Experiments for Backbone and Sidechain Assignments of Uniformly 13 C- and 15 N- Labeled Proteins
|
|
|
- Randolf Ramsey
- 7 years ago
- Views:
Transcription
1 Experiments for Backbone and Sidechain Assignments of Uniformly 13 C- and 15 N- Labeled Proteins
2 Class Outline Understanding 3D experiments Chemical shift assignment experiments Backbone Aliphatic sidechains Aromatic sidechains Stereospecific chemical shifts assignments
3 Resonance Assignment Strategies Depend on the Protein s MW Molecular Weight 8-10 KDa ~ 8-25 KDa 25 KDa Isotopic Labeling None Uniform 13 C, 15 N Uniform 13 C, 15 N, 2 H and/or selective labeling Approach 1 H homonuclear (COSY/TOCSY + NOESY) Triple Resonance 2 H-modified triple resonance As the molecular weight increases the number of peaks also increase, resulting in crowded and overlapped spectra. Additionally, the proteins tumble slower in solution which results in broader peaks.
4 What do we Mean by Resonance Assignment? First we make a list of the NMR active nuclei in the protein. Second, we identify their chemical shifts from NMR spectra. Note: In this example the protein should be isotopically labeled with 15 N and 13 C.
5 Linking Three NMR-active Nuclei Typical 2D experiment showing the correlation between two NMR active nuclei. We could use two 2D experiments: one linking 1 H to 15 N and the other linking 15 N to 13 C. However, it is more efficient to combine these two experiments into one.
6 3D NMR Experiments Each peak has 3 chemical shifts associated with it: HN, N, and CO In this example each peak contains information about the NMR active nuclei around the peptide bond.
7 Analysing 3D Data We can take 2D slices from the 3D cube along the 15 N dimension and associate HN with CO.
8 Backbone Assignments
9 15 N-HSQC: A Protein s fingerprint? The most common strategy nowadays for backbone chemical shift assignments is to use 15 N-edited 3D experiments.
10 The Protein Backbone 3D NMR experiments for chemical shift assignments are based on the ability to transfer magnetization through NMR active nuclei using J couplings. Many of these experiments have been designed to walk through the protein s backbone.
11 3D NMR Methods: HNCO Recorded chemical shifts: HN (i), N (i), CO (i-1) Nomenclature: -The experiment name lists the atoms whose chemical shifts are recorded. -When the magnetization transfer is through nuclei whose chemical shift isn t recorded, the atom is listed in parentheses. -When possible, avoid duplicating atom names: HN + N + CO will be HNNCO, instead it s abbreviated as HNCO.
12 3D NMR Methods: CBCA(CO)NH and HNCACB -These experiments are the workhorse for backbone assignments. -Both provide similar sets of data: CBCA(CO)NH Inter-residue connections only HNCACB Inter- and Intra-residue connections Chemical Shifts HN(i) N(i) C (i-1) C (i-1) HN(i) N(i) C (i-1, i) C (i-1, i)
13 (1) The Assignment Process
14 (2) The Assignment Process -Next we look for a pair of peaks in the CBCA(CO)NH experiment with 13 C chemical shifts of 30 and 57.5 ppm. This strip gives us the chemical shift of HN (i+1) and N (i+1) : 8.60 and ppm.
15 (3) The Assignment Process -To obtain the chemical shifts of C (i+1) and C (i+1) we have to find the HNCACB strip corresponding to 1 H = 8.60 ppm and 15 N = ppm. -Then we continue this way until a brake is found.
16 (4) The Assignment Process
17 Primary Sequence Identification -Until now we only know the order in which our anonymous spin systems (HN/N/C /C ) are arranged. However, we want to know the amino acid type to which each belongs. -We start by identifying those spin systems that have unique chemical shifts. For example: Gly: No C and C ~ 45ppm Ser/Thr: C is downfield of C (~65-75ppm) Ala: C is particularly upfield (~15-20ppm) J. Cavanagh, et al., Protein NMR Spectroscopy, 1996
18 Amino Acids 13 C Chemical Shifts Residue Gly (ppm) Ala Ser Thr Val Leu Ile ( 1) / 14-22( 2) 9-16 Lys Arg Pro Glu Gln Met Phe Tyr His Trp Cys Cys(S-S) Asp Asn Structure of Biological Macromolecules, Rizo and Brunch
19 Chemical shift Info: BMRB (BioMagResBank)
20 Residue Identification
21 More Options for BB Assignments HNCA / HN(CO)CA HNHA / H(CA)NH HNCO / HN(CA)CO
22 Sidechain Assignments
23 Aminoacid Sidechains
24 SC Assignment by 3D-NMR: H(CCO)NH- and (H)C(CO)NH-TOCSY -A variety of sidechaindirected experiments are available to identify sidechain chemical shifts. For example: H(CCO)NH and (H)C(CO)NH. Chemical Shifts HN(i) N(i) H (i-1) -These experiments correlate the 1 H and 13 C sidechain atoms of residue i-1 with the amide 1 H and 15 N of residue i. HN(i) N(i) C (i-1)
25 Sidechain Assignments
26 1 H/ 15 N-TOCSY-HSQC -This experiment allows us to observe intra-residue correlations between the sidechain protons and the amide 1 H/ 15 N.
27 Non HN-based Methods: HCCH- TOCSY -This experiment correlates a 1 H/ 13 C pair to all other protons in the same aminoacid sidechain. -Also, very useful for determining which 1 H is directly attached to which 13 C.
28 Aromatic Sidechains
29 Assignment Strategy #1 a) Link protons with aromatic ring protons: 2D-NOESY in D 2 O b) Assign ring protons: 2D-COSY, 2D-TOCSY in D 2 O c) Assign aromatic carbons: 1 H/ 13 C- HSQC
30 Example: Phenylanine a) b) c)
31 Assignment Strategy #2 It could be difficult to obtain complete assignments of aromatic residues when the aromatic protons have a high density of NOEs and poor chemical shift dispersion. Another strategy consists in correlations between the sidechain C and ring H / chemical shifts using J-couplings. Experiment names: (H )C (C C )H and (H )C (C C C )H Yamazaki, T., Forman-Kay, J.D, and Kay, L.E, JACS (1993), 115,
32 Stereospecific Assignments
33 ASN and GLN NH 2 Groups The sidechain CO-N bond has partial double bond character. Rotation around this bond is slow in the NMR time scale. The distance between the E proton and the (Asn) or (Glu) protons is smaller than for the Z proton. Use relative intensities in the 3D 15 N-NOESY experiment to stereospecifically assign them.
