Phase transformation
|
|
|
- Dylan Nichols
- 7 years ago
- Views:
Transcription
1 Phase transformation
2 Phase Transformation Topics o Basic principles relating to transformations o Kinetics of phase transformation: Nucleation and growth o Metastable and equilibrium state o Shape memory alloys
3 Phase Transformation Terminology Transformation rate reaction progress on time Phase transformation alteration of one or more phases to other phase(s) types of phase transformation: diffusion-independent transformations diffusion-dependent transformations diffusionless transformations
4 Phase Transformation Diffusion-independent transformations No change in either the number or composition of the phases present. solidification of a pure metal allotropic transformations (e.g.: α phase β phase) recrystallization and grain growth
5 Phase Transformation Diffusion-dependent transformations Some alteration in phase compositions and often in the number of phases present. The final microstructure ordinarily consists of two phases. e.g.: eutectoid reaction
6 Phase Transformation Diffusionless transformations The phase transformation occours without diffusion. A metastable phase is produced. Martensitic transformation
7 nucleation formation of numerous small particles new phase(s) Liquid phase Liquid phase growth increase in size until the transformation has reached completion Grain boundaries nuclei Growing crystals Grains (crystals) Liquid-to-solid and solid-state transformations
8 Nucleation Two types: homogeneous: heterogeneous: The new phase forms uniformly throughout the parent phase. The new phase(s) prefers structural inhomogeneities, such as container surfaces, insoluble impurities, grain boundaries, dislocations, etc. homogeneous heterogeneous
9 Homogeneous nucleation G: Gibbs free energy (G=H-TS) ΔG: change in free energy A transformation will occur spontaneously only when G has a negative value. Nuclei of the solid phase form in the interior of the liquid, atoms cluster together. Assumption: each nucleus is spherical with a radius r Gibbs free energy difference between the two states: liquid phase ΔG Solid phase + small crystals within liquid nucleus
10 Homogeneous nucleation 2 component of G: G v the energy difference between the liquid and solid phase ΔG v < 0 if T < T solidification γ 4 surface free energy 3 πr3 volume of the spherical nucleus 4πr 2 surface area of the nucleus G = 4 3 πr3 G v + 4πr 2 γ difference between the solid and liquid phases X volume formation of the solid liquid phase boundary
11 G = 4 3 πr3 G v + 4πr 2 γ
12 G = 4 3 πr3 G v + 4πr 2 γ critical radius r* r < r* Shrink and redissolve r > r* growth nucleus energy barrier to the nucleation process: critical free energy, ΔG* (activation free energy)
13 Homogeneous nucleation Critical radius and free energy: G = 4 3 πr3 G v + 4πr 2 γ d G dr = 4πr 2 G v + 8πr γ = 0 r = 2γ G v Critical radius of a stable solid particle G = 16πγ3 3 G v 2 Energy required for the formation of a stable nucleus
14 Homogeneous nucleation G v is a function of temperature G v = H f(t m T) T m H f is the latent heat T m melting temperature in K Lowering of temperature at temperatures below the equilibrium solidification temperature (T m ), nucleation occurs more readily. r = 2γT m H f (T m T) G = 16πγ 3 T m 2 3 H f 2 (T m T) 2
15 Homogeneous nucleation The number of stable nuclei n* (r > r*) is a function of temperature n = K 1 e G kt K 1 - constant 1) If T lowered below T m, the magnitude of n* increases. 2) Short-range diffusion during the formation of nuclei: temperature s effect on the rate of diffusion (D diffusion coefficient) D = D 0 e Q RT the frequency at which atoms from the liquid attach themselves to the solid nucleus, v d. v d = K 2 e Q kt
16 supercooling Nr of stable nuclei * diffusion = Nucleation rate n = K 1 e G kt v d = K 2 e Q kt
17 Homogeneous nucleation Nucleation rate The small activation driving force suppresses the nucleation rate The low atomic mobility suppresses the nucleation rate
18 Heterogeneous nucleation Nuclei form on a surfaces or interfaces Activation energy for nucleation is lowered when nuclei form on preexisting surfaces or interfaces, because the surface free energy is reduced. surface tension force balance: γ IL = γ SI +γ SL cos(θ) θ - wetting angle γ IL - Interface-liquid surface energy γ SI - Solid-Interface surface energy γ SL - Solid-liquid surface energy
19 Heterogeneous nucleation r = 2γ SL G v G = 16πγ SL G v S(θ)=0 1 S(θ) G het = G hom S(θ)
20 Growth growth of the new phase particles long-range atomic diffusion Growing nucleus diffusion through the original phase The growth process stops in any region where particles of the new phase meet the transformation is completed The growth rate is determined by the diffusion rate G = Ce Q kt
21 Growth Liquid phase Liquid phase Grain boundaries nuclei Growing crystals Grains (crystals)
22 Overal transformation rate
23 Solid-solid transformation The same general principles Time dependency Avrami equation typical kinetic behavior for most solid-state reactions y = 1 e ktn T=constant k, n parameters f(t,p)
24 Solid-solid transformation Percent recrystallization as a function of time and at constant temperature for pure copper.
