!" # $ % & ' ( ) * %!" ( %+..,..!" -!", - ( )!%. %# - ( % % 2008
|
|
|
- Kelly Riley
- 7 years ago
- Views:
Transcription
1 ..,
2 :543.42(075.8) : : /..,... : - -, ISBN () - (), ,, -, :543.42(075.8) : ,.. ISBN ,.., 2008, 2008.,
3
4 , -,. -,,,, -,. -, -,,., -, -. - ().. -,. 4
5 -. -, ,, ,., -., , ( ).,,..,,, , - -, ,.. -,. «-» , :. 5
6 ,, «-».., -, : -,,, -, -,, XX - :, -, ,,,,, ,. -,, , -,. 6
7 (,,, ). - (,,.), -.. -,, -.,,,,,. (,,..). (),. (SiO 2 ), (Al 2 O 3 ), (, CaCO 3 ), - (MgO).,,. -,,.. -,, -,. -,. : 1.,, ,, -, -. -,. - ( ) ( 7
8 ). () :,, , ,,,,,,,. -.,., ,, -,, ()., -., 2 %. - 2,4 2,8 % 4,5 5,0 %,.., -., -. -., Si, S, Mn, Cr, P, Cu. 8
9 ,., FeCO 3. - :,, -, % Fe () Fe 3 O 4 () % Fe - Fe 3 O 3 () % Fe 2Fe 2 O 3 3H 2 O () Fe 2 O 3 H 2 O () % Fe FeCO 3 () % Mn () -.. -,,...., 2 %.. -,, - -, , -.,,,.,,.. -., -,. - ( < 0,3 %), ( = 0,3 0,7 %) - ( > 0,7 %)., 1, 2, 3.., 08, 15, 20,
10 ,, -. - ; ; , : 2, :,,., 3, 3, 3.. :, , 2, 3 4 -, ,,,,,, (,, -, -, ). -.,., - ( 1 %) (08, 20, 75), 2 (30, 65). 0,66 1,34 % 0,58 %. ( - ). - :,, :, 7-0,7 %, 13 1,3 %,. 7, 8, 8,,,,,,. (10, 11, 10, 12, 13),, -,, ( ),. - () 0,25 % (11, 22, 43.), (12, 20, 40, 30), 0,2 %. :, 10
11 , -.,, 0,25 0,45 %. -,. - 2,5 %, 2,5 10 %, 10 %. -,,,,,,,. -,. - 0,5 % ,,,. - :, ( ) : 1. 15, 15, 20, 30, 30, 35, 38, 40, 45, 50 0,12...0,54 %, 0,17...0,37 %, 0,40...0,80 %, 0,70...1,30 % , 20, 25, 30, 35, 40, 102, 352, ,12...0,56 %, 0,17...0,37 %, 0,70...1,80 % , 18, 27, 30, 35, 25. 0,15...0,45 %, 0,17...0,37 %, 0,70...1,25 %, 0,40...1,30 % , 38, 40-0,29...0,45 %, 1,00...1,60 %, 0,30...0,60 %, 1,30...1,60 % , 20, 30, ,11...0,44 %, 0,30...0,70 %, 0,80...2,70 %, 0,15...0,55 %. 11
12 ,12...0,18 % 0,37...0,44 %, 0,17...0,37 %, 0,40...0,80 %, 0,80...1,10 % 0,06...0,12 % 0,10...0,18 % , 202-0,10...0,18 % 0,17...0,25 %, 0,17...0,37 %, 0,40...0,70 %, 1,50...1,90 %, 0,20...0,30 % , 40, 45, 50, 1224, ,09...0,45 %, 0,17...0,37 %, 0,30...0,80 %, 0,40...1,65 %, 0,80...3,65 % , 30, 35, ,17...0,39 %, 0,90...1,40 %, - 0,80...1,30 %, 0,80...1,40 %, 302 1,40...1,80 %.,,,,,, -,,,,, -,,. - 0,40 1,8 %, 1,0 %. 2,2 % 5 %, - 0,7 1,12 %, 2,5 18,5 %, 4,8 8,5 % 3,0 4,4 %; - 1,0 %, % 0,4 29 %;, 0,15 %, 7 %, %, 3,5 5,8 % % ; - 13 %, 20,0 %, 0,15 % 0,25 %. : (7, 9, 9, 9, 95, 65, 9, 12, 18, 63, 95, 910, 1852.), ; - (6, 91, 121,, 328, 482, 5, 4, 42, 663, ). 12
13 : 8,5 29 %, 0,15 6,2 %, 1,8 6,0 % 6 20 % (60, 55, 60, 70.); 14,0 23,0 %, 2,8 6,0 %, 0,25 3,2 %, 30,0 39, 0 % (35, 0628.); 33,5 80,0 %, 0,15 1,5 %, 0,3 1,1 % (50, 34, 50, 80.).,, -. -, - :,,,,,,,,,, -,,,,.,, -., - 1,5 %., c..,,, -..., - Fe 3 C ( ), ( ).., -,,., -. ( ), ( ),.,
14 () / 2 (10, 15, 21, 30.). -,,, -,,,.. -,.. (40, 50.) ,,,,, -.,,,. : - (), - (%) ( 30-6, 35-10, 45-7, 55-4.).,,,,,, -, (%) 5 : %; 99,0 99,90 %; 99,90 99,99 %; 99,99 99,999 %; 99,999 99,9999 %.,,, -,,,,,,,,, -,,,,,,,,,. 14
15 . - = 8,96 / ,,. -,,. : 00 (99,99 % Cu), 0 (99,95 % Cu), 1 (99,9 % Cu). :. -,,,,, -. -, : 96, 70, 68, 63, 62-1, 403, 303. :,,,,,,. -,.,,, -. -,, -. ( %) -,,,,,. (-,,,,, ).. : 5, 7, 9-2; 10-2, 9-4; , ,5 11 %. -, -,,. : 2; 1,7; 1,9 1,60 1,85 1,85 2,1 %. (0,2 0,5 %). -,,,,,. ( ) (5.). -,, -,,. 15
16 ,, - 7 % : 6,5 0,4; 6,5 0,15; 4 0,2; ,5; 8,0-0,30; 7-0,2; ,, -,, -. ( %). - (19, 30-0,1-1.), (15-20.), (13-3, 6-1,5). -,,, -., -,,. -,,,., , - ( = 2,7 / 3 ), -,,, (99,999 % Al), 995 (99,995 % Al), 99 (99,990 % Al), 97 (99,970 % Al), 95 (99,95 Al) 85 (99,85 % Al), 8 (99,80 % Al), 0 (99 % Al). -,. -,, -..,.,. 37 : 8, 13, 2, 9 (4), 19, 57 (10), 11, 25, 30.,. -,,.. 16
17 : 1, 16, 3,8 4,9 % ( ), ; 4-1, 6, 8., -,, ;,,, -,.,, -, (95, 65,, 1,.). -,,,,.. () 97., 450,, -. -,,., (,,..) = 1,7 / ,,, : 1) 3 Mg Al Zn Mn; 2) () () 5, 4, 5, 5, 6 Mg Al Zn Mn; 8 Mg Zn Zr Cd; 12 Mg Zn Zr; 15 Mg Zn Zr La; 3) 9, 10, : 1) 1 8; 2) 2, 2 1, 17.; 3) 5,
18 - = 4,5 / , -.,.. (Ti) -.,. 882, , , ,. - -,,,. -,,. -,. 6, 14.,,.,, -. 5, 3-1, 20, 21. = 8,9 / 3 - = , -.,, -,, ,5-1,5 (), -. 9 % 9,5 ( ). 20 % (2080-) (1560). 18
19 (78, 77.), -, , (,,,,, ).. 2 : 99,9 ( 99,90 %) 99,8 ( 99,80 %) 99,8 ( 99,80 %).,. 3 99,93, 99,9 99,8 (, 99,93, 99,90 99,80 % - ) 24. : 2 (99,9 99,8 99,9 99,8 % ) (999,9 999) 37.,,,, ,,.,,,..,, -,,.,,, -. -,.,,.. - : -, - 19
20 (), - -, -,. :, ; ; ; ;. :, - ;., -, -.,. -,, -. -,,. : - (1 5), (,, -2, -.), (-1-2), (1, 16.), (, 0, 3.), (65,, , 948.), -, (-1, -2, -3, -4, -1, -2.),, (6, 8, 15, 8-, 20-, 712, 304, 108-.). 1.2., -,. :,,,,, -,, -. :, -,,,,,,,. 20
21 -. -,,. :, -,,, -,,. -.,, -.. -, ().. -,... ( ). -,,,, -, ,,.,., 1 2, 1-1.,
22 , : -,,,,,,,. - (b) ( m ). () -. m, -. M mhm. (1) b H M, (2) m b. (, ) b Hm, 1 -. (, - ) -, -. - m ( - ).. b r,, - s. -, - 0 max. b. 22
23 - ( - ).. -, ,, -, -, , -.,, , ( ), -. -,.,,., -, ( )
24 -,, - (,, - ).. :., -,.,, ,., (, -, )..,. 1.? 2.? 3.? 4.? 24
25 2.,..,.,., -,...,,,.,,. -. -, :,.. - ( ) -. -,, , , - -..,. -,. 25
26 ,, -.,. -, -, -. (- )., (, - ). -. ( ). -,.,.. -,. -,,,,, -,. ( ),,,,. -.,.. -..,. -, -.,, -., 26
27 , -.,,, , (. 1) : (, );. -., - -.,. -, (),,.. 27
28 ,, ,,,. - -,, -.., : (- -,.. - ). 3.,, -, ,. - -.,, -.,. -., -,,,.. :, -,,,, -. 28
29 , -.,,, -..,, % % %. - ( ). -, -,, -. -.,, %., -,. -,, %. -.,, -., -,,. - :,, ;. - -.,. - 29
30 . -. (0,1 1 %) -, -,,.,, ,,. -.,. VIII. : 1. : 1/ (1 ) e. (, ). 1/16 16 ( 1.. ). 1 = 1, , 1... = 1, = 1, , (, 2 18 = ) N = /. - ( ), , - 18., : m ,,,. 30
31 : = 1 / 3, 1 - = 18 /, /. : /., V0 310., ,, -. ( - ),,., ( )., -, 10,.. 10.,,,...., -, , 16., -, Z.., -, («-» ). -.,. -,. : = 19 / 3, = 238, 4,310 8, , V : = 0,97 / 3, = 23, 6,310 8, V 3 231, , ,97 31
32 , -. = 4, CGSE, m = 9, ,, ,,, ,.,...., ,, - (). -. m 0 - r. :,. -.,. - n, 1, 2, 3 n = 1,,. - n., - 32
33 -. n.. 1- (n = 1) -; 2- (n = 2) L-; 3- (n = 3) M-; 4- (n = 4) N-; 5- (n = 5) O-; 6- (n = 6) P-.,. n- E n me z, (3) 2 2 nh 2 2 nh rn. (4) me z - l, 0 n 1 n1; l 0; n 2; l 0; l 1; n 3; l 0; l 1; l 2;.. l -.,,. l = 0 s-; l = 1 p-; l = 2 d-; l = 3 f-..,, n = 3 l = 1 3.,. 2. l. 33
34 . 2., n + l -., - n. : 4s(n + l = = 4), 3d, 3d(n + l = = 5), 4, 4(n + l = = 5), 3d,.. n = :. - s, 1 1 : s s. 2 2., nl. n, l, s.,,.. l.,,. -. m, +l l., l = 2, m = +2; +1; 0; 1; 2. -, l. 34
35 ,., -, (n), - (l) (m), 2-,.. : z = 1., 1, n = 1 (-). l = 0,.. -, m = s : s s , : z = L l 0 m 0 s ; ; (2 S) ; ; n = 2 l m s (2 p) 6 (2 ) m0 s ; ; (2 ) m1 s ;. (2 ) n = l 0 m 0 s ; 2 (3 S) l 1 m 12 (3 p) 1 m 02 6 m 12 35
36 l 2m 22 m 12 m m 12 m K 8 L. 3. -,,, ( ).., -.,, - 82., (- ), -. -, 82 ( 82 ).., Al, ,, M
37 .. - (- ). -,.., (. 4).. 4. ;,, -,,, (),.,. - ( ) ( ),. -. -, -.,, -,,. -.,,., -., 37
38 (). -, , -,., -,. 4s - 3p 3d - 3p 5s - 3p 4d - 3p 5d - 3p n=3 n=4 n=5 n=6 E() s 5s 4s 3961,5 3944,1 2660,4 3961,5 6p 5p 4p 2652,5 3944,1 3092,8 3082,1 2373,1 2575,1 5d 4d 3d 2373,4 3092,8 2367,1 2575,4 2568,0 3082,2 2660,4 2652,5 2575,1 2575,4 2568,0 2373,4 2367,1 5f 4f 0 3p. 5.,. 5.,. 4s., -, 3,
39 3944,1 Å 3961,5 Å. -, 3,1,.,..,, = 2652,5 Å 2660,4 Å, 5s, 4,7. -., -.,, -..,., l. s-, - s b.., 4 3.,, -... : 3961,5 Å, 3944,1 Å ( 4s3), 3092,7 Å 3082,2 Å ( 3d3)., -,....., -., -, - ()., , -. 3,9,, 24,6,. 39
40 (), ().,... n. l, 3s, 3p 3d,. 21.., d- f-.., : s-, d- f ,,, -. ( ) -.,. ( ) :. - 40
41 , - :,. sini n. (1) sin r ( ), ( - ). -,, -. -,, -. -.,, -,. -,, ,.. -, -...,, -. -.,,.,. -., -, (), -.,, (). 1. (2) T,. -, () -,..,. 41
42 1 4,, T,.. -,,.,. 360, 2 -,., -, 0, 1 T = 90, /2, 1 T 4 2 = 180,,..,,. 0,,, 180,.,, -, / = /. -, -,. ( ) ( )., ( ),. c ct. (3) ( ) -.,,,,,,., : 1,33, -, n = 1,33. -,,. - 42
43 .. -,., ,,. -.. : 1. -, ,. - ( ). -,. -.,.,. -. E h, (4) h = 6, ; (). 43
44 0, 0. (. 6). x 0 X. 6. J N. J N h. (5) -. -,, -. -., -. -,. :,,., -. -,, (). 1,, 1. c. (6) (Å), - (), (), (). : 44
45 c10 ; ; ,7 ; ; 1 h ; (7) h 4, , ,6210 ; ; , ; , ; , ,. ( ). (7)., h, (8),.. 1 ; (9) h h E P. (10) c c h. (11) P m, P m ( c )., h, (12) m = 2, /., m 0 = 9, E ev
46 2 m 2 0 E ev. 2 m0 Pm 2m E, 0 0 h h h Å. 2mE 0 2meV 0 2me 0 V V.. V 150 ( Å). V ,5 10 1,2210 Å 0,122 Å 1,2210 1, : 7 / = /, 11 / = / Pm / h 6, P 6, ,, -. -,, , -...,,. -.,,.. -, 46
47 ,. -,. -.. :, ( - ) 0,75 (7 500 Å). ( < 25) ( > 25). -, Å.,,., -.,,.. - : (4 000 Å Å), (2 300 Å Å) - (1 850 Å 50 Å),.,, -, -. -, -. 47
48 () () - () () () (Å) 1 = 10 6 = 10 9 = Å 1 = 10 6 = 10 3 = 10 4 Å 1 = 10 9 = 10 3 = 10 Å 1 Å =10 10 = 10 4 = 10 1 c ; hc ; ( Å) ; E E () 8 10 ( Å) ; 1 ( ) ; () 1 ( ) () -1 () 1 = 10 6 c ; ; c h 48
49 . 1 ( -1 ) ( 1 ) 1 1 = = ; hc 1 ( ) 8066,0 (); 1 ; ( ) ; () Å () () () 1 = 10 7 = 6, = 10 7 = 6, = 1, = = 1, h ; hc E ; , 2397 E() ( ) () ; h ; 1 ( ) E() 8066, ? 4., -? 5.? 49
50 ,., -., -,,... -.,,,,. -. ( )., -.., -.,,.,..,, - 4,9,,, 4,9., 4,9. = 2536,5 Å. 50
51 ,, -. 10,4 ( 10,4 ), , -. -, , E kt, (13) k = 1, / = 8, / ;. ( = 300 ): 5 3 E 8, ,026.,, -, -. - (13). -,. -,
52 N(V) N max Vmax V(E). 8. : N,, V,,. - 0, ,0005..,,. = 2000 ( 0, 2 ), -,, - : 2,1., (4,9 Hg, 6,2 N 2 ). = ,, 5,1. 10,4, - Hg ,, -. E E g E g N AN kt 0 e AN0 e, (14) g0 g0 N 0 ; N, - ; g g 0, -, ; ; A. 52
53 -.., -. (14), J.,. : 0 E kt g J AN 0 e h. (15) g -, - : E J AC e kt, (16) N 0 -. (16), -.,, , ,, , -.. ()
54 R : A2 E 1 kt J A Ce R Be J E 2 Ce kt 54 E E kt 2 1, (17) A1 B. A 2 2 1, ( ), ,. E kt B 1 E 2 C2 A kt 2C2e J A C e C R J. (18) -,,. -. ( - ), -., , -., -.
55 , (16),.,. - (18).. - (. 9)., -..,,,,. -, -., b J a C, (19) ;, E, a A e kt ; b. 0 JJ I II II III III b=1b 1> b1>b>0 >0 b=0b C 55 I- I ( ( ) ) II- J J ( ( ) III-. 9. ( ) J A C e kt., - b. - E
56 , -. lg J lg ab lg C. (20),, b = 1. b. -, - (. 10). lgj lgj lga lga lgc lgc ,. -.,,. -.,. -, 0,1 Å
57 (. 11). -., -,, -. -,. J C C C ,,. -,,,,,,... -,. -,.,., , 57
58 .. -.,,. -,, , -, -,, -, -, -. -,. -.,.. -,, ,, ,. 58
59 , -.,, 100.,, -, : P iu, (21) i ; U. -,..,. -,.. -.,,,, :, , ,,,.,. 59
60 ,,, , -,. - -.,, ,.. -,.,.,,, (. 13) ,,,. -, 60
61 , -., -. -, (. 14). R. 14..,. -. -,. -,..., -.,,.,,, -. -, -,., ,,,... 61
62 -. -.,., -. -,,, -,.,..,... ( - ), , ,
63 .,, -.., -., , (. 15). -,. -..,, - : Q CU. (22) - t, Q it, (23) i, ; t,. -.,., -. (. 16, ) -..,.. R. 16.,,, 63
64 (. 16, )., -. - (. 16, ), -., : T 2 LC, (24) 1 1, (25) T 2 LC, ;, ; L, ;, (. 17), -,., -..,, 10 4.,, -.,. -., - 64
65 . ( 200 ). -.,,. (- ), -.,. - -., -. ( ),.,. ( ), :,, , -,, ,
66 ~ R v~ V. 18., 40, 5 6., ,,,, -., ( ). -. :, -, 1.,, 2-20 ( ). - -,..,,.,., - -.,.,. 18, 66
67 ., -. 50, 100.,,.,.,, -, (- ).,.. 100, -.,. T 2 ~ R=400 R=40 0,5 C 1 1 L L 2 1 T1 C , -.. (. 19) , -., 1. -, L 2., 1. -, L
68 1. R c 2 C , -.,.. 1/50 (. 20). B, +300 I II III IV V 0, ( I) II -... III,.. III., -. IV,., 68
69 .,,,,., , -,,. -,., , ,, -. 1.? 2.?
70 ,, n. -.,, -.,.., - (. 22, ) > 2> 3 1> , : ;, - (. 22, ) , -.,,,., ;, -,,, -,. 2 70
71 . 23. (, )...,,. -,. i1 r2, r1 i2,.. (. 23, ).. -,.. ( 1 ), ( 2 ) (. 23, ).,,, ( ), -.,,., - 71
72 .,. 2sin 2 n. (26) n sin 2. -,., Å,., - - -, A Å : -,,,.. -,.., Å. -,. - ( 1,9) -
73 ,, (),. -,. -.,. -,.,,. -. (CaF 2 ) (LiF) Å Å, -..,.., 3,. 5,5,. ( 25 ).,..,., 60. -, ( ) -,. 30, ( ). -., -,,. : 30 -,, 73
74 (. 25, )., 60 -., : ; ; ;, , , b ( )
75 . - ( 180 ).. b B A. 26., -., -,. (.. ). -. AB m,, -, m ; AB bsin, bsin m. (27),, -,,,.. -,.,
76 . -,, (27). m. m = 0,, ( 0), -. m = 1,,..,,, -. m = , Å -, Å. -. -,, -. -, -..,. : m. (28) b cos m -, cos,. -,, -,., / /. -,.,.,. 76
77 4.3., -,. -,.,, (. 27).,,,.,., -, ,... -.,,, -. -., -., -.,. - -,,. 77
78 ( );, -,. - d.,,,, -. d, f 1. f1,, -,.,. - (. 28) , -,,.,,,. -,. -., 90,,. 78
79 ,,, -. f 2 ; f 2 -.,.,,. -, -, -., : , , -, , Å. -..,,
80 2.. - l, -, -.,,. l f 2 sin. (29), - sin, l f2. (30),. f l 2. (31) sin -, - : dl f2 d. (32) d sin d - ( ),, -. f 2 = 1,5 5, 13 Å/.,., - : dl l. (33) d f 2 f 1. 80
81 f g. (34) f g = : S 2 1 g a, (35)., S., ,. 2 1.,., S dl ga dl d d. (36) 5. -.,,, ,,., -. -.,,. 0, (37) d d., f S 2 f2 d, (38),. 81
82 ,.. ga f2. (39) d f.. g 2, f 1 f a 1. (40) d,,., -,. -,,, (38). 4.5.,. -, - -, (. 29) -, Cr, Ni, W, V, Zn, Fe, Pb, Sn, Al, Cu, Mg, Mo, Mn, Si -.,,.,,, Cr, W, Mn, V, Mo, Ni, Co, Ti, Al, Nb, Zr, Si, Cu ; Zn, Ni, Mn, Fe, Pb, Sn, Al, Be, Si ; Mg, Cu, Mn, Fe, Si, Zn., -,,,.. -,, - 82
83 -, : 1 ; 2 ; 3 ; 4 ; 5, -. -, -, -., - -., : ; : (519,146; 519,235 ); () 11,2; ; 83
84 - 459, ; 2,31,2;, : 322,2; 28,8; ;, : 6,5; , 10 %...+5 % 2 %...+2 % : 6,0 8,0 ; 3,0 4,0 ; ;, : 25 ; 30.., -, (- ),, ,. ;, -, -, (. 30),. 84
85 , -.,, - (,..). - : 0,1 %, 0,1 %, 0,02 %. -,,, -, : ; 13,5 20 ; 0,015., - : R 250 ; N /; 3,2. : 220 ±10 %; 50 ± 0,1; 2,2 ; : ; ; : (, ); - 85
86 ( - ); ( ) (. 31) ,,.,,. -,,, -, -.,. : ;, /: 390 1,65; 470 3,5; ,34; 0,089 ; 322,2 ; 0,01 ; 7 ; 3,5 ; 86
87 930 ; 220, 50 ;, : ; ; ; 35., Test-Master PRO. - (. 32) ( - ) Test-Master PRO,, WAS AG. :,,,,,,,,, -, - -,,. 87
88 , - 100% Test-aster, -. DSP ( ). 14 CCD- -, est-master,.. 5. METALSCAN 3010/20/30. Arun Technology,. METALSCAN 3010/20/30 (. 33) - - -,,. 3020, METALSCAN 3010/20/30 Metalscan , -, -. - ( -, )
89 -. : (17 ) ( ),. : ;, ; CCD-; ; : ; - ( 1 3 ), ;. : - ; - ( ); - ( 200 ); ; LCD, TFT - ; (330MHz, OS Windows 98, 32Mb RAM, 2Gb HDD, FDD) - ;. :, Windows 98;,, ;, , 50/60.,,
90 (. 34) -,. -71,,, ,, ± 0,5,, , ,,. -, - 90
91 , «-400» CRL-., -. -,,. - : - ;, 4,, ; - ; - ; ,,., ( 6 ). : -,.. ;, - ;,. IBM- -. WinQuant, -,,,. 91
92 : : - 1 ;,, , 2400 /; -,, , 1800 /; (Na), 670 (), 766 (Li); -, ; : ; ; ; ; ; 0,0001 % %; ( ) 0,5 5 %; ; 120.; 36.; HAMAMATSU, ; ; 350 ; ; : 15 25, - ± 5 /, < 80 %; : 220 ± 22V 50, 2,5 ; 99,995 %. 1.? 2. -? 3.? 4.? 5.? ? 92
93 , (),,, 22.,,,,,,,, -. (),, - -., - (-, -.),. - ~ 1 2 8,, ,, - ()., -. ( ), ()..,,., 1 1,5, 0,
94 -. - ( 20, 2, 3), -. -, , -.. -, (- ),,. -,,,. -, -,, -, - 450,, ,. -.,, -,. -,,,. -., V1 «1». -, -., -., -, -. «1». 94
95 , V V 3, Å 4379, , , , (), Å , , , ,33 V ,20 1 Cr Cr 4 Cr 6 Cr , , , , , , , , , , Ni , , , , , , , , , , , , , , , , , , , , , , , , ,28
96 Ni 2. 2 (), Å, Å , ,24 Ti , , , , , , , , , , , , , , ,30 W ,61 W , W , n , n , , , , ,17 Nb , ,85 Nb ,94 Nb 3 Co , , , ,22 Si , , , , , , ,02 2. :, - «=», 1 = 2; -, «<» «>», 1 < 2 1 > 2; -, : «<<» «>>», 1<<2 1>>2.
97 ,, -,. -. -,,, ,. -,, - 60., -,. - 2, Å...,, : ; 1 3 ();,, (). 97
98 :,,,,,,,,,. 1.. : V1, V3, V4. V1 -. V3 - V4. V1 0,15 0,5 %, V3 0,8 2,5 %. V4 -, 0,1 % V1 V V1, % 0,15 1 = 4 0,30 2 = 4 0,50 3 =
99 . 37. V3 V V3, % 0,8 1 = 2 1,5 1 = 3 2,5 1 = 4 4 «1V» V4 V 0,1 % V4 99
100 2.. : r1, Cr4, Cr6 Cr7. Cr4 Cr r1 r6 -. r6 -, - ; Cr1 Cr Cr1, % 0,05 1 = 4 0, ,
101 . 40. Cr Cr6 Cr Cr4, % 1,0 1 = 2 2,5 1 = 3 5,
102 Cr Cr6, % = > 2; 1 < = Cr7 Cr Cr7, % 0,3 1 = 7 0,7 2 = 7 1,0 1 = 6; 2 7 1,5 1 < 5; 1 6; 2 > 7 2,5 2 = ; 2 = = 4; 2 = > 4; > 4;
103 9, % r1 0,05 0,2 0,3 % r % r % r7 0,3 30 2,5 % : Mo1, Mo2. Mo1 -,, Mo2. Mo1 0,15 1,2 % Mo2 0,25 2 %. 10 Mo Mo1, % 0, ,15 0,30 1 = 4; 2 7 0,30 0,60 1 6; 2 8 0,60 1,2 1 5; 2 = 6 1,2 1 > 5; Mo1 103
104 . 44. Mo2 11 Mo Mo2, % 0,25 1 = 4 0,30 1 > 2; 1 < 3 0,70 1 = 3 2,0 1 > : Ni1 Ni2. Ni1. 0,2 5 %. 0,2 0,5 -. Ni2 -, 1,5 20 %. 1 2 %,, - Ni1. 10 %. 104
105 . 45. Ni1 12 Ni Ni1, % 0, ,5 1 = 2 3,0 1 > 2; 1 < 4 5,0 1 = Ni2 105
106 13 Ni Ni2, % 1,5 1 3,0 1 < = > Ti1, -. Ti1 0,05 1,5 % Ti1 14 Ti Ti1, % 0,05 2 = 3 0,1 0,15 2 > 3 0, ,35 1 = 4 0,8 1 = 5 1 1,
107 6.. - : W1, W2 W3. W1, W2 -, -, W3. W %, W % W3 1 5 % W W2 107
108 15 W W1, % 1 1 < 4 2,5 1 = ; = >> 3; 2 >> 4 16 W W2, % 5 1 = >> W3 17 W W3, % 1,0 1 = 3 2, ,5 1 > 3; 1 4 5,0 1 > 4 108
109 7.. : Mn1 Mn3. Mn1, - -, Mn3. Mn1 0,3 4 %, Mn3 0,4 1 %. Mn1 3 % Mn1 18 Mn Mn1, % 0,3 0,5 1 < 2 0,6 0,7 1 = 2 1,0 2,0 1 > 2 3,0 4,0 1 = 3 19 Mn Mn3, % 0,4 2 < 4 0,7 2 > 4; 1 < 3 1,0 1 = 3 109
110 . 52. Mn3 8.. : Nb1, Nb2 Nb3. Nb1, Nb2, -, Nb Nb1 0,1 1,5 %. 1Nb 2Nb, 1Nb 4671,09 Å, 1 %, - 2Nb 4675,12 Å, 0,2 %. - 0,2 % 1 %, - Nb1. 110
111 . 53. Nb Nb2 «1» Nb2 0,3 %. 111
112 20 Nb Nb1, % 0,1 0, ,6 1,0 1 > 3; 1 4 1,5 1 > Nb3 0,2 %. Nb2 Nb3. Nb2, 0,3 % ,80 Å, 2Nb. Nb3 0,2 %, - 4 %. Nb3, - Cr7. Nb1. 112
113 9.. Co1, % Co1 21 Co Co1, % 2 1 < = > Si2 0,1 4 %. Si Si Si1, % 0,1 0, ,3 1 = 4 0,6 1 = 6; 2 = 3 1,2 1,4 1 > 6; 1 7 1,8 2,0 1 7; 2 < 6 3,0 4,0 1 > 7;
114 . 57. Si , 6, 0,7, 0,8. 30., -, 20 %, 1 %, - 0,8 1,2 %.., -, -., -.. -, - 114
115 .,, M; 12MX 12MX; 15XM 20; ; ( 0,03 %) (- Ti1), ; 44 -,, Cr7 - Nb3 : 1. 0,15 % - 0,1 % Mo2 V4, MX 15XM. 2. -,, 50 -.,, , -., 115
116 -, , :, -, ,, -. -,, -., -,, ,,, -, -., (), ,. -. -, 116
117 .,.. (- ) -.,,,, -. :, - ;, -,, ; -,.,,, - 3., 60,, ,..,, ,, -, -. -,..., -, ,,. 117
118 , :,,.,, -,, : ; ; ; ; (. 56). -, - :, «69 70» «69 70», «65 66» « »..,.. - -, (. 57) -,.. 118
119 : 1 12,,,,,.,, (100 %), -., -, -,. -.,,, epxoce,,, , , ,,,,,,, -., 20., 119
120 ,,. 6, 6.,, -. - ( ), -. 1.? 2. 1V3 = 3? 3.? 4., -? 5.? 120
121 1.....:, :. - -., ,.. -..:, :, :, ,..,.. -..: -, ,..,....:, / - -.., :, /.., ,.. -. / «-»., : -, /.., : -, : -, : -, : -,
122 /16.. XEROX.... 7, , NATIONAL QUALITY ASSURANCE - ISO 9001: ,.,.,
B I N G O B I N G O. Hf Cd Na Nb Lr. I Fl Fr Mo Si. Ho Bi Ce Eu Ac. Md Co P Pa Tc. Uut Rh K N. Sb At Md H. Bh Cm H Bi Es. Mo Uus Lu P F.
 Hf Cd Na Nb Lr Ho Bi Ce u Ac I Fl Fr Mo i Md Co P Pa Tc Uut Rh K N Dy Cl N Am b At Md H Y Bh Cm H Bi s Mo Uus Lu P F Cu Ar Ag Mg K Thomas Jefferson National Accelerator Facility - Office of cience ducation
Hf Cd Na Nb Lr Ho Bi Ce u Ac I Fl Fr Mo i Md Co P Pa Tc Uut Rh K N Dy Cl N Am b At Md H Y Bh Cm H Bi s Mo Uus Lu P F Cu Ar Ag Mg K Thomas Jefferson National Accelerator Facility - Office of cience ducation
ELECTRON CONFIGURATION (SHORT FORM) # of electrons in the subshell. valence electrons Valence electrons have the largest value for "n"!
 179 ELECTRON CONFIGURATION (SHORT FORM) - We can represent the electron configuration without drawing a diagram or writing down pages of quantum numbers every time. We write the "electron configuration".
179 ELECTRON CONFIGURATION (SHORT FORM) - We can represent the electron configuration without drawing a diagram or writing down pages of quantum numbers every time. We write the "electron configuration".
Chem 115 POGIL Worksheet - Week 4 Moles & Stoichiometry Answers
 Key Questions & Exercises Chem 115 POGIL Worksheet - Week 4 Moles & Stoichiometry Answers 1. The atomic weight of carbon is 12.0107 u, so a mole of carbon has a mass of 12.0107 g. Why doesn t a mole of
Key Questions & Exercises Chem 115 POGIL Worksheet - Week 4 Moles & Stoichiometry Answers 1. The atomic weight of carbon is 12.0107 u, so a mole of carbon has a mass of 12.0107 g. Why doesn t a mole of
Electronegativity and Polarity
 and Polarity N Goalby Chemrevise.org Definition: is the relative tendency of an atom in a molecule to attract electrons in a covalent bond to itself. is measured on the Pauling scale (ranges from 0 to
and Polarity N Goalby Chemrevise.org Definition: is the relative tendency of an atom in a molecule to attract electrons in a covalent bond to itself. is measured on the Pauling scale (ranges from 0 to
Sustainable energy products Simulation based design for recycling
 Sustainable energy products Simulation based design for recycling Markus A. Reuter (Prof. Dr. Dr. hc) Director: Technology Management, Outotec Oyj Aalto University (Finland), Central South University (China),
Sustainable energy products Simulation based design for recycling Markus A. Reuter (Prof. Dr. Dr. hc) Director: Technology Management, Outotec Oyj Aalto University (Finland), Central South University (China),
CLASS TEST GRADE 11. PHYSICAL SCIENCES: CHEMISTRY Test 6: Chemical change
 CLASS TEST GRADE PHYSICAL SCIENCES: CHEMISTRY Test 6: Chemical change MARKS: 45 TIME: hour INSTRUCTIONS AND INFORMATION. Answer ALL the questions. 2. You may use non-programmable calculators. 3. You may
CLASS TEST GRADE PHYSICAL SCIENCES: CHEMISTRY Test 6: Chemical change MARKS: 45 TIME: hour INSTRUCTIONS AND INFORMATION. Answer ALL the questions. 2. You may use non-programmable calculators. 3. You may
Chem 115 POGIL Worksheet - Week 4 Moles & Stoichiometry
 Chem 115 POGIL Worksheet - Week 4 Moles & Stoichiometry Why? Chemists are concerned with mass relationships in chemical reactions, usually run on a macroscopic scale (grams, kilograms, etc.). To deal with
Chem 115 POGIL Worksheet - Week 4 Moles & Stoichiometry Why? Chemists are concerned with mass relationships in chemical reactions, usually run on a macroscopic scale (grams, kilograms, etc.). To deal with
From Quantum to Matter 2006
 From Quantum to Matter 006 Why such a course? Ronald Griessen Vrije Universiteit, Amsterdam AMOLF, May 4, 004 vrije Universiteit amsterdam Why study quantum mechanics? From Quantum to Matter: The main
From Quantum to Matter 006 Why such a course? Ronald Griessen Vrije Universiteit, Amsterdam AMOLF, May 4, 004 vrije Universiteit amsterdam Why study quantum mechanics? From Quantum to Matter: The main
100% ionic compounds do not exist but predominantly ionic compounds are formed when metals combine with non-metals.
 2.21 Ionic Bonding 100% ionic compounds do not exist but predominantly ionic compounds are formed when metals combine with non-metals. Forming ions Metal atoms lose electrons to form +ve ions. Non-metal
2.21 Ionic Bonding 100% ionic compounds do not exist but predominantly ionic compounds are formed when metals combine with non-metals. Forming ions Metal atoms lose electrons to form +ve ions. Non-metal
All answers must use the correct number of significant figures, and must show units!
 CHEM 10113, Quiz 2 September 7, 2011 Name (please print) All answers must use the correct number of significant figures, and must show units! IA Periodic Table of the Elements VIIIA (1) (18) 1 2 1 H IIA
CHEM 10113, Quiz 2 September 7, 2011 Name (please print) All answers must use the correct number of significant figures, and must show units! IA Periodic Table of the Elements VIIIA (1) (18) 1 2 1 H IIA
Ionizing Radiation, Czech Republic, CMI (Czech Metrology Institute)
 Ionizing Radiation, Czech Republic, (Czech Metrology Institute) Calibration or Measurement RADIOACTIVITY 1.0E+00 1.0E+02 Bq cm -2 C-14 1.0E+01 1.0E+02 Bq cm -2 Co-60 1.0E+01 1.0E+02 Bq cm -2 Sr-90 1.0E+01
Ionizing Radiation, Czech Republic, (Czech Metrology Institute) Calibration or Measurement RADIOACTIVITY 1.0E+00 1.0E+02 Bq cm -2 C-14 1.0E+01 1.0E+02 Bq cm -2 Co-60 1.0E+01 1.0E+02 Bq cm -2 Sr-90 1.0E+01
It takes four quantum numbers to describe an electron. Additionally, every electron has a unique set of quantum numbers.
 So, quantum mechanics does not define the path that the electron follows; rather, quantum mechanics works by determining the energy of the electron. Once the energy of an electron is known, the probability
So, quantum mechanics does not define the path that the electron follows; rather, quantum mechanics works by determining the energy of the electron. Once the energy of an electron is known, the probability
EXPERIMENT 4 The Periodic Table - Atoms and Elements
 EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
EXPERIMENT 4 The Periodic Table - Atoms and Elements INTRODUCTION Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements
Victims Compensation Claim Status of All Pending Claims and Claims Decided Within the Last Three Years
 Claim#:021914-174 Initials: J.T. Last4SSN: 6996 DOB: 5/3/1970 Crime Date: 4/30/2013 Status: Claim is currently under review. Decision expected within 7 days Claim#:041715-334 Initials: M.S. Last4SSN: 2957
Claim#:021914-174 Initials: J.T. Last4SSN: 6996 DOB: 5/3/1970 Crime Date: 4/30/2013 Status: Claim is currently under review. Decision expected within 7 days Claim#:041715-334 Initials: M.S. Last4SSN: 2957
Distribution of Chemical Elements In Urban Sediments in Slovenia (Extended Abstract)
 Robert SAJN and Simon PIRC Distribution of Chemical Elements In Urban Sediments in Slovenia (Extended Abstract) The goal of the study work was to assess the distribution of chemical elements in anthropogenic
Robert SAJN and Simon PIRC Distribution of Chemical Elements In Urban Sediments in Slovenia (Extended Abstract) The goal of the study work was to assess the distribution of chemical elements in anthropogenic
= 11.0 g (assuming 100 washers is exact).
 CHAPTER 8 1. 100 washers 0.110 g 1 washer 100. g 1 washer 0.110 g = 11.0 g (assuming 100 washers is exact). = 909 washers 2. The empirical formula is CFH from the structure given. The empirical formula
CHAPTER 8 1. 100 washers 0.110 g 1 washer 100. g 1 washer 0.110 g = 11.0 g (assuming 100 washers is exact). = 909 washers 2. The empirical formula is CFH from the structure given. The empirical formula
CATALOGUE REFERENCE MATERIALS
 IPT Institute for Technological Research CATALOGUE REFERENCE MATERIALS 2014 Laboratory of Metrological References IPT s Reference Materials www.ipt.br/nmr.htm Av. Prof. Almeida Prado n0 532 Predio 31 Cidade
IPT Institute for Technological Research CATALOGUE REFERENCE MATERIALS 2014 Laboratory of Metrological References IPT s Reference Materials www.ipt.br/nmr.htm Av. Prof. Almeida Prado n0 532 Predio 31 Cidade
SUS. Company Profile. Ulrich Nell, Feldstr.23, D - 46149 Oberhausen, Tel. 0049(0)208/658535 Fax 0049(0)208/658536
 SUS Ulrich Nell, Feldstr.23, D - 46149 Oberhausen, Tel. 0049(0208/658535 Fax 0049(0208/658536 Company Profile SUS was founded in 1986 in Oberhausen in the Ruhr area (close to Düsseldorf, in order to meet
SUS Ulrich Nell, Feldstr.23, D - 46149 Oberhausen, Tel. 0049(0208/658535 Fax 0049(0208/658536 Company Profile SUS was founded in 1986 in Oberhausen in the Ruhr area (close to Düsseldorf, in order to meet
Data Analysis and Validation Support for PM2.5 Chemical Speciation Networks- #82
 Data Analysis and Validation Support for PM2.5 Chemical Speciation Networks- #82 Max R. Peterson and Edward E. Rickman Research Triangle Institute 3040 Cornwallis Road P.O. Box 12194 Research Triangle
Data Analysis and Validation Support for PM2.5 Chemical Speciation Networks- #82 Max R. Peterson and Edward E. Rickman Research Triangle Institute 3040 Cornwallis Road P.O. Box 12194 Research Triangle
Chapter 8 Atomic Electronic Configurations and Periodicity
 Chapter 8 Electron Configurations Page 1 Chapter 8 Atomic Electronic Configurations and Periodicity 8-1. Substances that are weakly attracted to a magnetic field but lose their magnetism when removed from
Chapter 8 Electron Configurations Page 1 Chapter 8 Atomic Electronic Configurations and Periodicity 8-1. Substances that are weakly attracted to a magnetic field but lose their magnetism when removed from
Decomposition. Composition
 Decomposition 1. Solid ammonium carbonate is heated. 2. Solid calcium carbonate is heated. 3. Solid calcium sulfite is heated in a vacuum. Composition 1. Barium oxide is added to distilled water. 2. Phosphorus
Decomposition 1. Solid ammonium carbonate is heated. 2. Solid calcium carbonate is heated. 3. Solid calcium sulfite is heated in a vacuum. Composition 1. Barium oxide is added to distilled water. 2. Phosphorus
Find a pair of elements in the periodic table with atomic numbers less than 20 that are an exception to the original periodic law.
 Example Exercise 6.1 Periodic Law Find the two elements in the fifth row of the periodic table that violate the original periodic law proposed by Mendeleev. Mendeleev proposed that elements be arranged
Example Exercise 6.1 Periodic Law Find the two elements in the fifth row of the periodic table that violate the original periodic law proposed by Mendeleev. Mendeleev proposed that elements be arranged
B) atomic number C) both the solid and the liquid phase D) Au C) Sn, Si, C A) metal C) O, S, Se C) In D) tin D) methane D) bismuth B) Group 2 metal
 1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
1. The elements on the Periodic Table are arranged in order of increasing A) atomic mass B) atomic number C) molar mass D) oxidation number 2. Which list of elements consists of a metal, a metalloid, and
Sampling and preparation of heterogeneous waste fuels?
 Sampling and preparation of heterogeneous waste fuels? Is it possible to accomplish a representative and relevant composition data? Evalena Wikström, Lennart Gustavsson and Jolanta Franke Aim of the project
Sampling and preparation of heterogeneous waste fuels? Is it possible to accomplish a representative and relevant composition data? Evalena Wikström, Lennart Gustavsson and Jolanta Franke Aim of the project
CHAPTER 21 ELECTROCHEMISTRY
 Chapter 21: Electrochemistry Page 1 CHAPTER 21 ELECTROCHEMISTRY 21-1. Consider an electrochemical cell formed from a Cu(s) electrode submerged in an aqueous Cu(NO 3 ) 2 solution and a Cd(s) electrode submerged
Chapter 21: Electrochemistry Page 1 CHAPTER 21 ELECTROCHEMISTRY 21-1. Consider an electrochemical cell formed from a Cu(s) electrode submerged in an aqueous Cu(NO 3 ) 2 solution and a Cd(s) electrode submerged
SEATTLE CENTRAL COMMUNITY COLLEGE DIVISION OF SCIENCE AND MATHEMATICS. Oxidation-Reduction
 SEATTLE CENTRAL COMMUNITY COLLEGE DIVISION OF SCIENCE AND MATHEMATICS OxidationReduction Oxidation is loss of electrons. (Oxygen is EN enough to grab e away from most elements, so the term originally meant
SEATTLE CENTRAL COMMUNITY COLLEGE DIVISION OF SCIENCE AND MATHEMATICS OxidationReduction Oxidation is loss of electrons. (Oxygen is EN enough to grab e away from most elements, so the term originally meant
M. Bouchard, S. Rivard, and C.O. Arsenault Corporation Scientifique Claisse, Quebec City, Canada S. Ness Intertek Genalysis, Perth, Australia
 Iron Ore Composition Determination and Total Iron Quantification by Borate fusion and WDXRF A Simplified Analytical Method to the Prevailing ISO 9516-1 M. Bouchard, S. Rivard, and C.O. Arsenault Corporation
Iron Ore Composition Determination and Total Iron Quantification by Borate fusion and WDXRF A Simplified Analytical Method to the Prevailing ISO 9516-1 M. Bouchard, S. Rivard, and C.O. Arsenault Corporation
APPENDIX B: EXERCISES
 BUILDING CHEMISTRY LABORATORY SESSIONS APPENDIX B: EXERCISES Molecular mass, the mole, and mass percent Relative atomic and molecular mass Relative atomic mass (A r ) is a constant that expresses the ratio
BUILDING CHEMISTRY LABORATORY SESSIONS APPENDIX B: EXERCISES Molecular mass, the mole, and mass percent Relative atomic and molecular mass Relative atomic mass (A r ) is a constant that expresses the ratio
Nomenclature of Ionic Compounds
 Nomenclature of Ionic Compounds Ionic compounds are composed of ions. An ion is an atom or molecule with an electrical charge. Monatomic ions are formed from single atoms that have gained or lost electrons.
Nomenclature of Ionic Compounds Ionic compounds are composed of ions. An ion is an atom or molecule with an electrical charge. Monatomic ions are formed from single atoms that have gained or lost electrons.
Steven M. Ho!and. Department of Geology, University of Georgia, Athens, GA 30602-2501
 PRINCIPAL COMPONENTS ANALYSIS (PCA) Steven M. Ho!and Department of Geology, University of Georgia, Athens, GA 30602-2501 May 2008 Introduction Suppose we had measured two variables, length and width, and
PRINCIPAL COMPONENTS ANALYSIS (PCA) Steven M. Ho!and Department of Geology, University of Georgia, Athens, GA 30602-2501 May 2008 Introduction Suppose we had measured two variables, length and width, and
Chapter 8 - Chemical Equations and Reactions
 Chapter 8 - Chemical Equations and Reactions 8-1 Describing Chemical Reactions I. Introduction A. Reactants 1. Original substances entering into a chemical rxn B. Products 1. The resulting substances from
Chapter 8 - Chemical Equations and Reactions 8-1 Describing Chemical Reactions I. Introduction A. Reactants 1. Original substances entering into a chemical rxn B. Products 1. The resulting substances from
OXIDATION REDUCTION. Section I. Cl 2 + 2e. 2. The oxidation number of group II A is always (+) 2.
 OXIDATION REDUCTION Section I Example 1: Na Example 2: 2C1 Example 3: K + + e Na + + e Cl 2 + 2e K Example 4: C1 2 + 2e 2Cl 1. The oxidation number of group I A is always (+) 1. 2. The oxidation number
OXIDATION REDUCTION Section I Example 1: Na Example 2: 2C1 Example 3: K + + e Na + + e Cl 2 + 2e K Example 4: C1 2 + 2e 2Cl 1. The oxidation number of group I A is always (+) 1. 2. The oxidation number
Qualitätsmanagement-Handbuch
 U r Kalibrier- und Messmöglichkeiten Qualitätsmanagement-Handbuch Relative erweiterte Messunsicherheit, die sich aus der Standardmessunsicherheit durch Multiplikation mit dem Erweiterungsfaktor k = 2 ergibt.
U r Kalibrier- und Messmöglichkeiten Qualitätsmanagement-Handbuch Relative erweiterte Messunsicherheit, die sich aus der Standardmessunsicherheit durch Multiplikation mit dem Erweiterungsfaktor k = 2 ergibt.
SCP SCIENCE www.scpscience.com. Certified Reference Materials. Materials
 Certified Reference 47 EnviroMAT and AgroMAT Certifi ed Reference (CRMs) are designed to complement existing Performance Evaluation Programs in environmental and agricultural analysis. EnviroMAT CRMs allow
Certified Reference 47 EnviroMAT and AgroMAT Certifi ed Reference (CRMs) are designed to complement existing Performance Evaluation Programs in environmental and agricultural analysis. EnviroMAT CRMs allow
8. Relax and do well.
 CHEM 1314 3:30 pm Section Exam II ohn II. Gelder October 16, 2002 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last three pages include a periodic
CHEM 1314 3:30 pm Section Exam II ohn II. Gelder October 16, 2002 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last three pages include a periodic
Role of Hydrogen Bonding on Protein Secondary Structure Introduction
 Role of Hydrogen Bonding on Protein Secondary Structure Introduction The function and chemical properties of proteins are determined by its three-dimensional structure. The final architecture of the protein
Role of Hydrogen Bonding on Protein Secondary Structure Introduction The function and chemical properties of proteins are determined by its three-dimensional structure. The final architecture of the protein
Nomenclature and Formulas of Ionic Compounds. Section I: Writing the Name from the Formula
 Purpose: Theory: Nomenclature and Formulas of Ionic Compounds 1. To become familiar with the rules of chemical nomenclature, based on the classification of compounds. 2. To write the proper name of the
Purpose: Theory: Nomenclature and Formulas of Ionic Compounds 1. To become familiar with the rules of chemical nomenclature, based on the classification of compounds. 2. To write the proper name of the
Chemistry CP Unit 2 Atomic Structure and Electron Configuration. Learning Targets (Your exam at the end of Unit 2 will assess the following:)
 Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
Chemistry CP Unit 2 Atomic Structure and Electron Learning Targets (Your exam at the end of Unit 2 will assess the following:) 2. Atomic Structure and Electron 2-1. Give the one main contribution to the
Redox and Electrochemistry
 Name: Thursday, May 08, 2008 Redox and Electrochemistry 1. A diagram of a chemical cell and an equation are shown below. When the switch is closed, electrons will flow from 1. the Pb(s) to the Cu(s) 2+
Name: Thursday, May 08, 2008 Redox and Electrochemistry 1. A diagram of a chemical cell and an equation are shown below. When the switch is closed, electrons will flow from 1. the Pb(s) to the Cu(s) 2+
How to read your Oil Analysis Report
 How to read your Oil Analysis Report A better understanding Petroleum Technologies Group, LLC. 4665 Broadmoor S.E. Ste#15 Grand Rapids, MI 49512 Phone # 616/698 9399 Fax # 616/698 9395 www.oil lab.com
How to read your Oil Analysis Report A better understanding Petroleum Technologies Group, LLC. 4665 Broadmoor S.E. Ste#15 Grand Rapids, MI 49512 Phone # 616/698 9399 Fax # 616/698 9395 www.oil lab.com
EXPERIMENT 8: Activity Series (Single Displacement Reactions)
 EPERIMENT 8: Activity Series (Single Displacement Reactions) PURPOSE a) Reactions of metals with acids and salt solutions b) Determine the activity of metals c) Write a balanced molecular equation, complete
EPERIMENT 8: Activity Series (Single Displacement Reactions) PURPOSE a) Reactions of metals with acids and salt solutions b) Determine the activity of metals c) Write a balanced molecular equation, complete
Neutralization of Acid Mine Drainage Using Stabilized Flue Gas Desulfurization Material
 Neutralization of Acid Mine Drainage Using Stabilized Flue Gas Desulfurization Material W. Wolfe 1, C.-M. Cheng 1, R. Baker 1, T. Butalia 1, J. Massey-Norton 2 1 The Ohio State University, 2 American Electric
Neutralization of Acid Mine Drainage Using Stabilized Flue Gas Desulfurization Material W. Wolfe 1, C.-M. Cheng 1, R. Baker 1, T. Butalia 1, J. Massey-Norton 2 1 The Ohio State University, 2 American Electric
Analyses on copper samples from Micans
 PDF rendering: DokumentID 1473479, Version 1., Status Godkänt, Sekretessklass Öppen Analyses on copper samples from Micans P. Berastegui, M. Hahlin, M. Ottosson, M. Korvela, Y. Andersson, R. Berger and
PDF rendering: DokumentID 1473479, Version 1., Status Godkänt, Sekretessklass Öppen Analyses on copper samples from Micans P. Berastegui, M. Hahlin, M. Ottosson, M. Korvela, Y. Andersson, R. Berger and
Nuclear ZPE Tapping. Horace Heffner May 2007
 ENERGY FROM UNCERTAINTY The uncertainty of momentum for a particle constrained by distance Δx is given, according to Heisenberg, by: Δmv = h/(2 π Δx) but since KE = (1/2) m v 2 = (1/(2 m) ) (Δmv) 2 ΔKE
ENERGY FROM UNCERTAINTY The uncertainty of momentum for a particle constrained by distance Δx is given, according to Heisenberg, by: Δmv = h/(2 π Δx) but since KE = (1/2) m v 2 = (1/(2 m) ) (Δmv) 2 ΔKE
REVIEW QUESTIONS Chapter 8
 Chemistry 101 ANSWER KEY REVIEW QUESTIONS Chapter 8 Use only a periodic table to answer the following questions. 1. Write complete electron configuration for each of the following elements: a) Aluminum
Chemistry 101 ANSWER KEY REVIEW QUESTIONS Chapter 8 Use only a periodic table to answer the following questions. 1. Write complete electron configuration for each of the following elements: a) Aluminum
Name: Teacher: Pd. Date:
 Name: Teacher: Pd. Date: STAAR Tutorial : Energy and Matter: Elements, Compounds, and Chemical Equations: 6.5C Differentiate between elements and compounds on the most basic level. 8.5F Recognize whether
Name: Teacher: Pd. Date: STAAR Tutorial : Energy and Matter: Elements, Compounds, and Chemical Equations: 6.5C Differentiate between elements and compounds on the most basic level. 8.5F Recognize whether
ORTEC DET-SW-UPG. Latest Software Features. Ease of Use. Source Location with the Detective V3 Software
 ORTEC DET-SW-UPG Latest Software Features Three Search Modes: Gamma/Neutron total count rate. SNM search mode. Sliding average "monitor" mode. (NEW) User choice of identification schemes: Classify mode
ORTEC DET-SW-UPG Latest Software Features Three Search Modes: Gamma/Neutron total count rate. SNM search mode. Sliding average "monitor" mode. (NEW) User choice of identification schemes: Classify mode
1.- L a m e j o r o p c ió n e s c l o na r e l d i s co ( s e e x p li c a r á d es p u é s ).
 PROCEDIMIENTO DE RECUPERACION Y COPIAS DE SEGURIDAD DEL CORTAFUEGOS LINUX P ar a p od e r re c u p e ra r nu e s t r o c o rt a f u e go s an t e un d es a s t r e ( r ot u r a d e l di s c o o d e l a
PROCEDIMIENTO DE RECUPERACION Y COPIAS DE SEGURIDAD DEL CORTAFUEGOS LINUX P ar a p od e r re c u p e ra r nu e s t r o c o rt a f u e go s an t e un d es a s t r e ( r ot u r a d e l di s c o o d e l a
Electrochemistry Worksheet
 Electrochemistry Worksheet 1. Assign oxidation numbers to each atom in the following: a. P 4 O 6 b. BiO 3 c. N 2 H 4 d. Mg(BrO 4 ) 2 e. MnSO 4 f. Mn(SO 4 ) 2 2. For each of the reactions below identify
Electrochemistry Worksheet 1. Assign oxidation numbers to each atom in the following: a. P 4 O 6 b. BiO 3 c. N 2 H 4 d. Mg(BrO 4 ) 2 e. MnSO 4 f. Mn(SO 4 ) 2 2. For each of the reactions below identify
Universal Data Acquisition (UDA)
 Universal Data Acquisition (UDA) I C P - O P T I C A L E M I S S I O N P R O D U C T N O T E Introduction Historically, Inductively Coupled Plasma (ICP) spectroscopy has been used for multiple analyte
Universal Data Acquisition (UDA) I C P - O P T I C A L E M I S S I O N P R O D U C T N O T E Introduction Historically, Inductively Coupled Plasma (ICP) spectroscopy has been used for multiple analyte
Name period AP chemistry Unit 2 worksheet Practice problems
 Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
Name period AP chemistry Unit 2 worksheet Practice problems 1. What are the SI units for a. Wavelength of light b. frequency of light c. speed of light Meter hertz (s -1 ) m s -1 (m/s) 2. T/F (correct
Elemental composition of commercial seasalts
 Elemental composition of commercial seasalts M. J. Atkinson, Hawaii Institute of Marine Biology, PO Box 1346, Kanoehe, Hawaii 96744, and C. Bingman, Department of Biochemistry and Molecular Biophysics,
Elemental composition of commercial seasalts M. J. Atkinson, Hawaii Institute of Marine Biology, PO Box 1346, Kanoehe, Hawaii 96744, and C. Bingman, Department of Biochemistry and Molecular Biophysics,
2. DECOMPOSITION REACTION ( A couple have a heated argument and break up )
 TYPES OF CHEMICAL REACTIONS Most reactions can be classified into one of five categories by examining the types of reactants and products involved in the reaction. Knowing the types of reactions can help
TYPES OF CHEMICAL REACTIONS Most reactions can be classified into one of five categories by examining the types of reactants and products involved in the reaction. Knowing the types of reactions can help
Standard Operation Procedure. Elemental Analysis of Solution samples with Inductively Coupled Plasma Mass Spectrometry
 Standard Operation Procedure Elemental Analysis of Solution samples with Inductively Coupled Plasma Mass Spectrometry Soil & Plant Analysis Laboratory University of Wisconsin Madison http://uwlab.soils.wisc.edu
Standard Operation Procedure Elemental Analysis of Solution samples with Inductively Coupled Plasma Mass Spectrometry Soil & Plant Analysis Laboratory University of Wisconsin Madison http://uwlab.soils.wisc.edu
Quantification of Non-Fibrous and Fibrous Particulates in Human Lungs: Twenty Year Update on Pneumoconiosis Database
 Ann. occup. Hyg., Vol. 46, Supplement 1, pp. 397 401, 2002 2002 British Occupational Hygiene Society Published by Oxford University Press DOI: 10.1093/annhyg/mef694 Quantification of Non-Fibrous and Fibrous
Ann. occup. Hyg., Vol. 46, Supplement 1, pp. 397 401, 2002 2002 British Occupational Hygiene Society Published by Oxford University Press DOI: 10.1093/annhyg/mef694 Quantification of Non-Fibrous and Fibrous
MODERN ATOMIC THEORY AND THE PERIODIC TABLE
 CHAPTER 10 MODERN ATOMIC THEORY AND THE PERIODIC TABLE SOLUTIONS TO REVIEW QUESTIONS 1. Wavelength is defined as the distance between consecutive peaks in a wave. It is generally symbolized by the Greek
CHAPTER 10 MODERN ATOMIC THEORY AND THE PERIODIC TABLE SOLUTIONS TO REVIEW QUESTIONS 1. Wavelength is defined as the distance between consecutive peaks in a wave. It is generally symbolized by the Greek
3. What would you predict for the intensity and binding energy for the 3p orbital for that of sulfur?
 PSI AP Chemistry Periodic Trends MC Review Name Periodic Law and the Quantum Model Use the PES spectrum of Phosphorus below to answer questions 1-3. 1. Which peak corresponds to the 1s orbital? (A) 1.06
PSI AP Chemistry Periodic Trends MC Review Name Periodic Law and the Quantum Model Use the PES spectrum of Phosphorus below to answer questions 1-3. 1. Which peak corresponds to the 1s orbital? (A) 1.06
Electrochemistry Voltaic Cells
 Electrochemistry Voltaic Cells Many chemical reactions can be classified as oxidation-reduction or redox reactions. In these reactions one species loses electrons or is oxidized while another species gains
Electrochemistry Voltaic Cells Many chemical reactions can be classified as oxidation-reduction or redox reactions. In these reactions one species loses electrons or is oxidized while another species gains
 C o a t i a n P u b l i c D e b tm a n a g e m e n t a n d C h a l l e n g e s o f M a k e t D e v e l o p m e n t Z a g e bo 8 t h A p i l 2 0 1 1 h t t pdd w w wp i j fp h D p u b l i c2 d e b td S t
C o a t i a n P u b l i c D e b tm a n a g e m e n t a n d C h a l l e n g e s o f M a k e t D e v e l o p m e n t Z a g e bo 8 t h A p i l 2 0 1 1 h t t pdd w w wp i j fp h D p u b l i c2 d e b td S t
NET IONIC EQUATIONS. A balanced chemical equation can describe all chemical reactions, an example of such an equation is:
 NET IONIC EQUATIONS A balanced chemical equation can describe all chemical reactions, an example of such an equation is: NaCl + AgNO 3 AgCl + NaNO 3 In this case, the simple formulas of the various reactants
NET IONIC EQUATIONS A balanced chemical equation can describe all chemical reactions, an example of such an equation is: NaCl + AgNO 3 AgCl + NaNO 3 In this case, the simple formulas of the various reactants
CODES FOR PHARMACY ONLINE CLAIMS PROCESSING
 S FOR PHARMACY ONLINE CLAIMS PROCESSING The following is a list of error and warning codes that may appear when processing claims on the online system. The error codes are bolded. CODE AA AB AI AR CB CD
S FOR PHARMACY ONLINE CLAIMS PROCESSING The following is a list of error and warning codes that may appear when processing claims on the online system. The error codes are bolded. CODE AA AB AI AR CB CD
Lecture 5. elements (Figure 1). In addition, there are many ways of classifying trace elements.
 Lecture 5 Nomenclature for Trace Element Classification We have already grouped elements into two classes, major elements and trace elements (Figure 1). In addition, there are many ways of classifying
Lecture 5 Nomenclature for Trace Element Classification We have already grouped elements into two classes, major elements and trace elements (Figure 1). In addition, there are many ways of classifying
Multicrystalline solar silicon production for development of photovoltaic industry
 Multicrystalline solar silicon production for development of photovoltaic industry A.I. Nepomnyaschikh Institute of Geochemistry, Siberian Branch, Russian Academy of Sciences, E-mail: ainep@igc.irk.ru
Multicrystalline solar silicon production for development of photovoltaic industry A.I. Nepomnyaschikh Institute of Geochemistry, Siberian Branch, Russian Academy of Sciences, E-mail: ainep@igc.irk.ru
2. Write the chemical formula(s) of the product(s) and balance the following spontaneous reactions.
 1. Using the Activity Series on the Useful Information pages of the exam write the chemical formula(s) of the product(s) and balance the following reactions. Identify all products phases as either (g)as,
1. Using the Activity Series on the Useful Information pages of the exam write the chemical formula(s) of the product(s) and balance the following reactions. Identify all products phases as either (g)as,
RESEARCH PAPERS FACULTY OF MATERIALS SCIENCE AND TECHNOLOGY IN TRNAVA SLOVAK UNIVERSITY OF TECHNOLOGY IN BRATISLAVA
 RESEARCH PAPERS FACULTY OF MATERIALS SCIENCE AND TECHNOLOGY IN TRNAVA SLOVAK UNIVERSITY OF TECHNOLOGY IN BRATISLAVA 2010 Number 28 EFFECT OF REDUCTION IN THREE-DRAWN AND TWO-DRAWN SINGLE-RUN TECHNOLOGY
RESEARCH PAPERS FACULTY OF MATERIALS SCIENCE AND TECHNOLOGY IN TRNAVA SLOVAK UNIVERSITY OF TECHNOLOGY IN BRATISLAVA 2010 Number 28 EFFECT OF REDUCTION IN THREE-DRAWN AND TWO-DRAWN SINGLE-RUN TECHNOLOGY
47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25. 4 Atoms and Elements
 47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25 4 Atoms and Elements 4.1 a. Cu b. Si c. K d. N e. Fe f. Ba g. Pb h. Sr 4.2 a. O b. Li c. S d. Al e. H f. Ne g. Sn h. Au 4.3 a. carbon b. chlorine c. iodine d.
47374_04_p25-32.qxd 2/9/07 7:50 AM Page 25 4 Atoms and Elements 4.1 a. Cu b. Si c. K d. N e. Fe f. Ba g. Pb h. Sr 4.2 a. O b. Li c. S d. Al e. H f. Ne g. Sn h. Au 4.3 a. carbon b. chlorine c. iodine d.
5.111 Principles of Chemical Science
 MIT OpenCourseWare http://ocw.mit.edu 5.111 Principles of Chemical Science Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Page 1 of 10 pages
MIT OpenCourseWare http://ocw.mit.edu 5.111 Principles of Chemical Science Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Page 1 of 10 pages
Steps for balancing a chemical equation
 The Chemical Equation: A Chemical Recipe Dr. Gergens - SD Mesa College A. Learn the meaning of these arrows. B. The chemical equation is the shorthand notation for a chemical reaction. A chemical equation
The Chemical Equation: A Chemical Recipe Dr. Gergens - SD Mesa College A. Learn the meaning of these arrows. B. The chemical equation is the shorthand notation for a chemical reaction. A chemical equation
Dimethylglyoxime Method Method 10220 0.1 to 6.0 mg/l Ni TNTplus 856
 Nickel DOC316.53.01065 Dimethylglyoxime Method Method 10220 0.1 to 6.0 mg/l Ni TNTplus 856 Scope and application: For water and wastewater. Test preparation Instrument-specific information Table 1 shows
Nickel DOC316.53.01065 Dimethylglyoxime Method Method 10220 0.1 to 6.0 mg/l Ni TNTplus 856 Scope and application: For water and wastewater. Test preparation Instrument-specific information Table 1 shows
Naming Compounds Handout Key
 Naming Compounds Handout Key p. 2 Name each of the following monatomic cations: Li + = lithium ion Ag + = silver ion Cd +2 = cadmium ion Cu +2 = copper (II) ion Al +3 = aluminum ion Mg +2 = magnesium ion
Naming Compounds Handout Key p. 2 Name each of the following monatomic cations: Li + = lithium ion Ag + = silver ion Cd +2 = cadmium ion Cu +2 = copper (II) ion Al +3 = aluminum ion Mg +2 = magnesium ion
Chalcophile and Key Element Distribution in the Eastern Goldfields: seismic traverse EGF01. Aleks Kalinowski Geoscience Australia, pmdcrc Y2 project
 pmd CR C Chalcophile and Key Element Distribution in the Eastern Goldfields: seismic traverse EGF01 predictive mineral discovery Aleks Kalinowski Geoscience Australia, pmdcrc Y2 project Aleks.Kalinowski@ga.gov.au
pmd CR C Chalcophile and Key Element Distribution in the Eastern Goldfields: seismic traverse EGF01 predictive mineral discovery Aleks Kalinowski Geoscience Australia, pmdcrc Y2 project Aleks.Kalinowski@ga.gov.au
The Role of Triads in the Evolution of the Periodic Table: Past and Present
 The Role of Triads in the Evolution of the Periodic Table: Past and Present Eric Scerri Department of Chemistry and Biochemistry, University of California, Los Angeles, Los Angeles, CA 90095; scerri@chem.ucla.edu
The Role of Triads in the Evolution of the Periodic Table: Past and Present Eric Scerri Department of Chemistry and Biochemistry, University of California, Los Angeles, Los Angeles, CA 90095; scerri@chem.ucla.edu
Multi-elemental determination of gasoline using Agilent 5100 ICP-OES with oxygen injection and a temperature controlled spray chamber
 Multi-elemental determination of gasoline using Agilent 5100 ICP-OES with oxygen injection and a temperature controlled spray chamber Application note Energy & chemicals, petrochemicals Authors Elizabeth
Multi-elemental determination of gasoline using Agilent 5100 ICP-OES with oxygen injection and a temperature controlled spray chamber Application note Energy & chemicals, petrochemicals Authors Elizabeth
Chapter 6 Notes Science 10 Name:
 6.1 Types of Chemical Reactions a) Synthesis (A + B AB) Synthesis reactions are also known as reactions. When this occurs two or more reactants (usually elements) join to form a. A + B AB, where A and
6.1 Types of Chemical Reactions a) Synthesis (A + B AB) Synthesis reactions are also known as reactions. When this occurs two or more reactants (usually elements) join to form a. A + B AB, where A and
AP Chemistry 2008 Free-Response Questions
 AP Chemistry 008 Free-Response Questions The College Board: Connecting Students to College Success The College Board is a not-for-profit membership association whose mission is to connect students to college
AP Chemistry 008 Free-Response Questions The College Board: Connecting Students to College Success The College Board is a not-for-profit membership association whose mission is to connect students to college
Portable X-ray fluorescence Spectroscopy. Michael A. Wilson Research Soil Scientist USDA-NRCS National Soil Survey Center Lincoln, NE
 Portable X-ray fluorescence Spectroscopy Michael A. Wilson Research Soil Scientist USDA-NRCS National Soil Survey Center Lincoln, NE OBJECTIVES Background of the method Features of the instrument Applications
Portable X-ray fluorescence Spectroscopy Michael A. Wilson Research Soil Scientist USDA-NRCS National Soil Survey Center Lincoln, NE OBJECTIVES Background of the method Features of the instrument Applications
Chem 1101. Highlights of last lecture. This lecture. Australian Mining Sites. A/Prof Sébastien Perrier. Metallurgy: (Extracting metal from ore)
 Chem 111 A/Prof Sébastien Perrier Room: 351 Phone: 9351-3366 Email: s.perrier@chem.usyd.edu.au Unless otherwise stated, all images in this file have been reproduced from: Blackman, Bottle, Schmid, Mocerino
Chem 111 A/Prof Sébastien Perrier Room: 351 Phone: 9351-3366 Email: s.perrier@chem.usyd.edu.au Unless otherwise stated, all images in this file have been reproduced from: Blackman, Bottle, Schmid, Mocerino
Instrumentation. (Figure 2)
 X-Ray Fluorescence Lab Report Nydia Esparza Victoria Rangel Physics of XRF XRF is a non destructive analytical technique that is used for elemental and chemical analysis. X-Ray Fluorescence Spectroscopy
X-Ray Fluorescence Lab Report Nydia Esparza Victoria Rangel Physics of XRF XRF is a non destructive analytical technique that is used for elemental and chemical analysis. X-Ray Fluorescence Spectroscopy
Dinamica degli inquinanti
 Dinamica degli inquinanti Characterization techniques Renato Baciocchi University of Rome Tor Vergata, Rome, Italy Characterization techniques Preparation Water content Loss on Ignition, LOI Alkaline extraction
Dinamica degli inquinanti Characterization techniques Renato Baciocchi University of Rome Tor Vergata, Rome, Italy Characterization techniques Preparation Water content Loss on Ignition, LOI Alkaline extraction
Molecules, Atoms, Grams and Mole Calculation Practice
 Molecules, Atoms, Grams and Mole Calculation Practice Helpful HINTS: In these problems look for two things: 1) From what unit to what unit? 2) Does the object stay the same, or does the object change?
