Sunitinib and other targeted therapies for renal cell carcinoma
|
|
|
- Willa Barber
- 7 years ago
- Views:
Transcription
1 British Journal of Cancer (2011), 1 5 All rights reserved /11 Minireview Sunitinib and other targeted therapies for renal cell carcinoma T Powles*,1, S Chowdhury 3, R Jones 2, M Mantle 1, P Nathan 5, A Bex 6, L Lim 1 and T Hutson 4 1 Department of Medical Oncology, St Bartholomew s Hospital London, Barts and the London NHS trust, Charterhouse Square, London, UK; 2 Department of Medical Oncology, The Beatson Hospital Glasgow, UK; 3 Department of Medical Oncology, Guy s and St Thomas Hospital, London, UK; 4 Department of Medical Oncology, Baylor University Medical Centre, Dallas, TX, USA; 5 Department of Medical Oncology, Mount Vernon Hospital, London, UK; 6 Department of Medical Oncology, National Cancer Institute, Amsterdam, The Netherlands Targeted therapy has radically altered the way metastatic renal cancer is treated. Six drugs are now licensed in this setting, with several other agents under evaluation. Sunitinib is currently the most widely used in the first line setting with impressive efficacy and an established toxicity profile. However, as further randomised studies report and as newer drugs become available this may change. In this review, we address our current understanding of targeted therapy in renal cancer. We also discuss areas in which our knowledge is incomplete, including the identification of correlative biomarkers and mechanisms of drug resistance. Finally, we will describe the major areas of clinical research that will report over the next few years. British Journal of Cancer advance online publication, 25 January 2011; doi: /sj.bjc Keywords: renal cancer; sunitinib; mtor; VEGF The treatment of metastatic clear cell renal carcinoma (RCC) has dramatically changed over the last 5 years. This has been driven by two groups of targeted agents; namely vascular endothelial growth factor (VEGF)-targeted therapies and mammalian target of rapamycin (mtor) inhibitors. Both, first and second line treatment is of proven benefit and these agents have replaced immune therapies that were previously standard of care for metastatic RCC (Motzer and Bukowski, 2006; Motzer et al, 2009). Sunitinib is a multitargeted tyrosine kinase inhibitor (TKI) that predominantly targets VEGF (Figure 1). It also has off target effects, involving other tyrosine kinases that may account for some of its activity and toxicity (Motzer and Bukowski, 2006). The pivotal trial of sunitinib was published in 2006; and subsequently it has established itself as standard first line therapy (Motzer et al, 2009). The exact mechanism of its activity in RCC remains unknown, and it is not yet possible to identify specific cohorts of patients who benefit from therapy. Additionally, sunitinib and the other drugs are only effective in controlling the disease for a limited period before progression occurs. Therefore, an important area of research is investigating mechanisms of resistance. Sunitinib is one of a number of VEGF-targeted agents with activity in this setting. Four drugs including sunitinib have been approved by the FDA for the treatment of metastatic RCC. The other three agents are sorafenib, pazopanib and bevacizumab (Escudier et al, 2007a, b; Rini et al, 2008a, b; Sternberg et al, 2009). Newer agents under investigation in ongoing randomised trials include tivozinib, dovitinib and axtitinib. It is hoped that these agents will show improved activity, decreased toxicity or set a benchmark as second or third line therapy. However, none of these *Correspondence: Dr T Powles; thomas.polwes@bartsandthelondon.nhs.uk Received 16 June 2010; revised 3 September 2010; accepted 29 November 2010 trials are aimed at specific subsets of patients with metastatic clear cell RCC. The other major group of targeted agents in renal cancer is the mtor inhibitors. These include temsirolimus and everolimus that are both widely used and have proven activity (Hudes et al, 2007; Motzer et al, 2008a, b). Once again there has been a failure to identify specific groups of patients that benefit from these agents, and although these agents offer initial benefit, resistance occurs. This review addresses our current understanding of the role of targeted therapy in renal cancer. It will also highlight areas in which our understanding is incomplete and finally will describe the major areas of clinical research, which will report over the next few years. HISTOLOGY AND PROGNOSIS IN METASTATIC RENAL CANCER Renal cell carcinoma consists of a number of histological subtypes, the most common of which is clear cell RCC. It is characterised by a mutation to the Von Hippel Lindau protein that is linked to both regulation of HIF and VEGF (Na et al, 2003) (Figure 1). Papillary RCC accounts for the bulk of the remaining non-clear cell tumours. The majority of trials focus on the clear cell population in which HIF/VEGF appears to be the dominant tumour biology (Rini et al, 2008a, b). The most commonly used method for predicting outcome of patients with metastatic renal cancer is the Memorial Sloan- Kettering Prognostic score. It stratifies patients into three groups (good, intermediate and poor) depending on a number of factors, including lactate dehydrogenase, performance status, serum calcium, haemoglobin and time since diagnosis to treatment (Motzer and Bukowski, 2006). Although designed before the introduction of targeted therapy it has been validated in the TKI era (Motzer et al, 2008; Heng et al, 2009).
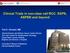
2 2 TKIs ENDOTHELIAL CELL TKIs Drug target PDGF receptor VEGF receptor Bevacizumab Activation of transcription Growth factor receptor (cell stimulus) P70S6K HIFα PI3K mtor Cyclin D1 C-Myc SUNITINIB IN METASTATIC RENAL CANCER mtor inhibitors Cell growth VHL mutation upregulates HIF-1α TUMOUR CELLS Figure 1 Diagrammatic representation of the mechanism of action of targeted therapy in clear cell renal cancer. This figure shows how HIF-1 is unregulated within the clear cell renal cancer tumour cell by two mechanisms. This upregulation in turn stimulates receptors on endothelial cells (VEGF and PDGF). Dotted lines denote the drug targets. Off-target effects on the stroma and tumour cells are not represented. Sunitinib is superior to interferon in the first line treatment of clear cell RCC. In the pivotal study the progression-free survival (PFS) was 11 months for sunitinib vs 5 months for interferon (hazard ratio (HR): 0.53; (95% CI: ); Po0.001) (Motzer et al, 2007, 2009). Most of the study patients were MSKCC good and intermediate risk, had a good performance status and a nephrectomy before treatment. The majority of patients on the interferon arm subsequently received sunitinib (33%) or other VEGF-signalling inhibitors (32%) confounding the overall survival (OS) data (26.4 months for sunitinib vs 21.8 months for interferon (HR: 0.821; 95% CI: ; P ¼ 0.051). Subsequent analysis of those patients who did not cross over to sunitinib showed an OS of 14 months for interferon and 28 months for sunitinib (Flanigan et al, 2001; Motzer et al, 2009). The benefits of sunitinib were present in all three MSKCC prognostic groups, although the numbers in the poor-risk category were small (o10% of the study population) resulting in statistically insignificant data. Subsequent data from a large expanded access series reproduced the PFS results (10.9 months; 95% CI: months), with an OS of 18.4 months (95%CI: months) underlining the practise changing nature of these results (Gore et al, 2009). There is a lack of randomised data for sunitinib in the non-clear cell population. The most comprehensive data comes from the expanded access cohort in which the PFS and OS were 7.8 (95% CI: ) months and 13.4 (95% CI: ) months, respectively (Gore et al, 2009). A further retrospective analysis supported the use of sunitinib in this setting with a PFS of 11.9 months (Choueiri et al, 2008). The most common toxicities encountered with sunitinib included (grade 3 or more) hypertension (12%), fatigue (11%), diarrhoea (9%) and hand foot syndrome (9%). In the landmark phase III study 50% of the patients required a dose reduction of sunitinib due to these events (Motzer et al, 2008a, b). A recent meta-analysis identified a relationship between steady-state area under the curve of sunitinib and time to tumour progression, toxicity and OS (Houk et al, 2009) suggesting increased exposure to sunitinib appears to be associated with improved clinical outcomes, as well as increased risk of adverse effects. Therefore, attempts should initially be made to address toxicity before dose reductions are made. Sunitinib is currently given at 50 mg for 4 consecutive weeks followed by a 2-week treatment-free interval. Phase II data investigating continuous dosing at 37.5 mg showed similar toxicity profile to the 50 mg intermittent dose (Escudier et al, 2009a, b, c). A randomized phase II study comparing the standard 50 mg regimen with continuous 37.5 mg dosing is exploring this regimen formally and will report in PREDICTING CLINICAL BENEFIT WITH TARGETED THERAPY A number of prognostic biomarkers have been identified that correlate with a poor outcome with sunitinib. These include the MSKCC prognostic criteria, raised platelet/neutrophil levels at diagnosis (Heng et al, 2009; Motzer et al, 2008a, b). However, it has not been possible to identify biomarkers or imaging modalities that predict clinical benefit with specific agents. Sunitinib causes dynamic changes to cytokine expression and growth factors; however, it remains unclear whether these predict clinical benefit (Rini et al, 2008a, b). Further work in this area is required. Although widely used, response rates using CT (RECIST criteria) do not correlate with outcome (Kontovinis et al, 2009). Other methods of correlating CT changes with outcome, such as the Choi criteria, which measures tumour attenuation (Hounsfield units), as well as changes in two-dimensional size, are under investigation and hold promise (van der Veldt et al, 2010). Other imagining modalities, such as dynamic contrast enhanced MRI and FDG- PET, are also being investigated (Hahn et al, 2008). Perhaps the most promising current biomarker is the presence of treatment-associated hypertension, which in retrospective analysis, appears to correlate with outcome (Rixe et al, 2008). This finding is under investigation in prospective studies with axitinib. It raises a number of issues, for example, is this finding a pharmacokinetic or pharmacodynamic affect and if the hypertension is associated with clinical benefit how aggressively should it be managed? The optimal way of identifying predictive rather than prognostic biomarkers in this setting is with prospective studies comparing VEGF-targeted agents with other classes of drug, such as the mtor inhibitors. Using this approach it is possible to determine whether subgroups benefit from the specific agents rather than just identifying prognostic markers. Clinical trials such as RECORD 3 that compares sunitinib and everolimus are the closest we have to this design, although the identification of biomarkers in not the primary endpoint and the translational component is, therefore, not comprehensive. OTHER VEGF-TARGETED AGENTS (Table 1) Sorafenib and bevacizumab were both developed at approximately the same time as sunitinib in RCC. Sorafenib is associates with a PFS benefit as second line therapy after interferon failure (Escudier et al, 2007a, b). However, a first line sorafenib vs interferon randomised phase II study showed no benefit for sorafenib, making it less attractive in this setting (Escudier et al, 2009a, b, c). Two randomised phase III studies investigated bevacizumab in combination with interferon vs interferon alone as first line treatment for metastatic RCC (Escudier et al, 2007a, b; Rini et al, 2008a, b). These studies focused on the clear cell population and consisted of predominantly good and intermediate risk patients. Both studies showed a benefit for the combination in terms of PFS (5.1 vs 8.5; 5.4 vs 10.2), with the OS for in the bevacizumab arm of 18.3 and 22.9 months ref. More recently Pazopanib has received FDA and EMA approval. In the pivotal randomised phase III study the PFS for the pazopanib was 11.1 months compared with 2.8 months for placebo