25 Metastable vs equilibrium states Equilibrium states Infinite slow cooling / heating Phase diagrams Metastabele states supercooling or superheating For other than infinite slow cooling / heating, transformations are shifted to lower / higher temperatures than indicated by the phase diagram Isothermal transformation diagrams Continuous cooling transformation diagrams
26 Metastable vs equilibrium states Isothermal transformation diagrams Isothermal diagram for an eutectoid iron carbon alloy
27 Metastable vs equilibrium states Continuous transformation diagrams cooling Continuous cooling diagram for an eutectoid iron carbon alloy
28 Martensitic transformation Diffusionless transformation Martensite: non-equilibrium single-phase structure (iron alloys) Face centered cubic phase transforms to a body-centered tetragonal (BCT) martensite. Cooperative movements of atom without diffusion, very fast, when the material is cooled to the transformation temperature
29 Martensitic transformation Model of the transformation
30 Martensitic transf. Shape Memory alloys Diffusionless transformation shape-memory alloy: shape-memory effect: may have two crystal structures (or phases) involves phase transformation. One phase (austenite phase) body-centered cubic structure that exists at elevated Temperatures. Upon cooling, the austenite transforms to a (a body-centered tetragonal) martensite phase. Martensite is heavily twinned, deformation occurs by the migration of twin boundaries. Twin boundary: atoms on one side of the boundary are located in mirror-image positions
31 Shape Memory alloys
Chapter Outline: Phase Transformations in Metals
 Chapter Outline: Phase Transformations in Metals Heat Treatment (time and temperature) Microstructure Mechanical Properties Kinetics of phase transformations Multiphase Transformations Phase transformations
Chapter Outline: Phase Transformations in Metals Heat Treatment (time and temperature) Microstructure Mechanical Properties Kinetics of phase transformations Multiphase Transformations Phase transformations
Iron-Carbon Phase Diagram (a review) see Callister Chapter 9
 Iron-Carbon Phase Diagram (a review) see Callister Chapter 9 University of Tennessee, Dept. of Materials Science and Engineering 1 The Iron Iron Carbide (Fe Fe 3 C) Phase Diagram In their simplest form,
Iron-Carbon Phase Diagram (a review) see Callister Chapter 9 University of Tennessee, Dept. of Materials Science and Engineering 1 The Iron Iron Carbide (Fe Fe 3 C) Phase Diagram In their simplest form,
Kinetics of Phase Transformations: Nucleation & Growth
 Kinetics of Phase Transformations: Nucleation & Growth Radhika Barua Department of Chemical Engineering Northeastern University Boston, MA USA Thermodynamics of Phase Transformation Northeastern University
Kinetics of Phase Transformations: Nucleation & Growth Radhika Barua Department of Chemical Engineering Northeastern University Boston, MA USA Thermodynamics of Phase Transformation Northeastern University
Phase Transformations in Metals and Alloys
 Phase Transformations in Metals and Alloys THIRD EDITION DAVID A. PORTER, KENNETH E. EASTERLING, and MOHAMED Y. SHERIF ( г йс) CRC Press ^ ^ ) Taylor & Francis Group Boca Raton London New York CRC Press
Phase Transformations in Metals and Alloys THIRD EDITION DAVID A. PORTER, KENNETH E. EASTERLING, and MOHAMED Y. SHERIF ( г йс) CRC Press ^ ^ ) Taylor & Francis Group Boca Raton London New York CRC Press
Introduction to Materials Science, Chapter 9, Phase Diagrams. Phase Diagrams. University of Tennessee, Dept. of Materials Science and Engineering 1
 Phase Diagrams University of Tennessee, Dept. of Materials Science and Engineering 1 Chapter Outline: Phase Diagrams Microstructure and Phase Transformations in Multicomponent Systems Definitions and basic
Phase Diagrams University of Tennessee, Dept. of Materials Science and Engineering 1 Chapter Outline: Phase Diagrams Microstructure and Phase Transformations in Multicomponent Systems Definitions and basic
Lecture 19: Eutectoid Transformation in Steels: a typical case of Cellular
 Lecture 19: Eutectoid Transformation in Steels: a typical case of Cellular Precipitation Today s topics Understanding of Cellular transformation (or precipitation): when applied to phase transformation
Lecture 19: Eutectoid Transformation in Steels: a typical case of Cellular Precipitation Today s topics Understanding of Cellular transformation (or precipitation): when applied to phase transformation
Heat Treatment of Steel
 Heat Treatment of Steel Steels can be heat treated to produce a great variety of microstructures and properties. Generally, heat treatment uses phase transformation during heating and cooling to change
Heat Treatment of Steel Steels can be heat treated to produce a great variety of microstructures and properties. Generally, heat treatment uses phase transformation during heating and cooling to change
The atomic packing factor is defined as the ratio of sphere volume to the total unit cell volume, or APF = V S V C. = 2(sphere volume) = 2 = V C = 4R
 3.5 Show that the atomic packing factor for BCC is 0.68. The atomic packing factor is defined as the ratio of sphere volume to the total unit cell volume, or APF = V S V C Since there are two spheres associated
3.5 Show that the atomic packing factor for BCC is 0.68. The atomic packing factor is defined as the ratio of sphere volume to the total unit cell volume, or APF = V S V C Since there are two spheres associated
SOLIDIFICATION. (a)formation of stable nuclei. Growth of a stable nucleus. (c) Grain structure
 SOLIDIFICATION Most metals are melted and then cast into semifinished or finished shape. Solidification of a metal can be divided into the following steps: Formation of a stable nucleus Growth of a stable
SOLIDIFICATION Most metals are melted and then cast into semifinished or finished shape. Solidification of a metal can be divided into the following steps: Formation of a stable nucleus Growth of a stable
Lecture: 33. Solidification of Weld Metal
 Lecture: 33 Solidification of Weld Metal This chapter presents common solidification mechanisms observed in weld metal and different modes of solidification. Influence of welding speed and heat input on
Lecture: 33 Solidification of Weld Metal This chapter presents common solidification mechanisms observed in weld metal and different modes of solidification. Influence of welding speed and heat input on
Lecture 12: Heterogeneous Nucleation: a surface catalyzed process
 Lecture 1: Heterogeneous Nucleation: a surface catalyzed process Today s topics What is heterogeneous nucleation? What implied in real practice of materials processing and phase transformation? Heterogeneous
Lecture 1: Heterogeneous Nucleation: a surface catalyzed process Today s topics What is heterogeneous nucleation? What implied in real practice of materials processing and phase transformation? Heterogeneous
9.11 Upon heating a lead-tin alloy of composition 30 wt% Sn-70 wt% Pb from 150 C and utilizing Figure
 9-13 9.8: 9.11 Upon heating a lead-tin alloy of composition 30 wt% Sn-70 wt% Pb from 150 C and utilizing Figure (a) The first liquid forms at the temperature at which a vertical line at this composition
9-13 9.8: 9.11 Upon heating a lead-tin alloy of composition 30 wt% Sn-70 wt% Pb from 150 C and utilizing Figure (a) The first liquid forms at the temperature at which a vertical line at this composition
Defects Introduction. Bonding + Structure + Defects. Properties
 Defects Introduction Bonding + Structure + Defects Properties The processing determines the defects Composition Bonding type Structure of Crystalline Processing factors Defects Microstructure Types of
Defects Introduction Bonding + Structure + Defects Properties The processing determines the defects Composition Bonding type Structure of Crystalline Processing factors Defects Microstructure Types of
BINARY SYSTEMS. Definition of Composition: Atomic (molar) fraction. Atomic percent. Mass fraction. Mass percent (weight percent)
 BINARY SYSTEMS Definition of Composition: Atomic (molar) fraction Atomic percent Mass fraction Mass percent (weight percent) na =, x i n = A i i i Weight percent mainly in industry! x at % A = x 100 A
BINARY SYSTEMS Definition of Composition: Atomic (molar) fraction Atomic percent Mass fraction Mass percent (weight percent) na =, x i n = A i i i Weight percent mainly in industry! x at % A = x 100 A
Phase Equilibria & Phase Diagrams
 Phase Equilibria & Phase Diagrams Week7 Material Sciences and Engineering MatE271 1 Motivation Phase diagram (Ch 9) Temperature Time Kinematics (Ch 10) New structure, concentration (mixing level) (at what
Phase Equilibria & Phase Diagrams Week7 Material Sciences and Engineering MatE271 1 Motivation Phase diagram (Ch 9) Temperature Time Kinematics (Ch 10) New structure, concentration (mixing level) (at what
Chapter Outline Dislocations and Strengthening Mechanisms
 Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
Chapter 8. Phase Diagrams
 Phase Diagrams A phase in a material is a region that differ in its microstructure and or composition from another region Al Al 2 CuMg H 2 O(solid, ice) in H 2 O (liquid) 2 phases homogeneous in crystal
Phase Diagrams A phase in a material is a region that differ in its microstructure and or composition from another region Al Al 2 CuMg H 2 O(solid, ice) in H 2 O (liquid) 2 phases homogeneous in crystal
Strengthening. Mechanisms of strengthening in single-phase metals: grain-size reduction solid-solution alloying strain hardening
 Strengthening The ability of a metal to deform depends on the ability of dislocations to move Restricting dislocation motion makes the material stronger Mechanisms of strengthening in single-phase metals:
Strengthening The ability of a metal to deform depends on the ability of dislocations to move Restricting dislocation motion makes the material stronger Mechanisms of strengthening in single-phase metals:
MSE 528 - PRECIPITATION HARDENING IN 7075 ALUMINUM ALLOY
 MSE 528 - PRECIPITATION HARDENING IN 7075 ALUMINUM ALLOY Objective To study the time and temperature variations in the hardness and electrical conductivity of Al-Zn-Mg-Cu high strength alloy on isothermal
MSE 528 - PRECIPITATION HARDENING IN 7075 ALUMINUM ALLOY Objective To study the time and temperature variations in the hardness and electrical conductivity of Al-Zn-Mg-Cu high strength alloy on isothermal
The mechanical properties of metal affected by heat treatment are:
 Training Objective After watching this video and reviewing the printed material, the student/trainee will learn the basic concepts of the heat treating processes as they pertain to carbon and alloy steels.