34 VAL and LEU Biosynthesis Pro-R Pro-S Pro-R Pro-S
35 CT- 13 C/ 1 H-HSQC Spectra
36 Summary of Assignment Experimets Backbone CBCA(CO)NH, HNCACB, HNCO, HNHA Aliphatics and aromatics sidechains (H)C(CO)NH, H(CCO)NH, HCCH_TOCSY, 15 N-edited- TOCSY, 2D-NOESY, 2D-TOCSY, 2D-COSY, 13 C-HSQC, (H )C (C C )H, and (H )C (C C C )H Stereospecific assignments: -NH 2 (Asn, Gln), Methyls (Val, Leu) 15 N-edited NOESY, CT- 13 C-HSQC
37 Assignment Problem
Lecture #7 (2D NMR) Utility of Resonance Assignments
 Lecture #7 (2D NMR) Basics of multidimensional NMR (2D NMR) 2D NOESY, COSY and TOCSY 2/23/15 Utility of Resonance Assignments Resonance Assignments: Assignment of frequency positions of resonances (peaks)
Lecture #7 (2D NMR) Basics of multidimensional NMR (2D NMR) 2D NOESY, COSY and TOCSY 2/23/15 Utility of Resonance Assignments Resonance Assignments: Assignment of frequency positions of resonances (peaks)
NMR Nuclear Magnetic Resonance
 NMR Nuclear Magnetic Resonance Nuclear magnetic resonance (NMR) is an effect whereby magnetic nuclei in a magnetic field absorb and re-emit electromagnetic (EM) energy. This energy is at a specific resonance
NMR Nuclear Magnetic Resonance Nuclear magnetic resonance (NMR) is an effect whereby magnetic nuclei in a magnetic field absorb and re-emit electromagnetic (EM) energy. This energy is at a specific resonance
Amino Acids. Amino acids are the building blocks of proteins. All AA s have the same basic structure: Side Chain. Alpha Carbon. Carboxyl. Group.
 Protein Structure Amino Acids Amino acids are the building blocks of proteins. All AA s have the same basic structure: Side Chain Alpha Carbon Amino Group Carboxyl Group Amino Acid Properties There are
Protein Structure Amino Acids Amino acids are the building blocks of proteins. All AA s have the same basic structure: Side Chain Alpha Carbon Amino Group Carboxyl Group Amino Acid Properties There are
IV. -Amino Acids: carboxyl and amino groups bonded to -Carbon. V. Polypeptides and Proteins
 IV. -Amino Acids: carboxyl and amino groups bonded to -Carbon A. Acid/Base properties 1. carboxyl group is proton donor! weak acid 2. amino group is proton acceptor! weak base 3. At physiological ph: H
IV. -Amino Acids: carboxyl and amino groups bonded to -Carbon A. Acid/Base properties 1. carboxyl group is proton donor! weak acid 2. amino group is proton acceptor! weak base 3. At physiological ph: H
Peptide bonds: resonance structure. Properties of proteins: Peptide bonds and side chains. Dihedral angles. Peptide bond. Protein physics, Lecture 5
 Protein physics, Lecture 5 Peptide bonds: resonance structure Properties of proteins: Peptide bonds and side chains Proteins are linear polymers However, the peptide binds and side chains restrict conformational
Protein physics, Lecture 5 Peptide bonds: resonance structure Properties of proteins: Peptide bonds and side chains Proteins are linear polymers However, the peptide binds and side chains restrict conformational
Amino Acids, Peptides, Proteins
 Amino Acids, Peptides, Proteins Functions of proteins: Enzymes Transport and Storage Motion, muscle contraction Hormones Mechanical support Immune protection (Antibodies) Generate and transmit nerve impulses
Amino Acids, Peptides, Proteins Functions of proteins: Enzymes Transport and Storage Motion, muscle contraction Hormones Mechanical support Immune protection (Antibodies) Generate and transmit nerve impulses
Advanced Medicinal & Pharmaceutical Chemistry CHEM 5412 Dept. of Chemistry, TAMUK
 Advanced Medicinal & Pharmaceutical Chemistry CHEM 5412 Dept. of Chemistry, TAMUK Dai Lu, Ph.D. dlu@tamhsc.edu Tel: 361-221-0745 Office: RCOP, Room 307 Drug Discovery and Development Drug Molecules Medicinal
Advanced Medicinal & Pharmaceutical Chemistry CHEM 5412 Dept. of Chemistry, TAMUK Dai Lu, Ph.D. dlu@tamhsc.edu Tel: 361-221-0745 Office: RCOP, Room 307 Drug Discovery and Development Drug Molecules Medicinal
Proton Nuclear Magnetic Resonance Spectroscopy
 Proton Nuclear Magnetic Resonance Spectroscopy Introduction: The NMR Spectrum serves as a great resource in determining the structure of an organic compound by revealing the hydrogen and carbon skeleton.
Proton Nuclear Magnetic Resonance Spectroscopy Introduction: The NMR Spectrum serves as a great resource in determining the structure of an organic compound by revealing the hydrogen and carbon skeleton.
BOC334 (Proteomics) Practical 1. Calculating the charge of proteins
 BC334 (Proteomics) Practical 1 Calculating the charge of proteins Aliphatic amino acids (VAGLIP) N H 2 H Glycine, Gly, G no charge Hydrophobicity = 0.67 MW 57Da pk a CH = 2.35 pk a NH 2 = 9.6 pi=5.97 CH
BC334 (Proteomics) Practical 1 Calculating the charge of proteins Aliphatic amino acids (VAGLIP) N H 2 H Glycine, Gly, G no charge Hydrophobicity = 0.67 MW 57Da pk a CH = 2.35 pk a NH 2 = 9.6 pi=5.97 CH
Pipe Cleaner Proteins. Essential question: How does the structure of proteins relate to their function in the cell?
 Pipe Cleaner Proteins GPS: SB1 Students will analyze the nature of the relationships between structures and functions in living cells. Essential question: How does the structure of proteins relate to their
Pipe Cleaner Proteins GPS: SB1 Students will analyze the nature of the relationships between structures and functions in living cells. Essential question: How does the structure of proteins relate to their
Part A: Amino Acids and Peptides (Is the peptide IAG the same as the peptide GAI?)