3 Table 1 Randomised studies in the first line setting in metastatic clear cell renal cancer 3 Reference Histology MSKCC risk groups Study drug vs control arm Progression-free survival (months) a Overall survival (months) a Motzer et al, 2007 Clear cell All Sunitinib vs interferon 11 vs vs 21.8 Hudes et al, 2007 All types Poor Temsirolimus vs interferon 5.5 vs vs 7.3 Escudier et al, 2007a, b Clear cell All Bevacizumab + interferon vs interferon 10.2 vs vs 20.6 Rini et al, 2008a, b Clear cell All Bevacizumab +interferon vs interferon 8.5 vs vs 17.4 Sternberg et al, 2009 Clear cell All Pazopanib vs placebo 11.1 vs 2.8 NA a The first figure is that of the study arm the second figure is for the control arm. for previously untreated patients (HR: 0.40; 95% CI: 0.27, 0.60; Po0.001) (Sternberg et al, 2009). The toxicity profile for pazopanib may be different to the other VEGF TKIs with the most common adverse event being diarrhoea (52%; 4% Grade 3 or 4), hypertension (40%; 4% Grade 3 or 4), hair colour changes (38%) and nausea (26%; o1% Grade 3 or 4). The incidence of fatigue and hand and foot syndrome appears to be relatively low (grade 3 or more in 5 and 2%, respectively). However, abnormalities in liver function tests (43 normal) occurred in 20% of patients (Sternberg et al, 2009; Hutson et al, 2010). These factors may help select the optimal agent for individuals. A pivotal study comparing pazopanib and sunitinib in the first line setting has closed recruitment and will report in Although direct comparison of the above agents is not currently possible because of the lack of direct randomized studies in the first line setting, indirect comparison is possible but flawed in nature (Table 1). Nevertheless sunitinib has set a benchmark for both overall and PFS here, although the PFS for pazopanib is also 11 month and is, therefore, promising. In view of the fact that sorafenib did not appear superior to interferon it is more difficult to recommend this agent above those with proven superiority to interferon, such as bevacizumab (with interferon) and sunitinib (Escudier et al, 2009a, b, c). mtor INHIBITORS Everolimus and temsirolimus are both mtor inhibitors (TORC1) that have been investigated and are widely used in metastatic RCC (Hudes et al, 2007; Motzer et al, 2009) (Figure 1). Temsirolimus was investigated in untreated poor risk disease. Results of this study showed prolonged OS for temsirolimus compared with interferon (HR for death: 0.73; 95% CI: ; P ¼ 0.01). However, the combination of temsirolimus and interferon together did not show significant benefit over temsirolimus alone. It is speculated that this may be because of the dose intensity achieved in this arm of the study. Subset analysis showed significant benefit for non-clear cell population that is of particular interest and warrants further investigation. The drug was relatively well tolerated with fewer serious adverse events in the temsirolimus group than in the interferon group (P ¼ 0.02). Everolimus is the only agent with positive randomised data after TKI failure in RCC (Motzer et al, 2009). RECORD 1 compared the effects of everolimus and placebo in patients who had previously received at least one line of targeted therapy. The PFS strongly favoured everolimus (HR: 0.30; 95% CI: ; Po0.0001). Perhaps, the most significant toxicity seen was pneumonitis (any grade 8%), which requires particular attention with using this agent. VEGF INHIBITORS AS SECOND LINE THERAPY IN RCC Although phase III data support the use of sorafenib as second line therapy, this study conducted in the era of immune therapy and (Escudier et al, 2007a, b), there is no randomised data to support further VEGF TKI therapy after failure of initial targeted therapy. However, phase II data suggest that there may be non-cross resistance between these agents (Rini et al, 2009; Sablin et al, 2009). Therefore, the use of sequential VEGF-targeted therapy may be beneficial. A number of randomised phase II and III studies, which investigate a switch from one TKI to another, are ongoing. Pazopanib, sorafenib, axitinib, cediranib and sunitinib are all being investigated in this setting. Data with axitinib is likely to be the first of these studies to report. Axitinib is a potent VEGF-specific target TKI with few off target effects. Results in the post interferon setting are impressive (PFS of 15.7 months (95% CI: )) and a phase II study that investigated axitinib post sorafenib are particularly striking (PFS and OS were 7.4 months (95% CI: ) and 13.6 months (95% CI: ), respectively) (Rixe et al, 2007; Rini et al, 2009). A randomised phase III second line study, comparing sorafenib and axitinib post VEGF-targeted therapy is now closed and results are awaited. The occurrence of hypertension with axitinib may predict clinical benefit, which is being investigated formally in clinical trials (Rixe et al, 2008). OTHER AGENTS UNDER INVESTIGATION Tivozinib is a potent VEGF TKI with impressive clinical data. In a randomised discontinuation study 88% of patients obtained a clinical benefit from the drug (Bhargava et al, 2009). A randomised phase III study comparing tivozinib and sorafenib in patients not previously exposed to targeted therapy will close in December Dovitinib is another promising TKI that targets FGF-2, as well as VEGF. It is being investigated in the third line setting in mtor refractory disease. COMBINATION THERAPY A logical step in improving outcomes is to investigate these drugs in combination. However, combining sunitinib with other forms of targeted therapy has been difficult because of excessive toxicity (Feldman et al, 2009). This may not be the case with bevacizumab, which has been shown to be tolerable in combination with other agents such as everolimus. In a phase II study of bevacizumab and everolimus the median PFS for this combination was 9.1 months with response rates of 30% (Hainsworth et al, 2010). A recent randomised phase II study comparing sunitinib v.s. bevacizumab and interferon v.s bevacizumab and temsirolimus demonstrated significant toxicity for the latter combination without any efficacy advantage (Escudier et al, 2010). Perhaps, the most early awaited combination data is RECORD II that compares bevacizumab and everolimus vs bevacizumab and interferon. The potential disadvantages of combination therapy include additional toxicity, cost and the theoretical risk of multidrug resistance. Early results in this setting have been disappointing.
4 4 MECHANISMS OF RESISTANCE TO THERAPY The mutation to the VHL gene and subsequent activation of HIF and VEGF are hall marks of clear cell renal cancer. The VEGFtargeted therapy is thought to work by blocking the angiogenic effects of this overactive pathway (Gossage and Eisen, 2010). Only a small minority of renal tumours initially progress through VEGFtargeted therapy, and the majority of patients initially obtain clinical benefit with these drugs (Motzer et al, 2009). However, resistance occurs, which is usually referred to as acquired resistance (Rini and Atkins, 2009). The onset of this acquired resistance is variable both in terms of time and clinical pattern, and our understanding of it at the molecular level is in its infancy. This is largely due to the lack of sequential tissue for comparative analysis. Unlike in other malignancies, the acquisition of specific mutation to the target of the drug is not thought to be responsible for resistance in RCC (Valent, 2008; Rini et al, 2009). This is because in RCC the main target for sunitinib is the VEGF receptors on the vascular endothelium that are genetically stable. There are currently two main theories to account for acquired resistance. First, it is speculated that angiogenic escape occurs, in which with time the initial targeted therapy becomes ineffective at blocking the VEGF axis. This hypothesis is supported by the observation that switching from one VEGF-targeted therapy to another potentially more potent agent results in further responses (Rini et al, 2009). It is speculated that this may be due to upregulation of associated growth factors, such as HIF1-a (Rini et al, 2009). The second area of investigation is based around the emerging preclinical data that support the role of alternative pathways and growth factors in propagating tumour growth in acquired resistance. Specifically, FGF-2, tie2/ang2, il-8 and the Src family have been implicated in this process (Oliner et al, 2004; Cenni et al, 2005; Bergers and Hanahan 2008). Clinical trials have been designed to target all these protein with dovotinib, AMG386 and saracatinib. Resistance also occurs universally with the mtor inhibitors. The current drugs in mrcc only target TORC1 and it appears that inhibition of this in isolation leads to compensatory upregulation of PI3 kinase (O Reilly et al, 2006). The development of combined TORC1 and 2 inhibitors could potentially overcome this and will be investigated in mrcc (Sarbassov et al, 2006). CLINICAL TRIALS IN THE FUTURE The two main goals over the next 5 10 years in mrcc are to firmly establish the most effective agents in each class and identify predictive biomarkers associated with clinical benefit to specific agents. To achieve these goals two different trial designs are required. Randomised phase III studies comparing newer agents with benchmark controls (currently sunitinib for the first line and everolimus for second line) are required to redefine standard therapy. The COMPARZ study, a non-inferiority study, which compares pazopanib and sunitinib, has