Training Objective After watching this video and reviewing the printed material, the student/trainee will learn the basic concepts of the heat treating processes as they pertain to carbon and alloy steels.
Formation of solids from solutions and melts
 Formation of solids from solutions and melts Solids from a liquid phase. 1. The liquid has the same composition as the solid. Formed from the melt without any chemical transformation. Crystallization and
Formation of solids from solutions and melts Solids from a liquid phase. 1. The liquid has the same composition as the solid. Formed from the melt without any chemical transformation. Crystallization and
Module 34. Heat Treatment of steel IV. Lecture 34. Heat Treatment of steel IV
 Module 34 Heat reatment of steel IV Lecture 34 Heat reatment of steel IV 1 Keywords : Austenitization of hypo & hyper eutectoid steel, austenization temperature, effect of heat treatment on structure &
Module 34 Heat reatment of steel IV Lecture 34 Heat reatment of steel IV 1 Keywords : Austenitization of hypo & hyper eutectoid steel, austenization temperature, effect of heat treatment on structure &
Alloys & Their Phase Diagrams
 Alloys & Their Phase Diagrams Objectives of the class Gibbs phase rule Introduction to phase diagram Practice phase diagram Lever rule Important Observation: One question in the midterm Consider the Earth
Alloys & Their Phase Diagrams Objectives of the class Gibbs phase rule Introduction to phase diagram Practice phase diagram Lever rule Important Observation: One question in the midterm Consider the Earth
Solidification, Crystallization & Glass Transition
 Solidification, Crystallization & Glass Transition Cooling the Melt solidification Crystallization versus Formation of Glass Parameters related to the formaton of glass Effect of cooling rate Glass transition
Solidification, Crystallization & Glass Transition Cooling the Melt solidification Crystallization versus Formation of Glass Parameters related to the formaton of glass Effect of cooling rate Glass transition
Lecture 18 Strain Hardening And Recrystallization
 -138- Lecture 18 Strain Hardening And Recrystallization Strain Hardening We have previously seen that the flow stress (the stress necessary to produce a certain plastic strain rate) increases with increasing
-138- Lecture 18 Strain Hardening And Recrystallization Strain Hardening We have previously seen that the flow stress (the stress necessary to produce a certain plastic strain rate) increases with increasing
Chapter Outline. Diffusion - how do atoms move through solids?
 Chapter Outline iffusion - how do atoms move through solids? iffusion mechanisms Vacancy diffusion Interstitial diffusion Impurities The mathematics of diffusion Steady-state diffusion (Fick s first law)
Chapter Outline iffusion - how do atoms move through solids? iffusion mechanisms Vacancy diffusion Interstitial diffusion Impurities The mathematics of diffusion Steady-state diffusion (Fick s first law)
Heat Treatment of Steels : Spheroidize annealing. Heat Treatment of Steels : Normalizing
 Heat Treatment of Steels :Recrystallization annealing The carbon and alloy steels were treated at a temperature of about 700 C, which is about 20 C below the eutectoid temperature. The holding time should
Heat Treatment of Steels :Recrystallization annealing The carbon and alloy steels were treated at a temperature of about 700 C, which is about 20 C below the eutectoid temperature. The holding time should
DIFFUSION IN SOLIDS. Materials often heat treated to improve properties. Atomic diffusion occurs during heat treatment
 DIFFUSION IN SOLIDS WHY STUDY DIFFUSION? Materials often heat treated to improve properties Atomic diffusion occurs during heat treatment Depending on situation higher or lower diffusion rates desired
DIFFUSION IN SOLIDS WHY STUDY DIFFUSION? Materials often heat treated to improve properties Atomic diffusion occurs during heat treatment Depending on situation higher or lower diffusion rates desired
Chapter Outline Dislocations and Strengthening Mechanisms
 Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
Chapter Outline Dislocations and Strengthening Mechanisms What is happening in material during plastic deformation? Dislocations and Plastic Deformation Motion of dislocations in response to stress Slip
Martensite in Steels
 Materials Science & Metallurgy http://www.msm.cam.ac.uk/phase-trans/2002/martensite.html H. K. D. H. Bhadeshia Martensite in Steels The name martensite is after the German scientist Martens. It was used
Materials Science & Metallurgy http://www.msm.cam.ac.uk/phase-trans/2002/martensite.html H. K. D. H. Bhadeshia Martensite in Steels The name martensite is after the German scientist Martens. It was used
Experiment: Heat Treatment - Quenching & Tempering
 Experiment: Heat Treatment - Quenching & Tempering Objectives 1) To investigate the conventional heat treatment procedures, such as quenching and annealing, used to alter the properties of steels. SAE
Experiment: Heat Treatment - Quenching & Tempering Objectives 1) To investigate the conventional heat treatment procedures, such as quenching and annealing, used to alter the properties of steels. SAE
Chapter 12 - Liquids and Solids
 Chapter 12 - Liquids and Solids 12-1 Liquids I. Properties of Liquids and the Kinetic Molecular Theory A. Fluids 1. Substances that can flow and therefore take the shape of their container B. Relative
Chapter 12 - Liquids and Solids 12-1 Liquids I. Properties of Liquids and the Kinetic Molecular Theory A. Fluids 1. Substances that can flow and therefore take the shape of their container B. Relative
Materials Issues in Fatigue and Fracture
 Materials Issues in Fatigue and Fracture 5.1 Fundamental Concepts 5.2 Ensuring Infinite Life 5.3 Finite Life 5.4 Summary FCP 1 5.1 Fundamental Concepts Structural metals Process of fatigue A simple view
Materials Issues in Fatigue and Fracture 5.1 Fundamental Concepts 5.2 Ensuring Infinite Life 5.3 Finite Life 5.4 Summary FCP 1 5.1 Fundamental Concepts Structural metals Process of fatigue A simple view