 ChemActivity 46 Amino Acids, Polypeptides and Proteins 1 ChemActivity 46 Part A: Amino Acids and Peptides (Is the peptide IAG the same as the peptide GAI?) Model 1: The 20 Amino Acids at Biological p See
ChemActivity 46 Amino Acids, Polypeptides and Proteins 1 ChemActivity 46 Part A: Amino Acids and Peptides (Is the peptide IAG the same as the peptide GAI?) Model 1: The 20 Amino Acids at Biological p See
AMINO ACIDS & PEPTIDE BONDS STRUCTURE, CLASSIFICATION & METABOLISM
 AMINO ACIDS & PEPTIDE BONDS STRUCTURE, CLASSIFICATION & METABOLISM OBJECTIVES At the end of this session the student should be able to, recognize the structures of the protein amino acid and state their
AMINO ACIDS & PEPTIDE BONDS STRUCTURE, CLASSIFICATION & METABOLISM OBJECTIVES At the end of this session the student should be able to, recognize the structures of the protein amino acid and state their
PROTON NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY (H-NMR)
 PROTON NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY (H-NMR) WHAT IS H-NMR SPECTROSCOPY? References: Bruice 14.1, 14.2 Introduction NMR or nuclear magnetic resonance spectroscopy is a technique used to determine
PROTON NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY (H-NMR) WHAT IS H-NMR SPECTROSCOPY? References: Bruice 14.1, 14.2 Introduction NMR or nuclear magnetic resonance spectroscopy is a technique used to determine
Organic Chemistry Tenth Edition
 Organic Chemistry Tenth Edition T. W. Graham Solomons Craig B. Fryhle Welcome to CHM 22 Organic Chemisty II Chapters 2 (IR), 9, 3-20. Chapter 2 and Chapter 9 Spectroscopy (interaction of molecule with
Organic Chemistry Tenth Edition T. W. Graham Solomons Craig B. Fryhle Welcome to CHM 22 Organic Chemisty II Chapters 2 (IR), 9, 3-20. Chapter 2 and Chapter 9 Spectroscopy (interaction of molecule with
For example: (Example is from page 50 of the Thinkbook)
 SOLVING COMBINED SPECTROSCOPY PROBLEMS: Lecture Supplement: page 50-53 in Thinkbook CFQ s and PP s: page 216 241 in Thinkbook Introduction: The structure of an unknown molecule can be determined using
SOLVING COMBINED SPECTROSCOPY PROBLEMS: Lecture Supplement: page 50-53 in Thinkbook CFQ s and PP s: page 216 241 in Thinkbook Introduction: The structure of an unknown molecule can be determined using
The Computer Aided Resonance Assignment Tutorial
 The Computer Aided Resonance Assignment Tutorial Rochus L.J. Keller Institute for Molecular Biology and Biophysics The Swiss Federal Institute of Technology Zürich, Switzerland The authors and publishers
The Computer Aided Resonance Assignment Tutorial Rochus L.J. Keller Institute for Molecular Biology and Biophysics The Swiss Federal Institute of Technology Zürich, Switzerland The authors and publishers
BUILDING BLOCKS FOR MULTIDIMENSIONAL NMR AND SPECIAL CONSIDERATIONS FOR BIOLOGICAL APPLICATIONS OF NMR
 09-Pochapsky-09-cpp 3/7/06 2:9 pm Page 24 9 BUILDING BLOCKS FOR MULTIDIMENSIONAL NMR AND SPECIAL CONSIDERATIONS FOR BIOLOGICAL APPLICATIONS OF NMR The development of multidimensional NMR (three or more
09-Pochapsky-09-cpp 3/7/06 2:9 pm Page 24 9 BUILDING BLOCKS FOR MULTIDIMENSIONAL NMR AND SPECIAL CONSIDERATIONS FOR BIOLOGICAL APPLICATIONS OF NMR The development of multidimensional NMR (three or more
Nuclear Magnetic Resonance Spectroscopy
 Nuclear Magnetic Resonance Spectroscopy Nuclear magnetic resonance spectroscopy is a powerful analytical technique used to characterize organic molecules by identifying carbonhydrogen frameworks within
Nuclear Magnetic Resonance Spectroscopy Nuclear magnetic resonance spectroscopy is a powerful analytical technique used to characterize organic molecules by identifying carbonhydrogen frameworks within
Nuclear Magnetic Resonance Spectroscopy
 Nuclear Magnetic Resonance Spectroscopy Introduction NMR is the most powerful tool available for organic structure determination. It is used to study a wide variety of nuclei: 1 H 13 C 15 N 19 F 31 P 2
Nuclear Magnetic Resonance Spectroscopy Introduction NMR is the most powerful tool available for organic structure determination. It is used to study a wide variety of nuclei: 1 H 13 C 15 N 19 F 31 P 2
CHAPTER 15: ANSWERS TO SELECTED PROBLEMS
 CHAPTER 15: ANSWERS T SELECTED PRBLEMS SAMPLE PRBLEMS ( Try it yourself ) 15.1 ur bodies can carry out the second reaction, because it requires less energy than we get from breaking down a molecule of
CHAPTER 15: ANSWERS T SELECTED PRBLEMS SAMPLE PRBLEMS ( Try it yourself ) 15.1 ur bodies can carry out the second reaction, because it requires less energy than we get from breaking down a molecule of
Background A nucleus with an odd atomic number or an odd mass number has a nuclear spin that can be observed by NMR spectrometers.
 NMR Spectroscopy I Reading: Wade chapter, sections -- -7 Study Problems: -, -7 Key oncepts and Skills: Given an structure, determine which protons are equivalent and which are nonequivalent, predict the
NMR Spectroscopy I Reading: Wade chapter, sections -- -7 Study Problems: -, -7 Key oncepts and Skills: Given an structure, determine which protons are equivalent and which are nonequivalent, predict the
Name: Date: Problem How do amino acid sequences provide evidence for evolution? Procedure Part A: Comparing Amino Acid Sequences
 Name: Date: Amino Acid Sequences and Evolutionary Relationships Introduction Homologous structures those structures thought to have a common origin but not necessarily a common function provide some of
Name: Date: Amino Acid Sequences and Evolutionary Relationships Introduction Homologous structures those structures thought to have a common origin but not necessarily a common function provide some of
NMR Spectroscopy of Aromatic Compounds (#1e)
 NMR Spectroscopy of Aromatic Compounds (#1e) 1 H NMR Spectroscopy of Aromatic Compounds Erich Hückel s study of aromaticity in the 1930s produced a set of rules for determining whether a compound is aromatic.
NMR Spectroscopy of Aromatic Compounds (#1e) 1 H NMR Spectroscopy of Aromatic Compounds Erich Hückel s study of aromaticity in the 1930s produced a set of rules for determining whether a compound is aromatic.
Ionization of amino acids
 Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
Amino Acids 20 common amino acids there are others found naturally but much less frequently Common structure for amino acid COOH, -NH 2, H and R functional groups all attached to the a carbon Ionization
Recap. Lecture 2. Protein conformation. Proteins. 8 types of protein function 10/21/10. Proteins.. > 50% dry weight of a cell
 Lecture 2 Protein conformation ecap Proteins.. > 50% dry weight of a cell ell s building blocks and molecular tools. More important than genes A large variety of functions http://www.tcd.ie/biochemistry/courses/jf_lectures.php
Lecture 2 Protein conformation ecap Proteins.. > 50% dry weight of a cell ell s building blocks and molecular tools. More important than genes A large variety of functions http://www.tcd.ie/biochemistry/courses/jf_lectures.php
Nuclear Magnetic Resonance notes
 Reminder: These notes are meant to supplement, not replace, the laboratory manual. Nuclear Magnetic Resonance notes Nuclear Magnetic Resonance (NMR) is a spectrometric technique which provides information
Reminder: These notes are meant to supplement, not replace, the laboratory manual. Nuclear Magnetic Resonance notes Nuclear Magnetic Resonance (NMR) is a spectrometric technique which provides information
Proton Nuclear Magnetic Resonance ( 1 H-NMR) Spectroscopy
 Proton Nuclear Magnetic Resonance ( 1 H-NMR) Spectroscopy Theory behind NMR: In the late 1940 s, physical chemists originally developed NMR spectroscopy to study different properties of atomic nuclei,
Proton Nuclear Magnetic Resonance ( 1 H-NMR) Spectroscopy Theory behind NMR: In the late 1940 s, physical chemists originally developed NMR spectroscopy to study different properties of atomic nuclei,
Protein Physics. A. V. Finkelstein & O. B. Ptitsyn LECTURE 1
 Protein Physics A. V. Finkelstein & O. B. Ptitsyn LECTURE 1 PROTEINS Functions in a Cell MOLECULAR MACHINES BUILDING BLOCKS of a CELL ARMS of a CELL ENZYMES - enzymatic catalysis of biochemical reactions
Protein Physics A. V. Finkelstein & O. B. Ptitsyn LECTURE 1 PROTEINS Functions in a Cell MOLECULAR MACHINES BUILDING BLOCKS of a CELL ARMS of a CELL ENZYMES - enzymatic catalysis of biochemical reactions
The Four Questions to Ask While Interpreting Spectra. 1. How many different environments are there?