Molecules, Atoms, Grams and Mole Calculation Practice Helpful HINTS: In these problems look for two things: 1) From what unit to what unit? 2) Does the object stay the same, or does the object change?
1332 CHAPTER 18 Sample Questions
 1332 CHAPTER 18 Sample Questions Couple E 0 Couple E 0 Br 2 (l) + 2e 2Br (aq) +1.06 V AuCl 4 + 3e Au + 4Cl +1.00 V Ag + + e Ag +0.80 V Hg 2+ 2 + 2e 2 Hg +0.79 V Fe 3+ (aq) + e Fe 2+ (aq) +0.77 V Cu 2+
1332 CHAPTER 18 Sample Questions Couple E 0 Couple E 0 Br 2 (l) + 2e 2Br (aq) +1.06 V AuCl 4 + 3e Au + 4Cl +1.00 V Ag + + e Ag +0.80 V Hg 2+ 2 + 2e 2 Hg +0.79 V Fe 3+ (aq) + e Fe 2+ (aq) +0.77 V Cu 2+
Sample Analysis Design Isotope Dilution
 Isotope Dilution Most accurate and precise calibration method available Requires analyte with two stable isotopes Monoisotopic elements cannot be determined via isotope dilution Spike natural sample with
Isotope Dilution Most accurate and precise calibration method available Requires analyte with two stable isotopes Monoisotopic elements cannot be determined via isotope dilution Spike natural sample with
CHEMICAL REACTIONS. Chemistry 51 Chapter 6
 CHEMICAL REACTIONS A chemical reaction is a rearrangement of atoms in which some of the original bonds are broken and new bonds are formed to give different chemical structures. In a chemical reaction,
CHEMICAL REACTIONS A chemical reaction is a rearrangement of atoms in which some of the original bonds are broken and new bonds are formed to give different chemical structures. In a chemical reaction,
Electrochemistry - ANSWERS
 Electrochemistry - ANSWERS 1. Using a table of standard electrode potentials, predict if the following reactions will occur spontaneously as written. a) Al 3+ + Ni Ni 2+ + Al Al 3+ + 3e - Al E = -1.68
Electrochemistry - ANSWERS 1. Using a table of standard electrode potentials, predict if the following reactions will occur spontaneously as written. a) Al 3+ + Ni Ni 2+ + Al Al 3+ + 3e - Al E = -1.68
MILESTONE CLEAN CHEMISTRY LINE DUOPUR TRACECLEAN ULTRA-TRACE INSERTS. A Breakthrough in Reduction and Control of the Analytical Blank MILESTONE
 MILESTONE CLEAN CHEMISTRY LINE A Breakthrough in Reduction and Control of the Analytical Blank DUOPUR TRACECLEAN ULTRA-TRACE INSERTS MILESTONE MILESTONE CLEAN CHEMISTRY LINE An innovative and complete
MILESTONE CLEAN CHEMISTRY LINE A Breakthrough in Reduction and Control of the Analytical Blank DUOPUR TRACECLEAN ULTRA-TRACE INSERTS MILESTONE MILESTONE CLEAN CHEMISTRY LINE An innovative and complete
CVD SILICON CARBIDE. CVD SILICON CARBIDE s attributes include:
 CVD SILICON CARBIDE CVD SILICON CARBIDE is the ideal performance material for design engineers. It outperforms conventional forms of silicon carbide, as well as other ceramics, quartz, and metals in chemical
CVD SILICON CARBIDE CVD SILICON CARBIDE is the ideal performance material for design engineers. It outperforms conventional forms of silicon carbide, as well as other ceramics, quartz, and metals in chemical
UNIT (2) ATOMS AND ELEMENTS
 UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
UNIT (2) ATOMS AND ELEMENTS 2.1 Elements An element is a fundamental substance that cannot be broken down by chemical means into simpler substances. Each element is represented by an abbreviation called
CHEMISTRY B- FACTOR LABEL PACKET NAME: HR: PAGE 1. Chemistry B. Factor Label Packet
 CHEMISTRY B- FACTOR LABEL PACKET NAME: HR: PAGE 1 Chemistry B Factor Label Packet CHEMISTRY B- FACTOR LABEL PACKET NAME: HR: PAGE 2 PERIODIC TABLE OF ELEMENTS WITH OXIDATION NUMBERS +1 0 H +2 +3-3 He Li
CHEMISTRY B- FACTOR LABEL PACKET NAME: HR: PAGE 1 Chemistry B Factor Label Packet CHEMISTRY B- FACTOR LABEL PACKET NAME: HR: PAGE 2 PERIODIC TABLE OF ELEMENTS WITH OXIDATION NUMBERS +1 0 H +2 +3-3 He Li
Chemistry 122 Mines, Spring 2014
 Chemistry 122 Mines, Spring 2014 Answer Key, Problem Set 9 1. 18.44(c) (Also indicate the sign on each electrode, and show the flow of ions in the salt bridge.); 2. 18.46 (do this for all cells in 18.44
Chemistry 122 Mines, Spring 2014 Answer Key, Problem Set 9 1. 18.44(c) (Also indicate the sign on each electrode, and show the flow of ions in the salt bridge.); 2. 18.46 (do this for all cells in 18.44
Quartz Glass. Tubes and Rods
 Quartz Glass Tubes and Rods PH 300, GE 214, QI PN GVB Solutions in Glass Schlackstrasse 3 52080 Aachen Germany +49-241/9108588 +49-241/9108589 E- info@g-v-b.de www.g-v-b.de Table of contents General Information
Quartz Glass Tubes and Rods PH 300, GE 214, QI PN GVB Solutions in Glass Schlackstrasse 3 52080 Aachen Germany +49-241/9108588 +49-241/9108589 E- info@g-v-b.de www.g-v-b.de Table of contents General Information
AP Chemistry 2009 Free-Response Questions Form B
 AP Chemistry 009 Free-Response Questions Form B The College Board The College Board is a not-for-profit membership association whose mission is to connect students to college success and opportunity. Founded
AP Chemistry 009 Free-Response Questions Form B The College Board The College Board is a not-for-profit membership association whose mission is to connect students to college success and opportunity. Founded
Review: Balancing Redox Reactions. Review: Balancing Redox Reactions
 Review: Balancing Redox Reactions Determine which species is oxidized and which species is reduced Oxidation corresponds to an increase in the oxidation number of an element Reduction corresponds to a
Review: Balancing Redox Reactions Determine which species is oxidized and which species is reduced Oxidation corresponds to an increase in the oxidation number of an element Reduction corresponds to a
Session 1: Introduction and Overview
 Session 1: Introduction and Overview I. Data Validation Overview II. General Approach to Data Validation III. Data Validation Levels IV. Examples V. Resources VI. Key Internet Sites October 2011 o It is
Session 1: Introduction and Overview I. Data Validation Overview II. General Approach to Data Validation III. Data Validation Levels IV. Examples V. Resources VI. Key Internet Sites October 2011 o It is
b. N 2 H 4 c. aluminum oxalate d. acetic acid e. arsenic PART 2: MOLAR MASS 2. Determine the molar mass for each of the following. a. ZnI 2 b.
 CHEMISTRY DISCOVER UNIT 5 LOTS OF PRACTICE ON USING THE MOLE!!! PART 1: ATOMIC MASS, FORMULA MASS, OR MOLECULAR MASS 1. Determine the atomic mass, formula mass, or molecular mass for each of the following
CHEMISTRY DISCOVER UNIT 5 LOTS OF PRACTICE ON USING THE MOLE!!! PART 1: ATOMIC MASS, FORMULA MASS, OR MOLECULAR MASS 1. Determine the atomic mass, formula mass, or molecular mass for each of the following
Periodic Table, Valency and Formula
 Periodic Table, Valency and Formula Origins of the Periodic Table Mendelѐѐv in 1869 proposed that a relationship existed between the chemical properties of elements and their atomic masses. He noticed
Periodic Table, Valency and Formula Origins of the Periodic Table Mendelѐѐv in 1869 proposed that a relationship existed between the chemical properties of elements and their atomic masses. He noticed
CHAPTER 9. 9.1 Naming Ions. Chemical Names and Formulas. Naming Transition Metals. Ions of Transition Metals. Ions of Transition Metals
 CHAPTER 9 Chemical Names and Formulas 9.1 Naming Ions Monatomic Ions: a single atom with a positive or negative charge Cation (rules): listed first Anion (rules): ide ending Transition Metals have a varying
CHAPTER 9 Chemical Names and Formulas 9.1 Naming Ions Monatomic Ions: a single atom with a positive or negative charge Cation (rules): listed first Anion (rules): ide ending Transition Metals have a varying
4. Using the data from Handout 5, what is the standard enthalpy of formation of BaO (s)? What does this mean?
 HOMEWORK 3A 1. In each of the following pairs, tell which has the higher entropy. (a) One mole of liquid water or one mole of water vapor (b) One mole of dry ice or one mole of carbon dioxide at 1 atm
HOMEWORK 3A 1. In each of the following pairs, tell which has the higher entropy. (a) One mole of liquid water or one mole of water vapor (b) One mole of dry ice or one mole of carbon dioxide at 1 atm
Web Typography Sucks
 Web Typography Sucks Richard Rutter Mark Boulton RR: We re here to talk about web typography sucks - how it does, why it needn t, when it doesn t and how we can all do something about it. Let s talk about
Web Typography Sucks Richard Rutter Mark Boulton RR: We re here to talk about web typography sucks - how it does, why it needn t, when it doesn t and how we can all do something about it. Let s talk about
Global discovery trends 1950-2009: What, where and who found them
 Global discovery trends 1950-2009: What, where and who found them Richard Schodde Managing Director, Panel Discussion: Exploration expenditures are increasing, but discoveries are not. Why? PDAC 2010,
Global discovery trends 1950-2009: What, where and who found them Richard Schodde Managing Director, Panel Discussion: Exploration expenditures are increasing, but discoveries are not. Why? PDAC 2010,
Earthquake Hazard Zones: The relative risk of damage to Canadian buildings
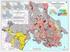 Earthquake Hazard Zones: The relative risk of damage to Canadian buildings by Paul Kovacs Executive Director, Institute for Catastrophic Loss Reduction Adjunct Research Professor, Economics, Univ. of Western
Earthquake Hazard Zones: The relative risk of damage to Canadian buildings by Paul Kovacs Executive Director, Institute for Catastrophic Loss Reduction Adjunct Research Professor, Economics, Univ. of Western