Ch. 4: Imperfections in Solids Part 1. Dr. Feras Fraige
 Ch. 4: Imperfections in Solids Part 1 Dr. Feras Fraige Outline Defects in Solids 0D, Point defects vacancies Interstitials impurities, weight and atomic composition 1D, Dislocations edge screw 2D, Grain
Ch. 4: Imperfections in Solids Part 1 Dr. Feras Fraige Outline Defects in Solids 0D, Point defects vacancies Interstitials impurities, weight and atomic composition 1D, Dislocations edge screw 2D, Grain
LABORATORY EXPERIMENTS TESTING OF MATERIALS
 LABORATORY EXPERIMENTS TESTING OF MATERIALS 1. TENSION TEST: INTRODUCTION & THEORY The tension test is the most commonly used method to evaluate the mechanical properties of metals. Its main objective
LABORATORY EXPERIMENTS TESTING OF MATERIALS 1. TENSION TEST: INTRODUCTION & THEORY The tension test is the most commonly used method to evaluate the mechanical properties of metals. Its main objective
Lecture 4: Thermodynamics of Diffusion: Spinodals
 Materials Science & Metallurgy Master of Philosophy, Materials Modelling, Course MP6, Kinetics and Microstructure Modelling, H. K. D. H. Bhadeshia Lecture 4: Thermodynamics of Diffusion: Spinodals Fick
Materials Science & Metallurgy Master of Philosophy, Materials Modelling, Course MP6, Kinetics and Microstructure Modelling, H. K. D. H. Bhadeshia Lecture 4: Thermodynamics of Diffusion: Spinodals Fick
Lecture 22: Spinodal Decomposition: Part 1: general description and
 Lecture 22: Spinodal Decomposition: Part 1: general description and practical implications Today s topics basics and unique features of spinodal decomposition and its practical implications. The relationship
Lecture 22: Spinodal Decomposition: Part 1: general description and practical implications Today s topics basics and unique features of spinodal decomposition and its practical implications. The relationship
RAPIDLY SOLIDIFIED COPPER ALLOYS RIBBONS
 Association of Metallurgical Engineers of Serbia AMES Scientific paper UDC:669.35-153.881-412.2=20 RAPIDLY SOLIDIFIED COPPER ALLOYS RIBBONS M. ŠULER 1, L. KOSEC 1, A. C. KNEISSL 2, M. BIZJAK 1, K. RAIĆ
Association of Metallurgical Engineers of Serbia AMES Scientific paper UDC:669.35-153.881-412.2=20 RAPIDLY SOLIDIFIED COPPER ALLOYS RIBBONS M. ŠULER 1, L. KOSEC 1, A. C. KNEISSL 2, M. BIZJAK 1, K. RAIĆ
Introduction To Materials Science FOR ENGINEERS, Ch. 5. Diffusion. MSE 201 Callister Chapter 5
 Diffusion MSE 21 Callister Chapter 5 1 Goals: Diffusion - how do atoms move through solids? Fundamental concepts and language Diffusion mechanisms Vacancy diffusion Interstitial diffusion Impurities Diffusion
Diffusion MSE 21 Callister Chapter 5 1 Goals: Diffusion - how do atoms move through solids? Fundamental concepts and language Diffusion mechanisms Vacancy diffusion Interstitial diffusion Impurities Diffusion
HEAT TREATMENT OF STEEL
 HEAT TREATMENT OF STEEL Heat Treatment of Steel Most heat treating operations begin with heating the alloy into the austenitic phase field to dissolve the carbide in the iron. Steel heat treating practice
HEAT TREATMENT OF STEEL Heat Treatment of Steel Most heat treating operations begin with heating the alloy into the austenitic phase field to dissolve the carbide in the iron. Steel heat treating practice
Phase. Gibbs Phase rule
 Phase diagrams Phase A phase can be defined as a physically distinct and chemically homogeneous portion of a system that has a particular chemical composition and structure. Water in liquid or vapor state
Phase diagrams Phase A phase can be defined as a physically distinct and chemically homogeneous portion of a system that has a particular chemical composition and structure. Water in liquid or vapor state
KINETIC MOLECULAR THEORY OF MATTER
 KINETIC MOLECULAR THEORY OF MATTER The kinetic-molecular theory is based on the idea that particles of matter are always in motion. The theory can be used to explain the properties of solids, liquids,
KINETIC MOLECULAR THEORY OF MATTER The kinetic-molecular theory is based on the idea that particles of matter are always in motion. The theory can be used to explain the properties of solids, liquids,
Chemical Building Blocks: Chapter 3: Elements and Periodic Table
 Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
Name: Class: Date: Chemical Building Blocks: Chapter 3: Elements and Periodic Table Study Guide Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
WJM Technologies excellence in material joining
 Girish P. Kelkar, Ph.D. (562) 743-7576 girish@welding-consultant.com www.welding-consultant.com Weld Cracks An Engineer s Worst Nightmare There are a variety of physical defects such as undercut, insufficient
Girish P. Kelkar, Ph.D. (562) 743-7576 girish@welding-consultant.com www.welding-consultant.com Weld Cracks An Engineer s Worst Nightmare There are a variety of physical defects such as undercut, insufficient
Massachusetts Institute of Technology Department of Mechanical Engineering Cambridge, MA 02139
 Massachusetts Institute of Technology Department of Mechanical Engineering Cambridge, MA 02139 2.002 Mechanics and Materials II Spring 2004 Laboratory Module No. 5 Heat Treatment of Plain Carbon and Low
Massachusetts Institute of Technology Department of Mechanical Engineering Cambridge, MA 02139 2.002 Mechanics and Materials II Spring 2004 Laboratory Module No. 5 Heat Treatment of Plain Carbon and Low
Chapter 5: Diffusion. 5.1 Steady-State Diffusion
 : Diffusion Diffusion: the movement of particles in a solid from an area of high concentration to an area of low concentration, resulting in the uniform distribution of the substance Diffusion is process
: Diffusion Diffusion: the movement of particles in a solid from an area of high concentration to an area of low concentration, resulting in the uniform distribution of the substance Diffusion is process
We will study the temperature-pressure diagram of nitrogen, in particular the triple point.
