Chemistry 20 Chapters 13 Lipids
|
|
|
- Audra Lucas
- 7 years ago
- Views:
Transcription
1 Chemistry 20 Chapters 13 ipids ipids: are family of biomolecules that have the common property of being soluble in organic solvents but not in water. Role of lipids: they have three important roles in nature: 1. They store energy and protect and insulate internal organs. In human bodies they are stored as fat cells and in animal bodies as starch. 2. They are found in nerve fibers and hormones as steroids, which act as chemical messengers. 3. Because they are not soluble in water, a major function of lipids is to build the cell membranes that separate the internal contents of cells from the surrounding aqueous environment. ipids are divides into the four groups: 1. Simple lipids (waxes, fats and oils). 2. Complex lipids (glycerophospholipids). 3. Steroid (Cholesterol and steroid hormones). 4. Prostaglandins. s (review): a fatty acid contains a long chain attached to carboxylic acid group at one end. They are insoluble in water because the size of the nonpolar portion is bigger than the size of polar portion (carboxyl group). Normally, fatty acid contains an even number of carbon atoms, usually between 12 and 20. They can be either saturated fatty acids with only single bonds between the carbons or unsaturated fatty acids with one (monounsaturated fatty acid) or more (polyunsaturated fatty acid) double bonds. Note (review): Unsaturated fatty acids can be cis or trans (trans is rare). Unsaturated fatty acids are liquid at room temperature because the cis double bond causes the carbon chain to bend, which gives the molecules an irregular shape. As a result, fewer attractions occur between carbon chain and they have lower melting point. n the other hand, saturated fatty acids are solid at room temperature because they are linear (no bend) and they can pack together in close parallel alignment and the have higher ondon dispersion forces than unsaturated fatty acids. Essential fatty acids: human body is capable of synthesizing most fatty acids from carbohydrates or other fatty acids. However, humans do not synthesize sufficient amounts of fatty acids that have more than one double bond. These fatty acids are called essential fatty acids because they must be provided by the diet (a deficiency of essential fatty acids can cause skin dermatitis in infants. Waxes: are found in many plants and animals. Coating of carnauba wax on fruits and the leaves and stems of plants help to prevent loss of water and damage from pests. Waxes on the skin, fur, and feathers of animals and birds provide a waterproof coating. A wax is an ester of a saturated fatty acid and a long-chain alcohol, each containing from 14 to 30 carbon atoms.
2 ong-chain alcohol Ester bond Note: Beeswax obtained from honeycombs and carnauba wax obtained from palm trees are used to give a protective coating to furniture, cars, and floors. Jojoba wax is used making candles and cosmetics such as lipstick. anolin, a mixture of waxes obtained from wool, is used in hand and facial lotions to aid retention of water, which softens the skin. Fats and oils (Triacylglycerols): in the body, fatty acids are stored as fats and oils known as triacylglycerols (or triglycerides). Triglycerides are trimesters of glycerol (a trihyroxyl alcohol) and fatty acids. A triacylglycerol is produced by esterification (a reaction in which the hydroxyl groups of glycerol form ester bonds with the carboxyl groups of fatty acids. Most fats and oils are mixed triacylglycerols that contain two or three different fatty acids. Note: Triacylglycerols are the major form of energy storage for animals. Animal that hibernate eat large quantities of plants, seed, and nuts that contain high levels of fats and oils. They gain as much as 14 kilograms a week. As the external temperature drop, the animal goes into hibernation. The body temperature drops to nearly freezing and there is a dramatic reduction in cellular activity, respiration, and heart rate. They will hibernate for 4-7 months and during this time, stored fat is the only source of energy. Difference between fat and oil: a fat is a triacylglycerol that is solid at room temperature, such as fats in meat, whole milk, butter, and cheese. Most fats come from animal sources. An oil is a triacylglycerol that is usually liquid at room temperature. The most commonly used oils come from plant sources. live oil and peanut oil are monounsaturated (one double bond C=C). ils from corn, cottonseed, safflower, and sunflower are polyunsaturated (two or more double bond C=C). A few oils such as palm oil and coconut oil are solid at room temperature because they consist mostly of saturated fatty acids (single bond C-C). Animal fats usually contain more saturated fatty acids than do vegetable oils. Dr. Behrang Madani Chemistry 20 Mt SAC
3 Chemical properties of triacylglycerols: the chemical reactions of the triacylglycerols (fats and oils) are the same as we discussed for alkenes, carboxylic acids and esters: 1. Hydrogenation: hydrogenation of unsaturated fats converts carbon-carbon double bonds to single bonds. The hydrogen gas bubbled through the heated oil the presence of a nickel catalyst (or another transition metal). Note: Most of the time the liquid oil hydrogenate to produce solid oil. This process has two advantages: increasing the period of consumption and transporting easer the solid product. In commercial hydrogenation, the addition of hydrogen is stopped before all the double bonds in an oil become completely saturated. As a result, we can obtain a soft and semisolid product instead of a brittle product. This control of hydrogenation gives the various products such as soft margarines, solid stick margarine, and solid shortenings. 2. Hydrolysis: triacylglycerol are hydrolysed (split by water) in the presence of strong acids or digestive enzymes called lipases. The products of hydrolysis of the ester bonds are glycerol and three fatty acids. 3. Saponification: when a fat is heated with a strong base such as sodium hydroxide, saponification of the fat gives glycerol and the sodium salts of the fatty acids, which are soaps. KH produces a softer, liquid soap. ils that are polyunsaturated produce softer soaps. Names like coconut or avocado shampoo tell you the sources of the oil used in the reaction. Fat or oil + Strong base glycerol + salts of fatty acids (soaps) Dr. Behrang Madani Chemistry 20 Mt SAC
4 Glycerophospholipids: are similar to triacylglycerols except that one hydroxyl group of glycerol is replaced by the ester of phosphoric acid and amino alcohol, bonded through a phosphodiester bond. G Y C E R Triacylglycerol (triglyceride) G Y C E R phosphate Glycerophospholipid amino alcohol Three amino alcohols found in glycerophospholipids are clorine, serine, and ethanolamine. Because these compounds exist in the body at physiological ph of 7.4, they are ionized. CH 3 + NH 3 H-CH 2 -CH 2 -N + -CH 3 H-CH 2 -CH-C - + H-CH 2 -CH 2 -NH 3 P CH 3 - Choline Serine Ethanolamine Phosphate Glycerophospholipids contain both polar and nonpolar regions, which allow them to interact with both polar and nonpolar substances. The ionized alcohol and phosphate portion called the head is polar and can hydrogen bond with water. The two fatty acids connected to the glycerol molecule represent the nonpolar tails of the phospholipids. Glycerophospholipids are the most abundant lipids in cell membranes, where they play an important role in cellular permeability. In the body fluids, they combine with the less polar triglycerides and cholesterol to make them more soluble as they are transported in the body. Steroids: are compounds containing the steroid nucleus, which consists of three cyclohexane rings and one cyclopentane ring fused together (no fatty acids). The four rings in the steroid nucleus are designated A, B, C, and D. The carbon atoms are numbered beginning with the carbons in ring A and, in types of steroids like cholesterol, ending with two methyl groups (attached groups make a wide variety of steroid compounds).
