of Neutropenia in Chemotherapy Registry, which collects data on patients treated with chemotherapy at 137 clinical sites across the United States. Chemotherapy-induced neutropenia occurred in 43% of 2,106 patients treated with chemotherapy for a variety of malignancies, including colon cancer, lung cancer, breast cancer, ovarian cancer, and lymphoma, and FN occurred in 14%. The likelihoods of CIN and FN were highest in the first cycle of chemotherapy in all tumor types, and more than half of the first occurrences of CIN and FN were in the first cycle.[3] There are several possible reasons for the risk of CIN and FN being highest in the first cycle, and the relative lack of supportive care in the first cycle is an important one. In patients with NHL treated with CHOP or CHOP-R, for example, 41% of patients who were not given CSFs in the first and subsequent cycles experienced FN.[25] In addition, a number of studies have shown that the absence of proactive CSF therapy is a significant predictor of FN.[16,24,26] Whatever the cause of risk in the first cycle, CIN that occurs in the first cycle has the potential for severe consequences. In a study in 267 elderly patients with NHL treated with CHOP, there were 35 treatment-related deaths. The majority of these deaths, 63%, occurred in the first cycle, and 82% of all treatment-related deaths were infection-related.[27] Page 4 of 8
5 The occurrence of CIN in the first cycle also substantially affects the long-term course of treatment. There were dose reductions or delays in 61% of 1,111 patients treated with a variety of chemotherapy regimens for early-stage breast cancer, and the occurrence of one such treatment alteration predicted later alterations, the incidence of additional delays being 57% and of additional reductions being 79% (Figure 4).[15] The same pattern was seen in 492 patients with intermediate-grade NHL who were treated with CHOP or CNOP, the incidence of additional delays being 41% and of additional reductions being 68% (see Figure 4).[16] What is also striking in the Picozzi study is the apparent underuse of CSFs to prevent later dose reductions and delays, even though the majority of those treatment alterations were necessitated by the occurrence of neutropenia. Only 2% to 3% of patients in whom there was a dose delay or reduction were subsequently treated with CSFs to lessen the likelihood of later delays or reductions.[16] Chemotherapy-induced neutropenia in the first cycle is also associated with premature termination of the chemotherapy. In a study in 124 patients with intermediate-grade NHL, treatment with six to eight cycles of CHOP was planned an optimal number of cycles but 20% of the patients were treated with fewer than six cycles, and those patients who were hospitalized for FN in the first cycle were more than four times as likely to be treated with fewer than six cycles as were those who were not hospitalized for FN.[28] That the occurrence of neutropenia in the first cycle can compromise the course of cancer treatment should be considered in the context of treatment efficacy and long-term survival. In the study of 124 patients with NHL discussed above, overall survival at 5 years was significantly lower in patients aged 60 to 74 years who were treated with fewer than six cycles of CHOP (hazard ratio for death, 6.0; 95% CI, ). Overall survival at 5 years was also lower in the patients aged 75 years and older who were treated with fewer than six cycles, but the number of patients was too small for significance to be shown.[28] As discussed earlier, treatment with less-than-optimal RDI has also been associated with significantly lower long-term survival in much larger studies.[18,19,21] Conclusions Chemotherapy-induced neutropenia is a major dose-limiting toxicity in patients treated with chemotherapy, with significant clinical consequences in terms of increased morbidity and early mortality.[1,2] Hospitalization is often required in patients with FN, and it can be both lengthy and expensive.[1] In some high-risk patients in whom serious infections occur, mortality can exceed 50% even with prompt anti-infective treatment.[29,30] The most effective approach to managing neutropenia, therefore, is to prevent FN. As discussed by Rader in this supplement,[31] effective supportive care measures such as CSFs are available for managing neutropenia. Treatment with CSFs can not only reduce the incidence of FN Page 5 of 8
6 but also reduce infections and increase the proportion of patients who are treated with chemotherapy at full dose and on schedule.[26] The finding that FN occurs most often in the first cycle of chemotherapy has important implications for the use of supportive care in oncology, and in particular for the use of therapies for managing neutropenia and the continuing development of clinical guidelines for their use. As discussed by Crawford in this supplement,[32] patients who are at increased risk for FN should be treated with CSFs starting in the first cycle of chemotherapy. Disclosures: Dr. Ozer has served on speakers bureaus for and received research grants from Amgen, Genentech, and Sanofi-Aventis, and he also has received grants from Lilly and Novartis. References: 1. Kuderer NM, Dale DC, Crawford J, et al: Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer In press. 2. Lyman GH: Guidelines of the National Comprehensive Cancer Network on the use of myeloid growth factors with cancer chemotherapy: A review of the evidence. J Natl Comp Cancer Netw 3: , Crawford J, Wolff DA, Culakova E, et al: First cycle risk of severe and febrile neutropenia in cancer patients receiving systemic chemotherapy: Results from a prospective nationwide study (abstract 2210). Blood 104:607a-608a, Caggiano V, Weiss RV, Rickert TS, et al: Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer 103: , Cosler LE, Calhoun EA, Agboola O, et al: Effects of indirect and additional direct costs on the risk threshold for prophylaxis with colony-stimulating factors in patients at risk for severe neutropenia from cancer chemotherapy. Pharmacotherapy 24: , Lyman GH, Kuderer NM: Filgrastim in patients with neutropenia. Potential effects on quality of life. Drugs 62(suppl 1):65-78, Fortner BV, Stolshek B, Schwartzberg LS, et al: Decline in absolute neutrophil count (ANC) is associated with lower quality of life (QOL) in cancer patients receiving docetaxel (abstract 2808). Proc Am Soc Clin Oncol 21:247b, Okon TA, Fortner BV, Schwartzberg L, et al: Quality of life (QOL) in patients with grade IV chemotherapy-induced neutropenia (CIN) (abstract 2920). Proc Am Soc Clin Oncol 21:275b, Fortner BV, Tauer KW, Okon T, et al: Experiencing neutropenia: Quality of life interviews with adult cancer patients. BMC Nurs 4:4, Glaspy J, Hackett J, Flyer P, et al: Febrile neutropenia is associated with an increase in the incidence, duration, and severity of chemotherapy toxicities (abstract 1812). Blood 98:432a, Martin M, Lluch A, Segui MA, et al: Prophylactic growth factor (GF) support with adjuvant docetaxel, doxorubicin, and cyclophosphamide (TAC) for node-negative breast cancer (BC): An interim safety analysis of the GEICAM 9805 study (abstract 620). Proc Am Soc Clin Oncol 23:32, Martin M, Lluch A, Seguí MA, et al: Toxicity and health-related quality of life (HRQoL) in node-negative breast cancer (BC) patients (pts) receiving adjuvant treatment with TAC (docetaxel, doxorubicin, cyclophoshamide) or FAC (5-fluorouracil, doxorubicin, cyclophosphamide): Impact of adding prophylactic growth factors (GF) to TAC. GEICAM study 9805 (abstract 604) J Clin Oncol 23(suppl):29s, Page 6 of 8