 K4. Triple Point of Nitrogen I. OBJECTIVE OF THE EXPERIMENT We will study the temperature-pressure diagram of nitrogen, in particular the triple point. II. BAKGROUND THOERY States of matter Matter is made
K4. Triple Point of Nitrogen I. OBJECTIVE OF THE EXPERIMENT We will study the temperature-pressure diagram of nitrogen, in particular the triple point. II. BAKGROUND THOERY States of matter Matter is made
Test 5 Review questions. 1. As ice cools from 273 K to 263 K, the average kinetic energy of its molecules will
 Name: Thursday, December 13, 2007 Test 5 Review questions 1. As ice cools from 273 K to 263 K, the average kinetic energy of its molecules will 1. decrease 2. increase 3. remain the same 2. The graph below
Name: Thursday, December 13, 2007 Test 5 Review questions 1. As ice cools from 273 K to 263 K, the average kinetic energy of its molecules will 1. decrease 2. increase 3. remain the same 2. The graph below
Phase Diagrams and Phase Separation. MF Ashby and DA Jones, Engineering Materials Vol 2, Pergamon. G Strobl, The Physics of Polymers, Springer
 and Phase Separation Books MF Ashby and DA Jones, Engineering Materials Vol 2, Pergamon P Haasen, Physical Metallurgy, G Strobl, The Physics of Polymers, Springer Introduction Mixing two (or more) components
and Phase Separation Books MF Ashby and DA Jones, Engineering Materials Vol 2, Pergamon P Haasen, Physical Metallurgy, G Strobl, The Physics of Polymers, Springer Introduction Mixing two (or more) components
In order to solve this problem it is first necessary to use Equation 5.5: x 2 Dt. = 1 erf. = 1.30, and x = 2 mm = 2 10-3 m. Thus,
 5.3 (a) Compare interstitial and vacancy atomic mechanisms for diffusion. (b) Cite two reasons why interstitial diffusion is normally more rapid than vacancy diffusion. Solution (a) With vacancy diffusion,
5.3 (a) Compare interstitial and vacancy atomic mechanisms for diffusion. (b) Cite two reasons why interstitial diffusion is normally more rapid than vacancy diffusion. Solution (a) With vacancy diffusion,
HIGH STRENGTH DUCTILE IRON PRODUCED BY THE ENGINEERED COOLING: PROCESS CONCEPT
 IJMC14-244-2 HIGH STRENGTH DUCTILE IRON PRODUCED BY THE ENGINEERED COOLING: PROCESS CONCEPT Copyright 215 American Foundry Society Abstract Simon N. Lekakh Missouri University of Science and Technology,
IJMC14-244-2 HIGH STRENGTH DUCTILE IRON PRODUCED BY THE ENGINEERED COOLING: PROCESS CONCEPT Copyright 215 American Foundry Society Abstract Simon N. Lekakh Missouri University of Science and Technology,
Binary phase diagrams
 inary phase diagrams inary phase diagrams and ibbs free energy curves inary solutions with unlimited solubility Relative proportion of phases (tie lines and the lever principle) Development of microstructure
inary phase diagrams inary phase diagrams and ibbs free energy curves inary solutions with unlimited solubility Relative proportion of phases (tie lines and the lever principle) Development of microstructure
Chapter 10 Phase equilibrium
 Chapter 10 Phase equilibrium It is a familiar fact that pure substances tend to exist in one of three distinct states: solid, liquid, and gas. Take water, for example. As ice is heated at atmospheric pressure,
Chapter 10 Phase equilibrium It is a familiar fact that pure substances tend to exist in one of three distinct states: solid, liquid, and gas. Take water, for example. As ice is heated at atmospheric pressure,
6 Cloud droplet formation and Köhler theory
 6 Cloud droplet formation and Köhler theory This chapter discusses the details of cloud droplet formation. Cloud droplet formation requires phase changes. Some phase changes require a nucleation process,
6 Cloud droplet formation and Köhler theory This chapter discusses the details of cloud droplet formation. Cloud droplet formation requires phase changes. Some phase changes require a nucleation process,
CHAPTER 7 DISLOCATIONS AND STRENGTHENING MECHANISMS PROBLEM SOLUTIONS
 7-1 CHAPTER 7 DISLOCATIONS AND STRENGTHENING MECHANISMS PROBLEM SOLUTIONS Basic Concepts of Dislocations Characteristics of Dislocations 7.1 The dislocation density is just the total dislocation length
7-1 CHAPTER 7 DISLOCATIONS AND STRENGTHENING MECHANISMS PROBLEM SOLUTIONS Basic Concepts of Dislocations Characteristics of Dislocations 7.1 The dislocation density is just the total dislocation length
Study the following diagrams of the States of Matter. Label the names of the Changes of State between the different states.
 Describe the strength of attractive forces between particles. Describe the amount of space between particles. Can the particles in this state be compressed? Do the particles in this state have a definite
Describe the strength of attractive forces between particles. Describe the amount of space between particles. Can the particles in this state be compressed? Do the particles in this state have a definite
Mark Asta Mat Sci 103 Spring, 2016 mdasta@berkeley.edu Phase Transformations & Kinetics LOGISTICS
 UNIVERSITY OF CALIFORNIA College of Engineering Department of Materials Science & Engineering Mark Asta Mat Sci 103 Spring, 2016 mdasta@berkeley.edu Phase Transformations & Kinetics LOGISTICS Course Website
UNIVERSITY OF CALIFORNIA College of Engineering Department of Materials Science & Engineering Mark Asta Mat Sci 103 Spring, 2016 mdasta@berkeley.edu Phase Transformations & Kinetics LOGISTICS Course Website
Warm-Up 9/9. 1. Define the term matter. 2. Name something in this room that is not matter.
