Maximizing Therapeutic Success in an Era of Increasing Antibiotic Drug Resistance
|
|
|
- Bathsheba Russell
- 7 years ago
- Views:
Transcription
1 Maximizing Therapeutic Success in an Era of Increasing Antibiotic Drug Resistance Authors: David L. Paterson, MD, PhD Complete author affiliations and disclosures are at the end of this activity. Release Date: April 27, 2006; Valid for credit through April 27, 2007 Target Audience This activity is intended for infectious disease specialists, internists, and other physicians and healthcare professionals who treat infected patients with parenteral antibiotics in hospitals and intensive care units as well as nurses who work in hospitals and healthcare facilities where serious bacterial infections are treated with parenteral agents. Goal The goal of this activity is to provide a strategy to optimize antibiotic use, including defining the role of newer antibiotic options in treating serious hospital infections caused by emerging gram-negative and gram-positive pathogens. Learning Objectives Upon completion of this activity, participants will be able to: 1. Review current data in regard to the prevalence, clinical impact, and management challenges of antimicrobial drug resistance in hospital settings. 2. Use expert recommendations concerning antibiotic selection and use for empiric therapy to determine optimum antibiotics in clinical practice. 3. Appropriately incorporate both older and newer antibiotics in the management of serious bacterial infections, particularly those encountered in hospital settings. Contents of This CME/CE Activity 1. Maximizing Therapeutic Success in an Era of Increasing Antibiotic Drug Resistance Introduction The Prevalence of Antibiotic Resistance in the Hospital Setting 1
2 Antibiotic Selection for Specific Pathogens Empiric Antibiotic Selection Self-Assessment Case 1: Nosocomial Postappendectomy Secondary Peritonitis Self-Assessment Case 1 (Continued) Self-Assessment Case 2: Ventilator-Associated Pneumonia With MRSA: Empiric Therapy in General and Empiric Therapy Based on Organism Self-Assessment Case 2 (Continued) Summary References Maximizing Therapeutic Success in an Era of Increasing Antibiotic Drug Resistance Introduction Resistance of bacteria to antibiotics has been noted ever since the discovery of penicillin by Fleming and its subsequent production by Florey. Antibiotic resistance may be intrinsic: This implies that the antibiotic is unable to have an effect on "wild-type" bacteria previously unexposed to the antibiotic in question. For example, vancomycin is not active against gram-negative bacteria, nor has it ever been. Antibiotic resistance may also be either mutational or acquired. This implies changes in the bacteria that prevent the antibiotic from exerting its effect on the bacterial target, which may have resulted from either (1) mutation of existing genetic material within the bacteria or (2) acquisition of new genetic material from other bacteria. For example, Escherichia coli in its natural state may be susceptible to both ampicillin and ciprofloxacin. However, the mutation of existing genetic material may lead to ciprofloxacin resistance, and the acquisition of genes that encode for beta-lactamase production may lead to resistance of E coli to ampicillin. The problems of antibiotic resistance are typically magnified in a hospital setting. Exposure to antibiotics while a patient is in the hospital may lead to genetic mutations that contribute to antibiotic resistance. Patients may be inadvertently exposed to the bacterial flora of other patients (usually due to a breakdown in basic infection control precautions). As a result, antibiotic-resistant bacteria may colonize multiple patients: Exposure of these patients to antibiotics may eliminate all but the most resistant bacteria. These resistant organisms may transfer antibiotic resistance genes to other bacteria, thereby multiplying the problem. Antibiotic resistance is therefore a major issue for clinicians, but especially those who practice in hospital settings. This Clinical Update concentrates on 3 areas: (1) current data in regard to the prevalence of antibiotic resistance in the hospital setting, (2) recommendations in regard to antibiotic selection for specific pathogens, and (3) recommendations in regard to empiric antibiotic selection for common serious infections. 2
3 1. How do the rates of antibiotic resistance at your institution compare with national or international averages? Lower in general About the same Higher in general Explanation: One of the 3 is probably true, depending on the type of institution (teaching or community, for example). 2. Which of the following is the most important barrier in the optimal management of a patient with nosocomial infection? (Select 1 answer.) Lack of effective treatment options for multidrug-resistant pathogens Lack of hospital-specific cumulative antibiotic susceptibility report Lack of unit-specific cumulative antibiotic susceptibility report Patient's comorbidities 3. How confident are you that you are up-to-date in the optimal management of nosocomial infections? (Select 1 answer.) Not at all confident Somewhat confident Confident Very confident 3
4 4. How do you use knowledge of local rates of resistance in choosing antibiotic therapy for a patient with a life-threatening infection? Follow published treatment guidelines Follow hospital treatment protocols Initiate broad-spectrum antibiotic regimen and de-escalate when possible Initiate narrow-spectrum antibiotic regimen and add antibiotics as necessary Individualize treatment Explanation: "Individualize treatment" is the most politically correct answer; however, both "Follow hospital treatment protocols" and "Initiate broad-spectrum antibiotic regimen and de-escalate when possible" are also appropriate responses. The Prevalence of Antibiotic Resistance in the Hospital Setting All of us who work in the hospital setting understand that antibiotic resistance is an important issue. However, the epidemiology of antibiotic-resistant organisms varies from one geographic area to another. Furthermore, even in an individual hospital, there is variation in the occurrence of resistant organisms. The Unit-Specific or Hospital-Specific Cumulative Antibiotic Susceptibility Report or "Antibiogram" For any individual patient with an infection, an antibiotic susceptibility report is typically issued, detailing the susceptibility of the particular organism to multiple antibiotics. In an individual institution or a unit within that institution, cumulative antibiotic susceptibility reports on multiple patients ("antibiograms") can be constructed in order to aid with appropriate antibiotic choice. Antibiotic use can be classified into 4 categories (Table 1). First, antibiotic use may be prophylactic, such as in the perioperative use of antibiotics to prevent surgical wound infection. Second, antibiotics may be used as empiric treatment. This implies the use of antibiotics directed at a particular syndrome -- such as community-acquired urinary tract infection or ventilator-associated pneumonia -- without precise knowledge of the 4
5 organisms causing the infection. Third, antibiotic use may be pathogen-directed. In other words, the organism causing the infection (for example, Staphylococcus aureus) is known, but the susceptibility of the organism is unknown. Finally, antibiotic therapy may be susceptibility-guided. In this case, both the identity of the organism causing infection and the susceptibility profile of the organism are known. Table 1. Types of Antibiotic Therapy and How Antibiograms Can Assist in Choice of Therapy Type of Therapy Description Use of Antibiograms Prophylaxis Empiric Antibiotics used to prevent infection Organism is unknown but syndrome is known Pathogen-directed Organism is known but susceptibility is unknown Susceptibilityguided Organism is known and susceptibility is known Selection of antibiotic Selection of antibiotic or combinations of antibiotics Selection of antibiotic Cumulative antibiogram not useful The provision of cumulative antibiotic susceptibility reports (antibiograms) is particularly helpful for choosing empiric and pathogen-directed treatment regimens. In contrast, the provision of antibiotic susceptibility reports on individual patients is clearly of use to ensure that antimicrobial treatment was adequate for the organism causing the infection. It also assists in antibiotic "streamlining" -- the process by which excessively broadspectrum empiric antibiotic therapy can be switched to narrower spectrum therapy aimed only at the implicated pathogen(s). Cumulative Antibiotic Susceptibility Reports and Choice of Empiric Therapy A major role for cumulative antibiotic susceptibility reports is in guiding antibiotic choice for empiric therapy. A large proportion of antibiotic therapy is empiric, be it antibiotic therapy commenced in the community or in the hospital. In most circumstances, the clinician will make a diagnosis of the anatomic site of infection on the basis of the patient's symptoms and signs. In some circumstances, additional laboratory tests or radiologic studies will be needed in order to make the diagnosis. Thus, a clinician may diagnose conditions, such as uncomplicated urinary tract infection, community-acquired pneumonia, late-onset ventilator-associated pneumonia, etc. Because empiric therapy occurs in the absence of knowledge of the organism causing the infection, the choice of antibiotics rests on the likely organisms causing that particular type of infection. For example, for uncomplicated urinary tract infection, E coli predominates; for communityacquired pneumonia, Streptococcus pneumoniae; and for ventilator-associated pneumonia, Pseudomonas aeruginosa, S aureus, Enterobacter cloacae, and Klebsiella pneumoniae are most frequently isolated. 5
6 Empiric antibiotic therapy that is based on an antibiogram is most likely to be helpful when the antibiogram closely reflects the source of organisms in the patient. Thus, an antibiogram for community-acquired E coli isolates from women with uncomplicated urinary tract infection is more likely to be useful in guiding therapy for an individual patient with uncomplicated urinary tract infection than is a hospital antibiogram for E coli. The geographic source of organisms is also relevant. Thus, an antibiogram for S pneumoniae isolates produced by the Georgia State Department of Health is of questionable relevance in treating an individual patient with community-acquired pneumonia in Boston, Massachusetts. This is the important question that this issue raises: "How can clinicians obtain antibiograms that are meaningful to their patients?" Laboratories processing specimens from outpatients rarely produce antibiograms. Furthermore, it may be difficult for such laboratories to distinguish whether samples are from patients in nursing homes or truly from the community. This is important because antibiotic pressures and infection control considerations are likely to lead to a greater extent of antibiotic resistance in nursing homes than elsewhere in the community. Important issues also arise for the hospital clinical microbiology laboratory constructing an antibiogram. It may be difficult for a microbiologist to determine whether organisms are community-acquired or hospital-acquired. Hospital laboratories should know the ward in which the patient was residing at the time when the isolate was collected. This, however, may not be the source of the infection. For example, a patient admitted from home to a medical intensive care unit (ICU) with sepsis of urinary tract origin may actually have a community-acquired pathogen rather than an ICU-acquired pathogen. Isolates from patients in emergency departments of tertiary care referral centers may be particularly misleading. For example, it may be tempting to assess the advent of community-acquired methicillin-resistant S aureus (MRSA) in a particular locality by determining the proportion of oxacillin resistance in S aureus isolates collected from patients in the hospital's emergency department. However, this may be grossly misleading because more than 50% of such isolates may be from patients with recent hospital admission and discharge, who are representing to the hospital with complications. Despite these caveats, antibiograms can potentially provide useful information for antibiotic prescribers. It is known that for patients in ICUs, mortality rises if the empiric antibiotic therapy chosen does not cover the pathogens causing the infection. [1-4] Kollef and colleagues [1] showed that infection-related mortality was 17.7% in those patients who received appropriate empiric antibiotic therapy vs 42.0% in those patients who received inappropriate empiric antibiotic therapy. The most common reason why empiric antibiotic therapy was inappropriate was resistance of the bacteria to the antibiotic chosen. In an effort to improve the adequacy of their antibiotic selection, Ibrahim and colleagues [5] reviewed the antibiogram for their particular ICU and created a clinical guideline for antibiotic selection in that unit. The adequacy of empiric antibiotic selection for ventilator-associated pneumonia for patients in their ICU increased from 48.0% before the creation of antibiotic guidelines to 94.2% with the use of their guidelines. 6