 1 H NMR Spectroscopy (#1c) The technique of 1 H NMR spectroscopy is central to organic chemistry and other fields involving analysis of organic chemicals, such as forensics and environmental science. It
1 H NMR Spectroscopy (#1c) The technique of 1 H NMR spectroscopy is central to organic chemistry and other fields involving analysis of organic chemicals, such as forensics and environmental science. It
PROTEINS STRUCTURE AND FUNCTION (DR. TRAISH)
 Introduction to Proteins - Proteins are abundant and functionally diverse molecules - They participate in cell regulation at all levels - They share a common structural feature: all are linear polymers
Introduction to Proteins - Proteins are abundant and functionally diverse molecules - They participate in cell regulation at all levels - They share a common structural feature: all are linear polymers
VTT TECHNICAL RESEARCH CENTRE OF FINLAND
 Figure from: http://www.embl.de/nmr/sattler/teaching Why NMR (instead of X ray crystallography) a great number of macromolecules won't crystallize) natural environmant (water) ligand binding and inter
Figure from: http://www.embl.de/nmr/sattler/teaching Why NMR (instead of X ray crystallography) a great number of macromolecules won't crystallize) natural environmant (water) ligand binding and inter
Used to determine relative location of atoms within a molecule Most helpful spectroscopic technique in organic chemistry Related to MRI in medicine
 Structure Determination: Nuclear Magnetic Resonance CHEM 241 UNIT 5C 1 The Use of NMR Spectroscopy Used to determine relative location of atoms within a molecule Most helpful spectroscopic technique in
Structure Determination: Nuclear Magnetic Resonance CHEM 241 UNIT 5C 1 The Use of NMR Spectroscopy Used to determine relative location of atoms within a molecule Most helpful spectroscopic technique in
Nuclear Magnetic Resonance
 Nuclear Magnetic Resonance NMR is probably the most useful and powerful technique for identifying and characterizing organic compounds. Felix Bloch and Edward Mills Purcell were awarded the 1952 Nobel
Nuclear Magnetic Resonance NMR is probably the most useful and powerful technique for identifying and characterizing organic compounds. Felix Bloch and Edward Mills Purcell were awarded the 1952 Nobel
Introduction to NMR spectroscopy. Swiss Institute of Bioinformatics I.Phan & J. Kopp
 Introduction to NMR spectroscopy Swiss Institute of Bioinformatics I.Phan & J. Kopp NMR: the background Complex technique. Requires knowledge in: Mathematics Physics Chemistry Biology (Medicin) Involves
Introduction to NMR spectroscopy Swiss Institute of Bioinformatics I.Phan & J. Kopp NMR: the background Complex technique. Requires knowledge in: Mathematics Physics Chemistry Biology (Medicin) Involves
(c) How would your answers to problem (a) change if the molecular weight of the protein was 100,000 Dalton?
 Problem 1. (12 points total, 4 points each) The molecular weight of an unspecified protein, at physiological conditions, is 70,000 Dalton, as determined by sedimentation equilibrium measurements and by
Problem 1. (12 points total, 4 points each) The molecular weight of an unspecified protein, at physiological conditions, is 70,000 Dalton, as determined by sedimentation equilibrium measurements and by
1 Introduction to NMR Spectroscopy
 Introduction to NMR Spectroscopy Tremendous progress has been made in NMR spectroscopy with the introduction of multidimensional NMR spectroscopy and pulse Fourier transform NMR spectroscopy. For a deeper
Introduction to NMR Spectroscopy Tremendous progress has been made in NMR spectroscopy with the introduction of multidimensional NMR spectroscopy and pulse Fourier transform NMR spectroscopy. For a deeper
Solving Spectroscopy Problems
 Solving Spectroscopy Problems The following is a detailed summary on how to solve spectroscopy problems, key terms are highlighted in bold and the definitions are from the illustrated glossary on Dr. Hardinger
Solving Spectroscopy Problems The following is a detailed summary on how to solve spectroscopy problems, key terms are highlighted in bold and the definitions are from the illustrated glossary on Dr. Hardinger
Chapter 13 Spectroscopy NMR, IR, MS, UV-Vis
 Chapter 13 Spectroscopy NMR, IR, MS, UV-Vis Main points of the chapter 1. Hydrogen Nuclear Magnetic Resonance a. Splitting or coupling (what s next to what) b. Chemical shifts (what type is it) c. Integration
Chapter 13 Spectroscopy NMR, IR, MS, UV-Vis Main points of the chapter 1. Hydrogen Nuclear Magnetic Resonance a. Splitting or coupling (what s next to what) b. Chemical shifts (what type is it) c. Integration
NMR for Physical and Biological Scientists Thomas C. Pochapsky and Susan Sondej Pochapsky Table of Contents
 Preface Symbols and fundamental constants 1. What is spectroscopy? A semiclassical description of spectroscopy Damped harmonics Quantum oscillators The spectroscopic experiment Ensembles and coherence
Preface Symbols and fundamental constants 1. What is spectroscopy? A semiclassical description of spectroscopy Damped harmonics Quantum oscillators The spectroscopic experiment Ensembles and coherence
NMR SPECTROSCOPY. Basic Principles, Concepts, and Applications in Chemistry. Harald Günther University of Siegen, Siegen, Germany.
 NMR SPECTROSCOPY Basic Principles, Concepts, and Applications in Chemistry Harald Günther University of Siegen, Siegen, Germany Second Edition Translated by Harald Günther JOHN WILEY & SONS Chichester
NMR SPECTROSCOPY Basic Principles, Concepts, and Applications in Chemistry Harald Günther University of Siegen, Siegen, Germany Second Edition Translated by Harald Günther JOHN WILEY & SONS Chichester
Shu-Ping Lin, Ph.D. E-mail: splin@dragon.nchu.edu.tw
 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute te of Biomedical Engineering ing E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ edu tw/pweb/users/splin/ Date: 10.13.2010
Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute te of Biomedical Engineering ing E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ edu tw/pweb/users/splin/ Date: 10.13.2010
Peptide Bond Amino acids are linked together by peptide bonds to form polypepetide chain.
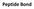 Peptide Bond Peptide Bond Amino acids are linked together by peptide bonds to form polypepetide chain. + H 2 O 2 Peptide bonds are strong and not broken by conditions that denature proteins, such as heating.
Peptide Bond Peptide Bond Amino acids are linked together by peptide bonds to form polypepetide chain. + H 2 O 2 Peptide bonds are strong and not broken by conditions that denature proteins, such as heating.
Chapter 11 Structure Determination: Nuclear Magnetic Resonance Spectroscopy. Nuclear Magnetic Resonance Spectroscopy. 11.1 Nuclear Magnetic Resonance
 John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 11 Structure Determination: Nuclear Magnetic Resonance Spectroscopy 11.1 Nuclear Magnetic Resonance Spectroscopy Many atomic nuclei behave
John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 11 Structure Determination: Nuclear Magnetic Resonance Spectroscopy 11.1 Nuclear Magnetic Resonance Spectroscopy Many atomic nuclei behave
INTRODUCTION TO PROTEIN STRUCTURE
 Name Class: Partner, if any: INTRODUCTION TO PROTEIN STRUCTURE PRIMARY STRUCTURE: 1. Write the complete structural formula of the tripeptide shown (frame 10). Circle and label the three sidechains which
Name Class: Partner, if any: INTRODUCTION TO PROTEIN STRUCTURE PRIMARY STRUCTURE: 1. Write the complete structural formula of the tripeptide shown (frame 10). Circle and label the three sidechains which
HOMEWORK PROBLEMS: IR SPECTROSCOPY AND 13C NMR. The peak at 1720 indicates a C=O bond (carbonyl). One possibility is acetone:
 HMEWRK PRBLEMS: IR SPECTRSCPY AND 13C NMR 1. You find a bottle on the shelf only labeled C 3 H 6. You take an IR spectrum of the compound and find major peaks at 2950, 1720, and 1400 cm -1. Draw a molecule
HMEWRK PRBLEMS: IR SPECTRSCPY AND 13C NMR 1. You find a bottle on the shelf only labeled C 3 H 6. You take an IR spectrum of the compound and find major peaks at 2950, 1720, and 1400 cm -1. Draw a molecule
4. It is possible to excite, or flip the nuclear magnetic vector from the α-state to the β-state by bridging the energy gap between the two. This is a
 BASIC PRINCIPLES INTRODUCTION TO NUCLEAR MAGNETIC RESONANCE (NMR) 1. The nuclei of certain atoms with odd atomic number, and/or odd mass behave as spinning charges. The nucleus is the center of positive
BASIC PRINCIPLES INTRODUCTION TO NUCLEAR MAGNETIC RESONANCE (NMR) 1. The nuclei of certain atoms with odd atomic number, and/or odd mass behave as spinning charges. The nucleus is the center of positive
Academic Nucleic Acids and Protein Synthesis Test
 Academic Nucleic Acids and Protein Synthesis Test Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Each organism has a unique combination
Academic Nucleic Acids and Protein Synthesis Test Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Each organism has a unique combination
Determination of Equilibrium Constants using NMR Spectrscopy
 CHEM 331L Physical Chemistry Laboratory Revision 1.0 Determination of Equilibrium Constants using NMR Spectrscopy In this laboratory exercise we will measure a chemical equilibrium constant using key proton
CHEM 331L Physical Chemistry Laboratory Revision 1.0 Determination of Equilibrium Constants using NMR Spectrscopy In this laboratory exercise we will measure a chemical equilibrium constant using key proton
Introduction to Nuclear Magnetic Resonance (NMR) And. NMR Metabolomics
 Introduction to Nuclear Magnetic Resonance (NMR) And NMR Metabolomics Acknowledgment: Some slides from talks by Natalia Serkova, Wimal Pathmasiri, and from many internet sources (e.g., U of Oxford, Florida
Introduction to Nuclear Magnetic Resonance (NMR) And NMR Metabolomics Acknowledgment: Some slides from talks by Natalia Serkova, Wimal Pathmasiri, and from many internet sources (e.g., U of Oxford, Florida
NMR SPECTROSCOPY A N I N T R O D U C T I O N T O... Self-study booklet NUCLEAR MAGNETIC RESONANCE. 4 3 2 1 0 δ PUBLISHING
 A N I N T R O D U T I O N T O... NMR SPETROSOPY NULEAR MAGNETI RESONANE 4 3 1 0 δ Self-study booklet PUBLISING NMR Spectroscopy NULEAR MAGNETI RESONANE SPETROSOPY Origin of Spectra Theory All nuclei possess
A N I N T R O D U T I O N T O... NMR SPETROSOPY NULEAR MAGNETI RESONANE 4 3 1 0 δ Self-study booklet PUBLISING NMR Spectroscopy NULEAR MAGNETI RESONANE SPETROSOPY Origin of Spectra Theory All nuclei possess
The Experiment Some nuclei have nuclear magnetic moments; just as importantly, some do not
 Chemistry 2600 Lecture Notes Chapter 15 Nuclear Magnetic Resonance Spectroscopy Page 1 of 23 Structure Determination in Organic Chemistry: NMR Spectroscopy Three main techniques are used to determine the
Chemistry 2600 Lecture Notes Chapter 15 Nuclear Magnetic Resonance Spectroscopy Page 1 of 23 Structure Determination in Organic Chemistry: NMR Spectroscopy Three main techniques are used to determine the
Nuclear Shielding and 1. H Chemical Shifts. 1 H NMR Spectroscopy Nuclear Magnetic Resonance
 NMR Spectroscopy Nuclear Magnetic Resonance Nuclear Shielding and hemical Shifts What do we mean by "shielding?" What do we mean by "chemical shift?" The electrons surrounding a nucleus affect the effective
NMR Spectroscopy Nuclear Magnetic Resonance Nuclear Shielding and hemical Shifts What do we mean by "shielding?" What do we mean by "chemical shift?" The electrons surrounding a nucleus affect the effective
Structural Bioinformatics (C3210) Experimental Methods for Macromolecular Structure Determination
 Structural Bioinformatics (C3210) Experimental Methods for Macromolecular Structure Determination Introduction Knowing the exact 3D-structure of bio-molecules is essential for any attempt to understand
Structural Bioinformatics (C3210) Experimental Methods for Macromolecular Structure Determination Introduction Knowing the exact 3D-structure of bio-molecules is essential for any attempt to understand
A. A peptide with 12 amino acids has the following amino acid composition: 2 Met, 1 Tyr, 1 Trp, 2 Glu, 1 Lys, 1 Arg, 1 Thr, 1 Asn, 1 Ile, 1 Cys
 Questions- Proteins & Enzymes A. A peptide with 12 amino acids has the following amino acid composition: 2 Met, 1 Tyr, 1 Trp, 2 Glu, 1 Lys, 1 Arg, 1 Thr, 1 Asn, 1 Ile, 1 Cys Reaction of the intact peptide
Questions- Proteins & Enzymes A. A peptide with 12 amino acids has the following amino acid composition: 2 Met, 1 Tyr, 1 Trp, 2 Glu, 1 Lys, 1 Arg, 1 Thr, 1 Asn, 1 Ile, 1 Cys Reaction of the intact peptide
Covalent bonds are the strongest chemical bonds contributing to the protein structure A peptide bond is formed between with of the following?