just closed and is due to report in 2011/12. Results are eagerly awaited by further studies with tivozinib and axitinib vs a benchmark are required before there is clarity about the optimal first line VEGF TKI. The assumption that these agents are more active in the first-line setting without the appropriate studies is flawed, despite their impressive efficacies in other settings. Sorafenib should not be considered an adequate benchmark control in the first line setting (Escudier et al, 2009a, b, c). There is also a need for smaller biomarker studies, which take sequential tissue, plasma and functional imaging during therapy. These studies are aimed at gaining a better understanding of how each drug works. The true goal for these studies is to identify predictive biomarkers for specific agents, which requires randomisation against other agents. CONCLUSIONS Targeted therapy has changed the way metastatic renal cancer is treated and the OS for these patients is now greater than 2 years in prospective studies. Sequential therapy with VEGF-targeted therapy and mtor inhibitors are currently the standard of care. Data on specific combinations are eagerly awaited. Correlative markers associated with clinical benefit remain elusive and are urgently required to develop treatment for this disease. This will help us to move away from the current one size fits all approach and help develop truly individualised targeted therapy. REFERENCES Bergers G, Hanahan D (2008) Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 8: Bhargava P, Esteves B, Nosov DA, Lipatov ON, Lyulko AA, Anischenko AA, Chacko RT, Lee P, Al-Adhami M, Ryan J (2009) Updated activity and safety results of a phase II randomized discontinuation trial (RDT) of AV-951, a potent and selective VEGFR1, 2, and 3 kinase inhibitor, in patients with renal cell carcinoma (RCC). ASCO Ann Meet Proc 27 (Suppl): 15S (abstract 5032) Cenni E, Perut F, Zuntini M, Granchi D, Amato I, Avnet S, Brandi ML, Giunti A, Baldini N (2005) Inhibition of angiogenic activity of renal carcinoma by an antisense oligonucleotide targeting fibroblast growth factor-2. Anticancer Res 25(2A): Choueiri TK, Plantade A, Elson P, Negrier S, Ravaud A, Oudard S, Zhou M, Rini BI, Bukowski RM, Escudier B (2008) Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol 26: Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM, TARGET Study Group (2007a) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356: Escudier B, Negrier S, Gravis G, Chevreau C, Delva R, Bay J, Geoffrois L, Legouffe E (2010) Can the combination of temsirolimus and bevacizumab improve the treatment of metastatic renal cell carcinoma (mrcc)? Results of the randomized TORAVA phase II trial. J Clin Oncol 28(Suppl): 7s (abstract 4516) Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar B, Bajetta E, Gorbunova V, Bay JO, Bodrogi I, Jagiello-Gruszfeld A, Moore N, AVOREN Trial investigators (2007b) Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 370: Escudier B, Roigas J, Gillessen S, Harmenberg U, Srinivas S, Mulder SF, Fountzilas G, Peschel C, Flodgren P, Maneval EC, Chen I, Vogelzang NJ (2009a) Phase II study of sunitinib administered in a continuous once-daily dosing regimen in patients with cytokine-refractory metastatic renal cell carcinoma. J Clin Oncl 27: Escudier B, Szczylik C, Hutson TE, Demkow T, Staehler M, Rolland F, Negrier S, Laferriere N, Scheuring UJ, Cella D, Shah S, Bukowski RM (2009b) Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol 27: Escudier B, Szczylik C, Hutson TE, Demkow T, Staehler M, Rolland F, Negrier S, Laferriere N, Scheuring UJ, Cella D, Shah S, Bukowski RM (2009c) Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol 27: Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, Caton Jr JR, Munshi N, Crawford ED (2001) Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med 345:
5 Gore ME, Szczylik C, Porta C, Bracarda S, Bjarnason GA, Oudard S, Hariharan S, Lee SH, Haanen J, Castellano D, Vrdoljak E, Schöffski P, Mainwaring P, Nieto A, Yuan J, Bukowski R (2009) Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol 10: Gossage L, Eisen T (2010) Alterations in VHL as potential biomarkers in renal-cell carcinoma. Nat Rev Clin Oncol 7: Hahn OM, Yang C, Medved M, Karczmar G, Kistner E, Karrison T, Manchen E, Mitchell M, Ratain MJ, Stadler WM (2008) Dynamic contrast-enhanced magnetic resonance imaging pharmacodynamic biomarker study of sorafenib in metastatic renal carcinoma. J Clin Oncol 26: Hainsworth JD, Spigel DR, Burris III HA, Waterhouse D, Clark BL, Whorf R (2010) II Phase trial of bevacizumab and everolimus in patients with advanced renal cell carcinoma. J Clin Oncol 28: Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, Venner P, Knox JJ, Chi KN, Kollmannsberger C, McDermott DF, Oh WK, Atkins MB, Bukowski RM, Rini BI, Choueiri TK (2009) Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 27(34): Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ (2009) Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/ pharmacodynamic meta-analysis. Cancer Chemother Pharmacol 66: Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O Toole T, Lustgarten S, Moore L, Motzer RJ, Global ARCC Trial (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. NEnglJMed356: Hutson TE, Davis ID, Machiels JP, De Souza PL, Rottey S, Hong BF, Epstein RJ, Baker KL, McCann L, Crofts T, Pandite L, Figlin RA (2010) Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol 28: Kontovinis LF, Papazisis KT, Touplikioti P, Andreadis C, Mouratidou D, Kortsaris AH (2009) Sunitinib treatment for patients with clear-cell metastatic renal cell carcinoma: clinical outcomes and plasma angiogenesis markers. BMC Cancer 9: 82 Motzer RJ, Bukowski RM, Figlin RA, Hutson TE, Michaelson MD, Kim ST, Baum CM, Kattan MW. Prognostic nomogram for sunitinib in patients with metastatic renal cell carcinoma. Cancer (2008a); 113: Motzer RJ, Bukowski RM (2006) Targeted therapy for metastatic renal cell carcinoma. J Clin Oncol 24: Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA, Hollaender N, Urbanowitz G, Berg WJ, Kay A, Lebwohl D, Ravaud A, RECORD-1 Study Group. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet (2008b); 372: Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, Garcia-del-Muro X, Sosman JA, Solska E, Wilding G, Thompson JA, Kim ST, Chen I, Huang X, Figlin RA (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. JClinOncol 27: Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356: Na X, Wu G, Ryan CK, Schoen SR, di Santagnese PA, Messing EM (2003) Overproduction of vascular endothelial growth factor related to von Hippel-Lindau tumor suppressor gene mutations and hypoxia-inducible factor-1 alpha expression in renal cell carcinomas. J Urol 170(2 Pt 1): O Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N (2006) mtor inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66: Oliner J, Min H, Leal J, Yu D, Rao S, You E, Tang X, Kim H, Meyer S, Han SJ, Hawkins N, Rosenfeld R, Davy E, Graham K, Jacobsen F, Stevenson S, Ho J, Chen Q, Hartmann T, Michaels M, Kelley M, Li L, Sitney K, Martin F, Sun JR, Zhang N, Lu J, Estrada J, Kumar R, Coxon A, Kaufman S, Pretorius J, Scully S, Cattley R, Payton M, Coats S, Nguyen L, Desilva B, Ndifor A, Hayward I, Radinsky R, Boone T, Kendall R (2004) Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell 6(5): Rini BI, Atkins MB (2009) Resistance to targeted therapy in renal-cell carcinoma. Lancet Oncol 10(10): Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou SS, Archer L, Atkins JN, Picus J, Czaykowski P, Dutcher J, Small EJ (2008a) Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB J Clin Oncol 26: Rini BI, Michaelson MD, Rosenberg JE, Bukowski RM, Sosman JA, Stadler WM, Hutson TE, Margolin K, Harmon CS, DePrimo SE, Kim ST, Chen I, George DJ (2008b) Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol 26: Rini BI, Wilding G, Hudes G, Stadler WM, Kim S, Tarazi J, Rosbrook B, Trask PC, Wood L, Dutcher JP (2009) Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J Clin Oncol 27: Rixe O, Bukowski RM, Michaelson MD, Wilding G, Hudes GR, Bolte O, Motzer RJ, Bycott P, Liau KF, Freddo J, Trask PC, Kim S, Rini BI (2007) Axitinib treatment in patients with cytokine-refractory metastatic renalcell cancer: a phase II study. Lancet Oncol 8: Rixe O, Dutcher J, Motzer R, Wilding G, Stadler W, Garrett M, Pithavala Y, Kim S, Tarazi J, Rini B (2008) Association between diastolic blood pressure (DBP) G) X90 mm Hg and efficacy in patients with metastatic renal cell carcinoma receiving axitinib (AG ). Ann Oncol 19(suppl 8): viii189(abstr 587) PD 2009 Sablin MP, Negrier S, Ravaud A, Oudard S, Balleyguier C, Gautier J, Celier C, Medioni J, Escudier B (2009) Sequential sorafenib and sunitinib for renal cell carcinoma. J Urol 182: Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM (2006) Prolonged rapamycin treatment inhibits mtorc2 assembly and Akt/PKB. Mol Cell 22: Sternberg C, Szczylik E, Lee PV, Salman J, Mardiak ID, Davis L, Pandite M, Chen L, McCann L, Hawkins R (2009) A randomized, double-blind phase III study of pazopanib in treatment-naive and cytokine-pretreated patients with advanced renal cell carcinoma (RCC). J Clin Oncol 27(Suppl): 15s (abstract 5021) Valent P (2008) Emerging stem cell concepts for imatinib-resistant chronic myeloid leukaemia: implications for the biology, management, and therapy of the disease. Br J Haematol 142: van der Veldt AA, Meijerink MR, van den Eertwegh AJ, Haanen JB, Boven E (2010) Choi response criteria for early prediction of clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Br J Cancer 102:
Stage IV Renal Cell Carcinoma. Changing Management in A Comprehensive Community Cancer Center. Susquehanna Health Cancer Center
 Stage IV Renal Cell Carcinoma Changing Management in A Comprehensive Community Cancer Center Susquehanna Health Cancer Center 2000 2009 Warren L. Robinson, MD, FACP January 27, 2014 Introduction 65,150
Stage IV Renal Cell Carcinoma Changing Management in A Comprehensive Community Cancer Center Susquehanna Health Cancer Center 2000 2009 Warren L. Robinson, MD, FACP January 27, 2014 Introduction 65,150
Precision oncology: identifying predictive biomarkers for the treatment of metastatic renal cell carcinoma
 Perspective Precision oncology: identifying predictive biomarkers for the treatment of metastatic renal cell carcinoma Parth K. Modi 1, Nicholas J. Farber 1, Eric A. Singer 1,2 1 Division of Urology, Rutgers
Perspective Precision oncology: identifying predictive biomarkers for the treatment of metastatic renal cell carcinoma Parth K. Modi 1, Nicholas J. Farber 1, Eric A. Singer 1,2 1 Division of Urology, Rutgers
Background. t 1/2 of 3.7 4.7 days allows once-daily dosing (1.5 mg) with consistent serum concentration 2,3 No interaction with CYP3A4 inhibitors 4
 Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Abstract No. 4501 Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial R. Motzer, D.