5 Cholesterols: is one of the most important and abundant steroids in the body. Cholesterol has methyl groups, alkyl chain and hydroxyl group (-H) attached to the steroid nucleus. Cholesterol in the body: Cholesterol is a component of cellular membranes, myelin sheath, and brain and nerve tissue. It is also found in the liver, bile salts, and skin, where it forms vitamin D. In the adrenal gland, it is used to synthesize steroid hormones. Cholesterol in the body in obtained from eating meats, milk, and eggs, and it is also synthesized by the liver from fats, carbohydrates, and proteins. There is no cholesterol in vegetable and plant products. If the diet is high in cholesterol, the liver produces less. A typical American daily diet includes mg of cholesterol (one of the highest in the world). However, we should consume no more than 300 mg of cholesterol a day. Note: When cholesterol exceeds its saturation level in the bile, gallstones may form. High levels of cholesterol are also associated with the accumulation of lipid deposits (plaque) that line and narrow the coronary arteries. Some research indicates that saturated fats in the diet may stimulate the production of cholesterol by the liver. Steroid hormones: hormones are chemical messengers that serve as a kind of communication system from one part of the body to another. The steroid hormones, which include the sex hormones are closely related in structure to cholesterol and depend on cholesterol for their synthesis. Two important male sex hormones, testosterone and androsterone, promote the growth of muscle and of facial hair and the maturation of the male sex organs and of sperm. The estrogens, a group of female sex hormones, direct the development of female characteristics: the uterus increases in size, fat is deposited in the breasts, and the pelvis broadens. Progesterone prepares the uterus for the implantation of a fertilized egg. ipoproteins: in the body, lipids must be transported through the bloodstream to tissues where they are stored, used for energy, or to make hormones. However, most lipids are nonpolar and insoluble in the aqueous environment of blood. They are made more soluble by combining them with phospholipids and proteins to form water-soluble complexes called lipoproteins. Dr. Behrang Madani Chemistry 20 Mt SAC
6 In general, lipoproteins are spherical particles with an outer surface of polar proteins and phospholipids that surround hundreds of nonpolar molecules of triacylglycerols and cholesteryl esters (formed by the esterification of the hydroxyl group in cholesterol with a fatty acid). Triacylglycerols There are several types of lipoproteins that differ in density, lipid composition, and function: 1. Chylomicrons 2. Very-low-density lipoprotein (VD) 3. ow-density lipoprotein (D) 4. High-density lipoprotein (HD). The chylomicrons formed in the mucosal cells of the small intestine and the VDs formed in the liver transport triacylglycerol, phospholipids, and cholesterol to the tissues for storage or to the muscles for energy. The Ds transport cholesterol to tissues to be used for the synthesis of cell membranes, steroid hormones, and bile salts. When the level of D exceeds the amount of cholesterol needed by the tissues, the Ds deposit cholesterol in the arteries and/or myocardial infarctions (heart stacks). This is why D cholesterol is called bad cholesterol. The HDs remove excess cholesterol from the tissues and carry it to the liver where it is converted to bile salts and eliminated. When HD levels are high, cholesterol that is not needed by the tissues is carried to the liver for elimination rather than deposited in the arteries, which gives the HDs the name of good cholesterol. Most of the cholesterol in the body is synthesized in the liver, although some comes from the diet. Higher HD levels are found in people who exercise regularly and eat less saturated fat. VD Intestine and elimination iver Fat storage cells D HD Heart and muscle Energy Cell membranes: the membrane of a cell separates the contents of a cell from the external fluids. It is semipermeable so that nutrients can enter the cell and waste products can leave. There are two rows of phospholipids in a cell membrane, that they are arranged like a sandwich. Their nonpolar tails, which are hydrophobic (water-fearing), move to the center, while their polar heads, which are hydrophilic (water-loving) align on the outer edge of the membrane. This double row arrangement of phospholipids is called a lipid bilayer.
7 Most of the phospholipids in the lipid bilayer contain unsaturated fatty acids. Due to the kinks in the carbon chains at the cis double bonds, the phospholipids do not fit closely together. As a result, the lipid bilayer is not a rigid, fixed structure, but one that is dynamic and fluid-like. In this liquid-like bilayer, there are also proteins, carbohydrates, and cholesterol molecules. For this reason, the model of biological membranes is referred to as the fluid mosaic model of membranes. Note: In the fluid mosaic model, proteins known as peripheral proteins emerge on just one of the surface, outer or inner. The integral proteins extend through the entire lipid bilayer and appear on both surfaces of membrane. Some carbohydrates are attached to proteins and lipids and they are responsible for cell recognition and communication with chemical messengers such as hormones. Carbohydrate Phospholipid bilayer Nonpolar Polar
Lipids. Classes of Lipids. Types of Lipids. Saturated and Unsaturated Fatty Acids. Fatty Acids. 15.1 Lipids 15.2 Fatty Acids
 hapter 15 15.1 15.2 Fatty Acids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but not in water. named for the Greek word lipos, which means fat. extracted
hapter 15 15.1 15.2 Fatty Acids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but not in water. named for the Greek word lipos, which means fat. extracted
Lipids. There are 2 types of lipids; those that contain the structural component of a fatty acid; and
 Lipids Lipids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but not in water. named for the Greek word lipos, which means fat. extracted from cells using
Lipids Lipids are biomolecules that contain fatty acids or a steroid nucleus. soluble in organic solvents, but not in water. named for the Greek word lipos, which means fat. extracted from cells using
CHEM 121. Chapter 19, Name: Date:
 CHEM 121. Chapter 19, Name: Date: 1. A lipid is any substance of biochemical origin that is A) soluble in water but insoluble in nonpolar solvents B) insoluble in both water and nonpolar solvents C) insoluble
CHEM 121. Chapter 19, Name: Date: 1. A lipid is any substance of biochemical origin that is A) soluble in water but insoluble in nonpolar solvents B) insoluble in both water and nonpolar solvents C) insoluble
(Woods) Chem-131 Lec-19 09-4 Lipids 1. Lipids:
 (Woods) Chem-131 Lec-19 09-4 Lipids 1 Lipids Classifying Lipids Triacylglycerols (triglycerides): a storage form of energy not required for immediate use. Phospholipids, p sphingolipids, p and cholesterol
(Woods) Chem-131 Lec-19 09-4 Lipids 1 Lipids Classifying Lipids Triacylglycerols (triglycerides): a storage form of energy not required for immediate use. Phospholipids, p sphingolipids, p and cholesterol
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids
 The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
The Structure and Function of Macromolecules: Carbohydrates, Lipids & Phospholipids The FOUR Classes of Large Biomolecules All living things are made up of four classes of large biological molecules: Carbohydrates
Ch24_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch24_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Substances originating in plant or animal material and soluble in non-polar organic solvents
Ch24_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Substances originating in plant or animal material and soluble in non-polar organic solvents
Waxes. From the head of sperm whales Structural material of beehives Coating on the leaves of Brazilian palm. Fats and Oils
 Lipids Lipids are organic compounds that contain hydrocarbons which are the foundation for the structure and function of living cells. Lipids are non polar so they are soluble in nonpolar environments
Lipids Lipids are organic compounds that contain hydrocarbons which are the foundation for the structure and function of living cells. Lipids are non polar so they are soluble in nonpolar environments
I The THREE types of LIPIDS
 LECTURE OUTLINE Chapter 5 The Lipids: Fats, Oils, Phospholipids and Sterols I The THREE types of LIPIDS A. Triglycerides (fats & oils)- the MAJOR type of lipid in food and humans. 1. 2 parts of triglyceridesa)
LECTURE OUTLINE Chapter 5 The Lipids: Fats, Oils, Phospholipids and Sterols I The THREE types of LIPIDS A. Triglycerides (fats & oils)- the MAJOR type of lipid in food and humans. 1. 2 parts of triglyceridesa)
Carbohydrates, proteins and lipids
 Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Carbohydrates, proteins and lipids Chapter 3 MACROMOLECULES Macromolecules: polymers with molecular weights >1,000 Functional groups THE FOUR MACROMOLECULES IN LIFE Molecules in living organisms: proteins,
Exam 4 Outline CH 105 Spring 2012
 Exam 4 Outline CH 105 Spring 2012 You need to bring a pencil and your ACT card. Chapter 24: Lipids 1. Describe the properties and types of lipids a. All are hydrophobic b. Fatty acid-based typically contain
Exam 4 Outline CH 105 Spring 2012 You need to bring a pencil and your ACT card. Chapter 24: Lipids 1. Describe the properties and types of lipids a. All are hydrophobic b. Fatty acid-based typically contain
Lipids. Classifying Lipids
 (Woods) Chem-131 Lec-19 09-4 Lipids 1 Lipids Triacylglycerols (triglycerides): a storage form of energy not required for immediate use. Phospholipids, sphingolipids, and cholesterol (together with proteins)
(Woods) Chem-131 Lec-19 09-4 Lipids 1 Lipids Triacylglycerols (triglycerides): a storage form of energy not required for immediate use. Phospholipids, sphingolipids, and cholesterol (together with proteins)
Biochemistry of Cells
 Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
Biochemistry of Cells 1 Carbon-based Molecules Although a cell is mostly water, the rest of the cell consists mostly of carbon-based molecules Organic chemistry is the study of carbon compounds Carbon
BIOLOGICAL MOLECULES OF LIFE
 BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
BIOLOGICAL MOLECULES OF LIFE C A R B O H Y D R A T E S, L I P I D S, P R O T E I N S, A N D N U C L E I C A C I D S The Academic Support Center @ Daytona State College (Science 115, Page 1 of 29) Carbon
Chapter 3 Molecules of Cells
 Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Bio 100 Molecules of cells 1 Chapter 3 Molecules of Cells Compounds containing carbon are called organic compounds Molecules such as methane that are only composed of carbon and hydrogen are called hydrocarbons
Nutrients: Carbohydrates, Proteins, and Fats. Chapter 5 Lesson 2
 Nutrients: Carbohydrates, Proteins, and Fats Chapter 5 Lesson 2 Carbohydrates Definition- the starches and sugars found in foods. Carbohydrates are the body s preferred source of energy providing four
Nutrients: Carbohydrates, Proteins, and Fats Chapter 5 Lesson 2 Carbohydrates Definition- the starches and sugars found in foods. Carbohydrates are the body s preferred source of energy providing four
Organic Compounds. Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for?
 Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Organic Compounds Essential Questions: What is Organic? What are the 4 major Organic Compounds? How are they made? What are they used for? Aristotle: Francesco Redi: What do we already know? Spontaneous
Elements in Biological Molecules
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
How To Understand The Chemistry Of Organic Molecules
 CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
Disaccharides consist of two monosaccharide monomers covalently linked by a glycosidic bond. They function in sugar transport.
 1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
1. The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism s cells. As a basis for understanding this concept: 1.
UNIT (11) MOLECULES OF LIFE: LIPIDS AND PROTEINS
 UNIT (11) MOLECULES OF LIFE: LIPIDS AND PROTEINS 11.1 Types of Lipids Lipids are also biochemical compounds that contain carbon, hydrogen, and oxygen. But lipids, unlike carbohydrates, share no common
UNIT (11) MOLECULES OF LIFE: LIPIDS AND PROTEINS 11.1 Types of Lipids Lipids are also biochemical compounds that contain carbon, hydrogen, and oxygen. But lipids, unlike carbohydrates, share no common
A disaccharide is formed when a dehydration reaction joins two monosaccharides. This covalent bond is called a glycosidic linkage.
 CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
CH 5 Structure & Function of Large Molecules: Macromolecules Molecules of Life All living things are made up of four classes of large biological molecules: carbohydrates, lipids, proteins, and nucleic
Chapter 5. The Structure and Function of Macromolecule s
 Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Chapter 5 The Structure and Function of Macromolecule s Most Macromolecules are polymers: Polymer: (poly: many; mer: part) Large molecules consisting of many identical or similar subunits connected together.
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Unit 2: Cells, Membranes and Signaling CELL MEMBRANE. Chapter 5 Hillis Textbook
 Unit 2: Cells, Membranes and Signaling CELL MEMBRANE Chapter 5 Hillis Textbook HOW DOES THE LAB RELATE TO THE NEXT CHAPTER? SURFACE AREA: the entire outer covering of a cell that enables materials pass.
Unit 2: Cells, Membranes and Signaling CELL MEMBRANE Chapter 5 Hillis Textbook HOW DOES THE LAB RELATE TO THE NEXT CHAPTER? SURFACE AREA: the entire outer covering of a cell that enables materials pass.
Biological molecules:
 Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Biological molecules: All are organic (based on carbon). Monomers vs. polymers: Monomers refer to the subunits that, when polymerized, make up a larger polymer. Monomers may function on their own in some
Chapter 3: Biological Molecules. 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids
 Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Chapter 3: Biological Molecules 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Elements in Biological Molecules Biological macromolecules are made almost entirely of just 6 elements: Carbon (C)
Figure 3-1-1: Alkaline hydrolysis (saponification) of oil to make soaps.
 Chapter 3 CHEMICAL MODIFICATION OF OILS AND FATS From the fats and oils obtained from natural resources, the majority of them are used directly or just after refinement. While the others are used after
Chapter 3 CHEMICAL MODIFICATION OF OILS AND FATS From the fats and oils obtained from natural resources, the majority of them are used directly or just after refinement. While the others are used after
An introduction to the biochemistry of diet.
 An introduction to the biochemistry of diet. SEPA BioScience Montana Module 3 Introduction: The following provides a basic introduction to the biochemistry of three major nutritional components of your
An introduction to the biochemistry of diet. SEPA BioScience Montana Module 3 Introduction: The following provides a basic introduction to the biochemistry of three major nutritional components of your
Chemical Basis of Life Module A Anchor 2
 Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Chemical Basis of Life Module A Anchor 2 Key Concepts: - Water is a polar molecule. Therefore, it is able to form multiple hydrogen bonds, which account for many of its special properties. - Water s polarity
Lab 2 Biochemistry. Learning Objectives. Introduction. Lipid Structure and Role in Food. The lab has the following learning objectives.
 1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
1 Lab 2 Biochemistry Learning Objectives The lab has the following learning objectives. Investigate the role of double bonding in fatty acids, through models. Developing a calibration curve for a Benedict
Organic Chemistry Lab Experiment 4 Preparation and Properties of Soap
 Organic Chemistry Lab Experiment 4 Preparation and Properties of Soap Introduction A soap is the sodium or potassium salt of a long-chain fatty acid. The fatty acid usually contains 12 to 18 carbon atoms.
Organic Chemistry Lab Experiment 4 Preparation and Properties of Soap Introduction A soap is the sodium or potassium salt of a long-chain fatty acid. The fatty acid usually contains 12 to 18 carbon atoms.
Chapter 5: The Structure and Function of Large Biological Molecules
 Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
Name Period Concept 5.1 Macromolecules are polymers, built from monomers 1. The large molecules of all living things fall into just four main classes. Name them. 2. Circle the three classes that are called
The Molecules of Cells
 The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
The Molecules of Cells I. Introduction A. Most of the world s population cannot digest milk-based foods. 1. These people are lactose intolerant because they lack the enzyme lactase. 2. This illustrates
CORPORATE HEALTH LOWERING YOUR CHOLESTEROL & BLOOD PRESSURE
 CORPORATE HEALTH LOWERING YOUR CHOLESTEROL & BLOOD PRESSURE What is Cholesterol? What s wrong with having high cholesterol? Major risk factor for cardiovascular disease Higher the cholesterol higher the
CORPORATE HEALTH LOWERING YOUR CHOLESTEROL & BLOOD PRESSURE What is Cholesterol? What s wrong with having high cholesterol? Major risk factor for cardiovascular disease Higher the cholesterol higher the
Fats, Oils, and Other Lipids
 Chapter 5 Fats, Oils, and Other Lipids Slide Show developed by: Richard C. Krejci, Ph.D. Professor of Public Health Columbia College 9.30.15 Objectives for Chapter 5 1. Describe the three classifications
Chapter 5 Fats, Oils, and Other Lipids Slide Show developed by: Richard C. Krejci, Ph.D. Professor of Public Health Columbia College 9.30.15 Objectives for Chapter 5 1. Describe the three classifications
Reactions of Fats and Fatty Acids
 Reactions of Fats and Fatty Acids Outline Fats and Oils Fatty Acid Biosynthesis Biodiesel Homework We hear quite a lot about the place of fats and oils in human nutrition. Foods high in fat are at the
Reactions of Fats and Fatty Acids Outline Fats and Oils Fatty Acid Biosynthesis Biodiesel Homework We hear quite a lot about the place of fats and oils in human nutrition. Foods high in fat are at the
Carbon-organic Compounds
 Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Elements in Cells The living substance of cells is made up of cytoplasm and the structures within it. About 96% of cytoplasm and its included structures are composed of the elements carbon, hydrogen, oxygen,
Fatty Acids carboxylic acids
 Triglycerides (TG) should actually be called triacylglycerols (TAG). TG or TAG are molecules with a glycerol (a carbohydrate) backbone to which are attached three acyl groups. They represent a concentrated
Triglycerides (TG) should actually be called triacylglycerols (TAG). TG or TAG are molecules with a glycerol (a carbohydrate) backbone to which are attached three acyl groups. They represent a concentrated
Cholesterol and Triglycerides What You Should Know
 Cholesterol and Triglycerides What You Should Know Michael T. McDermott MD Professor of Medicine Endocrinology Practice Director Division of Endocrinology, Metabolism and Diabetes University of Colorado
Cholesterol and Triglycerides What You Should Know Michael T. McDermott MD Professor of Medicine Endocrinology Practice Director Division of Endocrinology, Metabolism and Diabetes University of Colorado
Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids
 VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
VEA Bringing Learning to Life Program Support Notes Macromolecules 1 Carbohydrates, Lipids & Nucleic Acids Grades 10 - College 25mins Teacher Notes by Sue Wright, B. Sc., Dip. Ed. Produced by VEA Pty Ltd
Let s Talk Oils and Fats!
 Lesson Overview Lesson Participants: School Nutrition Assistants/Technicians, School Nutrition Managers, Child and Adult Care Food Program Staff, Teachers Type of Lesson: Short, face-to-face training session
Lesson Overview Lesson Participants: School Nutrition Assistants/Technicians, School Nutrition Managers, Child and Adult Care Food Program Staff, Teachers Type of Lesson: Short, face-to-face training session
Chapter 2. The Chemistry of Life Worksheets
 Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Chapter 2 The Chemistry of Life Worksheets (Opening image courtesy of David Iberri, http://en.wikipedia.org/wiki/file:camkii.png, and under the Creative Commons license CC-BY-SA 3.0.) Lesson 2.1: Matter
Carbohydrates Lipids Proteins Nucleic Acids
 Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
Carbohydrates Lipids Proteins Nucleic Acids Carbon The element of life! All living things contain the element carbon. Organic means it contains carbon The reason for this is because of carbon s ability
4. Which carbohydrate would you find as part of a molecule of RNA? a. Galactose b. Deoxyribose c. Ribose d. Glucose
 1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
1. How is a polymer formed from multiple monomers? a. From the growth of the chain of carbon atoms b. By the removal of an OH group and a hydrogen atom c. By the addition of an OH group and a hydrogen
1. The diagram below represents a biological process
 1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
1. The diagram below represents a biological process 5. The chart below indicates the elements contained in four different molecules and the number of atoms of each element in those molecules. Which set
Worksheet 13.1. Chapter 13: Human biochemistry glossary
 Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Worksheet 13.1 Chapter 13: Human biochemistry glossary α-helix Refers to a secondary structure of a protein where the chain is twisted to form a regular helix, held by hydrogen bonds between peptide bonds
Name: Hour: Elements & Macromolecules in Organisms
 Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
Name: Hour: Elements & Macromolecules in Organisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight. All compounds
BSC 2010 - Exam I Lectures and Text Pages. The Plasma Membrane Structure and Function. Phospholipids. I. Intro to Biology (2-29) II.
 BSC 2010 - Exam I Lectures and Text Pages I. Intro to Biology (2-29) II. Chemistry of Life Chemistry review (30-46) Water (47-57) Carbon (58-67) Macromolecules (68-91) III. Cells and Membranes Cell structure
BSC 2010 - Exam I Lectures and Text Pages I. Intro to Biology (2-29) II. Chemistry of Life Chemistry review (30-46) Water (47-57) Carbon (58-67) Macromolecules (68-91) III. Cells and Membranes Cell structure
Nomenclature of fatty acids. Fatty Acids. Chapter 9: Lipids. Fatty acids are carboxylic acids with a long hydrocarbon chain
 Chapter 9: Lipids Definition: those molecules which can be extracted from biological tissue with a nonpolar solvent Structural relationships of major lipid classes Lipids are essential components of all
Chapter 9: Lipids Definition: those molecules which can be extracted from biological tissue with a nonpolar solvent Structural relationships of major lipid classes Lipids are essential components of all
Keystone Review Practice Test Module A Cells and Cell Processes. 1. Which characteristic is shared by all prokaryotes and eukaryotes?
 Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Keystone Review Practice Test Module A Cells and Cell Processes 1. Which characteristic is shared by all prokaryotes and eukaryotes? a. Ability to store hereditary information b. Use of organelles to control
Endocrine System: Practice Questions #1
 Endocrine System: Practice Questions #1 1. Removing part of gland D would most likely result in A. a decrease in the secretions of other glands B. a decrease in the blood calcium level C. an increase in
Endocrine System: Practice Questions #1 1. Removing part of gland D would most likely result in A. a decrease in the secretions of other glands B. a decrease in the blood calcium level C. an increase in
Overview of Lipid Metabolism
 Overview of Lipid Metabolism Learning Objectives By the end of this lecture the students should be able to understand: Classification of Lipids The digestion, absorption and utilization of dietary lipids
Overview of Lipid Metabolism Learning Objectives By the end of this lecture the students should be able to understand: Classification of Lipids The digestion, absorption and utilization of dietary lipids
I. Chapter 5 Summary. II. Nucleotides & Nucleic Acids. III. Lipids
 I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
I. Chapter 5 Summary A. Simple Sugars (CH 2 O) n : 1. One C contains a carbonyl (C=O) rest contain - 2. Classification by functional group: aldoses & ketoses 3. Classification by number of C's: trioses,
Chapter 48. Nutrients in Food. Carbohydrates, Proteins, and Lipids. Carbohydrates, Proteins, and Lipids, continued
 Carbohydrates, Proteins, and Lipids The three nutrients needed by the body in the greatest amounts are carbohydrates, proteins, and lipids. Nutrients in Food All of these nutrients are called organic compounds,
Carbohydrates, Proteins, and Lipids The three nutrients needed by the body in the greatest amounts are carbohydrates, proteins, and lipids. Nutrients in Food All of these nutrients are called organic compounds,
1. Essay: The Digestive and Absorption Processes of Macronutrients
 Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Jenny Kim Professor Rosario Nutrition: Macronutrients Project June 26, 2014 1. Essay: The Digestive and Absorption Processes of Macronutrients Whenever we eat, the foods we ingest in our bodies undergo
Health Maintenance: Controlling Cholesterol
 Sacramento Heart & Vascular Medical Associates February 18, 2012 500 University Ave. Sacramento, CA 95825 Page 1 What is cholesterol? Cholesterol is a fatty substance. It has both good and bad effects
Sacramento Heart & Vascular Medical Associates February 18, 2012 500 University Ave. Sacramento, CA 95825 Page 1 What is cholesterol? Cholesterol is a fatty substance. It has both good and bad effects
Determination of Specific Nutrients in Various Foods. Abstract. Humans need to consume food compounds such as carbohydrates, proteins, fats,
 Determination of Specific Nutrients in Various Foods Abstract Humans need to consume food compounds such as carbohydrates, proteins, fats, and vitamins to meet their energy requirements. In this lab, reagents
Determination of Specific Nutrients in Various Foods Abstract Humans need to consume food compounds such as carbohydrates, proteins, fats, and vitamins to meet their energy requirements. In this lab, reagents
Elements & Macromolecules in Organisms
 Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Name: Date: Per: Table # Elements & Macromolecules in rganisms Most common elements in living things are carbon, hydrogen, nitrogen, and oxygen. These four elements constitute about 95% of your body weight.
Chapter 25: Metabolism and Nutrition
 Chapter 25: Metabolism and Nutrition Chapter Objectives INTRODUCTION 1. Generalize the way in which nutrients are processed through the three major metabolic fates in order to perform various energetic
Chapter 25: Metabolism and Nutrition Chapter Objectives INTRODUCTION 1. Generalize the way in which nutrients are processed through the three major metabolic fates in order to perform various energetic
Anatomy and Physiology Placement Exam 2 Practice with Answers at End!
 Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
Anatomy and Physiology Placement Exam 2 Practice with Answers at End! General Chemical Principles 1. bonds are characterized by the sharing of electrons between the participating atoms. a. hydrogen b.
10.1 The function of Digestion pg. 402
 10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
10.1 The function of Digestion pg. 402 Macromolecules and Living Systems The body is made up of more than 60 % water. The water is found in the cells cytoplasm, the interstitial fluid and the blood (5
Lab 3 Organic Molecules of Biological Importance
 Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
Name Biology 3 ID Number Lab 3 Organic Molecules of Biological Importance Section 1 - Organic Molecules Section 2 - Functional Groups Section 3 - From Building Blocks to Macromolecules Section 4 - Carbohydrates
BIOLOGICAL MEMBRANES: FUNCTIONS, STRUCTURES & TRANSPORT
 BIOLOGICAL MEMBRANES: FUNCTIONS, STRUCTURES & TRANSPORT UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY BMLS II / B Pharm II / BDS II VJ Temple
BIOLOGICAL MEMBRANES: FUNCTIONS, STRUCTURES & TRANSPORT UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY BMLS II / B Pharm II / BDS II VJ Temple
1.1.2. thebiotutor. AS Biology OCR. Unit F211: Cells, Exchange & Transport. Module 1.2 Cell Membranes. Notes & Questions.
 thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
thebiotutor AS Biology OCR Unit F211: Cells, Exchange & Transport Module 1.2 Cell Membranes Notes & Questions Andy Todd 1 Outline the roles of membranes within cells and at the surface of cells. The main
OVERVIEW OF LIPID METABOLISM
 VERVIEW F LIPID METABLISM Date: September 20, 2005 * Time: 8:00 am 8:50 am * Room: G202 Biomolecular Building Lecturer: Steve Chaney 515A Mary Ellen Jones Building stephen_chaney@med.unc.edu 9663286 *Please
VERVIEW F LIPID METABLISM Date: September 20, 2005 * Time: 8:00 am 8:50 am * Room: G202 Biomolecular Building Lecturer: Steve Chaney 515A Mary Ellen Jones Building stephen_chaney@med.unc.edu 9663286 *Please
Blood clot in atheroma. help make vitamin D and hormones, like oestrogen and testosterone, in your body.
 CHOLESTEROL This factsheet explains what cholesterol is and why too much cholesterol in your blood is harmful. It also provides information regarding cholesterol testing and tips to help reduce your blood
CHOLESTEROL This factsheet explains what cholesterol is and why too much cholesterol in your blood is harmful. It also provides information regarding cholesterol testing and tips to help reduce your blood
Elevated Cholesterol and Homocysteine
 Elevated Cholesterol and Homocysteine The evidence linking inflammation of the blood vessels and heart disease/hardening of the arteries is well documented. There is considerable debate about the role
Elevated Cholesterol and Homocysteine The evidence linking inflammation of the blood vessels and heart disease/hardening of the arteries is well documented. There is considerable debate about the role
Six major functions of membrane proteins: Transport Enzymatic activity
 CH 7 Membranes Cellular Membranes Phospholipids are the most abundant lipid in the plasma membrane. Phospholipids are amphipathic molecules, containing hydrophobic and hydrophilic regions. The fluid mosaic
CH 7 Membranes Cellular Membranes Phospholipids are the most abundant lipid in the plasma membrane. Phospholipids are amphipathic molecules, containing hydrophobic and hydrophilic regions. The fluid mosaic
1. (U4C1L4:G9) T or F: The human body is composed of 60 to 70 percent water. 2. (U4C1L4:G13) Another name for fiber in a diet is.
 Cadet Name: Date: 1. (U4C1L4:G9) T or F: The human body is composed of 60 to 70 percent water. A) True B) False 2. (U4C1L4:G13) Another name for fiber in a diet is. A) vegetables B) laxative C) fruit D)
Cadet Name: Date: 1. (U4C1L4:G9) T or F: The human body is composed of 60 to 70 percent water. A) True B) False 2. (U4C1L4:G13) Another name for fiber in a diet is. A) vegetables B) laxative C) fruit D)
CELL MEMBRANES, TRANSPORT, and COMMUNICATION. Teacher Packet
 AP * BIOLOGY CELL MEMBRANES, TRANSPORT, and COMMUNICATION Teacher Packet AP* is a trademark of the College Entrance Examination Board. The College Entrance Examination Board was not involved in the production
AP * BIOLOGY CELL MEMBRANES, TRANSPORT, and COMMUNICATION Teacher Packet AP* is a trademark of the College Entrance Examination Board. The College Entrance Examination Board was not involved in the production
Lipid Metabolism. Dr. Howaida Nounou Biochemistry department Sciences college
 Lipid Metabolism Dr. Howaida Nounou Biochemistry department Sciences college Lipids - Heterogenous group of biomolecules - Water insoluble (hydrophobic) - Soluble in organic and non-polar solvents acetone,
Lipid Metabolism Dr. Howaida Nounou Biochemistry department Sciences college Lipids - Heterogenous group of biomolecules - Water insoluble (hydrophobic) - Soluble in organic and non-polar solvents acetone,
Laboratory 28: Properties of Lipids
 Introduction Lipids are naturally occuring substances that are arbitrarily grouped together on the basis of their insolubility in water (a polar solvent) and solubility in nonpolar solvents. Lipids include
Introduction Lipids are naturally occuring substances that are arbitrarily grouped together on the basis of their insolubility in water (a polar solvent) and solubility in nonpolar solvents. Lipids include
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES
 LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
LAB 3: DIGESTION OF ORGANIC MACROMOLECULES INTRODUCTION Enzymes are a special class of proteins that lower the activation energy of biological reactions. These biological catalysts change the rate of chemical
Chemistry 20 Chapters 15 Enzymes
 Chemistry 20 Chapters 15 Enzymes Enzymes: as a catalyst, an enzyme increases the rate of a reaction by changing the way a reaction takes place, but is itself not changed at the end of the reaction. An
Chemistry 20 Chapters 15 Enzymes Enzymes: as a catalyst, an enzyme increases the rate of a reaction by changing the way a reaction takes place, but is itself not changed at the end of the reaction. An
Chapter 2 Chemical Principles
 Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Chapter 2 Chemical Principles I. Chemistry. [Students should read this section on their own]. a. Chemistry is the study of the interactions between atoms and molecules. b. The atom is the smallest unit
Sinclair Community College, Division of Allied Health Technologies
 Sinclair Community College, Division of Allied Health Technologies Health Promotion for Community Health Workers Cardiovascular disease, stroke, and cancer Class #5 High Blood Cholesterol (date) Course
Sinclair Community College, Division of Allied Health Technologies Health Promotion for Community Health Workers Cardiovascular disease, stroke, and cancer Class #5 High Blood Cholesterol (date) Course
The Molecules of Life - Overview. The Molecules of Life. The Molecules of Life. The Molecules of Life
 The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
The Molecules of Life - Overview The Molecules of Life The Importance of Carbon Organic Polymers / Monomers Functions of Organic Molecules Origin of Organic Molecules The Molecules of Life Water is the
Cholesterol made simple!
 Cholesterol made simple! Cholesterol is the biggest risk factor for heart disease and also increases your risk of stroke and circulatory disease - Heart UK The Cholesterol Charity What is Cholesterol and
Cholesterol made simple! Cholesterol is the biggest risk factor for heart disease and also increases your risk of stroke and circulatory disease - Heart UK The Cholesterol Charity What is Cholesterol and
Biology 12 June 2003 Provincial Examination
 Biology 12 June 2003 rovincial Examination ANWER KEY / CORING GUIDE CURRICULUM: Organizers 1. Cell Biology 2. Cell rocesses and Applications 3. Human Biology ub-organizers A, B, C, D E, F, G, H I, J, K,
Biology 12 June 2003 rovincial Examination ANWER KEY / CORING GUIDE CURRICULUM: Organizers 1. Cell Biology 2. Cell rocesses and Applications 3. Human Biology ub-organizers A, B, C, D E, F, G, H I, J, K,
Ch. 8 - The Cell Membrane
 Ch. 8 - The Cell Membrane 2007-2008 Phospholipids Phosphate head hydrophilic Fatty acid tails hydrophobic Arranged as a bilayer Phosphate attracted to water Fatty acid repelled by water Aaaah, one of those
Ch. 8 - The Cell Membrane 2007-2008 Phospholipids Phosphate head hydrophilic Fatty acid tails hydrophobic Arranged as a bilayer Phosphate attracted to water Fatty acid repelled by water Aaaah, one of those
FIGURE 2.18. A. The phosphate end of the molecule is polar (charged) and hydrophilic (attracted to water).