7 13. Lyman GH, Dale DC, Crawford J: Incidence and predictors of low dose intensity in adjuvant breast cancer chemotherapy: A nationwide study of community practices. J Clin Oncol 21: , Lyman GH, Dale DC, Friedberg J, et al: Incidence and predictors of low chemotherapy dose-intensity in aggressive non-hodgkin's lymphoma: A nationwide study. J Clin Oncol 22: , Link BK, Budd GT, Scott S, et al: Delivering adjuvant chemotherapy to women with early-stage breast carcinoma: Current patterns of care. Cancer 92: , Picozzi VJ, Pohlman BL, Morrison VA, et al: Patterns of chemotherapy administration in patients with intermediate-grade non-hodgkin's lymphoma. Oncology (Williston Park) 15: , Winton T, Livingston R, Johnson D, et al: Vinorelbine plus cisplatin vs observation in resected non-small-cell lung cancer. N Engl J Med 352: , Bonadonna G, Moliterni A, Zambetti M, et al: 30 years follow up of randomised studies of adjuvant CMF in operable breast cancer: Cohort study. BMJ 330:217, Kwak LW, Halpern J, Olshen RA, et al: Prognostic significance of actual dose intensity in diffuse large-cell lymphoma: Results of a tree-structured survival analysis. J Clin Oncol 8: , Ardizzoni A, Favaretto A, Boni L, et al: Platinum-etoposide chemotherapy in elderly patients with small-cell lung cancer: Results of a randomized multicenter phase II study assessing attenuated-dose or full-dose with lenograstim prophylaxis-a Forza Operativa Nazionale Italiana Carcinoma Polmonare and Gruppo Studio Tumori Polmonari Veneto (FONICAP-GSTPV) study. J Clin Oncol 23: , Citron ML, Berry DA, Cirrincione C, et al: Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup trial C9741/Cancer and Leukemia Group B trial 9741 [erratum in J Clin Oncol 21:2226, 2003]. J Clin Oncol 21: , Hudis C, Citron M, Berry D, et al: Five year follow-up of INT C9741: Dose-dense (DD) chemotherapy (CRx) is safe and effective (abstract 41). Presented at the 28th Annual San Antonio Breast Cancer Symposium, December 8-11, 2005, San Antonio, Texas. 23. Burstein HJ, Parker LM, Keshaviah A, et al: Efficacy of pegfilgrastim and darbepoetin alfa as hematopoietic support for dose-dense every-2-week adjuvant breast cancer chemotherapy. J Clin Oncol 23: , Lyman GH, Morrison VA, Dale DC, et al: Risk of febrile neutropenia among patients with intermediate-grade non-hodgkin's lymphoma receiving CHOP chemotherapy. Leuk Lymphoma 44: , Morrison VA, Weller EA, Habermann TM, et al: Patterns of growth factor (GF) usage and febrile neutropenia (FN) among older patients (pts) with diffuse large B-cell lymphoma (DLBCL) treated with CHOP or R-CHOP: An Intergroup Experience (CALGB 9793, ECOG-SWOG 4494) (abstract 3309). Blood 104:904a, Kuderer NM, Crawford J, Dale DC, et al: Meta-analysis of prophylactic granulocyte colony-stimulating factor (G-CSF) in cancer patients receiving chemotherapy (abstract 8117). J Clin Oncol 23(suppl):758s, Gomez H, Hidalgo M, Casanova L, et al. Risk factors for treatment-related death in elderly patients with aggressive non-hodgkin's lymphoma: Results of a multivariate analysis. J Clin Oncol 16: , Chrischilles EA, Link BK, Scott SD, et al: Factors associated with early termination of CHOP Page 7 of 8
8 therapy and the impact on survival among patients with chemosensitive intermediate-grade non-hodgkin's lymphoma. Cancer Control 10: , Lyman GH, Kuderer NM: Epidemiology of febrile neutropenia. Support Cancer Ther 1:1-12, Lyman GH, Kuderer NM: Cost effectiveness of myeloid growth factors in cancer chemotherapy. Curr Hematol Rep 2: , Rader M: Granulocyte colony-stimulating factors use in patients with chemotherapy-induced neutropenia: clinical and economic benefits. Oncology (suppl) TK:TK-TK, (this supplement) 32. Crawford J: Risk assessment and guidelines for first-cycle colony-stimulating factor use in the management of chemotherapy-induced neutropenia. Oncology (suppl) TK:TK-TK, (this supplement) Source URL: Links: [1] [2] Page 8 of 8
J Clin Oncol 22:4302-4311. 2004 by American Society of Clinical Oncology INTRODUCTION
 VOLUME 22 NUMBER 21 NOVEMBER 1 2004 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T Incidence and Predictors of Low Chemotherapy Dose-Intensity in Aggressive Non-Hodgkin s Lymphoma: A Nationwide
VOLUME 22 NUMBER 21 NOVEMBER 1 2004 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T Incidence and Predictors of Low Chemotherapy Dose-Intensity in Aggressive Non-Hodgkin s Lymphoma: A Nationwide
Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003)
 Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003) Reeder CB et al. Proc ASCO 2010;Abstract 8037. Introduction > Patients (pts) with low-grade
Lenalidomide (LEN) in Patients with Transformed Lymphoma: Results From a Large International Phase II Study (NHL-003) Reeder CB et al. Proc ASCO 2010;Abstract 8037. Introduction > Patients (pts) with low-grade
The Impact of Taxotere on Adjuvant Breast Cancer
 The Impact of Taxotere on Adjuvant Breast Cancer a report by Pierre Fumoleau and Henri Roché Centre Georges François Leclerc, Dijon, and Institut Claudius Regaud, Toulouse, France DOI: 10.17925/EOH.2005.0.0.1l
The Impact of Taxotere on Adjuvant Breast Cancer a report by Pierre Fumoleau and Henri Roché Centre Georges François Leclerc, Dijon, and Institut Claudius Regaud, Toulouse, France DOI: 10.17925/EOH.2005.0.0.1l
Chemobrain. Halle C.F. Moore, MD The Cleveland Clinic October 3, 2015
 Chemobrain Halle C.F. Moore, MD The Cleveland Clinic October 3, 2015 Terminology Chemotherapy-associated cognitive dysfunction Post-chemotherapy cognitive impairment Cancer treatment-associated cognitive
Chemobrain Halle C.F. Moore, MD The Cleveland Clinic October 3, 2015 Terminology Chemotherapy-associated cognitive dysfunction Post-chemotherapy cognitive impairment Cancer treatment-associated cognitive
original article P. Jenkins 1 * & S. Freeman 2 introduction patients and methods original article
 Annals of Oncology 20: 34 40, 2009 doi:10.1093/annonc/mdn560 Published online 13 August 2008 Pretreatment haematological laboratory values predict for excessive myelosuppression in patients receiving adjuvant
Annals of Oncology 20: 34 40, 2009 doi:10.1093/annonc/mdn560 Published online 13 August 2008 Pretreatment haematological laboratory values predict for excessive myelosuppression in patients receiving adjuvant
MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS
 MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS All prescriptions for antineoplastic drugs must be accompanied by the MOH special form. All the attachments mentioned on this form shall be submitted
MOH Policy for dispensing NEOPLASTIC DISEASES DRUGS All prescriptions for antineoplastic drugs must be accompanied by the MOH special form. All the attachments mentioned on this form shall be submitted
Activity of pemetrexed in thoracic malignancies
 Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Activity of pemetrexed in thoracic malignancies Results of phase III clinical studies of pemetrexed in malignant pleural mesothelioma and non-small cell lung cancer show benefit P emetrexed (Alimta) is
Chemotherapy or Not? Anthracycline or Not? Taxane or Not? Does Density Matter? Chemotherapy in Luminal Breast Cancer: Choice of Regimen.