 Warm-Up 9/9 1. Define the term matter. 2. Name something in this room that is not matter. Warm-Up 9/16 1. List the three most important rules of lab safety. 2. Would you classify jello as a solid or a
Warm-Up 9/9 1. Define the term matter. 2. Name something in this room that is not matter. Warm-Up 9/16 1. List the three most important rules of lab safety. 2. Would you classify jello as a solid or a
Experiment: Crystal Structure Analysis in Engineering Materials
 Experiment: Crystal Structure Analysis in Engineering Materials Objective The purpose of this experiment is to introduce students to the use of X-ray diffraction techniques for investigating various types
Experiment: Crystal Structure Analysis in Engineering Materials Objective The purpose of this experiment is to introduce students to the use of X-ray diffraction techniques for investigating various types
Short range ordering and microstructure property relationship in amorphous alloys
 Short range ordering and microstructure property relationship in amorphous alloys Dissertation zur Erlangung des Doktorgrades der Mathematisch-Naturwissenschaftlichen Fakultäten der Georg-August-Universität
Short range ordering and microstructure property relationship in amorphous alloys Dissertation zur Erlangung des Doktorgrades der Mathematisch-Naturwissenschaftlichen Fakultäten der Georg-August-Universität
States of Matter CHAPTER 10 REVIEW SECTION 1. Name Date Class. Answer the following questions in the space provided.
 CHAPTER 10 REVIEW States of Matter SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Identify whether the descriptions below describe an ideal gas or a real gas. ideal gas
CHAPTER 10 REVIEW States of Matter SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Identify whether the descriptions below describe an ideal gas or a real gas. ideal gas
14:635:407:02 Homework III Solutions
 14:635:407:0 Homework III Solutions 4.1 Calculate the fraction of atom sites that are vacant for lead at its melting temperature of 37 C (600 K). Assume an energy for vacancy formation of 0.55 ev/atom.
14:635:407:0 Homework III Solutions 4.1 Calculate the fraction of atom sites that are vacant for lead at its melting temperature of 37 C (600 K). Assume an energy for vacancy formation of 0.55 ev/atom.
Fundamentals of the Heat Treating of Steel
 CHAPTER 2 Fundamentals of the Heat Treating of Steel BEFORE CONSIDERATION can be given to the heat treatment of steel or other iron-base alloys, it is helpful to explain what steel is. The common dictionary
CHAPTER 2 Fundamentals of the Heat Treating of Steel BEFORE CONSIDERATION can be given to the heat treatment of steel or other iron-base alloys, it is helpful to explain what steel is. The common dictionary
Name Date Class STATES OF MATTER. SECTION 13.1 THE NATURE OF GASES (pages 385 389)
 13 STATES OF MATTER SECTION 13.1 THE NATURE OF GASES (pages 385 389) This section introduces the kinetic theory and describes how it applies to gases. It defines gas pressure and explains how temperature
13 STATES OF MATTER SECTION 13.1 THE NATURE OF GASES (pages 385 389) This section introduces the kinetic theory and describes how it applies to gases. It defines gas pressure and explains how temperature
Continuous Cooling Transformation (CCT) Diagrams
 Continuous Cooling Transformation (CCT) Diagrams R. Manna Assistant Professor Centre of Advanced Study Department of Metallurgical Engineering Institute of Technology, Banaras Hindu University Varanasi-221
Continuous Cooling Transformation (CCT) Diagrams R. Manna Assistant Professor Centre of Advanced Study Department of Metallurgical Engineering Institute of Technology, Banaras Hindu University Varanasi-221
Each grain is a single crystal with a specific orientation. Imperfections
 Crystal Structure / Imperfections Almost all materials crystallize when they solidify; i.e., the atoms are arranged in an ordered, repeating, 3-dimensional pattern. These structures are called crystals
Crystal Structure / Imperfections Almost all materials crystallize when they solidify; i.e., the atoms are arranged in an ordered, repeating, 3-dimensional pattern. These structures are called crystals
Properties and Classifications of Matter
 PS-3.1 Distinguish chemical properties of matter (including reactivity) from physical properties of matter (including boiling point, freezing/melting point, density [with density calculations], solubility,
PS-3.1 Distinguish chemical properties of matter (including reactivity) from physical properties of matter (including boiling point, freezing/melting point, density [with density calculations], solubility,
FUNDAMENTALS OF ENGINEERING THERMODYNAMICS
 FUNDAMENTALS OF ENGINEERING THERMODYNAMICS System: Quantity of matter (constant mass) or region in space (constant volume) chosen for study. Closed system: Can exchange energy but not mass; mass is constant
FUNDAMENTALS OF ENGINEERING THERMODYNAMICS System: Quantity of matter (constant mass) or region in space (constant volume) chosen for study. Closed system: Can exchange energy but not mass; mass is constant
8. Technical Heat Treatment
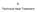 8. Technical Heat Treatment 8. Technical Heat Treatment 95 6 cm 4 2 0-2 -4 C 400 C C -6-14 -12-10 -8-6 -4-2 0 2 cm 6 723 C temperature C 1750 750 250 When welding a workpiece, not only the weld itself,
8. Technical Heat Treatment 8. Technical Heat Treatment 95 6 cm 4 2 0-2 -4 C 400 C C -6-14 -12-10 -8-6 -4-2 0 2 cm 6 723 C temperature C 1750 750 250 When welding a workpiece, not only the weld itself,
CHAPTER 8. Phase Diagrams 8-1
 CHAPTER 8 Phase Diagrams 8-1 Introducción Fase: Una region en un material que difiere en estructura y función de otra región. Diagramas de fase : Representan las fases presentes en el metal a diferentes
CHAPTER 8 Phase Diagrams 8-1 Introducción Fase: Una region en un material que difiere en estructura y función de otra región. Diagramas de fase : Representan las fases presentes en el metal a diferentes
THREE MAIN SOLIDIFICATION REACTIONS OF VANADIUM MODIFIED T1 TUNGSTEN HIGH SPEED TOOL STEEL. Hossam Halfa
 THREE MAIN SOLIDIFICATION REACTIONS OF VANADIUM MODIFIED T1 TUNGSTEN HIGH SPEED TOOL STEEL Hossam Halfa Steel Technology Department, Central Metallurgical R&D Institute (CMRDI), Helwan, Egypt, hossamhalfa@cmrdi.sci.eg;
THREE MAIN SOLIDIFICATION REACTIONS OF VANADIUM MODIFIED T1 TUNGSTEN HIGH SPEED TOOL STEEL Hossam Halfa Steel Technology Department, Central Metallurgical R&D Institute (CMRDI), Helwan, Egypt, hossamhalfa@cmrdi.sci.eg;