Targeted Molecular Therapy for Renal Cell Carcinoma: Impact on Existing Treatment Paradigms
 Targeted Molecular Therapy for Renal Cell Carcinoma: Impact on Existing Treatment Paradigms Wolfram Samlowski, MD For a CME/CEU version of this article please go to www.namcp.org/cmeonline.htm, and then
Targeted Molecular Therapy for Renal Cell Carcinoma: Impact on Existing Treatment Paradigms Wolfram Samlowski, MD For a CME/CEU version of this article please go to www.namcp.org/cmeonline.htm, and then
BJUI. Optimal management of metastatic renal cell carcinoma: an algorithm for treatment
 2009 The Authors; Journal compilation 2009 BJU International Mini-review Article MANAGEMENT ALGORITHMS FOR METASTATIC RCC BELLMUNT et al. BJUI BJU INTERNATIONAL Optimal management of metastatic renal cell
2009 The Authors; Journal compilation 2009 BJU International Mini-review Article MANAGEMENT ALGORITHMS FOR METASTATIC RCC BELLMUNT et al. BJUI BJU INTERNATIONAL Optimal management of metastatic renal cell
Successes and Limitations of Targeted Therapies in Renal Cell Carcinoma
 g Tumor Res. Basel, Karger, 2014, vol 41, pp 98 112 (DOI: 10.1159/000355906) Successes and Limitations of Targeted Therapies in Renal Cell Carcinoma Marc Pracht Dominik Berthold Medical Oncology Unit,
g Tumor Res. Basel, Karger, 2014, vol 41, pp 98 112 (DOI: 10.1159/000355906) Successes and Limitations of Targeted Therapies in Renal Cell Carcinoma Marc Pracht Dominik Berthold Medical Oncology Unit,
Title: Making Optimal Therapeutic Decisions in Patients with Advanced Renal Cell Carcinoma
 Overall Aim and Objectives: Program Synopsis Title: Making Optimal Therapeutic Decisions in Patients with Advanced Renal Cell Carcinoma Clinical Synopsis: New options for targeted treatment of metastatic
Overall Aim and Objectives: Program Synopsis Title: Making Optimal Therapeutic Decisions in Patients with Advanced Renal Cell Carcinoma Clinical Synopsis: New options for targeted treatment of metastatic
Sorafenib. Bernard ESCUDIER Institut Gustave Roussy Villejuif, France
 Sorafenib for renal cell carcinoma Bernard ESCUDIER Institut Gustave Roussy Villejuif, France Renal Cell Carcinoma: Drugs and Targets pvhl = HIFα CCI-779 Bevacizumab VEGF KDR PDGF PDGFR TGFα EGFR Sunitinib,
Sorafenib for renal cell carcinoma Bernard ESCUDIER Institut Gustave Roussy Villejuif, France Renal Cell Carcinoma: Drugs and Targets pvhl = HIFα CCI-779 Bevacizumab VEGF KDR PDGF PDGFR TGFα EGFR Sunitinib,
54,390 estimated new cases of RCC 13,010 estimated deaths. Incidence is increasing 2.0% per year
 Advanced Renal Cell Carcinoma Individualizing Treatment Selection in the Era of Targeted Therapy Renal Cell Carcinoma In the United States in 28: 54,9 estimated new cases of RCC, estimated deaths Median
Advanced Renal Cell Carcinoma Individualizing Treatment Selection in the Era of Targeted Therapy Renal Cell Carcinoma In the United States in 28: 54,9 estimated new cases of RCC, estimated deaths Median
Tumori rari del rene: trattamento per stadio ed istologia Dr. Camillo Porta
 Tumori rari del rene: trattamento per stadio ed istologia Dr. Camillo Porta S.C. di Oncologia Medica, Fondazione I.R.C.C.S. Policlinico San Matteo, Pavia Non-Clear Cell Renal Cell Carcinoma (nccrcc) nccrcc
Tumori rari del rene: trattamento per stadio ed istologia Dr. Camillo Porta S.C. di Oncologia Medica, Fondazione I.R.C.C.S. Policlinico San Matteo, Pavia Non-Clear Cell Renal Cell Carcinoma (nccrcc) nccrcc
What is the Optimal Front-Line Treatment for mrcc? Michael B. Atkins, MD Deputy Director, Georgetown-Lombardi Comprehensive Cancer Center
 What is the Optimal Front-Line Treatment for mrcc? Michael B. Atkins, MD Deputy Director, Georgetown-Lombardi Comprehensive Cancer Center The Case for Immunotherapy in mrcc 1. Achieves patient s goal 2.
What is the Optimal Front-Line Treatment for mrcc? Michael B. Atkins, MD Deputy Director, Georgetown-Lombardi Comprehensive Cancer Center The Case for Immunotherapy in mrcc 1. Achieves patient s goal 2.
PROSPETTIVE FUTURE NEL TRATTAMENTO. Cinzia Ortega Dipartimento di Oncologia Medica Fondazione del Piemonte per l Oncologia I.R.C.C.S.
 PROSPETTIVE FUTURE NEL TRATTAMENTO MEDICO DEL mrcc Cinzia Ortega Dipartimento di Oncologia Medica Fondazione del Piemonte per l Oncologia I.R.C.C.S. Candiolo Future strategies in mrcc Improve therapeutic
PROSPETTIVE FUTURE NEL TRATTAMENTO MEDICO DEL mrcc Cinzia Ortega Dipartimento di Oncologia Medica Fondazione del Piemonte per l Oncologia I.R.C.C.S. Candiolo Future strategies in mrcc Improve therapeutic
Published Ahead of Print on January 9, 2012 as 10.1200/JCO.2011.37.2516. J Clin Oncol 30. 2012 by American Society of Clinical Oncology INTRODUCTION
 Published Ahead of Print on January 9, 2012 as 10.1200/JCO.2011.37.2516 The latest version is at http://jco.ascopubs.org/cgi/doi/10.1200/jco.2011.37.2516 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R
Published Ahead of Print on January 9, 2012 as 10.1200/JCO.2011.37.2516 The latest version is at http://jco.ascopubs.org/cgi/doi/10.1200/jco.2011.37.2516 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R
How To Understand The Effects Of A Drug On Your Health
 Farmacologia degli inibitori TK e mtor Romano Danesi Professore ordinario di Farmacologia UOC Farmacologia Universitaria Azienda Ospedaliero-Universitaria Pisana Dipartimento di Medicina Interna Università
Farmacologia degli inibitori TK e mtor Romano Danesi Professore ordinario di Farmacologia UOC Farmacologia Universitaria Azienda Ospedaliero-Universitaria Pisana Dipartimento di Medicina Interna Università
Immunotherapy for Metastatic Renal Cell Carcinoma
 Immunotherapy for Metastatic Renal Cell Carcinoma Timothy M. Kuzel, MD Professor of Medicine and Dermatology Feinberg School of Medicine Northwestern University Chicago, Ilinois ICLIO 1 st Annual National
Immunotherapy for Metastatic Renal Cell Carcinoma Timothy M. Kuzel, MD Professor of Medicine and Dermatology Feinberg School of Medicine Northwestern University Chicago, Ilinois ICLIO 1 st Annual National
Sequential Treatment Strategies and Combination Therapy Regimens in Metastatic Renal Cell Carcinoma
 Sequential Treatment Strategies and Combination Therapy Regimens in Metastatic Renal Cell Carcinoma Sumanta Kumar Pal, MD, and Nicholas J. Vogelzang, MD Dr. Pal is an Assistant Professor of Genitourinary
Sequential Treatment Strategies and Combination Therapy Regimens in Metastatic Renal Cell Carcinoma Sumanta Kumar Pal, MD, and Nicholas J. Vogelzang, MD Dr. Pal is an Assistant Professor of Genitourinary
Patient with metastatic renal cell carcinoma treated successfully with pazopanib for four years
 Teaching with case studies: interdisciplinary oncology Patient with metastatic renal cell carcinoma treated successfully with pazopanib for four years Agnieszka Słowik, MD 1, Joanna Streb, MD 1, Robert
Teaching with case studies: interdisciplinary oncology Patient with metastatic renal cell carcinoma treated successfully with pazopanib for four years Agnieszka Słowik, MD 1, Joanna Streb, MD 1, Robert
New therapeutic developments in renal cell cancer
 Critical Reviews in Oncology/Hematology 69 (2009) 56 63 New therapeutic developments in renal cell cancer Hans Prenen a,b,, Thierry Gil b, Ahmad Awada b a Department of General Medical Oncology, University
Critical Reviews in Oncology/Hematology 69 (2009) 56 63 New therapeutic developments in renal cell cancer Hans Prenen a,b,, Thierry Gil b, Ahmad Awada b a Department of General Medical Oncology, University
Renal cell carcinoma (RCC) represents 2% of all
 Predictors of Response to Targeted Therapy in Renal Cell Carcinoma Laurie J. Eisengart, MD; Gary R. MacVicar, MD; Ximing J. Yang, MD, PhD N Context. The prognosis for patients with metastatic renal cell
Predictors of Response to Targeted Therapy in Renal Cell Carcinoma Laurie J. Eisengart, MD; Gary R. MacVicar, MD; Ximing J. Yang, MD, PhD N Context. The prognosis for patients with metastatic renal cell