7 It must be acknowledged that an antibiogram-based guideline does not have an unlimited duration of utility. It is likely that shifts in antibiotic usage engendered by the creation of the guideline will, over time, lead to a change in resistance patterns. Thus, it is prudent to update antibiograms and antibiogram-based antibiotic guidelines on a regular basis. A yearly review should be regarded as a bare minimum. "Rolling" antibiograms, which are constantly updated, are probably optimal if an institution has the information technology resources to create them. An important consideration is whether hospital-wide antibiograms give an accurate indication of antibiotic resistance in a particular unit within the hospital. Namias and colleagues [6] showed that ICU antibiograms differed substantially from the antibiogram for the entire hospital. I have observed a similar finding in my own institution: A recent antibiogram for the entire hospital showed that 69% of P aeruginosa isolates were susceptible to gentamicin, whereas just 34% of such isolates from one particular ICU were susceptible to gentamicin. In contrast, in another ICU in my hospital, 79% of P aeruginosa isolates were susceptible to gentamicin. It appears important for prescribers in ICUs to request unit-specific antibiograms so that empiric antibiotic prescribing can be optimized. Even in such ICUs, care needs to be exercised when extrapolating recommendations from unit-specific antibiograms to specific patients. Some patients have a long duration of stay in ICUs, during which time they have colonization with multiply resistant organisms and receive therapy with multiple antibiotics. El Amari and colleagues [7] found that the greatest risk factor for antibiotic resistance in P aeruginosa was prior use of the particular antibiotic to which the organism is known to be resistant. Thus, in patients with prolonged ICU stay (arbitrarily defined as ICU length of stay exceeding 14 days), empiric therapy may better be individualized on the basis of avoidance of antibiotics that the patient has previously received. A second principle is avoidance of antibiotics to which colonizing organisms are known to be resistant. A frequently raised issue concerns the level of resistance that should denote that a particular antibiotic should be avoided as empiric therapy. Opinions about this vary widely. For example, in the empiric treatment of gonorrhoea, it is often stated that an antibiotic should not be used empirically if there is greater than 5% resistance to that particular antibiotic in Neisseria gonorrhoeae strains from that community. [8] Such a standard would never be practical for many hospital-acquired organisms. For example, in many hospitals P aeruginosa strains have, cumulatively, greater than 5% resistance to every antibiotic. The most scientifically valid way to assess this situation is to perform a decision analysis. Information required to perform such an analysis includes the consequences of empiric therapy to which the organism is found to be either susceptible or resistant. It is well known that not all patients treated with an antibiotic to which the organism is resistant will do poorly, nor will every patient treated with an antibiotic to which an organism is susceptible do well. [9] A decision analysis assigns literature-based probabilities for various outcomes and often assigns costs to various outcomes. An example is that of Le and Miller [10] who showed that when more than 22% of E coli urinary isolates in a community are resistant to trimethoprim-sulfamethoxazole (TMP- 7
8 SMX), empiric quinolone therapy for uncomplicated urinary tract infection becomes less costly than TMP-SMX. Studies such as this are lacking for hospital-acquired infections. In general, unit-specific hospital antibiograms serve more utility in showing which antibiotics should be avoided for specific infections rather than which antibiotics should be used. For example, if cumulative susceptibilities for P aeruginosa in a particular ICU are meropenem 82%, cefepime 80%, piperacillin-tazobactam 79%, and aztreonam 50%, it would be logical to exclude aztreonam as an empiric antibiotic choice for patients with suspected pseudomonal infections. Such susceptibilities may not distinguish between meropenem, cefepime, and piperacillin-tazobactam as a treatment of choice. However, they do serve as an indication that combination beta-lactam/aminoglycoside or beta-lactam/quinolone therapy be used empirically, because even the most active antibiotic lacks activity against a substantial proportion of strains. Cumulative Antibiotic Susceptibility Reports and Choice of Pathogen-Directed Therapy In many situations, the identification of the infecting bacterial pathogen will be known before the antibiotic susceptibility results. Usually the delay is just 24 hours, but this may represent an important window for optimization of antibiotic therapy in critically ill patients. There is therefore an opportunity for clinical microbiology laboratories to work with providers to develop pathogen-specific guidelines. Thus, if a clinician receives a report that a patient has E cloacae growing in a particular specimen, then a reasoned antibiotic choice could be made for that patient by consulting with a unit-based antibiogram. An example of such a pathogen-specific guideline is given in Table 2 and in the following list: [11-15] Recommendations for treatment of Enterobacter infections when susceptibilities are not yet available: o o o Bacteremia -- cefepime 1 g every 12 hours intravenously (IV) or levofloxacin 500 mg every 24 hours IV Pneumonia -- cefepime 1 g every 12 hours IV or levofloxacin 500 mg every 24 hours IV Urinary tract infection -- TMP-SMX (3 mg/kg per dose of the TMP component every 8 hours) or 1 double-strength tablet every 12 hours by mouth Role of combination therapy: Two large studies of serious Enterobacter infections have been performed. In neither study did combination therapy result in a significant improvement in survival or a reduction in emergence of resistance 8
9 Therapy once susceptibilities are known: Streamlining to TMP-SMX should be performed: The drug is inexpensive and has yielded excellent results in the treatment of serious Enterobacter infections. Third-generation cephalosporins (eg, ceftriaxone, cefotaxime, and ceftazidime) should be avoided in serious Enterobacter infections, regardless of apparent in vitro susceptibility, because there is a significant risk for relapse of infection. Table 2. An Example of a Pathogen-Directed, Unit-Specific Antibiogram Antibiotic Number of Isolates ICU Percentage Susceptible Ampicillin-sulbactam Aztreonam Cefepime Ceftriaxone Cefuroxime Gentamicin Levofloxacin Piperacillin TMP-SMX Piperacillin-tazobactam ICU = intensive care unit; TMP-SMX = trimethoprim-sulfamethoxazole This antibiogram represents Enterobacter cloacae, Enterobacter aerogenes, and other rarer species combined. E aerogenes is more likely to be susceptible to some antibiotics than E cloacae. Such an antibiogram can be posted on the Web site of an ICU or be part of a guidelines booklet for an institution or unit. National and International Data on the Extent of Antibiotic Resistance In some institutions, antibiograms are not available. In this instance, it is necessary to obtain national data sets to determine the most likely extent of antibiotic resistance of a particular bacterial pathogen. Even if antibiograms are produced by a particular institution, it is useful to review national data for 2 reasons. First, it allows for a comparison of local data with national data to determine whether the extent of resistance is better or worse than national averages. Second, it provides the ability to presage future trends in antibiotic resistance: If resistance is rising for a particular pathogen at a national level, it may be only a matter of time before resistance rates rise locally. 9
10 Data on antibiotic resistance in the United States are collected by the US Centers for Disease Control and Prevention (CDC) via the National Nosocomial Infections Surveillance (NNIS) System. [16-18] This system was established in 1970 when selected hospitals in the United States began to routinely report their nosocomial infection surveillance data for aggregation into a national database. Nearly 300 hospitals participate in the NNIS System. The NNIS data presented here are predominantly from patients in ICUs. With regard to gram-positive organisms, 28.5% of enterococci associated with nosocomial infections in ICU patients in 2003 were resistant to vancomycin. This represented a 12% increase compared with aggregate data from 1998 to About 59.5% of S aureus strains and 89.1% of coagulase-negative staphylococci from the same study were methicillin-resistant. This represented an 11% increase in methicillin resistance in S aureus compared with Clearly, MRSA is a substantial problem that necessitates the use of alternative antibiotics, such as vancomycin, linezolid, daptomycin, or tigecycline, in many clinical situations. This is discussed in greater detail in subsequent sections. The greatest extent of increase in resistance of any organism to antibiotics was "resistance" of K pneumoniae to third-generation cephalosporins. Resistance of K pneumoniae or E coli to third-generation cephalosporins is actually defined as nonsusceptibility to either aztreonam or third-generation cephalosporins, and is a surrogate marker for the production of extended-spectrum beta lactamases (ESBLs). These beta lactamases are a major threat to the routine use of cephalosporins and penicillins. Over one fifth (20.6%) of K pneumoniae strains associated with nosocomial infections in ICU patients in 2003 were nonsusceptible to either aztreonam or thirdgeneration cephalosporins: This represented a 47% increase compared with aggregate data from the 5 previous years. In contrast, just 5.8% of E coli strains were nonsusceptible to aztreonam or third-generation cephalosporins. Resistance of Enterobacter strains to third-generation cephalosporins, exhibited by 31.1% of strains in 2003, is a marker of hyperproduction of the AmpC beta lactamase. This beta lactamase, which differs structurally from ESBLs, is an important mechanism of resistance to cephalosporins and penicillins of Enterobacter species, Serratia species, Citrobacter species, and other related organisms. P aeruginosa is an important pathogen that is associated with hospital-acquired infections. Unfortunately, the organism is notable for intrinsic resistance to multiple antibiotics plus the ability to develop mutational resistance or acquire new mechanisms of resistance. Resistance of an individual P aeruginosa strain to multiple antibiotics is frequently encountered. In the NNIS System survey in 2003, the resistance rates of P aeruginosa were 31.9% (to ceftazidime), 29.5% (to quinolones), and 21.1% (to imipenem). The NNIS System has not published rates of resistance of Acinetobacter species or Stenotrophomonas maltophilia; however, these organisms are also notable for their intrinsic, mutational, and acquired mechanisms of resistance to multiple antibiotics. 10
11 5. Which of the following antibiotics would you consider first-line for therapy of serious infections due to E coli, Klebsiella species, or Enterobacter species? Ertapenem Imipenem-cilastatin Levofloxacin Linezolid Tigecycline Explanation: It depends on the hospital. At a teaching hospital, imipenem, ertapenem, and tigecycline are appropriate choices. At a community hospital, levofloxacin would be acceptable. The broad-spectrum coverage of imipenem is not necessary for these pathogens, and linezolid does not cover gram negatives, such as those listed. Antibiotic Selection for Specific Pathogens S aureus As previously noted, the prevalence of MRSA in hospital-acquired infections is high. Community-acquired MRSA is also on the increase, and may be "imported" into hospitals. If S aureus isolates are methicillin-susceptible, then semisynthetic penicillins, such as nafcillin or oxacillin, should be used in preference to alternative agents. For many years, vancomycin has been regarded as the treatment of choice for MRSA infections. However, there are concerns that outcomes with vancomycin are not as favorable as could be potentially achieved with newer alternative agents (linezolid, daptomycin, or tigecycline). It is probable that the conventional vancomycin dosing regimen of 1 g every 12 hours IV is inadequate for infection types, such as pneumonia or bloodstream infection related to endocarditis, osteomyelitis, or other deep-tissue sites. In such situations, I would suggest aiming for vancomycin trough levels of at least mcg/ml or mg/l. Whether these levels are best achieved by continuous infusion or intermittent infusion at a higher dose is a matter of debate. A randomized trial of continuous-infusion vancomycin (target serum concentration, mg/l) vs 11