Science Standard Articulated by Grade Level Strand 5: Physical Science
 Concept 1: Properties of Objects and Materials Classify objects and materials by their observable properties. Kindergarten Grade 1 Grade 2 Grade 3 Grade 4 PO 1. Identify the following observable properties
Concept 1: Properties of Objects and Materials Classify objects and materials by their observable properties. Kindergarten Grade 1 Grade 2 Grade 3 Grade 4 PO 1. Identify the following observable properties
The study about as cast microstructure and solidification
 The study about as cast microstructure and solidification process of Al-Mg-Si alloys Introduction High-strength and high formability aluminum alloys are the highly demanded alloy in automobile industry,
The study about as cast microstructure and solidification process of Al-Mg-Si alloys Introduction High-strength and high formability aluminum alloys are the highly demanded alloy in automobile industry,
CHAPTER 14 THE CLAUSIUS-CLAPEYRON EQUATION
 CHAPTER 4 THE CAUIU-CAPEYRON EQUATION Before starting this chapter, it would probably be a good idea to re-read ections 9. and 9.3 of Chapter 9. The Clausius-Clapeyron equation relates the latent heat
CHAPTER 4 THE CAUIU-CAPEYRON EQUATION Before starting this chapter, it would probably be a good idea to re-read ections 9. and 9.3 of Chapter 9. The Clausius-Clapeyron equation relates the latent heat
Determination of the heat storage capacity of PCM and PCM objects as a function of temperature
 Determination of the heat storage capacity of PCM and PCM objects as a function of temperature E. Günther, S. Hiebler, H. Mehling ZAE Bayern, Walther-Meißner-Str. 6, 85748 Garching, Germany Outline Introduction
Determination of the heat storage capacity of PCM and PCM objects as a function of temperature E. Günther, S. Hiebler, H. Mehling ZAE Bayern, Walther-Meißner-Str. 6, 85748 Garching, Germany Outline Introduction
LN 10. 3.091 Introduction to Solid State Chemistry. Lecture Notes No. 10 PHASE EQUILIBRIA AND PHASE DIAGRAMS
 3.091 Introduction to Solid State Chemistry Lecture Notes No. 10 PHASE EQUILIBRIA AND PHASE DIAGRAMS * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * Sources
3.091 Introduction to Solid State Chemistry Lecture Notes No. 10 PHASE EQUILIBRIA AND PHASE DIAGRAMS * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * Sources
Chemistry 13: States of Matter
 Chemistry 13: States of Matter Name: Period: Date: Chemistry Content Standard: Gases and Their Properties The kinetic molecular theory describes the motion of atoms and molecules and explains the properties
Chemistry 13: States of Matter Name: Period: Date: Chemistry Content Standard: Gases and Their Properties The kinetic molecular theory describes the motion of atoms and molecules and explains the properties
Lecture 12. Physical Vapor Deposition: Evaporation and Sputtering Reading: Chapter 12. ECE 6450 - Dr. Alan Doolittle
 Lecture 12 Physical Vapor Deposition: Evaporation and Sputtering Reading: Chapter 12 Evaporation and Sputtering (Metalization) Evaporation For all devices, there is a need to go from semiconductor to metal.
Lecture 12 Physical Vapor Deposition: Evaporation and Sputtering Reading: Chapter 12 Evaporation and Sputtering (Metalization) Evaporation For all devices, there is a need to go from semiconductor to metal.
Review - After School Matter Name: Review - After School Matter Tuesday, April 29, 2008
 Name: Review - After School Matter Tuesday, April 29, 2008 1. Figure 1 The graph represents the relationship between temperature and time as heat was added uniformly to a substance starting at a solid
Name: Review - After School Matter Tuesday, April 29, 2008 1. Figure 1 The graph represents the relationship between temperature and time as heat was added uniformly to a substance starting at a solid
Chapter 13 - LIQUIDS AND SOLIDS
 Chapter 13 - LIQUIDS AND SOLIDS Problems to try at end of chapter: Answers in Appendix I: 1,3,5,7b,9b,15,17,23,25,29,31,33,45,49,51,53,61 13.1 Properties of Liquids 1. Liquids take the shape of their container,
Chapter 13 - LIQUIDS AND SOLIDS Problems to try at end of chapter: Answers in Appendix I: 1,3,5,7b,9b,15,17,23,25,29,31,33,45,49,51,53,61 13.1 Properties of Liquids 1. Liquids take the shape of their container,
FATIGUE PROPERTIES OF P/M MATERIALS. Robert C. O'Brien. Hoeganaes Corporation River Road Riverton, New Jersey 08077
 FATIGUE PROPERTIES OF P/M MATERIALS Robert C. O'Brien Hoeganaes Corporation River Road Riverton, New Jersey 08077 Presented at the SAE Congress Detroit, Michigan, February 29-March 4, 1988 Abstract The
FATIGUE PROPERTIES OF P/M MATERIALS Robert C. O'Brien Hoeganaes Corporation River Road Riverton, New Jersey 08077 Presented at the SAE Congress Detroit, Michigan, February 29-March 4, 1988 Abstract The
ME 612 Metal Forming and Theory of Plasticity. 1. Introduction
 Metal Forming and Theory of Plasticity Yrd.Doç. e mail: azsenalp@gyte.edu.tr Makine Mühendisliği Bölümü Gebze Yüksek Teknoloji Enstitüsü In general, it is possible to evaluate metal forming operations
Metal Forming and Theory of Plasticity Yrd.Doç. e mail: azsenalp@gyte.edu.tr Makine Mühendisliği Bölümü Gebze Yüksek Teknoloji Enstitüsü In general, it is possible to evaluate metal forming operations
Factors Affecting Precipitation of Calcium Carbonate
 Factors Affecting Precipitation of Calcium Carbonate John A. Wojtowicz Chemcon Laboratory tests with clear solutions showed that precipitation of calcium carbonate does not occur in the ph range 7.5 to
Factors Affecting Precipitation of Calcium Carbonate John A. Wojtowicz Chemcon Laboratory tests with clear solutions showed that precipitation of calcium carbonate does not occur in the ph range 7.5 to
Mechanical Properties of Metals Mechanical Properties refers to the behavior of material when external forces are applied
 Mechanical Properties of Metals Mechanical Properties refers to the behavior of material when external forces are applied Stress and strain fracture or engineering point of view: allows to predict the
Mechanical Properties of Metals Mechanical Properties refers to the behavior of material when external forces are applied Stress and strain fracture or engineering point of view: allows to predict the
The first law: transformation of energy into heat and work. Chemical reactions can be used to provide heat and for doing work.