2. Background This was the fourth submission for everolimus requesting listing for clear cell renal carcinoma.
 PUBLIC SUMMARY DOCUMENT Product: Everolimus, tablets, 5 mg and 10 mg, Afinitor Sponsor: Novartis Pharmaceuticals Australia Pty Ltd Date of PBAC Consideration: November 2011 1. Purpose of Application To
PUBLIC SUMMARY DOCUMENT Product: Everolimus, tablets, 5 mg and 10 mg, Afinitor Sponsor: Novartis Pharmaceuticals Australia Pty Ltd Date of PBAC Consideration: November 2011 1. Purpose of Application To
Clinical Management Guideline Management of locally advanced or recurrent Renal cell carcinoma. Protocol for Planning and Treatment
 Protocol for Planning and Treatment The process to be followed in the management of: LOCALLY ADVANCED OR METASTATIC RENAL CELL CARCINOMA Patient information given at each stage following agreed information
Protocol for Planning and Treatment The process to be followed in the management of: LOCALLY ADVANCED OR METASTATIC RENAL CELL CARCINOMA Patient information given at each stage following agreed information
Come valutare la risposta o la progressione nei pazienti in trattamento con terapie biologiche
 Come valutare la risposta o la progressione nei pazienti in trattamento con terapie biologiche Andrea Busolo U.O. di Radiologia Rocco De Vivo U.O. di Oncologia Medica ULSS 6 - Vicenza Activity and efficacy
Come valutare la risposta o la progressione nei pazienti in trattamento con terapie biologiche Andrea Busolo U.O. di Radiologia Rocco De Vivo U.O. di Oncologia Medica ULSS 6 - Vicenza Activity and efficacy
Per gentile concessione del Dr. P. Zucali
 La seconda linea di trattamento: scenario attuale e prospettive future Paolo Andrea Zucali Dipartimento di Oncologia HUMANITAS CANCER CENTER Rozzano - Milano mrcc: second line SECOND-LINE SYSTEMIC THERAPY
La seconda linea di trattamento: scenario attuale e prospettive future Paolo Andrea Zucali Dipartimento di Oncologia HUMANITAS CANCER CENTER Rozzano - Milano mrcc: second line SECOND-LINE SYSTEMIC THERAPY
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES
 PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GENITOURINARY RENAL CELL CARCINOMA GU Site Group Renal Cell Carcinoma Date Guideline Created: June 2012 Author: Dr. Charles Catton 1. INTRODUCTION
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GENITOURINARY RENAL CELL CARCINOMA GU Site Group Renal Cell Carcinoma Date Guideline Created: June 2012 Author: Dr. Charles Catton 1. INTRODUCTION
Targeted Therapies in Renal Cell Carcinoma mammalian Target of Rapamycin (mtor) Inhibitors
 Targeted Therapies in Renal Cell Carcinoma mammalian Target of Rapamycin (mtor) Inhibitors Kristen Hehr, Pharm.D. PGY2 Oncology Pharmacy Resident South Texas Veterans Health Care System University of Texas
Targeted Therapies in Renal Cell Carcinoma mammalian Target of Rapamycin (mtor) Inhibitors Kristen Hehr, Pharm.D. PGY2 Oncology Pharmacy Resident South Texas Veterans Health Care System University of Texas
BNC105 PHASE II RENAL CANCER TRIAL RESULTS
 ABN 53 075 582 740 ASX ANNOUNCEMENT 19 March 2014 BNC105 PHASE II RENAL CANCER TRIAL RESULTS Results show BNC105 utility in patients with advanced disease Identified biomarkers which correlate with patient
ABN 53 075 582 740 ASX ANNOUNCEMENT 19 March 2014 BNC105 PHASE II RENAL CANCER TRIAL RESULTS Results show BNC105 utility in patients with advanced disease Identified biomarkers which correlate with patient
Actual role of multitargeted therapy in renal cell carcinoma (RCC) Review Article
 Cancer Therapy Vol 6, page 445 Cancer Therapy Vol 6, 445-456, 2008 Actual role of multitargeted therapy in renal cell carcinoma (RCC) Review Article Alessandro Sciarra*, Giovanni B. Di Pierro, Andrea Alfarone,
Cancer Therapy Vol 6, page 445 Cancer Therapy Vol 6, 445-456, 2008 Actual role of multitargeted therapy in renal cell carcinoma (RCC) Review Article Alessandro Sciarra*, Giovanni B. Di Pierro, Andrea Alfarone,
Metastatic renal cell carcinoma to the left maxillary sinus
 Case Report Metastatic renal cell carcinoma to the left maxillary sinus Y.-F. He 1, J. Chen 1, W.-Q. Xu 2, C.-S. Ji 1, J.-P. Du 1, H.-Q. Luo 1 and B. Hu 1 1 Department of Medical Oncology, The Provincial
Case Report Metastatic renal cell carcinoma to the left maxillary sinus Y.-F. He 1, J. Chen 1, W.-Q. Xu 2, C.-S. Ji 1, J.-P. Du 1, H.-Q. Luo 1 and B. Hu 1 1 Department of Medical Oncology, The Provincial
Renal Cell Carcinoma (Event Driven)
 Brochure More information from http://www.researchandmarkets.com/reports/2365661/ Renal Cell Carcinoma (Event Driven) Description: The Renal Cell Carcinoma market is highly lucrative. We anticipate that
Brochure More information from http://www.researchandmarkets.com/reports/2365661/ Renal Cell Carcinoma (Event Driven) Description: The Renal Cell Carcinoma market is highly lucrative. We anticipate that
Maintenance therapy in in Metastatic NSCLC. Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai
 Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
Maintenance therapy in in Metastatic NSCLC Dr Amit Joshi Associate Professor Dept. Of Medical Oncology Tata Memorial Centre Mumbai Definition of Maintenance therapy The U.S. National Cancer Institute s
National Horizon Scanning Centre. Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer. December 2007
 Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
Vandetanib (Zactima) for advanced or metastatic non-small cell lung cancer December 2007 This technology summary is based on information available at the time of research and a limited literature search.
Confirming the Role of mtors in Oncology: Treatment of Renal Cell Carcinoma
 Confirming the Role of mtors in Oncology: Treatment of Renal Cell Carcinoma Program Director Jose Baselga, MD Chief, Medical Oncology Service Vall d Hebron University Hospital Barcelona, Spain Faculty
Confirming the Role of mtors in Oncology: Treatment of Renal Cell Carcinoma Program Director Jose Baselga, MD Chief, Medical Oncology Service Vall d Hebron University Hospital Barcelona, Spain Faculty
Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer
 LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Everolimus plus exemestane for second-line endocrine treatment of oestrogen receptor positive metastatic breast cancer Everolimus plus exemestane for second-line
Horizon Scanning in Oncology
 Horizon Scanning in Oncology Pazopanib (Votrient ) for the treatment of locally advanced and/or metastatic renal cell carcinoma DSD: Horizon Scanning in Oncology Nr. 013 ISSN online 2076-5940 Horizon
Horizon Scanning in Oncology Pazopanib (Votrient ) for the treatment of locally advanced and/or metastatic renal cell carcinoma DSD: Horizon Scanning in Oncology Nr. 013 ISSN online 2076-5940 Horizon
Carcinoma papilar renal, cromófobo y otras histologías. Maria José Méndez Vidal Servicio de oncología Medica Hospital Reina Sofía Córdoba
 Carcinoma papilar renal, cromófobo y otras histologías. Maria José Méndez Vidal Servicio de oncología Medica Hospital Reina Sofía Córdoba Europe 121 629 new cases RCC 2012, 75 676 affected men Slide 3
Carcinoma papilar renal, cromófobo y otras histologías. Maria José Méndez Vidal Servicio de oncología Medica Hospital Reina Sofía Córdoba Europe 121 629 new cases RCC 2012, 75 676 affected men Slide 3
Molecular markers and clinical trial design parallels between oncology and rare diseases?
 Molecular markers and clinical trial design parallels between oncology and rare diseases?, Harriet Sommer Institute for Medical Biometry and Statistics, University of Freiburg Medical Center 6. Forum Patientennahe
Molecular markers and clinical trial design parallels between oncology and rare diseases?, Harriet Sommer Institute for Medical Biometry and Statistics, University of Freiburg Medical Center 6. Forum Patientennahe
Targeted Therapy of Kidney Cancer: Keeping the Art Around the Algorithms
 To use targeted therapies optimally for treatment of metastatic kidney cancer, clinical issues besides those addressed in pivotal treatment trials should be recognized. Michele R. Sassi. Gondolas, Venice,
To use targeted therapies optimally for treatment of metastatic kidney cancer, clinical issues besides those addressed in pivotal treatment trials should be recognized. Michele R. Sassi. Gondolas, Venice,
Drug/Drug Combination: Bevacizumab in combination with chemotherapy
 AHFS Final Determination of Medical Acceptance: Off-label Use of Bevacizumab in Combination with Chemotherapy for the Treatment of Metastatic Breast Cancer Previously Treated with Cytotoxic Chemotherapy
AHFS Final Determination of Medical Acceptance: Off-label Use of Bevacizumab in Combination with Chemotherapy for the Treatment of Metastatic Breast Cancer Previously Treated with Cytotoxic Chemotherapy
The New Kid on the Block for Advanced Renal Cell Carcinoma
 The New Kid on the Block for Advanced Renal Cell Carcinoma Wyeth Pharmaceuticals recently launched Torisel (temsirolimus), a targeted, first-in-class mtor inhibitor. This new treatment for metastatic renal
The New Kid on the Block for Advanced Renal Cell Carcinoma Wyeth Pharmaceuticals recently launched Torisel (temsirolimus), a targeted, first-in-class mtor inhibitor. This new treatment for metastatic renal
Ovarian Cancer and Modern Immunotherapy: Regulatory Strategies for Drug Development
 Ovarian Cancer and Modern Immunotherapy: Regulatory Strategies for Drug Development Sanjeeve Bala, MD, MPH Ovarian Cancer Endpoints Workshop FDA White Oak September 3, 2015 Overview Immune agents from
Ovarian Cancer and Modern Immunotherapy: Regulatory Strategies for Drug Development Sanjeeve Bala, MD, MPH Ovarian Cancer Endpoints Workshop FDA White Oak September 3, 2015 Overview Immune agents from
NCCN Task Force Report: Optimizing Treatment of Advanced Renal Cell Carcinoma With Molecular Targeted Therapy
 S-1 NCCN Task Force Report: Optimizing Treatment of Advanced Renal Cell Carcinoma With Molecular Targeted Therapy Gary R. Hudes, MD; Michael A. Carducci, MD; Toni K. Choueiri, MD; Peg Esper, MSN, MSA,
S-1 NCCN Task Force Report: Optimizing Treatment of Advanced Renal Cell Carcinoma With Molecular Targeted Therapy Gary R. Hudes, MD; Michael A. Carducci, MD; Toni K. Choueiri, MD; Peg Esper, MSN, MSA,
GUIDELINES ADJUVANT SYSTEMIC BREAST CANCER
 GUIDELINES ADJUVANT SYSTEMIC BREAST CANCER Author: Dr Susan O Reilly On behalf of the Breast CNG Written: December 2008 Agreed at CNG: June 2009 & June 2010 Review due: June 2011 Guidelines Adjuvant Systemic
GUIDELINES ADJUVANT SYSTEMIC BREAST CANCER Author: Dr Susan O Reilly On behalf of the Breast CNG Written: December 2008 Agreed at CNG: June 2009 & June 2010 Review due: June 2011 Guidelines Adjuvant Systemic
Kidney Cancer Research: Developing a New Vision for the Future
 Kidney Cancer Research: Developing a New Vision for the Future A symposium for young investigators, hosted by the Kidney Cancer Association, September 7, 2011 Introduction Since the founding of the Kidney
Kidney Cancer Research: Developing a New Vision for the Future A symposium for young investigators, hosted by the Kidney Cancer Association, September 7, 2011 Introduction Since the founding of the Kidney
Histopathology and prognosis in renal cancer
 Histopathology and prognosis in renal cancer Granular cell Granular cell Granular cell Granular cell Clear cell Chromophobe cell Papillary type 2 Luca Mazzucchelli Istituto cantonale di patologia, Locarno
Histopathology and prognosis in renal cancer Granular cell Granular cell Granular cell Granular cell Clear cell Chromophobe cell Papillary type 2 Luca Mazzucchelli Istituto cantonale di patologia, Locarno
Pharmacogenomic markers in EGFR-targeted therapy of lung cancer
 Pharmacogenomic markers in EGFR-targeted therapy of lung cancer Rafal Dziadziuszko, MD, PhD University of Colorado Cancer Center, Aurora, CO, USA Medical University of Gdansk, Poland EMEA Workshop on Biomarkers,
Pharmacogenomic markers in EGFR-targeted therapy of lung cancer Rafal Dziadziuszko, MD, PhD University of Colorado Cancer Center, Aurora, CO, USA Medical University of Gdansk, Poland EMEA Workshop on Biomarkers,
Targeted Therapy What the Surgeon Needs to Know
 Targeted Therapy What the Surgeon Needs to Know AATS Focus in Thoracic Surgery 2014 David R. Jones, M.D. Professor & Chief, Thoracic Surgery Memorial Sloan Kettering Cancer Center I have no disclosures
Targeted Therapy What the Surgeon Needs to Know AATS Focus in Thoracic Surgery 2014 David R. Jones, M.D. Professor & Chief, Thoracic Surgery Memorial Sloan Kettering Cancer Center I have no disclosures
Avastin in Metastatic Breast Cancer
 Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
Non-interventional study Avastin in Metastatic Breast Cancer ML 21165 / 2007 Clinical Study Report Synopsis ROCHE ML21165 / WiSP Project RH09 / V. 1.0 / 24.06.2013 ROCHE ML21165-2 - Name of Sponsor Roche
Renal Cell Carcinoma: Prognostic Factors and Patient Selection
 european urology supplements 6 (2007) 477 483 available at www.sciencedirect.com journal homepage: www.europeanurology.com Renal Cell Carcinoma: Prognostic Factors and Patient Selection Arie S. Belldegrun
european urology supplements 6 (2007) 477 483 available at www.sciencedirect.com journal homepage: www.europeanurology.com Renal Cell Carcinoma: Prognostic Factors and Patient Selection Arie S. Belldegrun
Management of low grade glioma s: update on recent trials
 Management of low grade glioma s: update on recent trials M.J. van den Bent The Brain Tumor Center at Erasmus MC Cancer Center Rotterdam, the Netherlands Low grades Female, born 1976 1 st seizure 2005,
Management of low grade glioma s: update on recent trials M.J. van den Bent The Brain Tumor Center at Erasmus MC Cancer Center Rotterdam, the Netherlands Low grades Female, born 1976 1 st seizure 2005,
Avastin in breast cancer: Summary of clinical data
 Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
SEOM clinical guidelines for the treatment of renal cell carcinoma
 SEOM clinical guidelines for the treatment of renal cell carcinoma The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation
SEOM clinical guidelines for the treatment of renal cell carcinoma The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation
Lung cancer is not just one disease. There are two main types of lung cancer:
 1. What is lung cancer? 2. How common is lung cancer? 3. What are the risk factors for lung cancer? 4. What are the signs and symptoms of lung cancer? 5. How is lung cancer diagnosed? 6. What are the available
1. What is lung cancer? 2. How common is lung cancer? 3. What are the risk factors for lung cancer? 4. What are the signs and symptoms of lung cancer? 5. How is lung cancer diagnosed? 6. What are the available
Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
 Efficacy Results from the ToGA Trial: A Phase III Study of Trastuzumab Added to Standard Chemotherapy in First-Line HER2- Positive Advanced Gastric Cancer Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
Efficacy Results from the ToGA Trial: A Phase III Study of Trastuzumab Added to Standard Chemotherapy in First-Line HER2- Positive Advanced Gastric Cancer Van Cutsem E et al. Proc ASCO 2009;Abstract LBA4509.
What is New in Oncology. Michael J Messino, MD Cancer Care of WNC An affiliate of Mission hospitals
 What is New in Oncology Michael J Messino, MD Cancer Care of WNC An affiliate of Mission hospitals Personalized Medicine Personalized Genomics Genomic Medicine Precision Medicine Definition Application
What is New in Oncology Michael J Messino, MD Cancer Care of WNC An affiliate of Mission hospitals Personalized Medicine Personalized Genomics Genomic Medicine Precision Medicine Definition Application
Clinical Trials in non-clear cell RCC: ESPN, ASPEN and beyond
 Clinical Trials in non-clear cell RCC: ESPN, ASPEN and beyond Toni K. Choueiri, MD Clinical Director and Kidney Cancer Center Director The Lank Center for Genitourinary Oncology Dana-Farber Cancer Institute
Clinical Trials in non-clear cell RCC: ESPN, ASPEN and beyond Toni K. Choueiri, MD Clinical Director and Kidney Cancer Center Director The Lank Center for Genitourinary Oncology Dana-Farber Cancer Institute
Avastin in breast cancer: Summary of clinical data
 Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Avastin in breast cancer: Summary of clinical data Worldwide, over one million people are diagnosed with breast cancer every year 1. It is the most frequently diagnosed cancer in women 1,2, and the leading
Kidney cancer, predominantly renal cell carcinoma
 CONSENSUS GUIDELINE Management of kidney cancer: Canadian Kidney Cancer Forum Consensus Update Canadian Kidney Cancer Forum 2009 Can Urol Assoc J 2009;3(3):200-4 Kidney cancer, predominantly renal cell
CONSENSUS GUIDELINE Management of kidney cancer: Canadian Kidney Cancer Forum Consensus Update Canadian Kidney Cancer Forum 2009 Can Urol Assoc J 2009;3(3):200-4 Kidney cancer, predominantly renal cell
mrcc: Tratamiento HOY de segunda línea Pablo Maroto Htal Sant Pau
 mrcc: Tratamiento HOY de segunda línea Pablo Maroto Htal Sant Pau El CCRm precisa de una estrategia de tratamiento Los agentes anti-diana constituyen el tratamiento estandar del CCRm. Pero.. Cómo combinarlos?
mrcc: Tratamiento HOY de segunda línea Pablo Maroto Htal Sant Pau El CCRm precisa de una estrategia de tratamiento Los agentes anti-diana constituyen el tratamiento estandar del CCRm. Pero.. Cómo combinarlos?