12 intermittent infusion (target trough, mg/l) has been performed. [11] Persistent bacteremia after 5 days was lower in the continuous-infusion group than in the every 12 hours group (28% vs 35%), but the sample size was too small (119 total) to adequately determine whether this was a statistically significant difference. Alternatives to vancomycin for MRSA infections include linezolid, daptomycin, and tigecycline. For methicillin-susceptible strains, all indications are semisynthetic penicillin (eg, nafcillin and oxacillin). The alternatives for MRSA are the following (doses are for patients with normal renal function): Skin and soft-tissue infections: o o Preferred: vancomycin 1 g every 12 hours IV Alternatives for outpatient therapy: linezolid 600 mg every 12 hours by mouth or daptomycin 4 mg/kg every 24 hours IV o Alternative for mixed infections: tigecycline 100 mg loading dose, then 50 mg every 12 hours IV Pneumonia: o Preferred: vancomycin mg/kg every 12 hours IV or linezolid 600 mg every 12 hours IV o Alternative for mixed infections: tigecycline 100 mg loading dose, then 50 mg every 12 hours IV Bacteremia: o Preferred: daptomycin 6 mg/kg every 24 hours or vancomycin mg/kg every 12 hours IV Linezolid has potential utility for skin and soft-tissue infections treated out of hospital because both have IV and oral formulations. Linezolid has also been proven to be effective in hospital-acquired pneumonia due to MRSA. Daptomycin also has potential utility for skin and soft-tissue infections treated out of hospital because it need only be given once daily, and blood for serum concentrations does not need to be drawn. Daptomycin is rapidly bactericidal and is effective for some cases of MRSA bloodstream infection. The antibiotic is ineffective for treating pneumonia because it is inactivated by surfactant found in the lungs. Tigecycline has in vitro activity against MRSA and is effective for skin and soft-tissue and intra-abdominal infections. These infections may be polymicrobial, in which case tigecycline has an advantage over other anti-mrsa antibiotics because it has activity against a broad spectrum of gram-negative and anaerobic organisms as well. Vancomycin, linezolid, and daptomycin lack activity against gram-negative organisms. 12
13 Enterococci The treatment of choice for serious infections, such as bacteremia due to enterococci, is ampicillin plus gentamicin if the organism is confirmed as susceptible to these antibiotics. If high-level resistance to gentamicin is found, streptomycin may be combined with ampicillin, presuming that high-level resistance to this antibiotic is not also found. Vancomycin is an alternative to ampicillin in patients with penicillin allergy. Unfortunately, vancomycin-resistant enterococci (VRE) are typically also resistant to ampicillin. Linezolid, daptomycin, and tigecycline are all active in vitro against the majority of VRE strains. The greatest clinical experience is with linezolid; however, coinciding with the greater use of linezolid than the other alternative agents has been the advent of linezolid resistance. In some institutions, 10% to 20% of VRE isolates are linezolid-resistant. There are little published data on the use of daptomycin or tigecycline for infections with VRE, although most strains are susceptible in vitro. Because most VRE infections arise from an intra-abdominal source and may be polymicrobial, tigecycline may have some utility given its concomitant activity against gram-negative bacilli and anaerobes. Enterobacteriaceae The members of the Enterobacteriaceae are gram-negative bacilli, which are usually resident in the gastrointestinal tract. Examples of such organisms include E coli, K pneumoniae, E cloacae, Proteus mirabilis, and Citrobacter freundii. In patients hospitalized in ICUs, the Enterobacteriaceae account for approximately one third of all cases of ICU-acquired pneumonia, one third of all cases of ICU-acquired urinary tract infection, and 10% to 15% of ICU-acquired bloodstream infections. [12,13] Options for treatment of the Enterobacteriaceae include beta-lactam antibiotics (penicillin; cephalosporins; carbapenems; and the monobactam, aztreonam), beta-lactam antibiotics combined with beta-lactamase inhibitors, quinolones, TMP-SMX, aminoglycosides, and tigecycline. Beta-lactamase production is the most common mechanism of resistance of the Enterobacteriaceae to penicillins, cephalosporins, or aztreonam. The beta lactamases inactivate these antibiotics by splitting the amide bond of the antibiotic's beta-lactam ring. Over 300 different beta lactamases have been described. As mentioned previously, most strains of Enterobacter species, Citrobacter species, Providencia species, Morganella morganii, and Serratia species that are resistant to third-generation cephalosporins produce an AmpC beta lactamase. Characteristically, AmpC beta lactamases can inactivate first- and second-generation cephalosporins (including the cephamycins, such as cefoxitin and cefotetan) and third-generation cephalosporins. These beta lactamases are not inhibited by beta-lactamase inhibitors, such as clavulanic acid. An important characteristic of AmpC beta lactamases is that their production can be increased by exposure of the bacteria to certain antibiotics. This phenomenon is known as induction. The amount of beta-lactamase production depends on the concentration of the antibiotic and the time of exposure. Penicillin, ampicillin, most first-generation cephalosporins, 13
14 cefoxitin, and imipenem are strong inducers of AmpC beta lactamases. However, all of these antibiotics except the carbapenem will be inactivated by the AmpC beta lactamase induced. In most populations of organisms, such as Enterobacter species, mutants exist that permanently hyperproduce the AmpC type 1 beta lactamase. [14] These mutants usually occur at frequencies of 10-5 to Their presence at this low frequency is not enough to result in frank resistance to antibiotics, such as the third-generation cephalosporins. However, there are important clinical implications of these mutants. Antibiotic therapy with agents that are not inducers of transient beta-lactamase production (for example, third-generation cephalosporins) will kill all organisms in the colony except the permanently hyperproducing mutants. These mutants therefore become the dominant population at a site of infection and lead to frank resistance to third-generation cephalosporins. This can result in the emergence of resistance during therapy. Organisms, such as K pneumoniae, E coli, or P mirabilis, do not characteristically hyperproduce the AmpC type 1 beta lactamase. Occasionally, they may acquire plasmidmediated AmpC beta lactamases. However, much more commonly they may acquire ESBLs. These beta lactamases have important differences compared with AmpC beta lactamases. It is not appropriate to designate the AmpC beta lactamases as ESBLs because they are not derivatives of a parent beta lactamase with more limited spectra of activity (for example, TEM or SHV beta lactamases). The ESBLs also differ from the AmpC beta lactamases in that they are not able to inactivate the cephamycins (for example, cefoxitin or cefotetan) and are inactivated in vitro by the beta-lactamase inhibitor clavulanic acid. Given the prevalence of beta lactamases produced by the Enterobacteriaceae, I do not consider penicillins, third-generation cephalosporins, or aztreonam as appropriate firstline therapy for serious hospital-acquired infections due to these organisms. Cefepime (a fourth-generation cephalosporin) is less likely to be inactivated by ESBLs or AmpC beta lactamases than third-generation cephalosporins. However, certain ESBL types and combinations of ESBL and AmpC production by bacteria may compromise the activity of cefepime. Piperacillin-tazobactam, ticarcillin-clavulanate, and ampicillin-sulbactam are examples of the combination of a beta lactam with a beta-lactamase inhibitor. The theoretical advantage of the addition of a beta-lactamase inhibitor is that this protects the beta-lactam antibiotic from the destructive effects of the beta lactamase. The beta-lactamase inhibitors in use are active against the beta lactamases produced by anaerobic organisms, such as Bacteroides fragilis, and many beta lactamases commonly produced by E coli and K pneumoniae as well as the penicillinases produced by S aureus. Thus, these antibiotics remain useful options for the treatment of Enterobacteriaceae family. Unfortunately, multiple mechanisms of resistance may work together in producing resistance to a given class of antibiotics. The entry of any beta-lactam antibiotic into the bacterial cell is via outer membrane proteins, which function as doors through which the 14
15 antibiotics pass. These proteins may be lost, contributing to decreased entry of the antibiotic and reduced antimicrobial activity. In some sites of infection with high organism load (for example, intra-abdominal abscesses or severe cases of ventilatorassociated pneumonia), the huge amounts of beta-lactamase production by a high inoculum of organisms may overcome the effects of beta-lactamase inhibitors. Finally, as noted above, the AmpC beta lactamase (produced by organisms, such as Enterobacter species) is not susceptible to the effects of beta-lactamase inhibitors, and therefore may be inherently resistant to beta-lactam/beta-lactamase inhibitor combinations. Quinolone antibiotics, such as ciprofloxacin and levofloxacin, are usually highly active in vitro against the Enterobacteriaceae family. However, the rates of resistance appear to be rising. There is an increased probability of resistance of ESBL-producing organisms to quinolones compared with non-esbl-producing organisms of the same species. [15] The reasons for this coresistance are not entirely clear. Plasmid-mediated quinolone resistance has been reported and may contribute in some cases. [19] The usual mechanism of resistance to quinolones is mutation of the genes that encode the target enzymes (DNA gyrase and topoisomerase IV) for quinolones. Stepwise increases in resistance occur if there are mutations in one and then two of the genes encoding these enzymes. Although not yet fully characterized, alterations in outer membrane proteins coupled with active efflux pumps (which pump antibiotics out of the bacterial cell) appear to be important additional mechanisms of bacterial resistance to quinolones. Carbapenems are often used as drugs of last resort in the treatment of serious infections due to gram-negative bacilli. Resistance of the Enterobacteriaceae to carbapenems is generally rare, but may be mediated by the combination of outer membrane protein deficiency coupled with the production of beta lactamases. Rates of resistance to ertapenem are generally higher than to imipenem or meropenem. A new type of beta lactamase, termed KPC, has become widely prevalent in Enterobacteriaceae in New York, NY, and neighboring areas. This beta lactamase leads to resistance to all carbapenems. Other geographic areas have developed problems with production by Enterobacteriaceae of metallo-beta lactamases (MBLs). Both the KPCs and MBLs may lead to resistance of these organisms to virtually all beta-lactam antibiotics. Antibiotics that do not possess a beta-lactam ring, such as polymyxins or tigecycline, may be the only therapeutic alternative in this situation. In view of the comments above, serious infections due to the Enterobacteriaceae family should be managed with a beta lactam or quinolone antibiotic, which is active in vitro against the infecting organism. An important caveat is that ESBL-producing organisms may appear susceptible to third-generation cephalosporins (ceftazidime, cefotaxime, or ceftriaxone) or cefepime, yet be functionally resistant to these agents. [20] High clinical failure rates are observed when cephalosporins are used to treat bacteremia or pneumonia due to ESBL-producing Klebsiella species or, more rarely, ESBL-producing E coli. Carbapenems (imipenem or meropenem) are the antibiotics of choice for serious infections due to ESBL producers. [21] Polymyxins or tigecycline may be useful for infections that are resistant to carbapenems. 15
16 A second important finding to reemphasize is that third-generation cephalosporins are associated with a significant risk for relapse of infection when used to treat AmpCproducing organisms, such as Enterobacter species. [22] Again, carbapenems or quinolones are the most reliable agents against Enterobacter species, Serratia species, or Citrobacter species. There is no evidence that combination therapy improves outcome or reduces the advent of resistance of the Enterobacteriaceae family. P aeruginosa P aeruginosa is a frequent and often the most troublesome of the gram-negative bacilli. It is a particular problem as a cause of ventilator-associated pneumonia. P aeruginosa is also a common cause of both bloodstream infection and cholangitis. Antibiotic resistance is a major problem posed by P aeruginosa; the organism displays a diverse array of antibiotic resistance mechanisms. [23] Resistance to beta-lactam antibiotics is usually, but not exclusively, mediated by beta lactamases. P aeruginosa produces a chromosomally encoded AmpC beta lactamase, which can hydrolyze antipseudomonal penicillins, aztreonam, and third-generation cephalosporins. Derepressed mutants grossly hyperproduce this beta lactamase. A number of acquired beta lactamases can also be produced. MBLs have been frightening because they can hydrolyze carbapenems and all other beta lactams, with the exception of aztreonam. [24] However, the frequent presence of other beta lactamases in these bacteria usually results in resistance to aztreonam. The most common mechanism of carbapenem resistance is not mediated by beta lactamase, but rather is loss of OprD, which is a porin or outer membrane protein. Loss of OprD results in resistance to imipenem and reduced susceptibility (but usually not frank resistance) to meropenem. OprD may be coregulated with an efflux pump called MexEF- OprN. [23] Use of imipenem can select for loss of OprD, but not for upregulation of the efflux pump. In contrast, use of quinolones can select for upregulation of the efflux pump and reduced OprD (resulting in resistance to both quinolones and imipenem). Frank resistance to meropenem usually requires both the loss of OprD and upregulation of an efflux pump known as MexAB-OprM. [25] The efflux pumps are an important mechanism of multidrug resistance, because they may confer resistance to quinolones, antipseudomonal penicillins, cephalosporins, and sometimes aminoglycosides. Tigecycline is ineffective against P aeruginosa because of the presence of efflux pumps. Quinolone resistance in P aeruginosa may also be mediated by mutations to the chromosomally mediated topoisomerases II and IV, whereas aminoglycoside resistance may be mediated by outer membrane impermeability or by aminoglycoside-modifying enzymes. The end result of these multiple mechanisms of resistance may be P aeruginosa isolates resistant to all antibiotics except for the polymyxins (colistin and polymyxin B) Resistance has even been noted to these toxic antibiotics, especially when patients have received the nebulized drug for long periods of time. Prevention of P aeruginosa pneumonia with nebulized colisitin/polymyxin B is not recommended because of the risk for infection with resistant organisms. [26] 16
17 There has been long-standing debate over the value of combination therapy in the treatment of serious P aeruginosa infections. Combination therapy had been considered the mainstay of therapy for many years, but proponents of monotherapy have emerged. Much of the support for combination therapy emanated from the study by Hilf and colleagues. [27] In a prospective observational study of 200 consecutive patients with P aeruginosa bacteremia, mortality was significantly higher in patients given monotherapy (47%) than in patients given combination therapy (27%). It should be noted that the most common combination used was piperacillin or ticarcillin combined with tobramycin or gentamicin. The monotherapy group was dominated by patients given an aminoglycoside alone. Few patients received cephalosporins, aztreonam, carbapenems, or quinolones. [27] More recently, a prospective observational study from Israel evaluated monotherapy vs beta-lactam-aminoglycoside combination therapy for gram-negative bacteremia. [28] Of the 2165 patients in the study, 16% had P aeruginosa. In this study, 34% (21 of 61) patients with P aeruginosa bacteremia died on beta-lactam monotherapy, whereas 28% (11 of 39) patients died after receipt of combination therapy. This corresponded to an odds ratio of 0.7 with 95% confidence intervals of [28] A meta-analysis of results from randomized controlled trials of antibiotic therapy for P aeruginosa did not show any benefit from combination therapy. [29] When combination therapy is used, a combination of antipseudomonal beta lactam plus aminoglycoside is typically given. Minimization of the aminoglycoside component of this regimen to 3-5 days may minimize the risk for toxicity. [30] Combinations of beta lactams and quinolones are used increasingly often, but the clinical data to support such combinations are sparse. A randomized trial comparing ciprofloxacin plus piperacillin vs tobramycin plus piperacillin for empiric therapy in febrile neutropenic patients has been performed. [31] However, just 4 of 543 febrile episodes were due to P aeruginosa bacteremia. Combinations of 2 beta lactams are not widely recommended. Double betalactam therapy has proved inferior to the beta-lactam plus aminoglycoside combination in animal models. [32] A study in humans showed the emergence of resistance in 40% (2 of 5) of cases in a series of patients with P aeruginosa infection treated with double beta lactams. [33] Dosing of antimicrobial agents in therapy of serious P aeruginosa infections, such as ventilator-associated pneumonia or bacteremia, should be aggressive. For ciprofloxacin, an IV dose of 400 mg every 8 hours is recommended instead of standard 400 mg every 12 hours. Pharmacodynamically, levofloxacin 750 mg/day may be more effective than 500 mg/day for serious pseudomonal infections. Aminoglycosides, even when in combination therapy, should be dosed once daily when used against P aeruginosa. Aminoglycosides exhibit concentration-dependent bactericidal activity, and they produce prolonged postantibiotic effects. Tobramycin or gentamicin at 5-7 mg/kg/day and amikacin at mg/kg/day are recommended for patients with normal renal function. For beta-lactam antibiotics, the time-dependent killing activity of the antibiotic class may be optimized by continuous IV infusion of the antibiotic. At this time, this approach remains to be validated in large clinical studies, but has little downside except for the need to dedicate an IV line for antibiotic administration. Carbapenems are not stable for a 17
18 full 24 hours, and prolonged (3-4 hour) infusions may be used as an alternative in order to optimize their time-dependent killing activity. An unfortunate situation sometimes arises whereby P aeruginosa isolates are resistant to all commercially available antibiotics except the polymyxins (colistin and polymyxin B). The polymyxins act primarily on the bacterial cell wall, leading to rapid permeability changes in the cytoplasmic membrane. Unfortunately, most pharmacokinetic studies on colistin were performed more than 30 years ago with intramuscular administration of the drug. Therefore, currently recommended doses of colistin may be suboptimal, and pharmacokinetic studies are urgently needed. A number of in vitro studies have suggested that use of colistin as part of combination therapy may result in greater killing of P aeruginosa than monotherapy. Combinations have included colistin plus rifampin. [34] This should be regarded as experimental, salvage therapy until robust clinical data become available. Acinetobacter Species Acinetobacter species may also be capable of virtually complete antibiotic resistance. As is the case with P aeruginosa, resistance of Acinetobacter species may be mediated by a combination of beta lactamases and outer membrane protein deficiencies. The role of efflux pumps is largely unexplored in Acinetobacter species, but may be important. A clinically useful observation has been the in vitro efficacy of ampicillin-sulbactam in the face of resistance to almost all other drug classes. Sulbactam is able to bind to penicillinbinding protein 2 and therefore can impart direct antimicrobial activity against Acinetobacter species. [35] Carbapenems (for example, imipenem or meropenem) are often potent agents in the treatment of severe infections due to Acinetobacter species. This has been borne out in studies of Acinetobacter bacteremia. [36] Ampicillin-sulbactam may represent a viable option to carbapenems. [37] In patients with Acinetobacter strains that are resistant to virtually all currently available antibiotics, tigecycline or colistin may be the only viable option. [38] As noted above, colistin has been combined with rifampin and other antibiotics, but reports of the success of these regimens are anecdotal only at this stage. Stenotrophomonas maltophilia Stenotrophomonas maltophilia is intrinsically resistant to carbapenems because of the production of carbapenem-hydrolyzing beta lactamases. S maltophilia usually harbors 2 types of beta lactamase: L1, an MBL that hydrolyzes all beta lactams except aztreonam and is not inhibited by clavulanic acid, and L2, an inducible beta lactamase that hydrolyzes aztreonam but is inhibited by clavulanic acid. S maltophilia strains harboring these beta lactamases hydrolyze almost all beta lactams and beta-lactam/beta-lactamase inhibitor combinations. However, the majority of strains are susceptible to ticarcillinclavulanate, but not to ampicillin-sulbactam or piperacillin-tazobactam. S maltophilia is frequently resistant to all aminoglycosides, probably due to impermeability of the outer membrane. 18
19 There are no randomized controlled trials that can guide therapy of S maltophilia. TMP- SMX should be considered the primary therapeutic agent. Combination therapy is warranted in severe infections, such as bacteremia or pneumonia. However, it must be recognized that S maltophilia may be a colonizer of the airways, in which case treatment is not needed. Appropriate antibiotics to use in combination with TMP-SMX include ticarcillin-clavulanate or ceftazidime. [39] Minocycline or tigecycline are active in vitro, but clinical data are sparse. Quinolones should be avoided as monotherapy for S maltophilia. 6. When should combination therapy be used for treatment of infections with P aeruginosa? Almost always Usually Sometimes Rarely Almost never Explanation: "Almost always" is the best answer because this pathogen is often resistant to 1 or more of the antipseudomonal antibiotics, but "Usually" is also reasonable. Empiric Antibiotic Selection Appropriate empiric antibiotic selections can be made if the likely pathogens (and their susceptibility profiles) at any particular infection site are known. Cumulative antibiotic susceptibility reports on multiple patients (antibiograms) or national susceptibility data can be useful in guiding empiric antibiotic therapy. Additionally, empiric antibiotic selection should be individualized by taking into account recent antibiotic use and known bacterial colonization status. For example, specific antibiotics should not be used if they have been administered to the patient. Additionally, if a patient is known to have been colonized with highly resistant organisms, the antibiotic choice should be modified to reflect this. 19
Management of Extended Spectrum Beta- Lactamase (ESBL) Producing Enterobacteriaceae in health care settings
 Management of Extended Spectrum Beta- Lactamase (ESBL) Producing Enterobacteriaceae in health care settings Dr. Mary Vearncombe PIDAC-IPC February 2012 Objectives: To provide an overview of the RP/AP Annex
Management of Extended Spectrum Beta- Lactamase (ESBL) Producing Enterobacteriaceae in health care settings Dr. Mary Vearncombe PIDAC-IPC February 2012 Objectives: To provide an overview of the RP/AP Annex
Urinary Tract Infections
 Urinary Tract Infections Overview A urine culture must ALWAYS be interpreted in the context of the urinalysis and patient symptoms. If a patient has no signs of infection on urinalysis, no symptoms of
Urinary Tract Infections Overview A urine culture must ALWAYS be interpreted in the context of the urinalysis and patient symptoms. If a patient has no signs of infection on urinalysis, no symptoms of
Practice Guidelines. Updated Guideline on Diagnosis and Treatment of Intra-abdominal Infections
 Updated Guideline on Diagnosis and Treatment of Intra-abdominal Infections CARRIE ARMSTRONG Guideline source: Surgical Infection Society, Infectious Diseases Society of America Literature search described?
Updated Guideline on Diagnosis and Treatment of Intra-abdominal Infections CARRIE ARMSTRONG Guideline source: Surgical Infection Society, Infectious Diseases Society of America Literature search described?
GUIDELINES EXECUTIVE SUMMARY
 GUIDELINES Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for Developing an Institutional Program to Enhance Antimicrobial Stewardship Timothy
GUIDELINES Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for Developing an Institutional Program to Enhance Antimicrobial Stewardship Timothy
Nosocomial Antibiotic Resistance in Multiple Gram-Negative Species: Experience at One Hospital with Squeezing the Resistance Balloon at Multiple Sites
 INVITED ARTICLE HEALTHCARE EPIDEMIOLOGY Robert A. Weinstein, Section Editor Nosocomial Antibiotic Resistance in Multiple Gram-Negative Species: Experience at One Hospital with Squeezing the Resistance
INVITED ARTICLE HEALTHCARE EPIDEMIOLOGY Robert A. Weinstein, Section Editor Nosocomial Antibiotic Resistance in Multiple Gram-Negative Species: Experience at One Hospital with Squeezing the Resistance
ANTIBIOTICS IN SEPSIS
 ANTIBIOTICS IN SEPSIS Jennifer Curello, PharmD, BCPS Clinical Pharmacist, Infectious Diseases Antimicrobial Stewardship Program Ronald Reagan UCLA Medical Center October 27, 2014 The power of antibiotics
ANTIBIOTICS IN SEPSIS Jennifer Curello, PharmD, BCPS Clinical Pharmacist, Infectious Diseases Antimicrobial Stewardship Program Ronald Reagan UCLA Medical Center October 27, 2014 The power of antibiotics
Date: November 30, 2010
 Department of Health and Human Services Public Health Service Food and Drug Administration Center for Drug Evaluation and Research Date: November 30, 2010 To: Through: From: Subject: Drug Name(s): Application
Department of Health and Human Services Public Health Service Food and Drug Administration Center for Drug Evaluation and Research Date: November 30, 2010 To: Through: From: Subject: Drug Name(s): Application
Beta-lactam antibiotics - Cephalosporins
 Beta-lactam antibiotics - Cephalosporins Targets - PBP s Activity - Cidal - growing organisms (like the penicillins) Principles of action - Affinity for PBP s Permeability ypropertiesp Stability to bacterial
Beta-lactam antibiotics - Cephalosporins Targets - PBP s Activity - Cidal - growing organisms (like the penicillins) Principles of action - Affinity for PBP s Permeability ypropertiesp Stability to bacterial
Drug Use Review. Edward Cox, M.D. Director Office of Antimicrobial Products
 Department of Health and Human Services Public Health Service Food and Drug Administration Center for Drug Evaluation and Research Drug Use Review Date: April 5, 2012 To: Through: Edward Cox, M.D. Director
Department of Health and Human Services Public Health Service Food and Drug Administration Center for Drug Evaluation and Research Drug Use Review Date: April 5, 2012 To: Through: Edward Cox, M.D. Director
Antibiotic resistance does it matter in paediatric clinical practice? Jette Bangsborg Department of Clinical Microbiology Herlev Hospital
 Antibiotic resistance does it matter in paediatric clinical practice? Jette Bangsborg Department of Clinical Microbiology Herlev Hospital Background The Department of Clinical Microbiology at Herlev Hospital
Antibiotic resistance does it matter in paediatric clinical practice? Jette Bangsborg Department of Clinical Microbiology Herlev Hospital Background The Department of Clinical Microbiology at Herlev Hospital
British Society for Antimicrobial Chemotherapy
 British Society for Antimicrobial Chemotherapy BSAC to actively support the EUCAST Disc Diffusion Method for Antimicrobial Susceptibility Testing in preference to the current BSAC Disc Diffusion Method
British Society for Antimicrobial Chemotherapy BSAC to actively support the EUCAST Disc Diffusion Method for Antimicrobial Susceptibility Testing in preference to the current BSAC Disc Diffusion Method
2013 Indiana Healthcare Provider and Hospital Administrator Multi-Drug Resistant Organism Survey
 2013 Indiana Healthcare Provider and Hospital Administrator Multi-Drug Resistant Organism Survey Antibiotic resistance is a global issue that has significant impact in the field of infectious diseases.
2013 Indiana Healthcare Provider and Hospital Administrator Multi-Drug Resistant Organism Survey Antibiotic resistance is a global issue that has significant impact in the field of infectious diseases.
NewYork-Presbyterian Hospital Sites: Columbia University Medical Center Guideline: Medication Use Manual Page 1 of 12
 Page 1 of 12 TITLE: ANTIBIOTICS IN ADULT PATIENTS EMPIRIC USE GUIDELINES, COLUMBIA UNIVERSITY MEDICAL CENTER MEDICATION GUIDELINE PURPOSE: These are the 2011 guidelines for the empiric use of antibiotics
Page 1 of 12 TITLE: ANTIBIOTICS IN ADULT PATIENTS EMPIRIC USE GUIDELINES, COLUMBIA UNIVERSITY MEDICAL CENTER MEDICATION GUIDELINE PURPOSE: These are the 2011 guidelines for the empiric use of antibiotics
How To Treat Mrsa From A Dead Body
 HUSRES Annual Report 2012 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara 2013 1 The basis of this HUSRES 2012 report is the HUSLAB/Whonet database 2012, which contains susceptibility data on
HUSRES Annual Report 2012 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara 2013 1 The basis of this HUSRES 2012 report is the HUSLAB/Whonet database 2012, which contains susceptibility data on
ANTIMICROBIAL AGENT CLASSES AND SUBCLASSES
 ANTIMICROBIAL AGENT CLASSES AND SUBCLASSES FOR USE WITH CLSI DOCUMENTS M2 AND M7 Beta-lactams: penicillins e penicillin a penicillin beta-lactam/beta-lactamase inhibitor combinations aminopenicillin a
ANTIMICROBIAL AGENT CLASSES AND SUBCLASSES FOR USE WITH CLSI DOCUMENTS M2 AND M7 Beta-lactams: penicillins e penicillin a penicillin beta-lactam/beta-lactamase inhibitor combinations aminopenicillin a
General Trends in Infectious Disease
 General Trends in Infectious Disease Four phenomena underline the increase in ID problems: Aging population Increasing numbers of immunocompromised patients Increased mobility of the population Newly emerging
General Trends in Infectious Disease Four phenomena underline the increase in ID problems: Aging population Increasing numbers of immunocompromised patients Increased mobility of the population Newly emerging
APPENDIX B: UWHC SURGICAL ANTIMICROBIAL PROPHYLAXIS GUIDELINES
 APPENDIX B: UWHC SURGICAL ANTIMICROBIAL PROPHYLAXIS GUIDELINES Principles of prophylaxis 1) Use antimicrobials for surgical procedures where prophylactic antimicrobials have been found to be beneficial.
APPENDIX B: UWHC SURGICAL ANTIMICROBIAL PROPHYLAXIS GUIDELINES Principles of prophylaxis 1) Use antimicrobials for surgical procedures where prophylactic antimicrobials have been found to be beneficial.
HUSRES Annual Report 2010 Martti Vaara www.huslab.fi www.intra.hus.fi
 HUSRES Annual Report 2010 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara, 2/2011 1 The basis of this HUSRES 2010 report is the HUSLAB/Whonet database 2010, which contains susceptibility data
HUSRES Annual Report 2010 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara, 2/2011 1 The basis of this HUSRES 2010 report is the HUSLAB/Whonet database 2010, which contains susceptibility data
SHEA Position Paper. Vol. 18 No. 4 INFECTION CONTROL AND HOSPITAL EPIDEMIOLOGY 275
 Vol. 18 No. 4 INFECTION CONTROL AND HOSPITAL EPIDEMIOLOGY 275 SHEA Position Paper Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Joint Committee on the Prevention
Vol. 18 No. 4 INFECTION CONTROL AND HOSPITAL EPIDEMIOLOGY 275 SHEA Position Paper Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Joint Committee on the Prevention
The Five Intravenous Antibiotics You Need to Know
 The Five Intravenous Antibiotics You Need to Know Ray Geyer, DO Infectious Disease Specialist Medical Director, Benefis Infection Prevention Empirical Antimicrobial Prescribing: Save the Patient and Kill
The Five Intravenous Antibiotics You Need to Know Ray Geyer, DO Infectious Disease Specialist Medical Director, Benefis Infection Prevention Empirical Antimicrobial Prescribing: Save the Patient and Kill
Guidelines for Antimicrobial Stewardship in Hospitals in Ireland. A Strategy for the Control of Antimicrobial Resistance in Ireland
 A Strategy for the Control of Antimicrobial Resistance in Ireland Guidelines for Antimicrobial Stewardship in Hospitals in Ireland Hospital Antimicrobial Stewardship Working Group Guidelines for Antimicrobial
A Strategy for the Control of Antimicrobial Resistance in Ireland Guidelines for Antimicrobial Stewardship in Hospitals in Ireland Hospital Antimicrobial Stewardship Working Group Guidelines for Antimicrobial
Intra-abdominal abdominal Infections
 Intra-abdominal abdominal Infections Marnie Peterson, Pharm.D., BCPS Dept. of Pediatric Infectious Diseases Medical School University of Minnesota Intra-abdominal abdominal Infections Intra-abdominal abdominal
Intra-abdominal abdominal Infections Marnie Peterson, Pharm.D., BCPS Dept. of Pediatric Infectious Diseases Medical School University of Minnesota Intra-abdominal abdominal Infections Intra-abdominal abdominal
HUSRES Annual Report 2008 Martti Vaara. www.huslab.fi www.intra.hus.fi
 HUSRES Annual Report 2008 Martti Vaara www.huslab.fi www.intra.hus.fi The basis of this HUSRES 2008 report is the HUSLAB/Whonet database 2008, which contains susceptibility data on about 180.000 bacteria
HUSRES Annual Report 2008 Martti Vaara www.huslab.fi www.intra.hus.fi The basis of this HUSRES 2008 report is the HUSLAB/Whonet database 2008, which contains susceptibility data on about 180.000 bacteria
Global Spread of Carbapenemase- Producing Klebsiella pneumoniae
 Global Spread of Carbapenemase- Producing Klebsiella pneumoniae These pathogens arose in the mid-1990s and continue to spread, leaving few options for treating infected patients John Quale John Quale is
Global Spread of Carbapenemase- Producing Klebsiella pneumoniae These pathogens arose in the mid-1990s and continue to spread, leaving few options for treating infected patients John Quale John Quale is
Staphylococcus aureus Bloodstream Infection Treatment Guideline
 Staphylococcus aureus Bloodstream Infection Treatment Guideline Purpose: To provide a framework for the evaluation and management patients with Methicillin- Susceptible (MSSA) and Methicillin-Resistant
Staphylococcus aureus Bloodstream Infection Treatment Guideline Purpose: To provide a framework for the evaluation and management patients with Methicillin- Susceptible (MSSA) and Methicillin-Resistant
TREATMENT OF PERITONEAL DIALYSIS (PD) RELATED PERITONITIS. General Principles
 WA HOME DIALYSIS PROGRAM (WAHDIP) GUIDELINES General Principles 1. PD related peritonitis is an EMERGENCY early empiric treatment followed by close review is essential 2. When culture results and sensitivities
WA HOME DIALYSIS PROGRAM (WAHDIP) GUIDELINES General Principles 1. PD related peritonitis is an EMERGENCY early empiric treatment followed by close review is essential 2. When culture results and sensitivities
IDSA GUIDELINES EXECUTIVE SUMMARY
 IDSA GUIDELINES Diagnosis and Management of Complicated Intra-abdominal Infection in Adults and Children: Guidelines by the Surgical Infection Society and the Infectious Diseases Society of America Joseph
IDSA GUIDELINES Diagnosis and Management of Complicated Intra-abdominal Infection in Adults and Children: Guidelines by the Surgical Infection Society and the Infectious Diseases Society of America Joseph
Urinary Tract Infections
 Urinary Tract Infections Leading cause of morbidity and health care expenditures in persons of all ages. An estimated 50 % of women report having had a UTI at some point in their lives. 8.3 million office
Urinary Tract Infections Leading cause of morbidity and health care expenditures in persons of all ages. An estimated 50 % of women report having had a UTI at some point in their lives. 8.3 million office
How To Treat Mrsa In Finnish
 HUSRES Annual Report 2013 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara 2014 1 The basis of this HUSRES 2013 report is the HUSLAB/Whonet database 2013, which contains susceptibility data on
HUSRES Annual Report 2013 Martti Vaara www.huslab.fi www.intra.hus.fi Martti Vaara 2014 1 The basis of this HUSRES 2013 report is the HUSLAB/Whonet database 2013, which contains susceptibility data on
Antimicrobial Activity and Spectrum of PPI-0903M (T-91825), a Novel Cephalosporin, Tested against a Worldwide Collection of Clinical Strains
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 2005, p. 3501 3512 Vol. 49, No. 8 0066-4804/05/$08.00 0 doi:10.1128/aac.49.8.3501 3512.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved.
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Aug. 2005, p. 3501 3512 Vol. 49, No. 8 0066-4804/05/$08.00 0 doi:10.1128/aac.49.8.3501 3512.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved.
Version 12 May 2013. BSAC Methods for Antimicrobial Susceptibility Testing. All enquiries to: Mandy Wootton. Email: Mandy.Wootton@wales.nhs.
 BSAC Methods for Antimicrobial Susceptibility Testing Version 12 May 2013 All enquiries to: Mandy Wootton Email: Mandy.Wootton@wales.nhs.uk Telephone: +44 (0) 2920 746581 Contents Page Working Party members
BSAC Methods for Antimicrobial Susceptibility Testing Version 12 May 2013 All enquiries to: Mandy Wootton Email: Mandy.Wootton@wales.nhs.uk Telephone: +44 (0) 2920 746581 Contents Page Working Party members
HUSRES Annual Report 2015
 HUSRES Annual Report 2015 1 This report is based on the HUSLAB/Whonet database 2015, which contains data on more than 190.000 microbes. EUCAST 2015 clinical breakpoints have mainly been used. The doses
HUSRES Annual Report 2015 1 This report is based on the HUSLAB/Whonet database 2015, which contains data on more than 190.000 microbes. EUCAST 2015 clinical breakpoints have mainly been used. The doses
www.biochemj.org/bj/330/0581/bj3300581.htm
 Ribosomes as Antibiotic Targets www.biochemj.org/bj/330/0581/bj3300581.htm Ware, Bioscience in the 21 st Century, 2009 PERSPECTIVE Widespread use of antibiotics after WWII improved human health globally
Ribosomes as Antibiotic Targets www.biochemj.org/bj/330/0581/bj3300581.htm Ware, Bioscience in the 21 st Century, 2009 PERSPECTIVE Widespread use of antibiotics after WWII improved human health globally
Appendix M: Guidelines for the Prophylaxis and Management of Intraabdominal, Biliary, and Appendiceal Infections
 Appendix M: Guidelines for the Prophylaxis and Management of Intraabdominal, Biliary, and Appendiceal Infections University of Wisconsin Hospital and Clinics Intraabdominal Infections Prophylaxis and Management
Appendix M: Guidelines for the Prophylaxis and Management of Intraabdominal, Biliary, and Appendiceal Infections University of Wisconsin Hospital and Clinics Intraabdominal Infections Prophylaxis and Management
Antimicrobial Resistance and Human Health
 Antimicrobial Resistance and Human Health Dearbháile Morris, Discipline of Bacteriology, School of Medicine, National University of Ireland, Galway The microbial world The is a gene Talk cloud in a The
Antimicrobial Resistance and Human Health Dearbháile Morris, Discipline of Bacteriology, School of Medicine, National University of Ireland, Galway The microbial world The is a gene Talk cloud in a The
CEFA-DROPS AND CEFA-TABS
 Page 1 of 5 FORT DODGE ANIMAL HEALTH Division of Wyeth 800-5TH STREET N.W., P.O. BOX 518 FORT DODGE IA 50501 USA Telephone: 515-955-4600 Fax: 515-955-3730 Order Desk Telephone: 800-685-5656 Order Desk
Page 1 of 5 FORT DODGE ANIMAL HEALTH Division of Wyeth 800-5TH STREET N.W., P.O. BOX 518 FORT DODGE IA 50501 USA Telephone: 515-955-4600 Fax: 515-955-3730 Order Desk Telephone: 800-685-5656 Order Desk
Oscar E. Guzman, PharmD, BCPS
 P Chapter 32 Antibiotic Streamlining Oscar E. Guzman, PharmD, BCPS Introduction Antibiotic streamlining is one of the most important methods pharmacists can use to encourage appropriate antibiotic use,
P Chapter 32 Antibiotic Streamlining Oscar E. Guzman, PharmD, BCPS Introduction Antibiotic streamlining is one of the most important methods pharmacists can use to encourage appropriate antibiotic use,
Vancomycin. Beta-lactams. Beta-lactams. Vancomycin (Glycopeptide) Rifamycins (rifampin) MID 4
 Antibiotic Classes Introduction to Antimicrobials Rachel J. Gordon, MD, MPH Assistant Professor of Clinical Medicine and Epidemiology Beta-lactams Inhibit cell wall synthesis Penicillins Cephalosporins
Antibiotic Classes Introduction to Antimicrobials Rachel J. Gordon, MD, MPH Assistant Professor of Clinical Medicine and Epidemiology Beta-lactams Inhibit cell wall synthesis Penicillins Cephalosporins
Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs
 Journal of Antimicrobial Chemotherapy (2006) 57, 326 330 doi:10.1093/jac/dki463 Advance Access publication 29 December 2005 Adjustment of antibiotic treatment according to the results of blood cultures
Journal of Antimicrobial Chemotherapy (2006) 57, 326 330 doi:10.1093/jac/dki463 Advance Access publication 29 December 2005 Adjustment of antibiotic treatment according to the results of blood cultures
PRIORITY RESEARCH TOPICS
 PRIORITY RESEARCH TOPICS Understanding all the issues associated with antimicrobial resistance is probably impossible, but it is clear that there are a number of key issues about which we need more information.
PRIORITY RESEARCH TOPICS Understanding all the issues associated with antimicrobial resistance is probably impossible, but it is clear that there are a number of key issues about which we need more information.
ANTIBIOTIC RESISTANCE THREATS. in the United States, 2013
 ANTIBIOTIC RESISTANCE THREATS in the United States, 2013 TABLE OF CONTENTS Foreword... 5 Executive Summary.... 6 Section 1: The Threat of Antibiotic Resistance... 11 Introduction.... 11 National Summary
ANTIBIOTIC RESISTANCE THREATS in the United States, 2013 TABLE OF CONTENTS Foreword... 5 Executive Summary.... 6 Section 1: The Threat of Antibiotic Resistance... 11 Introduction.... 11 National Summary
ANTIBIOTICS FROM HEAD TO TOE: PART 5 - URINARY TRACT INFECTIONS LAUREN HYNICKA, PHARM.D.
 ANTIBIOTICS FROM HEAD TO TOE: PART 5 - URINARY TRACT INFECTIONS LAUREN HYNICKA, PHARM.D. ANTIBIOTICS FROM HEAD TO TOE: PART 5 - URINARY TRACT INFECTIONS ACTIVITY DESCRIPTION Antibiotic use in the safest
ANTIBIOTICS FROM HEAD TO TOE: PART 5 - URINARY TRACT INFECTIONS LAUREN HYNICKA, PHARM.D. ANTIBIOTICS FROM HEAD TO TOE: PART 5 - URINARY TRACT INFECTIONS ACTIVITY DESCRIPTION Antibiotic use in the safest
Lecture Outline. Quinolones
 Lecture Outline Quinolones Trimethoprim/Sulfamethoxazole Miscellaneous antimicrobials - Metronidazole Daptomycin Cases Quinolones Bactericidal broad spectrum antibiotics Increasingly used because of their
Lecture Outline Quinolones Trimethoprim/Sulfamethoxazole Miscellaneous antimicrobials - Metronidazole Daptomycin Cases Quinolones Bactericidal broad spectrum antibiotics Increasingly used because of their
Urinary Tract Infection and Asymptomatic Bacteriuria Guidance
 Urinary Tract Infection and Asymptomatic Bacteriuria Guidance Urinary tract infection (UTI) is the most common indication for antimicrobial use in hospitals and a significant proportion of this use is
Urinary Tract Infection and Asymptomatic Bacteriuria Guidance Urinary tract infection (UTI) is the most common indication for antimicrobial use in hospitals and a significant proportion of this use is
Version 11.1 May 2012
 BSAC Methods for Antimicrobial Susceptibility Testing Version 11.1 May 2012 All enquiries to: Jenny Andrews at: + 44 (0) 121 507 5693 Email: jenny.andrews1@nhs.net Contents Page Working Party members 5
BSAC Methods for Antimicrobial Susceptibility Testing Version 11.1 May 2012 All enquiries to: Jenny Andrews at: + 44 (0) 121 507 5693 Email: jenny.andrews1@nhs.net Contents Page Working Party members 5
Frequently Asked Questions
 Guidelines for Testing and Treatment of Gonorrhea in Ontario, 2013 Frequently Asked Questions Table of Contents Background... 1 Treatment Recommendations... 2 Treatment of Contacts... 4 Administration
Guidelines for Testing and Treatment of Gonorrhea in Ontario, 2013 Frequently Asked Questions Table of Contents Background... 1 Treatment Recommendations... 2 Treatment of Contacts... 4 Administration
Hemodialysis catheter infection
 Hemodialysis catheter infection Scary facts In 2006, 82% of patients in the United States initiated dialysis via a catheter The overall likelihood of Tunneled cuffed catheters use was 35% greater in 2005
Hemodialysis catheter infection Scary facts In 2006, 82% of patients in the United States initiated dialysis via a catheter The overall likelihood of Tunneled cuffed catheters use was 35% greater in 2005
Introduction to Infection Control
 CHAPTER 3 Introduction to Infection Control George Byrns and Mary Elkins Learning Objectives 1 Define terms used in infection control. 2. Review significant risk factors for infection. 3. Identify the
CHAPTER 3 Introduction to Infection Control George Byrns and Mary Elkins Learning Objectives 1 Define terms used in infection control. 2. Review significant risk factors for infection. 3. Identify the
Antimicrobial Stewardship Programs
 A Hospital Pharmacist s Guide to Antimicrobial Stewardship Programs For more information on antimicrobial stewardship, please visit this initiative s website at www.ashpadvantage.com/stewardship Developed
A Hospital Pharmacist s Guide to Antimicrobial Stewardship Programs For more information on antimicrobial stewardship, please visit this initiative s website at www.ashpadvantage.com/stewardship Developed
Outpatient Parenteral Antimicrobial Therapy
 Outpatient Parenteral Antimicrobial Therapy Jason E. Bowling, MD a,b, *, James S. Lewis II, PharmD c,d, Aaron D. Owens, MD a,e KEYWORDS Outpatient parenteral antimicrobial therapy Antibiotics Adverse events
Outpatient Parenteral Antimicrobial Therapy Jason E. Bowling, MD a,b, *, James S. Lewis II, PharmD c,d, Aaron D. Owens, MD a,e KEYWORDS Outpatient parenteral antimicrobial therapy Antibiotics Adverse events
C-Difficile Infection Control and Prevention Strategies
 C-Difficile Infection Control and Prevention Strategies Adrienne Mims, MD MPH VP, Chief Medical Officer Adrienne.Mims@AlliantQuality.org 1/18/2016 1 Disclosure This educational activity does not have commercial
C-Difficile Infection Control and Prevention Strategies Adrienne Mims, MD MPH VP, Chief Medical Officer Adrienne.Mims@AlliantQuality.org 1/18/2016 1 Disclosure This educational activity does not have commercial
James T. Dwyer DO, FACOI
 Antibiotics in the Surgical Patient James T. Dwyer DO, FACOI Objectives Define current prophylactic recommendations for the use of antibiotics in the surgical patient List current antibiotics available
Antibiotics in the Surgical Patient James T. Dwyer DO, FACOI Objectives Define current prophylactic recommendations for the use of antibiotics in the surgical patient List current antibiotics available
THERAPY OF ANAEROBIC INFECTIONS LUNG ABSCESS BRAIN ABSCESS
 THERAPY OF ANAEROBIC INFECTIONS Douglas Black, Pharm.D. Associate Professor School of Pharmacy University of Washington dblack@u.washington.edu LUNG ABSCESS A lung abscess is a localized pus cavity in
THERAPY OF ANAEROBIC INFECTIONS Douglas Black, Pharm.D. Associate Professor School of Pharmacy University of Washington dblack@u.washington.edu LUNG ABSCESS A lung abscess is a localized pus cavity in
bactericidal Gram+ streptococci, enterococci, meningococci, treponema pallidum (syphilis), most anaerobes poor activity against Gram- rods
 Penicillin binds penicillin binding beta-lactamase proteins (transpeptidases, cleaves beta-lactam carboxypeptidases); ring and inactivates enzymes involved in cell drug wall (peptidoglycan) (chromosomal
Penicillin binds penicillin binding beta-lactamase proteins (transpeptidases, cleaves beta-lactam carboxypeptidases); ring and inactivates enzymes involved in cell drug wall (peptidoglycan) (chromosomal
Introduction to Antimicrobial Therapy
 Introduction to Antimicrobial Therapy Christine Kubin, Pharm.D., BCPS Clinical Pharmacist, Infectious Diseases Case #1 L.G. is a 78 yo woman admitted for cardiac cath. 3-vessel disease was identified and
Introduction to Antimicrobial Therapy Christine Kubin, Pharm.D., BCPS Clinical Pharmacist, Infectious Diseases Case #1 L.G. is a 78 yo woman admitted for cardiac cath. 3-vessel disease was identified and
Treatment of skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus in adults
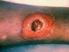 1 of 6 9/24/2010 11:16 AM Official reprint from UpToDate www.uptodate.com 2010 UpToDate Treatment of skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus in adults Author
1 of 6 9/24/2010 11:16 AM Official reprint from UpToDate www.uptodate.com 2010 UpToDate Treatment of skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus in adults Author
American Thoracic Society Documents
 American Thoracic Society Documents Guidelines for the Management of Adults with Hospital-acquired, Ventilator-associated, and Healthcare-associated Pneumonia This official statement of the American Thoracic
American Thoracic Society Documents Guidelines for the Management of Adults with Hospital-acquired, Ventilator-associated, and Healthcare-associated Pneumonia This official statement of the American Thoracic
skin and soft tissue infections (skinfold pyoderma, impetigo, folliculitis, furunculosis, cellulitis) caused by susceptible strains of organisms.
 Part II SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MARBOCYL P 5 mg tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active Ingredient Marbofloxacin 5mg per tablet
Part II SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT MARBOCYL P 5 mg tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active Ingredient Marbofloxacin 5mg per tablet
Liofilchem - Antibiotic Disk Interpretative Criteria and Quality Control - F14013 - Rev.7 / 20.02.2013
 Liofilchem Antibiotic Disk Interpretative Criteria and Quality Control F0 Rev.7 /.02. Amikacin AK Amoxicillin + Clavulanic acid AUG (+) ATCC 352 Coagulasenegative staphylococci Amoxicillin + Clavulanic
Liofilchem Antibiotic Disk Interpretative Criteria and Quality Control F0 Rev.7 /.02. Amikacin AK Amoxicillin + Clavulanic acid AUG (+) ATCC 352 Coagulasenegative staphylococci Amoxicillin + Clavulanic
WARNING LETTER. According to its approved product labeling (PI) (in pertinent part, emphasis original):
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993-0002 TRANSMITTED BY FACSIMILE Sapan A. Shah, Ph.D. President and Chief Executive Officer
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993-0002 TRANSMITTED BY FACSIMILE Sapan A. Shah, Ph.D. President and Chief Executive Officer
Therapeutic protocoles for adults concerning ESBL Enterobacteria infections in health care institutions
 Therapeutic protocoles for adults concerning ESBL Enterobacteria infections in health care institutions CYSTITIS The growing importance of resistance to quinolones, their environmental impact and reduced
Therapeutic protocoles for adults concerning ESBL Enterobacteria infections in health care institutions CYSTITIS The growing importance of resistance to quinolones, their environmental impact and reduced
Urinary tract infections caused by extended spectrum β-lactamase (ESBL) producing Escherichia coli and Klebsiella pneumoniae
 African Journal of Biotechnology Vol. 10(73), pp. 16661-16666, 21 November, 2011 Available online at http://www.academicjournals.org/ajb DOI: 10.5897/AJB11.2449 ISSN 1684 5315 2011 Academic Journals Full
African Journal of Biotechnology Vol. 10(73), pp. 16661-16666, 21 November, 2011 Available online at http://www.academicjournals.org/ajb DOI: 10.5897/AJB11.2449 ISSN 1684 5315 2011 Academic Journals Full
Why Do Some Antibiotics Fail?
 Why Do Some Antibiotics Fail? Patty W. Wright, M.D. April 2010 Objective To outline common reasons why antibiotic therapy is not successful and how this can be avoided. And to teach you a little bit about
Why Do Some Antibiotics Fail? Patty W. Wright, M.D. April 2010 Objective To outline common reasons why antibiotic therapy is not successful and how this can be avoided. And to teach you a little bit about
Nursing college, Second stage Microbiology Dr.Nada Khazal K. Hendi L14: Hospital acquired infection, nosocomial infection
 L14: Hospital acquired infection, nosocomial infection Definition A hospital acquired infection, also called a nosocomial infection, is an infection that first appears between 48 hours and four days after
L14: Hospital acquired infection, nosocomial infection Definition A hospital acquired infection, also called a nosocomial infection, is an infection that first appears between 48 hours and four days after
Received 22 March 2006/Returned for modification 2 May 2006/Accepted 14 September 2006
 JOURNAL OF CLINICAL MICROBIOLOGY, Nov. 2006, p. 4085 4094 Vol. 44, No. 11 0095-1137/06/$08.00 0 doi:10.1128/jcm.00614-06 Copyright 2006, American Society for Microbiology. All Rights Reserved. Two-Center
JOURNAL OF CLINICAL MICROBIOLOGY, Nov. 2006, p. 4085 4094 Vol. 44, No. 11 0095-1137/06/$08.00 0 doi:10.1128/jcm.00614-06 Copyright 2006, American Society for Microbiology. All Rights Reserved. Two-Center
SEA-HLM-415 Distribution: General. Establishment of national laboratory-based surveillance of antimicrobial resistance
 SEA-HLM-415 Distribution: General Establishment of national laboratory-based surveillance of antimicrobial resistance World Health Organization 2011 All rights reserved. Requests for publications, or for
SEA-HLM-415 Distribution: General Establishment of national laboratory-based surveillance of antimicrobial resistance World Health Organization 2011 All rights reserved. Requests for publications, or for
SECTION 6 THERAPEUTIC DRUG MONITORING
 SECTION 6 THERAPEUTIC DRUG MONITORING Kieran Hand Consultant Pharmacist Anti-infectives The objectives of this section are: To test your ability to monitor serum levels for drugs with a narrow therapeutic
SECTION 6 THERAPEUTIC DRUG MONITORING Kieran Hand Consultant Pharmacist Anti-infectives The objectives of this section are: To test your ability to monitor serum levels for drugs with a narrow therapeutic
IDSA GUIDELINES EXECUTIVE SUMMARY
 IDSA GUIDELINES International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and
IDSA GUIDELINES International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and
BD Phoenix Automated Microbiology System
 BD Phoenix Automated Microbiology System Resistance Detection Workflow Efficiency Analysis and Communication BD Diagnostics 7 Loveton Circle Sparks, MD 115-0999 800.638.8663 www.bd.com/ds CHROMagar is
BD Phoenix Automated Microbiology System Resistance Detection Workflow Efficiency Analysis and Communication BD Diagnostics 7 Loveton Circle Sparks, MD 115-0999 800.638.8663 www.bd.com/ds CHROMagar is
PRMCE ANTI-INFECTIVES SELECTION GUIDELINE FOR ADULTS
 PRMCE ANTI-INFECTIVES SELECTION GUIDELINE FOR ADULTS SKIN AND SOFT TISSUE INFECTIONS: Mild A. Cellulitis: MRSA uncommonly causes cellulitis in the absence of a wound abscess. Add empiric anti-mrsa therapy
PRMCE ANTI-INFECTIVES SELECTION GUIDELINE FOR ADULTS SKIN AND SOFT TISSUE INFECTIONS: Mild A. Cellulitis: MRSA uncommonly causes cellulitis in the absence of a wound abscess. Add empiric anti-mrsa therapy
SURGICAL PROPHYLAXIS: ANTIBIOTIC RECOMMENDATIONS FOR ADULT PATIENTS
 Page 1 of 8 TITLE: SURGICAL PROPHYLAXIS: ANTIBIOTIC RECOMMENDATIONS FOR ADULT PATIENTS GUIDELINE: Antibiotics are administered prior to surgical procedures to prevent surgical site infections. PURPOSE:
Page 1 of 8 TITLE: SURGICAL PROPHYLAXIS: ANTIBIOTIC RECOMMENDATIONS FOR ADULT PATIENTS GUIDELINE: Antibiotics are administered prior to surgical procedures to prevent surgical site infections. PURPOSE:
World Health Organization Department of Communicable Disease Surveillance and Response
 WHO/CDS/CSR/DRS/2001.7 Infection control programmes to contain antimicrobial resistance Lindsay E. Nicolle World Health Organization Department of Communicable Disease Surveillance and Response This document
WHO/CDS/CSR/DRS/2001.7 Infection control programmes to contain antimicrobial resistance Lindsay E. Nicolle World Health Organization Department of Communicable Disease Surveillance and Response This document
Recommendations for Metrics for Multidrug-Resistant Organisms in Healthcare Settings: SHEA/HICPAC Position Paper
 infection control and hospital epidemiology october 2008, vol. 29, no. 10 shea/hicpac position paper Recommendations for Metrics for Multidrug-Resistant Organisms in Healthcare Settings: SHEA/HICPAC Position
infection control and hospital epidemiology october 2008, vol. 29, no. 10 shea/hicpac position paper Recommendations for Metrics for Multidrug-Resistant Organisms in Healthcare Settings: SHEA/HICPAC Position
Open Access Article pissn 0976 3325 eissn 2229 6816
 Original Article A STUDY OF EXTENDED SPECTRUM β-lactamase (ESBL) AND AmpC β-lactamase PRODUCING KLEBSIELLA PNEUMONIAE IN NEONATAL INTENSIVE CARE UNIT AT TERTIARY CARE HOSPITAL, AHMEDABAD Modi Dhara J 1,
Original Article A STUDY OF EXTENDED SPECTRUM β-lactamase (ESBL) AND AmpC β-lactamase PRODUCING KLEBSIELLA PNEUMONIAE IN NEONATAL INTENSIVE CARE UNIT AT TERTIARY CARE HOSPITAL, AHMEDABAD Modi Dhara J 1,
Treatment of Fever and Infection in Children with Transfusion Dependent Thalassaemia
 Treatment of Fever and Infection in Children with Transfusion Dependent Thalassaemia Document Information Version: 2 Date: June 2014 Authors (incl. job title): Professor David Rees, Sue Height (consultant
Treatment of Fever and Infection in Children with Transfusion Dependent Thalassaemia Document Information Version: 2 Date: June 2014 Authors (incl. job title): Professor David Rees, Sue Height (consultant
National Quality Forum (NQF) Endorsed Set of 34 Safe Practices*
 NQF Endorsed Set of Safe Practices (released 2009) 1. Leadership Structures and Systems Leadership structures and systems must be established to ensure that there is organization-wide awareness of patient
NQF Endorsed Set of Safe Practices (released 2009) 1. Leadership Structures and Systems Leadership structures and systems must be established to ensure that there is organization-wide awareness of patient
Chapter 20: Antimicrobial Drugs
 Chapter 20: Antimicrobial Drugs 1. Overview of Antimicrobial Drugs 2. Antibacterial Drugs 3. Antiviral Drugs 4. Drugs for Eukaryotic Pathogens 1. Overview of Antimicrobial Drugs Antibiotics An antibiotic
Chapter 20: Antimicrobial Drugs 1. Overview of Antimicrobial Drugs 2. Antibacterial Drugs 3. Antiviral Drugs 4. Drugs for Eukaryotic Pathogens 1. Overview of Antimicrobial Drugs Antibiotics An antibiotic
 Skin and Soft tissue Infections: new bugs, old drugs Disclosure Statement Sponsor: Goodman Photographic Presented by: Dr. Kristopher Wiebe, MD, CCFP (EM) Presented to: BC Chapter, Canadian Society of Hospital
Skin and Soft tissue Infections: new bugs, old drugs Disclosure Statement Sponsor: Goodman Photographic Presented by: Dr. Kristopher Wiebe, MD, CCFP (EM) Presented to: BC Chapter, Canadian Society of Hospital
5 Measuring the performance
 5 Measuring the performance of antimicrobial stewardship programs Authors: David Looke and Margaret Duguid 5.1 Key points Part I Measuring the performance of antimicrobial stewardship programs Monitoring
5 Measuring the performance of antimicrobial stewardship programs Authors: David Looke and Margaret Duguid 5.1 Key points Part I Measuring the performance of antimicrobial stewardship programs Monitoring
Antibiotic-Associated Diarrhea, Clostridium difficile- Associated Diarrhea and Colitis
 Antibiotic-Associated Diarrhea, Clostridium difficile- Associated Diarrhea and Colitis ANTIBIOTIC-ASSOCIATED DIARRHEA Disturbance of the normal colonic microflora Leading to alterations in bacterial degradation
Antibiotic-Associated Diarrhea, Clostridium difficile- Associated Diarrhea and Colitis ANTIBIOTIC-ASSOCIATED DIARRHEA Disturbance of the normal colonic microflora Leading to alterations in bacterial degradation
ANTIMICROBIAL USE GUIDELINES
 ANTIMICROBIAL USE GUIDELINES University of Wisconsin Hospital and Clinics Pharmacy and Therapeutics Committee Department of Pharmacy Drug Policy Program July 2011 to June 2012 Twenty-First Edition PREFACE
ANTIMICROBIAL USE GUIDELINES University of Wisconsin Hospital and Clinics Pharmacy and Therapeutics Committee Department of Pharmacy Drug Policy Program July 2011 to June 2012 Twenty-First Edition PREFACE
Optimizing Antimicrobial Susceptibility Test Reporting
 JOURNAL OF CLINICAL MICROBIOLOGY, Sept. 2011, p. S15 S19 0095-1137/11/$12.00 doi:10.1128/jcm.00712-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. VOL. 49, NO. 9 SUPPL. Optimizing
JOURNAL OF CLINICAL MICROBIOLOGY, Sept. 2011, p. S15 S19 0095-1137/11/$12.00 doi:10.1128/jcm.00712-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. VOL. 49, NO. 9 SUPPL. Optimizing
Dosing information in renal impairment
 No. Drug name Usual dose Adjustment for Renal failure estimated CrCl (ml/min) Aminoglycoside antibiotics 1 Amikacin 2 Gentamicin 7.5 mg /kg q 12 hr > 50-90 7.5 mg/kg q 12 hr 10-50 7.5 mg/kg q 24 hr < 10
No. Drug name Usual dose Adjustment for Renal failure estimated CrCl (ml/min) Aminoglycoside antibiotics 1 Amikacin 2 Gentamicin 7.5 mg /kg q 12 hr > 50-90 7.5 mg/kg q 12 hr 10-50 7.5 mg/kg q 24 hr < 10
growing problem By: Jena Cummins, Doctor of Pharmacy Candidate
 Clostridium difficile: A growing problem By: Jena Cummins, Doctor of Pharmacy Candidate Introduction The word difficile il is Latin for the word difficult. Clostridium difficile, affectionately known as
Clostridium difficile: A growing problem By: Jena Cummins, Doctor of Pharmacy Candidate Introduction The word difficile il is Latin for the word difficult. Clostridium difficile, affectionately known as
PACKAGE LEAFLET. CLINDAMYCIN capsules Clidamycin. One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride).
 PACKAGE LEAFLET CLINDAMYCIN capsules Clidamycin COMPOSITION One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride). One capsule of 150 mg contains 150 mg Clindamycin (as hydrochloride). PROPERTIES
PACKAGE LEAFLET CLINDAMYCIN capsules Clidamycin COMPOSITION One capsule of 75 mg contains 75 mg Clindamycin (as hydrochloride). One capsule of 150 mg contains 150 mg Clindamycin (as hydrochloride). PROPERTIES
The 14th EURL-AR Proficiency Test - enterococci, staphylococci and E. coli 2013. Lina Cavaco Susanne Karlsmose Rene S. Hendriksen Frank M.
 The 14th EURL-AR Proficiency Test - enterococci, staphylococci and E. coli 2013 Lina Cavaco Susanne Karlsmose Rene S. Hendriksen Frank M. Aarestrup The 14TH EURL-AR Proficiency Test Enterococci, Staphylococci
The 14th EURL-AR Proficiency Test - enterococci, staphylococci and E. coli 2013 Lina Cavaco Susanne Karlsmose Rene S. Hendriksen Frank M. Aarestrup The 14TH EURL-AR Proficiency Test Enterococci, Staphylococci
Review of Healthcare-Associated Infection (HAI) and Multidrug-Resistant Organism (MDRO) Reporting Requirements in the United States PRESENTED BY:
 Review of Healthcare-Associated Infection (HAI) and Multidrug-Resistant Organism (MDRO) Reporting Requirements in the United States PRESENTED BY: BYRAN DAI MASTERS OF HEALTH SCIENCE CANDIDATE 14 JHSPH
Review of Healthcare-Associated Infection (HAI) and Multidrug-Resistant Organism (MDRO) Reporting Requirements in the United States PRESENTED BY: BYRAN DAI MASTERS OF HEALTH SCIENCE CANDIDATE 14 JHSPH
McDonald s Global Vision for Antimicrobial Stewardship in Food Animals* I
 McDonald s Global Vision for Antimicrobial Stewardship in Food Animals* I Preserving antimicrobial effectiveness in the future through ethical practices today As the body of scientific evidence grows,
McDonald s Global Vision for Antimicrobial Stewardship in Food Animals* I Preserving antimicrobial effectiveness in the future through ethical practices today As the body of scientific evidence grows,
Originally published as:
 Originally published as: Dedeic-Ljubovic, A., Hukic, M., Pfeifer, Y., Witte, W., Padilla, E., López-Ramis, I., Albertí, S. Emergence of CTX-M-15 extended-spectrum β-lactamase-producing Klebsiella pneumoniae
Originally published as: Dedeic-Ljubovic, A., Hukic, M., Pfeifer, Y., Witte, W., Padilla, E., López-Ramis, I., Albertí, S. Emergence of CTX-M-15 extended-spectrum β-lactamase-producing Klebsiella pneumoniae
Principles of Disease and Epidemiology. Copyright 2010 Pearson Education, Inc.
 Principles of Disease and Epidemiology Pathology, Infection, and Disease Disease: An abnormal state in which the body is not functioning normally Pathology: The study of disease Etiology: The study of
Principles of Disease and Epidemiology Pathology, Infection, and Disease Disease: An abnormal state in which the body is not functioning normally Pathology: The study of disease Etiology: The study of
SURGICAL ANTIBIOTIC PROPHYLAXIS. Steve Johnson, PharmD, BCPS Prime Therapeutics, Inc
 SURGICAL ANTIBIOTIC PROPHYLAXIS Steve Johnson, PharmD, BCPS Prime Therapeutics, Inc OBJECTIVES Discuss antibiotic use as prophylaxis vs presumptive therapy vs treatment of infections. Discuss risk factors
SURGICAL ANTIBIOTIC PROPHYLAXIS Steve Johnson, PharmD, BCPS Prime Therapeutics, Inc OBJECTIVES Discuss antibiotic use as prophylaxis vs presumptive therapy vs treatment of infections. Discuss risk factors
Omega-3 fatty acids improve the diagnosis-related clinical outcome. Critical Care Medicine April 2006;34(4):972-9
 Omega-3 fatty acids improve the diagnosis-related clinical outcome 1 Critical Care Medicine April 2006;34(4):972-9 Volume 34(4), April 2006, pp 972-979 Heller, Axel R. MD, PhD; Rössler, Susann; Litz, Rainer
Omega-3 fatty acids improve the diagnosis-related clinical outcome 1 Critical Care Medicine April 2006;34(4):972-9 Volume 34(4), April 2006, pp 972-979 Heller, Axel R. MD, PhD; Rössler, Susann; Litz, Rainer
Highlights of the Revised Official ICD-9-CM Guidelines for Coding and Reporting Effective October 1, 2008
 Highlights of the Revised Official ICD-9-CM Guidelines for Coding and Reporting Effective October 1, 2008 Please refer to the complete ICD-9-CM Official Guidelines for Coding and Reporting posted on this
Highlights of the Revised Official ICD-9-CM Guidelines for Coding and Reporting Effective October 1, 2008 Please refer to the complete ICD-9-CM Official Guidelines for Coding and Reporting posted on this
SAMPLE ANTIMICROBIAL STEWARDSHIP POLICY
 SAMPLE ANTIMICROBIAL STEWARDSHIP POLICY FOR A LOCAL HEALTH DISTRICT OR NETWORK Purpose of this document The development of an official policy for Antimicrobial Stewardship (AMS) is a fundamental step towards
SAMPLE ANTIMICROBIAL STEWARDSHIP POLICY FOR A LOCAL HEALTH DISTRICT OR NETWORK Purpose of this document The development of an official policy for Antimicrobial Stewardship (AMS) is a fundamental step towards
FACULTY OF MEDICAL SCIENCE
 Doctor of Philosophy Program in Microbiology FACULTY OF MEDICAL SCIENCE Naresuan University 171 Doctor of Philosophy Program in Microbiology The time is critical now for graduate education and research
Doctor of Philosophy Program in Microbiology FACULTY OF MEDICAL SCIENCE Naresuan University 171 Doctor of Philosophy Program in Microbiology The time is critical now for graduate education and research
Diagnosis, Treatment and Prevention of Typhoid Fever in the Children of Bangladesh: A Microbiologist s View.
 Diagnosis, Treatment and Prevention of Typhoid Fever in the Children of Bangladesh: A Microbiologist s View. Samir K Saha, Ph.D. Department of Microbiology Dhaka Shishu Hospital Bangladesh Typhoid Fever:Salmonella
Diagnosis, Treatment and Prevention of Typhoid Fever in the Children of Bangladesh: A Microbiologist s View. Samir K Saha, Ph.D. Department of Microbiology Dhaka Shishu Hospital Bangladesh Typhoid Fever:Salmonella
EAST CAROLINA UNIVERSITY INFECTION CONTROL POLICY. Methicillin-resistant Staph aureus: Management in the Outpatient Setting
 EAST CAROLINA UNIVERSITY INFECTION CONTROL POLICY Methicillin-resistant Staph aureus: Management in the Outpatient Setting Date Originated: Date Reviewed: Date Approved: Page 1 of Approved by: Department
EAST CAROLINA UNIVERSITY INFECTION CONTROL POLICY Methicillin-resistant Staph aureus: Management in the Outpatient Setting Date Originated: Date Reviewed: Date Approved: Page 1 of Approved by: Department
Tuberculosis Exposure Control Plan for Low Risk Dental Offices
 Tuberculosis Exposure Control Plan for Low Risk Dental Offices A. BACKGROUND According to the CDC, approximately one-third of the world s population, almost two billion people, are infected with tuberculosis.
Tuberculosis Exposure Control Plan for Low Risk Dental Offices A. BACKGROUND According to the CDC, approximately one-third of the world s population, almost two billion people, are infected with tuberculosis.