 The first law: transformation of energy into heat and work Chemical reactions can be used to provide heat and for doing work. Compare fuel value of different compounds. What drives these reactions to proceed
The first law: transformation of energy into heat and work Chemical reactions can be used to provide heat and for doing work. Compare fuel value of different compounds. What drives these reactions to proceed
CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING
 CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING Essential Standard: STUDENTS WILL UNDERSTAND THAT THE PROPERTIES OF MATTER AND THEIR INTERACTIONS ARE A CONSEQUENCE OF THE STRUCTURE OF MATTER,
CHEMISTRY STANDARDS BASED RUBRIC ATOMIC STRUCTURE AND BONDING Essential Standard: STUDENTS WILL UNDERSTAND THAT THE PROPERTIES OF MATTER AND THEIR INTERACTIONS ARE A CONSEQUENCE OF THE STRUCTURE OF MATTER,
THERMAL STABILITY OF Al-Cu-Fe QUASICRYSTALS PREPARED BY SHS METHOD
 This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ or send
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/ or send
CHAPTER 3: MATTER. Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64
 CHAPTER 3: MATTER Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64 3.1 MATTER Matter: Anything that has mass and occupies volume We study
CHAPTER 3: MATTER Active Learning Questions: 1-6, 9, 13-14; End-of-Chapter Questions: 1-18, 20, 24-32, 38-42, 44, 49-52, 55-56, 61-64 3.1 MATTER Matter: Anything that has mass and occupies volume We study
Thermodynamic database of the phase diagrams in copper base alloy systems
 Journal of Physics and Chemistry of Solids 66 (2005) 256 260 www.elsevier.com/locate/jpcs Thermodynamic database of the phase diagrams in copper base alloy systems C.P. Wang a, X.J. Liu b, M. Jiang b,
Journal of Physics and Chemistry of Solids 66 (2005) 256 260 www.elsevier.com/locate/jpcs Thermodynamic database of the phase diagrams in copper base alloy systems C.P. Wang a, X.J. Liu b, M. Jiang b,
Atoms and Elements. Outline Atoms Orbitals and Energy Levels Periodic Properties Homework
 Atoms and the Periodic Table The very hot early universe was a plasma with cationic nuclei separated from negatively charged electrons. Plasmas exist today where the energy of the particles is very high,
Atoms and the Periodic Table The very hot early universe was a plasma with cationic nuclei separated from negatively charged electrons. Plasmas exist today where the energy of the particles is very high,
EUTECTIC TRANSFORMATION IN DUCTILE CAST IRON. PART I THEORETICAL BACKGROUND
 METALLURGY AND FOUNDRY ENGINEERING Vol. 31, 005, No. Edward Fraœ *, Marcin Górny **, Hugo F. López *** EUTECTIC TRANSFORMATION IN DUCTILE CAST IRON. PART I THEORETICAL BACKGROUND SYMBOLS Symbols Meaning
METALLURGY AND FOUNDRY ENGINEERING Vol. 31, 005, No. Edward Fraœ *, Marcin Górny **, Hugo F. López *** EUTECTIC TRANSFORMATION IN DUCTILE CAST IRON. PART I THEORETICAL BACKGROUND SYMBOLS Symbols Meaning
2 MATTER. 2.1 Physical and Chemical Properties and Changes
 2 MATTER Matter is the material of which the universe is composed. It has two characteristics: It has mass; and It occupies space (i.e., it has a volume). Matter can be found in three generic states: Solid;
2 MATTER Matter is the material of which the universe is composed. It has two characteristics: It has mass; and It occupies space (i.e., it has a volume). Matter can be found in three generic states: Solid;
Objectives/Introduction Extraction of zinc Physical properties of zinc Zinc casting alloys Wrought zinc alloys Engineering design with zinc alloys
 Lecture 7 Zinc and its alloys Subjects of interest Objectives/Introduction Extraction of zinc Physical properties of zinc Zinc casting alloys Wrought zinc alloys Engineering design with zinc alloys Objectives
Lecture 7 Zinc and its alloys Subjects of interest Objectives/Introduction Extraction of zinc Physical properties of zinc Zinc casting alloys Wrought zinc alloys Engineering design with zinc alloys Objectives
Chemistry. The student will be able to identify and apply basic safety procedures and identify basic equipment.
 Chemistry UNIT I: Introduction to Chemistry The student will be able to describe what chemistry is and its scope. a. Define chemistry. b. Explain that chemistry overlaps many other areas of science. The
Chemistry UNIT I: Introduction to Chemistry The student will be able to describe what chemistry is and its scope. a. Define chemistry. b. Explain that chemistry overlaps many other areas of science. The
Indiana's Academic Standards 2010 ICP Indiana's Academic Standards 2016 ICP. map) that describe the relationship acceleration, velocity and distance.
 .1.1 Measure the motion of objects to understand.1.1 Develop graphical, the relationships among distance, velocity and mathematical, and pictorial acceleration. Develop deeper understanding through representations
.1.1 Measure the motion of objects to understand.1.1 Develop graphical, the relationships among distance, velocity and mathematical, and pictorial acceleration. Develop deeper understanding through representations
Name Class Date. In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question.
 Assessment Chapter Test A Chapter: States of Matter In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. The kinetic-molecular
Assessment Chapter Test A Chapter: States of Matter In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. The kinetic-molecular
Problems in Welding of High Strength Aluminium Alloys
 Singapore Welding Society Newsletter, September 1999 Problems in Welding of High Strength Aluminium Alloys Wei Zhou Nanyang Technological University, Singapore E-mail: WZhou@Cantab.Net Pure aluminium has
Singapore Welding Society Newsletter, September 1999 Problems in Welding of High Strength Aluminium Alloys Wei Zhou Nanyang Technological University, Singapore E-mail: WZhou@Cantab.Net Pure aluminium has
Thermodynamics. Thermodynamics 1
 Thermodynamics 1 Thermodynamics Some Important Topics First Law of Thermodynamics Internal Energy U ( or E) Enthalpy H Second Law of Thermodynamics Entropy S Third law of Thermodynamics Absolute Entropy
Thermodynamics 1 Thermodynamics Some Important Topics First Law of Thermodynamics Internal Energy U ( or E) Enthalpy H Second Law of Thermodynamics Entropy S Third law of Thermodynamics Absolute Entropy
Define the notations you are using properly. Present your arguments in details. Good luck!
 Umeå Universitet, Fysik Vitaly Bychkov Prov i fysik, Thermodynamics, 0-0-4, kl 9.00-5.00 jälpmedel: Students may use any book(s) including the textbook Thermal physics. Minor notes in the books are also
Umeå Universitet, Fysik Vitaly Bychkov Prov i fysik, Thermodynamics, 0-0-4, kl 9.00-5.00 jälpmedel: Students may use any book(s) including the textbook Thermal physics. Minor notes in the books are also
Recrystallization II 23
 Recrystallization II 23 Chem 355 Jasperse RECRYSTALLIZATIN-Week 2 1. Mixed Recrystallization of Acetanilide 2. Mixed Recrystallization of Dibenzylacetone 3. Recrystallization of an Unknown Background Review:
Recrystallization II 23 Chem 355 Jasperse RECRYSTALLIZATIN-Week 2 1. Mixed Recrystallization of Acetanilide 2. Mixed Recrystallization of Dibenzylacetone 3. Recrystallization of an Unknown Background Review:
