CHIRALITY, SYMMETRY PLANES AND ENANTIOMERS
|
|
|
- Marcus Bond
- 7 years ago
- Views:
Transcription
1 CHIRALITY, SYMMETRY PLANES AND ENANTIOMERS Craig Wheelock October 17th, (copies of slides can be downloaded from my homepage)
2 WHICH OBJECTS ARE SYMMETRIC? (mirror image is identical) asym sym (outside) sym asym asym asym sym sym
3 PLANES OF SYMMETRY
4 A SYMMETRIC OBJECT HAS A PLANE OF SYMMETRY - ALSO CALLED A MIRROR PLANE plane of symmetry mirror plane
5 IF AN OBJECT HAS A PLANE OF SYMMETRY, ITS MIRROR IMAGE WILL BE IDENTICAL IDENTICAL MIRROR IMAGES WILL SUPERIMPOSE (MATCH EXACTLY WHEN PLACED ON TOP OF EACH OTHER)
6 CHIRALITY
7 An object without symmetry is CHIRAL no symmetry The mirror image of a chiral object is different and will not superimpose on the original object. OBJECTS WHICH ARE CHIRAL HAVE A SENSE OF HANDEDNESS AND EXIST IN TWO FORMS
8 An object with symmetry is ACHIRAL (not chiral) The mirror image of an achiral object is identical and will superimpose on the original object. plane of symmetry
9 Cl ENANTIOMERS non-superimposable mirror images Cl rotate H F F H this molecule is chiral Cl H F note that the fluorine and bromine have been interchanged in the enantiomer
10 non-superimposable
11 OPTICAL ACTIVITY PLANE-POLARIZED LIGHT
12 PLANE-POLARIZED POLARIZED LIGHT BEAM single ray or photon wavelength λ All sine waves (rays) in the beam aligned in same plane. SIDE VIEW A beam is a collection of these rays. frequency ( ν ) ν = c λ c = speed of light unpolarized beam. polarized beam NOT PLANE-POLARIZED Sine waves are not aligned in the same plane. END VIEW
13 Optically Active Refers to molecules that interact with plane-polarized light Jean Baptiste Biot French Physicist He discovered that some natural substances (glucose, nicotine, sucrose) rotate the plane of plane-polarized light and that others did not.
14 Optical Activity angle of rotation, α α incident polarized light sample cell (usually quartz) transmitted light (rotated) a solution of the substance to be examined is placed inside the cell
15 TYPES OF OPTICAL ACTIVITY new older Dextrorotatory (+)- d- Rotates the plane of plane-polarized light to the right. new older Levorotatory (-)- l- Rotates the plane of plane-polarized light to the left.
16 t [α] D = Specific Rotation [α] D α cl α = observed rotation c = concentration ( g/ml ) l = length of cell ( dm ) D = yellow light from sodium lamp t = temperature ( Celsius ) This equation corrects for differences in cell length and concentration. Specific rotation calculated in this way is a physical property of an optically active substance. You always get the same t value of [α] D
17 SPECIFIC ROTATIONS OF BIOACTIVE COMPOUNDS COMPOUND [α] D cholesterol cocaine -16 morphine -132 codeine -136 heroin -107 epinephrine -5.0 progesterone +172 testosterone +109 sucrose β-d-glucose α-d-glucose +112 oxacillin +201
18 PASTEUR S DISCOVERY Louis Pasteur 1848 Sorbonne, Paris HOOC CH CH COOH + Na OOC CH CH COO 2- NH 4 + OH OH OH OH tartaric acid ( found in wine must ) sodium ammonium tartrate Pasteur crystallized this substance on a cold day
19 Crystals of Sodium Ammonium Tartrate hemihedral faces Pasteur found two different crystals. mirror images Biot s results : (+) (-) Louis Pasteur separated these and gave them to Biot to measure.
20 Enantiomers non-superimposable mirror images (also called optical isomers) W W X C Z Y Y Z C X Pasteur decided that the molecules that made the crystals, just as the crystals themselves, must be mirror images. Each crystal must contain a single type of enantiomer.
21 Pasteur s hypothesis eventually led to the discovery that tetravalent carbon atoms are tetrahedral. Van t Hoff and LeBel (1874) C tetrahedral carbon Only tetrahedral geometry can lead to mirror image molecules: C C Square planar, square pyrimidal or trigonal pyramid will not work: C C C
22 ENANTIOMERS HAVE EQUAL AND OPPOSITE ROTATIONS W Enantiomers W X C Y Y C X Z Z (+)-nn o dextrorotatory (-)-nn o levorotatory ALL OTHER PHYSICAL PROPERTIES ARE THE SAME
23 TARTARIC ACID from fermentation of wine OH OH Enantiomers OH OH HOOC H H COOH H HOOC COOH H (+)-tartaric acid (-)-tartaric acid HOOC H OH OH meso COOH H meso -tartaric acid ALSO FOUND (as a minor component) [α] D = 0 more about this compound later
24 TARTARIC ACID (-) - tartaric acid (+) - tartaric acid [α] D = o [α] D = o mp o solubility of 1 g 0.75 ml H 2 O 1.7 ml methanol 250 ml ether mp o solubility of 1 g 0.75 ml H 2 O 1.7 ml methanol 250 ml ether insoluble CHCl 3 d = g/ml insoluble CHCl 3 d = g/ml [α] D = 0 o mp 140 o d = g/ml meso - tartaric acid solubility of 1 g 0.94 ml H 2 O insoluble CHCl 3
25 RACEMIC MIXTURE an equimolar (50/50) mixture of enantiomers [α] D = 0 o the effect of each molecule is cancelled out by its enantiomer
26 STEREOISOMERS ENANTIOMERS are a type of STEREOISOMER Stereoisomers are the same constitutional isomer, but differ in the way they are arranged in 3-D space at one or more of their atoms.
27 STEREOCENTERS One of the ways a molecule can be chiral is to have a stereocenter A stereocenter is an atom, or a group of atoms, that can potentially cause a molecule to be chiral stereocenters - can give rise to chirality
28 STEREOGENIC CARBONS ( called chiral carbons in older literature ) Cl This is one type of. stereocenter. others are possible H F A stereogenic carbon is tetrahedral and has four different groups attached. H F Cl
29 F Cl ACHIRAL The plane of the paper is a plane of symmetry F F Cl Cl Cl Cl TWO IDENTICAL GROUPS RENDERS A TETRAHEDRAL CARBON ACHIRAL
30 ONE PAIR OF ATOMS ATTACHED TO A TETRAHEDRAL CARBON IS IN A PLANE PERPENDICULAR TO THE OTHER PAIR plane 1 plane 2 F Cl Look at your model set!!!!
31 TWO VIEWS OF THE PLANE OF SYMMETRY F plane of symmetry F Cl Cl Cl F Cl Cl side view edge view
32 CONFIGURATION ABSOLUTE CONFIGURATION ( R / S )
33 CONFIGURATION The three dimensional arrangement of the groups attached to an atom Stereoisomers differ in the configuration at one or more of their atoms.