Clinical Trial Results Database Page 1
 Clinical Trial Results Database Page Sponsor Novartis Generic Drug Name BGT6 Therapeutic Area of Trial Advanced solid malignancies Approved Indication Investigational Study Number CBGT6A0 Title A phase
Clinical Trial Results Database Page Sponsor Novartis Generic Drug Name BGT6 Therapeutic Area of Trial Advanced solid malignancies Approved Indication Investigational Study Number CBGT6A0 Title A phase
GENETIC PROFILES AND TARGETED TREATMENT OF CANCER - PERSONALIZED MEDICINE
 GENETIC PROFILES AND TARGETED TREATMENT OF CANCER - PERSONALIZED MEDICINE Branko Zakotnik MD, PhD Department of Medical Oncology Institute of Oncology Ljubljana 1 I have no conflict of interest to declare
GENETIC PROFILES AND TARGETED TREATMENT OF CANCER - PERSONALIZED MEDICINE Branko Zakotnik MD, PhD Department of Medical Oncology Institute of Oncology Ljubljana 1 I have no conflict of interest to declare
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases
 Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
Role of taxanes in the treatment of advanced NHL patients: A randomized study of 87 cases R. Shraddha, P.N. Pandit Radium Institute, Patna Medical College and Hospital, Patna, India Abstract NHL is a highly
Come è cambiata la storia naturale della malattia
 Malattia Metastatica del Carcinoma del Grosso Intestino Tecniche e terapie Innovative Come è cambiata la storia naturale della malattia Antonio Frassoldati Oncologia Clinica - Ferrara 29 ottobre 2011 Colorectal
Malattia Metastatica del Carcinoma del Grosso Intestino Tecniche e terapie Innovative Come è cambiata la storia naturale della malattia Antonio Frassoldati Oncologia Clinica - Ferrara 29 ottobre 2011 Colorectal
Miquel Àngel Seguí Palmer
 Miquel Àngel Seguí Palmer HER2+ Breast Cancer is characterized by overexpression of HER2 receptors HER2+ Breast Cancer is characterized by overexpression of HER2 receptors HER2+ status is associated with
Miquel Àngel Seguí Palmer HER2+ Breast Cancer is characterized by overexpression of HER2 receptors HER2+ Breast Cancer is characterized by overexpression of HER2 receptors HER2+ status is associated with
KIDNEY CANCER PATHOLOGY IN THE NEW CONTEXT OF TARGETED THERAPY. Key words: Renal cell carcinoma ; targeted therapy; VHL; HIF; VEGF; mtor
 KIDNEY CANCER PATHOLOGY IN THE NEW CONTEXT OF TARGETED THERAPY Yves Allory 1,2, Stéphane Culine 1,3, Alexandre de la Taille 1,4 1, INSERM, U955, Team 7 Translational research in genito-urinary oncogenesis,
KIDNEY CANCER PATHOLOGY IN THE NEW CONTEXT OF TARGETED THERAPY Yves Allory 1,2, Stéphane Culine 1,3, Alexandre de la Taille 1,4 1, INSERM, U955, Team 7 Translational research in genito-urinary oncogenesis,
Mechanism Of Action of Palbociclib & PFS Benefit
 A Phase II Randomized Controlled Trial of Palbociclib & Tamoxifen/Fulvestrant in Postmenopausal Women and Men With Hormone-Receptor Positive, HER2- Negative Metastatic Breast Cancer (MBC) Protocol Chair:
A Phase II Randomized Controlled Trial of Palbociclib & Tamoxifen/Fulvestrant in Postmenopausal Women and Men With Hormone-Receptor Positive, HER2- Negative Metastatic Breast Cancer (MBC) Protocol Chair:
RENAL CELL CARCINOMA
 RENAL CELL CARCINOMA Effective Date: June 2013 The recommendations contained in this guideline are a consensus of the Alberta Provincial Genitourinary Tumour Team and are a synthesis of currently accepted
RENAL CELL CARCINOMA Effective Date: June 2013 The recommendations contained in this guideline are a consensus of the Alberta Provincial Genitourinary Tumour Team and are a synthesis of currently accepted
Avastin (Renal Cell Carcinoma) - Analysis and Forecasts to 2022
 Brochure More information from http://www.researchandmarkets.com/reports/2228475/ Avastin (Renal Cell Carcinoma) - Analysis and Forecasts to 2022 Description: Avastin (Renal Cell Carcinoma) Analysis and
Brochure More information from http://www.researchandmarkets.com/reports/2228475/ Avastin (Renal Cell Carcinoma) - Analysis and Forecasts to 2022 Description: Avastin (Renal Cell Carcinoma) Analysis and
Guidelines for Management of Renal Cancer
 Guidelines for Management of Renal Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Versions 2 and 3 Section 5 updated bullets 5.3 and 5.4 Section 6 updated
Guidelines for Management of Renal Cancer Date Approved by Network Governance July 2012 Date for Review July 2015 Changes Between Versions 2 and 3 Section 5 updated bullets 5.3 and 5.4 Section 6 updated
BNC105 CANCER CLINICAL TRIALS REACH KEY MILESTONES CLINICAL PROGRAM TO BE EXPANDED
 ASX ANNOUNCEMENT 3 August 2011 ABN 53 075 582 740 BNC105 CANCER CLINICAL TRIALS REACH KEY MILESTONES CLINICAL PROGRAM TO BE EXPANDED Data from renal cancer trial supports progression of the trial: o Combination
ASX ANNOUNCEMENT 3 August 2011 ABN 53 075 582 740 BNC105 CANCER CLINICAL TRIALS REACH KEY MILESTONES CLINICAL PROGRAM TO BE EXPANDED Data from renal cancer trial supports progression of the trial: o Combination
A disease of populations of cells that live, divide, invade and spread without regard to normal limits
 1 Targeted Cancer Therapies Mark McKeage Medical Oncology Specialist Professor in Clinical Pharmacology 2 Cancer Definition- A disease of populations of cells that live, divide, invade and spread without
1 Targeted Cancer Therapies Mark McKeage Medical Oncology Specialist Professor in Clinical Pharmacology 2 Cancer Definition- A disease of populations of cells that live, divide, invade and spread without
Biomarker Trends in Breast Cancer Research
 WHITE PAPER Biomarker Trends in Breast Cancer Research Jason Hill, PhD, Associate Director, External Science Affairs, Quintiles Quintiles examines the novel drug combinations and mechanisms of action that
WHITE PAPER Biomarker Trends in Breast Cancer Research Jason Hill, PhD, Associate Director, External Science Affairs, Quintiles Quintiles examines the novel drug combinations and mechanisms of action that
Translocation Renal Cell Carcinomas
 Translocation Renal Cell Carcinomas Cora N. Sternberg, MD, FACP Chair, Department of Medical Oncology San Camillo and Forlanini Hospitals Rome, Italy Kidney cancer is not a single disease Clear cell (75%)
Translocation Renal Cell Carcinomas Cora N. Sternberg, MD, FACP Chair, Department of Medical Oncology San Camillo and Forlanini Hospitals Rome, Italy Kidney cancer is not a single disease Clear cell (75%)
NCCN Non-Small Cell Lung Cancer V.1.2011 Update Meeting 07/09/10
 Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
Guideline Page and Request NSCL-3 Stage IA, margins positive delete the recommendation for chemoradiation. Stage IB, IIA, margins positive delete the recommendation for chemoradiation + Stage IIA, Stage
Prognostic factors in patients receiving third-line targeted therapy for metastatic renal cell carcinoma.
 Prognostic factors in patients receiving third-line targeted therapy for metastatic renal cell carcinoma. Iacovelli R 1, Farcomeni A 2, Sternberg CN 3, Carteni G 4, Milella M 5, Santoni M 6, Cerbone L
Prognostic factors in patients receiving third-line targeted therapy for metastatic renal cell carcinoma. Iacovelli R 1, Farcomeni A 2, Sternberg CN 3, Carteni G 4, Milella M 5, Santoni M 6, Cerbone L
Phase 3 Trial of Everolimus for Metastatic Renal Cell Carcinoma
 Original Article Phase 3 Trial of Everolimus for Metastatic Renal Cell Carcinoma Final Results and Analysis of Prognostic Factors Robert J. Motzer, MD 1 ; Bernard Escudier, MD 2 ; Stephane Oudard, MD,
Original Article Phase 3 Trial of Everolimus for Metastatic Renal Cell Carcinoma Final Results and Analysis of Prognostic Factors Robert J. Motzer, MD 1 ; Bernard Escudier, MD 2 ; Stephane Oudard, MD,
Management and systemic treatment of clear cell metastatic renal cell carcinoma: BSMO expert panel recommendations
 Management and systemic treatment of clear cell metastatic renal cell carcinoma: BSMO expert panel recommendations Z. El Ali, MD, PhD, D. Van Brummelen, MD 2, P. Wolter, MD 3, S. Rottey, MD, PhD 4, S.
Management and systemic treatment of clear cell metastatic renal cell carcinoma: BSMO expert panel recommendations Z. El Ali, MD, PhD, D. Van Brummelen, MD 2, P. Wolter, MD 3, S. Rottey, MD, PhD 4, S.
Anti-PD1 Agents: Immunotherapy agents in the treatment of metastatic melanoma. Claire Vines, 2016 Pharm.D. Candidate
 + Anti-PD1 Agents: Immunotherapy agents in the treatment of metastatic melanoma Claire Vines, 2016 Pharm.D. Candidate + Disclosure I have no conflicts of interest to disclose. + Objectives Summarize NCCN
+ Anti-PD1 Agents: Immunotherapy agents in the treatment of metastatic melanoma Claire Vines, 2016 Pharm.D. Candidate + Disclosure I have no conflicts of interest to disclose. + Objectives Summarize NCCN
OI PARP ΑΝΑΣΤΟΛΕΙΣ ΣΤΟΝ ΚΑΡΚΙΝΟ ΤΟΥ ΜΑΣΤΟΥ ΝΙΚΟΛΑΙΔΗ ΑΔΑΜΑΝΤΙΑ ΠΑΘΟΛΟΓΟΣ-ΟΓΚΟΛΟΓΟΣ Β ΟΓΚΟΛΟΓΙΚΗ ΚΛΙΝΙΚΗ ΝΟΣ. ΜΗΤΕΡΑ
 OI PARP ΑΝΑΣΤΟΛΕΙΣ ΣΤΟΝ ΚΑΡΚΙΝΟ ΤΟΥ ΜΑΣΤΟΥ ΝΙΚΟΛΑΙΔΗ ΑΔΑΜΑΝΤΙΑ ΠΑΘΟΛΟΓΟΣ-ΟΓΚΟΛΟΓΟΣ Β ΟΓΚΟΛΟΓΙΚΗ ΚΛΙΝΙΚΗ ΝΟΣ. ΜΗΤΕΡΑ Study Overview Inhibition of poly(adenosine diphosphate [ADP]-ribose) polymerase
OI PARP ΑΝΑΣΤΟΛΕΙΣ ΣΤΟΝ ΚΑΡΚΙΝΟ ΤΟΥ ΜΑΣΤΟΥ ΝΙΚΟΛΑΙΔΗ ΑΔΑΜΑΝΤΙΑ ΠΑΘΟΛΟΓΟΣ-ΟΓΚΟΛΟΓΟΣ Β ΟΓΚΟΛΟΓΙΚΗ ΚΛΙΝΙΚΗ ΝΟΣ. ΜΗΤΕΡΑ Study Overview Inhibition of poly(adenosine diphosphate [ADP]-ribose) polymerase
journal of medicine The new england Sunitinib versus Interferon Alfa in Metastatic Renal-Cell Carcinoma Abstract
 The new england journal of medicine established in 1812 january 11, 2007 vol. 356 no. 2 versus Interferon Alfa in Metastatic Renal-Cell Carcinoma Robert J. Motzer, M.D., Thomas E. Hutson, D.O., Pharm.D.,
The new england journal of medicine established in 1812 january 11, 2007 vol. 356 no. 2 versus Interferon Alfa in Metastatic Renal-Cell Carcinoma Robert J. Motzer, M.D., Thomas E. Hutson, D.O., Pharm.D.,
Predictive Biomarkers for Tumor Immunotherapy: Are we ready for clinical implementation? Howard L. Kaufman Rush University
 Predictive Biomarkers for Tumor Immunotherapy: Are we ready for clinical implementation? Howard L. Kaufman Rush University Changing Paradigms in Cancer Treatment Potential Uses of Biomarkers Adverse event
Predictive Biomarkers for Tumor Immunotherapy: Are we ready for clinical implementation? Howard L. Kaufman Rush University Changing Paradigms in Cancer Treatment Potential Uses of Biomarkers Adverse event
ESCMID Online Lecture Library. by author
 Do statins improve outcomes of patients with sepsis and pneumonia? Jordi Carratalà Department of Infectious Diseases Statins for sepsis & community-acquired pneumonia Sepsis and CAP are major healthcare
Do statins improve outcomes of patients with sepsis and pneumonia? Jordi Carratalà Department of Infectious Diseases Statins for sepsis & community-acquired pneumonia Sepsis and CAP are major healthcare
Clinical Trial Design. Sponsored by Center for Cancer Research National Cancer Institute
 Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
Clinical Trial Design Sponsored by Center for Cancer Research National Cancer Institute Overview Clinical research is research conducted on human beings (or on material of human origin such as tissues,
Cáncer Renal avanzado. Nuevas estrategias para el tratamiento individualizado.