 Chemotherapy in Luminal Breast Cancer: Choice of Regimen Andrew D. Seidman, MD Attending Physician Breast Cancer Medicine Service Memorial Sloan Kettering Cancer Center Professor of Medicine Weill Cornell
Chemotherapy in Luminal Breast Cancer: Choice of Regimen Andrew D. Seidman, MD Attending Physician Breast Cancer Medicine Service Memorial Sloan Kettering Cancer Center Professor of Medicine Weill Cornell
FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma
 Media Release Basel, 31 January 2011 FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma Approval provides option that improves the length of time people with incurable
Media Release Basel, 31 January 2011 FDA approves Rituxan/MabThera for first-line maintenance use in follicular lymphoma Approval provides option that improves the length of time people with incurable
La Chemioterapia Adiuvante Dose-Dense. Lo studio GIM 2. Alessandra Fabi
 La Chemioterapia Adiuvante Dose-Dense Lo studio GIM 2 Alessandra Fabi San Antonio Breast Cancer Symposium -December 10-14, 2013 GIM 2 study Epirubicin and Cyclophosphamide (EC) followed by Paclitaxel (T)
La Chemioterapia Adiuvante Dose-Dense Lo studio GIM 2 Alessandra Fabi San Antonio Breast Cancer Symposium -December 10-14, 2013 GIM 2 study Epirubicin and Cyclophosphamide (EC) followed by Paclitaxel (T)
Costs Associated with Neutropenia in Elderly Patients Treated First-Line for Advanced Non-Small Cell Lung Cancer
 Costs Associated with Neutropenia in Elderly Patients Treated First-Line for Advanced Non-Small Cell Lung Cancer Michael Stokes United BioSource Corporation ISPOR 13 th Annual International Meeting Toronto,
Costs Associated with Neutropenia in Elderly Patients Treated First-Line for Advanced Non-Small Cell Lung Cancer Michael Stokes United BioSource Corporation ISPOR 13 th Annual International Meeting Toronto,
Adjuvant Therapy Non Small Cell Lung Cancer. Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015
 Adjuvant Therapy Non Small Cell Lung Cancer Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015 No Disclosures Number of studies Studies Per Month 12 10 8 6 4 2 0 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3
Adjuvant Therapy Non Small Cell Lung Cancer Sunil Nagpal MD Director, Thoracic Oncology Jan 30, 2015 No Disclosures Number of studies Studies Per Month 12 10 8 6 4 2 0 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3
Avastin in breast cancer: Summary of clinical data
 Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group
 REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group In the 2002 edition of the ASCO meeting, a total of 315 abstracts in the field of respiratory
REPORT ASCO 2002 ORLANDO : LUNG CANCER Johan F. Vansteenkiste, MD, PhD, Univ. Hospital and Leuven Lung Cancer Group In the 2002 edition of the ASCO meeting, a total of 315 abstracts in the field of respiratory
Clair Clark, Cancer Care Pharmacist Beatson West of Scotland Cancer Centre
 Clair Clark, Cancer Care Pharmacist Beatson West of Scotland Cancer Centre An audit of neutropenic complications in breast cancer patients receiving adjuvant or neo-adjuvant chemotherapy with FEC-D in
Clair Clark, Cancer Care Pharmacist Beatson West of Scotland Cancer Centre An audit of neutropenic complications in breast cancer patients receiving adjuvant or neo-adjuvant chemotherapy with FEC-D in
One of the most mature trials that examined PROCEEDINGS. Hormone Therapy in Postmenopausal Women With Breast Cancer * William J.
 Hormone Therapy in Postmenopausal Women With Breast Cancer * William J. Gradishar, MD ABSTRACT *Based on a presentation given by Dr Gradishar at a roundtable symposium held in Baltimore on June 28, 25.
Hormone Therapy in Postmenopausal Women With Breast Cancer * William J. Gradishar, MD ABSTRACT *Based on a presentation given by Dr Gradishar at a roundtable symposium held in Baltimore on June 28, 25.
Avastin in breast cancer: Summary of clinical data
 Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
UNDO and Neutenia in the Treatment of Cancer
 EU INSIGHT Volume 1 Issue 3 Winter 2003/04 IMPACT OF NEUTROPENIA IN CHEMOTHERAPY EUROPEAN STUDY GROUP Major Prospective Neutropenia Study Close to Initiation To ensure that you are kept informed of the
EU INSIGHT Volume 1 Issue 3 Winter 2003/04 IMPACT OF NEUTROPENIA IN CHEMOTHERAPY EUROPEAN STUDY GROUP Major Prospective Neutropenia Study Close to Initiation To ensure that you are kept informed of the
Cancer Care in the Elderly: A Guide to Economic Performance
 Cancer Economics CANCER CARE IN THE ELDERLY: COST AND QUALITY-OF-LIFE CONSIDERATIONS Gary H. Lyman, MD, MPH, 1 Nicole M. Kuderer, 2 and Lodovico Balducci, MD 1 The Departments of Internal Medicine and
Cancer Economics CANCER CARE IN THE ELDERLY: COST AND QUALITY-OF-LIFE CONSIDERATIONS Gary H. Lyman, MD, MPH, 1 Nicole M. Kuderer, 2 and Lodovico Balducci, MD 1 The Departments of Internal Medicine and
NATIONAL CANCER DRUG FUND PRIORITISATION SCORES
 NATIONAL CANCER DRUG FUND PRIORITISATION SCORES Drug Indication Regimen (where appropriate) BORTEZOMIB In combination with dexamethasone (VD), or with dexamethasone and thalidomide (VTD), is indicated
NATIONAL CANCER DRUG FUND PRIORITISATION SCORES Drug Indication Regimen (where appropriate) BORTEZOMIB In combination with dexamethasone (VD), or with dexamethasone and thalidomide (VTD), is indicated
EVIDENCE IN BRIEF OVERALL CLINICAL BENEFIT
 perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
perc also deliberated on the alignment of bendamustine with patient values. perc noted that bendamustine has a progression-free survival advantage, may be less toxic than currently available therapies
Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
 Efficacy Results from the ToGA Trial: A Phase III Study of Trastuzumab Added to Standard Chemotherapy in First-Line HER2- Positive Advanced Gastric Cancer Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
Efficacy Results from the ToGA Trial: A Phase III Study of Trastuzumab Added to Standard Chemotherapy in First-Line HER2- Positive Advanced Gastric Cancer Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
Prior Authorization Guideline
 Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
Prior Authorization Guideline Guideline: PS Inj - Alimta Therapeutic Class: Antineoplastic Agents Therapeutic Sub-Class: Antifolates Client: PS Inj Approval Date: 8/2/2004 Revision Date: 12/5/2006 I. BENEFIT
CHAPTER 26 LATE BREAKING DEVELOPMENTS: IMPACT OF ANTI-CD20 MONOCLONAL ANTIBODIES ON LYMPHOMA THERAPY
 CHAPTER 26 LATE BREAKING DEVELOPMENTS: IMPACT OF ANTI-CD20 MONOCLONAL ANTIBODIES ON LYMPHOMA THERAPY 26.1 Introduction rituximab Subsequent to the completion of drafts for the guidelines earlier in 2004,