 PLASMA MEMBRANE 1. The plasma membrane is the outermost part of a cell. 2. The main component of the plasma membrane is phospholipids. FIGURE 2.18 A. The phosphate end of the molecule is polar (charged)
PLASMA MEMBRANE 1. The plasma membrane is the outermost part of a cell. 2. The main component of the plasma membrane is phospholipids. FIGURE 2.18 A. The phosphate end of the molecule is polar (charged)
How To Understand The Human Body
 Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Introduction to Biology and Chemistry Outline I. Introduction to biology A. Definition of biology - Biology is the study of life. B. Characteristics of Life 1. Form and size are characteristic. e.g. A
Chapter 6. Solution, Acids and Bases
 Chapter 6 Solution, Acids and Bases Mixtures Two or more substances Heterogeneous- different from place to place Types of heterogeneous mixtures Suspensions- Large particles that eventually settle out
Chapter 6 Solution, Acids and Bases Mixtures Two or more substances Heterogeneous- different from place to place Types of heterogeneous mixtures Suspensions- Large particles that eventually settle out
Digestive System Why is digestion important? How is food digested? Physical Digestion and Movement
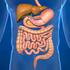 Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
Digestive System The digestive system is made up of the digestive tract a series of hollow organs joined in a long, twisting tube from the mouth to the anus and other organs that help the body break down
Membrane Structure and Function
 Membrane Structure and Function -plasma membrane acts as a barrier between cells and the surrounding. -plasma membrane is selective permeable -consist of lipids, proteins and carbohydrates -major lipids
Membrane Structure and Function -plasma membrane acts as a barrier between cells and the surrounding. -plasma membrane is selective permeable -consist of lipids, proteins and carbohydrates -major lipids
Biological cell membranes
 Unit 14: Cell biology. 14 2 Biological cell membranes The cell surface membrane surrounds the cell and acts as a barrier between the cell s contents and the environment. The cell membrane has multiple
Unit 14: Cell biology. 14 2 Biological cell membranes The cell surface membrane surrounds the cell and acts as a barrier between the cell s contents and the environment. The cell membrane has multiple
Types of Cooking Fats and Oils - Smoking Points of Fats and Oils
 Types of Cooking Fats and s - Smoking Points of Fats and s Not all fats are the same. Following are some basics on the various types of fats to help you make sense of what is best for your own body. Saturated
Types of Cooking Fats and s - Smoking Points of Fats and s Not all fats are the same. Following are some basics on the various types of fats to help you make sense of what is best for your own body. Saturated
Using the Nutrition Facts Label
 Using the Nutrition Facts Label A How-To Guide for Older Adults Inside Why Nutrition Matters For You...1 At-A-Glance: The Nutrition Facts Label...2 3 Key Areas of Importance...4 Your Guide To a Healthy
Using the Nutrition Facts Label A How-To Guide for Older Adults Inside Why Nutrition Matters For You...1 At-A-Glance: The Nutrition Facts Label...2 3 Key Areas of Importance...4 Your Guide To a Healthy
Plasma Membrane hydrophilic polar heads
 The Parts of the Cell 3 main parts in ALL cells: plasma membrane, cytoplasm, genetic material this is about the parts of a generic eukaryotic cell Plasma Membrane -is a fluid mosaic model membrane is fluid
The Parts of the Cell 3 main parts in ALL cells: plasma membrane, cytoplasm, genetic material this is about the parts of a generic eukaryotic cell Plasma Membrane -is a fluid mosaic model membrane is fluid
Overview of Fat Digestion and Metabolism in Dairy Cows
 Overview of Fat Digestion and Metabolism in Dairy Cows James K. Drackley Professor of Animal Sciences University of Illinois, Urbana email: drackley@uiuc.edu Introduction Over the last 25 years, the use
Overview of Fat Digestion and Metabolism in Dairy Cows James K. Drackley Professor of Animal Sciences University of Illinois, Urbana email: drackley@uiuc.edu Introduction Over the last 25 years, the use
18.2 Protein Structure and Function: An Overview
 18.2 Protein Structure and Function: An Overview Protein: A large biological molecule made of many amino acids linked together through peptide bonds. Alpha-amino acid: Compound with an amino group bonded
18.2 Protein Structure and Function: An Overview Protein: A large biological molecule made of many amino acids linked together through peptide bonds. Alpha-amino acid: Compound with an amino group bonded
Nutritional Glossary. Index of Contents
 Nutritional Glossary This glossary provides nutrition information about the nutrients commonly found in fruits, vegetables, and other plant foods Each glossary definition has a long and a short version.
Nutritional Glossary This glossary provides nutrition information about the nutrients commonly found in fruits, vegetables, and other plant foods Each glossary definition has a long and a short version.
Organic Molecules of Life - Exercise 2
 Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Organic Molecules of Life - Exercise 2 Objectives -Know the difference between a reducing sugar and a non-reducing sugar. -Distinguish Monosaccharides from Disaccharides and Polysaccharides -Understand
Preliminary MFM Quiz
 Preliminary MFM Quiz 1. The major carrier of chemical energy in all cells is: A) adenosine monophosphate B) adenosine diphosphate C) adenosine trisphosphate D) guanosine trisphosphate E) carbamoyl phosphate
Preliminary MFM Quiz 1. The major carrier of chemical energy in all cells is: A) adenosine monophosphate B) adenosine diphosphate C) adenosine trisphosphate D) guanosine trisphosphate E) carbamoyl phosphate
Introduction to Biodiesel Chemistry Terms and Background Information
 Introduction to Biodiesel Chemistry Terms and Background Information Basic rganic Chemistry rganic chemistry is the branch of chemistry that deals with organic compounds. rganic compounds are compounds
Introduction to Biodiesel Chemistry Terms and Background Information Basic rganic Chemistry rganic chemistry is the branch of chemistry that deals with organic compounds. rganic compounds are compounds
Macromolecules in my food!!
 Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
Macromolecules in my food!! Name Notes/Background Information Food is fuel: All living things need to obtain fuel from something. Whether it is self- made through the process of photosynthesis, or by ingesting
Cell Membrane Coloring Worksheet
 Cell Membrane Coloring Worksheet Composition of the Cell Membrane & Functions The cell membrane is also called the plasma membrane and is made of a phospholipid bilayer. The phospholipids have a hydrophilic
Cell Membrane Coloring Worksheet Composition of the Cell Membrane & Functions The cell membrane is also called the plasma membrane and is made of a phospholipid bilayer. The phospholipids have a hydrophilic
Trans Fats Lessons Learned
 Trans Fats Lessons Learned Karen Omichinski, B.H.Ec.,., RD, CDE North Eastman Health Association CDEN Workshop February 9, 2007 Trans Fats: Outline Chemistry History Health Risks of Industrial Trans Fats
Trans Fats Lessons Learned Karen Omichinski, B.H.Ec.,., RD, CDE North Eastman Health Association CDEN Workshop February 9, 2007 Trans Fats: Outline Chemistry History Health Risks of Industrial Trans Fats
Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly)
 Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly) 6 basic nutrients - 4 food groups (milk, meat, fruit and vegetable,
Chapter 49 - Nutrients and the Digestive System I. Nutrients (chemical substances necessary for organisms to grow and function properly) 6 basic nutrients - 4 food groups (milk, meat, fruit and vegetable,
The chemical reactions inside cells are controlled by enzymes. Cells may be specialised to carry out a particular function.
 12.1 What are animals and plants built from? All living things are made up of cells. The structures of different types of cells are related to their functions. to relate the structure of different types
12.1 What are animals and plants built from? All living things are made up of cells. The structures of different types of cells are related to their functions. to relate the structure of different types