 Cáncer Renal avanzado. Nuevas estrategias para el tratamiento individualizado. Daniel Castellano Oncología Médica.Unidad de Tumores GenitoUrinarios Hospital Universitario 12 de Octubre. I + 12 Research
Cáncer Renal avanzado. Nuevas estrategias para el tratamiento individualizado. Daniel Castellano Oncología Médica.Unidad de Tumores GenitoUrinarios Hospital Universitario 12 de Octubre. I + 12 Research
Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer
 Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer Review Article [1] December 01, 2003 By George W. Sledge, Jr, MD [2] Gemcitabine (Gemzar) and paclitaxel show good activity as single
Gemcitabine, Paclitaxel, and Trastuzumab in Metastatic Breast Cancer Review Article [1] December 01, 2003 By George W. Sledge, Jr, MD [2] Gemcitabine (Gemzar) and paclitaxel show good activity as single
Clinical Trial Designs for Firstline Hormonal Treatment of Metastatic Breast Cancer
 Clinical Trial Designs for Firstline Hormonal Treatment of Metastatic Breast Cancer Susan Honig, M.D. Patricia Cortazar, M.D. Rajeshwari Sridhara, Ph.D. Acknowledgements John Johnson Alison Martin Grant
Clinical Trial Designs for Firstline Hormonal Treatment of Metastatic Breast Cancer Susan Honig, M.D. Patricia Cortazar, M.D. Rajeshwari Sridhara, Ph.D. Acknowledgements John Johnson Alison Martin Grant
Victims Compensation Claim Status of All Pending Claims and Claims Decided Within the Last Three Years
 Claim#:021914-174 Initials: J.T. Last4SSN: 6996 DOB: 5/3/1970 Crime Date: 4/30/2013 Status: Claim is currently under review. Decision expected within 7 days Claim#:041715-334 Initials: M.S. Last4SSN: 2957
Claim#:021914-174 Initials: J.T. Last4SSN: 6996 DOB: 5/3/1970 Crime Date: 4/30/2013 Status: Claim is currently under review. Decision expected within 7 days Claim#:041715-334 Initials: M.S. Last4SSN: 2957
KIDNEY FUNCTION RELATION TO SIZE OF THE TUMOR IN RENAL CELL CANCINOMA
 KIDNEY FUNCTION RELATION TO SIZE OF THE TUMOR IN RENAL CELL CANCINOMA O.E. Stakhvoskyi, E.O. Stakhovsky, Y.V. Vitruk, O.A. Voylenko, P.S. Vukalovich, V.A. Kotov, O.M. Gavriluk National Canсer Institute,
KIDNEY FUNCTION RELATION TO SIZE OF THE TUMOR IN RENAL CELL CANCINOMA O.E. Stakhvoskyi, E.O. Stakhovsky, Y.V. Vitruk, O.A. Voylenko, P.S. Vukalovich, V.A. Kotov, O.M. Gavriluk National Canсer Institute,
Acknowledgements. PAH in Children: Natural History. The Sildenafil Saga
 The Sildenafil Saga David L. Wessel, MD Executive Vice President Chief Medical Officer Ikaria Distinguished Professor of Critical Care Children s National Medical Center Washington, DC, USA Acknowledgements
The Sildenafil Saga David L. Wessel, MD Executive Vice President Chief Medical Officer Ikaria Distinguished Professor of Critical Care Children s National Medical Center Washington, DC, USA Acknowledgements
Cancer Treatments Subcommittee of PTAC Meeting held 2 March 2012. (minutes for web publishing)
 Cancer Treatments Subcommittee of PTAC Meeting held 2 March 2012 (minutes for web publishing) Cancer Treatments Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Cancer Treatments Subcommittee of PTAC Meeting held 2 March 2012 (minutes for web publishing) Cancer Treatments Subcommittee minutes are published in accordance with the Terms of Reference for the Pharmacology
Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study
 Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
Turkish Journal of Cancer Volume 34, No.1, 2004 19 Is the third-line chemotherapy feasible for non-small cell lung cancer? A retrospective study MUSTAFA ÖZDO AN, MUSTAFA SAMUR, HAKAN BOZCUK, ERKAN ÇOBAN,
If several different trials are mentioned in one publication, the data of each should be extracted in a separate data extraction form.
 General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
General Remarks This template of a data extraction form is intended to help you to start developing your own data extraction form, it certainly has to be adapted to your specific question. Delete unnecessary
NOUVEAUTES THERAPEUTIQUES DANS LES TUMEURS NEUROENDOCRINES DIGESTIVES (Radiothérapie vectorisée et loco-régionale exclue) Philippe RUSZNIEWSKI
 NOUVEAUTES THERAPEUTIQUES DANS LES TUMEURS NEUROENDOCRINES DIGESTIVES (Radiothérapie vectorisée et loco-régionale exclue) Réunion APRAMEN, Paris, 2 février 2013 Philippe RUSZNIEWSKI Pôle des Maladies de
NOUVEAUTES THERAPEUTIQUES DANS LES TUMEURS NEUROENDOCRINES DIGESTIVES (Radiothérapie vectorisée et loco-régionale exclue) Réunion APRAMEN, Paris, 2 février 2013 Philippe RUSZNIEWSKI Pôle des Maladies de
Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment.
 1 Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment. John T. Carpenter, M.D. University of Alabama at Birmingham NP 2508 1720 Second Avenue South Birmingham, AL 35294-3300
1 Low dose capecitabine is effective and relatively nontoxic in breast cancer treatment. John T. Carpenter, M.D. University of Alabama at Birmingham NP 2508 1720 Second Avenue South Birmingham, AL 35294-3300
Preliminary Results from a Phase 2 Study of ARQ 197 in Patients with Microphthalmia Transcription Factor Family (MiT) Associated Tumors
 Preliminary Results from a Phase 2 Study of ARQ 197 in Patients with Microphthalmia Transcription Factor Family (MiT) Associated Tumors John Goldberg 1 *, George Demetri 2, Edwin Choy 3, Lee Rosen 4, Alberto
Preliminary Results from a Phase 2 Study of ARQ 197 in Patients with Microphthalmia Transcription Factor Family (MiT) Associated Tumors John Goldberg 1 *, George Demetri 2, Edwin Choy 3, Lee Rosen 4, Alberto
Development of Bone Metastases in Men With Prostate Cancer
 Development of Bone Metastases in Men With Prostate Cancer Explore the Causes Understand the Consequences Natural History of Prostate Cancer Progression Many prostate tumors may become castrate-resistant
Development of Bone Metastases in Men With Prostate Cancer Explore the Causes Understand the Consequences Natural History of Prostate Cancer Progression Many prostate tumors may become castrate-resistant
A Comprehensive Overview of Targeted Therapy in Metastatic Renal Cell Carcinoma
 Current Cancer Drug Targets, 2012, 12, 857-872 857 A Comprehensive verview of Targeted Therapy in Metastatic Renal Cell Carcinoma Z. Mihály 1, Z. Sztupinszki 1, P. Surowiak 2 and B. Gy rffy 1, * 1 Research
Current Cancer Drug Targets, 2012, 12, 857-872 857 A Comprehensive verview of Targeted Therapy in Metastatic Renal Cell Carcinoma Z. Mihály 1, Z. Sztupinszki 1, P. Surowiak 2 and B. Gy rffy 1, * 1 Research
Clinical Trial Designs for Incorporating Multiple Biomarkers in Combination Studies with Targeted Agents
 Clinical Trial Designs for Incorporating Multiple Biomarkers in Combination Studies with Targeted Agents J. Jack Lee, Ph.D. Department of Biostatistics 3 Primary Goals for Clinical Trials Test safety and
Clinical Trial Designs for Incorporating Multiple Biomarkers in Combination Studies with Targeted Agents J. Jack Lee, Ph.D. Department of Biostatistics 3 Primary Goals for Clinical Trials Test safety and
Relative Risk (Sokal & Hasford): Relationship with Treatment Results. Michele Baccarani
 Relative Risk (Sokal & Hasford): Relationship with Treatment Results Michele Baccarani European LeukemiaNet EVOLVING CONCEPTS IN THE MANAGEMENT OF CHRONIC MYELOID LEUKEMIA VENICE 8 9 MAY 2006 Disease risk
Relative Risk (Sokal & Hasford): Relationship with Treatment Results Michele Baccarani European LeukemiaNet EVOLVING CONCEPTS IN THE MANAGEMENT OF CHRONIC MYELOID LEUKEMIA VENICE 8 9 MAY 2006 Disease risk
Critical Pathways Forward in the Treatment of Renal Cell Carcinoma
 Critical Pathways Forward in the Treatment of Renal Cell Carcinoma Based on an Expert Summit convened in Washington, DC, July 30-31, 2012 Key Points 1. The approval of seven agents, all targeting angiogenesis,
Critical Pathways Forward in the Treatment of Renal Cell Carcinoma Based on an Expert Summit convened in Washington, DC, July 30-31, 2012 Key Points 1. The approval of seven agents, all targeting angiogenesis,