CHAPTER 26 LATE BREAKING DEVELOPMENTS: IMPACT OF ANTI-CD20 MONOCLONAL ANTIBODIES ON LYMPHOMA THERAPY 26.1 Introduction rituximab Subsequent to the completion of drafts for the guidelines earlier in 2004,
Frequency of NHL Subtypes in Adults
 Chemotherapy Options Stephanie A. Gregory, M.D. The Elodia Kehm Professor of Medicine Director, Section of Hematology Rush University Medical Center Chicago, Illinois Frequency of NHL Subtypes in Adults
Chemotherapy Options Stephanie A. Gregory, M.D. The Elodia Kehm Professor of Medicine Director, Section of Hematology Rush University Medical Center Chicago, Illinois Frequency of NHL Subtypes in Adults
Evidence-based use of taxanes in the adjuvant setting of breast cancer. A review of randomized phase III trials
 Cancer Treatment Reviews (2007) 33, 474 483 available at www.sciencedirect.com journal homepage: www.elsevierhealth.com/journals/ctrv ANTI-TUMOUR TREATMENT Evidence-based use of taxanes in the adjuvant
Cancer Treatment Reviews (2007) 33, 474 483 available at www.sciencedirect.com journal homepage: www.elsevierhealth.com/journals/ctrv ANTI-TUMOUR TREATMENT Evidence-based use of taxanes in the adjuvant
Drug/Drug Combination: Bevacizumab in combination with chemotherapy
 AHFS Final Determination of Medical Acceptance: Off-label Use of Bevacizumab in Combination with Chemotherapy for the Treatment of Metastatic Breast Cancer Previously Treated with Cytotoxic Chemotherapy
AHFS Final Determination of Medical Acceptance: Off-label Use of Bevacizumab in Combination with Chemotherapy for the Treatment of Metastatic Breast Cancer Previously Treated with Cytotoxic Chemotherapy
Sonneveld, P; de Ridder, M; van der Lelie, H; et al. J Clin Oncology, 13 (10) : 2530-2539 Oct 1995
 Comparison of Doxorubicin and Mitoxantrone in the Treatment of Elderly Patients with Advanced Diffuse Non-Hodgkin's Lymphoma Using CHOP Versus CNOP Chemotherapy. Sonneveld, P; de Ridder, M; van der Lelie,
Comparison of Doxorubicin and Mitoxantrone in the Treatment of Elderly Patients with Advanced Diffuse Non-Hodgkin's Lymphoma Using CHOP Versus CNOP Chemotherapy. Sonneveld, P; de Ridder, M; van der Lelie,
I will be having surgery and radiation treatment for breast cancer. Do I need drug treatment too?
 What is node-positive breast cancer? Node-positive breast cancer means that cancer cells from the tumour in the breast have been found in the lymph nodes (sometimes called glands ) in the armpit area.
What is node-positive breast cancer? Node-positive breast cancer means that cancer cells from the tumour in the breast have been found in the lymph nodes (sometimes called glands ) in the armpit area.
Advances In Chemotherapy For Hormone Refractory Prostate Cancer. TAX 327 study results & SWOG 99-16 study results presented at ASCO 2004
 Ronald de Wit Rotterdam Cancer Institute The Netherlands Advances In Chemotherapy For Hormone Refractory Prostate Cancer TAX 327 study results & SWOG 99-16 study results presented at Slide 1 Prostate Cancer
Ronald de Wit Rotterdam Cancer Institute The Netherlands Advances In Chemotherapy For Hormone Refractory Prostate Cancer TAX 327 study results & SWOG 99-16 study results presented at Slide 1 Prostate Cancer
Type of intervention Treatment. Economic study type Cost-effectiveness analysis.
 Impact of uncertainty on cost-effectiveness analysis of medical strategies: the case of highdose chemotherapy for breast cancer patients Marino P, Siani C, Roche H, Moatti J P Record Status This is a critical
Impact of uncertainty on cost-effectiveness analysis of medical strategies: the case of highdose chemotherapy for breast cancer patients Marino P, Siani C, Roche H, Moatti J P Record Status This is a critical
Diffuse large B-cell lymphoma: the curable disease?
 Hematology Meeting Reports 2007; 1(5):77 86 Diffuse large B-cell lymphoma: the curable disease? Michael Pfreundschuh Med Klinik I, Saarland University Medical School, Homburg/Saar, Germany Corresponding
Hematology Meeting Reports 2007; 1(5):77 86 Diffuse large B-cell lymphoma: the curable disease? Michael Pfreundschuh Med Klinik I, Saarland University Medical School, Homburg/Saar, Germany Corresponding
Supplementary Online Content
 Supplementary Online Content Huang H, Li X, Zhu J, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving
Supplementary Online Content Huang H, Li X, Zhu J, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving
Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer
 Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer Background: A non-anthracycline based regimen for high-risk, HER 2 positive breast cancer in the adjuvant setting (BCIRG 006). Patient
Docetaxel + Carboplatin + Trastuzumab (TCH) Adjuvant Breast Cancer Background: A non-anthracycline based regimen for high-risk, HER 2 positive breast cancer in the adjuvant setting (BCIRG 006). Patient
January 2013 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW. Summary. Contents
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/paclitaxel for cancer Paclitaxel albumin (Abraxane ) as a substitute for docetaxel/ paclitaxel for
David Loew, LCL MabThera
 MabThera The star continues to rise David Loew, LCL MabThera MabThera the star continues to raise Group sales (CHF bn) 4,5 4,0 3,5 3,0 2,5 2,0 1,5 1,0 0,5 0,0 2001 2002 2003 2004 2005 Outstanding clinical
MabThera The star continues to rise David Loew, LCL MabThera MabThera the star continues to raise Group sales (CHF bn) 4,5 4,0 3,5 3,0 2,5 2,0 1,5 1,0 0,5 0,0 2001 2002 2003 2004 2005 Outstanding clinical
Inpatient Oncology Length of Stay and Hospital Costs: Implications for Rising Inpatient Expenditures
 Inpatient Oncology Length of Stay and Hospital Costs: Implications for Rising Inpatient Expenditures Katie J. Suda, PharmD* Susannah E. Motl, PharmD John C. Kuth, PharmD * University of Tennessee, College
Inpatient Oncology Length of Stay and Hospital Costs: Implications for Rising Inpatient Expenditures Katie J. Suda, PharmD* Susannah E. Motl, PharmD John C. Kuth, PharmD * University of Tennessee, College
Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma Bendamustine with rituximab for the first-line
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Bendamustine with rituximab for the first-line treatment of advanced indolent non-hodgkin's and mantle cell lymphoma Bendamustine with rituximab for the first-line
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases
 Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
Out-Patient Chemotherapy for Lung Cancer
 Lung Cancer Out-Patient Chemotherapy for Lung Cancer Principles and practice JMAJ 46(12): 542 546, 2003 Shuichi YONEDA Director, Department of Pulmonary Medicine, Saitama Cancer Center Abstract: Recent
Lung Cancer Out-Patient Chemotherapy for Lung Cancer Principles and practice JMAJ 46(12): 542 546, 2003 Shuichi YONEDA Director, Department of Pulmonary Medicine, Saitama Cancer Center Abstract: Recent
Mantle Cell Lymphoma Understanding Your Treatment Options
 New Developments in Mantle Cell Lymphoma John P. Leonard, M.D. Richard T. Silver Distinguished Professor of Hematology and Medical Oncology Associate Dean for Clinical Research Vice Chairman, Department
New Developments in Mantle Cell Lymphoma John P. Leonard, M.D. Richard T. Silver Distinguished Professor of Hematology and Medical Oncology Associate Dean for Clinical Research Vice Chairman, Department
New Treatment Options for Breast Cancer
 New Treatment Options for Breast Cancer Brandon Vakiner, PharmD., BCOP Clinical Pharmacy Specialist - Oncology The University of Iowa Hospitals and Clinics Assistant Professor (Clinical) University of
New Treatment Options for Breast Cancer Brandon Vakiner, PharmD., BCOP Clinical Pharmacy Specialist - Oncology The University of Iowa Hospitals and Clinics Assistant Professor (Clinical) University of
Implications of dose rounding intravenous chemotherapy at a community based hospital
 Implications of dose rounding intravenous chemotherapy at a community based hospital 1 2 ABSTRACT OBJECTIVES: To quantify and evaluate the total number of pharmacist interventions completed for dose rounding
Implications of dose rounding intravenous chemotherapy at a community based hospital 1 2 ABSTRACT OBJECTIVES: To quantify and evaluate the total number of pharmacist interventions completed for dose rounding
Dose-Dense Chemotherapy in High-Risk Breast Cancer: Treatment Outcome and Toxicity
 original article < Dose-Dense Chemotherapy in High-Risk Breast Cancer: Treatment Outcome and Toxicity Salah El-Mesidy, MD, Mohsen Mokhtar, MD, Amr El-Kashif, MD, Loay Kasem, M.Sc Department of Clinical
original article < Dose-Dense Chemotherapy in High-Risk Breast Cancer: Treatment Outcome and Toxicity Salah El-Mesidy, MD, Mohsen Mokhtar, MD, Amr El-Kashif, MD, Loay Kasem, M.Sc Department of Clinical
Lymphoma: The Roleof Nurses in the Treatment Process
 Lymphoma: The Roleof Nurses in the Treatment Process Sarah Liptrott MSc,BN(Hons), RN Istituto Europeo di Oncologia, Milan (IT) EBMT Swiss Study Day 2014, Zurich, Switzerland LymphomaManagement Watch &
Lymphoma: The Roleof Nurses in the Treatment Process Sarah Liptrott MSc,BN(Hons), RN Istituto Europeo di Oncologia, Milan (IT) EBMT Swiss Study Day 2014, Zurich, Switzerland LymphomaManagement Watch &
LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW. FOLFIRINOX for first line treatment of advanced pancreatic cancer January 2012
 Background LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW FOLFIRINOX for first line treatment of advanced pancreatic cancer January 2012 The incidence of pancreatic cancer in the UK is 9.4/100,000. It is
Background LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW FOLFIRINOX for first line treatment of advanced pancreatic cancer January 2012 The incidence of pancreatic cancer in the UK is 9.4/100,000. It is
Treatment of Low Risk MDS. Overview. Myelodysplastic Syndromes (MDS)
 Overview Amy Davidoff, Ph.D., M.S. Associate Professor Pharmaceutical Health Services Research Department, Peter Lamy Center on Drug Therapy and Aging University of Maryland School of Pharmacy Clinical
Overview Amy Davidoff, Ph.D., M.S. Associate Professor Pharmaceutical Health Services Research Department, Peter Lamy Center on Drug Therapy and Aging University of Maryland School of Pharmacy Clinical
4/8/13. Pre-test Audience Response. Prostate Cancer 2012. Screening and Treatment of Prostate Cancer: The 2013 Perspective
 Pre-test Audience Response Screening and Treatment of Prostate Cancer: The 2013 Perspective 1. I do not offer routine PSA screening, and the USPSTF D recommendation will not change my practice. 2. In light
Pre-test Audience Response Screening and Treatment of Prostate Cancer: The 2013 Perspective 1. I do not offer routine PSA screening, and the USPSTF D recommendation will not change my practice. 2. In light
J Clin Oncol 24:4888-4894. 2006 by American Society of Clinical Oncology INTRODUCTION
 VOLUME 24 NUMBER 30 OCTOBER 20 2006 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T Impact on Survival of Time From Definitive Surgery to Initiation of Adjuvant Chemotherapy for Early-Stage Breast
VOLUME 24 NUMBER 30 OCTOBER 20 2006 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T Impact on Survival of Time From Definitive Surgery to Initiation of Adjuvant Chemotherapy for Early-Stage Breast
Section 3 G-CSF in neutropenia guidelines. Implementation Toolkit
 Section 3 G-CSF in neutropenia guidelines Guidelines Implementation Toolkit Contents Section 3 G-CSF in neutropenia guidelines Introduction to Section 3 3.1 Introduction Overall Goal Specific Targets and
Section 3 G-CSF in neutropenia guidelines Guidelines Implementation Toolkit Contents Section 3 G-CSF in neutropenia guidelines Introduction to Section 3 3.1 Introduction Overall Goal Specific Targets and
Non-Hodgkin s lymphomas (NHLs) are a
 Oncology 33 Non-Hodgkin s lymphoma in the elderly The incidence of non-hodgkin s lymphoma (NHL) is increasing, and this increase is even more rapid in the older population. Although treatment of NHL in
Oncology 33 Non-Hodgkin s lymphoma in the elderly The incidence of non-hodgkin s lymphoma (NHL) is increasing, and this increase is even more rapid in the older population. Although treatment of NHL in
Cytotoxic Therapy in Metastatic Breast Cancer
 Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer Cytotoxic Therapy in Metastatic Breast Cancer Cytotoxic Therapy in Metastatic Breast Cancer Version 2002: von Minckwitz Versions
Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer Cytotoxic Therapy in Metastatic Breast Cancer Cytotoxic Therapy in Metastatic Breast Cancer Version 2002: von Minckwitz Versions
Michael Crump MD. Lymphoma Site Leader Princess Margaret Hospital University of Toronto
 Evolution of Lymphoma Therapy: What can we expect for the rest of the millenium decade? Michael Crump MD Lymphoma Site Leader Princess Margaret Hospital University of Toronto disclaimers Served on advisory
Evolution of Lymphoma Therapy: What can we expect for the rest of the millenium decade? Michael Crump MD Lymphoma Site Leader Princess Margaret Hospital University of Toronto disclaimers Served on advisory
How valuable is a cancer therapy? It depends on who you ask.
 How valuable is a cancer therapy? It depends on who you ask. Comparing and contrasting the ESMO Magnitude of Clinical Benefit Scale with the ASCO Value Framework in Cancer Ram Subramanian Kevin Schorr
How valuable is a cancer therapy? It depends on who you ask. Comparing and contrasting the ESMO Magnitude of Clinical Benefit Scale with the ASCO Value Framework in Cancer Ram Subramanian Kevin Schorr
DECISION AND SUMMARY OF RATIONALE
 DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Clofarabine in the treatment of relapsed acute myeloid leukaemia (AML) The application was for clofarabine to remain in
DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Clofarabine in the treatment of relapsed acute myeloid leukaemia (AML) The application was for clofarabine to remain in
Lymphomas after organ transplantation
 Produced 21.03.2011 Revision due 21.03.2011 Lymphomas after organ transplantation People who have undergone an organ transplant are more at risk of developing lymphoma known as post-transplant lymphoproliferative
Produced 21.03.2011 Revision due 21.03.2011 Lymphomas after organ transplantation People who have undergone an organ transplant are more at risk of developing lymphoma known as post-transplant lymphoproliferative
BCCA Protocol Summary for Advanced Therapy for Relapsed Testicular Germ Cell Cancer Using PACLitaxel, Ifosfamide and CISplatin (TIP)
 BCCA Protocol Summary for Advanced Therapy for Relapsed Testicular Germ Cell Cancer Using PACLitaxel, Ifosfamide and CISplatin (TIP) Protocol Code Tumour Group Contact Physician UGUTIP Genitourinary Dr.
BCCA Protocol Summary for Advanced Therapy for Relapsed Testicular Germ Cell Cancer Using PACLitaxel, Ifosfamide and CISplatin (TIP) Protocol Code Tumour Group Contact Physician UGUTIP Genitourinary Dr.
Treatment of low-grade non-hodgkin lymphoma
 Produced 28.02.2011 Due for revision 28.02.2013 Treatment of low-grade non-hodgkin lymphoma Lymphomas are described as low grade if the cells appear to be dividing slowly. There are several kinds of low-grade
Produced 28.02.2011 Due for revision 28.02.2013 Treatment of low-grade non-hodgkin lymphoma Lymphomas are described as low grade if the cells appear to be dividing slowly. There are several kinds of low-grade
Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer
 Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer Review Article [1] December 01, 2003 By George W. Sledge, Jr, MD [2] Gemcitabine (Gemzar) and paclitaxel show good activity as single
Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer Review Article [1] December 01, 2003 By George W. Sledge, Jr, MD [2] Gemcitabine (Gemzar) and paclitaxel show good activity as single
Hospital Outpatient Coding and Billing Information Sheet for Neulasta and NEUPOGEN
 Hospital Outpatient Coding and Billing Information Sheet for Neulasta and Neulasta Delivery Kit Neulasta Prefilled Syringe For assistance contact 1-844-MYNEULASTA (1-844-696-3852) or visit www.amgenassistonline.com
Hospital Outpatient Coding and Billing Information Sheet for Neulasta and Neulasta Delivery Kit Neulasta Prefilled Syringe For assistance contact 1-844-MYNEULASTA (1-844-696-3852) or visit www.amgenassistonline.com
Sequential adjuvant docetaxel and anthracycline chemotherapy for node positive breast cancers: a retrospective study
 JBUON 2013; 18(2): 314-320 ISSN: 1107-0625 www.jbuon.com E-mail: info@jbuon.com ORIGINAL ARTICLE Sequential adjuvant docetaxel and anthracycline chemotherapy for node positive breast cancers: a retrospective
JBUON 2013; 18(2): 314-320 ISSN: 1107-0625 www.jbuon.com E-mail: info@jbuon.com ORIGINAL ARTICLE Sequential adjuvant docetaxel and anthracycline chemotherapy for node positive breast cancers: a retrospective
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER
 GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
GUIDELINES FOR THE MANAGEMENT OF LUNG CANCER BY Ali Shamseddine, MD (Coordinator); as04@aub.edu.lb Fady Geara, MD Bassem Shabb, MD Ghassan Jamaleddine, MD CLINICAL PRACTICE GUIDELINES FOR THE TREATMENT
NCCN Non-Small Cell Lung Cancer V.1.2011 Update Meeting 07/09/10
 Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
Aggressive lymphomas. Michael Crump Princess Margaret Hospital
 Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
Aggressive lymphomas Michael Crump Princess Margaret Hospital What are the aggressive lymphomas? Diffuse large B cell Mediastinal large B cell Anaplastic large cell Burkitt lymphoma (transformed lymphoma:
The Impact of Palliative Care and Hospice Services in the Care of Patients with Advanced Stage Non-Small Cell Lung Cancer
 The Impact of Palliative Care and Hospice Services in the Care of Patients with Advanced Stage Non-Small Cell Lung Cancer Richard C. Stephenson, MD Hospice & Palliative CareCenter Amy H. Hughes Forsyth
The Impact of Palliative Care and Hospice Services in the Care of Patients with Advanced Stage Non-Small Cell Lung Cancer Richard C. Stephenson, MD Hospice & Palliative CareCenter Amy H. Hughes Forsyth
J Clin Oncol 23:8447-8452. 2005 by American Society of Clinical Oncology INTRODUCTION
 VOLUME 23 NUMBER 33 NOVEMBER 20 2005 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T New Treatment Options Have Changed the Survival of Patients With Follicular Lymphoma Richard I. Fisher, Michael
VOLUME 23 NUMBER 33 NOVEMBER 20 2005 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T New Treatment Options Have Changed the Survival of Patients With Follicular Lymphoma Richard I. Fisher, Michael
Cycle frequency: Every four weeks Total number of cycles: 6-8
 Fludarabine Low Grade non-hodgkin s Lymphoma and CLL Fludarabine 25mg/m 2 oral Days 1-5 Cycle frequency: Every four weeks Total number of cycles: 6-8 Anti-emetic group Low Prophylactic co-trimoxazole and
Fludarabine Low Grade non-hodgkin s Lymphoma and CLL Fludarabine 25mg/m 2 oral Days 1-5 Cycle frequency: Every four weeks Total number of cycles: 6-8 Anti-emetic group Low Prophylactic co-trimoxazole and
DIFFUSE LARGE B-CELL LYMPHOMA
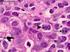 DIFFUSE LARGE B-CELL LYMPHOMA Executive Summary Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-hodgkin lymphoma (NHL), constituting up to 40% of all cases globally.[1] This subtype
DIFFUSE LARGE B-CELL LYMPHOMA Executive Summary Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-hodgkin lymphoma (NHL), constituting up to 40% of all cases globally.[1] This subtype
Controversies in Management of. Inoperable NSCLC. Inoperable NSCLC. Introduction:
 Inoperable NSCLC Controversies in Management of Inoperable NSCLC Introduction: It is difficult to overemphasize the magnitude of lung cancer as Public Health Problem in our society. - In US, Lung cancer
Inoperable NSCLC Controversies in Management of Inoperable NSCLC Introduction: It is difficult to overemphasize the magnitude of lung cancer as Public Health Problem in our society. - In US, Lung cancer
The Expanding Role of Epirubicin in the Treatment of Breast Cancer
 Regimens containing higher doses of epirubicin prolong relapse-free and overall survival rates compared with standard therapies. Michele Sassi. La Digue Island, c. 2001. Seychelles Islands, Indian Ocean.
Regimens containing higher doses of epirubicin prolong relapse-free and overall survival rates compared with standard therapies. Michele Sassi. La Digue Island, c. 2001. Seychelles Islands, Indian Ocean.
As you know, the CPT Editorial Panel developed two new codes to describe complex ACP services for CY 2015.
 December 30, 2014 SUBMITTED ELECTRONICALLY VIA http://www.regulations.gov Marilyn Tavenner Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS
December 30, 2014 SUBMITTED ELECTRONICALLY VIA http://www.regulations.gov Marilyn Tavenner Administrator Centers for Medicare and Medicaid Services Department of Health and Human Services Attention: CMS
Can I have FAITH in this Review?
 Can I have FAITH in this Review? Find Appraise Include Total Heterogeneity Paul Glasziou Centre for Research in Evidence Based Practice Bond University What do you do? For an acutely ill patient, you do
Can I have FAITH in this Review? Find Appraise Include Total Heterogeneity Paul Glasziou Centre for Research in Evidence Based Practice Bond University What do you do? For an acutely ill patient, you do
Controversites: Screening for Prostate Cancer in Older Adults
 Controversites: Screening for Prostate Cancer in Older Adults William Dale, MD, PhD University of Chicago Sections of Geriatrics & Palliative Medicine and Hematology/Oncology Director, Specialized Oncology
Controversites: Screening for Prostate Cancer in Older Adults William Dale, MD, PhD University of Chicago Sections of Geriatrics & Palliative Medicine and Hematology/Oncology Director, Specialized Oncology
Management of Peritoneal Metastases (PM) from colorectal cancers: New Perspectives. Dominique ELIAS
 Management of Peritoneal Metastases (PM) from colorectal cancers: New Perspectives Dominique ELIAS Declaration of interest BOARDS Congress and teaching 0 Merck 0 Ipsen Novartis Sanofi Trials The peritoneum
Management of Peritoneal Metastases (PM) from colorectal cancers: New Perspectives Dominique ELIAS Declaration of interest BOARDS Congress and teaching 0 Merck 0 Ipsen Novartis Sanofi Trials The peritoneum
Stage IV Renal Cell Carcinoma. Changing Management in A Comprehensive Community Cancer Center. Susquehanna Health Cancer Center
 Stage IV Renal Cell Carcinoma Changing Management in A Comprehensive Community Cancer Center Susquehanna Health Cancer Center 2000 2009 Warren L. Robinson, MD, FACP January 27, 2014 Introduction 65,150
Stage IV Renal Cell Carcinoma Changing Management in A Comprehensive Community Cancer Center Susquehanna Health Cancer Center 2000 2009 Warren L. Robinson, MD, FACP January 27, 2014 Introduction 65,150
Cancer research in the Midland Region the prostate and bowel cancer projects
 Cancer research in the Midland Region the prostate and bowel cancer projects Ross Lawrenson Waikato Clinical School University of Auckland MoH/HRC Cancer Research agenda Lung cancer Palliative care Prostate
Cancer research in the Midland Region the prostate and bowel cancer projects Ross Lawrenson Waikato Clinical School University of Auckland MoH/HRC Cancer Research agenda Lung cancer Palliative care Prostate
Screening Mammography for Breast Cancer: American College of Preventive Medicine Practice Policy Statement
 Screening Mammography for Breast Cancer: American College of Preventive Medicine Practice Policy Statement Rebecca Ferrini, MD, Elizabeth Mannino, MD, Edith Ramsdell, MD and Linda Hill, MD, MPH Burden
Screening Mammography for Breast Cancer: American College of Preventive Medicine Practice Policy Statement Rebecca Ferrini, MD, Elizabeth Mannino, MD, Edith Ramsdell, MD and Linda Hill, MD, MPH Burden
J Clin Oncol 27:2474-2481. 2009 by American Society of Clinical Oncology INTRODUCTION
 VOLUME 27 NUMBER 15 MAY 2 29 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T Phase III Trial Evaluating the Addition of Paclitaxel to Doxorubicin Followed by Cyclophosphamide, Methotrexate, and
VOLUME 27 NUMBER 15 MAY 2 29 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T Phase III Trial Evaluating the Addition of Paclitaxel to Doxorubicin Followed by Cyclophosphamide, Methotrexate, and
Effects of Chemotherapy on Quality of Life for Patients with Lung Cancer
 CLINICAL STUDY Effects of Chemotherapy on Quality of Life for Patients with Lung Cancer Ahmet Bircan, MD 1 ; M. Bahad r Berktafl, MD 1 ; Hülya Bay z, MD 1 ; Nihal Baflay, MD 1 ; Sema Bircan, MD 2 ; Mine
CLINICAL STUDY Effects of Chemotherapy on Quality of Life for Patients with Lung Cancer Ahmet Bircan, MD 1 ; M. Bahad r Berktafl, MD 1 ; Hülya Bay z, MD 1 ; Nihal Baflay, MD 1 ; Sema Bircan, MD 2 ; Mine
Chemotherapy in Ovarian Cancer. Dr R Jones Consultant Medical Oncologist South Wales Gynaecological Oncology Group
 Chemotherapy in Ovarian Cancer Dr R Jones Consultant Medical Oncologist South Wales Gynaecological Oncology Group Adjuvant chemotherapy for early stage EOC Fewer than 30% women present with FIGO stage
Chemotherapy in Ovarian Cancer Dr R Jones Consultant Medical Oncologist South Wales Gynaecological Oncology Group Adjuvant chemotherapy for early stage EOC Fewer than 30% women present with FIGO stage
New Trends & Current Research in the Treatment of Lung Cancer, Pt. II
 New Trends & Current esearch in the Treatment of Lung Cancer, Pt. II Howard (Jack) West, MD President & CEO, GACE Medical Director, Thoracic Oncology Program Swedish Cancer Institute Seattle, WA Cancer
New Trends & Current esearch in the Treatment of Lung Cancer, Pt. II Howard (Jack) West, MD President & CEO, GACE Medical Director, Thoracic Oncology Program Swedish Cancer Institute Seattle, WA Cancer
HIV Case Conference: HIV Associated Lymphoma
 F/C AETC Faculty General HIV Tuesday, July 22, 2014 12:30 1:30pm (EDT) Facilitator Todd S. Wills, MD University of South Florida Didactic Presenter Jose Castro, MD University of Miami Case Discussant(s)
F/C AETC Faculty General HIV Tuesday, July 22, 2014 12:30 1:30pm (EDT) Facilitator Todd S. Wills, MD University of South Florida Didactic Presenter Jose Castro, MD University of Miami Case Discussant(s)
Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment.
 1 Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment. John T. Carpenter, M.D. University of Alabama at Birmingham NP 2508 1720 Second Avenue South Birmingham, AL 35294-3300
1 Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment. John T. Carpenter, M.D. University of Alabama at Birmingham NP 2508 1720 Second Avenue South Birmingham, AL 35294-3300
Rituximab for the first-line maintenance treatment of follicular non-hodgkin s lymphoma
 Issue date: June 2011 Rituximab for the first-line maintenance treatment of follicular non-hodgkin s lymphoma This guidance was developed using the the single technology appraisal process NICE technology
Issue date: June 2011 Rituximab for the first-line maintenance treatment of follicular non-hodgkin s lymphoma This guidance was developed using the the single technology appraisal process NICE technology
Proceedings of the World Small Animal Veterinary Association Sydney, Australia 2007
 Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress Rescue Chemotherapy Protocols for Dogs with Lymphoma Kenneth M. Rassnick, DVM, DACVIM (Oncology) Cornell University
Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress Rescue Chemotherapy Protocols for Dogs with Lymphoma Kenneth M. Rassnick, DVM, DACVIM (Oncology) Cornell University
Clinical Trial Design. Sponsored by Center for Cancer Research National Cancer Institute
 Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN
 + IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
+ IF AT FIRST YOU DON T SUCCEED: TRIAL, TRIAL AGAIN Rena Buckstein MD FRCPC Head Hematology Site Group Sunnybrook Odette Cancer Center (OCC) Head of Hematology Clinical Trials Group at OCC + Outline Start
Management of stage III A-B of NSCLC. Hamed ALHusaini Medical Oncologist
 Management of stage III A-B of NSCLC Hamed ALHusaini Medical Oncologist Global incidence, CA cancer J Clin 2011;61:69-90 Stage III NSCLC Includes heterogeneous group of patients with differences in the
Management of stage III A-B of NSCLC Hamed ALHusaini Medical Oncologist Global incidence, CA cancer J Clin 2011;61:69-90 Stage III NSCLC Includes heterogeneous group of patients with differences in the
Real-world experience with adjuvant fec-d chemotherapy in four Ontario regional cancer centres
 MEDICAL ONCOLOGY Real-world experience with adjuvant fec-d chemotherapy in four Ontario regional cancer centres Y. Madarnas md,* S.F. Dent md, S.F. Husain md,* A. Robinson md, S. Alkhayyat md, W.M. Hopman
MEDICAL ONCOLOGY Real-world experience with adjuvant fec-d chemotherapy in four Ontario regional cancer centres Y. Madarnas md,* S.F. Dent md, S.F. Husain md,* A. Robinson md, S. Alkhayyat md, W.M. Hopman
Therapeutic Options in Refractory or Relapsed CD20-positive Follicular Lymphoma
 a report by Martin Dreyling Therapeutic Options in Refractory or Relapsed CD20-positive Follicular Lymphoma Head, Lymphoma Section, Department of Medicine III, University Hospital Großhadern, Ludwig Maximilians-University
a report by Martin Dreyling Therapeutic Options in Refractory or Relapsed CD20-positive Follicular Lymphoma Head, Lymphoma Section, Department of Medicine III, University Hospital Großhadern, Ludwig Maximilians-University
Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer
 LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
Stage II breast cancer
 CHAPTER 10 Stage II breast cancer Lori Jardines, MD, Bruce G. Haffty, MD, and Melanie Royce, MD, PhD This chapter focuses on the treatment of stage II breast cancer, which encompasses primary tumors >
CHAPTER 10 Stage II breast cancer Lori Jardines, MD, Bruce G. Haffty, MD, and Melanie Royce, MD, PhD This chapter focuses on the treatment of stage II breast cancer, which encompasses primary tumors >
Neutropenic Fever Module Appendices
 Neutropenic Fever Module Appendices Examples of Chemotherapy Regimens with Medium to High Risk of Febrile Neutropenia... 2 Examples of Chemotherapy Regimens with a High Risk of Febrile Neutropenia (> 20%)...
Neutropenic Fever Module Appendices Examples of Chemotherapy Regimens with Medium to High Risk of Febrile Neutropenia... 2 Examples of Chemotherapy Regimens with a High Risk of Febrile Neutropenia (> 20%)...
Breast cancer treatment for elderly women: a systematic review
 Breast cancer treatment for elderly women: a systematic review Gerlinde Pilkington Rumona Dickson Anna Sanniti Funded by the NCEI and POI Elderly people less likely to receive chemotherapy than younger
Breast cancer treatment for elderly women: a systematic review Gerlinde Pilkington Rumona Dickson Anna Sanniti Funded by the NCEI and POI Elderly people less likely to receive chemotherapy than younger
When the diagnosis is cancer, many people
 Hard decisions about cancer 5 tests and treatments to question When the diagnosis is cancer, many people understandably want to pull out all the stops to treat it. But some tests, treatments, and procedures
Hard decisions about cancer 5 tests and treatments to question When the diagnosis is cancer, many people understandably want to pull out all the stops to treat it. But some tests, treatments, and procedures
Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials
 Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 5-year survival: an overview of the randomised trials Early Breast Cancer Trialists Collaborative Group (EBCTCG)*
Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 5-year survival: an overview of the randomised trials Early Breast Cancer Trialists Collaborative Group (EBCTCG)*
Komorbide brystkræftpatienter kan de tåle behandling? Et registerstudie baseret på Danish Breast Cancer Cooperative Group
 Komorbide brystkræftpatienter kan de tåle behandling? Et registerstudie baseret på Danish Breast Cancer Cooperative Group Lotte Holm Land MD, ph.d. Onkologisk Afd. R. OUH Kræft og komorbiditet - alle skal
Komorbide brystkræftpatienter kan de tåle behandling? Et registerstudie baseret på Danish Breast Cancer Cooperative Group Lotte Holm Land MD, ph.d. Onkologisk Afd. R. OUH Kræft og komorbiditet - alle skal
DECISION AND SUMMARY OF RATIONALE
 DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Everolimus in combination with exemestane hormone therapy for oestrogen receptor positive locally advanced or metastatic
DECISION AND SUMMARY OF RATIONALE Indication under consideration Clinical evidence Everolimus in combination with exemestane hormone therapy for oestrogen receptor positive locally advanced or metastatic
Pharmacogenetic Activities in SWOG Breast Cancer
 Pharmacogenetic Activities in SWOG Breast Cancer Pharmacogenomics: Future Plans S8897 Adjuvant CMF vs. CAF/ no Treatment Ambrosone RO1: Other genes (TBCI approved, analyses ongoing) S0221 Adjuvant Dose
Pharmacogenetic Activities in SWOG Breast Cancer Pharmacogenomics: Future Plans S8897 Adjuvant CMF vs. CAF/ no Treatment Ambrosone RO1: Other genes (TBCI approved, analyses ongoing) S0221 Adjuvant Dose
SMALL CELL LUNG CANCER
 Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
Protocol for Planning and Treatment The process to be followed in the management of: SMALL CELL LUNG CANCER Patient information given at each stage following agreed information pathway 1. DIAGNOSIS New