34 CONFIGURATION - relates to the three dimensional sense of attachment for groups attached to a chiral atom or group of atoms (i.e., attached to a stereocenter). clockwise 1 4 C C 1 counter clockwise view with substituent of lowest priority in back 3 R (rectus) S 3 (sinister)
35 SPECIFICATION OF CONFIGURATION Enantiomers are assigned a CONFIGURATION using the same priority rules we developed for E / Z stereoisomers. 1. Higher atomic number has higher priority. 2. If priority cannot be decided based on the first atom attached move to the next atom, following the path having the highest prioity atom. 3. Expand multiple bonds by replicating the atoms attached to each end of the bond. CAHN-INGOLD-PRELOG SEQUENCE RULES
36 1 R 4 2 (R)-2-Butanol 3
37 omochlorofluoroiodomethane atomic number H F Cl I 1 I I 1 4 F Cl 3 C 2 4 F 2 C 3 Cl R Enantiomers S
38 NUMBER OF STEREOISOMERS POSSIBLE
39 How Many Stereoisomers Are Possible? maximum number of stereoisomers sometimes fewer than this number will exist = 2 n, where n = number of stereocenters (sterogenic carbons)
40 CH 3 CH 2 OH * OH C CH 2 CH CH CH 3 * CH 3 CH 3 R R R S S R S S 2 2 = 4 stereoisomers CH 3 * * * OH R R R R R S R S R S R R R S S S R S S S R S S S CH 3 CH CH3 2 3 = 8 stereoisomers
41 FINDING STEREOCENTERS (STEREOGENIC CARBONS) O O O * CH 3 * CH 3 CH 3 plane ( indicates no stereoisomers ) stereoisomers (max) : 2 0 = = = 2
42 FINDING STEREOCENTERS (STEREOGENIC CARBONS) O O O * * * * CH 3 CH 3 CH 3 CH 3 CH3 * * CH 3 plane ( but only if cis ) stereoisomers (max) : 2 2 = = = 4
43 FINDING STEREOCENTERS (CHIRAL CARBONS) CH 3 CH 3 * HO * CH 3 * * * * * * CH 3 CH 3 H n = = 256
44 MOLECULES WITH TWO STEREOCENTERS DIASTEREOMERS / MESO
45 2-omo-3-chlorobutane * * CH 3 CHCHCH Cl = 4 stereoisomers possible SR RS SS RR When comparing butanes, the comparisons are done best using the eclipsed conformation. The relationships and planes of symmetry are not easily seen in other conformations.
46 PRIORITIES AND DETERMINING R and S 3 1 Cl 2 CH 3 CH 3 H H 4 rotate 4 2 H H 1 Cl CH 3 3 CH 3 S 2 Cl 1 CH 3 CH 3 H H 4 3 rotate 4 H 1 CH CH 3 H Cl R
47 diastereomers 2-omo-3-chlorobutane S Cl R CH 3 CH 3 H H Cl CH 3 H H CH 3 mirror enantiomers 1 enantiomers 2 S Cl R CH 3 CH 3 H H Cl S S R R H CH 3 CH 3 H
48 Models of the Isomers of 2-omo2 omo-3-chlorobutane (methyl groups are reduced to a single red atom) Cl CH 3 H enantiomers-1 diastereomers enantiomers-2
49 MESO ISOMER plane of symmetry Cl Cl * * CH 3 CH 3 H H S CH 3 H Cl mirror Cl R CH 3 H Meso isomer - has a plane of symmetry and the mirror image is identical to the original molecule. - must have 2 stereocenters
50 Models of the Isomers of 2,3-Dichlorobutane (methyl groups are reduced to a single red atom) Cl CH 3 H meso diastereomers enantiomers
51 CIS / TRANS RINGS DIASTEREOMERS
52 1-omo-2-chlorocyclopropane note that the cis / trans isomers are also diastereomers Cl R S Cl R S cis diastereomers enantiomers R R S S Cl Cl trans enantiomers
53 1,2-Dibromocyclopropane mirror image identical cis diastereomers meso R R S S trans enantiomers
54 DISUBSTITUTED CYCLOHEXANES USING PLANAR RINGS FOR STEREOCHEMICAL ANALYSIS
55 1-omo-2-chlorocyclohexane cyclohexanes may be analyzed using planar rings diastereomers Cl Cl S R S R enantiomers cis trans S S R R Cl enantiomers Cl
56 1,2-dichlorocyclohexane mirror image identical Cl Cl Cl Cl cis diastereomers meso Cl Cl trans S S R R Cl enantiomers Cl
57 CONCLUSION 2 n = the maximum number When a molecule has 1. multiple stereocenters and 2. there is a possibility of an arrangement with a plane of symmetry you will not always find all of the 2 n stereoisomers that are possible. Some of the stereoisomers may be meso isomers, and their mirror images will be superimposable (identical) - this will eliminate at least one of the possible stereoisomers, and sometimes more.
58 PHYSICAL PROPERTIES OF ENANTIOMERS, DIASTEREOMERS AND MESO COMPOUNDS
59 REMEMBER : ENANTIOMERS HAVE EQUAL AND OPPOSITE ROTATIONS W Enantiomers W X C Z Y Y Z C X (+)-nn o dextrorotatory (-)-nn o levorotatory ALL OTHER PHYSICAL PROPERTIES ARE THE SAME
60 IN CONTRAST : DIASTEREOMERS MAY HAVE DIFFERENT ROTATIONS AND ALSO DIFFERENT PHYSICAL PROPERTIES
61 RELATIONSHIPS AMONG STEREOISOMERS
62 COMPARING TWO STEREOISOMERS Two Stereoisomers nonsuperimposable mirror images not mirror images Enantiomers Diastereomers
63 DETERMINING CHIRALITY / OPTICAL ACTIVITY no plane of symmetry molecule has one or more stereocenters no mirror image superimposes yes plane of symmetry has an enantiomer is meso is chiral is achiral optically active optically inactive
64 ISOMERS Different compounds with the same molecular formula STEREOISOMERS Isomers with the same order of attachment, but a different configuration (3D arrangement) of groups on one or more of the atoms double bond or ring cis/trans ISOMERS (geometric) each isomer could have stereoisomers with a ring both can apply CONSTITUTIONAL ISOMERS Isomers with a different order of attachment of the atoms in their molecules ENANTIOMERS Stereoisomers whose molecules are nonsuperimposible mirror images of each other DIASTEREOMERS Stereoisomers whose molecules are not mirror images of each other TYPES OF ISOMERISM
65
66 Biological role of stereochemistry (S)-amino acid (R)-amino acid Only one of the 2 amino acid enantiomers can achieve 3-point binding with the enzyme binding site
67 Importance of stereochemistry Thalidomide (Neurosedyn) S teratogenic and causes birth defects Ibuprofen (Ipren) S active form R effective against morning sickness R inactive
68 To recapitulate... chirality and optical activity plane of symmetry enantiomers and diastereomers R and S use Cahn-Ingold-Prelog priority rules meso compounds contain stereocenters, but are achiral stereoisomers can have significant biological effects
69 Web resources ISIS Draw Structure Drawing Software Chime Molecular Display RasMol Molecular Display Software Jmol Java viewer for 3D chemical structures General resource for organic chemistry Spartan computational chemistry
70 PROBLEM: ARE THESE IDENTICAL OR ARE THEY ENANTIOMERS? CH 3 C H C YOU CAN USE INTERCHANGES F H CH 3 F 1 CH 3 2 H H C C C C F H CH 3 ENANTIOMER F H CH 3 SAME F 3 CH 3 F ENANTIOMER
71 What is the best term to describe the relationship between A and each of the other molecules? Constitutional isomers? Conformations? Enantiomers? Diastereomers? Identical? Are any of these molecules optically active?
72 ANSWERS A-C and E are all the same constitutional isomer, 1,3,5-trichloro-, but F is 1,2,4-trichloro- F is a constitutional isomer to A and to any of the others. A (e,e,a) and B (a,a,e) are conformations A (e,e,a) and C (e,e,e) are diastereomers A and D are diastereomers (D is a conformation of C) A and E are diastereomers (E is identical to D, turned left-to-right) A and G are identical (G is the same as A turned left-to-right) Only F is optically active, all the rest are meso molecules! [α] D = 0 [α] D = 0
Isomers Have same molecular formula, but different structures
 Isomers ave same molecular formula, but different structures Constitutional Isomers Differ in the order of attachment of atoms (different bond connectivity) Stereoisomers Atoms are connected in the same
Isomers ave same molecular formula, but different structures Constitutional Isomers Differ in the order of attachment of atoms (different bond connectivity) Stereoisomers Atoms are connected in the same
Molecule Projections
 Key Definitions ü Stereochemistry refers to the chemistry in 3 dimensions (greek stereos = solid). This science was created by Pasteur (1860), van Hoff et LeBel (1874). ü Stereisomers are isomeric molecules
Key Definitions ü Stereochemistry refers to the chemistry in 3 dimensions (greek stereos = solid). This science was created by Pasteur (1860), van Hoff et LeBel (1874). ü Stereisomers are isomeric molecules
IR Applied to Isomer Analysis
 DiscovIR-LC TM Application Note 025 April 2008 Deposition and Detection System IR Applied to Isomer Analysis Infrared spectra provide valuable information about local configurations of atoms in molecules.