 MCAT Question Covalent bonds are the strongest chemical bonds contributing to the protein structure A peptide bond is formed between with of the following? A. Carboxylic group and amino group B. Two carboxylic
MCAT Question Covalent bonds are the strongest chemical bonds contributing to the protein structure A peptide bond is formed between with of the following? A. Carboxylic group and amino group B. Two carboxylic
Patrick, An Introduction to Medicinal Chemistry 4e Chapter 13 Drug design: optimizing target interactions. Pyrrole ring N H
 Patrick, An Introduction to dicinal hemistry 4e hapter 13 Drug design: optimizing target interactions Answers to end-of-chapter questions 1) The pyrrole ring of DU 122290 serves to increase the rigidity
Patrick, An Introduction to dicinal hemistry 4e hapter 13 Drug design: optimizing target interactions Answers to end-of-chapter questions 1) The pyrrole ring of DU 122290 serves to increase the rigidity
Determining the Structure of an Organic Compound
 Determining the Structure of an Organic Compound The analysis of the outcome of a reaction requires that we know the full structure of the products as well as the reactants In the 19 th and early 20 th
Determining the Structure of an Organic Compound The analysis of the outcome of a reaction requires that we know the full structure of the products as well as the reactants In the 19 th and early 20 th
13C NMR Spectroscopy
 13 C NMR Spectroscopy Introduction Nuclear magnetic resonance spectroscopy (NMR) is the most powerful tool available for structural determination. A nucleus with an odd number of protons, an odd number
13 C NMR Spectroscopy Introduction Nuclear magnetic resonance spectroscopy (NMR) is the most powerful tool available for structural determination. A nucleus with an odd number of protons, an odd number
The peptide bond is rigid and planar
 Level Description Bonds Primary Sequence of amino acids in proteins Covalent (peptide bonds) Secondary Structural motifs in proteins: α- helix and β-sheet Hydrogen bonds (between NH and CO groups in backbone)
Level Description Bonds Primary Sequence of amino acids in proteins Covalent (peptide bonds) Secondary Structural motifs in proteins: α- helix and β-sheet Hydrogen bonds (between NH and CO groups in backbone)
Structure Determination
 5 Structure Determination Most of the protein structures described and discussed in this book have been determined either by X-ray crystallography or by nuclear magnetic resonance (NMR) spectroscopy. Although
5 Structure Determination Most of the protein structures described and discussed in this book have been determined either by X-ray crystallography or by nuclear magnetic resonance (NMR) spectroscopy. Although
Mutation. Mutation provides raw material to evolution. Different kinds of mutations have different effects
 Mutation Mutation provides raw material to evolution Different kinds of mutations have different effects Mutational Processes Point mutation single nucleotide changes coding changes (missense mutations)
Mutation Mutation provides raw material to evolution Different kinds of mutations have different effects Mutational Processes Point mutation single nucleotide changes coding changes (missense mutations)
Insulin therapy in various type 1 diabetes patients workshop
 Insulin therapy in various type 1 diabetes patients workshop Bruce H.R. Wolffenbuttel, MD PhD Dept of Endocrinology, UMC Groningen website: www.umcg.net & www.gmed.nl Twitter: @bhrw Case no. 1 Male of
Insulin therapy in various type 1 diabetes patients workshop Bruce H.R. Wolffenbuttel, MD PhD Dept of Endocrinology, UMC Groningen website: www.umcg.net & www.gmed.nl Twitter: @bhrw Case no. 1 Male of
Cours RMN Master 2 Chimie et physico-chimie: des molécules aux bio-systèmes
 Monique CHAN-HUOT Monique.chanhuot@ens.fr Cours RMN Master 2 Chimie et physico-chimie: des molécules aux bio-systèmes Séance 4 et 5: 21/11/2011 et 28/11/2011 RMN et biologie structurale (stratégies de
Monique CHAN-HUOT Monique.chanhuot@ens.fr Cours RMN Master 2 Chimie et physico-chimie: des molécules aux bio-systèmes Séance 4 et 5: 21/11/2011 et 28/11/2011 RMN et biologie structurale (stratégies de
The Organic Chemistry of Amino Acids, Peptides, and Proteins
 Essential rganic Chemistry Chapter 16 The rganic Chemistry of Amino Acids, Peptides, and Proteins Amino Acids a-amino carboxylic acids. The building blocks from which proteins are made. H 2 N C 2 H Note:
Essential rganic Chemistry Chapter 16 The rganic Chemistry of Amino Acids, Peptides, and Proteins Amino Acids a-amino carboxylic acids. The building blocks from which proteins are made. H 2 N C 2 H Note:
Hydration and Hydrogen Bonds by NMR
 COURSE#1022: Biochemical Applications of NMR Spectroscopy http://www.bioc.aecom.yu.edu/labs/girvlab/nmr/course/ Hydration and Hydrogen Bonds by NMR LAST UPDATE: 5/5/2010 1 Outline Hydrogen Bonds References:
COURSE#1022: Biochemical Applications of NMR Spectroscopy http://www.bioc.aecom.yu.edu/labs/girvlab/nmr/course/ Hydration and Hydrogen Bonds by NMR LAST UPDATE: 5/5/2010 1 Outline Hydrogen Bonds References:
NMR Spectroscopy in Notre Dame
 NMR Spectroscopy in Notre Dame University of Notre Dame College of Science Department of Chemistry and Biochemistry Nuclear Magnetic Resonance Facility http://www.nd.edu/~nmr Reservation system for spectrometers
NMR Spectroscopy in Notre Dame University of Notre Dame College of Science Department of Chemistry and Biochemistry Nuclear Magnetic Resonance Facility http://www.nd.edu/~nmr Reservation system for spectrometers
NMR - Basic principles
 NMR - Basic principles Subatomic particles like electrons, protons and neutrons are associated with spin - a fundamental property like charge or mass. In the case of nuclei with even number of protons
NMR - Basic principles Subatomic particles like electrons, protons and neutrons are associated with spin - a fundamental property like charge or mass. In the case of nuclei with even number of protons
Rapid and Reproducible Amino Acid Analysis of Physiological Fluids for Clinical Research Using LC/MS/MS with the atraq Kit
 Rapid and Reproducible Amino Acid Analysis of Physiological Fluids for Clinical Research Using LC/MS/MS with the atraq Kit Fast, simple and cost effective analysis Many areas of biochemical research and
Rapid and Reproducible Amino Acid Analysis of Physiological Fluids for Clinical Research Using LC/MS/MS with the atraq Kit Fast, simple and cost effective analysis Many areas of biochemical research and
Guidelines for Writing a Scientific Paper
 Guidelines for Writing a Scientific Paper Writing an effective scientific paper is not easy. A good rule of thumb is to write as if your paper will be read by a person who knows about the field in general
Guidelines for Writing a Scientific Paper Writing an effective scientific paper is not easy. A good rule of thumb is to write as if your paper will be read by a person who knows about the field in general
Chapter 26 Biomolecules: Amino Acids, Peptides, and Proteins
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 26 Biomolecules: Amino Acids, Peptides, and Proteins Proteins Amides from Amino Acids Amino acids contain a basic amino group and an acidic carboxyl
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 26 Biomolecules: Amino Acids, Peptides, and Proteins Proteins Amides from Amino Acids Amino acids contain a basic amino group and an acidic carboxyl
Examination of Proton NMR Spectra
 Examination of Proton NMR Spectra What to Look For 1) Number of Signals --- indicates how many "different kinds" of protons are present. 2) Positions of the Signals --- indicates something about magnetic
Examination of Proton NMR Spectra What to Look For 1) Number of Signals --- indicates how many "different kinds" of protons are present. 2) Positions of the Signals --- indicates something about magnetic
Rapid resonance assignment is a prerequisite for highthroughput
 Reduced-dimensionality NMR spectroscopy for high-throughput protein resonance assignment Thomas Szyperski*, Deok C. Yeh*, Dinesh K. Sukumaran*, Hunter N. B. Moseley, and Gaetano T. Montelione *Departments
Reduced-dimensionality NMR spectroscopy for high-throughput protein resonance assignment Thomas Szyperski*, Deok C. Yeh*, Dinesh K. Sukumaran*, Hunter N. B. Moseley, and Gaetano T. Montelione *Departments
H H N - C - C 2 R. Three possible forms (not counting R group) depending on ph
 Amino acids - 0 common amino acids there are others found naturally but much less frequently - Common structure for amino acid - C, -N, and functional groups all attached to the alpha carbon N - C - C
Amino acids - 0 common amino acids there are others found naturally but much less frequently - Common structure for amino acid - C, -N, and functional groups all attached to the alpha carbon N - C - C
INFRARED SPECTROSCOPY (IR)
 INFRARED SPECTROSCOPY (IR) Theory and Interpretation of IR spectra ASSIGNED READINGS Introduction to technique 25 (p. 833-834 in lab textbook) Uses of the Infrared Spectrum (p. 847-853) Look over pages
INFRARED SPECTROSCOPY (IR) Theory and Interpretation of IR spectra ASSIGNED READINGS Introduction to technique 25 (p. 833-834 in lab textbook) Uses of the Infrared Spectrum (p. 847-853) Look over pages
Chemical Exchange in NMR Spectroscopy
 COURSE#1022: Biochemical Applications of NMR Spectroscopy http://www.bioc.aecom.yu.edu/labs/girvlab/nmr/course/ Chemical Exchange in NMR Spectroscopy LAST UPDATE: 3/28/2012 1 References Bain, A. D. (2003).
COURSE#1022: Biochemical Applications of NMR Spectroscopy http://www.bioc.aecom.yu.edu/labs/girvlab/nmr/course/ Chemical Exchange in NMR Spectroscopy LAST UPDATE: 3/28/2012 1 References Bain, A. D. (2003).
Organic Spectroscopy. UV - Ultraviolet-Visible Spectroscopy. !! 200-800 nm. Methods for structure determination of organic compounds:
 Organic Spectroscopy Methods for structure determination of organic compounds: X-ray rystallography rystall structures Mass spectroscopy Molecular formula -----------------------------------------------------------------------------
Organic Spectroscopy Methods for structure determination of organic compounds: X-ray rystallography rystall structures Mass spectroscopy Molecular formula -----------------------------------------------------------------------------
UV-Vis Vis spectroscopy. Electronic absorption spectroscopy
 UV-Vis Vis spectroscopy Electronic absorption spectroscopy Absortpion spectroscopy Provide information about presence and absence of unsaturated functional groups Useful adjunct to IR Determination of
UV-Vis Vis spectroscopy Electronic absorption spectroscopy Absortpion spectroscopy Provide information about presence and absence of unsaturated functional groups Useful adjunct to IR Determination of
Determination of Equilibrium Constants using NMR Spectroscopy
 CHEM 331L Physical Chemistry Laboratory Revision 2.1 Determination of Equilibrium Constants using NMR Spectroscopy In this laboratory exercise we will measure the equilibrium constant for the cis-trans
CHEM 331L Physical Chemistry Laboratory Revision 2.1 Determination of Equilibrium Constants using NMR Spectroscopy In this laboratory exercise we will measure the equilibrium constant for the cis-trans
18.2 Protein Structure and Function: An Overview
 18.2 Protein Structure and Function: An Overview Protein: A large biological molecule made of many amino acids linked together through peptide bonds. Alpha-amino acid: Compound with an amino group bonded
18.2 Protein Structure and Function: An Overview Protein: A large biological molecule made of many amino acids linked together through peptide bonds. Alpha-amino acid: Compound with an amino group bonded
Time out states and transitions
 Time out states and transitions Spectroscopy transitions between energy states of a molecule excited by absorption or emission of a photon hn = DE = E i - E f Energy levels due to interactions between
Time out states and transitions Spectroscopy transitions between energy states of a molecule excited by absorption or emission of a photon hn = DE = E i - E f Energy levels due to interactions between
Spin-Lattice Relaxation Times
 Spin-Lattice Relaxation Times Reading Assignment: T. D. W. Claridge, High Resolution NMR Techniques in Organic Chemistry, Chapter 2; E. Breitmaier, W. Voelter, Carbon 13 NMR Spectroscopy,3rd Ed., 3.3.2.
Spin-Lattice Relaxation Times Reading Assignment: T. D. W. Claridge, High Resolution NMR Techniques in Organic Chemistry, Chapter 2; E. Breitmaier, W. Voelter, Carbon 13 NMR Spectroscopy,3rd Ed., 3.3.2.
Outline. Market & Technology Trends. LifeTein Technology Portfolio. LifeTein Services
 1 Outline Market & Technology Trends LifeTein Technology Portfolio LifeTein Services 2 Synthetic Therapeutic Peptides More than 60 synthetic therapeutic peptides under 50 amino acids in size have reached
1 Outline Market & Technology Trends LifeTein Technology Portfolio LifeTein Services 2 Synthetic Therapeutic Peptides More than 60 synthetic therapeutic peptides under 50 amino acids in size have reached
Application Note AN4
 TAKING INVENTIVE STEPS IN INFRARED. MINIATURE INFRARED GAS SENSORS GOLD SERIES UK Patent App. No. 2372099A USA Patent App. No. 09/783,711 World Patents Pending INFRARED SPECTROSCOPY Application Note AN4
TAKING INVENTIVE STEPS IN INFRARED. MINIATURE INFRARED GAS SENSORS GOLD SERIES UK Patent App. No. 2372099A USA Patent App. No. 09/783,711 World Patents Pending INFRARED SPECTROSCOPY Application Note AN4
PROTEIN SEQUENCING. First Sequence
 PROTEIN SEQUENCING First Sequence The first protein sequencing was achieved by Frederic Sanger in 1953. He determined the amino acid sequence of bovine insulin Sanger was awarded the Nobel Prize in 1958
PROTEIN SEQUENCING First Sequence The first protein sequencing was achieved by Frederic Sanger in 1953. He determined the amino acid sequence of bovine insulin Sanger was awarded the Nobel Prize in 1958
M.Sc. in Nano Technology with specialisation in Nano Biotechnology
 M.Sc. in Nano Technology with specialisation in Nano Biotechnology Nanotechnology is all about designing, fabricating and controlling materials, components and machinery with dimensions on the nanoscale,
M.Sc. in Nano Technology with specialisation in Nano Biotechnology Nanotechnology is all about designing, fabricating and controlling materials, components and machinery with dimensions on the nanoscale,
NMR Spectroscopy. Applications. Drug design MRI. Food quality. Structural biology. Metabonomics
 Applications Drug design MRI Food qualit Metabonomics Structural biolog Basic Principles N.M.R. = Nuclear Magnetic Resonance Spectroscopic technique, thus relies on the interaction between material and
Applications Drug design MRI Food qualit Metabonomics Structural biolog Basic Principles N.M.R. = Nuclear Magnetic Resonance Spectroscopic technique, thus relies on the interaction between material and
Protein Dynamics by NMR. Why NMR is the best!
