Molecular Modeling using Spartan 06
|
|
|
- Edward Rose
- 7 years ago
- Views:
Transcription
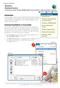
1 Molecular Modeling using Spartan 06 The purpose of this laboratory is to help you visualize different types of bonds found within and between biologically relevant molecules. This first page is a guide to some of the basic functions of Spartan 06. Subsequent pages provide step-by-step directions to help you construct and analyze several different molecules. Use the data you find to complete the worksheet at the end of this document. Once you have completed this exercise you may build more molecules on your own. The following is a view of the main window when Spartan 06 is open: The icons in the tool bar at the top are displayed below: New- Opens new molecule window Open- Opens previously saved window Close- Closes current window without closing program Save- Saves current window View- Finalized molecule Add- Allows addition of new atoms to current molecule Remove- Removes atoms Add Bond Remove Bond Minimize- Calculates most stable structure Distance- measure length of bond between two selected atoms Angles- measure angle between three selected atoms
2 Begin by using your lecture notes to answer questions 1 and 2 of the Chemistry and Molecular Modeling Worksheet. When you re ready, use the following instructions to open Spartan Double click Spartan 06 icon on desktop - Click on the New icon ( ), the entry model kit will appear at the right of the screen (shown at right). 1) Let s begin by exploring bonds between hydrogen and oxygen atoms. To do this you will build and analyze a water molecule (H 2 O) - Click the Add ( ) icon on the top toolbar. - Click on the button displaying an oxygen atom with two single bonds on the entry model kit. - Click on the main screen. An oxygen atom with two available single bonds will appear. - Click the hydrogen button from the entry model kit. - Add a hydrogen atom to each of the available single bonds by clicking on each bond (available bonds should appear yellow on the main screen). - Now that your molecule is built, click the Minimize ( ) icon on the top toolbar to put your molecule in its most stable form. - Click the View ( ) icon to finalize your molecule. - Once finalized, look at several different representations of your molecule by using the Model menu at the top of the screen and selecting from different views such as wire, ball and wire, tube, or space filling, from the dropdown menu. - Return to ball and spoke view. - Left click, hold, and drag the mouse on the main molecule screen to rotate the molecule in three dimensions. Right click, hold, and drag the mouse to move the molecule to a different location on the screen. Right click, hold, and drag the mouse while holding down the shift key to zoom in and out. - Next we will examine the polarity of the O-H bonds in water, but in order to do this you must first save the molecule you have built by going to File>Save As. Name this file H2O and save it to the desktop. - Now click on Setup>Surfaces. In the Surfaces window that pops up, click Add. Use the drop-down menus to set Surface to density, Property to potential, and Resolution to medium. This calculation will determine which parts of the molecule will interact most favorably with positive charges. Click OK and close the Surfaces window. - Next click on Setup>Submit. A window will pop-up, indicating that Spartan has begun calculating the electrostatic potential of the molecule. Click OK. You may have to wait briefly while Spartan completes the calculations. When the window indicating that the calculations are complete appears, click OK.
3 - To view the electrostatic potential of various portions of the molecule, click Display>Surfaces. Click on the yellow box next to density and close the Surfaces window. - Now set the color scheme by clicking on Display>Properties. Then click on the water molecule and set the Property Range on the left (red) to -261 and the Property Range on the right (blue) to While leaving the Surface Properties window open, rotate the molecule to view all surfaces of it, and notice that some portions of the molecule are red, indicating that they interact favorably with positive charges (such as sodium ions). Are such parts of the molecule more likely to be electron-poor or electron-rich? - Blue parts of the molecule repulse positive charges. Are such parts of the molecule likely to be electron-poor or electron rich? - To view the ball and spoke model and the electrostatic potential map simultaneously, use the drop-down menu in the Surface Properties window to set the Style to Transparent. Rotate the molecule as desired to gain the information needed to answer questions 3-5 on the worksheet. 2) Now let s examine a covalent bond that forms between identical atoms, namely the double bond between the oxygen atoms in O 2. - Begin by closing the H2O file ( ) and opening a new molecule window ( ). - Click the Add ( ) icon on the top toolbar. - Click on the button displaying an oxygen atom with a double bond (=O) on the entry model kit. - Complete the molecule by adding the same type of oxygen atom to the available (yellow) double bond. - Use the Minimize ( ) icon on the top toolbar to put your molecule in its most stable form. - Save this file as O2, then calculate and map its electrostatic potential by following the same steps as you did for water (be sure to set the Property Range to -261 to 246). After viewing the molecule, use what you learned to answer questions 6 and 7 on the worksheet. 3) Now let s examine a covalent bond that forms between atoms with similar electronegativity, namely the bonds between carbon and hydrogen atoms in the simple molecule methane (CH 4 ). - Begin by closing the O2 file ( ) and opening a new molecule window ( ). - Click the Add ( ) icon on the top toolbar. - Click on the entry model kit button displaying a carbon atom with four single bonds. - Complete the molecule by adding a hydrogen atom to each available (yellow) single bond. - Use the Minimize ( ) icon on the top toolbar to put your molecule in its most stable form. - Save this file as CH4, then calculate and map its electrostatic potential by following the same steps as you did for water (be sure to set the Property Range to to 246). When finished re-open the H2O and O2 files, and display the
4 electrostatic potential map for each molecule. Compare the structures and color scheme of each molecule, and use what you learned to answer questions 8-10 on the worksheet. 4) Now let s explore a larger molecule with several different types of bonds, specifically C-H, C-C, C-O, and O-H bonds. To do this you will build and analyze a molecule of ethanol (shown to the right). - Begin by closing the H2O, O2, and CH4 files ( ) and opening a new molecule window ( ). - Click the Add ( ) icon on the top toolbar. - Click on the button displaying a carbon atom with four single bonds on the entry model kit. - Click on the main screen. A carbon atom with four available single bonds will appear. Attach another carbon atom to one of these available (yellow) bonds by clicking on it. - Click on the button displaying an oxygen atom with two single bonds on the entry model kit. - Add an oxygen atom to one of the carbon atoms by clicking on one of the available (yellow) bonds. - Add a hydrogen atom to each of the remaining available (yellow) single bonds. - Now that your molecule is built, click the Minimize ( ) icon on the top toolbar to put your molecule in its most stable form. - Rotate this structure to view it in its entirety. - Save the molecule you have built by going to File>Save As. Name this file ethanol and save it to the desktop. - Now click on Setup>Surfaces. In the Surfaces window that pops up, click Add. Use the drop-down menus to set Surface to density, Property to potential, and Resolution to medium. This calculation will determine which parts of the molecule will interact most favorably with positive charges. Click OK and close the Surfaces window. - Next click on Setup>Submit. A window will pop-up, indicating that Spartan has begun calculating the electrostatic potential of the molecule. Click OK. You may have to wait briefly while Spartan completes the calculations. When the window indicating that the calculations are complete appears, click OK. - To view the electrostatic potential of various portions of the molecule, click Display>Surfaces. Click on the yellow box next to density and close the Surfaces window. - To view the ball and spoke model and the electrostatic potential map simultaneously, click on Display> Properties. Then click on the water molecule, and use the drop-down menu to set the Style to Transparent. Rotate the molecule as desired to gain the information needed to answer questions 11 and 12 on the worksheet.