DiscovIR-LC TM Application Note 025 April 2008 Deposition and Detection System IR Applied to Isomer Analysis Infrared spectra provide valuable information about local configurations of atoms in molecules.
ORGANIC COMPOUNDS IN THREE DIMENSIONS
 (adapted from Blackburn et al., Laboratory Manual to Accompany World of hemistry, 2 nd ed., (1996) Saunders ollege Publishing: Fort Worth) Purpose: To become familiar with organic molecules in three dimensions
(adapted from Blackburn et al., Laboratory Manual to Accompany World of hemistry, 2 nd ed., (1996) Saunders ollege Publishing: Fort Worth) Purpose: To become familiar with organic molecules in three dimensions
2.4 Enantiomeric Isomers
 2.4 Enantiomeric Isomers There is an additional type of isomer that can exist when we have an atom with 4 different groups attached to that atom in tetrahedral geometry. Consider a methane molecule with
2.4 Enantiomeric Isomers There is an additional type of isomer that can exist when we have an atom with 4 different groups attached to that atom in tetrahedral geometry. Consider a methane molecule with
Survival Organic Chemistry Part I: Molecular Models
 Survival Organic Chemistry Part I: Molecular Models The goal in this laboratory experience is to get you so you can easily and quickly move between empirical formulas, molecular formulas, condensed formulas,
Survival Organic Chemistry Part I: Molecular Models The goal in this laboratory experience is to get you so you can easily and quickly move between empirical formulas, molecular formulas, condensed formulas,
Absolute Structure Absolute Configuration
 Absolute Structure Absolute Configuration Some definitions Absolute Configuration -> spatial arrangement of the atoms for a chiral molecule (R/S, P/M or D/L assignment). Absolute Structure -> spatial arrangement
Absolute Structure Absolute Configuration Some definitions Absolute Configuration -> spatial arrangement of the atoms for a chiral molecule (R/S, P/M or D/L assignment). Absolute Structure -> spatial arrangement
Chemistry 1110 Organic Chemistry IUPAC Nomenclature
 hemistry 1110 rganic hemistry IUPA Nomenclature 1 f the approximately 32 million unique chemical compounds presently known, over 95% of them can be classified as organic; i.e., containing carbon. The IUPA
hemistry 1110 rganic hemistry IUPA Nomenclature 1 f the approximately 32 million unique chemical compounds presently known, over 95% of them can be classified as organic; i.e., containing carbon. The IUPA
EXPERIMENT 1: Survival Organic Chemistry: Molecular Models
 EXPERIMENT 1: Survival Organic Chemistry: Molecular Models Introduction: The goal in this laboratory experience is for you to easily and quickly move between empirical formulas, molecular formulas, condensed
EXPERIMENT 1: Survival Organic Chemistry: Molecular Models Introduction: The goal in this laboratory experience is for you to easily and quickly move between empirical formulas, molecular formulas, condensed
Molecular Models Experiment #1
 Molecular Models Experiment #1 Objective: To become familiar with the 3-dimensional structure of organic molecules, especially the tetrahedral structure of alkyl carbon atoms and the planar structure of
Molecular Models Experiment #1 Objective: To become familiar with the 3-dimensional structure of organic molecules, especially the tetrahedral structure of alkyl carbon atoms and the planar structure of
Chapter 4 Lecture Notes
 Chapter 4 Lecture Notes Chapter 4 Educational Goals 1. Given the formula of a molecule, the student will be able to draw the line-bond (Lewis) structure. 2. Understand and construct condensed structural
Chapter 4 Lecture Notes Chapter 4 Educational Goals 1. Given the formula of a molecule, the student will be able to draw the line-bond (Lewis) structure. 2. Understand and construct condensed structural
Combinatorial Biochemistry and Phage Display
 Combinatorial Biochemistry and Phage Display Prof. Valery A. Petrenko Director - Valery Petrenko Instructors Galina Kouzmitcheva and I-Hsuan Chen Auburn 2006, Spring semester COMBINATORIAL BIOCHEMISTRY
Combinatorial Biochemistry and Phage Display Prof. Valery A. Petrenko Director - Valery Petrenko Instructors Galina Kouzmitcheva and I-Hsuan Chen Auburn 2006, Spring semester COMBINATORIAL BIOCHEMISTRY
Stereochemistry Tutorial: Drawing Enantiomers and Diastereomers
 tereochemistry Tutorial: Drawing Enantiomers and Diastereomers Definitions for vocabulary words can be found in the Illustrated Glossary of rganic Chemistry, available on the course web site. A. Discussion
tereochemistry Tutorial: Drawing Enantiomers and Diastereomers Definitions for vocabulary words can be found in the Illustrated Glossary of rganic Chemistry, available on the course web site. A. Discussion
5. Structure, Geometry, and Polarity of Molecules
 5. Structure, Geometry, and Polarity of Molecules What you will accomplish in this experiment This experiment will give you an opportunity to draw Lewis structures of covalent compounds, then use those
5. Structure, Geometry, and Polarity of Molecules What you will accomplish in this experiment This experiment will give you an opportunity to draw Lewis structures of covalent compounds, then use those
Organic Functional Groups Chapter 7. Alcohols, Ethers and More
 Organic Functional Groups Chapter 7 Alcohols, Ethers and More 1 What do you do when you are in Pain? What do you do when you are in a lot of pain? 2 Functional Groups A functional group is an atom, groups
Organic Functional Groups Chapter 7 Alcohols, Ethers and More 1 What do you do when you are in Pain? What do you do when you are in a lot of pain? 2 Functional Groups A functional group is an atom, groups
CHEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) Page 1
 CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
CEM 121. Chapter 18. Name: Date: 1. Which of the following compounds is both an aldose and a hexose? A) B) C) D) Page 1 2. Which of the following structures is that of an L-monosaccharide? A) B) C) D)
1. What is the hybridization of the indicated atom in the following molecule?
 Practice Final Exam, Chemistry 2210, rganic Chem I 1. What is the hybridization of the indicated atom in the following molecule? A. sp 3 B. sp 2 C. sp D. not hybridized 2. Name the functional groups in
Practice Final Exam, Chemistry 2210, rganic Chem I 1. What is the hybridization of the indicated atom in the following molecule? A. sp 3 B. sp 2 C. sp D. not hybridized 2. Name the functional groups in
Assessment Schedule 2013 Chemistry: Demonstrate understanding of the properties of organic compounds (91391)
 NCEA Level 3 Chemistry (91391) 2013 page 1 of 8 Assessment Schedule 2013 Chemistry: Demonstrate understanding of the properties of organic compounds (91391) Evidence Statement Q Evidence Achievement Achievement
NCEA Level 3 Chemistry (91391) 2013 page 1 of 8 Assessment Schedule 2013 Chemistry: Demonstrate understanding of the properties of organic compounds (91391) Evidence Statement Q Evidence Achievement Achievement
Solid Phase Peptide Synthesis
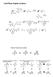 Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
Solid Phase Peptide Synthesis Fischer Projections revisited C C C Remember that group rotations are always allowed C C 80 rotations of entire molecule are allowed C 80 C 90 rotations of entire molecule
Molecular Geometry and Chemical Bonding Theory
 Chapter 10 Molecular Geometry and Chemical Bonding Theory Concept Check 10.1 An atom in a molecule is surrounded by four pairs of electrons, one lone pair and three bonding pairs. Describe how the four
Chapter 10 Molecular Geometry and Chemical Bonding Theory Concept Check 10.1 An atom in a molecule is surrounded by four pairs of electrons, one lone pair and three bonding pairs. Describe how the four
The Synthesis of trans-dichlorobis(ethylenediamine)cobalt(iii) Chloride
 CHEM 122L General Chemistry Laboratory Revision 2.0 The Synthesis of trans-dichlorobis(ethylenediamine)cobalt(iii) Chloride To learn about Coordination Compounds and Complex Ions. To learn about Isomerism.
CHEM 122L General Chemistry Laboratory Revision 2.0 The Synthesis of trans-dichlorobis(ethylenediamine)cobalt(iii) Chloride To learn about Coordination Compounds and Complex Ions. To learn about Isomerism.
Sugar Identification using Polarimetry
 Sugar Identification using Polarimetry Safety Concerns: No special safety precautions need to be implemented. Standard organic laboratory safety measures need to be in place. Purpose The purpose of this
Sugar Identification using Polarimetry Safety Concerns: No special safety precautions need to be implemented. Standard organic laboratory safety measures need to be in place. Purpose The purpose of this
TRANSITION METALS AND COORDINATION CHEMISTRY
 CHAPTER TWENTY-ONE TRANSITION METALS AND COORDINATION CHEMISTRY For Review 1. Chromium ([Ar]:4s 0 3d 5 ) and copper [Ar]:4s 1 3d 10 ) have electron configurations which are different from that predicted
CHAPTER TWENTY-ONE TRANSITION METALS AND COORDINATION CHEMISTRY For Review 1. Chromium ([Ar]:4s 0 3d 5 ) and copper [Ar]:4s 1 3d 10 ) have electron configurations which are different from that predicted
Unit Vocabulary: o Organic Acid o Alcohol. o Ester o Ether. o Amine o Aldehyde
 Unit Vocabulary: Addition rxn Esterification Polymer Alcohol Ether Polymerization Aldehyde Fermentation Primary Alkane Functional group Saponification Alkene Halide (halocarbon) Saturated hydrocarbon Alkyne
Unit Vocabulary: Addition rxn Esterification Polymer Alcohol Ether Polymerization Aldehyde Fermentation Primary Alkane Functional group Saponification Alkene Halide (halocarbon) Saturated hydrocarbon Alkyne
Final Examination, Organic Chemistry 1 (CHEM 2210) December 2000 Version *A* A. B. C. D.
 Final Examination, rganic hemistry 1 (EM 2210) December 2000 Version *A* 1. What are the hybridization of, and the geometrical shape around, the nitrogen atom in the following molecule? N 3 3 A. sp, linear
Final Examination, rganic hemistry 1 (EM 2210) December 2000 Version *A* 1. What are the hybridization of, and the geometrical shape around, the nitrogen atom in the following molecule? N 3 3 A. sp, linear
EXPERIMENT # 17 CHEMICAL BONDING AND MOLECULAR POLARITY
 EXPERIMENT # 17 CHEMICAL BONDING AND MOLECULAR POLARITY Purpose: 1. To distinguish between different types of chemical bonds. 2. To predict the polarity of some common molecules from a knowledge of bond
EXPERIMENT # 17 CHEMICAL BONDING AND MOLECULAR POLARITY Purpose: 1. To distinguish between different types of chemical bonds. 2. To predict the polarity of some common molecules from a knowledge of bond
STANDARD ANSWERS AND DEFINITIONS
 Evidence for Kekule s model to be wrong: STANDARD ANSWERS AND DEFINITIONS All C-C bond lengths are the same length, between C-C and C=C. Only reacts with Br2 with a halogen carrier Benzene is lower in
Evidence for Kekule s model to be wrong: STANDARD ANSWERS AND DEFINITIONS All C-C bond lengths are the same length, between C-C and C=C. Only reacts with Br2 with a halogen carrier Benzene is lower in
Dr.B.R.AMBEDKAR OPEN UNVERSITY FACULTY OF SCIENCE M.Sc. I year -CHEMISTRY (2013-14) Course I: Inorganic Chemistry
 M.Sc. I year -CHEMISTRY (2013-14) Course I: Inorganic Chemistry Maximum Marks 15 Minimum Marks - 06 Section A 1X10=10 Answer any One question from the following Two questions a. What is symmetry operation?
M.Sc. I year -CHEMISTRY (2013-14) Course I: Inorganic Chemistry Maximum Marks 15 Minimum Marks - 06 Section A 1X10=10 Answer any One question from the following Two questions a. What is symmetry operation?
Amount of Substance. http://www.avogadro.co.uk/definitions/elemcompmix.htm
 Page 1 of 14 Amount of Substance Key terms in this chapter are: Element Compound Mixture Atom Molecule Ion Relative Atomic Mass Avogadro constant Mole Isotope Relative Isotopic Mass Relative Molecular
Page 1 of 14 Amount of Substance Key terms in this chapter are: Element Compound Mixture Atom Molecule Ion Relative Atomic Mass Avogadro constant Mole Isotope Relative Isotopic Mass Relative Molecular
PRACTICE PROBLEMS, CHAPTERS 1-3
 PRATIE PRBLEMS, APTERS 1-3 (overed from h. 3: Alkane and Alkyl alide nomenclature only) 1. The atomic number of boron is 5. The correct electronic configuration of boron is: A. 1s 2 2s 3 B. 1s 2 2p 3.
PRATIE PRBLEMS, APTERS 1-3 (overed from h. 3: Alkane and Alkyl alide nomenclature only) 1. The atomic number of boron is 5. The correct electronic configuration of boron is: A. 1s 2 2s 3 B. 1s 2 2p 3.
Laboratory 11: Molecular Compounds and Lewis Structures
 Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
Introduction Laboratory 11: Molecular Compounds and Lewis Structures Molecular compounds are formed by sharing electrons between non-metal atoms. A useful theory for understanding the formation of molecular
Chapter 1 Structure and Bonding. Modified by Dr. Daniela Radu
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 1 Structure and Bonding Modified by Dr. Daniela Radu What is Organic Chemistry? Living things are made of organic chemicals Proteins that make
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 1 Structure and Bonding Modified by Dr. Daniela Radu What is Organic Chemistry? Living things are made of organic chemicals Proteins that make
Alkanes. Chapter 1.1
 Alkanes Chapter 1.1 Organic Chemistry The study of carbon-containing compounds and their properties What s so special about carbon? Carbon has 4 bonding electrons. Thus, it can form 4 strong covalent bonds
Alkanes Chapter 1.1 Organic Chemistry The study of carbon-containing compounds and their properties What s so special about carbon? Carbon has 4 bonding electrons. Thus, it can form 4 strong covalent bonds
7) How many electrons are in the second energy level for an atom of N? A) 5 B) 6 C) 4 D) 8
 HOMEWORK CHEM 107 Chapter 3 Compounds Putting Particles Together 3.1 Multiple-Choice 1) How many electrons are in the highest energy level of sulfur? A) 2 B) 4 C) 6 D) 8 2) An atom of phosphorous has how
HOMEWORK CHEM 107 Chapter 3 Compounds Putting Particles Together 3.1 Multiple-Choice 1) How many electrons are in the highest energy level of sulfur? A) 2 B) 4 C) 6 D) 8 2) An atom of phosphorous has how
Chapter 5 Classification of Organic Compounds by Solubility
 Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Chapter 5 Classification of Organic Compounds by Solubility Deductions based upon interpretation of simple solubility tests can be extremely useful in organic structure determination. Both solubility and
Chemistry 151 Final Exam
 Chemistry 151 Final Exam Name: SSN: Exam Rules & Guidelines Show your work. No credit will be given for an answer unless your work is shown. Indicate your answer with a box or a circle. All paperwork must
Chemistry 151 Final Exam Name: SSN: Exam Rules & Guidelines Show your work. No credit will be given for an answer unless your work is shown. Indicate your answer with a box or a circle. All paperwork must
Symmetry and group theory
 Symmetry and group theory or How to Describe the Shape of a Molecule with two or three letters Natural symmetry in plants Symmetry in animals 1 Symmetry in the human body The platonic solids Symmetry in
Symmetry and group theory or How to Describe the Shape of a Molecule with two or three letters Natural symmetry in plants Symmetry in animals 1 Symmetry in the human body The platonic solids Symmetry in
Chem 11 Oa-Section 2. Exam #2. 1 October 2004
 " Chem 11 Oa-Section 2 Exam #2 1 October 2004 Name: K~ Instructions Please read each question carefully and answer it completely and clearly. Do the problems in the order that is easiest for you. Point
" Chem 11 Oa-Section 2 Exam #2 1 October 2004 Name: K~ Instructions Please read each question carefully and answer it completely and clearly. Do the problems in the order that is easiest for you. Point
An Introduction to Organic Chemistry
 An Introduction to Organic Chemistry 81 Organic Chemistry Organic chemistry is the study of compounds containing carbon with the exception of simple compounds e.g. carbonates (CO 3 2- ), carbon dioxide
An Introduction to Organic Chemistry 81 Organic Chemistry Organic chemistry is the study of compounds containing carbon with the exception of simple compounds e.g. carbonates (CO 3 2- ), carbon dioxide
Chapter 13 Organic Chemistry
 Chapter 13 Organic Chemistry Introduction Organic chemistry is the study of carbon based compounds. The structural and genetic materials of living organisms are organic compounds. Many of the substances
Chapter 13 Organic Chemistry Introduction Organic chemistry is the study of carbon based compounds. The structural and genetic materials of living organisms are organic compounds. Many of the substances
Chapter 2: The Chemical Context of Life
 Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Chapter 2: The Chemical Context of Life Name Period This chapter covers the basics that you may have learned in your chemistry class. Whether your teacher goes over this chapter, or assigns it for you
Page 1. 6. Which hydrocarbon is a member of the alkane series? (1) 1. Which is the structural formula of methane? (1) (2) (2) (3) (3) (4) (4)
 1. Which is the structural formula of methane? 6. Which hydrocarbon is a member of the alkane series? 7. How many carbon atoms are contained in an ethyl group? 1 3 2 4 2. In the alkane series, each molecule
1. Which is the structural formula of methane? 6. Which hydrocarbon is a member of the alkane series? 7. How many carbon atoms are contained in an ethyl group? 1 3 2 4 2. In the alkane series, each molecule
2/10/2011. Stability of Cycloalkanes: Ring Strain. Stability of Cycloalkanes: Ring Strain. 4.3 Stability of Cycloalkanes: Ring Strain
 4.3 Stability of Cycloalkanes: Ring Strain Angle strain The strain induced in a molecule when bond angles are forced to deviate from the ideal 109º tetrahedral value (Adolf von Baeyer 1885) Stability of
4.3 Stability of Cycloalkanes: Ring Strain Angle strain The strain induced in a molecule when bond angles are forced to deviate from the ideal 109º tetrahedral value (Adolf von Baeyer 1885) Stability of
Chapter 9. Chemical reactivity of molecules depends on the nature of the bonds between the atoms as well on its 3D structure
 Chapter 9 Molecular Geometry & Bonding Theories I) Molecular Geometry (Shapes) Chemical reactivity of molecules depends on the nature of the bonds between the atoms as well on its 3D structure Molecular
Chapter 9 Molecular Geometry & Bonding Theories I) Molecular Geometry (Shapes) Chemical reactivity of molecules depends on the nature of the bonds between the atoms as well on its 3D structure Molecular
Chapter 12 Organic Compounds with Oxygen and Sulfur
 Chapter 12 Organic Compounds with Oxygen and Sulfur 1 Alcohols An alcohol contains a hydroxyl group ( OH) that replaces a hydrogen atom in a hydrocarbon. A phenol contains a hydroxyl group ( OH) attached
Chapter 12 Organic Compounds with Oxygen and Sulfur 1 Alcohols An alcohol contains a hydroxyl group ( OH) that replaces a hydrogen atom in a hydrocarbon. A phenol contains a hydroxyl group ( OH) attached
Answer Key, Problem Set 11
 Chemistry 122 Mines, Spring 2010 Answer Key, Problem Set 11 1. Write the electron configuration for each of the following atoms and ions (use the noble gas abbreviation): (a) Mn: [Ar] 4s 2 3d 5 (Note:
Chemistry 122 Mines, Spring 2010 Answer Key, Problem Set 11 1. Write the electron configuration for each of the following atoms and ions (use the noble gas abbreviation): (a) Mn: [Ar] 4s 2 3d 5 (Note:
C has 4 valence electrons, O has six electrons. The total number of electrons is 4 + 2(6) = 16.
 129 Lewis Structures G. N. Lewis hypothesized that electron pair bonds between unlike elements in the second (and sometimes the third) row occurred in a way that electrons were shared such that each element
129 Lewis Structures G. N. Lewis hypothesized that electron pair bonds between unlike elements in the second (and sometimes the third) row occurred in a way that electrons were shared such that each element
Self Assessment_Ochem I
 UTID: 2013 Objective Test Section Identify the choice that best completes the statement or answers the question. There is only one correct answer; please carefully bubble your choice on the scantron sheet.
UTID: 2013 Objective Test Section Identify the choice that best completes the statement or answers the question. There is only one correct answer; please carefully bubble your choice on the scantron sheet.
CHEMISTRY BONDING REVIEW
 Answer the following questions. CHEMISTRY BONDING REVIEW 1. What are the three kinds of bonds which can form between atoms? The three types of Bonds are Covalent, Ionic and Metallic. Name Date Block 2.
Answer the following questions. CHEMISTRY BONDING REVIEW 1. What are the three kinds of bonds which can form between atoms? The three types of Bonds are Covalent, Ionic and Metallic. Name Date Block 2.
pre -TEST Big Idea 2 Chapters 8, 9, 10
 Name: AP Chemistry Period: Date: R.F. Mandes, PhD, NBCT Complete each table with the appropriate information. Compound IMF Compound IMF 1 NiCl 3 7 ClCH 2 (CH 2 ) 3 CH 3 2 Fe 8 H 2 CF 2 3 Ar 9 H 2 NCH 2
Name: AP Chemistry Period: Date: R.F. Mandes, PhD, NBCT Complete each table with the appropriate information. Compound IMF Compound IMF 1 NiCl 3 7 ClCH 2 (CH 2 ) 3 CH 3 2 Fe 8 H 2 CF 2 3 Ar 9 H 2 NCH 2
ACE PRACTICE TEST Chapter 8, Quiz 3
 ACE PRACTICE TEST Chapter 8, Quiz 3 1. Using bond energies, calculate the heat in kj for the following reaction: CH 4 + 4 F 2 CF 4 + 4 HF. Use the following bond energies: CH = 414 kj/mol, F 2 = 155 kj/mol,
ACE PRACTICE TEST Chapter 8, Quiz 3 1. Using bond energies, calculate the heat in kj for the following reaction: CH 4 + 4 F 2 CF 4 + 4 HF. Use the following bond energies: CH = 414 kj/mol, F 2 = 155 kj/mol,
Chemistry Workbook 2: Problems For Exam 2
 Chem 1A Dr. White Updated /5/1 1 Chemistry Workbook 2: Problems For Exam 2 Section 2-1: Covalent Bonding 1. On a potential energy diagram, the most stable state has the highest/lowest potential energy.
Chem 1A Dr. White Updated /5/1 1 Chemistry Workbook 2: Problems For Exam 2 Section 2-1: Covalent Bonding 1. On a potential energy diagram, the most stable state has the highest/lowest potential energy.
Chapter 11 Homework and practice questions Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations
 Chapter 11 Homework and practice questions Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations SHORT ANSWER Exhibit 11-1 Circle your response in each set below. 1. Circle the least
Chapter 11 Homework and practice questions Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations SHORT ANSWER Exhibit 11-1 Circle your response in each set below. 1. Circle the least
Chemistry Diagnostic Questions
 Chemistry Diagnostic Questions Answer these 40 multiple choice questions and then check your answers, located at the end of this document. If you correctly answered less than 25 questions, you need to
Chemistry Diagnostic Questions Answer these 40 multiple choice questions and then check your answers, located at the end of this document. If you correctly answered less than 25 questions, you need to
CORK INSTITUTE OF TECHNOLOGY INSTITIÚID TEICNEOLAÍOCHTA CHORCAÍ
 CORK INSTITUTE OF TECHNOLOGY INSTITIÚID TEICNEOLAÍOCHTA CHORCAÍ Module Title: Topics in Organic Chemistry Module Code: CHEO 7003 School : Science Programme Title: Bachelor of Science in Analytical & Pharmaceutical
CORK INSTITUTE OF TECHNOLOGY INSTITIÚID TEICNEOLAÍOCHTA CHORCAÍ Module Title: Topics in Organic Chemistry Module Code: CHEO 7003 School : Science Programme Title: Bachelor of Science in Analytical & Pharmaceutical
CHEMISTRY 101 EXAM 3 (FORM B) DR. SIMON NORTH
 1. Is H 3 O + polar or non-polar? (1 point) a) Polar b) Non-polar CHEMISTRY 101 EXAM 3 (FORM B) DR. SIMON NORTH 2. The bond strength is considerably greater in HF than in the other three hydrogen halides
1. Is H 3 O + polar or non-polar? (1 point) a) Polar b) Non-polar CHEMISTRY 101 EXAM 3 (FORM B) DR. SIMON NORTH 2. The bond strength is considerably greater in HF than in the other three hydrogen halides
Draft agreed by the QWP February 2015. Draft adopted by the CHMP for release for consultation March 2015. Draft endorsed by the CMD(h) March 2015
 1 2 3 26 March 2015 EMA/CHMP/QWP/104223/2015 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Reflection paper on the chemical structure and properties criteria to be considered for the evaluation
1 2 3 26 March 2015 EMA/CHMP/QWP/104223/2015 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Reflection paper on the chemical structure and properties criteria to be considered for the evaluation
Vocabulary: VSEPR. 3 domains on central atom. 2 domains on central atom. 3 domains on central atom NOTE: Valence Shell Electron Pair Repulsion Theory
 Vocabulary: VSEPR Valence Shell Electron Pair Repulsion Theory domain = any electron pair, or any double or triple bond is considered one domain. lone pair = non-bonding pair = unshared pair = any electron
Vocabulary: VSEPR Valence Shell Electron Pair Repulsion Theory domain = any electron pair, or any double or triple bond is considered one domain. lone pair = non-bonding pair = unshared pair = any electron
1.15 Bonding in Methane and Orbital Hybridization
 1.15 Bonding in Methane and Orbital Hybridization Structure of Methane tetrahedral bond angles = 109.5 bond distances = 110 pm but structure seems inconsistent with electron configuration of carbon Electron
1.15 Bonding in Methane and Orbital Hybridization Structure of Methane tetrahedral bond angles = 109.5 bond distances = 110 pm but structure seems inconsistent with electron configuration of carbon Electron
the double or triple bond. If the multiple bond is CH 3 C CCHCCH 3
 Alkenes, Alkynes, and Aromatic ompounds Alkenes and Alkynes Unsaturated contain carbon-carbon double and triple bond to which more hydrogen atoms can be added. Alkenes: carbon-carbon double bonds Alkynes:
Alkenes, Alkynes, and Aromatic ompounds Alkenes and Alkynes Unsaturated contain carbon-carbon double and triple bond to which more hydrogen atoms can be added. Alkenes: carbon-carbon double bonds Alkynes:
Advanced Medicinal & Pharmaceutical Chemistry CHEM 5412 Dept. of Chemistry, TAMUK
 Advanced Medicinal & Pharmaceutical Chemistry CHEM 5412 Dept. of Chemistry, TAMUK Dai Lu, Ph.D. dlu@tamhsc.edu Tel: 361-221-0745 Office: RCOP, Room 307 Drug Discovery and Development Drug Molecules Medicinal
Advanced Medicinal & Pharmaceutical Chemistry CHEM 5412 Dept. of Chemistry, TAMUK Dai Lu, Ph.D. dlu@tamhsc.edu Tel: 361-221-0745 Office: RCOP, Room 307 Drug Discovery and Development Drug Molecules Medicinal
 2814 hains, Rings and Spectroscopy June 2003 Mark Scheme 2814 Mark Scheme June 2003 The following annotations may be used when marking: X = incorrect response (errors may also be underlined) ^ = omission
2814 hains, Rings and Spectroscopy June 2003 Mark Scheme 2814 Mark Scheme June 2003 The following annotations may be used when marking: X = incorrect response (errors may also be underlined) ^ = omission
CHEM 121. Chapter 19, Name: Date:
 CHEM 121. Chapter 19, Name: Date: 1. A lipid is any substance of biochemical origin that is A) soluble in water but insoluble in nonpolar solvents B) insoluble in both water and nonpolar solvents C) insoluble
CHEM 121. Chapter 19, Name: Date: 1. A lipid is any substance of biochemical origin that is A) soluble in water but insoluble in nonpolar solvents B) insoluble in both water and nonpolar solvents C) insoluble
EXPERIMENT 5: DIPEPTIDE RESEARCH PROJECT
 EXPERIMENT 5: DIPEPTIDE RESEARCH PROJECT Pre-Lab Questions: None. 64 I. Background Information DIPEPTIDE RESEARCH PROJECT Methods developed by organic chemists for the synthesis of biopolymers have had
EXPERIMENT 5: DIPEPTIDE RESEARCH PROJECT Pre-Lab Questions: None. 64 I. Background Information DIPEPTIDE RESEARCH PROJECT Methods developed by organic chemists for the synthesis of biopolymers have had
DCI for Electronegativity. Data Table:
 DCI for Electronegativity Data Table: Substance Ionic/covalent EN value EN Value EN NaCl ionic (Na) 0.9 (Cl) 3.0 2.1 KBr (K) 0.8 (Br) 2.8 MgO (Mg) 1.2 (O) 3.5 HCl (H) 2.1 (Cl) 3.0 HF (H) 2.1 (F) 4.0 Cl
DCI for Electronegativity Data Table: Substance Ionic/covalent EN value EN Value EN NaCl ionic (Na) 0.9 (Cl) 3.0 2.1 KBr (K) 0.8 (Br) 2.8 MgO (Mg) 1.2 (O) 3.5 HCl (H) 2.1 (Cl) 3.0 HF (H) 2.1 (F) 4.0 Cl
EXPERIMENT 17 : Lewis Dot Structure / VSEPR Theory
 EXPERIMENT 17 : Lewis Dot Structure / VSEPR Theory Materials: Molecular Model Kit INTRODUCTION Although it has recently become possible to image molecules and even atoms using a high-resolution microscope,
EXPERIMENT 17 : Lewis Dot Structure / VSEPR Theory Materials: Molecular Model Kit INTRODUCTION Although it has recently become possible to image molecules and even atoms using a high-resolution microscope,
methyl RX example primary RX example secondary RX example secondary RX example tertiary RX example
 ucleophilic Substitution & Elimination hemistry 1 eginning patterns to knowfor S and E eactions - horizontal and vertical templates for practice Example 1 - two possible perspectives (deuterium and tritium
ucleophilic Substitution & Elimination hemistry 1 eginning patterns to knowfor S and E eactions - horizontal and vertical templates for practice Example 1 - two possible perspectives (deuterium and tritium
In the box below, draw the Lewis electron-dot structure for the compound formed from magnesium and oxygen. [Include any charges or partial charges.
 Name: 1) Which molecule is nonpolar and has a symmetrical shape? A) NH3 B) H2O C) HCl D) CH4 7222-1 - Page 1 2) When ammonium chloride crystals are dissolved in water, the temperature of the water decreases.
Name: 1) Which molecule is nonpolar and has a symmetrical shape? A) NH3 B) H2O C) HCl D) CH4 7222-1 - Page 1 2) When ammonium chloride crystals are dissolved in water, the temperature of the water decreases.
Benzene Benzene is best represented as a resonance hybrid:
 Electrophilic Aromatic Substitution (EAS) is a substitution reaction usually involving the benzene ring; more specifically it is a reaction in which the hydrogen atom of an aromatic ring is replaced as
Electrophilic Aromatic Substitution (EAS) is a substitution reaction usually involving the benzene ring; more specifically it is a reaction in which the hydrogen atom of an aromatic ring is replaced as
CONFORMATIONAL ANALYSIS PRACTICE EXERCISES. 1) Draw a Newman projection of the most stable conformation of 2-methylpropane.
 CONFORMATIONAL ANALYSIS PRACTICE EXERCISES 1) Draw a Newman projection of the most stable conformation of 2-methylpropane. 2) The structures below are: C 3 C 3 C 3 C 3 A) not isomers. B) conformational
CONFORMATIONAL ANALYSIS PRACTICE EXERCISES 1) Draw a Newman projection of the most stable conformation of 2-methylpropane. 2) The structures below are: C 3 C 3 C 3 C 3 A) not isomers. B) conformational
Molecular Models in Biology
 Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
Molecular Models in Biology Objectives: After this lab a student will be able to: 1) Understand the properties of atoms that give rise to bonds. 2) Understand how and why atoms form ions. 3) Model covalent,
A. Essentially the order of Pt II affinity for these ligands (their Lewis basicity or nucleophilicity)
 (c) Entering Ligand Effects 284 The second order rate constant k 2 in square planar complexes in the rate law below that we just discussed is strongly dependent on the nature of Y, the entering ligand
(c) Entering Ligand Effects 284 The second order rate constant k 2 in square planar complexes in the rate law below that we just discussed is strongly dependent on the nature of Y, the entering ligand
How To Understand The Chemistry Of Organic Molecules
 CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
CHAPTER 3 THE CHEMISTRY OF ORGANIC MOLECULES 3.1 Organic Molecules The chemistry of carbon accounts for the diversity of organic molecules found in living things. Carbon has six electrons, four of which
cyclohexane cyclopentane Nomenclature Follows same rules as for stright-chain alkanes. Examples: name the following
 Structure and Stereochemistry of Alkanes Reading: Wade chapter 3, sections 3-10- 3-16 Study Problems: 3-43, 3-44, 3-45, 3-46 Key oncepts and Skills: ompare the energies of cycloalkanes, and explain ring
Structure and Stereochemistry of Alkanes Reading: Wade chapter 3, sections 3-10- 3-16 Study Problems: 3-43, 3-44, 3-45, 3-46 Key oncepts and Skills: ompare the energies of cycloalkanes, and explain ring
3/5/2014. iclicker Participation Question: A. MgS < AlP < NaCl B. MgS < NaCl < AlP C. NaCl < AlP < MgS D. NaCl < MgS < AlP
 Today: Ionic Bonding vs. Covalent Bonding Strengths of Covalent Bonds: Bond Energy Diagrams Bond Polarities: Nonpolar Covalent vs. Polar Covalent vs. Ionic Electronegativity Differences Dipole Moments
Today: Ionic Bonding vs. Covalent Bonding Strengths of Covalent Bonds: Bond Energy Diagrams Bond Polarities: Nonpolar Covalent vs. Polar Covalent vs. Ionic Electronegativity Differences Dipole Moments
Enantiomers: Synthesis, characterization, and resolution of tris(ethylenediamine)cobalt(iii) chloride Introduction:
 Enantiomers: Synthesis, characterization, and resolution of tris(ethylenediamine)cobalt(iii) chloride Introduction: The development of coordination chemistry prior to 1950 involved the synthesis and characterization
Enantiomers: Synthesis, characterization, and resolution of tris(ethylenediamine)cobalt(iii) chloride Introduction: The development of coordination chemistry prior to 1950 involved the synthesis and characterization
Lecture Overview. Hydrogen Bonds. Special Properties of Water Molecules. Universal Solvent. ph Scale Illustrated. special properties of water
 Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Lecture Overview special properties of water > water as a solvent > ph molecules of the cell > properties of carbon > carbohydrates > lipids > proteins > nucleic acids Hydrogen Bonds polarity of water
Draft agreed by the QWP February 2015. Draft adopted by the CHMP for release for consultation March 2015. Draft endorsed by the CMD(h) March 2015
 17 December 2015 EMA/CHMP/QWP/104223/2015 Committee for Medicinal Products for Human Use (CHMP) Reflection paper on the chemical structure and properties criteria to be considered for the evaluation of
17 December 2015 EMA/CHMP/QWP/104223/2015 Committee for Medicinal Products for Human Use (CHMP) Reflection paper on the chemical structure and properties criteria to be considered for the evaluation of
Exercises Topic 2: Molecules
 hemistry for Biomedical Engineering. Exercises Topic 2 Authors: ors: Juan Baselga & María González Exercises Topic 2: Molecules 1. Using hybridization concepts and VSEPR model describe the molecular geometry
hemistry for Biomedical Engineering. Exercises Topic 2 Authors: ors: Juan Baselga & María González Exercises Topic 2: Molecules 1. Using hybridization concepts and VSEPR model describe the molecular geometry
A Grignard reagent formed would deprotonate H of the ethyl alcohol OH.
 216 S11-E2 Page 2 Name Key I. (9 points) Answer in the boxes below the following questions for the Grignard reagent C 3 -Mg. (1) (2 points) Is the carbon atom associated with magnesium electrophilic or
216 S11-E2 Page 2 Name Key I. (9 points) Answer in the boxes below the following questions for the Grignard reagent C 3 -Mg. (1) (2 points) Is the carbon atom associated with magnesium electrophilic or
Polarity. Andy Schweitzer
 Polarity Andy Schweitzer What does it mean to be polar? A molecule is polar if it contains + and somewhere in the molecule. Remember: Protons can not move. So for a molecule to get a +/- it must somehow
Polarity Andy Schweitzer What does it mean to be polar? A molecule is polar if it contains + and somewhere in the molecule. Remember: Protons can not move. So for a molecule to get a +/- it must somehow
Exam 4 Outline CH 105 Spring 2012
 Exam 4 Outline CH 105 Spring 2012 You need to bring a pencil and your ACT card. Chapter 24: Lipids 1. Describe the properties and types of lipids a. All are hydrophobic b. Fatty acid-based typically contain
Exam 4 Outline CH 105 Spring 2012 You need to bring a pencil and your ACT card. Chapter 24: Lipids 1. Describe the properties and types of lipids a. All are hydrophobic b. Fatty acid-based typically contain
Preliminary MFM Quiz
 Preliminary MFM Quiz 1. The major carrier of chemical energy in all cells is: A) adenosine monophosphate B) adenosine diphosphate C) adenosine trisphosphate D) guanosine trisphosphate E) carbamoyl phosphate
Preliminary MFM Quiz 1. The major carrier of chemical energy in all cells is: A) adenosine monophosphate B) adenosine diphosphate C) adenosine trisphosphate D) guanosine trisphosphate E) carbamoyl phosphate
Molecular Geometry and VSEPR We gratefully acknowledge Portland Community College for the use of this experiment.
 Molecular and VSEPR We gratefully acknowledge Portland ommunity ollege for the use of this experiment. Objectives To construct molecular models for covalently bonded atoms in molecules and polyatomic ions
Molecular and VSEPR We gratefully acknowledge Portland ommunity ollege for the use of this experiment. Objectives To construct molecular models for covalently bonded atoms in molecules and polyatomic ions
Chapter 2 The Chemical Context of Life
 Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
Chapter 2 The Chemical Context of Life Multiple-Choice Questions 1) About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living
PROTON NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY (H-NMR)
 PROTON NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY (H-NMR) WHAT IS H-NMR SPECTROSCOPY? References: Bruice 14.1, 14.2 Introduction NMR or nuclear magnetic resonance spectroscopy is a technique used to determine
PROTON NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY (H-NMR) WHAT IS H-NMR SPECTROSCOPY? References: Bruice 14.1, 14.2 Introduction NMR or nuclear magnetic resonance spectroscopy is a technique used to determine
Chem 112 Intermolecular Forces Chang From the book (10, 12, 14, 16, 18, 20,84,92,94,102,104, 108, 112, 114, 118 and 134)
 Chem 112 Intermolecular Forces Chang From the book (10, 12, 14, 16, 18, 20,84,92,94,102,104, 108, 112, 114, 118 and 134) 1. Helium atoms do not combine to form He 2 molecules, What is the strongest attractive
Chem 112 Intermolecular Forces Chang From the book (10, 12, 14, 16, 18, 20,84,92,94,102,104, 108, 112, 114, 118 and 134) 1. Helium atoms do not combine to form He 2 molecules, What is the strongest attractive
EXPERIMENT 9 Dot Structures and Geometries of Molecules
 EXPERIMENT 9 Dot Structures and Geometries of Molecules INTRODUCTION Lewis dot structures are our first tier in drawing molecules and representing bonds between the atoms. The method was first published
EXPERIMENT 9 Dot Structures and Geometries of Molecules INTRODUCTION Lewis dot structures are our first tier in drawing molecules and representing bonds between the atoms. The method was first published
Health Science Chemistry I CHEM-1180 Experiment No. 15 Molecular Models (Revised 05/22/2015)
 (Revised 05/22/2015) Introduction In the early 1900s, the chemist G. N. Lewis proposed that bonds between atoms consist of two electrons apiece and that most atoms are able to accommodate eight electrons
(Revised 05/22/2015) Introduction In the early 1900s, the chemist G. N. Lewis proposed that bonds between atoms consist of two electrons apiece and that most atoms are able to accommodate eight electrons
On Factoring Chirality and Stereoisomerism
 Topics in Stereochemisty, Volume 14 Edited by Ernest L. Eliel, Norman L. Allinger, Samuel H. Wilen Copyright 1983 by John Wiley & Sons, Inc. On Factoring Chirality and Stereoisomerism HANS HIRSCHMANN Department
Topics in Stereochemisty, Volume 14 Edited by Ernest L. Eliel, Norman L. Allinger, Samuel H. Wilen Copyright 1983 by John Wiley & Sons, Inc. On Factoring Chirality and Stereoisomerism HANS HIRSCHMANN Department
We emphasize Lewis electron dot structures because of their usefulness in explaining structure of covalent molecules, especially organic molecules.
 Chapter 10 Bonding: Lewis electron dot structures and more Bonding is the essence of chemistry! Not just physics! Chemical bonds are the forces that hold atoms together in molecules, in ionic compounds,
Chapter 10 Bonding: Lewis electron dot structures and more Bonding is the essence of chemistry! Not just physics! Chemical bonds are the forces that hold atoms together in molecules, in ionic compounds,
POLARITY AND MOLECULAR SHAPE WITH HYPERCHEM LITE
 POLARITY AND MOLECULAR SHAPE WITH HYPERCHEM LITE LAB MOD4.COMP From Gannon University SIM INTRODUCTION Many physical properties of matter, such as boiling point and melting point, are the result of the
POLARITY AND MOLECULAR SHAPE WITH HYPERCHEM LITE LAB MOD4.COMP From Gannon University SIM INTRODUCTION Many physical properties of matter, such as boiling point and melting point, are the result of the
Bonding & Molecular Shape Ron Robertson
 Bonding & Molecular Shape Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\00bondingtrans.doc The Nature of Bonding Types 1. Ionic 2. Covalent 3. Metallic 4. Coordinate covalent Driving
Bonding & Molecular Shape Ron Robertson r2 n:\files\courses\1110-20\2010 possible slides for web\00bondingtrans.doc The Nature of Bonding Types 1. Ionic 2. Covalent 3. Metallic 4. Coordinate covalent Driving
Which substance contains positive ions immersed in a sea of mobile electrons? A) O2(s) B) Cu(s) C) CuO(s) D) SiO2(s)
 BONDING MIDTERM REVIEW 7546-1 - Page 1 1) Which substance contains positive ions immersed in a sea of mobile electrons? A) O2(s) B) Cu(s) C) CuO(s) D) SiO2(s) 2) The bond between hydrogen and oxygen in
BONDING MIDTERM REVIEW 7546-1 - Page 1 1) Which substance contains positive ions immersed in a sea of mobile electrons? A) O2(s) B) Cu(s) C) CuO(s) D) SiO2(s) 2) The bond between hydrogen and oxygen in
Modelling Compounds. 242 MHR Unit 2 Atoms, Elements, and Compounds
 6.3 Figure 6.26 To build the Michael Lee-Chin Crystal at the Royal Ontario Museum, models were used at different stages to convey different types of information. Modelling Compounds The Michael Lee-Chin
6.3 Figure 6.26 To build the Michael Lee-Chin Crystal at the Royal Ontario Museum, models were used at different stages to convey different types of information. Modelling Compounds The Michael Lee-Chin
Chemistry B11 Chapter 6 Solutions and Colloids
 Chemistry B11 Chapter 6 Solutions and Colloids Solutions: solutions have some properties: 1. The distribution of particles in a solution is uniform. Every part of the solution has exactly the same composition
Chemistry B11 Chapter 6 Solutions and Colloids Solutions: solutions have some properties: 1. The distribution of particles in a solution is uniform. Every part of the solution has exactly the same composition
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
 Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
Basic Chemistry Why do we study chemistry in a biology course? All living organisms are composed of chemicals. To understand life, we must understand the structure, function, and properties of the chemicals
A pure covalent bond is an equal sharing of shared electron pair(s) in a bond. A polar covalent bond is an unequal sharing.
 CHAPTER EIGHT BNDING: GENERAL CNCEPT or Review 1. Electronegativity is the ability of an atom in a molecule to attract electrons to itself. Electronegativity is a bonding term. Electron affinity is the
CHAPTER EIGHT BNDING: GENERAL CNCEPT or Review 1. Electronegativity is the ability of an atom in a molecule to attract electrons to itself. Electronegativity is a bonding term. Electron affinity is the
A REVIEW OF GENERAL CHEMISTRY: ELECTRONS, BONDS AND MOLECULAR PROPERTIES
 A REVIEW OF GENERAL CEMISTRY: ELECTRONS, BONDS AND MOLECULAR PROPERTIES A STUDENT SOULD BE ABLE TO: 1. Draw Lewis (electron dot and line) structural formulas for simple compounds and ions from molecular
A REVIEW OF GENERAL CEMISTRY: ELECTRONS, BONDS AND MOLECULAR PROPERTIES A STUDENT SOULD BE ABLE TO: 1. Draw Lewis (electron dot and line) structural formulas for simple compounds and ions from molecular
Structures and Properties of Substances. Introducing Valence-Shell Electron- Pair Repulsion (VSEPR) Theory
 Structures and Properties of Substances Introducing Valence-Shell Electron- Pair Repulsion (VSEPR) Theory The VSEPR theory In 1957, the chemist Ronald Gillespie and Ronald Nyholm, developed a model for
Structures and Properties of Substances Introducing Valence-Shell Electron- Pair Repulsion (VSEPR) Theory The VSEPR theory In 1957, the chemist Ronald Gillespie and Ronald Nyholm, developed a model for