 Protein Dynamics by NMR Why NMR is the best! Key Points NMR dynamics divided into 2 regimes: fast and slow. How protein mobons affect NMR parameters depend on whether they are faster or slower than the
Protein Dynamics by NMR Why NMR is the best! Key Points NMR dynamics divided into 2 regimes: fast and slow. How protein mobons affect NMR parameters depend on whether they are faster or slower than the
Biochemistry 462a Hemoglobin Structure and Function Reading - Chapter 7 Practice problems - Chapter 7: 1-6; Proteins extra problems
 Biochemistry 462a Hemoglobin Structure and Function Reading - Chapter 7 Practice problems - Chapter 7: 1-6; Proteins extra problems Myoglobin and Hemoglobin Oxygen is required for oxidative metabolism
Biochemistry 462a Hemoglobin Structure and Function Reading - Chapter 7 Practice problems - Chapter 7: 1-6; Proteins extra problems Myoglobin and Hemoglobin Oxygen is required for oxidative metabolism
Part ONE. a. Assuming each of the four bases occurs with equal probability, how many bits of information does a nucleotide contain?
 Networked Systems, COMPGZ01, 2012 Answer TWO questions from Part ONE on the answer booklet containing lined writing paper, and answer ALL questions in Part TWO on the multiple-choice question answer sheet.
Networked Systems, COMPGZ01, 2012 Answer TWO questions from Part ONE on the answer booklet containing lined writing paper, and answer ALL questions in Part TWO on the multiple-choice question answer sheet.
Workshop IIc. Manual interpretation of MS/MS spectra. Ebbing de Jong. Center for Mass Spectrometry and Proteomics Phone (612)625-2280 (612)625-2279
 Workshop IIc Manual interpretation of MS/MS spectra Ebbing de Jong Why MS/MS spectra? The information contained in an MS spectrum (m/z, isotope spacing and therefore z ) is not enough to tell us the amino
Workshop IIc Manual interpretation of MS/MS spectra Ebbing de Jong Why MS/MS spectra? The information contained in an MS spectrum (m/z, isotope spacing and therefore z ) is not enough to tell us the amino
The Hydrogen Atom Is a Magnet. http://www.seed.slb.com/en/scictr/watch/gashydrates/detecting.htm
 The Hydrogen Atom Is a Magnet Nuclear Magnetic Resonance Spectroscopy (NMR) Proton NMR A hydrogen nucleus can be viewed as a proton, which can be viewed as a spinning charge. As with any spinning charge,
The Hydrogen Atom Is a Magnet Nuclear Magnetic Resonance Spectroscopy (NMR) Proton NMR A hydrogen nucleus can be viewed as a proton, which can be viewed as a spinning charge. As with any spinning charge,
Table of contents. Bibliografische Informationen http://d-nb.info/1006571213. digitalisiert durch
 1. Research scope: The role of structure rigid'tf'ication in nature and chemistry 1 2. Establishing a Dha=Tap backbone scan in order to elucidate structural properties of the N-terminusofNPY 5 2.1 Introduction.
1. Research scope: The role of structure rigid'tf'ication in nature and chemistry 1 2. Establishing a Dha=Tap backbone scan in order to elucidate structural properties of the N-terminusofNPY 5 2.1 Introduction.
(21) Appl. No.: 09/120,044
 US 20010014332A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2001/0014332 A1 MINETTI et al. (43) Pub. Date: Aug. 16, 2001 (54) MODIFIED IMMUNOGENIC PNEUMOLYSIN COMPOSITIONS
US 20010014332A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2001/0014332 A1 MINETTI et al. (43) Pub. Date: Aug. 16, 2001 (54) MODIFIED IMMUNOGENIC PNEUMOLYSIN COMPOSITIONS
Lecture 15: Enzymes & Kinetics Mechanisms
 ROLE OF THE TRANSITION STATE Lecture 15: Enzymes & Kinetics Mechanisms Consider the reaction: H-O-H + Cl - H-O δ- H Cl δ- HO - + H-Cl Reactants Transition state Products Margaret A. Daugherty Fall 2004
ROLE OF THE TRANSITION STATE Lecture 15: Enzymes & Kinetics Mechanisms Consider the reaction: H-O-H + Cl - H-O δ- H Cl δ- HO - + H-Cl Reactants Transition state Products Margaret A. Daugherty Fall 2004
AMINO ACIDS QUANTITATION IN BIOLOGICAL MEDIA. Monica Culea
 STUDIA UNIVERSITATIS BABEŞ BLYAI, PHYSICA, L, 4b, 25 AMIN ACIDS QUANTITATIN IN BILGICAL MEDIA Monica Culea Univ. Babes Bolyai, Biomedical Physics Dept., 1 Kogalniceanu str, 34 Cluj Napoca, Romania e mail:
STUDIA UNIVERSITATIS BABEŞ BLYAI, PHYSICA, L, 4b, 25 AMIN ACIDS QUANTITATIN IN BILGICAL MEDIA Monica Culea Univ. Babes Bolyai, Biomedical Physics Dept., 1 Kogalniceanu str, 34 Cluj Napoca, Romania e mail:
Protein Structure Determination. Why Bother With Structure?
 Protein Structure Determination How are these structures determined? Why Bother With Structure? The amino acid sequence of a protein contains interesting information. A protein sequence can be compared
Protein Structure Determination How are these structures determined? Why Bother With Structure? The amino acid sequence of a protein contains interesting information. A protein sequence can be compared
Proton Nuclear Magnetic Resonance Spectroscopy
 CHEM 334L Organic Chemistry Laboratory Revision 2.0 Proton Nuclear Magnetic Resonance Spectroscopy In this laboratory exercise we will learn how to use the Chemistry Department's Nuclear Magnetic Resonance
CHEM 334L Organic Chemistry Laboratory Revision 2.0 Proton Nuclear Magnetic Resonance Spectroscopy In this laboratory exercise we will learn how to use the Chemistry Department's Nuclear Magnetic Resonance
Amino Acids as Acids, Bases and Buffers:
 Amino Acids as Acids, Bases and Buffers: - Amino acids are weak acids - All have at least 2 titratable protons (shown below as fully protonated species) and therefore have 2 pka s o α-carboxyl (-COOH)
Amino Acids as Acids, Bases and Buffers: - Amino acids are weak acids - All have at least 2 titratable protons (shown below as fully protonated species) and therefore have 2 pka s o α-carboxyl (-COOH)
Determination of Molecular Structure by MOLECULAR SPECTROSCOPY
 Determination of Molecular Structure by MOLEULAR SPETROSOPY hemistry 3 B.Z. Shakhashiri Fall 29 Much of what we know about molecular structure has been learned by observing and analyzing how electromagnetic
Determination of Molecular Structure by MOLEULAR SPETROSOPY hemistry 3 B.Z. Shakhashiri Fall 29 Much of what we know about molecular structure has been learned by observing and analyzing how electromagnetic
NMR and other Instrumental Techniques in Chemistry and the proposed National Curriculum.
 NMR and other Instrumental Techniques in Chemistry and the proposed National Curriculum. Dr. John Jackowski Chair of Science, Head of Chemistry Scotch College Melbourne john.jackowski@scotch.vic.edu.au
NMR and other Instrumental Techniques in Chemistry and the proposed National Curriculum. Dr. John Jackowski Chair of Science, Head of Chemistry Scotch College Melbourne john.jackowski@scotch.vic.edu.au