5 5) Now let s move on and look at an even more complicated structure. During this part of the lab we will also explore how two small molecules can interact via hydrogen bonds. To begin this part of the exercise, you will first need to construct the nitrogenous base cytosine, a component of DNA. - Begin by closing the ethanol file ( ) and opening a new molecule window ( ). - Click the Add ( ) icon on the top toolbar. - Click on the button displaying a nitrogen atom with three single bonds on the entry model kit. cytosine - Click on the main screen. A nitrogen atom with three available single bonds will appear. This corresponds with the bottom-most atom in the picture of cytosine provided above. We ll continue building this molecule by proceeding in a clockwise fashion around the ring. - Go back to the entry model kit and click on the button displaying a carbon atom with a double bond and two single bonds. Add this carbon atom to one of the available (yellow) bonds of your nitrogen atom. - Add the same type of carbon to the available (yellow) double bond of the first carbon atom you attached. - Keep building this linear chain of atoms by adding the same type of carbon (one double bond and two single bonds) to one of the available (yellow) single bonds of the last carbon you added. Now you should have a chain of four atoms, specifically, a single nitrogen followed by three carbon atoms. - Next add the second nitrogen atom that will be found in the ring, by going back to the entry model kit and clicking on the nitrogen button which has a double bond and a single bond. Add this nitrogen atom to the available (yellow) double bond of the last carbon you added. - Again, go back to the entry model kit and click on the carbon button with two single bonds and a double bond. Add one such carbon to the available single bond of the most recently added nitrogen. - Click the Minimize ( ) icon on the top toolbar to better visualize all the atoms and bonds you have built thus far. - Close the ring by clicking on the Add Bond button ( ) at the top of the page. Next click on one of the available single bonds from your first nitrogen atom and then the available single bond from your last carbon atom. - Click the Minimize ( ) icon on the top toolbar to better visualize the ring. Is the ring six-sided? And does it have all the necessary atoms in the appropriate order as shown in the diagram above? - Now add the remaining atoms to your cytosine molecule. - Go to the entry model kit and click on the button corresponding to oxygen with a double bond. Add one such oxygen to the available (yellow) double bond of your molecule. - Use the appropriate buttons on the entry tool kit to add the NH 2 and other remaining atoms. - Now that your molecule is built, click the Minimize ( ) icon on the top toolbar to put your molecule in its most stable form.
6 - Click the View ( ) icon to finalize your molecule. - Once finalized, look at several different representations of your molecule, rotating it and zooming in and so you can see all of its relevant features. - Save the molecule you have built by going to File>Save As. Name this file cytosine and save it to the desktop. - Now click on Setup>Surfaces. In the Surfaces window that pops up, click Add. Use the drop-down menus to set Surface to density, Property to potential, and Resolution to medium. This calculation will determine which parts of the molecule will interact most favorably with positive charges. Click OK and close the Surfaces window. - Next click on Setup>Submit. A window will pop-up, indicating that Spartan has begun calculating the electrostatic potential of the molecule. Click OK. You may have to wait briefly while Spartan completes the calculations. When the window indicating that the calculations are complete appears, click OK. - To view the electrostatic potential of various portions of the molecule, click Display>Surfaces. Click on the yellow box next to density and close the Surfaces window. - To view the ball and spoke model and the electrostatic potential map simultaneously, click on Display> Properties. Then click on the water molecule, and use the drop-down menu to set the Style to Transparent. Rotate the molecule as desired to gain the information needed to answer question 13 on the worksheet. - Do NOT close the cytosine document. 4) Now open the Spartan document titled guanine. This document can be found on the desktop of the computer you are using. - Now click on Setup>Surfaces. In the Surfaces window that pops up, click Add. Use the drop-down menus to set Surface to density, Property to potential, and Resolution to medium. This calculation will determine which parts of the molecule will interact most favorably with positive charges. Click OK and close the Surfaces guanine window. - Next click on Setup>Submit. A window will pop-up, indicating that Spartan has begun calculating the electrostatic potential of the molecule. Click OK. You may have to wait briefly while Spartan completes the calculations. When the window indicating that the calculations are complete appears, click OK. - To view the electrostatic potential of various portions of the molecule, click Display>Surfaces. Click on the yellow box next to density and close the Surfaces window. - To view the ball and spoke model and the electrostatic potential map simultaneously, click on Display> Properties. Then click on the water molecule, and use the drop-down menu to set the Style to Transparent. - If necessary, right click, hold, and drag the mouse to pull the guanine molecule to a part of the screen that is away from the cytosine. Rotate the guanine as desired to gain the information needed to answer question 14 on the worksheet.
7 - With both the guanine and cytosine files open, take a moment to refresh your memory about what a hydrogen bond is, referring to your lecture notes if necessary. Record your answer on the worksheet. - Within the structure of double-stranded DNA, cytosine and guanine form three hydrogen bonds with each other. In Spartan, orient the cytosine and guanine molecules so that you can easily visualize where the three hydrogen bonds will form. What do you notice? Does red in one molecule line up with blue in the other? Print out this image, write on it to indicate where the hydrogen bonds form, then staple this page to your worksheet. Turn in both documents.
8 Name: BI 149: Chemistry and Molecular Modeling Worksheet 1. Carbon, hydrogen, nitrogen, and oxygen are the most common elements making up the human body. Using your handout from today s lecture, what is the difference in electronegativity between carbon and hydrogen? carbon and oxygen? carbon and nitrogen? oxygen and hydrogen? nitrogen and hydrogen? 2. Water readily dissolves table salt (NaCl). In a salt solution, which atom of a water molecule (hydrogen or oxygen) will be attracted to a sodium ion (Na + ) from the salt? Why? 3. The electrostatic potential of a molecule, or part of a molecule, is a measure of how favorably the atom(s) under study interact with (or are attracted to) positive charges. Which atom of a water molecule, hydrogen or oxygen, will interact favorably with (be attracted to) positive charges? Why? Which atom of a water molecule, hydrogen or oxygen, will interact unfavorably with (be repulsed from) positive charges? Why? 4. When looking at the electrostatic potential map for water, what color is this surface near the oxygen atom? Given that red shading indicates portions of the molecule that interact favorably with positive charges, do you think this part of the molecule is electron-rich or electronpoor? Briefly explain your answer. 5. When looking at the electrostatic potential map for water, what color is this surface near the hydrogen atoms? Given that blue shading indicates portions of the molecule that repulse positive charges, do you think this part of the molecule is electron-rich or electron-poor? Explain your answer.
9 6. Molecular oxygen (O 2 ) is formed when two oxygen atoms are covalently attached to each other through a double bond. How many electrons are shared between two atoms involved in a double bond? How many electrons are donated from each atom? (feel free to use your lecture notes or textbook to look up this answer!) 7. Describe the electrostatic potential map for molecular oxygen. (what do you notice? does the molecule have red parts? blue parts? is it all basically the same color?) What does this tell you about the nature of O 2? Is this molecule polar or nonpolar? 8. Methane (CH4) is formed when one carbon atom is covalently attached to four hydrogen atoms. How many electrons are shared between two atoms involved in a single bond? How many electrons are donated from each atom? (feel free to use your lecture notes or textbook to look up this answer!) 9. Describe the electrostatic potential map for methane. (what do you notice? does the molecule have red parts? blue parts? is it all basically the same color?) What does this tell you about the nature of methane? Is this molecule polar or nonpolar? 10. When comparing the structures of water, molecular oxygen, and methane, which molecule is the most polar (i.e.- has red and blue parts)? Which molecule is the least polar? How do these results correlate with the differences in electronegativity that you calculated when answering question #1? 11. When looking at the electrostatic potential map for ethanol, which atom will interact most favorably with positive charges (i.e.- which atom has a red electrostatic potential? Which atom will repulse positive charges (i.e.- which atom has a blue electrostatic potential? 12. Which bond in this molecule is the most polar? In other words, which bond is between an atom with a red electrostatic potential and a blue electrostatic potential? Which bonds appear to be the least polar? What color is used to represent nonpolar portions of the molecule?
10 13. What color is being used to represent the electrostatic potential of the nitrogen atom that doesn t have any hydrogens directly bonded to it? What does this tell you about this atom? Is it electron-rich or electron poor? Will this nitrogen attract or repulse positive charges? 14. Which hydrogens in the structures of cytosine and guanine are the most likely to repulse positive charges (i.e.- which have the bluest electrostatic potential?)?... the hydrogen atoms bonded to carbon atoms or the hydrogen atoms bonded to nitrogen atoms? Why do you think this might be the case? 15. What is the definition of a hydrogen bond? 16. In DNA, three hydrogen bonds form between guanine and cytosine. Indicate where these bonds form on the print-out of the structures from Spartan.
POLARITY AND MOLECULAR SHAPE WITH HYPERCHEM LITE
 POLARITY AND MOLECULAR SHAPE WITH HYPERCHEM LITE LAB MOD4.COMP From Gannon University SIM INTRODUCTION Many physical properties of matter, such as boiling point and melting point, are the result of the
POLARITY AND MOLECULAR SHAPE WITH HYPERCHEM LITE LAB MOD4.COMP From Gannon University SIM INTRODUCTION Many physical properties of matter, such as boiling point and melting point, are the result of the
Molecular Models in Biology
 Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Visualizing Molecular Orbitals: A MacSpartan Pro Experience
 Introduction Name(s) Visualizing Molecular Orbitals: A MacSpartan Pro Experience In class we have discussed Lewis structures, resonance, VSEPR, hybridization and molecular orbitals. These concepts are
Introduction Name(s) Visualizing Molecular Orbitals: A MacSpartan Pro Experience In class we have discussed Lewis structures, resonance, VSEPR, hybridization and molecular orbitals. These concepts are
Section Activity #1: Fill out the following table for biology s most common elements assuming that each atom is neutrally charged.
 LS1a Fall 2014 Section Week #1 I. Valence Electrons and Bonding The number of valence (outer shell) electrons in an atom determines how many bonds it can form. Knowing the number of valence electrons present
LS1a Fall 2014 Section Week #1 I. Valence Electrons and Bonding The number of valence (outer shell) electrons in an atom determines how many bonds it can form. Knowing the number of valence electrons present
EXPERIMENT 17 : Lewis Dot Structure / VSEPR Theory
 EXPERIMENT 17 : Lewis Dot Structure / VSEPR Theory Materials: Molecular Model Kit INTRODUCTION Although it has recently become possible to image molecules and even atoms using a high-resolution microscope,
EXPERIMENT 17 : Lewis Dot Structure / VSEPR Theory Materials: Molecular Model Kit INTRODUCTION Although it has recently become possible to image molecules and even atoms using a high-resolution microscope,
CHEMISTRY BONDING REVIEW
 Answer the following questions. CHEMISTRY BONDING REVIEW 1. What are the three kinds of bonds which can form between atoms? The three types of Bonds are Covalent, Ionic and Metallic. Name Date Block 2.
Answer the following questions. CHEMISTRY BONDING REVIEW 1. What are the three kinds of bonds which can form between atoms? The three types of Bonds are Covalent, Ionic and Metallic. Name Date Block 2.
Health Science Chemistry I CHEM-1180 Experiment No. 15 Molecular Models (Revised 05/22/2015)
 (Revised 05/22/2015) Introduction In the early 1900s, the chemist G. N. Lewis proposed that bonds between atoms consist of two electrons apiece and that most atoms are able to accommodate eight electrons
(Revised 05/22/2015) Introduction In the early 1900s, the chemist G. N. Lewis proposed that bonds between atoms consist of two electrons apiece and that most atoms are able to accommodate eight electrons
Chemistry 111 Laboratory Experiment 4: Visualizing Molecular Orbitals with MacSpartan Pro (This experiment will be conducted in OR341)
 Chemistry 111 Laboratory Experiment 4: Visualizing Molecular Orbitals with MacSpartan Pro (This experiment will be conducted in OR341) Introduction In class we have discussed Lewis structures, resonance,
Chemistry 111 Laboratory Experiment 4: Visualizing Molecular Orbitals with MacSpartan Pro (This experiment will be conducted in OR341) Introduction In class we have discussed Lewis structures, resonance,
EXPERIMENT 9 Dot Structures and Geometries of Molecules
 EXPERIMENT 9 Dot Structures and Geometries of Molecules INTRODUCTION Lewis dot structures are our first tier in drawing molecules and representing bonds between the atoms. The method was first published
EXPERIMENT 9 Dot Structures and Geometries of Molecules INTRODUCTION Lewis dot structures are our first tier in drawing molecules and representing bonds between the atoms. The method was first published
Laboratory 11: Molecular Compounds and Lewis Structures
 Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
Survival Organic Chemistry Part I: Molecular Models
 Survival Organic Chemistry Part I: Molecular Models The goal in this laboratory experience is to get you so you can easily and quickly move between empirical formulas, molecular formulas, condensed formulas,
Survival Organic Chemistry Part I: Molecular Models The goal in this laboratory experience is to get you so you can easily and quickly move between empirical formulas, molecular formulas, condensed formulas,
Chapter 2 Polar Covalent Bonds: Acids and Bases
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 2 Polar Covalent Bonds: Acids and Bases Modified by Dr. Daniela R. Radu Why This Chapter? Description of basic ways chemists account for chemical
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 2 Polar Covalent Bonds: Acids and Bases Modified by Dr. Daniela R. Radu Why This Chapter? Description of basic ways chemists account for chemical
Molecular Models Experiment #1
 Molecular Models Experiment #1 Objective: To become familiar with the 3-dimensional structure of organic molecules, especially the tetrahedral structure of alkyl carbon atoms and the planar structure of
Molecular Models Experiment #1 Objective: To become familiar with the 3-dimensional structure of organic molecules, especially the tetrahedral structure of alkyl carbon atoms and the planar structure of
Modelling Compounds. 242 MHR Unit 2 Atoms, Elements, and Compounds
 6.3 Figure 6.26 To build the Michael Lee-Chin Crystal at the Royal Ontario Museum, models were used at different stages to convey different types of information. Modelling Compounds The Michael Lee-Chin
6.3 Figure 6.26 To build the Michael Lee-Chin Crystal at the Royal Ontario Museum, models were used at different stages to convey different types of information. Modelling Compounds The Michael Lee-Chin
Which substance contains positive ions immersed in a sea of mobile electrons? A) O2(s) B) Cu(s) C) CuO(s) D) SiO2(s)
 BONDING MIDTERM REVIEW 7546-1 - Page 1 1) Which substance contains positive ions immersed in a sea of mobile electrons? A) O2(s) B) Cu(s) C) CuO(s) D) SiO2(s) 2) The bond between hydrogen and oxygen in
BONDING MIDTERM REVIEW 7546-1 - Page 1 1) Which substance contains positive ions immersed in a sea of mobile electrons? A) O2(s) B) Cu(s) C) CuO(s) D) SiO2(s) 2) The bond between hydrogen and oxygen in
Chapter 4, Lesson 4: Energy Levels, Electrons, and Covalent Bonding
 Chapter 4, Lesson 4: Energy Levels, Electrons, and Covalent Bonding Key Concepts The electrons on the outermost energy level of the atom are called valence electrons. The valence electrons are involved
Chapter 4, Lesson 4: Energy Levels, Electrons, and Covalent Bonding Key Concepts The electrons on the outermost energy level of the atom are called valence electrons. The valence electrons are involved
H 2O gas: molecules are very far apart
 Non-Covalent Molecular Forces 2/27/06 3/1/06 How does this reaction occur: H 2 O (liquid) H 2 O (gas)? Add energy H 2O gas: molecules are very far apart H 2O liquid: bonding between molecules Use heat
Non-Covalent Molecular Forces 2/27/06 3/1/06 How does this reaction occur: H 2 O (liquid) H 2 O (gas)? Add energy H 2O gas: molecules are very far apart H 2O liquid: bonding between molecules Use heat
Directions: T. Trimpe 2005 http://sciencespot.net/
 Candy Compounds Teacher Information I use this activity after we have discussed ionic and covalent bonds to give my students a chance to practice bonding. I walk around the classroom as students work on
Candy Compounds Teacher Information I use this activity after we have discussed ionic and covalent bonds to give my students a chance to practice bonding. I walk around the classroom as students work on
Chapter 2: The Chemical Context of Life
 Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chemistry Workbook 2: Problems For Exam 2
 Chem 1A Dr. White Updated /5/1 1 Chemistry Workbook 2: Problems For Exam 2 Section 2-1: Covalent Bonding 1. On a potential energy diagram, the most stable state has the highest/lowest potential energy.
Chem 1A Dr. White Updated /5/1 1 Chemistry Workbook 2: Problems For Exam 2 Section 2-1: Covalent Bonding 1. On a potential energy diagram, the most stable state has the highest/lowest potential energy.
Intermolecular and Ionic Forces
 Intermolecular and Ionic Forces Introduction: Molecules are attracted to each other in the liquid and solid states by intermolecular, or attractive, forces. These are the attractions that must be overcome
Intermolecular and Ionic Forces Introduction: Molecules are attracted to each other in the liquid and solid states by intermolecular, or attractive, forces. These are the attractions that must be overcome
EXPERIMENT 1: Survival Organic Chemistry: Molecular Models
 EXPERIMENT 1: Survival Organic Chemistry: Molecular Models Introduction: The goal in this laboratory experience is for you to easily and quickly move between empirical formulas, molecular formulas, condensed
EXPERIMENT 1: Survival Organic Chemistry: Molecular Models Introduction: The goal in this laboratory experience is for you to easily and quickly move between empirical formulas, molecular formulas, condensed
3/5/2014. iclicker Participation Question: A. MgS < AlP < NaCl B. MgS < NaCl < AlP C. NaCl < AlP < MgS D. NaCl < MgS < AlP
 Today: Ionic Bonding vs. Covalent Bonding Strengths of Covalent Bonds: Bond Energy Diagrams Bond Polarities: Nonpolar Covalent vs. Polar Covalent vs. Ionic Electronegativity Differences Dipole Moments
Today: Ionic Bonding vs. Covalent Bonding Strengths of Covalent Bonds: Bond Energy Diagrams Bond Polarities: Nonpolar Covalent vs. Polar Covalent vs. Ionic Electronegativity Differences Dipole Moments
Name: Date: Period: Presentation #4. Covalent compounds continued practice with drawing them. Modeling covalent compounds in 3D
 Homework Activities Name: Date: Period: This week we will practice creating covalent compounds through drawings and 3D models. We will also look at polar and non-polar molecules to see how their structures
Homework Activities Name: Date: Period: This week we will practice creating covalent compounds through drawings and 3D models. We will also look at polar and non-polar molecules to see how their structures
DNA Worksheet BIOL 1107L DNA
 Worksheet BIOL 1107L Name Day/Time Refer to Chapter 5 and Chapter 16 (Figs. 16.5, 16.7, 16.8 and figure embedded in text on p. 310) in your textbook, Biology, 9th Ed, for information on and its structure
Worksheet BIOL 1107L Name Day/Time Refer to Chapter 5 and Chapter 16 (Figs. 16.5, 16.7, 16.8 and figure embedded in text on p. 310) in your textbook, Biology, 9th Ed, for information on and its structure
Molecular Geometry & Polarity
 Name AP Chemistry Molecular Geometry & Polarity Molecular Geometry A key to understanding the wide range of physical and chemical properties of substances is recognizing that atoms combine with other atoms
Name AP Chemistry Molecular Geometry & Polarity Molecular Geometry A key to understanding the wide range of physical and chemical properties of substances is recognizing that atoms combine with other atoms
ATOMS AND BONDS. Bonds
 ATOMS AND BONDS Atoms of elements are the simplest units of organization in the natural world. Atoms consist of protons (positive charge), neutrons (neutral charge) and electrons (negative charge). The
ATOMS AND BONDS Atoms of elements are the simplest units of organization in the natural world. Atoms consist of protons (positive charge), neutrons (neutral charge) and electrons (negative charge). The
5. Structure, Geometry, and Polarity of Molecules
 5. Structure, Geometry, and Polarity of Molecules What you will accomplish in this experiment This experiment will give you an opportunity to draw Lewis structures of covalent compounds, then use those
5. Structure, Geometry, and Polarity of Molecules What you will accomplish in this experiment This experiment will give you an opportunity to draw Lewis structures of covalent compounds, then use those
Bonding & Molecular Shape Ron Robertson
 Bonding & Molecular Shape Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\00bondingtrans.doc The Nature of Bonding Types 1. Ionic 2. Covalent 3. Metallic 4. Coordinate covalent Driving
Bonding & Molecular Shape Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\00bondingtrans.doc The Nature of Bonding Types 1. Ionic 2. Covalent 3. Metallic 4. Coordinate covalent Driving
Protein Studies Using CAChe
 Protein Studies Using CAChe Exercise 1 Building the Molecules of Interest, and Using the Protein Data Bank In the CAChe workspace, click File / pen, and navigate to the C:\Program Files\Fujitsu\ CAChe\Fragment
Protein Studies Using CAChe Exercise 1 Building the Molecules of Interest, and Using the Protein Data Bank In the CAChe workspace, click File / pen, and navigate to the C:\Program Files\Fujitsu\ CAChe\Fragment
Chapter 2 Polar Covalent Bonds; Acids and Bases
 John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 2 Polar Covalent Bonds; Acids and Bases Javier E. Horta, M.D., Ph.D. University of Massachusetts Lowell Polar Covalent Bonds: Electronegativity
John E. McMurry http://www.cengage.com/chemistry/mcmurry Chapter 2 Polar Covalent Bonds; Acids and Bases Javier E. Horta, M.D., Ph.D. University of Massachusetts Lowell Polar Covalent Bonds: Electronegativity
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Hydrogen Bonds The electrostatic nature of hydrogen bonds
 Hydrogen Bonds Hydrogen bonds have played an incredibly important role in the history of structural biology. Both the structure of DNA and of protein a-helices and b-sheets were predicted based largely
Hydrogen Bonds Hydrogen bonds have played an incredibly important role in the history of structural biology. Both the structure of DNA and of protein a-helices and b-sheets were predicted based largely
Chapter 8 Concepts of Chemical Bonding
 Chapter 8 Concepts of Chemical Bonding Chemical Bonds Three types: Ionic Electrostatic attraction between ions Covalent Sharing of electrons Metallic Metal atoms bonded to several other atoms Ionic Bonding
Chapter 8 Concepts of Chemical Bonding Chemical Bonds Three types: Ionic Electrostatic attraction between ions Covalent Sharing of electrons Metallic Metal atoms bonded to several other atoms Ionic Bonding
EXPERIMENT # 17 CHEMICAL BONDING AND MOLECULAR POLARITY
 EXPERIMENT # 17 CHEMICAL BONDING AND MOLECULAR POLARITY Purpose: 1. To distinguish between different types of chemical bonds. 2. To predict the polarity of some common molecules from a knowledge of bond
EXPERIMENT # 17 CHEMICAL BONDING AND MOLECULAR POLARITY Purpose: 1. To distinguish between different types of chemical bonds. 2. To predict the polarity of some common molecules from a knowledge of bond
INTERMOLECULAR FORCES
 INTERMOLECULAR FORCES Intermolecular forces- forces of attraction and repulsion between molecules that hold molecules, ions, and atoms together. Intramolecular - forces of chemical bonds within a molecule
INTERMOLECULAR FORCES Intermolecular forces- forces of attraction and repulsion between molecules that hold molecules, ions, and atoms together. Intramolecular - forces of chemical bonds within a molecule
Molecular Geometry and VSEPR We gratefully acknowledge Portland Community College for the use of this experiment.
 Molecular and VSEPR We gratefully acknowledge Portland ommunity ollege for the use of this experiment. Objectives To construct molecular models for covalently bonded atoms in molecules and polyatomic ions
Molecular and VSEPR We gratefully acknowledge Portland ommunity ollege for the use of this experiment. Objectives To construct molecular models for covalently bonded atoms in molecules and polyatomic ions
CHEMISTRY 101 EXAM 3 (FORM B) DR. SIMON NORTH
 1. Is H 3 O + polar or non-polar? (1 point) a) Polar b) Non-polar CHEMISTRY 101 EXAM 3 (FORM B) DR. SIMON NORTH 2. The bond strength is considerably greater in HF than in the other three hydrogen halides
1. Is H 3 O + polar or non-polar? (1 point) a) Polar b) Non-polar CHEMISTRY 101 EXAM 3 (FORM B) DR. SIMON NORTH 2. The bond strength is considerably greater in HF than in the other three hydrogen halides
Click on various options: Publications by Wizard Publications by Design Blank Publication
 Click on various options: Publications by Wizard Publications by Design Blank Publication Select the Blank Publications Tab: Choose a blank full page Click on Create New Page Insert > Page Select the number
Click on various options: Publications by Wizard Publications by Design Blank Publication Select the Blank Publications Tab: Choose a blank full page Click on Create New Page Insert > Page Select the number
Bonding Practice Problems
 NAME 1. When compared to H 2 S, H 2 O has a higher 8. Given the Lewis electron-dot diagram: boiling point because H 2 O contains stronger metallic bonds covalent bonds ionic bonds hydrogen bonds 2. Which
NAME 1. When compared to H 2 S, H 2 O has a higher 8. Given the Lewis electron-dot diagram: boiling point because H 2 O contains stronger metallic bonds covalent bonds ionic bonds hydrogen bonds 2. Which
Self Assessment_Ochem I
 UTID: 2013 Objective Test Section Identify the choice that best completes the statement or answers the question. There is only one correct answer; please carefully bubble your choice on the scantron sheet.
UTID: 2013 Objective Test Section Identify the choice that best completes the statement or answers the question. There is only one correct answer; please carefully bubble your choice on the scantron sheet.
In this example, Mrs. Smith is looking to create graphs that represent the ethnic diversity of the 24 students in her 4 th grade class.
 Creating a Pie Graph Step-by-step directions In this example, Mrs. Smith is looking to create graphs that represent the ethnic diversity of the 24 students in her 4 th grade class. 1. Enter Data A. Open
Creating a Pie Graph Step-by-step directions In this example, Mrs. Smith is looking to create graphs that represent the ethnic diversity of the 24 students in her 4 th grade class. 1. Enter Data A. Open
Elements in the periodic table are indicated by SYMBOLS. To the left of the symbol we find the atomic mass (A) at the upper corner, and the atomic num
 . ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
. ATOMIC STRUCTURE FUNDAMENTALS LEARNING OBJECTIVES To review the basics concepts of atomic structure that have direct relevance to the fundamental concepts of organic chemistry. This material is essential
Excel Tutorial. Bio 150B Excel Tutorial 1
 Bio 15B Excel Tutorial 1 Excel Tutorial As part of your laboratory write-ups and reports during this semester you will be required to collect and present data in an appropriate format. To organize and
Bio 15B Excel Tutorial 1 Excel Tutorial As part of your laboratory write-ups and reports during this semester you will be required to collect and present data in an appropriate format. To organize and
Test Bank - Chapter 4 Multiple Choice
 Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Test Bank - Chapter 4 The questions in the test bank cover the concepts from the lessons in Chapter 4. Select questions from any of the categories that match the content you covered with students. The
Section I Using Jmol as a Computer Visualization Tool
 Section I Using Jmol as a Computer Visualization Tool Jmol is a free open source molecular visualization program used by students, teachers, professors, and scientists to explore protein structures. Section
Section I Using Jmol as a Computer Visualization Tool Jmol is a free open source molecular visualization program used by students, teachers, professors, and scientists to explore protein structures. Section
Chemical Bonding: Covalent Systems Written by Rebecca Sunderman, Ph.D Week 1, Winter 2012, Matter & Motion
 Chemical Bonding: Covalent Systems Written by Rebecca Sunderman, Ph.D Week 1, Winter 2012, Matter & Motion A covalent bond is a bond formed due to a sharing of electrons. Lewis structures provide a description
Chemical Bonding: Covalent Systems Written by Rebecca Sunderman, Ph.D Week 1, Winter 2012, Matter & Motion A covalent bond is a bond formed due to a sharing of electrons. Lewis structures provide a description
Chemistry 1050 Chapter 13 LIQUIDS AND SOLIDS 1. Exercises: 25, 27, 33, 39, 41, 43, 51, 53, 57, 61, 63, 67, 69, 71(a), 73, 75, 79
 Chemistry 1050 Chapter 13 LIQUIDS AND SOLIDS 1 Text: Petrucci, Harwood, Herring 8 th Edition Suggest text problems Review questions: 1, 5!11, 13!17, 19!23 Exercises: 25, 27, 33, 39, 41, 43, 51, 53, 57,
Chemistry 1050 Chapter 13 LIQUIDS AND SOLIDS 1 Text: Petrucci, Harwood, Herring 8 th Edition Suggest text problems Review questions: 1, 5!11, 13!17, 19!23 Exercises: 25, 27, 33, 39, 41, 43, 51, 53, 57,
Description for structure of substances by computer 3D animation in chemical education
 Description for structure of substances by computer 3D animation in chemical education Guo Liping, Liu Xiaoqing, Lei Jiaheng, Cheng Yongxi (Department of Applied Chemistry, Wuhan University of Technology,
Description for structure of substances by computer 3D animation in chemical education Guo Liping, Liu Xiaoqing, Lei Jiaheng, Cheng Yongxi (Department of Applied Chemistry, Wuhan University of Technology,
NaCl Lattice Science Activities
 NaCl Lattice Science Activities STEM: The Science of Salt Using a Salt Lattice Model Teacher Notes Science Activities A Guided-Inquiry Approach Using the 3D Molecular Designs NaCl Lattice Model Classroom
NaCl Lattice Science Activities STEM: The Science of Salt Using a Salt Lattice Model Teacher Notes Science Activities A Guided-Inquiry Approach Using the 3D Molecular Designs NaCl Lattice Model Classroom
To Begin Customize Office
 To Begin Customize Office Each of us needs to set up a work environment that is comfortable and meets our individual needs. As you work with Office 2007, you may choose to modify the options that are available.
To Begin Customize Office Each of us needs to set up a work environment that is comfortable and meets our individual needs. As you work with Office 2007, you may choose to modify the options that are available.
Chapter 5, Lesson 3 Why Does Water Dissolve Salt?
 Chapter 5, Lesson 3 Why Does Water Dissolve Salt? Key Concepts The polarity of water molecules enables water to dissolve many ionically bonded substances. Salt (sodium chloride) is made from positive sodium
Chapter 5, Lesson 3 Why Does Water Dissolve Salt? Key Concepts The polarity of water molecules enables water to dissolve many ionically bonded substances. Salt (sodium chloride) is made from positive sodium
Summary. Load and Open GaussView to start example
 Summary This document describes in great detail how to navigate the Linux Red Hat Terminal to bring up GaussView, use GaussView to create a simple atomic or molecular simulation input file, and then use
Summary This document describes in great detail how to navigate the Linux Red Hat Terminal to bring up GaussView, use GaussView to create a simple atomic or molecular simulation input file, and then use
CHAPTER 6 Chemical Bonding
 CHAPTER 6 Chemical Bonding SECTION 1 Introduction to Chemical Bonding OBJECTIVES 1. Define Chemical bond. 2. Explain why most atoms form chemical bonds. 3. Describe ionic and covalent bonding.. 4. Explain
CHAPTER 6 Chemical Bonding SECTION 1 Introduction to Chemical Bonding OBJECTIVES 1. Define Chemical bond. 2. Explain why most atoms form chemical bonds. 3. Describe ionic and covalent bonding.. 4. Explain
Computer Modeling of Organic Compounds
 Computer Modeling of Organic Compounds Molecular Modeling of Organic Compounds Structure, Energy, and Conformation 1 Models and Modeling Chemists must use models to conceptualize the structures and properties
Computer Modeling of Organic Compounds Molecular Modeling of Organic Compounds Structure, Energy, and Conformation 1 Models and Modeling Chemists must use models to conceptualize the structures and properties
The elements of the second row fulfill the octet rule by sharing eight electrons, thus acquiring the electronic configuration of neon, the noble gas o
 2. VALENT BNDING, TET RULE, PLARITY, AND BASI TYPES F FRMULAS LEARNING BJETIVES To introduce the basic principles of covalent bonding, different types of molecular representations, bond polarity and its
2. VALENT BNDING, TET RULE, PLARITY, AND BASI TYPES F FRMULAS LEARNING BJETIVES To introduce the basic principles of covalent bonding, different types of molecular representations, bond polarity and its
A pure covalent bond is an equal sharing of shared electron pair(s) in a bond. A polar covalent bond is an unequal sharing.
 CHAPTER EIGHT BNDING: GENERAL CNCEPT or Review 1. Electronegativity is the ability of an atom in a molecule to attract electrons to itself. Electronegativity is a bonding term. Electron affinity is the
CHAPTER EIGHT BNDING: GENERAL CNCEPT or Review 1. Electronegativity is the ability of an atom in a molecule to attract electrons to itself. Electronegativity is a bonding term. Electron affinity is the
CHAPTER 10: INTERMOLECULAR FORCES: THE UNIQUENESS OF WATER Problems: 10.2, 10.6,10.15-10.33, 10.35-10.40, 10.56-10.60, 10.101-10.
 CHAPTER 10: INTERMOLECULAR FORCES: THE UNIQUENESS OF WATER Problems: 10.2, 10.6,10.15-10.33, 10.35-10.40, 10.56-10.60, 10.101-10.102 10.1 INTERACTIONS BETWEEN IONS Ion-ion Interactions and Lattice Energy
CHAPTER 10: INTERMOLECULAR FORCES: THE UNIQUENESS OF WATER Problems: 10.2, 10.6,10.15-10.33, 10.35-10.40, 10.56-10.60, 10.101-10.102 10.1 INTERACTIONS BETWEEN IONS Ion-ion Interactions and Lattice Energy
Reading Preview. Key Terms covalent bond molecule double bond triple bond molecular compound polar bond nonpolar bond
 Section 4 4 bjectives After this lesson, students will be able to L.1.4.1 State what holds covalently bonded s together. L.1.4.2 Identify the properties of molecular compounds. L.1.4.3 Explain how unequal
Section 4 4 bjectives After this lesson, students will be able to L.1.4.1 State what holds covalently bonded s together. L.1.4.2 Identify the properties of molecular compounds. L.1.4.3 Explain how unequal
Experiment 13 Molecular Models on a Computer
 Experiment 13 Models on a Computer PRE-LABORATORY QUESTIONS The following preparatory questions should be answered before coming to laboratory. They are intended to introduce you to several ideas that
Experiment 13 Models on a Computer PRE-LABORATORY QUESTIONS The following preparatory questions should be answered before coming to laboratory. They are intended to introduce you to several ideas that
4.5 Physical Properties: Solubility
 4.5 Physical Properties: Solubility When a solid, liquid or gaseous solute is placed in a solvent and it seems to disappear, mix or become part of the solvent, we say that it dissolved. The solute is said
4.5 Physical Properties: Solubility When a solid, liquid or gaseous solute is placed in a solvent and it seems to disappear, mix or become part of the solvent, we say that it dissolved. The solute is said
Using Microsoft Word. Working With Objects
 Using Microsoft Word Many Word documents will require elements that were created in programs other than Word, such as the picture to the right. Nontext elements in a document are referred to as Objects
Using Microsoft Word Many Word documents will require elements that were created in programs other than Word, such as the picture to the right. Nontext elements in a document are referred to as Objects
4/18/2011. 9.8 Substituent Effects in Electrophilic Substitutions. Substituent Effects in Electrophilic Substitutions
 9.8 Substituent effects in the electrophilic substitution of an aromatic ring Substituents affect the reactivity of the aromatic ring Some substituents activate the ring, making it more reactive than benzene
9.8 Substituent effects in the electrophilic substitution of an aromatic ring Substituents affect the reactivity of the aromatic ring Some substituents activate the ring, making it more reactive than benzene
Elements in Biological Molecules
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
3D structure visualization and high quality imaging. Chimera
 3D structure visualization and high quality imaging. Chimera Vincent Zoete 2008 Contact : vincent.zoete@isb sib.ch 1/27 Table of Contents Presentation of Chimera...3 Exercise 1...4 Loading a structure
3D structure visualization and high quality imaging. Chimera Vincent Zoete 2008 Contact : vincent.zoete@isb sib.ch 1/27 Table of Contents Presentation of Chimera...3 Exercise 1...4 Loading a structure
Name Lab #3: Solubility of Organic Compounds Objectives: Introduction: soluble insoluble partially soluble miscible immiscible
 Lab #3: Solubility of rganic Compounds bjectives: - Understanding the relative solubility of organic compounds in various solvents. - Exploration of the effect of polar groups on a nonpolar hydrocarbon
Lab #3: Solubility of rganic Compounds bjectives: - Understanding the relative solubility of organic compounds in various solvents. - Exploration of the effect of polar groups on a nonpolar hydrocarbon
Chapter 10 Molecular Geometry and Chemical Bonding Theory
 Chem 1: Chapter 10 Page 1 Chapter 10 Molecular Geometry and Chemical Bonding Theory I) VSEPR Model Valence-Shell Electron-Pair Repulsion Model A) Model predicts Predicts electron arrangement and molecular
Chem 1: Chapter 10 Page 1 Chapter 10 Molecular Geometry and Chemical Bonding Theory I) VSEPR Model Valence-Shell Electron-Pair Repulsion Model A) Model predicts Predicts electron arrangement and molecular
Lab 3 Organic Molecules of Biological Importance
 Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
TxEIS on Internet Explorer 7
 TxEIS on Internet Explorer 7 General Set Up Recommendations: Several modifications will need to be made to the computer settings in Internet Explorer to ensure TxEIS runs smoothly, reports pop up as desired,
TxEIS on Internet Explorer 7 General Set Up Recommendations: Several modifications will need to be made to the computer settings in Internet Explorer to ensure TxEIS runs smoothly, reports pop up as desired,
3D-GIS in the Cloud USER MANUAL. August, 2014
 3D-GIS in the Cloud USER MANUAL August, 2014 3D GIS in the Cloud User Manual August, 2014 Table of Contents 1. Quick Reference: Navigating and Exploring in the 3D GIS in the Cloud... 2 1.1 Using the Mouse...
3D-GIS in the Cloud USER MANUAL August, 2014 3D GIS in the Cloud User Manual August, 2014 Table of Contents 1. Quick Reference: Navigating and Exploring in the 3D GIS in the Cloud... 2 1.1 Using the Mouse...
In the box below, draw the Lewis electron-dot structure for the compound formed from magnesium and oxygen. [Include any charges or partial charges.
 Name: 1) Which molecule is nonpolar and has a symmetrical shape? A) NH3 B) H2O C) HCl D) CH4 7222-1 - Page 1 2) When ammonium chloride crystals are dissolved in water, the temperature of the water decreases.
Name: 1) Which molecule is nonpolar and has a symmetrical shape? A) NH3 B) H2O C) HCl D) CH4 7222-1 - Page 1 2) When ammonium chloride crystals are dissolved in water, the temperature of the water decreases.
Chapter 13 - LIQUIDS AND SOLIDS
 Chapter 13 - LIQUIDS AND SOLIDS Problems to try at end of chapter: Answers in Appendix I: 1,3,5,7b,9b,15,17,23,25,29,31,33,45,49,51,53,61 13.1 Properties of Liquids 1. Liquids take the shape of their container,
Chapter 13 - LIQUIDS AND SOLIDS Problems to try at end of chapter: Answers in Appendix I: 1,3,5,7b,9b,15,17,23,25,29,31,33,45,49,51,53,61 13.1 Properties of Liquids 1. Liquids take the shape of their container,
POLAR COVALENT BONDS Ionic compounds form repeating. Covalent compounds form distinct. Consider adding to NaCl(s) vs. H 2 O(s):
 POLAR COVALENT BONDS Ionic compounds form repeating. Covalent compounds form distinct. Consider adding to NaCl(s) vs. H 2 O(s): Sometimes when atoms of two different elements form a bond by sharing an
POLAR COVALENT BONDS Ionic compounds form repeating. Covalent compounds form distinct. Consider adding to NaCl(s) vs. H 2 O(s): Sometimes when atoms of two different elements form a bond by sharing an
DCI for Electronegativity. Data Table:
 DCI for Electronegativity Data Table: Substance Ionic/covalent EN value EN Value EN NaCl ionic (Na) 0.9 (Cl) 3.0 2.1 KBr (K) 0.8 (Br) 2.8 MgO (Mg) 1.2 (O) 3.5 HCl (H) 2.1 (Cl) 3.0 HF (H) 2.1 (F) 4.0 Cl
DCI for Electronegativity Data Table: Substance Ionic/covalent EN value EN Value EN NaCl ionic (Na) 0.9 (Cl) 3.0 2.1 KBr (K) 0.8 (Br) 2.8 MgO (Mg) 1.2 (O) 3.5 HCl (H) 2.1 (Cl) 3.0 HF (H) 2.1 (F) 4.0 Cl
Chapter 2 The Chemical Context of Life
 Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Metatrader 4 Tutorial
 Metatrader 4 Tutorial Thank you for your interest in Best Metatrader Broker! This tutorial goes in detail about how to install and trade with your new Metatrader Forex trading platform. With Best Metatrader
Metatrader 4 Tutorial Thank you for your interest in Best Metatrader Broker! This tutorial goes in detail about how to install and trade with your new Metatrader Forex trading platform. With Best Metatrader
1. Right click using your mouse on the desktop and select New Shortcut.
 offers 3 login page styles: Standard Login, List Login or Quick Time Punch. Each login page can be saved as a shortcut to your desktop or as a bookmark for easy fast login access. For quicker access to
offers 3 login page styles: Standard Login, List Login or Quick Time Punch. Each login page can be saved as a shortcut to your desktop or as a bookmark for easy fast login access. For quicker access to
NNIN Nanotechnology Education
 NNIN Nanotechnology Education Teacher s Preparatory Guide Lesson #1 Polarity and Solubility of Molecules This is a series of four lessons which build upon each other to explore the use of nanotechnology
NNIN Nanotechnology Education Teacher s Preparatory Guide Lesson #1 Polarity and Solubility of Molecules This is a series of four lessons which build upon each other to explore the use of nanotechnology
Chapter 4 Lecture Notes
 Chapter 4 Lecture Notes Chapter 4 Educational Goals 1. Given the formula of a molecule, the student will be able to draw the line-bond (Lewis) structure. 2. Understand and construct condensed structural
Chapter 4 Lecture Notes Chapter 4 Educational Goals 1. Given the formula of a molecule, the student will be able to draw the line-bond (Lewis) structure. 2. Understand and construct condensed structural
PROTONS AND ELECTRONS
 reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
reflect Imagine that you have a bowl of oranges, bananas, pineapples, berries, pears, and watermelon. How do you identify each piece of fruit? Most likely, you are familiar with the characteristics of
Non-Covalent Bonds (Weak Bond)
 Non-Covalent Bonds (Weak Bond) Weak bonds are those forces of attraction that, in biological situations, do not take a large amount of energy to break. For example, hydrogen bonds are broken by energies
Non-Covalent Bonds (Weak Bond) Weak bonds are those forces of attraction that, in biological situations, do not take a large amount of energy to break. For example, hydrogen bonds are broken by energies
Chapter 5 Student Reading
 Chapter 5 Student Reading THE POLARITY OF THE WATER MOLECULE Wonderful water Water is an amazing substance. We drink it, cook and wash with it, swim and play in it, and use it for lots of other purposes.
Chapter 5 Student Reading THE POLARITY OF THE WATER MOLECULE Wonderful water Water is an amazing substance. We drink it, cook and wash with it, swim and play in it, and use it for lots of other purposes.
Choosing your Preferred Colours in Windows
 Choosing your Preferred Colours in Windows Some people will occasionally find certain text and background combinations difficult to read, while others prefer to always have a certain colour combination
Choosing your Preferred Colours in Windows Some people will occasionally find certain text and background combinations difficult to read, while others prefer to always have a certain colour combination
KEY for Unit 1 Your Chemical Toolbox: Scientific Concepts, Fundamentals of Typical Calculations, the Atom and Much More
 KEY for Unit 1 Your Chemical Toolbox: Scientific Concepts, Fundamentals of Typical Calculations, the Atom and Much More The Modern Periodic Table The Periodic Law - when elements are arranged according
KEY for Unit 1 Your Chemical Toolbox: Scientific Concepts, Fundamentals of Typical Calculations, the Atom and Much More The Modern Periodic Table The Periodic Law - when elements are arranged according
FOR TEACHERS ONLY. The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION PHYSICAL SETTING/CHEMISTRY
 FOR TEACHERS ONLY PS CH The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION PHYSICAL SETTING/CHEMISTRY Wednesday, January 29, 2003 9:15 a.m. to 12:15 p.m., only SCORING KEY AND RATING
FOR TEACHERS ONLY PS CH The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION PHYSICAL SETTING/CHEMISTRY Wednesday, January 29, 2003 9:15 a.m. to 12:15 p.m., only SCORING KEY AND RATING
Where Is My Lone Pair?
 Where Is My Lone Pair? Goal: In this tutorial we'll learn how to determine which orbital contains a lone pair. This is important for resonance, conjugation, and aromaticity. To master this subject you'll
Where Is My Lone Pair? Goal: In this tutorial we'll learn how to determine which orbital contains a lone pair. This is important for resonance, conjugation, and aromaticity. To master this subject you'll
Chapter 17. How are acids different from bases? Acid Physical properties. Base. Explaining the difference in properties of acids and bases
 Chapter 17 Acids and Bases How are acids different from bases? Acid Physical properties Base Physical properties Tastes sour Tastes bitter Feels slippery or slimy Chemical properties Chemical properties
Chapter 17 Acids and Bases How are acids different from bases? Acid Physical properties Base Physical properties Tastes sour Tastes bitter Feels slippery or slimy Chemical properties Chemical properties
Geometries and Valence Bond Theory Worksheet
 Geometries and Valence Bond Theory Worksheet Also do Chapter 10 textbook problems: 33, 35, 47, 49, 51, 55, 57, 61, 63, 67, 83, 87. 1. Fill in the tables below for each of the species shown. a) CCl 2 2
Geometries and Valence Bond Theory Worksheet Also do Chapter 10 textbook problems: 33, 35, 47, 49, 51, 55, 57, 61, 63, 67, 83, 87. 1. Fill in the tables below for each of the species shown. a) CCl 2 2
Scanned image. If multiple scanner installed in the computer then click here to select desired scanner. Select Resolution, Color, and Scan Type.
 Objectives & Goals Scanning & Document Management Opening & Understanding Multi Scan/Select Scanning & Drag/Drop Documents Set Document Details Set Reminders, Actions and Links Create New Document From
Objectives & Goals Scanning & Document Management Opening & Understanding Multi Scan/Select Scanning & Drag/Drop Documents Set Document Details Set Reminders, Actions and Links Create New Document From
Micro Cam Software. User Manual V1.3
 Micro Cam Software User Manual V1.3 CONTENT CHAPTER 1: MICRO CAM SOFTWARE INSTALLATION AND CONNECTION... - 1-1.1 SOFTWARE MICRO CAM INSTALLATION... - 1-1.2 WIRED DEVICE CONNECTION... - 4-1.3 SOFTWARE OPERATION
Micro Cam Software User Manual V1.3 CONTENT CHAPTER 1: MICRO CAM SOFTWARE INSTALLATION AND CONNECTION... - 1-1.1 SOFTWARE MICRO CAM INSTALLATION... - 1-1.2 WIRED DEVICE CONNECTION... - 4-1.3 SOFTWARE OPERATION
States of Matter CHAPTER 10 REVIEW SECTION 1. Name Date Class. Answer the following questions in the space provided.
 CHAPTER 10 REVIEW States of Matter SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Identify whether the descriptions below describe an ideal gas or a real gas. ideal gas
CHAPTER 10 REVIEW States of Matter SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Identify whether the descriptions below describe an ideal gas or a real gas. ideal gas
WebEx Sharing Resources
 WebEx Sharing Resources OTS PUBLICATION: WX0 REVISED: 4/8/06 04 TOWSON UNIVERSITY OFFICE OF TECHNOLOGY SERVICES =Shortcut =Advice =Caution Introduction During a WebEx session, the host has the ability
WebEx Sharing Resources OTS PUBLICATION: WX0 REVISED: 4/8/06 04 TOWSON UNIVERSITY OFFICE OF TECHNOLOGY SERVICES =Shortcut =Advice =Caution Introduction During a WebEx session, the host has the ability
Chapter 6 An Overview of Organic Reactions
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 6 An Overview of Organic Reactions Why this chapter? To understand organic and/or biochemistry, it is necessary to know: -What occurs -Why and
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 6 An Overview of Organic Reactions Why this chapter? To understand organic and/or biochemistry, it is necessary to know: -What occurs -Why and
Chapter 7. Comparing Ionic and Covalent Bonds. Ionic Bonds. Types of Bonds. Quick Review of Bond Types. Covalent Bonds
 Comparing Ionic and Covalent Bonds Chapter 7 Covalent Bonds and Molecular Structure Intermolecular forces (much weaker than bonds) must be broken Ionic bonds must be broken 1 Ionic Bonds Covalent Bonds
Comparing Ionic and Covalent Bonds Chapter 7 Covalent Bonds and Molecular Structure Intermolecular forces (much weaker than bonds) must be broken Ionic bonds must be broken 1 Ionic Bonds Covalent Bonds
User Tutorial on Changing Frame Size, Window Size, and Screen Resolution for The Original Version of The Cancer-Rates.Info/NJ Application
 User Tutorial on Changing Frame Size, Window Size, and Screen Resolution for The Original Version of The Cancer-Rates.Info/NJ Application Introduction The original version of Cancer-Rates.Info/NJ, like
User Tutorial on Changing Frame Size, Window Size, and Screen Resolution for The Original Version of The Cancer-Rates.Info/NJ Application Introduction The original version of Cancer-Rates.Info/NJ, like
Use the Force! Noncovalent Molecular Forces
 Use the Force! Noncovalent Molecular Forces Not quite the type of Force we re talking about Before we talk about noncovalent molecular forces, let s talk very briefly about covalent bonds. The Illustrated
Use the Force! Noncovalent Molecular Forces Not quite the type of Force we re talking about Before we talk about noncovalent molecular forces, let s talk very briefly about covalent bonds. The Illustrated
AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts
 AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts 8.1 Types of Chemical Bonds A. Ionic Bonding 1. Electrons are transferred 2. Metals react with nonmetals 3. Ions paired have lower energy
AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts 8.1 Types of Chemical Bonds A. Ionic Bonding 1. Electrons are transferred 2. Metals react with nonmetals 3. Ions paired have lower energy
CHAPTER 10 THE SHAPES OF MOLECULES
 ATER 10 TE AE MLEULE 10.1 To be the central atom in a compound, the atom must be able to simultaneously bond to at least two other atoms. e,, and cannot serve as central atoms in a Lewis structure. elium
ATER 10 TE AE MLEULE 10.1 To be the central atom in a compound, the atom must be able to simultaneously bond to at least two other atoms. e,, and cannot serve as central atoms in a Lewis structure. elium
ChemPad3. a tutorial. Ben Shine and Dana Tenneson. May 21, 2008
 ChemPad3 a tutorial Ben Shine and Dana Tenneson May 21, 2008 1 Welcome to ChemPad! ChemPad is a Tablet PC application for students learning introductory organic chemistry. ChemPad allows students to draw
ChemPad3 a tutorial Ben Shine and Dana Tenneson May 21, 2008 1 Welcome to ChemPad! ChemPad is a Tablet PC application for students learning introductory organic chemistry. ChemPad allows students to draw
